Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
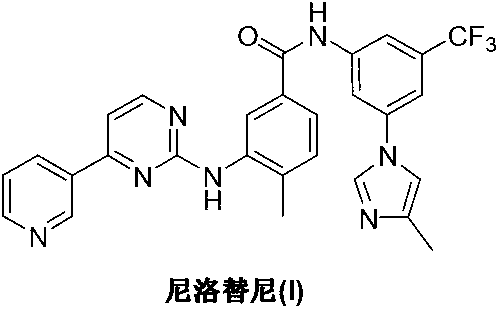
75 results about "Nilotinib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nilotinib is used to treat a certain type of blood cancer (chronic myelogenous leukemia-CML).
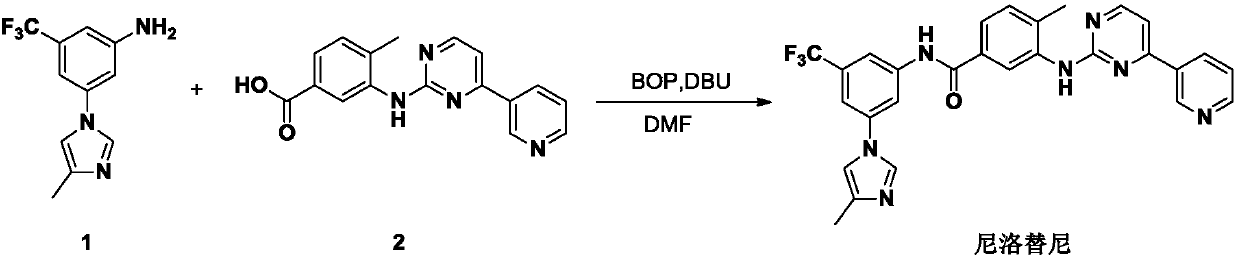
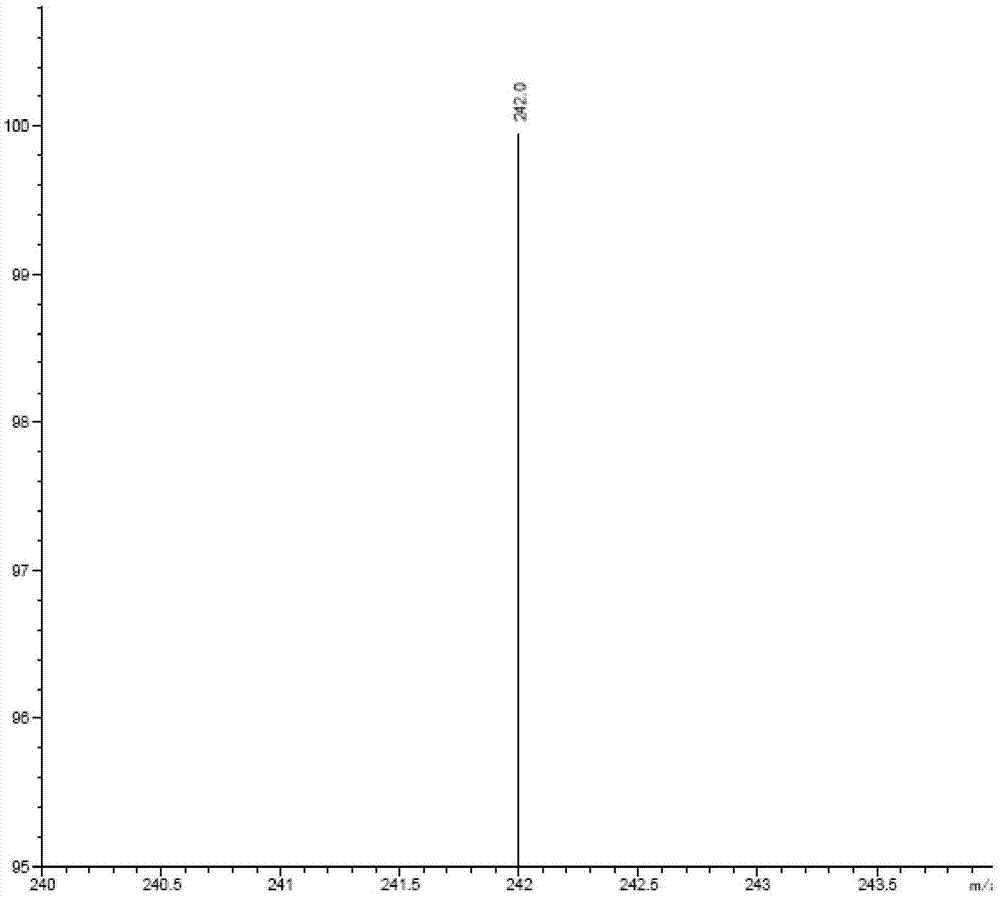
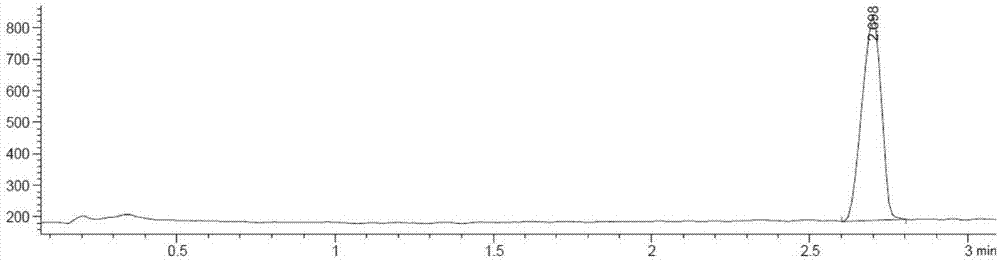
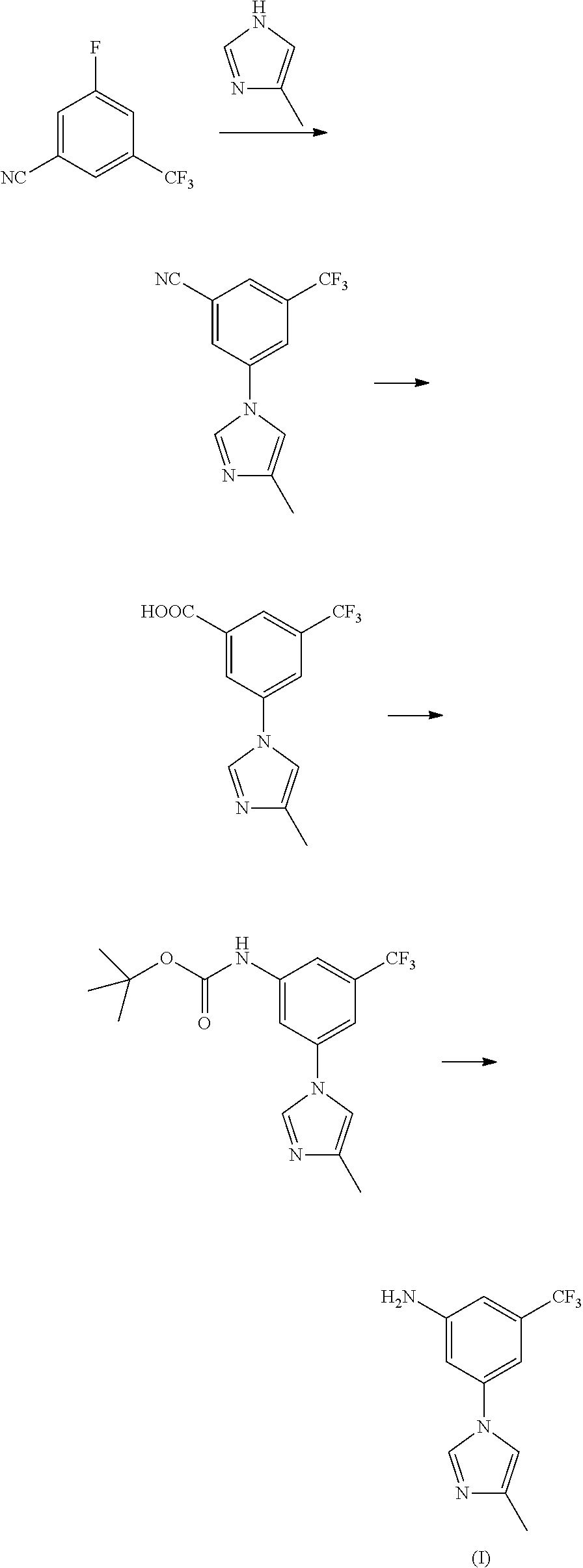
Process for the preparation of nilotinib
ActiveUS20130210847A1High yieldDelayed reaction timeOrganic active ingredientsBiocideNilotinibMedicinal chemistry
Owner:LANXESS DEUTDCHLAND GMBH +1
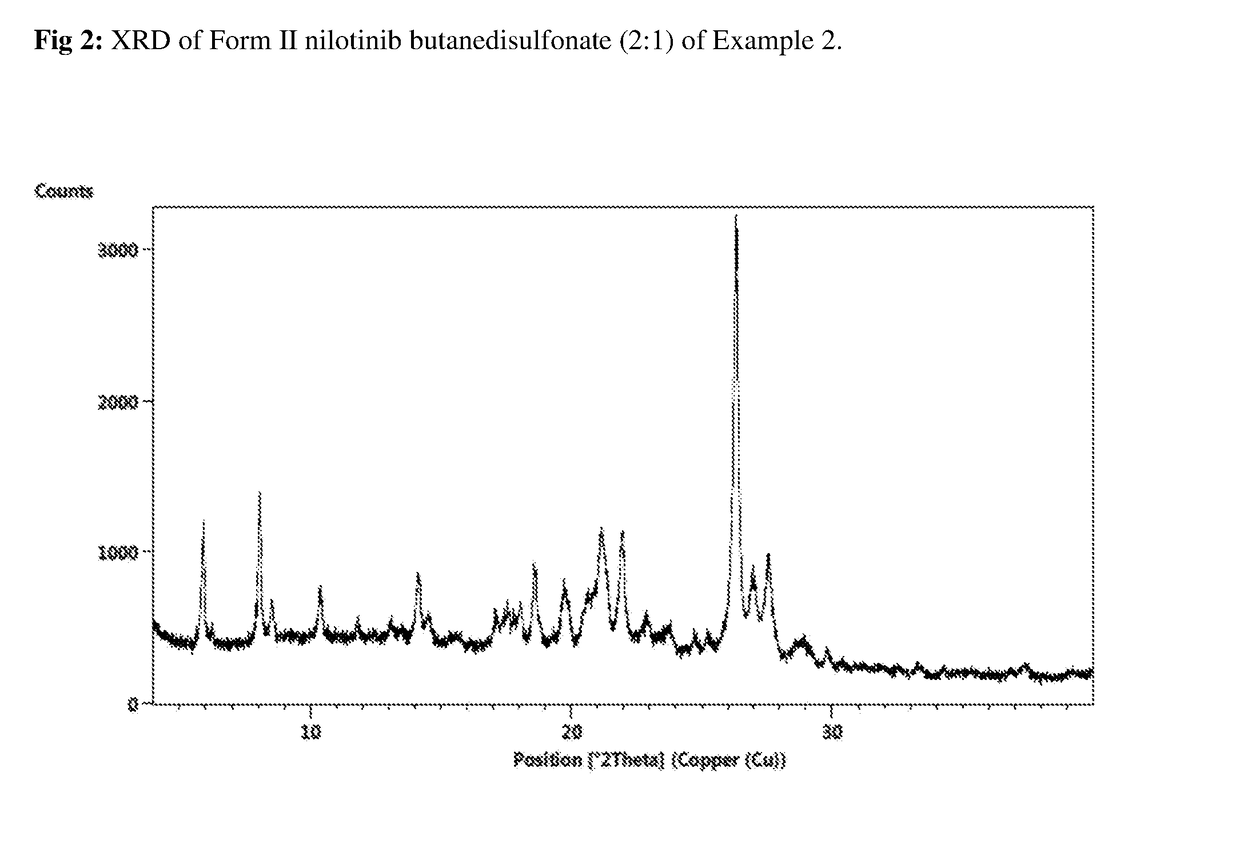
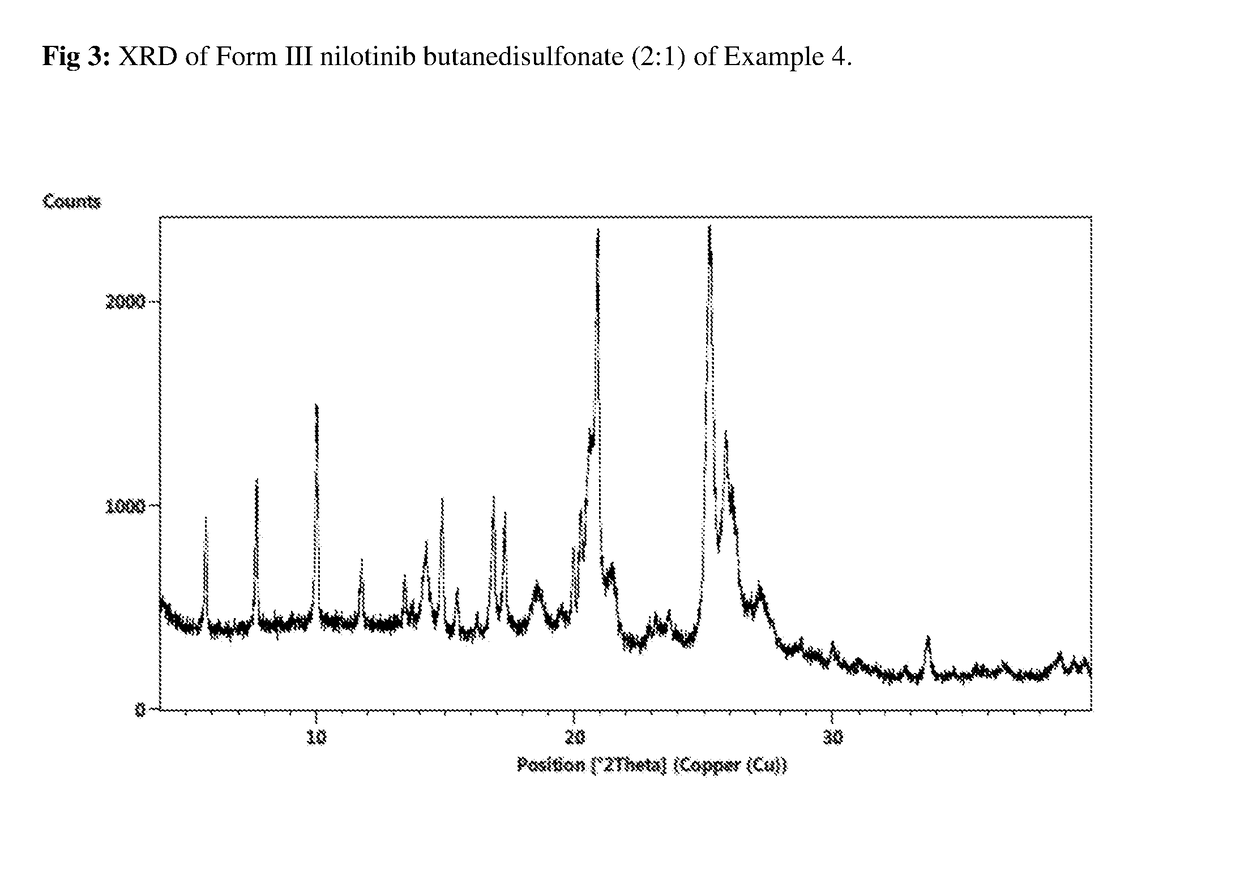
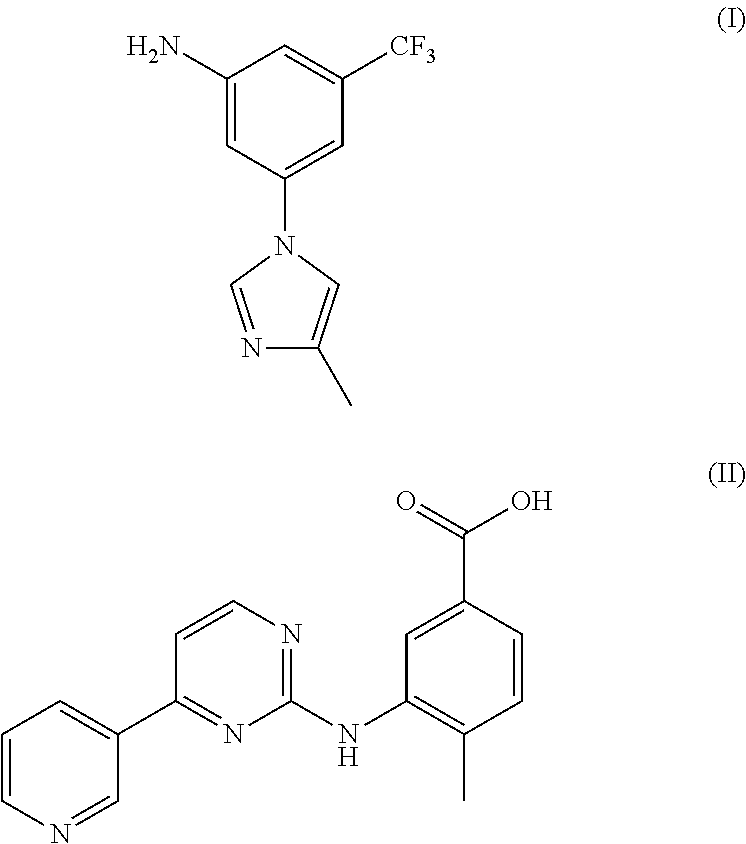
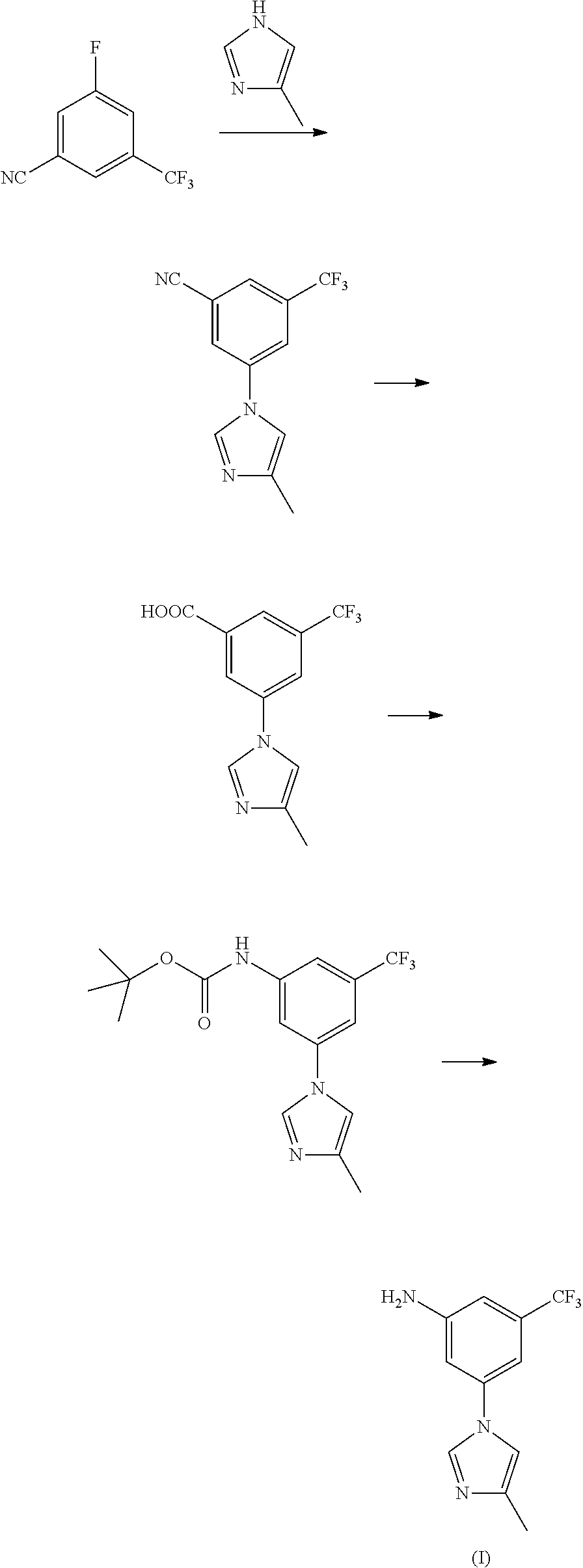
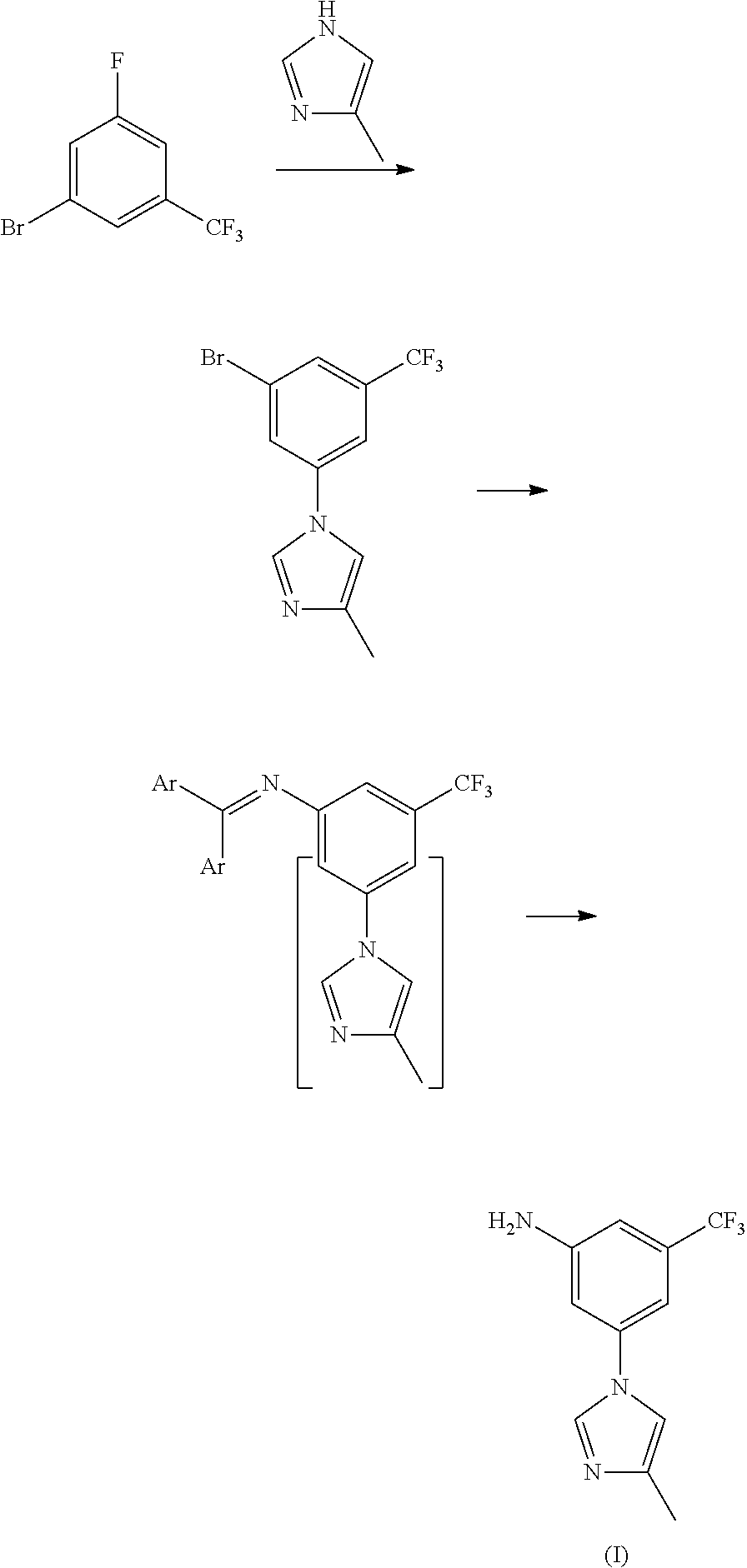
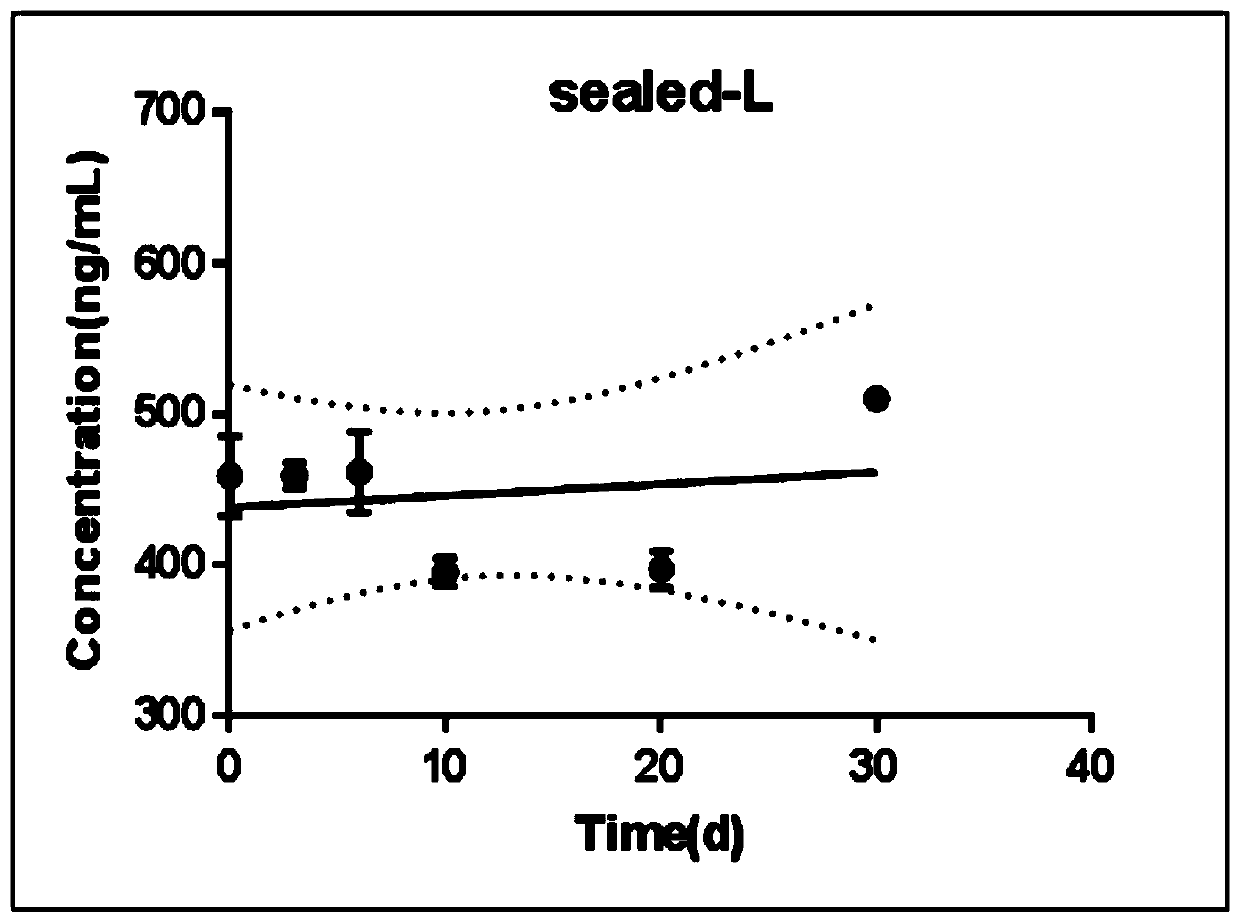
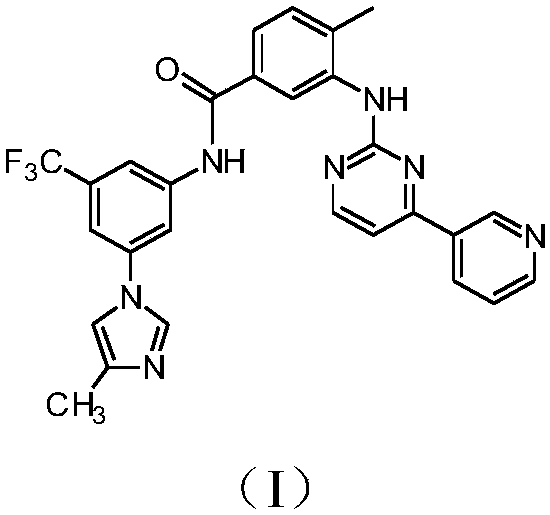
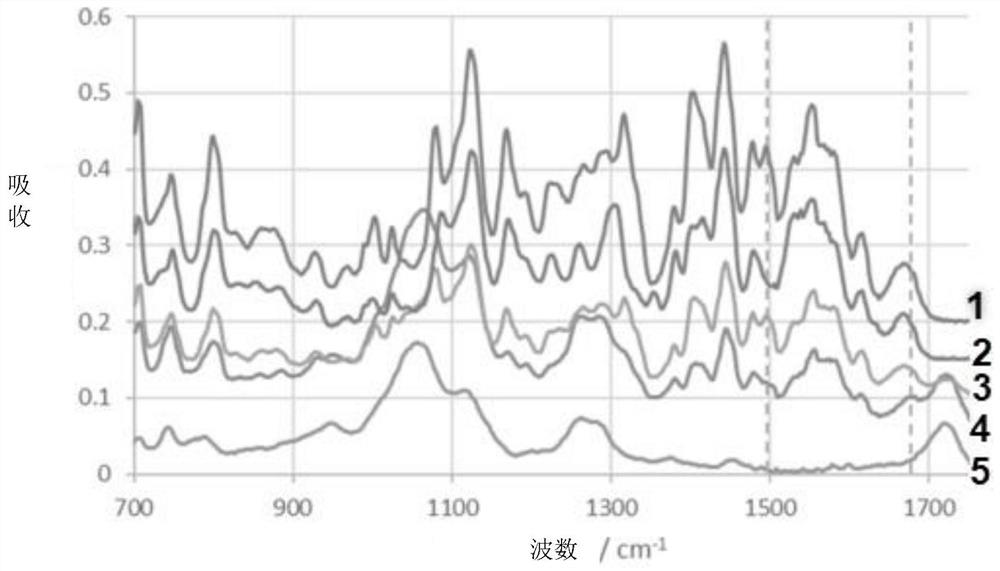
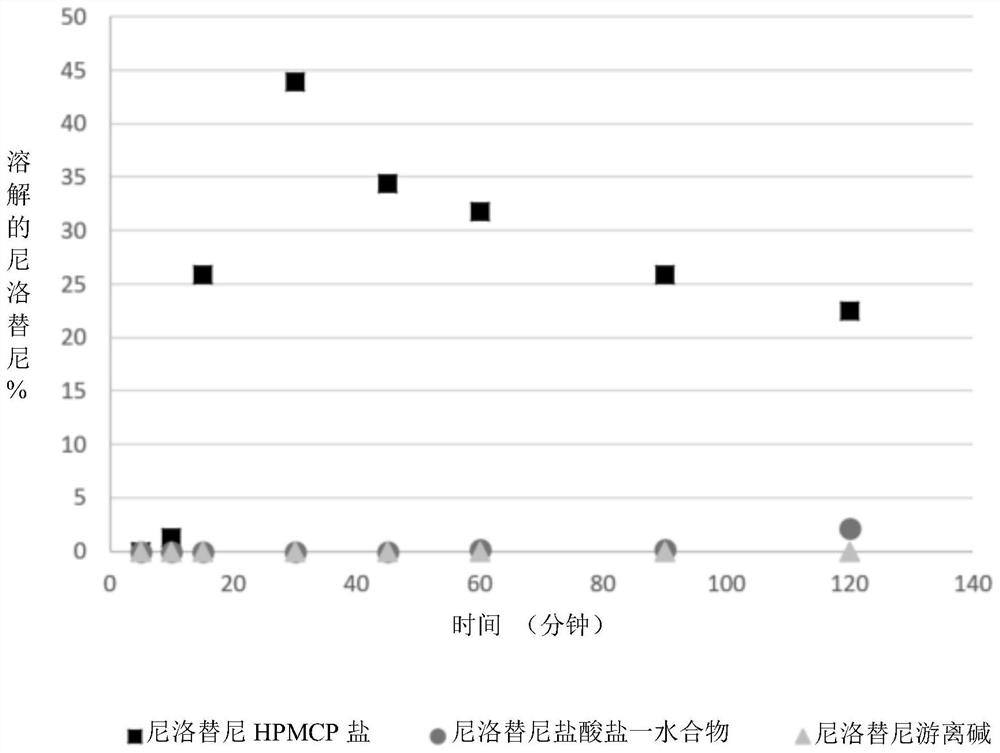
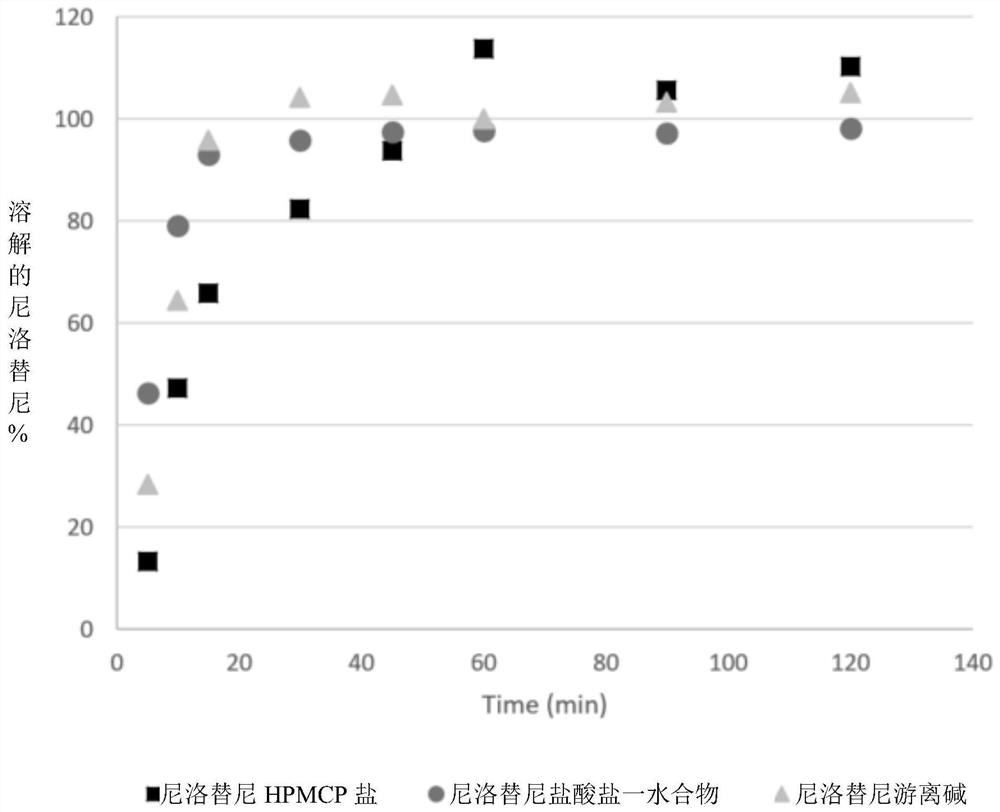
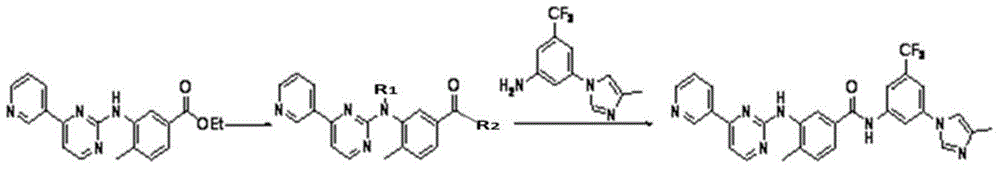
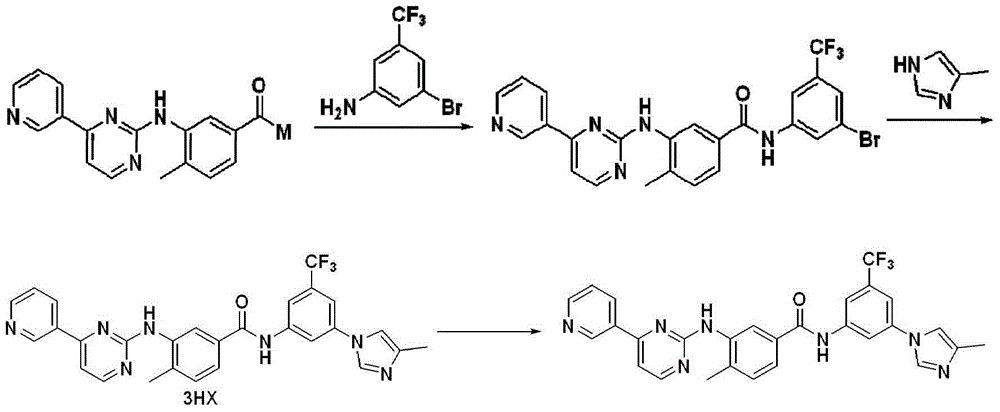
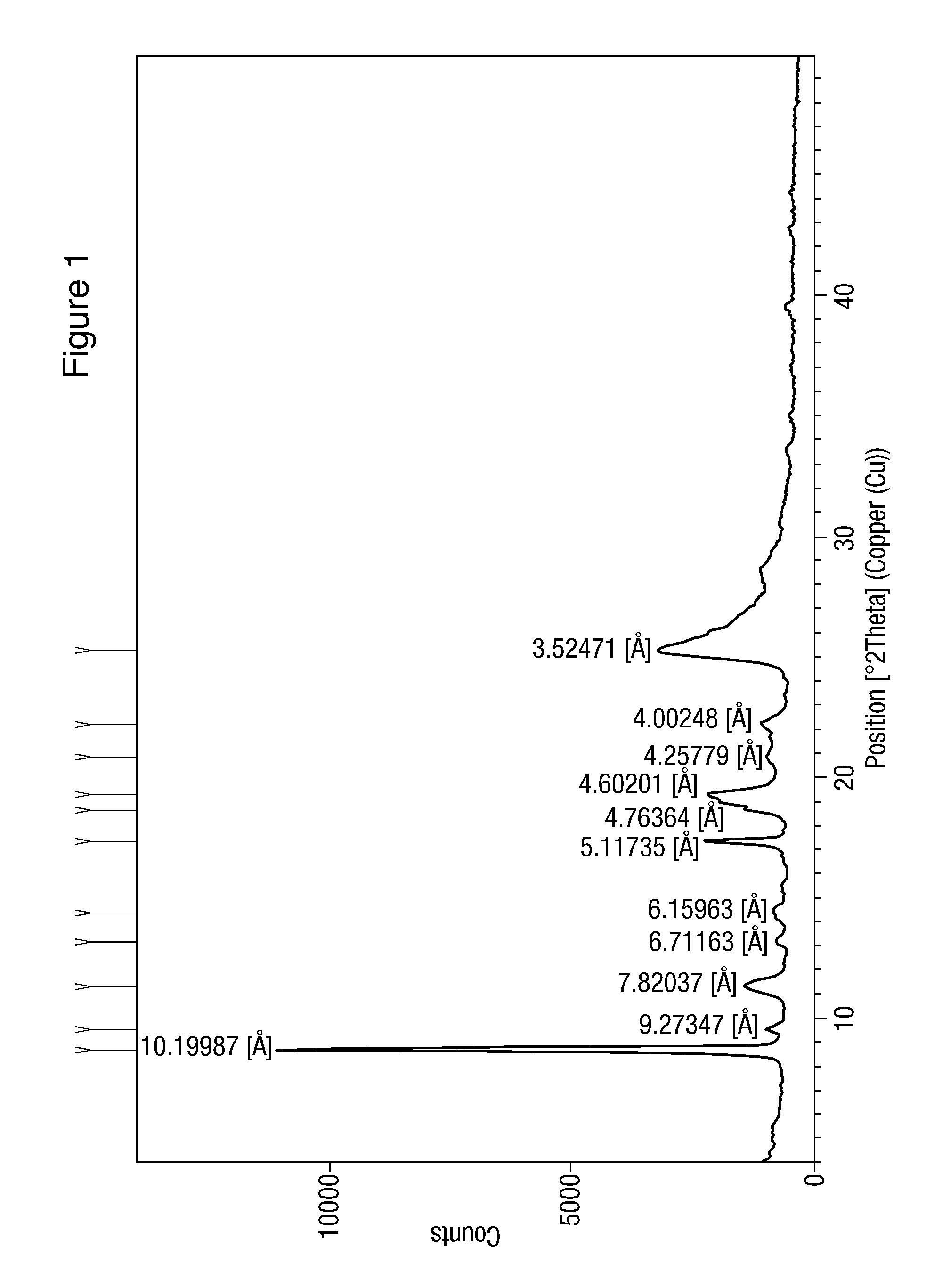
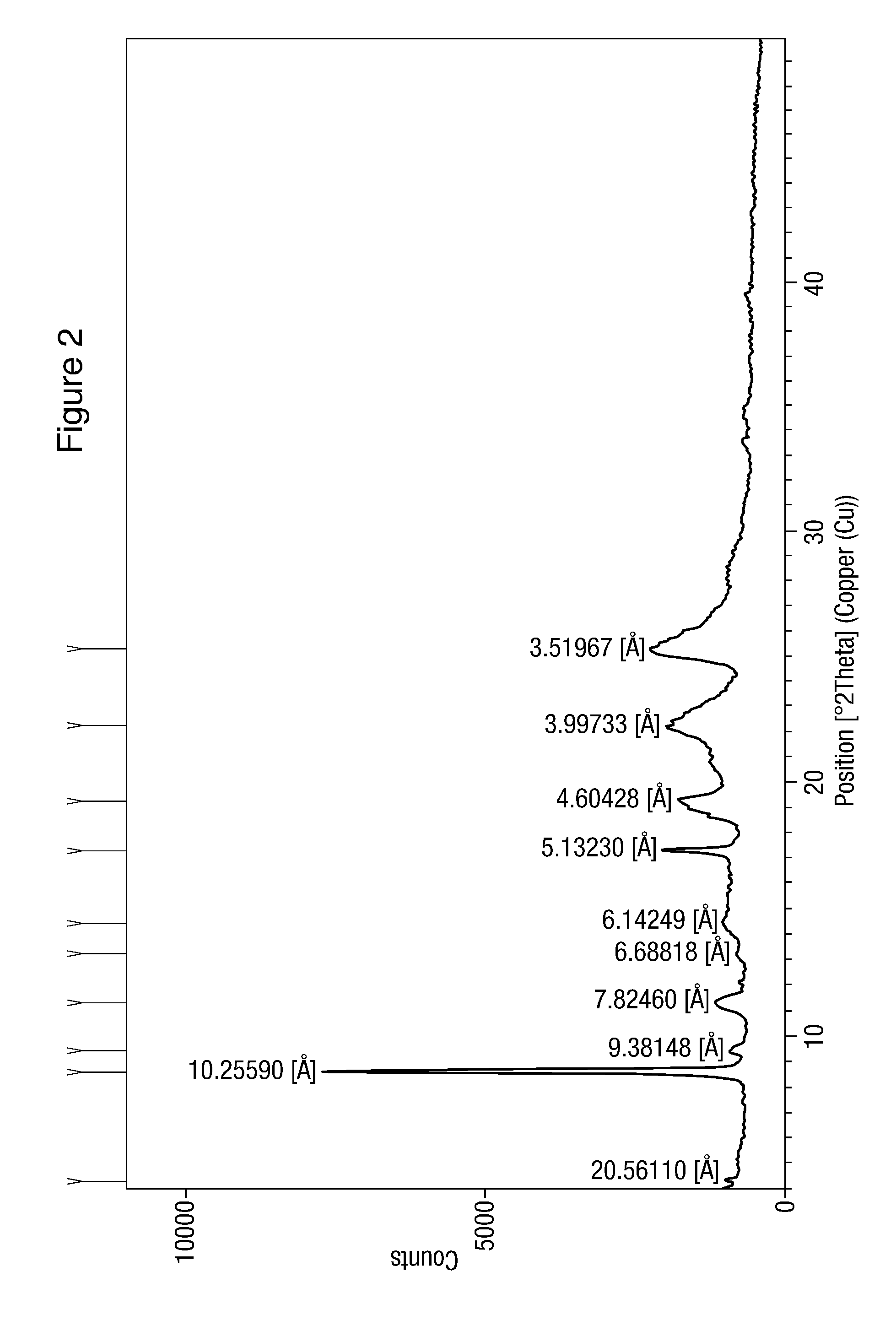
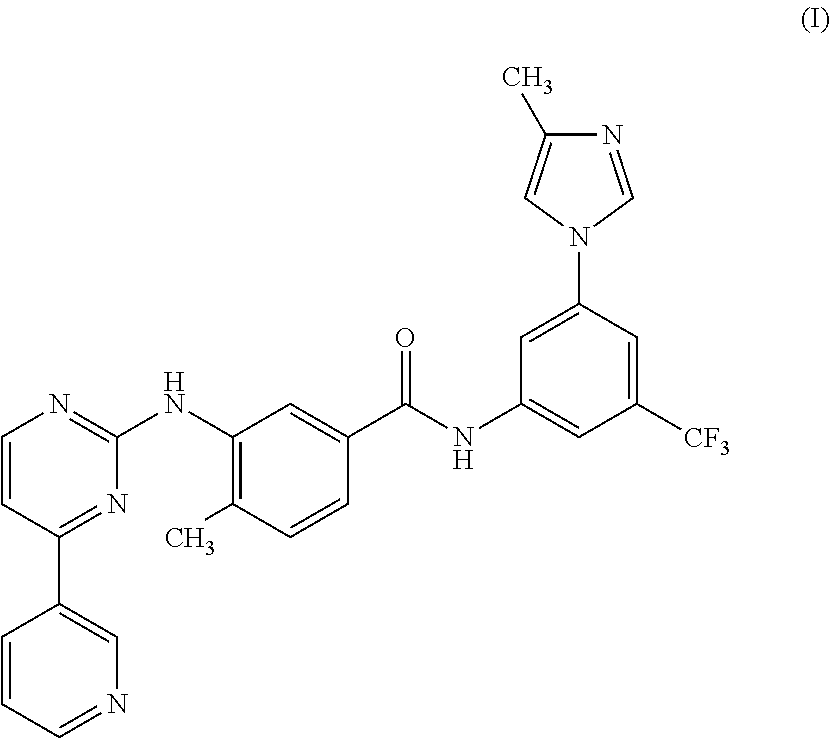
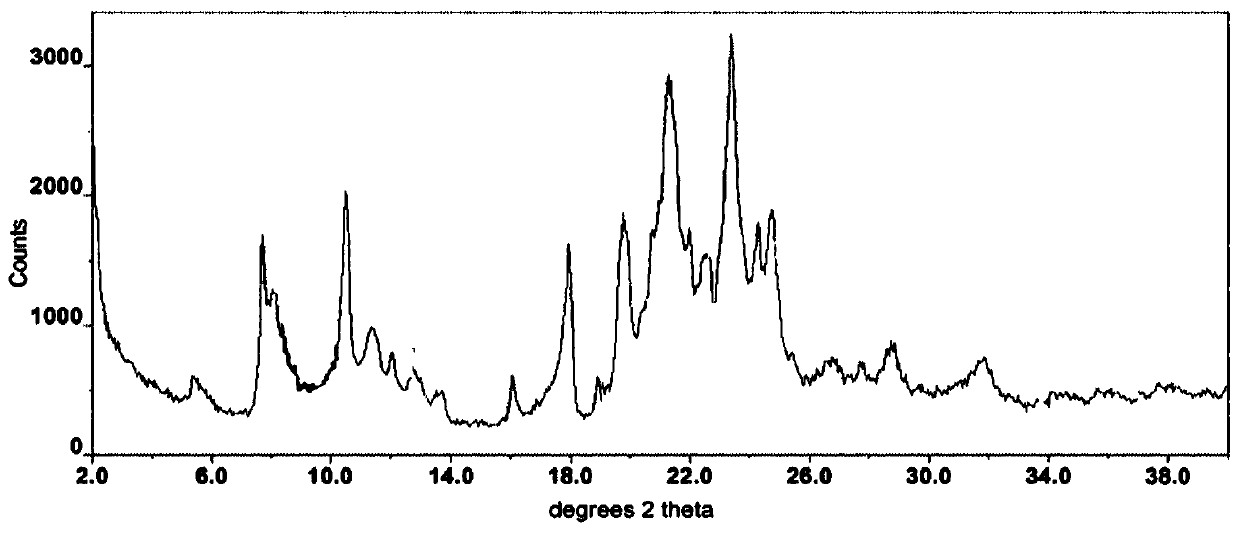
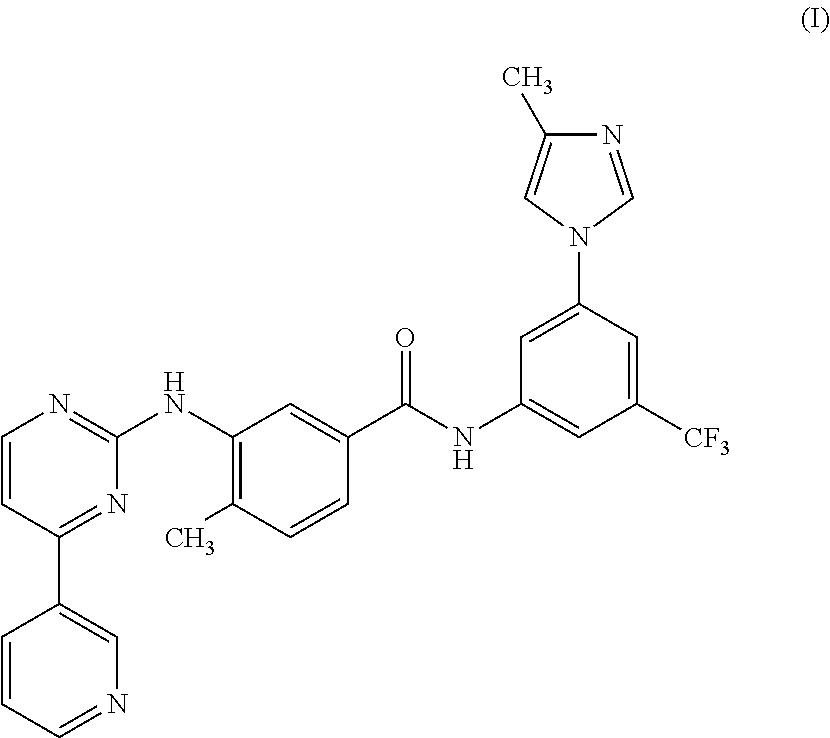
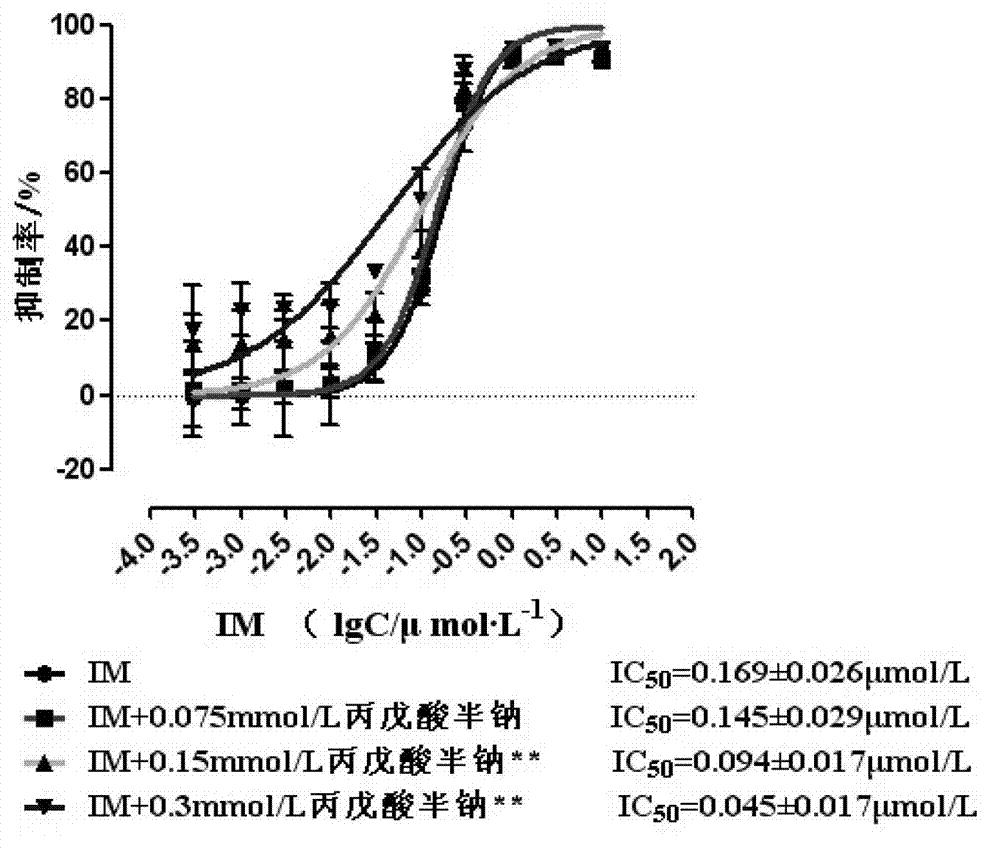
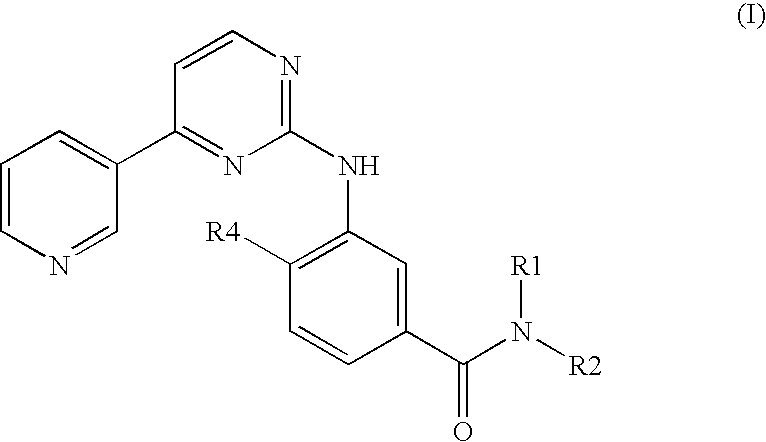
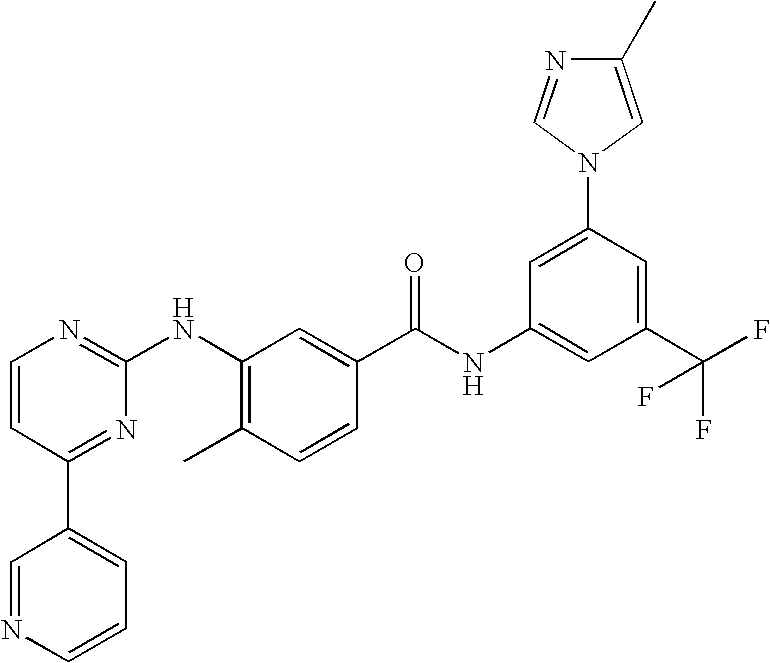
Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-[5-(4-methyl-1h-imidazol-1-yl)-3-(triflouoromethyl)phenyl] benzamide solubilized using organic acids
