Ezetimible intermediate and synthetic method of ezetimible
A technology of ezetimibe and its intermediates, which is applied in the field of ezetimibe synthesis, and can solve the problems of long synthetic route, high dose of chiral reducing agent, low yield and purity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
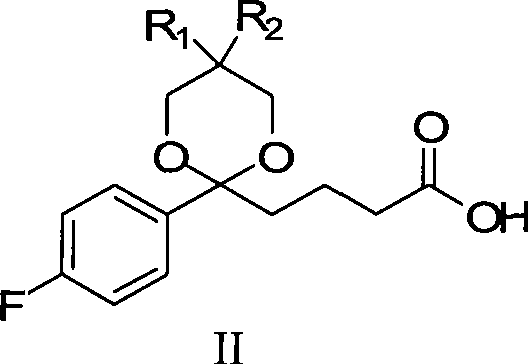
[0093] The synthetic method of formula II compound (wherein R1=H, R2=H)
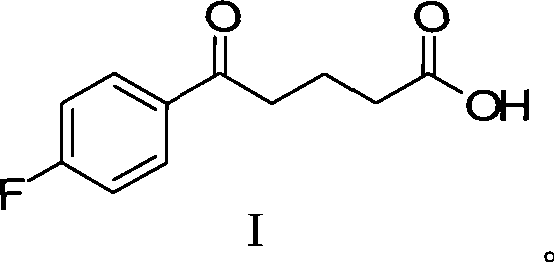
[0094] Add 21.0g (0.1mol) of compound I and 50mL of dichloromethane into a 500mL reaction flask, and add 14.3mL (14.81g, 0.195mol) of 1,3-propanediol, 12mL of trimethyl orthoformate and 0.15mL of concentrated Sulfuric acid, the system was stirred and reacted at 40°C for 2 hours, and the reaction was complete by TLC; 25g of sodium bicarbonate solid was added, and after stirring for 15 minutes, 15mL of methanol was added to the residue, and 200mL of 10% sodium hydroxide was slowly added to the reaction system under the condition of ice-water bath cooling The solution was stirred continuously at a temperature of 20-25° C., and the reaction was carried out for 5 hours. TLC detected that the hydrolysis reaction was complete. Slowly add 150 mL of 10% citric acid solution to the reaction solution under cooling in an ice-water bath to make the pH value 1-2. Continue to stir for 30 minutes, add 200mL ethyl aceta...
Embodiment 2
[0096] The synthetic method of formula II compound (wherein R1=H, R2=H)
[0097] Add 21.0g (0.1mol) formula I compound, 50mL dichloromethane to 500mL reaction bottle, under continuous stirring, add 12.47.mL (12.92g, 0.17mol) 1,3-propanediol, 12mL trimethyl orthoformate and 0.15 mL of concentrated sulfuric acid, the system was stirred and reacted at 60°C for 3h, and the reaction was complete by TLC; 25g of sodium bicarbonate solid was added, and after stirring for 15min, 15mL of methanol was added to the residue, and 200mL of 10% The sodium hydroxide solution was stirred continuously at a temperature of 20-25° C., and the reaction was carried out for 5 hours. TLC detected that the hydrolysis reaction was complete. Slowly add 150 mL of 10% citric acid solution to the reaction solution under cooling in an ice-water bath to make the pH value 1-2. Continue to stir for 30 minutes, add 200mL ethyl acetate for extraction, then extract the aqueous solution twice with 50-50mL ethyl ace...
Embodiment 3
[0099] The synthetic method of formula II compound (wherein R1=H, R2=H)
[0100]Add 21.0g (0.1mol) formula I compound, 50mL dichloromethane to 500mL reaction bottle, under continuous stirring, add 18.34.mL (19g, 0.25mol) 1,3-propanediol, 12mL trimethyl orthoformate and 0.15mL Concentrated sulfuric acid, the system was stirred and reacted at 30°C for 4h, and the reaction was complete by TLC; 25g of sodium bicarbonate solid was added, and after stirring for 15min, 15mL of methanol was added to the residue, and 200mL of 10% hydroxide was slowly added to the reaction system under the condition of ice-water bath cooling. The sodium solution was stirred continuously at a temperature of 20-25° C., and the reaction was carried out for 5 hours. TLC detected that the hydrolysis reaction was complete. Slowly add 150 mL of 10% citric acid solution to the reaction solution under cooling in an ice-water bath to make the pH value 1-2. Continue to stir for 30 minutes, add 200mL ethyl acetate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com