Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
156 results about "Botulinum neurotoxin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method and compositions for detecting botulinum neurotoxin
ActiveUS20060134722A1Antibacterial agentsNervous disorderSurface plasmon resonance imagingFluorophore
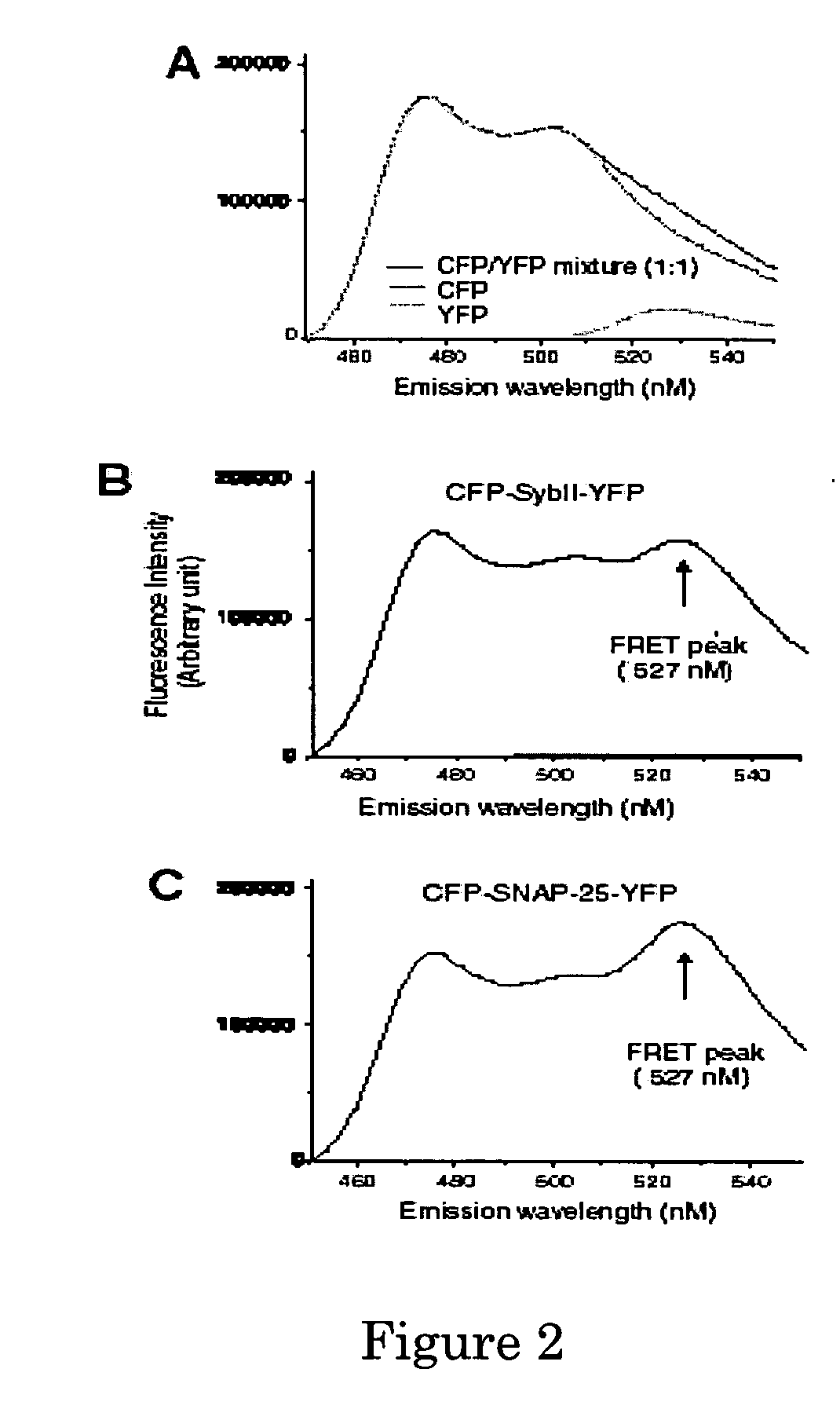
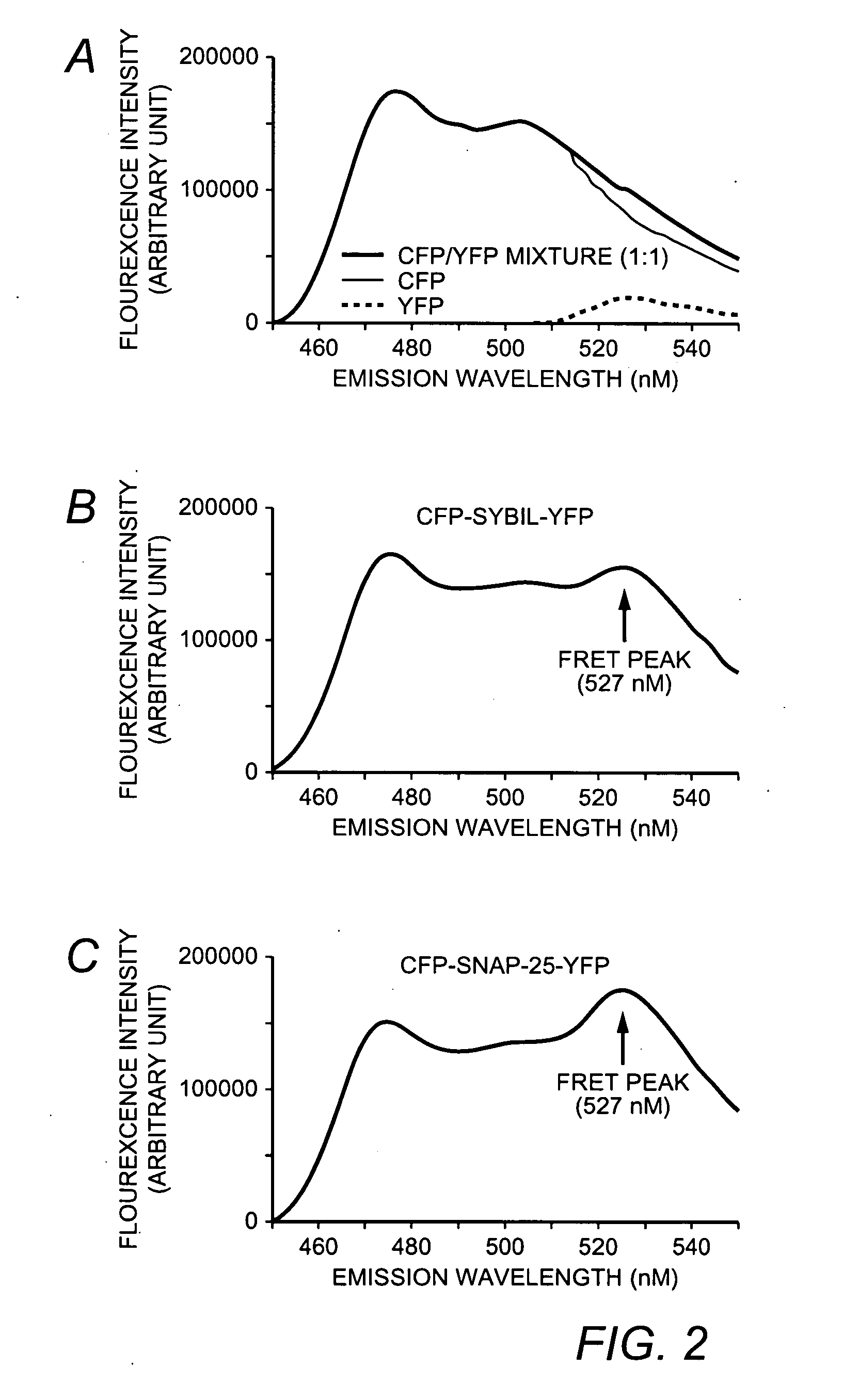
A molecular construct capable of fluorescent resonance energy transfer (FRET), comprising a linker peptide, a donor fluorophore moiety and an acceptor fluorophore moiety, wherein the linker peptide is a substrate of a botulinum neurotoxin selected from the group consisting of synaptobrevin, syntaxin and SNAP-25, or a fragment thereof capable being cleaved by the botulinum neurotoxin, and separates the donor and acceptor fluorophores by a distance of not more than 10 nm, and wherein emission spectrum of the donor fluorophore moiety overlaps with the excitation spectrum of the acceptor fluorophore moiety; or wherein the emission spectra of the fluorophores are detectably different. Also provided are isolated nucleic acid expressing the construct, kits comprising said construct and cell lines comprising said nucleic acid. Further provided are methods of detecting a BoNT using the above described construct via FRET, and methods for detecting a BoNT using surface plasmon resonance imaging.
