Rotigotine derivatives and preparation and application thereof
A technology of derivatives and saturated alkanes, which is applied in the field of medicine, can solve problems such as inability to achieve therapeutic effects, and achieve the effects of improving patient compliance, reducing costs, and high conversion rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
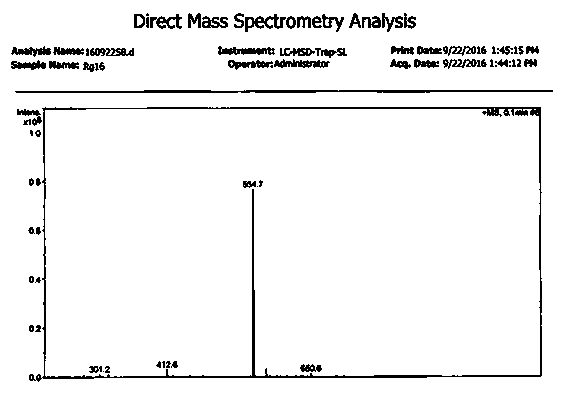
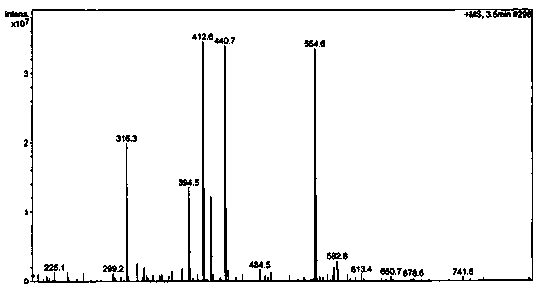
Image
Examples
Embodiment 1
[0059] The purification of embodiment 1 palmitic acid
[0060] Accurately weigh an appropriate amount of palmitic acid (48.30g) into a 500mL eggplant-shaped bottle, add 200mL of n-heptane, stir and reflux in an oil bath at 45°C until completely dissolved, and continue stirring for about 1h. After slowly cooling to about 30°C, stir in an ice-water bath for 20 minutes, place in a refrigerator at 4°C overnight, filter the crystals with suction, wash with 100 mL of n-heptane, and dry in vacuum at 45°C for 2 hours. (yield 35.10g, yield 72.7%)
Embodiment 2
[0061] The purification of embodiment 2 stearic acid
[0062] Accurately weigh an appropriate amount of stearic acid (99.10g) into a 1000mL eggplant-shaped bottle, add 500mL of chloroform, stir and reflux in an oil bath at 45°C until completely dissolved, and continue stirring for about 2h. Stir in an ice-water bath for 20 minutes, place in a refrigerator at 4°C for refrigeration, take it out and filter it quickly at low temperature, rinse with 30 mL of chloroform, and dry in vacuum at 45°C for 2 hours. (yield 43.22g, yield 43.6%)
Embodiment 3
[0063] The preparation of embodiment 3 rotigotine palmitate
[0064] Weigh the purified palmitic acid (4.06g, 15.83mmol, 1.0eq) into a 250mL eggplant-shaped bottle filled with about 100mL of anhydrous dichloromethane, and add EDC·HCl (3.64g, 18.99mmol , 1.2eq), DMAP (0.77g, 6.30mmol, 0.4eq) and SOCl 2 (1723μL, 23.73mmol, 1.5eq), after stirring evenly, add 2 drops of DMF for catalysis, and reflux at 50°C for 2h. Rotary steaming at 40°C, after being nearly spin-dry, add a small amount of toluene, spin-dry again, and repeat the operation once to obtain the acid chloride product. After adding a small amount of dichloromethane to it to re-dissolve, add pre-dissolved rotigotine (4.99g, 15.82mmol, 1.0eq), and add dropwise TEA (6.6mL, 47.45mmol, 3.0eq), N 2 Protected and stirred for 24h (TLC monitoring, developer petroleum ether: ethyl acetate = 10:1). After the reaction stopped, add 100mL 0.1M Na 2 HCO 3 solution, stirred for 10 min, separated the organic layer, and repeated the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com