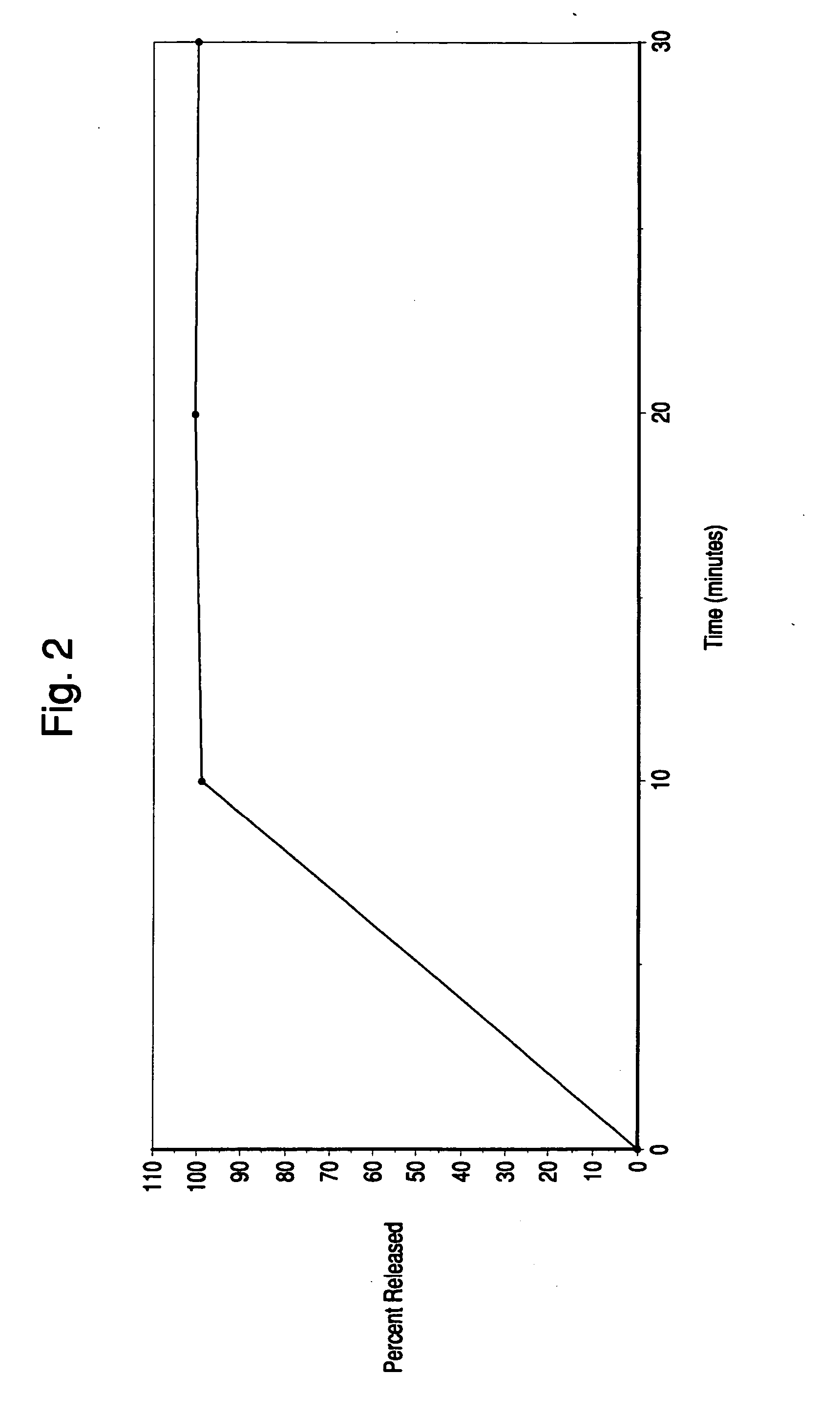
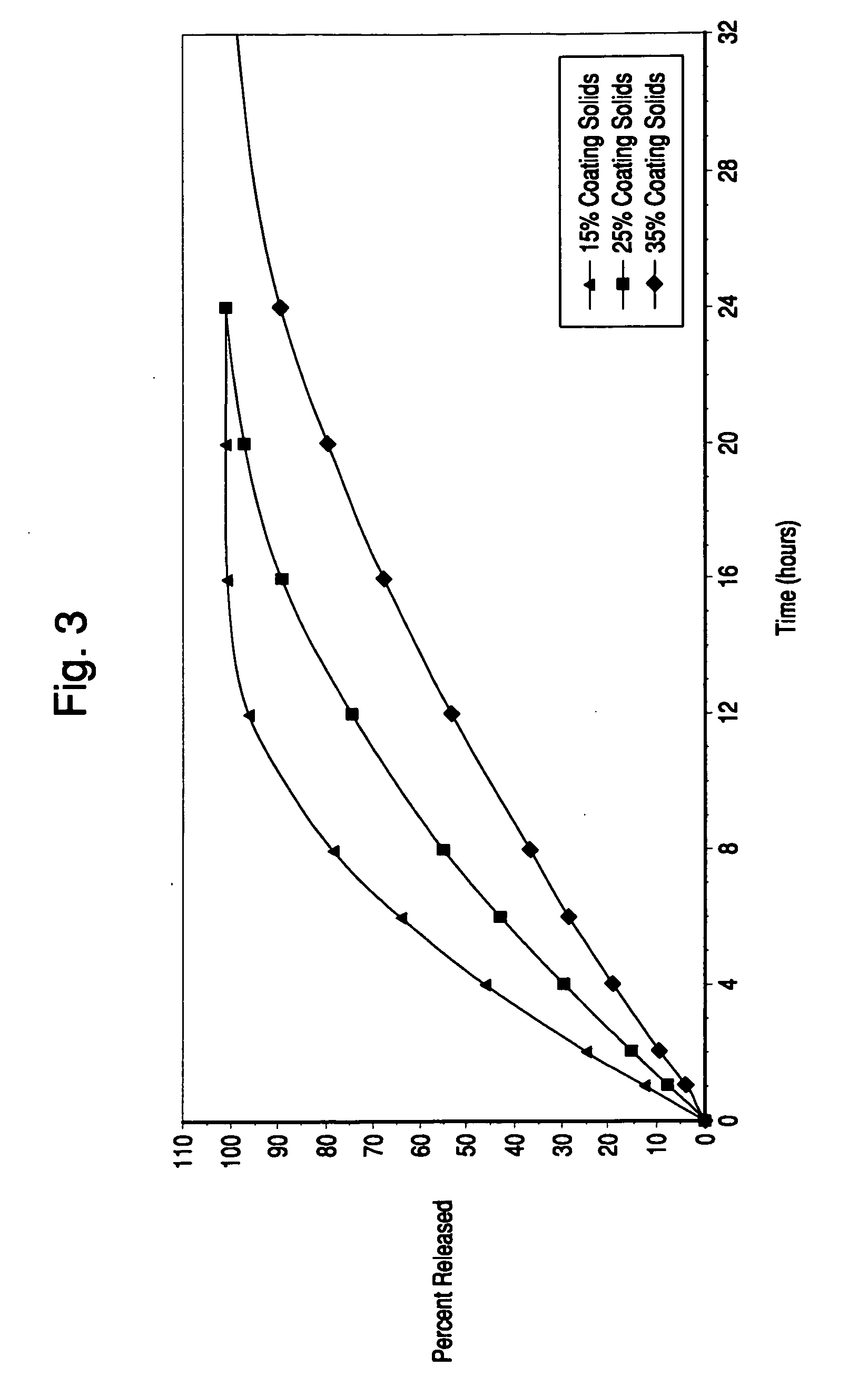
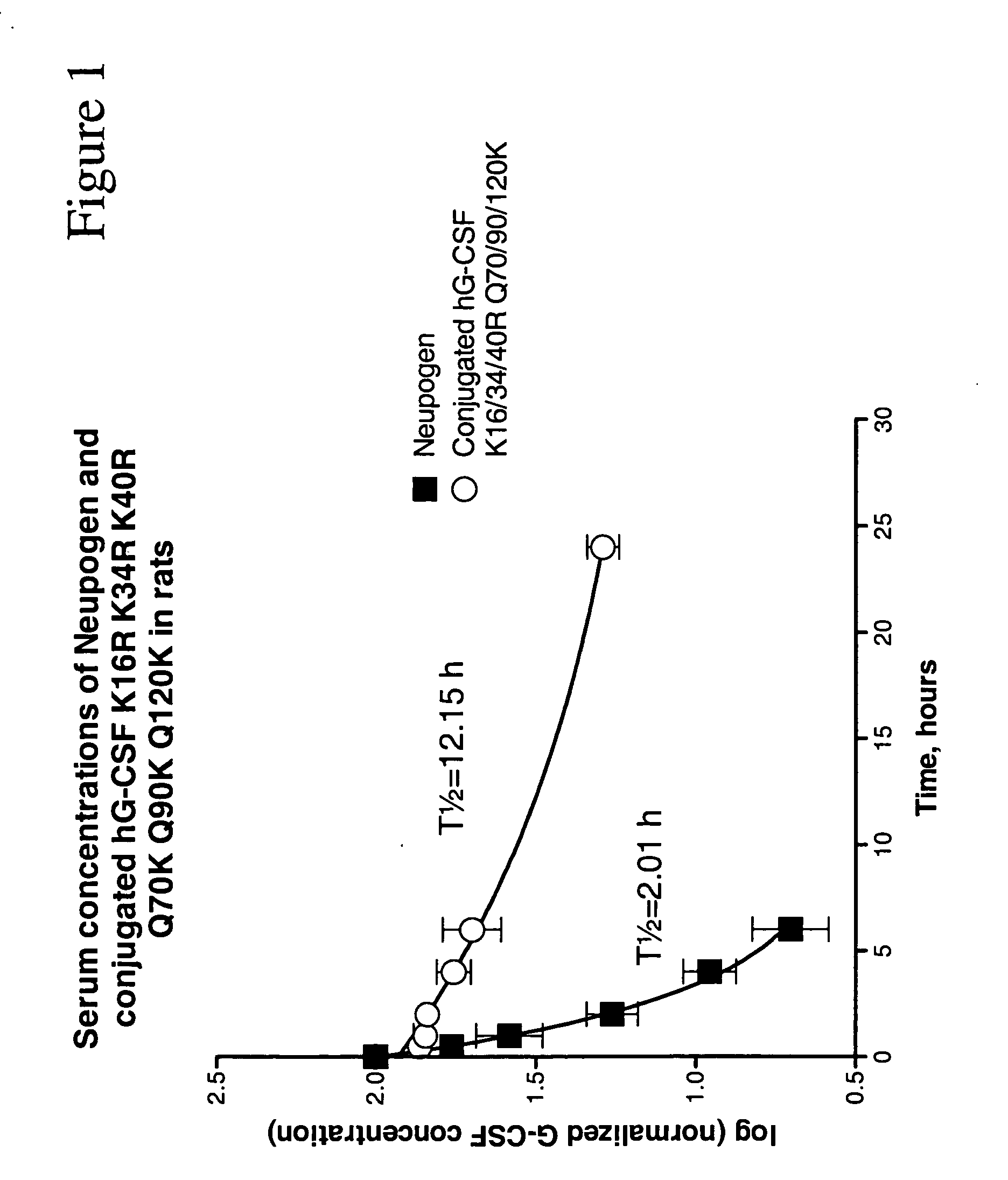
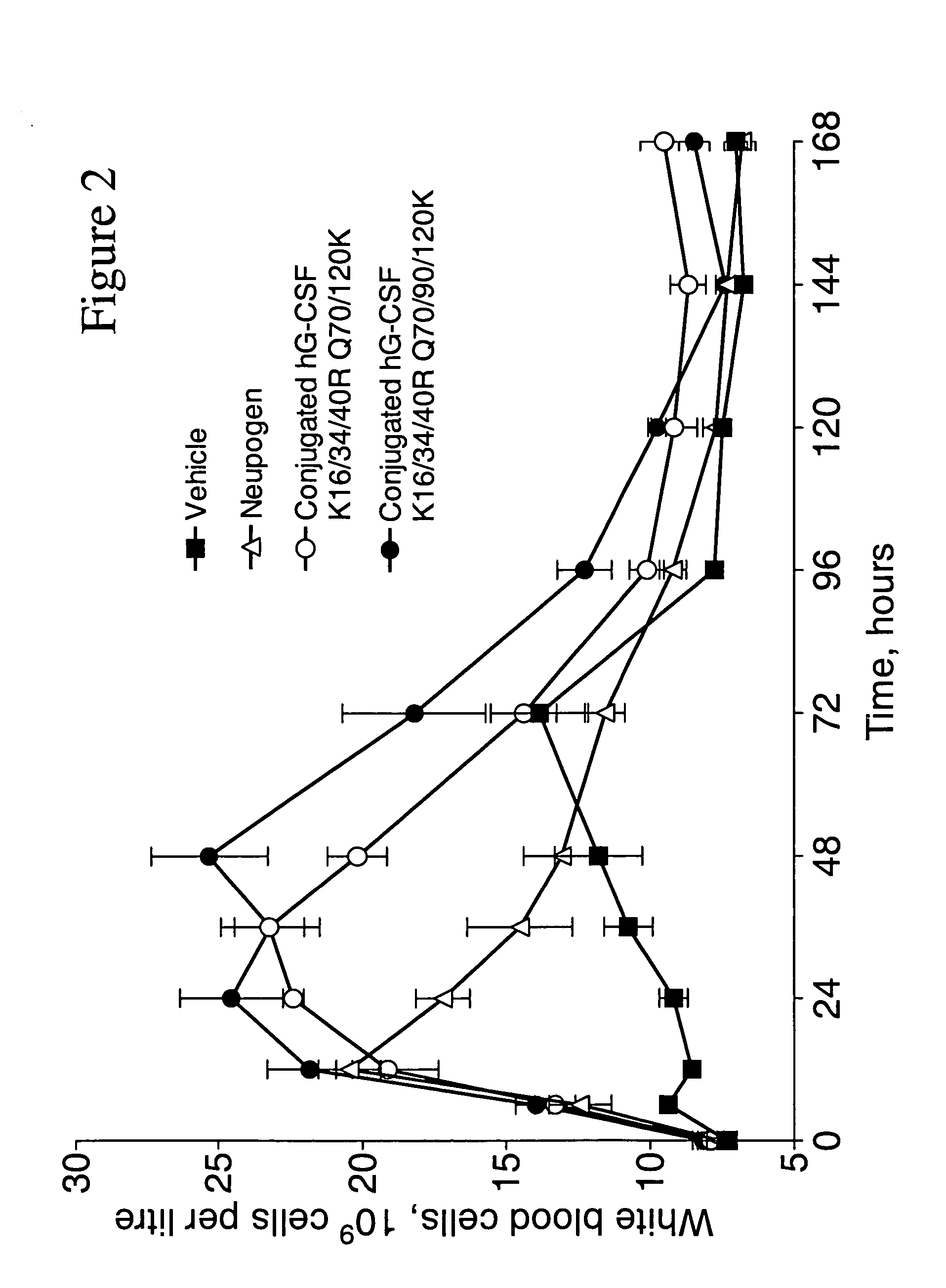
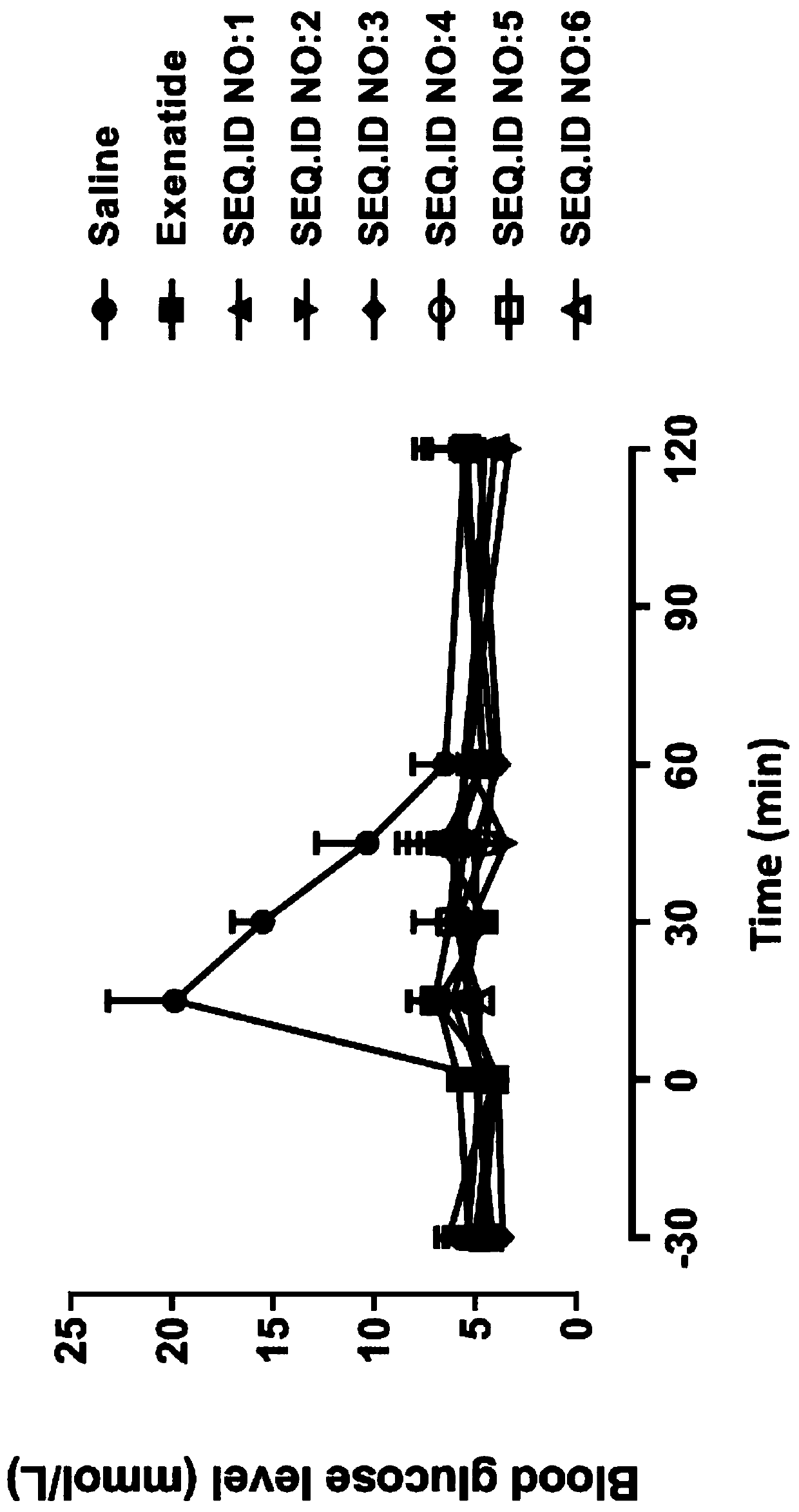
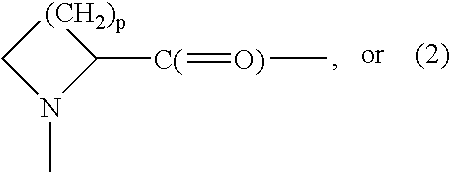Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "Biological half-life" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The biological half-life of a biological substance is the time it takes for half to be removed by biological processes. This concept is used when the rate of removal is roughly exponential. It is often denoted by the abbreviation t₁/₂. This is used to measure the removal of things such as metabolites, drugs, and signalling molecules from the body. Typically, the biological half-life refers to the body's natural cleansing through the function of the liver and through the excretion of the measured substance through the kidneys and intestines.
