Preparation method for nilotinib
A nilotinib and compound technology, which is applied in the field of preparation of nilotinib, can solve the problems of high cost and long steps of synthetic nilotinib route, and achieve the goal of reducing preparation cost, increasing yield and short synthesis route Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
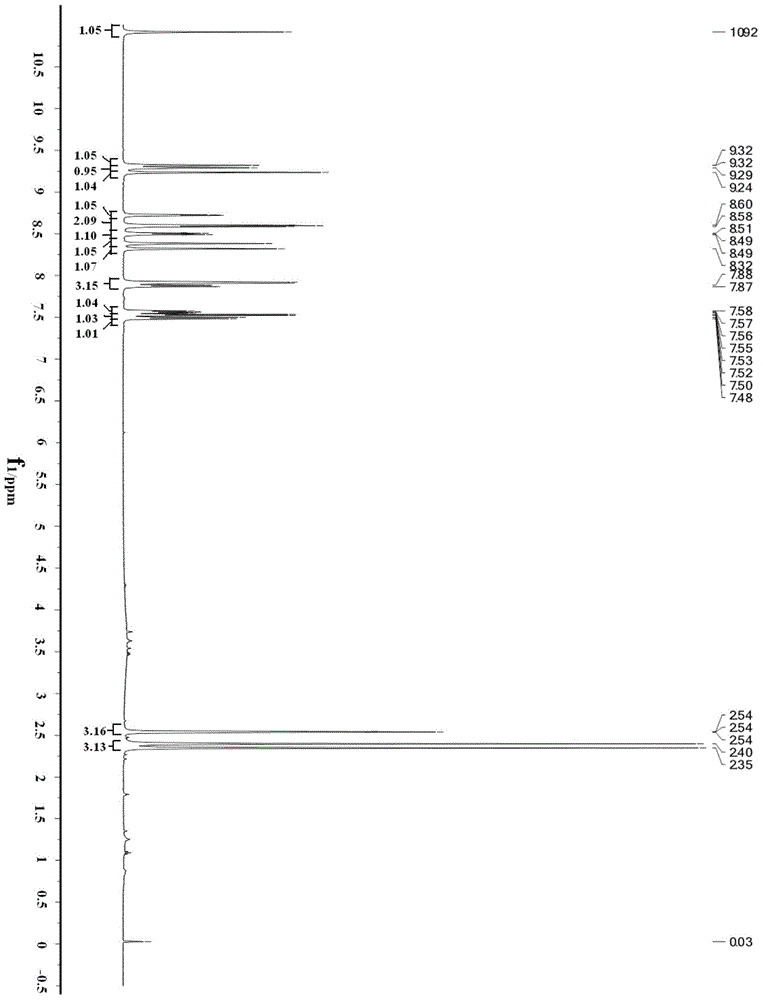
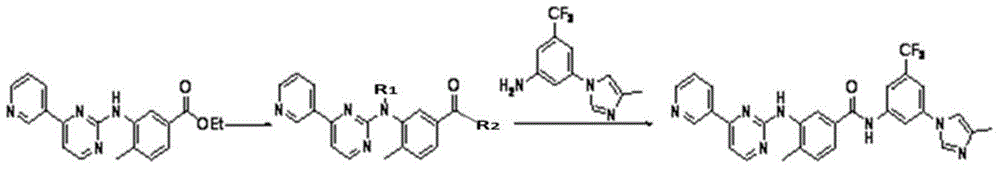
[0039] As described in the background technology section, to solve the problems of long route and high cost of synthesizing nilotinib in the existing technology. In order to solve this problem, the present invention provides a kind of preparation method of Nilotinib, and this preparation method comprises the following steps: compound A and 3-(4-methyl-1H-imidazol-1-yl)-5- (Trifluoromethyl) aniline is subjected to carbonyl insertion amination reaction to obtain an amination product; and the above amination product is subjected to R group deprotection treatment to obtain nilotinib; wherein, compound A has a structure shown in formula I:
[0040]
[0041] In formula I, the R group includes but not limited to benzyl, -COCF 3 , -CHO or -CO 2 R', wherein the R' group includes but is not limited to C 1 ~C 10 Alkyl, C 1 ~C 3 Alkoxyethyl or C 7 ~C 19 Aralkyl.
