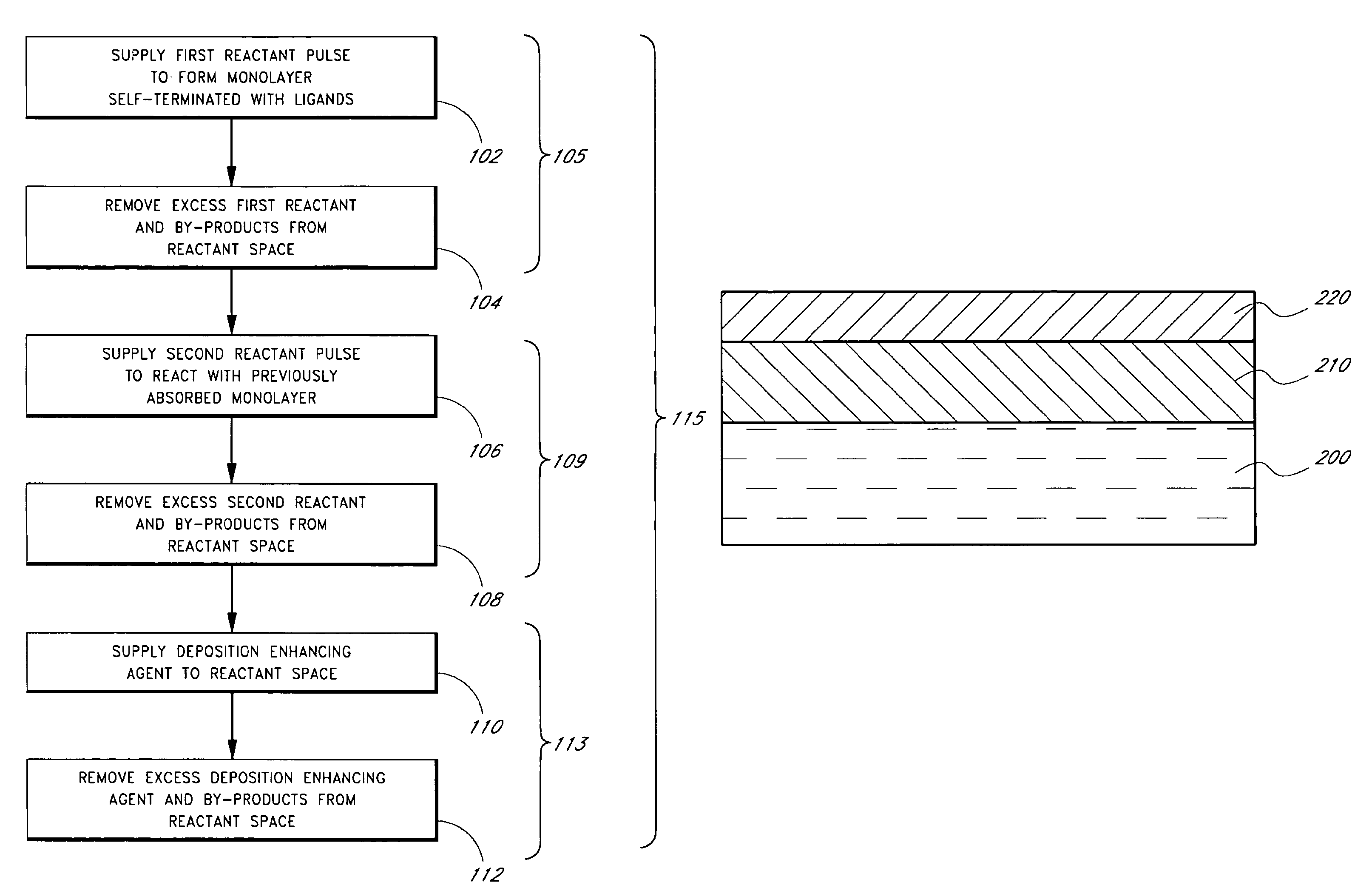
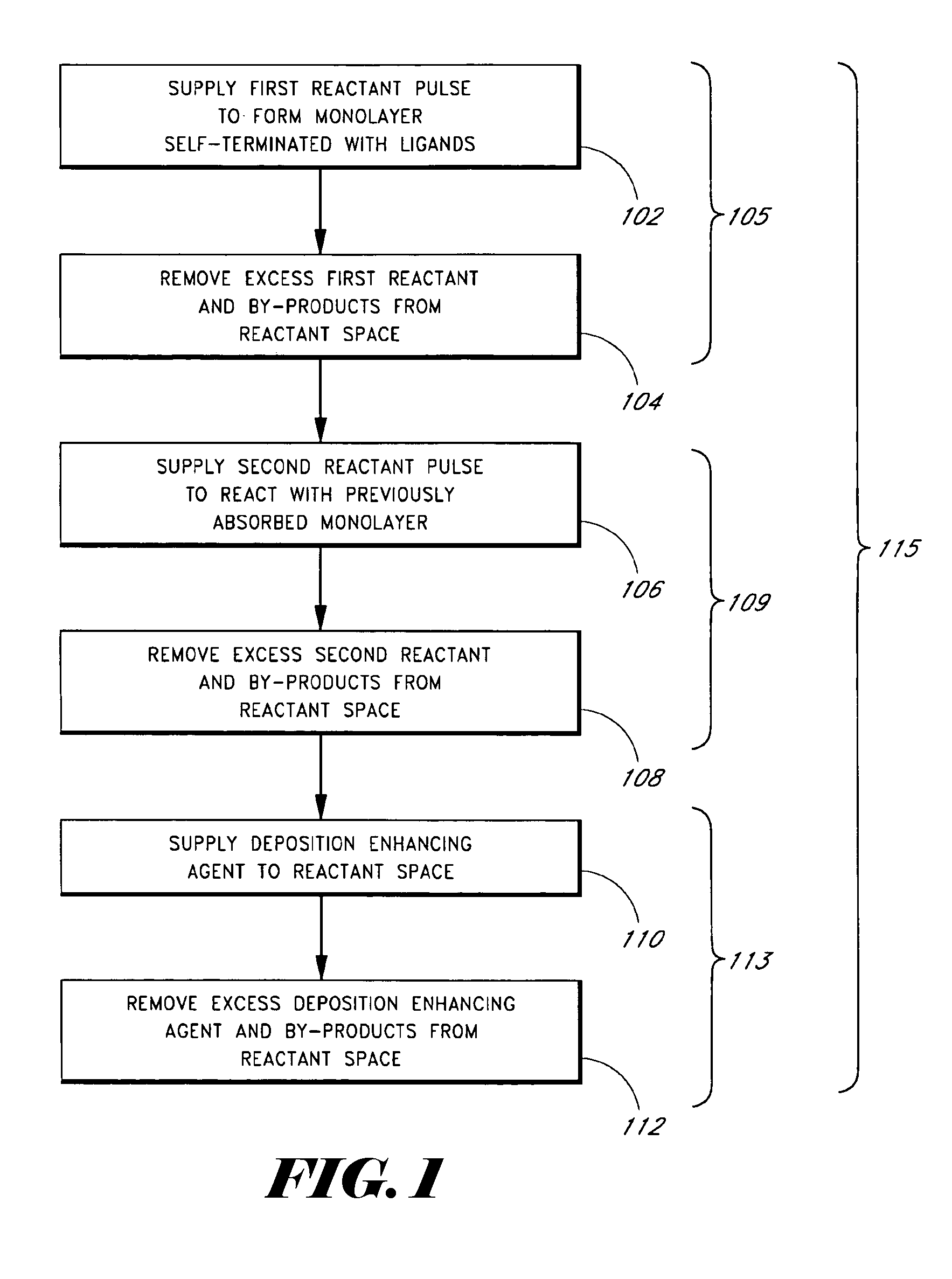
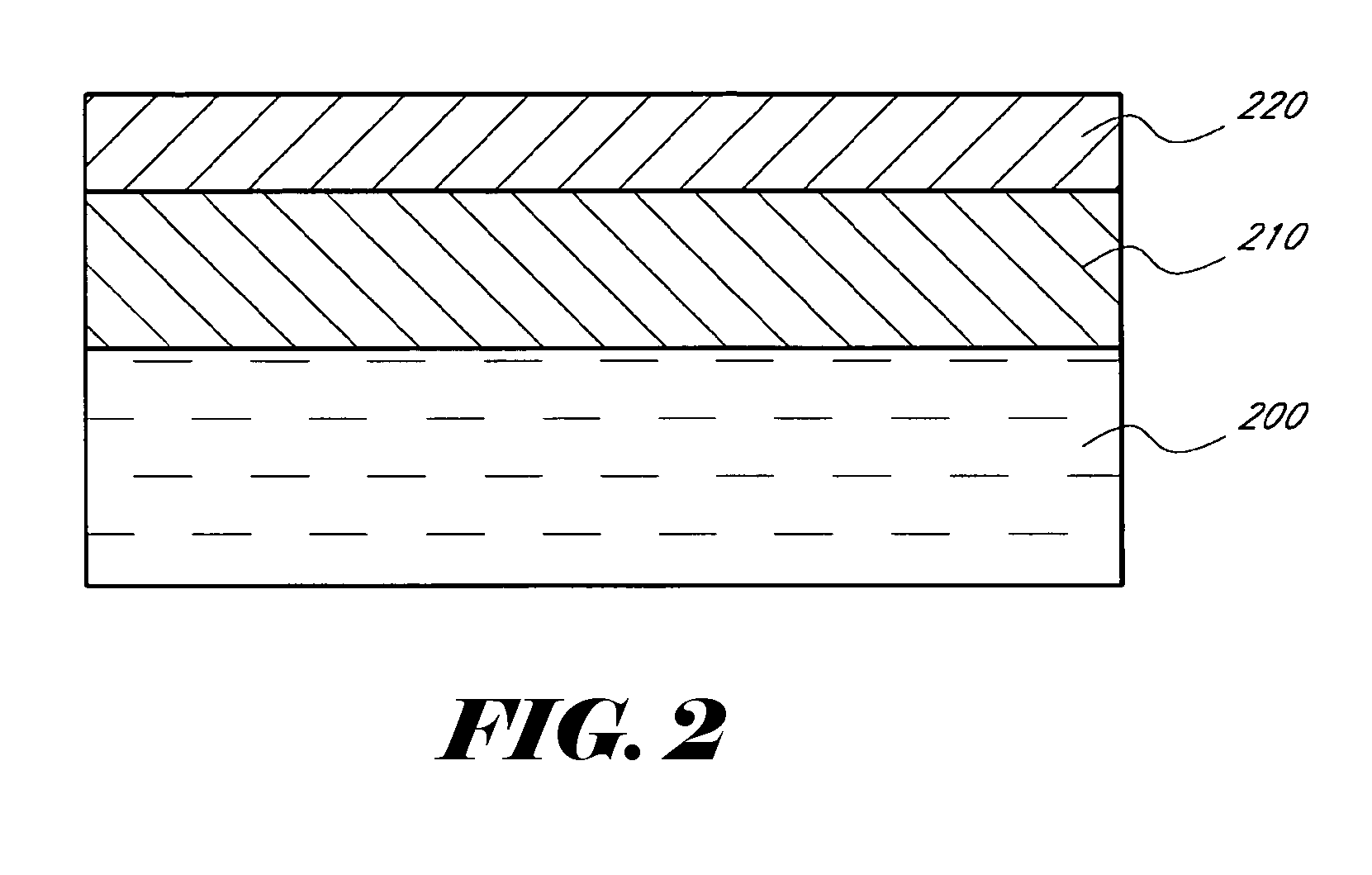
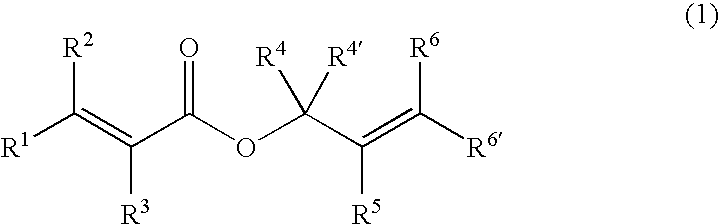
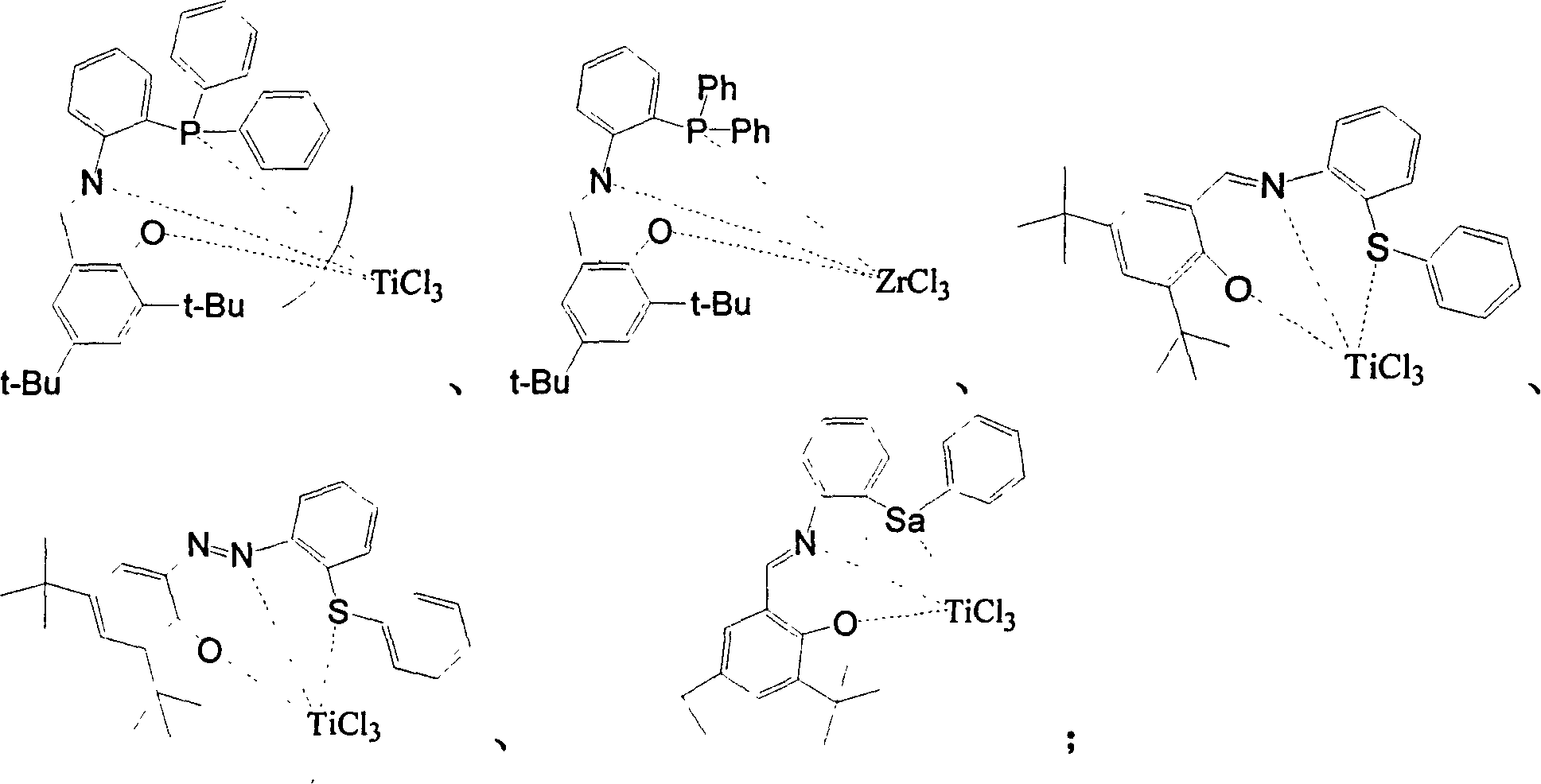
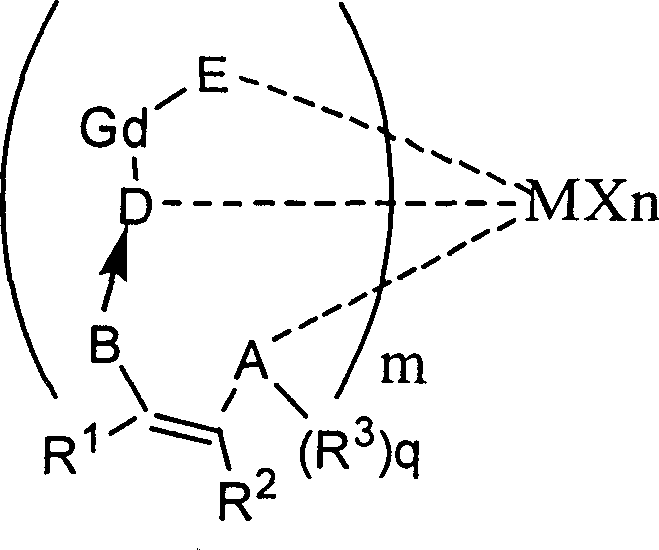
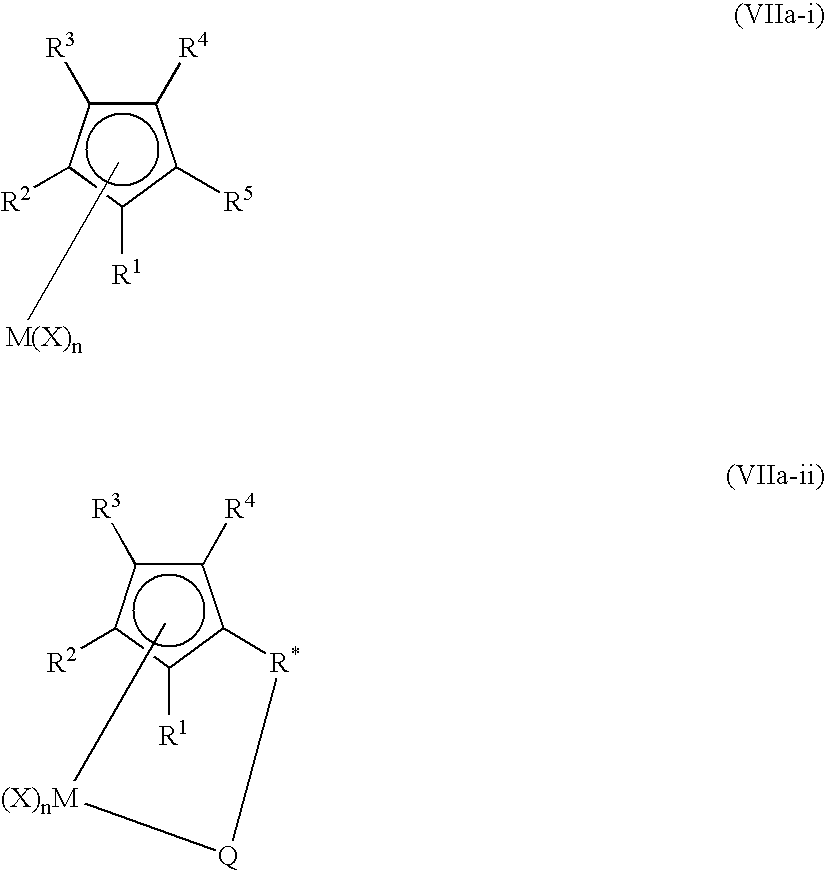
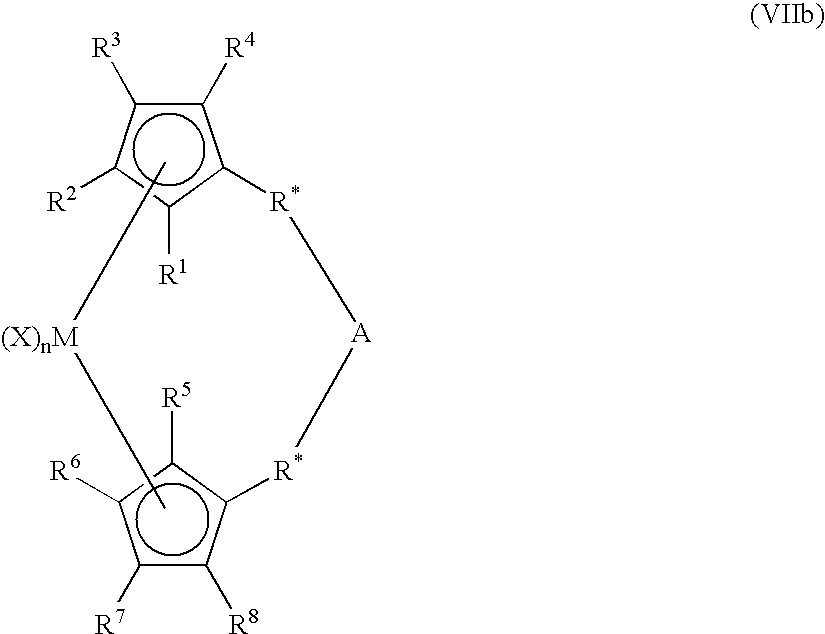
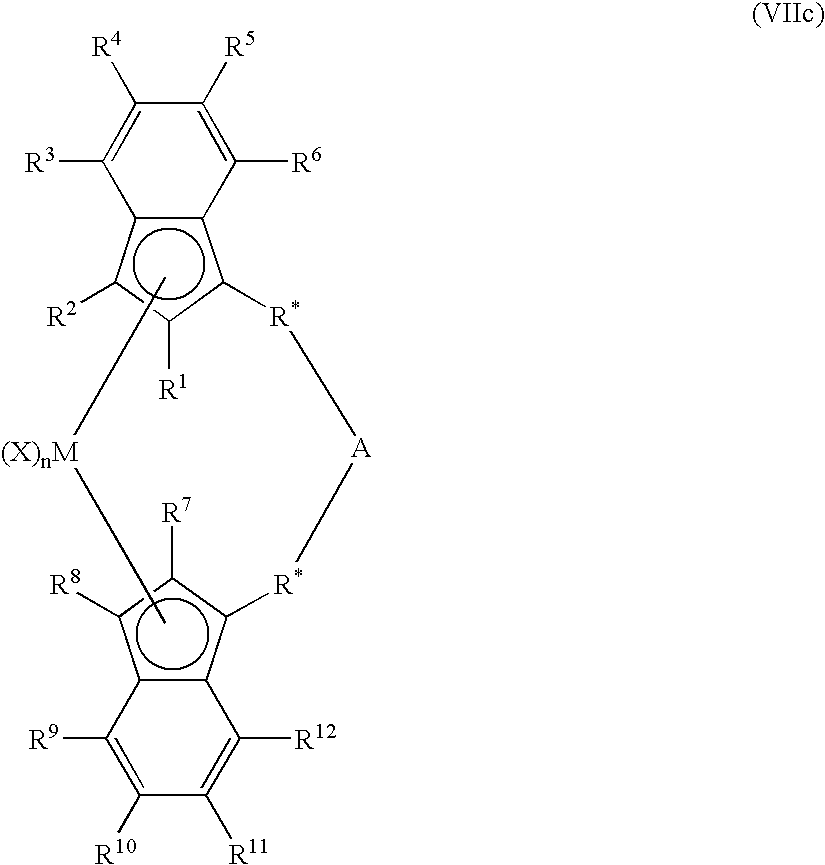
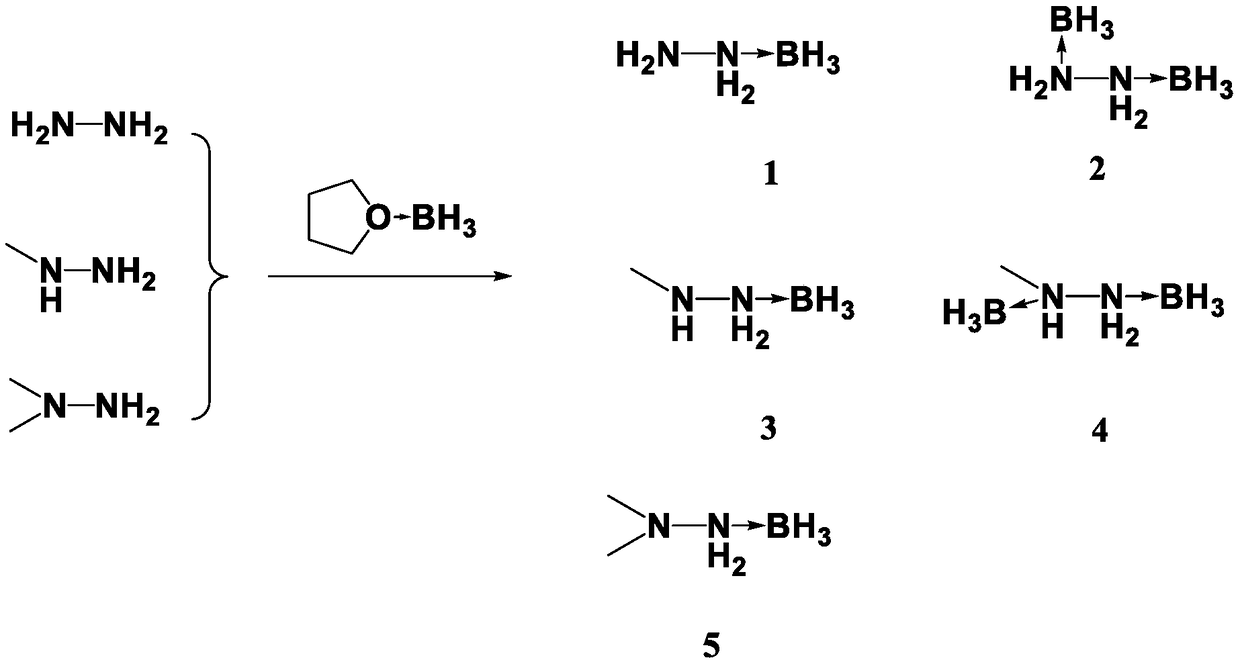
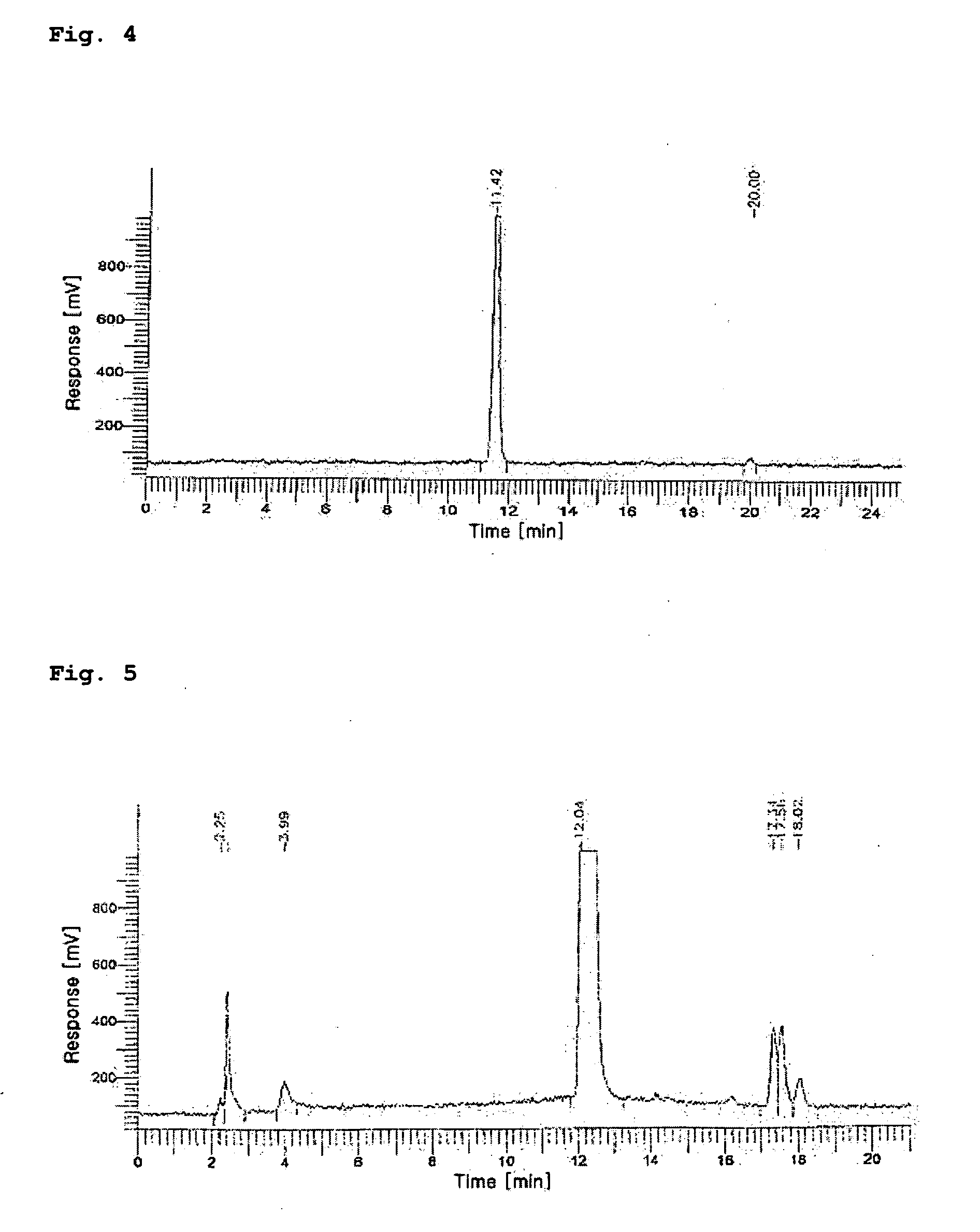
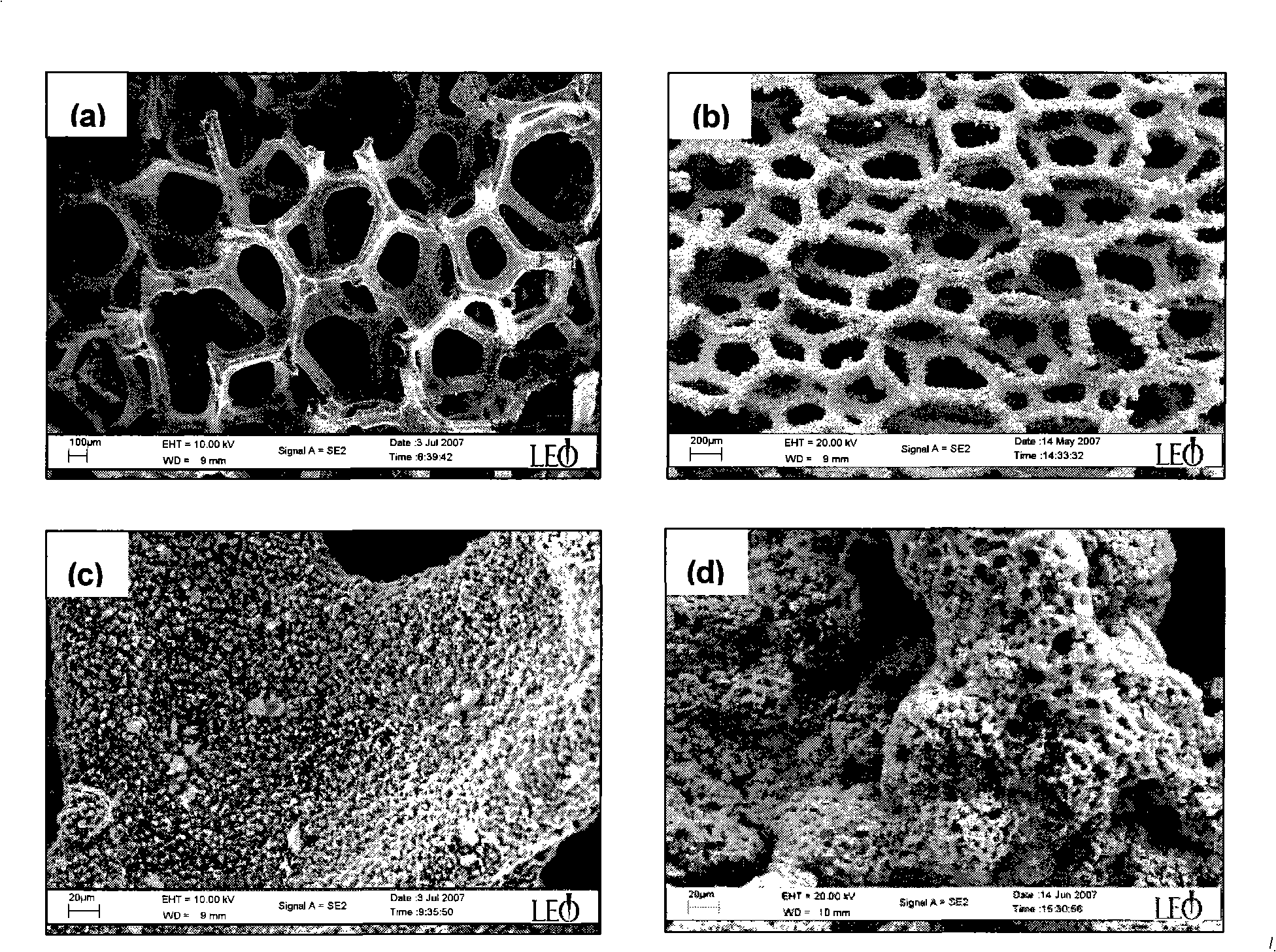
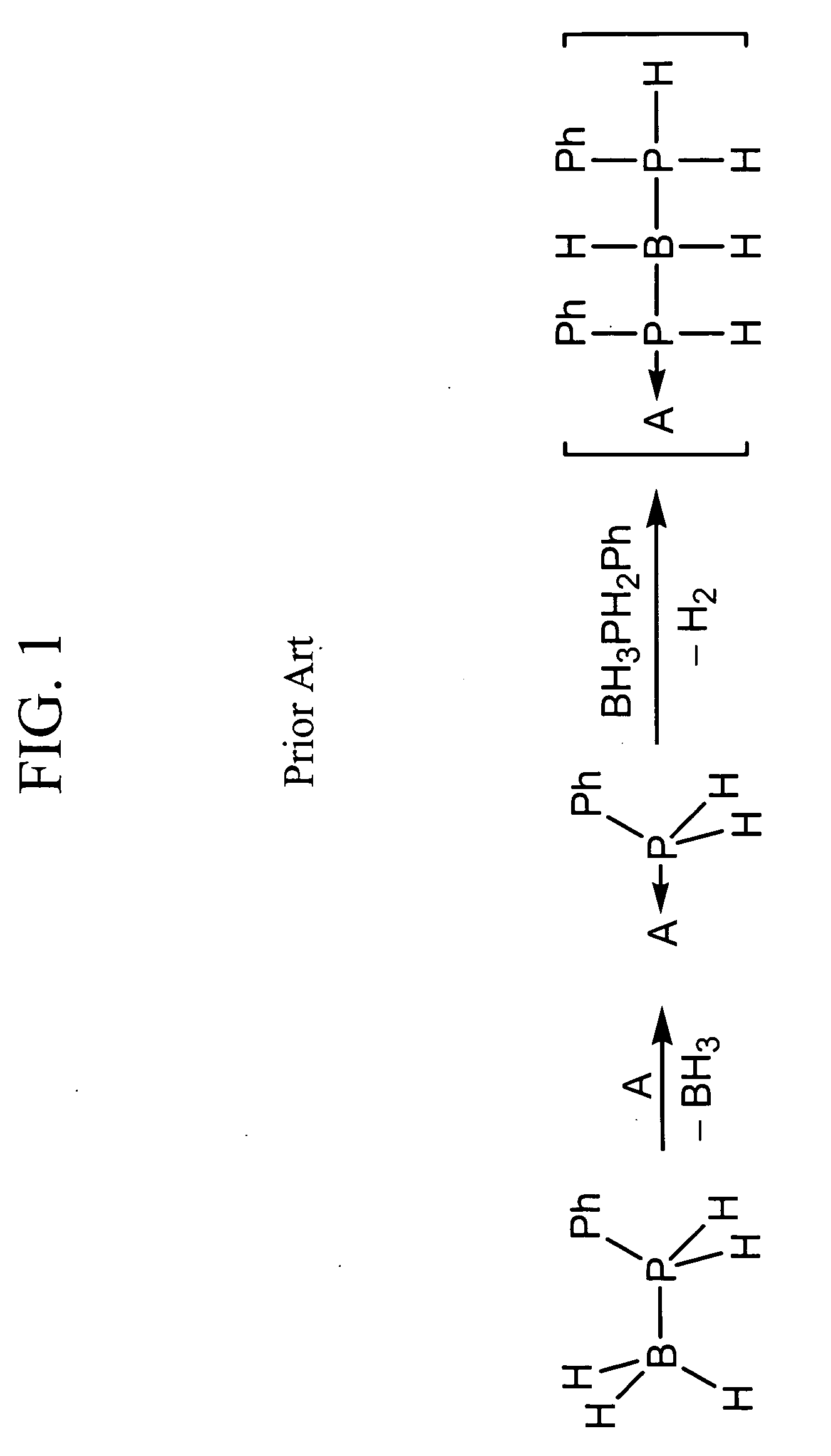
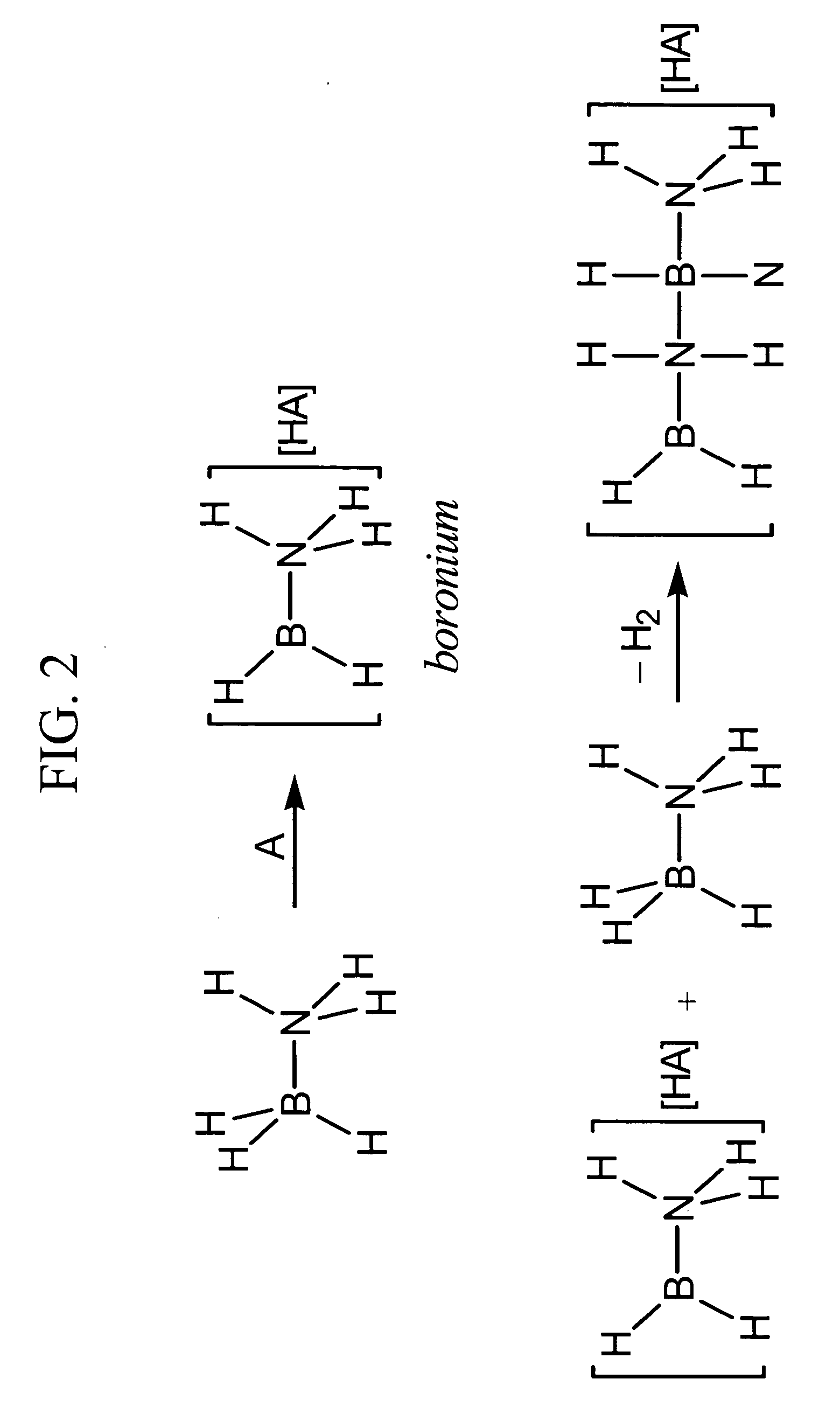
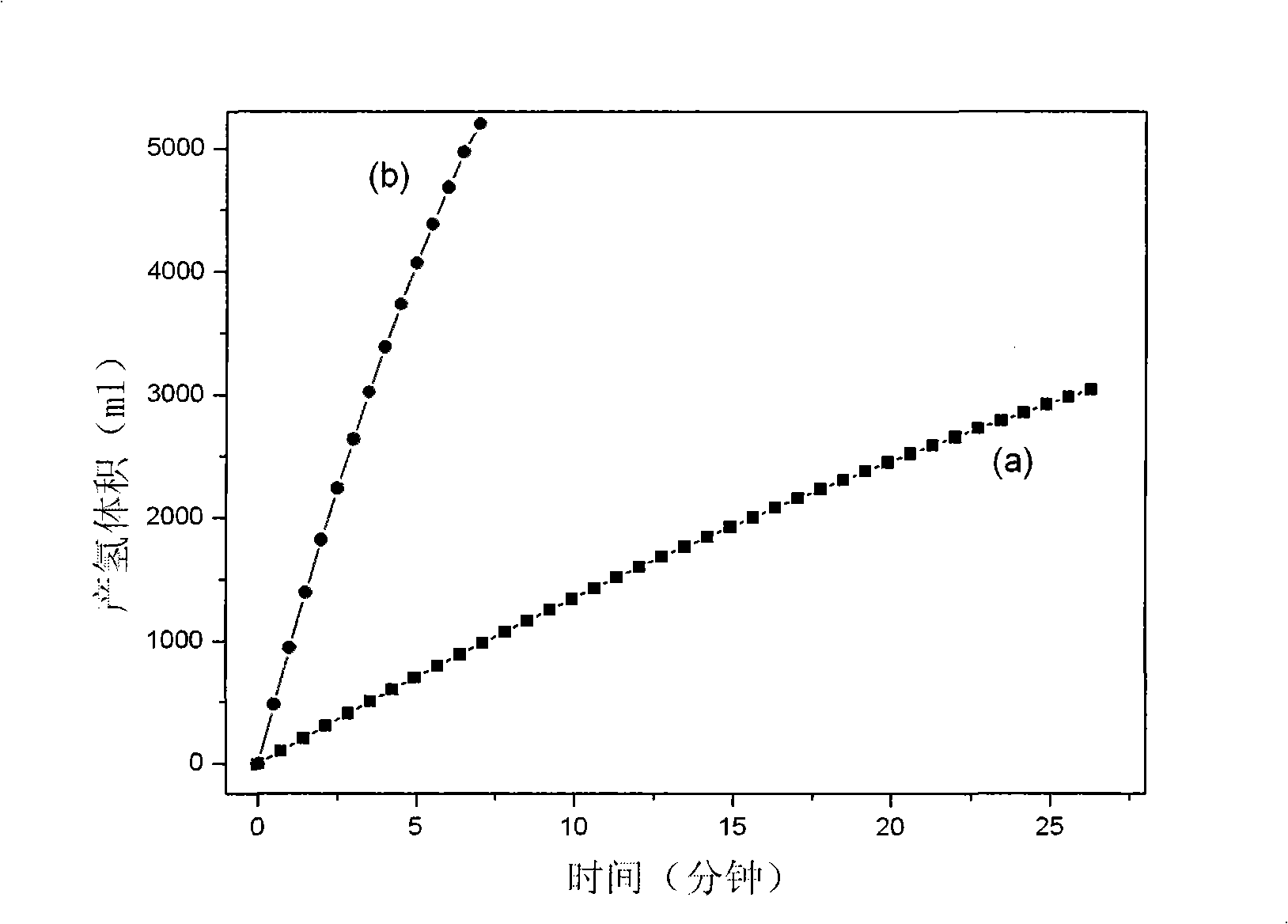Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1055 results about "Borane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Borane, also called borine, is an inorganic compound with the chemical formula BH 3. It is a colourless gas that only persists at elevated temperatures or in dilution. Borane is the simplest member of the boranes.
Silane and borane treatments for titanium carbide films
ActiveUS8841182B1Reduce oxidized portionPrevent oxidationSemiconductor/solid-state device manufacturingSilanesCompound (substance)
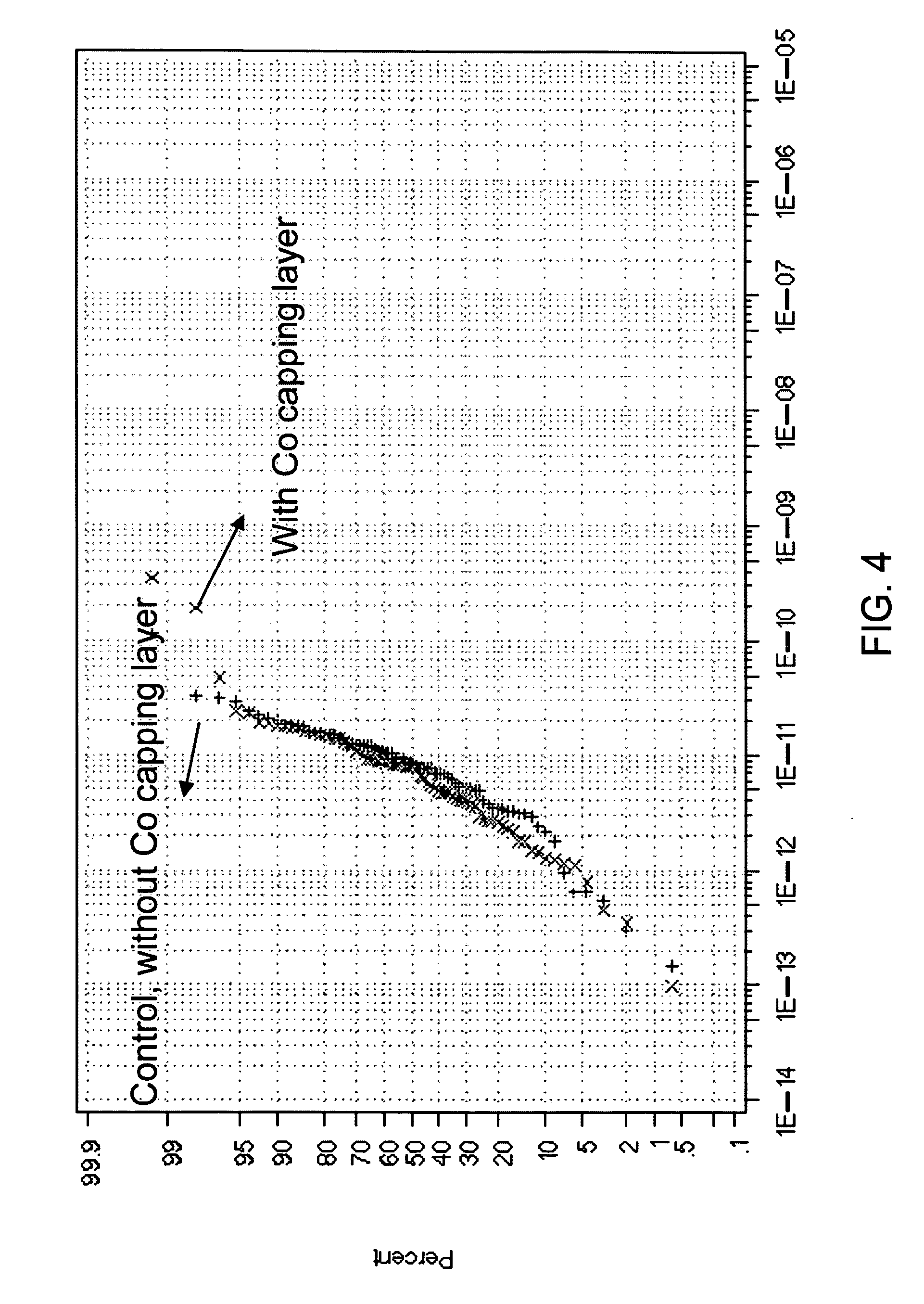
Methods of treating metal-containing thin films, such as films comprising titanium carbide, with a silane / borane agent are provided. In some embodiments a film including titanium carbide is deposited on a substrate by an atomic layer deposition (ALD) process. The process may include a plurality of deposition cycles involving alternating and sequential pulses of a first source chemical that includes titanium and at least one halide ligand, a second source chemical that includes metal and carbon, where the metal and the carbon from the second source chemical are incorporated into the thin film, and a third source chemical, where the third source chemical is a silane or borane that at least partially reduces oxidized portions of the titanium carbide layer formed by the first and second source chemicals. The treatment can form a capping layer on the metal carbide film.
