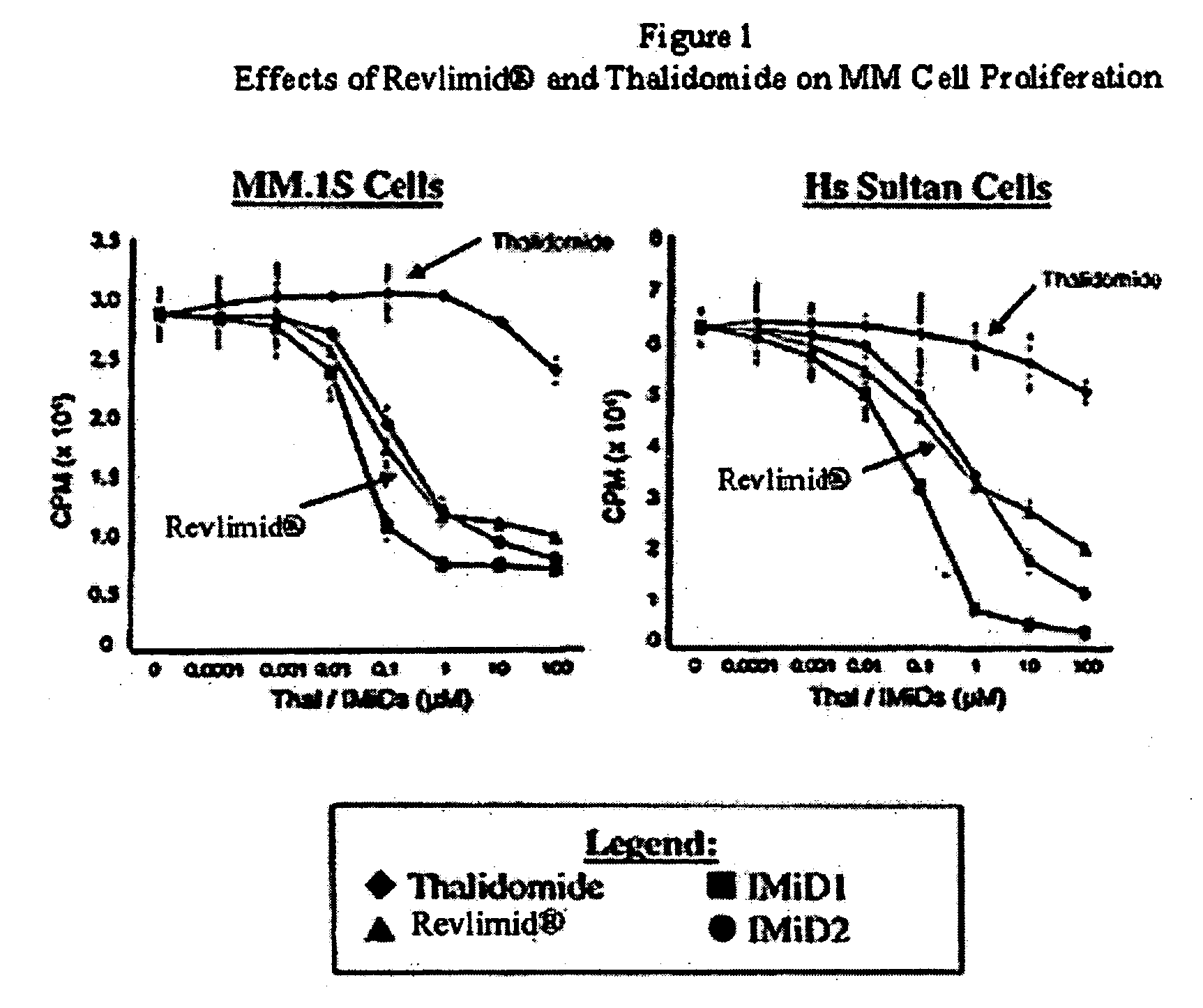
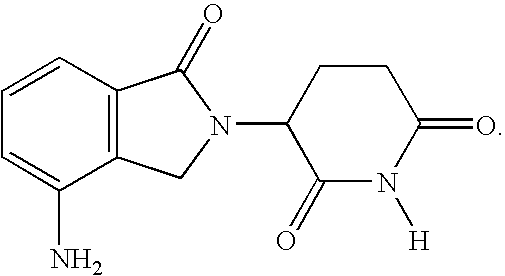
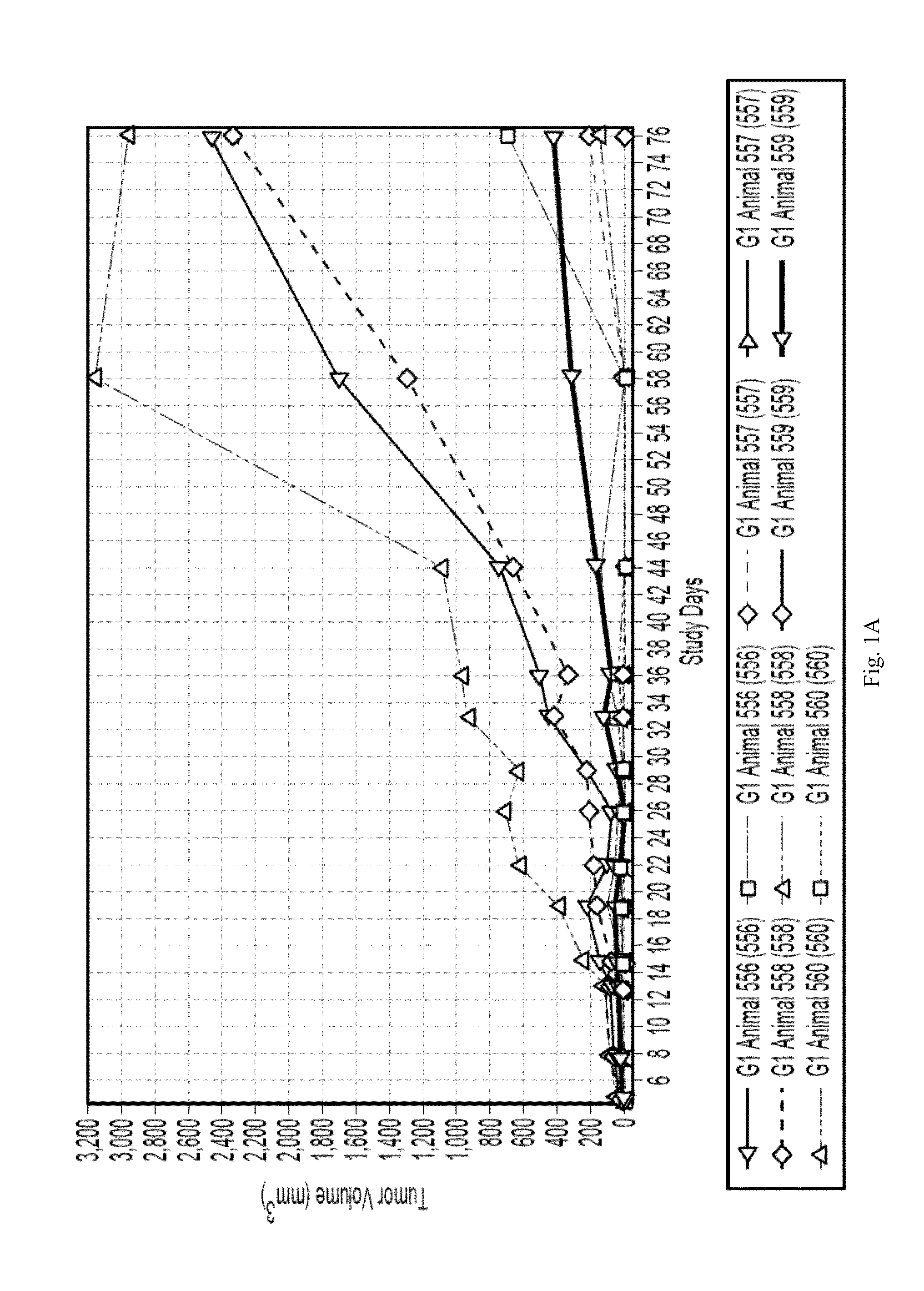
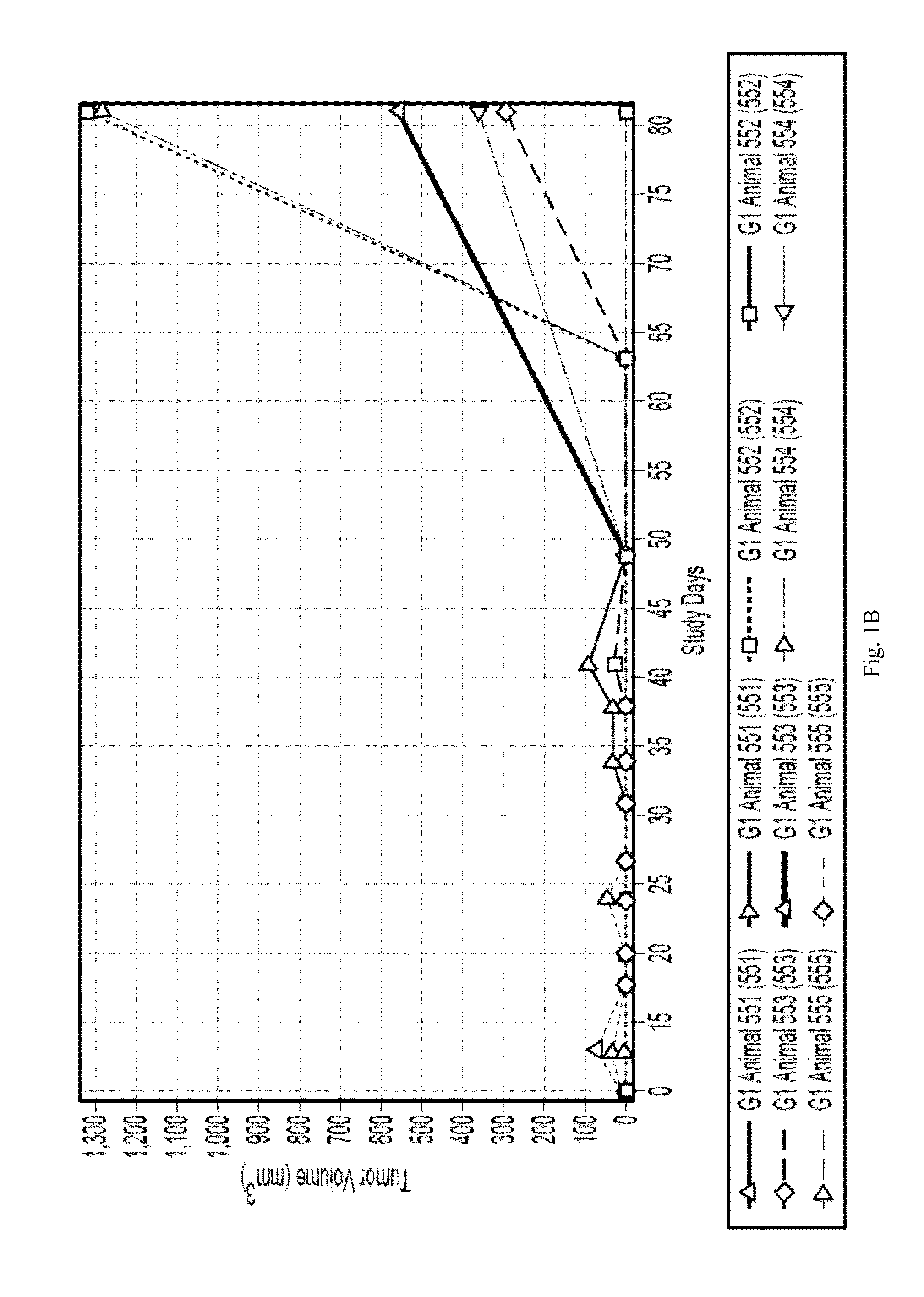
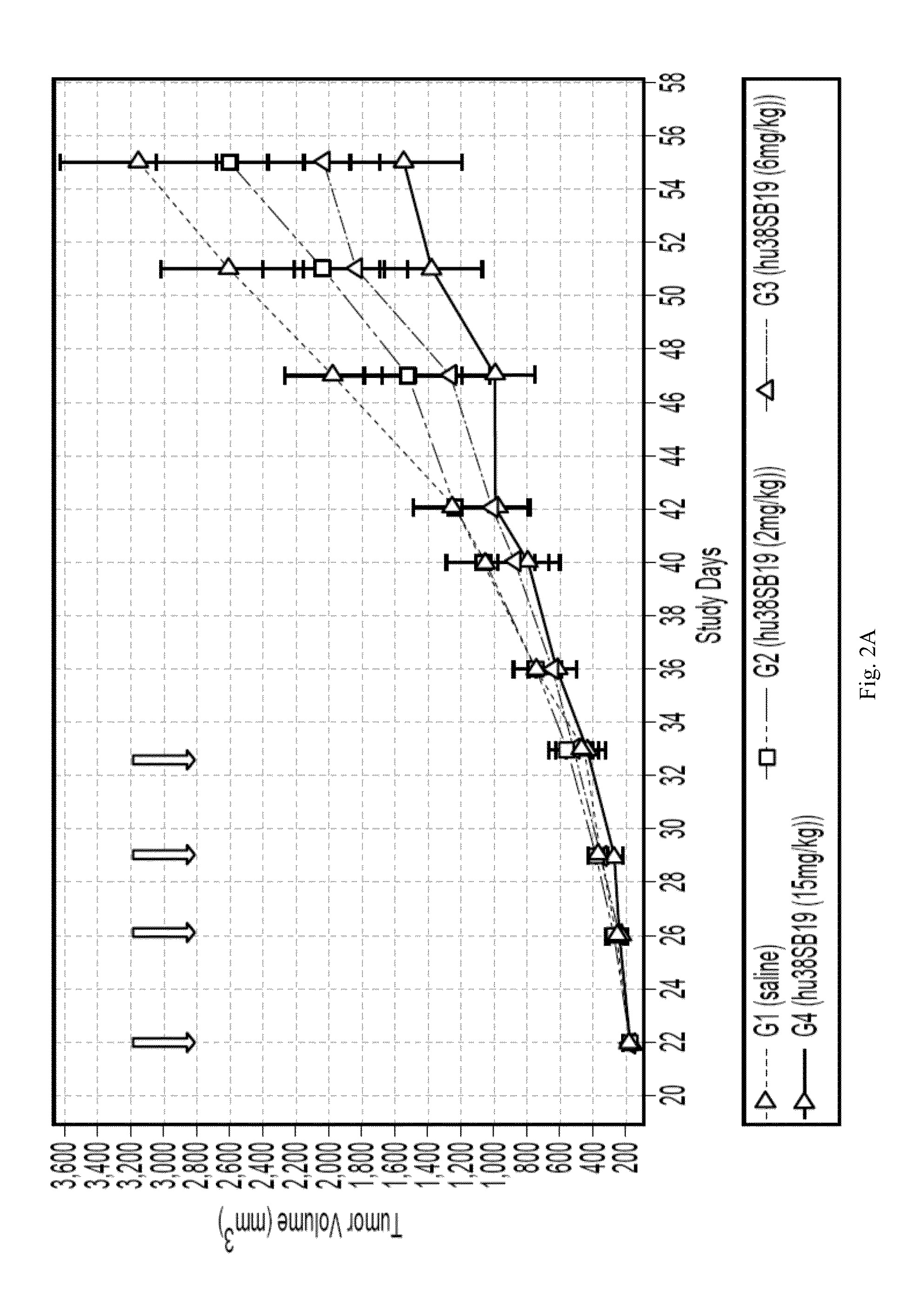
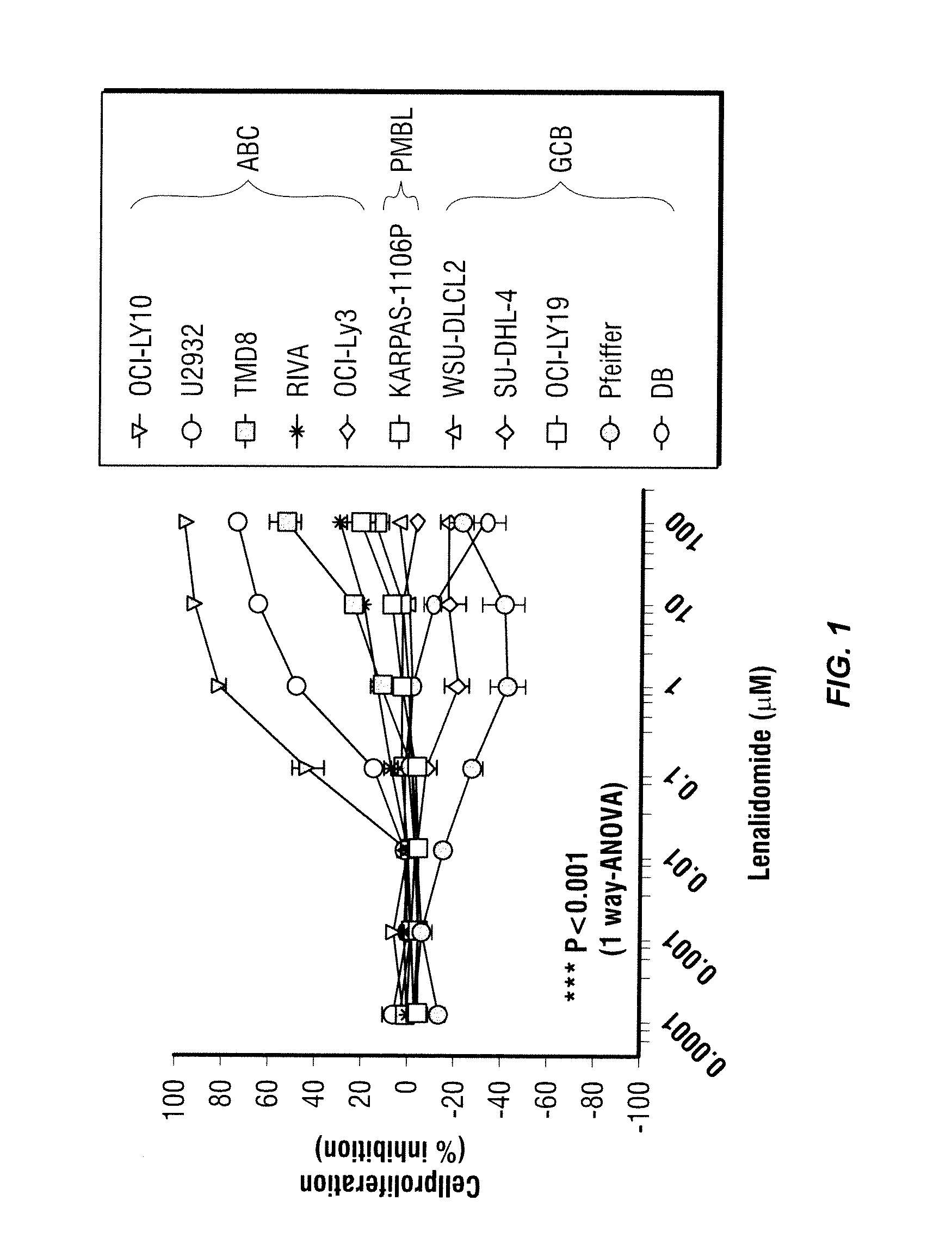
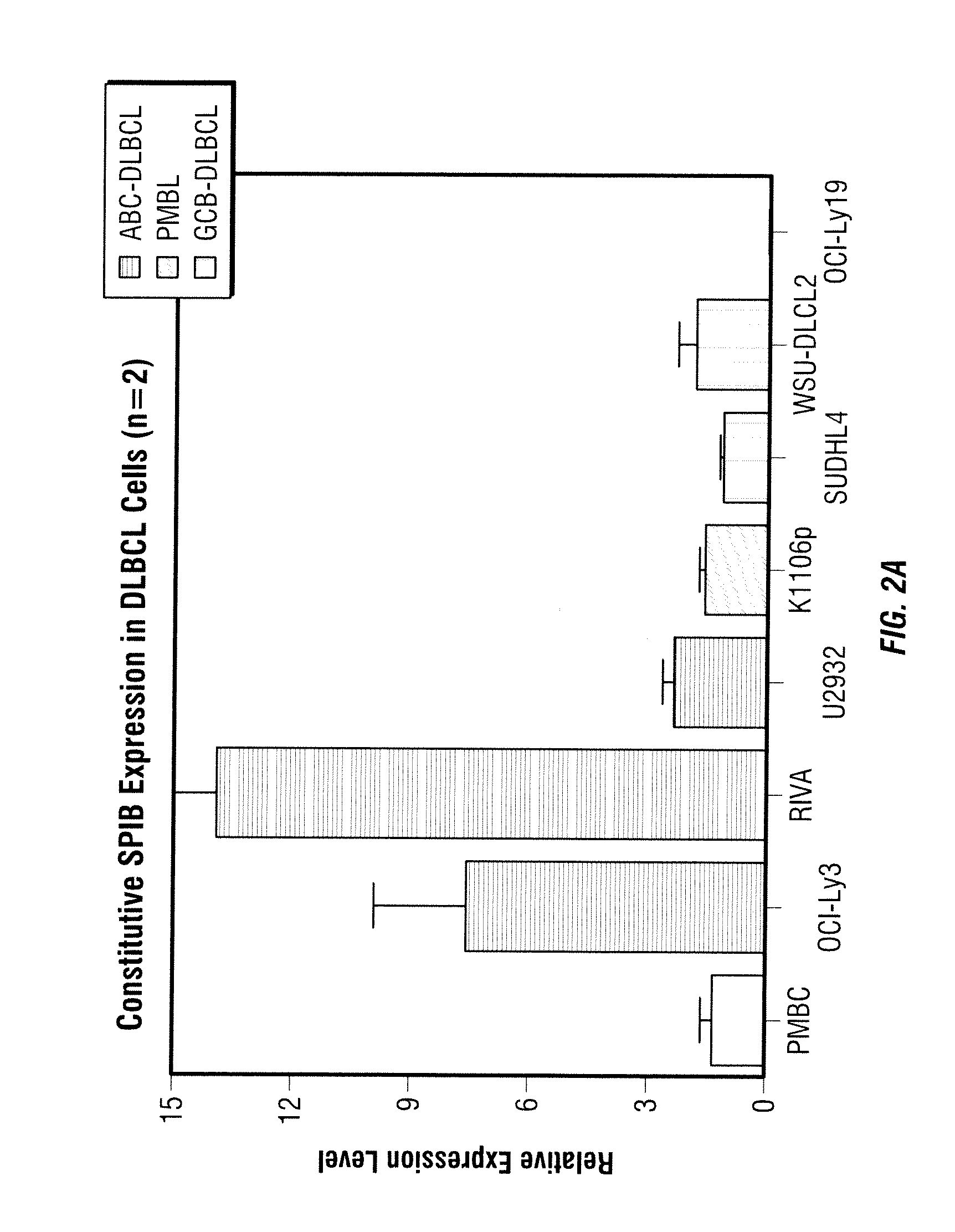
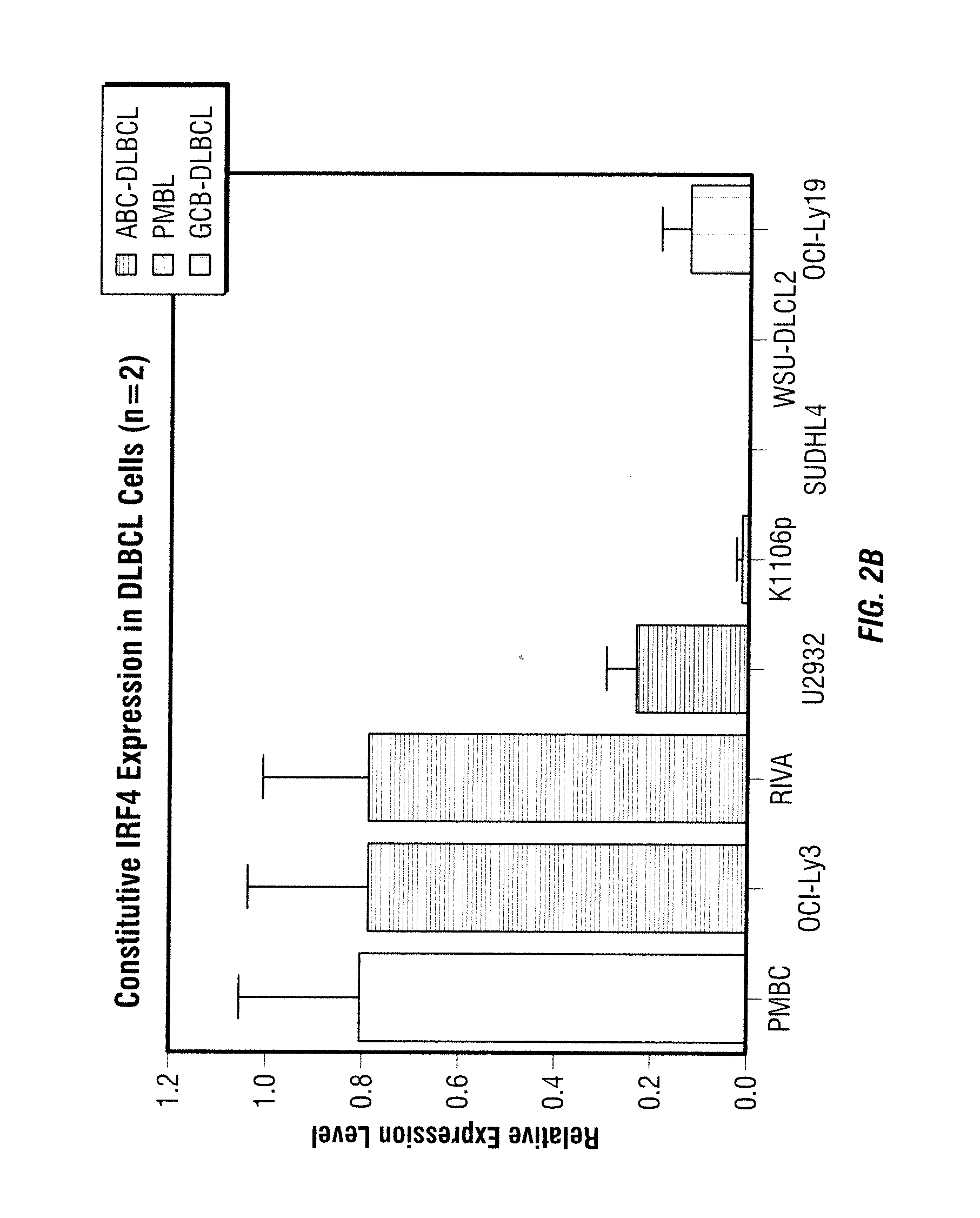
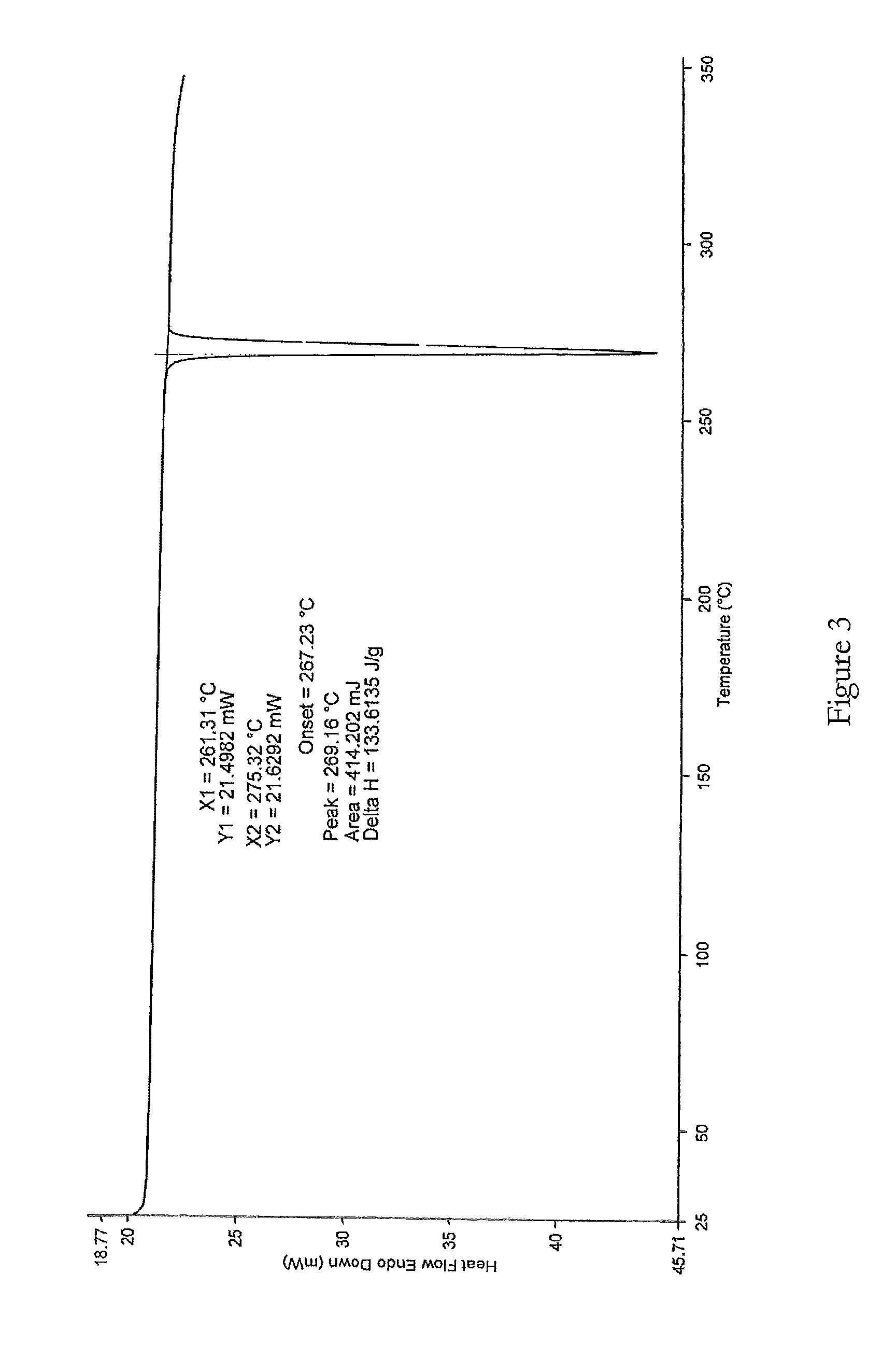
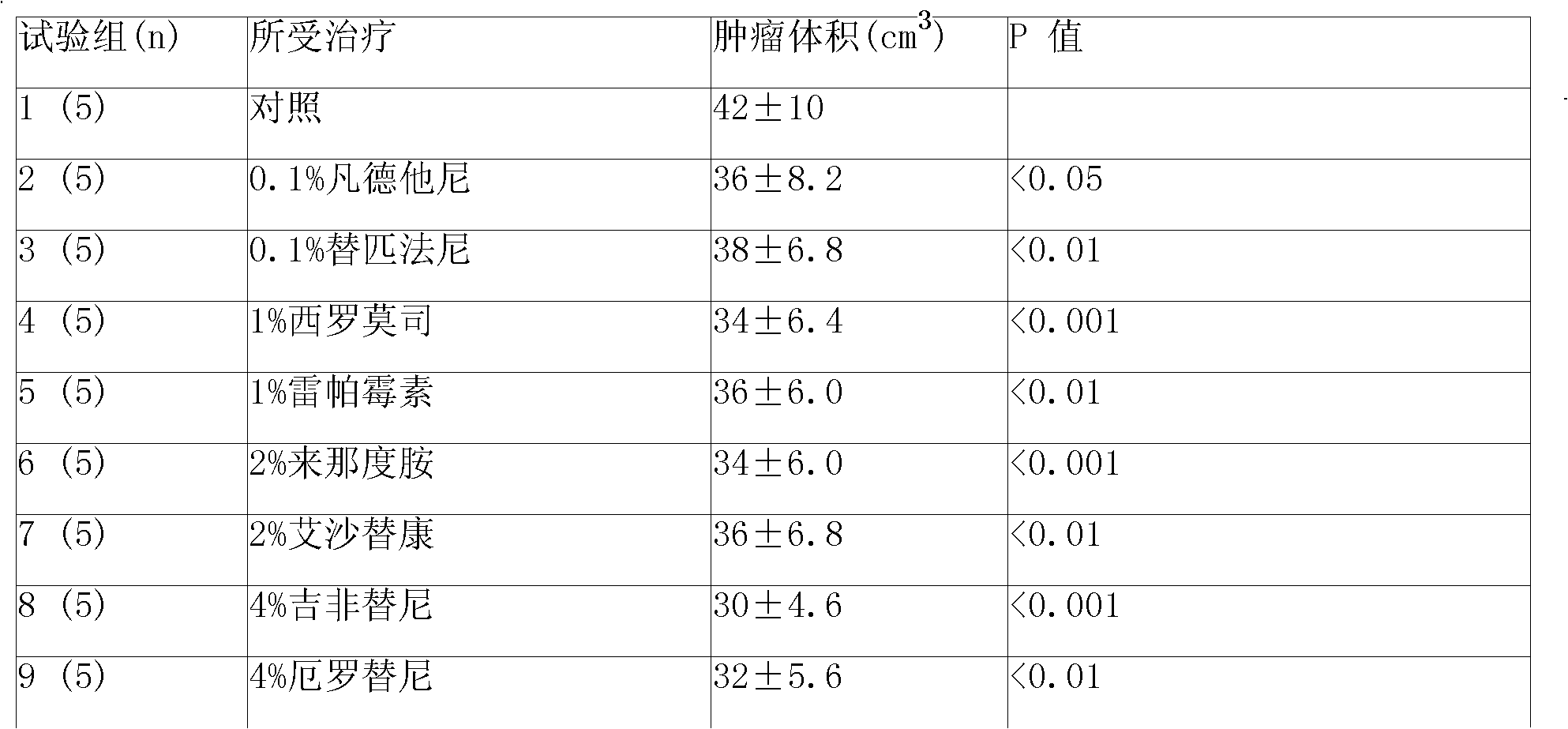
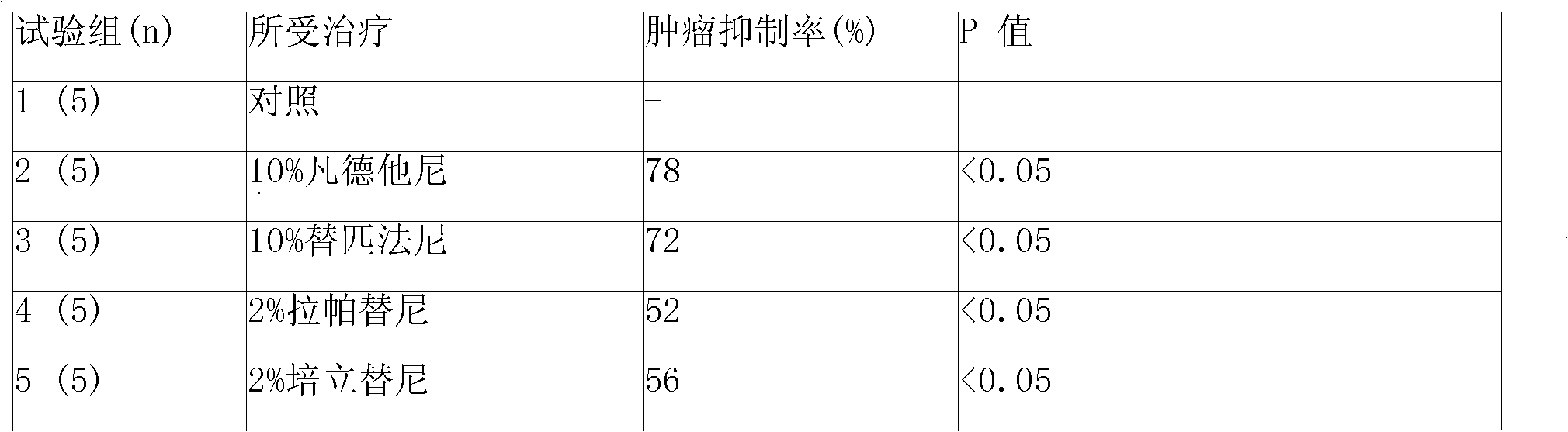
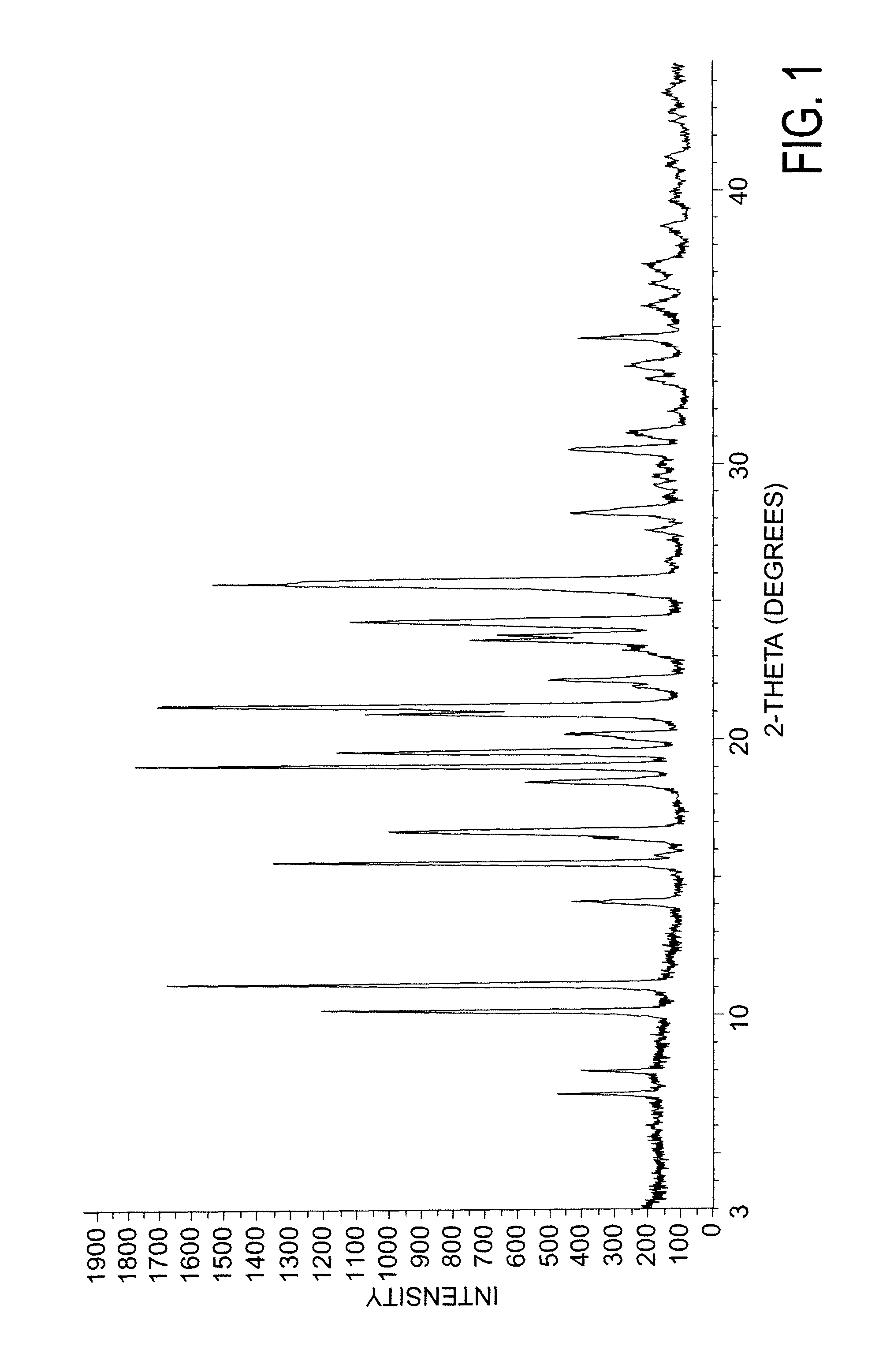
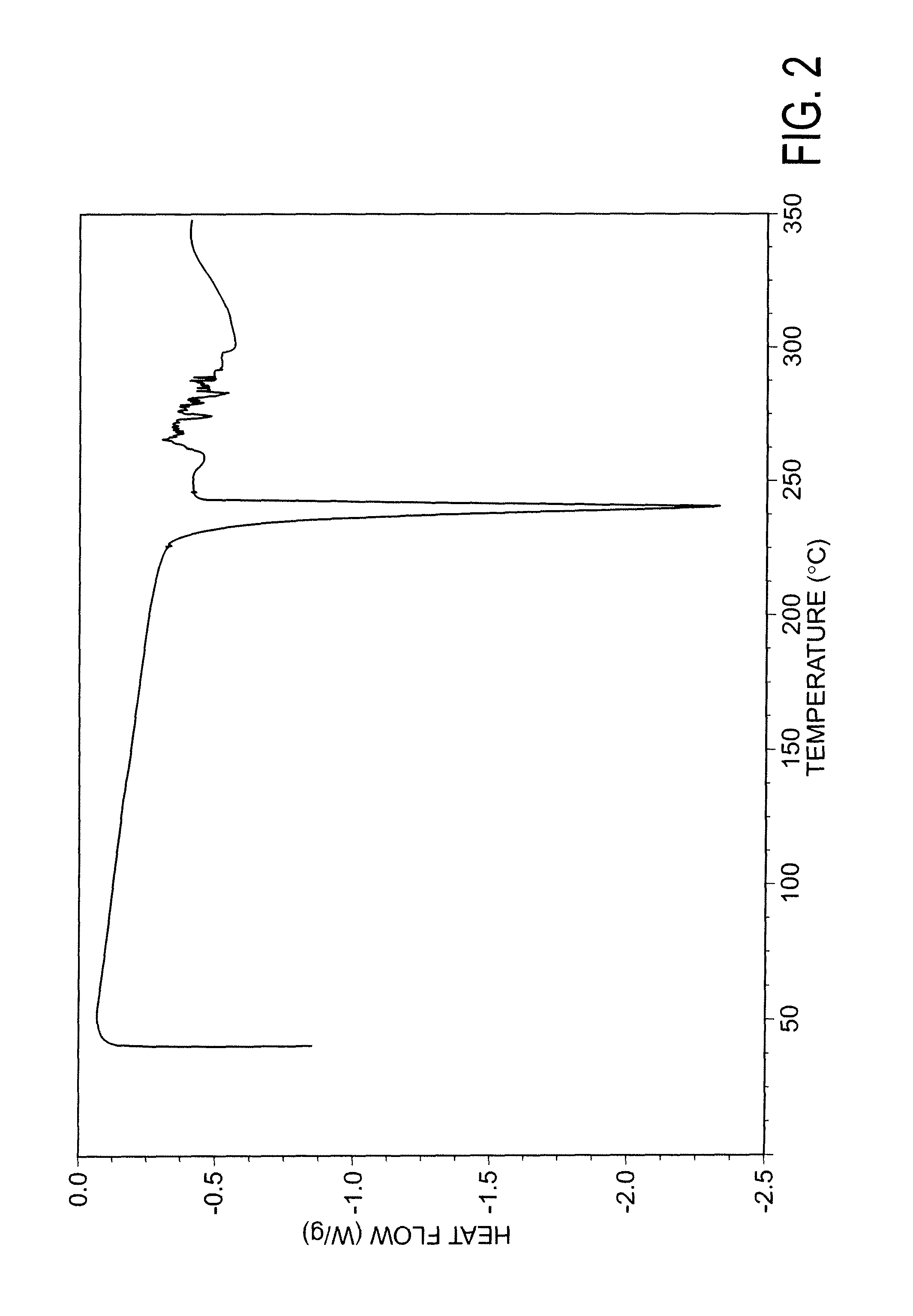
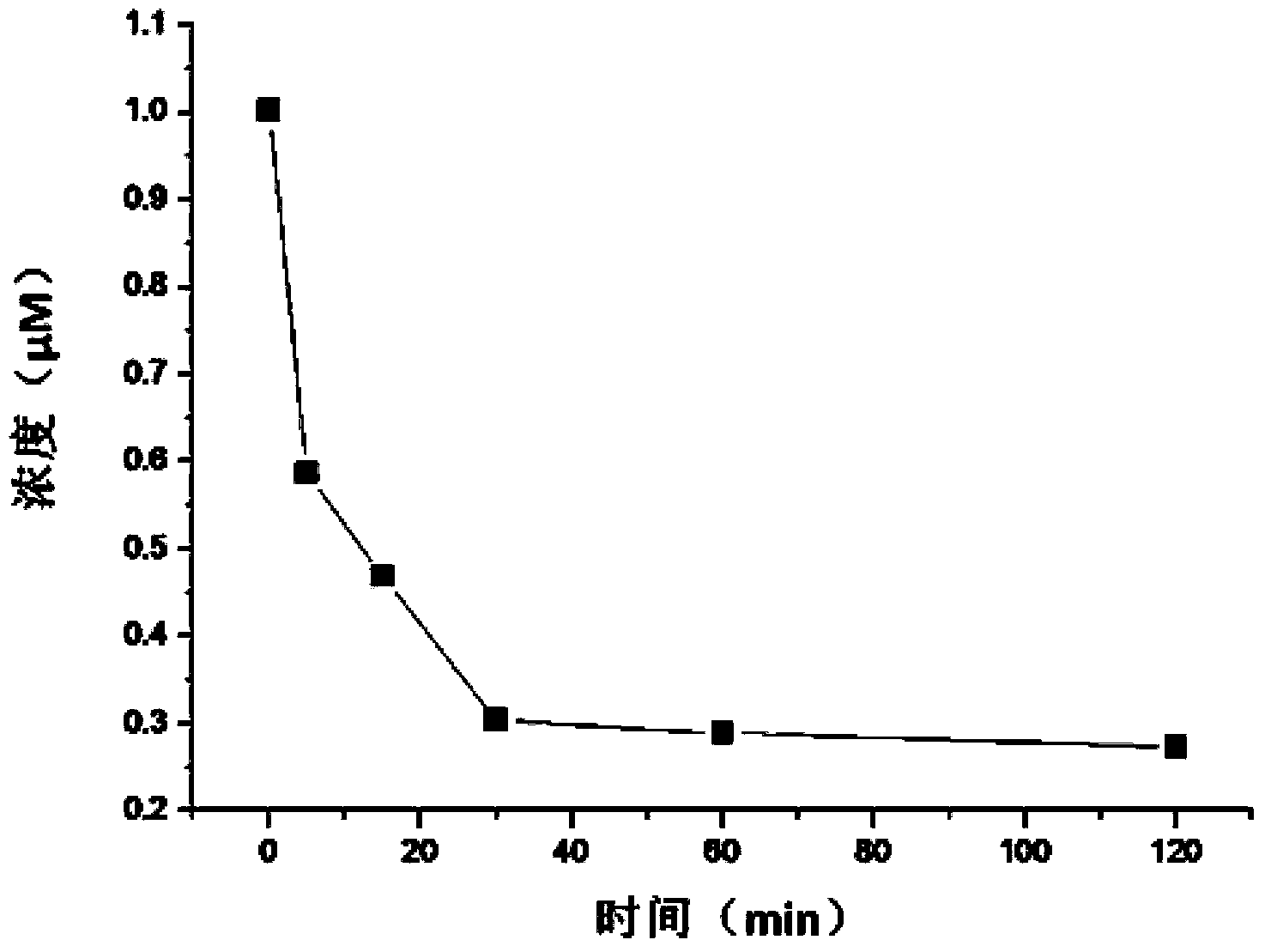
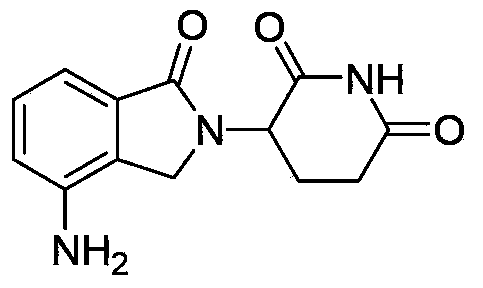
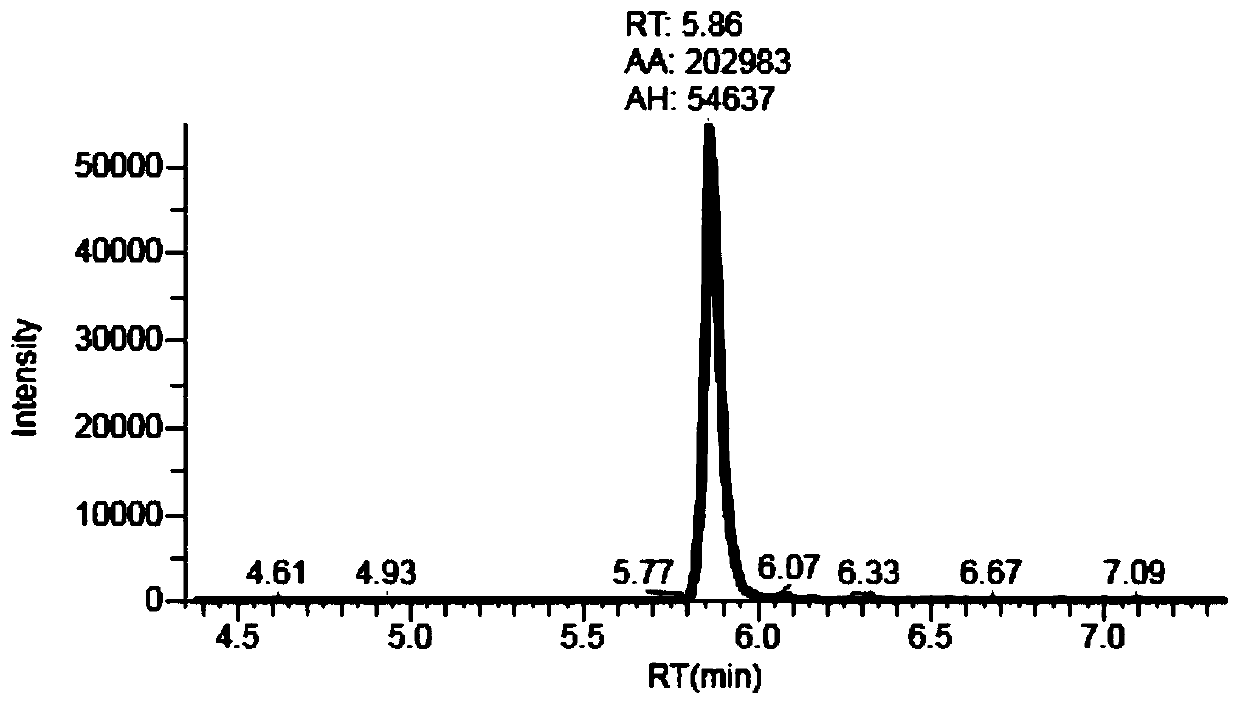
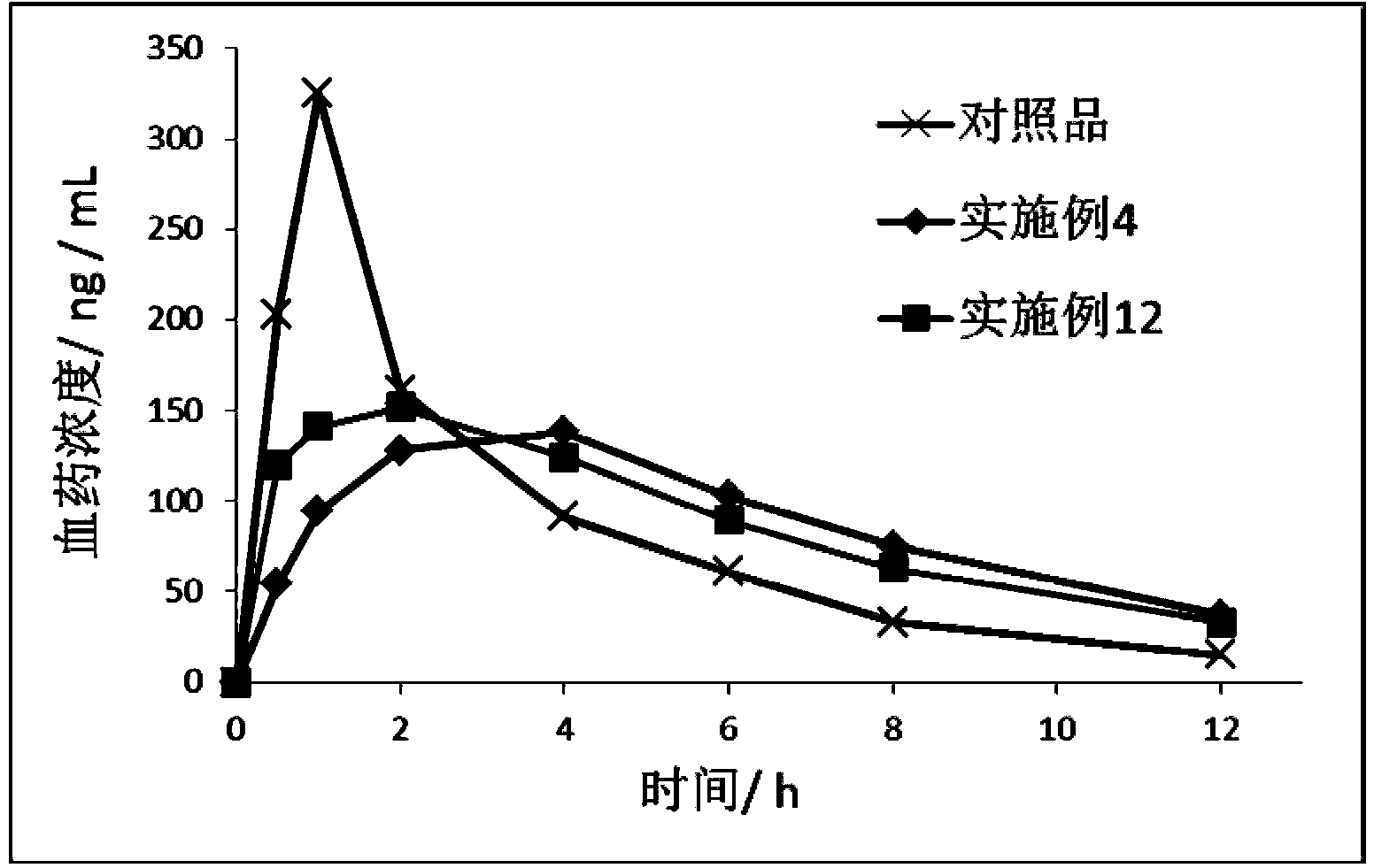