InactiveUS20150273070A1Improve bioavailabilitySuppressing food effectBiocideOrganic active ingredientsOrganic acidFOOD EFFECT
Soluble pharmaceutical compositions of nilotinib or a pharmaceutically acceptable salt thereof were invented using one or more organic acids that function as a solubilizing agent, increasing the bioavailability of nilotinib and supressing the food effect associated with certain compositions of nilotinib. The pharmaceutical compositions are in the form of solid oral dosage forms, including capsules and tablets.
Owner:NOVARTIS AG
Eutectic complex composed of resveratrol and protein kinase inhibitor, and composition comprising eutectic complex
InactiveCN110283052AHydroxy compound active ingredientsOrganic chemistry methodsLenvatinibPTK Inhibitors
The invention provides a eutectic complex composed of resveratrol and a protein kinase inhibitor, and a composition comprising the eutectic complex. The eutectic complex is characterized in that the protein kinase inhibitor is selected from one of imatinib, gefitinib, erlotinib, sunitinib, sorafenib, dasatinib, lapatinib, nilotinib, pazopanib, afatinib, crizotinib, axitinib, regorafenib, ibrutinib, lenvatinib, palbociclib, osimertinib and alectinib or pharmaceutically acceptable salt thereof. The eutectic complex provided by the invention can produce synergistic effects of inhibiting histamine release.
Owner:黄泳华
Neratinib sustained-release implant for treating solid tumor
InactiveCN101185633AOrganic active ingredientsPharmaceutical delivery mechanismProstate cancerTherapeutic effect
