Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
127 results about "Citalopram" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
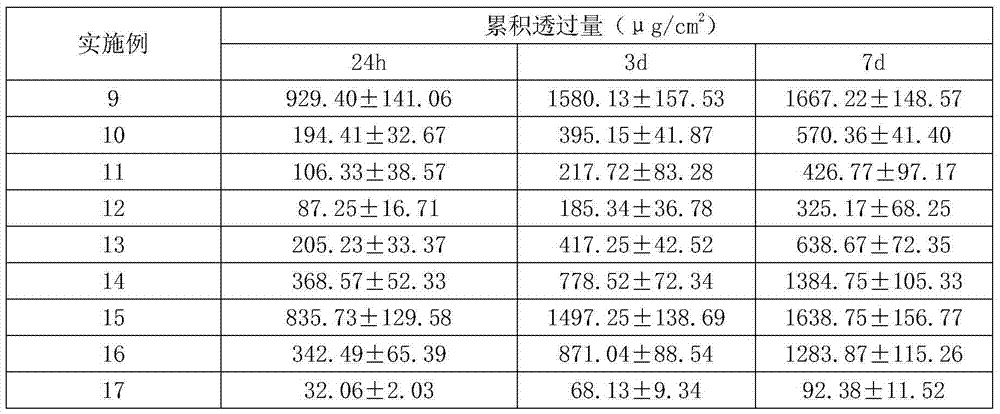
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Citalopram is used to treat depression.
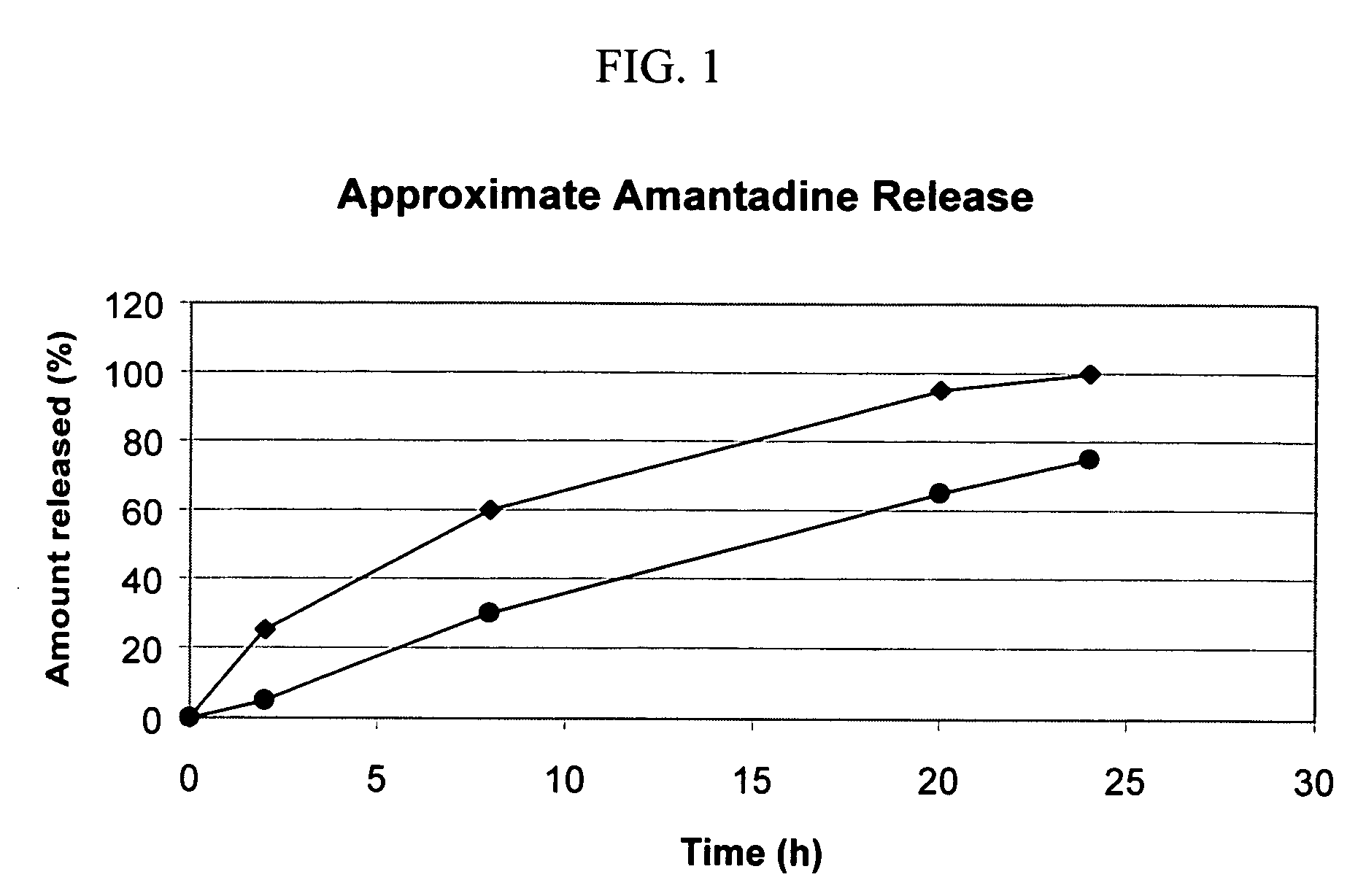
Formulation for sustained delivery
Disclosed is an extended or controlled release dosage form of citalopram or its related forms and other newer antidepressants for oral administration to treat chronic patients suffering from depression and to minimize the side effects associated with the current drug treatment.
Owner:CHALLAPALLI PRASAD V N +2
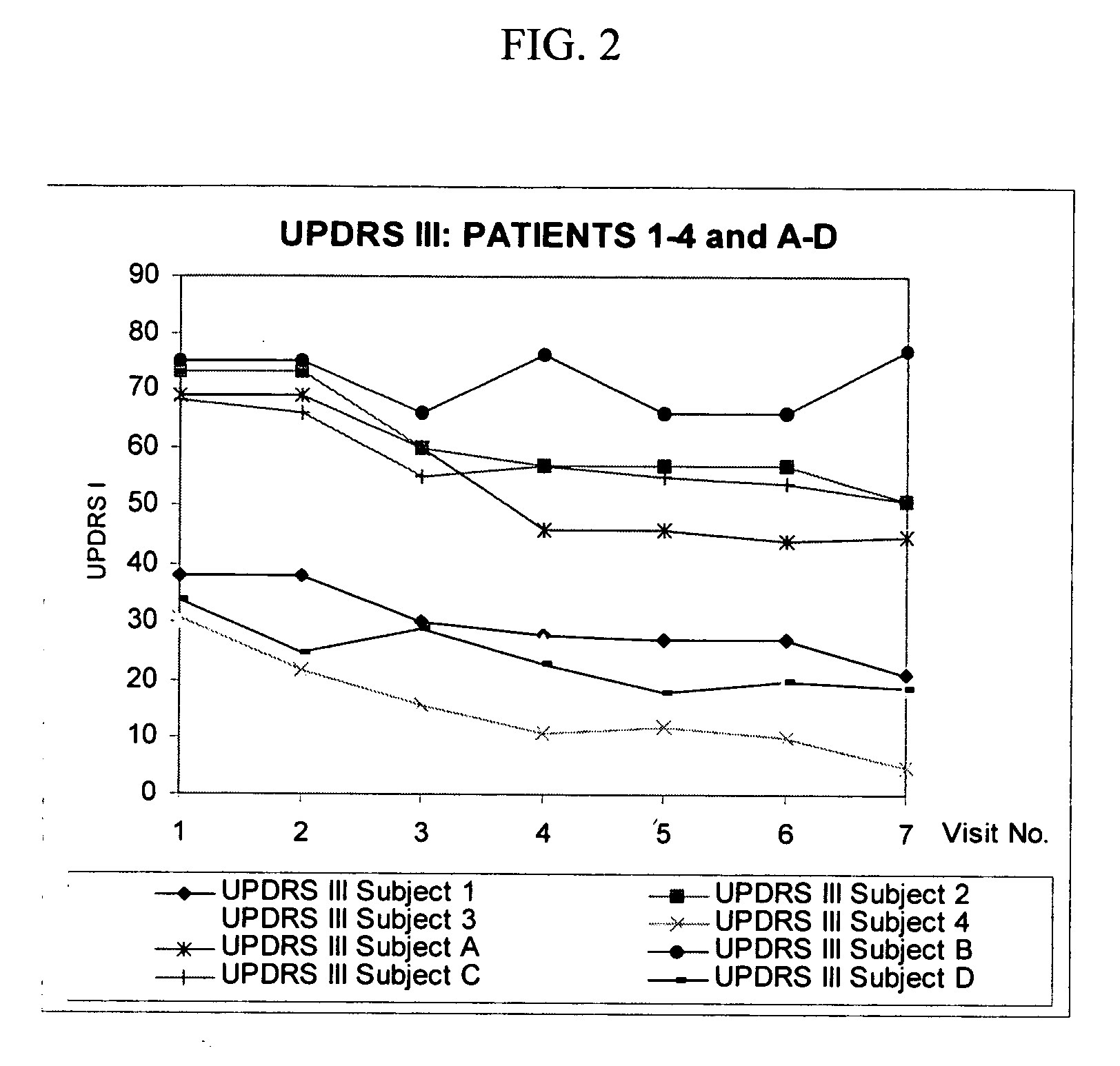
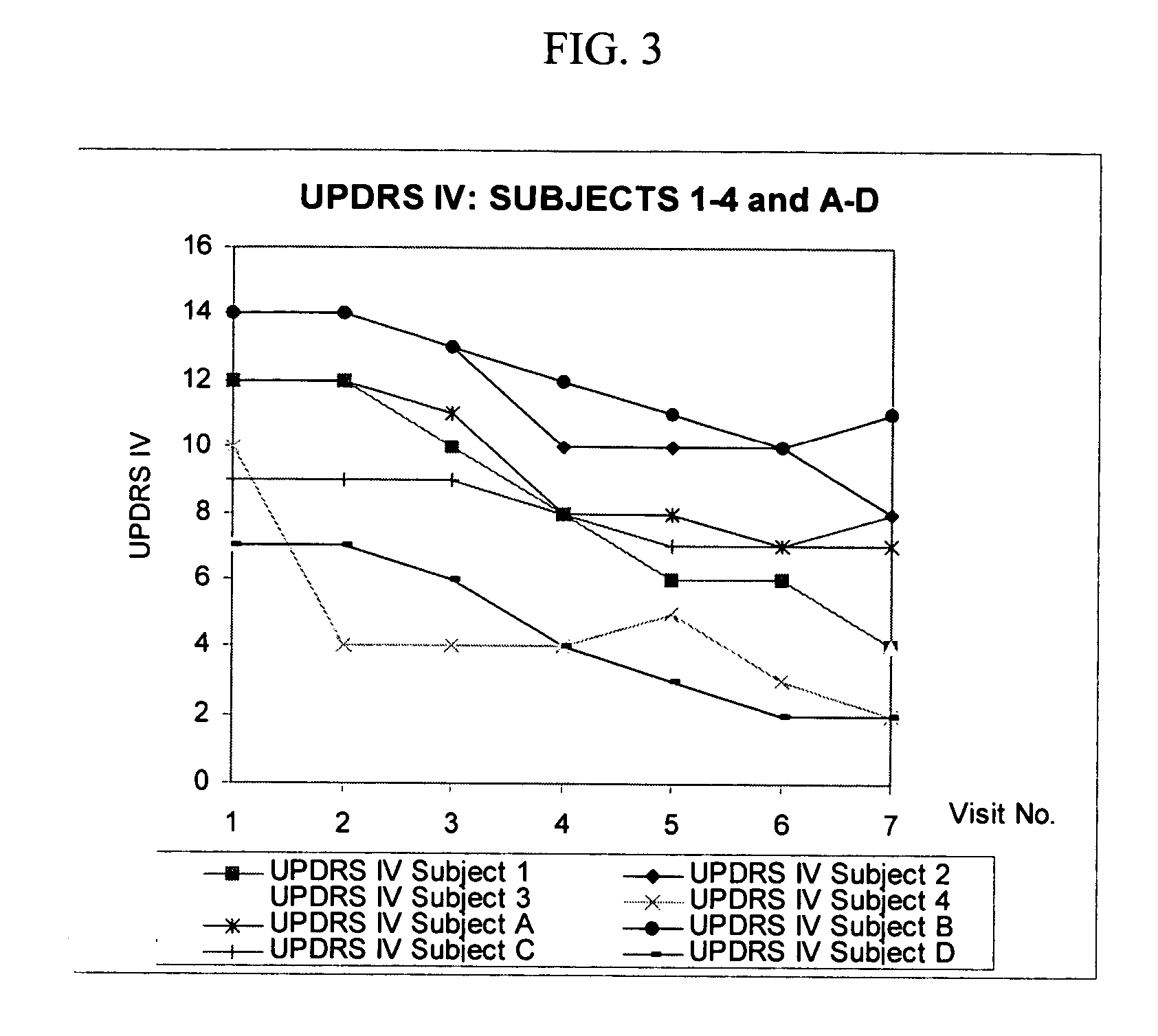
Combination treatment for impaired motor function in parkinson's disease
InactiveUS20060063810A1Shorten the timeIncreased “ on ” timeBiocideNervous disorderNR1 NMDA receptorNMDA receptor
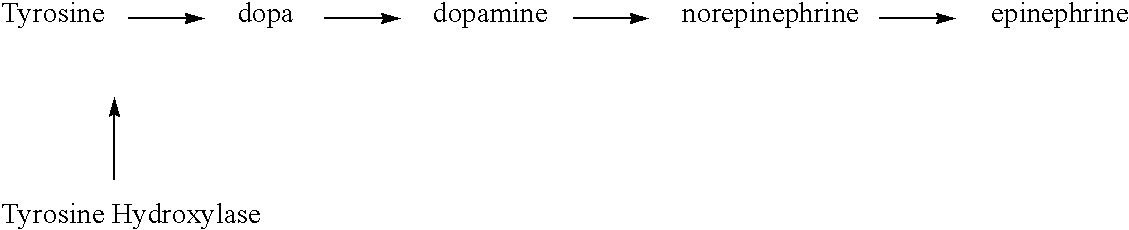
The invention provides a method, and dosage form therefor, of treating impaired motor function associated with Parkinson's disease, anti-Parkinson's drug treatment, e.g. L-Dopa therapy, and / or dementia associated with Parkinson's disease. The invention includes the combined administration of an NMDA receptor antagonist and an antidepressant, e.g., the combination of amantadine and citalopram or venlafaxine, or an NMDA receptor antagonist and an anxiolytic agent, e.g., amantadine and buspirone or trazodone, for the amelioration of undesired tremors, akinesia, dyskinesia, or bradykinesia associated with one or more different disorders or diseases. The drugs can be included in a single dosage form. One embodiment includes a combination dosage form containing each drug in controlled release forms. Another embodiment includes a combination dosage form providing a controlled release of an NMDA receptor antagonist and a rapid release of a neuroactive agent after administration to a subject.
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO
Comprehensive pharmacologic therapy for treatment of obesity including cysteine
InactiveUS20050065190A1Simple and inexpensive designWithout fear of injury to personsBiocidePeptide/protein ingredientsDiethylpropionVitamin C
