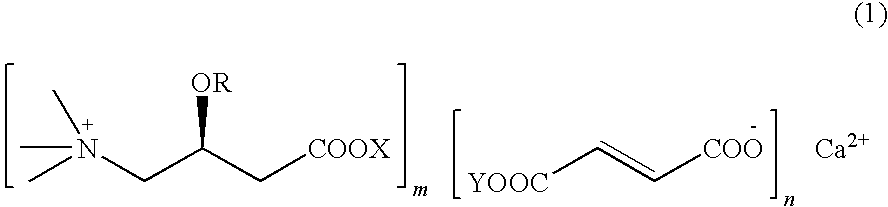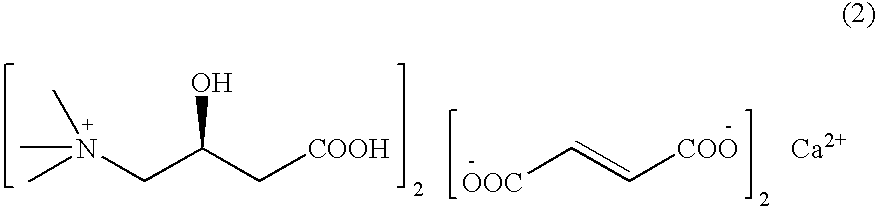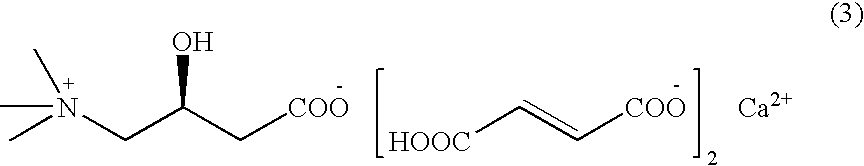Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
86results about How to "Suitable for oral" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
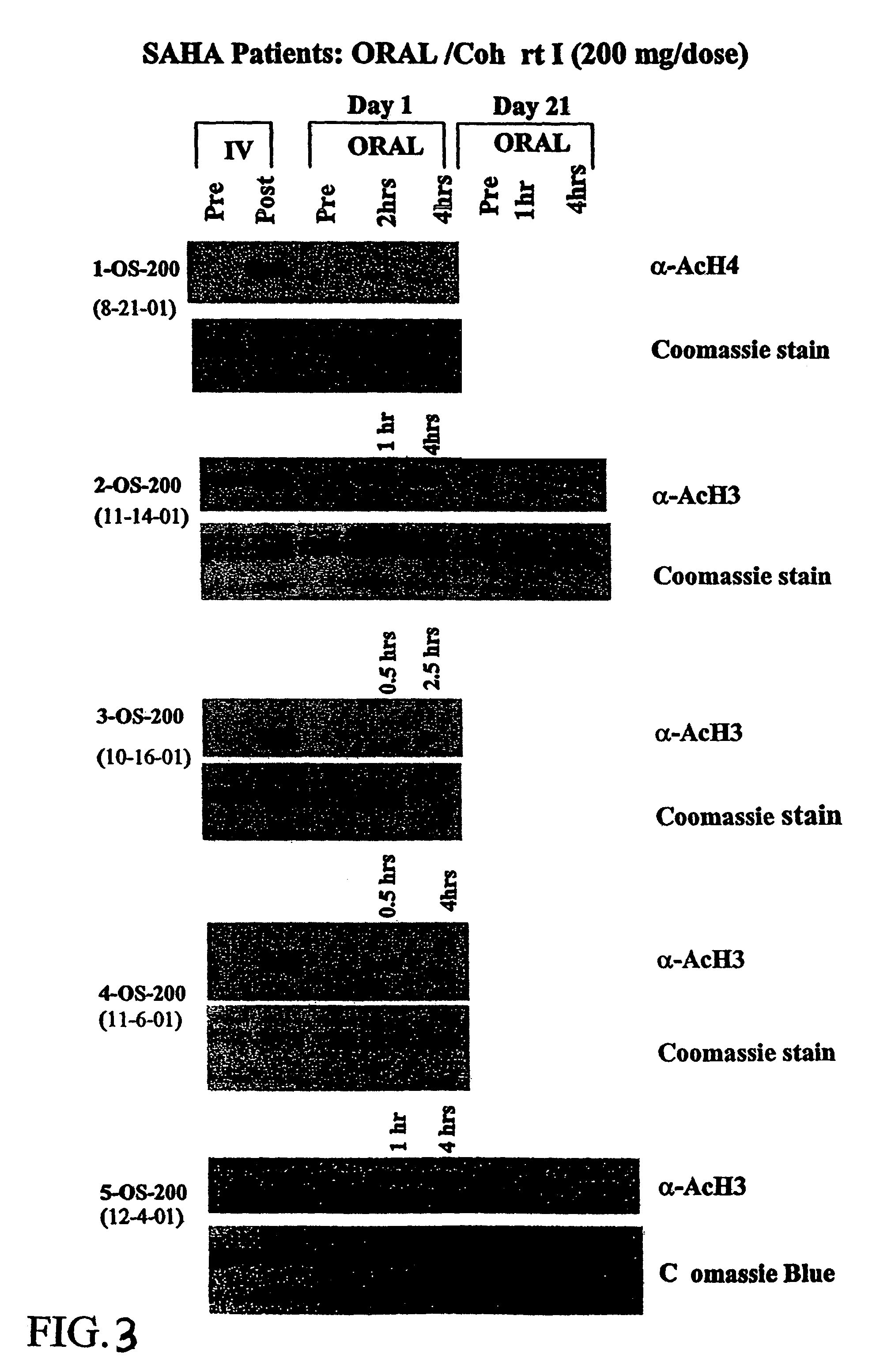
Methods of treating cancer with HDAC inhibitors
InactiveUS20060167103A1Easy to followSimple processBiocideOrganic chemistryDosing regimenGrowth cell
The present invention provides methods of treating cancers, chemoprevention, selectively inducing terminal differentiation, cell growth arrest and / or apoptosis of neoplastic cells, and / or inhibiting histone deacetylase (HDAC) by administration of pharmaceutical compositions comprising potent HDAC inhibitors. The oral bioavailability of the active compounds in the pharmaceutical compositions of the present invention is surprisingly high. Moreover, the pharmaceutical compositions unexpectedly give rise to high, therapeutically effective blood levels of the active compounds over an extended period of time. The present invention further provides a safe, daily dosing regimen of these pharmaceutical compositions, which is easy to follow, and which results in a therapeutically effective amount of the HDAC inhibitors in vivo.
Owner:MERCK HDAC RESEARCH LLC +1
Methods of treating cancer with HDAC inhibitors
The present invention provides methods of treating cancers, chemoprevention, selectively inducing terminal differentiation, cell growth arrest and / or apoptosis of neoplastic cells, and / or inhibiting histone deacetylase (HDAC) by administration of pharmaceutical compositions comprising potent HDAC inhibitors. The oral bioavailability of the active compounds in the pharmaceutical compositions of the present invention is surprisingly high. Moreover, the pharmaceutical compositions unexpectedly give rise to high, therapeutically effective blood levels of the active compounds over an extended period of time. The present invention further provides a safe, daily dosing regimen of these pharmaceutical compositions, which is easy to follow, and which results in a therapeutically effective amount of the HDAC inhibitors in vivo.
Owner:BACOPOULOS NICHOLAS G +4
Bifidobacterium cect 7765 and use thereof in the prevention and/or treatment of overweight, obesity and associated pathologies
InactiveUS20140369965A1Prevention and treatment of overweightSmall sizeAntibacterial agentsBiocideAcute hyperglycaemiaDyslipidemia
The present invention relates to the Bifidobacterium CECT 7765 strain, to its cell components, metabolites, and secreted molecules, to the combinations thereof with other microorganisms, and to compositions comprising the aforementioned products, as well as to the use of a strain of the Bifidobacterium pseudocatenulatum species, or to using the CECT 7765 strain for the prevention and / or treatment of obesity, overweight, hyperglycemia and diabetes, preferably type 2 diabetes mellitus, hepatic steatosis or fatty liver, dyslipidemia, metabolic syndrome, immune system dysfunction associated with obesity and overweight; and an unbalanced composition of the intestinal microbiota associated with obesity and overweight.
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC)
Methods of treating mesothelioma with suberoylanilide hydroxamic acid
InactiveUS7148257B2Simple processPotent inhibitor of histone deacetylaseBiocideOrganic chemistryMesotheliomaCancer research
Methods for treating mesothelioma comprising administering the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) are disclosed.