A sustained release implant includes 0.1%-50% (w / w) nilotinib, 50-99% sustained release excipients and 0-15% sustained release moderator. Sustained release excipients are mainly one or combination of poly (L-lactide-co-ethyl phosphate), poly (L-lactide-co- phosphoric acid propyl), polylactic acid, the copolymer of polylactic acid and hydroxyacetic acid and polifeprosan; sustained release moderator is one or combination of mannitol, sorbic alcohol and chondroitin; sustained release implant applied in local tumor can slowly release nilotinib onto local tumor, thus maintaining effective drug concentration of local tumor as well as significantly reducing overall toxic reaction; the invention not only reduces overall toxic reaction of nilotinib, but also selectively improves drug concentration in local tumor, enhancing the therapeutic effects of non-operative therapy such as chemotherapy drugs and radiotherapy. The implant can be used for treating solid tumors including lung cancer, esophageal carcinoma, gastric cancer, liver cancer, breast cancer, ovarian cancer, prostatic carcinoma, pancreatic cancer, bladder carcinoma, cerebroma, and colorectal cancer.
Owner:SHANDONG LANJIN PHARMA +1
Pharmaceutical compositions of nilotinib
ActiveUS20200261449A1Improve bioavailabilityReduced strength/dosePowder deliveryOrganic active ingredientsFOOD EFFECTOral medication
Amorphous solid dispersions of nilotinib fumarate or nilotinib tartrate are provided, as well as pharmaceutical compositions thereof, wherein the compositions exhibit enhanced bioavailability in the fasted state. Preferably, the compositions may be orally administered