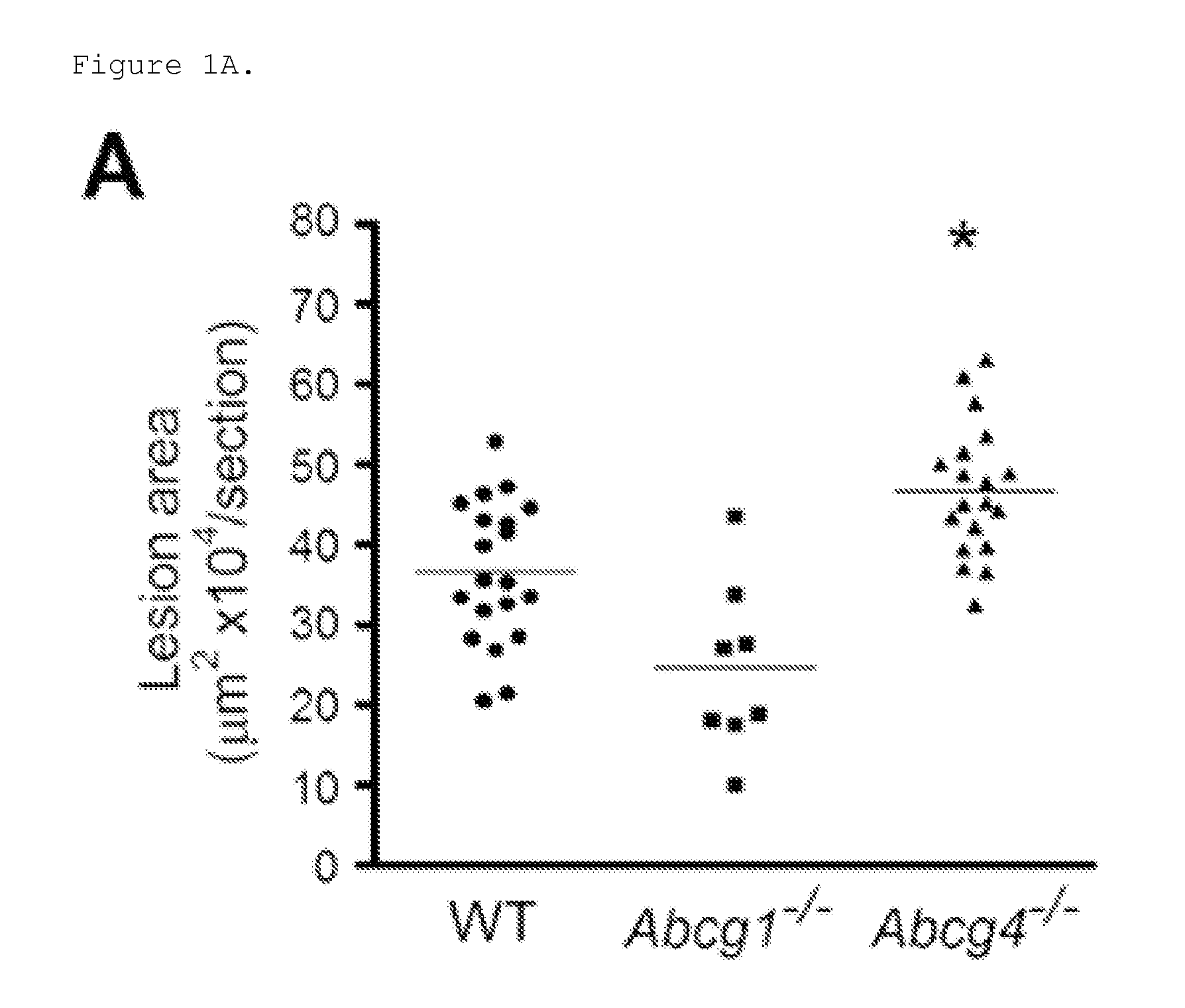
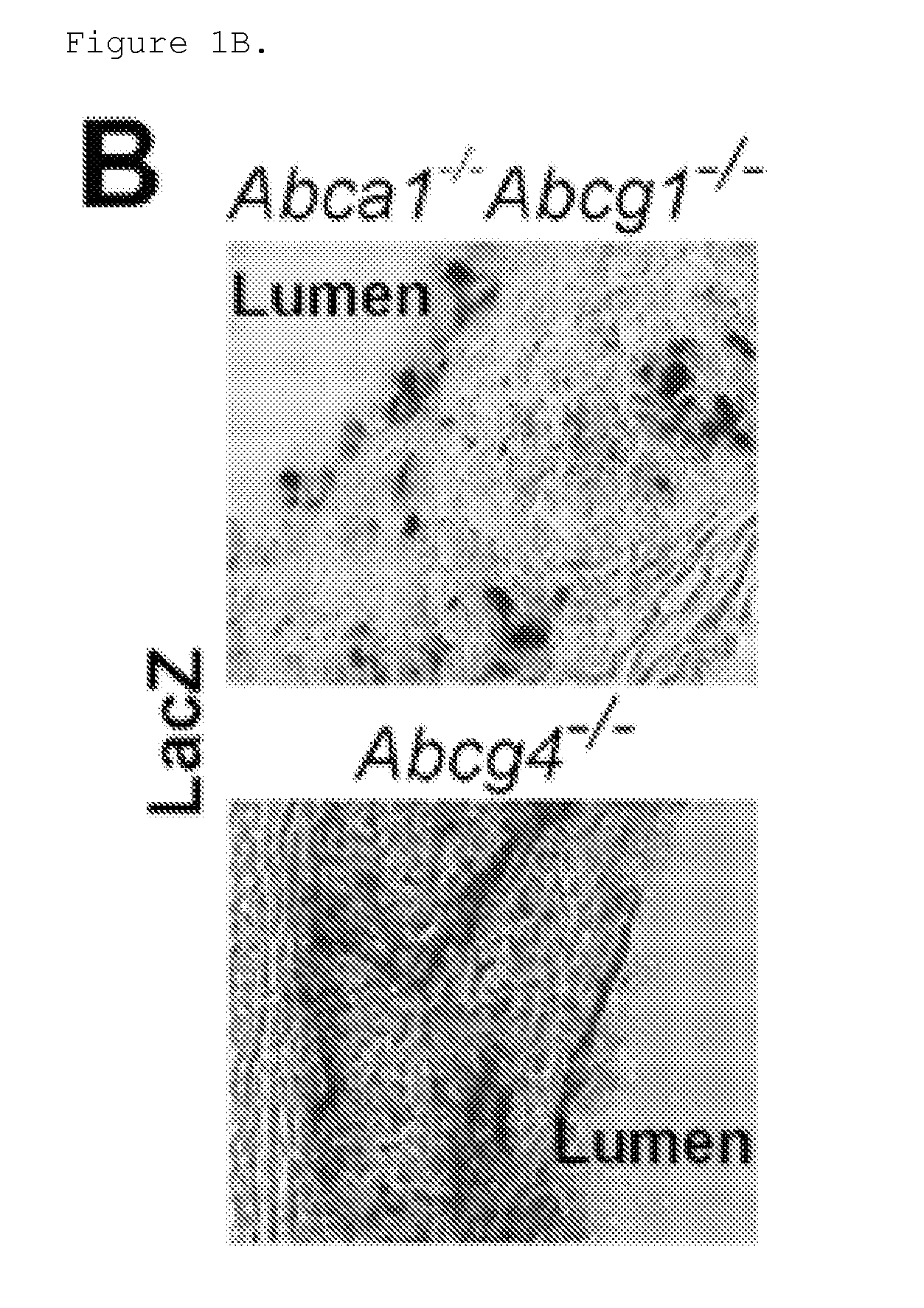
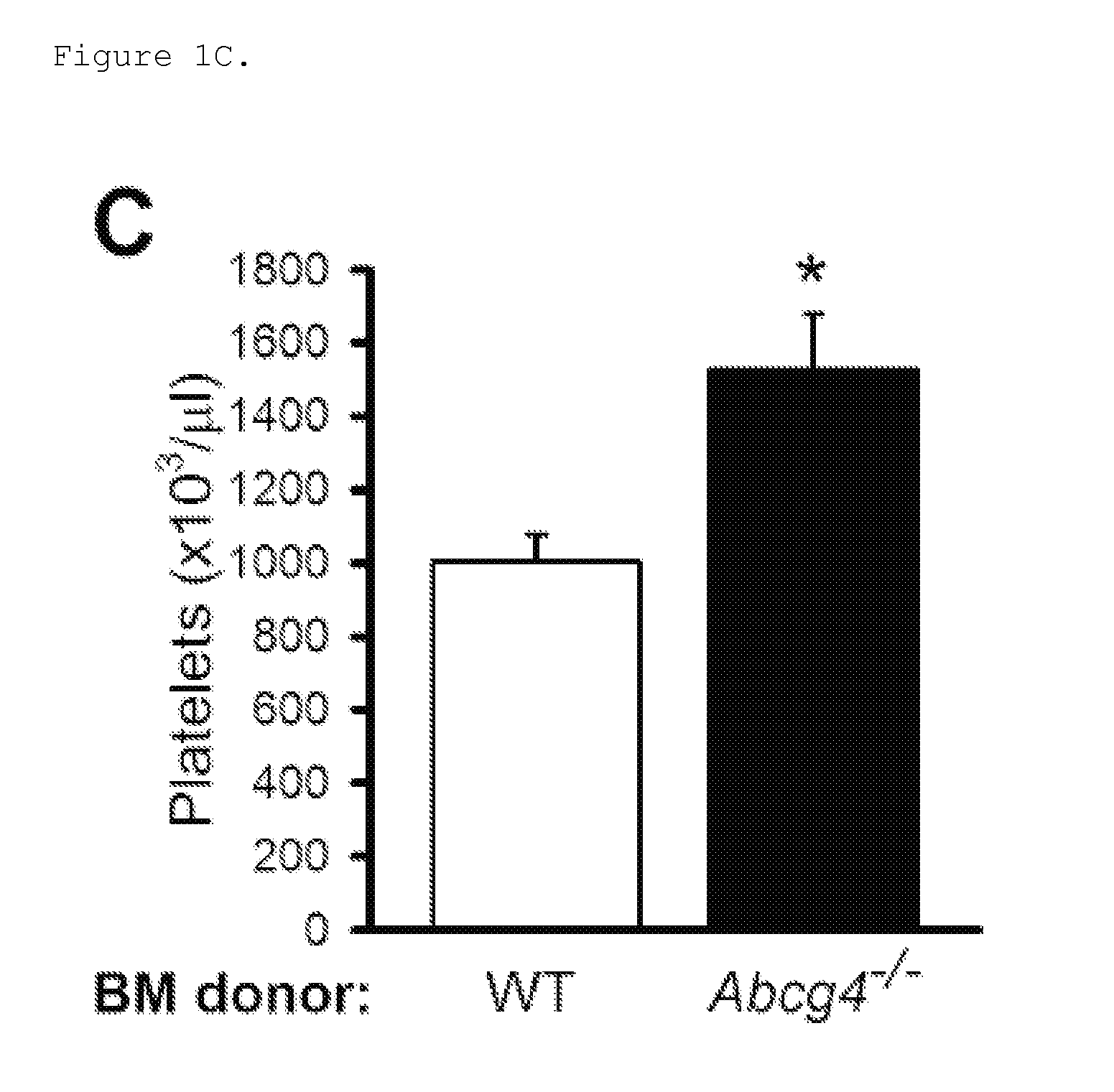
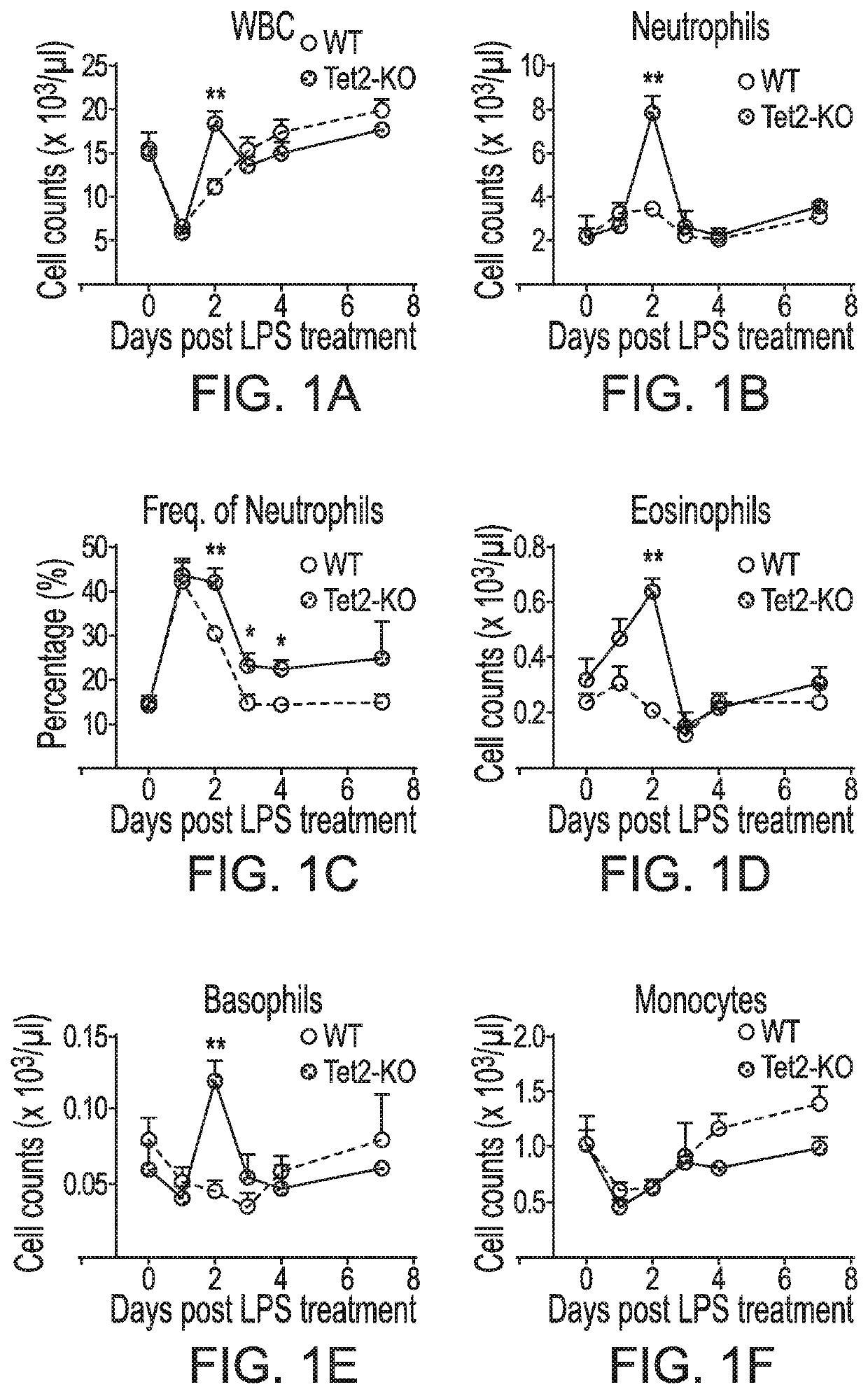
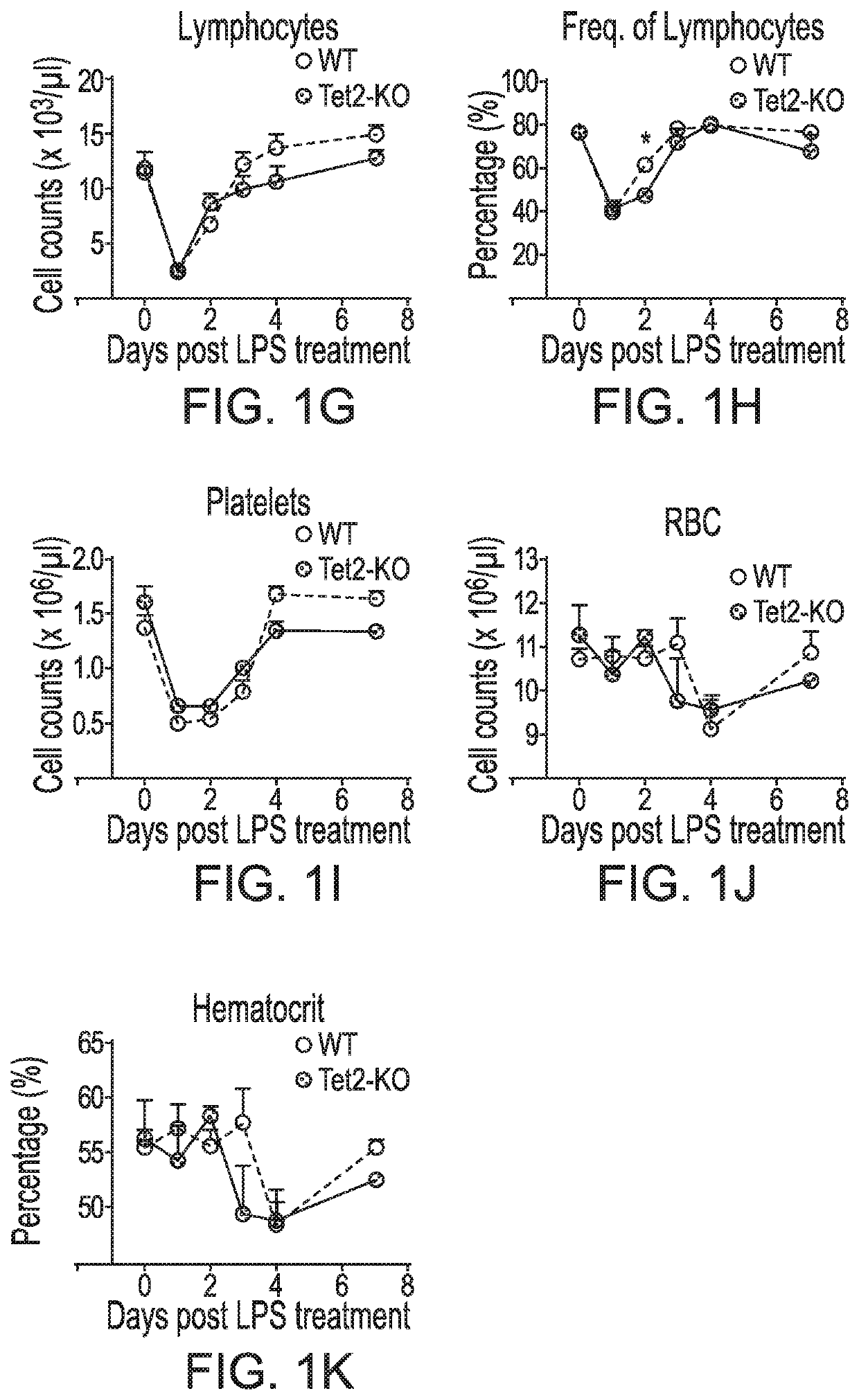
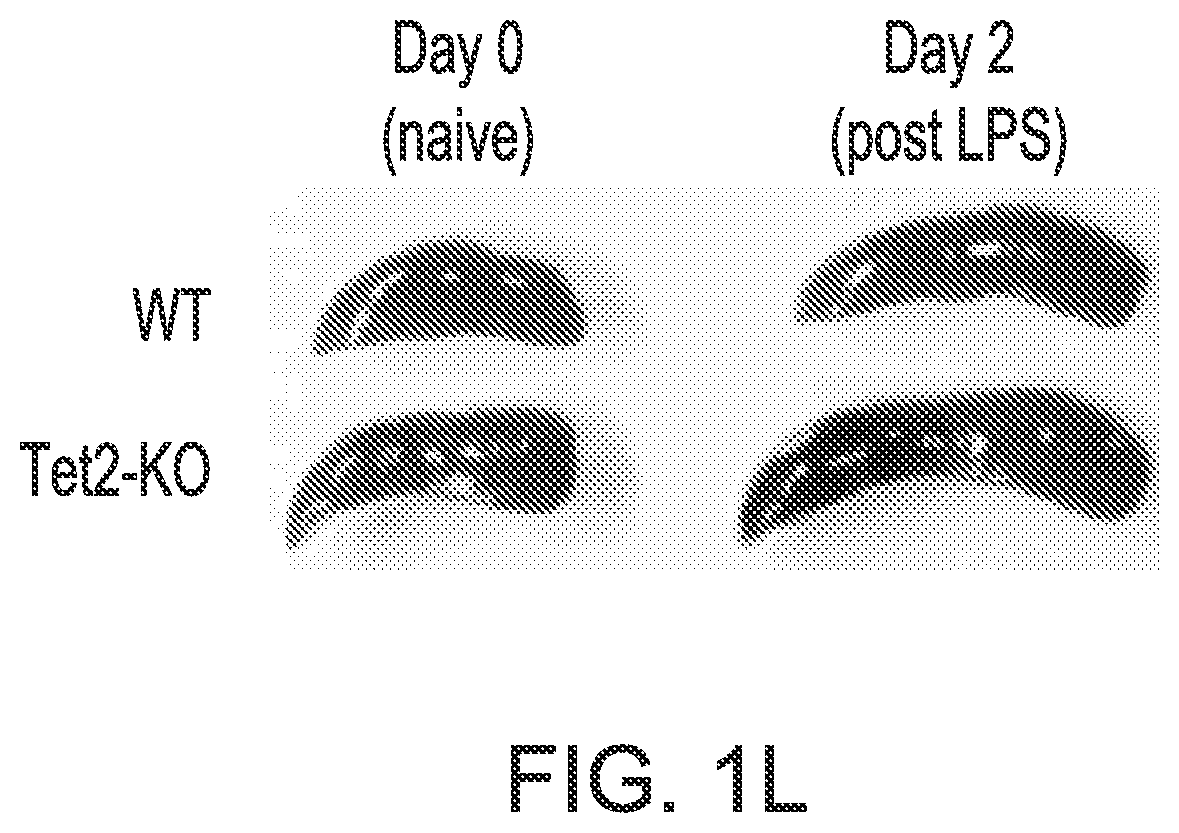
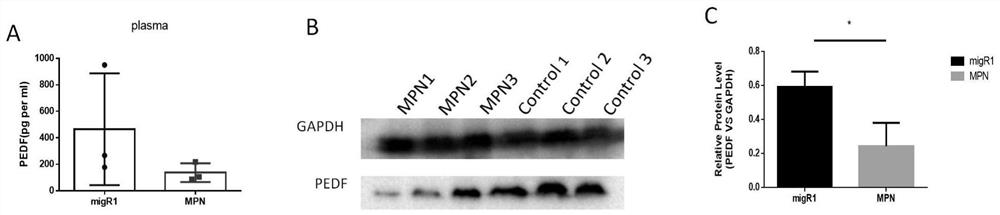
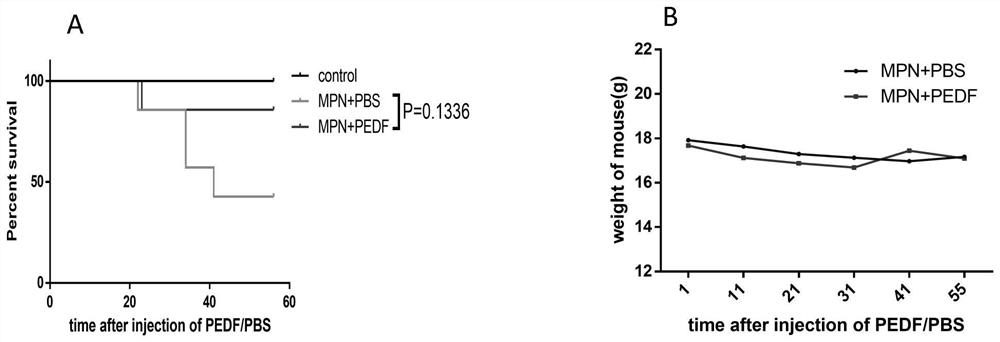
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "Myeloproliferative neoplasm" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The myeloproliferative neoplasms (MPNs), previously myeloproliferative diseases (MPDs), are a group of diseases of the bone marrow in which excess cells are produced. They are related to, and may evolve into, myelodysplastic syndrome and acute myeloid leukemia, although the myeloproliferative diseases on the whole have a much better prognosis than these conditions. The concept of myeloproliferative disease was first proposed in 1951 by the hematologist William Dameshek. In the most recent World Health Organization classification of hematologic malignancies, this group of diseases was renamed from "myeloproliferative diseases" to "myeloproliferative neoplasms". This reflects the underlying clonal genetic changes that are a salient feature of this group of disease.
