Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
191 results about "Isobenzofuran" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
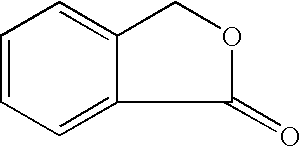
Isobenzofuran is a heterocyclic compound consisting of fused benzene and furan rings. It is isomeric with benzofuran. Isobenzofuran is highly reactive and rapidly polymerizes; however, it has been identified and prepared by thermolysis of suitable precursors and trapped at low temperature.
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
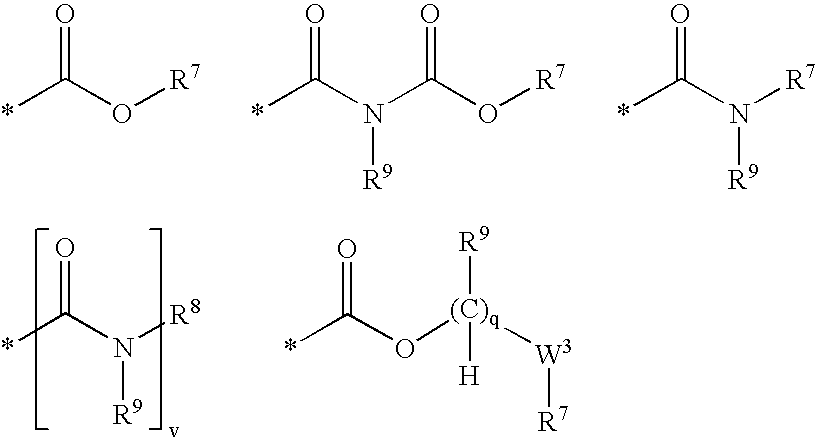
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
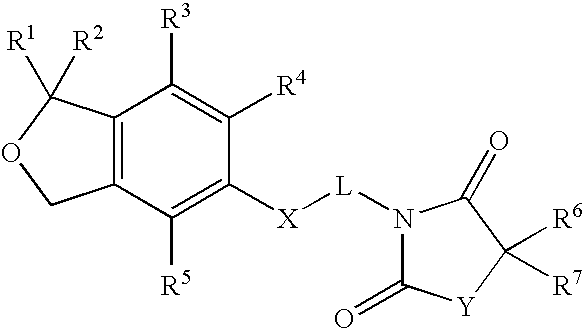
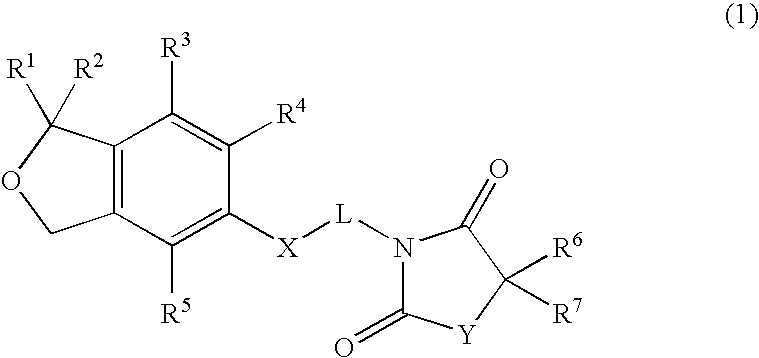
Spiro[isobenzofuran-1,4′-piperidin]-3-ones and 3H-spiroisobenzofuran-1,4′-piperidines
Substituted spiro[isobenzofuran-1,4′-piperidin]-3-ones and 3H-spiroisobenzofuran-1,4′-piperidines capable of modulating NPY5 receptor activity are provided. Such compounds may be used to modulate ligand binding to NPY5 receptors in vivo or in vitro, and are particularly useful in the treatment of a variety of disorders (e.g., eating disorders such as obesity or bulimia, psychiatric disorders, diabetes and cardiovascular disorders such as hypertension) in humans, domesticated companion animals and livestock animals. Pharmaceutical compositions and methods for treating such disorders are provided, as are methods for using such compounds for detecting NPY5 receptors.
Owner:NEUROGEN
Spiro[isobenzofuran-1,4'-piperidin]-3-ones and 3H-spiroisobenzofuran-1,4'-piperidines
Substituted spiro[isobenzofuran-1,4′-piperidin]-3-ones and 3H-spiroisobenzofuran-1,4′-piperidines capable of modulating NPY5 receptor activity are provided. Such compounds may be used to modulate ligand binding to NPY5 receptors in vivo or in vitro, and are particularly useful in the treatment of a variety of disorders (e.g., eating disorders such as obesity or bulimia, psychiatric disorders, diabetes and cardiovascular disorders such as hypertension) in humans, domesticated companion animals and livestock animals. Pharmaceutical compositions and methods for treating such disorders are provided, as are methods for using such compounds for detecting NPY5 receptors.
Owner:BAKTHAVATCHALAM RAJAGOPAL +9
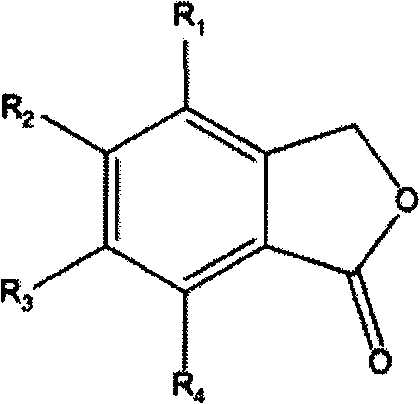
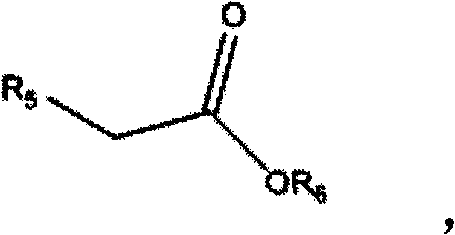
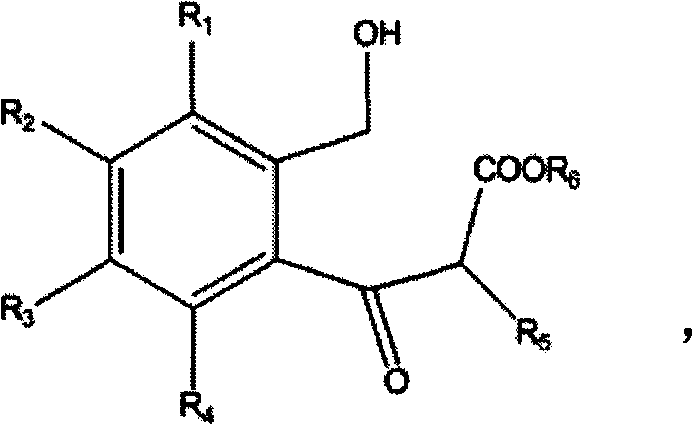
1,3-dihydroisobenzofuran derivatives
The object is to provide a novel LXRβ agonist that is useful as a preventative and / or therapeutic agent for atherosclerosis; arteriosclerosis such as those resulting from diabetes; dyslipidemia; hypercholesterolemia; lipid-related diseases; inflammatory diseases that are caused by inflammatory cytokines; skin diseases such as allergic skin diseases; diabetes; or Alzheimer's disease. The solving means is a 1,3-dihydroisobenzofuran derivative represented by the following general formula (1) or salt thereof, or their solvate.
Owner:KOWA CO LTD
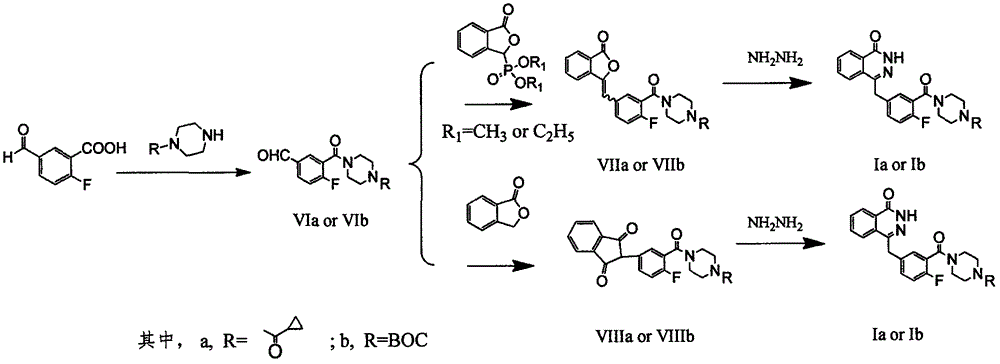
Preparation method of Olaparib and analogue of Olaparib
InactiveCN105085407ARaw materials are easy to getSimple processOrganic chemistryBenzoic acidIsobenzofuran
The invention discloses a preparation method of Olaparib and an analogue of the Olaparib. 2-fluoro-5-formyl benzoic acid is taken as a raw material and reacts with 1-substitutent piperazine to produce 3-(4-substitutent)piperazine-1-carbonyl)-4-fluorobenzalde which reacts with (3-oxo-1,3-dihydro-isobenzofuran-1-yl)dialkyl phosphate to produce 1-(substitutent)-4-[5-(3-oxo-3H-isobenzofuran-1-yl-methylene)-2-fluorobenzoyl]piperazine, then the 1-(substitutent)-4-[5-(3-oxo-3H-isobenzofuran-1-yl-methylene)-2-fluorobenzoyl]piperazine reacts with hydrazine hydrate to produce the Olaparib (Ia,R=cyclopropyl formyl) and the analogue (Ib,R=BOC) of the Olaparib; or 3-(4-substitutent)paperazine-1-carbonyl)-4-fluorobenzalde reacts with phthalide to produce 1-(substituent)-4-[5-(2,3-dihydro-1,3-dioxo-1H-indene-2-yl)-2-fluorobenzoyl]piperazine which reacts with the hydrazine hydrate to produce the Olaparib (Ia,R=cyclopropyl formyl) and the analogue (Ib,R=BOC) of the Olaparib.
Owner:GUANGZHOU YOUMIJIAN PHARMA TECH CO LTD
1'-[4-[1-(4-fluorophenyl)-1H-indole-3-yl]-1-butyl]-spiro[isobenzofuran-1(3H),4'-piperidine] hydrohalogenides
The present invention relates to a hydrohalogenide of 1′-[4-[1-(4-fluorophenyl)-1H-indole-3-yl]-1-butyl]-spiro[isobenzofuran-1(3H),4′-piperidine], pharmaceutical compositions containing the acid addition salts and the use thereof for the treatment of psychic and neurological disorders.
Owner:H LUNDBECK AS
Black leuco dyes for use in CD/DVD labeling
Systems and methods of labeling optical disk recording media using black leuco dyes in an electromagnetic radiation sensitive composition are described. Black leuco dyes containing isobenzofuranone are prepared in various compositions that can include activators, radiation absorbers, non-leuco colorants, and / or a variety of carriers. The leuco dye compositions can be applied and prepared to achieve a desired visual effect upon development of the black leuco dye.
Owner:HEWLETT PACKARD DEV CO LP
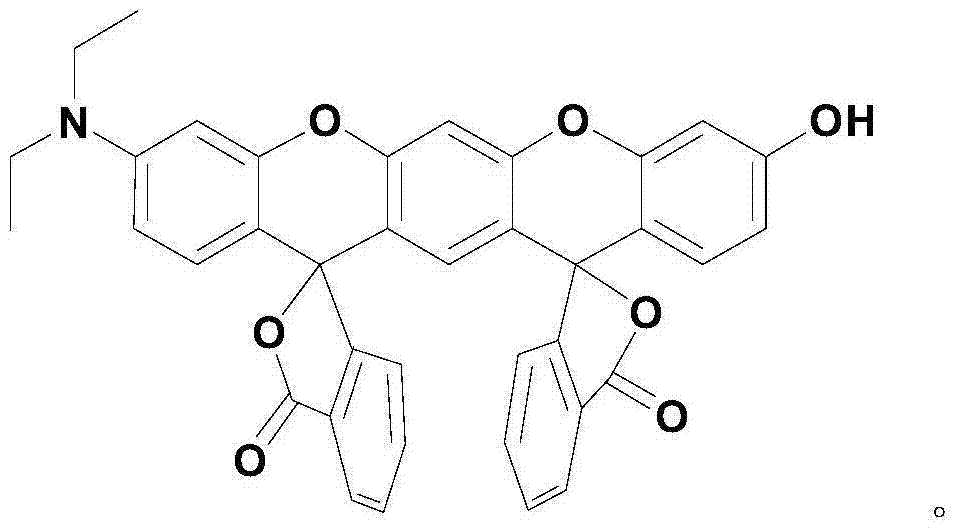
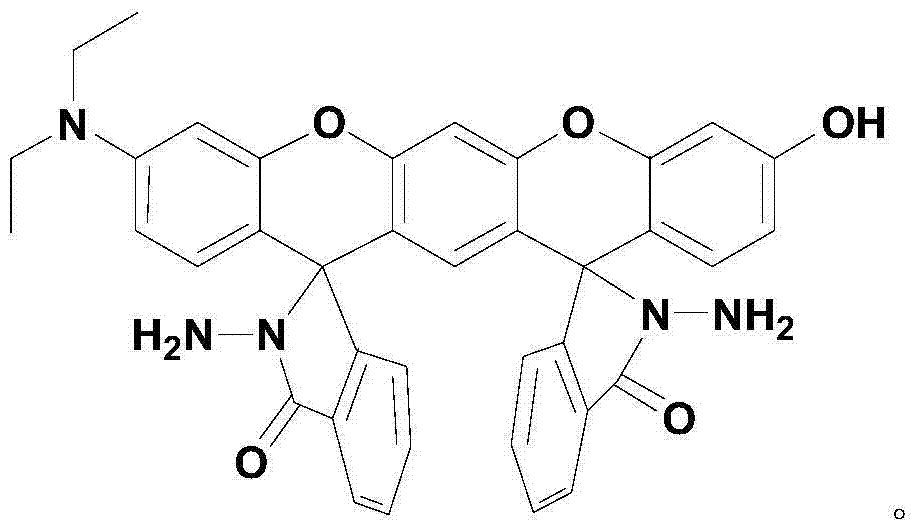
Xanthene fluorescence dye, preparation method and applications thereof
InactiveCN104327536ASimple manufacturing methodLow costOrganic chemistryAzo dyesQuinoneIsobenzofuran
The invention relates to a xanthene fluorescence dye, a preparation method and applications thereof, wherein the fluorescence dye is 3',3''-bis(oxospiroisobenzofuran)-3-hydroxy-7-(diethylamino)benzopyran-xanthene. According to the present invention, the xanthene fluorescence dye is designed and synthesized, the dye has the double-spiro six-membered ring fused system, the optical signal release of the dye is controlled by the double-spiro structure, the opening / closing of the spiro structure regulates the fluorophore xanthene conjugation system (the quinone type system forms or disappears) in the whole molecular structure, and the absorption wavelength and the emission wavelength are expanded so as to make the optical signal intensity accordingly change; and the isothiocyanate derivative molecule fluorescence probe synthesized through further derivatization provides characteristics of rapid response, high selectivity, high detection sensitivity and low detection limitation on Hg<2+>. In summary, the present invention provides the xanthene fluorescence dye design synthesis method, and the dye is adopted to design the Hg<2+> detecting molecule fluorescence probe for the optical signal report gene.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Isobenzofuran compound capable of improving cigarette smoking effect and preparing method and application of isobenzofuran compound
ActiveCN106858710AReduce stepsIncrease aromaOrganic chemistryTobacco treatmentChromatographic separationIsobenzofuran
The invention relates to an isobenzofuran compound capable of improving a cigarette smoking effect and a preparing method and application of the isobenzofuran compound, and belongs to the technical field of phytochemistry. The compound is obtained by separating cigarette, and is named 6,8-dihydro-5-methoxy-2,2-dimethyl-2H-furo [3.4-g] chromene, the molecular formula is C14H16O3, and the molecular formula is shown in the formula I; the preparing method comprises the steps of adopting the cigarette as a raw material, conducting extract extraction, conducting organic solvent extraction, conducting MCI de-coloring, conducting silica gel column chromatography and conducting efficient liquid-phase chromatographic separation. The isobenzofuran compound capable of improving the cigarette smoking effect is added into a cigarette filter tip, the fragrance of the cigarette can be improved, the sweetness of cigarette smoke is improved, the fluid generation feeling is highlighted, and the smoking quality of the cigarette is improved.
