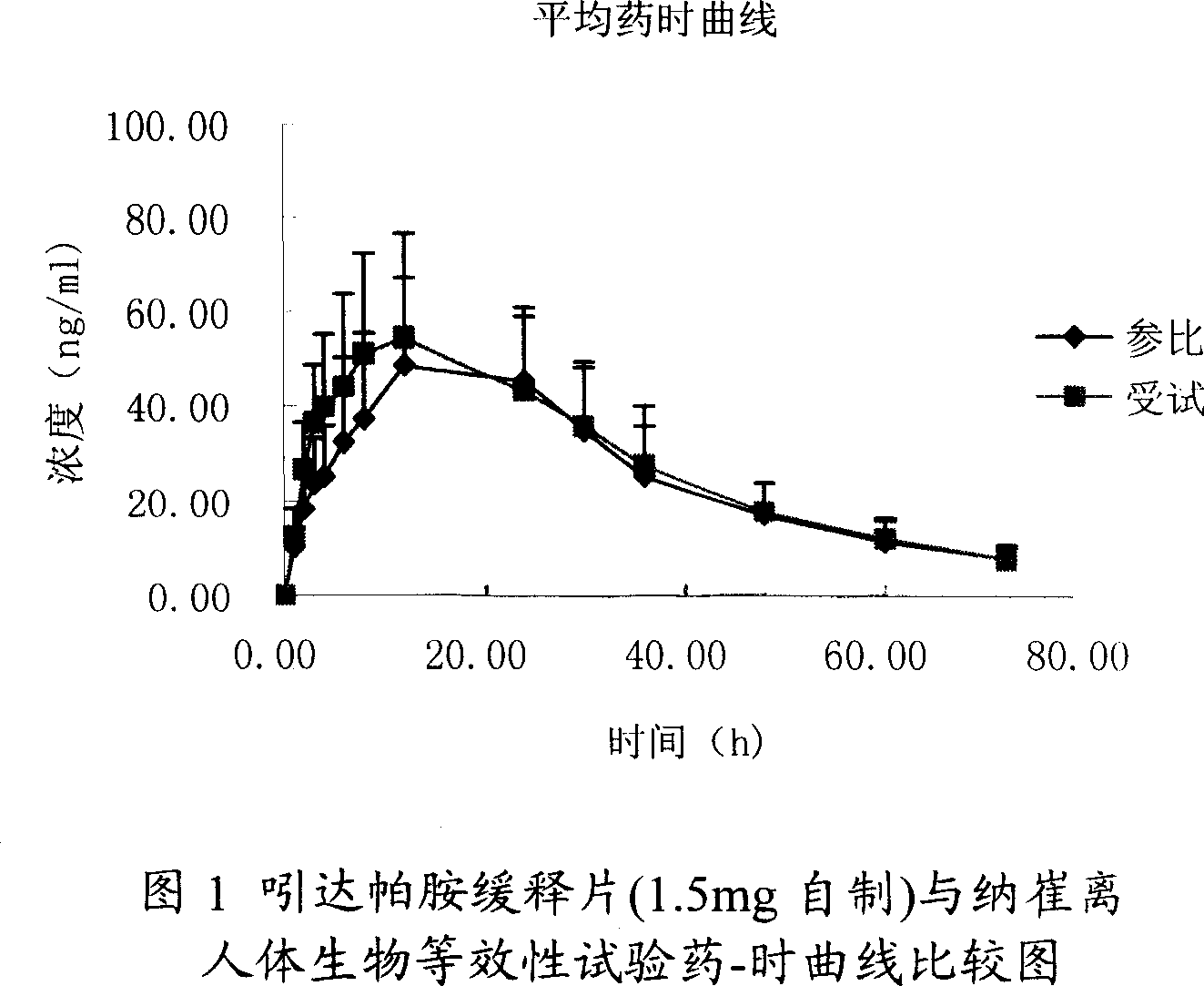
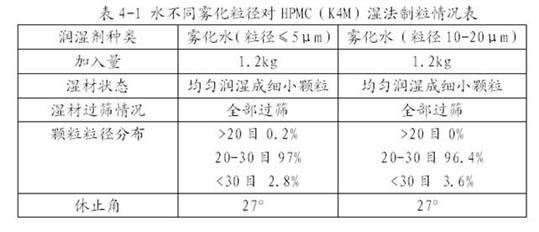
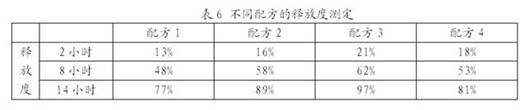
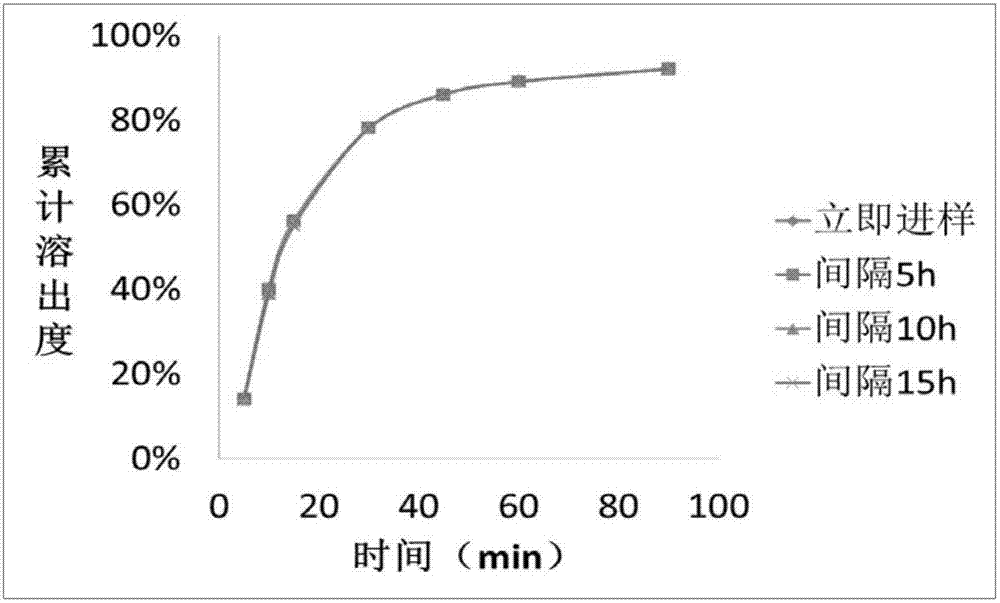
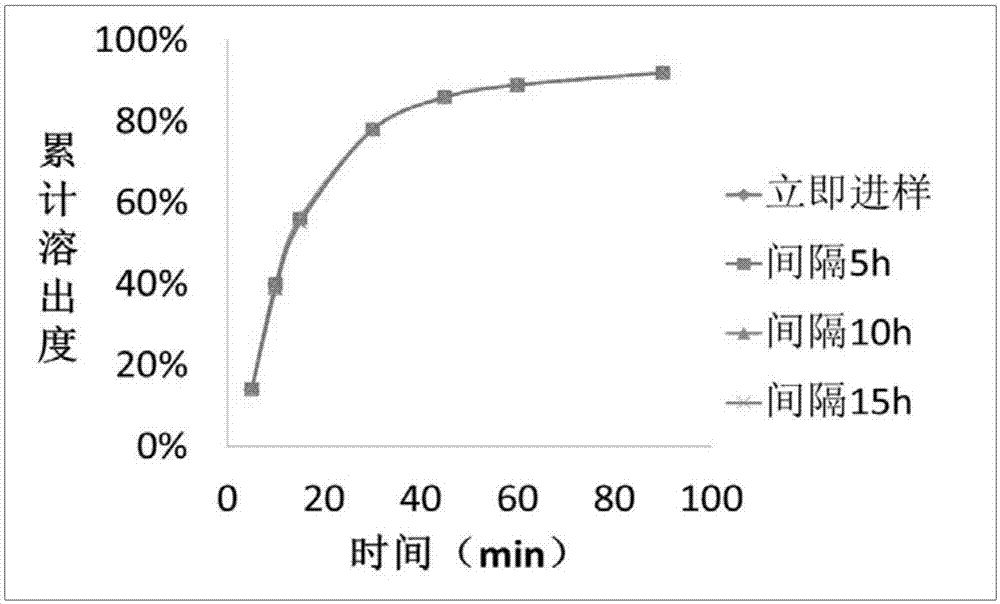
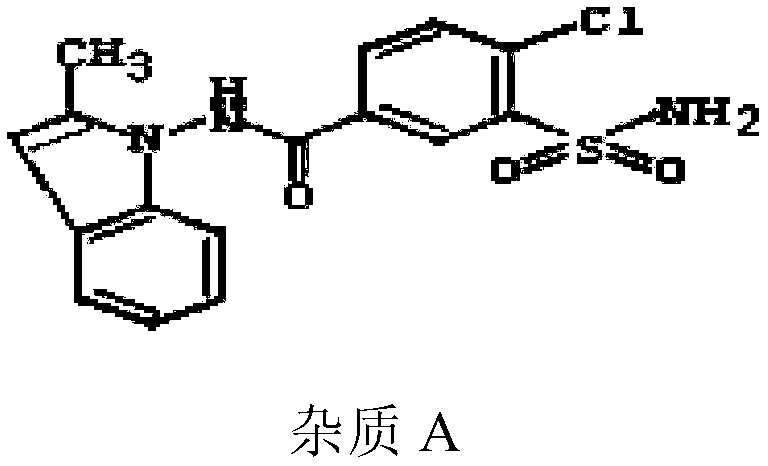
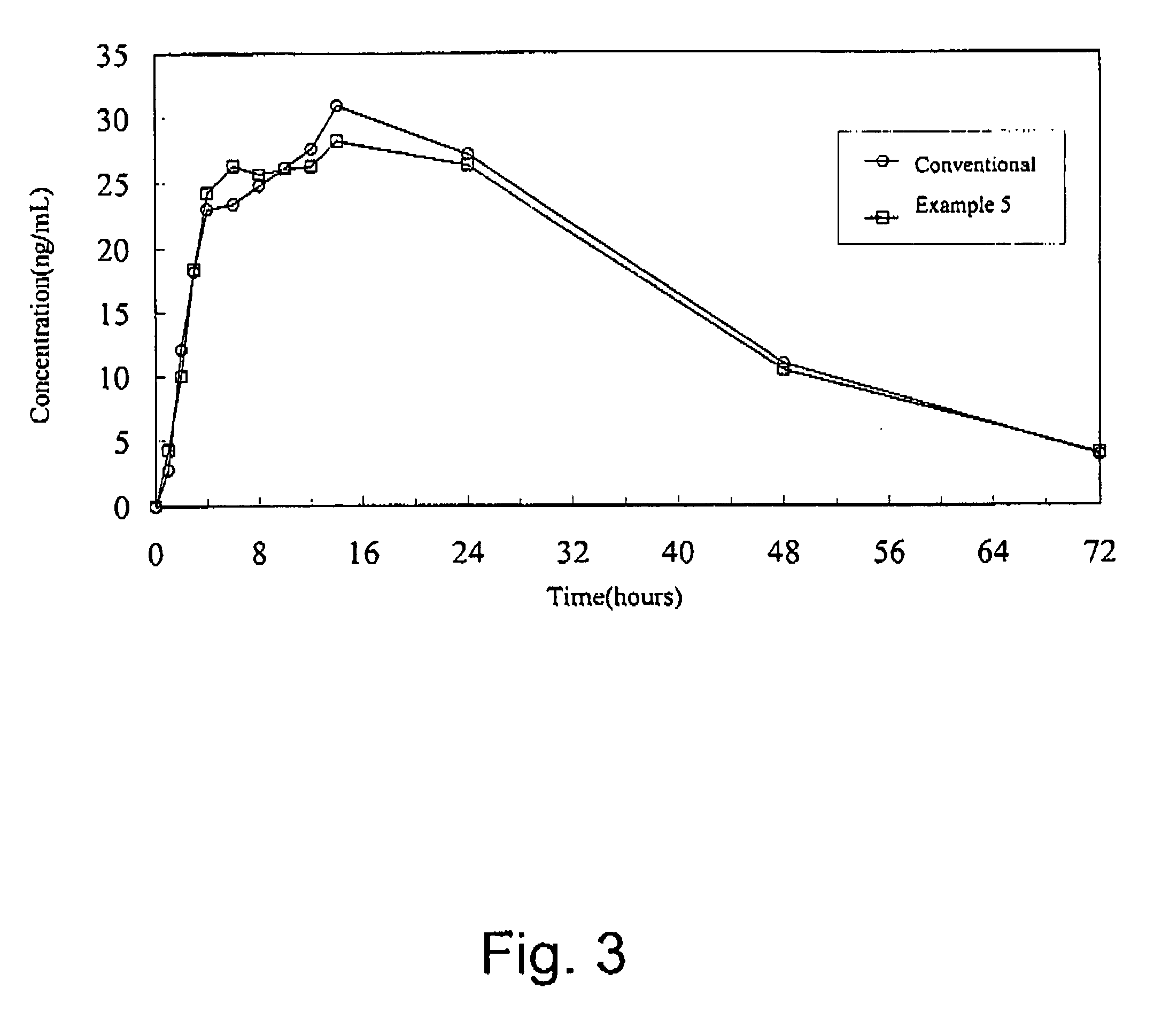
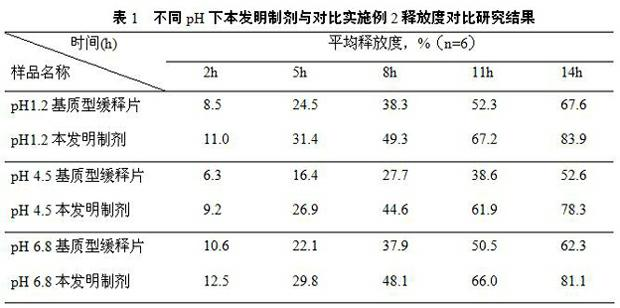
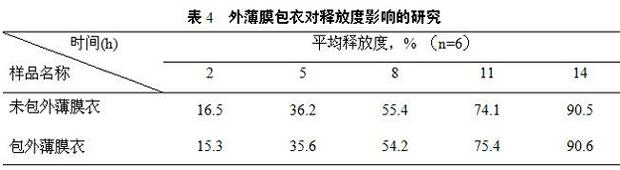
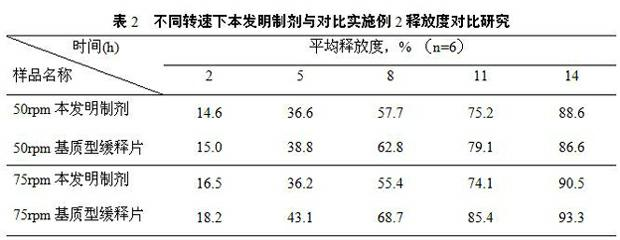
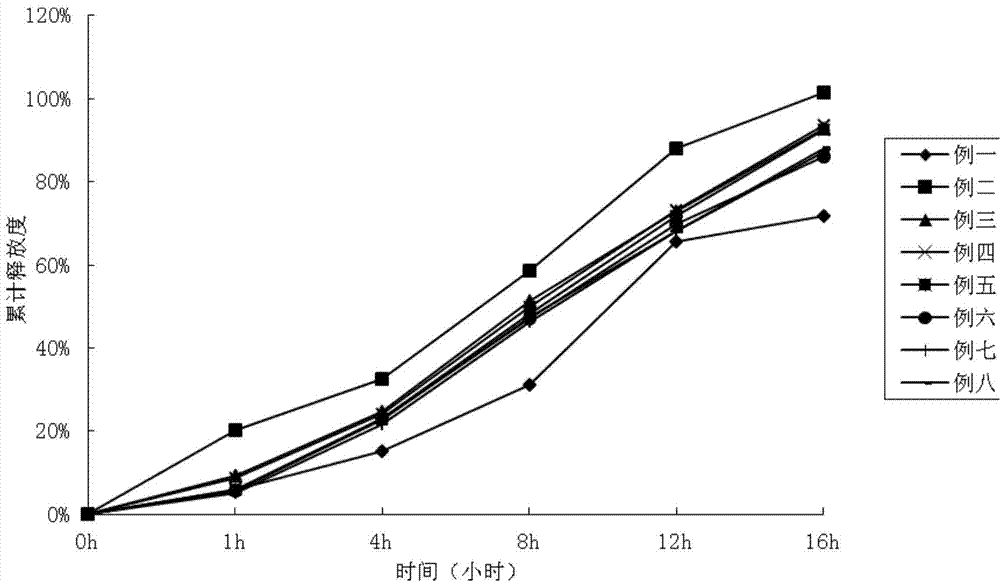
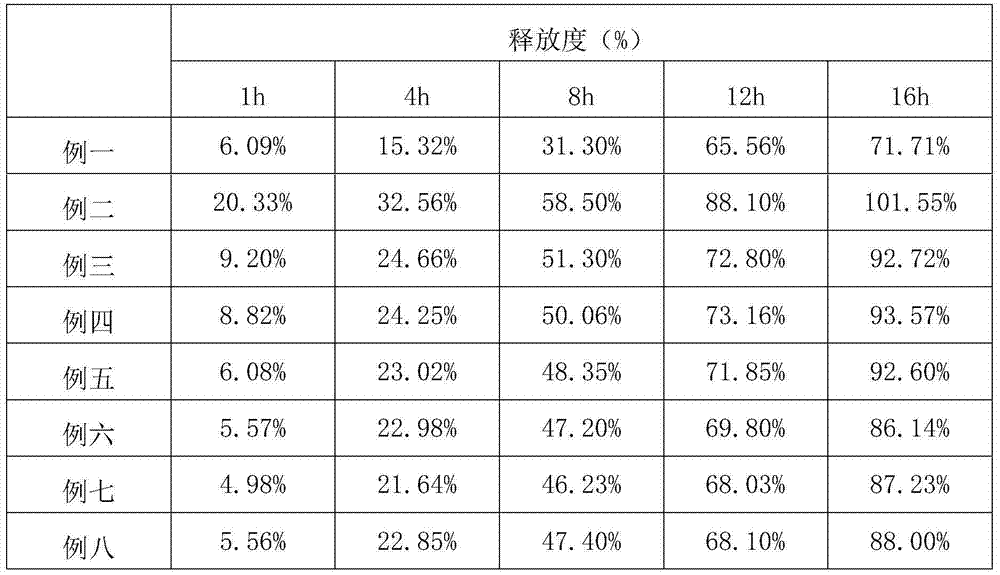
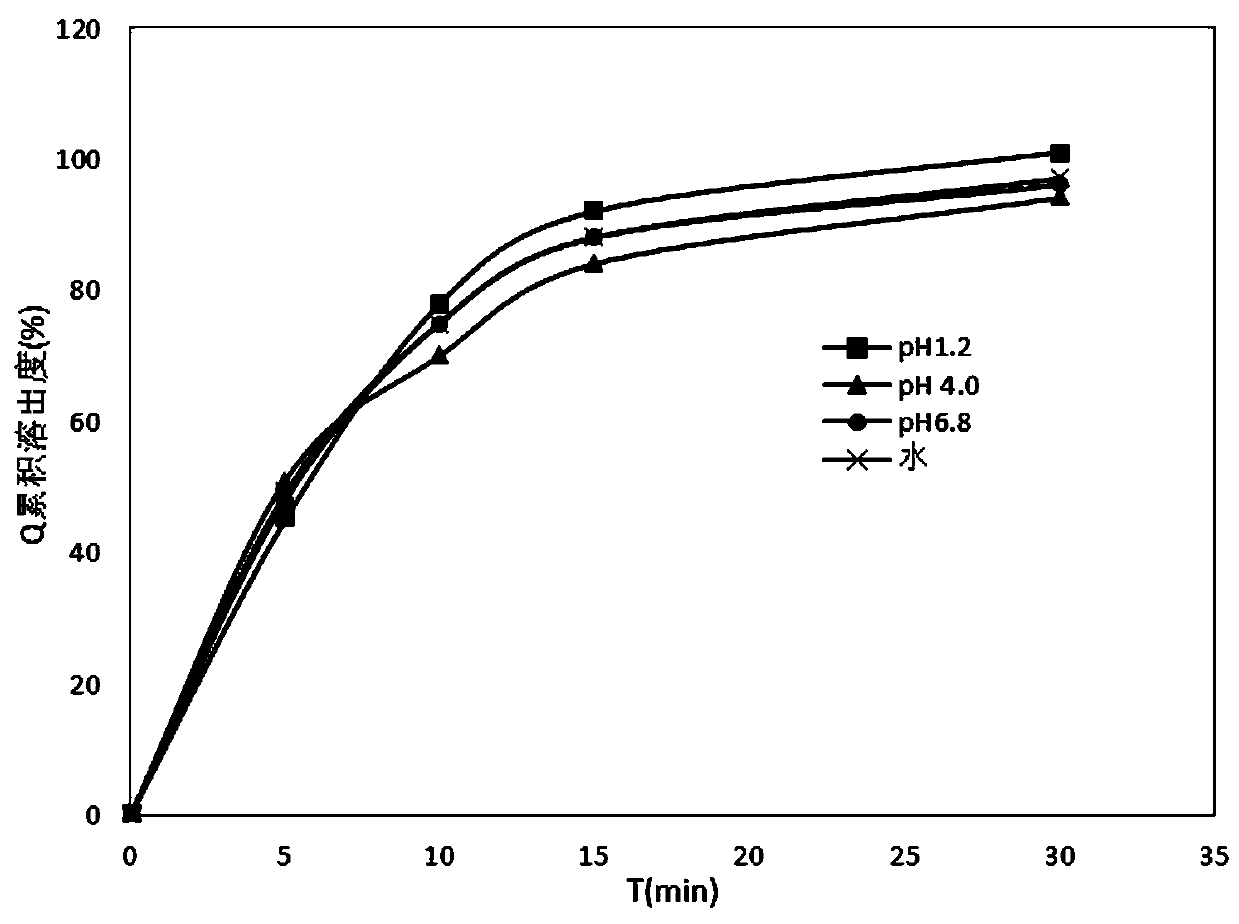
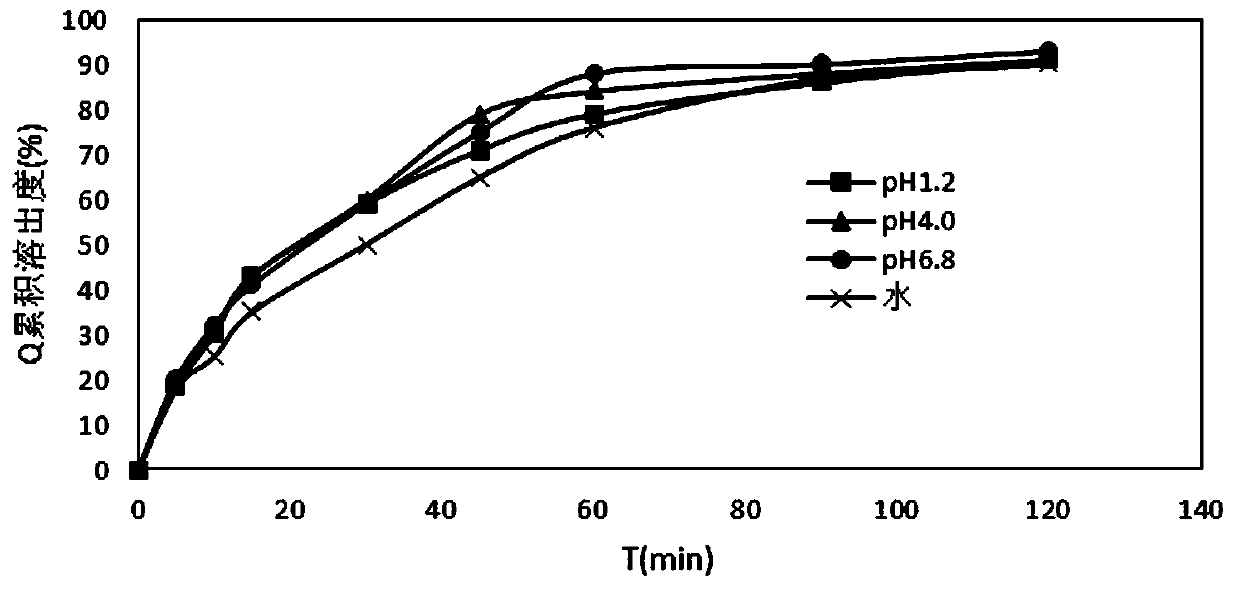
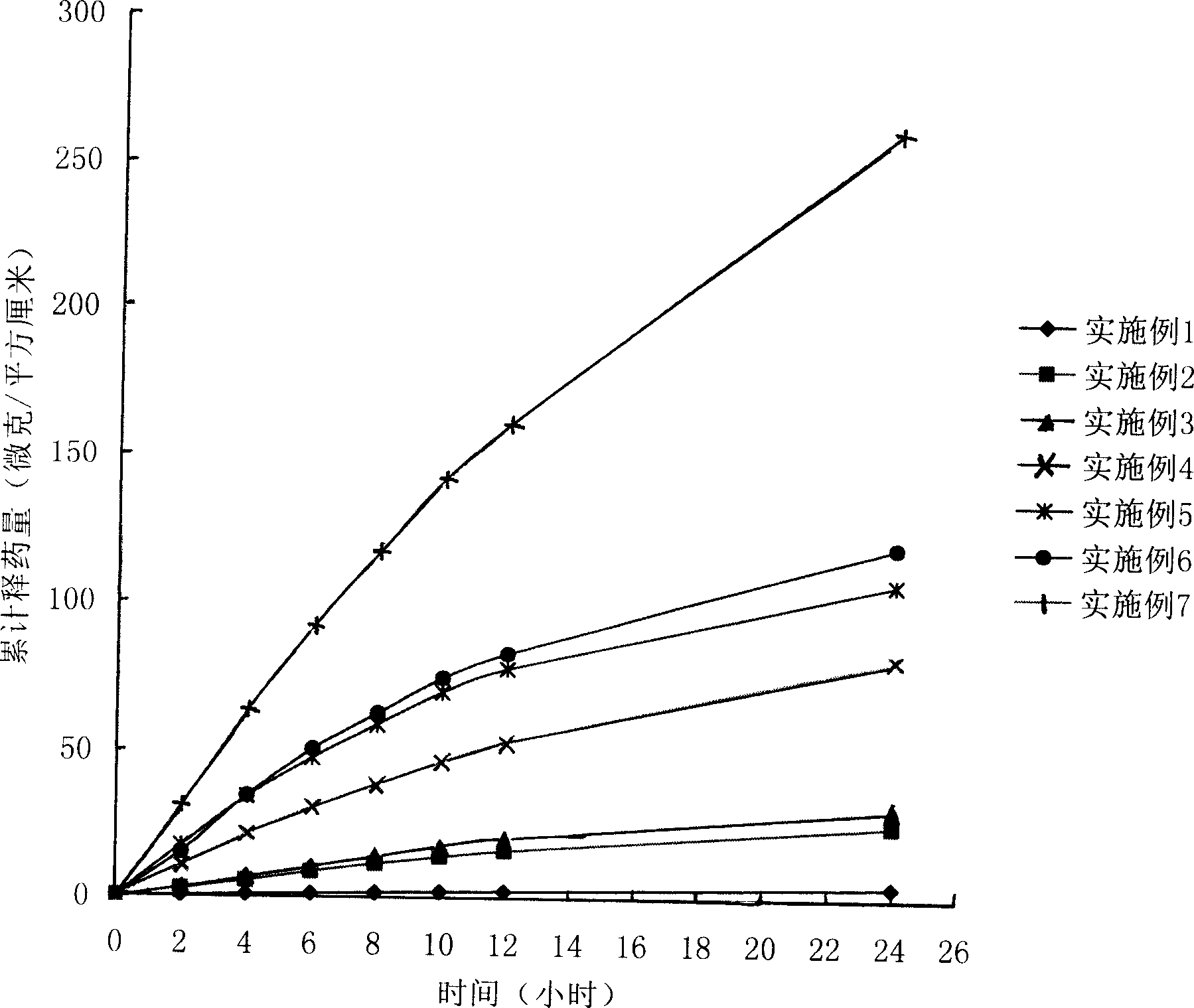
