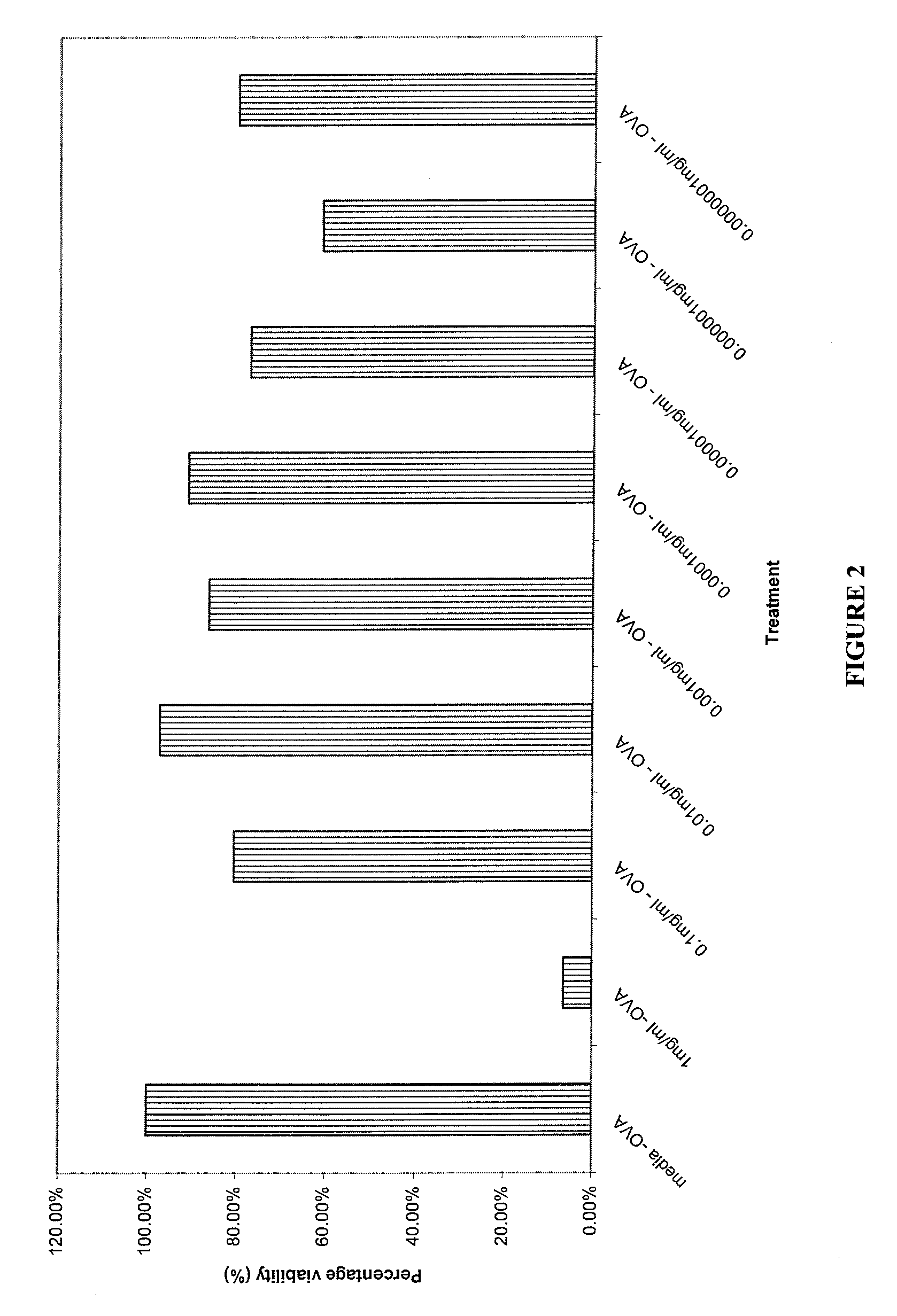
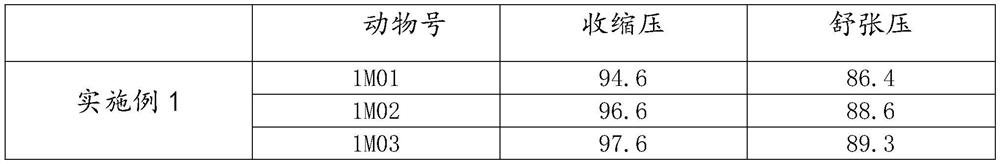
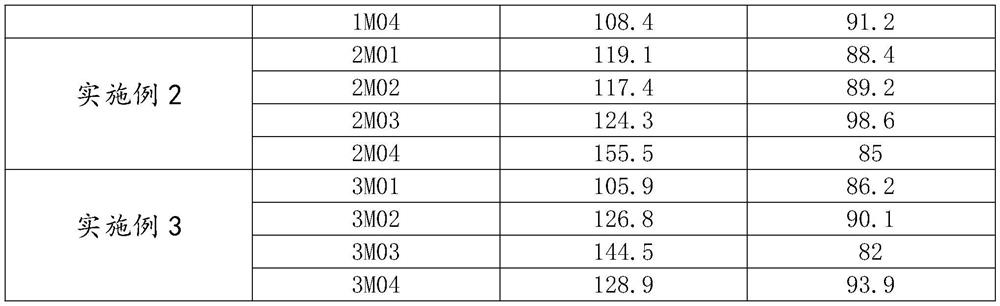
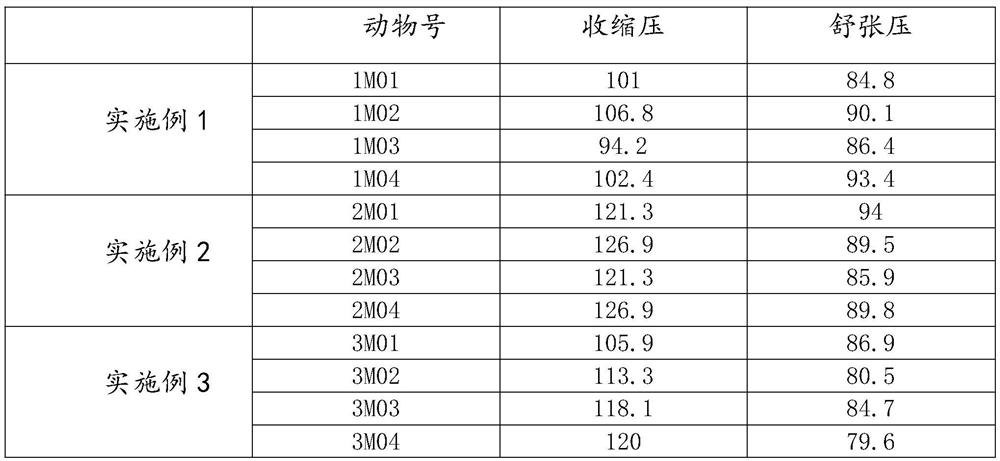
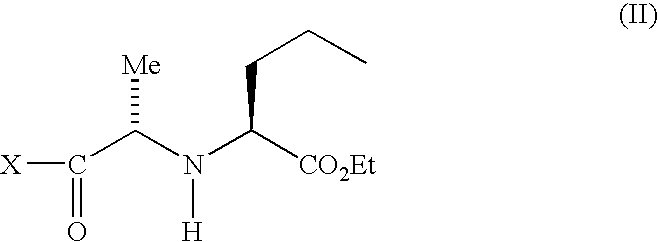Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
62 results about "Perindopril" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Perindopril is used to treat high blood pressure.
Carbonyl reductase, gene and mutant and application thereof to asymmetrical reduced carbonyl compound
ActiveCN102618513AHigh optical purityMild reaction conditionsBacteriaMicroorganism based processesHigh concentrationMethyl o-chloromandelate
The invention discloses a novel carbonyl reductase, a gene, a mutant thereof, a recombinant expression vector containing the gene and the mutant, a recombinant expression transformant, a recombinase preparation method, and applications of the carbonyl reductase and recombinase to preparation of active chiral alcohols with a chiral carbonyl compound before asymmetrical reduction. The carbonyl reductase is derived from candida glabrata, is applied to preparation of a plurality of optically-active chiral alcohols such as (R)-chloromandelic acid methyl ester, (R)-2-hydroxy-4-phenyl ethyl butyrate, (R)-4-chlorin-3-phenyl ethyl butyrate and the like. Compared with other preparation methods, a product prepared through the method has high concentration, does not require additionally or slightly adding any expensive coenzyme, has high optical purity, and has the advantages of mild reaction conditions, easiness and convenience for operating, easiness for amplifying and the like, and has a good industrial application prospect in the production of clopidogrel, L-carnitine and perindopril antihypertensive medicinal intermediates.