Owner:MERCK HDAC RESEARCH LLC +1
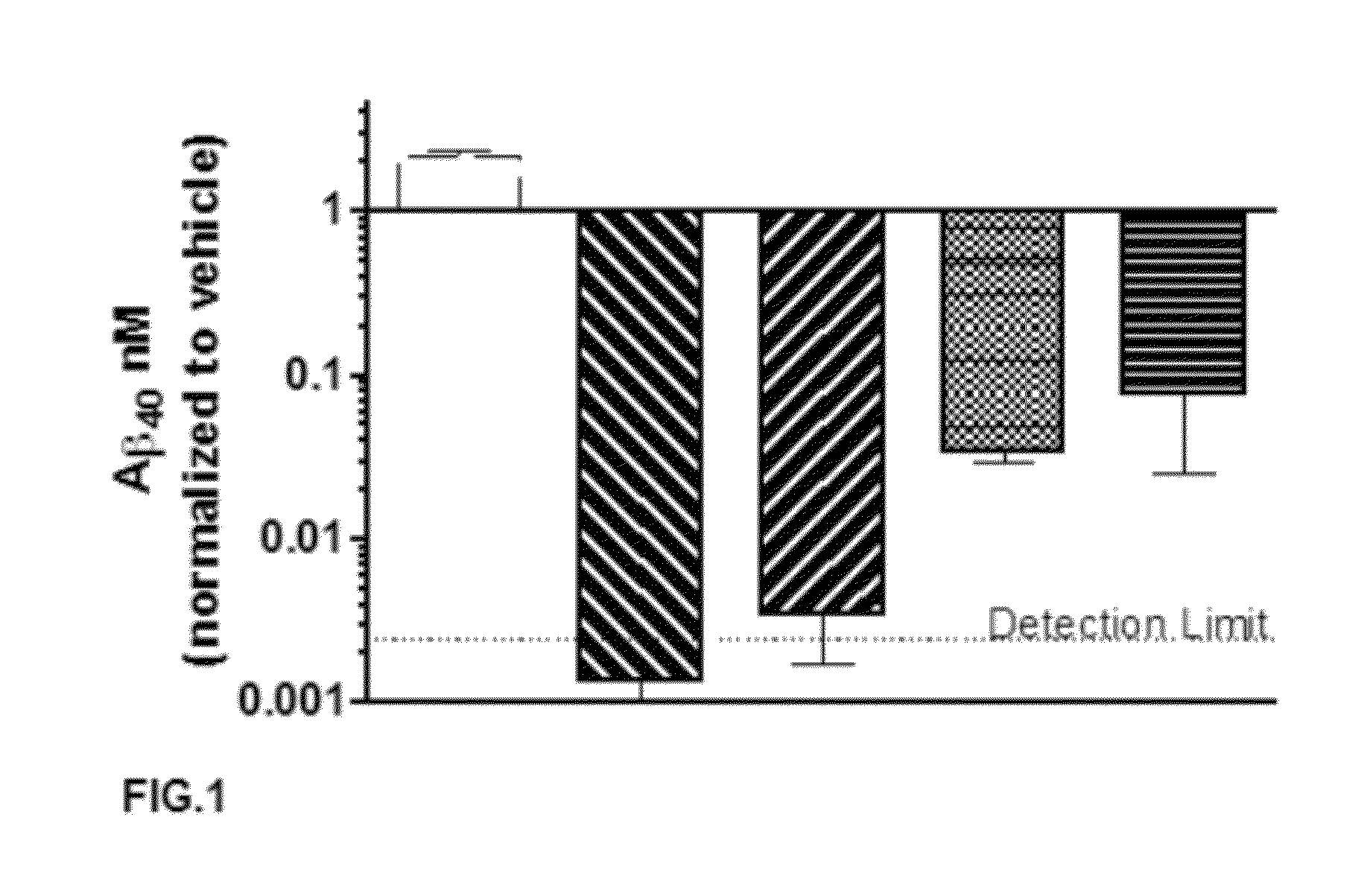
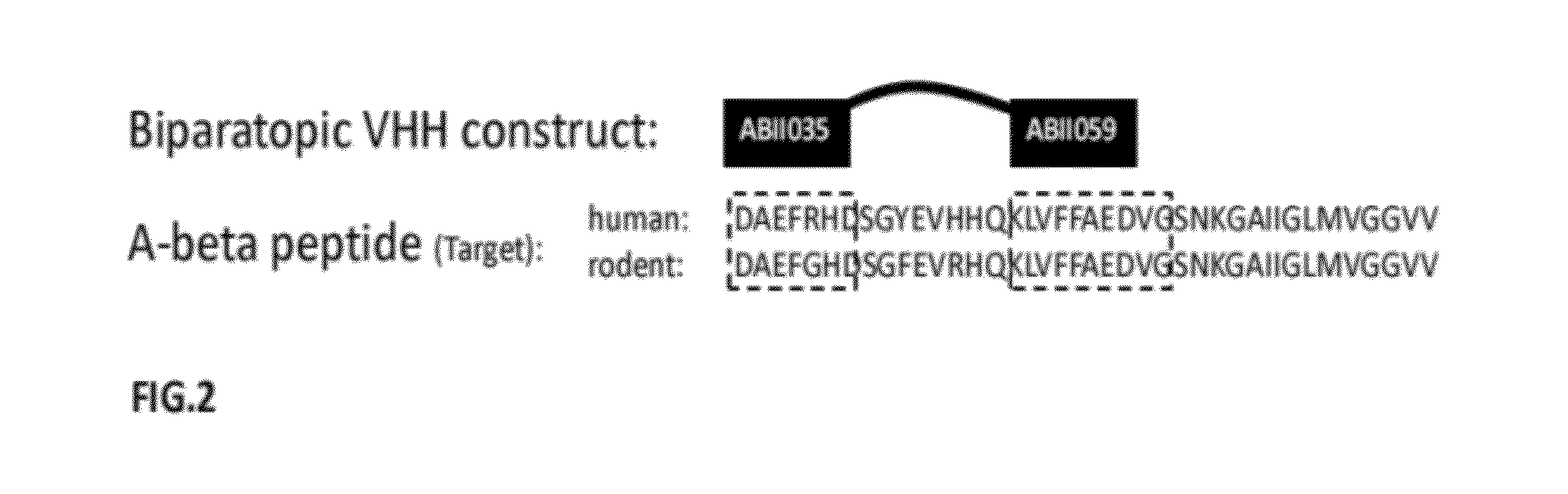
A-beta binding polypeptides
ActiveUS8337845B2Shorten the progressVision lossSenses disorderNervous disorderDiseaseIntravenous gammaglobulin
The invention relates to biparatopic A-beta binding polypeptides and, more specifically, to biparatopic A-beta binding polypeptides comprising at least two immunoglobulin single variable domains binding to different epitopes of A-beta. The invention also relates to specific sequences of such polypeptides, methods of their production, and methods of using them, including methods of treatment of diseases such as Alzheimer's Disease.
Owner:ABLYNX NV
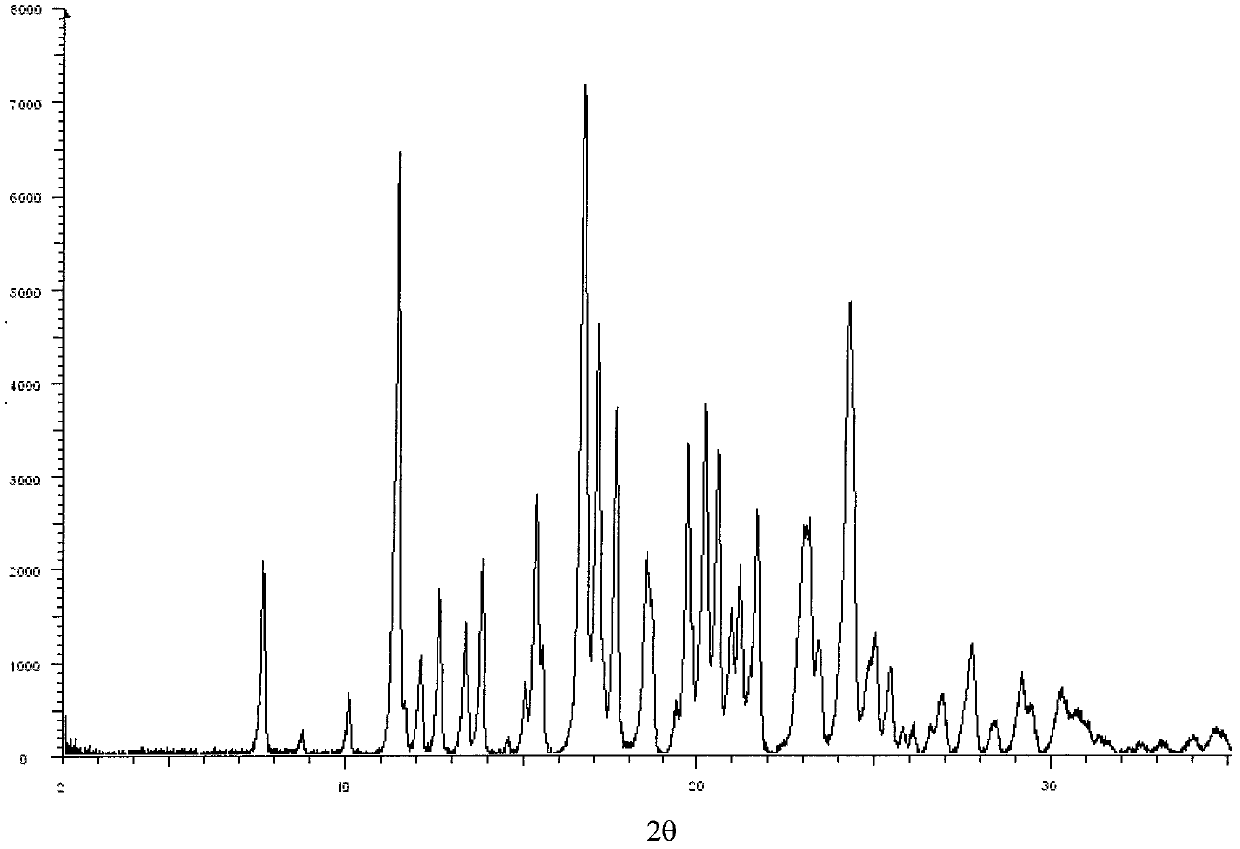
Levulorotation carnitine calcium fumarate and its preparing method and use
ActiveCN101209975ASuitable for oralNot easy to absorb moistureNervous disorderPeptide/protein ingredientsSolubilityFiltration
The invention relates to an L-carnitine calcium fumarate and a preparation method and usages thereof. The L-carnitine calcium fumarate is characterized by oral intake, stability and no-hygroscopicity, and has stronger and more functions of nutrition and treatment, compared with corresponding internal salt and good water solubility; the preparation method is: the calcium furmarate is dissolved in the water and added with calcic alkali for reaction with the temperature increasing to 70 to 90 DEG C for 2 to 8 hours and then water is evaporated by reducing pressure. The solid obtained by drying is added into ethanol and evenly mixed, with the L-carnitine added for reaction with the temperature at 60 to 70 DEG C for 1 to 6 hours, then the mixture is put into a refrigerator for refrigeration for 2 to 8 hours and the L-carnitine calcium fumarate is finally obtained by suction and filtration; the composition containing the L-carnitine calcium fumarate can be made into one or more excipients acceptable on pharmacology, particularly solid and liquid oral intake preparation, such as powdered drug, granules, tablets, capsules, oral liquid, etc., is preferred and the solid and liquid oral intake preparation can be used for food / nutrition additives for people, or feed additives for animals, including additives for calcium supplement.