Owner:ASM IP HLDG BV
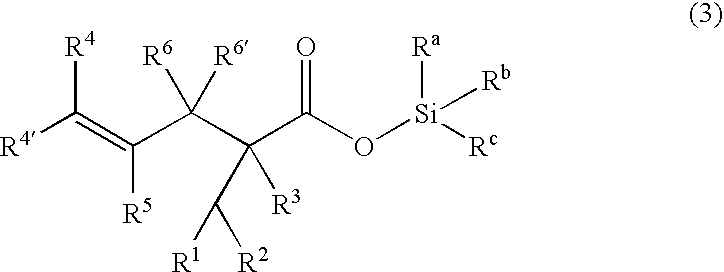
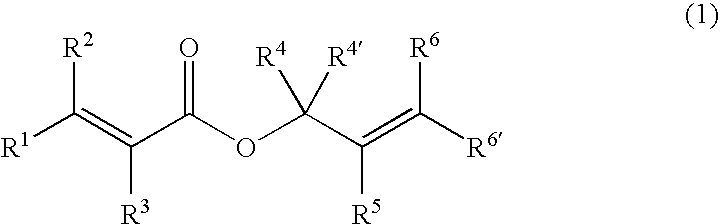
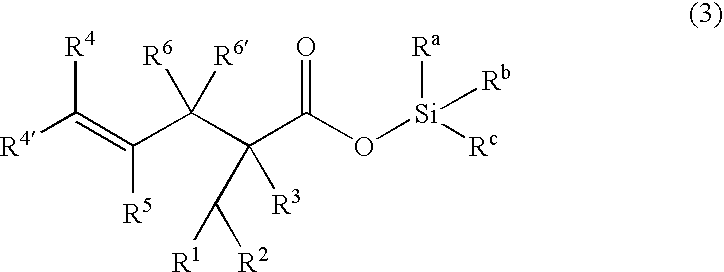
Processes of making gamma,delta-unsaturated carboxylic acid and silyl ester thereof, carboxyl group-containing organosilicon compound and process of making
ActiveUS7307178B2Few stepsHigh yieldSilicon organic compoundsPreparation from carboxylic acid esters/lactonesCarboxyl radicalPerylene derivatives
A γ,δ-unsaturated carboxylic acid silyl ester is prepared by reacting an α,β-unsaturated carboxylic acid ester with a hydrosilane or hydrosiloxane in the presence of tris(pentafluorophenyl)borane. γ,δ-Unsaturated carboxylic acid derivatives are readily prepared through fewer steps and in high yields.
Owner:SHIN ETSU CHEM CO LTD
Processes of making gamma,delta-unsaturated carboxylic acid and silyl ester thereof, carboxyl group-containing organosilicon compound and process of making
ActiveUS20050070729A1High yieldFew stepsSilicon organic compoundsPreparation from carboxylic acid esters/lactonesCarboxyl radicalPerylene derivatives
A γ,δ-unsaturated carboxylic acid silyl ester is prepared by reacting an α,β-unsaturated carboxylic acid ester with a hydrosilane or hydrosiloxane in the presence of tris(pentafluorophenyl)borane. γ,δ-Unsaturated carboxylic acid derivatives are readily prepared through fewer steps and in high yields.
Owner:SHIN ETSU CHEM IND CO LTD
Olefinic polymerization and copolymerization method of supported non-metallocene catalyst
A method of alkene polymerization and copolymarization with f load non- metallocene catalyst: emplying the load non- metallocene catalyst and catalyst promoter forming the catalytic system, introducing polymer monomer or comonomer for alkene polymerization or copolymarization; adding the load non- metallocene catalyst into dissolvent and mixing with catalyst promoter and then transferring the mixture into polymerization reactor, or adding the load non- metallocene catalyst and catalyst promoter into the polymerization reactor in order or simultaneously. The catalyst promoter is chosen from: alkyl aluminium, aluminium oxane, Lewis acid, boralotano, alkyl borane or alkyl borane ammonium salt. The load non- metallocene catalyst is loaded to multiple poral solid by employing methods of solution dipping, equivoluminal dipping or solution dipping-before-equivoluminal dipping to form organic integral of property stable and strong binding force. The invention is characterized by the high alkene polymerization active, high fusion point and good normalization of the produced polymer, and the sastification of the demand of high level product production.
Owner:YANGZI PETROCHEM
Activated catalyst systems from substituted dialuminoxane complexes
InactiveUS6958306B2Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationArylHalogen
A catalyst composition and method for preparing a supported catalyst system for olefin polymerization is provided. In one aspect, the catalyst composition includes a reaction product of a dialuminoxane and a halogen substituted aryl borane, wherein the reaction takes place on a support and at conditions sufficient to exchange one or more ligands on the dialuminoxane for one or more ligands on the halogen substituted aryl borane while on the support. In one embodiment, the method for preparing the supported catalyst system includes combining a dialuminoxane with a support to form a treated catalyst support, and combining a halogen substituted aryl borane with the treated catalyst support at conditions sufficient to exchange one or more ligands on the dialuminoxane for one or more ligands on the halogen substituted aryl borane while on the support to form a supported activator. The method further includes reacting one or more polymerization catalysts with the supported activator.
Owner:UNIVATION TECH LLC
Amine organoborane complex initiated polymerizable compositions containing siloxane polymerizable components
InactiveUS6777512B1Easy to useBroaden applicationOrganic-compounds/hydrides/coordination-complexes catalystsGroup 3/13 element organic compoundsAminosilochromeOligomer
In one embodiment the invention is a polymerizable composition comprising a) an organoborane amine complex; b) one or more of monomers, oligomers or polymers having olefinic unsaturation which is capable of polymerization by free radical polymerization; c) one or more compounds, oligomers or prepolymers having a siloxane backbone and reactive moieties capable of polymerization; and d) a catalyst for the polymerization of the one or more compounds, oligomers or prepolymers having a siloxane backbone and reactive moieties capable of polymerization. This composition may further comprise a compound which causes the organoborane amine complex to disassociate. In a preferred embodiment, the two part composition further comprises a compound which is reactive with both the b) one or more of monomers, oligomers or polymers having olefinic unsaturation which is capable of polymerization by free radical polymerization; and the c) one or more compounds, oligomers or prepolymers having a siloxane backbone and reactive moieties capable of polymerization. This composition can be polymerized by contacting the two parts of the composition. In another embodiment the invention is an organoborane amine complex comprising an alkyl borane having ligands which are alkyl, cycloalkyl or both and an amino siloxane.
Owner:DOW GLOBAL TECH LLC
Preparation method for propellant fuel
InactiveCN108910843AShorter ignition delay timeHazard reductionOrganic chemistryHydrazineEvaporationIgnition delay
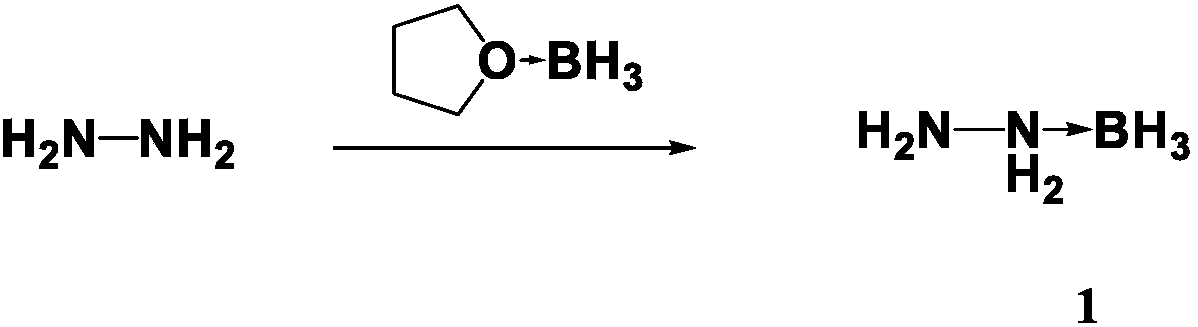
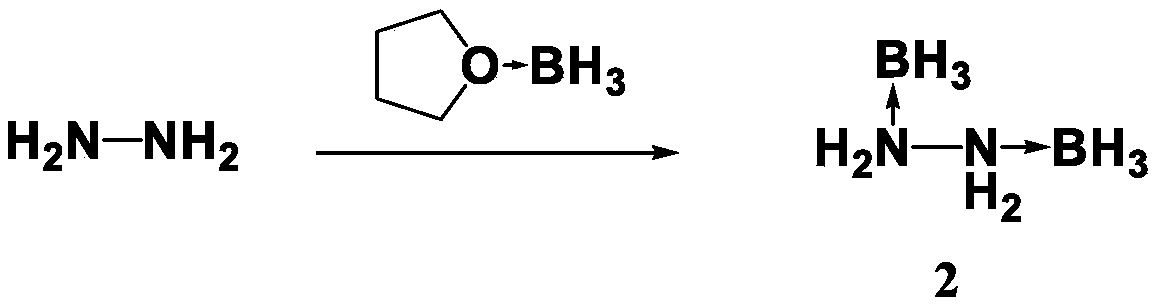
The invention discloses a preparation method for propellant fuel. The preparation method comprises the following steps: uniformly mixing hydrazine or a methyl derivative thereof with a tetrahydrofuransolution of borane to perform a reaction, and after the reaction, performing rotary evaporation on the tetrahydrofuran solution to obtain a first product; adding a first solvent into the first product, then performing washing, and then performing rotary evaporation to remove the first solvent, so as to obtain a hydrazinoborane derivative. According to the method, hydroboration is carried out on ahighly-volatile hydrazine fuel and a methylated derivative of hydrazine, the obtained borohydride of the hydrazine is a colorless non-volatile liquid or a white solid, has no volatility and greatly reduces hazard of the hydrazine fuel, and is a novel aerospace propellant fuel since the ignition delay time of the hydrazine borofluoride is extremely short.
Owner:INST OF CHEM MATERIAL CHINA ACADEMY OF ENG PHYSICS
Solid chemical hydride dispenser for generating hydrogen gas
InactiveUS20070020172A1Molybdeum compoundsSynthetic resin layered productsCompound (substance)Hydrogen evolution
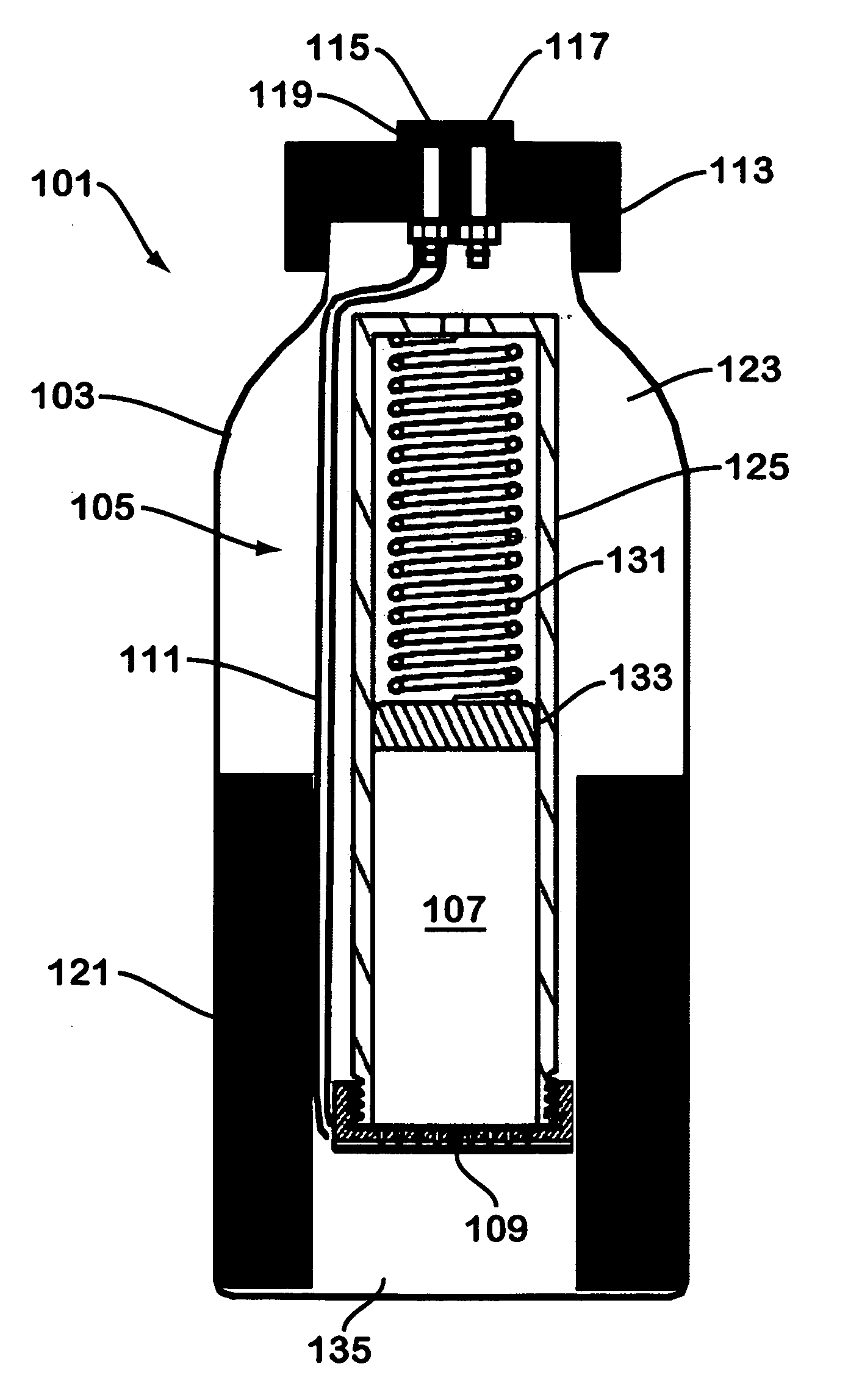
A device for generating hydrogen gas is provided. The device (101) comprises a first hydrogen-containing composition (107) that reacts with a second composition to evolve hydrogen gas; a dispenser (105) adapted to apply the first composition to a first porous member (109); and a conduit (111) adapted to supply the second composition to the first porous member. In a preferred embodiment, the first composition is selected from the group consisting of hydrides, borohydrides and boranes, the second composition is water, and the dispenser is spring-loaded and is charged with the first composition. As the first composition reacts with water at the interface to evolve hydrogen gas, the dispenser forces the reaction product across the interface and out of the dispenser, where it will not interfere with the progress of the hydrogen evolution reaction.