Owner:EAST CHINA UNIV OF SCI & TECH
Stable pharmaceutical compositions containing an ACE inhibitor
InactiveUS6869963B2Extended shelf lifeMinimize impactBiocidePill deliveryAlkaline earth metalInstability
A stable pharmaceutical composition comprising about 1 wt. % to about 80 wt. % of an ACE inhibitor or a pharmaceutical acceptable salt thereof, about 1 wt. % to about 70 wt. % of an alkali or alkaline earth metal carbonate, and about 1 wt. % to about 80 wt. % of hydroxypropyl cellulose, wherein the ACE inhibitor is selected from the group consisting of quinapril, enalapril, spirapril, ramipril, perindopril, indolapril, lisinopril, alacepril, trandolapril, benazapril, libenzapril, delapril, cilazapril and combinations thereof; wherein the formation of an internal cyclization product, and / or ester hydrolysis product, and / or oxidation product, has been reduced or eliminated, and the weight percents are based on the total weight of the pharmaceutical composition. The stabilized pharmaceutical compositions of the invention exhibit a number of advantages as follows: (i) the ACE inhibitor or a pharmaceutical acceptable salt thereof present in the compositions is preserved from degradation; (ii) the compositions exhibit extended shelf-life under normal storage conditions; (iii) the effect of moisture on the compositions is minimized; (iv) the compositions exhibit minimal, if any, discoloration over a significant period of time; and (v) the compositions exhibit minimal, if any, instability when employed in the presence of colorants.
Owner:SANDOZ AG
Stable pharmaceutical compositions containing an ace inhibitor
InactiveUS20050009806A1Extended shelf lifeMinimize impactBiocidePill deliveryAlkaline earth metalDepressant
A stable pharmaceutical composition comprising about 1 wt. % to about 80 wt. % of an ACE inhibitor or a pharmaceutical acceptable salt thereof, about 1 wt. % to about 70 wt. % of an alkali or alkaline earth metal carbonate, and about 1 wt. % to about 80 wt. % of hydroxypropyl cellulose, wherein the ACE inhibitor is selected from the group consisting of quinapril, enalapril, spirapril, ramipril, perindopril, indolapril, lisinopril, alacepril, trandolapril, benazapril, libenzapril, delapril, cilazapril and combinations thereof; wherein the formation of an internal cyclization product, and / or ester hydrolysis product, and / or oxidation product, has been reduced or eliminated, and the weight percents are based on the total weight of the pharmaceutical composition. The stabilized pharmaceutical compositions of the invention exhibit a number of advantages as follows: (i) the ACE inhibitor or a pharmaceutical acceptable salt thereof present in the compositions is preserved from degradation; (ii) the compositions exhibit extended shelf-life under normal storage conditions; (iii) the effect of moisture on the compositions is minimized; (iv) the compositions exhibit minimal, if any, discoloration over a significant period of time; and (v) the compositions exhibit minimal, if any, instability when employed in the presence of colorants.
Owner:SANDOZ AG
Method for treating tea tree seeds
InactiveCN106717280APromote germinationImprove germination rateBiocidePlant growth regulatorsFreeze-dryingZoology
The invention mainly relates to the technical field of planting, and discloses a method for treating tea tree seeds. The method comprises the following steps: preserving seeds; soaking the seeds; sterilizing the seeds; accelerating germination of the seeds; sowing the seeds. The method is simple, the germination rate of the tea tree seeds can be up to 91%, the pretreatment time before seeding can be shortened to 5-6 days, the sprouting time after direct seeding can be shortened to 8-9 days, the survival rate can be up to 94%, and the economic income can be increased by 18.3%; after being picked, tea tree fruits are soaked in a chitosan liquid, then seed coats can be softened, nutrition can be provided, dormancy completion can be accelerated through freeze drying, and germination can be accelerated; as the seeds are soaked in the chitosan liquid, softening of the seed coats can be accelerated, the pH value can be reduced, germ activity can be stimulated, and germination can be accelerated; after bactericide is sprayed, pathogenic microorganism multiplication can be inhibited; through plant extraction, multiple nutrient components can be available, and the survival rate can be increased; due to addition of zeatin and perindopril, seed germination can be promoted; the seeds are put into a germination accelerating tank, and a plant ash solution is sprayed, so that balanced nutrition can be provided, and the stress resistance can be improved.
Owner:吴萍
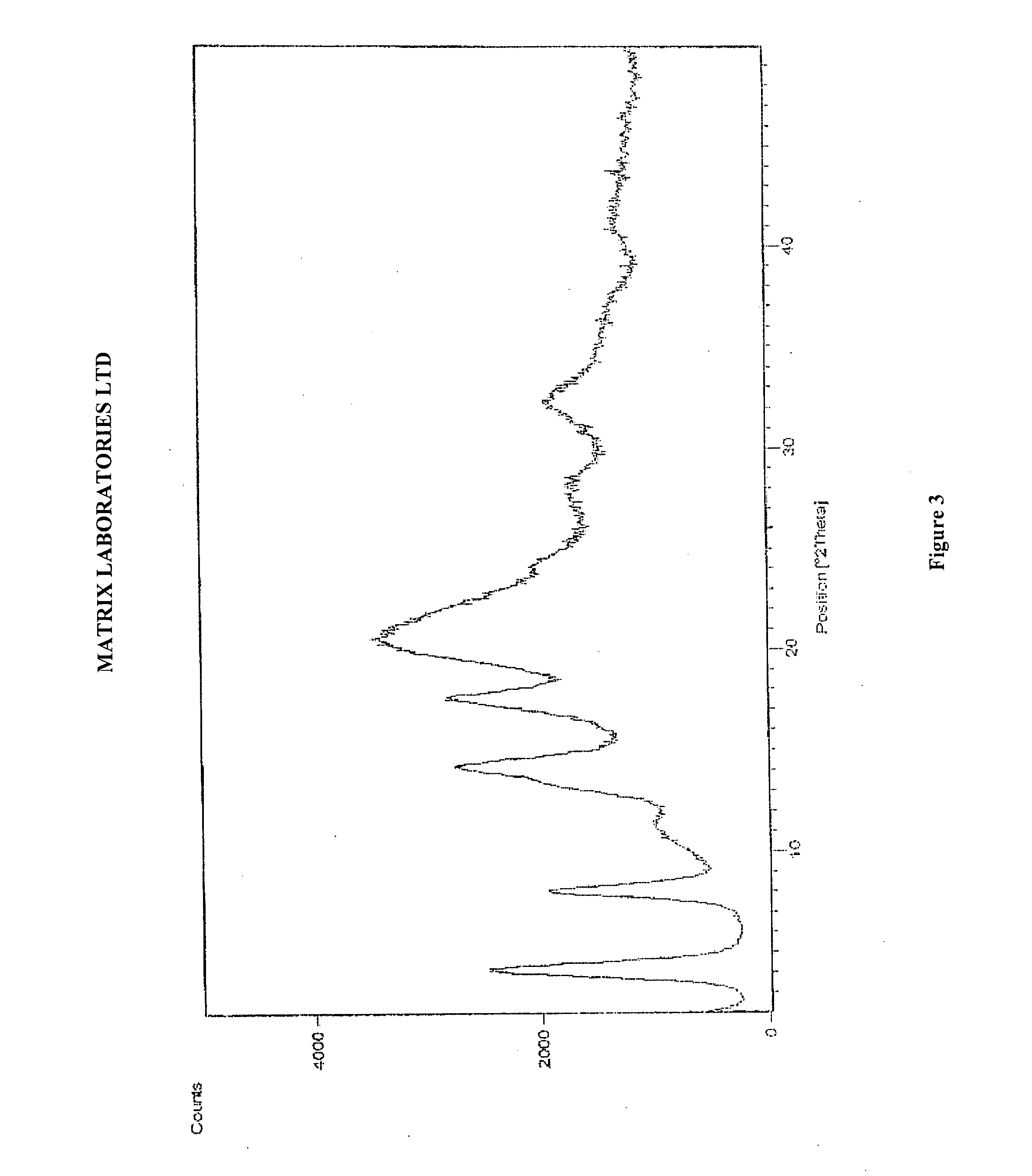
Novel polymorphic forms of perindopril (l)-arginine and process for the preparation thereof
InactiveUS20110301357A1Good physical and chemical stabilityOrganic chemistryOrganic compound preparationArginineBiology
The present invention relates to novel and stable polymorphic forms of Perindopril (L)-Arginine designated as Form γ and amorphous form and processes for their preparation. The present invention also provides the novel polymorph Form γ with greater stability to heat and humidity and can be prepared on large scale by an efficient, economic and reproducible process.
