Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
130 results about "ABL" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
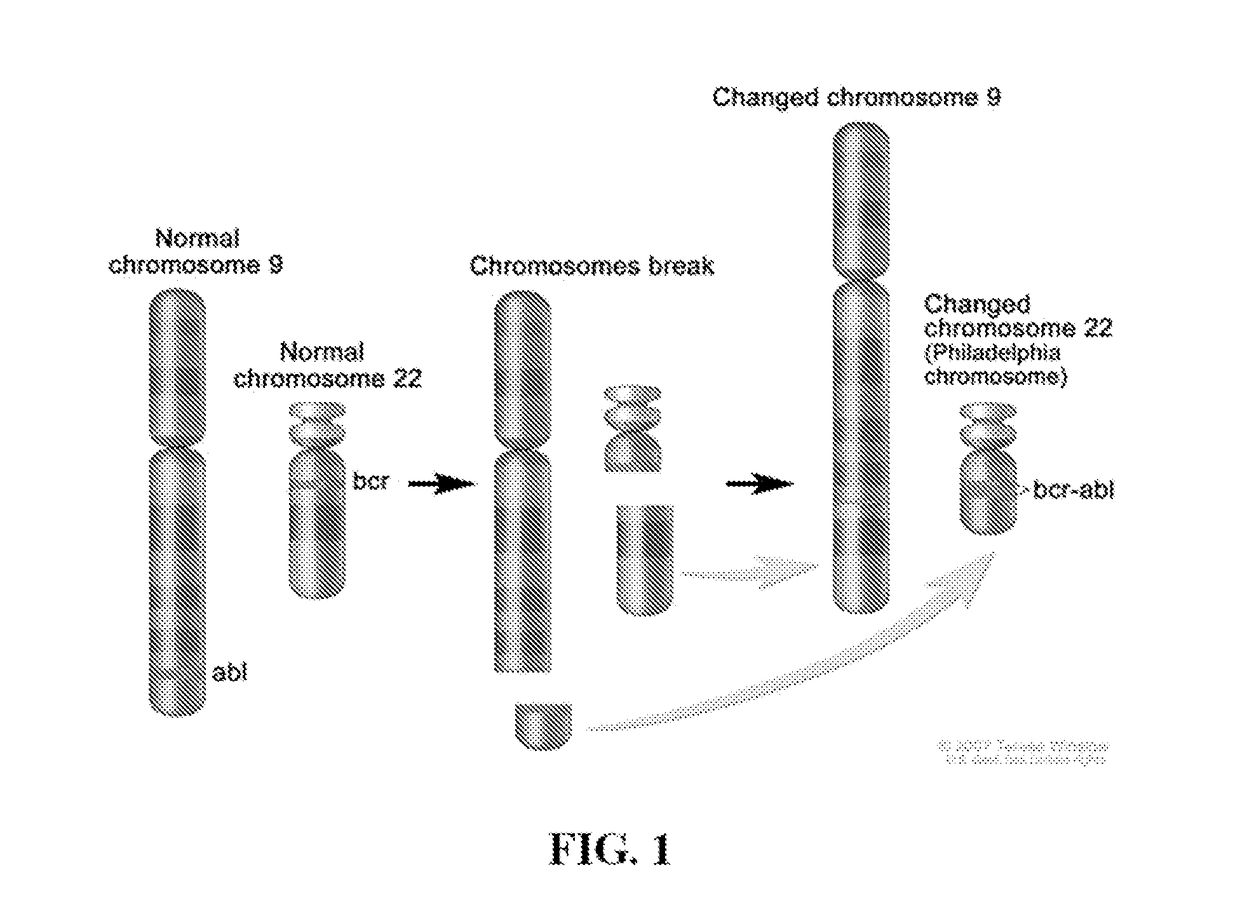
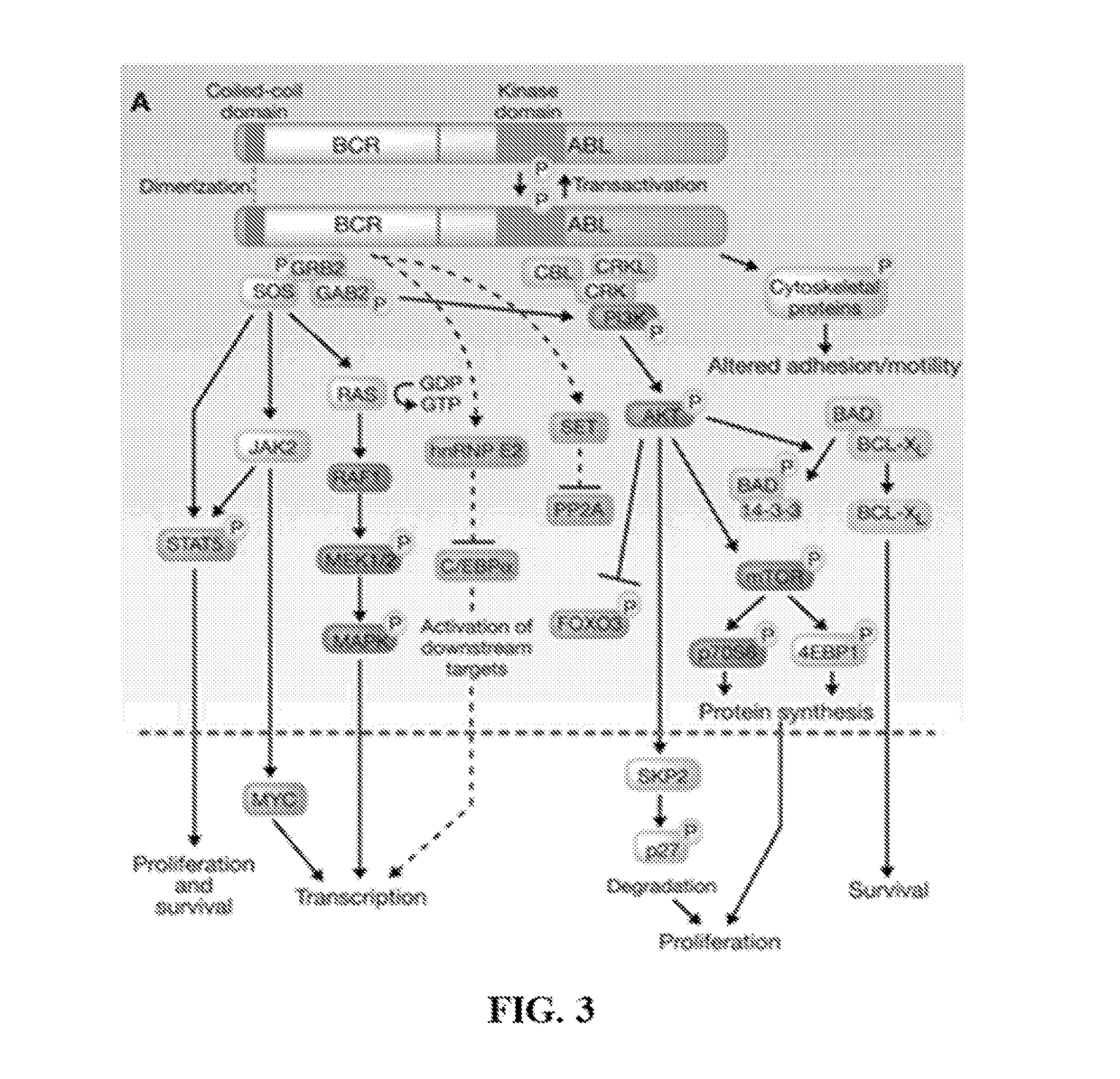
Abelson murine leukemia viral oncogene homolog 1 also known as ABL1 is a protein that, in humans, is encoded by the ABL1 gene (previous symbol ABL) located on chromosome 9. c-Abl is sometimes used to refer to the version of the gene found within the mammalian genome, while v-Abl refers to the viral gene.
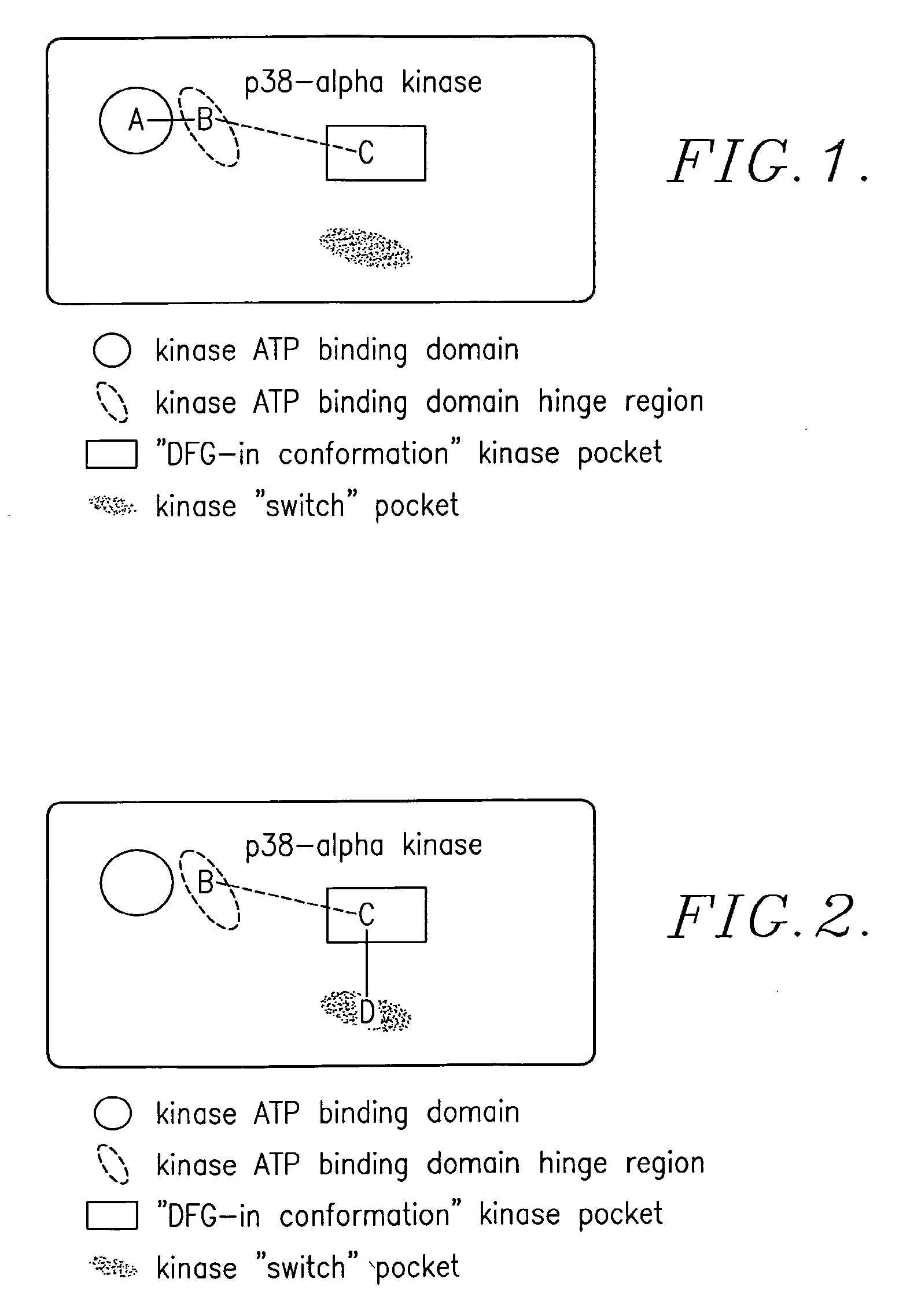
Methods and compositions for the treatment of myeloproliferative diseases and other proliferative diseases
Compounds of the present invention, alone and in combination with other active agents, find utility in the treatment of hyperproliferative diseases, mammalian cancers and especially human cancers including but not limited to for example malignant melanomas, myeloproliferative diseases, chronic myelogenous leukemia, acute lymphocytic leukemia, a disease caused by c-ABL kinase, oncogenic forms thereof, aberrant fusion proteins thereof and polymorphs thereof.
Owner:DECIPHERA PHARMA LLC
Enzyme modulators and treatments
ActiveUS20080113967A1Inhibitory activityImprove securityAntibacterial agentsBiocideDiseaseEnzyme modulator
Novel compounds and methods of using those compounds for the treatment of inflammatory conditions, hyperproliferative diseases, cancer, and diseases characterized by hyper-vascularization are provided. In a preferred embodiment, modulation of the activation state of p38 kinase protein, abl kinase protein, ber-abl kinase protein, braf kinase protein, VEGFR kinase protein, or PDGFR kinase protein comprises the step of contacting said kinase protein with the novel compounds.
Owner:DECIPHERA PHARMA LLC
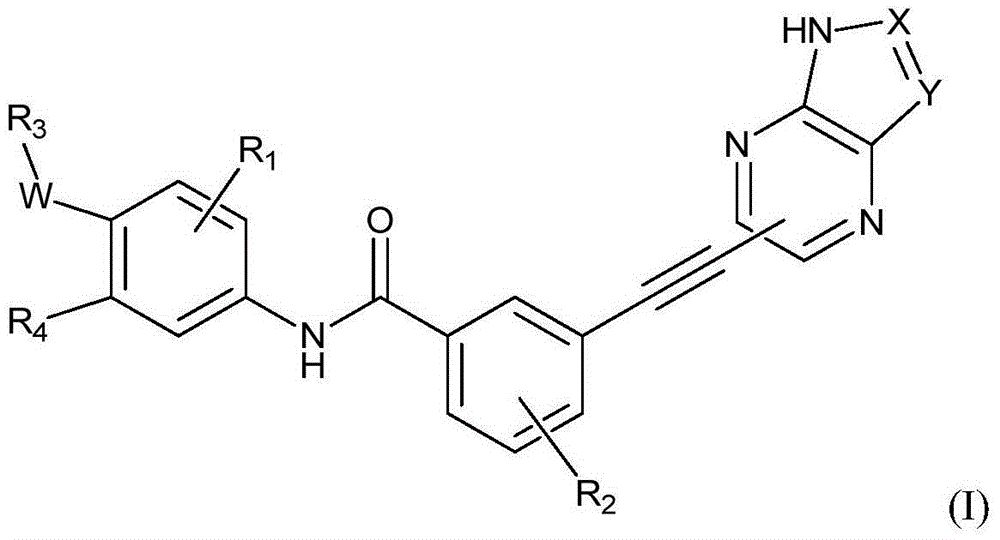
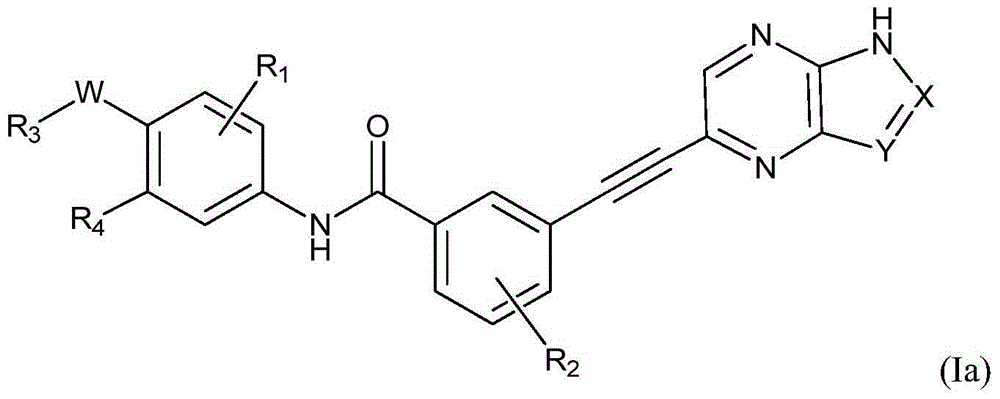
Pyrimidine Derivatives As Kinase Modulators and Method of Use
The invention provides compounds and methods for inhibition of kinases, more specifically IGF 1 R kinases. The invention also provides compounds and methods for inhibition of wildtype Abl. The invention provides compounds for modulating protein kinase enzymatic activity for modulating cellular activities such as proliferation, differentiation, programmed cell death, migration and chemoinvasion. Compounds of the invention inhibit, regulate and / or modulate kinase receptor signal transduction pathways related to the changes in cellular activities as mentioned above, and the invention includes compositions which contain these compounds, and methods of using them to treat kinase-dependent diseases and conditions. A compound of formula (I), or a pharmaceutically acceptable salt, hydrate, or prodrug thereof, wherein, V is NR1R1a, or O—R1, wherein X is H, halo, C1-C6 alkyl, NO2, mono-, di-, or tri-halo substituted methyl, NR13R,14. C(O)O—C1-C6 