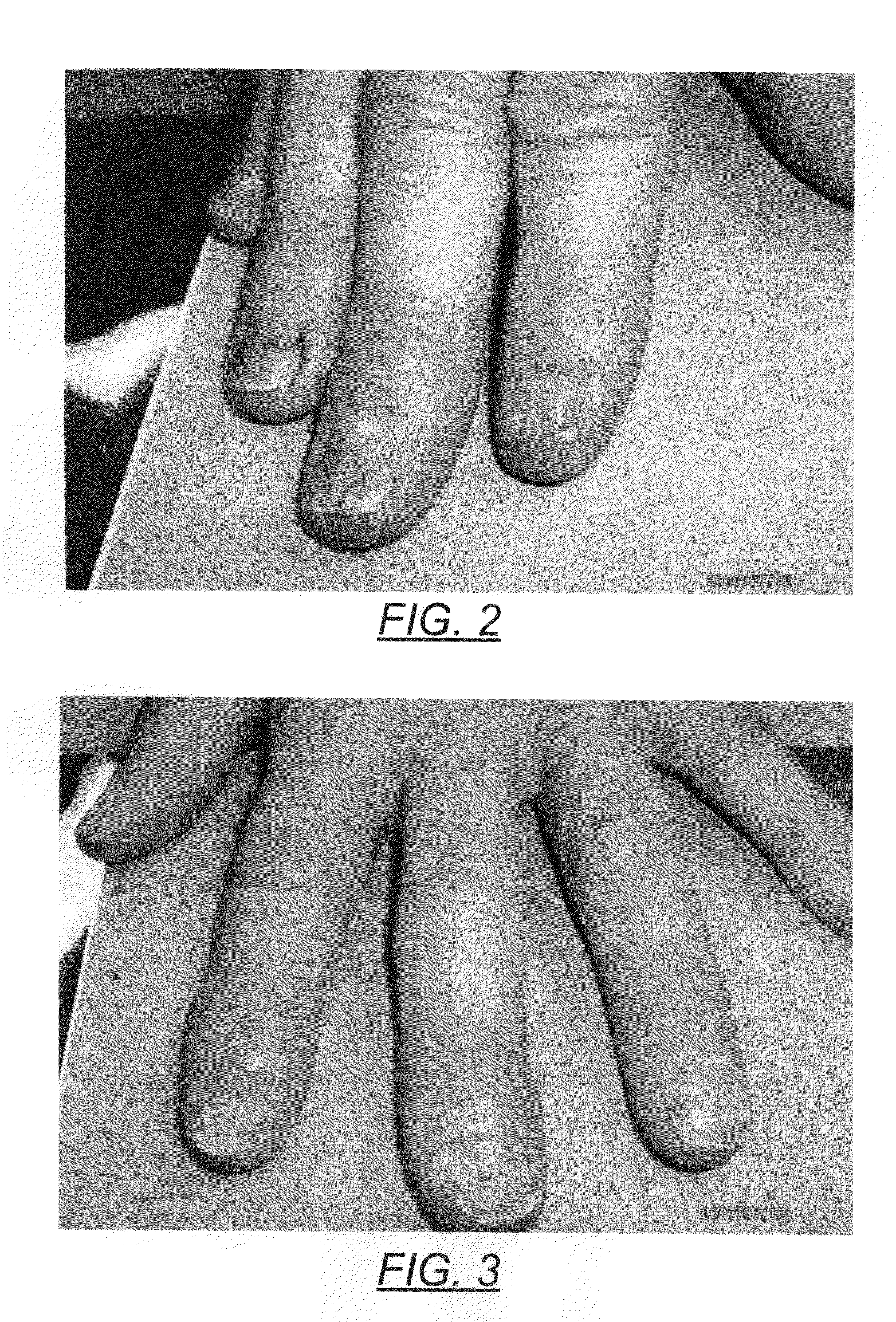
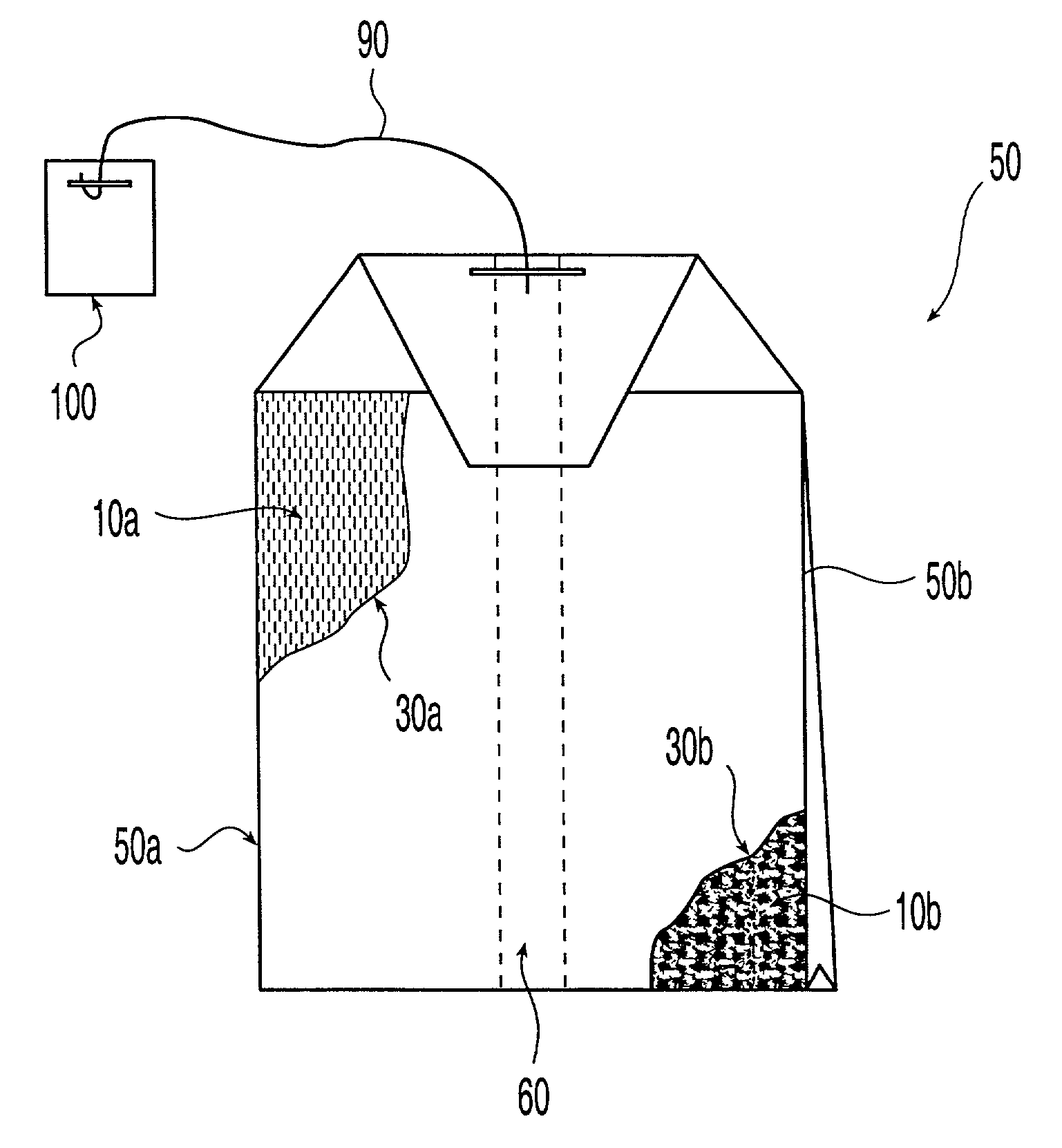
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
780 results about "Buccal administration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Buccal administration is a topical route of administration by which drugs held or applied in the buccal (/ˈbʌkəl/) area (in the cheek) diffuse through the oral mucosa (tissues which line the mouth) and enter directly into the bloodstream. Buccal administration may provide better bioavailability of some drugs and a more rapid onset of action compared to oral administration because the medication does not pass through the digestive system and thereby avoids first pass metabolism.
Smart adapter for infusion devices
ActiveUS20160030683A1Improve safety and efficacyInfusion syringesMicroneedlesPen InjectorDrug administration
Smart sensors are employed to determine one or more of drug identification, dose, flow rate, concentration, agglomeration, and degradation and / or other characteristics of drug administration that can be detected via sensing technology. A smart sensor(s) can be coupled to or retrofitted onto injection pen injectors and / or drug delivery cartridges and / or infusion sets or cannulae, enabling infusion sets, pen injector systems or drug delivery cartridges to improve tracking of drug self-administration and stop medication errors that occur primarily through self or automated injection (e.g., due to incorrect or incomplete dosing, excessive dose or rate, incorrect drug, or drug degradation).
Owner:BECTON DICKINSON & CO
Methods and compositions for oral administration of exenatide
InactiveUS20110046053A1Reducing food intakeDecreased gastric motilityPeptide/protein ingredientsMetabolism disorderDiabetes mellitusOral medication
This invention provides compositions comprising a byetta, fish oil, and a protease inhibitor, method for treating diabetes mellitus, comprising administering same, and methods for oral or rectal administration of a byetta.
Owner:ORAMED
Delayed release formulations for oral administration of a polypeptide therapeutic agent and methods of using same
InactiveUS20040126358A1Increase ionic strengthReduced strengthAntipyreticAnalgesicsOral medicationWhite blood cell
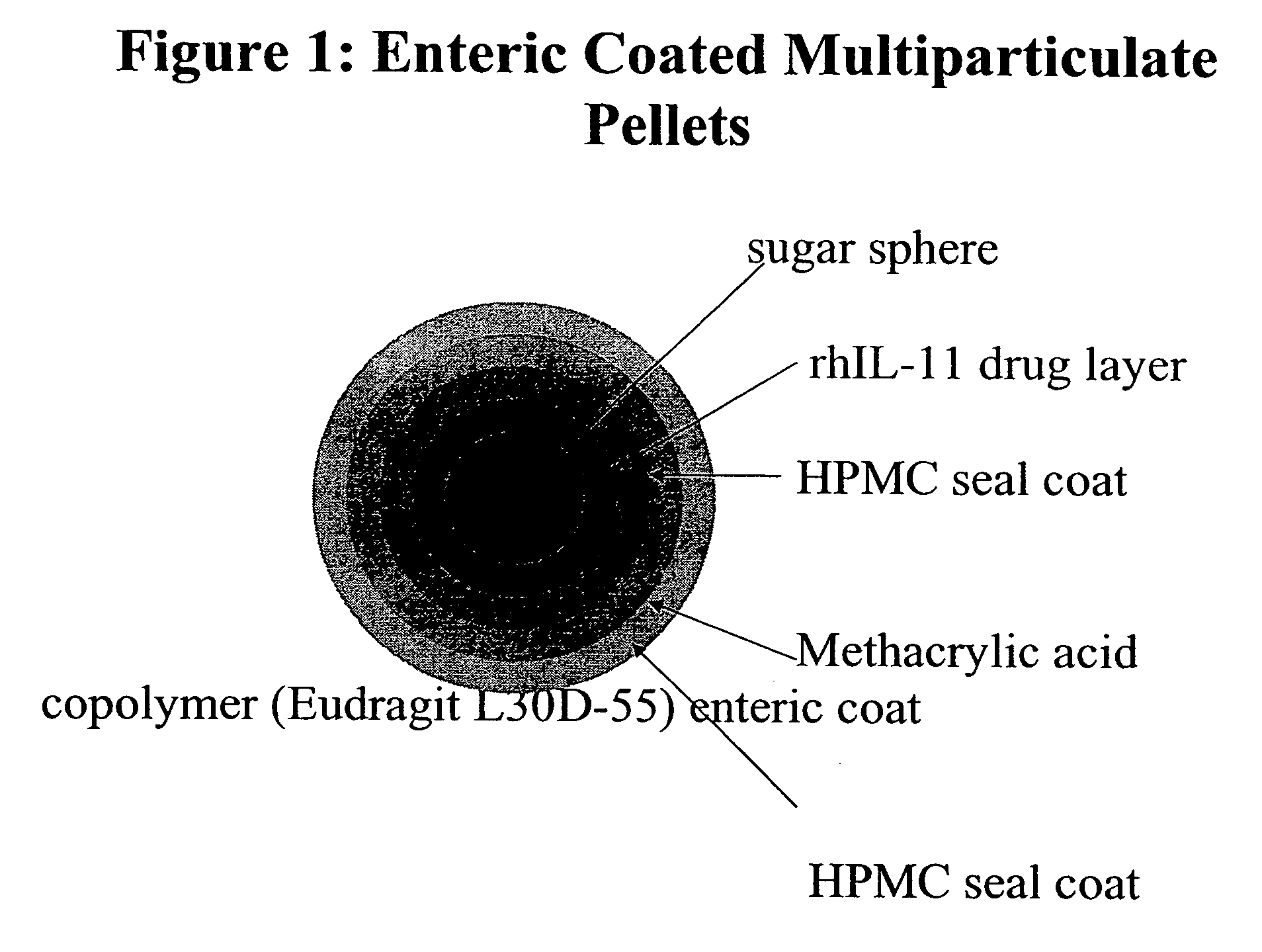
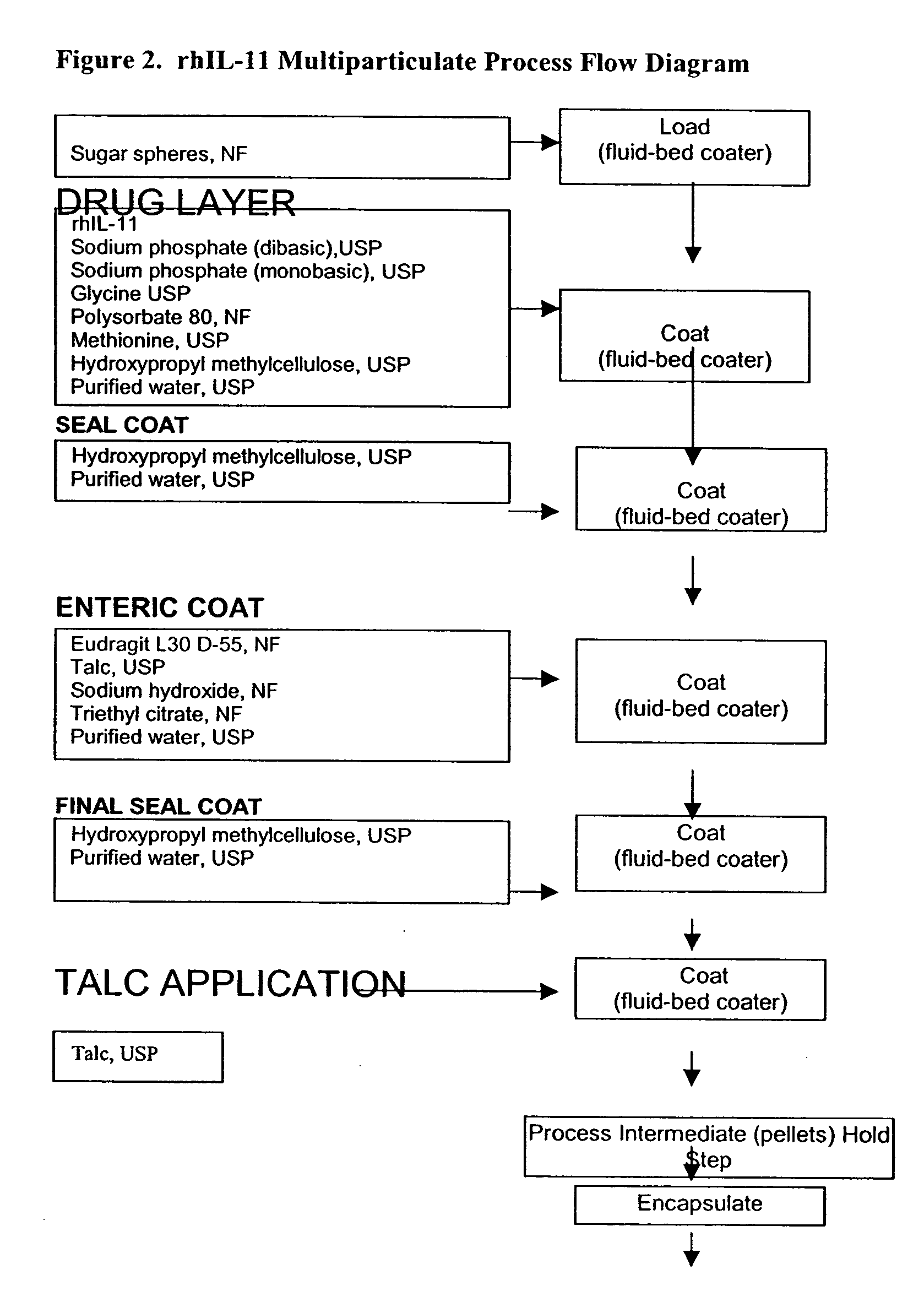
The invention provides compositions containing polypeptides, including therapeutic polypeptides such as interleukin-11, that are suitable for oral administration.