Owner:リャオニンコンセプヌトラシーオーエルティーディー
Aseptically packaged nutritional concentrate
InactiveUS20120258209A1Effectively processReduce heat densityMilk preparationFruit and vegetables preservationChemistryEmulsion
Disclosed are packaged compositions comprising an aseptically sterilized container and a sterilized, concentrated, nutritional liquid emulsion that is aseptically packaged and sealed within the container. Also disclosed are methods for making and using the packaged compositions. In some embodiments, the aseptically packaged, concentrated, nutritional liquid emulsions have a desirable flavor and aroma and have increased emulsion stability.
Owner:ABBOTT LAB INC
Veterinary compositions
InactiveUS20120322782A1Prolonged gastric retentionHigh molecular weightAntibacterial agentsBiocideControlled releaseBuccal administration
The present invention relates to veterinary compositions in a form of an orally deliverable tablet, and more particularly to a controlled-release composition that provides sufficiently long duration to permit once daily administration.
Owner:ZOETIS SERVICE LLC
Microorganisms as carriers of nucleotide sequences coding for antigens and protein toxins, process of manufacturing and uses thereof
ActiveUS8669091B2Efficient tumor therapyEnhance immune responseAntibacterial agentsBacteriaAntigenTransport system
A Escherichia, Salmonella, Yersinia, Vibrio, Listeria, Shigella, or Pseudomonas bacterium that has the following components: (I) a polynucleotide encoding a heterologous antigenic determinant that induces a CTL response against a tumor cell; (II) a polynucleotide encoding a heterologous protein toxin or toxin subunit; and (III) (a) a polynucleotide encoding a transport system that expresses the products of (I) and (II) on the outer surface of the bacterium or that secretes products of (I) and (II) from the bacterium; and (IV) a polynucleotide that activates the expression of one or more of (I). (II), and / Or (III) in the bacterium wherein polynucleotides (I), (II), (III) and (IV) are different from each other and polynucleotides (I), (II) and (III) encode proteins that are different from each other.
Owner:SOCIUM THERAPEUTICS INC
Glycyrrhetinic acid solid lipid nanoparticles and preparation method for same
InactiveCN102512369AUniform particle sizeHigh encapsulation efficiencyOrganic active ingredientsDigestive systemDiseaseActive agent
The invention relates to glycyrrhetinic acid solid lipid nanoparticles and a preparation method for the same, belonging to the field of medicinal preparation. The main ingredients of the glycyrrhetinic acid solid lipid nanoparticles disclosed by the invention comprise active raw material glycyrrhetinic acid, medicinal phospholipid, a lipid material and a surfactant. The glycyrrhetinic acid solid lipid nanoparticle solution and the freeze-dried powder injection thereof prepared by the preparation method disclosed by the invention are small in particle diameter, high in entrapment efficiency, good in stability and capable of being used for a plurality of administration routes such as oral administration and injection administration. The glycyrrhetinic acid solid lipid nanoparticles disclosed by the invention can reduce dosage, enhance curative effect and reduce the toxic and side effects of medicine, as well as are suitable for treating a plurality of diseases such as hepatitis, liver cancer, lung cancer, ovarian cancer, gastritis, gastric cancer, leukaemia and aids.
Owner:WUHAN UNIV
Pharmaceutical composition containing nicotinic acid and/or nicotinamide and/or tryptophan for positively influencing the intestinal microbiota
ActiveUS20150126462A1Positively influenceSuitable for oralBiocideOrganic chemistryIntestinal microorganismsLarge intestine
The present invention relates to a new pharmaceutical composition containing nicotinic acid, nicotinamide, tryptophanor related compounds for positively influencing the intestinal microbiota. In certain embodiments, the pharmaceutical composition is partially or entirely released into the small intestine or large intestine.
Owner:CONARIS RES INST
Oligonucleotide-Containing Pharmacological Compositions And Their Use
InactiveUS20080167257A1Reduce expressionSuitable for oralAntibacterial agentsOrganic active ingredientsPharmacologyOligonucleotide
Owner:LAKEWOOD AMEDEX
Oligonucleotide-Containing Pharmacological Compositions And Their Use
InactiveUS20080161257A1Good for healthReduce expressionAntibacterial agentsOrganic active ingredientsOligonucleotidePharmacology
Owner:LAKEWOOD AMEDEX
Alpha-d-galactoside inhibitors of galectins
ActiveUS20170349619A1High affinityMaintain good propertiesOrganic active ingredientsSenses disorderMedicineIsrapafant
An embodiment of the present invention relates to a compound of the general formula. The compound of formula is suitable for use in a method for treating a disorder relating to the binding of a galectin, such as galectin-3 to a ligand in a mammal, such as a human. Furthermore an embodiment of the present invention concerns a method for treatment of a disorder relating to the binding of a galectin, such as galectin-3 to a ligand in a mammal, such as a human.
Owner:GALECTO BIOTECH
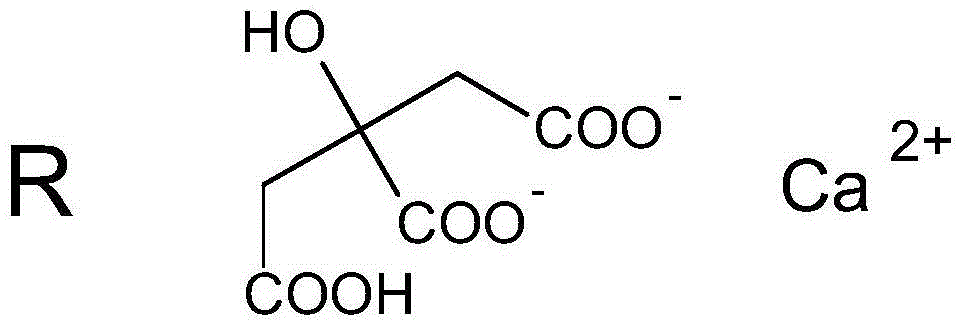
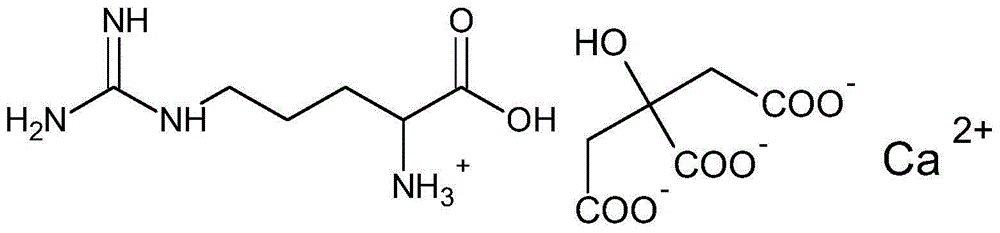
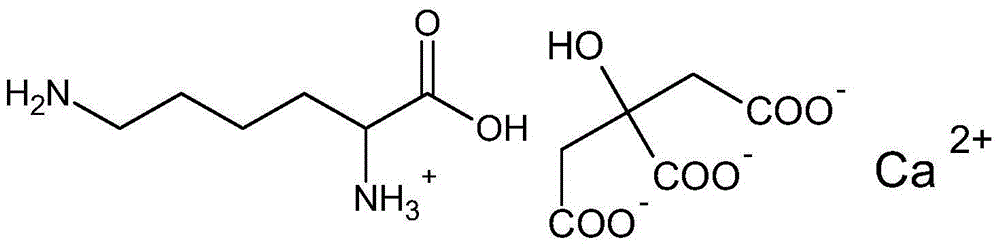
Calcium citrate salt and preparation method and application thereof
ActiveCN105777534ASuitable for oralNot easy to absorb moistureOrganic active ingredientsOrganic compound preparationParticulates(R)-Carnitine
A calcium citrate salt and its preparation method and application are disclosed. The calcium citrate salt has stronger nutritional and therapeutical effects than simple calcium citrate. The preparation method comprises the following steps: dissolving citric acid in water, adding calcium oxide or calcium hydrate or calcium carbonate, filtering to remove foreign materials after the solution is dissolved to be transparent, adding L-arginine or L-lysine or acetyl L-carnitine or propionyl l-carnitine into a filtrate, reacting at 0-100 DEG C for 2-8 h, carrying out vacuum concentration, cooling, carrying out crystallization by adding anhydrous ethanol, filtering and drying, and detecting to be qualified so as to obtain the product. A composition containing the calcium citrate salt can be made into one or more pharmacologically acceptable excipients, especially a solid-liquid oral preparation, such as pulvis, a particulate agent, a tablet, capsules and an oral liquid, etc., or directly made into a beverage, and can be used as dietary / nutritional supplement for human or a feed supplement for animals, including nutritional supplements for supplementing L-arginine, L-lysine, acetyl L-carnitine and propionyl L-carnitine.
Owner:リャオニンコンセプヌトラシーオーエルティーディー
Medicine composition for treating diabetes
InactiveCN103735843AShorten the timeLow recurrence rateMetabolism disorderPlant ingredientsDrugOral medication
The invention discloses a medicine composition for treating diabetes. The medicine comprises the following components in parts by weight: 5-15 parts of longan seeds, 5-15 parts of lichee seeds, 5-10 parts of mulberry, 3-8 parts of pawpaw seeds, 3-10 parts of radix astragali and 5-10 parts of rhizoma polygonati. The components of the formulation of the medicine composition for treating diabetes are simple and scientifically, rationally formulated, the medicine has remarkable efficacy, short course, small toxic and side effect and long efficacy, is suitable for diabetic patients of variable types for administration, is suitable for oral administration, and is convenient, safe and reliable.