alkyl, or N(R13)—C(O)—C1-C6 alkyl; Y is H, halo, OH, C1-C6 alkyl, C0-C6alkyl-NR,15R16, NR15R,6, C1-C6 alkoxy, —N(R13)—(CH2)n-NR15R16, —C(O)O—C1-C6 alkyl, —O—(CH2)n—NR15R16, —C(O)—C1-C6 alkyl, —C0-C6-alkyl-R21, —O—R21, —C(O)—R21, —O—(CH2)n—R21, —C(O)—NR13R14, —C(O)—N(R13)-aryl, —C(O)—N(R13)(CH2)n—NR15R16, —C(O)—N(R13)—(CH2)n-aryl —C(O)—N(R13)—(CH2)n-heterocyclyl; or X and Y together with the atoms to which they are attached form a 4-7 membered heterocyclyl or heteroaryl group containing one or two heteroatoms independently selected from O, N, and S. Z is H, NR2R3, —S—R2a, or —O—R2a
Owner:EXELIXIS INC
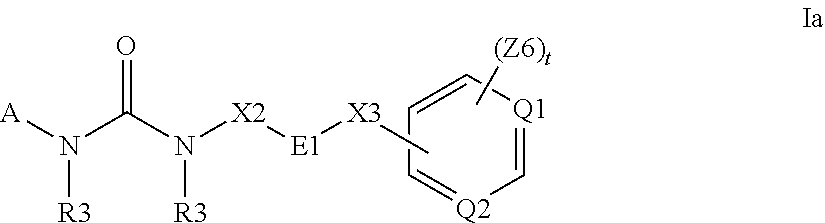
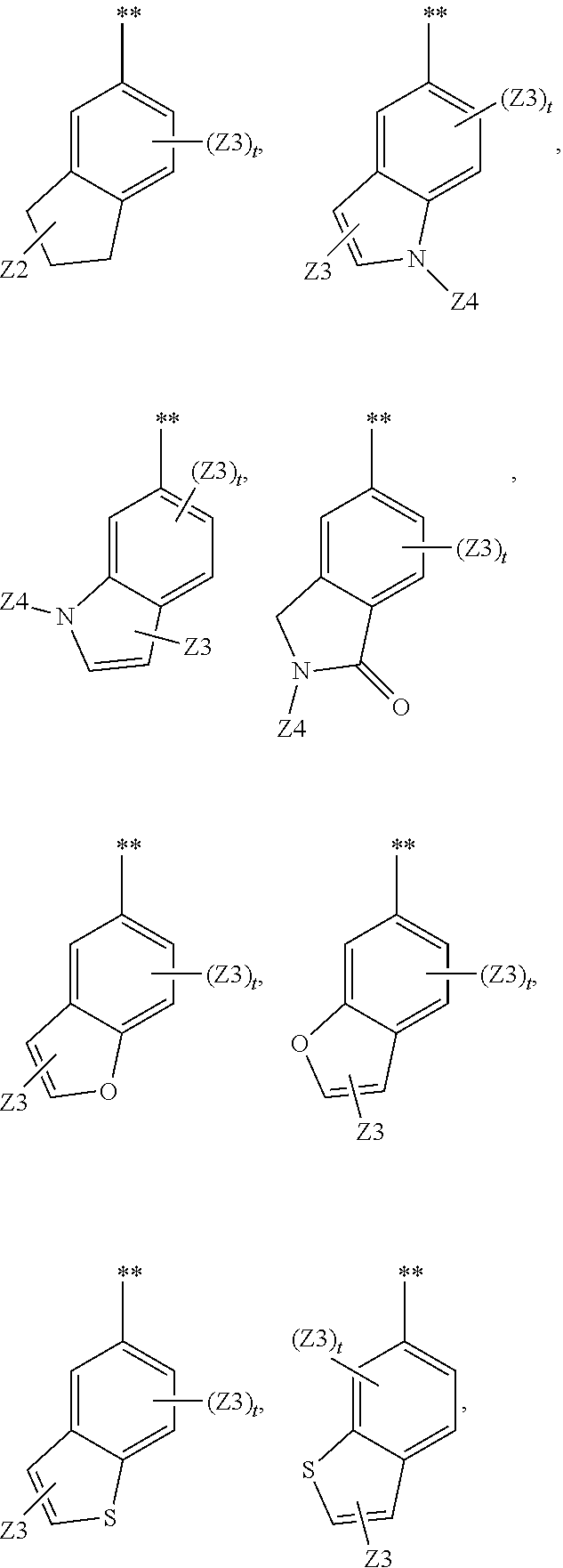
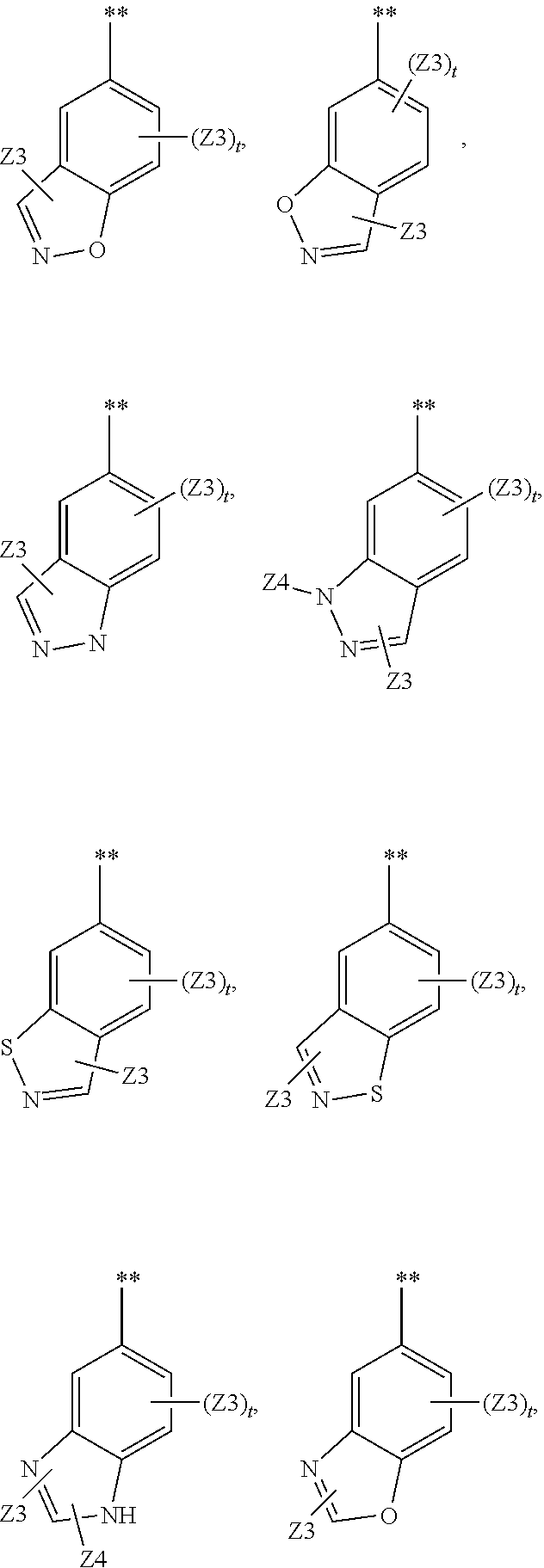
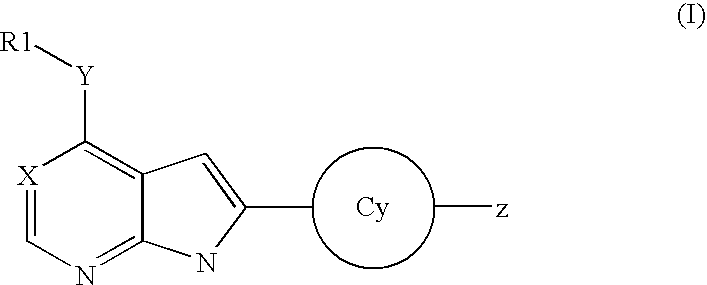
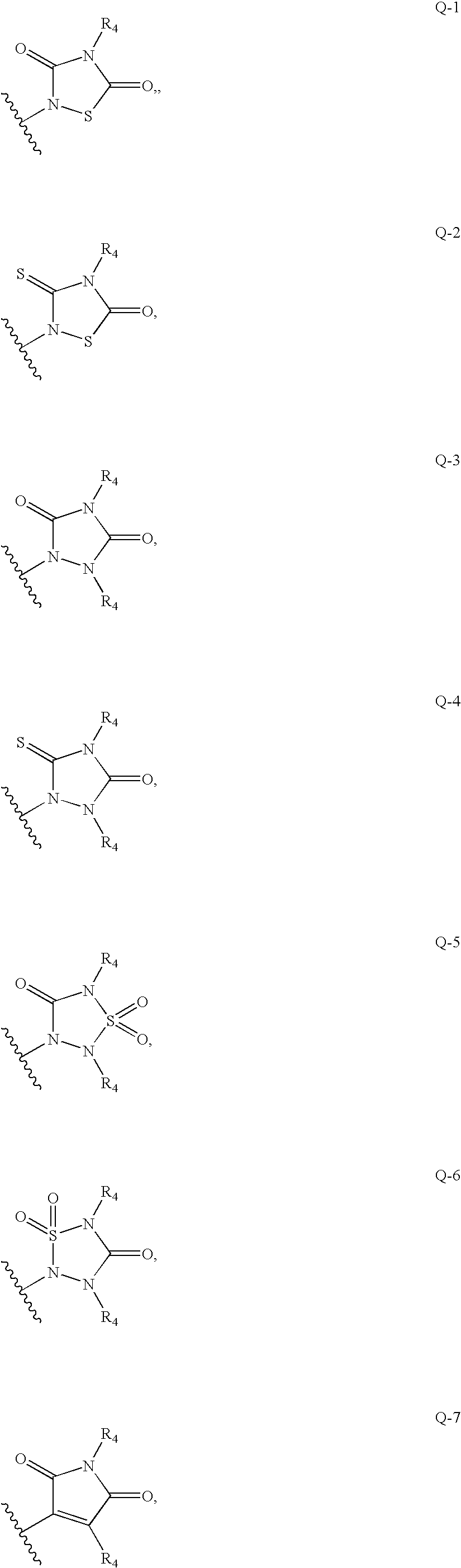
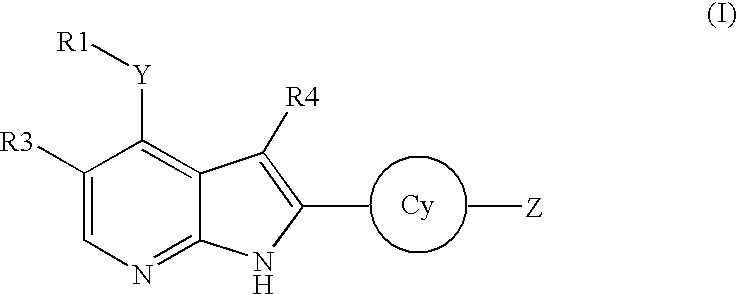
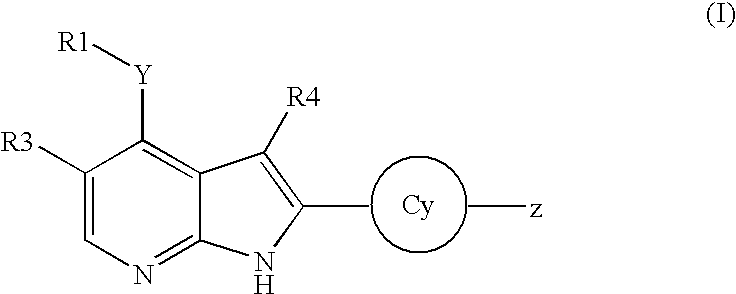
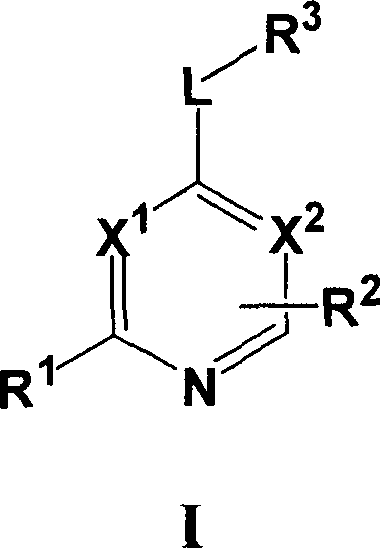
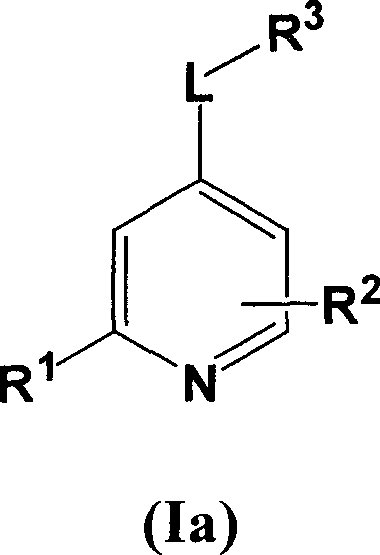
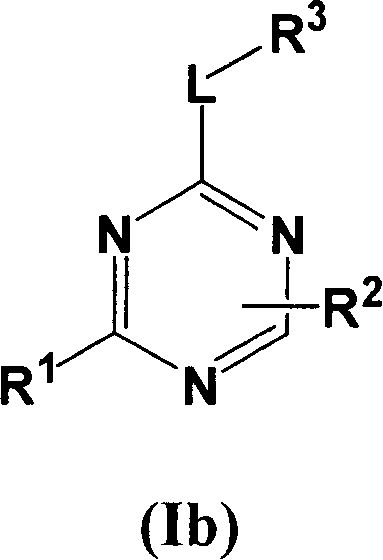
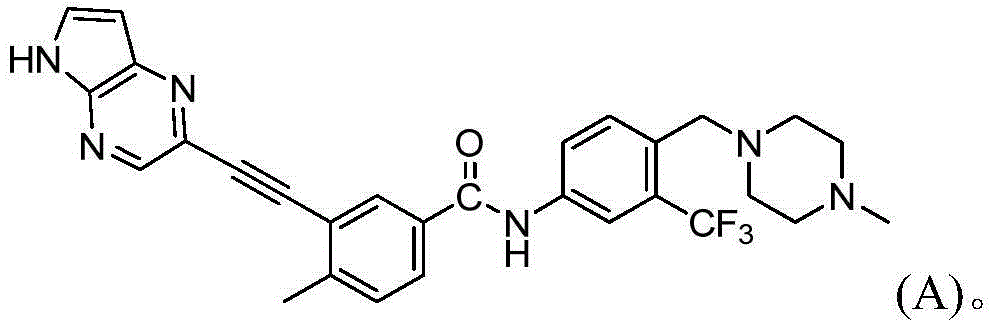
Substituted pyrrolo[2.3-B]pyridines
Compounds represented by Formula (I):or stereoisomers or pharmaceutically acceptable salts thereof, are inhibitors of least two of the Abl, Aurora-A, Blk, c-Raf, cSRC, Src, PRK2, FGFR3, Flt3, Lck, Mek1, PDK-1, GSK3β, EGFR, p70S6K, BMX, SGK, CaMKII, Tie-2, IGF-1R, Ron, Ret, and KDR kinases in animals, including humans, for the treatment and / or prevention of various diseases and conditions such as cancer.
Owner:OSI PHARMA INC
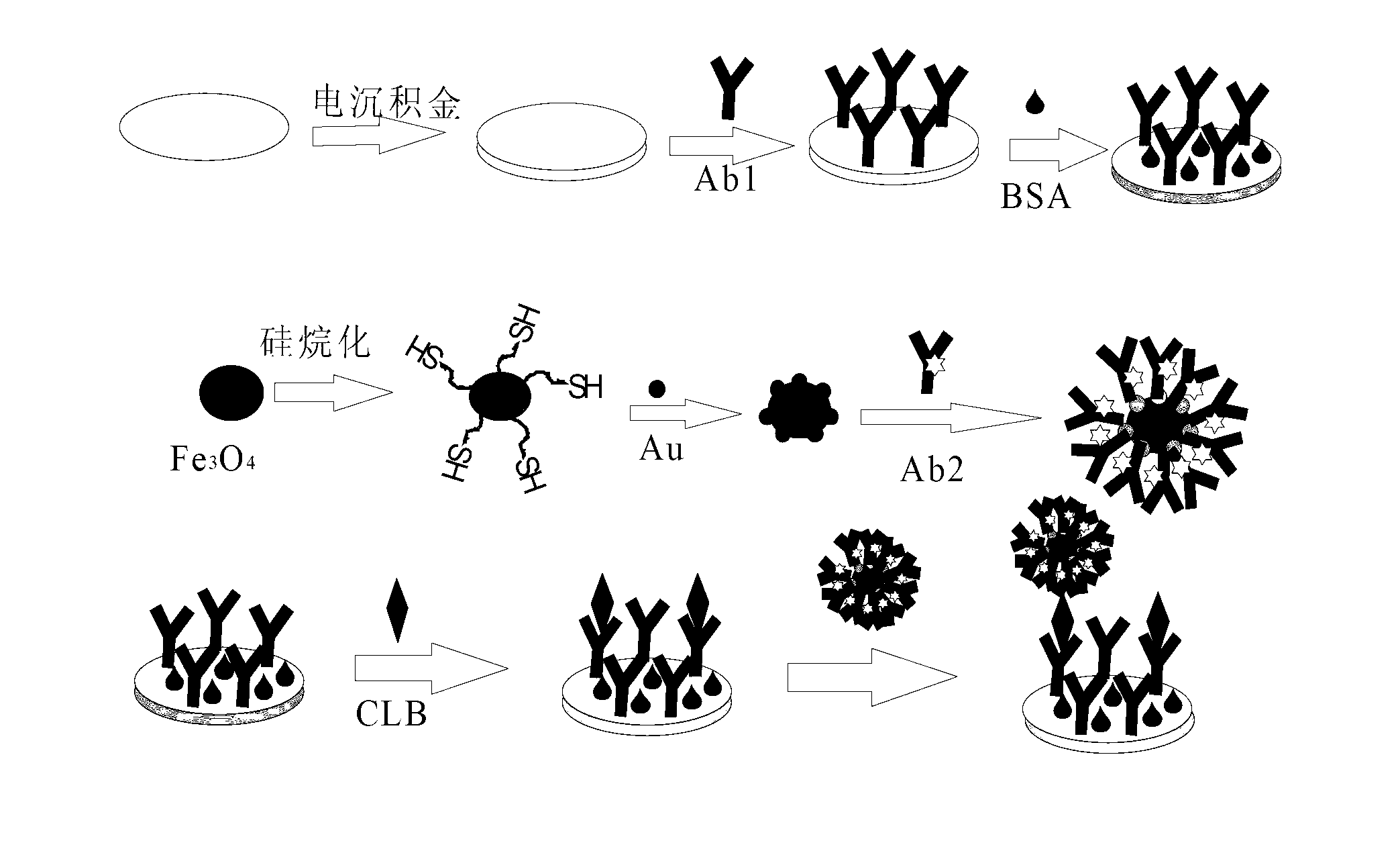
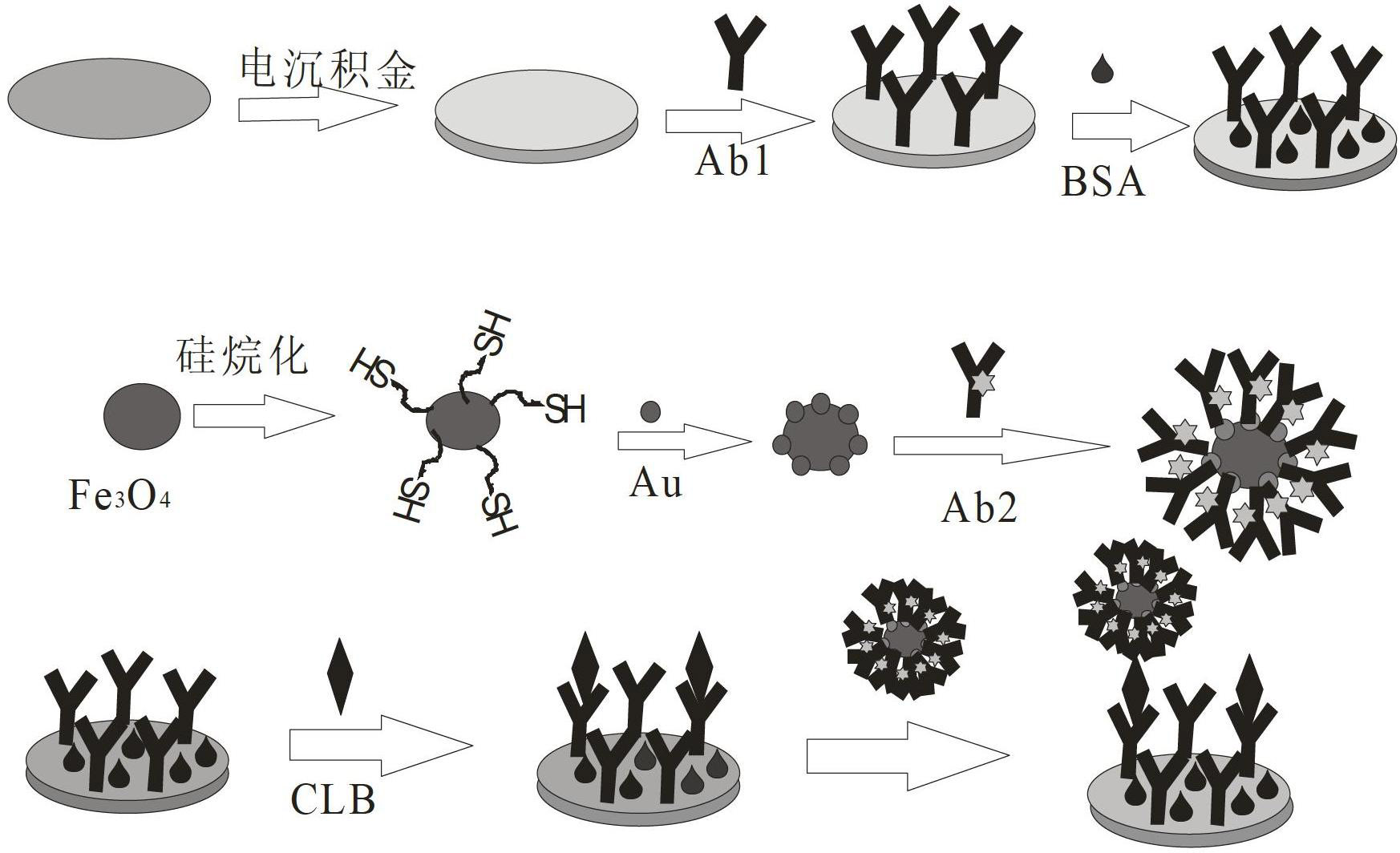
Preparation method and application of magnetic sandwich nano immunosensor
The invention discloses a preparation method of a magnetic sandwich nano immunosensor and application of the preparation method. The preparation method is characterized by comprising the following steps: mixing and stirring a collaurum solution and silane ferriferrous oxide into a colorless and transparent solution, and carrying out magnetic separation by externally applying a magnet to obtain FE3O4 / Au colloid nano magnetic beads; loading horse radish peroxidase (HRP) and secondary antibodies of an object to be measured to the FE3O4 / Au colloid nano magnetic beads to obtain a secondary antibody probe Fe3O4 / Au-HRP-Ab2; electrically depositing Au to a glassy carbon electrode, loading primary antibodies of the object to be measured, and then closing non-specific active sites by using protein to obtain an Abl / Au / GCE electrode; and finally, loading antigen to the Abl / Au / GCE electrode, and then obtaining the sandwich nano immunosensor by combining the magnet probe. The magnetic sandwich nano immunosensor can be used for measuring the concentration of melamine, tonyred, clenbuterol or estrogen in food and has the advantages of high detection speed, high accuracy and sensitivity and low cost.