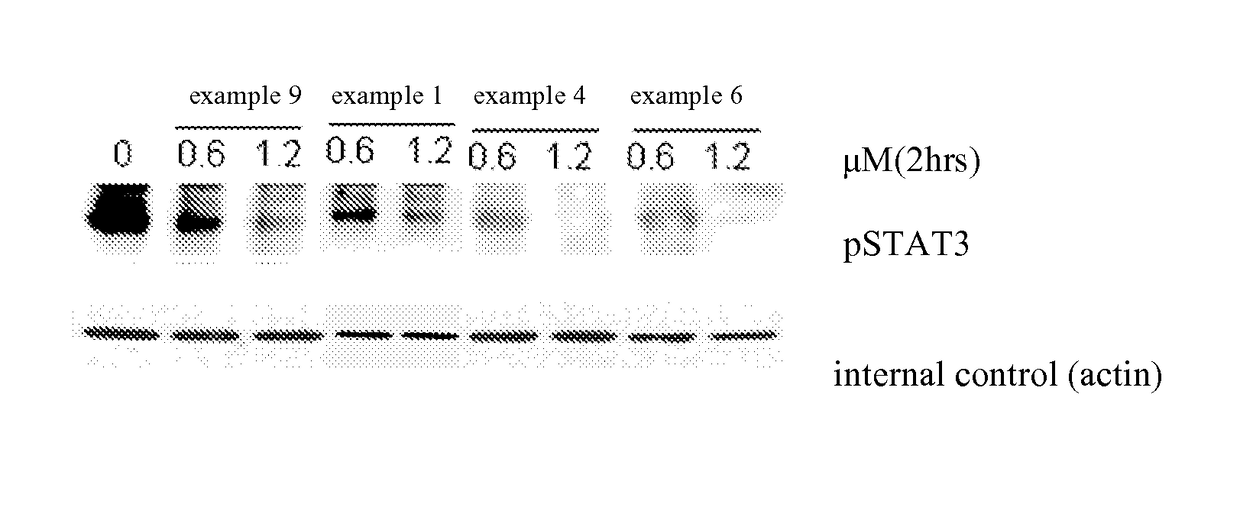
Methods and compositions for the treatment of myeloproliferative diseases and other proliferative diseases
Compounds of the present invention, alone and in combination with other active agents, find utility in the treatment of hyperproliferative diseases, mammalian cancers and especially human cancers including but not limited to for example malignant melanomas, myeloproliferative diseases, chronic myelogenous leukemia, acute lymphocytic leukemia, a disease caused by c-ABL kinase, oncogenic forms thereof, aberrant fusion proteins thereof and polymorphs thereof.
Owner:DECIPHERA PHARMA LLC
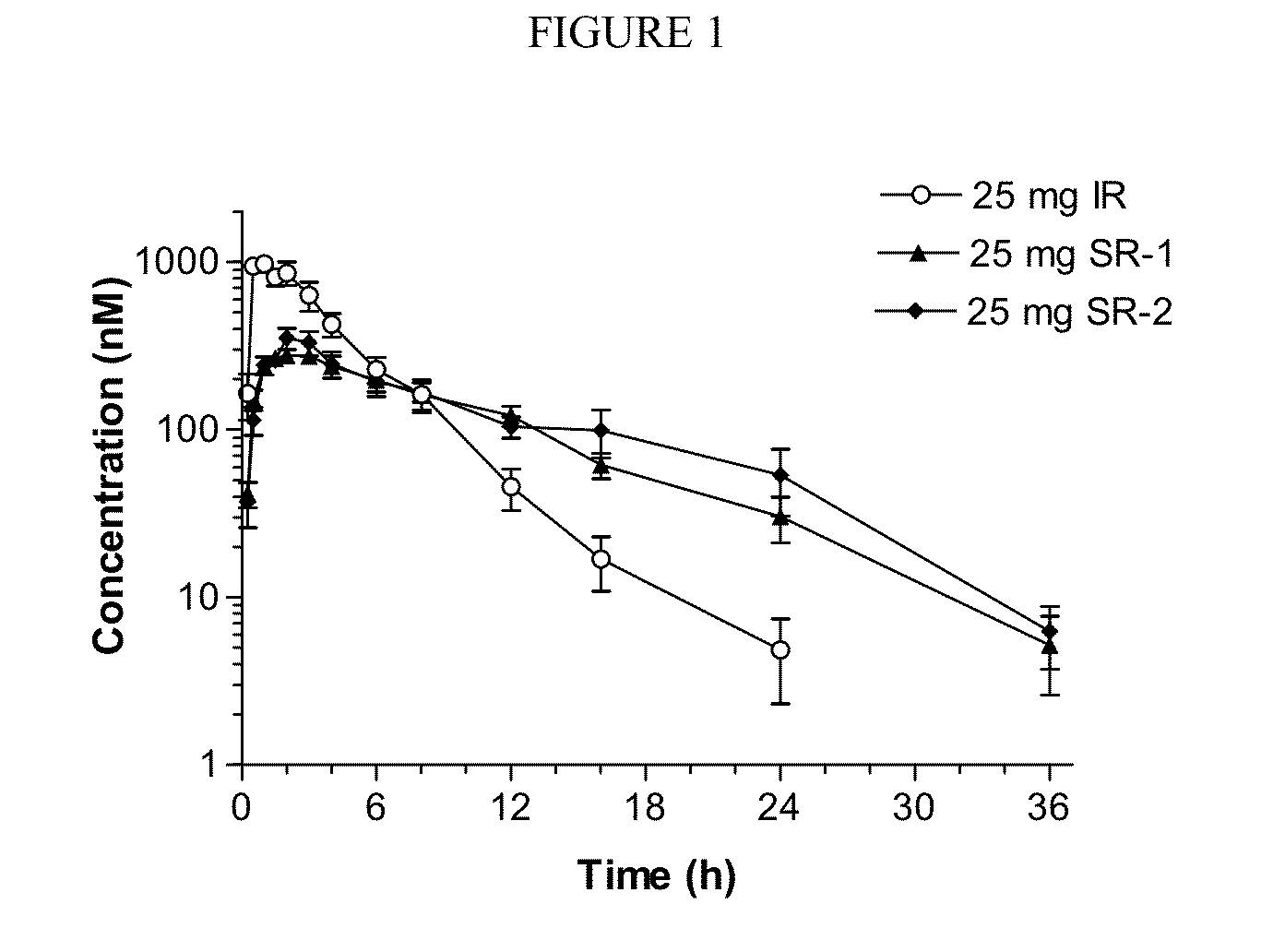
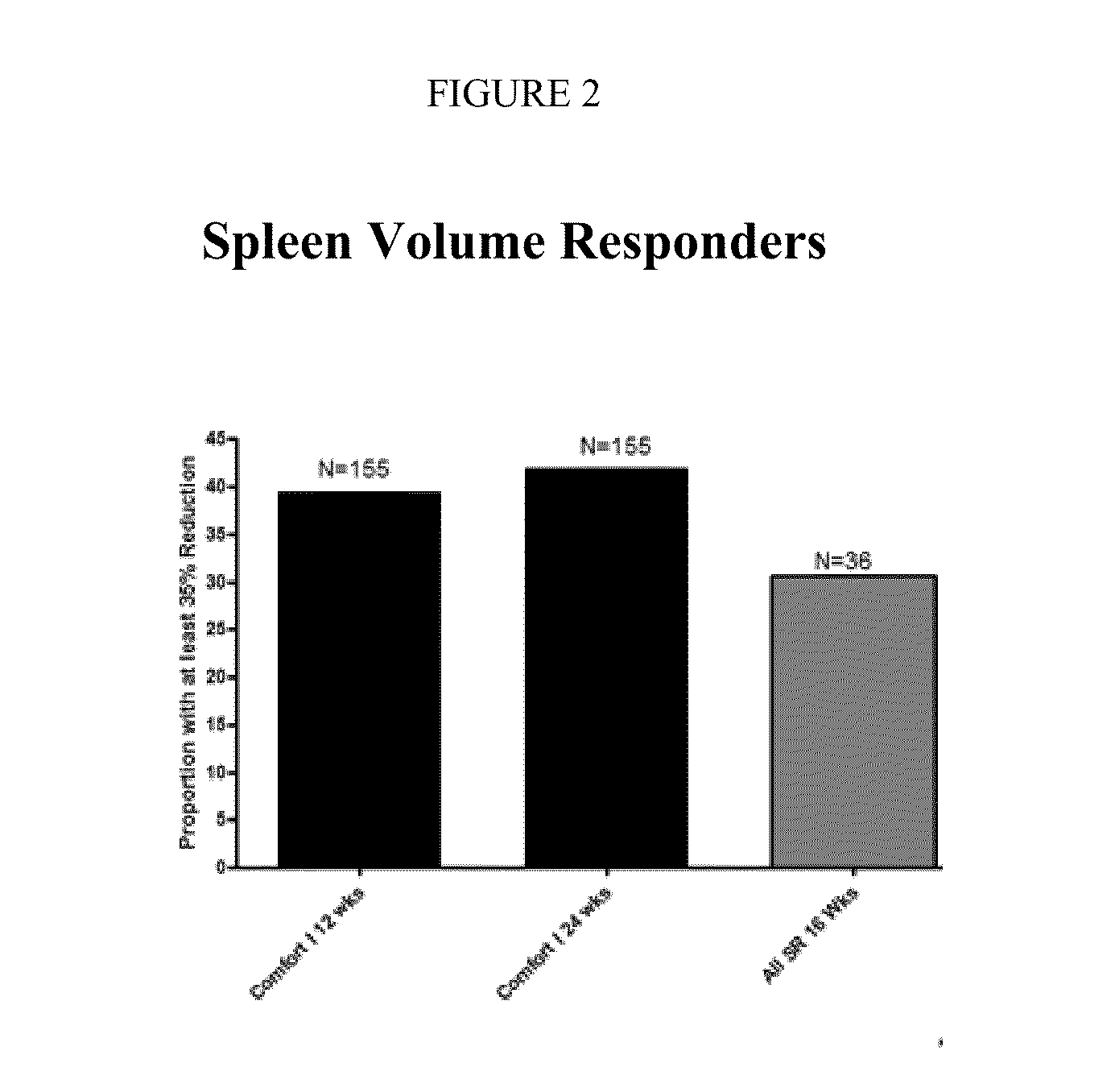
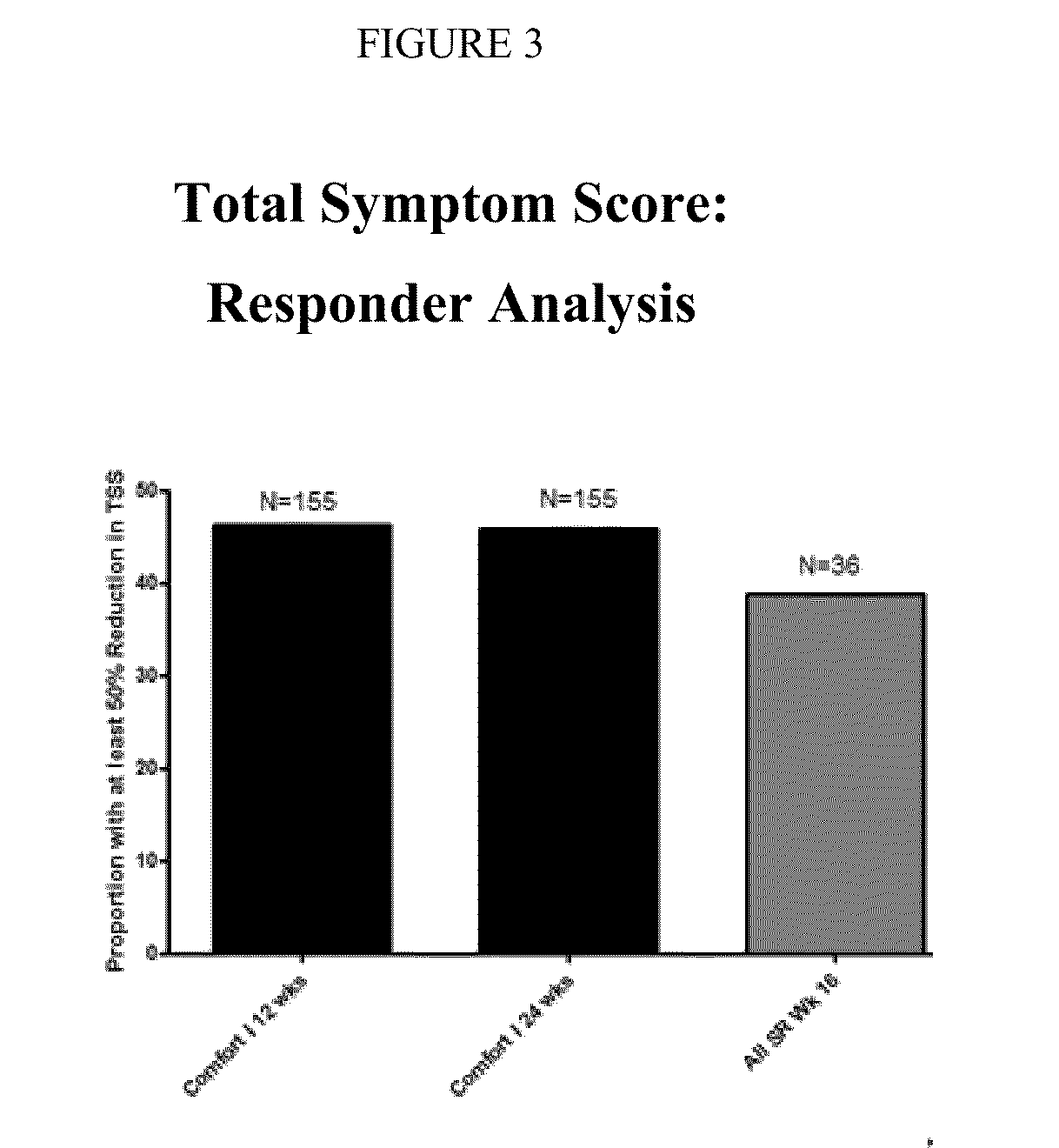
Sustained-release dosage forms of ruxolitinib
The present invention relates to sustained-release formulations and dosage forms of ruxolitinib, or a pharmaceutically acceptable salt thereof, which are useful in the treatment of Janus kinase-associated diseases such as myeloproliferative disorders.
Owner:INCYTE HLDG & INCYTE
N-acyl ureas exhibiting anti-cancer and anti-proliferative activities
Compounds of the present invention find utility in the treatment of mammalian cancers and especially human cancers including, but not limited to, malignant melanomas, solid tumors, glioblastomas, ovarian cancer, pancreatic cancer, prostate cancer, lung cancers, breast cancers, kidney cancers, hepatic cancers, cervical carcinomas, metastasis of primary tumor sites, myeloproliferative diseases, chronic myelogenous leukemia, leukemias, papillary thyroid carcinoma, non-small cell lung cancer, mesothelioma, hypereosinophilic syndrome, gastrointestinal stromal tumors, colonic cancers, ocular diseases characterized by hyperproliferation leading to blindness including various retinopathies, diabetic retinopathy, rheumatoid arthritis, asthma, chronic obstructive pulmonary disease, mastocytosis, mast cell leukemia, and diseases caused by PDGFR-α kinase, PDGFR-β kinase, c-KIT kinase, cFMS kinase, c-MET kinase, and oncogenic forms, aberrant fusion proteins and polymorphs of any of the foregoing kinases.
Owner:DECIPHERA PHARMA LLC
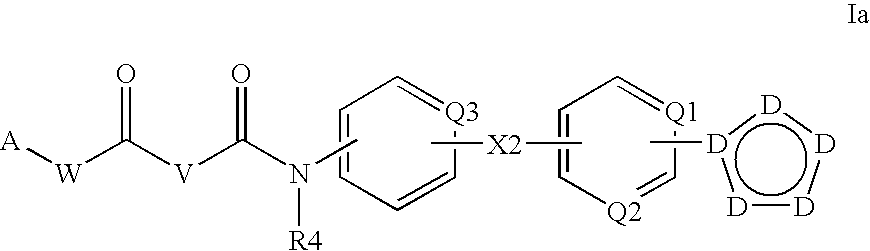
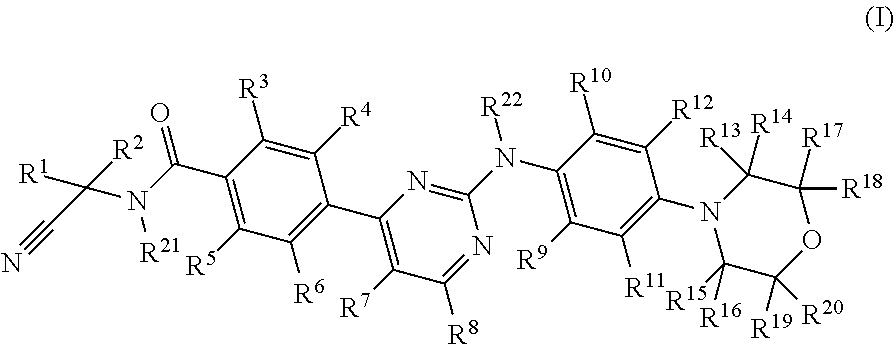
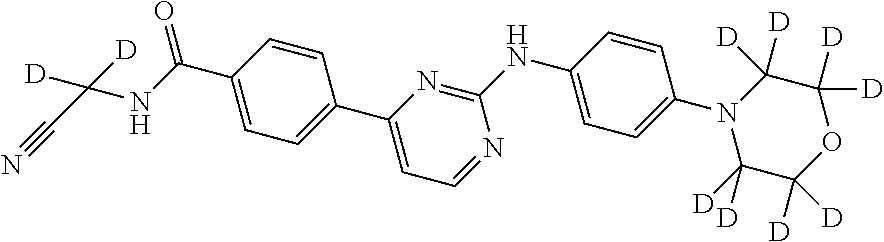
Deuterated phenylamino pyrimidine compound and drug composition containing the same
The present invention relates to a deuterated phenylamino pyrimidine compound and a drug composition containing the deuterated phenylamino pyrimidine compound. Particularly the present invention discloses a deuterated phenylamino pyrimidine compound represented by a formula (I), and a drug composition containing the deuterated phenylamino pyrimidine compound, or a crystal form, a pharmaceutically acceptable salt, a hydrate or a solvate thereof. The compound can be used for treating and / or preventing JAK kinase-associated diseases such as myeloproliferative diseases, cancers, immunologic diseases and the like.
