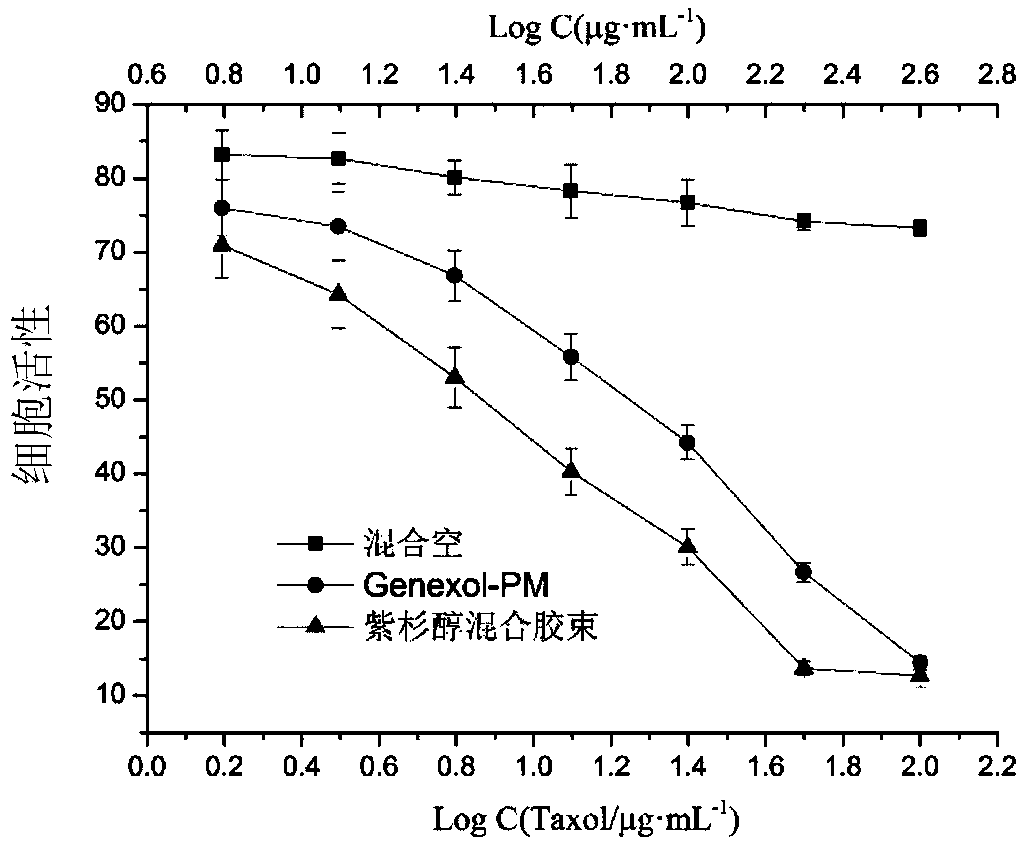
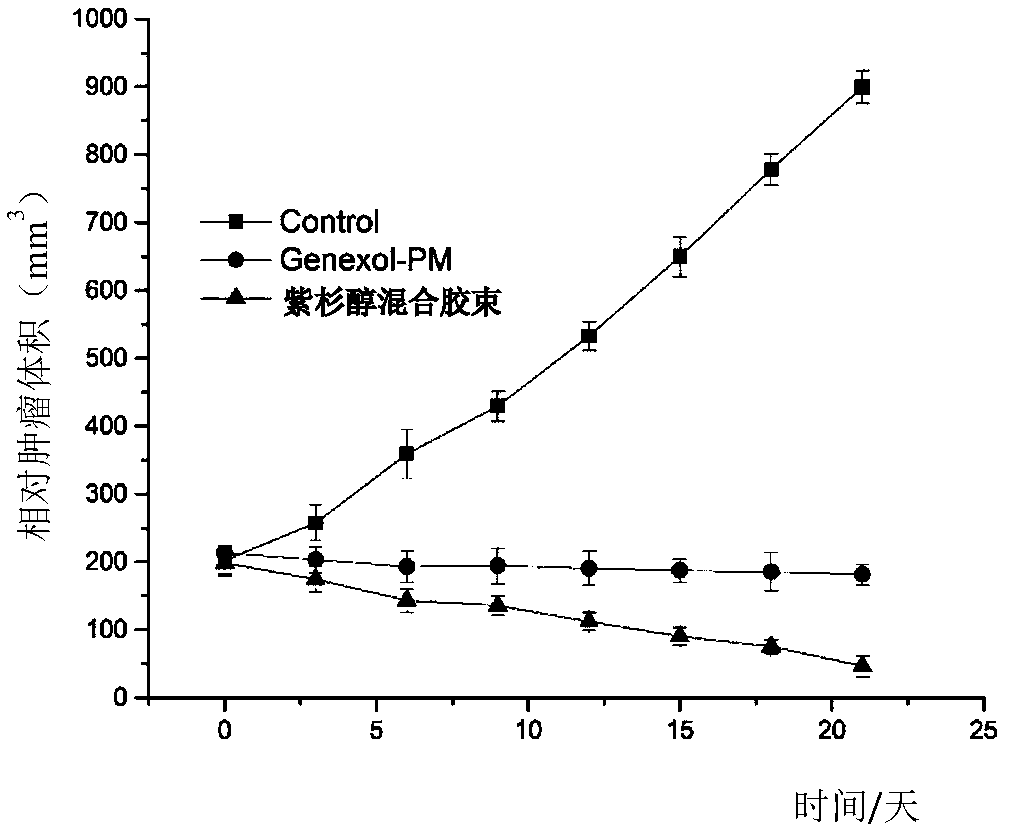
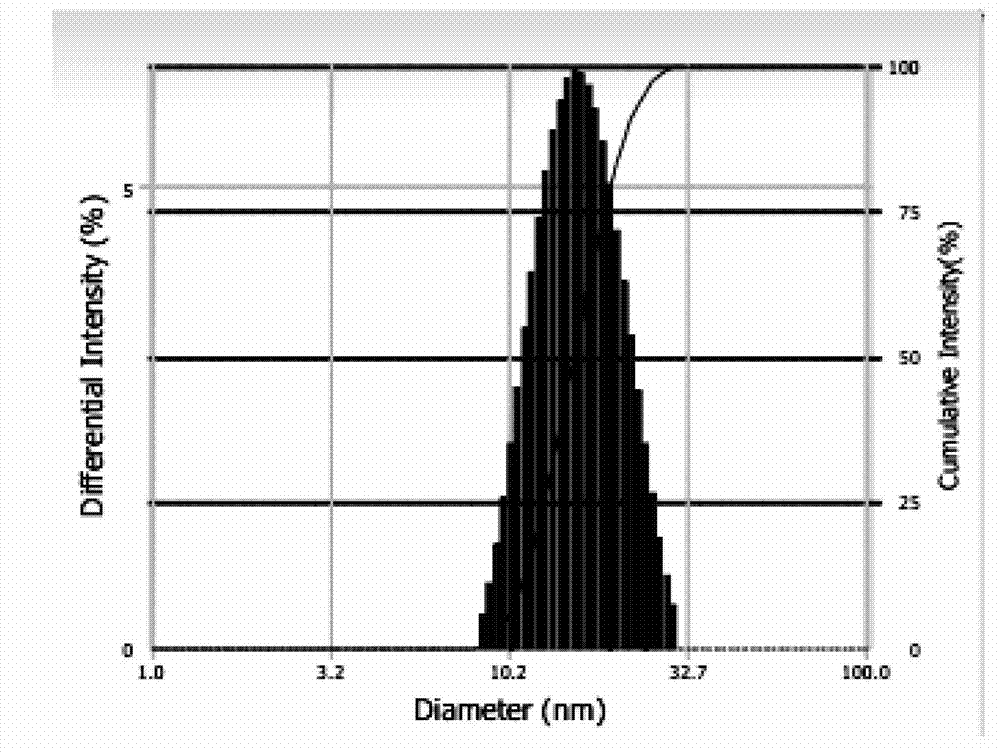
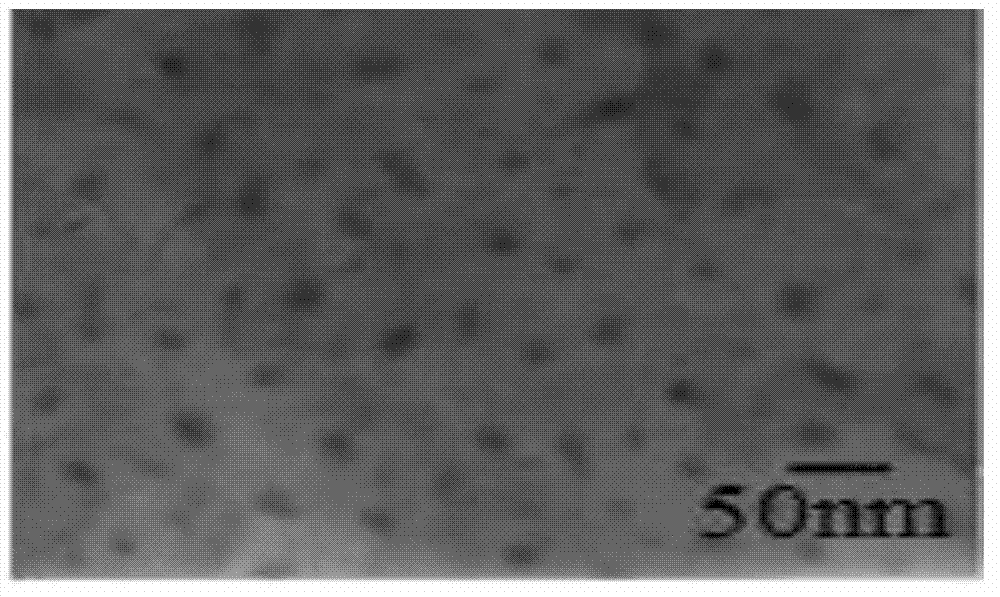
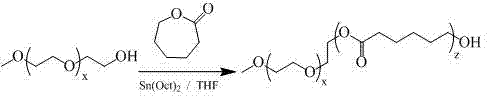
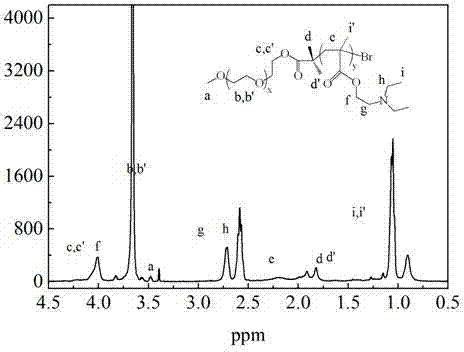
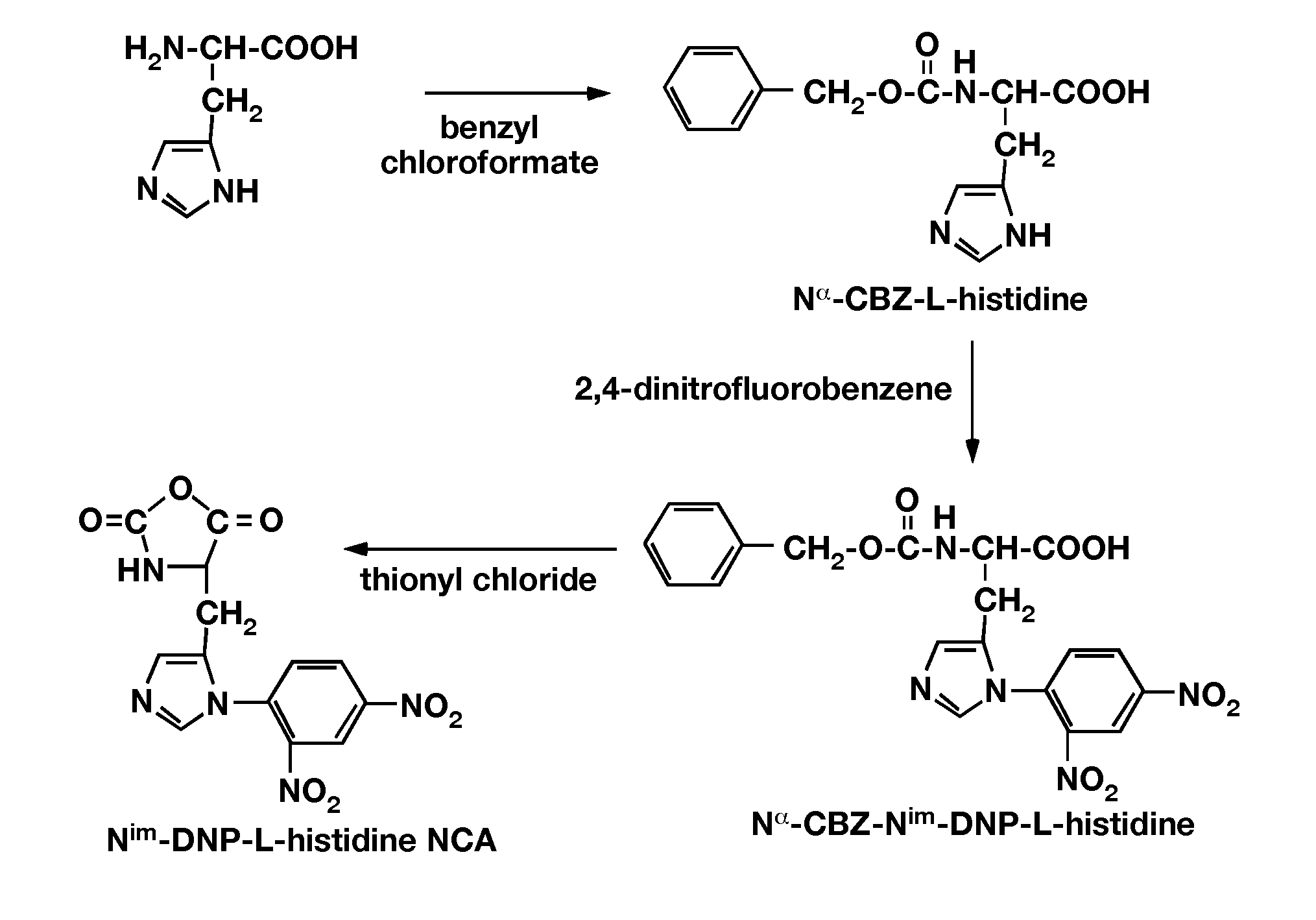
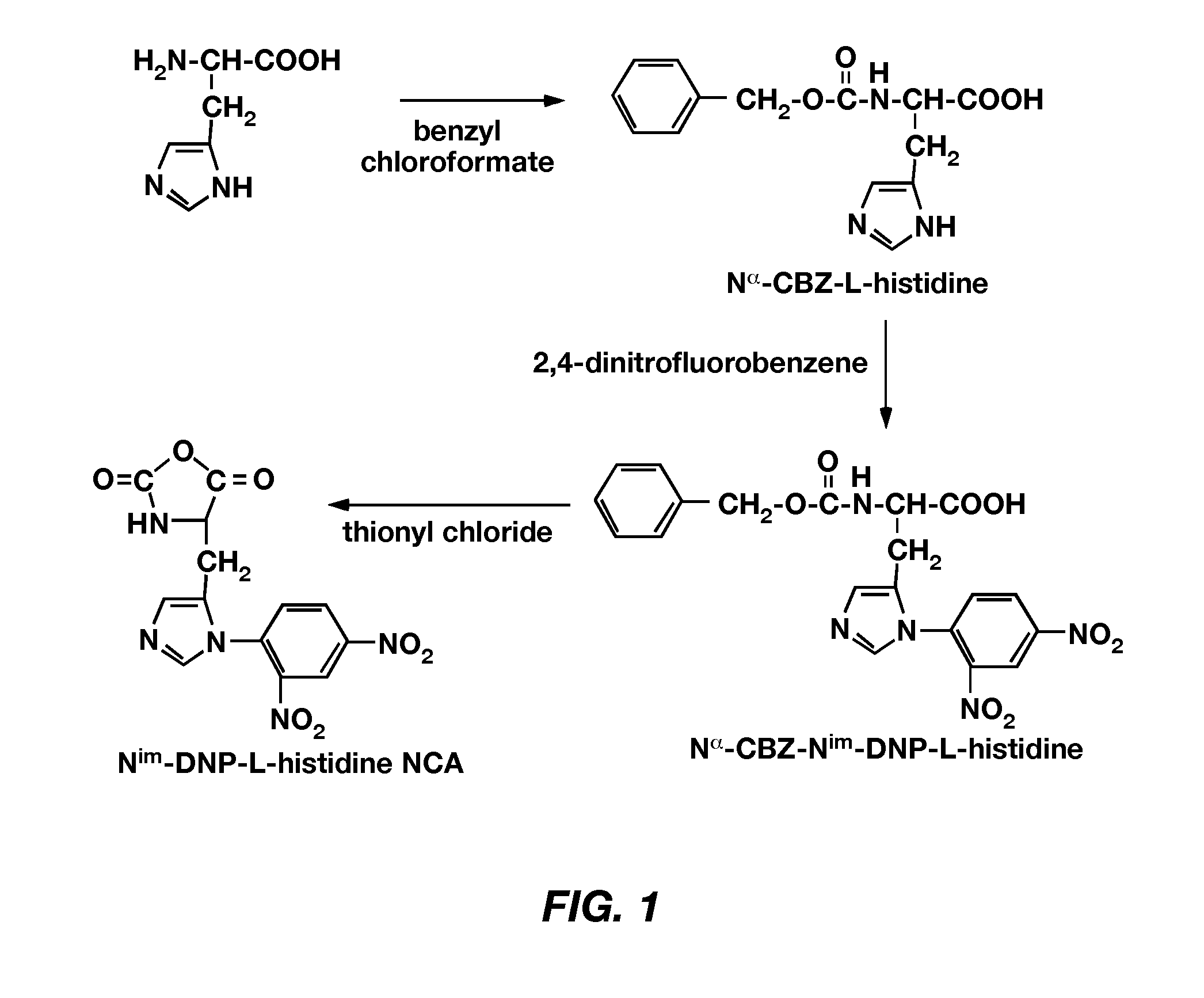
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
146 results about "Mixed micelle" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
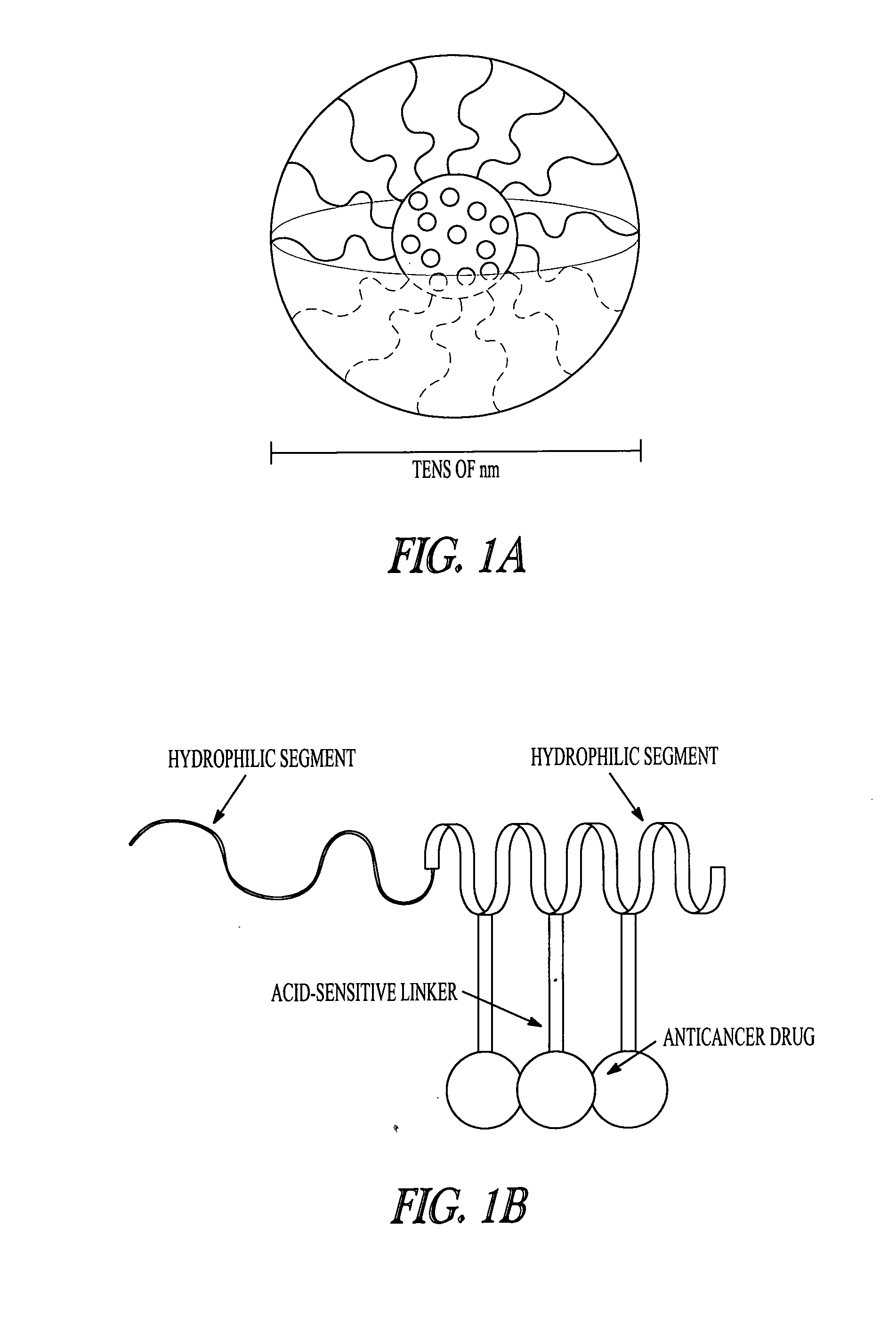
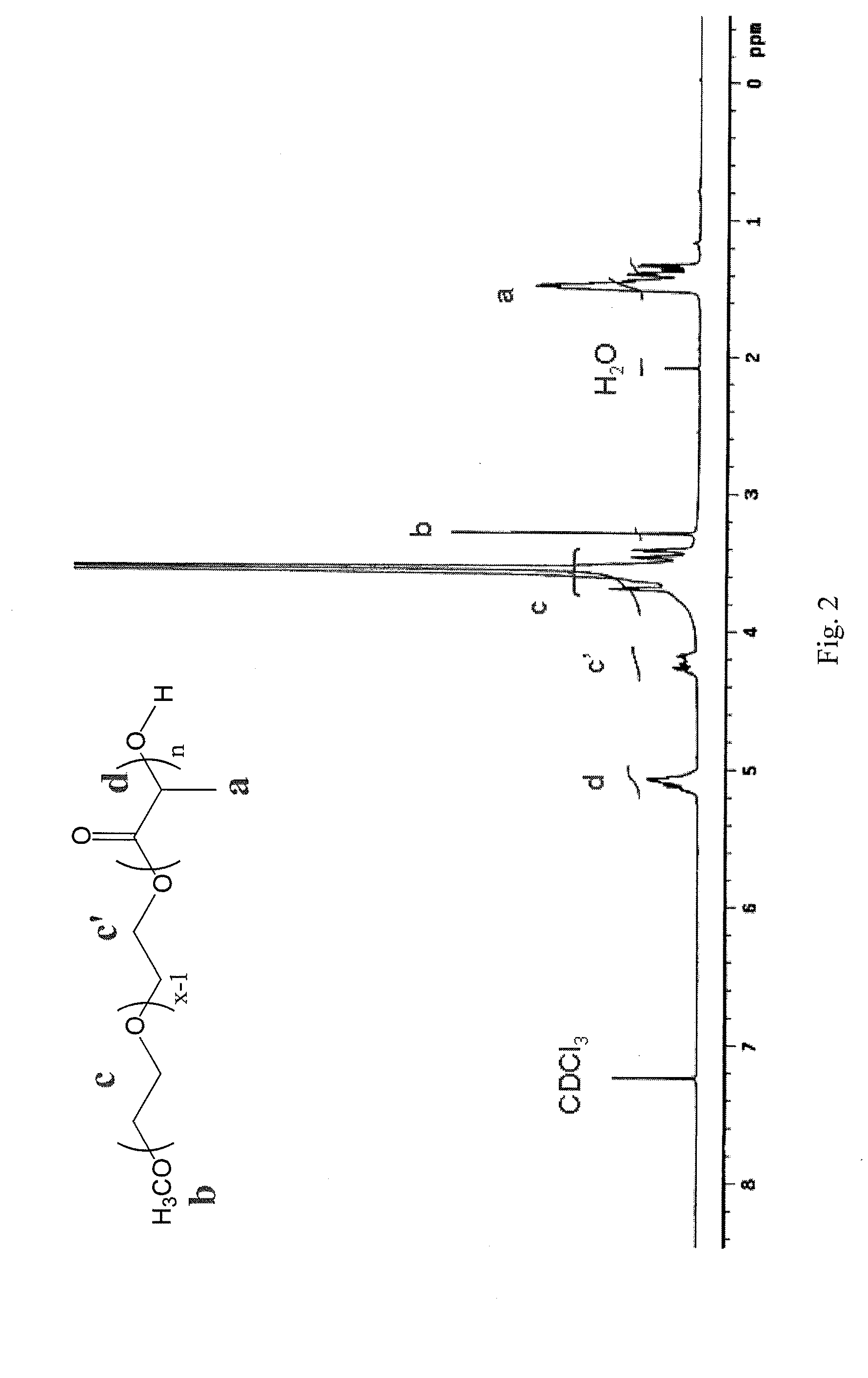
Mixed micelles can be composed of various compounds that are not very soluble in water. These compounds are dissolved in the center of the sphere where they can blend in with the hydrophobic tails.
Mixed micellar drug deliver system and method of preparation
Pharmaceutical compositions comprising a macromolecular pharmaceutical agent in micellar form are disclosed. The micelles are formed from an alkali metal alkyl sulfate, and at least one additional micelle-forming compound as described in the specification. An alkali metal salicylate and a pharmaceutically acceptable edetate are also included in the composition. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed.
Owner:GENEREX PHARMA
Pharmaceutical compositions for buccal and pulmonary application
Pharmaceutical compositions comprising a macromolecular pharmaceutical agent in mixed micellar form are disclosed. The mixed micelles are formed from an alkali metal alkyl sulfate, and at least three different micelle-forming compounds as described in the specification. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed. A preferred method for administering the present composition is through the buccal region of the mouth.
Owner:GENEREX PHARMA INC +1
Personal care formulations
InactiveUS6861060B1Increase stickinessIncrease load capacityCosmetic preparationsBiocideLipid formationPersonal care
Personal care and hygiene formulations for topical application to mucosal surfaces. These formulations include an amphiphilic lipid carrier in the form of a colloidal composition which can include a micellar aggregate or mixed micelles dispersed in a continuous aqueous phase, or an emulsion of lipid droplets suspended in a continuous aqueous phase, and an active agent which is an anti-microbial agent. The lipid carrier has high adhesiveness to mucous membranes such as the soft tissues of the oral cavity. The lipid carrier also has a high load capacity for the active agent to be carried to these tissues. These formulations have the desirable properties of carrying a large amount of active agent for controlled and prolonged release thereof at the desired site, such as mucous membrane surfaces and surrounding tissue. Accordingly, the present invention provides a formulation for oral or topical application including an anti-microbial agent and a lipid. The agent is held by