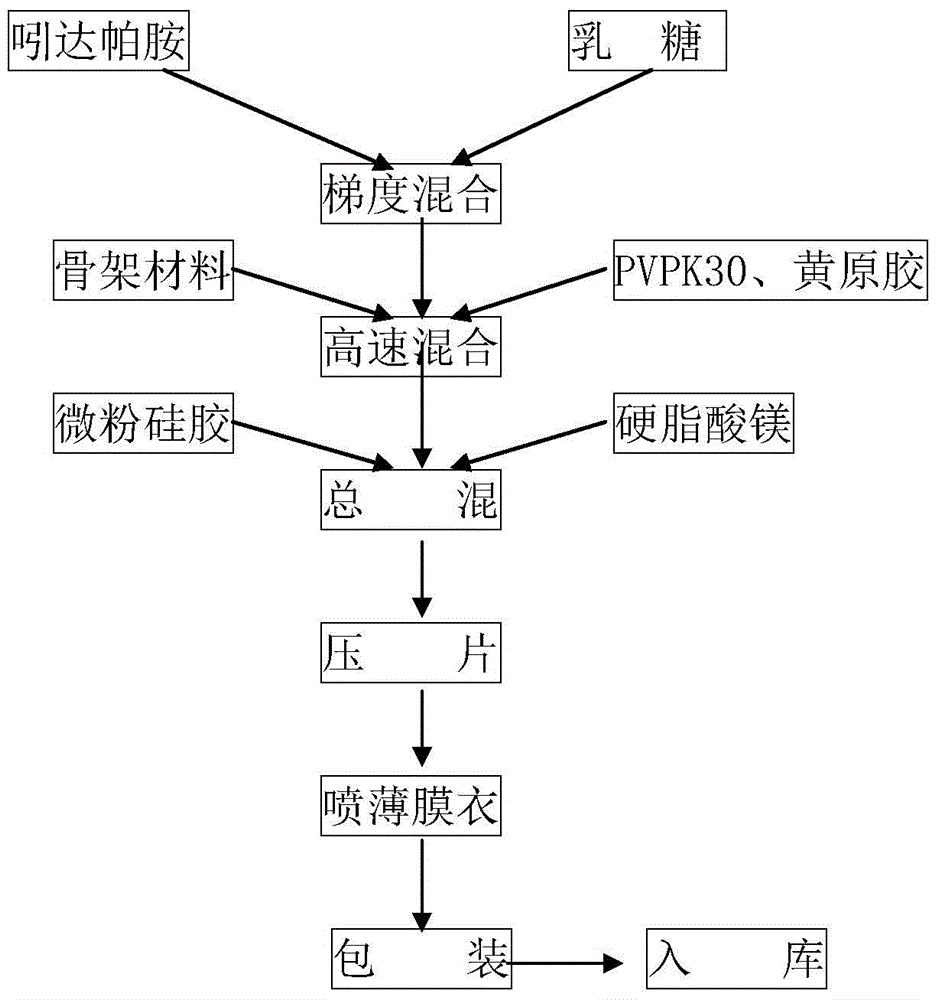
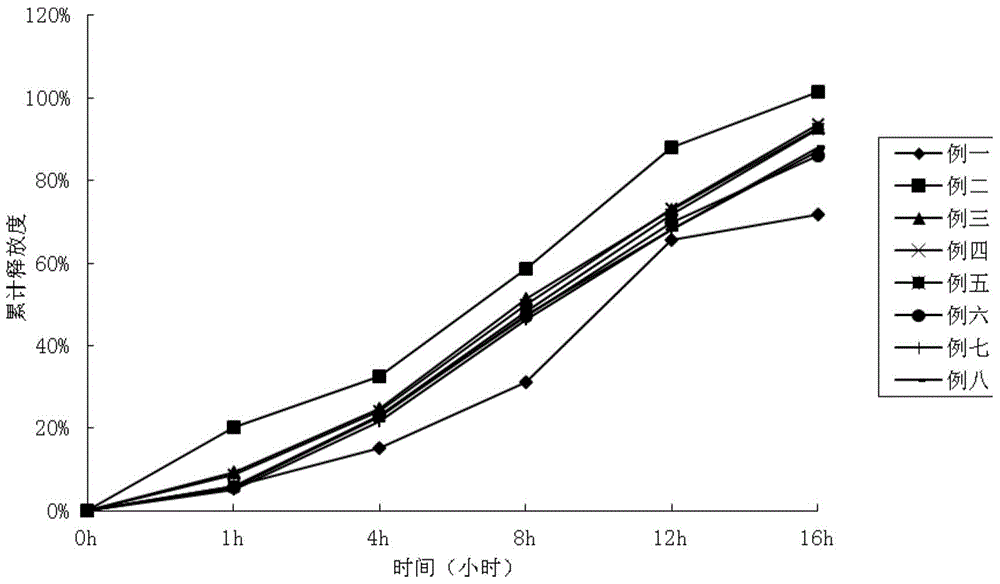
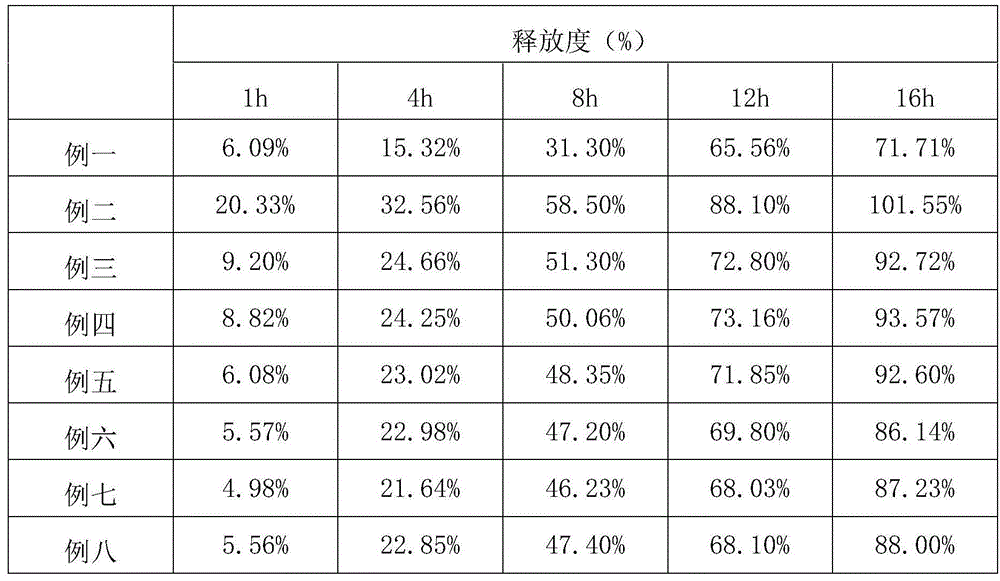
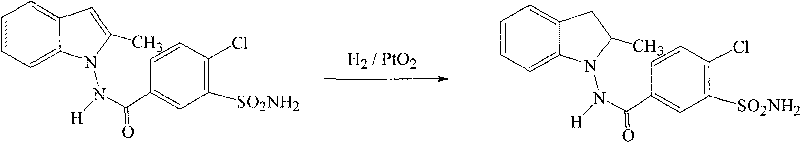Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
98 results about "Indapamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
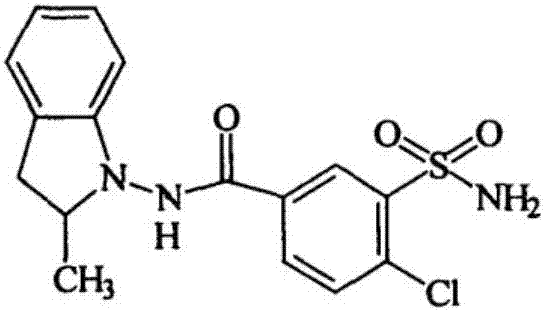
This medication is used to treat high blood pressure. Indapamide is also used to reduce extra fluid in the body (edema) caused by heart failure.
Compositions and methods for treatment of renal disease
InactiveUS20100249081A1Prevent kidney damageReduce riskBiocideOrganic active ingredientsDiseaseGlomerular diseases
The invention relates to methods, compositions, and kits for the treatment of proteinuria and / or hypertension (e.g., proteinuria and / or hypertension arising from primary renal disease (e.g., focal segmental glomerulosclerosis, glomerular disease) or secondary to other conditions (e.g., diabetes, diabetic nephropathy, liver disease). Specifically, the invention relates to methods involving combination therapy wherein an indoline (e.g., indapamide) is administered in combination with an anti-aldosterone agent (e.g., spironolactone and / or epleronone).