Owner:WYETH LLC
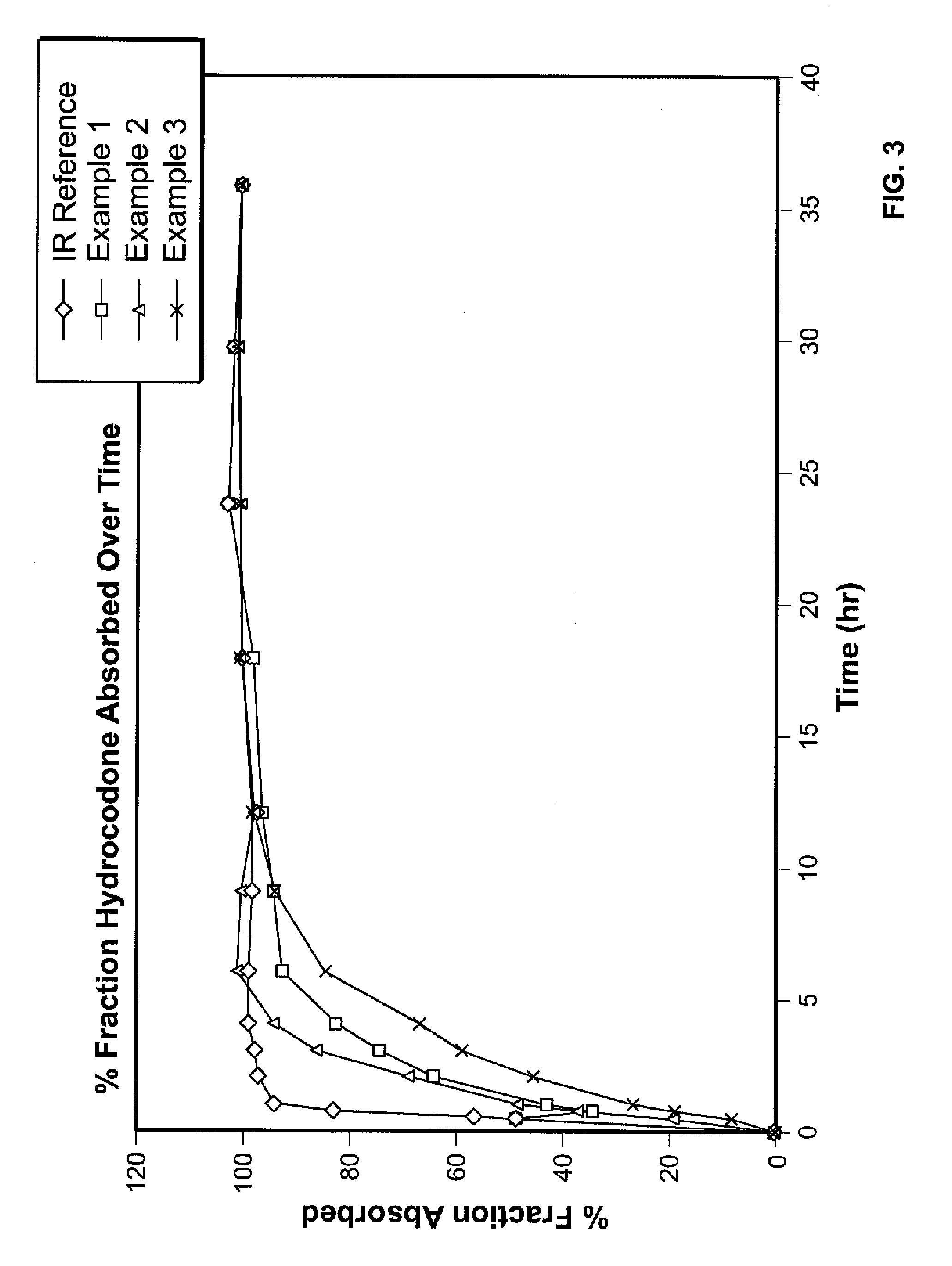
Controlled Release Hydrocodone Formulations
InactiveUS20110262532A1Improve efficiency and qualityGood effectPowder deliveryBiocideControlled releaseHuman patient
A solid oral controlled-release oral dosage form of hydrocodone is disclosed. The dosage form comprising an analgesically effective amount of hydrocodone or a pharmaceutically acceptable salt thereof, and a sufficient amount of a controlled release material to render the dosage form suitable for twice-a-day administration to a human patient, the dosage form providing a C12 / Cmax ratio of 0.55 to 0.85, said dosage form providing a therapeutic effect for at least about 12 hours.
Owner:PURDUE PHARMA LP
Tetracycline compositions for topical administration
Multi-part pharmaceutical formulations containing tetracycline for topical administration, as well as methods of making and administering the same, are disclosed.
Owner:WARNER CHILCOTT CO LLC
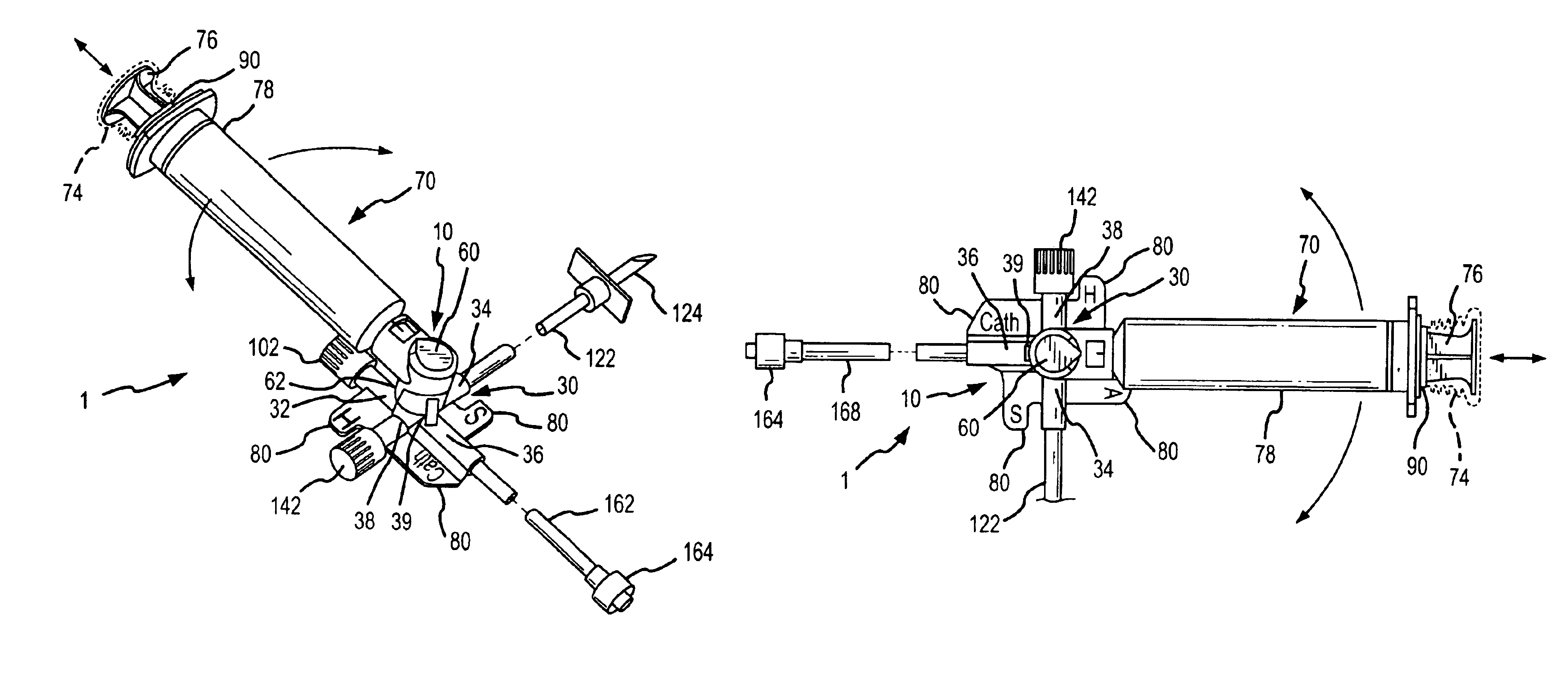
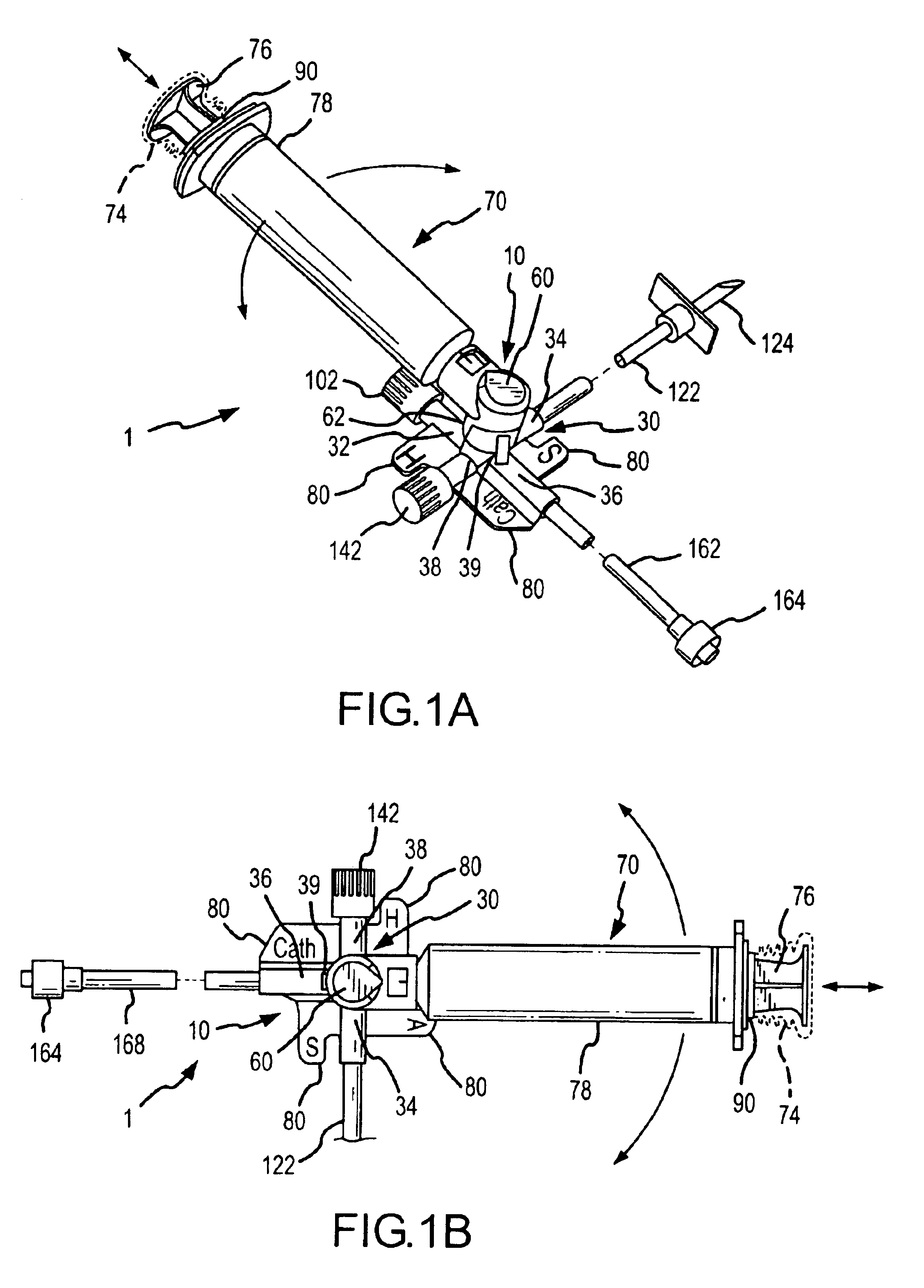
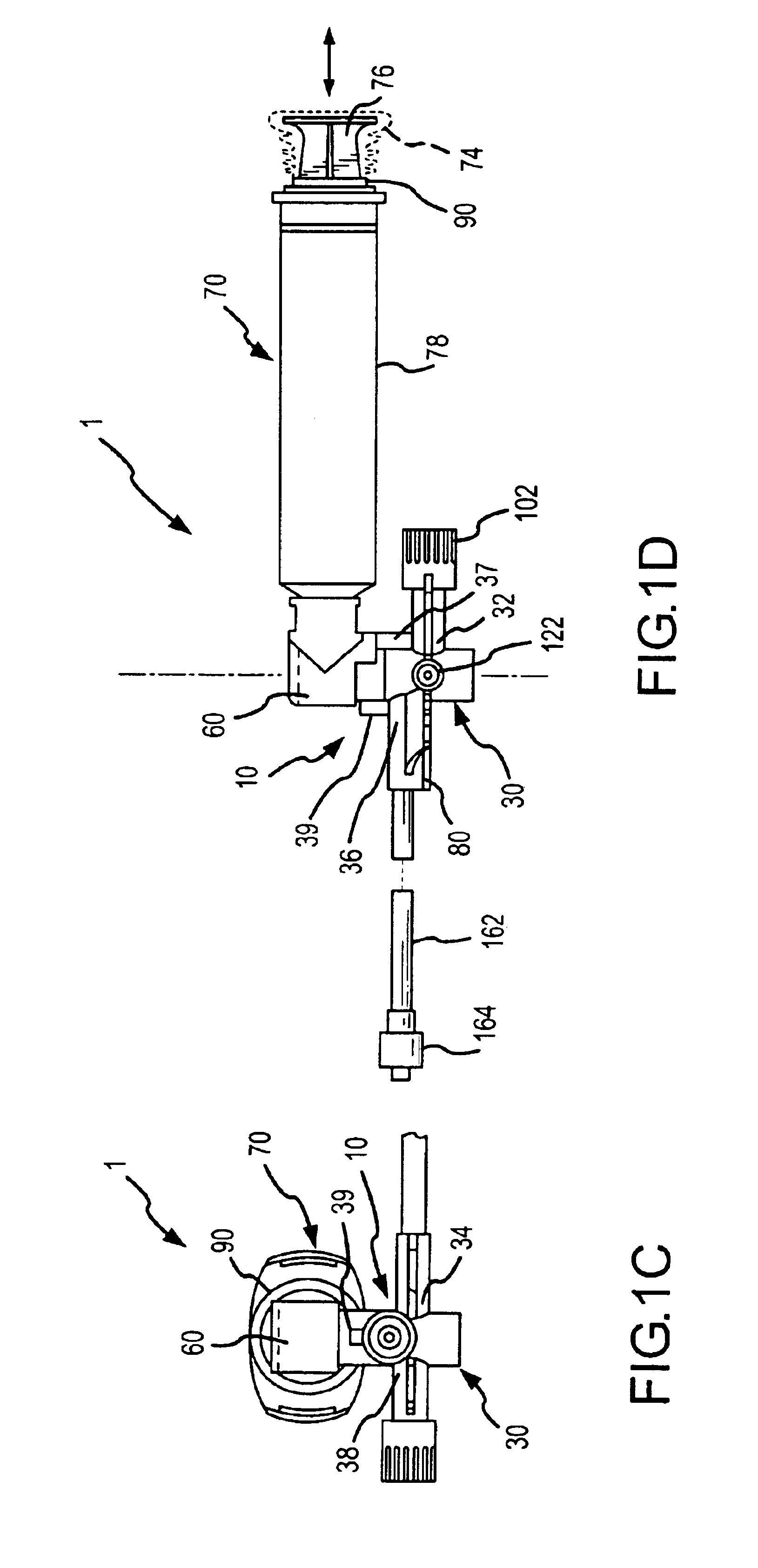
Apparatus and method for administration of IV liquid medication and IV flush solutions
InactiveUS6953450B2Reduce the number of stepsShorten the timeDiaphragm valvesYielding couplingInterconnectionBuccal administration
The present invention provides a medical liquid administration apparatus and administration method that are particularly apt for intravenous (IV) applications. More particularly, the administration apparatus and method may be employed in conjunction with the administration of liquid medication and one or more flush solutions from multi-dose sources, wherein fluid interconnections between at least one flush solution source and the administration apparatus may be established a single time at the outset of a given procedure. The administration apparatus may include a valve having a control member selectively positionable to provide any selected one of a plurality of closed flow paths through the valve, and a syringe interconnected to the control member for clockwise / counterclockwise co-rotation therewith (e.g. to establish the selected flow path).
