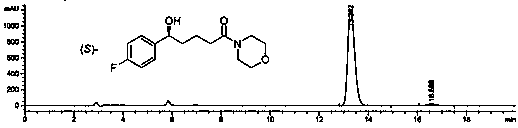
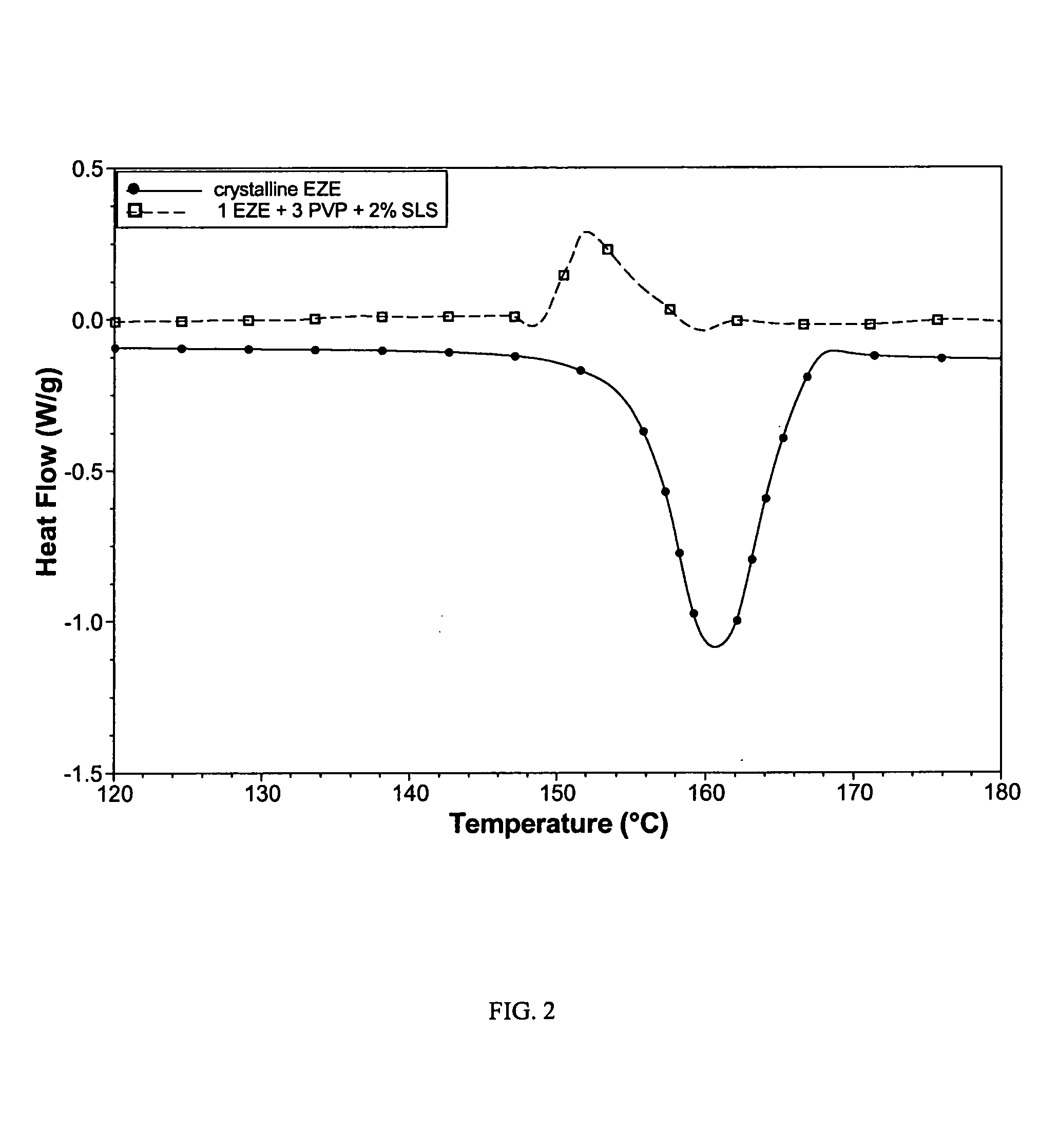
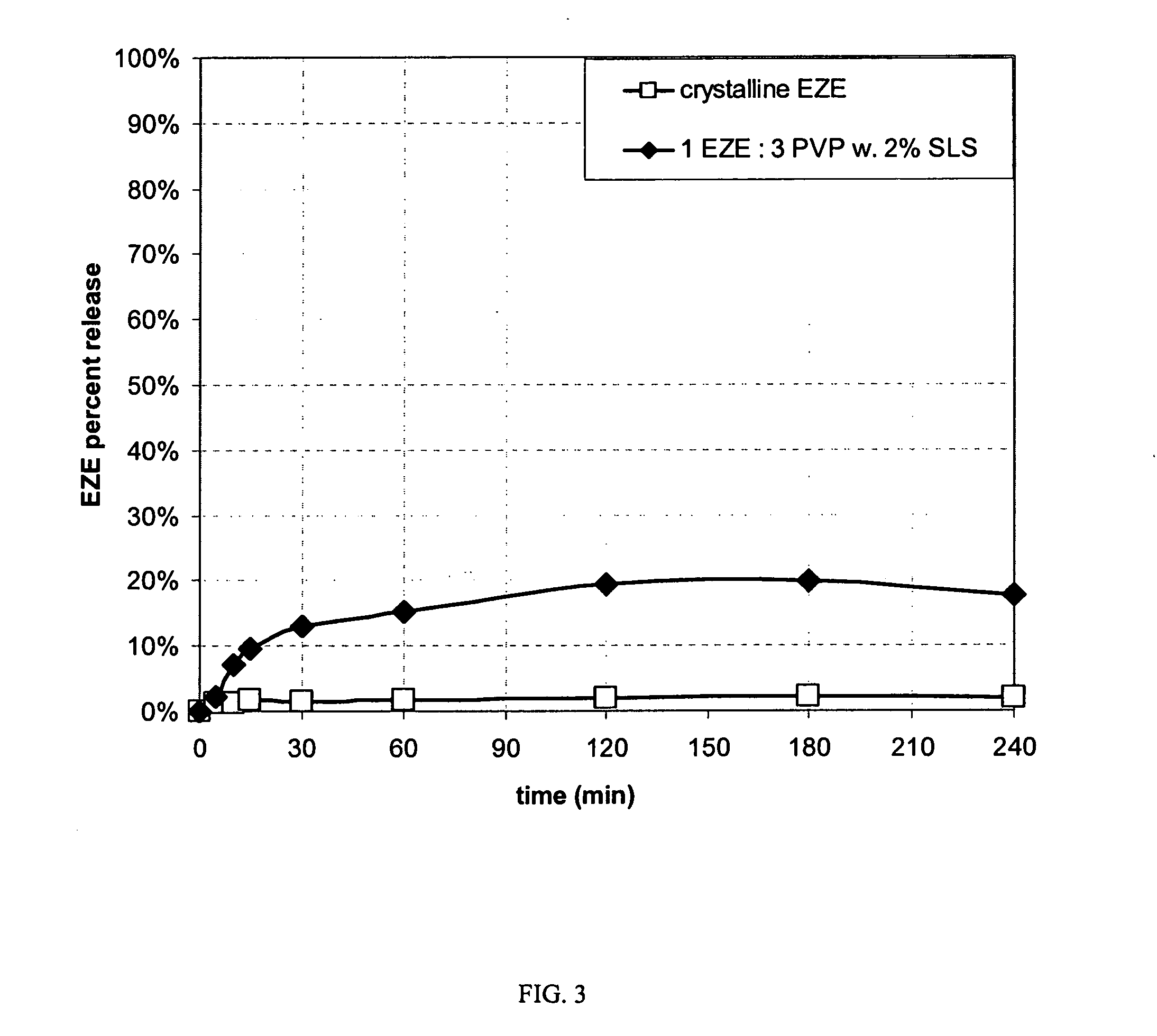
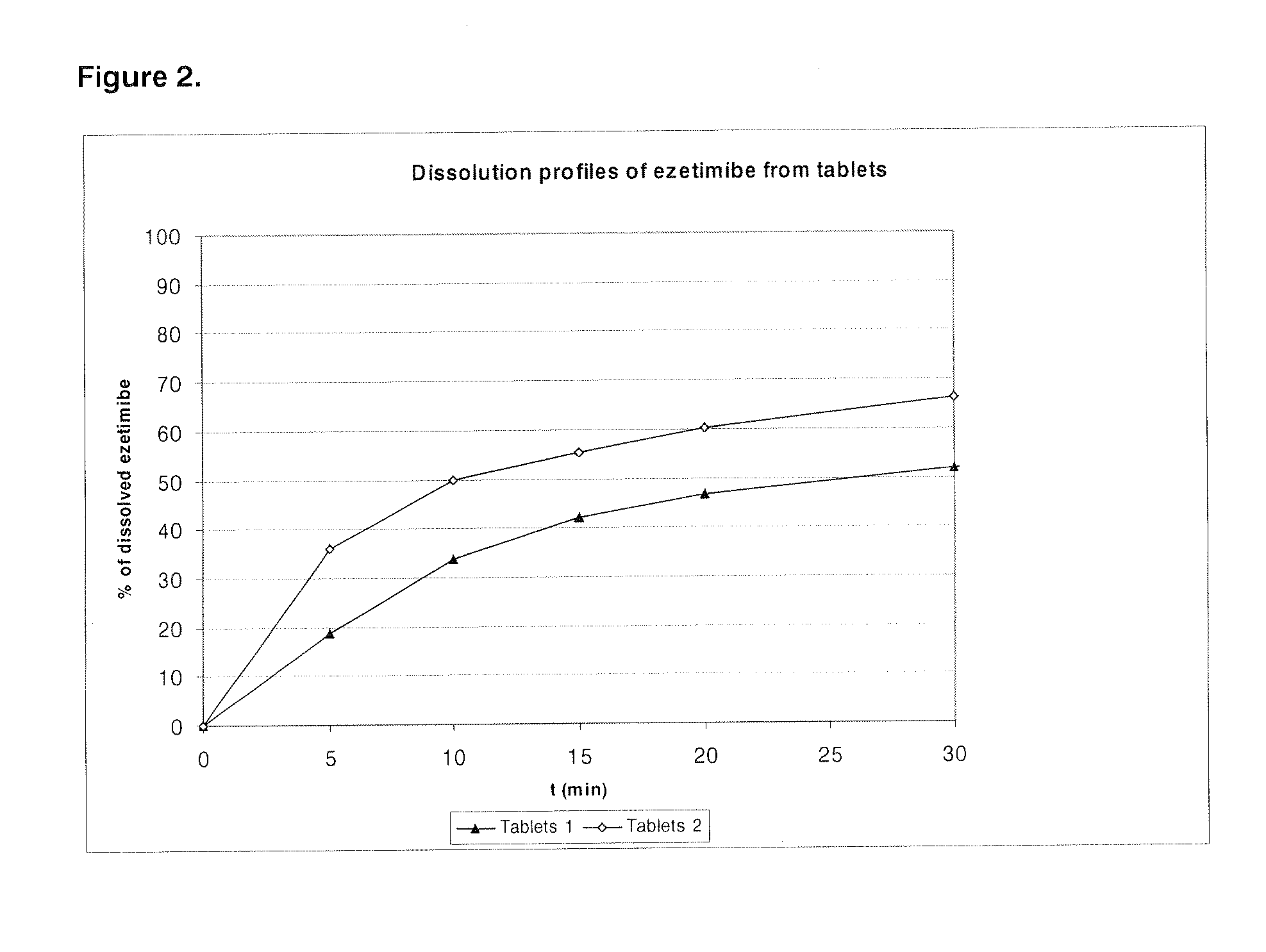
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
123 results about "Ezetimibe" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
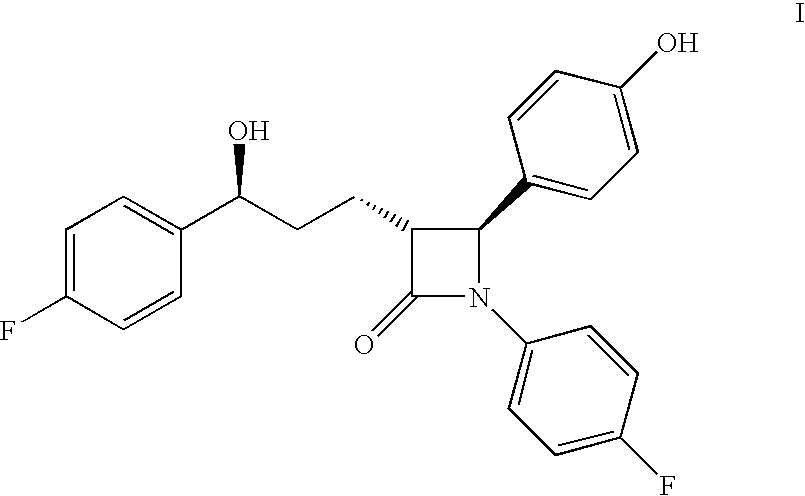
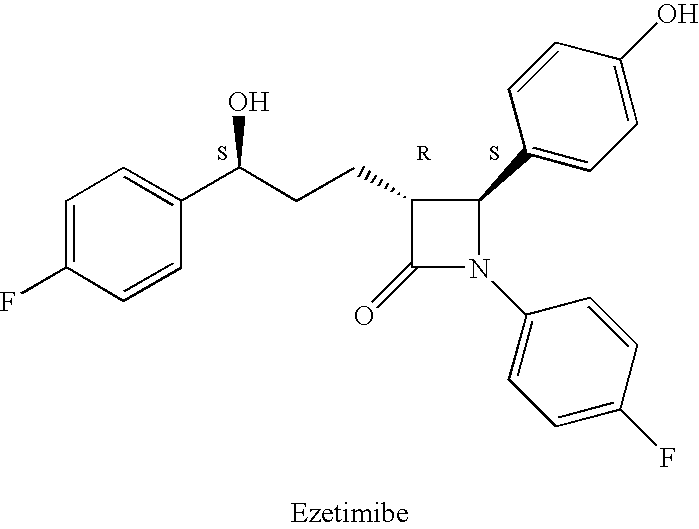
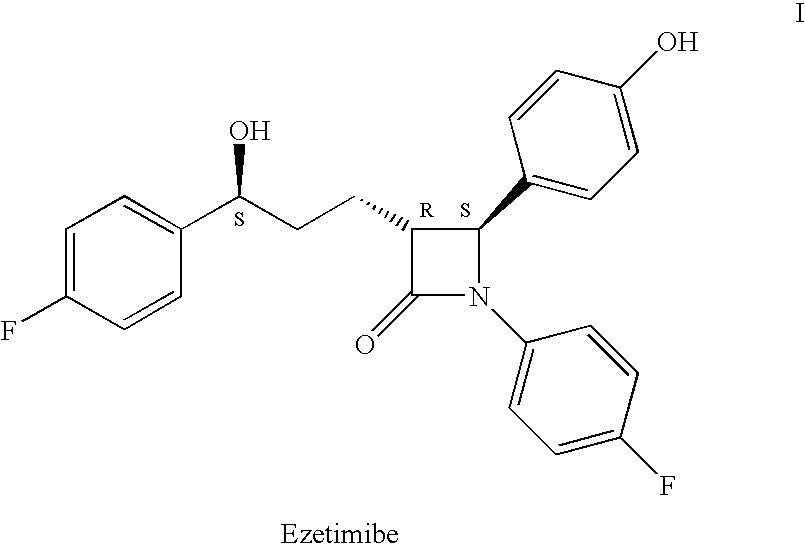
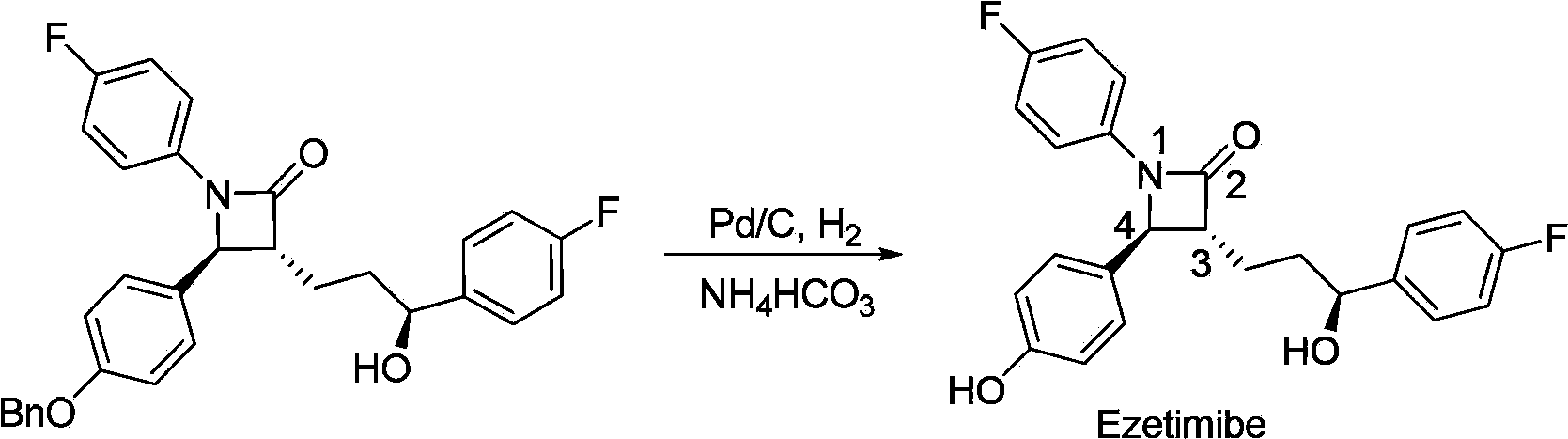
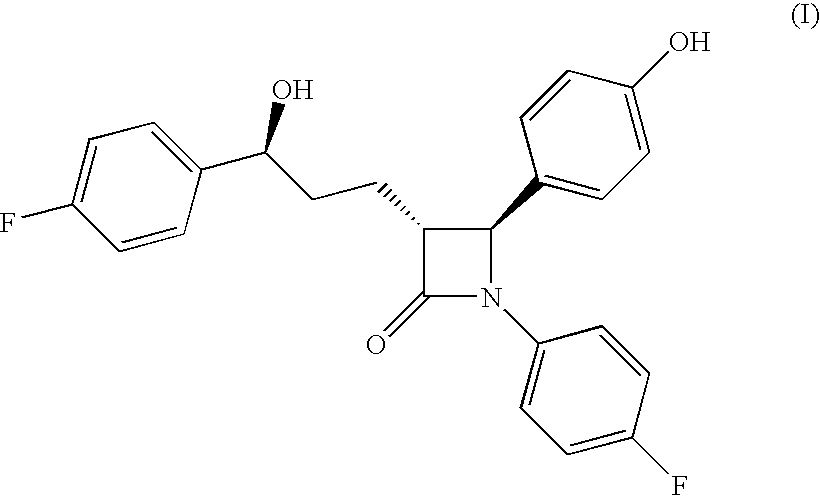
Ezetimibe is used along with a low cholesterol/low fat diet and exercise to help lower cholesterol in the blood. Ezetimibe may be used alone or with other drugs (such as "statins" or fibrates).