Owner:唐树青
Formulations and Methods for Modulating Satiety
InactiveUS20080254108A1Improve bioavailabilityModulate/induce satietyMetabolism disorderTetrapeptide ingredientsOral medicationSuppressed appetite
This disclosure relates to formulations and methods of suppressing appetite and eliciting satiety (sense of being filled) in mammals through the oral administration of an effective amount of an appetite suppressing moiety.
Owner:NATURALPHARM
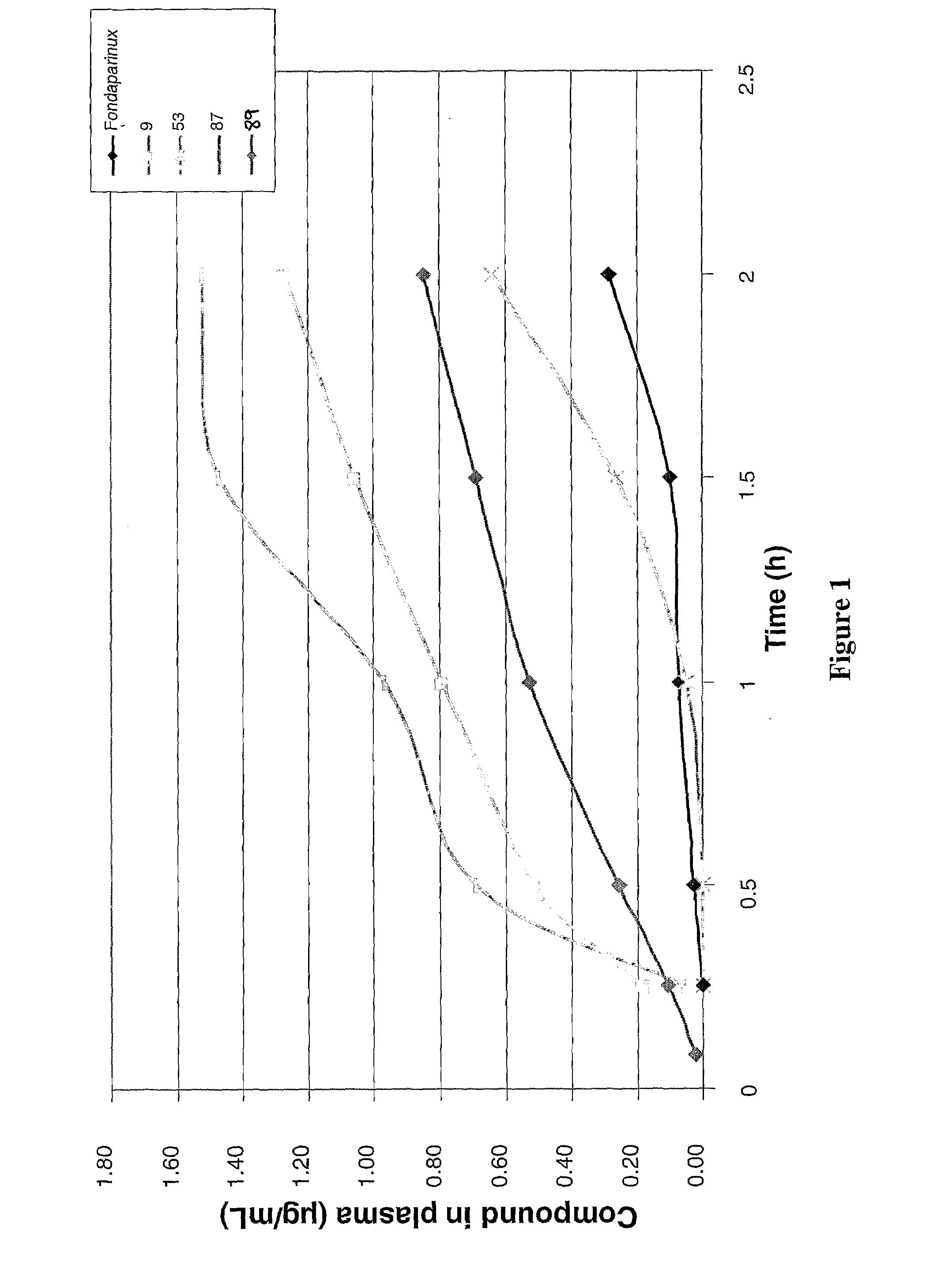
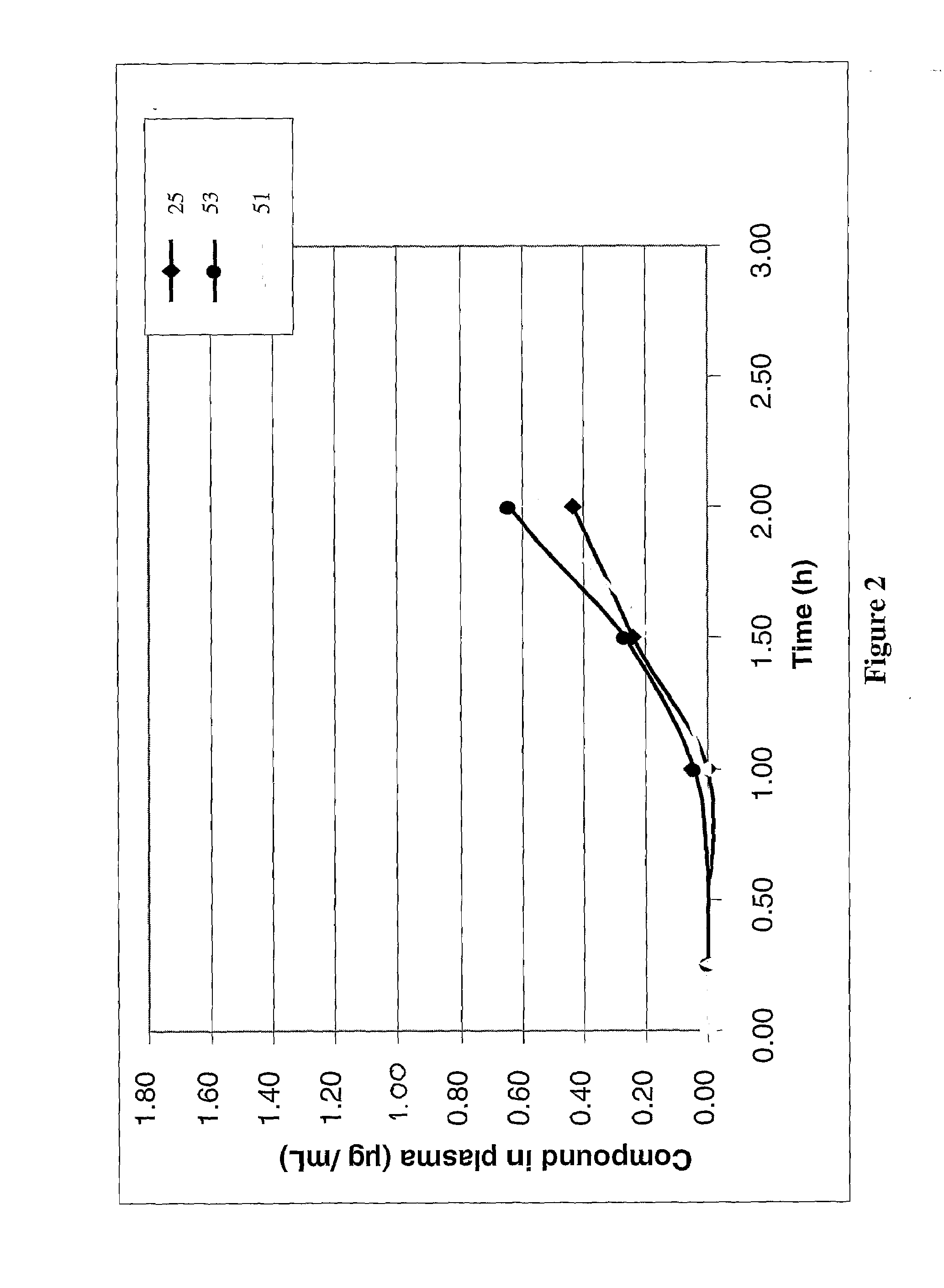
Anticoagulant compounds
InactiveUS20100081708A1Suitable for oralEsterified saccharide compoundsOrganic active ingredientsMedicineAnticoagulant
Owner:ENDOTIS PHARMA
Oral emulsion rich in [alpha]-linolenic acid and preparation method of oral emulsion
ActiveCN107441217AImprove bioavailabilityGreat tasteSenses disorderNervous disorderAdjuvantSide effect
The invention discloses an oral emulsion rich in [alpha]-linolenic acid. The oral emulsion is prepared from the following components in percentage by weight: 1-25% of plant oil containing the [alpha]-linolenic acid, 1-25% of an emulsifier, 0.1-20% of adjuvants, 0.001-5% of an antioxidant, 0-1.5% of flavoring agent and the balance of purified water; and the plant oil containing the [alpha]-linolenic acid is extracted from the following components in parts by weight: 20-25 parts of eucommia ulmoides oliver seeds, 10-15 parts of perilla seeds, 10-15 parts of kiwi fruit seeds, 12-17 parts of peony seeds, 10-16 parts of linseed and 7-12 parts of Chinese prickly ash seeds. The invention also discloses a preparation method of the oral emulsion rich in the [alpha]-linolenic acid. The oral emulsion containing the [alpha]-linolenic acid prepared by the invention is stable in ingredient, high in bioavailability, free from toxic and side effects, obvious in curative effect, broad in application to indications and obvious in social and economic values, and bad smell and taste of the oil can be masked.