Owner:WISCONSIN ALUMNI RES FOUND
Therapeutic treatments using botulinum neurotoxin
ActiveUS20090232850A1Eliminates sensory inputImprove developmentBacterial antigen ingredientsNervous disorderDiseaseObstructive Pulmonary Diseases
Methods for treating a coronary risk factor (such as hypertension, diabetes, hyperlipidemia and obesity) and / or a respiratory disorder (such as asthma, chronic obstructive pulmonary disease and bronchitis) and / or arthritis by local administration of a botulinum neurotoxin to at least one of a head, neck or shoulder location (for example, by subdermal, subcutaneous or intramuscular administration of the botulinum neurotoxin) of a patient with a coronary risk factor, respiratory disorder or arthritis.
Owner:ALLERGAN INC
Systems and methods for delivery of biologically active agents
InactiveUS20100196445A1Efficient deliveryPeptide/protein ingredientsMicroneedlesCell-Extracellular MatrixActive agent
The invention provides methods and devices for the delivery of therapeutic or biologically active agents to tissue, for example by facilitating the transport of said agents through the skin of a human or animal. Therapeutic agents include various botulinum neurotoxin (BoNT) and other biologically active agents, which can be delivered across the skin and into the dermis using multiple strategies including microneedle drug delivery, transport moieties, or penetration enhancers such as extracellular matrix-digestive enzymes.
Owner:THE SCRIPPS RES INST +1
Recombinant vaccine against botulinum neurotoxin
InactiveUS7081529B2Fast and efficient purificationBacterial antigen ingredientsBacteriaVaccinationRecombinant vaccines
This invention is directed to preparation and expression of synthetic genes encoding polypeptides containing protective epitopes of botulinum neurotoxin (BoNT). The invention is also directed to production of immunogenic peptides encoded by the synthetic genes, as weel as recovery and purification of the immunogenic peptides from recombinant organisms. The invention is also directed to methods of vaccination against botulism using the expressed peptides.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
