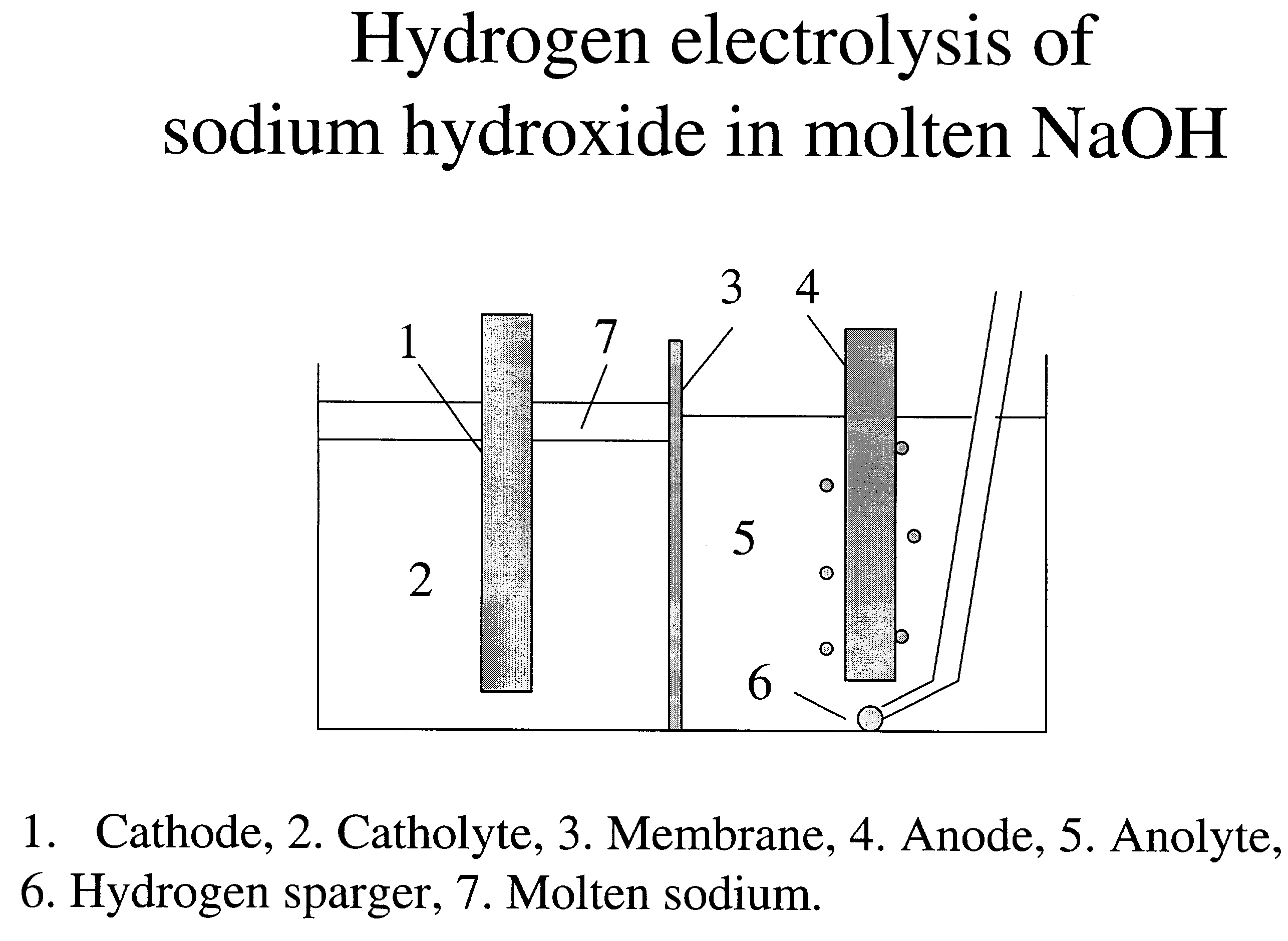
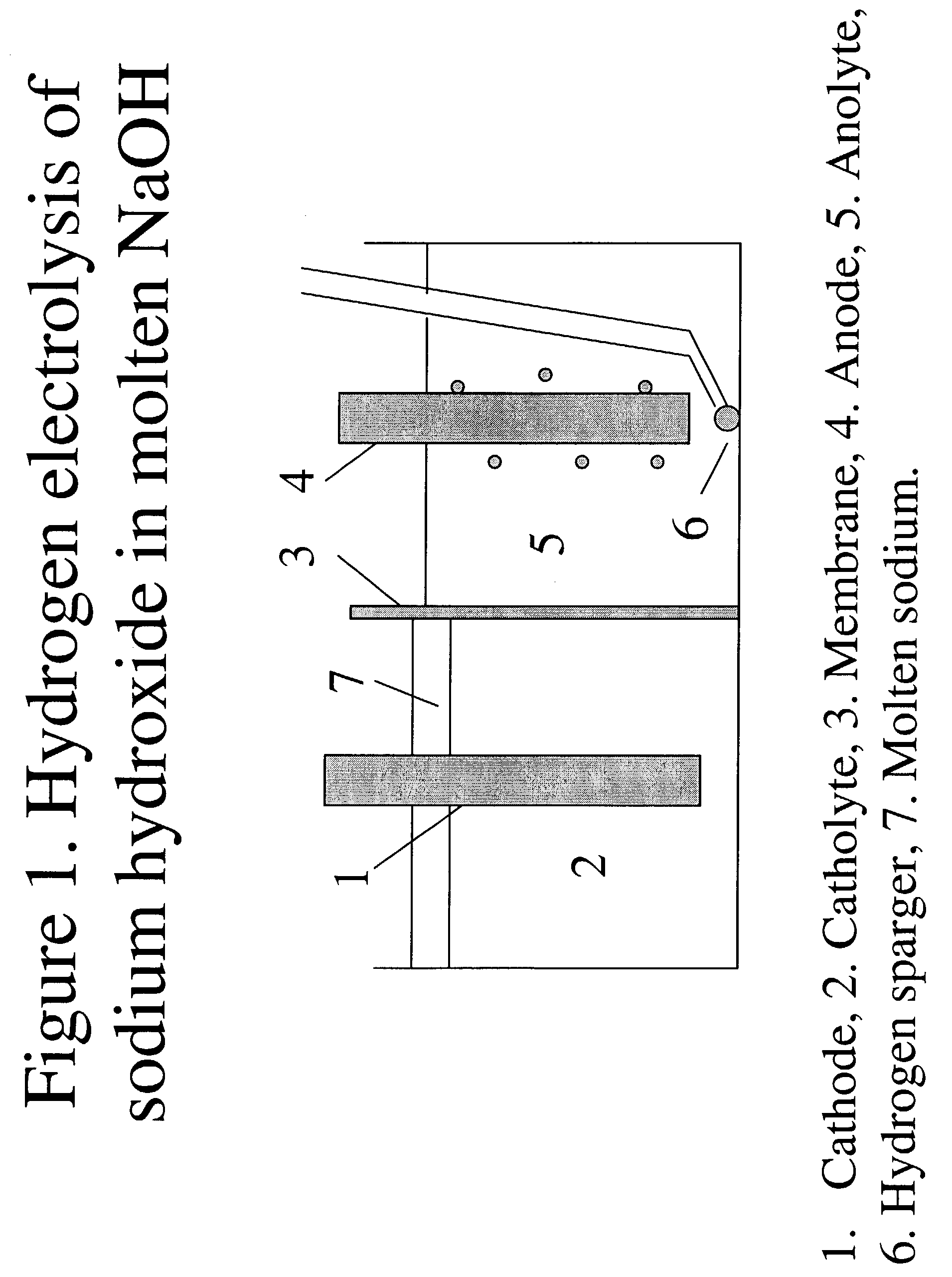
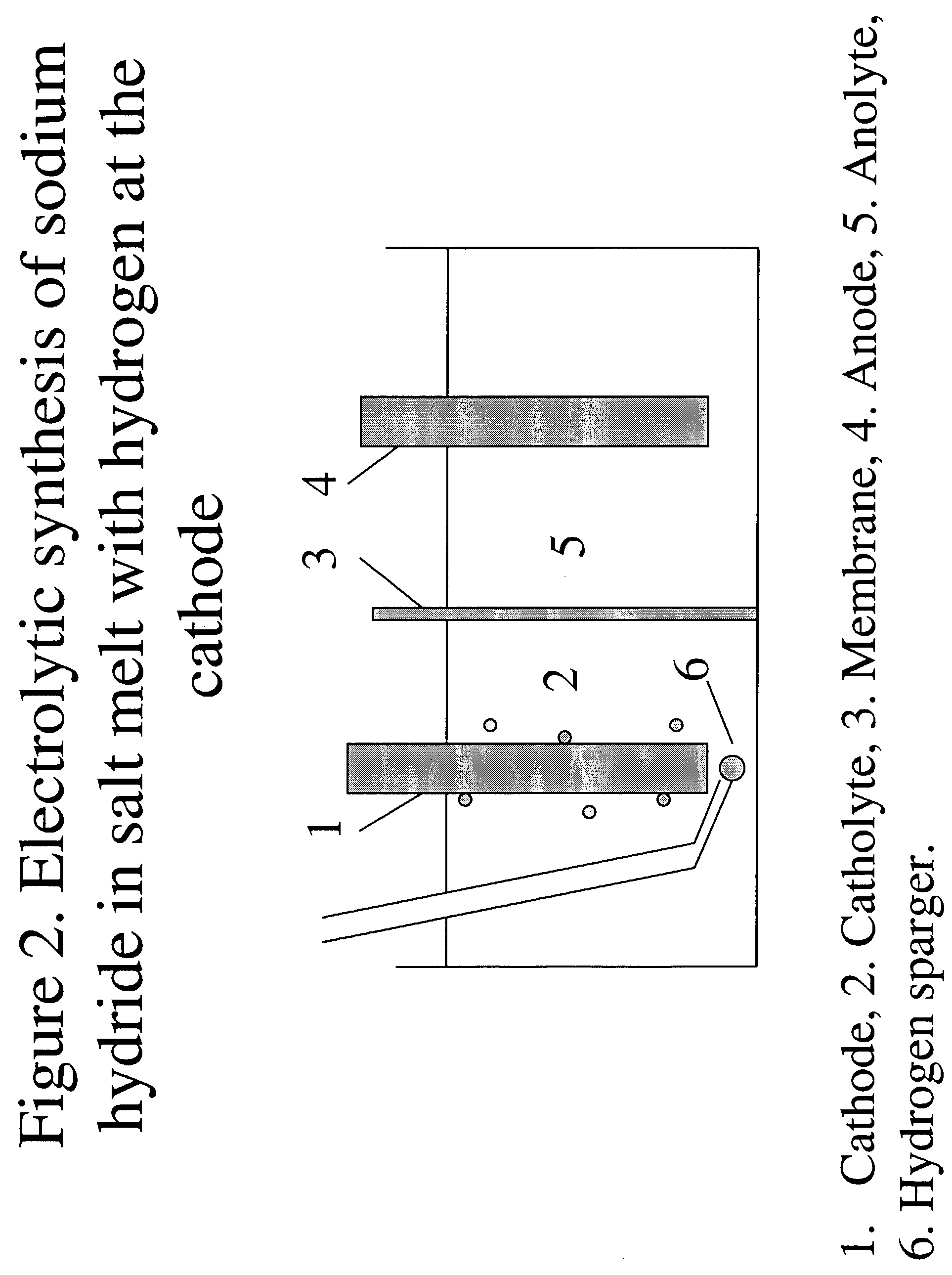
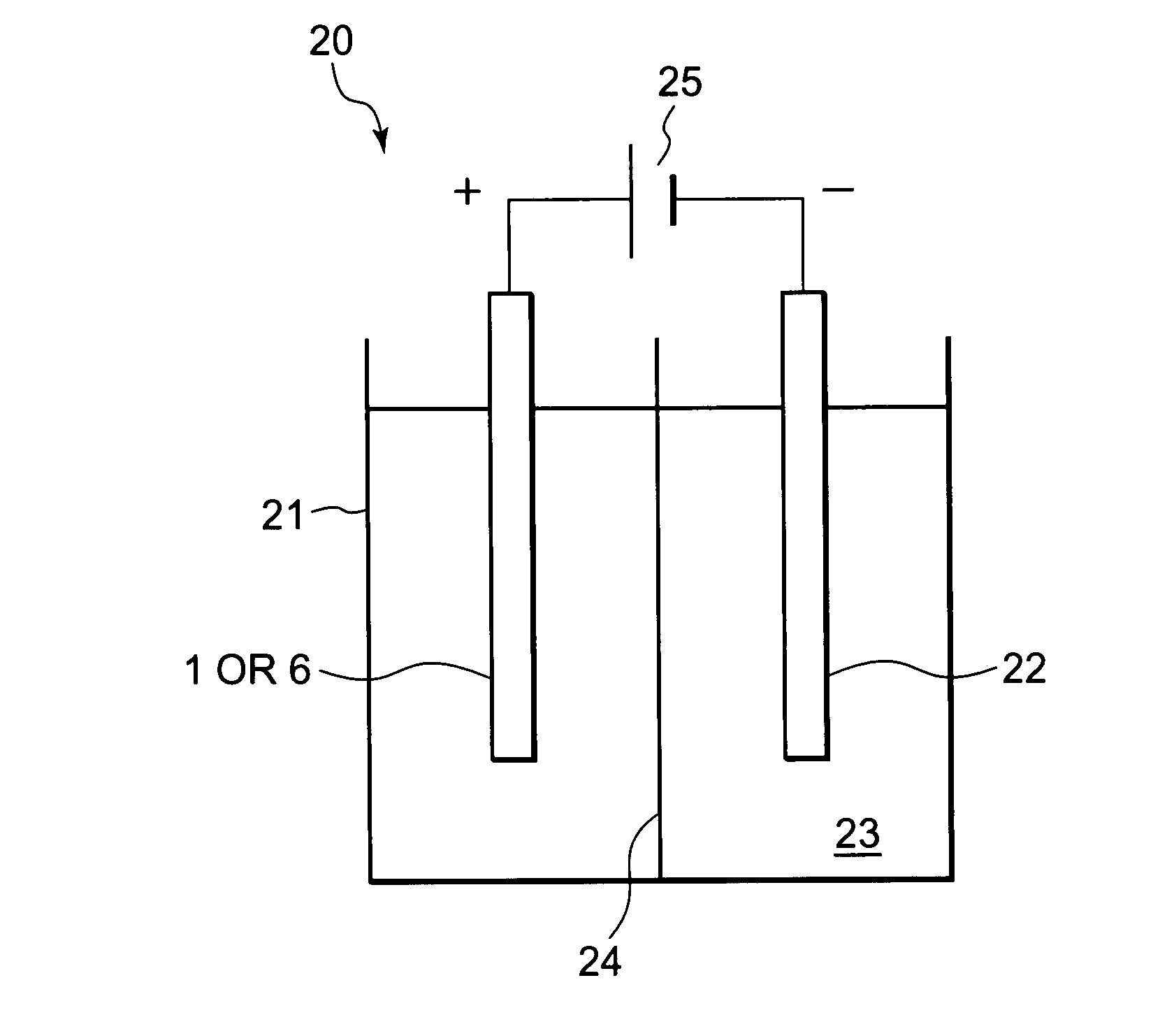
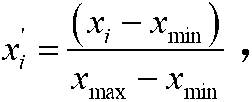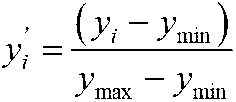Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1347 results about "Electrolytic process" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An electrolytic process is the use of electrolysis industrially to refine metals or compounds at a high purity and low cost. Some examples are the Hall-Héroult process used for aluminium, or the production of hydrogen from water. Electrolysis is usually done in bulk using hundreds of sheets of metal connected to an electric power source. In the production of copper, these pure sheets of copper are used as starter material for the cathodes, and are then lowered into a solution such as copper sulphate with the large anodes that are cast from impure (97% pure) copper. The copper from the anodes are electroplated on to the cathodes, while any impurities settle to the bottom of the tank. This forms cathodes of 99.999% pure copper.