to a patient in either the fed or fasted state, with a decrease or elimination of the food effect. Preferably, following oral administration of the pharmaceutical compositions, there is no substantial difference in the pharmacokinetic parameters (e.g., Cmax, AUC0-t and / or AUC0-infinity) of nilotinib, regardless of whether the pharmaceutical compositions are administered to a subject in the fed or fasted state.
Owner:SLAYBACK PHARMA LLC
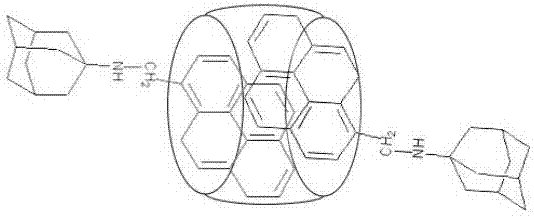
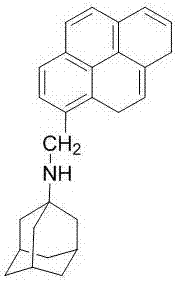
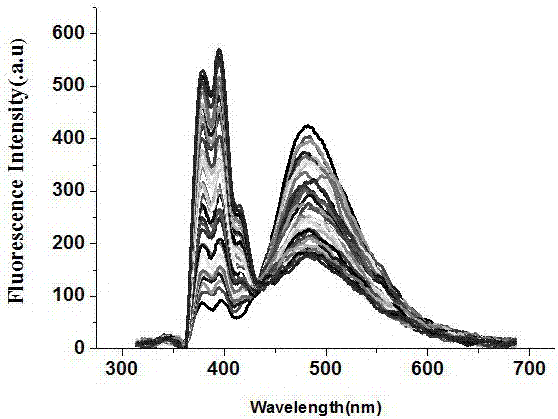
Ratio fluorescence probe for identification of nilotinib, preparation and identification method thereof
ActiveCN107502339AReduce detection and analysis costsEasy to operateFluorescence/phosphorescenceLuminescent compositionsFluorescenceNilotinib
The invention discloses a ratio fluorescence probe for identification of nilotinib, and a preparation and identification method thereof. With a molecular formula of C114H118O20N42, the ratio fluorescence probe is prepared from cucurbit[10]uril and a guest B with a molecular formula of C27H29N, during identification, the probe is dissolved in water to prepare a reagent, a to-be-identified sample is dripped into the reagent to obtain a sample solution, the sample solution is subjected to fluorescence excitation, and the fluorescence excited fluorescence wavelength is detected. The ratio fluorescence probe provided by the invention has the characteristics of low detection and analysis cost, simple operation, simple preparation process, visible test result and more direct analysis.