Owner:BAXTER ENGLEWOOD
Controlled release pharmaceutical compositions comprising a fumaric acid ester
The present invention relates to controlled release pharmaceutical compositions comprising fumaric acid ester(s) as active substance(s). The compositions are suitable for use in the treatment of e.g. psoriasis or other hyperproliferative, inflammatory or autoimmune disorders and are designated to release the fumaric acid ester in a controlled manner so that local high concentrations of the active substance within the gastrointestinal tract upon oral administration can be avoided and, thereby, enabling a reduction in gastro-intestinal related side-effects.
Owner:BIOGEN SWISS MFG GMBH
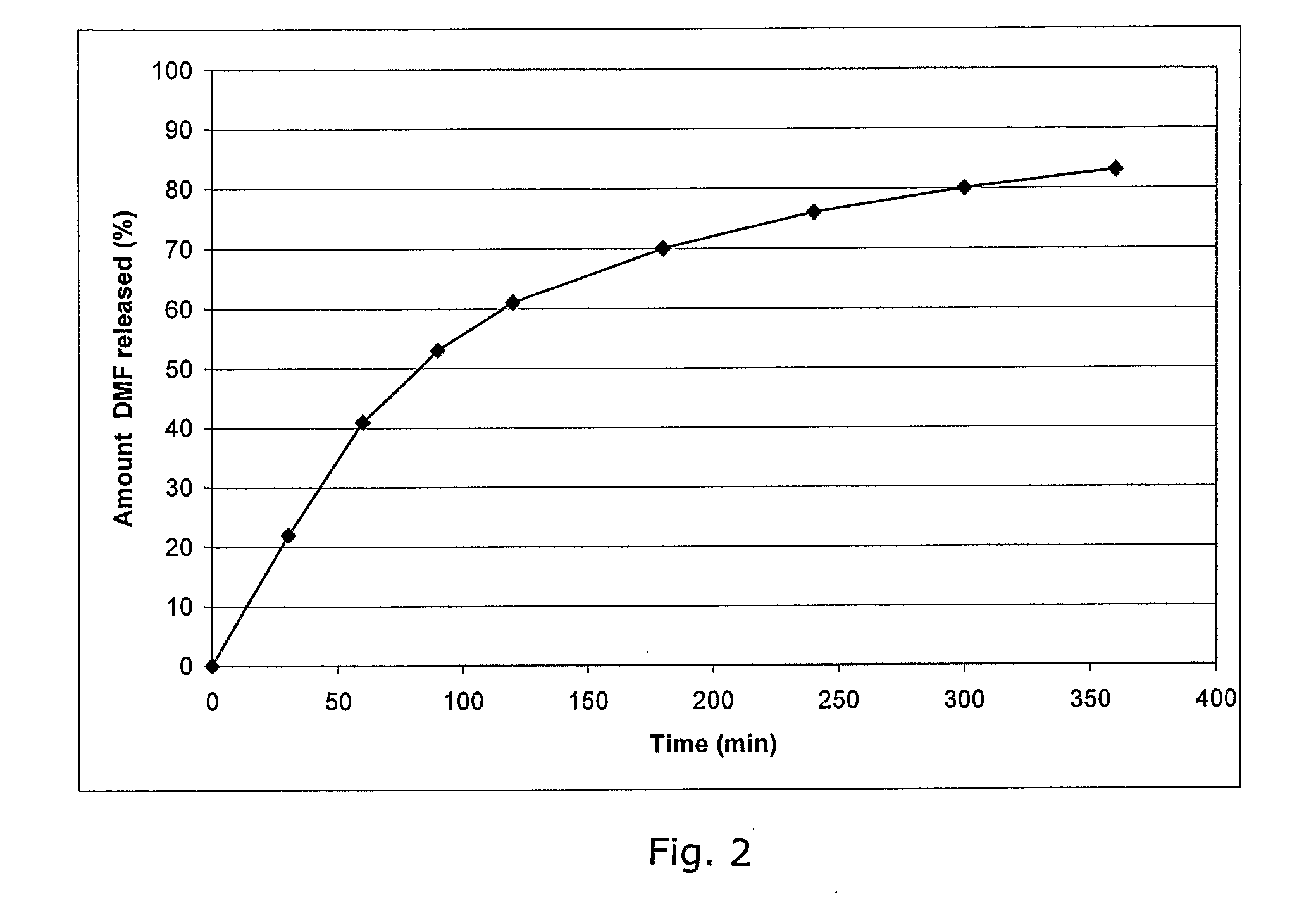
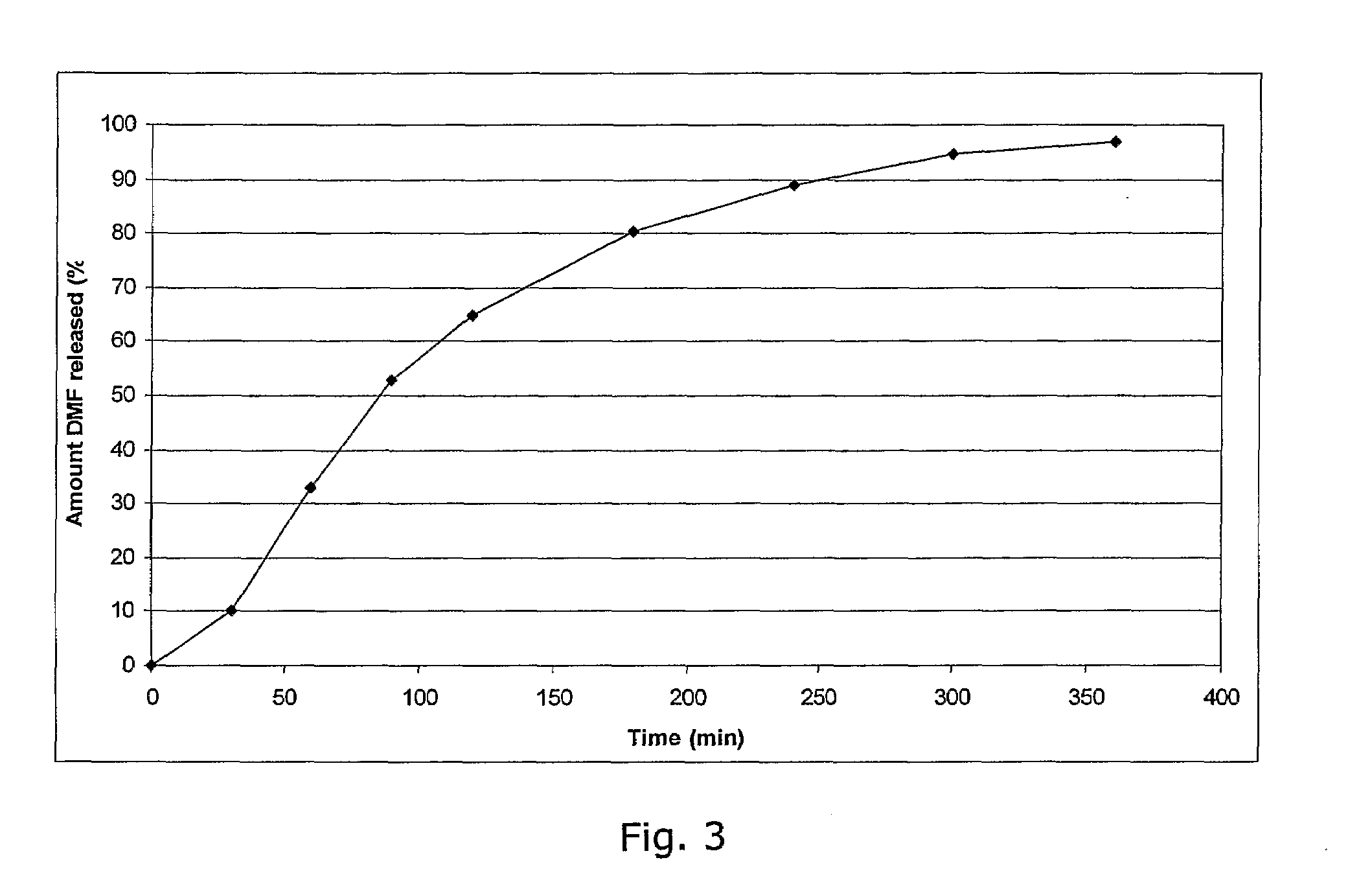
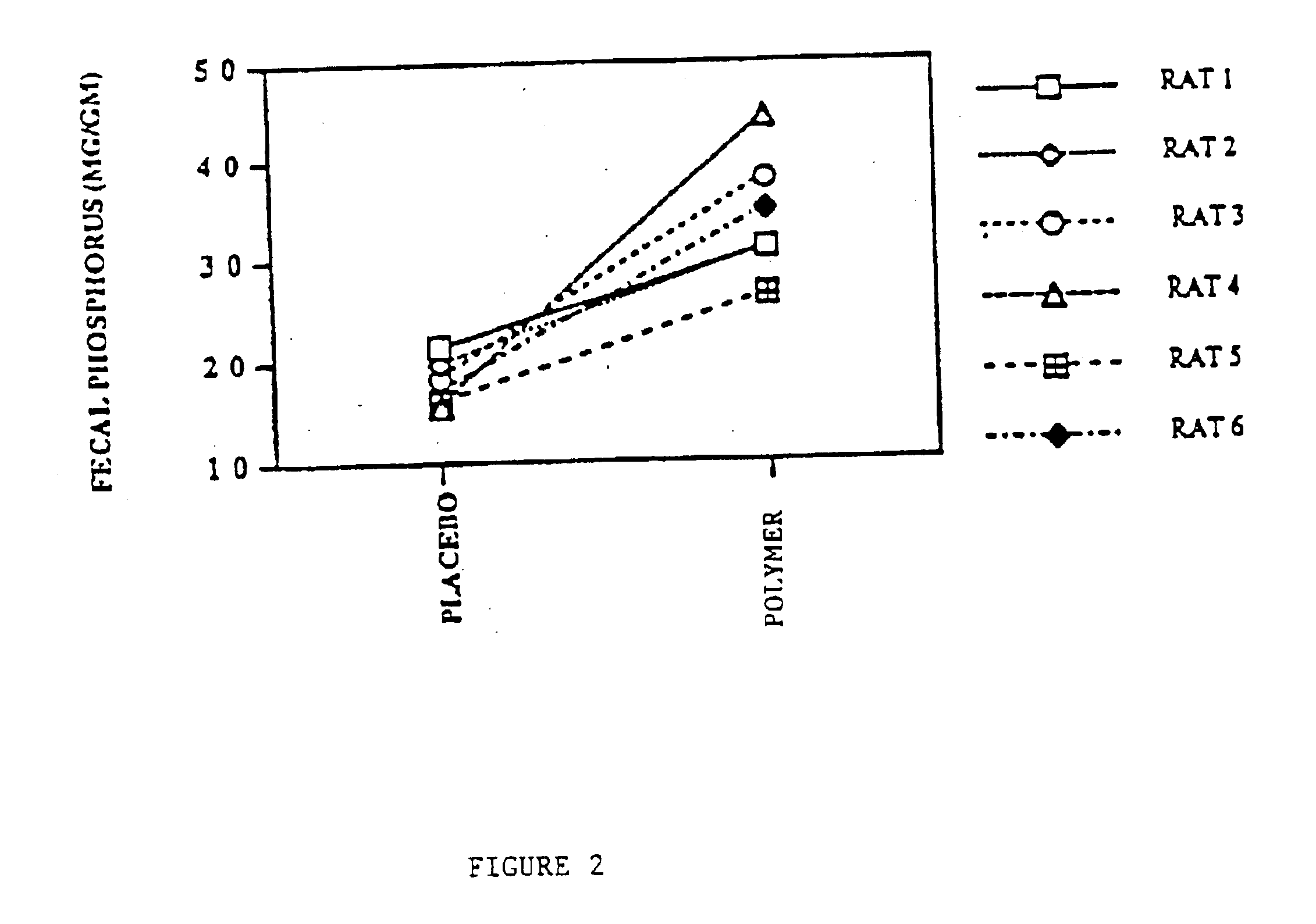
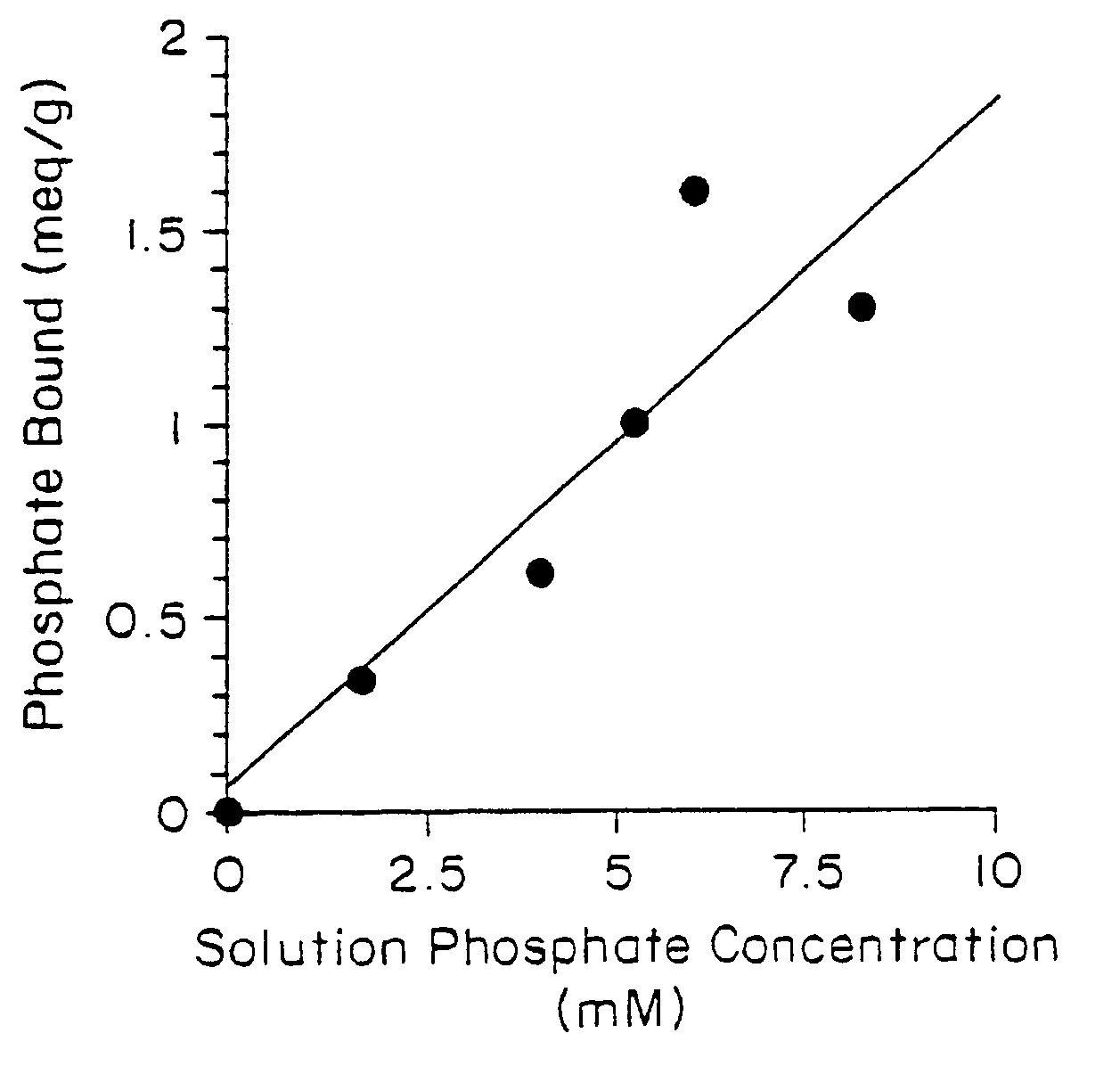
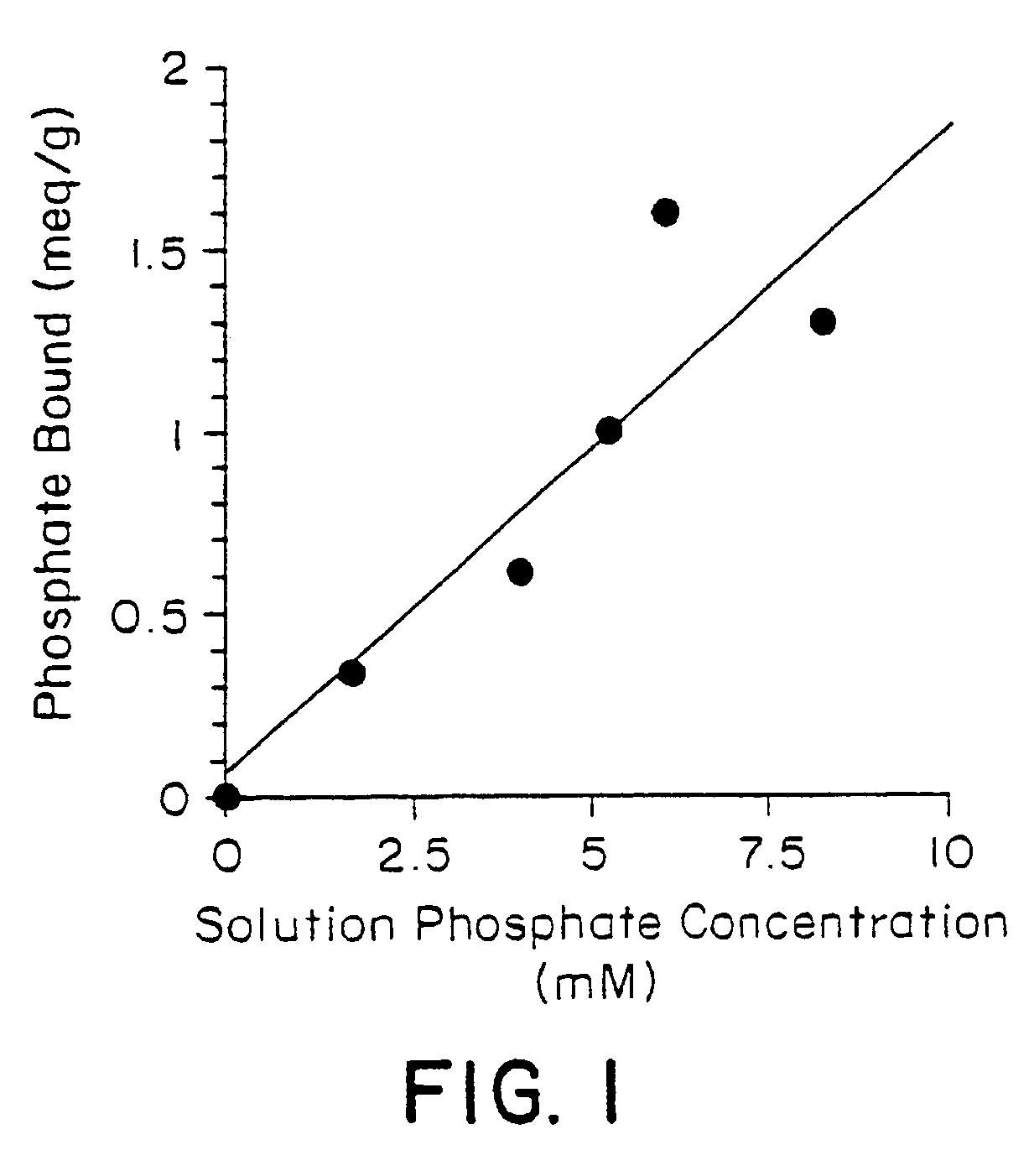
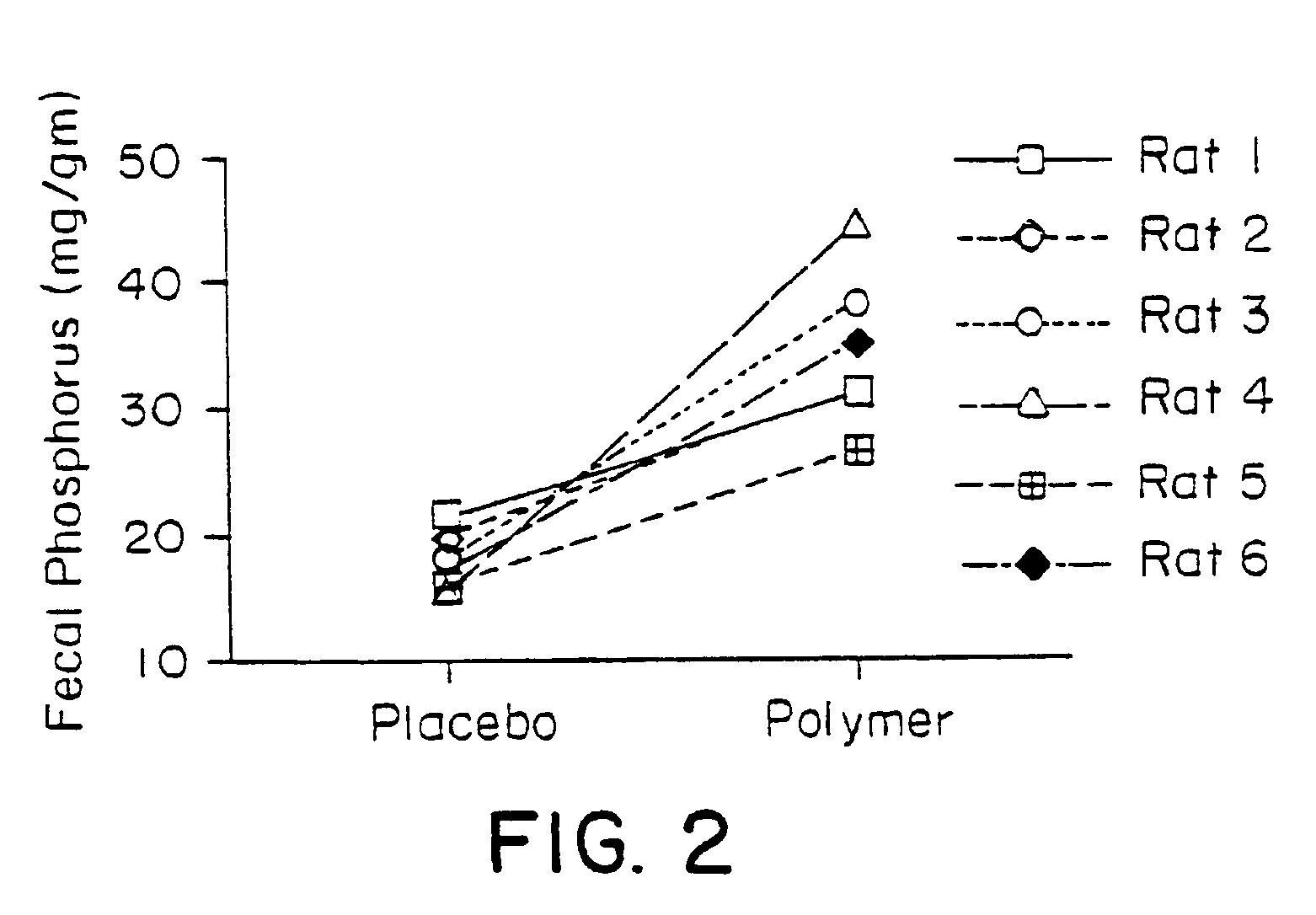
Method of making phosphate-binding polymers for oral administration
InactiveUS6858203B2Low serum levelsPromote absorptionPowder deliveryMetabolism disorderOral medicationBuccal administration