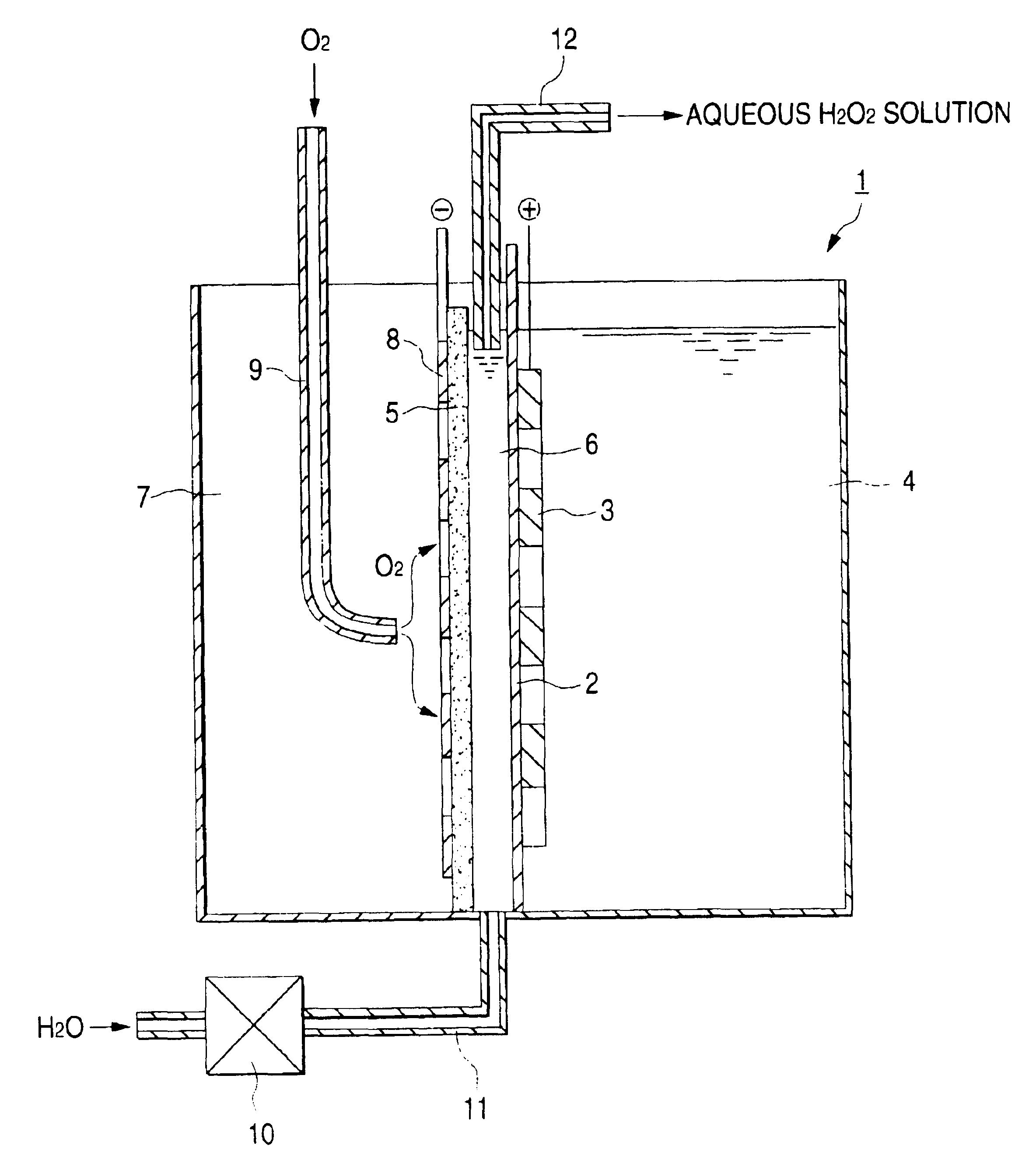
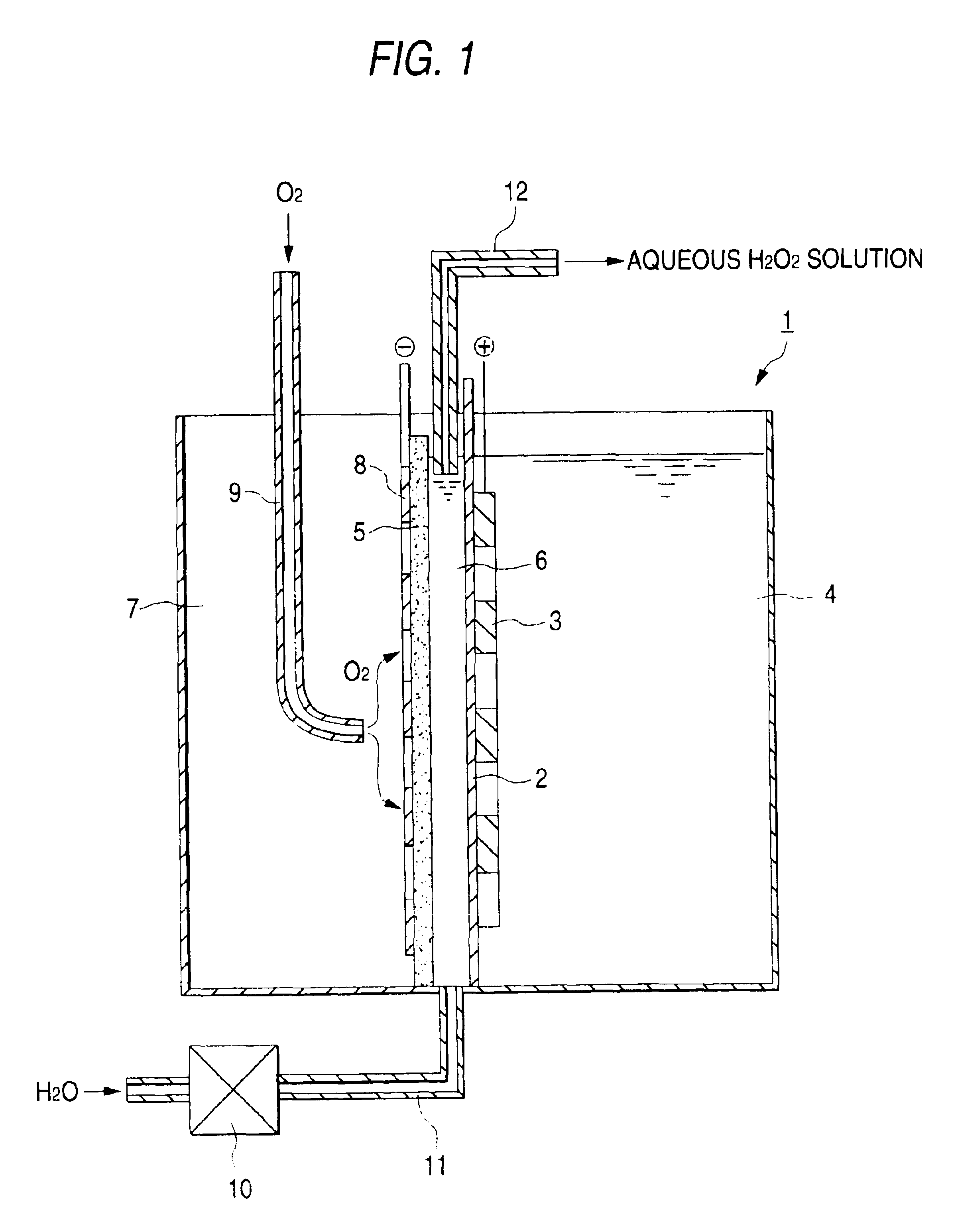
Electrolytic cell for hydrogen peroxide production and process for producing hydrogen peroxide
InactiveUS6767447B2CellsPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesSodium sulfateNuclear chemistry
An electrolytic cell and method of electrolysis for producing hydrogen peroxide at a moderate current density while preventing metal deposition on the cathode surface. A feed water from which multivalent metal ions have been removed and in which a salt of a univalent metal, e.g., sodium sulfate, has been dissolved in a given concentration is prepared with an apparatus for removing multivalent metal ions and dissolving a salt in low concentration. The feed water is supplied to an electrolytic cell. Even when electrolysis is continued, almost no deposition of a hydroxide or carbonate occurs on the cathode because multivalent metal ions are not present in the electrolytic solution. Due to the dissolved salt, a sufficient current density is secured to prevent an excessive load from being imposed on the electrodes, etc. Thus, stable production of hydrogen peroxide is possible over a long period of time.
Owner:DE NORA PERMELEC LTD
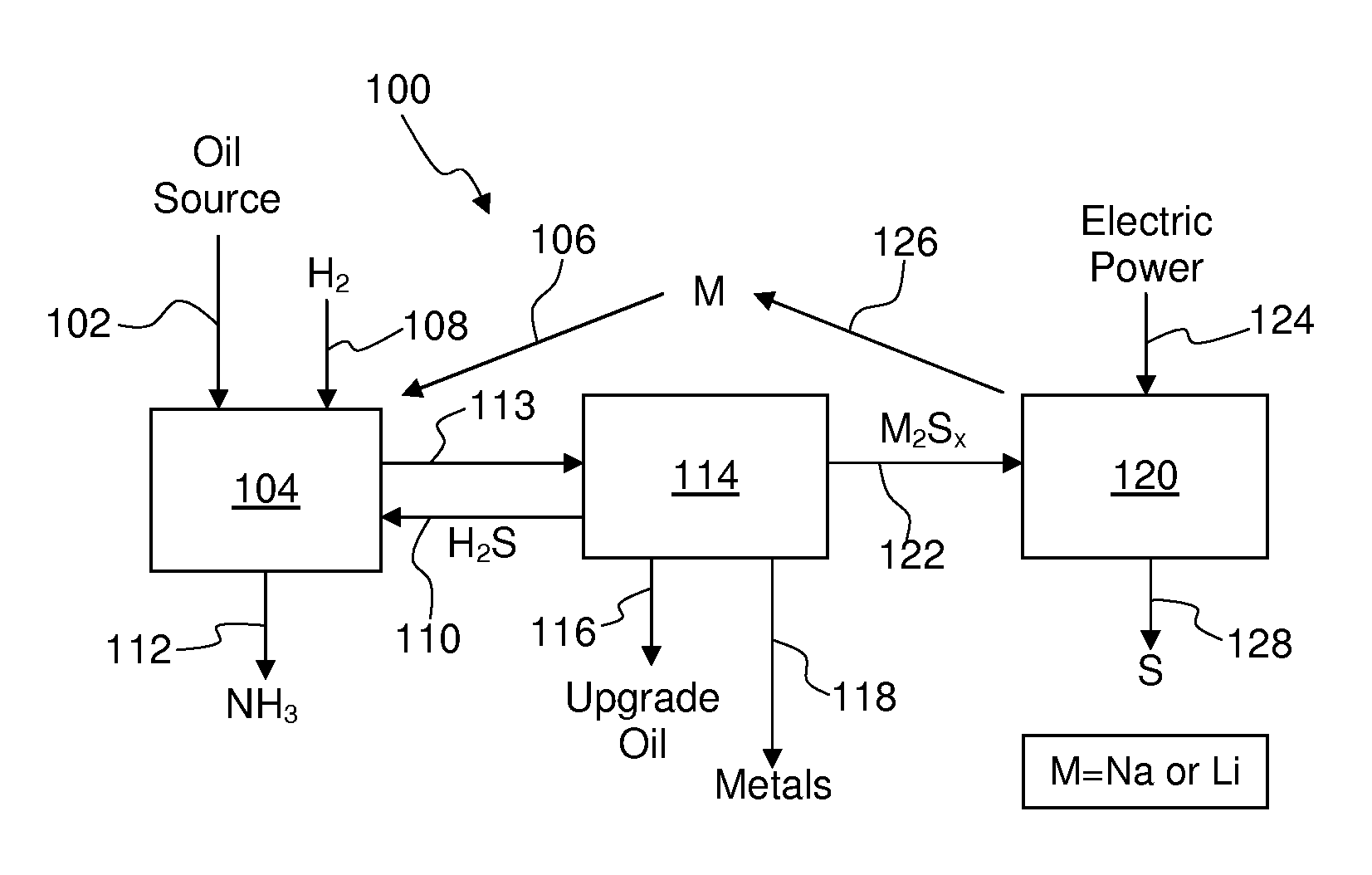
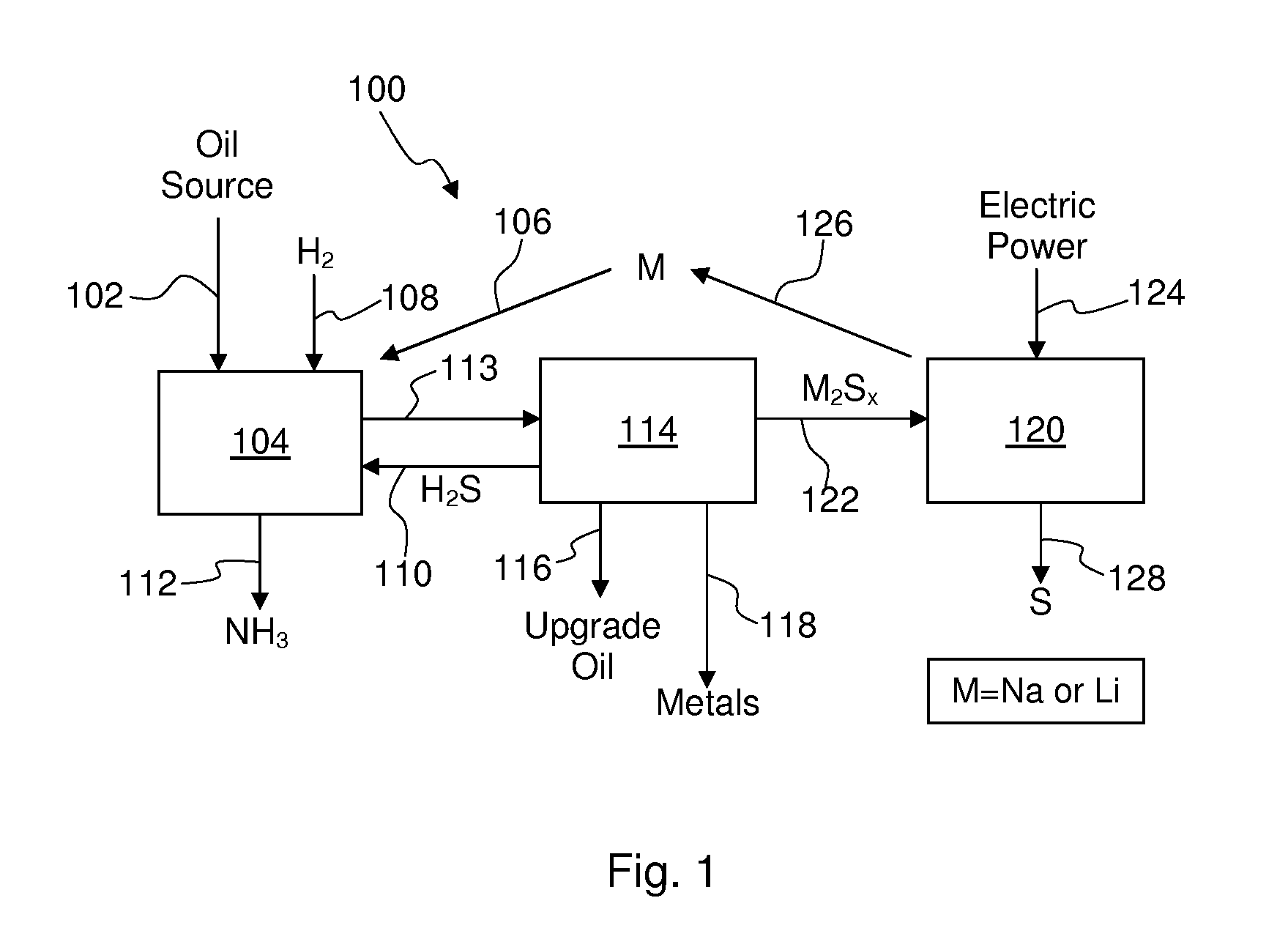
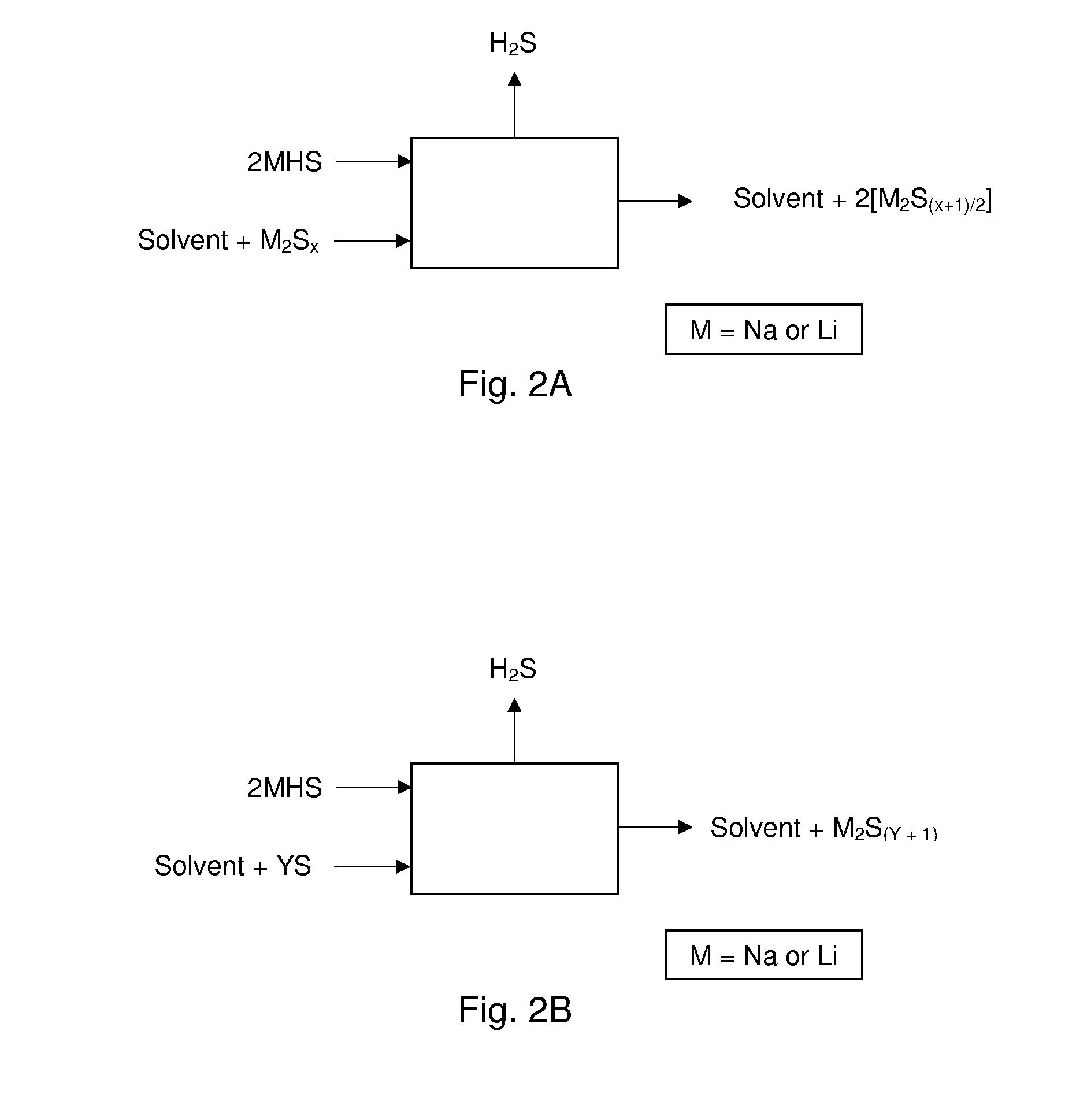
Process For Recovering Alkali Metals and Sulfur From Alkali Metal Sulfides and Polysulfides
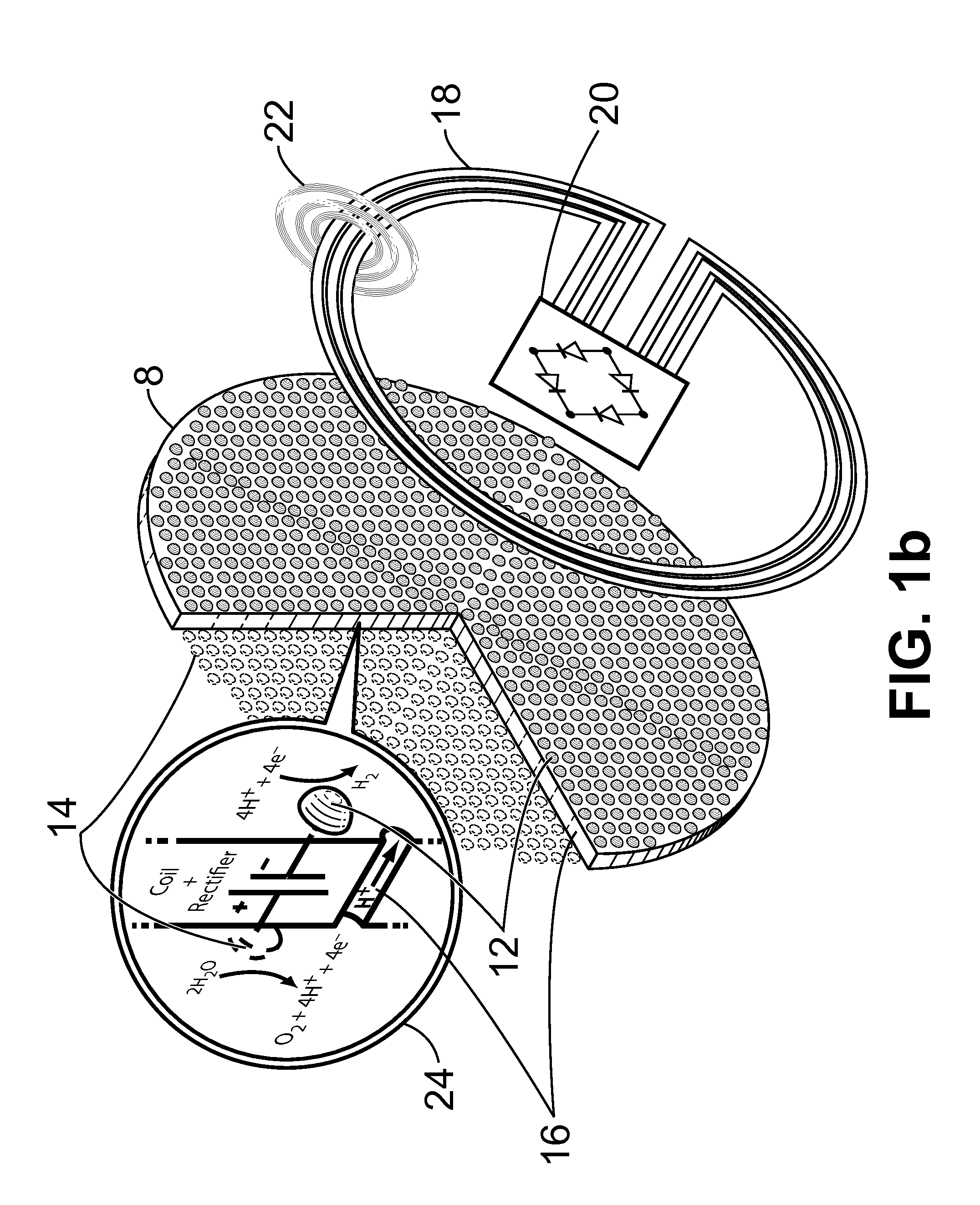
Alkali metals and sulfur may be recovered from alkali polysulfides in an electrolytic process that utilizes an electrolytic cell having an alkali ion conductive membrane. An anolyte solution includes an alkali polysulfide and a solvent that dissolves elemental sulfur. A catholyte solution includes alkali metal ions and a catholyte solvent. Applying an electric current oxidizes sulfur in the anolyte compartment, causes alkali metal ions to pass through