Owner:HENAN UNIVERSITY
Compositions comprising amino acids for prevention and/or treatment of renal disorders
InactiveUS20130237577A1Improve toleranceSuitable for oralBiocidePeptide/protein ingredientsThreonineTherapeutic treatment
Composition comprising leucine, isoleucine, valine, threonine and lysine for use in prophylactic and / or therapeutic treatment of renal disorders in a subject, preferably an elderly subject.
Owner:DETERMINANTS OF METABOLISM RES LAB
Lactobacillus plantarum 550 with constipation relieving and sleep aiding functions and application of lactobacillus plantarum 550
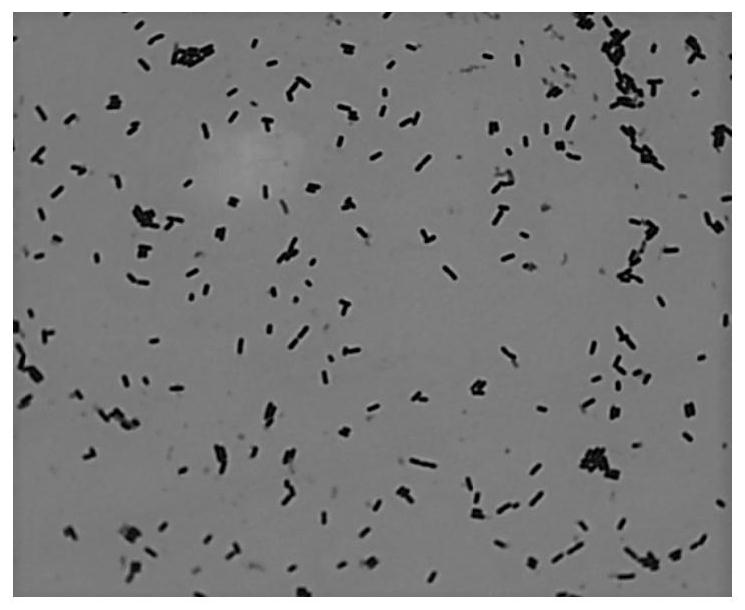
PendingCN113462591APromote growthIncrease acidityAntibacterial agentsNervous disorderBiotechnologyIntestino-intestinal
The invention discloses lactobacillus plantarum 550 with constipation relieving and sleep aiding functions and application of lactobacillus plantarum 550, belonging to the technical field of biology. The lactobacillus plantarum 550 provided by the invention is preserved in the China Center for Type Culture Collection on December 21, 2007, wherein the accession number of the lactobacillus plantarum 550 is CCTCC No. M 207202. The lactobacillus plantarum 550 provided by the invention is applied to preparation of functional food and medicines for relieving constipation, or / and helping sleep, or / and reducing cholesterol, or / and inhibiting pathogenic bacteria, or / and degrading nitrite. The lactobacillus plantarum 550 grows well and quickly on an MRS agar culture medium, is high in acid production capacity, has good gastric acid and cholate tolerance, and can be colonized in intestinal tracts. The lactobacillus plantarum 550 has a good bowel relaxing effect, strong capability of producing gamma-aminobutyric acid, the effect of reducing cholesterol in vitro, a good inhibition effect on common pathogenic bacteria, capability of rapidly degrading nitrite and the good safety.
Owner:SICHUAN GAOFUJI BIOLOGICAL TECH
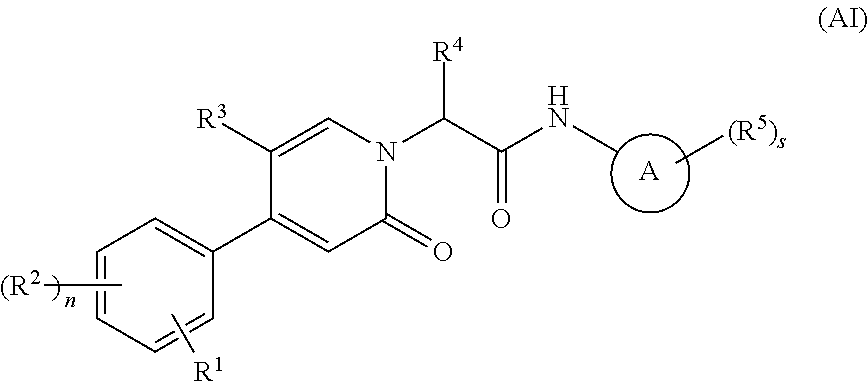
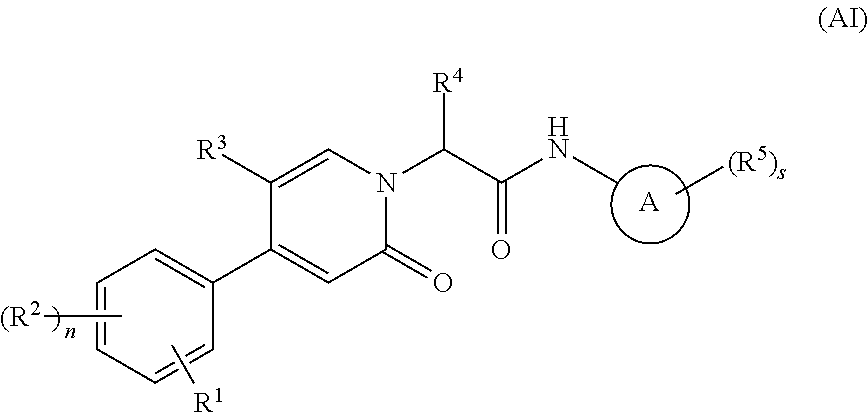
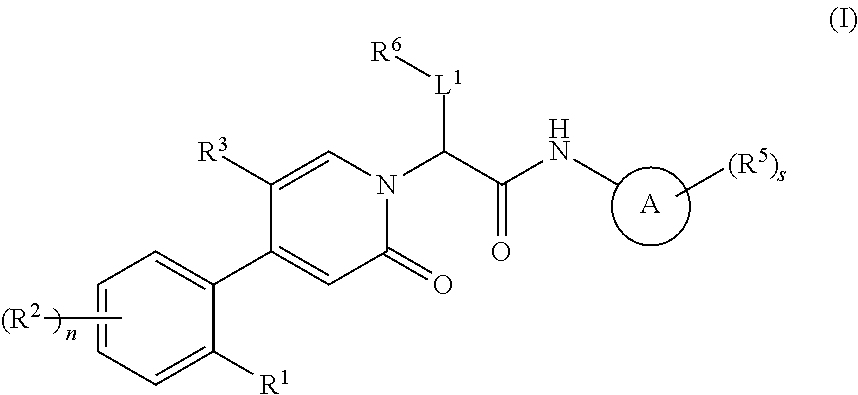
Oxopicolinamide derivative, preparation method therefor and pharmaceutical use thereof
ActiveUS10633375B2Pleasing and palatableSuitable for oralOrganic active ingredientsOrganic chemistryDiseaseThrombus
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
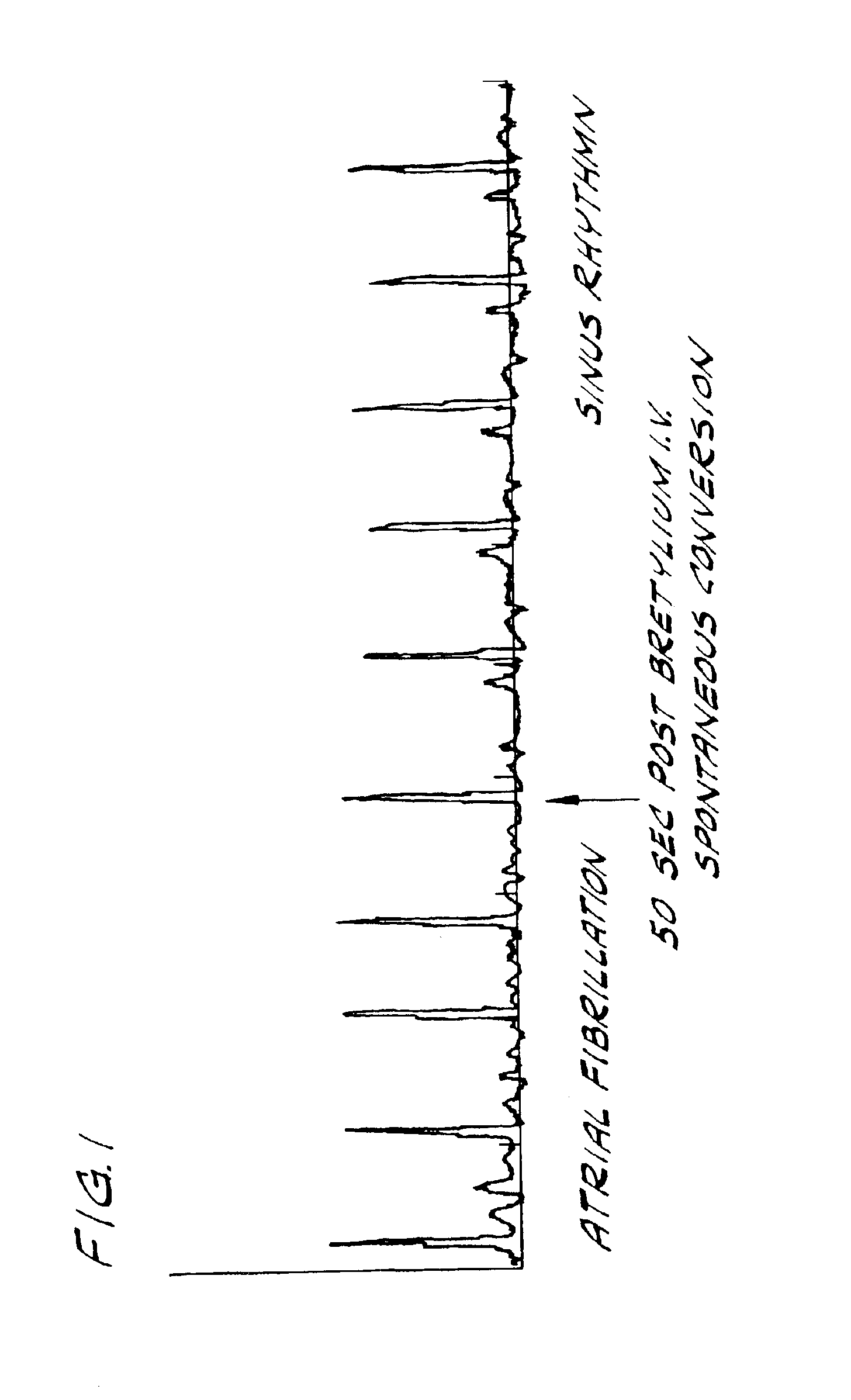
Bretylium compositions and kits and their use in preventing and treating cardiovascular conditions
InactiveUS6884792B2High activityImprove effectivenessBiocideSalicyclic acid active ingredientsPharmaceutical drugBULK ACTIVE INGREDIENT
The present invention is directed to novel pharmaceutical combinations including compositions and kits comprising bretylium as the active ingredient, as well as methods for preventing and / or treating conditions related to the cardiovascular system using such novel pharmaceutical combinations.
