Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
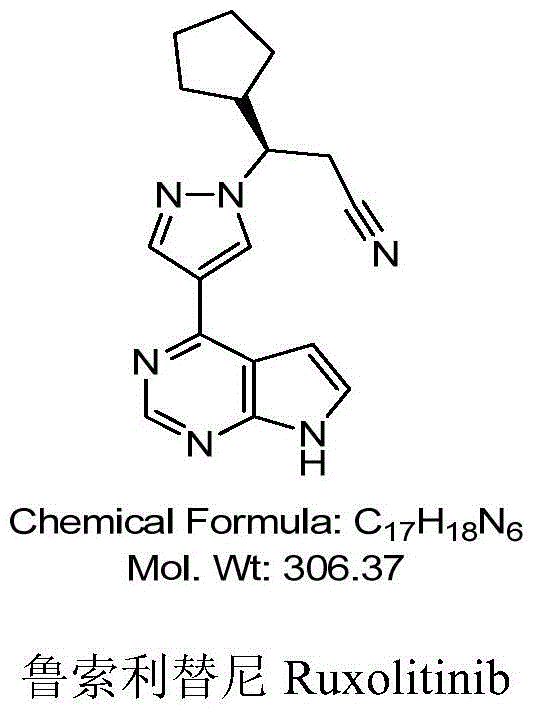
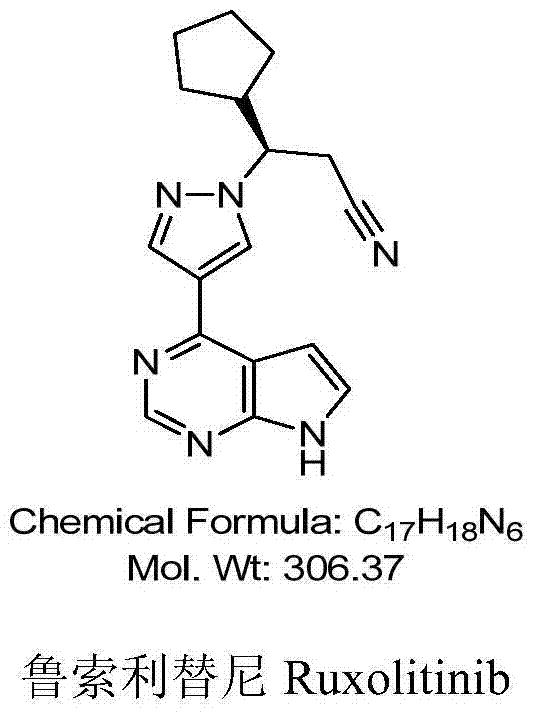
43 results about "Ruxolitinib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat certain bone marrow disorders (myelofibrosis, polycythemia vera).
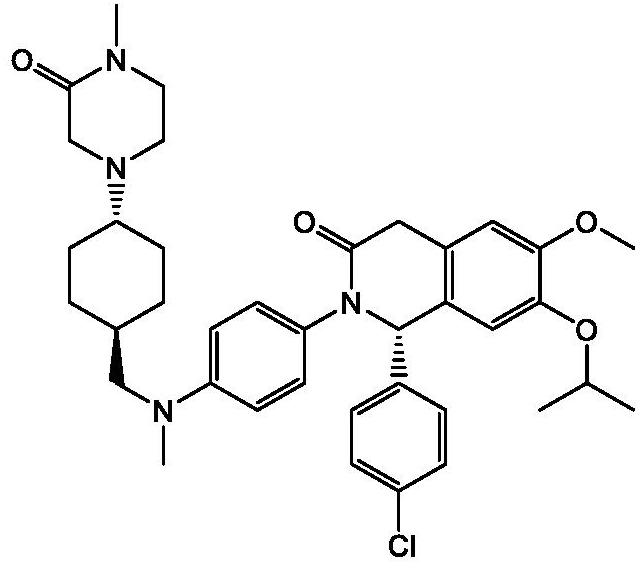
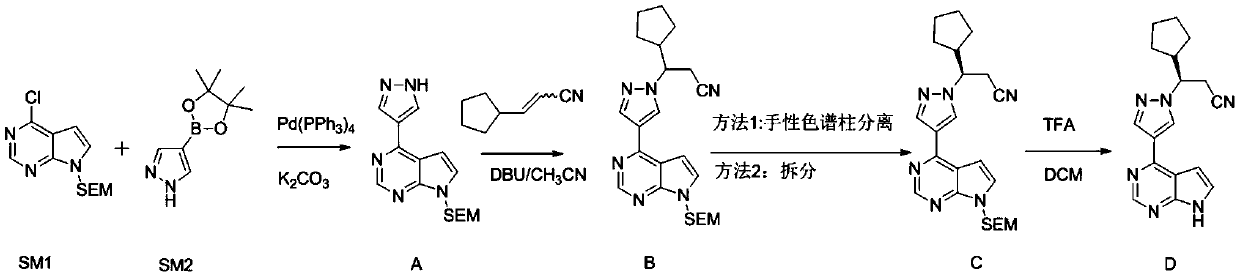
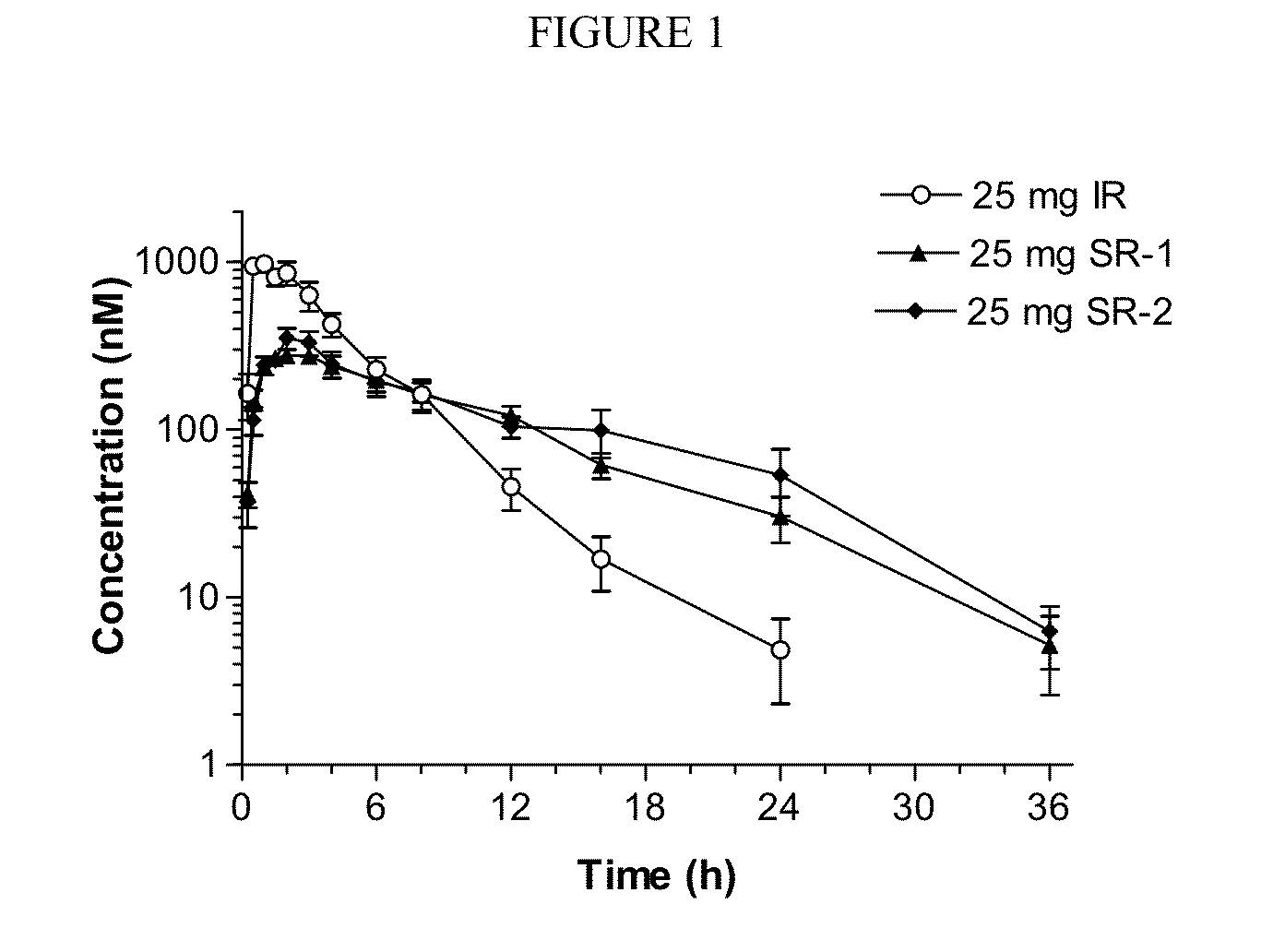
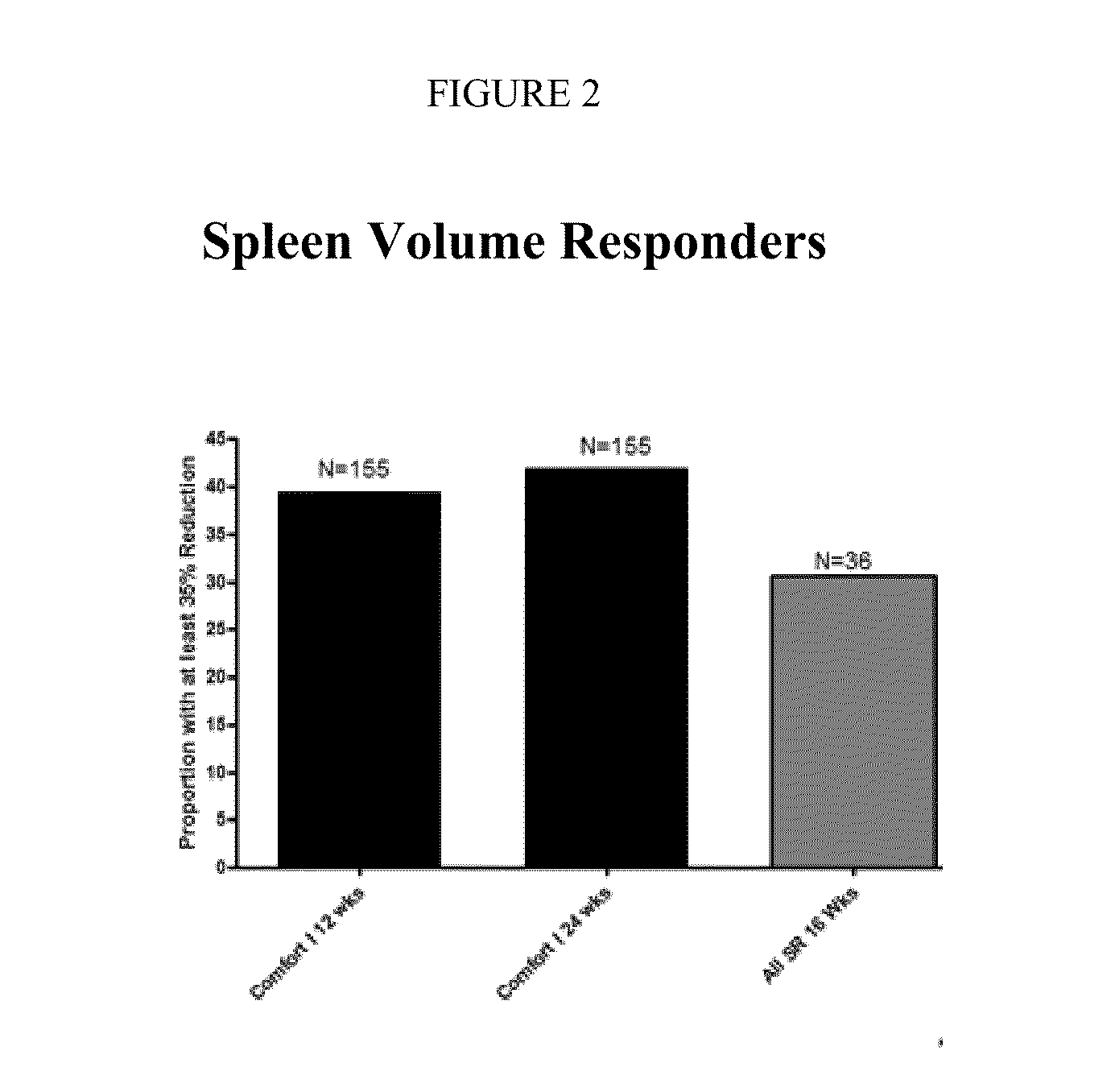
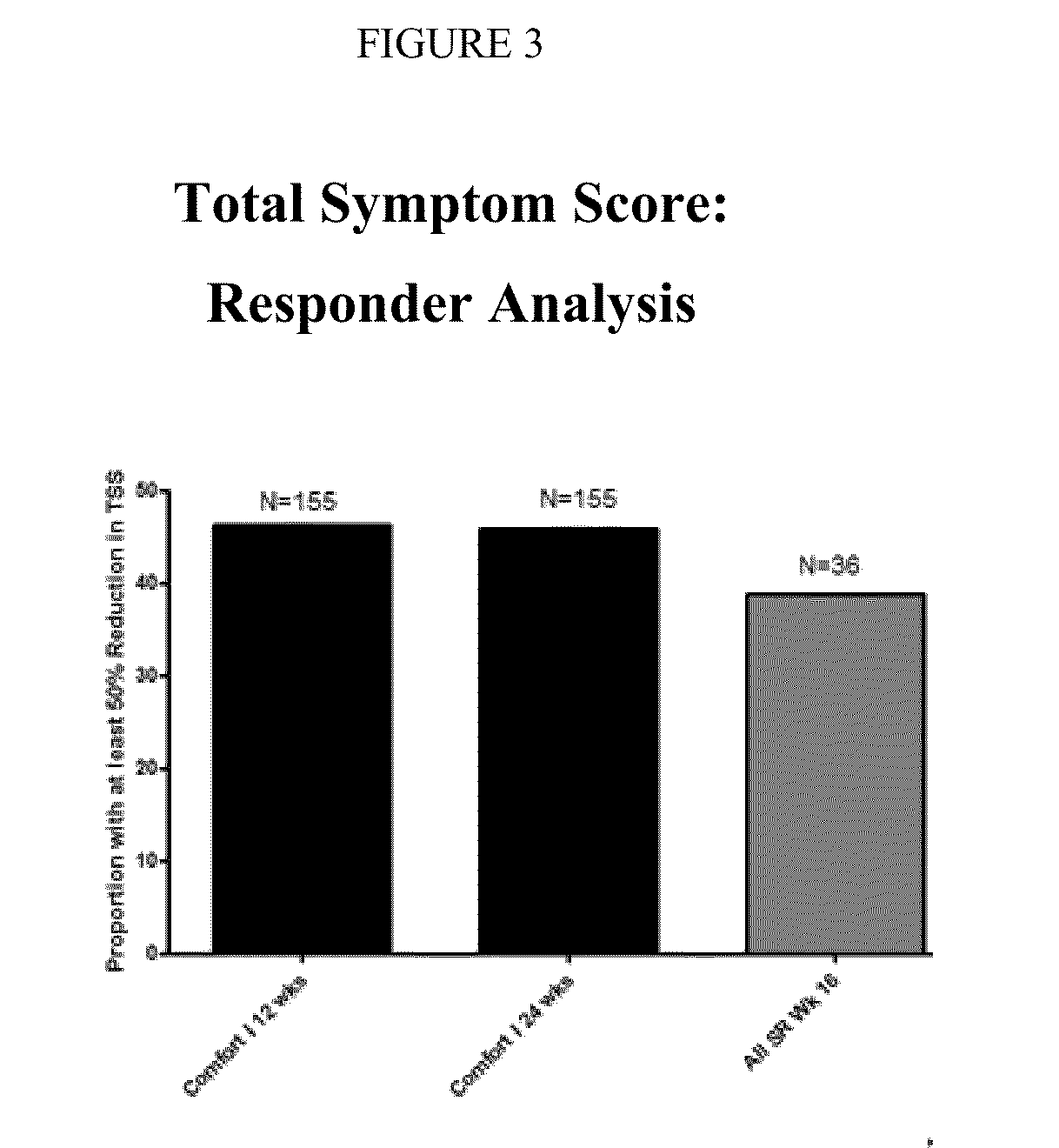
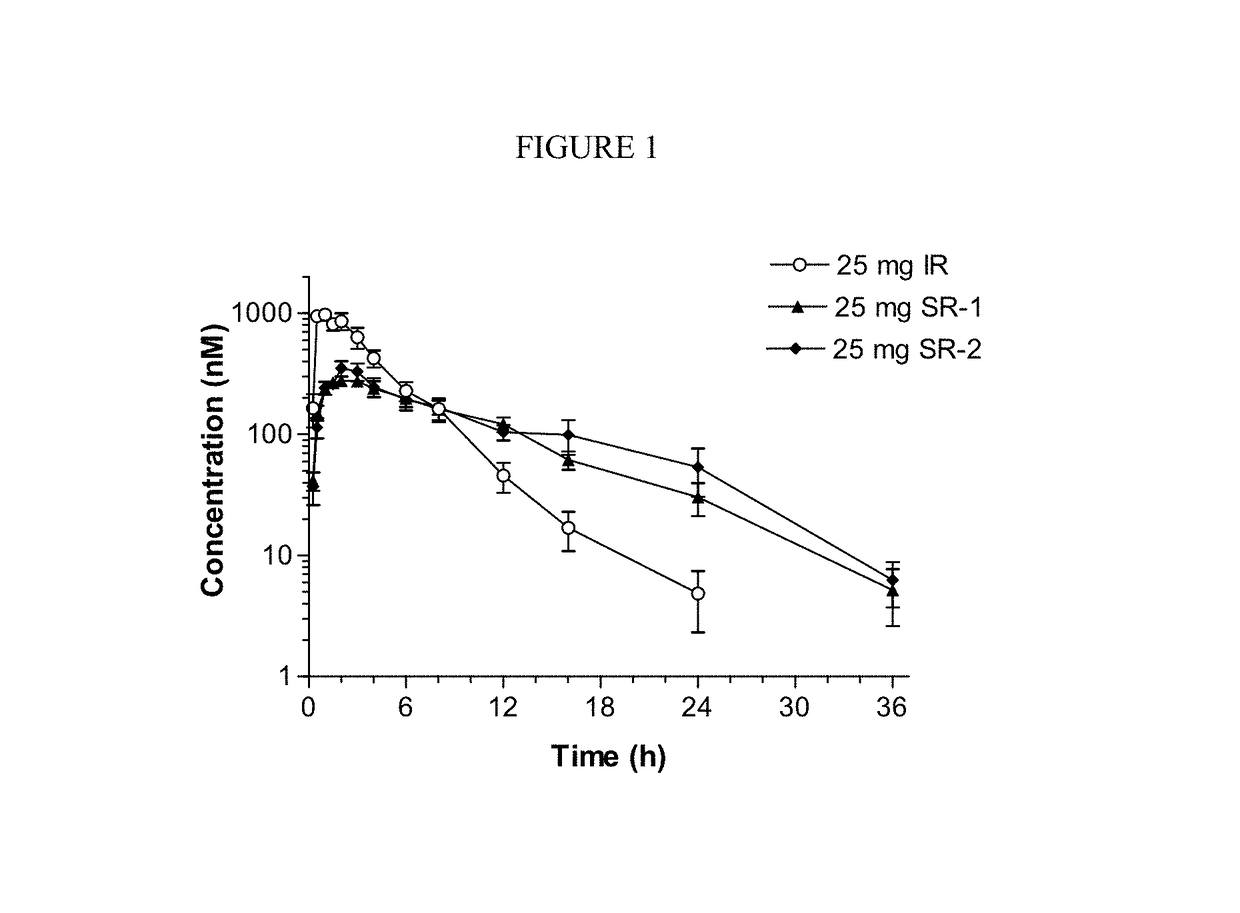
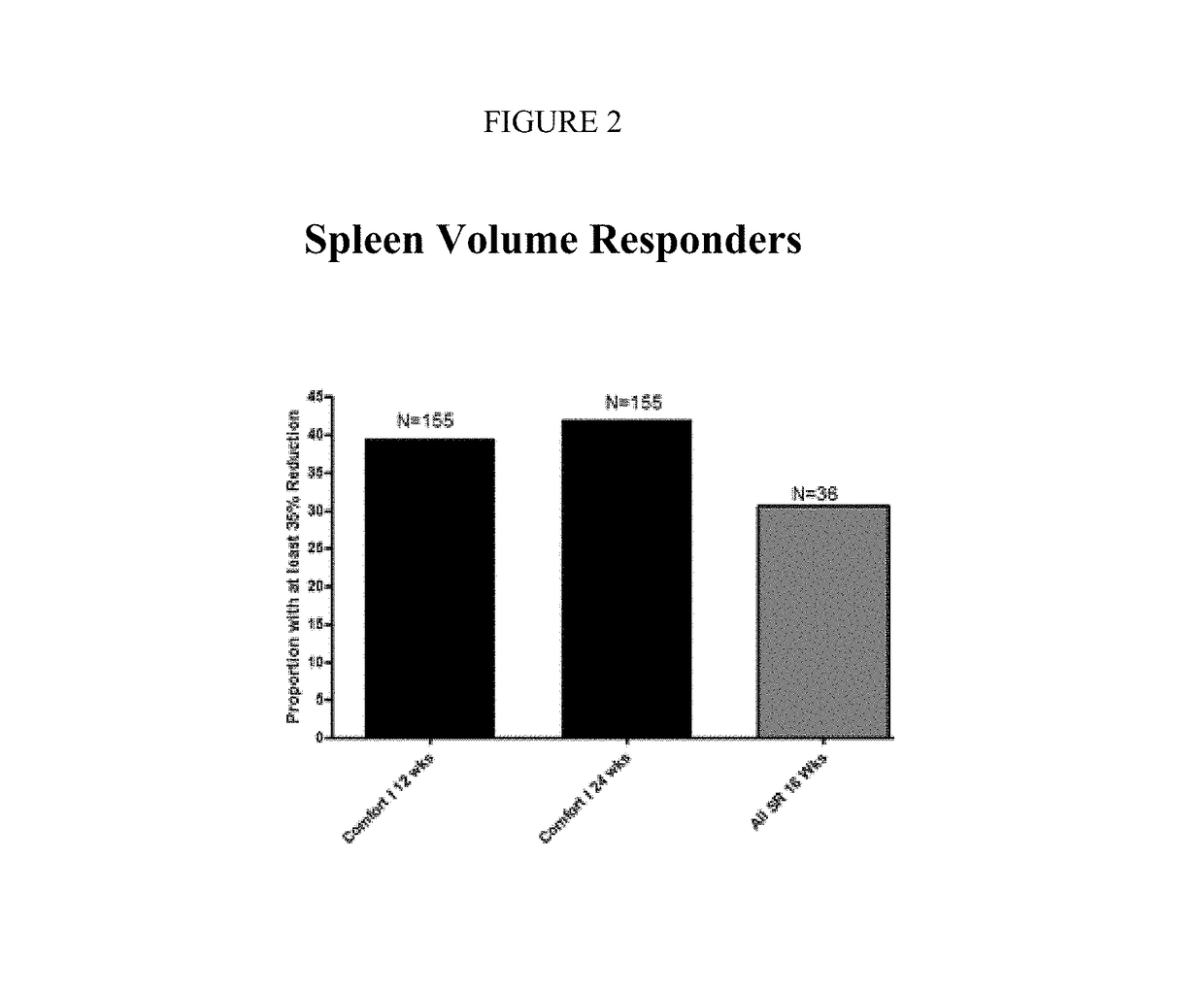
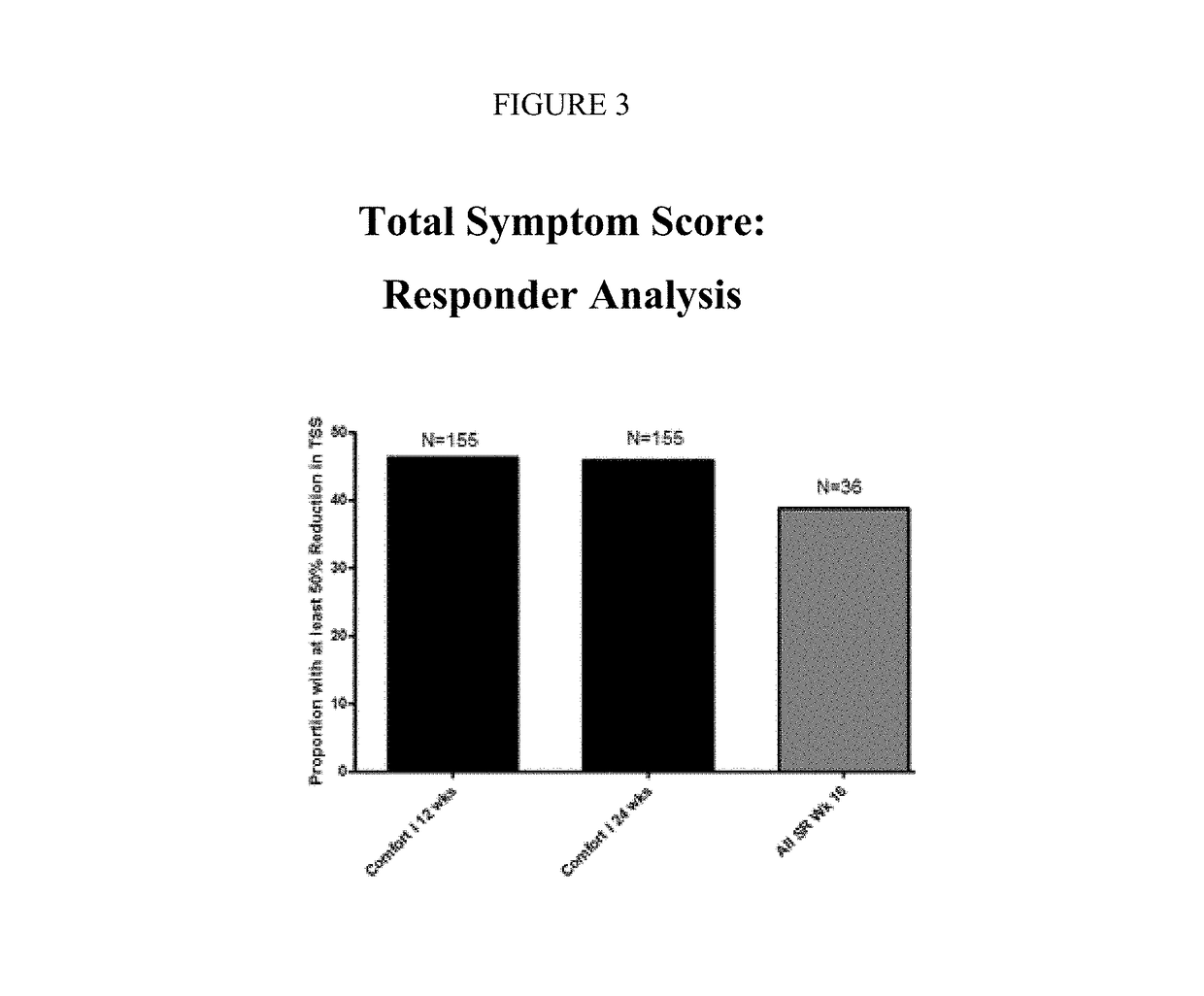
Sustained-release dosage forms of ruxolitinib
The present invention relates to sustained-release formulations and dosage forms of ruxolitinib, or a pharmaceutically acceptable salt thereof, which are useful in the treatment of Janus kinase-associated diseases such as myeloproliferative disorders.
Owner:INCYTE HLDG & INCYTE
Sustained-release dosage forms of ruxolitinib
ActiveUS10166191B2Lower the volumeOrganic active ingredientsSenses disorderDiseaseSustained release drug
The present invention relates to sustained-release formulations and dosage forms of ruxolitinib, or a pharmaceutically acceptable salt thereof, which are useful in the treatment of Janus kinase-associated diseases such as myeloproliferative disorders.
Owner:INCYTE HLDG & INCYTE
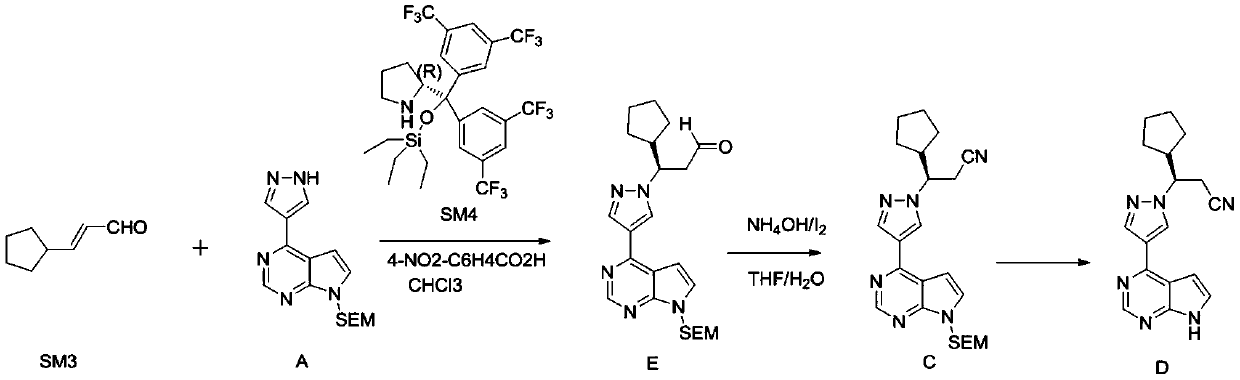
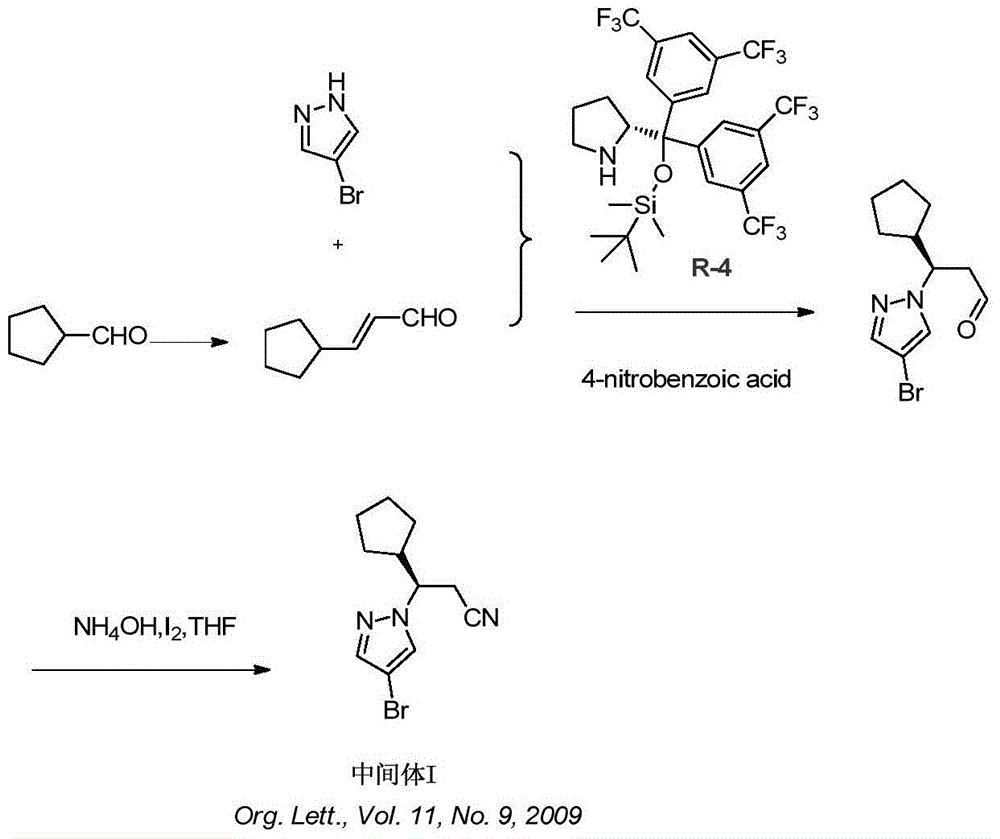
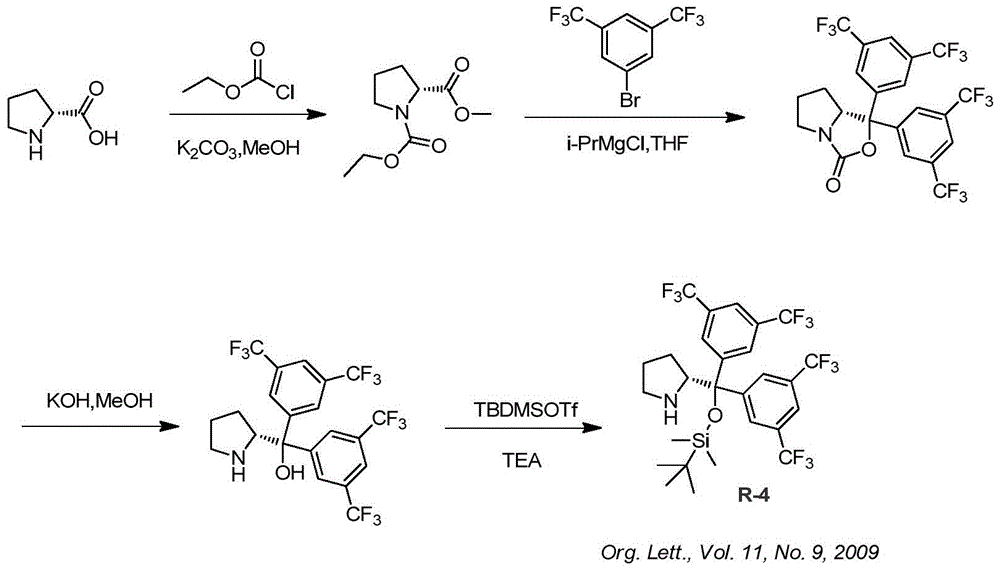
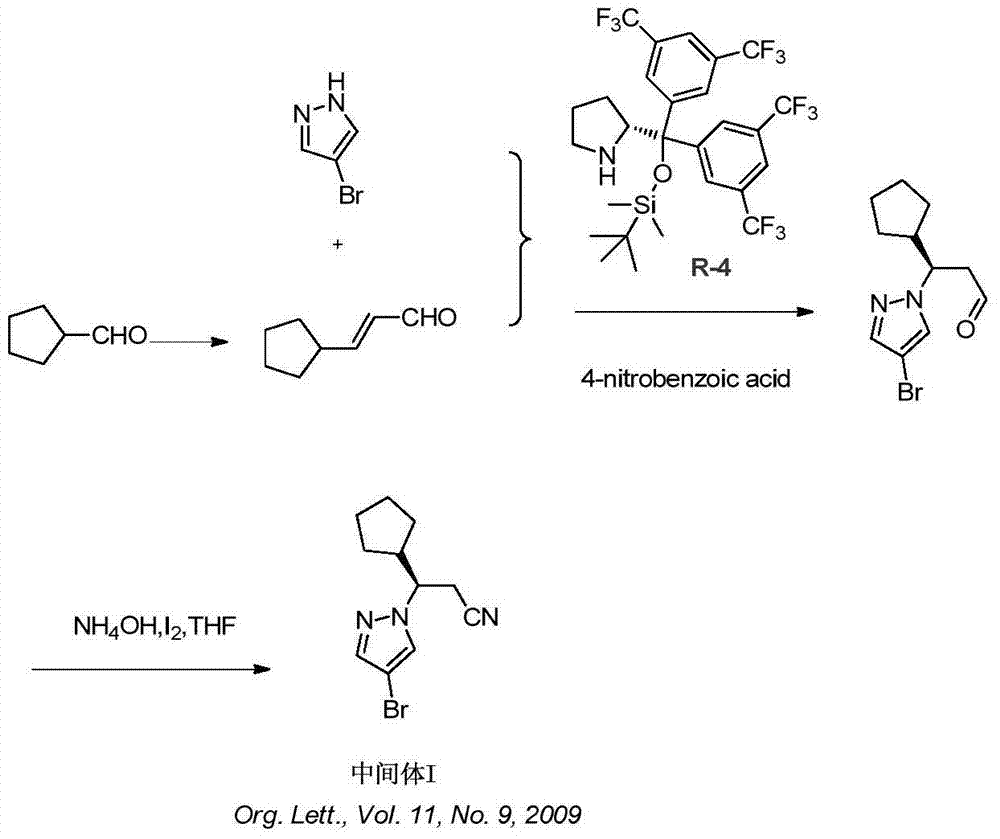
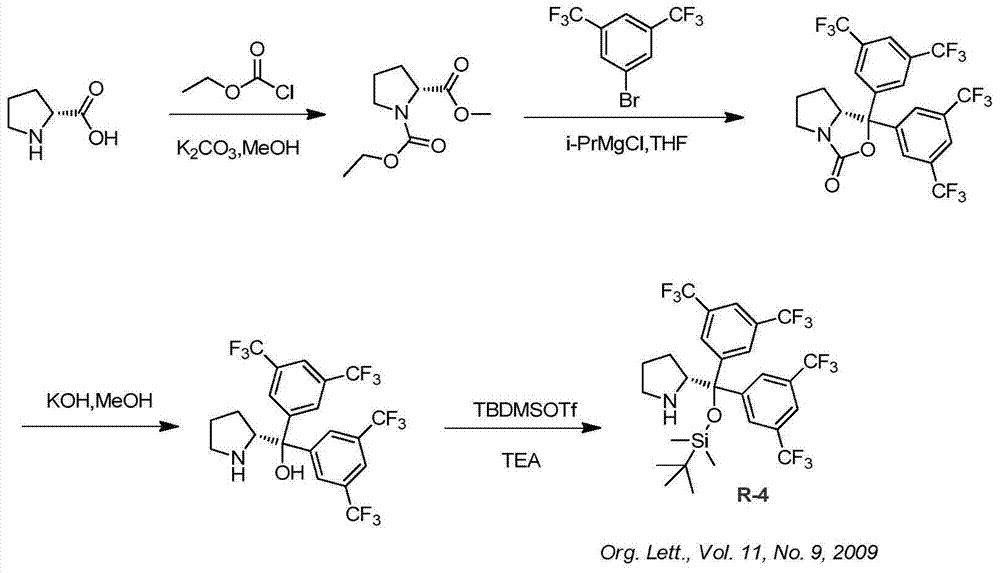
Synthesis method of ruxolitinib intermediate
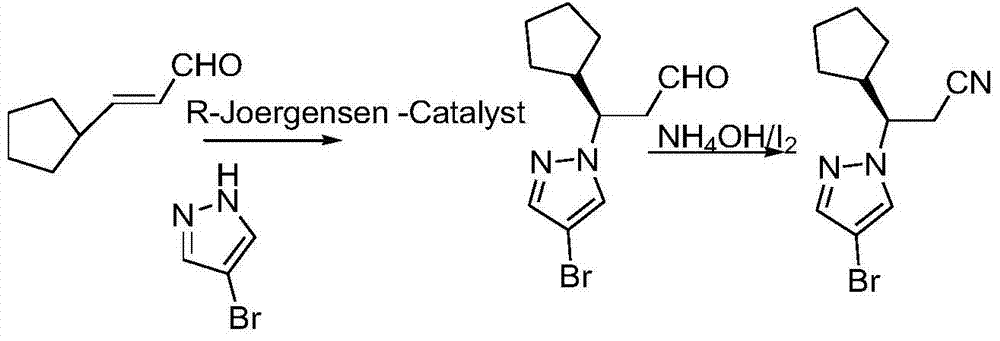
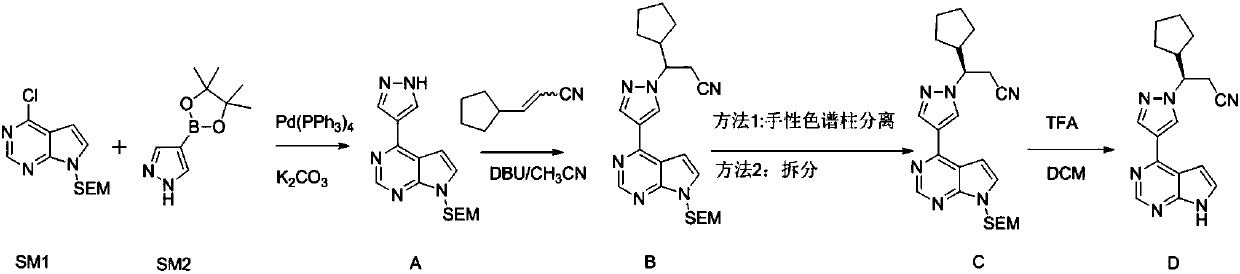
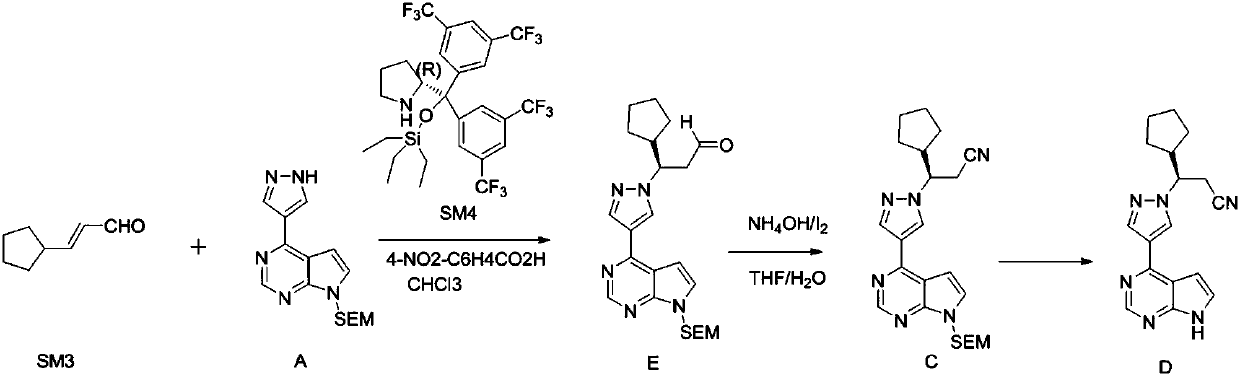
The invention relates to a synthesis method of a ruxolitinib intermediate. The method comprises the following steps: firstly, carrying out catalytic reaction on cyclopentane methyl formate and acetonitrile to prepare 3-cyclopentyl-3-oxypropionitrile; carrying out enzymatic asymmetric reduction on 3-cyclopentyl-3-oxypropionitrile