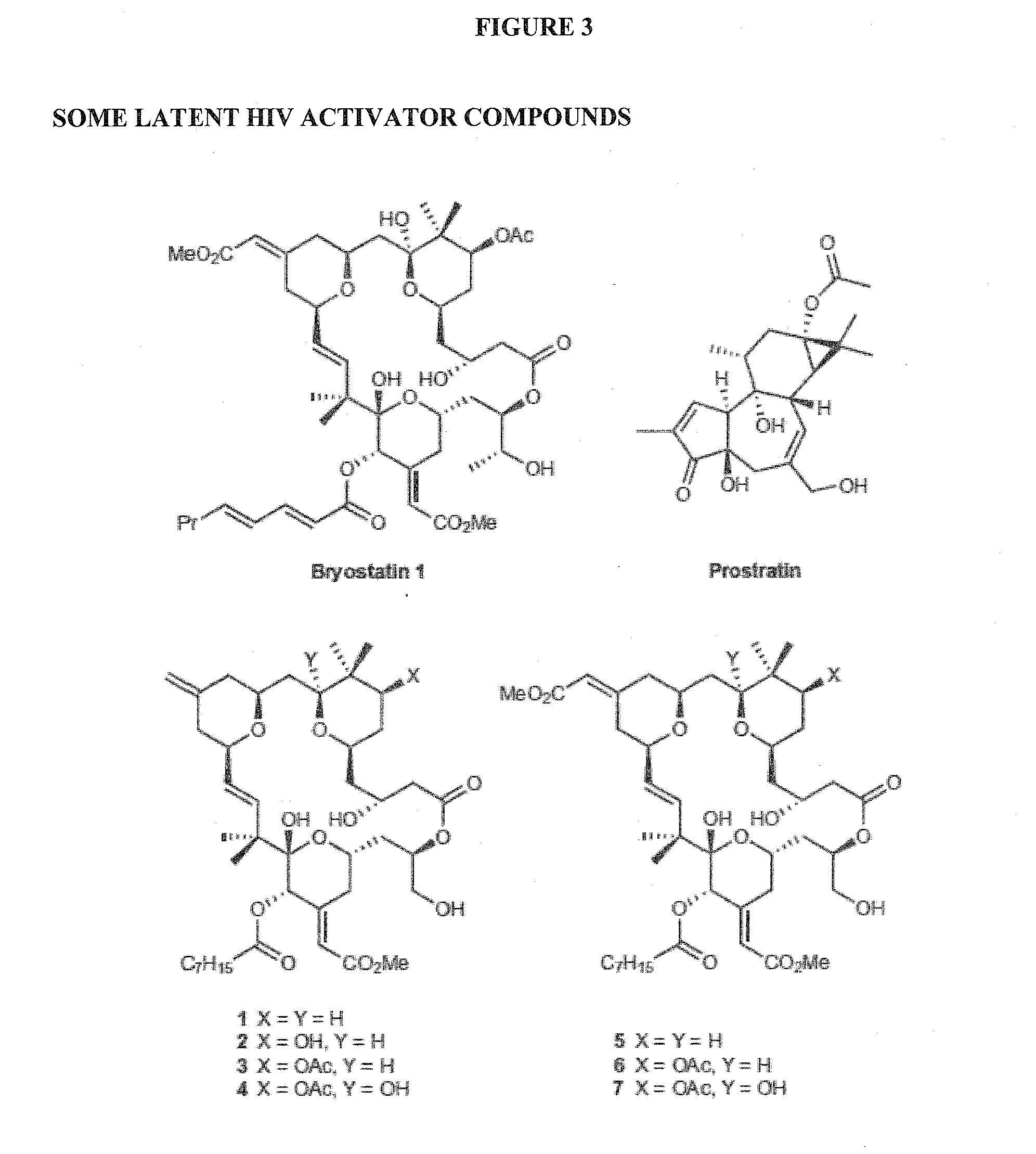
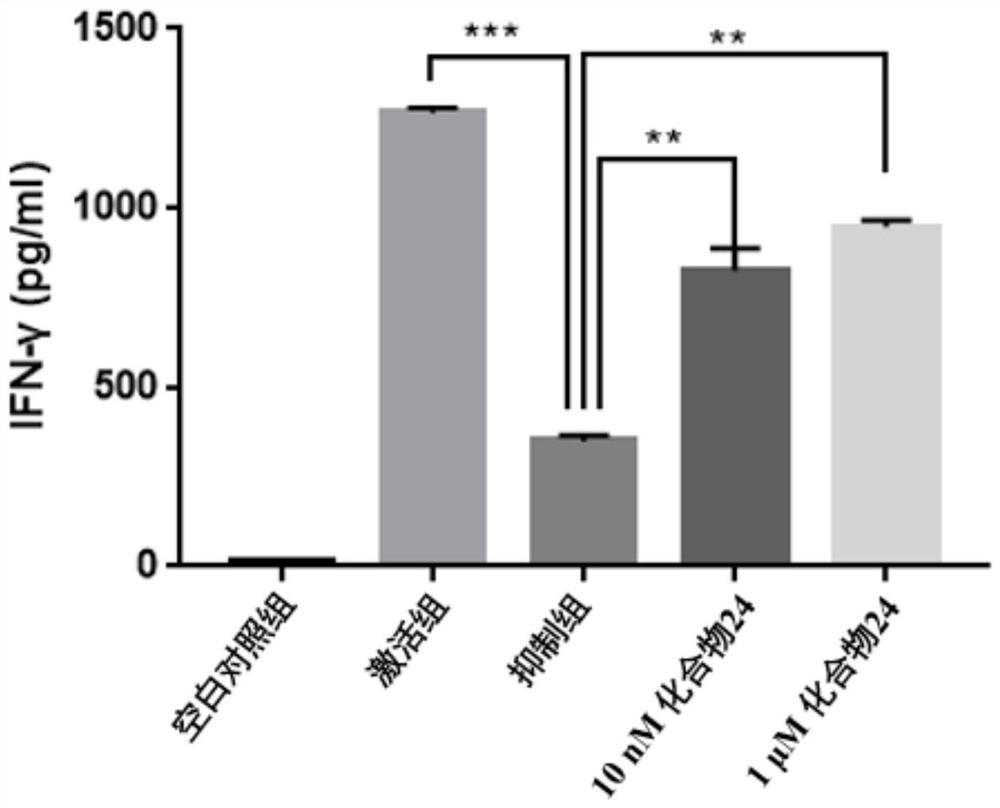
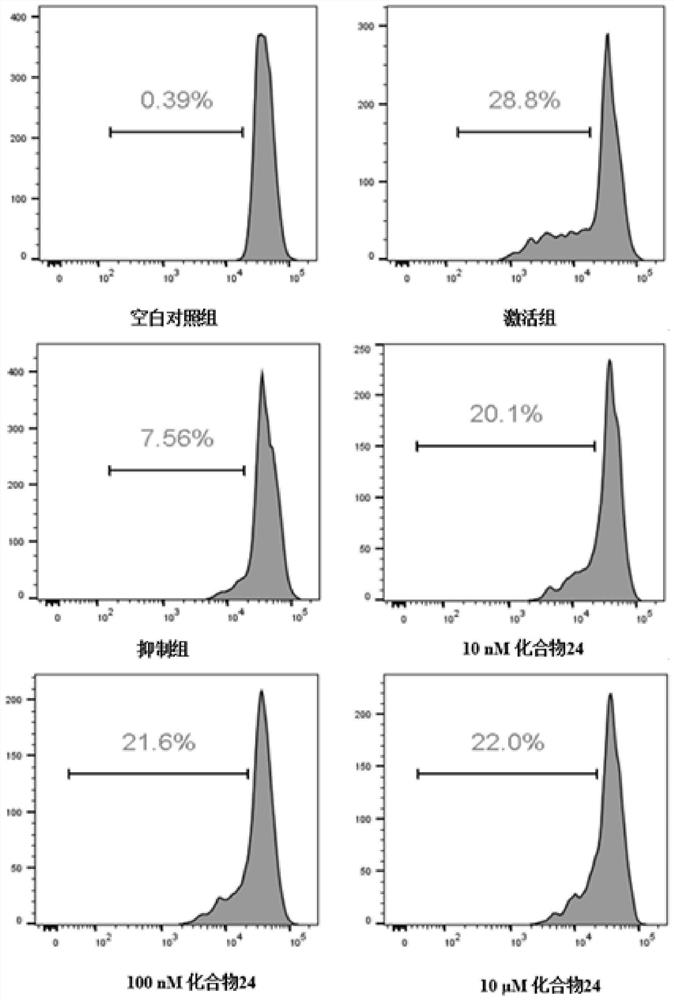
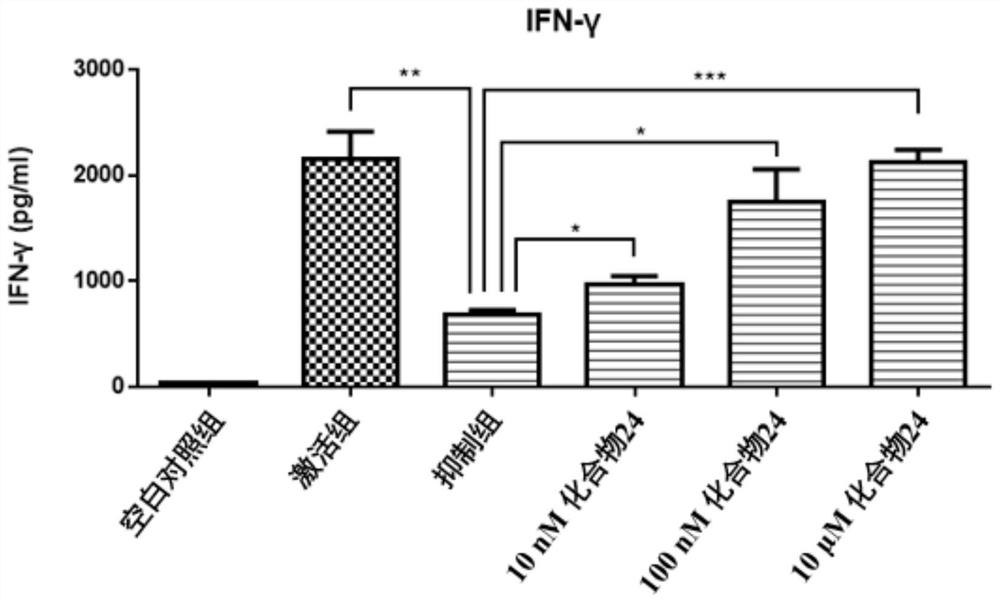
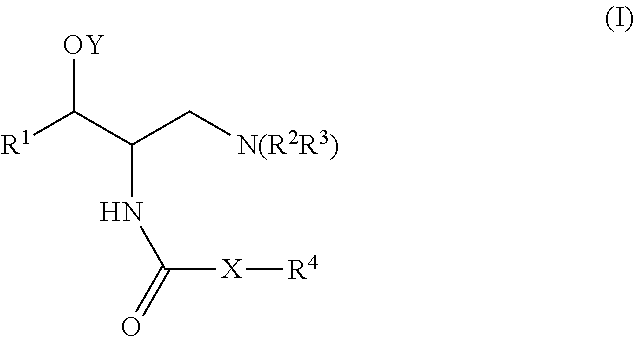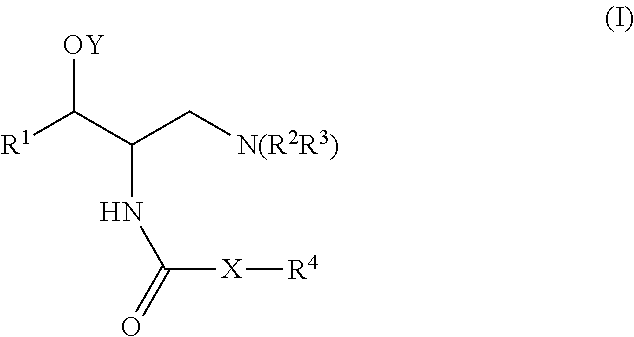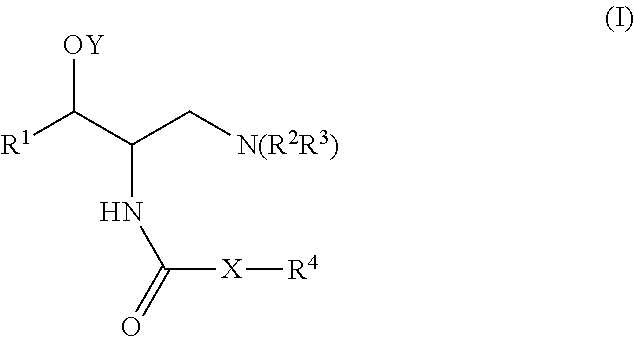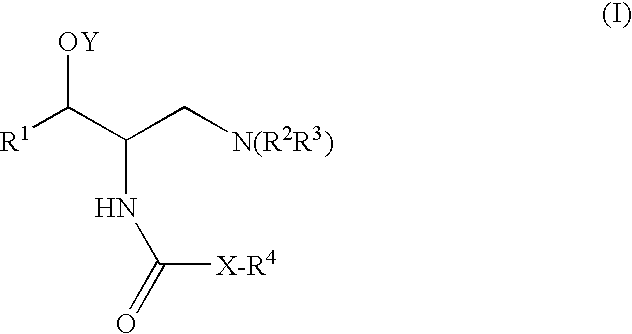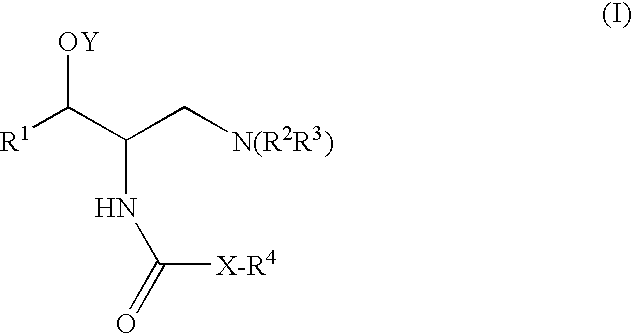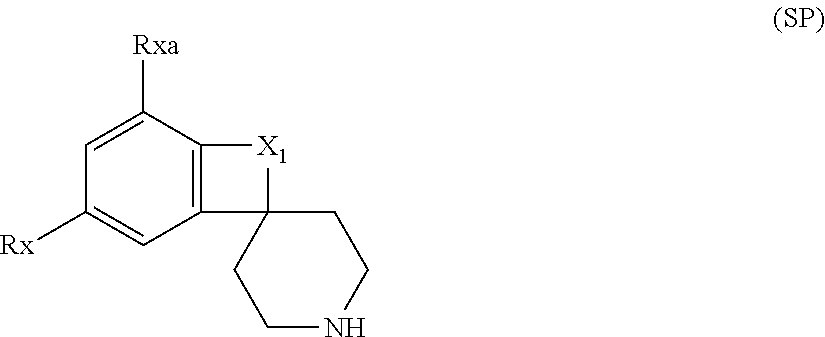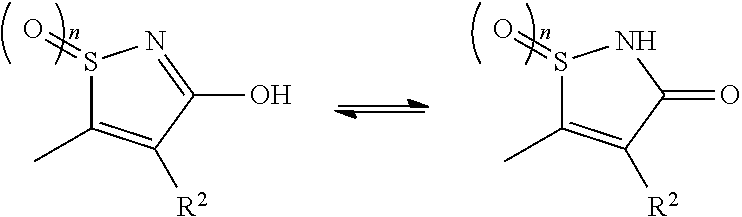Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
126results about How to "High metabolic stability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
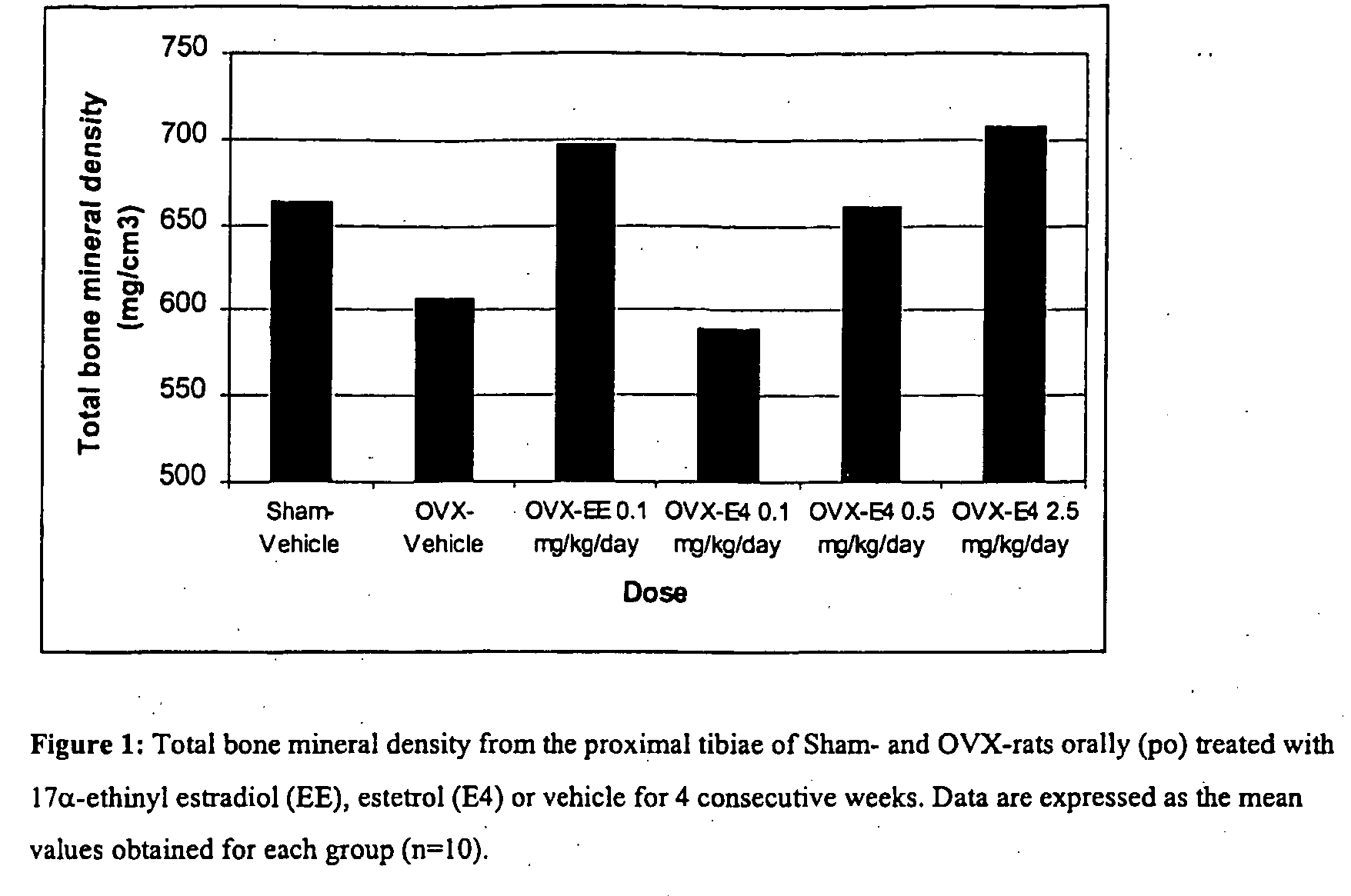
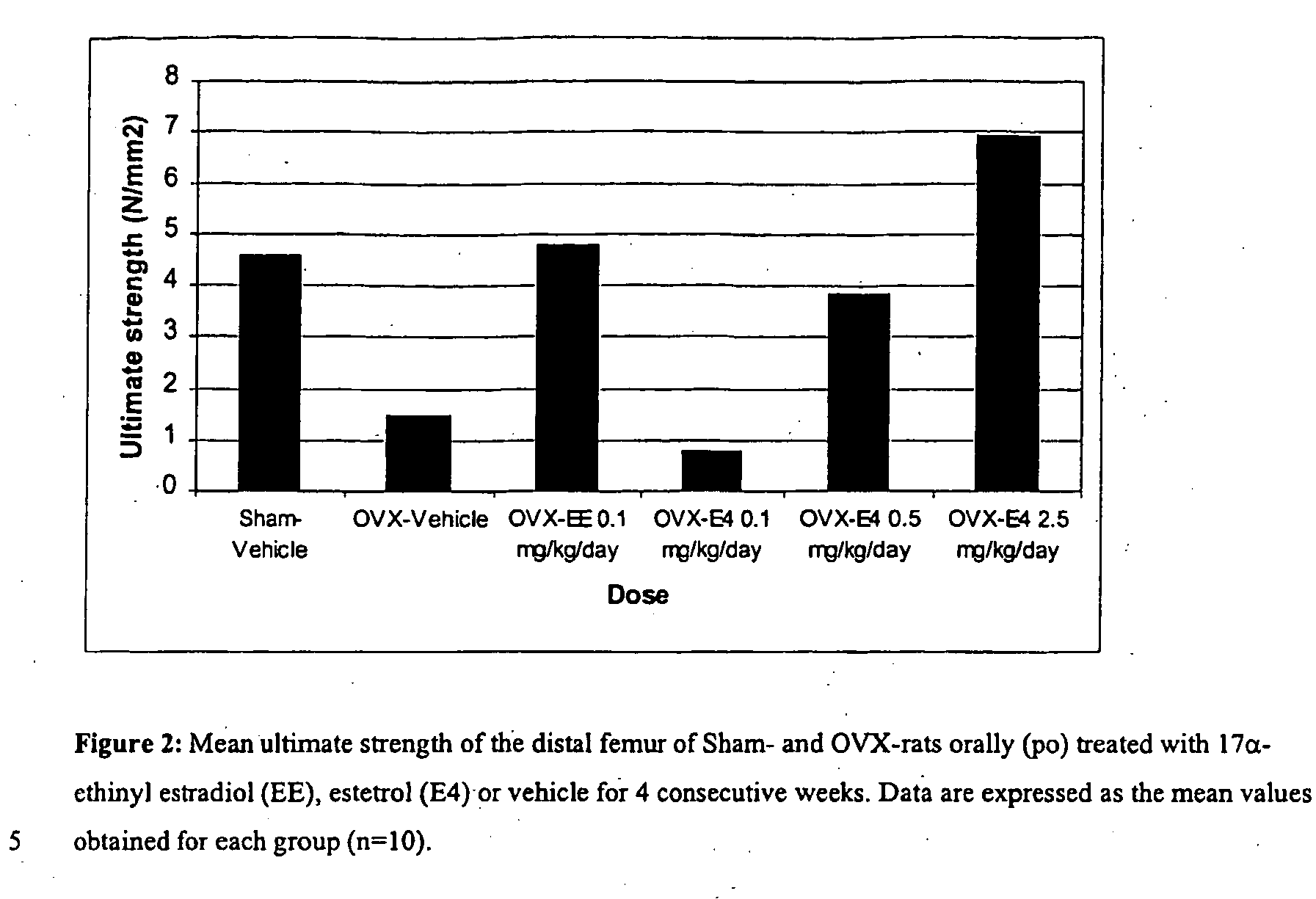
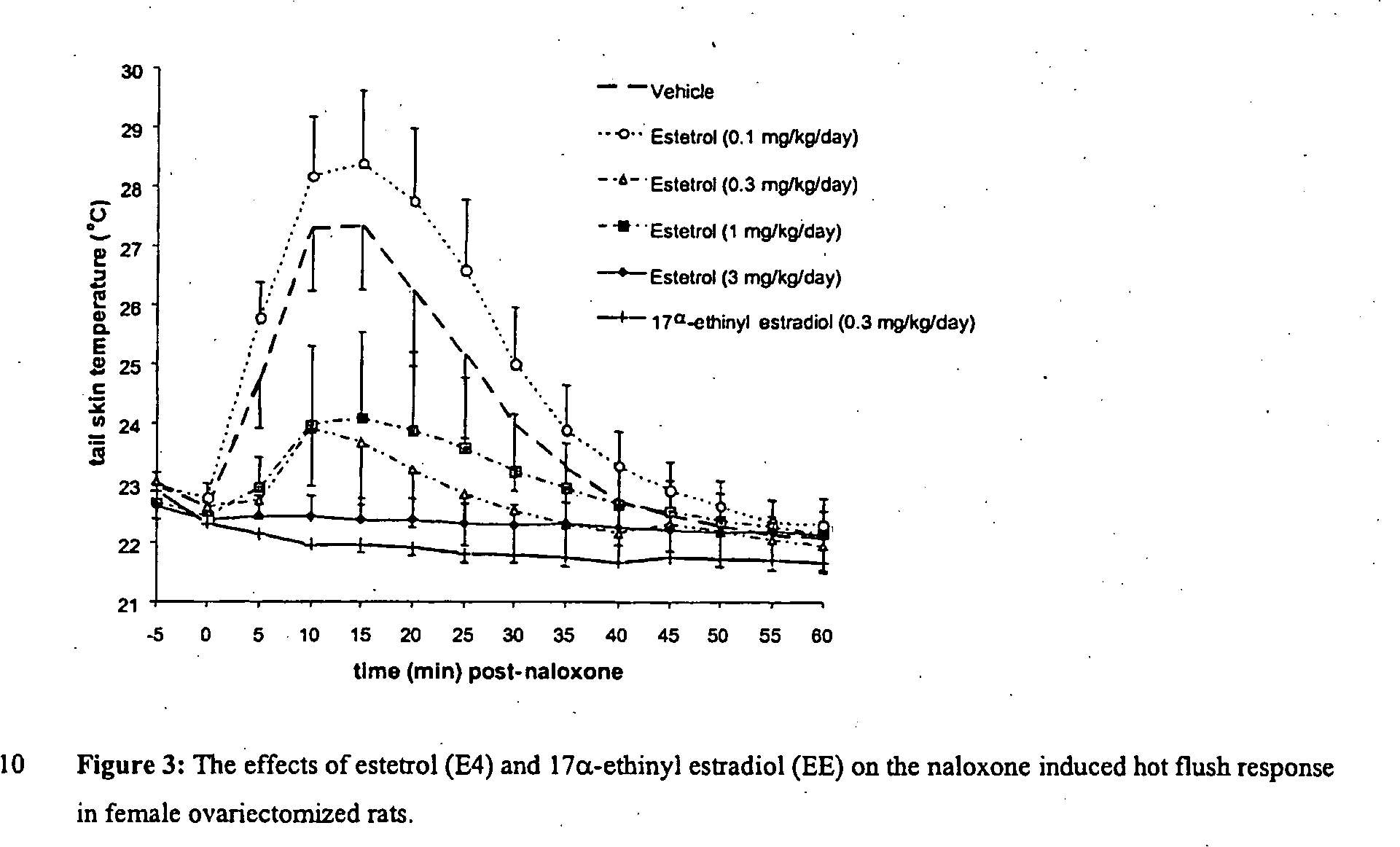
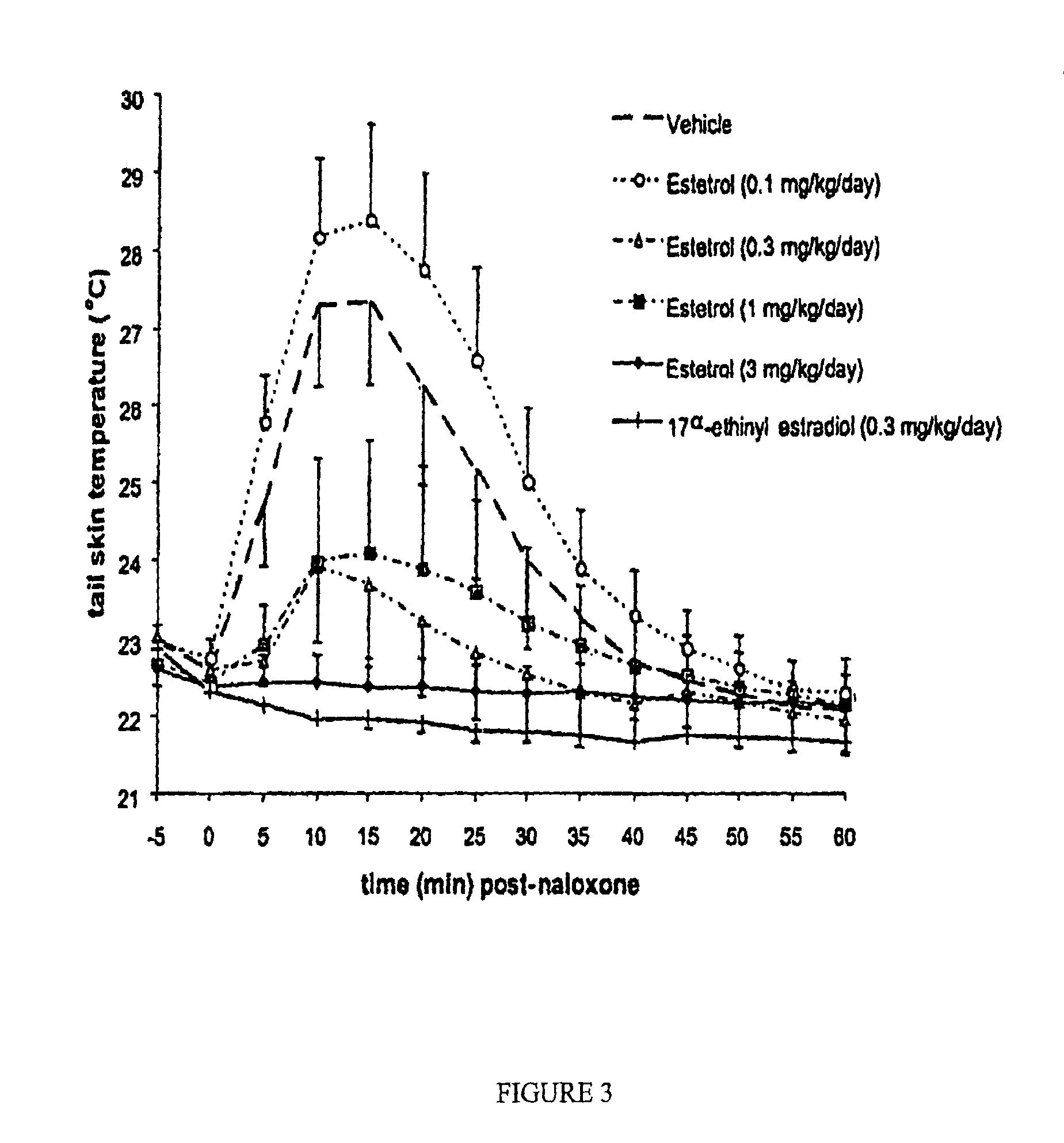