Owner:LYNNTECH POWER SYST
Methods for depositing tungsten layers employing atomic layer deposition techniques
InactiveUS7405158B2Solid-state devicesSemiconductor/solid-state device manufacturingDisilaneGas phase
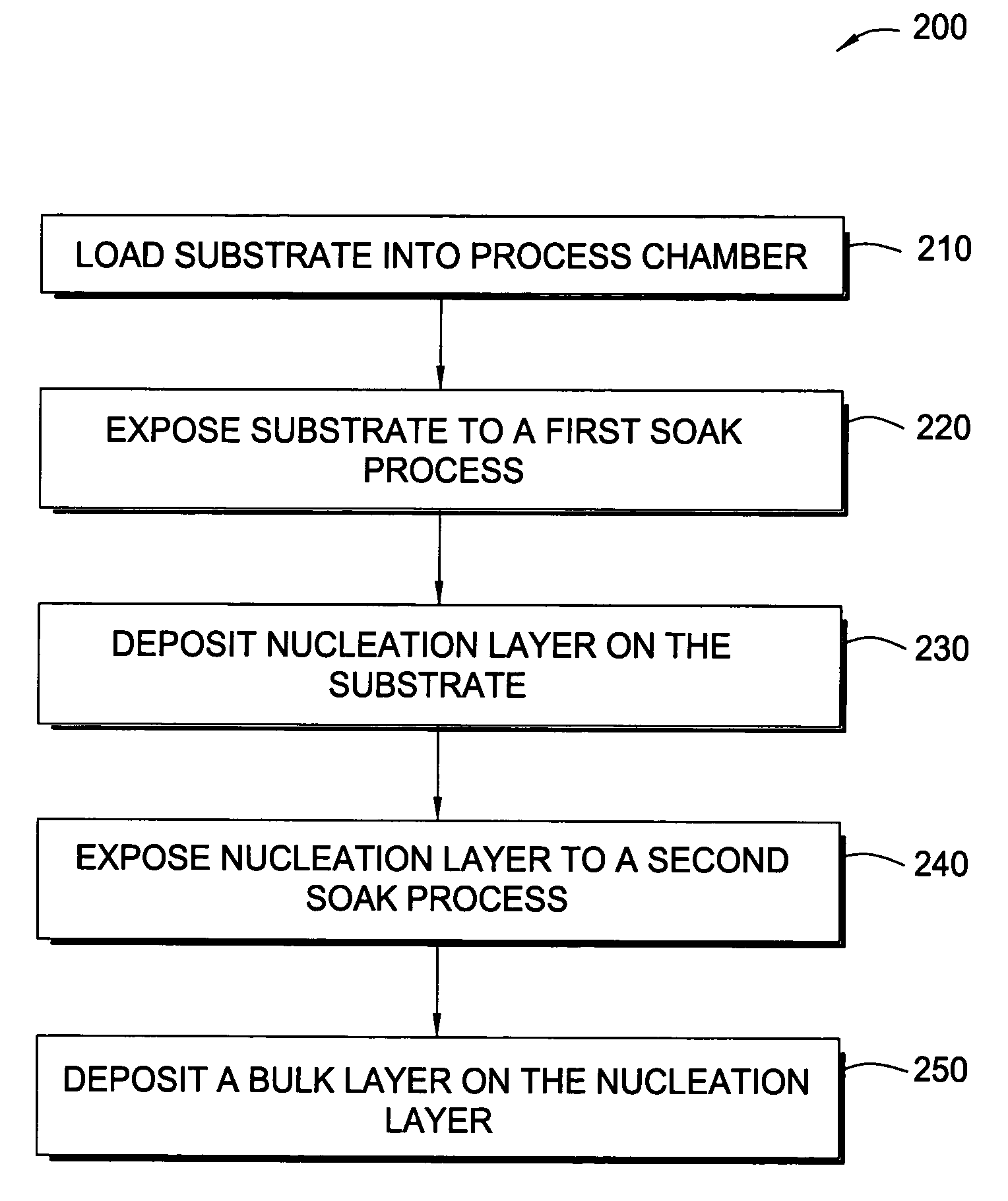
In one embodiment of the invention, a method for forming a tungsten-containing layer on a substrate is provided which includes positioning a substrate containing a barrier layer disposed thereon in a process chamber, exposing the substrate to a first soak process for a first time period and depositing a nucleation layer on the barrier layer by flowing a tungsten-containing precursor and a reductant into the process chamber. The method further includes exposing the nucleation layer to a second soak process for a second time period and depositing a bulk layer on the nucleation layer. In one example, the barrier layer contains titanium nitride, the first and second soak processes independently comprise at least one reducing gas selected from the group consisting of hydrogen, silane, disilane, dichlorosilane, borane, diborane, derivatives thereof and combinations thereof and the nucleation layer may be deposited by an atomic layer deposition process or a pulsed chemical vapor deposition process while the bulk layer may be deposited by a chemical vapor deposition process or a physical vapor deposition process.
Owner:APPLIED MATERIALS INC
Methods for depositing tungsten layers employing atomic layer deposition techniques
InactiveUS20060009034A1Solid-state devicesSemiconductor/solid-state device manufacturingDisilaneGas phase
In one embodiment of the invention, a method for forming a tungsten-containing layer on a substrate is provided which includes positioning a substrate containing a barrier layer disposed thereon in a process chamber, exposing the substrate to a first soak process for a first time period and depositing a nucleation layer on the barrier layer by flowing a tungsten-containing precursor and a reductant into the process chamber. The method further includes exposing the nucleation layer to a second soak process for a second time period and depositing a bulk layer on the nucleation layer. In one example, the barrier layer contains titanium nitride, the first and second soak processes independently comprise at least one reducing gas selected from the group consisting of hydrogen, silane, disilane, dichlorosilane, borane, diborane, derivatives thereof and combinations thereof and the nucleation layer may be deposited by an atomic layer deposition process or a pulsed chemical vapor deposition process while the bulk layer may be deposited by a chemical vapor deposition process or a physical vapor deposition process.
Owner:APPLIED MATERIALS INC
Method of preparing rhenium-tricarbonyl complex and its precursor
InactiveUS20070140959A1High yieldInhibit productionIn-vivo radioactive preparationsRhenium compounds preparationScavengerPhosphate
Disclosed herein is a method of preparing a 188Re-tricarbonyl complex for radiopharmaceutical use and of preparing a precursor thereof, and a contrast agent using the same. Particularly, this invention provides a method of preparing a 188Re-tricarbonyl precursor by reacting perrhenate with borane-ammonia (BH3.NH3), potassium boranocarbonate (K2[H3BCO2]) and phosphate in the presence of borohydride exchange resin as a reducing agent, and a method of preparing a 188Re-tricarbonyl complex by reacting the 188Re-tricarbonyl precursor with a ligand. According to the method of this invention, the borohydride exchange resin is used as a reducing agent and as an anion scavenger, thereby obtaining the 188Re-tricarbonyl precursor and complex having high radiolabeling yield and high purity. In addition, the 188Re-tricarbonyl complex can be used as a contrast agent having excellent plasma stability.
Owner:KOREA ATOMIC ENERGY RES INST
Processes for synthesizing alkali metal borohydride compounds
Processes for synthesizing borohydride compounds with reduced energy requirements and high efficiency are disclosed. The processes include the reaction of a base with a borane complex or diborane to produce a borohydride compound of formula YBH4, where Y is a monovalent cationic moiety.
Owner:MILLENNIUM CELL
Catalyst for hydrogen production by catalyzing and hydrolyzing borohydride and preparation method thereof
InactiveCN101347736AFast deposition rateIncrease concentrationMetal/metal-oxides/metal-hydroxide catalystsMetal hydridesChemical platingRare earth
The invention relates to hydrogen production and hydrogen storage technologies and materials, in particular to a catalyst for catalytic hydrolysis of borane for the hydrogen production and a preparation method thereof, thereby solving the problems that the direct application of powder catalyst in a catalytic hydrolysis solid-liquid reaction system can cause the loss of the catalyst, the catalytic hydrolysis reaction is difficult to control and the hydrolysis by-products are difficult to be recovered, etc. The catalyst is composed of an active component and a carrier; the active component is a binary, ternary or multinary alloy or a single precious metal or the combination thereof which is composed of one or more transition metals, rare earth metals or precious metals and metalloids; the active component is deposited on the carrier through the improved chemical plating technology, the surface thereof is rough and porous, and the structure of the prepared catalyst is the amorphous or the nanocrystalline structure. The preparation method has simple preparation process, high preparation efficiency and convenient large-scale preparation; the sources of the used raw materials are rich; the catalytic activity of the prepared supported catalyst is high, the real-time control of the catalytic hydrolysis reaction of the borane can be realized, the catalytic performance is stable, and the catalyst can be repeatedly used for a plurality of times.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Fuel blends for hydrogen generators
InactiveUS20050132640A1Easy to optimizeEfficient hydrolysisLiquid carbonaceous fuelsHydrogen productionFuel cellsHydrolysis
The present invention relates to improved aqueous fuels for hydrogen generators and a method for using them in the production of hydrogen. The present invention also relates to a system of using the subject aqueous fuels to generate hydrogen gas for use in a fuel cell or other device. The subject fuels contain a mixture of boron hydrides, at least one of which is a metal salt, including metal borohydrides, higher boranes and metal higher boron hydrides. The subject aqueous fuels contain a mixture of boron hydrides having a positive ionic charge (+IC) to boron ratio of between 0.2 and 0.4 or between 0.6 and 0.99. Preferred fuels contain a mixture of boron hydrides having an (+IC) to boron ratio between 0.2 and 0.3 or between 0.7 and 0.8. Mixtures containing a metal borohydride also contain a metal hydroxide to stability it against premature hydrolysis in the aqueous fuel media.
Owner:MILLENNIUM CELL
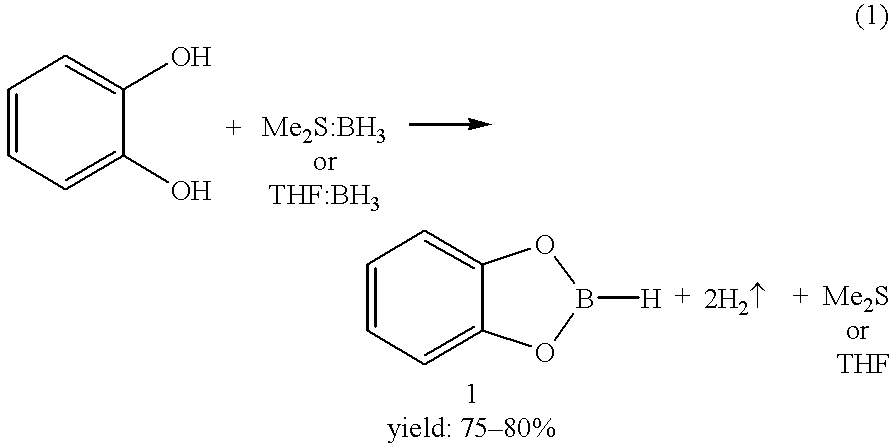
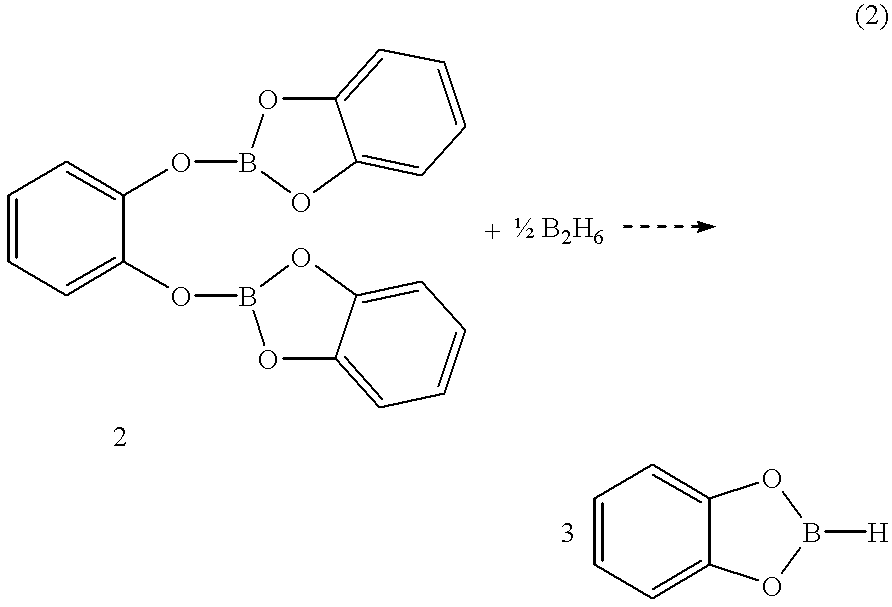
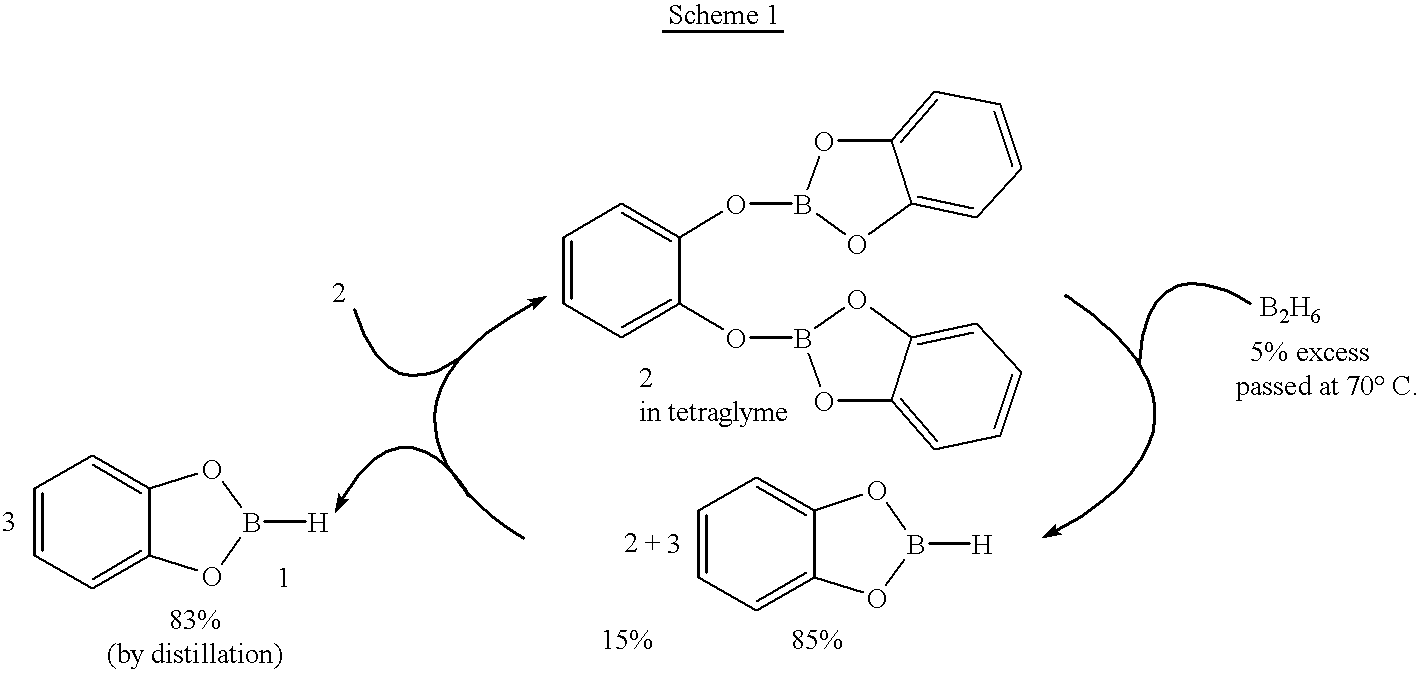
Economical and convenient procedures for the synthesis of catecholborane
New, economical and convenient procedures for the preparation of catecholborane in high chemically pure form using tri-O-phenylene bis borate readily prepared from the reaction of catechol with boric acid, and diborane or borane-Lewis base complexes.
