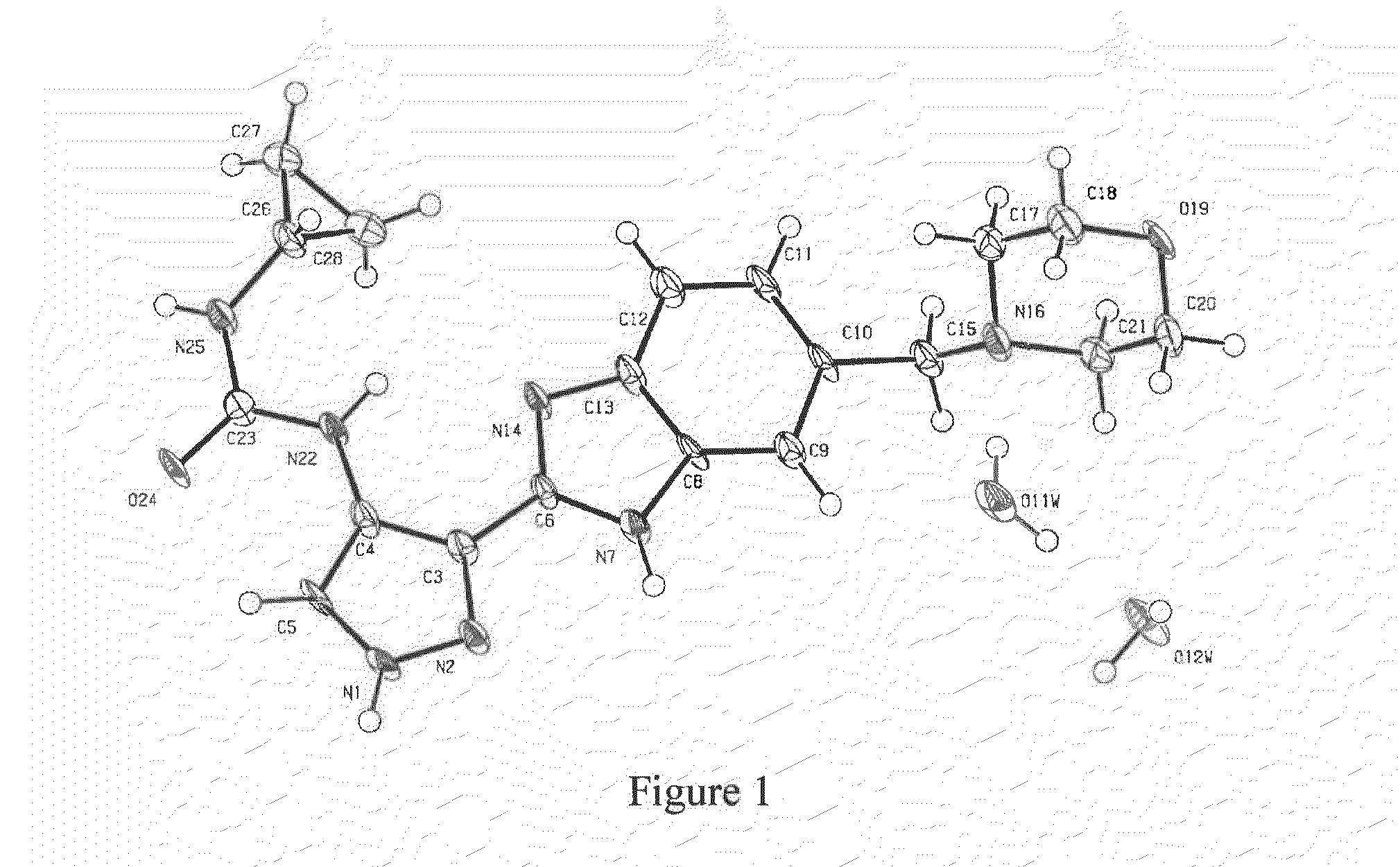
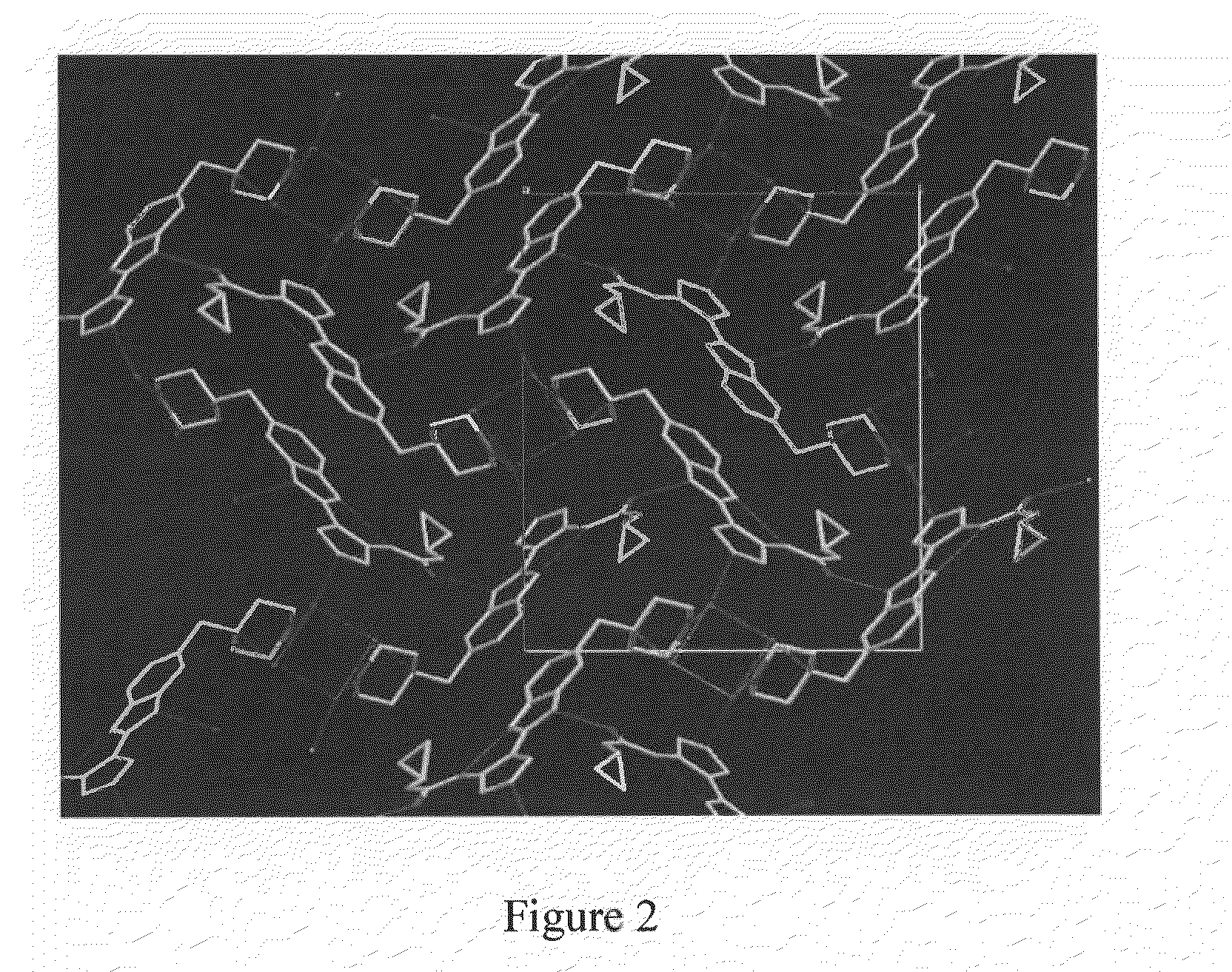
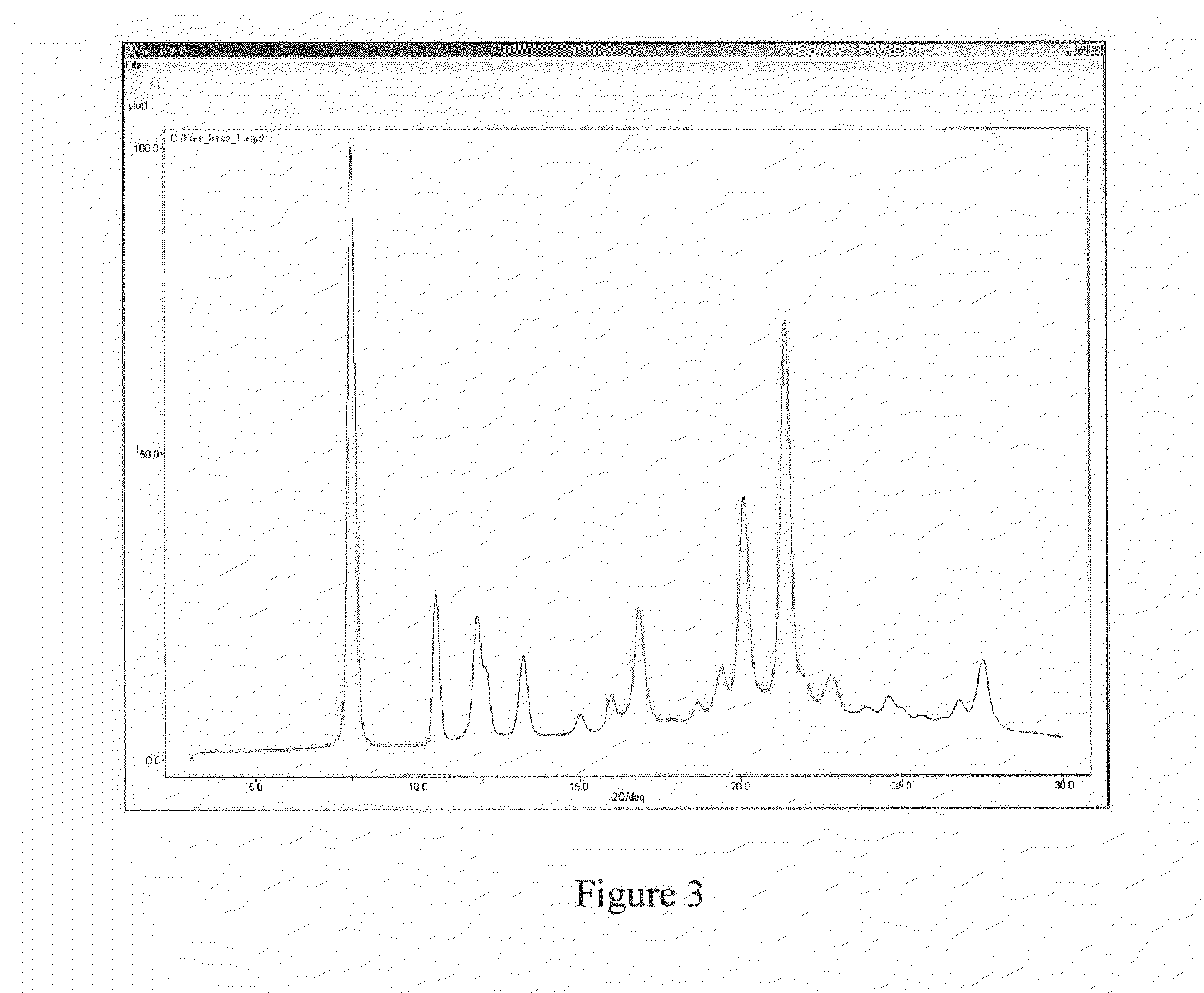
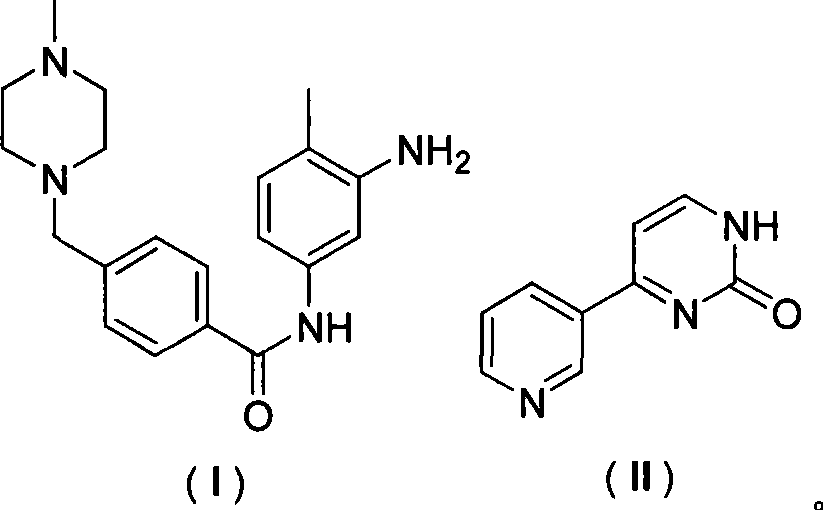
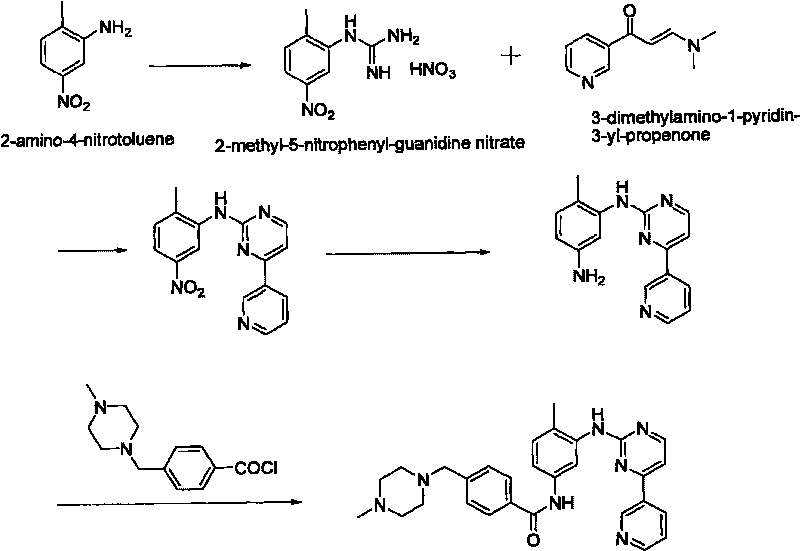
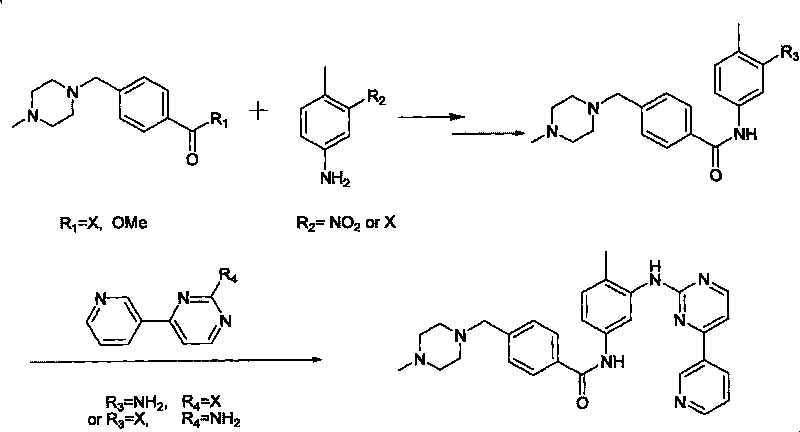
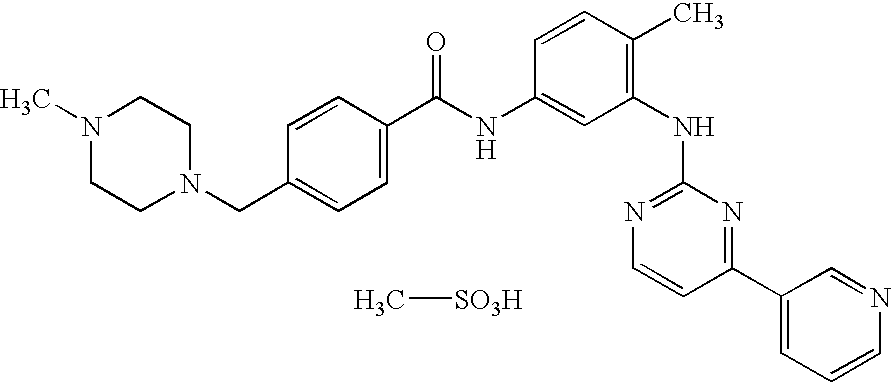
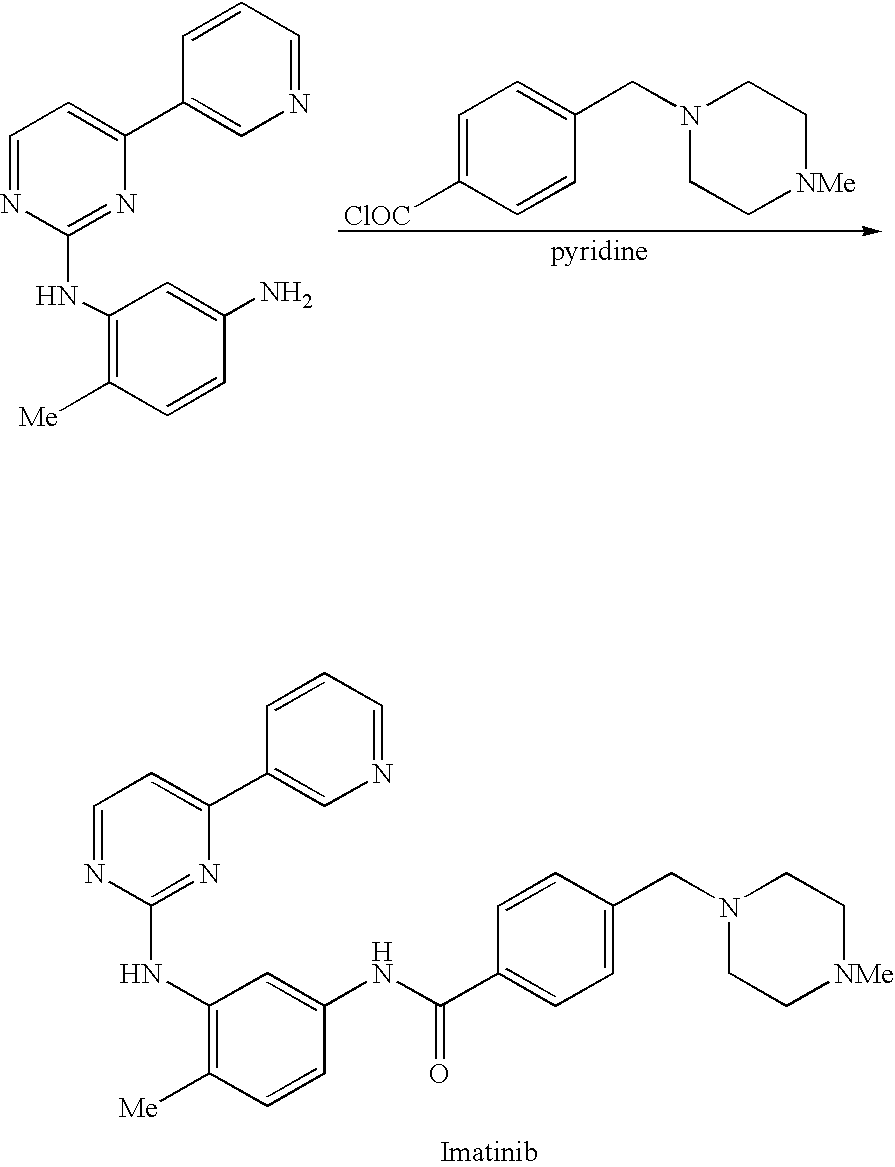
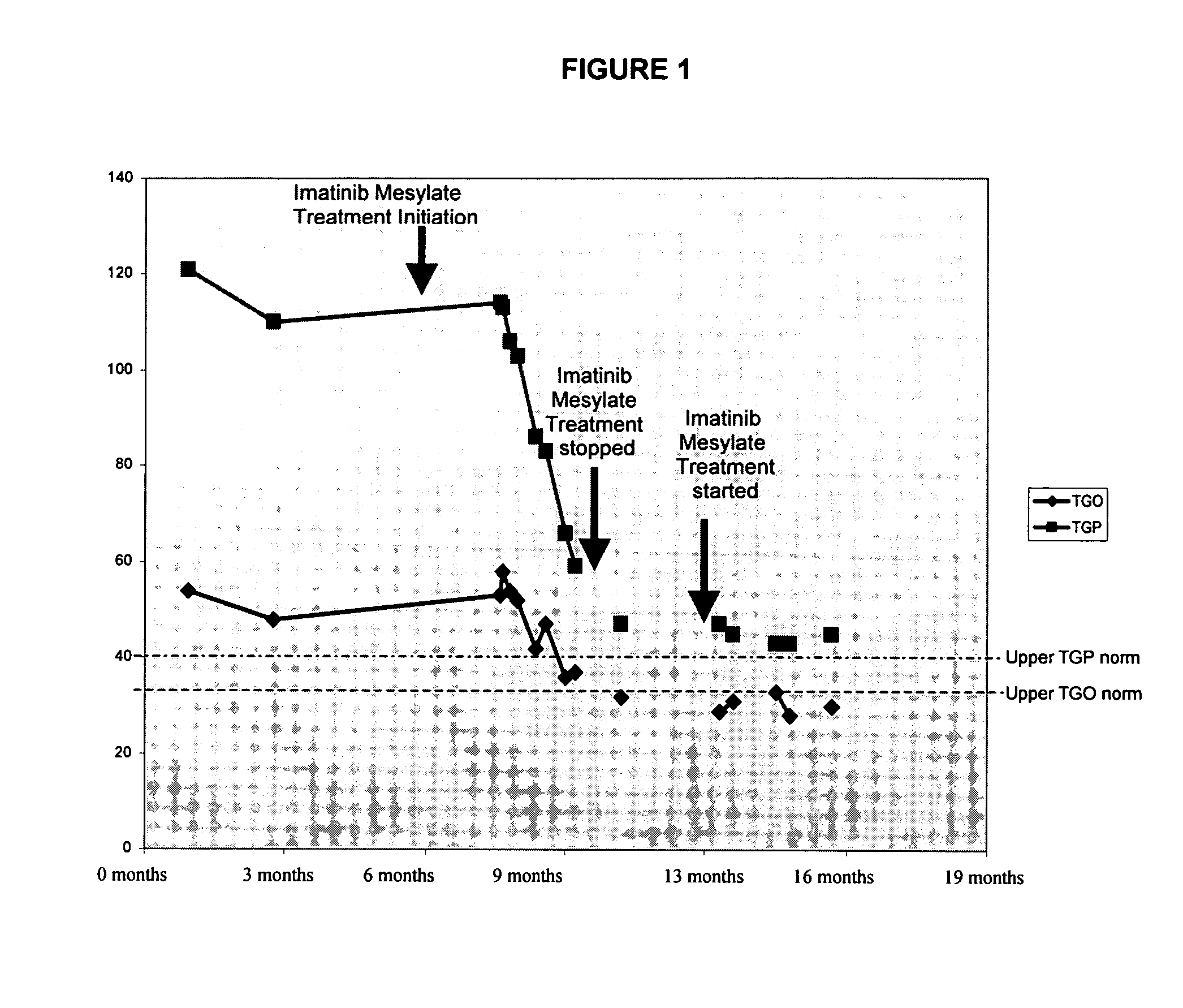
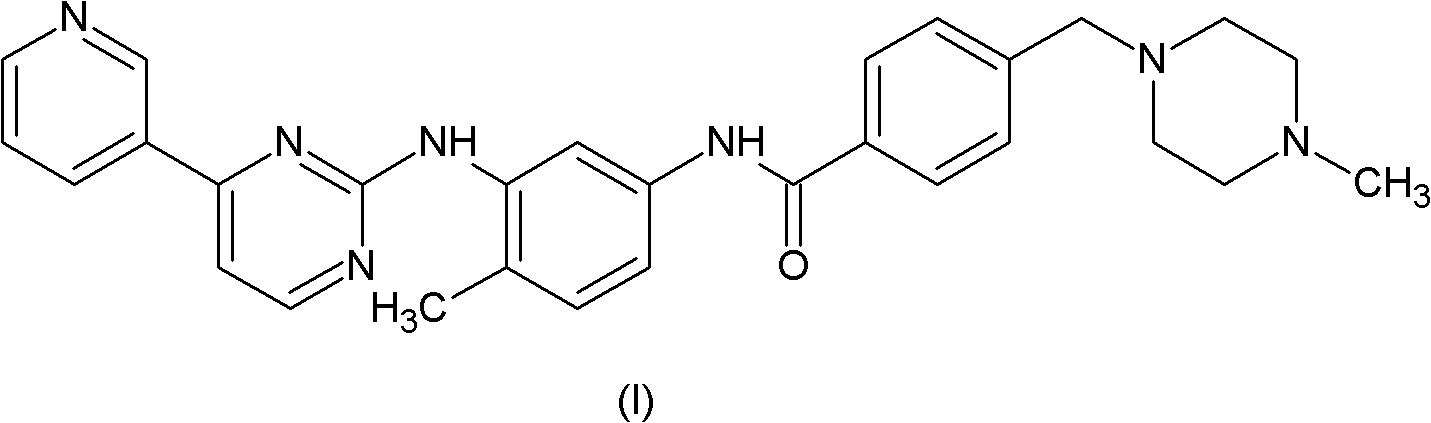
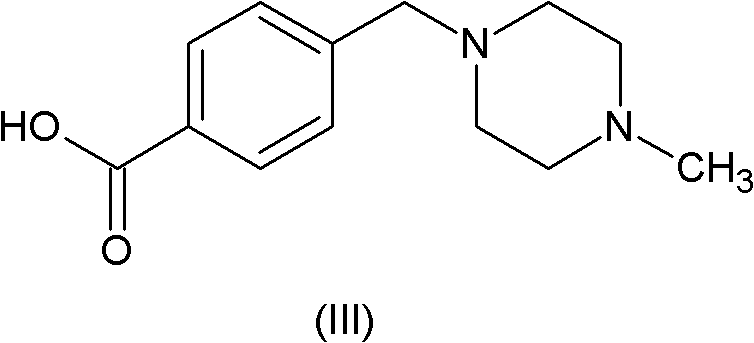
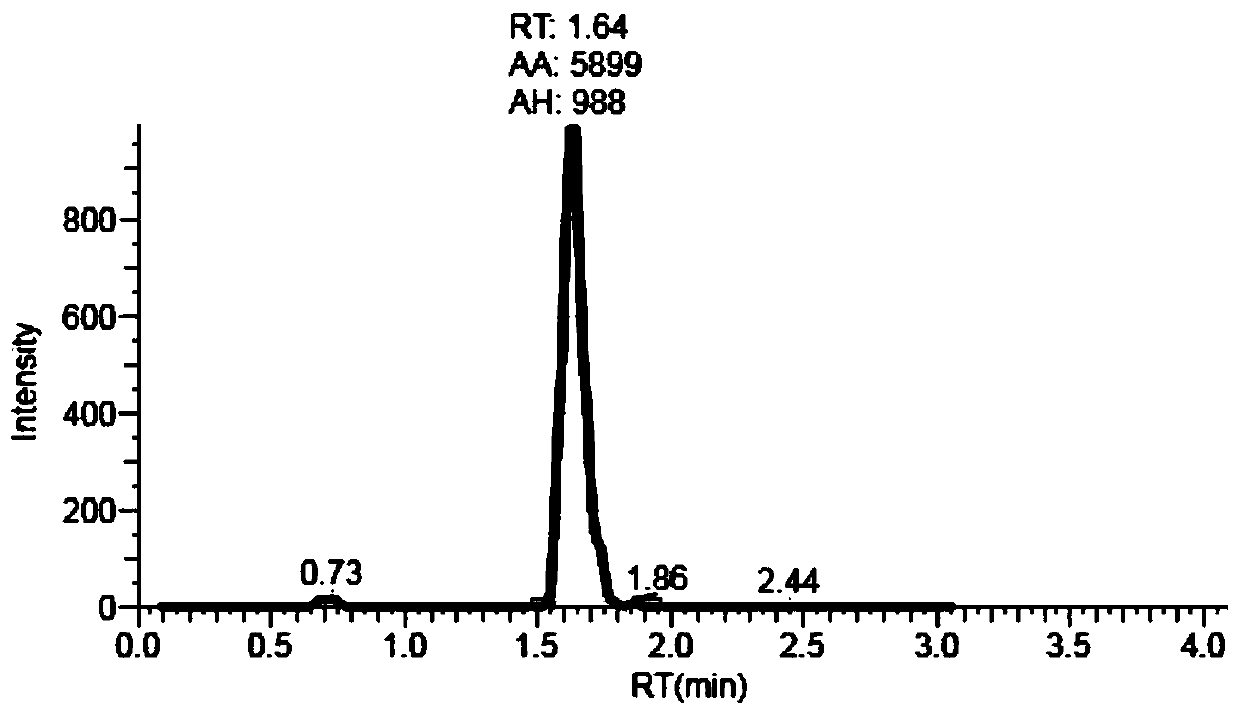
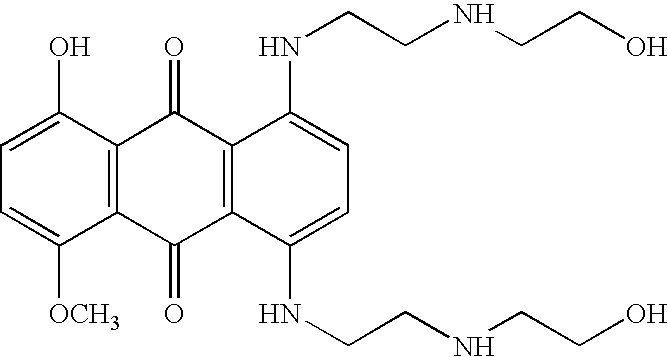
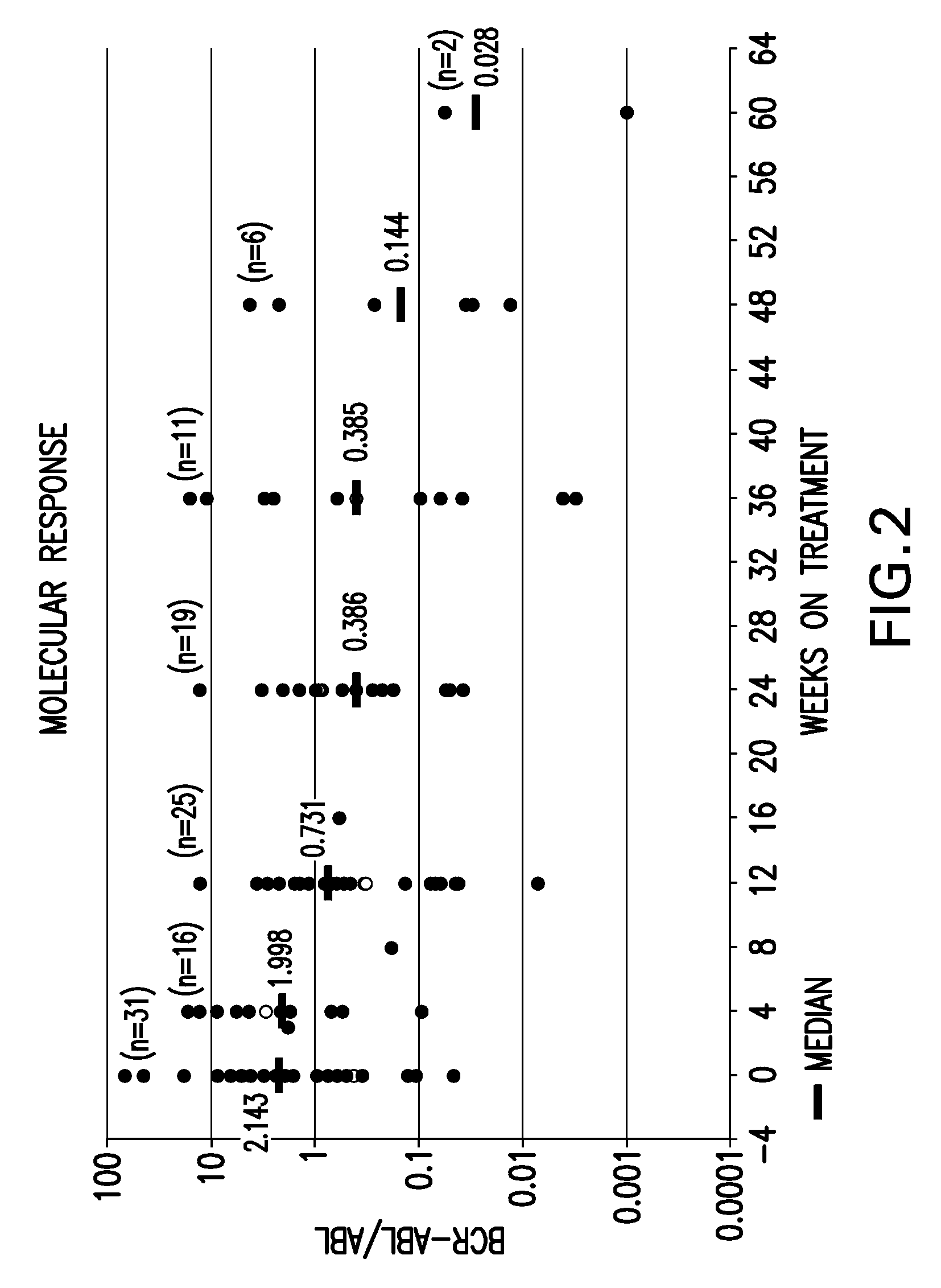
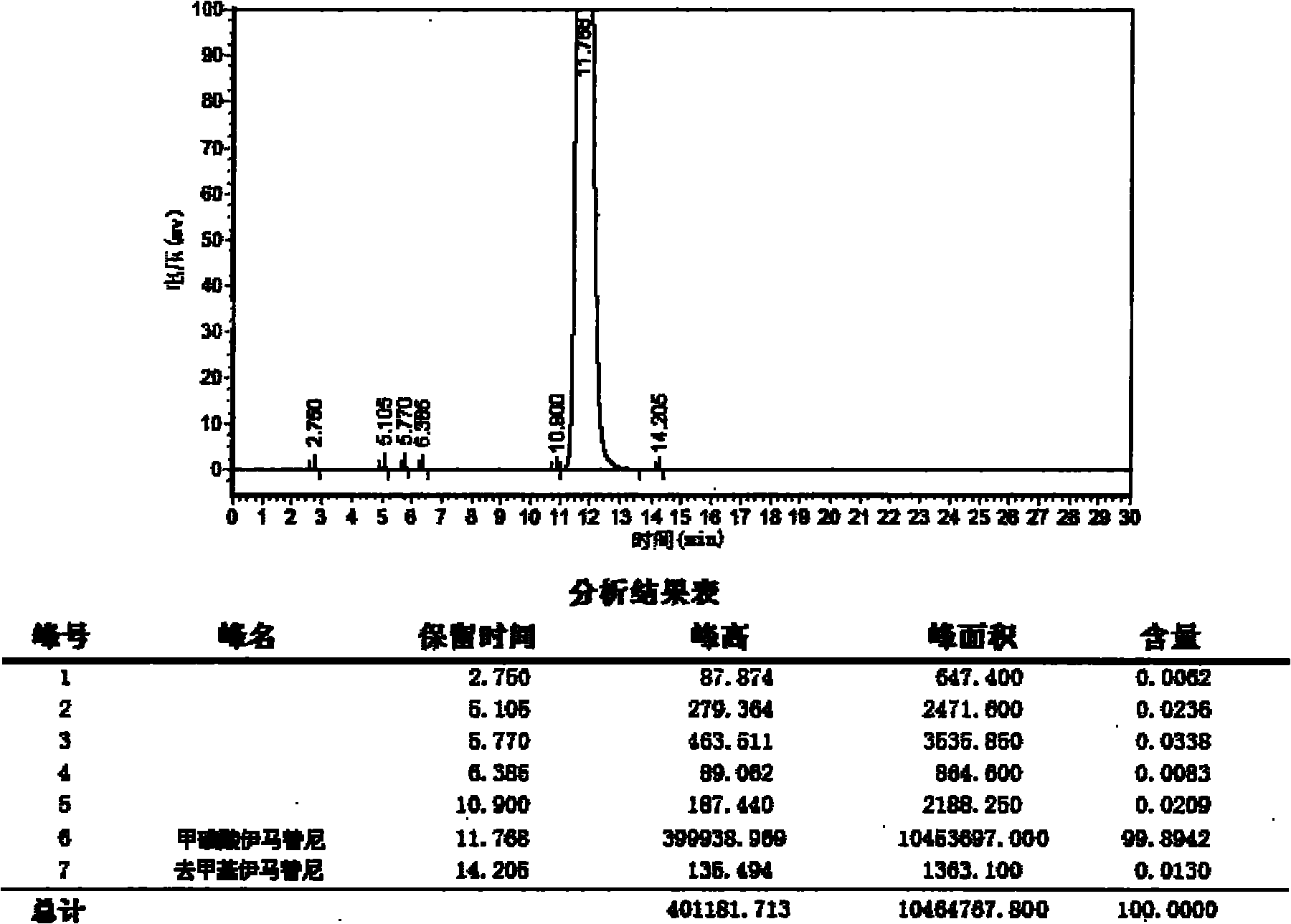
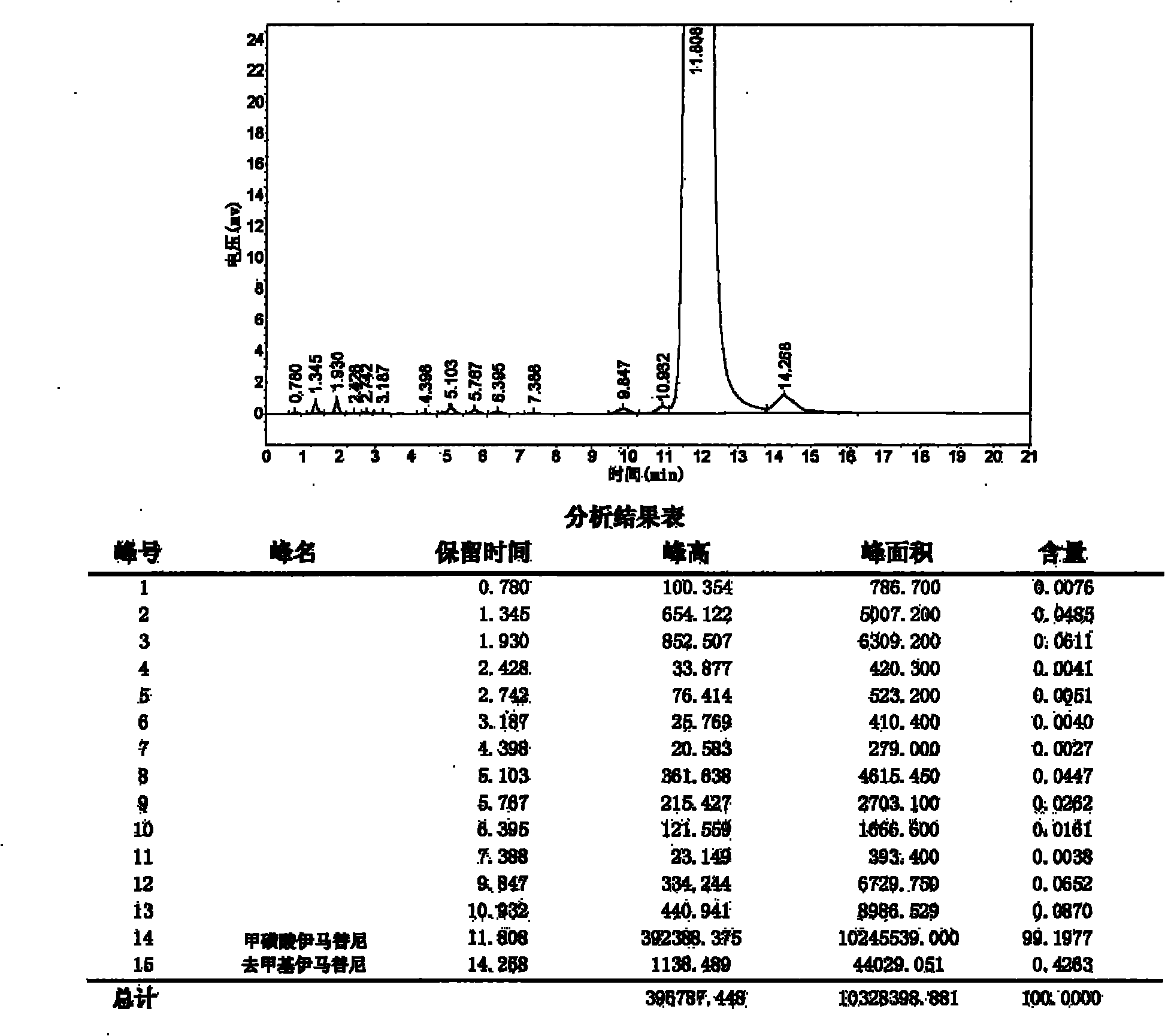
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
258 results about "Imatinib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat certain types of cancer (such as acute lymphoblastic leukemia, chronic myeloid leukemia, gastrointestinal stromal tumors, and myelodysplastic/myeloproliferative diseases).
Method for judging sensibility to imatinib
InactiveUS20060246436A1Medical cost incurredWaste of medical incurredMicrobiological testing/measurementRecombinant DNA-technologyImatinib treatmentImatinib
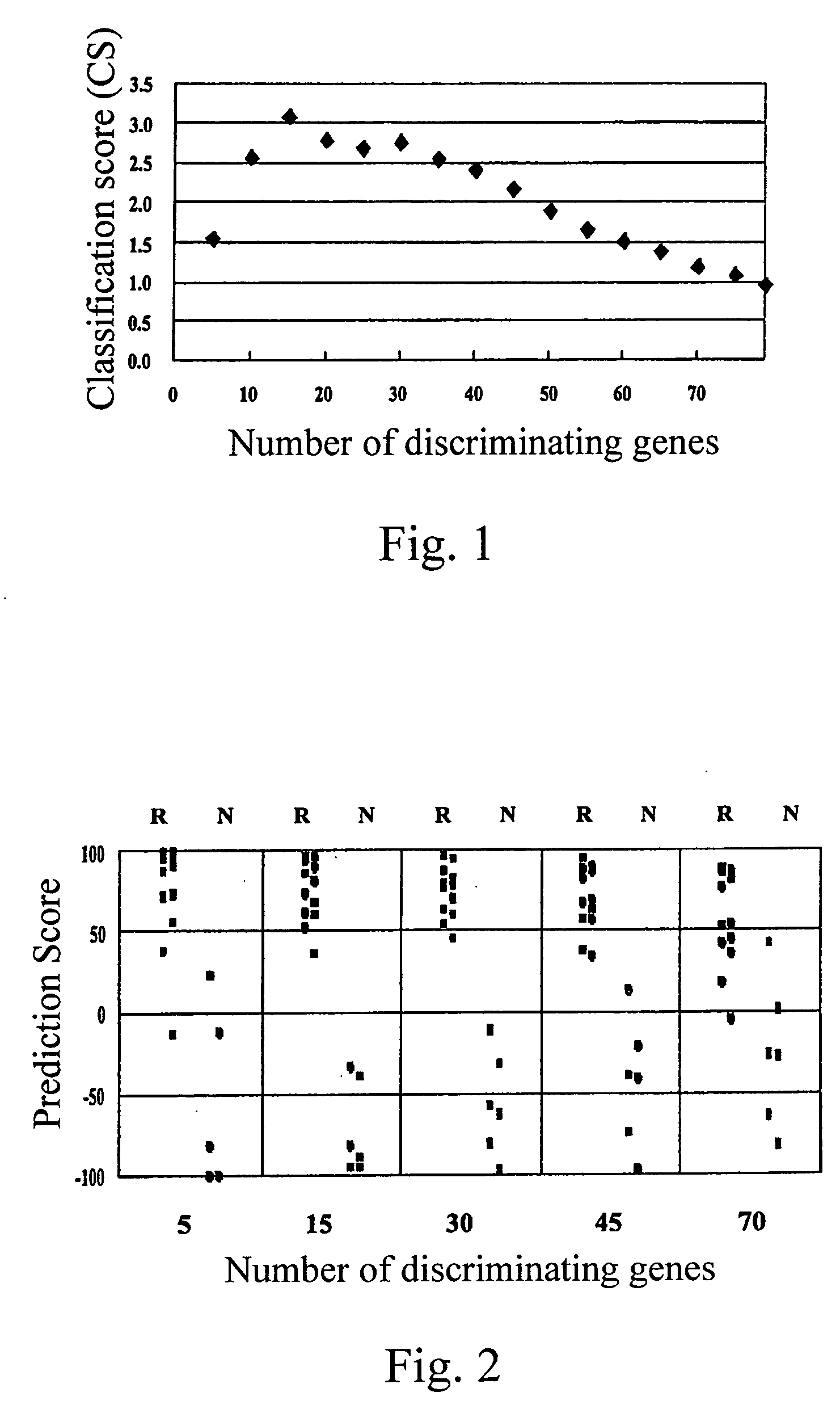
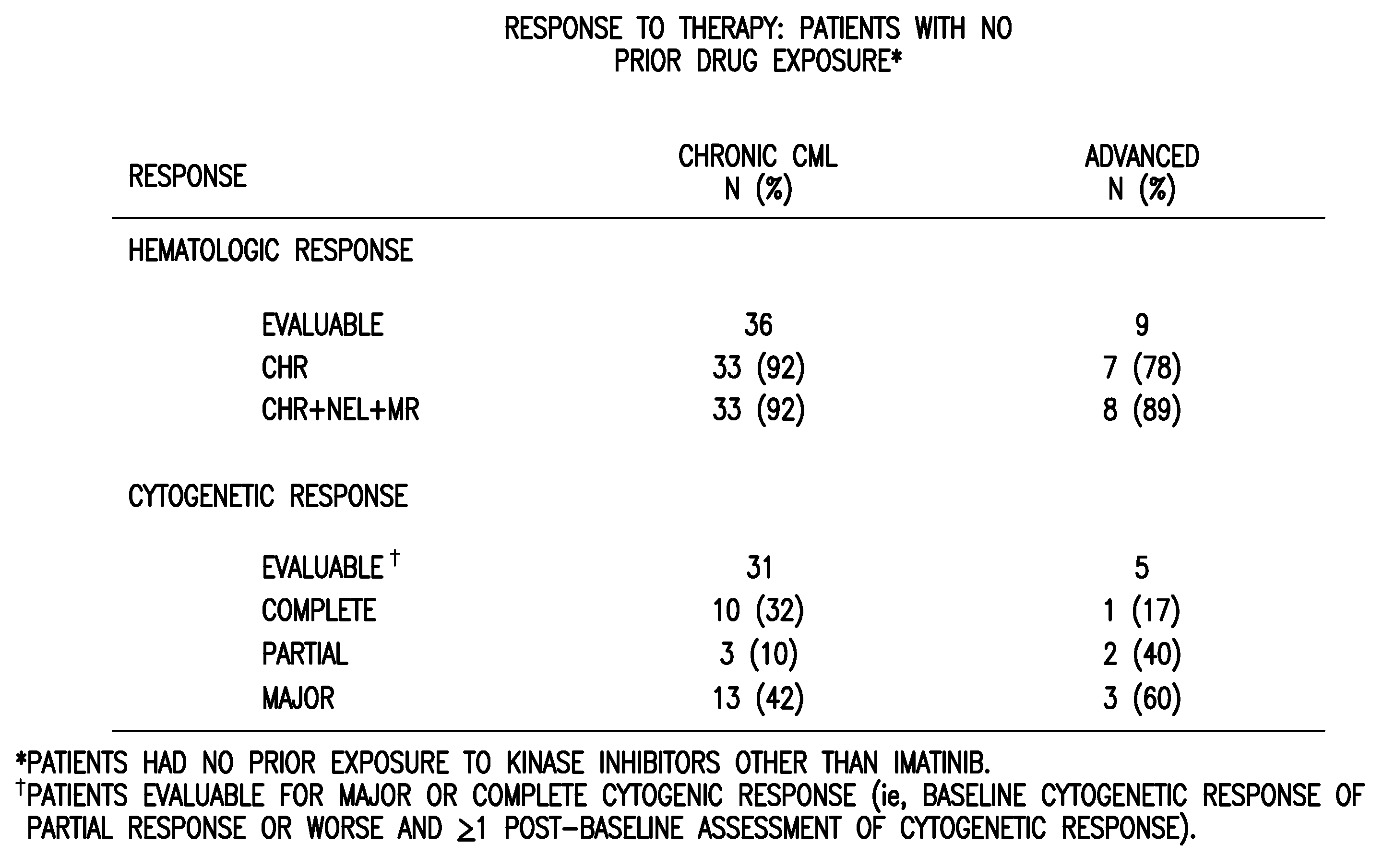
A method of judging whether a patient is sensitive to imatinib or not, in case where the patient is suffering from a disease such as CML to be treated by administration of imatinib, that is, a method for judging whether the administration of imatinib is effective for the therapy of the disease