Owner:NINGBO UNIV
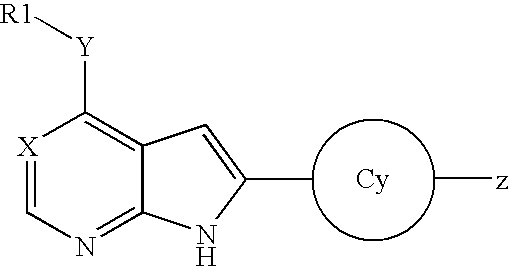
Aryl-amino substituted pyrrolopyrimidine multi-kinase inhibiting compounds
Compounds represented by Formula (I): or stereoisomers or pharmaceutically acceptable salts thereof, are inhibitors of least two of the Abl, Aurora-A, Blk, c-Raf, cSRC, Src, PRK2, FGFR3, Flt3, Lck, Mek1, PDK-1, GSK3β, EGFR, p70S6K, BMX, SGK, CaMKII, Tie-2, IGF-1R, Ron, Ret, and KDR kinases in animals, including humans, for the treatment and / or prevention of various diseases and conditions such as cancer.
Owner:OSI PHARMA INC
Anti-inflammatory medicaments
Novel compounds and methods of using those compounds for the treatment of inflammatory conditions, hyperproliferative diseases, cancer, and diseases characterized by hyper-vascularization are provided. In a preferred embodiment, modulation of the activation state of p38 kinase protein, abl kinase protein, bcr-abl kinase protein, braf kinase protein, VEGFR kinase protein, or PDGFR kinase protein comprises the step of contacting said kinase protein with the novel compounds.
Owner:DECIPHERA PHARMA LLC
Pharmaceutical Compounds
InactiveUS20100004232A1Reduce morbidityPatient compliance is goodBiocideSenses disorderDiseaseImatinib resistant
The use of a compound for the manufacture of a medicament for the prophylaxis or treatment of: A. a disease state or condition mediated by a kinase which is BCR-abl, VEGFR, PDGFR, EGFR, Flt3, JAK (e.g. JAK2 or JAK3), C-abl, PDK1, Chk (e.g. Cbk1 or Chk2), FGFR (e.g. FGFR3), Ret, Eph (e.g. EphB2 or EphB4), or Src (e.g. cSrc); or B. a cancer in which the cancer cells thereof contain a drug resistant kinase mutation which is: (a) a threonine gatekeeper mutation; or (b) a drug-resistant gatekeeper mutation; or (c) an imatinib resistant mutation; or (d) a nilotinib resistant mutation; or (e) a dasatinib resistant mutation; or (f) a T670I mutation in KIT; or (g) a T674I mutation in PDGFR; or (h) T790M mutation in EGFR; or (i) a T315I mutation in abl; or C. a cancer which expresses a mutated molecular target which is a mutated form of BCRabl, c-kit, PDGF, EGF receptor or ErbB2; or D. a disease mediated by a kinase containing a mutation in a region of the protein that binds to or interacts with other cancer agents but does not bind to or interact with the compounds of formula (I) or (I′), for example a mutated kinase selected from c-abl, c-kit, PDGFR including PDGFR-beta and PDGFR-alpha, and ErbB family members such as EGFR (ErbB1), HER2 (ErbB2), ErbB3, and ErbB4, members of the Ephrin receptor family including EphA1, EphA2, EphA3, EphA4, EphA5, EphA8, EphA10, EphB1, EphB2, EphB3, EphB5, EphB6, c-Src and kinases of the JAK family such as TYK2; wherein the compound is a compound of the formula (I or I′): or a salt, solvate, tautomer or N-oxide thereof wherein R0′, R1, R1′, R2′, R3′, R4′, A′, X′, E, A and M are as defined in the claims.
Owner:ASTEX THERAPEUTICS LTD
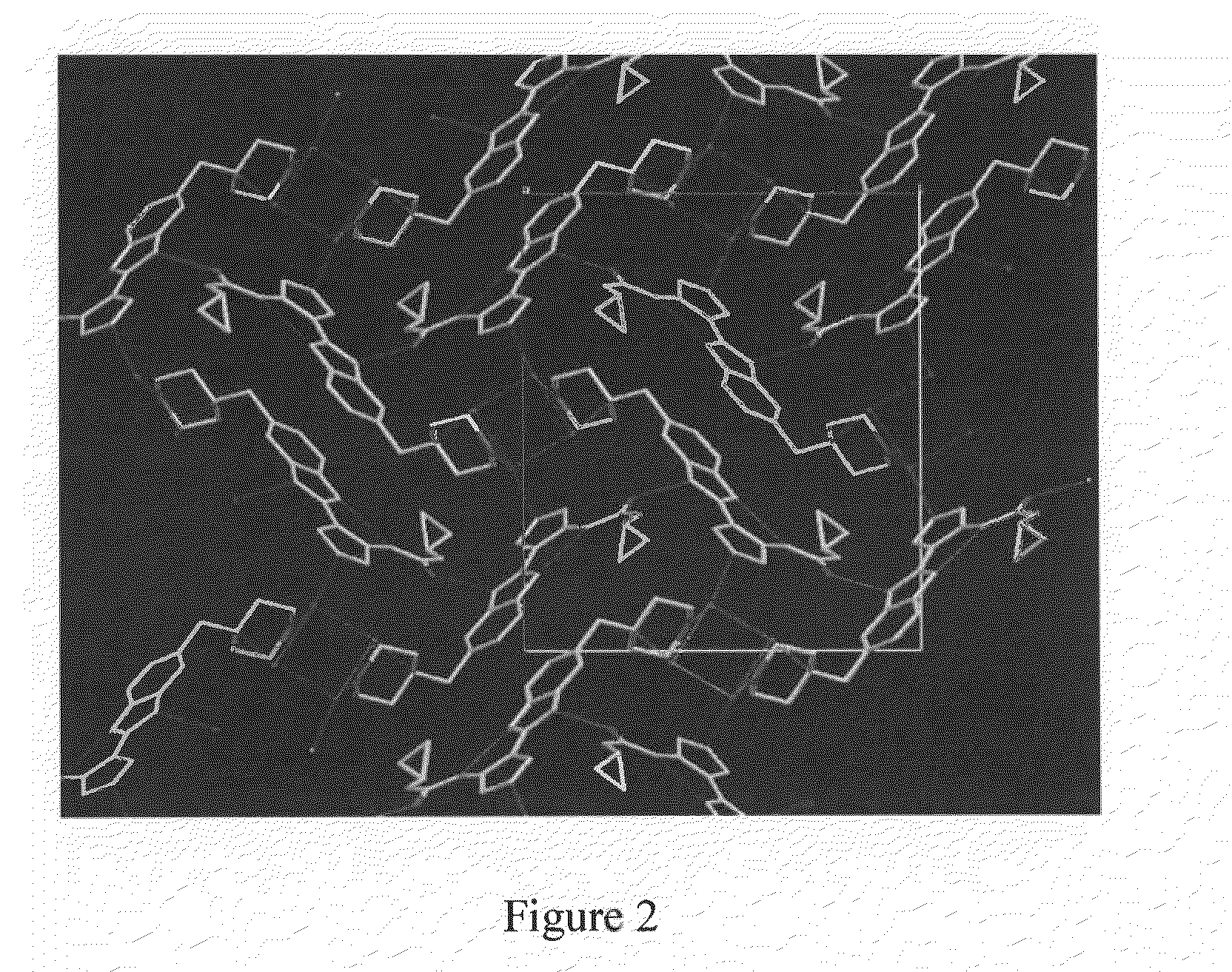
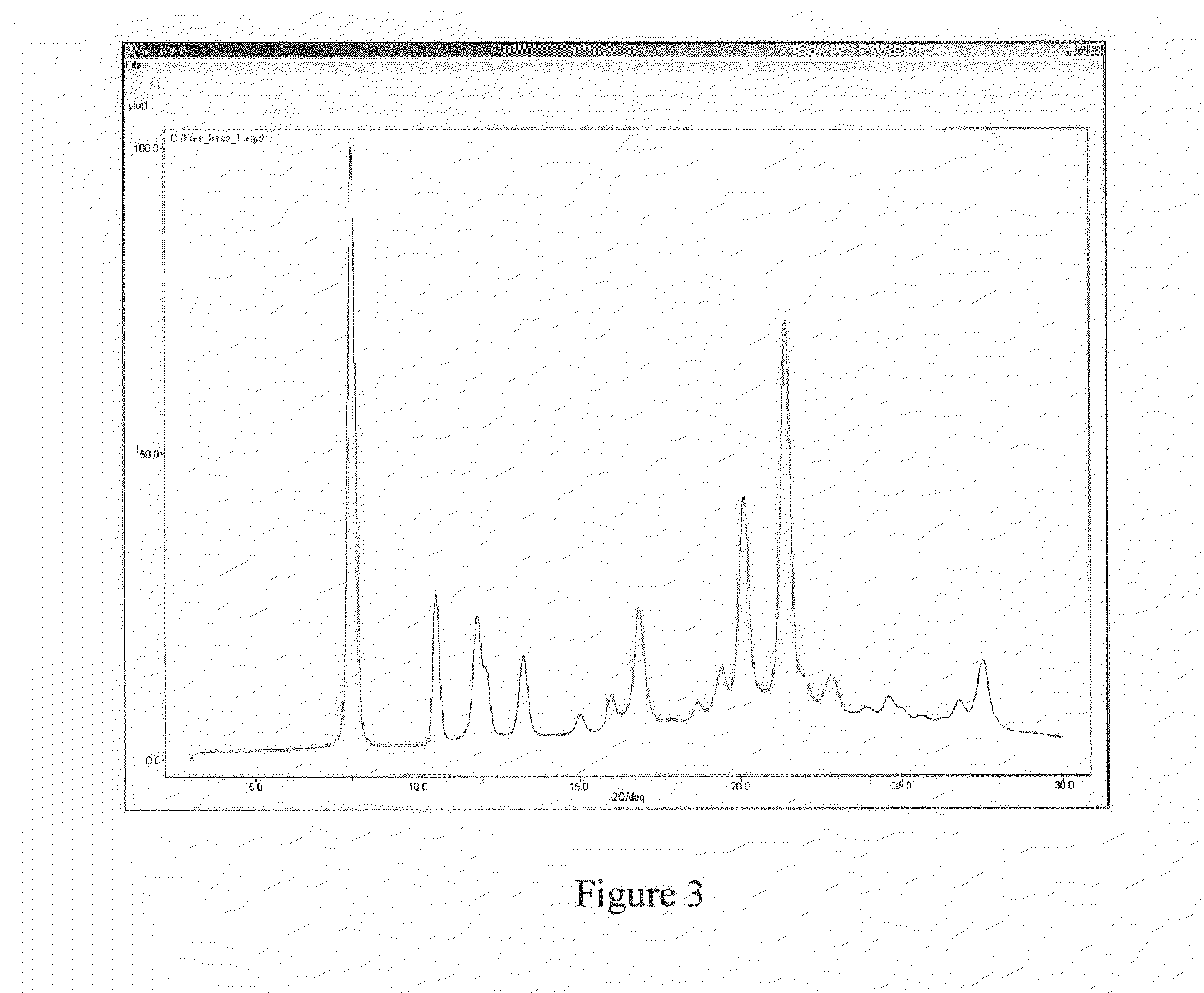
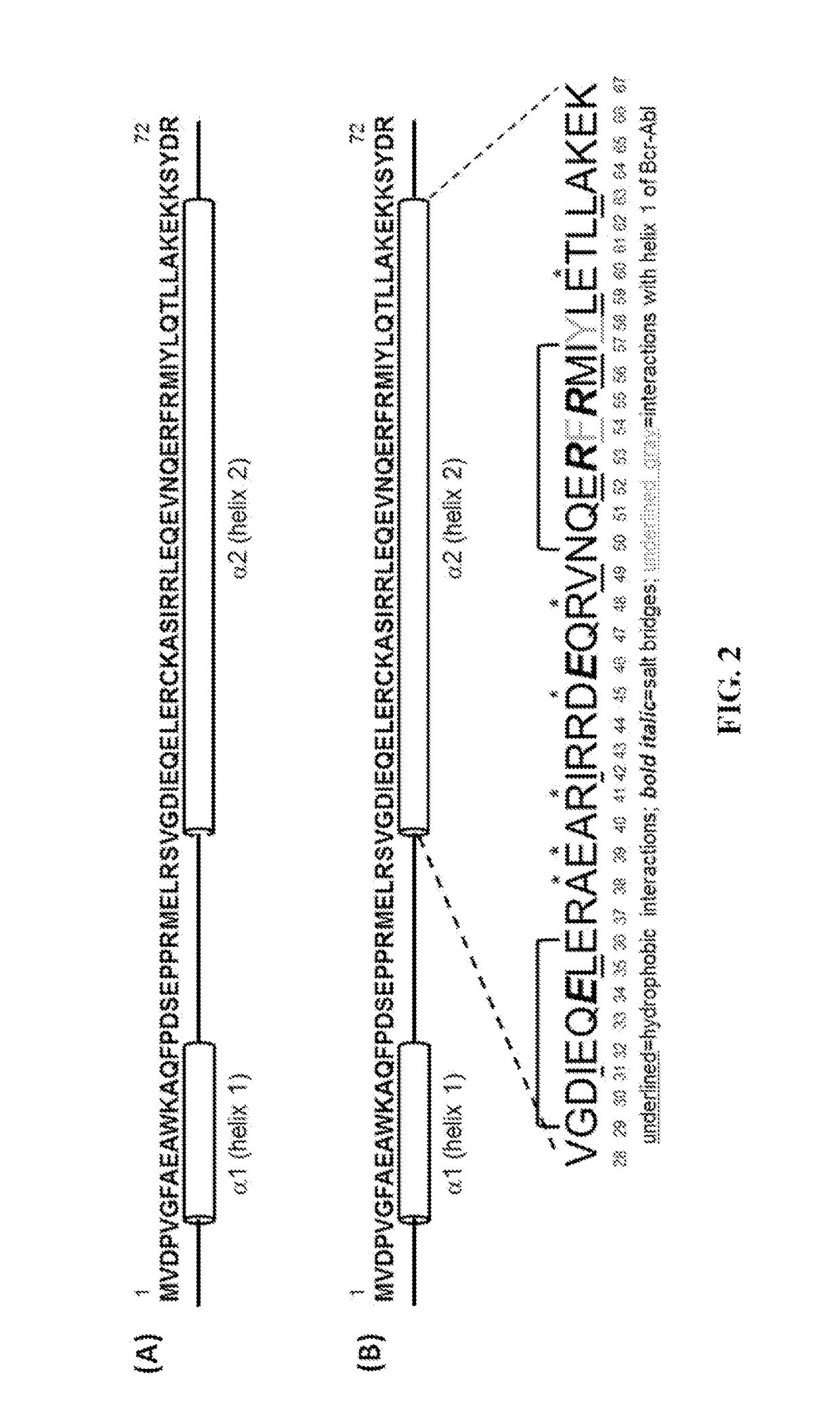
Peptide Inhibitors of BCR-ABL Oligomerization
In one aspect, the invention relates to peptides comprising the Bcr-Abl coiled-coil oligomerization domain and an alpha helix stabilizing moiety, mutant forms thereof, truncated forms thereof, derivatives thereof, and related peptides, which are useful as inhibitors of the Bcr-Abl chimeric protein; pharmaceutical compositions comprising the compounds; and methods of treating hyperproliferative disorders associated with Bcr-Abl using the compounds and compositions. This abstract is intended as a scanning tool for purposes of searching in the particular art and is not intended to be limiting of the present invention.
Owner:UNIV OF UTAH RES FOUND
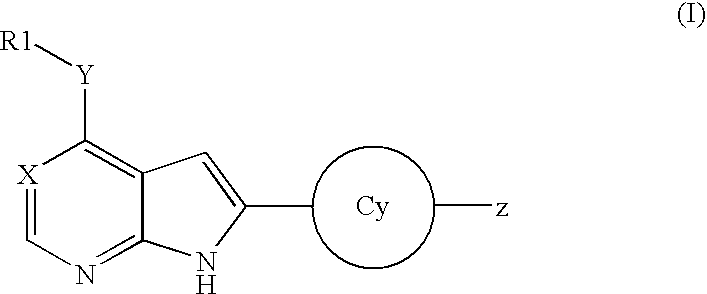
Pyrrolopyridine kinase inhibiting compounds
Compounds represented by Formula (I): or stereoisomers or pharmaceutically acceptable salts thereof, are inhibitors of least one of the Abl, Aurora-A, Blk, c-Raf, cSRC, Src, PRK2, FGFR3, Flt3, Lck, Mek1, PDK-1, GSK3β, EGFR, p70S6K, BMX, SGK, CaMKII, Tie-2, IGF-1R, Ron, Met, and KDR kinases in animals, including humans, for the treatment and / or prevention of various diseases and conditions such as cancer.
Owner:OSI PHARMA INC
Anti-tumor compound preparation method
InactiveCN101597284AGood curative effectLittle side effectsOrganic chemistryAntineoplastic agentsSide effectTyrosine
The invention provides an anti-tumor compound preparation method, namely, a preparation method of a compound shown in general formula (I) or a salt of same which can be accepted in pharmacology; wherein, R is selected from H, optional substitutional alkyl, optional substitutional cycloalkyl, optional substitutional aryl, optional substitutional aralkyl or optional substitutional heterocyclic radical. The compound shown in the general formula (I) prepared by the invention can inhibit tyrosine kinase Bcr-Abl and other kinases (Lck, Fyn, SrC, Hck) of SRC family after decomposition in vivo so that the compound prepared by the invention shown in the general formula (I) or the salt can be accepted in pharmacology and can be used in the medical application of cancer treatment. Compared with the existing anti-tumor compounds, the compound of the invention has better curative effect and lower side effect.