The comprehensive pharmacologic therapy for treatment of obesity including Cysteine is a procedure which involves the administration of a desired therapeutic range of Diethylpropion and / or Phentermine in combination with a SSRI medication and nutritional supplementation for brief and long durations which may be 12 months or more. The preferred procedure involves the administration of drugs in combination which are identified as: Citalopram (Celexa) and Phentermine; Citalopram (Celexa) and Diethylpropion; Citalopram (Celexa), Phentermine, and Diethylpropion. In addition nutritional supplementation such as a multivitamin, 5-Hydroxytryptophan, Cysteine, vitamin B6, vitamin C, Tyrosine, Calcium, and Lysine may be used to enhance the performance of the weight loss treatment program.
Owner:FEDERAL LAW ENFORCEMENT DEV SERVICES
Combination of an NMDA receptor antagonist and a selective serotonin reuptake inhibitor for the treatment of depression and other mood disorders
The present invention provides a method for the treatment of depression, including treatment-resistant depression, and other mood disorders using a combination of an NMDA receptor antagonist and a SSRI that is citalopram or escitalopram. It has unexpectedly been shown that the combination has a synergistic and potentiated effect of either compound as monotherapy, resulting in an enhanced therapeutic effect at lower doses.
Owner:FOREST LAB HLDG LTD +1
Skin irritation suppressant and transdermal preparation
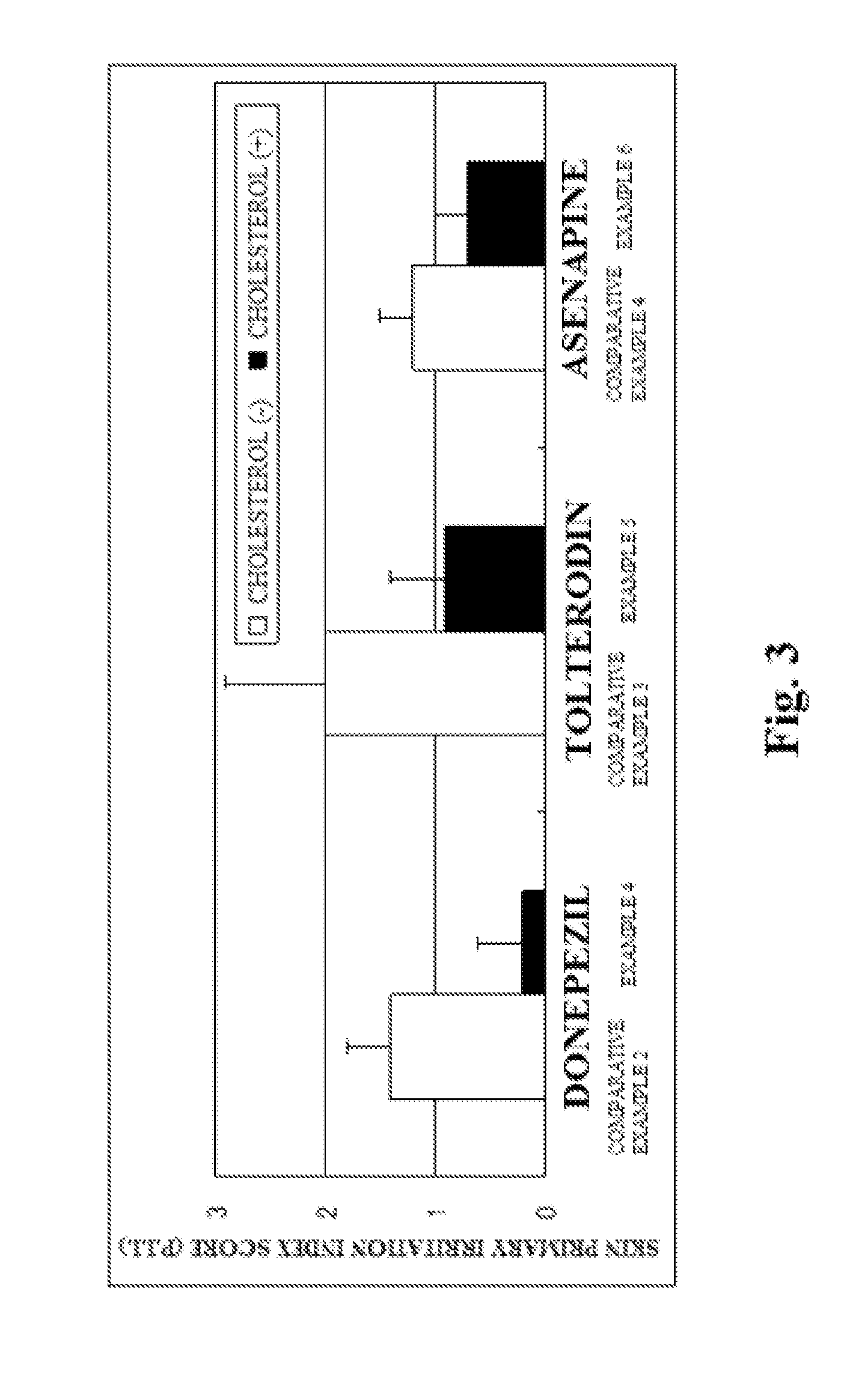
InactiveUS20130053357A1Sufficient reduction effectReduce skin effectOrganic active ingredientsBiocideCholesterol derivativeCitalopram
Provided is a skin irritation suppressant for transdermal preparations, having a sufficient reduction effect of skin irritation due to a drug. Also provided is a transdermal preparation comprising the skin irritation suppressant. One embodiment of the invention is a skin irritation suppressant for suppressing the skin irritation due to a drug and a pharmaceutical ingredient to be used in a transdermal preparation other than the drug, the skin irritation suppressant comprising a sterol compound selected from the group consisting of cholesterol, cholesterol derivatives and cholesterol analogs, and the drug is one or more basic drugs selected from the group consisting of tolterodine, asenapine, bisoprolol, risperidone, nicotine and citalopram, and their pharmaceutically acceptable salts.
