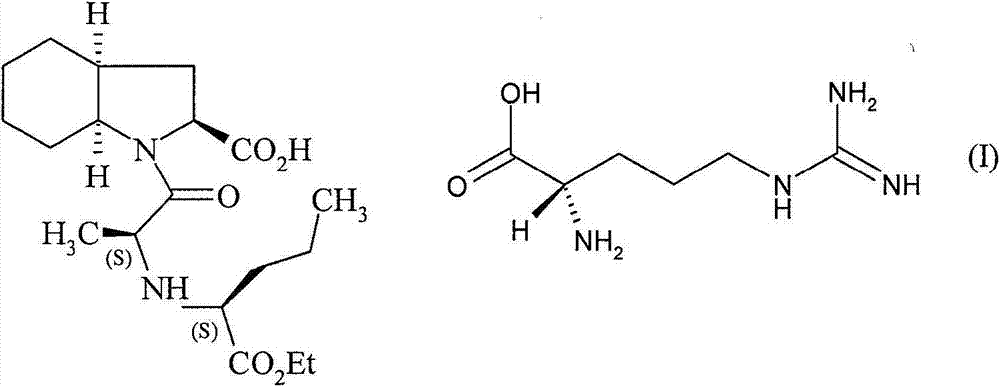
Process for the preparation of perindopril L-arginine salt
A technology of arginine salt and arginine, applied in the field of preparation of L-arginine salt of perindopril, which can solve difficult processing problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 : L-arginine salt of perindopril - starting from perindopril (free acid) in a binary mixture of acetonitrile / DMSO 25 / 75 without seeding
[0046] Perindopril (12.5 g, 1 eq.) and L-arginine (5.32 g-0.9 eq) were suspended in a mixture of acetonitrile (20 g, d=0.787) and DMSO (61 g, d=1.100). The reaction mixture was heated at 50 °C overnight. The product was then isolated by filtration through a frit. Wash and dry the filter cake.
[0047] Perindopril arginine (14.5 g) was obtained with a yield of 79% relative to perindopril. The separated crystalline phase is the delta phase. The HPLC quality of the isolated product was greater than 99.0%.
[0048] The filtration rate of mother liquor is about 6000kg / h / m 2 .
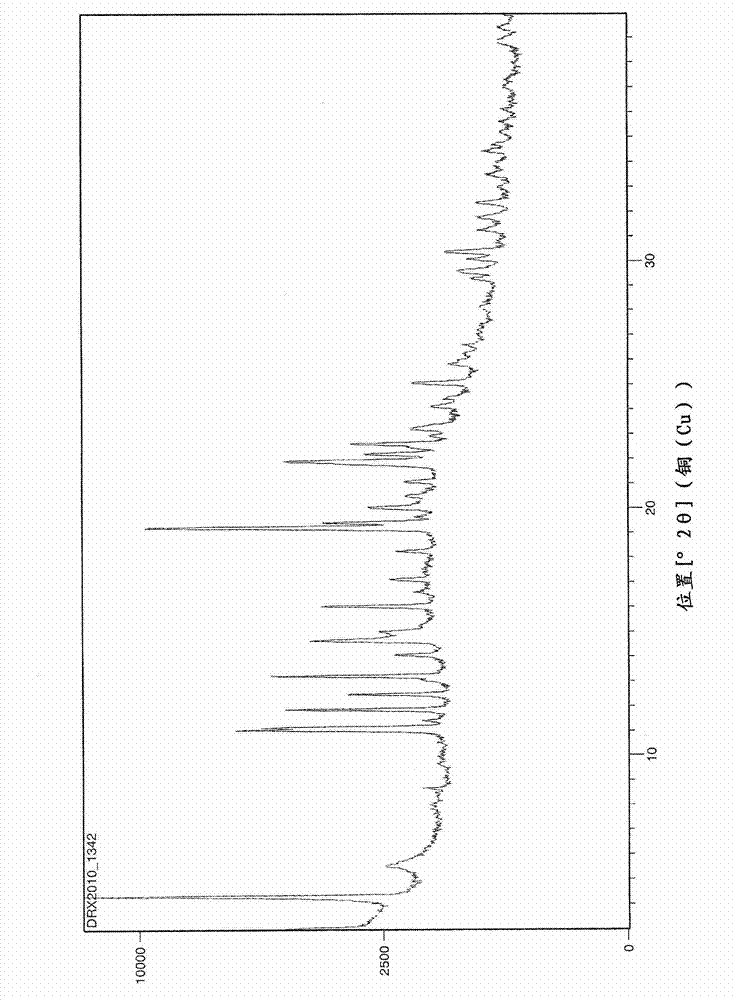
[0049] The L-arginine salt of perindopril thus obtained was in the delta crystal form. This crystalline form has the following X-ray powder diffraction pattern, determined using a diffractometer with a copper counter-cathode and expressed as inter...
