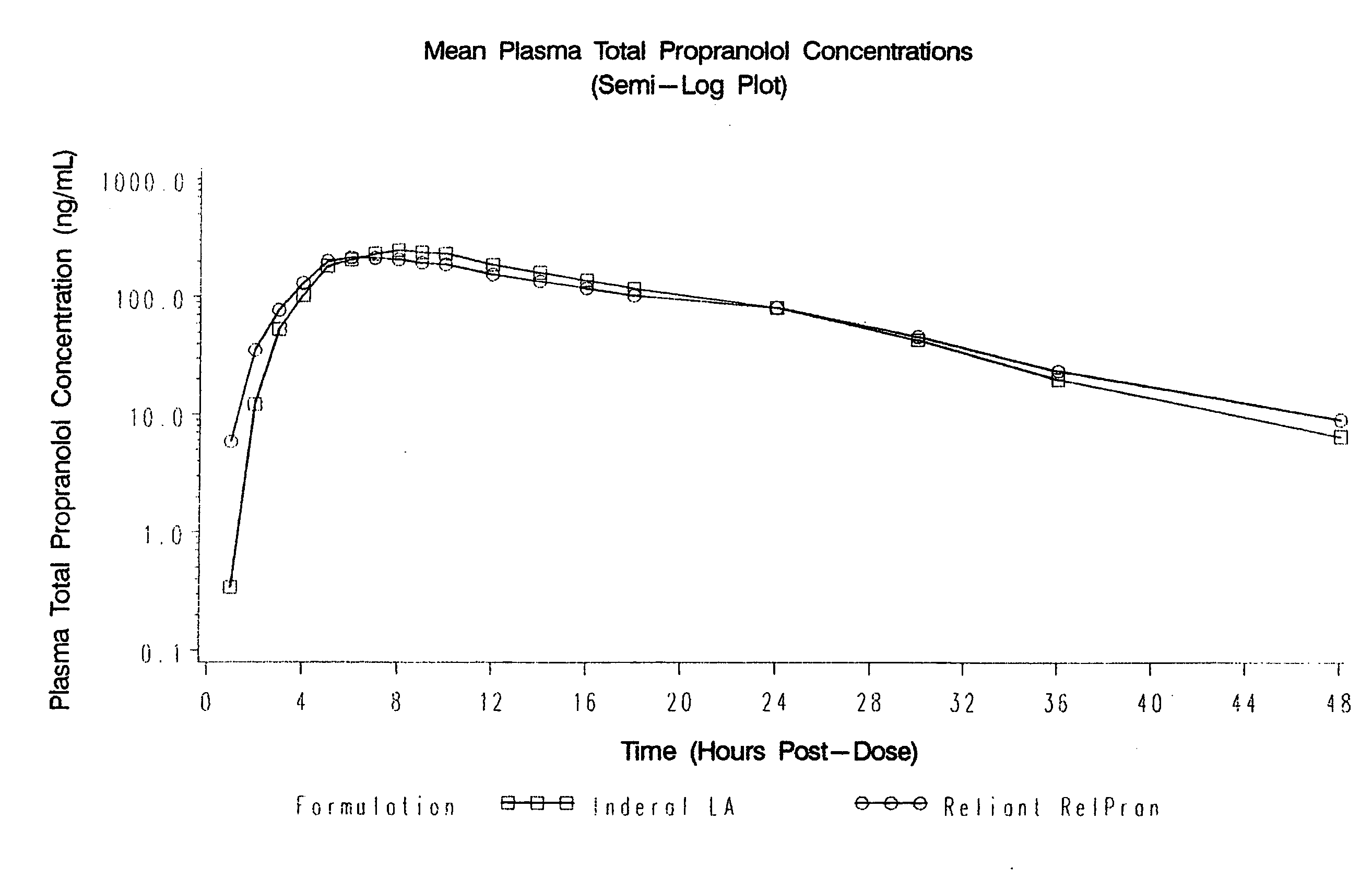
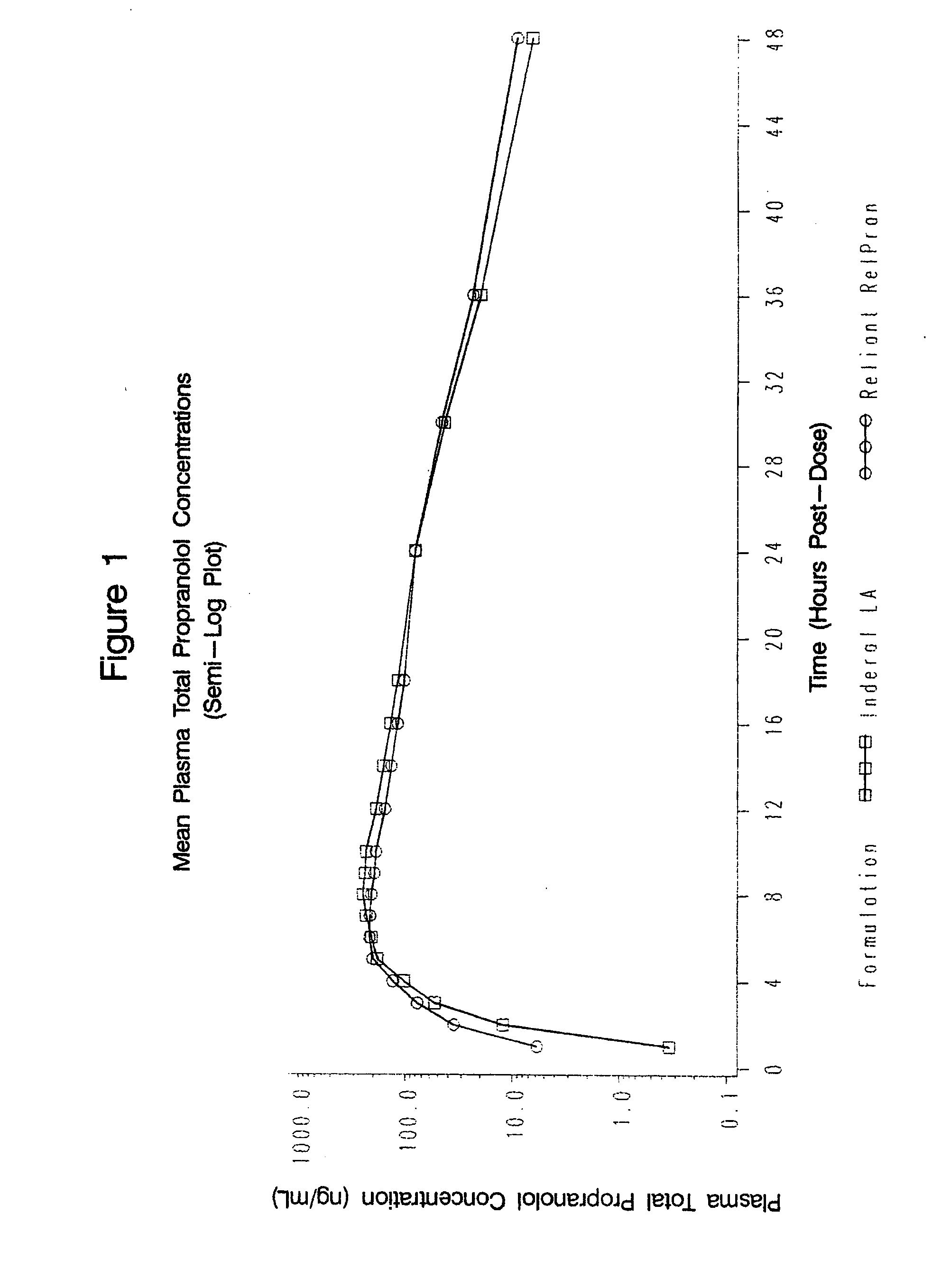
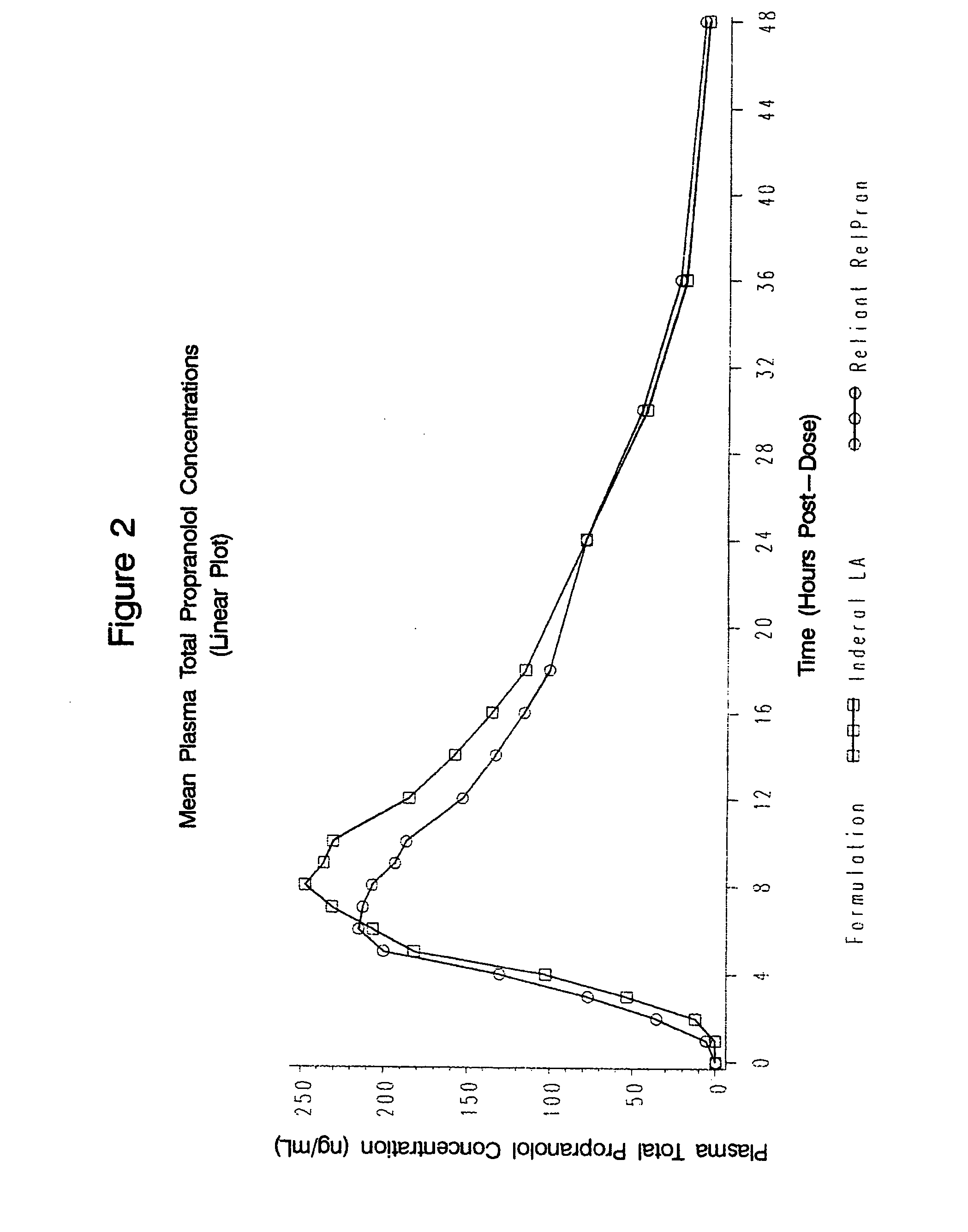
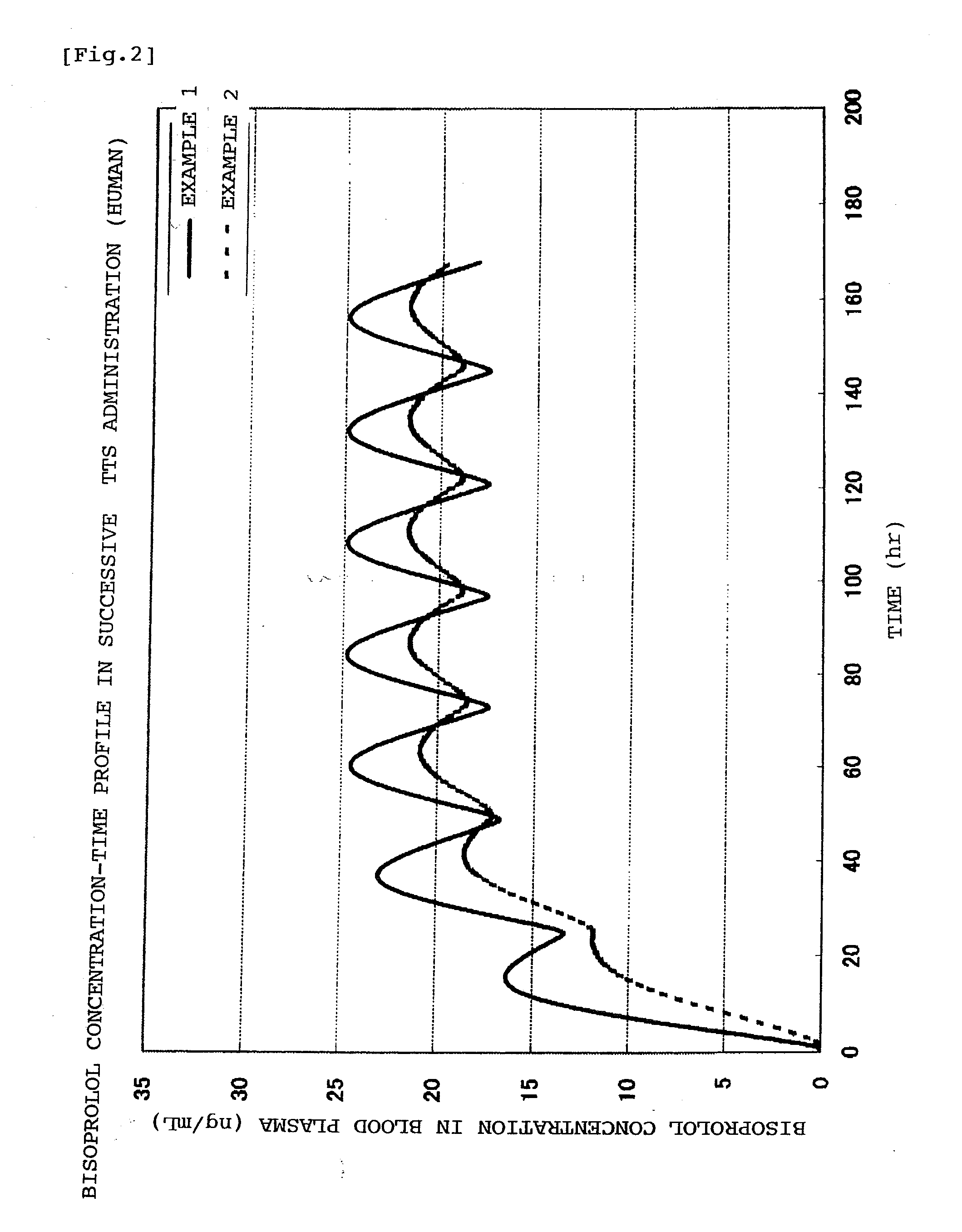
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Bisoprolol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bisoprolol is used with or without other medications to treat high blood pressure (hypertension).