Owner:SUZHOU ZELGEN BIOPHARML
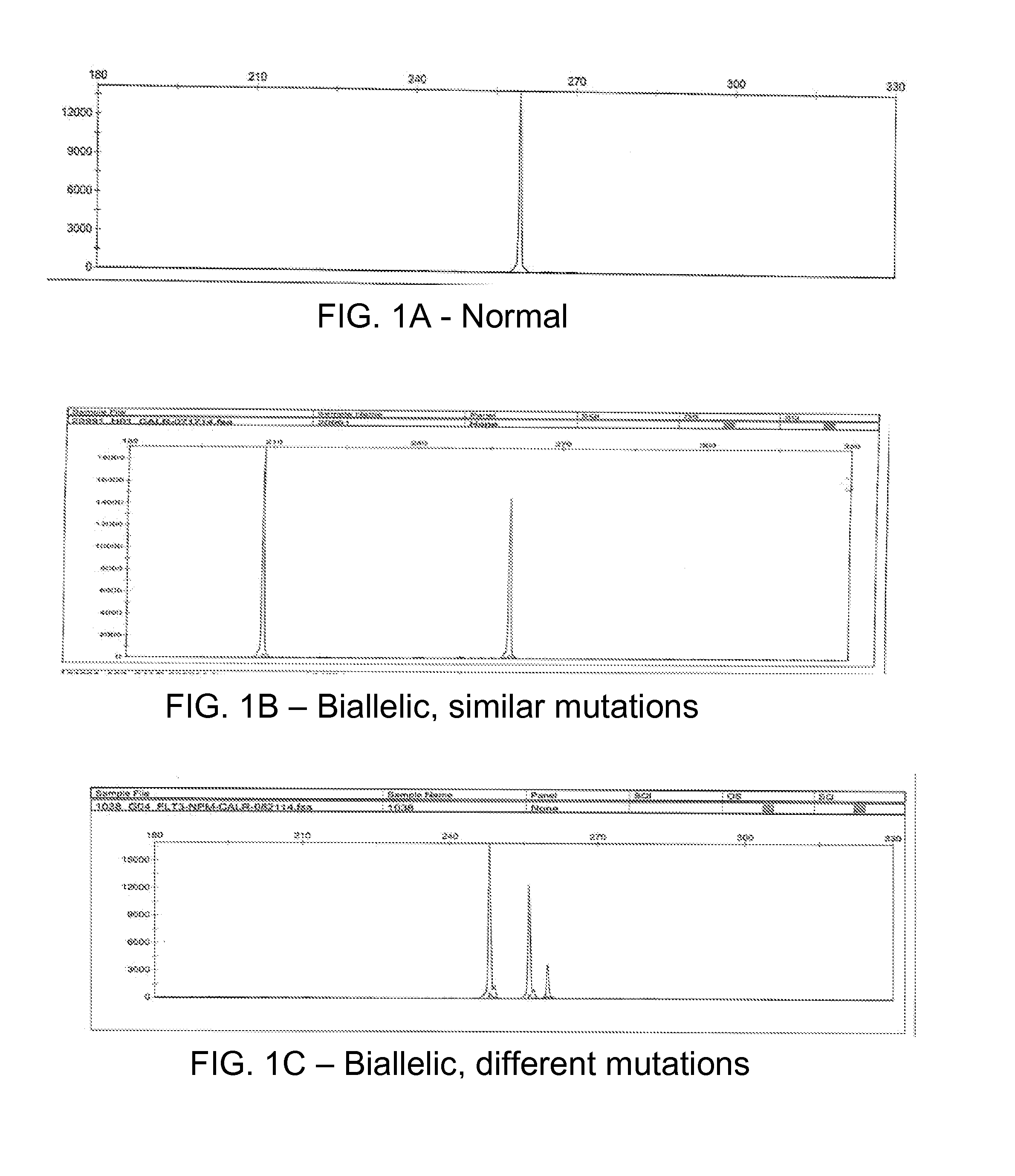
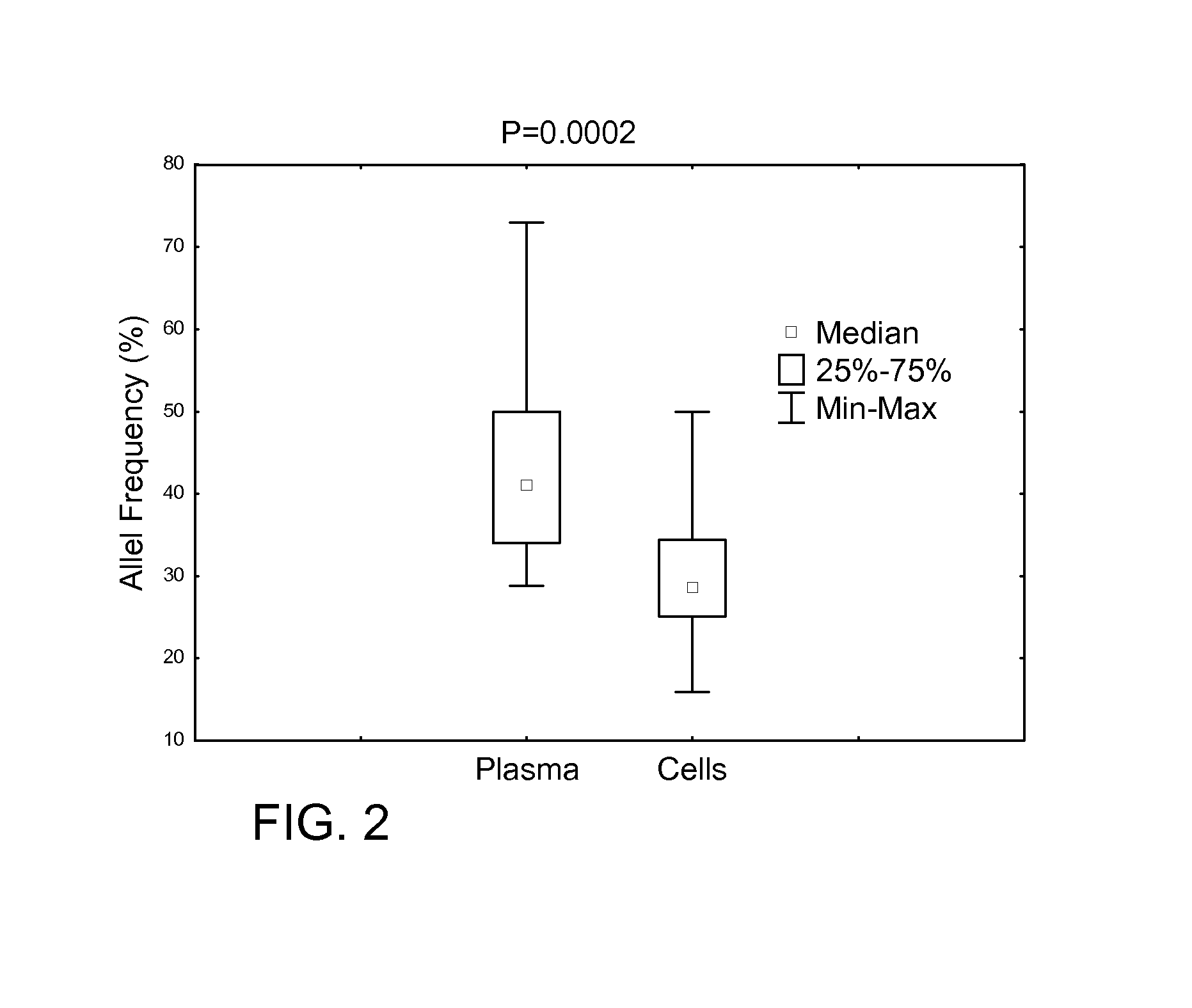
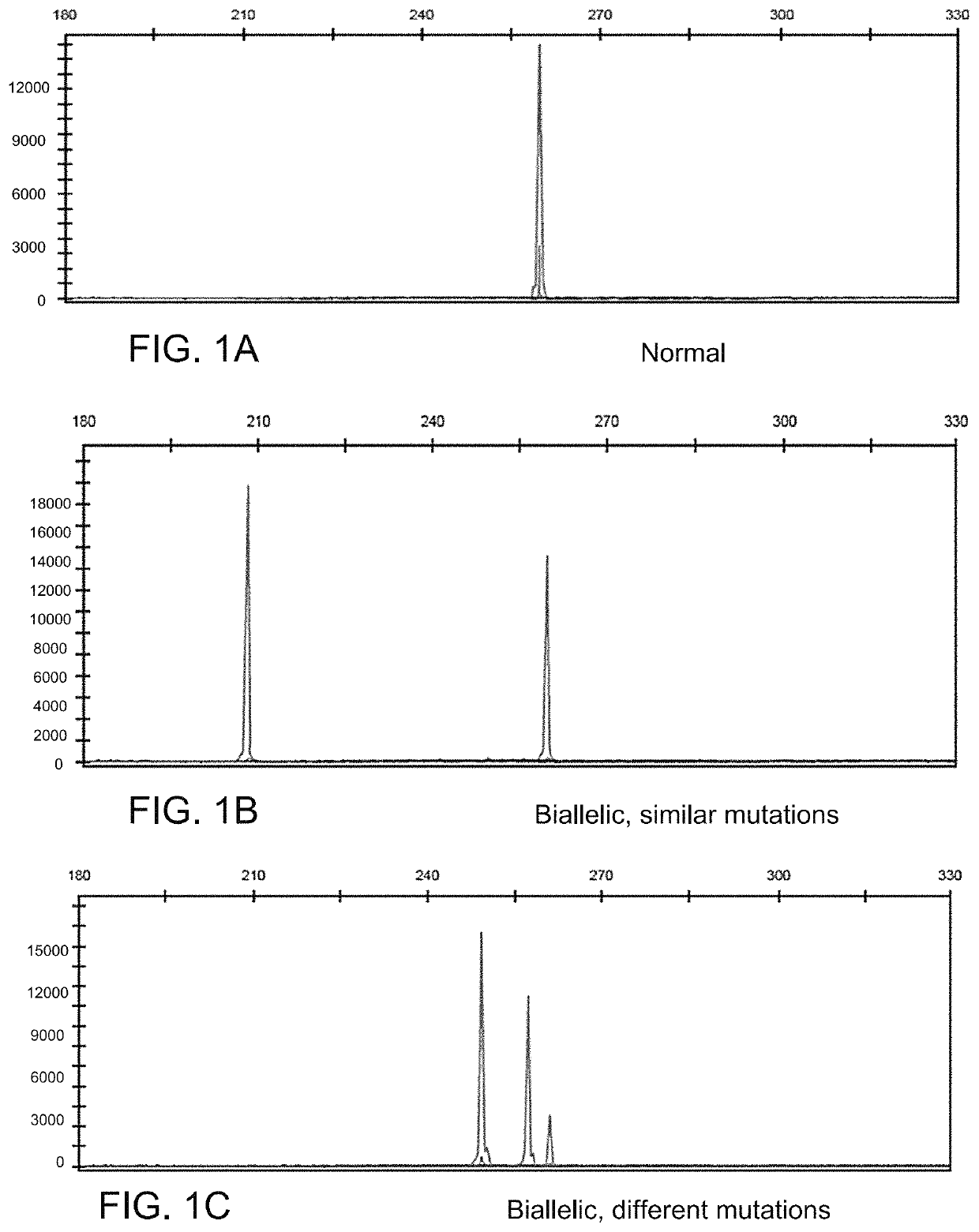
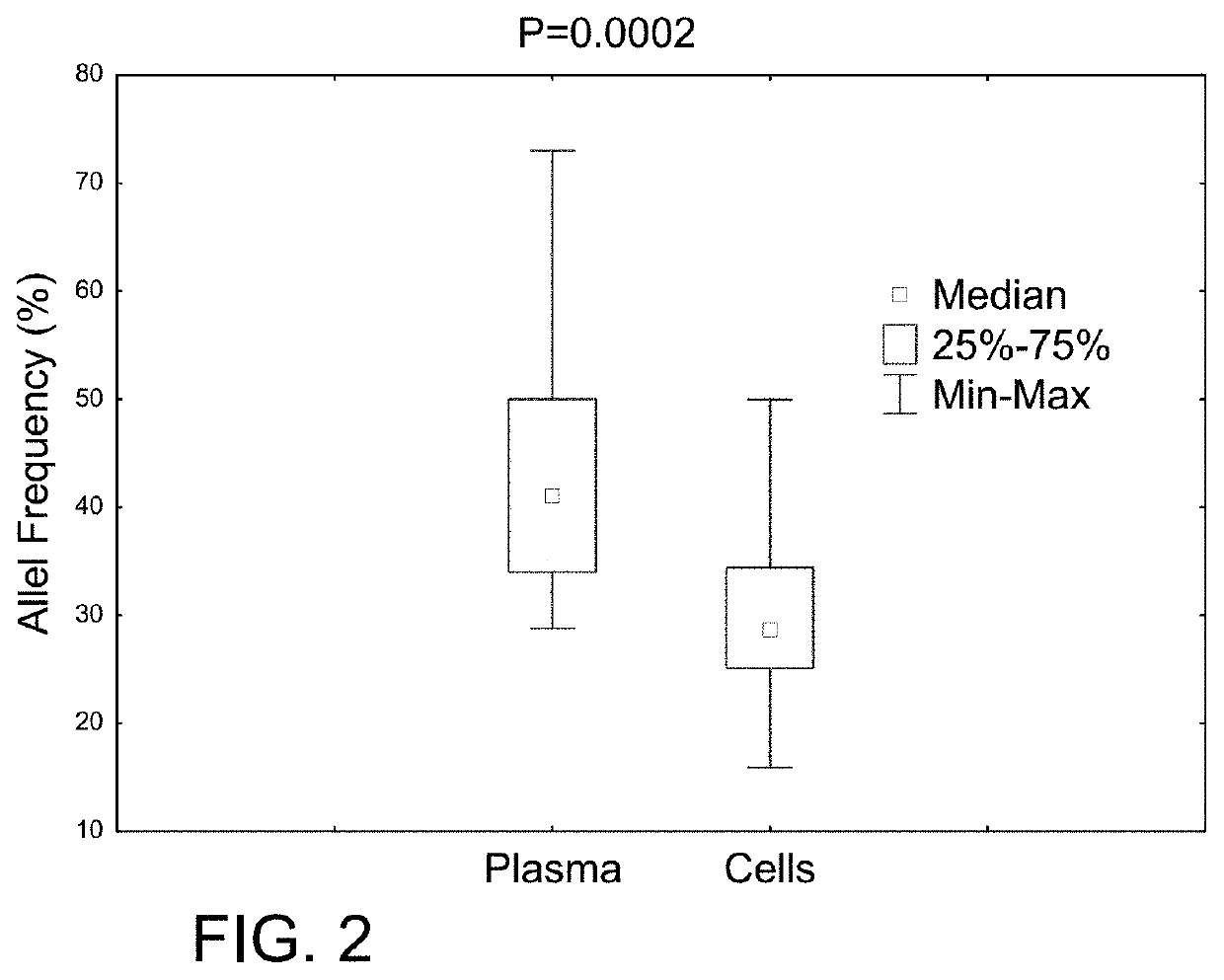
Determining tumor load and biallelic mutation in patients with calr mutation using peripheral blood plasma
ActiveUS20160130664A1Efficiently quantizedBiocideMicrobiological testing/measurementTumor LoadPlasma composition
Compositions and fragment length analysis methods are provided for detecting CALR mutations and determining tumor load in patients with myeloproliferative neoplasms.
Owner:NEOGENOMICS LAB
Using method and compositions comprising immunomodulatory compounds for treatment and management of myeloproliferative diseases
Methods of treating, preventing and / or managing a myeloproliferative disease are disclosed. Specific methods encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent, and / or the transplantation of blood or cells. Particular second active agents are capable of suppressing the overproduction of hematopoietic stem cells or ameliorating one or more of the symptoms of a myeloproliferative disease. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Substituted fused tricyclic compounds, compositions and medicinal applications thereof
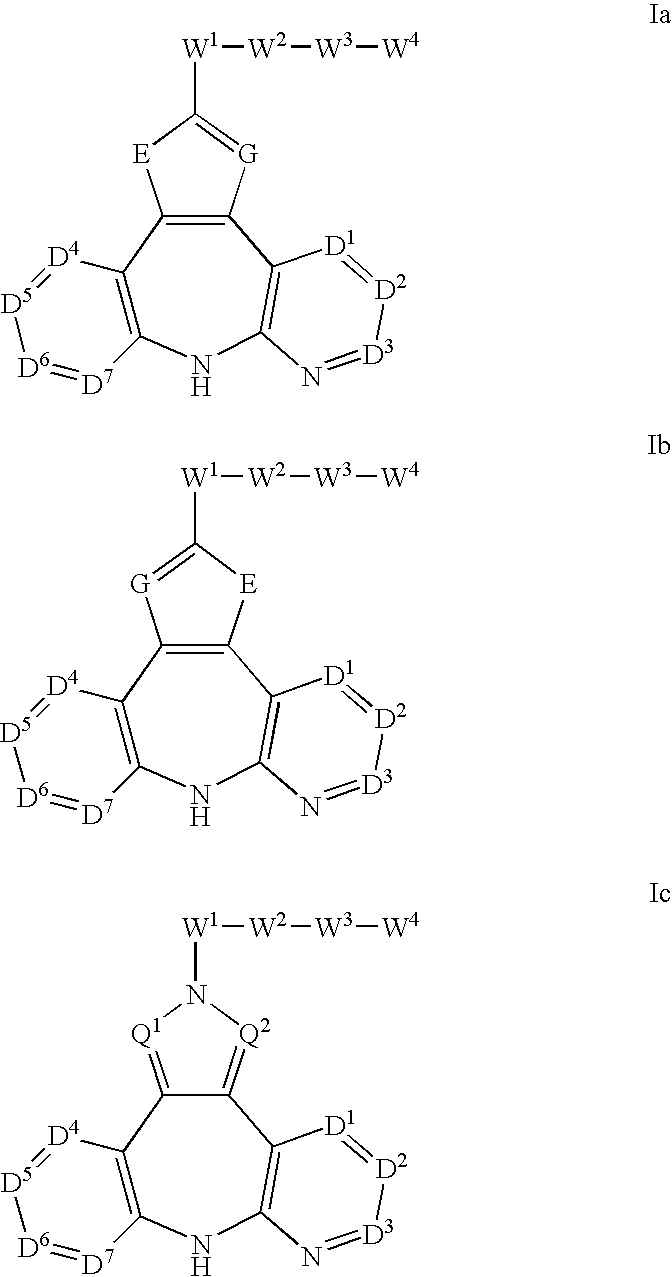
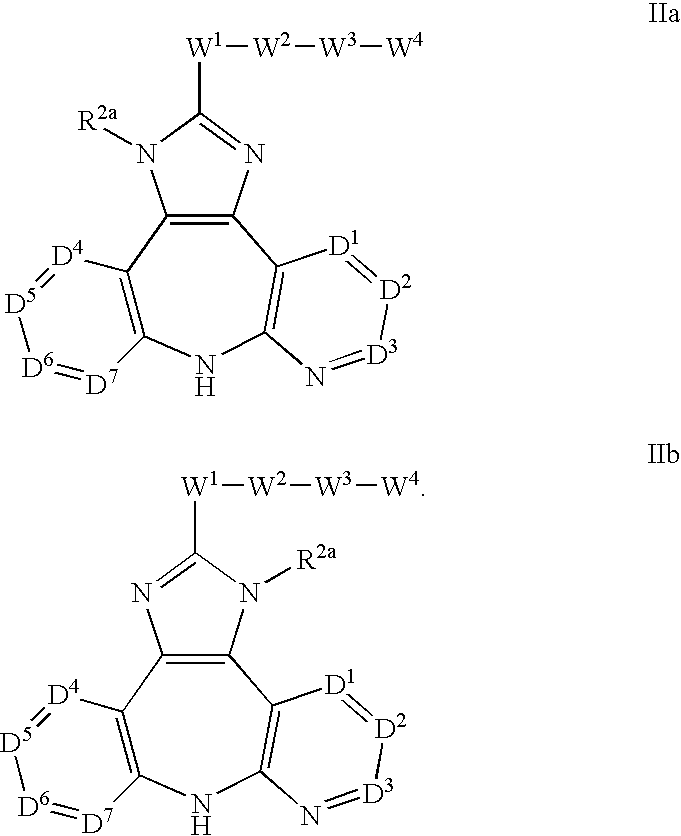
The present invention relates to substituted fused tricyclic compounds of formula (I) or (Ia), their tautomers, polymorphs, stereoisomers, prodrugs, solvates, co-crystals, pharmaceutically acceptable salts, pharmaceutical compositions containing them and methods of treating conditions and diseases that are mediated by JAK activity. The compounds of the present invention are useful in the treatment, prevention or suppression of diseases and disorders mediated by JAK activity. Such conditions include, but not limited to, arthritis, Alzheimer's disease, autoimmune thyroid disorders, cancer, diabetes, leukemia, T-cell prolymphocytic leukemia, lymphoma, myleoproliferation disorders, lupus, multiple myeloma, multiple sclerosis, osteoarthritis, sepsis, psoriatic arthritis, prostate cancer, T-cell autoimmune disease, inflammatory diseases, chronic and acute allograft transplant rejection, bone marrow transplant, stroke, asthma, chronic obstructive pulmonary disease, allergy, bronchitis, viral diseases, or Type I diabetes, complications from diabetes, rheumatoid arthritis, asthma, Crohn's disease, dry eye, uveitis, inflammatory bowel disease, organ transplant rejection, psoriasis and ulcerative colitis. The present disclosure also relates to process for the preparation of such compounds, and to pharmaceutical compositions containing them.
Owner:IMPETIS BIOSCI LTD
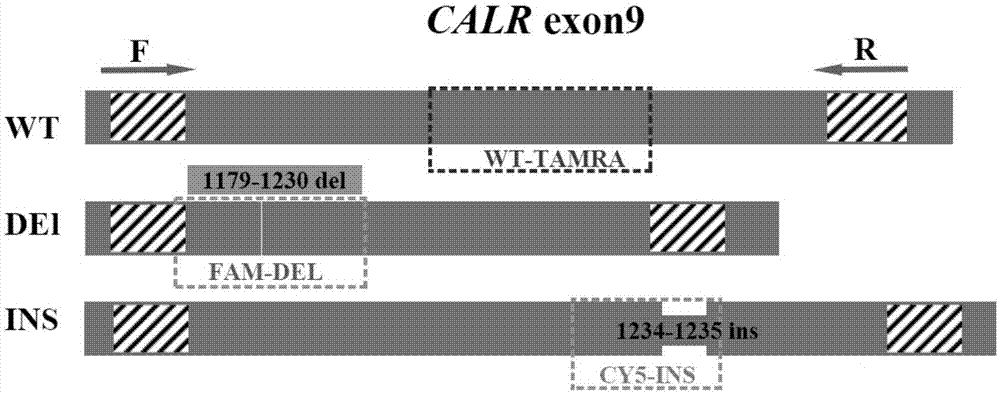
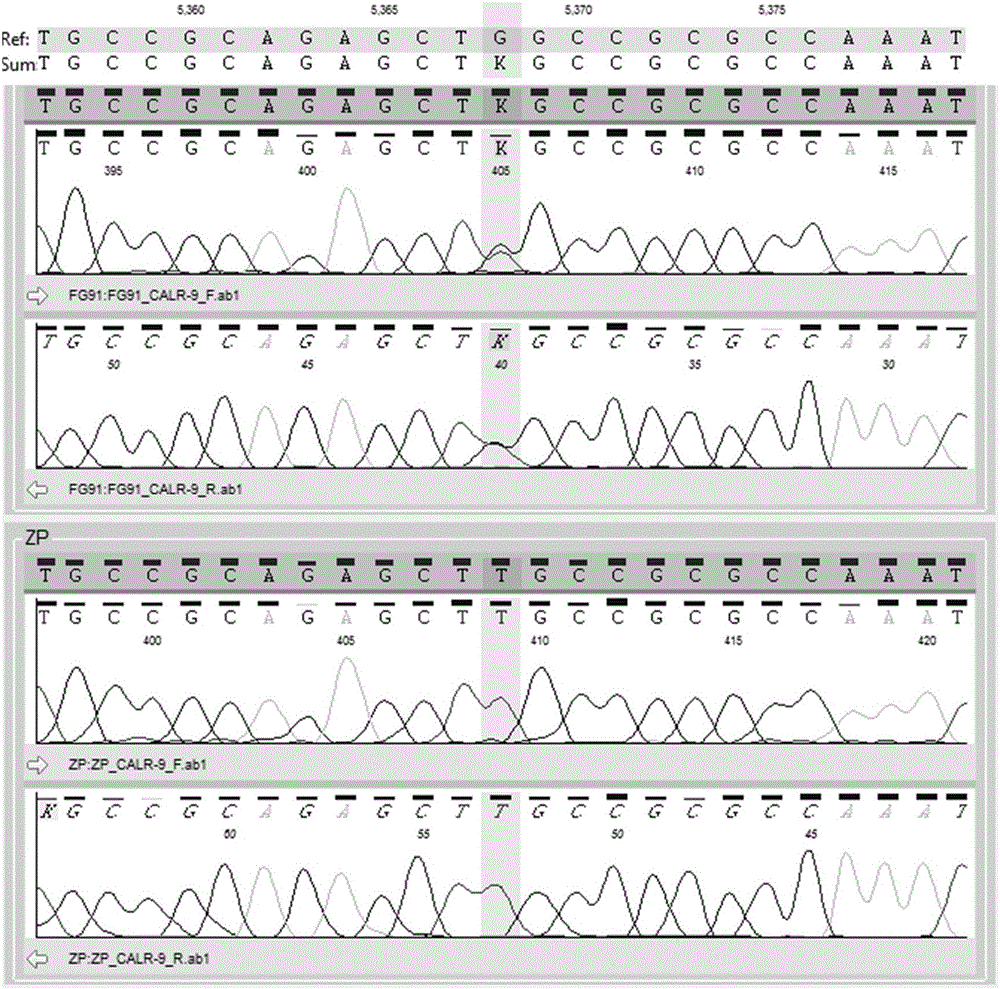
Detection method for CALR (Calreticulin) gene deletion and insertion mutation and kit
InactiveCN105441562AAccurate detectionImmediate observation of test resultsMicrobiological testing/measurementDNA/RNA fragmentationCalr geneSpecific detection
The invention provides a detection method for deletion and insertion mutation of L367fs*46 (c.1179-1230del) and K385fs*4(c.1234-1235insTTGTC) of the ninth exon of a CALR (Calreticulin) gene. Universal amplification primers are designed according to deletion and insertion mutation areas of the ninth exon of the CALR gene, specific detection fluorescence probes are designed according to amplified fragments, real-time fluorescence quantification PCR (polymerase chain reaction) is performed on a to-be-detected sample, and deletion and insertion mutation of the CALR gene are identified through multiple single-tube reactions. The invention further provides applications of the universal amplification primers and the specific detection fluorescence probes in preparation of myeloproliferative neoplasm detection reagents as well as a kit for detecting myeloproliferative neoplasms.