Owner:MYLAN LAB
Drug combination containing perindopril
InactiveCN101766598AImprove stabilityOrganic active ingredientsCardiovascular disorderActive componentExcipient
The invention discloses a drug combination containing perindopril. The drug combination comprises perindopril or salts thereof as the active components and contains one or more medicinal excipients. The drug combination of the invention can effectively improve the stability of perindopril, particularly, the content of the active components remains 98.86% after the accelerated test of capsules for 6 months.
Owner:SHANGHAI PHARMA DONGYING JIANGSU PHARMA CO LTD
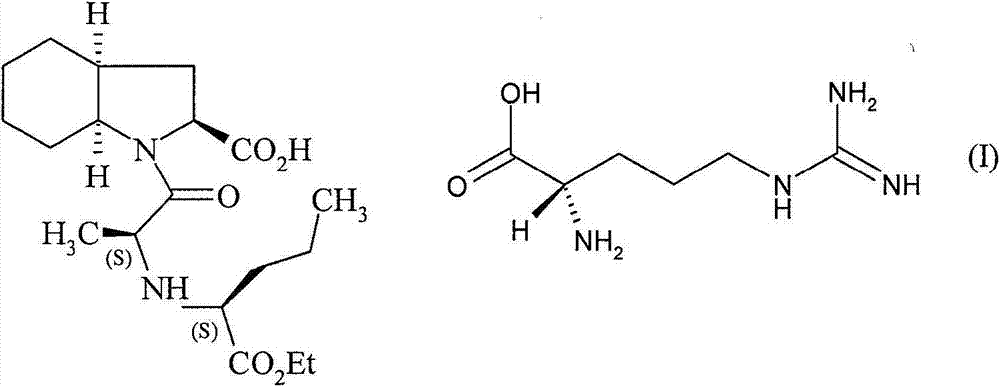
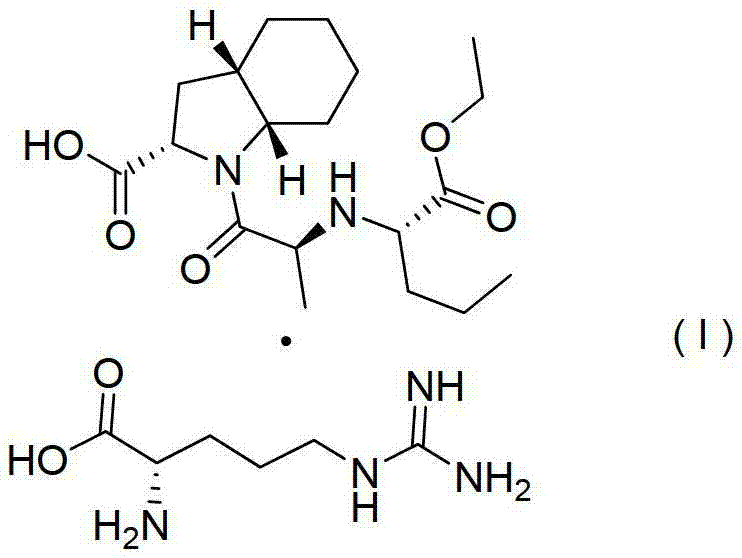
Process for the preparation of perindopril L-arginine salt
The present invention relates to a process for the preparation of perindopril L-arginine salt of formula (I): by means of reaction between perindopril and L-arginine in a solvent system selected from: a binary mixture of acetonitrile and dimethyl sulphoxide, a binary mixture of ethyl acetate and dimethyl sulphoxide, a ternary mixture of acetonitrile, dimethyl sulphoxide and toluene, at a temperature from 10 to 100 DEG C, preferably from 40 to 80 DEG C, followed by isolation by means of filtration of the L-arginine salt thereby obtained.
Owner:LES LAB SERVIER
Compound blood pressure reducing prepn containing angiotonin converzyme inhibitor, calcium ion agonist and Estazolam
InactiveCN1526398AGood curative effectLittle side effectsOrganic active ingredientsPill deliveryCaptoprilSide effect
The present invention provides one new kind of compound blood pressure reducing preparation containing angiotonin converzyme inhibitor, calcium ion agonist, Estazolam and pharmaceutically acceptable carrier. The angiotonin converzyme inhibitor is selected from Enalapril, Ramipril, Benalapril, Lisinopril, Acertil, etc. as well as their mixture; and the calcium ion agonist is selected from Nitrendpine, Amlodipine Besylate, Nifedipine, Felodipine, etc. as well as their mixture. The present invention utilizes the synergistic effect between different medicines to raise the blood pressure lowering effect, reduce side effect and improve the compliance of patient.
Owner:杜晓锋
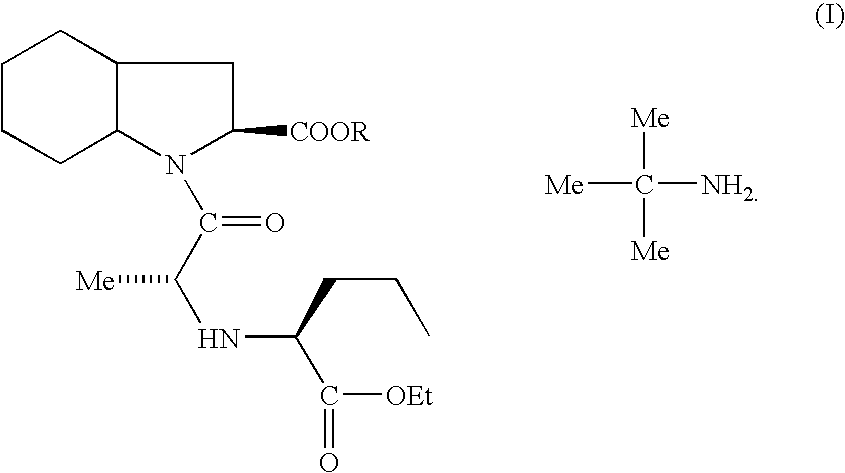
Process for making (2S, 3aS, 7aS)-1-[(2S)-2-[[(1S)-1-(ethoxycarbonyl) butyl] amino]-1-oxopropyl] octahydro-1H-indole-2-carboxylic acid
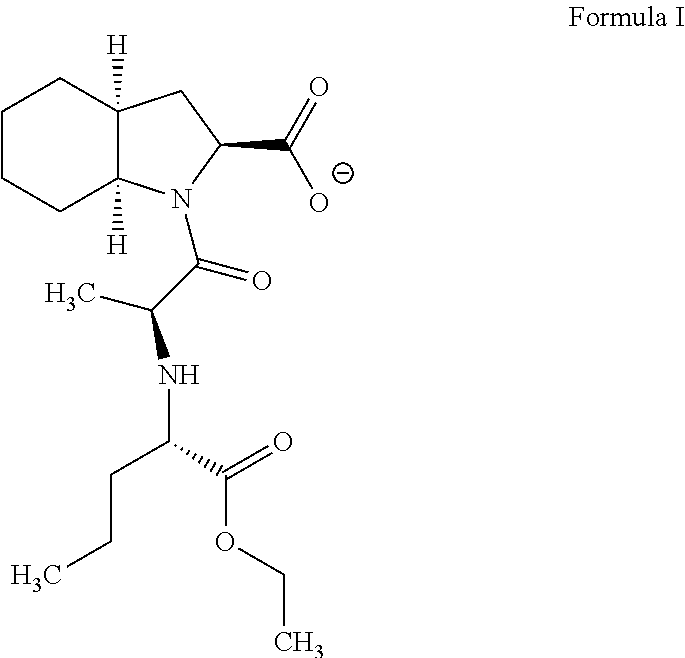
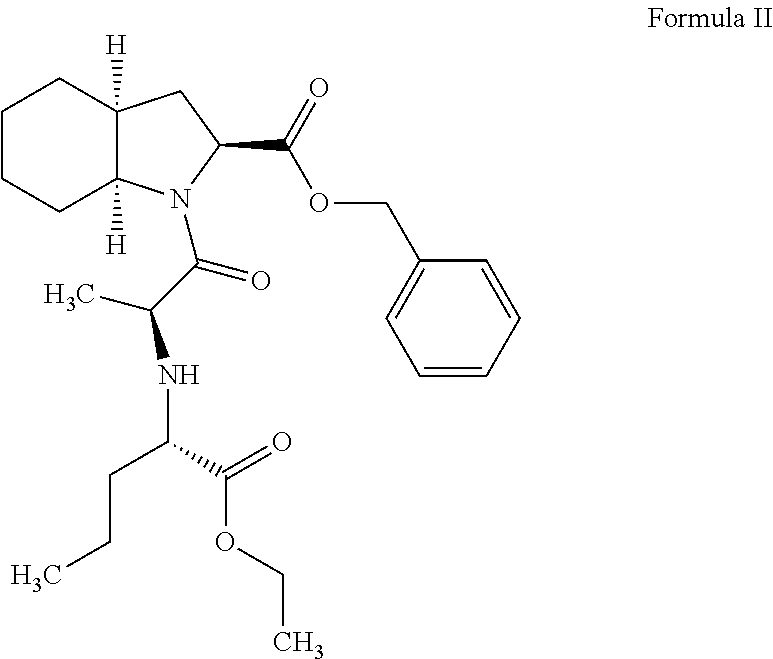
InactiveUS20060178422A1High purityReduce the presence of impuritiesBiocideOrganic chemistryCarboxylic acidEthyl acetate
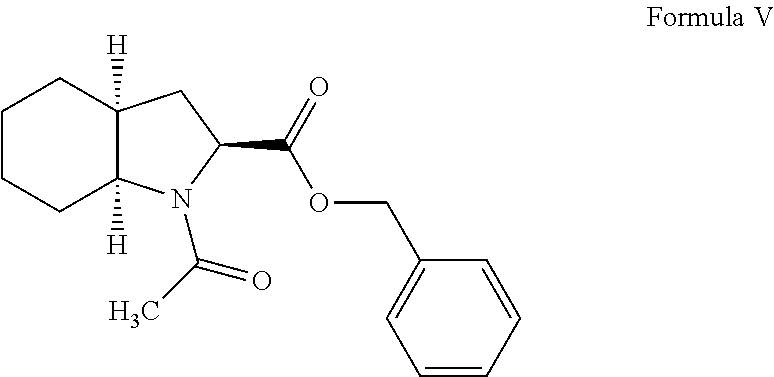