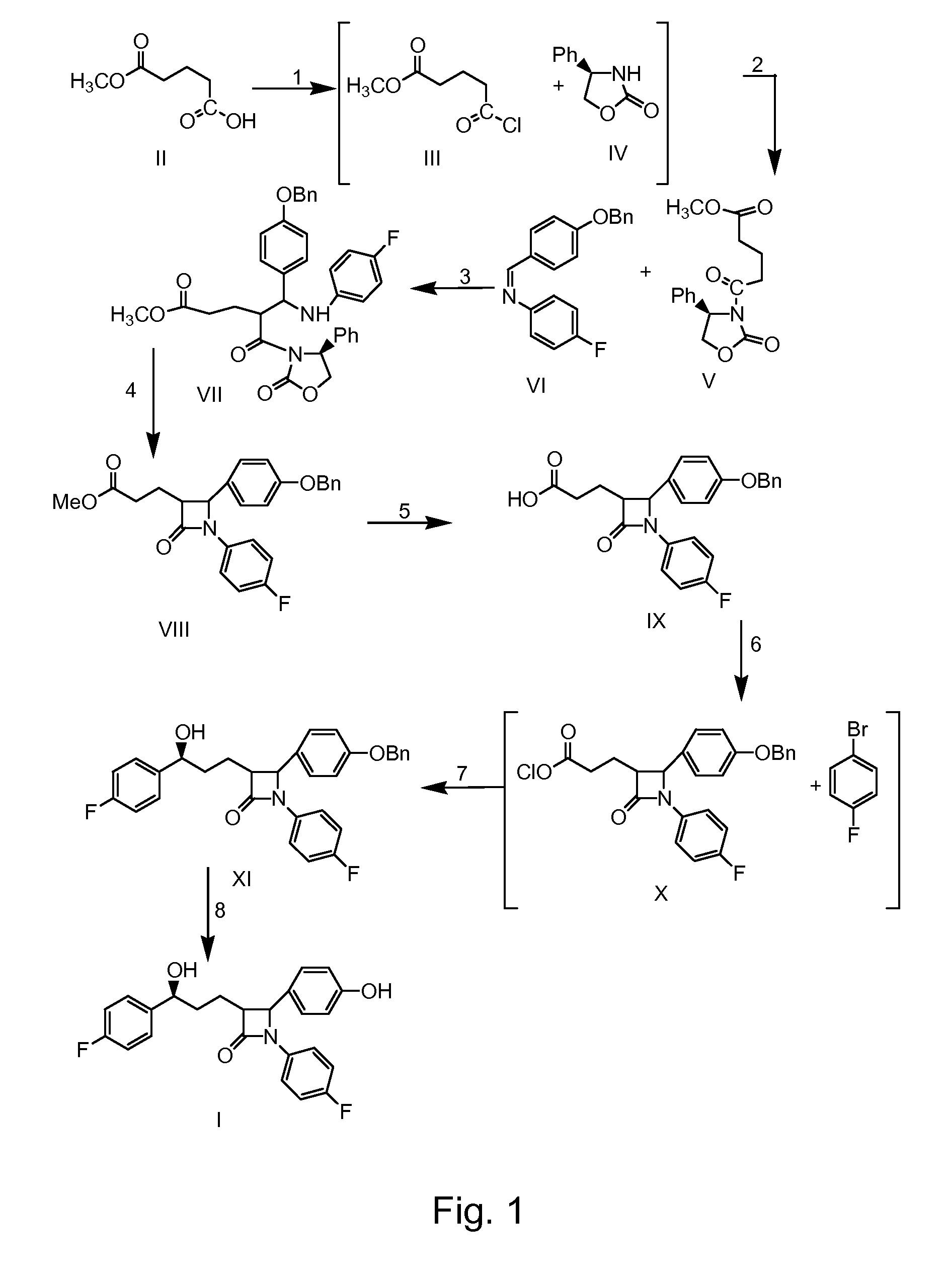
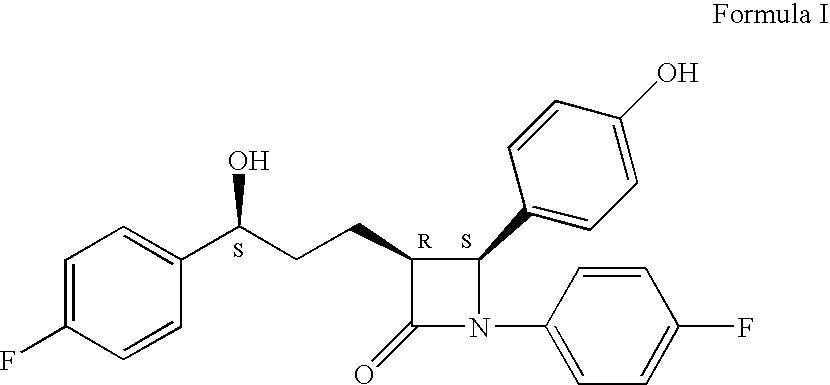
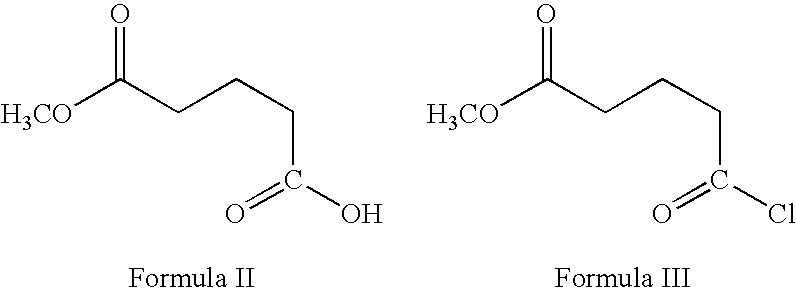
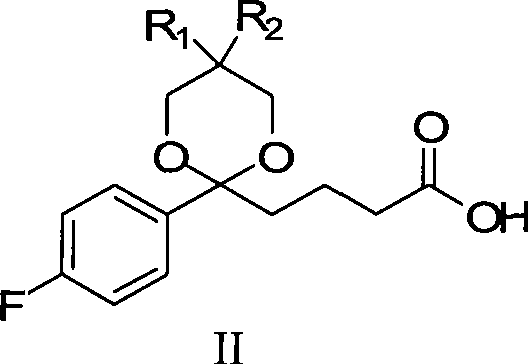
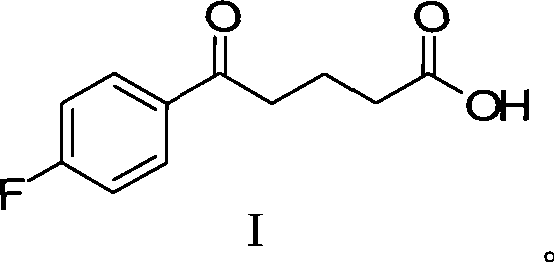
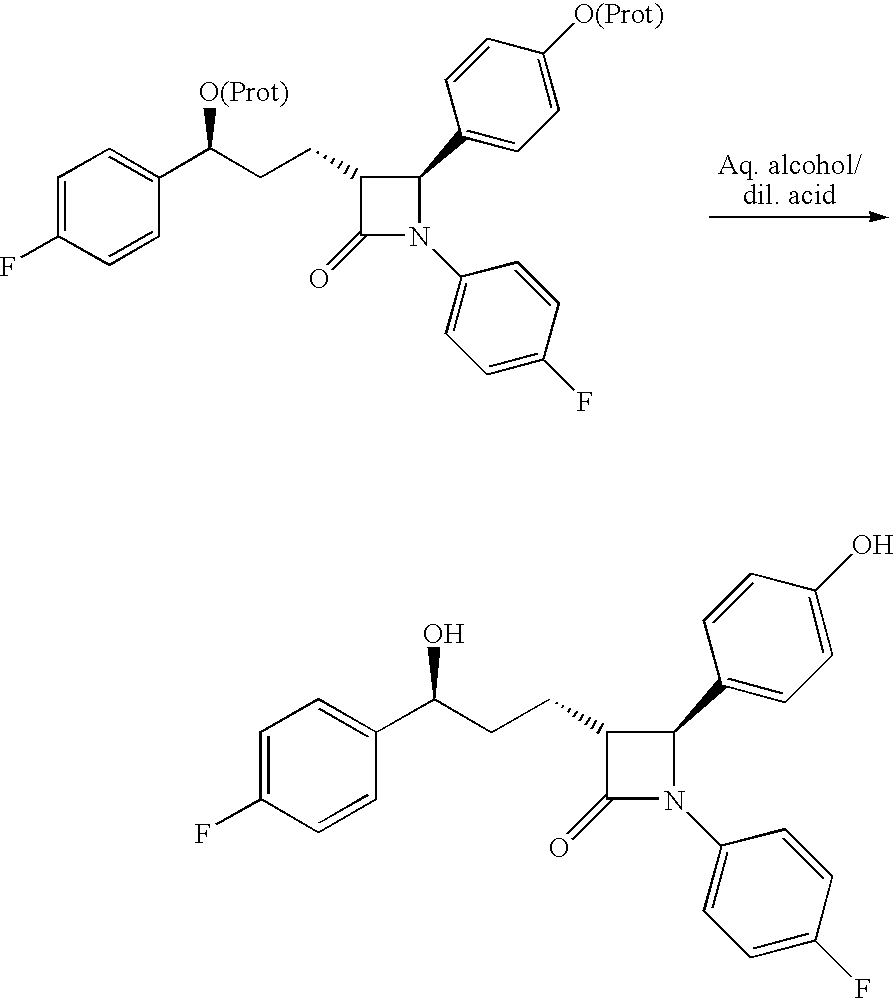
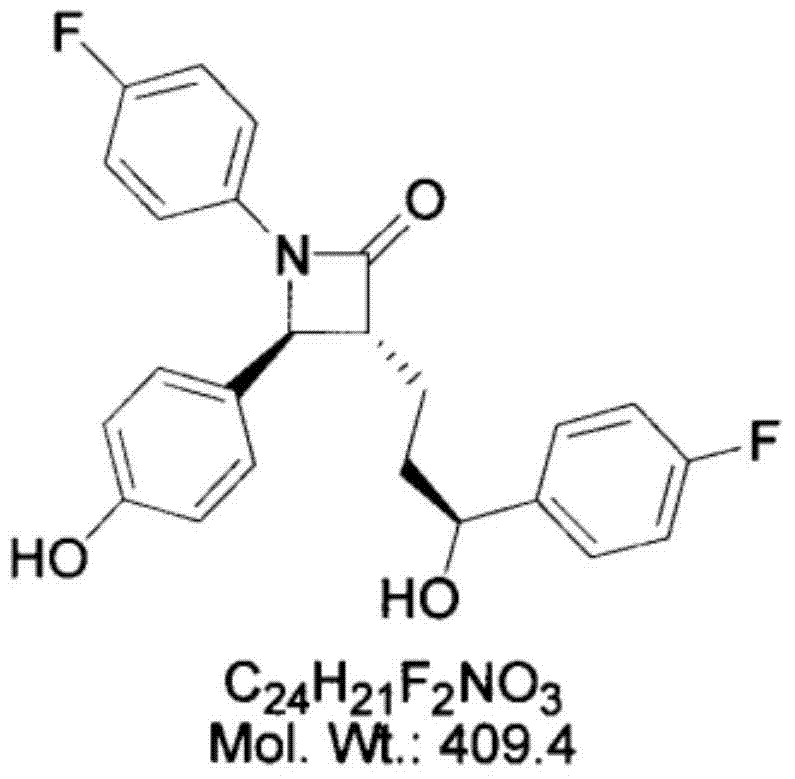
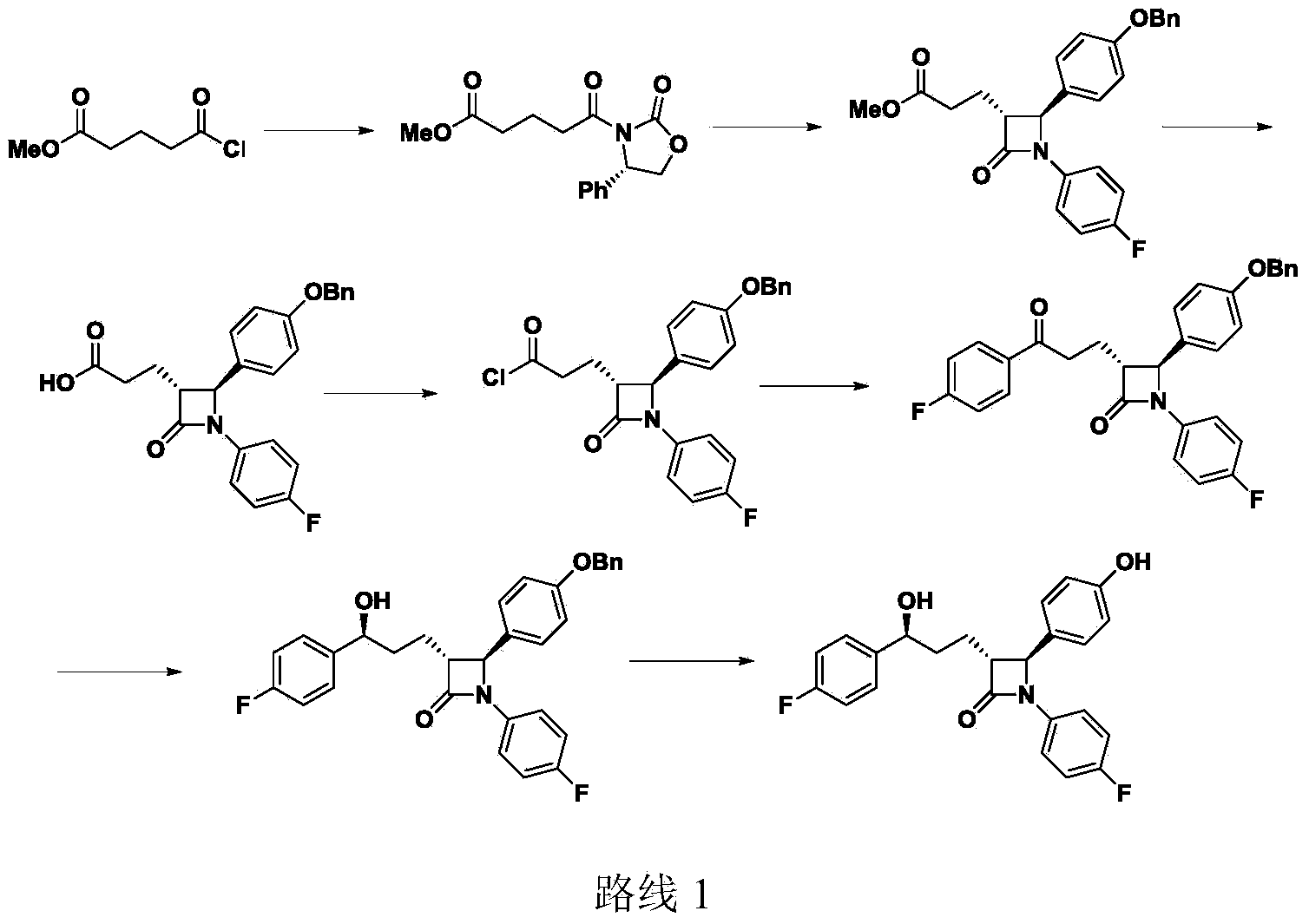
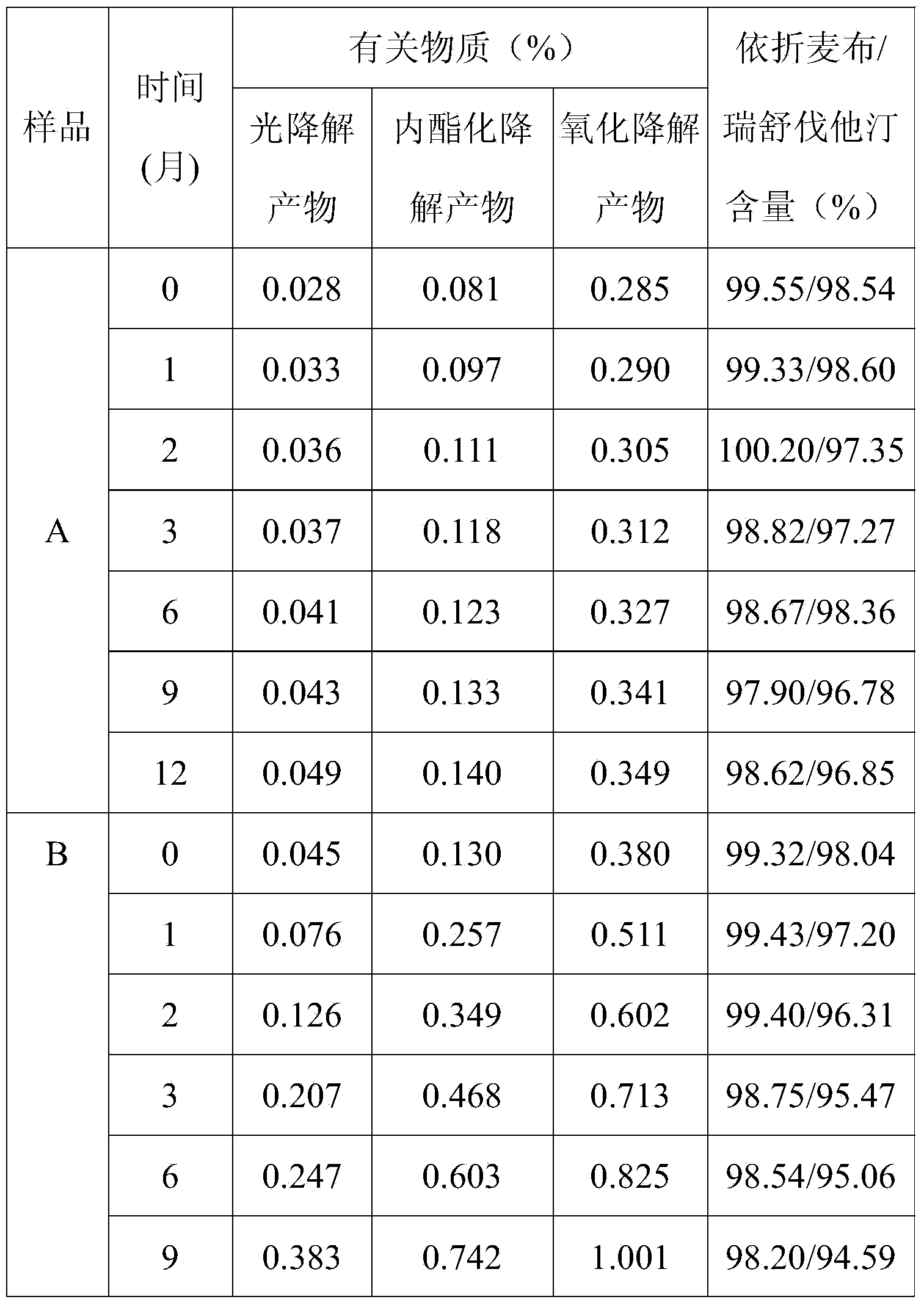
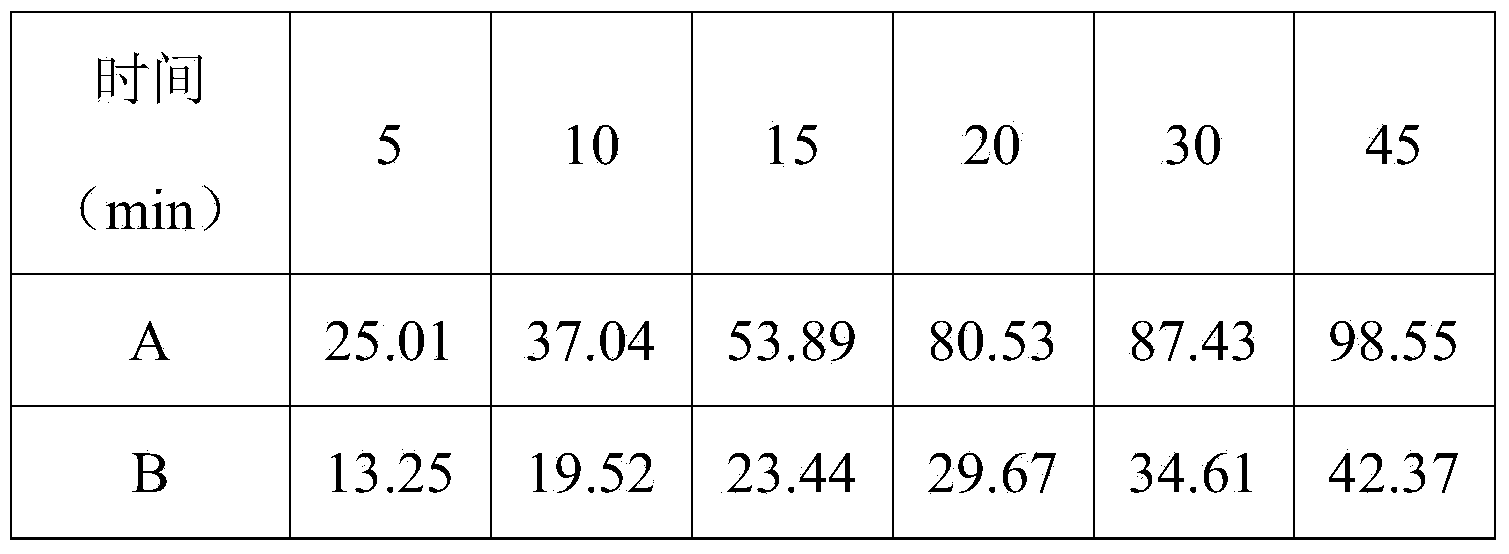
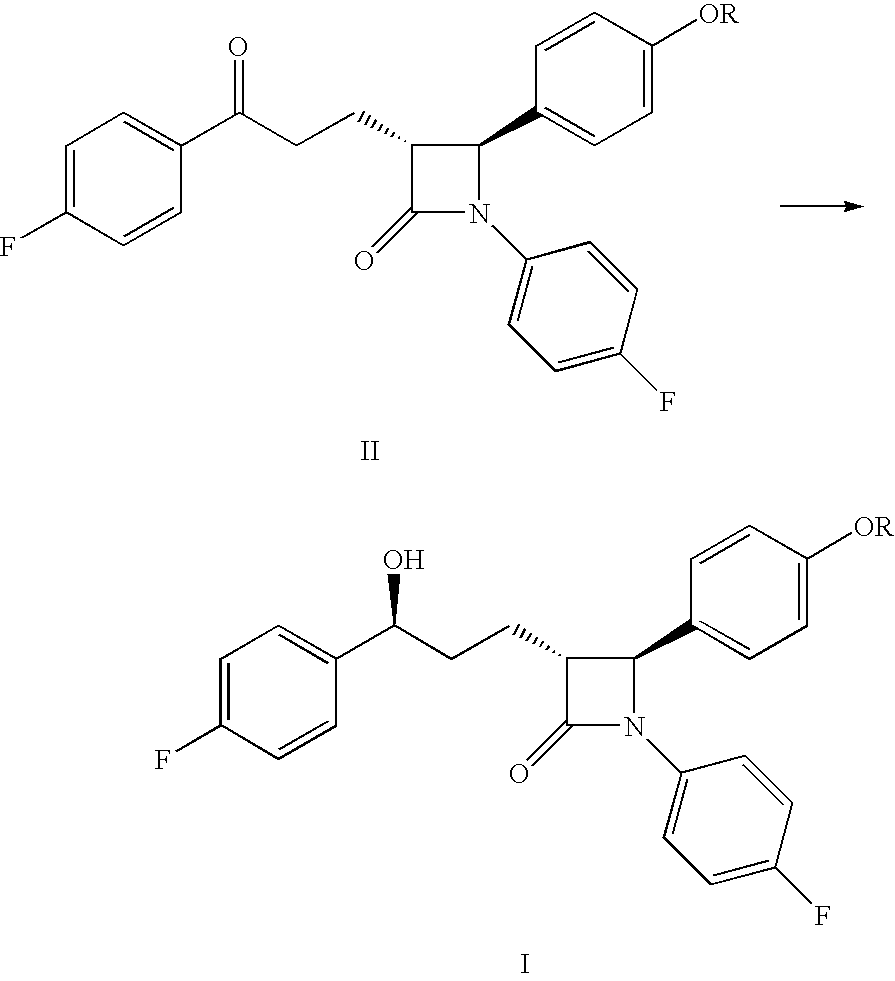
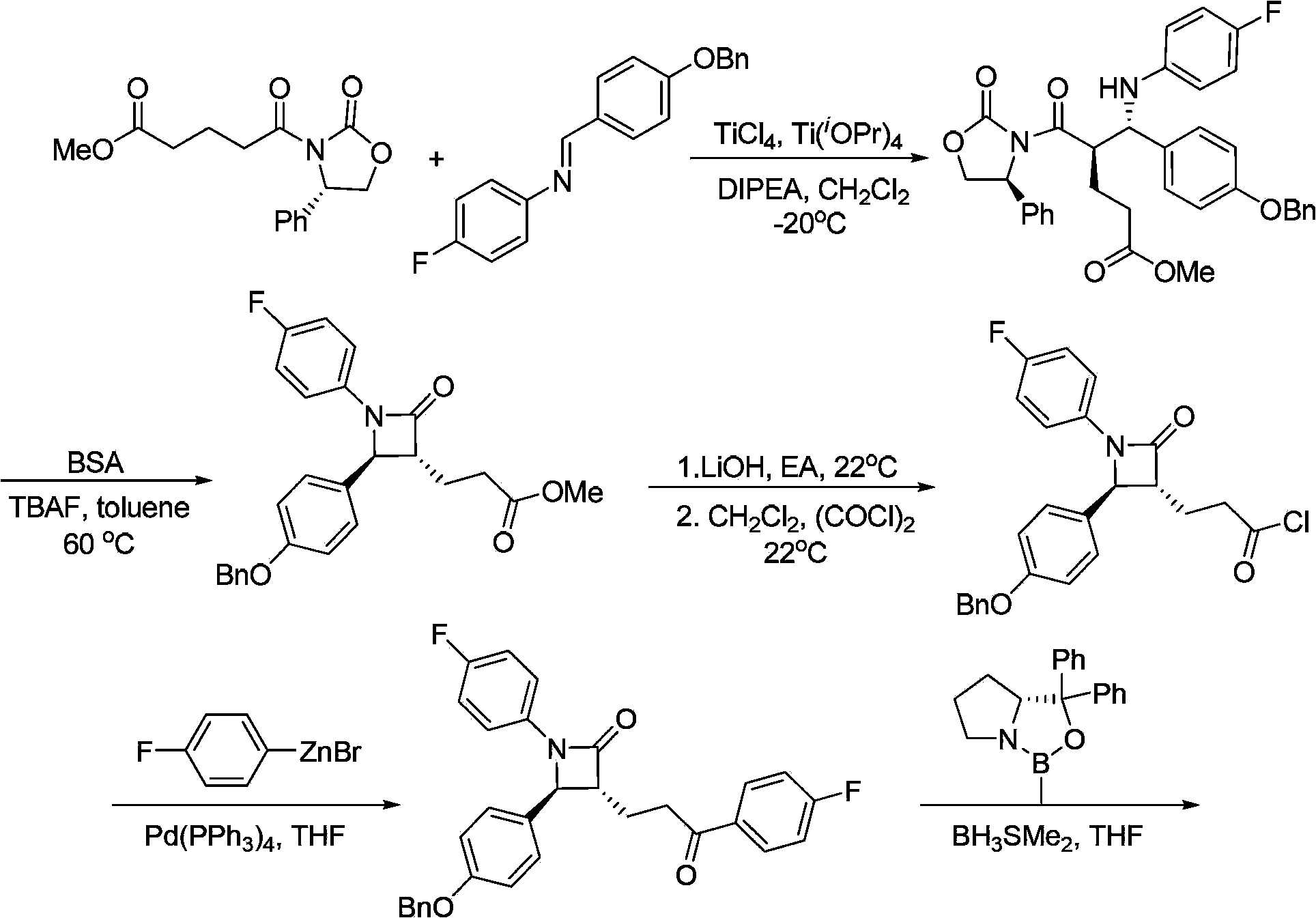
Preparation of ezetimibe
InactiveUS20070049748A1Improve scalabilitySafe handlingOrganic chemistryEzetimibeMedicinal chemistry
Owner:DR REDDYS LAB LTD +1
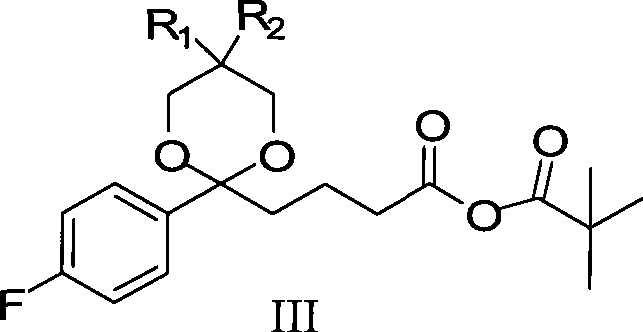
Ezetimible intermediate and synthetic method of ezetimible
InactiveCN101423511AReduce pollutionEasy to operateOrganic chemistryMetabolism disorderCompound aSynthesis methods
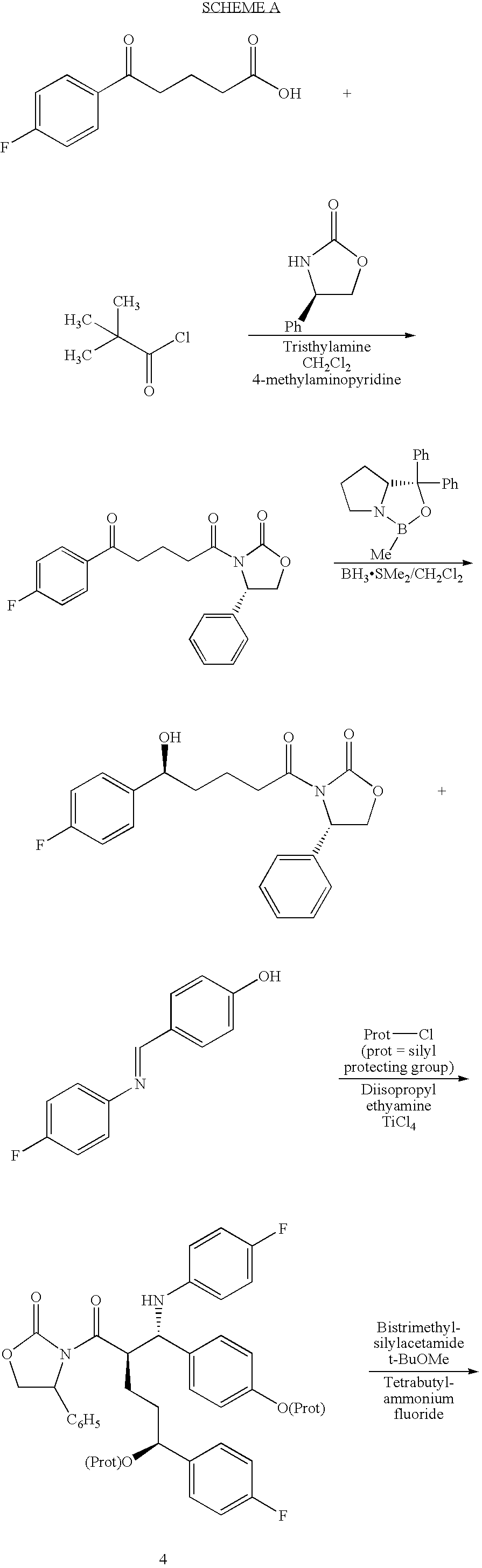
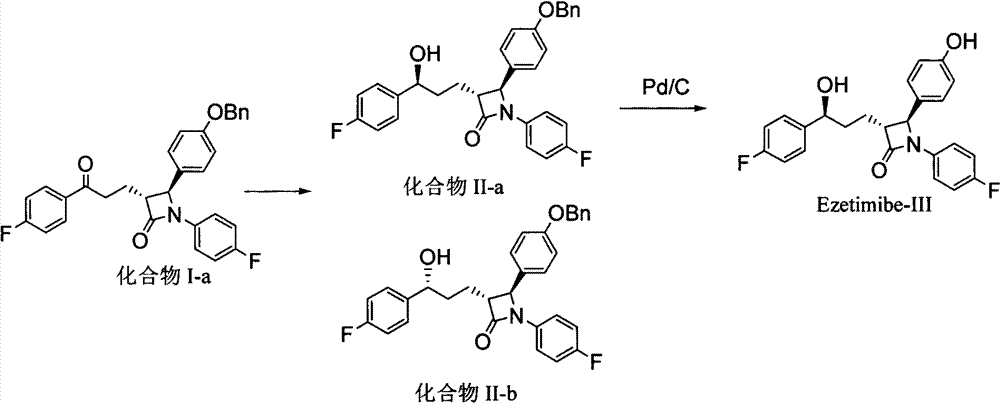
The invention provides an intermediate for synthesizing ezetimibe and a preparation process thereof, and also provides a method for synthesizing the ezetimibe by the intermediate. The synthesizing method has short route, and comprises the following concrete steps: a compound I and substituted 1, 3-propanediol react to generate a compound II; the compound II and pivalyl chloride react to generate a compound III; the compound III and a compound A react to generate a compound IV; the compound IV and a compound V react to generate a compound VI under the condition of a titanium compound catalyst; the compound VI is re-ringed to generate a compound VII with beta-lactam; the compound VII is hydrolyzed to produce a compound VIII; and the compound VII is reduced to a compound IX ezetimibe by a borane chiral reducing agent. The synthesization has short route and mild reaction condition; and the produced intermediate and final product has high yield and high purity.
