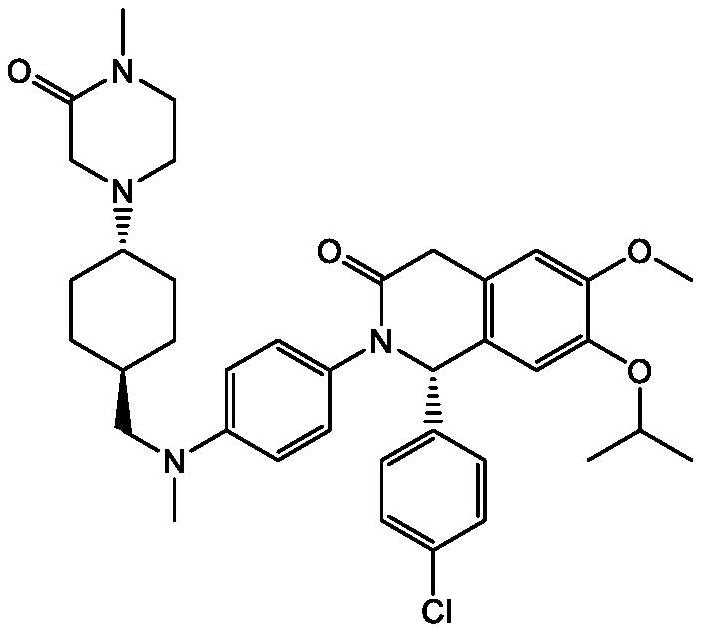
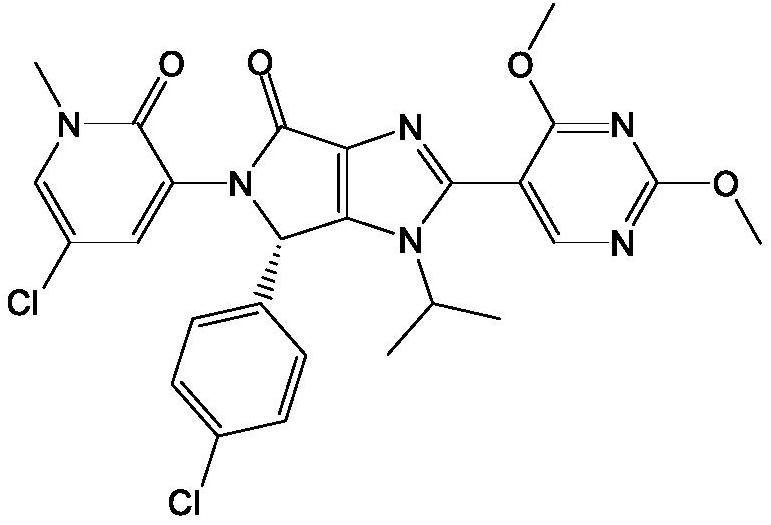
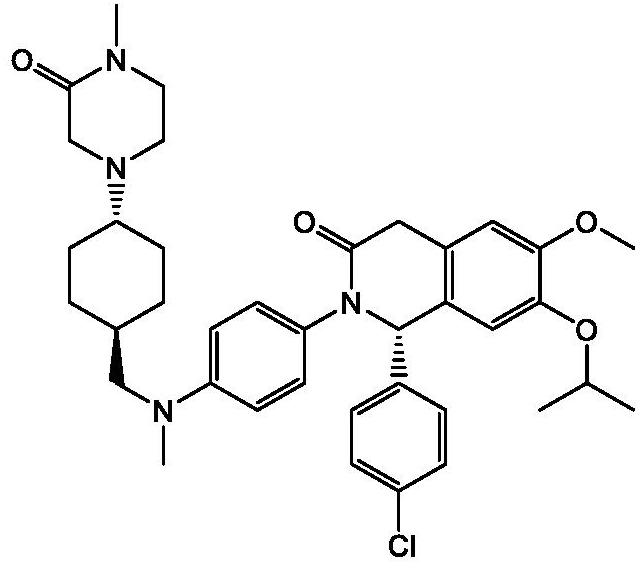
Use of MDM2 inhibitors for treatment of myelofibrosis
A technology of myelofibrosis and MDM2, applied in the field of drug combination for treating MF
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] Certain terms used herein are described below. The compounds or biological agents of the invention are described using standard nomenclature. Unless defined otherwise, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs.
[0030] As used herein, the term "combination", "therapeutic combination" or "pharmaceutical combination" refers to a fixed combination in one dosage unit form, or a non-fixed combination, or a kit of parts for administration in combination , wherein two or more therapeutic agents may be administered independently at the same time or within time intervals, especially where these time intervals allow the combination partners to exhibit a cooperative (eg, synergistic) effect.
[0031] The term "combination therapy" refers to the administration of two or more therapeutic agents to treat the conditions or disorders being treated described in this di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com