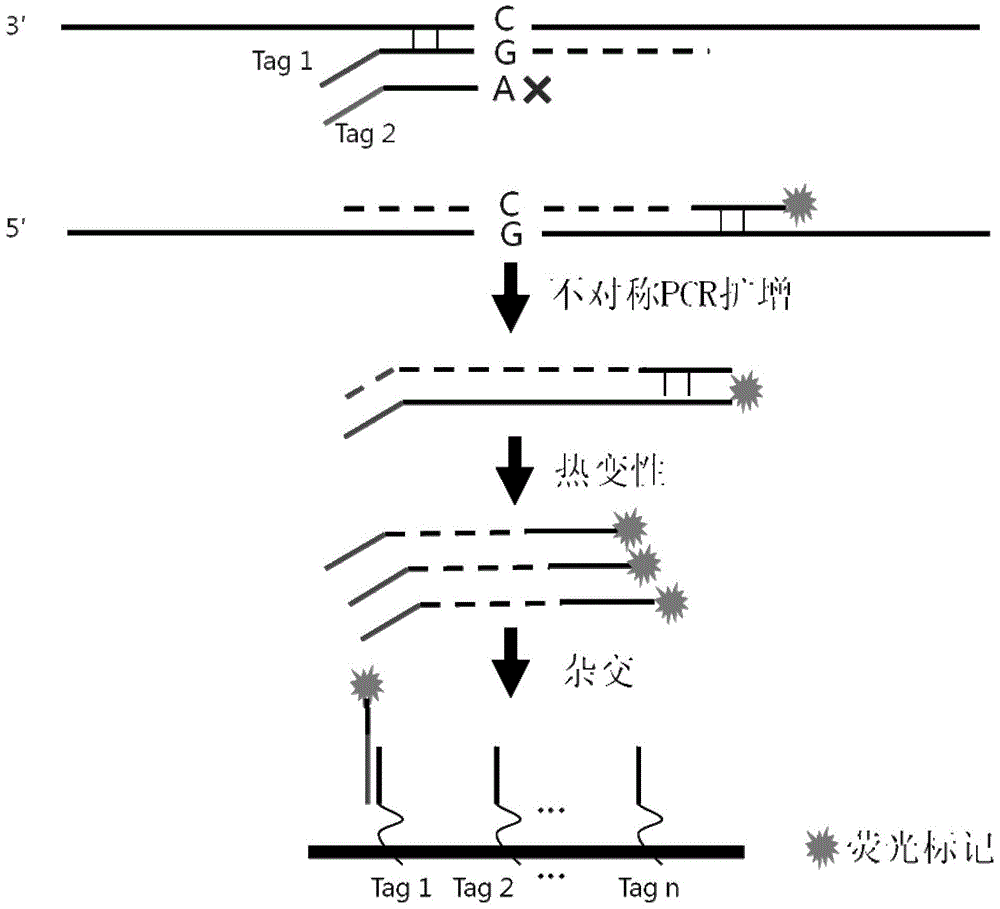
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
293 results about "Clopidogrel" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
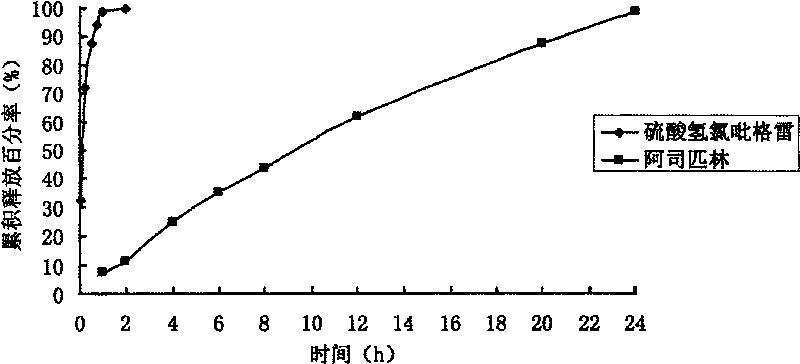
Clopidogrel is used to prevent heart attacks and strokes in persons with heart disease (recent heart attack), recent stroke, or blood circulation disease (peripheral vascular disease). It is also used with aspirin to treat new/worsening chest pain (new heart attack, unstable angina) and to keep blood vessels open and prevent blood clots after certain procedures (such as cardiac stent).
Nanoparticulate clopidogrel and aspirin combination formulations
InactiveUS20070003615A1Reduced bioavailabilityHigh dissolution ratePowder deliveryBiocideControl releasePharmaceutical drug
The present invention is directed to compositions comprising a nanoparticulate clopidogrel and aspirin combination, or salts or derivatives thereof, having improved clopidogrel bioavailability. The nanoparticulate clopidogrel particles, and optionally the nanoparticulate aspirin particles, of the composition have an effective average particle size of less than about 2000 nm and are useful in the prevention and treatment of pathologies induced by platelet aggregation. The clopidogrel and aspirin particles may also be formulated as a controlled release polymeric coating or matrix drug delivery system.
Owner:ELAN PHRMA INT LTD
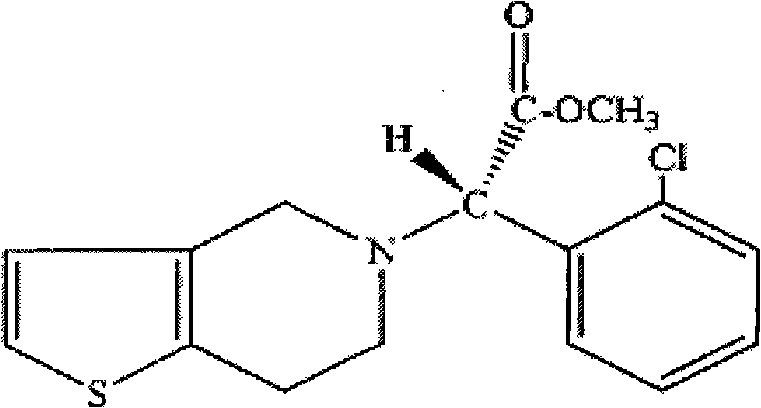
Method for preparing clopidogrel and its derivatives
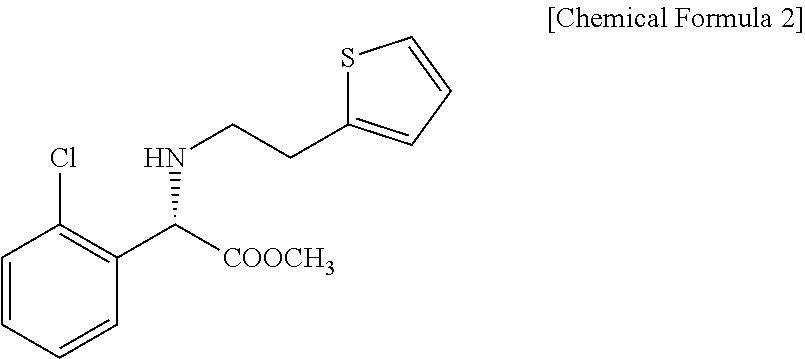
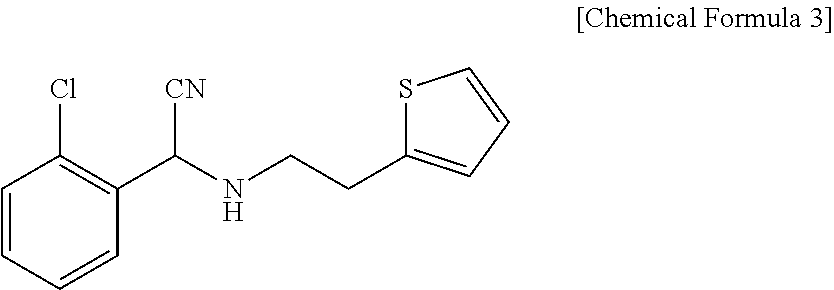
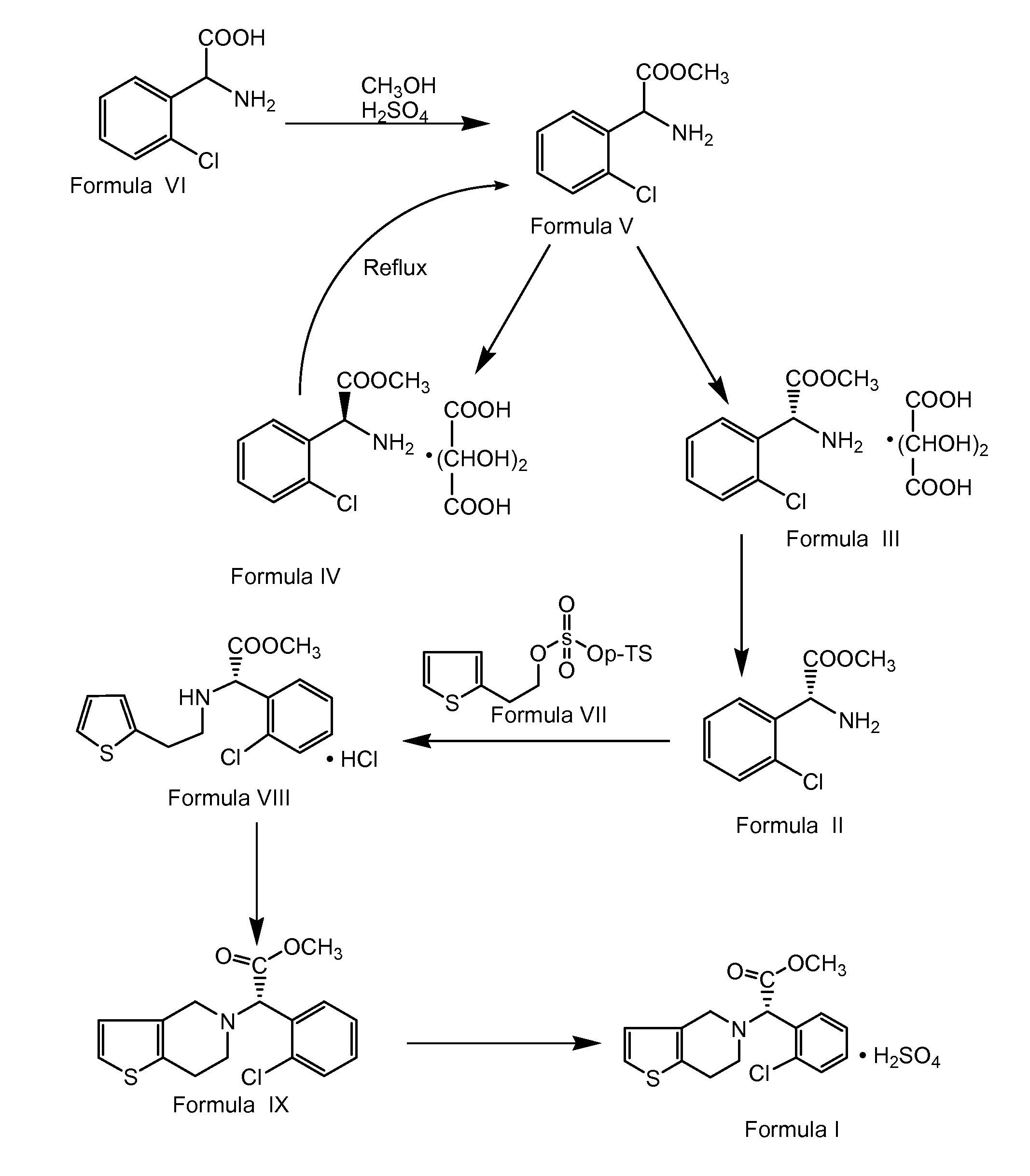
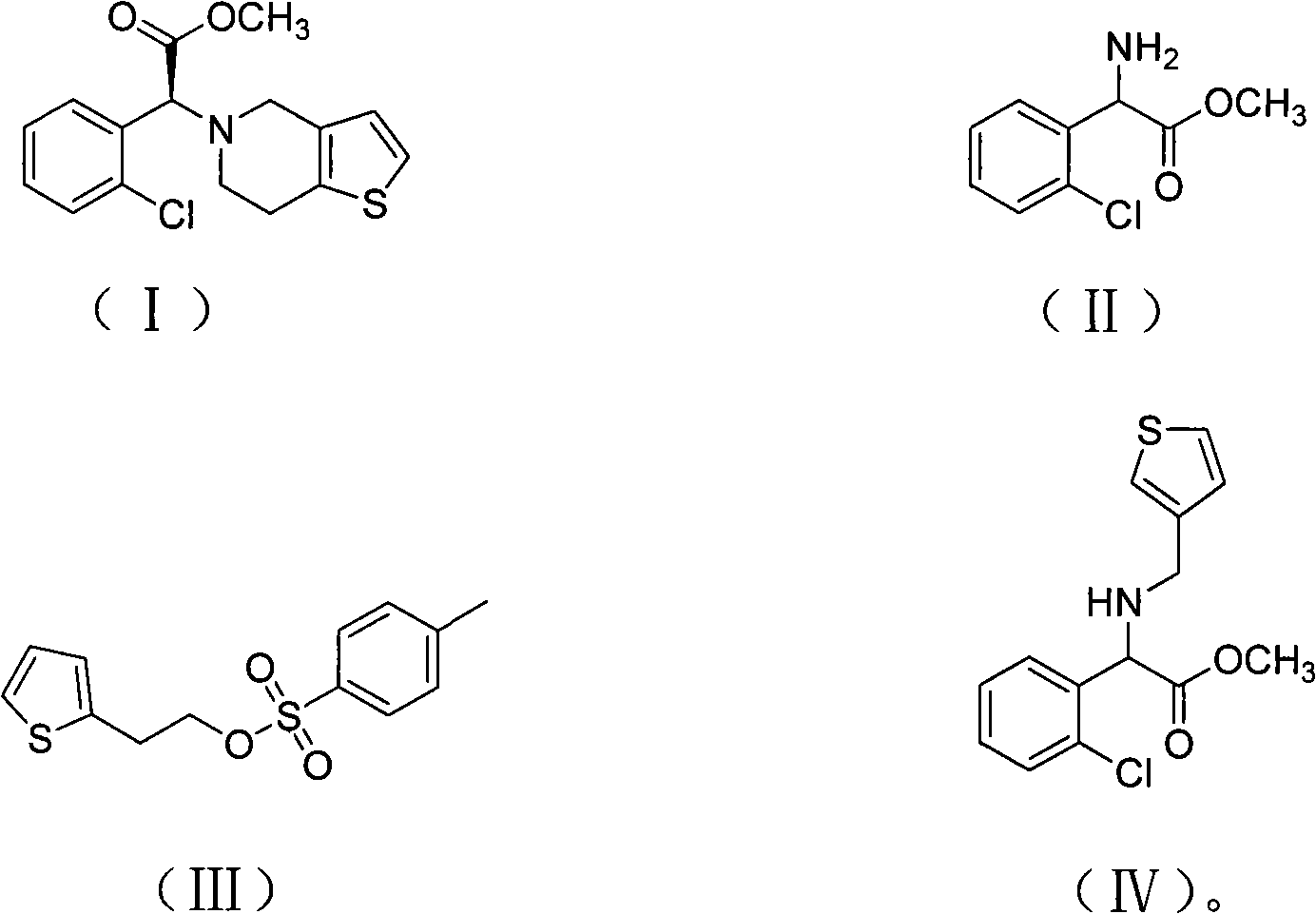
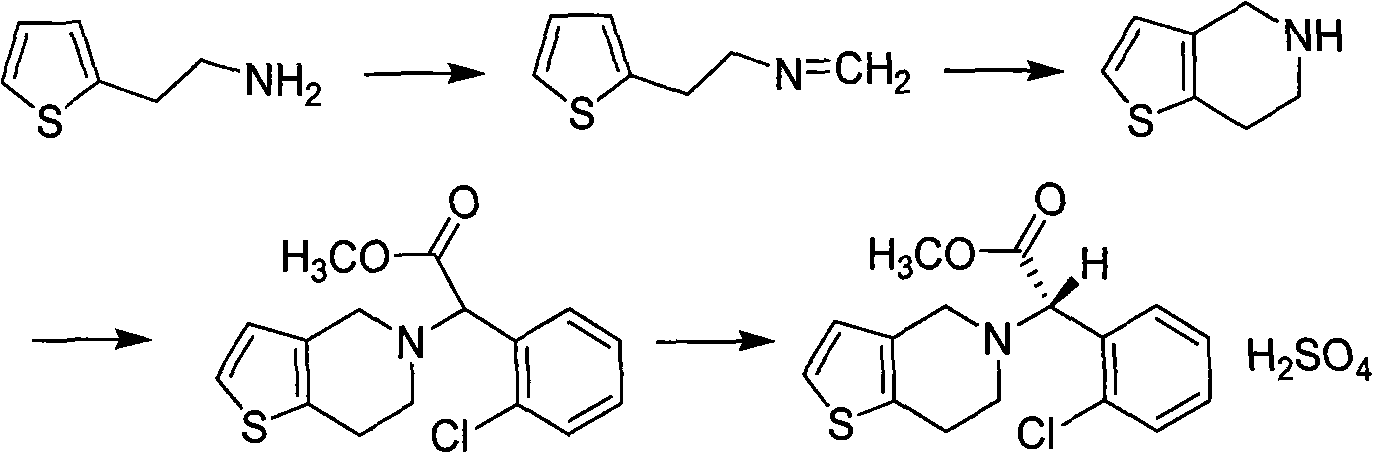
The present invention relates to a method for preparing Clopidogrel and its derivatives. More particularly, the present invention is a method for preparation of (S)-2-Clopidogrel and its derivatives, which are active inhibitors of platelet aggregation, from an optically active (S)-2-chlorophenyl glycine alkyl ester through hydrolysis of racemic 2-chlorophenylglycine alkyl esters using an enzyme. The present invention employs a simple procedure to prepare Clopidogrel and its derivatives. Because no chiral resolving agents are used except for a small amount of enzyme, the cost of preparation can be reduced. In addition, the present invention is suitable for synthesizing highly optical-active Clopidogrel and its derivatives on a large scale by using optically active (S)-2-chlorophenylglycine alkyl ester obtained in high yield as an intermediate, and is also environmentally friendly since no highly toxic reagents are employed.