to generate chiral alcohol (S)-3-cyclopentyl-3-oxypropionitrile; and carrying out Mitsunobu reaction and 4-bromopyrazole coupling on (S)-3-cyclopentyl-3-oxypropionitrile to obtain ruxolitinib intermediate (3R)-3-(4-bromo-1H-pyrazole-1-yl)-3-cyclopentane propionitrile. The synthesis method has the advantages of short route, low cost, mild condition and good stereoselectivity, and is suitable for industrialized mass production.
Owner:BIOCOMPOUNDS PHARMACEUTICAL INC +1
Synthetic method of 4-chloro-7H-pyrrolo[2,3-d] pyrimidine
The invention discloses a synthetic method of 4-chloro-7H-pyrrolo[2,3-d] pyrimidine. The compound is an important intermediate for synthesizing rheumatoid arthritis JAK inhibitors: ruxolitinib and tofacitinib. The synthetic method comprises the following steps: by taking a compound I (4,6-dichloro-5-allyl pyrimidine) as an initial raw material, performing a nucleophilic substitution reaction with ammonia water to generate a compound II; then performing a reaction on the compound II and ozone and performing a reduction reaction to generate a compound III; and finally, performing self ring-closing reaction in an acidic environment to generate a compound IV, that is, the 4-chloro-7H-pyrrolo[2,3-d] pyrimidine. The synthetic route is as shown in the formula in the description. According to the synthetic process provided by the invention, the raw material is cheap and easily available, the synthetic route is simple, the cost is low, the yield is high, and the synthetic method is easy for industrial production.
Owner:EAST CHINA NORMAL UNIV +1
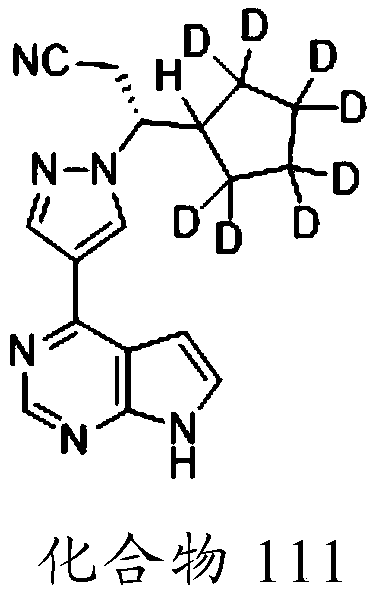
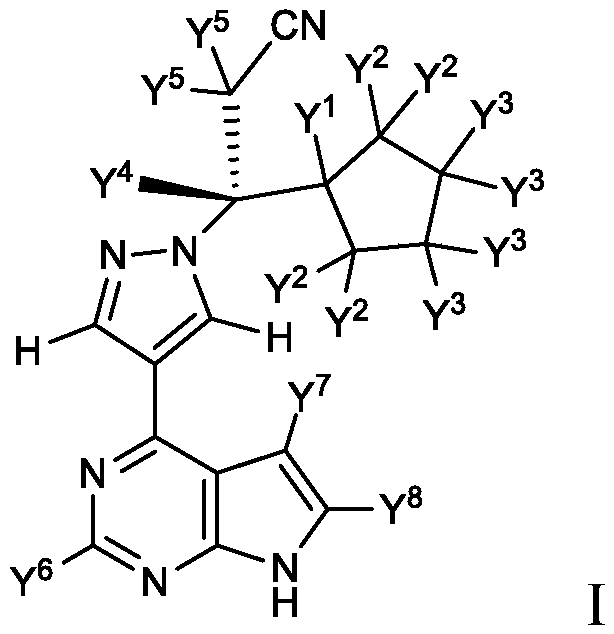
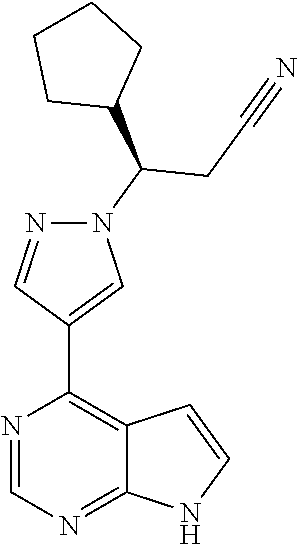
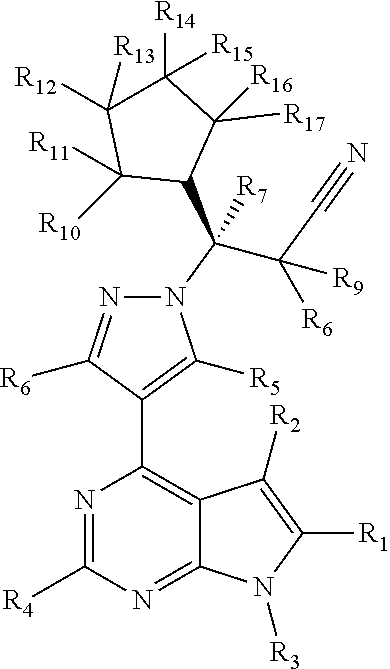
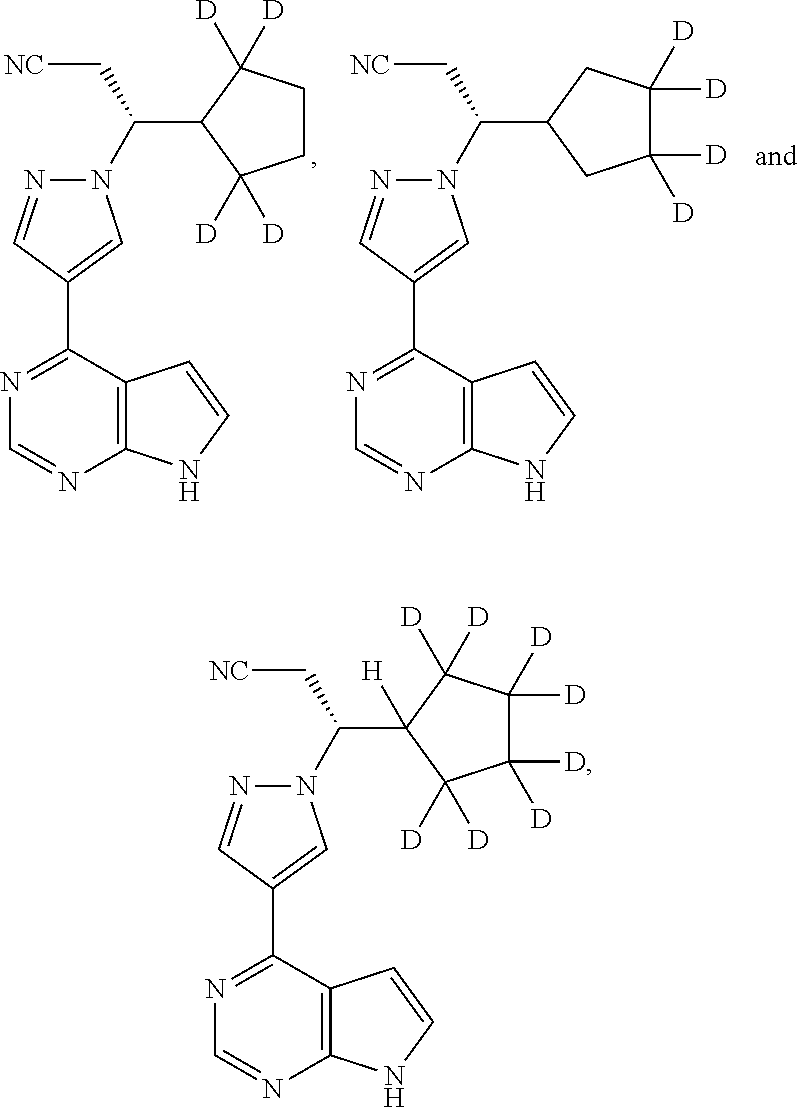
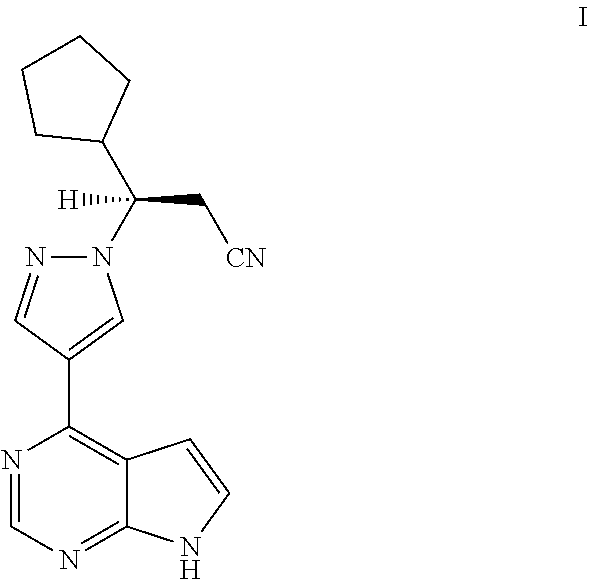
Deuterated Derivatives of Ruxolitinib
Owner:SUN PHARMA IND INC
Deuterated derivatives of ruxolitinib
Owner:CONCERT PHARMA INC
4-chloro-7H-pyrrolo[2,3-d]pyrimidine synthetic method