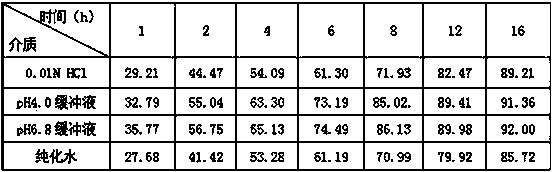
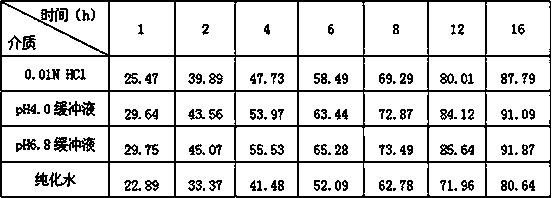
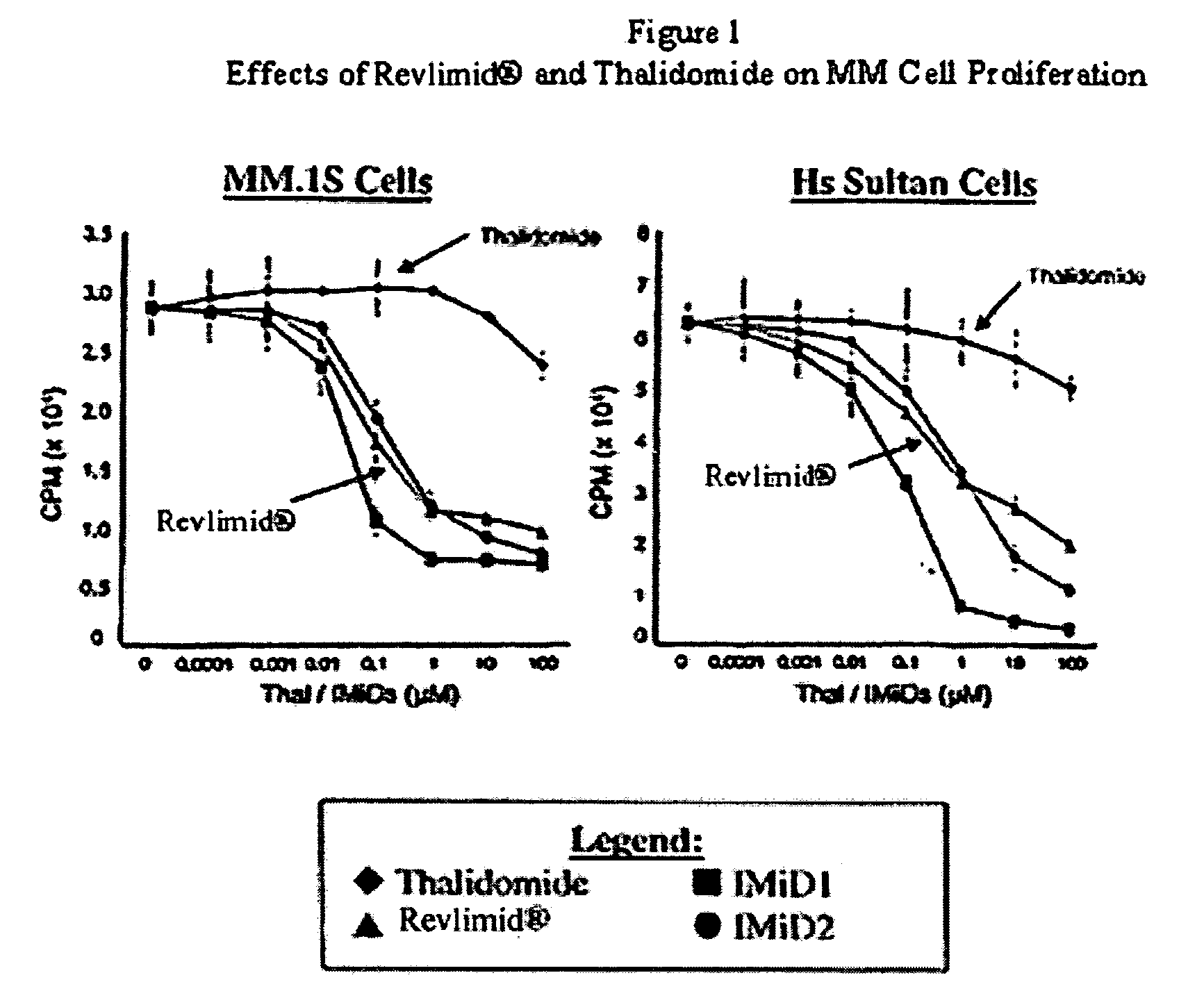
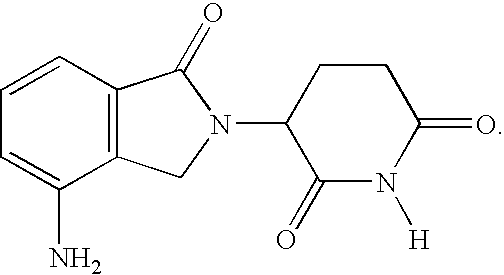
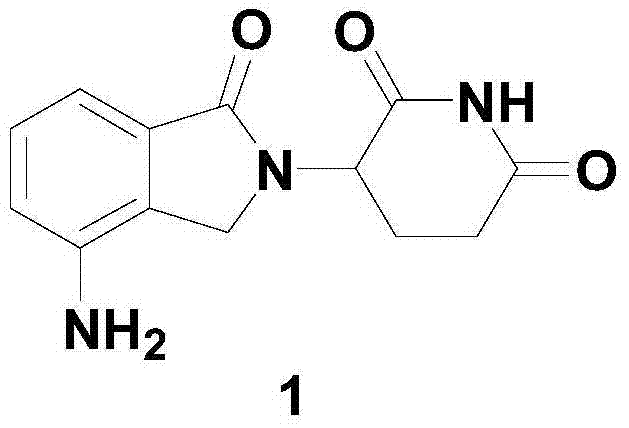
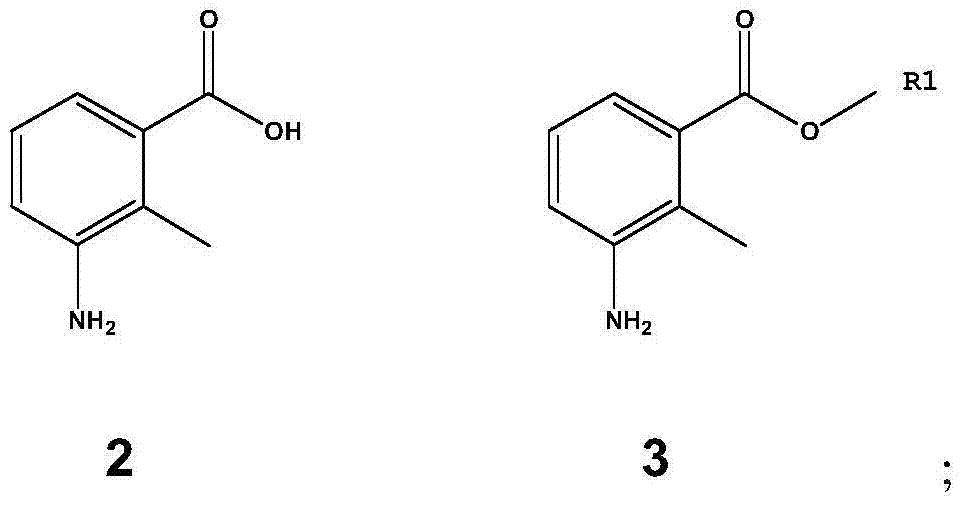
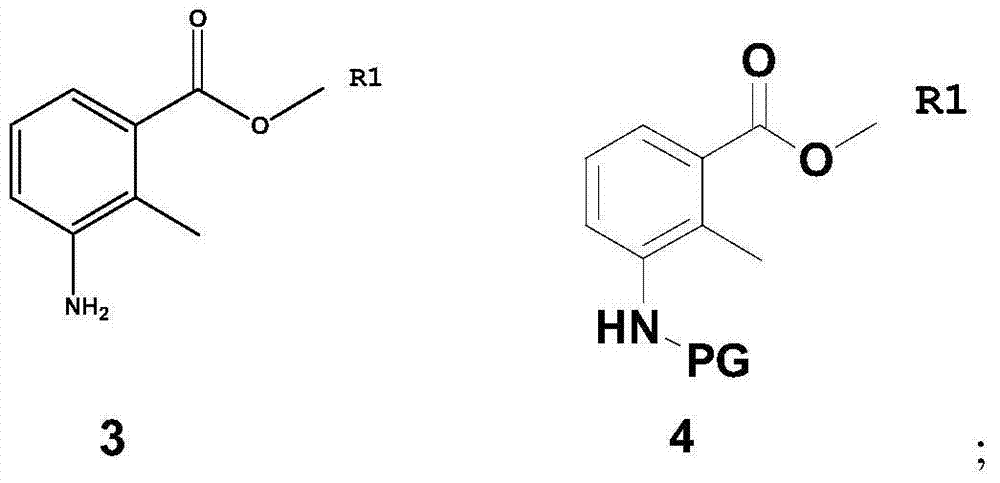
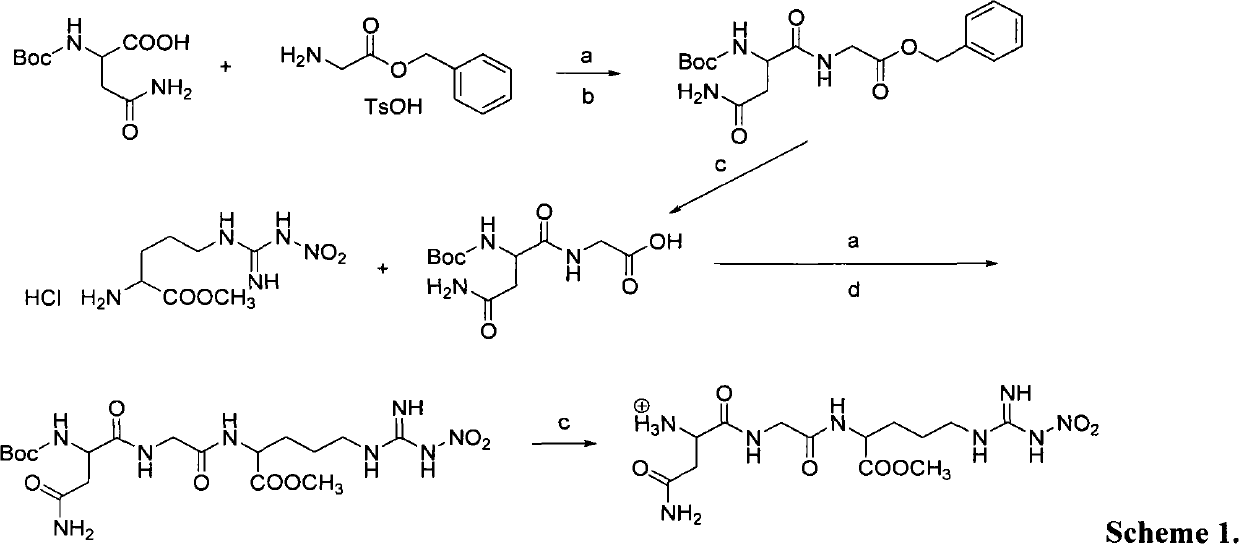
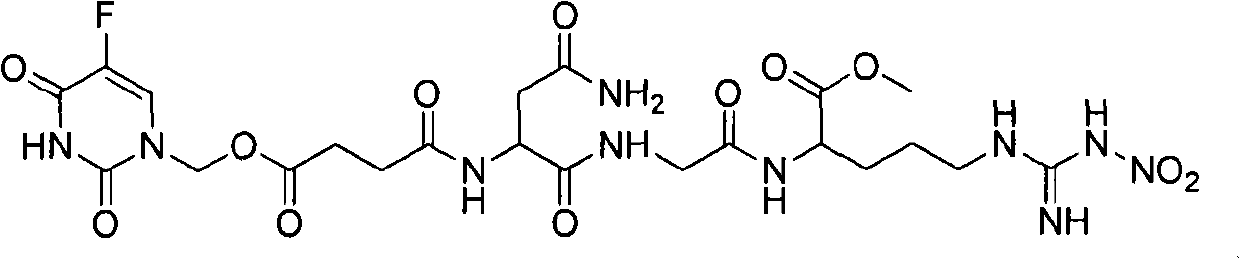
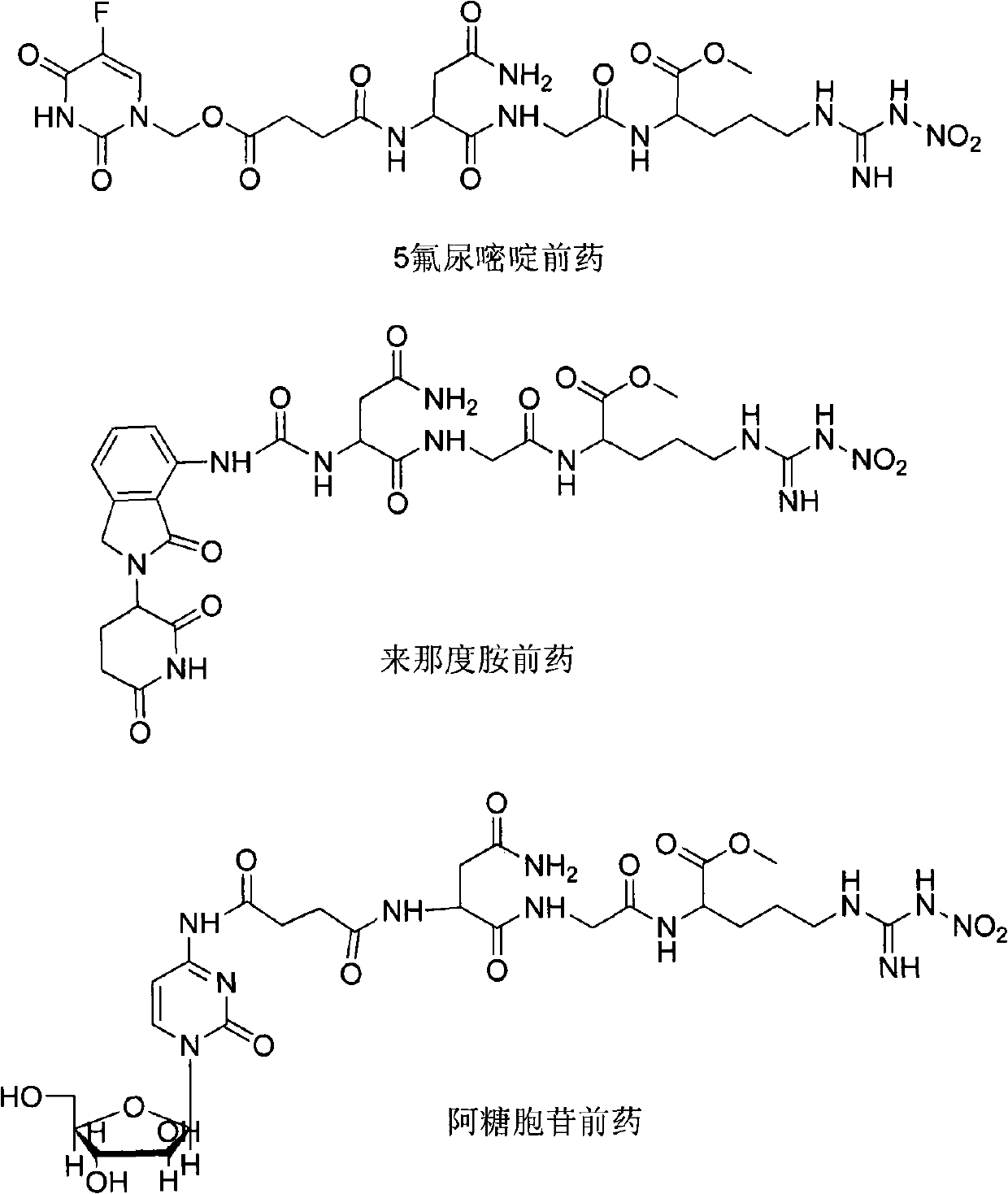
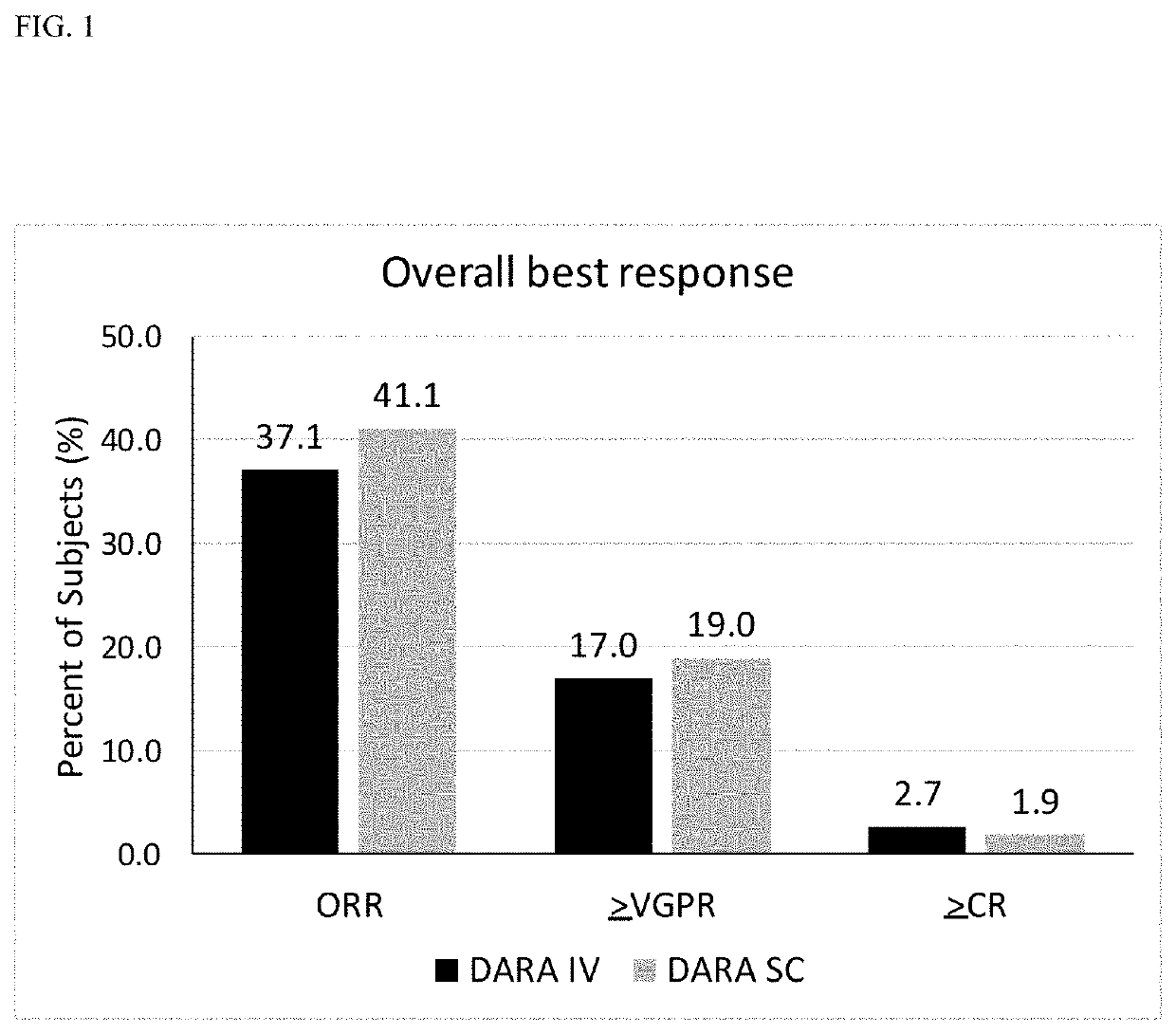
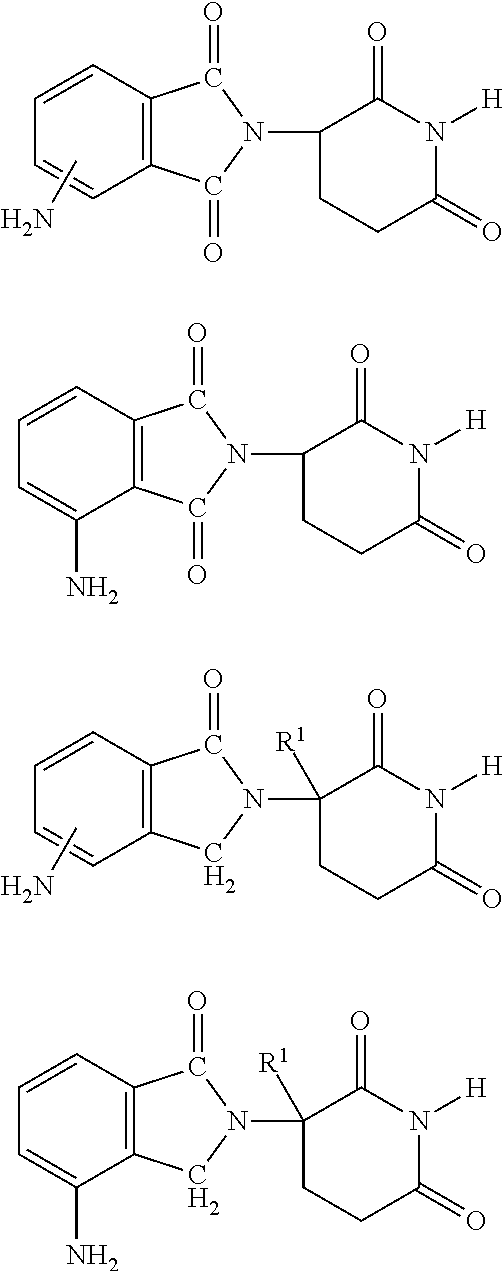
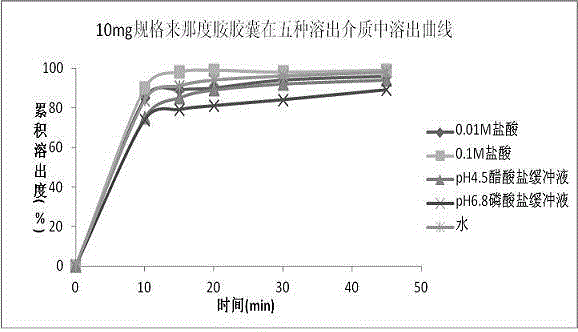
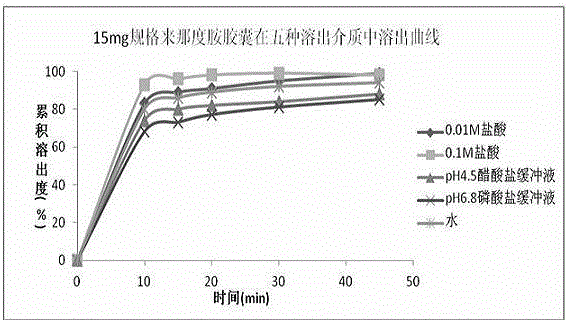
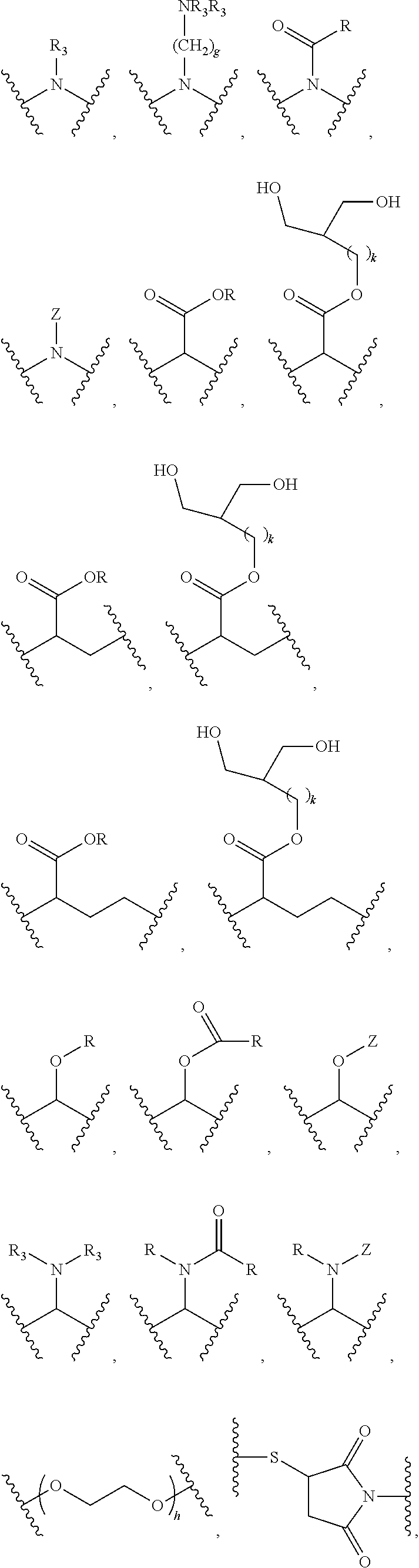
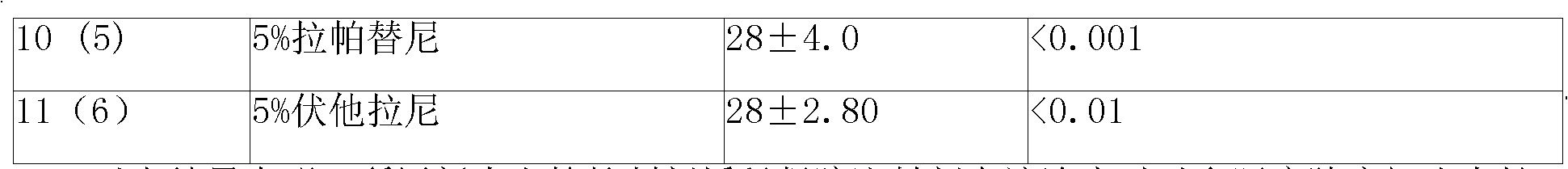Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
