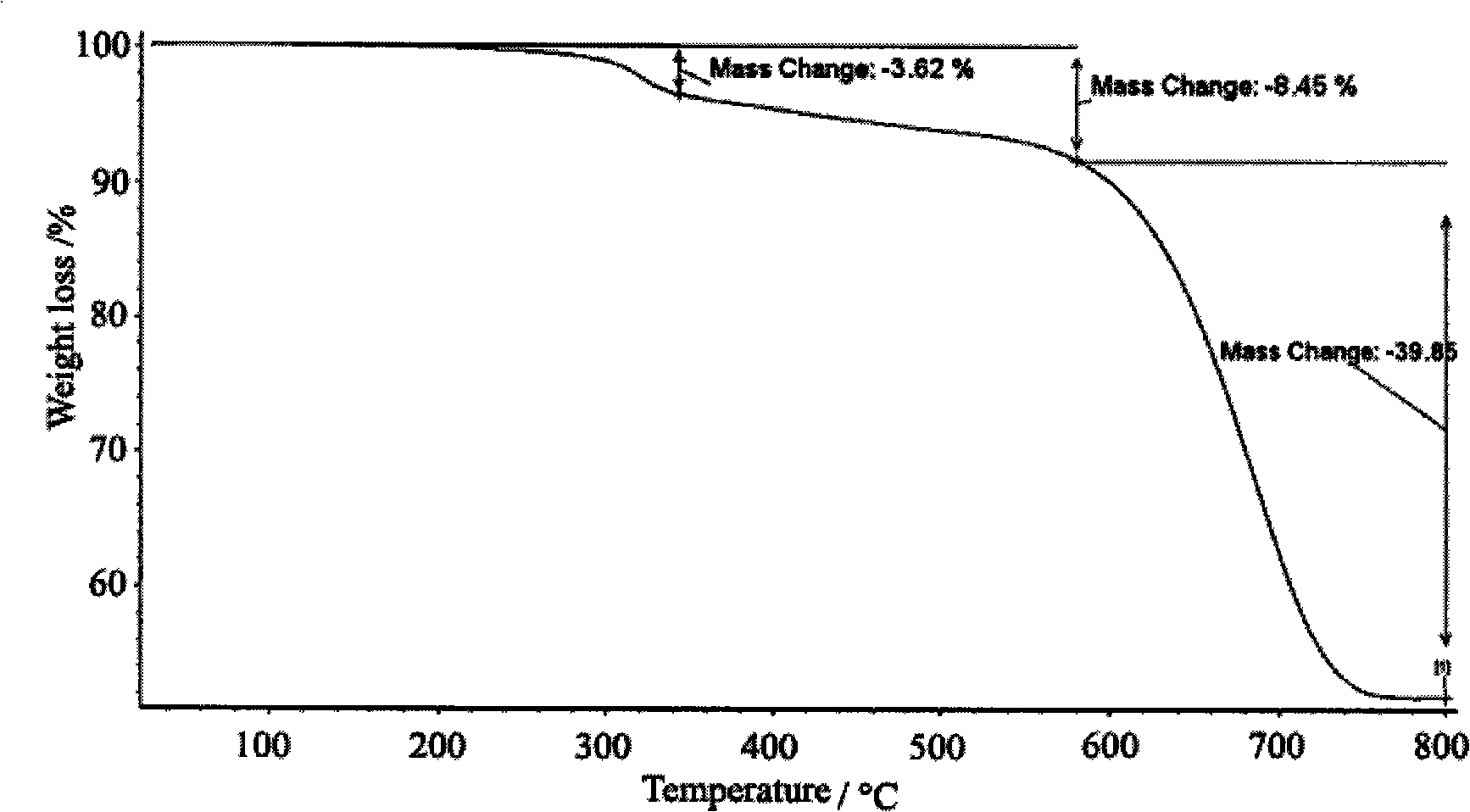
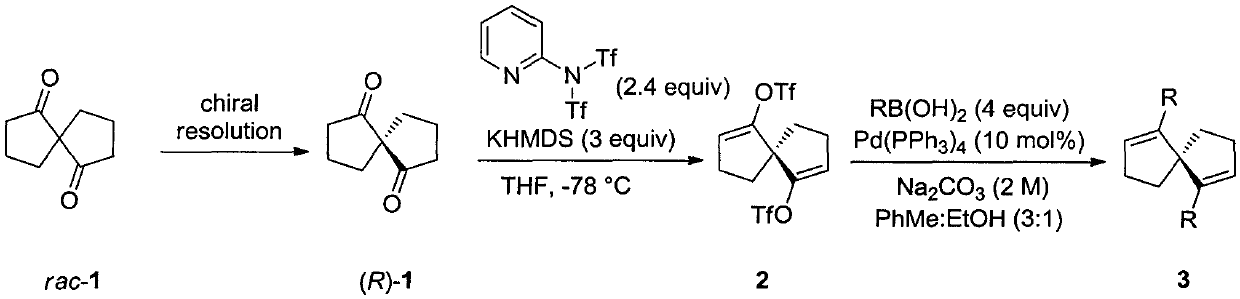
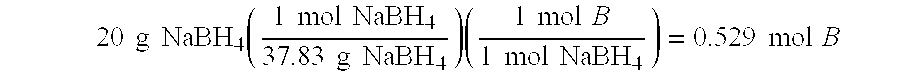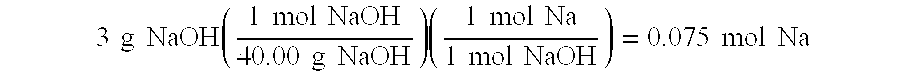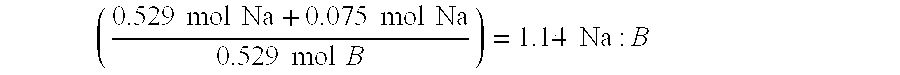Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Boron Hydrides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fuel blends for hydrogen generators
InactiveUS20050132640A1Easy to optimizeEfficient hydrolysisLiquid carbonaceous fuelsHydrogen productionFuel cellsHydrolysis
The present invention relates to improved aqueous fuels for hydrogen generators and a method for using them in the production of hydrogen. The present invention also relates to a system of using the subject aqueous fuels to generate hydrogen gas for use in a fuel cell or other device. The subject fuels contain a mixture of boron hydrides, at least one of which is a metal salt, including metal borohydrides, higher boranes and metal higher boron hydrides. The subject aqueous fuels contain a mixture of boron hydrides having a positive ionic charge (+IC) to boron ratio of between 0.2 and 0.4 or between 0.6 and 0.99. Preferred fuels contain a mixture of boron hydrides having an (+IC) to boron ratio between 0.2 and 0.3 or between 0.7 and 0.8. Mixtures containing a metal borohydride also contain a metal hydroxide to stability it against premature hydrolysis in the aqueous fuel media.