the carrier through a hydrophobic interaction and is released from the carrier in a controlled manner over a prolonged period of time. The lipid is also characterized by having a high adhesive capability towards mucous membrane surfaces. The lipid and the agent are preferably present in a ratio in a range of from about 1:10 to 10:1, more preferably from about 1:5 to about 5:1, and most preferably from about 1:3 to about 3:1 in the formulation.
Owner:LURIYA ELENA +1
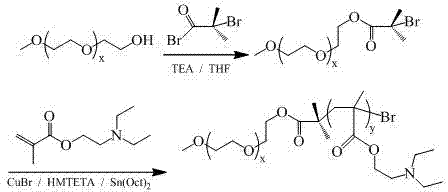
Multifunctional mixed micelle of graft and block copolymers and preparation thereof
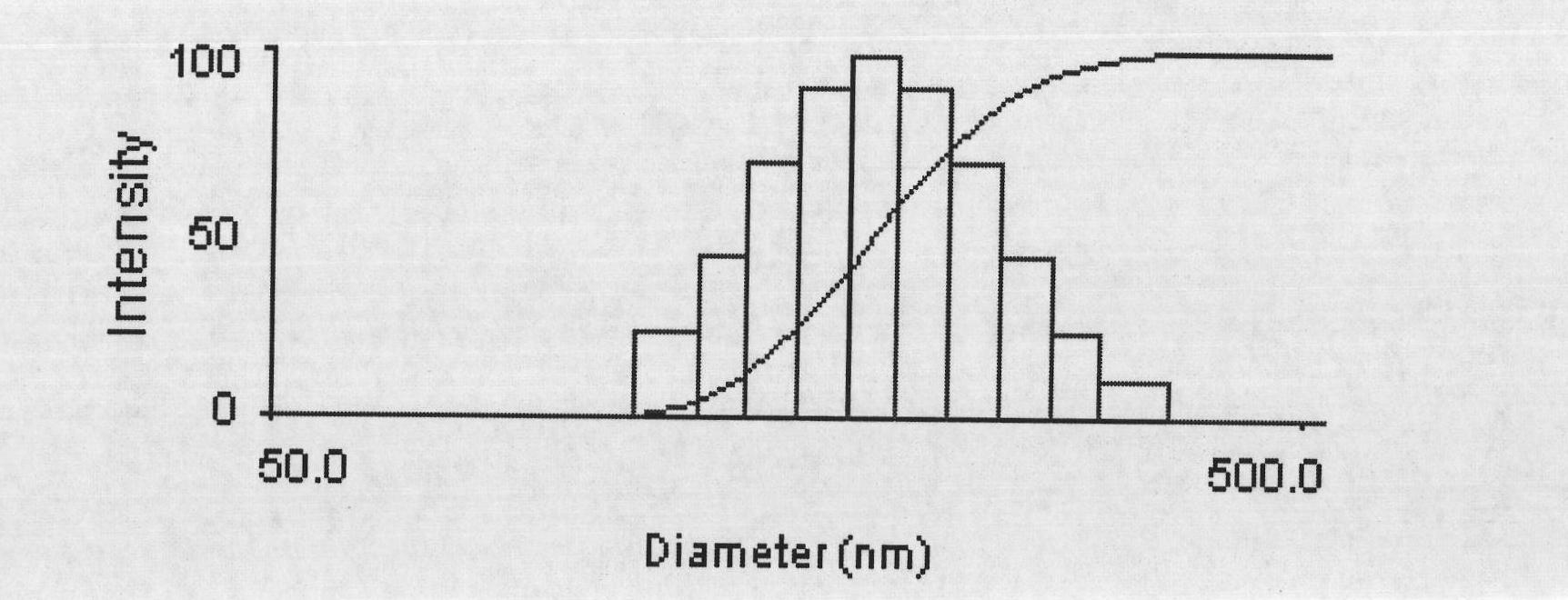
The present invention discloses a novel mixed micelle structure with a functional inner core and hydrophilic outer shells self-assembled from a graft macromolecule and one or more block copolymer, and preferably from a graft copolymer and two or more diblock copolymers. The micelle synthesized in the present invention has a size of about 50-200 nm, which can be used as a cancer diagnosis agent and a cancer drug delivery carrier.
Owner:NATIONAL TSING HUA UNIVERSITY
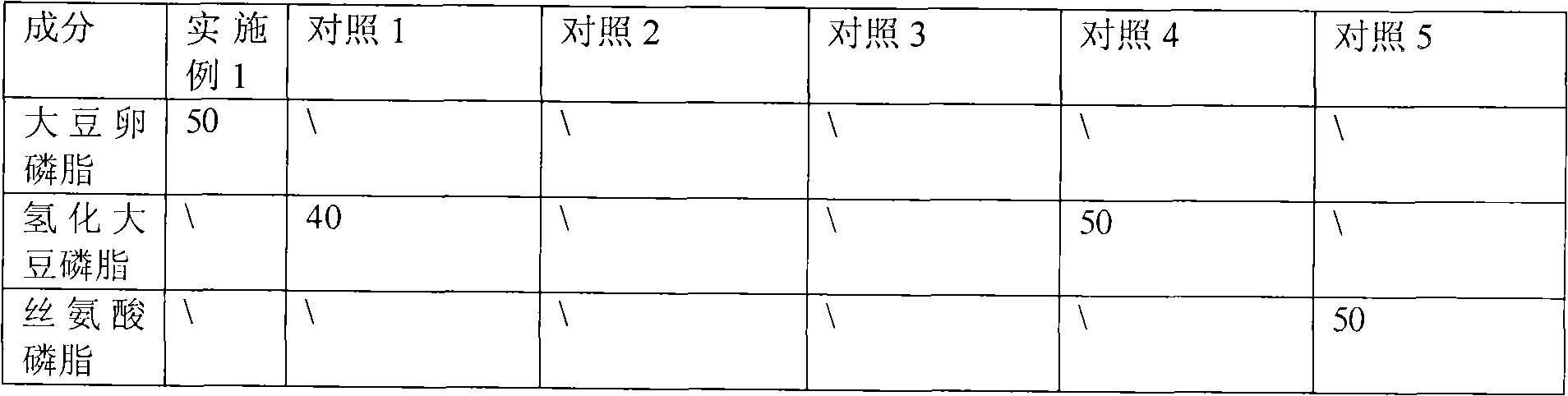
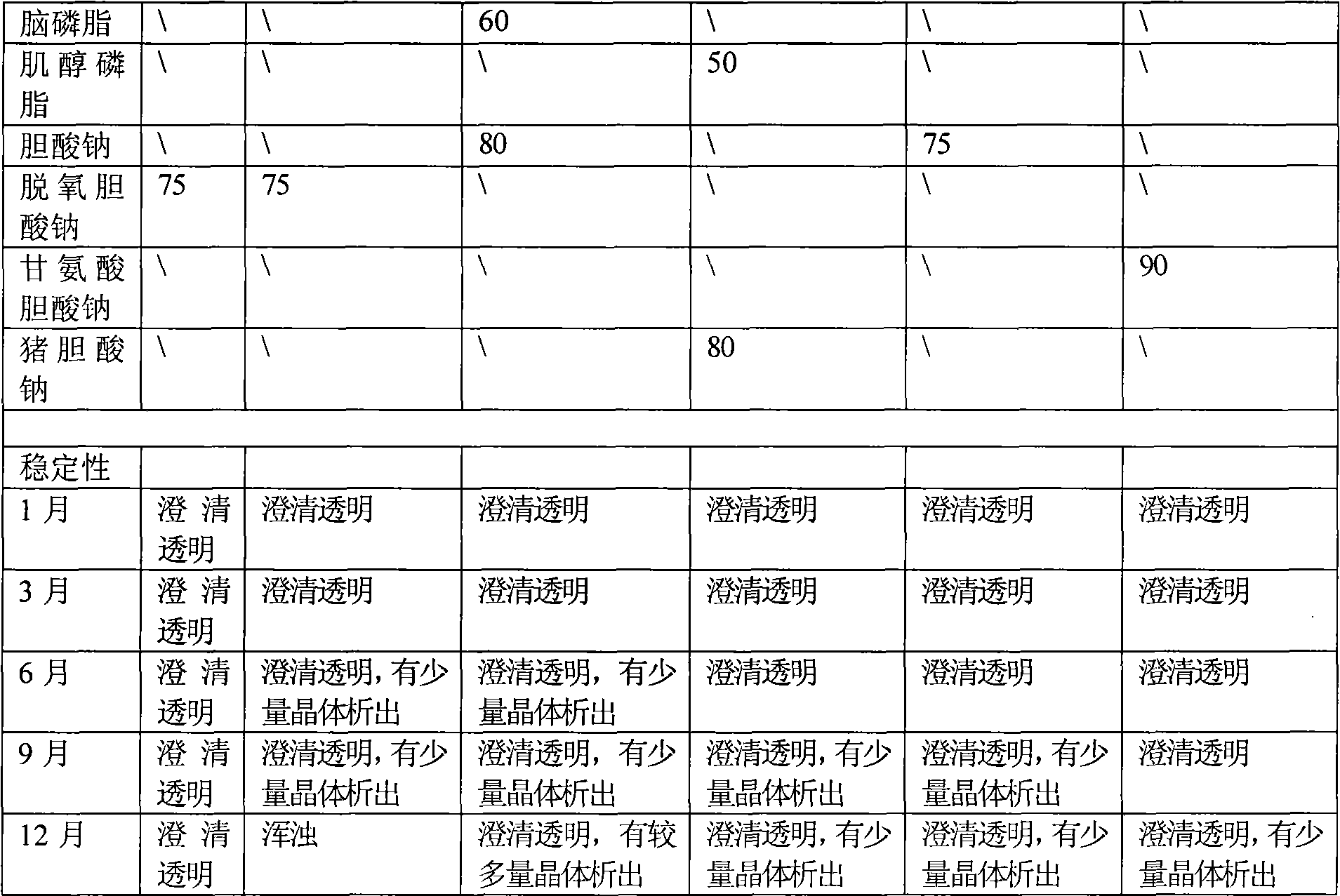
Mixed glue bundle pharmaceutical preparations produced in combination use of multiple surfactant and processes for their preparation
InactiveCN101138550AStrong dilution stabilityReduce viscosityPharmaceutical non-active ingredientsLiposomal deliverySolubilityMixed micelle
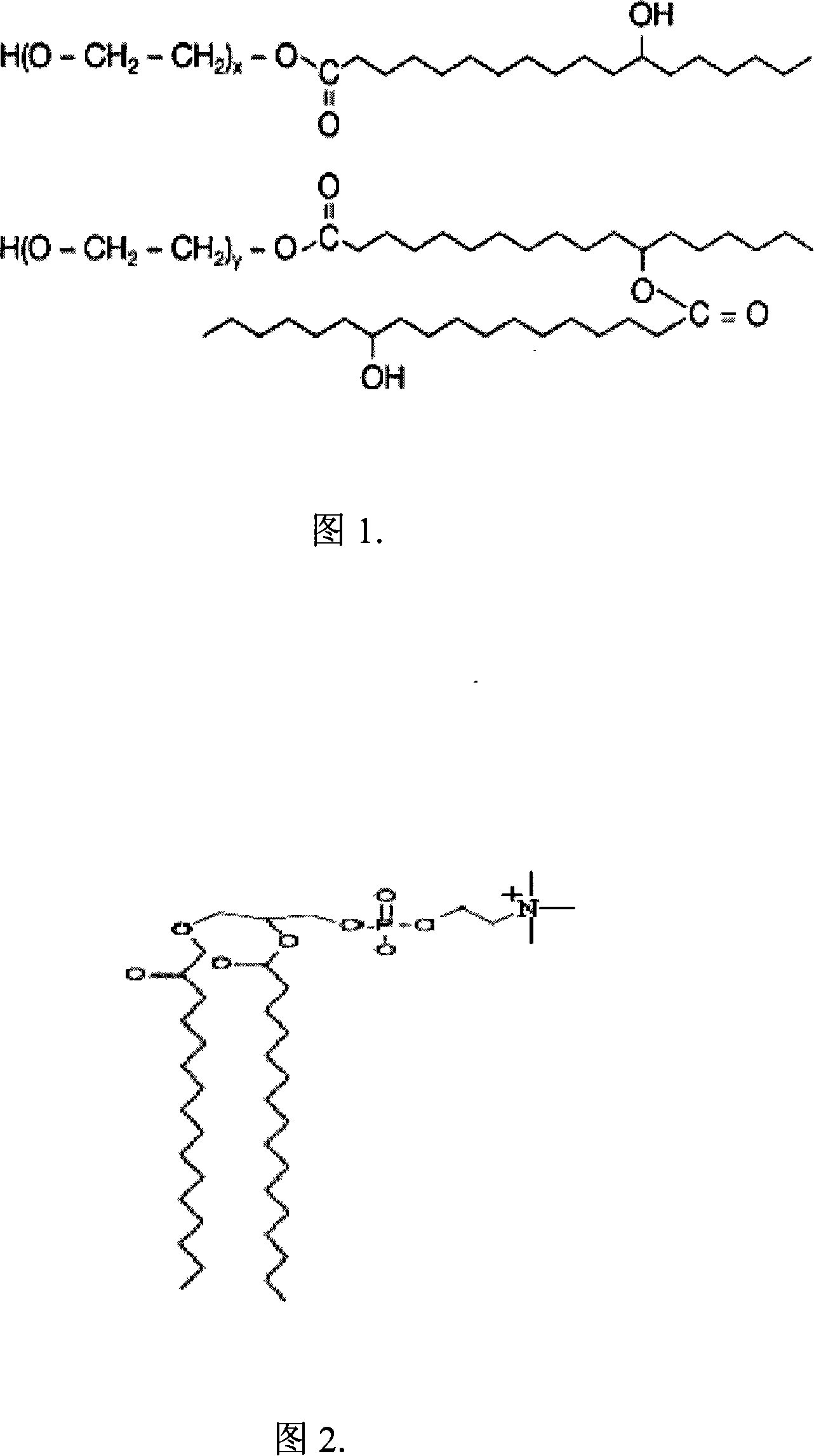
The present invention relates to a mixed micelle medicine preparation and a preparation method, which is prepared by the combination of various kinds of surface acting agents. The mixed micelle consists of the polyethylene glycol-12-hydroxy stearate and the other surface acting agents of one kind or various kinds. The other surface acting agents comprise phospholipid, VE Macrogol succinate, Macrogol-VE-carbonate and Macrogol-VE-succinate. In addition, the mixed micelle also comprises drugs, solvent, a stabilizer with or without other components and a PH conditioner. The amount of the polyethylene glycol-12-hydroxy stearate in the prescription is 4 percentage to 40 percentage, W / V: the amount of the phospholipid is 0 percentage to 30 percentage, W / V: the amount of the activator is 0 percentage to 30 percentage, W / V: the amount of the drug is 0.001 percentage to 10 percentage, W / V: the amount of the solvent is 0 percentage to 90 percentage, W / V. The medicine comprises the hydrophobicity drug and the lip solubility drug, but the medicine is not restricted by the both kinds of drugs. The present invention has the following advantages. Firstly, the preparation has good dilution stability, which can improve the defect in the present preparation and can meet the demanding for clinical drug administration. Secondly, the toxicity is low and the chemical stability is excellent.
