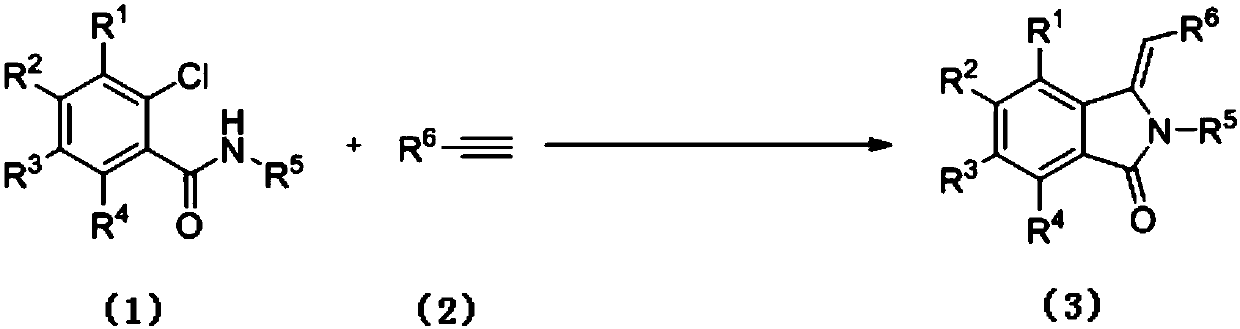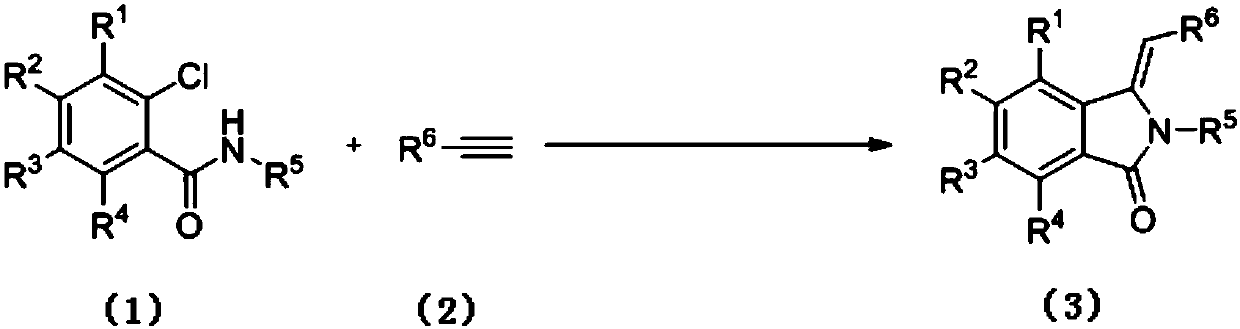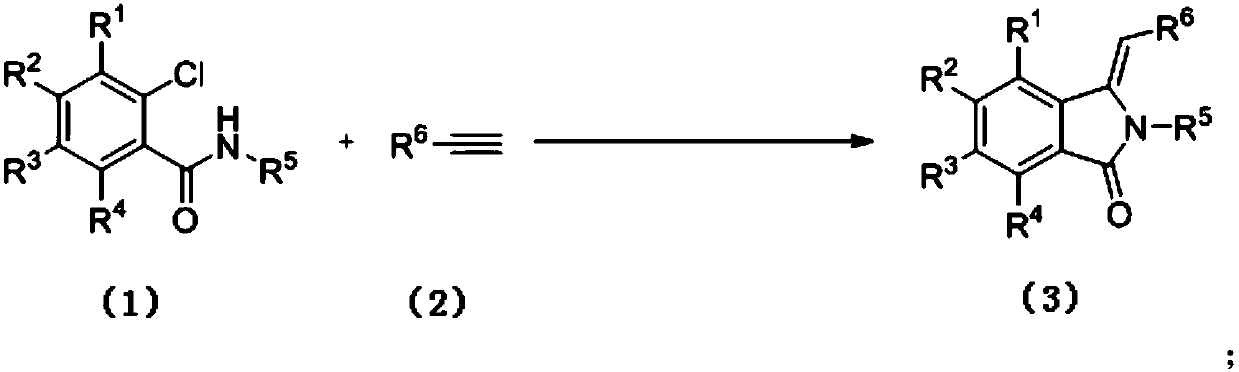Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
176 results about "Isoindoles" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Benzopyrroles with the nitrogen at the number two carbon, in contrast to INDOLES which have the nitrogen adjacent to the six-membered ring.
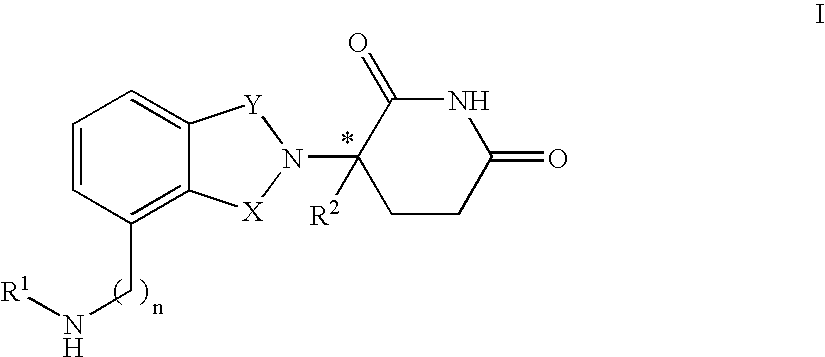
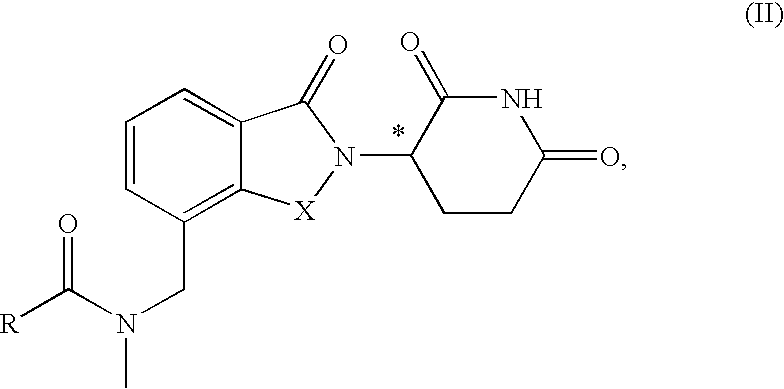
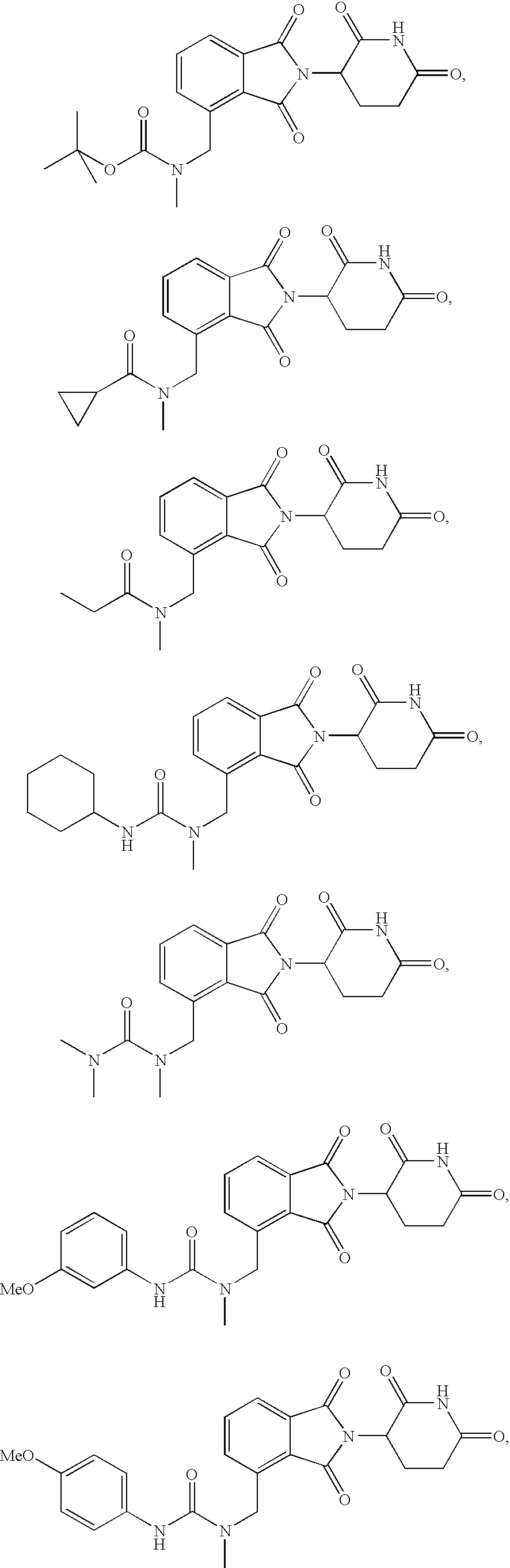
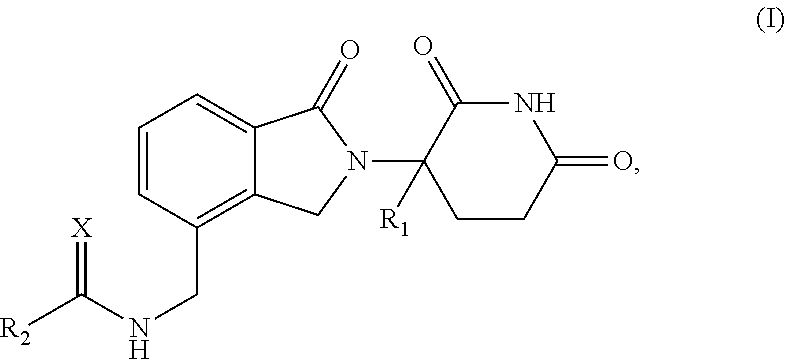
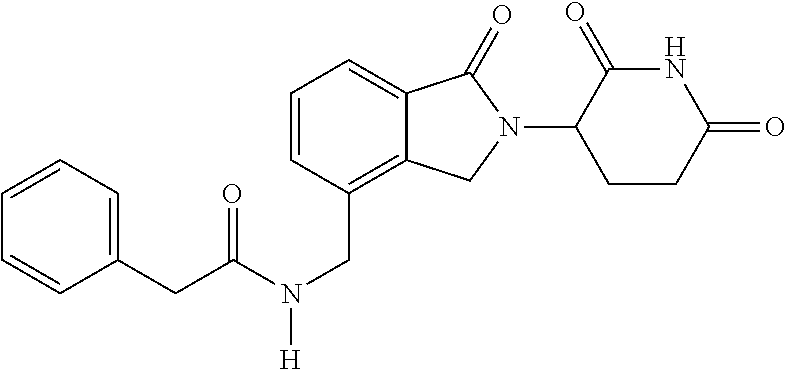
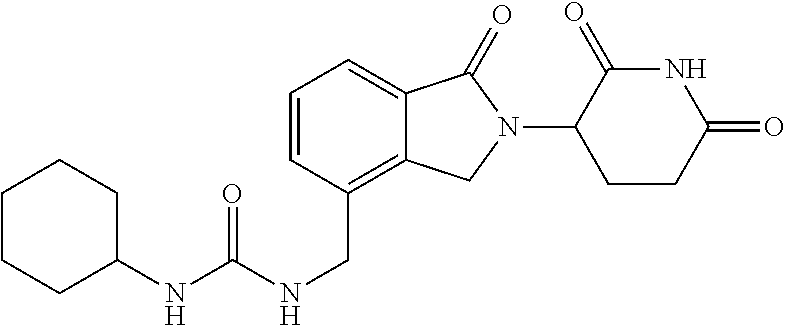
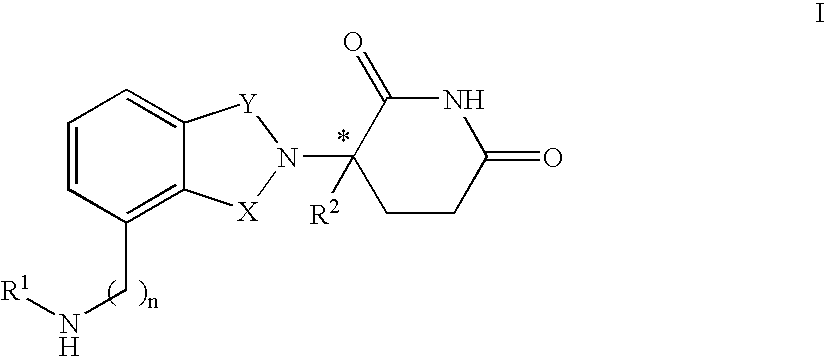
Isoindole-imide compounds, compositions, and uses thereof
The invention relates to isoindole-imide compounds and pharmaceutically acceptable salts, hydrates, solvates, clathrates, enantiomers, diastereomers, racemates, or mixtures of stereoisomers thereof, pharmaceutical compositions comprising these isoindole-imide compounds, and methods for reducing the level of cytokines and their precursors in mammals. In particular, the invention pertains to isoindole-imide compounds that are potent inhibitors of the production of TNF-α in mammals. The isoindole-imides described herein are useful for treating or preventing diseases or disorders in mammals, for example, cancers, such as solid tumors and blood-born tumors; heart disease, such as congestive heart failure; osteoporosis; and genetic, inflammatory; allergic; and autoimmune diseases.
Owner:CELGENE CORP
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
N-methylaminomethyl isoindole compounds and compositions comprising and methods of using the same
This invention relates to N-methylaminomethyl-isoindoline compounds, and pharmaceutically acceptable salts, solvates, stereoisomers, and prodrugs thereof. Methods of use, and pharmaceutical compositions of these compounds are disclosed.
Owner:CELGENE CORP
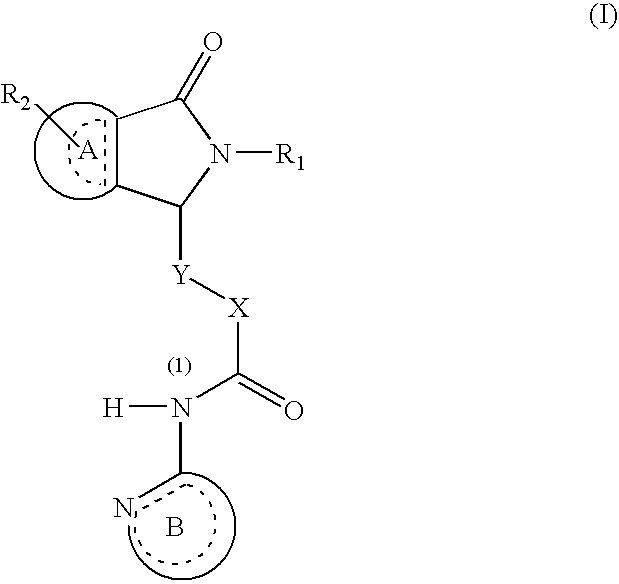
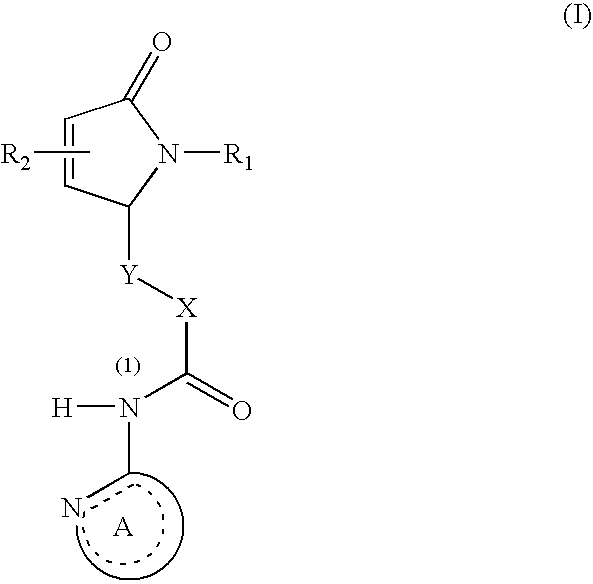
Dihydroisoindolones As Allosteric Modulators Of Glucokinase
The present invention relates to compounds of Formula (I), methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating glucokinase mediated disorders. More particularly, the compounds of the present invention are glucokinase modulators useful for treating disorders including, but not limited to, type II diabetes.