Owner:HISAMITSU PHARM CO INC
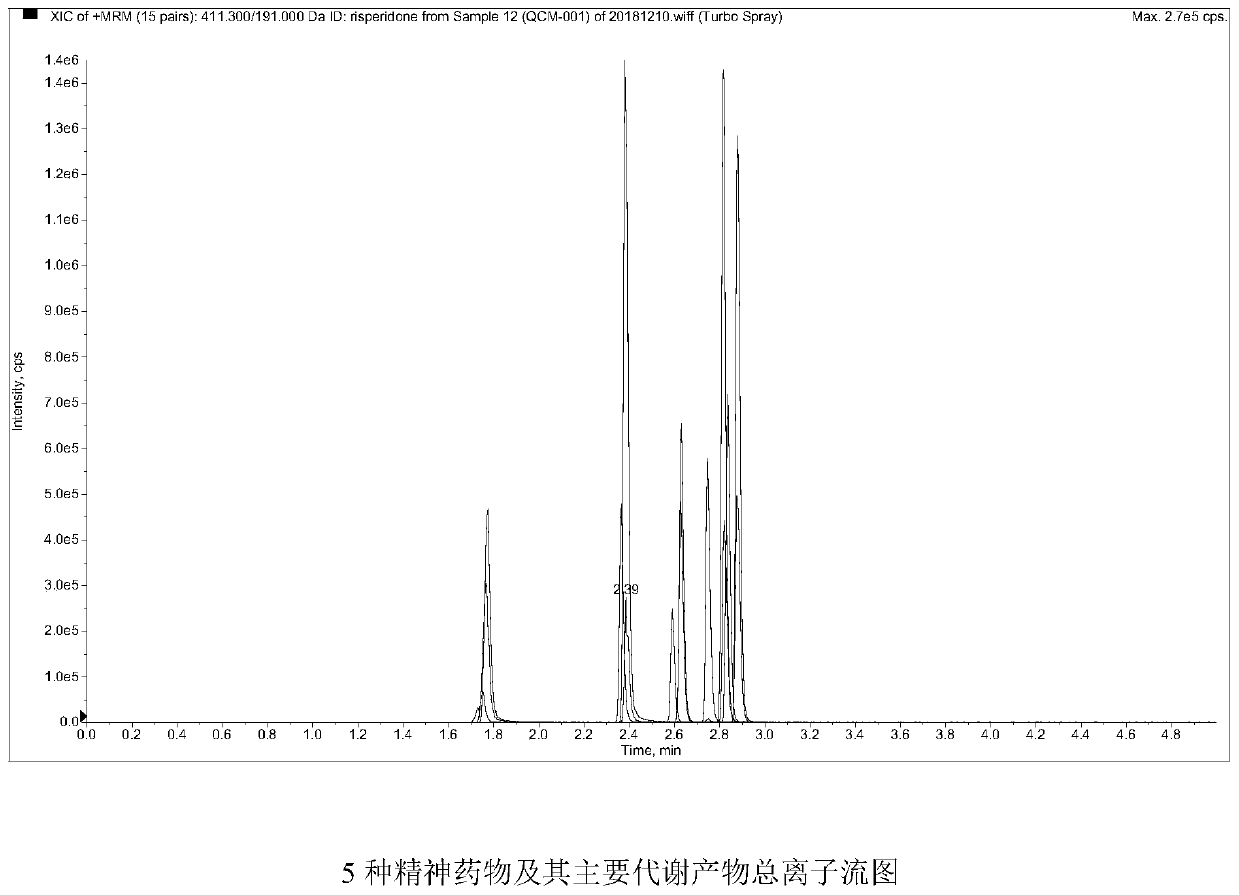
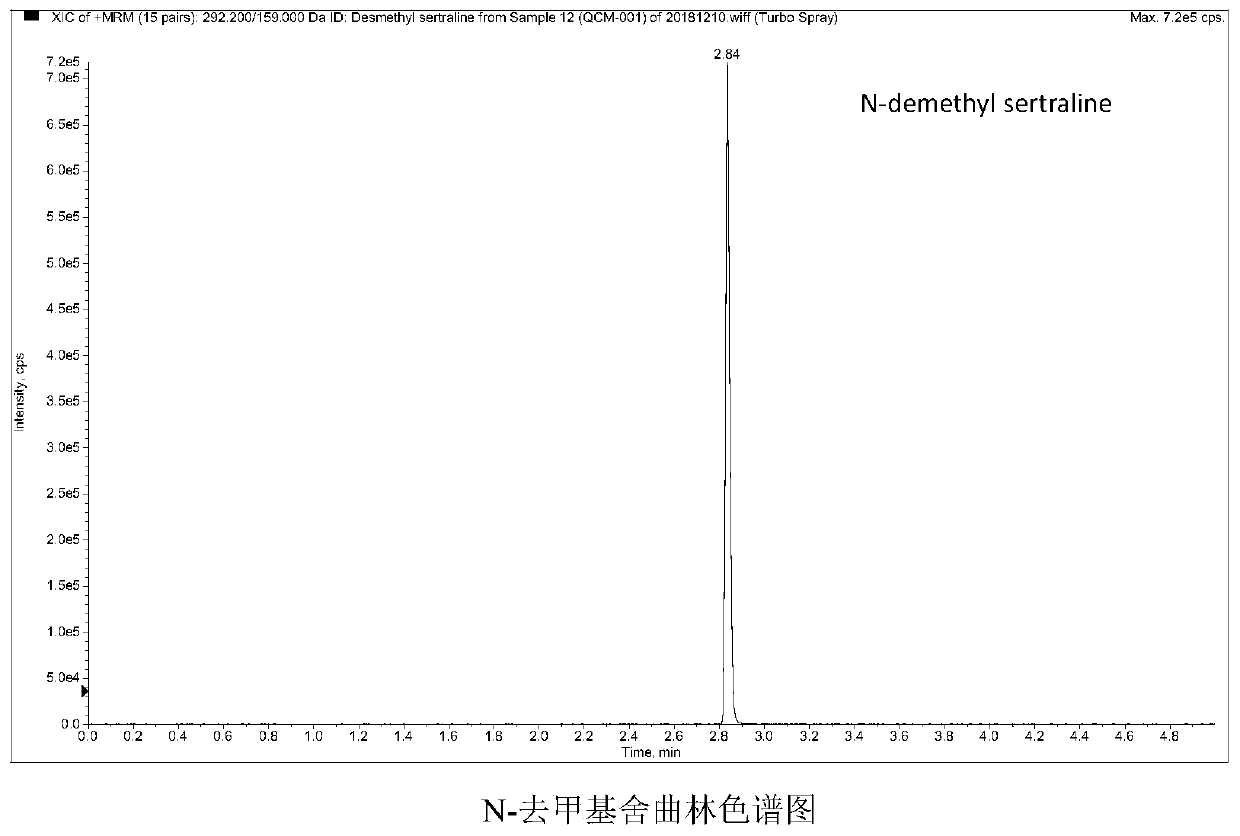
Method and kit for detecting five psychotropic drugs and main metabolites thereof in blood
The invention belongs to the field of drug detection, and particularly relates to a method and a kit for detecting five psychotropic drugs and main metabolites thereof in blood. The five psychotropicdrugs and the main metabolites thereof comprise: olanzapine and demethyl olanzapine, risperidone and 9-hydroxy risperidone, aripiprazole and dehydrogenated aripiprazole, Escitalopram and demethyl citalopram, sertraline and N-demethyl sertraline. Accoridng to the method provided by the invention, a pair of quantitative ion pairs is respectively selected for each detection substance, a relative retention time thereof is used as a qualitative basis, and a standard curve is made by using a standard product for quantification; furthermore, the accuracy and effectiveness of the method are evaluatedfrom quality control of three low, middle and high levels, thereby avoiding distortion of the detection result; and meanwhile, an internal standard working solution is applied to correction, so that matrix effects can be avoided, and accurate quantification is realized. The method provided by the invention has the advantages of simple and rapid operation, high flux and low cost, and can be appliedto the therapeutic drug monitoring of the psychotropic drugs in the clinical work of the psychiatry department.
Owner:BEIJING HUILONGGUAN HOSPITAL +1
Kit for detecting anti-depressant drugs in serum and plasma by liquid chromatography tandem mass spectrometry and application thereof
InactiveCN109085264APharmacologically activeInterpret blood levelsComponent separationSertralineTandem mass spectrometry
The invention provides a kit for detecting anti-depressant drugs in serum and plasma by liquid chromatography tandem mass spectrometry. The kit comprises drug standard substances, drug internal standardization compounds, drug extraction compositions, negative plasma and a diluent. The drug standard substances comprise amfebutamone, oxybupropion, citalopram, Escitalopram, venlafaxine, O-desmethylvenlafaxine, duloxetine, fluoxetine, norfloxetine, fluvoxamine, mirtazapine, paroxetine, sertraline and trazodone. The drug internal standardization compounds comprise amfebutamone-d9, oxybupropion-d6,citalopram-d6, venlafaxine-d6, O-desmethylvenlafaxine-d6, duloxetine-d3, fluoxetine-d6, norfloxetine-d6, fluvoxamine-d4, mirtazapine-d3, paroxetine-d6, sertraline-d3 and trazodone-d6. The drug extraction compositions comprise, by volume, 60% of methanol solution, 20% of acetonitrile solution, 10% of isopropyl alcohol solution and 10% of purified water. The diluent comprises 50 % of methanol waterfluid. The kit can be used for simultaneous detection of the anti-depressant drugs and active metabolites, the detection time is short, and flux is high.