Owner:MILLENNIUM CELL
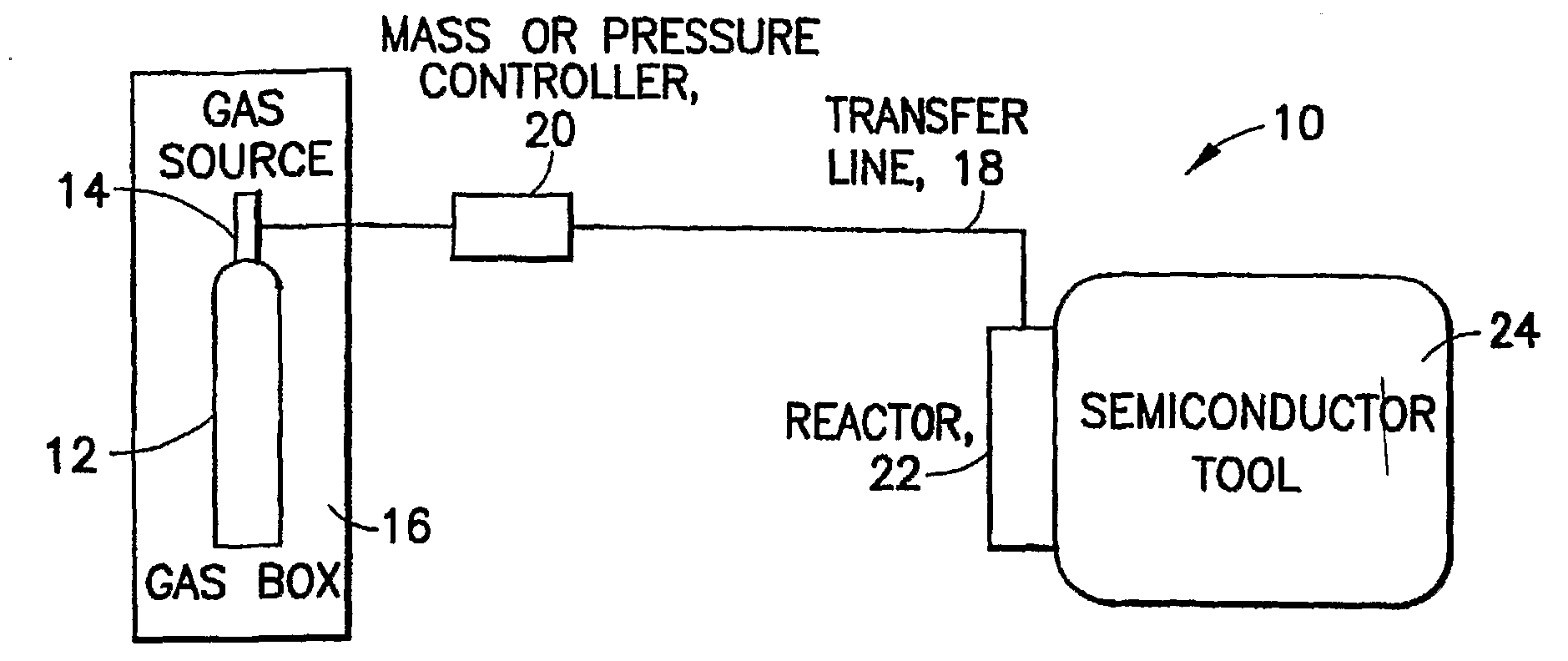
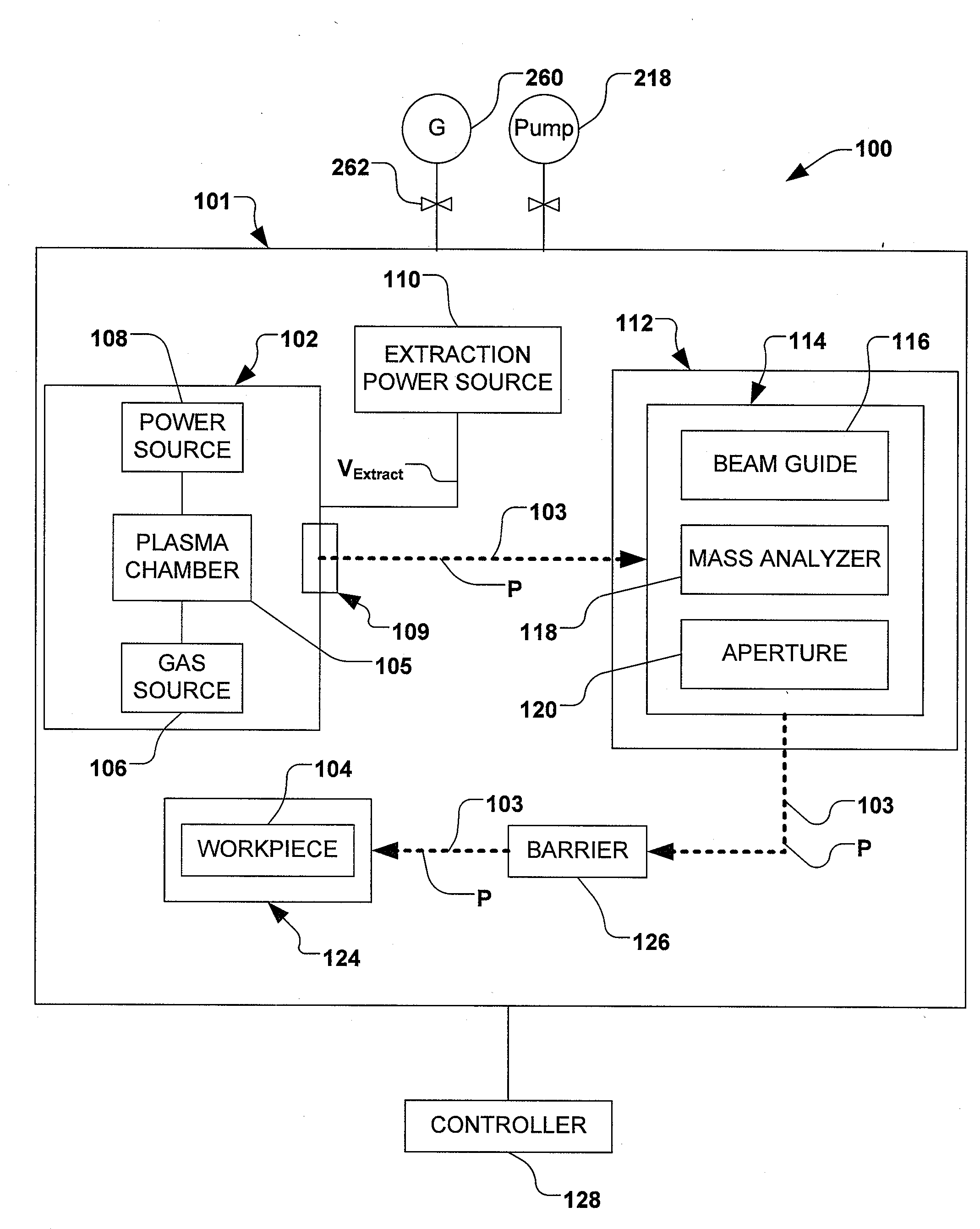
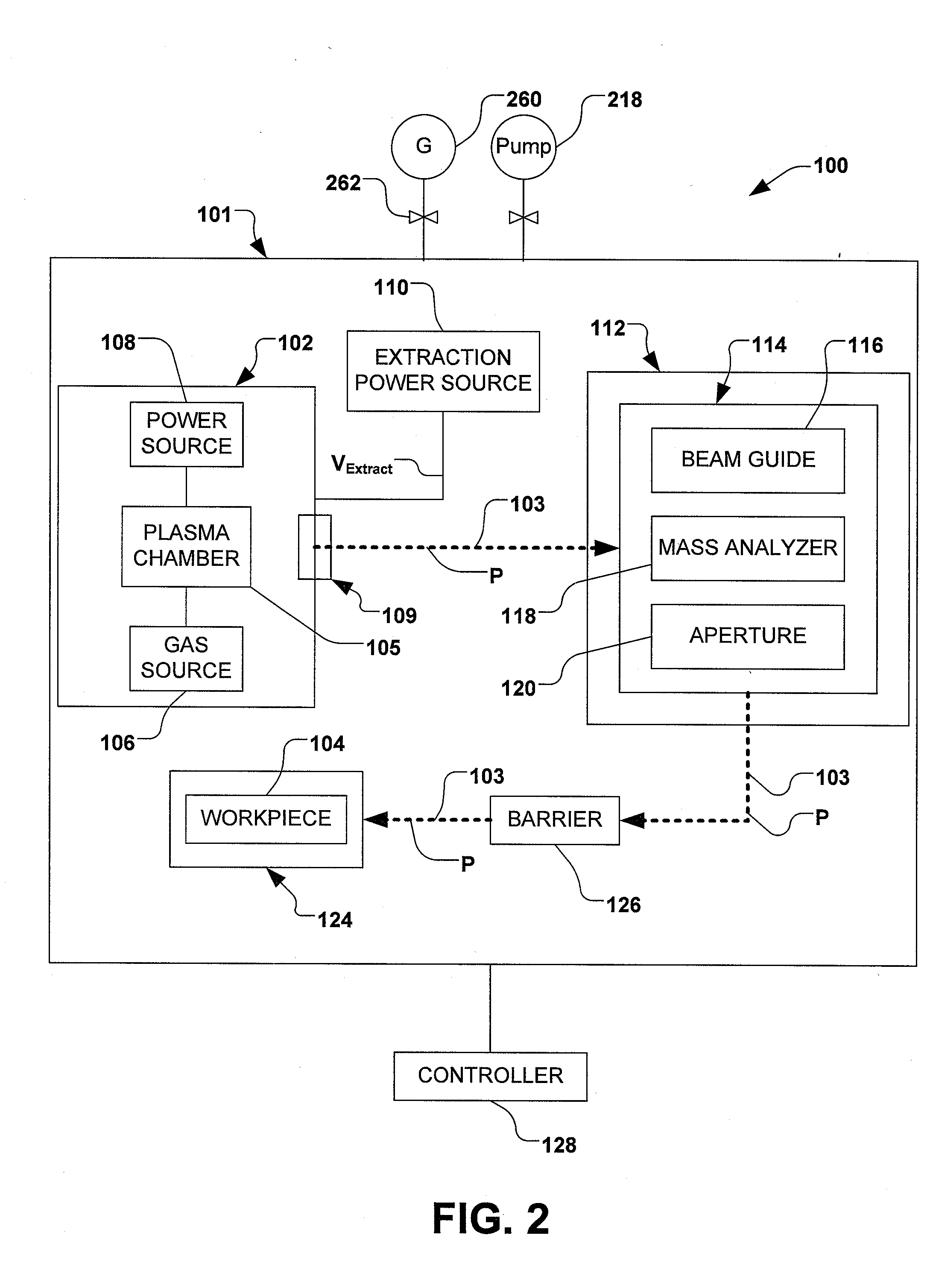
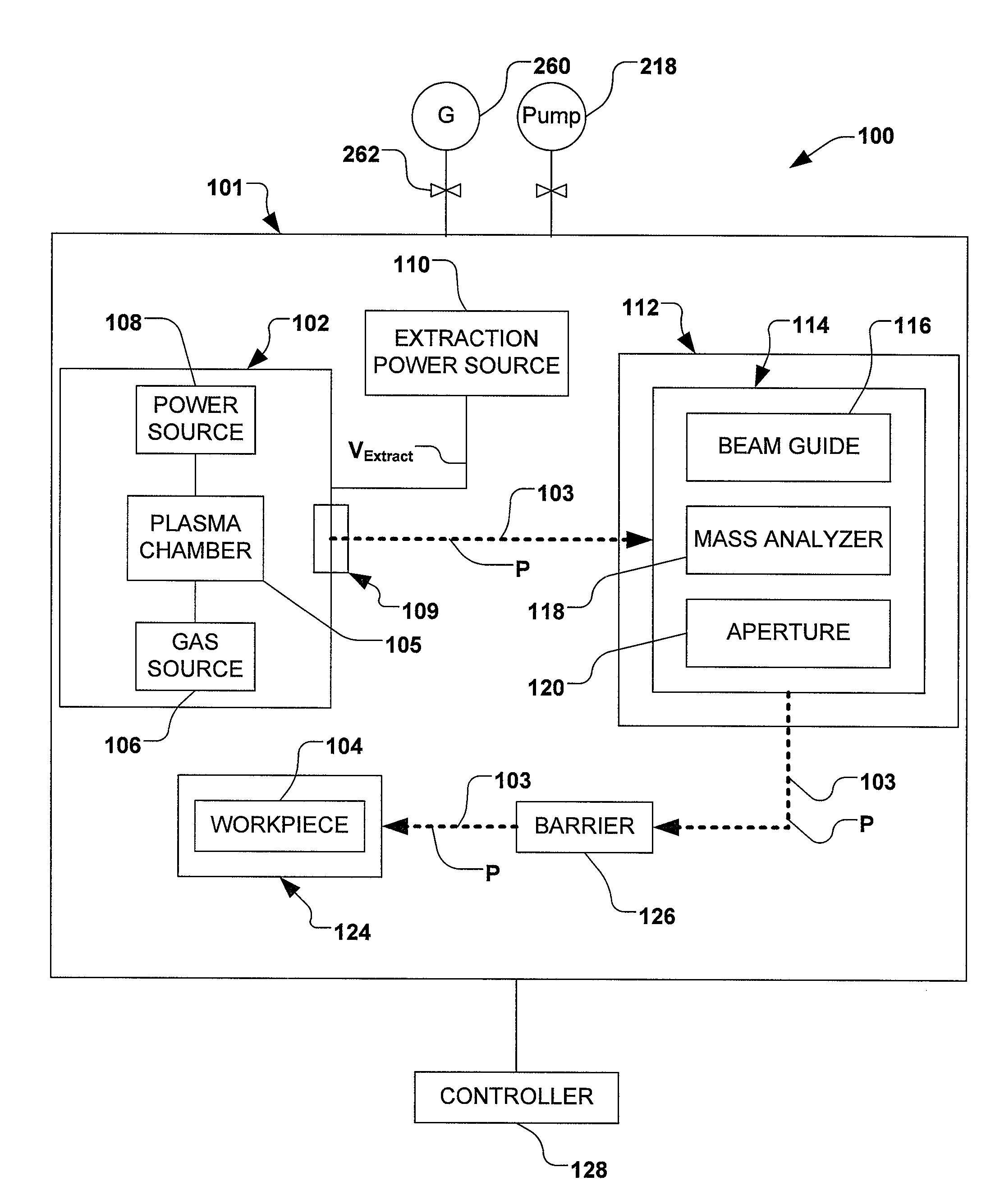
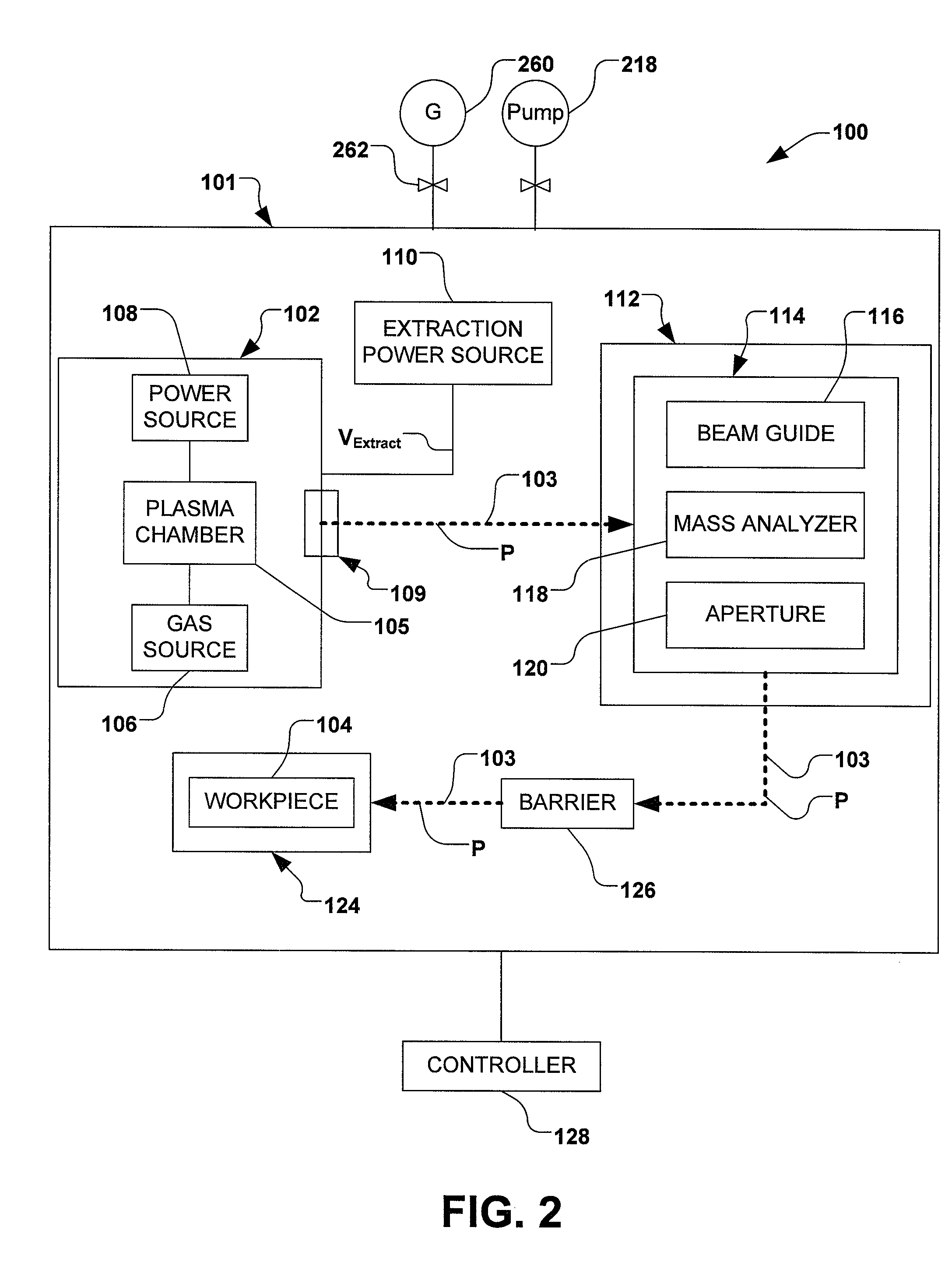
Boron Ion Implantation Using Alternative Fluorinated Boron Precursors, and Formation of Large Boron Hydrides for Implanation
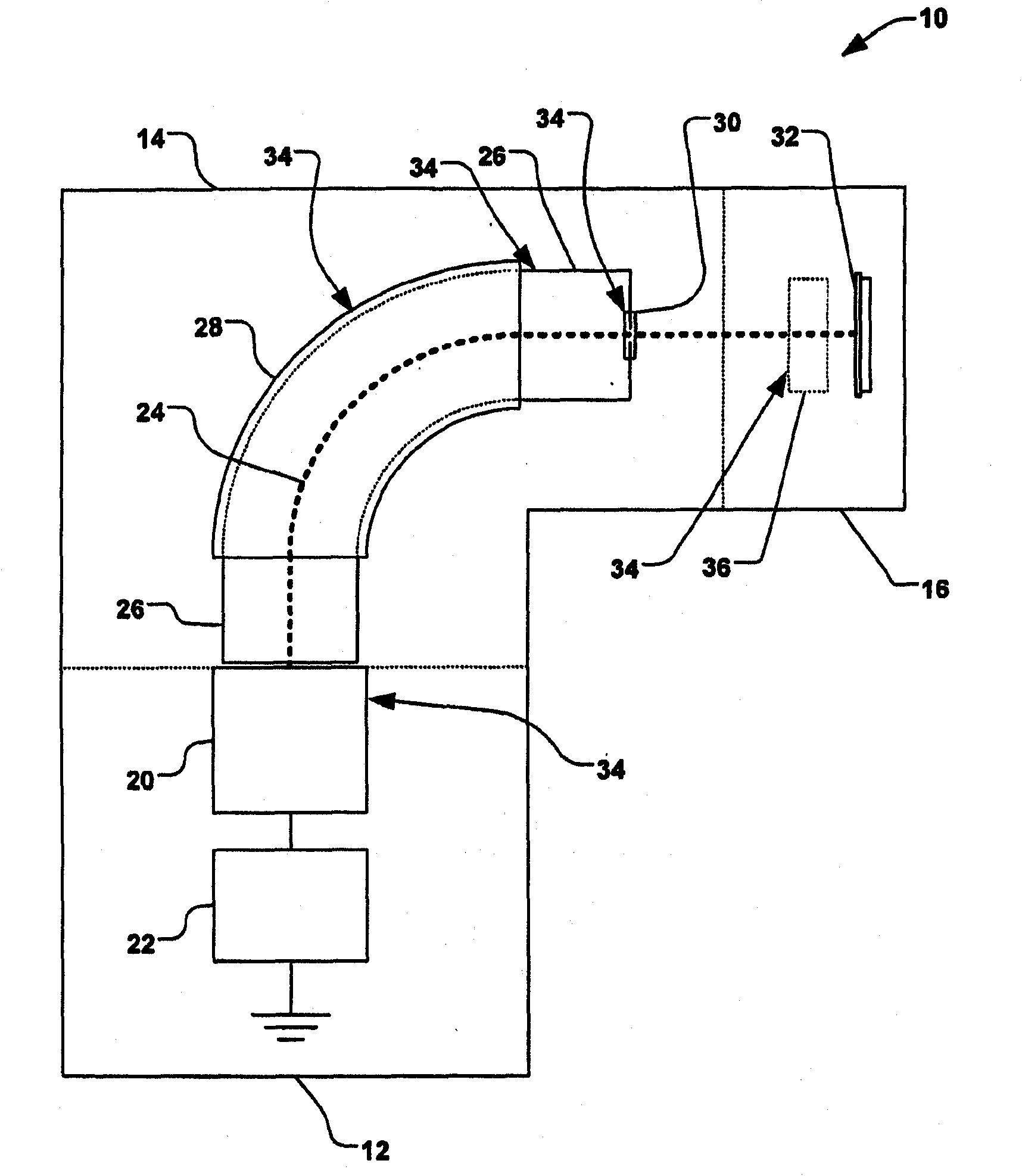
ActiveUS20080248636A1Easy to cutImprove efficiencyOther chemical processesElectric discharge tubesBoron trifluorideIon implantation
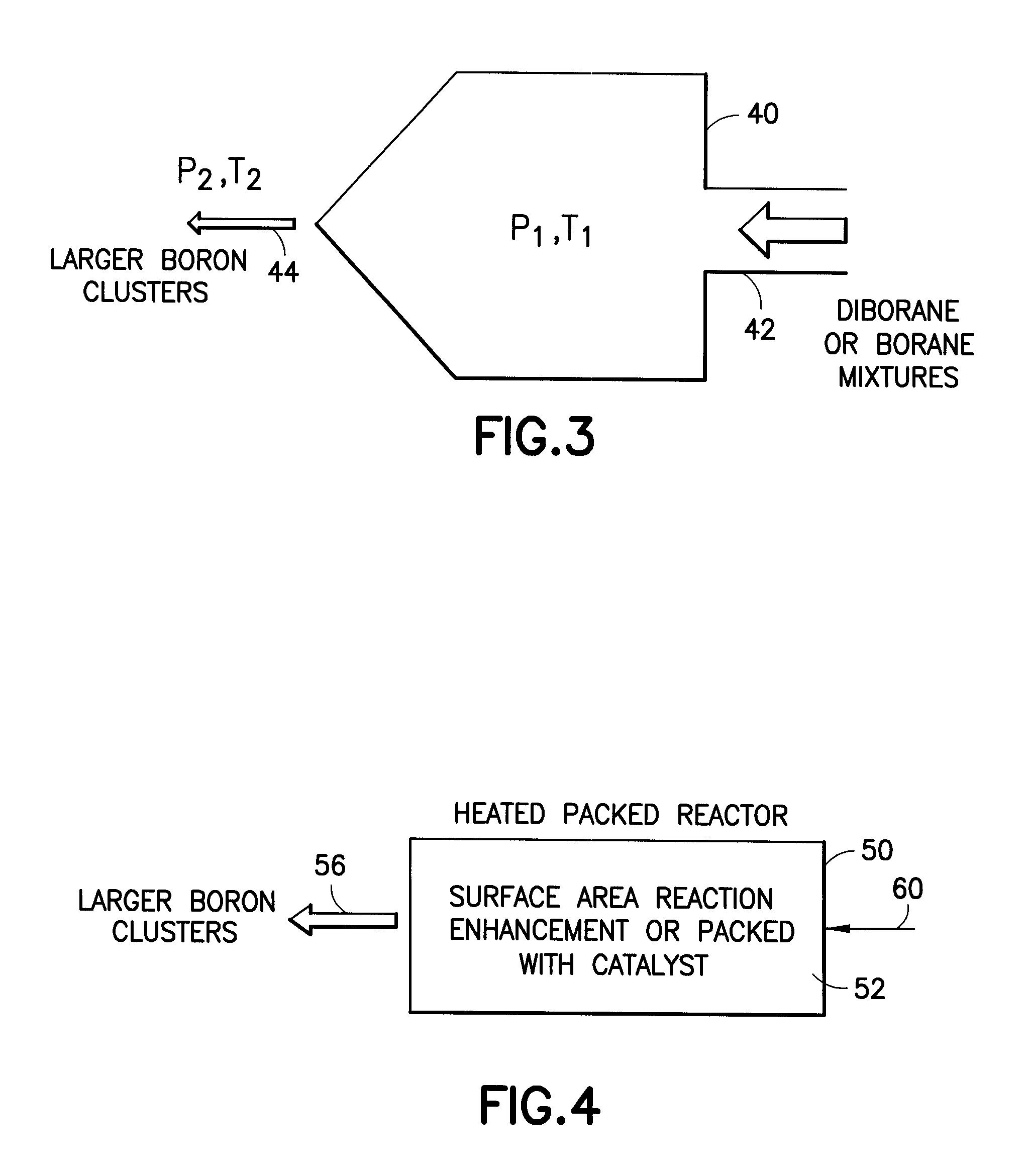
Methods of implanting boron-containing ions using fluorinated boron-containing dopant species that are more readily cleaved than boron trifluoride. A method of manufacturing a semiconductor device including implanting boron-containing ions using fluorinated boron-containing dopant species that are more readily cleaved than boron trifluoride. Also disclosed are a system for supplying a boron hydride precursor, and methods of forming a boron hydride precursor and methods for supplying a boron hydride precursor. In one implementation of the invention, the boron hydride precursors are generated for cluster boron implantation, for manufacturing semiconductor products such as integrated circuitry.
Owner:ENTEGRIS INC
Control of particles on semiconductor wafers when implanting boron hydrides
ActiveUS20090294698A1Reduce particle pollutionElectric discharge tubesCleaning using gasesMulti pollutantIon beam
A method for reducing particle contamination during implantation of ions comprises providing an implantation system for implanting ions into a workpiece via an ion beam, wherein one or more components are under selective vacuum and have one or more contaminants in a first state disposed thereon. A gas is introduced to the implantation system, wherein the gas generally reacts with at least a portion of the one or more contaminants, therein transforming the at least a portion of the one or more contaminants into a second state The at least a portion of the one or more contaminants in the second state remain disposed on the one or more components, and wherein the at least a portion of the second state of the one or more contaminants generally does not produce particle contamination on the one or more workpieces.