Owner:BEIJING LABSOLUTIONS PHARMA
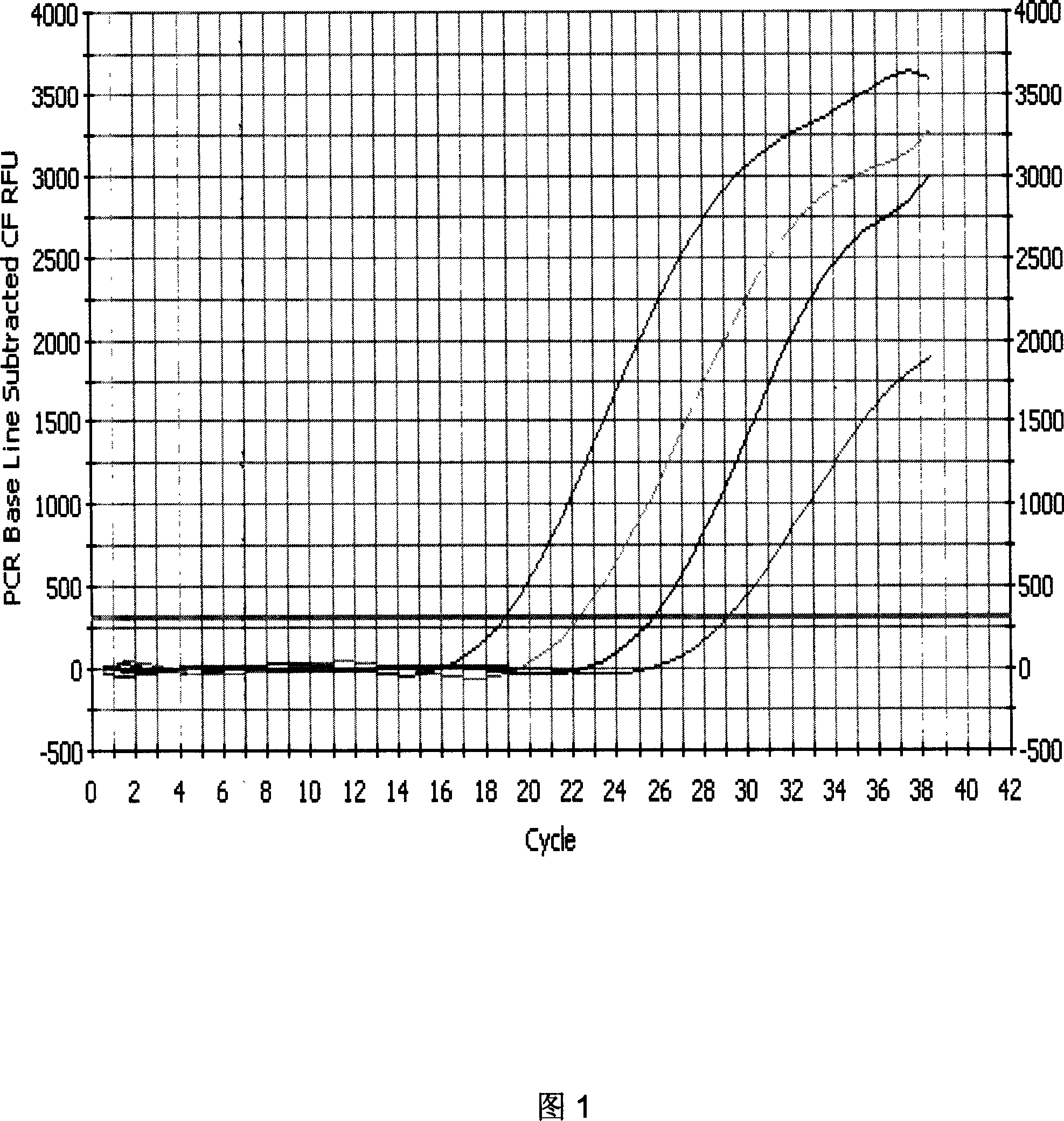
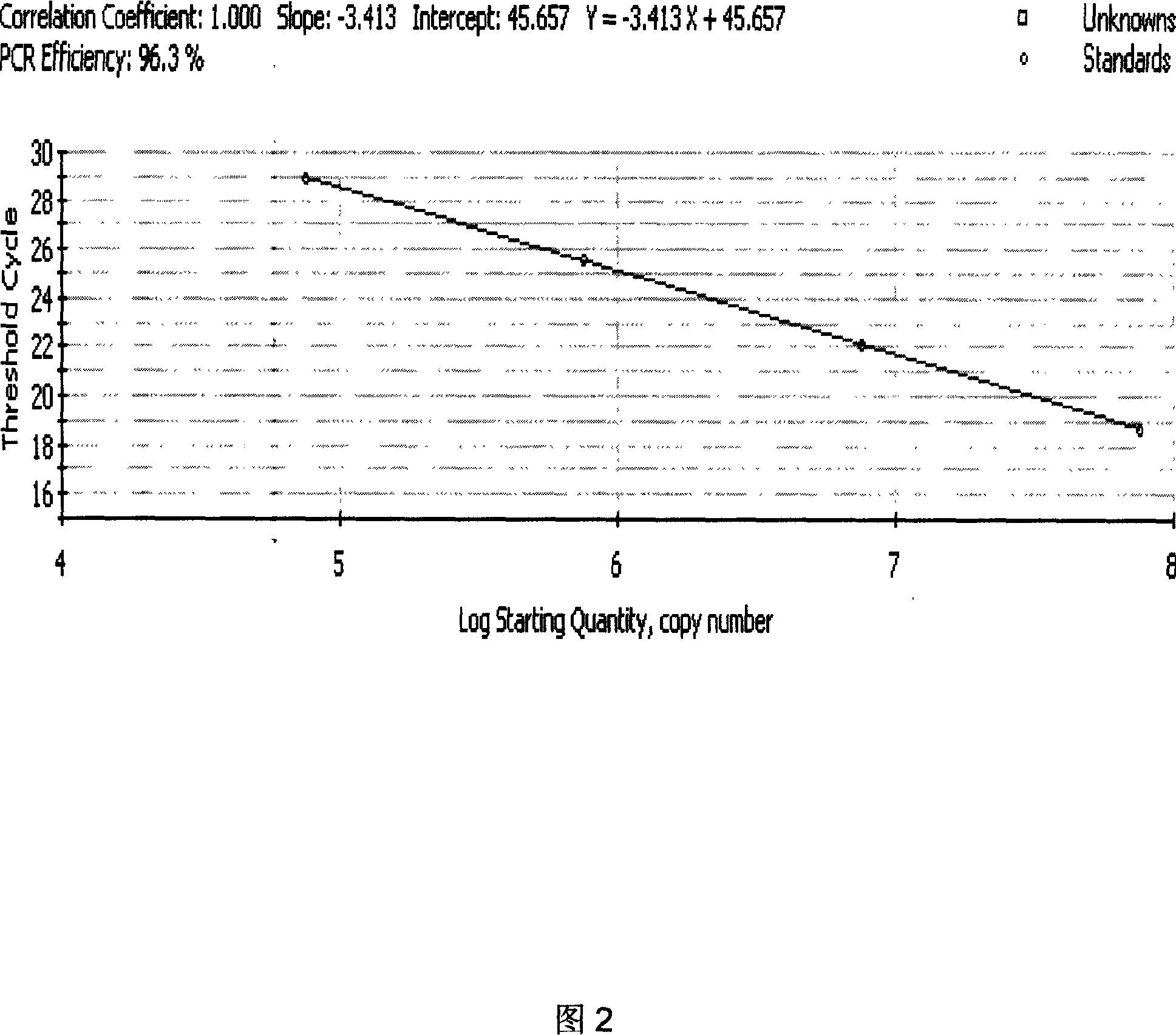
PML-RARa gene fluorescence quantitative RT-PCR primer and probe and reagent kit
InactiveCN1995385AImprove induction rateReduce early deathMicrobiological testing/measurementReference genesFluorescence
The invention discloses a quantitative RT-PCR primer and probe and agent box of PML-RARa fusing gene mRNA fluorescence with BCR-ABL fusing gene primer and probe sequence as SEQ ID NO1-4 and internal reference gene primer and probe sequence as SEQ ID NO5-7, wherein the agent box contains cell cracking liquid, water, RT-PCR reacting liquid, internal reference G6PDHRT-PCR reacting liquid, BCR-ABL fusing gene detecting probe, G6PDH internal reference gene testing probe, composite enzyme, standard material and comparing material; the invention measures the expressive level of L-pattern, S-pattern and V-pattern mRNA simultaneously, rapidly and precisely, which improves inducing buffer rate and lessens early bleeding death.
Owner:SHANGHAI FOSUN PHARMA (GROUP) CO LTD +1
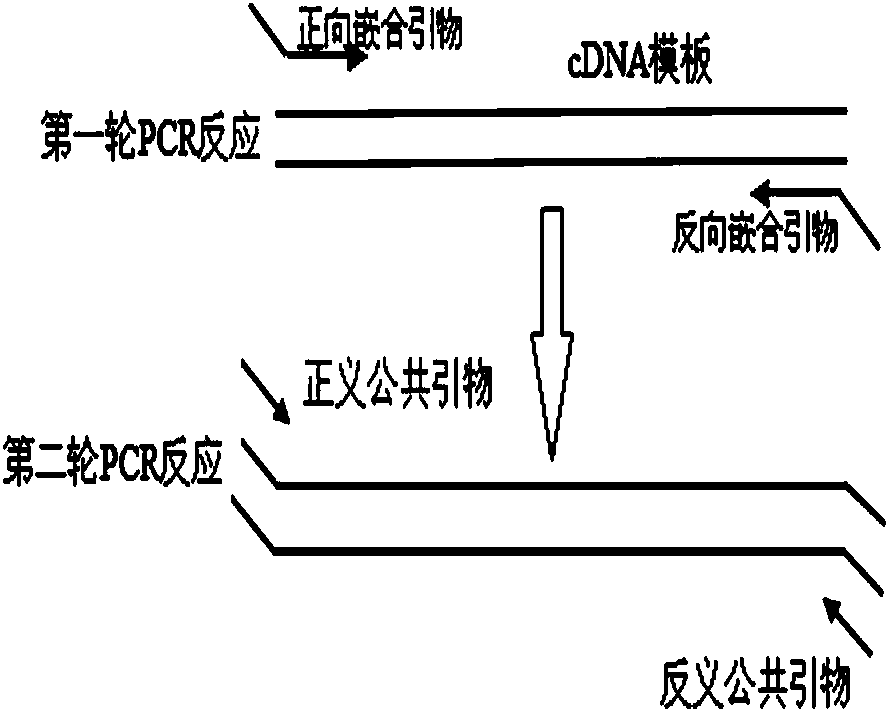
Multiplex PCR (polymerase chain reaction) kit for detecting leukemia fusion genes
The invention belongs to the field of gene detection, and in particular relates to a multiplex PCR (polymerase chain reaction) kit for detecting leukemia fusion genes. The kit comprises a conventional multiplex PCR component, and chimeric primer and common primer pairs aiming at the common leukemia fusion genes. Chimeric primers are sequences of adding common primers to the 5' ends of specific primers of seven shear isomers of the common leukemia fusion genes BCR / ABL, PML / RARalpha, AML1 / ETO and E2A / PBX1. By using the multiplex PCR kit for detecting the leukemia fusion genes, seven leukemia fusion genes including AML1 / ETO, BCR / ABLe1a2, BCR / ABLe13a2, BCR / ABLe14a2, PML / RARalphaL, PML / RARalphaS and E2A / PBX11 with high incidence can be simultaneously detected, so that the consumption of reagents can be saved, the detection cost can be reduced, and meanwhile, the detection efficiency is improved. Moreover, the highest detection sensitivity of the kit can reach 10 copies per 25mu L, and the kit is simple in operation and high in clinical detection rate, so that the kit has clinical popularization and application values.
Owner:SUZHOU UNIV
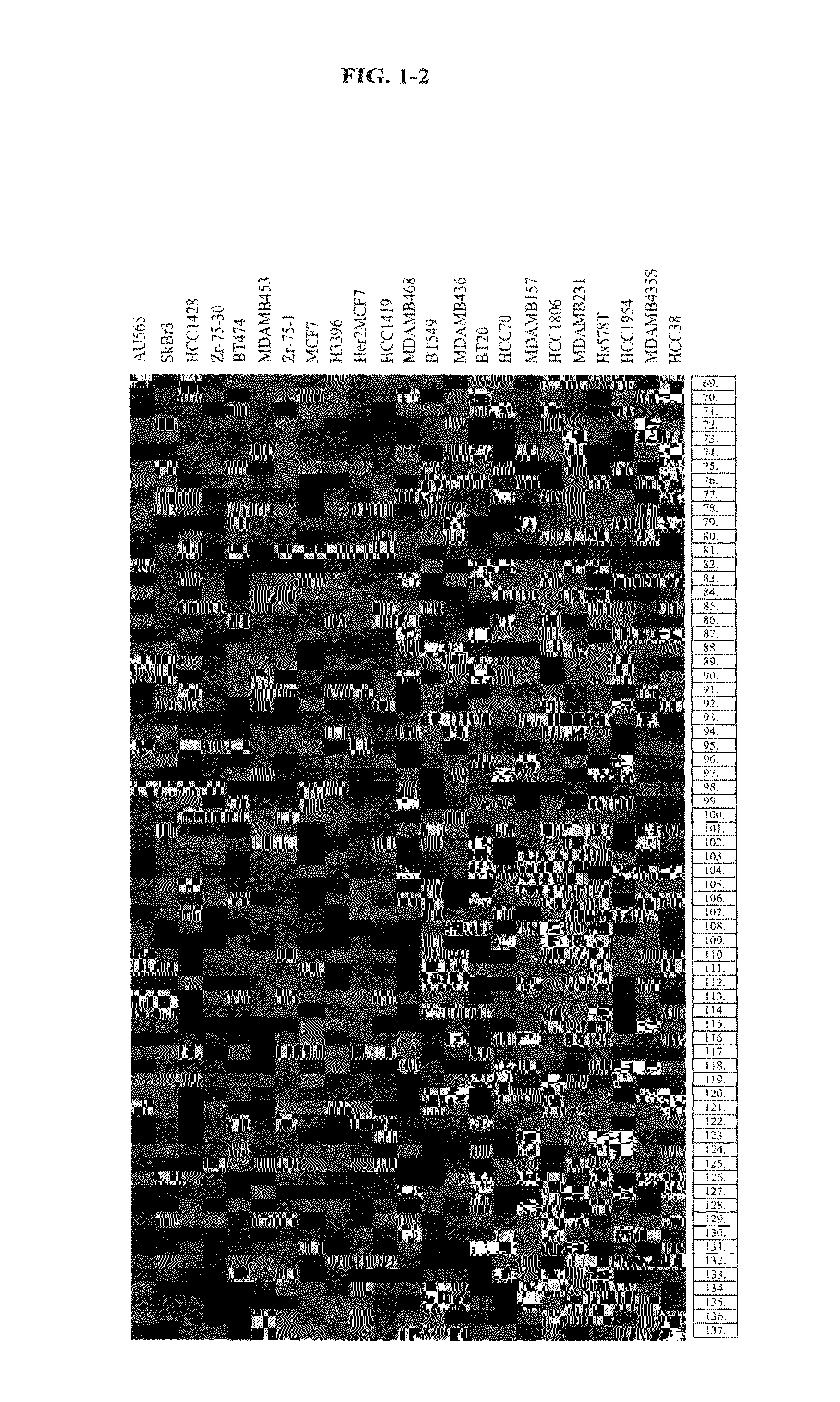
Identification of polynucleotides for predicting activity of compounds that interact with and/or modulate protein tyrosine kinases and/or protein tyrosine kinase pathways in breast cells
InactiveUS7537891B2Improved prognosisContinue treatmentSugar derivativesPeptide/protein ingredientsDisease areaProtein-Tyrosine Kinases
The present invention describes polynucleotides that have been discovered to correlate to the relative sensitivity or resistance of cells, e.g., breast cell lines, to treatment with compounds that interact with and modulate, e.g., inhibit, protein tyrosine kinases, such as, for example, members of the Src family of tyrosine kinases, e.g., Src, Fgr, Fyn, Yes, Blk, Hck, Lck and Lyn, as well as other protein tyrosine kinases, including, Bcr-abl, Jak, PDGFR, c-kit and Eph receptors. These polynucleotides have been shown to have utility in predicting the resistance and sensitivity of breast cell lines to the compounds. Such polynucleotides comprise polynucleotide predictor or marker sets useful in methods of predicting drug response, and as prognostic or diagnostic indicators in disease management, particularly in those disease areas, e.g., breast cancer, in which signaling through one or more of the aforementioned Src tyrosine and protein tyrosine kinases is involved with the disease process.
Owner:BRISTOL MYERS SQUIBB CO
Primers, probes and kit for detecting leukemia-related fusion genes
InactiveCN105296637AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationMicrobiologyFluorescent pcr
The invention discloses primers, probes and a kit for detecting leukemia-related fusion genes. The primers and probes are designed according to specificity of eight types of fusion genes such as BCR / ABL, PML / RAR alpha, AML1 / ETO and TEL / AML1. The primers, probes and kit have high sensitivity and good specificity. The primers, probes and kit can detect 10 copies of a mutational sample. The kit utilizes a one-step real-time fluorescent quantitative reverse transcription polymerase chain reaction method to detect a sample to be detected, realizes reverse transcription and quantitative PCR in a reaction system, only needs one-step fluorescent PCR amplification, is free of a reagent and pipe cover opening in the reaction process, has detection time of 90min and is operated simply. The kit has the advantages of high sensitivity, good specificity, low price, fastness and simple operation and has a high scientific research value and a high clinical application value.
Owner:武汉海吉力生物科技有限公司
Novel compounds and compositions as protein kinase inhibitors
The invention provides a novel class of compounds, pharmaceutical compositions comprising such compounds and methods of using such compounds to treat or prevent diseases or disorders associated with abnormal or deregulated tyrosine kinase activity, particularly diseases associated with the activity of PDGF-R, c-Kit and Bcr-abl.
Owner:IRM
Irf-4 as a tumor suppressor and uses thereof
The invention relates to methods for treating BCR / ABL mediated disorders. The methods of the invention also include monitoring progression of or sensitivity to treatment of BCR / ABL mediated disorders as well as identifying subjects for the treatment methods of the invention. Screening assays and related products and kits are also encompassed within the invention.
Owner:BRANDEIS UNIV
Hydrochloride of pyrrolo-pyrazine compound and application of hydrochloride
ActiveCN104557939AImprove solubilityGood efficacy in vivoOrganic active ingredientsOrganic chemistryDiseaseBcr abl kinase
The present invention relates to the field of chemical medicines, in particular to compounds as represented by formula I having BCR-ABL kinase inhibitory activity, or pharmaceutically acceptable salt, isomer, solvate, crystal or prodrug thereof, and pharmaceutical composition containing the compounds, and application of the compounds or compositions in drug preparation. The compounds of the present invention have strong inhibitory effect on BCR-ABL kinase, and can be used to treat diseases such as tumors.