GFP-SNAP25 fluorescence release assay for botulinum neurotoxin protease activity
The present invention provides a nucleic acid molecule which contains a nucleotide sequence encoding a SNAP-25 substrate which includes (i) a green fluorescent protein; (ii) a first partner of an affinity couple; and (iii) a portion of SNAP-25 that includes a BoNT / A, BoNT / C1 or BoNT / E recognition sequence containing a cleavage site, where the cleavage site intervenes between the green fluorescent protein and the first partner of the affinity couple. Further provided herein is a nucleic acid molecule which contains a nucleotide sequence encoding a tagged toxin substrate which includes (i) a fluorescent protein; (ii) a first partner of an affinity couple; and (iii) a clostridial toxin recognition sequence containing a cleavage site, where the cleavage site intervenes between the fluorescent protein and the first partner of the affinity couple.
Owner:ALLERGAN INC
Antibodies for Botulinum Neurotoxins
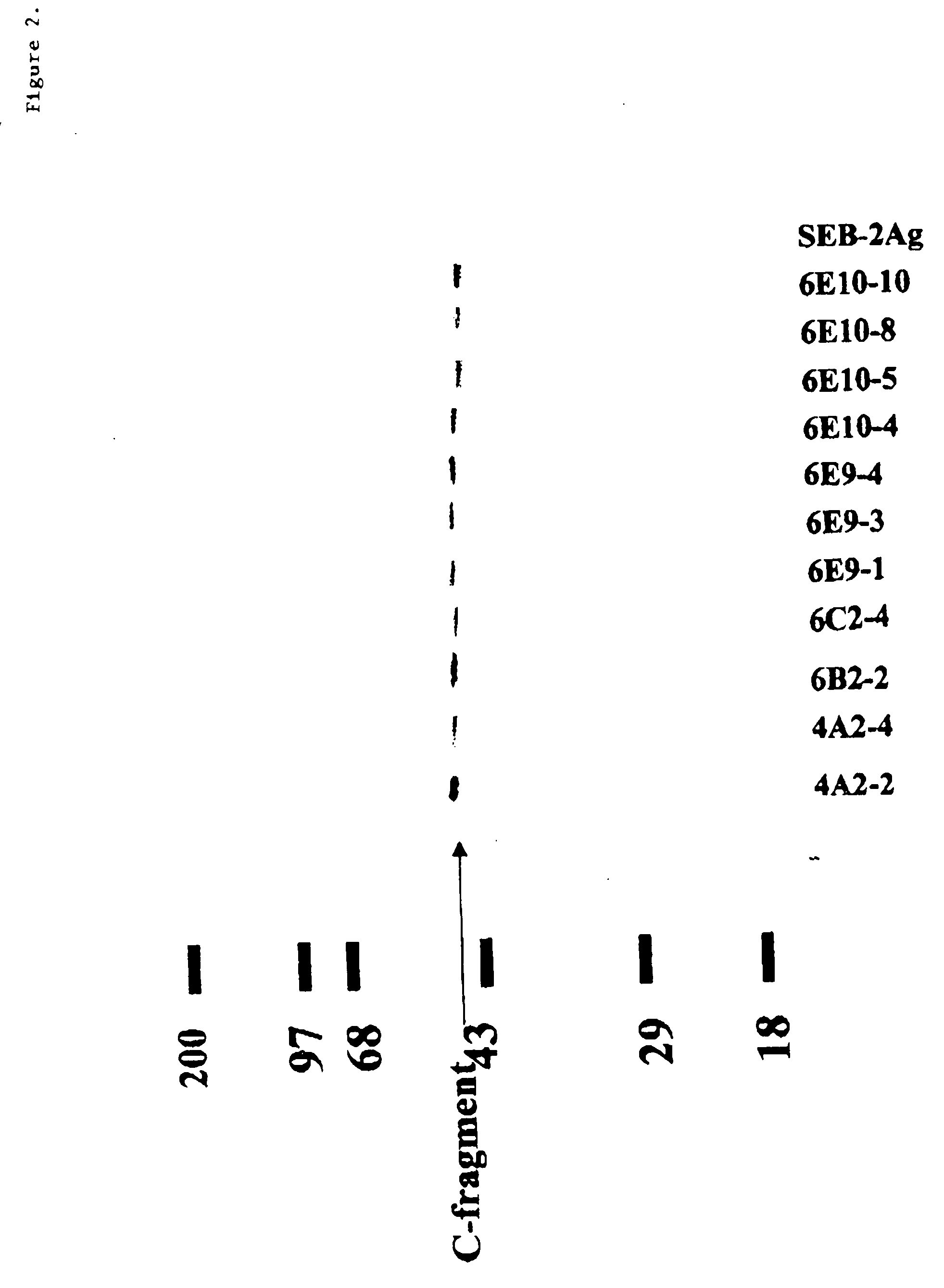
The present disclosure provides antibodies that specifically bind to botulinum neurotoxins (e.g., BoNT / A, BoNT / B, BoNT / C, BoNT / D, BoNT / E, BoNT / F, BoNT / G, etc.) and the epitopes bound by those antibodies. The antibodies and derivatives thereof that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
Therapeutic monoclonal antibodies that neutralize botulinum neurotoxins
InactiveUS7563874B2Mitigate and eliminate symptomBiocidePeptide/protein ingredientsMedicineBotulinum Neurotoxin Type A
Owner:RGT UNIV OF CALIFORNIA
Method of targeting pharmaceuticals to motor neurons
A method of targeting therapeutic molecules to motor neurons is disclosed. In one embodiment, this method comprises the steps of (a) synthesizing a prodrug comprising a therapeutic molecule covalently bound to a polymeric delivery vehicle, and (b) conjugating the prodrug to a botulinum neurotoxin heavy chain.