Owner:ENANTIOTECH CORP
Solid Pharmaceutical Dosage Form
InactiveUS20110028456A1High drug loadingEasy to manufacturePowder deliveryBiocideValsartanTrenbolone
A pharmaceutical composition comprising a solid unit dosage form comprising: one or more of pharmaceutically active ingredients selected from valacyclovir, olanzapine, voriconazole, topotecan, artesunate, amodiaquine, guggulosterone, ramipril, telmisartan, tibolone, atorvastatin, simvastatin, amlodipine, ezetimibe, fenofibrate, tacrolimus, valgancyclovir, valsartan, clopidrogel, estradiol, trenbolone, efavirenz, metformin, pseudoephedrine, verapamil, felodipine, valproic acid / sodium valproate, mesalamine, hydrochlorothiazide, levosulpiride, nelfinavir, cefixime and cefpodoxime proxetil in combination with a water insoluble polymer and / or a water soluble polymer. Methods for making the pharmaceutical composition are also disclosed.
Owner:CIPLA LTD
Process for ezetimibe intermediate
The invention provides a process for preparing intermediate of ezetimibe, which shows hypocholesterolemic activity. Thus 3-[5-(4-fluorophenyl)-1,5-dioxopentyl]-4-phenyl-2-oxazolidinone is reduced with (−)-DIP chloride to obtain 3-[(5S)-5-(4-fluorophenyl)-5-hydroxy-1-oxopentyl]-4-phenyl-2-oxazolidinone.
Owner:HETERO DRUGS LTD
Preparation method of ezetimibe tablet
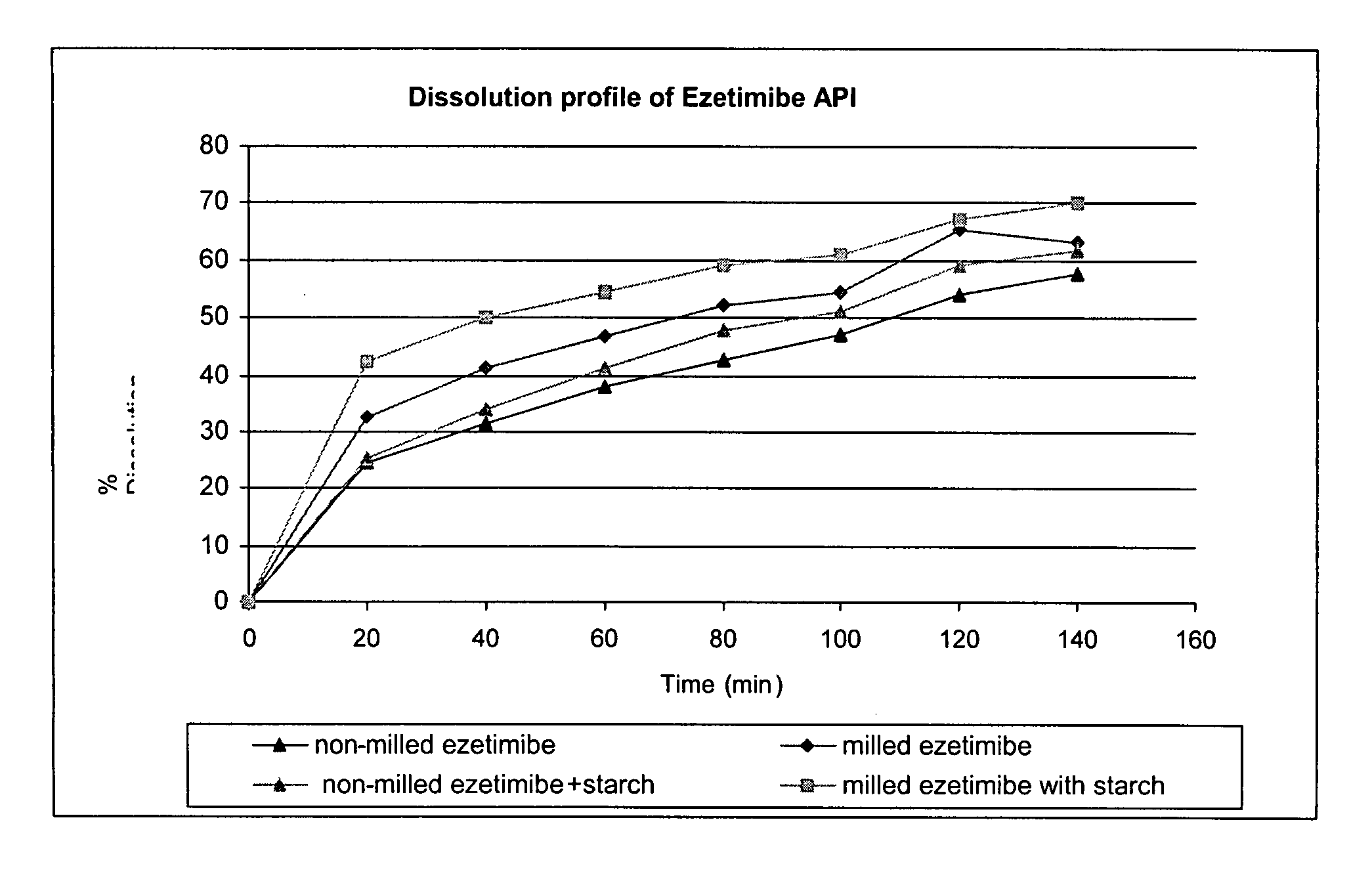
The invention discloses a preparation method of an ezetimibe tablet. The preparation method comprises the following steps of (1) dissolving hydroxypropyl cellulose and ezetimibe into ethanol, and dissolving a carrier material mannitol into a water solution; (2) respectively introducing a supercritical carbon dioxide fluid and the two solutions into a coaxial three-channel nozzle by setting the pressure and temperature of the supercritical carbon dioxide fluid to 10-50 MPa and 35-60 DEG C, and acquiring ultrafine granule mixed powder which contains the ezetimibe, the hydroxypropyl cellulose and the mannitol by utilizing the anti-solvent effect of the supercritical carbon dioxide fluid; (3) directly tabletting the ultrafine granule mixed powder and pharmaceutically acceptable auxiliary materials. The method disclosed by the invention can be used for fast dissolving the ezetimibe, can achieve the dissolution rate of the ezetimibe tablet by 95% for 5 minutes and does not contain the surface active agent.
Owner:QINGDAO UNIV OF SCI & TECH
Preparation method of ezetimibe medicine composition
InactiveCN103655453AEasy to operateEasy to scale up productionOrganic active ingredientsMetabolism disorderMedicineEzetimibe
The invention relates to a preparation method of an ezetimibe medicine composition. The preparation method comprises the following steps: 1, suspending ezetimibe in a proper solvent to prepare an uniform ezetimibe micron-sized suspension; 2, adding the suspension into a diluent of the medicine composition in a spraying manner and drying to prepare particles and powder of the ezetimibe medicine composition; and 3, preparing the particles and the powder into the ezetimibe medicinal minimum dose unit. Firstly, the medicine is prepared into the micron-sized suspension, granulation is carried out in a one-step granulation manner, the medicine is added into the diluent and finally, the medicinal minimum dose unit is prepared. The preparation method has the benefits that the effect of the preparation method is superior to that of a method in which micronization is carried out after the slightly soluble medicament is separately micronized; and the operation is simple and the large scale production is easy.
Owner:CHINA RESOURCES SAIKE PHARMA
Ezetimibe compositions
InactiveUS20100234342A1Improve bioavailabilityImprove solubilityBiocideOrganic active ingredientsEzetimibeDissolution
This invention is a novel pharmaceutical composition of ezetimibe or a pharmaceutically acceptable salt thereof comprising one or more pharmaceutically acceptable excipients having high bioavailability with improved solubility and dissolution rate which is stable throughout the shelflife, methods for their preparation, and methods for treatment using the same.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI
Ezetimibe compositions
InactiveUS20070275075A1Improve solubilityImprove bioavailabilityPowder deliveryBiocideEzetimibeBioavailability
Provided are ezetimibe compositions with improved solubility and increased bioavailability, methods of their preparation, and method of treatment using the same. An ezetimibe composition may be prepared, for example, by co-milling ezetimibe with at least one hydrophilic excipient.
Owner:TEVA PHARM USA INC
Preparation method of ezetimibe and its intermediate
InactiveCN102952055AHigh catalytic efficiencyD.e. high valueOrganic chemistryBulk chemical productionDiphosphinesOxygen
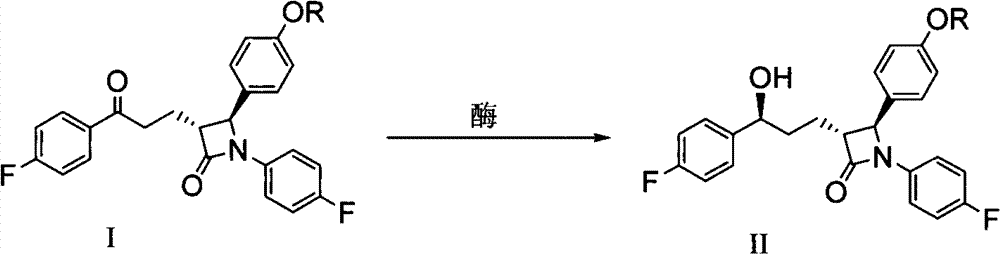
The invention discloses a preparation method of an ezetimibe intermediate compound as shown in formula II. The method includes the step of: in an organic solvent, in the absence of water and oxygen, and under the effects of an asymmetric hydrogenation catalyst and an organic alkali, subjecting compound I and hydrogen to an asymmetric catalytic hydrogenation reaction as shown in the following. Specifically, R is a conventional protecting group in the field, can protect a phenolic hydroxyl group and is stable under alkaline conditions. The asymmetric hydrogenation catalyst is a ruthenium-diphosphine-diamine catalyst invented by Ryoji Noyori, a Japanese scientist and a Nobel Prize winner. The preparation method provided in the invention has mild reaction conditions, can prepare products with high optical purity, yield and purity, and has after-treatment that is convenient to operate, thus being easy to realize industrialized production.
Owner:CHIRAL QUEST (SUZHOU) CO LTD
Inhibition of angiogenesis
InactiveUS20090208448A1Inhibiting prostateLower circulating cholesterolBiocideOrganic active ingredientsDiseaseHuman tumor
Cholesterol-uptake-blocking drugs inhibit angiogenesis and are useful to inhibit diseases perpetuated by angiogenesis. Cholesterol reduction with the use of the drugs increases the intratumoral level of thrombospondin-1, an angiogenesis inhibitor. Ezetimibe (Zetia®), a specific cholesterol-uptake blocking drug, also retards the growth of human tumors, most preferably in combination with low-cholesterol diet. The pharmacologic reduction in serum cholesterol retards prostate cancer growth by inhibiting tumor angiogenesis to combat the growth of prostatic tumors which are directly accelerated by hypercholesterolemia.