The invention discloses a 4-chloro-7H-pyrrolo[2,3-d]pyrimidine synthetic method. A compound is an important intermediate for synthesizing ruxolitinib and tofacitinib as a JAK inhibitor for treating rheumatoid arthritis. The 4-chloro-7H-pyrrolo[2,3-d]pyrimidine synthetic method comprises the following steps of by taking a compound I (4,6-dichloro-5-allyl pyrimidine) as a starting material, performing oxidation reaction on the compound I and ozone to produce a compound II; then performing nucleophilic substitution reaction on the compound II and triethyl orthoformate to produce a compound III; then performing nucleophilic substitution reaction on the compound III and ammonia gas to produce a compound IV; and finally, performing ring closing on the compound IV self in an acid environment to produce a compound V, i.e., 4-chloro-7H-pyrrolo[2,3-d]pyrimidine, wherein a synthetic route is shown as the following formula (described in the description). The synthetic method disclosed by the invention is cheap and available in raw materials, simple and short in synthetic route, low in cost, high in yield and easy in industrial production.
Owner:EAST CHINA NORMAL UNIVERSITY +1
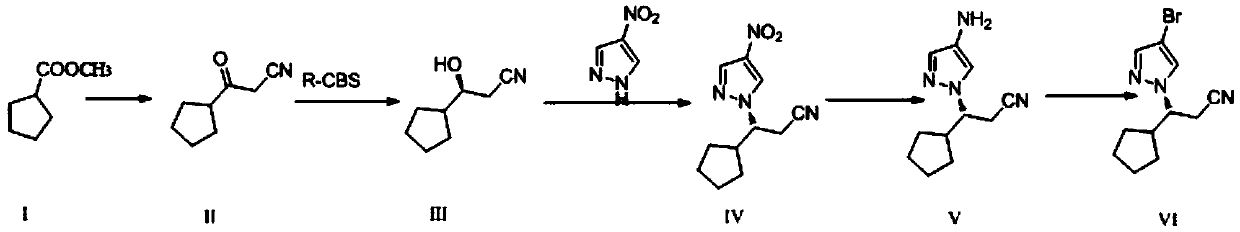
Preparation method of ruxolitinib intermediate (3R)-3-(4-Br-1H-pyrazole-1-yl)-cyclopentyl propanenitrile
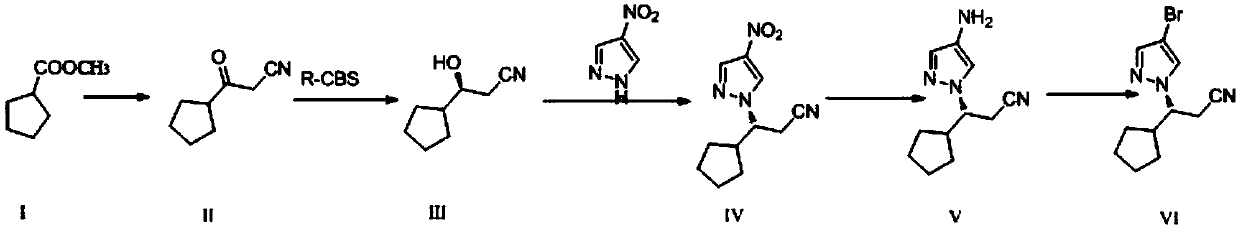
The present invention relates to a preparation method of ruxolitinib intermediate (3R)-3-(4-Br-1H-pyrazole-1-yl)-cyclopentyl propanenitrile, and the method comprises the following steps: (1) synthesisof 3-oxo-3-cyclopentyl propionitrile (II); (2) synthesis of (S)-3-cyclopentyl-3-hydroxypropionitrile (III); (3) synthesis of (3R)-3-(4-nitro-1H-pyrazole-1-yl)-cyclopentyl propionitrile (IV); (4) synthesis of (3R)-3-(4-amino-1H-pyrazole-1-yl)-cyclopentyl propionitrile (V); and (5) (3R)-3-(4-Br-1H-pyrazole-1-yl)-cyclopentyl propanenitrile (VI). The method has the advantages of good stereoselectivity, low cost, mild reaction conditions, no requirement on harsh reaction such as high temperature, high pressure and ultra-low temperature.