Owner:SHENYANG PHARMA UNIVERSITY
Pharmaceutical compositions for buccal and pulmonary administration comprising an alkali metal alkyl sulfate and at least three micelle-forming compounds
Pharmaceutical compositions comprising a macromolecular pharmaceutical agent in mixed micellar form are disclosed. The mixed micelles are formed from an alkali metal alkyl sulfate, and at least three different micelle-forming compounds as described in the specification. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed. A preferred method for administering the present composition is through the buccal region of the mouth.
Owner:GENEREX PHARMA
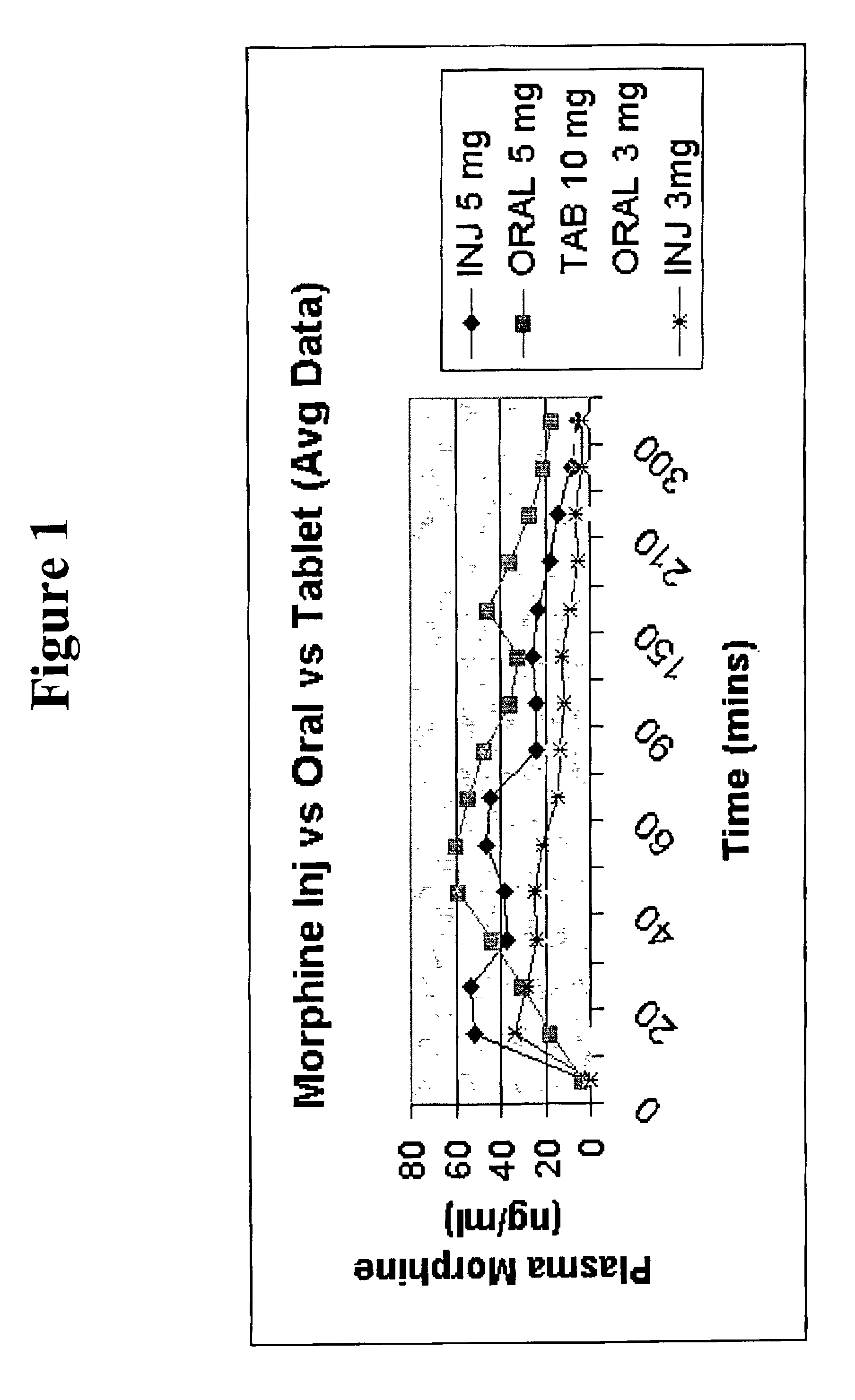
Pharmaceutical compositions for buccal delivery of pain relief medications