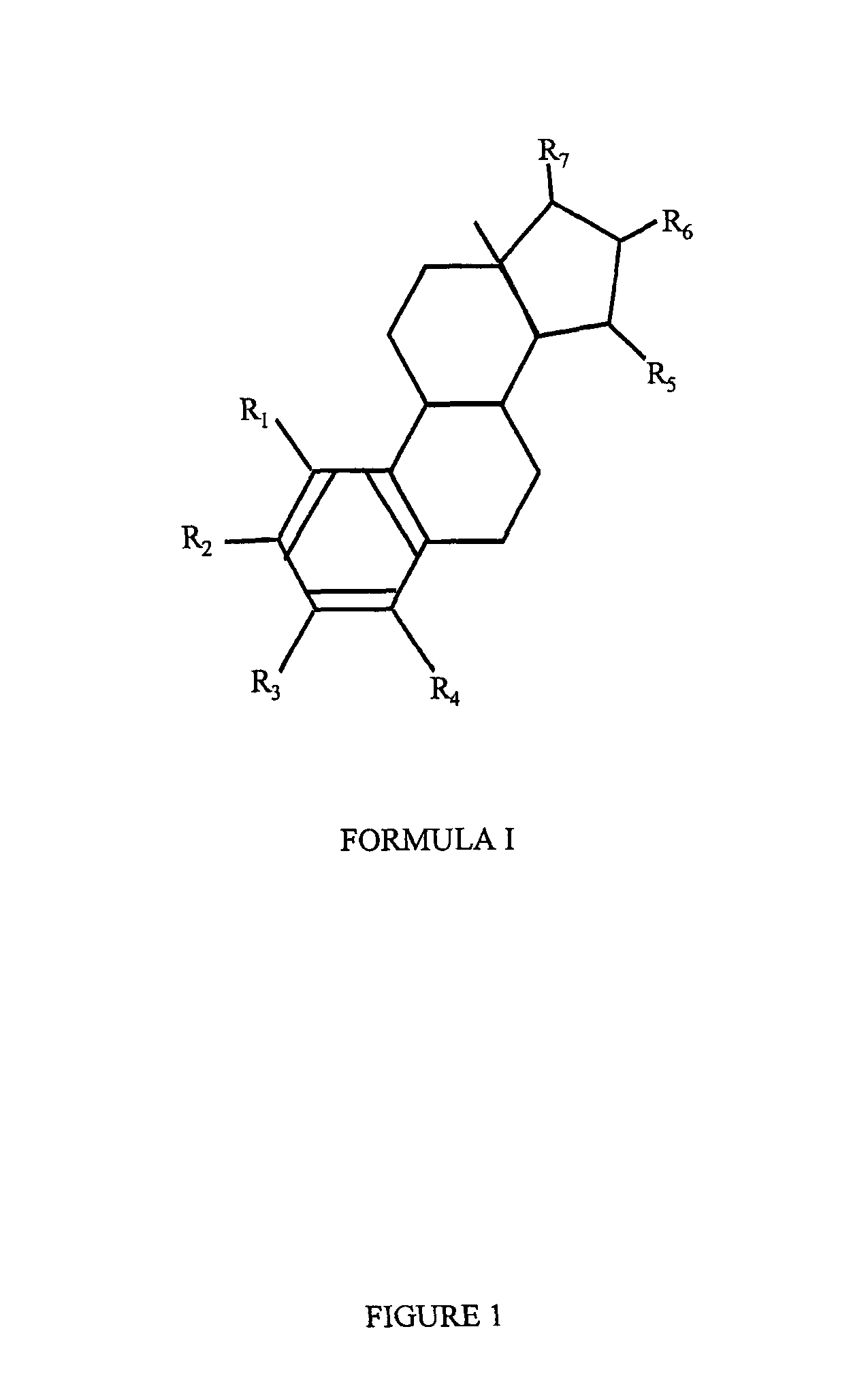
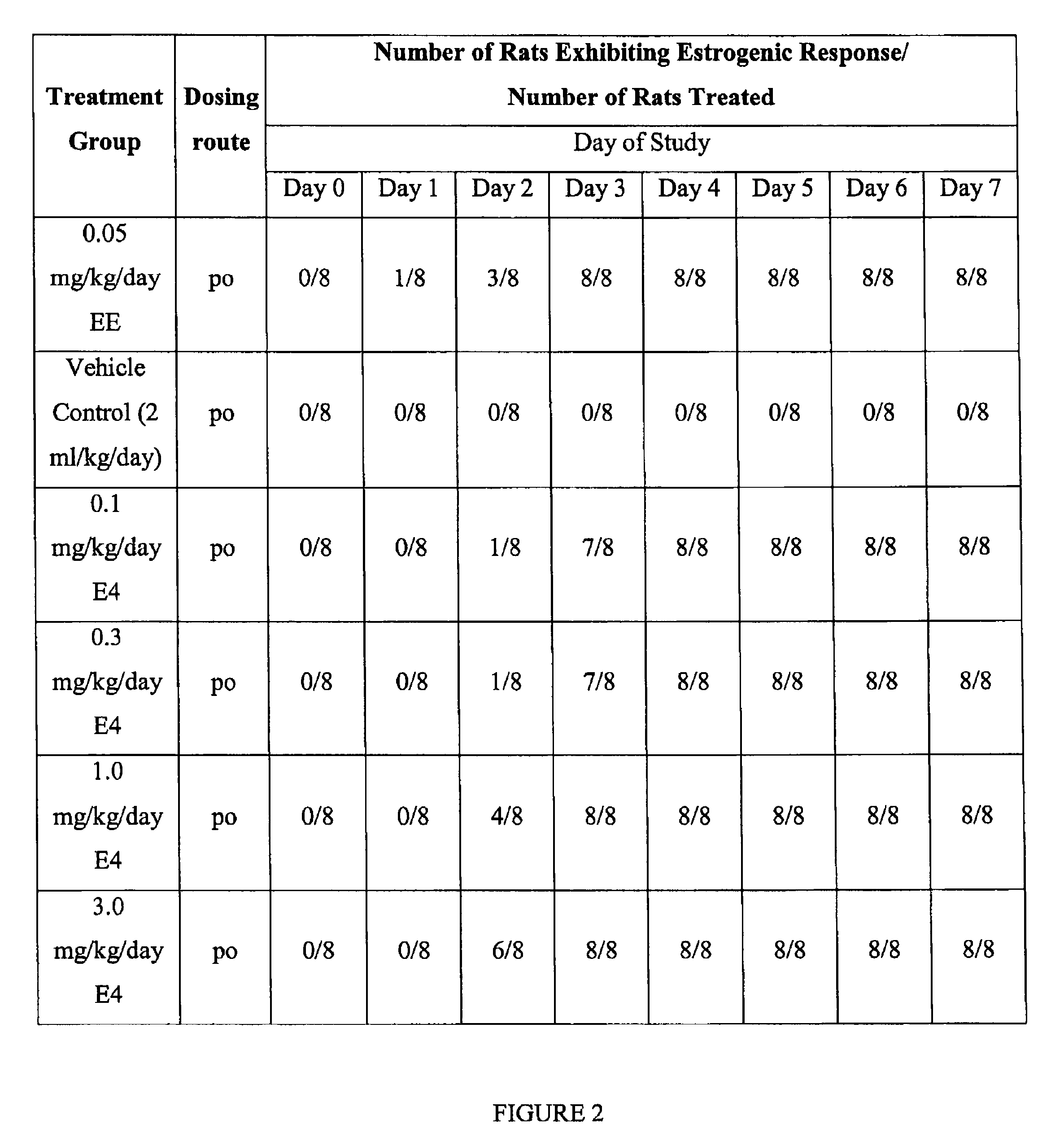
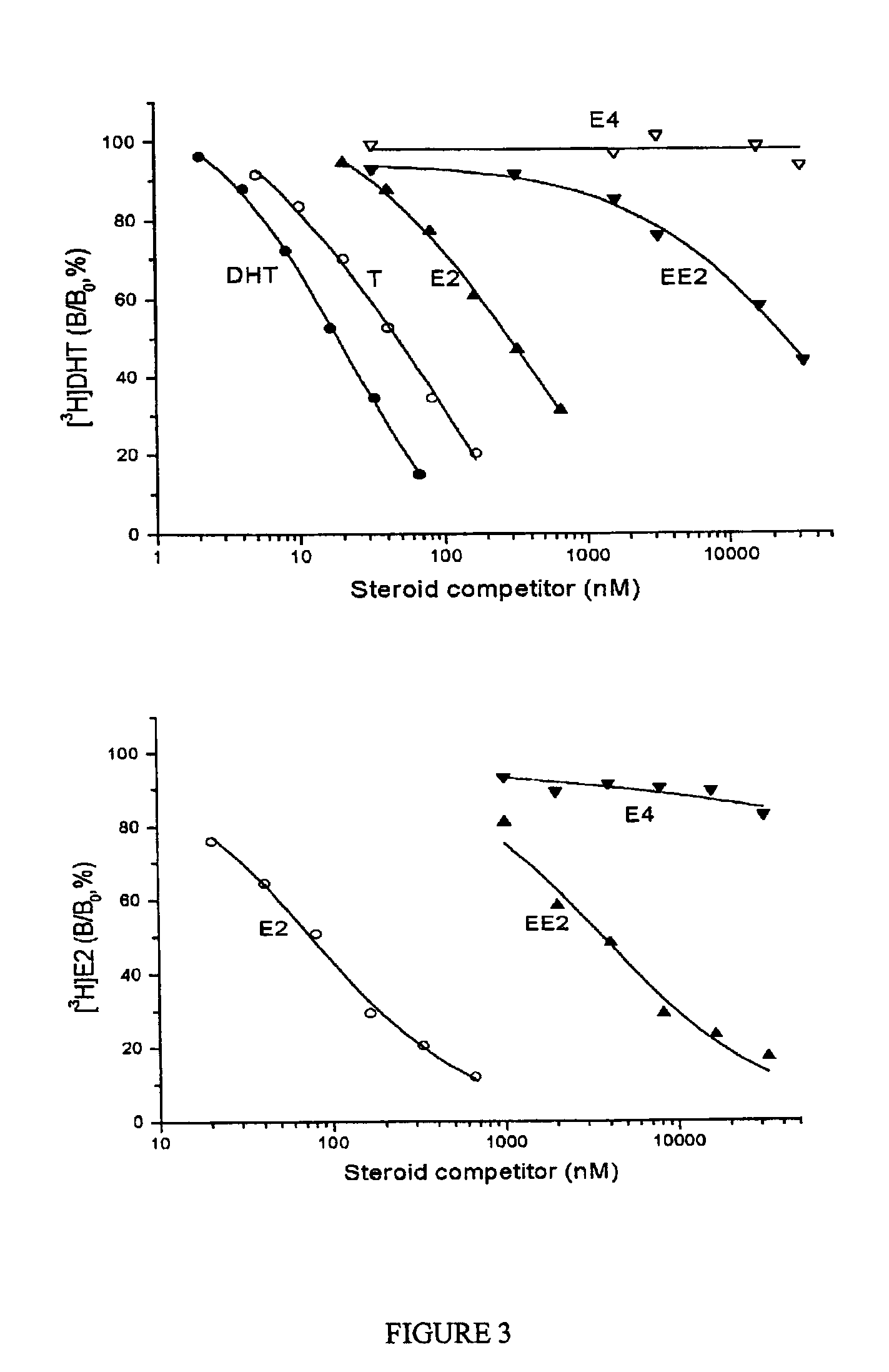
Estrogenic compounds in combination with progestogenic compounds in hormone-replacement therapy
ActiveUS20050070488A1Reliable efficacyImprove oral bioavailabilityBiocideOrganic active ingredientsProgestinCompound (substance)
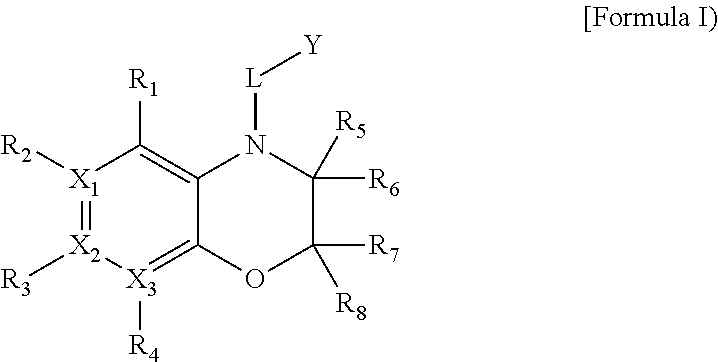
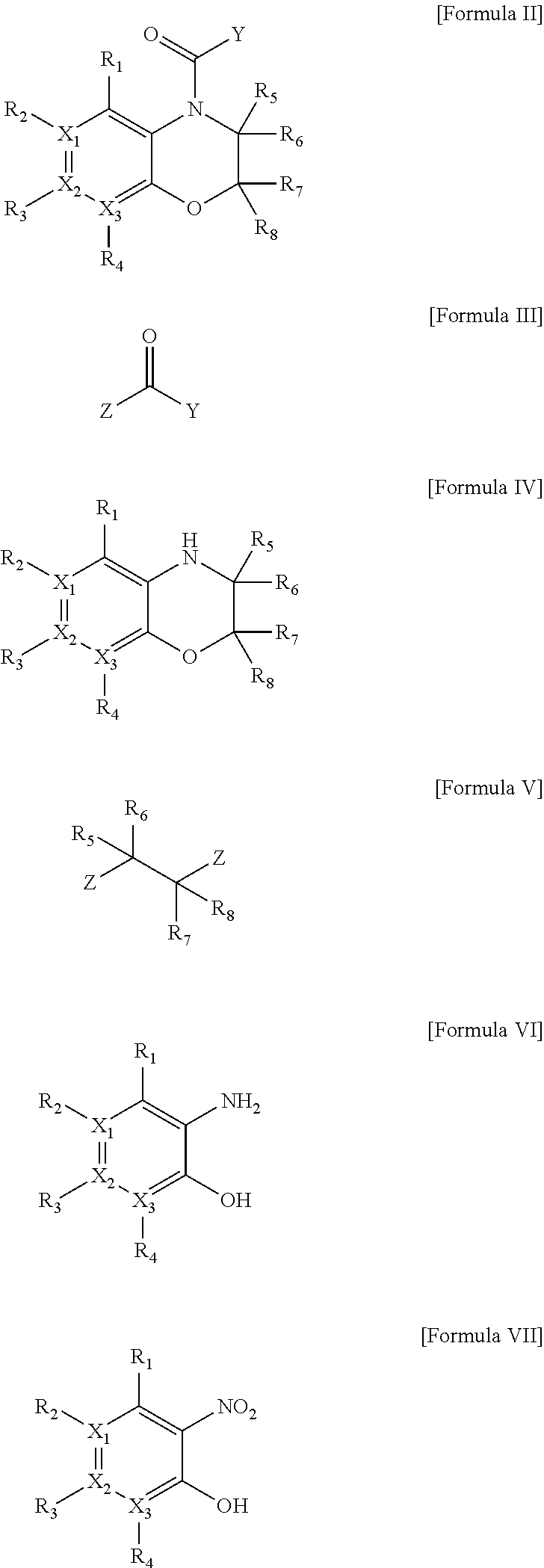
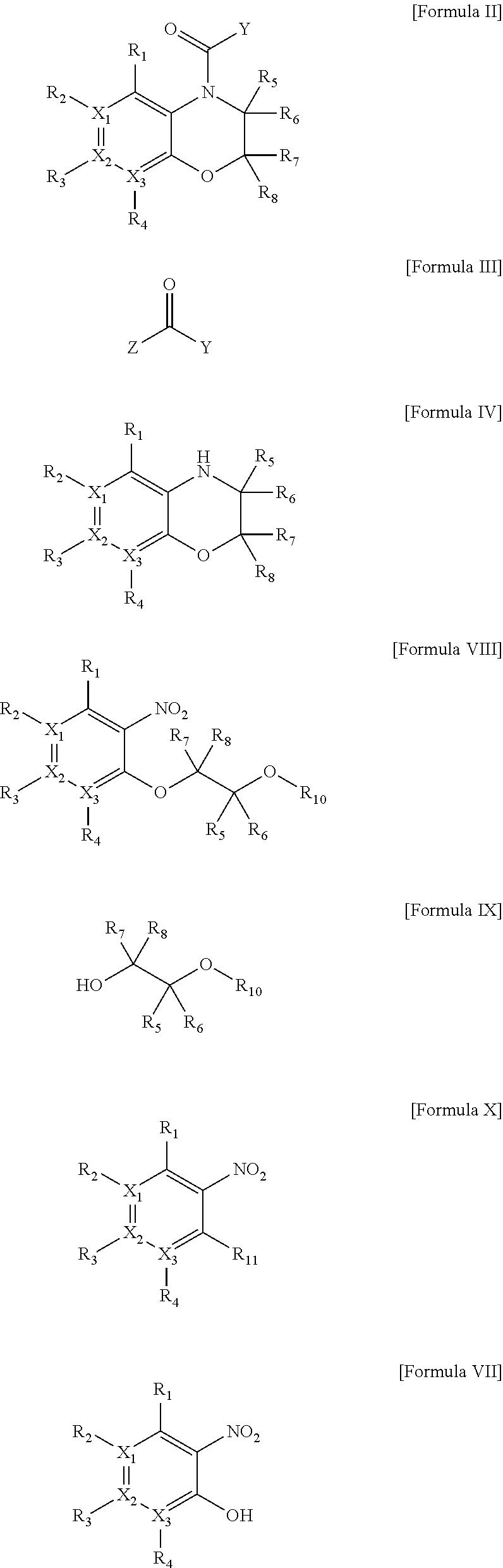
One aspect of the present invention relates to a method of hormone replacement in mammals, which method comprises the oral administration of an estrogenic component and a progestogenic component to a mammal in an effective amount to prevent or treat symptoms of hypoestrogenism, wherein the estrogenic component is selected from the group consisting of substances represented by the above formula in which formula R1, R2, R3, R4 independently are a hydrogen atom, a hydroxyl group or an alkoxy group with 1-5 carbon atoms; each of R5, R6, R7 is a hydroxyl group; and no more than 3 of R1, R2, R3, R4 are hydrogen atoms; precursors capable of liberating a substance according to the aforementioned formula when used in the present method; and mixtures of one or more of the aforementioned substances and / or precursors. Another aspect of the invention concerns a pharmaceutical kit comprising oral dosage units that contain the aforementioned estrogenic component and a progestogenic component as well as an androgenic component.