Owner:DIASYS DIAGNOSTIC SYST SHANGHAI
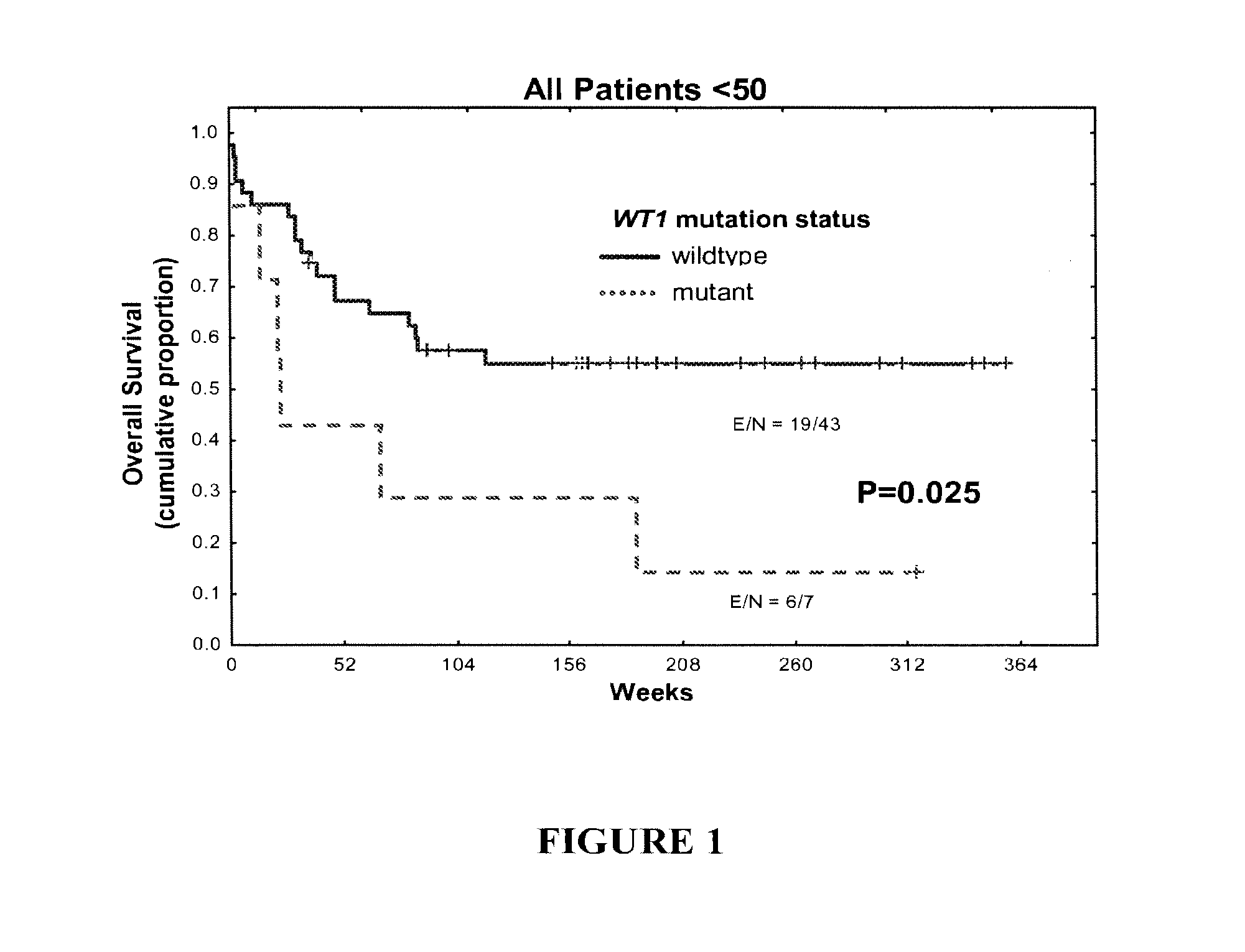
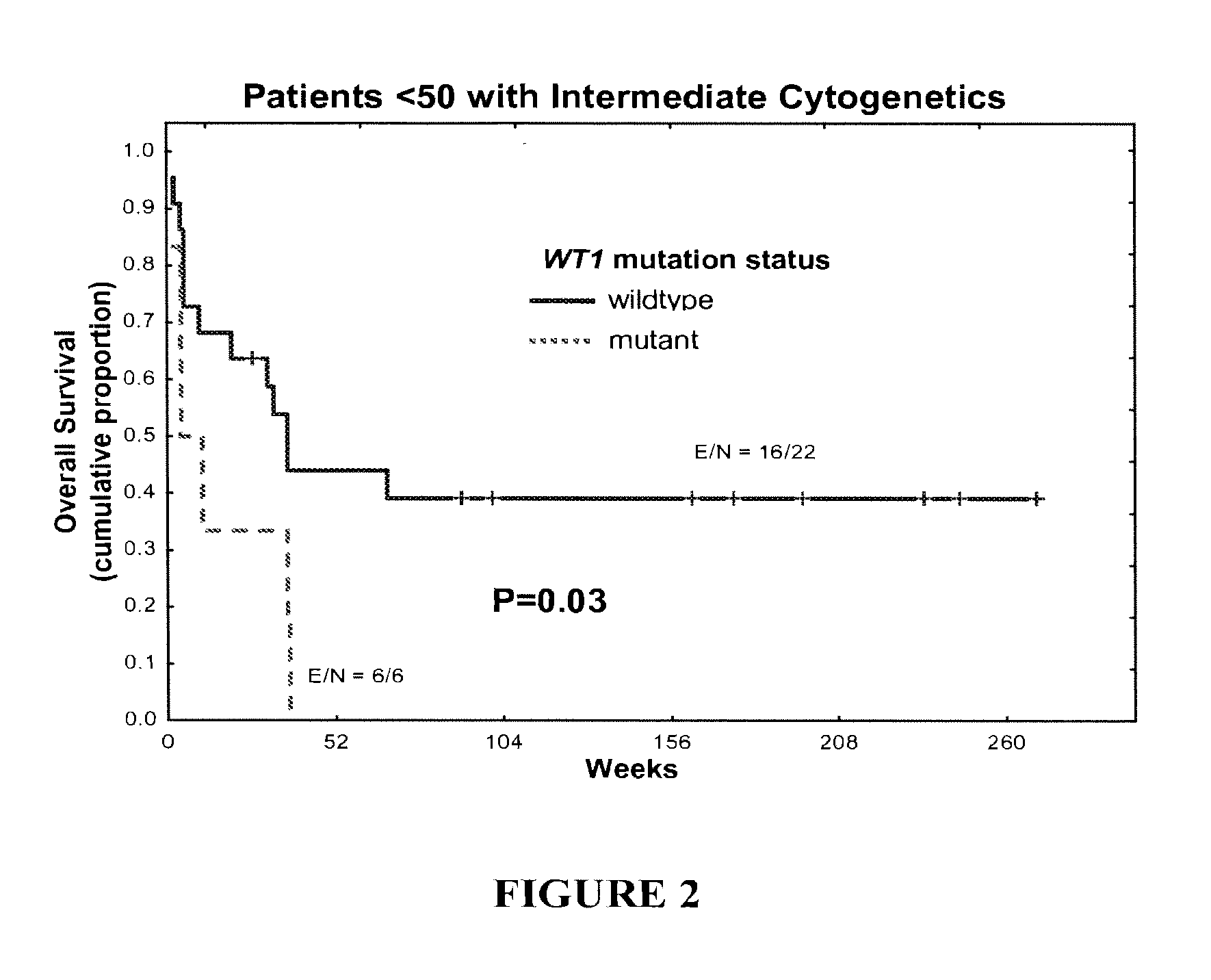
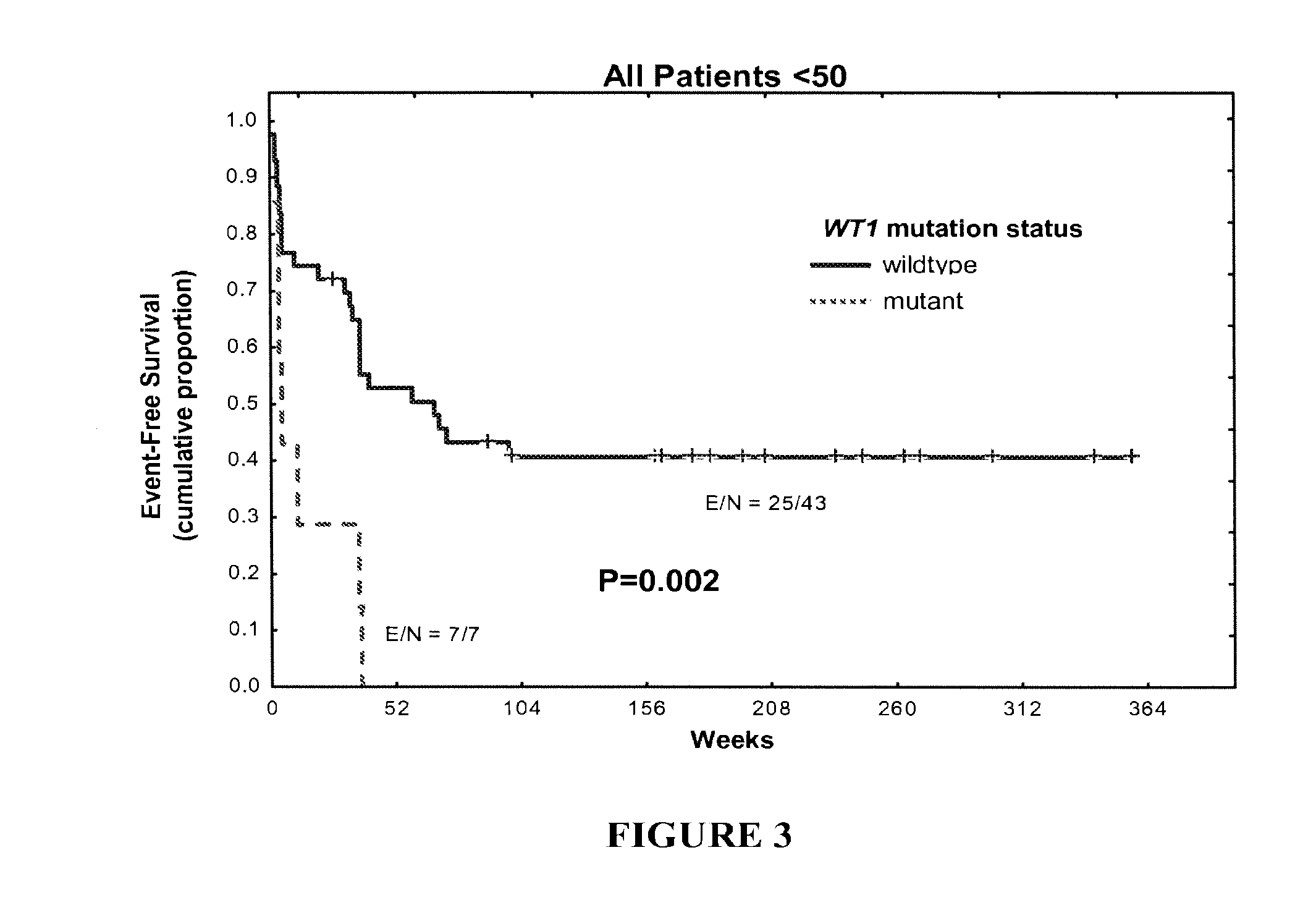
WT1 mutations for prognosis of myeloproliferative disorders
InactiveUS20160201141A1Poor outcomeShorten the construction periodMicrobiological testing/measurementMyeloproliferative DisordersLeukemia
The invention provides methods for determining the prognosis of a patient diagnosed with a leukemia, including B-cell chronic lymphocytic leukemia, by measuring mutations of the WT1 gene in a biological sample. The invention also relates to the diagnosis of leukemia, including B-cell chronic lymphocytic leukemia.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
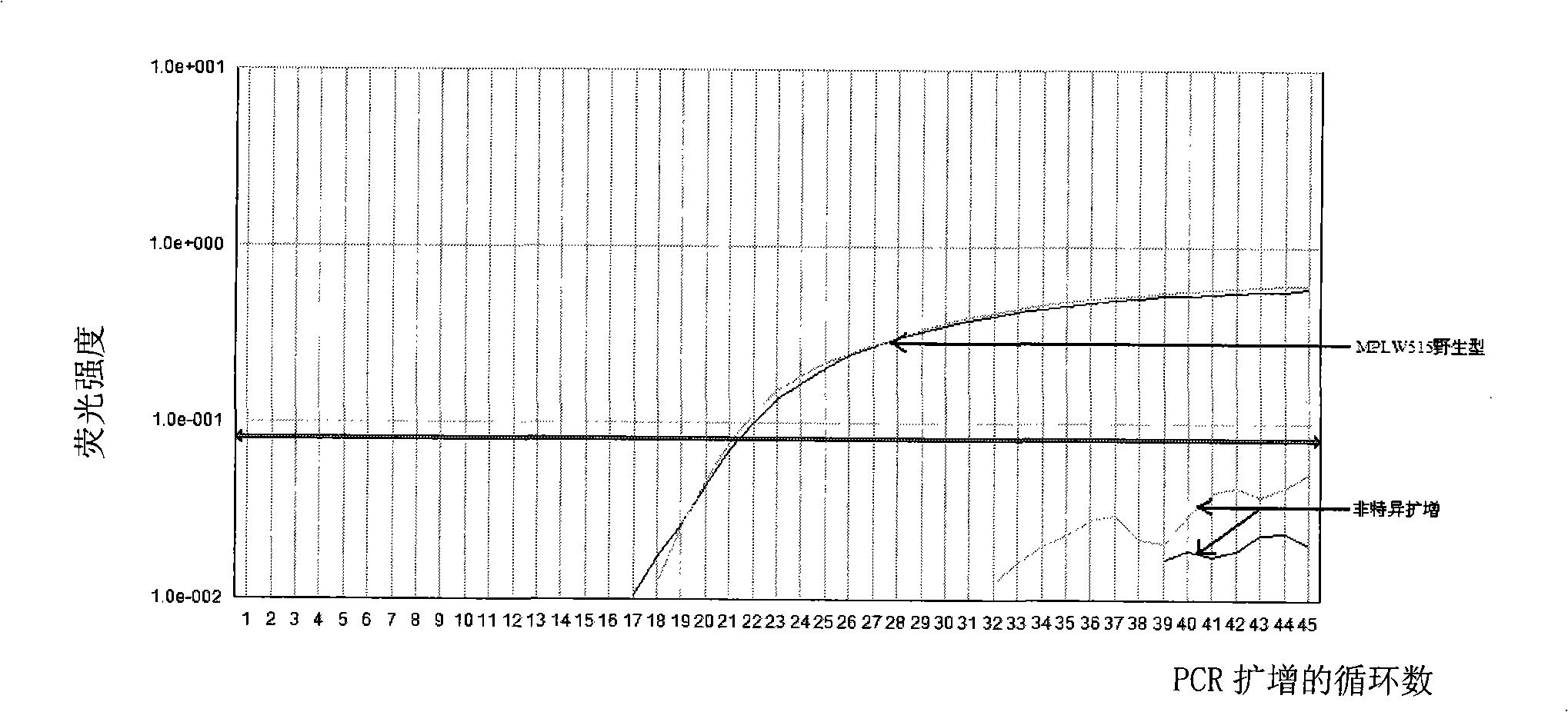
Reagent kit for detecting bone marrow proliferative diseases MPLW515L mutation, special-purpose primer and probe thereof
InactiveCN101403009AFast new wayReliable new wayMicrobiological testing/measurementAnalysis by subjecting material to chemical reactionMpl geneMyeloid proliferation
The invention discloses a kit and a special primer and probe thereof which are used for detecting the MPLW515L mutation of myeloid proliferation diseases. The kit provided by the invention comprises a primer and a TaqMan-MGB probe; wherein, the nucleotide sequence of the primer is shown by SEQ ID NO:1 and SEQ ID NO:2 in a sequence table; the nucleotide sequence of the TaqMan-MGB probe is shown by SEQ ID NO: 3 and SEQ ID NO:4 in the sequence table. The kit provided by the invention is used for detecting the MPLW515L mutation in clinic and scientific research, which not only provides a fast, reliable and accurate new way for diagnosing MPDs, but also can furthermore provide the exact ratio of the mutation genes of MPLW515L and the genes of wild MPL, thus providing proof for curative effect observation and the dynamic observation of minimal residual diseases.