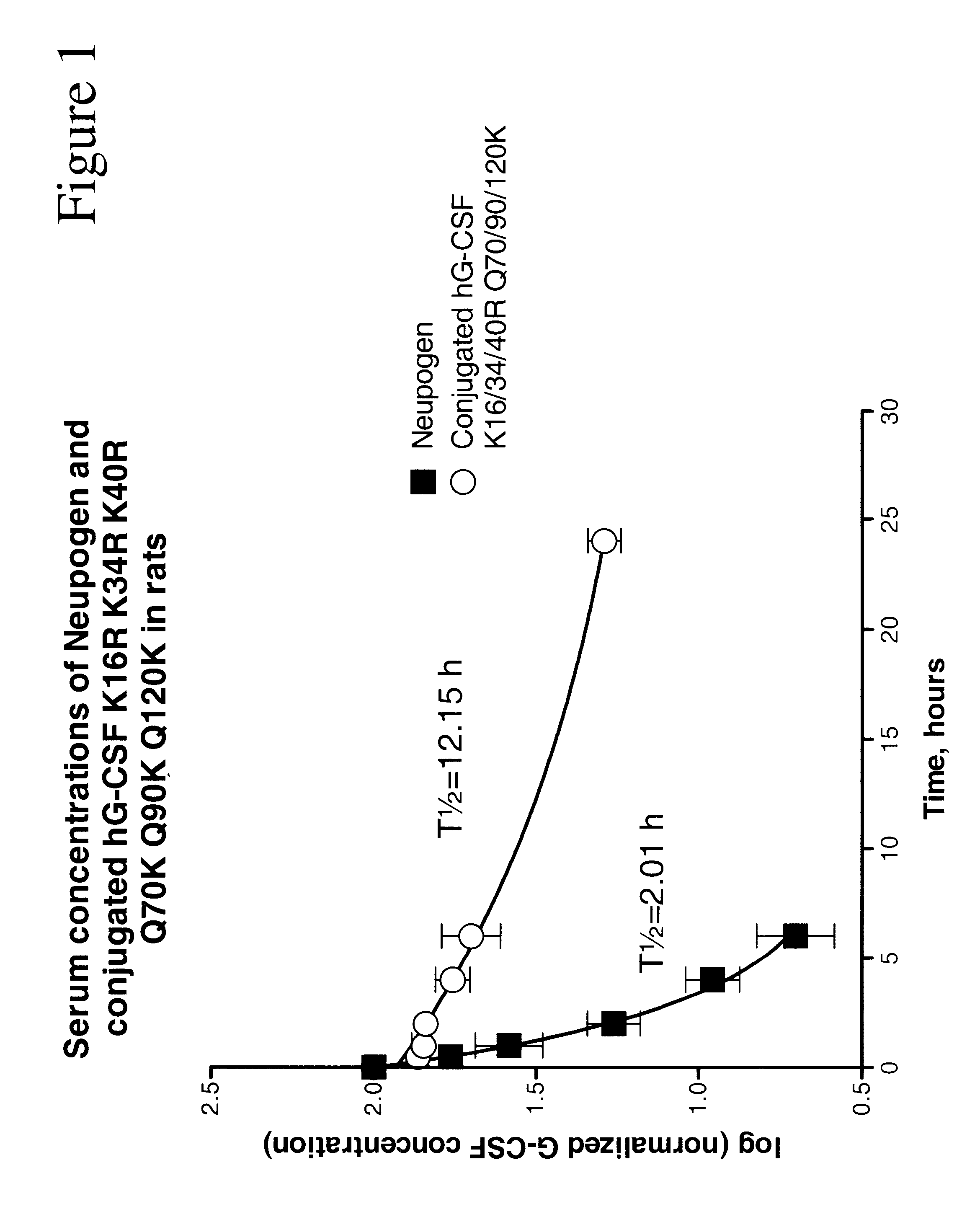
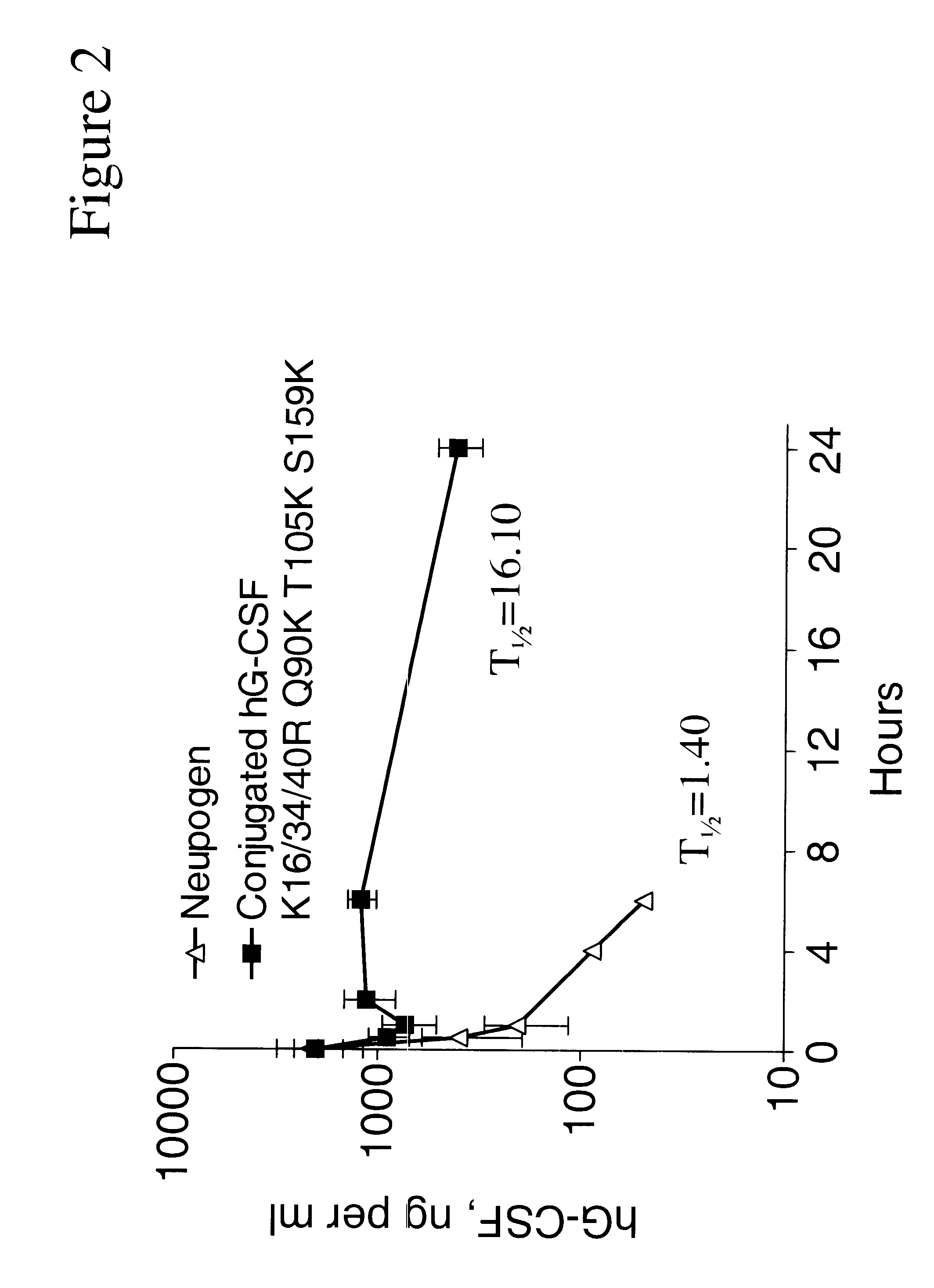
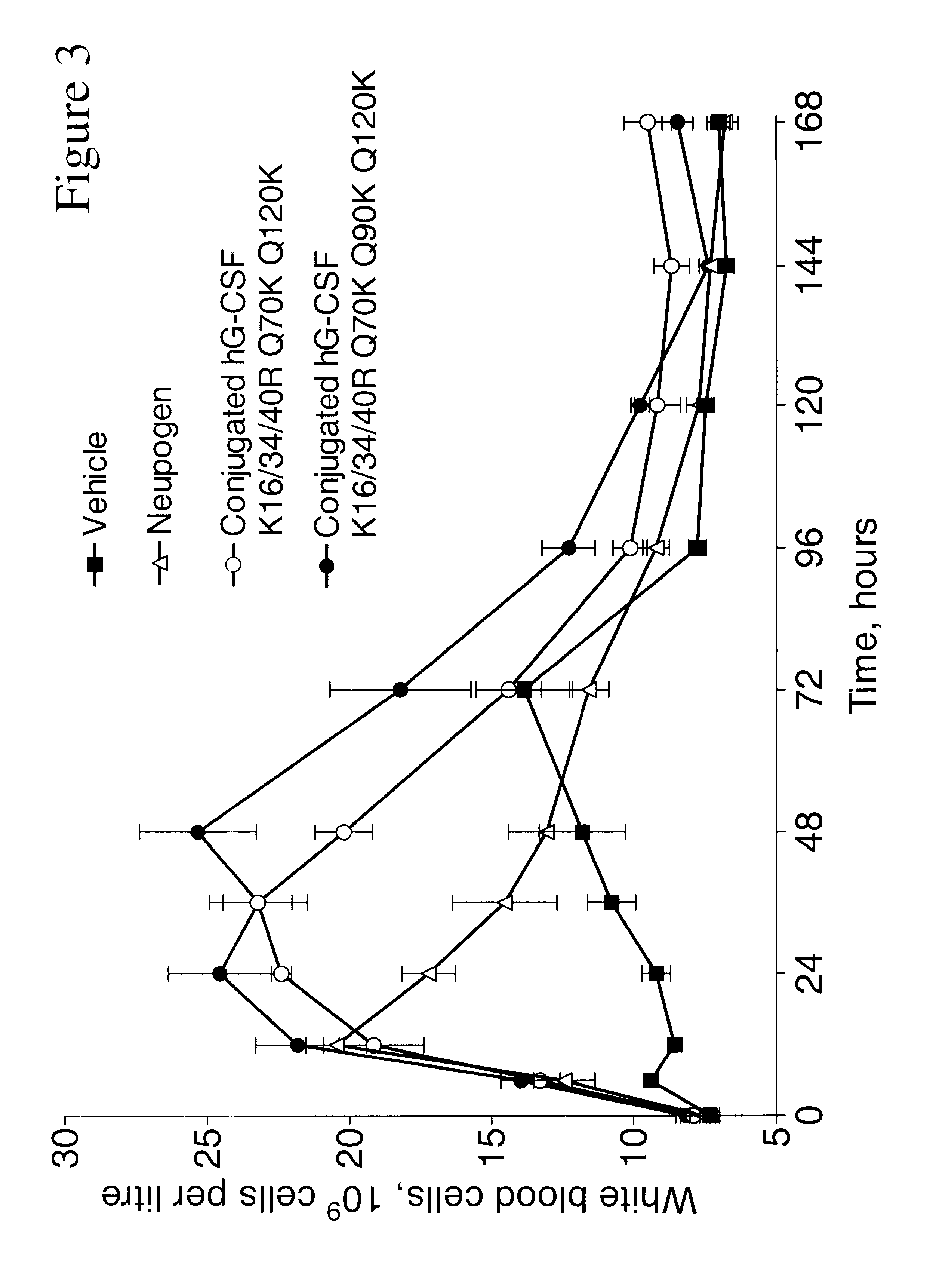
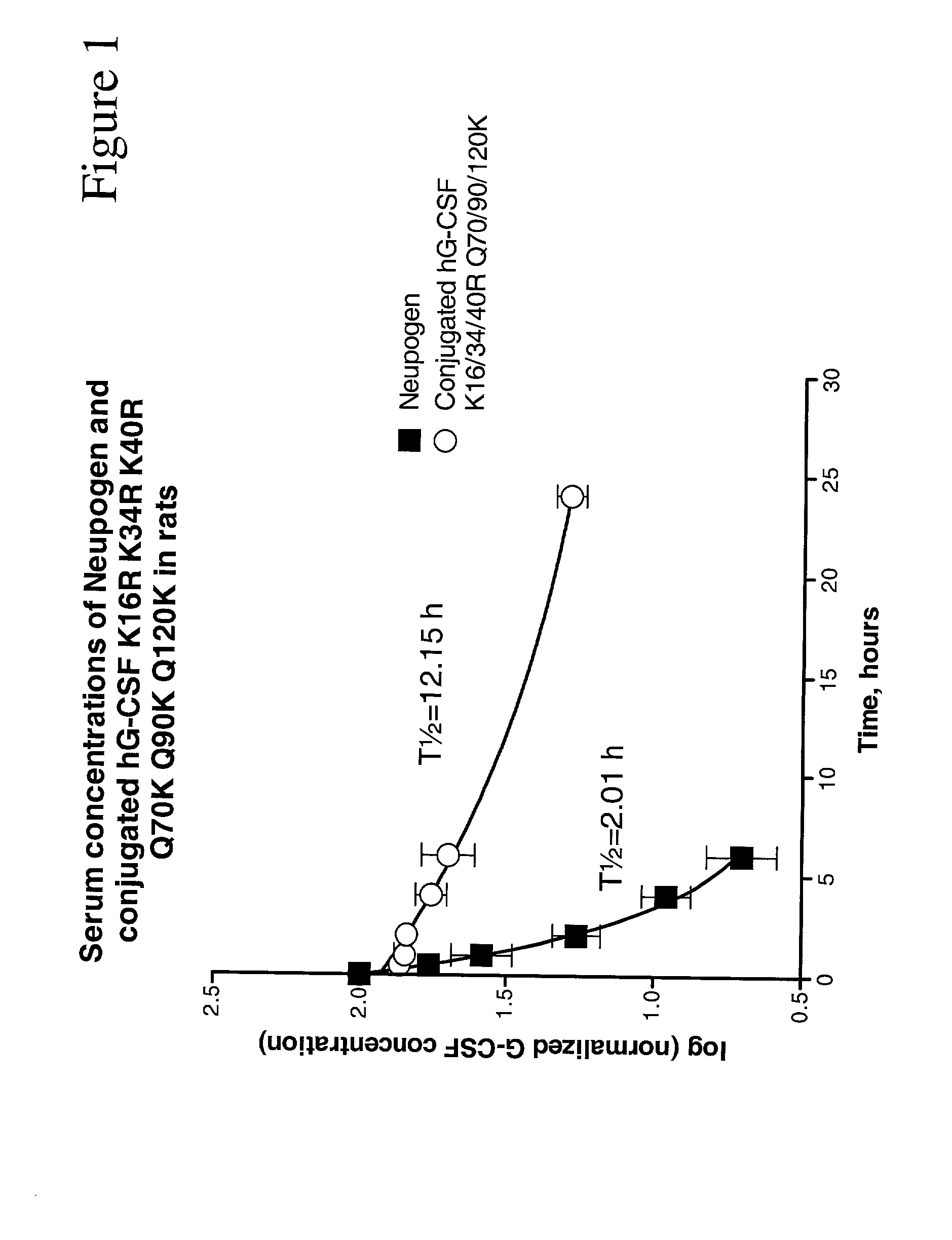
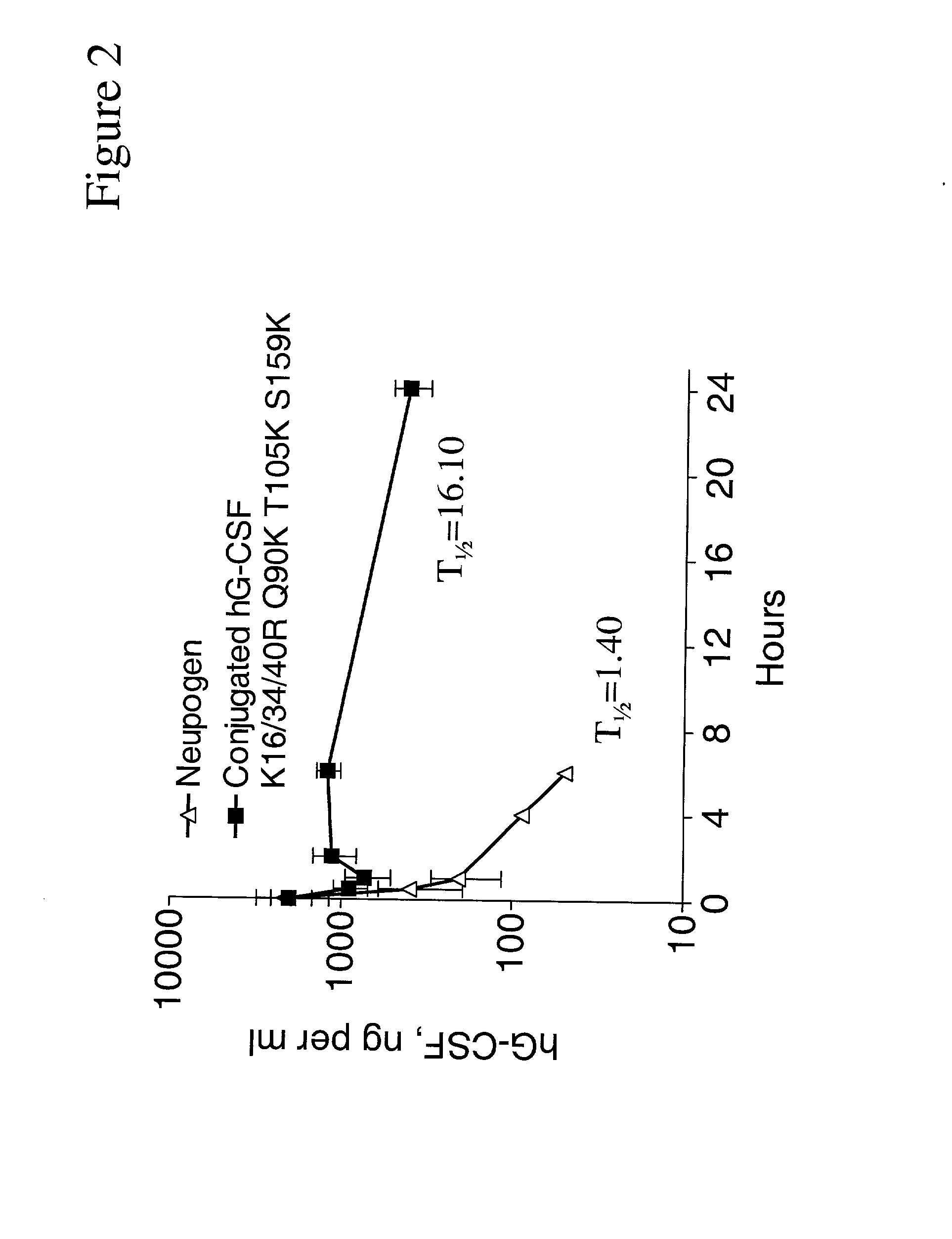
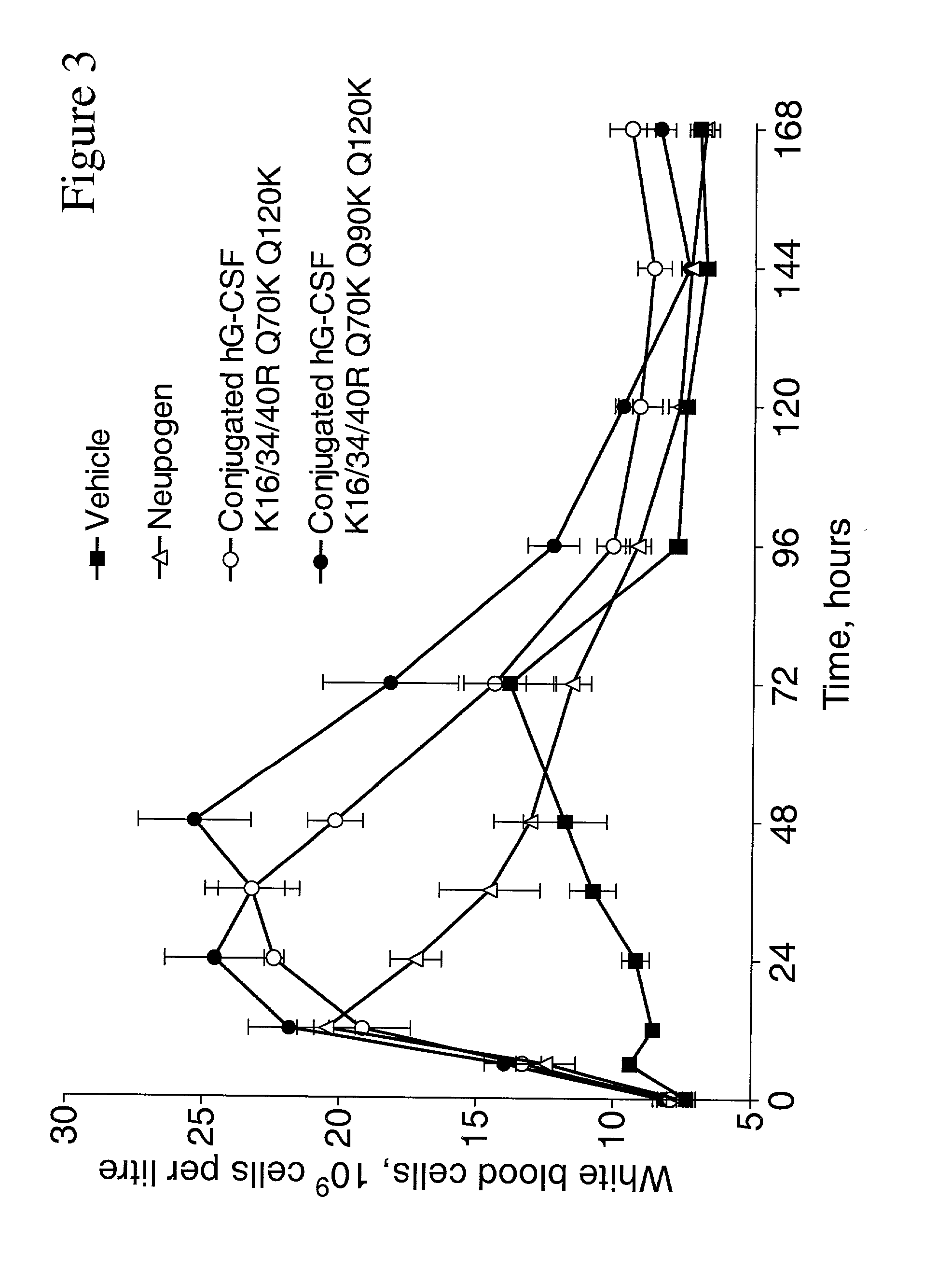
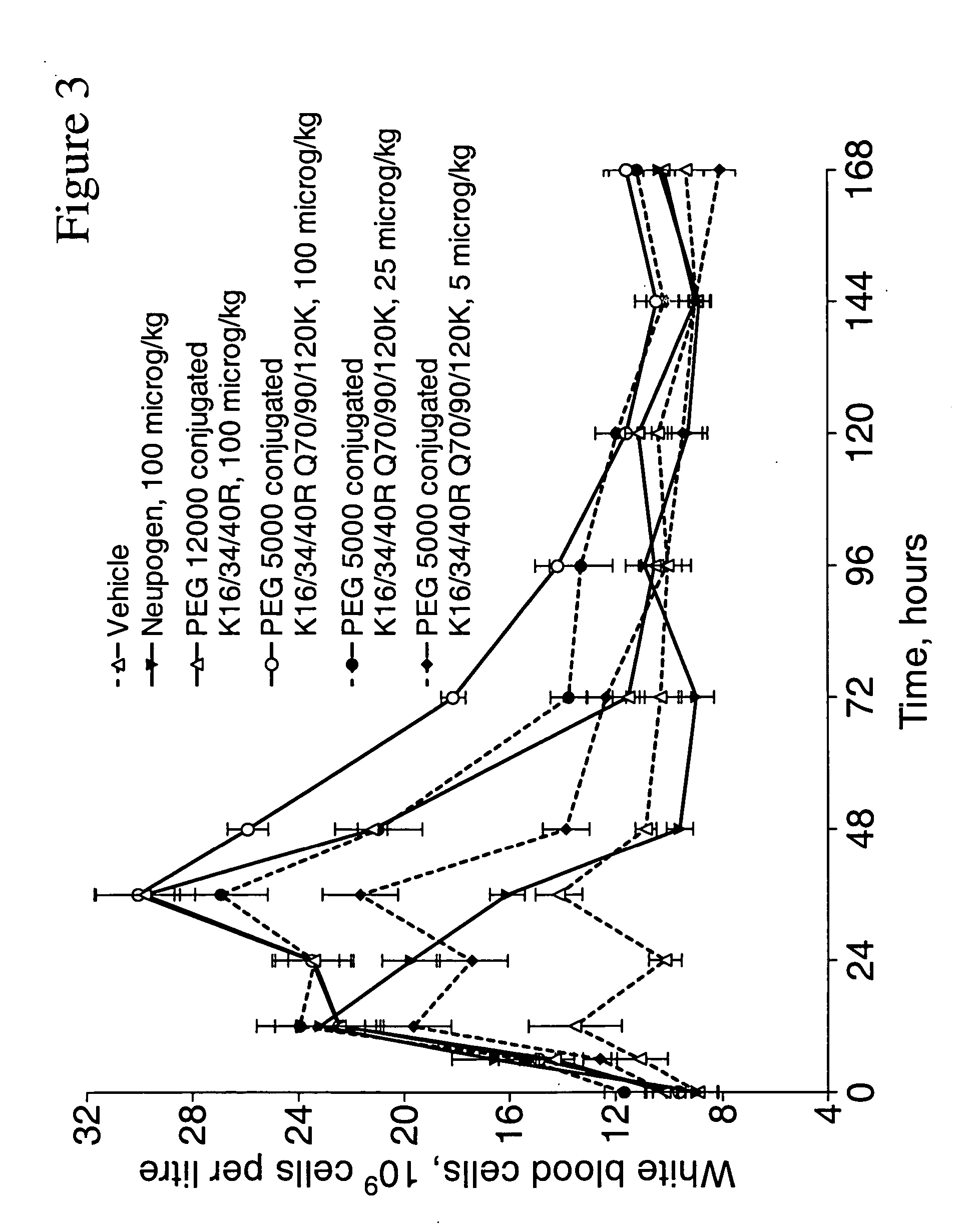
G-CSF conjugates
InactiveUS6555660B2Improved propertyReduced in vitroBiocidePeptide/protein ingredientsHalf-lifePolyethylene glycol
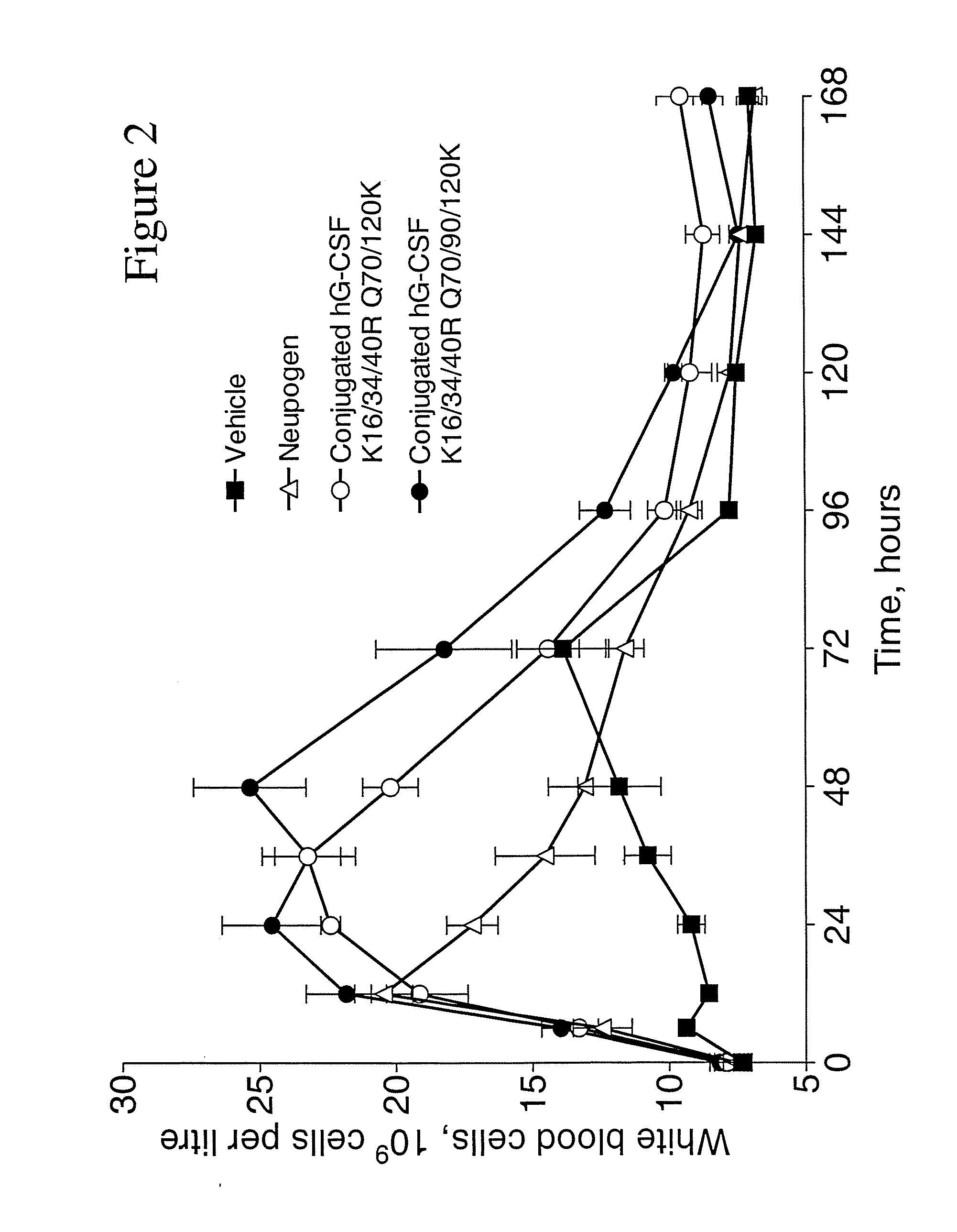
The invention relates to polypeptide conjugates comprising a polypeptide exhibiting G-CSF activity and having an amino acid sequence that differs from the amino acid sequence of human G-CSF in at least one specified introduced and / or removed amino acid residue comprising an attachment group for a non-polypeptide moiety, and having at least one non-polypeptide moiety attached to an attachment group of the polypeptide. The attachment group may e.g. be a lysine, cysteine, aspartic acid or glutamic acid residue or a glycosylation site, and the non-polypeptide moiety may e.g. be a polymer such as polyethylene glycol or an oligosaccharide. The conjugate, which has a reduced in vitro bioactivity compared to hG-CSF, has one or more improved properties such as increased biological half-life and increased stimulation of neutrophils.
Owner:MAXYGEN
Methods for glucagon suppression using modified exendins
InactiveUS7153825B2Saccharide peptide ingredientsVasoactive intestinal peptideDiseaseBiological half-life
We claim a method of lowering plasma glucagon in a subject in need thereof comprising administering to the subject a composition comprising a modified exendin or modified exendin analog, wherein said modification comprises one or more molecule linked to an exendin or the exendin analog wherein said molecule is selected from the group consisiting of polyethylene glycol, gelatin and / or albumin. The modified exendin or the modified exendin analog has activity of suppressing glucagon secretion and / or lowering glucagon levels in the subject and possesses increased biological half-life compared to unmodified exendin or unmodified exendin analog. The method is useful in treating hyperglucagonemia and other disorders that would be benefited by lowering plasma glucagon or suppressing glucagon secretion.