Owner:BACANER MARVIN B +1
Flumatinib mesylate crystal form B and preparation method and use thereof
ActiveCN102816147ASuitable for oralOral route of administration preferredOrganic active ingredientsOrganic chemistryMedicineFlumatinib Mesylate
The invention relates to flumatinib mesylate crystal form B and a preparation method and the use thereof. One crystal form of the flumatinib mesylate, the preparation method thereof, medicine combination of the compound and with treatment effective quantity and application of the crystal form in preparation of medicine for treating chronic myelogenous leukemia are further provided.
Owner:JIANGSU HANSOH PHARMA CO LTD
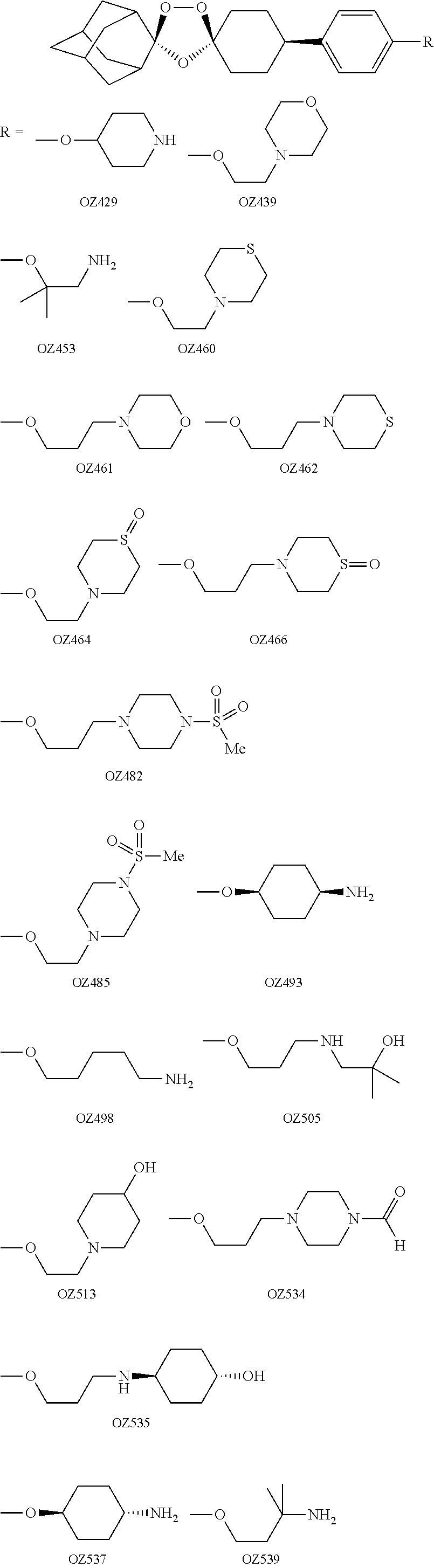
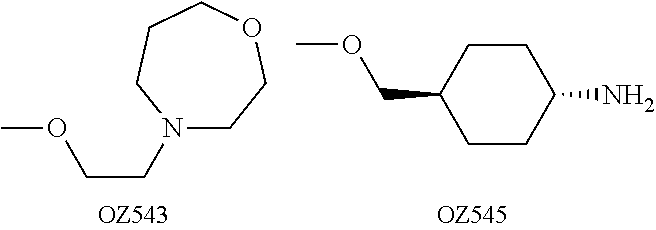
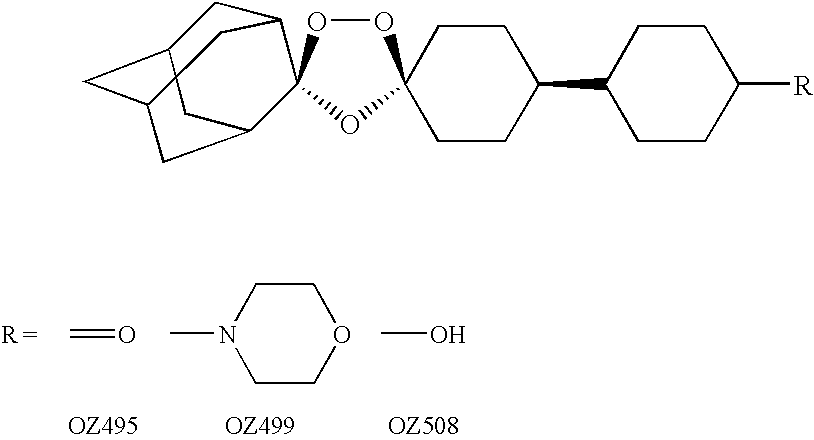
Dispiro 1,2,4-trioxolane antimalarials
A means and method for treating malaria, schistosomiasis, and cancer using a spiro or dispiro 1,2,4-trioxolane is described. The preferred 1,2,4-trioxolanes include a spiroadamantane group on one side of the trioxolane group, and a spirocyclohexyl on the other side of the trioxolane group. In comparison to artemisinin semisynthetic derivatives, the compounds of this invention are structurally simple, easy to synthesize, non-toxic, and potent against malarial parasites. The compounds of the invention unexpectedly provide a single-dose cure for malaria, as well as prophylactic activity against the same. The compounds are also active against schistosomiasis and cancer.
Owner:MMV MEDICINES FOR MALARIA VENTURE
Artemether oral micro-emulsion in-situ gel and preparation method thereof
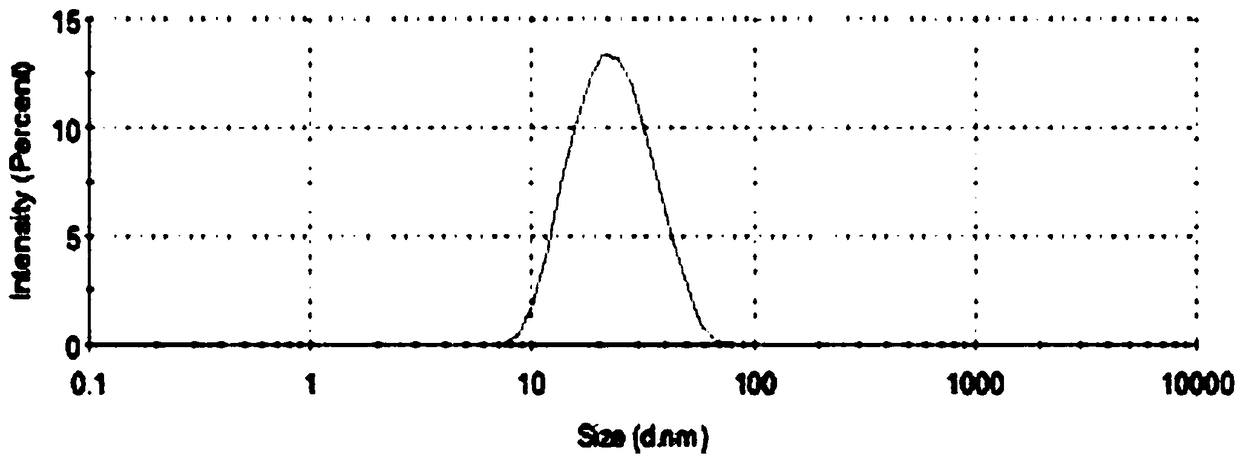
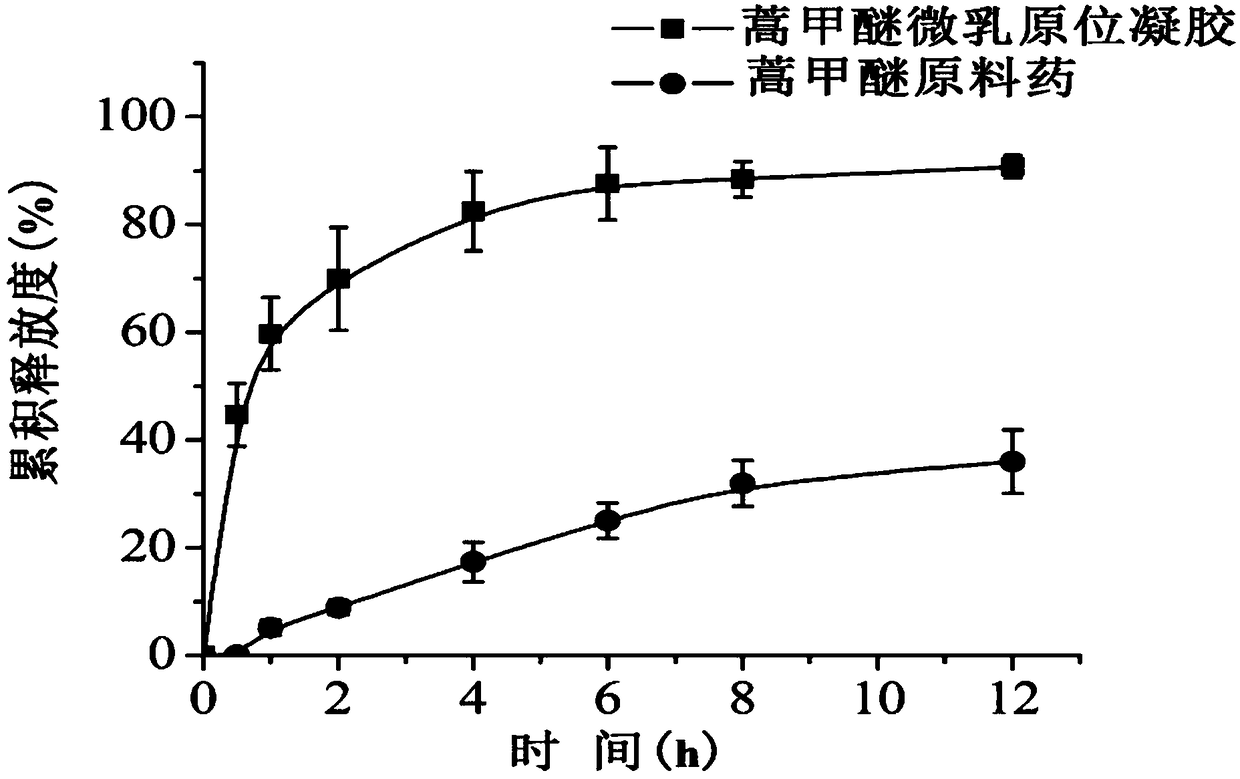
ActiveCN108578356AImprove solubilityFacilitated releaseOrganic active ingredientsAerosol deliverySolubilityOral medication
The invention belongs to the technical field of medicines and particularly relates to an artemether oral micro-emulsion in-situ gel and a preparation method thereof. The artemether oral micro-emulsionin-situ gel is prepared from the following components of 0.1-0.8% of artemether, 0.9-7.2% of oil phases, 0.4-12% of surfactants, 0.4-12% of cosurfactants, 0.23-0.3% of in-situ gel substrates, 0-0.1%of adhesives and the balance water. The average particle size of emulsion drops of the artemether oral micro-emulsion in-situ gel is 20.90 nm, the emulsion drops are uniform in size, the solubility and releasing rate of the artemether can be increased, and the artemether oral micro-emulsion in-situ gel is stable at the room temperature; the artemether oral micro-emulsion in-situ gel is low in viscosity and good in flowability, the gel is formed immediately in a stomach after oral administration, the detention time of the artemether in the stomach can be prolonged, treatment of a stomach cancerand improving of the bioavailability of the artemether are facilitated; and the preparation technique is easy, operation and filling are easy, and industrial production is facilitated.