Owner:HANGZHOU BAICHEN MEDICAL INSTR CO LTD +1
Crystalline composition containing escitalopram
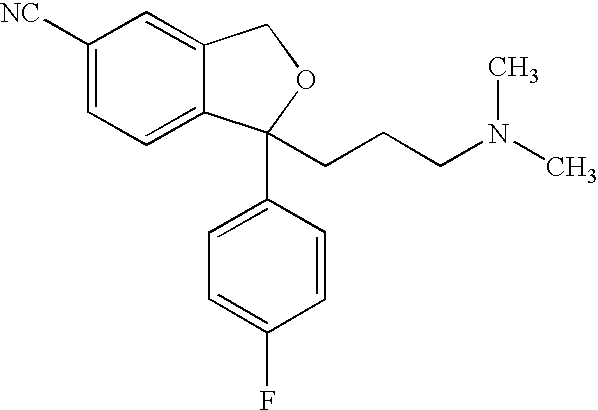
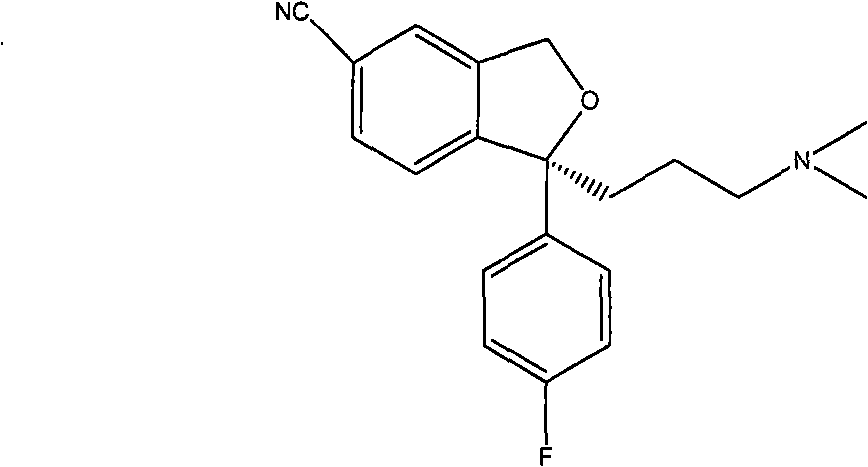
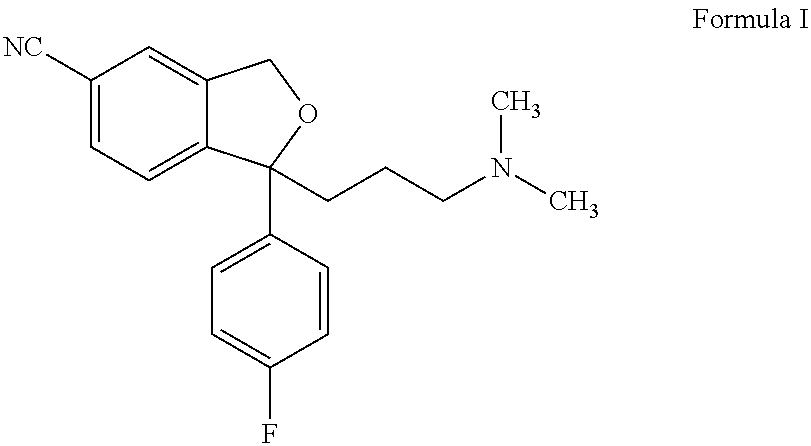
The present invention discloses crystalline particles of escitalopram oxalate which either have a broad particle size distribution or comprise at least 0.01% (w / w) of Z-4-(4-dimethylamino-1-(4-fluorophenyl)-but-1-enyl)-3-hydroxymethyl-benzonitrile, said particles being suitable for use in direct compression. Furthermore, the invention discloses a novel pharmaceutical unit dosage form containing such crystalline particles of escitalopram oxalate as well as methods for manufacture of such crystalline particles of escitalopram oxalate Finally, the invention provides a method for reduction of the amount of hydroxyl containing impurities in a solution of citalopram or escitalopram.
Owner:H LUNDBECK AS
Preparation method of citalopram intermediate
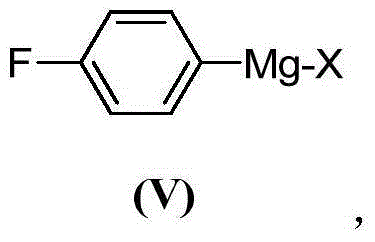
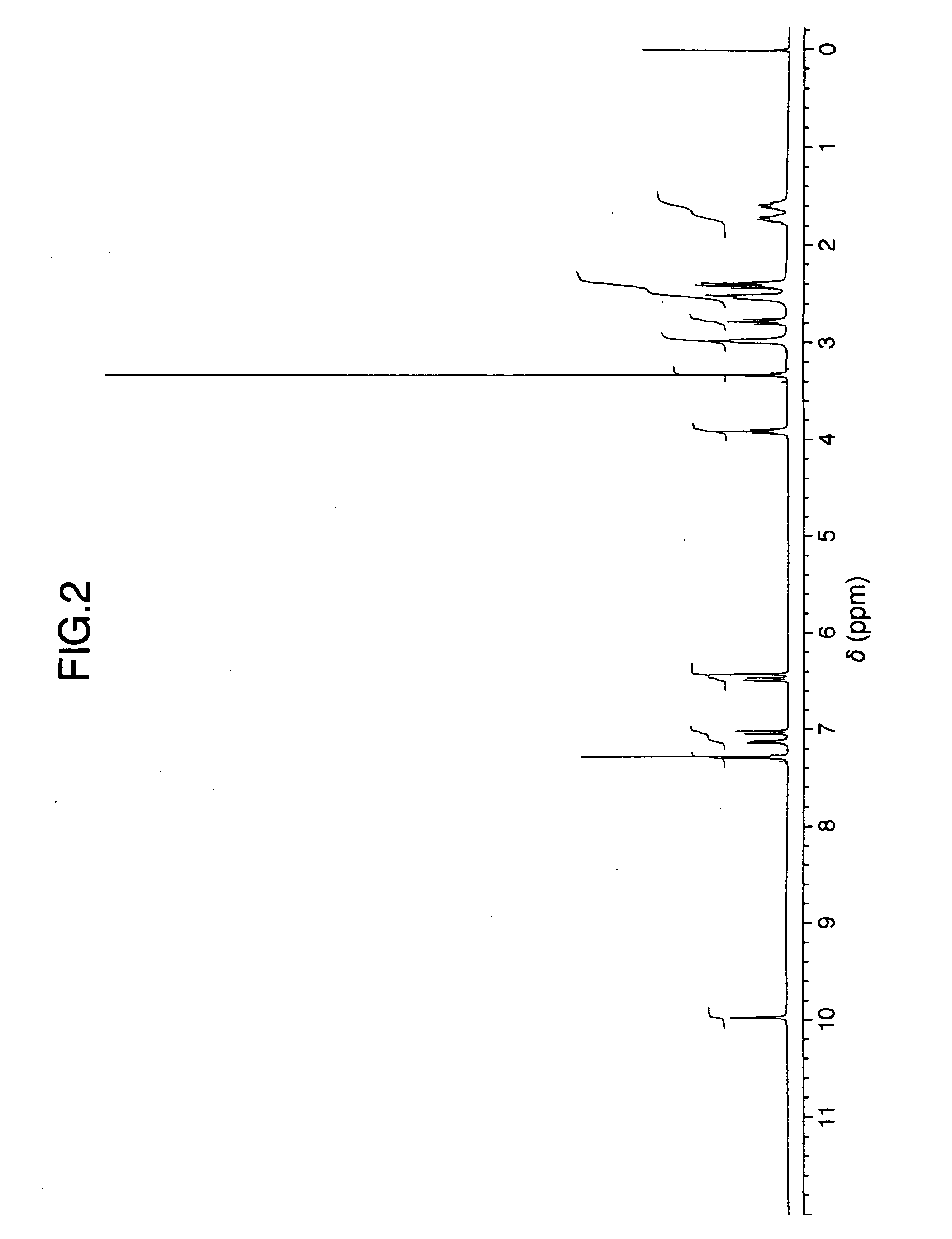
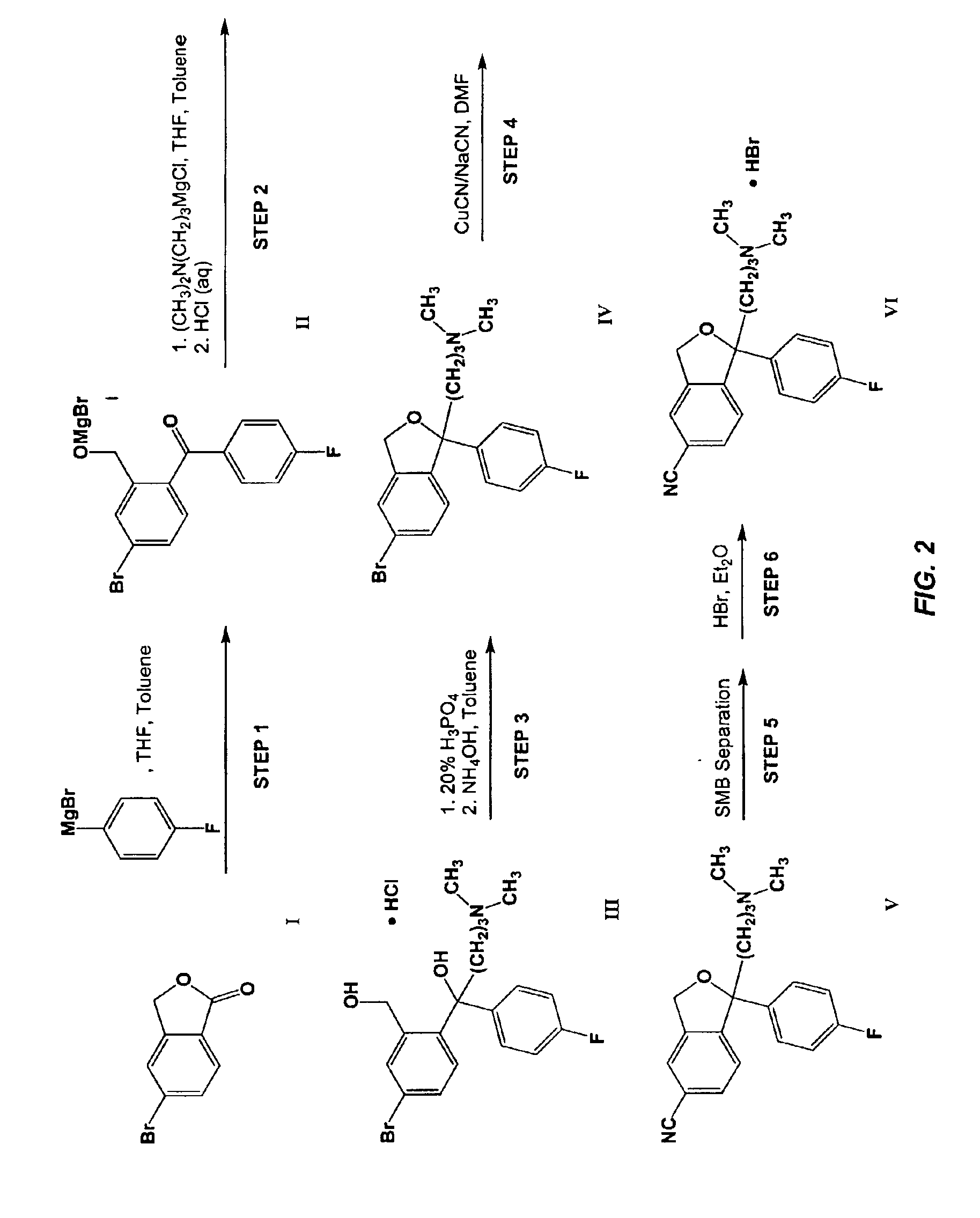
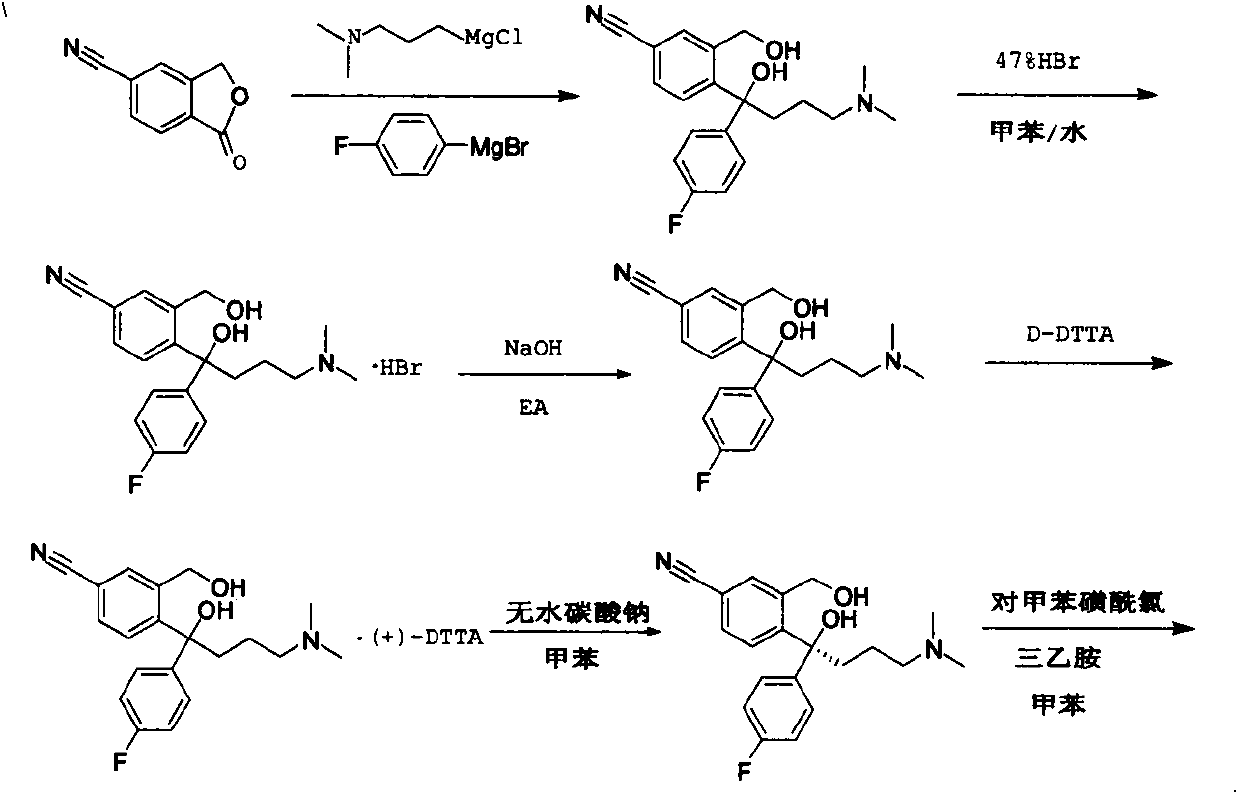
InactiveCN105294496ACarboxylic acid nitrile preparationOrganic compound preparationGrignard reagentNitrogen
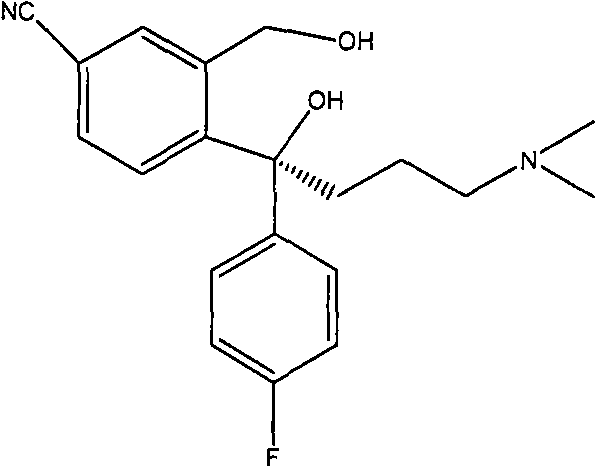
The invention provides a preparation method of a citalopram intermediate, and belongs to the technical field of pharmaceuticals. In the method, 2-methyltetrahydrofuran is taken as a reaction solvent. Under the protection of nitrogen, a 4-fluorophenylmagnesium bromide solution Grignard reagent, 5-cyanophthalein and a N,N-dimethylpropyl magnesium chloride Grignard reagent are taken as raw materials. A reaction is carried out to synthesize 4-(4-(dimethylamino)-1-(4-fluorophenyl)-1-hydroxybutyl)-3-(hydroxymethyl)benzonitrilehydrobromide. The method is characterized by high yield and high purity, and is suitable for industrial production.
Owner:SUN YAT SEN UNIV +1
Comprehensive pharmacologic therapy for treatment of a dysfunction
InactiveUS20050233008A1Promote resultsHigh expectation of weight lossBiocidePeptide/protein ingredientsDiethylpropionVitamin C
The comprehensive pharmacologic therapy for treatment of obesity is a procedure which involves the administration of a desired therapeutic range of Diethylpropion and / or Phentermine in combination with a SSRI medication and nutritional supplementation for brief and long durations which may be 12 months or more. The preferred procedure involves the administration of drugs in combination which are identified as: Citalopram (Celexa) and Phentermine; Citalopram (Celexa) and Diethylpropion; Citalopram (Celexa), Phentermine, and Diethylpropion. In addition nutritional supplementation such as a multivitamin, 5-Hydroxytryptophan, vitamin B6, vitamin C, Tyrosine, Calcium, and Lysine may be used to enhance the performance of the weight loss treatment program.
Owner:FEDERAL LAW ENFORCEMENT DEV SERVICES
Comprehensive pharmacologic therapy for treatment of obesity including cysteine