Owner:ALDRICH CHEM
Organoborane amine complex polymerization initiators and polymerizable compositions
InactiveUS6740716B2Improve temperature resistanceSafe handlingPolyureas/polyurethane adhesivesSynthetic resin layered productsOligomerNitrogen
The invention is a two part composition useful for initiating cure of one or more polymerizable monomers which cure when exposed to free radicals comprising in one part an organoboraneamine complex and in a second part an isocyanate which is capable of decomplexing the organoborane complex wherein the ratio of amine nitrogen atoms to boron atoms is greater than 4.0:1.0. In another embodiment the invention is a two part polymerizable composition comprisingpart 1, a) an organoborane amine complex; andpart 2, b) one or more of monomers, oligomers or polymers having olefinic unsaturation which is capable of polymerization by free radical polymerizationc) an effective amount of a compound which causes the complex to disassociate thereby freeing the borane to initiate polymerization of the one or more monomers, oligomers or polymers having olefinic unsaturation wherein the compound which causes disassociation of the complex is kept separate from the complex until initiation of polymerization is desired; andd) a material which manages the heat of the polymerization reaction such that adhesion to the substrate is maintained, which material can be located in either part 1, part 2 or both parts.
Owner:DOW GLOBAL TECH LLC
Selective self-initiating electroless capping of copper with cobalt-containing alloys
InactiveUS20050136193A1Reduced metal concentrationLiquid surface applicatorsSemiconductor/solid-state device manufacturingElectroless depositionAlloy
Embodiments of the invention generally provide compositions of plating solutions, methods to mix plating solutions and methods to deposit capping layers with plating solutions. The plating solutions described herein may be used as electroless deposition solutions to deposit capping layers on conductive features. The plating solutions are rather dilute and contain strong reductants to self-initiate on the conductive features. The plating solutions may provide in-situ cleaning processes for the conductive layer while depositing capping layers free of particles. In one embodiment, a method for forming an electroless deposition solution is provided which includes forming a conditioning buffer solution with a first pH value and comprising a first complexing agent, forming a cobalt-containing solution with a second pH value and comprising a cobalt source, a tungsten source and a second complexing agent, forming a buffered reducing solution with a third pH value and comprising a hypophosphite source and a borane reductant, combining the conditioning buffer solution, the cobalt-containing solution and the buffered reducing solution to form the electroless deposition solution. The electroless deposition solution includes the cobalt source in a concentration range from about 1 mM to about 30 mM, the tungsten source in a concentration range from about 0.1 mM to about 5 mM, the hypophosphite source in a concentration range from about 5 mM to about 50 mM, the borane reductant in a concentration range from about 5 mM to about 50 mM, and has a total pH value in a range from about 8 to about 10.
Owner:APPLIED MATERIALS INC
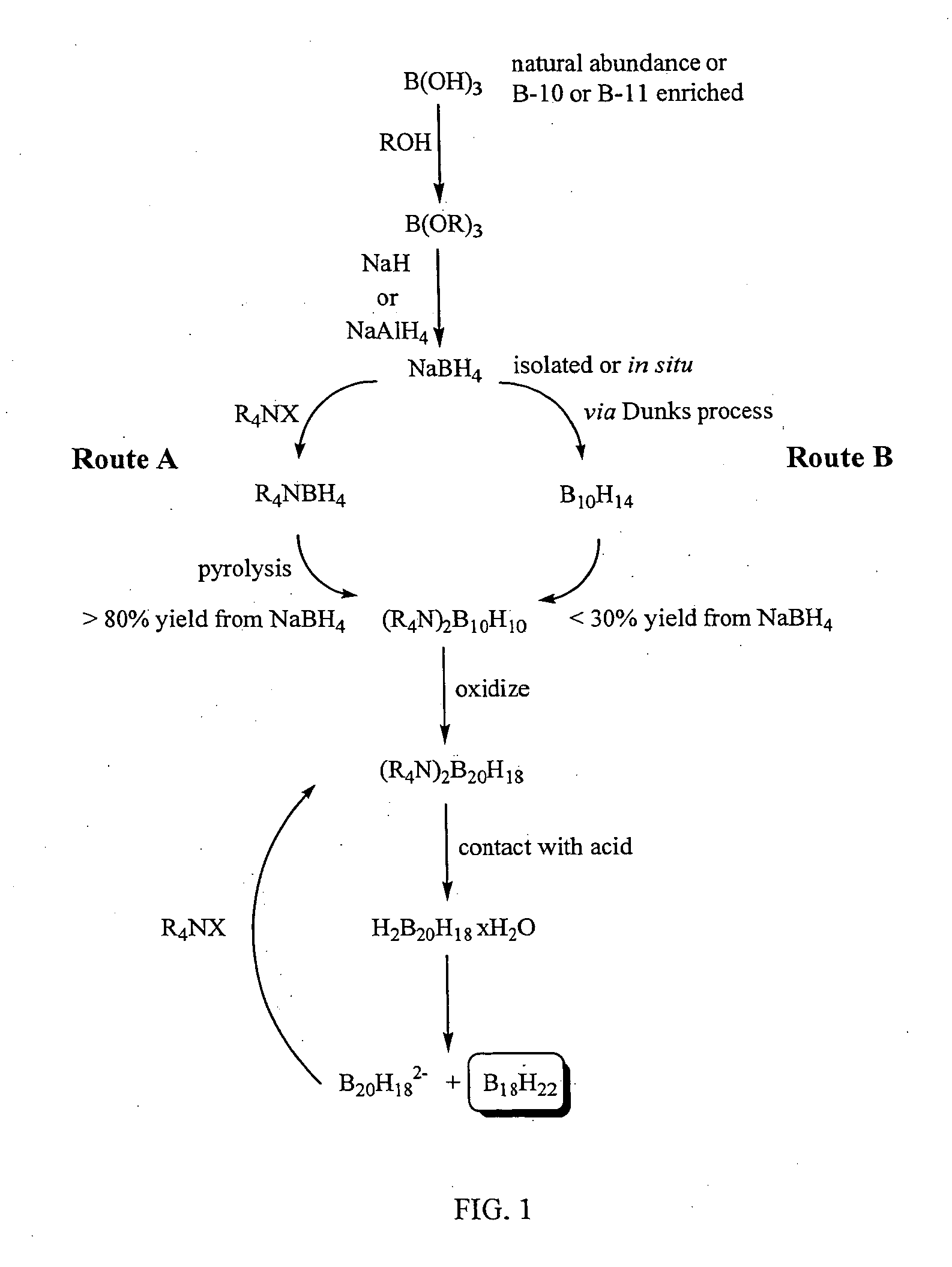
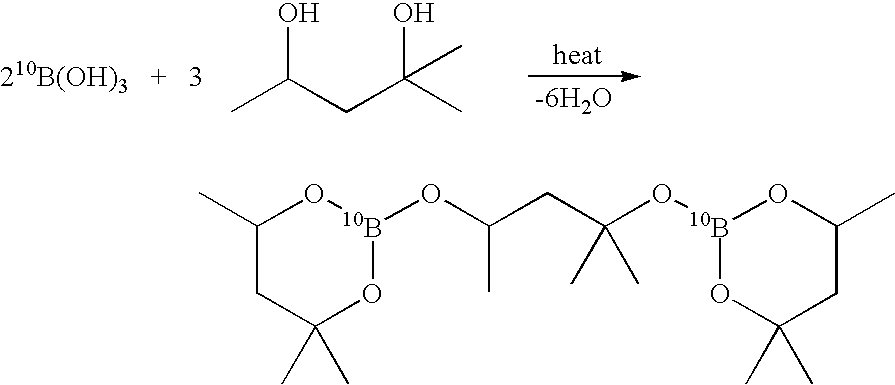
Isotopically-enriched boranes and methods of preparing them
InactiveUS20050163693A1High synthetic yieldHigh isolation yieldSemiconductor/solid-state device manufacturingChemical recyclingIsotopeConjugate acid
Owner:SEMEQUIP
Method of production of B10H102-ammonium salts and methods of production of B18H22
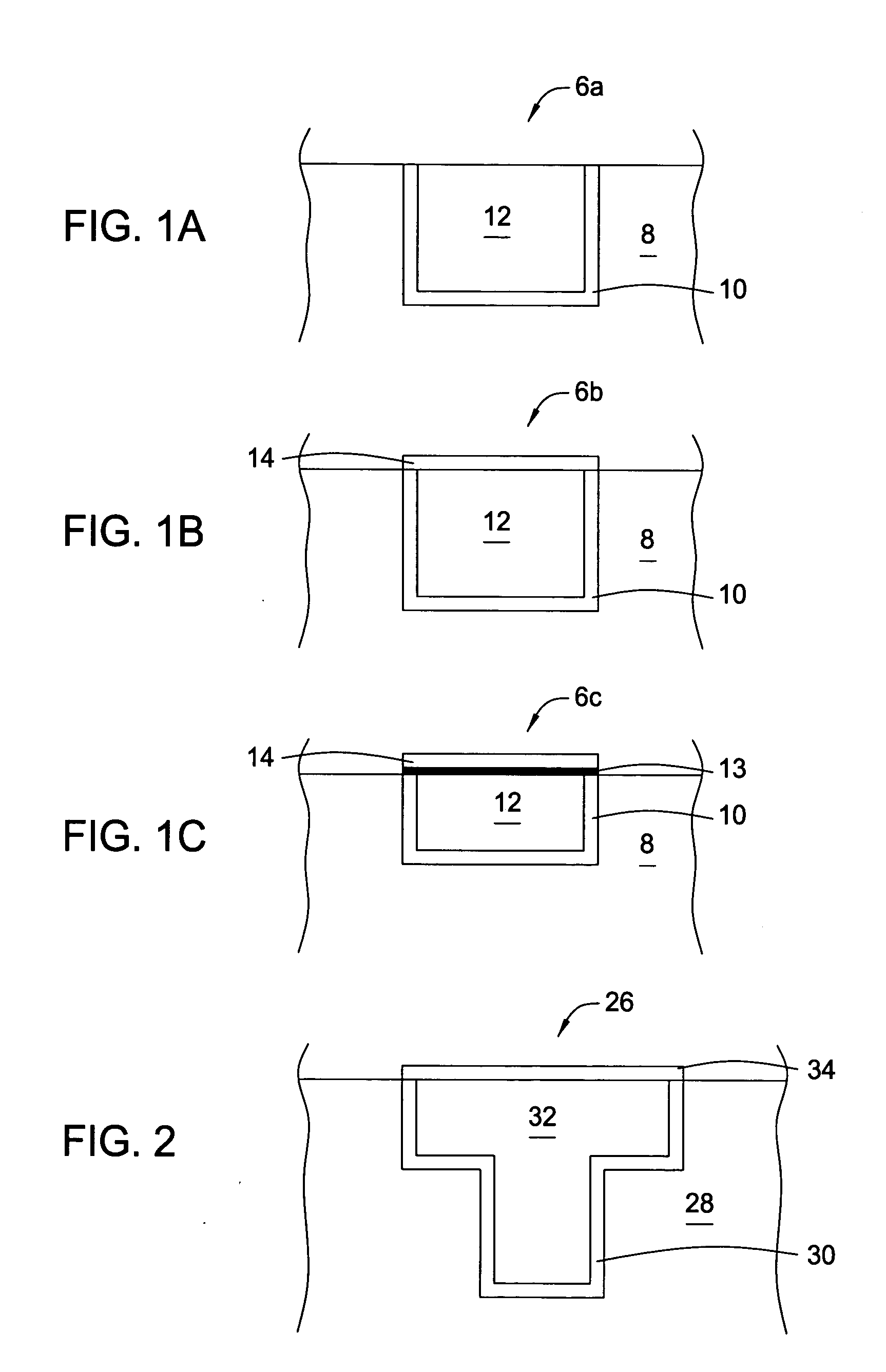
InactiveUS20050169828A1High yieldSemiconductor/solid-state device manufacturingGroup 3/13 element organic compoundsMethods of productionOctadecaborane
The invention provides new methods for synthesis of B9H9−, B10H102−, B11H14−, and B12H122− salts, particularly alkylammonium salts of B9H9−, B10H102−, B11H14−, and B12H122−. More particularly, the invention provides methods of preparing tetraalkylamronium salts of B9H9−, B10H102−, B11H14−, and B12H122− by pyrolysis of tetraalkylammonium borohydrides under controlled conditions. The invention additionally provides methods of preparing, in an atom efficient process, octadecaborane from the tetraalkylammonium salts of the invention. Preferred methods of the invention are suitable for preparation of isotopically enriched boranes, particularly isotopically enriched 10B18H22 and 11B18H22.
Owner:SEMEQUIP
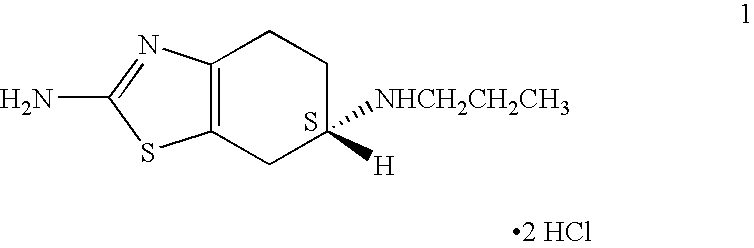
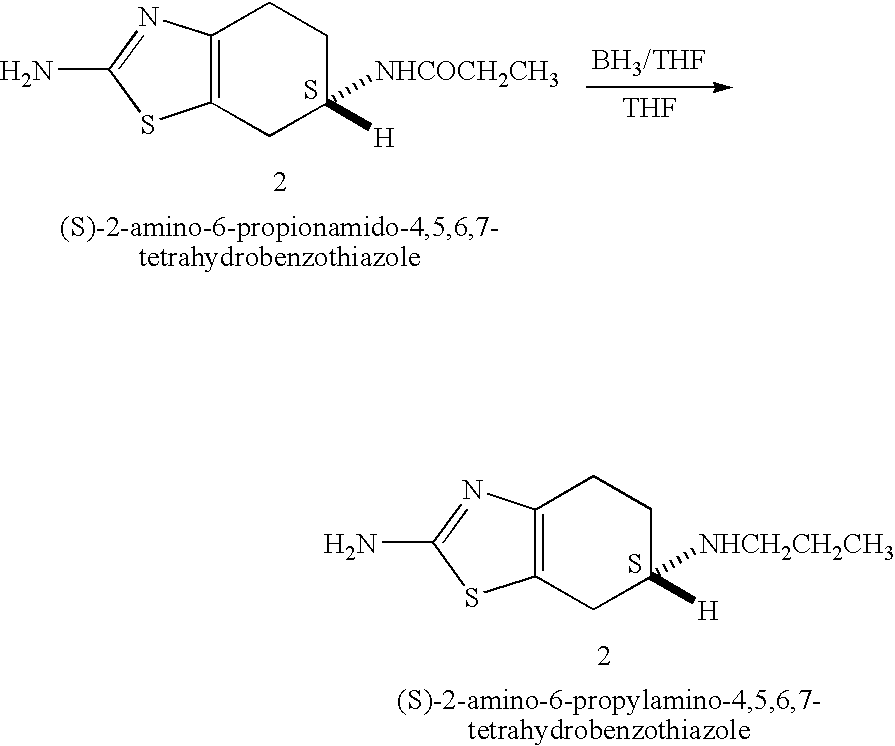
Process for the reduction of (S)-2-amino-6-propionamido-4,5,6,7-tetrahydrobenzo-thiazole
A process is disclosed for the reduction of (S)-2-amino-6-propionamido-4,5,6,7-tetrahydrobenzothiazole, which comprises reacting (S)-2-amino-6-propionamido-4,5,6,7-tetrahydrobenzothiazole with a borane reagent in the presence of suitable organic solvent to yield (S)-2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole base, which may be converted to an acid addition salt thereof. The process provided herein can be easily, conveniently and inexpensively scaled-up.