Owner:PEOPLES HOSPITAL PEKING UNIV
Methods for treating Janus kinase-associated disorders by administering soluble transforming growth factor beta type II receptor
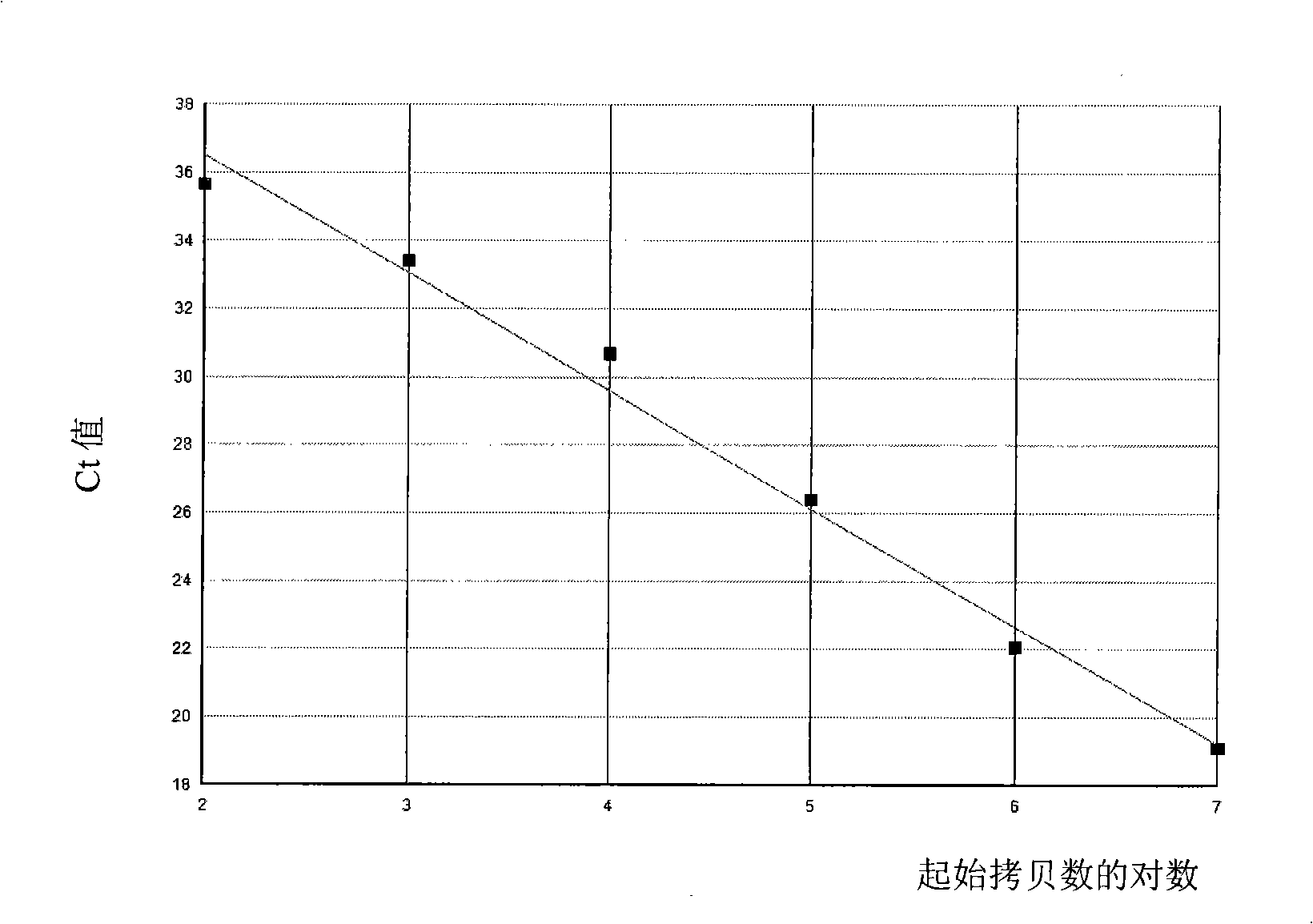
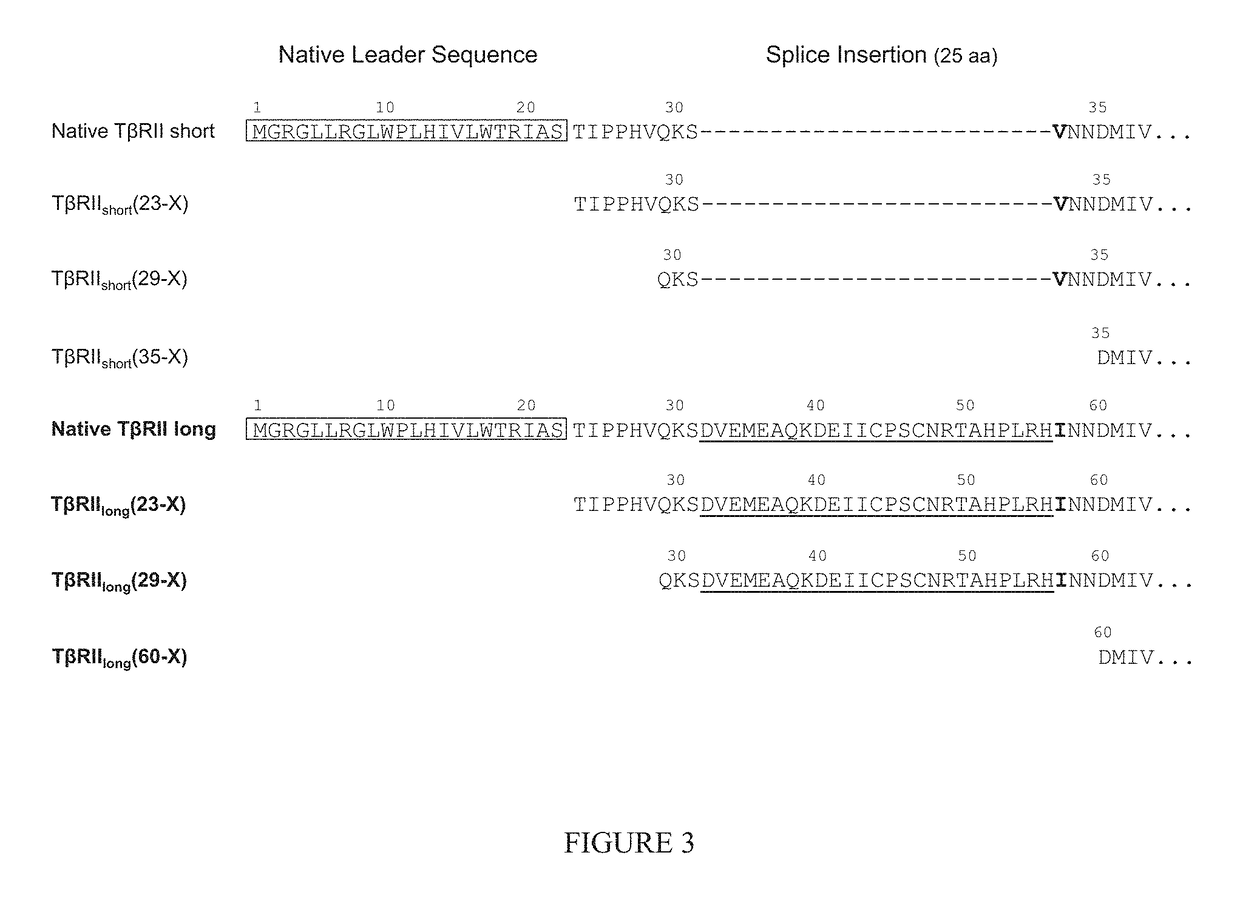
In part, the present disclosure relates methods for treating, preventing, or reducing the severity of a myeloproliferative disorder (e.g., polycythemia vera, essential thrombocythemia, and myelofibrosis) or one or more complications of a myeloproliferative disorder. The present disclosure further relates methods for treating, preventing, or reducing the severity of a Janus kinase-associated disorder or one or more complications of a Janus kinase-associated disorder. In certain aspects the disclosure provides TβRII antagonists for treating, preventing, or reducing the severity of a myeloproliferative disorder (e.g., polycythemia vera, essential thrombocythemia, and myelofibrosis) or a Janus kinase-associated disorder or one or more complications of a myeloproliferative disorder or a Janus kinase-associated disorder.
Owner:ACCELERON PHARMA INC
Detection kit for detecting MDS/MPN (myelodysplastic syndrome/myeloproliferative neoplasms) related gene group
InactiveCN107868823AImprove detection efficiencyImprove the detection rateMicrobiological testing/measurementFlow cytometryRelated gene
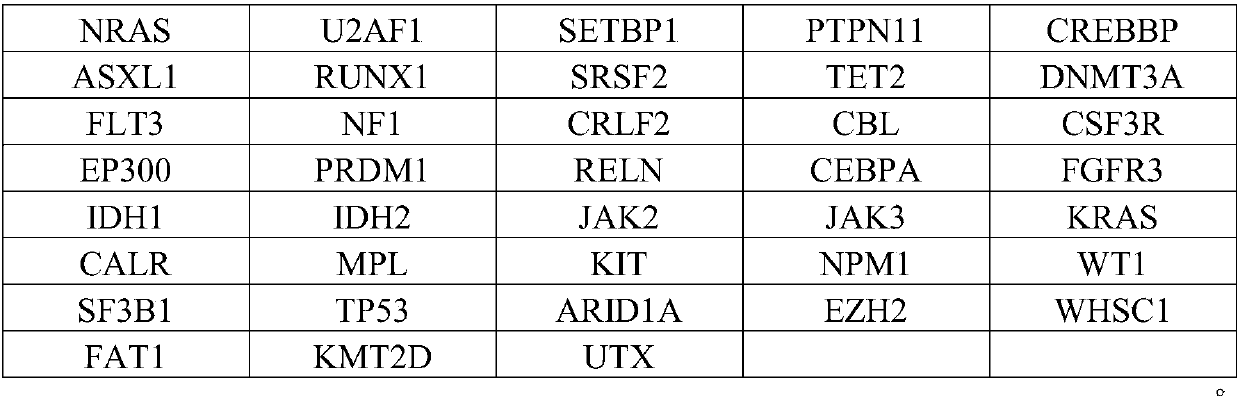
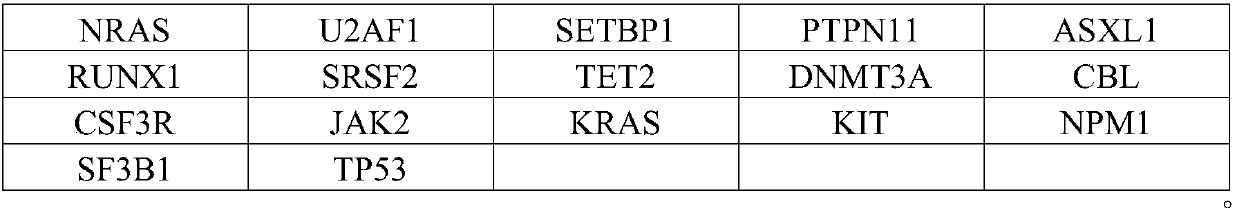
The invention relates to a detection kit for detecting an MDS / MPN (myelodysplastic syndrome / myeloproliferative neoplasms) related gene group. According to the technical scheme, firstly, a gene group used for screening MDS / MPN related gene mutation is determined, and 17 genes with high directivity are screened out. On the basis, a high-throughput sequencing technology is combined, auxiliary diagnosis of the MDS / MPN beyond the morphology and the flow cytometry can be realized, and particularly, the detection kit has special advantages in evaluation of disease prognosis and anticipation of actioneffects on related target medicines. The provided detection kit of the gene group for used for screening MDS / MPN related gene mutation has the advantages of high detection efficiency, high detectionrate and the like.
Owner:天津见康华美医学诊断技术有限公司
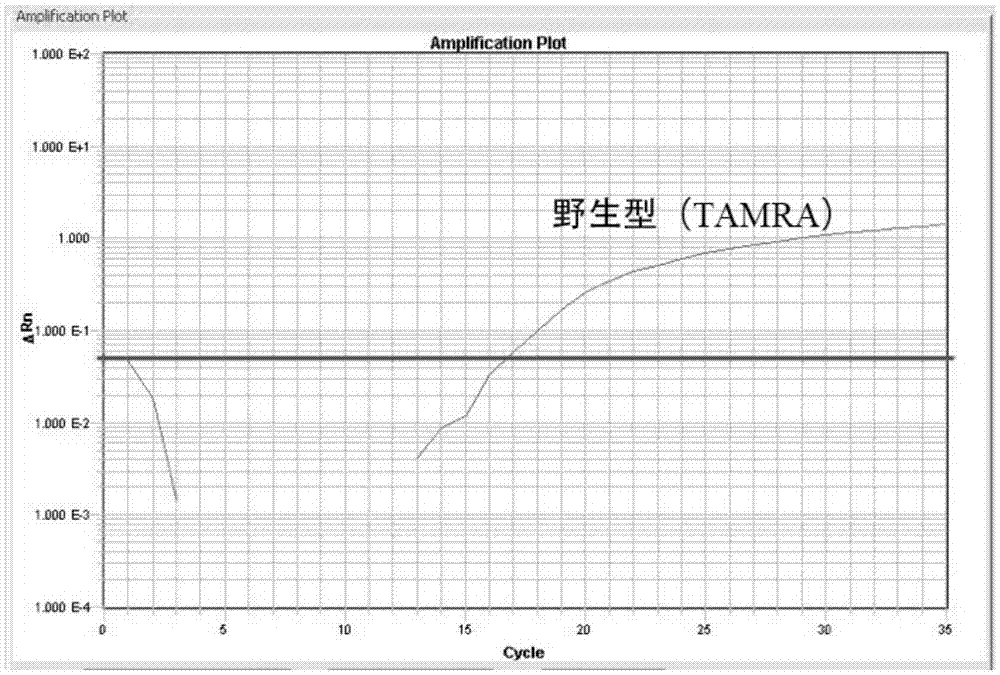
Method of detecting JAK2V617F mutation and its special primer and TaqMan MGB probe
InactiveCN1932038AMicrobiological testing/measurementDNA/RNA fragmentationMRD NegativeAgricultural science
The present invention discloses method of detecting JAK2V617F mutation and its special primer and TaqMan-MGB probe. The primer for detecting the genome DNA JAK2V617F mutation of myeloproliferative diseases is the primer pair shown in the SEQ ID No. 1 and SEQ ID No. 2 in the sequence list. The primer for detecting cDNA JAK2V617F mutation of myeloproliferative diseases is the primer pair shown in the SEQ ID No. 3 and SEQ ID No. 4 in the sequence list. The TaqMan-MGB probe has the DNA sequence shown in the SEQ ID No. 5 and SEQ ID No. 6 in the sequence list; and the DNA sequence has reporter fluorophore of different colors in the 5' end, and quenching group marked in the 3' end and connected dihydro clyclic indol porphyrin-tripeptide. The present invention provides one fast, reliable and accurate way for the diagnosis of MPDs, and can further provide the determined ratio between JAK2 mutation gene and wild JAK2 gene, so as to provide basis for treating observation.
Owner:PEOPLES HOSPITAL PEKING UNIV
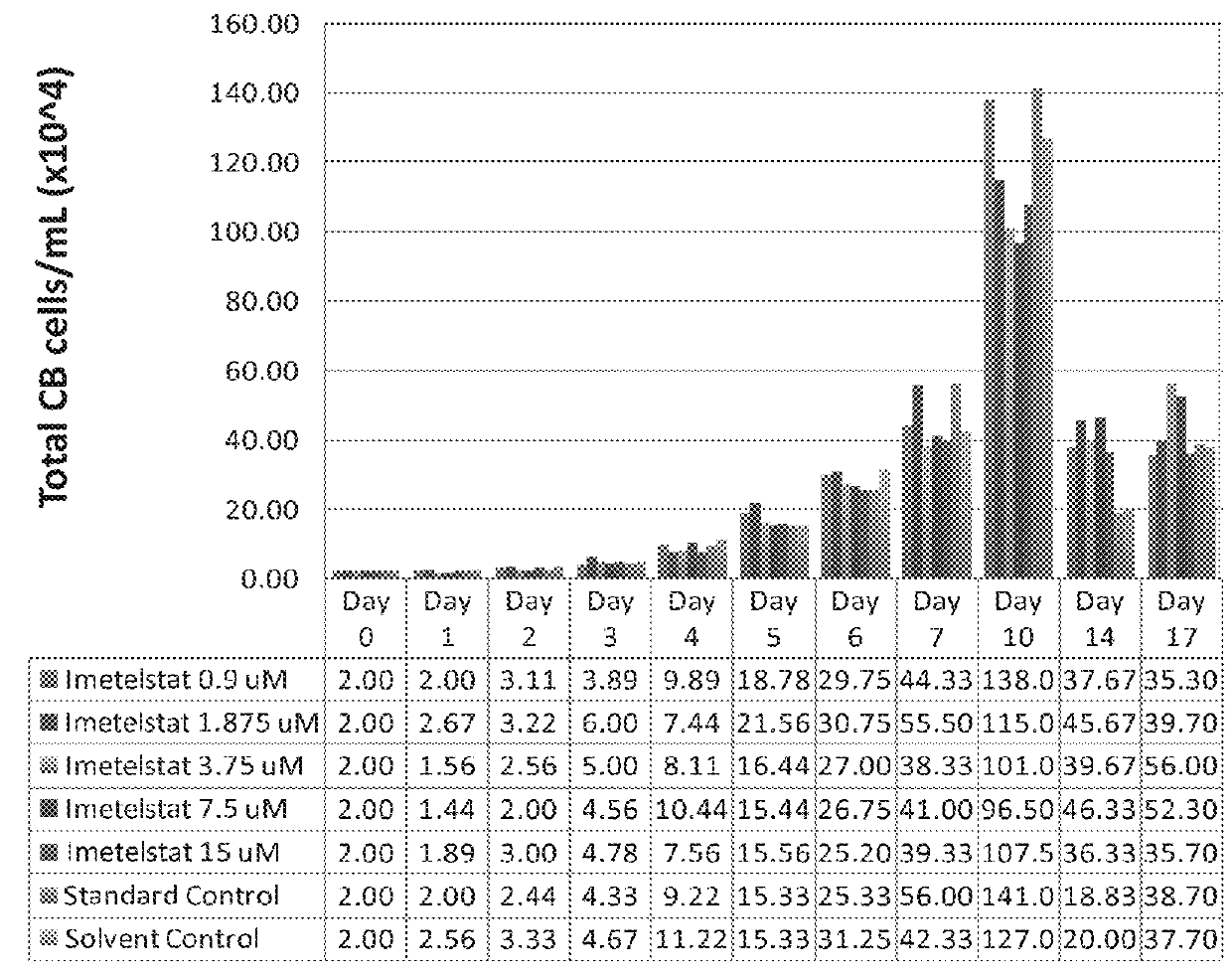
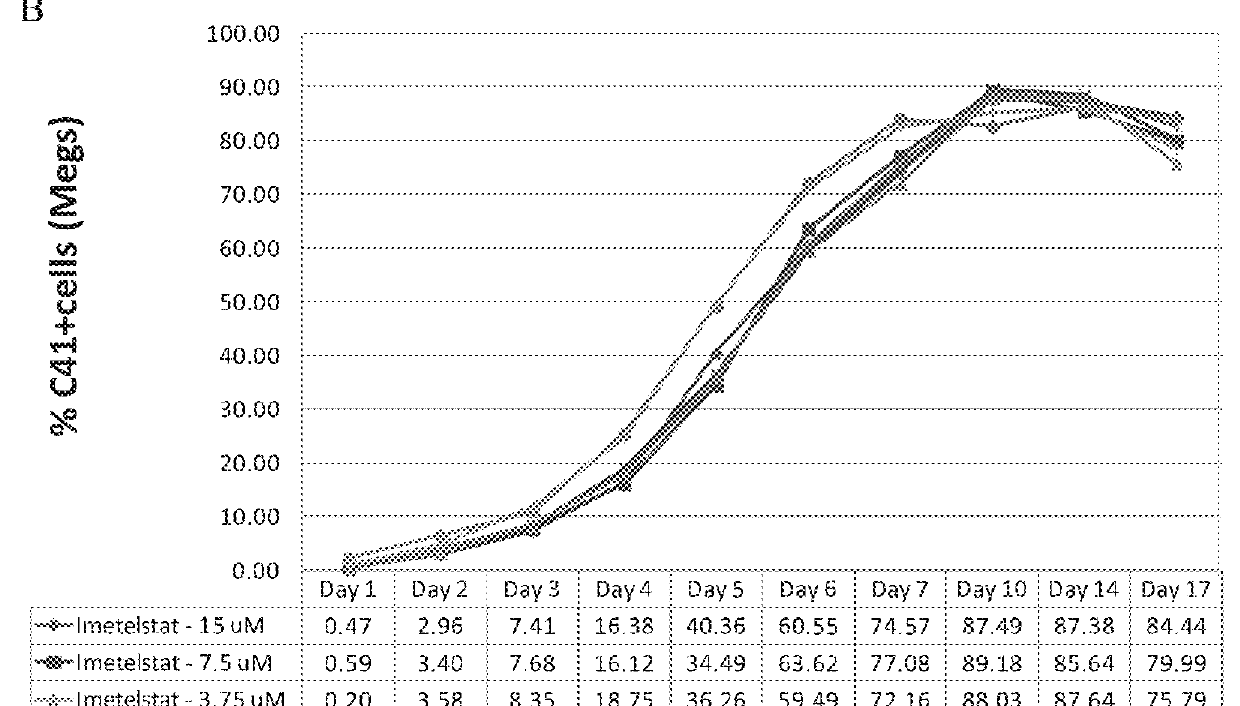