Time-sustained-release formulations comprising a beta-blocker
InactiveUS20080131517A1Providing therapyAvoid problemsPowder deliveryOrganic active ingredientsCarteololBeta blocker
The present invention relates to compositions and methods of treating human subjects with a beta-adrenergic receptor blocking agent (“beta-blocker”) provided in a time-sustained-release delivery system. The time-sustained-release drug delivery systems includes at least three populations of beads, where each population of beads includes a beta-blocker. The beads may be selected from immediate-release beads, enteric-release beads, sustained-release beads, and time-sustained-release beads. The beta-blocker may be selected from acebutolol, atenolol, betaxolol, bisoprolol, esmolol, metoprolol, nebivolol, butoxamine, carteolol, carvedilol, labetalol, nadolol, oxprenolol, penbutolol, propranolol, pindolol, sotalol, and timolol. According to presently preferred embodiments, the beta-blocker is propranolol. The dosage forms of the present invention are useful for treating conditions including hypertension, angina pectoris due to coronary atherosclerosis, hypertrophic subaortic stenosis, congestive heart failure, arrhythmias, angina, anxiety, glaucoma, migraines, esophageal varices, alcohol withdrawal syndrome, irregular heartbeat, tachycardia, tremor, and neuroleptic-induced akathisia. They are also useful in the prophylaxis of migraine headaches.
Owner:RELIANT PHARMACEUTICALS INC
Patch
InactiveUS20090012181A1Little irritationImprove skinBiocideOrganic active ingredientsSide effectMaximum level
A Bisoprolol patch, wherein the skin penetration rate of Bisoprolol after 24 hours is 15 to 60% of the maximum skin penetration rate thereof, shows a little difference between the maximum level of the concentration in blood and the minimum level thereof in repeated administration and, therefore, scarcely exhibits side effects. Moreover, it achieves the quick development of the drug effect owing to the stabilization of the concentration in blood within a short time.
Owner:HISAMITSU PHARM CO INC
Adhesive composition for patch and use thereof
InactiveUS20100092544A1Improve skinImprove cohesionFilm/foil adhesivesVinyl aromatic copolymer adhesivesElastomerPolymer science
The present invention provides an adhesive composition for patch, containing a rubber elastomer and a tackifier having a weight average molecular weight of 1200-2500, a patch having a support and an adhesive layer containing the composition, which is provided on at least one surface of the support, and a patch preparation having an adhesive layer containing a percutaneously absorbable drug (excluding bisoprolol).
Owner:NITTO DENKO CORP
Adhesive pharmaceutical preparation containing bisoprolol