Owner:NANJING SANHOME PHARM RES & DEV CO LTD
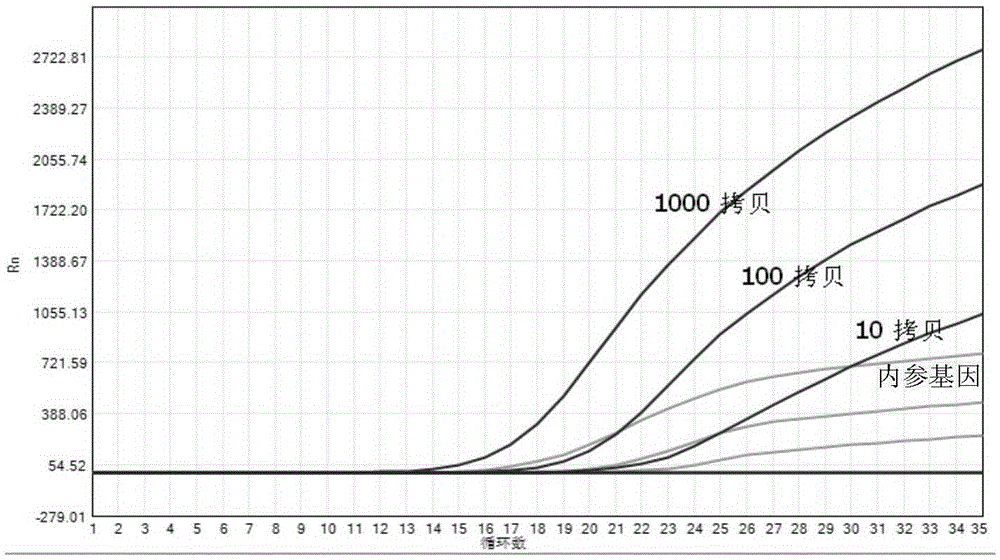
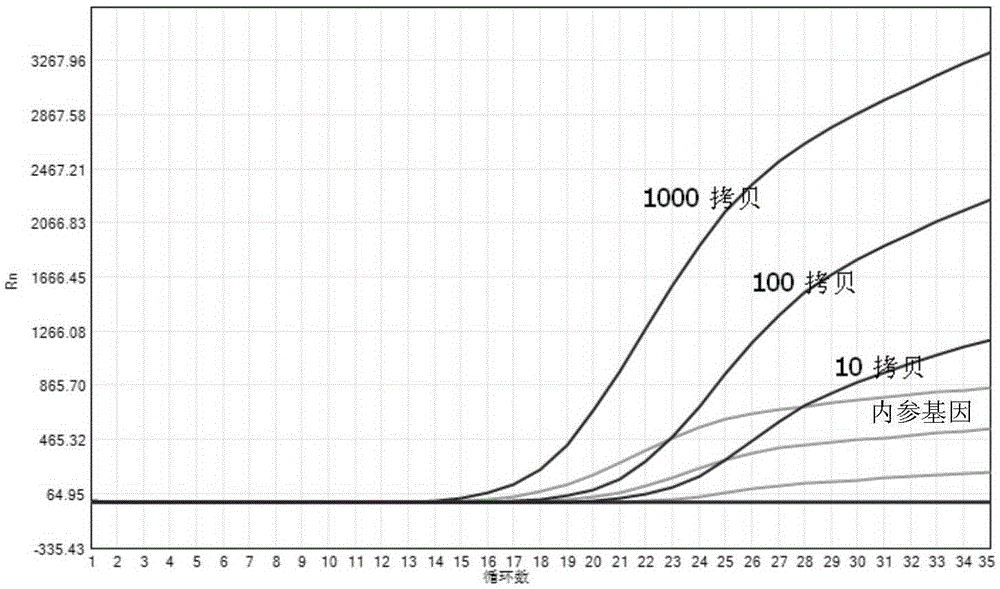
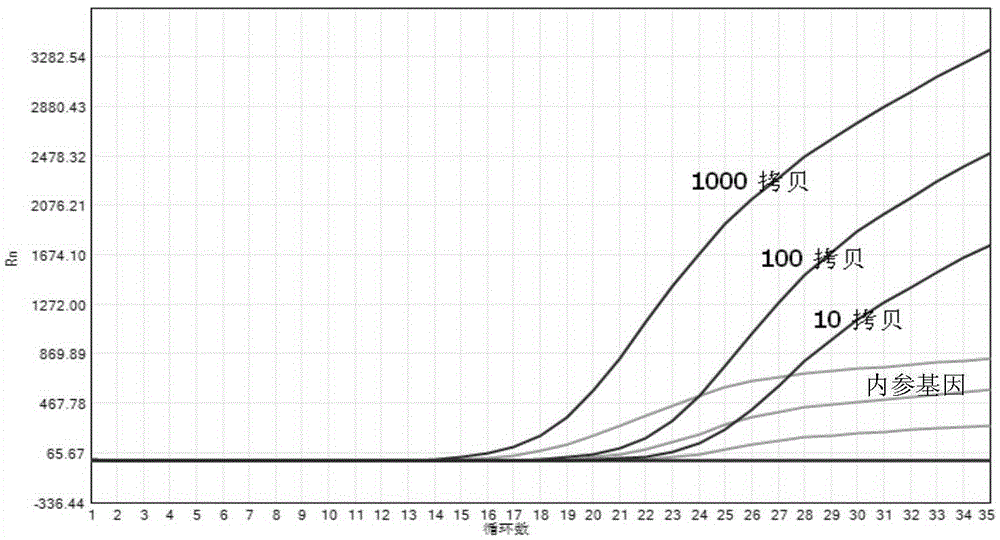
Method and kit for quantitative detection of BCR-ABL fusion genes
InactiveCN109554474AReduce pollutionReduce riskMicrobiological testing/measurementDNA/RNA fragmentationReference genesFluorescence
The invention provides a method and kit for quantitative detection of BCR-ABL fusion genes. The specific technical scheme comprises that two BCR-ABL fusion genetypes are detected only by two differentupstream primers, a common downstream primer, a fluorescent labeled probe and a set of ABL internal reference gene detection system. With use of the specific primers and probes provided by the invention, the two fusion types of BCR-ABL can be detected only by one tube of a material, and the kit has the advantages of high specificity, good sensitivity and high accuracy.
Owner:DAAN GENE CO LTD
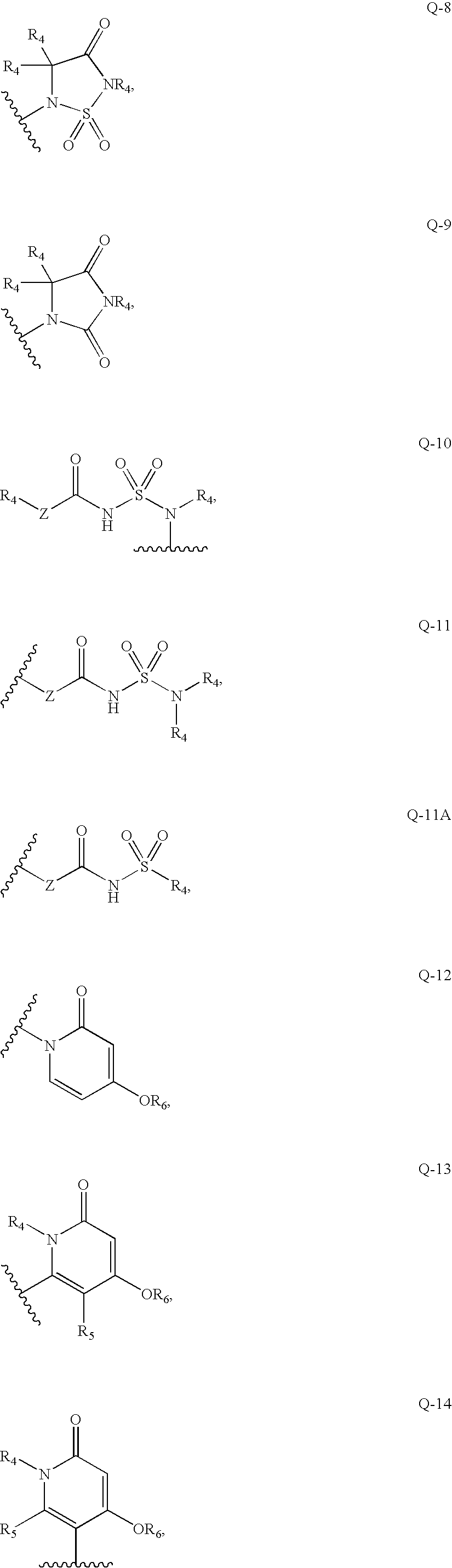
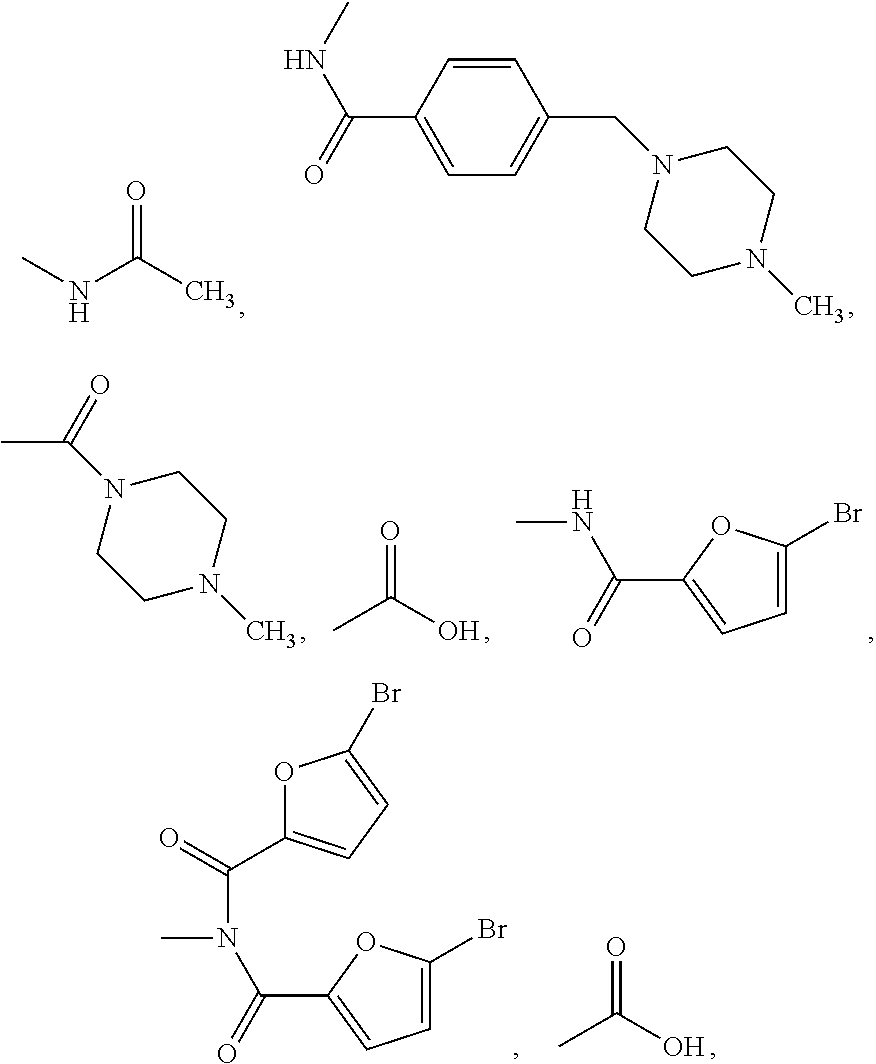
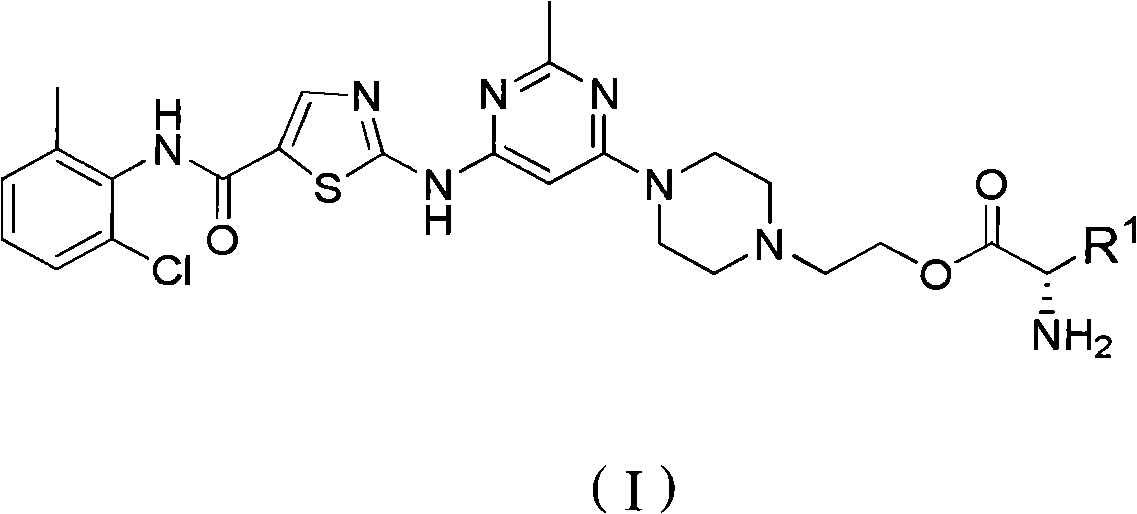
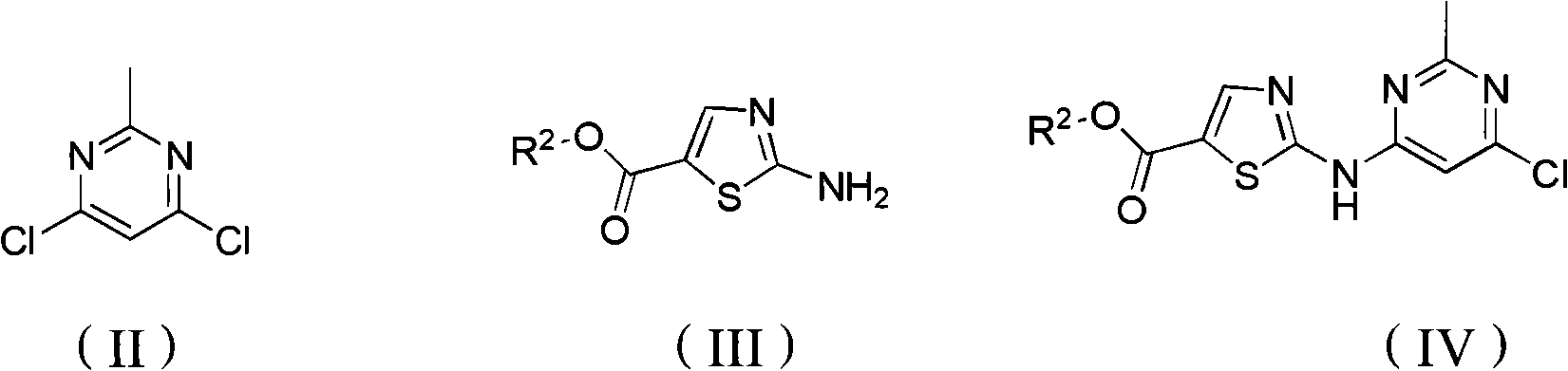
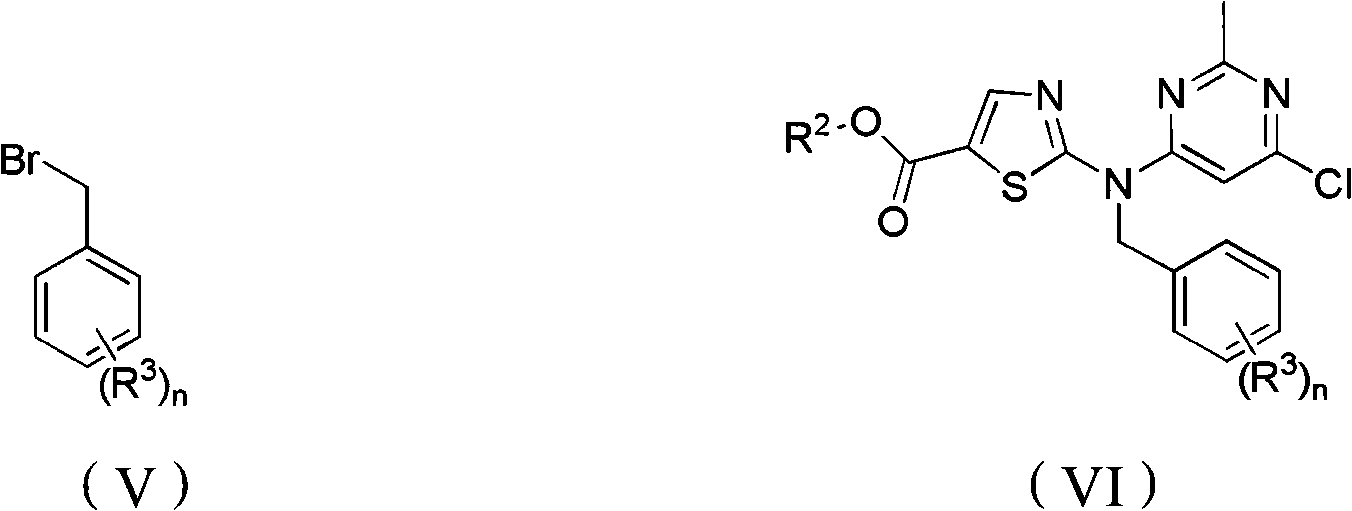
N-(2-chloro-6-methylphenyl)-2[(2-methylpyrimidine-4-group) amino] thiazole-5-formamide compound as well as preparation method and application thereof
The invention belongs to the field of medicament synthesis, relates to an N-(2-chloro-6-methylphenyl)-2[(2-methylpyrimidine-4-group) amino] thiazole-5-formamide compound shown in a general formula (b), and in particular relates to a pyrimidine 6-bit four-membered heterocyclic ring or spiro ring substituted N-(2-chloro-6-methylphenyl)-2[(2-methylpyrimidine-4-group) amino] thiazole-5-formamide compound, a preparation method of the pyrimidine 6-bit four-membered heterocyclic ring or spiro ring substituted N-(2-chloro-6-methylphenyl)-2[(2-methylpyrimidine-4-group) amino] thiazole-5-formamide compound, and an application of the pyrimidine 6-bit four-membered heterocyclic ring or spiro ring substituted N-(2-chloro-6-methylphenyl)-2[(2-methylpyrimidine-4-group) amino] thiazole-5-formamide compound in medical science. The compound disclosed by the invention is subjected to in-vitro ABL kinase inhibitory activity and anti-tumor activity tests, and results show that the compound has good ABL kinase inhibitory activity and anti-tumor activity and can be further used for preparing a new anti-tumor medicament.
Owner:FUDAN UNIV
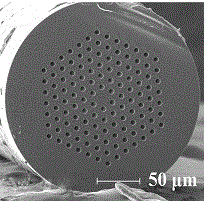
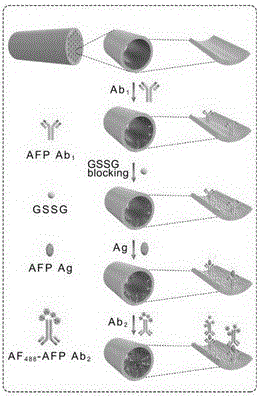
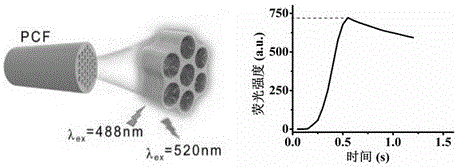
Immunosensor based on photonic crystal fiber and application thereof
ActiveCN106290896AEfficient collectionLarge specific surface areaBiological testingAntigenPhotonic crystal
The invention belongs to the technical field of immunoassay analysis and particularly relates to an immunosensor based on photonic crystal fibers as well as a manufacturing method and application thereof. The manufacturing method comprises the following steps: combining an alpha-fetoprotein antibody Abl on the PCF inner surface through a covalent bond, adding an antigen and a fluorescent labeled second antibody (Ab2) sequentially, forming a sandwich structure Ab1-AFP-Ab2 through the interaction between the antibody and the antigen, and quantitatively detecting a tumor marker AFP of primary hepatic carcinoma (PHC). Compared with an existing product and the prior art, the photonic crystal optical fiber immunosensor has the benefits that the whole preparation process is simple and suitable for industrial production; the antigen concentration is positively correlated with the fluorescence intensity, so that the alpha-fetoprotein can be qualitatively and quantitatively detected; the operation in the detection process is very simple and convenient, and the needs of higher detection sensitivity can be met, therefore, an important clinical diagnostic significance is realized.