Owner:AXCELIS TECHNOLOGIES
Boron ion implantation using alternative fluorinated boron precursors, and formation of large boron hydrides for implantation
ActiveUS20110065268A1Easy to cutImprove efficiencyElectric discharge tubesVacuum evaporation coatingBoron trifluorideBoron containing
Methods of implanting boron-containing ions using fluorinated boron-containing dopant species that are more readily cleaved than boron trifluoride. A method of manufacturing a semiconductor device including implanting boron-containing ions using fluorinated boron-containing dopant species that are more readily cleaved than boron trifluoride. Also disclosed are a system for supplying a boron hydride precursor, and methods of forming a boron hydride precursor and methods for supplying a boron hydride precursor. In one implementation of the invention, the boron hydride precursors are generated for cluster boron implantation, for manufacturing semiconductor products such as integrated circuitry.
Owner:ENTEGRIS INC
Hydrogen generation catalysts and systems for hydrogen generation
Supported catalysts are provided to promote hydrogen generation from the hydrolysis of boron hydrides. The supported catalyst is a supported metallic mixture comprising a first transition metal selected from the group consisting of cobalt, ruthenium, zinc, molybdenum, manganese, titanium, tin, cadmium, and iridium, in an amount of from about 0.1 to about 20% by weight, and a second metal selected from the group consisting of cobalt, ruthenium, zinc, molybdenum, manganese, titanium, tin, cadmium, boron, and iridium, in an amount of from about 0.05 to about 25% by weight of the supported catalyst.
Owner:MILLENNIUM CELL
Method for preparing boron nitride coating carbon nano-tube/nano-wire and boron nitride nano-tube
Owner:SHANDONG UNIV
Manufacture of silver-copper-germanium alloy
InactiveCN101218361ANot commercially viableWelding/cutting media/materialsSoldering mediaImpuritySilver copper
Silver alloys containing copper and germanium e.g. about 1 wt % Ge and of very low copper content e.g. about 0.8 wt % Cu can be precipitation hardened to 65 HV or above, whereas alloys of similar copper content and not containing germanium remain soft. In an embodiment, a silver alloy comprises 92.5-97 wt % Ag, 1-4.5 wt % Cu, 0.4-4 wt % Zn, 0.8-1.5 wt % Ge, 0 to 0.2 wt % Si, In or Sn and 0-0.2 wt % Mn, the balance being boron as grain refiner, incidental ingredients and impurities. The said alloy preferably comprises boron as grain refiner added as a boron hydride, e.g. sodium borohydride. A further group of alloys comprises a ternary alloy of silver, copper and germanium containing from more than 93.5 wt % to 95.5 wt % Ag, from 0.5 to 3 wt % Ge and the remainder, apart from incidental ingredients (if any), impurities and grain refiner, copper, the grain refiner being sodium borohydride or another boron hydride. Silicon-containing casting grain that gives rise to bright as-cast products is also disclosed. In a further embodiment, a zinc-containing silver alloy resistant to tarnish under severe conditions e.g. exposure to human sweat or French dressing comprises 1-5 wt % Zn, 0.7-3 wt % Cu, 0.1-3 wt % Ge, 0-0.3 wt % Mn, 0-0.25 wt % Si, B in an amount effective for grain refinement, up to 0.5 wt % incidental ingredients, the balance being Ag in an amount of 92.5-96 wt %, and impurities. A preferred manufacturing method giving an alloy with favourable physical properties involves melting together the ingredients, and incorporating boron by dispersing into molten silver alloy to foirn the whole or a precursor pait of said alloy a compound selecting fiom alkyl boron compounds, boron hydrides, boron halides, boron-containing metal hydrides, boron-containing metal halides and mixtures thereof The alloy is particularly suitable for castings which may be hardened in an oven e.g. at about 300 DEG C. for 30-45 min.
Owner:ARGENTIUM INT
Metal alloy manufacturing
Silver alloys containing copper and germanium e.g. about 1 wt % Ge and of very low copper content e.g. about 0.8 wt % Cu can be precipitation hardened to 65 HV or above, whereas alloys of similar copper content and not containing germanium remain soft. In an embodiment, a silver alloy comprises 92.5-97 wt % Ag, 1-4.5 wt % Cu, 0.4-4 wt % Zn, 0.8-1.5 wt % Ge, 0 to 0.2 wt % Si, In or Sn and 0-0.2 wt % Mn, the balance being boron as grain refiner, incidental ingredients and impurities. The said alloy preferably comprises boron as grain refiner added as a boron hydride, e.g. sodium borohydride. A further group of alloys comprises a ternary alloy of silver, copper and germanium containing from more than 93.5 wt % to 95.5 wt % Ag, from 0.5 to 3 wt % Ge and the remainder, apart from incidental ingredients (if any), impurities and grain refiner, copper, the grain refiner being sodium borohydride or another boron hydride. Silicon-containing casting grain that gives rise to bright as-cast products is also disclosed. In a further embodiment, a zinc-containing silver alloy resistant to tarnish under severe conditions e.g. exposure to human sweat or French dressing comprises 1-5 wt % Zn, 0.7-3 wt % Cu, 0.1-3 wt % Ge, 0-0.3 wt % Mn, 0-0.25 wt % Si, B in an amount effective for grain refinement, up to 0.5 wt % incidental ingredients, the balance being Ag in an amount of 92.5-96 wt %, and impurities. A preferred manufacturing method giving an alloy with favourable physical properties involves melting together the ingredients, and incorporating boron by dispersing into molten silver alloy to foirn the whole or a precursor pait of said alloy a compound selecting fiom alkyl boron compounds, boron hydrides, boron halides, boron-containing metal hydrides, boron-containing metal halides and mixtures thereof The alloy is particularly suitable for castings which may be hardened in an oven e.g. at about 300° C. for 30-45 min.
Owner:ARGENTIUM INT
Hydrogen generation catalysts and methods for hydrogen generation
Supported catalyst methods are provided to promote hydrogen generation from the hydrolysis of boron hydrides. The methods use a supported catalyst which is a supported metallic mixture comprising a first transition metal selected from the group consisting of cobalt, ruthenium, zinc, molybdenum, manganese, titanium, tin, cadmium, and iridium, in an amount of from about 0.1 to about 20% by weight, and a second metal selected from the group consisting of cobalt, ruthenium, zinc, molybdenum, manganese, titanium, tin, cadmium, boron, and iridium, in an amount of from about 0.05 to about 25% by weight of the supported catalyst.
Owner:MILLENNIUM CELL
Fuel blends for hydrogen generators
InactiveCN1918268ASolubility maximizationReduces premature curing problemsLiquid carbonaceous fuelsHydrogen productionFuel cellsPhysical chemistry
The present invention relates to improved aqueous fuels for hydrogen generators and a method for using them in the production of hydrogen. The present invention also relates to a system of using the subject aqueous fuels to generate hydrogen gas for use in a fuel cell or other device. The subject fuels contain a mixture of boron hydrides, at least one of which is a metal salt, including metal borohydrides, higher boranes and metal higher boron hydrides. The subject aqueous fuels contain a mixture of boron hydrides having a positive ionic charge (<+>IC) to boron ratio of between 0.2 and 0.4 or between 0.6 and 0.99. Preferred fuels contain a mixture of boron hydrides having an (<+>IC) to boron ratio between 0.2 and 0.3 or between 0.7 and 0.8. Mixtures containing a metal borohydride also contain a metal hydroxide to stability it against premature hydrolysis in the aqueous fuel media.
Owner:MILLENNIUM CELL
Control of particles on semiconductor wafers when implanting boron hydrides
InactiveCN102047376AElectric discharge tubesSemiconductor/solid-state device manufacturingPhysical chemistryIon beam
A method for reducing particle contamination during implantation of ions comprises providing an implantation system (200) for implanting ions into a workpiece (228) via an ion beam (210), wherein one or more components are under selective vacuum and have one or more contaminants in a first state disposed thereon. A gas is introduced to the implantation system (at 200), wherein the gas generally reacts with at least a portion of the one or more contaminants, therein transforming the at least a portion of the one or more contaminants into a second state. The at least a portion of the one or more contaminants in the second state remain disposed on the one or more components, and wherein the at least a portion of the second state of the one or more contaminants generally does not produce particle contamination on the one or more workpieces.
Owner:AXCELIS TECHNOLOGIES
Hydrogen generation catalysts and systems for hydrogen generation
Supported catalysts and methods are provided to promote hydrogen generation from the hydrolysis of boron hydrides. The supported catalysts contain a supported metal comprising at least one transition metal selected from the group consisting of cobalt, ruthenium, zinc, molybdenum, manganese, titanium, tin, cadmium, and iridium, in an amount of from about 0.1 to about 20% by weight of the supported catalyst.
Owner:ZHANG QINGLIN +3
Catalyst for preparing boron hydride by electrolysis and preparing method for catalytic electrode
This invention relates to a catalyst for preparing boron-hydrogen compounds in an electrolytic process and manufacture of catalytic electrodes containing it. The catalyst comprises one of hydrogen-storing alloy, carbon materials and metal borides or their mixture. It is prepared by: mixing catalyst powders 60 - 90 parts, additives 0 - 20 parts and binding agent 1 - 10 parts uniformly, adding solution to form films, and pressing the films onto current-collecting Ni or steel nets; or adding solution to form pastes, coating them onto surfaces or internal sides of Ni or steel nets in a certain thickness, and drying to obtain catalytic electrodes. The catalyst and catalytic electrodes can be used to prepare boron hydrides with high performance of electric current >50% in alkali solution by electrolysis with low cost.
Owner:WUHAN UNIV
Control of particles on semiconductor wafers when implanting boron hydrides
A method for reducing particle contamination during implantation of ions comprises providing an implantation system for implanting ions into a workpiece via an ion beam, wherein one or more components are under selective vacuum and have one or more contaminants in a first state disposed thereon. A gas is introduced to the implantation system, wherein the gas generally reacts with at least a portion of the one or more contaminants, therein transforming the at least a portion of the one or more contaminants into a second state The at least a portion of the one or more contaminants in the second state remain disposed on the one or more components, and wherein the at least a portion of the second state of the one or more contaminants generally does not produce particle contamination on the one or more workpieces.
Owner:AXCELIS TECHNOLOGIES
Method for producing boron hydrides by electrolysis
InactiveCN101070597AIncrease the areaReduce concentration polarizationElectrolysis componentsElectrochemical responseElectrolysis
The invention relates to a method of electrolyzing production borohydride. Electrolyser adopts blown film as the barrier film. The mixed liquor of metaborate and alkali is loaded in anode chamber and cathode chamber. Organic amine solution exists in cathode chamber. The material of anode or cathode is the granular material of slaty or fluidized bed. The pressure of anode chamber solution should be higher than that of cathode chamber solution at the time of electrolysis. The pressure difference is above 100Pa, so that it can prevent the BH4- of cathode chamber moving to the anode chamber. The mosaic electrode can largely enhance the surface area of electrode, improve the condition of mass transfer, and enhance the speed of electrochemical reaction. Pulse electrolytic technology is adopted to improve the electrochemical reaction speed and the current efficiency, which realizes metaborate directly regenerating to borohydride. It provides a more inexpensive source of hydrogen material for hydrogen supply system, and it also largely reduces the operating cost.