Owner:KPC PHARM INC
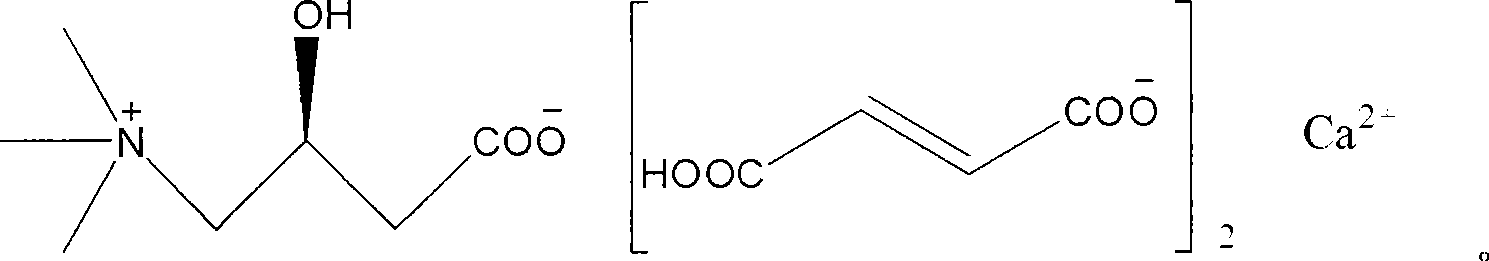
L-carnitine calcium fumarate, preparation method and application for the same
InactiveUS20090281183A1Good fluidityGood water solubilityOrganic active ingredientsBiocideSolubilityOral medication
L-carnitine calcium fumarate and its preparation and applications are disclosed in the present invention. Having good water solubility as well as better and stronger nutritious and treating functions than the corresponding inner salts, the L-carnitine calcium fumarate as proposed having the advantages of being non-hygroscopic, stable in chemical property, and suitable for oral administration. A preparing method is as follow: fumaric acid is suspended in water and calcium base is added. The resulting mixture is heated up to 70˜90° C. and kept under stirring for 2˜8 hours, and then concentrated under reduced pressure. The resulting dried substance is added into ethanol, and the mixture is vigorously stirred. The inner salt of L-carnitine is added, and the mixture is reacted for 1-6 hours at 60˜70° C. and then cooled for 2˜8 hours. L-carnitine calcium fumarate is obtained by filtration.
Owner:SHENYANG KONCEPNUTRA
Traditional Chinese medicine composition for treating chronic prostatitis and preparation method thereof
InactiveCN108114266AImprove immunityRestore physical functionOrganic active ingredientsHydrolysed protein ingredientsChlorogenic acidSide effect
The invention relates to a traditional Chinese medicine composition for treating chronic prostatitis and a preparation method thereof. The traditional Chinese medicine composition is prepared from thefollowing raw materials in parts by weight: 8 to 12 parts of cortex eucommiae chlorogenic acid, 6 to 10 parts of cortex cinnamomi polyphenol, 13 to 17 parts of oyster peptide, 8 to 12 parts of maca,8 to 12 parts of cordyceps militaris, 13 to 17 parts of silk peptide, 2 to 6 parts of herba abelmoschi esculenti, 2 to 6 parts of cortex pausinystaliae macroceras, 2 to 6 parts of catuaba, 2 to 6 parts of herba epimedii, 2 to 6 parts of herba gynostemmatis, 2 to 6 parts of herba rhodiolae, 2 to 6 parts of radix salviae miltiorrhizae and 2 to 6 parts of semen persicae. The traditional Chinese medicine composition disclosed by the invention is simple in preparation and remarkable in curative effect, has no side effect, is safe and effective, is low in cost and is suitable for oral administration.
Owner:江苏优曦特斯生物制品有限公司
Novel galactoside inhibitor of galectins
ActiveUS20190359643A1High affinityMaintain good propertiesOrganic active ingredientsSenses disorderMedicineIsrapafant
An embodiment of the present invention relates to a compound of the general formula. The compound of formula is suitable for use in a method for treating a disorder relating to the binding of a galectin, such as galectin-3 to a ligand in a mammal, such as a human. Furthermore an embodiment of the present invention concerns a method for treatment of a disorder relating to the binding of a galectin, such as galectin-3 to a ligand in a mammal, such as a human.
Owner:GALECTO BIOTECH
Flumatinib mesylate crystal form A and preparation method and use thereof
InactiveCN102816146ASuitable for oralOral route of administration preferredOrganic active ingredientsOrganic chemistryMedicineFlumatinib Mesylate
The invention relates to flumatinib mesylate crystal form A and a preparation method and the use thereof. Specifically, one crystal form of the flumatinib mesylate, the preparation method thereof, medicine combination of the compound and with treatment effective quantity and application of the crystal form in preparation of medicine for treating chronic myelogenous leukemia are further provided.