Owner:NORTHEAST NORMAL UNIVERSITY
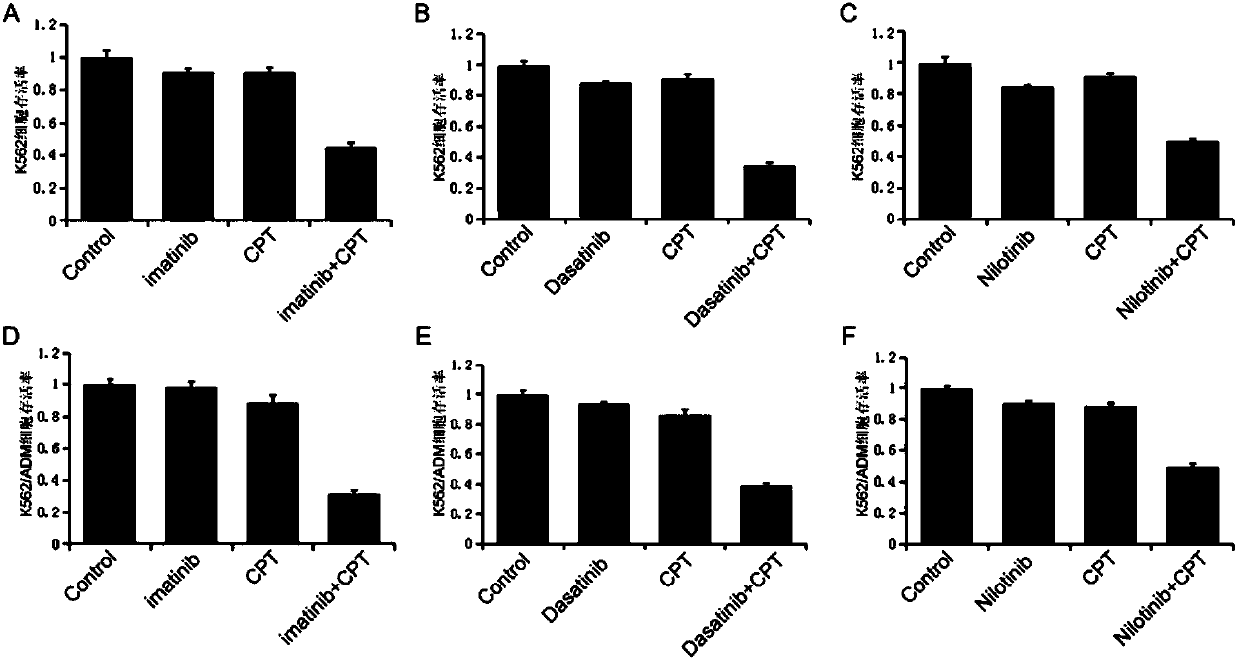
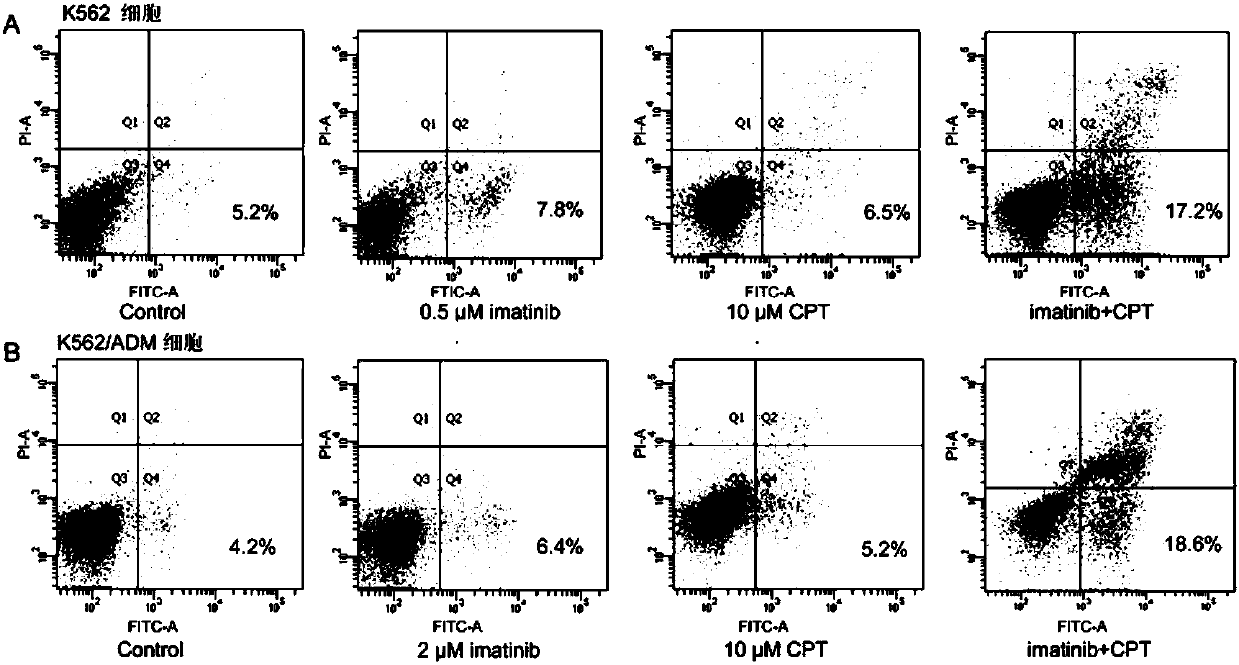
Cryptotanshinone pharmaceutical composition and application thereof in preparation of CML (chronic myeloid leukemia) treatment drug
InactiveCN107583054AIncreased sensitivityGood synergyOrganic active ingredientsAntineoplastic agentsMyeloid leukemiaSide effect
The invention discloses cryptotanshinone pharmaceutical composition and an application thereof in preparation of a CML (chronic myeloid leukemia) treatment drug. The cryptotanshinone pharmaceutical composition contains cryptotanshinone and a tyrosine kinase inhibitor, with the adoption of cryptotanshinone for treatment, protein expression level of Bcr-Abl genes in CML K562 and multi-drug resistantstrains K562 / ADM cells can be remarkably reduced, cell proliferation inhibition ratio of the tyrosine kinase inhibitor is increased, and apoptosis rate of CML K562 cells and K562 / ADM cells can be remarkably increased through combination of cryptotanshinone and the tyrosine kinase inhibitor. With the adoption of the cryptotanshinone pharmaceutical composition, chemosensitivity of tyrosine kinase can be improved, chemotherapy drug dosage of a patient is reduced, toxic and side effects on a human body are reduced, and a new treatment scheme is provided for clinical treatment of CML.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
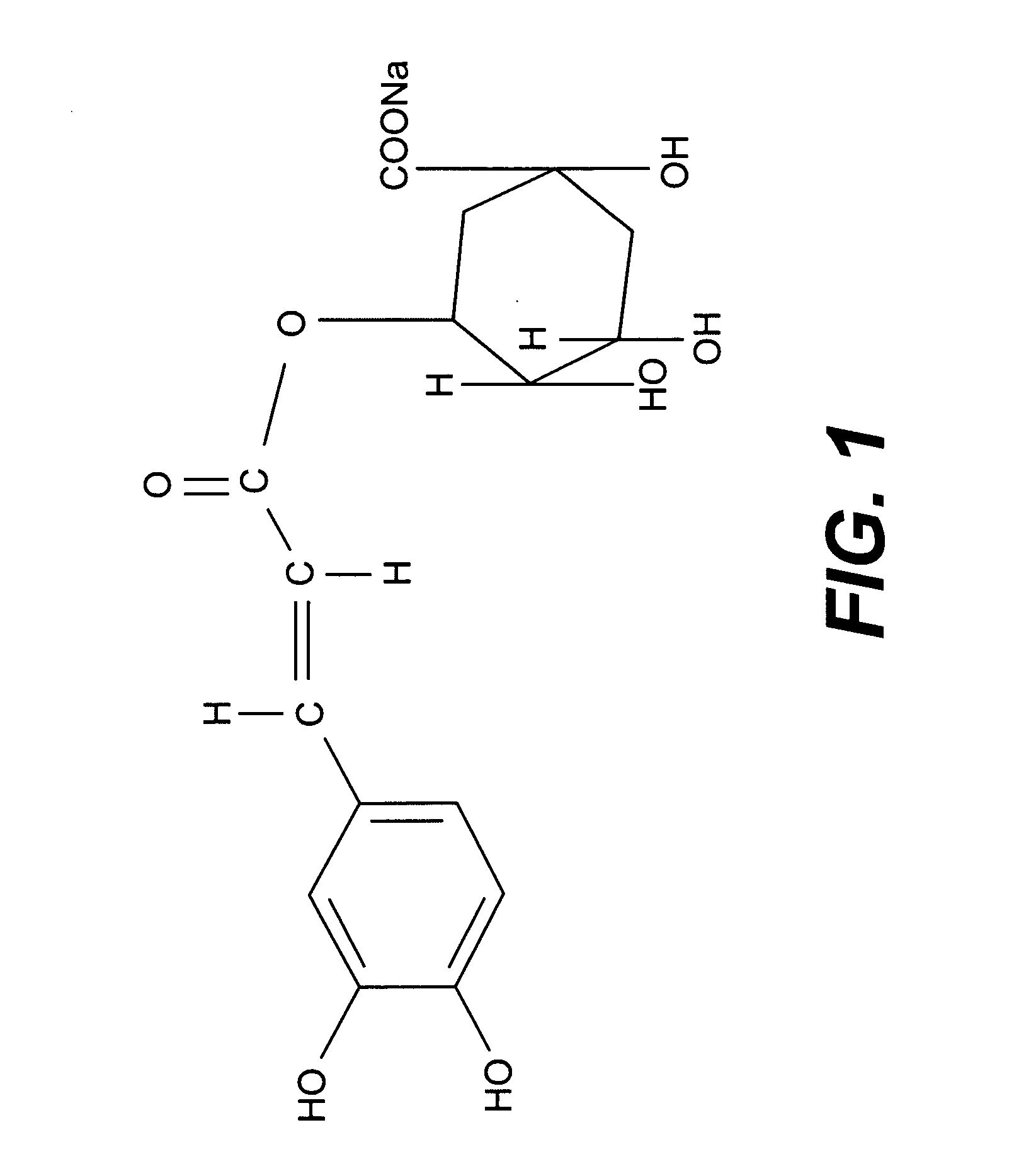
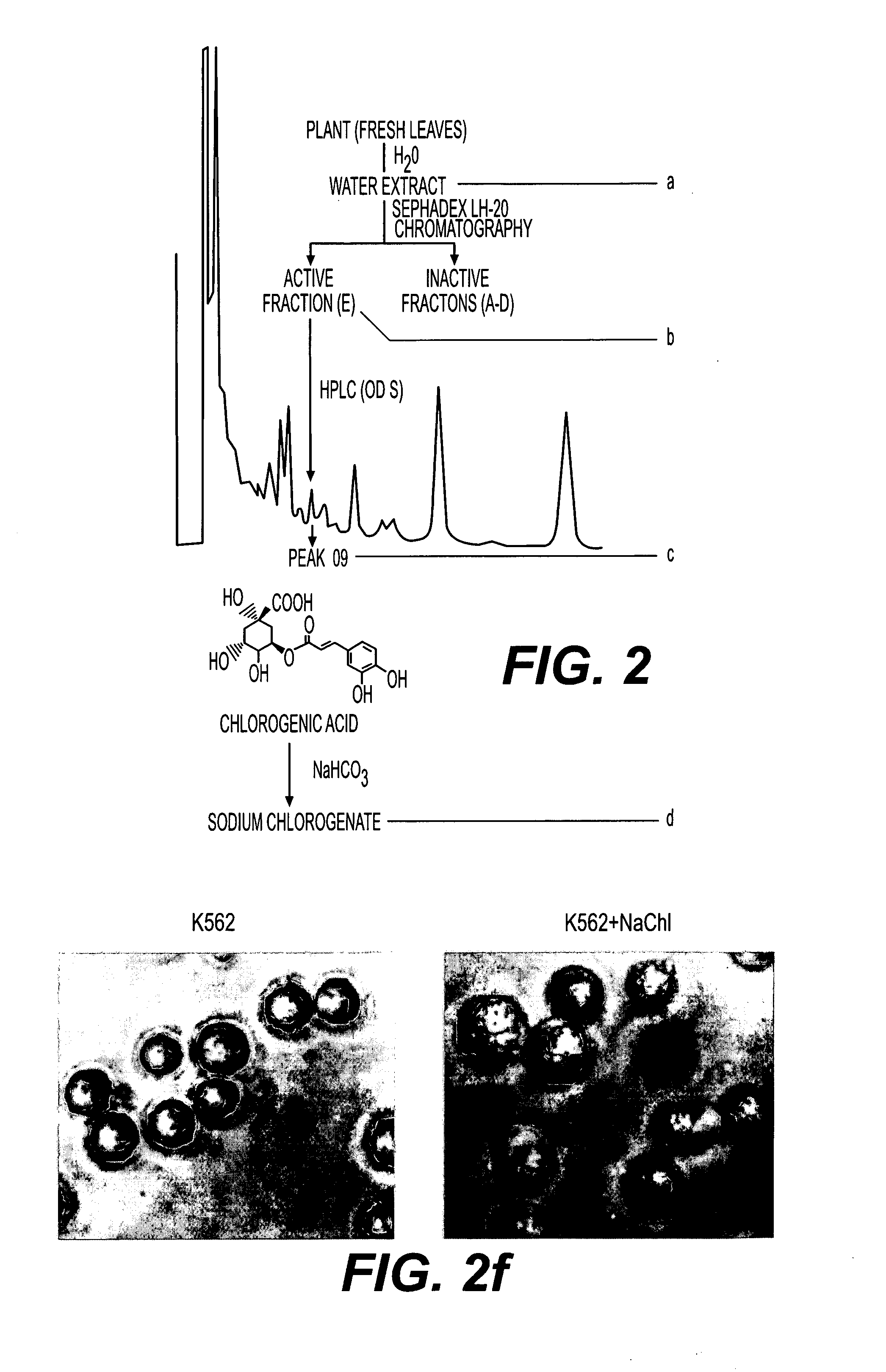
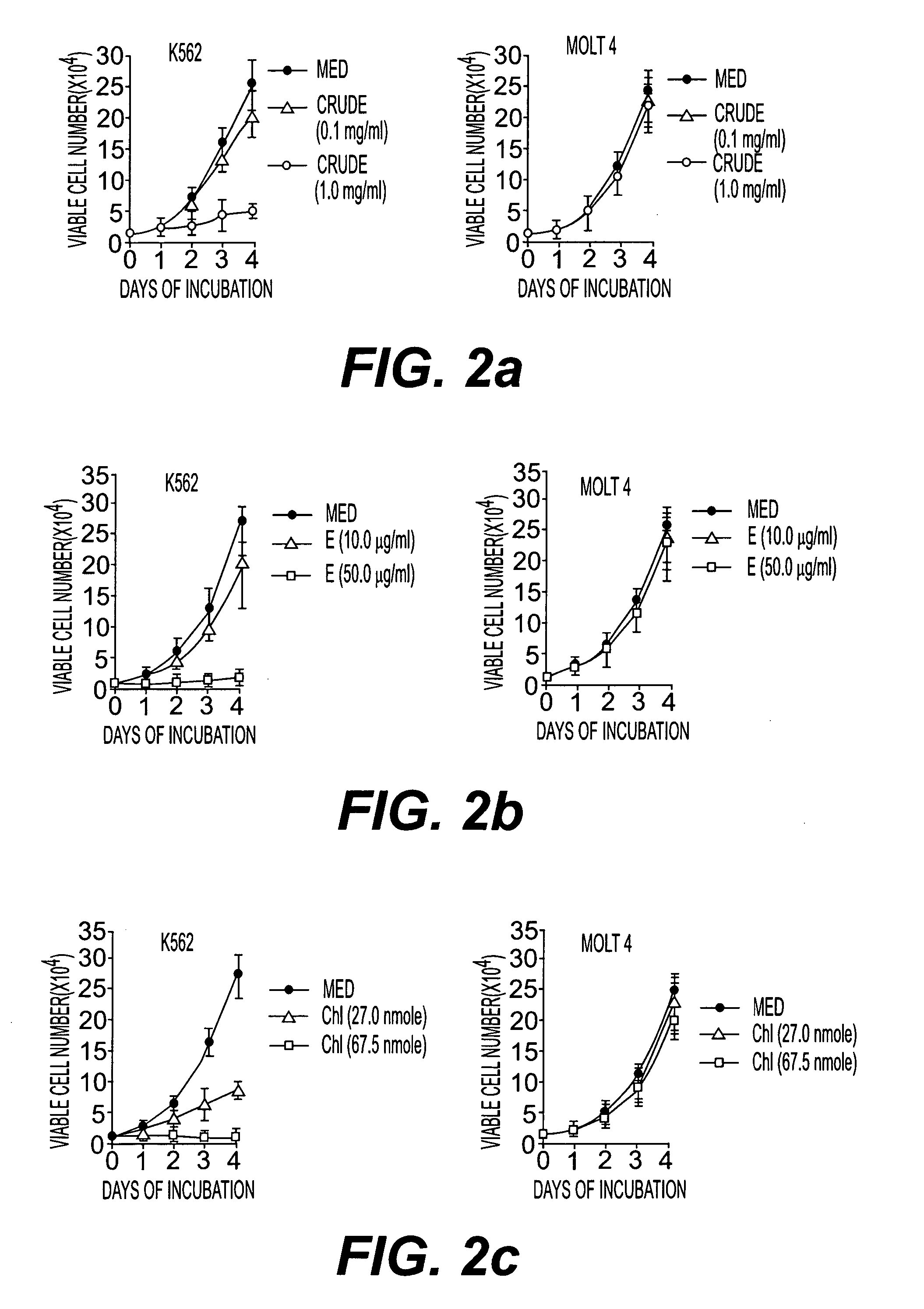
Pharmaceutical composition useful for treating chronic myeloid leukemia
This invention relates to a pharmaceutical composition useful for treating chronic myeloid leukemia where Bcr-Abl kinase is constitutively expressed in animals and humans, and a treatment of chronic myeloid leukemia (CML) by a composition comprising an effective amount of analogs and / or salts of chlorogenic acid. The analogs are preferably sodium chlorogenate (Na-Chl) or potassium or ammonium salts, which were prepared from Chlorogenic acid or its analogs.