or not, is disclosed. Amounts of a plurality of genes selected from the group consisting of the specific 77 genes in sample cells separated from body are measured; and the measured amounts are compared with those of responders and non-responders to imatinib or a derivative thereof or a pharmaceutically acceptable salt thereof:
Owner:THE UNIV OF TOKYO
Mutations in KIT confer imatinib resistance in gastrointestinal stromal tumors
InactiveUS20060019280A1Easy to determineGood choiceSugar derivativesMicrobiological testing/measurementNucleotideImatinib
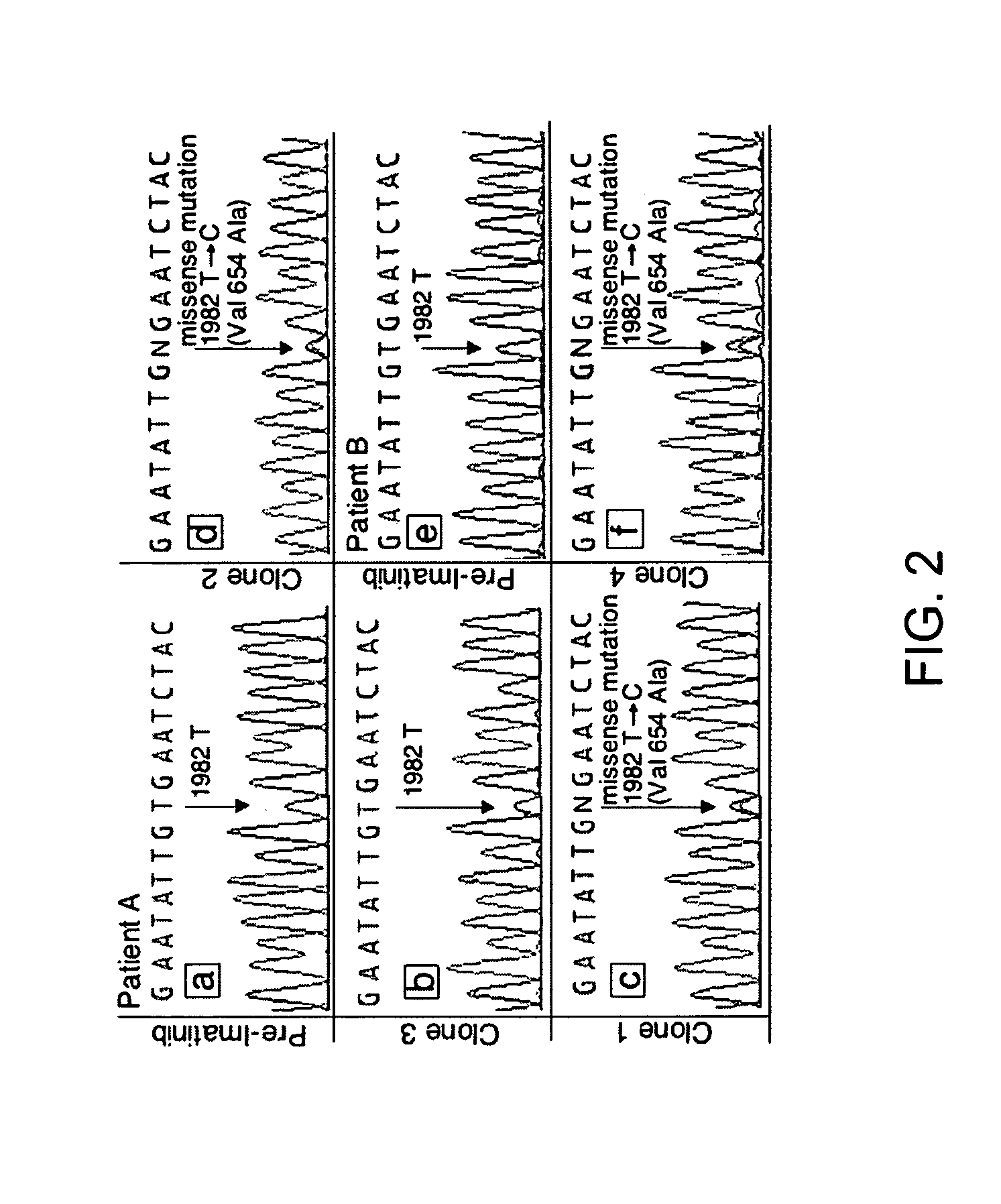
The present invention relates to methods and compositions concerning resistance to a drug for cancer comprising aberrant KIT signal, such as aberrant KIT sequence or expression. In a specific embodiment, the cancer is also initially responsive to imatinib therapy, such as in gastrointestinal stromal tumors (GISTs). In particular embodiments, a mutation in a KIT polynucleotide confers resistance to imatinib treatment, and in specific embodiments the exemplary mutation is at 1982T→C. Thus, the invention provides a means to adjust for or circumvent the resistance to imatinib drug treatment.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Pharmaceutical Compounds
InactiveUS20100004232A1Reduce morbidityPatient compliance is goodBiocideSenses disorderDiseaseImatinib resistant
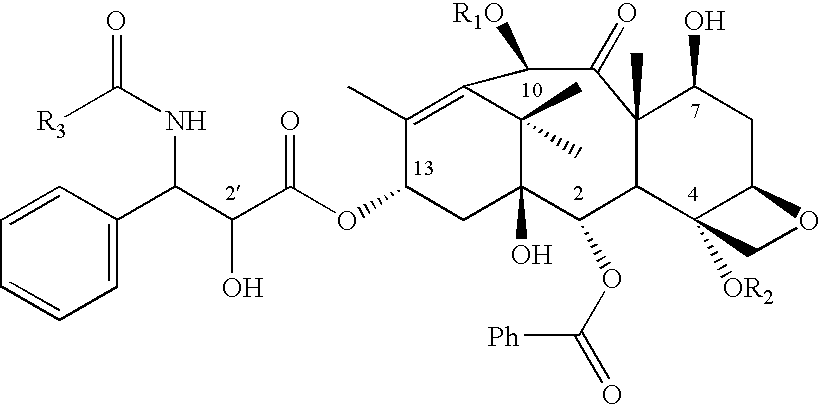