Owner:CHINA TOBACCO YUNNAN IND
Processing-friendly dianhydride hardener for epoxy resin systems based on 5,5'-carbonylbis(isobenzofuran-1,3-dione)
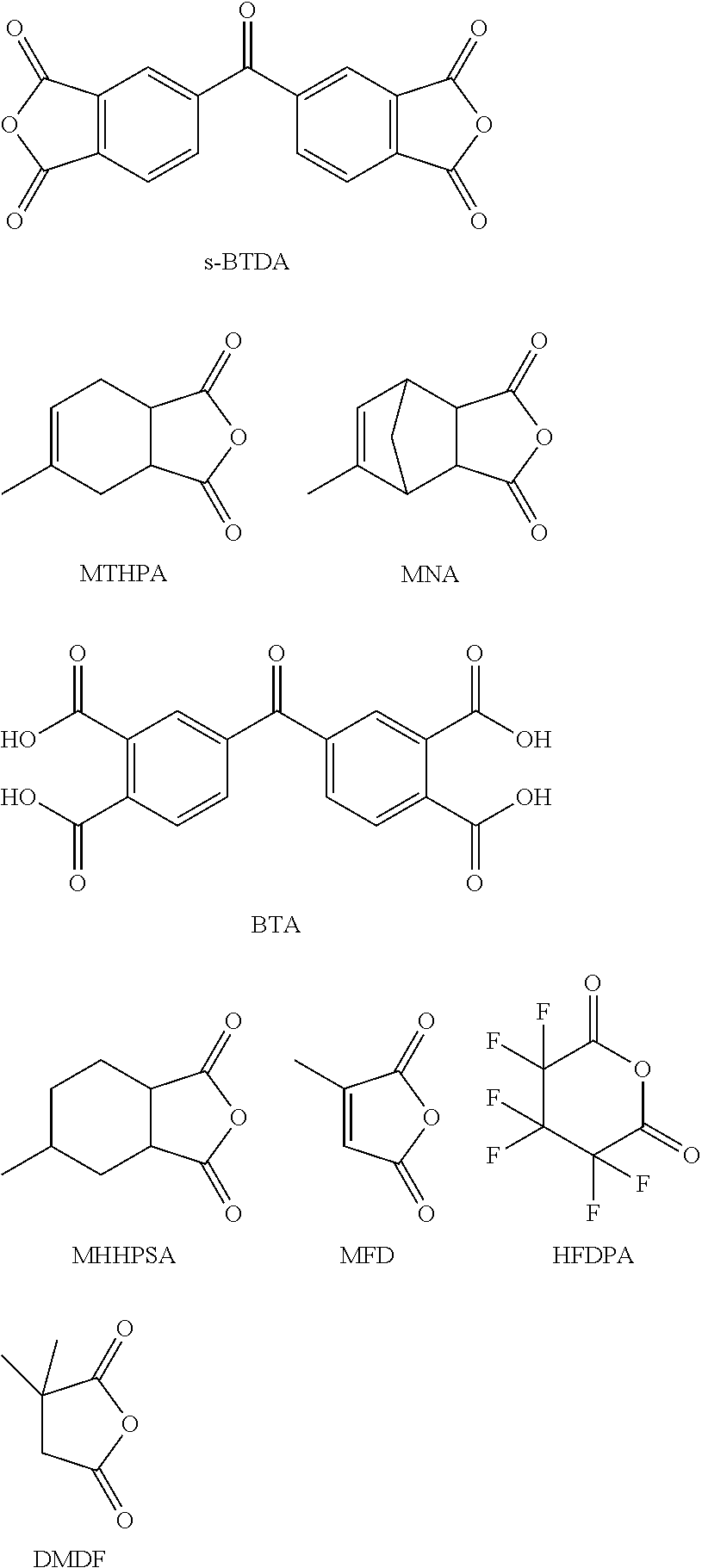
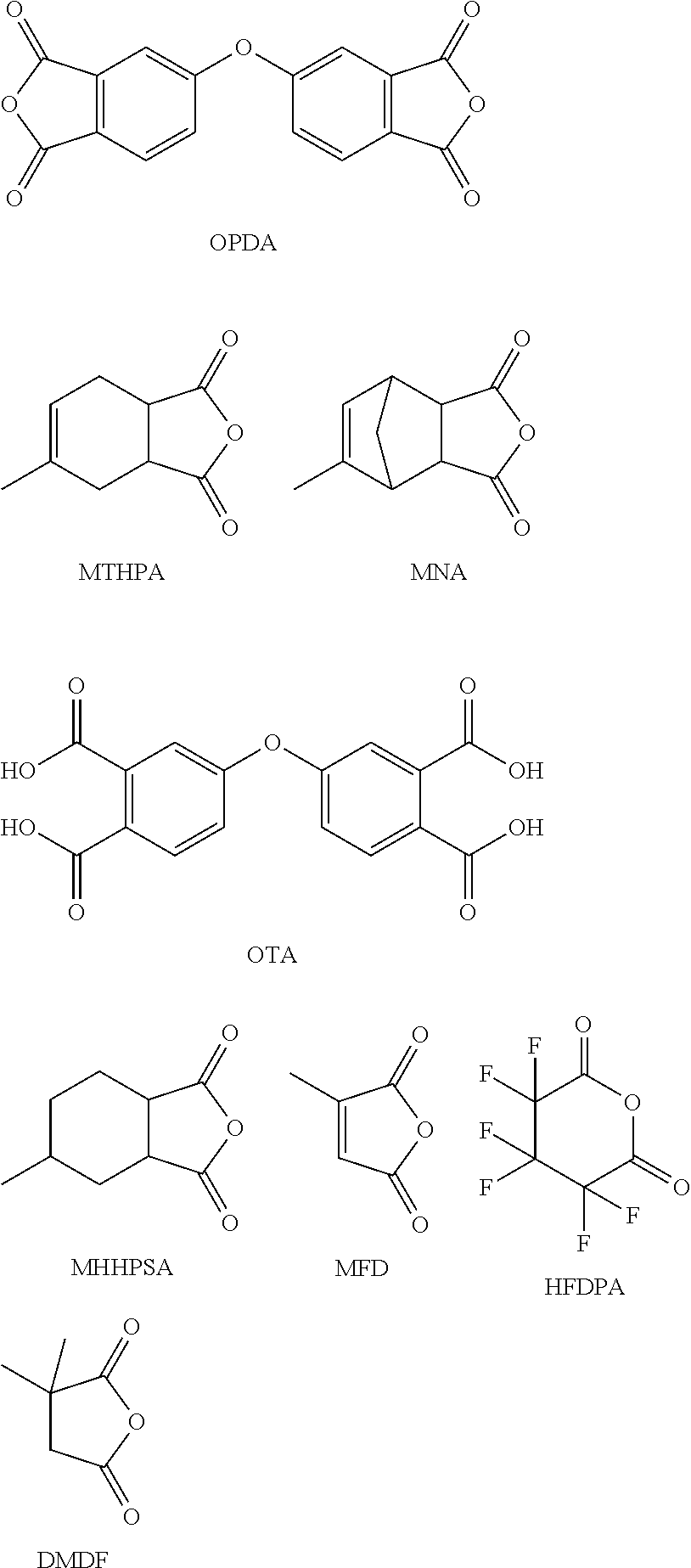
The present invention provides a composition comprising 5,5′-carbonylbis(isobenzofuran-1,3-dione), 3,3′,4,4′-benzophenonetetracarboxylic acid and at least one monoanhydride compound selected from the group consisting of methylhexahydroisobenzofuran-1,3-dione, 5-methyl-3a,4,7,7a-tetrahydro-4,7-methanoisobenzofuran-1,3-dione, 5-methyl-3a,4,7,7a-tetrahydroisobenzofuran-1,3-dione, 3-methylfuran-2,5-dione, 3,3,4,4,5,5-hexafluorodihydro-2H-pyran-2,6(3H)-dione and 3,3-dimethyldihydrofuran-2,5-dione. The invention also provides a hardener system for an epoxy resin, said hardener system comprising said composition. The invention also provides a method for hardening an epoxy resin employing the inventive composition.
Owner:EVONIK DEGUSSA GMBH
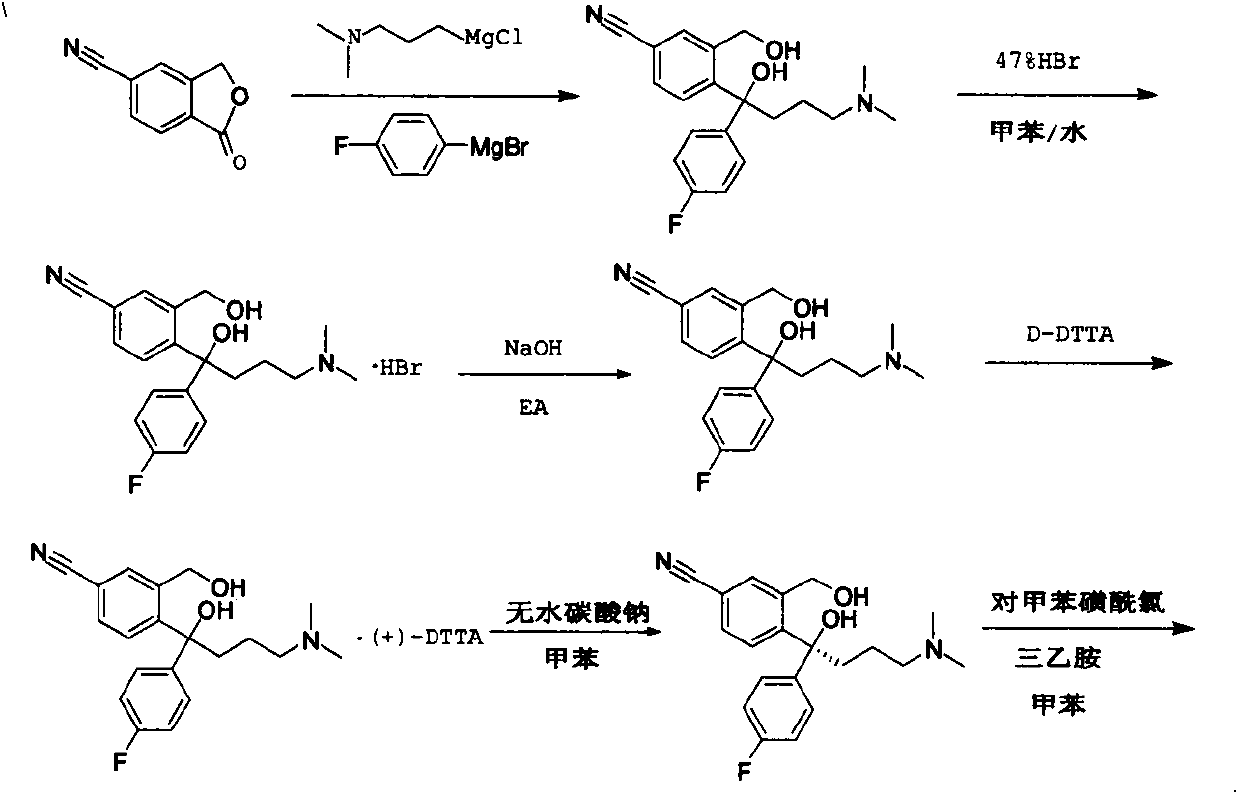
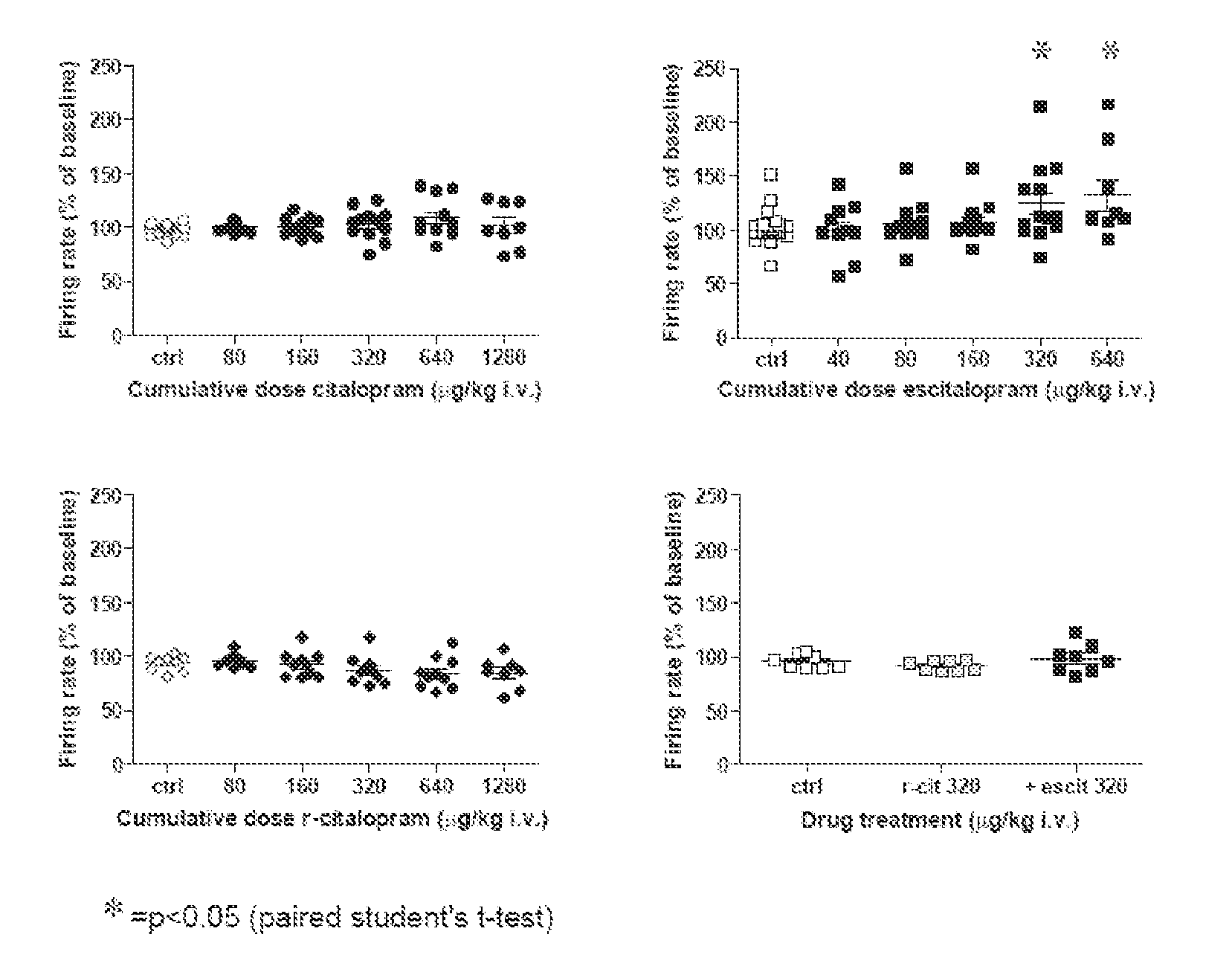