Owner:GUPTA AJAY
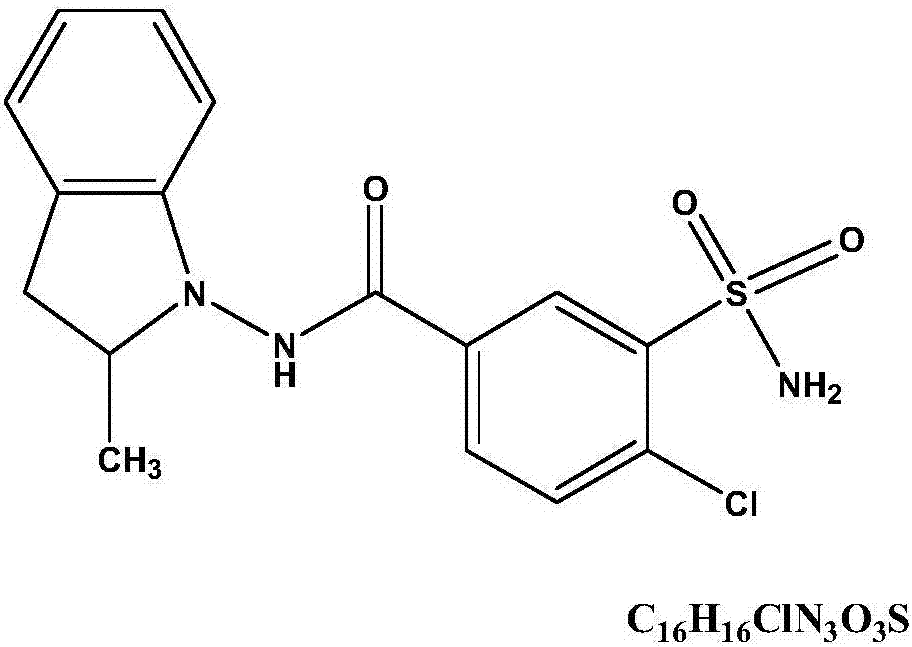
Method for synthesizing indapamide
ActiveCN101717359ACheap and effectiveGood reaction selectivityOrganic active ingredientsOrganic chemistryHydrobromideOrganic base
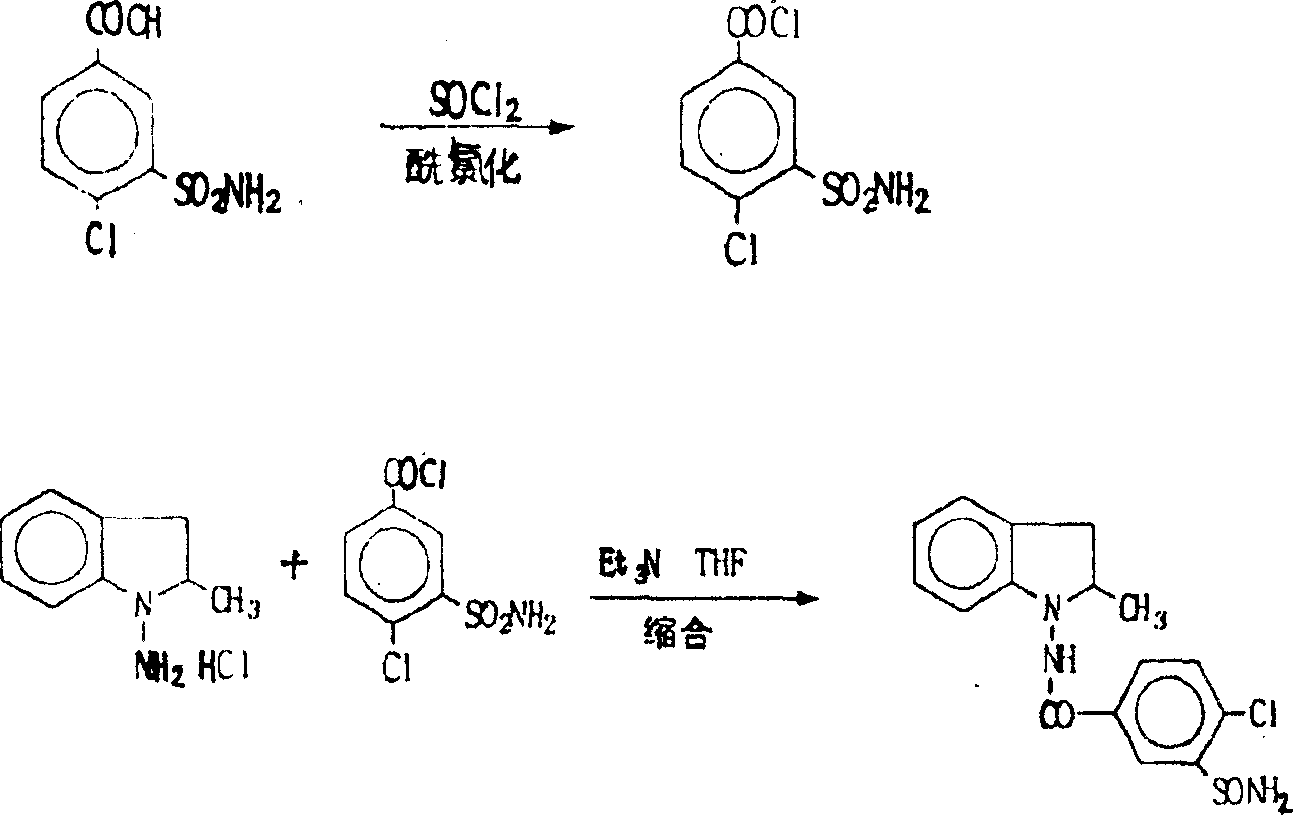
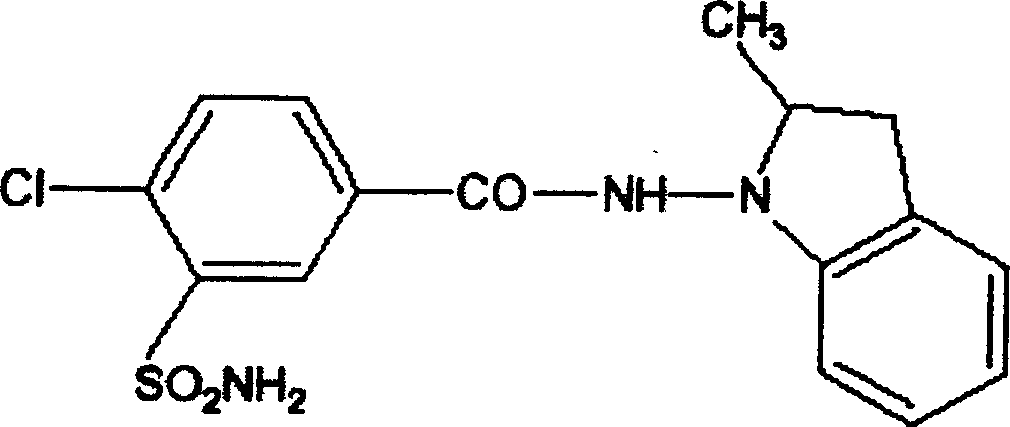
The invention discloses a method for preparing indapamide from chloro-1,3-dimethyl-2-climiqualine as a condensing agent, comprising the following concrete steps of: under the action of a condensing agent of chloro-1,3-dimethyl-2-climiqualine and organic base, reacting 4-chloro-3-sulfamoylbenzoic acid with N-amido-2-methylene indoline or corresponding salts thereof in inert solvent at room temperature and carrying out purification treatment to obtain a target product of the indapamide, wherein the corresponding salts of the 4-chloro-3-sulfamoylbenzoic acid and the N-amido-2-methylene indoline are hydrochloride, hydrobromide, mesylate or paraaminomethyl benzene sulfonate. The invention has the advantages of high yield in the technological process, low cost and simple and convenient operation, and can meet the requirement of large-scale production.
Owner:BEIJING CHENGYU PHARMA
Indapamide slow release tablet and its preparing method
InactiveCN1943564AImprove complianceAvoid side effectsOrganic active ingredientsPill deliveryProlonged-release tabletPotassium
The invention relates to a indapamide retard tablet and its preparation process, said retard tablet comprising indapamide, bone material, filler and lubricant, in each said tablet containing controllable 0.5-1.5mg( each tablet containing 0.15 g. or 150g / 1000 tablet ).
Owner:KANGYA OF NINGXIA PHARMA
Preparation method for slow-release tablet of indapamide-containing medicament
InactiveCN102579382AThe particles are dense and roundLarge specific surface areaOrganic active ingredientsPharmaceutical product form changeProlonged-release tabletMethyl cellulose
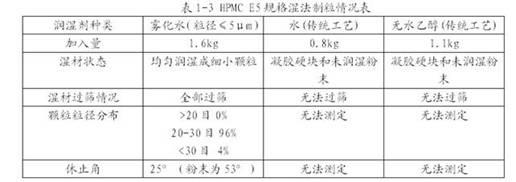
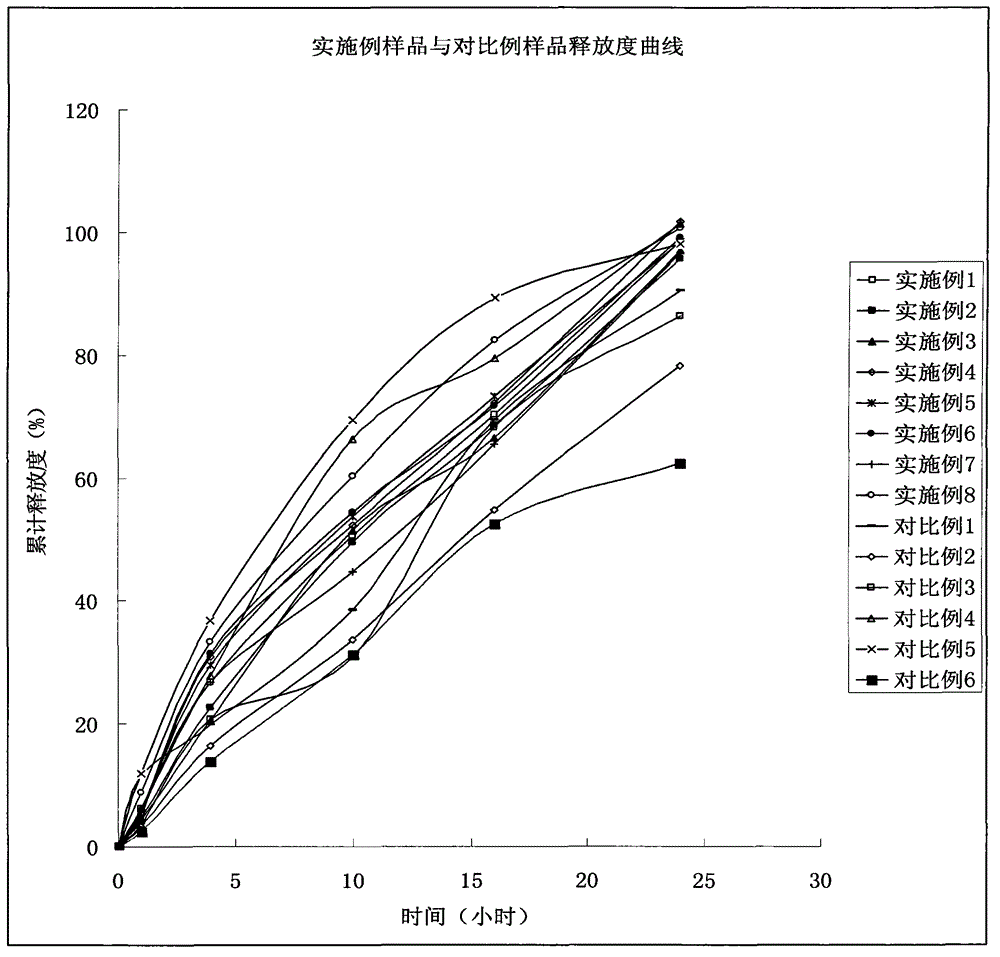
The invention aims to provide a preparation method for a slow-release tablet of an indapamide-containing medicament. In the method, water is taken as a wetting agent for performing HPMC (Hydroxy Propyl Methyl Cellulose) wet granulation, and the prepared HPMC particles are compact and regular, are porous on the surface and have high liquidity. HPMC particles and a small amount of indapamide can be uniformly mixed by adopting the conventional mixing way, so that preparation of the slow-release tablet is finished. A plurality of other auxiliary materials are not required to be added additionally, an organic solvent is not required for granulating, the slow release controlling effect on the releasing degree of the prepared tablet is good, and granulation can be completed by adopting the conventional granulation equipment.
Owner:CHONGQING KERUI PHARMA GRP
Synthesis method of indapamide
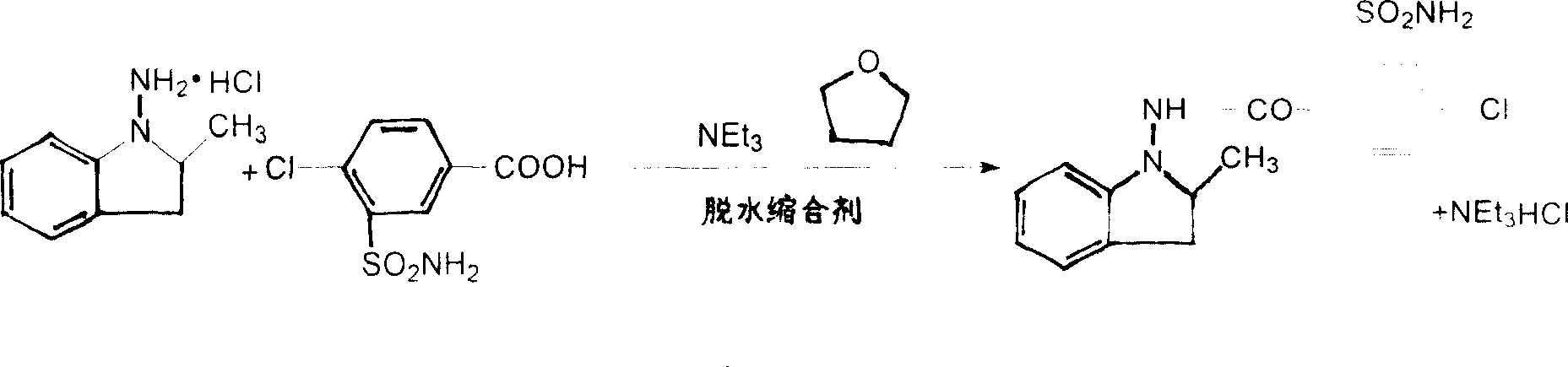
The present invention relates to Indapamide synthesizing process. The synthesis process includes the following steps: dissolving N-amido-2-methyl indoline hydrochloride in non-protonic organic solvent, and dropping triethylamine via stirring; the subsequent adding 4-chloro-3-sulphonylamino -benzoic acid and dewatering condensing agent and reaction at 0-20 deg.c for 2.0-20 hr to obtain coarse Indapamide product; and final refining in isopropanol-water to obtain Indapamide product. Compared with traditional process, the present invention has raised safety, less pollution and corrosion to environment and apparatus, lowered cost and raised yield.