Owner:CHEMAGIS
Selective self-initiating electroless capping of copper with cobalt-containing alloys
InactiveUS20050095830A1Reduced metal concentrationSemiconductor/solid-state device manufacturingLiquid/solution decomposition chemical coatingElectroless depositionAlloy
Embodiments of the invention generally provide compositions of plating solutions, methods to mix plating solutions and methods to deposit capping layers with plating solutions. The plating solutions described herein may be used as electroless deposition solutions to deposit capping layers on conductive features. The plating solutions are rather dilute and contain strong reductants to self-initiate on the conductive features. The plating solutions may provide in-situ cleaning processes for the conductive layer while depositing capping layers free of particles. In one embodiment, a method for forming an electroless deposition solution is provided which includes forming a conditioning buffer solution with a first pH value and comprising a first complexing agent, forming a cobalt-containing solution with a second pH value and comprising a cobalt source, a tungsten source and a second complexing agent, forming a buffered reducing solution with a third pH value and comprising a hypophosphite source and a borane reductant, combining the conditioning buffer solution, the cobalt-containing solution and the buffered reducing solution to form the electroless deposition solution. The electroless deposition solution includes the cobalt source in a concentration range from about 1 mM to about 30 mM, the tungsten source in a concentration range from about 0.1 mM to about 5 mM, the hypophosphite source in a concentration range from about 5 mM to about 50 mM, the borane reductant in a concentration range from about 5 mM to about 50 mM, and has a total pH value in a range from about 8 to about 10.
Owner:APPLIED MATERIALS INC
Solid chemical hydride dispenser for generating hydrogen gas
InactiveUS7666386B2Molybdeum compoundsSynthetic resin layered productsCompound (substance)Hydrogen evolution
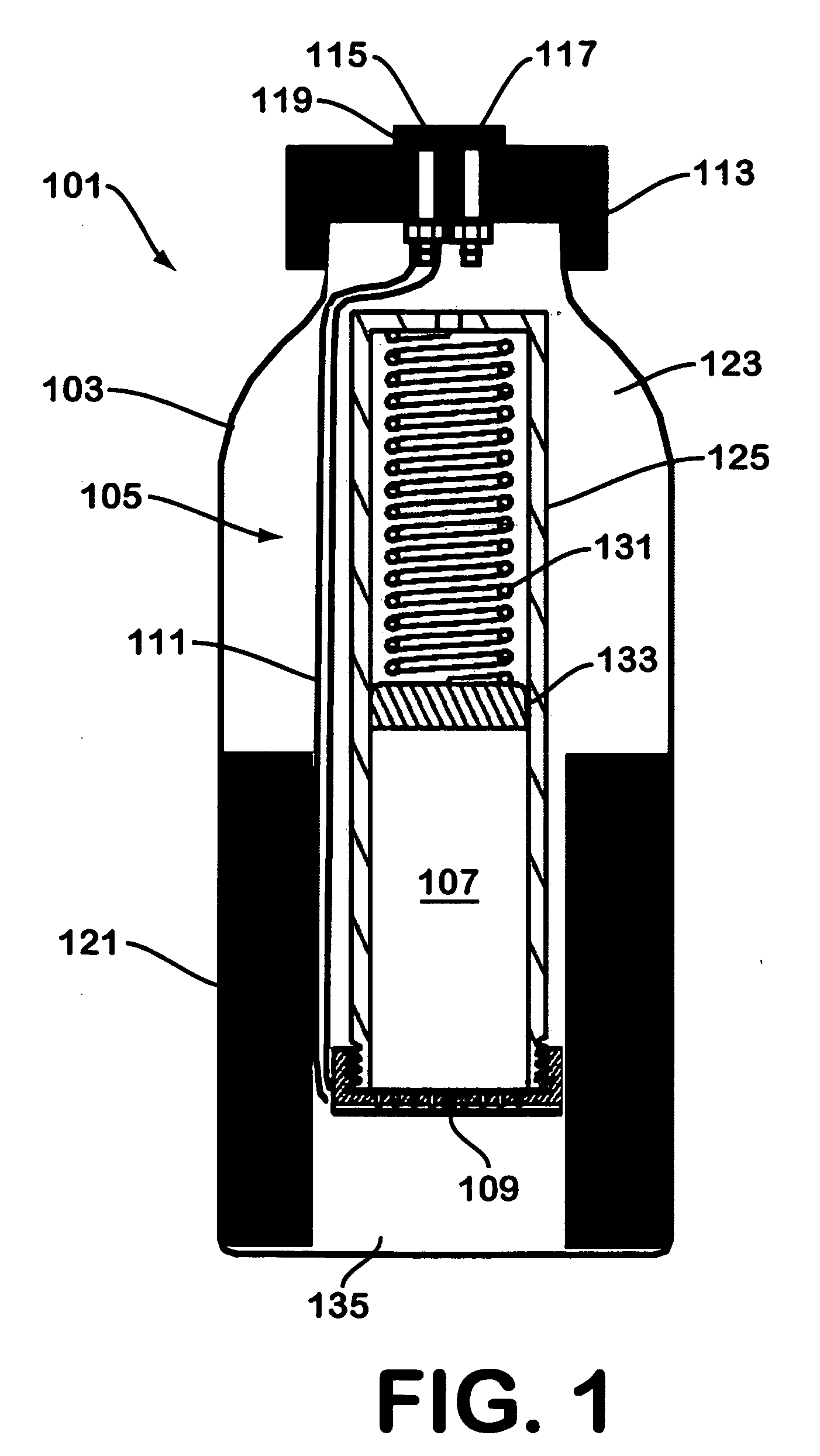
A device for generating hydrogen gas is provided. The device (101) comprises a first hydrogen-containing composition (107) that reacts with a second composition to evolve hydrogen gas; a dispenser (105) adapted to apply the first composition to a first porous member (109); and a conduit (111) adapted to supply the second composition to the first porous member. In a preferred embodiment, the first composition is selected from the group consisting of hydrides, borohydrides and boranes, the second composition is water, and the dispenser is spring-loaded and is charged with the first composition. As the first composition reacts with water at the interface to evolve hydrogen gas, the dispenser forces the reaction product across the interface and out of the dispenser, where it will not interfere with the progress of the hydrogen evolution reaction.
Owner:LYNNTECH POWER SYST
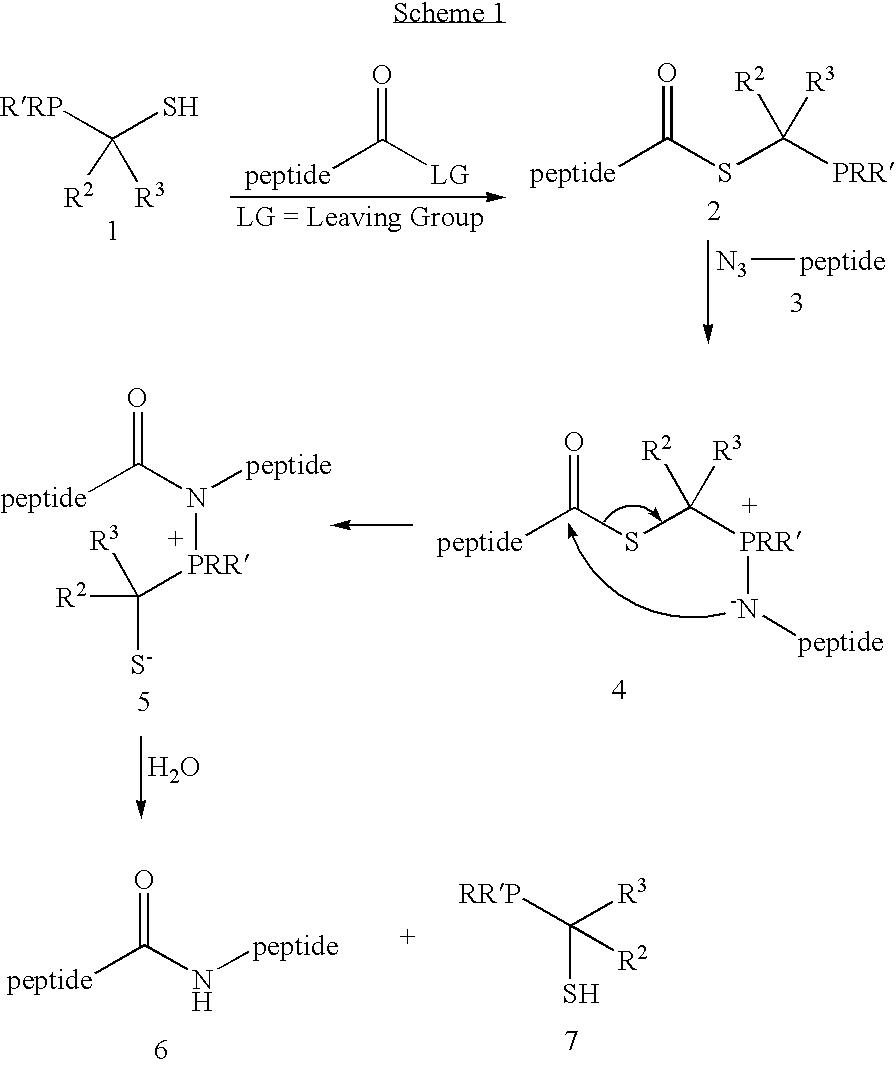
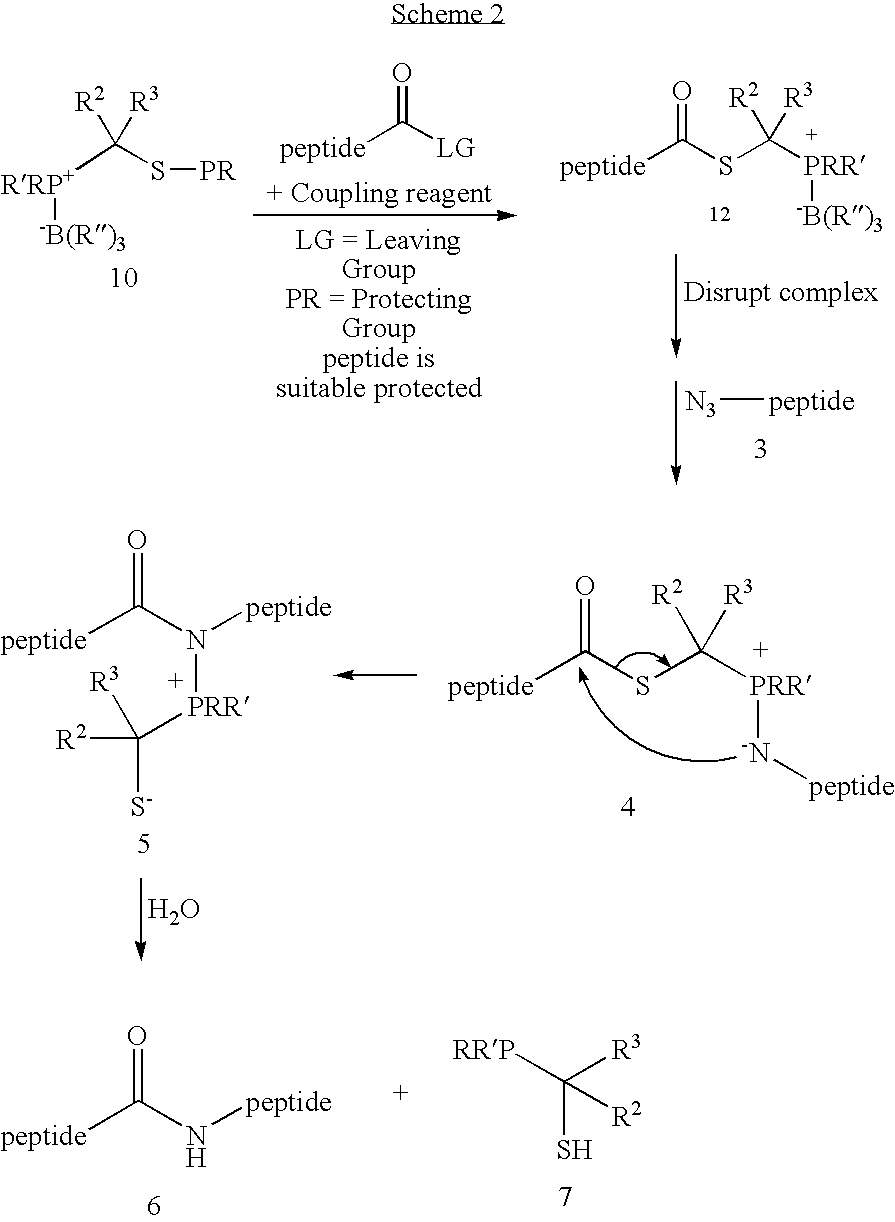
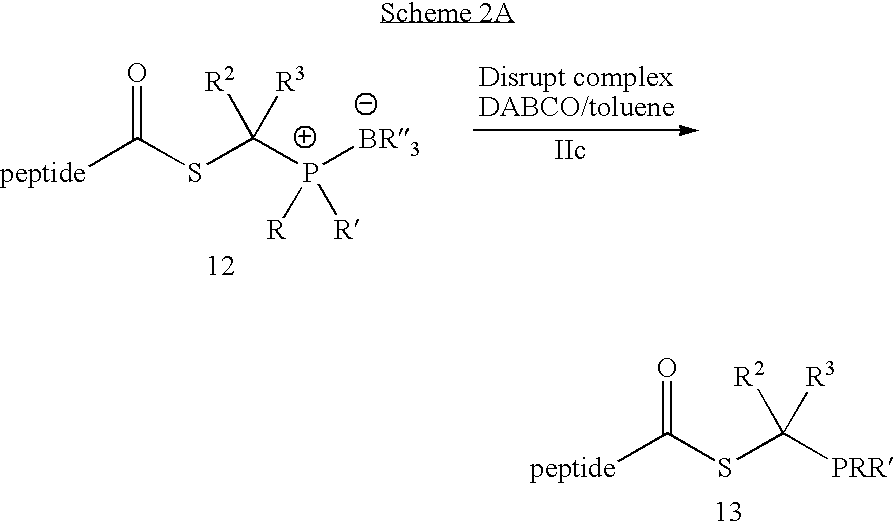
Chemical synthesis of reagents for peptide coupling
The present invention provides improved methods for synthesis of phosphinothiol reagents, as well as novel protected reagents, for use in formation of amide bonds, and particularly for peptide ligation. The invention provides phosphine-borane complexes useful as reagents in the formation of amide bonds, particularly for the formation of an amide bond between any two of an amino acid, a peptide, or a protein.
Owner:WISCONSIN ALUMNI RES FOUND
Process for forming a low carbon, low resistance metal film during the manufacture of a semiconductor device and systems including same
InactiveUS20060261441A1Reduce pollutionImprove conductivitySemiconductor/solid-state device detailsSolid-state devicesDevice materialLow resistance
A method for forming a conductive feature comprises forming a metal film such as ruthenium then annealing the film in an atmosphere comprising a hydrogen-rich gas such as ammonia, hydrogen, borane, or diborane, or in another gas such as carbon monoxide. The anneal may decrease the carbon content of the film and results in a metal layer having a lower resistance than the preannealed metal film.