InactiveUS20090291126A1Improve adhesionLess irritatingBiocideOrganic active ingredientsIrritationAdditive ingredient
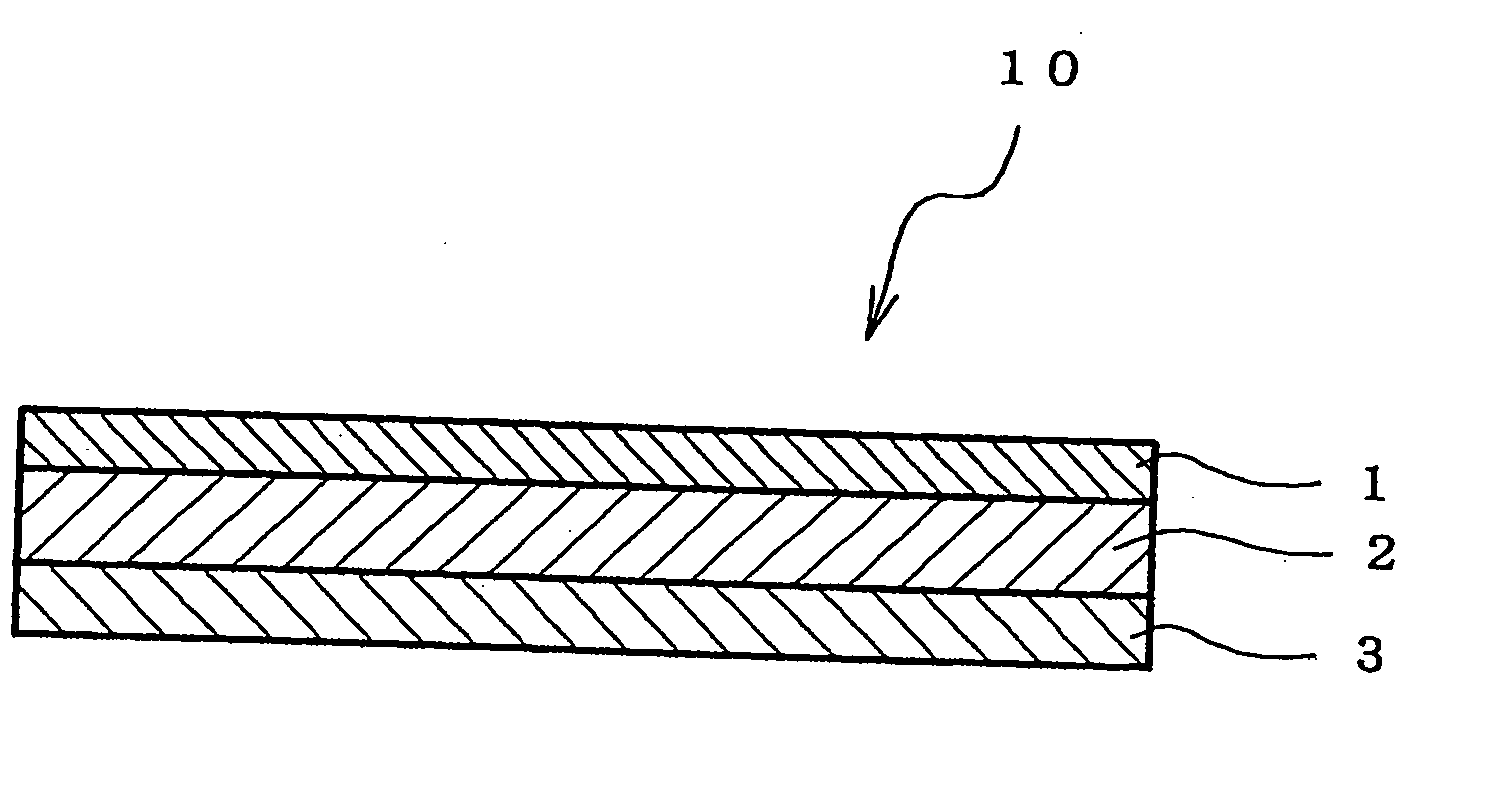
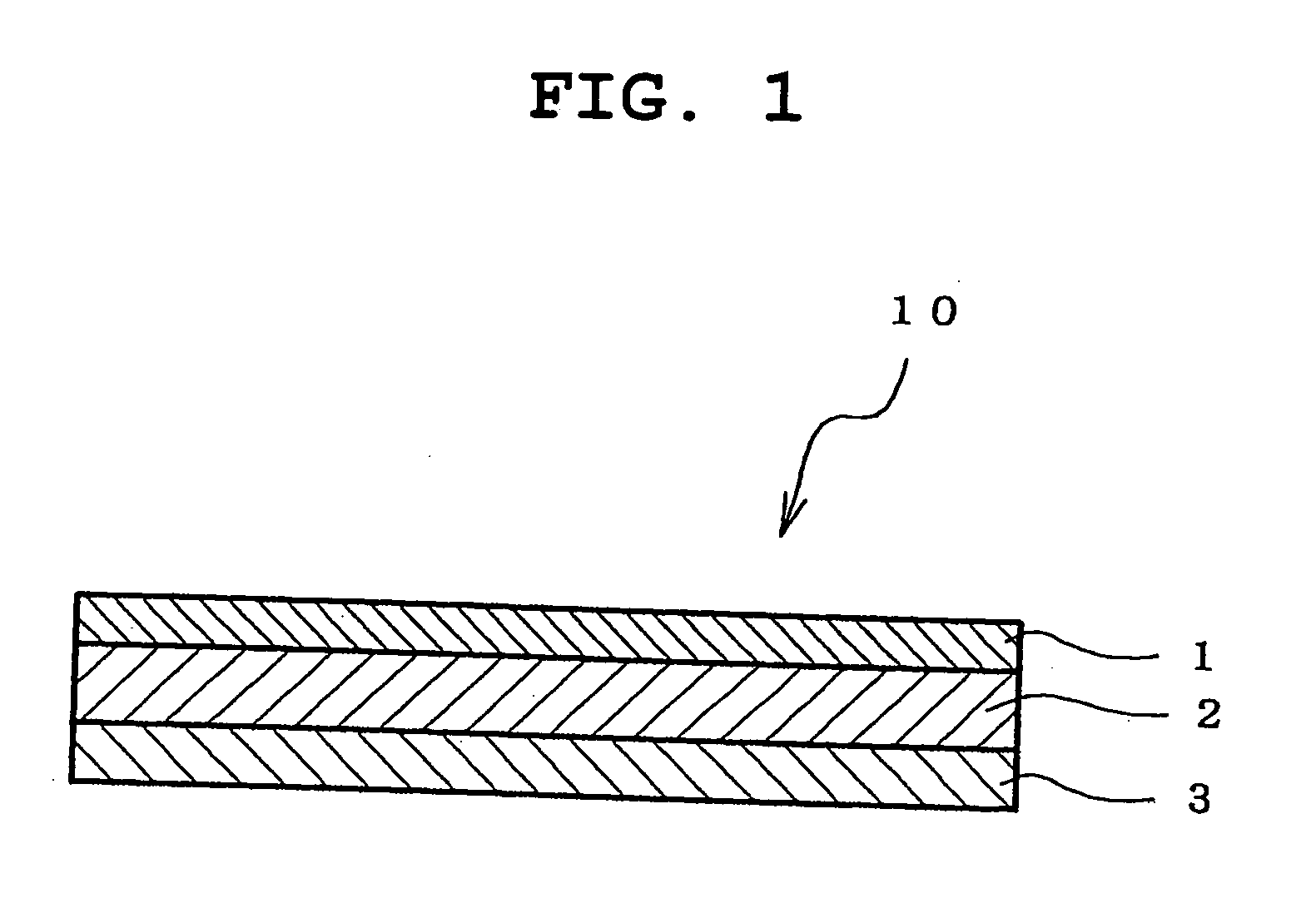
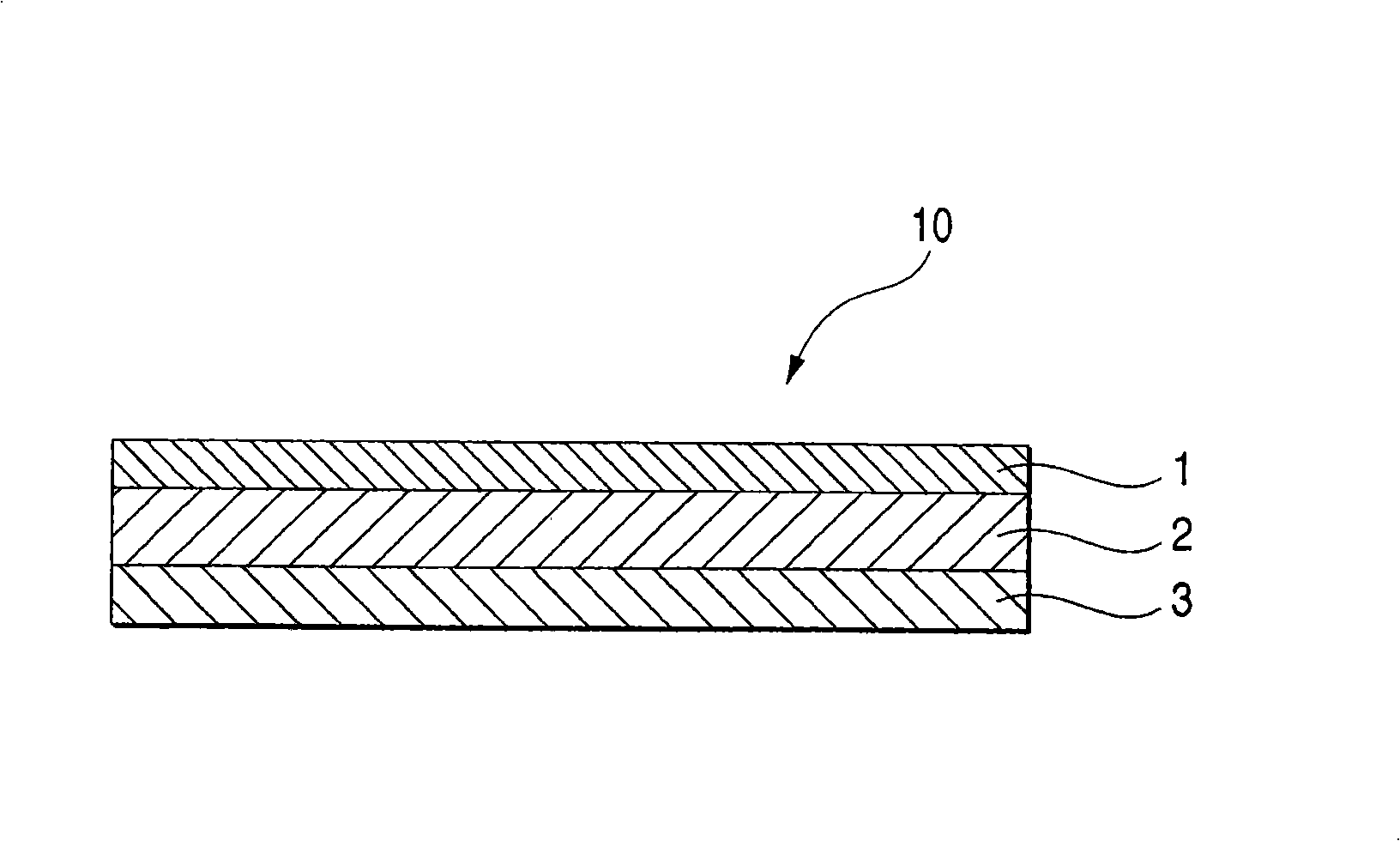
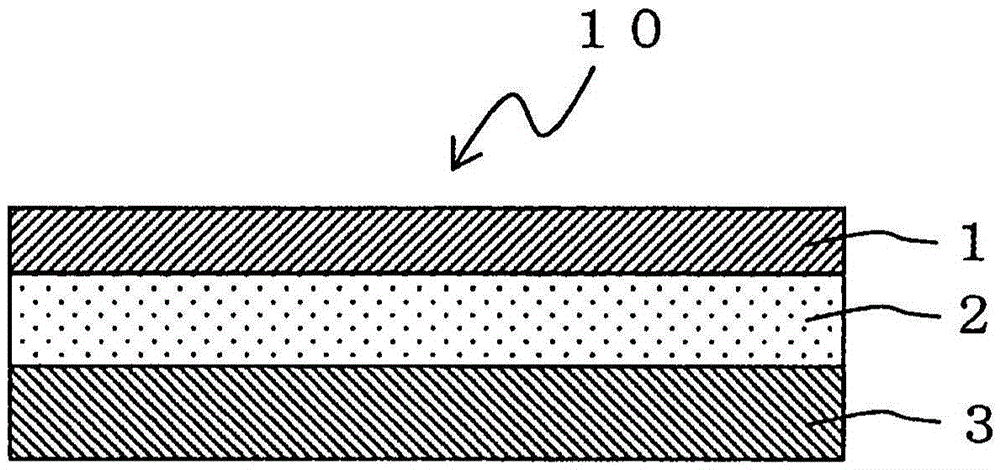
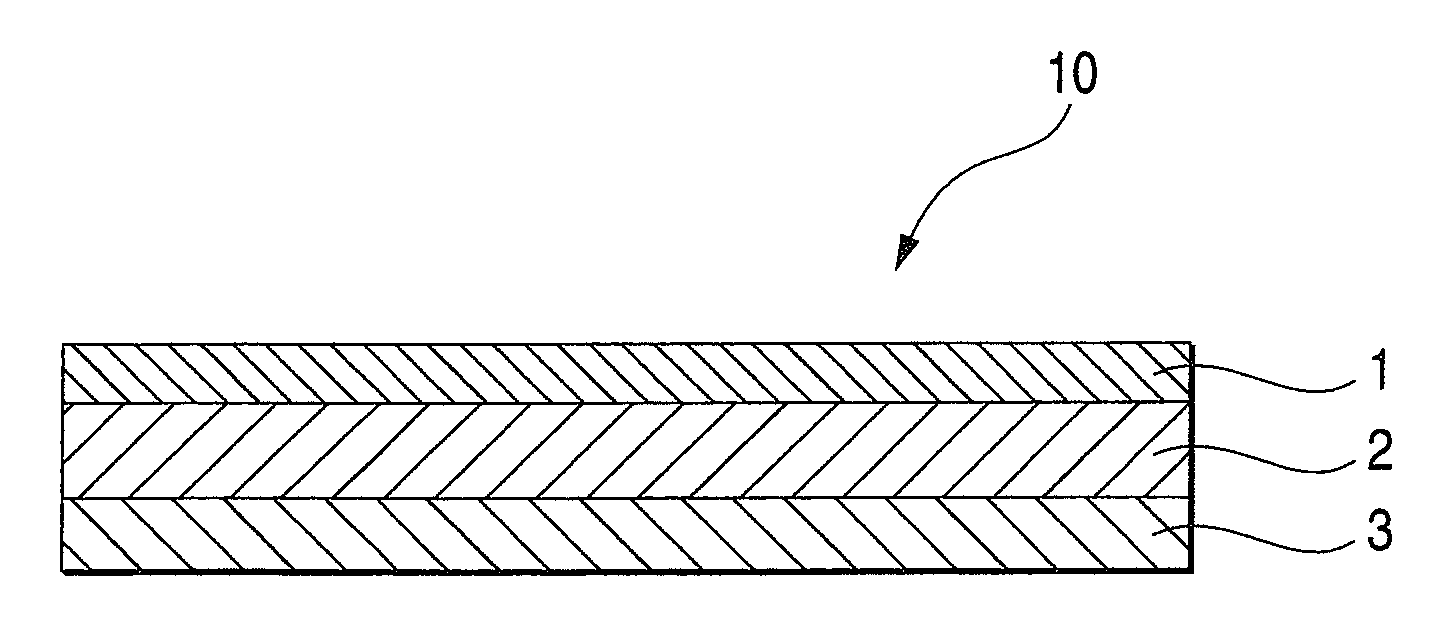
An adhesive pharmaceutical preparation which has a less irritation to the skin surface, keeps excellent stability of bisoprolol in the preparation, and allows continuous administration of a pharmacologically effective amount of bisoprolol into the living body, is provided. The adhesive preparation 10 comprises a support 1, an adhesive layer 2 laminated on one surface of the support 1. The adhesive layer 2 is characterized by containing bisoprolol, polyisobutylene, tackifier, and an organic liquid ingredient compatible to polyisobutylene and tackifier. Thus, an adhesive pharmaceutical preparation which has a good adhesiveness with less irritation to the skin and which gives almost no pain at peel-off or removal from the skin surface with leaving almost no paste, can be provided. In addition, bisoprolol is very stably maintained in the preparation and a pharmacologically effective amount of bisoprolol can be administered continuously into the living body through the skin surface.
Owner:NITTO DENKO CORP +1
Percutaneous administration device of bisoprolol
InactiveUS20100098747A1Suppress irritationReduce skin irritationOrganic active ingredientsBiocideBisoprololLiving body
The present invention relates to a percutaneous administration device of bisoprolol, which includes a backing; and a pressure-sensitive adhesive layer containing bisoprolol, which is laminated on one surface of the backing, wherein the maximum value of a release rate of bisoprolol during a period of from immediately after the application on skin until a lapse of 24 hours is 30 μg / cm2 / hr or less; and wherein the release rate of bisoprolol at the time of a lapse of 24 hours after the application on skin is 10 μg / cm2 / hr or less. The percutaneous administration device of the present invention is reduced in the skin irritation during the application, especially at the time of peeling, and is capable of persistently administrating a therapeutically or preventively effective amount of bisoprolol into a living body.
Owner:NITTO DENKO CORP +1
Patch
InactiveUS20100227932A1Little irritationImprove skinOrganic active ingredientsBiocideMaximum levelSide effect
A Bisoprolol patch, wherein the skin penetration rate of Bisoprolol after 24 hours is 15 to 60% of the maximum skin penetration rate thereof, shows a little difference between the maximum level of the concentration in blood and the minimum level thereof in repeated administration and, therefore, scarcely exhibits side effects. Moreover, it achieves the quick development of the drug effect owing to the stabilization of the concentration in blood within a short time.