Owner:CHILDRENS MEDICAL CENT CORP +1
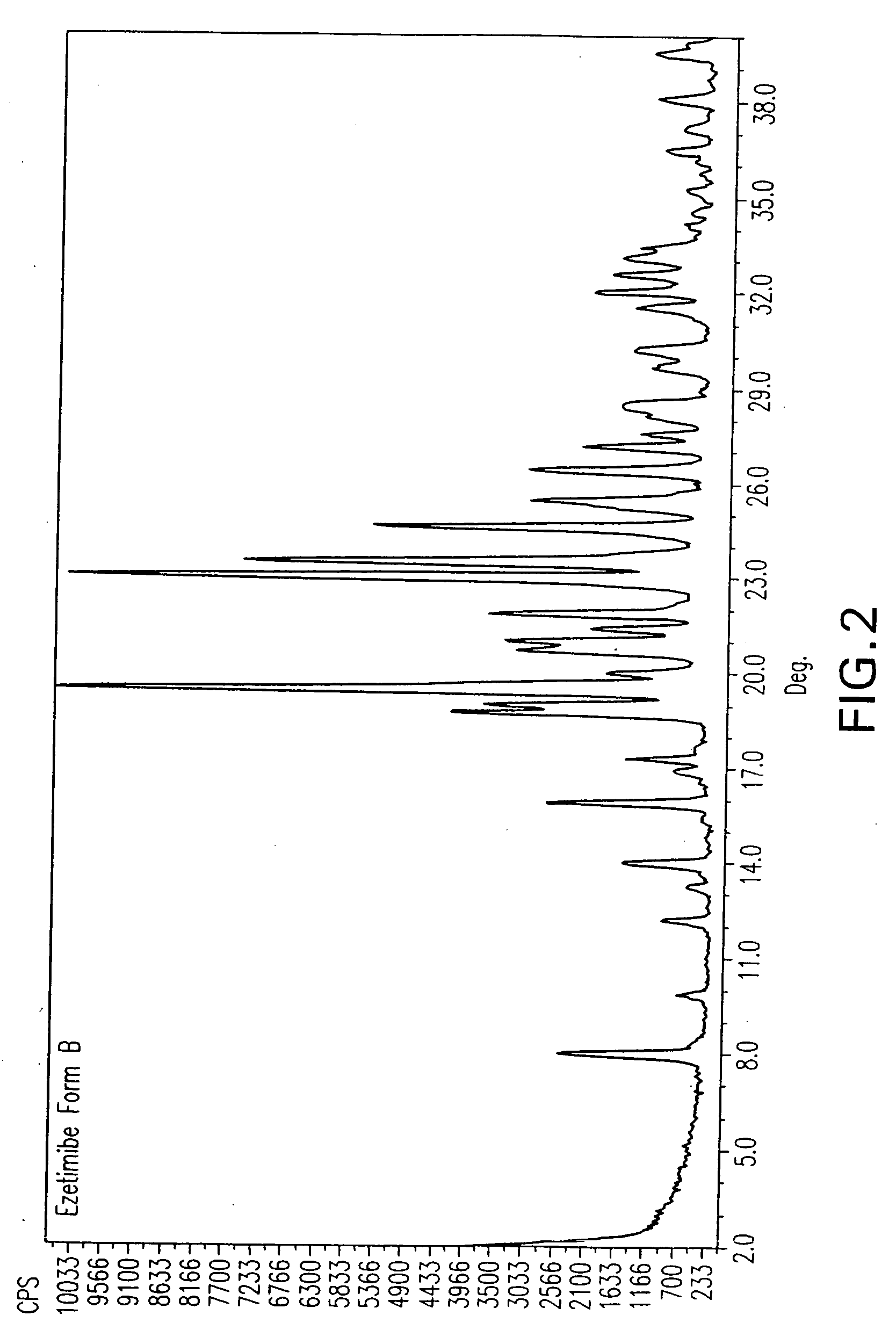
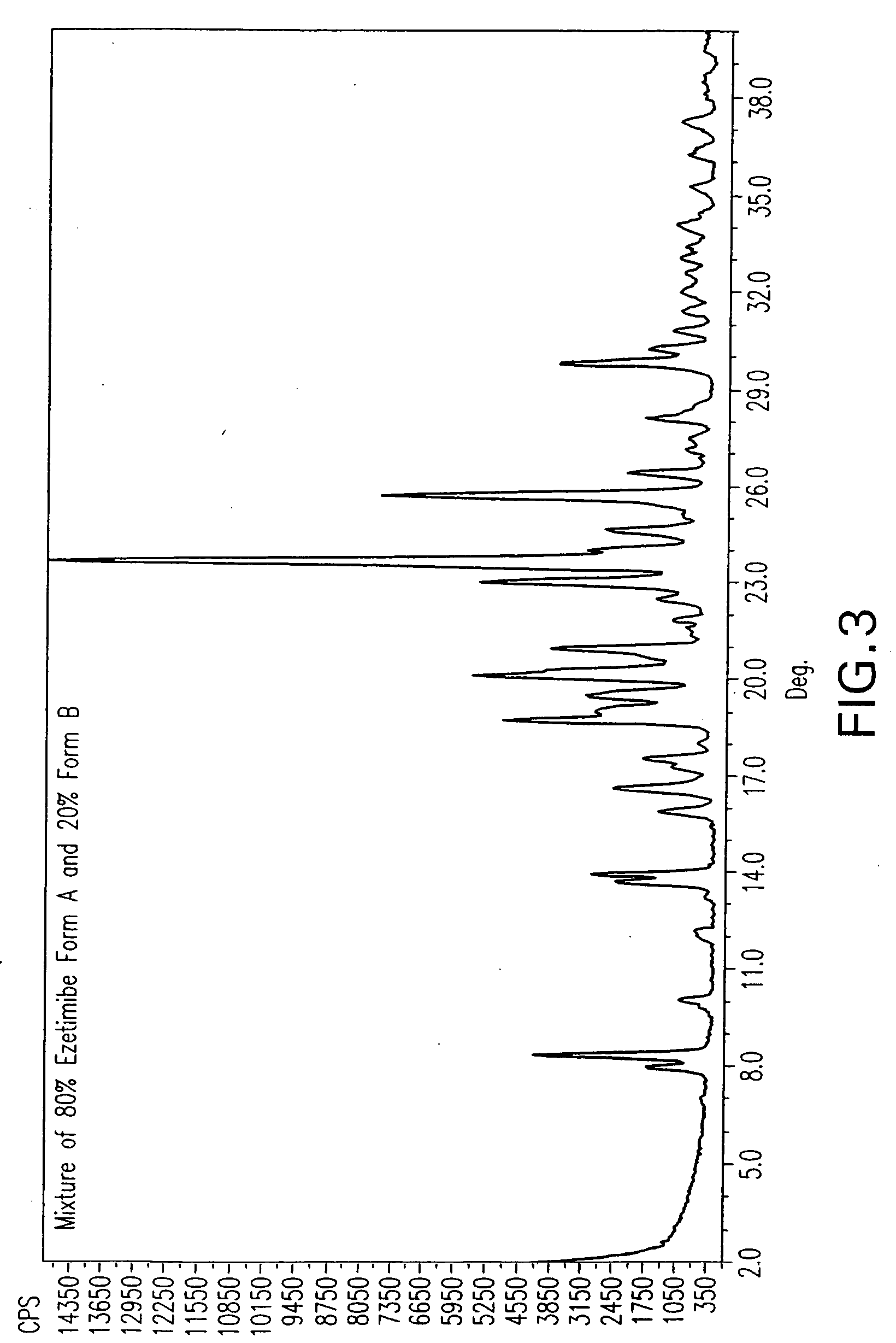
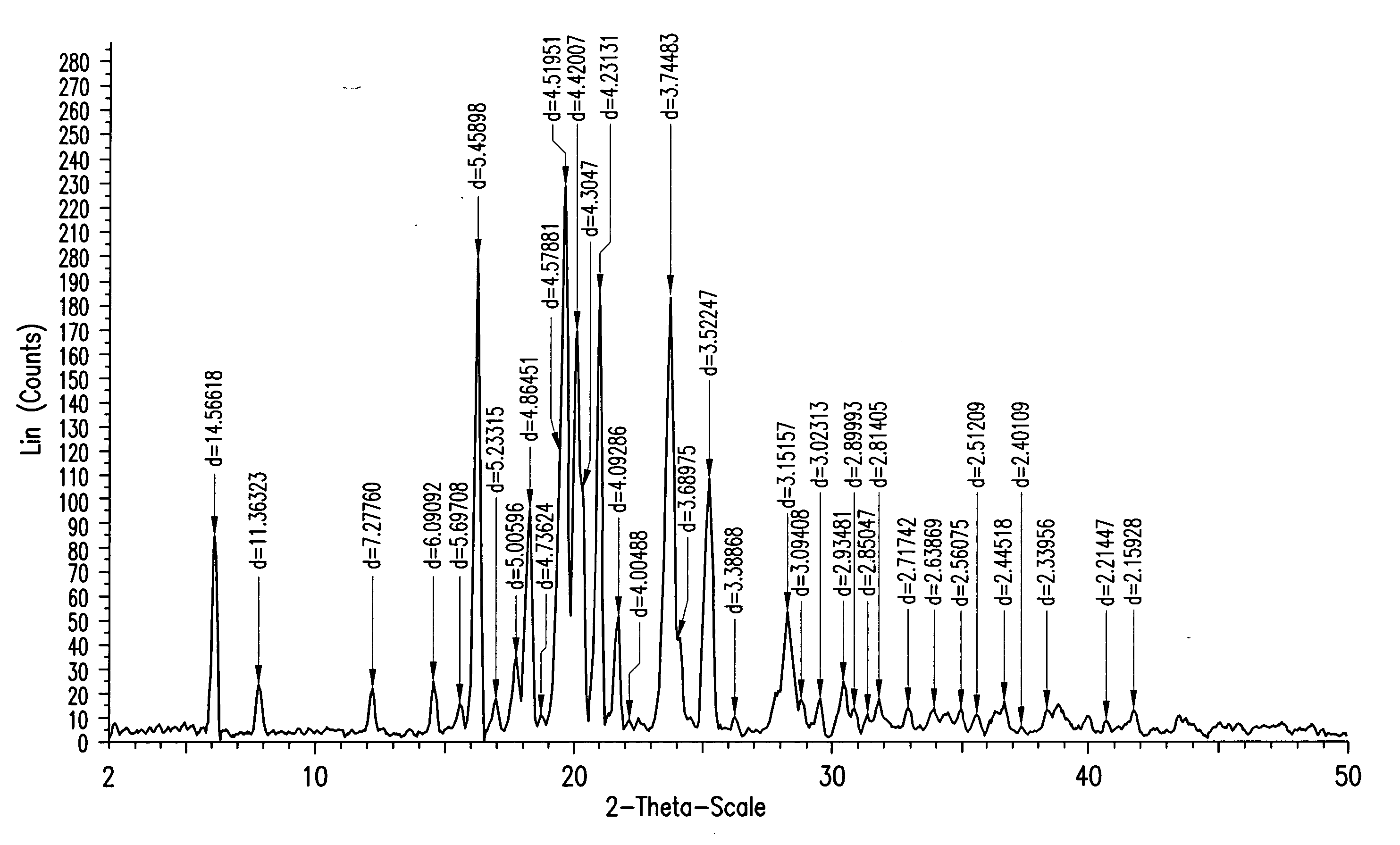
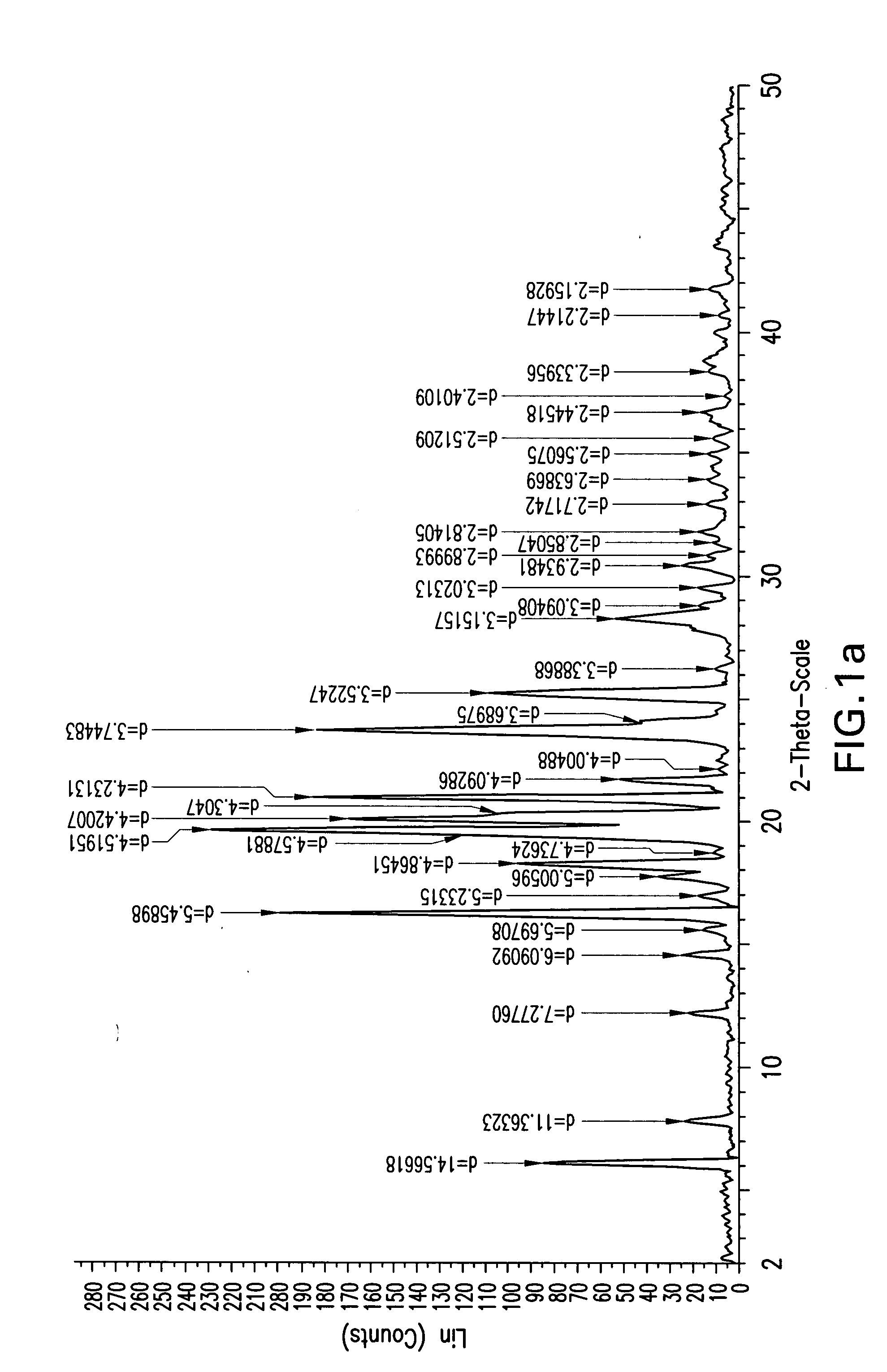
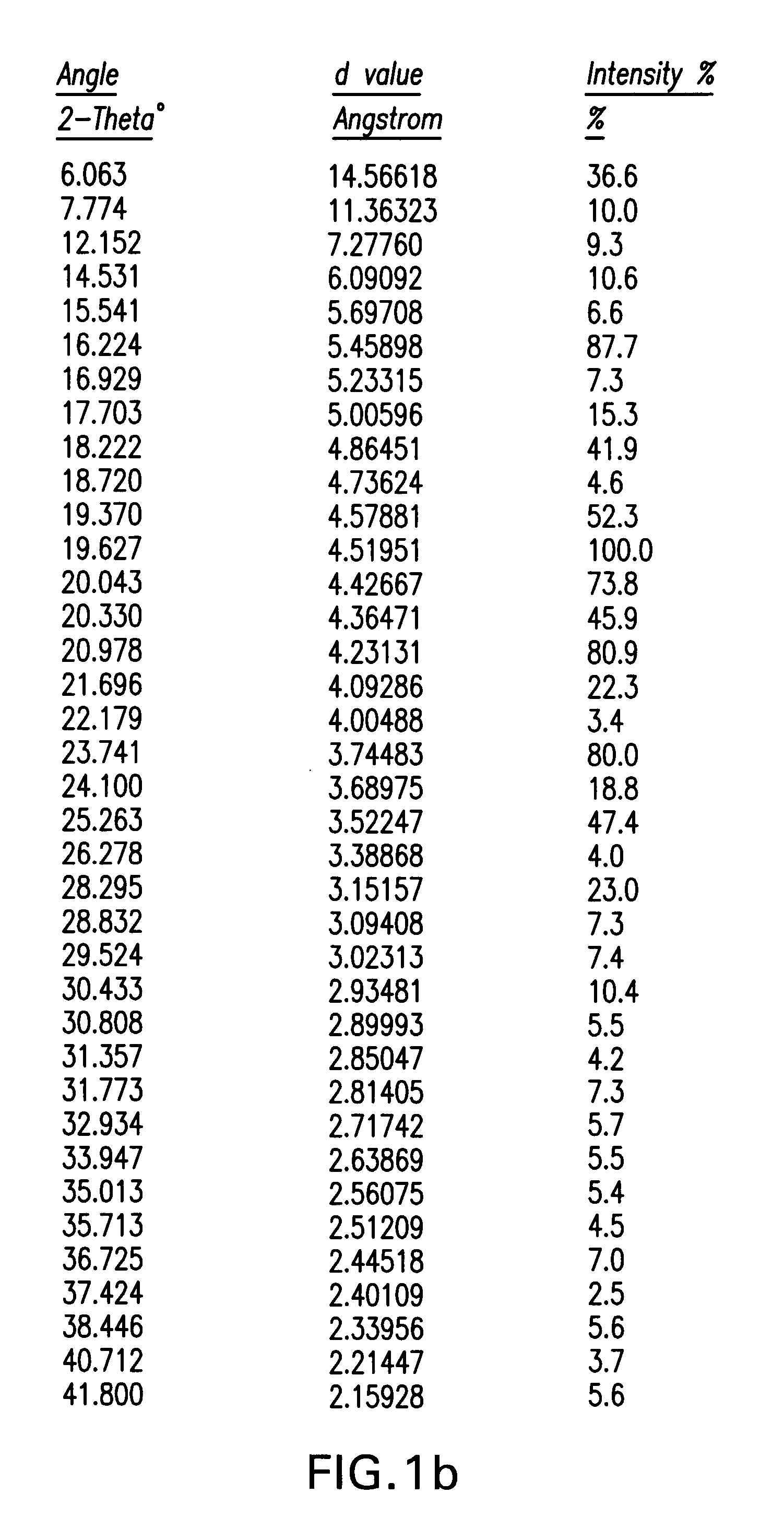
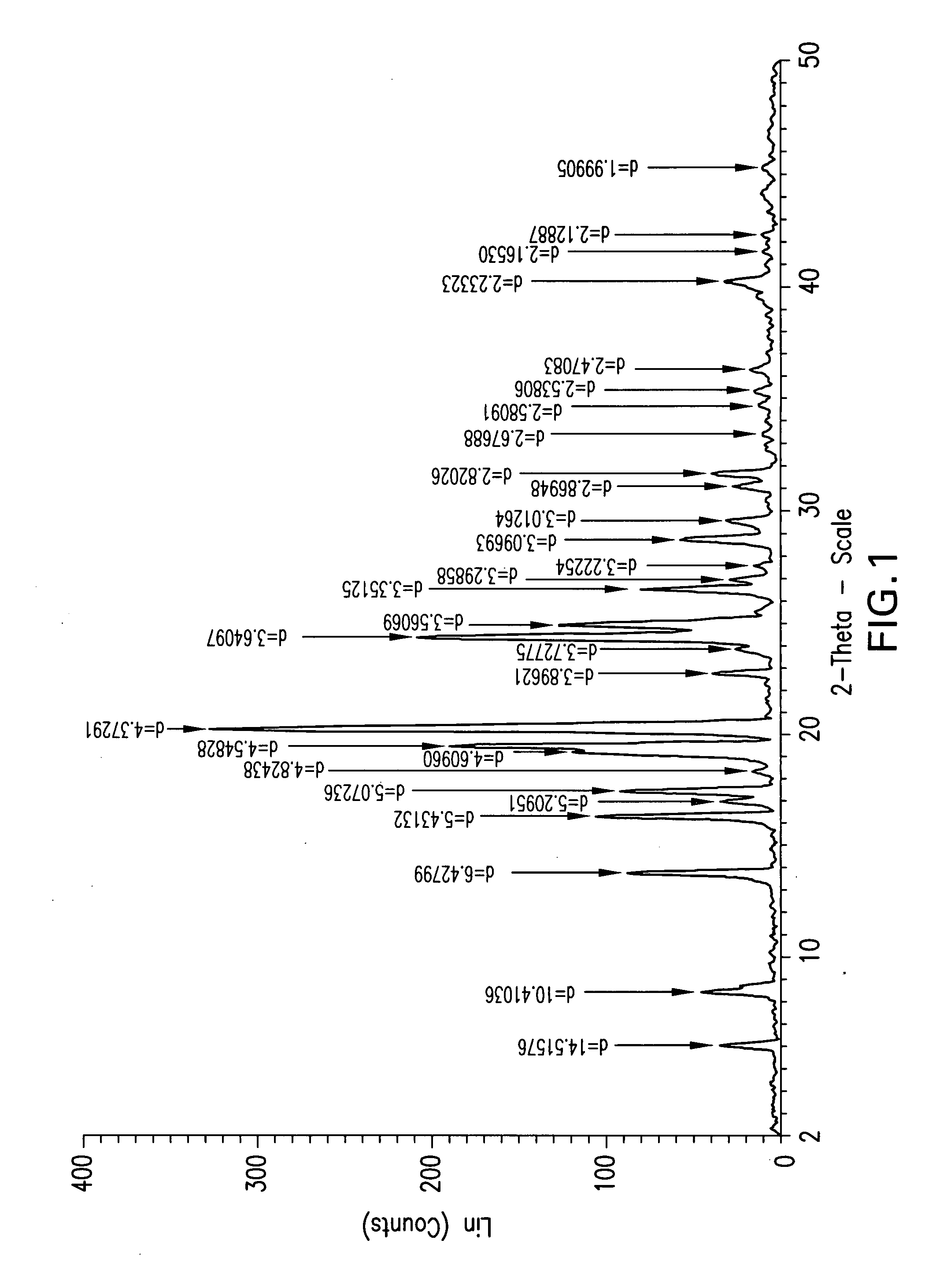
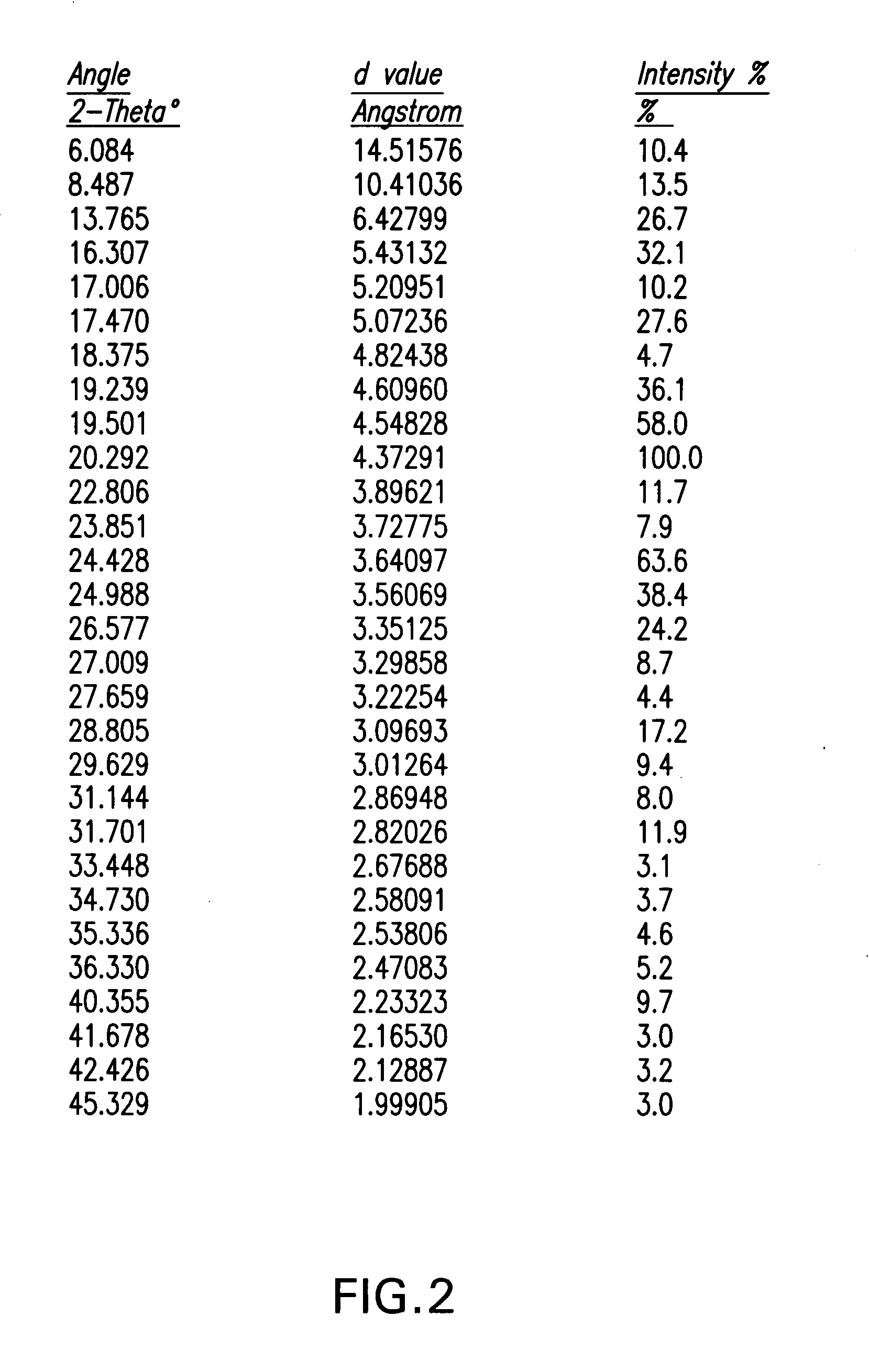
Ezetimibe polymorphs
Provided are processes for preparing crystalline forms of ezetimibe, such as ezetimibe Form A or Form B, for example, by precipitating ezetimibe from selected solvents. Alternatively, some forms may be transformed into different forms at elevated temperatures or under various humidity conditions, or by micronization. Also provided are micronized ezetimibe Form A, micronized ezetimibe Form B, and ezetimibe having a plate morphology. Pharmaceutical compositions containing these forms are particularly useful in reducing cholesterol in patients in need thereof.
Owner:TEVA PHARM USA INC
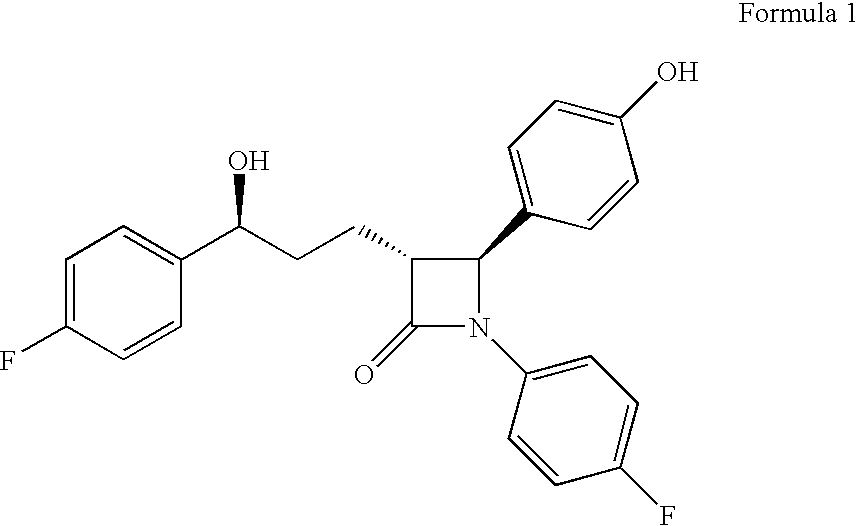
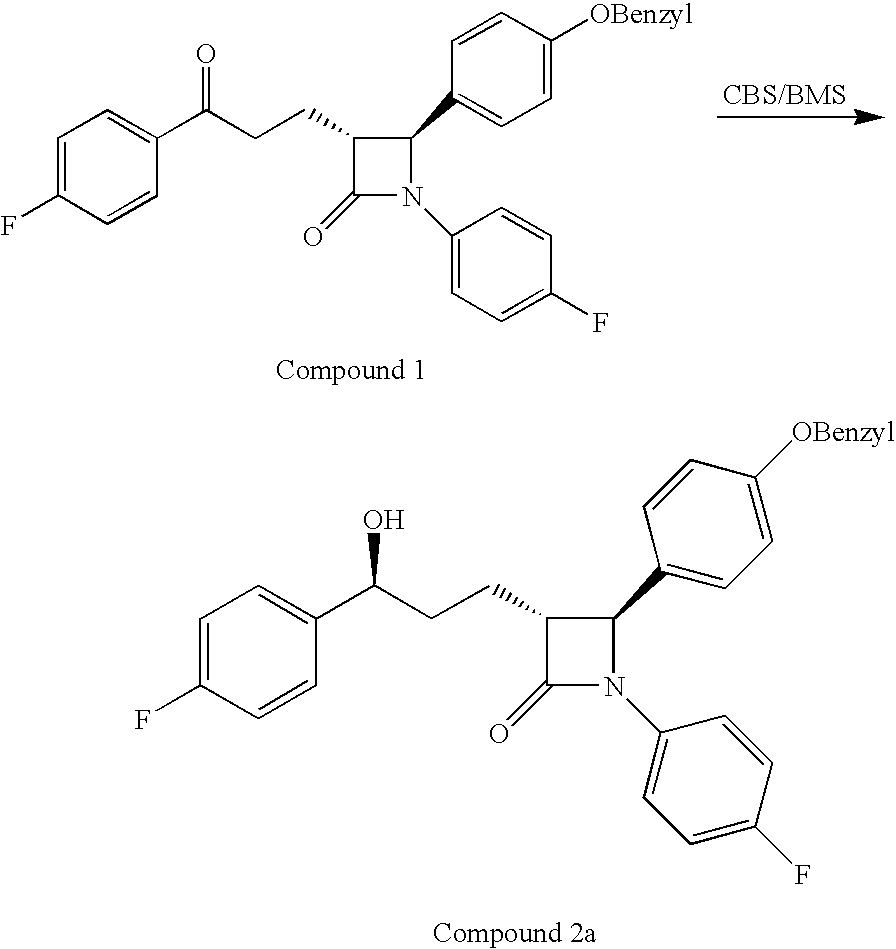
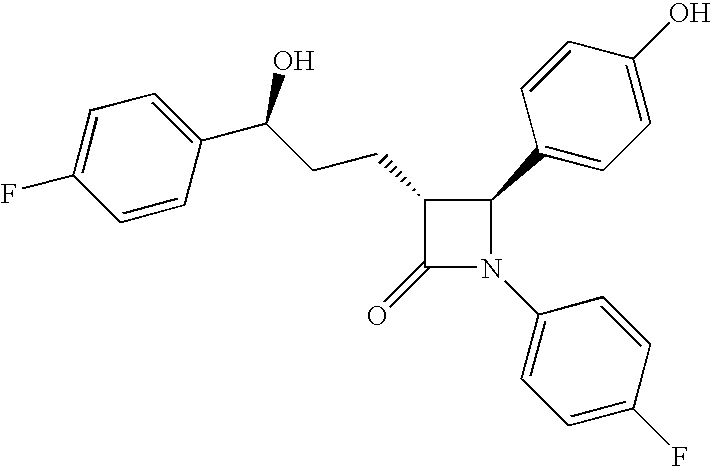
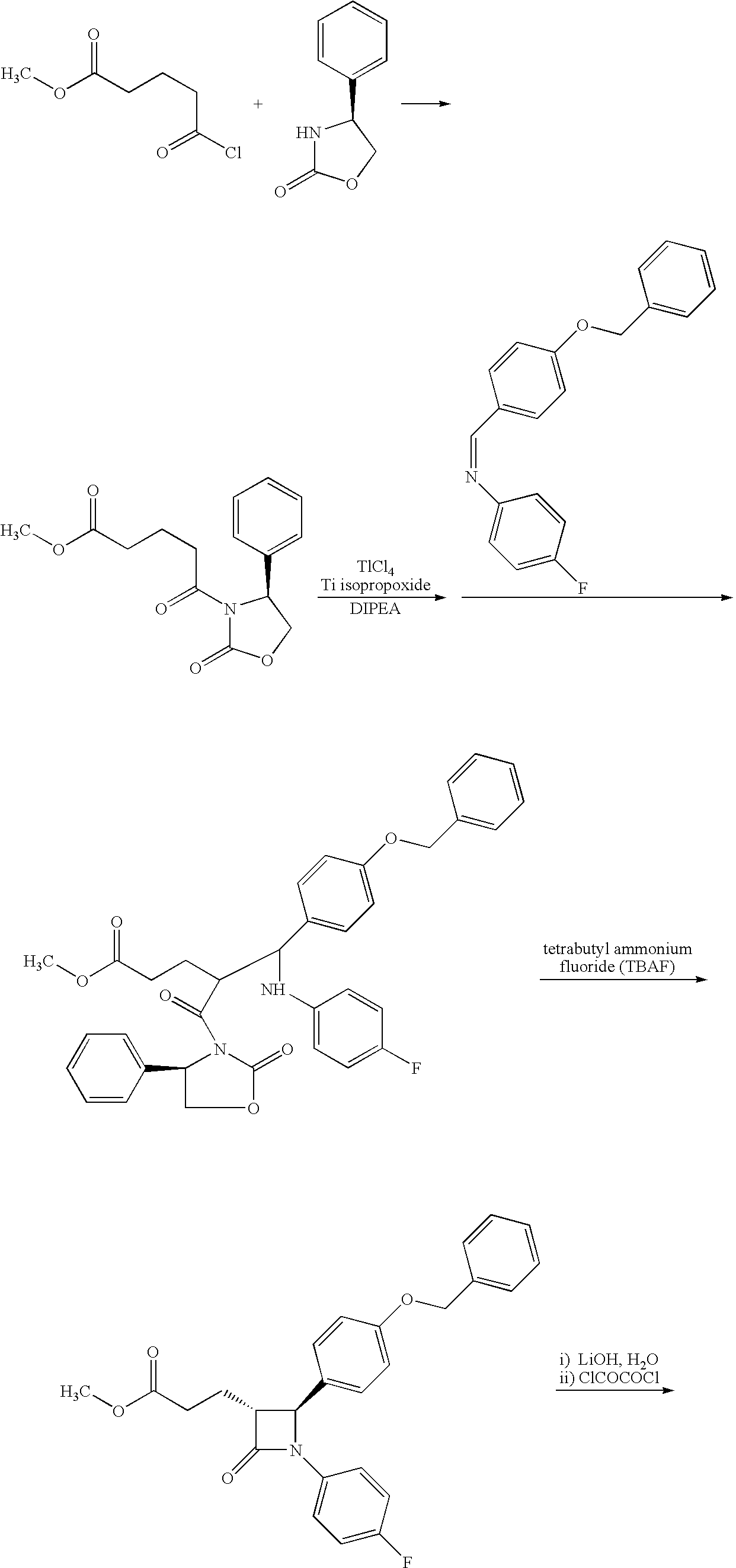
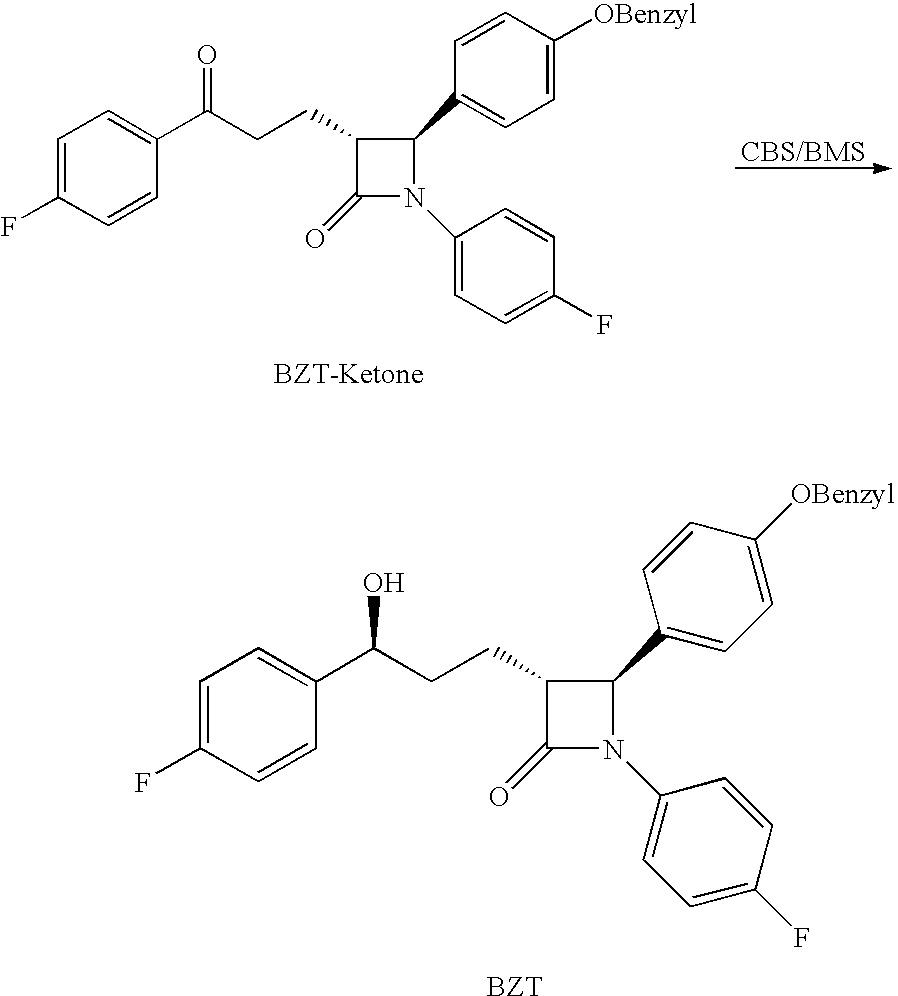
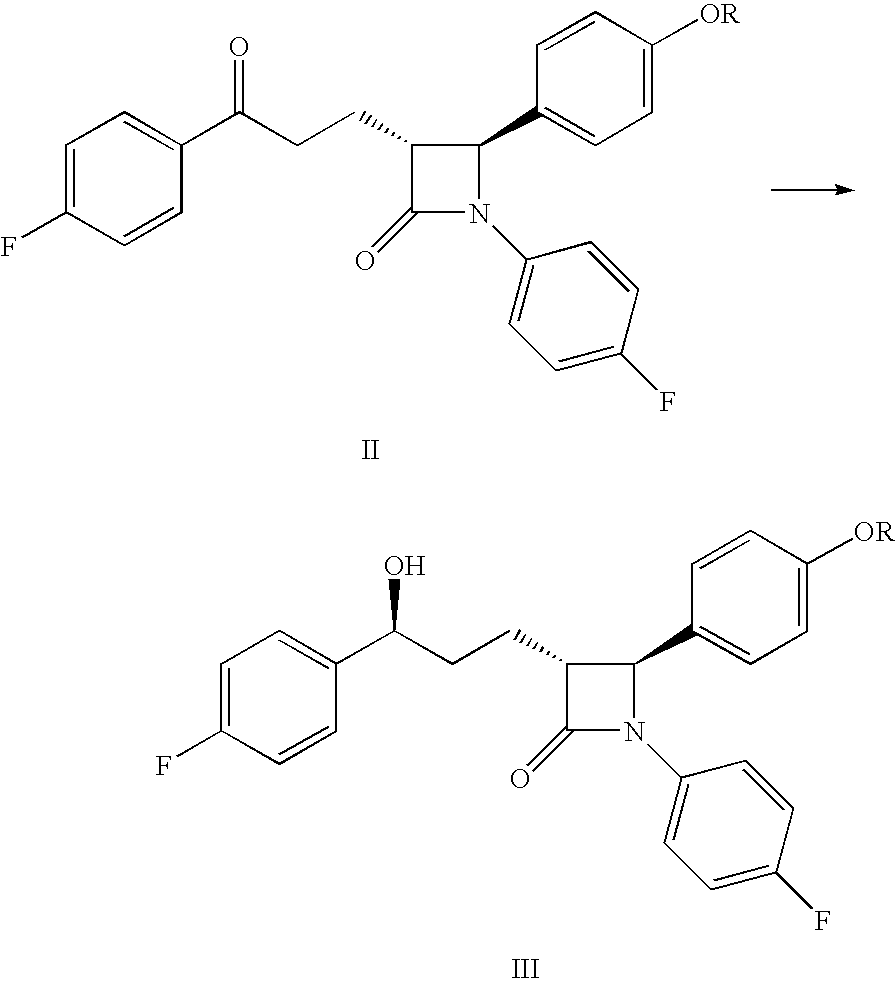
Processes for the preparation of (3R,4S)-4-((4-benzyloxy)phenyl)-1-(4-fluorophenyl)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-2-azetidinone, an intermediate for the synthesis of ezetimibe