Owner:海化生命(厦门)科技有限公司
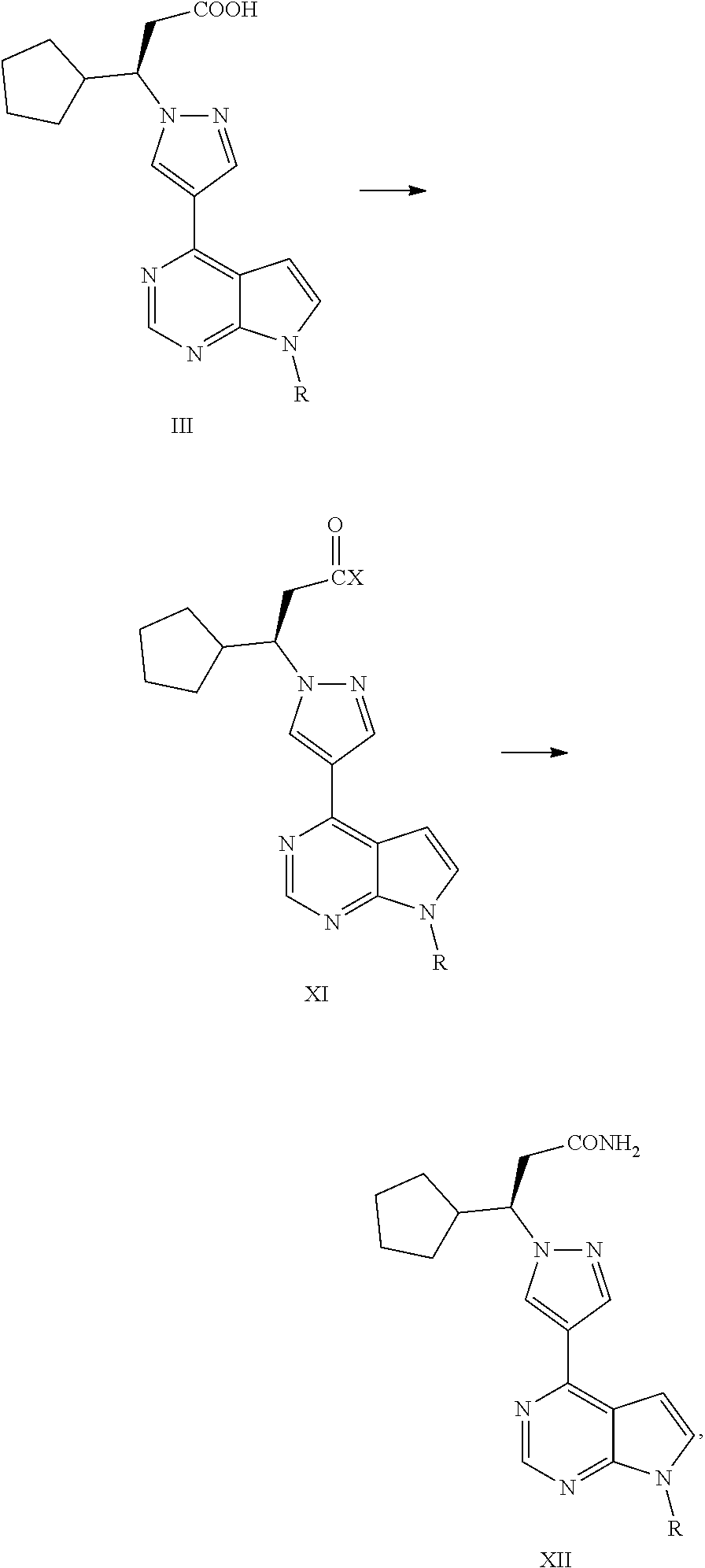
Preparation methods of JAK inhibitor and salt thereof
ActiveCN107759601AHigh yieldHigh reaction yieldPreparation by aldehyde oxidation-reductionGroup 3/13 element organic compoundsHalogenRuxolitinib
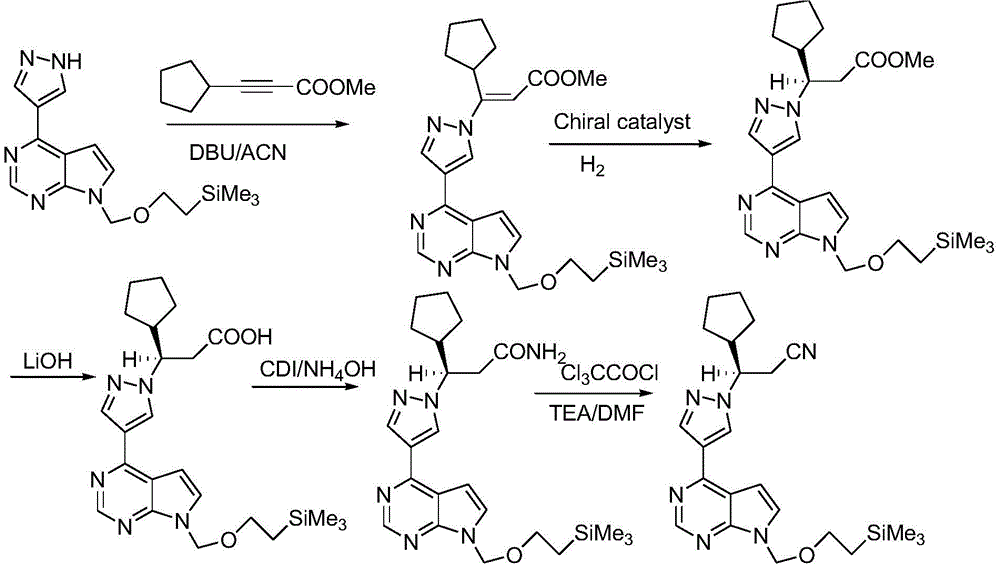
The present invention relates to preparation methods of a JAK inhibitor and a salt thereof. The preparation method comprises: (1) carrying out a Suzuki coupling reaction on (R)-3-(4-boronic acid-1H-pyrazol-1-yl)-3-cyclopentylpropionitrile and 6-halogen-5-(2-methoxyvinyl)pyrimidin-4-ylamine to generate (3R)-cyclopentyl-3-[4-(5-(2-methoxyvinyl)pyrimidin-4-ylamine)pyrazol-1-yl]propionitrile; and (2)carrying out a protection group removing and ring-closure reaction on the (3R)-cyclopentyl-3-[4-(5-(2-methoxyvinyl)pyrimidin-4-ylamine)pyrazol-1-yl]propionitrile to generate a JAK inhibitor ruxolitinib. According to the present invention, the new ruxolitinib preparation route is provided, wherein each reaction of the route has the high yield, the total yield of the route is high, the purity of theobtained product is good, the post-treatment of the reaction is simple, and column chromatography is not required; by adopting the route, the required raw materials or catalysts and other materials are relatively easy to obtain; and compared to the method in the prior art, the method of the present invention is economical and is suitable for industrial production.
Owner:SUZHOU VIGONVITA LIFE SCIENCES CO LTD
Intermediate of JAK inhibitor, and preparation method thereof
ActiveCN107759623AHigh yieldHigh reaction yieldGroup 3/13 element organic compoundsState of artRuxolitinib
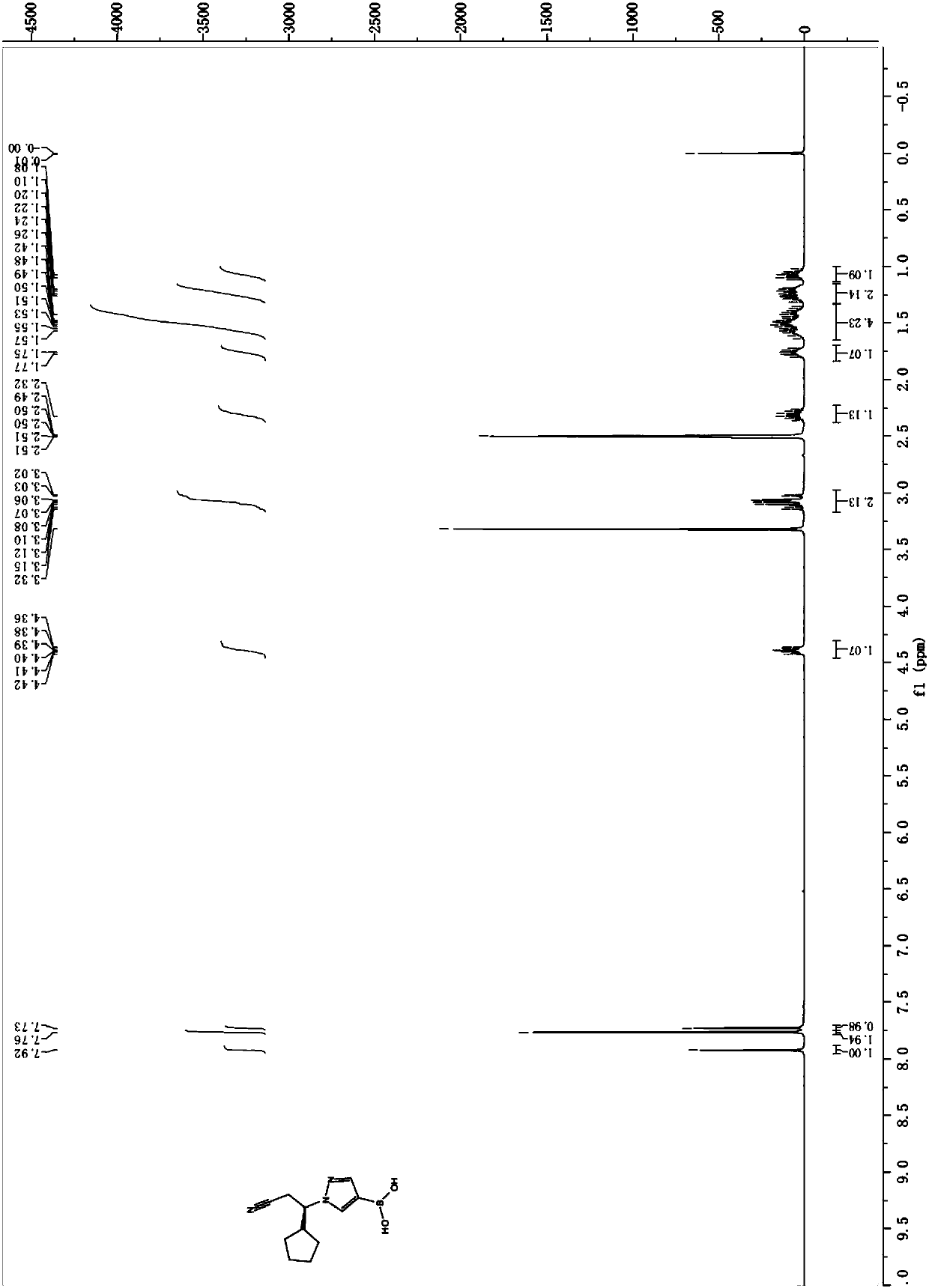
The present invention relates to a novel key intermediate of a JAK inhibitor ruxolitinib, and a preparation method thereof, wherein the chemical name of the intermediate is (R)-3-(4-boric acid-1H-pyrazole-1-yl)-3-cyclopentylpropionitrile. According to the present invention, the new ruxolitinib preparation route is provided, wherein each reaction of the route has the high yield, the total yield ofthe route is high, the purity of the obtained product is good, the post-treatment of the reaction is simple, and column chromatography is not required; by adopting the route, the required raw materials or catalysts and other materials are relatively easy to obtain; and compared to the method in the prior art, the method of the present invention is economical and is suitable for industrial production.
Owner:SUZHOU VIGONVITA LIFE SCIENCES CO LTD
New application of sorafenib, regorafenib and analogues or derivatives thereof
ActiveCN111870600AExempt from transplantSmall burdenAntineoplastic agentsHeterocyclic compound active ingredientsChemical synthesisRed blood cell
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
Reagent composition for improving cell transfection efficiency
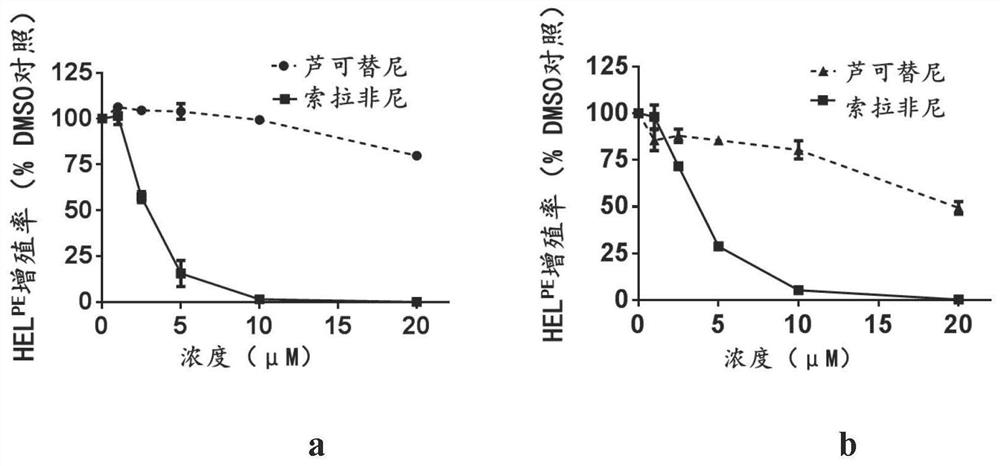
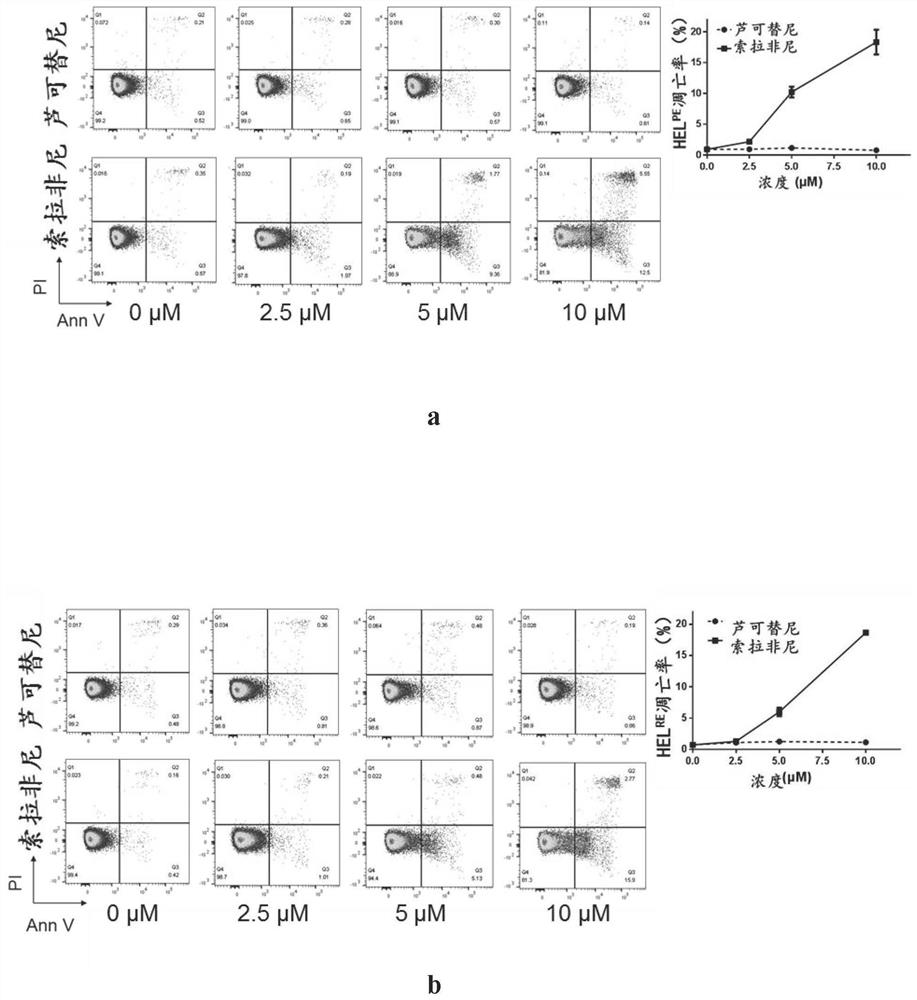
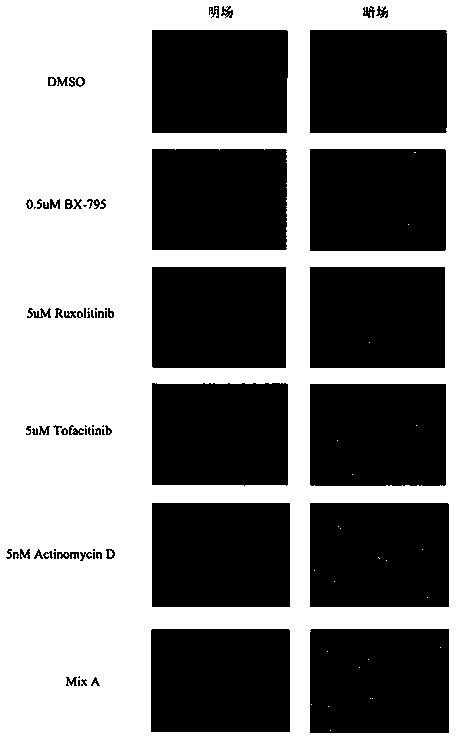
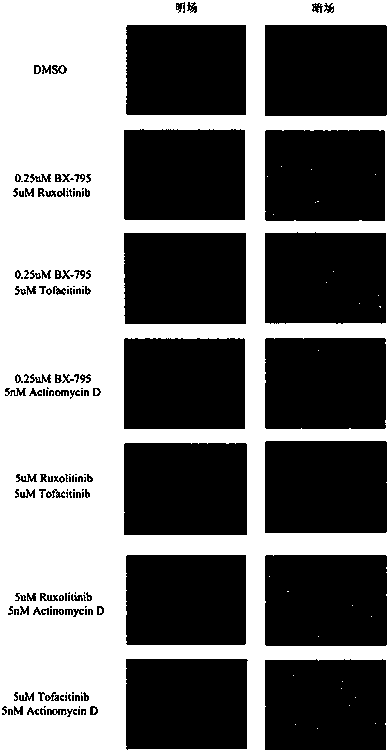
ActiveCN108715865AEasy to operateLow biological toxicityMicroencapsulation basedVector-based foreign material introductionTofacitinibRuxolitinib
The invention relates to a reagent composition for improving the cell transfection efficiency, and belongs to the technical field of biology. The reagent composition comprises two or more of a compound BX975 which is 0.01 to 10 micrometers, a compound Ruxolitinib which is 0.1 to 100 micrometers, a compound Tofacitinib which is 0.1 to 100 micrometers, and a compound Actinomycin D which is 0.1 to 100 nM. Reagent mixed liquor and compounds containing NDA and transfection reagents or lentivirus packaged with destination vector are respectively added into cell culture fluid, so that the cell transfection efficiency is improved. The reagent composition is simple to operate, high in repeatability and large in application range, and is successfully applied to cell lines, such as L929 and BI, and primary cell, such as mice primary lung fibrocyte and T lymphocyte.