The present invention discloses a process for the synthesis and isolation of (2S, 3aS, 7aS)-1-[(2S)-2-[[(1S)-1-(ethoxycarbonyl)butyl]amino]-1-oxopropyl] octahydro-1H-indole-2-carboxylic acid and its tert-butylamine salt, by condensing (2S, 3aS, 7aS)-octahydroindole-2-carboxylic acid benzyl ester and N[(S)1-carboxybutyl]-(S)-alanine ethyl ester in nonreactive solvents in turn avoiding the formation of impurity viz. N-acetyl (2S,3aS,7aS)-octahydroindole-2-carboxylic acid benzyl ester (Formula V). The de-protection of benzyl ester group is optimized and then isolation of the product from aqueous layer by extraction using an organic solvent, which eliminates the need of lyophilization. The process of the present invention yields perindopril erbumnine salt of Formula 1B free of contaminants derivable from dicyclohexylcarbodiimide and impurities originated by the use of ethyl acetate.
Owner:IPCA LAB LTD
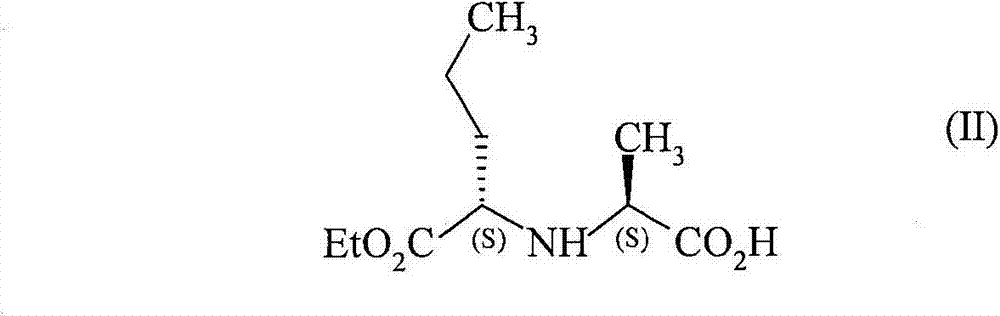
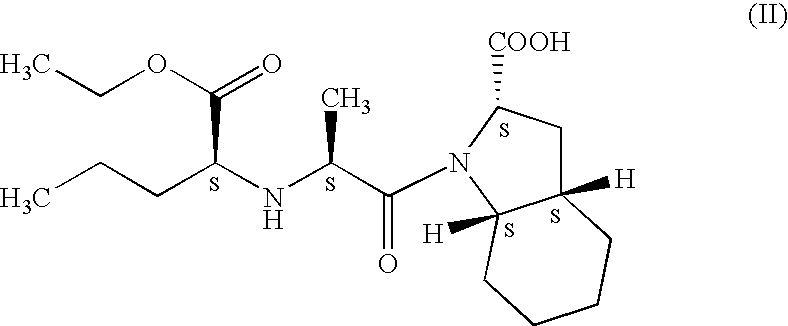
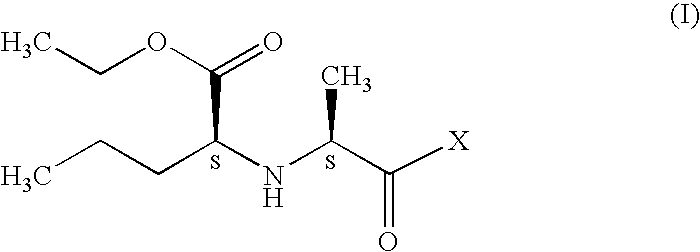
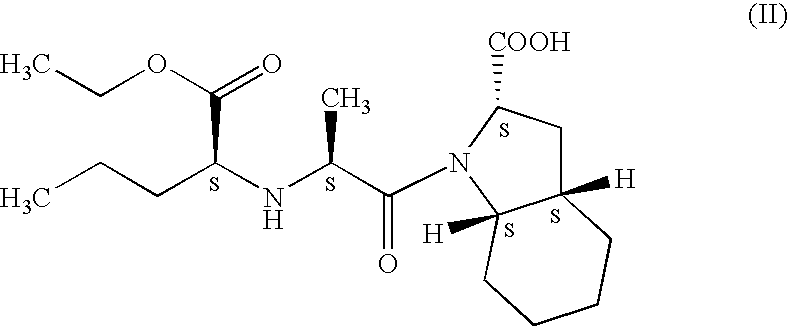
Process for preparation of perindopril and salts thereof
InactiveUS7521566B2Simple, safe, selective and cost-effectiveHigh purityOrganic compound preparationAmino-carboxyl compound preparationCost effectivenessBromine
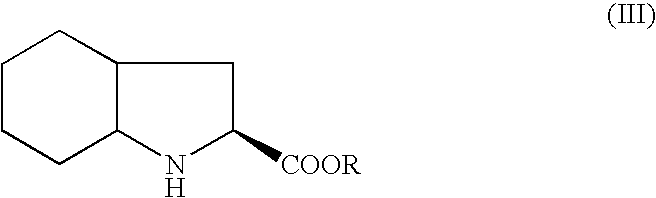
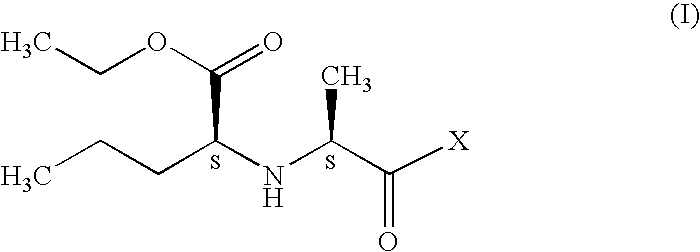
A process for preparation of perindopril of formula (II) and salts thereofwhich is simple, safe, convenient and cost-effective.The process involves reaction of compound of formula (I),wherein X is chlorine or brominewith compound of formula (VII)wherein A signifies that the six-membered ring of the bicyclic system is either saturated or unsaturated to give compound of formula (VIII),wherein A is as defined above,followed by catalytic hydrogenation of the compound of formula (VIII) thus obtained to give the perindopril of formula (II).The above process for the manufacture of perindopril would specifically avoid the use of harmful chemicals like phosgene or costly coupling agents like dicyclohexylcarbodiimide and 1-hydroxybenxotriazole usually used for such manufacture. The process would also not require any intervention of a catalyst and does not require any alkaline or acidic reaction conditions. Importantly, the process provides for manufacture of perindopril with high stereoselectively giving perindopril (II) having (S)-configuration in all the five chiral centres of the molecule, conforming to pharmacoepeial specifications.The invention also relates to a method for preparation of the compound of formula (I) and also to a method for preparation of N-[(S)-1-carbethoxybutyl]-(S)-alanine of formula (III) used in the process.