Owner:ESTETRA SRL
Oral modified release formulations
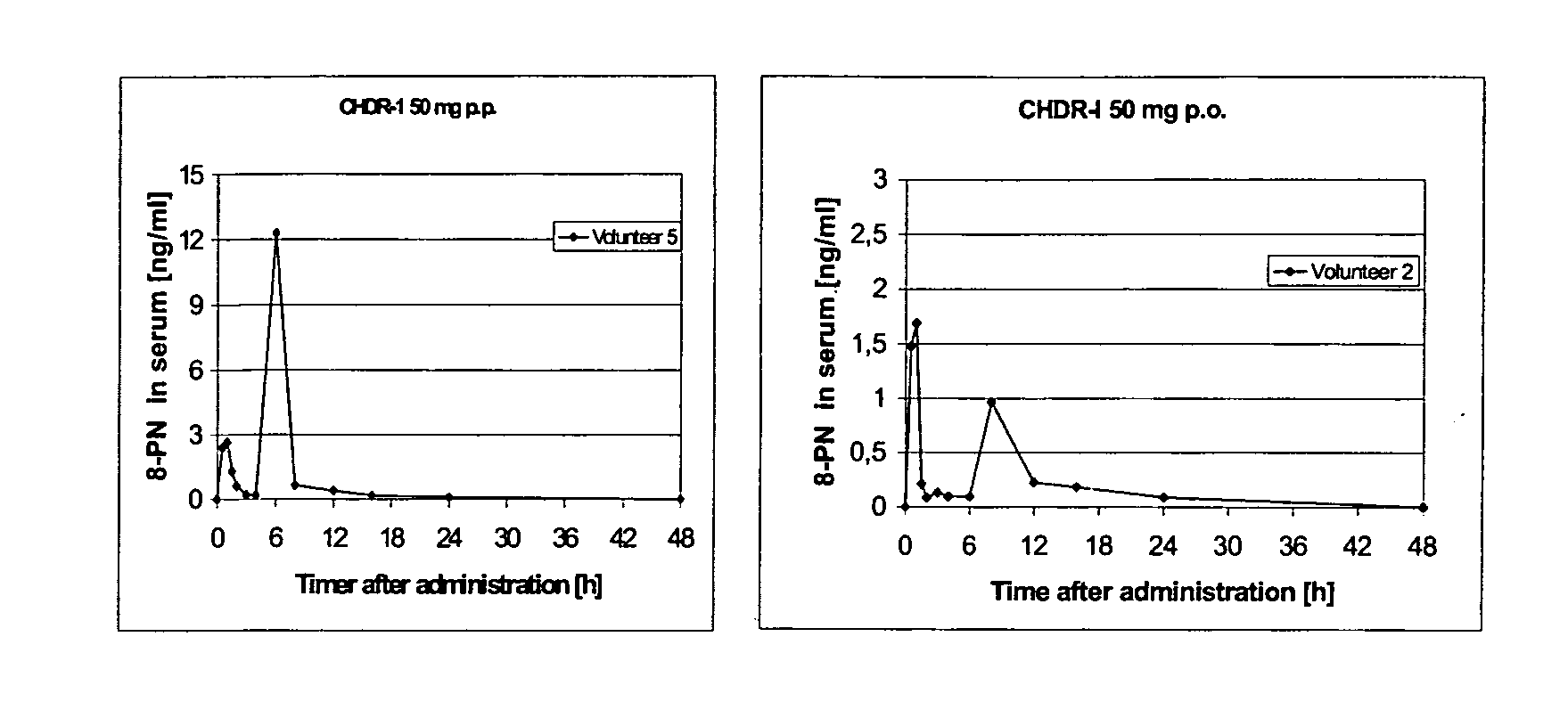
InactiveUS20100086599A1High dose of drugReduce doseBiocidePowder deliveryDrospirenoneImmediate release
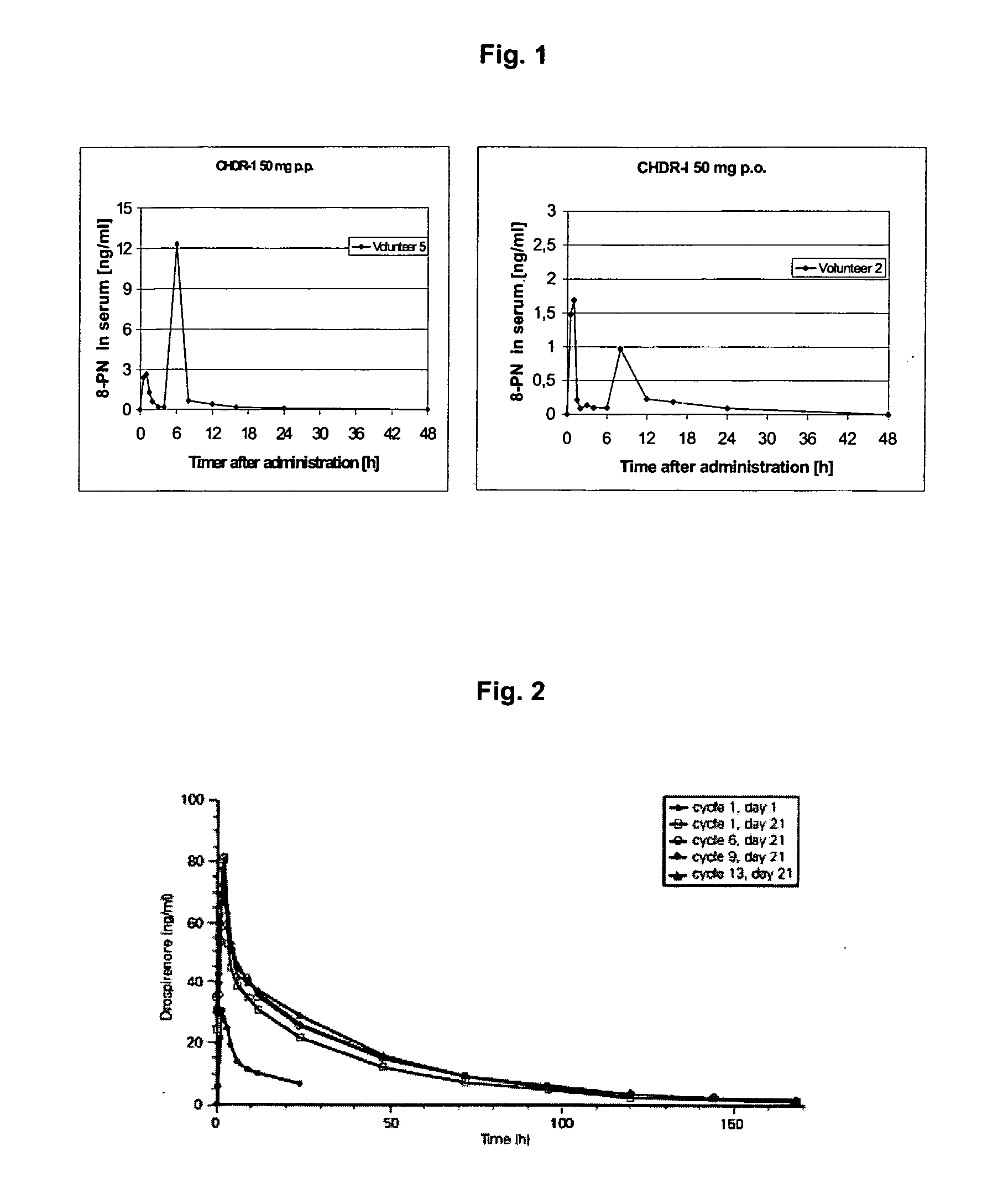
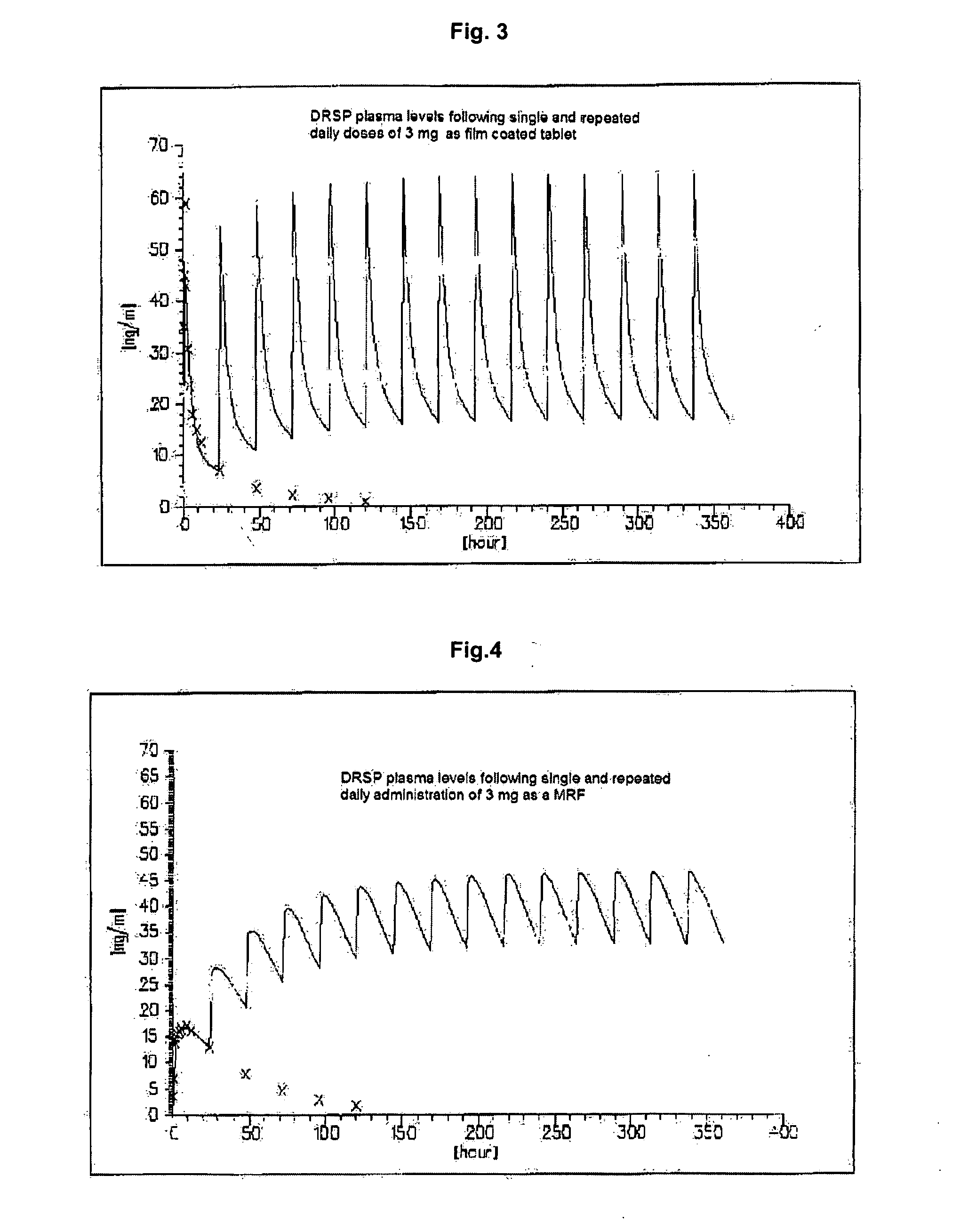
This invention is directed to an oral modified release formulation of the phytoestrogen 8-Prenylnaringenin in combination with a progestin, preferably with Drospirenone, and several uses thereof. In another aspect of the invention an oral modified formulation of 8-Prenylnaringenin with an immediately releasing progestin, like Drospirenone, is provided as well as several uses thereof.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Peptides For Skin Rejuvenation And Methods Of Using The Same
ActiveUS20140309173A1Improvement factorMore lipophilicCosmetic preparationsOrganic active ingredientsDiseaseCell-Extracellular Matrix
The invention provides compositions for stimulating the formation of one or more extracellular matrix components that contain a lipoaminoacid derivative of the tripeptide carnosine such as N-Octanoyl Carnosine. Also provided are compositions containing N-Octanoyl Carnosine in combination with selected tripeptide and / or tetrapeptides as well as pharmaceutical and / or cosmetic compositions containing such compositions. The invention further provides methods of using the compositions and compositions of the invention to treat, alleviate, and / or ameliorate a symptom, condition, disorder, or disease of the skin or mucosa, wherein the symptom, condition, disorder, or disease is associated with changes in extracellular matrix components.
Owner:ANTEIS SA
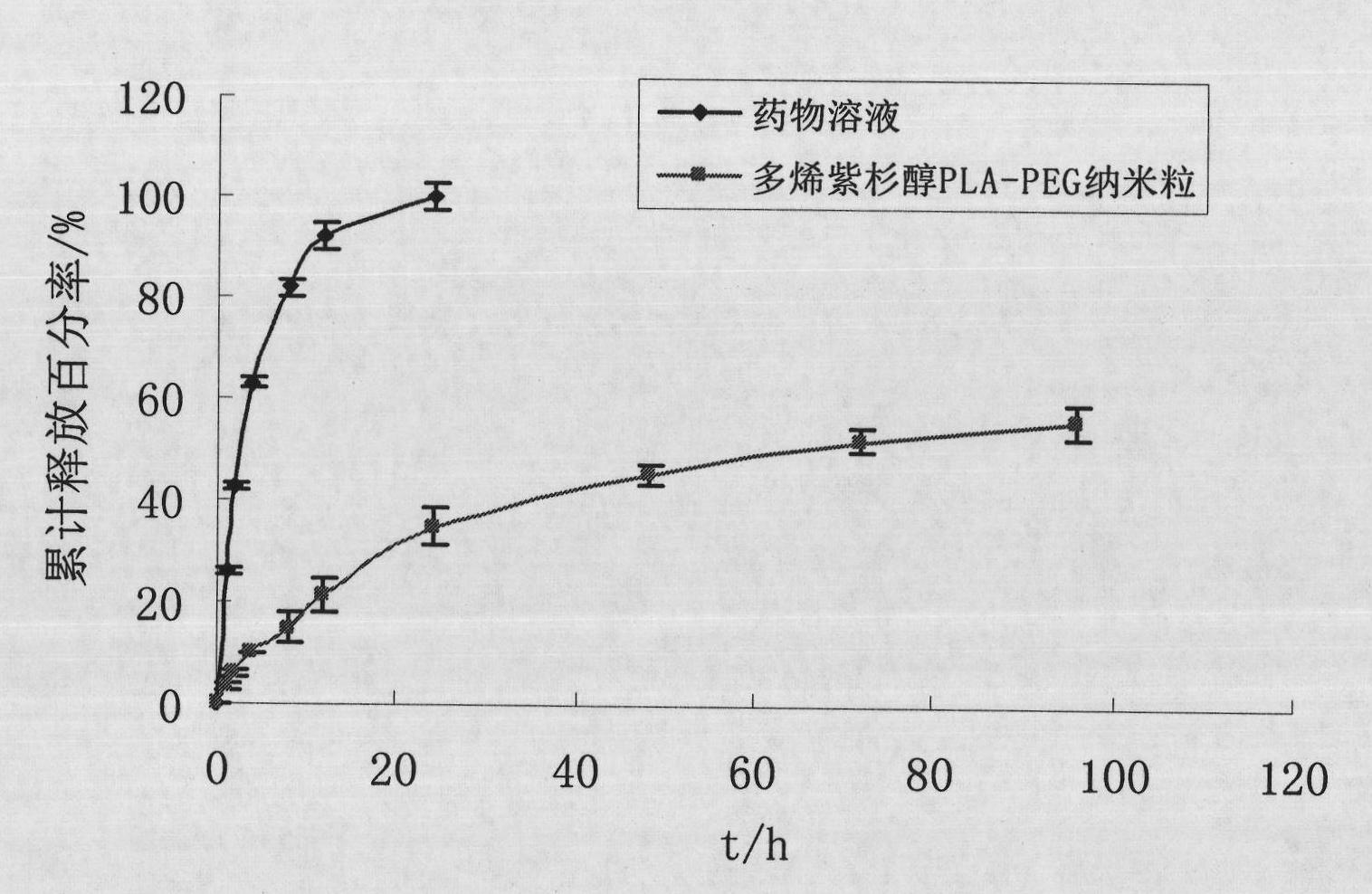
Preparation method of polyene-containing taxol nanoparticle mixed micelle preparation and freeze-drying agent
InactiveCN101804021AImprove solubilityHigh metabolic stabilityOrganic active ingredientsPowder deliveryMixed micelleFreeze-drying
The invention discloses a preparation method of a polyene-containing taxol nanoparticle mixed micelle preparation and a freeze-drying agent, which prepares docetaxel PLA-PEG nanoparticles or micelle or nanoparticle mixed micelle through a modified solvent evaporation method, takes PLA-PEG copolymer as a carrier, and wraps docetaxel in a PLA hydrophobic core. When in use, the docetaxel PLA-PEG containing long cycle freeze-dried preparation only needs to be added with water and is dissolved, and uniform nanoparticle suspension, micellar solution or mixed micellar nanoparticle suspension can be prepared. The preparation method does not need tween-80 and ethanol solubilization, only takes the biodegradable PLA-PEG as the carrier, and does not contain any surfactant; and compared with the docetaxel injection on sale, the preparation can reduce the toxicity and the adverse reactions of the medicine, and improve the clinical application safety of the medicine.