Owner:AMYLIN PHARMA INC
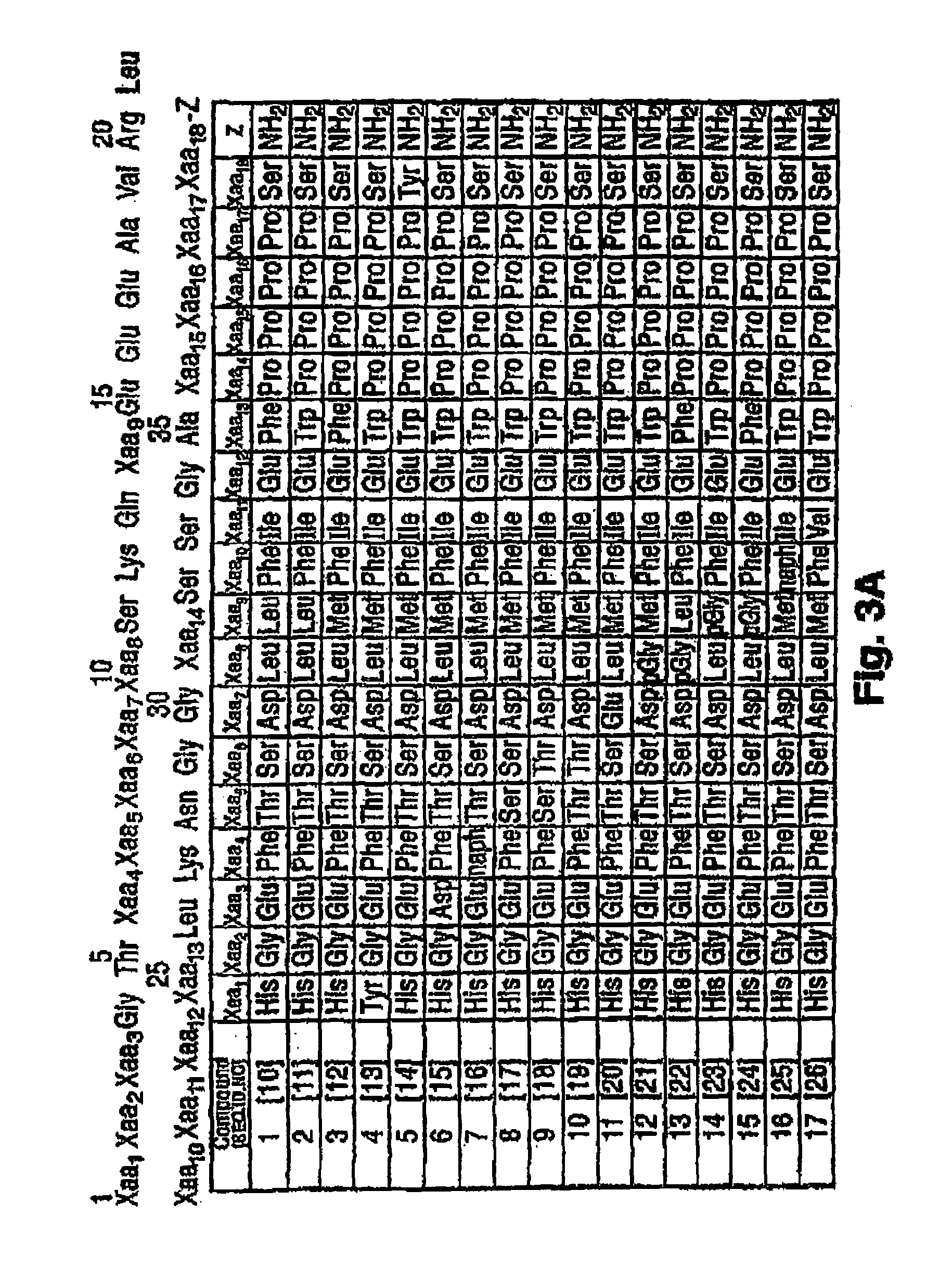
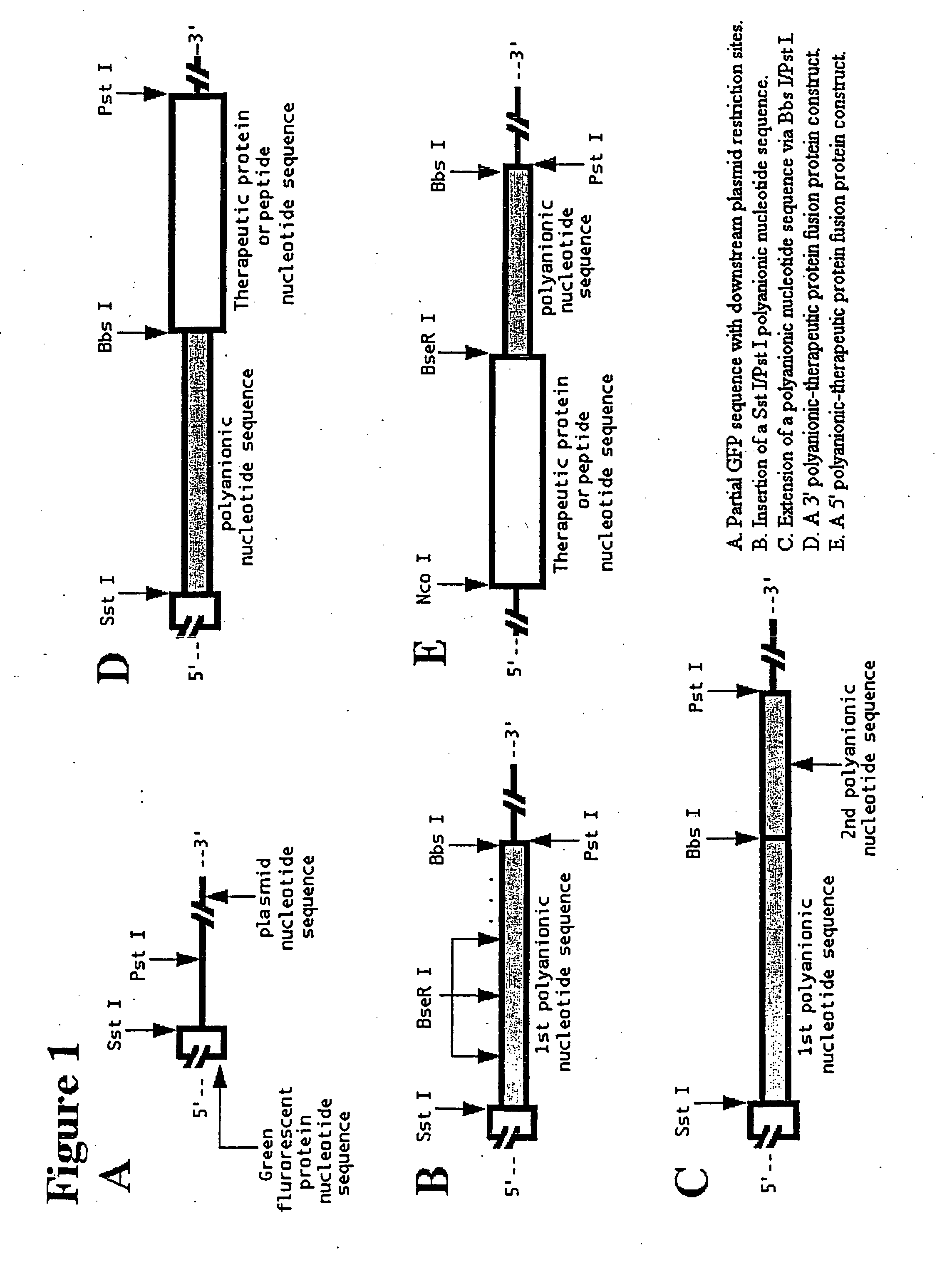
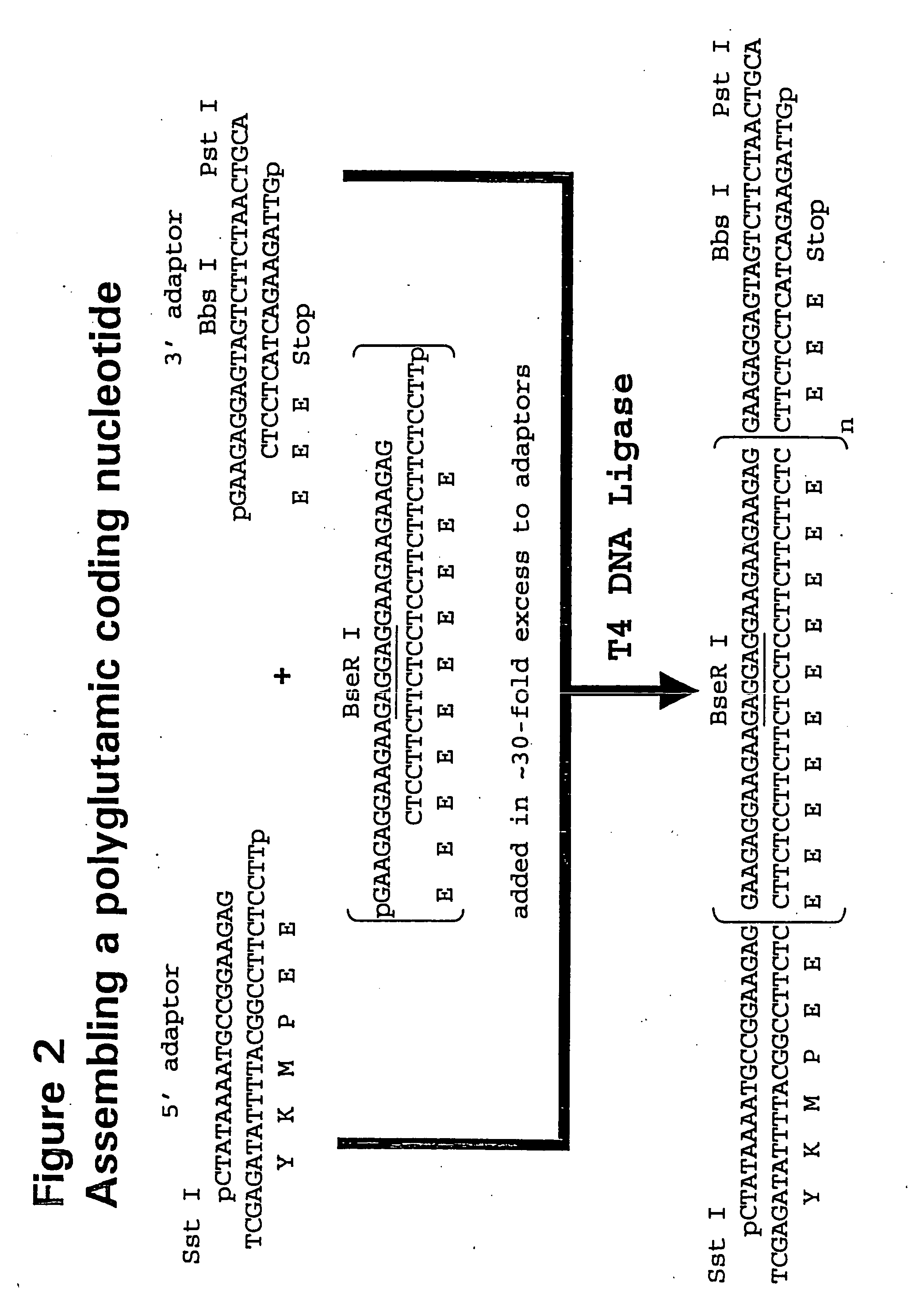
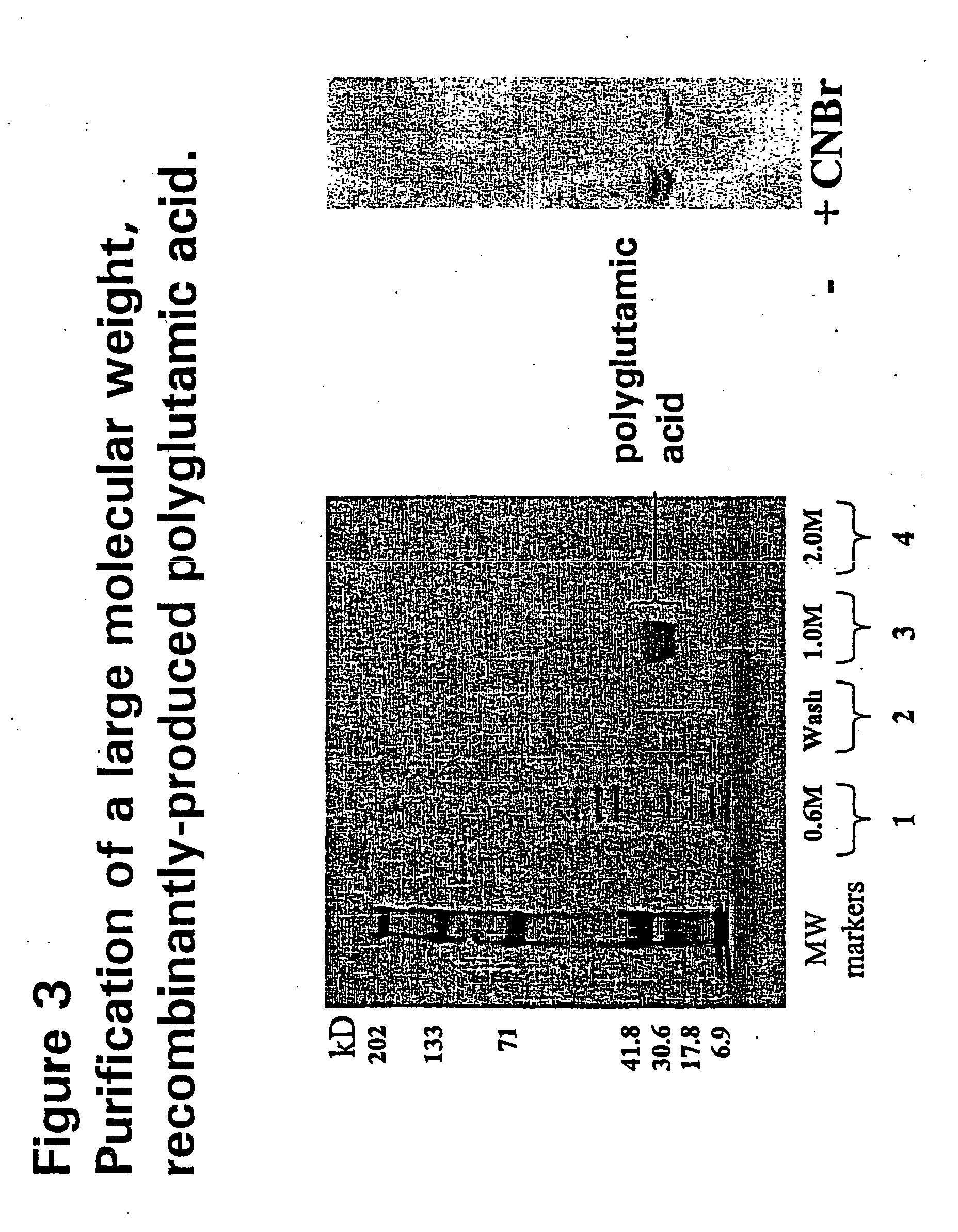
Recombinant production of polyanionic polymers, and uses thereof
InactiveUS20050118136A1Peptide-nucleic acidsPeptide/protein ingredientsSolubilityBiological half-life
A polyanionic polymer can improve the bioactivity and water-solubility properties of a drug to which it is joined. The inventive method provides a monodispersed preparation of a recombinantly-produced polyanionic polymer that can be easily manipulated, such as lengthened. An active moiety may be chemically or recombinantly joined to a polyanionic polymer to increase its biological half-life and / or solubility. The instant invention also provides a method for targeting the delivery of a polyanionic polymer conjugate or fusion protein to a specific cell type or tissue.
Owner:CELL THERAPUETICS INC
Antitumor drug PEGylation and applications of antitumor drug PEGylation in reversal of tumor multidrug resistance
InactiveCN104689330AImprove solubilityLow immunogenicityPharmaceutical non-active ingredientsAntineoplastic agentsWater insolubleHalf-life
The present invention discloses antitumor drug PEGylation and applications of the antitumor drug PEGylation in reversal of tumor multidrug resistance. The PEGylated antitumor drug is prepared by conjugating activated PEG onto an antitumor drug, and can be used for preparing the drug for reversal of tumor multidrug resistance. According to the present invention, the antitumor drugs (particularly water insoluble drugs) containing amino, carboxyl, hydroxyl and other modifiable groups are PEGylated so as to increase the water solubility, such that excellent characteristics of micelle self-assembly forming, production of invisible nanoparticles or active targeting tumor nanoparticles, drug release behavior regulation, system toxicity reducing, biological half-life improving, EPR effect production, tumor passive / active targeting production, tumor multidrug resistance reversal and the like are provided, and good clinical application prospects are provided.
Owner:SHANGHAI JIAO TONG UNIV
G-CSF conjugates
InactiveUS20030064922A1Improve propertiesIncrease stimulationPeptide/protein ingredientsPharmaceutical delivery mechanismPolyethylene glycolNeutrophil granulocyte
The invention relates to polypeptide conjugates comprising a polypeptide exhibiting G-CSF activity and having an amino acid sequence that differs from the amino acid sequence of human G-CSF in at least one specified introduced and / or removed amino acid residue comprising an attachment group for a non-polypeptide moiety, and having at least one non-polypeptide moiety attached to an attachment group of the polypeptide. The attachment group may e.g. be a lysine, cysteine, aspartic acid or glutamic acid residue or a glycosylation site, and the non-polypeptide moiety may e.g. be a polymer such as polyethylene glycol or an oligosaccharide. The conjugate, which has a reduced in vitro bioactivity compared to hG-CSF, has one or more improved properties such as increased biological half-life and increased stimulation of neutrophils.