Pharmaceutical compositions comprising a narcotic analgesic in mixed micellar form are disclosed. The mixed micelles are formed from an alkali metal alkyl sulfate, and other micelle-forming compounds as described in the specification. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed. A preferred method for administering the present composition is through the buccal mucosa of the mouth.
Owner:GENEREX PHARMA
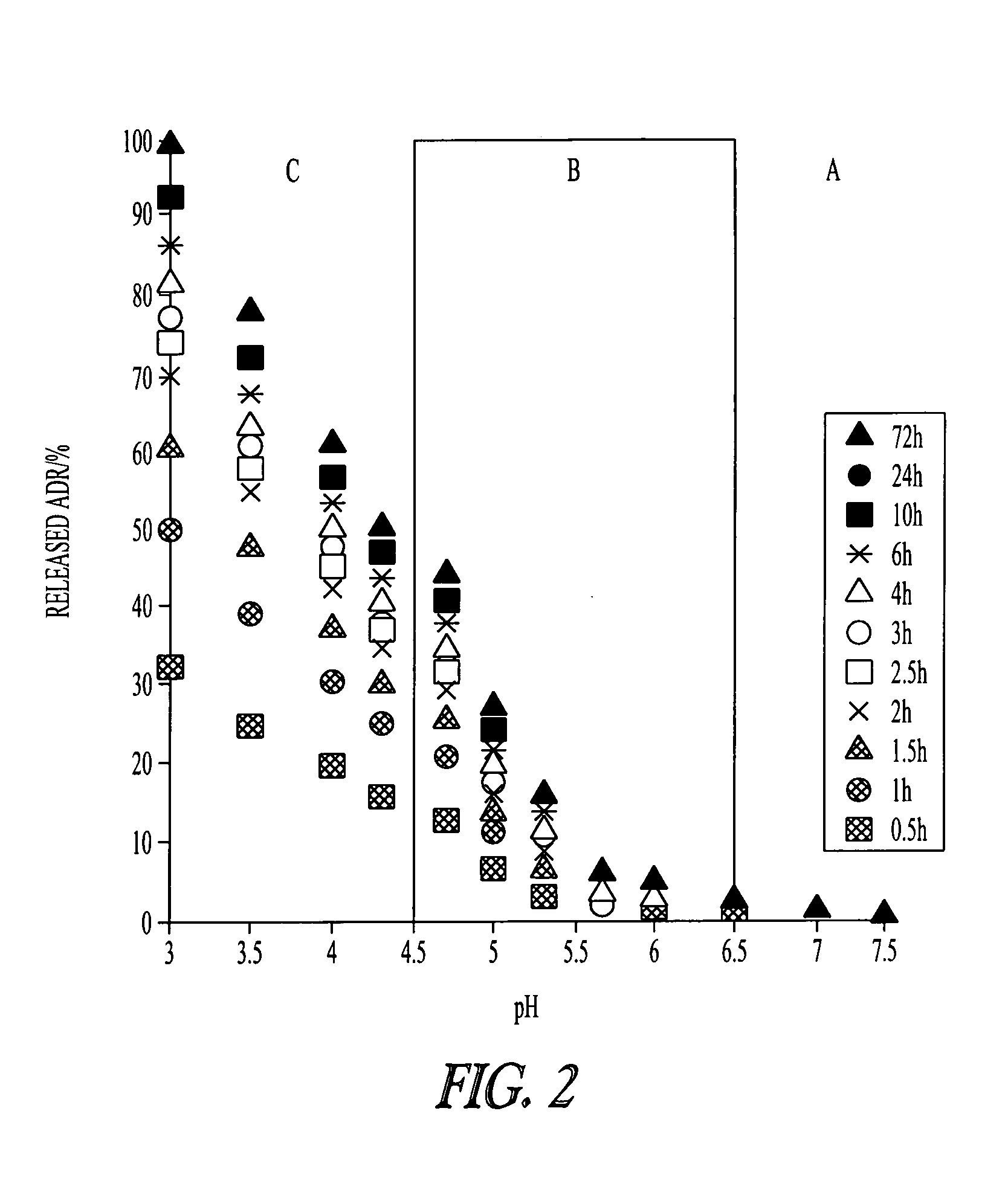
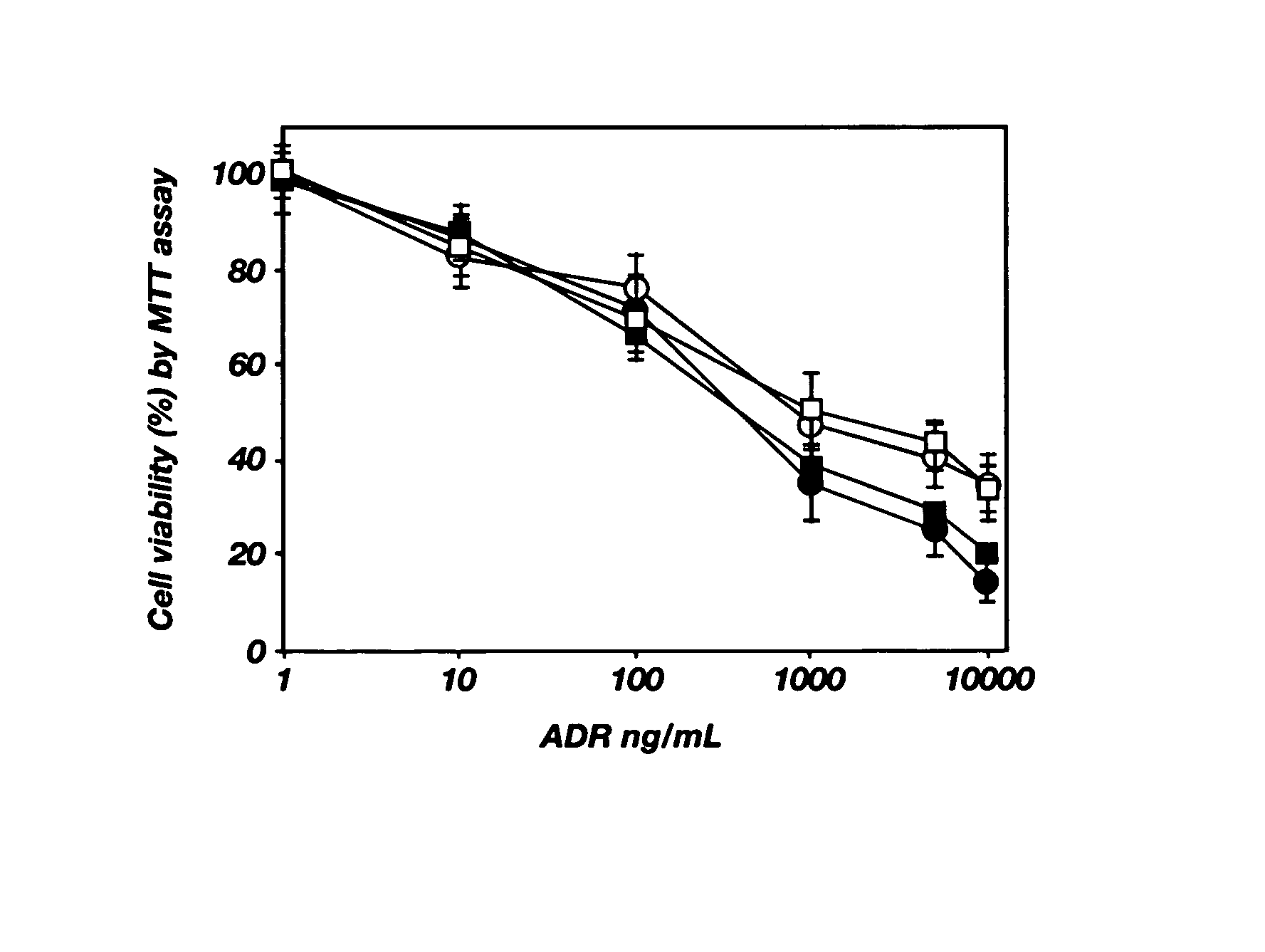
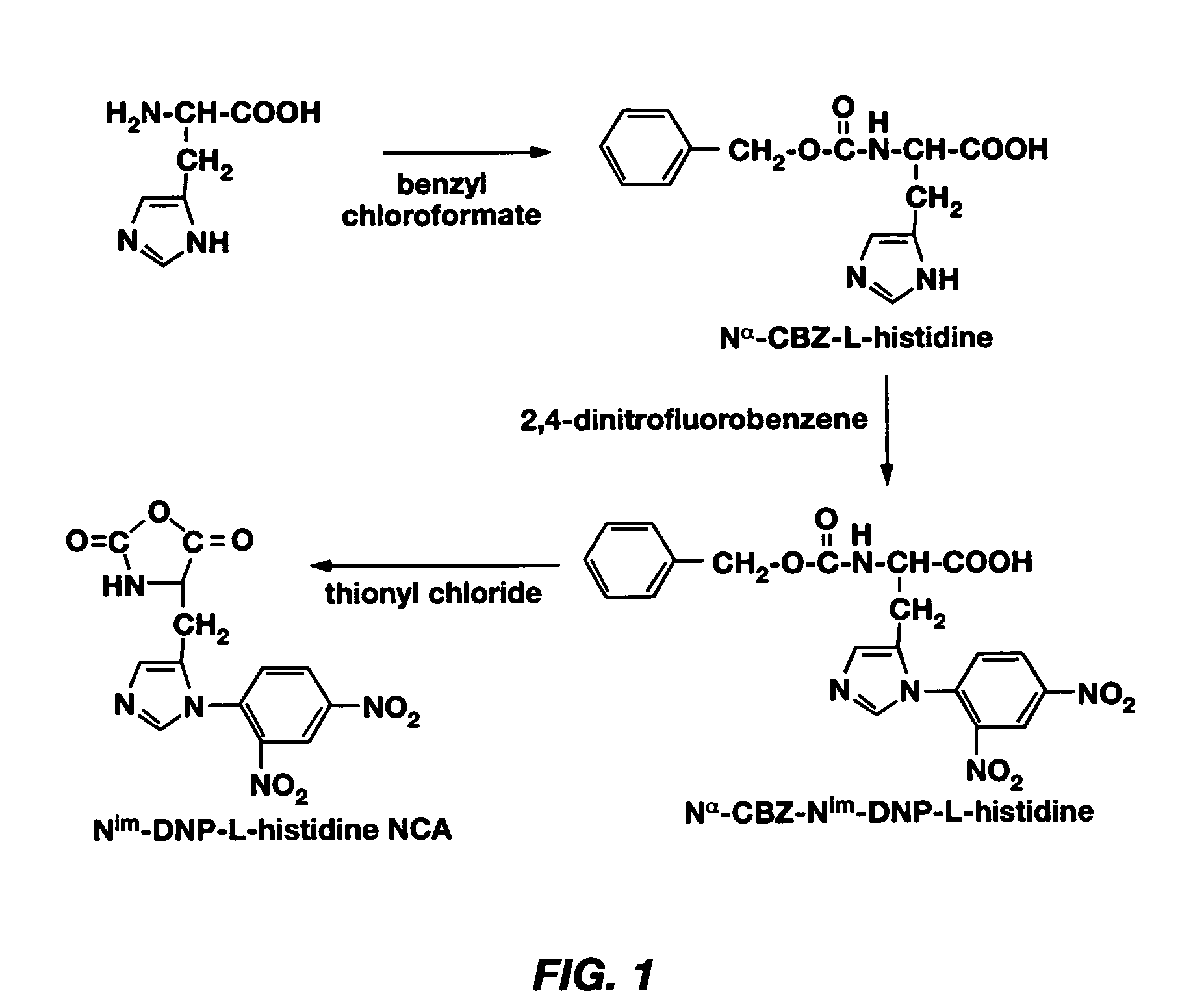
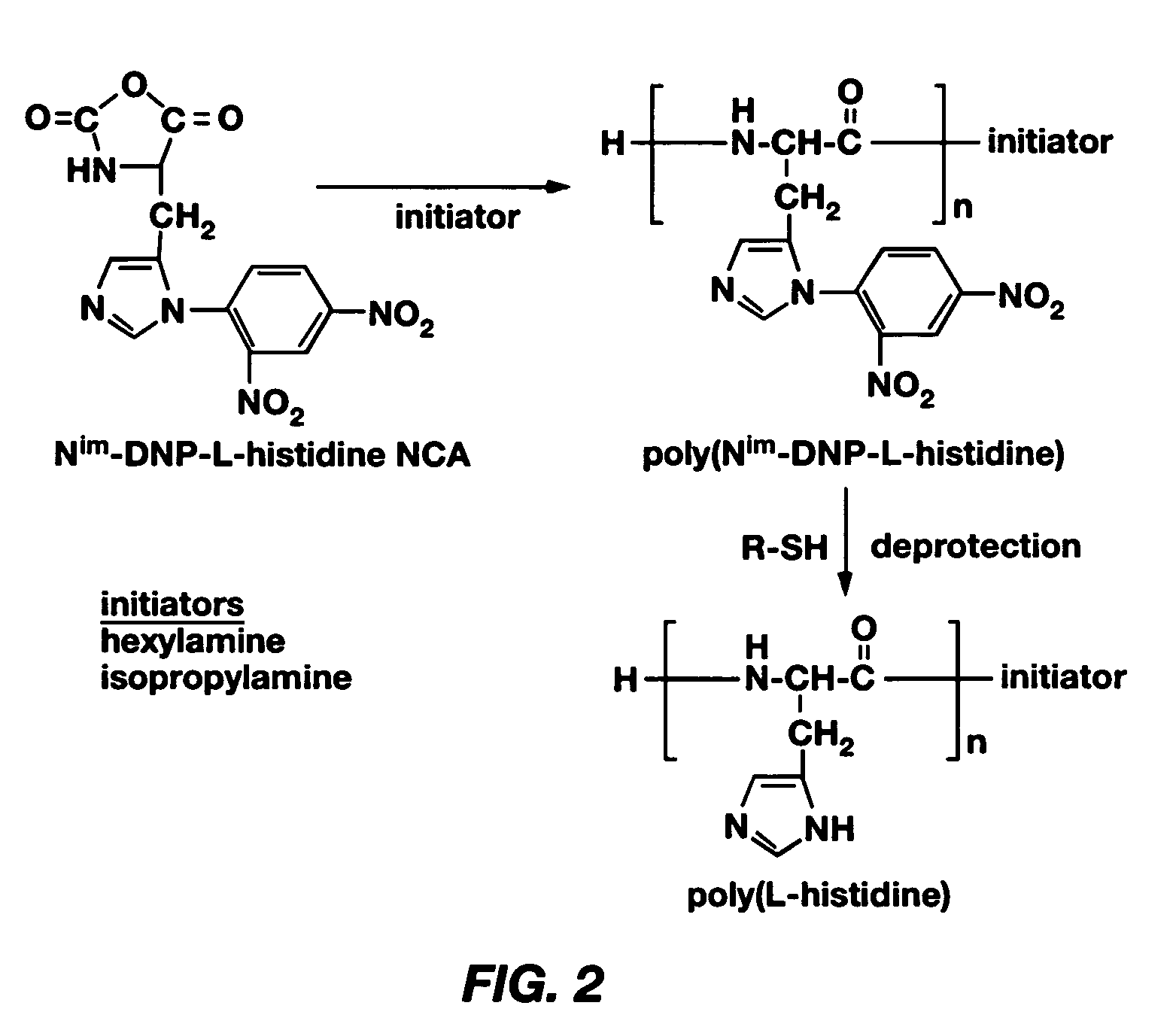
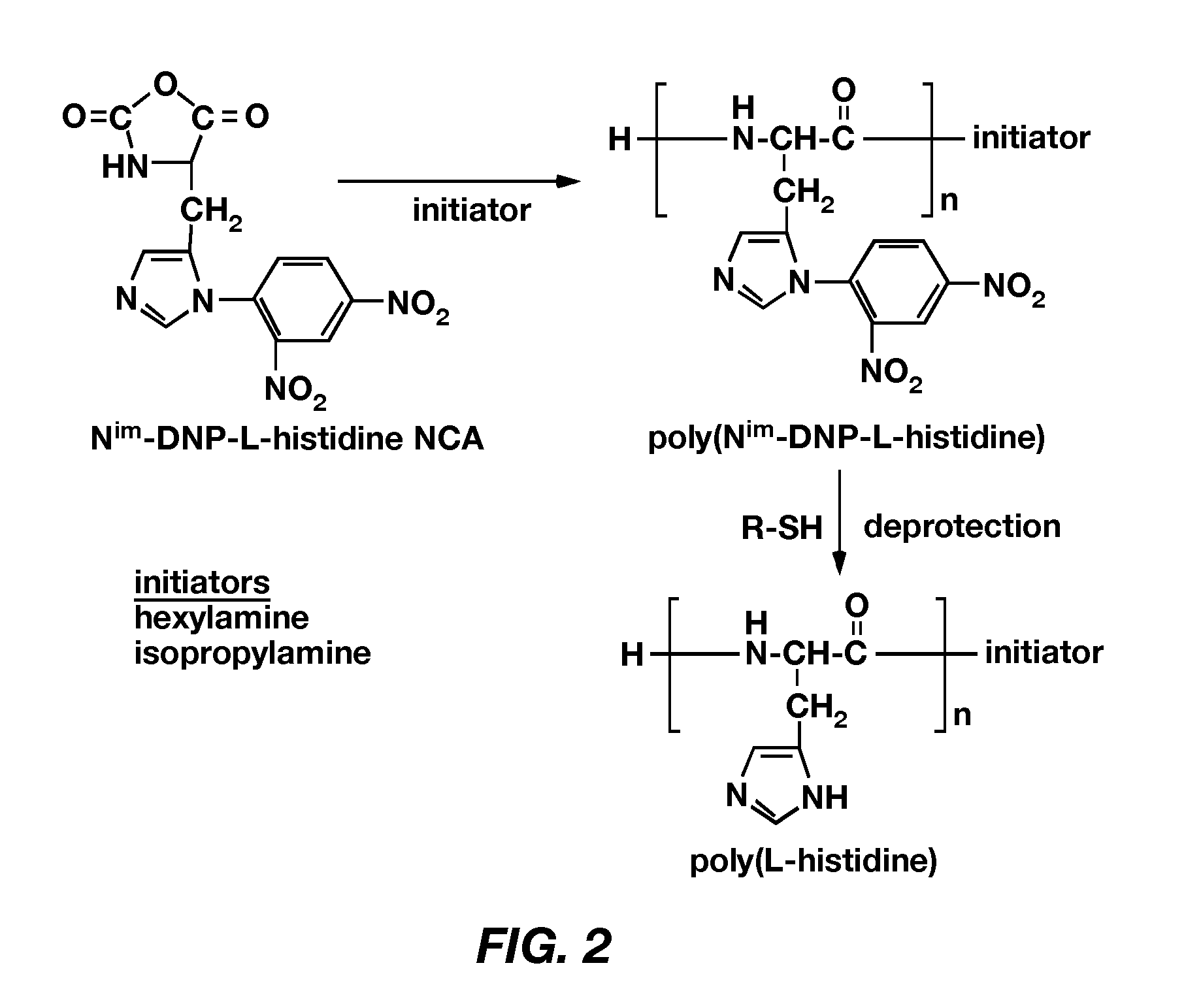
PH-sensitive polymeric micelles for drug delivery
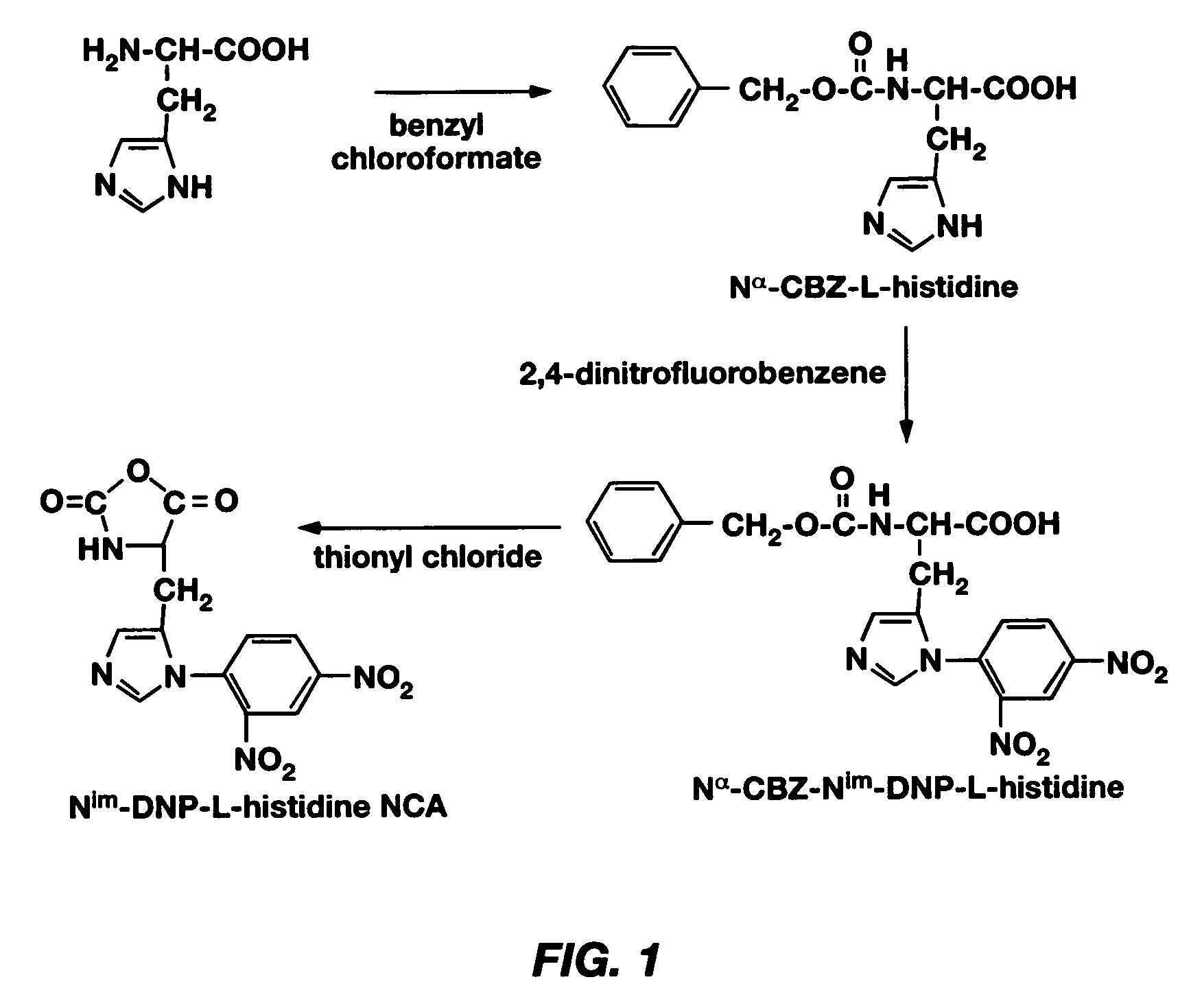
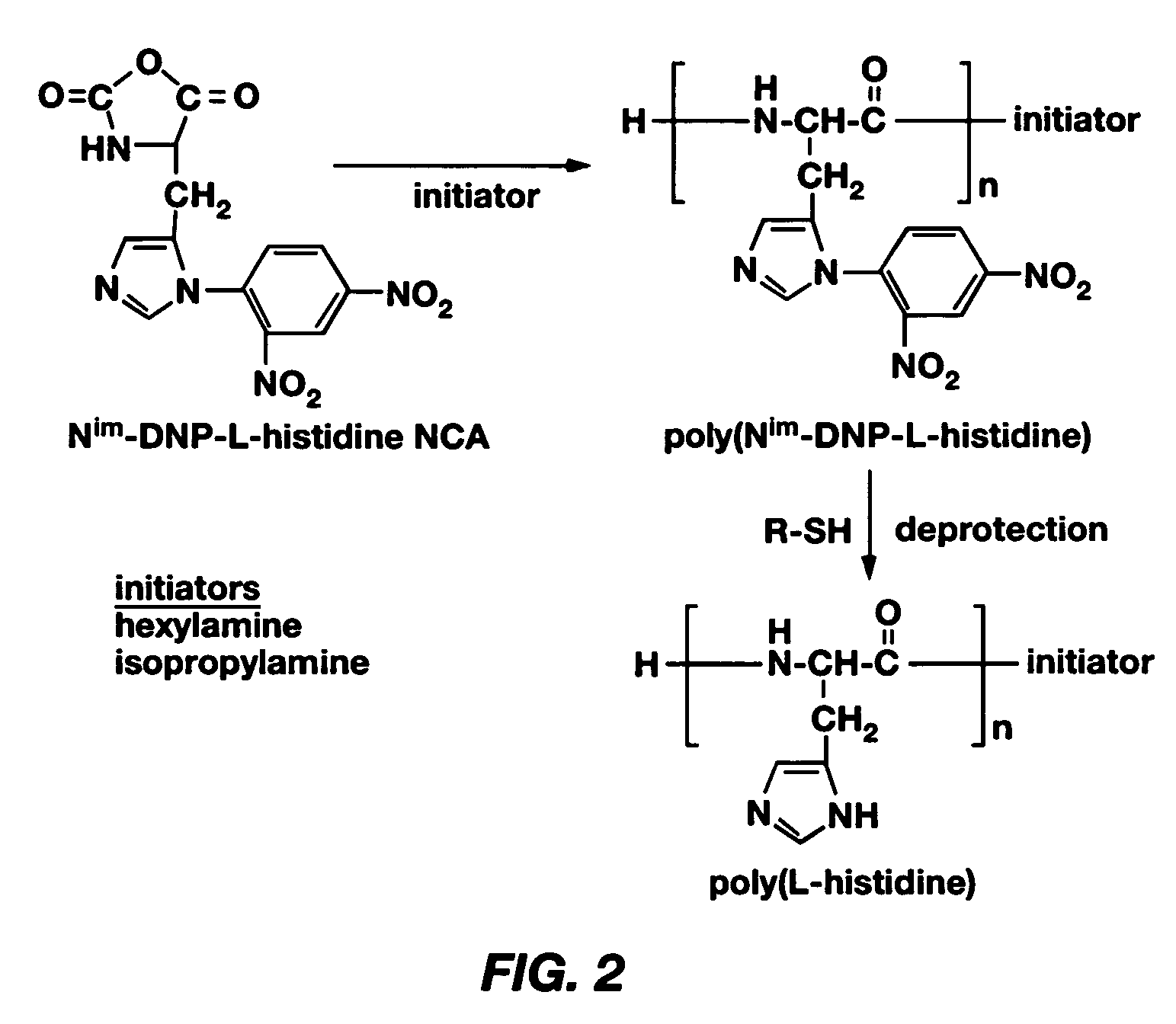
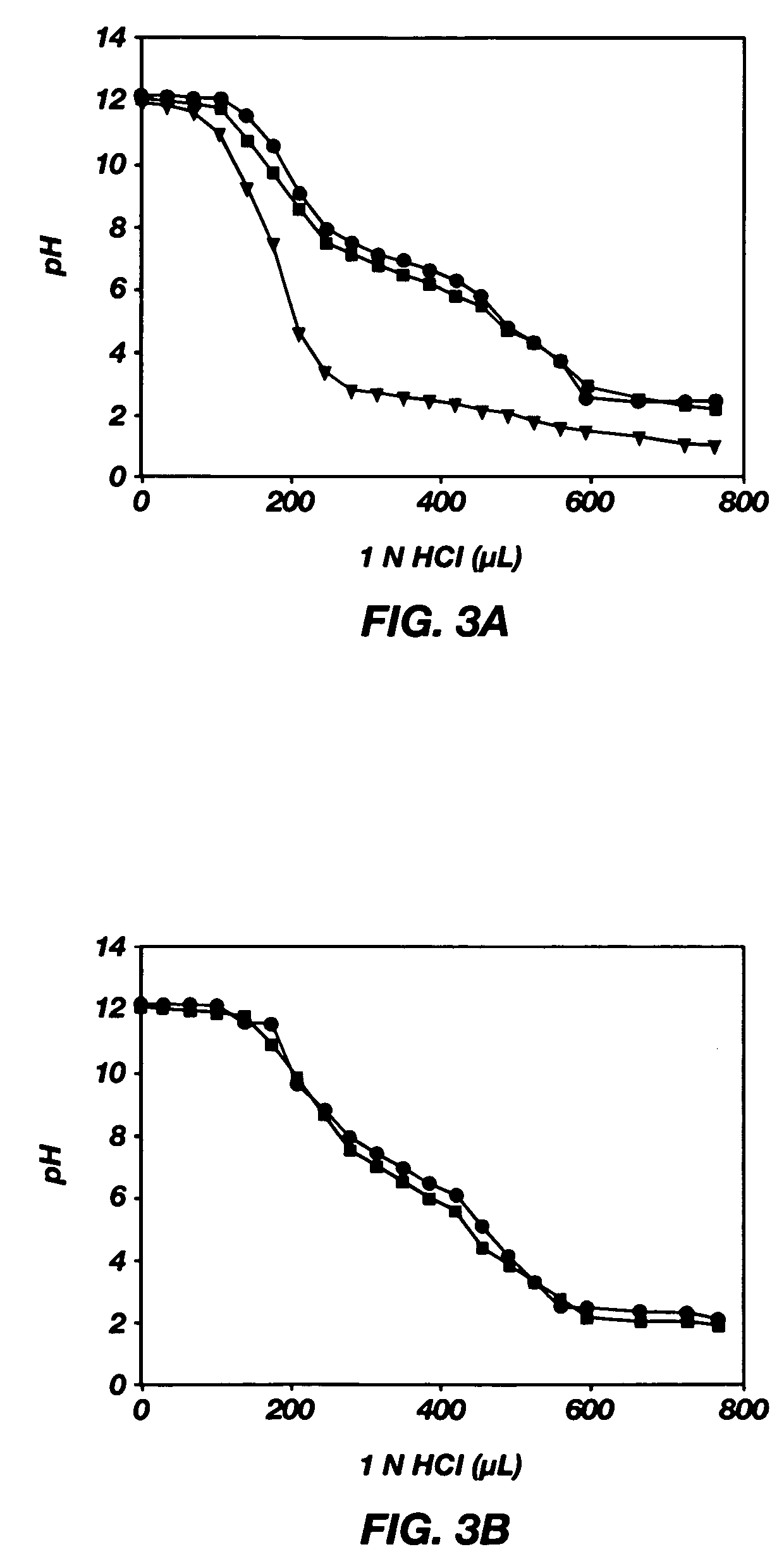
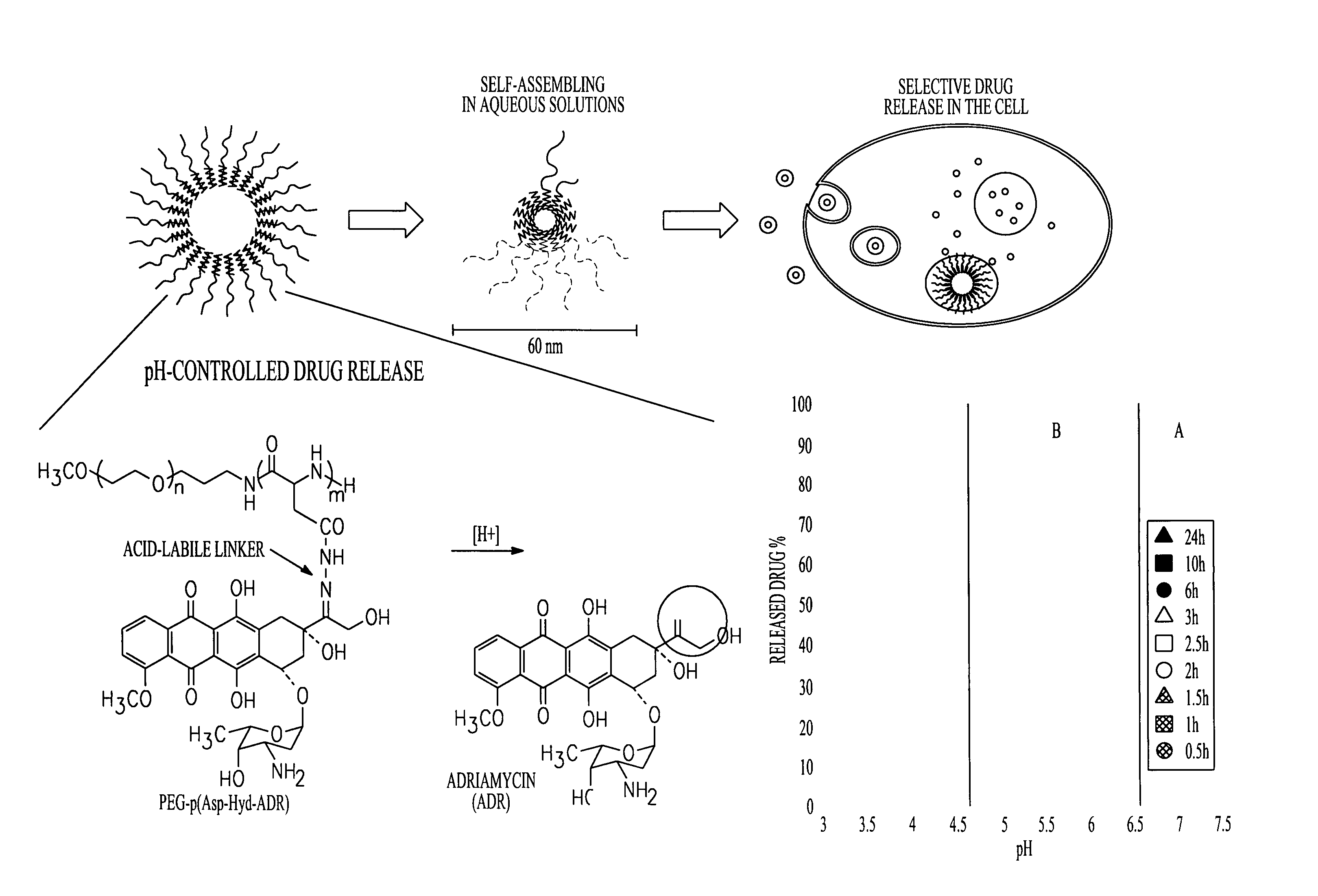
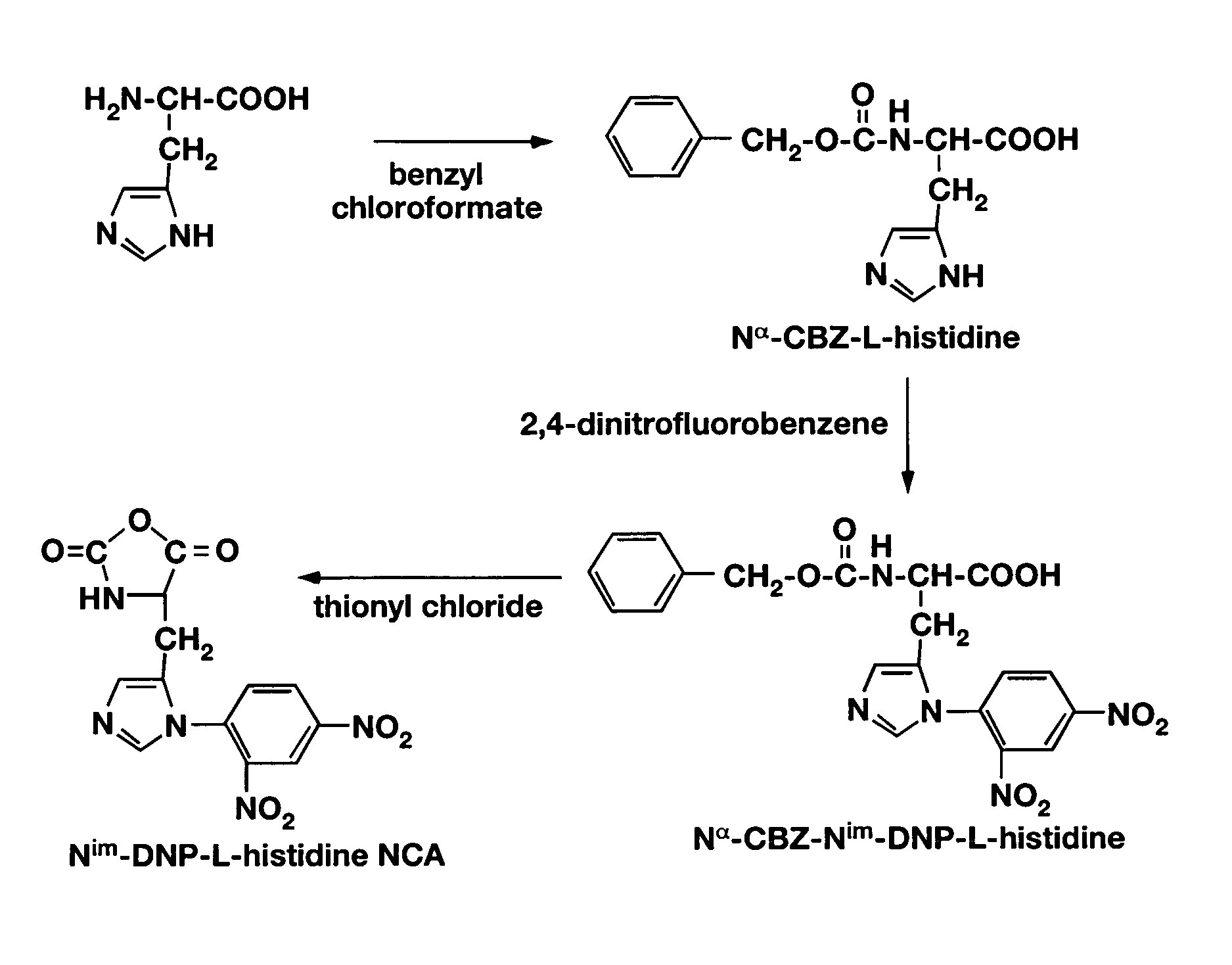