the alkali ion conductive membrane to the catholyte compartment, and reduces the alkali metal ions in the catholyte compartment. Sulfur is recovered by removing and cooling a portion of the anolyte solution to precipitate solid phase sulfur. Operating the cell at low temperature causes elemental alkali metal to plate onto the cathode. The cathode may be removed to recover the alkali metal in batch mode or configured as a flexible band to continuously loop outside the catholyte compartment to remove the alkali metal.
Owner:ENLIGHTEN INNOVATIONS INC
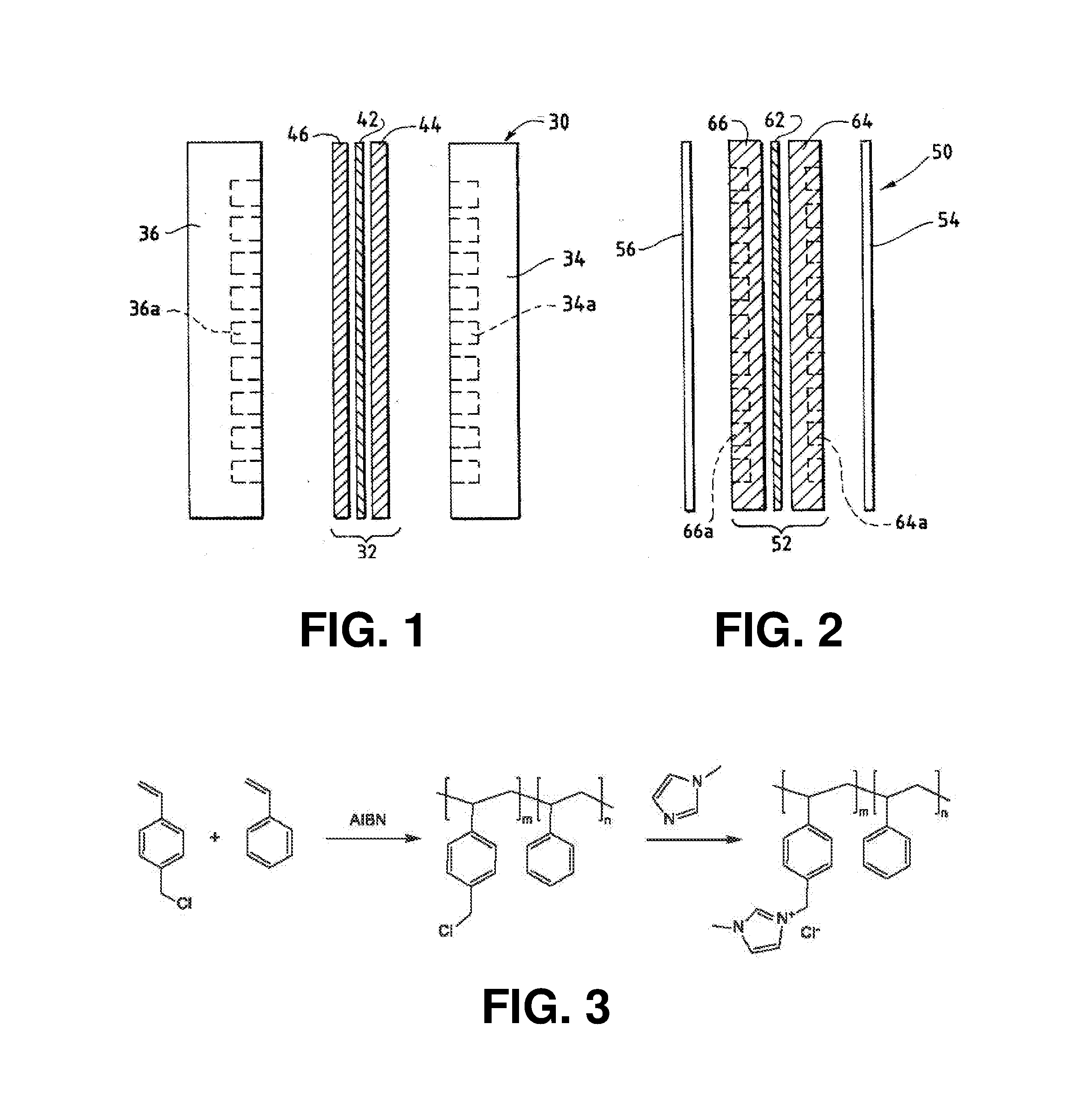
Method And System For Electrochemical Production Of Formic Acid From Carbon Dioxide
An electrochemical device converts carbon dioxide to a formic acid reaction product. The device includes an anode and a cathode, each comprising a quantity of catalyst. The anode and cathode each have reactant introduced thereto. Two membranes, a cation exchange polymer electrolyte membrane and an anion exchange polymer electrolyte membrane, are interposed between the anode and the cathode, forming a central flow compartment where a carbon dioxide reduction product, such as formic acid, can be recovered. At least a portion of the cathode catalyst is directly exposed to gaseous carbon dioxide during electrolysis. The average current density at the membrane is at least 20 mA / cm2, measured as the area of the cathode gas diffusion layer that is covered by catalyst, and formate ion selectivity is at least 50% at a cell potential difference of 3.0 V. In some embodiments, at least one polymer electrolyte membrane comprises a polymer in which a constituent monomer is (p-vinylbenzyl)-R, where R is selected from the group consisting of imidazoliums, pyridiniums and phosphoniums. In some embodiments, the polymer electrolyte membrane is a Helper Membrane comprising a polymer containing an imidazolium ligand, a pyridinium ligand, or a phosphonium ligand.