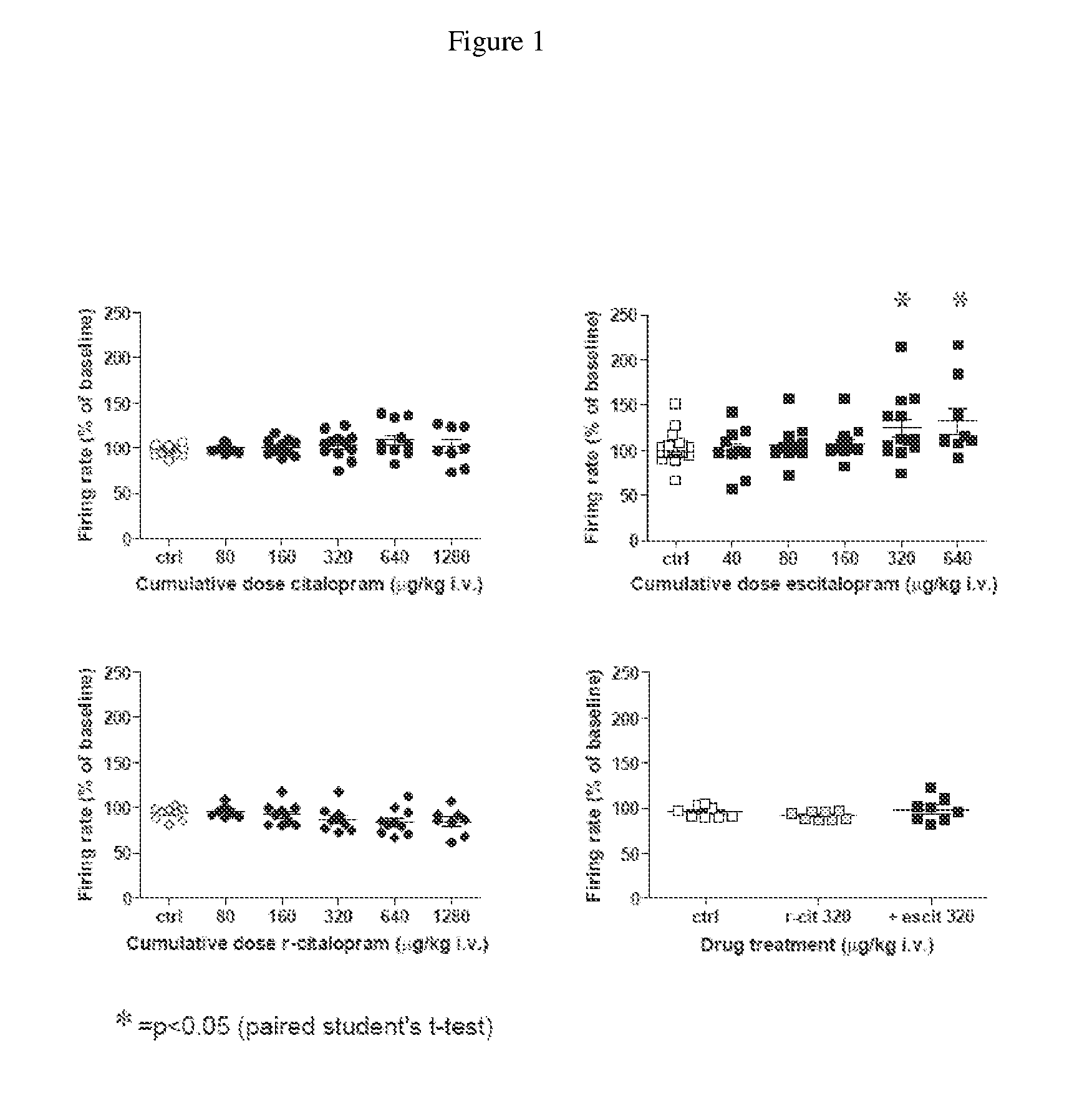
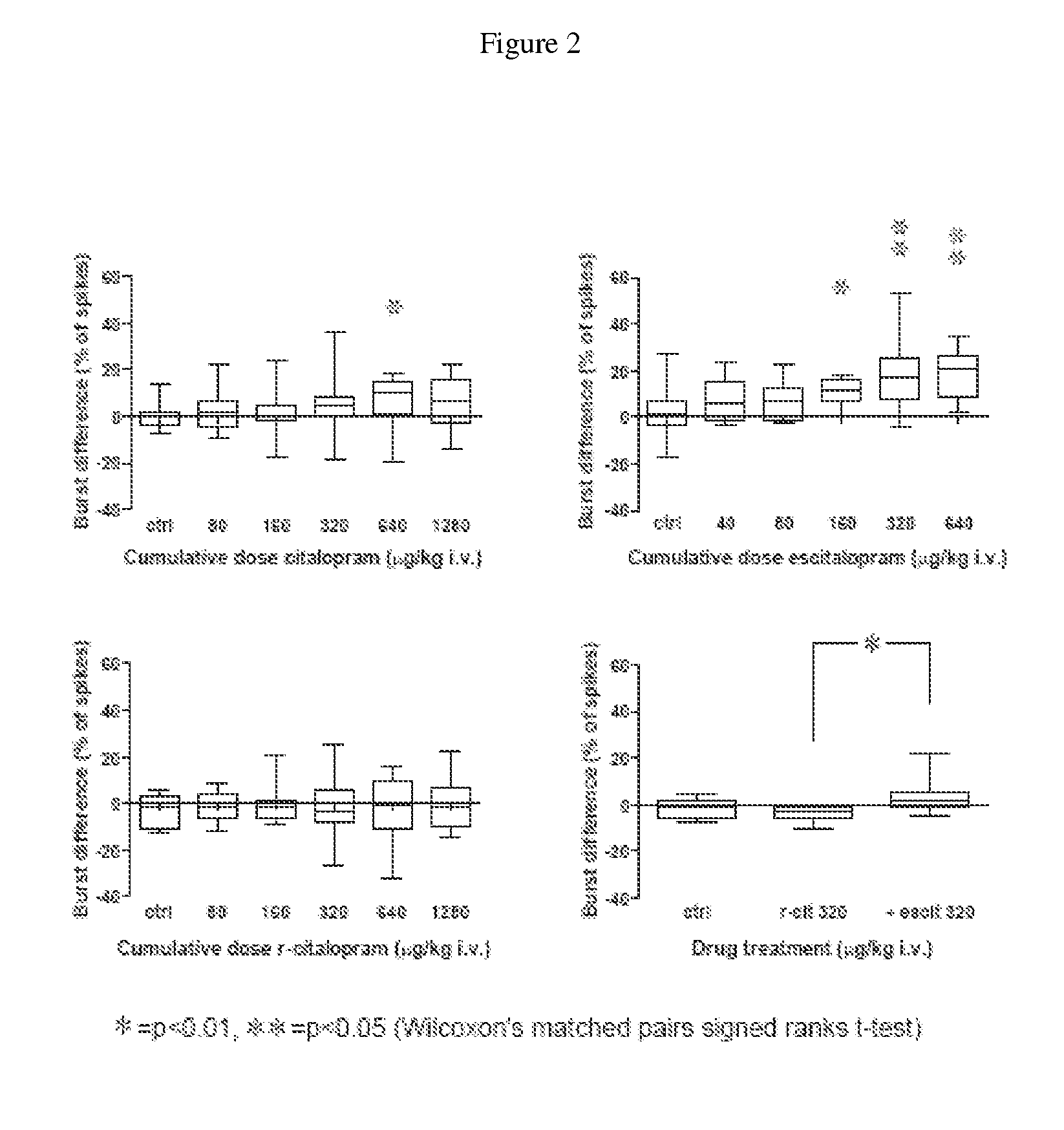
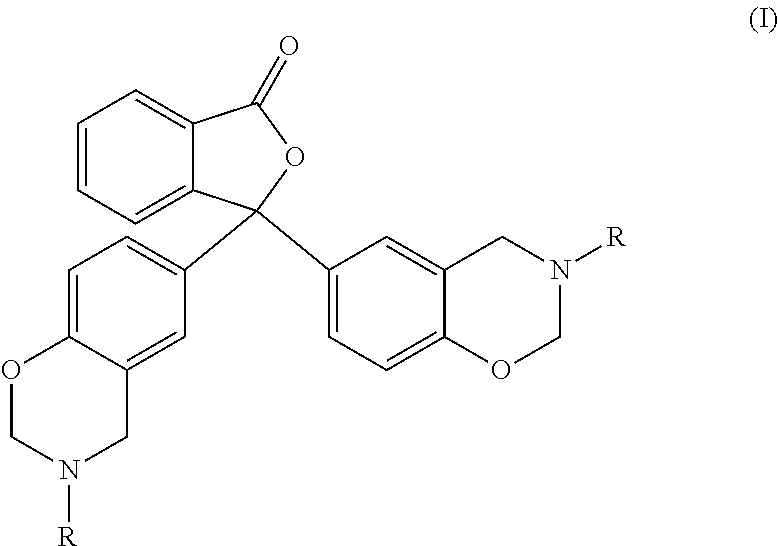
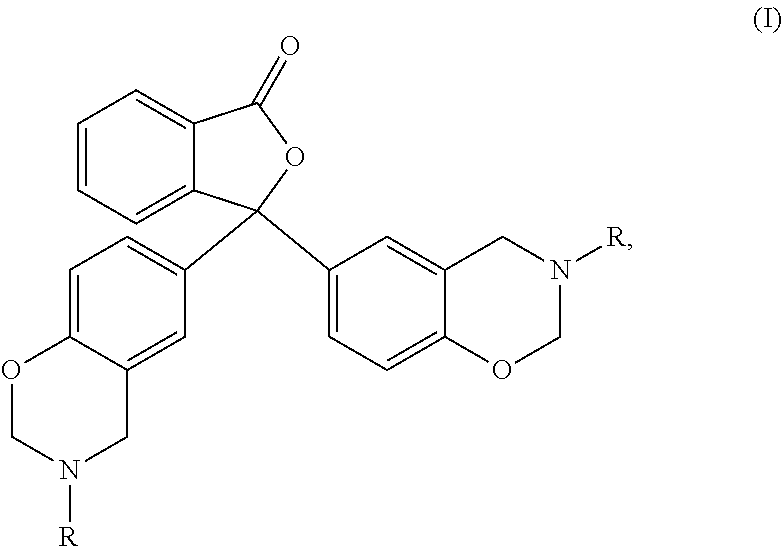
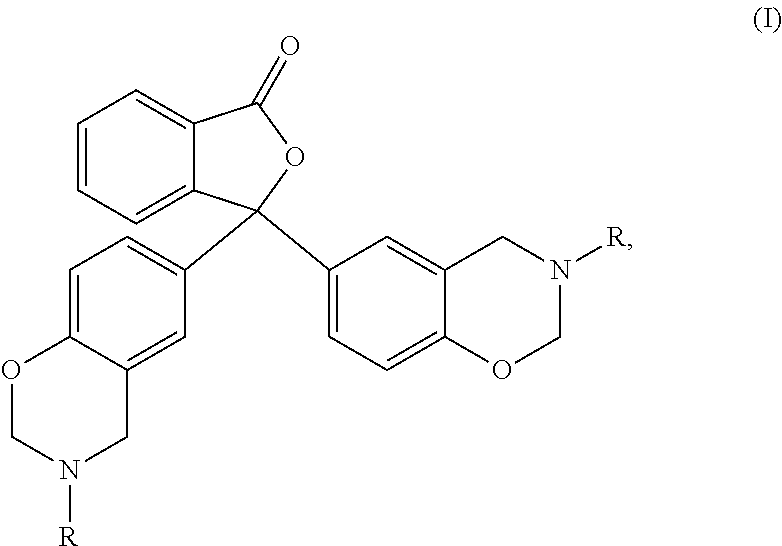
Uses of escitalopram
The present invention relates to the use of the compound escitalopram (INN-name), i.e. (S)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzofurancarbonitrile, or a pharmaceutically acceptable salt thereof for the preparation of a medicament for improving cognition in a condition where the cognitive processes are diminished.
Owner:H LUNDBECK AS
Benzoxazine Compounds Derivated From Phenolphtalein Having Flame-Retardant Properties And A Process For Their Preparation
The instant invention relates to 3,3′-bis(3,4-dihydro-3-phenyl-2H-1,3-benzoxazin-6-yl)-1(3H)-isobenzofuranone and analogues based on phenolphthalein, formaldehyde and a primary amine. Such compounds are, when cured to form polymeric networks, difficultly inflammable and resistant to high temperatures. Such compounds may especially be used in the production of printed wiring boards.
