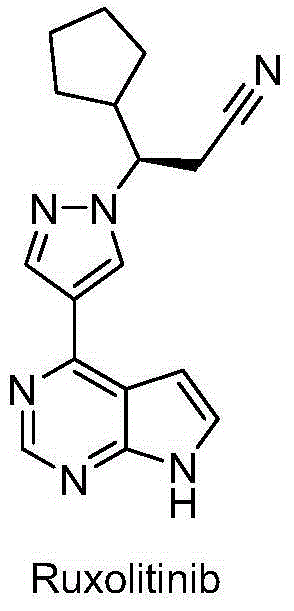
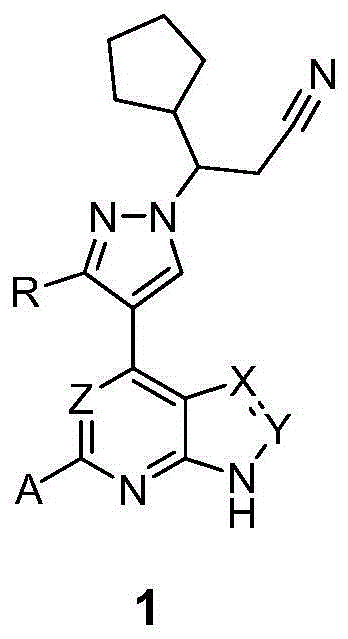
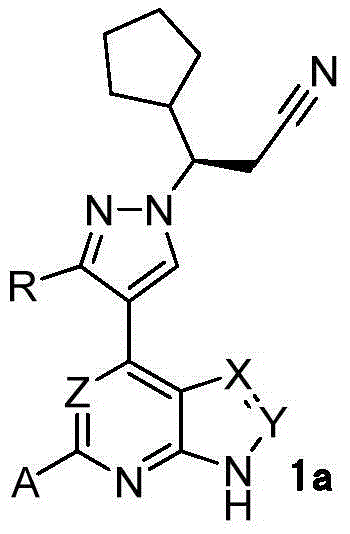
Novel heterocyclic compound and preparation method therefor and use thereof as kinase inhibitor
A compound and inert solvent technology, which is applied in the field of new heterocyclic compounds and its preparation, can solve the problems that patients cannot take medicine on time, affect the effect of drug treatment, and the patient's drug concentration fluctuates too much.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] A particularly preferred preparation process is as follows:
[0050]
[0051](1) At a certain temperature (such as 70-100°C), in an inert solvent (such as acetonitrile, DMF, DMSO, THF, etc.), in a base (such as DBU, triethylamine, diisopropylethylamine, pyridine, K 2 CO 3 、Na 2 CO 3 , NaOH, KOH, etc.), compound 2 and compound 3 were subjected to Michael addition reaction for a period of time (such as 4-20 hours) to obtain compound 4;
[0052] (2) At a certain temperature (such as 70-100 ° C), in an inert solvent (such as dioxane and water, toluene and water, DMSO, THF, DMF, etc.), in a catalyst (such as tetrakis (triphenylphosphine) ) palladium, three (dibenzylideneacetone) dipalladium (Pd 2 (dba) 3 ), bis(dibenzylideneacetone)palladium, dichlorobis(triphenylphosphine)palladium, triphenylphosphinepalladium acetate, bis(tri-orthophenylmethylphosphine)palladium dichloride, 1,2-bis( Diphenylphosphino) ethane dichloride palladium, etc.) and bases (such as potassiu...
Embodiment 1
[0075]
[0076] Compound 2 (250mg, 2.1mmol) and compound 10 (400mg, 2.1mmol) were placed in a 50mL single-necked bottle, under nitrogen protection, DBU (1g, 4.2mmol) was added dropwise at room temperature, and stirred evenly. Then heated to 80 ° C, the reaction 8h. After the reaction was completed, the solvent was spin-dried, and column chromatography (ethyl acetate:petroleum ether=5:1) gave 250 mg of a white solid with a yield of 36%.
[0077] 1 HNMR (CDCl 3 , 400MHz) δppm7.67 (s, 1H), 4.04 (td, 1H, J = 10.2Hz, 4.0Hz), 3.03 (dd, 1H, J = 8.4Hz, 17.2Hz), 2.84 (dd, J = 4Hz, 17.2Hz),2.49(m,1H),2.38(s,3H),1.89(m,1H),1.45-1.70(m,4H),1.20-1.32(m,15H).
[0078]
[0079] Compound 11 (160mg, 0.5mmol), compound 12 (153mg, 0.5mmol), tetrakistriphenylphosphine palladium (29mg, 0.025mmol) and potassium carbonate (207mg, 1.5mmol) were placed in a 50mL single-port reaction flask, nitrogen Under protection, 1,4-dioxane (4 mL) and H 2 O (1 mL), stirred until uniformly dispersed in t...
Embodiment 2
[0087]
[0088] Compound 17 (190 mg, 0.7 mmol) and compound 2 (169 mg, 1.4 mmol) were placed in a 50 mL single-necked bottle, and DBU (425 mg, 2.8 mmol) was added dropwise at room temperature under nitrogen protection, and stirred evenly. Then heated to 80 ° C, the reaction 8h. After the reaction was completed, the solvent was spin-dried, and column chromatography (ethyl acetate:petroleum ether=5:1) gave 60 mg of a colorless oil with a yield of 17%.
[0089] 1 HNMR (CDCl 3 , 400MHz) δppm7.56(s, 1H), 4.21(td, 1H, J=10.2Hz, 4.0Hz), 3.05(m, 1H), 2.85(m, 1H), 2.53(m, 1H), 1.93( m,1H),1.45-1.80(m,4H),1.20-1.32(m,15H).
[0090]
[0091] Compound 18 (60mg, 0.16mmol), compound 12 (48mg, 0.16mmol), tetrakistriphenylphosphine palladium (9mg, 0.008mmol) and potassium carbonate (207mg, 1.5mmol) were placed in a 50mL single-port reaction flask, nitrogen Under protection, 1,4-dioxane (4 mL) and H 2 O (1 mL), stirred until uniformly dispersed in the system, then heated to 80°C, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com