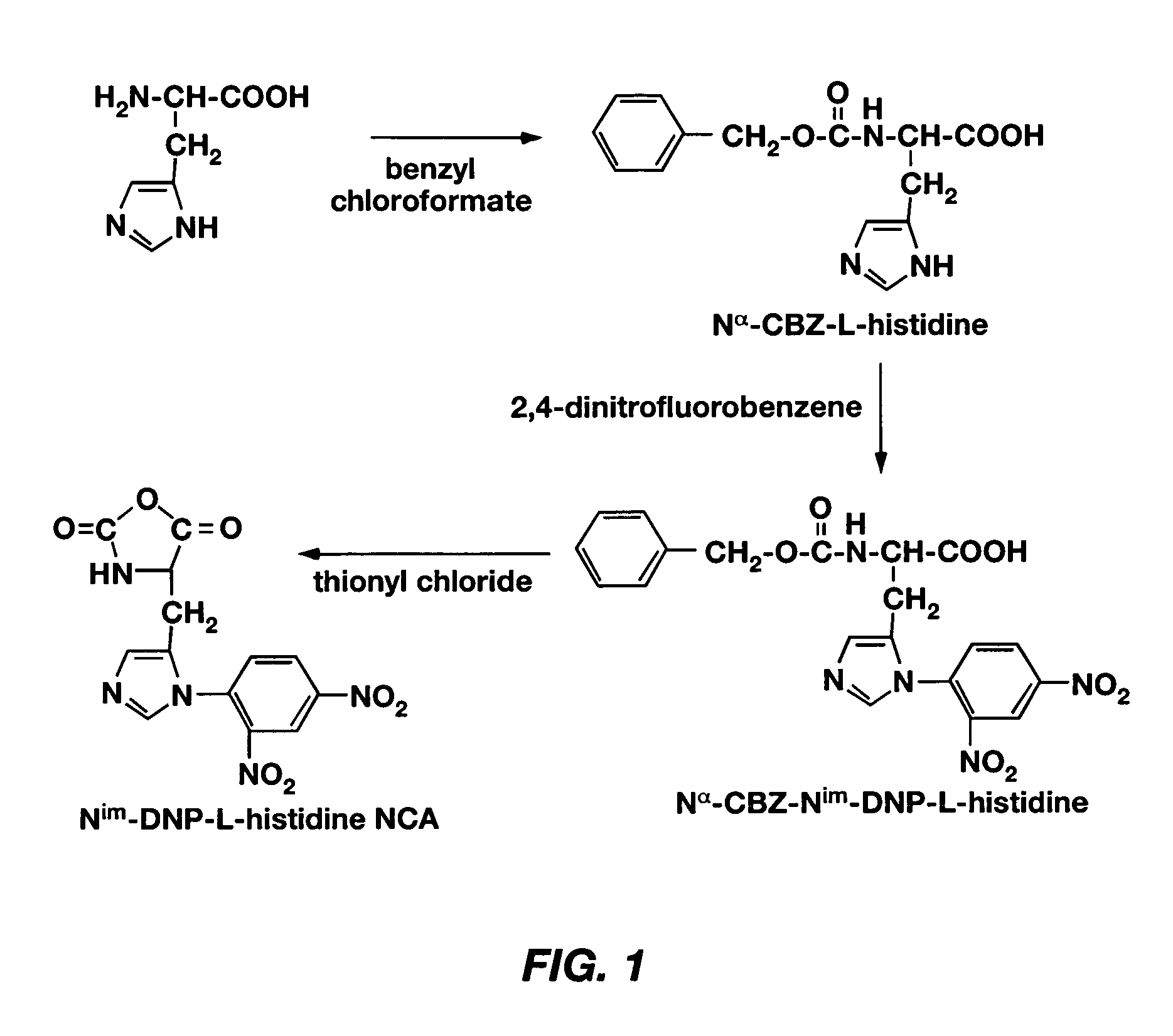
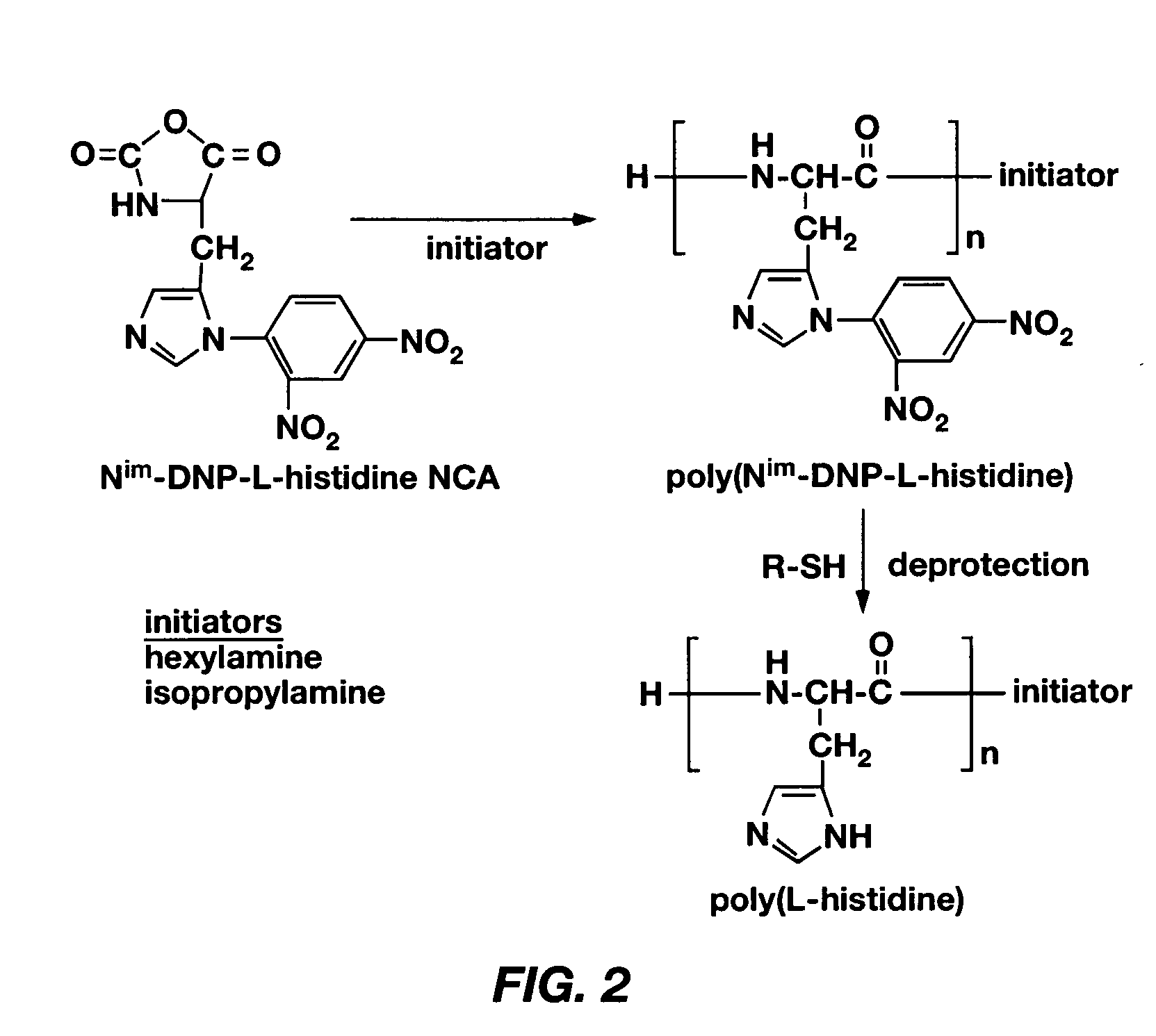
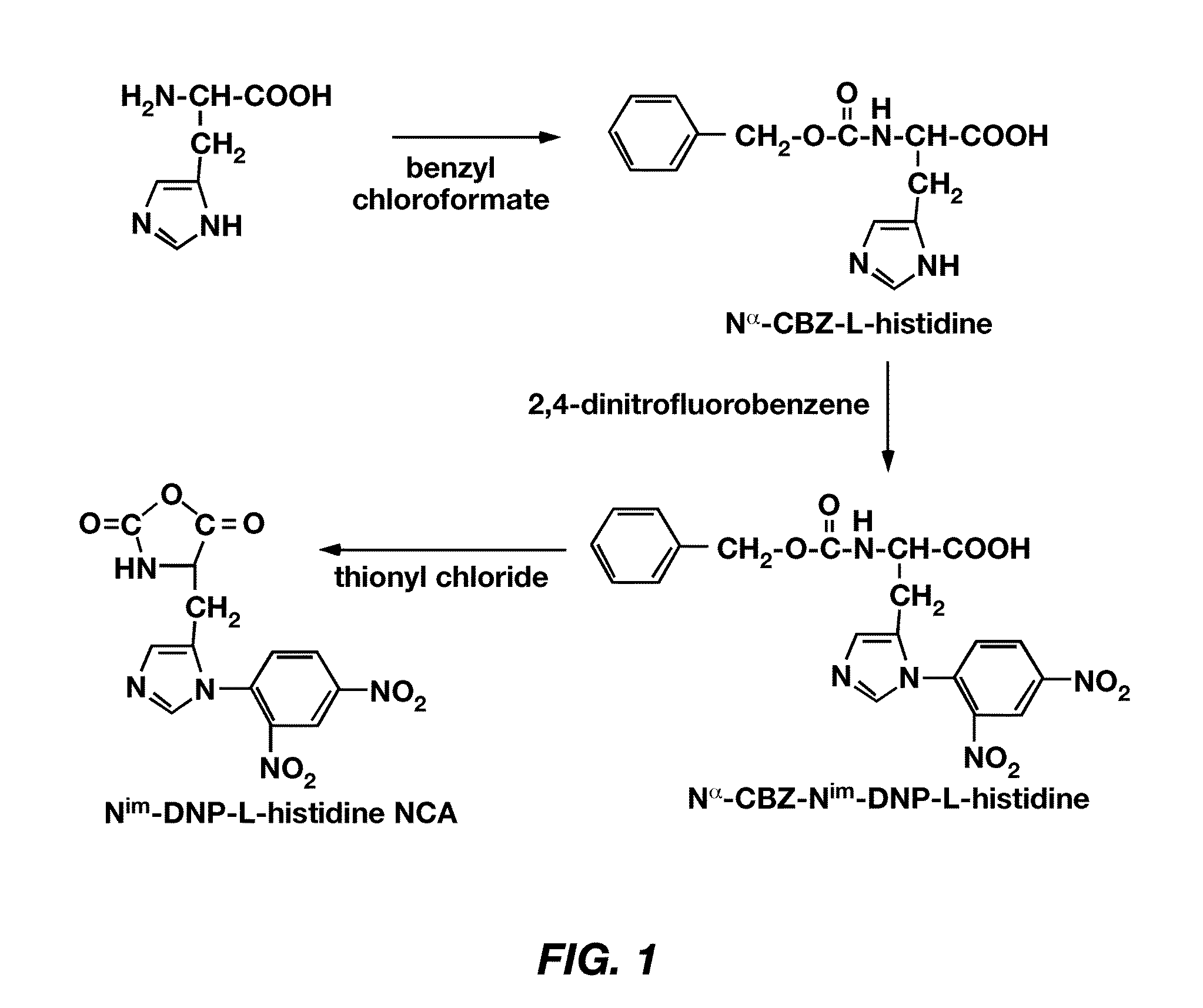
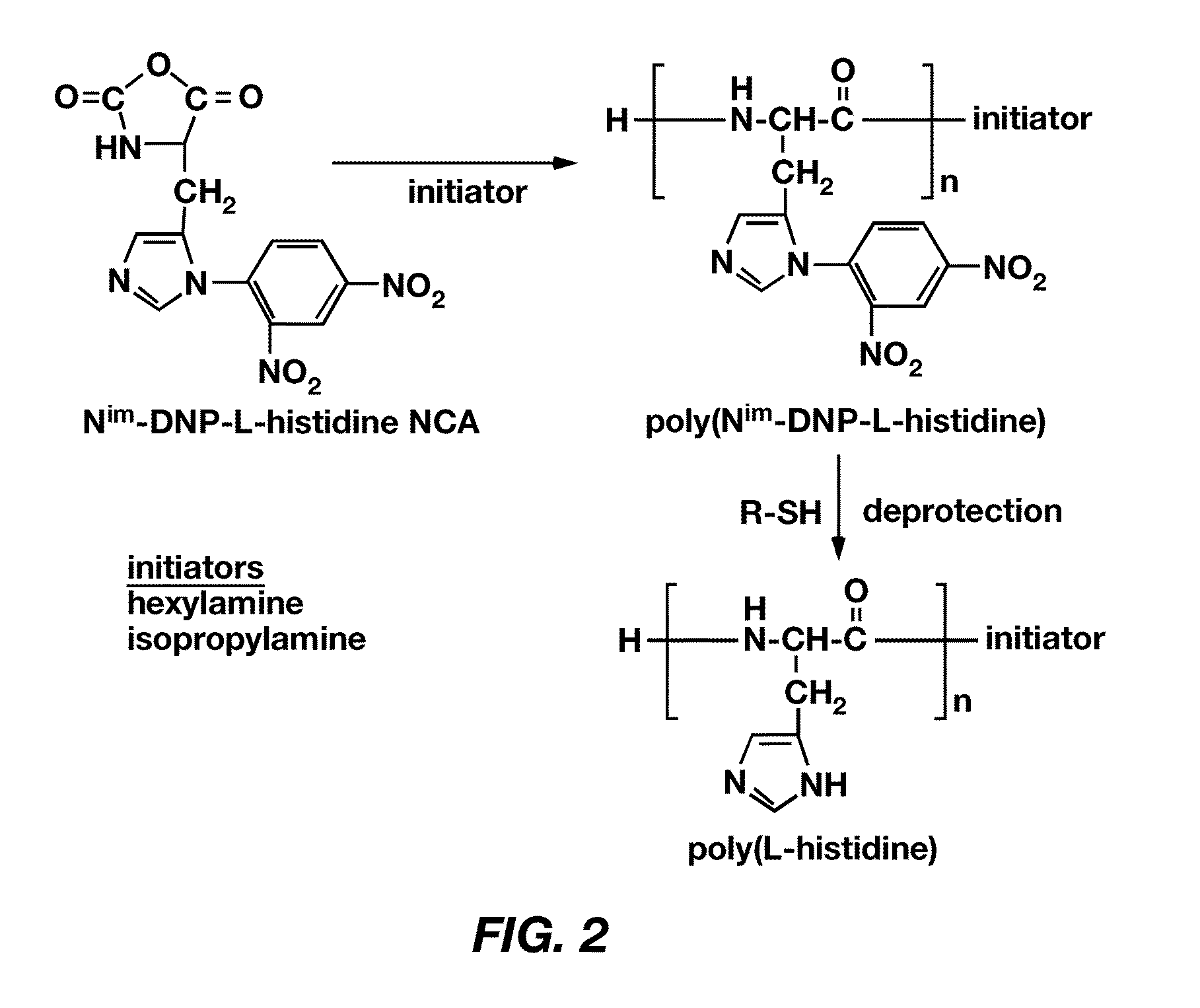
Mixed micelles containing poly(L-histidine)-poly(ethylene glycol) block copolymer and poly(L-lactic acid)-poly(ethylene glycol) block copolymer are a pH-sensitive drug carrier that release the drug in an acidic microenvironment, but not in the blood. Since the microenvironment of solid tumors is acidic, these mixed micelles are useful for treating cancer, including those cancers exhibiting multidrug resistance. Targeting ligands, such as folate, can also be attached to the mixed micelles for enhancing drug delivery into cells. Methods of making poly(L-histidine), synthetic intermediates, and block copolymers are also described.