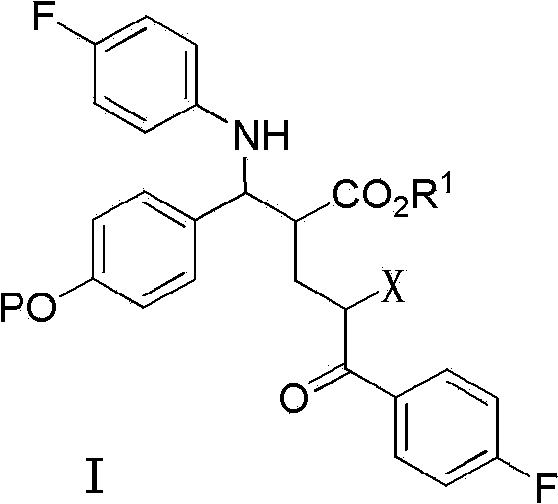
The invention encompasses (3R,4S)-4-((4-benzyloxy)phenyl)-1-(4-fluorophenyl)-3-(3-(4-fluorophenyl)-3-oxopropyl)-2-azetidinone (Compound 2a) having an enantiomeric purity of at least about 97.5%. The invention also encompasses Compound 2a having a chemical purity of at least about 97%. The invention further encompasses processes for preparing Compound 2a from Compound 1 having the following formula: The invention also encompasses processes for preparing a compound having the following formula: from a compound having the following formula: wherein R is selected from the group consisting of: H or a hydroxyl protecting group. The invention also encompasses processes for preparing Compound 2a, preferably to form Compound 2a-Form 01. Also included are processes for preparing ezetimibe from Compound 2a-Form 01 or Compound 2a prepared according to the invention, compositions containing such ezetimibe, and methods for reducing cholesterol using such compositions.
Owner:TEVA PHARM USA INC
Process for the synthesis of azetidinone
InactiveUS20080032964A1Reduce cholesterolBiocideOrganic active ingredientsEzetimibeCombinatorial chemistry
Owner:TEVA PHARM USA INC
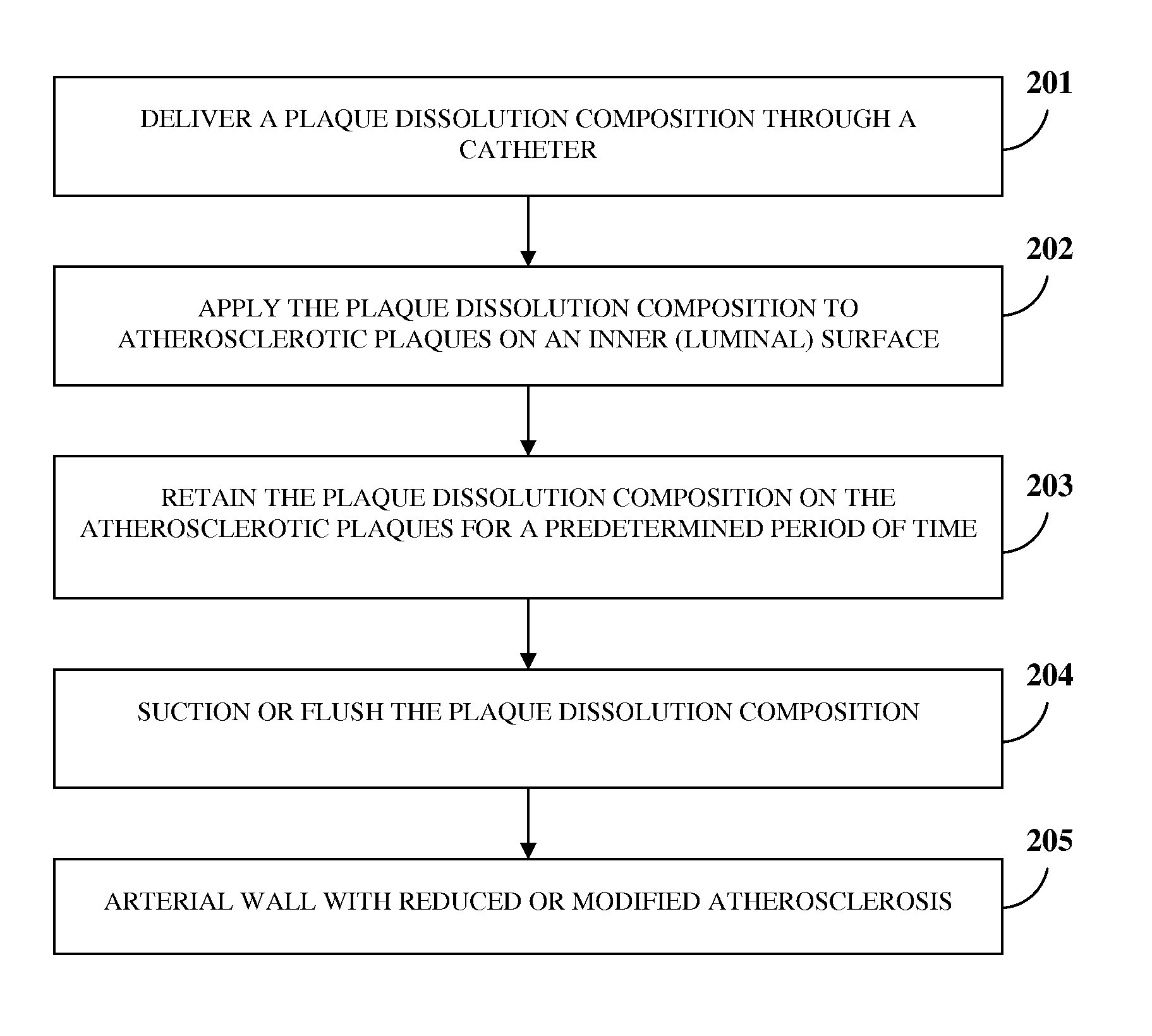
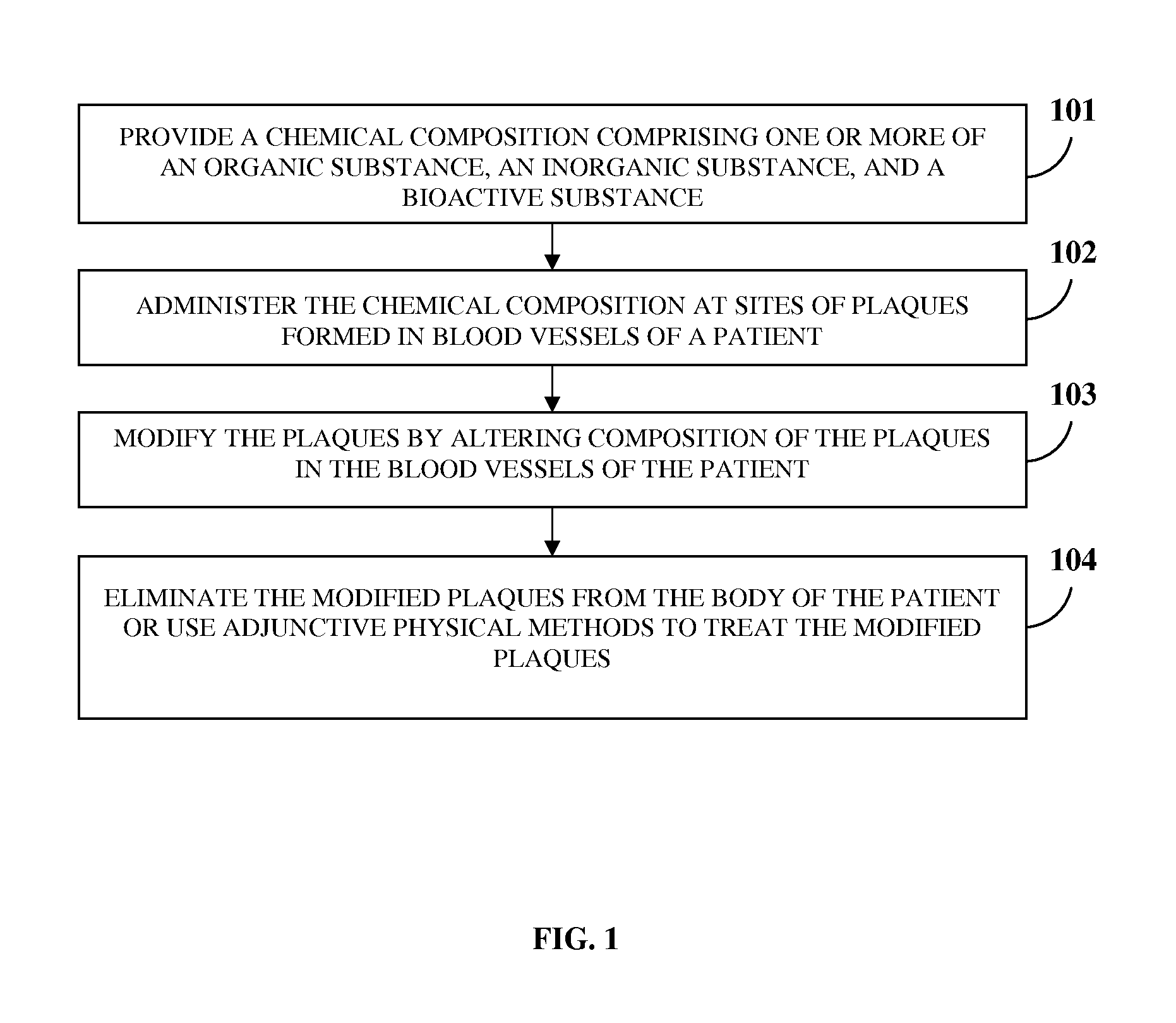