Owner:WISCONSIN ALUMNI RES FOUND
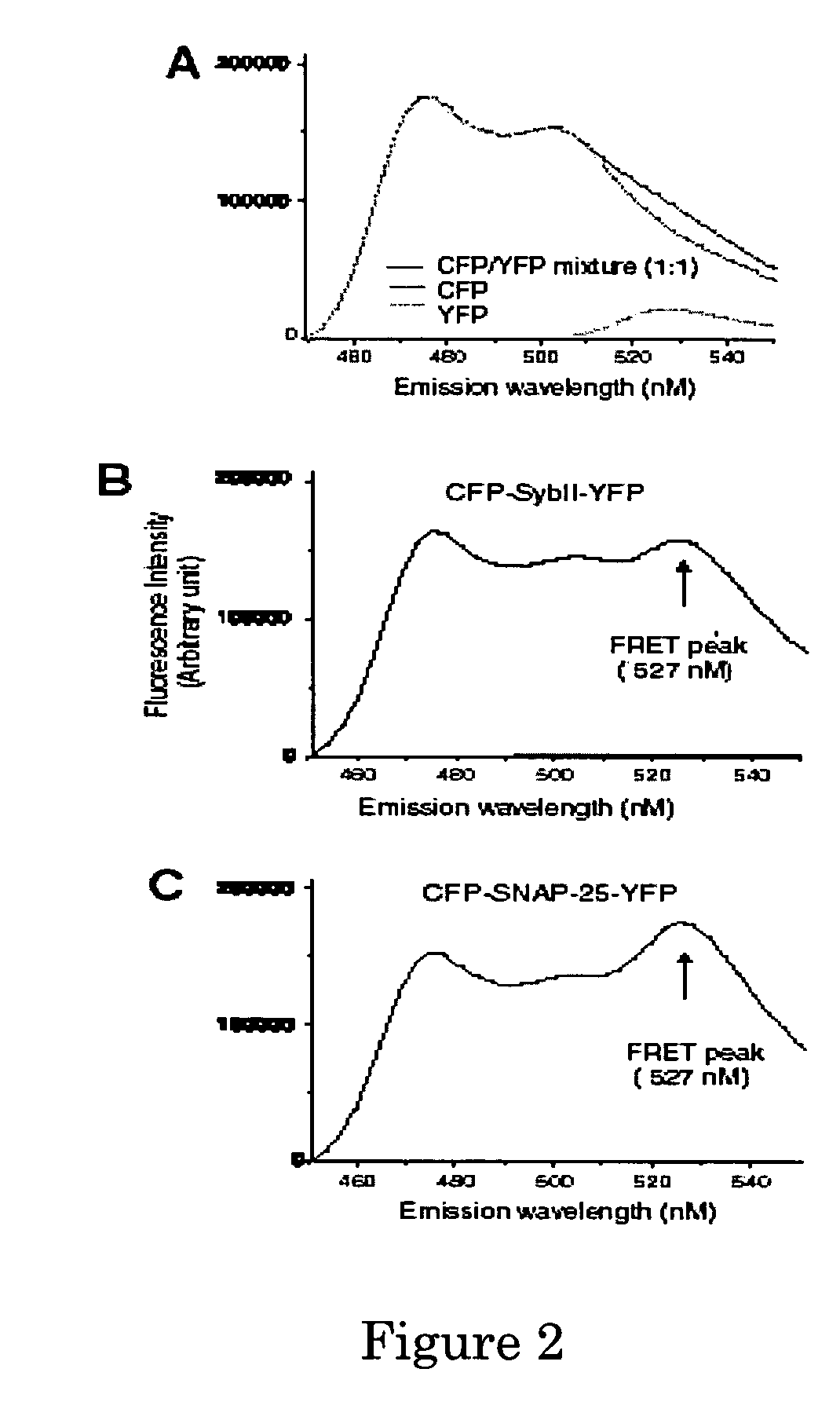
Resonance Energy Transfer Assay with Cleavage Sequence and Spacer
ActiveUS20110033866A1Improving sensitivity of energyHigh sensitivityMicrobiological testing/measurementBiological material analysisEnergy transferCoupling
A molecular construct comprises a donor label, an acceptor label, a linker peptide disposed between the donor and the acceptor, the linker having a cleavage site sequence, and a spacer between at least one of (a) the donor and the cleavage site sequence and (b) the acceptor and the cleavage site sequence. Preferably, the construct is selected from the group consisting of CFP-(SGLRSRA)-SNAP-25-(SNS)-YFP, and CFP-(SGLRSRA)-synaptobrevin-(SNS)-YFP. In preferred embodiments, the linker peptide is a substrate of a botulinum neurotoxin selected from the group consisting of synaptobrevin (VAMP), syntaxin and SNAP-25, or a fragment thereof that can be recognized and cleaved by the botulinum neurotoxin. Advantageously, the spacer increases the electronic coupling between the donor label and the acceptor label relative to a corresponding construct without the spacer.
Owner:BIOMADISON
Method of Preparing an Immunologically-Active Adjuvant-Bound Dried Vaccine Composition
ActiveUS20100158951A1Reduce concentrationAntibacterial agentsBacterial antigen ingredientsAdjuvantBotulinum neurotoxin
The disclosure provides a method of preparing an immunologically-active adjuvant-bound freeze dried vaccine composition. A specific embodiment provides a stable vaccine composition comprising an aluminum-salt adjuvant, a recombinant Clostridium botulinum neurotoxin protein and a glass-forming agent. These vaccine compositions are useful in the treatment of humans and other animals at risk of infection from Clostridium botulinum neurotoxin.