Owner:DIOXIDE MATERIALS
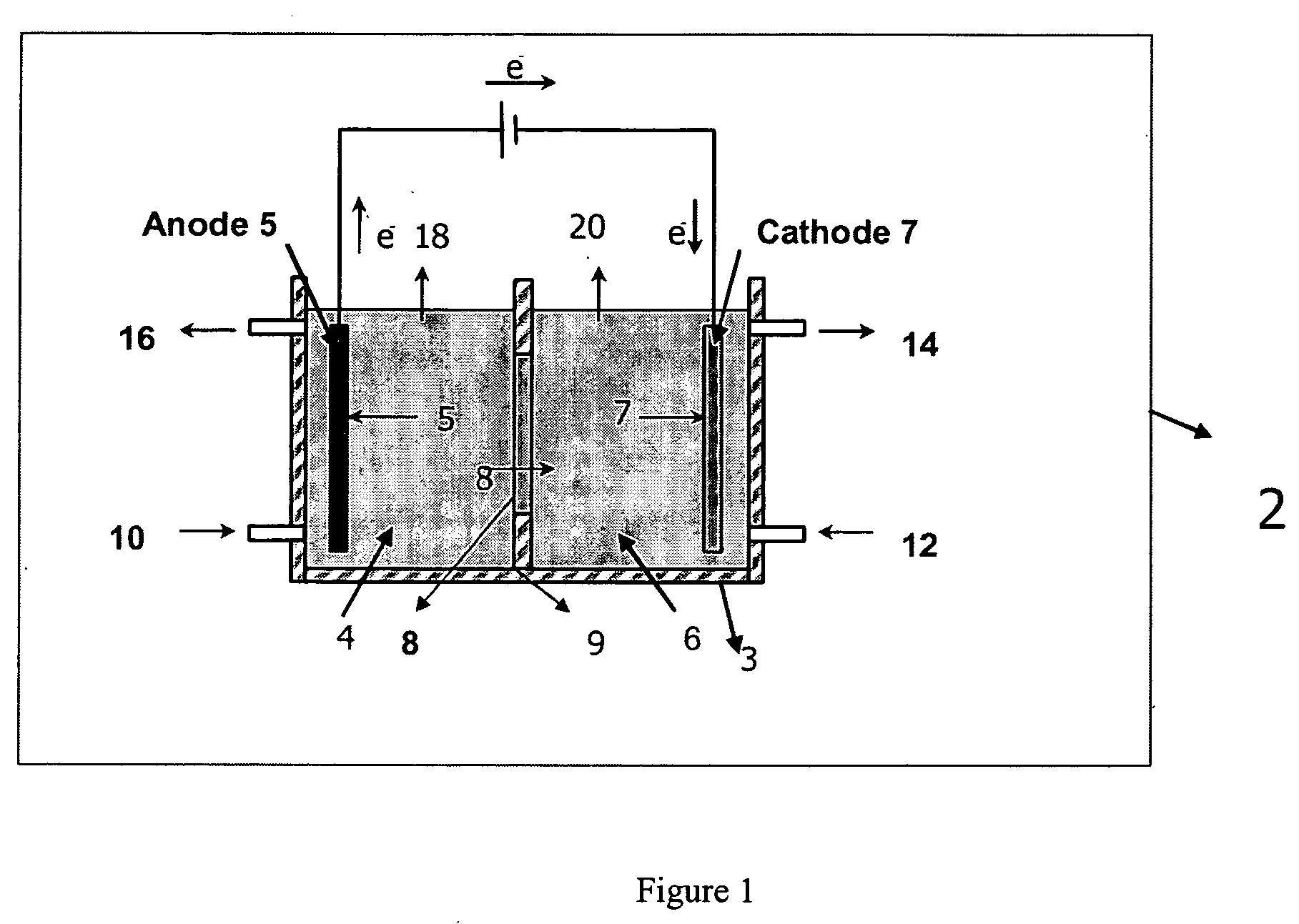
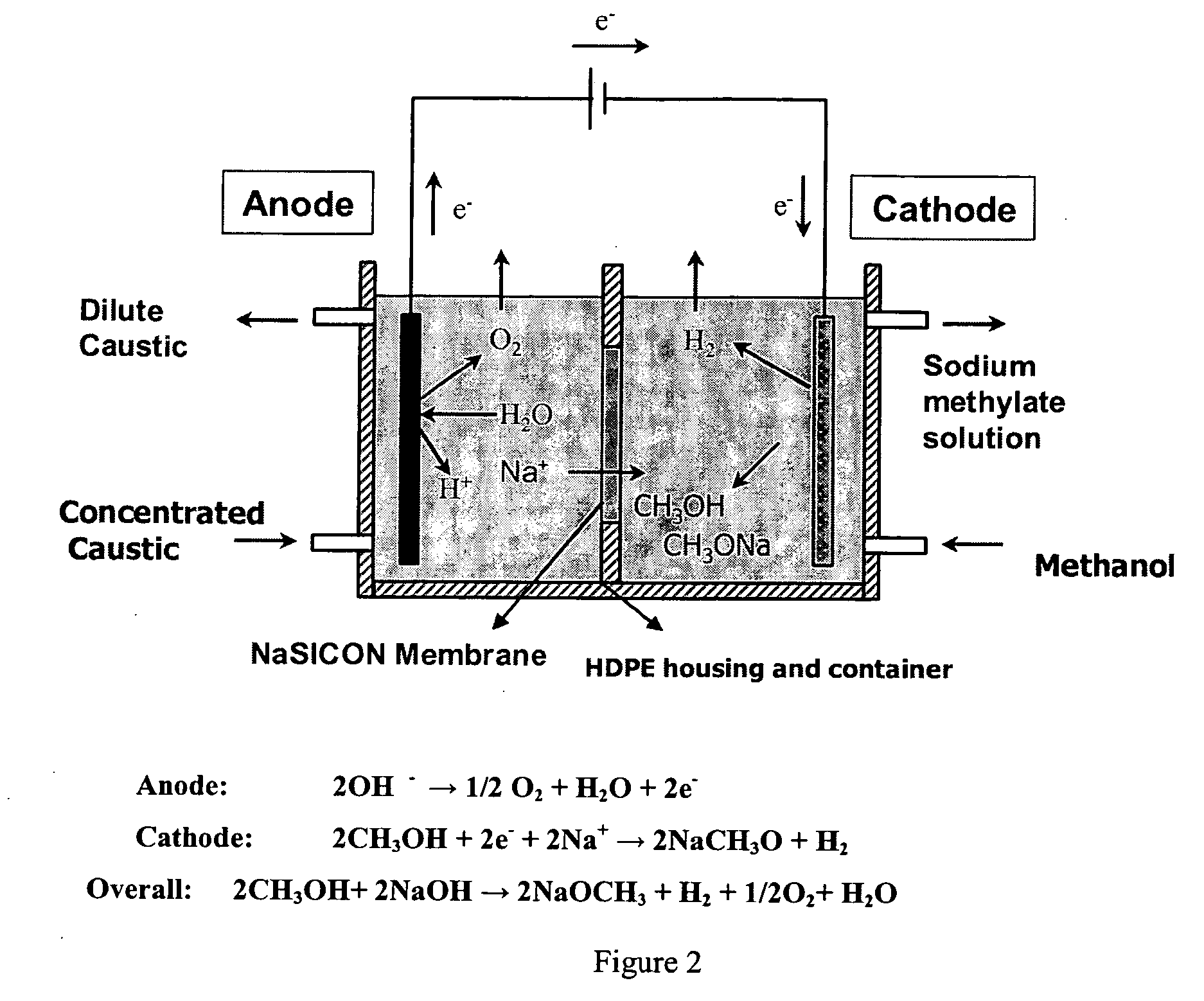
Electrolytic method to make alkali alcoholates using ceramic ion conducting solid membranes
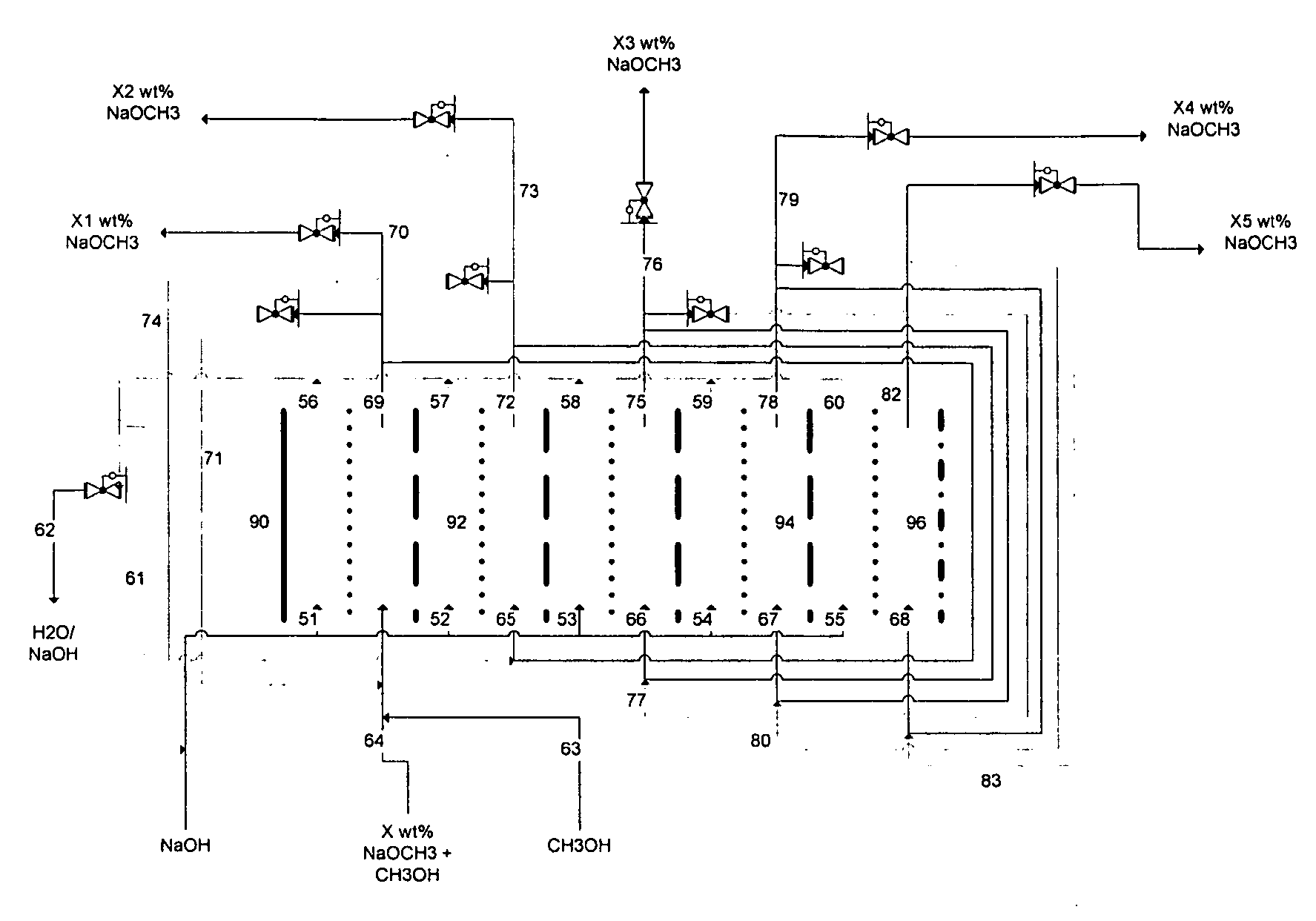
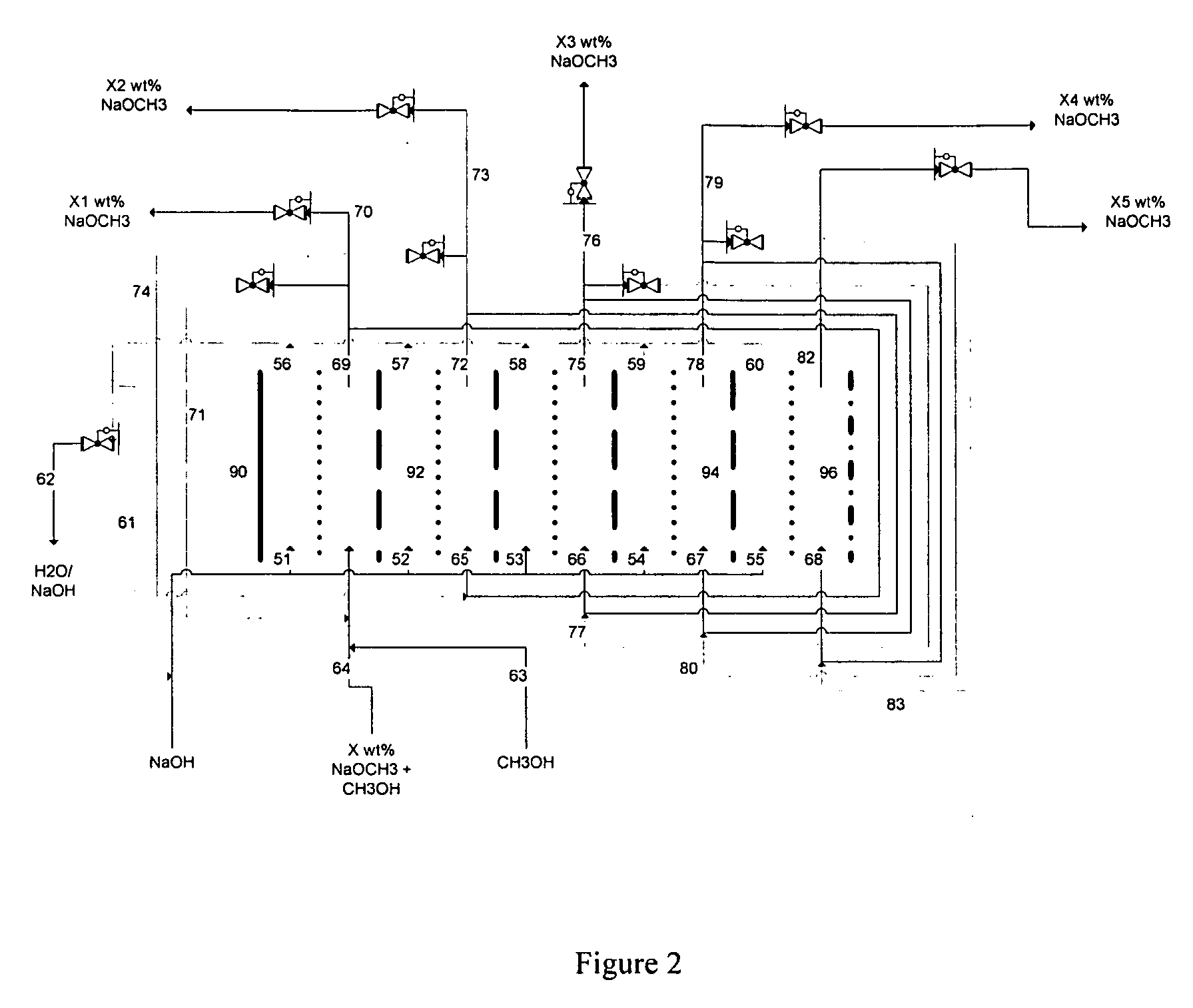
Disclosed are processes of making solutions of alkali alkoxides in their corresponding alcohols using an electrolytic process. In one embodiment, sodium methoxide in methanol is made from methanol and aqueous sodium hydroxide solution, where the aqueous sodium hydroxide solution is present in the anolyte compartment and a solution of sodium methoxide in methanol is present in the catholyte compartment, the two compartments are separated by a ceramic membrane that selectively transports sodium ions under the influence of an electric potential, and wherein the composition of the solution of sodium methoxide in methanol in the catholyte compartment of the electrolytic cell comprises between at least about 2% by weight sodium methoxide and at most about 20% by weight sodium methoxide.