Owner:AMANO SATOSHI +2
Adhesive patch containing bisoprolol
InactiveCN102770130AInhibition of attachmentEasy to take outOrganic active ingredientsPlastersDrug contentBisoprolol
Disclosed is an adhesive patch which effectively inhibits the occurrence of oozing or squeezing out of the adhesive layer component from the exposed area of the adhesive layer of the adhesive patch during storage, and also inhibits the occurrence of oozing of bisoprolol or salts of same from the adhesive layer thereby preventing reduction of drug content. A backing material, a release liner and an adhesive layer respectively, along with the entire adhesive patch, are shaped into a planar rectangle wherein the corners of the adhesive patch are formed into convex shapes on the rear surface on the backing material side. Further, the adhesive patch has a central part and edges wherein the corners of the rectangular shape of the central part can also be formed into convex shapes, and furthermore it can be provided with built-up articulated sections between two or more adjacent convex parts wherein the thickness of the adhesive patch is thinner than the thickness of the adhesive layer at each convex part. If the release liner is provided with a split at the back, the split should not traverse the convex parts.
Owner:NITTO DENKO CORP +1
Adhesive medicament preparation containing bisoprolol
InactiveCN101262858AImprove adhesionLess irritatingOrganic active ingredientsPharmaceutical non-active ingredientsSkin surfaceIrritation
It is intended to provide an adhesive preparation which has a low irritation to the skin surface, is excellent in stability of bisoprolol in the preparation and further is capable of continuously administrating a pharmacologically effective amount of bisoprolol to the living body. The adhesive preparation 10 has a support 1 and an adhesive layer 2 which is layered on one surface of the support 1. The adhesive layer 2 is characterized by containing bisoprolol, polyisobutylene, a tackifier and an organic liquid component which is compatible with polyisobutylene and the tackifier. By this, the adhesive preparation having good skin adhesiveness, low skin irritation and reduced pain or adhesive residue at the time of removal from the skin surface can be provided. Further, the adhesive preparation in which the stability of bisoprolol in the adhesive preparation is excellent and the pharmacologically effective amount of bisoprolol can be continuously administrated to the living body through the skin surface is provided.