Owner:MAXYGEN
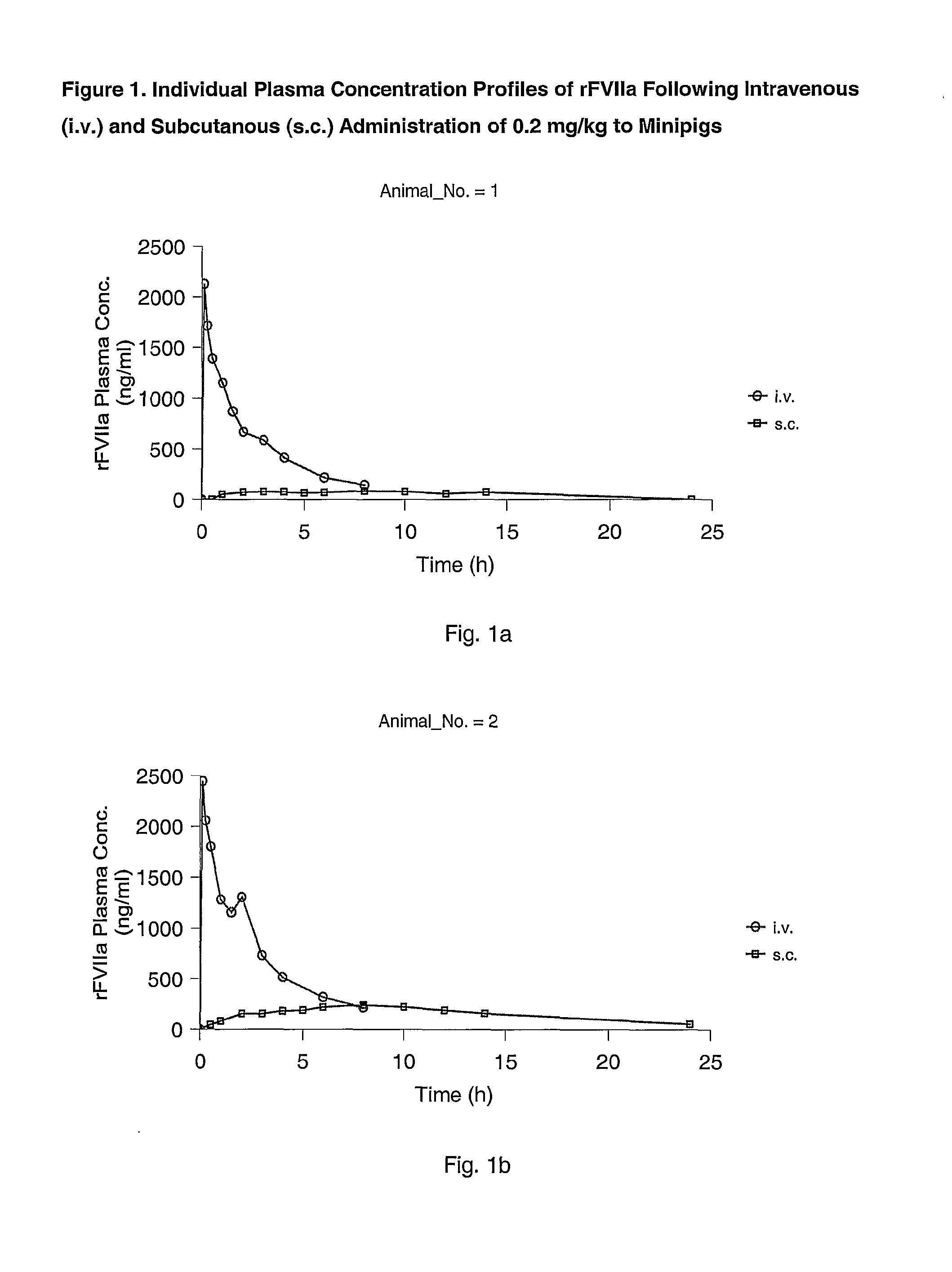
Subcutaneous administration of coagulation factor VII
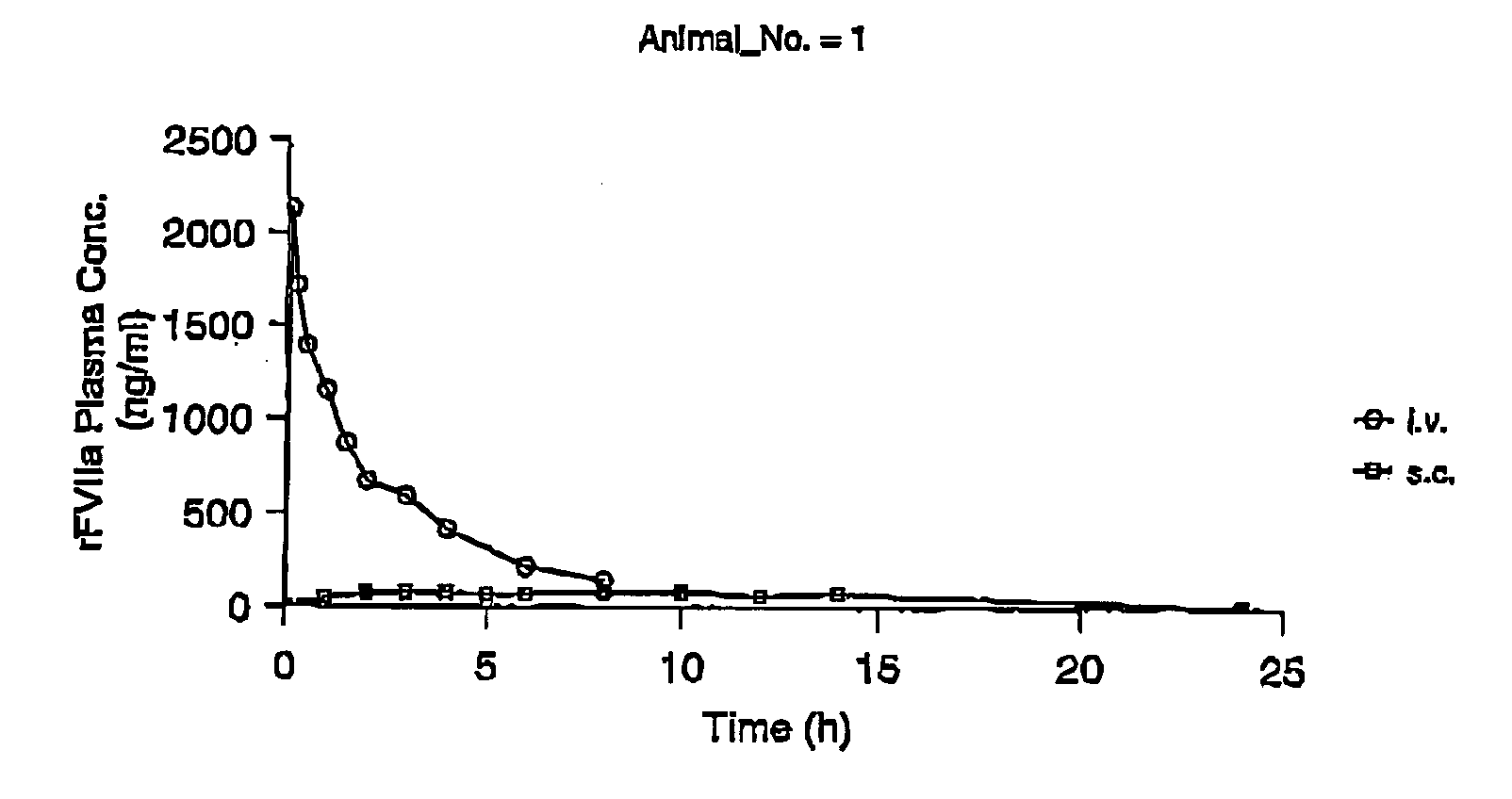
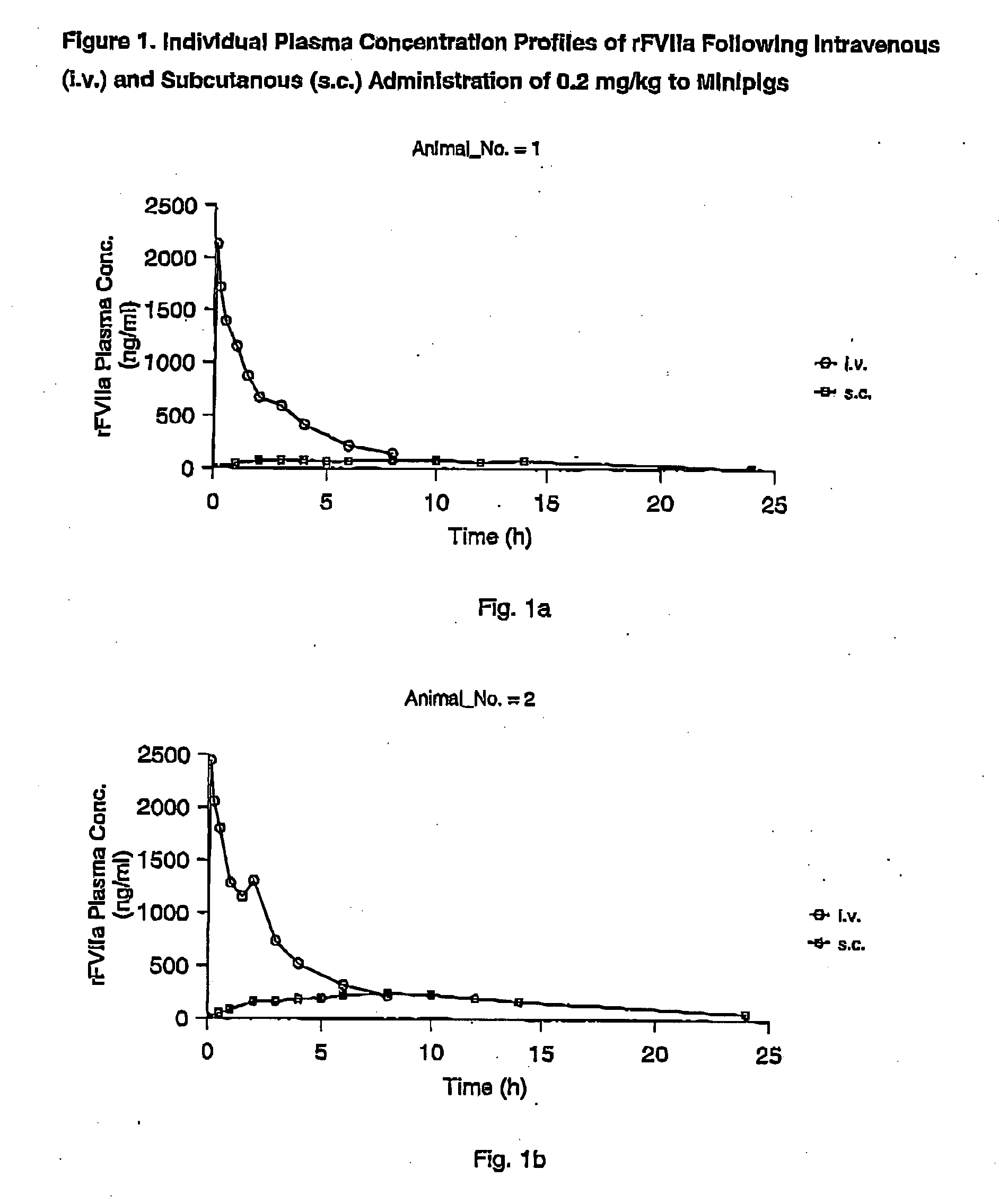
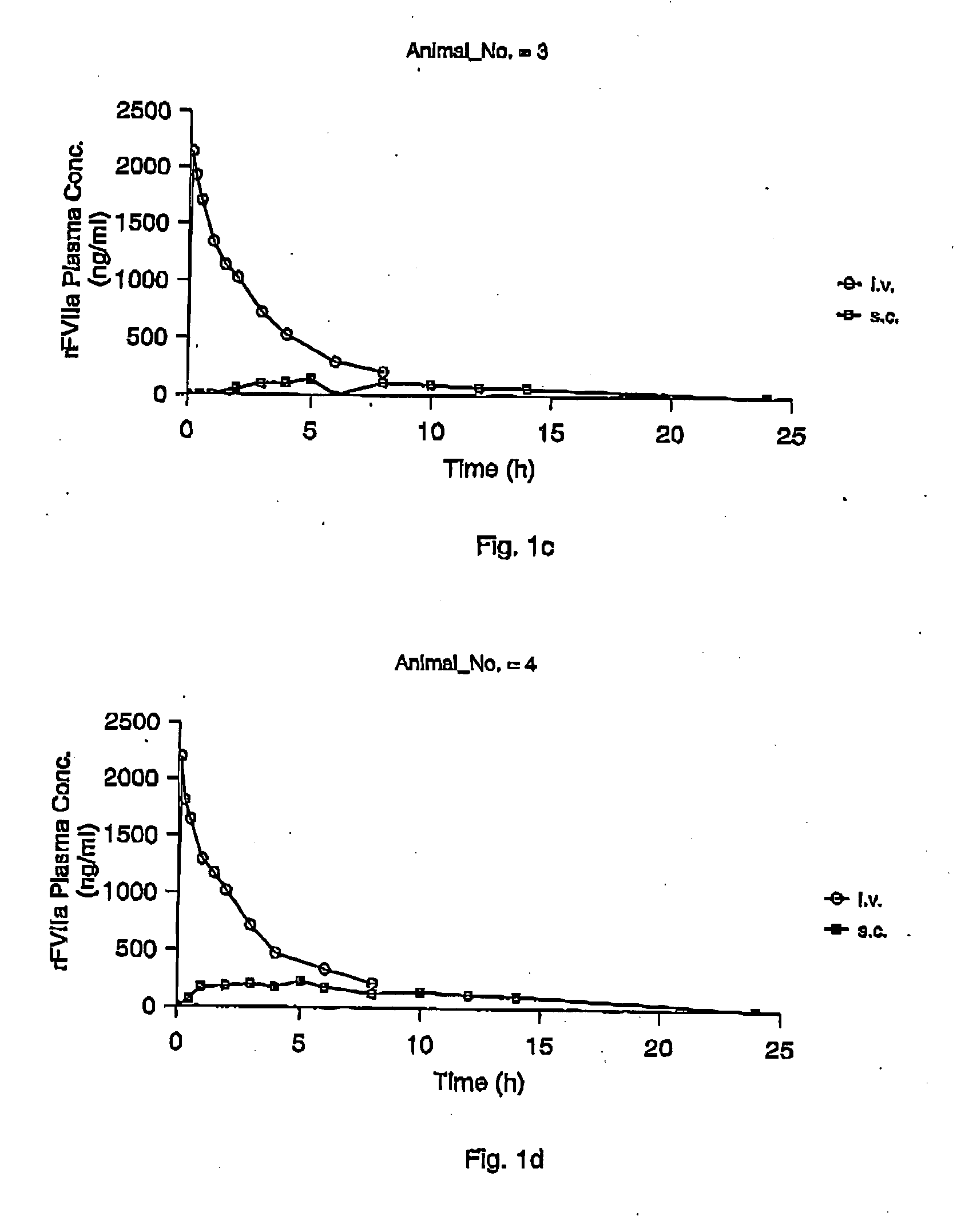
InactiveUS7786070B2Acceptable absorptionImprove the level ofPeptide/protein ingredientsPharmaceutical delivery mechanismFactor VIIaBiological half-life
The invention relates to the use of a Factor VIIa for the manufacture of a medicament for treatment of a condition affectable by Factor VIIa, said medicament being for subcutaneous, intramuscular or intradermal administration, and to the use of a Factor VIIa for the manufacture of a medicament for treatment of a condition affectable by Factor VIIa, wherein said medicament, when administered subcutaneously, intradermally or intramuscularly, shows a prolonged biological half-life.