Owner:GUIZHOU UNIV
Preparation method of nilotinib
InactiveCN103288804AProduction is easy to controlImprove product qualityOrganic chemistryBenzoic acidOrganic base
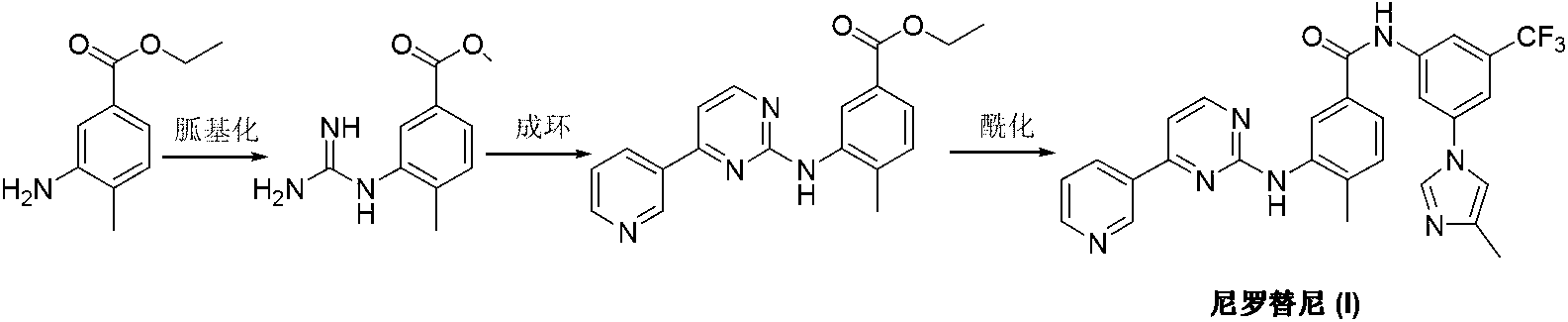
The invention discloses a preparation method of nilotinib, which comprises the following steps of: under the effect of organic base and a condensing agent, performing a one-step condensation reaction of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid (II) and 5-(4-methyl-1H-imidazole-1-yl)-3-trifluoromethyl phenylamine (III) to obtain nilotinib (I). The preparation method disclosed by the invention has the advantages of easily-available raw materials, simple process, mild conditions, environment optimization and quality improvement, is suitable for industrial production and promotes the development of the economic technology of raw material medicines.
Owner:SUZHOU MIRACPHARMA TECH +1
Process for the preparation of Nilotinib
ActiveUS9061028B2Difficult to handleEfficient and economical and viable for scale operationBiocideOrganic active ingredientsNilotinibMedicinal chemistry
Owner:LANXESS DEUTDCHLAND GMBH +1
Oral nilotinib nano-formulation and preparation method thereof
ActiveCN107320460AEfficient removalImprove solubilityOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityNilotinib
The invention discloses an oral nilotinib nano-formulation which comprises an active ingredient nilotinib, a main auxiliary material, an additional auxiliary material and a stabilizer, wherein the mass content of the nilotinib is 30-55%; the mass content of the main auxiliary material is 20-40%, the mass content of the additional auxiliary material is 2-10%, the mass content of the stabilizer is 2-20%, and the particle size of the oral nilotinib nano-formulation is less than 1 micron. The invention further discloses a preparation method of the oral nilotinib nano-formulation. Due to the prepared oral nilotinib nano-formulation, the problems that the medicine has low dissolubility in water and low bioavailability in a human body and the like are solved, the solubleness and permeability of the nilotinib are improved, and finally the aim of improving the bioavailability is achieved. Therefore, the digestion performance of the medicine is greatly improved, and the drug dissolution reaches 93-97% within 15 minutes.
Owner:BEIJING UNIV OF CHEM TECH
Medicine composition of tyrosine kinase inhibitor and histone deacetylase inhibitor
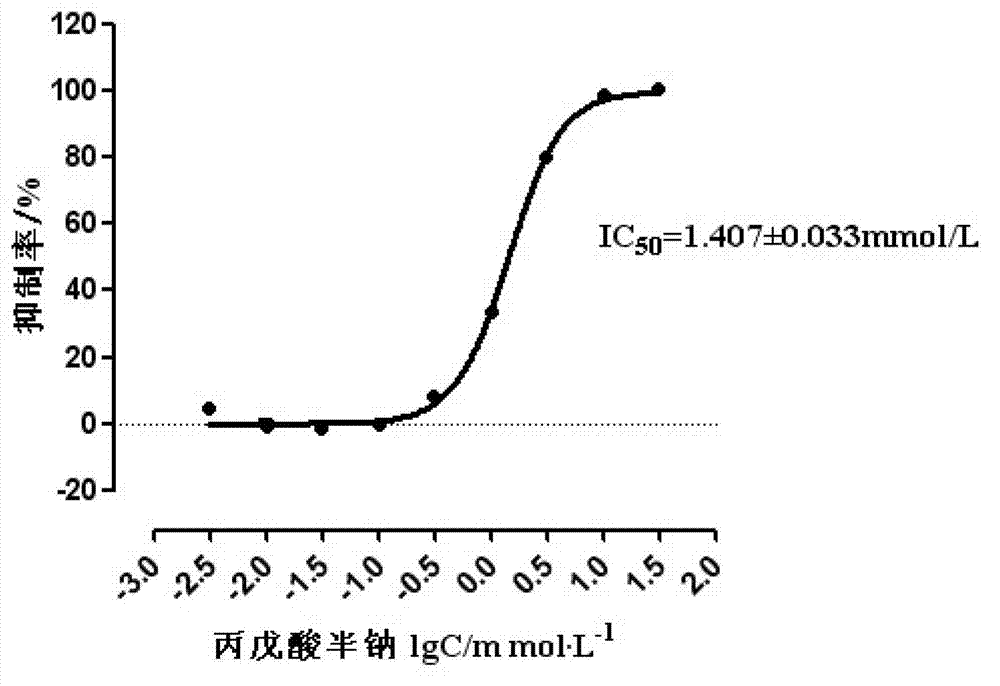
InactiveCN103083671ASignificant synergyDelayed drug resistanceOrganic active ingredientsAntineoplastic agentsTreatment effectHydroxamic acid
The invention discloses a medicine composition of a tyrosine kinase inhibitor and a histone deacetylase inhibitor. The prepared medicine composition comprises a tyrosine kinase inhibitor and a histone deacetylase inhibitor according to a mass ratio of (0.5:1)-(1:4). The tyrosine kinase inhibitor is selected from imatinib, dasatinib, nilotinib, gefitinib and the like; and the histone deacetylase inhibitor is selected from short-chain fatty acid such as butyric acids and valproic acids, hydroxamic acids such as trichostatin A, cyclic tetrapeptides and benzamides. Compared with the tyrosine kinase inhibitor of treating tumors by one target, the medicine composition has the advantages of strong treatment effect, high medicine tolerance, and small clinical medicine dosage, and is applied clinically by using a medicine preparation form.
Owner:毛幼桦 +1