Owner:COUNCIL OF SCI & IND RES
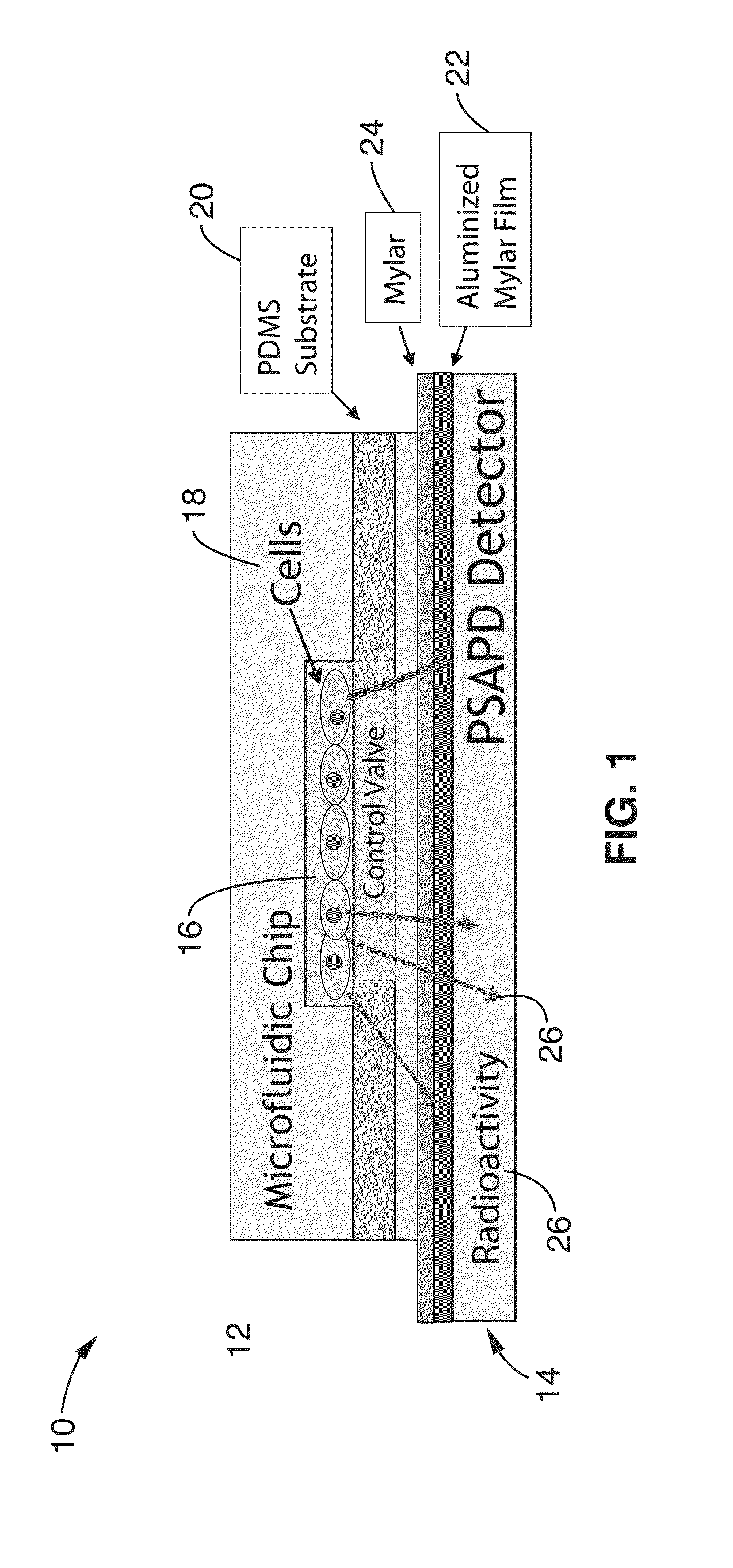
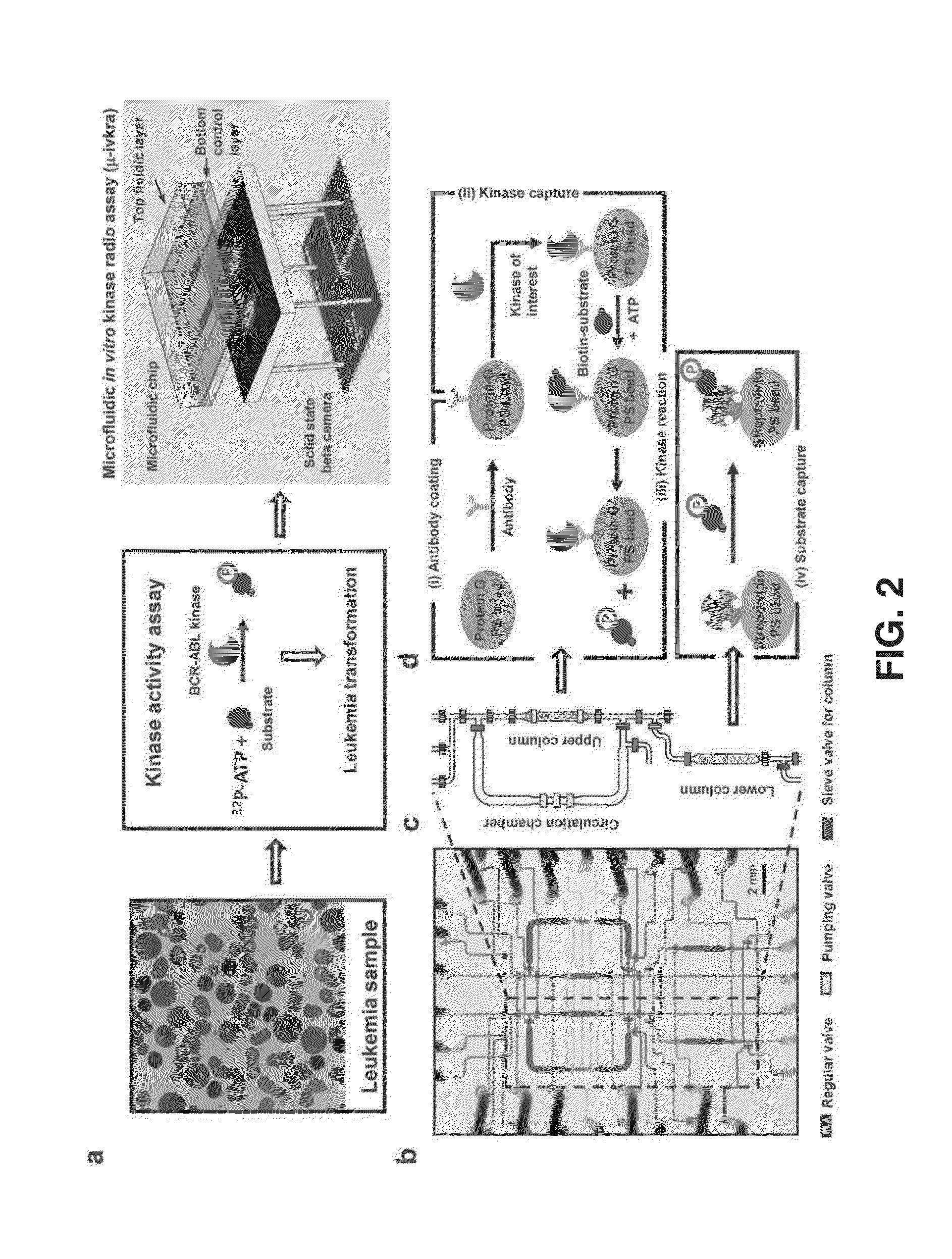
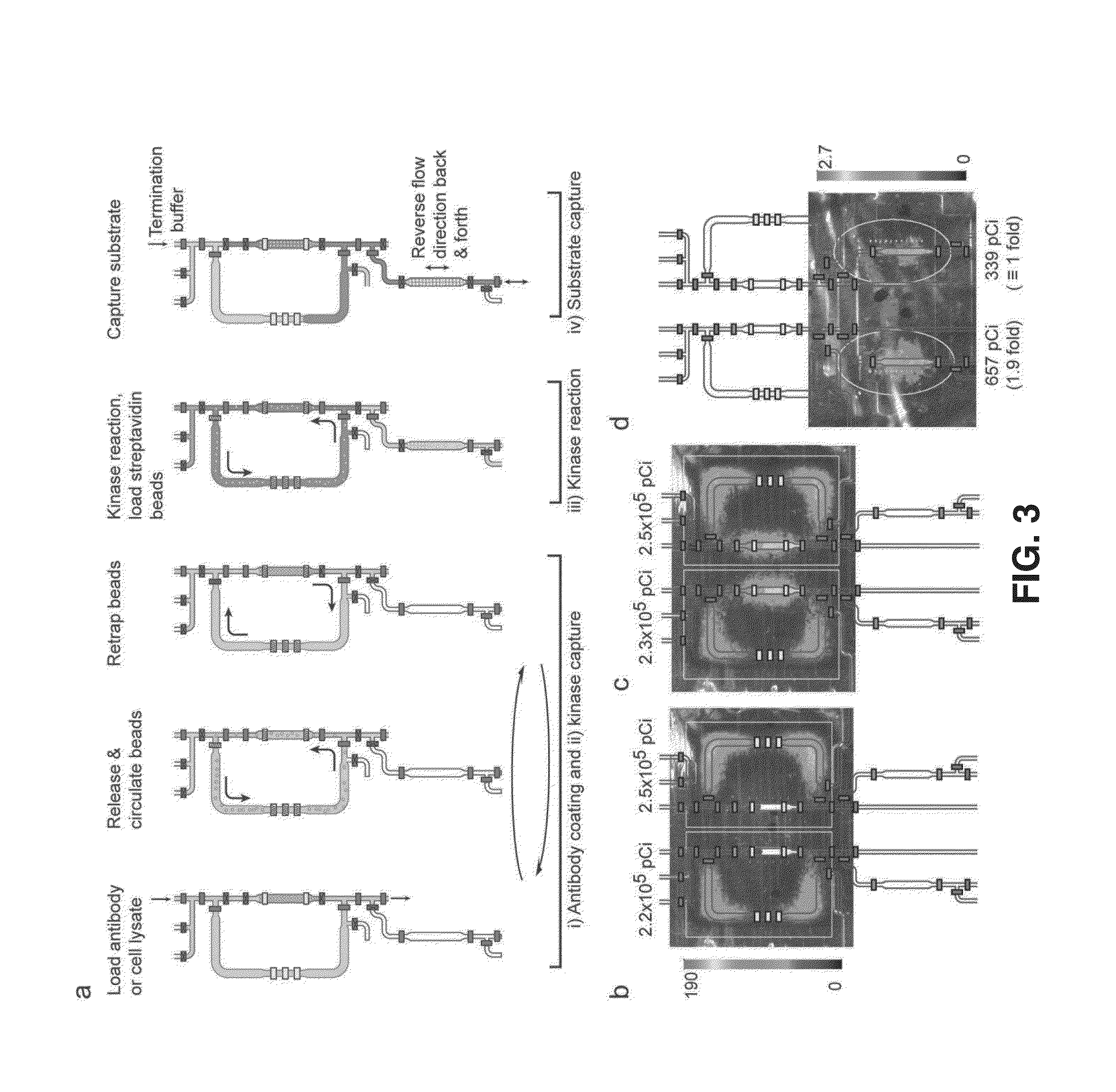
Integrated microfluidic radioassay and imaging platform for small sample analysis
ActiveUS20130244257A1Low costWork has been limitedBioreactor/fermenter combinationsBiological substance pretreatmentsCell lysatesIntegrated devices
An immunocapture-based in vitro kinase assay on an integrated polydimethylsiloxane (PDMS) microfluidics platform that can reproducibly measure kinase activity from as few as 3,000 cells is described. For this platform, the standard radiometric 32P-ATP labeled phosphate transfer assay was adopted. Implementation on a microfluidic device required the development of methods for repeated trapping and mixing of solid-phase affinity micro beads. A solid state beta-particle camera imbedded directly below the microfluidic device was used to provide real-time quantitative detection of the signal from this and other microfluidic radio bioassays. The integrated device can measure ABL protein kinase activity from BCR-ABL positive leukemia patient samples, and can measure the small molecule phosphorylation such as phosphorylation of the deoxycytidine analog 18F-FAC by deoxycytidine kinase (dCK) isolated from cell lysates.
Owner:RGT UNIV OF CALIFORNIA
Oligonucleotide, method and kit for detecting relative expression of MLL5 gene in sample
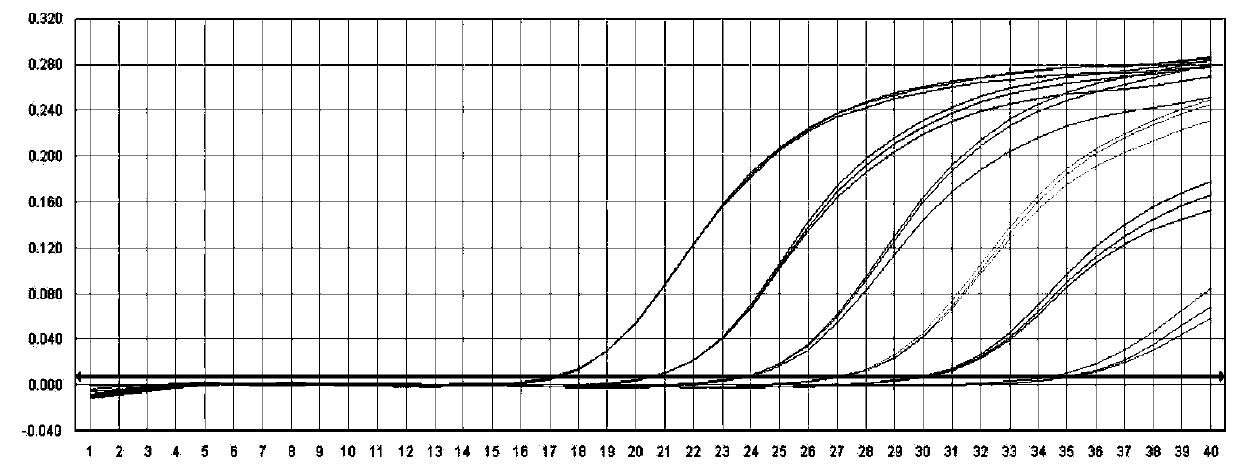
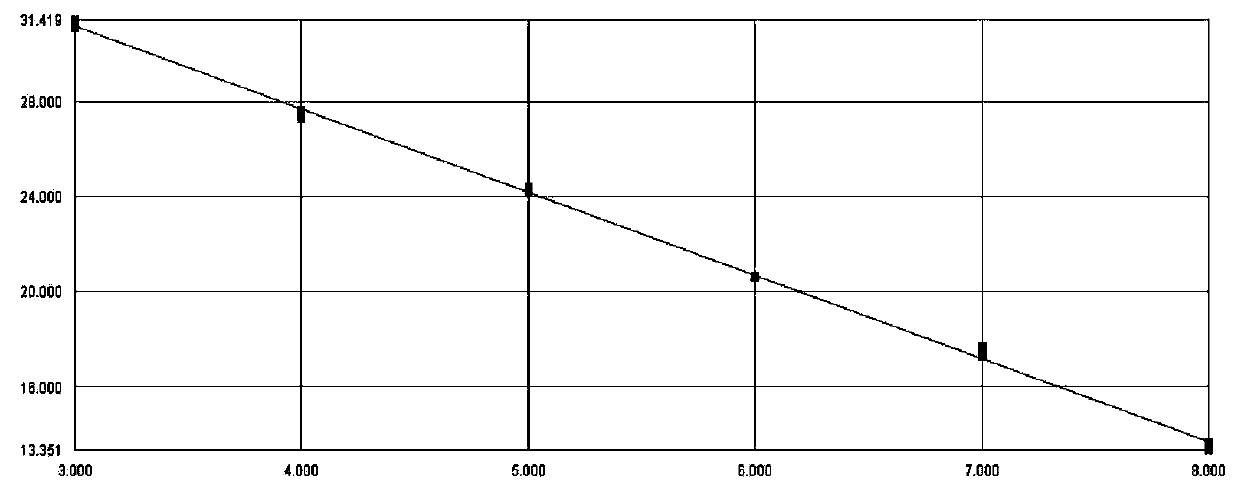
InactiveCN110699454AGood amplification efficiencyIncrease speedMicrobiological testing/measurementDNA/RNA fragmentationReference genesABL
The invention provides a method, a kit and an oligonucleotide for detecting relative expression of the MLL5 gene in a sample, and all relate to an upstream primer MLL5-F for detection, an upstream primer MLL5-R for detection and a probe MLL5-Probe for detection, and an upstream primer ABL-F for the internal reference gene ABL, a downstream primer ABL-R and a probe ABL-Probe. The relative expression of the MLL5 gene in clinical samples can be rapidly detected. The invention can be applied to evaluation of therapeutic effect and prediction of prognosis.
Owner:北京艾迪康医学检验实验室有限公司
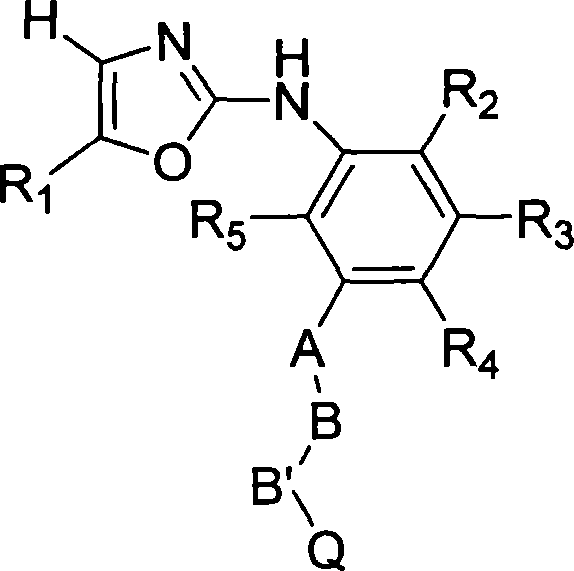
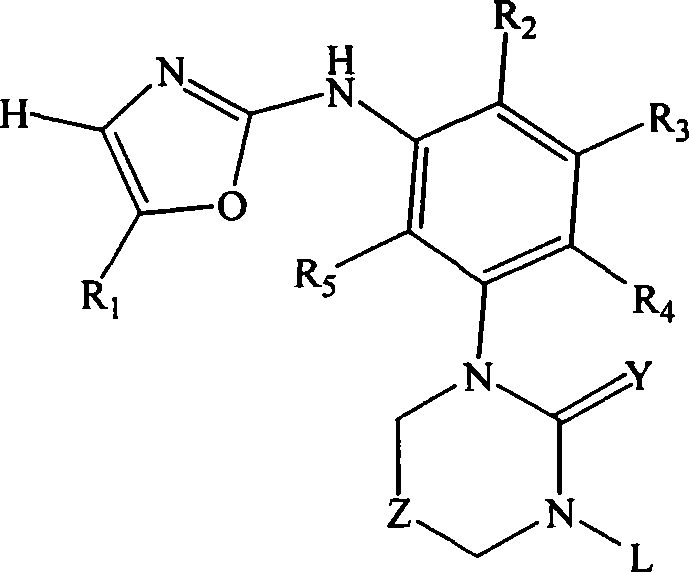
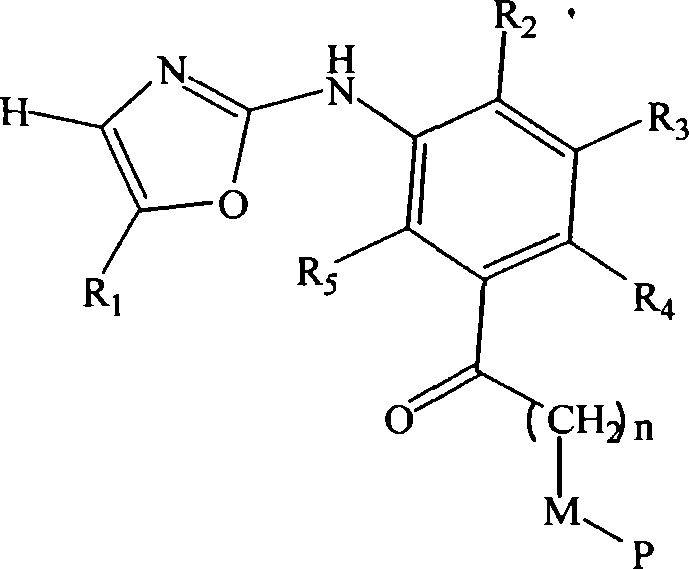
Substituted oxazole derivatives and their use as tyrosine kinase inhibitors
The present invention relates to novel compounds selected from substituted oxazole derivatives that selectively modulate, regulate, and / or inhibit signal transduction mediated by certain native and / or mutant tyrosine kinases implicated in a variety of human and animal diseases such as cell proliferative, metabolic, allergic, and degenerative disorders. More particularly, these compounds are potent and selective c-kit, bcr-abl and Flt-3 inhibitors.