The use of a compound for the manufacture of a medicament for the prophylaxis or treatment of: A. a disease state or condition mediated by a kinase which is BCR-abl, VEGFR, PDGFR, EGFR, Flt3, JAK (e.g. JAK2 or JAK3), C-abl, PDK1, Chk (e.g. Cbk1 or Chk2), FGFR (e.g. FGFR3), Ret, Eph (e.g. EphB2 or EphB4), or Src (e.g. cSrc); or B. a cancer in which the cancer cells thereof contain a drug resistant kinase mutation which is: (a) a threonine gatekeeper mutation; or (b) a drug-resistant gatekeeper mutation; or (c) an imatinib resistant mutation; or (d) a nilotinib resistant mutation; or (e) a dasatinib resistant mutation; or (f) a T670I mutation in KIT; or (g) a T674I mutation in PDGFR; or (h) T790M mutation in EGFR; or (i) a T315I mutation in abl; or C. a cancer which expresses a mutated molecular target which is a mutated form of BCRabl, c-kit, PDGF, EGF receptor or ErbB2; or D. a disease mediated by a kinase containing a mutation in a region of the protein that binds to or interacts with other cancer agents but does not bind to or interact with the compounds of formula (I) or (I′), for example a mutated kinase selected from c-abl, c-kit, PDGFR including PDGFR-beta and PDGFR-alpha, and ErbB family members such as EGFR (ErbB1), HER2 (ErbB2), ErbB3, and ErbB4, members of the Ephrin receptor family including EphA1, EphA2, EphA3, EphA4, EphA5, EphA8, EphA10, EphB1, EphB2, EphB3, EphB5, EphB6, c-Src and kinases of the JAK family such as TYK2; wherein the compound is a compound of the formula (I or I′): or a salt, solvate, tautomer or N-oxide thereof wherein R0′, R1, R1′, R2′, R3′, R4′, A′, X′, E, A and M are as defined in the claims.