Owner:HUNTSMAN ADVANCED MATERIALS LICENSING SWITZERLAND GMBH +1
Processing-friendly dianhydride hardener for epoxy resin systems based on 5,5'-oxybis(isobenzofuran-1,3-dione)
The present invention provides a composition comprising 5,5′-oxybis(isobenzofuran-1,3-dione), 4,4′-oxybis(ortho-phthalic acid) and at least one monoanhydride compound selected from the group consisting of methylhexahydroisobenzofuran-1,3-dione, 5-methyl-3a,4,7,7a-tetrahydro-4,7-methanoisobenzofuran-1,3-dione, 5-methyl-3 a,4,7,7a-tetrahydroisobenzofuran-1,3-dione, 3-methylfuran-2,5-dione, 3,3,4,4,5,5-hexafluorodihydro-2H-pyran-2,6(3H)-dione, and 3,3-dimethyldihydrofuran-2,5-dione. The invention also provides a hardener system for epoxy resins, said hardener system comprising said composition. The invention also provides a method for hardening of epoxy resins employing the inventive composition.
Owner:EVONIK DEGUSSA GMBH
Process for the Synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isobenzofuran-1,9'-xanthen]-3-one (Rose Bengal) and Related Xanthenes
A new process for the manufacture of iodinated xanthenes in high purity includes a cyclization step followed by an iodination step. No extraction, chromatographic or solvent concentration steps are required, and the intermediate as well as final compounds are isolated via filtration or similar means. The process requires a single organic solvent, and the steps are completed at temperatures below 100° C. The exclusion of chloride ions, of chloride free-radicals, hypochlorite ions, or hypochlorous acid as reagents or from reagents that may generate these species in situ in the presence of oxidants, prevents undesirable impurity formation. Several new compounds have been conceived and isolated using these methods. These new compounds are also formed into new medicaments.
Owner:PROVECTUS PHARMATECH
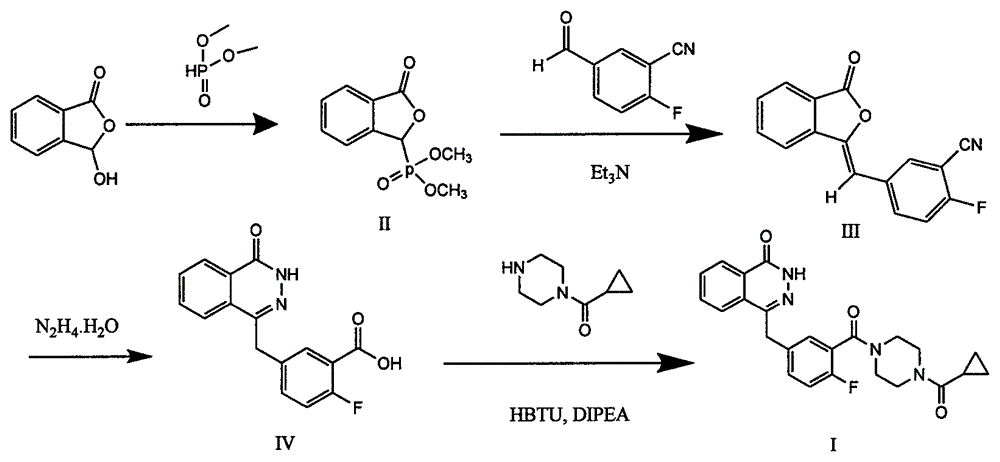
Preparation method of Olaparib intermediate
InactiveCN105085408ARaw materials are easy to getSimple processOrganic chemistryBenzoic acidIsobenzofuran
The invention relates to a preparation method of an Olaparib intermediate (IV), namely, 2-fluoro-5-[(4-oxo-3,4-dihydrophthalazin-1-yl)methyl]benzoic acid. The method comprises steps as follows: 1, 2-fluoro-5-formyl benzoic acid is used as a raw material and reacts with (3-oxo-1,3-dihydro-isobenzofuran-1-yl)dialkyl phosphate to produce 2-fluoro-5-(3-oxo-3H-isobenzofuran-1-yl-methylene)benzoic acid, namely, an intermediate (V); or 2-fluoro-5-formyl benzoic acid reacts with phthalide to produce 5-(2,3-dihydro-1,3-dioxo-1H-indene-2-yl)-2-fluobenzoic acid, namely, an intermediate (VI); 2, the intermediate V or the intermediate VI reacts with hydrazine hydtaye to produce an Olaparib intermediate (IV). The preparation method is concise in process, environment-friendly, economical and suitable for the industrial key Olaparib intermediate, raw materials are easy to obtain, and purification is easy.
Owner:GUANGZHOU YOUMIJIAN PHARMA TECH CO LTD
Bisphenol type substituted template molecularly imprinted polymer and preparation therefor and application thereof
ActiveCN105085813AHigh selectivityThere is no template leakage problemIon-exchange process apparatusOther chemical processesPerturbateurs endocriniensIsobenzofuran
The invention provides a bisphenol type substituted template molecularly imprinted polymer and a preparation method therefor and an application method thereof. The bisphenol type substituted template molecularly imprinted polymer (DMIP) is prepared by adopting a mass polymerization method by taking 3,3-bis(4-hydroxyl phenyl)-1(3H)-isobenzofuranone (phenolphthalein) as a substituted template molecule. The polymer represents high affinity and selectivity on ninth bisphenol type endocrine disrupters such as bisphenol S, bisphenol F, bisphenol A and the like. By using phenolphthalein which is low in price and low in toxicity as the bisphenol type substituted template molecule, the problem of an inaccurate result caused by template leakage when the molecularly imprinted polymer (MIP) prepared by taking a target compound as the template is used for a solid phase extraction material is solved. The prepared DMIP represents remarkable group selectivity on the bisphenol type endocrine disrupters and the DMIP serving as a sample pre-treatment material for the bisphenol type endocrine disrupters has a wide application prospect.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
DOPO (9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide) derivative fire retardant and preparation method and application thereof
ActiveCN107739453AImprove flame retardant performanceEnhanced oxygen and heat insulation capacityGroup 5/15 element organic compoundsArylDiketone
