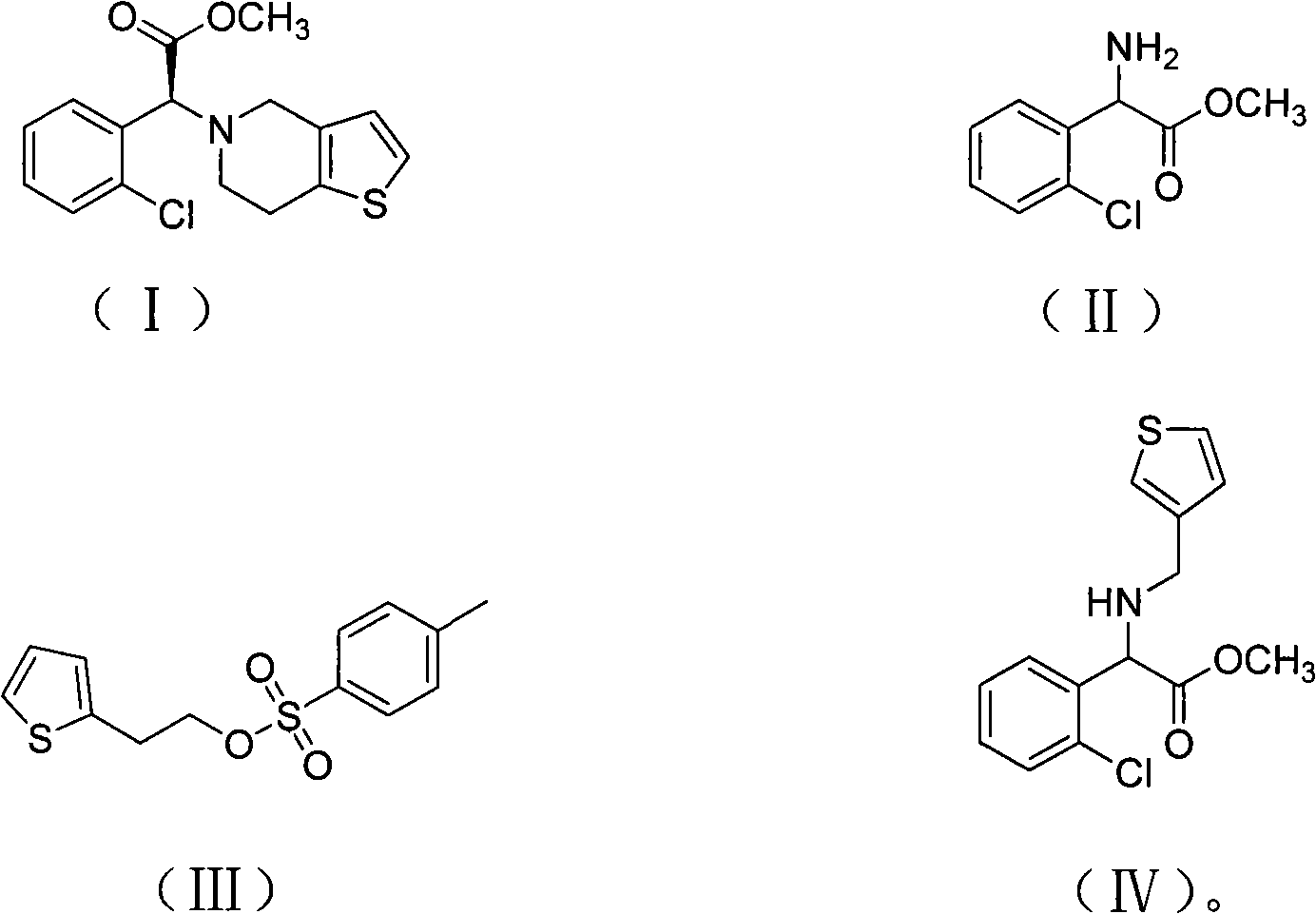
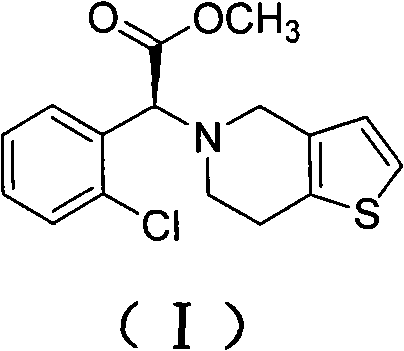
Method for preparing clopidogrel and salts thereof
A technology of clopidogrel salt and clopidogrel, which is applied in the field of medicine and chemical industry, can solve the problems of difficulty in purifying the target product, affecting the purity of the target product, and low melting point of the compound, and achieves a product with mild process conditions, good quality, and abundant sources. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Add 400g of methanol and 193g of o-chlorophenylglycine to the reaction bottle, add 220g of concentrated sulfuric acid dropwise to the material under stirring, drop it in about 0.5-1.0 hours, heat to reflux, and keep warm for 20 hours under reflux. After the reaction, methanol was evaporated. When the internal temperature reached 85°C, after cooling down to room temperature, 400g crushed ice and 800g chloroform were added, and sodium hydroxide was added dropwise under stirring to adjust the pH value to 8.5. The chloroform was extracted twice and incorporated into the chloroform layer, the chloroform layer was washed twice with water, and dried by adding anhydrous magnesium sulfate. The dried chloroform layer was distilled under reduced pressure until no chloroform came out to obtain a red oily product o-chlorophenylglycine methyl ester (II), 165 g, content 97.2%.
[0045] In another reaction bottle, drop into 800g acetonitrile, 165g o-chlorophenylglycine methyl ester pre...
Embodiment 2
[0051] The preparation process of o-chlorophenylglycine methyl ester (II) is the same as that of Example 1, and will not be repeated.
[0052] Add 500g butanone, 165g of the above-prepared o-chlorophenylglycine methyl ester, 140g oxalic acid and 50g salicylaldehyde into the reaction flask, heat up to 80°C, drop in 148g (+) tartaric acid, and keep stirring for 6 hours. Cool down to room temperature and filter. The filter cake was recrystallized with 2.5 times the weight of methanol, cooled to 0°C and filtered to obtain 125 g of (+) o-chlorophenylglycine methyl ester tartrate in the form of white crystals, [α]D / 20=85°. Raise the temperature of the butanone mother liquor to 100°C, keep it warm for 0.5 hours, cool down to 80°C, add 72g (+) tartaric acid, repeat the above resolution steps, and obtain 74g of (+) o-chlorophenylglycine methyl ester tartrate. The mother liquor can continue to repeat Use it, or save it for the next batch to apply.
[0053] Put 64g of thiophenethanol a...
Embodiment 3
[0058] The preparation process of o-chlorophenylglycine methyl ester (II) is the same as that of Example 1, and will not be repeated.
[0059] 400g of acetone, 165g of o-chlorophenylglycine methyl ester prepared above, 80g of formic acid and 60g of salicylaldehyde were added to the reaction flask, the temperature was raised to 75°C, 103g (+) tartaric acid was dropped, and the mixture was incubated and stirred for 10 hours. Cool down to room temperature and filter. The filter cake was recrystallized with 2.5 times the weight of methanol, cooled to 0°C and filtered to obtain 126 g of (+) o-chlorophenylglycine methyl ester tartrate in the form of white crystals, [α]D / 20=85°. Raise the temperature of the acetone mother liquor to 110°C, keep it warm for 0.5 hours, cool down to 75°C, add 50g of (+) tartaric acid, and repeat the above separation steps to obtain 72g of (+) o-chlorophenylglycine methyl ester tartrate. The mother liquor can be reused , and can also be reserved for the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com