Owner:NANJING UNIV OF TECH
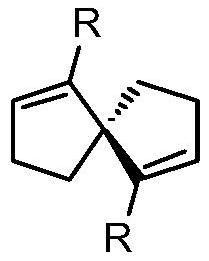
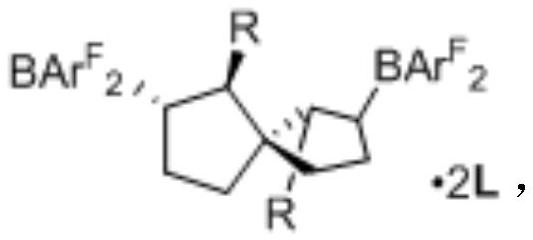
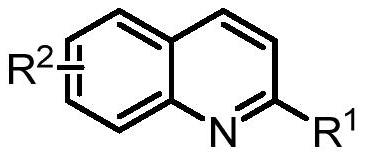
Synthesis of spiro-ring bis-boron catalyst and application thereof in hydrogenation reaction
ActiveCN109575060AIncrease conversionsHigh enantioselectivityOrganic-compounds/hydrides/coordination-complexes catalystsOrganic chemistry methodsHydrogenation reactionQuinoline
The invention relates to a synthesis of spiro-ring diene compound with C2 symmetry, and a a series of chirality spiro-ring bis-boron catalysts prepared by virtue of the reaction of the spiro-ring diene compound and boron hydrides. The spiro-ring bis-boron catalysts have high activity and enantioselectivity in the asymmetric hydrogenation reaction of quinoline compounds, and belong to the technicalfield of application. By adopting the synthesis of spiro-ring bis-boron catalyst and the application thereof in hydrogenation reaction, the problems of the traditional quinoline asymmetric hydrogenation reaction method that precious metal catalysts are used and the functional groups are poor in tolerance can be mainly solved, the nonmetal catalytic quinoline asymmetric hydrogenation reaction canbe realized, the reaction substrate range is wide, and the functional group tolerance is high. The synthesis of spiro-ring bis-boron catalyst and the application thereof in hydrogenation reaction areused in the medicine research and chemical production.
Owner:NANKAI UNIV
Method for preparing two-dimensional boron hydride nanosheet
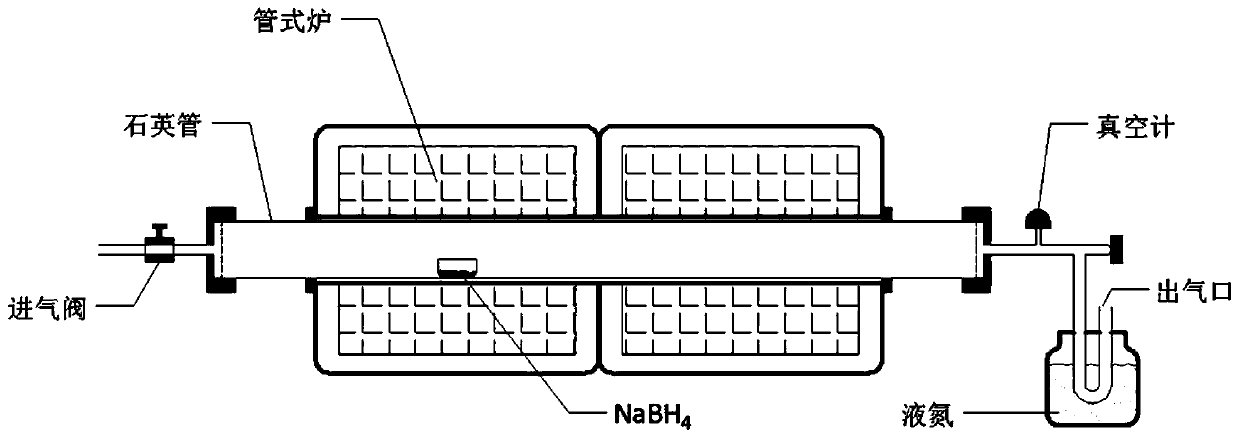
The invention discloses a method for preparing a two-dimensional boron hydride nanosheet, and the method comprises the following steps of: placing a boron source in a vacuum reaction furnace, and obtaining the high-yield and super-stable two-dimensional boron hydride nanosheet in a quartz boat in a reducing atmosphere through a strict two-step or three-step sectional heating procedure under the condition of no metal substrate. According to the method, a step-by-step heating mode is adopted, under the condition that no metal substrate or other epitaxial substrates exist, the boron hydride nanosheet is obtained in the mode that the solid boron source is controllably decomposed through a two-step or three-step segmented heating method, and therefore the method for preparing the high-yield andsuper-stable two-dimensional boron hydride nanosheet is provided. The two-dimensional boron hydride nanosheet obtained by the method can be applied to multiple technical fields, including solar cells, energy storage and conversion devices, transistor devices, sensors and memristors.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
Method for preparing boron nitride coating carbon nano-tube/nano-wire and boron nitride nano-tube
ActiveCN100590069CNo pollution in the processNo pollutionNitrogen compoundsNanowireReaction temperature
Owner:SHANDONG UNIV
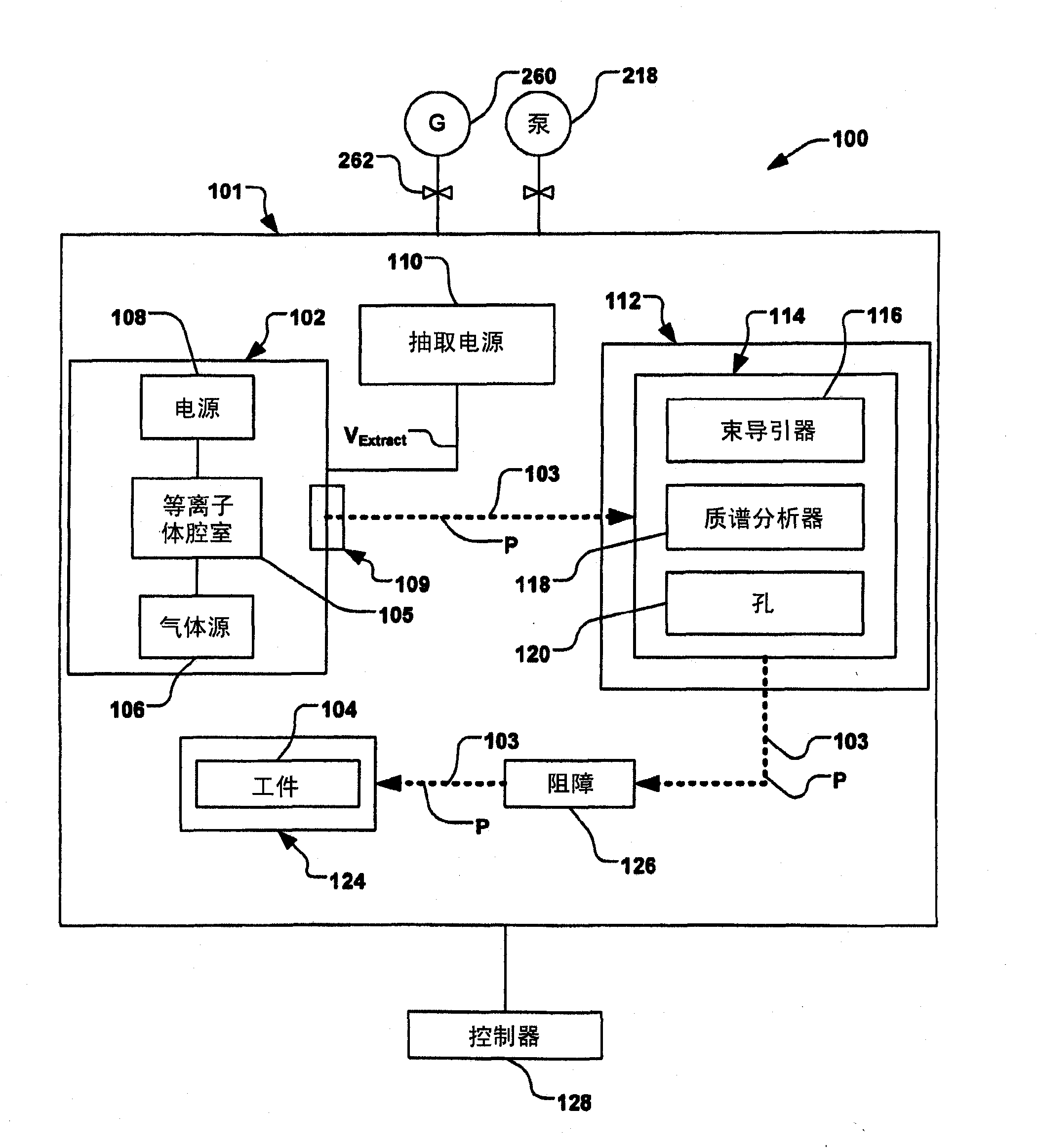
Hydrogen generation systems and methods
InactiveUS20080286195A1Reduce and eliminate foamingSimulator controlHydrogen productionPhosphoric acidBorohydride
Systems and methods for hydrogen generation that convert a boron hydride fuel to hydrogen by contacting the fuel with an acidic reagent, i.e., a reagent having a pH less than about 7, in the presence of water, are provided. The fuel may comprise a boron hydride in solid or slurry form, either utilized individually or as a mixture of two of more boron hydrides. The acidic reagent may comprise inorganic acids such as hydrochloric acid, sulfuric acid, and phosphoric acid, and organic acids such as acetic acid, formic acid, maleic acid, malic acid, citric acid, and tartaric acid, or mixtures thereof, and at least one additive.
Owner:MILLENNIUM CELL
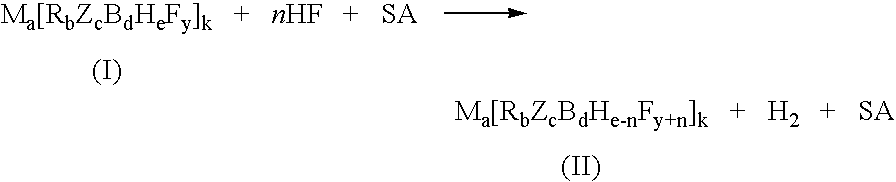
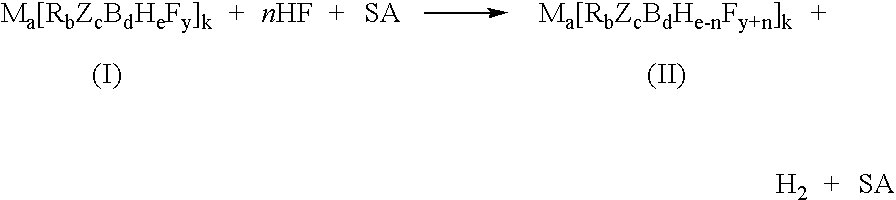
Process for the fluorination of boron hydrides
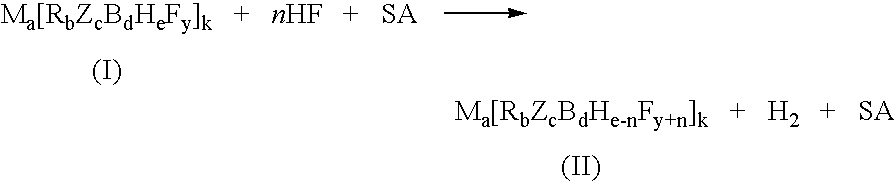
InactiveUS7429671B2Selective and controlled fluorinationImprove the level ofGroup 3/13 element organic compoundsPhosphorus organic compoundsSuperacidBorohydride
A process for fluorination of borohydride salts including providing a reaction medium comprising HF and a superacid. A borohydride salt compound is added to the reaction medium. The borohydride salt is reacted with the reaction medium under conditions to form a fluorinated borohydride salt. In addition, reactor vessels may be provided for reacting the HF, superacid additive and borohydride that are fabricated from materials resistant to superacid compositions.
Owner:AIR PROD & CHEM INC
Preparation methods of rare earth and alkaline earth hexaboride nanowires, nanorods and nanotubes
InactiveCN105271281BLow equipment requirementsReduced stabilityMaterial nanotechnologyMetal boridesMetal chlorideAlkaline earth metal
The invention relates to a preparation method of rare earth and alkaline earth hexaboride nanowires, nanorods and nanotubes. Traditional methods for preparing hexaboride nanowires have disadvantages such as high cost, high toxicity, and strong corrosion. The invention discloses a new reaction process on a substrate, using one of rare earth metal chlorides or alkaline earth metal chlorides or a combination thereof as a metal source, and borohydride as a boron source, at 900-1050°C Synthesis of hexaboride nanowires, nanorods and nanotubes under favorable conditions. The method does not need reducing gas and catalyst, and has the advantages of simple process conditions, good crystallinity, high yield and the like.