The invention discloses a DOPO (9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide) derivative fire retardant and a preparation method and application thereof. The DOPO derivative fire retardant contains a structure connected by a basic unit A-M-B and an additional unit, wherein the additional unit is an M unit, an M-A unit, an M-B unit, a DOPO derivative unit, a nitrile group, a nitrile group substituted DOPO derivate unit and / or DOPS derivate unit, and the condition is that A is a terminal unit, and B is an amido substituted DOPO derivate unit; A is a 1,3-diketone group-isobenzofuran-5-group-methanoyl monovalence group disclosed in the following formula (I); B is a bivalence amine group disclosed in the following formula (II) or (III); R1 and R2 are independently hydrogen, C1-C15 alkyl group or C6-C12 aryl groups, each m is independently 1,2,3 or 4, and M expresses direction connection or C6-C12 aryl groups. The DOPO derivative novel fire retardant is provided with a functional groupcompatible with an organic polymer, and the mechanical property and the antiflaming effect of a composite material which contains the fire retardant are improved. The formulas are shown in the description.
Owner:GUIZHOU MATERIAL IND TECH INSTITUE
Method for detecting phenothiazine-derivative color and color-developer reagent used therein
ActiveUS20100112622A1Good colorIncrease the scope of applicationMonoazo dyesAnalysis using chemical indicatorsIsobenzofuranPhenothiazine derivative
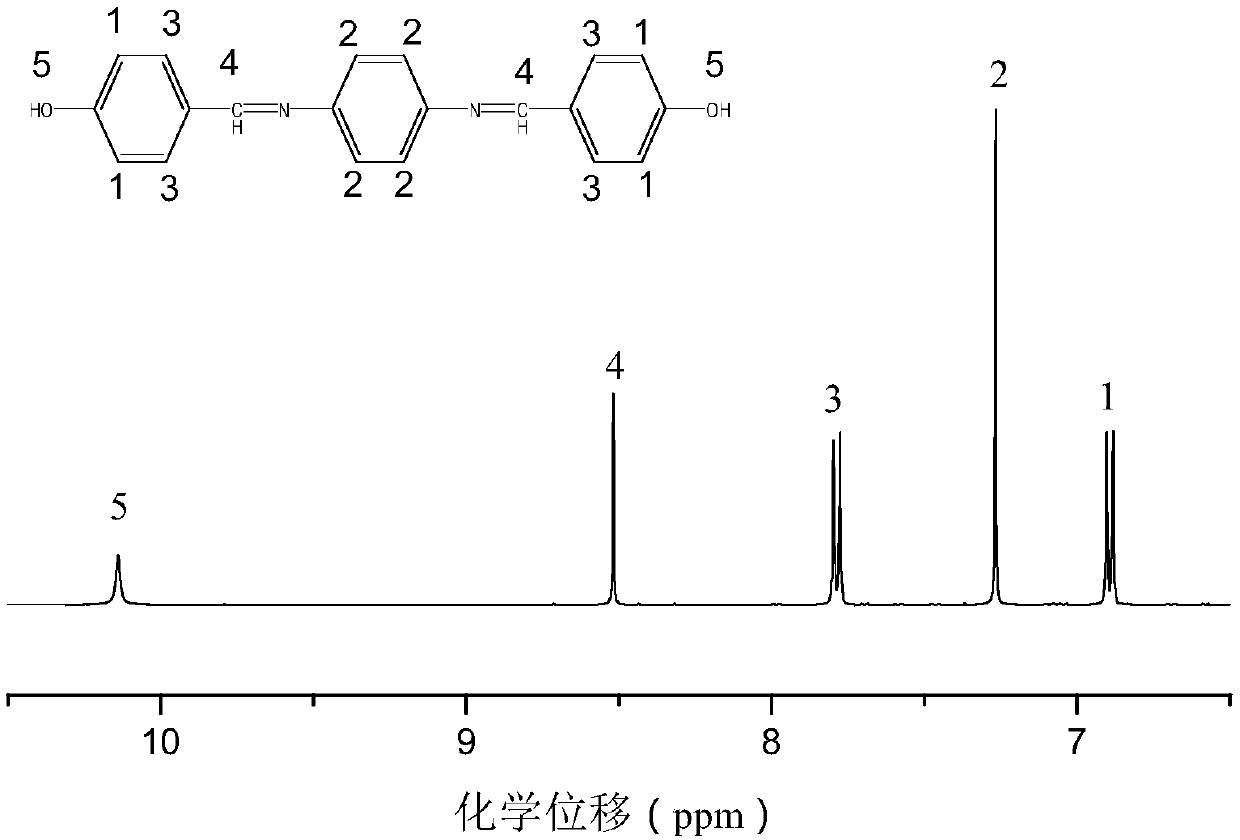
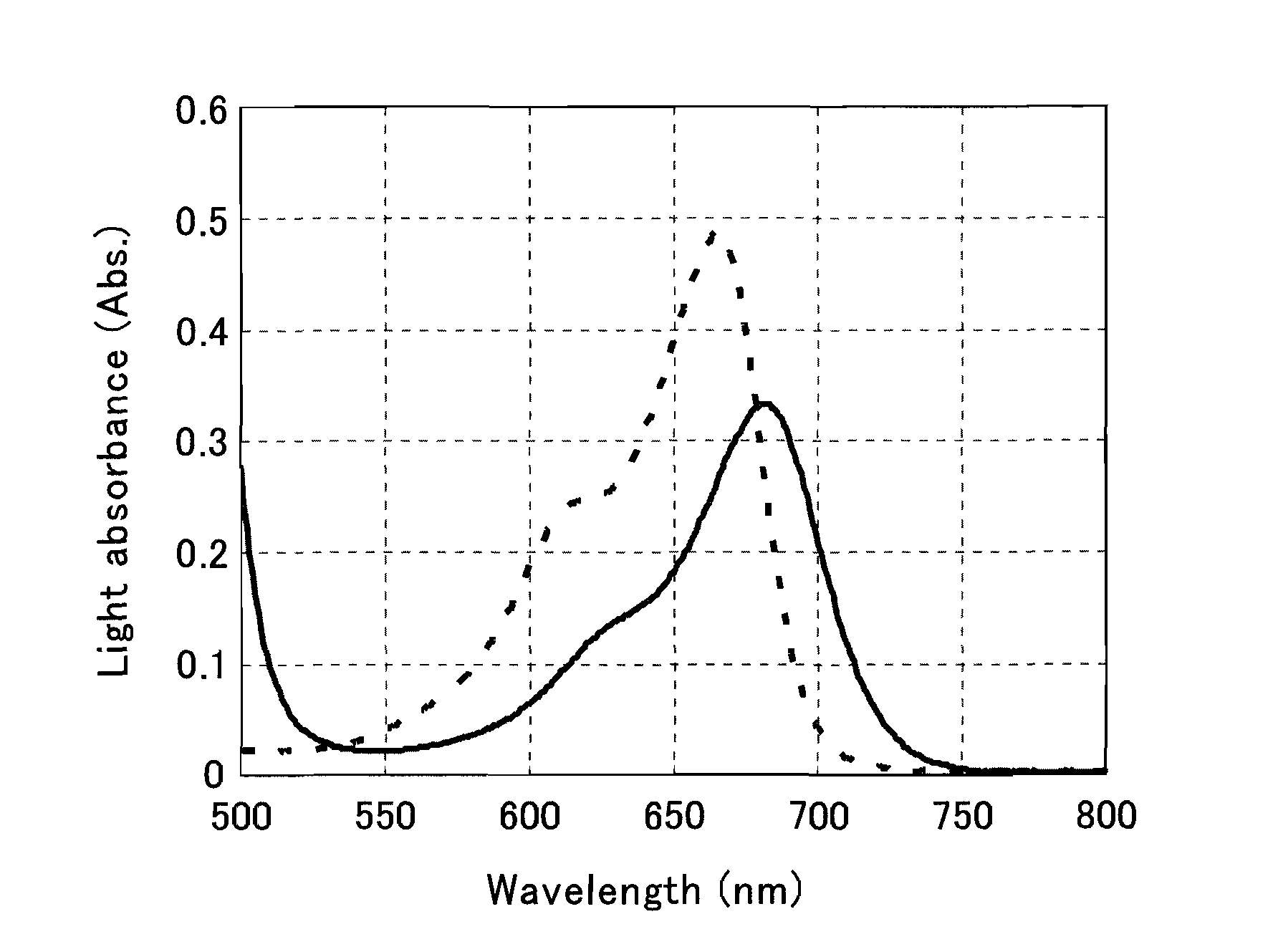
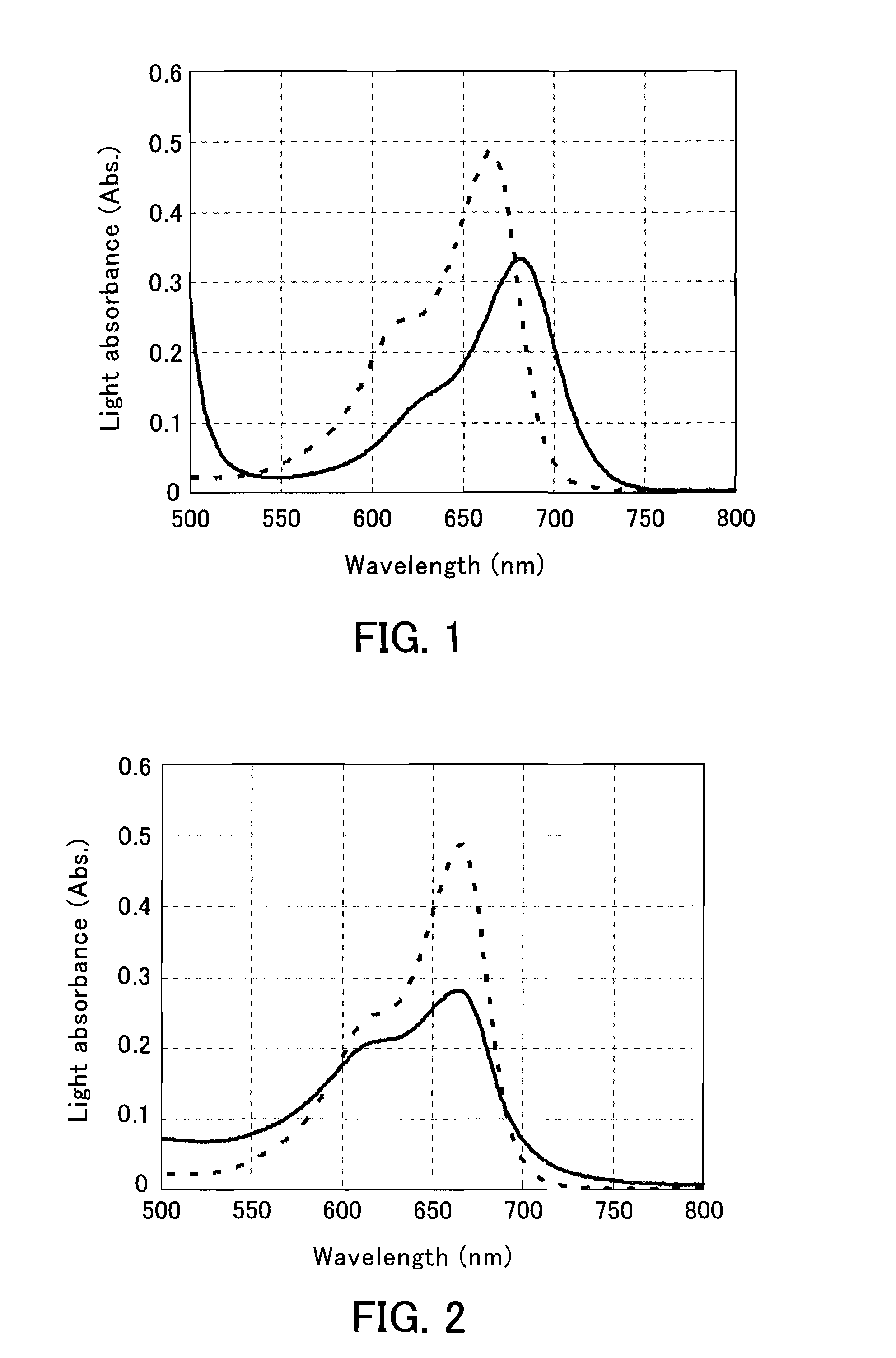
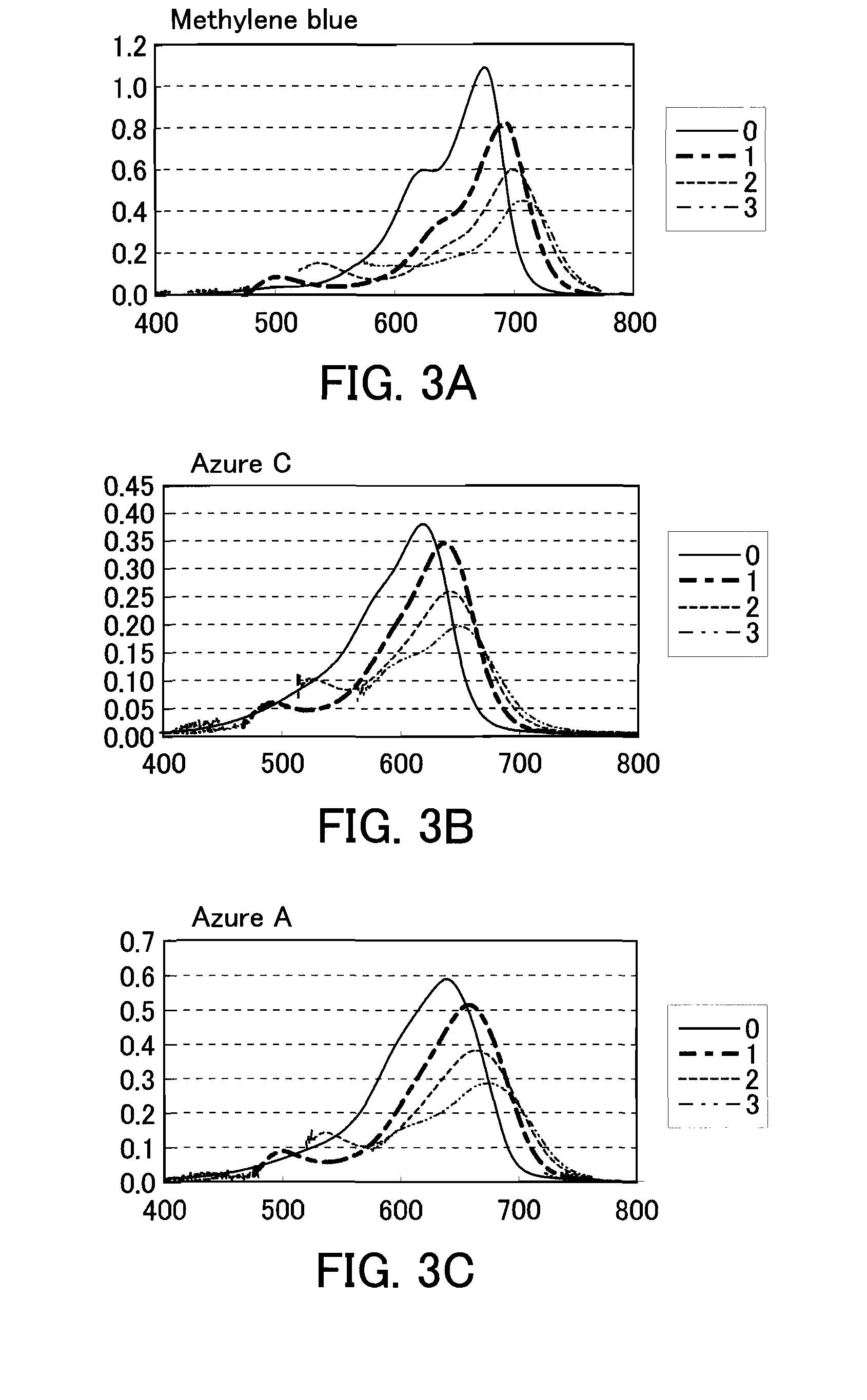
The present invention provides a phenothiazine-derivative color-measuring method that can detect a phenothiazine-derivative color even at a wavelength longer than the wavelength that exhibits maximum absorption. A phenothiazine-derivative color is detected, by adding a 5-hydroxy-1-(4-sulfophenyl)-4-(4-sulfophenylazo)pyrazole-3-carboxylic acid salt, 6-hydroxy-5-(4-sulfophenylazo)-2-naphthalenesulfonic acid salt, 3-hydroxy-4-(4-sulfonaphthylazo)-2,7-naphthalenedisulfonic acid salt, 7-hydroxy-8-(4-sulfonaphthylazo)-1,3-naphthalenedisulfonic acid salt, 3′,6′-dihydroxy-2′,4′,5′,7′-tetraiodospiro[isobenzofuran-1(3H),9′-(9H)xanthene]-3-one salt, 3′,6′-dihydroxy-2′,4′,5′,7′-tetrabromo-4,5,6,7,-tetrachlorospiro[isobenzofuran-1(3H),9′-[9H]xanthene]-3-one salt, 4,5,6,7-tetrachloro-3′,6′-dihydroxy-2′,4′,5′,7′-tetraiodospiro[isobenzofuran-1(3H),9′-[9H]xanthene]-3-one salt or flavonoid-based color to the reaction system containing a phenothiazine-derivative color, and then measuring the light absorbance at a wavelength of 610 to 730 nm.
Owner:ARKRAY INC
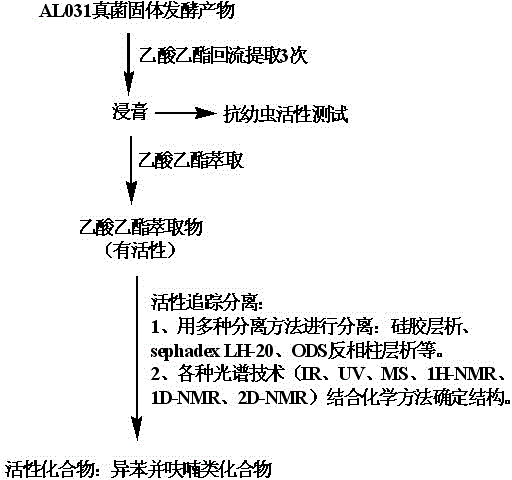
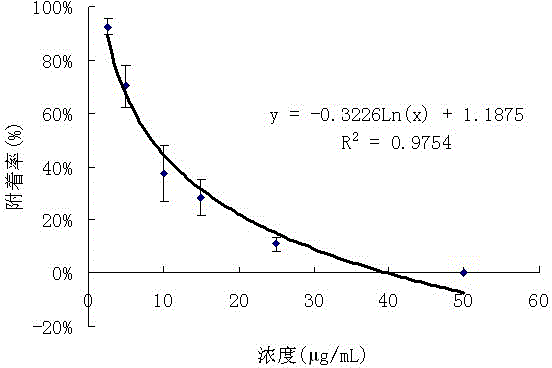
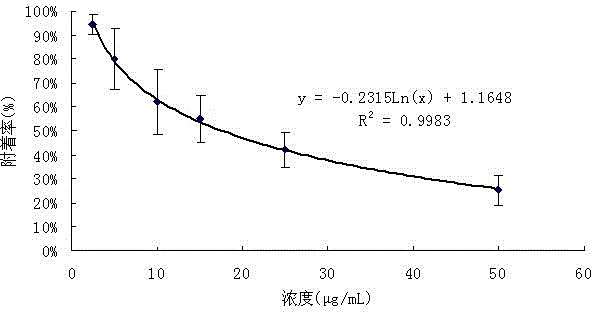
Application for isobenzofuran type compounds in marine biofouling prevention and preparation method thereof
InactiveCN102617529AStrong anti-biofouling activityEasy to degradeOrganic chemistryAntifouling/underwater paintsIsobenzofuranChemical compound
The invention discloses an application for isobenzofuran type compounds in marine biofouling prevention and a preparation method thereof, which belongs to the field of marine fouling organism repellency and mainly solves the problem of marine biofouling. The application for isobenzofuran type compounds in marine biofouling prevention can be used for preventing underwater structure surfaces from being attached and / or fouled by marine organisms. The sobenzofuran type compounds are all obtained from isolation of fungus which is named cephalosporium cordaAL 031 in the strain classification, and the preservation serial number of the fungus strain is CCTCC Number M2010140. Extracts of secondary metabolites of the fungus and two isobenzofuran type compounds are performed a fouling resistance activity screening, the experiment result indicates that the extracts of secondary metabolites of the fungus and two isobenzofuran compounds show high fouling resistance activity, attachment of marine fouling organisms can be inhibited, and the application for isobenzofuran type compounds in marine biofouling prevention and the preparation method thereof can be used for prevention and treatment of marine biofouling and have a good application perspective.
Owner:YUNNAN MINZU UNIV
Method for synthetizing indanone compound
ActiveCN102491886ASolve operational problemsSolve processingOrganic compound preparationCarbonyl compound preparationLiquid wasteIsobenzofuran
The invention relates to a method for a synthetizing indanone compound, which comprises the steps of using substituted isobenzofuran-1(3H)-ketone as a raw material, reacting the substituted isobenzofuran-1(3H)-ketone and an ester compound containing alpha-methylene to produce a 1,3-dicarbonyl compound, and further synthetizing the substituted indanone compound under the action of a catalyst. Compared with the prior art, the problems including difficult operation due to large usage of acid compounds containing phosphorus or aluminium trichloride and the like, large amounts of liquid waste due to after-treatment and the like by means of the existing indanone compound synthetizing technology are mainly solved, reaction operation is simple, reaction conditions are easy to achieve, produced liquid waste is little in the after-treatment process, the pollution of the entire reaction process environment is low, and industrialization and sustainable development are easy to achieve.