Owner:SHANDONG UNIV
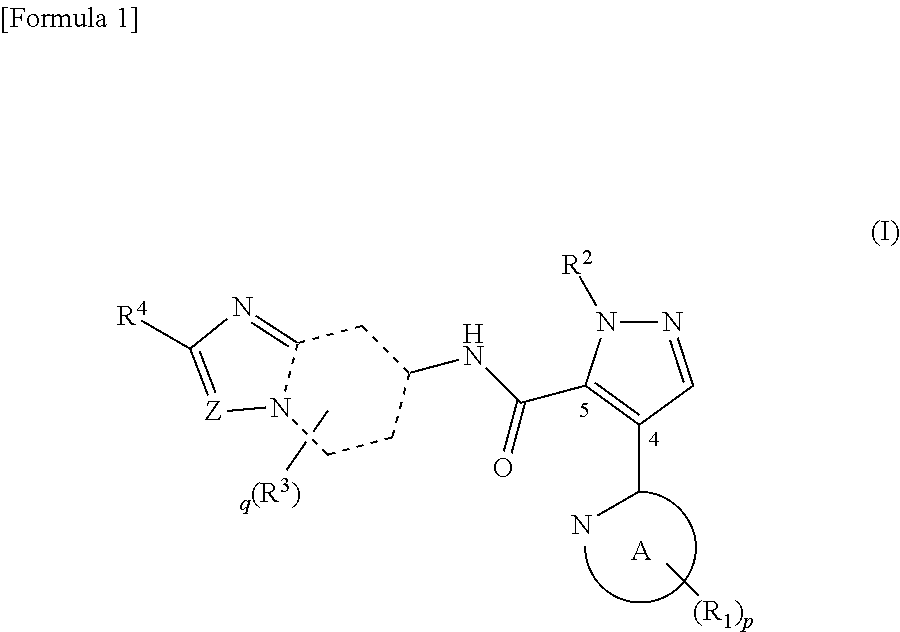
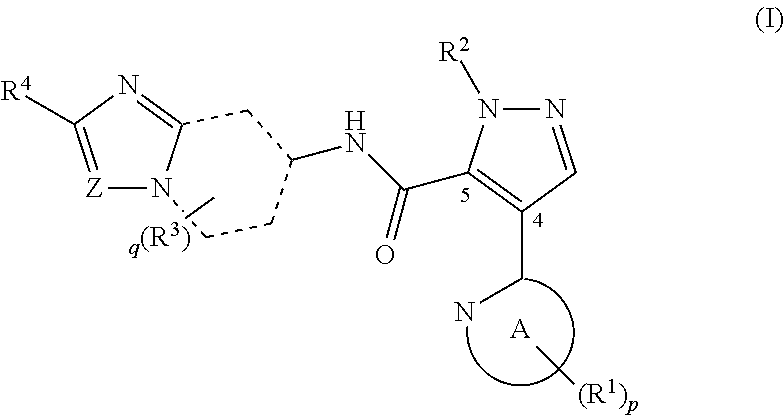
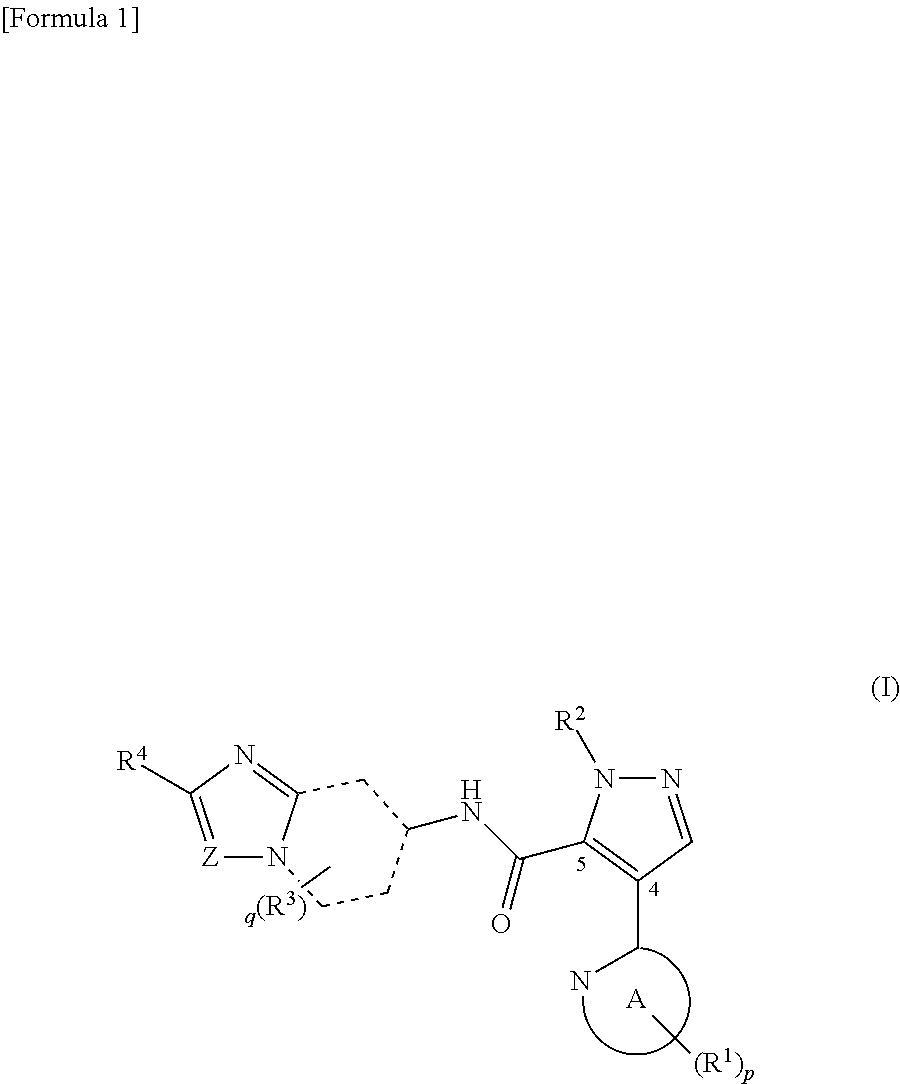
Novel pyrazole derivative
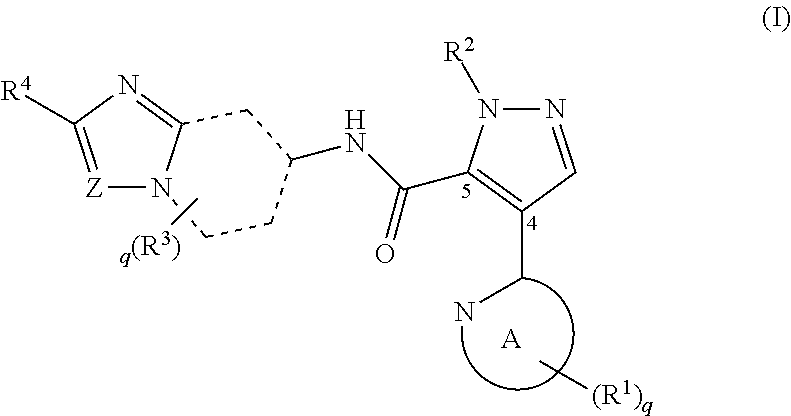
It has been desired to develop a pharmaceutical composition, which is used in agents for preventing and / or treating various diseases related to PDE10 (e.g. mental disorder and neurodegenerative disorder). The present invention provides: compounds having PDE10 inhibitory effect, in particular, compounds having a 4-heteroarylpyrazole-5-carboxylic acid amide structure represented by the following formula (I), or their pharmaceutically acceptable salts, or their solvates; pharmaceutical compositions comprising, as active ingredients, the compounds, or their pharmaceutically acceptable salts, or their solvates; and medical use of the compounds, or their pharmaceutically acceptable salts, or their solvates.
Owner:MOCHIDA PHARM CO LTD
2-Acylaminopropoanol-Type Glucosylceramide Synthase Inhibitors
InactiveUS20110184021A1Inhibit synthesisHigh metabolic stabilityBiocideOrganic chemistryUrologyGlucosylceramide Synthase Inhibitors
A compound for use in treating polycystic kidney disease is represented by Structural Formula (I): or a pharmaceutically acceptable salt thereof. A pharmaceutical composition comprises a compound represented by Structural Formula (I) or a pharmaceutically acceptable salt thereof. A method of treating polycystic kidney disease in a subject in need thereof comprises administering to the subject a therapeutically effective amount of a compound represented by Structural Formula (I) or a pharmaceutically acceptable salt thereof. Methods of treating in polycystic kidney disease in a subject in need thereof respectively comprise administering to the subject a therapeutically effective amount of a compound represented by Structural Formula (I) or a pharmaceutically acceptable salt thereof.
Owner:GENZYME CORP
Method for preparing block polymer micelle freeze-drying preparation carrying docetaxel
InactiveCN101732234AImprove solubilityHigh metabolic stabilityOrganic active ingredientsPharmaceutical delivery mechanismMetabolic stabilityOrganic solvent
The invention discloses a method for preparing a block polymer micelle freeze-drying preparation carrying docetaxel, which uses a PEO-PPO-PEO block polymer as a micelle carrier to wrap the docetaxel inside the micelle and comprises the following steps: (1) dissolving the carrier and the docetaxel into an organic solvent to make the PEO-PPO-PEO block polymer and the docetaxel fully dissolved in the organic solvent, and then preparing aqueous solution of the docetaxel micelle by using the PEO-PPO-PEO block polymer as the carrier; and (2) adding a freeze-drying protective agent into the aqueous solution of the PEO-PPO-PEO block polymer carrying micelle, and filtering, sterilizing and freeze-drying the mixture to obtain freeze-drying preparation of the carrier micelle system. The block polymer micelle freeze-drying preparation carrying the docetaxel can increase the dissolubility, metabolic stability and in vivo circulation time of the docetaxel, reduce the toxicity and improve the bioavailability, and is more suitable for clinical application.
Owner:SHANDONG UNIV
2-acylaminopropoanol-type glucosylceramide synthase inhibitors
InactiveUS8309593B2Inhibit synthesisHigh metabolic stabilityBiocideOrganic chemistryStructural formulaGLYCOCERAMIDES
A compound for use in treating polycystic kidney disease is represented by Structural Formula (I): or a pharmaceutically acceptable salt thereof. A pharmaceutical composition comprises a compound represented by Structural Formula (I) or a pharmaceutically acceptable salt thereof. A method of treating polycystic kidney disease in a subject in need thereof comprises administering to the subject a therapeutically effective amount of a compound represented by Structural Formula (I) or a pharmaceutically acceptable salt thereof. Methods of treating in polycystic kidney disease in a subject in need thereof respectively comprise administering to the subject a therapeutically effective amount of a compound represented by Structural Formula (I) or a pharmaceutically acceptable salt thereof.
Owner:GENZYME CORP
Estrogenic compounds in combination with progestogenic compounds in hormone-replacement therapy
ActiveUS8026228B2Suppress ovarian estrogenAvoid symptomsBiocideOrganic active ingredientsPresent methodMammal
One aspect of the present invention relates to a method of hormone replacement in mammals, which method comprises the oral administration of an estrogenic component and a progestogenic component to a mammal in an effective amount to prevent or treat symptoms of hypoestrogenism, wherein the estrogenic component is selected from the group consisting of substances represented by the above formula in which formula R1, R2, R3, R4 independently are a hydrogen atom, a hydroxyl group or an alkoxy group with 1-5 carbon atoms; each of R5, R6, R7 is a hydroxyl group; and no more than 3 of R1, R2, R3, R4 are hydrogen atoms; precursors capable of liberating a substance according to the aforementioned formula when used in the present method; and mixtures of one or more of the aforementioned substances and / or precursors. Another aspect of the invention concerns a pharmaceutical kit comprising oral dosage units that contain the aforementioned estrogenic component and a progestogenic component as well as an androgenic component.
Owner:ESTETRA SRL
Total tanshinone composite preparation
InactiveCN102100741AMild preparation conditionsOptimal control methodAntibacterial agentsPowder deliverySalvia miltiorrhizaOral medication
The invention relates to a total tanshinone composite preparation, in particular to a self-microemulsion medicine carrying composite preparation for improving the oral administration bioavailability of total tanshinone, which comprises the following components in part by mass: 0.5 to 5 parts of tanshinone, 25 to 75 parts of oil phase, 35 to 70 parts of surfactant and 0 to 25 parts of cosurfactant. In addition, the preparation can be prepared into a solid preparation or a liquid preparation by combining with other solid materials or water, and after entering a gastrointestinal tract, the solidpreparation or the liquid preparation can be emulsified automatically to form microemulsion of which the grain diameter is less than 100 nanometers when contacting water so as to increase the dissolution speed of the tanshinone, promote the absorption of the tanshinone and improve the oral administration bioavailability of the tanshinone; and the preparation conditions of the total tanshinone composite preparation is mild, a control method is simple and convenient, and special equipment is not required.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
2-acylaminopropoanol-type glucosylceramide synthase inhibitors
InactiveUS8304447B2Inhibit synthesisHigh metabolic stabilityBiocideOrganic chemistryDiseaseLow glucose
Owner:GENZYME CORP
Drug delivery system comprising a tetrahydroxilated estrogen for use in hormonal contraception
ActiveUS7732430B2Improve oral bioavailabilityLow potencyOrganic active ingredientsBiocidePresent methodPhysiology
A method of contraception in mammalian females, which method comprises the oral administration of an estrogenic component and a progestogenic component to a female of childbearing capability in an amount effective to inhibit ovulation, wherein the estrogenic component is selected from the group consisting of substances represented by the following formula (1)in which R1, R2, R3, R4 independently are a hydrogen atom, a hydroxyl group or an alkoxy group with 1-5 carbon atoms; each of R5, R6, R7 is a hydroxyl group; and no more than 3 of R1, R2, R3, R4 are hydrogen atoms; precursors capable of liberating a substance according to the aforementioned formula when used in the present method; and mixtures of one or more of the aforementioned substances and / or precursors. Another aspect of the invention concerns a pharmaceutical kit comprising oral dosage units that contain the aforementioned estrogenic component and / or a progestogenic component.
Owner:ESTETRA SRL
Conjugate comprising angiostation or its fragment, the method for producing the conjugate and use thereof
InactiveUS20100184661A1Prolong half-life in vivoInhibit migrationPeptide/protein ingredientsDepsipeptidesPeptideDrug
The present invention provides an anti-tumor or anti-angiogenesis medicament, the combination or kit containing the medicament, and the method for producing the same. The anti-tumor or anti-angiogenesis medicament contains a conjugate comprising a modifying agent and the angiostatin or its fragments, wherein the conjugate exhibits prolonged in vivo half-life as compared to an unmodified angiostatin or its fragments. The modifying agent is selected from the group consisting of macromolecular polymers, protein molecules or fragments thereof, peptides, small molecules, or chemical substances of any other forms.
Owner:TSINGHUA UNIV
Heterocyclic derivatives
ActiveUS20110028467A1Strong inhibitory activityReduce dosageOrganic active ingredientsOrganic chemistryPerylene derivativesUric acid
The present invention relates to heterocyclic derivatives, and more particularly, to novel heterocyclic derivatives useful for the preparation of medicaments for treating diseases related to uric acid.
Owner:C&C RES LAB
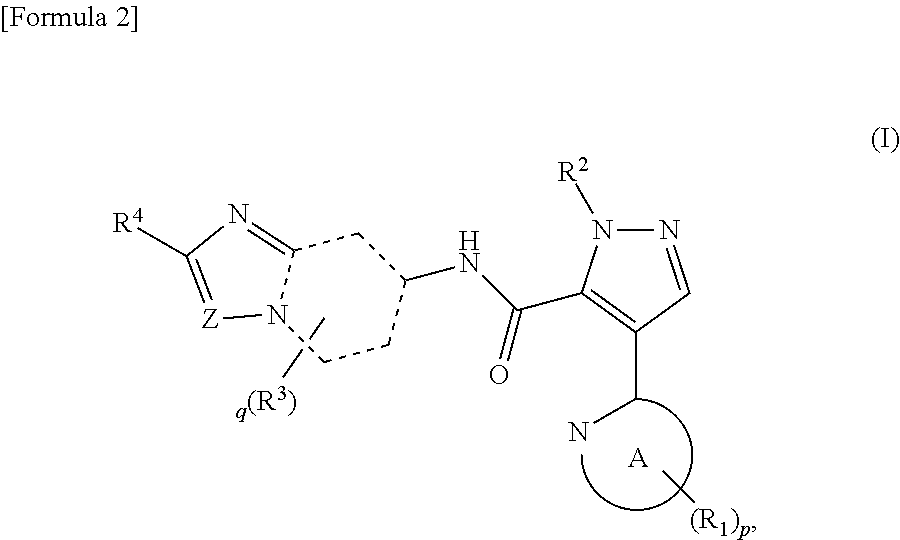
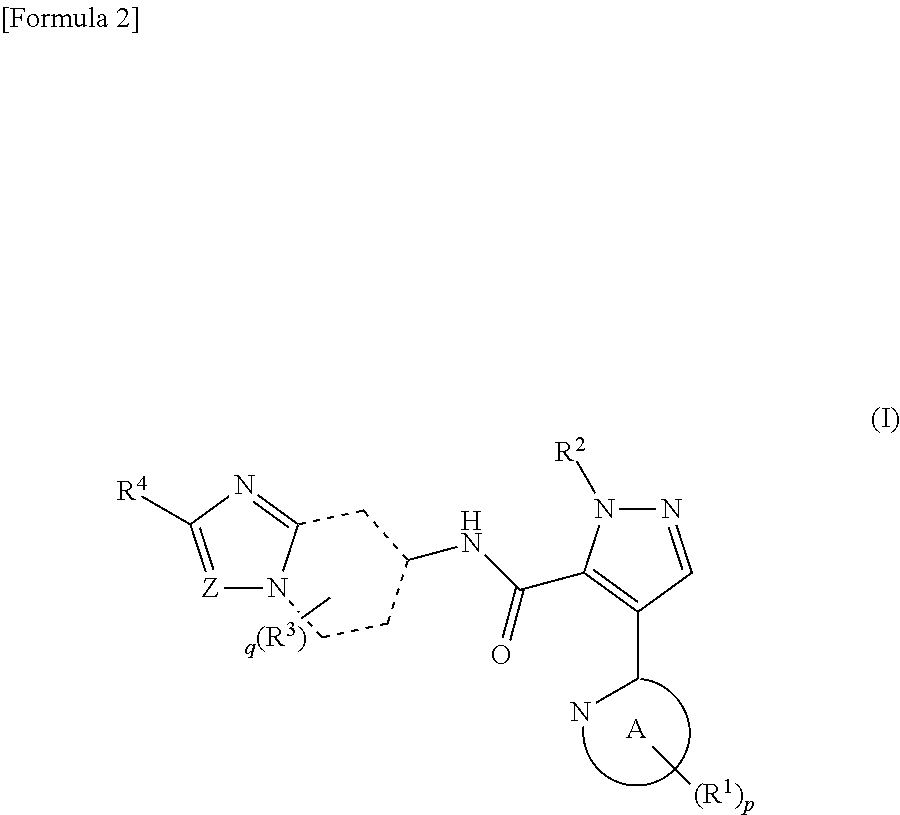
Pyrazole derivative
ActiveUS8980889B2Avoid hydrolysisImprove diseaseOrganic active ingredientsNervous disorderDiseaseCarboxylic acid
It has been desired to develop a pharmaceutical composition, which is used in agents for preventing and / or treating various diseases related to PDE10 (e.g. mental disorder and neurodegenerative disorder). The present invention provides: compounds having PDE10 inhibitory effect, in particular, compounds having a 4-heteroarylpyrazole-5-carboxylic acid amide structure represented by the following formula (I), or their pharmaceutically acceptable salts, or their solvates; pharmaceutical compositions comprising, as active ingredients, the compounds, or their pharmaceutically acceptable salts, or their solvates; and medical use of the compounds, or their pharmaceutically acceptable salts, or their solvates.
Owner:MOCHIDA PHARM CO LTD
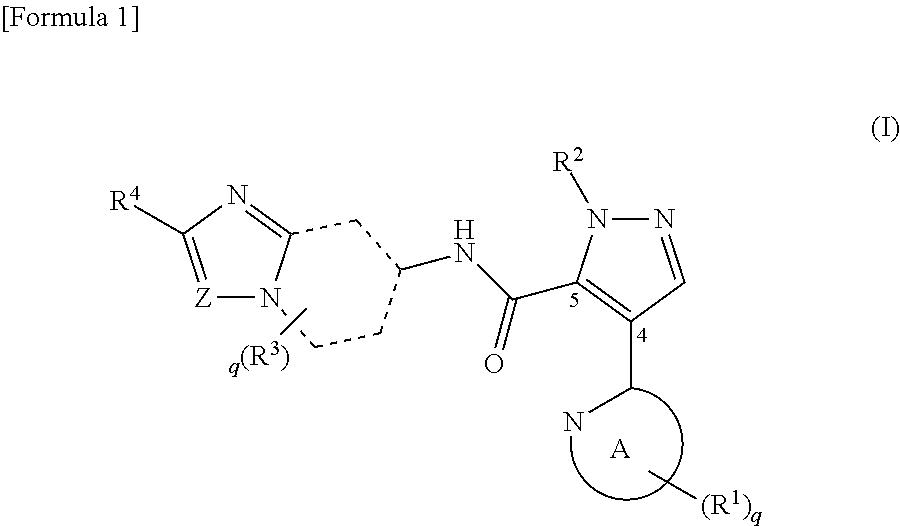
Agents for treating disorders involving modulation of ryanodine receptors
ActiveUS20140088171A1Excellent propertyHigh metabolic stabilityOrganic active ingredientsBiocideDiseaseRyanodine receptor
The present invention relates to 1,4-benzothiazepine derivatives and their use to treat conditions, disorders and diseases associated with ryanodine receptors (RyRs) that regulate calcium channel functioning in cells. The invention also discloses pharmaceutical compositions comprising the compounds and uses thereof to treat diseases and conditions associated with RyRs, in particular cardiac, musculoskeletal and central nervous system (CNS) disorders.