[0042]The present invention innovatively applies the carboxylation reaction to the synthetic route of nilotinib. S...
Embodiment 1
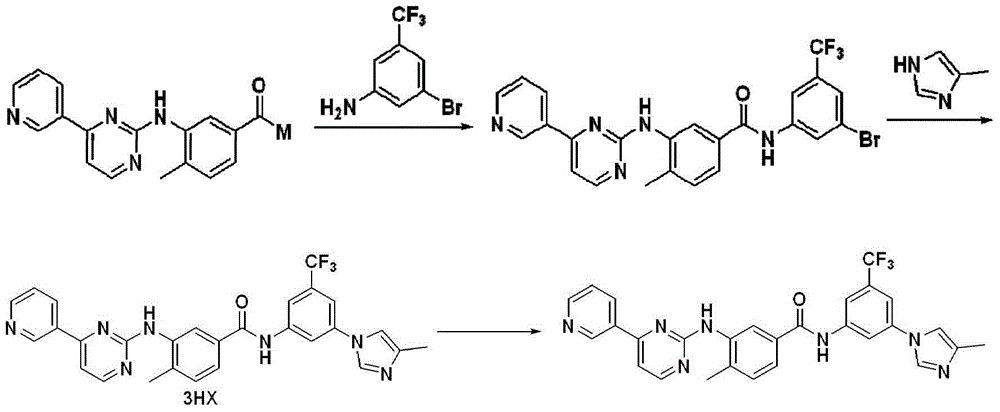
[0060] Dimethylformamide (1 L), 3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline (aromatic amine, 0.54 mol), (5 -Bromo-2-methylphenyl(4-(pyridin-3-yl)pyrimidin-2-yl)-tert-butyl carbamate (0.648mol), 1,1'-bis(diphenylphosphino)dicene Iron dichloride palladium dichloromethane complex (0.016mol), triphenylphosphine (0.032mol), phenol (2.5g, 0.027mol), triethylamine (1.62mol) and 4A molecular sieve (100g) were stirred evenly. Introduce nitrogen to replace the air, then introduce carbon monoxide to replace the nitrogen and make the pressure in the kettle reach 0.8MPa. Heat up to 90-105°C for reaction. After 42 hours of reaction, a product system containing aminated product is obtained. Cool the product system to 50 Below ℃, evacuate carbon monoxide and replace with nitrogen.
[0061] The above product system was diluted with methyl tert-butyl ether (1 L), and molecular sieves were filtered off to obtain a filtrate. Add mass concentration to the filtrate and be 1% K 2 CO ...
Embodiment 2
[0063] To the autoclave was added dimethylformamide (10 mL), 3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline (aromatic amine, 4.5 mmol), (5 -Bromo-2-methylphenyl(4-(pyridin-3-yl)pyrimidin-2-yl)-tert-butyl carbamate (5.4mmol), 1,1'-bis(diphenylphosphino)dicene Iron dichloride palladium dichloromethane complex (0.135mmol), triphenylphosphine (0.27mmol), phenol (0.225mmol), triethylamine (13.5mmol) and 4A molecular sieve (1g) are stirred evenly.Burse into nitrogen Replace the air, then introduce carbon monoxide to replace the nitrogen and make the pressure in the kettle reach 0.8MPa. Heat up to 90-105°C for reaction. After 72 hours of reaction, a product system containing aminated product is obtained. Cool the product system to below 50°C, Evacuate carbon monoxide and replace with nitrogen.
[0064] The above system was diluted with methyl tert-butyl ether (10 mL), and molecular sieves were filtered off to obtain a filtrate. Add mass concentration to the filtrate and b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com