Owner:NITTO DENKO CORP +1
Patch preparation
InactiveCN106256348AReduce biasImprove permeabilityEster polymer adhesivesPharmaceutical non-active ingredientsMeth-Bisoprolol
The invention provides a patch preparation, which includes a support and a pressure-sensitive adhesive layer formed on one surface of the support. The pressure-sensitive adhesive layer includes: (A) a polymer prepared by copolymerizing monomer components including a hydroxyl group-containing monomer and an alkyl (meth)acrylate monomer; (B) a polymer prepared by copolymerizing monomer components including a methyl methacrylate monomer and a butyl methacrylate monomer; and (C) a basic drug, provided that bisoprolol and a salt thereof are excluded. The patch preparation is capable of inhibiting a deviation in pressure-sensitive adhesive properties of the pressure-sensitive adhesive layer even elapsing a long period of time until it is used after production and is capable of keeping favorable pressure-sensitive adhesive properties.
Owner:NITTO DENKO CORP
Indapamide bisoprolol percutaneous patch and preparation method thereof
The invention belongs to the technical field of medicine and relates to an indapamide bisoprolol percutaneous patch and a preparation method thereof. The indapamide bisoprolol percutaneous patch is composed of a back liner layer, a medicine-carrying pressure-sensitive adhesive layer and an anti-bonding layer. The medicine-carrying pressure-sensitive adhesive layer includes indapamide, bisoprolol free alkali or a organic acid ion pair compound thereof, a pressure-sensitive adhesive and a percutaneous absorption promoter, wherein a total medicine amount of the indapamide, bisoprolol free alkali or the organic acid ion pair compound thereof accounts for 0.5-5.5 wt% of the total weight of the medicine-carrying pressure-sensitive adhesive layer and a molar ratio of the indapamide to the bisoprolol free alkali or the organic acid ion pair compound thereof is 0.5:1-2:1. According to the invention, two-way regulation of a percutaneous penetrating capability of indapamide and bisoprolol in the percutaneous patch can be achieved and equal-speed penetration of the indapamide and the bisoprolol from the percutaneous patch is achieved.
Owner:SHENYANG PHARMA UNIVERSITY
Bisoprolol-containing patch preparation
InactiveCN105530925ALess rolled upGood adhesivenessOrganic active ingredientsNervous disorderShear stressAdhesive
The present invention pertains to a bisoprolol-containing patch preparation containing a support body and an adhesive layer laminated to one surface of the support body and containing an adhesive agent and free bisoprolol, the stress relaxation percentage of the adhesive layer being 20-90%, and the shear stress of the adhesive layer being 1.0-6.5 N / 30 mm width. The bisoprolol-containing patch preparation has suppressed peeling during use, has superior skin adhesiveness, and suppresses skin irritation during delamination. As the adhesive agent, a rubber-based adhesive is preferable, and a polyisobutylene and / or styrene-isoprene-styrene block copolymer is more preferable.
Owner:NITTO DENKO CORP +1
Bisolol non intestine medicinal preparation
InactiveCN1650849AOrganic active ingredientsPharmaceutical delivery mechanismIntestinal structureAngina
A bisoprolol in the form of powdered or liquid injection for treating primary hypertension and angina pectoris is prepared from the physiologically acceptable salt of bisoprolol and additive.
Owner:尚宝虎 +1
Adhesive pharmaceutical preparation containing bisoprolol
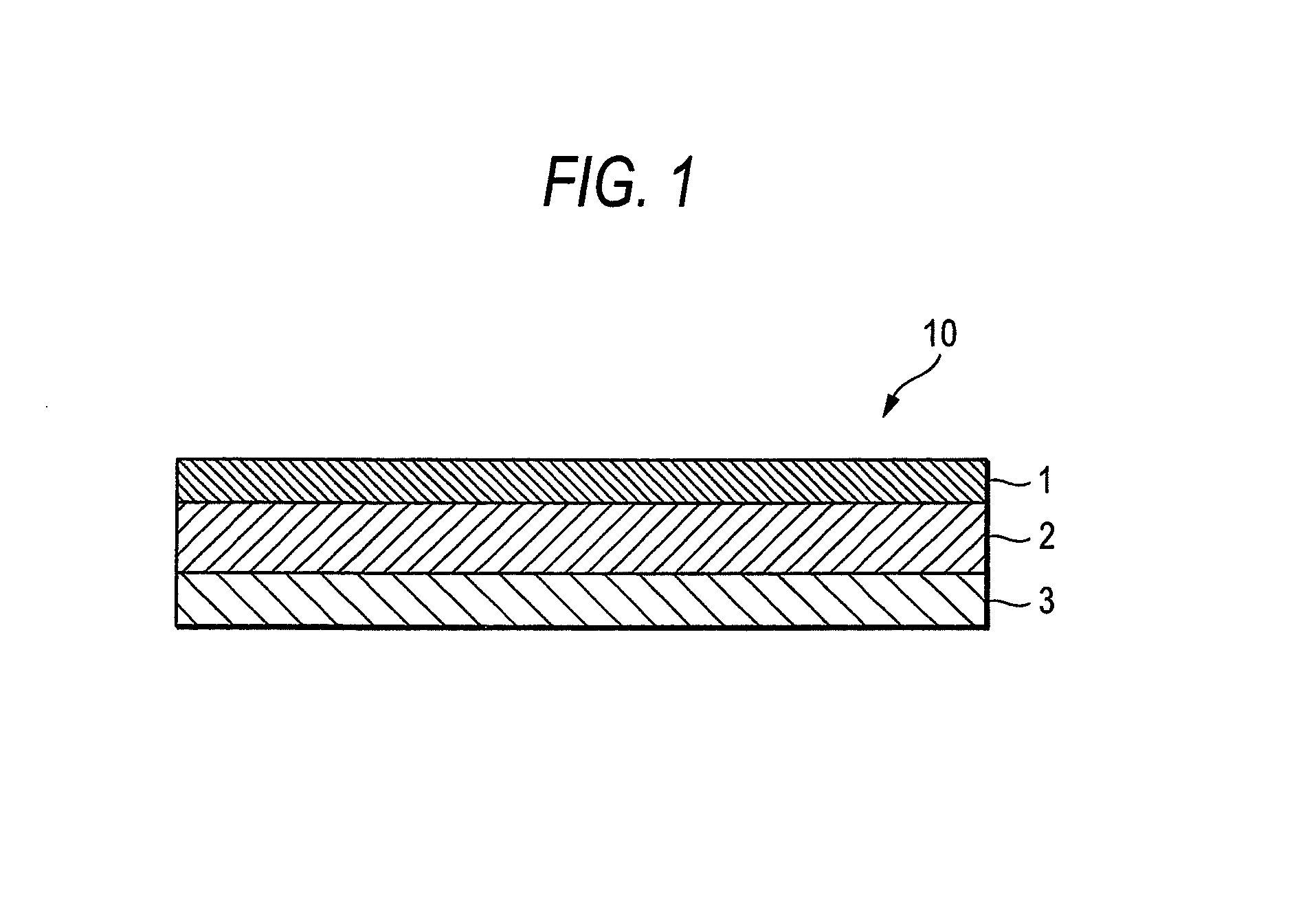
InactiveUS8808731B2Improve adhesionLess irritatingBiocideOrganic active ingredientsIrritationMedicine
An adhesive pharmaceutical preparation which has a less irritation to the skin surface, keeps excellent stability of bisoprolol in the preparation, and allows continuous administration of a pharmacologically effective amount of bisoprolol into the living body, is provided. The adhesive preparation 10 comprises a support 1, an adhesive layer 2 laminated on one surface of the support 1. The adhesive layer 2 is characterized by containing bisoprolol, polyisobutylene, tackifier, and an organic liquid ingredient compatible to polyisobutylene and tackifier. Thus, an adhesive pharmaceutical preparation which has a good adhesiveness with less irritation to the skin and which gives almost no pain at peel-off or removal from the skin surface with leaving almost no paste, can be provided. In addition, bisoprolol is very stably maintained in the preparation and a pharmacologically effective amount of bisoprolol can be administered continuously into the living body through the skin surface.