Phosphate-binding polymers are provided for removing phosphate from the gastrointestinal tract. The polymers are orally administered, and are useful for the treatment of hyperphosphatemia.
Owner:GENZYME CORP
Topical administration of basic antifungal compositions to treat fungal infections of the nails
Methods and topical pharmaceutical formulations are provided for the treatment of nail fungus (onychomycosis). The invention involves a pharmacologically active antifungal agent, plus a pharmaceutically acceptable base in a formulation having a pH of 7.5 to about 13.0, preferably about 8.0 to 11.5, and most preferably about 8.5 to 10.5. These basic formulations permeate the nail and are effective in treating fungal infections of the nail and surrounding tissues.
Owner:DERMATRENDS INC
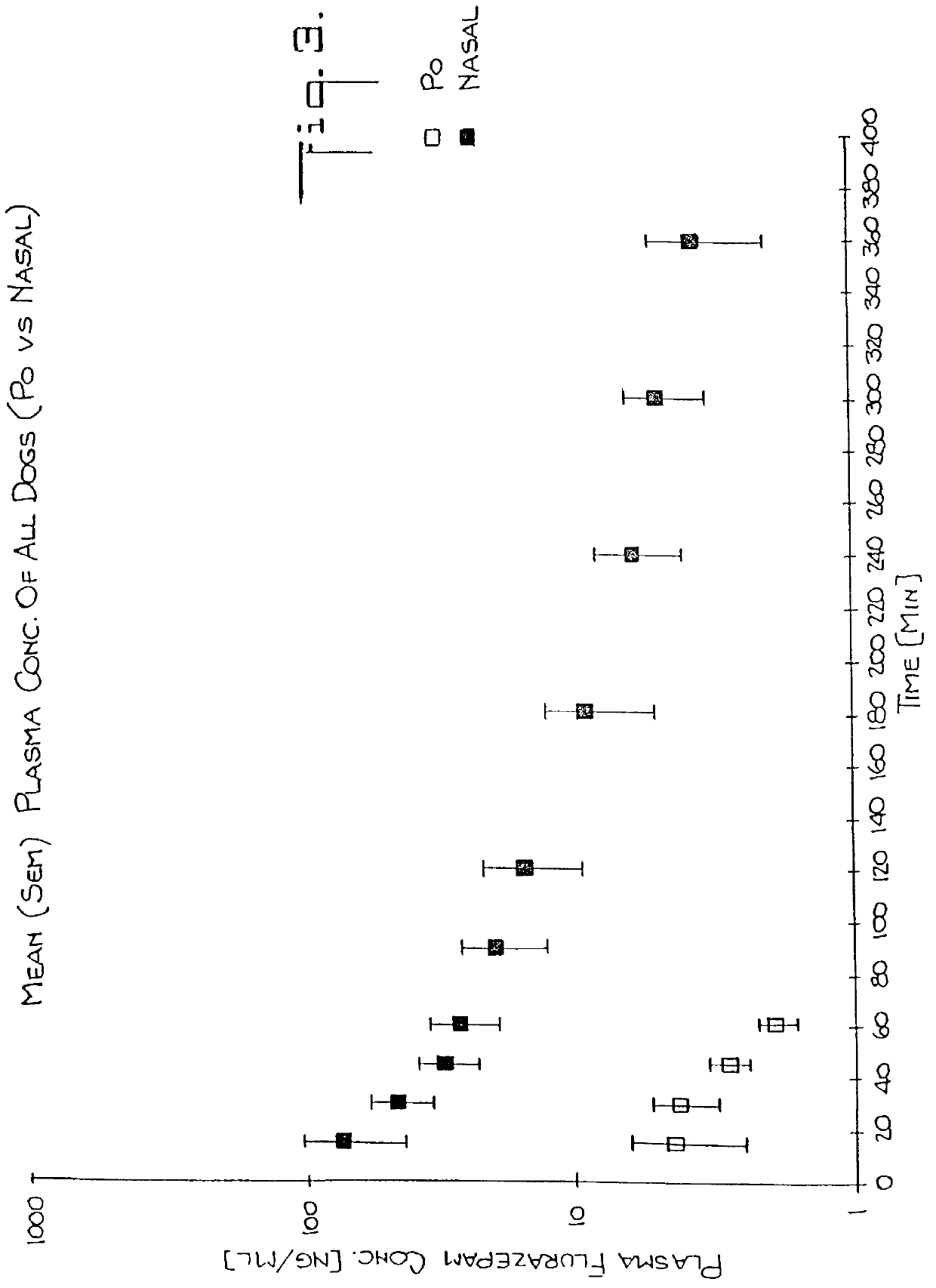
Nasal administration of benzodiazepine hypnotics
Nasal administration of benzodiazepines is described as providing improved therapeutic effects as compared to conventional delivery techniques. The compositions comprise a benzodiazepine hypnotic in a pharmaceutically acceptable nasal carrier.