Owner:NOVO NORDISK HEALTH CARE AG
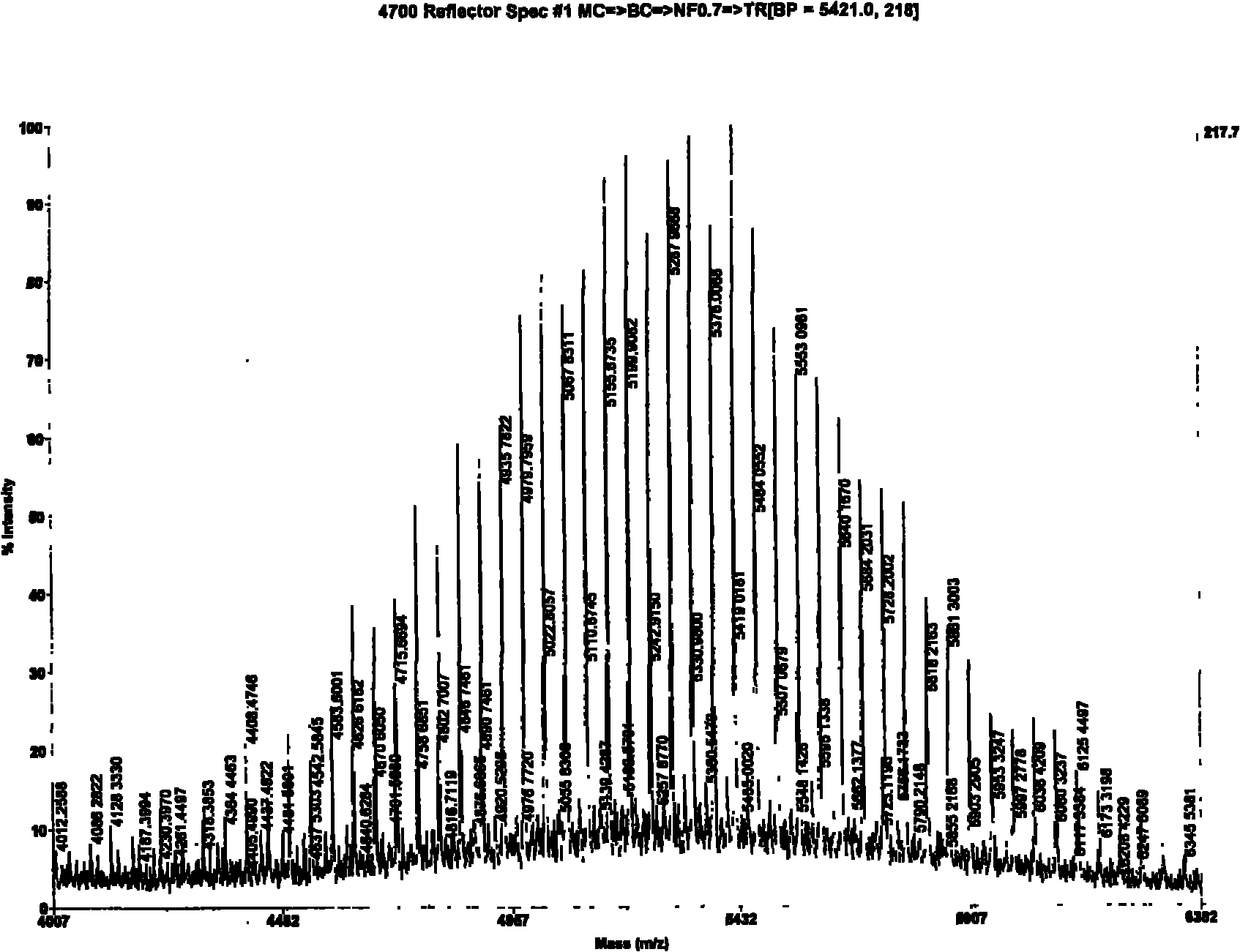
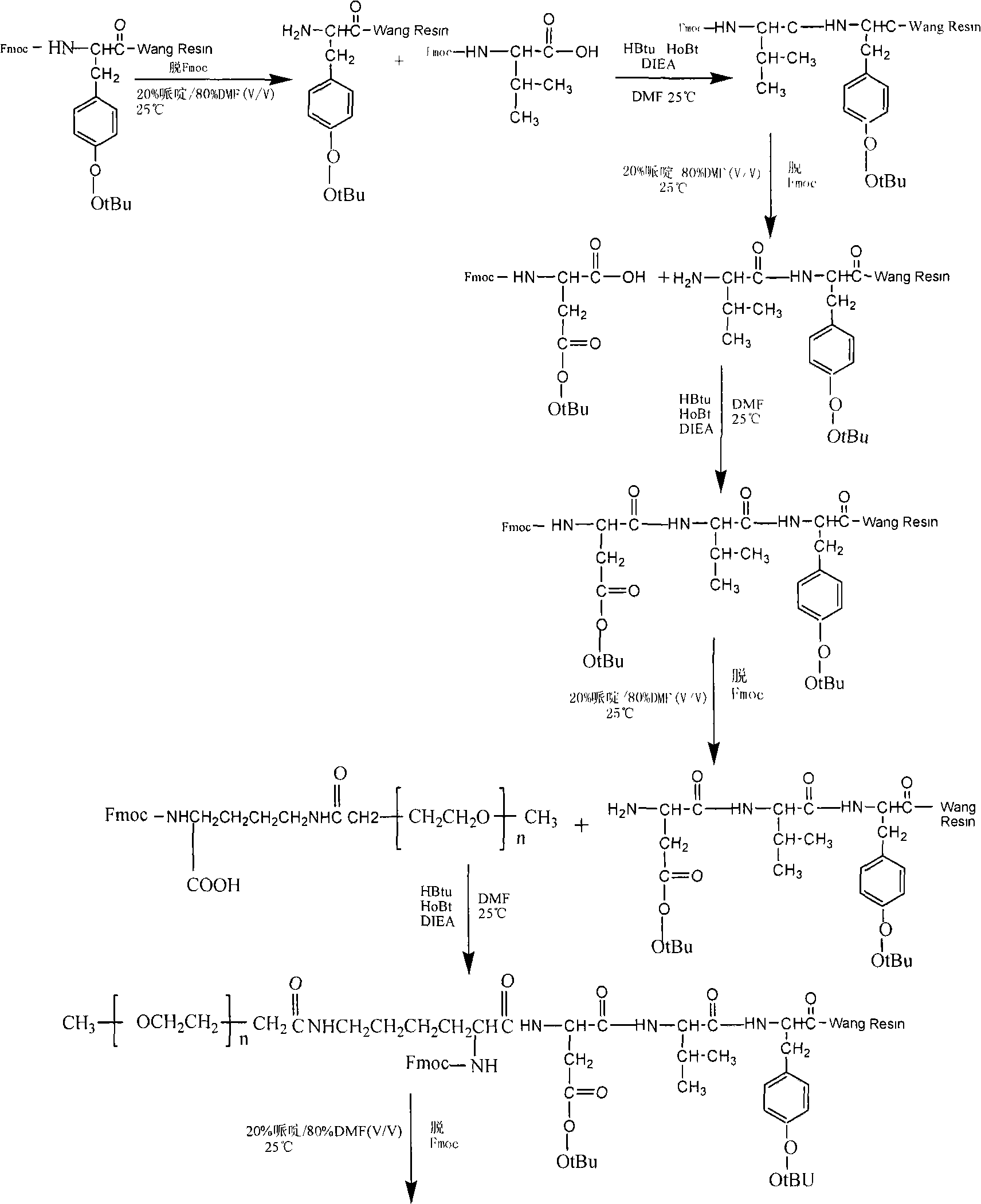
Method for synthesizing mono pegylation-thymopentin by solid phase and liquid phase combination
ActiveCN101870732APrecise structureEasy to operateCarrier-bound/immobilised peptidesSide chainMass spectrometry
The invention relates to a method for synthesizing mono pegylation-thymopentin (mPEG-TP5) by solid phase and liquid phase combination. The method comprises the following steps of: connecting modified polyethylene glycol (mPEG-SCM) to side chain amino of lysine protected by amino by adopting liquid-phase synthesis technology; synthesizing mPEG-TP5 by adopting solid-phase synthesis technology; analyzing and purifying high-efficiency liquid phase through dialysis; and identifying the product structure through nuclear magnetism and mass spectrum. The mono pegylation-thymopentin synthesized by themethod improves the defect that the conventional parent medicament thymopentin has quick biodegradation, poor digesting stability, short biological half-life and the like, and has good application prospect.
Owner:LANZHOU UNIVERSITY
Slow-released dosage form of sodium ferulate and preparation process thereof
InactiveCN1385151AReduce releaseReduce the number of dosesPharmaceutical delivery mechanismAnhydride/acid/halide active ingredientsBiological half-lifeGlycerol
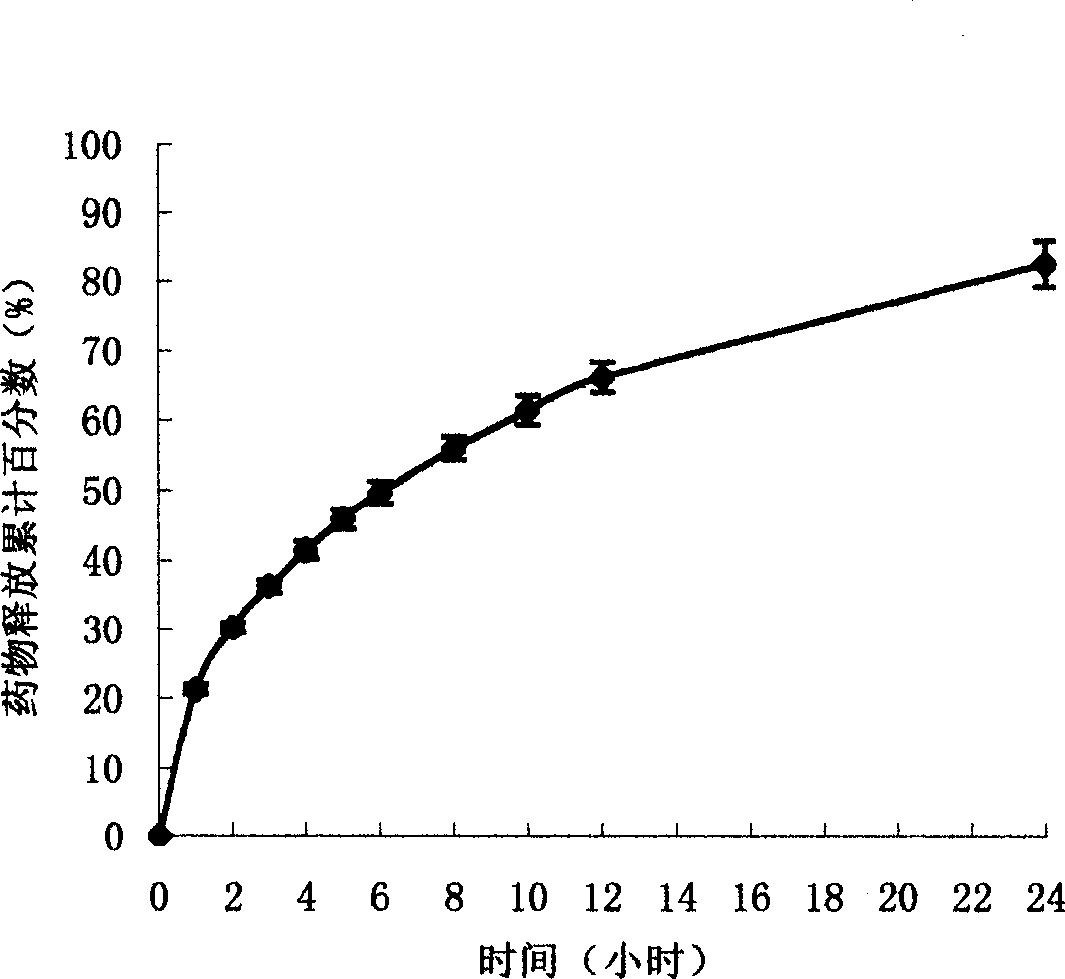
The present invention uses hydroxypropyl methylcellulose or glycerol behenate as slow-seleased auxiliary material, and adopts tableting process and solid dispersion technique to make sodium ferulate into various slow-released dosage forms whose slow-releasing effect can be up to 12-24 hr, so as to reduce frequency of administration and make blood medicine concentration retain smoothly and stably in longer time.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Orally administrable extended release pellet and tablet formulations of a highly water soluble compound
InactiveUS20070134315A1Suitable for oralOral convenienceBiocidePill deliveryOral medicationActive component
Pharmaceutical compositions comprising an extended release formulation of active compounds effective in the treatment of various pathological conditions are provided. More particularly, the invention provides methods of making and using extended release formulations comprising active compounds that present formulation challenges such as short biological half-life, instability, highly water soluble and / or high dose requirements. Specifically, orally administrable extended release pellet and tablet formulations of isovaleramide are preferred.
Owner:SUPERNUS PHARM INC
G-CSF polypeptides and conjugates
InactiveUS20060084793A1Improve propertiesProlong half-life in vivoPeptide/protein ingredientsPharmaceutical delivery mechanismCysteine thiolateSide effect
The invention relates to polypeptide conjugates comprising a polypeptide exhibiting G-CSF activity and having an amino acid sequence that differs from the amino acid sequence of human G-CSF in at least one specified introduced and / or removed amino acid residue comprising an attachment group for a non-polypeptide moiety, and having at least one non-polypeptide moiety attached to an attachment group of the polypeptide. The attachment group may e.g., be a lysine, cysteine, aspartic acid or glutamic acid residue or a glycosylation site, and the non-polypeptide moiety may e.g., be a polymer such as polyethylene glycol or an oligosaccharide. The conjugate has one or more improved properties such as increased biological half-life and reduced side effects.
Owner:MAXYGEN HLDG
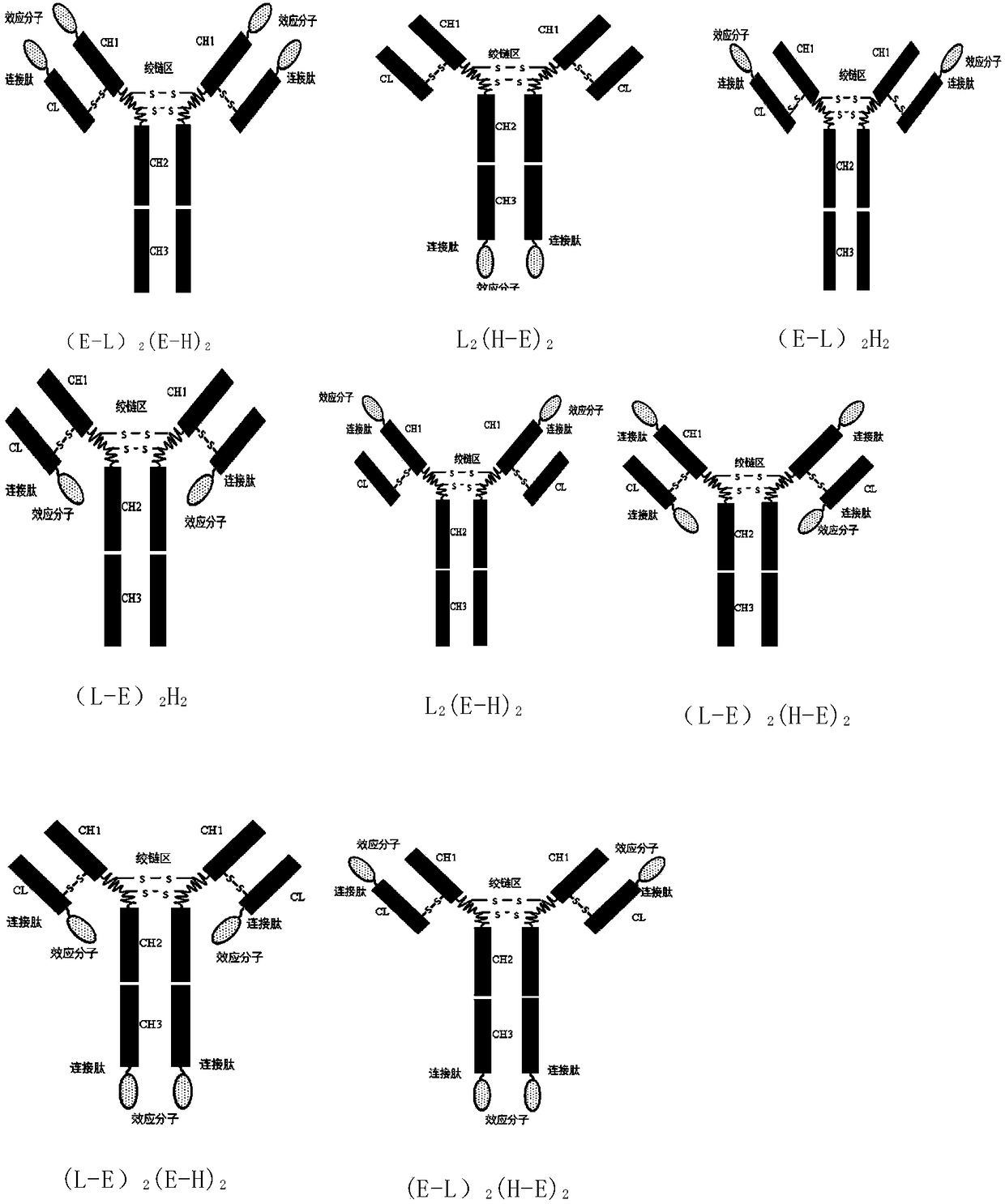
IgG-like long-acting immunological fusion protein and applications thereof
ActiveCN108623691AProlong biological half-lifeHigh affinityPeptide/protein ingredientsMetabolism disorderDiseaseAutoimmune disease
The invention discloses an IgG-like long-acting immunological fusion protein and applications thereof. The IgG-like long-acting immunological fusion protein comprises an effector molecule and an IgG antibody constant region, wherein the effector molecule is linked to the IgG antibody constant region through a linker peptide, the effector molecule is a protein capable of exerting physiological functions in vivo, and the IgG antibody constant region is a structure obtained by removing two heavy chain variable regions and two light chain variable regions from an IgG antibody. According to the present invention, the IgG-like immunological fusion protein can effectively prolong the biological half-life of the protein drug (effector molecule) under the premise of the ensuring of the high affinity to the targeting molecule and the good in vivo activity, is far better than the similar Fc immunological fusion protein, and can be used for the treatment of diabetes, tumors, autoimmune diseases, endocrine and various diseases.
Owner:BEIJING BIYANG BIOTECH
Fusion polypeptides and methods of use thereof
InactiveUS20150175675A1Increased glycosylationImprove stabilityTumor rejection antigen precursorsPeptide/protein ingredientsDiseaseAntibody fragments
The present invention also provides fusion polypeptides with a carboxy-terminal or N-terminal peptide domain (e.g., Fc, CTP, or Fc-CTP), and nucleic acid molecules encoding these polypeptides. The present invention further provides for methods of using the compositions of the invention for treatment of cancer and fibrotic diseases. The present invention also provides isolated polypeptides with a carboxy-terminal peptide (CTP) domain fused to an antibody fragment, and nucleic acid molecules encoding these polypeptides. The present invention also provides isolated polypeptides with a carboxy-terminal peptide (CTP) domain fused to the ectodomain of a receptor, and nucleic acid molecules encoding these polypeptides. Also provided are isolated fusion polypeptide molecules, with an isolated polypeptide attached to a carboxy terminus of a second polypeptide, and to nucleic acid molecules encoding these isolated fusion polypeptide molecules. Finally, methods of increasing a biological half-life of a polypeptide, methods of stabilizing a polypeptide, pharmaceutical compositions including the polypeptides and fusion polypeptides, and methods of treating or preventing a disorder using these polypeptides are provided
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Carbasalate calcium sustained release preparation and preparation method thereof
InactiveCN108014077AThe "peak valley" fluctuation of blood drug concentration is smallAvoid side effectsOrganic active ingredientsAntipyreticMedicineBiological half-life
The invention provides a carbasalate calcium sustained release preparation and a preparation method thereof. The carbasalate calcium sustained release preparation comprises the following components byweight percentage: 20-80% of carbasalate calcium, and 10-60% of a sustained release material, wherein the sustained release material is selected from one or more of an erodible skeleton material, aninsoluble skeleton material and an enteric material. The preparation method includes: heating the sustained release material till complete dissolution, then mixing the dissolved sustained material with carbasalate calcium, and putting the mixture into a swing machine for granulation. The sustained release preparation provided by the invention solves the problem of short biological half-life periodof carbasalate calcium itself, and has long-term antipyretic and fever-reducing effects.