Owner:ENZYTECH LTD
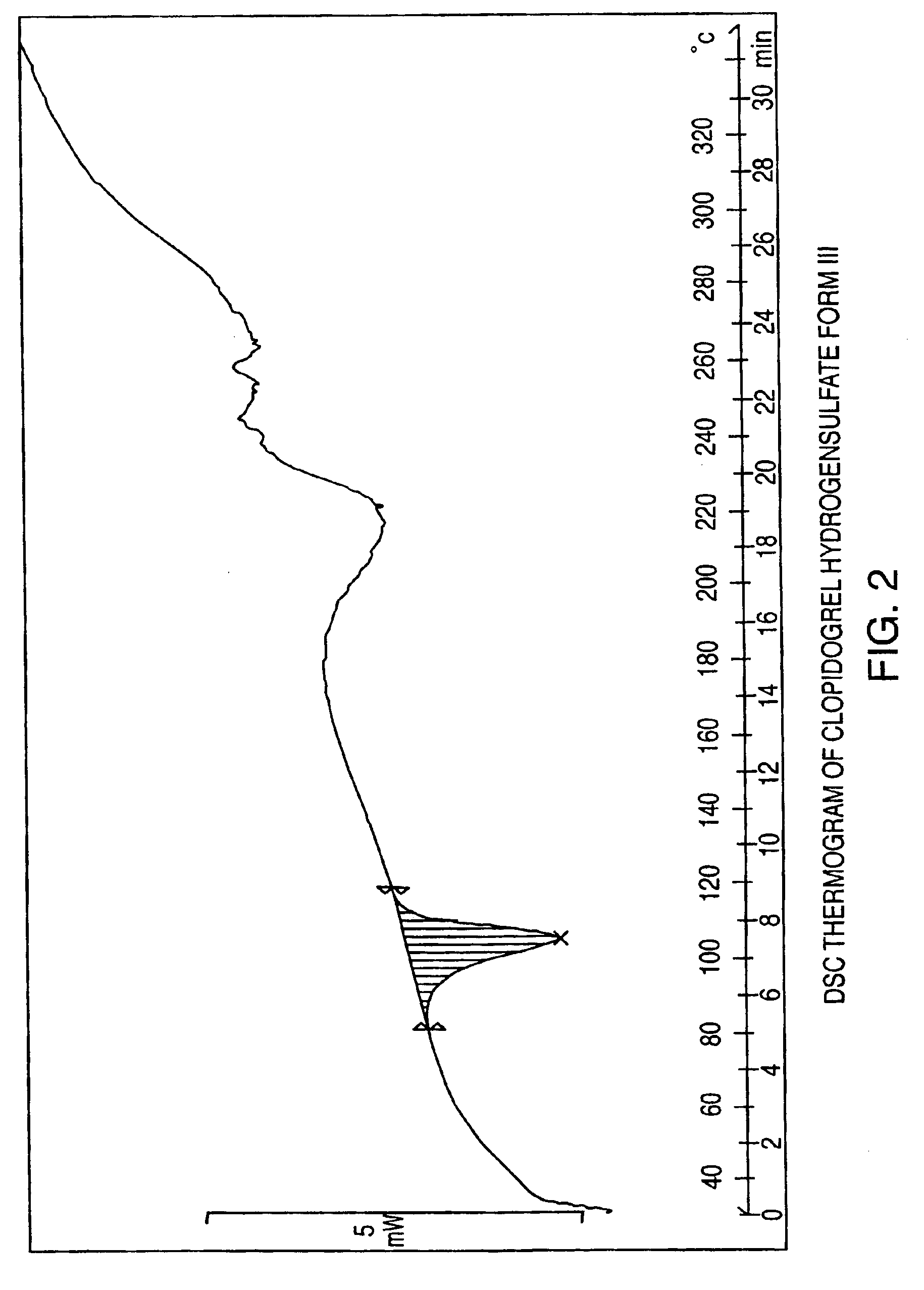
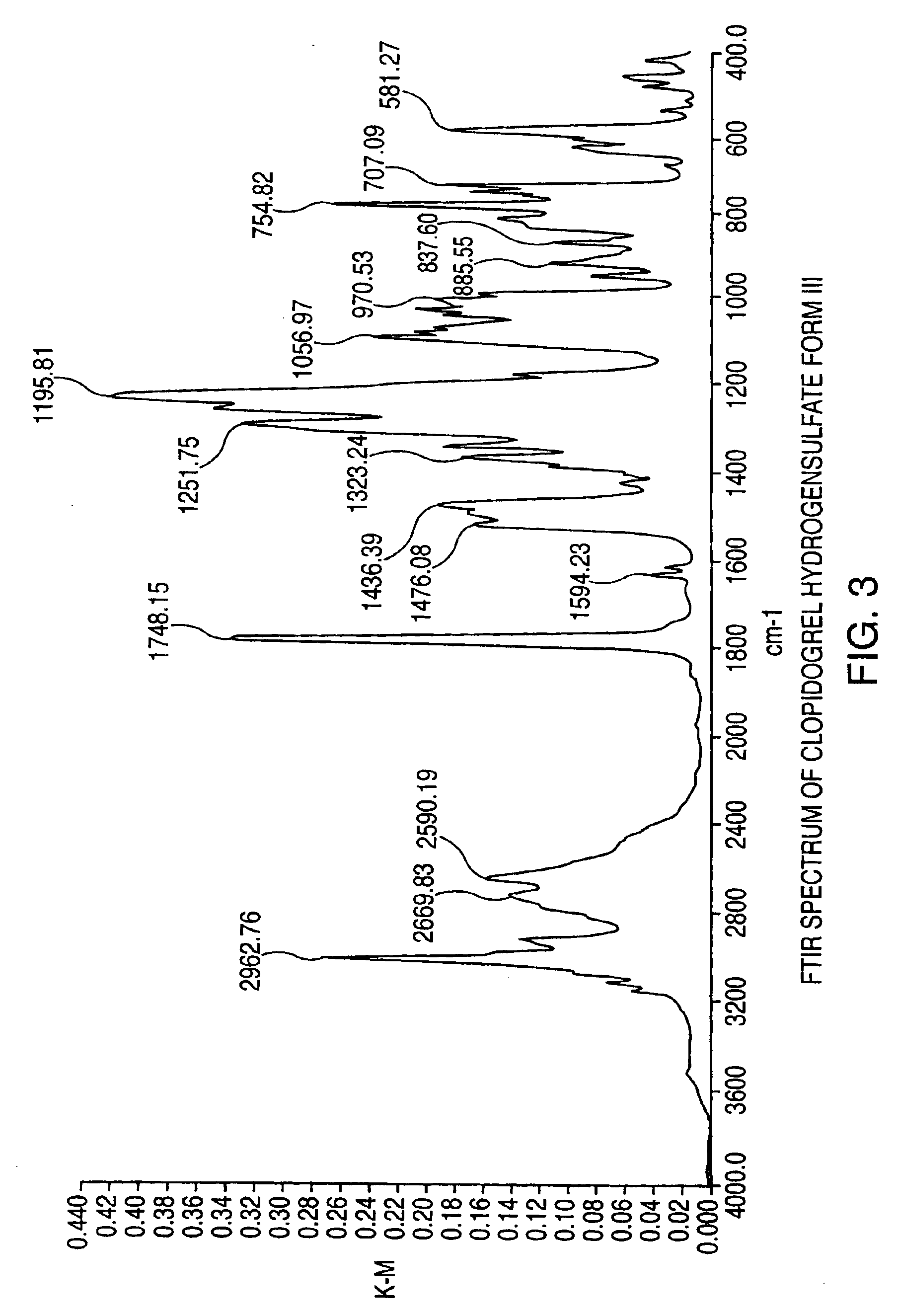
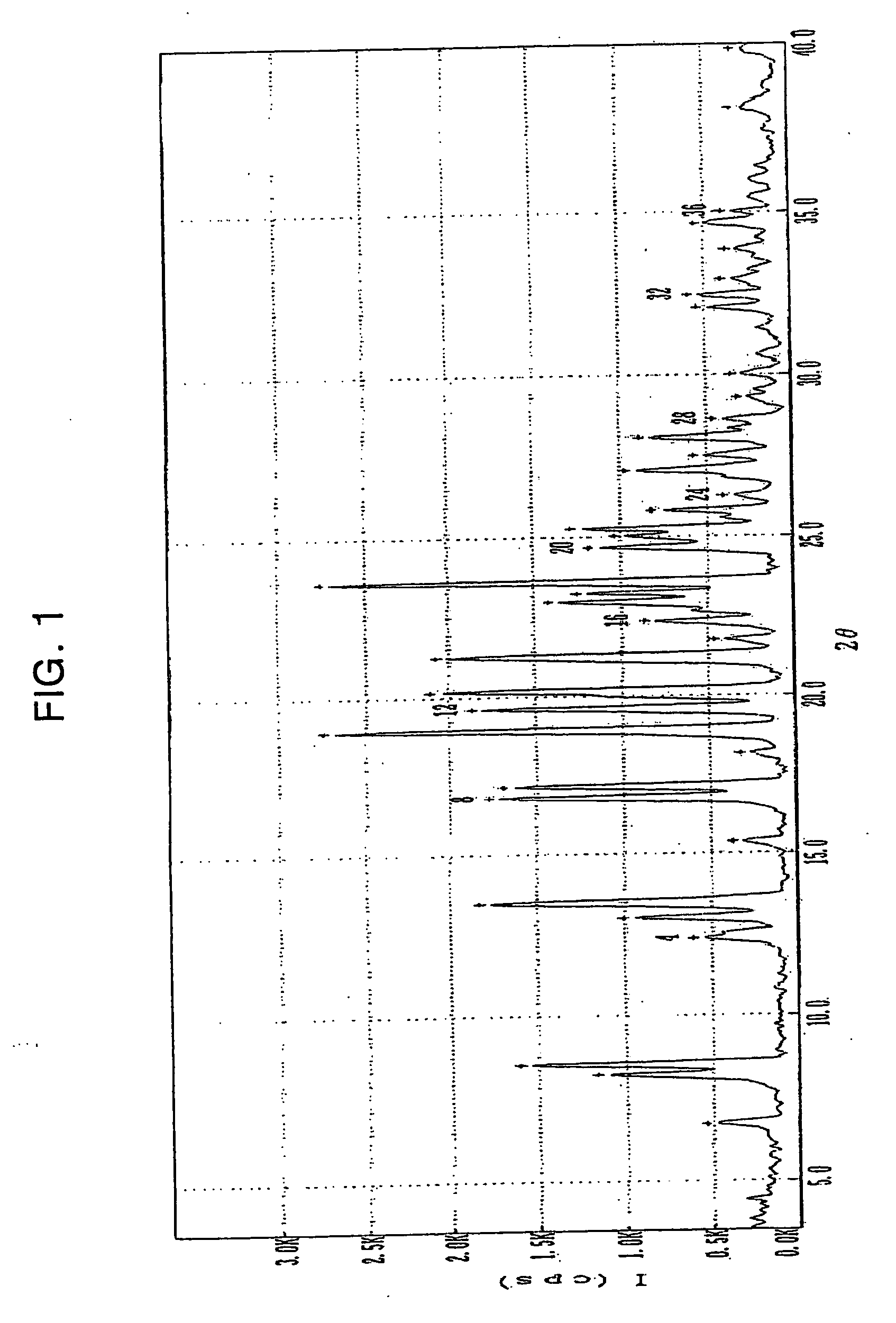
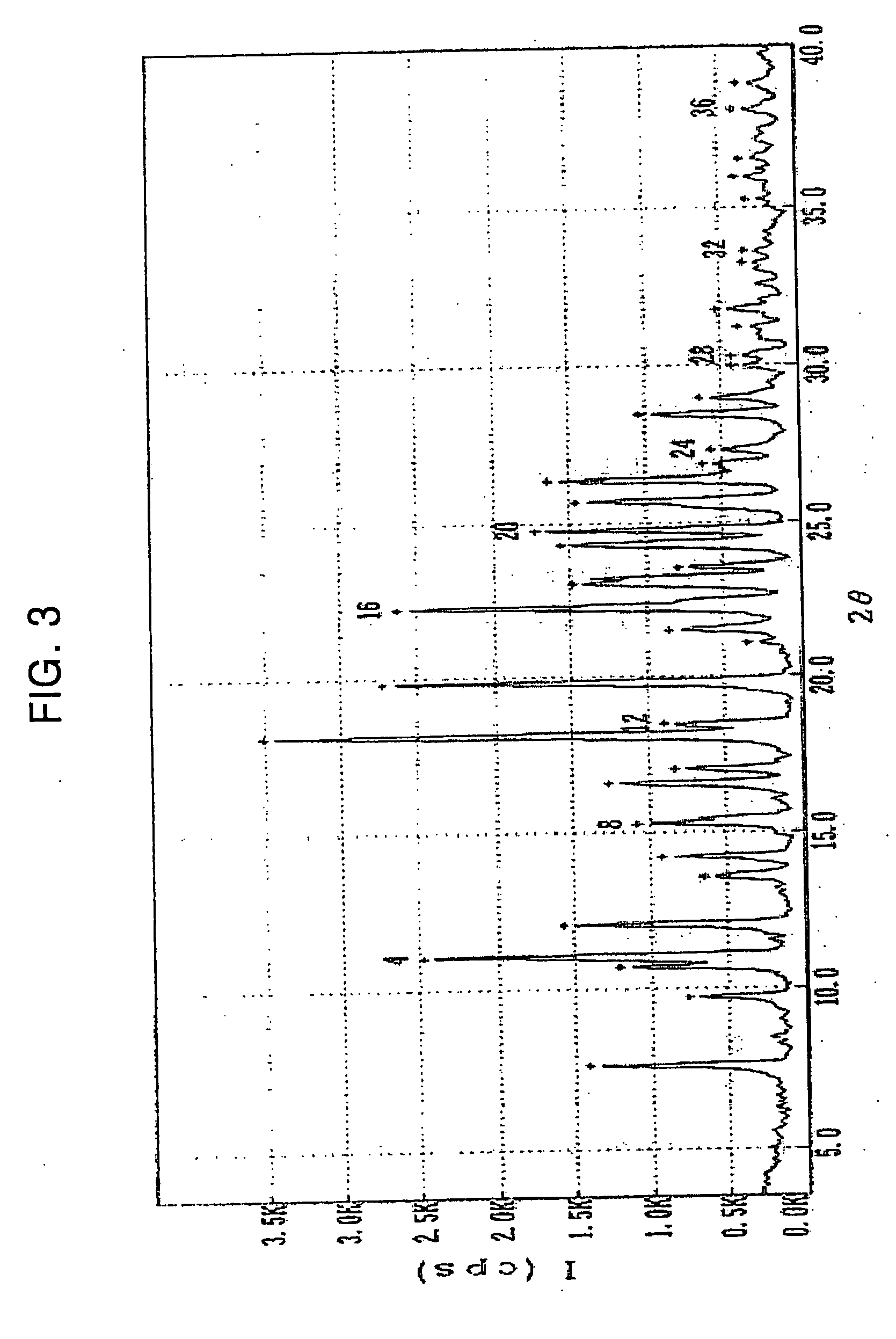
Polymorphs of clopidogrel hydrogensulfate
Provided are new crystalline Forms III, IV, V and VI of clopidogrel hydrogensulfate and the amorphous form of clopidogrel hydrogensulfate, as well as their pharmaceutical compositions, and method of treatments with such compositions. Also provided are novel processes for preparation of clopidogrel hydrogensulfate Form I, Form II, Form III, Form IV, Form V, Form VI and amorphous form.