Owner:UNIV OF COLORADO THE REGENTS OF
Antibodies against type A botulinum neurotoxin
InactiveUS20060177881A1Narrow regionImmunoglobulins against bacteriaFermentationEpitopePassive Immunizations
Owner:US ARMY MEDICAL RES MATERIEL COMMAND USAMRMC
Antibodies that Neutralize Botulinum Neurotoxins
This disclosure provides antibodies that specifically bind to and typically neutralize botulinum neurotoxins (e.g., BoNT / A, BoNT / B, BoNT / E, etc.) and the epitopes bound by those antibodies. The antibodies and derivatives thereof and / or other antibodies that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
Recombinant derivatives of botulinum neurotoxins engineered for trafficking studies and neuronal delivery
InactiveUS20110206616A1Improve efficiencyImprove the level ofUltrasonic/sonic/infrasonic diagnosticsBacteriaDisulfide bondBotulinum toxin type C
This invention relates to isolated Clostridium botulinum propeptides and neurotoxins, isolated nucleic acid molecules encoding Clostridium botulinum propeptides and neurotoxins, methods of expression, treatment methods, and methods of detecting neurotoxin trafficking. The isolated Clostridium botulinum propeptides have a light chain region; a heavy chain region, where the light and heavy chain regions are linked by a disulfide bond; an intermediate region connecting the light and heavy chain regions and comprising a highly specific protease cleavage site; and an S6 peptide sequence according to SEQ ID NO:2 positioned upstream from, but not attached directly to, the N-terminus of the neurotoxin propeptide at the light chain region to enable site specific attachment of cargo.
Owner:NEW YORK UNIV
Compositions and Methods for Safe Treatment of Rhinitis
InactiveUS20160250302A1Minimize the possibilityPeptide/protein ingredientsPharmaceutical delivery mechanismNoseMedicine
Methods for treating rhinitis in a subject are provided herein. The methods of the present invention comprise intranasal administration of a topical composition comprising a purified botulinum neurotoxin, a carrier and a viscosity modifier to one or more inner surfaces of the nose. The methods disclosed herein provide alternative methods for delivery of botulinum neurotoxin to the nasal anatomy for the treatment of rhinitis.
Owner:REVANCE THERAPEUTICS INC
Methods and compounds for increasing sensitivity of botulinum assays
ActiveUS9303285B2Promote degradationReduce sensitivityMicrobiological testing/measurementBiological material analysisFluorophoreCell
Apparatus, systems and methods can provide improved detection of botulinum neurotoxins. In one aspect an isoquinolynyl compound can be used to enhance the sensitivity of both Förster resonance energy transfer (FRET) and non-FRET cell-based assays. In another aspect, non-FRET assays and constructs utilize a reporter that is not coupled with the second fluorophore in a manner that produces significant FRET. In that subject matter an environment cell can include an enzyme that facilitates degradation of the reporter significantly faster after the cleavage than before the cleavage, and presence of the Botulinum toxin correlates with reduction of the signal from a baseline signal. Where the environment is a cell, the cell can advantageously express both the construct that includes the reporter, and an enzyme that facilitates the degradation.
Owner:BIOMADISON
Therapeutic monoclonal antibodies that neutralize botulinum neurotoxins
ActiveUS20100166773A1Low toxicityExtension of timeAntibacterial agentsImmunoglobulins against bacteriaMedicineNeutralizing epitope
This invention provides antibodies that specifically bind to and typically neutralize botulinum neurotoxins (e.g., BoNT / A, BoNT / B, BoNT / E, etc.) and the epitopes bound by those antibodies. The antibodies and derivatives thereof and / or other antibodies that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
Mutant botulinum neurotoxin serotype A polypeptide and uses thereof
Modified polypeptides based on the botulinum neurotoxin A heavy chain containing the double mutation Trp-Tyr->Leu-Ser in the ganglioside binding motif Ser-X-Trp-Tyr do not bind polysialogangliosides and nerve endings. The polypeptides are useful in the preparation of nontoxic vaccines against the effects of C. botulinum infection. The modified polypeptides are also useful as vehicles for the transepithelial delivery of diagnostic and therapeutic entities, through formation of conjugates between the polypeptides and the diagnostic or therapeutic entities.