Owner:珠海天凯生物科技有限公司
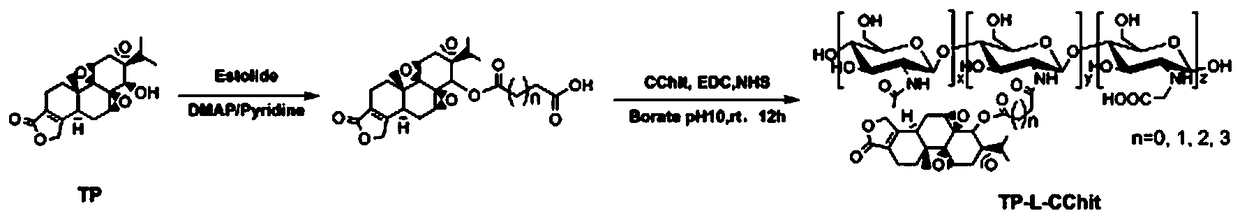
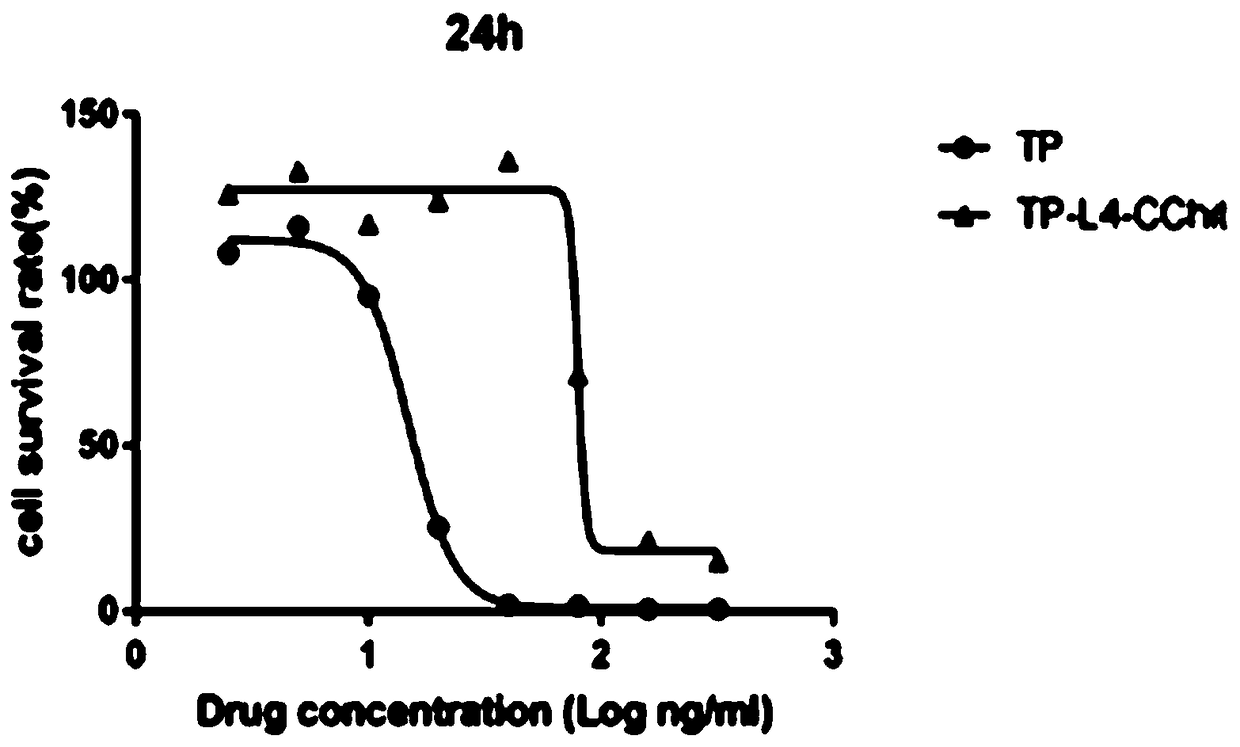
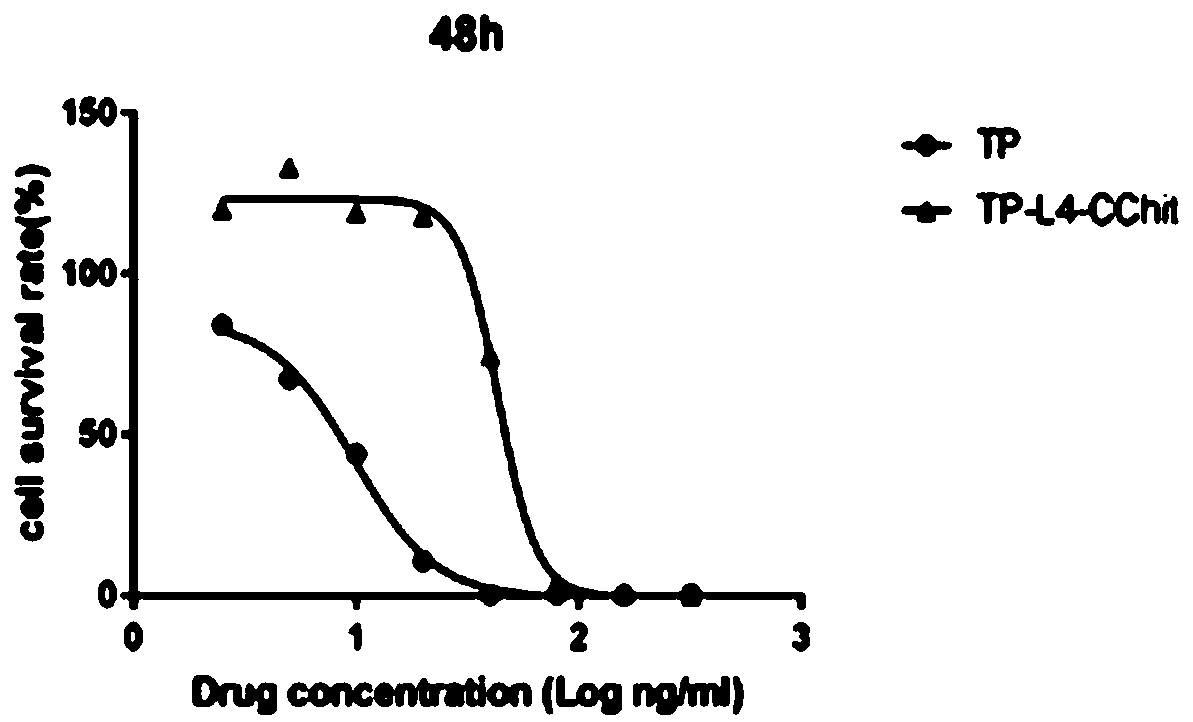
Preparation method and application of triptolide-carboxylation chitosan coupling drug
ActiveCN109464675ASimple methodEasy to produceOrganic active ingredientsNervous disorderSolubilityDisease
The invention discloses a preparation method and application of a triptolide-carboxylation chitosan coupling drug. The preparation method comprises the following steps: (1) synthesis of intermediate carboxylation triptolide (TP-L); and (2) synthesis of the triptolide-carboxylation chitosan coupling drug (TP-L-CChit). The triptolide-carboxylation chitosan coupling drug (TP-L-CChit) prepared by themethod, as a pro-drug, has application for preparing drugs for treating rheumatoid arthritis, tumors and Alzheimer's disease; the drug is simple in method, easy for production preparation, high in yield and rich and easily obtained in raw materials, the product is high in water solubility, long in biological half life period, high in availability, free of toxic or side effect and good in curativeeffect and is innovation for preparing drugs for treating rheumatoid arthritis, tumors and Alzheimer's disease, and the economic and social benefits are obvious.
Owner:HENAN UNIV OF CHINESE MEDICINE
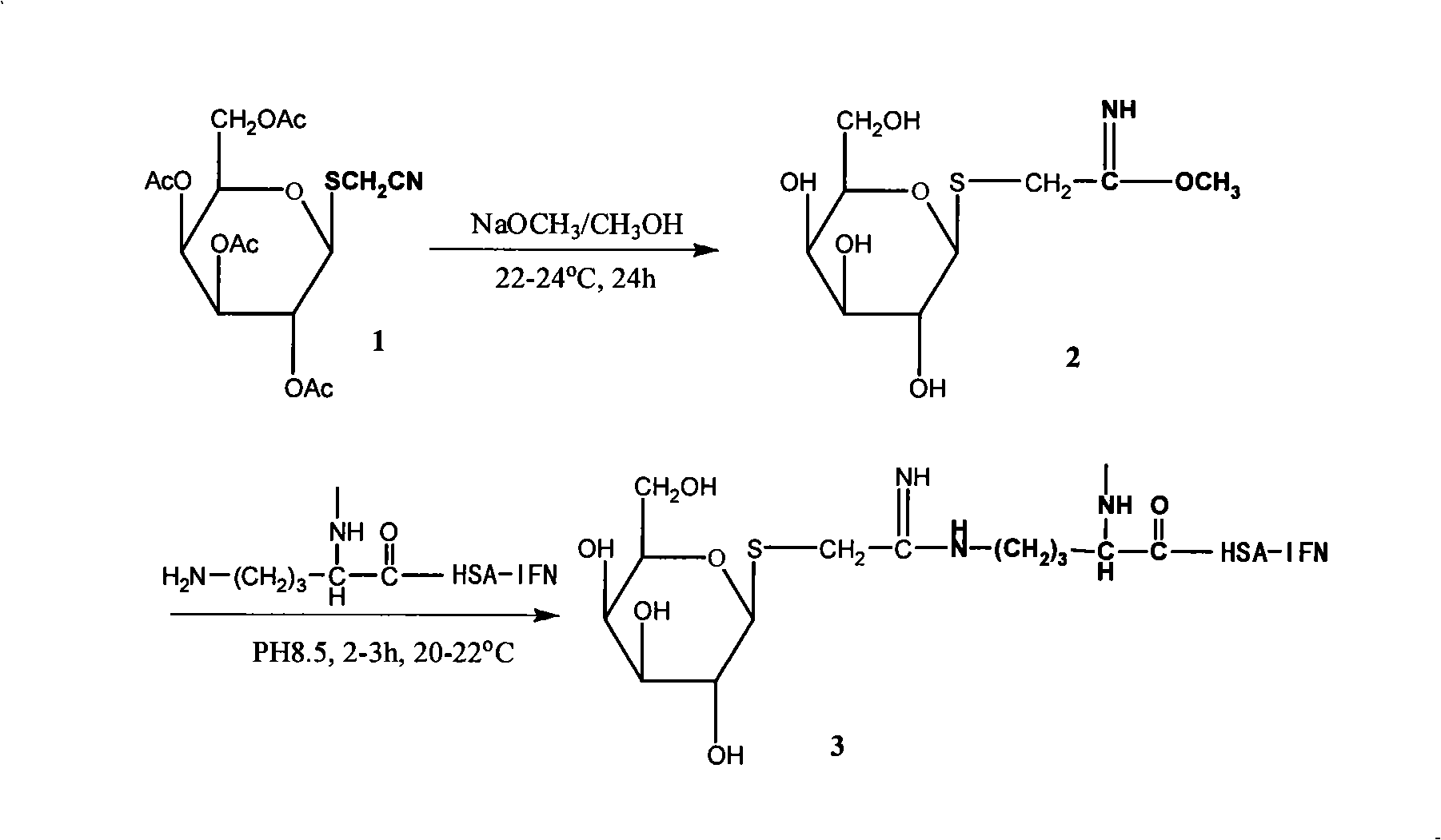
Preparation and use of galactosylated human serum albumin fused interferon
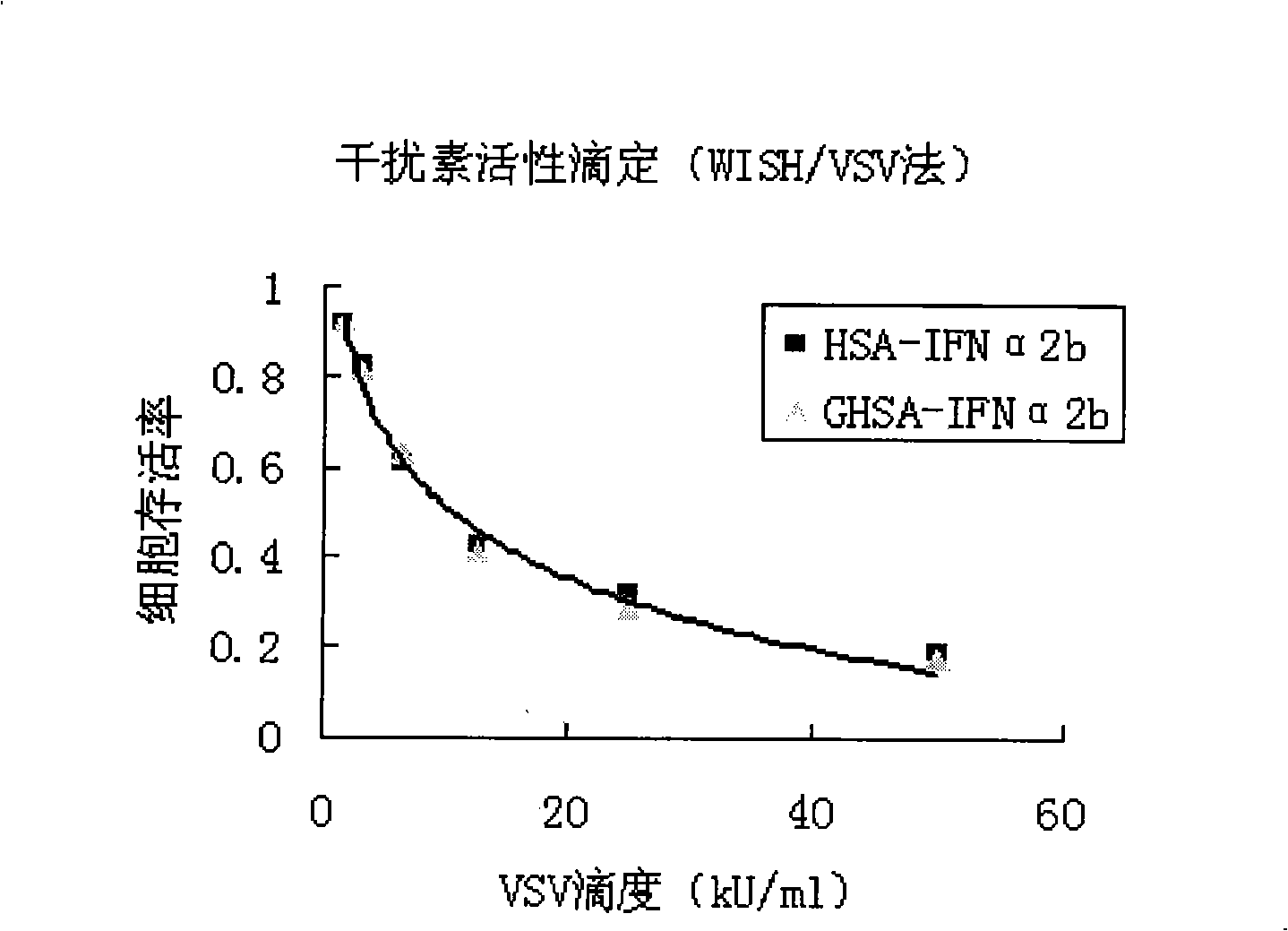
InactiveCN101328220AReduce the rate of degradation in the bodyProlong biological half-lifePeptide/protein ingredientsDigestive systemHalf-lifeInterferon alpha
The invention relates to a preparation method and an application of a galactosyl proserum fused interferon, belonging to the targeted biological medicine technical field. The invention takes a proserum fused interferon as a galactosyl precursor, can provide enough sites for glycosylation, and then more effectively prepare the galactosyl proserum fused interferon with different sugar densities (between 7 and 35). Compared with the proserum fused interferon, the compound has obvious liver targeting character and the same pharmacological activity, is hopeful to be used for preparing a novel virus hepatitis targeted therapeutic medicine, obviously improves the medicine concentration of IFN in the liver, prolongs the biological half-life of the galactosyl proserum fused interferon, effectively reduces the dosage of the clinic IFN, eases the pain of patients due to repeated injection, reduces generation of side reaction, greatly reduces the treatment cost of the patients, improves the treatment level of virus hepatitis in China, promotes excellent health of Chinese people, and has good social benefit and significant economic benefit.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
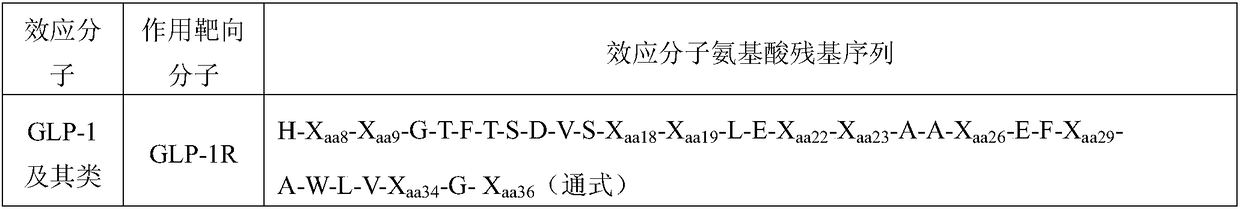
GLP-1(7-37) polypeptide analog
InactiveCN106608915ALonger duration of action than liraglutideLong duration of actionPeptide/protein ingredientsMetabolism disorderHalf-lifeBiological half-life
The present invention relates to a new GLP-1(7-37) analog, which has mutated A8S and mutated V33R, wherein the polypeptide analog has high biological activity and long biological half-life after the modification. The present invention further relates to a fatty acid modifier containing the new GLP-1(7-37) analog and applications of the fatty acid modifier in diabetes treatment.