Owner:ARMGO PHARMA
Polypeptide ligands specific for plasma kallikrein
ActiveUS10294274B2Good cross-reactivityEnhance ability to formulateAntibacterial agentsSenses disorderPeptide ligandKinin
The present invention relates to polypeptides which are covalently bound to molecular scaffolds such that two or more peptide loops are subtended between attachment points to the scaffold. In particular, the invention describes peptides which are specific for the human and rat protease plasma kallikrein and are modified in one or two peptide loops to enhance potency and / or protease resistance.
Owner:BICYCLERD LTD
Pyrazole derivative
It has been desired to develop a pharmaceutical composition, which is used in agents for preventing and / or treating various diseases related to PDE10 (e.g. mental disorder and neurodegenerative disorder). The present invention provides: compounds having PDE10 inhibitory effect, in particular, compounds having a 4-heteroarylpyrazole-5-carboxylic acid amide structure represented by the following formula (I), or their pharmaceutically acceptable salts, or their solvates; pharmaceutical compositions comprising, as active ingredients, the compounds, or their pharmaceutically acceptable salts, or their solvates; and medical use of the compounds, or their pharmaceutically acceptable salts, or their solvates.
Owner:MOCHIDA PHARM CO LTD
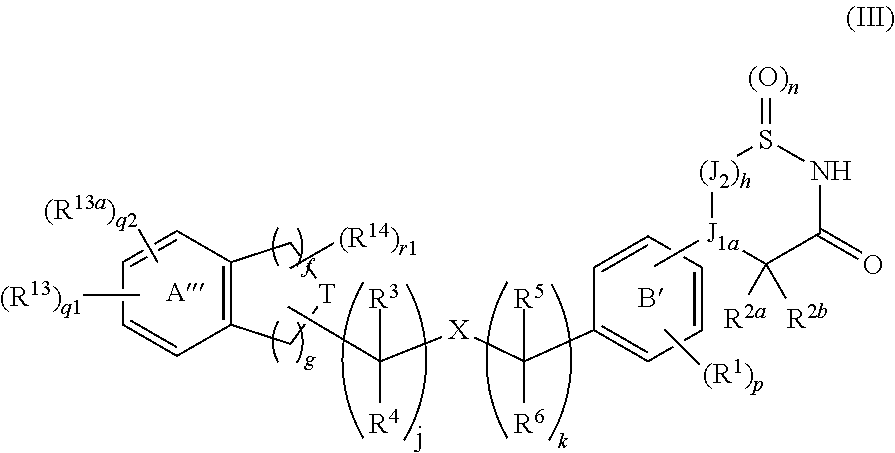
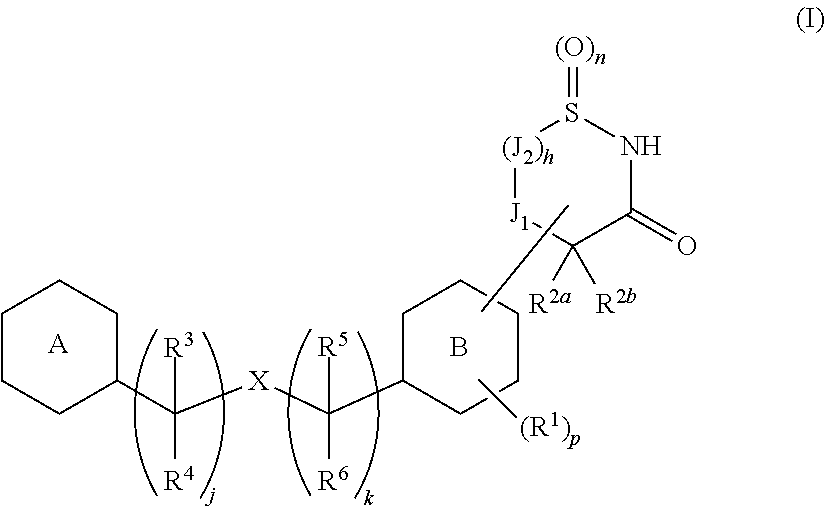
Cyclic amide derivative
InactiveUS20140057871A1Lower blood sugar levelsIncreased insulin secretionBiocidePowder deliveryPancreatic hormoneBULK ACTIVE INGREDIENT
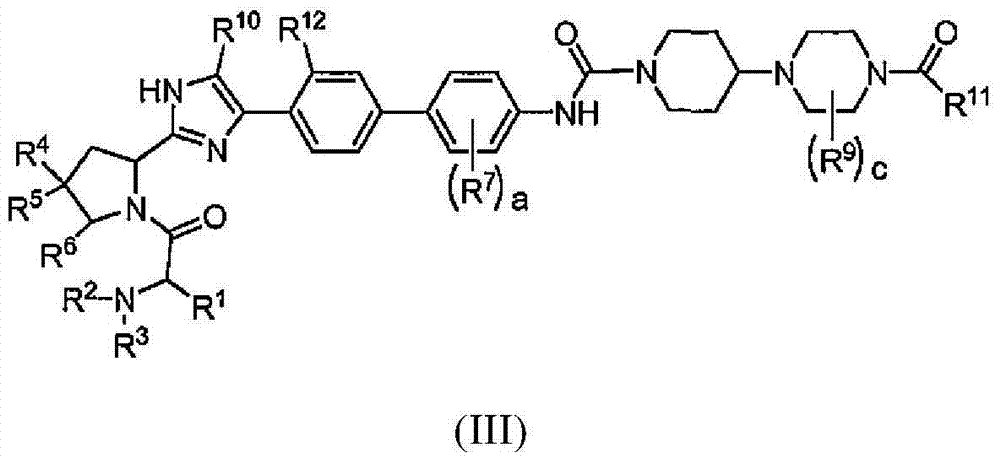
[Problem]To provide a GPR40 activating agent having, as an active ingredient, a novel compound having a GPR40 agonist action, a salt of the compound, a solvate of the salt or the compound, or the like, particularly, an insulin secretagogues and a prophylactic and / or therapeutic agent against diabetes, obesity, or the like.[Means of solving the problem]A compound of Formula (III):(where f is 0 to 2; g is 1 to 4; j is 0 to 3; k is 0 to 2; n is 0 to 2; p is 0 to 4; h is 0 to 3; q1 id 0 to 3; q2 is 0 or 1; r1 is 0 to 2 (with the proviso that q1+q2+r1 is 0 to 5); J1a is —CR11a—, N; J2 is —CR12aR12b—, —CR12c—; T is —CH2—, O, —S(O)i— (i is an integer of 0 to 2) or —NR7—; X is O, S, or —NR7—; ring A′″ is a benzene ring, a pyridine ring; ring B′ is a benzene ring, a pyridine ring, a pyrimidine ring; and R1 to R14 are specific groups),a salt of the compound, or a solvate of the salt or the compound.
Owner:MOCHIDA PHARM CO LTD
Peptides for skin rejuvenation and methods of using the same
ActiveUS9375398B2Improvement factorMore lipophilicCosmetic preparationsOrganic active ingredientsDiseaseDermatology
The invention provides compositions for stimulating the formation of one or more extracellular matrix components that contain a lipoaminoacid derivative of the tripeptide carnosine such as N-Octanoyl Carnosine. Also provided are compositions containing N-Octanoyl Carnosine in combination with selected tripeptide and / or tetrapeptides as well as pharmaceutical and / or cosmetic compositions containing such compositions. The invention further provides methods of using the compositions and compositions of the invention to treat, alleviate, and / or ameliorate a symptom, condition, disorder, or disease of the skin or mucosa, wherein the symptom, condition, disorder, or disease is associated with changes in extracellular matrix components.
Owner:ANTEIS SA
Novel heterocyclic compound and preparation method therefor and use thereof as kinase inhibitor
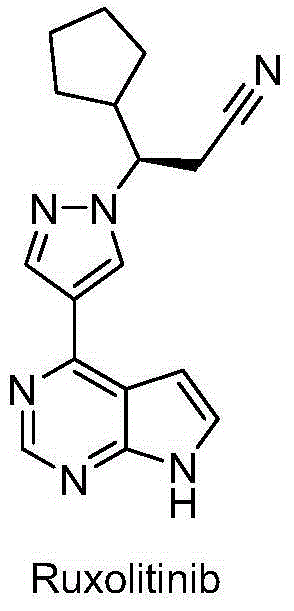
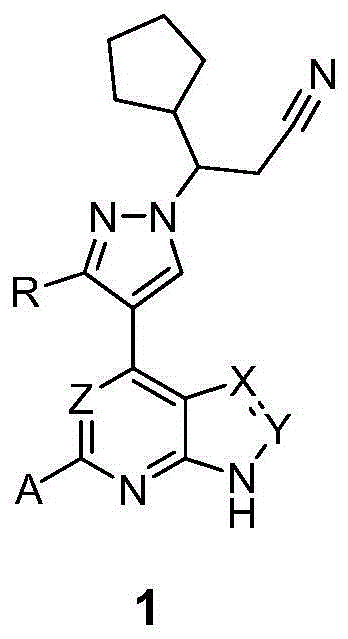
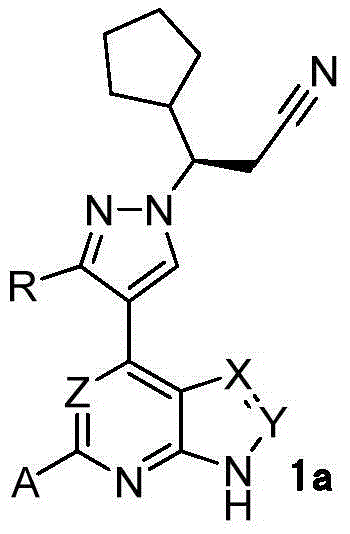
InactiveCN105218548AHigh metabolic stabilityIncrease exposureOrganic active ingredientsOrganic chemistryChemical compoundKinase
The invention relates to a novel heterocyclic compound and a preparation method therefor and use thereof as a kinase inhibitor. Specifically, the invention discloses a compound with a structure represented by a formula 1 shown in the description and a preparation method therefor. The compound is an effective kinase inhibitor and has very high bioavailability.
Owner:SHANGHAI HAIHE PHARMACEUTICAL CO LTD +1
Stabilized cell-penetrating peptide with hydrophobic side chain and preparation method and use of stabilized cell-penetrating peptide
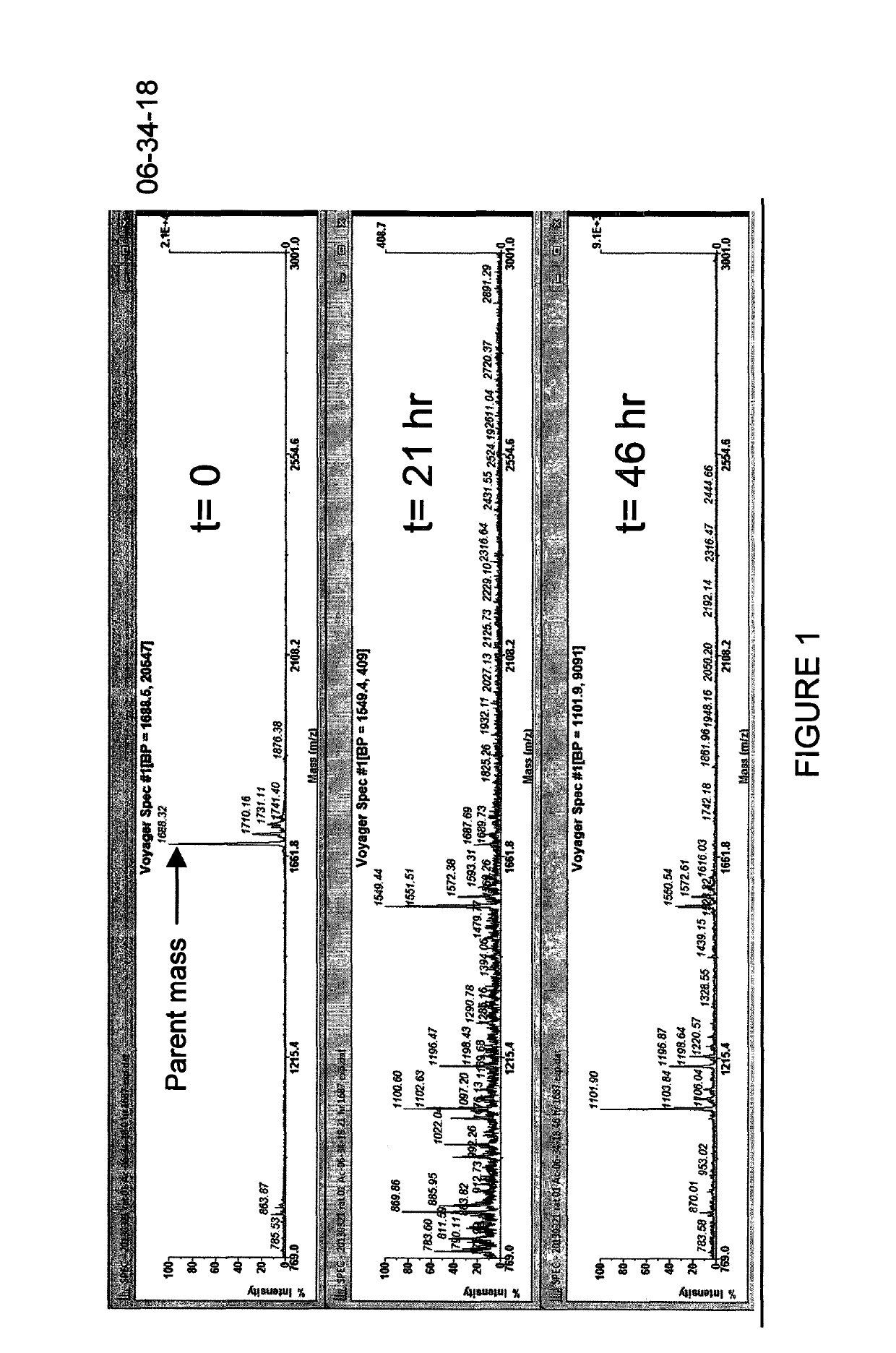