Owner:FUJIAN NORMAL UNIV
Method of enhancing immune-based therapy
PendingUS20220000872A1Good curative effectOrganic active ingredientsMammal material medical ingredientsRuxolitinibOncology
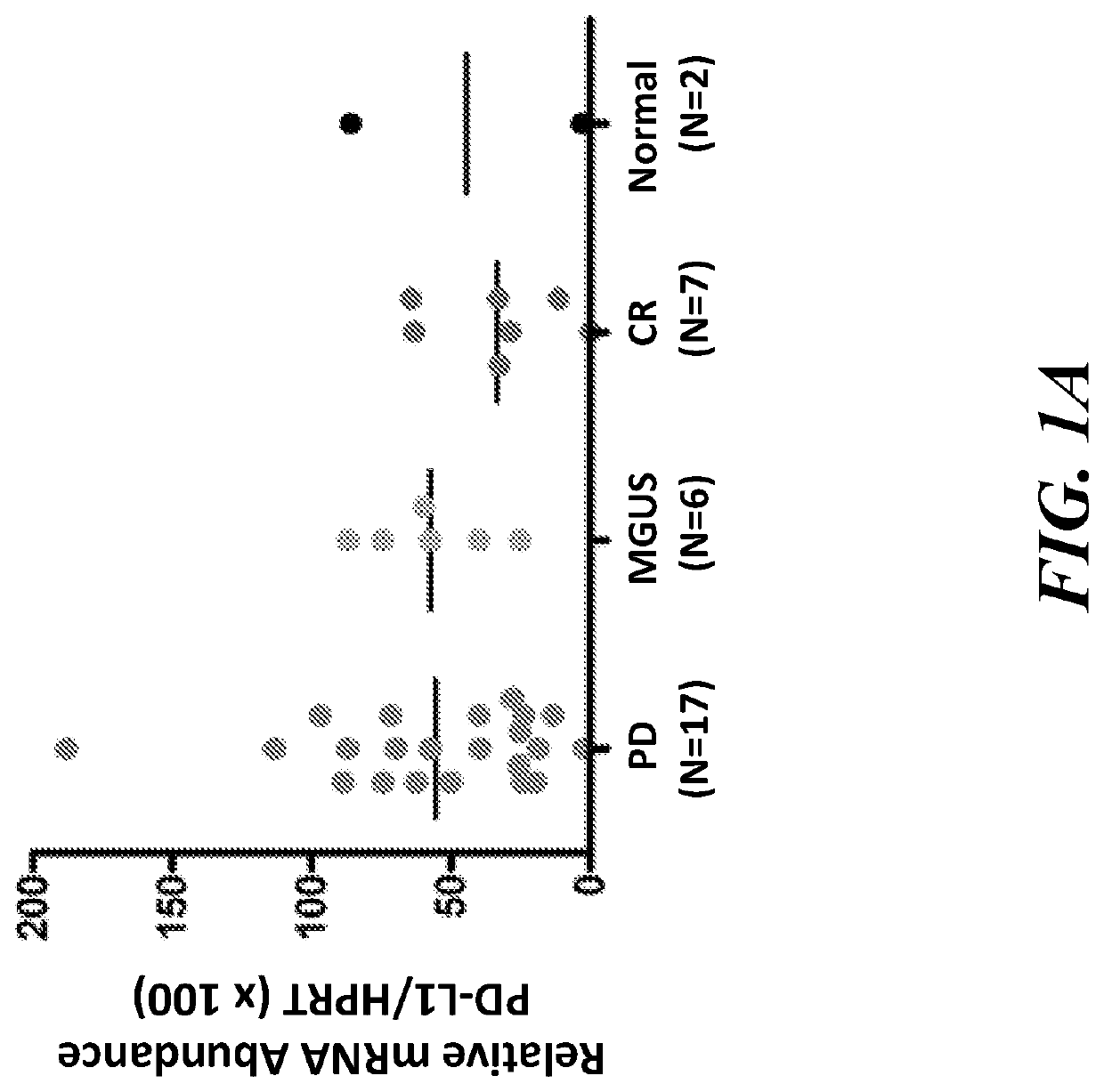
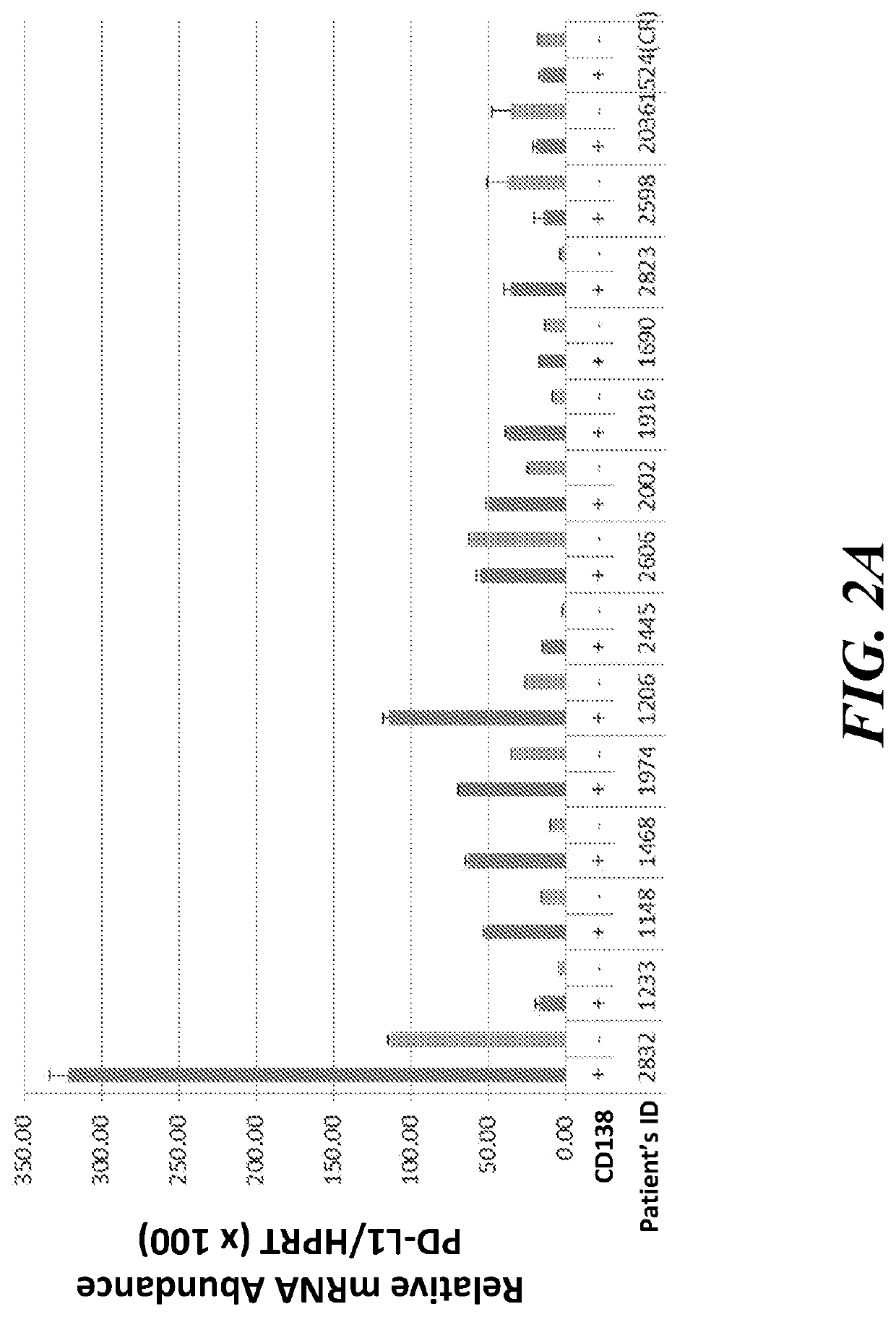
The present invention provides methods of treating and / or inhibiting cancer by administering a JAK1 / 2 inhibitor (e.g., ruxolitinib). The JAK1 / 2 inhibitor decreases expression of (or inhibits increased expression of) the checkpoint proteins PD-1, PD-L1, PD-L2, or B7 H3, and / or enhances T-cell killing of tumor cells, and / or enhances the anti-tumor effects of checkpoint inhibitors. The disclosed methods improve the efficacy of immune-based therapies used in treatment of cancer.
Owner:ONCOTRACKER INC
Synthesis process of ruxolitinib
ActiveUS20190023712A1Mild reaction conditionsShort stepsGroup 4/14 element organic compoundsBlood disorderDrugs synthesisRuxolitinib
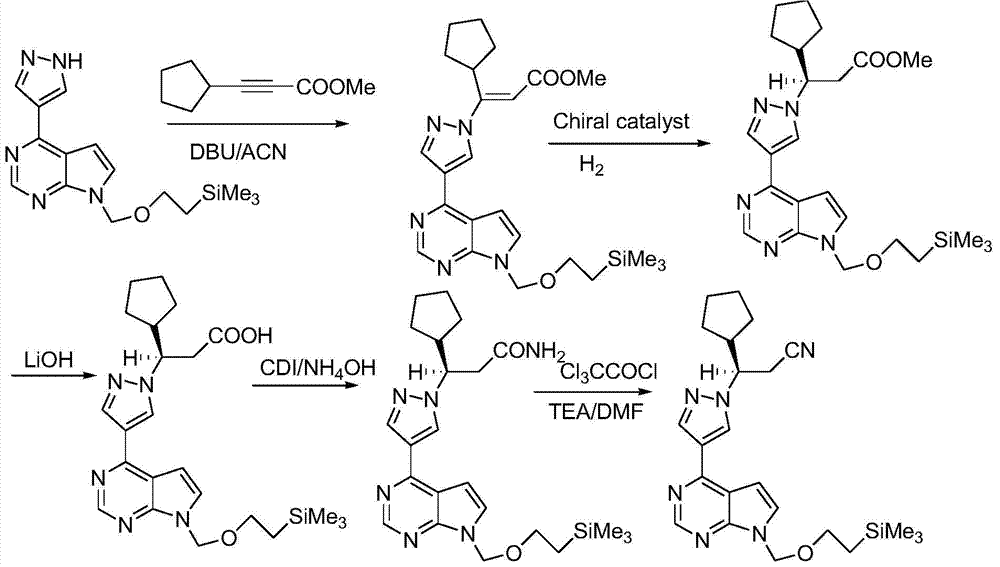
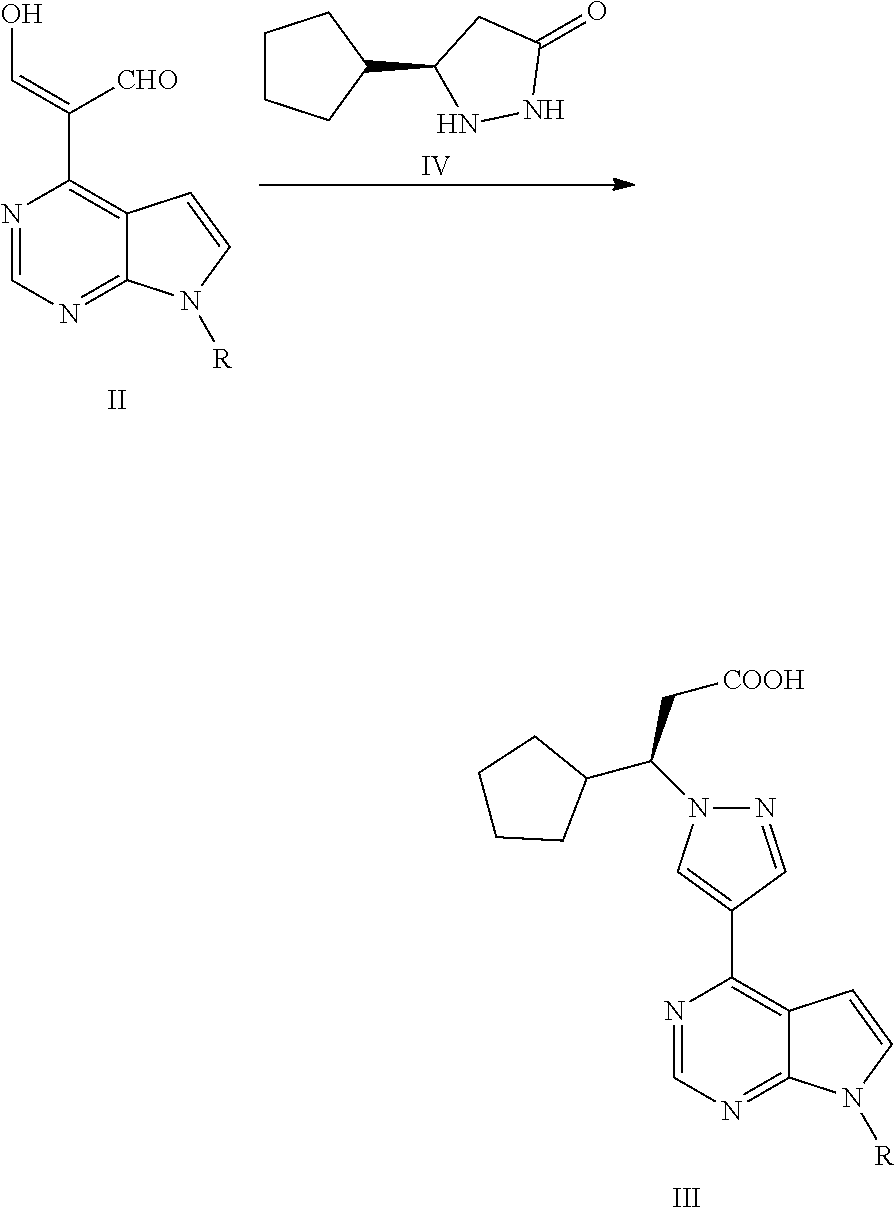
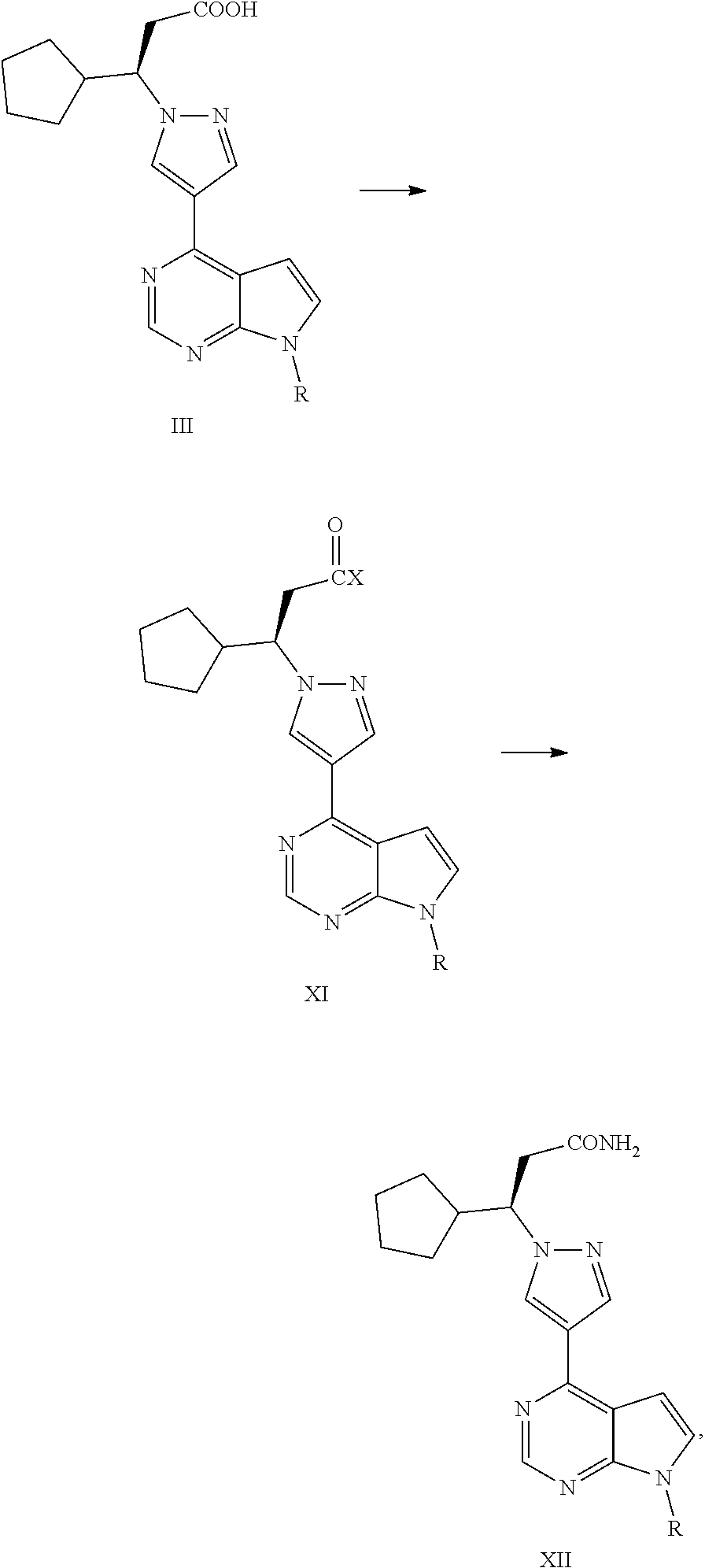
The present application falls within the field of drug synthesis, and in particular, the present application relates to a method for preparing ruxolitinib, and a method for preparing the intermediate and relevant intermediates used. The method comprises reacting a compound of formula II with a compound of formula IV or a salt thereof to obtain a compound of formula III, and then subjecting the compound of formula III to an acyl halogenation reaction, an amidation reaction, and a reaction dehydrating an amide to form a cyano group or removing the protecting group to prepare ruxolitinib. The method has the characteristics of brief steps, a high stereoselectivity, a high utilization ratio of atoms, mild reaction conditions and convenient post treatment. The method avoids using expensive asymmetric reaction catalysts, and is suitable for industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
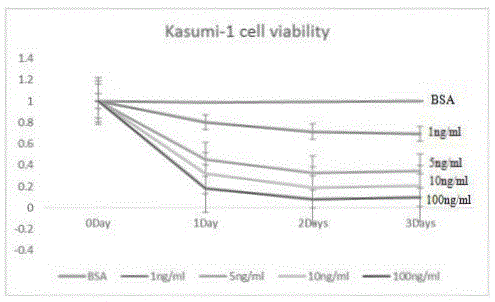
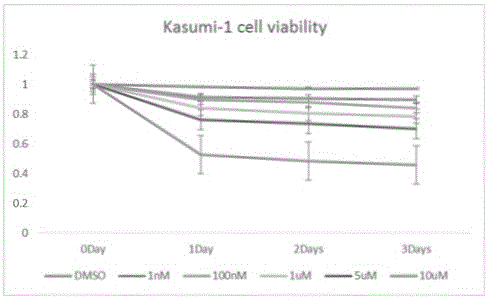
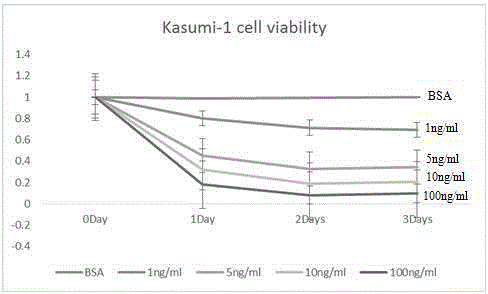
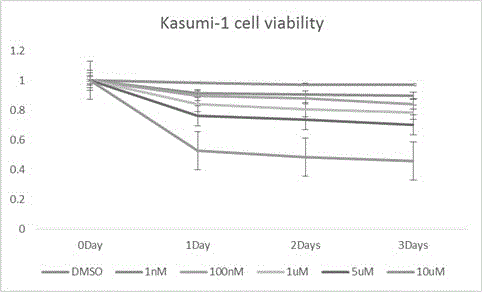
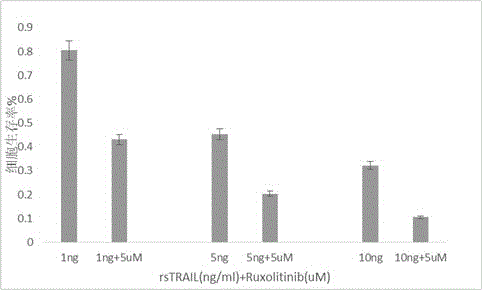
Use of Ruxolitinib in preparation of drug for treating M2 type acute myeloid leukemia
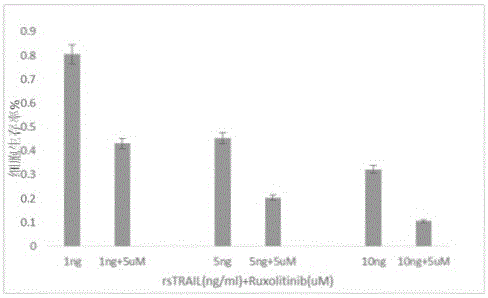
ActiveCN104398520AOvercoming TRAIL ResistanceIncreased sensitivityOrganic active ingredientsPeptide/protein ingredientsRuxolitinibAPOPTOGENIC PROTEIN
The invention relates to use of Ruxolitinib in preparation of a drug for treating M2 type acute myeloid leukemia with t (8; 21) chromosome translocation, and the in-vitro effective concentration is 1 * 10<-6>-1 *10<-5>M. Drug Ruxolitinib is not only itself has the effect of inhibiting growth and inducing apoptosis on leukemia cell Kasumi-1, and enhances the drug sensitivity of the leukemia cell Kasumi-1 on rsTRAI by up-regulation of mRNA and protein expression level of DR4, up-regulation of cell apoptosis protein Bax expression, activation of apoptosis protein caspase-3 and inhibition of NF-kappa B protein activity. The drug can be used for treating the M2 type acute myeloid leukemia with t (8; 21) chromosome translocation, the new drug for treating the M2 type acute myeloid leukemia with t (8; 21) chromosome translocation is provided, and a new approach for overcoming TRAIL resistance in leukemia cells can be provided.
