Method for determining contents of impurity compounds I in nilotinib
A technology for nilotinib and compounds, which can be used in measurement devices, instruments, scientific instruments, etc., and can solve the problems of inability to provide trace analysis results, specificity, sensitivity, method precision and repeatability defects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Instruments and Conditions:
[0037] High performance liquid chromatography mass spectrometry: Agilent 1260 infinity, MS detector.
[0038] Chromatographic column: Octadecylsilane bonded silica gel column
[0039] Mobile phase: A: 0.05%-0.2% formic acid or acetic acid or trifluoroacetic acid aqueous solution B: acetonitrile.
[0040] The isocratic is as follows: 0-8.0min, the proportion of the organic phase changes from 10% to 90%.
[0041] Column temperature: 35°C.
[0042] Flow rate: 1.0mL / min
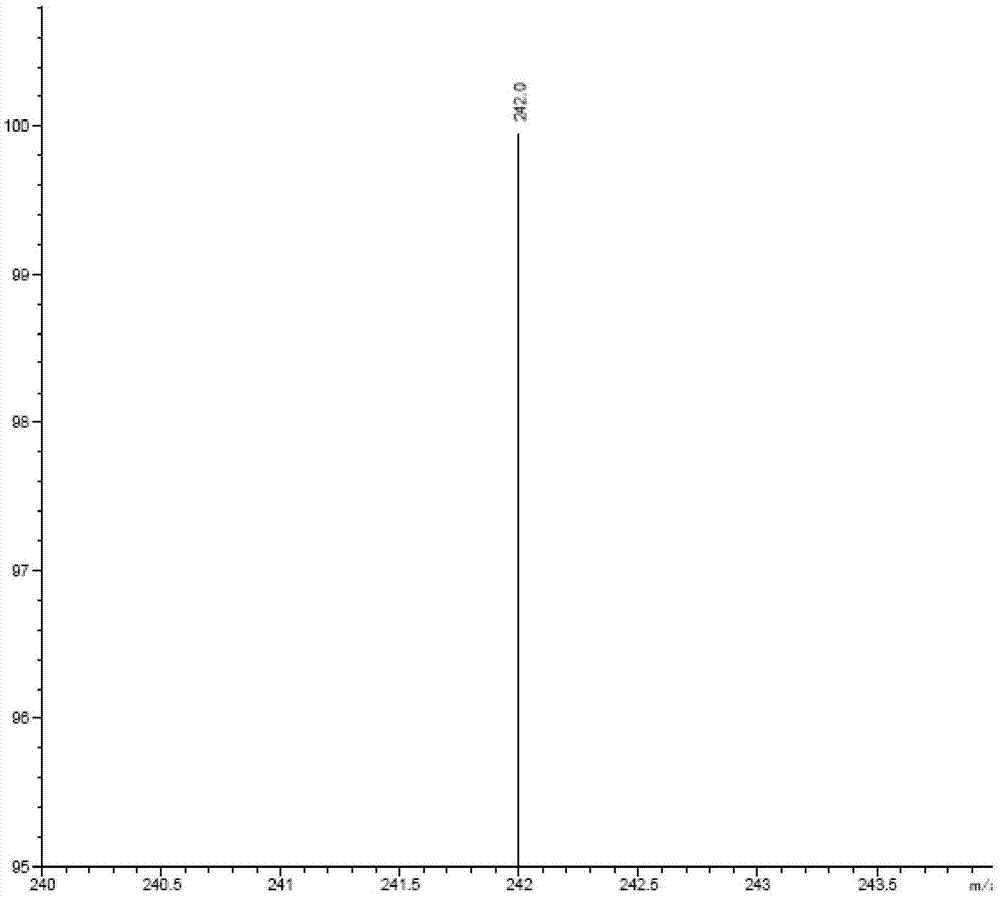
[0043] Select positive ions: 242
[0044] Injection volume: 5ul.
[0045] Experimental steps:
[0046] 1) Preparation of mobile phase A: Precisely measure 1.0 mL of trifluoroacetic acid and dissolve in 1000 mL of water, and mix well.
[0047] 2) Diluent B: Acetonitrile
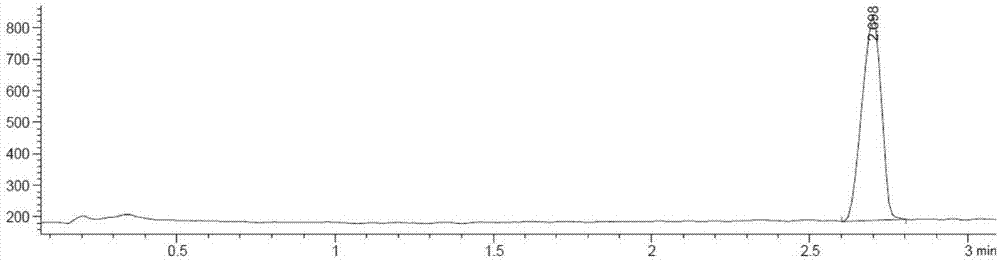
[0048] 3) Standard solution C: Accurately weigh about 28.5mg of compound I in a 100ml volumetric flask, add B to dissolve to volume, shake well, accurately measure 2.0ml and place it in another 100ml v...
Embodiment 2
[0050] Instruments and Conditions:
[0051] High performance liquid chromatography: Agilent 1260 infinity, MS detector.
[0052] Chromatographic column: Octadecylsilane bonded silica gel column
[0053] Mobile phase: A: 0.05%-0.2% formic acid or acetic acid or trifluoroacetic acid aqueous solution B: acetonitrile.
[0054] The isocratic is as follows: 0-8.0min, the proportion of the organic phase changes from 10% to 90%.
[0055] Column temperature: 35°C.
[0056] Flow rate: 1.0mL / min
[0057] Select positive ions: 242
[0058] Injection volume: 5ul.
[0059] Experimental steps:
[0060] 1) Preparation of mobile phase A: Precisely measure 1.0 mL of trifluoroacetic acid and dissolve in 1000 mL of water, and mix well.
[0061] 2) Diluent B: Acetonitrile
[0062] 3) Standard solution C: Accurately weigh about 28.5mg of compound I in a 100ml volumetric flask, add B to dissolve to volume, shake well, accurately measure 2.0ml and place it in another 100ml volumetric flask, a...
Embodiment 3
[0065] Instruments and Conditions:
[0066] High performance liquid chromatography: Agilent 1260 infinity, MS detector.
[0067] Chromatographic column: Octadecylsilane bonded silica gel column
[0068] Mobile phase: A: 0.05%-0.2% formic acid or acetic acid or trifluoroacetic acid aqueous solution B: acetonitrile.
[0069] The isocratic is as follows: 0-8.0min, the proportion of the organic phase changes from 10% to 90%.
[0070] Column temperature: 35°C.
[0071] Flow rate: 1.0mL / min
[0072] Select positive ions: 242
[0073] Injection volume: 5ul.
[0074] Experimental steps:
[0075] 1) Preparation of mobile phase A: Precisely measure 1.0 mL of trifluoroacetic acid and dissolve in 1000 mL of water, and mix well.
[0076] 2) Diluent B: Acetonitrile
[0077]3) Standard solution C: Accurately weigh about 28.5mg of compound I in a 100ml volumetric flask, add B to dissolve to volume, shake well, accurately measure 2.0ml and place it in another 100ml volumetric flask, ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com