Owner:TIANJIN LISHENG PHARM CO LTD
Indapamide sustained-release drug composite and preparation method thereof
InactiveCN103142529AAvoid sudden releaseRelease stabilityOrganic active ingredientsPharmaceutical delivery mechanismDrug release rateAdhesive
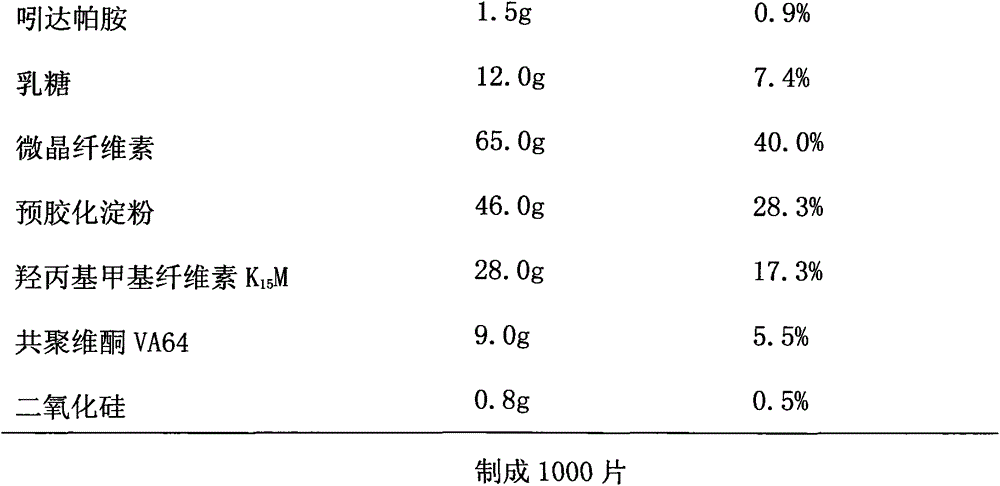
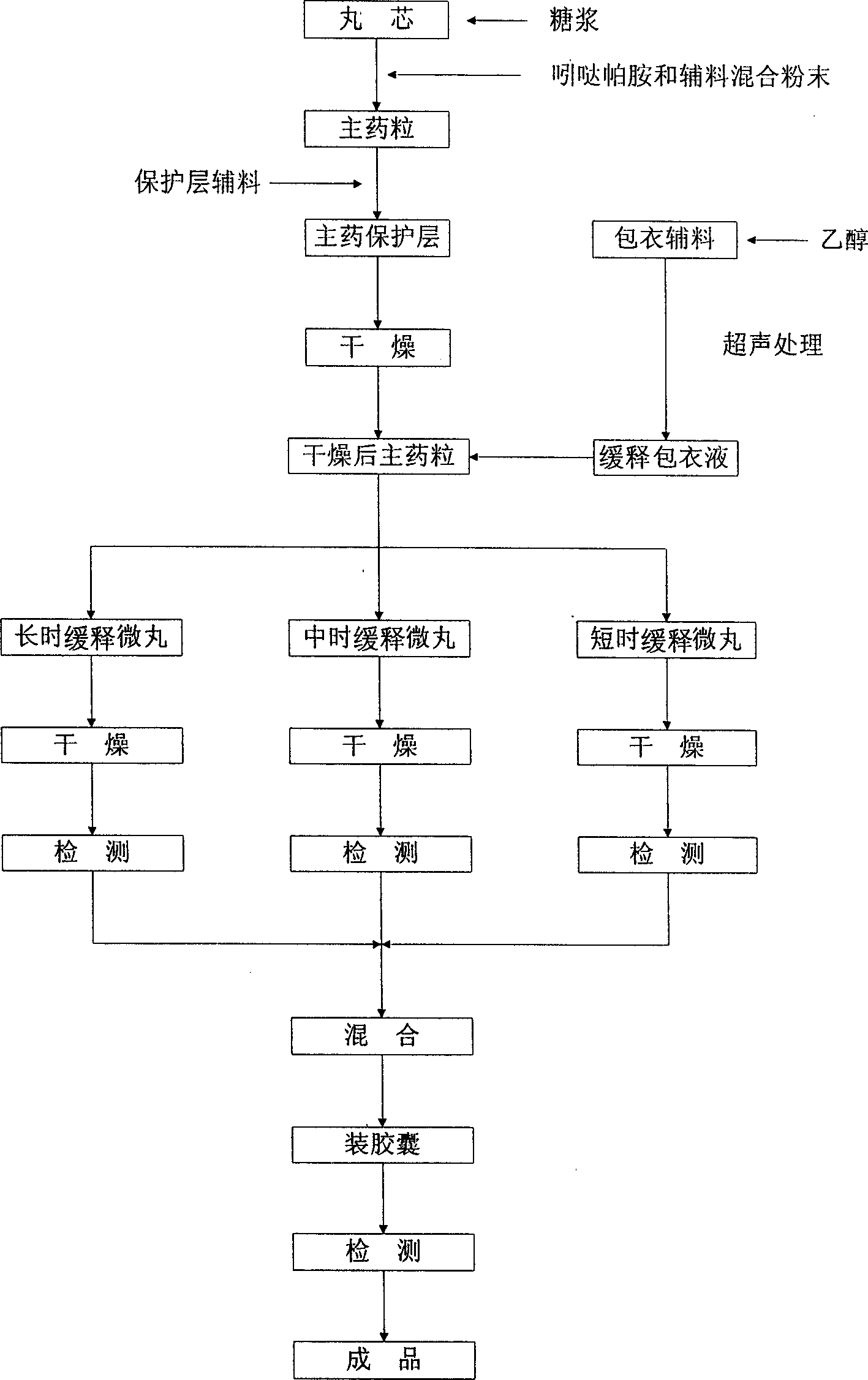
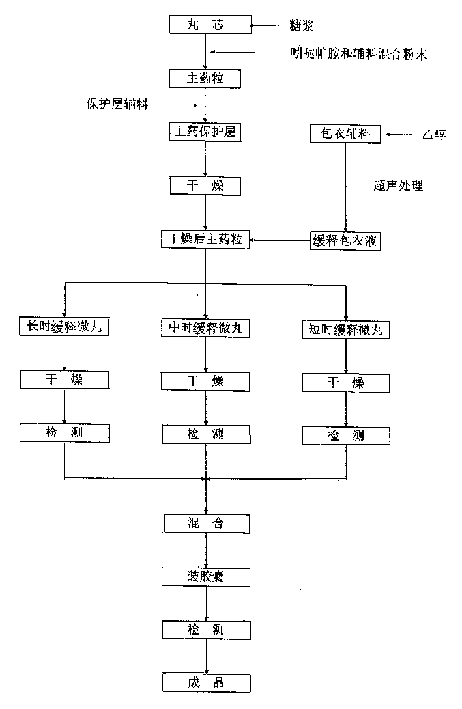
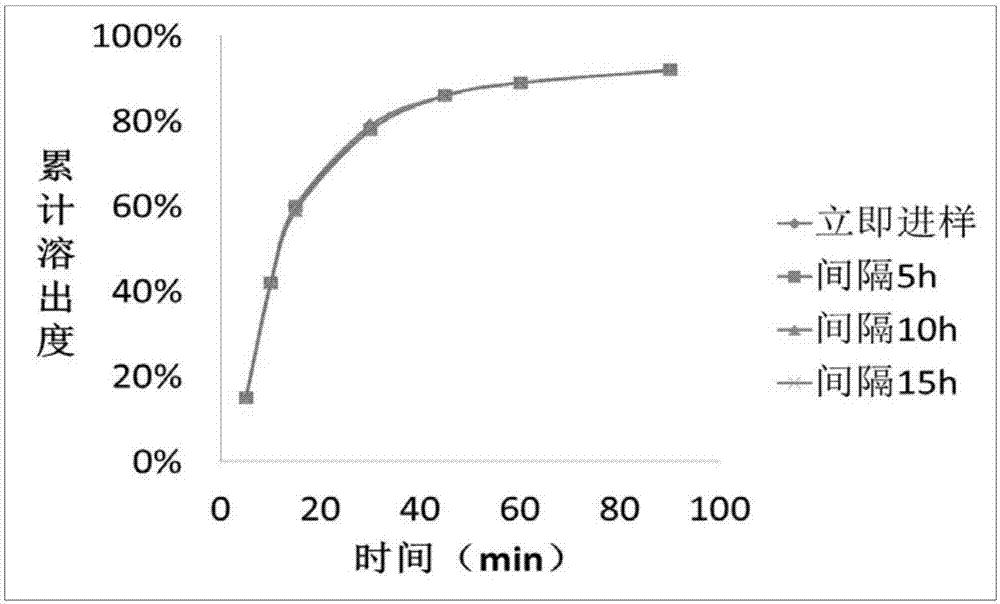
The invention discloses an indapamide sustained-release drug composite and a preparation method thereof. The indapamide sustained-release drug composite comprises the following ingredients: indapamide serving as an active ingredient and a proper amount of filler, framework material, lubricant and copovidone (VA64). The indapamide sustained-release tablet disclosed by the invention is used for treating primary hypertension and is characterized in that the VA64 is added in the prescription so that the burst release of the drug is prevented, the stable release of the drug is guaranteed and the hypokalemia caused by overhigh blood concentration is avoided; in the process, the indapamide is micronized to below 50 micrometers so that the release of the drug is improved and the delayed release of the drug is avoided; and the direct powder compression process is adopted so that the problems that the framework material coheres to form sticky balls due to adhesive and the homogeneity of drug releasing rate is influenced are avoided. The prepared indapamide sustained-release tablet has the advantages that the drug releasing rate is stable; the homogeneity of the releasing rate is good; the drug bioavailability is increased; and the drug quality is guaranteed.
Owner:KANGYA OF NINGXIA PHARMA
Indapamide slowly-releasing tablet
InactiveCN1394604ATake a small doseLittle side effectsOrganic active ingredientsPharmaceutical delivery mechanismAlcoholPEG 400
The indapamide slow-released tablet includes tablet core and coating, in which the tablet core is formed from indapamide, slow-releasing material, pore-forming agent, filling agent and lubricating agent in the ratio of 1.5g:40-150g:20-100g:10-100g:1-10g, and the coating is formed from film-forming amterial, antisticking agent, masking agent, polyethylene glycol 400, distrilled water and ethyl alcohol. The above-mentioned film-forming material, antisticking agent, masking agent, polyethylene glycol 400 and distilled water according to the ratio of 3-20g:2-15g:1-15g:5ml:140ml are added into the ethyl alcohol, and diluted with ethyl alcohol to 500ml to obtain the coating solution, then the outer layer of the tablet can be coated with said coating solution and dried so as to obtain the invented indapamide slow-release tablet.
Owner:严洁
Slow-released indapamide capsule and its prepn process
InactiveCN1468598ALittle side effectsFully absorbedOrganic active ingredientsPharmaceutical delivery mechanismSide effectCellulose acetate
The slow-released Indapamide capsule consists of Indapamide, slow-releasing material and supplementary material in the ratio of 0.5-3.0 to 5-20 to 131-165. The slow-releasing material is one to four of polyacrylic resin, hydroxypropyl methyl cellulose, ethyl cellulose, acetyl phthalate cellulose and PVP, and each capsule contains Indapamide in 0.5-3.0 mg. The slow-released Indapamide capsule may be prepared via rolling process. The preparation form of the present invention has lasting effective time, less side effect and high biological utilization, and through the different combination of the micro pellet, ideal release rate may be reached.