Owner:LES LAB SERVIER
Novel anti-hypertension compound slow release tablets and production process thereof
InactiveCN106924712AOrganic active ingredientsDipeptide ingredientsAnti hypertensionPinoresinol diglucoside
The invention relates to novel medical anti-hypertension compound slow release tablets. The tablets are mainly prepared from four bioactive components of 5 mg-20 mg of quercetin, 5 mg-20 mg of pinoresinol diglucoside (effective ingredient of the bark of eucommia), 0.4 mg-1.60 mg of sulfonamide diuretic indapamide and 2 mg-10 mg of angiotensin reductase II inhibitor (ACEI) perindopril salt and pharmaceutical adjuvants of 20 mg-60 mg of PVP-k30, 10 mg-30 mg of hydroxyl propyl cellulose (HPMC-K15M), 30 mg-60 mg of lactose, 53 mg-80 mg of microcrystalline cellulose (MCC), 1 mg-2 mg of magnesium stearate (lubricating agent), 2 mg-4 mg of low hydroxypropyl cellulose (LHPC, disintegrating agent), 1 mg-20 mg of starch and 1 mg-10 mg of talcum powder. The novel anti-hypertension compound slow release tablets provide a safe and effective medicine help for adjusting and stabilizing blood pressure of patients, improving heart functions, relieving heart and cerebral vessel lesions and avoiding occurrence of other cardiovascular disease risks.
Owner:SHANGHAI PHARMA DONGYING JIANGSU PHARMA CO LTD
Process for preparation of perindopril and salts thereof
InactiveUS20060276659A1Simple, safe, selective and cost-effectiveHigh purityOrganic compound preparationAmino-carboxyl compound preparationCost effectivenessBromine
A process for preparation of perindopril of formula (II) and salts thereof which is simple, safe convenient and cost-effective. The process involves reaction of compound of formula (I), wherein X is chlorine or bromine with compound of formula (VII) wherein A signifies that the six-membered ring of the bicyclic system is either saturated or unsaturated to give compound of formula (VIII), wherein A is as defined above, followed by catalytic hydrogenation of the compound of formula (VIII) thus obtained to give the perindopril of formula (II). The above process for the manufacture of perindopril would specifically avoid the use of harmful chemicals like phosgene or costly coupling agents like dicyclohexylcarbodiimide and 1-hydroxybenxotriazole usually used for such manufacture. The process would also not require any intervention of a catalyst and does not require any alkaline or acidic reaction conditions. Importantly, the process provides for manufacture of perindopril with high stereoselectively giving perindopril (II) having (S)-configuration in all the five chiral centres of the molecule, conforming to pharmacoepeial specifications. The invention also relates to a method for preparation of the compound of formula (I) and also to a method for preparation of N-[(S)-1-carbethoxybutyl]-(S)-alanine of formula (III) used in the process.
Owner:LES LAB SERVIER
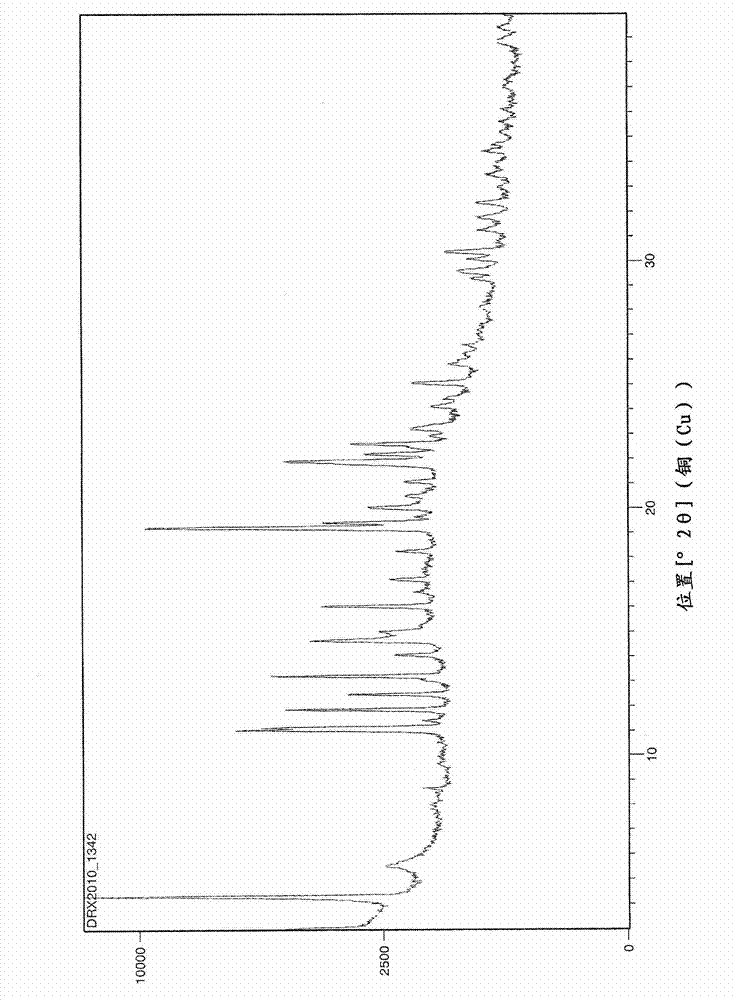
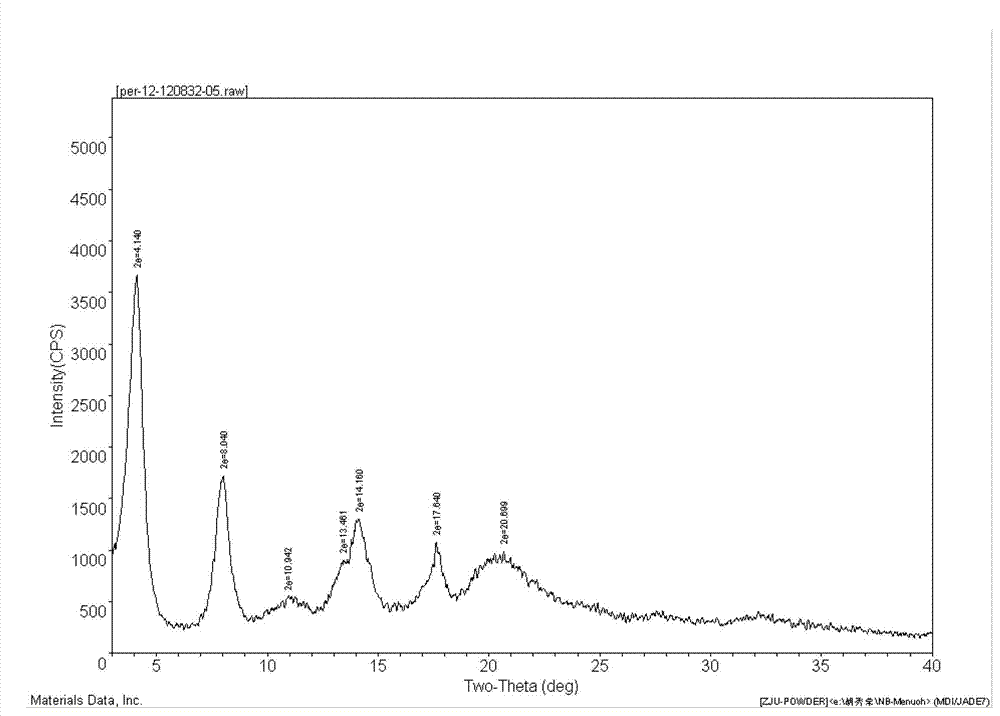
Preparation method of perindopril arginine salt of gamma-crystal form
ActiveCN103172696AHigh yieldEasy to filter and separatePeptide preparation methodsOrganic solventArginine
The invention discloses a preparation method of perindopril arginine salt of a gamma-crystal form. The preparation method comprises the following steps of: dissolving perindopril and L-arginine in water; adding an organic solvent after stirring and dissolving the perindopril and L-arginine; heating up the mixture until the mixture is refluxed; distributing water by utilizing a water distributor until no water enters the water distributor; cooling a reaction liquid to the room temperature for carrying out suction filtration; and drying the filter cake to obtain the perindopril arginine salt of the gamma-crystal form. According to the preparation method of the perindopril arginine salt of the gamma-crystal form disclosed by the invention, the organic solvent is added for refluxing and distributing water; the water is extracted by the solvent, so that no water exists in the reaction liquid, and therefore, the water molecule is not packaged by the target product to form a spherical shape, and the suction filtration and separation are easy; and meanwhile, the water is separated out, the yield of the product is 92% or higher.