Pharmaceutical compositions comprising nilotinib or its salt
A pharmaceutical composition, especially capsules. comprising granules containing nilotinib or a salt thereof with at least one pharmaceutically acceptable excipient. The granules may be produced by a wet granulation process.
Owner:NOVARTIS AG
Pharmaceutical compositions of nilotinib
ActiveUS10874671B2Improve bioavailabilityReduced strength/dosePowder deliveryOrganic active ingredientsFOOD EFFECTOral medication
Amorphous solid dispersions of nilotinib fumarate or nilotinib tartrate are provided, as well as pharmaceutical compositions thereof, wherein the compositions exhibit enhanced bioavailability in the fasted state. Preferably, the compositions may be orally administered to a patient in either the fed or fasted state, with a decrease or elimination of the food effect. Preferably, following oral administration of the pharmaceutical compositions, there is no substantial difference in the pharmacokinetic parameters (e.g., Cmax, AUC0-t and / or AUC0-infinity) of nilotinib, regardless of whether the pharmaceutical compositions are administered to a subject in the fed or fasted state.
Owner:SLAYBACK PHARMA LLC
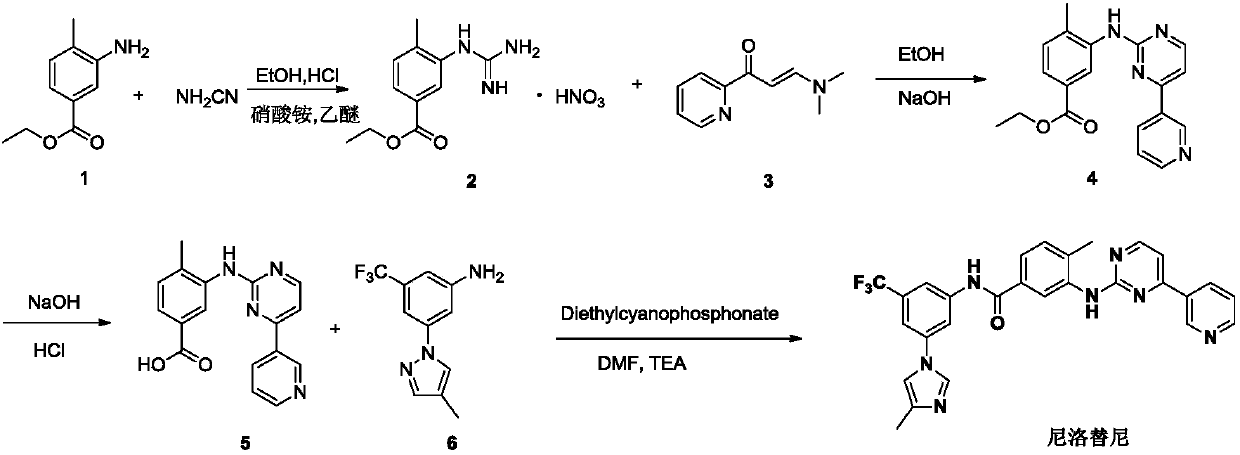
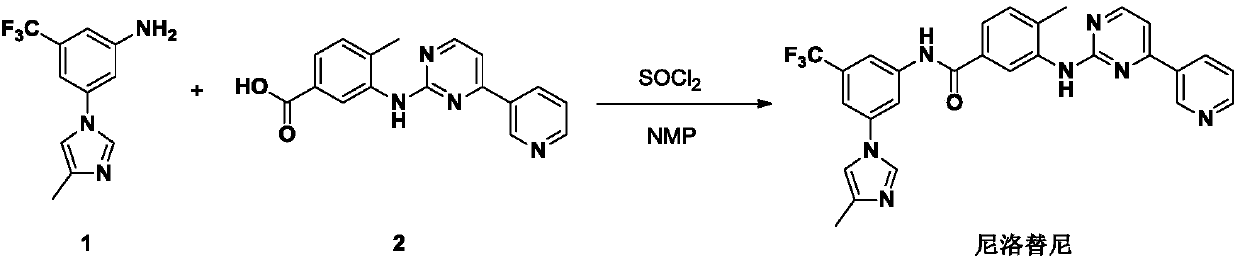
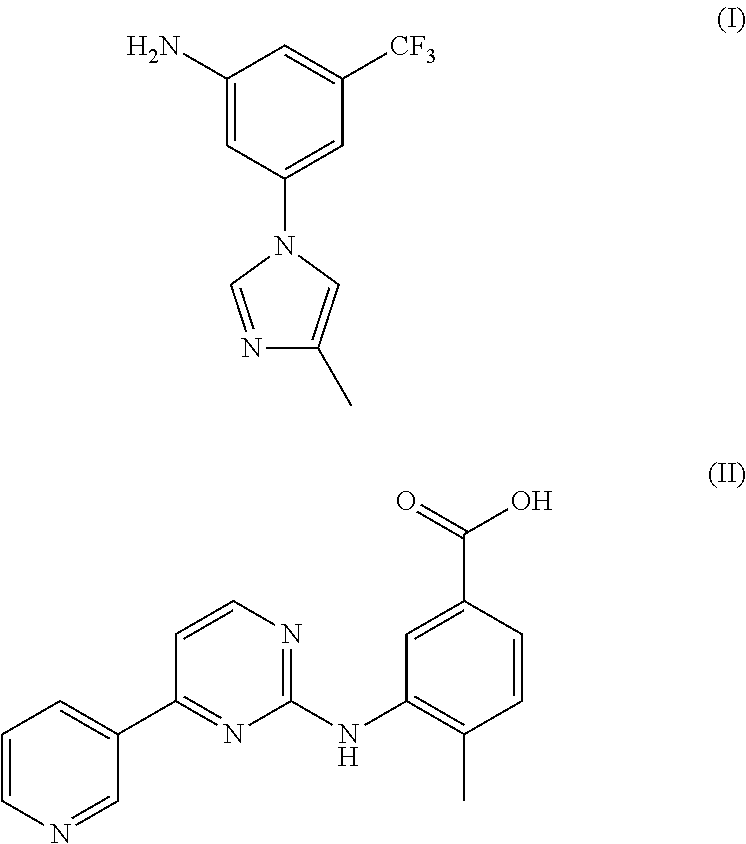
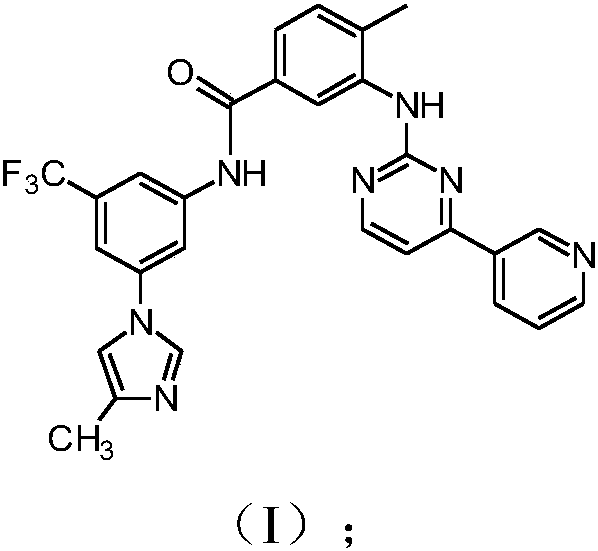
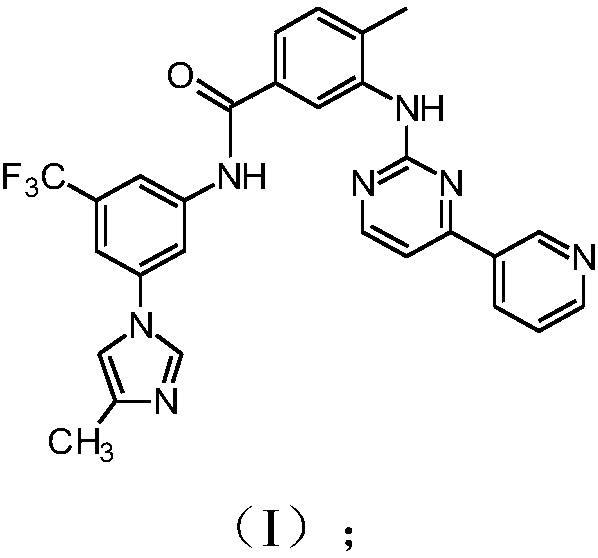
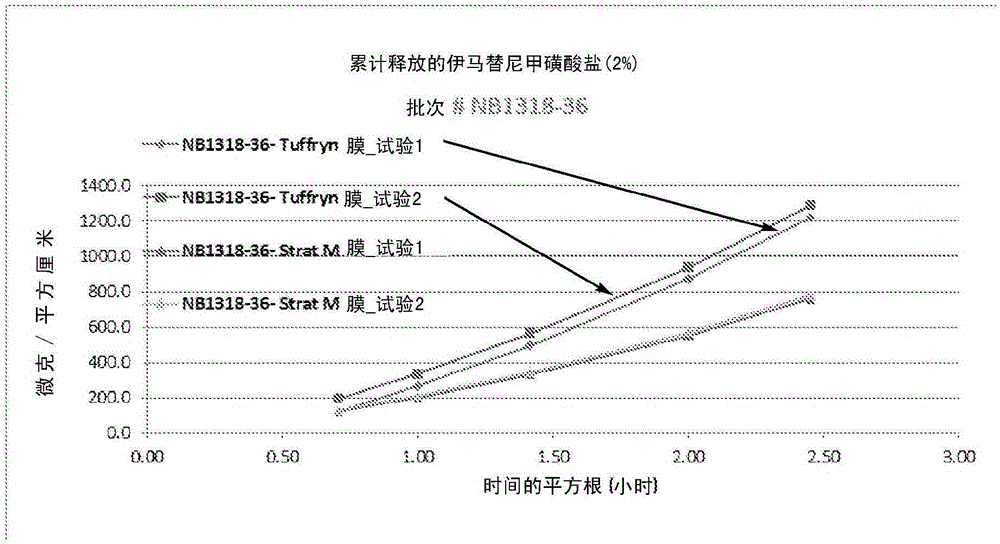
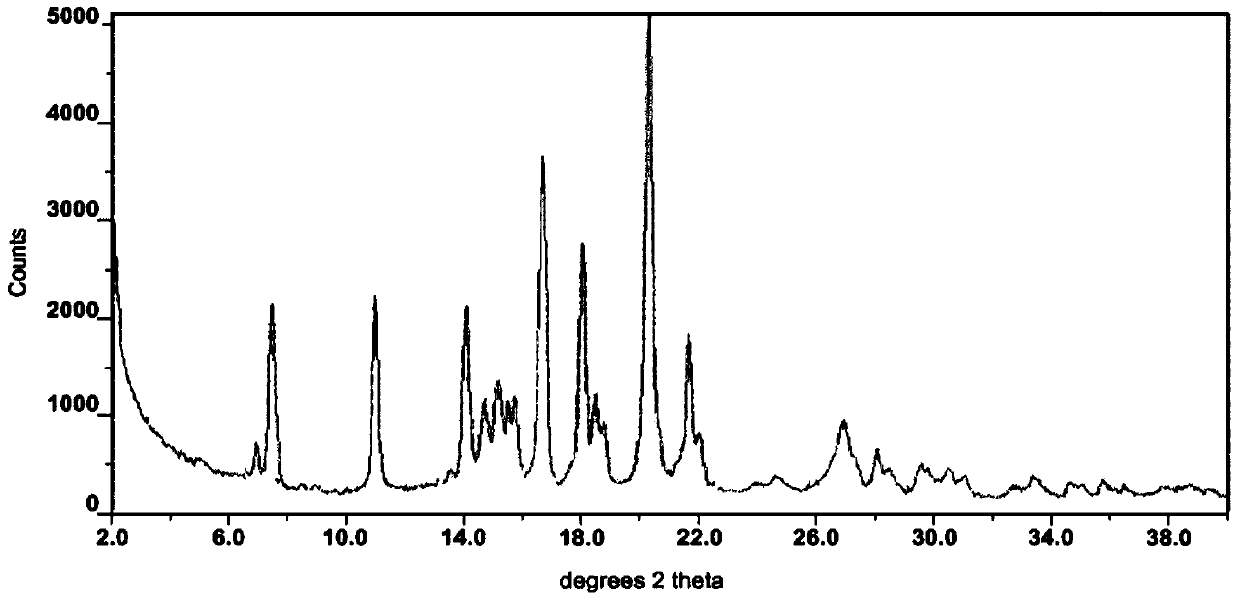
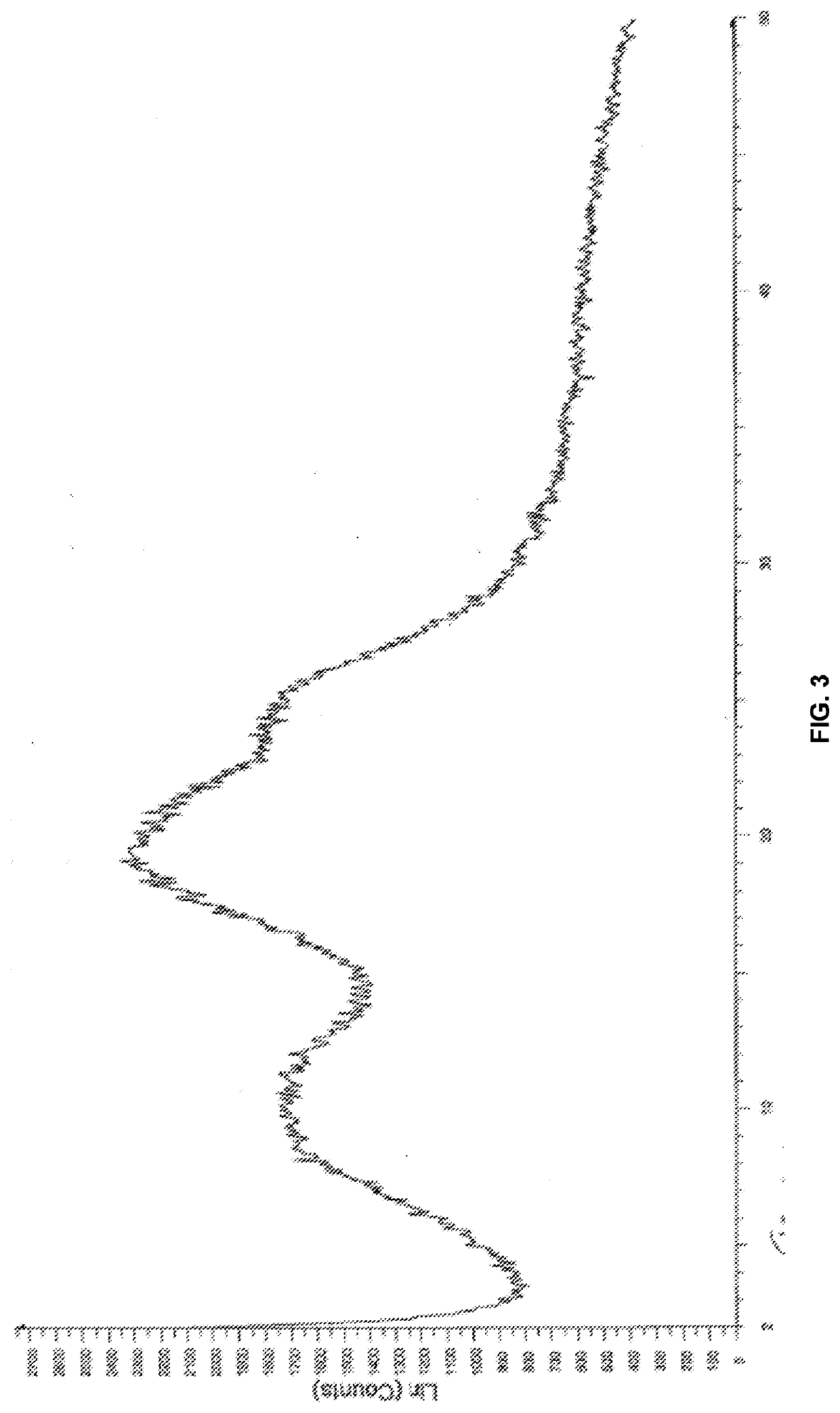
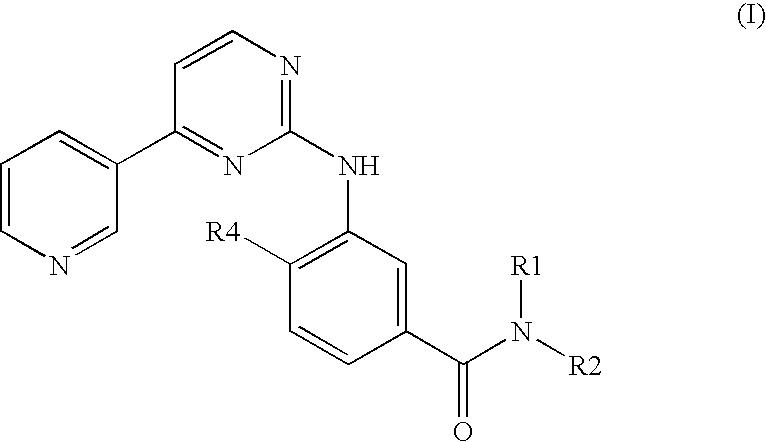
Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-5-(4-methyl-1h-imidazol-1-yl)-3-(trifluoromethyl)phenyl] benzamide solubilized using organic acids
Soluble pharmaceutical compositions of amorphous nilotinib or a pharmaceutically acceptable salt thereof were invented using one or more organic acids that function as a solubilizing agent, increasing the bioavailability of nilotinib and supressing the food effect associated with certain compositions of nilotinib. The pharmaceutical compositions are in th form of solid oral dosage forms, including capsules and tablets.
Owner:NOVARTIS AG
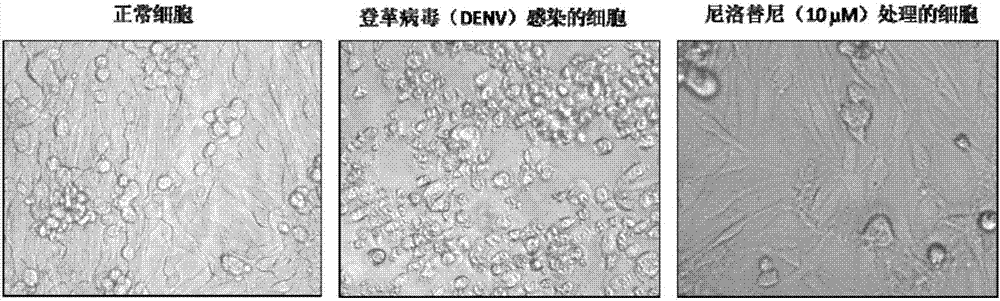
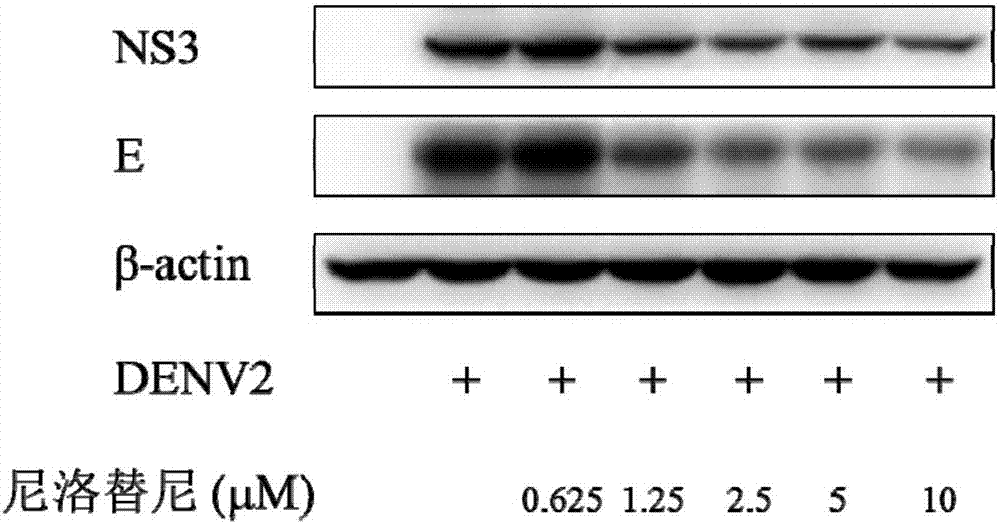
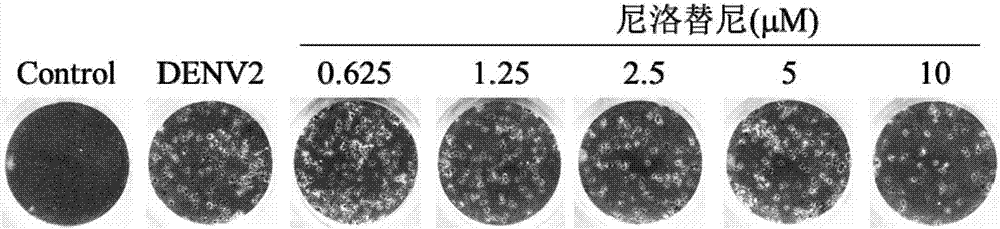
Nilotinib as medicine for treating dengue virus infection and pharmaceutical application thereof
InactiveCN107441094AGood effectHigh selectivityOrganic active ingredientsAntiviralsDiseaseInfection disease
Owner:SOUTHERN MEDICAL UNIVERSITY
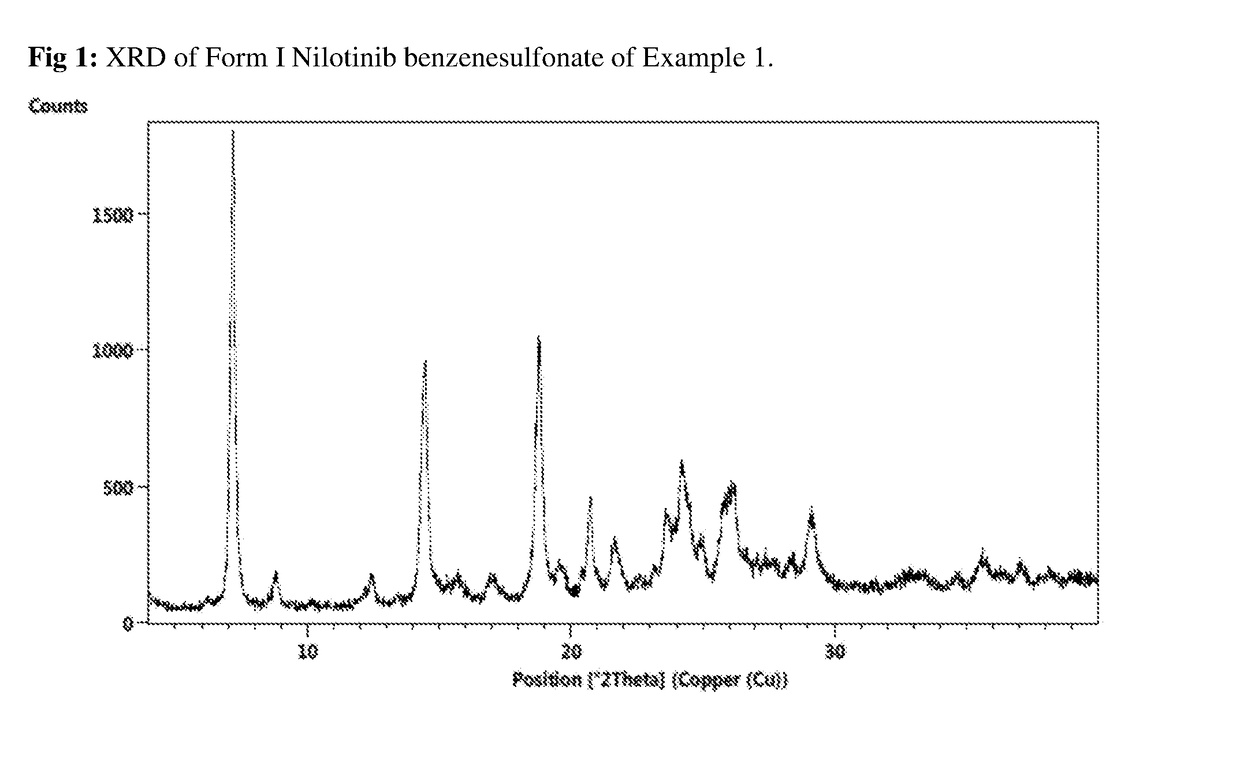
Salts of nilotinib and polymorphs thereof
ActiveUS10138221B2Promote dissolutionImprove solubilityOrganic chemistry methodsNilotinibBenzenesulfonic acid
Owner:SUN PHARMA INDS
Preparation method and intermediate of nilotinib
ActiveCN109666023AEasy to operateSimple and gentle process operationOrganic chemistrySolventNilotinib
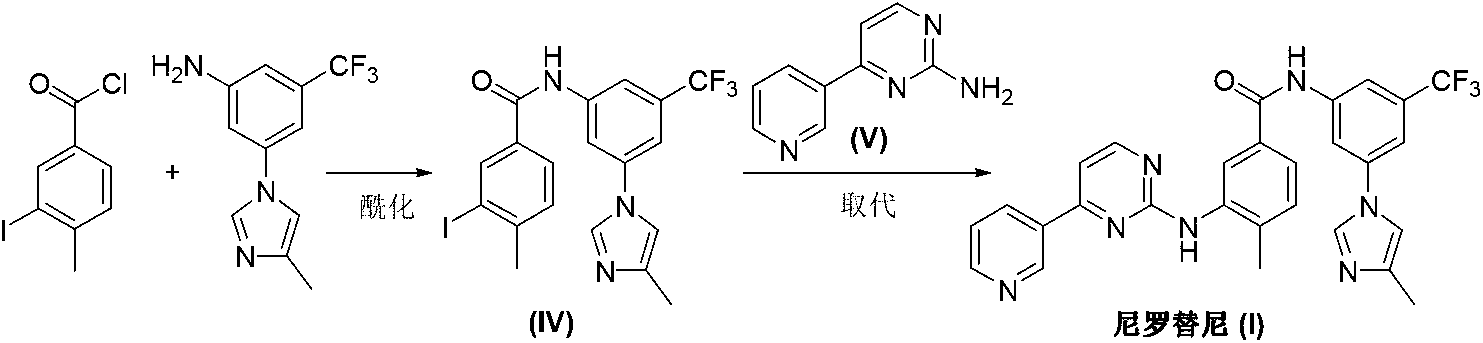
The invention discloses a nilotinib preparation method and an intermediate of nilotinib. The preparation method comprises: in a solvent, carrying out a reaction defined in the specification on a compound D or a hydrochloride salt thereof, and a compound SM3 under the action of an inorganic base to obtain the compound E nilotinib. According to the present invention, the preparation method has characteristics of simple reaction, easy operation, safe and environmentally friendly reagent, less side reaction and short reaction time. The reaction formula is defined in the specification.