Owner:THOMAS JEFFERSON UNIV
Mass spectrometry-based methods for detection and differentiation of botulinum neurotoxins
InactiveUS20060024763A1Microbiological testing/measurementBiological testingSpectroscopyMass Spectrometry-Mass Spectrometry
The present invention is directed to a method for detecting the presence of clostridial neurotoxins in a sample by mixing a sample with a peptide that can serve as a substrate for proteolytic activity of a clostridial neurotoxin; and measuring for proteolytic activity of a clostridial neurotoxin by a mass spectroscopy technique. In one embodiment, the peptide can have an affinity tag attached at two or more sites.
Owner:LOS ALAMOS NATIONAL SECURITY +1
Therapeutic monoclonal antibodies that neutralize botulinum neurotoxins
ActiveUS7700738B2Low toxicityExtension of timeAntibacterial agentsAntinoxious agentsAntiendomysial antibodiesBotulinum Neurotoxin Type A
This invention provides antibodies that specifically bind to and neutralize botulinum neurotoxin type A (BoNT / A) and the epitopes bound by those antibodies. The antibodies and derivatives thereof and / or other antibodies that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
Monoclonal antibodies that neutralize botulinum neurotoxin
InactiveUS20100222555A1Reduce development riskSugar derivativesImmunoglobulins against bacteriaMedicineBotulinum Neurotoxin Type B
This invention provides antibodies that specifically bind to botulinum neurotoxin type A (BoNT / A) and / or botulinum neurotoxin type B (BoNT / B) and the epitopes bound by those antibodies. The antibodies and derivatives thereof and / or other antibodies that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism. Also included in the invention are diagnostic and therapeutic assays directed to botulinum neurotoxins.
Owner:THOMAS JEFFERSON UNIV
Methods for Preventing or Treating Complications of Airway Control Devices
InactiveUS20090142430A1Minimizing and preventing and treating and enabling easy managementBiocidePowder deliveryAnesthesiaGastrointestinal tract
Disclosed in certain embodiments is a method of treating or preventing complications of airway control devices comprising administering to a patient having an airway control device a pharmaceutical composition comprising botulinum neurotoxin to one or more of the upper or lower aerodigestive secretory glands, the cricopharyngeus or the gastric or esophageal mucosal wall of the patient.
Owner:SANDERS +1
Method and compositions for detecting botulinum neurotoxin
A molecular construct capable of fluorescent resonance energy transfer (FRET), comprising a linker peptide, a donor fluorophore moiety and an acceptor fluorophore moiety, wherein the linker peptide is a substrate of a botulinum neurotoxin selected from the group consisting of synaptobrevin, syntaxin and SNAP-25, or a fragment thereof capable being cleaved by the botulinum neurotoxin, and separates the donor and acceptor fluorophores by a distance of not more than 10 nm, and wherein emission spectrum of the donor fluorophore moiety overlaps with the excitation spectrum of the acceptor fluorophore moiety; or wherein the emission spectra of the fluorophores are detectably different. Also provided are isolated nucleic acid expressing the construct, kits comprising said construct and cell lines comprising said nucleic acid. Further provided are methods of detecting a BoNT using the above described construct via FRET, and methods for detecting a BoNT using surface plasmon resonance imaging.