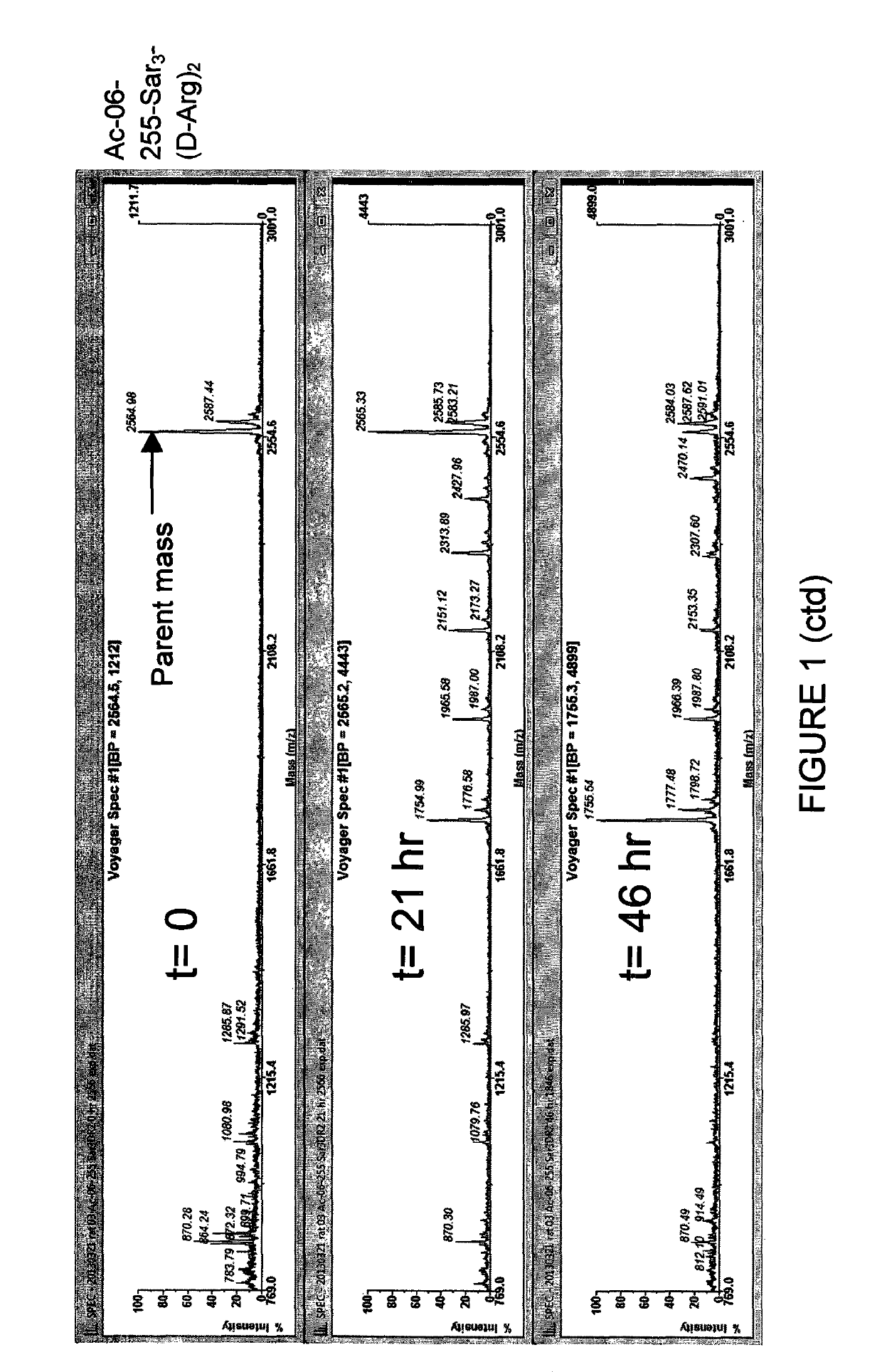
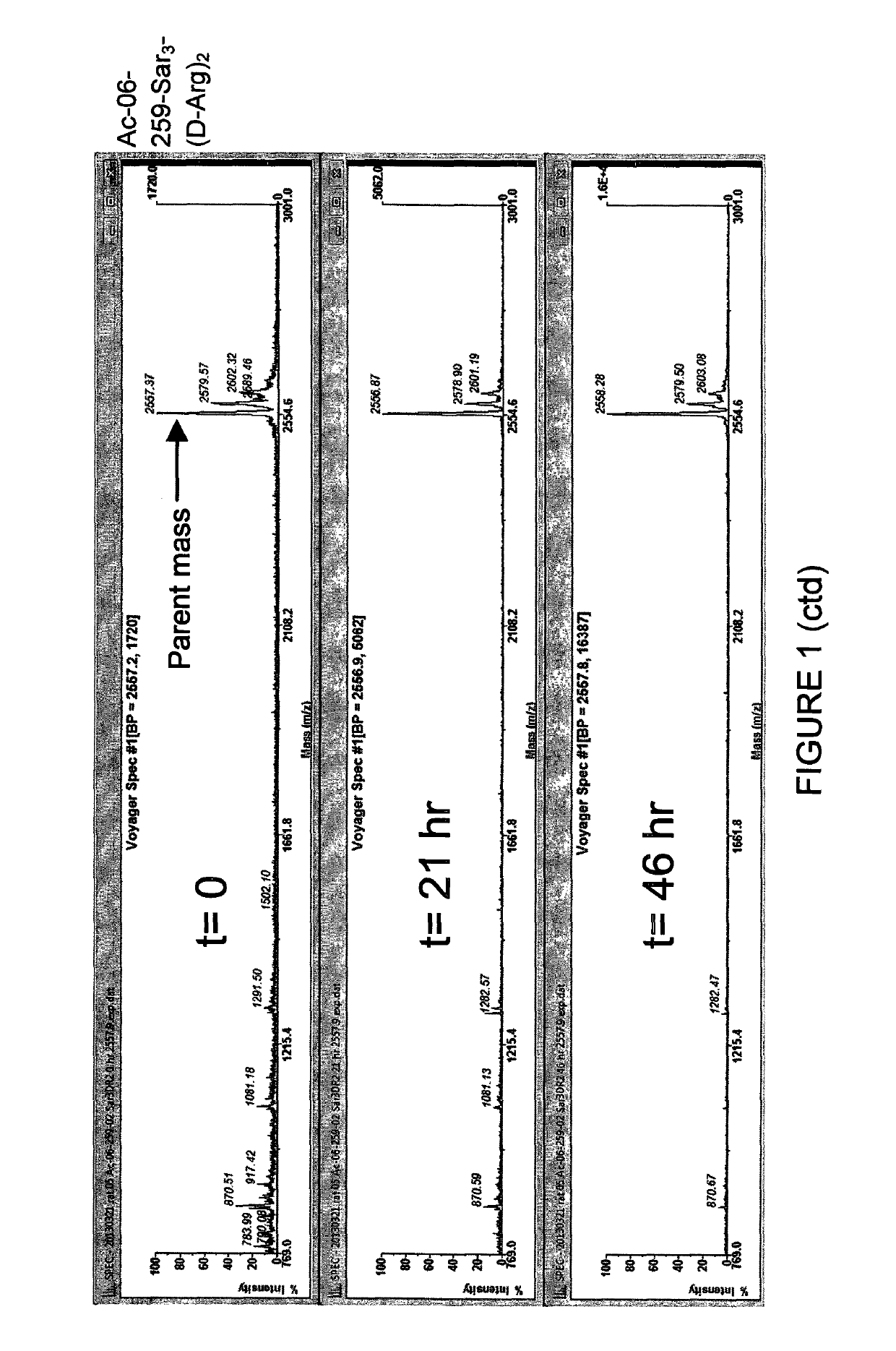
ActiveCN112245593AHigh metabolic stabilityThe synthesis method is simplePeptidesPharmaceutical non-active ingredientsCyclic peptideArginine
The present invention discloses a stabilized cell-penetrating peptide with a hydrophobic side chain and a preparation method and use of the stabilized cell-penetrating peptide. The stabilized cell-penetrating peptide comprises a cell-penetrating peptidyl peptide and the hydrophobic side chain, and the cell-penetrating peptidyl peptide is a cell-penetrating peptide rich in arginine; and the hydrophobic side chain contains hydrophobic small molecules, the stabilized cell-penetrating peptide is a cyclic peptide with a cyclized side chain and obtained by introducing two natural cysteines into a sequence of the cell-penetrating peptidyl peptide and crosslinking the cell-penetrating peptidyl peptide with the hydrophobic small molecules by an alkylation / arylation reaction of the cysteines. The preparation method of the stabilized cell-penetrating peptide is simple and effective, besides, metabolic stability and cell-penetrating capacity of the cell-penetrating peptide are remarkably improvedby adjusting conformation of the amino acids and hydrophilicity and hydrophobicity of the side chain, deep penetration of in-vitro cell spheres and living tumor tissues by the cell-penetrating peptideis realized, and the cell-penetrating peptide has a good application prospect in the field of medicine delivery.
Owner:SOUTHWEST JIAOTONG UNIV
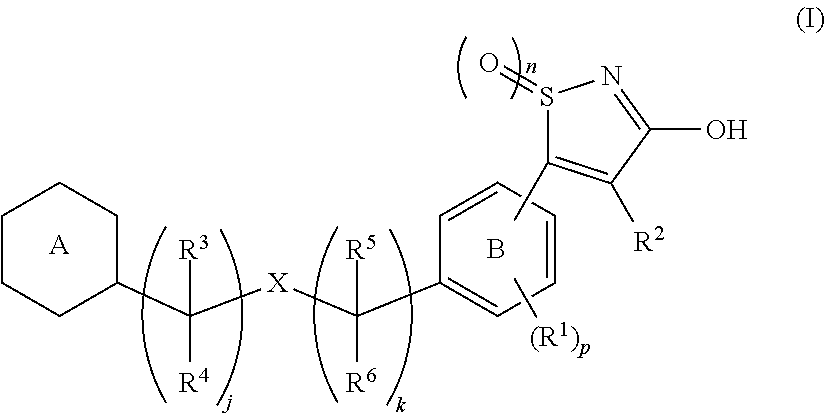
Novel 3-hydroxy-5-arylisothiazole derivative
InactiveUS20120157459A1Lower blood sugar levelsIncreased insulin secretionBiocideOrganic chemistryArylMedicine
[Problem]To provide a GPR40 activating agent having, as an active ingredient, a novel compound having a GPR40 agonist action, a salt of the compound, a solvate of the salt or the compound, or the like, particularly, an insulin secretagogue and a prophylactic and / or therapeutic agent against diabetes, obesity, or other diseases.[Means of solving the problem]A compound of Formula (I):(where n is 0 to 2; p is 0 to 4; j is 0 to 3; k is 0 to 2; a ring A is an aryl group which is optionally substituted with L or a heterocyclic group which is optionally substituted with L; a ring B is a benzene ring, a pyridine ring, or a pyrimidine ring; X is O, S, —NR7—; and R1 to R7 are specific groups),a salt of the compound, or a solvate of the salt or the compound.
Owner:MOCHIDA PHARM CO LTD
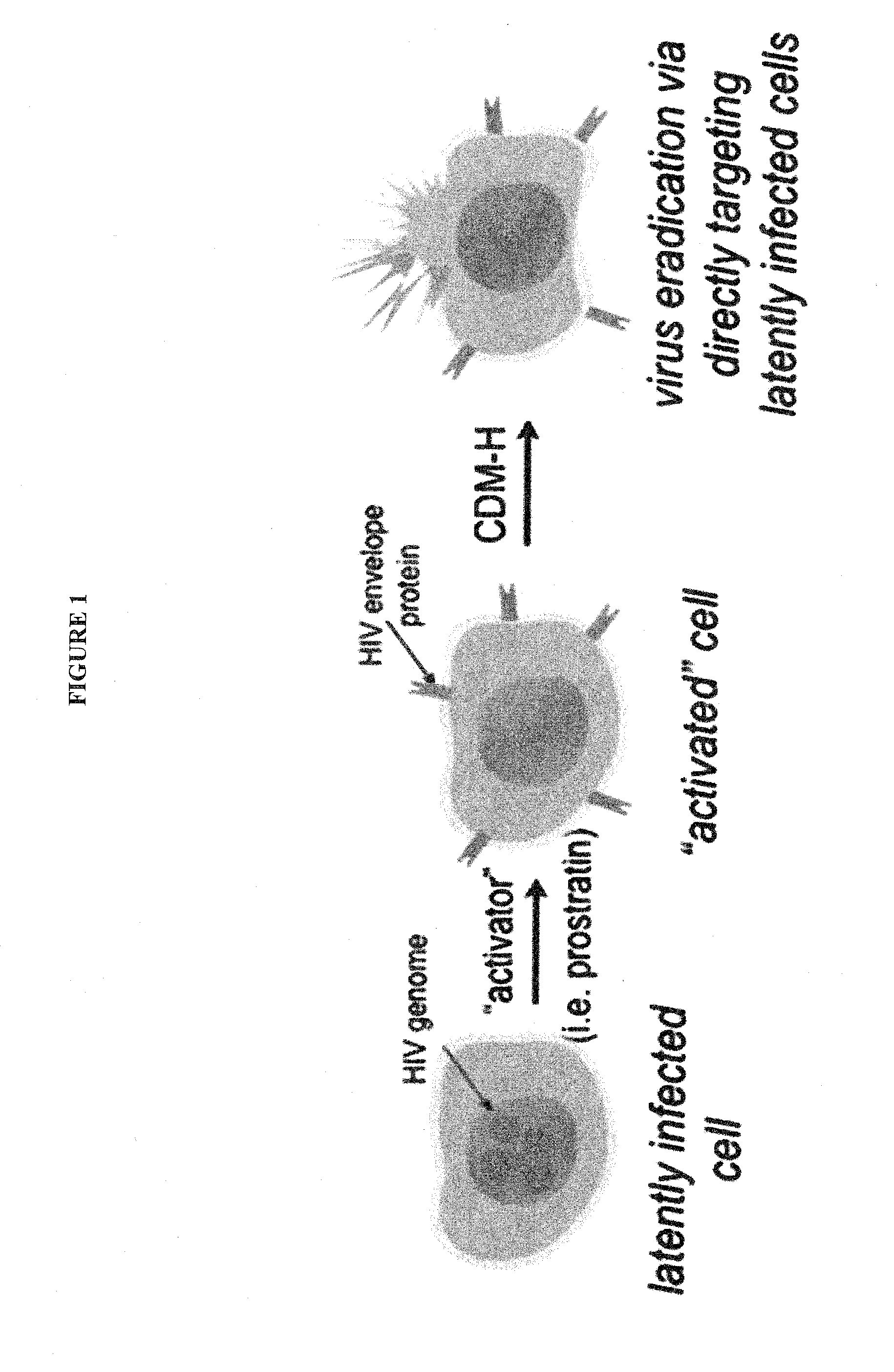
Cytotoxic-drug delivering molecules targeting HIV (cdm-hs), cytotoxic activity against the human immunodeficiency virus and methods of use
ActiveUS20150087609A1Vary amountReduce the possibilityBiocideNervous disorderCytotoxic drugHuman immunodeficiency
The present invention is directed to new bifunctional compounds and methods for treating HIV infections. The bifunctional small molecules, generally referred to as CDM-Hs, function through orthogonal pathways, by inhibiting the gp120-CD4 interaction, and by introducing cytotoxic moieties to gp120-expressing cells, thereby causing cell death and preventing cell infection and spread of HIV. It is shown that CDM-Hs bind to gp120 and gp-120 expressing cells competitively with CD4, and these compounds cause cell death of HIV-infected cells, thereby decreasing viral infectivity. Compounds and methods are described herein.
Owner:YALE UNIV
Biphenyl compound as well as preparation method and medical application thereof
ActiveCN111909108AAvoid interactionEnhance the efficacy of anti-tumor immunityOrganic active ingredientsOrganic chemistryAutoimmune conditionMetabolite
The invention discloses a biphenyl compound as well as a preparation method and medical application thereof, the structure of the biphenyl compound is shown as a formula (I) or a formula (II), and thebiphenyl compound or pharmaceutically acceptable salt, tautomer, meso-isomer, raceme, stereoisomer, metabolite, metabolite precursor, prodrug or solvate thereof is a PD-L1 inhibitor. The compound hasa remarkable inhibiting effect on the interaction of PD-1 and PD-L1 protein, so that the compound can be applied to the preparation of PD-L1 inhibitors and immunomodulator drugs for preventing or treating tumors, autoimmune diseases, organ transplant rejection, infectious diseases and inflammatory diseases.