Owner:AB SCIENCE +2
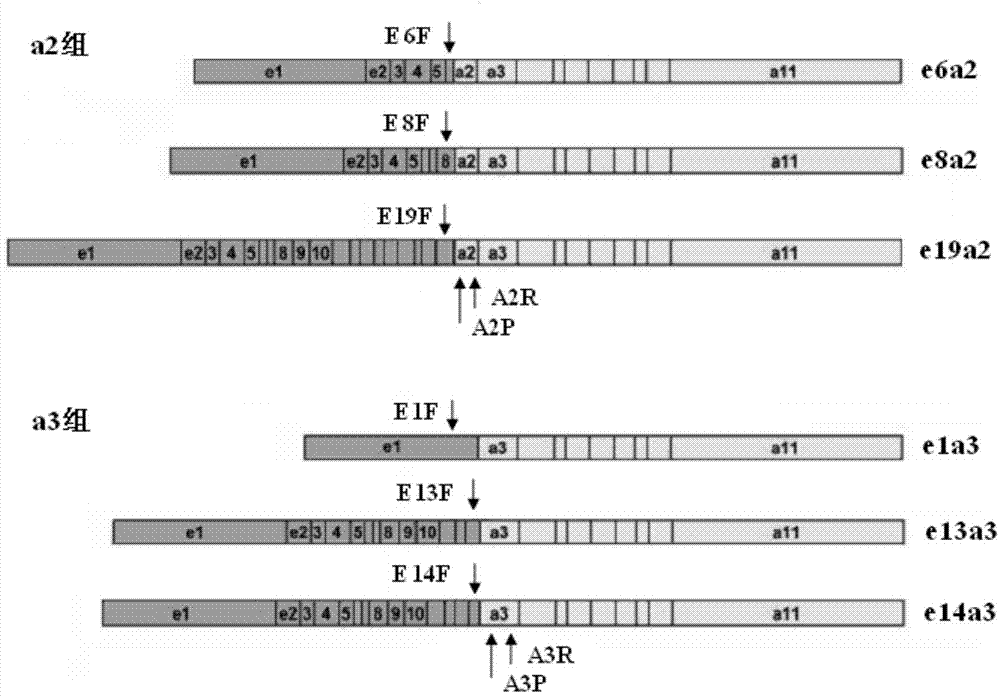
Method, primer, probe and kit for screening and identifying BCR-ABL unusual fusion types
ActiveCN103571945AReduce development costsLow costMicrobiological testing/measurementDNA/RNA fragmentationBCR-ABL Fusion GenePolymerase chain reaction
The invention discloses a method, primer, probe and kit for detecting six unusual fusion types of BCR-ABL fusion genes such as e6a2, e8a2, e19a2, e1a3, e13a3 and e14a3. The method comprises the following steps: performing primary screening on blood samples by adopting two tubes of fluorescent quantitative polymerase chain reaction (PCR), and quantitatively indentifying specific types of the blood samples belonging to the a2 or a3 group through primary screening judgment. Therefore, the screening cost is reduced, and the detection throughput is also improved. According to the detection method, primer, probe and kit, high sensitivity, high specificity and high detection throughput can be realized, and the method is a rapid and accurate type screening and identifying method.
Owner:南昌艾迪康医学检验实验室有限公司
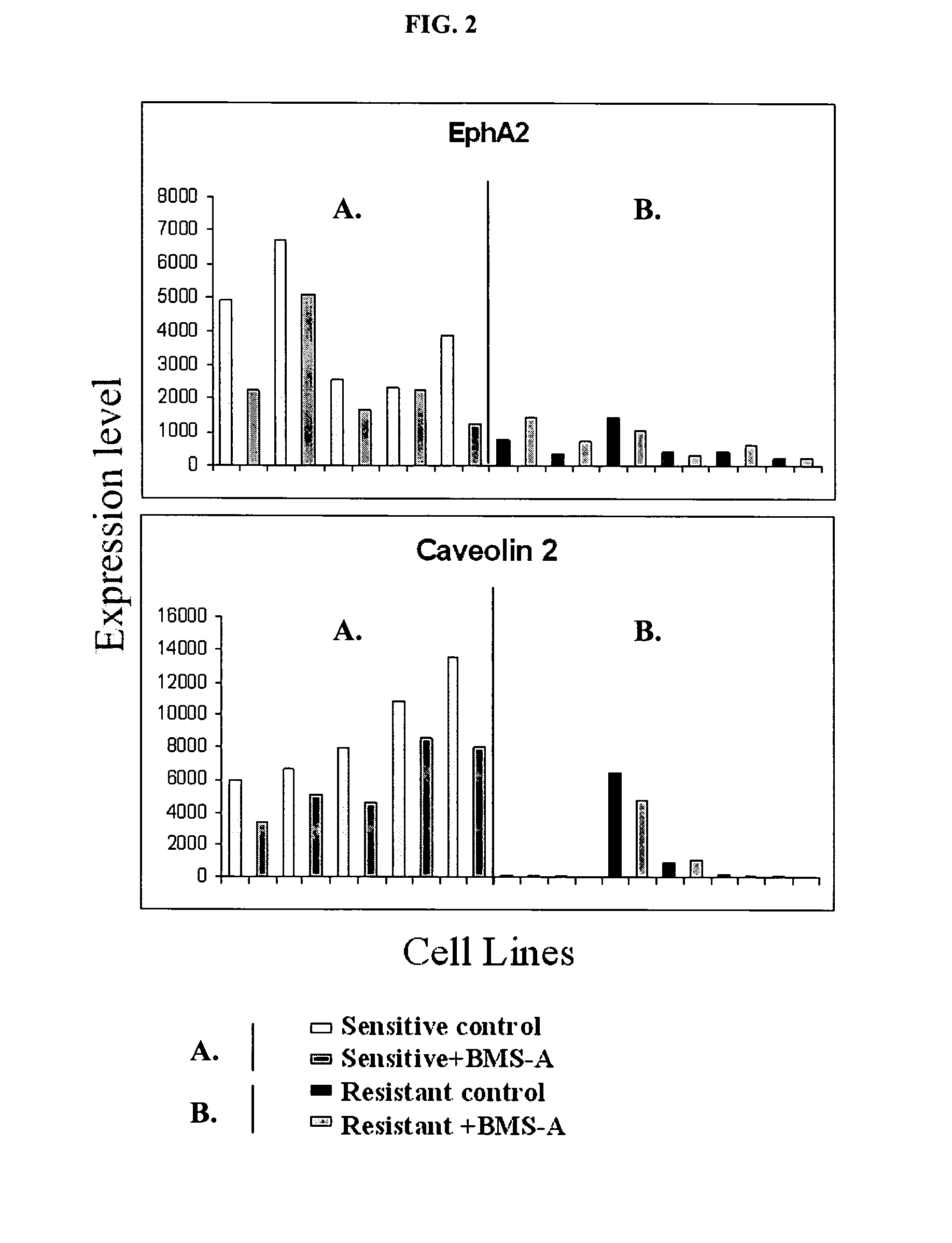
Methods of using EPHA2 for predicting activity of compounds that interact with and/or modulate protein tyrosine kinases and/or protein tyrosine kinase pathways in breast cells
InactiveUS7504211B2Modulate effectImproved prognosisPeptide/protein ingredientsMicrobiological testing/measurementDisease areaProtein-Tyrosine Kinases
The present invention describes polynucleotides that have been discovered to correlate to the relative sensitivity or resistance of cells, e.g., breast cell lines, to treatment with compounds that interact with and modulate, e.g., inhibit, protein tyrosine kinases, such as, for example, members of the Src family of tyrosine kinases, e.g., Src, Fgr, Fyn, Yes, Blk, Hck, Lck and Lyn, as well as other protein tyrosine kinases, including, Bcr-abl, Jak, PDGFR, c-kit and Eph receptors. These polynucleotides have been shown to have utility in predicting the resistance and sensitivity of breast cell lines to the compounds. Such polynucleotides comprise polynucleotide predictor or marker sets useful in methods of predicting drug response, and as prognostic or diagnostic indicators in disease management, particularly in those disease areas, e.g., breast cancer, in which signaling through one or more of the aforementioned Src tyrosine and protein tyrosine kinases is involved with the disease process.
Owner:BRISTOL MYERS SQUIBB CO
Mouse model of acute lymphoblastic leukemia and modeling method
ActiveCN106520805ASimplify the modeling processReduce the risk of accidental deathBlood/immune system cellsFermentationHematopoietic cellAcute lymphocytic leukemia
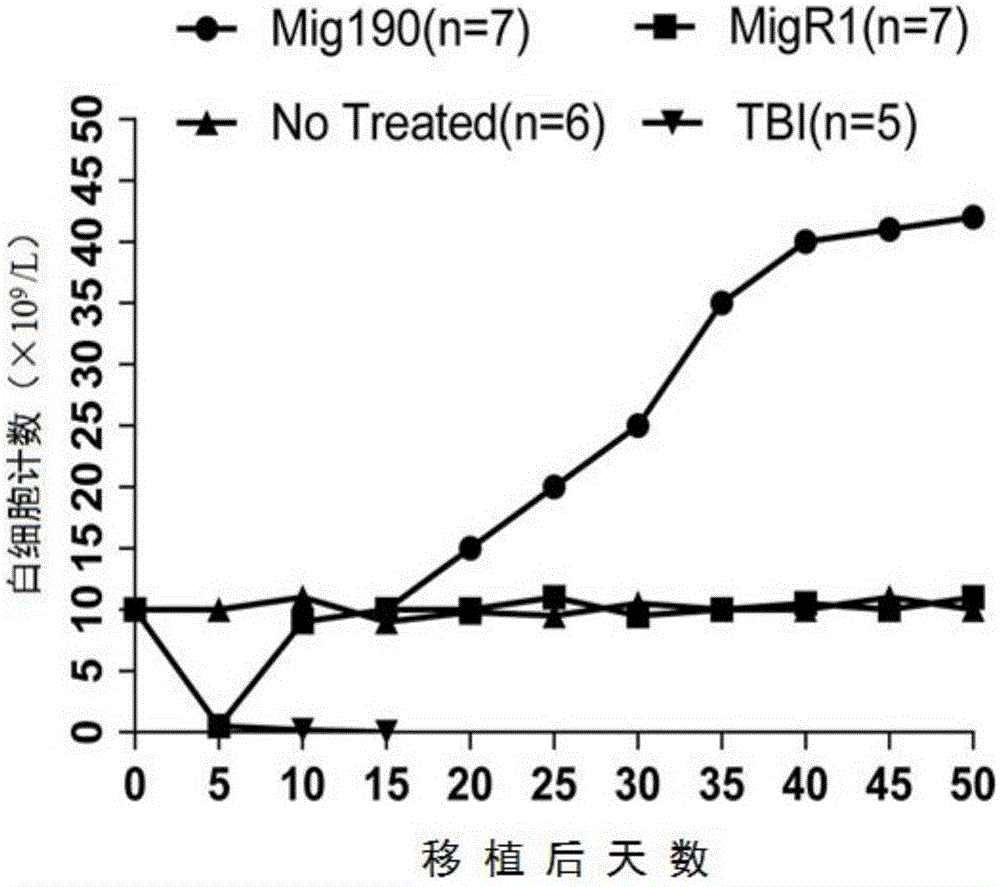
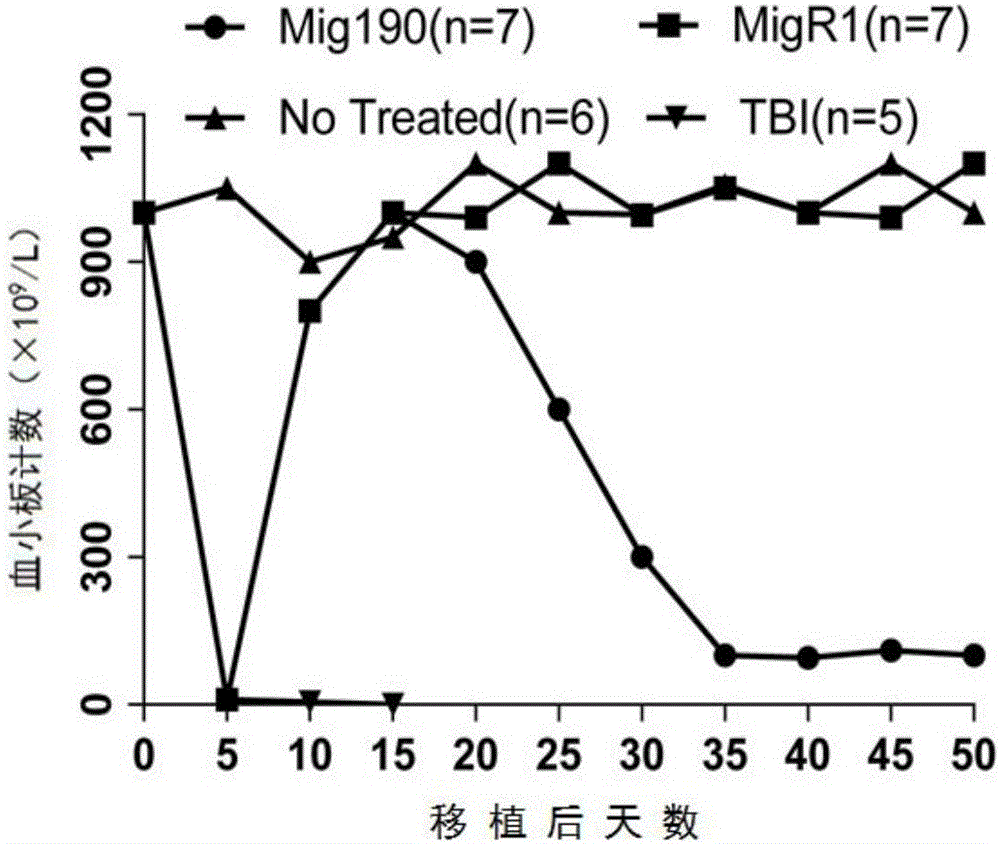
The invention provides a mouse model of acute lymphoblastic leukemia and a modeling method, and relates to the field of biotechnology. The preparation method comprises the following steps: infecting IL-7 stimulated hematopoietic cells with retroviruses with BCR-ABLP190; and transfusing the stimulated hematopoietic cells to syngenic mice. The method has the advantages of being simple and easy to implement in a modeling process and capable of establishing the ph+ALL mouse model in one step. According to the mouse model of the acute lymphoblastic leukemia provided by the invention, the sorted BCR-ABL+B cells are transfused to the syngenic mice for the second time without irradiation treatment, with performance consistent with that of mice of first episode; the model is good in stability; and in addition, the uniformity of recipient mice is enhanced and such risks as accidental death of the recipient mice due to irradiation are reduced.
Owner:XUZHOU MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




![Substituted pyrrolo[2.3-B]pyridines Substituted pyrrolo[2.3-B]pyridines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/731b3571-2c88-4f7a-b2a2-7e61bc0eb242/US07465726-20081216-C00001.png)
![Substituted pyrrolo[2.3-B]pyridines Substituted pyrrolo[2.3-B]pyridines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/731b3571-2c88-4f7a-b2a2-7e61bc0eb242/US07465726-20081216-C00002.png)
![Substituted pyrrolo[2.3-B]pyridines Substituted pyrrolo[2.3-B]pyridines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/731b3571-2c88-4f7a-b2a2-7e61bc0eb242/US07465726-20081216-C00003.png)
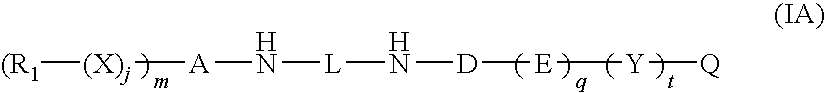



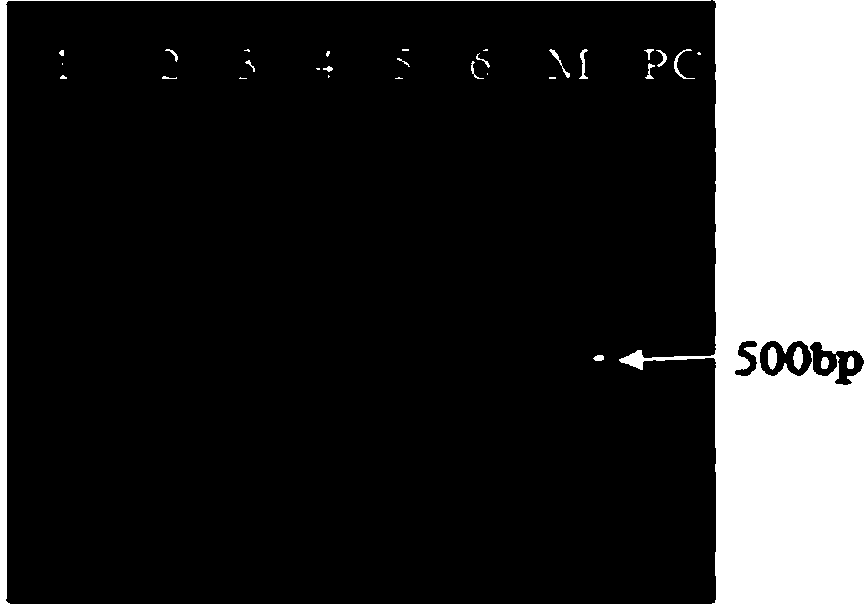






![N-(2-chloro-6-methylphenyl)-2[(2-methylpyrimidine-4-group) amino] thiazole-5-formamide compound as well as preparation method and application thereof N-(2-chloro-6-methylphenyl)-2[(2-methylpyrimidine-4-group) amino] thiazole-5-formamide compound as well as preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/beaa1544-4dc7-4772-b705-2fab2da474f5/2013101797399100002DEST_PATH_IMAGE001.PNG)
![N-(2-chloro-6-methylphenyl)-2[(2-methylpyrimidine-4-group) amino] thiazole-5-formamide compound as well as preparation method and application thereof N-(2-chloro-6-methylphenyl)-2[(2-methylpyrimidine-4-group) amino] thiazole-5-formamide compound as well as preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/beaa1544-4dc7-4772-b705-2fab2da474f5/218305DEST_PATH_IMAGE001.PNG)
![N-(2-chloro-6-methylphenyl)-2[(2-methylpyrimidine-4-group) amino] thiazole-5-formamide compound as well as preparation method and application thereof N-(2-chloro-6-methylphenyl)-2[(2-methylpyrimidine-4-group) amino] thiazole-5-formamide compound as well as preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/beaa1544-4dc7-4772-b705-2fab2da474f5/250988DEST_PATH_IMAGE002.PNG)