InactiveUS7268161B2Simple and inexpensive designEffective therapyBiocidePeptide/protein ingredientsDiethylpropionVitamin C
The comprehensive pharmacologic therapy for treatment of obesity including Cysteine is a procedure which involves the administration of a desired therapeutic range of Diethylpropion and / or Phentermine in combination with a SSRI medication and nutritional supplementation for brief and long durations which may be 12 months or more. The preferred procedure involves the administration of drugs in combination which are identified as: Citalopram (Celexa) and Phentermine; Citalopram (Celexa) and Diethylpropion; Citalopram (Celexa), Phentermine, and Diethylpropion. In addition nutritional supplementation such as a multivitamin, 5-Hydroxytryptophan, Cysteine, vitamin B6, vitamin C, Tyrosine, Calcium, and Lysine may be used to enhance the performance of the weight loss treatment program.
Owner:FEDERAL LAW ENFORCEMENT DEV SERVICES
Crystalline composition containing escitalopram
The present invention discloses crystalline particles of escitalopram oxalate which either have a broad particle size distribution or comprise at least 0.01% (w / w) of Z-4-(4-dimethylamino-1-(4-fluorophenyl)-but-1-enyl)-3-hydroxymethyl-benzonitrile, said particles being suitable for use in direct compression. Furthermore, the invention discloses a novel pharmaceutical unit dosage form containing such crystalline particles of escitalopram oxalate as well as methods for manufacture of such crystalline particles of escitalopram oxalate Finally, the invention provides a method for reduction of the amount of hydroxyl containing impurities in a solution of citalopram or escitalopram.
Owner:H LUNDBECK AS
Method for preparing (S)-citalopram intermediate S-type glycol
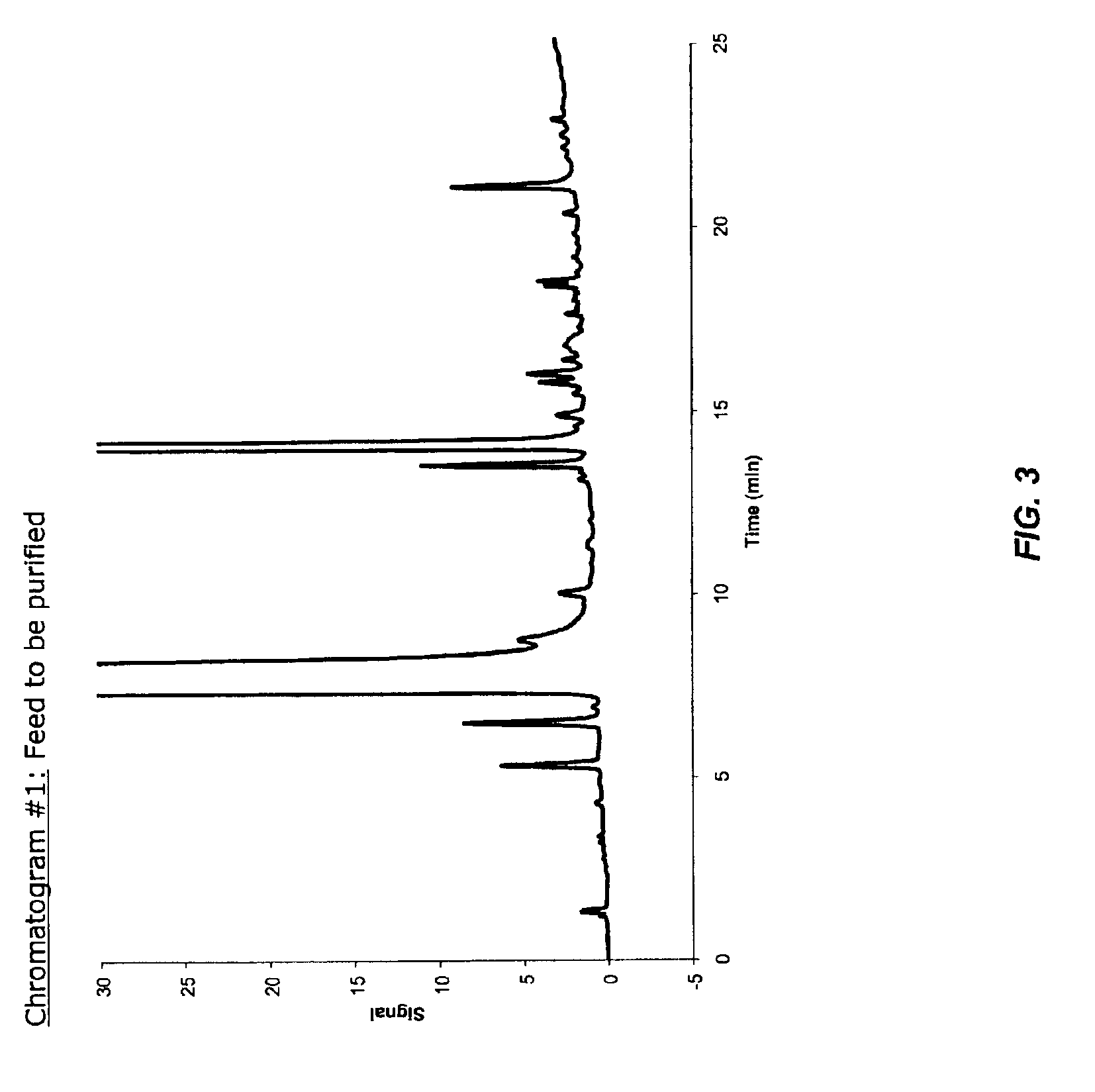
ActiveCN101265215ALow costEasy to operateOptically-active compound separationCarboxylic acid nitrile purification/separationSolventCitalopram
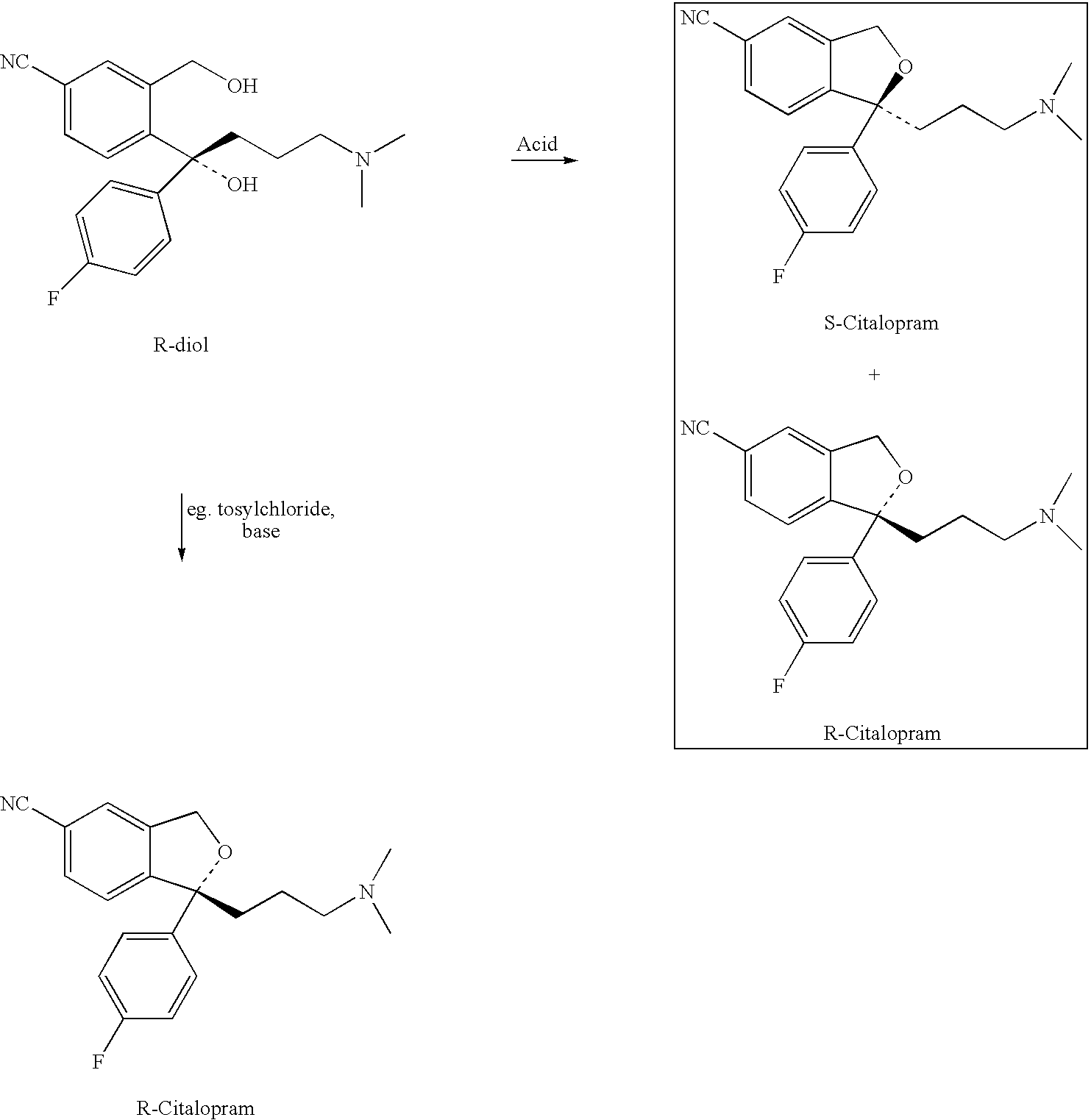
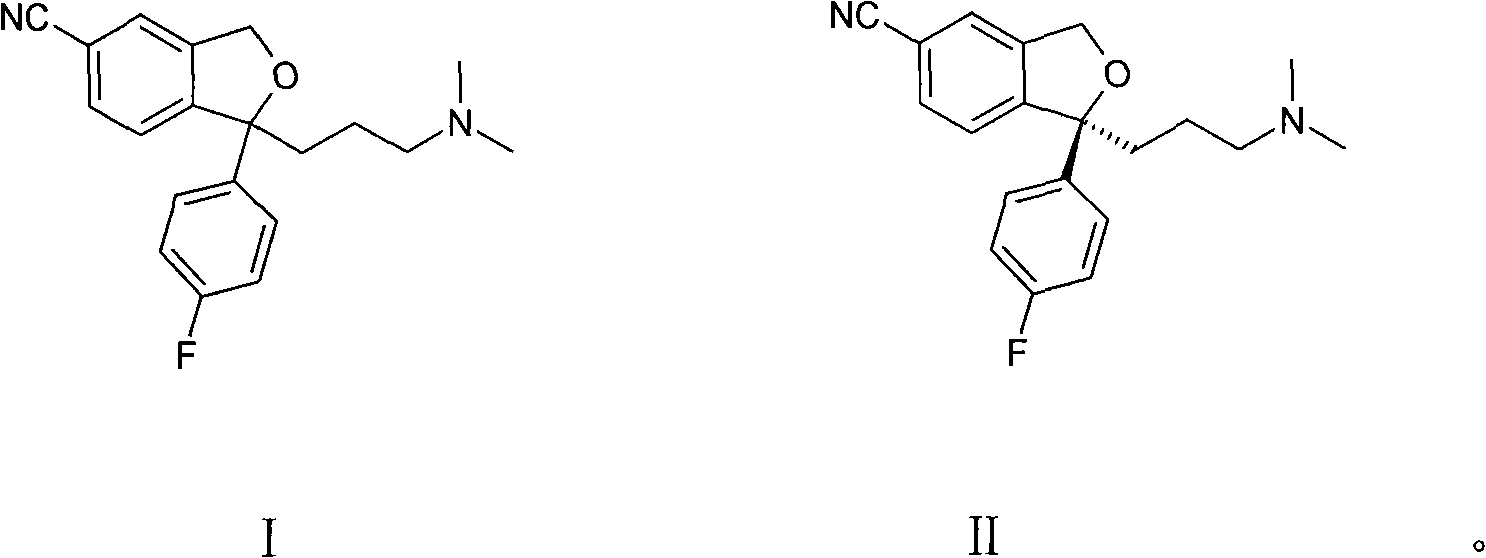
The invention relates to a method of preparing S-type diol that is a dextral citalopram intermediate product. Aiming at the disadvantages of high cost, low yield rate, fussy operation, high difficulty, and bad stability and repeatability in the existing method of preparing dextral citalopram that has single optical isomers, the invention provides a separation method of the S-type diol that is the dextral citalopram intermediate product which has low cost, convenient operation, and good stability and repeatability. The method is completed by the following way: racemic glycols (IV) are separated by adopting a separation method; resolving agent used for separation is organic acid that has the single optical isomers; resolving solvent used for separation is mixed solvent composed of halogenated hydrocarbon and absolute ethyl alcohol, or the mixed solvent composed of acetone and the absolute ethyl alcohol, or the acetone and isopropanol; when in separation, the racemic glycols (IV) are put in the mixed solution composed of the resolving agent and the resolving solvent, the salt of the S-type diol is crystallized and precipitated, and the S-type diol (I) which has single optical configurations is obtained through dissociation.