Owner:NITTO DENKO CORP +1
Chiral capillary electrochromatography open tube column based on gold nano-modification, preparation method and application
ActiveCN111707771AAchieve the purpose of separationEasy to separateComponent separationBulk chemical productionGlycidyl methacrylateCyclodextrin
The invention relates to a chiral capillary electrochromatography open tube column based on gold nano modification, a preparation method and an application. The method comprises the steps: bonding a glycidyl methacrylate (GMA) tentacle type polymer coating on the inner wall of a capillary tube; carrying out alkaline ring opening on an epoxy group of GMA under an alkaline condition by utilizing amino of reduced glutathione (GSH), and successfully synthesizing GSH on the capillary tube column; combining gold nanoparticles on the capillary tube column by utilizing the property that sulfydryl of glutathione can generate Au-S bonds with the gold nanoparticles, successfully introducing gold nanoparticles into a capillary stationary phase, and constructing a chiral resolution system based on carboxymethyl-beta-cyclodextrin. Experiments prove that compared with a hollow tubular column which is not modified with gold nanoparticles, the prepared capillary open tube column has the advantages thatthe separation degree and selectivity of three lol drugs (atenolol, sotalol and bisoprolol) are greatly improved, and the purpose of baseline separation is achieved.
Owner:济宁市第一人民医院
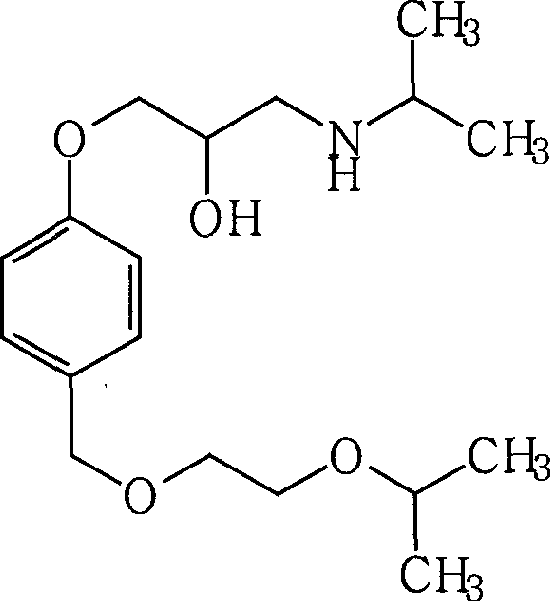
Synthesis technology of bisoprolol
InactiveCN101898972AShorten the timeSimple extraction processOrganic compound preparationChemical recyclingDistillationBisoprolol
The invention relates to a synthesis technology of bisoprolol. The technical scheme of the technology comprises the following steps: (1) using 4-hydroxybenzyl alcohol and isopropoxyethanol to perform the etherification reaction, using strongly acidic styrene type cation exchange resin as catalyst to ensure the alcoholic hydroxyl group etherification reaction to perform under the conditions of room temperature and no solvent; (2) adding sodium hydroxide and epoxychloropropane in the reaction product in the step (1) to ensure phenolic hydroxyl group to perform the etherification reaction; and (3) adding the reaction product in the step (2) in isopropamide, reacting by using boron hydride as catalyst, and preparing bisoprolol. By using the synthesis technology of the invention, the alcoholic hydroxyl group etherification reaction can be performed under the conditions of room temperature and no solvent, the processes of extraction, washing and reduced pressure distillation are simplified; boron hydride is used as catalyst to ensure the amination reaction to perform under a low temperature; the overall yield is increased to above 50%; and the synthesis technology is suitable for industrial production. The solvent used by the technology of the invention can be recycled through distillation; resinic solid acid catalyst can be recycled; and yield and product purity are high.
Owner:张综利
Adhesive pharmaceutical preparation containing bisoprolol
InactiveUS8298572B2Suppress bleedSufficient pressure-sensitive adhesion characteristicBiocideNervous disorderBisoprololPharmaceutical formulation
In the adhesive pharmaceutical preparation of the invention containing bisoprolol, a pressure-sensitive adhesive layer is laminated on one side of the backing. The pressure-sensitive adhesive layer contains a branched monoalcohol having from 12 to 28 carbon atoms, a free base of bisoprolol and a polyisobutylene pressure-sensitive adhesive. Accordingly, compatibility of the polyisobutylene pressure-sensitive adhesive with the free base of bisoprolol can be specifically increased. As a result, not only it becomes possible to increase blending amount of the free base of bisoprolol but also bleed of the free base of bisoprolol from the pressure-sensitive adhesive layer can be suppressed and, what is more, the pressure-sensitive adhesion characteristics sufficient from the practical point of view can be obtained.
Owner:NITTO DENKO CORP +1
Solid preparation of bisoprolol / hydrochlorothiazide medicine composition liposome
InactiveCN102614186BOrganic active ingredientsPharmaceutical non-active ingredientsSide effectHydrochlorothiazide
The invention discloses a solid preparation of a bisoprolol / hydrochlorothiazide medicine composition liposome and a preparation method thereof. Active components of bisoprolol fumarat, hydrochlorothiazide and specified compositions of hydrogenated granulesten, dilauroyl phosphatidylcholine, cholesterol and tween 60 are prepared into the liposome. Stability, dissolution rate and biological utilization rate of a medicine are greatly improved, functions are stable and durable, and curative effects are remarkable. The solid preparation of the bisoprolol / hydrochlorothiazide medicine composition liposome improves product quality and reduces toxic and side effects.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Percutaneous administration device of bisoprolol
InactiveUS8703178B2Suppress irritationReduce skin irritationOrganic active ingredientsBiocideBisoprololLiving body
The present invention relates to a percutaneous administration device of bisoprolol, which includes a backing; and a pressure-sensitive adhesive layer containing bisoprolol, which is laminated on one surface of the backing, wherein the maximum value of a release rate of bisoprolol during a period of from immediately after the application on skin until a lapse of 24 hours is 30 μg / cm2 / hr or less; and wherein the release rate of bisoprolol at the time of a lapse of 24 hours after the application on skin is 10 μg / cm2 / hr or less. The percutaneous administration device of the present invention is reduced in the skin irritation during the application, especially at the time of peeling, and is capable of persistently administrating a therapeutically or preventively effective amount of bisoprolol into a living body.
Owner:NITTO DENKO CORP +1
Bisoprolol-containing adhesive patch
InactiveCN105517544AInhibit coloringReduce adhesionOrganic active ingredientsSheet deliveryHalogenAdhesive
The present invention relates to a bisoprolol-containing adhesive patch that is provided with: a support body; and an adhesive layer that contains bisoprolol and an adhesive and that is stacked on one surface of the support body. The bisoprolol-containing adhesive patch is characterized by the halogen atom content within the adhesive layer being 0.01-100 weight ppm. The present invention makes it possible to minimize decreases in adhesive strength, discoloration, and the like in a bisoprolol-containing adhesive patch during storage thereof.