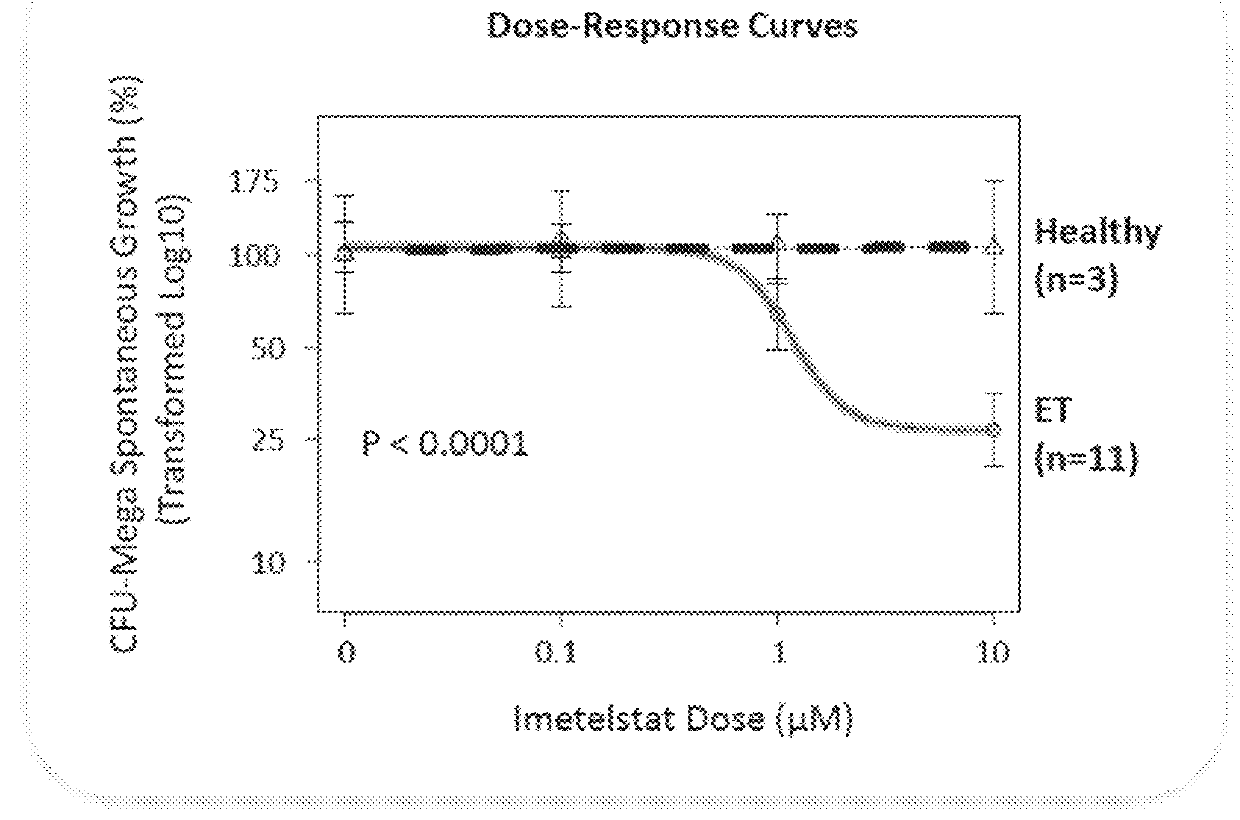
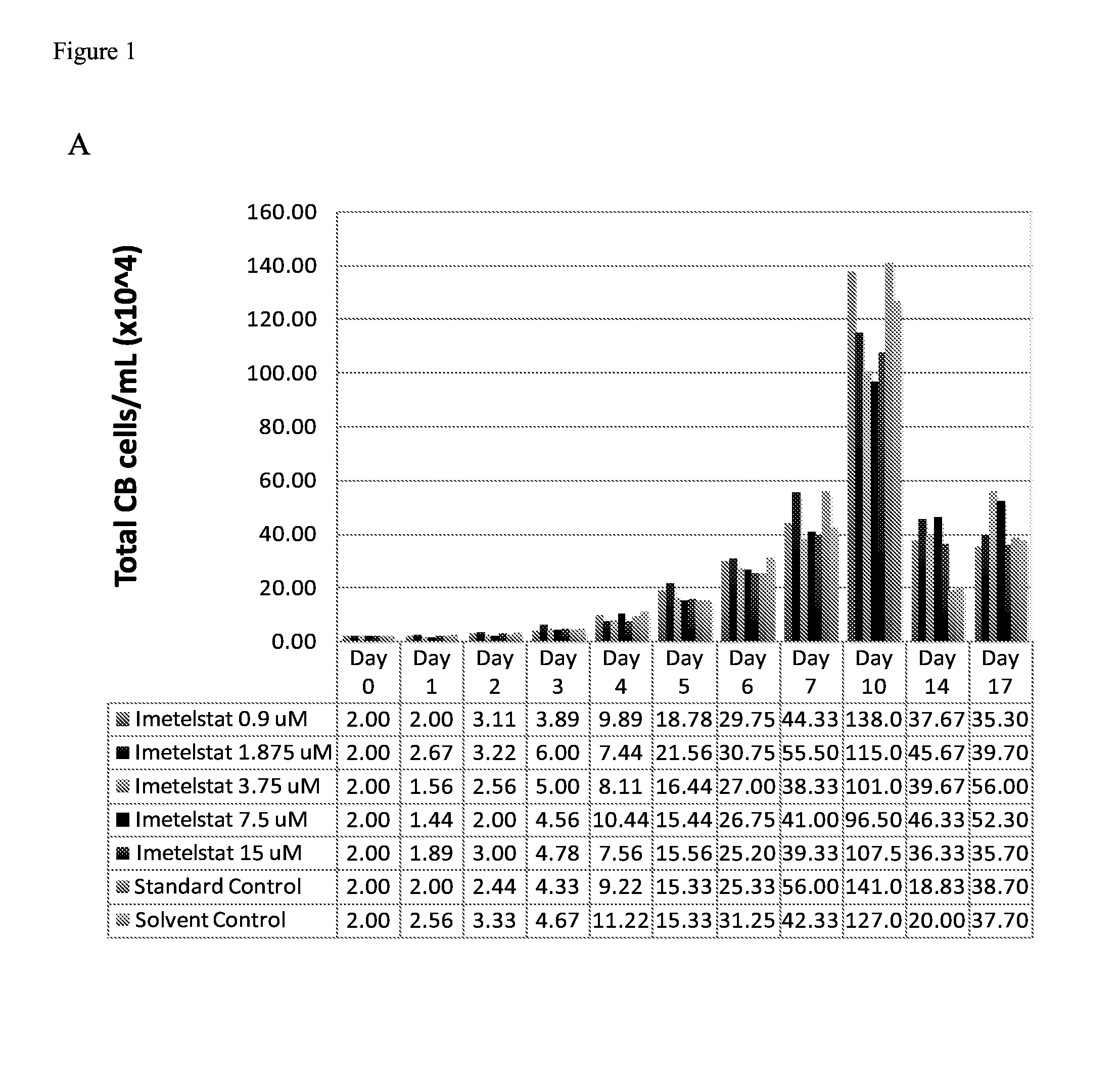
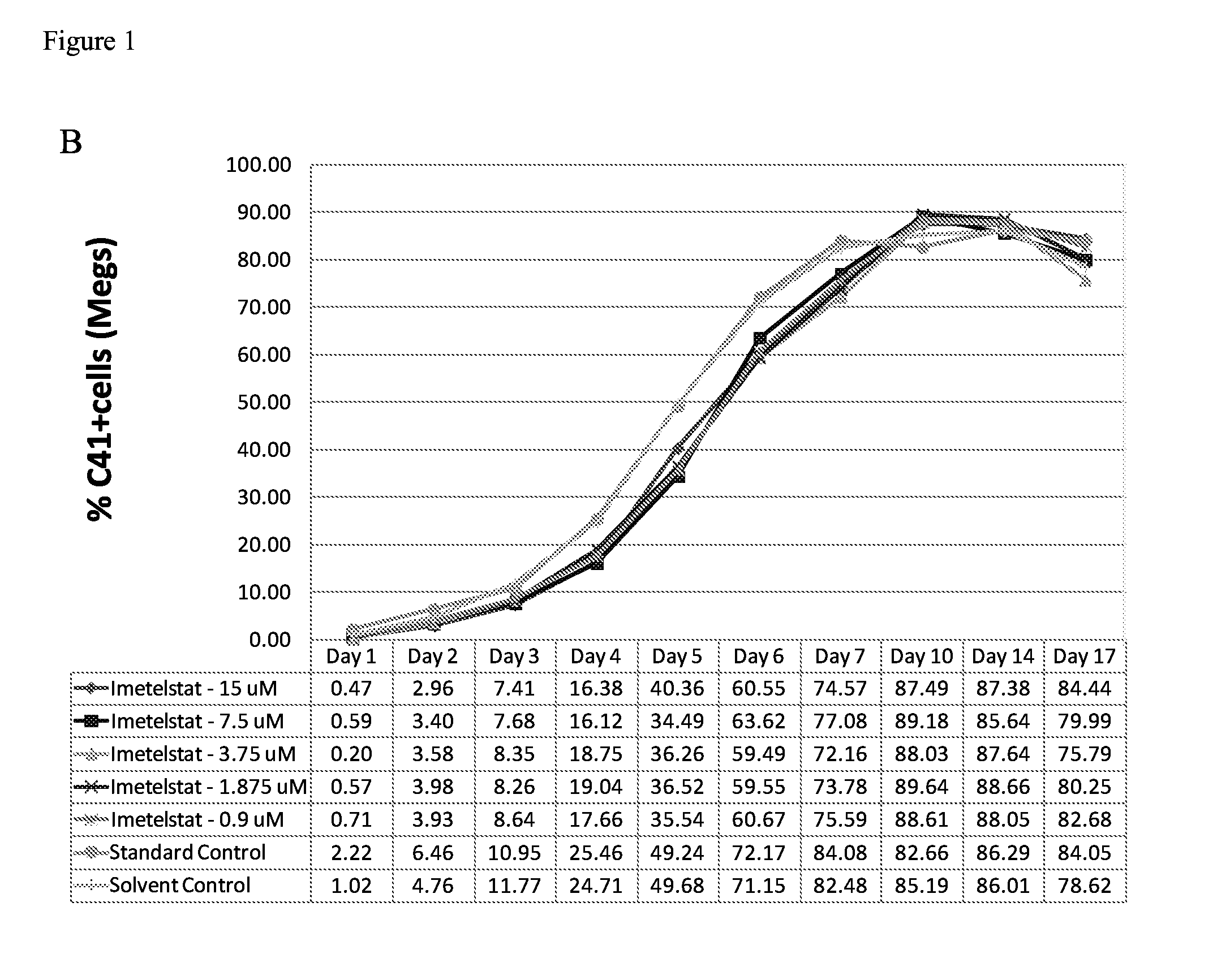
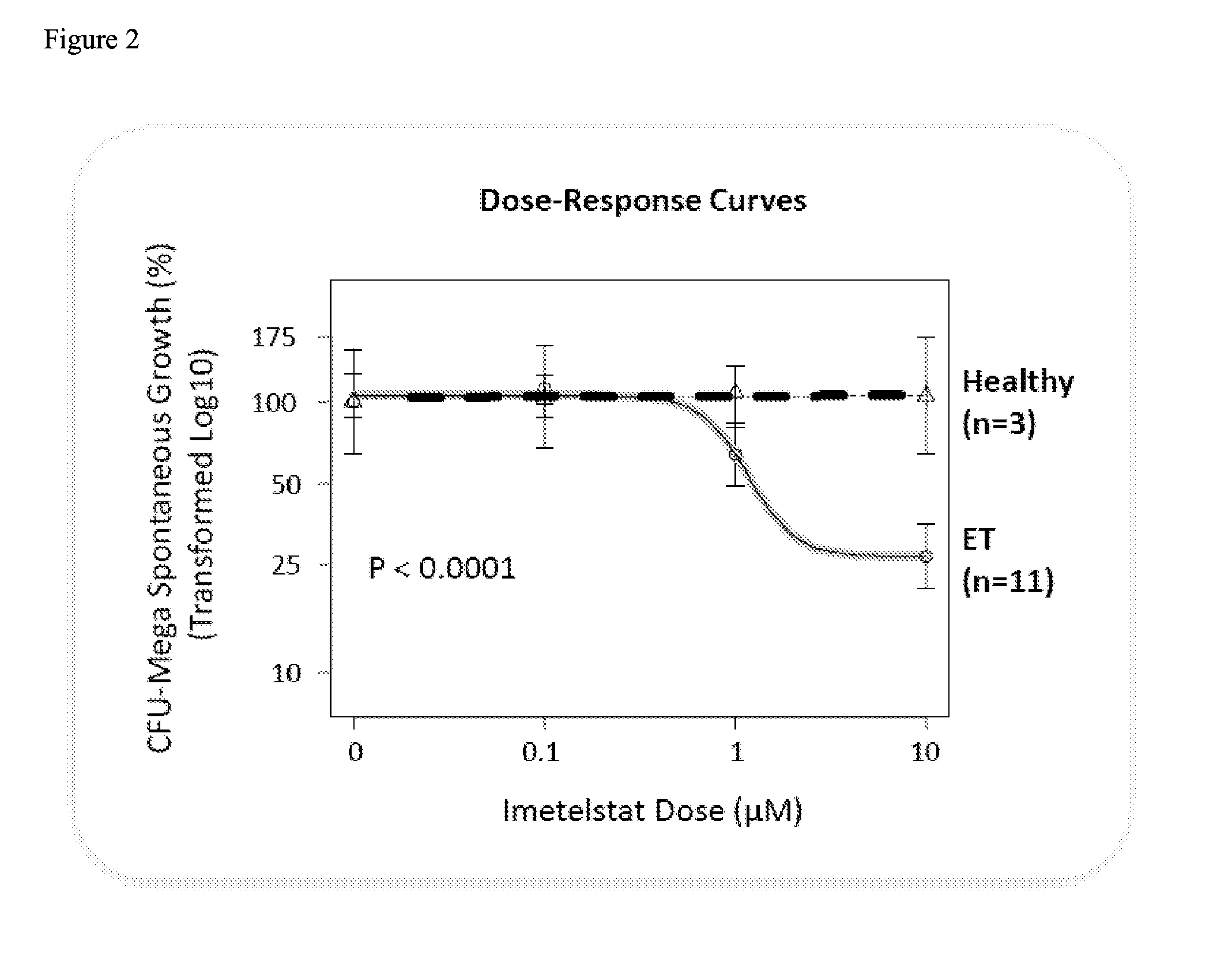
Use of telomerase inhibitors for the treatment of myeloproliferative disorders and myeloproliferative neoplasms
ActiveUS9375485B2Growth inhibitionRelieve symptomsPowder deliveryNanomedicineTelomeraseBlood platelet counts
Provided herein are methods for reducing neoplastic progenitor cell proliferation and alleviating symptoms associated in individuals diagnosed with or thought to have Essential Thrombocythemia (ET). Also provided herein are methods for using telomerase inhibitors for maintaining blood platelet counts at relatively normal ranges in the blood of individuals diagnosed with or suspected of having ET.
Owner:GERON CORPORATION
Reagent kit for detecting bone marrow proliferative diseases MPLW515K mutation, special-purpose primer and probe thereof
InactiveCN101403010AFast new wayReliable new wayMicrobiological testing/measurementFluorescence/phosphorescenceMRD NegativeMpl gene
The invention discloses a kit and a special primer and probe thereof which are used for detecting the MPLW515K mutation of myeloid proliferation diseases. The kit provided by the invention comprises a primer and a TaqMan-MGB probe; wherein, the nucleotide sequence of the primer is shown by SEQ ID NO:1 and SEQ ID NO:2 in a sequence table; the nucleotide sequence of the TaqMan-MGB probe is shown by SEQ ID NO: 3 and SEQ ID NO:4 in the sequence table. The kit provided by the invention can be used for detecting the MPLW515K mutation in clinic and scientific research, which not only provides a fast, reliable and accurate new way for diagnosing MPDs, but also can furthermore provide the exact ratio of the mutation genes of MPLW515K and the genes of wild MPL, thus providing proof for curative effect observation and the dynamic observation of minimal residual diseases.
Owner:PEOPLES HOSPITAL PEKING UNIV
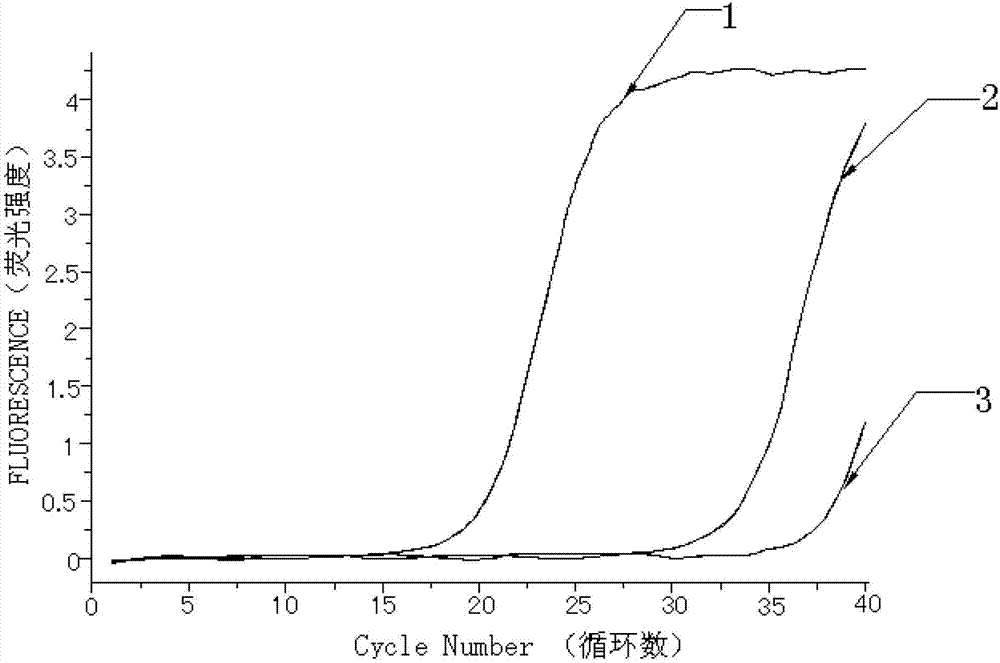
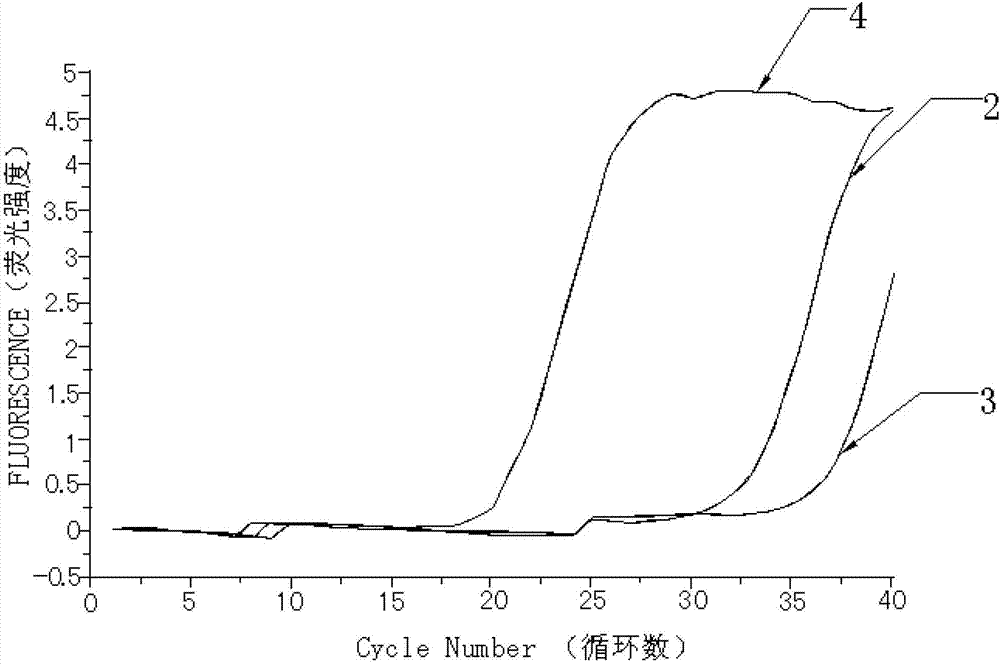
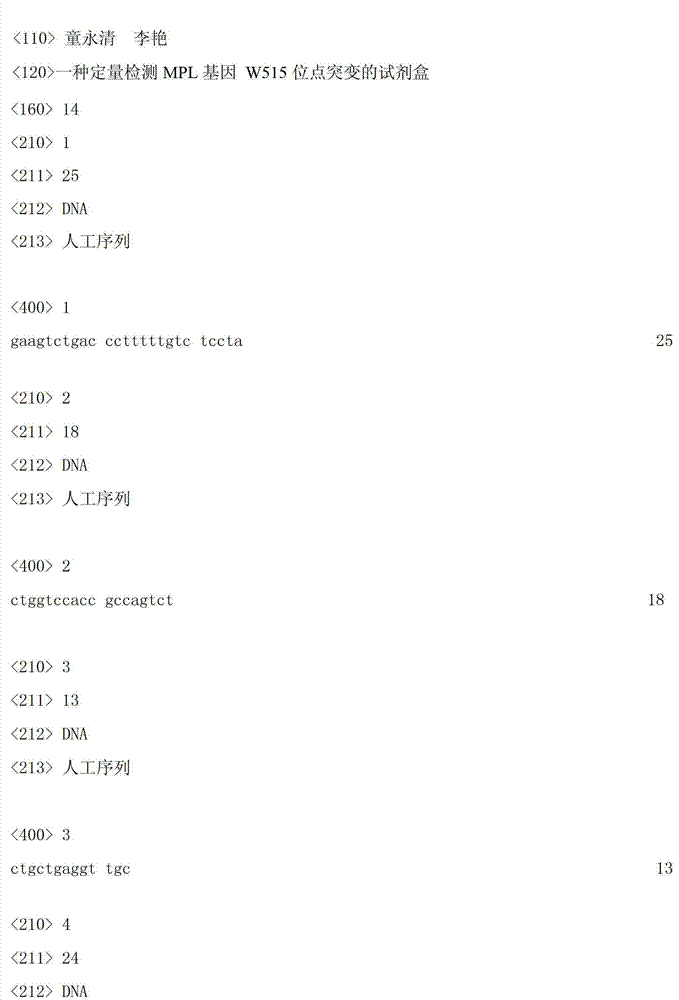
Kit for quantitatively detecting W515 site mutation of MPL genes
ActiveCN102925559AIncreased sensitivityStrong specificityMicrobiological testing/measurementGain of function mutationReference genes
The invention relates to a kit for quantitatively detecting the W515 site mutation of MPL genes, belonging to the field of biotechnology. The kit comprises at least one system of the W515L site mutation specific system of the MPL genes and the W515K site mutation specific system of the MPL genes, and an inner reference gene ABL system, wherein each system comprises an upstream primer, a downstream primer and a Taqman fluorescent probe. Research shows that the gain-of-function mutation rates of the W515L site mutation of the MPL genes and the W515K site mutation of the MPL genes are 10% and 3% in PMF and ET respectively, and are not found in PV. Therefore, the W515L site mutation of the MPL genes and the W515K site mutation of the MPL genes are mainly used for diagnosing the PMF and the ET. With the adoption of the fluorescent quantitative PCR (polymerase chain reaction) which is high in sensitivity and specificity for detecting the W515L site mutation of the MPL genes and the W515K site mutation of the MPL genes, the specificity and the sensitivity of the detection result are remarkably improved. Via the kit disclosed by the invention, a novel rapid, simple and convenient gene diagnosis technology is provided for prediction for myeloproliferative diseases.