Owner:SHANGHAI SUNTECH PHARMA
Pharmaceutical gastro-retentive solid oral dosage form of nilotinib
The present invention relates to a pharmaceutical gastro-retentive solid oral dosage form comprising nilotinib as the active ingredient. The invention is further related to methods of preparing said dosage form.
Owner:RANBAXY LAB LTD
Method for determining contents of impurity compounds I in nilotinib
The invention discloses a method for determining and analyzing the content of 3-(4-methyl-1H-imidazole-1-yl)-5-(trifluoromethyl) aniline in nilotinib. The content of the 3-(4-methyl-1H-imidazole-1-yl)-5-(trifluoromethyl) aniline in the nilotinib is directly measured by the aid of efficient liquid chromatography-mass spectrometers. Compared with the prior art, the method has the advantages of good specificity and repeatability, extremely high sensitivity, suitability for trace analysis and the like.
Owner:SHANGHAI AOBO PHARMTECH INC LTD
Method for preparing nilotinib intermediate
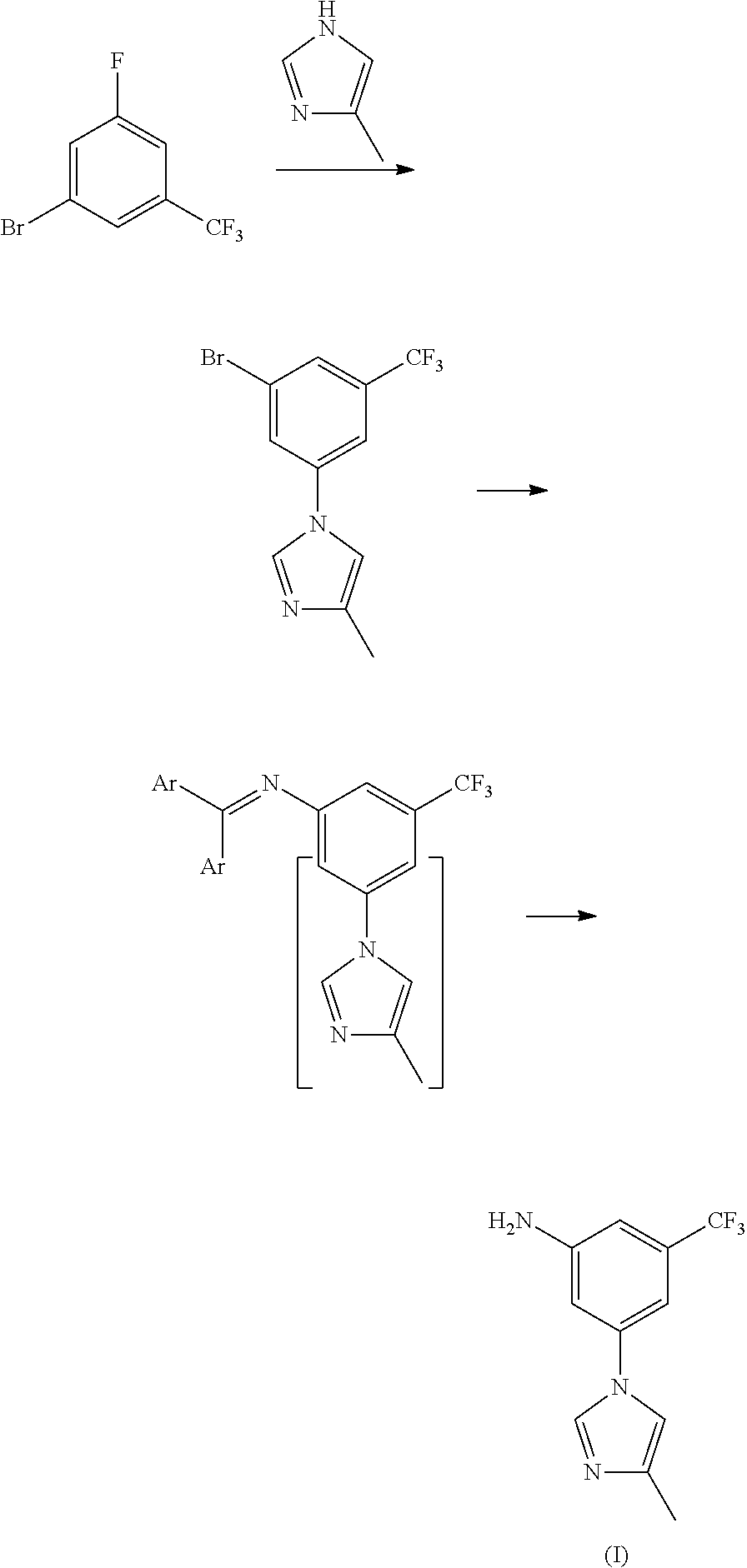
Disclosed in the present invention is a method for preparing nilotinib intermediate 3-(4-methyl-1H-imidazol-1-yl)-5trifluoromethyl phenylamine (I). The method comprises the following steps: taking trifluorotoluene as an initial material, and preparing the nilotinib intermediate (I) by nitration, bromization, condensation and reduction successively Compared with the prior art, the preparation method has the following advantages: a relatively high yield, the raw materials are easily obtained, a concise process and few side reactions, and is adapted to industrial production, so the development of an economic technology of the bulk drug is pro moted.
Owner:SUZHOU LIXIN PHARMA
Method for preparing nilotinib intermediate
Disclosed in the present invention is a method for preparing nilotinib intermediate 3-(4-methyl-1H-imidazol-1-yl)-5trifluoromethyl phenylamine (I). The method comprises the following steps: taking trifluorotoluene as an initial material, and preparing the nilotinib intermediate (I) by nitration, bromization, condensation and reduction successively Compared with the prior art, the preparation method has the following advantages: a relatively high yield, the raw materials are easily obtained, a concise process and few side reactions, and is adapted to industrial production, so the development of an economic technology of the bulk drug is promoted.
Owner:SUZHOU LIXIN PHARMA
Kit for detecting drug concentration of nilotinib in dried blood spots, and detection method implemented by kit
InactiveCN110045037ALess bloodAccurate concentrationComponent separationOrganic solventInternal standard
The invention discloses a kit for detecting the drug concentration of nilotinib in dried blood spots. The kit comprises an internal standard extraction reagent, and dried blood spot calibrators, wherein the internal standard extraction reagent adopts isotopically substituted nilotinib as an internal standard substance, and a solvent for the internal standard extraction reagent is a mixture of an organic solvent and water; the nilotinib dried blood spot calibrators are a group of nilotinib dried blood spot samples with different concentration gradients, the number of the dried blood spot samples is equal to or larger than 6, and the dried blood spot samples are fixed on a dried blood spot collection card; and the concentration range of the concentration gradients is between 50 ng / mL and 4,000 ng / mL. The invention further discloses a detection method implemented by the kit. The kit and the detection method provided by the invention have the advantages of small sampling volume, high sample stability, and high accuracy of detection results.
Owner:上海药明傲喆医学检验所有限公司
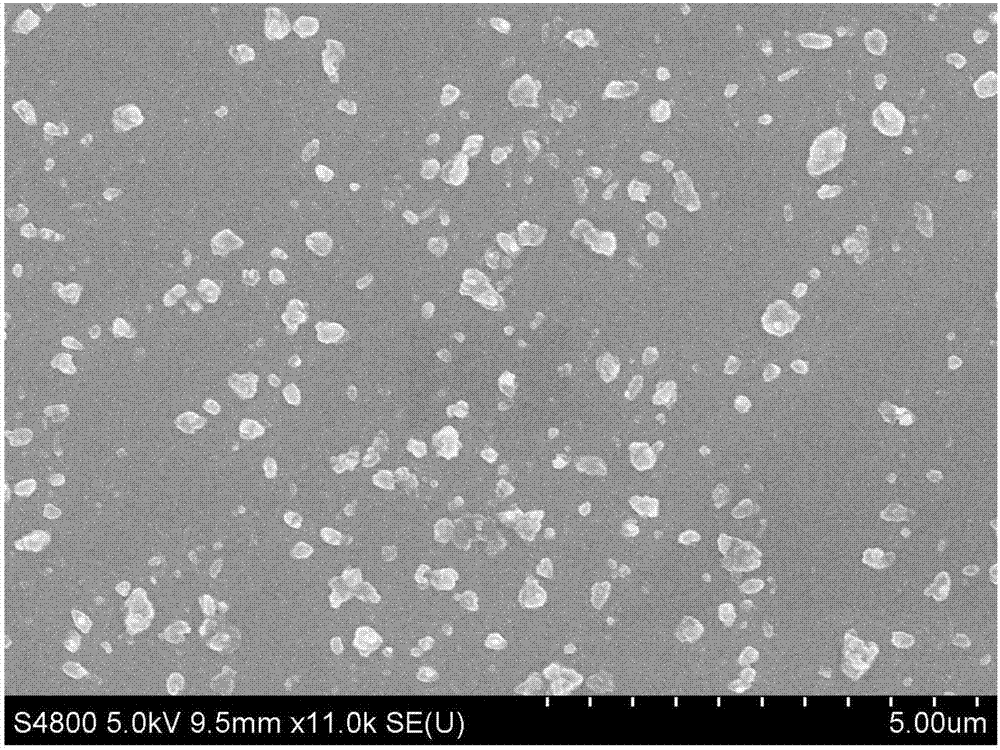
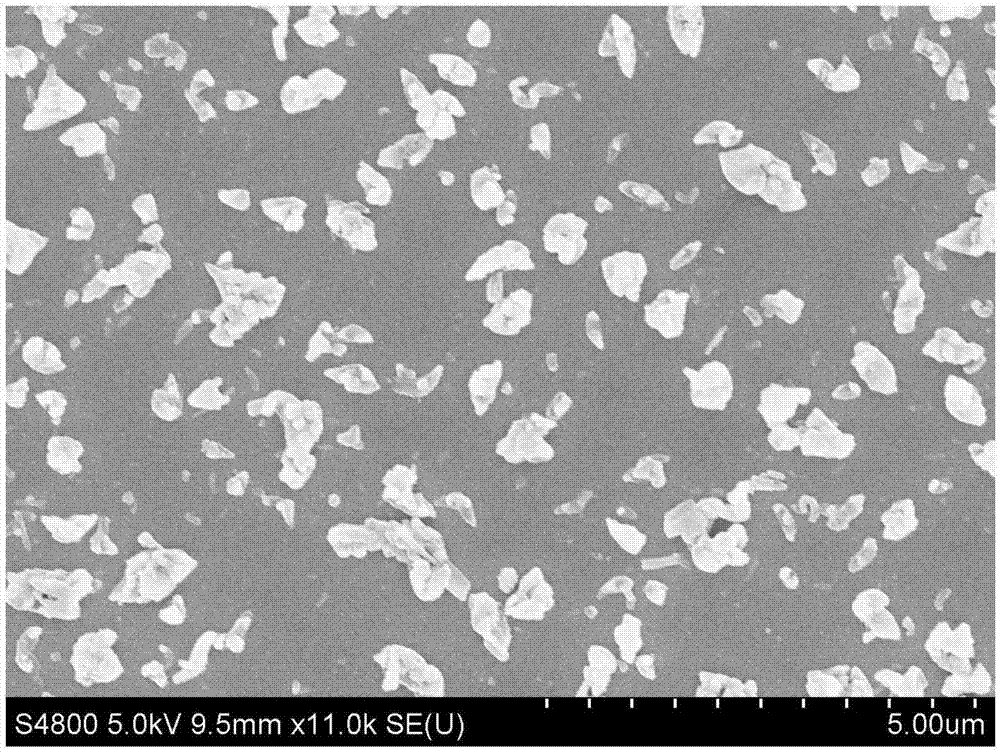
Purification method of small-grain size nilotinib
InactiveCN108586431AReduce pollutionImprove working environmentOrganic chemistryPurification methodsNilotinib
The invention relates to a purification method of small-grain size nilotinib, and belongs to the technical field of crude drug preparation. According to the technical scheme, firstly, a nilotinib ethyl acetate solution is prepared, then activated carbon and absolute ethyl alcohol are added for filtering, and through the steps of cooling, fed-batch of 50-70% ethanol water, stirring speed controlling and the like, the small-grain size nilotinib is prepared. The small-grain size nilotinib obtained through the method can be directly used for preparation technologies, and thus the requirements of the different technologies for the crude drug can be met.