Owner:TEVA PHARMA IND LTD
Carbonyl reductase, gene and mutant and application thereof to asymmetrical reduced carbonyl compound
ActiveCN102618513AHigh optical purityMild reaction conditionsBacteriaMicroorganism based processesHigh concentrationMethyl o-chloromandelate
The invention discloses a novel carbonyl reductase, a gene, a mutant thereof, a recombinant expression vector containing the gene and the mutant, a recombinant expression transformant, a recombinase preparation method, and applications of the carbonyl reductase and recombinase to preparation of active chiral alcohols with a chiral carbonyl compound before asymmetrical reduction. The carbonyl reductase is derived from candida glabrata, is applied to preparation of a plurality of optically-active chiral alcohols such as (R)-chloromandelic acid methyl ester, (R)-2-hydroxy-4-phenyl ethyl butyrate, (R)-4-chlorin-3-phenyl ethyl butyrate and the like. Compared with other preparation methods, a product prepared through the method has high concentration, does not require additionally or slightly adding any expensive coenzyme, has high optical purity, and has the advantages of mild reaction conditions, easiness and convenience for operating, easiness for amplifying and the like, and has a good industrial application prospect in the production of clopidogrel, L-carnitine and perindopril antihypertensive medicinal intermediates.
Owner:EAST CHINA UNIV OF SCI & TECH
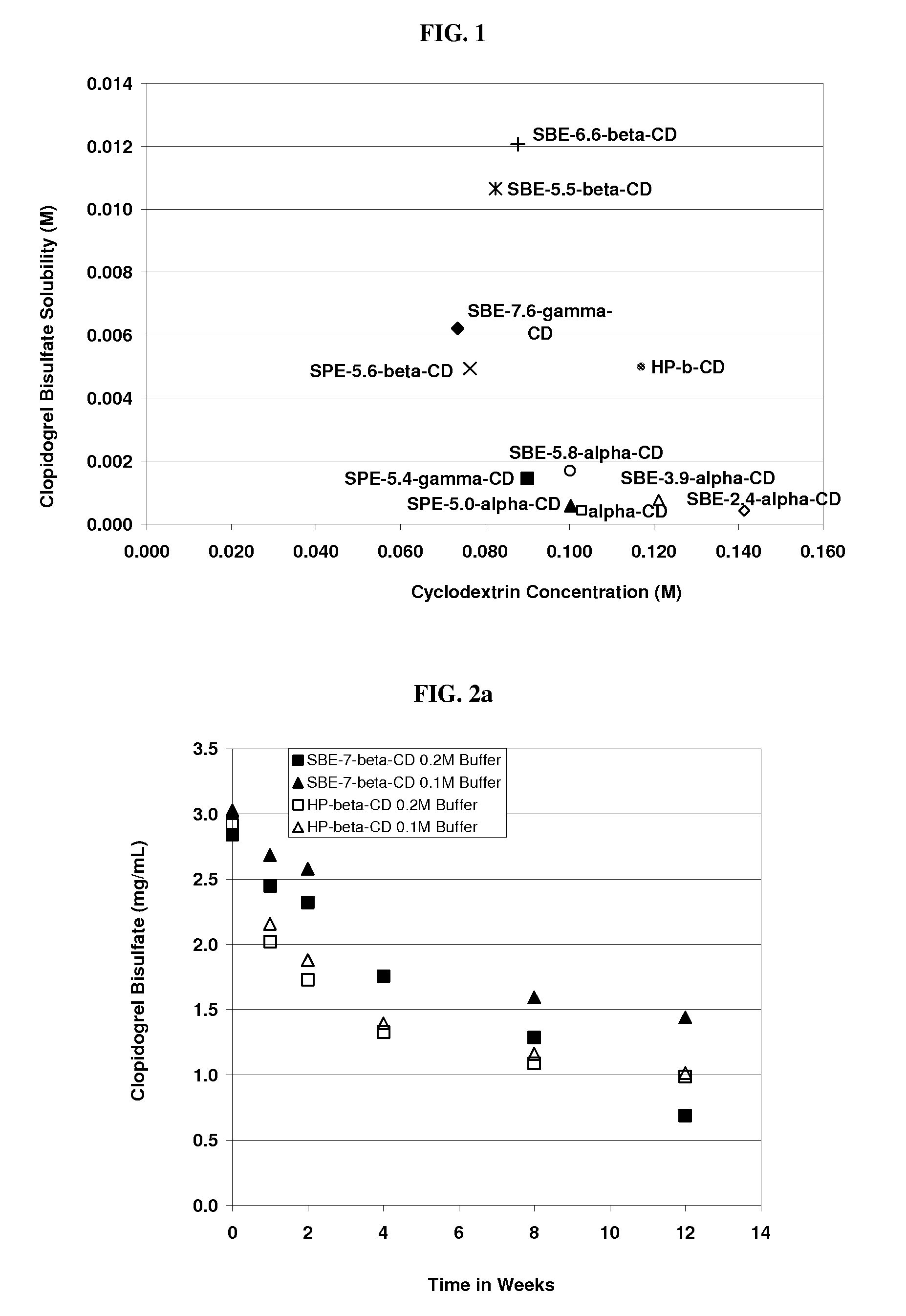
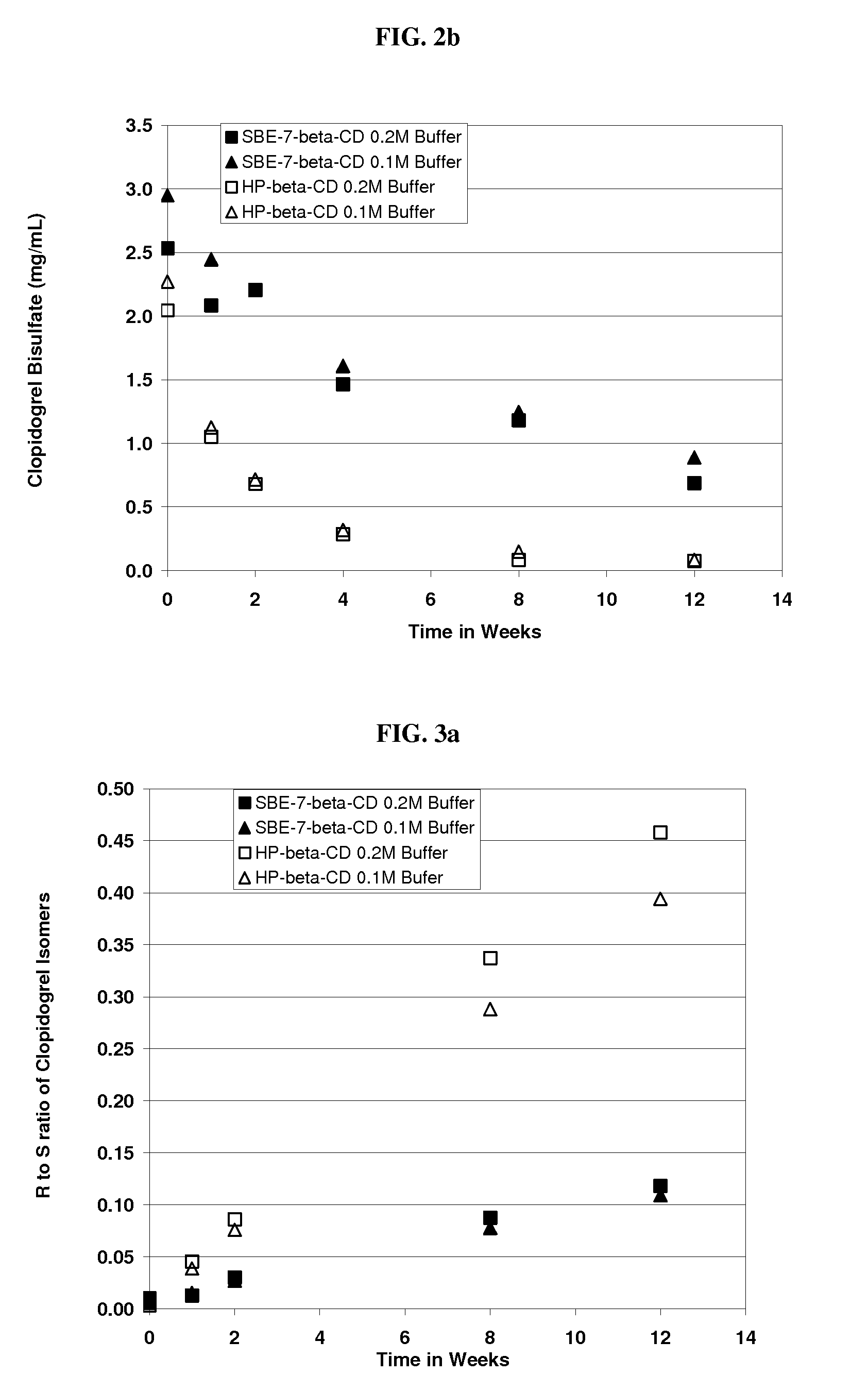
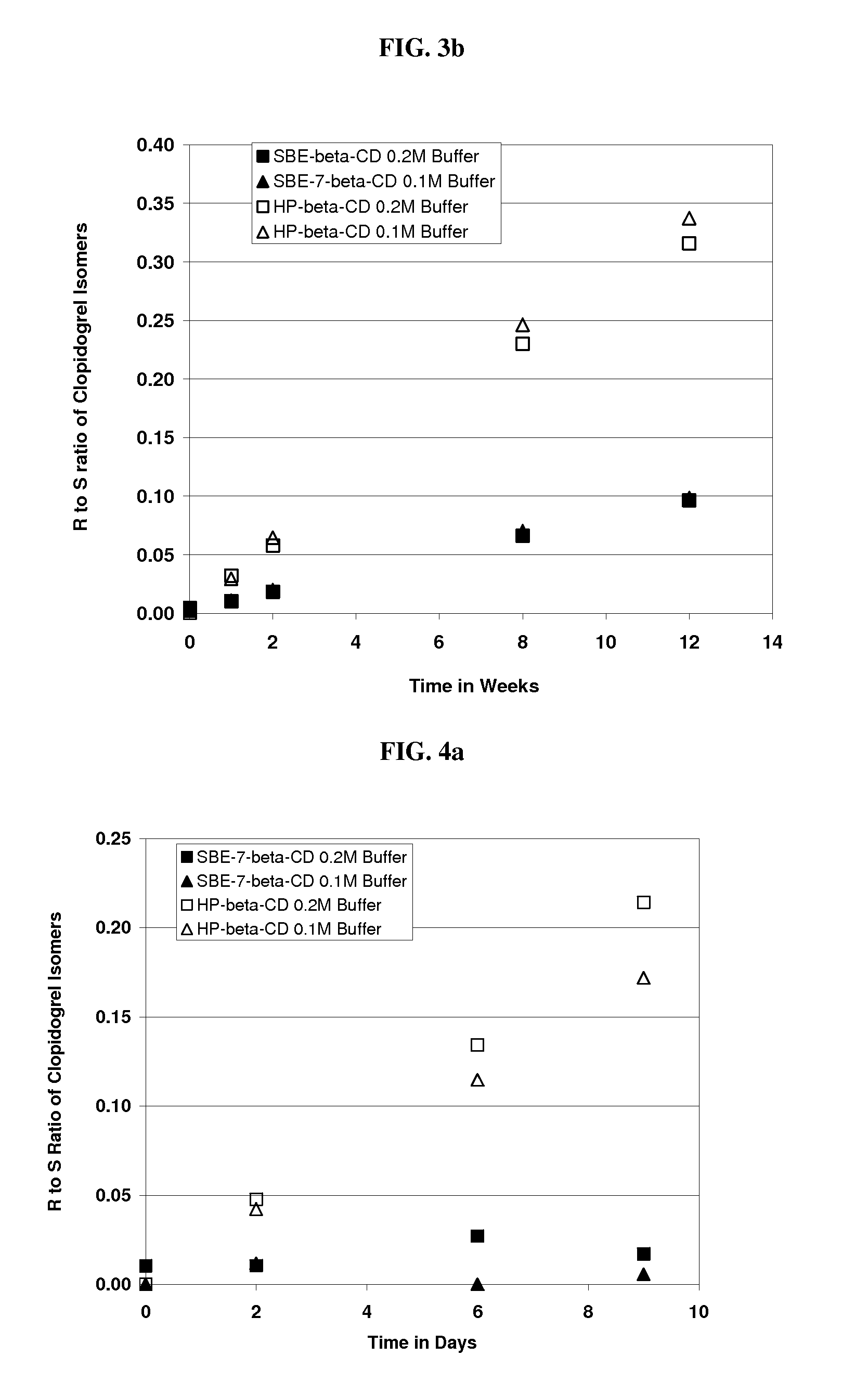
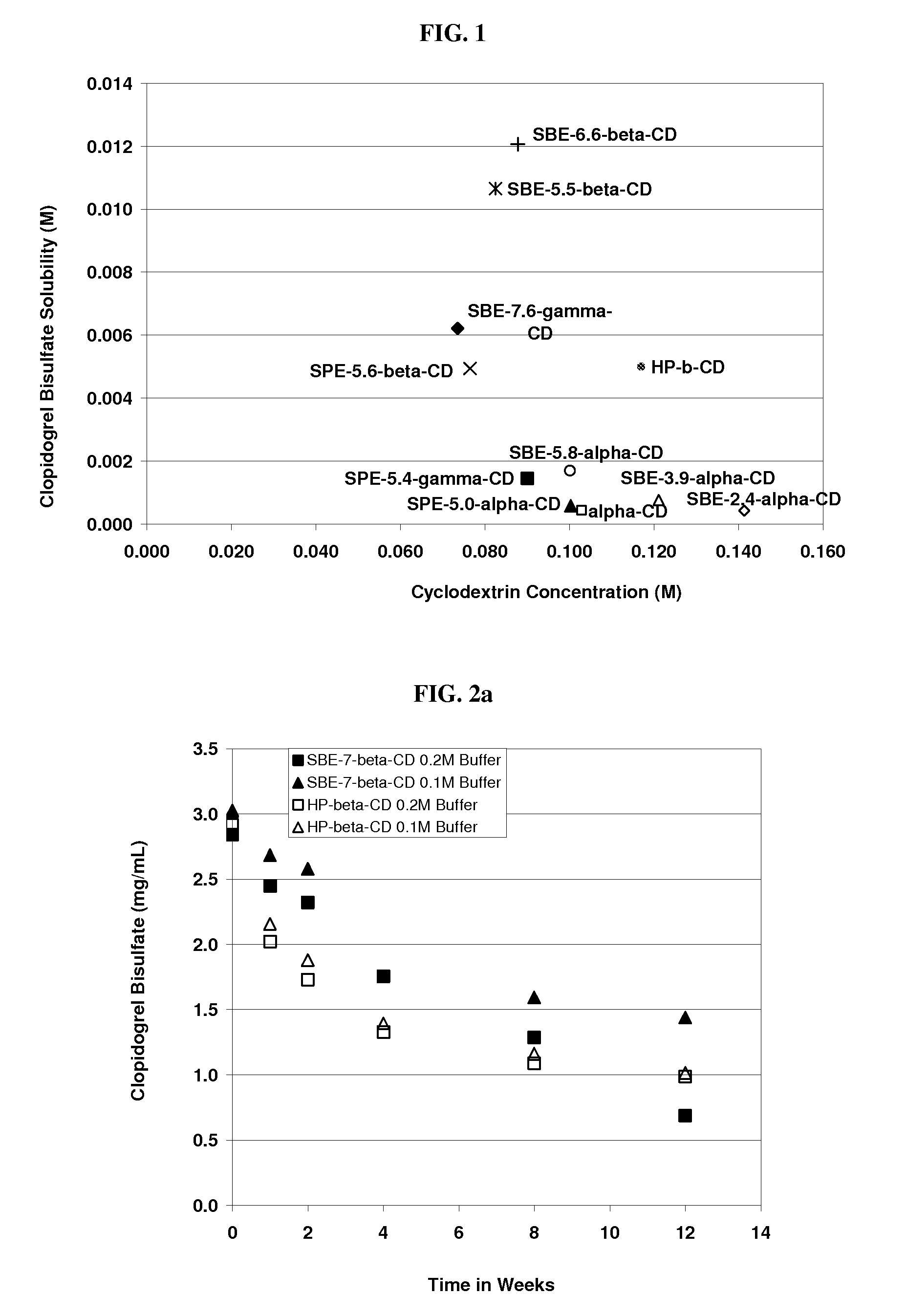
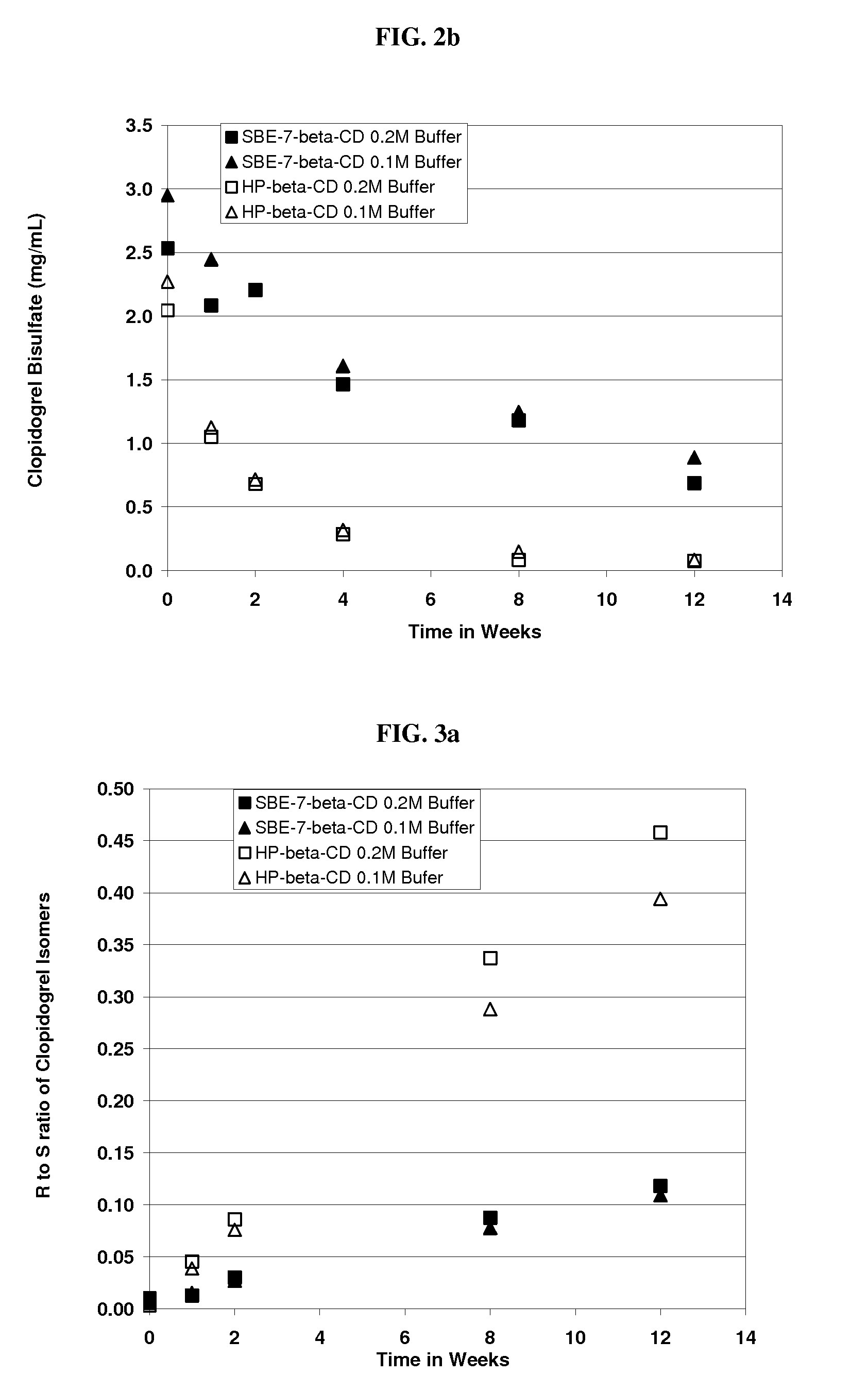
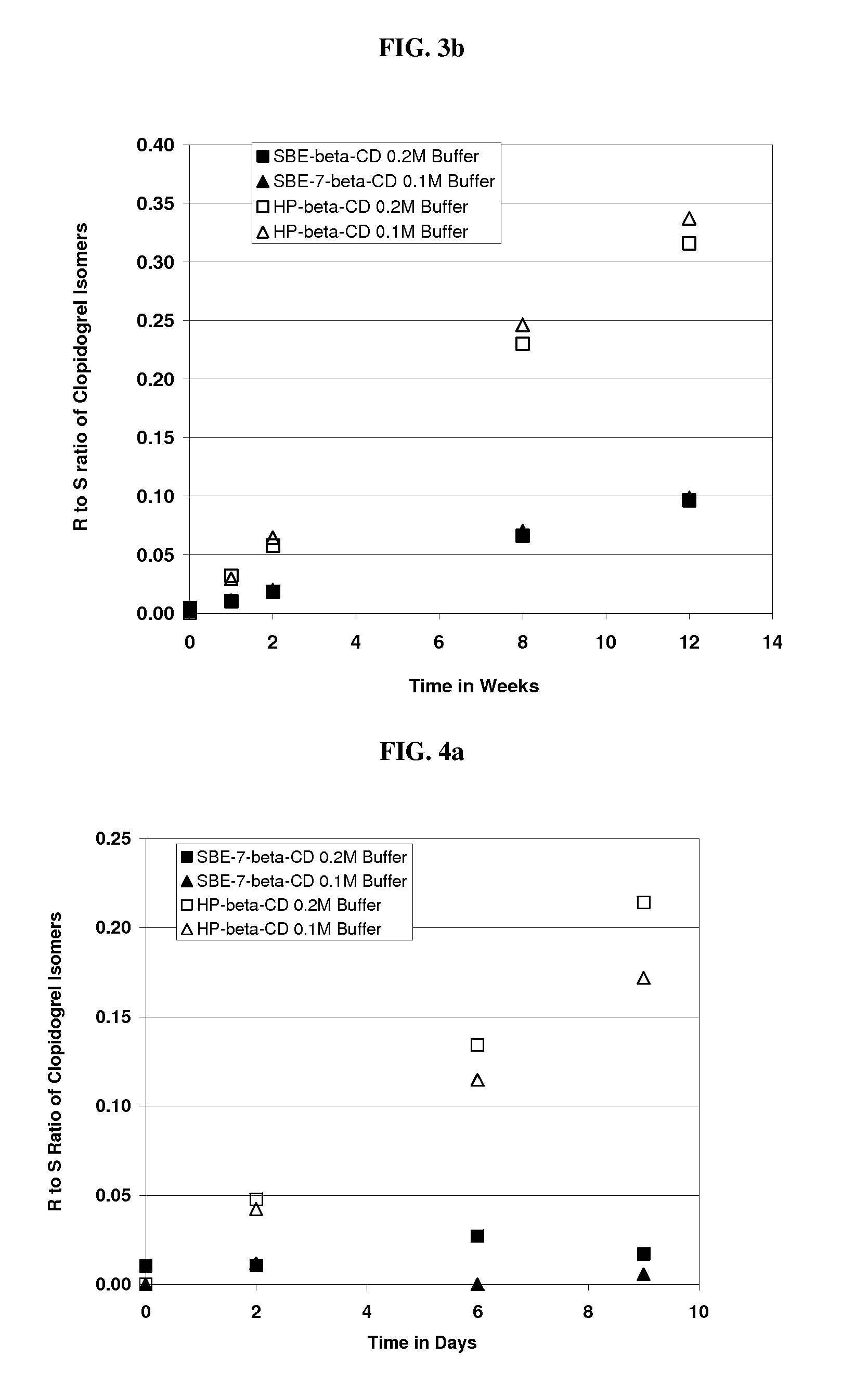
Formulations Containing Clopidogrel and Sulfoalkyl Ether Cyclodextrin and Methods of Use
ActiveUS20100292268A1Reduce chemical degradationReduce probabilityBiocideAntipyreticEtherCyclodextrin derivative
The present invention provides compositions containing clopidogrel, present as a free base or a pharmaceutically acceptable salt thereof, and sulfoalkyl ether cyclodextrin (SAE-CD). The compositions can be liquid, suspension or solid compositions. They can be adapted for oral, peroral or parenteral administration. The SAE-CD serves to aid in dissolution and stabilization of the clopidogrel in aqueous media. The