Owner:THE FIFTH PEOPLES HOSPITAL OF SHANGHAI FUDAN UNIV
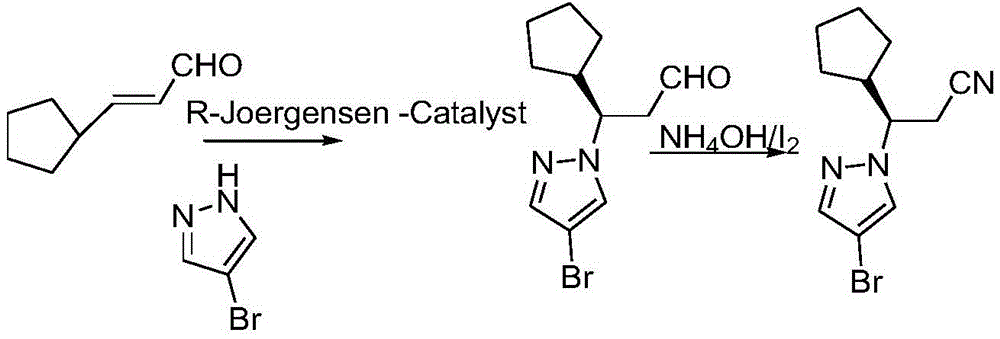
Method for synthesizing ruxolitinib intermediate (R)-3-(4-bromo-1H-pyrazol-1-yl)-3-cyclopentyl propionitrile
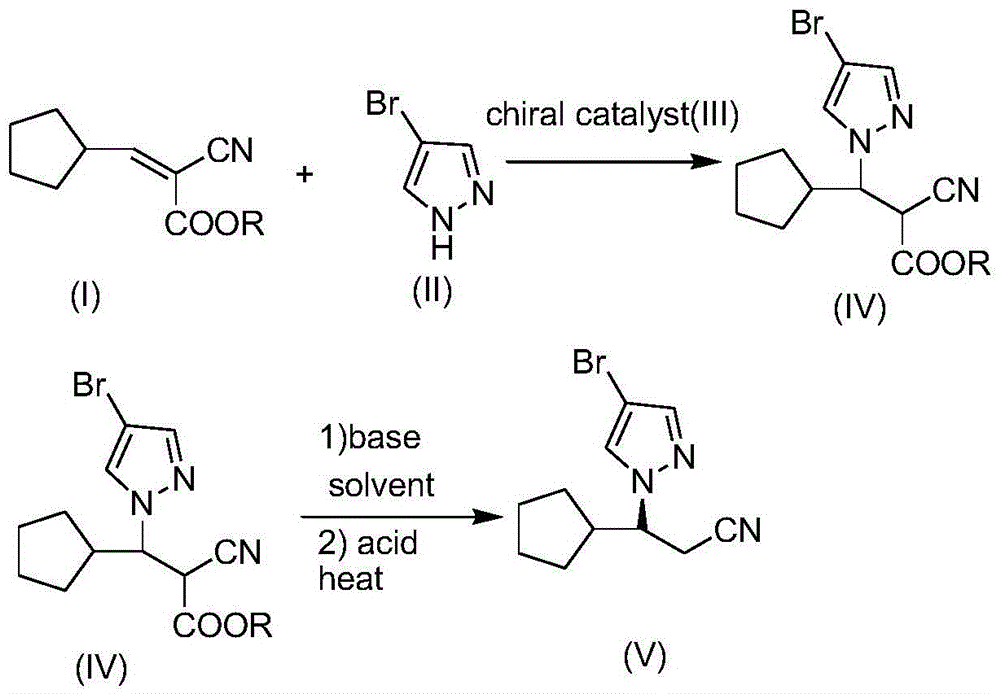
The invention relates to a method for synthesizing a ruxolitinib intermediate (R)-3-(4-bromo-1H-pyrazol-1-yl)-3-cyclopentyl propionitrile. The method includes the steps that 3-cyclopentyl-2-cyanoacrylate (I) serves as a raw material, the 3-cyclopentyl-2-cyanoacrylate (I) and 4-bromo-1H-pyrazol (II) are subjected to Michael addition under the condition of a chiral squaric acid amide catalyst (III), and (R)-3-(4-bromo-1H-pyrazol-1-yl)-2-cyano-3-cyclopentyl propanoic acid alkyl ester (IV) is obtained; the (R)-3-(4-bromo-1H-pyrazol-1-yl)-2-cyano-3-cyclopentyl propanoic acid alkyl ester (IV) is hydrolyzed and decarboxylated to obtain the (R)-3-(4-bromo-1H-pyrazol-1-yl)-3-cyclopentyl propionitrile (V). The synthesis method is simple, the product yield can be 80% or above, the enantioselectivity of the product is 90% or above, conditions are moderate, operation is easy, production cost is low, and the method can be used for industrial production.
Owner:滨州市帅博木业有限公司
Intermediate of jak inhibitor and preparation method thereof
ActiveCN107759623BHigh yieldHigh reaction yieldGroup 3/13 element organic compoundsPtru catalystRuxolitinib
The present invention relates to a novel key intermediate of JAK inhibitor ruxolitinib and a preparation method thereof. The chemical name of the intermediate is (R)-3-(4-boric acid-1H-pyrazole-1-yl)-3 ‑Cyclopentapropionitrile. The present invention provides a new route for the preparation of ruxolitinib, each reaction step of the route has relatively high yield, the total reaction yield is high, the obtained product has good purity, the post-treatment of the reaction is simple, and no column chromatography is required; Moreover, by adopting this route, the required raw materials or used catalysts and other substances are relatively easy to obtain. Compared with the prior art, the method of the present invention is more economical and more suitable for industrial production.
Owner:SUZHOU VIGONVITA LIFE SCIENCES CO LTD
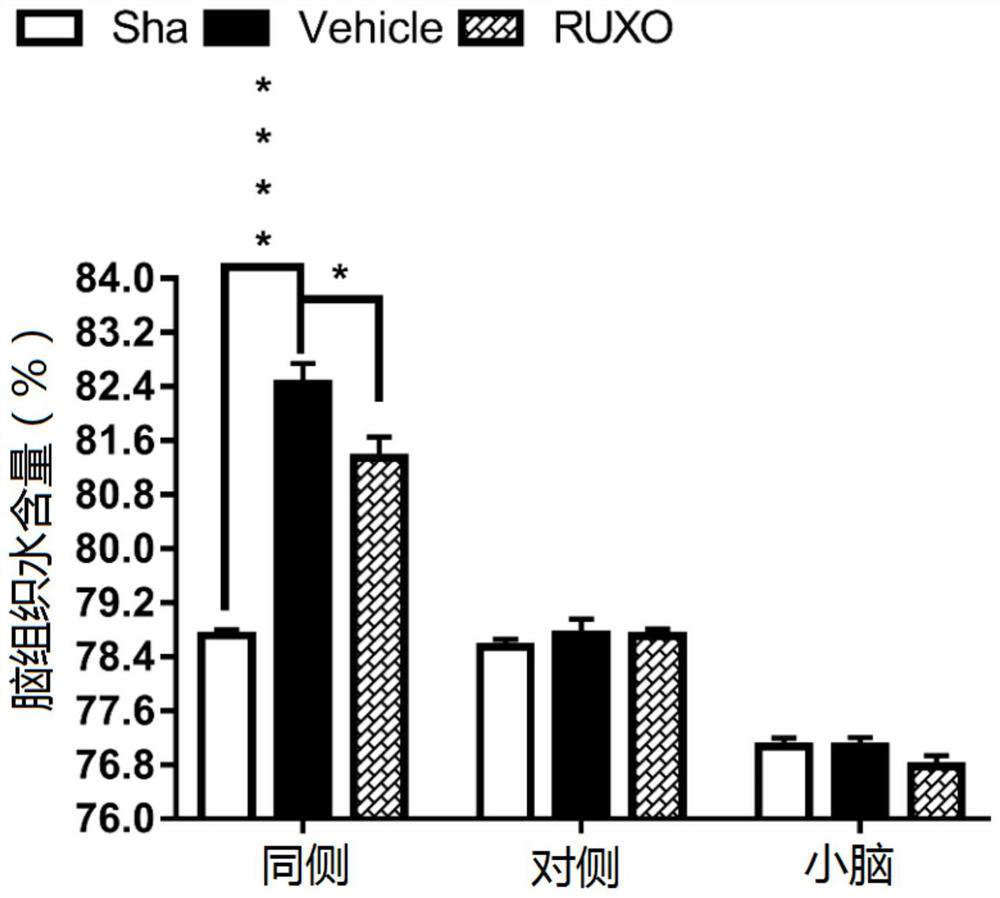
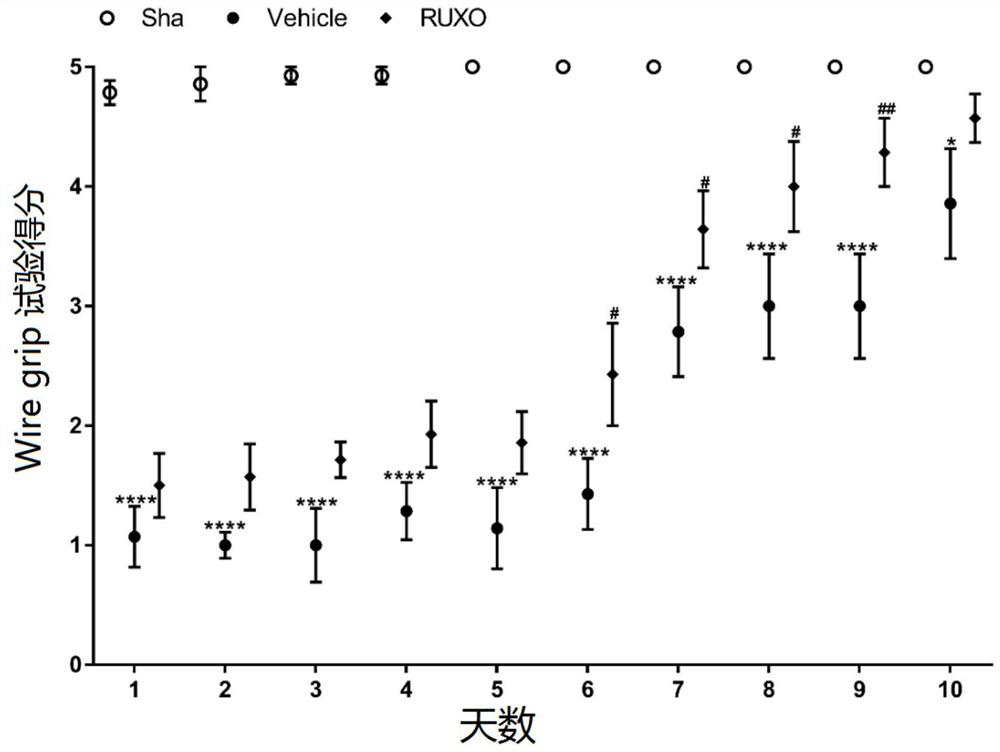
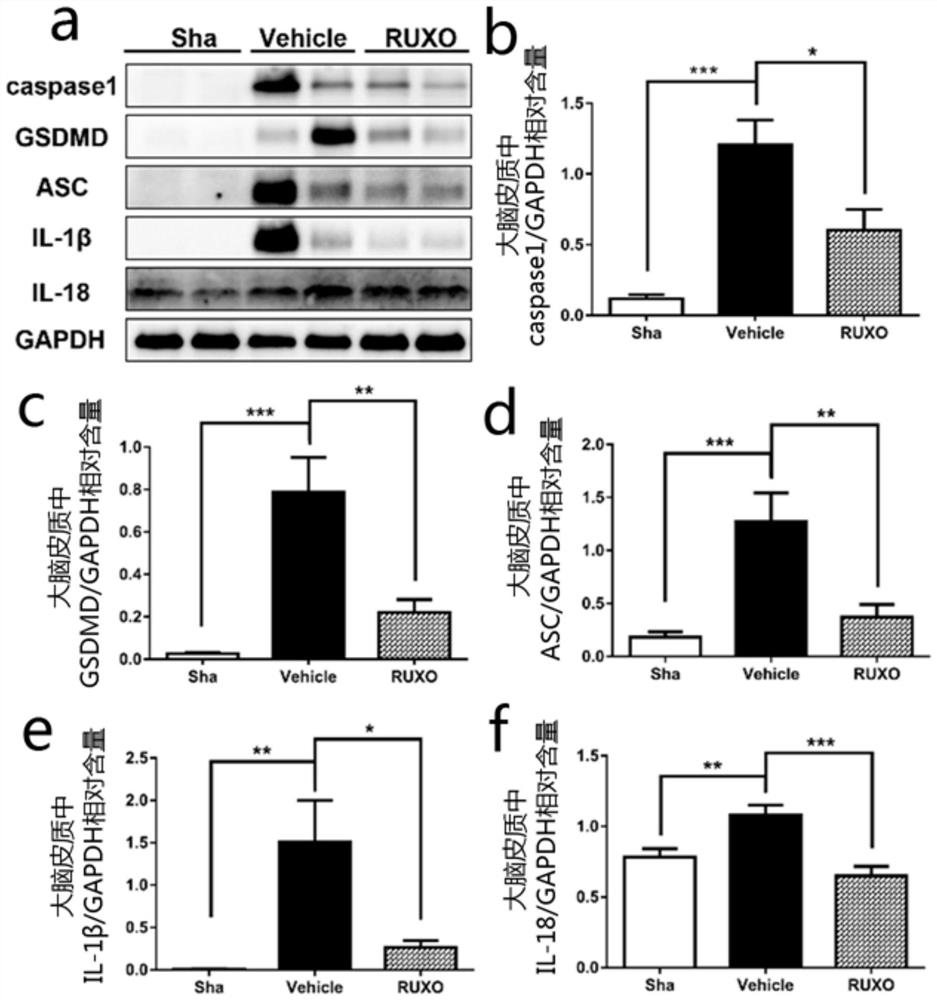
Application of jak-1 signal pathway inhibitor in preparation of medicine for treating brain injury
ActiveCN111067904BReduce formationImprovement of neurological impairmentOrganic active ingredientsNervous disorderBone marrow fibrosisInjury brain
The invention relates to the application of JAK-1 signaling pathway inhibitors in the preparation of drugs for treating brain injury and / or protecting brain nerves. Inhibitors of the JAK‑1 signaling pathway (one of which is Ruxolitinib, a clinical drug approved by the FDA for the treatment of myelofibrosis) exert neuroprotective effects by (but not limited to) inhibiting the process of pyroptosis. Injured patients provide new theoretical and technical means.