Owner:广州市宝创生物技术有限公司
Compositions and methods for treating cancer and biomarkers to detect cancer stem cell reprogramming and progression
PendingUS20190247413A1Slowing and stopping progressionOrganic active ingredientsHydrolasesReprogrammingBiomarker (petroleum)
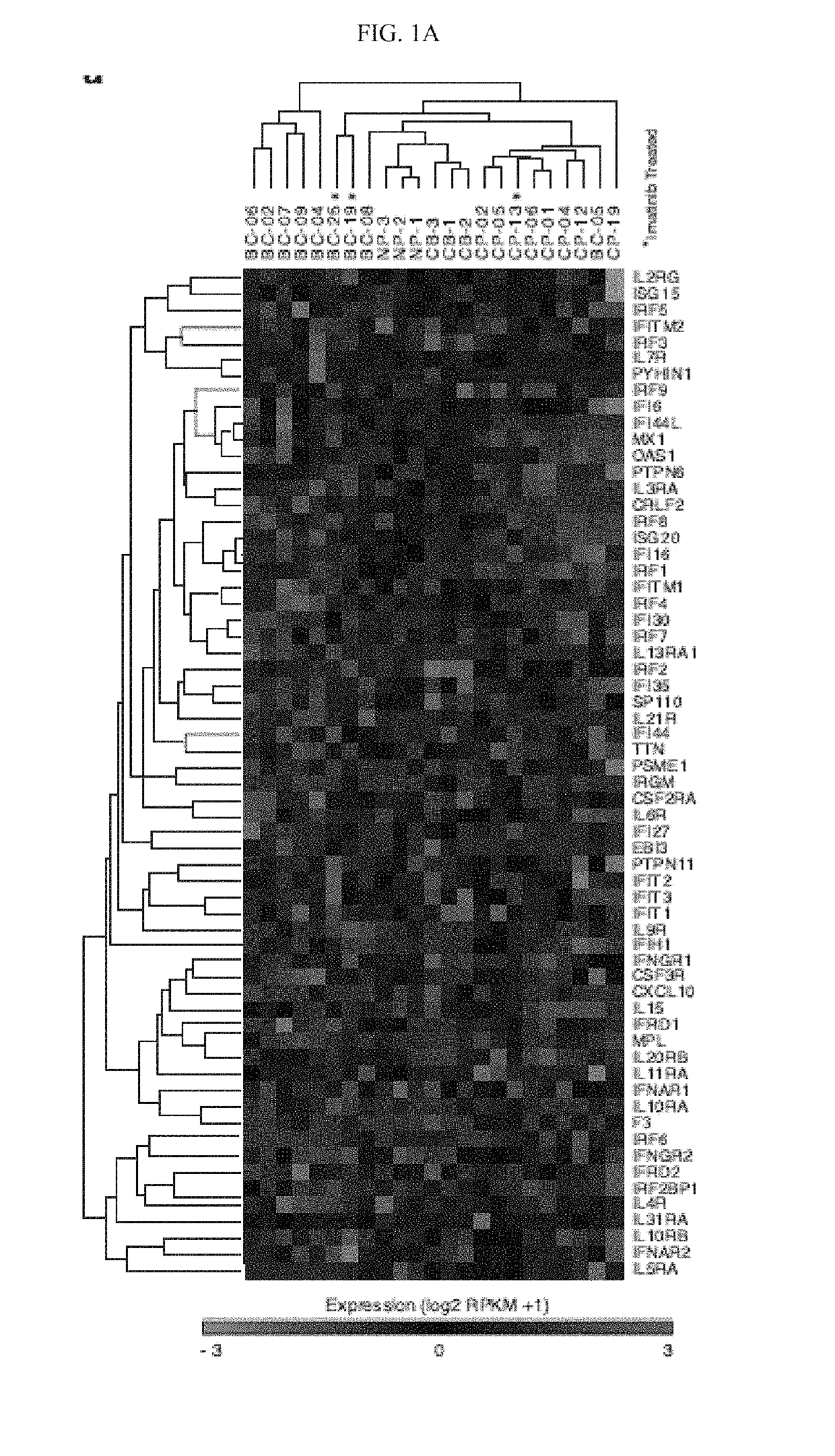
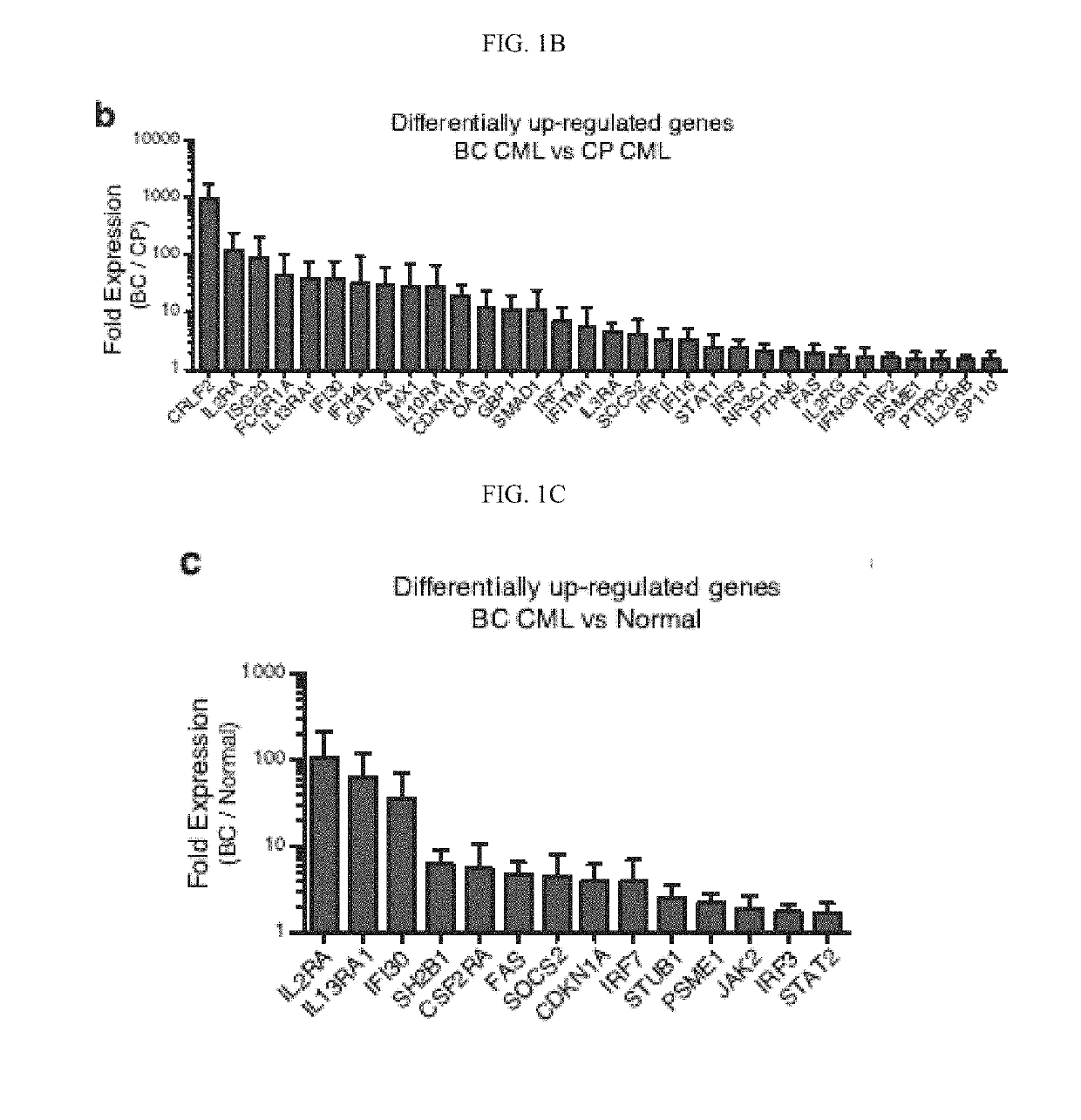
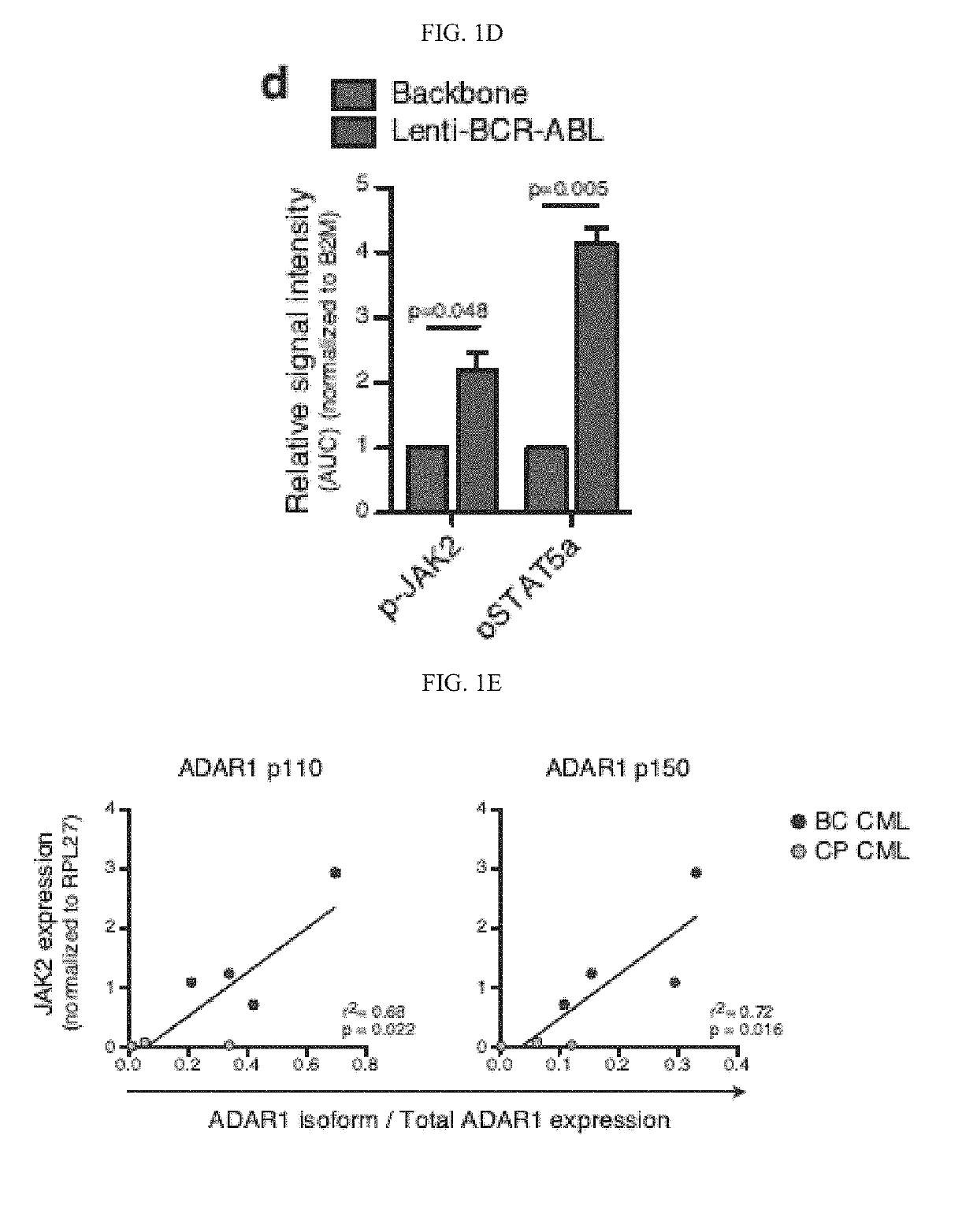
In alternative embodiment, provided are methods and compositions for treating, ameliorating or preventing diseases and conditions, such as cancer, including cancers associated with stem cells such as, without limitation, myelodysplastic syndrome (MDS) and a myeloproliferative neoplasm like chronic myeloid leukemia (CML) or acute myeloid leukemia (AML), and ablating or killing cancer stem cells. In alternative embodiment, provided are a new set of biomarkers to detect leukemia stem cell reprogramming and CML progression. In alternative embodiment, provided are therapeutic targets for treating myelodysplastic syndrome (MDS) and chronic myeloid leukemia (CML) by targeting edited let-7 transcripts.
Owner:RGT UNIV OF CALIFORNIA
Use of Telomerase Inhibitors for the Treatment of Myeloproliferative Disorders and Myeloproliferative Neoplasms
InactiveUS20150342982A1Reduced neoplastic progenitor cell proliferationReduce cell proliferationOrganic active ingredientsTransferasesTelomeraseBlood platelet counts
Provided herein are methods for reducing neoplastic progenitor cell proliferation and alleviating symptoms associated in individuals diagnosed with or thought to have myeloproliferative disorders, such as Essential Thrombocythemia (ET). Also provided herein are methods for using telomerase inhibitors for maintaining blood platelet counts at relatively normal ranges in the blood of individuals diagnosed with or suspected of having myeloproliferative disorders, such as ET.
Owner:GERON CORPORATION
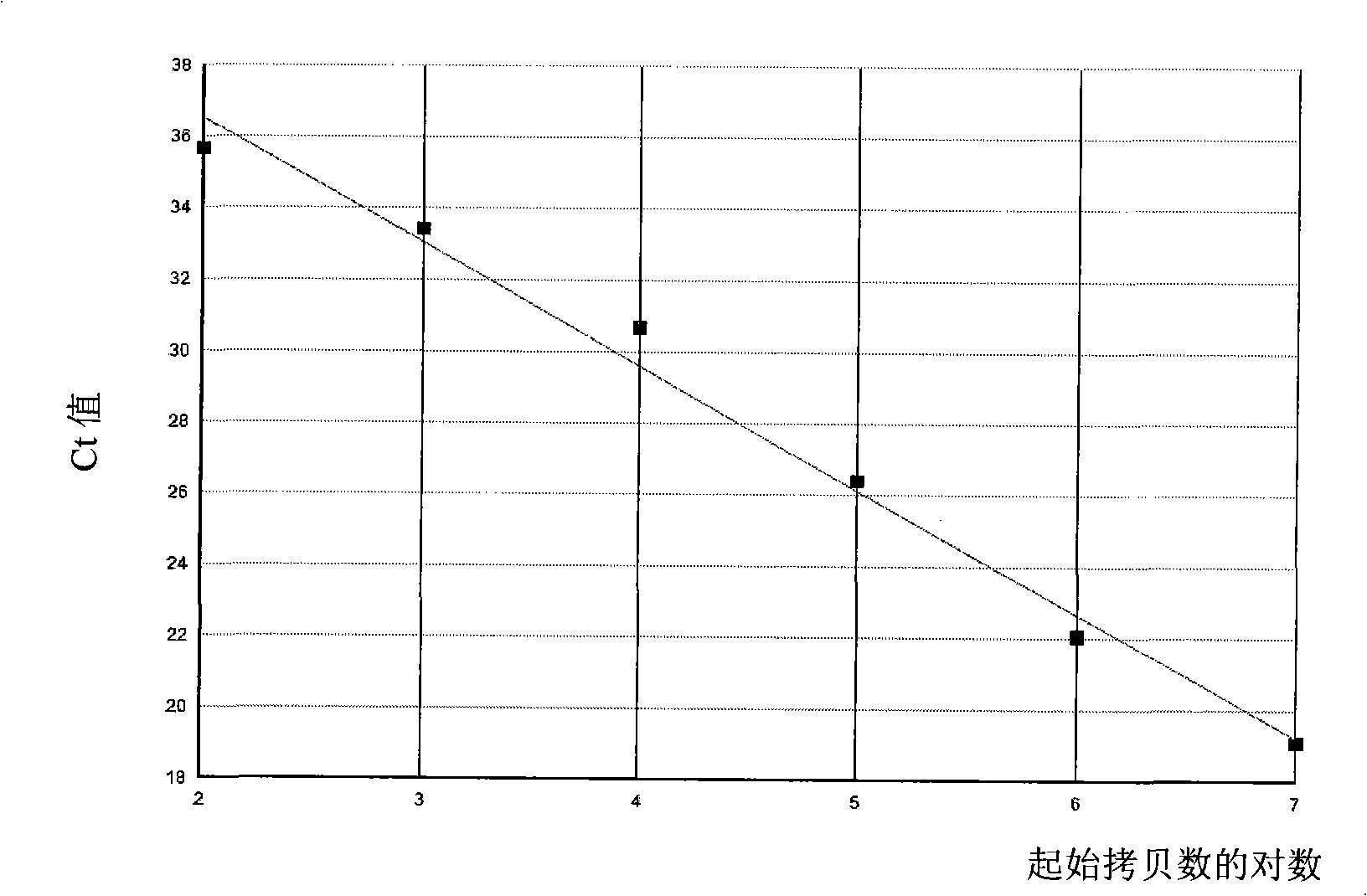
TaqMan-COLD-PCR method for detecting JAK2V617F mutation rate in peripheral blood
The invention relates to a TaqMan-COLD-PCR method for detecting the JAK2V617F mutation rate in peripheral blood. In the method, the DNA of a peripheral blood genome is used as a detection sample, the detection is completed within one reaction system, the JAK2V617F mutation percentage is quantitatively detected, and the lower detection limit is 0.1%; and the method has the characteristics of simple operation process, good repeatability, high flux of the detection sample, short report time and the like, and is especially applicable to the detection of a small quantity of mutant clone strains. The invention aims to overcome the defects of the background art and provide a method for sensitively detecting the JAK2V617F mutation rate in the DNA of a peripheral blood genome. As the unique molecular diagnosis marker of myeloproliferative diseases, the JAK2V617F mutation has been incorporated into the diagnosis standard of myeloproliferative diseases. The invention can obviously improve the diagnosis rate of myeloproliferative diseases. Meanwhile, the invention can be used for monitoring the curative effect in the treatment process of myeloproliferative diseases.
Owner:AEROSPACE CENT HOSPITAL
Deuterated phenyl amino pyrimidine compound and pharmaceutical composition containing the same
Owner:SUZHOU ZELGEN BIOPHARML
Primer, kit and method for detecting genetic mutation related to myeloproliferative neoplasms MPN
InactiveCN106434908AEfficient specificityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationGenetic engineeringBiology
The invention belongs to the technical field of genetic engineering and discloses a primer combination for genetic mutation related to myeloproliferative neoplasms (MPN), a kit containing the primer combination and a method for detecting the genetic mutation related to the MPN. Aiming at three types of MPN related genes, a primer sequence capable of efficiently and specifically amplifying the three types of genes is designed and has high specificity. The annealing temperature of all the designed primers is 58 DEG C and an amplification reaction can be finished for one time through utilizing the same PCR procedure. According to the primer combination, the kit and the method, disclosed by the invention, a presented sequencing peak diagram has a clear background and a signal value is high; the analysis difficulty of mutation is greatly reduced; and the primer, the kit and the detection method, designed according to the invention, are low in cost and high in specificity.
Owner:SHANGHAI TISSUEBANK MEDICAL LAB CO LTD
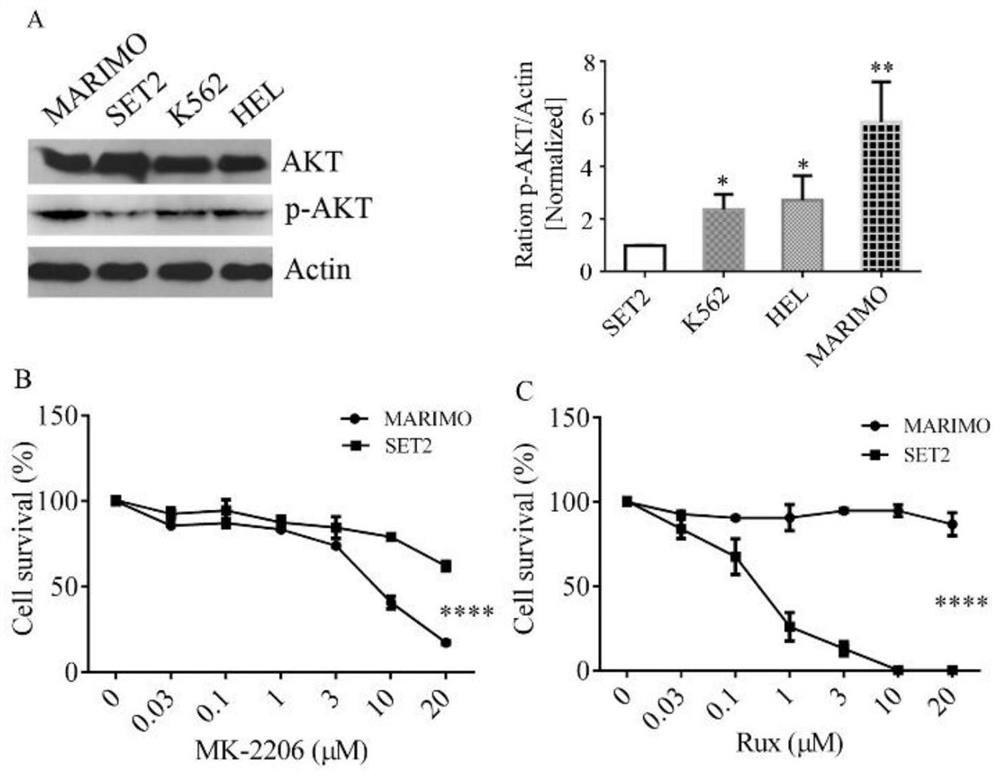
Pharmaceutical composition and application thereof in preparation of drugs for treating targeted calreticulin mutation type myeloproliferative diseases
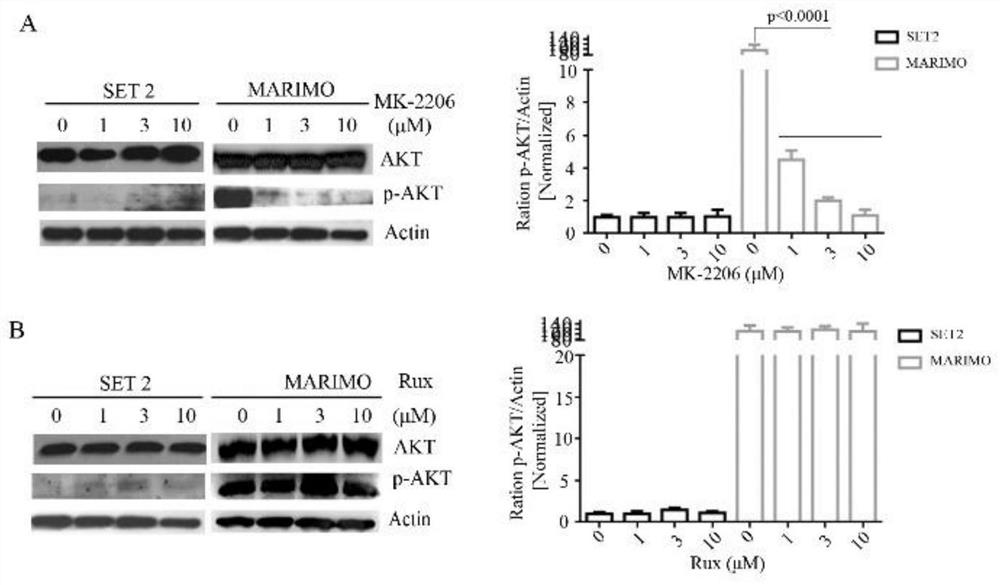
The invention discloses a pharmaceutical composition and an application thereof in preparation of drugs for treating targeted calreticulin mutation type myeloproliferative diseases, wherein the pharmaceutical composition is composed of MK-2206 and AZD 6244; CALR gene mutation type MARMO cells are adopted as research objects (the cells are specific unresponsive cells of rucotinib), a double-drug combination method capable of being applied to targeted CALR mutation type myeloproliferative diseases is explored, and a basis is provided for clinical drug combination treatment of patients who do not respond to rucotinib.