Owner:JANSSEN PHARMA NV
Preparation of mitiglinide calcium and its quality control method
InactiveCN1844096AImprove accuracyHigh sensitivityOrganic active ingredientsOrganic chemistryPropanoic acidMalonate
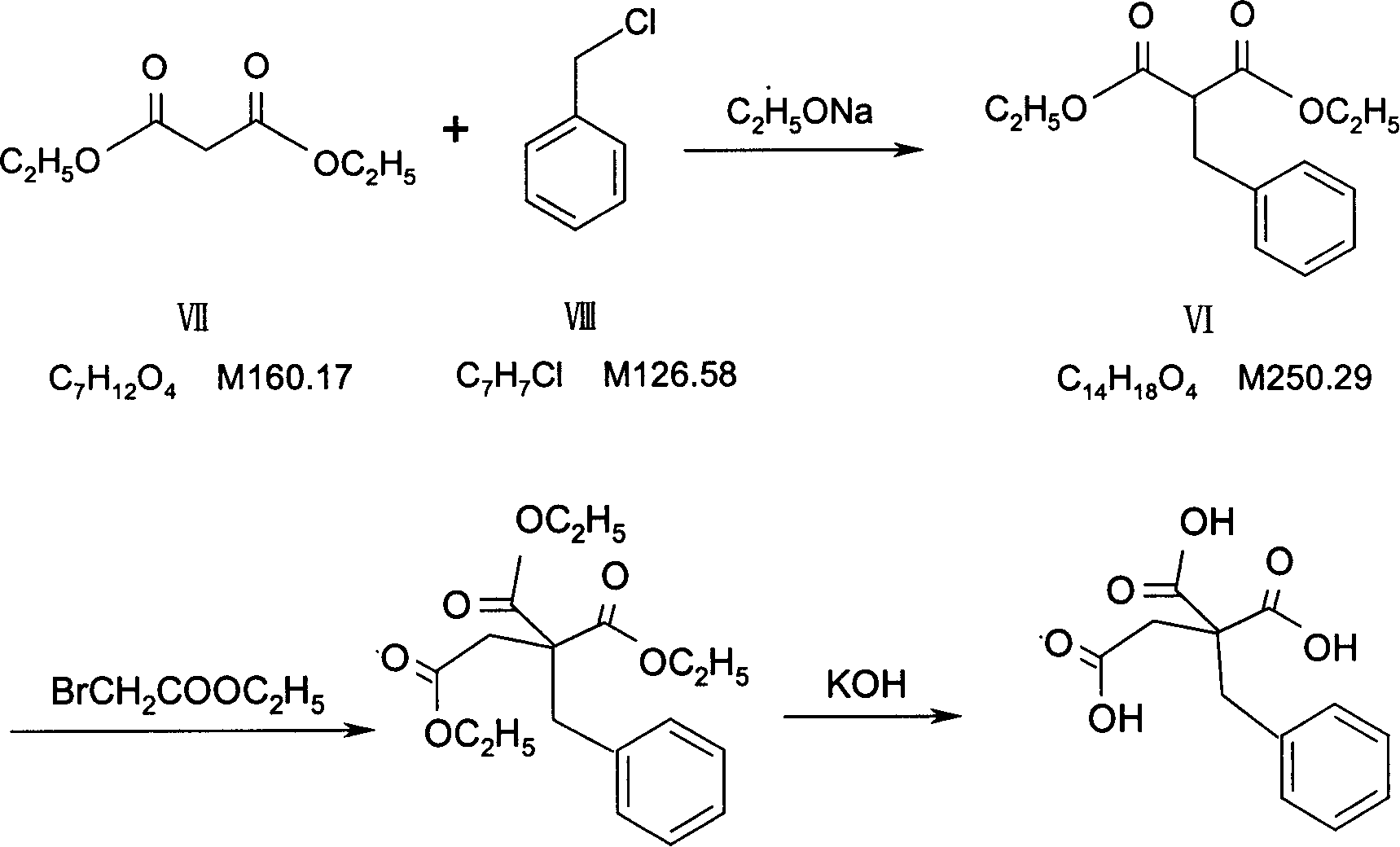
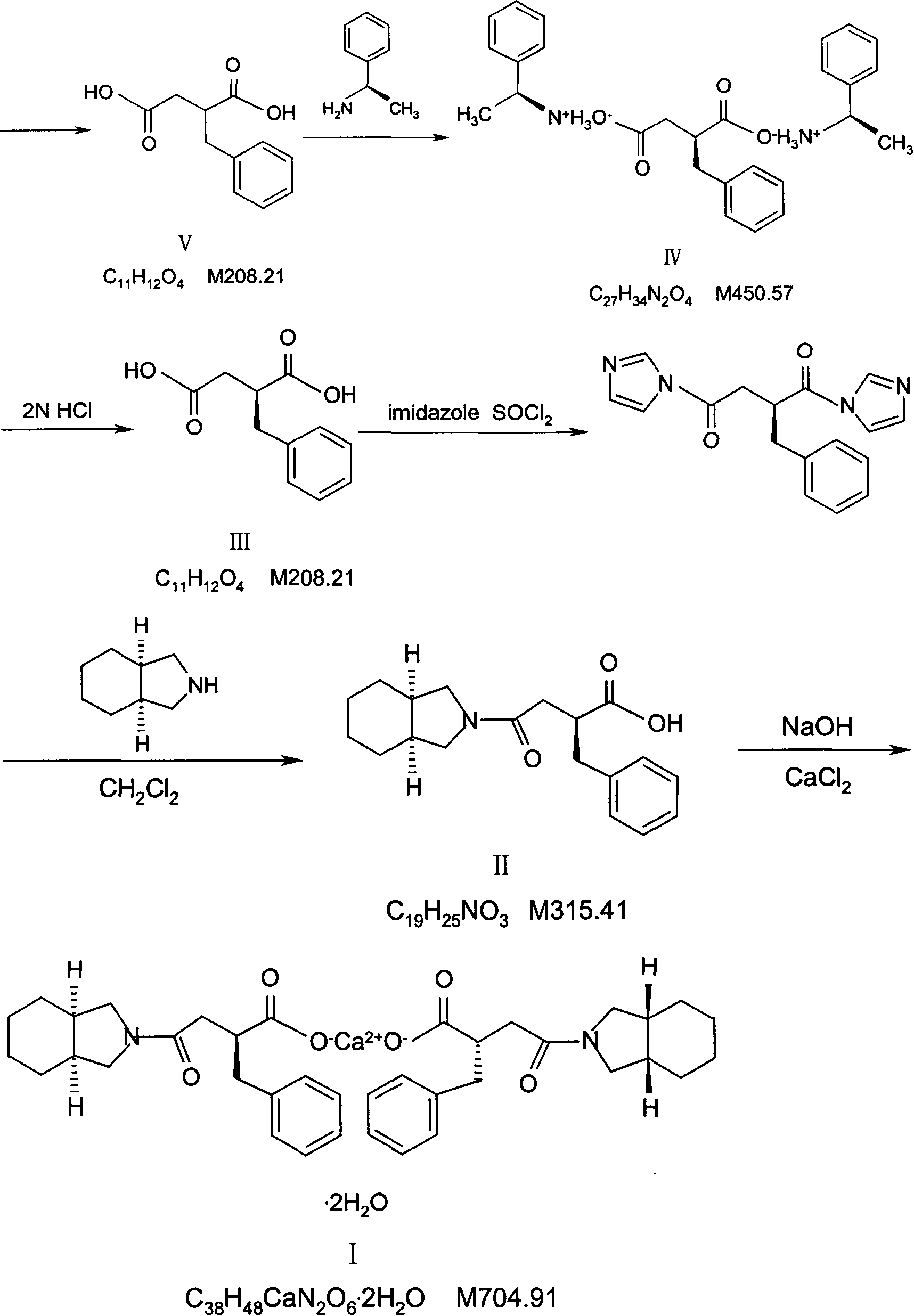
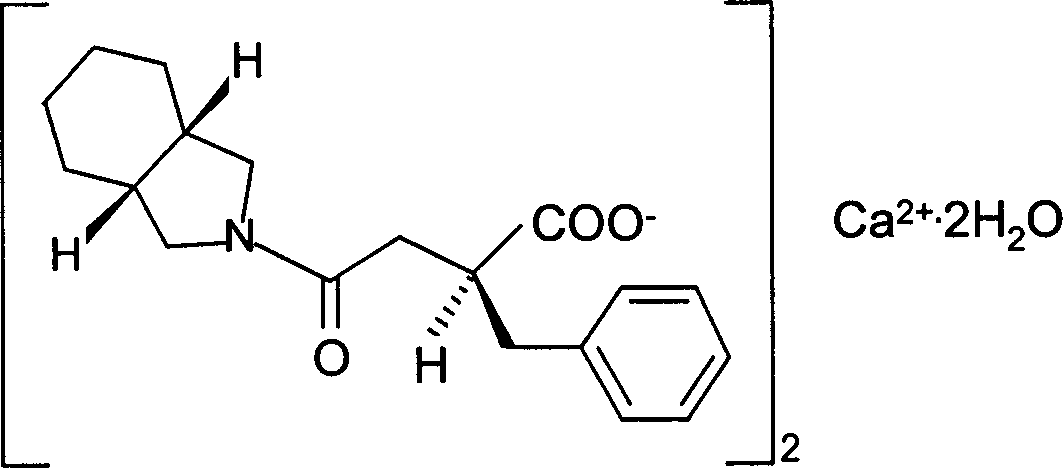
The invention relates to a preparation and quality control method of Mitiglinide Calcium comprising steps: 1,synthesis of cis- cyclohexyl-1,2-dimethylacid imide; 2,synthesis of cis-hexa-hydrogen isoindole; 3,synthesis of alpha-benzyldiethyl malonate; 4,synthesis of benzylsuccinic acid; 5,S- benzylsuccinic acid methylbenzylamine salt; 6,synthesis of s- benzylsuccinic acid; 7,synthesis of (2S)-2- benzyl-3-(cis-hexa-hydrogen isoindole-2- carbonyl) propanoic acid; 8,synthesis of Mitiglinide Calcium; 9,purity of Mitiglinide Calcium. This invention also contains the method for quality control of Mitiglinide Calcium comprising steps: watching deseription, messureing specific rotation, authenticating, checking, content messureing for Mitiglinide Calcium.
Owner:天津汉康医药生物技术有限公司
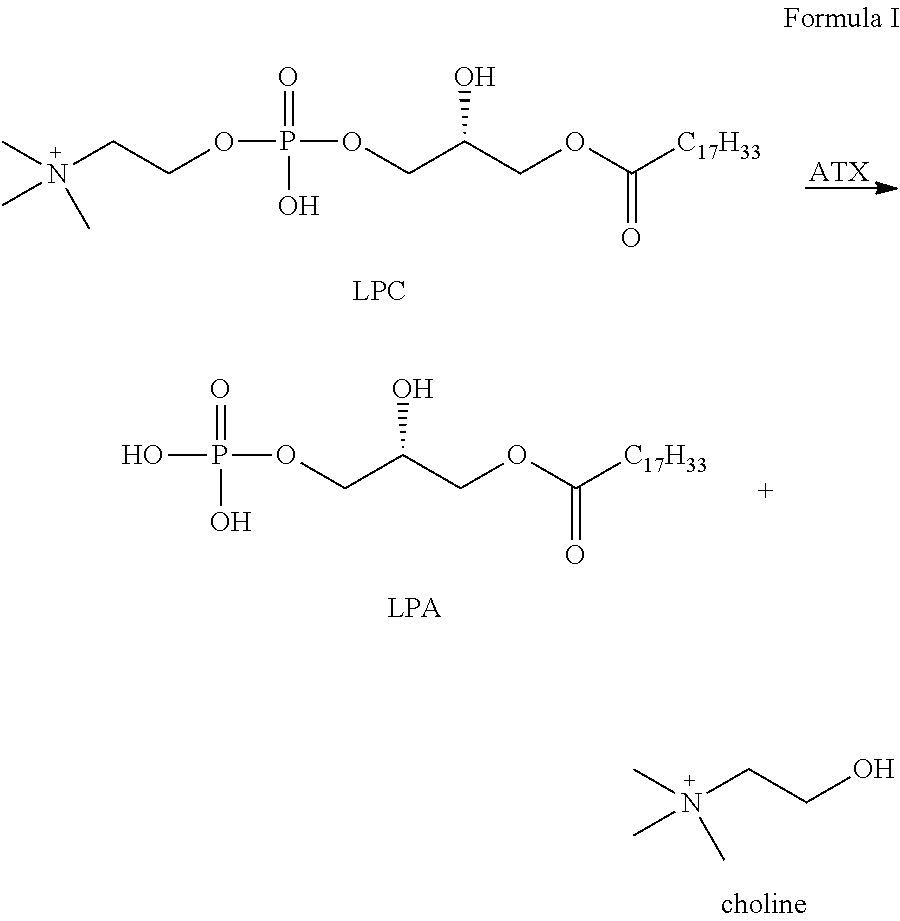
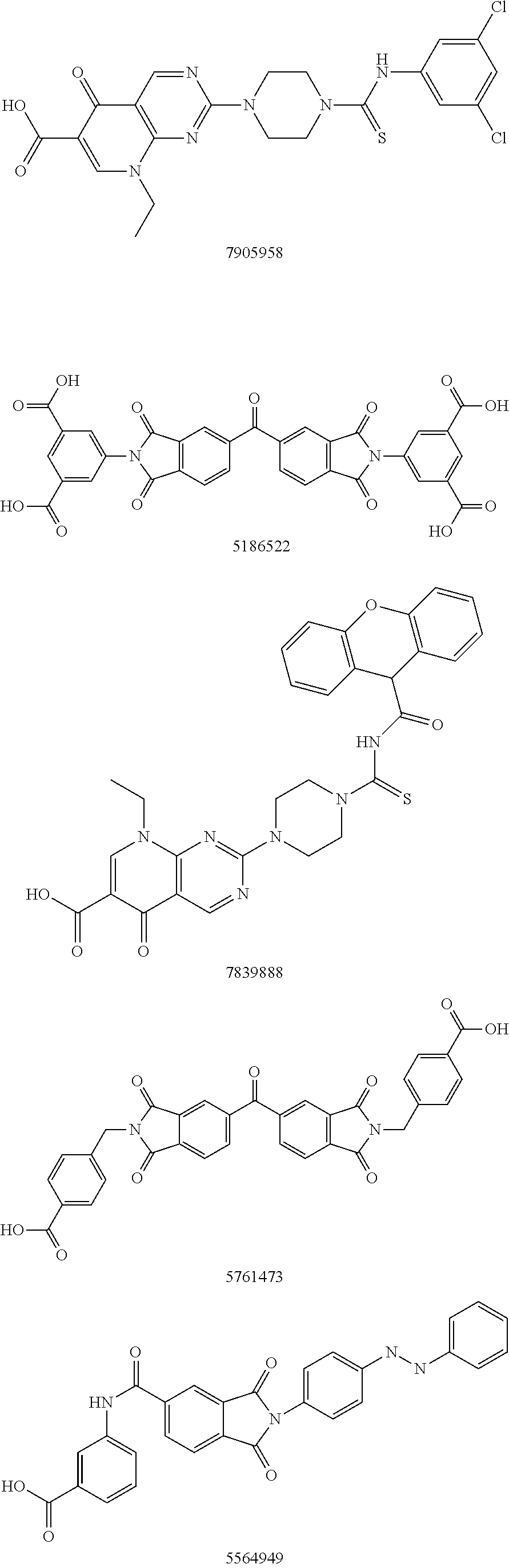
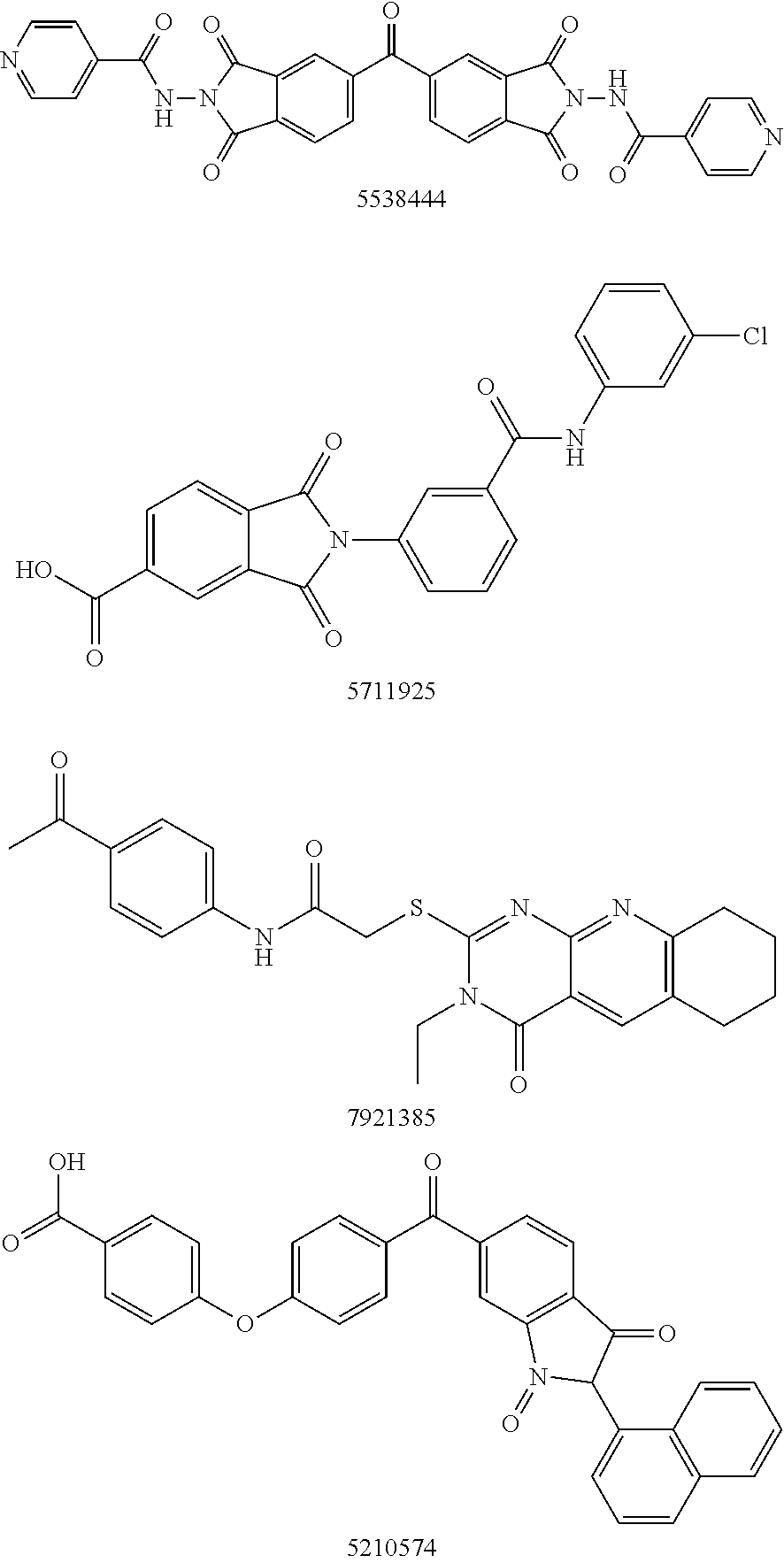
Autotaxin inhibitors
Classes of compounds that exhibit effective inhibition of autotaxin enzymes are provided. Such classes include thioureas, diphenyldiazerenes, xanthenes, and isoindoles and exhibit reactivity with autotaxin to ultimately reduce the size of the reactive sites thereon to prevent conversion of lysophosphatidyl choline to lysophophatidic acid. Furthermore, such compounds can be incorporated within delivery forms for human ingestion. As such, these compounds accord an excellent manner of potentially reducing generation of certain cancers attributable to the presence of naturally occurring autotaxin within the human body. Methods of inactivating autotaxin to certain degrees therewith such compounds are encompassed within invention as well.