stability of clopidogrel against hydrolytic degradation, thermal degradation, and photolytic degradation are improved. SAE-CD provides improved results over other cyclodextrin derivatives. The SAE-CD-containing composition of clopidogrel can be provided in liquid form, solid form or as a reconstitutable powder. Both ready-to-use and concentrated liquid compositions can be prepared. The liquid composition is optionally available as a clear solution. The compositions herein can be administered perorally or parenterally and provide substantial pharmacokinetic, pharmacodynamic and / or therapeutic advantages over a tablet composition administered perorally and excluding SAE-CD.
Owner:CYDEX PHARMACEUTICALS INC
Process for preparing clopidogrel
InactiveUS20070225320A1Economical, ecofriendlyHigh purityBiocideOrganic chemistryPolymer scienceClopidogrel
Owner:DR REDDYS LAB LTD +1
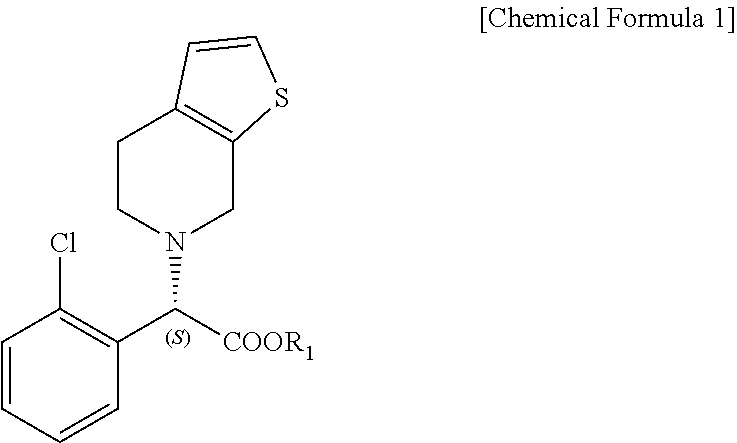
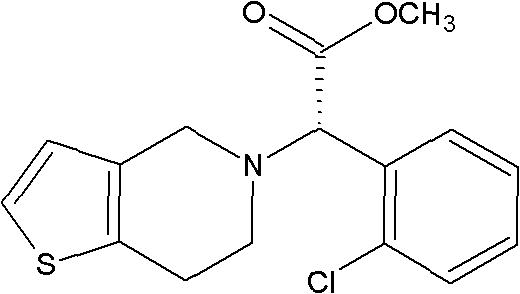
Preparation of (S)-Clopidogrel and related compounds
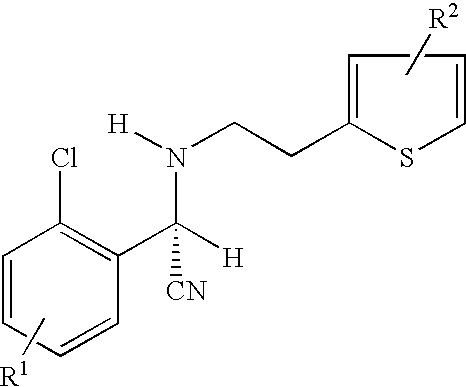
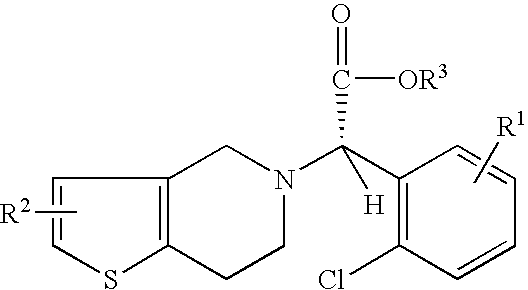
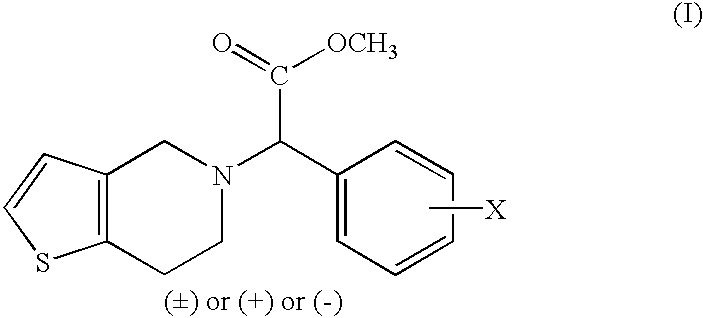
A process for producing enantiomerically enriched (S)-α-(2-chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5 (4H)-acetic acid hydrocarbyl ester, represented by the formula: Is provided, wherein R1 and R2 are hydrogens and R3 is methyl (i.e., (S)-Clopidogrel). The process includes the steps of: (a) contacting N-2-chlorobenz-aldehyde-ylidene-1-ethylamine-2(2-thiophenyl)imine and an HCN source, in the presence of a non-metallic asymmetric Strecker catalyst to form enantiomerically enriched (S)-α,α-(2-thiophenylethylamino)(2-chlorophenyl)acetonitrile; (b) contacting the enantiomerically enriched (S)-α,α-(2-thiophenylethylamino)(2-chlorophenyl)acetonitrile and a formaldehyde equivalent, in the presence of an acid catalyst to form enantiomerically enriched α-5(4,5,6,7-tetrahydro[3,2-c]thienopyridyl)(2-chlorobenzyl)-nitrile; and (c) contacting the enantiomerically enriched α-5(4,5,6,7-tetrahydro[3,2-c]thienopyridyl)(2-chlorobenzyl)-nitrile and a reagent capable of converting a cyano group into an ester group to form enantiomerically enriched hydrocarbyl ester of (S)-α-(2-chlorophenyl)-6,7-dihydrothieno-[3,2-c]pyridine-5(4H)-acetic acid.