Embodiment 2
[0053] Example 2 : L-arginine salt of perindopril - start with perindopril (free acid) in a binary mixture of acetonitrile / DMSO 25 / 75, seeded
[0054] Perindopril (100 g, 1 eq.) and L-arginine (42.6 g, 0.9 eq.) were suspended in a mixture of acetonitrile (220 g, d=0.787) and dimethylsulfoxide (630 g, d=1.100) middle. The reaction mixture was heated at 70°C for 3 hours, seeded with 2% delta phase, then cooled to 40°C over 1 hour. The mixture was kept at 40°C while stirring for 18 hours, then cooled to 20°C over 1 hour. The product was then isolated by filtration. Wash and dry the filter cake.
[0055] Perindopril (L)-arginine (119 g) was obtained at a yield of 79% relative to perindopril. The HPLC quality of the isolated product was greater than 99.0%.
[0056] The filtration rate of mother liquor is about 6000kg / h / m 2 .
Embodiment 3
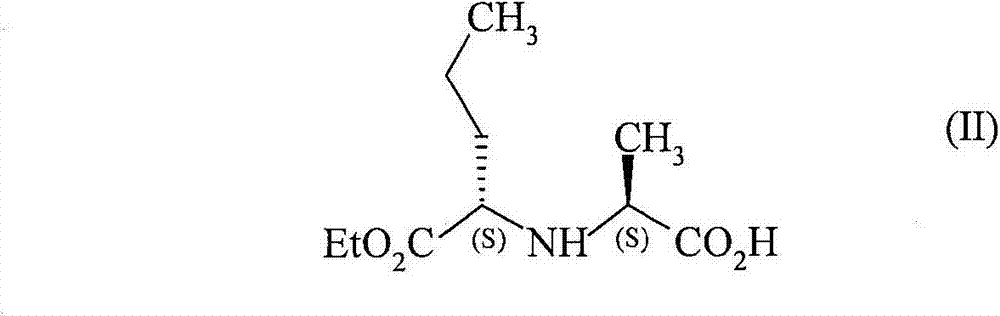
[0057] Example 3 : Starting from (2S, 3aS, 7aS)-2-carboxy-perhydroindole and N-[1-(S)-ethoxy-carbonyl-butyl]-(S)-alanine, by using N , General method of N'-carbonyl-diimidazole activation to generate perindopril (free acid)
[0058] N-[1-(S)-ethoxycarbonyl-butyl]-(S)-alanine (65g, 1eq.) and N,N'-carbonyldiimidazole (48g, 1eq.) were introduced, followed by acetonitrile (500g). The reaction mixture was then stirred for 3 hours at a temperature below +10°C.
[0059]The reaction mixture was poured into (2S, 3aS, 7aS)-2-carboxyperhydroindole (50 g, 1 eq.); an amount of fresh acetonitrile (80 g) was used to rinse the instrument.
[0060] The reaction mixture was then stirred at a temperature below +10°C for 5 hours and then clarified with a filter to obtain a clear solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com