Owner:UNIVERSITY OF MEMPHIS RESEARCH FOUNDATION
Process
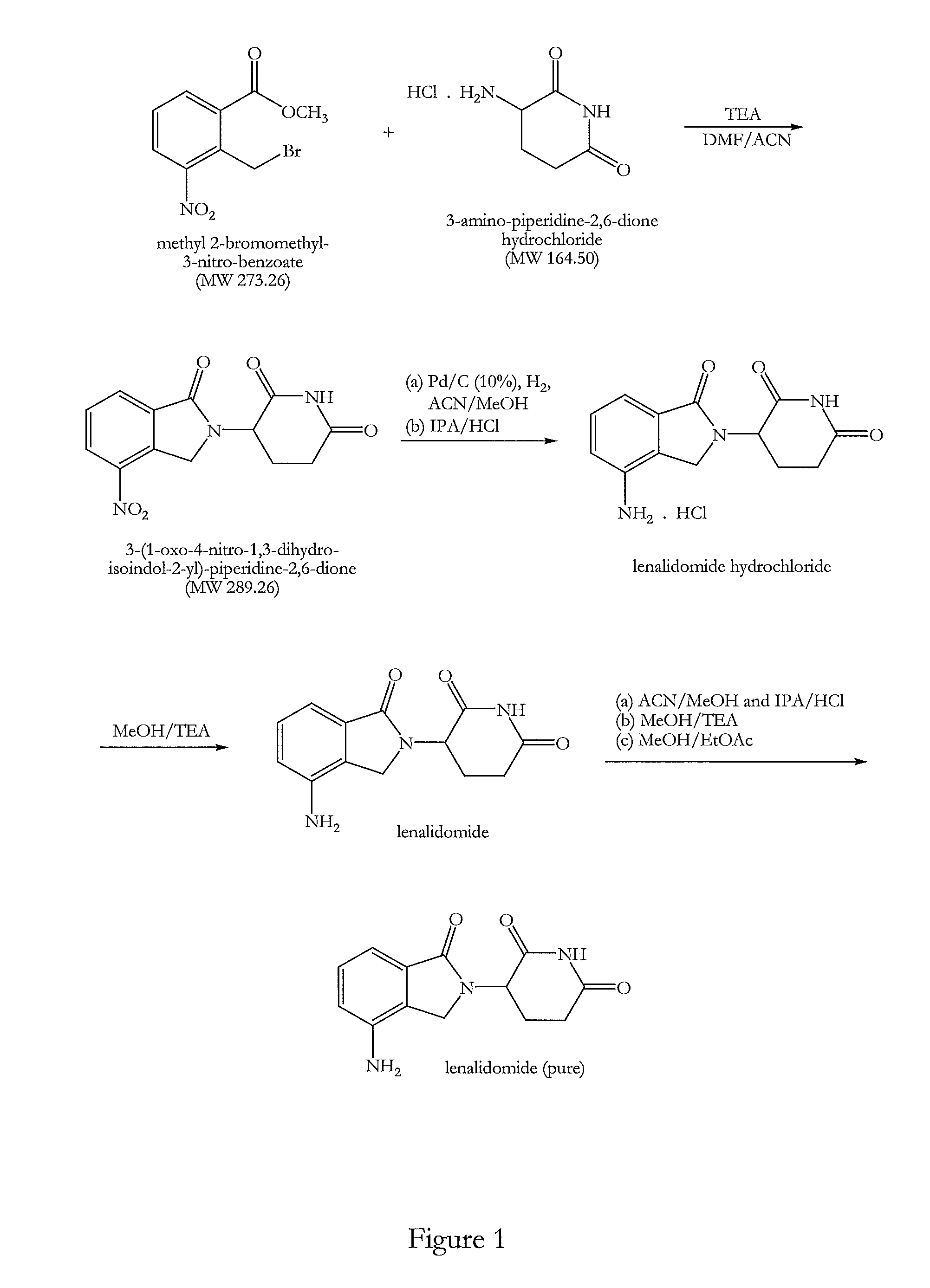
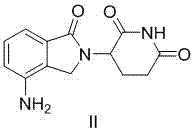
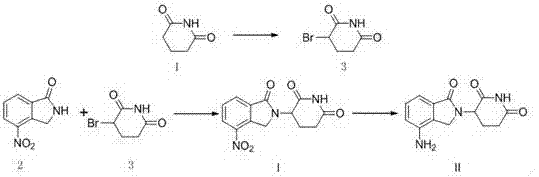
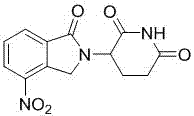
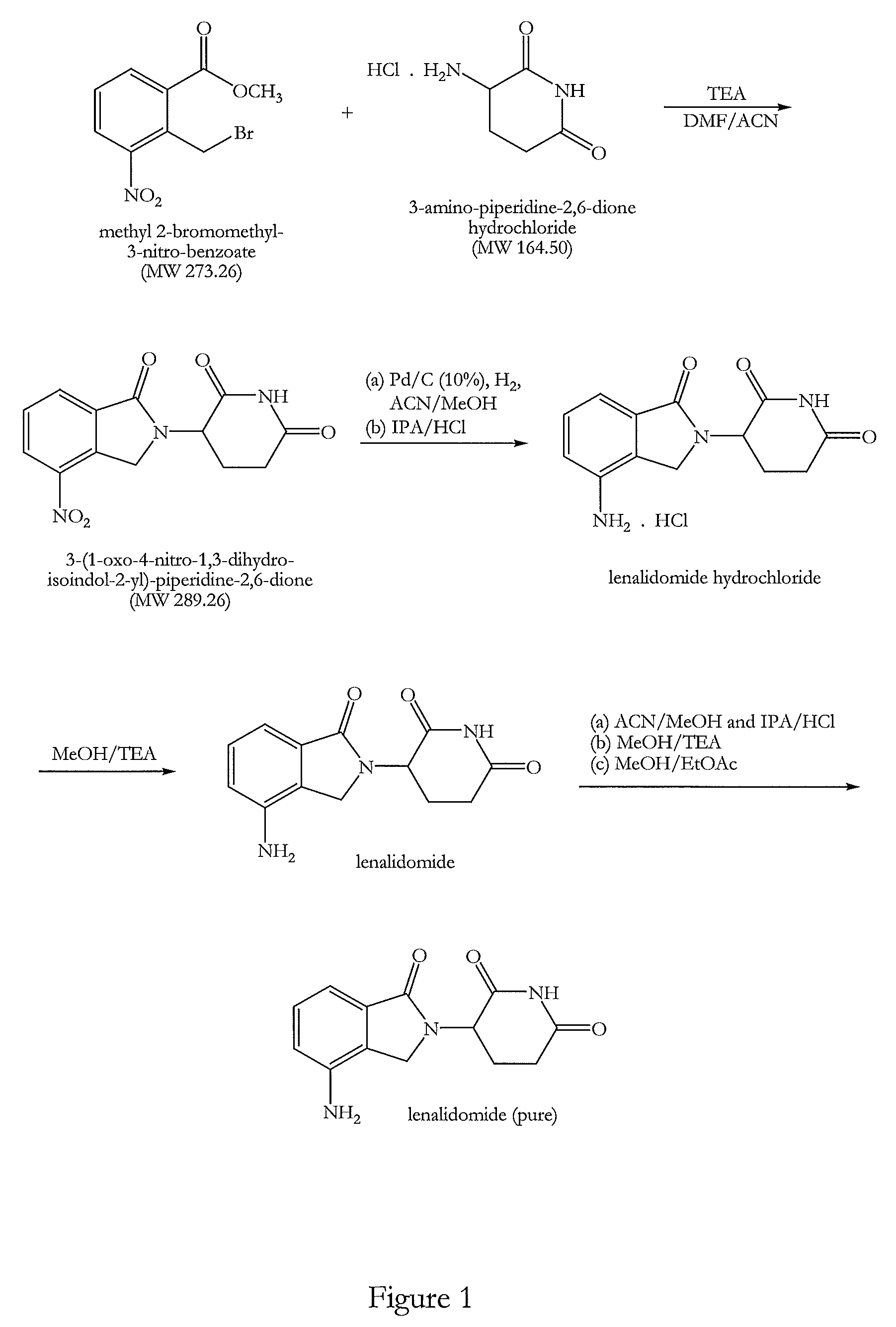
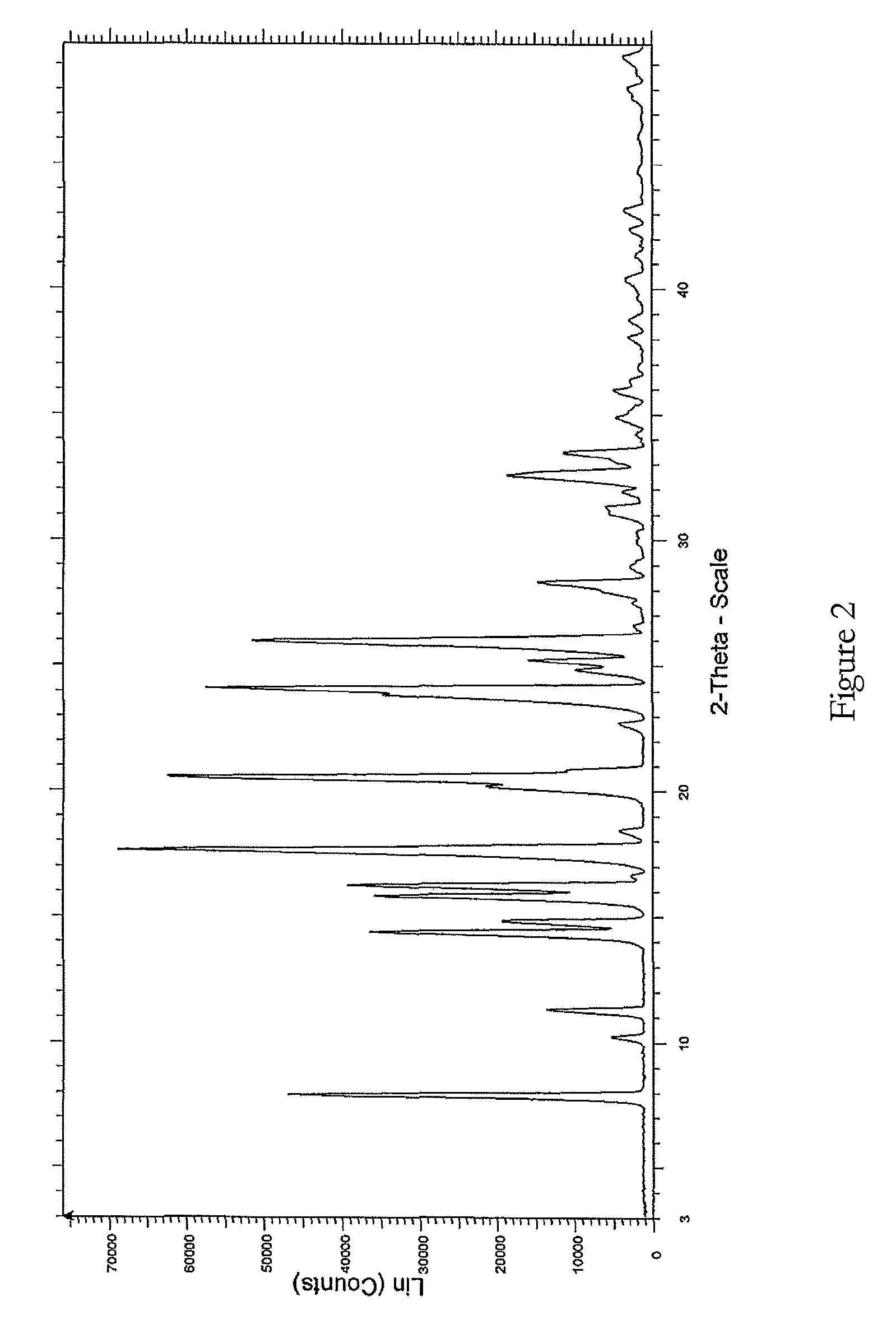
The present invention relates to improved processes for preparing 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione (I) (lenalidomide) and its intermediate 3-(1-oxo-4-nitro-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione. The present invention further relates to improved processes for preparing lenalidomide crystalline form A, use of said crystalline form A as an active pharmaceutical ingredient or as an intermediate in the preparation of further crystalline or amorphous forms of lenalidomide, compositions comprising lenalidomide crystalline form A and their use in the treatment of disease.
Owner:GENERICS UK LTD
Method for preparing lenalidomide
ActiveCN101665484ANovel process routeReasonable process conditionsOrganic chemistryDiketoneIsoindoles
Owner:SHANGHAI HAOYUAN MEDCHEMEXPRESS CO LTD
Use of isoindoles for the treatment of neurobehavioral disorders
InactiveUS20090318520A1Organic active ingredientsBiocideBehavioural disordersNorepinephrine transporter
The present invention generally relates to the use of drugs for the treatment of neurobehavioral disorders or symptoms of a neurobehavioral disorder associated with dysfunction of the trimonoamine modulating system (TMMS). More specifically, the invention describes methods for the treatment of a neurobehavioral disorder and / or treatment or prevention of symptoms of a neurobehavioral disorder by administering suitable Isoindole derivatives alone or in combination with other agents so as to provide relatively equal inhibitory effect on serotonin, dopamine and norepinephrine transporters.
Owner:LAPKO FOUND +1
Isoindole alkaloid compound in purslane, and extraction and separation method of isoindole alkaloid compound
ActiveCN107459477AHigh purityAnti-inflammatoryOrganic active ingredientsOrganic chemistryInfraredUltraviolet
The invention relates to the field of extraction and separation of traditional Chinese medicines, in particular to an isoindole alkaloid compound which is extracted, separated and identified from purslane, and an extraction and separation method of the isoindole alkaloid compound. The new alkaloid compound has a molecular formula of C28H23NO8 and is named as Oleraisoindole. The invention also provides the extraction and separation method of the isoindole alkaloid compound; the method comprises the steps of sequentially extracting by means of water boiling, extracting by using ethyl acetate, carrying out silica gel column chromatography, purifying by using an octadecylsilyl medium-pressure column and Sephadex LH-20, and carrying out liquid phase separation. The isoindole alkaloid compound is identified by using ultraviolet (UV), infrared ray (IR), HR-ESI-TOF-MS, hydrogen-1 nuclear magnetic resonance (1H-NMR), carbon-13 nuclear magnetic resonance (13C-NMR) and a two-dimensional nuclear magnetic spectrum analysis method. The compound has potential anti-inflammatory activity, anti-tumor activity, and the like; the invention also provides the preparation method of the compound, thus providing a lead compound and a theoretical basis for development of new medicines and new components.