Owner:NITTO DENKO CORP +1
Medicinal composition for treating cerebrovascular disease
InactiveCN100384416CLittle side effectsIncrease heart ratePowder deliverySolution deliveryVascular diseaseCoronary artery disease
A composite medicine in the various forms for treating cardiovascular disease such as coronary heat disease, hypertension, etc is prepared from bisoprolol and candesartan in the mole ratio of 1: (1-2).
Owner:BEIJING JINCHENG TAIER PHARMA CO LTD
Patch preparation
InactiveUS20160367494A1Avoid large deviationExcellent pressure-sensitive adhesive propertyEster polymer adhesivesPharmaceutical non-active ingredientsMeth-Bisoprolol
A patch preparation includes a support and a pressure-sensitive adhesive layer formed on one surface of the support. The pressure-sensitive adhesive layer includes: (A) a polymer prepared by copolymerizing monomer components including a hydroxyl group-containing monomer and an alkyl (meth)acrylate monomer; (B) a polymer prepared by copolymerizing monomer components including a methyl methacrylate monomer and a butyl methacrylate monomer; and (C) a basic drug, provided that bisoprolol and a salt thereof are excluded.
Owner:NITTO DENKO CORP
Bisoprolol-containing adhesive patch and package of same
InactiveCN105517543AInhibit hydrolysis over timeImproved stability over timeOrganic active ingredientsPharmaceutical containersBisoprololMoisture
The present invention relates to: a bisoprolol-containing adhesive patch which comprises a supporting body and an adhesive layer that is formed on at least one surface of the supporting body and contains bisoprolol, and wherein the adhesive layer has a moisture content of 10,000 ppm or less; and a package of a bisoprolol-containing adhesive patch, which is obtained by sealing the bisoprolol-containing adhesive patch in a bag that has resistance to water vapor permeation. A bisoprolol-containing adhesive patch according to the present invention achieves excellent long-term stability of bisoprolol that is contained in the adhesive layer. In addition, a package of a bisoprolol-containing adhesive patch according to the present invention has excellent usability and portability.
Owner:NITTO DENKO CORP +1
Adhesive preparation containing bisoprolol
ActiveUS20180194977A1Excellent pressure-sensitive adhesive propertyHigh dermal permeabilityOrganic active ingredientsNon-macromolecular adhesive additivesMeth-Bisoprolol
An adhesive preparation containing bisoprolol includes a backing and a pressure-sensitive adhesive layer formed on one side of the backing. The pressure-sensitive adhesive layer contains a polymer prepared through copolymerization of monomer components including a hydroxyl group-containing monomer and an alkyl (meth)acrylate monomer (component (A)), a polymer prepared through copolymerization of monomer components including a methyl methacrylate monomer and a butyl methacrylate monomer (component (B)), and bisoprolol (component (C)).
Owner:TOAEIYO
Compositions comrrising amlodipine and bisoprolol
ActiveCN102164585AEasy to useReduce usagePill deliveryCapsule deliveryBisoprololBULK ACTIVE INGREDIENT
Stable solid pharmaceutical composition containing amlodipine or pharmaceutical acceptable salts thereof and bisoprolol or pharmaceutical acceptable salts thereof as well as pharmaceutically accepted excipients, packaged in a damp- proof package and further comprising less than 0.5 % of the compound of the formula (3) based on the weight of the active ingredients.
Owner:EGIS GYOGYSZERGYAR NYILVANOSAN MUKODO RESZVENYTARSASAG (EGIS PHARMA PLC)
Enteric sustained-release preparation with aspirin and bisoprolol as active ingredients
The invention provides an enteric sustained-release preparation with aspirin and bisoprolol as active ingredients. The sustained-release oral solid preparation is produced from aspirin and bisoprolol which are as major ingredients and pharmaceutically acceptable auxiliaries through a preparation technology, and comprises but is not limited to a sustained-release tablet, a sustained-release two-layer tablet, a sustained-release capsule, a sustained-release granule and a sustained-release pill. The sustained-release oral solid preparation is characterized in that the bisoprolol is released in a quick release manner while the aspirin is released in a sustained release manner, and the sustained release manner can be realized through a sustained-release framework material and a framework forming agent or through a drug coating.
Owner:FUKANGREN BIO PHARMA
Bisoprolol-containing adhesive patch and packaging body therefor
InactiveCN105517545AEasy to removeWon't roll upOrganic active ingredientsPharmaceutical non-active ingredientsBisoprololPack material
The present invention relates to a bisoprolol-containing adhesive patch that is provided with: an adhesive patch body that comprises a support body and an adhesive layer that contains bisoprolol and that is formed on one surface of the support body; and a peeling liner that is temporarily attached to the adhesive surface of the adhesive layer of the adhesive patch body. The peel force between the adhesive layer and the peeling liner is equal to or less than 0.07 N / 24 mm width. This bisoprolol-containing adhesive patch minimizes the adhesion thereof to the inner surface of a packaging material, can be extracted with extreme ease at the time of use, and has good peeling liner peelability at the time of use.
Owner:NITTO DENKO CORP +1
Adhesive medicament preparation containing bisoprolol
InactiveCN101262858BImprove adhesionLess irritatingOrganic active ingredientsPharmaceutical non-active ingredientsSkin surfaceIrritation
It is intended to provide an adhesive preparation which has a low irritation to the skin surface, is excellent in stability of bisoprolol in the preparation and further is capable of continuously administrating a pharmacologically effective amount of bisoprolol to the living body. The adhesive preparation 10 has a support 1 and an adhesive layer 2 which is layered on one surface of the support 1. The adhesive layer 2 is characterized by containing bisoprolol, polyisobutylene, a tackifier and an organic liquid component which is compatible with polyisobutylene and the tackifier. By this, the adhesive preparation having good skin adhesiveness, low skin irritation and reduced pain or adhesive residue at the time of removal from the skin surface can be provided. Further, the adhesive preparation in which the stability of bisoprolol in the adhesive preparation is excellent and the pharmacologically effective amount of bisoprolol can be continuously administrated to the living body through the skin surface is provided.
Owner:NITTO DENKO CORP +1
Percutaneous administration device of bisoprolol
InactiveCN103784425AInhibit stimulusEnough securityOrganic active ingredientsNervous disorderBisoprololLiving body
The present invention relates to a percutaneous administration device of bisoprolol. The percutaneous administration device includes a substrate material and a pressure-sensitive adhesive layer which is laminated on one surface of the substrate material and contains bisoprolol, wherein the maximum value of a release rate of bisoprolol during a period of from immediately after the application on skin until a lapse of 24 hours is 30 [mu]g / cm<2> / hr or less; and wherein the release rate of bisoprolol at the time of a lapse of 24 hours after the application on skin is 10 [mu]g / cm<2> / hr or less. The percutaneous administration device of the present invention is reduced in the skin irritation during the application, especially at the time of peeling, and is capable of persistently administrating a therapeutically or preventively effective amount of bisoprolol into a living body.
Owner:NITTO DENKO CORP +1
Indapamide bisoprolol transdermal patch and preparation method thereof
ActiveCN104147002BSame speed throughOrganic active ingredientsSheet deliveryTransdermal patchMedicine
Owner:SHENYANG PHARMA UNIVERSITY
Medicinal composition for treating cerebrovascular disease
InactiveCN1823767ALittle side effectsIncrease heart ratePowder deliverySolution deliveryVascular diseaseCoronary artery disease
A composite medicine in the various forms for treating cardiovascular disease such as coronary heat disease, hypertension, etc is prepared from bisoprolol and candesartan in the mole ratio of 1: (1-2).
Owner:BEIJING JINCHENG TAIER PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com