Owner:SHASUN PHARMA SOLUTIONS LTD
Method for preparing clopidogrel and salts thereof
The invention provides a preparation method for clopidogrel and the salts of clopidogrel, belonging to the field of medical and chemical technology. The invention solves the problems of more reaction steps, long technical route, high cost and low purity of the existing preparation method for clopidogrel. The preparation method for clopidogrel comprises the following steps: a. synchronous resolution and racemization; b. preparation of (plus) alpha-(2 - thiophene triethylamine yl) -2 - (2 - chlorophenyl) methyl acetate; c. preparation of target product clopidogrel through cyclization reaction. The clopidogrel can generate medicinal salt with acids in a solvent. The preparation method for clopidogrel and the salts of clopidogrel has simple technology, easy operation, lower costs and higher product purity.
Owner:江苏八巨药业有限公司
Solid Pharmaceutical Dosage Form
InactiveUS20110028456A1High drug loadingEasy to manufacturePowder deliveryBiocideValsartanTrenbolone
A pharmaceutical composition comprising a solid unit dosage form comprising: one or more of pharmaceutically active ingredients selected from valacyclovir, olanzapine, voriconazole, topotecan, artesunate, amodiaquine, guggulosterone, ramipril, telmisartan, tibolone, atorvastatin, simvastatin, amlodipine, ezetimibe, fenofibrate, tacrolimus, valgancyclovir, valsartan, clopidrogel, estradiol, trenbolone, efavirenz, metformin, pseudoephedrine, verapamil, felodipine, valproic acid / sodium valproate, mesalamine, hydrochlorothiazide, levosulpiride, nelfinavir, cefixime and cefpodoxime proxetil in combination with a water insoluble polymer and / or a water soluble polymer. Methods for making the pharmaceutical composition are also disclosed.
Owner:CIPLA LTD
Solid preparation of clopidogrel and preparation method thereof
ActiveCN101390856AAvoid conversionOrganic active ingredientsMacromolecular non-active ingredientsMedicineClopidogrel
The invention relates to the clopidogrel solid preparation and the preparation method thereof, which belong to the medicine field. The invention solves the technical problem of preventing the dextro isomer of clopidogrel from transforming into laevo isomer, and preventing clopidogrel from degrading into clopidogrel acid. Concretely, clopidogrel or derivatives thereof and Beta-cyclodextrin are mixed and sieved, so as to prepare the clopidogrel solid preparation through the conventional solid preparation method. Beta-cyclodextrin used as principle medicine wrapping agent can effectively prevent the degrading problem of clopidogrel sulphate, and improve the stability of dextro isomer of clopidogrel sulphate. The result of stability experiment indicates that the clopidogrel laevo isomer and clopidogrel acid are not obviously increased when the clopidogrel solid preparation is stored for long time or used in an accelerated test process so that the clopidogrel solid preparation has good stability and safe clinical application.
Owner:CHONGQING LUMMY PHARMA
Process to prepare clopidogrel
The present invention relates to a process for the preparation of thieno[3,2-c]pyridine derivatives having pharmacologically significant anti-aggregating and anti-thrombotic properties.
Owner:CADILA HEALTHCARE LTD
Method for determination of platelet function under flow conditions
The invention lies in the area of platelet function diagnostics and relates to an in vitro method for the determination of platelet function under flow conditions. The method is particularly suitable for the determination of the effect of clopidogrel after oral intake and of other P2Y(12) antagonists with antithrombotic activity as well as the determination of P2Y(1) receptor antagonists with antithrombotic activity.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS PRODS
Clopidogrel base suitable for pharmaceutical formulation and preparation thereof
Provided is clopidogrel base suitable for pharmaceutical formulation, and processes for its preparation.
Owner:TEVA PHARM USA INC
Research and control method of impurity B control method in clopidogrel
The invention relates to a research and control method of impurity B control method in clopidogrel, the method comprises the following steps: (1) preparing a batch or multiple batches of 2-thiophene ethylamine; (2) measuring the 3-thiophene ethylamine in (1) by a GC method; (3) selecting 2-thiophene ethylamine with 3-thiophene ethylamine content not more than 0.40% based on the step (2) as initial materials: (4) obtaining the clopidogrel with less impurity B, adopting the 2-thiophene ethylamine in the step (3) by the preparation of 2-thiophene ethyl methylene amine and the preparation of 4, 5, 6, 7-tetrahydrothieno[3, 2-c]pyridine.
Owner:CHINA RESOURCES SAIKE PHARMA
Preparation process of clopidogre and its salt
InactiveCN100999525AEasy to operate and save timeShort reaction cycleOrganic chemistryAnticoagulant effectClopidogrel
The present invention provides one kind of compound with anticoagulant effect, and is especially new preparation process of clopidogrel and its pharmaceutical salt. The preparation process includes the following four steps: 1. resolving recemized methyl o-chlorophenyl glycinate to obtain (+)-methyl o-chlorophenyl glycinate and (-)-methyl o-chlorophenyl glycinate; 2. recemizing (-)-methyl o-chlorophenyl glycinate to obtain recemized (-)-methyl o-chlorophenyl glycinate; 3. repeating the steps 1 and 2; and 4. preparing clopidogrel with (+)-methyl o-chlorophenyl glycinate obtained in the step 1 and preparing the obtained clopidogrel into corresponding salt. The process of the present invention has simple path, short circulating period, low cost, high product purity, less toxic side effect and corrosion to the production apparatus.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD +1