Owner:SUZHOU UNIV
A kind of synthetic method of 4-chloro-7h-pyrrolo[2,3-d]pyrimidine
The invention discloses a synthetic method of 4-chloro-7H-pyrrolo[2,3-d] pyrimidine. The compound is an important intermediate for synthesizing rheumatoid arthritis JAK inhibitors: ruxolitinib and tofacitinib. The synthetic method comprises the following steps: by taking a compound I (4,6-dichloro-5-allyl pyrimidine) as an initial raw material, performing a nucleophilic substitution reaction with ammonia water to generate a compound II; then performing a reaction on the compound II and ozone and performing a reduction reaction to generate a compound III; and finally, performing self ring-closing reaction in an acidic environment to generate a compound IV, that is, the 4-chloro-7H-pyrrolo[2,3-d] pyrimidine. The synthetic route is as shown in the formula in the description. According to the synthetic process provided by the invention, the raw material is cheap and easily available, the synthetic route is simple, the cost is low, the yield is high, and the synthetic method is easy for industrial production.
Owner:EAST CHINA NORMAL UNIV +1
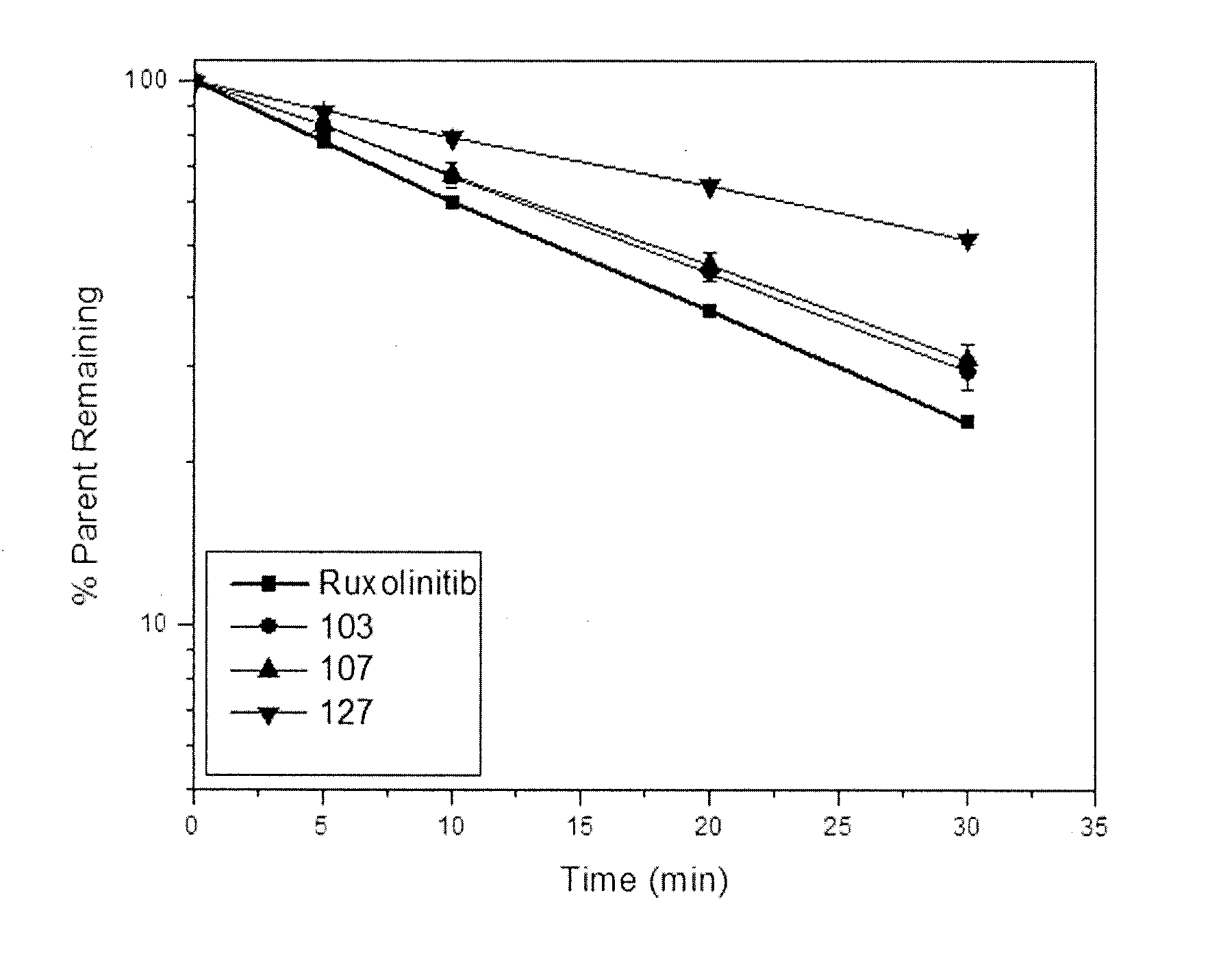
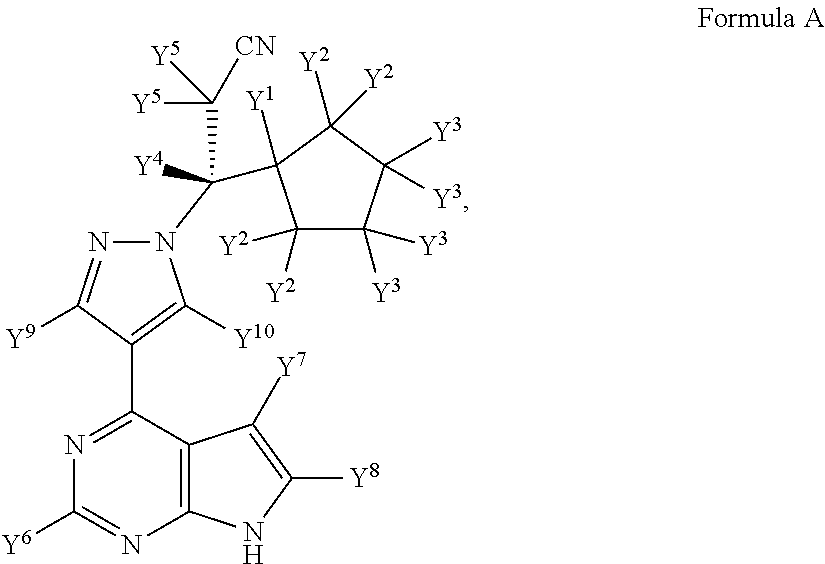
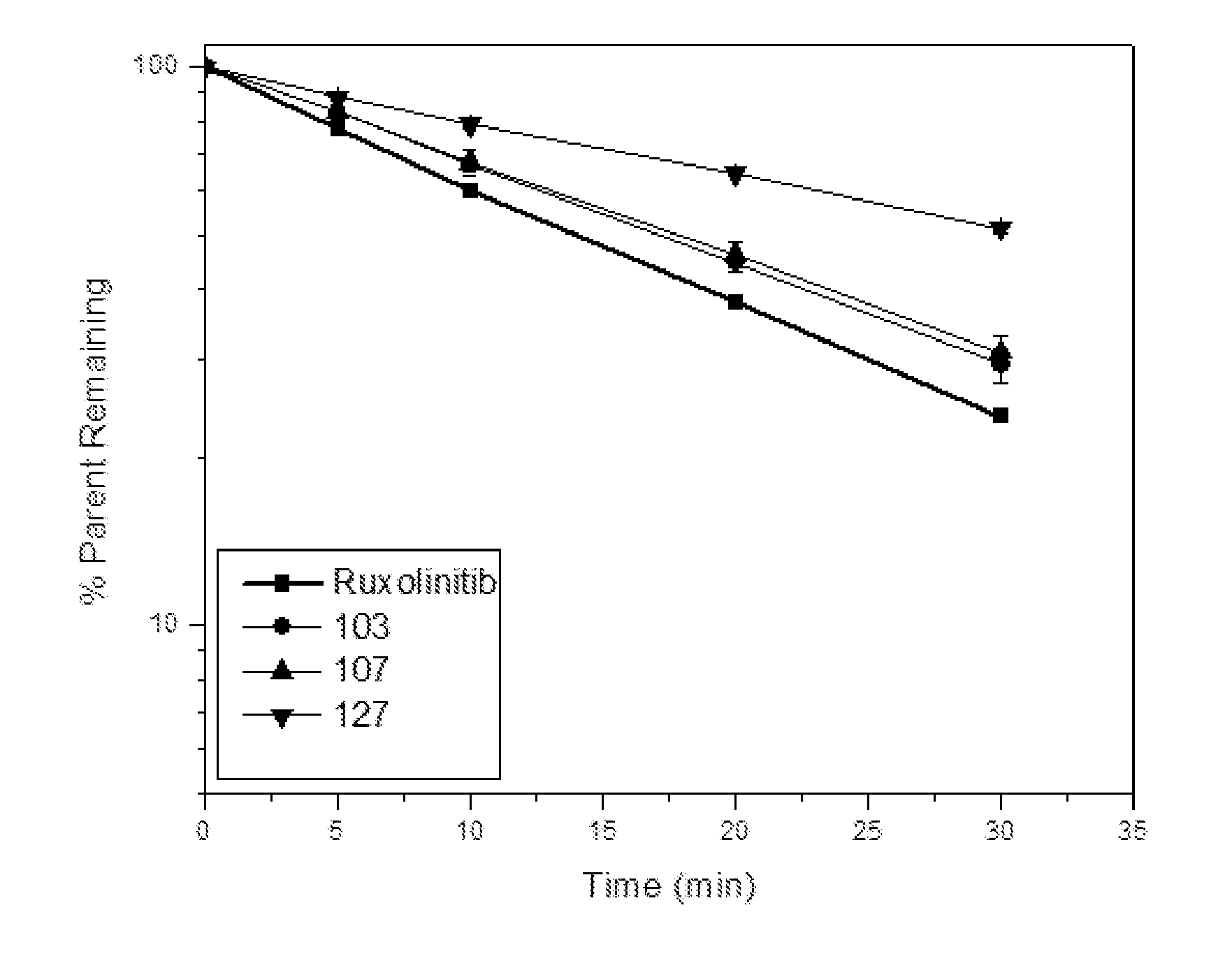
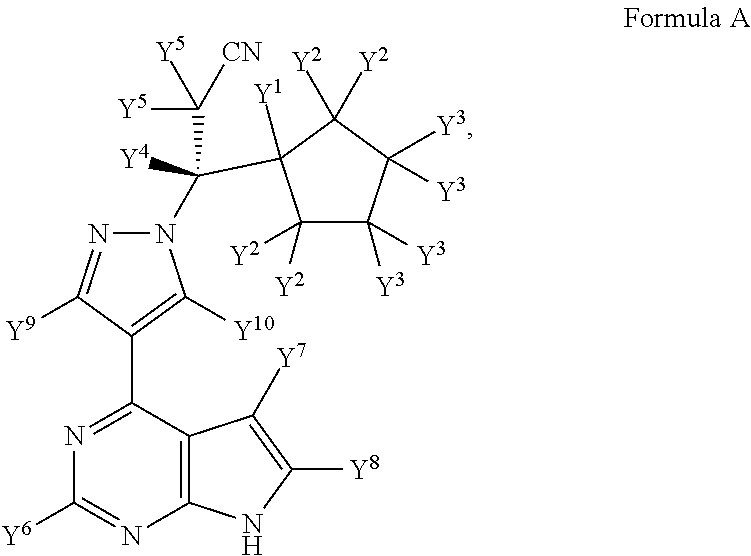
Deuterated derivative of ruxolitinib
The present invention discloses a deuterated derivative of ruxolitinib. One embodiment provides a compound of a formula A, or a pharmaceutically acceptable salt thereof, a pharmaceutical composition containing the compound, and a method for treating indications disclosed in the specification.
Owner:CONCERT PHARMA INC
Use of MDM2 inhibitors for treatment of myelofibrosis
The present invention relates to the use of MDM2 inhibitors in the treatment of myelofibrosis (MF). The invention also relates to a pharmaceutical combination comprising a) an MDM2 inhibitor, and b) at least one additional therapeutic agent, preferably rusotinib or a pharmaceutically acceptable salt thereof.
Owner:NOVARTIS AG
USE OF an anti-P-selectin antibody
InactiveUS20210002374A1Useful in treatmentOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesRuxolitinib
The invention relates to the use of an anti-P-selectin antibody or binding fragment thereof, suitably crizanlizumab or a binding fragment thereof in the treatment of myelofibrosis (MF). The invention also relates to a pharmaceutical combination comprising a) an anti-P-Selectin antibody and b) at least one further therapeutic agent, preferably ruxolitinib or a pharmaceutically acceptable salt thereof.
Owner:NOVARTIS AG
The preparation method of ruxolitinib intermediate (3r)-3-(4-bromo-1h-pyrazol-1-yl)-cyclopentylpropionitrile
The present invention relates to a preparation method of ruxolitinib intermediate (3R)-3-(4-Br-1H-pyrazole-1-yl)-cyclopentyl propanenitrile, and the method comprises the following steps: (1) synthesisof 3-oxo-3-cyclopentyl propionitrile (II); (2) synthesis of (S)-3-cyclopentyl-3-hydroxypropionitrile (III); (3) synthesis of (3R)-3-(4-nitro-1H-pyrazole-1-yl)-cyclopentyl propionitrile (IV); (4) synthesis of (3R)-3-(4-amino-1H-pyrazole-1-yl)-cyclopentyl propionitrile (V); and (5) (3R)-3-(4-Br-1H-pyrazole-1-yl)-cyclopentyl propanenitrile (VI). The method has the advantages of good stereoselectivity, low cost, mild reaction conditions, no requirement on harsh reaction such as high temperature, high pressure and ultra-low temperature.
Owner:海化生命(厦门)科技有限公司
A pharmaceutical composition and its application in the preparation of drugs targeting calreticulin mutations in myeloproliferative diseases
Owner:XUZHOU MEDICAL UNIV
The synthetic method of ruxolitinib intermediate (r)‑3‑(4‑bromo‑1h‑pyrazole‑1‑yl)‑3‑cyclopentylpropionitrile
The invention relates to a method for synthesizing a ruxolitinib intermediate (R)-3-(4-bromo-1H-pyrazol-1-yl)-3-cyclopentyl propionitrile. The method includes the steps that 3-cyclopentyl-2-cyanoacrylate (I) serves as a raw material, the 3-cyclopentyl-2-cyanoacrylate (I) and 4-bromo-1H-pyrazol (II) are subjected to Michael addition under the condition of a chiral squaric acid amide catalyst (III), and (R)-3-(4-bromo-1H-pyrazol-1-yl)-2-cyano-3-cyclopentyl propanoic acid alkyl ester (IV) is obtained; the (R)-3-(4-bromo-1H-pyrazol-1-yl)-2-cyano-3-cyclopentyl propanoic acid alkyl ester (IV) is hydrolyzed and decarboxylated to obtain the (R)-3-(4-bromo-1H-pyrazol-1-yl)-3-cyclopentyl propionitrile (V). The synthesis method is simple, the product yield can be 80% or above, the enantioselectivity of the product is 90% or above, conditions are moderate, operation is easy, production cost is low, and the method can be used for industrial production.
Owner:滨州市帅博木业有限公司
Jak inhibition as a novel therapy for preventing tumors in peutz-jeghers syndrome
ActiveUS20200129514A1Reduce partEnhanced signalOrganic active ingredientsPharmaceutical delivery mechanismRuxolitinibBiochemistry
Treatment with the Jak1 / 2 inhibitor ruxolitinib results in a dramatic decrease in polyposis for organisms with Peutz-Jeghers Syndrome.
Owner:CURINGENETICS LLC
Synthesis process of ruxolitinib
ActiveUS10562904B2Short stepsHigh stereoselectivityGroup 4/14 element organic compoundsOrganic active ingredientsPtru catalystRuxolitinib
The present application falls within the field of drug synthesis, and in particular, the present application relates to a method for preparing ruxolitinib, and a method for preparing the intermediate and relevant intermediates used. The method comprises reacting a compound of formula II with a compound of formula IV or a salt thereof to obtain a compound of formula III, and then subjecting the compound of formula III to an acyl halogenation reaction, an amidation reaction, and a reaction dehydrating an amide to form a cyano group or removing the protecting group to prepare ruxolitinib. The method has the characteristics of brief steps, a high stereoselectivity, a high utilization ratio of atoms, mild reaction conditions and convenient post treatment. The method avoids using expensive asymmetric reaction catalysts, and is suitable for industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Application of ruxolitinib in the preparation of drugs for the treatment of M2 type acute myeloid leukemia
ActiveCN104398520BOvercoming TRAIL ResistanceIncreased sensitivityOrganic active ingredientsPeptide/protein ingredientsMyeloid leukemiaRuxolitinib
The invention relates to use of Ruxolitinib in preparation of a drug for treating M2 type acute myeloid leukemia with t (8; 21) chromosome translocation, and the in-vitro effective concentration is 1 * 10<-6>-1 *10<-5>M. Drug Ruxolitinib is not only itself has the effect of inhibiting growth and inducing apoptosis on leukemia cell Kasumi-1, and enhances the drug sensitivity of the leukemia cell Kasumi-1 on rsTRAI by up-regulation of mRNA and protein expression level of DR4, up-regulation of cell apoptosis protein Bax expression, activation of apoptosis protein caspase-3 and inhibition of NF-kappa B protein activity. The drug can be used for treating the M2 type acute myeloid leukemia with t (8; 21) chromosome translocation, the new drug for treating the M2 type acute myeloid leukemia with t (8; 21) chromosome translocation is provided, and a new approach for overcoming TRAIL resistance in leukemia cells can be provided.