Owner:LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
Synthetic process of hydrochloric acid trientine
InactiveCN102924289AReduce usageReduce pollutionOrganic compound preparationAmino compound preparationPotassium cyanideKetone
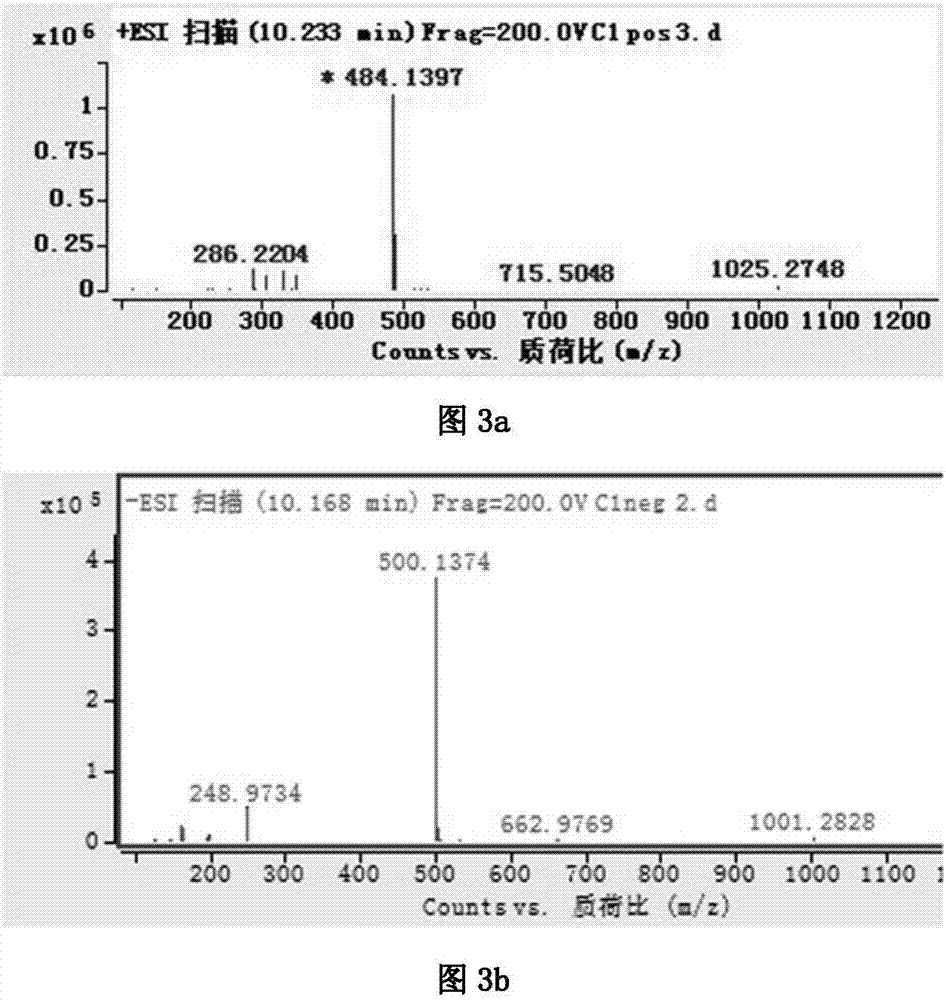
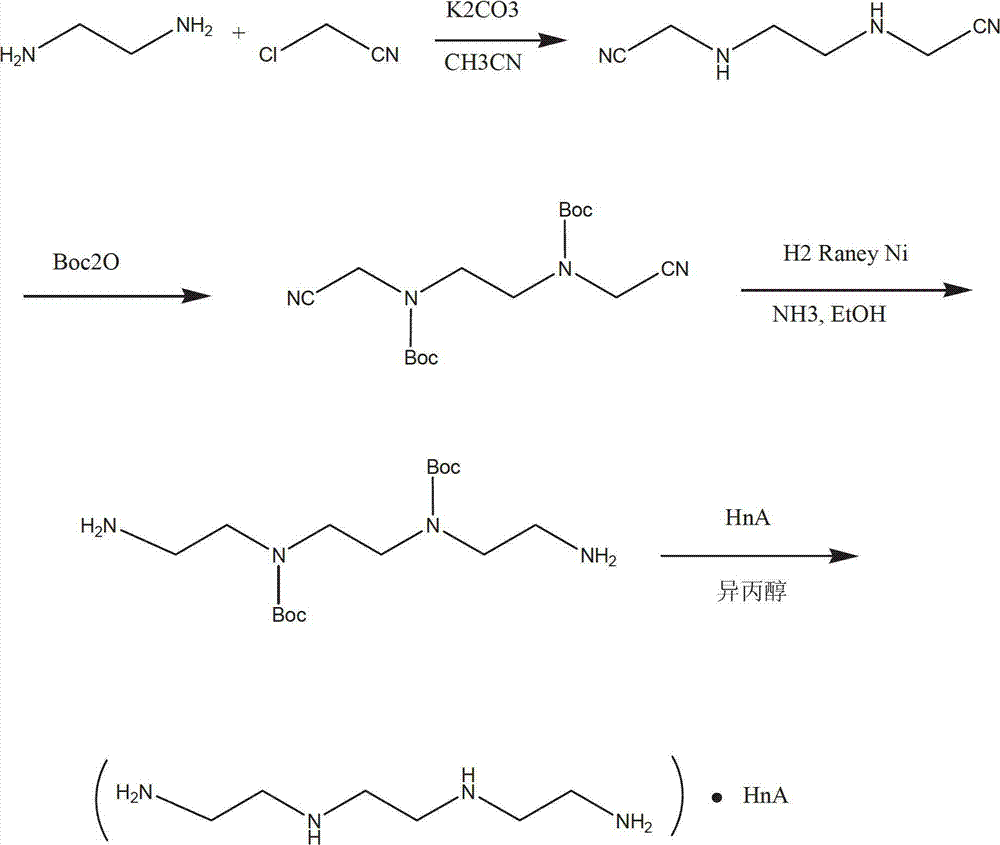
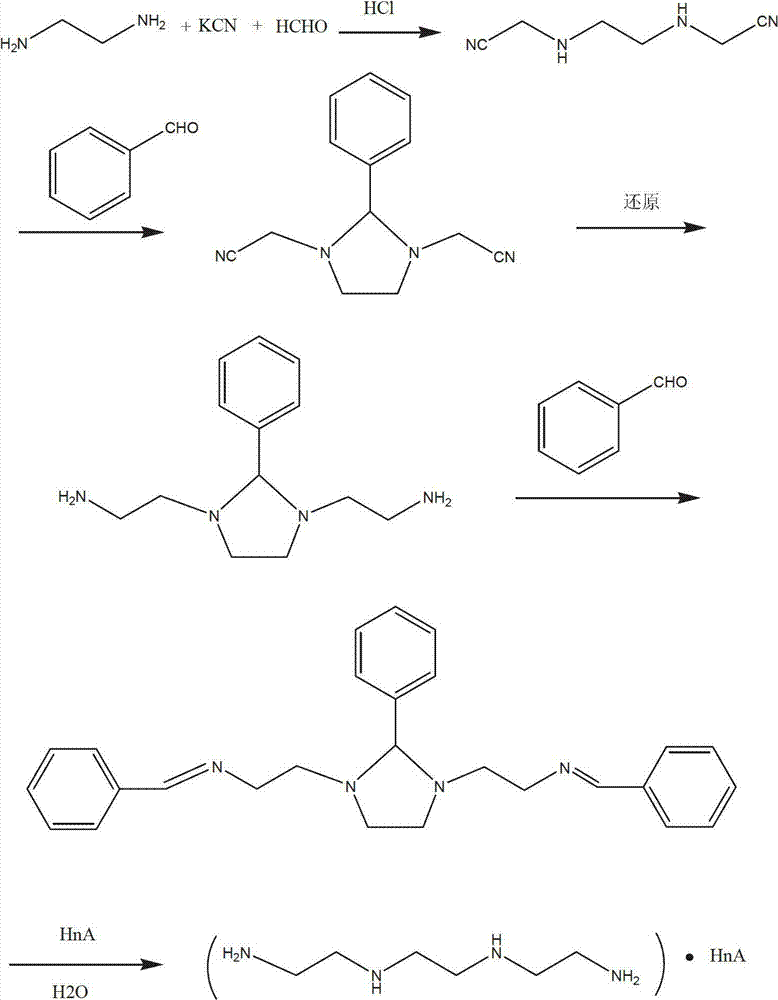
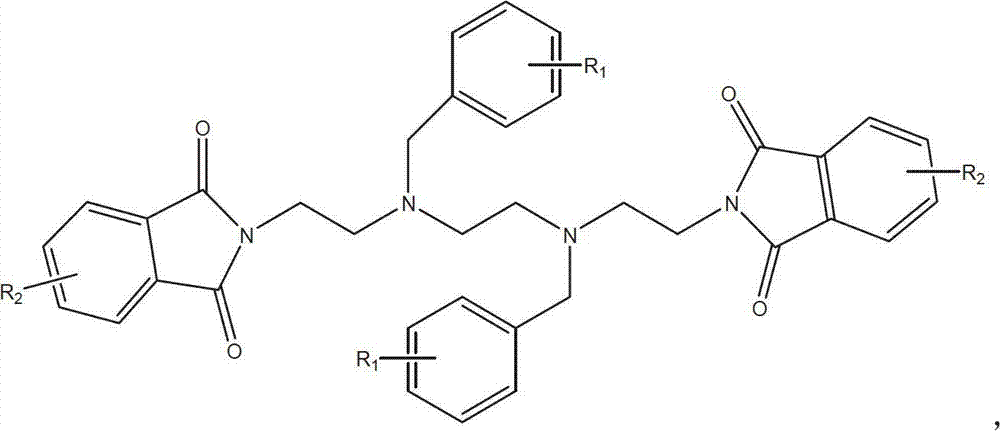
The invention discloses a novel synthetic process of hydrochloric acid trientine, and aims at providing the synthetic method of the hydrochloric acid trientine. The synthetic process can avoid using a highly-toxic material, namely potassium cyanide, and is temperate in reaction condition. A compound 2,2'-(2,2'-(ethane-1,2-diradical-bibenzyl-di (ethane-2,1-double radical)) bi isoindole-1,3-diketone is used as an initiator, after hydrazinolysis, an intermediate N1, N1'-(ethane-1,2-diradical) di(N1-nethyl ethane-1,2-diamine) is obtained. Through carbobenzoxy protection (Cbz protection), salt is generated,and dibenzyl 2,2'-(ethane-1,2-diradical-di (benzylamine-diradical)) di (ethane-2,1-diradical) dioctyl phthalatic acid aster hydrochloride is obtained so as to crystallize in ethanol through pressing catalytic hydrogenation deprotection to directly obtain the hydrochloric acid trientine. The synthetic process belongs to the organic synthetic technical field.
Owner:GUANGDONG AOERCHENG PHARMA
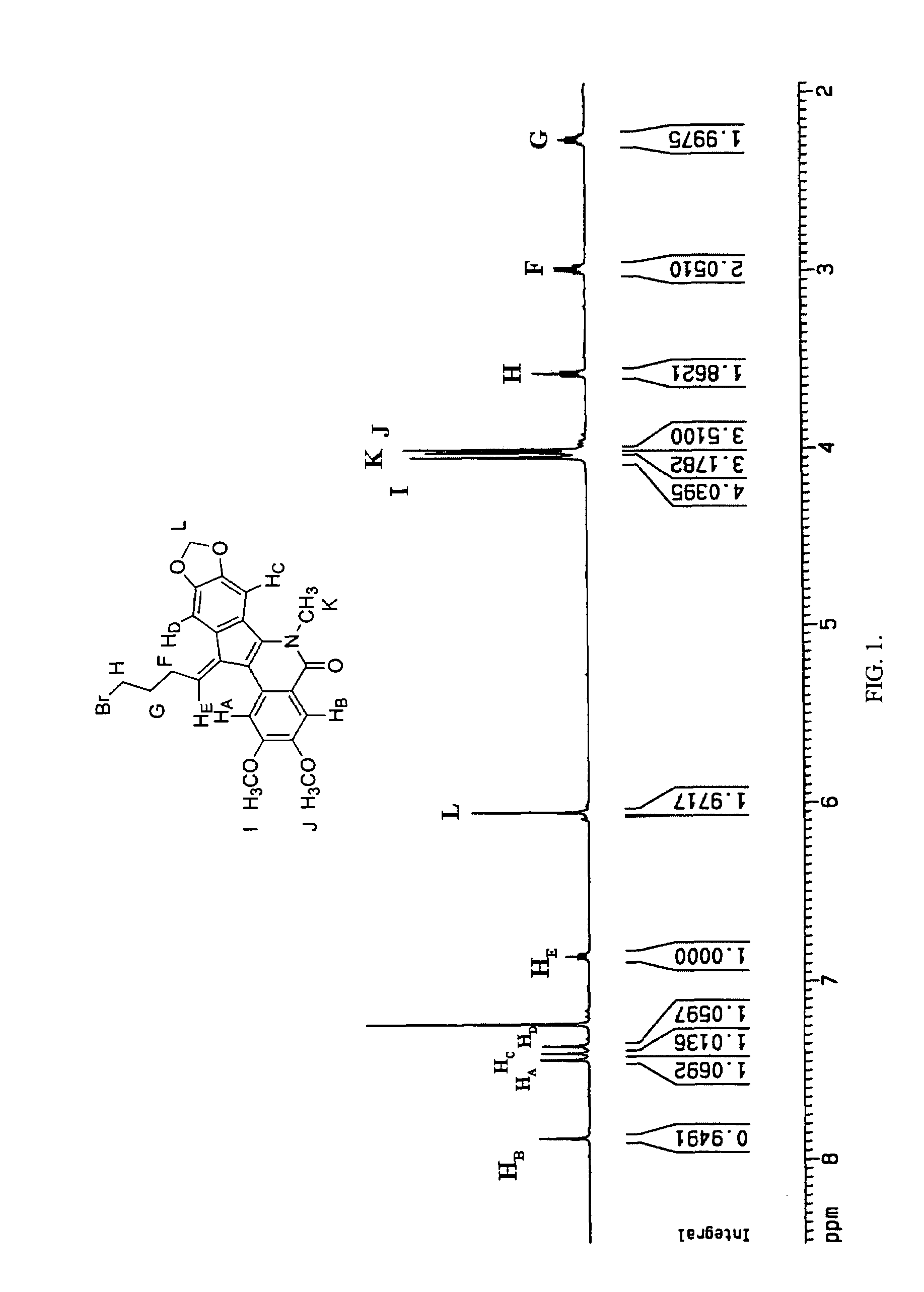
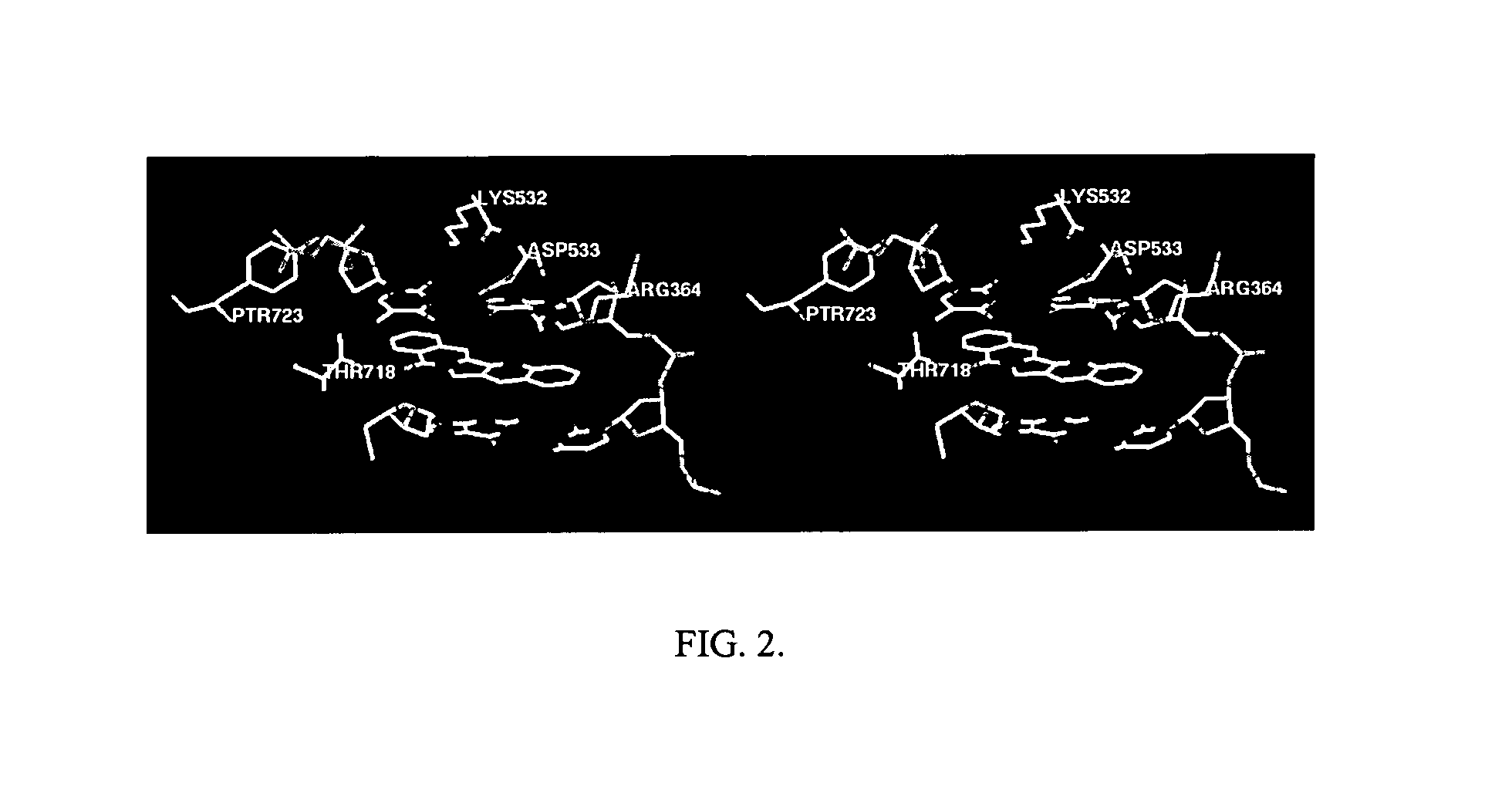
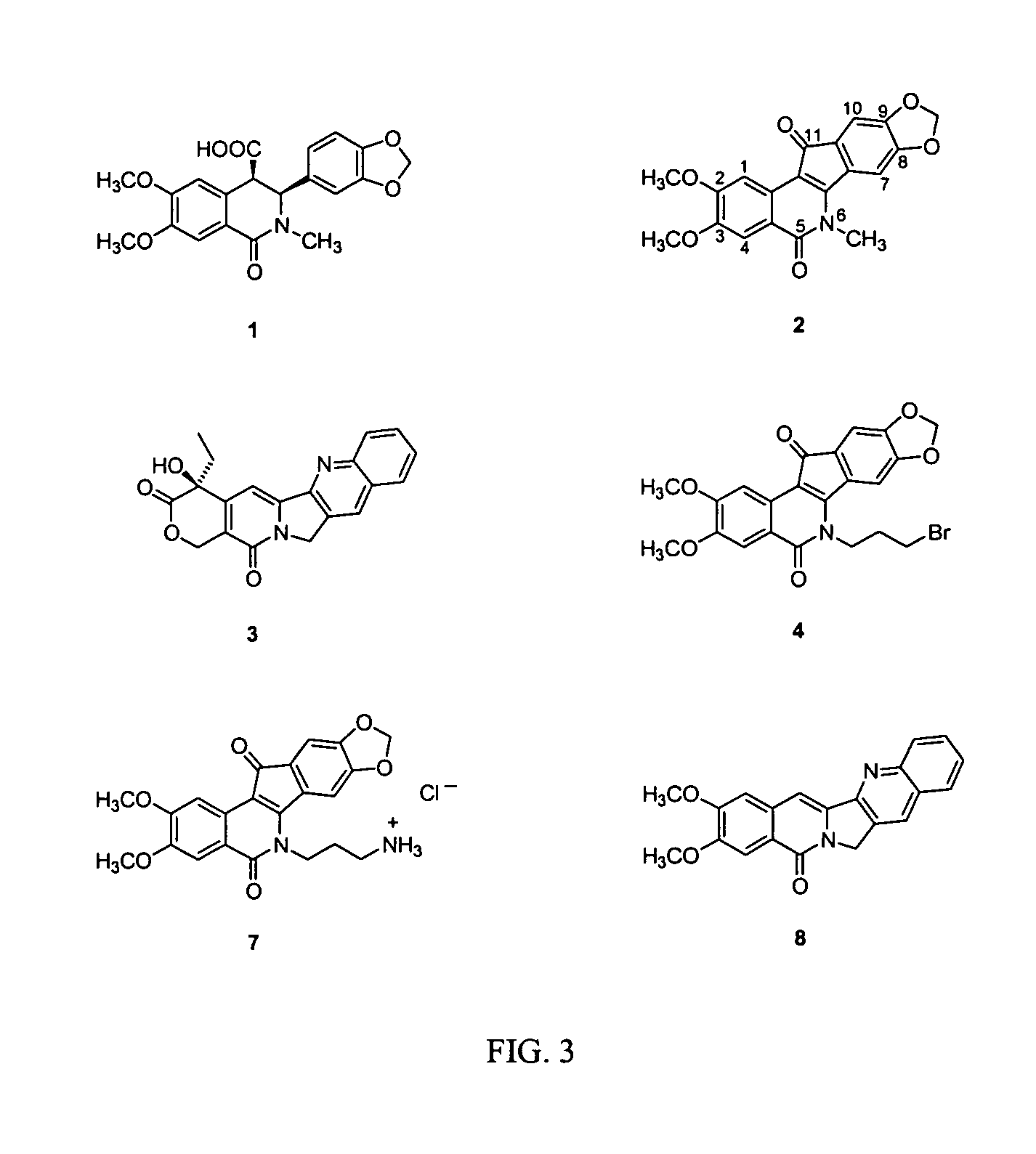
Cytotoxic indeno and isoindoloisoquinolones
The synthesis and biological activity of benzoisoindoloisoquinolone compounds are described. The synthesis and biological activity of C-11-substituted indenoisoquinolones are also described. Indenoisoquinolones substituted at C-11 are prepared by McMurry reactions of 11-ketoindenoisoquinolones with aldehydes.
Owner:UNITED STATES OF AMERICA +1
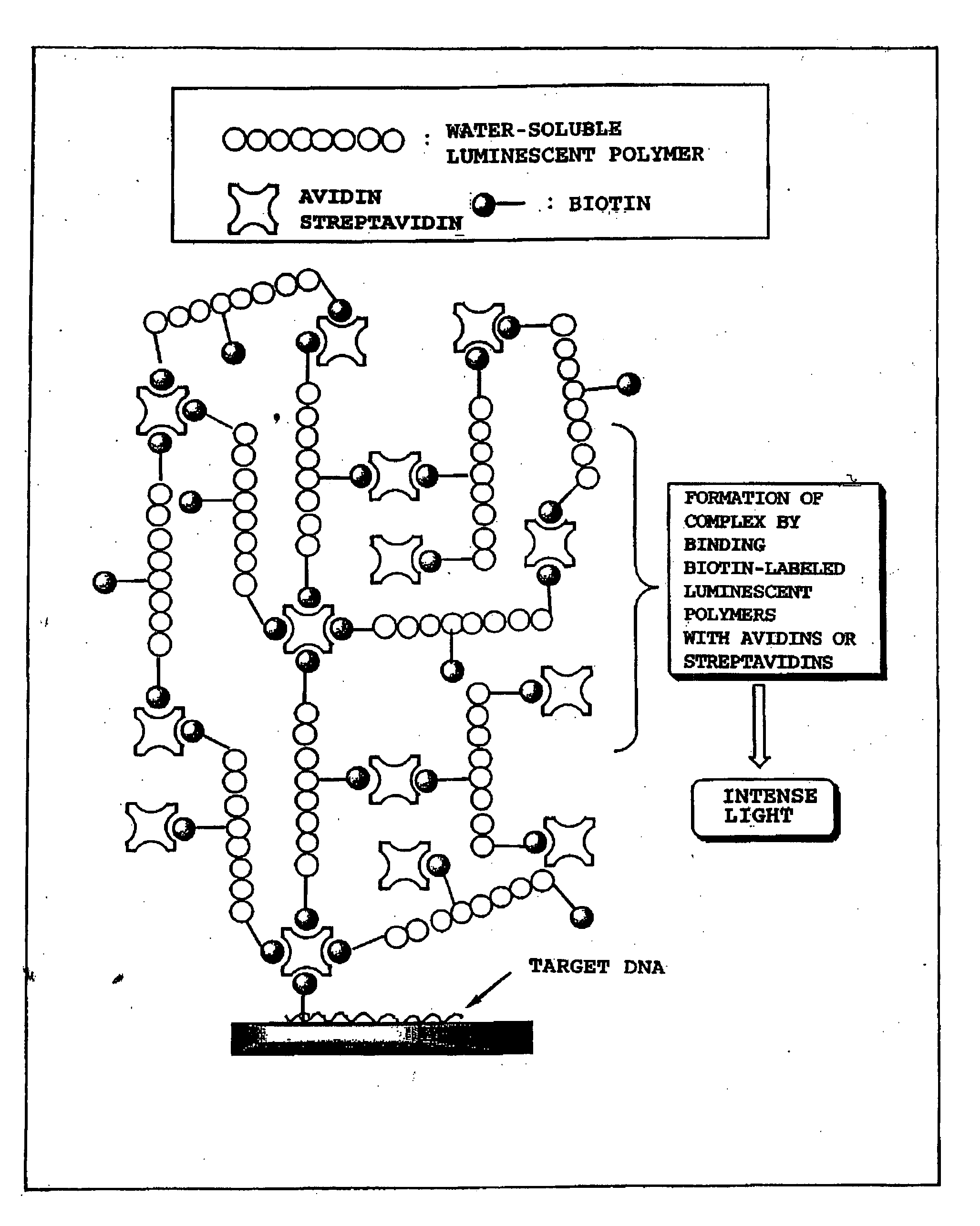
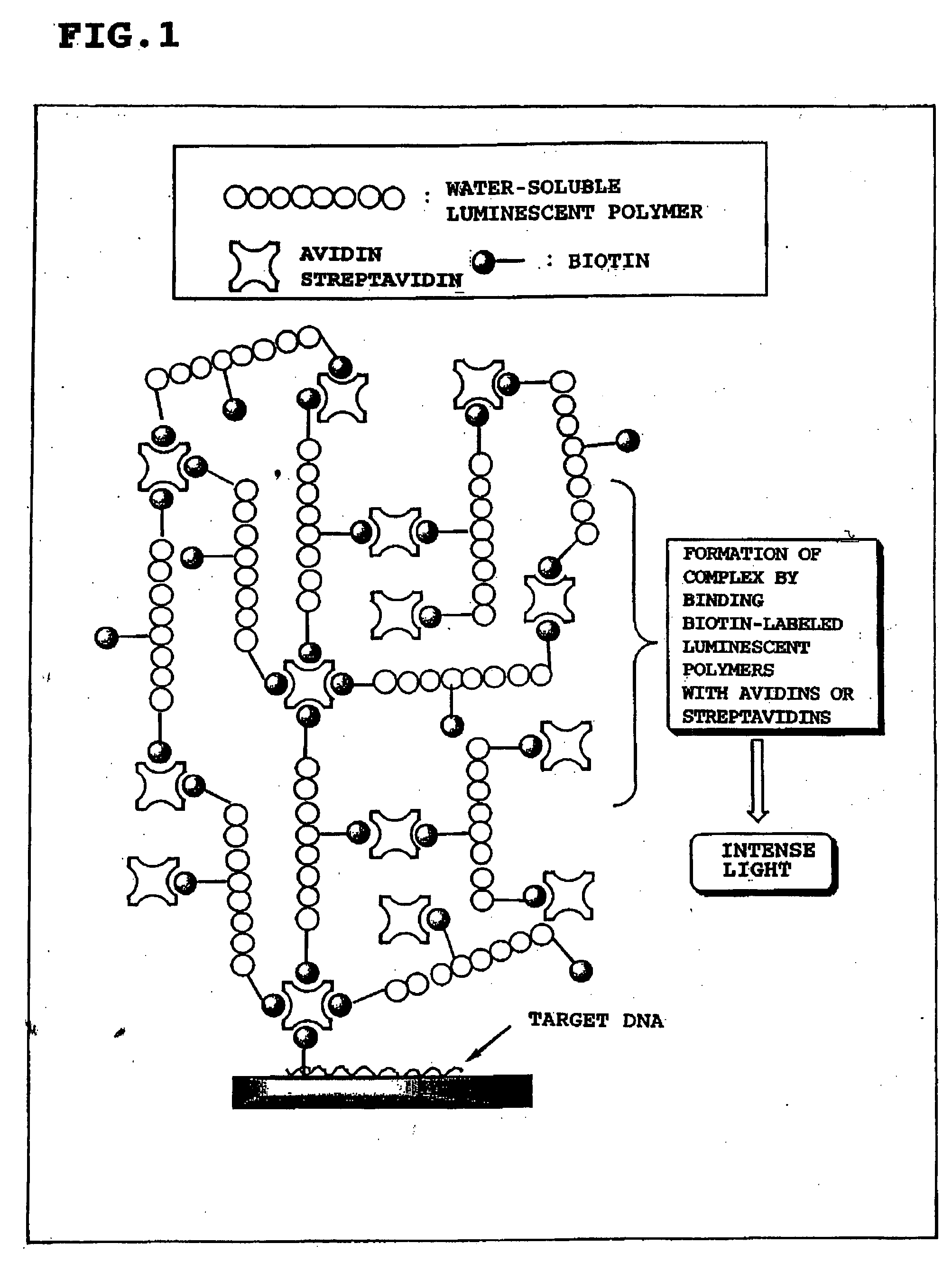