133 results about "Lenalidomide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
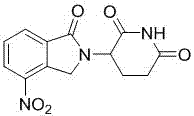
Lenalidomide is used to treat certain cancers (multiple myeloma, mantle cell lymphoma-MCL).
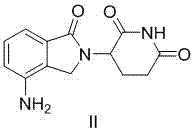
Method using 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione for treatment of certain leukemias
InactiveUS20060030594A1Prevent proliferationBiocidePeptide/protein ingredientsCompound (substance)Radiation therapy
Methods of treating, preventing or managing leukemias are disclosed. The methods encompass the administration of an immunomodulatory compound of the invention known as Revlimid® or lenalidomide. The invention further relates to methods of treatment using this compound with chemotherapy, radiation therapy, hormonal therapy, biological therapy or immunotherapy. Pharmaceutical compositions and single unit dosage forms suitable for use in the methods of the invention are also disclosed.
Owner:CELGENE CORP
Compositions Comprising Anti-CD38 Antibodies and Lenalidomide
ActiveUS20140161819A1Antibody ingredientsHeterocyclic compound active ingredientsCancer researchAntibody
Disclosed herein are compositions and kits which comprise anti-CD38 antibodies and lenalidomide compounds. Also disclosed are methods for treating cancers, such as multiple myeloma, in subjects with the compositions and kits.
Owner:RGT UNIV OF CALIFORNIA +1
Methods for the treatment of non-hodgkin's lymphomas using lenalidomide, and gene and protein biomarkers as a predictor
InactiveUS20110223157A1Monitoring effectiveness of treatmentBiocideMicrobiological testing/measurementADAMTS ProteinsFhit gene
Methods of treating or managing specific cancers, including non-Hodgkin's lymphoma, by the administration of 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione are disclosed. Methods of using gene and protein biomarkers as a predictor of non-Hodgkin's lymphoma response to treatment with 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione are also disclosed.