Owner:QUESTCOR PHARMA
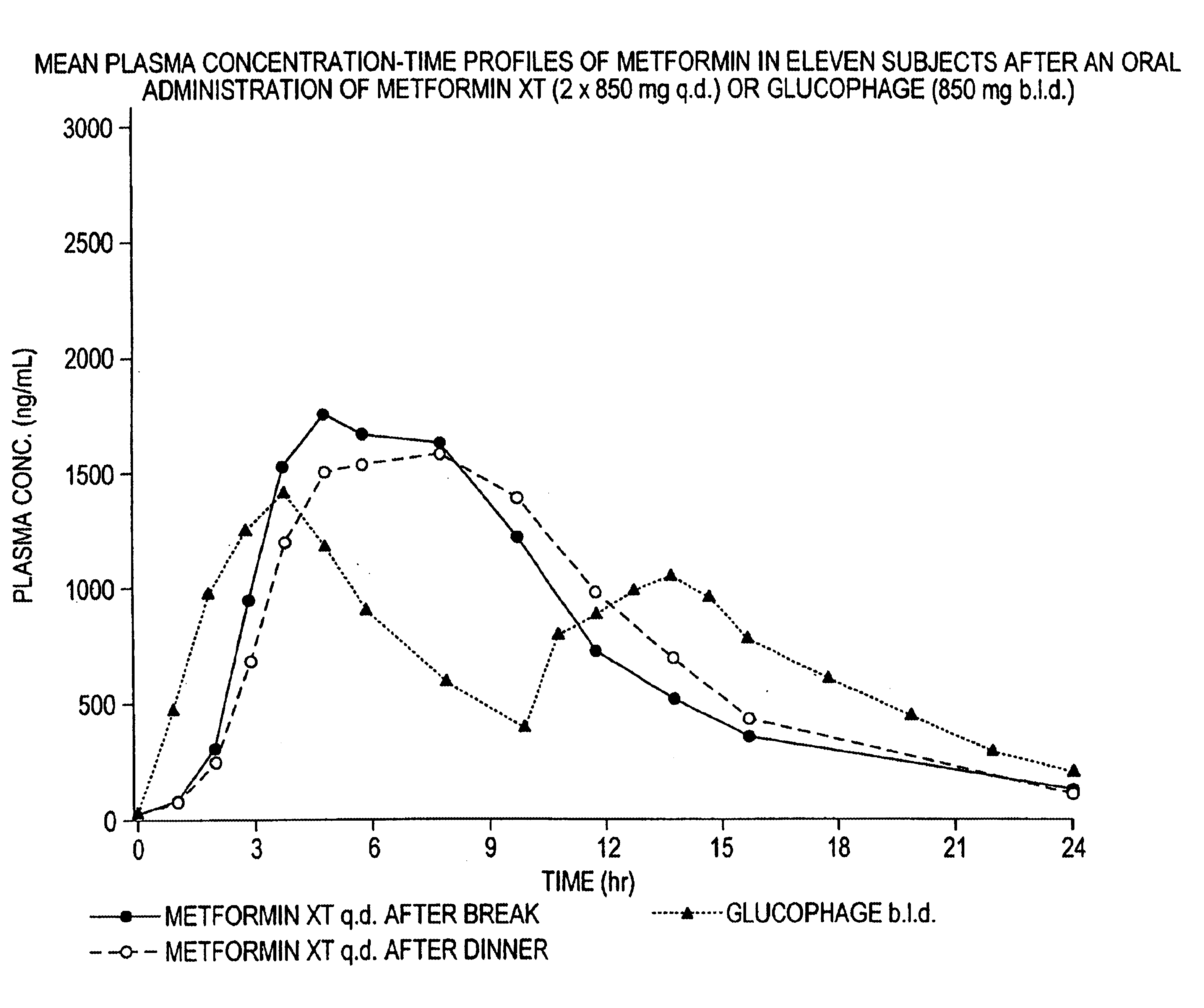
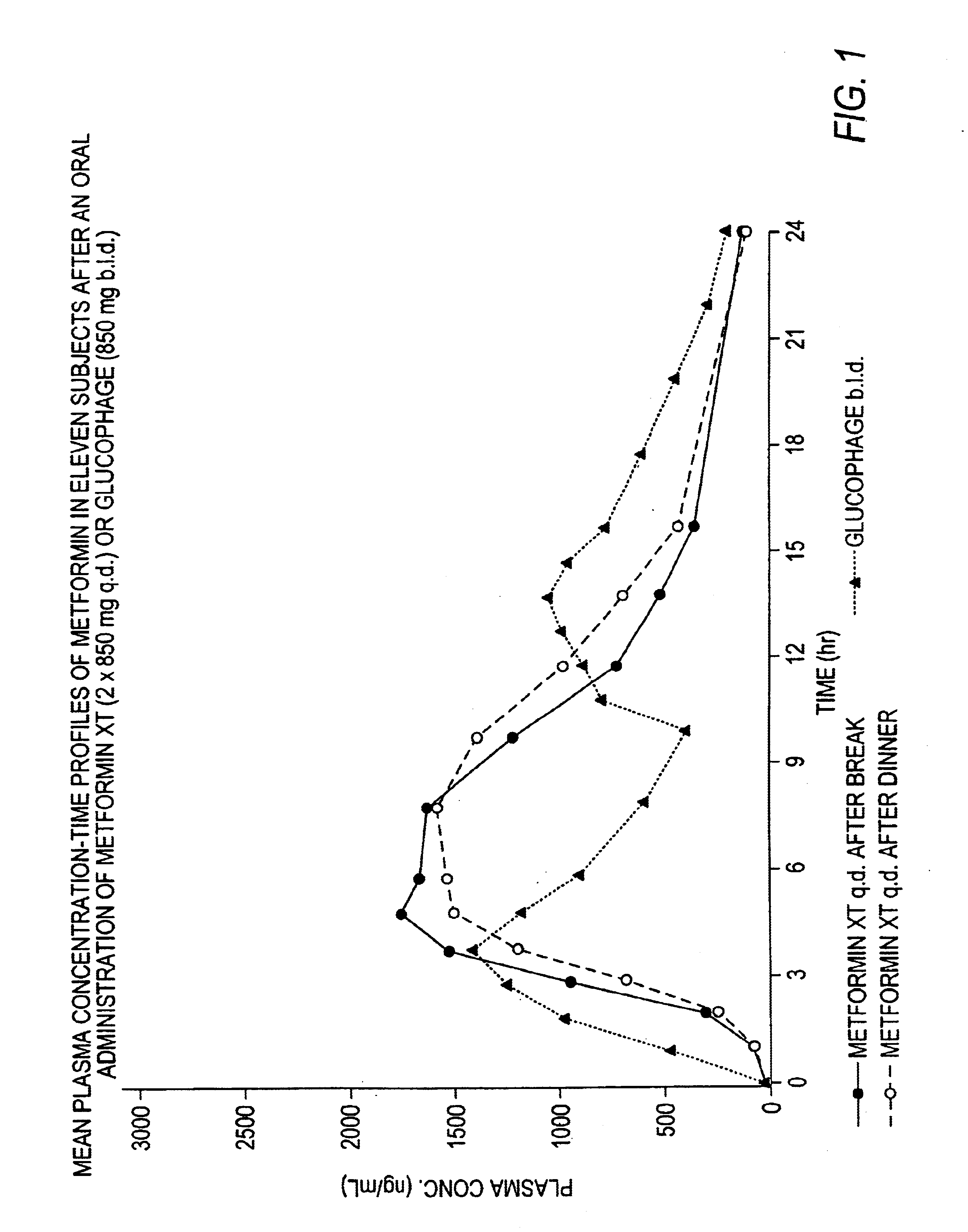
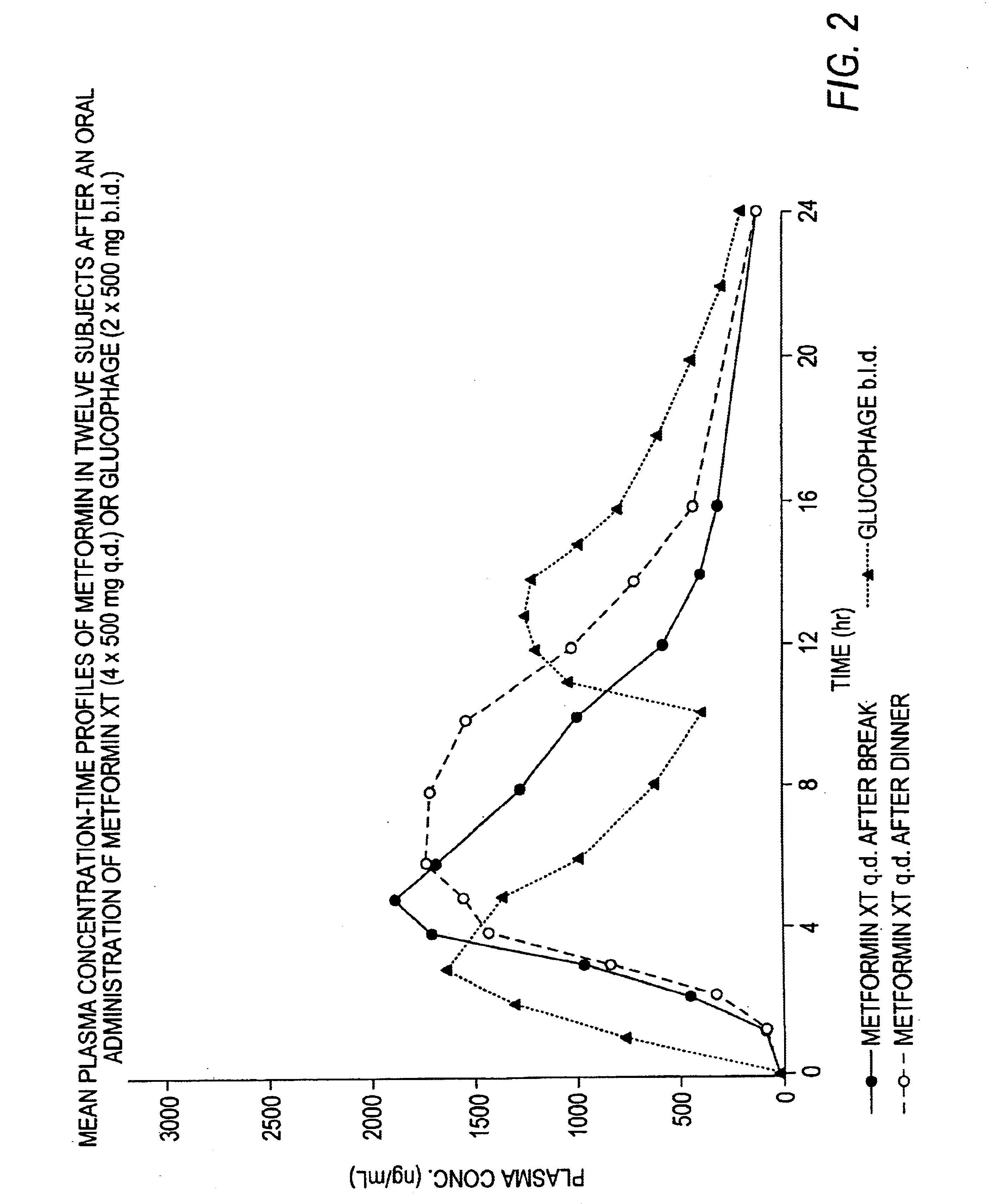
Controlled release metformin compositions
InactiveUS6866866B1Effective controlImprove bioavailabilityOrganic active ingredientsCoatingsCo administrationBlood plasma
A composition for treating patients having non-insulin-dependent diabetes mellitus (NIDDM) by administering a controlled release oral solid dosage form containing preferably a biguanide drug such as metformin, on a once-a-day basis. The dosage form provides a mean time to maximum plasma concentration (Tmax) of the drug which occurs at 5.5 to 7.5 hours after oral administration on a once-a-day basis to human patients. Preferably, the dose of drug is administered at dinnertime to a patient in the fed state.
Owner:ANDRX LABS
Oral administration form for an acid liable active proton pump inhibitor
Novel administration form for acid-labile active compounds are described. The novel administration forms have no enteric layers and are suitable for oral administration.
Owner:TAKEDA GMBH
Controlled Release Pharmaceutical Compositions Comprising a Fumaric Acid Ester
InactiveUS20080299196A1Decrease glass transition pointLow film forming temperatureBiocideSenses disorderDiseaseHigh concentration
The present invention relates to controlled release pharmaceutical compositions comprising fumaric acid ester(s) as active substance(s). The compositions are suitable for use in the treatment of e.g. psoriasis or other hyperproliferative, inflammatory or autoimmune disorders and are designed to release the fumaric acid ester in a controlled manner so that local high concentrations of the active substance within the gastrointestinal tract upon oral administration can be avoided and, thereby, enabling a reduction in gastro-intestinal related side-effects.
Owner:BIOGEN SWISS MFG GMBH
Composition and method for inhibiting platelet aggregation
The present invention provides novel compounds of dinucleotide polyphosphates and the method of preventing or treating diseases or conditions associated with platelet aggregation. The method comprises administering systemically to a patient a pharmaceutical comprising a purinergic P2τ receptor antagonist, in an amount effective to elevate its extracellular concentration to bind to P2τ receptors and inhibit P2τ receptor-mediated platelet aggregation. Methods of systemic administration include injection by intravenous, intramuscular, intrasternal and intravitreal routes, infusion, transdermal administration, oral administration, rectal administration and intra-operative instillation.