Owner:TIANJIN GUOYAO BOHAI BIOMEDICAL
Method for improving stability of indapamide tablets in acidic solution
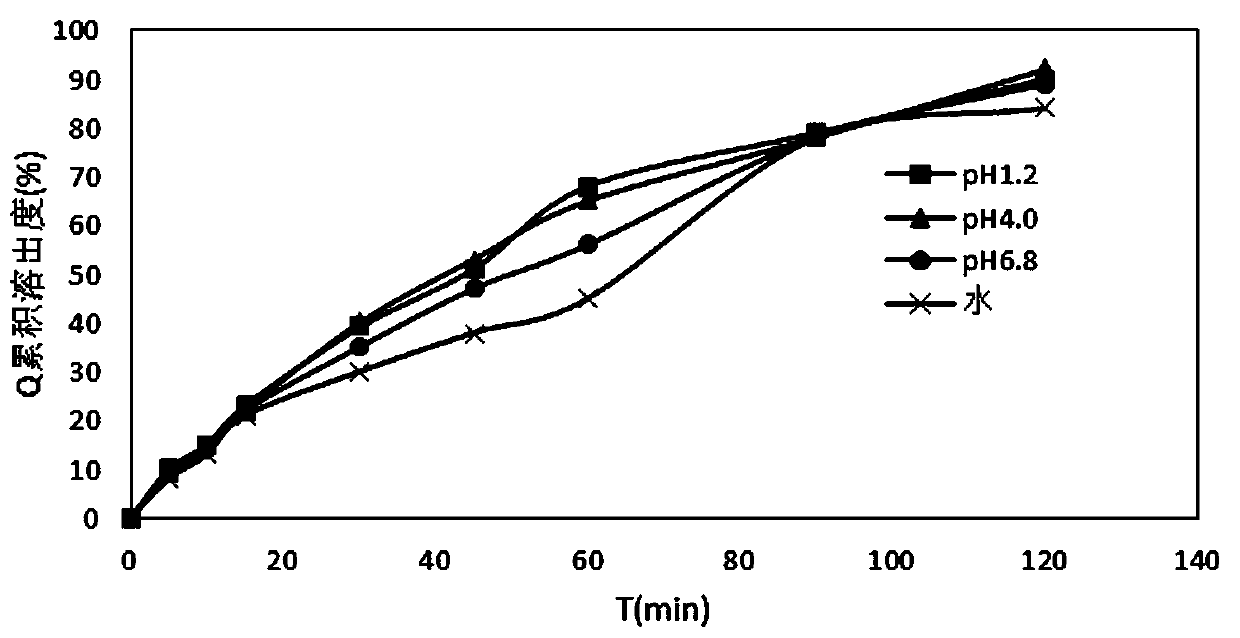
The invention discloses an acidic solution capable of improving the stability of indapamide tablets. The acidic solution is characterized by being a hydrochloric acid solution. The acidic solution also can contain vitamin C. After the vitamin C is added into the acidic solution, the stability of indapamide tablets is improved unexpectedly and remarkably, adverse impact on the dissolution rate of the indapamide tablets in the acid solution, for example the dissolution rate of the indapamide tablets in the acidic solution is not remarkably increased, is avoided, and the subsequent measurement of the dissolution rate of the indapamide tablets in is benefited.
Owner:GRAND PHARM (CHINA) CO LTD
Indapamide tablet and preparation method thereof
InactiveCN108653219AEasy to controlGuarantee the quality of preparationsOrganic active ingredientsPharmaceutical non-active ingredientsMedicineAdhesive
The invention discloses an indapamide tablet and a preparation method thereof. The indapamide tablet comprises a tablet core using indapamide as an active component and a coating layer, wherein the coating layer is formed at 36-45 DEG C; the tablet core is prepared from the following components in parts by weight: 1 part of the indapamide, 20-30 parts of a filling agent, 1-5 parts of an adhesive and 5-10 parts of a lubricating agent. Through control on the temperature of the coating, the impurity content of the indapamide tablet is obviously controlled, so that the quality of the preparation is guaranteed.
Owner:GRAND PHARM (CHINA) CO LTD
Indapamide tablet and preparation method thereof
InactiveCN107982235ARaise quality standardsDissolution rate is slowOrganic active ingredientsPill deliverySlurryDissolution
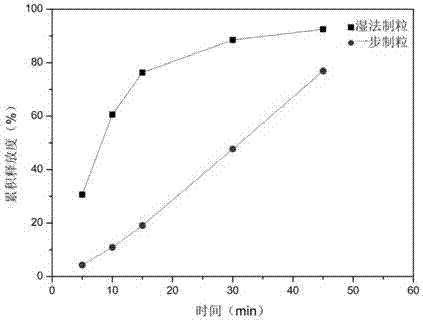
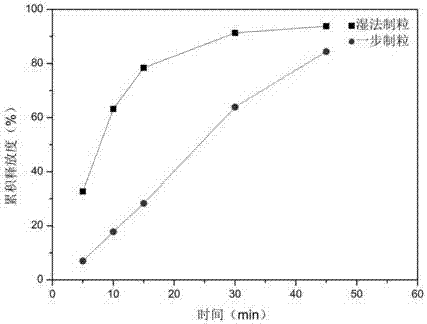
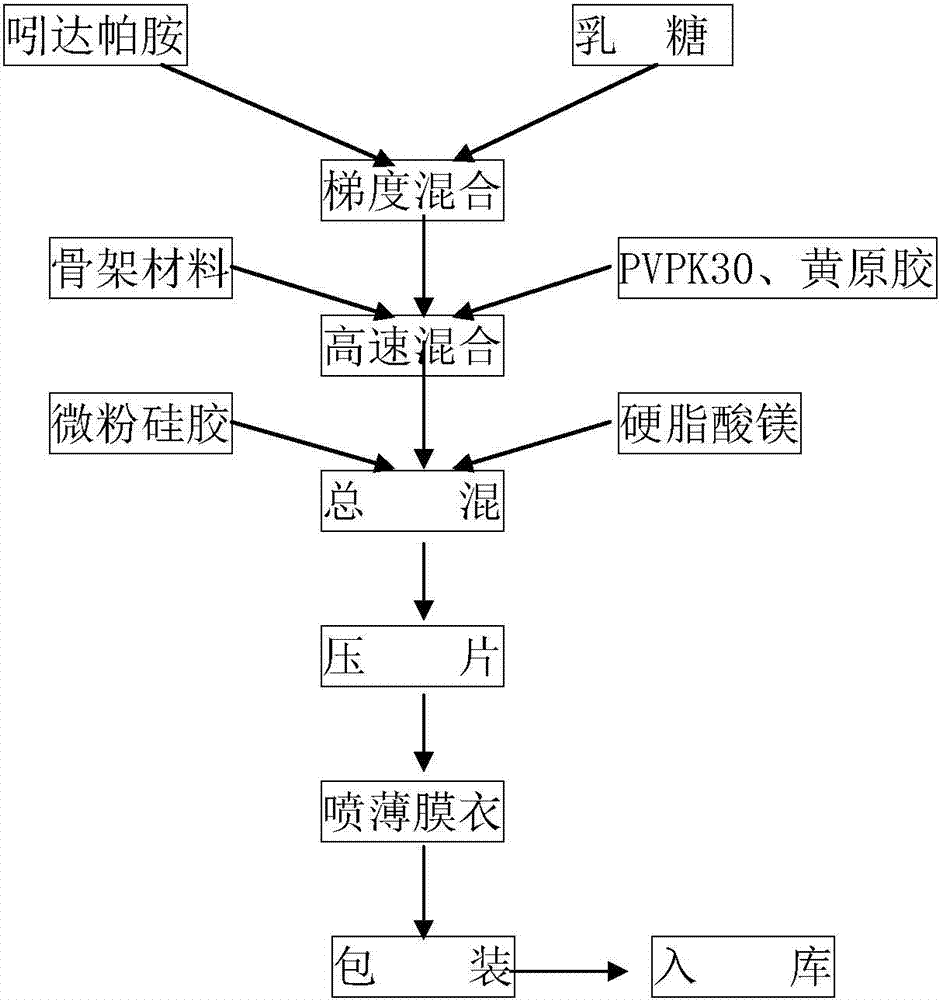
The invention provides an indapamide tablet. The indapamide tablet comprises the following components in parts by weight: 1% to 5% of indapamide, 50% to 75% of milk sugar, 10% to 35% of starch, 4% to8% of povidone, 2% to 5% of talcum powder and 0.2% to 1.5% of magnesium stearate, and the adhesive is a mixed solution of starch slurry and PVP-K30. The dissolution of the indapamide tablet is slower.The invention further provides a preparation method of the indapamide tablet. According to the preparation method, the indapamide tablet is palletized and prepared by adopting a one-step method, andthe preparation method is cleaner and more efficient.
Owner:GRAND PHARM (CHINA) CO LTD
Novel anti-hypertension compound slow release tablets and production process thereof
InactiveCN106924712AOrganic active ingredientsDipeptide ingredientsAnti hypertensionPinoresinol diglucoside
The invention relates to novel medical anti-hypertension compound slow release tablets. The tablets are mainly prepared from four bioactive components of 5 mg-20 mg of quercetin, 5 mg-20 mg of pinoresinol diglucoside (effective ingredient of the bark of eucommia), 0.4 mg-1.60 mg of sulfonamide diuretic indapamide and 2 mg-10 mg of angiotensin reductase II inhibitor (ACEI) perindopril salt and pharmaceutical adjuvants of 20 mg-60 mg of PVP-k30, 10 mg-30 mg of hydroxyl propyl cellulose (HPMC-K15M), 30 mg-60 mg of lactose, 53 mg-80 mg of microcrystalline cellulose (MCC), 1 mg-2 mg of magnesium stearate (lubricating agent), 2 mg-4 mg of low hydroxypropyl cellulose (LHPC, disintegrating agent), 1 mg-20 mg of starch and 1 mg-10 mg of talcum powder. The novel anti-hypertension compound slow release tablets provide a safe and effective medicine help for adjusting and stabilizing blood pressure of patients, improving heart functions, relieving heart and cerebral vessel lesions and avoiding occurrence of other cardiovascular disease risks.
Owner:SHANGHAI PHARMA DONGYING JIANGSU PHARMA CO LTD
Oral sustained-release pharmaceutical composition of indapamide, production and use thereof
InactiveUS20060182803A1Stable concentrationEliminate side effectsPill deliveryPharmacologyIndapamide
The present invention relates to an oral sustained-release pharmaceutical composition of indapamide and a process for producing the foregoing pharmaceutical composition. The pharmaceutical composition comprises indapamide in the amount 0.2% to 4% (w / w) of the composition, a hydrophilic polymer in the amount 10% to 30% (w / w) of the composition, a dry binding agent in the amount 2% to 20% (w / w) of the composition, and an erosion modifier in the amount 40% to 80% (w / w) of the composition. The present invention also relates to a method for delivering indapamide to a patient in need of indapamide-related therapies, which comprises administering to the patient a therapeutically effective amount of a sustained-release pharmaceutical composition in accordance with the present invention.
Owner:STANDARD CHEM & PHARMA
Indapamide osmotic pump preparation and preparation method thereof
ActiveCN102429886AImprove securityImprove effectivenessOrganic active ingredientsPharmaceutical delivery mechanismRelease modulatorProlonged-release tablet
The invention discloses an indapamide monolayer osmotic pump sustained-release tablet, which comprises a tablet core and a controlled-release film coating layer, wherein the tablet core contains 0.5-2% of indapamide as a monarch drug, 1-20% of filling agent, 50-90% of penetration enhancer, 1-15% of adhesive, 1-20% of release regulator and 0.5-3% of lubricant; the controlled-release film coating layer comprises a controlled-release film forming material, a plasticizer and / or a pore-forming agent; and the filling agent comprises agar powder.
Owner:HEFEI LIFEON PHARMA
Stable storage indapamide sustained release tablet and preparing process thereof
InactiveCN107951854AOrganic active ingredientsPharmaceutical non-active ingredientsSustained Release TabletMedicine
The invention discloses a stable storage indapamide sustained release tablet. The sustained release tablet comprises indapamide used as the active ingredient, an appropriate amount of filling agent, asustained-release material and a lubricating agent, and direct powder compression process is adopted. The stable storage indapamide sustained release tablet is characterized in that the ingredients of suitable kinds and the preparation process are selected, so that the genotoxicity impurity (2RS)-2-methyl-1-nitroso-2,3-dihydro-1H-indol is controlled at 20 ppm or below. The stable storage indapamide sustained release tablet can be produced in the mode of saving time and production cost.