Owner:THE FIFTH PEOPLES HOSPITAL OF SHANGHAI FUDAN UNIV
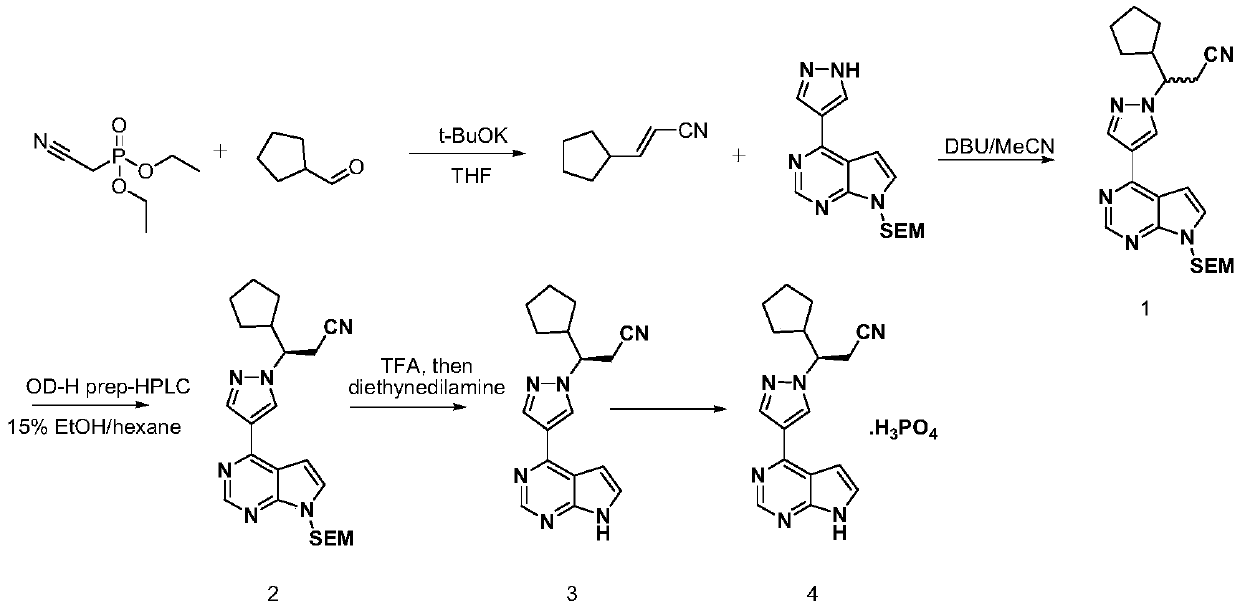
A kind of synthetic method of ruxolitinib intermediate
The invention relates to a synthesis method of a ruxolitinib intermediate. The method comprises the following steps: firstly, carrying out catalytic reaction on cyclopentane methyl formate and acetonitrile to prepare 3-cyclopentyl-3-oxypropionitrile; carrying out enzymatic asymmetric reduction on 3-cyclopentyl-3-oxypropionitrile to generate chiral alcohol (S)-3-cyclopentyl-3-oxypropionitrile; and carrying out Mitsunobu reaction and 4-bromopyrazole coupling on (S)-3-cyclopentyl-3-oxypropionitrile to obtain ruxolitinib intermediate (3R)-3-(4-bromo-1H-pyrazole-1-yl)-3-cyclopentane propionitrile. The synthesis method has the advantages of short route, low cost, mild condition and good stereoselectivity, and is suitable for industrialized mass production.
Owner:BIOCOMPOUNDS PHARMACEUTICAL INC +1
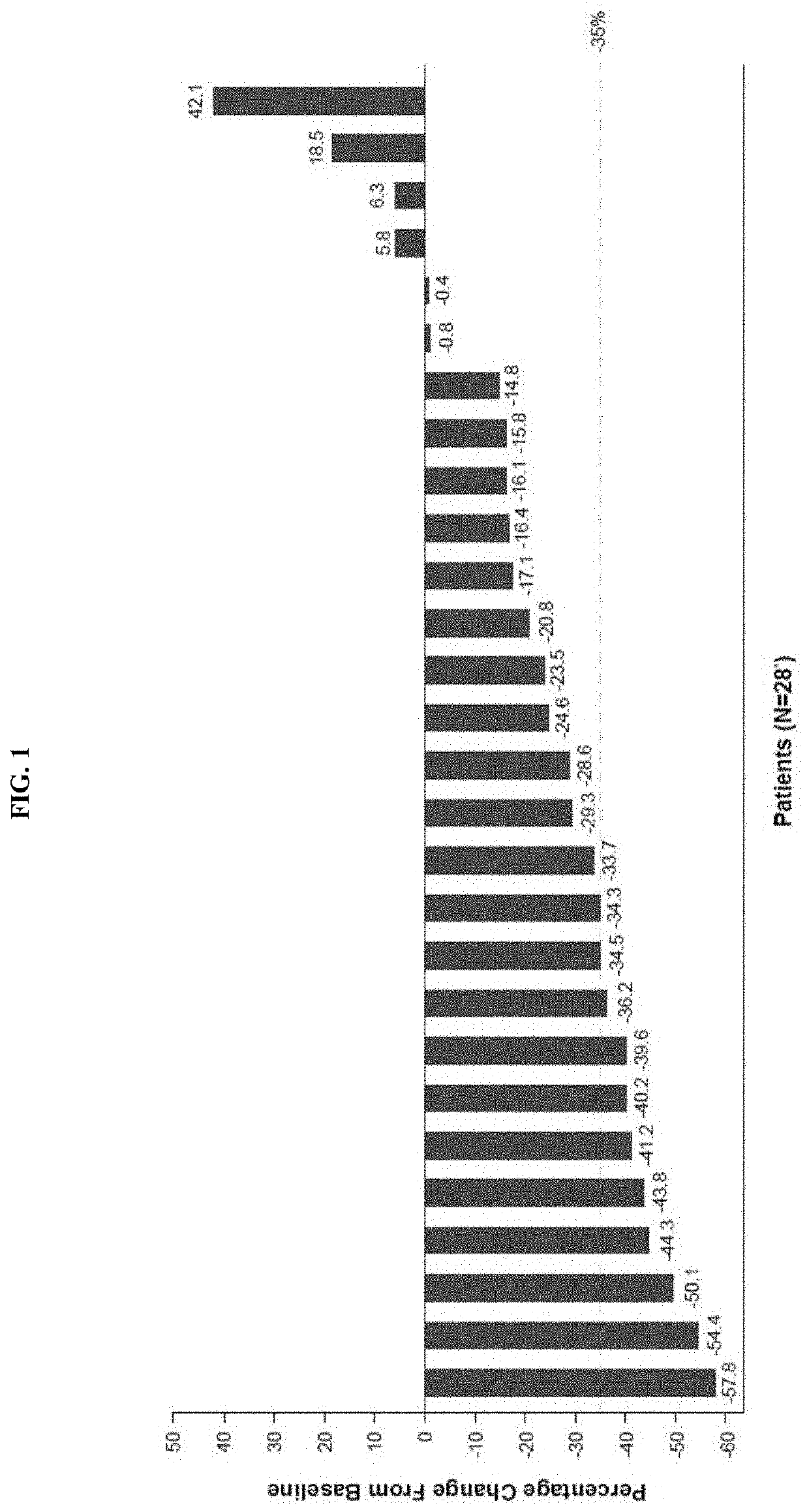
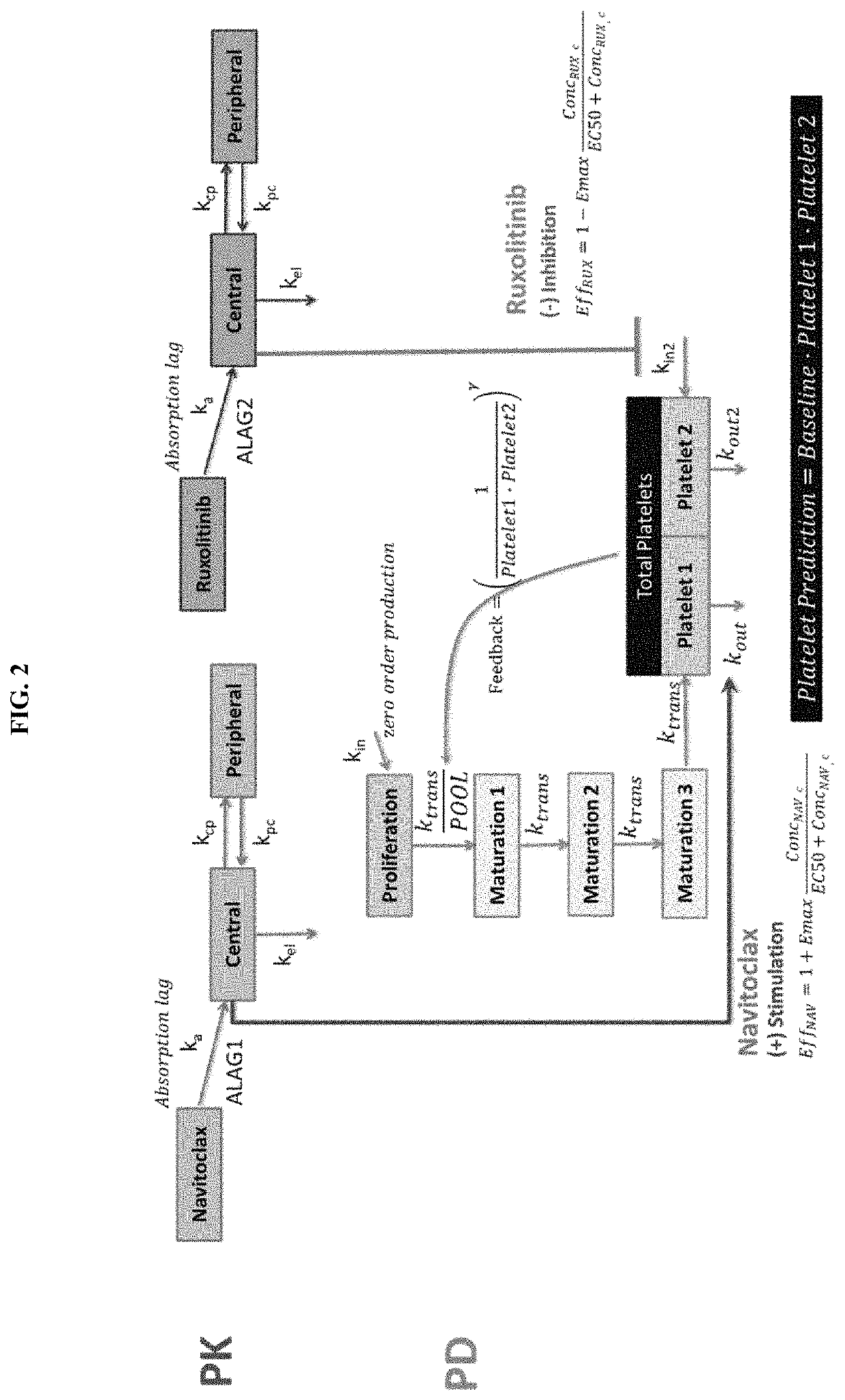
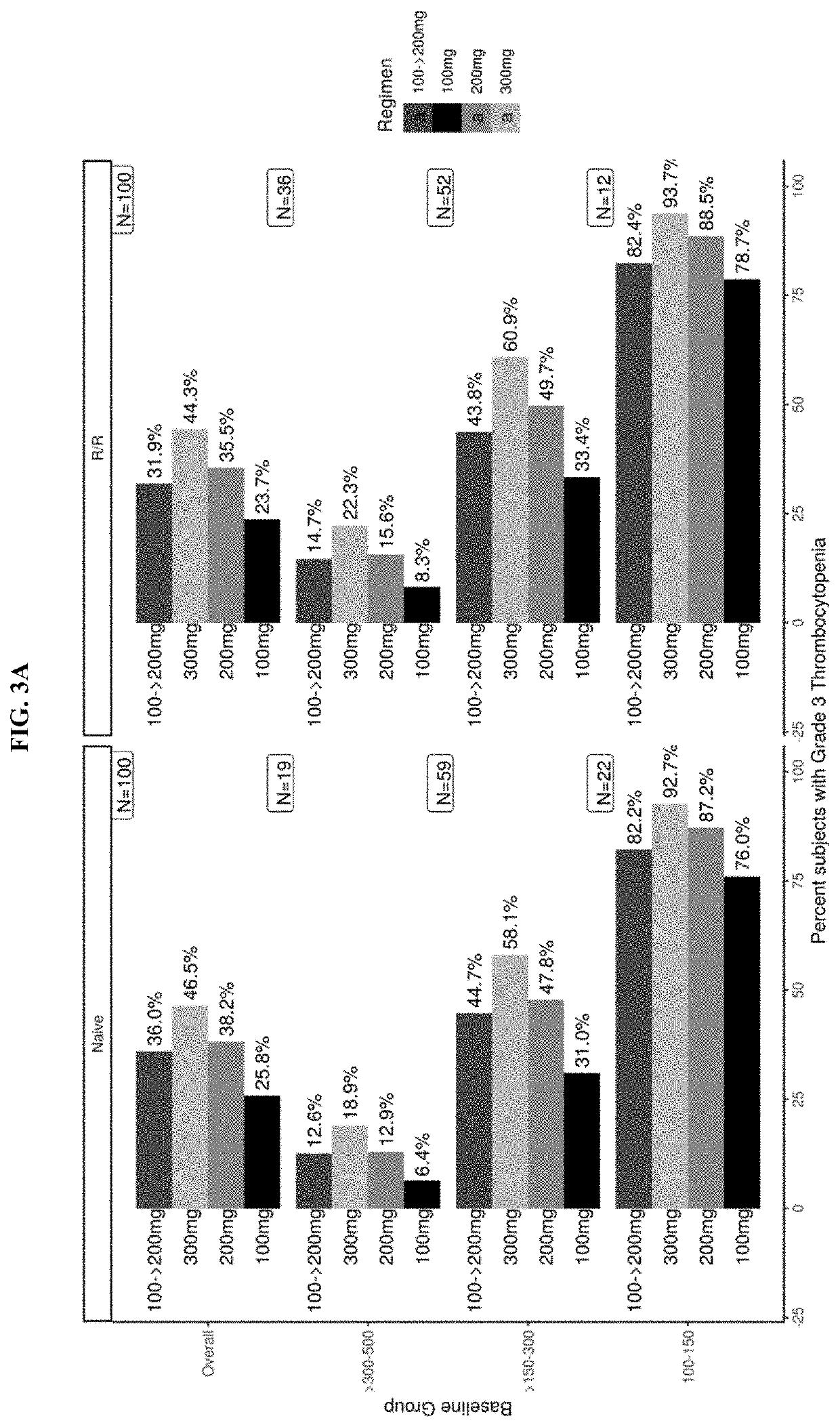
Dosing regimens for use in treating myelofibrosis and mpn-related disorders with navitoclax
ActiveUS20210128573A1Reduces effective amountAntineoplastic agentsHeterocyclic compound active ingredientsBone marrow fibrosisDosing regimen
The invention described herein relates to methods for treating a human subject with myelofibrosis or an MPN-related disorder, comprising administering navitoclax to the subject optionally in combination with ruxolitinib.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Synthetic method of 4-chloro-7H-pyrrolo[2,3-d] pyrimidine Synthetic method of 4-chloro-7H-pyrrolo[2,3-d] pyrimidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/29f751ef-119d-4de3-86cd-51a33e9e496d/BDA0001272290150000011.png)
![Synthetic method of 4-chloro-7H-pyrrolo[2,3-d] pyrimidine Synthetic method of 4-chloro-7H-pyrrolo[2,3-d] pyrimidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/29f751ef-119d-4de3-86cd-51a33e9e496d/BDA0001272290150000021.png)
![Synthetic method of 4-chloro-7H-pyrrolo[2,3-d] pyrimidine Synthetic method of 4-chloro-7H-pyrrolo[2,3-d] pyrimidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/29f751ef-119d-4de3-86cd-51a33e9e496d/BDA0001272290150000022.png)


![4-chloro-7H-pyrrolo[2,3-d]pyrimidine synthetic method 4-chloro-7H-pyrrolo[2,3-d]pyrimidine synthetic method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7f09348c-cd6f-4a14-a89f-f5d475eecaf2/BDA0001621800940000011.png)
![4-chloro-7H-pyrrolo[2,3-d]pyrimidine synthetic method 4-chloro-7H-pyrrolo[2,3-d]pyrimidine synthetic method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7f09348c-cd6f-4a14-a89f-f5d475eecaf2/BDA0001621800940000021.png)
![4-chloro-7H-pyrrolo[2,3-d]pyrimidine synthetic method 4-chloro-7H-pyrrolo[2,3-d]pyrimidine synthetic method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7f09348c-cd6f-4a14-a89f-f5d475eecaf2/BDA0001621800940000022.png)





![A kind of synthetic method of 4-chloro-7h-pyrrolo[2,3-d]pyrimidine A kind of synthetic method of 4-chloro-7h-pyrrolo[2,3-d]pyrimidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2a0562ac-68e7-4142-8f02-5b1c4cc88878/BDA0001272290150000011.png)
![A kind of synthetic method of 4-chloro-7h-pyrrolo[2,3-d]pyrimidine A kind of synthetic method of 4-chloro-7h-pyrrolo[2,3-d]pyrimidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2a0562ac-68e7-4142-8f02-5b1c4cc88878/BDA0001272290150000021.png)
![A kind of synthetic method of 4-chloro-7h-pyrrolo[2,3-d]pyrimidine A kind of synthetic method of 4-chloro-7h-pyrrolo[2,3-d]pyrimidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2a0562ac-68e7-4142-8f02-5b1c4cc88878/BDA0001272290150000022.png)