Owner:ZHEJIANG AOTUOKANG PHARMA GROUP
Preparation of Escitalopram, Its Salts and Intermediates
The present patent application relates to an improved process for the preparation of escitalopram, its salts and intermediates. It also relates to a novel crystalline form S of citalopram diol intermediate, process for preparation and its use in the preparation of citalopram, escitalopram and their salts.
Owner:SHODHANA LAB LTD
Carbostyril derivatives and serotonin reuptake inhibitors for treatment of mood disorders
The pharmaceutical composition of the present invention comprises (1) a carbostyril derivative and (2) a serotonin reuptake inhibitor in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof, which is a dopamine-serotonin system stabilizer. The serotonin reuptake inhibitor may be fluoxetine, duloxetine, venlafaxine, milnacipran, citalopram, fluvoxamine, paroxetine, sertraline or escitalopram. The pharmaceutical composition of the present invention is useful for treating patients with mood disorders, particularly depression or major depressive disorder.
Owner:OTSUKA PHARM CO LTD
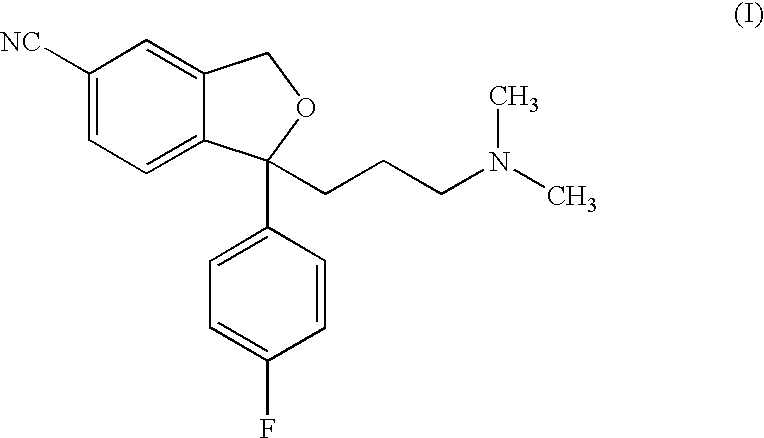
Process for the preparation of Citalopram intermediate
InactiveUS6967259B2Easy to prepareHigh yieldOrganic active ingredientsNervous disorderCitalopramStereochemistry
The present invention provides, inter alia, a novel process for the preparation of Citalopram, a known antidepressant.
Owner:PHARMACHEM TECH LTD
Process for the preparation of racemic citalopram and/or S-or R-citalopram by separation of a mixture of R-and S-citalopram
The invention relates to a process for the preparation of racemic citalopram free base or an acid addition salt thereof and / or R- or S-citalopram as the free base or an acid addition salt thereof by separation of a mixture of R- and S-citalopram with more than 50% of one of the enantiomers into a fraction consisting of racemic citalopram and / or a fraction of S-citalopram or R-citalopram characterized in that i) citalopram is precipitated from a solvent as the free base or as an acid addition salt thereof; ii) the precipitate formed is separated from the mother liquor; iia) if the precipitate is crystalline it is optionally recrystallised one or more times to form racemic citalopram, and then optionally converted into an acid addition salt thereof; iib) if the precipitate is not crystalline, steps i) and ii) are optionally repeated until a crystalline precipitate is obtained and the crystalline precipitate is recrystallised one or more times to form racemic citalopram, and then optionally converted into an acid addition salt thereof; iii) the mother liquor is optionally subjected to further purification and S-citalopram or R-citalopram is isolated from the mother liquor and optionally converted into an addition salt thereof.