Owner:UNIV OF UTAH RES FOUND
Polymeric micelles for combination drug delivery
InactiveUS20080248097A1Low water solubilityLower effective doesBiocideCarbohydrate active ingredientsCancer cellCombination drug therapy
The invention provides block polymers, micelles, and micelle formulations for combination drug therapy. Polyamide block polymers, such as those of formulas I and II are useful, for example, for preparation of mixed drug micelles, including simply mixed micelles, physically mixed micelles, and chemically mixed micelles. The invention further provides methods of treating cancer, and inhibiting and killing cancer cells. Also provided are methods for the preparation of polymer drug conjugates and intermediates for their synthesis.
Owner:WISCONSIN ALUMNI RES FOUND
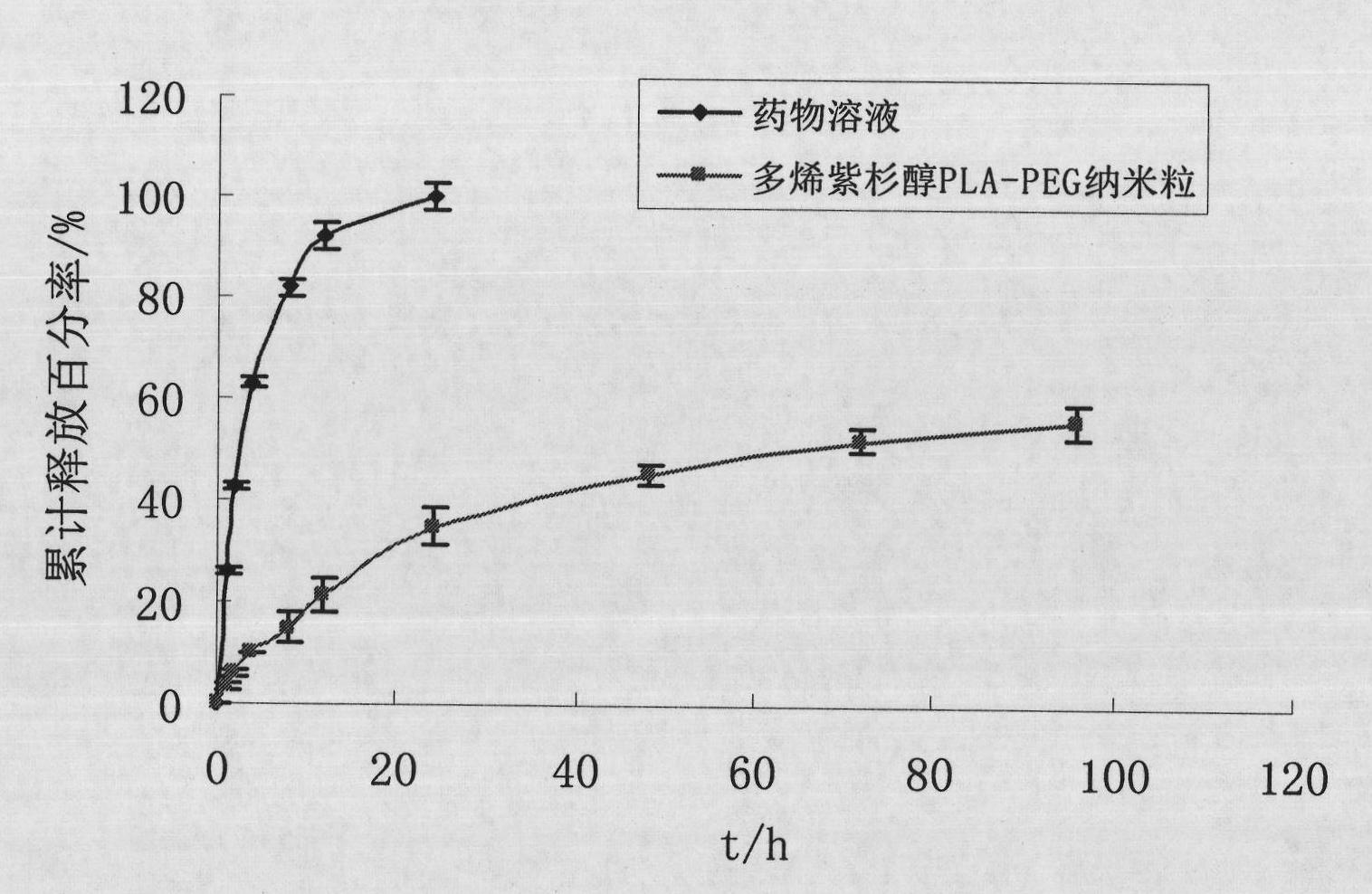
Preparation method of polyene-containing taxol nanoparticle mixed micelle preparation and freeze-drying agent
InactiveCN101804021AImprove solubilityHigh metabolic stabilityOrganic active ingredientsPowder deliveryMixed micelleFreeze-drying
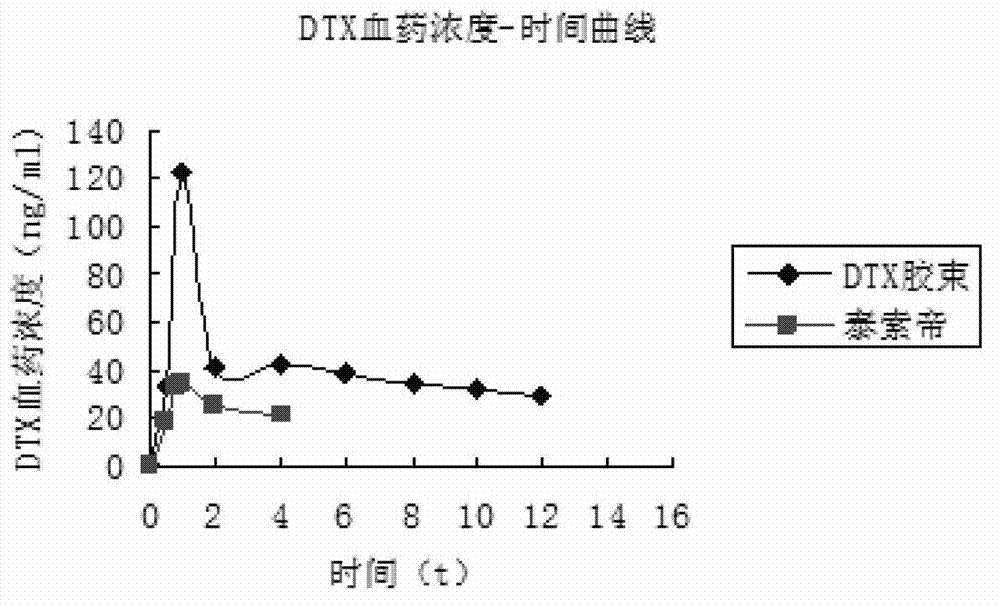
The invention discloses a preparation method of a polyene-containing taxol nanoparticle mixed micelle preparation and a freeze-drying agent, which prepares docetaxel PLA-PEG nanoparticles or micelle or nanoparticle mixed micelle through a modified solvent evaporation method, takes PLA-PEG copolymer as a carrier, and wraps docetaxel in a PLA hydrophobic core. When in use, the docetaxel PLA-PEG containing long cycle freeze-dried preparation only needs to be added with water and is dissolved, and uniform nanoparticle suspension, micellar solution or mixed micellar nanoparticle suspension can be prepared. The preparation method does not need tween-80 and ethanol solubilization, only takes the biodegradable PLA-PEG as the carrier, and does not contain any surfactant; and compared with the docetaxel injection on sale, the preparation can reduce the toxicity and the adverse reactions of the medicine, and improve the clinical application safety of the medicine.
Owner:SHANDONG UNIV
Mixed lipopeptide micelles for inducing an immune response
The invention concerns mixed micelles or micro-aggregates for inducing an immune response containing at least a first lipopeptide comprising a CTL epitope and at least a first lipid motif; and a second lipopeptide comprising at least an auxiliary T epitope and at least a lipid motif, whereof the type can be different from the first lipopeptide motif. Said micelles can be used as medicines and vaccines.
Owner:INSTITUT PASTEUR DE LILLE +2
Pharmaceutical compositions for buccal delivery of pain relief medications
Pharmaceutical compositions comprising a narcotic analgesic in mixed micellar form are disclosed. The mixed micelles are formed from an alkali metal alkyl sulfate, and other micelle-forming compounds as described in the specification. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed. A preferred method for administering the present composition is through the buccal mucosa of the mouth.
Owner:GENEREX PHARMA INC
pH-sensitive polymeric micelles for drug delivery
Mixed micelles containing poly(L-histidine)-poly(ethylene glycol) block copolymer and poly(L-lactic acid)-poly(ethylene glycol) block copolymer are a pH-sensitive drug carrier that release the drug in an acidic microenvironment, but not in the blood. Since the microenvironment of solid tumors is acidic, these mixed micelles are useful for treating cancer, including those cancers exhibiting multidrug resistance. Targeting ligands, such as folate, can also be attached to the mixed micelles for enhancing drug delivery into cells. Methods of making poly(L-histidine), synthetic intermediates, and block copolymers are also described.
Owner:UNIV OF UTAH RES FOUND
Paclitaxel mixed micelle preparation, and preparation method thereof
InactiveCN102198084AReduce toxic and side effectsImprove securityOrganic active ingredientsPharmaceutical delivery mechanismSolubilityPolyoxyethylene castor oil
The invention discloses a paclitaxel mixed micelle preparation, comprising 100 to 300 milligram of tocopherol polyethylene glycol succinate 1000 (TPGS), 0 to 50 milligram of phosphatide, 0.5 milliliter of anhydrous ethanol and 6 milligram of paclitaxel. The preparation method is as follows: the TPGS is dissolved in the anhydrous ethanol, or the TPGS and the phosphatide are dissolved in the anhydrous ethanol; the paclitaxel is added and dissolved under stirring; the mixture is filtered with a millipore filtration of 0.22 micrometer so as to obtain the paclitaxel mixed micelle preparation. In the invention, the TPGS and the phosphatide are used to form mixed micelles which have good stability and little toxic and side effects; the preparation method is simple and practicable, having a good application prospect. Compared to the prior art, polyoxyethylene castor oil in conventional prescription is substituted in the invention, thereby reducing toxic side effects of paclitaxel injections and greatly enhancing the safety of the injections on condition that solubility is guaranteed.
Owner:SHANDONG UNIV
Method for administering insulin to the buccal region
A mixed micellar pharmaceutical formulation includes a micellar proteinic pharmaceutical agent, an alkali metal C8 to C22 alkyl sulphate, alkali metal salicylate, a pharmaceutically acceptable edetate and at least one absorption enhancing compounds. The absorption enhancing compounds are selected from the group consisting of lecithin, hyaluronic acid, pharmaceutically acceptable salts of hyaluronic acid, octylphenoxypolyethoxyethanol, glycolic acid, lactic acid, chamomile extract, cucumber extract, oleic acid, linolenic acid, borage oil, evening of primrose oil, trihydroxy oxo cholanyiglycine, glycerin, polyglycerin, lysine, polylysine, triolein and mixtures thereof. The amount of each absorption enhancing compound is present in a concentration of from 1 to 10 wt: / wt. % of the total formulation, and the total concentration of absorption enhancing compounds are less than 50 wt. / wt. % of the formulation.