Owner:CHINA PHARM UNIV
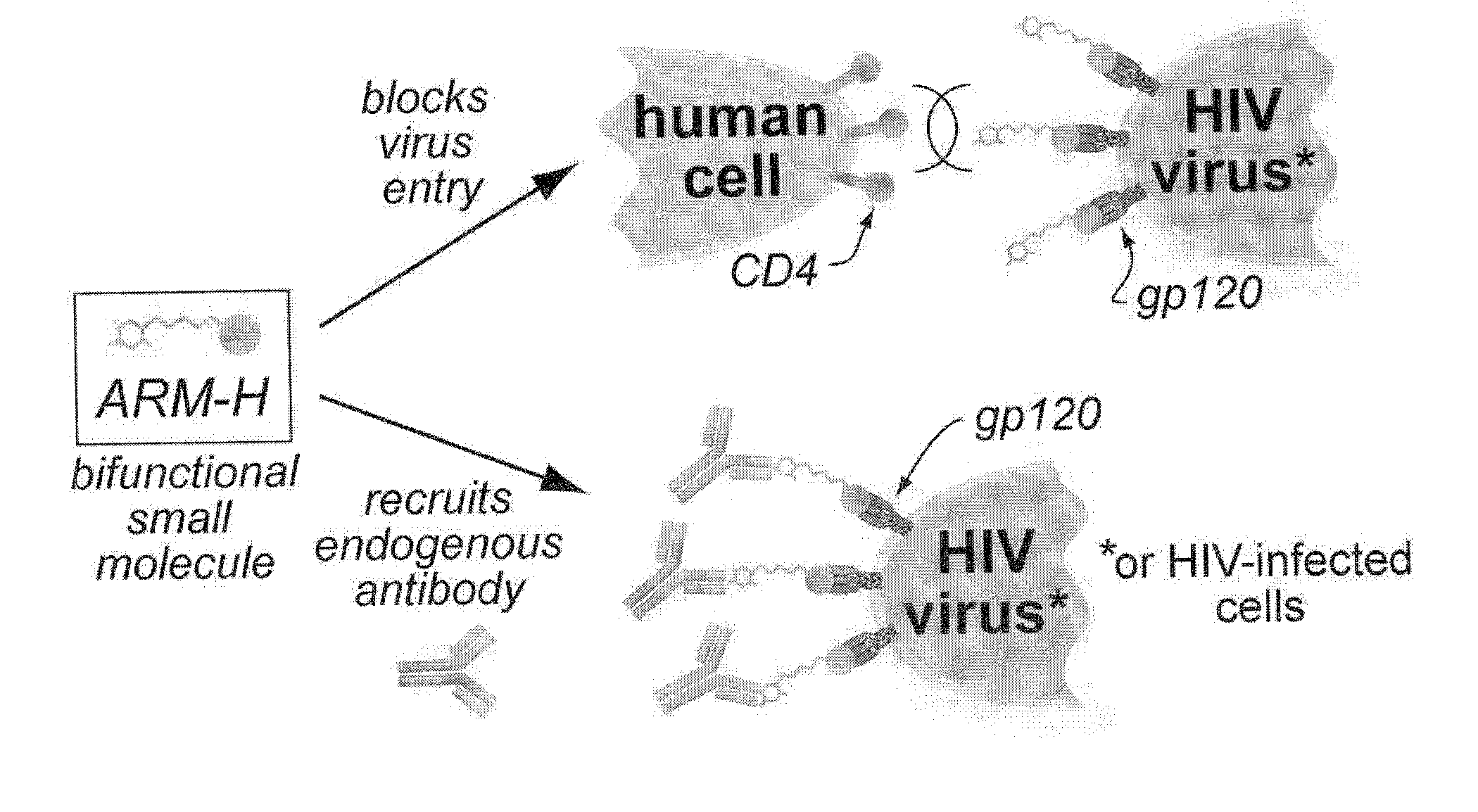
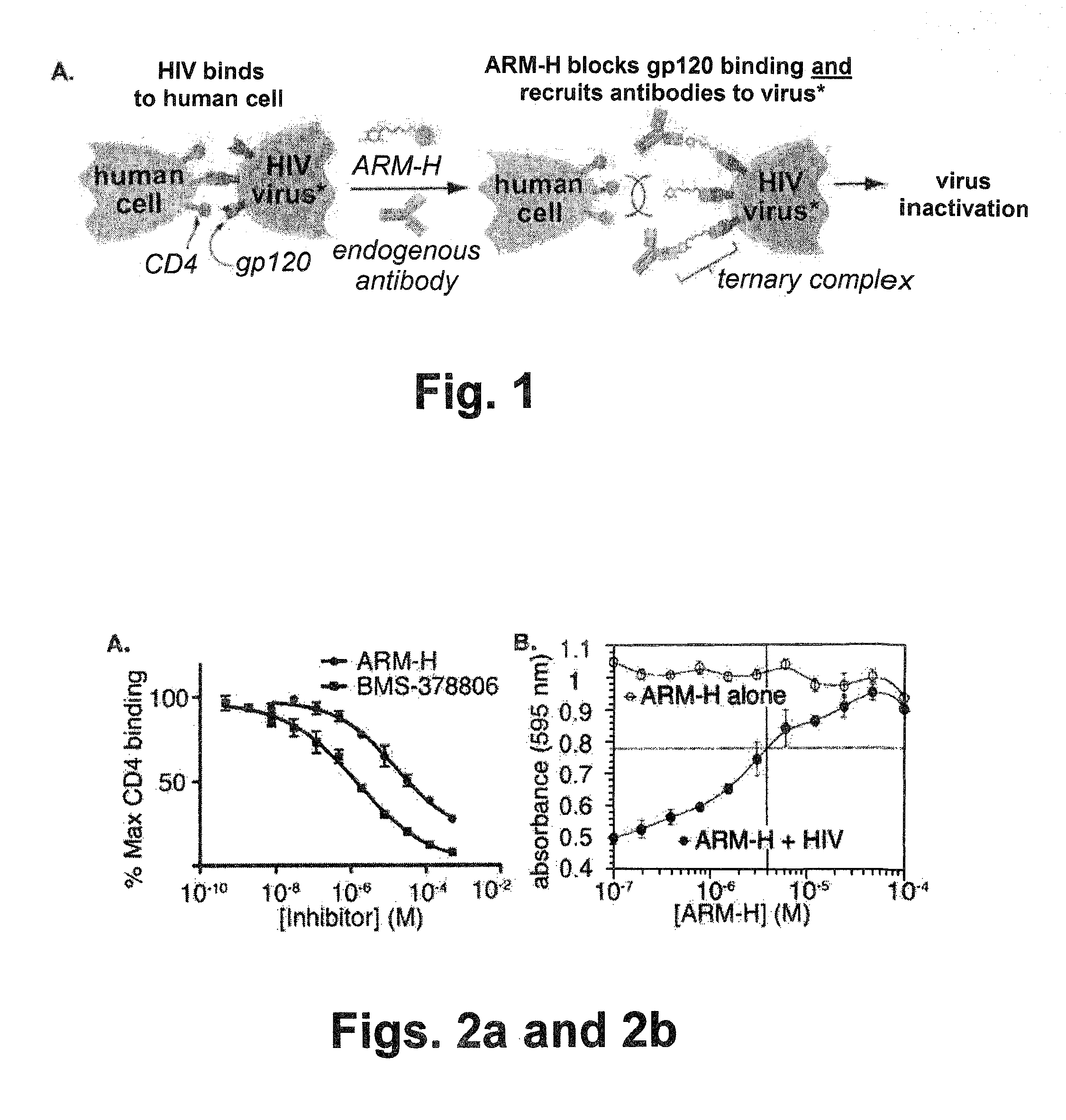
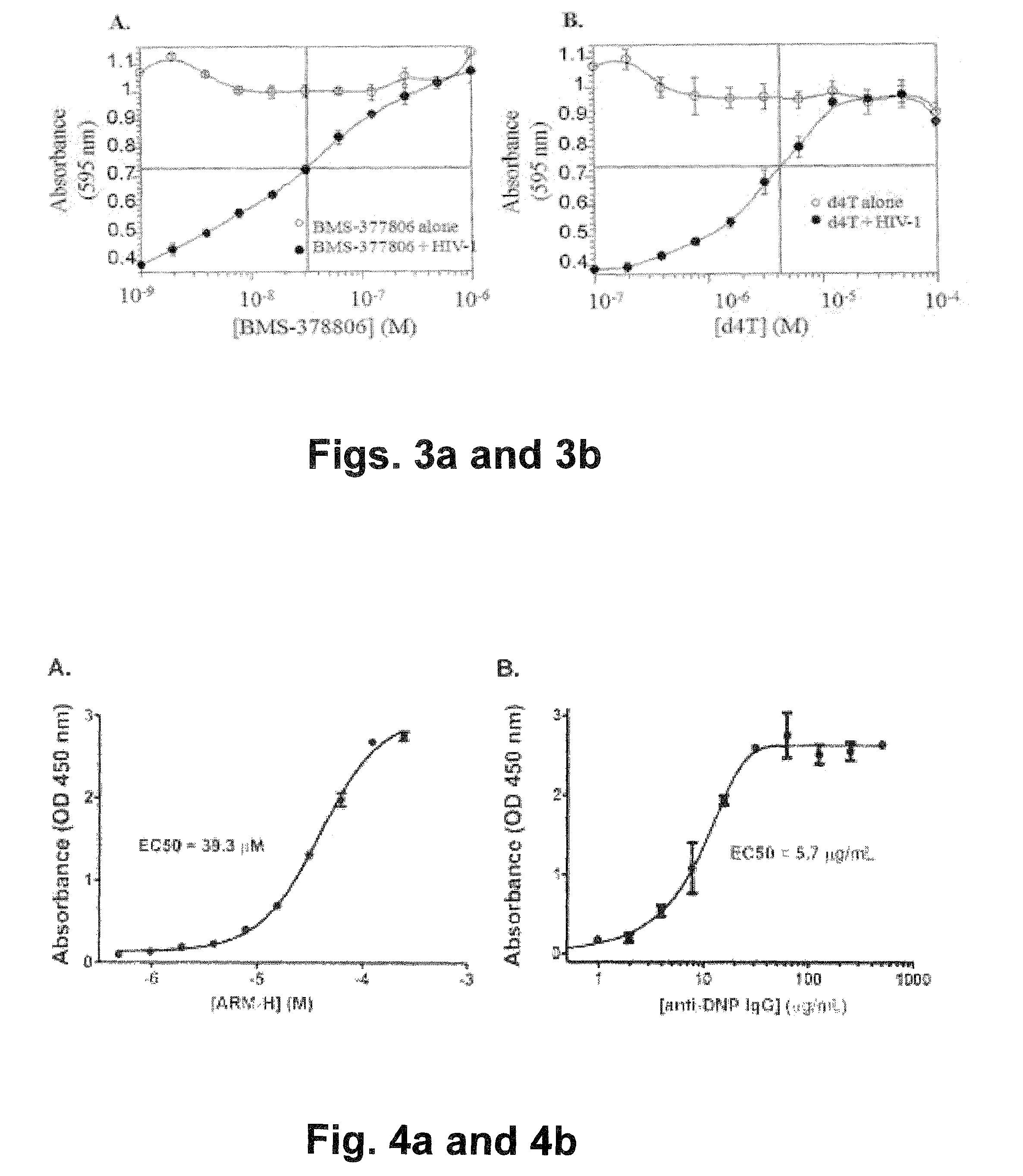
Bifunctional molecules with antibody-recruiting and entry inhibitory activity against the human immunodeficiency virus
ActiveUS9181224B2Reduce the possibilityAvoid infectionBiocideSugar derivativesTernary complexHuman immunodeficiency
The present invention is directed to new bifunctional compounds and methods for treating HIV infections. The bifunctional small molecules, generally referred to as ARM-H′ function through orthogonal pathways, by inhibiting the gp120-CD4 interaction, and by recruiting anti-DNP antibodies to gp120-expressing cells, thereby preventing cell infection and spread of HIV. It has been shown that ARM-H's bind to gp120 and gp-120 expressing cells competitively with CD4, thereby decreasing viral infectivity as shown by an MT-2 cell assay, the binding leading to formation of a ternary complex by recruiting anti-DNP antibodies to bind thereto, the antibodies present in the ternary complex promoting the complement-dependent destruction of the gp120-expressing cells. Compounds and methods are described herein.
Owner:YALE UNIV
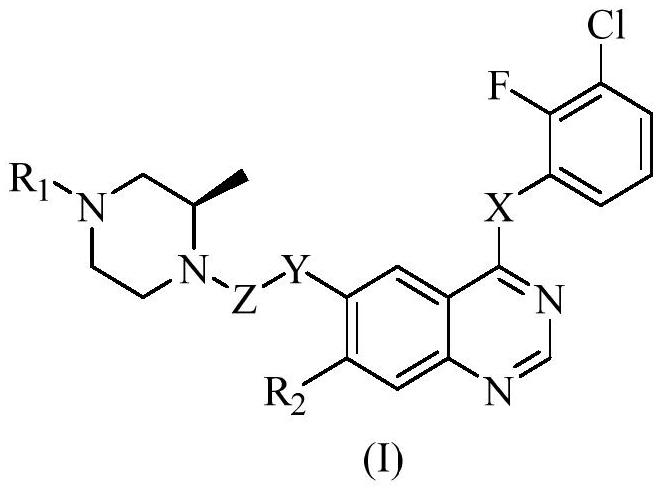
Polysubstituted quinazoline compound and application thereof
InactiveCN111848584AExcellent brain barrier penetration performanceGood pharmacodynamic propertiesOrganic active ingredientsOrganic chemistrySide effectMetabolic stability
The invention discloses a polysubstituted quinazoline compound and an application thereof, and belongs to the field of chemical medicines. The substituted quinazoline compound represented by the general formula (I) and the pharmaceutically acceptable salt thereof have excellent brain barrier permeability, enhanced metabolic stability and longer metabolic half-life period, show higher inhibitory activity on an activated or drug-resistant mutant form EGFR than a wild type EGFR, and can effectively reduce side effects.
Owner:JIANGNAN UNIV +1
Piperazine-piperidine compounds as hepatitis C virus inhibitors
The invention provides compounds of formula (I): wherein the variables are defined in the specification, or a pharmaceutically-acceptable salt thereof, that are inhibitors of replication of the hepatitis C virus. The invention also provides pharmaceutical compositions comprising such compounds, methods of using such compounds to treat hepatitis C viral infections, and processes and intermediates useful for preparing such compounds.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
Novel high-glycosylation erythropoietin immune fusion protein
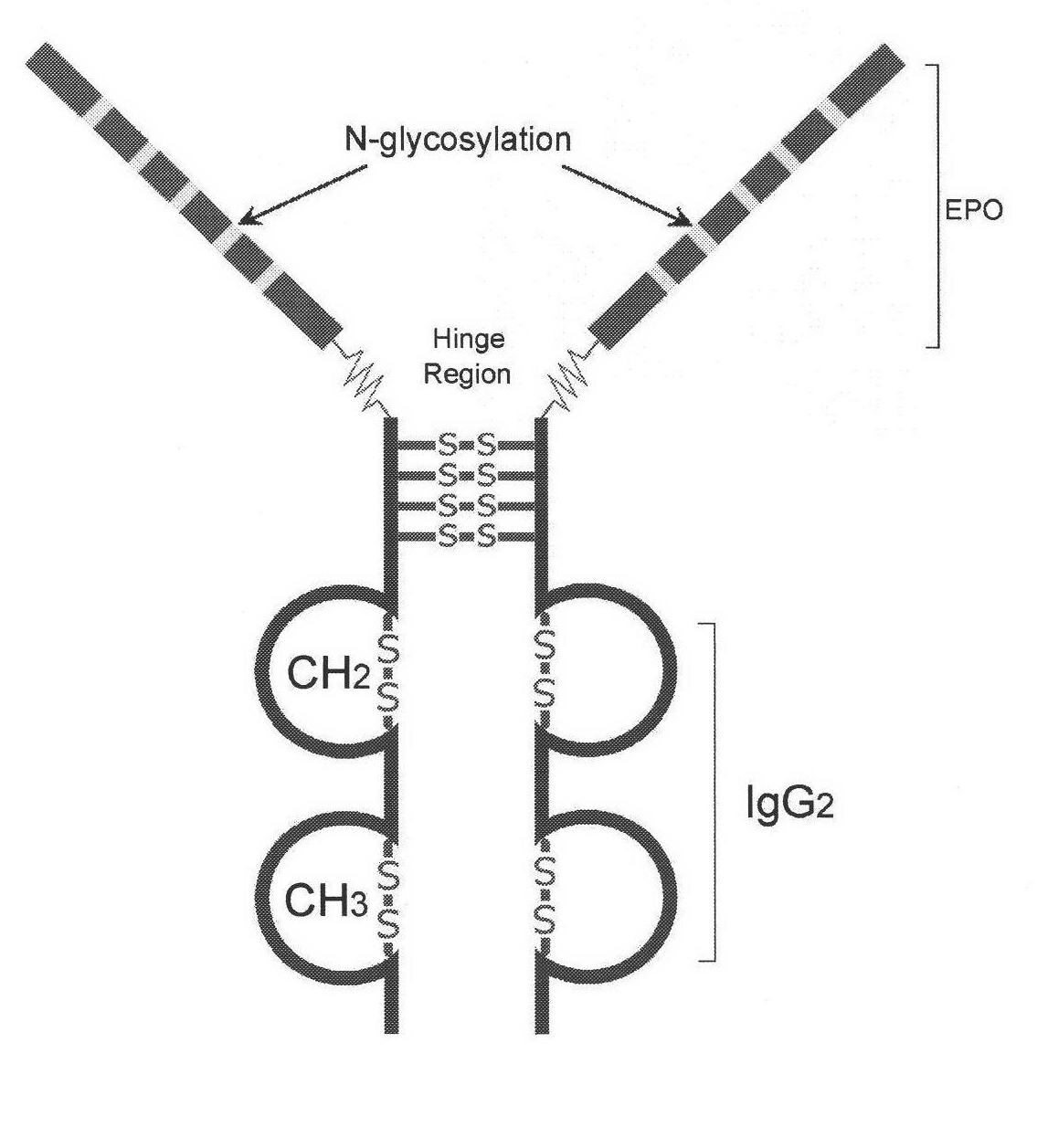
ActiveCN101870735AEffective treatmentHigh metabolic stabilityPeptide/protein ingredientsMacromolecular non-active ingredientsGenetic engineeringHalf-life
The invention discloses novel high-glycosylation erythropoietin immune fusion protein developed by a genetic engineering method, a polynucleotide for encoding the immune fusion protein and a method for preparing and purifying the immune fusion protein. The novel high-glycosylation erythropoietin immune fusion protein is dipolymer protein NESP-Fc formed by fusing new erythropoietin stimulating protein (NESP) with human IgG2-Fc. The immune fusion protein can be expressed efficiently in mammalian cells, has a simple purification process, and is favorable for further large-scale preparation. The immune fusion protein NESP-Fc has the biological activity which is similar to that of natural EPO, has long serum half-life period, and can be used for treating anemia caused by low EPO level.
Owner:BEIJING JINGYI TAIXIANG TECH DEV +1
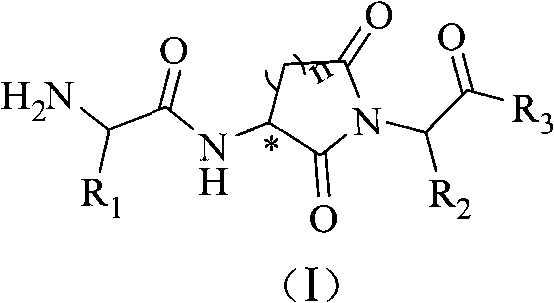
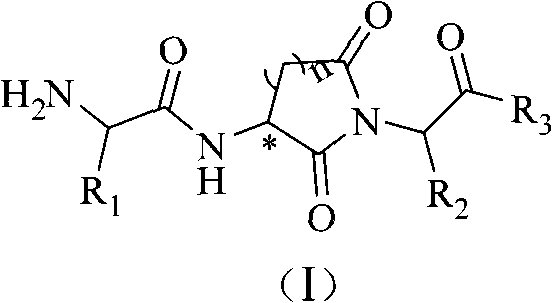
Alpha-amido acyl-ring imide peptoid metalloprotease inhibitor and application thereof
InactiveCN101538311AHigh selectivityGood compatibilityNervous disorderDipeptide ingredientsDiseaseImide
The invention relates to an alpha-amido acyl-ring imide peptoid metalloprotease inhibitor and an application thereof. The invention provides a powerful peptoid metalloprotease inhibitor which embodies outstanding selectivity between endopeptidases and exopeptidases so as to effectively treat diseases expressed by abnormal metalloprotease activity. Specifically, the invention relates to a peptoid compound with a structure of a general formula (I) and salt thereof acceptable on pharmacy. The invention also relates to a medical composition of the peptoid compound with the structure of the formula (I) and the pharmacy application thereof.
Owner:SHANDONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com