Luminescent polymer and use thereof in bioassay
ActiveUS20050019573A1High sensitivityThe method is simple and safeChemiluminescene/bioluminescenceGlass/slag layered productsLuminolLuminescent polymers
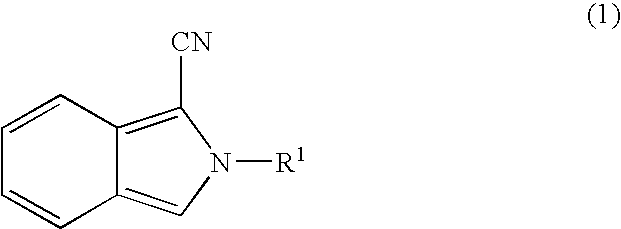
It is an object of the present invention to provide a luminescent polymer that is useful as a luminescent signal probe for labeling and detecting a target substance at high sensitivity in bioassay, and to provide the application of said luminescent polymer to bioassay. The luminescent polymer of the present invention comprises at least one biotin covalently attached to a polymer that includes monosaccharide or amino acid as a constituent monomer covalently attached to a luminescent substance. Preferably, two or more biotins are attached. Examples of the above-mentioned luminescent substance include cyanoisoindoles, luminols, and acridinium esters, and examples of the polymer include polysaccharides, polyamino acids, peptides, polypeptides, and proteins.
Owner:SEKISUI MEDICAL CO LTD +1
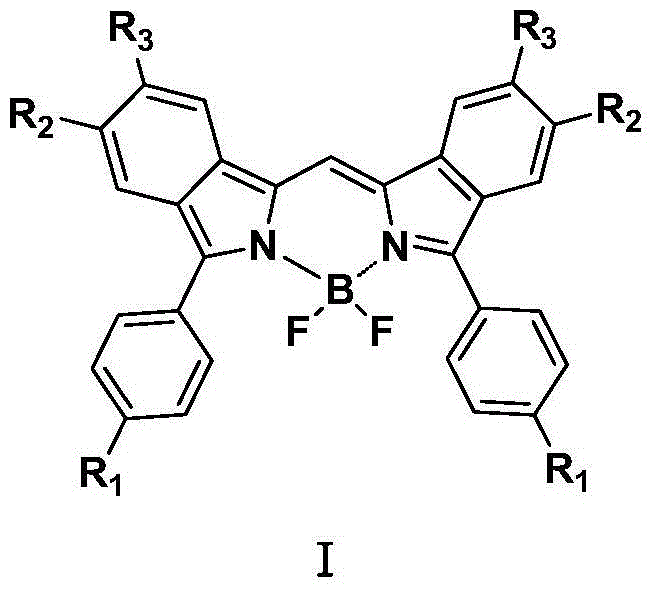
Fluoro-boron diisoindole compounds and preparation method thereof
InactiveCN104447824AChange photochemistryChange physical propertiesMethine/polymethine dyesGroup 3/13 element organic compoundsIsoindolesFluorescence
The invention relates to fluoro-boron diisoindole compounds and a preparation method thereof. Compared with the conventional fluoro-boron dipyrrole compounds, according to the fluoro-boron diisoindole compounds, pyrrole rings are directly replaced by isoindole rings, the conjugated range of pi bonds in a parent molecule is increased and thus the photochemical and physical properties of the compounds are changed, molecular absorption and emission wavelengths cause a 'red shift', the maximum ultraviolet absorption wavelength can reach 640nm-680nm and the maximum fluorescence emission wavelength is up to 670nm-713nm and the fluoro-boron diisoindole compounds are near-infrared fluorescent dyes.