Owner:GENEREX PHARMA INC
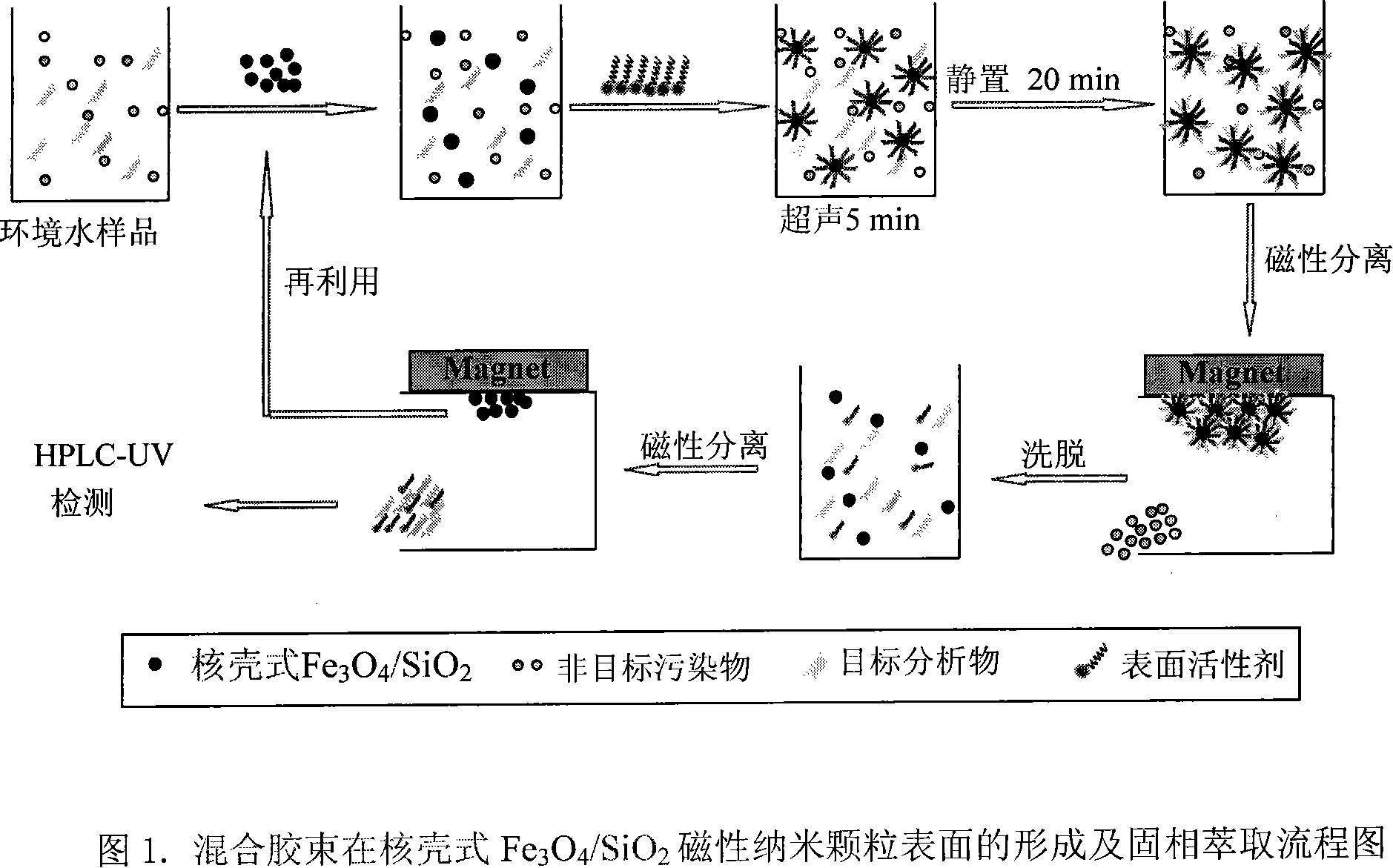
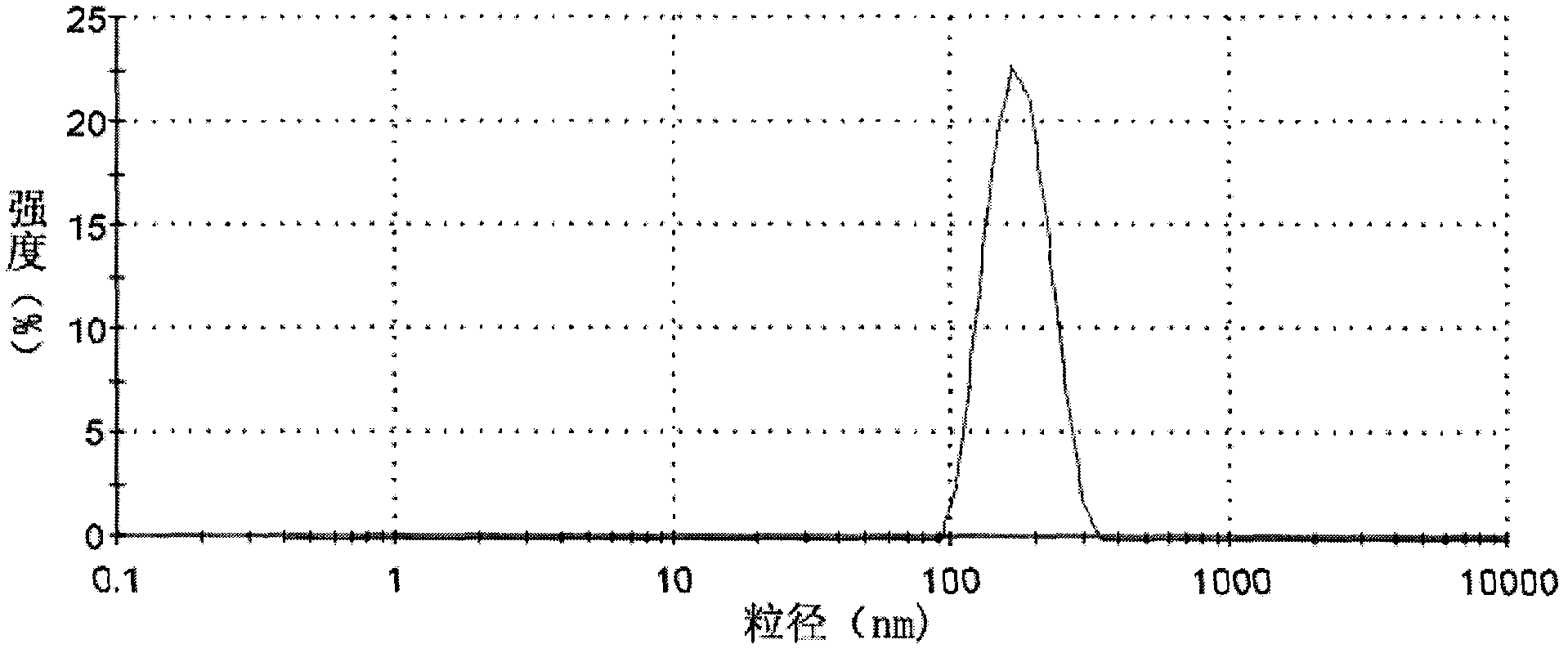
Preparation of mixed glue nucleus solitarius shell-type fe3o4 nano solid phase extractive agent and application of the same
InactiveCN101130157AImprove stabilityPrevent oxidationOther chemical processesWater contaminantsMixed micellePerfluorooctanoic acid
The invention relates to a new mixed micelle core-shell SiO2 / Fe3O4 nanometer solid phase extracting agent in the chemical analysis test instrument device field, which has the big specific surface area of the nanometer material, the strong magnetic separating property of the magnetic material, the good satiability of SiO2 shell layer and the good extraction property of the semi micelle and the absorption micelle, and is fit for the large scale batch preconditioning of bulk water sample, and has the small usage extracting agent, high efficient extraction, the simple operation, the high extracting speed, the friendly environment, the wide using range, the low cost and the reactivation property. Selecting bisphenol A(BPA), nonyl phenol(4-OP), octyl phenol (4-NP) as the represent of the hydrophobic pollution material and selecting perfluorine enanthic acid (PFHeA), perfluorooctanoic acid (PFOA), perfluorine octane sulfonic acid (PFOS), perfluorine n-nonanoic acid (PFDeA) or the like perfluorine compound as the represent of amphipathic nature pollution material, there are 0. 1g extracting agent and 50mg surface activator in 800ml environmental water simple, and the extracting ratio of the organic pollution materials is more than 90%.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
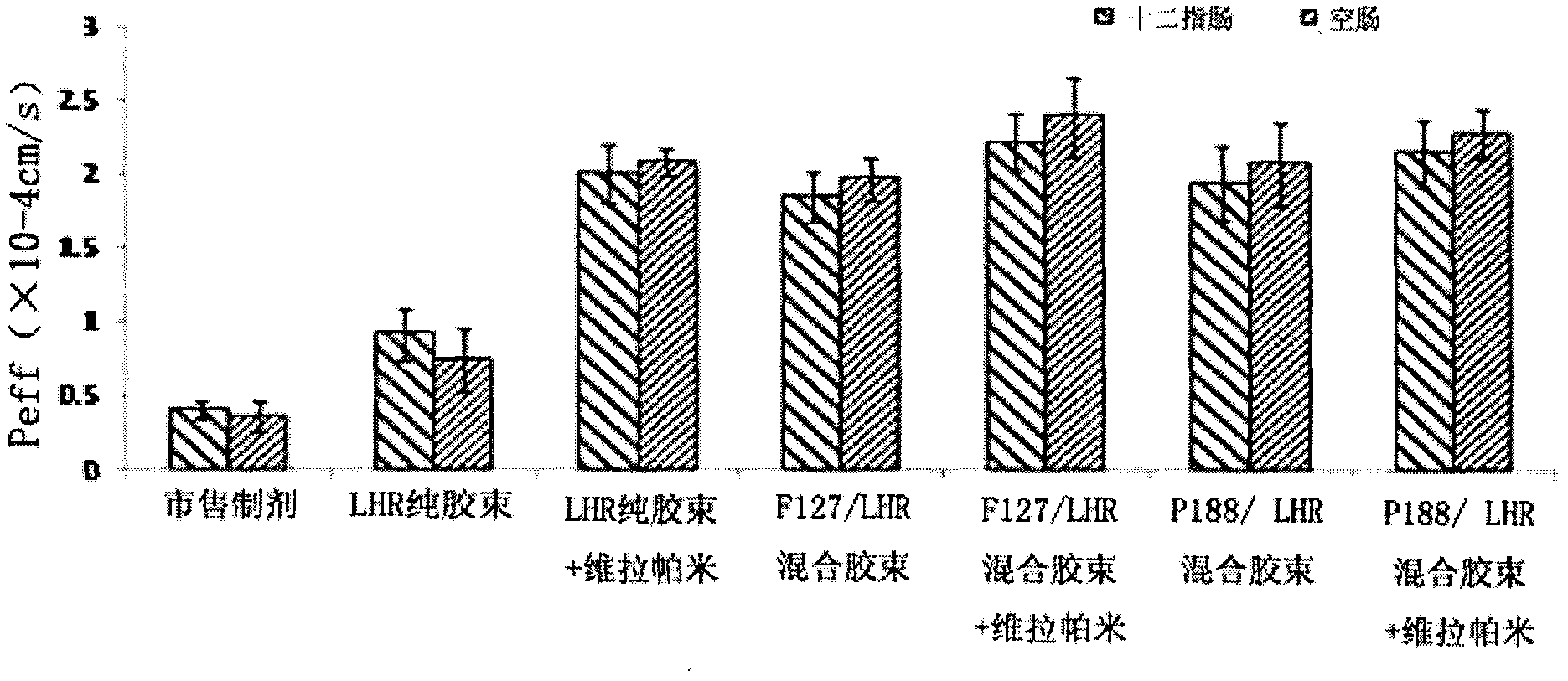
Preparation and application of insoluble drug-entrapped poloxamer/amphiphilic polysaccharide mixed micelle
InactiveCN102626518AOvercome the problems of high critical micelle concentration and low drug loadingImprove oral bioavailabilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsMixed micelleCytochrome p450 enzyme
The invention discloses preparation and application of an insoluble drug-entrapped poloxamer / amphiphilic polysaccharide mixed micelle. The insoluble drug-entrapped poloxamer / amphiphilic polysaccharide mixed micelle is prepared through a dialysis method or a solvent evaporation method. The mixed micelle is low in critical micelle concentration, is high in drug-loading rate, is capable of obviously prolonging the stabilization time and has the long-circulation function of a nanomicelle and has dual functions of restraining the metabolism of P-glycoprotein and cytochrome P450 enzyme and is capable of increasing the bioavailability of oral administration. The mixed micelle is simple in preparation method, is mature in process and is high in yield and can be prepared into preparations for the oral administration, such as tablets, capsules, pills and syrups.
Owner:CHINA PHARM UNIV
Orally Absorbed Pharmaceutical Formulation and Method of Administration
InactiveUS20090214657A1The process is simple and convenientIncrease glucose levelsPowder deliveryPeptide/protein ingredientsSolventSplit dose
A pharmaceutical formulation for absorption through oral mucosae comprising an effective amount of (a) a pharmaceutical agent in mixed micellar form, (b) at least one micelle-forming compound selected from the group comprising an alkali metal alkyl sulfate and a polyoxyethylene sorbitan monooleate, (c) a block copolymer of polyoxyethylene and polyoxypropylene, (d) at least one additional micelle-forming compound, and (e) a suitable solvent. The invention also provides a metered dose dispenser (aerosol or non-aerosol) containing the present formulation and a method of administering insulin using the metered dose dispenser comprising administering split doses of a formulation containing insulin before and after each meal.
Owner:GENEREX PHARMA
Blank mixed micelle, and preparation method and applications thereof
ActiveCN108420793AImprove uniformityReliable uniformityOrganic active ingredientsCosmetic preparationsMixed micelleCurative effect
The invention discloses blank mixed micelle, and a preparation method and applications thereof. The blank mixed micelle comprises an amphiphilic copolymer and ginsenoside represented by formula I, ishigh in efficiency, is safe, is stable, is high in targeting performance, is excellent in homogeneity, is stable in quality, is convention in preparation technology, can be used for coating one or a plurality of active substances in drugs or cosmetics and health care substances, and is capable of forming mixed micelle containing loaded active substances. Compared with conventional nanometer micelle, the blank mixed micelle possesses following advantages: the mixed micelle loaded with active substances is excellent in drug forming performance, multiple drug resistance, stability, homogeneity, and safety performance, and is small in particle size; and the curative effect of loaded active drugs on drug resistant cells is better.
Owner:XIAMEN GINPOSOME PHARM CO LTD
Docetaxel-loading mixed micelle preparation and preparation method thereof
InactiveCN102885772AImprove solubilityHigh drug loadingOrganic active ingredientsSolution deliverySolubilityDocetaxel
The invention discloses a docetaxel-loading mixed micelle preparation and a preparation method thereof. The mixed micelle preparation is prepared by the following steps of: preparing docetaxel mixed micelle by using a thin-film dehydration method; and coating the docetaxel with hydrophobic nuclei by taking amphiphilic TPGS, MPEG-PLA and CSO-SA as carrier materials of the mixed micelle. The docetaxel-loading mixed micelle preparation is prepared by using the amphiohilic materials of TPGS, MPEG-PLA and CSO-SA, so that drug-loading rate is increased, solubility and oral bioavailability are improved, and the problems of poor water solubility, low medicine release speed, improper particle size and the like when hydrophilic medicines are prepared are solved.
Owner:SHANDONG UNIV
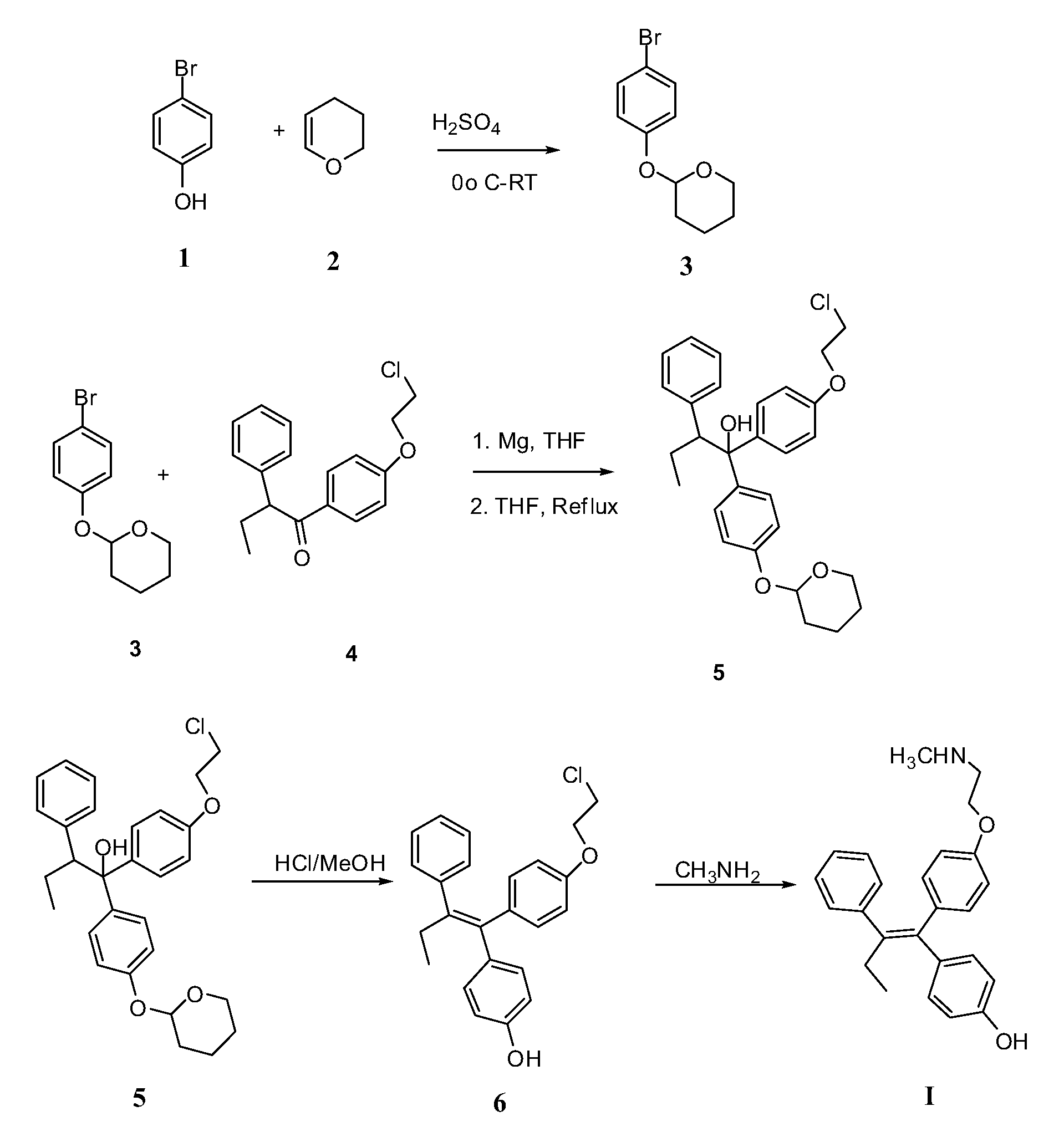
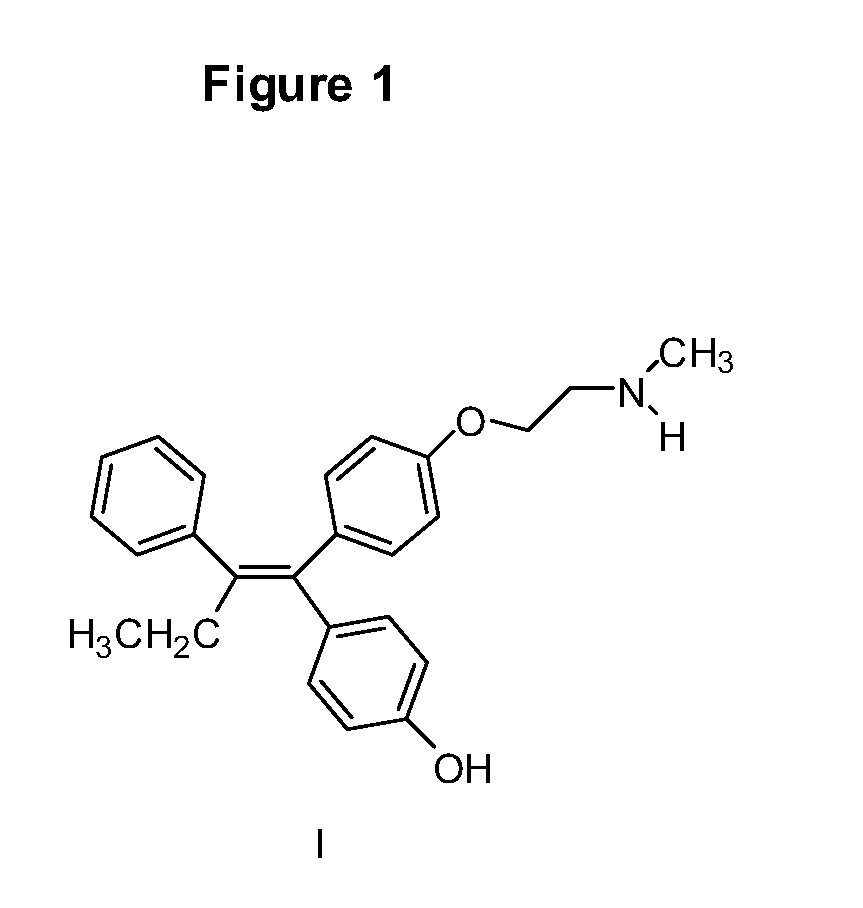
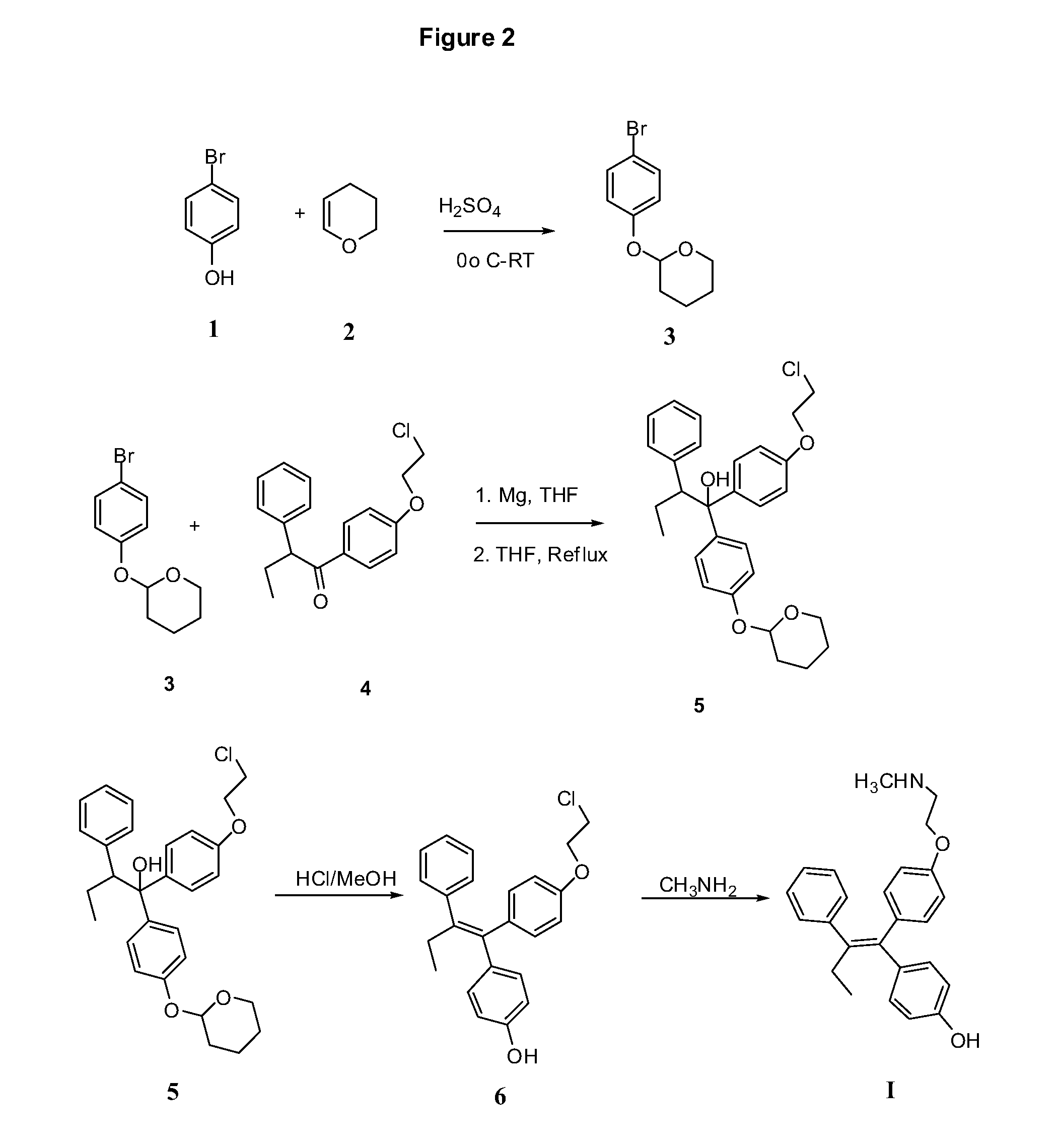
Endoxifen Compositions And Methods
The present invention provides compositions containing endoxifen, formulations and liposomes of endoxifen, methods of preparation of such agents and formulations, and use of such agents and formulations for the treatment of breast cancer and other breast diseases and diseases susceptible to endoxifen. In particular, the compositions of the present invention include liposomes, complexes, vesicles, emulsions, micelles and mixed micelles of endoxifen in which the compositions further contain any of a variety of neutral or charged lipids and desirably, cholesterol and cholesterol derivatives, sterols, Z- and E-guggulsterones, phospholipids, fatty acids, vitamin D, and vitamin E. The present invention also provides methods of preparing endoxifen. The present invention provides methods for treating and preventing breast cancer and other breast related diseases by administrating novel formulations or compositions comprising a therapeutically effective amount of endoxifen.