Owner:GUIZHOU INST OF TECH
Preparation process of duloxetine
Owner:武汉励合生物医药科技有限公司
A kind of preparation method of boronizing silicon nitride fiber
InactiveCN104831420BPerfect synthesis technologyHigh activityInorganic material artificial filamentsDehydrogenationOptoelectronics
The invention relates to a preparation method of a boronized silicon nitride fiber. The preparation method comprises the steps of firstly preparing a high-activity Si-N fiber by transforming an infusible polycarbosilane fiber, carrying out dehydrogenation coupling reaction on excessive N-H in the high-activity Si-N fiber and B-H compounds generated by virtue of pyrolysis of boron hydrides so as to chemically introduce an element B, and carrying out boron hydrides in ammonia gas, so as to prepare the boronized silicon nitride fiber. Compared with the prior art, the boronized silicon nitride fiber prepared by virtue of the preparation method is relatively high in boron content, nitrogen content and fiber strength and relatively uniform in boron distribution. The preparation method has the advantages that the process is simple and convenient, and the cost is low. Compared with SiBN fiber prepared by virtue of a polyborosilazane precursor route, the preparation method has relatively great cost advantage and is applicable to large-scale production, and existing decarburization-nitridation systems do not need to be changed.
Owner:NAT UNIV OF DEFENSE TECH
Process for the fluorination of boron hydrides
InactiveUS20080138269A1High level of fluorinationHigh yieldGroup 3/13 element organic compoundsPhosphorus organic compoundsSuperacidBorohydride