Owner:ASTEX THERAPEUTICS LTD
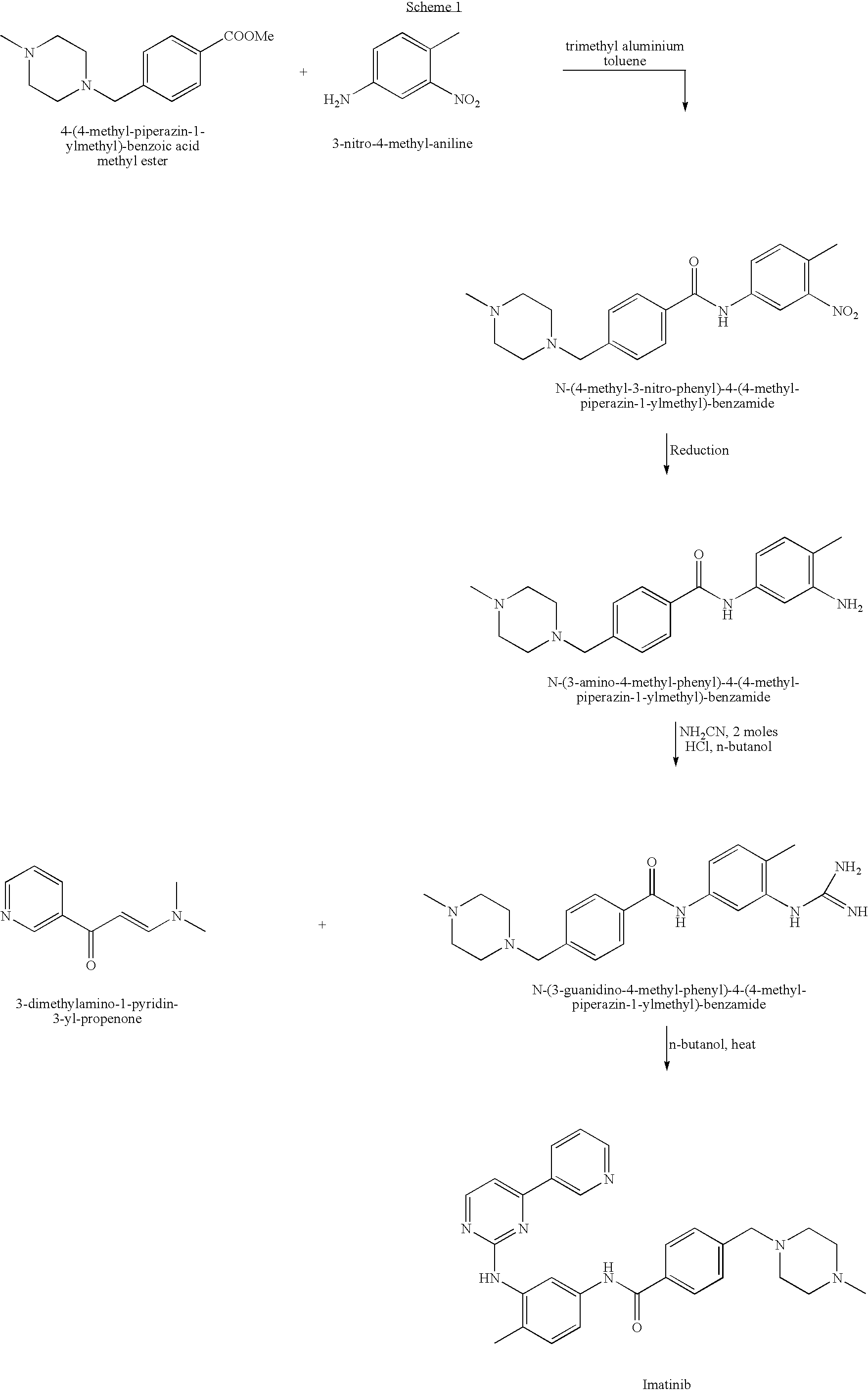
Process for synthesizing imatinib
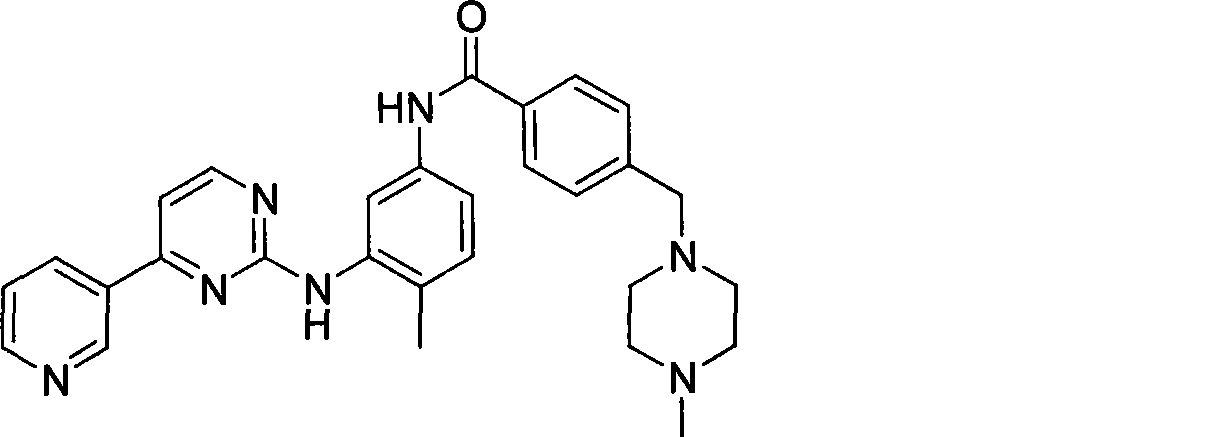
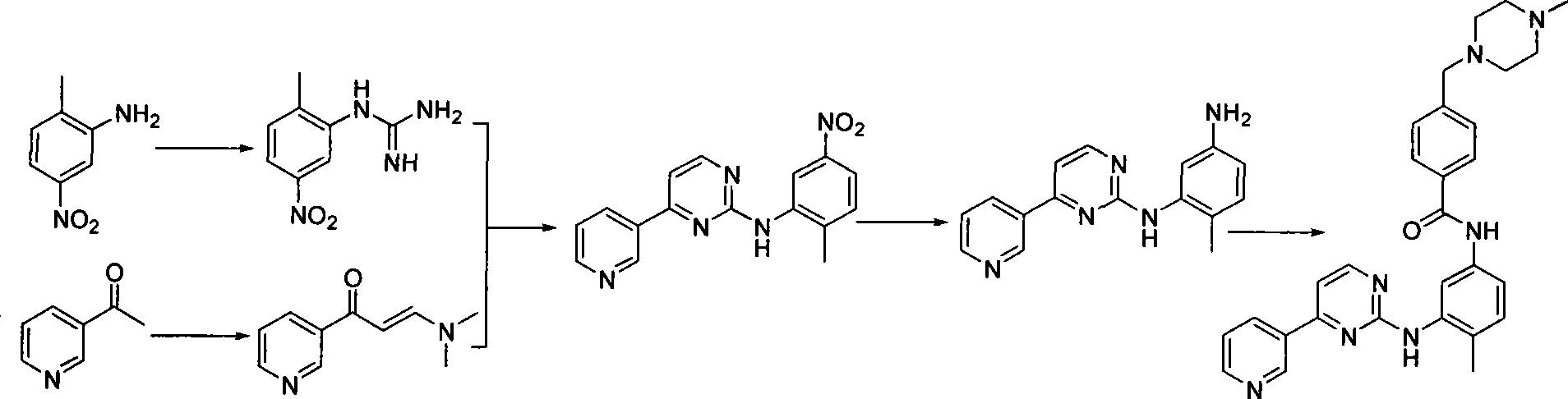
The invention discloses a method for synthesizing imatinib. The method comprises that: N-(4-methyl-3-3-aminophenyl)-4-(4-methyl-piperazinyl-1-methyl)-benzamide is used as a raw material, and reacts with 4-methyl-(3-pyridyl)-2-pyrimidone under actions of a polypeptide condensation agent and an organic alkali so as to generate the imatinib. The method has the advantages of mild reaction conditions, easy operation, high reaction yield and suitability for industrialized production.
Owner:FUJIAN SOUTH PHARMA CO LTD
Medicinal composition for treating non-small cell lung cancer and application thereof
InactiveCN103948689AReduce dosageLow toxicityAntineoplastic agentsHeavy metal compound active ingredientsSalvia miltiorrhizaCarboplatin
The invention discloses a medicinal composition for treating non-small cell lung cancer. The medicinal composition comprises a target medicament, a chemotherapeutic medicament and a traditional Chinese medicament, wherein the target medicament is one or more of bortezomib, imatinib, gefitinib and sunitinib; the chemotherapeutic medicament is one or more of 5-fluorouracil, carboplatin, epirubicin, adriamycin and fludarabine; and the traditional Chinese medicament is one or more of salvia miltiorrhiza, astragalus membranaceus, sappanwood, Chinese pulsatilla root and portulaca oleracea. The medicinal composition has a remarkable synergetic treatment effect when being used for treating the non-small cell lung cancer, and can be used for remarkably strengthening the cancer-inhibition effect compared with treatment of a single medicament, so that the medicament dosage can be reduced, and the toxic and side effects of chemotherapeutic medicaments can be reduced.
Owner:NORTHWEST A & F UNIV
Method for synthesizing Imatinib
Owner:TIANJIN WEIJIE TECH
Method for synthesizing Imatinib
ActiveCN101735196AThe aminolysis reaction is clean and completeHigh yieldOrganic chemistryAntineoplastic agentsImatinibCombinatorial chemistry
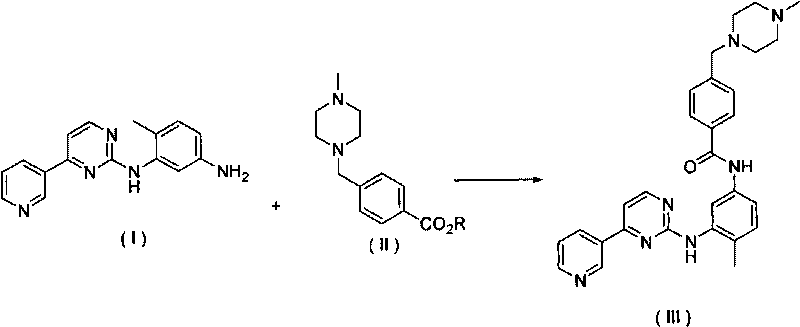
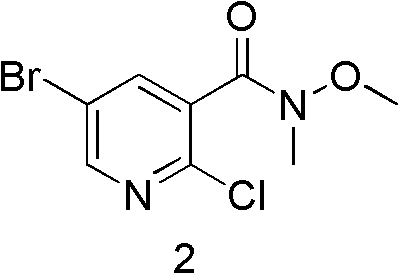
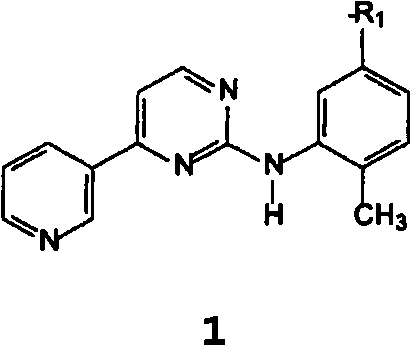
The invention discloses a method for synthesizing Imatinib, comprising the following steps: under the action of alkali, 4-methyl-N-3-(4-pyridine-3-radix-pyrimidine-2-radix)-1,3-phenylenediamine shown in the formula (I) reacts with 4-(4-methylpiperazine-1-methyl)-benzoate shown in the formula (II) in an aprotic inorganic solvent to form the Imatinib shown in the formula (III), namely 4-(4-methylpiperazine-1-methyl)-N-[4-methyl-3-[4-(3-pyridyl) pyrimidine-2-amino]-benzamide. In the above chemical structure general formula, R represents fatty alkyl, phenyl, substituted phenyl, benzyl or substituted benzyl containing 1-10 carbon atoms. The invention provides the new method for synthesizing Imatinib, which has mild reaction conditions and high yield and is environment-friendly.