Owner:JINA PHARMA
PH-sensitive polymeric micelles for drug delivery
Mixed micelles containing poly(L-histidine)-poly(ethylene glycol) block copolymer and poly(L-lactic acid)-poly(ethylene glycol) block copolymer are a pH-sensitive drug carrier that release the drug in an acidic microenvironment, but not in the blood. Since the microenvironment of solid tumors is acidic, these mixed micelles are useful for treating cancer, including those cancers exhibiting multidrug resistance. Targeting ligands, such as folate, can also be attached to the mixed micelles for enhancing drug delivery into cells. Methods of treating a warm-blooded animal with such a drug are disclosed.
Owner:UNIV OF UTAH RES FOUND
Ph-sensitive polymeric micelles for drug delivery
Mixed micelles containing poly(L-histidine-co-phenylalanine)-poly(ethylene glycol) block copolymer and poly(L-lactic acid)-poly(ethylene glycol) block copolymer are a pH-sensitive drug carrier that release the drug in an acidic microenvironment, but not in the blood. Since the microenvironment of solid tumors is acidic, these mixed micelles are useful for treating cancer, including those cancers exhibiting multidrug resistance. Targeting ligands, such as folate, can also be attached to the mixed micelles for enhancing drug delivery into cells. Methods of treating a warm-blooded animal with such a drug are disclosed.
Owner:UNIV OF UTAH RES FOUND
Endoxifen compositions and methods
The present invention provides compositions containing endoxifen, formulations and liposomes of endoxifen, methods of preparation of such agents and formulations, and use of such agents and formulations for the treatment of breast cancer and other breast diseases and diseases susceptible to endoxifen. In particular, the compositions of the present invention include liposomes, complexes, vesicles, emulsions, micelles and mixed micelles of endoxifen in which the compositions further contain any of a variety of neutral or charged lipids and desirably, cholesterol and cholesterol derivatives, sterols, Z- and E-guggulsterones, phospholipids, fatty acids, vitamin D, and vitamin E. The present invention also provides methods of preparing endoxifen. The present invention provides methods for treating and preventing breast cancer and other breast related diseases by administrating novel formulations or compositions comprising a therapeutically effective amount of endoxifen.
Owner:JINA PHARMA
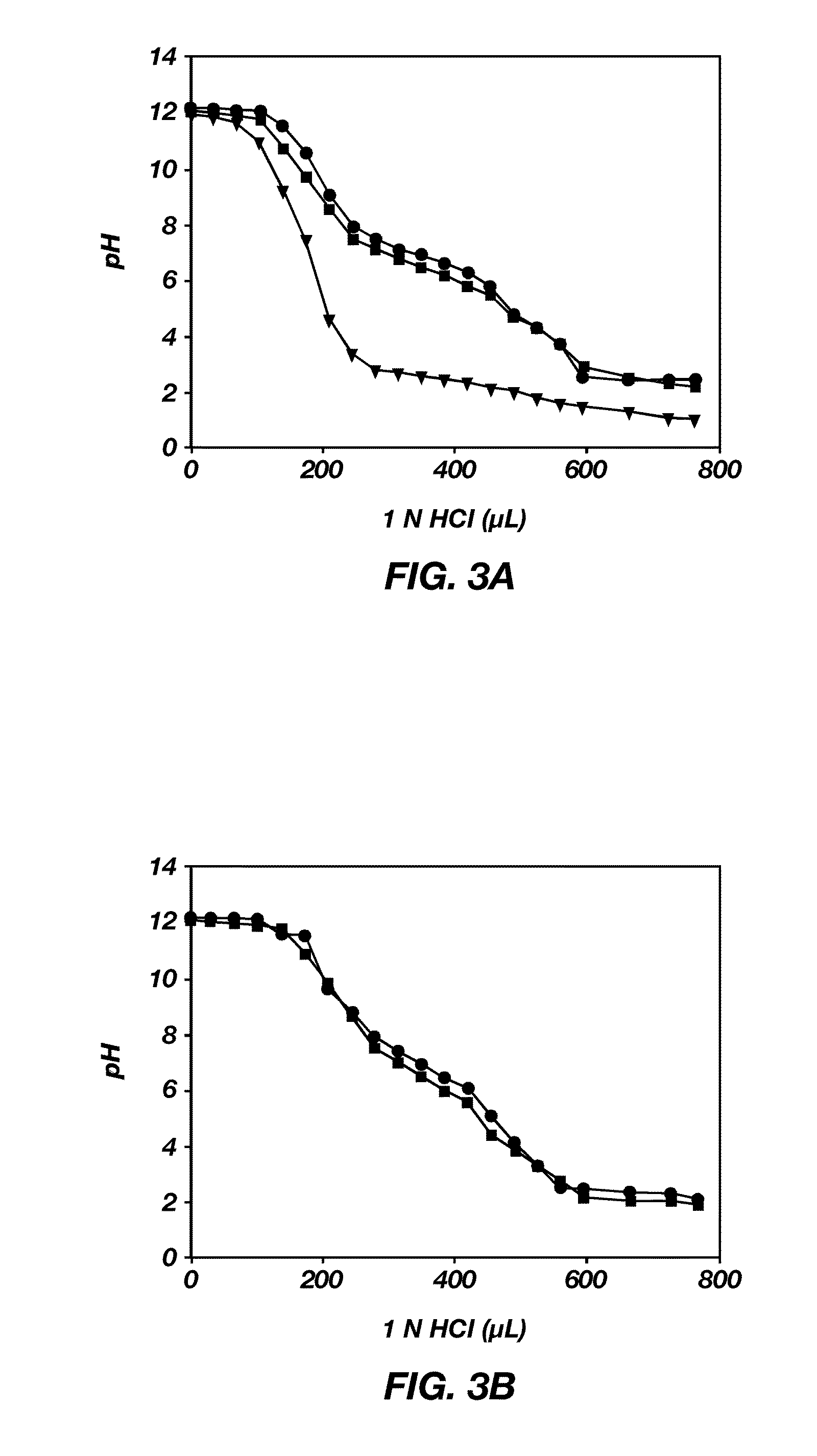
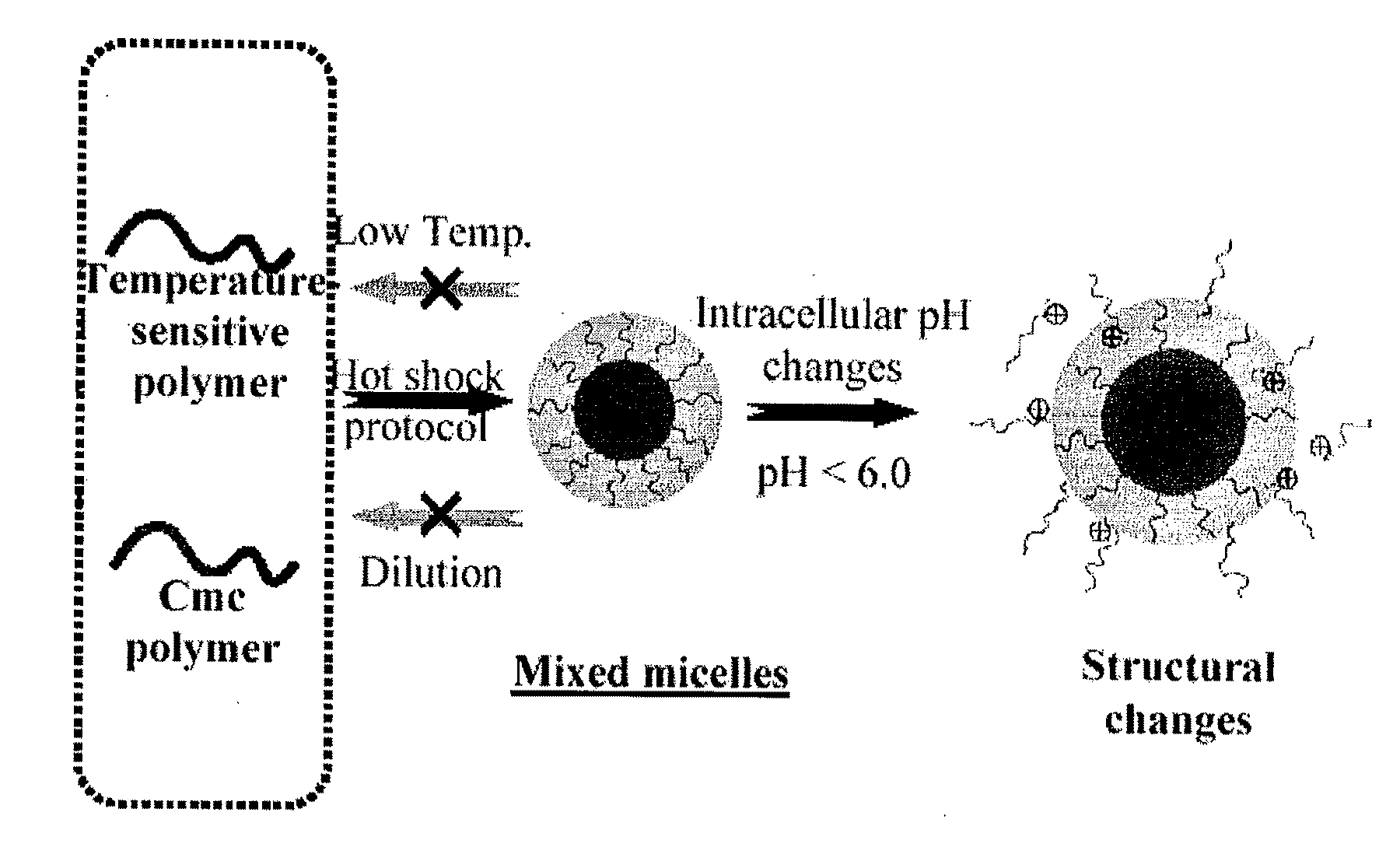
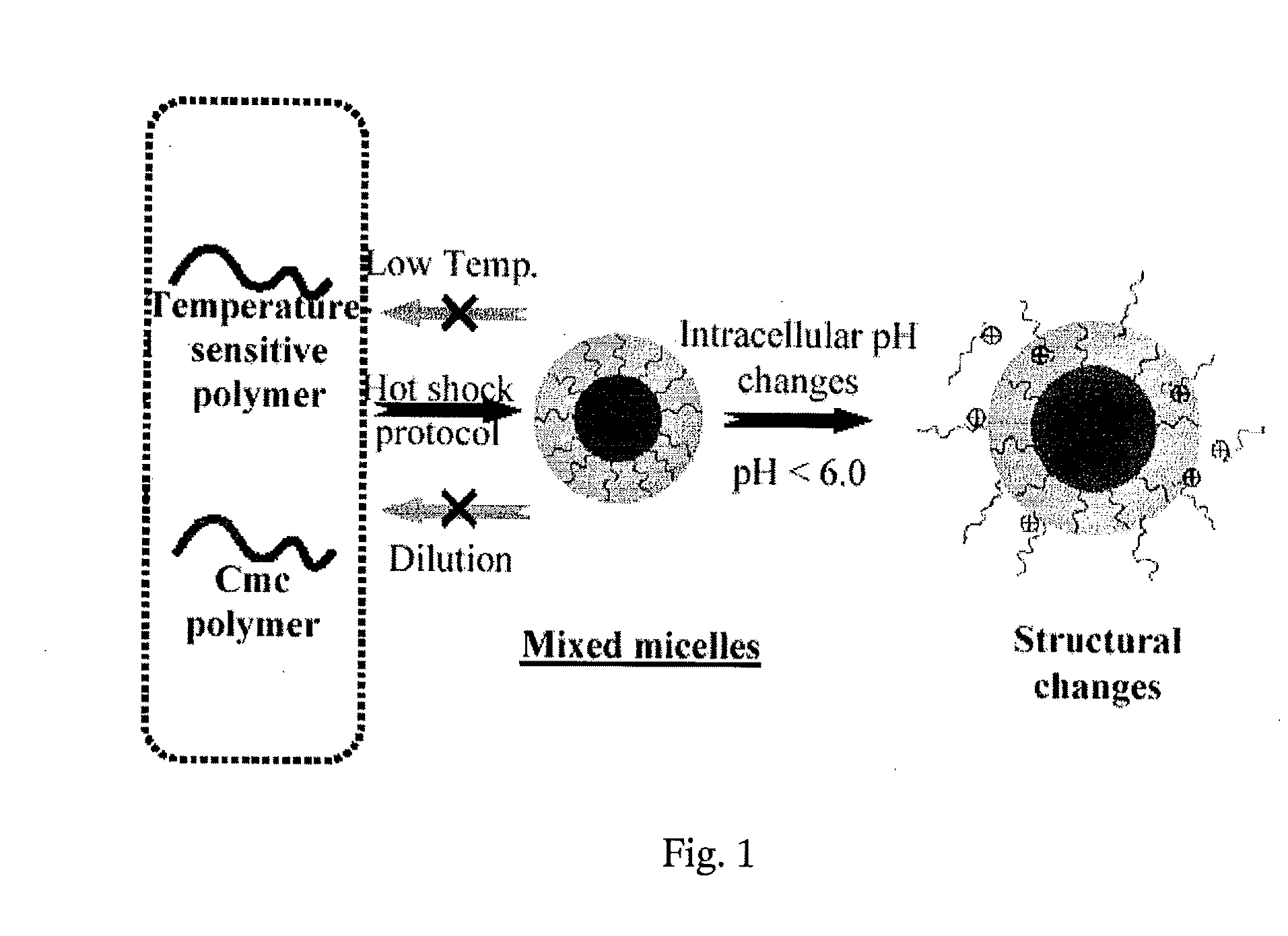
Stable micelles formed with diblock copolymers of critical micelle concentration copolymer and temperature-sensitive copolymer
InactiveUS20100247654A1Small sizeImprove stabilityBiocidePowder deliveryCritical micelle concentrationMixed micelle
A novel class of mixed micelles formed with critical micelle concentration (Cmc) character's diblock copolymer, and temperature-sensitive character's diblock copolymer were disclosed. The mixed micelles possess complementary effects in adjusting external temperature shift (storage vs. body temperature) and concentration change (dilution after intravenous injection). The mixed micelles of the present invention can serve as a potential injectable drug delivery system for anticancer drugs, such as doxorubicin and many others.
Owner:NATIONAL TSING HUA UNIVERSITY
Alpha-asarone mixed micelle injectio and preparation method thereof
The invention provides an alpha-asarone mixed micelle injectio, adopting alpha-asarone of which the chemical name is 2,4,5-trimethoxy-1- propenylbenzene as an active ingredient, and adopting a cholate / phospholipid mixed micelle system for preparing the injectio. The invention not only effectively solves the problem of hard water dissolution of the alpha-asarone, but also greatly increases the drug-loading rate of the micelle injectio. The mixed micelle injectio prepared by the invention has the advantages of favorable stability and simple preparation process.
Owner:SICHUAN UNIV
ph responsive polymer mixed micelle and application thereof
InactiveCN104758247AImprove bindingSolubilizePharmaceutical non-active ingredientsEmulsion deliveryMixed micellePh control
Owner:GUANGDONG UNIV OF TECH
Ph-sensitive polymeric micelles for drug delivery
Owner:UNIV OF UTAH RES FOUND
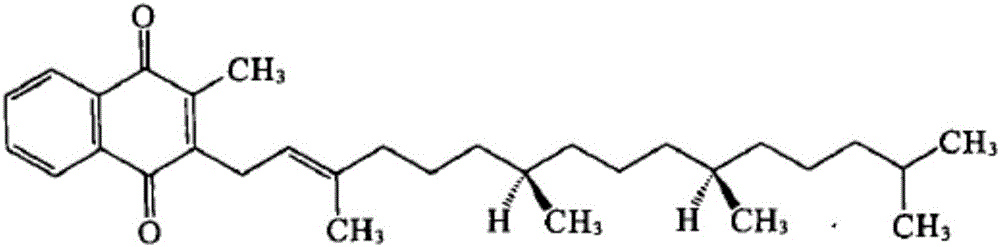
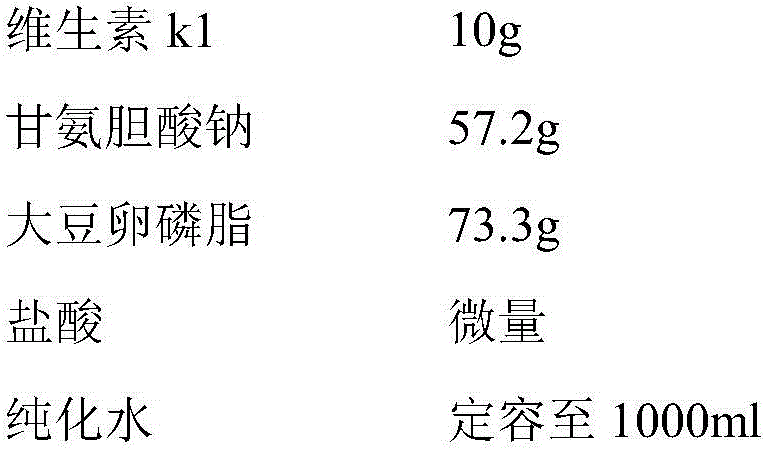
Vitamin K1 micelle injection and preparation method thereof
InactiveCN105997869AHigh encapsulation efficiencyIncrease concentrationOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityOrganic solvent
The invention discloses a vitamin K1 micelle injection and a preparation method thereof, and belongs to the field of micelle injection and preparation thereof. The invention discloses a vitamin K1 micelle for the first time. The vitamin K1 micelle is prepared from vitamin K1, cholate, phospholipid, a pH regulator and water for injection. The preparation method of the vitamin K1 micelle injection comprises the steps that an organic solvent freezing and drying method is adopted, a mixed micelle composed of phospholipid and cholate is adopted as a carrier, and the vitamin K1 is entrapped in hydrophobic nuclei of the mixed micelle. Due to the fact that the vitamin K1 is not dissolved into water, the solubility of the vitamin K1 in water is remarkably improved through the cholate / phospholipid mixed micelle preparation technology, polysorbate-80 and propylene glycol are not added into the vitamin K1 micelle injection, only phospholipid and cholate named as physiological excipient are adopted as auxiliary materials, and compared with vitamin K1 injections on sale currently, the preparation can reduce adverse drug effect and improve the clinic application safety.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com