Owner:NINGBO MENOVO PHARMA
Process for manufacture of pure (2S, 3aS, 7aS)-1-[(2S)-2-[[(1S)-1-(ethoxycarbonyl) butyl]amino]-1-oxopropyl] octahydro-1H-indole-2-carboxylic acid and its tert. butyl amine salt
New compounds useful as synthetic intermediates to synthesize perindopril, a new process for synthesizing perindopril, and new salts of perindopril.
Owner:IPCA LAB LTD
Preparation method of perindopril arginine
ActiveCN110283104ASimple purification processHigh yieldOrganic compound preparationPeptidesMedicinal chemistryPerindopril
The invention belongs to the field of synthesis of drugs, and relates to a preparation method of perindopril arginine. The preparation method of perindopril arginine is characterized in that an organic amine salt of perindopril reacts with L-arginine to obtain the perindopril arginine. The prepared perindopril arginine is a white powder, has a purity reaching 99.9% or above, and has a yield of 80% or above, and the method has the advantages of easiness in suction filtration in the technologic process, and no agglomeration phenomenon.
Owner:NANJING GRITPHARMA CO LTD
Capsule for the prevention of cardiovascular diseases
ActiveUS20110086094A1Improve the level ofLess impuritiesBiocideAntipyreticHigh risk populationsLisinopril
The invention relates to a capsule for the prevention of cardiovascular diseases which comprises coated tablets of acetylsalicylic acid, coated tablets of simvastatin or pravastatin, and coated tablets of lisinopril, ramiphl or perindopril. The capsules are used for the prevention of cardiovascular diseases in high-risk populations.
Owner:FERRER INT SA +1
Carbonyl reductase, gene and mutant and application thereof to asymmetrical reduced carbonyl compound
ActiveCN102618513BHigh optical purityMild reaction conditionsBacteriaMicroorganism based processesHigh concentrationEthyl butyrate
The invention discloses a novel carbonyl reductase, a gene, a mutant thereof, a recombinant expression vector containing the gene and the mutant, a recombinant expression transformant, a recombinase preparation method, and applications of the carbonyl reductase and recombinase to preparation of active chiral alcohols with a chiral carbonyl compound before asymmetrical reduction. The carbonyl reductase is derived from candida glabrata, is applied to preparation of a plurality of optically-active chiral alcohols such as (R)-chloromandelic acid methyl ester, (R)-2-hydroxy-4-phenyl ethyl butyrate, (R)-4-chlorin-3-phenyl ethyl butyrate and the like. Compared with other preparation methods, a product prepared through the method has high concentration, does not require additionally or slightly adding any expensive coenzyme, has high optical purity, and has the advantages of mild reaction conditions, easiness and convenience for operating, easiness for amplifying and the like, and has a good industrial application prospect in the production of clopidogrel, L-carnitine and perindopril antihypertensive medicinal intermediates.
Owner:EAST CHINA UNIV OF SCI & TECH
Process for the preparation of perindopril
A process for preparing perindopril is provided comprising condensing an N-[(S)-1-carbethoxybutyl]-(S)-alanyl halide of formula II:wherein X is a halide with an (2S,3aS,7aS)-2-carboxyperhydroindole of formula III:wherein R is hydrogen or a protecting group.
Owner:GLENMARK GENERRICS LTD
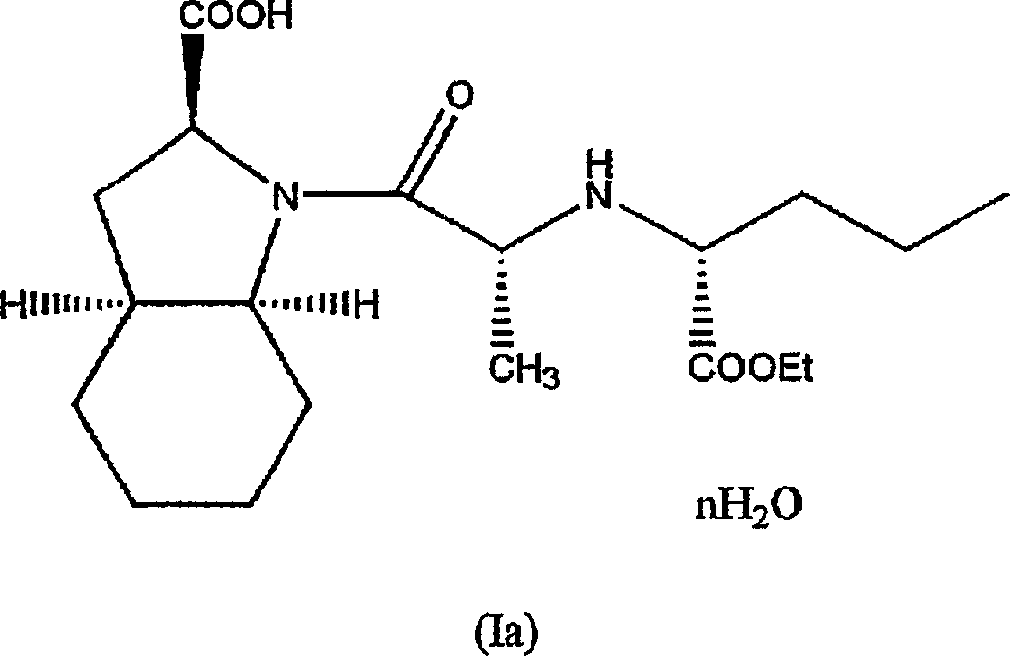
Hydrated perindopril salt, preparation method thereof and composition containing the same
InactiveCN1966519AHigh purityOrganic active ingredientsNervous disorderMedicinal chemistryPerindopril
The present invention relates to a pharmaceutically acceptable salt of hydrated perindopril of formula (1a) wherein n is an integer of 1 to 5, or a reciprocal of integers 2 to 5. The present invention also relates to a process for preparing the salt, the use and the pharmaceutical composition containing the same.
Owner:CIPLA LTD
Process for preparation of perindopril intermediate
InactiveUS20150252001A1Speed up the processInhibition formationPeptidesCarboxylic acidHydroxybenzotriazole
The present invention relates to an improved process for the preparation Of (2S,3aS,7aS)-1-[(2S)-2-[[(1S)-1-(ethoxycarbonyl)butyl]amino]3-S-oxopropyl]octahydro-1H-indole-2-carboxylic acid benzyl ester (the compound of formula II) comprising reacting (2S,3aS,7aS)-octahydro-1H-indole-2-carboxylic acid phenylmethyl ester 4-methylbenzenesulfonate (the compound of formula III) with N-[(S)-ethoxycarbonyl-1-butyl]-(S)-alanine (the compound of formula IV), using 1-hydroxybenzotriazole (HOBT), dicyclohexylcarbodimide (DCC) and triethylamine in the presence of toluene as a solvent at a temperature of 5-40° C.