Owner:INSPIRE PHARMA +1
Multimicroparticulate pharmaceutical forms for oral administration
InactiveUS20070264346A1Great therapeutic safetyGood effectOrganic active ingredientsPowder deliveryAlcohol freeMicroparticle
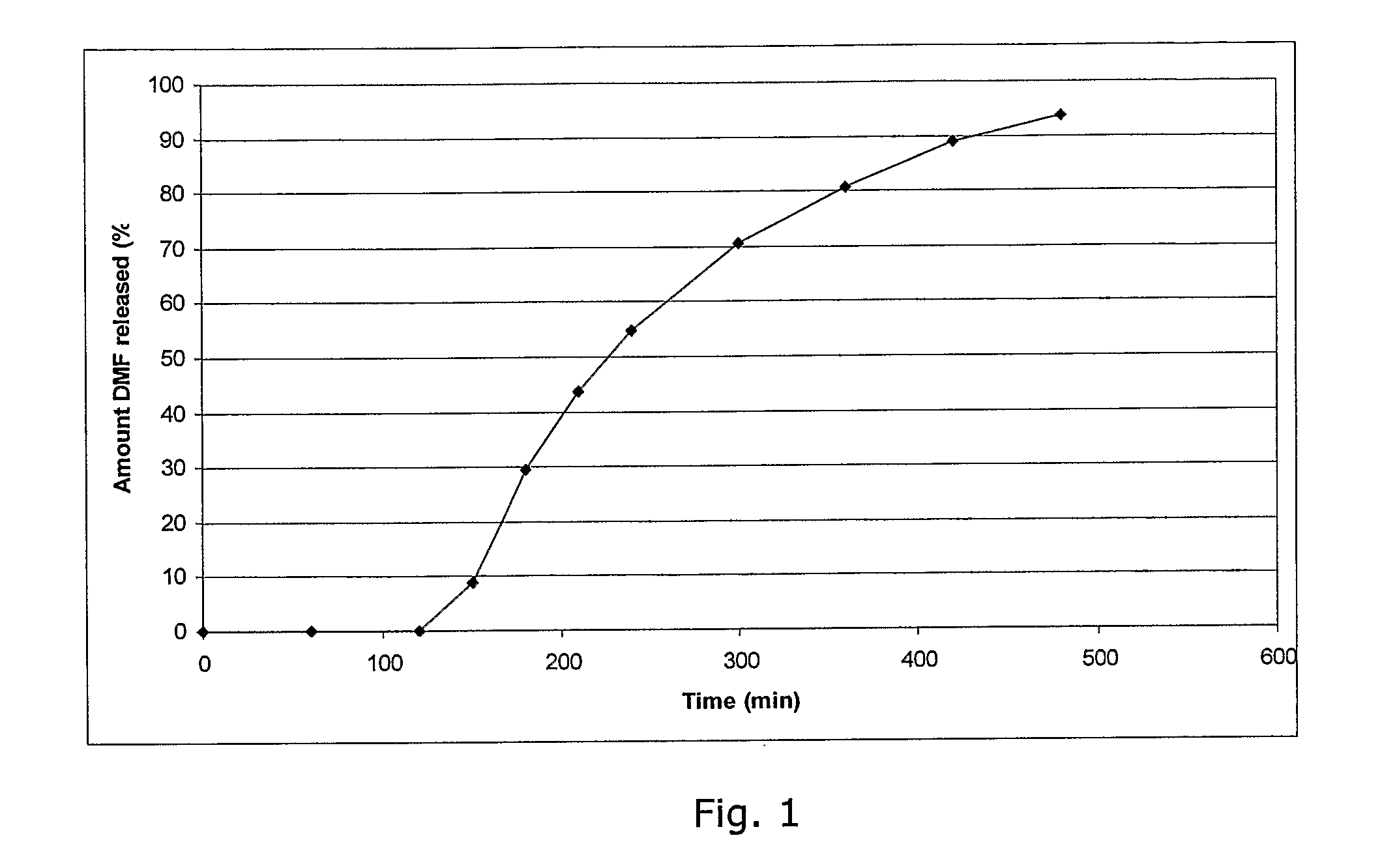
The object of the present invention is to minimize the risks of dose dumping associated with the concomitant consumption of alcohol and certain modified-release pharmaceutical or dietetic forms. The invention relates to an oral form comprising microparticles of the reservoir type for the modified release of at least one active principle (AP), characterized in that it is resistant to immediate dumping of the dose of AP in the presence of alcohol. In particular, the oral form according to the invention is characterized in that the time taken to release 50% of the AP in an alcoholic solution is not reduced more than 3-fold relative to the time taken to release 50% of the AP in an alcohol-free aqueous medium. The form comprises an agent D, which is a pharmaceutically acceptable compound whose hydration or solvation rate or capacity is greater in an alcohol-free aqueous medium than in alcoholic solution
Owner:FLAMEL IRELAND
Dosage unit for sublingual, buccal or oral administration of water-insoluble pharmaceutically active substances
ActiveUS20100008985A1Disperse fastEfficient packagingBiocidePowder deliveryWater insolubleProphylactic treatment
One aspect of the invention relates to a pharmaceutical dosage unit for sublingual, buccal, pulmonary or oral administration, said dosage unit having a weight of 20-500 mg and comprising 1-80 Wt. % of a microgranulate that is distributed throughout a solid hydrophilic matrix; said microgranulate being characterised in that it: has a volume weighted average diameter of 5-100 m; contains at least 0.01 wt. %, preferably at least 0.1 wt. % of one or more water-insoluble pharmaceutically active substances; contains at least 10 wt. %, preferably at least 20 wt. % of an emulsifier component; and is capable of forming a micro-emulsion upon contact with saliva or water. The dosage units of the present invention achieve the inherent benefits of oral delivery whilst at the same time realising a high transmucosal absorption rate of the cannabinoids contained therein. Other aspects of the present invention relate to the use of the aforementioned dosage units in the therapeutic or prophylactic treatment and to a process for the manufacture of said dosage units.
Owner:ECHO PHARM BV (NL)
Transnasal anticonvulsive compositions and modulated process
InactiveUS6627211B1Promote absorptionIncrease permeationBiocideNervous disorderCo administrationHigh plasma
A method of vehicle modulated administration of an anticonvulsive agent to the nasal mucous membranes of humans and animals is disclosed. The vehicle system is an aqueous pharmaceutical carrier comprising an aliphatic alcohol, a glycol and a biological surfactant such as a bile salt or a lecithin. The pharmaceutical composition provides a means to control and promote the rate and extent of transmucosal permeation and absorption of the medicaments via a single and multiple administration. Nasal administration of the pharmaceutical preparation produces a high plasma concentration of the anticonvulsant nearly as fast as intravenous administration. Such compositions are particularly suitable for a prompt and timely medication of patients in the acute and / or emergency treatment of status epilepticus and other fever-induced seizures.
Owner:BIOPHARM
Therapeutic treatments using botulinum neurotoxin
ActiveUS20090232850A1Eliminates sensory inputImprove developmentBacterial antigen ingredientsNervous disorderDiseaseObstructive Pulmonary Diseases
Methods for treating a coronary risk factor (such as hypertension, diabetes, hyperlipidemia and obesity) and / or a respiratory disorder (such as asthma, chronic obstructive pulmonary disease and bronchitis) and / or arthritis by local administration of a botulinum neurotoxin to at least one of a head, neck or shoulder location (for example, by subdermal, subcutaneous or intramuscular administration of the botulinum neurotoxin) of a patient with a coronary risk factor, respiratory disorder or arthritis.
Owner:ALLERGAN INC
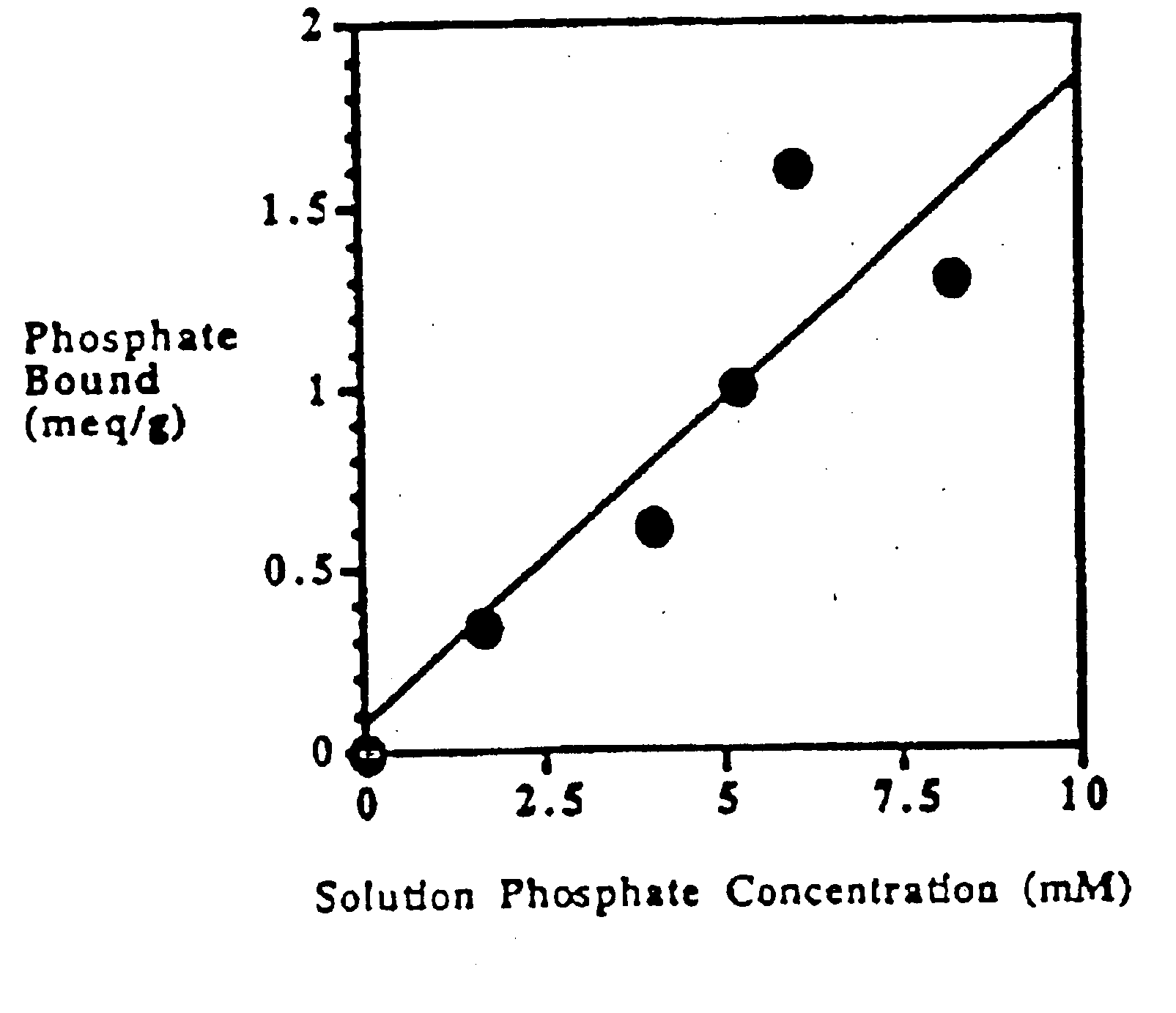
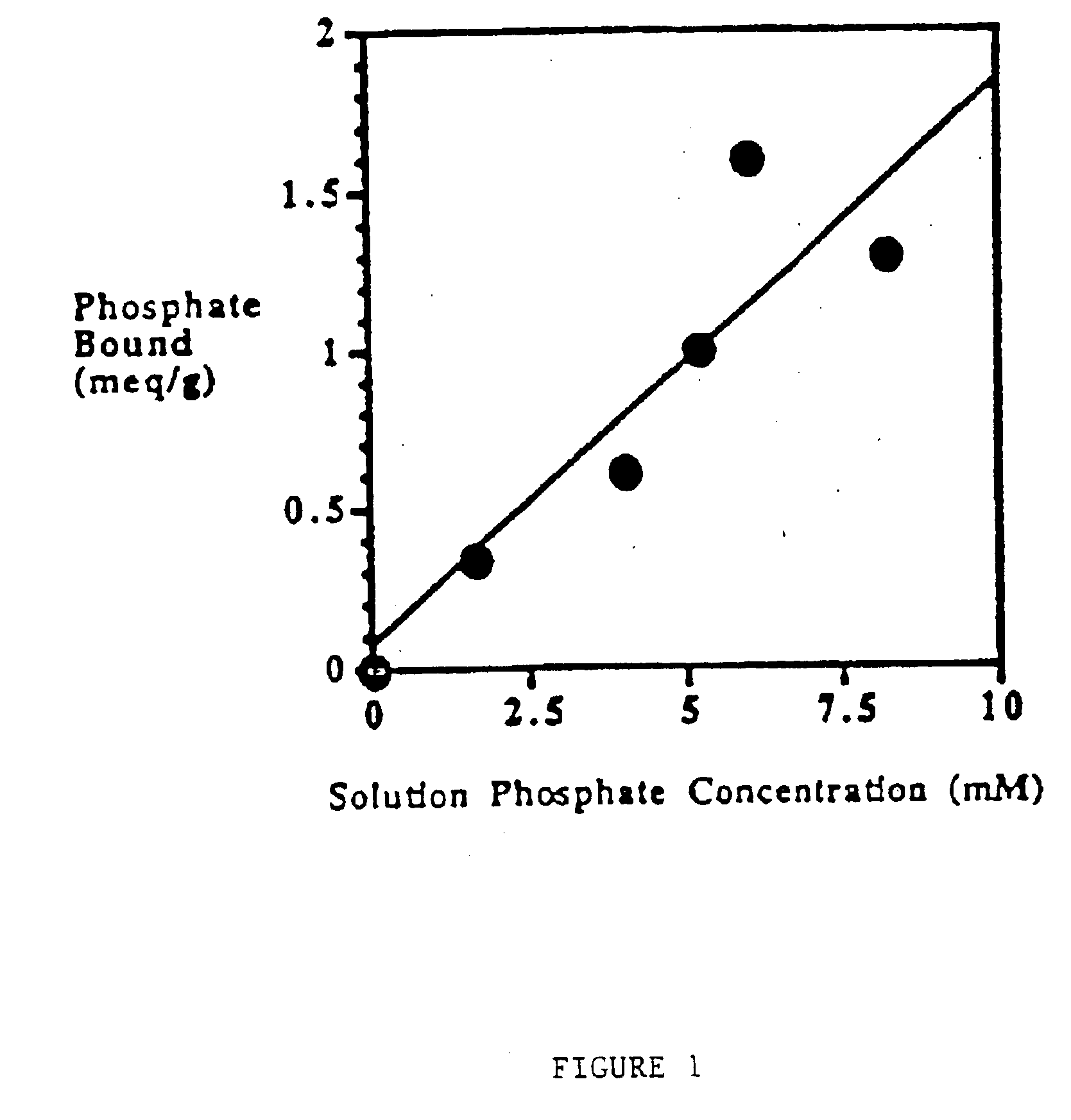
Phosphate-binding polymers for oral administration
InactiveUS7014846B2Low serum levelsPromote absorptionPowder deliveryMetabolism disorderOral medicationBuccal administration
Phosphate-binding polymers are provided for removing phosphate from the gastrointestinal tract. The polymers are orally administered, and are useful for the treatment of hyperphosphatemia.