Owner:ENLIGHTEN INNOVATIONS INC
Electrolytic method to make alkali alcoholates using ceramic ion conducting solid membranes
Disclosed are processes of making solutions of metal alcoholates in their corresponding alcohols using an electrolytic process. In a preferred embodiment, sodium methylate in methanol is made from methanol and sodium hydroxide solution. The sodium hydroxide solution is placed in the anolyte compartment and the methanol is placed in the catholyte compartment, and the two compartments are separated by a ceramic membrane that selectively transports sodium under the influence of current. In preferred embodiments, the process is cost-effective and not environmentally harmful.
Owner:ENLIGHTEN INNOVATIONS INC
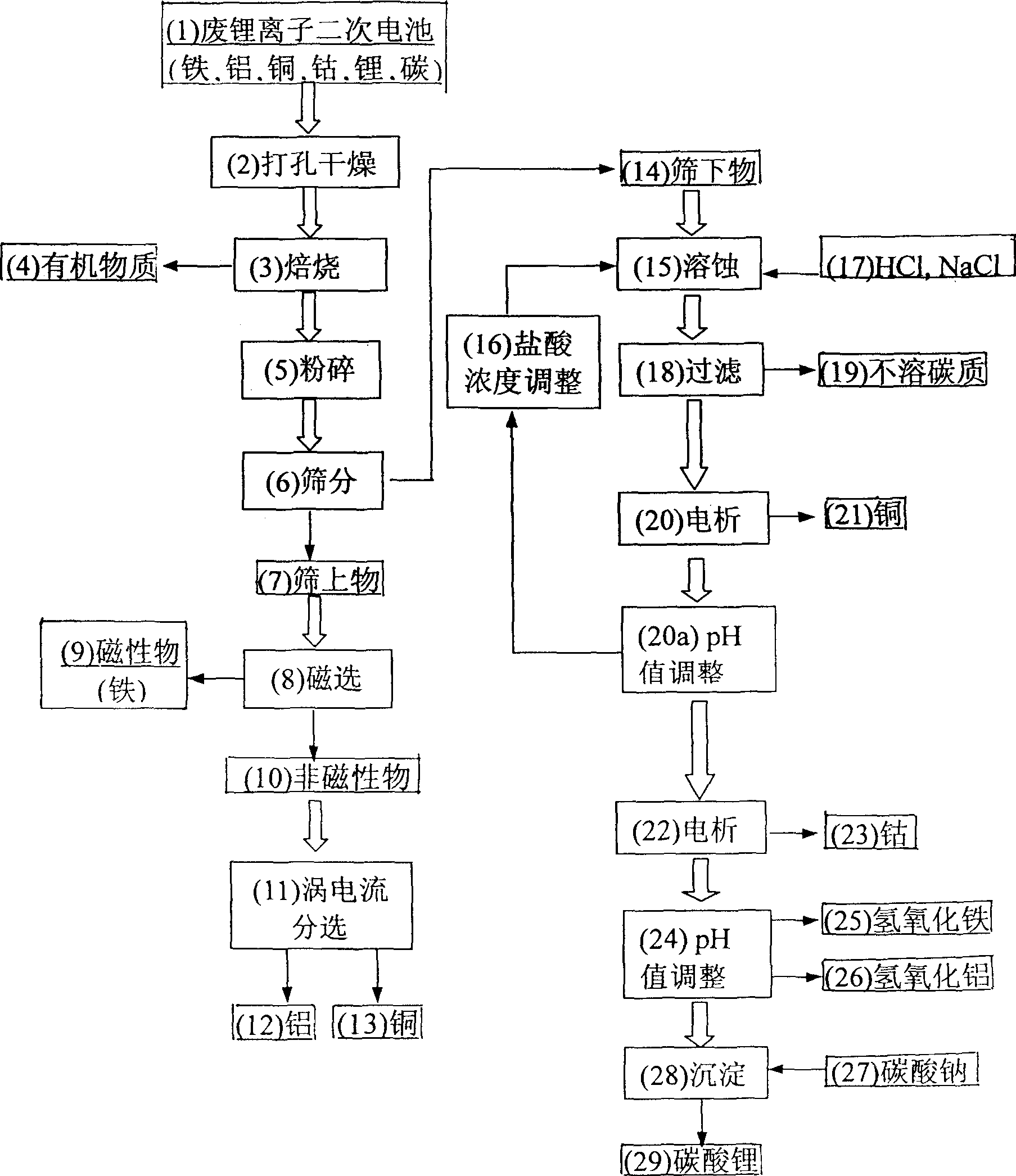
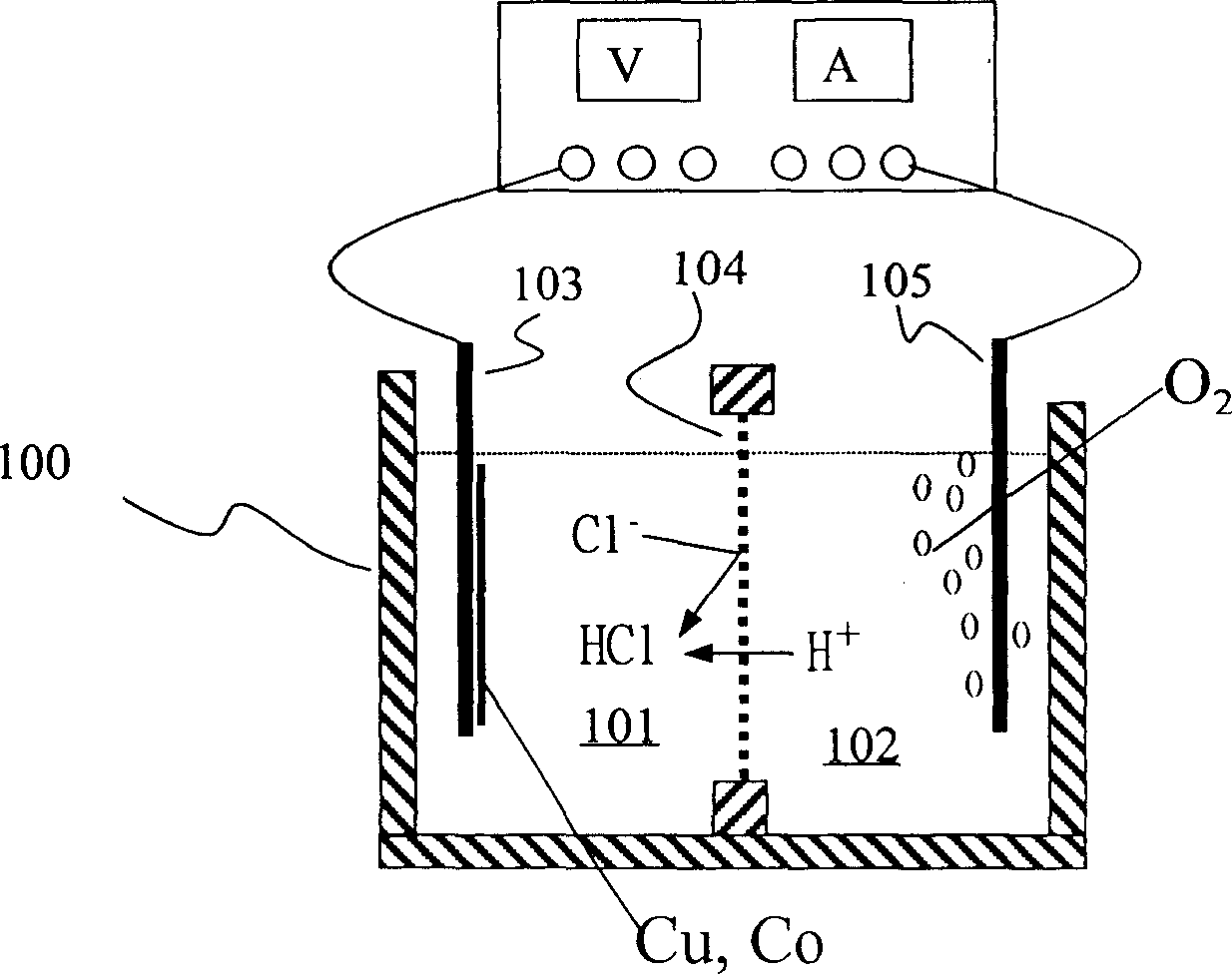
Method for recovering metal from used Li ion cell
InactiveCN1402376ATo achieve the purpose of separationReduce lossWaste accumulators reclaimingBattery recyclingDielectricCopper foil
The invented method includes the physical separation method combining with the preparation procedure of the cleaning wet recycle, providing the features of simple and high purity of the recovered metal. The invention includes following steps. With the disused lithium ion cells being burned in the high-temperature furnace, the organic dielectric is removed. After the smashing and sieving treatment, the oversize material is processed through the magnetic separation and the eddy current sorting so as to obtain the iron case, copper foil and aluminium foil etc. The undersize is processed through the steps of corrosion, filtering and electrolysis so as to obtain copper and cobalt. With carbonic acid radical being added to the solution richen in lithium ion, the high purity carbonate of lithiumis formed so as to recovery lithium.