Owner:PIRAMAL ENTERPRISES LTD
Pharmaceutic preparation for treating viral myocarditis
InactiveCN105079438ASynergistic effect is obviousNo obvious side effectsDipeptide ingredientsAntiviralsDiseaseSide effect
The invention discloses a pharmaceutic preparation for treating viral myocarditis. The pharmaceutic preparation is prepared from the following raw materials in parts by weight: 25-35 parts of astragalus membranaceus, 12-25 parts of radix ophiopogonis, 12-25 parts of red ginseng, 10-15 parts of pachyrhizua angulatus, 10-15 parts of folium apocyni veneti, 20-25 parts of spina date seeds, 12-25 parts of panax japonicus, 8-12 parts of licorice root, 5-10 parts of fructus choerospondiatis, 3-6 parts of muskroot-like semiaquilegia root, and 5-10 parts of perindopril. The invention further discloses a preparation method of the pharmaceutic preparation. According to the pharmaceutic preparation, the Chinese and western medicine components are mixed, so that the purpose of treating both symptoms and root causes of diseases is achieved, the treatment effect is good, and no toxic or side effect is generated.
Owner:SHANDONG UNIV
Myocarditis treatment tablet and preparation method
InactiveCN109078166AMake up for the vacancyReasonable ratio of ingredientsOrganic active ingredientsDipeptide ingredientsOtilonium bromideMedicine
The invention discloses a myocarditis treatment tablet and relates to the field of medicine science, for patients with myocarditis. The tablet is prepared from, by weight, 14-21 parts of otilonium bromide, 1.5-2.7 parts of perindopril, 3-8 parts of radix angelicae sinensis, 2.4-5.3 parts of radix ophiopogonis, 4-7 parts of radix polygalae, 2.4-3.7 parts of radix glycyrrhizae, and 16-23 parts of fresh folium agaves sisalanae. The tablet is prepared from components in reasonable proportion, has good treatment effect on heart diseases such as viral myocarditis under the synergistic effect of synergistic components, namely Otilonium Bromide and Perindopril, can effectively relieve symptoms and fill the gap of the like medicines, and has wide marketing prospect.
Owner:何凤姣
Perindopril dropping balls and their preparation
InactiveCN1981757ARapid dissolutionHigh dissolution rateOrganic active ingredientsPill deliveryPerindoprilPharmacology
A dripping pill of perindopril for treating hypertension and heart failure is prepared from perindopril and matrix. Its preparing process is also disclosed.
Owner:陈茜
Polymorphic forms of perindopril (L)-arginine and process for the preparation thereof
InactiveUS8686161B2Good physical and chemical stabilityIncrease heatOrganic chemistryOrganic compound preparationArginineMedicinal chemistry
The present invention relates to novel and stable polymorphic forms of Perindopril (L)-Arginine designated as Form γ and amorphous form and processes for their preparation. The present invention also provides the novel polymorph Form γ with greater stability to heat and humidity and can be prepared on large scale by an efficient, economic and reproducible process.
Owner:MYLAN LAB
Pharmaceutical composition for treating chronic heart failure
The invention relates to a pharmaceutical composition comprising an angiotensin converting enzyme inhibitor (ACEI), an enkephalinase inhibitor, a folic acid compound and a pharmaceutically acceptable carrier, wherein the ACEI is selected from the group consisting of enalapril, benazepril, ramipril, fosinopril, cilazapril, perindopril and the like, and the content is 1.25-75 mg; the enkephalinase inhibitor is sacubitril, and the content is 10-120 mg; and the folic acid compound is selected from the group consisting of folic acid, 5-methyltetrahydrofolate, calcium formyltetrahydrofolate, leucovorin, calcium levofolinate and the like, and the content is 0.1-5 mg. The invention provides the use of the pharmaceutical composition in the preparation of a medicament for the treatment of chronic heart failure and the prevention of stroke. By the implementation of the invention, the pharmaceutical composition can also improve the compliance of patients and improve the therapeutic effect by providing the pharmaceutical composition for a specific use to the patients.
Owner:SHENZHEN AUSA PHARM CO LTD +1
Pharmaceutical composition for treating hypertensive left ventricular hypertrophy and application thereof
InactiveCN103143020AAdvantages and Notable ImprovementsSignificant reversalOrganic active ingredientsDipeptide ingredientsCaptoprilLeft ventricular size
The invention discloses a pharmaceutical composition for treating hypertensive left ventricular hypertrophy; the pharmaceutical composition comprises total glucosides of paeony and at least one angiotensin converting enzyme inhibitor; the angiotensin converting enzyme inhibitor is captopril, enalapril, perindopril or fosinopril. The pharmaceutical composition has very significant reverse effect on hypertension induced ventricular hypertrophy; by adopting the pharmaceutical composition, the perfect synergy function on each index such as left ventricular index of myocardial hypertrophy is obtained.
Owner:海门市凤城旅游景点开发有限公司
Method for the treatment of cachexia
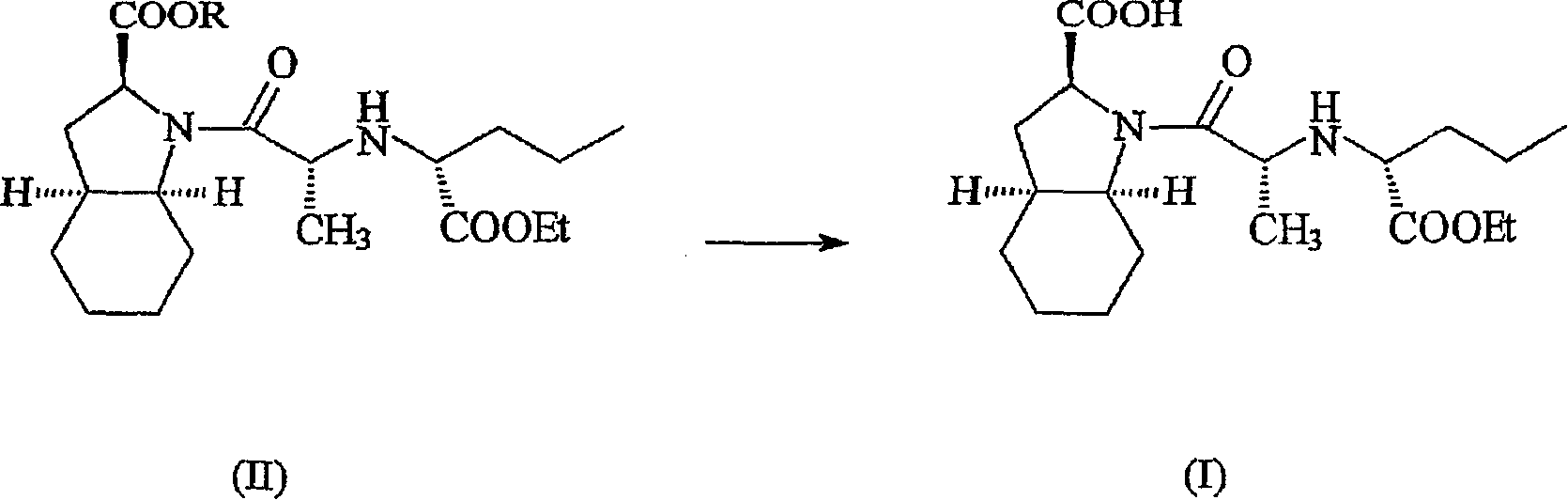
InactiveUS20070071838A1Suitable dissolution rateMaximize efficacyBiocideOrganic chemistryCompound (substance)Protecting group
A pharmaceutically acceptable salt of perindopril of formula (I) is made from a protected precursor compound of formula (II) wherein R represents a carboxyl protecting group, which process comprises subjecting a compound of formula (II) to deprotection of the carboxylic group COOR attached to the heterocyclic ring so as to yield the corresponding free acid, which deprotection is carried out in the presence of a base which forms a pharmaceutically acceptable salt with said free acid formed by the deprotection.