Owner:SCHAFER PETER H +3
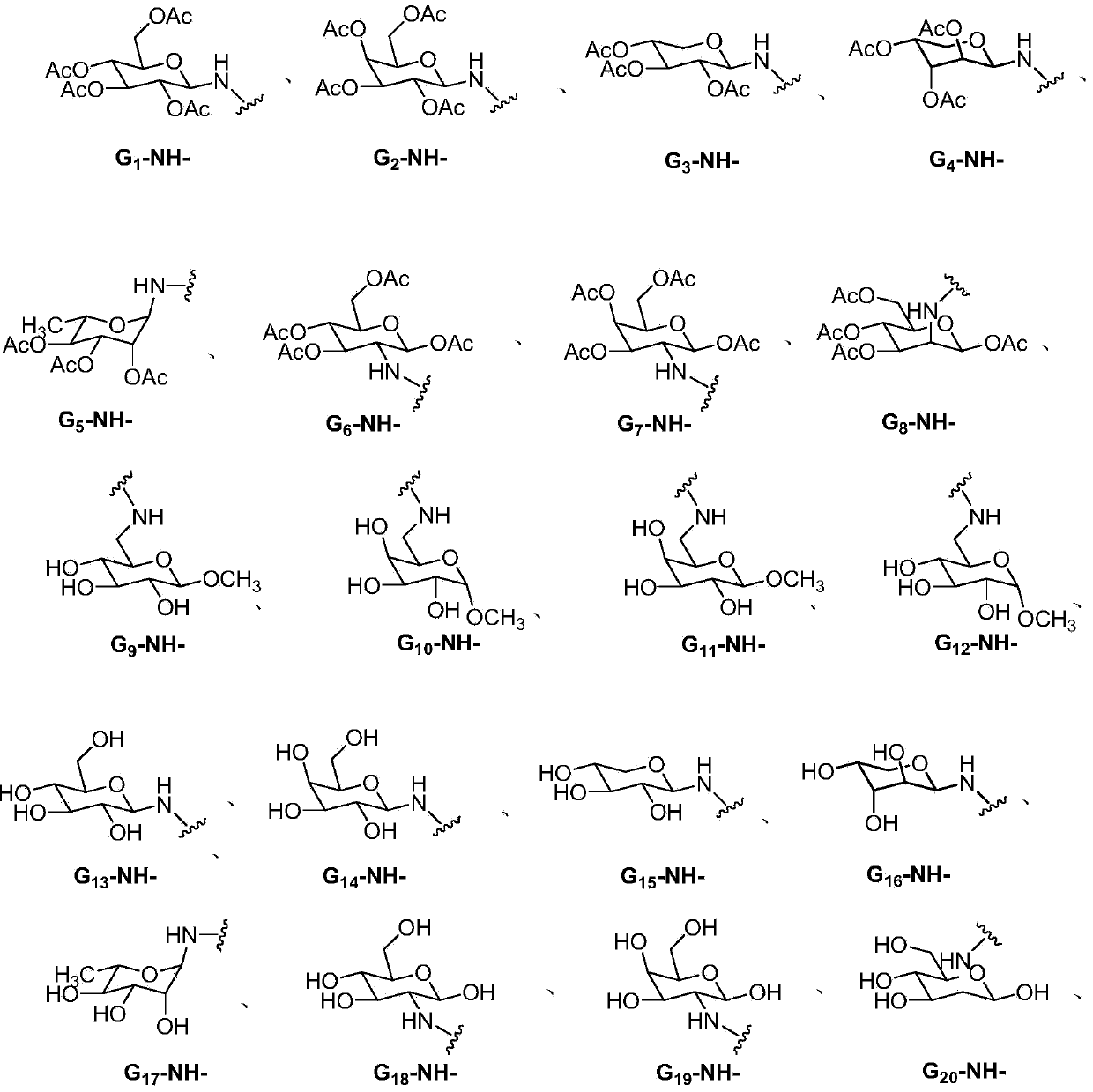
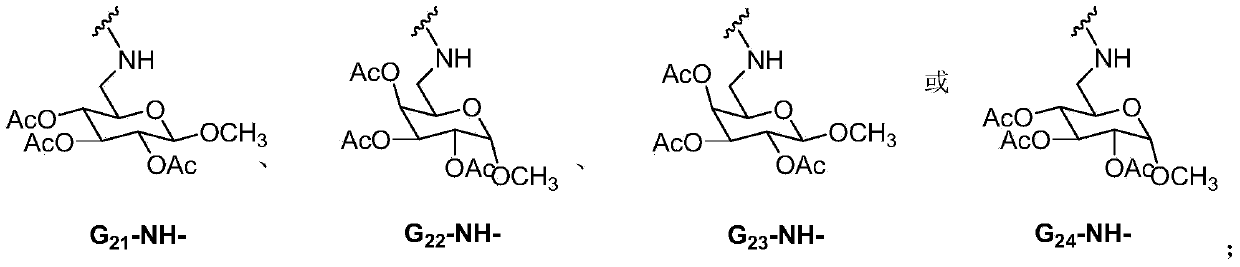
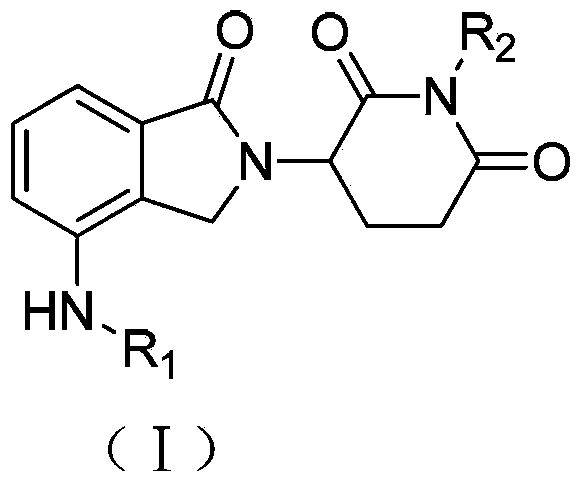
Lenalidomide derivative and preparation method and pharmaceutical application thereof
The invention relates to the field of pharmaceutical chemistry, and in particular to a lenalidomide derivative (I). G and n are defined in a direction book. Pharmacological tests show that the lenalidomide derivative plays an inhibiting role in vascular endothelial cell proliferation and can be used for clinical treatment of tumors or chronic inflammations.
Owner:CHINA PHARM UNIV
Process
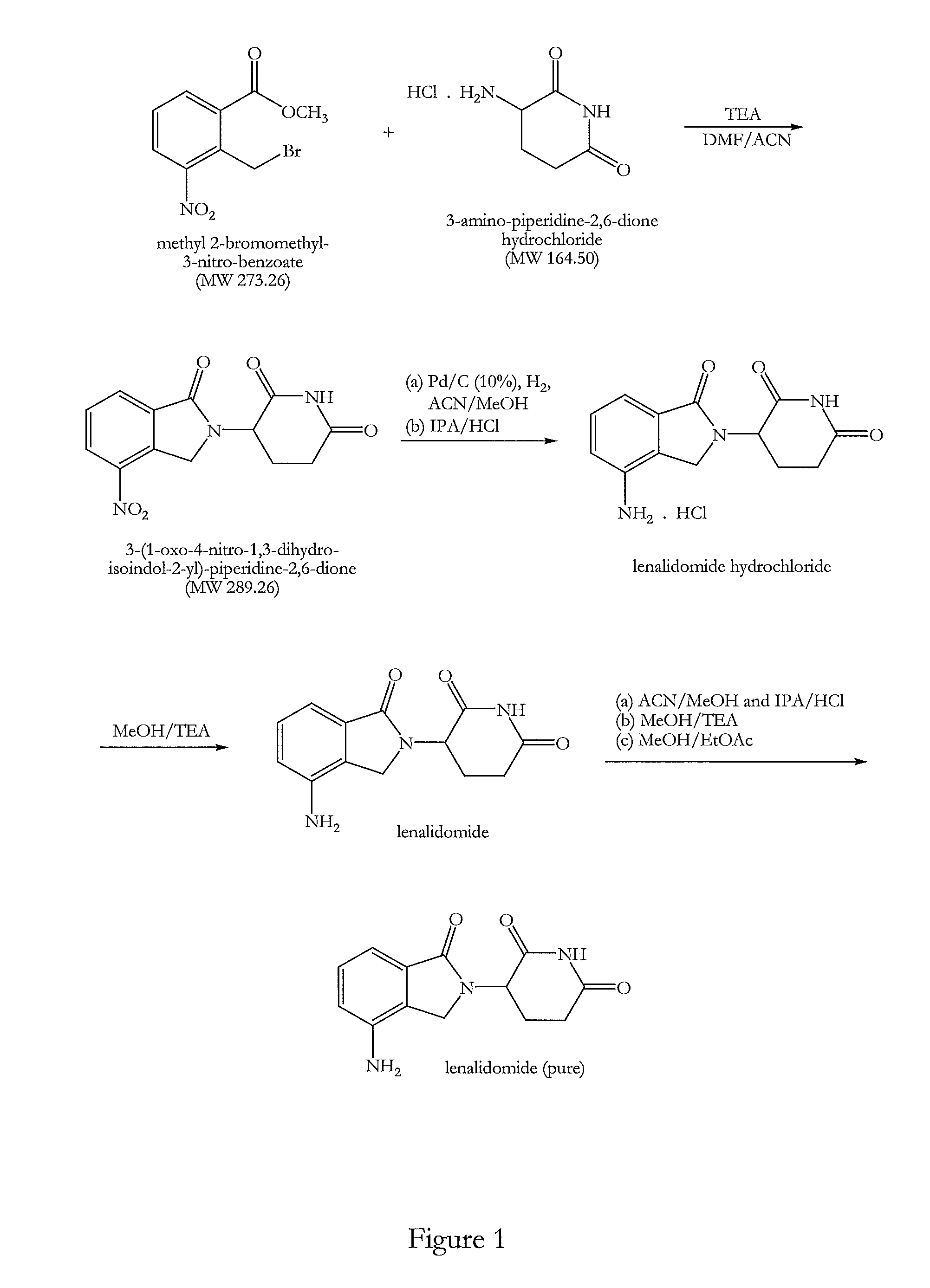
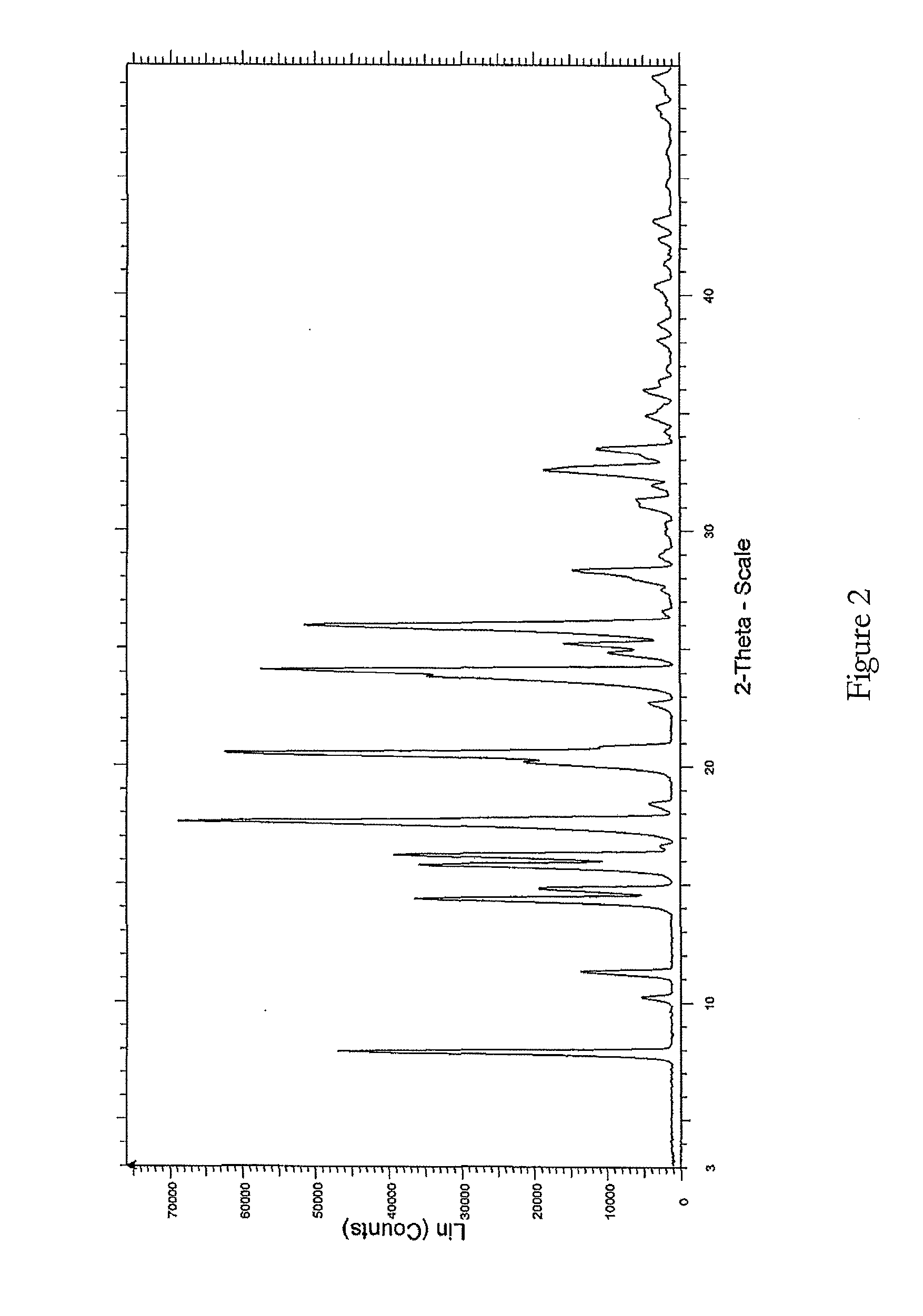
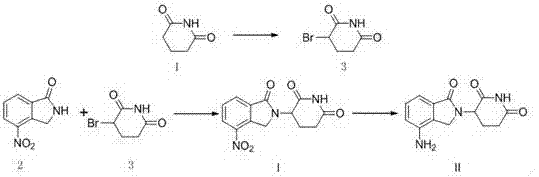
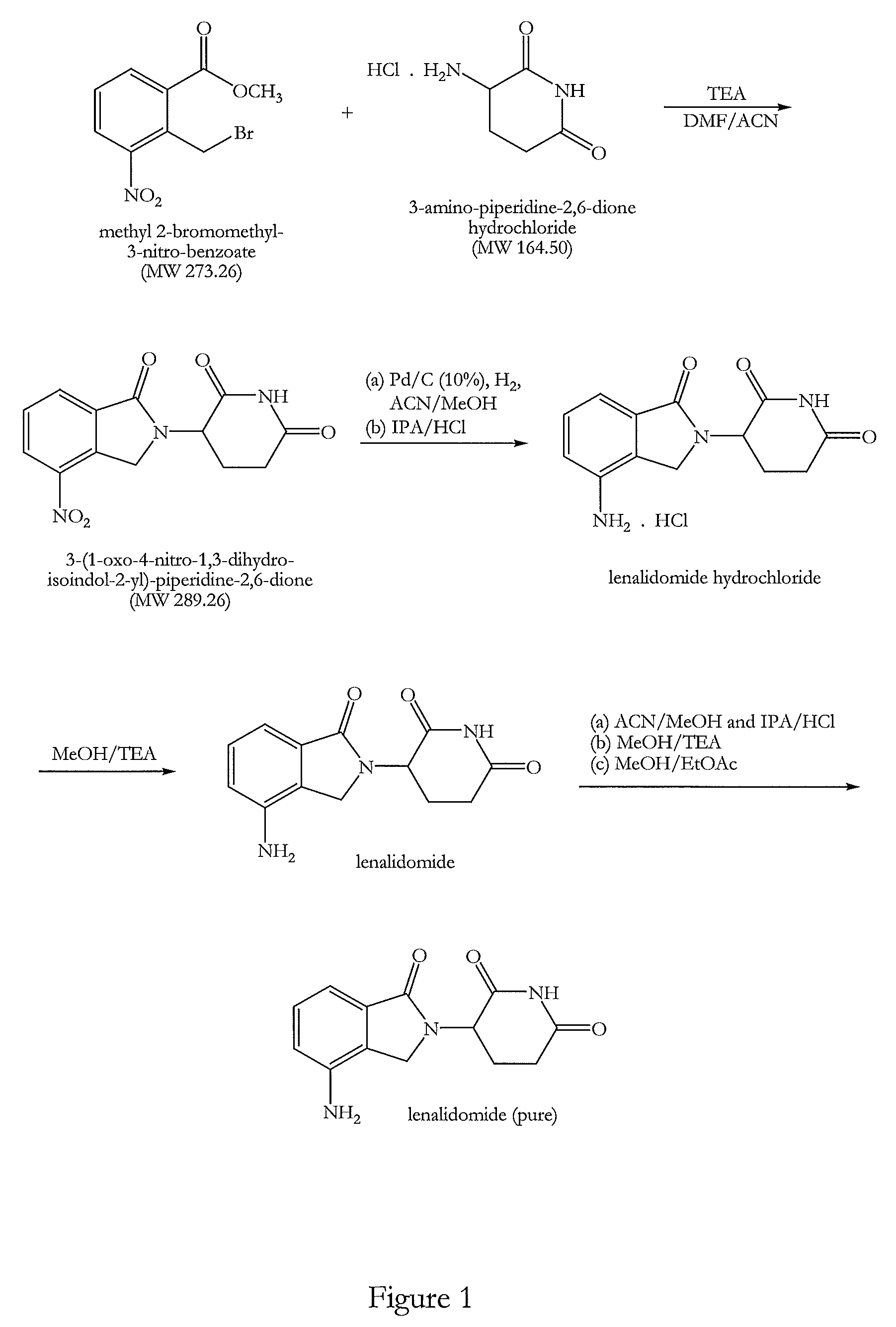
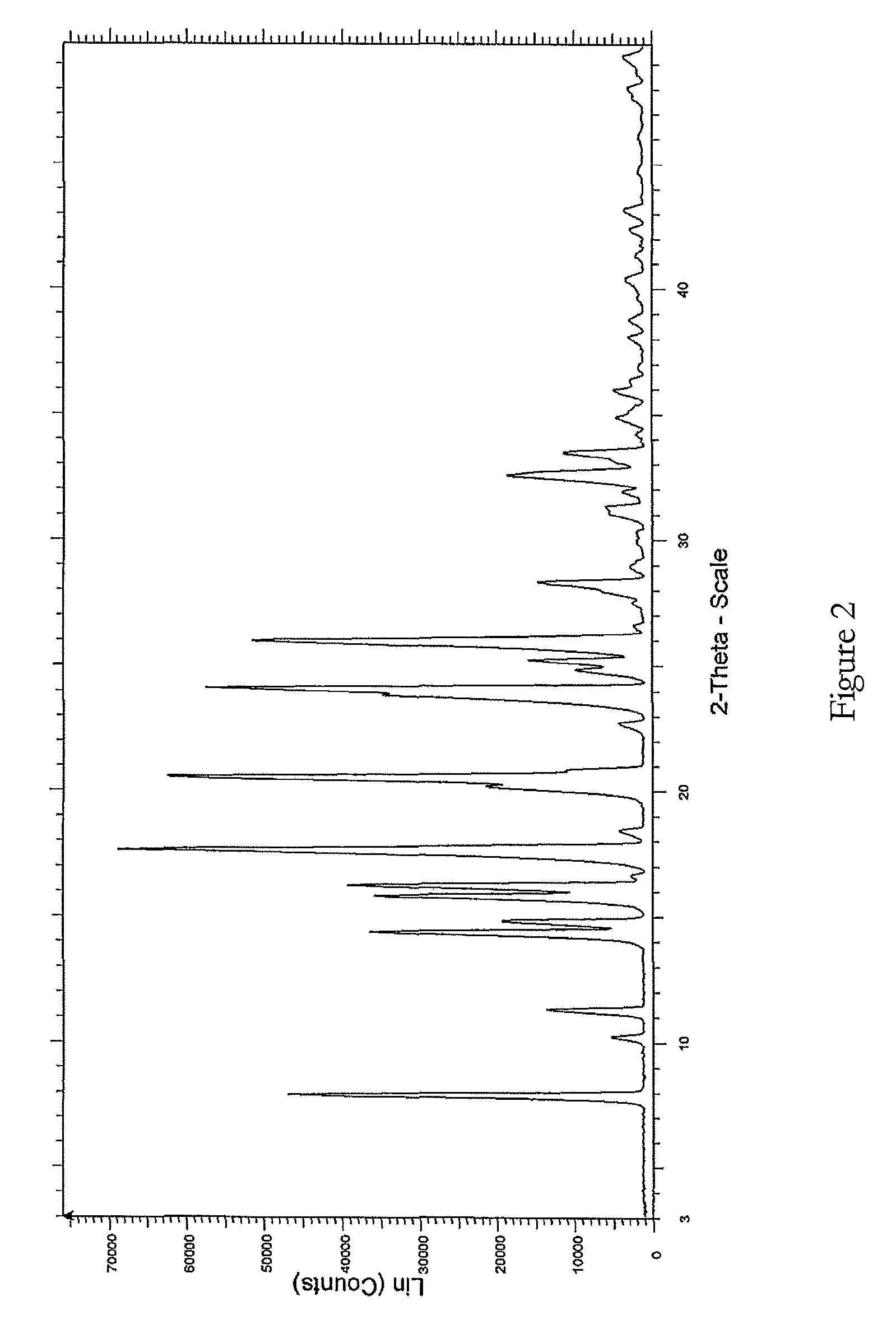
The present invention relates to improved processes for preparing 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione (I) (lenalidomide) and its intermediate 3-(1-oxo-4-nitro-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione. The present invention further relates to improved processes for preparing lenalidomide crystalline form A, use of said crystalline form A as an active pharmaceutical ingredient or as an intermediate in the preparation of further crystalline or amorphous forms of lenalidomide, compositions comprising lenalidomide crystalline form A and their use in the treatment of disease.