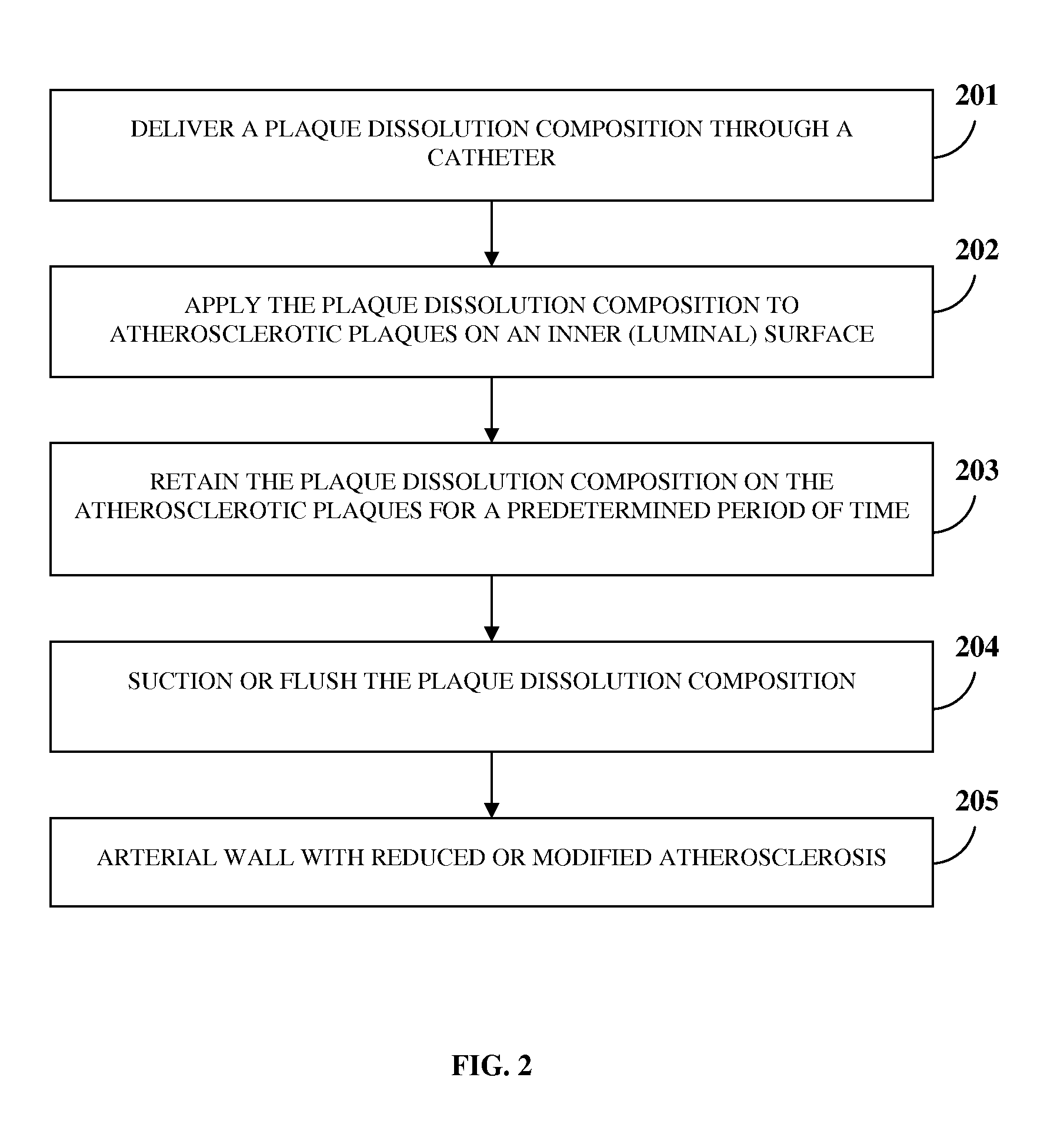
Atherosclerotic Plaque Dissolution Composition
InactiveUS20110196383A1High densityEasy to disassembleBiocideHalogenated hydrocarbon active ingredientsOctanoic AcidsBioactive substance
A method and chemical composition for modifying and dissolving atherosclerotic plaques formed in a patient's blood vessels is provided. A chemical composition comprising one or more of an organic substance, an inorganic substance, and a bioactive substance is administered at sites of the plaques. The chemical composition comprises one or more of d-limonene, propylene glycol, octanoic acid, 2-octane, glycerine, acetylsalicylic acid, acetic acid, omega-3 fatty acids, ethanol, methanol, ezetimibe, rosuvastatin, resveratrol, lactic acid, gluconic acid, chloroform, carbon disulfide, dichloromethane, toluene, lauryldimethyl hydroxysultaine, and any combination thereof. The chemical composition enables modification of plaques by altering their composition. The modification comprises partial dissolution, complete dissolution, or elimination of the plaques, and makes the plaques amenable to different forms of plaque treatment. The modified plaques are eliminated from the patient's body. The modification or elimination of the plaques facilitates treatment of cardiovascular diseases caused due to plaques formed in the blood vessels.