Owner:SHANGHAI RES INST OF CHEM IND +1
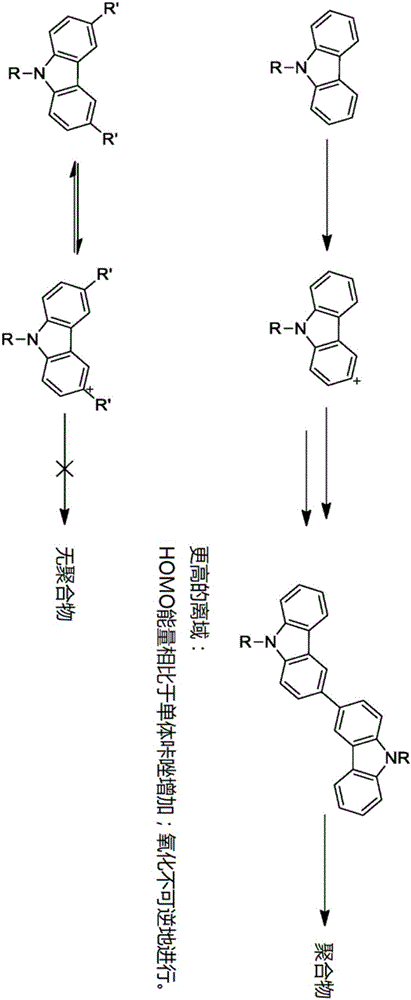
Crosslinkable host materials
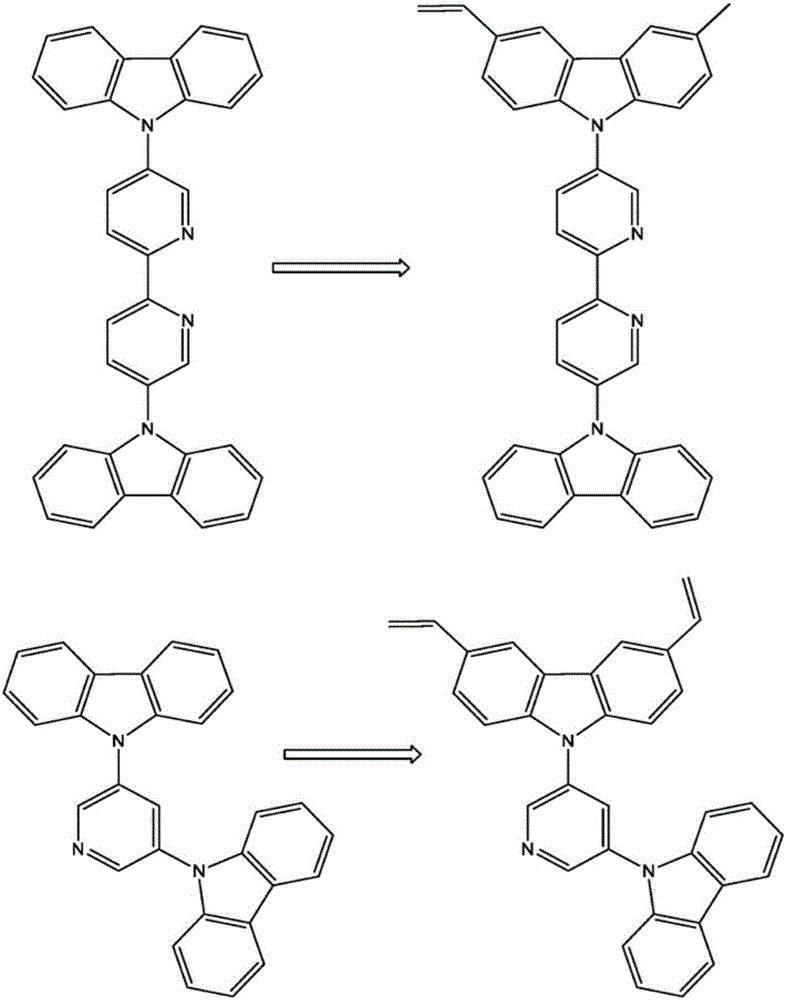
InactiveCN106715420AEasy to moveImprove efficiencyGroup 5/15 element organic compoundsFinal product manufacturePyridazinePhenanthroline
The invention relates to a crosslinkable organic molecule having a structure of the formula (1) and to the use thereof, wherein Ar is independently of one another, an unsaturated or aromatic carbo- or heterocyclic unit with 5 to 30 ring atoms, selected from the group consisting of naphthalene, anthracene, phenanthrene, pyrene, dihydropyrene, chrysene, perylene, fluoranthene, benzanthracene, tetracene, pentacene, benzpyrene, furan, benzofuran, isobenzofuran, thiophene, benzothiophene, isobenzothiophene, dibenzothiophene, pyrrole, indole, isoindole, carbazole, pyridine, quinoline, isoquinoline, acridine, phenanthridine, benzo-5,6-quinoline, benzo-6,7-quinoline, benzo-7,8-quinoline, phenothiazine, phenoxazine, pyrazole, indazole, imidazole, benzimidazol, naphthimidazole, phenanthrimidazole, pyridimidazole, pyrazine-imidazole, quinoxalinimidazole, oxazole, benzoxazole, naphthoxazole, anthroxazole, phenanthroxazole, isoxazole, isothiazole, 1,3-thiazole, benzothiazole, pyridazine, benzopyridazine, pyrimidine, benzpyrimidine, quinoxaline, pyrazine, phenazine, naphthyridine, azacarbazole, benzocarboline, phenanthroline, 1,2,3-triazole, 1,2,4-triazole, benzotriazole, 1,2,3-oxadiazole, 1,2,4-oxadiazole, 1,2,5-oxadiazole, 1,3,4-oxadiazole, 1,2,3-thiadiazole, 1,2,4-thiadiazole, 1,2,5-thiadiazole, 1,3,4-thiadiazole, 1,3,5-triazine, 1,2,4-triazine, 1,2,3-triazine, tetrazole, 1,2,3,4- oxatriazole, 1,2,3,4-oxatriazole, 1,2,4,5-tetrazine, 1,2,3,4-tetrazine, 1,2,3,5-tetrazin, purine, pteridine, indolizine, benzothiadiazole, indenocarbazole, indenofluorene, spirobifluorene, and indolocarbazole; D1 is a donor group having a structure of the formula (1a); and D2 is a donor group having a structure of the formula (1b).
Owner:SAMSUNG DISPLAY CO LTD
Preparation method of (S)-2-[1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonyl ethyl]-4-acetyl amino isoindoline-1, 3-diketone isomer
ActiveCN104529869AGuaranteed validityOrganic active ingredientsOrganic chemistryIsobenzofuranEthyl group
The invention discloses a preparation method of a (S)-2-[1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonyl ethyl]-4-acetyl amino isoindoline-1, 3-diketone isomer. The preparation method comprises the following steps: preparing 1-(3-ethoxy-4-methoxy-phenyl)-2-methylsulfonyl-ethylamine chiral amino acid salt by virtue of a mixed solvent reaction, and then reacting with N-(1,3-dioxo-1,3-dihydrogen-isobenzofuran-4-yl)-acetamide under the condition proper for generate a final product. According to the preparation method disclosed by the invention, the prepared (S)-2-[1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonyl ethyl]-4-acetyl amino isoindoline-1, 3-diketone isomer has the purity of at least 99 percent basically without containing other isomers, so that the effectiveness of a medicament preparation is ensured.
Owner:SUZHOU YABAO PHARMA R&D CO LTD
Isobenzofuran compound capable of improving cigarette suction throat comfort and preparation method and application of isobenzofuran compound
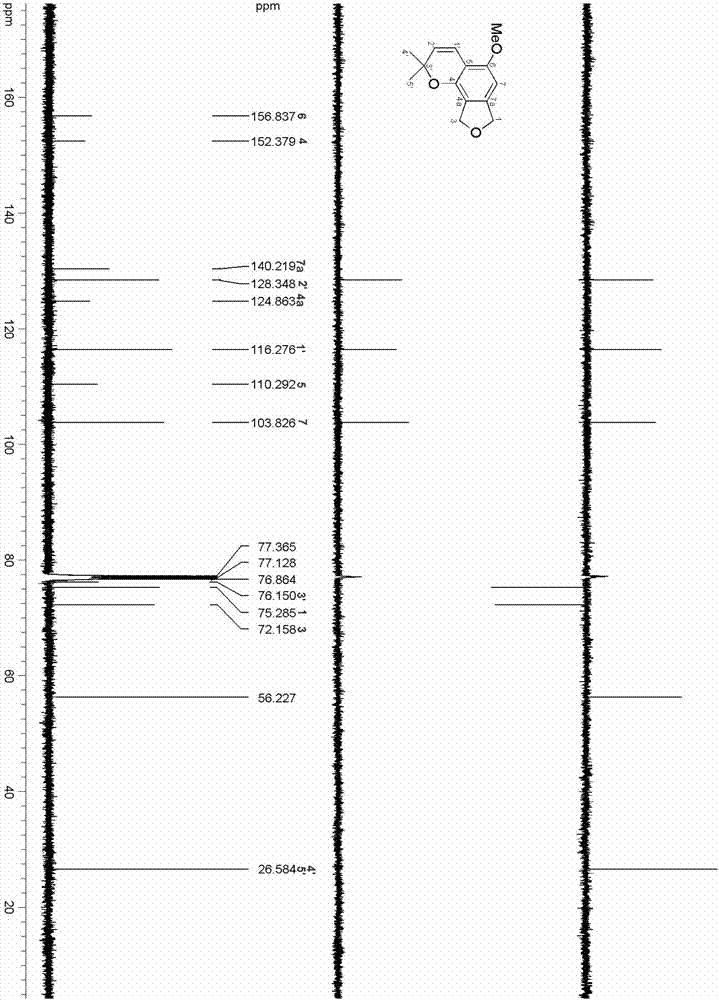
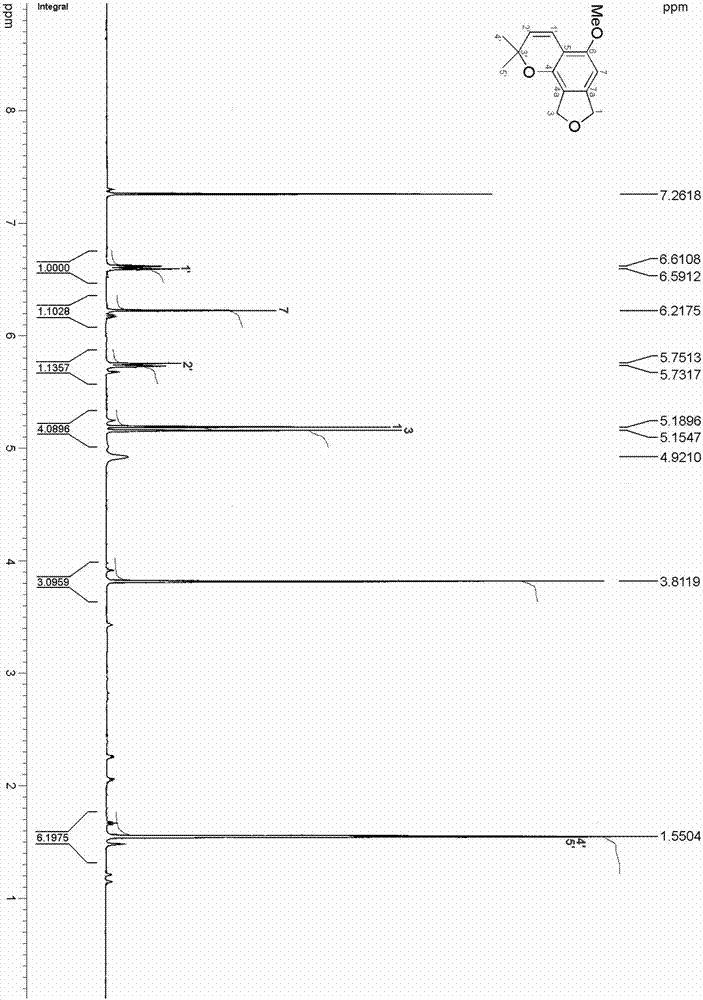
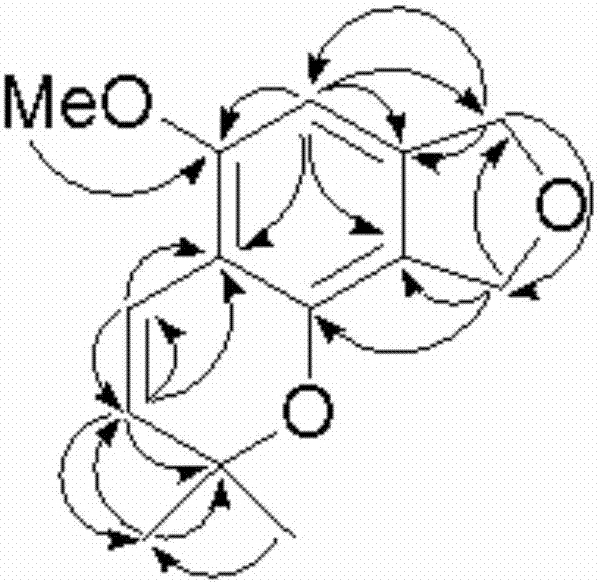
ActiveCN106957322ALess irritatingImprove comfortOrganic chemistryTobacco treatmentIsobenzofuranStructural formula
The invention relates to an isobenzofuran compound capable of improving the cigarette suction throat comfort and a preparation method and application of the isobenzofuran compound, and belongs to the technical field of phytochemistry. The compound is separated from cigarettes and tobaccos; the compound is named 5-methoxy-2,2-dimethyl-7,9-dihydro-2H-furo[3,4-]chromene, the English name is 5-methoxy-2,2-dimethyl-7,9-dihydro-2H-furo[3,4-]chromene, the molecular formula is C14H16O3 and the structural formula is as shown in a formula (I). According to the preparation method, the compound is prepared through extract extraction, organic solvent extraction, MCI decoloration, silica-gel column chromatography and high-performance liquid chromatography separation on the cigarettes and tobaccos as the raw material. By adding the compound to a cigarette filter, the cigarette suction throat comfort can be improved and the compound has an obvious throat moistening effect.