Owner:H LUNDBECK AS
Method for preparing citalopram and S-citalopram
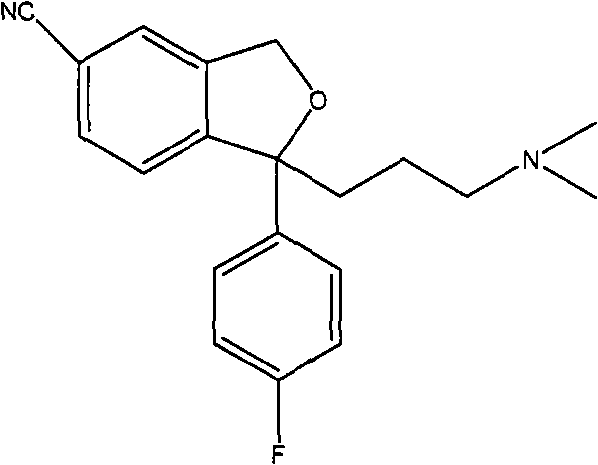
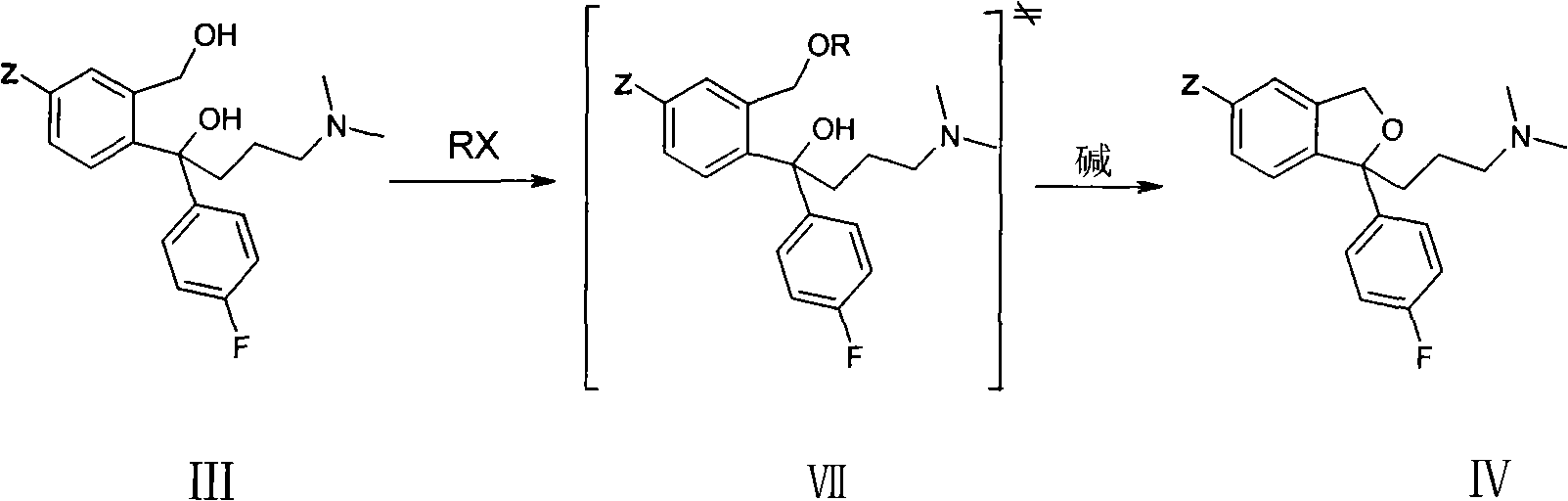
The invention provides a method for preparing citalopram and S-citalopram. In the method, 1-[2-(hydroxymethyl)-5-substituting group-]phenyl-4-(dimethylamino)-1-(4-fluorophenyl)-1- butyl alcohol undergoes a cyclization reaction with halogenated acylate in water and an organic solvent immiscible with water under the alkali condition to form a citalopram product. The method has high yield and high purity and is suitable for industrialized production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Treatment of neurotic disorders
Use of escitalopram (the S-(+)-enantiomer of citalopram) or a pharmaceutically acceptable salt thereof for the preparation of a medicament useful in the treatment of neurotic disorders is provided, including anxiety states, in particular generalised anxiety disorder and social anxiety disorder, post traumatic stress disorder, obsessive compulsive disorder and panic attacks.
Owner:H LUNDBECK AS
Skin irritation suppressant and transdermal preparation
InactiveCN102858372ALess irritatingOrganic active ingredientsNervous disorderCholesterol derivativePharmaceutical Adjuvants
Owner:HISAMITSU PHARM CO INC
Preparation methods for impurities of escitalopram oxalate
The invention relates to novel synthetic methods for three impurities of escitalopram oxalate. The methods have great significance for synthesis of the escitalopram oxalate with high purity. The invention mainly study syntheses of a citalopram amide impurity 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-isobenzofuran-5-formamide (II), a citalopram lactone impurity 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-3-oxo-1,3-dihydro-isobenzofuran-5-carbonitrile (III) and a citalopram-N-oxide impurity 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-isobenzofuran-5-cyano-N-oxide (IV). Specific synthetic routes of the impurities are showed as follows.
Owner:CHINA PHARM UNIV
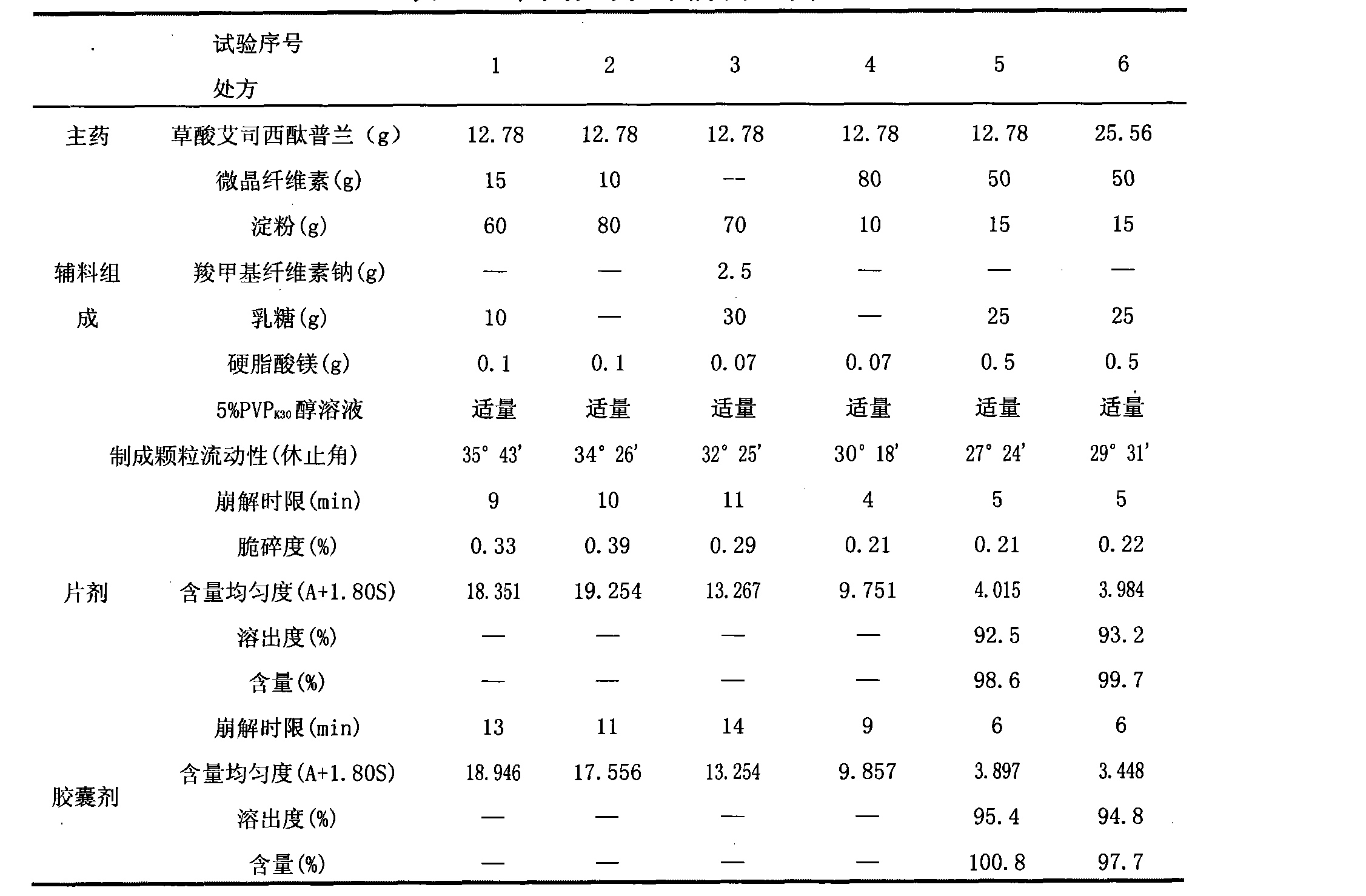
Oral solid preparation of escitalopram oxalate and preparation method thereof
The invention discloses an oxalic acid esmolol citalopram oral solid preparation and a preparation method. The oral solid preparation comprises raw materials of: 2-40 parts by weight of oxalic acid esmolol citalopram, 5-60 parts by weight of starch, 5-200 parts by weight of microcrystalline cellulose, 5-60 parts by weight of lactose, 0.05-5 parts by weight of binding agent and 0.05-3 parts by weight of lubricant; the binding agent is one or a plurality of mixed aqueous solutions or alcoholic solutions from the group consisting of polyvinyl pyrrolidone, hydroxyethyl methylcellulose, methylcellulose, hydroxypropyl cellulose and ethyl cellulose, the concentration of the binding agent is 0.1-10.0%; and the lubricant is stearic acid, magnesium stearate, calcium stearate, talcum or superfine silica gel powder.