Owner:FUJIAN SOUTH PHARMA CO LTD
Pyrazolopyridines alkynylbenzene compound and medicinal composition and application
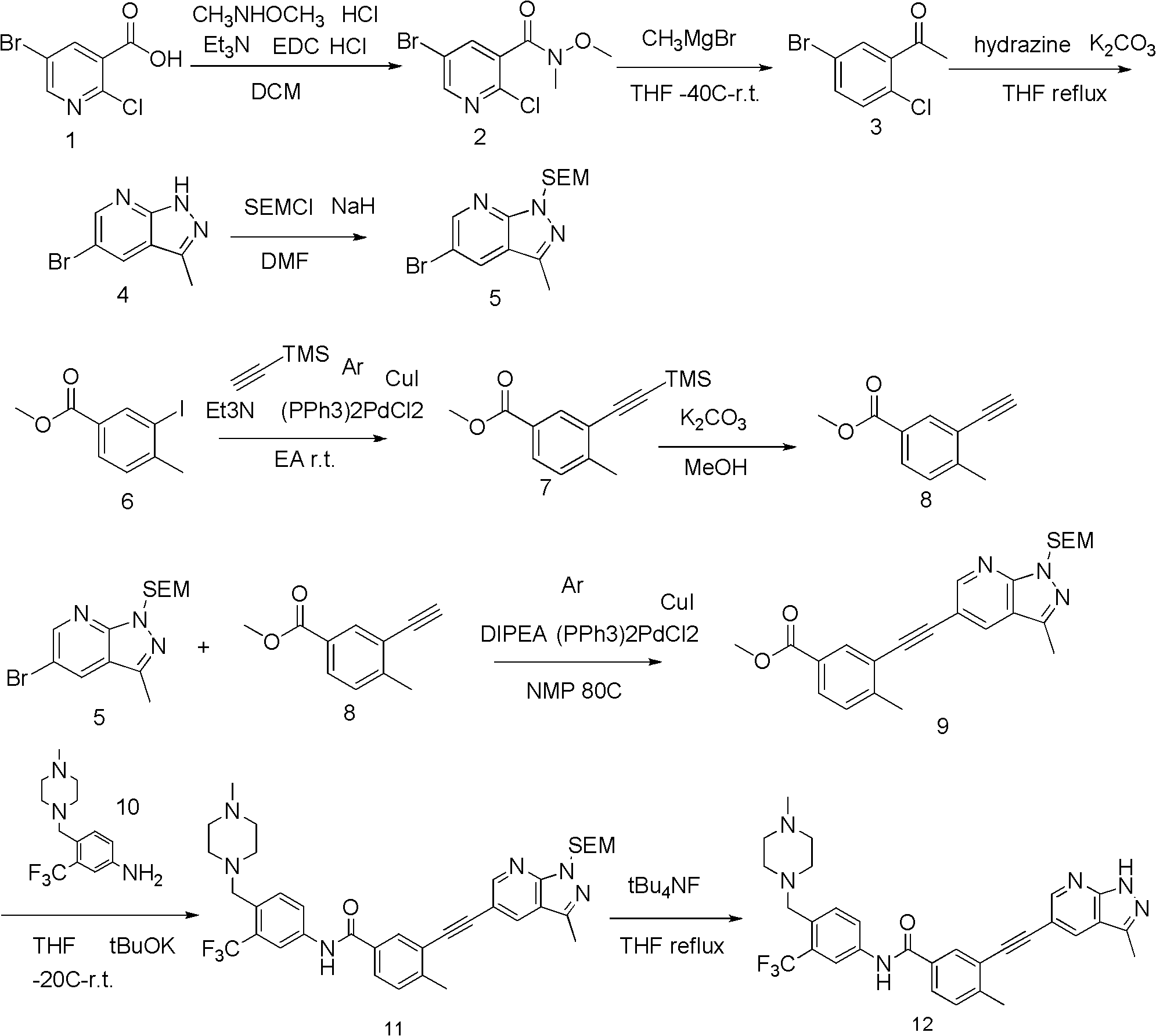
ActiveCN103214480AGrowth inhibitionOvercome drug resistanceOrganic active ingredientsOrganic chemistryImatinibPyrazole
The invention discloses a pyrazolopyridines alkynylbenzene compound having a structural characteristic shown in a formula (I) or its pharmaceutically acceptable salt or a stereisomer or prodrug molecules, and an application of the compound and its pharmaceutically acceptable salt or the stereisomer in preparation of medicines for treating or preventing tumor. Compared with a clinical used antitumor drug (imatinib), the compound has obvious advantage for resisting the activity of a plurality of tumor-derived type and drug resistance type cells, and the compound has the characteristics of good pharmacokinetics and low toxicity. The definitions of groups in the formula are disclosed in the specification.
Owner:ASCENTAGE PHARMA SUZHOU CO LTD
Process for the preparation of imatinib and intermediates thereof
Tthe present invention provides a process for the preparation of 4-methyl-N3- [4- (3-pyridinyl) -2- pyrimidinyl] -1, 3-benzenediamine and analogues thereof, intermediates useful for the synthesis of Imatinib, or 4- [ (4-methyl-l-piperazinyl) methyl] -N- [4-methyl-3- [ [4- (3- pyridinyl) -2-pyrimidinyl] amino] phenyl] benzamide.
Owner:F I S FAB ILTALIANA SINTETICI SPA
Use of imatinib to treat liver disorders and viral infections
The present invention relates to the use of imatinib for treating viral liver diseases and in particular for viral hepatitis. The invention provides the use of imatinib for inhibiting replication, transmission or both of hepatitis viruses. The invention further relates to the use of imatinib for inhibiting replication, transmission or both of other viruses including herpes virus, poxvirus, influenza virus, para influenza virus, respiratory syncytial virus, rhinovirus, yellow fever virus, west nile virus, and encephalitis virus.
Owner:BIONICHE LIFE SCI
Method for preparing imatinib
ActiveCN101921260AThe reaction steps are simpleShorten the production cycleOrganic chemistryBenzoic acidN dimethylformamide
The invention relates to a method for preparing imatinib, which comprises the following steps of: with a compound N-(5-Amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidineamine shown as the structural formula (II) and a compound 4-[(4-Methylpiperazin-l-yl)methyl]benzoic acid shown as the structural formula (III) as initial raw materials, dropwise adding phosphite ester at 50-90 DEG C for 1-2 hours inthe presence of a catalyst in an organic solvent; and continuously insulating and reacting at 50-90 DEG C to obtain the compound imatinib shown as the structural formula (I). The organic solvent is N,N-dimethylformamide, N,N-dimethylacetylamide or N-methylpyrrolidone. The catalyst is pyridine; and the phosphite ester is trimethyl phosphate, triethyl phosphate or triphenyl phosphate. The technical scheme of the invention has the advantages of simple reaction step, easy control of reaction, short production cycle, low toxicity of used raw materials, less pollution to the environment and higher product quality, and the yield can reach 95 percent, and the purity reaches 99.5 percent.
Owner:山东金城昆仑药业有限公司
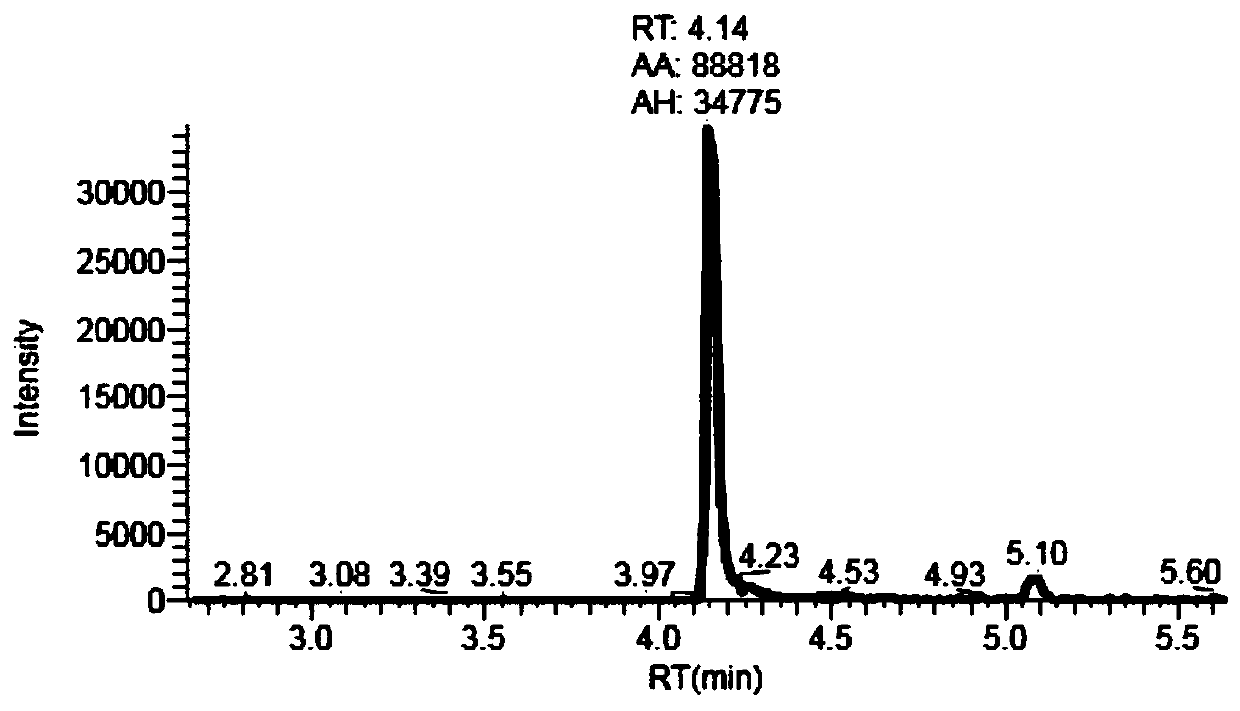
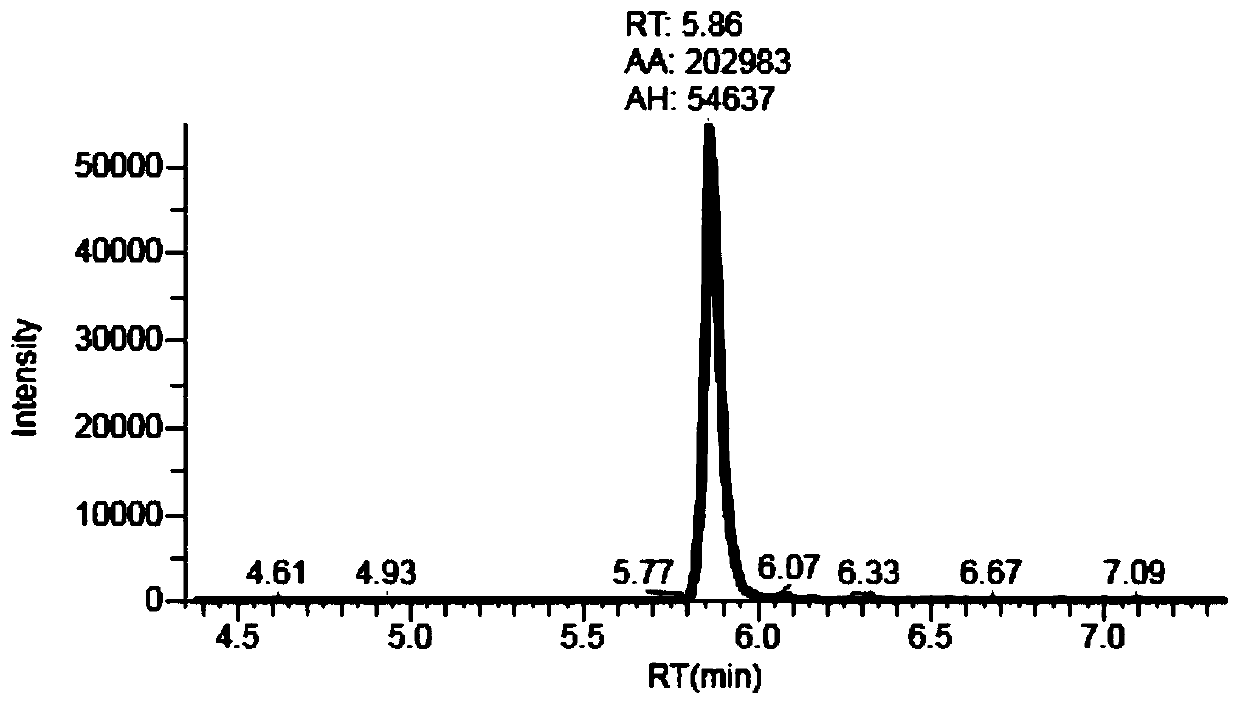
Method for simultaneously detecting multiple anti-tumor drugs in blood sample
InactiveCN110927297AEasy to handleImprove throughputComponent separationBusulfanTandem mass spectrometry
The invention discloses a method for simultaneously detecting multiple anti-tumor drugs in a blood sample. A pretreated sample to be detected is detected by adopting ultra-high performance liquid chromatography-tandem mass spectrometry (HPLC-MS / MS). The pretreatment process comprises the following steps: adding serum into a mixed solution of methanol and acetonitrile, oscillating and centrifuging,taking out the centrifuged supernatant, drying, dissolving the dried powder into a methanol aqueous solution, and filtering to obtain a sample to be detected. The method can be used for simultaneously detecting 13 kinds of anti-tumor drugs such as methotrexate, 5-fluorouracil, apatinib, busulfan, carboplatin, cyclophosphamide, docetaxel, gemcitabine, imatinib, illinotecan, lenalidomide, oxaliplatin, paclitaxel and the like in blood.
Owner:JINAN YING SHENG BIOTECH
Process for preparing Imatinib
Owner:CHEMAGIS
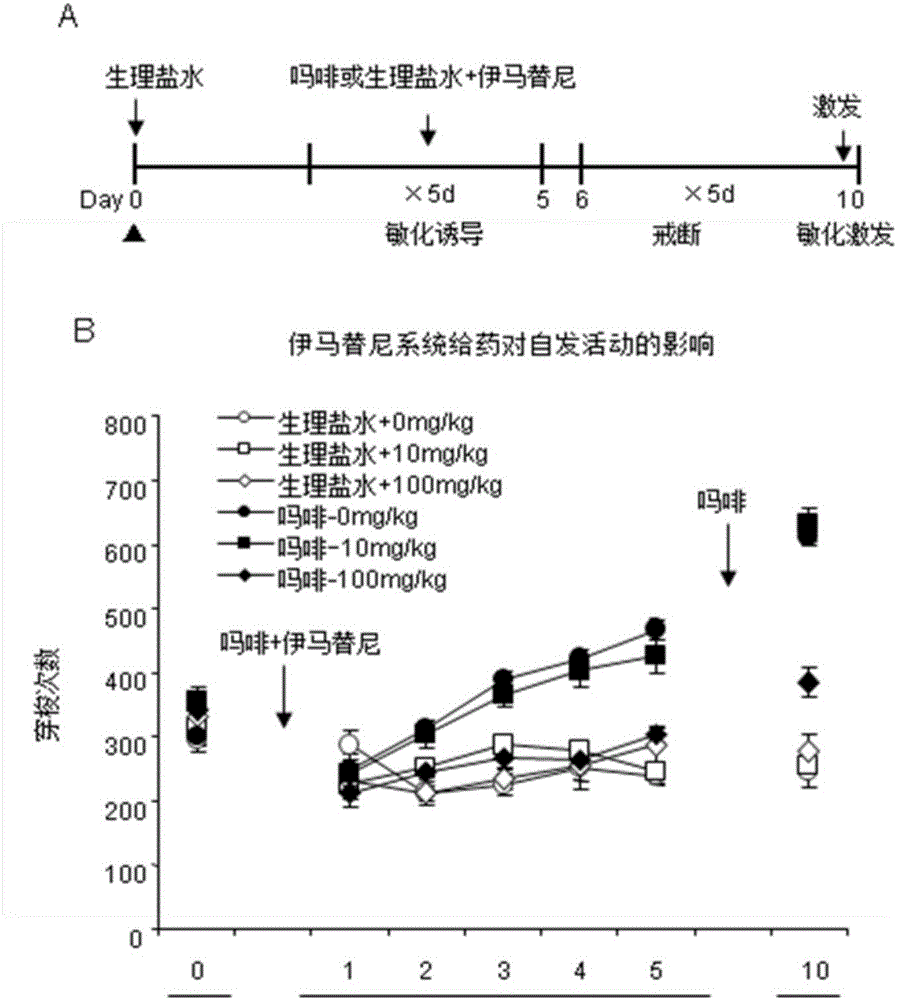
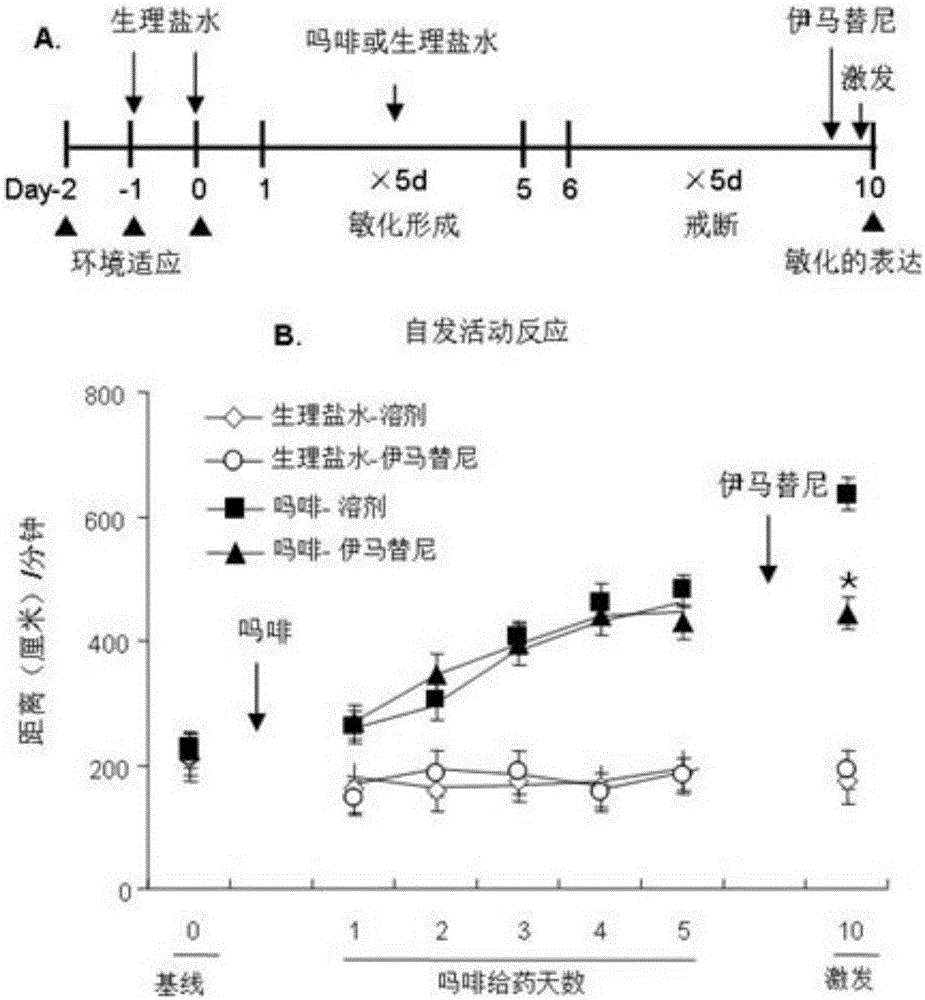
Novel application of imatinib and derivative thereof to preparation of drugs for treating drug addiction
ActiveCN106074555ALow costImprove bioavailabilityOrganic active ingredientsNervous disorderDrug withdrawalTherapy drug addiction
The invention discloses novel application of imatinib and a derivative thereof and provides novel application of imatinib and the derivative thereof to preparation of drugs for preventing and / or treating drug addiction and preparation of drugs for preventing and / or treating relapse after drug withdrawal. According to the novel application, a classic rat sensitization and conditioned place preference animal model for evaluating addiction is adopted, the influences of imatinib and the derivative methanesulfonic acid imatinib on expression of the recrudescence behavior and sensitization behavior appearing after withdrawal of the conditioned place preference of a rat is formed are observed respectively, and the result indicates that imatinib and the derivative methanesulfonic acid imatinib have an inhibitory effect on morphine addiction and have the effect of preventing relapse after withdrawal of morphine addiction.