Owner:JIANGSU HANSOH PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
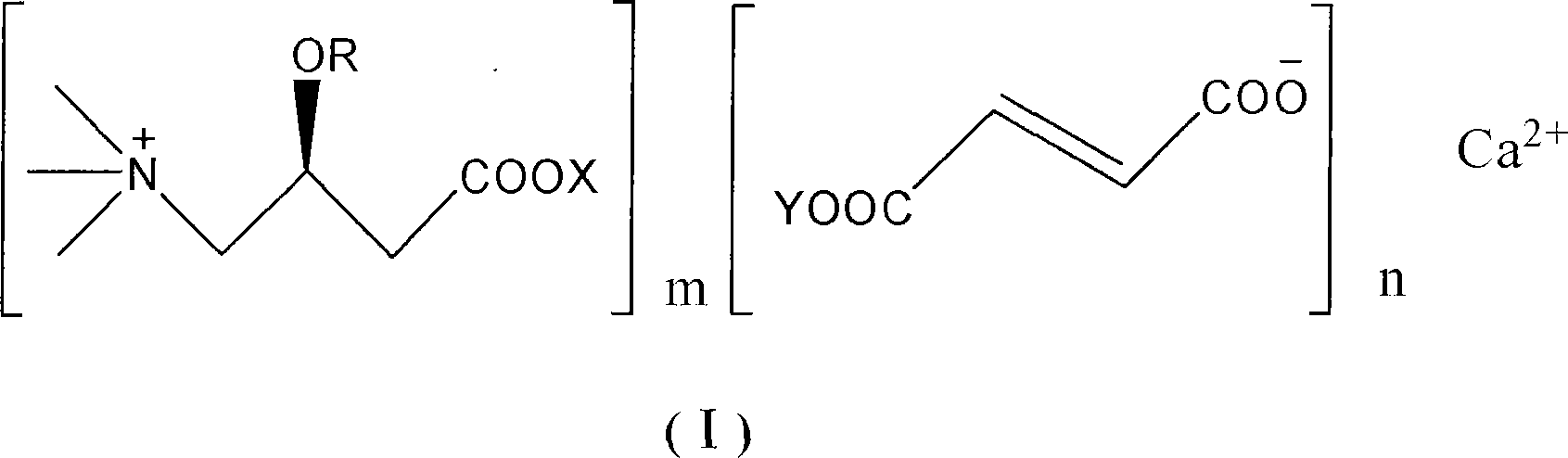
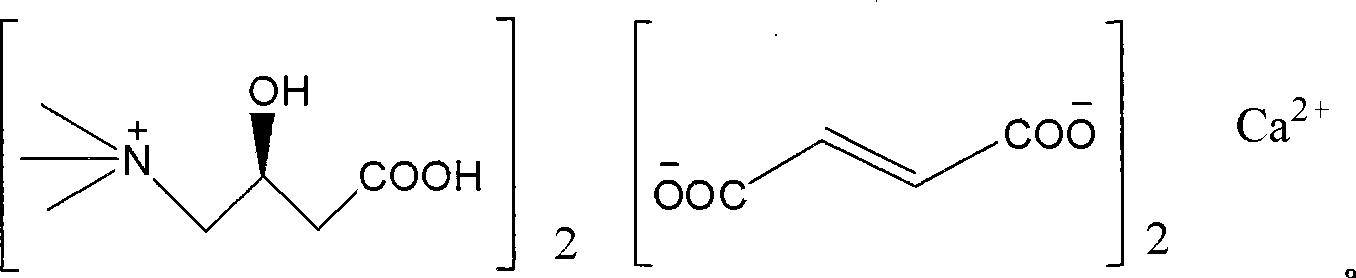
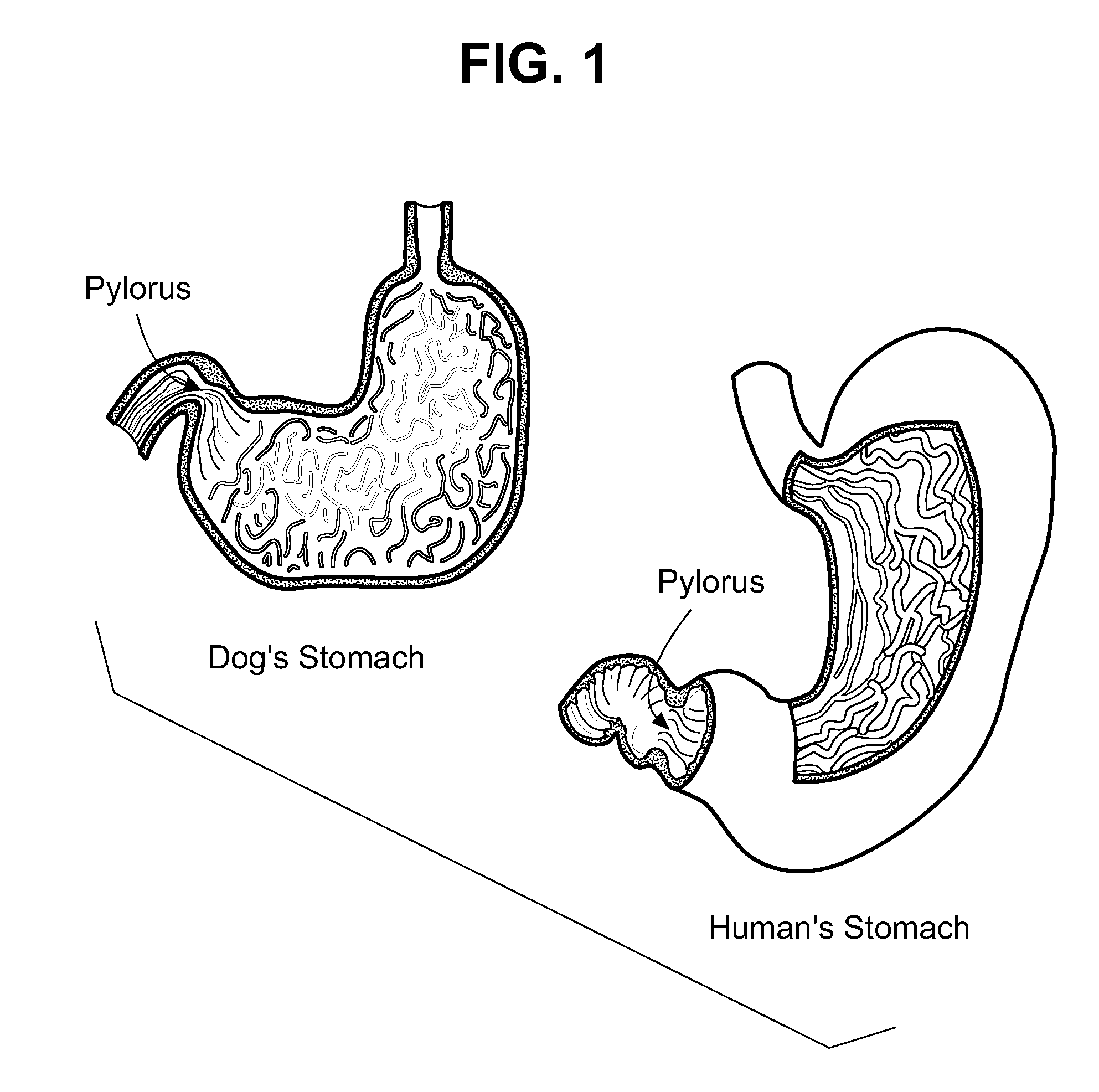



![Oral emulsion rich in [alpha]-linolenic acid and preparation method of oral emulsion Oral emulsion rich in [alpha]-linolenic acid and preparation method of oral emulsion](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/de71c532-5752-4017-bac2-ae6e02e0eb81/170626141909.png)
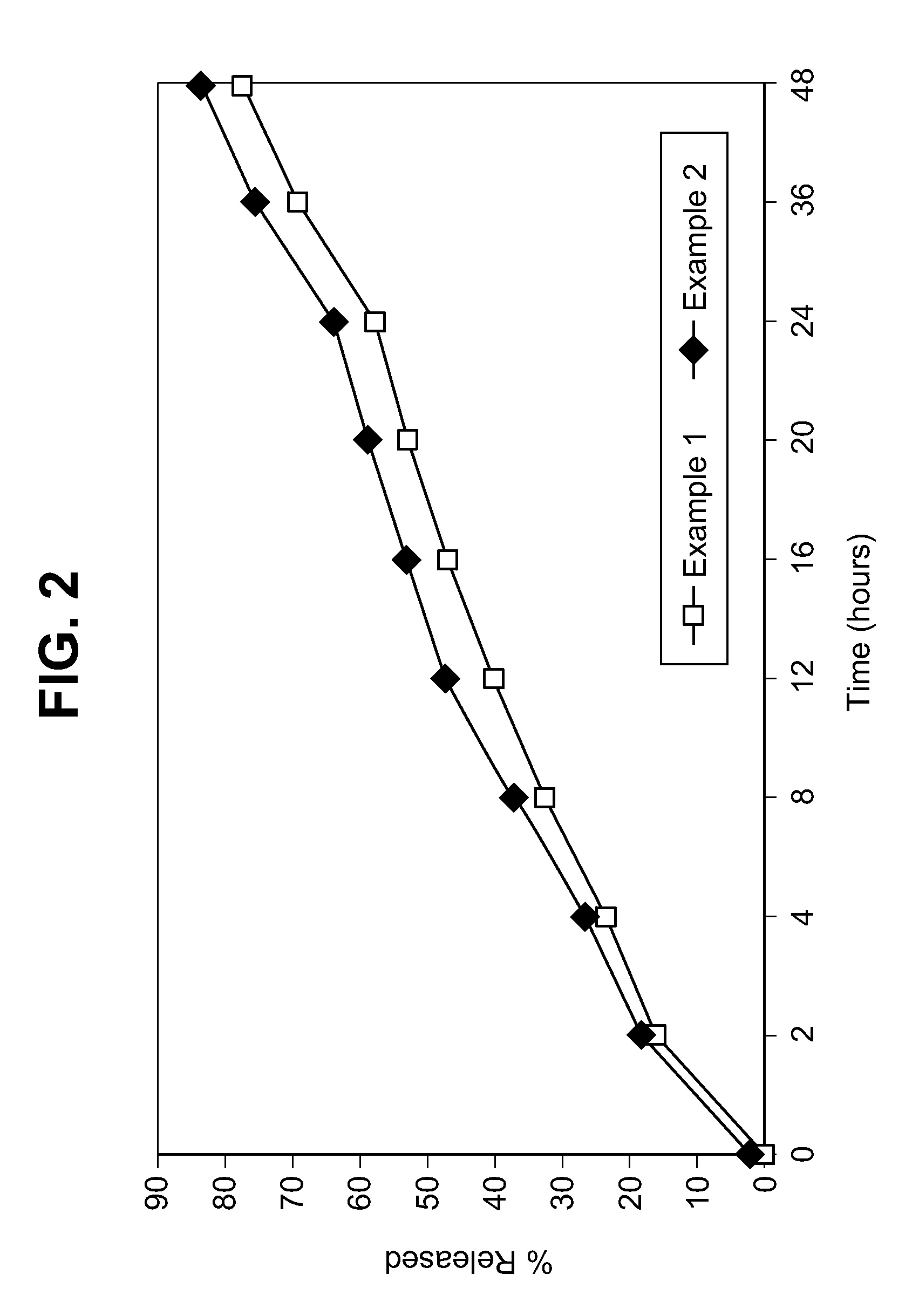
![Oral emulsion rich in [alpha]-linolenic acid and preparation method of oral emulsion Oral emulsion rich in [alpha]-linolenic acid and preparation method of oral emulsion](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/de71c532-5752-4017-bac2-ae6e02e0eb81/170626141914.png)
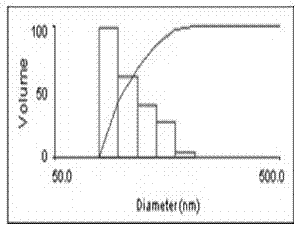
![Oral emulsion rich in [alpha]-linolenic acid and preparation method of oral emulsion Oral emulsion rich in [alpha]-linolenic acid and preparation method of oral emulsion](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/de71c532-5752-4017-bac2-ae6e02e0eb81/170626141919.png)