Owner:ATHEROLYSIS MEDICAL
Preparation midbody for Ezetimibe and preparation method of preparation midbody
ActiveCN104230978AEasy to manufactureGroup 4/14 element organic compoundsBulk chemical productionChlorobenzeneAcyl group
The invention provides a preparation midbody for Ezetimibe which is shown in a general formula I, wherein PG is an acetyl group, a T-butyloxycarbonyl group, a benzyl group, a benzyloxycarbonyl group, a trityl group, a trimethylsilyl group or a diphenylmethyl group silicon group. The invention further provides a preparation method of the midbody I, and the application for preparing the medicine Ezetimibe. The method for preparing the Ezetimibe by adopting the compounds shown in the general formula I is different from the method in the conventional document, the newer chirality assistant (S)-4-(2-chlorphenyl)-2-oxazolone is adopted, the productive rate reaches 91%, and the optical purity reaches 100%, so that the productive rate and the optical purity are higher than those of the previously applied (S)-4-phenyl-2-oxazolone. Besides, the selected chirality assistant (S)-4-(2-chlorphenyl)-2- oxazolone can be conveniently prepared by the original commercialized ((S)-2-chlorobenzene glycine potassium).
Owner:SHANGHAI SHYNDEC PHARMA CO LTD
Double-layer tablet containing ezetimibe and rosuvastatin and preparation method thereof
ActiveCN103585157AImprove the quality of lifeReduce adverse reactionsOrganic active ingredientsMetabolism disorderSolubilityAdditive ingredient
The invention relates to a double-layer tablet which contains ezetimibe and rosuvastatin serving as effective components and has a lipid-lowering effect and a preparation method thereof. Aiming at the problems poor water solubility of ezetimibe, instability of rosuvastatin to acid and oxygen and the like, the method adopts a micronization technology to improve the dissolution rate of ezetimibe and adopts an anti-oxidizing agent and a double-layer tabletting technology to improve the stability of rosuvastatin in a human body, further, ingredients of a medicine is enabled to take effect sufficiently, and the best synergistic effect is achieved; and a compound preparation agent is mainly applied to treatment of diseases relative to hypercholesteremia.
Owner:WUHAN WUYAO SCI & TECH
Pharmaceutical formulation comprising ezetimibe
The present invention relates to novel formulations comprising ezetimibe as active ingredient. In particular the invention relates to a pharmaceutical composition comprising 5 to 20 wt-% ezetimibe, 50 to 85 wt-% diluent, 3 to 25 wt-% disintegrant, 1 to 10 wt-% binder, and 0.5 to 1 wt-% lubricant, characterized in that the ezetimibe has a particle size distribution of d(0.9) of 5 μm to 35 μm and d(0.5) of 3 μm to 20 μm, as well as methods for preparing said formulations.
Owner:RATIOPHARM GMBH
Processes for preparing intermediate compounds useful for the preparation of ezetimibe
InactiveUS20090227786A1Easy to handleSilicon organic compoundsGroup 3/13 element organic compoundsEzetimibeKetone
The invention relates, in general, to an improved process for the preparation of the compounds (3R,4S)-4-(4-(benzyloxy)phenyl)-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]azetidin-2-one and (3R,4S)-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]-4-(4-hydroxyphenyl)-azetidin-2-one, which are key intermediates for the synthesis of ezetimibe, as well as the use of these intermediates for the preparation of ezetimibe.
Owner:MEDICHEM
Pharmaceutical composition of atorvastatin and ezetimibe
The present invention relates to an oral pharmaceutical composition comprising: a) a core comprising atorvastatin or a pharmaceutically acceptable salt thereof and an alkalizing agent; b) an intermediate coating over the core; and c) an outer coating comprising ezetimibe.
Owner:RANBAXY LAB LTD
Preparation method of ezetimibe chiral intermediate
ActiveCN103896700AReduce dosageIndium organic compoundsOrganic compound preparationPtru catalystCombinatorial chemistry
The present invention relates to the field of pharmaceutical synthesis, and specifically, to a method for preparing an Ezetimibe chiral intermediate. The method is as follows: a method for preparing a compound with a formula (b) structure comprises the following steps: obtaining the compound with the formula (b) structure by using a compound with a formula (a) structure under the effects of a chiral catalyst, alkali and hydrogen, R is NR1R2, OR3 or OH, R1 and R2 are separate alkyl groups, and R3 is an C1-C6 alkyl group or a benzyl group. The chiral catalyst is a compound with a formula (M) structure: DTB is shown as a formula DTB. The yield of the compound with the formula (b) prepared by using the method can exceed 95%, and the optical purity can reach 100% at the maximum.
Owner:浙江瑞博制药有限公司
Ezetimibe and atorvastatin calcium tablet and preparation method thereof
ActiveCN105832723AImprove distribution uniformityImprove hydrophilicityMetabolism disorderDrageesFiller ExcipientWater insoluble
The invention discloses an ezetimibe and atorvastatin calcium tablet and a preparation method thereof. The tablet is prepared from ezetimibe particles and atorvastatin calcium particles through double-layer tabletting, wherein the ezetimibe particles comprise the following components: ezetimibe, a binder, a surfactant, a water-soluble filling agent, a water-insoluble filling agent, a disintegrating agent and a lubricant; during pelletizing of the ezetimibe particles, the ezetimibe and the surfactant are dissolved or dispersed into a binder solution; the water-soluble filling agent is added to uniformly mix; and other auxiliary materials are added for pelletizing. During the preparation of the ezetimibe layer, the auxiliary materials are added in specific sequence, so that the in-vitro release problem of the ezetimibe is effectively solved.
Owner:ZHEJIANG JUTAI PHARMA
Methods for treating patients with familial hypercholesterolemia
InactiveUS20170312359A1Reducing lipidExtended time intervalMetabolism disorderPharmaceutical delivery mechanismAntigenLipid lowering drug
The present invention provides methods for treating patients suffering from familial hypercholesterolemia, including both HeFH and HoFH. The methods of the invention provide for lowering at least one lipid parameter in the patient by administering a therapeutically effective amount of an antibody or antigen-binding fragment thereof that specifically binds to ANGPTL3 in combination with a therapeutically effective amount of a statin, a first lipid lowering agent other than a statin, and a second lipid lowering agent other than a statin. The first non-statin lipid lowering agent is an agent that inhibits cholesterol uptake (e.g. ezetimibe) and the second non-statin lipid-lowering agent is an inhibitor of microsomal triglyceride transfer protein (e.g. lomitapide). The combination therapy is useful in treating hypercholesterolemia, as well as hyperlipidemia, hyperlipoproteinemia and dyslipidemia, including hypertriglyceridemia, chylomicronemia, and to prevent or treat diseases or disorders, for which abnormal lipid metabolism is a risk factor, such as cardiovascular diseases.
Owner:REGENERON PHARM INC
Amorphous ezetimibe and the production thereof
InactiveUS20080085315A1Reducing residual solvent contentIncrease in bulk powder densityBiocideOrganic active ingredientsSolubilityNon solvent
Ezetimibe compositions of enhanced bioavailability are described that contain ezetimibe with at least one solubility-enhancing polymer. Described methods to produce the bioenhanced products comprise solvent spray drying. One aspect of the method includes the steps of providing a mixture comprising ezetimibe, a solubility-enhancing polymer and a single solvent, a solvent blend or solvent / non-solvent blend and then evaporating the mixture to form amorphous ezetimibe.
Owner:ISP INVESTMENTS INC
Process for the preparation of a pharmaceutical composition comprising ezetimibe
ActiveUS20120135976A1Small particle sizeAvoid attenuationPowder deliveryBiocidePharmaceutical industryEzetimibe
The present invention belongs to the field of pharmaceutical industry and relates to a process for preparing dosage forms containing ezetimibe, comprising the steps of:a) providing a composition comprising ezetimibe,b) sieving a composition comprising a composition of step (a),c) shear mixing of the composition after step (b), preferably the mixing of the composition is carried out by high shear mixing,d) formulation into a dosage form.The present invention also relates to dosage forms containing ezetimibe and ezetimibe and simvastatin, which have been prepared according to the process according to the invention.
Owner:LEK PHARMA D D
Synthetic method of ezetimibe, and intermediate used in synthetic method
ActiveCN103570574AReduce usageHigh activityGroup 4/14 element organic compoundsIndium organic compoundsEzetimibeCarboxylic acid
The invention discloses a synthetic method of ezetimibe, and an intermediate used in the synthetic method. According to the synthetic method, allyl amination of Morita-Baylis-Hillman adducts is performed so as to realized production of a chiral beta-aromatic amido alpha-methylene carboxylic acid derivative with high activity and selectivity, and the chiral ezetimibe synthetic intermediate is obtained via simple conversion reaction; and chiral medicament ezetimibe can be synthesized further. According to the synthetic method, chirality of ezetimibe is realized via chiral catalysis, so that the use of chiral auxiliary base oxazolidinone is avoided, cost is low, and the synthetic method is friendly to the environment.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
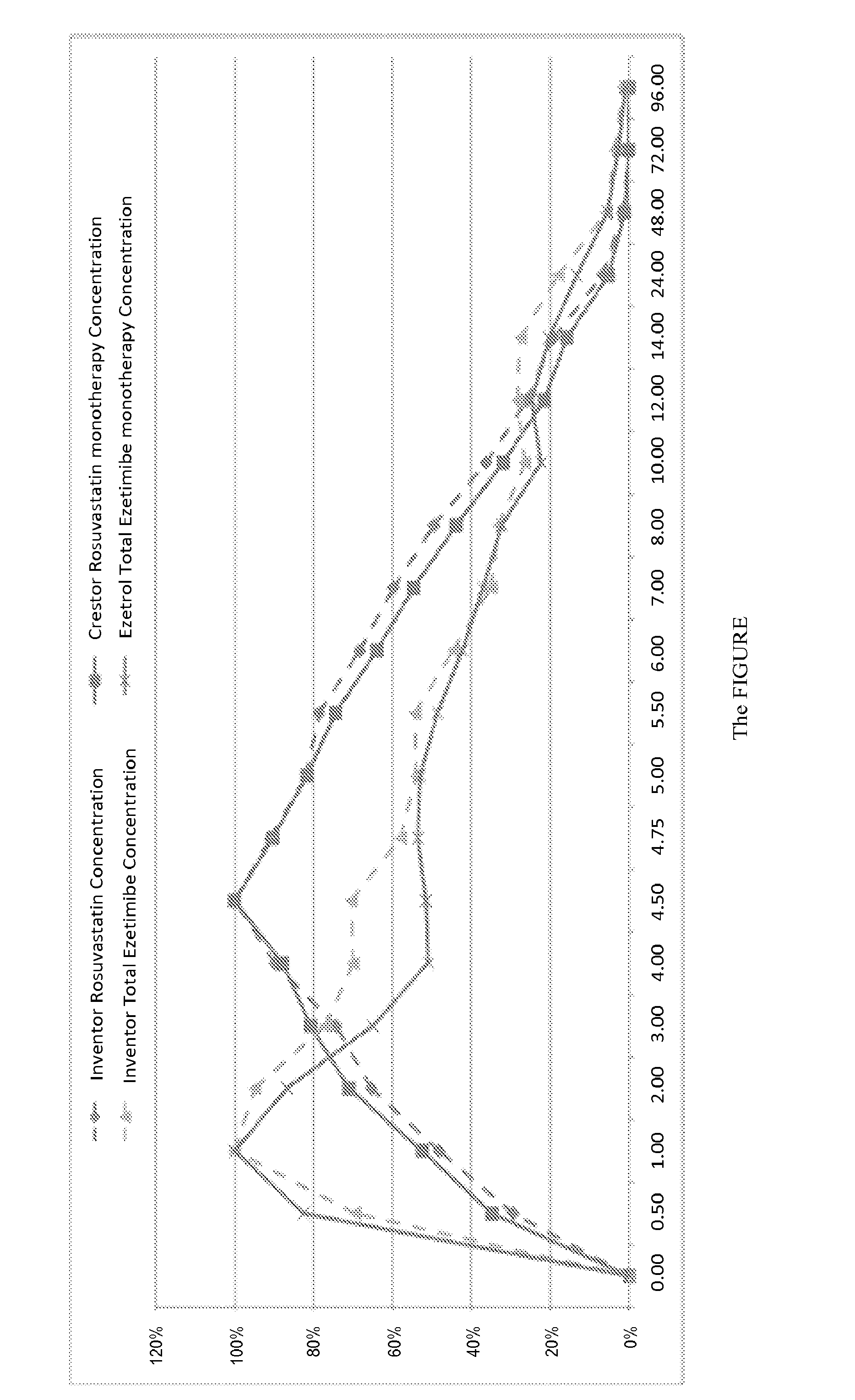
Oral Tablet Formulation Consisting Of Fixed Combination Of Rosuvastatin And Ezetimibe For Treatment Of Hyperlipidemia And Cardiovascular Diseases
ActiveUS20140287042A1Improve solubilityLower cholesterol levelsBiocideOrganic active ingredientsSecondary hyperlipidemiaEzetimibe
The present invention is an orally consumed fixed combination formulation of both rosuvastatin and ezetimibe in one tablet that is expected to have the same Area Under Curve as two active ingredients taken together individually orally, and pharmaceutically acceptable additives suitable for the preparation. In preferred embodiments of this invention, the rosuvastatin is in the form of rosuvastatin calcium and the pharmaceutically acceptable additives are selected from diluents, disintegrants, glidants, lubric ants, colorants and combinations thereof.
Owner:ALTHERA LIFE SCI
Processes for the preparation of (3R,4S)-4-((4-benzyloxy)phenyl)-1-(4-fluorophenyl)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-2-azetidinone, an intermediate for the synthesis of ezetimibe
The invention encompasses (3R,4S)-4-((4-benzyloxy)phenyl)-1-(4-fluorophenyl)-3-(3-(4-fluorophenyl)-3-oxopropyl)-2-azetidinone (Compound 2a) having an enantiomeric purity of at least about 97.5%. The invention also encompasses Compound 2a having a chemical purity of at least about 97%. The invention further encompasses processes for preparing Compound 2a from Compound 1 having the following formula:The invention also encompasses processes for preparing a compound having the following formula:from a compound having the following formula:wherein R is selected from the group consisting of: H or a hydroxyl protecting group. The invention also encompasses processes for preparing Compound 2a, preferably to form Compound 2a-Form 01. Also included are processes for preparing ezetimibe from Compound 2a-Form 01 or Compound 2a prepared according to the invention, compositions containing such ezetimibe, and methods for reducing cholesterol using such compositions
Owner:KANSAL VINOD KUMAR +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com