Owner:CHINA TOBACCO YUNNAN IND
Method for continuously preparing isobenzofuran compounds by using micro-channel reaction device
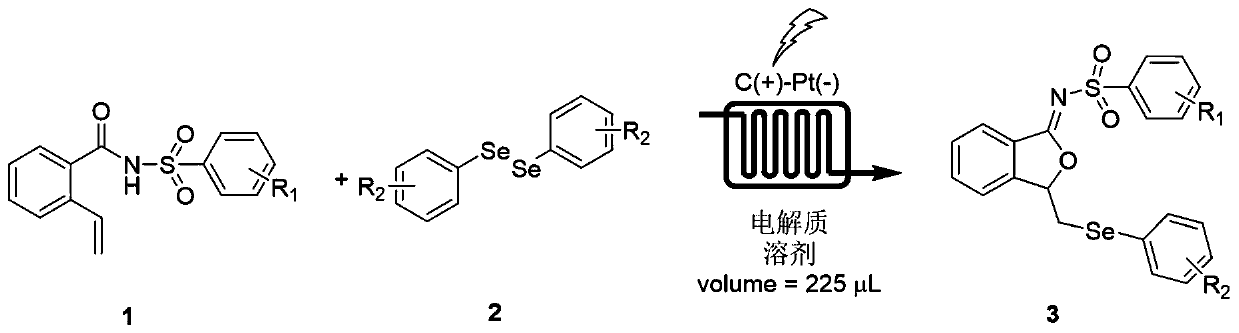
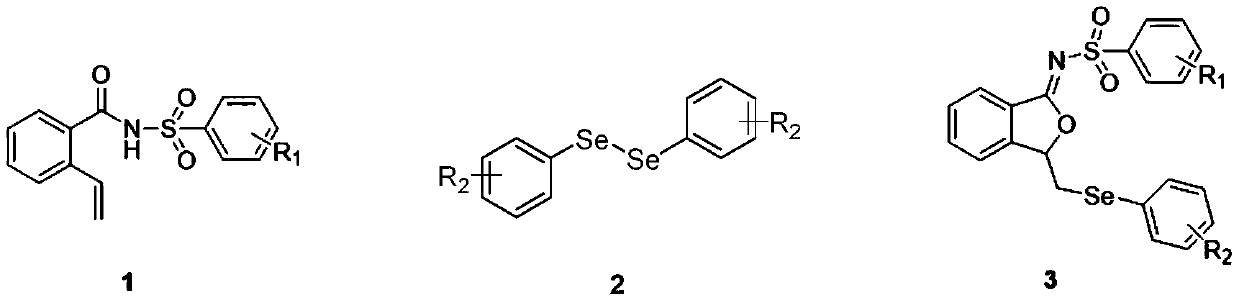
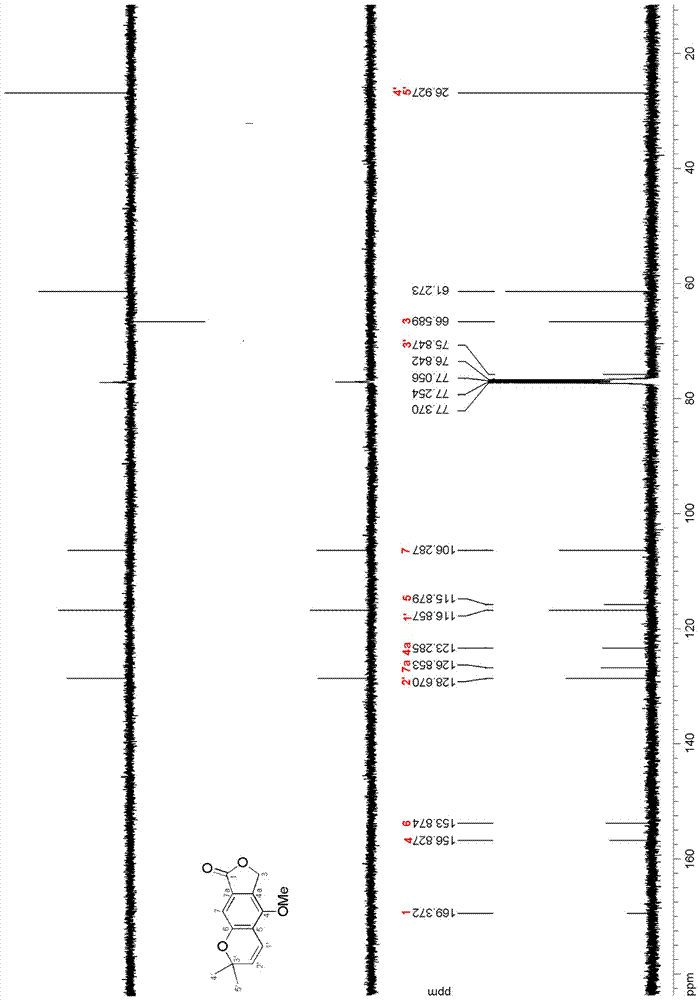
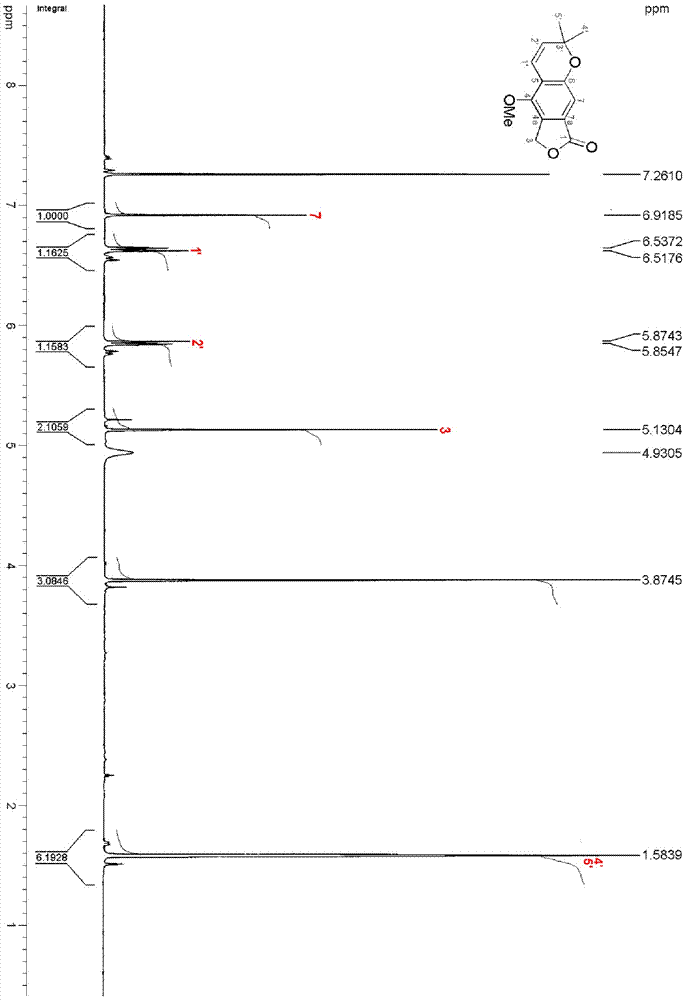
ActiveCN111560624AHigh yieldShort reaction timeOrganic chemistryElectrolytic organic productionIsobenzofuranPtru catalyst
The invention discloses a method for continuously preparing isobenzofuran compounds by using a micro-channel reaction device. The method comprises the following steps: dissolving an ortho-vinyl sulfonamide compound 1, diselenide 2 and an electrolyte in a solvent to obtain a homogeneous solution; pumping the homogeneous solution into the micro-channel reaction device, and carrying out a continuouselectrolytic reaction to obtain the isobenzofuran compound 3. The micro-channel reaction device is a micro-channel reaction device provided with an electrode. Compared with the prior art, the method has the following advantages: the micro-channel reaction device is adopted, so the reaction time is short, the product yield is high, and the reaction efficiency is remarkably improved; and meanwhile,expensive organic catalysts or metal catalysts do not need to be added, so operation is easy and convenient, environment friendliness and high efficiency are achieved, and cost is low.
Owner:NANJING TECH UNIV
Isobenzofuran compound with effect of removing free radicals in radix puerariae and application of isobenzofuran compounds in tobacco
ActiveCN106883243ASimple structureExact removal effectOrganic chemistryTobacco treatmentChromatographic separationIsobenzofuran
The invention discloses an isobenzofuran compound as well as a preparation method and application thereof. The isobenzofuran compound is obtained by separating from a traditional Chinese herbal medicine radix puerariae; the compound is named as 5-methoxy-2,2-dimethyl-2H-furo[3,4-g] chromen-8(6H)-one as an English name, and a molecular formula of the compound is C14H14O4; a structural formula of the compound is shown in the description. The preparation method of the isobenzofuran compound comprises the following steps: using the traditional Chinese herbal medicine radix puerariae as a raw material and carrying out extractum extraction, organic solvent extraction, MCI decolorizing, silica-gel column chromatography and high pressure liquid chromatography separation to obtain a finished product; the isobenzofuran compound is subjected to antioxidant activity and free radical removal activity screening, so the isobenzofuran compound has higher antioxidant and free radical removing activities; the compound is used for producing cigarettes and can be used for effectively removing the free radicals in smoke gas of the cigarettes.
Owner:YUNNAN MINZU UNIV
Preparation of isobenzofuran ketone compounds
The invention relates to a preparation method for obtaining isobenzofuran ketone compound from a crude extract of a fennelflower by adopting a method of high-speed countercurrent chromatography. A solvent used by the preparation method of the invention is petroleum ether or n-hexane or n-heptane or a four-component solvent system composed of n-pentane, acetic ether and methanol or ethanol or methyl cyanide and water and the isobenzofuran ketone compound is separated from the fennelflower by a separation step. The method has lager separation amount, no damage to samples, high recovery rate and moderate separation environment, and saves the solvent. Adoption of a countercurrent chromatograph can ensure that a large amount of crude samples can be brought in directly or compounded to a compound and the separation can realize higher purity and obtain good separation effect. The preparation method of the invention is not only applicable to preparing products with higher purity from the crude extract of plants but also applicable to purifying the crude extract of isobenzofuran ketone substances which are obtained through various means and separating the isobenzofuran ketone substances by the countercurrent chromatographs of different types.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
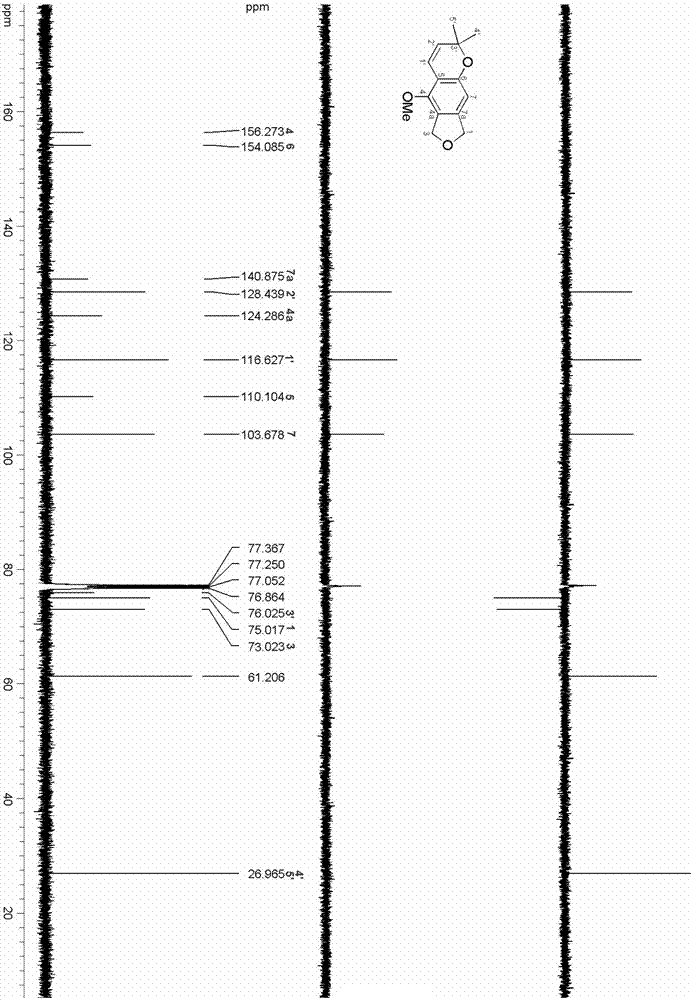
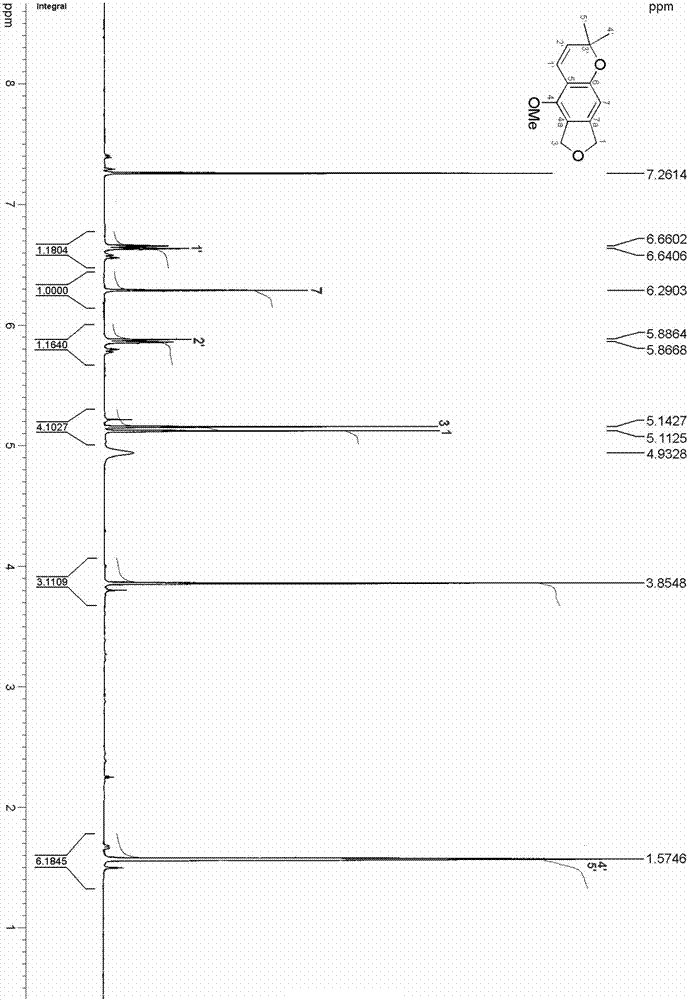
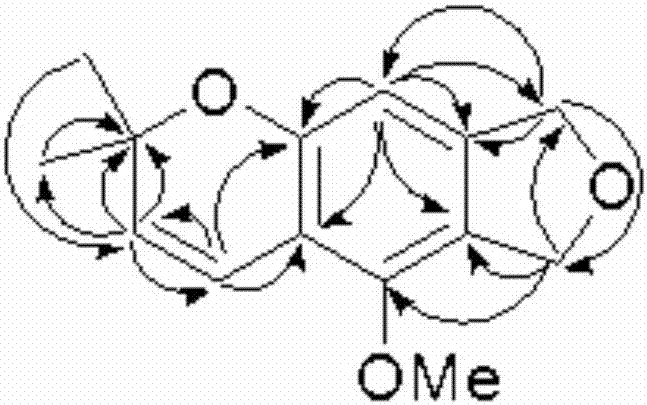
Thiophene[3,4-f]isobenzofuran-4,8-diketone polymer as well as preparation method and application thereof
ActiveCN109776766ASolid-state devicesSemiconductor/solid-state device manufacturingDiketoneIsobenzofuran
The invention provides a thiophene[3,4-f]isobenzofuran-4,8-diketone polymer as well as a preparation method and application thereof. The structure of the thiophene[3,4-f]isobenzofuran-4,8-diketone polymer is shown in the description, wherein n in the PBDFFTD is 24-25; n in the PBDFSFTD is 25-26. The PBDFFTD and the PBDFSFTD are high in spectral absorbability between 300-700 nm, and the optical band gaps of the PBDFFTD and the PBDFSFTD are respectively 1.81 eV and 1.77 eV; the PBDFFTD and the PBDFSFTD are matched with a non-fullerene receptor ITIC by absorption spectrum; the PBDFFTD and the PBDFSFTD are low in HOMO energy level; after primary optimization, the battery efficiency of the PBDFFTD is 7.00%, and the battery efficiency of the PBDFSFTD is 7.65%.