Owner:南京易亨制药有限公司
Medicinal composition of indapamide and folic acid and application of medicinal composition
InactiveCN104224788ALower levelImprove the level ofOrganic active ingredientsMetabolism disorderDiseasePlasma homocysteine
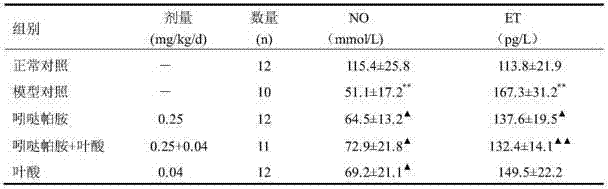
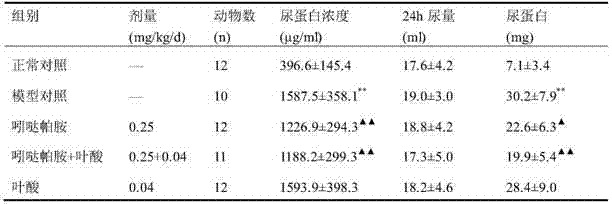
The invention relates to a medicinal composition prepared from indapamide, folic acid and a medicinally available carrier, and application of the composition for preparing a drug for treating hypertension with hyperhomocysteinemia and a disease of homocysteine rising. In the composition, the preferable content of indapamide is 0.625-2.5 mg, and the preferable content of folic acid is 0.4-0.8 mg. The medicinal composition disclosed by the invention has the benefits that indapamide and folic acid can be combined to reduce blood pressure, obviously lower level of homocysteine and raise the level of folic acid, so as to relieve damage of homocysteine to the blood vessel, strengthen targeted organ protection for a hypertension patient, and play a pharmacologic synergistic role. In addition, the medicinal composition can further allow the patient to take medicine conveniently and reduce the medical costs.
Owner:SHENZHEN AUSA PHARMA +1
Process for preparing indapamide sustained-release agent
ActiveCN104324013AImprove uniformityAvoid quality impactOrganic active ingredientsPharmaceutical delivery mechanismMedicineLactose
The invention relates to a method for preparing an indapamide sustained-release agent, and aims to enable the prepared indapamide sustained-release agent to have a better performance. The preparation process comprises the following steps: a, preparing the following materials in proportion: 0.5-2 percent of indapamide, 30-50 percent of a frame material, 14-28 percent of 100-mesh lactose, 20-35 percent of 200-mesh lactose, 10-20 percent of povidone K30 and 0.2-2 percent of lubricant; b, mixing the indapamide raw material and the lactose with two finenesses according to a gradient increasing method; c, mixing with the frame material and the povidone K30; d, adding the lubricant, and uniformly mixing; and e, tabletting. According to the method, a process of directly tabletting powder is adopted, so that the method has the advantages that the main drug is uniformly distributed in the tablet, the tablet has good uniformity (the uniformity index of a tablet with small content is needed to be controlled), influence of water and heating dryness caused by a traditional manufacture process on the quality of the main drug can be avoided in the manufacturing process, the production energy consumption can be reduced, and the production efficiency can be improved.
Owner:HUANGSHAN C KING PHARMA
Indapamide tablet and preparation method thereof
InactiveCN110538160ASolve the speed problemQuality improvementOrganic active ingredientsDrageesAdhesivePolyvinyl alcohol
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to an indapamide tablet and a preparation method thereof. The indapamide tablet is composed of a tablet core and a coating layer, and the tablet core is prepared from the following components in parts by weight: 0.5-2 parts of indapamide, 10-40 parts of a filler, 0.5-5 parts of an adhesive, and 0.5-10 parts of a lubricant, wherein the filler is any one or a combination of two or more of lactose anhydrous, lactose monohydrate, corn starch, microcrystalline cellulose lactose and starch lactose,the adhesive is any one or a composition of two or more of povidone K30, hydroxypropyl methylcellulose, polyvinyl alcohol and carboxymethylcellulose, and the lubricant is any one or a composition of two or more of talcum powder, magnesium stearate and silicon dioxide. The obtained indapamide tablet is a stable drug preparation which is lower in impurity content than a reference listed drug reference preparation, and has the bioequivalence.
Owner:TIANJIN PHARMA GROUP XINZHENG
Preparation technology of indapamide sustained-release tablets
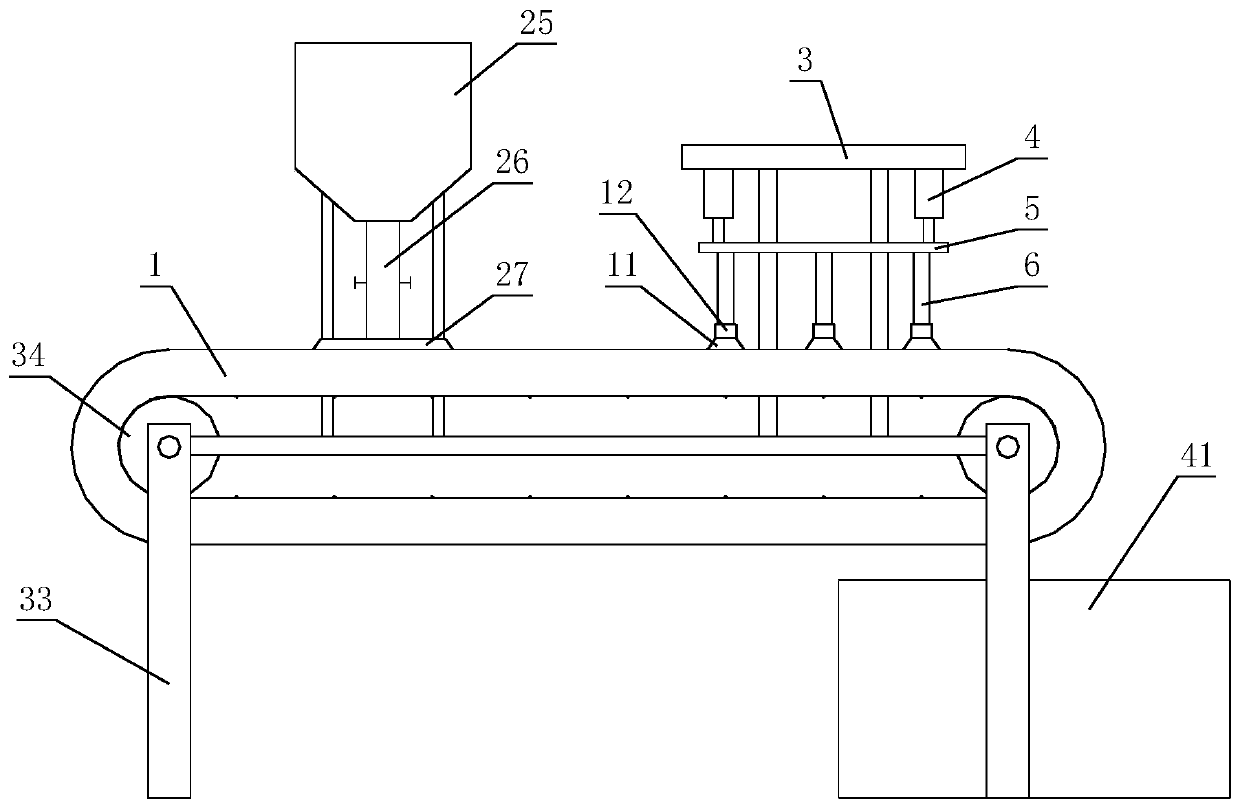
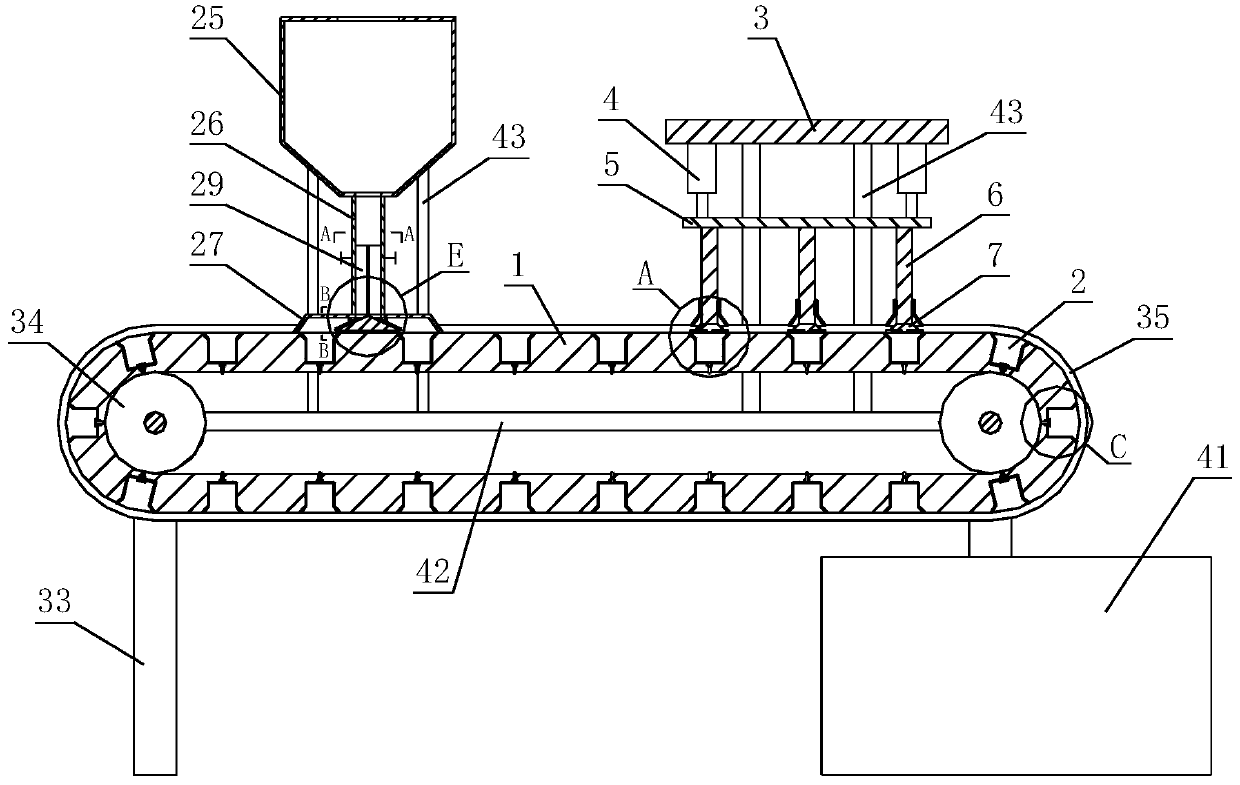
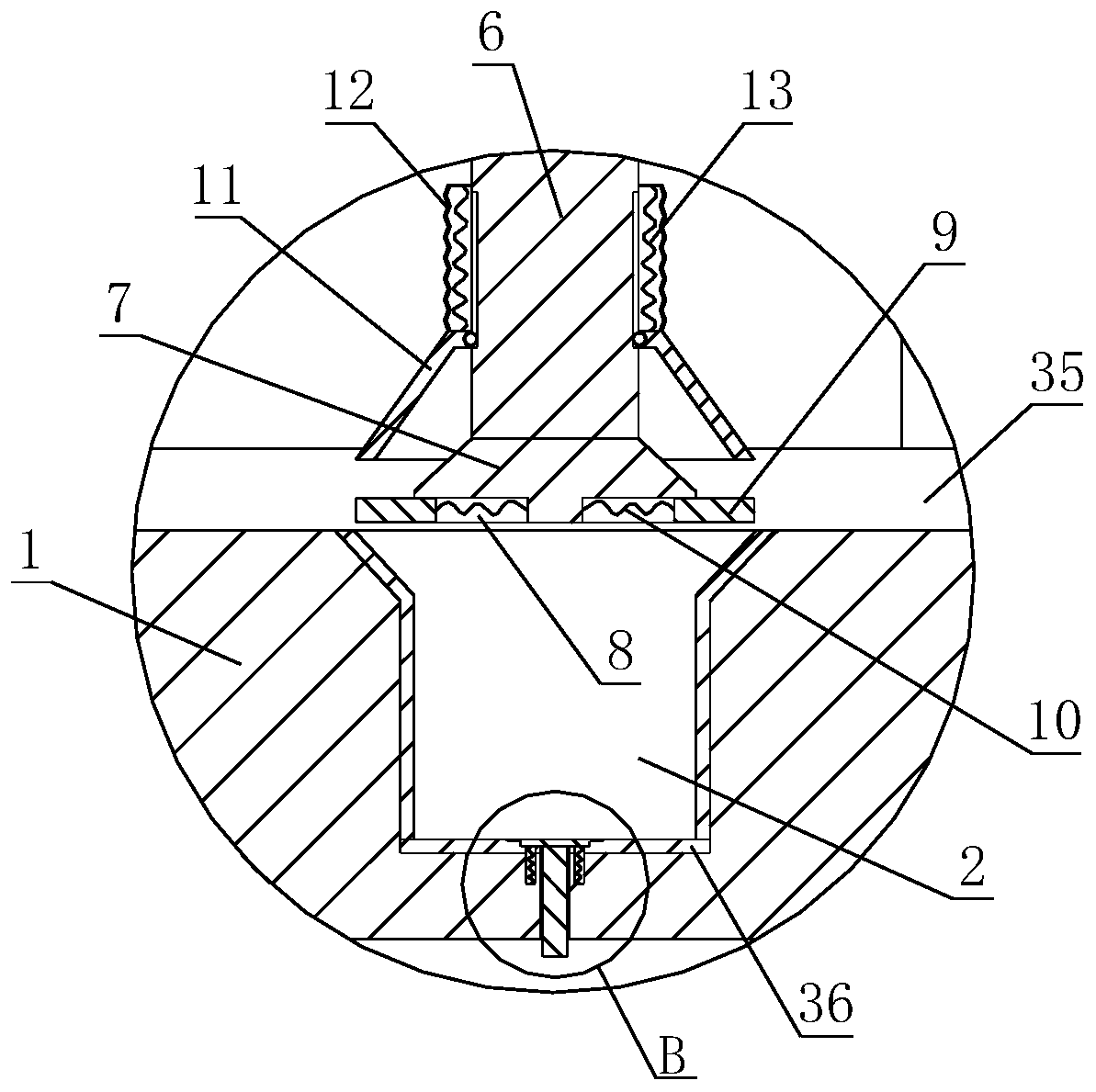
ActiveCN111267394AUniform qualityReduce lossesTransportation and packagingMixersProlonged-release tabletPharmaceutical drug
The invention relates to the technical field of chemical pharmaceutical, in particular to a preparation technology of indapamide sustained-release tablets. According to the specific technical scheme,the preparation technology of the indapamide sustained-release tablets comprises steps as follows: a sustained-release material, a filling agent, a lubricating agent and indapamide are sequentially added to a medicinal powder stirrer to be stirred and mixed; air is blown into the medicinal powder stirrer from the bottom and / or the top of the medicinal powder stirrer in a stirring and mixing process; mixed medicinal powder is added to a tabletting device for tabletting; and finally, the tablets obtained after tabletting are coated. According to the specific technology and equipment, the prepared indapamide sustained-release tablets have uniform and stable release rate, bioavailability of drugs is improved, little drug loss is produced in the tabletting process, and drug quality is uniform.