Formulations containing clopidogrel and sulfoalkyl ether cyclodextrin and methods of use
The present invention provides compositions containing clopidogrel, present as a free base or a pharmaceutically acceptable salt thereof, and sulfoalkyl ether cyclodextrin (SAE-CD). The compositions can be liquid, suspension or solid compositions. They can be adapted for oral, peroral or parenteral administration. The SAE-CD serves to aid in dissolution and stabilization of the clopidogrel in aqueous media. The stability of clopidogrel against hydrolytic degradation, thermal degradation, and photolytic degradation are improved. SAE-CD provides improved results over other cyclodextrin derivatives. The SAE-CD-containing composition of clopidogrel can be provided in liquid form, solid form or as a reconstitutable powder. Both ready-to-use and concentrated liquid compositions can be prepared. The liquid composition is optionally available as a clear solution. The compositions herein can be administered perorally or parenterally and provide substantial pharmacokinetic, pharmacodynamic and / or therapeutic advantages over a tablet composition administered perorally and excluding SAE-CD.
Owner:CYDEX PHARMACEUTICALS INC
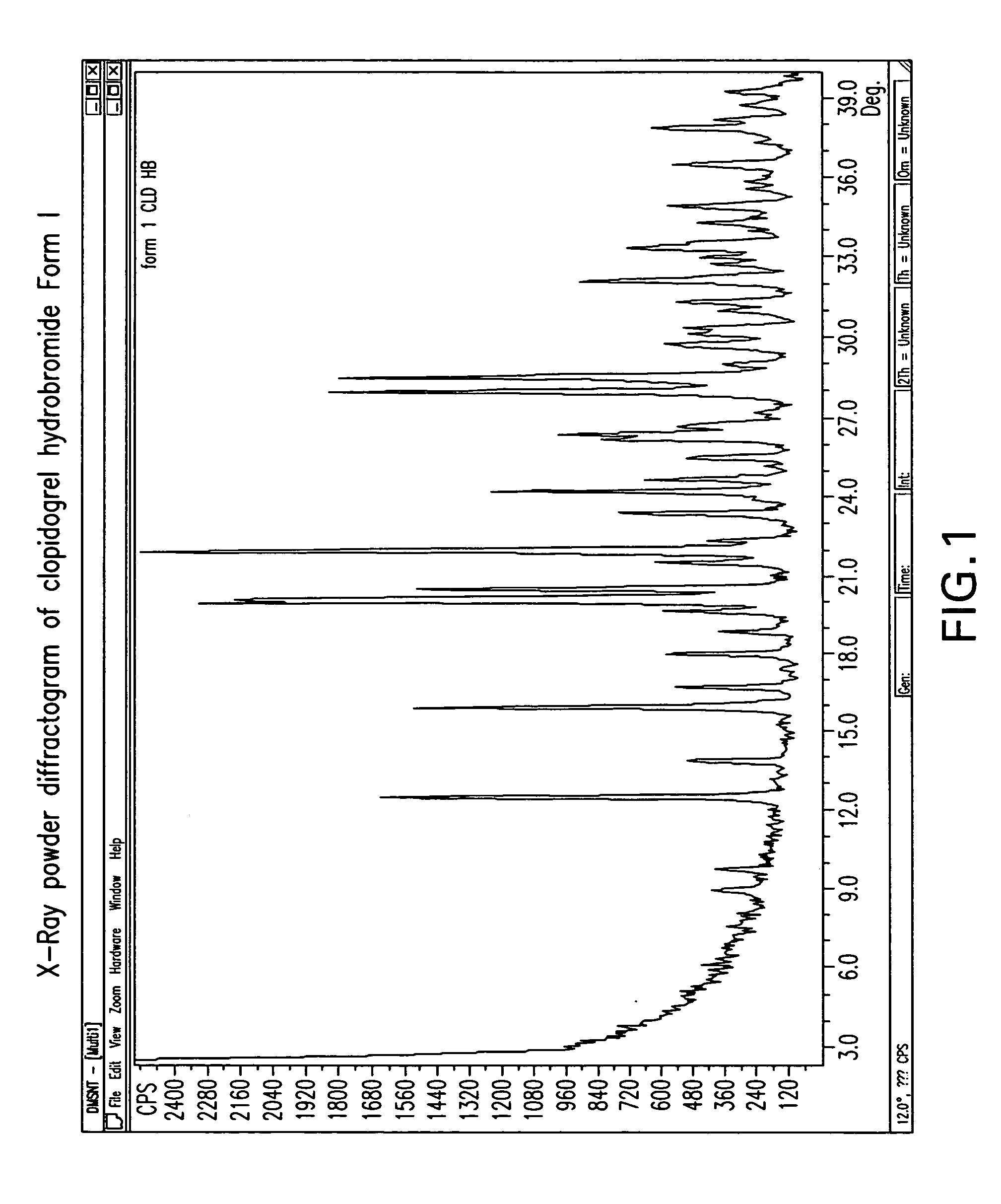
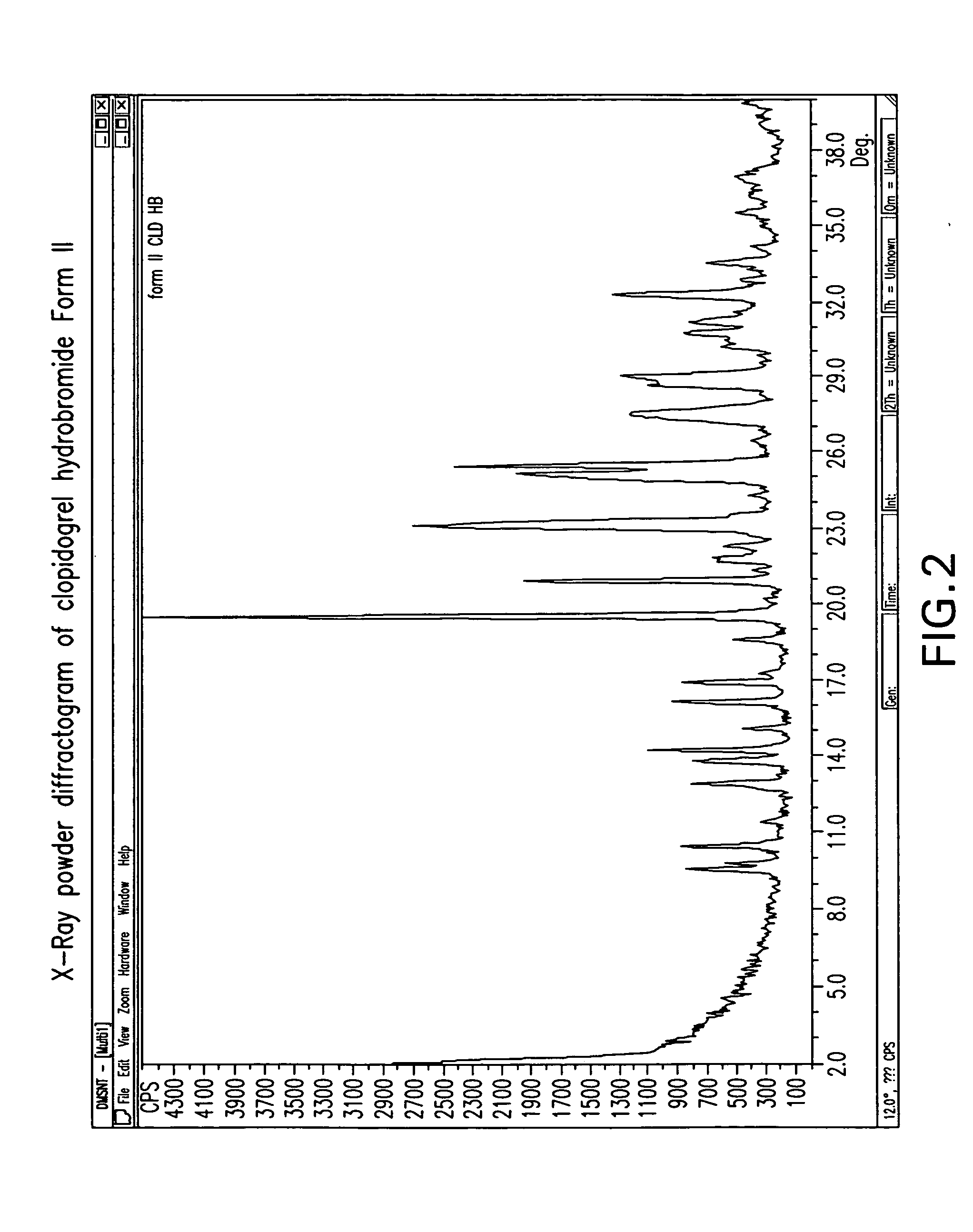
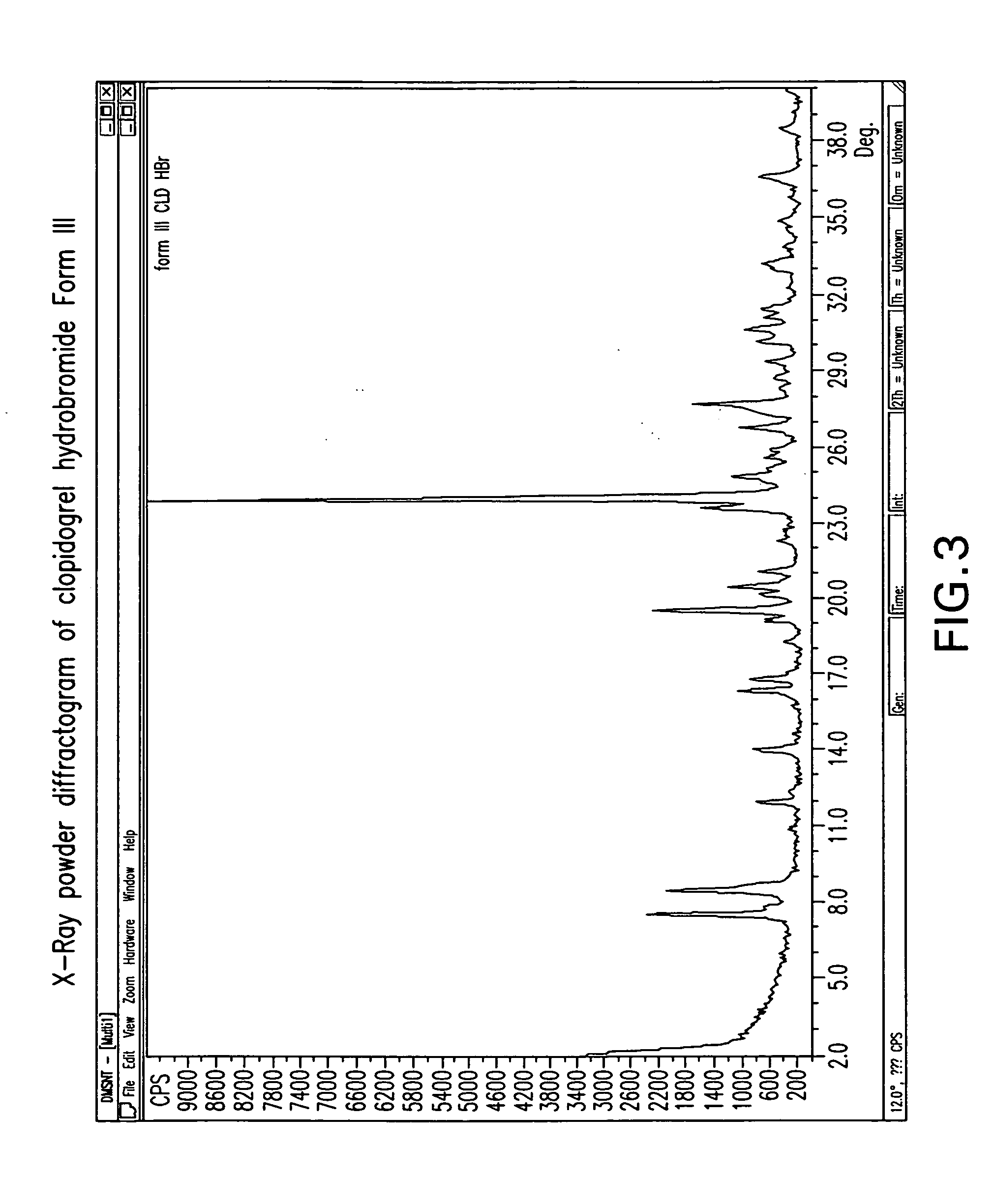
Crystalline clopidogrel hydrobromide and processes for preparation thereof
Owner:TEVA PHARM USA INC
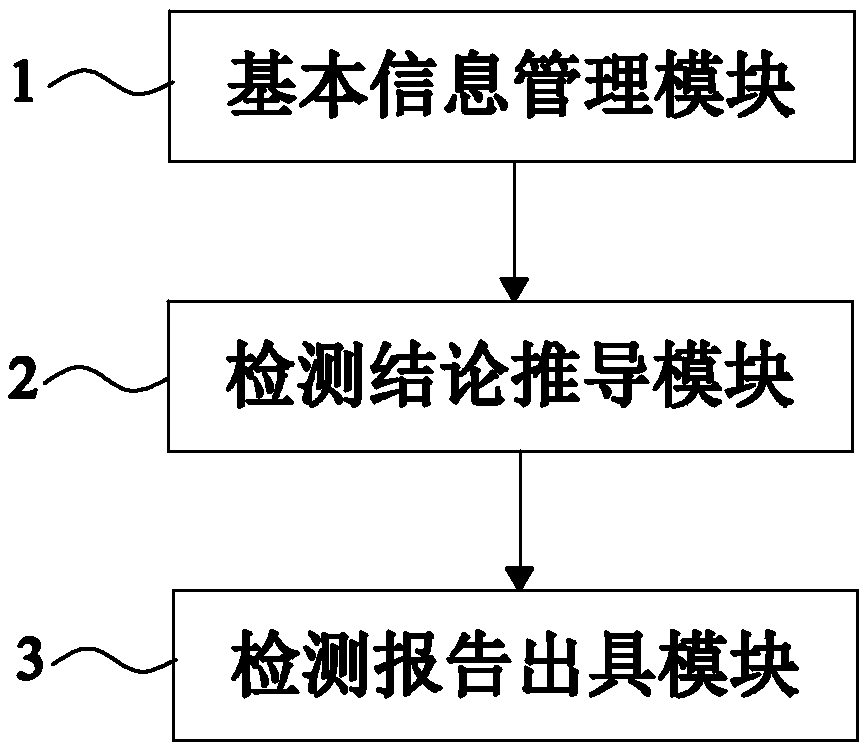
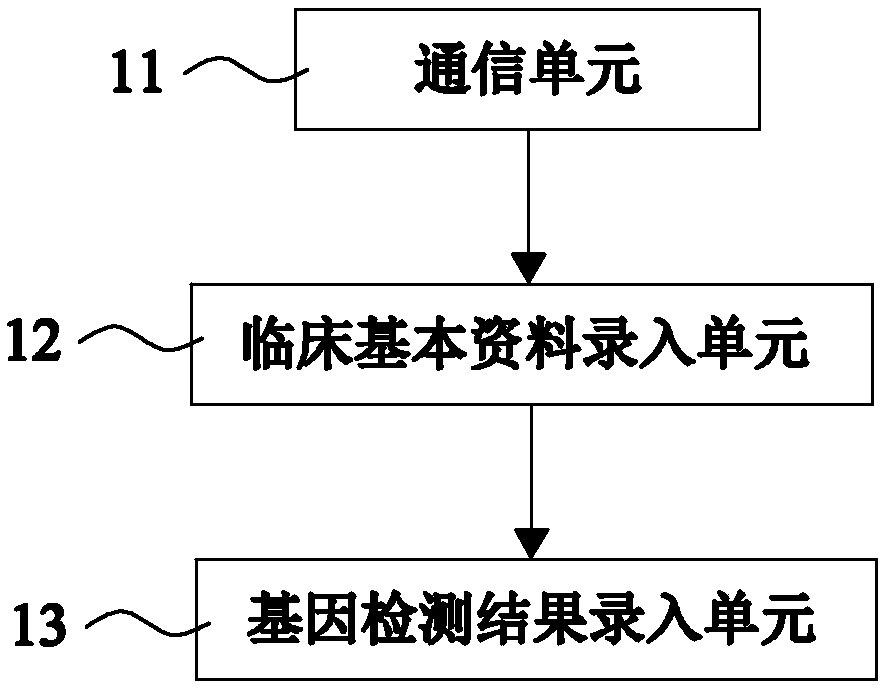
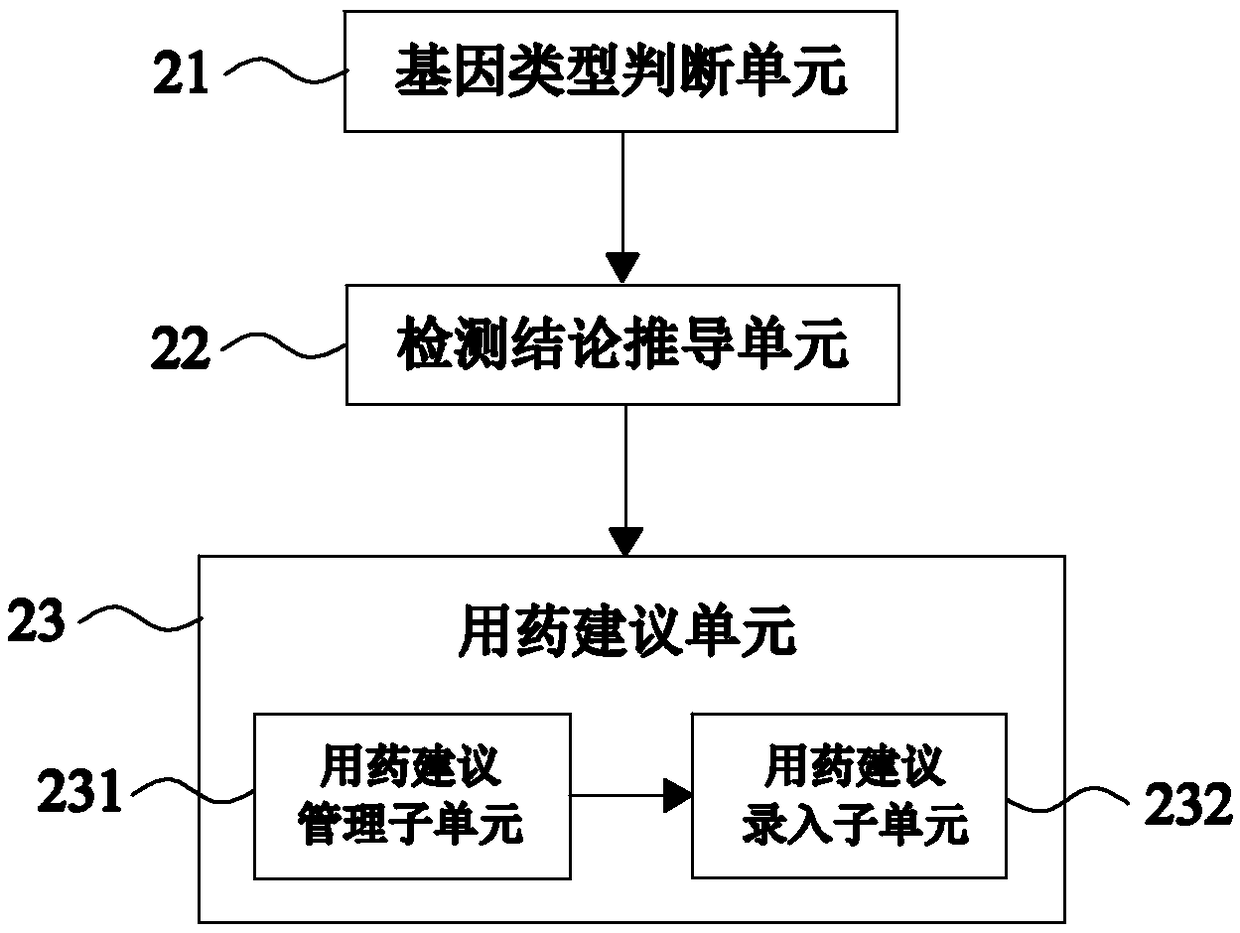
Automatic issuing system of clopidogrel medication related gene test reports
ActiveCN108715805AIssued quicklyIssued efficientBioreactor/fermenter combinationsBiological substance pretreatmentsPatient inputGenotype
Owner:SHANGHAI CONLIGHT MEDICAL LTD
Compound sustained-release preparation of aspirin and clopidogrel or pharmaceutically acceptable salt thereof
InactiveCN101703513AAvoid nauseaAvoid vomitingOrganic active ingredientsPharmaceutical delivery mechanismDiseaseMass ratio
The invention relates to a sustained-release preparation of a compound composite, in particular to a compound sustained-release preparation of aspirin and clopidogrel or an pharmaceutically acceptable salt thereof, comprising a basic remedy, an auxiliary material with sustained-release function and other auxiliary materials, wherein the mass ratio of the basic remedy to the auxiliary material with the sustained-release function is 1:0.01-1:20, and the mass ratio of the aspirin to the clopidogrel or the acceptable salt thereof is 0.1:1-10:1. The compound composite is used for preventing and treating diseases caused by platelet aggregation, and the sustained-release preparation prepared by the compound composite can reduce stimulation effect of medicaments on the gastrointestinal tract, improves patient compliance, avoids peak valley phenomenon in the blood after common preparations are taken, lowers the occurrence of adverse reaction, and enhances medication safety and curative effect, thereby achieving stable effects with sustainable action.
Owner:SHENYANG PHARMA UNIVERSITY
Detecting method for accurate risk early warning and accurate drug use of cardiovascular and cerebrovascular diseases and specific primer
ActiveCN106893783AStrong specificityReduce experiment costMicrobiological testing/measurementDNA/RNA fragmentationWarfarinSingle strand
The invention discloses a detecting method for accurate risk early warning and accurate drug use of cardiovascular and cerebrovascular diseases and a specific primer. The invention provides a specific primer for detecting SNP site of drug resistance of the cardiovascular and cerebrovascular diseases, which comprises a primer group 1 and a primer group 2; the primer group 1 is composed of primer pair 1-primer pair 13; a primer group 2 is composed of single stranded extended primer 1- single stranded extended primer 13; the method can efficiently detect and complete the accurate early warning of the attack of the cardiovascular and cerebrovascular diseases and the accurate drug use after being attacked, namely, four kinds of cardiovascular and cerebrovascular disease early warning with huge health risk and accurate drug use of four clinical common drugs (aspirin, nitroglycerin, warfarin, and clopidogrel) for prevention and treatment of cardiovascular and cerebrovascular diseases.
Owner:李爱娟
Method of preparing chloropy gra hydrogen sulphate type I
The present invention discloses a method for preparing I-type clopidogrel hydrosulfate. Said method includes the following steps: a), oblaining clopidogrel free alkali; b), adding ketone solvent into clopidogrel free alkali, and drop-adding sulfuric acid, after the drop-adding process is completed, heating to 20-50deg.C, heat-insulating and stirring, filtering and washing, vacuum drying wet product at 50-55deg.C so as to obtain the invented I-type clopidogrel hydrosulfate. The described ketone solvent is selected from five-carbon ketone or six0carbon ketone.
Owner:ZHEJIANG CHARIOTEER PHARMA
Polymorphs of clopidogrel hydrogensulfate
Provided are new crystalline Forms III, IV, V and VI of clopidogrel hydrogensulfate and the amorphous form of clopidogrel hydrogensulfate, as well as their pharmaceutical compositions, and method of treatments with such compositions. Also provided are novel processes for preparation of clopidogrel hydrogensulfate Form I, Form II, Form III, Form IV, Form V, Form VI and amorphous form.
Owner:TEVA PHARMA IND LTD
Crystalline clopidogrel naphthalenesulfonate or hydrate thereof, method for preparing same and pharmaceutical composition containing same
A crystalline clopidogrel naphthalenesulfonate or a hydrate thereof, a method for preparing same, and a pharmaceutical composition containing same are provided.