Owner:安徽安迪克莱斯医药有限责任公司
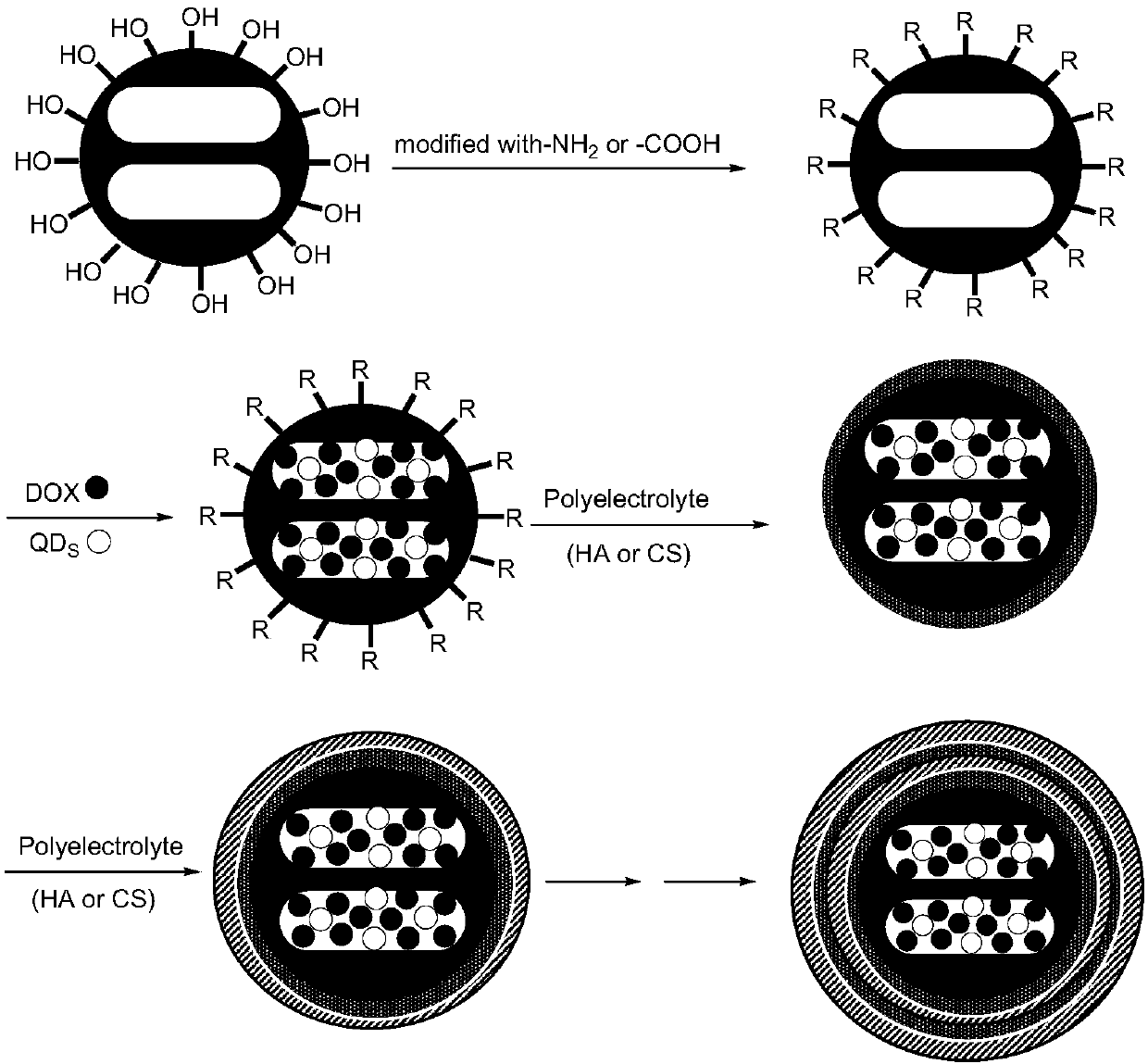
Preparation method and application of multifunctional membrane-controlled targeting nano-carrier integrating tracing and targeted drug delivery effects
ActiveCN107865972ARich in mesoporesRich surfaceOrganic active ingredientsPowder deliveryFluorescenceImatinib
The invention belongs to the technical fields of preparation and applications of nano-carriers, and in particular discloses a preparation method and an application of a multifunctional membrane-controlled targeting nano-carrier integrating tracing and targeted drug delivery effects. With nano mesoporous silica (MSN) as a drug 'warehouse', a positively charged high molecular material and a negatively charged high molecular material as preparation materials of a gating membrane, and adriamycin (DOX), cis-platinum, imatinib, taxol and the like as model anti-cancer drugs, research contents mainlyinclude optimization and improvement of natural materials, construction and process optimization of the gating membrane, structural characterization of a nano complex, drug release kinetic characteristics of drug molecules under the control of the gating membrane, and the like. Meanwhile, in the combination with the tracing imaging function of a fluorescent quantum dot, drug delivery behaviors andanti-tumor effectiveness of the membrane-controlled nano drug delivery system undergo preliminary evaluation through in-vitro experiments. Based upon research results, references are provided for thedesign and preparation of the novel membrane-controlled nano drug delivery system.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Preparation method of high-purity imatinib
The invention relates to a preparation method of high-purity imatinib, which comprises the following steps: taking 4-[(4-methyl-1-piperazine) methyl] benzoyl chloride dihydrochloride and N-(5-amino-2-methyl phenyl)-4-(3-pyridine)-2-pyrilamine as raw materials, taking acetonitrile or pyridine as a solvent, and adding triethylamine as an acid-binding agent when the acetonitrile is taken as the solvent to react at the reaction temperature of -5-50 DEG C; removing the solvent in the reaction liquid to recycle the solvent; adding water into the residues; extracting impurities by ethyl acetate; separating the organic phase from the water phase; then neutralizing the water phase by ammonia water until the PH is 9-10; and adding saturated fatty alcohol to precipitate imatinib crystals. Because the purity of the product obtained by the preparation method of the invention is higher than 99.5%, the single impurity is less than 0.10%, and the solvent has low dosage and can be recycled, the production cost can be greatly reduced, and the pollution on the environment can be reduced. Moreover, the method has simple operation processes and mild reaction conditions, thus the method is suitable for industrialized production.
Owner:TIANJIN WEIJIE TECH
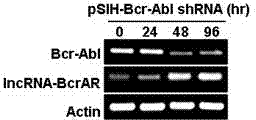
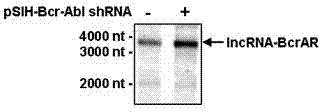
Long-chain non-coding RNA IncRNA-BcrAR and application thereof in cell canceration resistance
ActiveCN103789309APromote apoptosisGrowth inhibitionGenetic material ingredientsAntineoplastic agentsImatinibDrug target
The invention relates to a long-chain non-coding RNA IncRNA-BcrAR and application thereof in cell canceration resistance. IncRNA-BcrAR is in low-level expression in a human chronic myelogenous leukemia cell line K562 with positive Bcr-Abl, the K562 cell line with overexpressed IncRNA-BcrAR is constructed, the action of IncRNA-BcrAR on Bcr-Abl induced cell neoplastic transformation is observed, experiments prove that the IncRNA-BcrAR overexpression can obviously promote K562 cell apoptosis induced by Imatinib (therapeutic drug of Abl positive leukemia patients) and can remarkably inhibit tumor growth induced by K562 cells in a naked mouse body; and besides, the IncRNA-BcrAR overexpression can remarkably promote A-MuLV transformed mouse leukemia cell BC44 apoptosis induced by Imatinib. The IncRNA-BcrAR has important effect on Bcr-Abl and v-Abl mediated cell canceration resistance, and the long-chain non-coding RNA IncRNA-BcrAR provides new molecular marker and drug target for diagnosis and treatment of Abl induced leukemia.
Owner:FUJIAN AGRI & FORESTRY UNIV
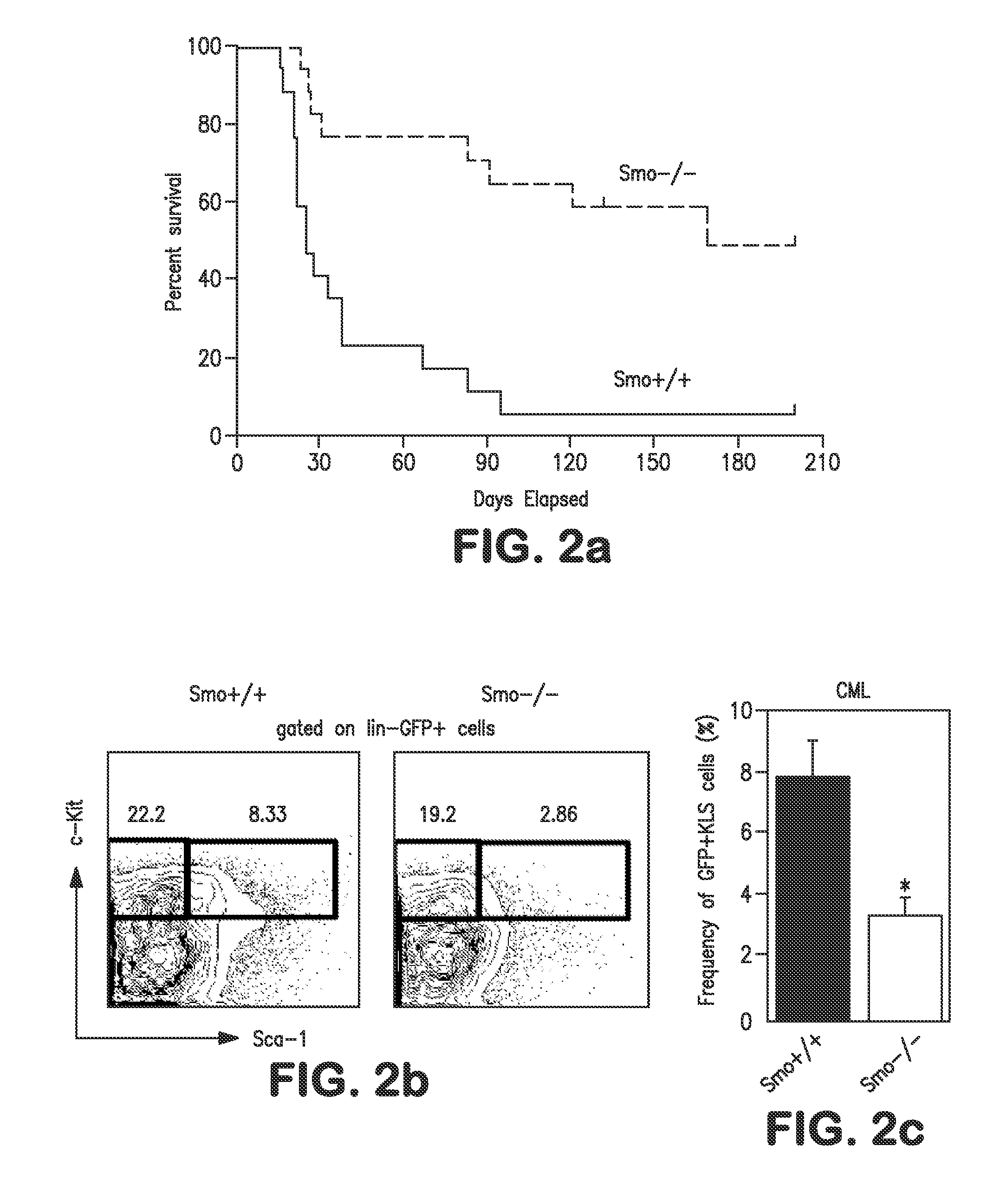
Hedgehog signaling and cancer stem cells in hematopoietic cell malignancies
InactiveUS20100080855A1Reduce in quantityBiocideInorganic active ingredientsHematopoietic cellImatinib
The present disclosure relates generally to methods of treating hematopoietic cell malignancy in a subject by administering to the subject a Hedgehog (Hh) pathway antagonist, such as a Smoothen (Smo) antagonist alone or in combination with an ABL-kinase antagonist. Specifically, the disclosure relates to a method of treating hematopoietic cell malignancy in a subject by administering the Hh pathway antagonist, such as the Smo antagonist cyclopamine, in combination with the ABL-kinase antagonist imatinib.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
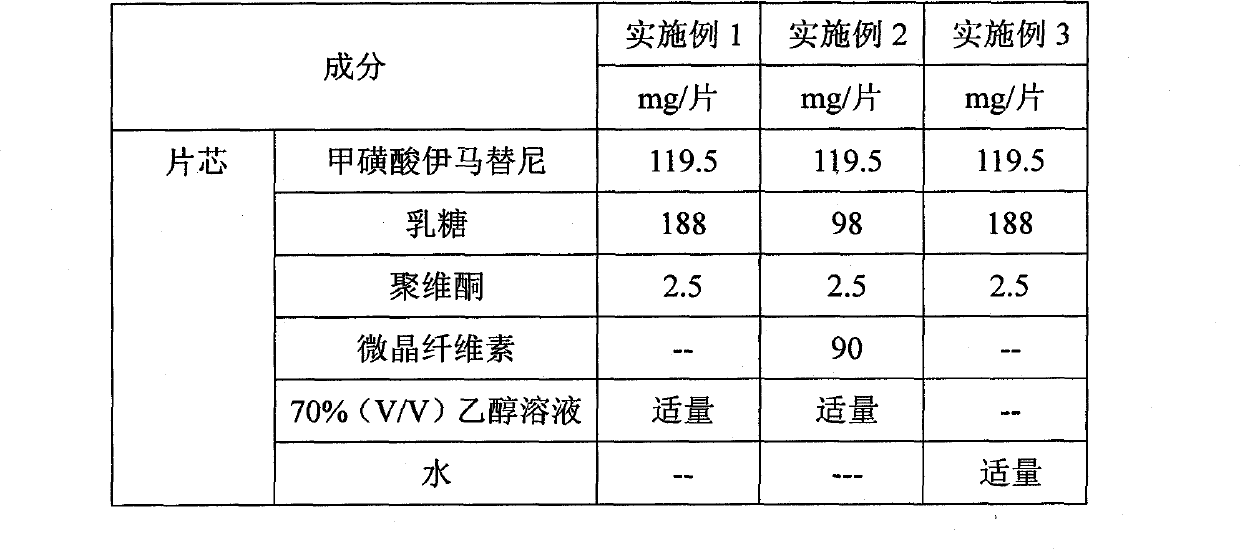
Preparation method of methylsulfonic acid imatinib tablet
The invention discloses a preparation method of methylsulfonic acid imatinib tablet. The invention takes organic solvent or organic solution with volume concentration bigger than 70% as pelletization solution, and the weight of water-insoluble filling agent in the tablet accounts for less than 20% of the total weight of the tablet. The invention has advantages of simple technical process, high yield and good dissolution of a product.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Application of c-Kit serving as drug addiction treatment target
ActiveCN105974131AEffective treatmentEffective excitabilityDisease diagnosisBiological testingSubstance dependenceAddiction treatment
The invention discloses new application of c-Kit, and provides application of the c-Kit serving as a drug target in screening of drugs for treating drug addiction and application of the c-Kit in screening of drugs for treating substance dependence addiction psychological craving and excitability of addiction drugs. A classical rat sensitization and conditioned place preference animal model for evaluating addiction is adopted for observing influences of an inhibitor imatinib of the c-Kit on renewed behaviors after formation and withdrawal of rat sensitization behavior expression and conditioned place preferences respectively, the inhibiting effect of imatinib on morphine addiction and an anti-relapse effect after withdrawal of morphine addiction is achieved are evaluated, and application of an acting target c-Kit receptor serving as the drug treatment target of drug addiction is determined. The c-Kit is good in effect and is expected to fundamentally treat drug addiction.
