Preparation method of perindopril arginine salt with gamma crystal form
A technology of indopril arginine salt and arginine, which is applied to the synthesis of arginine perindopril and the preparation of γ crystal form perindopril arginine salt, so as to save costs and reduce active ingredients. Churning, easy-to-manipulate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
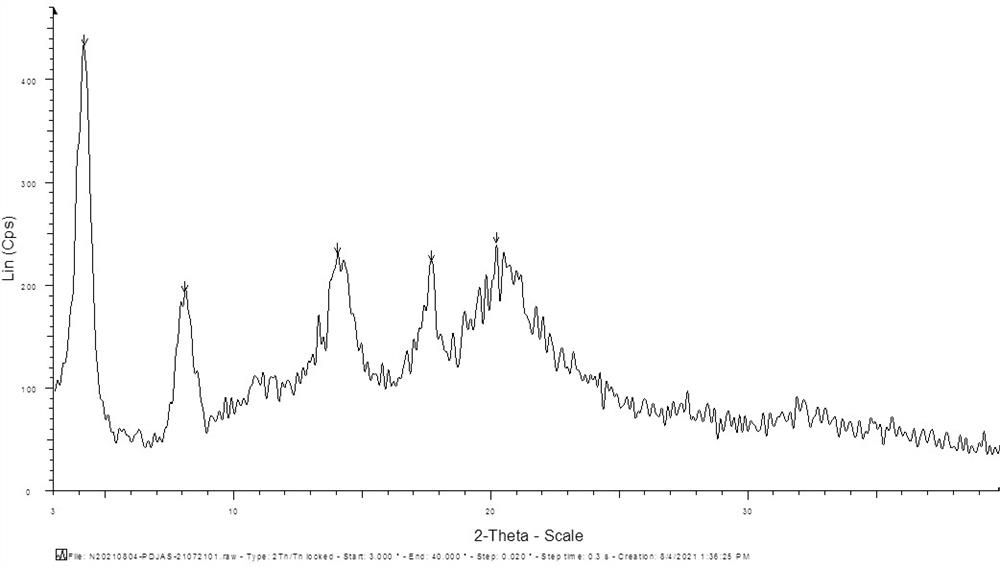
[0022] Add 5.0g of perindopril, 2.4g of L-arginine and 20ml of water into the reaction bottle, stir at room temperature until completely dissolved, filter, add 60ml of dimethyl sulfoxide dropwise to the filtrate, without temperature control during the dropwise addition, The temperature will not exceed 50°C. After the dropwise addition, cool down to 20~30°C, continue to stir for 1 hour, filter with suction, rinse the filter cake with 50ml acetone and 50ml n-heptane in sequence, drain it, and dry the filter cake to obtain Perindopro Arginine salt gamma crystal form. The XRD pattern of the gamma crystal form that this water / dimethyl sulfoxide system makes is as follows figure 1 shown.
Embodiment 2
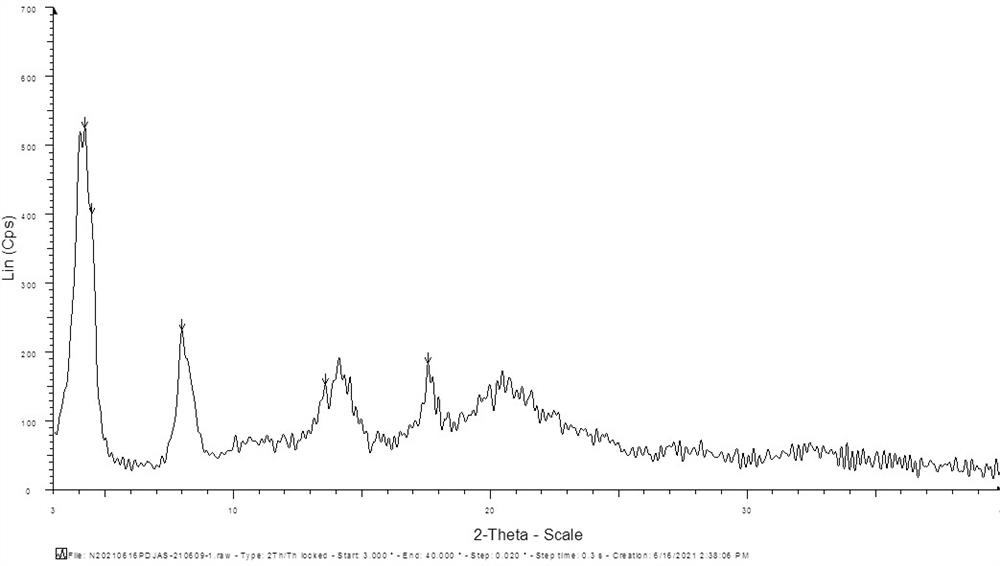
[0024] Add 5.0g of perindopril, 2.4g of L-arginine and 20ml of water into the reaction bottle, stir at room temperature until completely dissolved, filter, add 100ml of acetone dropwise to the filtrate, do not need to control the temperature during the dropwise addition, the temperature will not When the temperature exceeds 50°C, drop the temperature down to 20-30°C after the dropwise addition, continue to stir for 1 hour, filter with suction, rinse the filter cake with 50ml of n-heptane, drain it, and dry the filter cake to obtain perindopril arginine salt γ crystal type. The XRD pattern of the gamma crystal form thing that this water / acetone system makes is as follows figure 2 shown.
Embodiment 3
[0026] Add 11.8kg of perindopril, 5.5kg of L-arginine and 42.5kg of purified water into the reaction kettle, stir to dissolve, and filter. Add 240kg of dimethyl sulfoxide aqueous solution that has been cooled to below 10°C, start stirring, filter for 1 hour, wash with 118kg of acetone, filter for 20 minutes, then wash with 70kg of n-heptane, filter for 20 minutes, put the filter cake into a baking tray , vacuum drying at 45°C for 10 hours, and then vacuum drying at 80°C for 30 hours to obtain the industrially produced product of perindopril salt arginine gamma crystal form. (Purity: 99.62%, Impurity B: 0.081%, Impurity F: 0.205%)
[0027] The preparation process of the pharmaceutical composition of perindopril arginine salt gamma crystal form (10 mg / tablet) is as follows:
[0028] Prescription composition:
[0029] Perindopril Arginine 10mg
[0030] Lactose monohydrate 145mg
[0031] Magnesium Stearate 0.9mg
[0032] Maltodextrin 18mg
[0033] Colloidal silicon dioxide 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com