Owner:THE QUIGLEY
Subcutaneous implant rod for long-acting blood pressure reduction and preparation method of subcutaneous implant rod
PendingCN113476418AResolution timeEasy to prepareOrganic active ingredientsDipeptide ingredientsPolyethylene glycolPyrrolidinones
The invention discloses a subcutaneous implant rod for long-acting blood pressure reduction and a preparation method of the subcutaneous implant rod. A diblock copolymer is adopted as a carrier, and the formula comprises the following substances in parts by weight: 10-20 parts of polycaprolactone, 5-10 parts of polyethylene glycol, 1-5 parts of polyvinylpyrrolidone and 5-20 parts of bulk drugs; and wherein the raw material medicine is prepared by combining two medicines, namely perindopril and amlodipine, and the mass ratio of the perindopril to the amlodipine in the raw material medicine is (1-20): 1. After the implant rod of the invention is implanted subcutaneously, direct long-term sustained-release administration is performed to treat and stabilize blood pressure, the diblock copolymer is adopted as a carrier, the problem that an existing implant rod is short in loading action time is solved, the preparation method is simple, materials are easy to obtain, and the production process is simple and easy to master; and the prepared implant rod is implanted under the skin of a human body, has complete degradability, good biocompatibility and no toxicity according to biological materials, and is good in comfort level when implanted under the skin of the human body.
Owner:吕汇川
Medicinal composition for lowering blood pressure
InactiveCN101612156ACardiovascular disorderHeterocyclic compound active ingredientsCaptoprilAdditive ingredient
The invention relates to a medicinal composition for lowering blood pressure, which consists of trichlormethiazide and angiotensin converting enzyme inhibitor which serve as active ingredients and a pharmaceutical carrier, wherein the angiotensin converting enzyme inhibitor may be enalapril, cilazapril, benazepril, captopril, ramipril, perindopril and fosinopril. The unit dosage of trichlormethiazide is 0.5 to 8 milligrams, preferably 2 to 4 milligrams. The unit dosage of angiotensin converting enzyme inhibitor is 1.5 to 50 milligrams. The medicinal composition can be made into various oral preparations including conventional tablets, capsules, chewable tablets, dispersible tablets and effervescent tablets.
Owner:STAR LAKE BIOSCI CO INC ZHAOQING GUANGDONG
Preparation method of perindopril arginine salt with gamma crystal form
PendingCN114149357AReduce churnEasy to operateOrganic compound preparationOrganic chemistry methodsArgininePhysical chemistry
The invention provides a preparation method of gamma crystal form perindopril arginine salt, which comprises the following steps: adding perindopril and L-arginine into water, stirring and dissolving until the solution is clear, filtering, mixing the filtrate and an organic solvent in a dropwise adding manner, cooling to room temperature after dropwise adding, stirring and crystallizing for 1-2 hours, performing suction filtration to obtain a filter cake, leaching and draining the filter cake, and drying to obtain the gamma crystal form perindopril arginine salt. And drying the filter cake to obtain the gamma crystal form perindopril arginine salt. The method does not need low-temperature, freeze-drying and other operations, has no special requirements on equipment, also does not need reflux or heating operation, and is wide in crystallization temperature application range, and the obtained product is easy to filter, easy to separate and high in quality level.
Owner:JIANGSU SINOBIOPHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Process for making (2S, 3aS, 7aS)-1-[(2S)-2-[[(1S)-1-(ethoxycarbonyl) butyl] amino]-1-oxopropyl] octahydro-1H-indole-2-carboxylic acid Process for making (2S, 3aS, 7aS)-1-[(2S)-2-[[(1S)-1-(ethoxycarbonyl) butyl] amino]-1-oxopropyl] octahydro-1H-indole-2-carboxylic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/147bfe42-50cc-4784-9a73-32fcd70270b1/US20060178422A1-20060810-C00001.png)
![Process for making (2S, 3aS, 7aS)-1-[(2S)-2-[[(1S)-1-(ethoxycarbonyl) butyl] amino]-1-oxopropyl] octahydro-1H-indole-2-carboxylic acid Process for making (2S, 3aS, 7aS)-1-[(2S)-2-[[(1S)-1-(ethoxycarbonyl) butyl] amino]-1-oxopropyl] octahydro-1H-indole-2-carboxylic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/147bfe42-50cc-4784-9a73-32fcd70270b1/US20060178422A1-20060810-C00002.png)
![Process for making (2S, 3aS, 7aS)-1-[(2S)-2-[[(1S)-1-(ethoxycarbonyl) butyl] amino]-1-oxopropyl] octahydro-1H-indole-2-carboxylic acid Process for making (2S, 3aS, 7aS)-1-[(2S)-2-[[(1S)-1-(ethoxycarbonyl) butyl] amino]-1-oxopropyl] octahydro-1H-indole-2-carboxylic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/147bfe42-50cc-4784-9a73-32fcd70270b1/US20060178422A1-20060810-C00003.png)
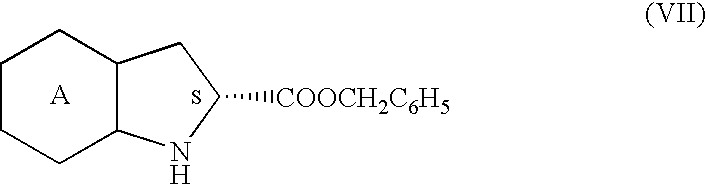



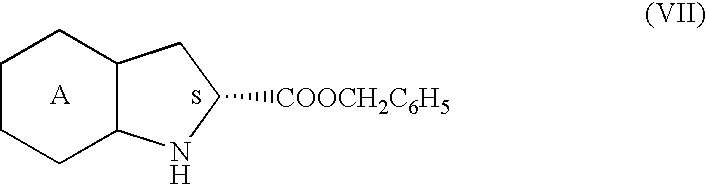
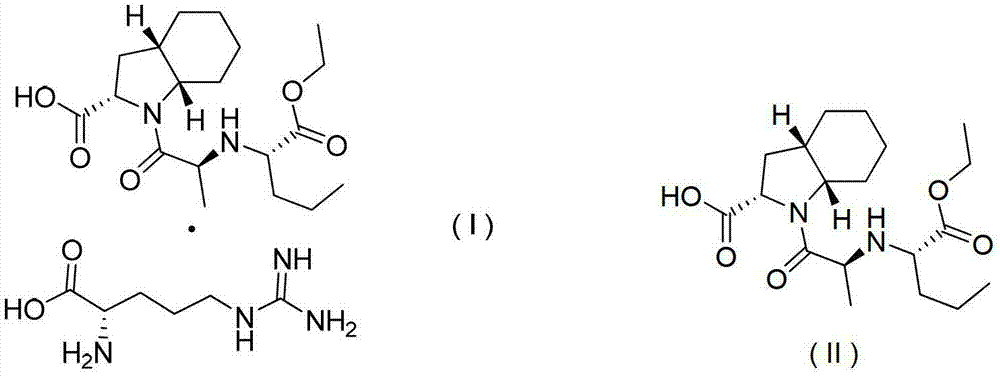
![Process for manufacture of pure (2S, 3aS, 7aS)-1-[(2S)-2-[[(1S)-1-(ethoxycarbonyl) butyl]amino]-1-oxopropyl] octahydro-1H-indole-2-carboxylic acid and its tert. butyl amine salt Process for manufacture of pure (2S, 3aS, 7aS)-1-[(2S)-2-[[(1S)-1-(ethoxycarbonyl) butyl]amino]-1-oxopropyl] octahydro-1H-indole-2-carboxylic acid and its tert. butyl amine salt](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d44bd65f-aad8-4e3a-b294-0bdf4bae20c0/US07615571-20091110-C00001.png)
![Process for manufacture of pure (2S, 3aS, 7aS)-1-[(2S)-2-[[(1S)-1-(ethoxycarbonyl) butyl]amino]-1-oxopropyl] octahydro-1H-indole-2-carboxylic acid and its tert. butyl amine salt Process for manufacture of pure (2S, 3aS, 7aS)-1-[(2S)-2-[[(1S)-1-(ethoxycarbonyl) butyl]amino]-1-oxopropyl] octahydro-1H-indole-2-carboxylic acid and its tert. butyl amine salt](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d44bd65f-aad8-4e3a-b294-0bdf4bae20c0/US07615571-20091110-C00002.png)
![Process for manufacture of pure (2S, 3aS, 7aS)-1-[(2S)-2-[[(1S)-1-(ethoxycarbonyl) butyl]amino]-1-oxopropyl] octahydro-1H-indole-2-carboxylic acid and its tert. butyl amine salt Process for manufacture of pure (2S, 3aS, 7aS)-1-[(2S)-2-[[(1S)-1-(ethoxycarbonyl) butyl]amino]-1-oxopropyl] octahydro-1H-indole-2-carboxylic acid and its tert. butyl amine salt](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d44bd65f-aad8-4e3a-b294-0bdf4bae20c0/US07615571-20091110-C00003.png)