A process for fluorination of borohydride salts including providing a reaction medium comprising HF and a superacid. A borohydride salt compound is added to the reaction medium. The borohydride salt is reacted with the with the reaction medium under conditions to form a fluorinated borohydride salt. In addition, reactor vessels may be provided for reacting the HF, superacid additive and borohydride that are fabricated from materials resistant to superacid compositions.
Owner:AIR PROD & CHEM INC
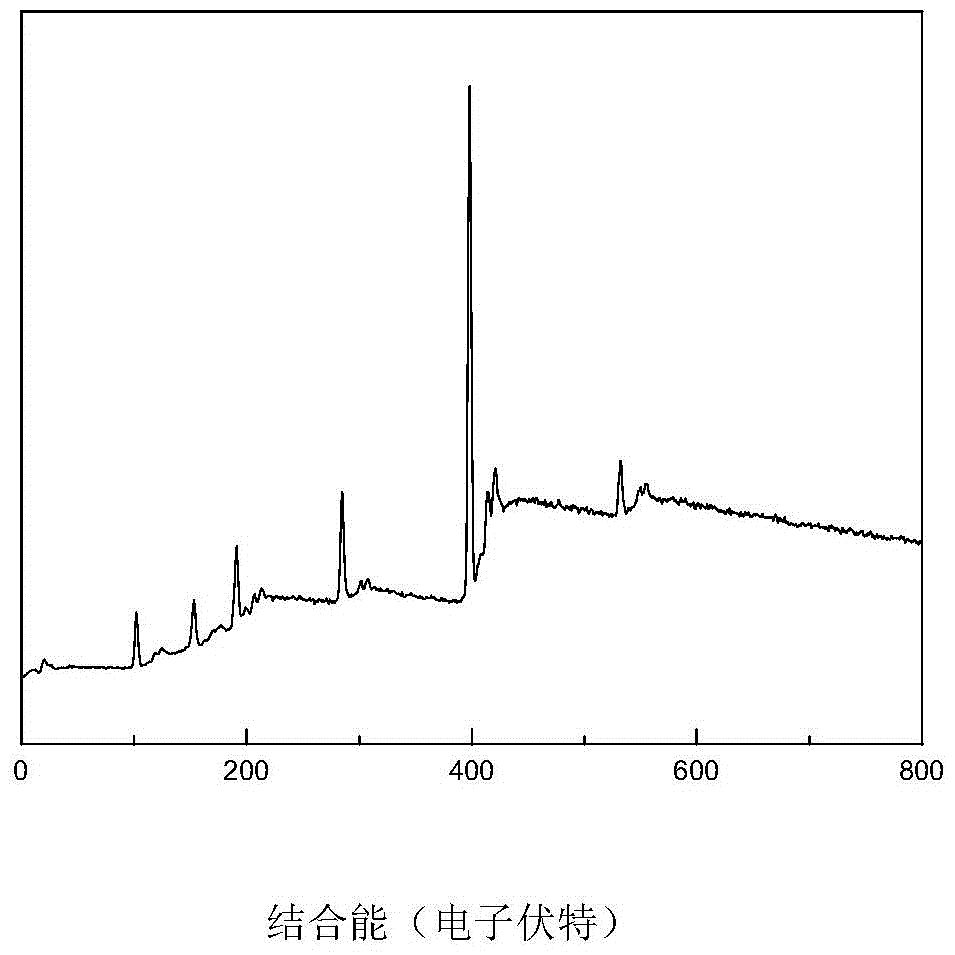
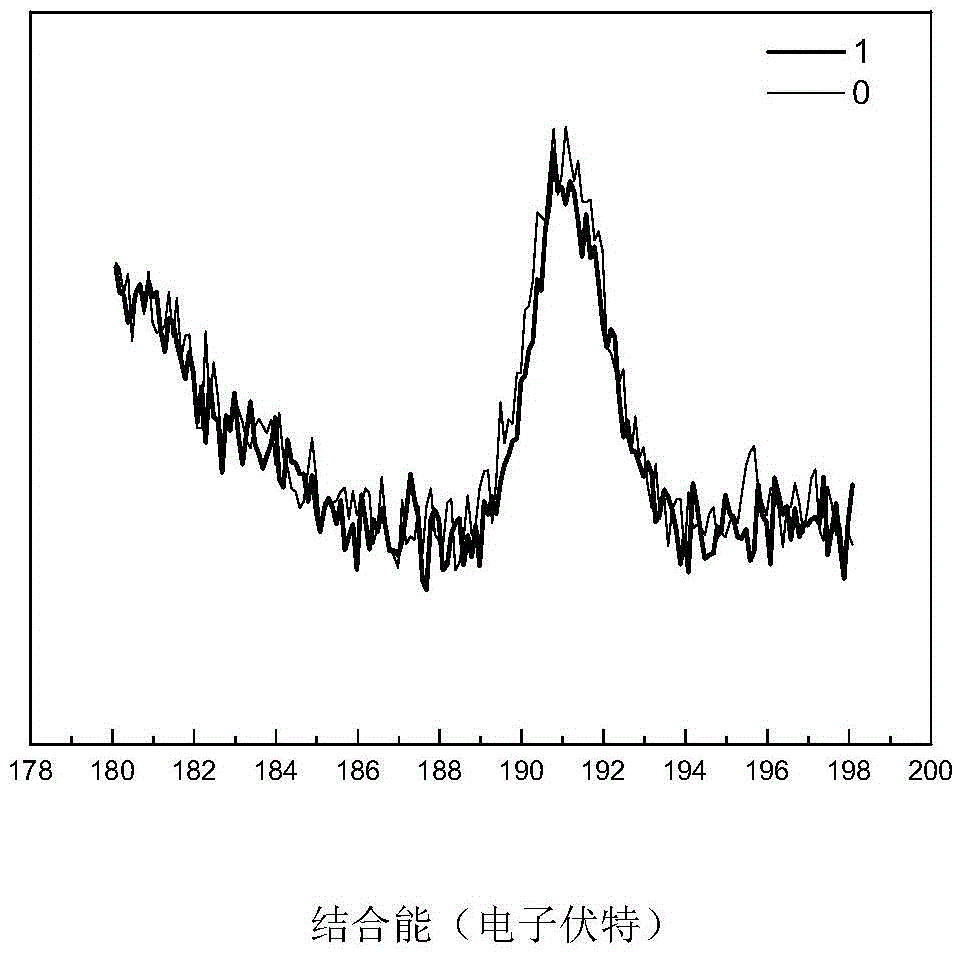
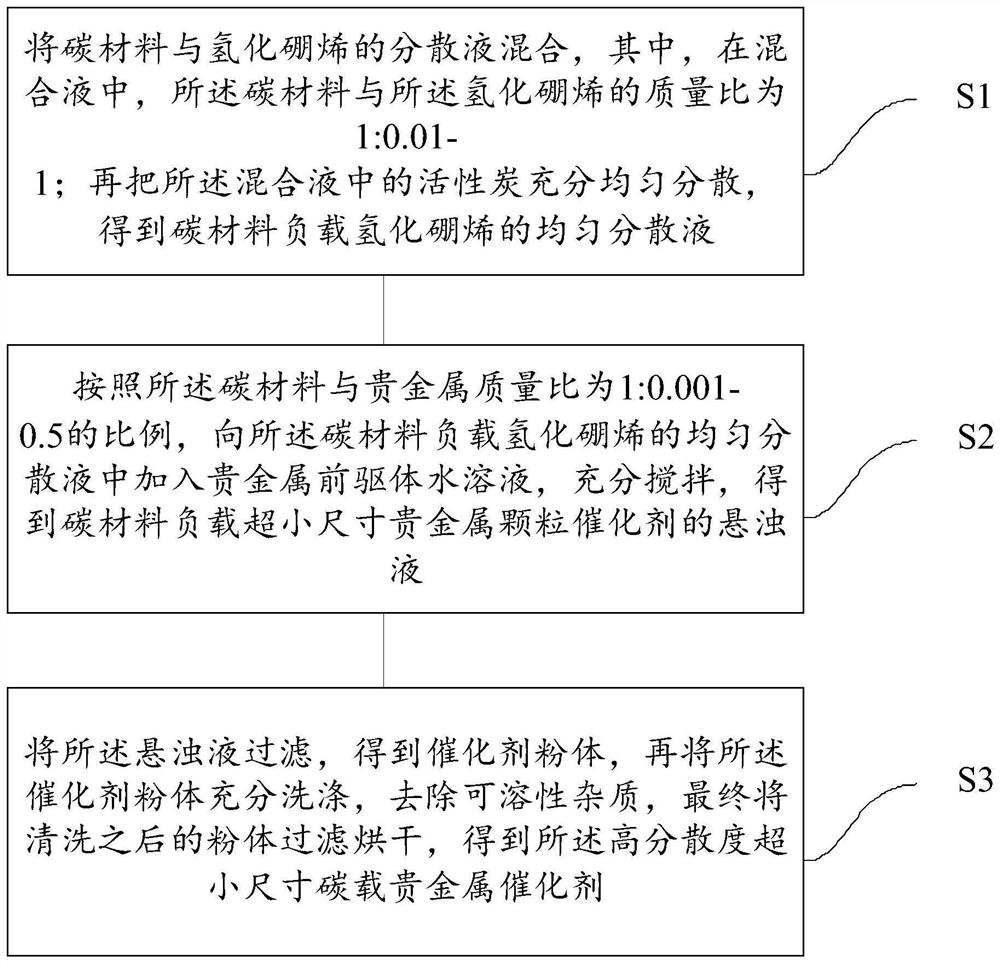
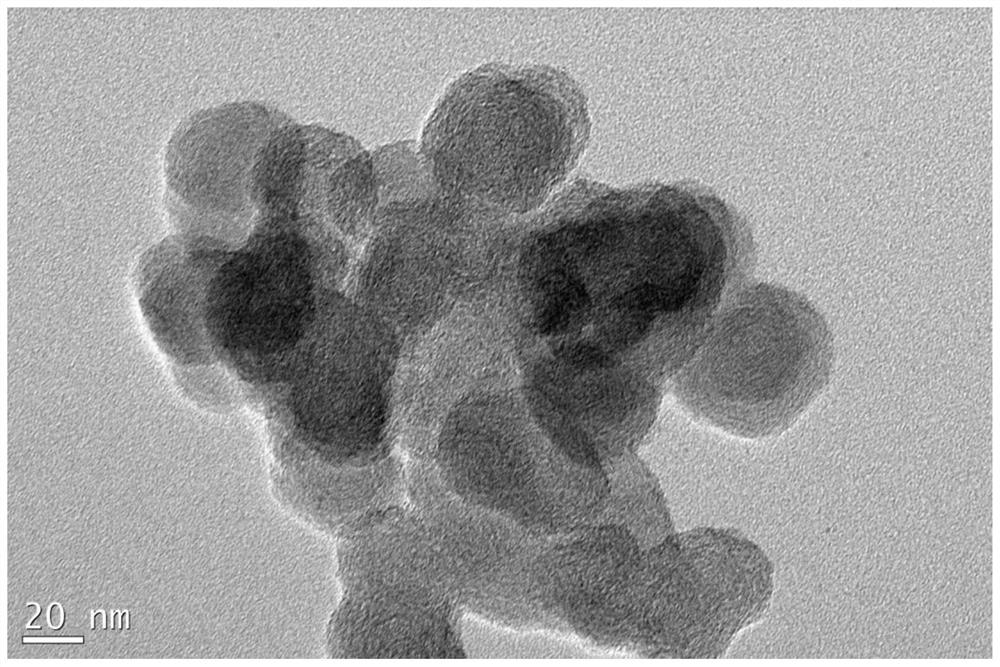
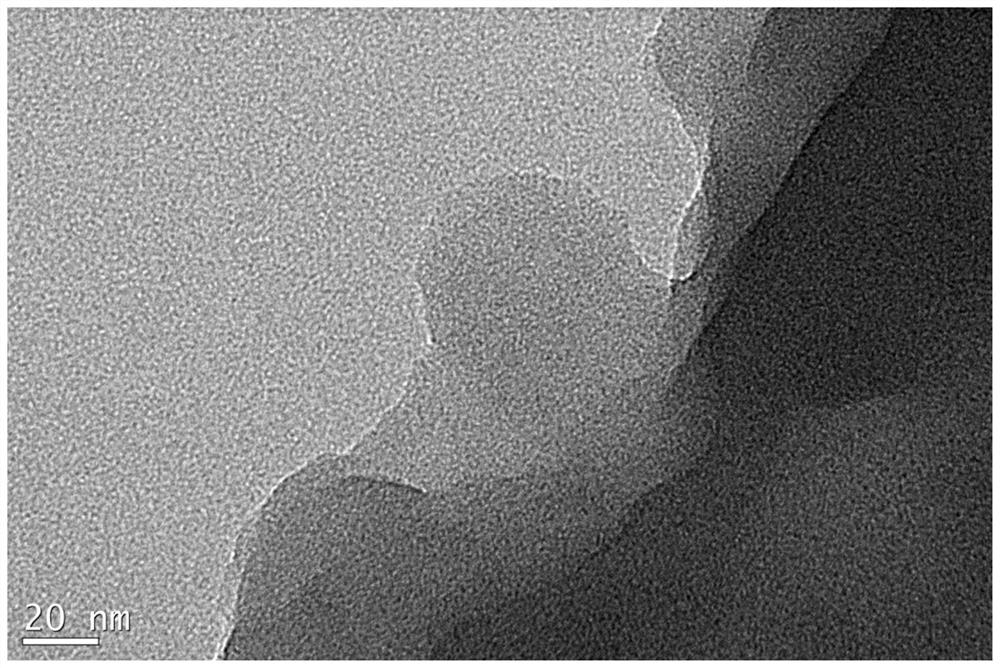
A kind of high-dispersion ultra-small size carbon-supported noble metal catalyst and preparation method thereof
InactiveCN110385126BHigh catalytic activityLow costCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsActivated carbonPtru catalyst
Owner:XI AN JIAOTONG UNIV +1
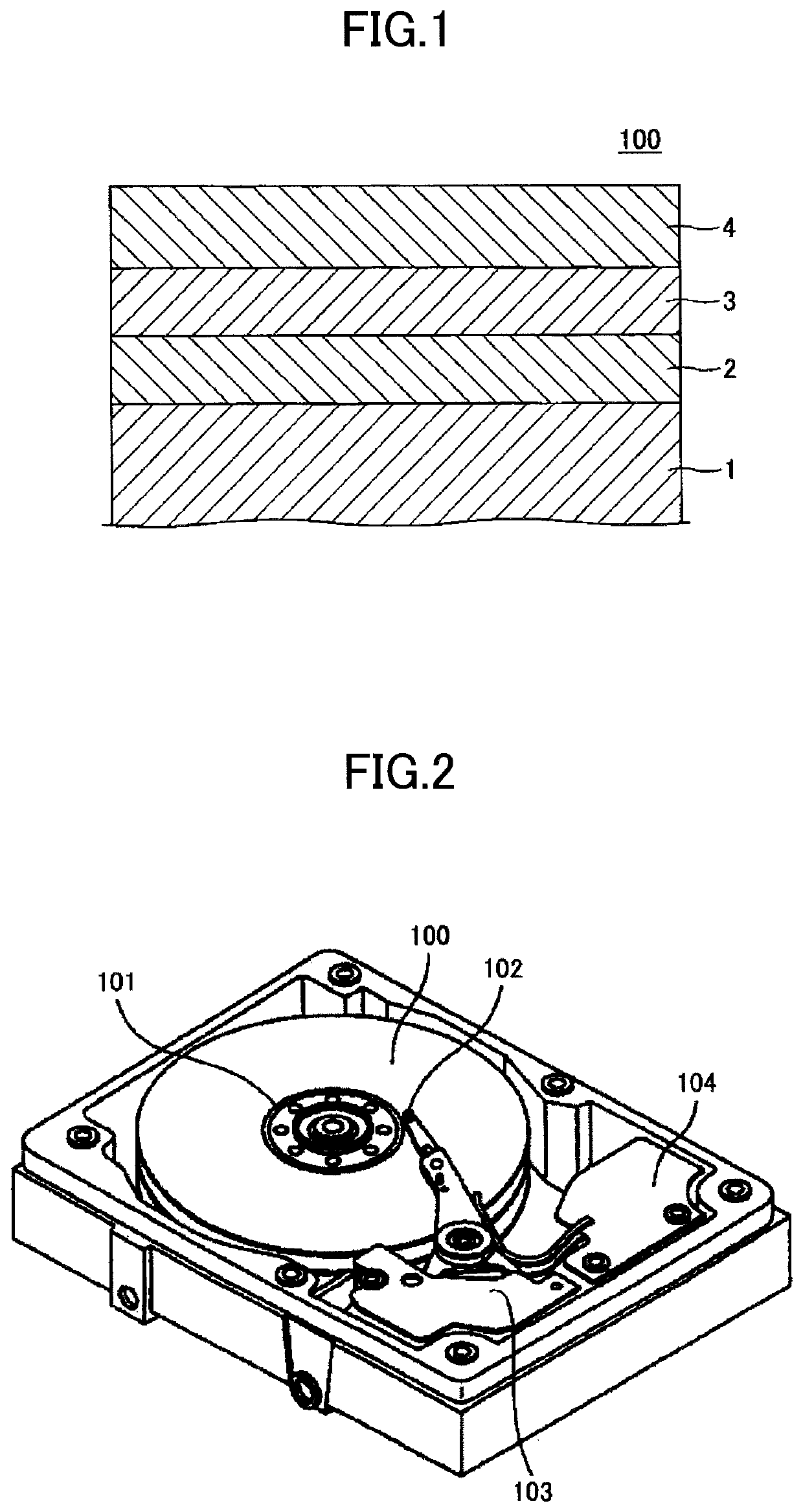
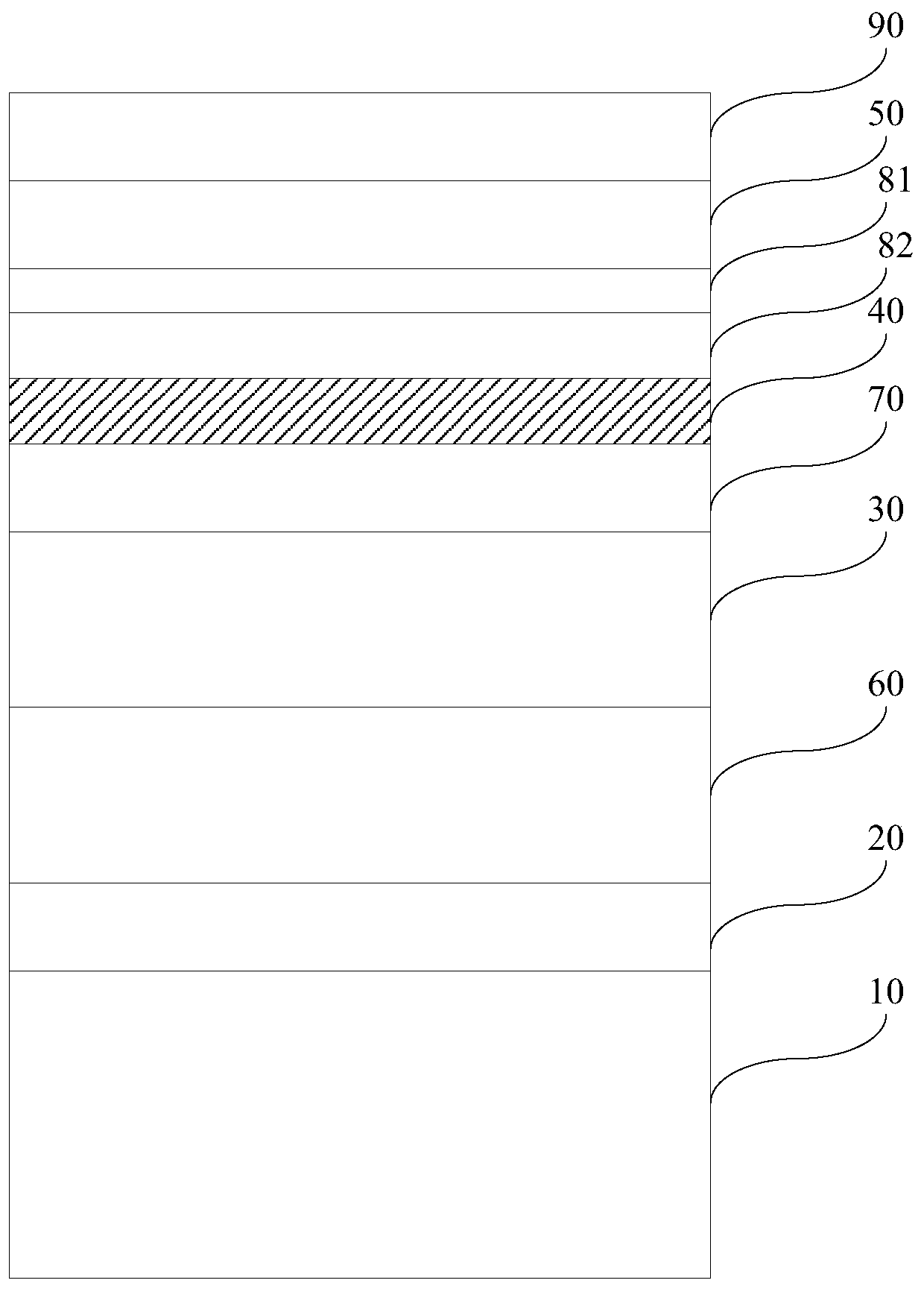
Magnetic recording medium and magnetic storage apparatus
ActiveUS10978103B2Improve signal-to-noise ratioRecord information storageMaterials with non-metallic substancesMagnetic storageBoron nitride
A magnetic recording medium includes: a substrate; an underlayer; and a magnetic layer including an alloy having a L10 structure and a (001) orientation, wherein the substrate, the underlayer, and the magnetic layer are stacked in the recited order, and wherein the magnetic layer has a granular structure and includes a carbon hydride, a boron hydride, or a boron nitride hydride.
