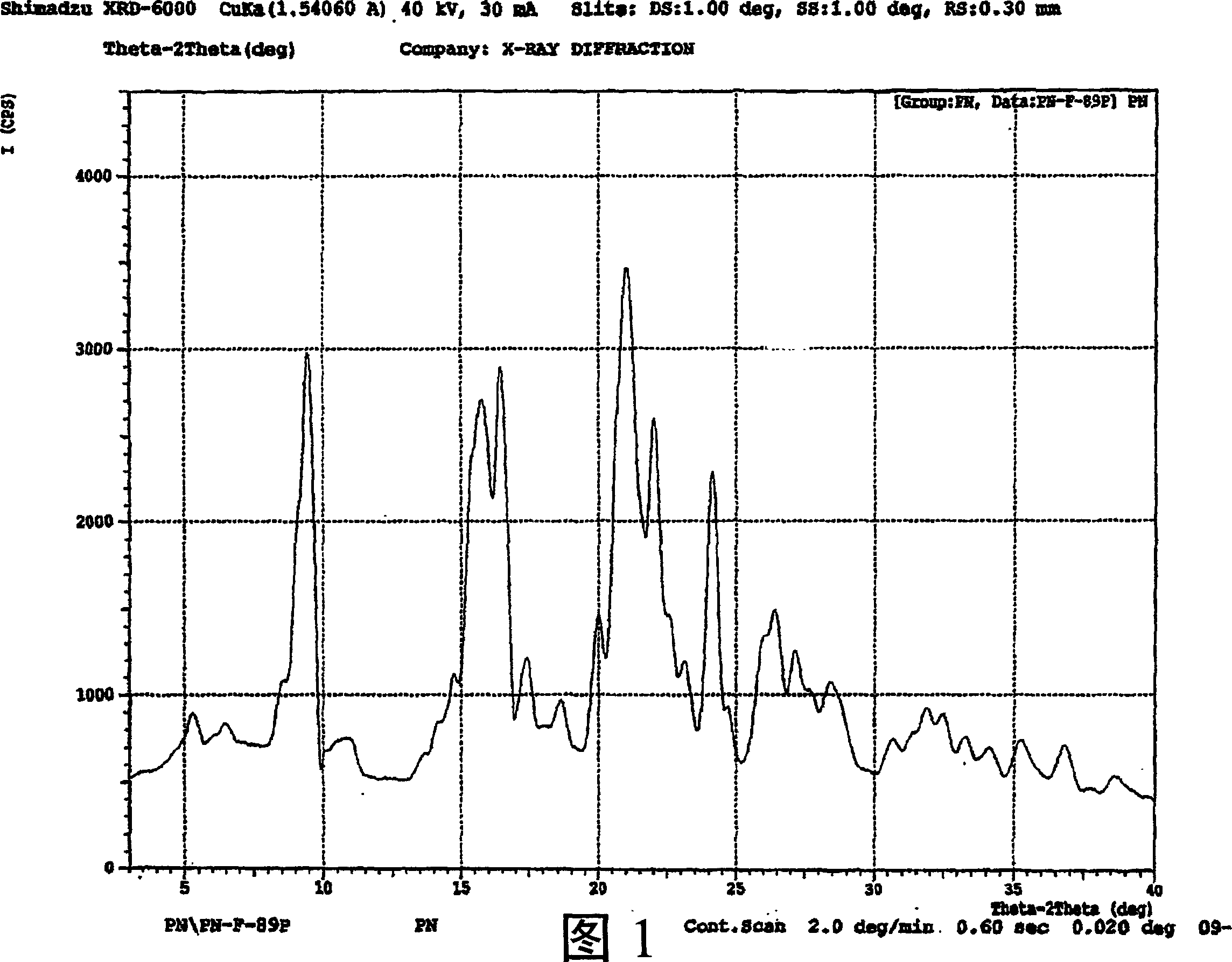
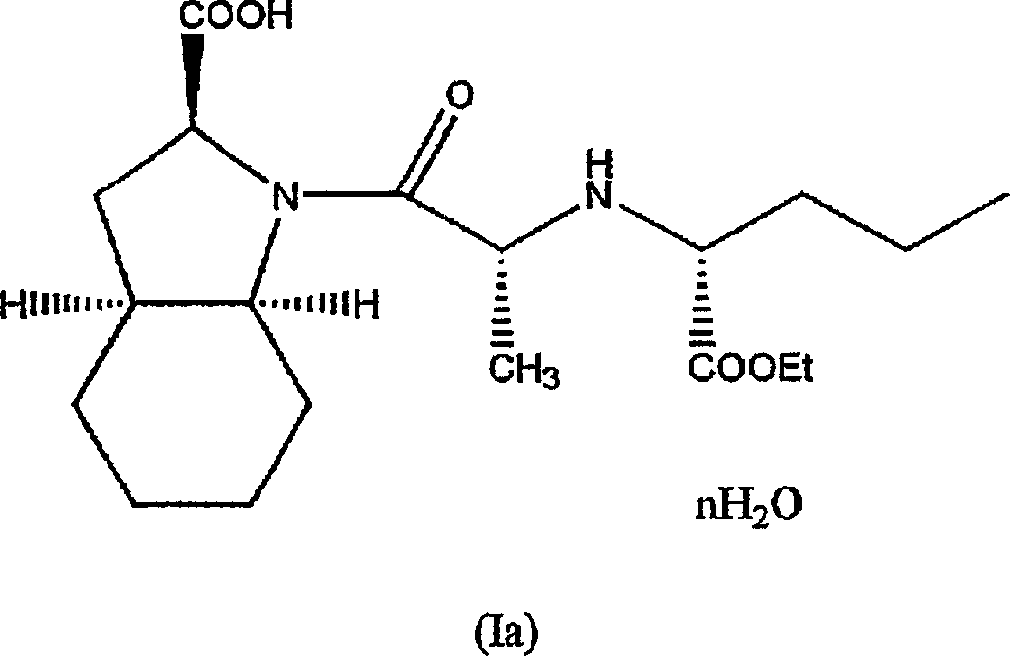
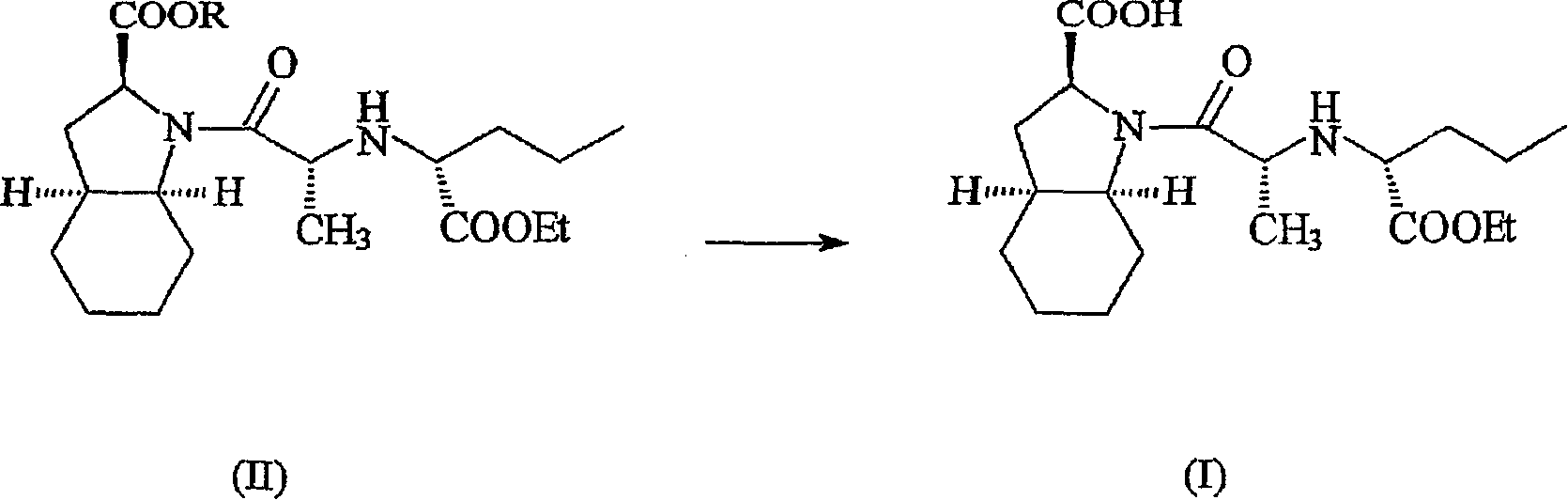
Hydrated perindopril salt, preparation method thereof and composition containing the same
A technology of perindopril and perindopril hydrate, which is applied in the field of perindopril hydrate salt, its preparation and use, and compositions containing it, and can solve the problems of time-consuming preparation methods and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The (2S, 3aS, 7aS)-1-{2-[1-(ethoxycarbonyl)-(S)-butylamino]-(S)-propionyl}-octahydroindole-2-carboxylic acid The benzyl ester, perindopril benzyl (10 g) was dissolved in isopropanol (100 mL). To the clear solution was added tert-butylamine (2.5g) and 10% w / w palladium on charcoal (2g). at 1kg / cm 2 The reaction mixture was hydrogenated under pressure for 2 hours.
[0050] The reaction mass was filtered to remove the catalyst. Concentrate in vacuo to remove solvent and replace isopropanol by concurrent addition of ethyl acetate. The obtained solid was cooled to 0EC and filtered to obtain perindopril (7.8g).
Embodiment 2
[0052] Perindopril (10 g) was suspended in acetone (80 mL). Water (0.4 mL) was added thereto, and the contents were heated until the solids dissolved and cooled to room temperature. The resulting slurry was filtered to obtain perindopril monohydrate (9.4 g).
Embodiment 3
[0054] Perindopril (20 g) was suspended in ethyl acetate (300 mL). To this was added water (1.5 mL) and the contents were heated until the solids dissolved and cooled to 10EC. The resulting slurry was filtered to obtain perindopril monohydrate (17 g).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com