Owner:EAST CHINA UNIV OF SCI & TECH
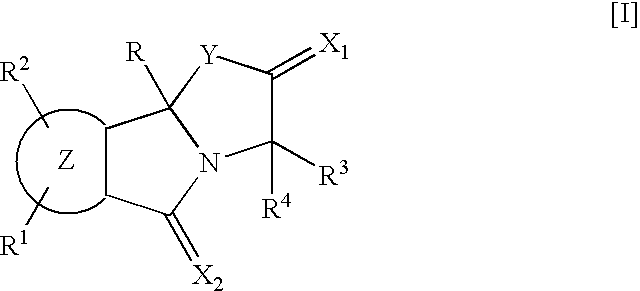
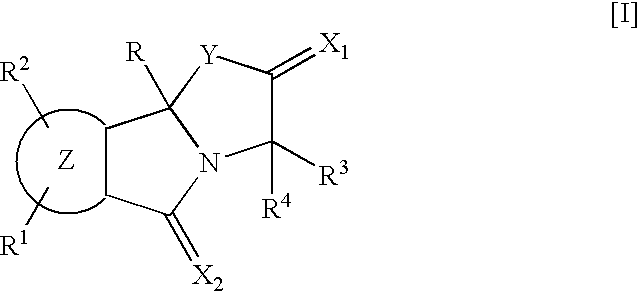
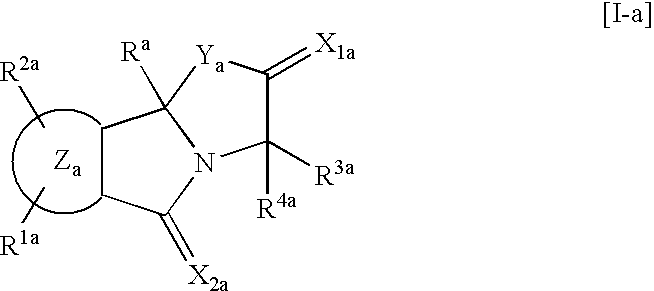
Isoindole derivatives
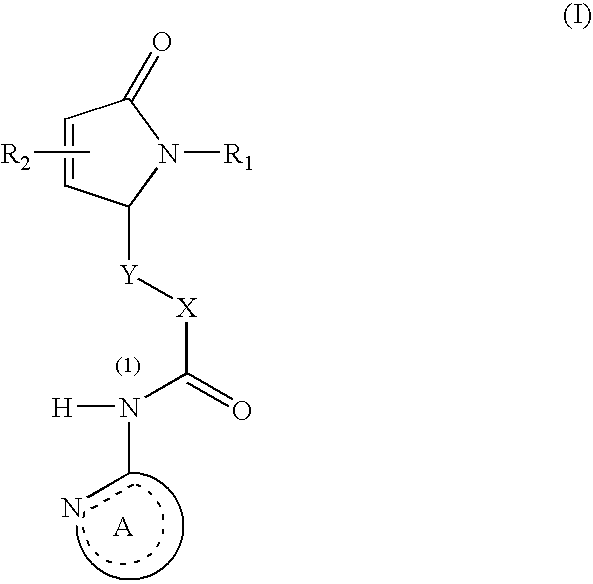
This invention relates to compounds represented by the general formula [I] wherein, R represents an azido group, etc., R1 and R2 are the same or different and represent hydrogen atoms, etc., R3 and R4 are the same or different and represent hydrogen atoms, etc., X1 represents an oxygen atom, etc., X2 represents an oxygen atom, etc., Y represents an oxygen atom, etc., and Z represents a condensed aryl group, etc., or a pharmaceutically acceptable salt thereof, preparation processes thereof, and an agent for treating diabetes, a prophylactic agent for chronic complications of diabetes or a drug against obesity, containing, as an effective ingredient, the compound or the pharmaceutically acceptable salt thereof.
Owner:BANYU PHARMA CO LTD
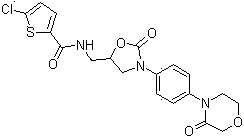
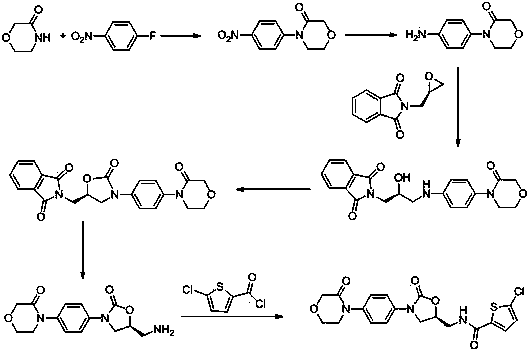
Method for preparing rivaroxaban
InactiveCN104031036ARaw materials are cheap and easy to getControllableOrganic chemistryKetoneCarboxylic acid
The invention discloses a method for preparing rivaroxaban, and is characterized in that the method comprises the following steps of synthesis of an intermediate I, synthesis of an intermediate II and synthesis of rivaroxaban, wherein the synthesis of the intermediate I comprises the synthetic steps of taking 4-(4-aminophenyl)-3-morpholinone and 2-[(2S)-2-oxiranyl-methyl)-1H-isoindole-1,3(2H)-diketone as raw materials, and in an alcohol solvent, carrying out a reaction with a condensating agent to obtain the intermediate I; the synthesis of the intermediate II comprises the synthetic steps of placing the intermediate I in a solvent, under the action of an amine reagent, carrying out a reaction to generate a primary amine compound, and forming an ammonium salt with an acid reagent to obtain the intermediate II; and the synthesis of rivaroxaban comprises the synthetic steps of placing 5-chlorothiophene carboxylic acid in a solvent, under the action of an acylation reagent, forming an acylated substance, then carrying out a reaction with the intermediate II under the action of an alkali reagent, and thus obtaining rivaroxaban. The method has the beneficial effects of cheap and easily obtained raw materials, simple and easily controlled operation, high reaction yield, high product purity, and low cost.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
Crosslinkable host materials
InactiveCN106715420AEasy to moveImprove efficiencyGroup 5/15 element organic compoundsFinal product manufacturePyridazinePhenanthroline
The invention relates to a crosslinkable organic molecule having a structure of the formula (1) and to the use thereof, wherein Ar is independently of one another, an unsaturated or aromatic carbo- or heterocyclic unit with 5 to 30 ring atoms, selected from the group consisting of naphthalene, anthracene, phenanthrene, pyrene, dihydropyrene, chrysene, perylene, fluoranthene, benzanthracene, tetracene, pentacene, benzpyrene, furan, benzofuran, isobenzofuran, thiophene, benzothiophene, isobenzothiophene, dibenzothiophene, pyrrole, indole, isoindole, carbazole, pyridine, quinoline, isoquinoline, acridine, phenanthridine, benzo-5,6-quinoline, benzo-6,7-quinoline, benzo-7,8-quinoline, phenothiazine, phenoxazine, pyrazole, indazole, imidazole, benzimidazol, naphthimidazole, phenanthrimidazole, pyridimidazole, pyrazine-imidazole, quinoxalinimidazole, oxazole, benzoxazole, naphthoxazole, anthroxazole, phenanthroxazole, isoxazole, isothiazole, 1,3-thiazole, benzothiazole, pyridazine, benzopyridazine, pyrimidine, benzpyrimidine, quinoxaline, pyrazine, phenazine, naphthyridine, azacarbazole, benzocarboline, phenanthroline, 1,2,3-triazole, 1,2,4-triazole, benzotriazole, 1,2,3-oxadiazole, 1,2,4-oxadiazole, 1,2,5-oxadiazole, 1,3,4-oxadiazole, 1,2,3-thiadiazole, 1,2,4-thiadiazole, 1,2,5-thiadiazole, 1,3,4-thiadiazole, 1,3,5-triazine, 1,2,4-triazine, 1,2,3-triazine, tetrazole, 1,2,3,4- oxatriazole, 1,2,3,4-oxatriazole, 1,2,4,5-tetrazine, 1,2,3,4-tetrazine, 1,2,3,5-tetrazin, purine, pteridine, indolizine, benzothiadiazole, indenocarbazole, indenofluorene, spirobifluorene, and indolocarbazole; D1 is a donor group having a structure of the formula (1a); and D2 is a donor group having a structure of the formula (1b).
Owner:SAMSUNG DISPLAY CO LTD
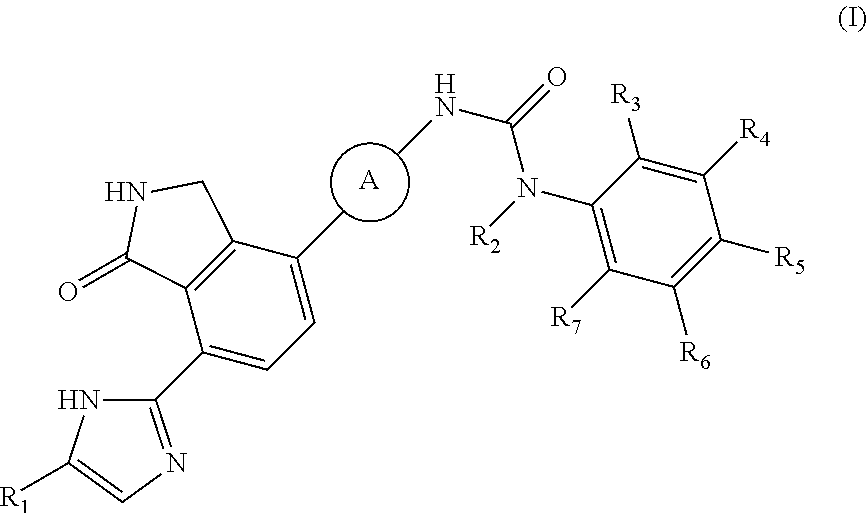
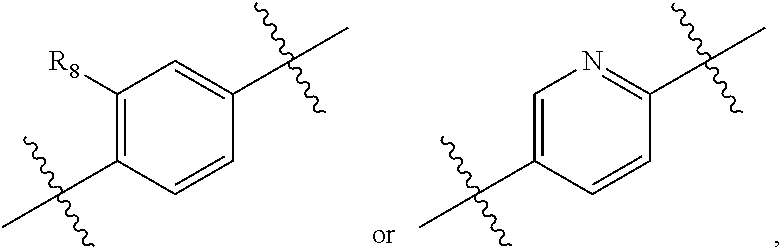
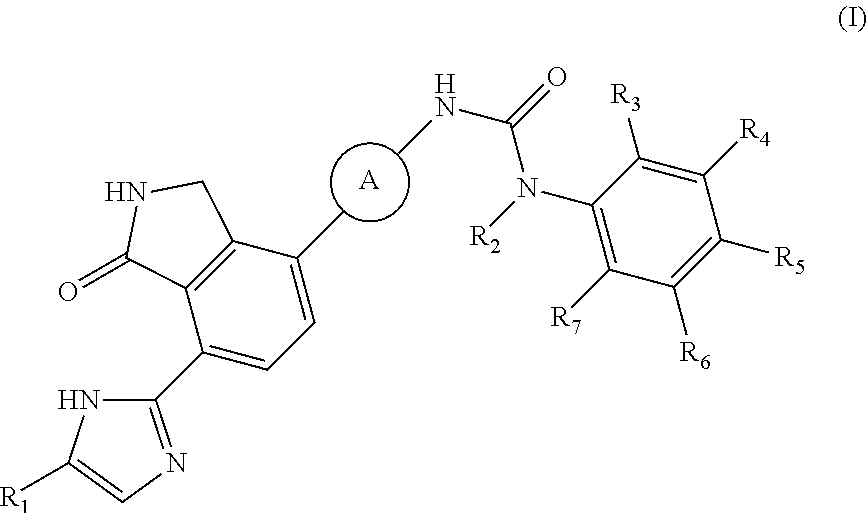
2,3-dihydro-isoindole-1-on derivative as btk kinase suppressant, and pharmaceutical composition including same
The present invention provides a compound selected from the group consisting of a compound of formula (I), pharmaceutically acceptable salts, esters, prodrugs, hydrates, solvates and isomers thereof; a use of the compound for the treatment, relief or prevention of diseases caused by abnormal or uncontrolled activation of protein kinase, and a use of the compound for the manufacture of a medicament for the treatment, relief or prevention of the diseases; a pharmaceutical composition comprising the compound as an active ingredient; and a method for the treatment, relief or prevention of the diseases using the compound. The inventive compound is useful for the treatment, relief or prevention of diseases caused by abnormal or uncontrolled activation of protein kinase.
Owner:CRYSTAL GENOMICS INC
Preparation method of lenalidomide
The invention relates to the field of drug synthesis, and particularly relates to a preparation method of a lenalidomide intermediate and lenalidomide. The compound is a drug for treating multiple myeloma. The method comprises the steps of adopting 2-bromomethyl-3-nitrobenzoate and 3-amino-2,6-piperidione hydrochloride as reaction substrates and an inorganic base as an acid-binding agent and obtaining a white to almost white key intermediate 3-(4-nitro-1-oxo-1,3-dihydro-2H-isoindole-2-yl) piperidine-2,6-diketone of the lenalidomide through simple post-treatment; and adopting a mixed solvent of an organic solvent and water as a reaction solvent and carrying out catalytic hydrogenation in the presence of palladium on carbon to prepare the lenalidomide (II). The process route is low in production cost, and a product is high in purity and friendly to environment, and has relatively great implement value and social and economical benefits.
Owner:CHANGZHOU PHARMA FACTORY
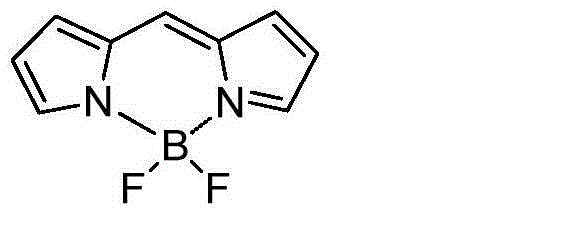
Near-infrared fluorine-boron dipyrrole fluorochrome and preparation method thereof
InactiveCN103952001AHigh yieldThe synthetic route is simpleMethine/polymethine dyesLuminescent compositionsQuantum yieldSolvent
The invention relates to a near-infrared fluorine-boron dipyrrole fluorochrome and a preparation method thereof. According to the method, halogenated isoindole imine and a boric acid reagent Suzuki are coupled, and then the near-infrared fluorine-boron dipyrrole fluorochrome is synthesized through acid catalysis condensation, wherein the emission wavelengths of the fluorochrome in various solvents are greater than 669nm, and the emission spectrum of the fluorochrome and derivatives thereof can reach 748nm. The fluorochrome has relatively high fluorescence quantum yield (0.67-1) and excellent optical physicochemical properties, such as light stability, and has a good application prospect in fields like laser dye and bioanalysis.
Owner:ANHUI NORMAL UNIV
Isoindole-imide compounds and compositions comprising and methods of using the same
Owner:CELGENE CORP
Process for the preparation of lenalidomide
The present invention relates to improved processes for preparing 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione (I) (lenalidomide) and its intermediate 3-(1-oxo-4-nitro-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione. The present invention further relates to improved processes for preparing lenalidomide crystalline form A, use of said crystalline form A as an active pharmaceutical ingredient or as an intermediate in the preparation of further crystalline or amorphous forms of lenalidomide, compositions comprising lenalidomide crystalline form A and their use in the treatment of disease.