Owner:XUZHOU MEDICAL UNIV
Compounds suitable for the treatment of myeloproliferative neoplasms as well as methods for the diagnosis/prognosis of myeloproliferative neoplasms
InactiveUS20160250163A1Meeting urgent needsPeptide/protein ingredientsHydroxy compound active ingredientsNeural regulationAdrenergic receptor agonists
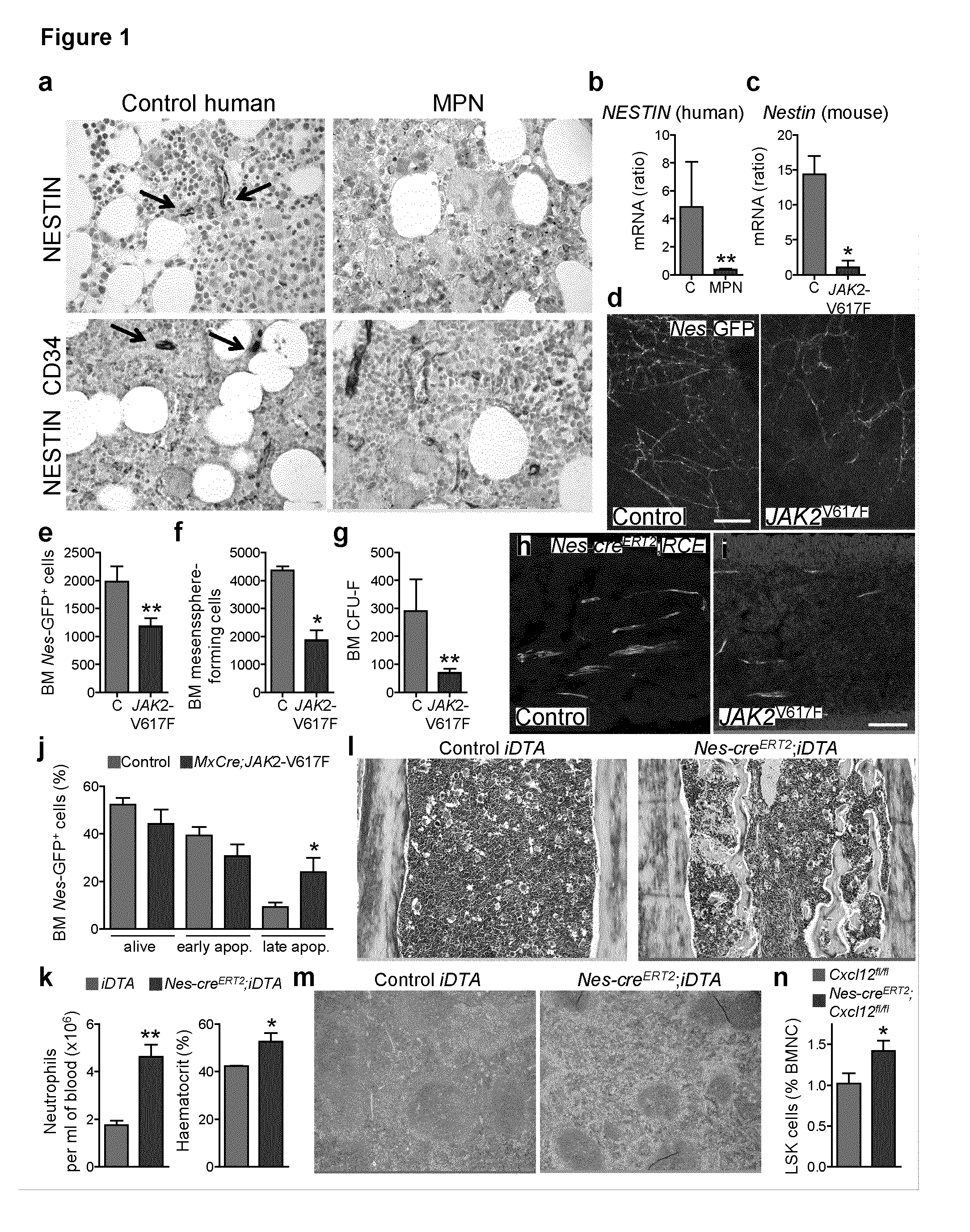
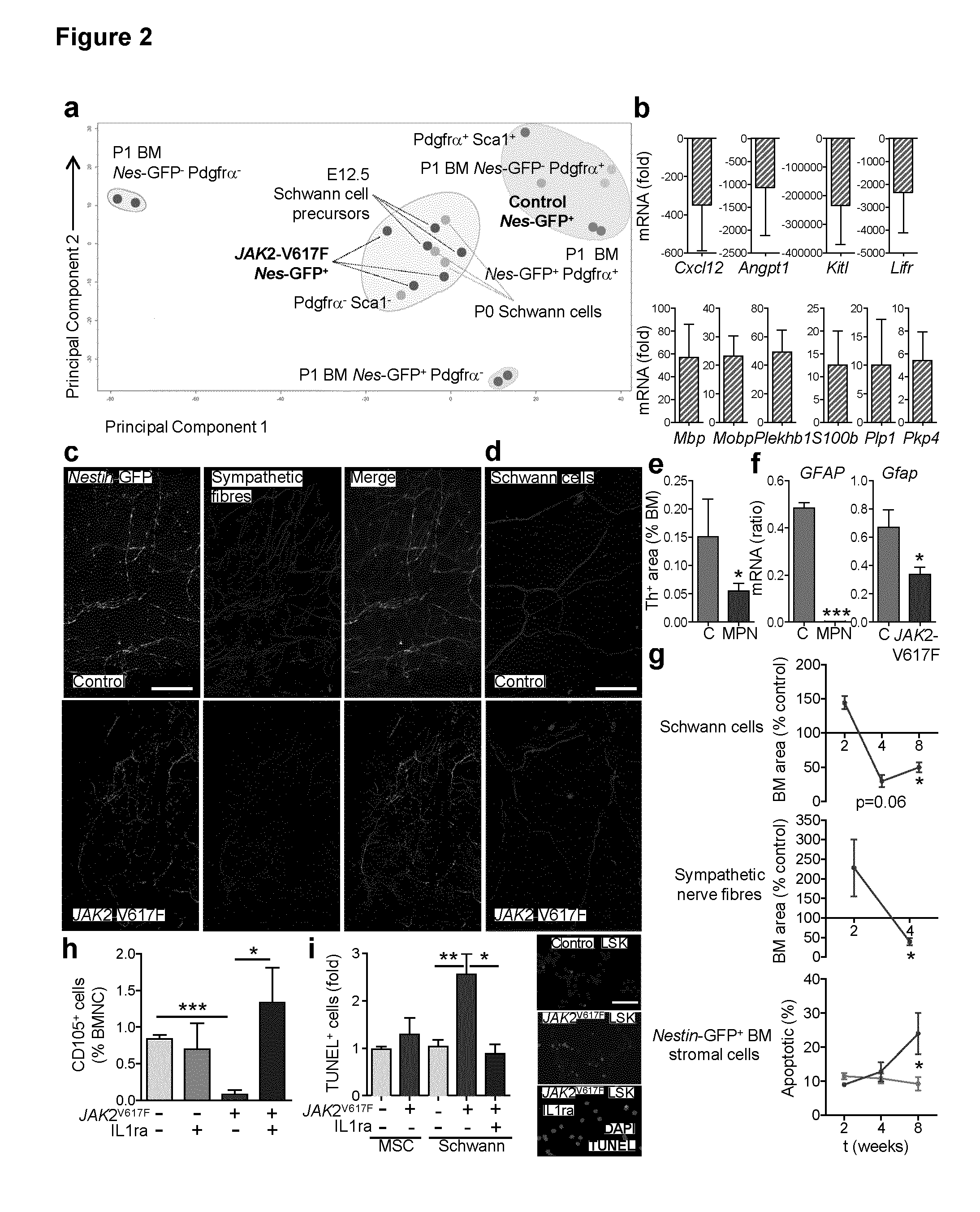
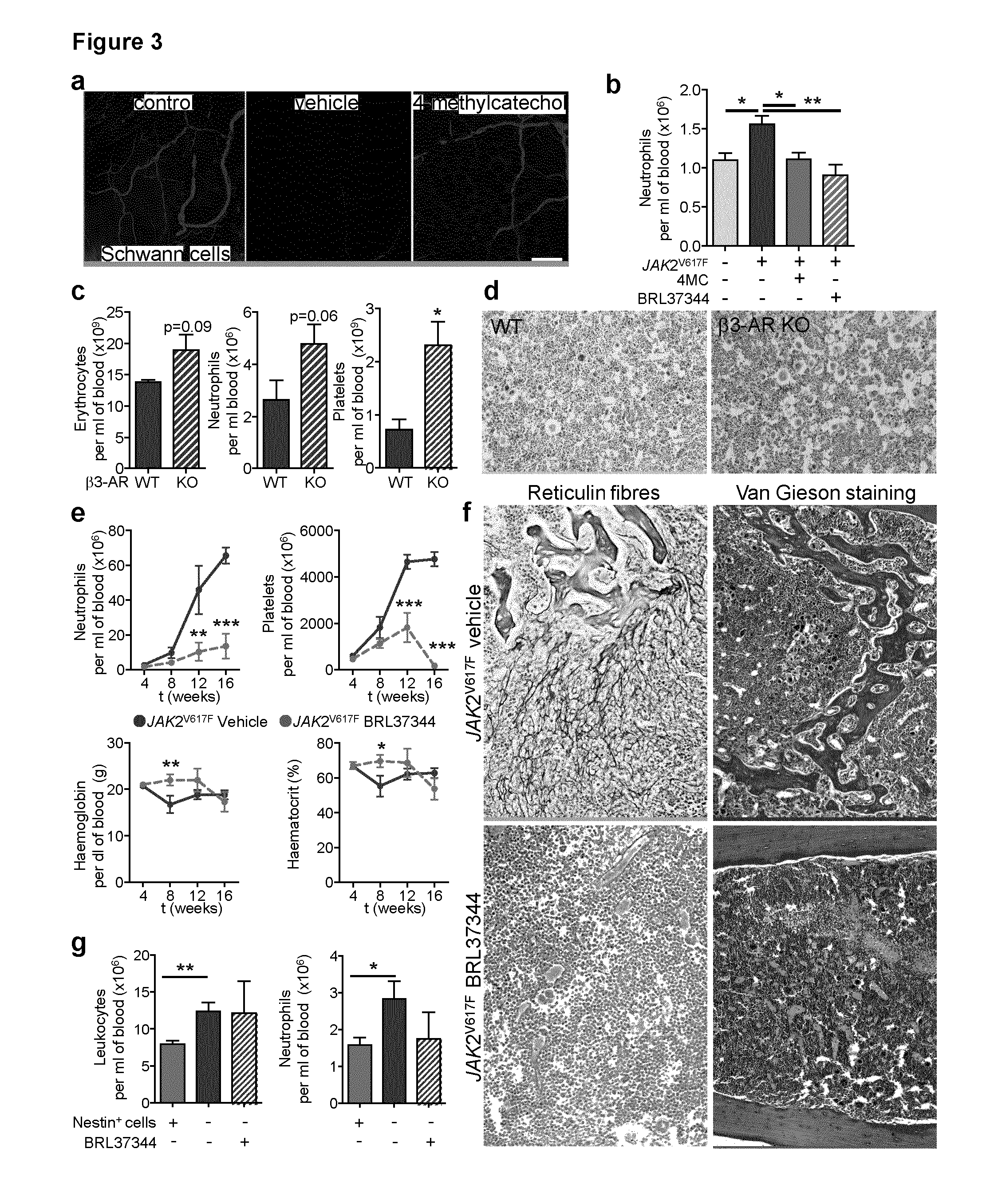
The present findings point to mutant HSCs as the cause of BM neuroglial damage that compromises MSC survival and function, critically contributing to MPN pathogenesis. In this sense, the present invention shows that the niche damage triggered by the mutant HSC is essential for the development of a haematopoietic malignancy previously considered to be caused by the HSC alone. Targeting HSC niche-forming MSCs and their neural regulation paves the way to more efficient therapeutic strategies in MPN. For this purpose, the present invention shows that an efficient therapeutic strategy for the treatment of MPN lies on the administration of neuroprotective compounds, such as 4-methylcatechol, capable of protecting BM sympathetic nerve fibres. Additionally, another efficient therapeutic strategy is shown herein as the administration of selective β3-adrenergic agonists such as BRL37344 or Mirabegron, since this strategy will compensate for deficient sympathetic stimulation of nestin+ MSCs.
Owner:FUNDACION CENT NACIONAL DE INVESTIGACIONES CARDIOVASCULARES CARLOS III CNIC
Methods of treating atherosclerosis or myeloproliferative neoplasms by administering a LYN kinase activator
This invention provides a method of treating a subject suffering from atherosclerosis or a myeloproliferative neoplasm which comprises administering to the subject an amount of a Lyn kinase activator effective to activate Lyn kinase so as to thereby treat the subject.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Prophylaxis treatment for acute myeloid leukemia
InactiveUS20190350885A1Increase signal levelImprove tissue repair abilityOrganic active ingredientsAntineoplastic agentsOncologyMyeloproliferative neoplasm
Compounds, and methods and uses of compounds, and pharmaceutical compositions thereof, are described herein for treating myeloid malignancies. In particular, compounds, and methods and uses of compounds, and pharmaceutical compositions thereof, are described herein for treating acute myeloid leukemia (AML), myeloproliferative neoplasm (MPN), and / or myelodysplastic syndrome (MDS).
Owner:INDIANA UNIV RES & TECH CORP
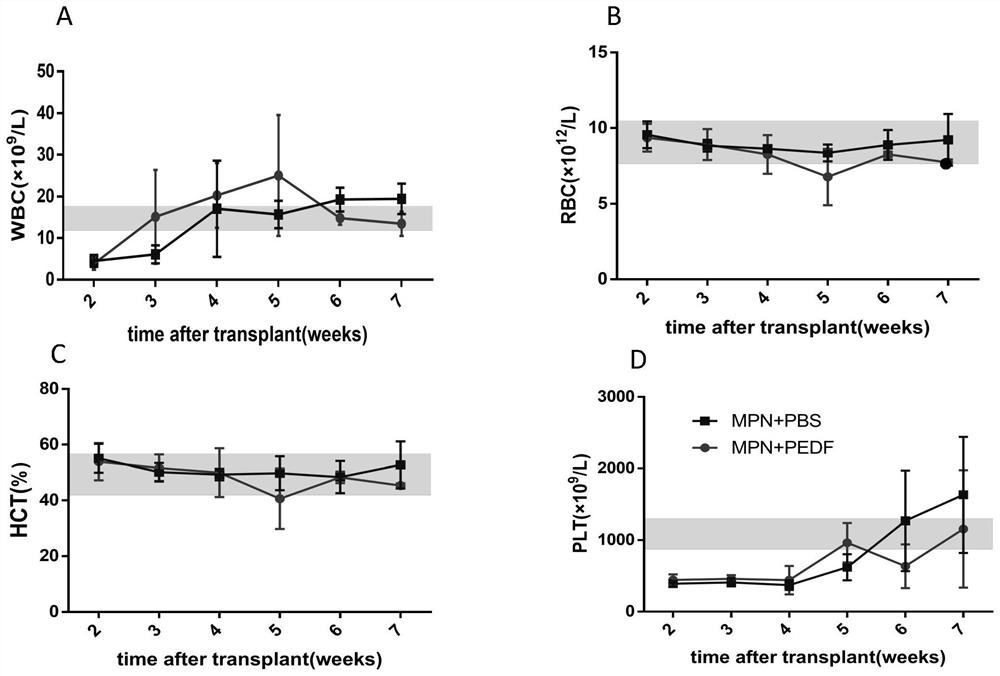
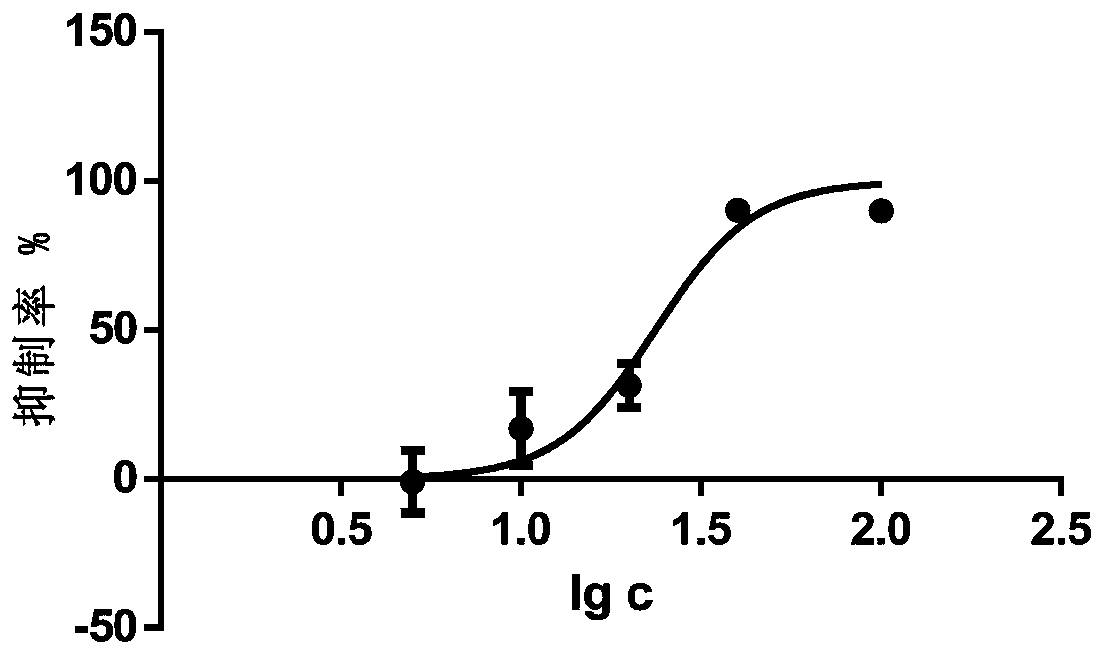
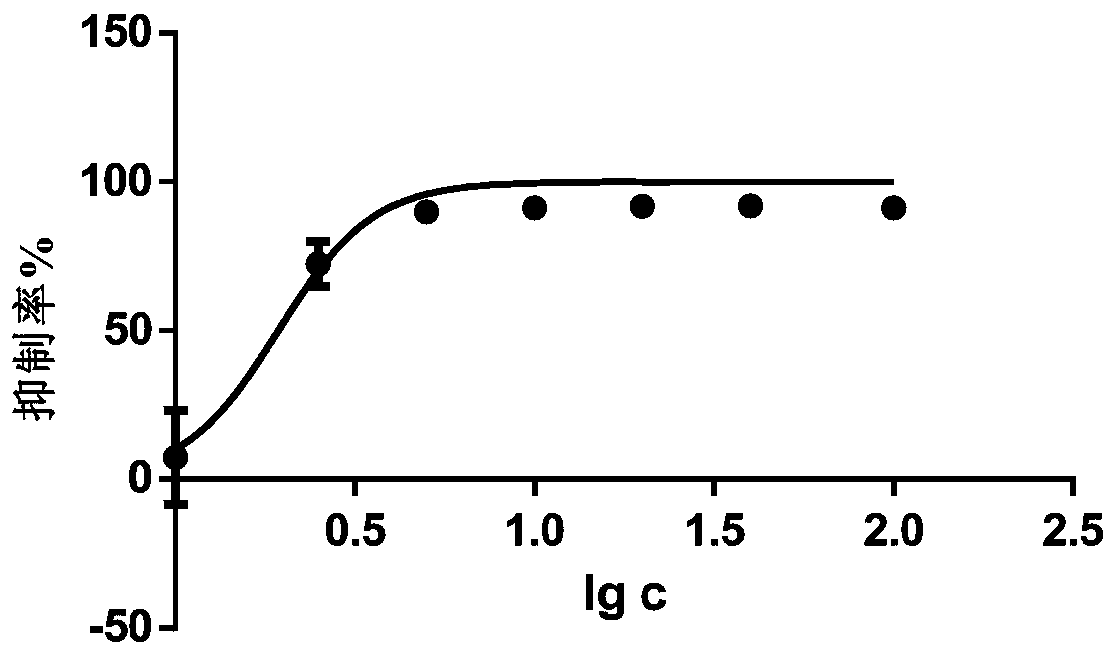
Application of pedf in preparation of medicine for treating myeloproliferative diseases
ActiveCN112516289BPromote proliferationPrevent proliferationPeptide/protein ingredientsAntineoplastic agentsDiseasePharmaceutical drug
Owner:XUZHOU MEDICAL UNIV
Application of ophiopogonin compound in preparation of drugs for preventing and treating tumors
ActiveCN111494397AHas broad-spectrum anti-cancer effectsOvercome the shortcomings of efficacyOrganic active ingredientsAntineoplastic agentsBenign tumoursDisease
The invention provides the application of an ophiopogonin compound in the preparation of drugs for preventing and treating tumors, wherein the tumor is a benign tumor or a malignant tumor, and the malignant tumor is selected from one or more of lung cancer, liver cancer, stomach cancer, breast cancer, colon cancer, leukemia, multiple myeloma, lymphoma, malignant melanoma, myeloproliferative disease solid tumor or hematological malignant tumor. The invention also provides an anti-tumor pharmaceutical composition, which contains a therapeutically effective amount of the compound shown in the formula (I) or pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier. As a compound capable of remarkably inhibiting tumor cell proliferation, the compound shown in the formula (I) or the pharmaceutically acceptable salt thereof has the advantages of small potential toxic and side effects, remarkable inhibition of tumor cell proliferation, broad-spectrum cancer resistance, easiness in preparation, low cost and the like, and has a wide anti-tumor application prospect clinically.
Owner:EXPERIMENTAL RES CENT CHINA ACAD OF CHINESE MEDICAL SCI
Determining tumor load and biallelic mutation in patients with CALR mutation using peripheral blood plasma
ActiveUS10550435B2Microbiological testing/measurementX-ray/gamma-ray/particle-irradiation therapyTumor LoadMyeloid hyperplasia
Compositions and fragment length analysis methods are provided for detecting CALR mutations and determining tumor load in patients with myeloproliferative neoplasms.
Owner:NEOGENOMICS LAB
Methods of Treating Cancer
PendingUS20210186946A1Organic chemistry methodsPharmaceutical delivery mechanismBone marrow fibrosisParanasal Sinus Carcinoma
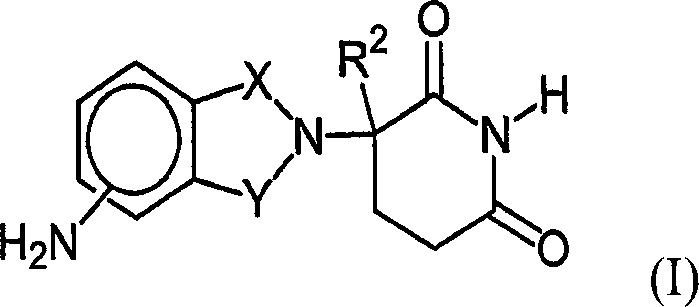
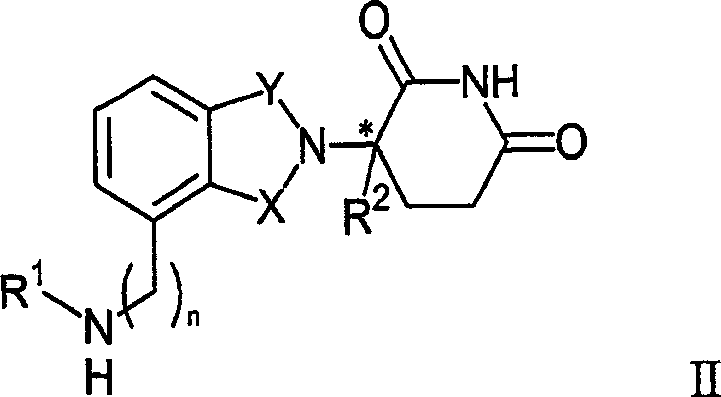
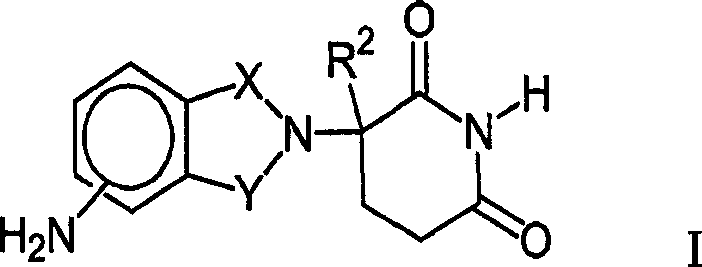
Therapeutic methods and pharmaceutical compositions for treating cancer including a myeloproliferative neoplasm (MPN), including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis in a human subject are described. In certain embodiments, the invention includes therapeutic methods of treating a MPN using a MDM2 inhibitor of Formula (I) or Formula (II).
Owner:KARTOS THERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com