Owner:GENERICS UK LTD
Method for preparing lenalidomide
ActiveCN101665484ANovel process routeReasonable process conditionsOrganic chemistryDiketoneIsoindoles
Owner:SHANGHAI HAOYUAN MEDCHEMEXPRESS CO LTD
Anticancer sustained-release gel injection
InactiveCN101336890APharmaceutical delivery mechanismPharmaceutical non-active ingredientsLestaurtinibSolvent
The invention relates to an anticancer slow-release gel injection which contains slow-release microspheres containing an angiogenesis inhibitor, an amphiphilic block polymer, a solvent and a slow-release agent, wherein the composition of the amphiphilic block polymer and the non-organic solvent exhibit the property of temperature-sensitive gelation. After the in vivo injection, the injection turns into a stagnant and biodegradable water-insoluble gel and the gel slowly releases the drug contained therein for a plurality of weeks to a plurality of months. After the intratumoral or peritumoral injection, the anticancer slow-release gel injection can significantly reduce the general drug reactions and is used for treating tumors of different stages. The angiogenesis inhibitor is selected from SU5416, SU6668, bosutinib, sprycel, erlotinib, vandetanib, gefitnib, canertinib, lapatinib, lestaurtinib, masitinib, vatalanib, mubritinib, tandutinib, nilotinib, marimastat, nilotinib, pelitinib, telatinib, sunitinib, sorafenib, zarnestra, sirolimus, imatinfb, lenalidomide and thalidomide.
Owner:济南基福医药科技有限公司
Preparation of lenalidomide
Processes for the preparation of substantially pure lenalidomide. The application also relates to an enriched, substantially pure, and pure amorphous form of lenalidomide and solid dispersions containing amorphous lenalidomide.
Owner:DR REDDYS LAB LTD
Lenalidomide derivative and use thereof as medicine
InactiveCN103396397AEasy to manufactureExtended half-lifeOrganic active ingredientsOrganic chemistryHalf-lifeMedicine
The invention relates to a lenalidomide derivative and a use thereof as a medicine, and in particular relates to a compound shown as a formula (I), a solvate, a hydrate, a stereoisomer or pharmaceutically acceptable salt thereof. The invention further relates to a pharmaceutical composition comprising the compound, and the use of the compound in preparing medicines for preventing or treating malignant tumors, in particular malignant tumors in the blood-lymphatic system. Compared with lenalidomide, the compound provided by the invention has a longer half-life period and can be used for preparing long-acting preparations.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
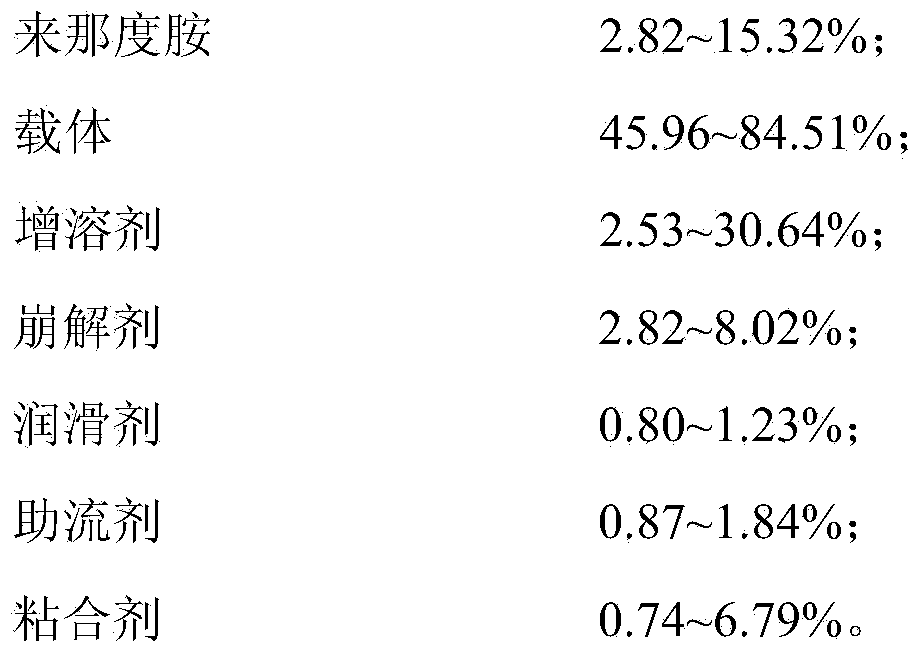
Composite for treating myelodysplastic syndrome and preparation method thereof
ActiveCN103705485ASimple granulation processControllable flyingOrganic active ingredientsPill deliverySolubilityAdhesive
The invention discloses a composite for treating myelodysplastic syndrome. The composite comprises the following components: lenalidomide, a carrier, a solubilizing agent, a disintegrating agent, a lubricating agent, a flow aid and an adhesive, wherein the carrier is a mixture of any one or several of a high-molecular water-soluble polymer, a water-soluble small molecule compound, a hydrophilic auxiliary material and an inorganic carrier; the solubilizing agent is a mixture of one or several of lauryl sodium sulfate, poloxamer, beta. cyclodextrin and a derivative thereof, polysorbate and polyoxyethylene alkyl ether. The invention also provides a preparation method of the composite, and the preparation method comprises the steps of grinding, mixing, dry granulation, total mixing and tabletting or capsule filling. The preparation method disclosed by the invention effectively enhances the water solubility and bioavailability of the lenalidomide; the dry granulation preparation process simplifies the preparation steps, reduces the cost, saves the energy resources, reduces the labor expenditure and realizes the energy conservation and environment protection in production.
Owner:AC PHARMA CO LTD
Method for preparing lenalidomide
InactiveCN104311536ASignificant technological progressOptimize the process routeCarbamic acid derivatives preparationOrganic compound preparationDicarbonateL-Glutamin
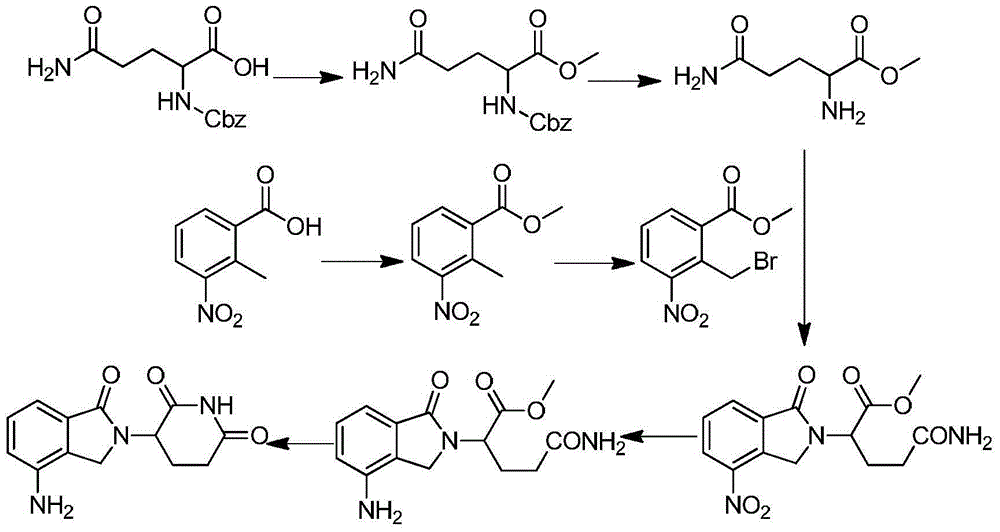
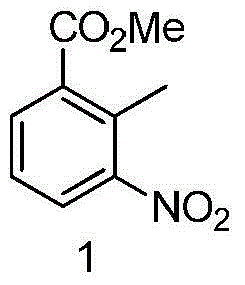
The invention discloses a method for preparing lenalidomide. The method comprises the following steps: firstly, etherifying 2-methyl-3-nitrobenzoic acid to obtain 2-methyl-3-nitrobenzoic acid methyl ester, brominating to obtain 2-brooethyl-3-nitrobenzoic acid methyl ester, reacting L-glutamine and tert-butyl dicarbonate to obtain N-Boc glutamic acid, acquiring 3-amino-2,6-piperidine diketone protected by Boc from N-Boc-glutamic acid in the presence of a condensing agent and a catalyst, further reacting with acid to prepare 3-amino-2,6-piperidine diketone hydrochloride, reacting 3-amino-2,6-piperidine diketone with 2-brooethyl-3-nitrobenzoic acid methyl ester so as to obtain 3-(4-nitryl-1,3 dihydro-1-oxo-2 hydrogen-isobenzazole-2-yl) piperidine-2,6-diketone, and finally reducing, thereby obtaining lenalidomide. The method disclosed by the invention is high in product yield.
Owner:SHANGHAI INST OF TECH
Method for simultaneously detecting multiple anti-tumor drugs in blood sample
InactiveCN110927297AEasy to handleImprove throughputComponent separationBusulfanTandem mass spectrometry
The invention discloses a method for simultaneously detecting multiple anti-tumor drugs in a blood sample. A pretreated sample to be detected is detected by adopting ultra-high performance liquid chromatography-tandem mass spectrometry (HPLC-MS / MS). The pretreatment process comprises the following steps: adding serum into a mixed solution of methanol and acetonitrile, oscillating and centrifuging,taking out the centrifuged supernatant, drying, dissolving the dried powder into a methanol aqueous solution, and filtering to obtain a sample to be detected. The method can be used for simultaneously detecting 13 kinds of anti-tumor drugs such as methotrexate, 5-fluorouracil, apatinib, busulfan, carboplatin, cyclophosphamide, docetaxel, gemcitabine, imatinib, illinotecan, lenalidomide, oxaliplatin, paclitaxel and the like in blood.
Owner:JINAN YING SHENG BIOTECH
Immunomodulator slow-release preparation and preparation method thereof
ActiveCN103610658AProlong the action timeUniform and constant action timeOrganic active ingredientsPill deliveryBlood concentrationProlonged-release tablet
The invention discloses an immunomodulator slow-release preparation and a preparation method thereof. A lenalidomide slow-release tablet is composed of a slow-release layer and an optional quick-release layer, wherein the slow-release layer contains active ingredients of lenalidomide and a slow-release framework material simultaneously; the quick-release layer does not contain the slow-release framework material. The lenalidomide slow-release tablet disclosed by the invention is capable of slowly and uniformly releasing medicines by virtue of the slow-release framework material, so as to reduce the release speed, delay the time to peak, prolong the action time of lenalidomide, and provide a uniform and constant blood concentration. Moreover, The lenalidomide slow-release tablet disclosed by the invention is simple in prescription and excellent in quality stability; the preparation process is simple to operate, free from special treatment and production equipment, low in production cost, and beneficial to batch-enlarged industrial production for the product; the preparation method is high in yield, the granulation and crushing procedures are simple and practicable to operate, the intermediate material is good in stability, flowability, compressibility and content uniformity, and completely meets the requirements of tabletting, and the surface of the prepared tablet is smooth and beautiful.
Owner:AC PHARMA CO LTD
Methods using 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione for treatment of certain leukemias
Methods of treating, preventing or managing leukemias are disclosed. The methods encompass the administration of an immunomodulatory compound of the invention known as Revlimid® or lenalidomide. The invention further relates to methods of treatment using this compound with chemotherapy, radiation therapy, hormonal therapy, biological therapy or immunotherapy. Pharmaceutical compositions and single unit dosage forms suitable for use in the methods of the invention are also disclosed.
Owner:CELGENE CORP
Method for preparing lenalidomide
ActiveCN103497175AHigh purityEfficient manufacturingOrganic chemistryBulk chemical productionPhotochemistry2-methylbenzoic acid
The invention discloses a method for preparing lenalidomide. The method comprises the step of synthesizing lenalidomide by taking 3-amino-2-methylbenzoic acid as an initial raw material. By utilizing the method for preparing lenalidomide, lenalidomide can be effectively prepared, the method is simple in technology, and high in synthesis efficiency, and the purity of prepared lenalidomide is higher. In addition, the preparation method has the advantages of being easily available for the initial raw material, simple for technological operation and mild in reaction conditions, dispensing with special reaction equipment, and having no difficultly separable compounds in the preparation process and the like, thus being more suitable for producing lenalidomide industrially on a large scale.
Owner:HUBEI BIO PHARMA IND TECHCAL INST
Novel anti-cancer medicaments using NGR(NO2) as targeting carrier, preparation thereof and use thereof
ActiveCN101948507AInhibition of anti-tumor effectImprove targetingTripeptide ingredientsPeptidesTumor targetCytarabine
The invention provides novel anti-cancer precursor medicaments using NGR(NO2) as a targeting carrier. The novel anti-cancer precursor medicaments are prepared by designing and synthesizing a 5-fluorouracil precursor medicament, a lenalidomide precursor medicament, a cytarabine precursor medicament, an epirubicin precursor medicament and a dasatinib precursor medicament. According to the initial research on the anti-tumor activity of the 5-fluorouracil precursor medicament, the 5-fluorouracil precursor medicament can inhibit the invasion and metastasis of tumor cells and the growth of solid tumors. The 5-fluorouracil precursor medicament is modified in both effectiveness and preparation compared with the 5-fluorouracil serving as a parent medicament and is widely applicable. Concretely, the invention mainly relates to three aspects: (1) design and antiangiogenic effect of a novel tumor-targeted tripeptide NGR(NO2); (2) preparation of anti-cancer precursor medicaments by coupling the tumor-targeted tripeptide NGR(NO2) and 5 anti-cancer medicaments through covalent bonds; and (3) antitumor and antiangiogenic medical use of the novel 5-fluorouracil precursor medicament.
Owner:廖年生 +1
Novel preparation method of lenalidomide
InactiveCN103601717AProcess raw materials are easy to getShort stepsOrganic chemistryBromineEconomic benefits
The invention discloses a novel preparation method of lenalidomide. Glutarimide and 4-nitro-isoindolin-1-one are used as raw materials of the lenalidomide. The lenalidomide is prepared by (1) performing bromination in the alpha position of the carbonyl of the glutarimide to obtain 3; (2) condensing 4-nitro-isoindolin-1-one and the 3 to obtain 4; and (3) subjecting the 4 to reduction to obtain 5, namely the lenalidomide. The preparation method has easily available raw materials, short steps, simple operation, high reaction yield and low production cost, and is environmental friendly. The preparation method has large implement value and social and economic benefit.
Owner:湖南华腾制药有限公司
Clinically Proven Subcutaneous Pharmaceutical Compositions Comprising Anti-CD38 Antibodies and Their Uses in Combination with Lenalidomide and Dexamethasone
InactiveUS20200308284A1Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesPharmaceutical drug
The present invention relates to clinically proven subcutaneous pharmaceutical compositions comprising anti-CD38 antibodies and methods of their uses in combination with lenalidomide and dexamethasone.