Owner:WEIHAI YUNRUI INFORMATION TECH CO LTD
Nilotinib sustained-release implant for treating solid tumor
InactiveCN101185627AOrganic active ingredientsPharmaceutical delivery mechanismProstate cancerTherapeutic effect
A sustained release implant includes 0.1%-50% (w / w) nilotinib, 50-99% sustained release excipients and 0-15% sustained release moderator. Sustained release excipients are one or the combination of poly (L-lactide-co-ethyl phosphate), poly (L-lactide-co- phosphoric acid propyl), glycolic acid and copolymer of glycolic acid and hydroxyacetic acid, and polifeprosan; sustained release moderator is one or combination of mannitol, sorbic alcohol and chondroitin; sustained release implant applied in local tumor can slowly release nilotinib onto local tumor, thus maintaining effective drug concentration of local tumor as well as significantly reducing overall toxic reaction, thus the invention not only reduces overall toxic reaction of nilotinib, but also selectively improves drug concentration in local tumor, enhancing the therapeutic effects of non-operative therapy such as chemotherapy drugs and radiotherapy. The implant can be used for treating solid tumors including lung cancer, esophageal carcinoma, gastric cancer, liver cancer, breast cancer, ovarian cancer, prostatic carcinoma, pancreatic cancer, bladder carcinoma, cerebroma, and colorectal cancer.
Owner:SHANDONG LANJIN PHARMA +1
Method for the preparation of pure nilotinib and its salt
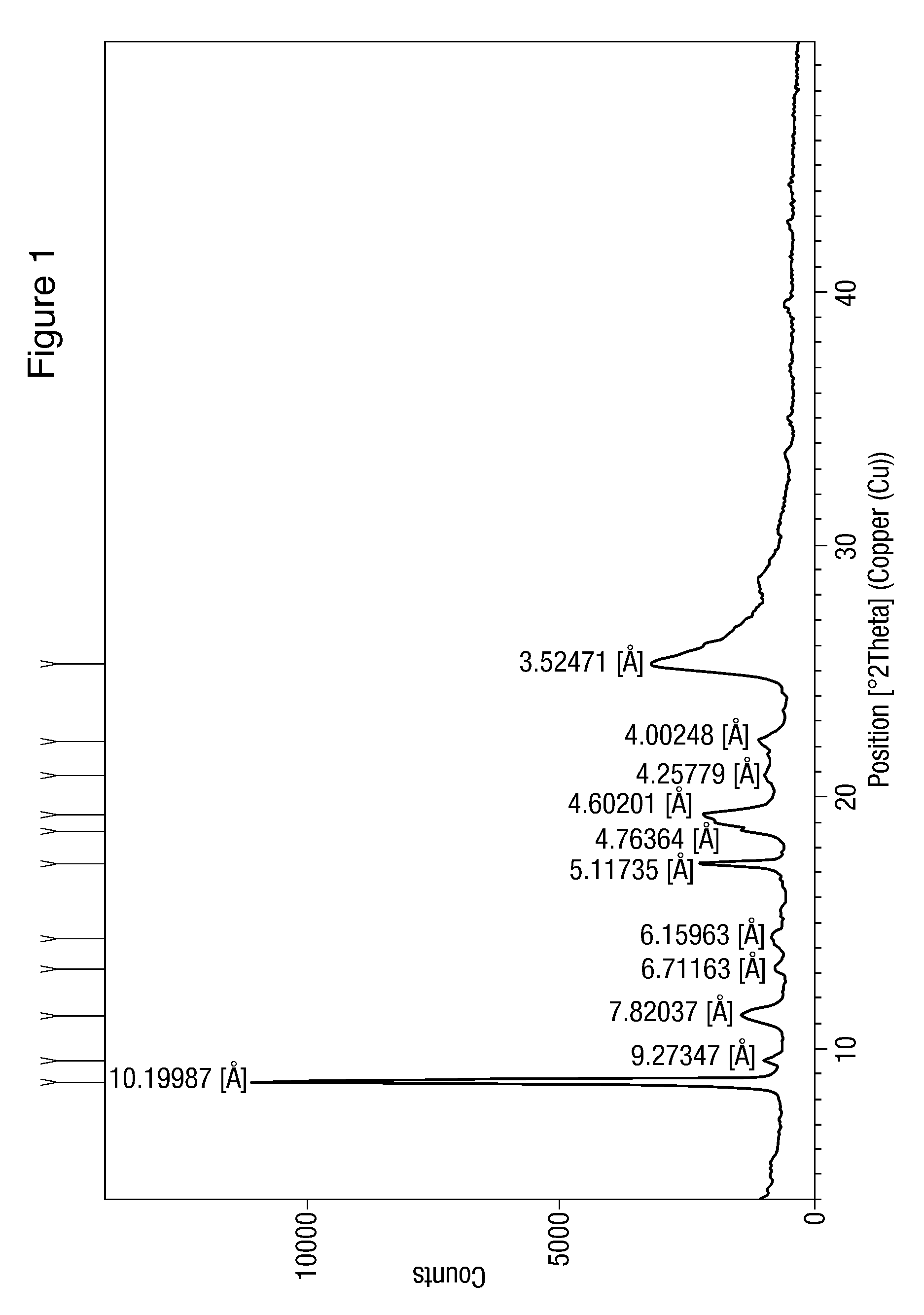
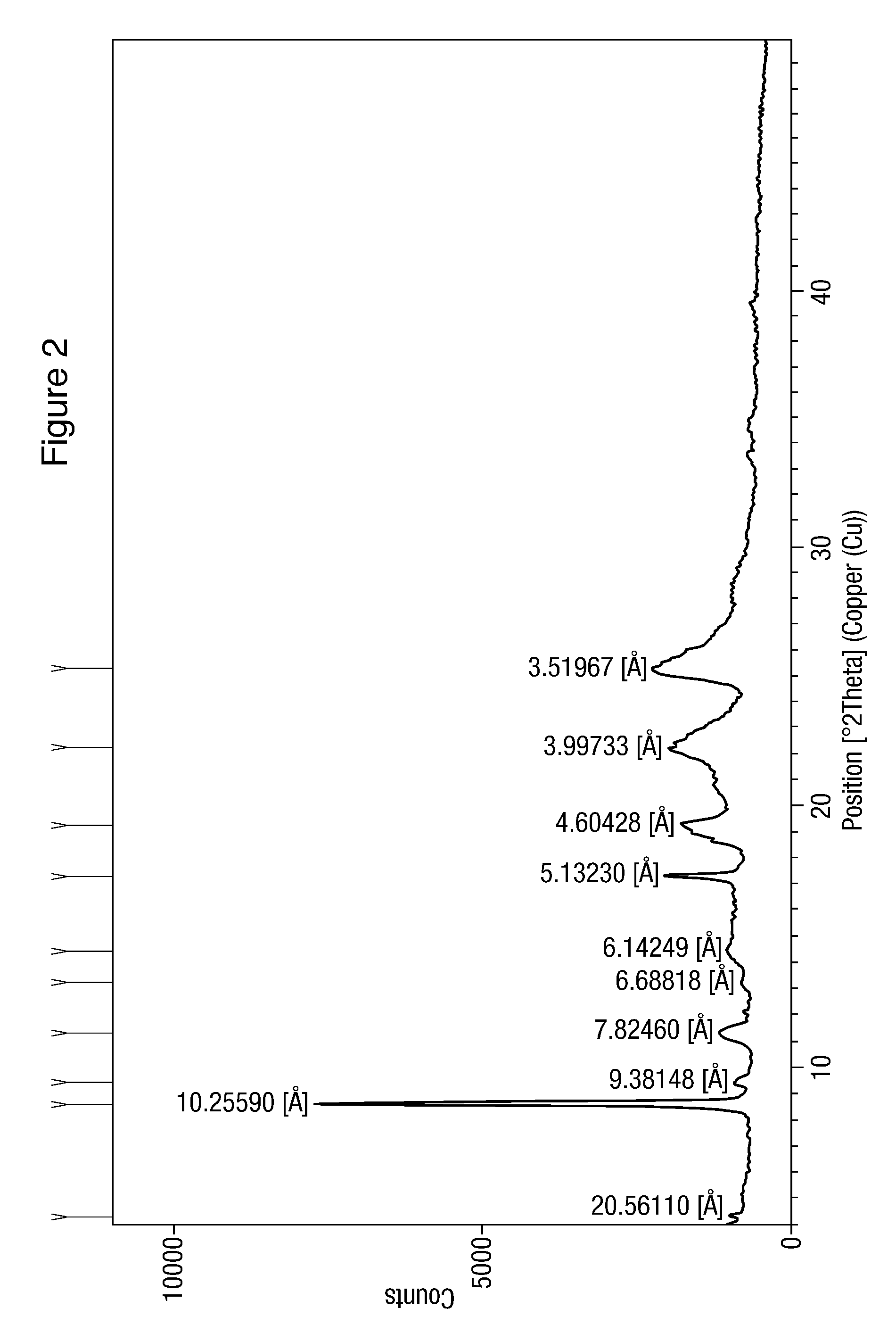
The present invention discloses a method for the preparation of pure nilotinib and its salt. The method comprises the following steps: (a) providing the solution of free alkali raw material of a chemical compound of the formula (I) in the mixture of C1-C4 alcohol and mineral acid; (b) adding alkali into the solution in the step (a) in order to acquire pH value of being more than 8, inoculating thesolution in the step (b); (c) inoculating the solution in the step (b) if appropriate; (d) acquiring a suspension solution of the chemical compound of the formula (I); (e) separating a product of theformula (I). The invention aims to the method to prepare pharmaceutical active ingredient Nilotinib free base or Nilotinib dihydrochloride dihydrate by means of an improved crystallization procedure.
Owner:F I S FAB ILTALIANA SINTETICI SPA
Nilotinib composition with improved solubility
The present invention provides compositions of nilotinib with a salt of a polymer. According to the nilotinib composition provided by the invention, the solubility and bioavailability are improved, and the nilotinib composition can be used for treating uncontrolled cell proliferation diseases. The invention also provides a preparation method and application of the nilotinib and polymer salt composition.
Owner:NEOFORM BIOPHARMACEUTICAL LTD
Preparation method of nilotinib intermediate-3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline
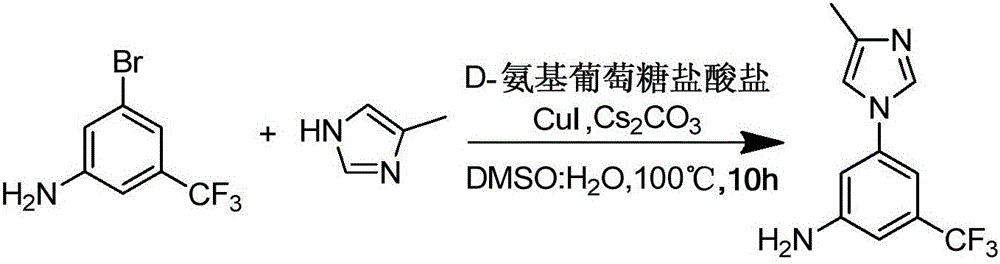
The invention provides a preparation method of a nilotinib intermediate-3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline. The preparation method comprises the following steps of adding 3-bromo-5-(trifluoromethyl)aniline, 4-methylimidazole, D-glucosamine hydrochloride, CuI, Cs2CO3, DMSO and water into a reaction vessel in sequence, and reacting for 8 to 12 hours at 90 to 110 DEG C; adding ethyl acetate into reaction liquid, taking a supernatant after centrifugal separation, and obtaining the 3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline after concentration and drying. The purity of a product prepared by the invention can reach 98 percent or above, the yield can reach 95 percent or above, and the preparation method provided by the invention has the advantages of being low in production cost, high in reaction yield, low in environmental pollution and the like, and is more suitable for industrial production.
Owner:ZHEJIANG ESIGMA BIOTECH CO LTD
Preparation method for nilotinib
ActiveCN104860929AImprove efficiencyAvoid complexation reactionsOrganic chemistryCompound aCombinatorial chemistry
The invention provides a preparation method for nilotinib. The preparation method comprises the following steps: producing a carbonyl insertion amination reaction of a compound A and 3-(4-methyl-1H-imidazole-1-yl)-5-(trifluoromethyl)phenylamine, and obtaining an amination product; performing R group deprotection treatment on the amination product, and obtaining nilotinib, wherein the compound A has a structure as shown in a formula I, in the formula I, R is selected from benzyl, -COCF3, -CHO or -CO2R', and R' is C1-C10 alkyl, C1-C3 alkoxy ethyl or C7-C19 aralkyl. The preparation method is short in synthetic route and mild in reaction condition, and due to the adoption of special raw materials, the preparation method reduces the process cost while increasing the carbonyl yield.
Owner:ASYMCHEM LAB TIANJIN +4
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-[5-(4-methyl-1h-imidazol-1-yl)-3-(triflouoromethyl)phenyl] benzamide solubilized using organic acids Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-[5-(4-methyl-1h-imidazol-1-yl)-3-(triflouoromethyl)phenyl] benzamide solubilized using organic acids](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/875af486-3c8c-4b61-8e3c-c06c6a1a32f3/US20150273070A1-20151001-D00001.PNG)
![Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-[5-(4-methyl-1h-imidazol-1-yl)-3-(triflouoromethyl)phenyl] benzamide solubilized using organic acids Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-[5-(4-methyl-1h-imidazol-1-yl)-3-(triflouoromethyl)phenyl] benzamide solubilized using organic acids](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/875af486-3c8c-4b61-8e3c-c06c6a1a32f3/US20150273070A1-20151001-D00002.PNG)
![Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-[5-(4-methyl-1h-imidazol-1-yl)-3-(triflouoromethyl)phenyl] benzamide solubilized using organic acids Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-[5-(4-methyl-1h-imidazol-1-yl)-3-(triflouoromethyl)phenyl] benzamide solubilized using organic acids](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/875af486-3c8c-4b61-8e3c-c06c6a1a32f3/US20150273070A1-20151001-D00003.PNG)











![Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-5-(4-methyl-1h-imidazol-1-yl)-3-(trifluoromethyl)phenyl] benzamide solubilized using organic acids Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-5-(4-methyl-1h-imidazol-1-yl)-3-(trifluoromethyl)phenyl] benzamide solubilized using organic acids](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fc3b0caa-94ba-475c-ad99-cceb5f1e418f/HDA0000438795460000011.PNG)
![Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-5-(4-methyl-1h-imidazol-1-yl)-3-(trifluoromethyl)phenyl] benzamide solubilized using organic acids Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-5-(4-methyl-1h-imidazol-1-yl)-3-(trifluoromethyl)phenyl] benzamide solubilized using organic acids](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fc3b0caa-94ba-475c-ad99-cceb5f1e418f/HDA0000438795460000021.PNG)
![Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-5-(4-methyl-1h-imidazol-1-yl)-3-(trifluoromethyl)phenyl] benzamide solubilized using organic acids Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-5-(4-methyl-1h-imidazol-1-yl)-3-(trifluoromethyl)phenyl] benzamide solubilized using organic acids](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fc3b0caa-94ba-475c-ad99-cceb5f1e418f/HDA0000438795460000022.PNG)