Owner:IND TECH RES INST
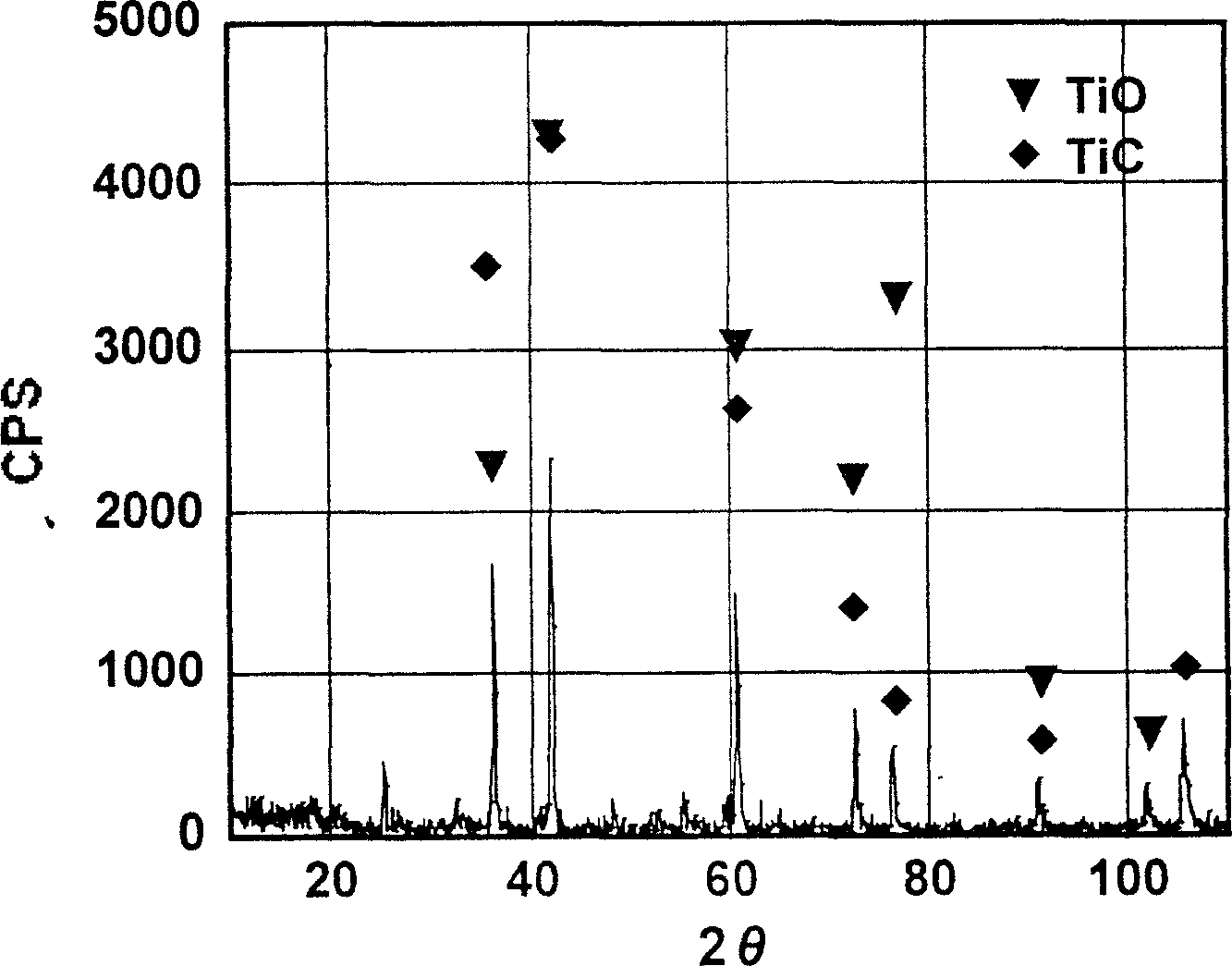
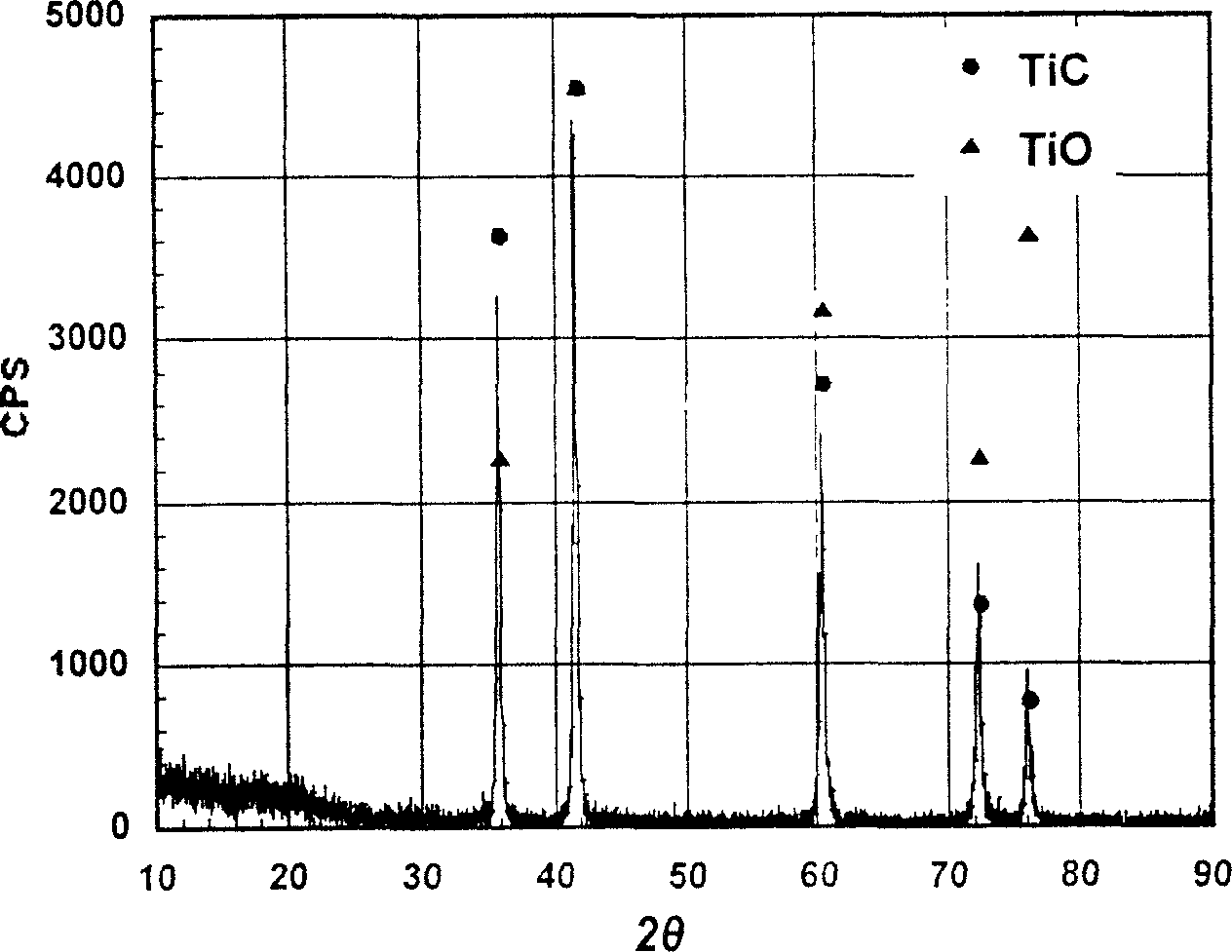
Pure titanium production from titanium monoxide/titanium carbide soluble solid anode electrolysis
A process of preparing purified Ti by the way of electrolysis directly from sosoloid positive pole, TiO?mTiC. Mix the powder of C and TiO2 or TiC as measurement of chemical reaction. Then press to be certain size to make positive pole, TiO?mTiC in airvoid at 600-1600íµ. Electrolyte is halide fused salt of alkali. Electrolysis at 400-1000íµ. C and O in the positive pole form CO, CO2 and O2. Purified Ti is obtained from negative pole.
Owner:鸿钛(北京)科技有限公司
Electrolytic process for producing chlorine dioxide
InactiveUS6203688B1Improved conversion efficiency per passMinimize or prevent any side anodic oxidation reactionsCellsChlorine dioxideElectrochemical cell
A process for converting in a single pass an aqueous alkaline pH, alkali metal chlorite solution into an aqueous chlorine dioxide-containing solution that involves the combination of (1) using an electrochemical acidification cell to lower the pH value of the aqueous alkali metal chlorite feed before it enters the anode compartment of an electrochemical oxidation cell where the chlorite is converted to chlorine dioxide with (2) using an anolyte flow pattern where the anolyte passes through a porous, high surface area electrode. This process results in a substantially improved conversion efficiency per pass.
Owner:STERLING PULP CHEM +2
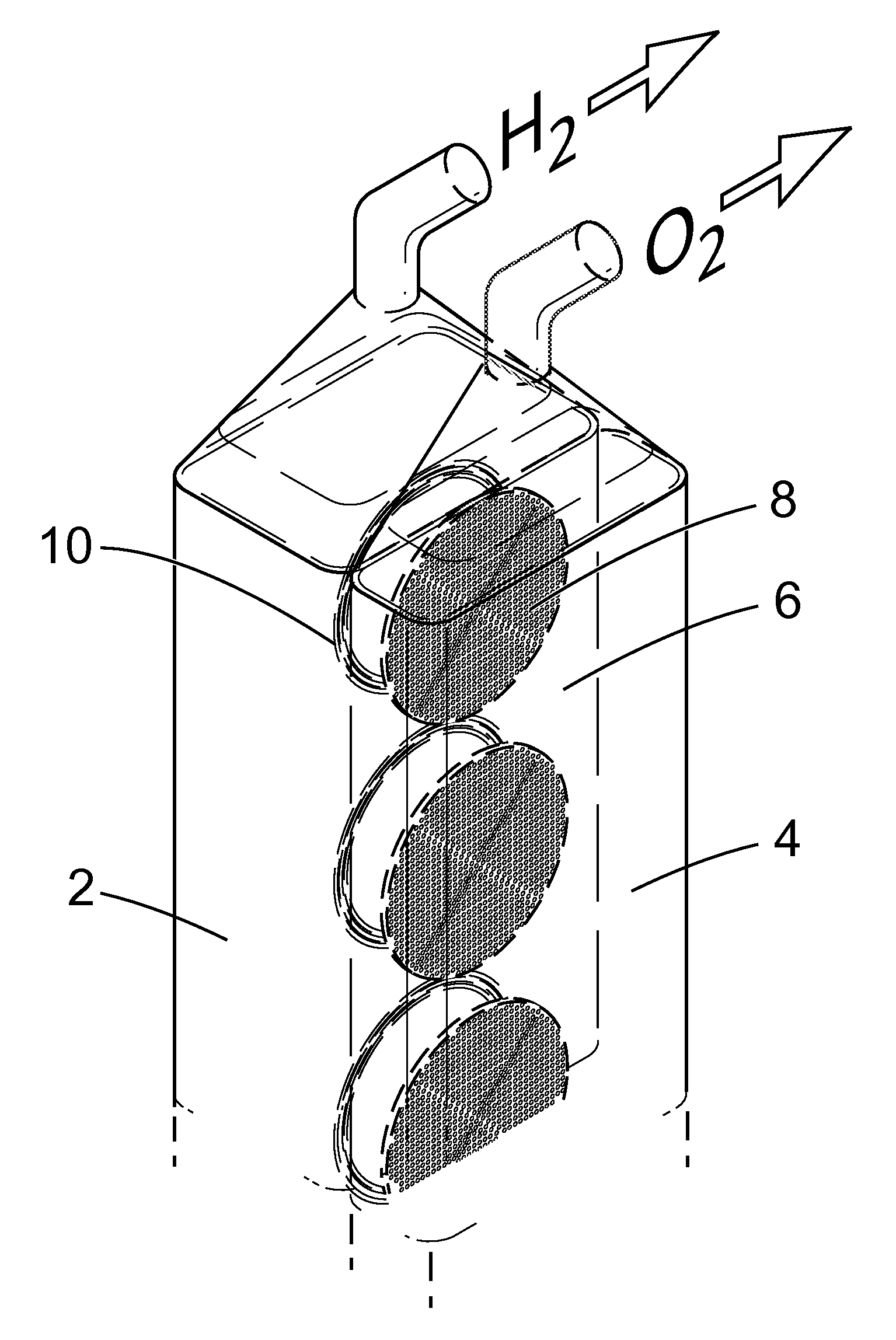
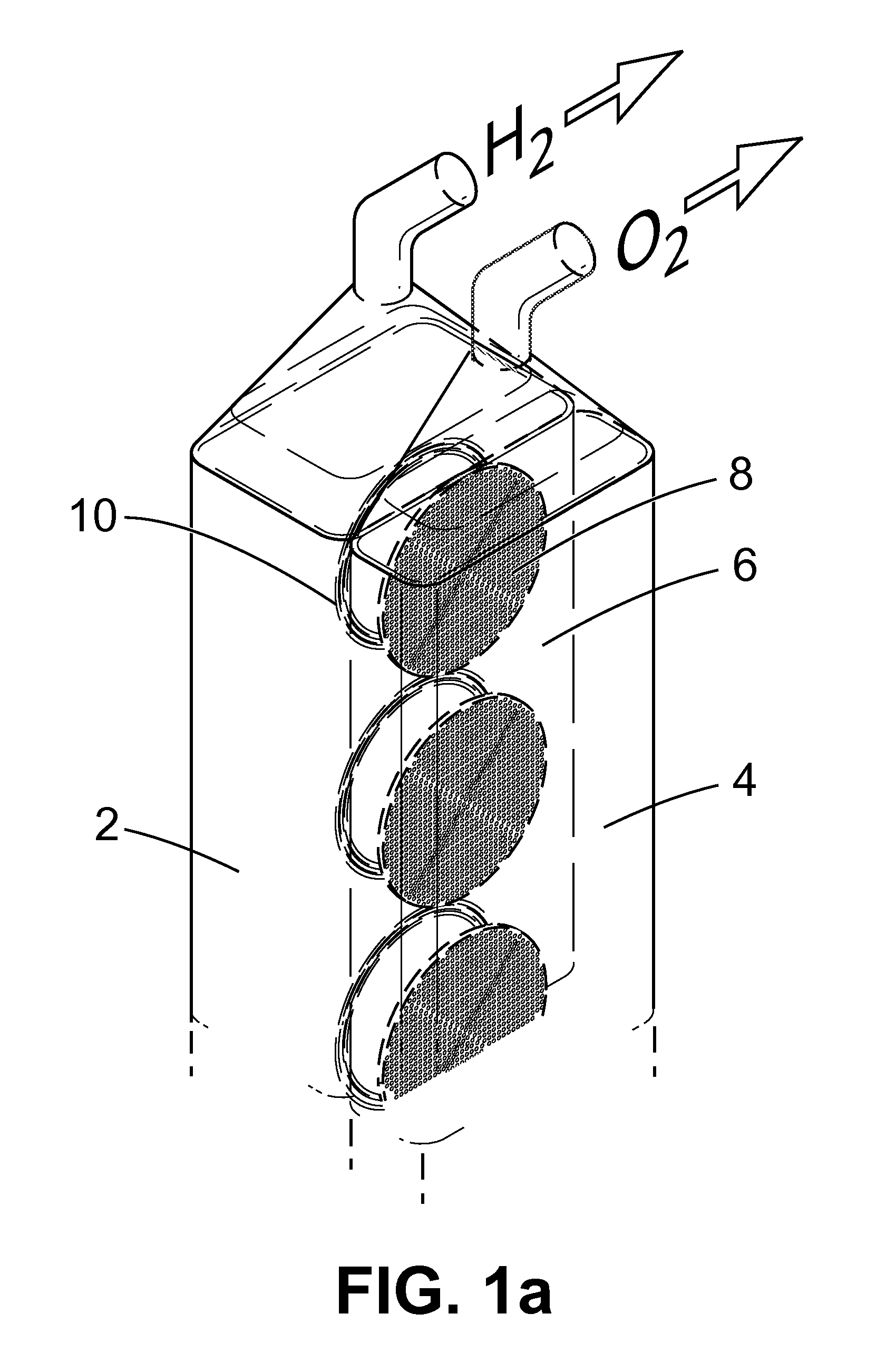
Apparatus and Method for the Electrolysis of Water
InactiveUS20120149789A1Reduce the amount requiredMaintain efficient productionPhotography auxillary processesHydrocarbon by hydrogenationHydrogenElectrolysed water
An apparatus for the electrolytic splitting of water into hydrogen and / or oxygen, the apparatus comprising: (i) at least one lithographically-patternable substrate having a surface; (ii) a plurality of microscaled catalytic electrodes embedded in said surface; (iii) at least one counter electrode in proximity to but not on said surface; (iv) means for collecting evolved hydrogen and / or oxygen gas; (v) electrical powering means for applying a voltage across said plurality of microscaled catalytic electrodes and said at least one counter electrode; and (vi) a container for holding an aqueous electrolyte and housing said plurality of microscaled catalytic electrodes and said at least one counter electrode. Electrolytic processes using the above electrolytic apparatus or functional mimics thereof are also described.