Owner:SICHUAN KELUN PHARMA CO LTD
Process for prepn. of racemic citalopram and/or S-or R-citalopram by separation of mixture of R-and S-citalopram
Disclosed is a process for the preparation of racemic citalopram free base or an acid addition salt thereof and / or R- or S-citalopram as the free base or an acid addition salt thereof by separation of a mixture of R- and S- citalopram with more than 50% of one of the enantiomers into a fraction consisting of racemic citalopram and a fraction of S-citalopram or R-citalopram characterised in that: i) citalopram is precipitated from a solvent as the free base or as an acid addition salt thereof; ii) the precipitate formed is separated from the mother liquor; iia) if the precipitate is crystalline it is optionally recrystallised one or more times to form racemic citalopram, and then optionally converted into an acid addition salt thereof; or iib) if the precipitate is not crystalline, steps i) and ii) are optionally repeated until a crystalline precipitate is obtained and the crystalline precipitate is optionally recrystallised one or more times to form racemic citalopram, and then optionally converted to an acid addition salt thereof; and iii) the mother liquor is optionally subjected to further purification and S-citalopram or R-citalopram is isolated from the mother liquor and optionally converted to an acid addition salt thereof.
Owner:H LUNDBECK AS
Method for the preparation of escitalopram
A novel method is provided for the manufacture of escitalopram. The method comprises chromatographic separation of the enantiomers of citalopram or an intermediate in the production of citalopram using a chiral stationary phase such as Chiralpak™ or Chiralcel™ OD. Novel chiral intermediates for the synthesis of Escitalopram made by said method are also provided.
Owner:H LUNDBECK AS
Process for preparation of pure citalopram
A method for preparing citalopram of general formula (I), wherein the compound of general formula (II), wherein Z is iodo, bromo, chloro or CF3-(CF2)n-SO2-O-, wherein n is 0, 1, 2, 3, 4, 5, 6, 7 or 8, a cyanide exchange reaction occurs. In the reaction, the group Z realizes the exchange with cyanide by reacting with the cyanide source; the crude citalopram produced The product is optionally subjected to some preliminary purification, and the crude citalopram base is then subjected to a liquid membrane distillation process; the citalopram product obtained is then optionally further purified, then worked up and converted to the base or a pharmaceutically acceptable salt thereof. seperate.
Owner:H LUNDBECK AS
Crystals of pharmaceutically acceptable salts of citalopram, methods of crystallization, and pharmaceutical compositions comprising them
InactiveUS20030232881A1Granulation avoidedReduce stepsBiocideOrganic chemistryCitalopramMedicinal chemistry
The invention is directed to methods of crystallizing pharmaceutically acceptable salts of citalopram, the resulting crystals, and pharmaceutical compositions comprising the crystals.
Owner:H LUNDBECK AS
Chemo-enzymatic process for the preparation of escitalopram
Owner:ADORKEN TECH
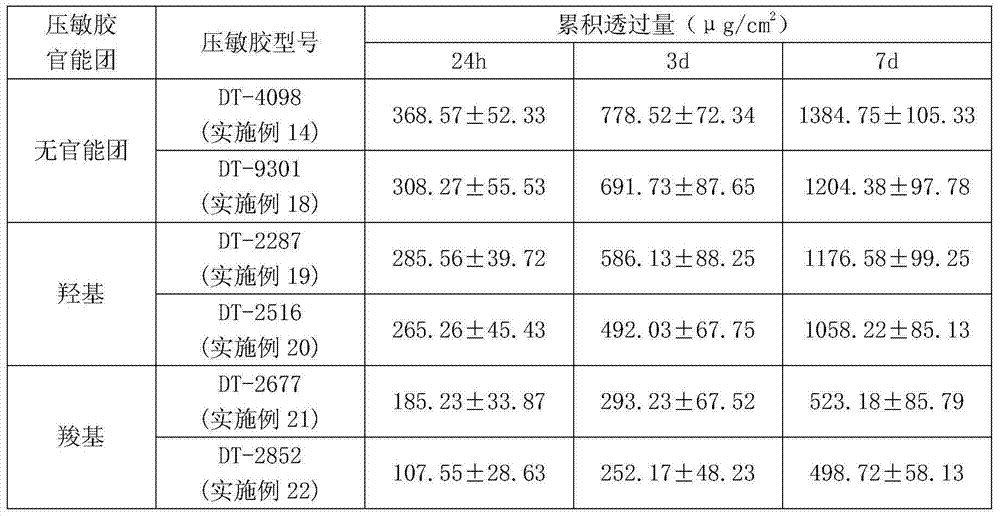
Escitalopram percutaneous patch and preparation method thereof
ActiveCN104840973AEffective regulation of transdermal penetration rateSimple technologyOrganic active ingredientsNervous disorderOrganic acidEscitalopram
The invention belongs to the technical field of medicine and relates to an escitalopram percutaneous patch and a preparation method thereof. The escitalopram percutaneous patch is composed of a backing layer, a medicine-carrying pressure-sensitive adhesive layer and an anti-sticking layer, the medicine-carrying pressure-sensitive adhesive layer comprises escitalopram free alkali or organic acid ion pair compound thereof, pressure-sensitive adhesive and percutaneous absorption promoter, the escitalopram free alkali or organic acid ion pair compound thereof accounts for 2.0-20wt% of total weight of the medicine-carrying pressure-sensitive adhesive layer, pressure-sensitive adhesive accounts for 77-97wt% of the total weight, the percutaneous absorption promoter accounts for 0-6.8wt% of the total weight, and in the escitalopram organic acid ion pair compound, a ratio of escitalopram free alkali and different organic acids is 0.5:1-2:1. Compared with an escitalopram organic acid ion pair compound percutaneous absorption patch and an escitalopram free alkali percutaneous absorption patch, the escitalopram percutaneous patch has the advantages that medicine release up to seven days and similar to constant rate can be provided, so that medication compliance of a patient is improved greatly.
Owner:SHENYANG PHARMA UNIVERSITY
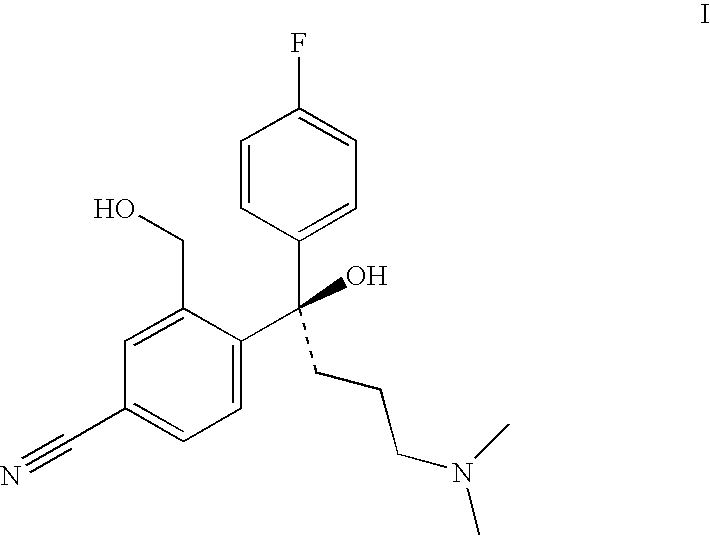
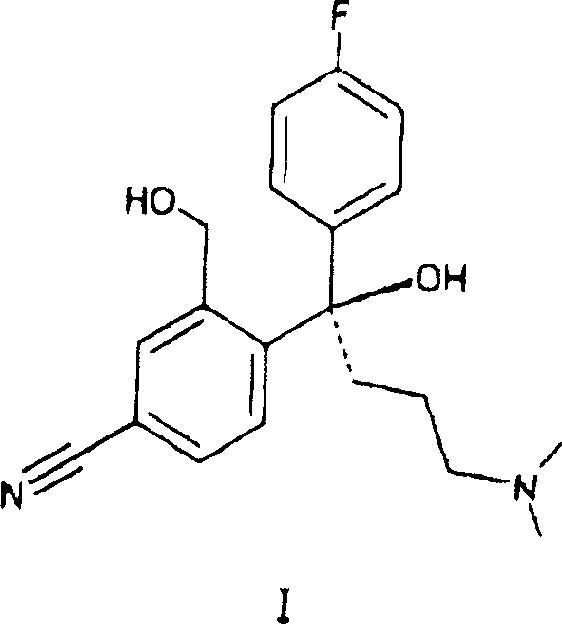
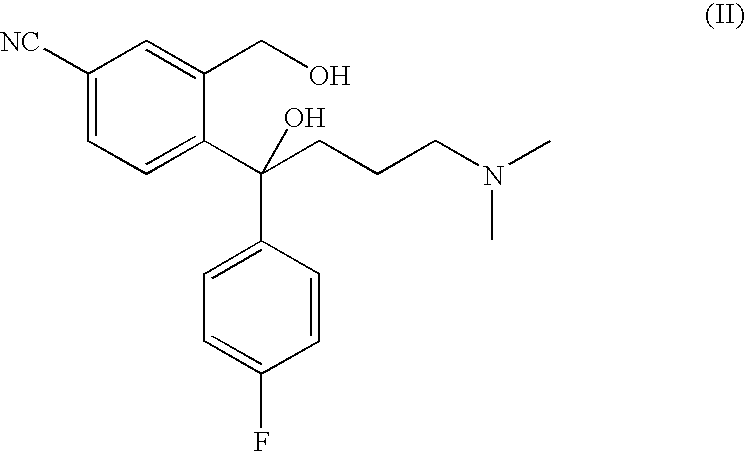
Crystalline citalopram diol intermediate alkali
The present invention relates to the diol intermediate of citalopram useful for treatment of depression, that is to say, the crystal of free alkali of 3-hydroxylmethyl-4-[1-(4-fluorophenyl)-1-hydroxyl-4-(dimethylamino)] butylbenzonitrile and the method of crystallization thereof. The present invention has disclosed the method to prepare pure citalopram and its purified salts through crystallization of the described alkali;the optical resolution method of citalopram diol intermediate, the method to prepare S-citalopram and its purified salts by crystals mentioned above. The present invention has also disclosed the method to prepare citalopram and its purified salts, S-citalopram and its purified salts, as well as pharmaceutical formulation thereof obtained. Using methods of the present invention, the quality and yield of the product can be signally improved, and the production cost of the medicinal material can be decreased.
Owner:ZHEJIANG HONGGUAN BIO PHARMA
Comprehensive pharmacologic therapy for treatment of obesity
InactiveUS20060135567A1High expectation of weight lossPromote resultsBiocidePeptide/protein ingredientsDiethylpropionVitamin C
The comprehensive pharmacologic therapy for treatment of obesity is a procedure which involves the administration of a desired therapeutic range of Diethylpropion and / or Phentermine in combination with a SSRI medication and nutritional supplementation for brief and long durations which may be 12 months or more. The preferred procedure involves the administration of drugs in combination which are identified as: Citalopram (Celexa) and Phentermine; Citalopram (Celexa) and Diethylpropion; Citalopram (Celexa), Phentermine, and Diethylpropion. In addition nutritional supplementation such as a multivitamin, 5-Hydroxytryptophan, vitamin B6, vitamin C, Tyrosine, Calcium, and Lysine may be used to enhance the performance of the weight loss treatment program.
Owner:HINZ MARTIN C
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com