Owner:WUHAN UNIV
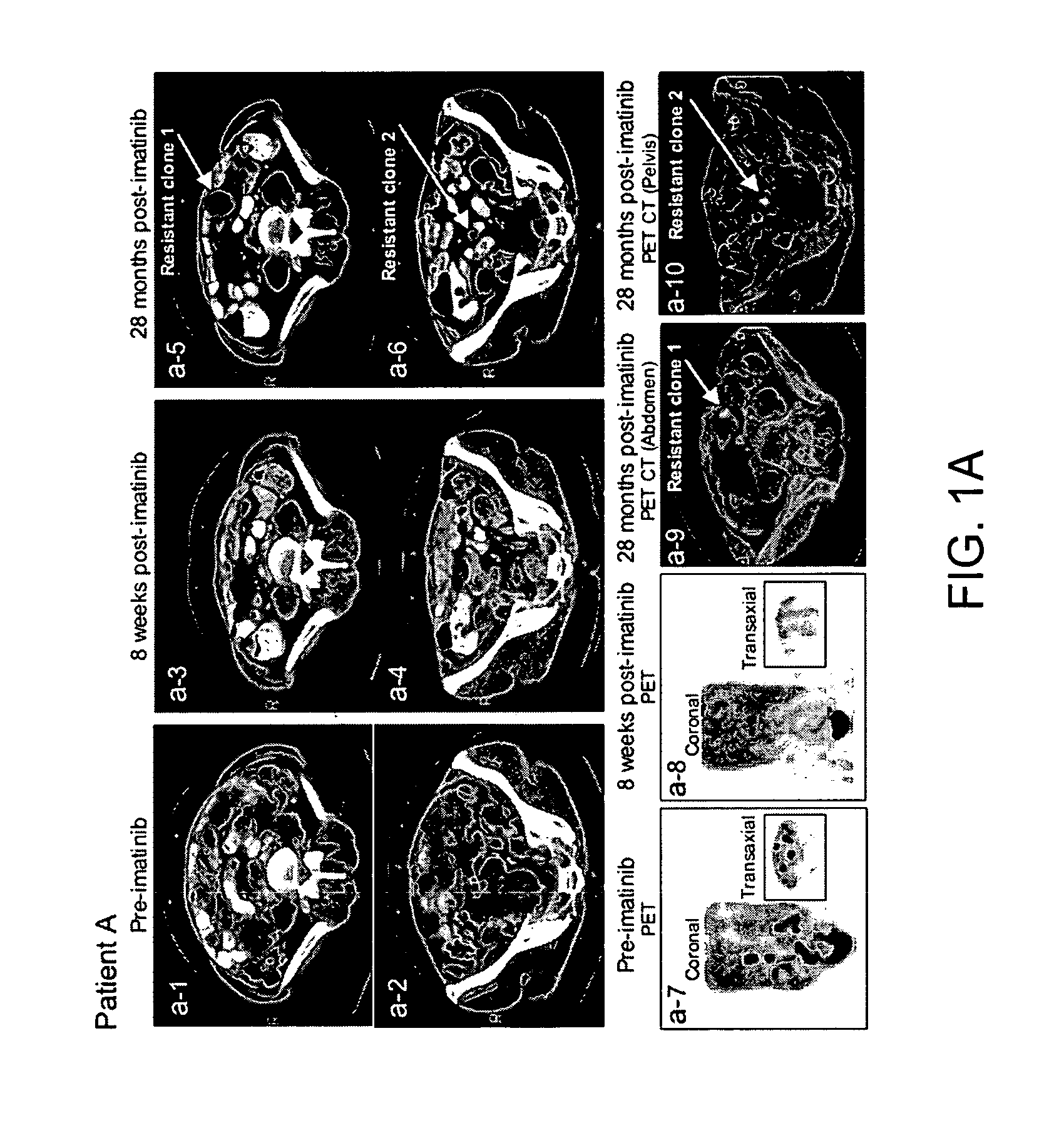
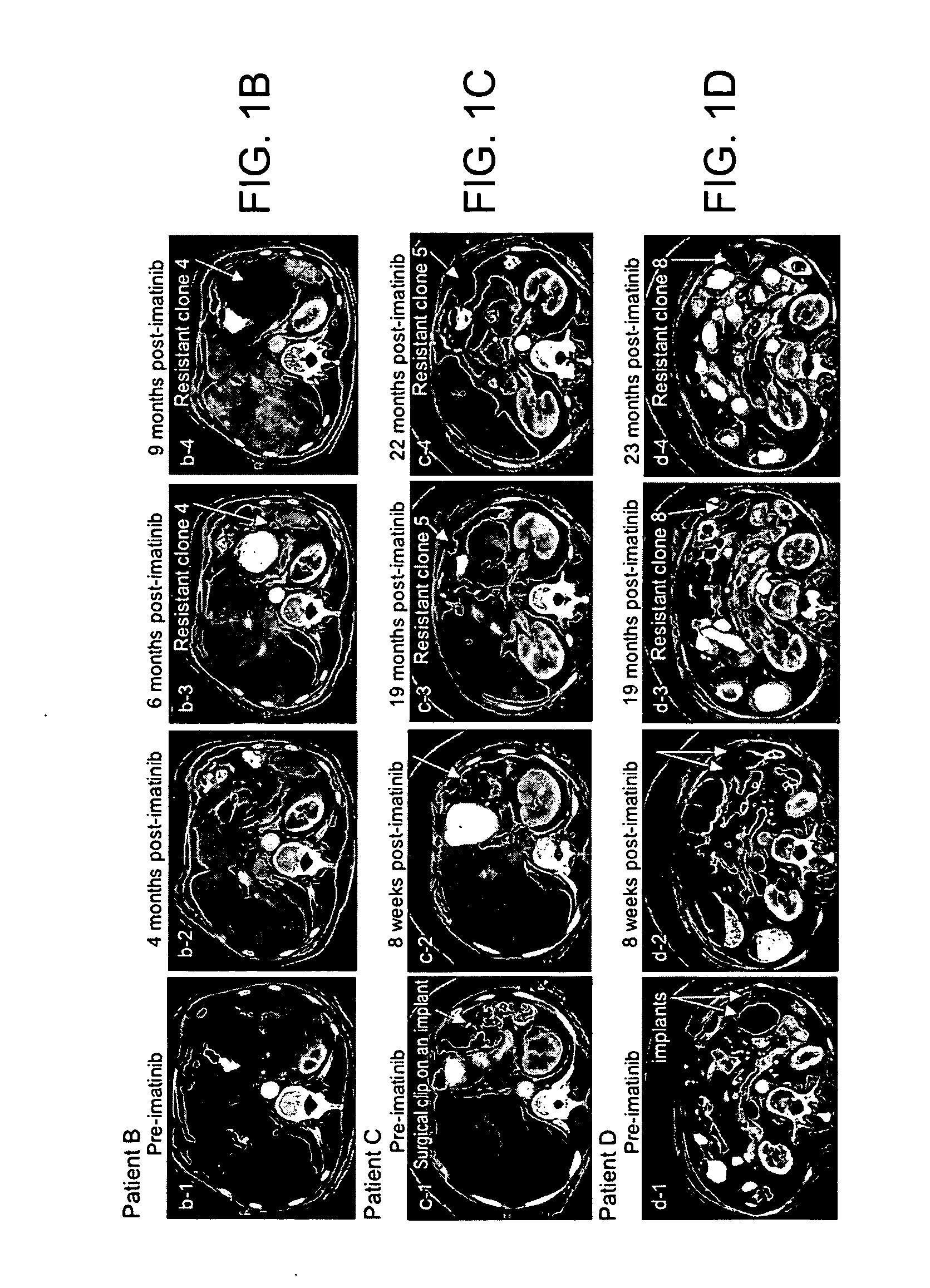
Imaging of drug accumulation as a guide to antitumor therapy
InactiveUS7175830B2Effective judgmentAvoid the needX-ray constrast preparationsRadioactive preparation carriersDocetaxel-PNPDocetaxel
The use of radio-labeled antitumor drugs in the treatment of solid tumors by the method of administering a radio-labeled anticancer drug to a patient and imaging at least a part of the patient using Positron Emission Tomography imaging is described. The method can be used to monitor delivery of antitumor drugs to tumors, to predict the effectiveness of therapy with a particular antitumor drug or combination of antitumor drugs, to assess the effectiveness of modulators of cellular accumulation, to individualize therapy and to evaluate the effectiveness of antitumor drugs with respect to particular cancers. Particularly preferred drugs are labeled taxanes, e.g., 11C-paclitaxel and 11C-docetaxel, labeled anthracyclines, e.g., 11C-doxorubicin and 11C-epirubicin, and other radiolabeled drugs, e.g. 11C-topotecan, 11C-SN-38, and 11C-imatinib. The invention further describes antitumor drugs labeled with the radioactive label 11C and methods of preparing radio-labeled drugs.
Owner:HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US REPRESENTED BY THE SEC
Imatinib compositions
Provided are compositions of imatinib, methods for their preparation, and methods for treatment using the same.
Owner:TEVA PHARMA IND LTD
Primer used for detecting c-kit gene mutation and application thereof
InactiveCN102061333AHigh reference significanceRaise the annealing temperatureMicrobiological testing/measurementDNA/RNA fragmentationTumor therapyA-DNA
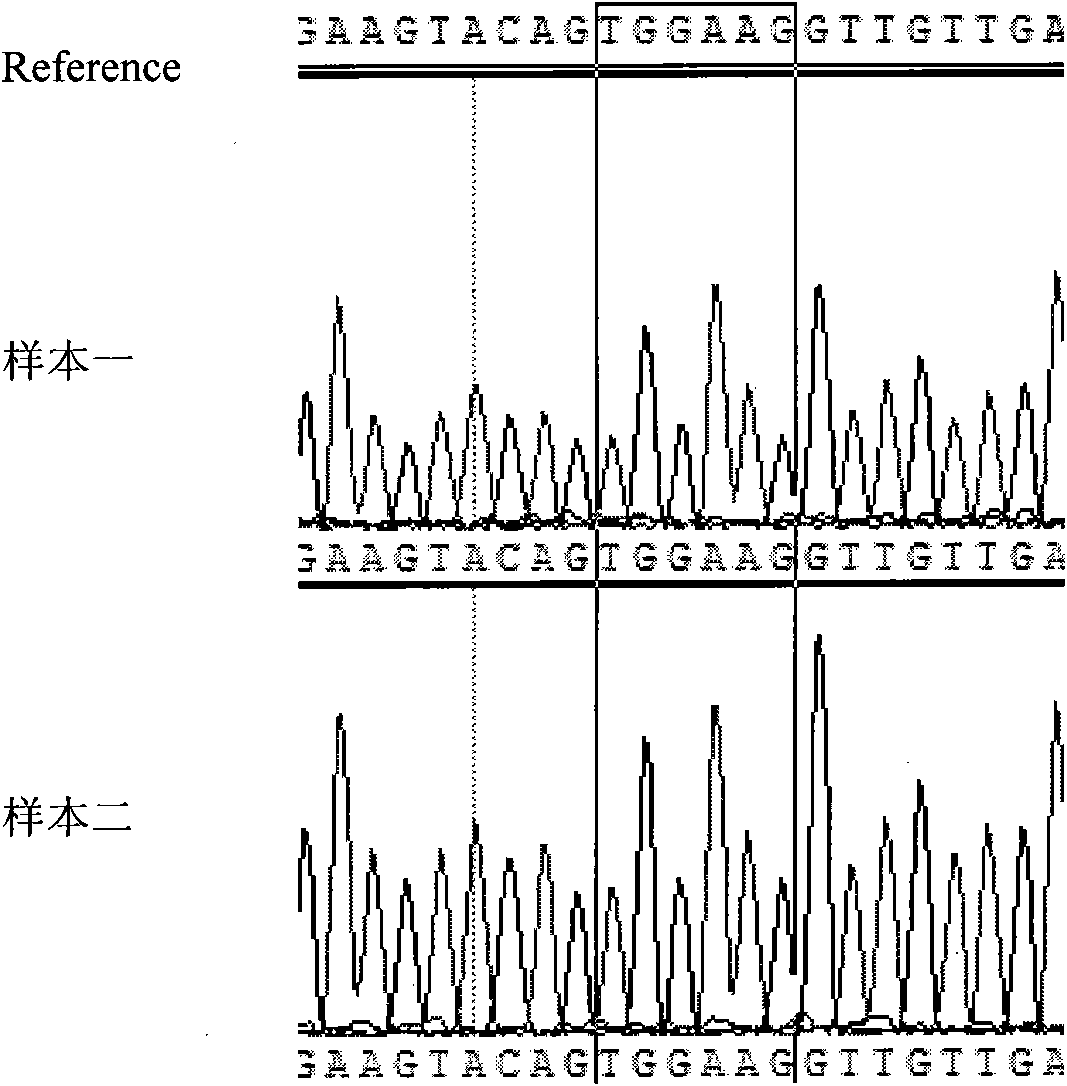
The invention relates to the technical field of molecule detection for guiding tumor therapy and in particular discloses a primer used for detecting mutation of exons 9 and 11 of a c-kit gene and application thereof to the identification of the sensitivity of a tumor patient to imatinib (gleevec) medicaments. First, a DNA is extracted from a tumor tissue or a blood sample of a patient; secondly, a target fragment is amplified by the designed primer; and finally, the mutation locuses of the kit exons 9 and 11 are detected by sequencing the target segment so as to guide the therapy on the tumor patient.
Owner:SHANGHAI BIOTECAN PHARMA
Method of treating cancers
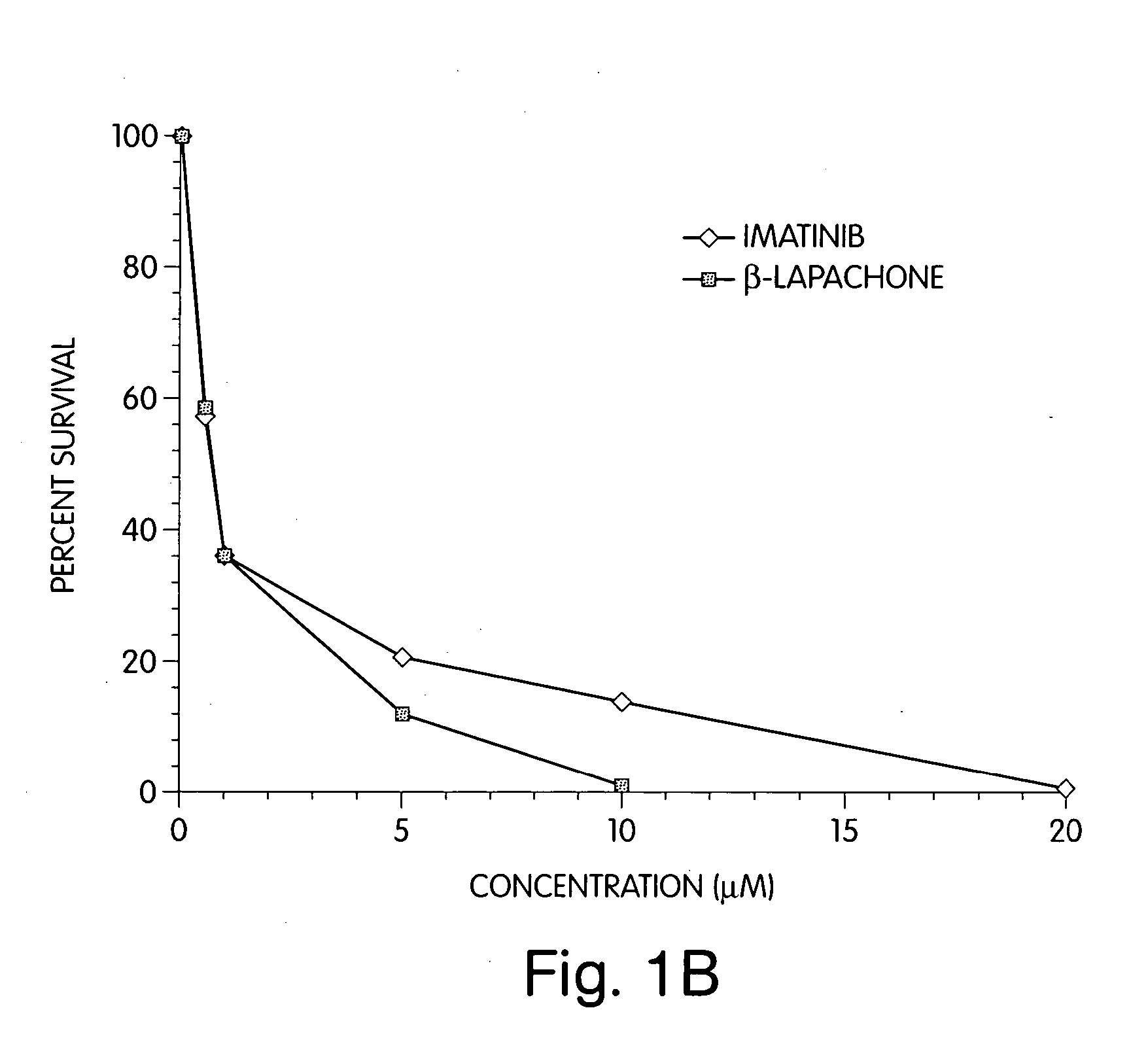
Cancers and / or malignancies can be treated by administration of a cell cycle checkpoint activator, which is preferably β-lapachone, or a derivative or analog thereof, combined with an oncogenic kinase modulator, preferably imatinib. This combination of the cell cycle checkpoint activator with the oncogenic kinase modulator results in an unexpectedly greater than additive (i.e., synergistic) apoptosis in cancer cells. The invention includes methods of treating cancers by administering the combination of the cell cycle checkpoint activator and the oncogenic kinase modulator, pharmaceutical compositions comprising the combination of drugs used in these methods, as well as pharmaceutical kits.