Owner:GENERICS UK LTD
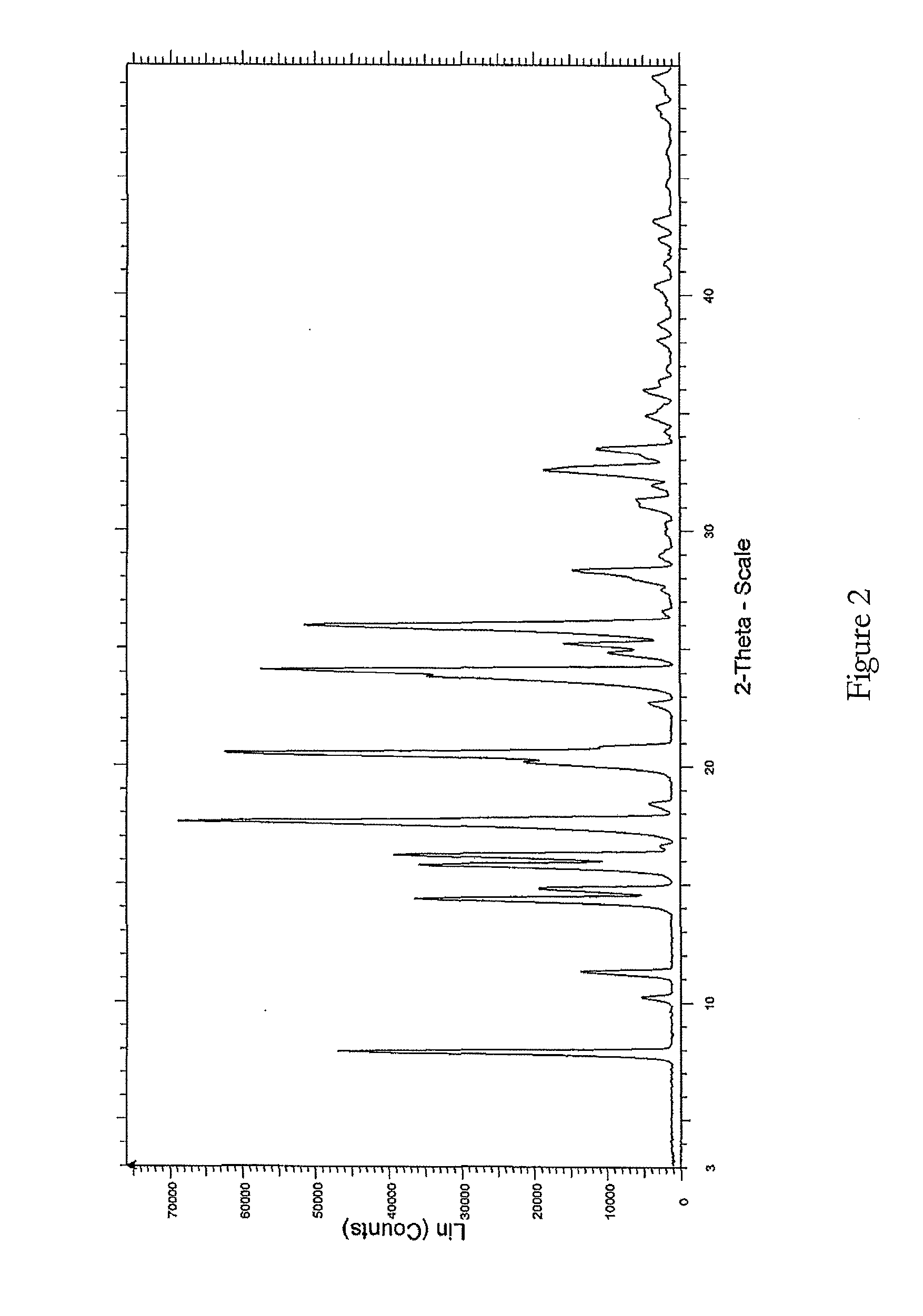
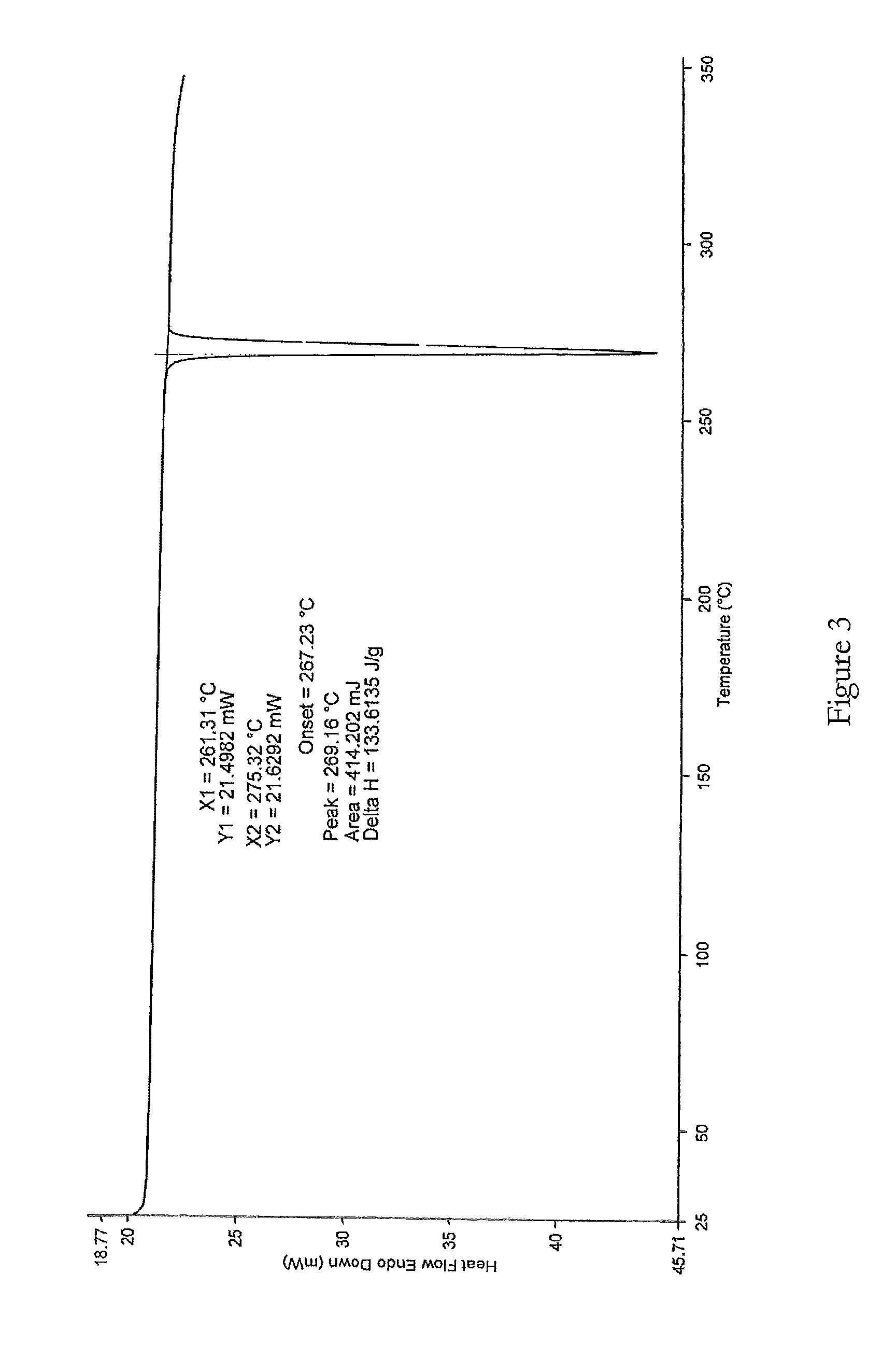
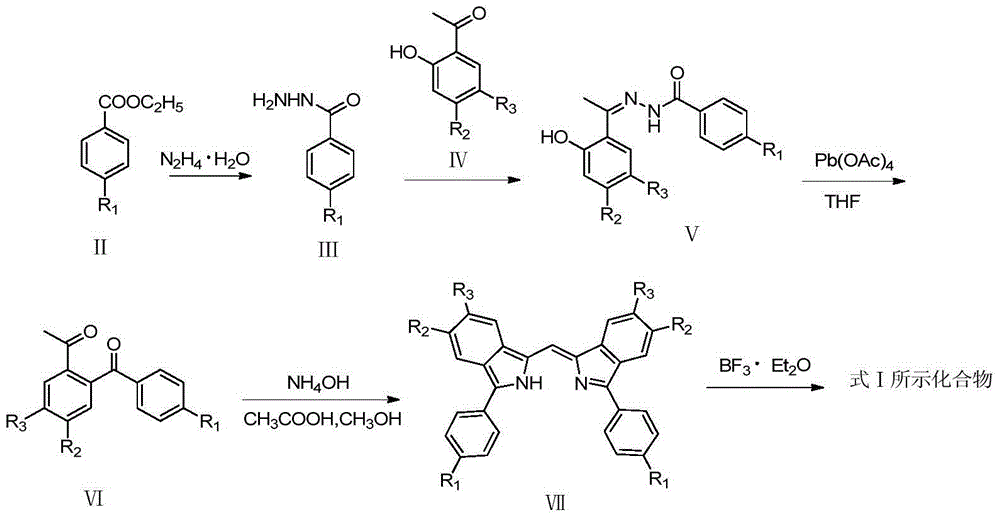
Method for normal-pressure efficient synthesis of 6H-isoindole [2,1-a] indole-6-ketone compounds
ActiveCN106117216AOvercome cumbersomeOvercome operational complexityOrganic chemistryOrganic solventIsoindoles
The invention discloses a method for normal-pressure efficient synthesis of 6H-isoindole [2,1-a] indole-6-ketone compounds. The technical scheme of the invention has the key points that by taking 2-(2-bromoaryl)-1H-indole compounds and CO as starting materials, under the effects of transition metal palladium catalysts, ligands and alkali, heating and stirring reaction is carried out at 100-140 DEG C in an organic solvent so as to obtain target products, namely the 6H-isoindole [2,1-a] indole-6-ketone compounds. The method disclosed by the invention has the advantages that reaction conditions are mild, the starting materials are simple and easy to prepare, substrates are wide in range of application, and the operation is simple.
Owner:HENAN NORMAL UNIV
Isoindole-imide compounds, compositions, and uses thereof
The invention relates to isoindole-imide compounds and pharmaceutically acceptable salts, hydrates, solvates, clathrates, enantiomers, diastereomers, racemates, or mixtures of stereoisomers thereof, pharmaceutical compositions comprising these isoindole-imide compounds, and methods for reducing the level of cytokines and their precursors in mammals. In particular, the invention pertains to isoindole-imide compounds that are potent inhibitors of the production of TNF-α in mammals. The isoindole-imides described herein are useful for treating or preventing diseases or disorders in mammals, for example, cancers, such as solid tumors and blood-born tumors; heart disease, such as congestive heart failure; osteoporosis; and genetic, inflammatory; allergic; and autoimmune diseases.
Owner:CELGENE CORP
Polymer for binding amine containing ligands and uses thereof
InactiveUS6680204B1Reduce generationComponent separationChemiluminescene/bioluminescenceCoated surfaceFluorescence
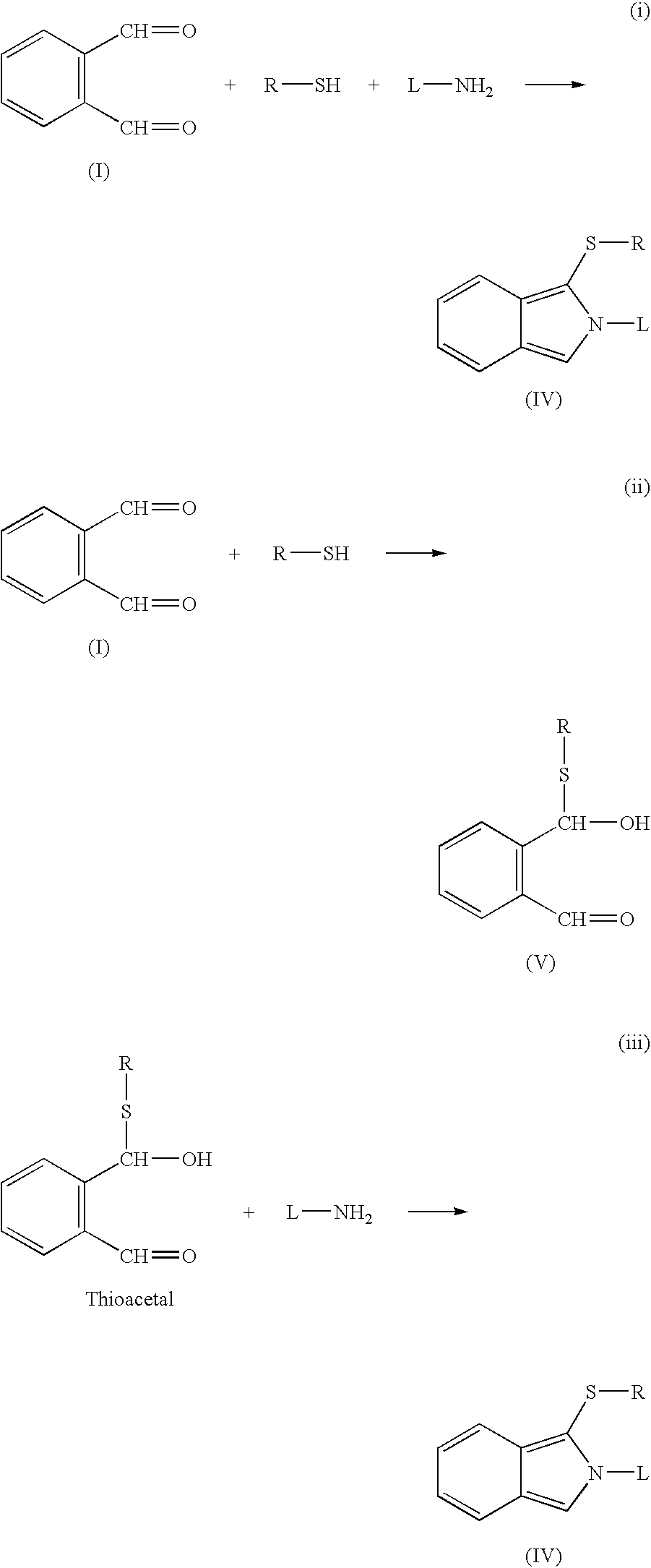
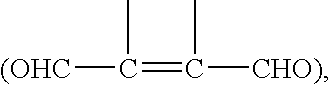
Reaction of a dialdehyde, particularly phthaldialdehyde (I), with R-Z where Z is a nucleophilic group (preferably SH) and R is polymerisable (e.g. allyl) gives a reactive thioacetal (V) which can react with an amine ligand L-NH2 to produce an isoindole (IV) which may be fluorescent. At some stage, generally before interaction with L-NH2, the R groups are polymerised, possibly leading to self-assembly of the polymer on a metal or SH-bearing surface. Such a coated surface is useful as a transducer in assays or as a binding medium e.g. for chromatography.
Owner:CRANFIELD UNIVERSITY
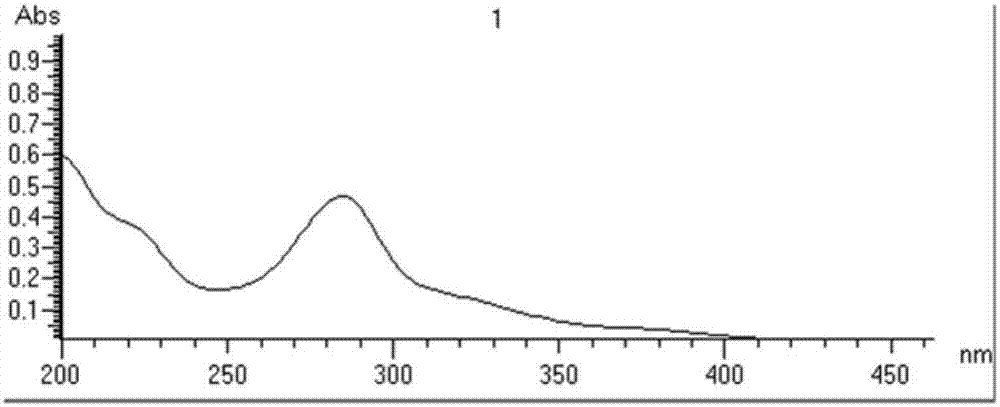
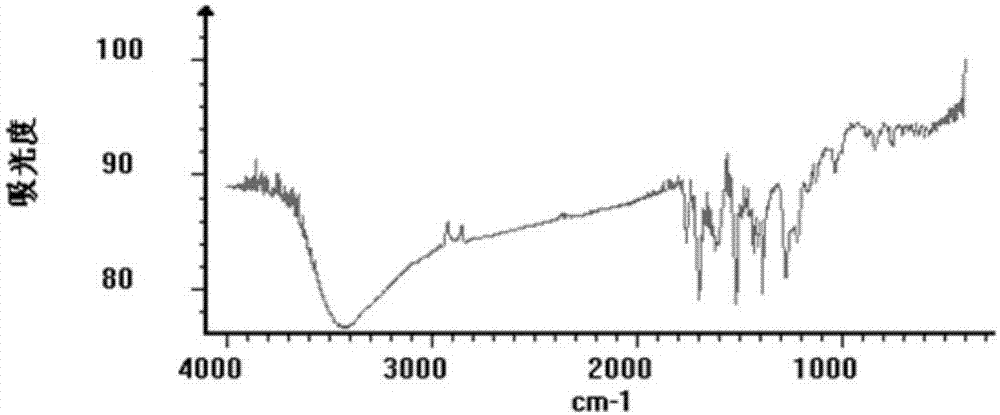
Dihydroisoindole-1H-pyrazolo[3,4-d]pyrimidone compound, and preparation method and application thereof
ActiveCN110872296AGood choiceGood blood-brain barrier permeabilityOrganic chemistryMetabolism disorderIsoindolesDepressant
The invention relates to a series of compounds represented as the formula (I), or pharmaceutically acceptable salts thereof, which can be used as new-generation Wee1 selective inhibitor. Compared withexisting Wee1 inhibitor, the compound has a higher selectivity on Wee1 kinase, so that the compound is safer and is higher in treatment index, and also has higher blood brain barrier permeability. The compound has better safety and a larger applicable range, and can be applied to treatment on various tumors, including brain tumor.