Owner:HANMI SCI CO LTD
Method for preparing clopidogrel
InactiveCN101845050APromote environmental protectionEliminate the splitting stepOrganic chemistrySulfonyl chlorideMethyl o-chloromandelate
The invention relates to a method for preparing clopidogrel. The conventional synthetic methods have the disadvantages of poor environmental protection, disadvantageous industrial production, low optical purity of final products and high cost. The technical scheme adopted by the invention comprises the following steps of: performing a reaction on a compound, namely, R,S-o-chloromandelic acid and methanol to produce R,S-chloromandelic acid methyl ester; performing the reaction on the R,S-chloromandelic acid methyl ester and benzene sulfonyl chloride under the action of an alkaline catalyst to produce 2-benzenesulfonic acyloxy-2(2-chlorphenyl) methyl acetate; performing an SN2 substitution reaction on the 2-benzenesulfonic acyloxy-2(2-chlorphenyl) methyl acetate and 4,5,6,7-tetrahydro-thiophene pyridine hydrochloride under an alkaline condition to produce R,S-clopidogrel free alkali; resolving the R,S-clopidogrel free alkali in resolving solvent by using a resolving agent; and dissociating the resolved R,S-clopidogrel free alkali to prepare the clopidogrel. In a synthetic route of the invention, reaction conditions are temperate, used reaction substrates are environmentally friendly, reaction yield in each step is high, the optical purity of a final product is up to over 99.5 percent, and pollution-free production can be realized.
Owner:SHANGYU JINGXIN PHARMA
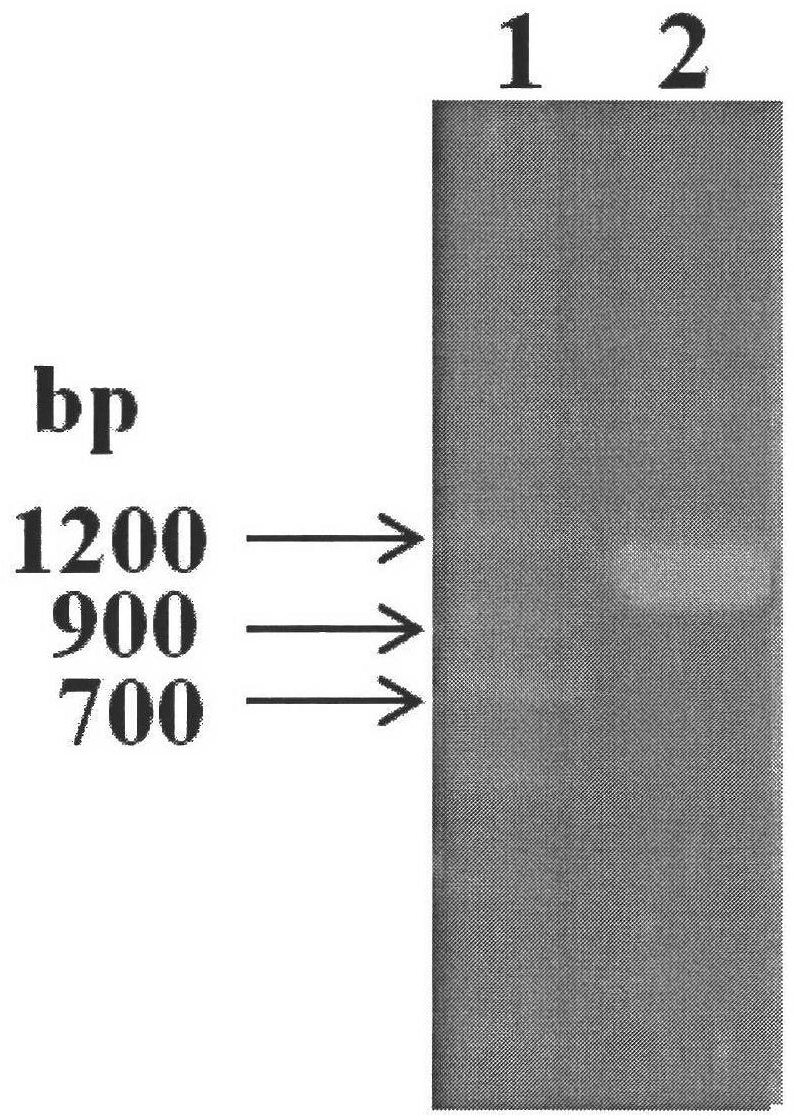
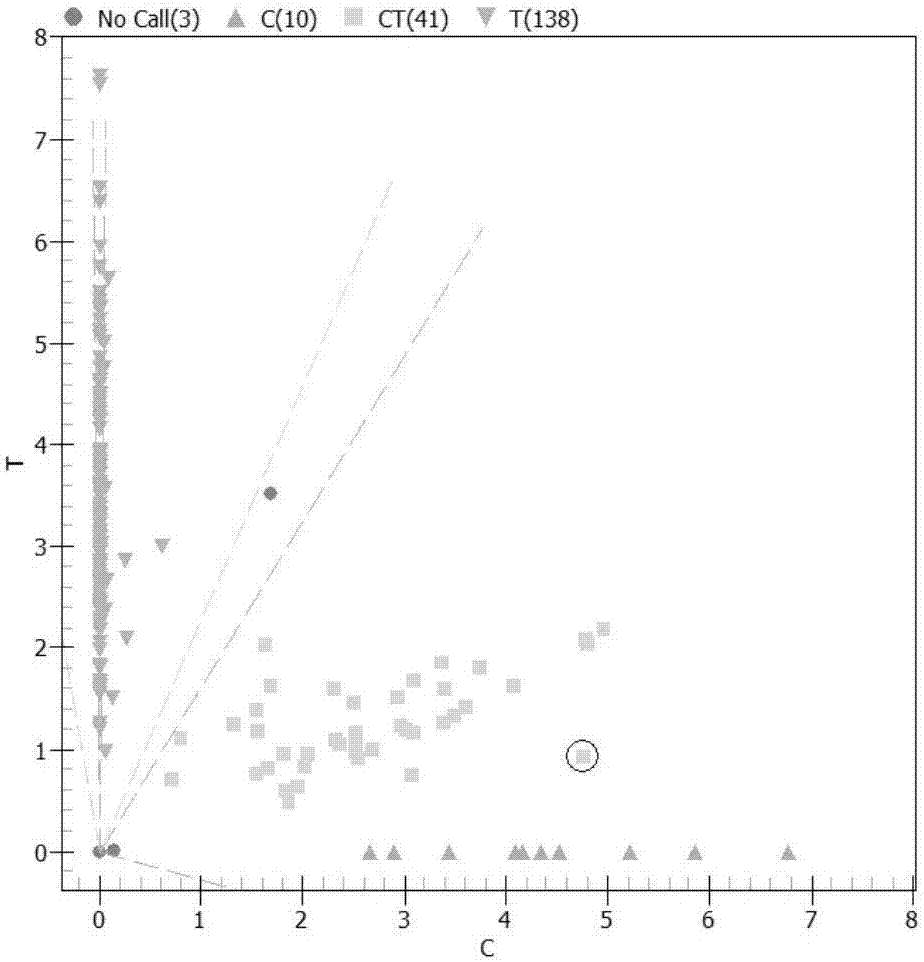
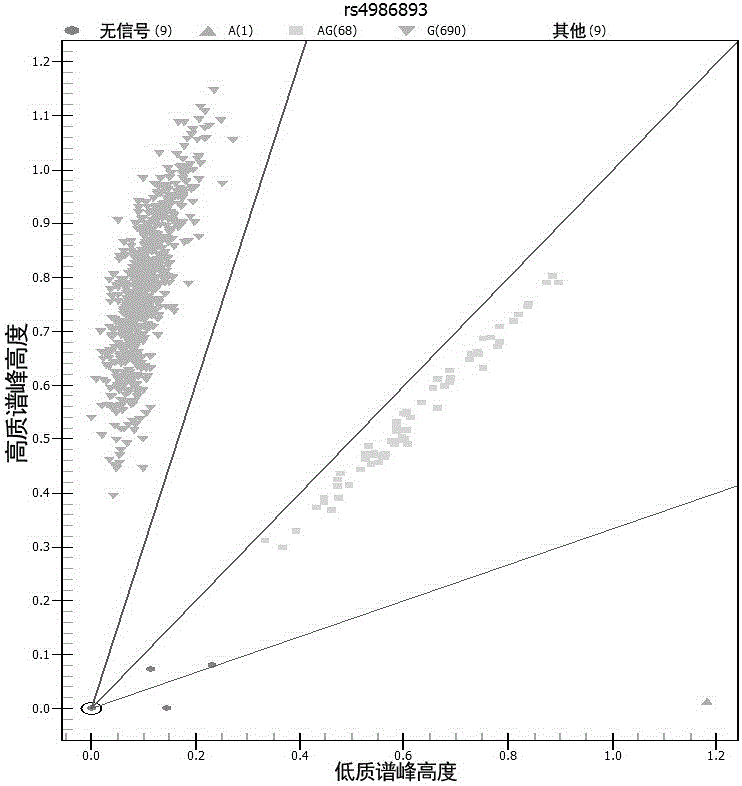
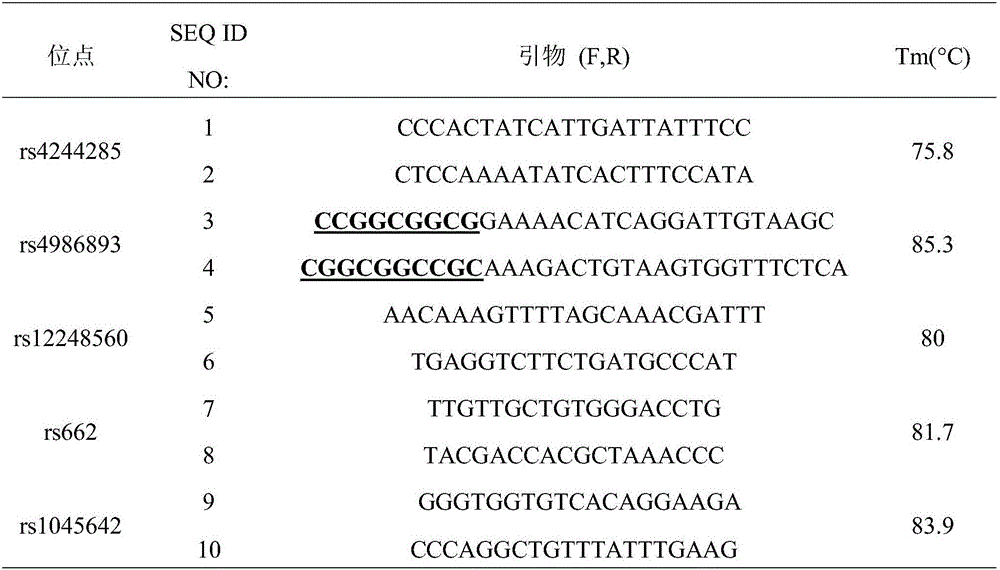
Kit for detecting efficient typing of gene loci related to clopidogrel drug resistance reaction
The invention belongs to the technical field of biology, and particularly relates to a kit for detecting efficient typing of gene loci related to clopidogrel drug resistance reaction. The kit is based on a Sequenom MassARRAY typing system, and comprises PCR (polymerase chain reaction) amplification primers and single-base extension primers of gene loci CYP2C19*2 (rs4244285) and CYP2C19*3 (rs4986893). The sequences of the PCR amplification primers of gene loci are respectively disclosed as SEQ ID NO.1, SEQ ID NO.2, SEQ ID NO.3 and SEQ ID NO.4. The sequences of the single-base extension primers of gene loci are respectively disclosed as SEQ ID NO.5 and SEQ ID NO.6. Before the subject takes clopidogrel, whether the gene loci of the subject are mutated is detected to instruct the individualized reasonable application of the subject, thereby providing references for individualized antiplatelet therapy.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Nanoparticulate clopidogrel and aspirin combination formulations
InactiveCN101237868AHigh dissolution rateSmall sizeSalicyclic acid active ingredientsPowder deliveryDiseaseControlled release
The present invention relates to compositions comprising nanoparticulate clopidogrel and aspirin combinations or salts or derivatives thereof, which have improved clopidogrel bioavailability. The nanoparticulate clopidogrel microparticles and optionally the nanoparticulate aspirin microparticles of the composition have an effective average particle size of less than about 2000 nm, which is used for the prevention and treatment of lesions caused by platelet aggregation. Clopidogrel and aspirin microparticles can also be formulated as a controlled release polymer coated delivery system or a matrix delivery system.
Owner:ELAN PHRMA INT LTD
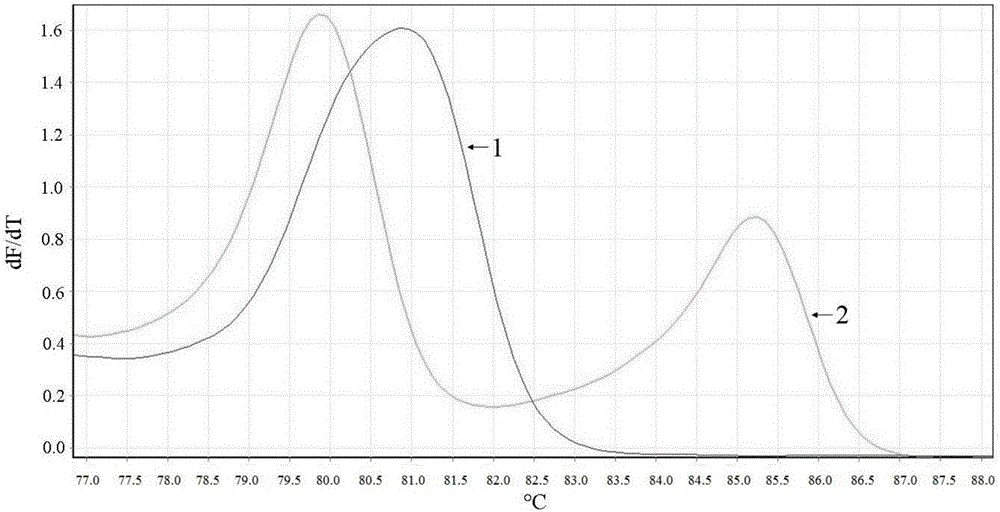
Clopidogrel drug-resistant gene detection method based on multiple HRM analysis
ActiveCN106636337AReduce dosageShorten detection timeMicrobiological testing/measurementDNA/RNA fragmentationResistant genesTyping
The invention relates to a clopidogrel drug-resistant gene detection method based on multiple HRM analysis. A primer in the method is shown as SEQ ID NO: 1-10. The invention further provides a primer combination related to HRM analysis and a kit. The clopidogrel drug-resistant gene detection method has the advantages that a method of HRM typing aiming at multiple SNP sites is established, three sites of rs4244285, rs4986893 and rs12248560 and / or two sites of rs1045642 and rs662 can be detected in a same reaction system, so that reagent consumption is reduced, and detection time is shortened. The method is used to detect samples of patients receiving percutaneous coronary interventional angiography, and a Sanger sequencing method is used for verification, so that accuracy is high.