Owner:BEIJING KAWIN TECH SHARE HLDG
Subcutaneous administration of coagulation Factor VII
InactiveUS20080145914A1Acceptable absorptionImprove the level ofPeptide/protein ingredientsPharmaceutical delivery mechanismFactor VIIaBiological half-life
The invention relates to the use of a Factor VIIa for the manufacture of a medicament for treatment of a condition affectable by Factor VIIa, said medicament being for subcutaneous, intramuscular or intradermal administration, and to the use of a Factor VIIa for the manufacture of a medicament for treatment of a condition affectable by Factor VIIa, wherein said medicament, when administered subcutaneously, intradermally or intramuscularly, shows a prolonged biological half-life.
Owner:NOVO NORDISK AS
Saponin nano micelle, preparing method, application and pharmaceutical composition thereof
ActiveUS9421269B2EffectivenessImprove drug loading capacityCosmetic preparationsBiocideOrganic solventAdditive ingredient
A saponin nano micelle, preparing method, application and pharmaceutical composition thereof is disclosed in the present invention. The saponin nano micelle comprises one or more of saponins represented by formula 1, in which, R1 and R2 are independently —H or a hydrophilic group, R3 is —H or —OH, R4 is a lipophilic group. The preparing method of the saponin nano micelle is that mixing the saponin with an organic solvent which can dissolve saponin, and then removing the organic solvent. The saponin micelle acts as a convey medium of the drug ingredients, and can replace conventional drug carriers such as pharmaceutical solubilizers or polymeric micelle, which has high safety and great significance. The pharmaceutical ingredients can prolong circulation time and biological half-life of drug in the blood, and increase the accumulation of drug in lesions, and reduce adverse reactions.
Owner:FUJIAN SOUTH PHARMA CO LTD
KGM modified lecithin loaded NADH transdermal ethosome and preparation, as well as preparation process and application thereof
ActiveCN108703951AEasy to be digested by enzymesAddressing the Biological Half-Life EndOrganic active ingredientsNervous disorderAlcoholCholesterol
The invention discloses a KGM modified lecithin loaded NADH transdermal ethosome, belonging to the technical field of medicinal preparation production. The ethosome is prepared from the following components by weight percent: 1.05-1 percent of NADH, 0.03-10 percent of konjac glucomannan, 1-10 percent of phospholipids, 0.02-1 percent of cholesterol, 0.1-0.5 percent of a stabilizing agent, 0-1 percent of an antioxidant, 5-50 percent of low molecular weight alcohol and the balance of water. The ethosome can be used for solving the problems that NADH is easily enzymolysed, has short biological half-life period and poor stability, has a novel multi-cell vesicle structure having a spherical or spheroidal shape, has more stable thermodynamic property, smaller grain size and higher encapsulation efficiency, has more rapid and stronger transdermal performance and skin tolerance, so that the dosage can be reduced, occurrence of adverse reaction can be reduced, and safety can be improved. The invention further discloses gel containing the ethosome and a preparation process of the ethosome and gel. The gel has fine texture and good human body absorption, and the preparation process is simple,has mild condition, and is suitable for industrial volume production.
Owner:HOBOOMLIFE BIO TECH SHENZHEN CO LTD
Saponin NANO micelle, preparing method, application and pharmaceutical composition thereof
ActiveUS20150297727A1EffectivenessImprove drug loading capacityCosmetic preparationsOrganic active ingredientsAdditive ingredientSolvent
A saponin nano micelle, preparing method, application and pharmaceutical composition thereof is disclosed in the present invention. The saponin nano micelle comprises one or more of saponins represented by formula 1, in which, R1 and R2 are independently —H or a hydrophilic group, R3 is —H or —OH, R4 is a lipophilic group. The preparing method of the saponin nano micelle is that mixing the saponin with an organic solvent which can dissolve saponin, and then removing the organic solvent. The saponin micelle acts as a convey medium of the drug ingredients, and can replace conventional drug carriers such as pharmaceutical solubilizers or polymeric micelle, which has high safety and great significance. The pharmaceutical ingredients can prolong circulation time and biological half-life of drug in the blood, and increase the accumulation of drug in lesions, and reduce adverse reactions.
Owner:FUJIAN SOUTH PHARMA CO LTD
Long-acting exenatide derivative, salt thereof and preparation method and application
ActiveCN110128526AEase of industrial productionThe synthesis steps are simplePeptide/protein ingredientsMetabolism disorderHalf-lifePharmacologic action
The invention relates to a long-acting exenatide derivative and belongs to the technical field of polypeptide compounds. The exenatide derivative long in pharmacologic action time is prepared by performing structural optimization on a exenatide sequence to allow the exenatide to have effects of lowering sugar and reducing weight, conjugating double-dose exenatide with single-dose fatty acid chain,and using the fatty acid chain to bring the serum albumin binding effect into play. The invention further discloses the preparation method of the exenatide derivative, the pharmaceutically acceptablesalt of the exenatide derivative, an exenatide derivative drug, a pharmaceutical composition and application of the exenatide derivative in the preparation of drugs for treating and / or preventing diabetes, obesity, hyperlipidemia and non-alcoholic fatty liver disease. The long-acting exenatide derivative can have a weight reducing effect on the basis that the sugar lowering activity of the exenatide derivative is kept, the biological half-life of the exenatide derivative is evidently prolonged as compared with the exenatide prototype, the biological half-life of part of the exenatide derivatives reaches more than 36 hours, and the sugar-lowering and weight-reducing action time is prolonged greatly.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD +1
Ultrafine powder of macrolide drug and preparation method for ultrafine powder
InactiveCN105294791AHigh drug loadingImprove bioavailabilityAntibacterial agentsPowder deliveryBiological half-lifeCurative effect
The invention relates to ultrafine powder of a macrolide drug and a preparation method for the ultrafine powder. The macrolide drugs are widely applied in clinic with definite curative effects. The drugs are wide in antibacterial spectrum, strong in antibacterial activity in vivo and long in biological half life period, and have good clinical use value. A method for preparing the ultrafine powder of the macrolide drug comprises: in a homogeneous solution containing the macrolide drug, applying ultrasound with frequency of 10kHz to 500kHz, power of 1mW to 5000W and acoustic intensity of 0.1mW / cm2 to 500W / cm2 to quickly obtain crystals of the macrolide drug; and then directly obtaining the ultrafine powder of the macrolide drug through conventional operations such as solid collection, washing and drying and the like. The ultrafine powder prepared according to the method provided by the invention does not contain a substrate, and has the advantages of high drug loading capacity, high dissolving speed and proneness to achieve better bioavailability, stability and safety, thereby meeting the requirements on bioavailability, dosage reduction and adverse effects of drugs, and having broad clinic application prospects.
Owner:无锡康福特药物科技有限公司 +1
Rotigotine derivatives and preparation and application thereof
ActiveCN108341798AHigh purityHigh reaction conversion rateOrganic active ingredientsNervous disorderAlkanePatient compliance
The invention relates to preparation and application of rotigotine derivatives, specifically to synthesis of a series of rotigotine derivatives. The rotigotine derivatives are applied to preparation of micrometer or nanometer drug suspension and are prepared by directly or indirectly acylating rotigotine with alkanes of different chain lengths or fatty acid thereof, olefins or fatty acid thereof,vitamins, polyethylene glycol, polylactic acid, amino acid and other groups containing hydroxyl groups, amino groups or carboxyl groups. According to the invention, the micrometer or nanometer drug suspensions of the rotigotine derivatives are prepared by using industrially common equipment used for reducing the particle sizes of drugs, e.g., a high-pressure homogenizer or a ball mill; and when used in an in-vivo administration manner, the prepared drug suspensions of the rotigotine derivatives greatly improve the biological half-life of rotigotine and prolong the action time of rotigotine, and can effectively reduce administration frequency and improve patient compliance during clinical administration.
Owner:SHENYANG PHARMA UNIVERSITY
Spray for treating burns, scalds and chronic ulcer and its prepn
InactiveCN1739775AImprove immunityAnti-inflammatoryAerosol deliveryDermatological disorderBiological half-lifePreservative
The spray for treating burns, scalds and chronic ulcer has PEG-aFGF decorated chemically with polyethylene glycol as acid fibroblast growth factor as main medicinal component. The spray contains mainly PEG-aFGF decorated chemically with polyethylene glycol as acid fibroblast growth factor 1-100 microgram, hot zedoary oil 1-100 mg, protecting agent 0.001-0.05 mg, preservative 0.001-0.01 mg, stabilizer 0.0001-0.001 mg and corrective 0.01-0.2 mg in each milliliter, and the rest is physiological saline or water for injection. The spray has high stability, long biological half time, high safety and other features.
Owner:WENZHOU MEDICAL UNIV
Ginkgolide B sodium chloride injection and preparation method thereof
InactiveCN105434338AImprove bioavailabilityImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsSolubilitySide effect
The invention discloses a ginkgolide B sodium chloride injection and a preparation method thereof. The ginkgolide B sodium chloride injection is the injection which is formed by mixing ginkgolide B lipid nanoparticles with a 5% sodium chloride solution and has the mass concentration of 0.02%. The ginkgolide B lipid nanoparticles are prepared from, by weight, 0.4-0.5% of ginkgolide B, 0.3-0.5% of glycerin monostearate, 0.2-0.3% of intravenous injection soyabean lecithin, 0.5-1% of ethyl acetate, 0.3-0.5% of polyoxyethylene 100-stearate, 0.2-0.3% of glycerinum and the balance deionized water. The ginkgolide B sodium chloride injection is stable and reliable in performance, has the slow release effect, is few in toxic and side effect, and solves the problems that existing ginkgolide B is poor in water solubility and short in biological half life period.
Owner:广东艾希德药业有限公司
G-CSF Conjugates
InactiveUS20060275257A1Improved propertyProlong half-life in vivoSugar derivativesPeptide/protein ingredientsCysteine thiolateSide effect
The invention relates to polypeptide conjugates comprising a polypeptide exhibiting G-CSF activity and having an amino acid sequence that differs from the amino acid sequence of human G-CSF in at least one specified introduced and / or removed amino acid residue comprising an attachment group for a non-polypeptide moiety, and having at least one non-polypeptide moiety attached to an attachment group of the polypeptide. The attachment group may e.g., be a lysine, cysteine, aspartic acid or glutamic acid residue or a glycosylation site, and the non-polypeptide moiety may e.g., be a polymer such as polyethylene glycol or an oligosaccharide. The conjugate has one or more improved properties such as increased biological half-life and reduced side effects.
Owner:MAXYGEN HLDG
Preparation method of anti-acne cream
InactiveCN108703901AImprove thermal stabilityGood chemical stabilityCosmetic preparationsToilet preparationsActive enzymePhosphate
The invention discloses a preparation method of anti-acne cream and belongs to the technical field of skin-care products. An ionic liquid is made with 1,1,3,3-tetramethylguanidine and is used as an extracting solvent; under cooperation with ultrasonic assistance, the extracting solvent penetrates in the cells of medicinal materials, effective ingredients are dissolved in the extracting solvent, and the effective ingredients of traditional Chinese medicine are protected; purslane herb is added to resist bacteria and diminish inflammation; Buchu helps control sebum secretion, fight senility, andrelieve pore blocking due to sebum; Mongolian milkvetch root and otherness are added to promote blood circulation, heal up sores and promote granulation; Chinese angelica root helps improve skin whiteness and transparency; phosphate materials made with VC can provide local whitening and acne fading; chitosan is degraded into chitooligosaccharide, and catalytic oxidization is carried out via laccase; synergy between carboxyl groups newly generated and original amino groups on C2 provides antioxidant protection for VC, keeps water of the smeared skin, maintains moisture and maintains water-sebum balance; active enzymes herein promote skin metabolism, accelerate acne healing, extend biological half-life and improve skin absorption rate.
Owner:吕莉
Research and application of a novel sustained release preparation adjuvant
InactiveCN105727302AReduce releasePharmaceutical delivery mechanismMacromolecular non-active ingredientsAdjuvantFreeze-drying
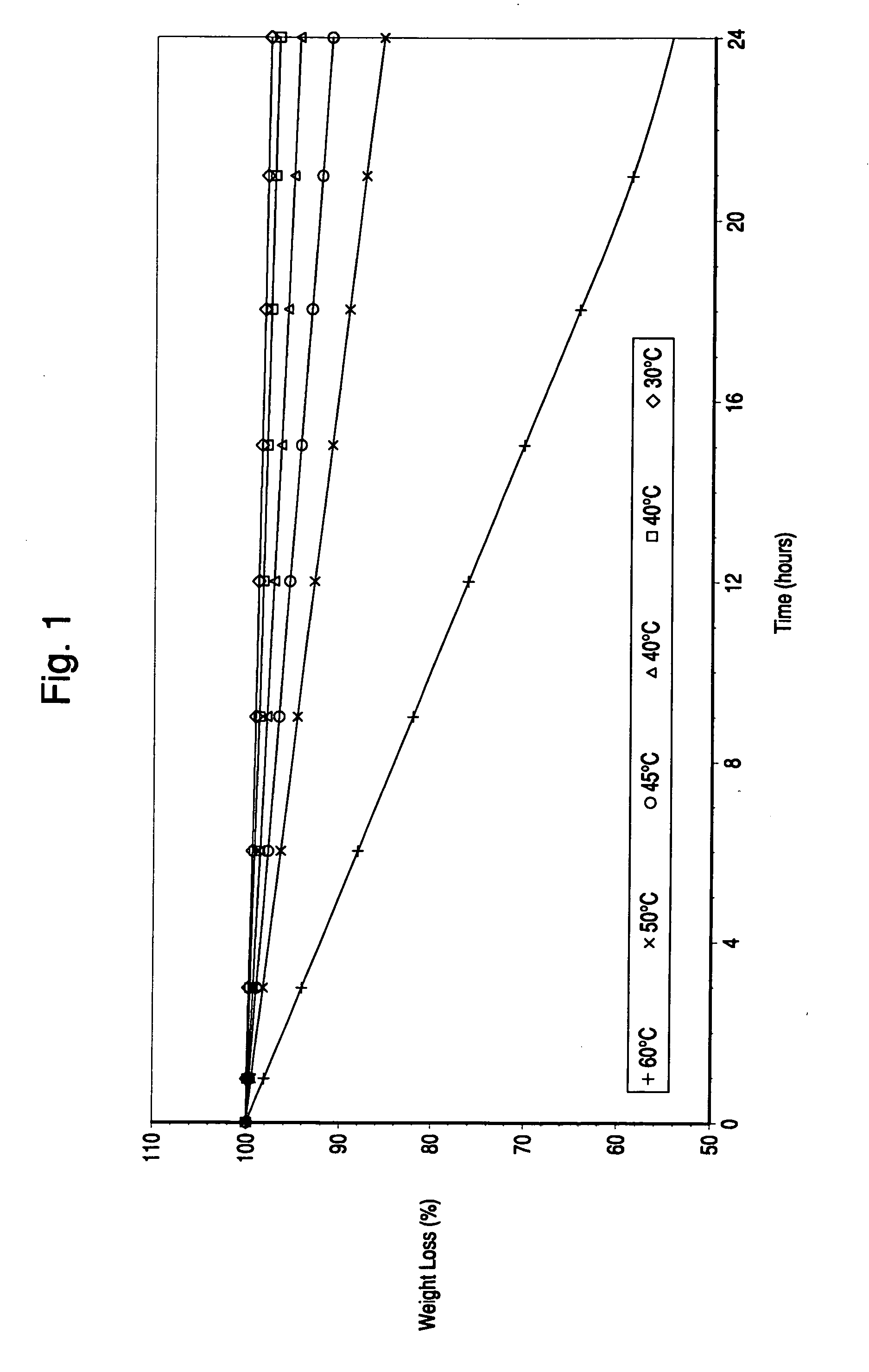
The invention discloses a novel, wide source, good biocompatibility, safe and nontoxic sustained-release preparation auxiliary material. This excipient is obtained from whey protein isolate, whey protein concentrate, or other mixtures containing one or more of the above proteins through succinylation or acetylation modification. release preparations. In the present invention, different acid anhydrides are used to acylate samples, and common drying techniques such as freeze drying, spray drying, blast drying and the like can be used to prepare auxiliary materials. The excipient enters the environment of the human body and can form a gel-like retardation layer outside the drug, thereby controlling the slow release of the drug in the human gastrointestinal tract, and is widely used in the preparation of sustained and controlled release preparations for drugs with short biological half-lives. and development.
Owner:SHENYANG PHARMA UNIVERSITY
Long-acting GLP-1 and preparation method and application thereof
InactiveCN109705207AGood hypoglycemic effectUnsatisfactory solutionPeptide/protein ingredientsMetabolism disorderTreatment effectBlood sugar
The invention relates to long-acting GLP-1 and a preparation method and application thereof, relates to the field of type 2 diabetes mellitus medicines, and solves the problems that GLP-1 is short inself biological half-life, poor in effect of reducing blood sugar, and difficult in exertion of effective treatment effects. The preparation method of the long-acting GLP-1 comprise the step that human umbilical cord mesenchymal stem cells are disinfected with recombinant adenovirus AdV-GLP-1 of 30MOI for 48h or above, so that the long-acting GLP-1 is obtained, and the infection efficiency is 90%or above. The invention relates to an application of the long-acting GLP-1 to preparation of the medicines for treating type 2 diabetes mellitus. The long-acting GLP-1 is used for modifying the humanumbilical cord mesenchymal stem cells, so that the long-acting GLP-1 having high blood sugar reducing activity and long pharmacological action time is obtained. The prepared long-acting GLP-1 is injected to mice suffering from the type 2 diabetes mellitus, and the long-acting GLP-1 achieves long-acting treatment effect in bodies of the mice.
Owner:许娜 +1
Conjugates of somatostatin analogues
ActiveUS20160271227A1Control releasePeptide/protein ingredientsPharmaceutical non-active ingredientsSomatostatin AnalogueBiological half-life
Conjugates of carriers and hydrogels for controlling the biological half-life of somatostatin and its analogs are disclosed.
Owner:PROLYNX LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com