Owner:JANSSEN BIOTECH INC
Combination therapy with lenalidomide and a cdk inhibitor for treating multiple myeloma
InactiveUS20140031325A1Improve efficacyImprove securityBiocideOrganic active ingredientsActive agentCDK inhibitor
Provided herein are methods for the treatment of multiple myeloma, wherein the methods comprise administration of lenalidomide and administration of a second active agent such as a CDK inhibitor.
Owner:CELGENE CORP
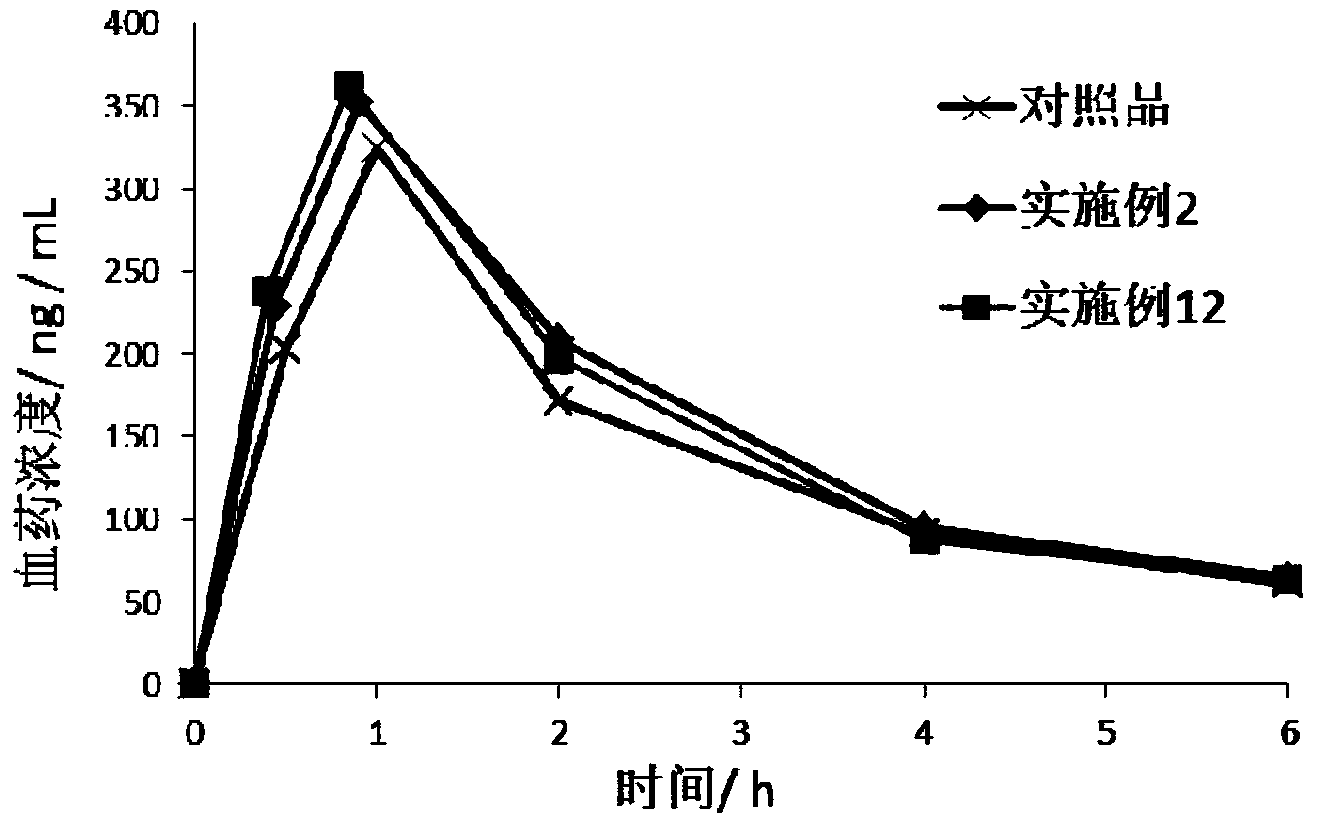
Pharmaceutical composition containing lenalidomide, and preparation method and medical application thereof
InactiveCN106309403AFast absorptionGood pharmacokinetic behaviorOrganic active ingredientsPharmaceutical non-active ingredientsMedicineOperability
The invention relates to a pharmaceutical composition containing lenalidomide, and a preparation method thereof. The pharmaceutical composition is prepared from the lenalidomide, a thinning agent, a disintegrating agent, a lubricating agent and an optional pharmaceutically acceptable carrier. The pharmaceutical composition containing the lenalidomide provided by the invention has favorable stability and drug dissolution rate, the product quality can be controlled, and the preparation method is simple in process, high in operability and suitable for industrialized mass production.
Owner:JIANGSU HANSOH PHARMA CO LTD
Fatty acid lenalidomide and their uses
InactiveUS20120283292A1Convenient treatmentUseful in treatmentBiocideOrganic chemistryFatty acidLenalidomide
The invention relates to fatty acid lenalidomide derivatives; compositions comprising an effective amount of a fatty acid lenalidomide derivative; and methods for treating or preventing a metabolic disease comprising the administration of an effective amount of a fatty acid lenalidomide derivative.
Owner:CATABASIS PHARMA
Methods for treating chronic lymphocytic leukemia and the use of biomarkers as a predictor of clinical sensitivity to immunomodulatory therapies
InactiveUS20170038387A1Organic active ingredientsMicrobiological testing/measurementCompound aOncology
A method of identifying a subject having chronic lymphocytic leukemia (CLL) who is likely to be responsive to a treatment compound, comprising obtaining a first sample and a second sample from the subject having CLL; administering 3-(5-amino-2-methyl-4-oxo-4H-quinazolin-3-yl)-piperidine-2,6-dione (Compound A) to the first sample and administering lenalidomide to the second sample; determining the level of a biomarker in the first sample and determining the level of the biomarker in the second sample; and diagnosing the subject as being likely to be responsive to the treatment compound if the level of the biomarker in the first sample is different from the level of the biomarker in the second sample.
Owner:CELGENE CORP
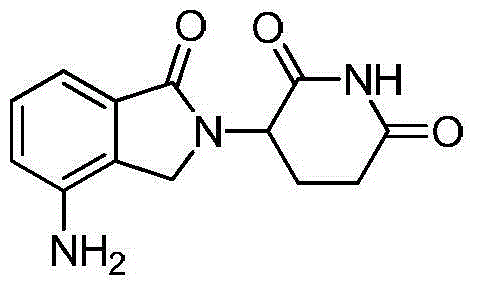
Preparation method of lenalidomide
The invention relates to the field of drug synthesis, and particularly relates to a preparation method of a lenalidomide intermediate and lenalidomide. The compound is a drug for treating multiple myeloma. The method comprises the steps of adopting 2-bromomethyl-3-nitrobenzoate and 3-amino-2,6-piperidione hydrochloride as reaction substrates and an inorganic base as an acid-binding agent and obtaining a white to almost white key intermediate 3-(4-nitro-1-oxo-1,3-dihydro-2H-isoindole-2-yl) piperidine-2,6-diketone of the lenalidomide through simple post-treatment; and adopting a mixed solvent of an organic solvent and water as a reaction solvent and carrying out catalytic hydrogenation in the presence of palladium on carbon to prepare the lenalidomide (II). The process route is low in production cost, and a product is high in purity and friendly to environment, and has relatively great implement value and social and economical benefits.
Owner:CHANGZHOU PHARMA FACTORY
Method for synthesizing lenalidomide
InactiveCN107033126AImprove recycling ratesQuick responseOrganic chemistryChemical recyclingNitro compoundActivated carbon
The invention provides a method for synthesizing lenalidomide, belongs to the field of anti-tumor and anti-leukemia medicines in organic synthesis, and particularly relates to the method for synthesizing lenalidomide. To solve the problems of low yield, low purity, environmental pollution caused by massive harmful waste, excessive cost for environment-friendly treatment, easily occurred risk of violent explosive, degradation caused by long reacting time at high temperature and low catalyst recycling frequency in a traditional lenalidomide synthesis reaction, the method comprises the following steps: 1, adding a lenalidomide precursor nitro compound into an organic solvent, adding an activated carbon catalyst having a Pd loading capacity of 10 percent, then leading the mixture serving as a material I into a pre-heating module of a microchannel reactor; and 2, respectively pumping preheated material I and a material II hydrogen into a reaction module of the microchannel reactor into a reactor, collecting outflowing reaction liquid, and treating to obtain the lenalidomide. The method is environment-friendly, and has the advantages of high reaction yield, high purity and good economical efficiency.
Owner:HEILONGJIANG XINCHUANG BIOLOGICAL TECH DEV CO LTD
Process for the preparation of lenalidomide
The present invention relates to improved processes for preparing 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione (I) (lenalidomide) and its intermediate 3-(1-oxo-4-nitro-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione. The present invention further relates to improved processes for preparing lenalidomide crystalline form A, use of said crystalline form A as an active pharmaceutical ingredient or as an intermediate in the preparation of further crystalline or amorphous forms of lenalidomide, compositions comprising lenalidomide crystalline form A and their use in the treatment of disease.
Owner:GENERICS UK LTD
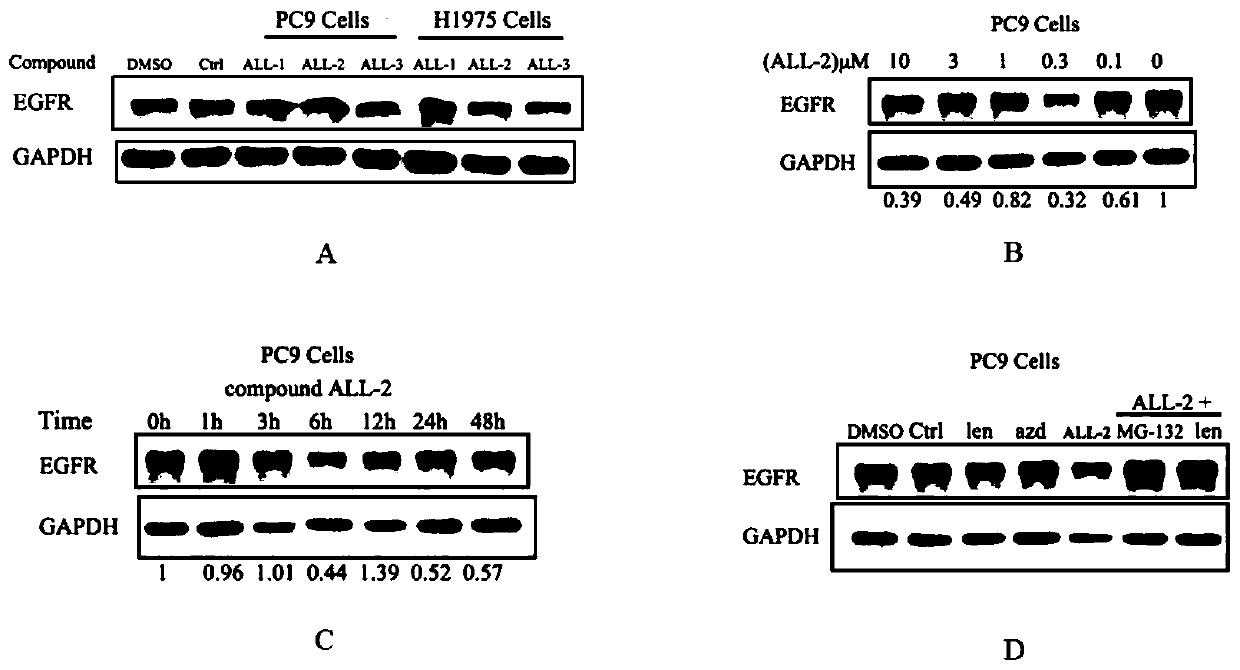
Lenalidomide-based small-molecular compound targeting to EGFR protein degradation, and preparation and application thereof
The invention relates to a lenalidomide-based small-molecular compound targeting to EGFR protein degradation, and preparation and application thereof, and provides a compound targeting to ubiquitination induced EGFR protein degradation and shown as a formula (I) which is described in the specification, and a preparation method and application thereof. Based on lenalidomide, chimeric small molecules capable of targeting to EGFR protein degradation are designed; in-vitro anti-tumor activity tests and in-vitro EGFR protein degradation activity show that the compound has good anti-tumor activity and EGFR protein degradation level, can be used for preventing or / and treating various cancers, and has good application prospects in the field of medicine.
Owner:ZHEJIANG UNIV OF TECH
Method for refining crude lenalidomide product
InactiveCN102993169AAid in crystallizationReduce volatilityOrganic chemistryActivated carbonMaterials science
The invention discloses a method for refining a crude lenalidomide product. The method comprises the following steps of: (1) adding the crude lenalidomide product to a mixed solution of isopropanol and water; (2) heating, after the crude lenalidomide product is completely dissolved, adding activated carbon and nanometer ceramic powder and carrying out heat preservation for the first time; (3) filtering when the solution is hot, and cooling filtrate till crystals precipitate; and (4) carrying out heat preservation for the second time, carrying out suction filtering after the crystal precipitation is finished, and finally drying. Due to adoption of the technical scheme, the method for refining the crude lenalidomide product has the beneficial effects of low isopropanol toxicity, high safety, simpleness in process, no need of special equipment and environment requirements, suitability for industrial production, high product content and high refining yield; and the purify of the lenalidomide is remarkably improved.
Owner:NANTONG YUANYA PRECISION MACHINERY
Methods of Treating Newly Diagnosed Multiple Myeloma with a Combination of An Antibody that Specifically Binds CD38, Lenalidomide and Dexamethasone
PendingUS20200268847A1Raise the possibilityReduce riskOrganic active ingredientsPeptide/protein ingredientsAntiendomysial antibodiesOncology
Disclosed herein are methods of treating multiple myeloma using an antibody that specifically binds CD38 in combination with lenalidomide and dexamethasone.
Owner:JANSSEN BIOTECH INC
Method for preparing lenalidomide
InactiveCN102838586AEasy to manufactureEfficient preparationOrganic chemistryChemical synthesisMethyl benzoate
The invention relates to the field of chemical synthesis, and discloses a method for preparing lenalidomide, which comprises the following steps: condensing 3-aminopiperidyl-2,6-diketohydrochloride with 2-halomethyl-3-nitro-methyl benzoate under weakly alkaline conditions, cooling the reaction solution to room temperature, adding into water, stirring, filtering to obtain a filter cake, sequentially washing with water and alcohol reagents, and drying to obtain 3-(7-nitro-3-oxo-1H-isoindazolyl-2-yl)piperidyl-2,6-dione; and carrying out nitro-amino reduction reaction, filtering the reaction solution, concentrating, crystallizing at -20 DEG C to room temperature, filtering, washing the filter cake with alcohol reagents, drying to obtain a lenalidomide crude product, and recrystallizing to obtain the lenalidomide finished product. By adjusting the after-treatment technique, the invention enhances the total yield of lenalidomide, and ensures the high purity; and the whole method has the advantages of mild reaction conditions and no high temperature or high pressure, and can be used for preparing lenalidomide in a simple and efficient way. 2-halomethyl-3-nitro-methyl benzoate.
Owner:CHONGQING TAIHAO PHARM CO LTD
Lenalidomide enantiomer supercritical fluid chromatographic separation method
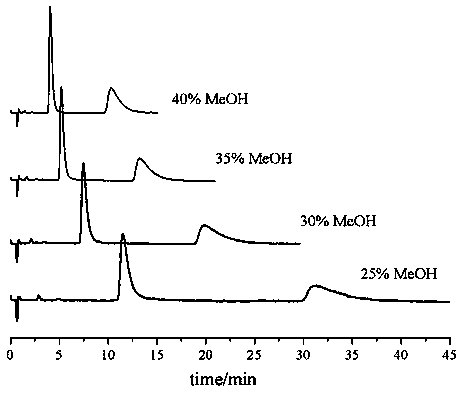
ActiveCN107703235AShort separation timeEasy to separateComponent separationChromatographic separationSilica gel
The present invention relates to a lenalidomide enantiomer supercritical fluid chromatographic separation method, which comprises: dissolving a lenalidomide sample in a lower alcohol, and carrying outchiral analysis separation at a chiral stationary phase by using a supercritical fluid chromatography method, wherein the mobile phase is the mixture of supercritical carbon dioxide and a lower alcohol, the lower alcohol is methanol or ethanol, a volume ratio of the supercritical carbon dioxide to the lower alcohol in the mobile phase is 60-75:40-25, and the chiral stationary phase is one selected from a silica gel surface coated or bonded tris(3,5-dimethylphenylcarbamylated)amylose chiral stationary phase, a silica gel surface bonded 4-chlorophenylcarbamylated-beta-cyclodextrin chiral stationary phase, and a silica gel surface bonded 3,5-dimethylphenylcarbamylated-beta-cyclodextrin chiral stationary phase. According to the present invention, with the supercritical fluid chromatographic separation method, the chiral chromatographic separation of the lenalidomide enantiomer can be well achieved, and under the preferred condition, the resolution of the lenalidomide enantiomer is 5.05.
Owner:GUANGDONG YANJIE PHARMA TECH CO LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com