Owner:上海弘翊生物科技有限公司 +1
Preparation method of 3-methylene isoindole-1-one derivatives
ActiveCN106699632ARich typeRaw materials are easy to getOrganic chemistry2-chlorobenzamideOrganic solvent
The invention relates to a preparation method of 3-methylene isoindole-1-one derivatives. The preparation method comprises the following steps: an N-substituted 2-chlorobenzamide derivative in formula (1), alkyne-terminated compounds in formula (2) and nitrogen-containing ligands react in an organic solvent at 80-100 DEG C in the presence of potassium carbonate and a copper salt catalyst, and the 3-methylene isoindole-1-one derivatives in formula (3) are obtained. The equation is shown in the specification, wherein R<1>, R<2>, R<3> and R<4> are hydrogen; R<5> is C1-C6 alkyl or aromatic, and aromatic is phenyl, benzyl or substituted benzyl; R<6> is phenyl, C2-C6 alkyl, acetoxy methylene or p-tolyloxy methylene. High-yield 3-methylene isoindole-1-one derivatives can be obtained with the method; reaction conditions are mild, reaction operation and a post-processing process are simple, and the method is suitable for large-scale production.
Owner:SUZHOU UNIV
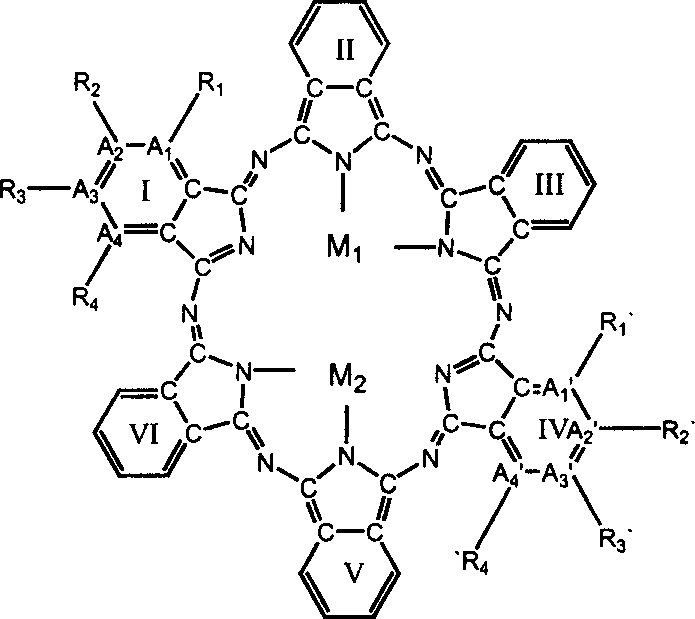
Super-phthalocyanine compound with six isoindole structure subunits in laver oxazine cycle and its synthesis and use
InactiveCN1548422ACopper organic compoundsNickel organic compoundsPhotoluminescenceSynthesis methods
The super-phthalocyanine compound with six isoindole structure subunits in laver oxazine cycle has completely new phthalocyanine compound structure, and has new properties, including three strong absorption peaks in UV-VIS-NIR absorption spectrum, especially characterized NIR peak at 1428 nm; surface photovoltaic characteristic; photoluminescence characteristic; electroluminescence; very high heat stability; excellent conductivity; etc. The unique properties make the compound possess important application in several fields. The present invention increases the basic laver oxazine cycle structures in phthalocyanine compound from 3 to 4 and increases one new family for phthalocyanine compounds.
Owner:杜锡光 +2
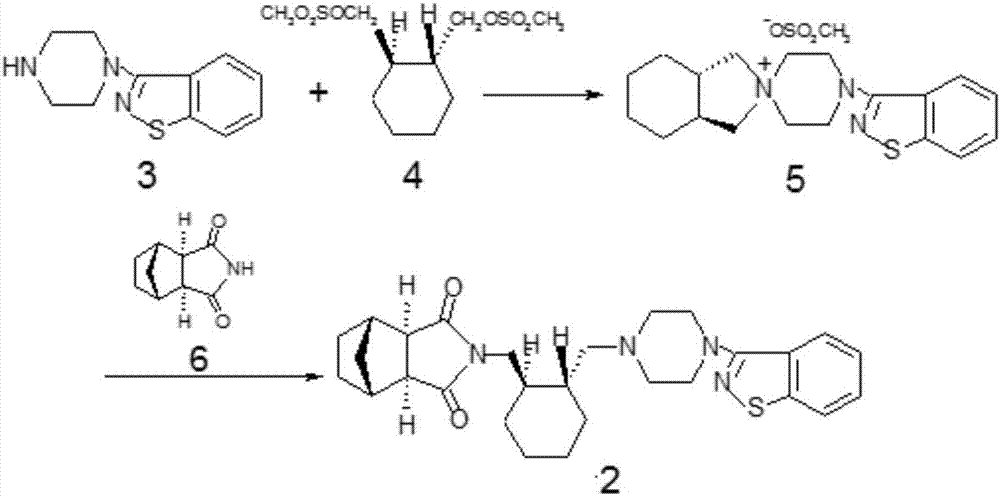
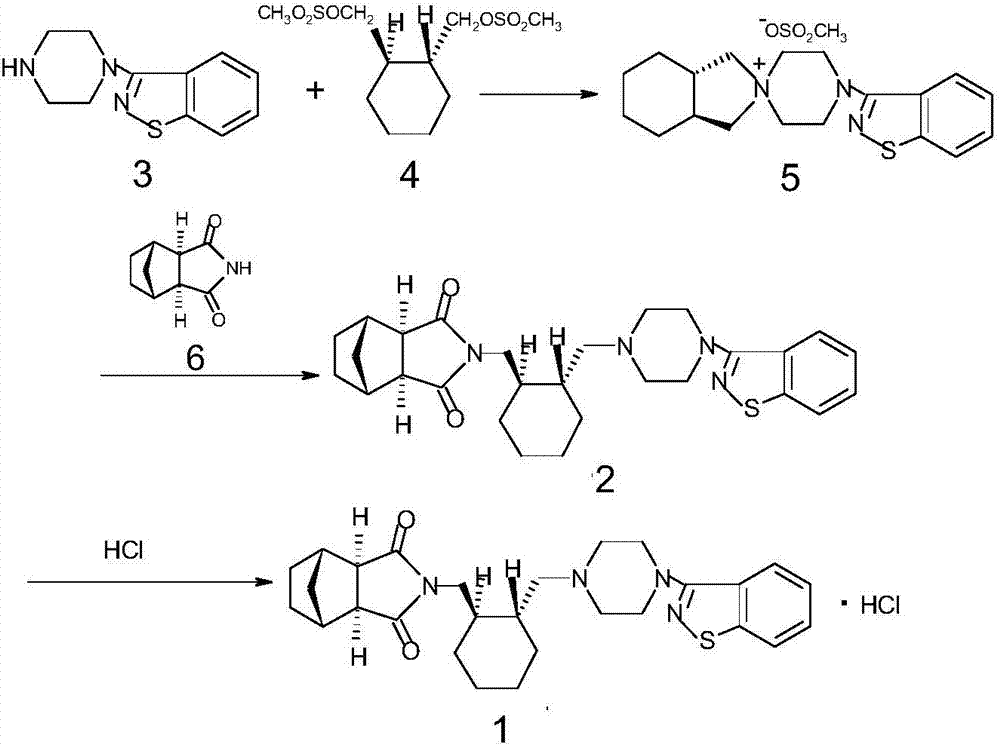
Lurasidone key intermediate preparation method
InactiveCN106946872AHigh yieldSimple and fast operationOrganic chemistry methodsLurasidoneIsoindoles
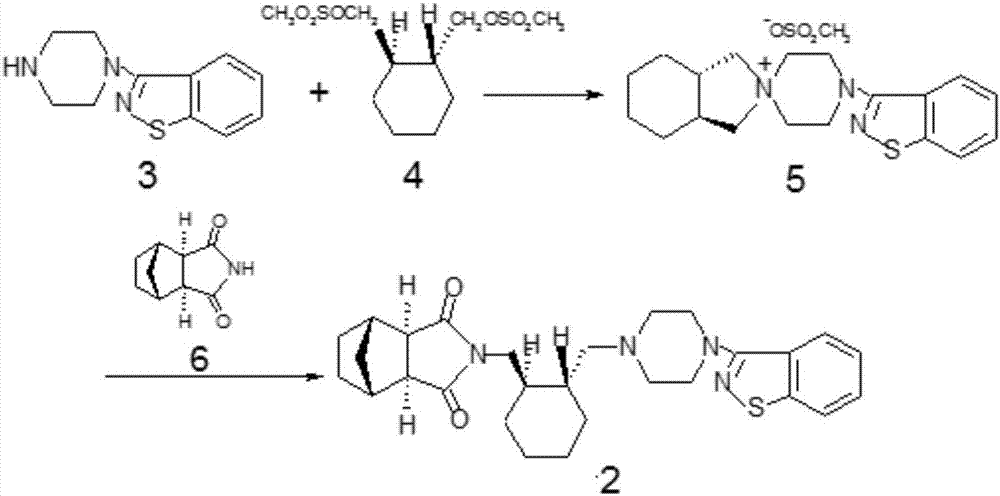
The present invention relates to a lurasidone key intermediate preparation method, and belongs to the technical field of compound synthesis. According to the method, when 4,-(1,2-benzisothiazol-3-yl)-(3aR,7aR)-octahydrospiro(2H-isoindole-2,1-piperazine)methanesulfonate is generated, 4-(1,2-benzisothiazol-3-yl)-1-piperazine is adopted as a raw material, the 4-(1,2-benzisothiazol-3-yl)-1-piperazine, (1R,2R)-1,2-bis(methanesulfonyloxymethyl)cyclohexane and potassium carbonate are subjected to a reaction in a solvent toluene, and a cyclodextrin phase transfer catalyst is added to the reaction system. According to the present invention, by using the cyclodextrin as the phase transfer catalyst, the incomplete reaction problem is solved, and the yield is substantially improved.
Owner:CHANGZHOU VOCATIONAL INST OF ENG
Integrated system for storing energy and generating power by using organic hydrogen storage material
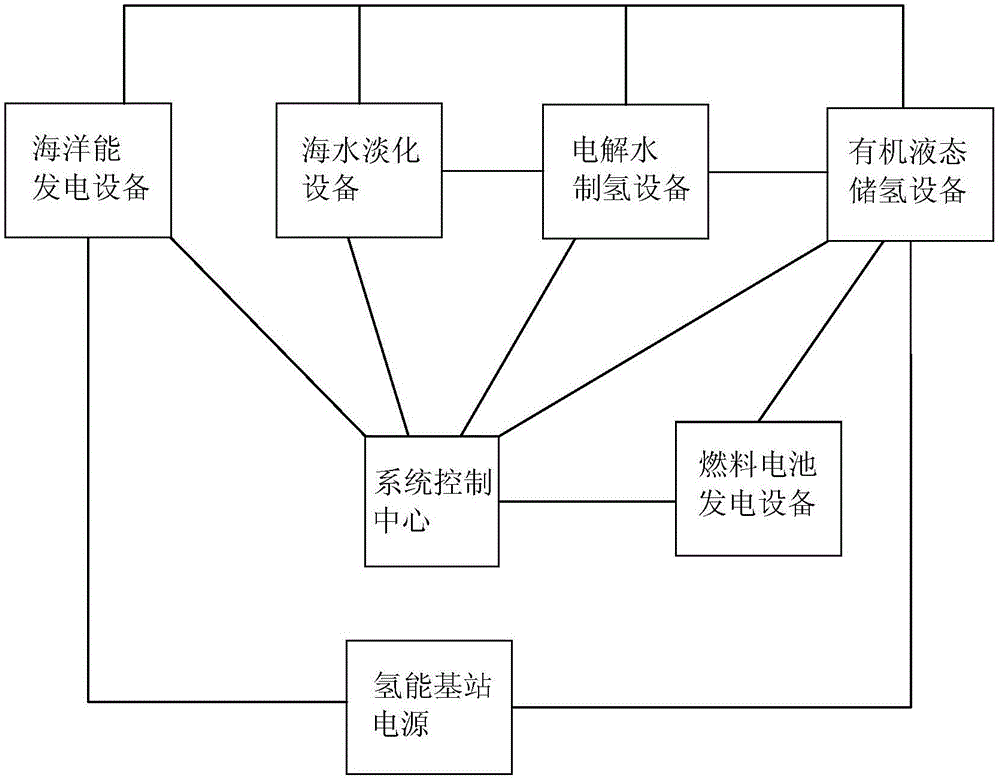
InactiveCN106356541AOvercome geographical limitationsAvoid lossHydrogenReactant parameters controlElectrolysisHigh energy
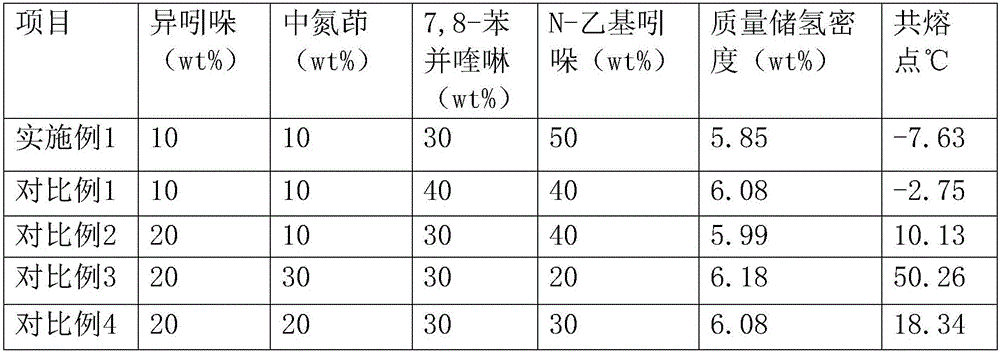
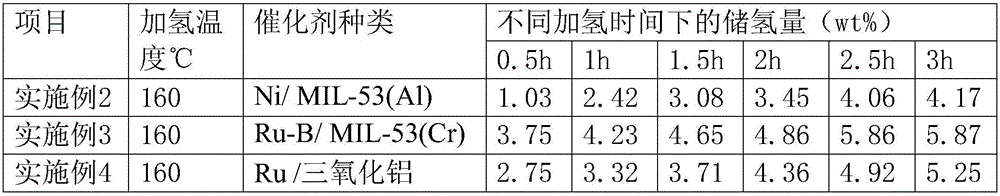
The invention discloses an integrated system for storing energy and generating power by using an organic hydrogen storage material. Power is generated by renewable sea energy, generated electric energy is used for supplying power for sea water desalting equipment, water electrolysis hydrogen preparation equipment and a power supply of a hydrogen energy base station, after sea water is filtered into fresh water, hydrogen is prepared by water electrolysis, renewable water resources are converted into hydrogen energy, obtained high-energy-density hydrogen is stored in the organic hydrogen storage material, the power supply of the hydrogen energy base station supplies power for organic hydrogen storage equipment, an obtained hydrogenation organic hydrogen storage material can be conveyed to any places through pipes, the organic hydrogen storage material consists of isoindole, purrocoline, 7,8-benzoquinoline and N-ethylindole, hydrogen separated from an organic hydrogen storage device is directly used for fuel cells, and the hydrogen energy is converted into electric energy or is directly used for heating through the hydrogen fuel cells. Compared with traditional ocean power generation, the integrated system for storing energy and generating power by using the organic hydrogen storage material overcomes geographical limitations of utilization of the sea energy, loss of the electric energy in a conveying process is avoided, and economy of utilization of energy is improved.
Owner:温州集智科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com











![Method for normal-pressure efficient synthesis of 6H-isoindole [2,1-a] indole-6-ketone compounds Method for normal-pressure efficient synthesis of 6H-isoindole [2,1-a] indole-6-ketone compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8f6616e4-7614-46fb-b3d6-89f4df5eb04f/BDA0001094280590000011.PNG)
![Method for normal-pressure efficient synthesis of 6H-isoindole [2,1-a] indole-6-ketone compounds Method for normal-pressure efficient synthesis of 6H-isoindole [2,1-a] indole-6-ketone compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8f6616e4-7614-46fb-b3d6-89f4df5eb04f/BDA0001094280590000021.PNG)
![Method for normal-pressure efficient synthesis of 6H-isoindole [2,1-a] indole-6-ketone compounds Method for normal-pressure efficient synthesis of 6H-isoindole [2,1-a] indole-6-ketone compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8f6616e4-7614-46fb-b3d6-89f4df5eb04f/BDA0001094280590000051.PNG)

![Dihydroisoindole-1H-pyrazolo[3,4-d]pyrimidone compound, and preparation method and application thereof Dihydroisoindole-1H-pyrazolo[3,4-d]pyrimidone compound, and preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/21cf7138-1159-4bb1-8979-ccebc699e72a/FDA0001785167610000011.png)
![Dihydroisoindole-1H-pyrazolo[3,4-d]pyrimidone compound, and preparation method and application thereof Dihydroisoindole-1H-pyrazolo[3,4-d]pyrimidone compound, and preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/21cf7138-1159-4bb1-8979-ccebc699e72a/FDA0001785167610000031.png)
![Dihydroisoindole-1H-pyrazolo[3,4-d]pyrimidone compound, and preparation method and application thereof Dihydroisoindole-1H-pyrazolo[3,4-d]pyrimidone compound, and preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/21cf7138-1159-4bb1-8979-ccebc699e72a/FDA0001785167610000041.png)