Owner:HENAN UNIVERSITY
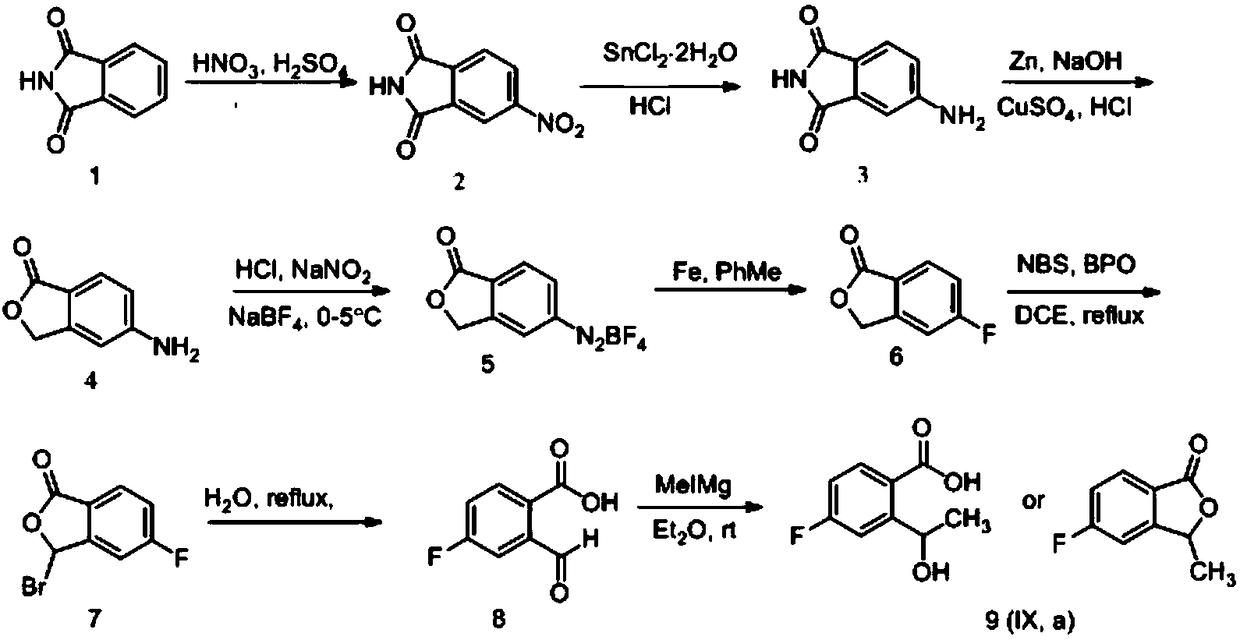
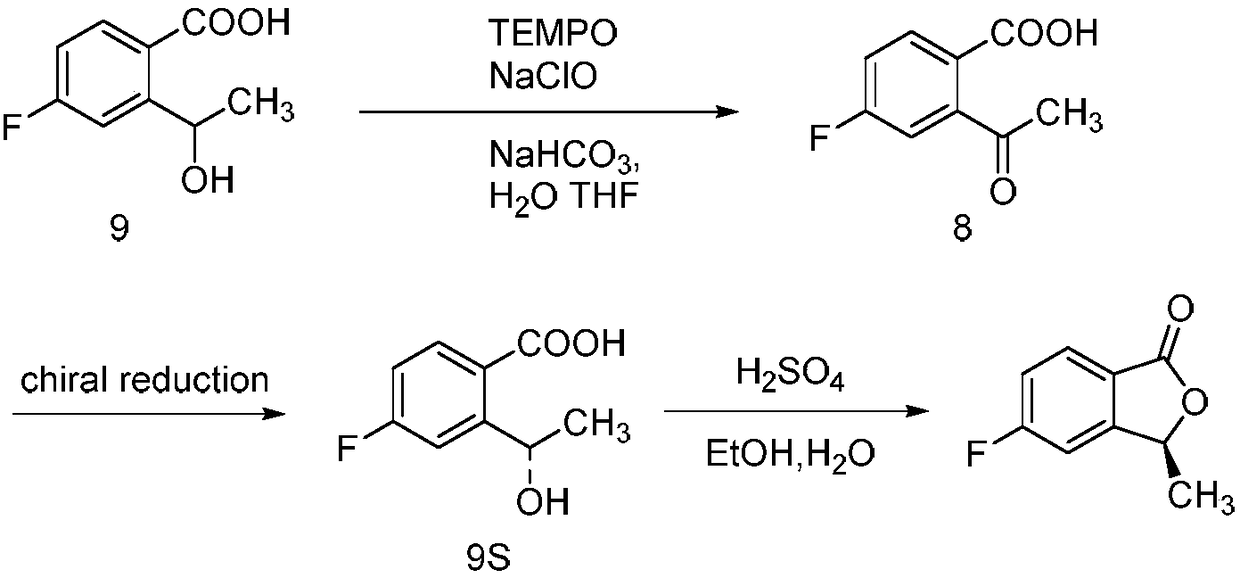
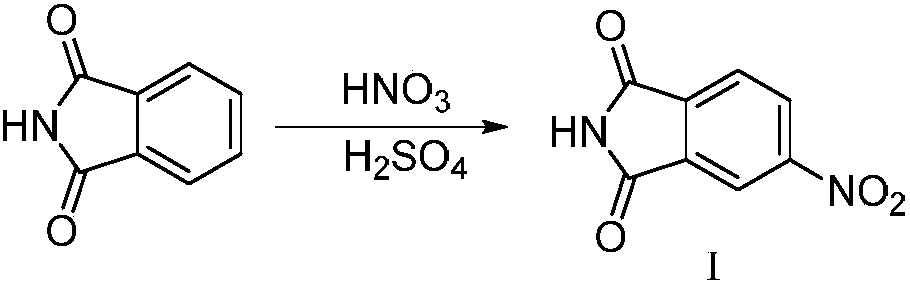
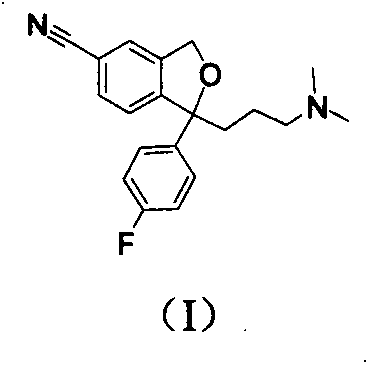
Synthesizing method of 5-fluoro-3-methyl isobenzofuran-1(3H)-ketone
ActiveCN109134410AEasy to operateEasy post-processingPreparation from carboxylic acid esters/lactonesOptically-active compound separationIsobenzofuranNitration
The invention relates to the technical field of medicine and relates to a synthesizing method of 5-fluoro-3-methyl isobenzofuran-1(3H)-ketone. The 5-fluoro-3-methyl isobenzofuran-1(3H)-ketone is synthesized by subjecting the initial raw material phthalimide to 8 steps such as nitration, reduction, cyclizing, diazotization, bromination and esterification. The method has the advantages that the preparation of the 5-fluoro-3-methyl isobenzofuran-1(3H)-ketone which is the key intermediate of the antitumor drug Lorlatinib (PF-06463922), total yield can reach 7.0% or above, and the method is simpleto operate, convenient in post-processing, low in time consumption, low in cost and beneficial to industrialization; the Lorlatinib is synthesized by subjecting the intermediate and 1-methyl-3-(( methylamino)methyl)-1H-pyrazol-5-nitrile to ammonolysis, substitution, coupling, chiral resolution and the like, and a new method is provided for the synthesizing of the antitumor drug Lorlatinib.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation methods for impurities of escitalopram oxalate
The invention relates to novel synthetic methods for three impurities of escitalopram oxalate. The methods have great significance for synthesis of the escitalopram oxalate with high purity. The invention mainly study syntheses of a citalopram amide impurity 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-isobenzofuran-5-formamide (II), a citalopram lactone impurity 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-3-oxo-1,3-dihydro-isobenzofuran-5-carbonitrile (III) and a citalopram-N-oxide impurity 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-isobenzofuran-5-cyano-N-oxide (IV). Specific synthetic routes of the impurities are showed as follows.
Owner:CHINA PHARM UNIV
Application and method for preparing isobenzofuran compound with antibacterial activity in tobaccos by supercritical fluid chromatography
ActiveCN106117171ARaw materials are easy to getSimple extraction methodOrganic chemistrySpecial paperIsobenzofuranKetone
The invention discloses an isobenzofuran compound. A structure of the isobenzofuran compound is as shown in the specification, and the isobenzofuran compound is named 2-hydroxyl-1-isopentenyl-isobenzofuran-5(3H)-ketone. The invention further discloses a preparation method of the isobenzofuran compound and application of the isobenzofuran compound to cigarette antibacterial tipping paper.
Owner:CHINA TOBACCO YUNNAN IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Spiro[isobenzofuran-1,4′-piperidin]-3-ones and 3H-spiroisobenzofuran-1,4′-piperidines Spiro[isobenzofuran-1,4′-piperidin]-3-ones and 3H-spiroisobenzofuran-1,4′-piperidines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/217491e2-bbca-42d9-b411-c2899095f074/US06943199-20050913-C00001.png)
![Spiro[isobenzofuran-1,4′-piperidin]-3-ones and 3H-spiroisobenzofuran-1,4′-piperidines Spiro[isobenzofuran-1,4′-piperidin]-3-ones and 3H-spiroisobenzofuran-1,4′-piperidines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/217491e2-bbca-42d9-b411-c2899095f074/US06943199-20050913-C00002.png)
![Spiro[isobenzofuran-1,4′-piperidin]-3-ones and 3H-spiroisobenzofuran-1,4′-piperidines Spiro[isobenzofuran-1,4′-piperidin]-3-ones and 3H-spiroisobenzofuran-1,4′-piperidines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/217491e2-bbca-42d9-b411-c2899095f074/US06943199-20050913-C00003.png)
![Spiro[isobenzofuran-1,4'-piperidin]-3-ones and 3H-spiroisobenzofuran-1,4'-piperidines Spiro[isobenzofuran-1,4'-piperidin]-3-ones and 3H-spiroisobenzofuran-1,4'-piperidines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f702d0e5-ed80-4a50-905a-7df7b2af92d5/US20060040964A1-20060223-C00001.png)
![Spiro[isobenzofuran-1,4'-piperidin]-3-ones and 3H-spiroisobenzofuran-1,4'-piperidines Spiro[isobenzofuran-1,4'-piperidin]-3-ones and 3H-spiroisobenzofuran-1,4'-piperidines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f702d0e5-ed80-4a50-905a-7df7b2af92d5/US20060040964A1-20060223-C00002.png)
![Spiro[isobenzofuran-1,4'-piperidin]-3-ones and 3H-spiroisobenzofuran-1,4'-piperidines Spiro[isobenzofuran-1,4'-piperidin]-3-ones and 3H-spiroisobenzofuran-1,4'-piperidines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f702d0e5-ed80-4a50-905a-7df7b2af92d5/US20060040964A1-20060223-C00003.png)


![1'-[4-[1-(4-fluorophenyl)-1H-indole-3-yl]-1-butyl]-spiro[isobenzofuran-1(3H),4'-piperidine] hydrohalogenides 1'-[4-[1-(4-fluorophenyl)-1H-indole-3-yl]-1-butyl]-spiro[isobenzofuran-1(3H),4'-piperidine] hydrohalogenides](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/288540d4-e1da-4b38-a92d-6e8bca376dea/US06844352-20050118-C00001.png)

![Process for the Synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isobenzofuran-1,9'-xanthen]-3-one (Rose Bengal) and Related Xanthenes Process for the Synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isobenzofuran-1,9'-xanthen]-3-one (Rose Bengal) and Related Xanthenes](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b6b35220-322c-4177-9cff-03dc2f7cfdc2/US20110071217A1-20110324-D00000.png)
![Process for the Synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isobenzofuran-1,9'-xanthen]-3-one (Rose Bengal) and Related Xanthenes Process for the Synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isobenzofuran-1,9'-xanthen]-3-one (Rose Bengal) and Related Xanthenes](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b6b35220-322c-4177-9cff-03dc2f7cfdc2/US20110071217A1-20110324-D00001.png)
![Process for the Synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isobenzofuran-1,9'-xanthen]-3-one (Rose Bengal) and Related Xanthenes Process for the Synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isobenzofuran-1,9'-xanthen]-3-one (Rose Bengal) and Related Xanthenes](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b6b35220-322c-4177-9cff-03dc2f7cfdc2/US20110071217A1-20110324-D00002.png)



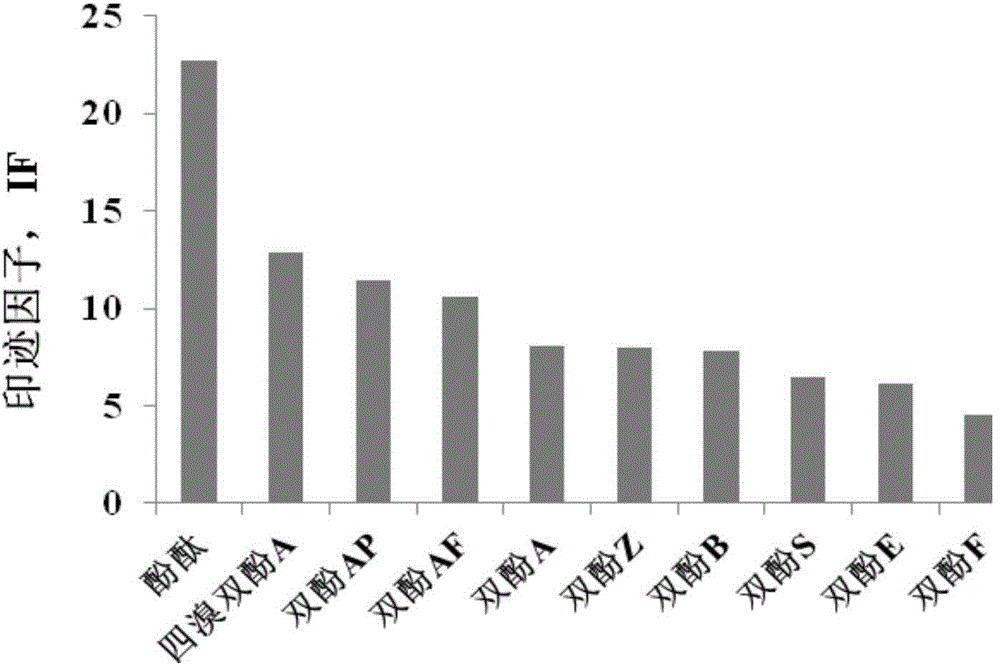





![Thiophene[3,4-f]isobenzofuran-4,8-diketone polymer as well as preparation method and application thereof Thiophene[3,4-f]isobenzofuran-4,8-diketone polymer as well as preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9e9dcf5a-82dd-48f6-8a9d-af1a4275f154/RE-1902121101021.png)
![Thiophene[3,4-f]isobenzofuran-4,8-diketone polymer as well as preparation method and application thereof Thiophene[3,4-f]isobenzofuran-4,8-diketone polymer as well as preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9e9dcf5a-82dd-48f6-8a9d-af1a4275f154/RE-1902121101022.png)
![Thiophene[3,4-f]isobenzofuran-4,8-diketone polymer as well as preparation method and application thereof Thiophene[3,4-f]isobenzofuran-4,8-diketone polymer as well as preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9e9dcf5a-82dd-48f6-8a9d-af1a4275f154/RE-1902121101023.png)