Skin irritation suppressant and transdermal preparation
A skin irritation and inhibitor technology, applied in the field of skin irritation inhibitors, can solve the problem that cholesterol does not have anti-inflammatory activity, and achieve the effect of reducing skin irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1)
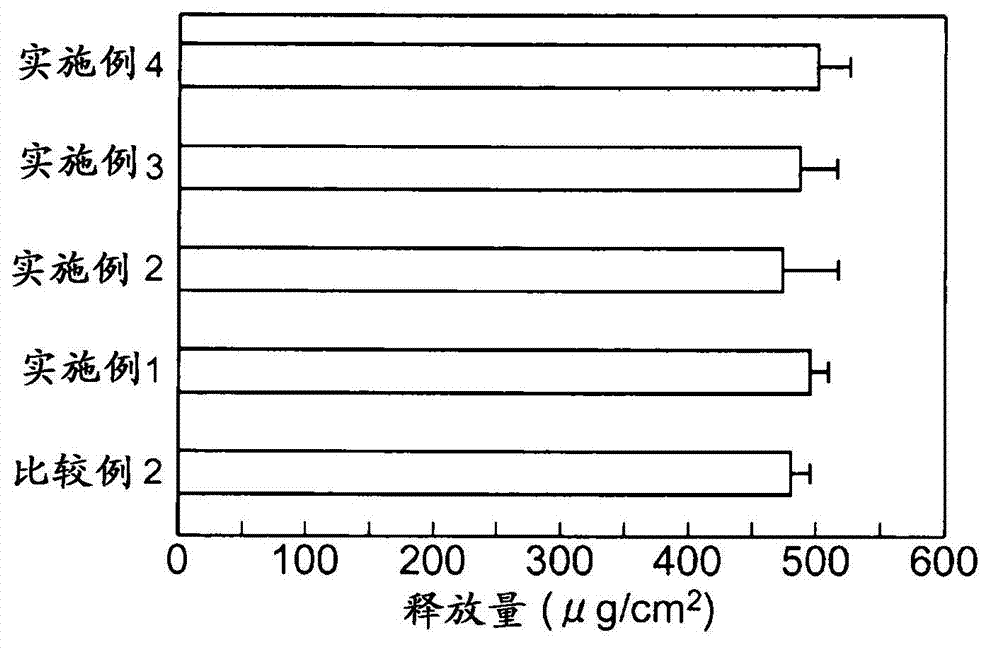
[0072] To study the changes in the amount of skin stimulating media produced by the test substance when the skin stimulating substance is applied to a human three-dimensional cultured epidermis model.
[0073] A human three-dimensional cultured epidermal model (LabCyteEPI-MODEL, manufactured by Japan Tissue Engineering Co., Ltd.) was used in which human normal epidermal cells were cultured in layers. LabCyte is cultured skin cultured in Transwell culture plates. When performing experiments, use both LabCyte and Transwell plates.
[0074] The test substance is a candidate for a skin irritation inhibitor, and cholesterol (manufactured by Wako Pure Chemical Industries, Ltd.), β-sitosterol (manufactured by Tama Biochemical Co., Ltd.), α-spinasterol (manufactured by ChromaDex), and ergosterol (manufactured by ChromaDex Corporation) were used. Wako Pure Chemical Industries, Ltd.), campesterol (Tama Biochemical Co., Ltd.), glycyrrhizic acid (Wako Pure Chemical Industries, Ltd.), gly...
experiment example 2)
[0089]
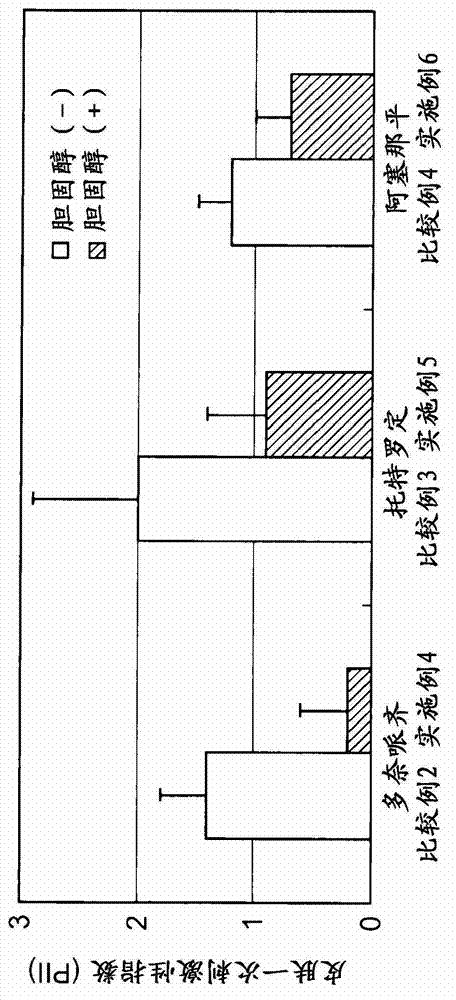
[0090] Cholesterol was used as the test substance. Drugs shown in Table 2 were used as skin irritating substances.
[0091] Experiments were performed using the following composition configurations.
[0092] • Drug addition group alone: a group in which only the drug was dispersed in the medium as a test solution. Drug concentrations are shown in Table 2.
[0093] · Drug+cholesterol addition group: a group in which a drug and 1% by mass of cholesterol were dispersed in a medium as a test solution. Drug concentrations are shown in Table 2.
[0094] The other conditions were the same as in Experimental Example 1, and the experiment was performed. The results are shown in Table 2. According to Table 2, cholesterol has the effect of inhibiting the production of skin-irritating mediators in a wide range of drugs.
[0095] Table 2
[0096]
experiment example 3)
[0098]
[0099]Using croton oil (Wako Pure Chemical Industries, Ltd.) as an edema-inducing substance, the suppression of ear edema in mice was studied. Experiments were performed using 7-week-old ddY female (SPF) mice. Cholesterol, glycyrrhizic acid, glycyrrhetinic acid, squalene, ergosterol, squalane, and lanosterol were used as test substances. Acetone (Wako Pure Chemical Industries, Ltd.) was used as the medium.
[0100] Experiments were performed using the following composition configurations.
[0101] · Positive control group: a group in which only the vehicle was administered as a test solution once, and a 2% croton oil solution was administered as a test solution twice after 1 hour.
[0102] · Test substance group: a group in which only the vehicle was administered as a test solution once, and a 2% croton oil solution containing 1% by mass of the test substance was administered as a test solution twice as a test solution one hour later.
[0103] The auricle edema i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com