Owner:HUANGSHAN C KING PHARMA
Indapamide percutaneous controlled releast plaster and its preparation method
InactiveCN1823765AImprove adhesionImprove flexibilityOrganic active ingredientsSheet deliveryPlasticizerCurative effect
A release-controllable percutaneous paster of indopamide for stable releasing within 24 hr is composed of substrate, layer, medicine bearing layer, and antisticking layer. Said medicine bearing layer contains indapamide, pressure sensitive adhesive, composite percutaneous absorption promoter, plasticizer, tackiness agent and antioxidant. Its preparing process is also disclosed.
Owner:SHENYANG PHARMA UNIVERSITY
Antihypertensive medicine containing amlodipine besylate
ActiveCN101229160APlay a synergistic roleReduce adverse reactionsOrganic active ingredientsCardiovascular disorderMedicineAmlodipine besilate
The invention discloses an antihypertensive composition comprising amlodipine besylate and consists of the components with the following weight ratios: 1 portion of amlodipine besylate and 2 to 8 portions of indapamide which both have the antihypertensive effect, but more adverse reactions are produced when the amlodipine besylate or the indapamide is used respectively. The invention which combines the amlodipine besylate and the indapamide which can have a better synergistic effect in a certain proportion scope not only can reduce the adverse reactions and the doses but also has significant antihypertensive effects, wide application prospects and worth popularization.
Owner:YIPINHONG PHARMACEUTICAL CO LTD
Indapamide slow release medicine containing composite lactose
ActiveCN104398482AImprove uniformityAvoid quality impactOrganic active ingredientsPharmaceutical delivery mechanismMedicineLactose
The invention relates to a preparation method of an indapamide slow release medicine in order to improve the slow release performance of the medicine. The medicine comprises 0.5-2wt% of indapamide, 30-50wt% of a skeletal material, 14-28wt% of 100 mesh lactose, 20-35wt% of 200 mesh lactose, 10-20wt% of polyvinylpyrrolidone K30 and 0.2-2wt% of a lubricant. The medicine is prepared by adopting a powder direct pressing technology, and has the advantages of uniform distribution of a main medicine in a tablet, god tablet uniformity (uniformity index control needed by low content tablets), avoiding of the influences of water, heating and drying brought by traditional preparation technologies on the quality of the main medicine in the preparation process, production energy consumption reduction, and production efficiency increase.
Owner:HUANGSHAN C KING PHARMA
Indapamide slow-release hypertension pill and preparation method thereof
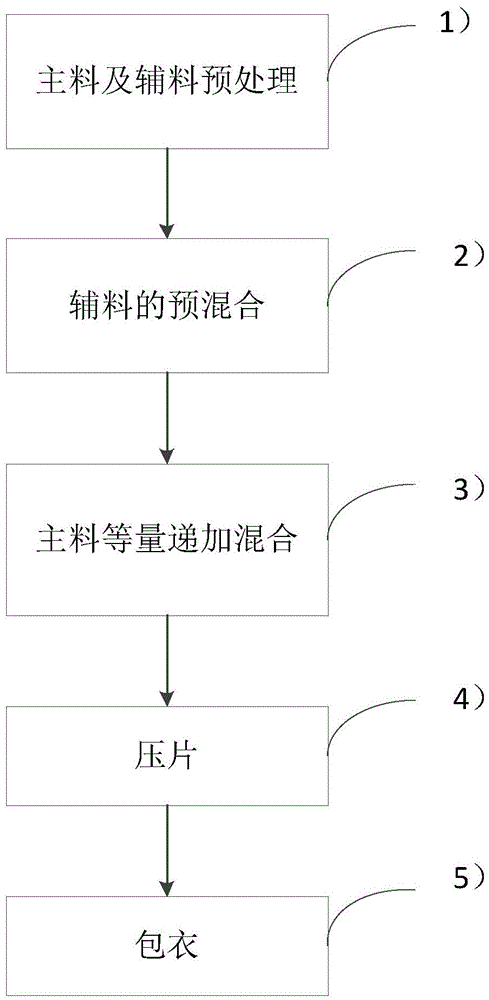
InactiveCN104906060AInhibition of burst releaseReduce financial burdenOrganic active ingredientsPharmaceutical delivery mechanismMedicineDiluent
The invention discloses an indapamide slow-release hypertension pill and a preparation method thereof. The indapamide slow-release hypertension pill comprises the following components in parts by weight: 0.75 part of indapamide, 40-55 parts of a framework material, 5-10 parts of a disintegration material, 40-50 parts of a diluent and 0.5 part of a lubricant. The indapamide slow-release hypertension pill is prepared by using a direct powder tabletting method which comprises the following steps: pretreating main materials and assistant materials, premixing the assistant materials, performing equivalent progressive increase mixing on the materials, tabletting, and coating. By adopting the indapamide slow-release hypertension pill disclosed by the invention, burst release can be relatively well inhibited, thorough later release can be ensured, the hypertension effect can be relatively stable and long-lasting, the production cost is reduced as a domestic framework material is adopted, and due to the adoption of a direct dry powder tabletting process, the phenomenon of too intense burst release caused by gelatinization of functional groups and water when a wet granulation method is adopted can be prevented, the pill can be prepared according to the formula ratio, the product quality can be relatively stable, and the in-batch product difference can be reduced.
Owner:HUBEI HUIRUI PHARMA
Composition containing indapamide and B vitamins as well as application thereof
InactiveCN101590040AAvoid damageAddress clinical gapsOrganic active ingredientsBlood disorderDiseaseHyperhomocysteinemia
The invention relates to a drug composition containing indapamide and B vitamins and an application thereof in preparing drugs for treating the HHCY-hyperhomocysteinemia and the elevated homocysteine. The composition of the indapamide and B vitamins can further reduce the Hcy level, and the effect is superior to that of single drug, so that the composition of the indapamide and the B vitamins can not only lower the Hcy level, but also has synergetic effect on reducing Hcy damages. The composition of the invention is a selection for treating Hcy, which provides a more effective treatment protocol for treating high homocysteine. The invention belongs to the pharmaceutical field.
Owner:北京奥萨医药研究中心有限公司 +1
Subcutaneous implant line for long effect pressure lowering
InactiveCN108498508AOrganic active ingredientsPharmaceutical delivery mechanismCarboxymethyl starchLacidipine
The invention discloses a subcutaneous implant line for long effect pressure lowering. The implant line is prepared from raw medicine of lacidipine, aldactone and indapamide and auxiliary materials ofcrosslinked povidone, polyethylene glycol and sodium carboxymethyl starch. The implant line is a cylindrical implant line prepared by mixing biodegradable materials as carriers and hypotensive medicine. The pressure lowering speed is high; the blood pressure can be stable after implantating for 3 days; the goal of stabilizing the blood pressure for a long time can be achieved. In addition, the degradation is slow; the complete degradation can be completed after about 3 years; the line implant line use time is long; the frequent operation implantation is not needed; the operation pain of patients is reduced.
Owner:朱军星
Compound preparation for treating hypertension
InactiveCN1579389AImprove toleranceSuitable for long-term useCardiovascular disorderTolerabilityLarge dose
The invention discloses a compound preparation for curing hypertension. The invention contains ingredients with following day doses: 0.5-2.5mg of indapamide and 1.25-5mg of piaster. The pressure relief effect is excellent than one single medicine with a large dose, and it can eliminate the bad reaction caused by one single medicine, prevents the compensation reaction caused by using one single medicine to relief blood pressure, upgrades the patience of sufferer, upgrades the compliance.
Owner:GUANGDONG CARDIOVASCULAR INSITITUTE
Process for preparing slow-releasing indapamide tablet
An antihypertensive slow-releasing indapamide tablet is prepared through mixing indapamide with slow-releasing material, pore-forming agent and filler, adding water, granulating, mixing with lubricant, tabletting to obtain tablet cores, preparing solution A fron distilled water, filming agent, antisticking agent and masking agent, preparing solution B from alcohol and polyethanediol 400, mixing A with B, diluting, coating on tablet cores, and drying.
Owner:TIANJIN HANRUI PHARMA
Indapamide bisoprolol percutaneous patch and preparation method thereof
The invention belongs to the technical field of medicine and relates to an indapamide bisoprolol percutaneous patch and a preparation method thereof. The indapamide bisoprolol percutaneous patch is composed of a back liner layer, a medicine-carrying pressure-sensitive adhesive layer and an anti-bonding layer. The medicine-carrying pressure-sensitive adhesive layer includes indapamide, bisoprolol free alkali or a organic acid ion pair compound thereof, a pressure-sensitive adhesive and a percutaneous absorption promoter, wherein a total medicine amount of the indapamide, bisoprolol free alkali or the organic acid ion pair compound thereof accounts for 0.5-5.5 wt% of the total weight of the medicine-carrying pressure-sensitive adhesive layer and a molar ratio of the indapamide to the bisoprolol free alkali or the organic acid ion pair compound thereof is 0.5:1-2:1. According to the invention, two-way regulation of a percutaneous penetrating capability of indapamide and bisoprolol in the percutaneous patch can be achieved and equal-speed penetration of the indapamide and the bisoprolol from the percutaneous patch is achieved.
Owner:SHENYANG PHARMA UNIVERSITY
Indapamide-containing medicine composition for treating hypertension and preparation method thereof
InactiveCN105796773AAvoid adverse reactionsLittle side effectsOrganic active ingredientsConiferophyta medical ingredientsTreatment effectFokienia hodginsii
The invention belongs to the technical field of medicine and particularly relates to an indapamide-containing medicine composition for treating hypertension and a preparation method thereof.The indapamide-containing medicine composition for treating hypertension is prepared from, by weight, 1.5-3.5 parts of indapamide, 2,000-4,000 parts of musa basjoo sieb et zucc., 2,000-4,000 parts of uncaria sessilifructus, 2,000-4,000 parts of fokienia hodginsii and 2,000-4,000 parts of herb of largeserrate mosla.The traditional Chinese medicine and indapamide are combined, the common gastrointestinal tract adverse reactions to indapamide can be avoided, and the treatment effect on hypertension can be improved.
Owner:JINAN BANGWEN MEDICAL TECH
Indapamide sustained-release drug composite and preparation method thereof
InactiveCN103142529BAvoid sudden releaseRelease stabilityOrganic active ingredientsPharmaceutical delivery mechanismDrug release rateAdhesive
The invention discloses an indapamide sustained-release drug composite and a preparation method thereof. The indapamide sustained-release drug composite comprises the following ingredients: indapamide serving as an active ingredient and a proper amount of filler, framework material, lubricant and copovidone (VA64). The indapamide sustained-release tablet disclosed by the invention is used for treating primary hypertension and is characterized in that the VA64 is added in the prescription so that the burst release of the drug is prevented, the stable release of the drug is guaranteed and the hypokalemia caused by overhigh blood concentration is avoided; in the process, the indapamide is micronized to below 50 micrometers so that the release of the drug is improved and the delayed release of the drug is avoided; and the direct powder compression process is adopted so that the problems that the framework material coheres to form sticky balls due to adhesive and the homogeneity of drug releasing rate is influenced are avoided. The prepared indapamide sustained-release tablet has the advantages that the drug releasing rate is stable; the homogeneity of the releasing rate is good; the drug bioavailability is increased; and the drug quality is guaranteed.
Owner:KANGYA OF NINGXIA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com