Owner:GENZYME CORP
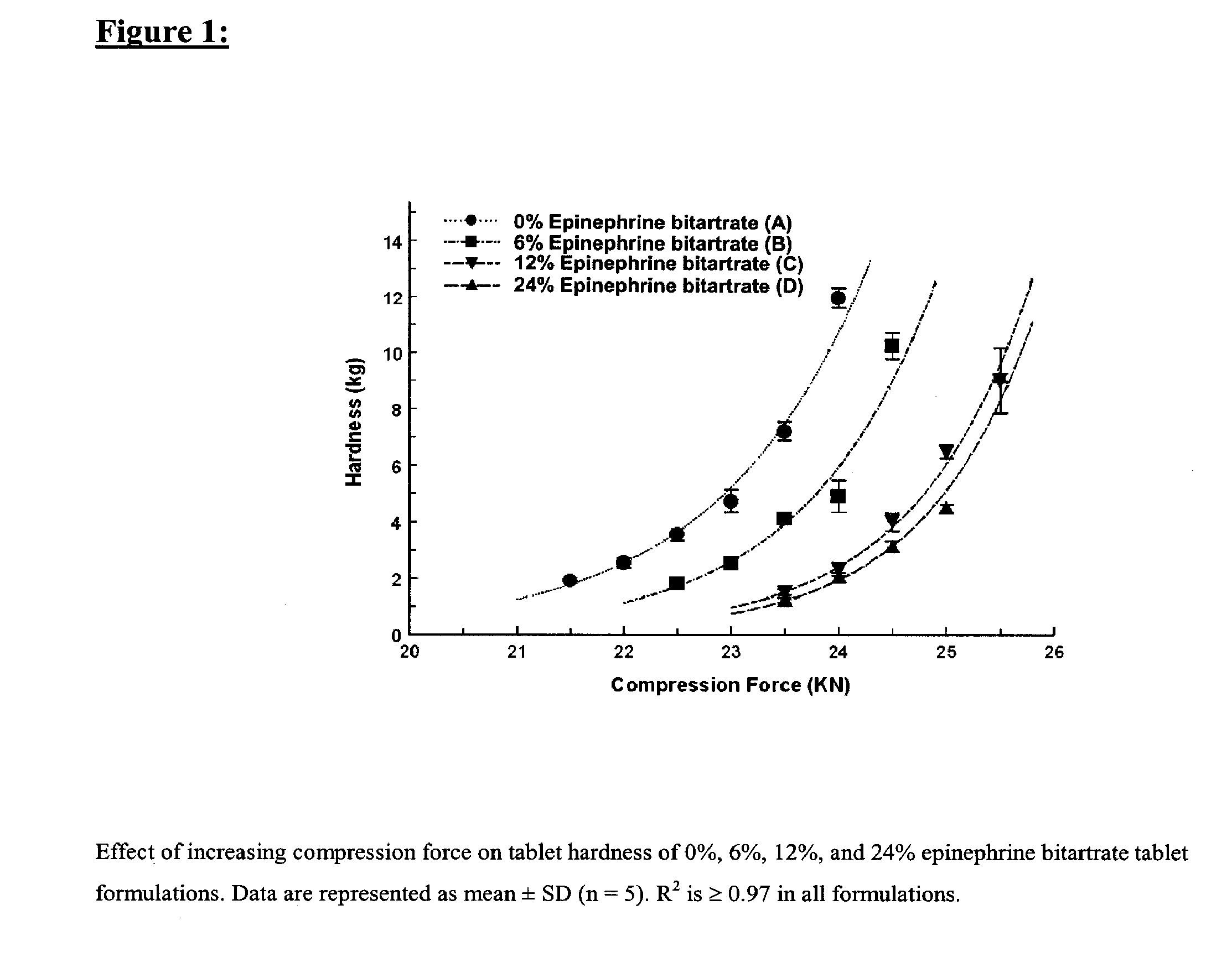
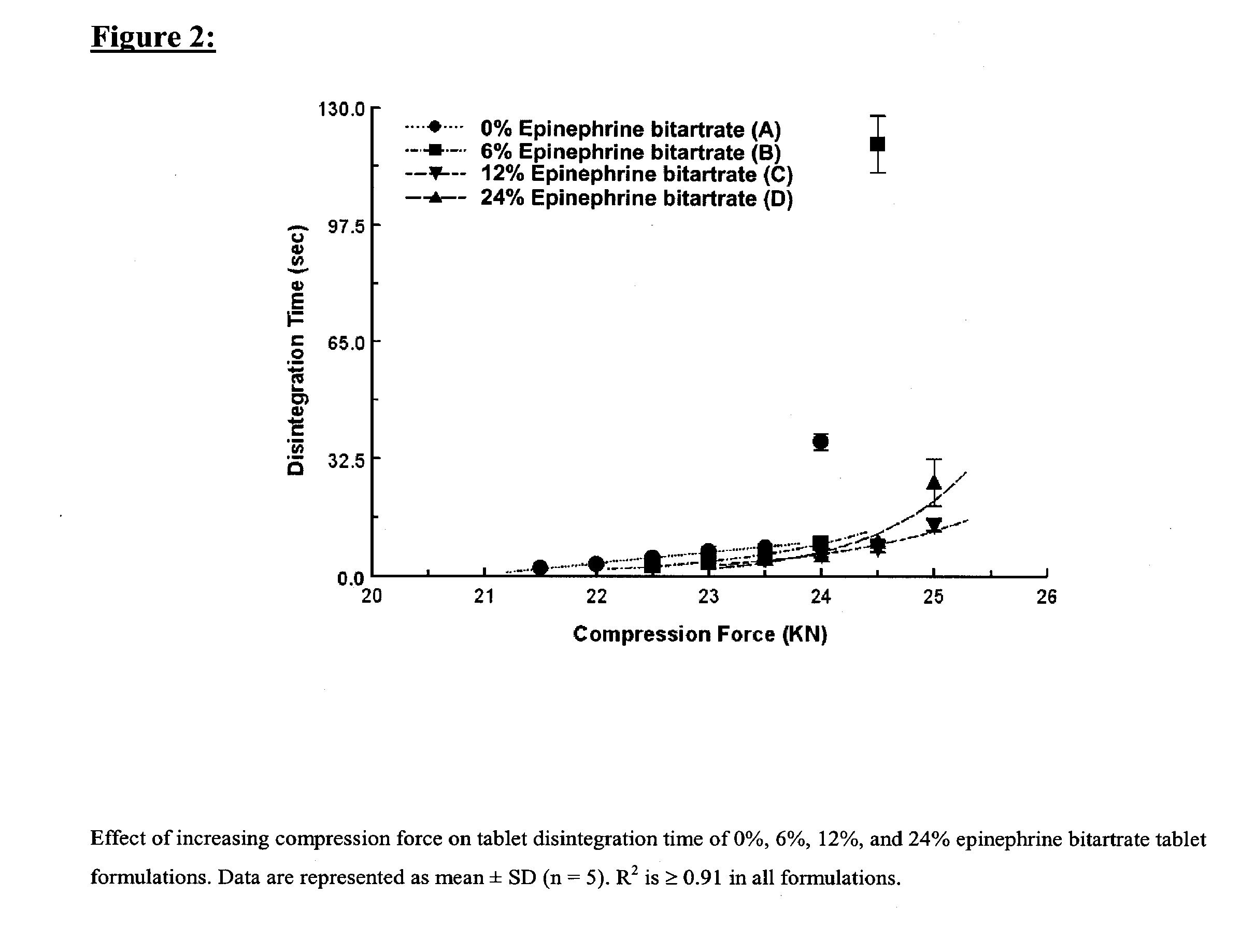
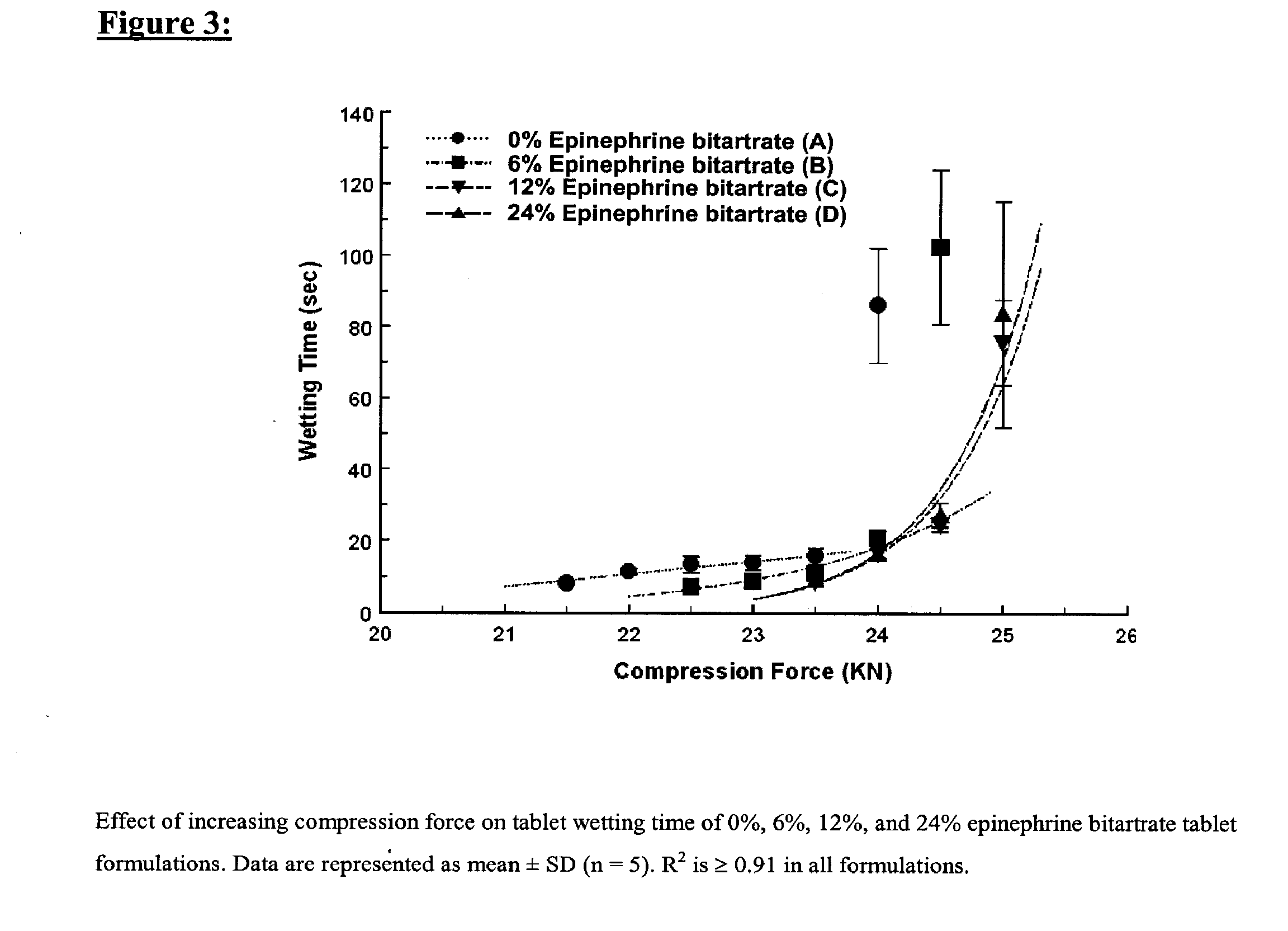
Fast-disintegrating epinephrine tablets for buccal or sublingual administration
Described herein are formulations for fast-disintegrating epinephrine tablets which can be prepared for buccal or sublingual administration, wherein the fast-disintegrating epinephrine tablets can produce plasma epinephrine concentrations similar to those achieved by an approximately 0.3 mg epinephrine dose in the thigh (Epi-Pen).
Owner:UNIVERSITY OF MANITOBA
Algae supplement and treatment method
InactiveUS20080124286A1Enhances epidermal stem cell productionExtend the life cycleCosmetic preparationsBiocidePhylum CyanobacteriaCuticle
This invention relates to a method and composition for enhancing epidermal and / or hair follicle stem cell production, cell renewal, and / or growth of the skin, hair and nails, by topical administration of a therapeutic dosage of cyanobacteria and green algae and / or both simultaneous enteral and topical administration of a therapeutic dosage of cyanobacteria and green algae.
Owner:LISSON JEROLD B
Coated filter bag material for oral administration of medicament in liquid and methods of making same
InactiveUS7090858B2Extended shelf lifeOrganic active ingredientsPowder deliveryOral medicationIntrathecal
Owner:JAYARAMAN SWAMINATHAN
Pharmaceutical Formulation Containing Opioid Agonist, Opioid Antagonist and Gelling Agent
InactiveUS20150031718A1Avoid abuseReduce releaseBiocidePharmaceutical non-active ingredientsOpioid antagonistPharmaceutical formulation
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Methods and compositions for oral administration of proteins
ActiveUS20110142800A1Promote absorptionPeptide/protein ingredientsMetabolism disorderDiabetes mellitusOral medication
This invention provides compositions comprising a protein, an absorption enhancer, a protease inhibitor, methods for treating diabetes mellitus, comprising administering same, and methods for oral administration of a protein with an enzymatic activity, comprising orally administering same.
Owner:ENTERA BIO LTD
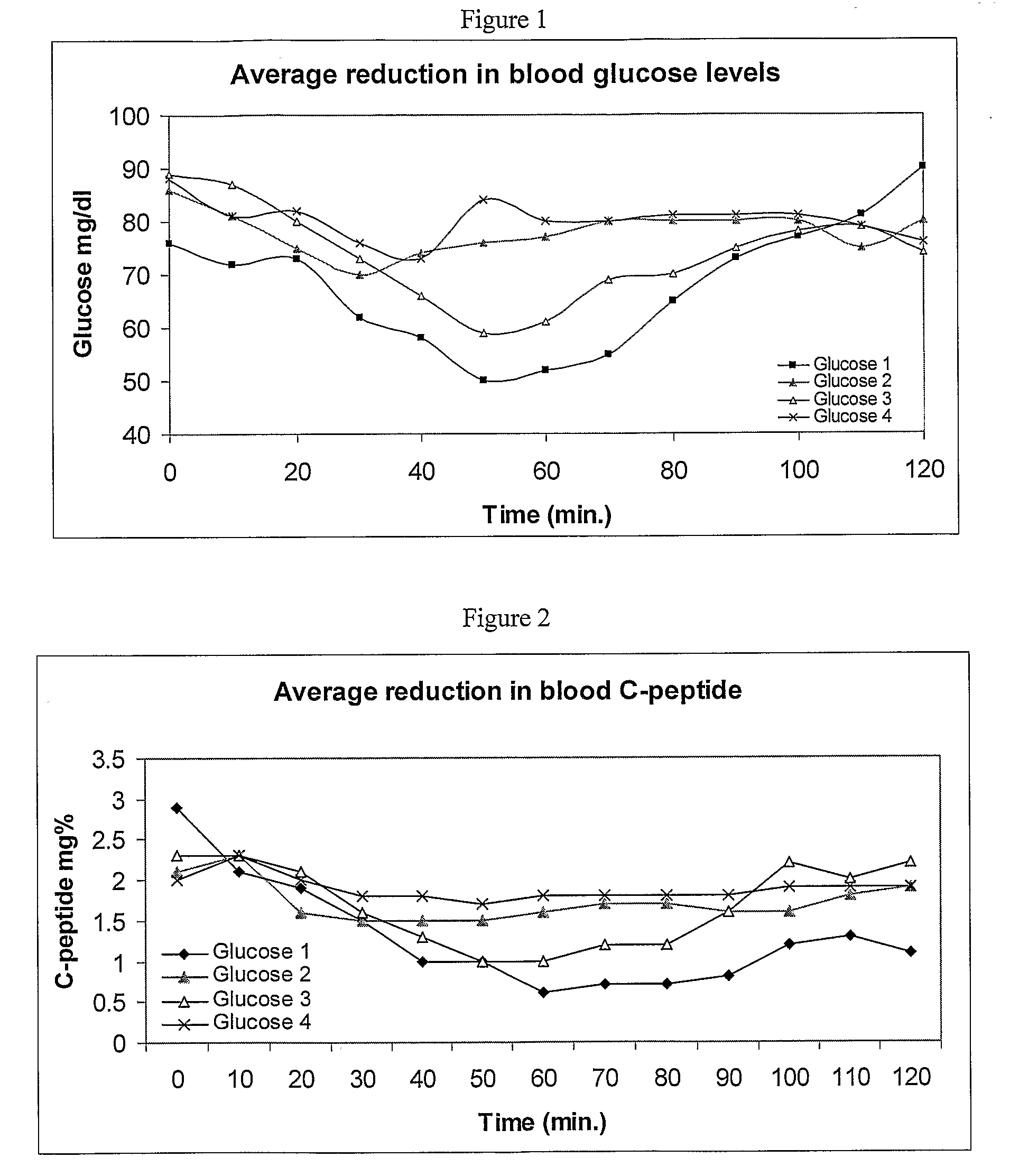
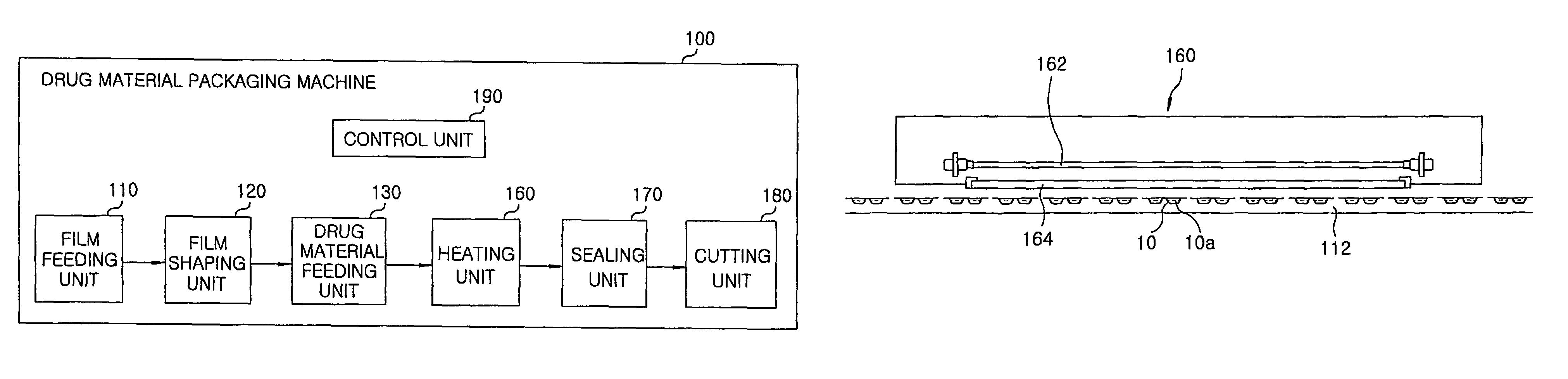
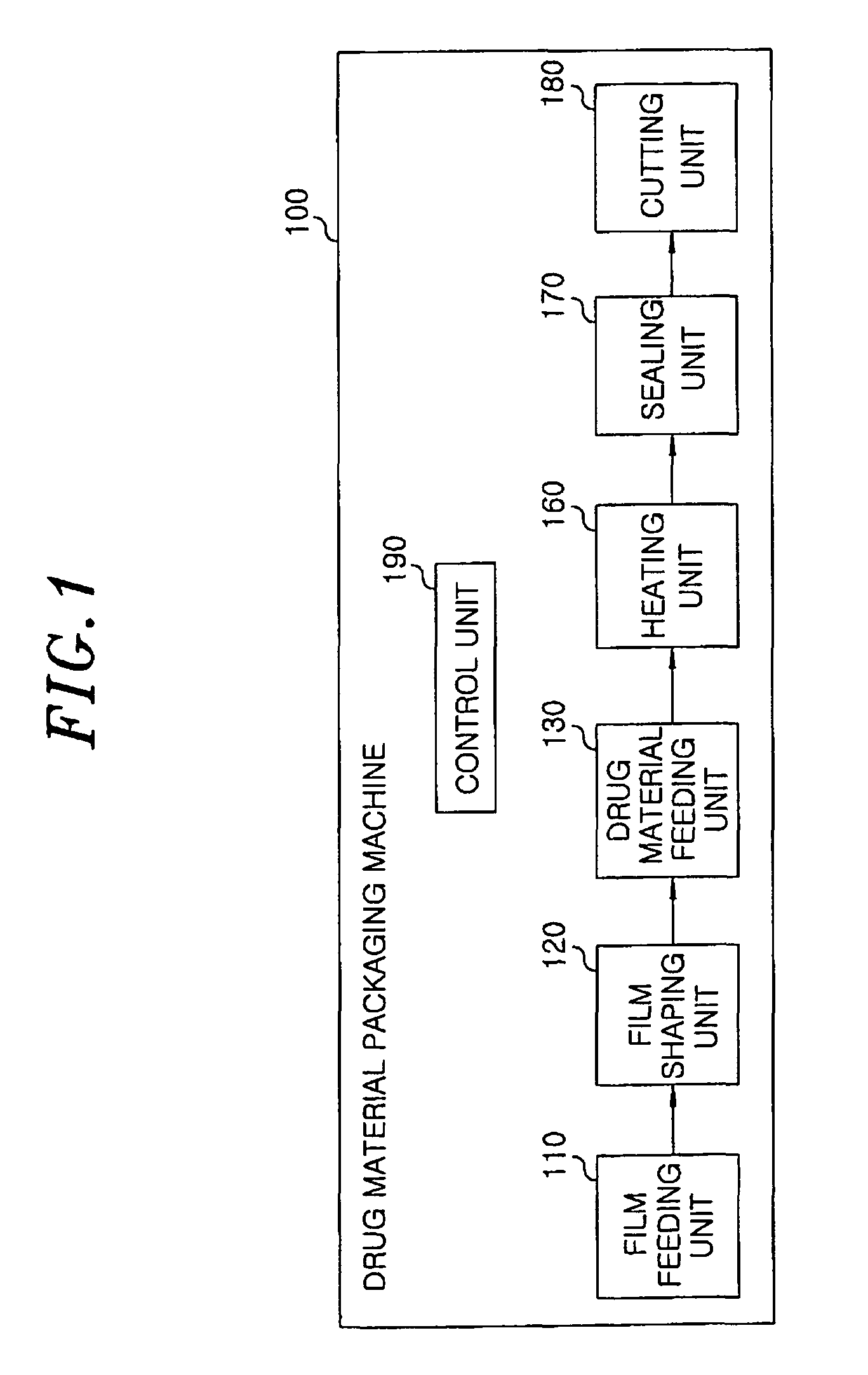
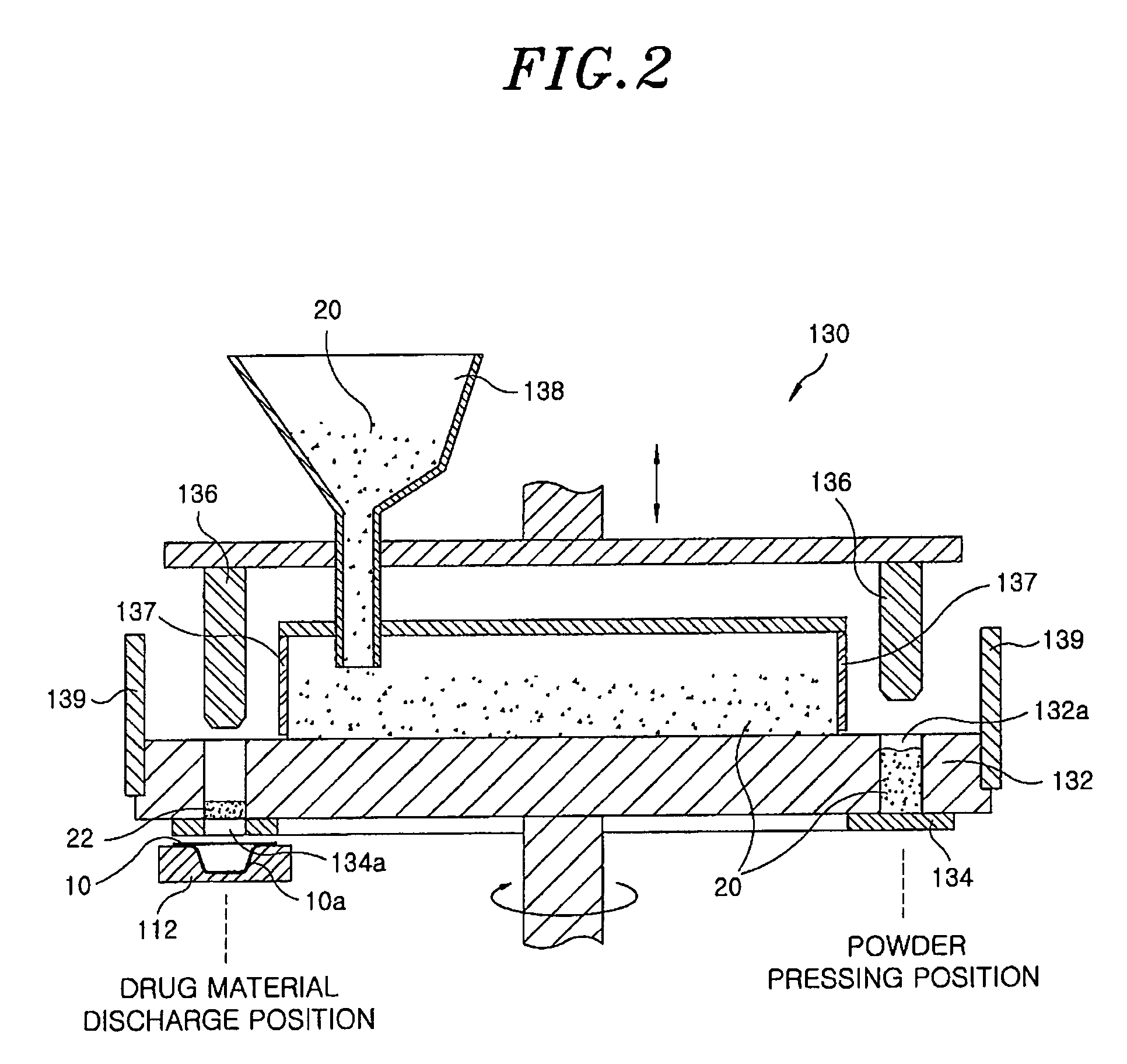
Method for preparing rapidly disintegrating formulation for oral administration and apparatus for preparing and packing the same
InactiveUS8127516B2Disintegrates quicklyEnhanced patient comfortAntibacterial agentsNervous disorderPowder mixtureOral medication
A method and packaging machine for preparing rapidly disintegrating formulations for oral administration are disclosed. The present invention is characterized in that a powdery mixture including a pharmaceutically active ingredient and a sugar or a sugar alcohol powder is filled into a packaging material and, thereafter, the mixture, filled in the packaging material, is heated. The present invention can simply and economically prepare an oral formulation which undergoes rapid disintegration in the oral cavity and provides for high-quality administration to patients.
Owner:HANMI PHARMA
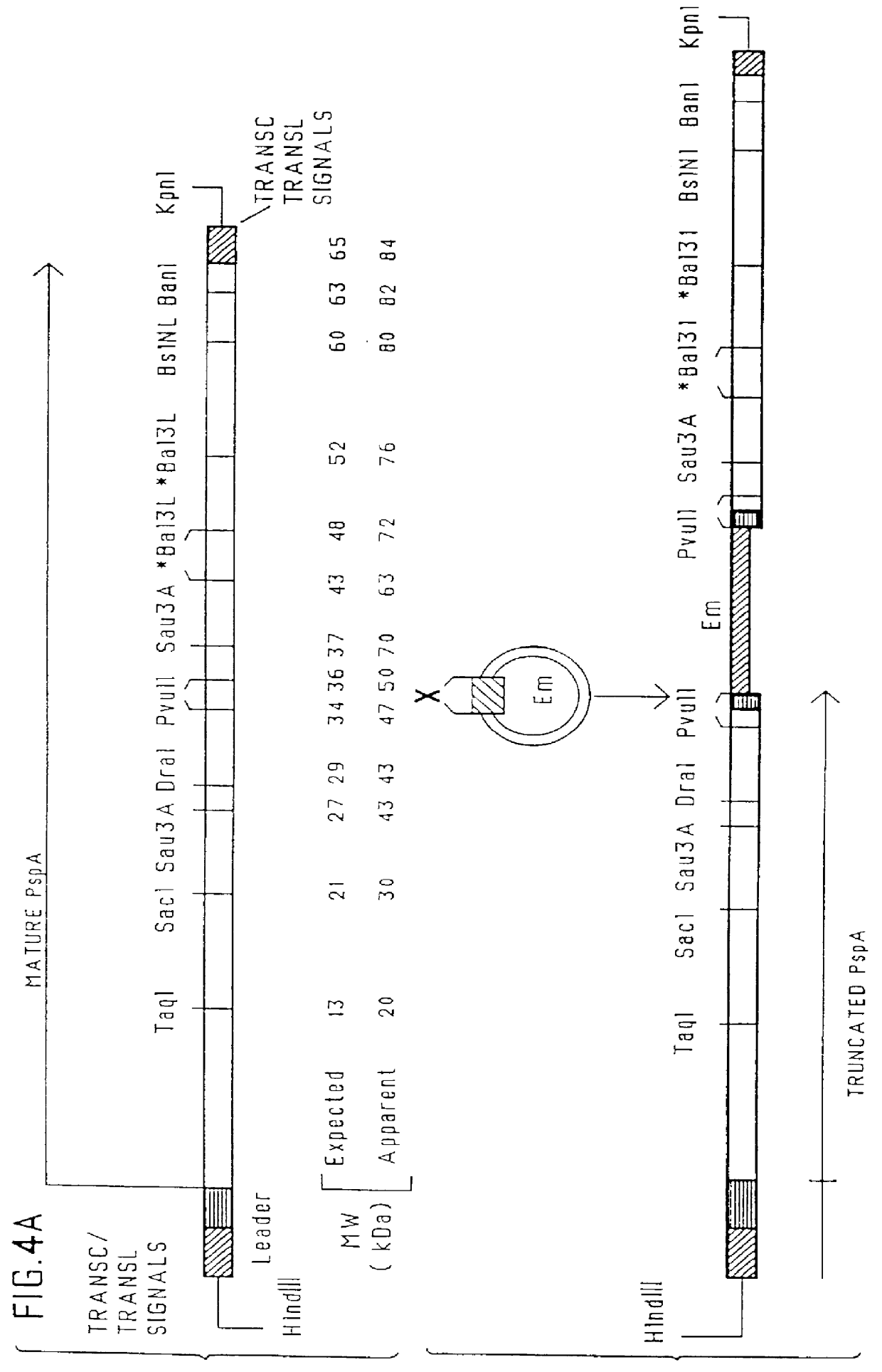