Owner:RESONAC CORPORATION
Catalyst for preparing boron hydride by electrolysis and preparing method for catalytic electrode
The invention discloses a catalyst for preparing borohydride by electrolysis and a method for preparing a catalytic electrode containing the catalyst. The catalyst is one of hydrogen storage alloys, carbon materials, and metal borides, or two or more of them mixture. Mix catalyst powder (60-100 parts), additives (0-20 parts), and binder (1-10 parts), add solvent to form a film first, and then press the film on the current-collecting nickel mesh or steel mesh; or Make slurry with a solvent, then coat or fill the surface or inside of porous nickel mesh or steel mesh, press to a certain thickness, and dry to obtain a catalytic electrode. The catalyst and the catalytic electrode of the invention are used for electrolytic preparation of borohydride, and have low price and high-efficiency performance, and the current efficiency can reach more than 50% in alkaline solution.
Owner:WUHAN UNIV
Synthesis of Spiro Bisboron Catalyst and Its Application in Hydrogenation
ActiveCN109575060BSolve residual problemsLow costOrganic-compounds/hydrides/coordination-complexes catalystsOrganic chemistry methodsPtru catalystHydrogenation reaction
The invention relates to a synthesis of spiro-ring diene compound with C2 symmetry, and a a series of chirality spiro-ring bis-boron catalysts prepared by virtue of the reaction of the spiro-ring diene compound and boron hydrides. The spiro-ring bis-boron catalysts have high activity and enantioselectivity in the asymmetric hydrogenation reaction of quinoline compounds, and belong to the technicalfield of application. By adopting the synthesis of spiro-ring bis-boron catalyst and the application thereof in hydrogenation reaction, the problems of the traditional quinoline asymmetric hydrogenation reaction method that precious metal catalysts are used and the functional groups are poor in tolerance can be mainly solved, the nonmetal catalytic quinoline asymmetric hydrogenation reaction canbe realized, the reaction substrate range is wide, and the functional group tolerance is high. The synthesis of spiro-ring bis-boron catalyst and the application thereof in hydrogenation reaction areused in the medicine research and chemical production.
Owner:NANKAI UNIV
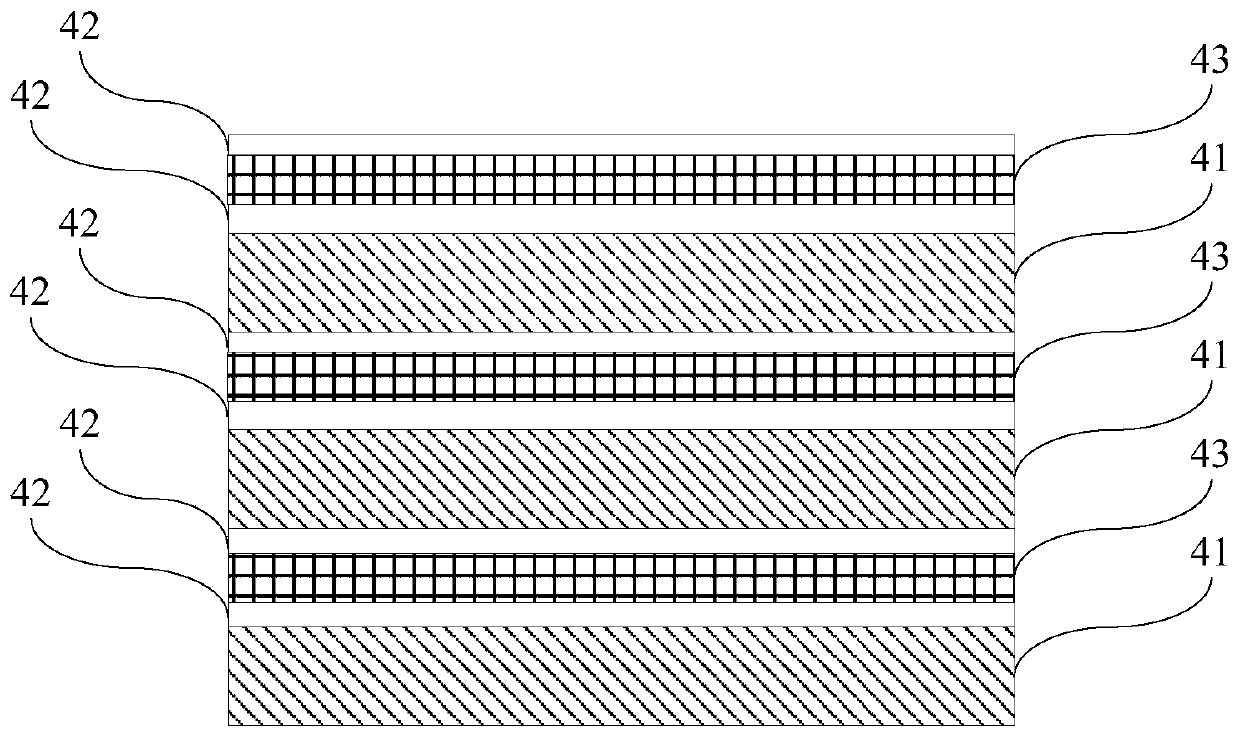
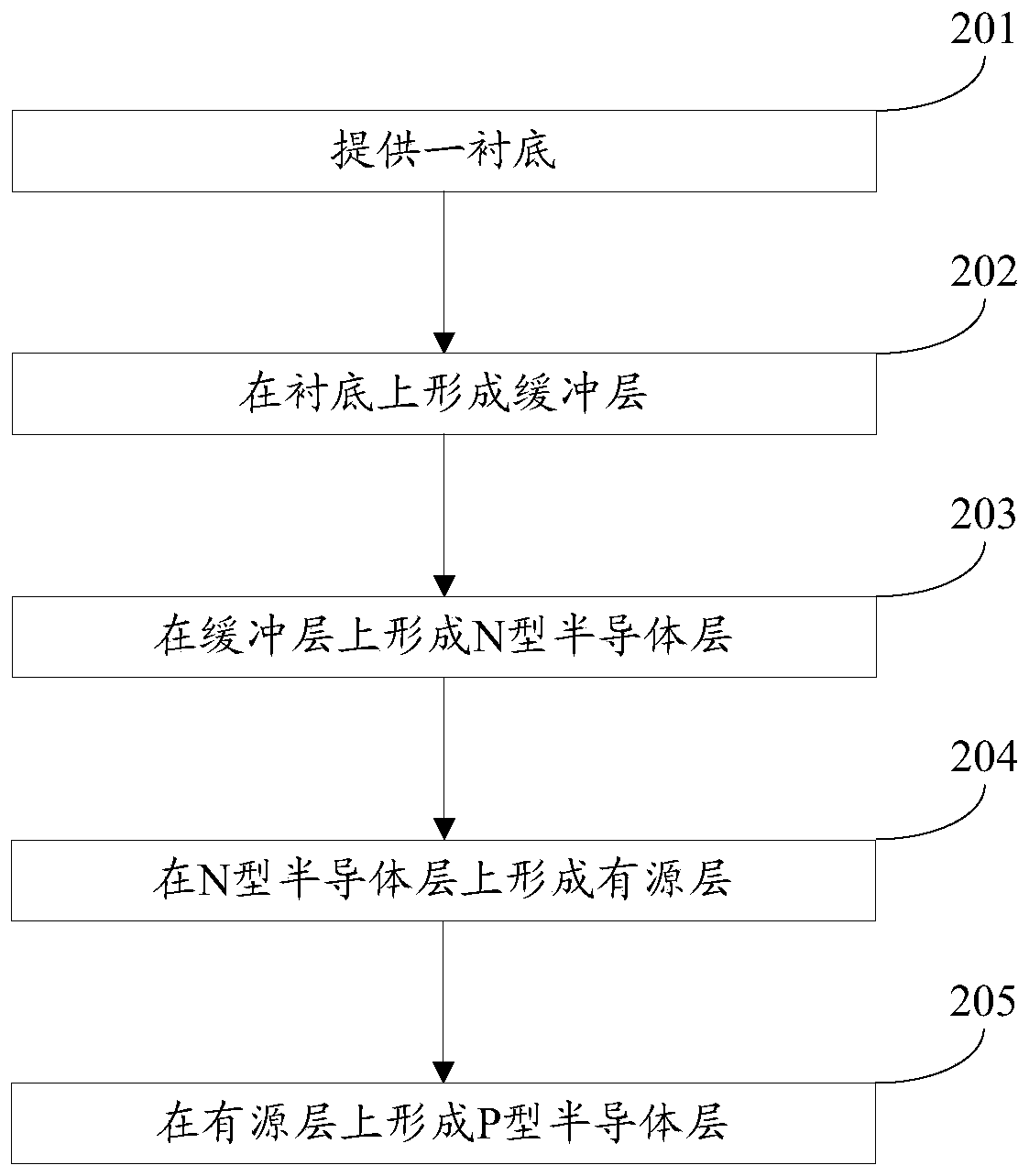
A gallium nitride-based light-emitting diode epitaxial wafer and its preparation method
ActiveCN109473522BPrevent junction temperature riseImprove composite luminous efficiencySemiconductor devicesJunction temperatureGallium nitride
The invention discloses a gallium nitride-based light emitting diode epitaxial wafer and a preparation method thereof, and belongs to the technical field of semiconductors. The gallium nitride-based light emitting diode epitaxial wafer comprises a substrate, a buffer layer, an N-type semiconductor layer, an active layer, and a P-type semiconductor layer, and the buffer layer, the N-type semiconductor layer, the active layer, and the P-type semiconductor layer are sequentially stacked on the substrate; the active layer comprises a plurality of quantum wells and a plurality of quantum barriers,and the plurality of quantum wells and the plurality of quantum barriers are alternately stacked; and at least one boron hydride layer is inserted into the quantum barrier. According to the epitaxialwafer and the preparation method in the invention, at least one boron hydride layer is inserted into the quantum barrier, and the thermal conductivity of the boron hydride layer is good, so the heat generated by combined luminescence of electrons and holes in the active layer can be conducted out in time to prevent the junction temperature of the active layer from rising, thereby facilitating theimprovement of combined luminous efficiency of the electrons and holes, and further improving the luminous efficiency of the LED, and the gallium nitride-based light emitting diode epitaxial wafer isparticularly suitable for LEDs at a high current density.
Owner:HC SEMITEK ZHEJIANG CO LTD
Process for the Fluorination of Boron Hydrides
InactiveUS20080279746A1Selective and controlled fluorinationImprove the level ofGroup 3/13 element organic compoundsPhosphorus organic compoundsSuperacidBorohydride
A process for fluorination of borohydride salts including providing a reaction medium comprising HF and a superacid. A borohydride salt compound is added to the reaction medium. The borohydride salt is reacted with the with the reaction medium under conditions to form a fluorinated borohydride salt. In addition, reactor vessels may be provided for reacting the HF, superacid additive and borohydride that are fabricated from materials resistant to superacid compositions.
Owner:AIR PROD & CHEM INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com