Owner:WISCONSIN ALUMNI RES FOUND
Recombinant light chains of botulinum neurotoxins and light chain fusion proteins for use in research and clinical therapy
Botulinum neurotoxins, the most potent of all toxins, induce lethal neuromuscular paralysis by inhibiting exocytosis at the neuromuscular junction. The light chains (LC) of these dichain neurotoxins are a new class of zinc-endopeptidases that specifically cleave the synaptosomal proteins, SNAP-25, VAMP, or syntaxin at discrete sites. The present invention relates to the construction, expression, purification, and use of synthetic or recombinant botulinum neutoroxin genes. For example, a synthetic gene for the LC of the botulinum neurotoxin serotype A (BoNT / A) was constructed and overexpressed in Escherichia coli. The gene product was purified from inclusion bodies. The methods of the invention can provide 1.1 g of the LC per liter of culture. The LC product was stable in solution at 4° C. for at least 6 months. This rBoNT / A LC was proteolytically active, specifically cleaving the Glu-Arg bond in a 17-residue synthetic peptide of SNAP-25, the reported cleavage site of BoNT / A. Its calculated catalytic efficiency kcat / Km was higher than that reported for the native BoNT / A dichain. Treating the rBoNT / A LC with mercuric compounds completely abolished its activity, most probably by modifying the cysteine-164 residue located in the vicinity of the active site. About 70% activity of the LC was restored by adding Zn2+ to a Zn2+-free, apo-LC preparation. The LC was nontoxic to mice and failed to elicit neutralizing epitope(s) when the animals were vaccinated with this protein. In addition, injecting rBoNT / A LC into sea urchin eggs inhibited exocytosis-dependent plasma membrane resealing.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
GFP-SNAP25 fluorescence release assay for botulinum neurotoxin protease activity
InactiveUS20060154314A9BacteriaMaterial analysis by observing effect on chemical indicatorFluorescenceNucleotide
The present invention provides a nucleic acid molecule which contains a nucleotide sequence encoding a SNAP-25 substrate which includes (i) a green fluorescent protein; (ii) a first partner of an affinity couple; and (iii) a portion of SNAP-25 that includes a BoNT / A, BoNT / C1 or BoNT / E recognition sequence containing a cleavage site, where the cleavage site intervenes between the green fluorescent protein and the first partner of the affinity couple. Further provided herein is a nucleic acid molecule which contains a nucleotide sequence encoding a tagged toxin substrate which includes (i) a fluorescent protein; (ii) a first partner of an affinity couple; and (iii) a clostridial toxin recognition sequence containing a cleavage site, where the cleavage site intervenes between the fluorescent protein and the first partner of the affinity couple.
Owner:ALLERGAN INC
Compositions and Methods for Safe Treatment of Rhinitis
InactiveUS20140120077A1Minimize the possibilityPeptide/protein ingredientsPharmaceutical delivery mechanismMedicineNose
Methods for treating rhinitis in a subject are provided herein. The methods of the present invention comprise intranasal administration of a topical composition comprising a purified botulinum neurotoxin, a carrier and a viscosity modifier to one or more inner surfaces of the nose. The methods disclosed herein provide alternative methods for delivery of botulinum neurotoxin to the nasal anatomy for the treatment of rhinitis.
Owner:REVANCE THERAPEUTICS INC
Antibodies that Neutralize Botulinum Neurotoxins
This disclosure provides antibodies that specifically bind to and typically neutralize botulinum neurotoxins (e.g., BoNT / A, BoNT / B, BoNT / E, etc.) and the epitopes bound by those antibodies. The antibodies and derivatives thereof and / or other antibodies that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
Methods for identifying inhibitors of botulinum neurotoxins
InactiveUS20060233836A1Compound screeningBacterial antigen ingredientsPeptide substrateStable cell line
A system and method for identifying a botulinum neurotoxin inhibitor employing a botulinum neurotoxin substrate complex having a peptide substrate, preferably SNAP-25, a reporter domain on one side of said peptide substrate and an immobilization domain on the opposite side of said peptide substrate. The botulinum neurotoxin inhibitor is identified by its ability to decrease the relative amount of cleaved complex, detected through measuring a decrease in complex bound to a solid support. The method of the present invention also utilizes novel cells that express a botulinum neurotoxin substrate complex. The methods of the present invention are adapted for cell based screening to monitor the catalytic activity of a BoNT in living cells and to identify molecules that inhibit the catalytic activity of a BoNT in living cells. Also provided are novel stable cell lines that express the botulinum toxin substrate complex and viral vectors capable of efficiently expressing an active light chain of the BoNT within mammalian cells.
Owner:BOTX TECH LLC
Remedy for hypermyotonia
InactiveUS20050163809A1Short timeSolve the real problemBacterial antigen ingredientsSenses disorderHypermyotoniaMedicine
A remedy for muscle hyperactivity, comprising a purified botulinum neurotoxin as an active ingredient.
Owner:JURIDICAL FOUND THE CHEMO SERO THERAPEUTIC RES INST
Novel Subtype of Closteridium Botulinum Neurotoxin Type A and Uses Thereof
ActiveUS20110171226A1Improving medicinal useNervous disorderPeptide/protein ingredientsMedicineBotulinum Neurotoxin Type A
A novel subtype of type A botulinum neurotoxin (BoNT / A) is disclosed in the application. Methods to purify the neurotoxin as well as uses thereof are also disclosed.
Owner:THE SCRIPPS RES INST +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com