Owner:ARQULE INC
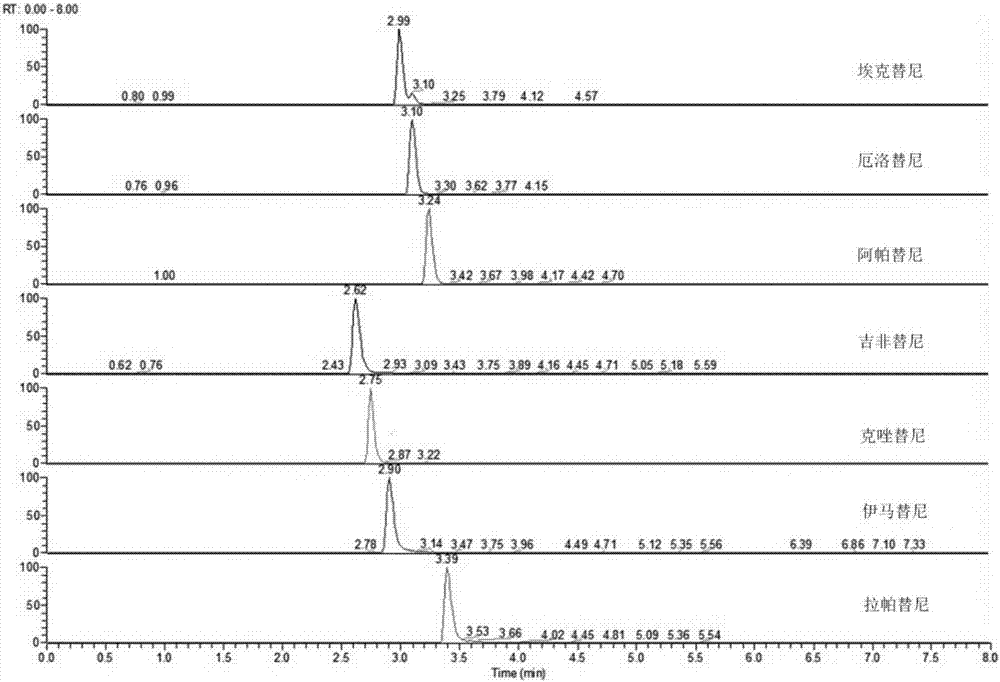
Method for simultaneously measuring concentrations of six tyrosine kinase inhibitors in blood plasma
ActiveCN106990185AHigh selectivityReduce false positive rateComponent separationTyrosine-kinase inhibitorTert-butyl methyl ether
The invention discloses a method for simultaneously measuring the concentrations of six tyrosine kinase inhibitors in blood plasma. Imatinib is adopted as an internal standard, a medicament in the blood plasma is extracted at first by virtue of a mixed solution of ethyl acetate and tert-butyl methyl ether, then a sample is separated by virtue of high performance liquid chromatography, medicament targeting detection is performed in a high resolution mass spectrometry parallel reaction monitoring mode, and secondary fragment ions of the medicament are used for quantification, so as to realize simultaneous analysis and measurement of the concentrations of the six tyrosine kinase inhibitors in the blood plasma. The method is quick, has the advantages of extremely high targeting performance, high speed, high throughput, high sensitivity, high specificity, high precision and accuracy, high stability, high extraction recovery rate, no obvious substrate effect or dilution effect, and the like, and can be used for plasma concentration monitoring of clinically common antitumor medicaments, i.e. tyrosine kinase inhibitors, and a nanogram-level detection limit can be achieved.
Owner:ZHEJIANG CANCER HOSPITAL +1
Process for preparing N-phenyl-2-pyrimidyl amine derivative
The present invention relates to new organic compound, and is especially the preparation process of N-(2-methyl-5-nitro) phenyl-4-(3-pyridyl) pyrimidiyl-2-amine as the key intermediate for imatinib, which is tyrosine protein kinase inhibitor for treating chronic myeloid leukemia and other diseases, and its analog N- phenyl-pyrimidiyl-2-amine derivative. The target product may be prepared through condensation of 4-aromatic heterocycle-2-halogenated pyrimidine and substituted aniline in the presence of catalyst. The preparation process has low material cost, scientific synthesis path, high product yield and other advantages.
Owner:ZHEJIANG ACAD OF MEDICAL SCI
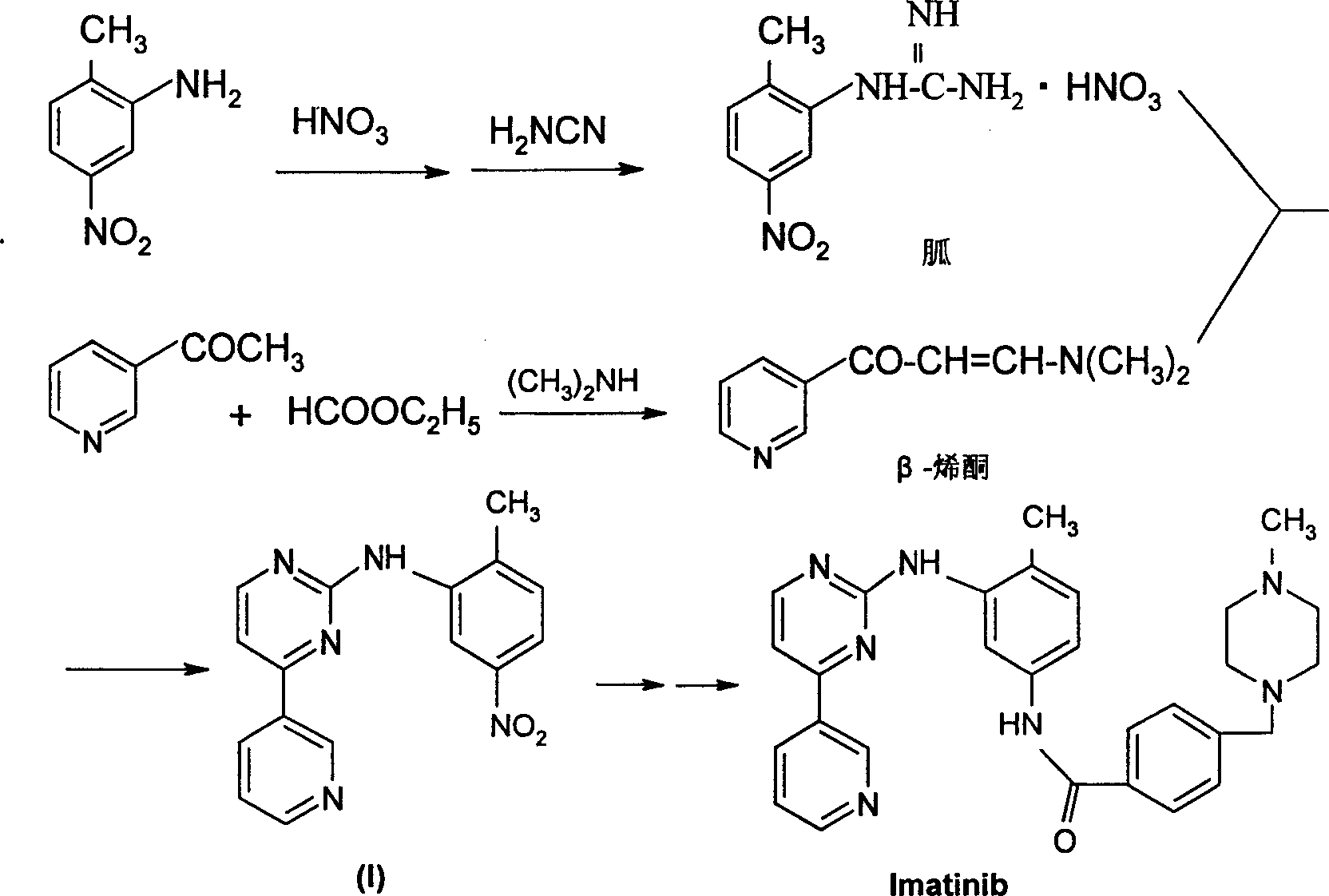
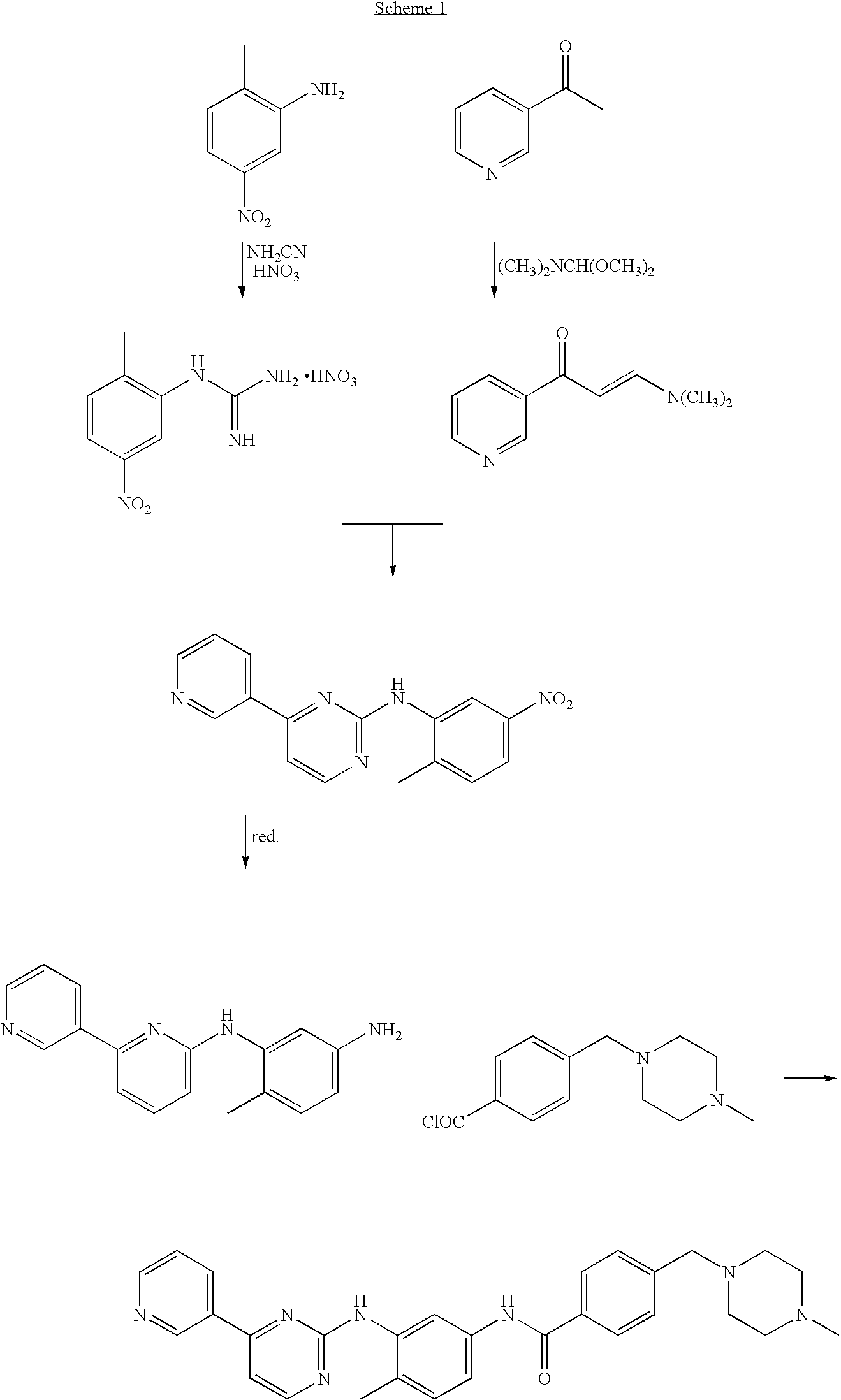
Process for preparation of imatinib base
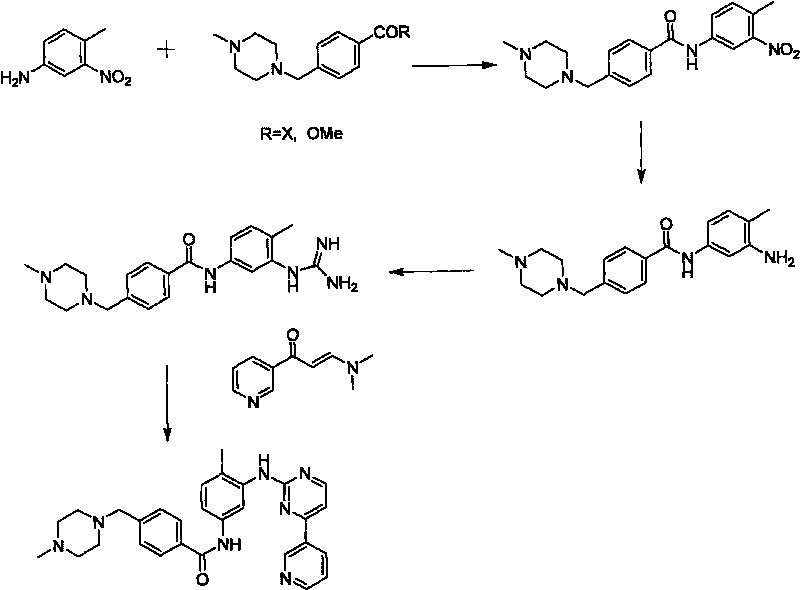
An improved process for the preparation of imatinib base and its pharmaceutically acceptable acid addition salts by (a) reacting 2-methyl-5-nitroaniline with cyanamide in the presence of hydrochloric acid to obtain 1-(2-methyl-5-nitrophenyl)guanidine hydrochloride; (b) converting 1-(2-methyl-5-nitrophenyl)guanidine hydrochloride to 1-(2-methyl-5-nitrophenyl)guanidine nitrate; (c) condensing 3-acetylpyridine with N,N-dimethylformamide dimethyl acetal to obtain 3-(dimethylamino)-1-(3-pyridinyl)-prop-2-en-1-one; (d) reacting 3-(dimethylamino)-1-(3-pyridinyl)-prop-2-en-1-one with 1-(2-methyl-5-nitrophenyl)guanidine nitrate to obtain N-(5-nitro-2-methylphenyl)-4-(3-pyridinyl)-2-pyrimidineamine; (e) reducing N-(5-nitro-2-methylphenyl)-4-(3-pyridinyl)-2-pyrimidineamine using hydrazine in the presence of Raney nickel to obtain N-(5-amino-2-methylphenyl)-4-(3-pyridinyl)-2-pyrimidine-amine; (f) condensing N-(5-amino-2-methylphenyl)-4-(3-pyridinyl)-2-pyrimidine-amine with 4-chloromethylbenzoyl chloride in the presence of an inorganic base to obtain 4-(chloromethyl)-N-(4-methyl-3-(4-(pyridin-3-yl)pyrimidin-2-ylamino)phenyl)benzamide; and (g) condensing 4-(chloromethyl)-N-(4-methyl-3-(4-(pyridin-3-yl)pyrimidin-2-ylamino)phenyl)benzamide with an excess of N-methylpiperazine to obtain imatinib base; and adding water or a mixture of water and an organic solvent; and isolating said imatinib base. The process allows for using simple starting materials, while simultaneously avoiding a laborious isolation and purification of intermediates and the final product, thereby facilitating scale-up.
Owner:INSTITUT FARMACEUTYCZNY
Convenient and quick method for preparing high-purity imatinib and mesylate thereof
The invention discloses a method for synthesizing imatinib, which comprises the following step of: in the presence of a urea cation condensing agent, reacting N(5-amino-2-methylphenyl)-4-(3-pyridyl)-pyrimithamine (namely a compound shown in a formula I) with 4-(4-methylpiperazin methyl)-benzoic acid in an organic solvent to generate the imatinib. By adopting a specific coupling agent, the invention provides a method for synthesizing imatinib and mesylate thereof, which has the advantages of high efficiency, simple treatment, high product purity, low content of impurities of methyl-removed imatinib, good quality, and suitability for industrial production.
Owner:NANJING CAVENDISH BIO ENG TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com