Owner:UT BATTELLE LLC
Multi-layer non-carbon metal-based anodes for aluminum production cells and method
InactiveUS6077415AImprove electrochemical activitySolution to short lifeMachining electrodesIsotope separationElectrical batteryOxygen ions
A composite, high-temperature resistant, non-carbon metal-based anode of a cell for the electrowinning of aluminium comprises a metal-based core structure of low electrical resistance, for connecting the anode to a positive current supply, coated with a series of superimposed, adherent, electrically conductive layers. These layers consist of at least one layer on the core structure constituting a barrier substantially impervious to monoatomic oxygen and molecular oxygen; one or more intermediate, protective layers on the oxygen barrier layer which remain inactive in the reactions for the evolution of oxygen gas; and an electrochemically active layer for the oxidation reaction of oxygen ions present at the anode / electrolyte interface into nascent monoatomic oxygen, as well as for subsequent reaction for the formation of gaseous biatomic oxygen. The active layer on the outermost intermediate layer is slowly consumable during electrolysis and protects the intermediate protective layer by inhibiting its dissolution into the electrolyte.
Owner:MOLTECH INVENT
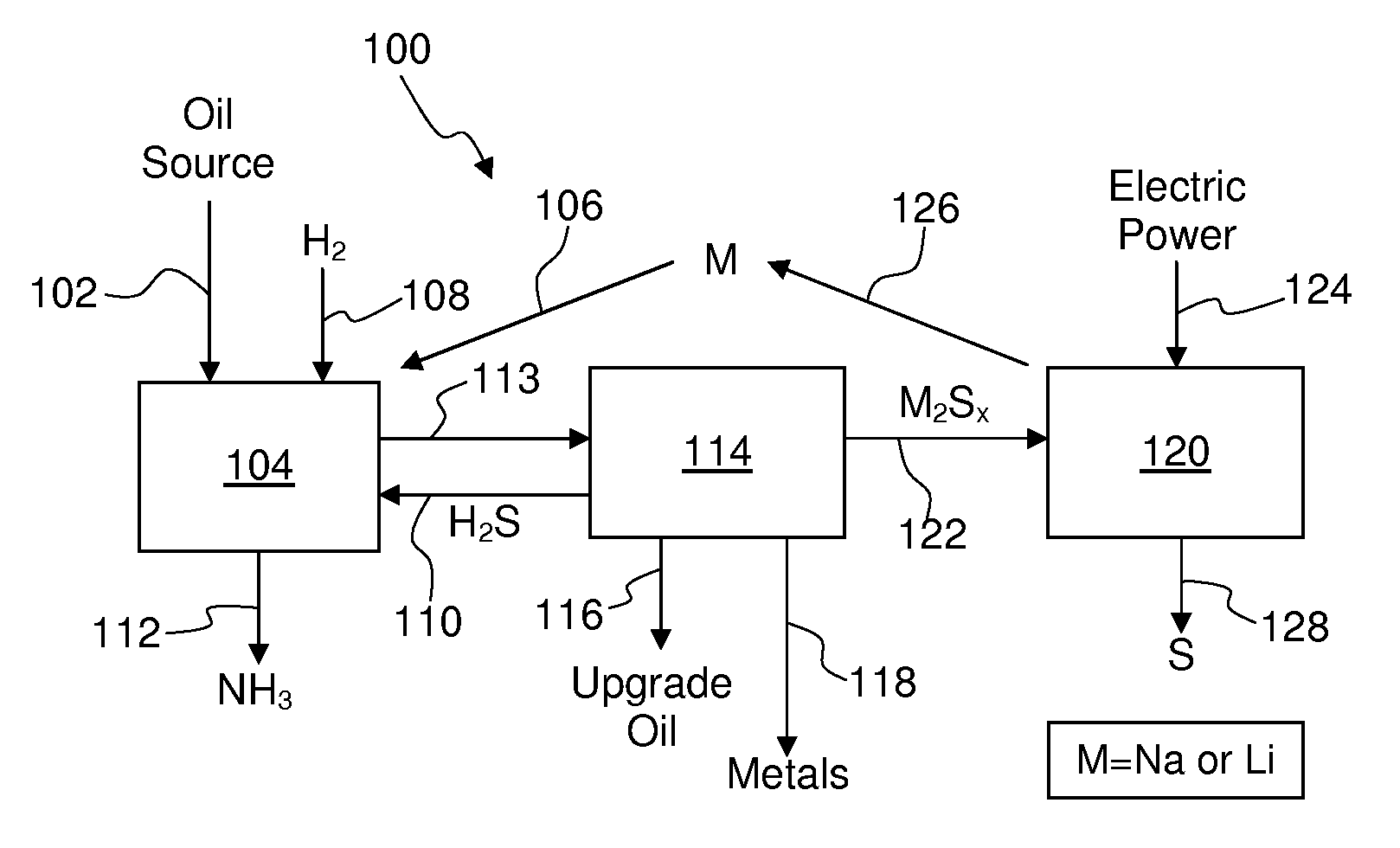
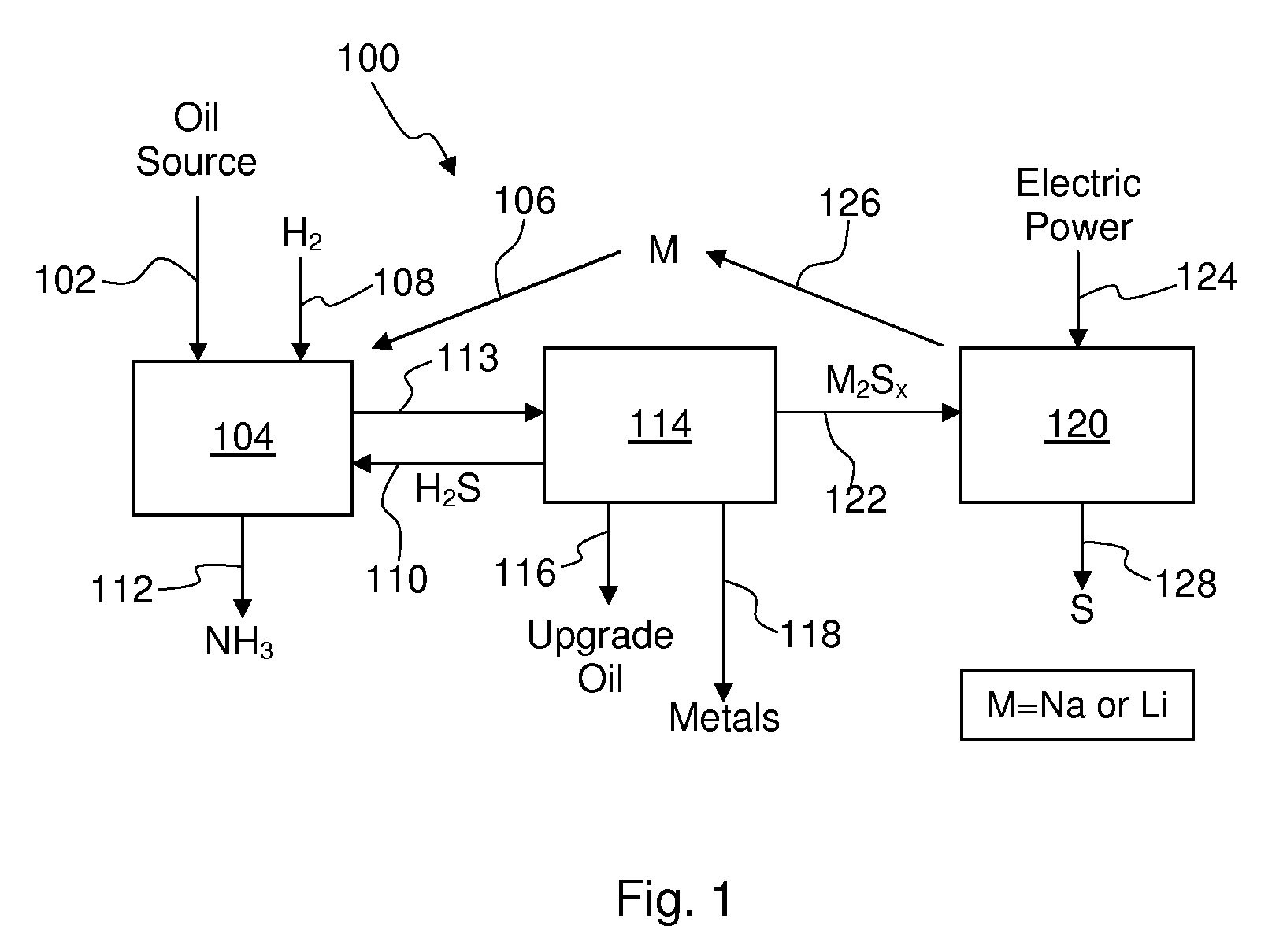
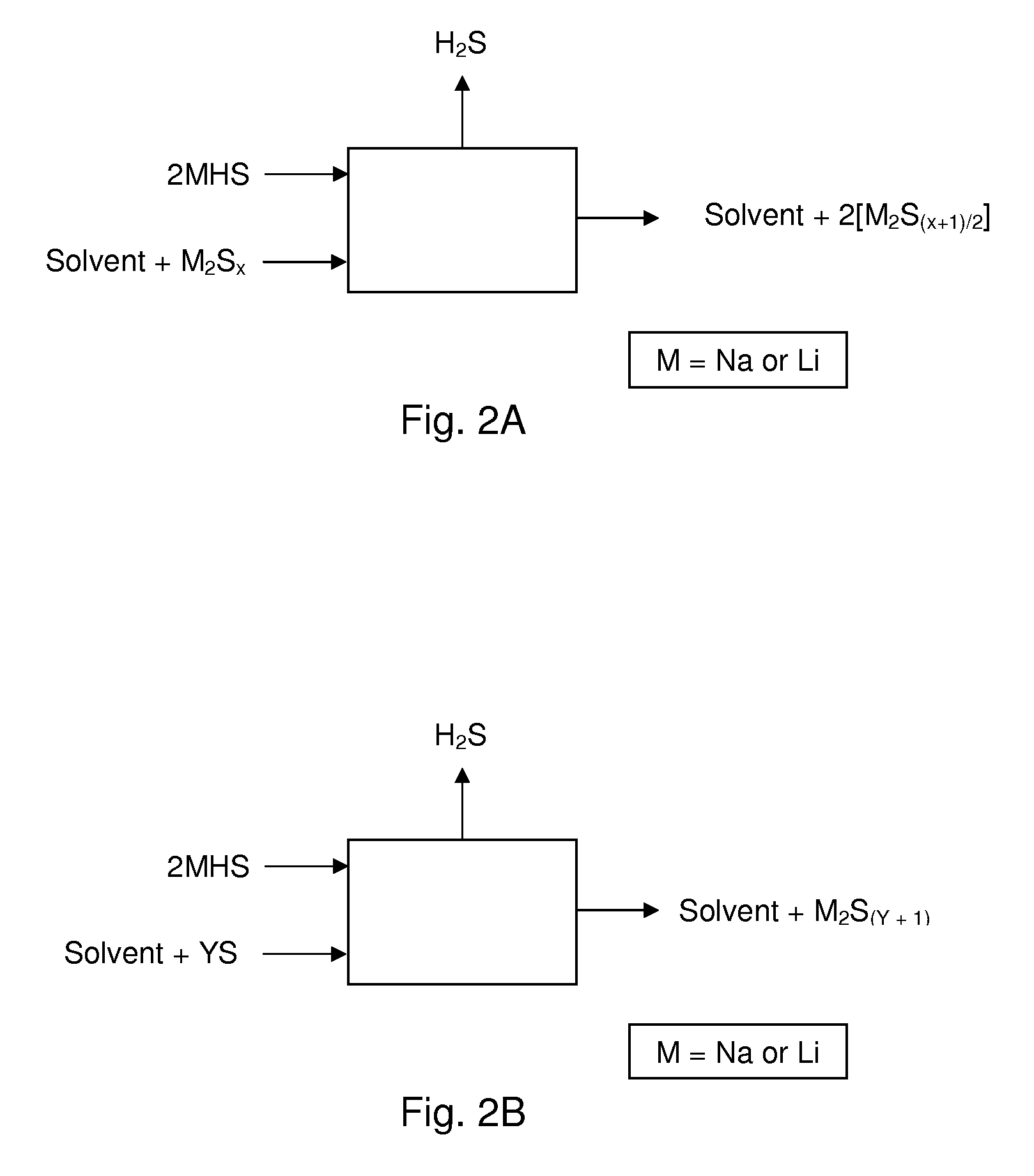
Process for recovering alkali metals and sulfur from alkali metal sulfides and polysulfides
Alkali metals and sulfur may be recovered from alkali polysulfides in an electrolytic process that utilizes an electrolytic cell having an alkali ion conductive membrane. An anolyte solution includes an alkali polysulfide and a solvent that dissolves elemental sulfur. A catholyte solution includes alkali metal ions and a catholyte solvent. Applying an electric current oxidizes sulfur in the anolyte compartment, causes alkali metal ions to pass through the alkali ion conductive membrane to the catholyte compartment, and reduces the alkali metal ions in the catholyte compartment. Sulfur is recovered by removing and cooling a portion of the anolyte solution to precipitate solid phase sulfur. Operating the cell at low temperature causes elemental alkali metal to plate onto the cathode. The cathode may be removed to recover the alkali metal in batch mode or configured as a flexible band to continuously loop outside the catholyte compartment to remove the alkali metal.