Owner:MICRON TECH INC
Organoboron luminescent compounds and methods of making and using same
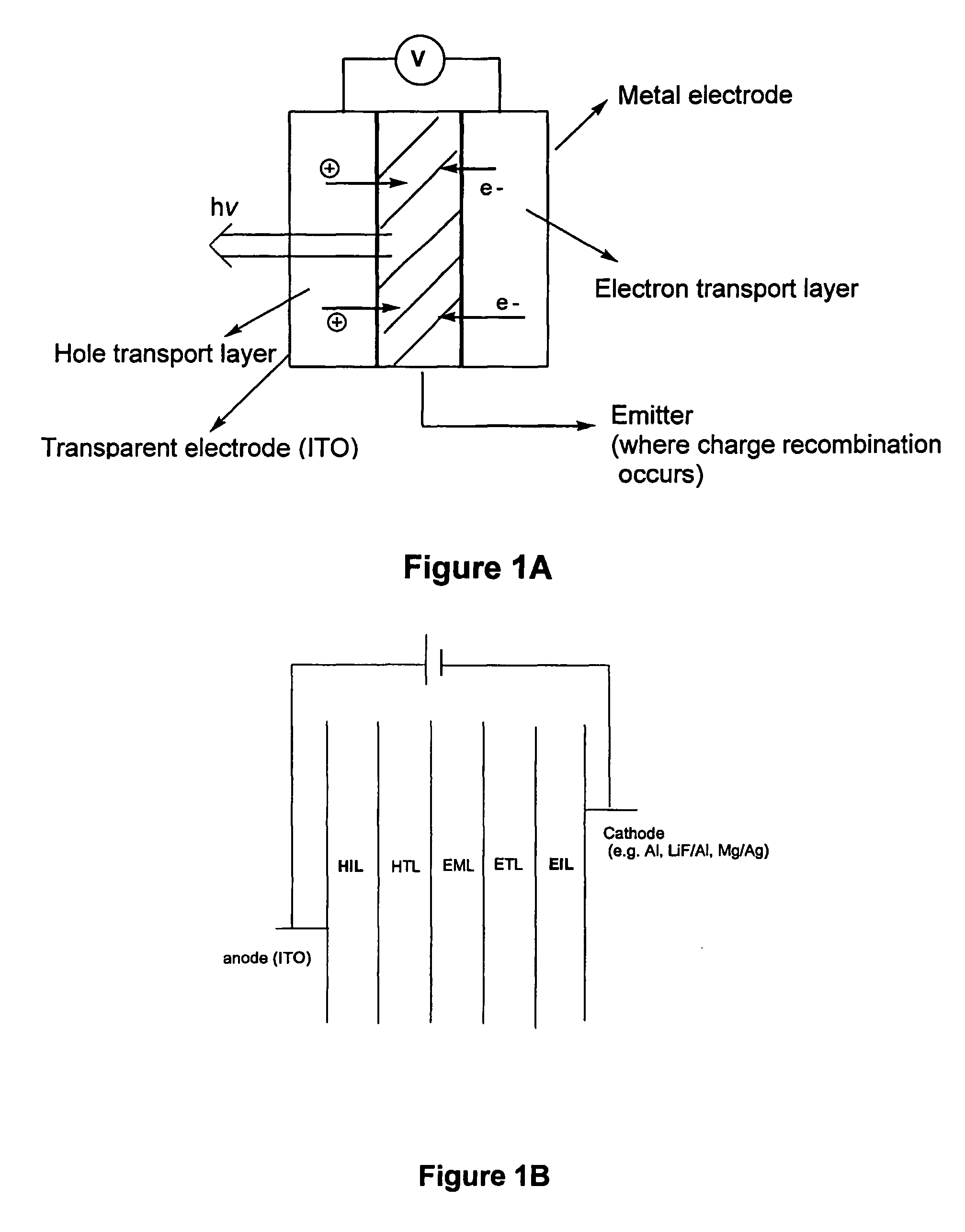
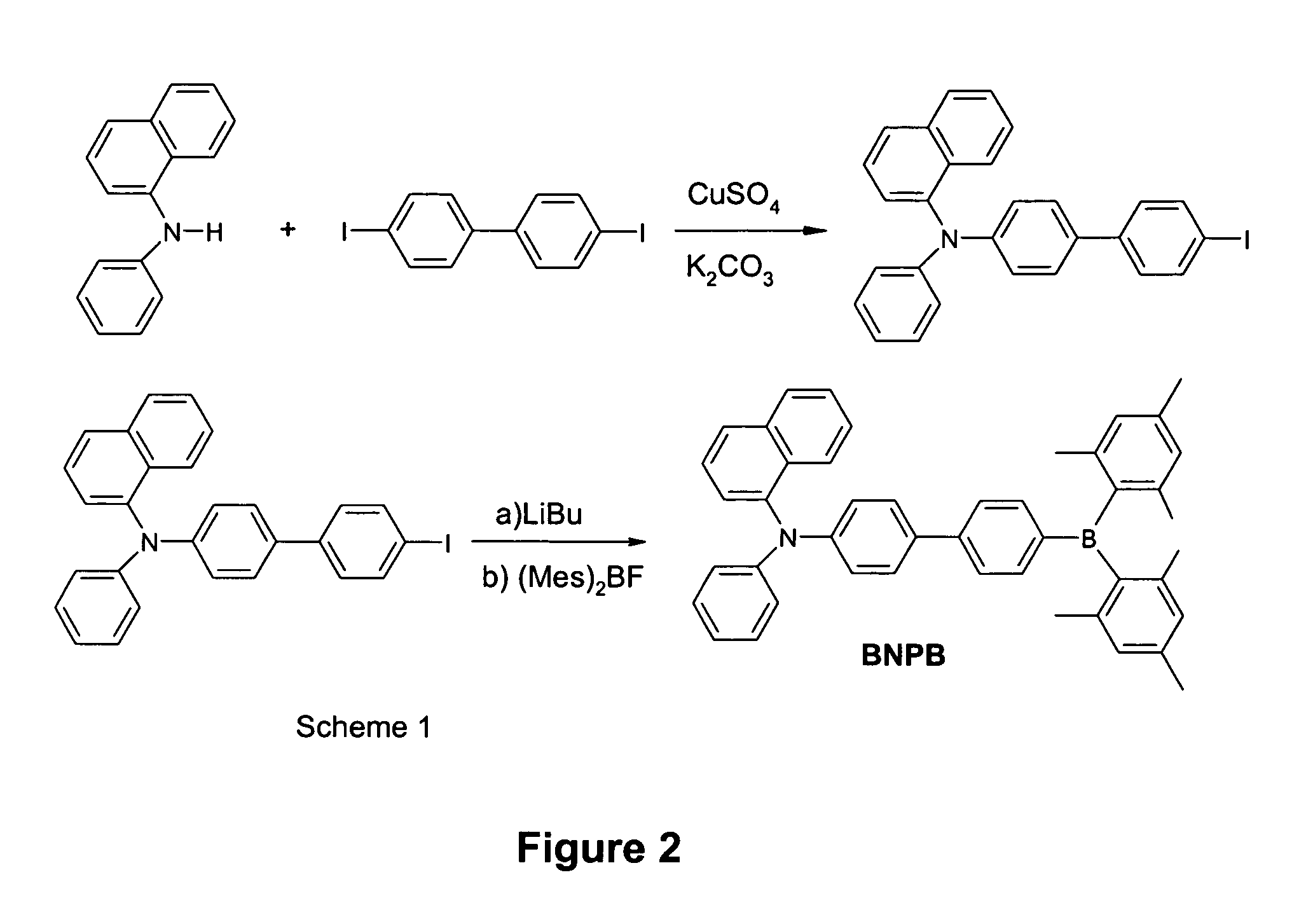
InactiveUS20060036114A1Discharge tube luminescnet screensLayered productsPhotoluminescenceOrganoboron compounds
The invention provides three-coordinated organoboron compounds that are useful for photoluminescence and electroluminescence. Compounds of the invention include light emitters, preferably emitting intense blue light, electron transporters, hole transporters and hole injectors. A particularly preferred such compound is p-(1-naphthylphenylamino)-4,4′-biphenyldimesitylborane (BNPB), which demonstrates all of these properties. The invention further provides methods of synthesizing such three-coordinated boron compounds, methods of producing photoluminescence and electroluminescence, methods for charge transports, methods for hole injection, methods of applying the compounds in thin films, and uses of the compounds of the invention in luminescent probes, and electroluminescent displays.
Owner:QUEENS UNIV OF KINGSTON
Method for producing doped, alloyed, and mixed-phase magnesium boride films
Conducting and superconducting doped, magnesium boride materials are formed by a process which combines physical vapor deposition with chemical vapor deposition by physically generating magnesium vapor in a deposition chamber and introducing a boron containing precursor and a dopant into the chamber which combines with the magnesium vapor to form the material. Embodiments include forming carbon-doped magnesium diboride film and powder with hybrid physical-chemical vapor deposition (HPCVD) by adding a carbon-containing metalorganic magnesium precursor, bis(methylcyclopentadienyl)magnesium, with a hydrogen carrier gas together with a borane precursor in a chamber having a source of magnesium vapor.
Owner:PENN STATE RES FOUND
Ezetimible intermediate and synthetic method of ezetimible
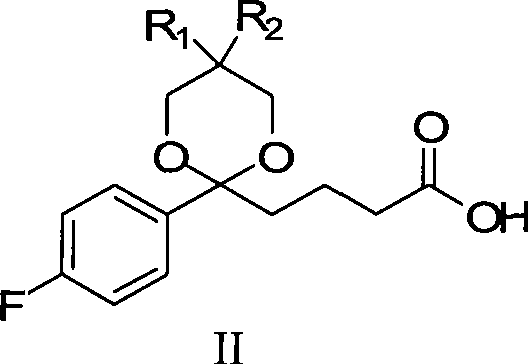
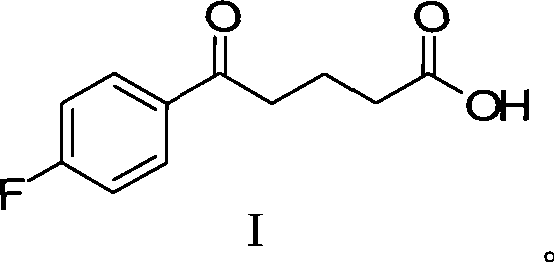
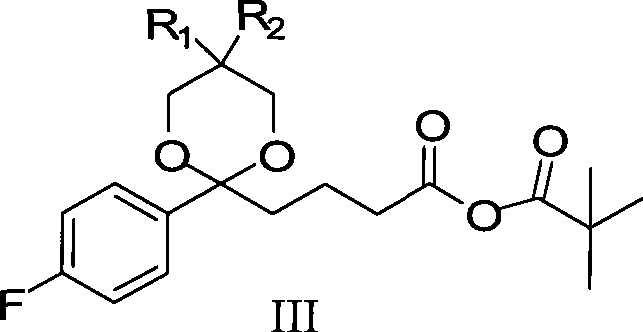
InactiveCN101423511AReduce pollutionEasy to operateOrganic chemistryMetabolism disorderCompound aSynthesis methods
The invention provides an intermediate for synthesizing ezetimibe and a preparation process thereof, and also provides a method for synthesizing the ezetimibe by the intermediate. The synthesizing method has short route, and comprises the following concrete steps: a compound I and substituted 1, 3-propanediol react to generate a compound II; the compound II and pivalyl chloride react to generate a compound III; the compound III and a compound A react to generate a compound IV; the compound IV and a compound V react to generate a compound VI under the condition of a titanium compound catalyst; the compound VI is re-ringed to generate a compound VII with beta-lactam; the compound VII is hydrolyzed to produce a compound VIII; and the compound VII is reduced to a compound IX ezetimibe by a borane chiral reducing agent. The synthesization has short route and mild reaction condition; and the produced intermediate and final product has high yield and high purity.
Owner:ENANTIOTECH CORP
Lithium ion battery electrolyte and battery and battery set containing the same
ActiveCN101494305AImprove consistencyImprove securitySecondary cellsHigh temperature storagePhysical chemistry
The invention provides an electrolyte of a lithium ion battery, which contains lithium salts, nonaqueous solvents and an addictive, wherein, the additive is selected from two or more than two varieties of an additive A, an additive B and an addictive C; the addictive A is one more types of fused ring compounds and condensed heterocyclic compounds; the addictive B is an aromatic compound containing alkoxy; the addictive C is a halogenated borane salt. The invention also provides a lithium ion battery and a battery set made by the electrolyte. The nonaqueous electrolytic solution can obviously raise the consistency of each single cell voltage in the discharging process of the battery set and the safety property of each single battery (and the battery set). The lithium ion battery containing the nonaqueous electrolytic solution has good low-temperature discharging property, high-temperature storage and cycling property.
Owner:BYD CO LTD
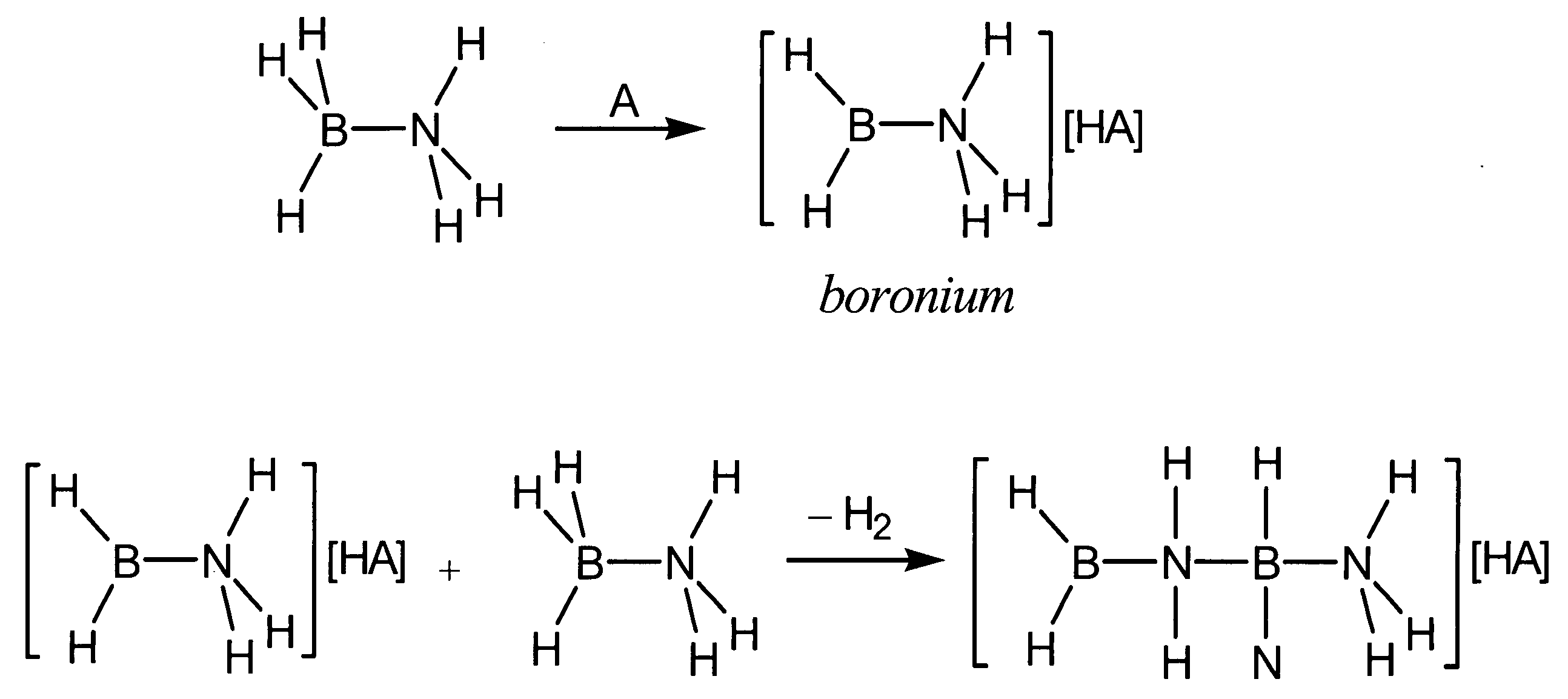
Acid-catalyzed dehydrogenation of amine-boranes
InactiveUS20060292068A1Regenerative fuel cellsCarburetting by solid carbonaceous material pyrolysisHydrogenAcid catalyzed
A method of dehydrogenating an amine-borane using an acid-catalyzed reaction. The method generates hydrogen and produces a solid polymeric [R1R2B—NR3R4]n product. The method of dehydrogenating amine-boranes may be used to generate H2 for portable power sources.
Owner:LOS ALAMOS NATIONAL SECURITY
Porous material supported nano alloy catalyst as well as preparation method and application thereof
ActiveCN104549555AHigh catalytic activityImprove stabilityCell electrodesMetal/metal-oxides/metal-hydroxide catalystsPtru catalystMetallurgy
The invention relates to a porous material supported nano alloy catalyst as well as a preparation method and application thereof and belongs to the technical field of nano material application and catalysis. The preparation method for the porous material supported nano alloy catalyst is characterized comprising the step of reaction through taking a metallic compound as a precursor, a porous material as a carrier and a borane substance as a reducing agent under the action of supercritical carbon dioxide used as a fluid medium; in the catalyst, the types of nano alloy are selected from Pt, Au, Ag, Pd, Ru, Rh, Pb, Fe, Co, Ni, Ir, Cu and the like and can be arbitrarily matched and proportioned; the size of each of nano alloy particles is about 1-2 nm. The nano particles not only can be distributed on the surface of the carrier, but also can be distributed in holes, even mesopores; therefore, the catalyst has higher catalytic activity and stability.
Owner:JIANGSU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com