Owner:SHANGHAI CHILDRENS MEDICAL CENT AFFILIATED TO SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Preparation of (S)-clopidogrel and related compounds
A process for producing enantiomerically enriched (S)-α-(2-chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5 (4H)-acetic acid hydrocarbyl ester, represented by the formula: Is provided, wherein R1 and R2 are hydrogens and R3 is methyl (i.e., (S)-Clopidogrel). The process includes the steps of: (a) contacting N-2-chlorobenz-aldehyde-ylidene-1-ethylamine-2 (2-thiophenyl)imine and an HCN source, in the presence of a non-metallic asymmetric Strecker catalyst to form enantiomerically enriched (S)-α,α-(2-thiophenylethylamino) (2-chlorophenyl) acetonitrile; (b) contacting the enantiomerically enriched (s)-α,α-(2-thiophenylethylamino) (2-chlorophenyl) acetonitrile and a formaldehyde equivalent, in the presence of an acid catalyst to form enantiomerically enriched α-5 (4,5,6,7-tetrahydro[3,2-c] thienopyridyl) (2-chlorobenzyl)-nitrile; and (c) contacting the enantiomerically enriched α-5(4,5,6,7-tetrahydro[3,2-c] thienopyridyl) (2-chlorobenzyl)-nitrile and a reagent capable of converting a cyano group into an ester group to form enantiomerically enriched hydrocarbyl ester of (S)-α-(2-chlorophenyl)-6,7-dihydrothieno-[3,2-c]pyridine-5(4H)-acetic acid.
Owner:SHASUN PHARMA SOLUTIONS LTD
Optically active 2-hydroxyltetrahydrothienopyridine derivative as well as preparation method and use thereof
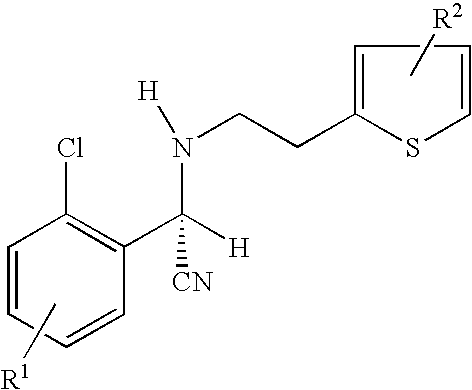
ActiveCN103665042ASolve the problem of resistanceImprove oral bioavailabilityOrganic active ingredientsGroup 5/15 element organic compoundsArylHalogen
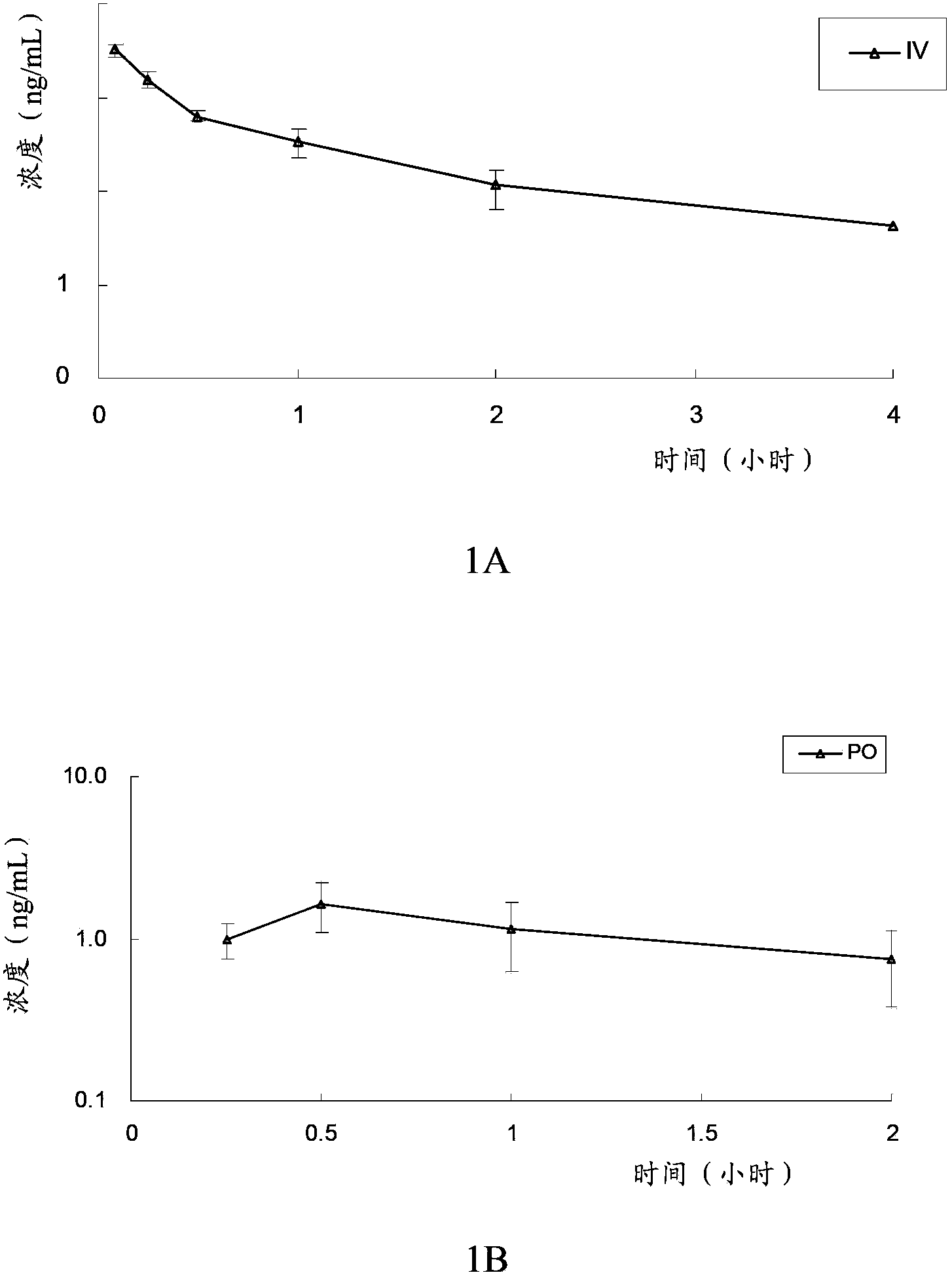
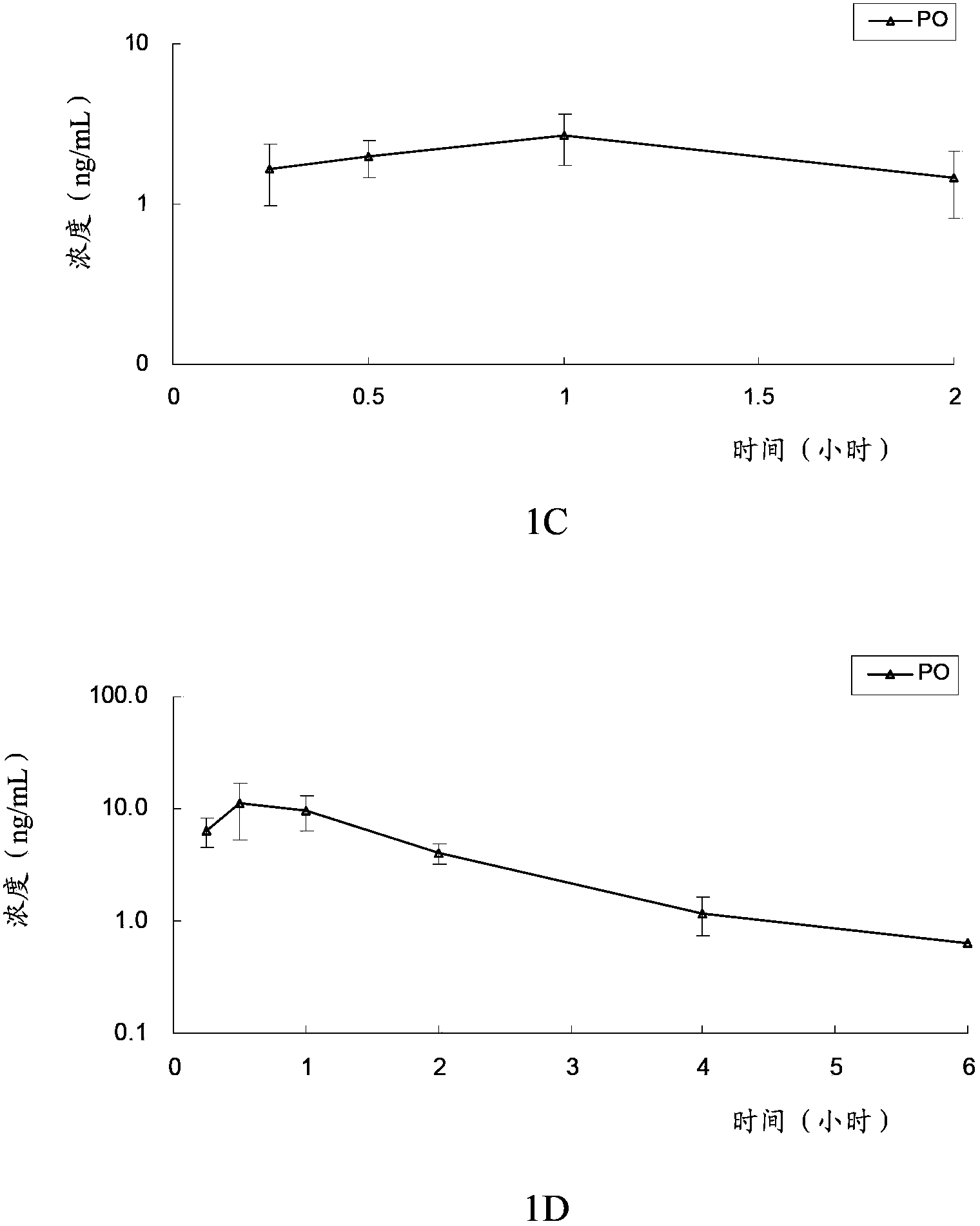
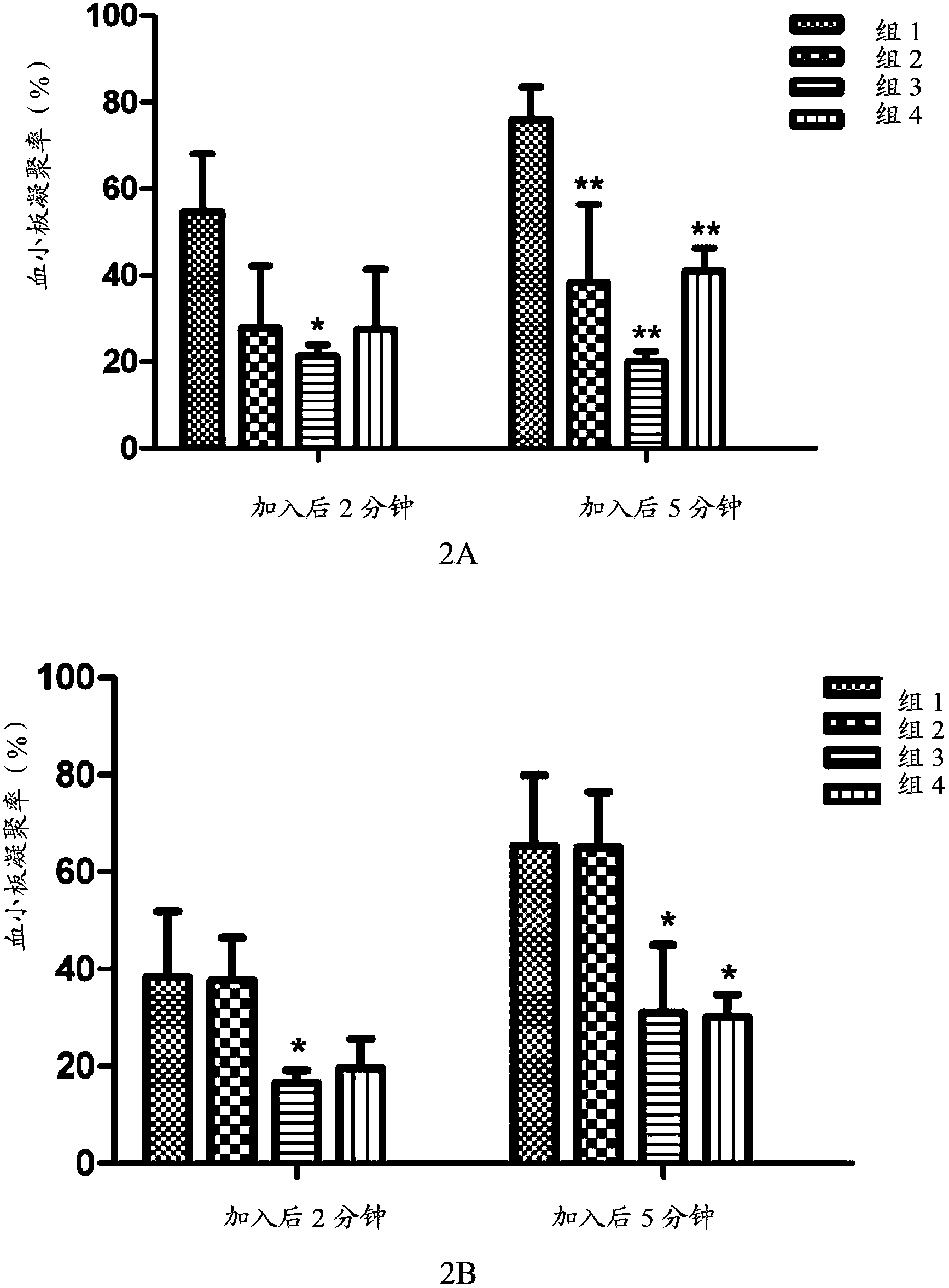
The invention provides an optically active 2-hydroxyltetrahydrothienopyridine derivative shown by formula I, or a pharmaceutically acceptable salt, solvate, polycrystal, enantiomer or racemization mixture thereof, wherein in the formula I, R1 is F, Cl, Br or I; m is 0 or 1; n is an integer from 1 to 6; R2 or R3 is independently hydrogen, C1-C6 alkyl or optionally substituted C1-6 alkyl; R4 or R5 is independently hydrogen, C1-C10 alkyl, C1-C10 alkenyl, C1-10 alkoxy, C1-10 aryl, halogen, acylamino, sulfmidyl, acyloxy or C(O)R', and R' is hydrogen, C1-C10 alkyl, C1-C10 alkenyl, C1-10 alkoxy, C1-10 aryl, halogen, acylamino, sulfmidyl or acyloxy. The compound has obvious platelet agglomeration resistance action, and the bioavailability of the compound is obviously higher than that of clopidogrel. The invention further provides a preparation method of the compound, a pharmaceutical composition containing the compound, and a pharmaceutical use of the compound and the pharmaceutical composition.
Owner:BEIJING PRELUDE PHARM SCI & TECH
Kit used for detecting polymorphism of genes related to Warfarin and Clopidogrel personalized medication and application thereof
ActiveCN105018583AHigh degree of integrationHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationWarfarinOperability
The invention discloses a kit used for detecting the polymorphism of genes related to Warfarin and Clopidogrel personalized medication and application thereof. The kit comprises a general chip, a first primer combination and a second primer combination, wherein each primer combination unit in the first primer combination is used for identifying one polymorphism site of the genes related to Warfarin medicine resistance, and each primer combination unit in the second primer combination is used for identifying one polymorphism site of the genes related to Clopidogrel medicine resistance. The kit has the advantages of being high in integration degree, sensitivity, specificity, reliability and operability and wide in application range, detection results are stable, a use method is fast and convenient, and automation of the kit is easy to achieve; the kit can be used for detecting gene mutation, analyzing gene polymorphism and the like and is suitable for gene analysis fields such as mutation detection of clinical diseases, pharmacogenomics analysis and medicolegal expertise.
Owner:BOAO BIOLOGICAL CO LTD +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com