Pharmaceutical composition and application thereof in preparation of drugs for treating targeted calreticulin mutation type myeloproliferative diseases
A technology for bone marrow proliferation and calreticulin, applied in the field of biomedicine, can solve the problems of patient recurrence and inability to eradicate mutant cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
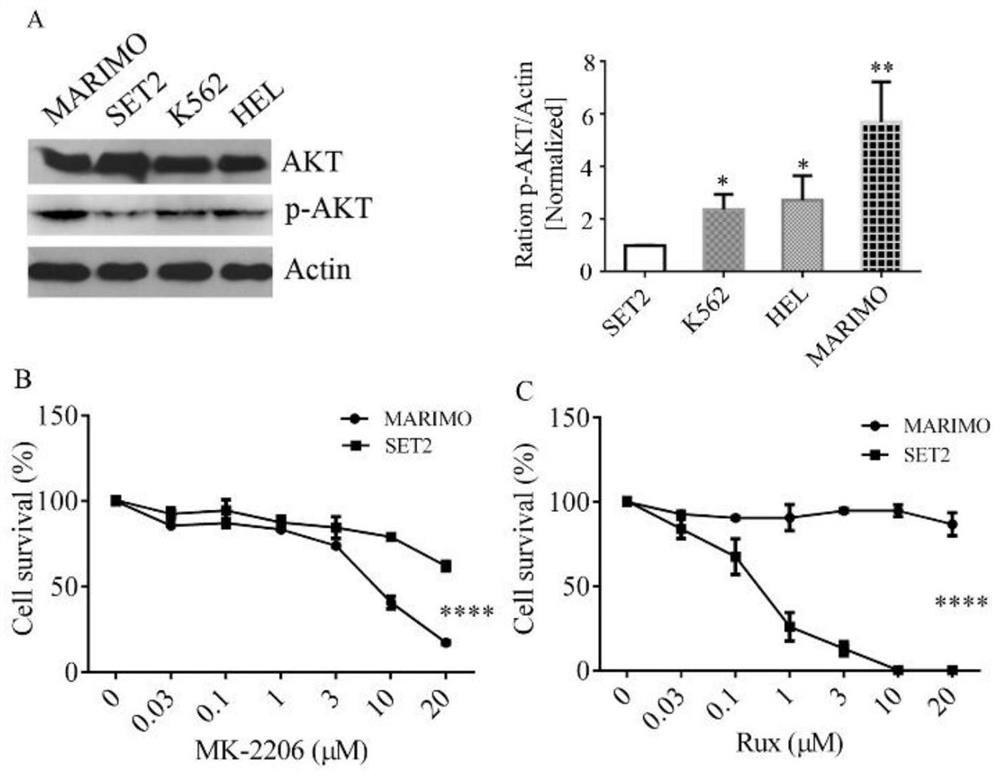
[0030] Reference attached figure 1 , detection of AKT and P-AKT levels in MARIMO cells, JAK2V617F mutant cell lines (HEL and SET2), and other myeloid cells of CML (K562) patients, the levels of phosphorylated AKT in MARIMO cells were significantly higher, while HEL and K562 Phosphorylated AKT levels were nearly 1.5-fold higher in cells and more than 5-fold higher in SET 2 cells ( figure 1 A). 10 μM MK-2206 exerted more than 50% growth inhibitory effect in MARIMO cells. The same treatment with 20 μM Rux did not reduce the survival of MARIMO cells while SET 2 cells were completely killed at 10 μM Rux ( figure 1 B and C). It shows that AKT is activated in MARIMO cells, and AKT inhibitors can inhibit the growth of MARIMO. JAK2 inhibitors did not affect the growth of MARIMO cells, but had a significant inhibitory effect on SET2 cells.
Embodiment 2
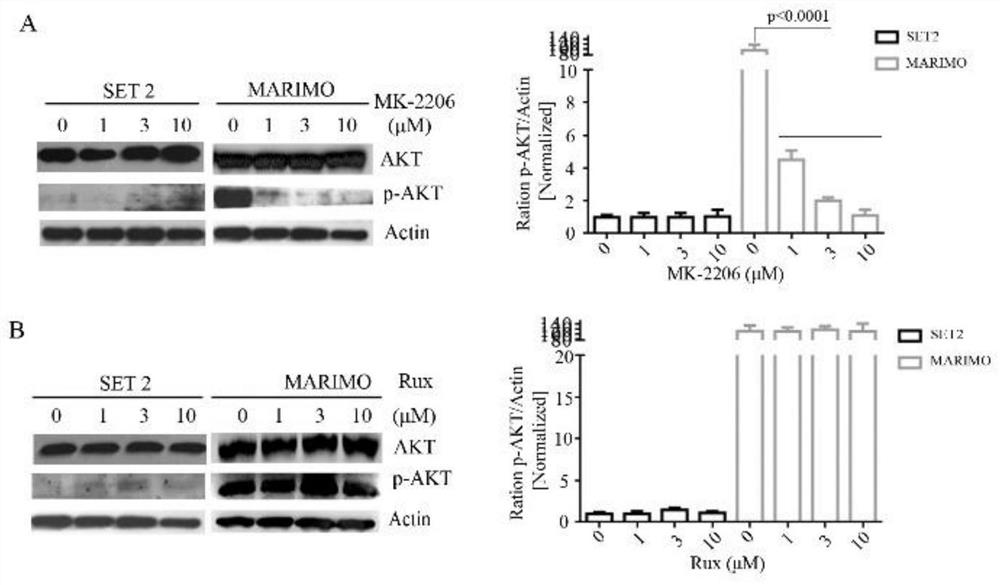
[0032] Reference attached figure 2 , after treating SET2 and MARIMO cells with 0μM, 1μM, 3μM, 10μM MK-2206 and JAK2 inhibitor Rux, respectively, the expression levels of AKT and P-AKT were detected by Western blot. The results of Western blot showed that MK-2206 could significantly reduce the level of P-AKT in MARIMO cells, and the expression of P-AKT in MARIMO cells did not change significantly after Rux treatment ( figure 2 A and B). It shows that MARIMO cells depend on AKT but not on JAK-STAT signaling pathway.
Embodiment 3
[0034] Reference attached image 3 , MARIMO cells were treated with 0 μM, 1 μM, 3 μM, and 10 μM MK-2206 for 48 hours, stained with EDU-Alexa Fluor647-A and DAPI flow cytometry antibodies, and detected the cell cycle. After the above treatment, the expression levels of CyclinD and CyclinE were detected by Western blot. The statistical results of flow cytometry showed that compared with the control group, cells in -G1 phase treated with 1 to 10 μM K-2206 were significantly arrested, and the proportion of cells in S phase labeled with EdU decreased from the control percentage in MARIMO cells are ~41%, ~39.5% and ~36% ( image 3 A and B). The expressions of CyclinD and CyclinE were also significantly decreased after MMK-2206 treatment, and Western blot quantification also showed that the down-regulation of CyclinD and CyclinE in the cell cycle was dose-dependent. It shows that AKT inhibition can induce MARIMO cell cycle arrest.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com