immunogenic compositions for mucosal administration of pneumococcal surface protein A (PspA)
InactiveUS6042838ALittle or no tropism for the GALTGood mucosal immunogenAntibacterial agentsBacterial antigen ingredientsCoccidiaPneumococcal surface protein A
Mucosal administration, particularly intranasally, of killed whole pneumococci, lysate of pneumococci and isolated and purified PspA, as well as immunogenic fragments thereof, particularly when administered with cholera toxin B subunit, provides protection in animals against pneumococcal colonization and systemic infection. The ability to elicit protection against pneumococcal colonization in a host prevents carriage among immunized individuals, which can lead to elimination of disease from the population as a whole.
Owner:UNIVERSITY OF ALABAMA
Transdermal anesthetic and vasodilator composition and methods for topical administration
A composition for topical application comprising a therapeutically effective amount of a topical anesthetic, a safe and effective amount of a pharmaceutically acceptable topical vasodilator and a pharmaceutically acceptable carrier and a method of administering the composition to a mammal are disclosed.
Owner:SAMUELS PAUL J +1
Dispersible pharmaceutical compositions
ActiveUS7842791B2Good dispersionEfficacious level of antibacterialAntibacterial agentsOrganic active ingredientsDiseaseMicrocrystalline wax
A pharmaceutical composition is provided comprising a vehicle that comprises (a) an amphipathic oil that is water dispersible and ethanol insoluble, (b) microcrystalline wax, and (c) a pharmaceutically acceptable non-aqueous carrier; and having an antibacterial substance in an antibacterially effective amount stably dispersed in the vehicle. The composition is suitable for administration by intramammary infusion to a milk producing animal for treatment and / or prevention of mastitis or other diseases of the udder, as well as for otic administration for treatment and / or prevention of an ear infection.
Owner:ZOETIS SERVICE LLC
Stabilized protein compositions for topical administration and methods of making same
InactiveUS7838011B2Easy to useReduce riskBacterial antigen ingredientsPeptide/protein ingredientsProtein compositionAdditive ingredient
A stabilizing composition that also enhances permeation is provided for the topical or transdermal administration of an active ingredient. The composition comprises collagen, elastin, sphingosine and cerebroside. Also provided are pharmaceutical or cosmetic formulations comprising an effective amount of an active agent and the stabilizing composition as well as methods of administering active agents topically or transdermally.
Owner:TRANSDERMAL
Pharmaceutical compositions for buccal delivery of pain relief medications
Pharmaceutical compositions comprising a narcotic analgesic in mixed micellar form are disclosed. The mixed micelles are formed from an alkali metal alkyl sulfate, and other micelle-forming compounds as described in the specification. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed. A preferred method for administering the present composition is through the buccal mucosa of the mouth.
Owner:GENEREX PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com