Owner:ENLIGHTEN INNOVATIONS INC
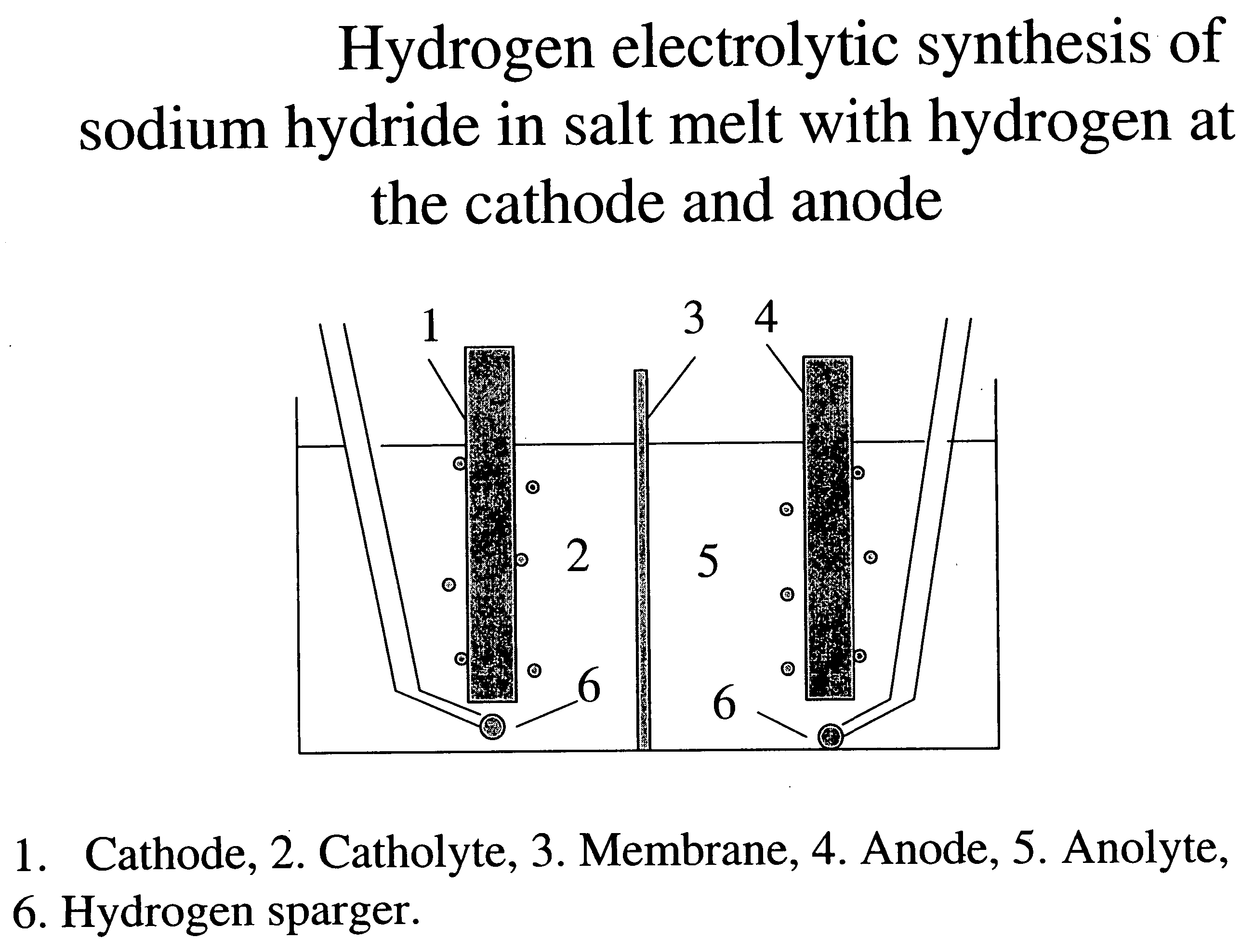
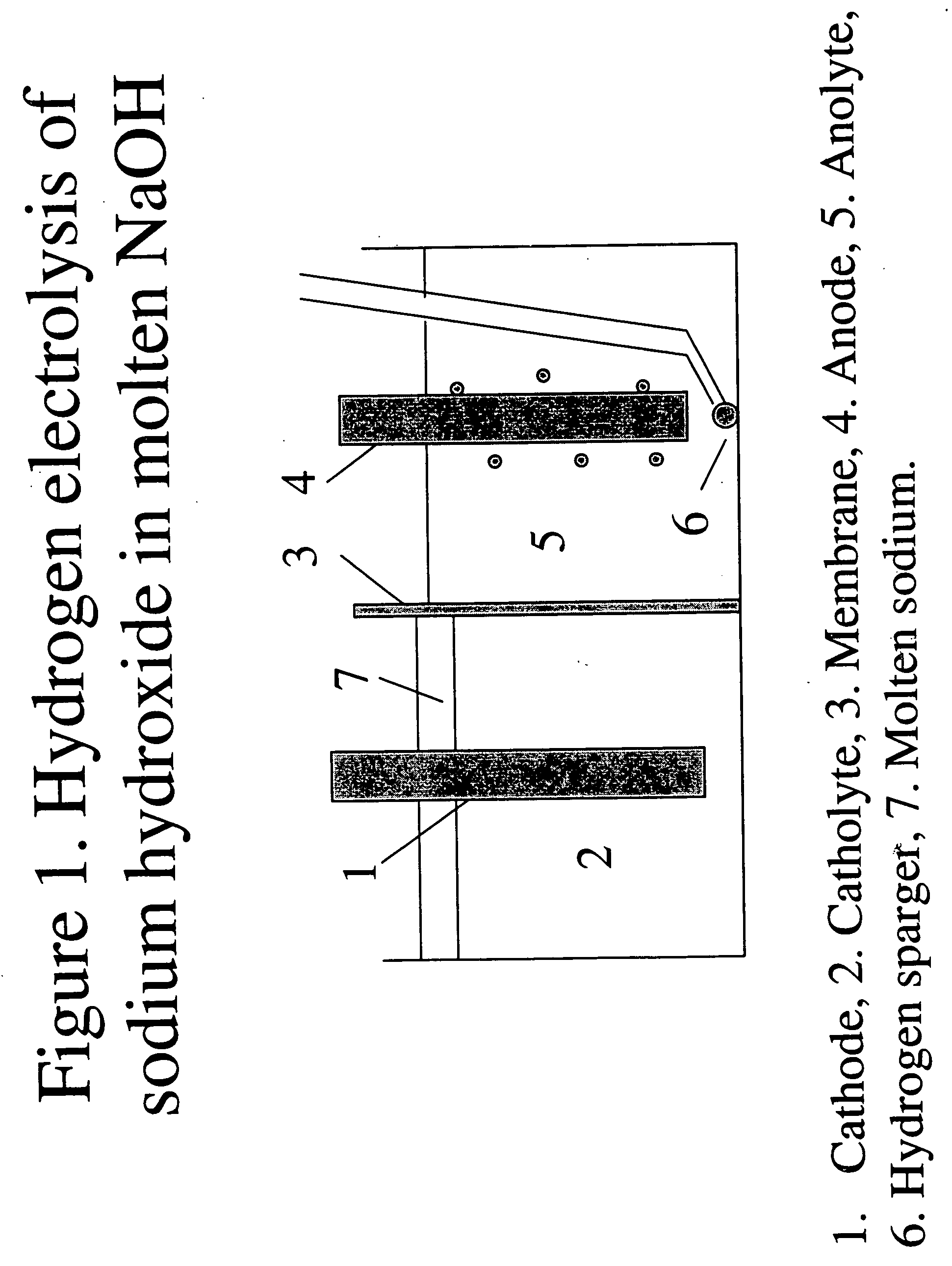
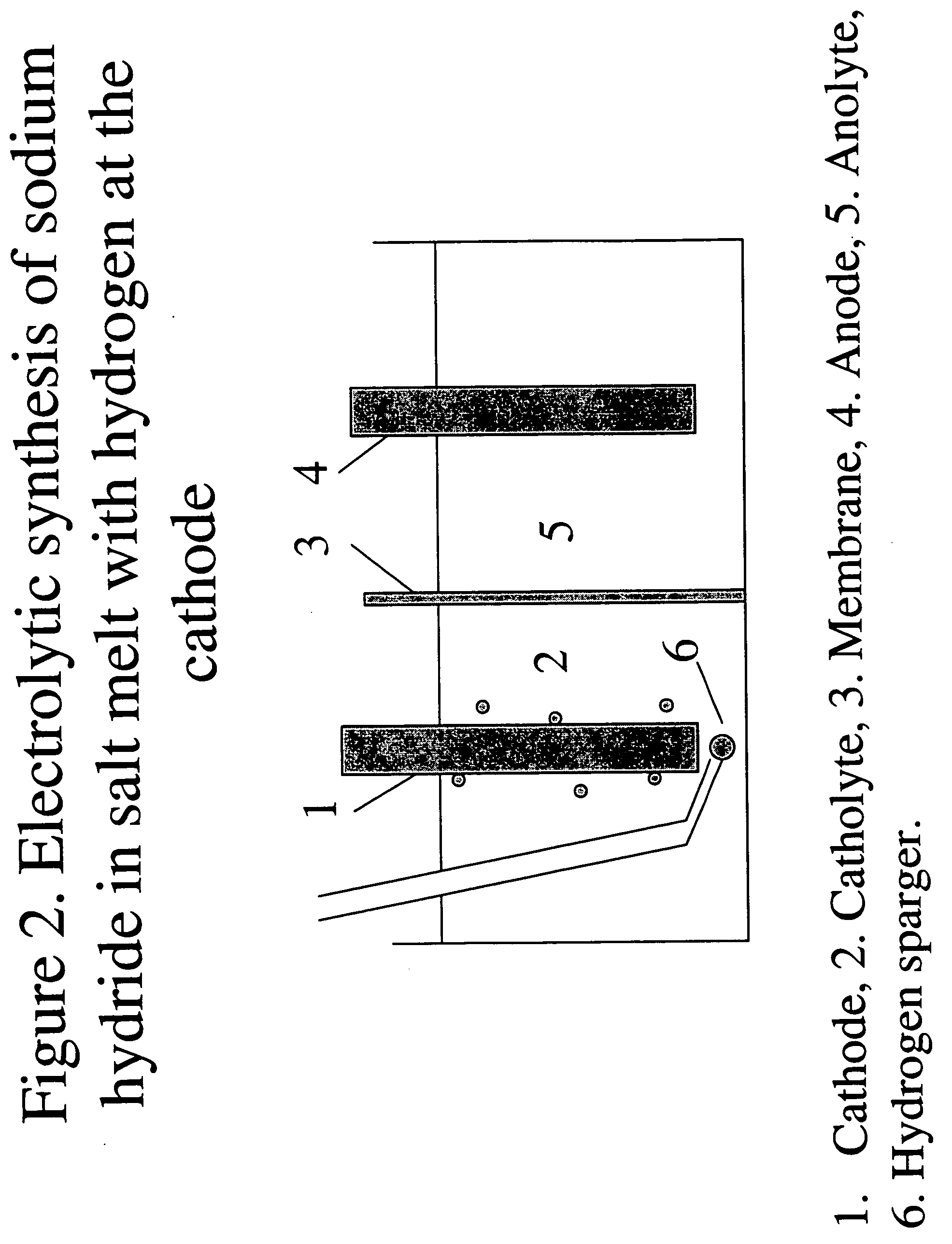
Hydrogen-assisted electrolysis processes
InactiveUS20060169593A1Reduce cell potentialEfficient and cost-effective processElectrolysis componentsPhotography auxillary processesHydrogenElectrical battery
A process and electrolytic cell for reducing in an ionic alkali metal compound, the cell containing anode and cathode electrodes, by supplying an electrolyte containing the alkali metal compound to the cell, applying an electric voltage to the cell to reduce said alkali metal compound at the cathode, and passing hydrogen or a hydrogen containing gas to at least one electrode while the compound is reduced at the cathode.
Owner:XU JIANGUO +4
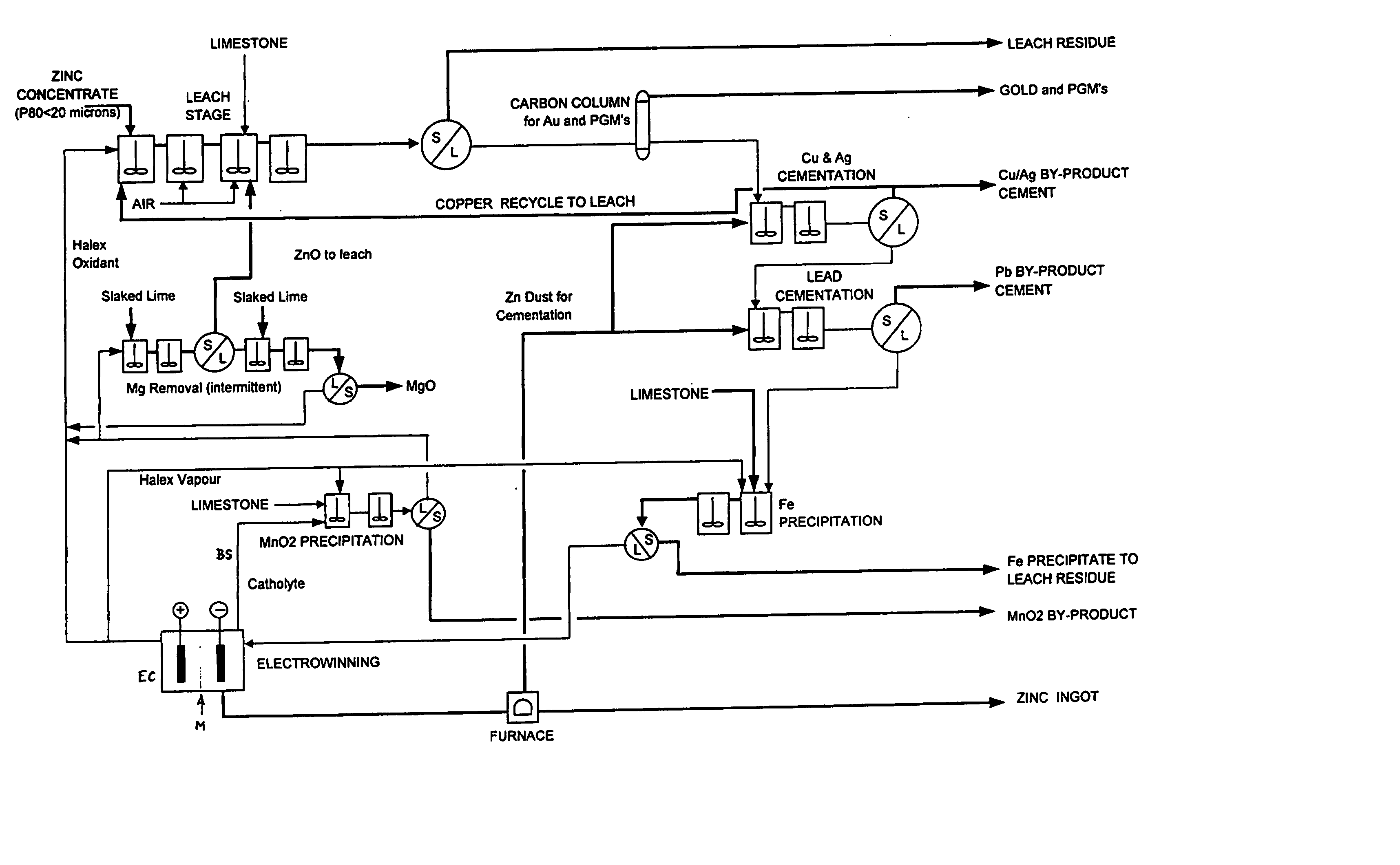
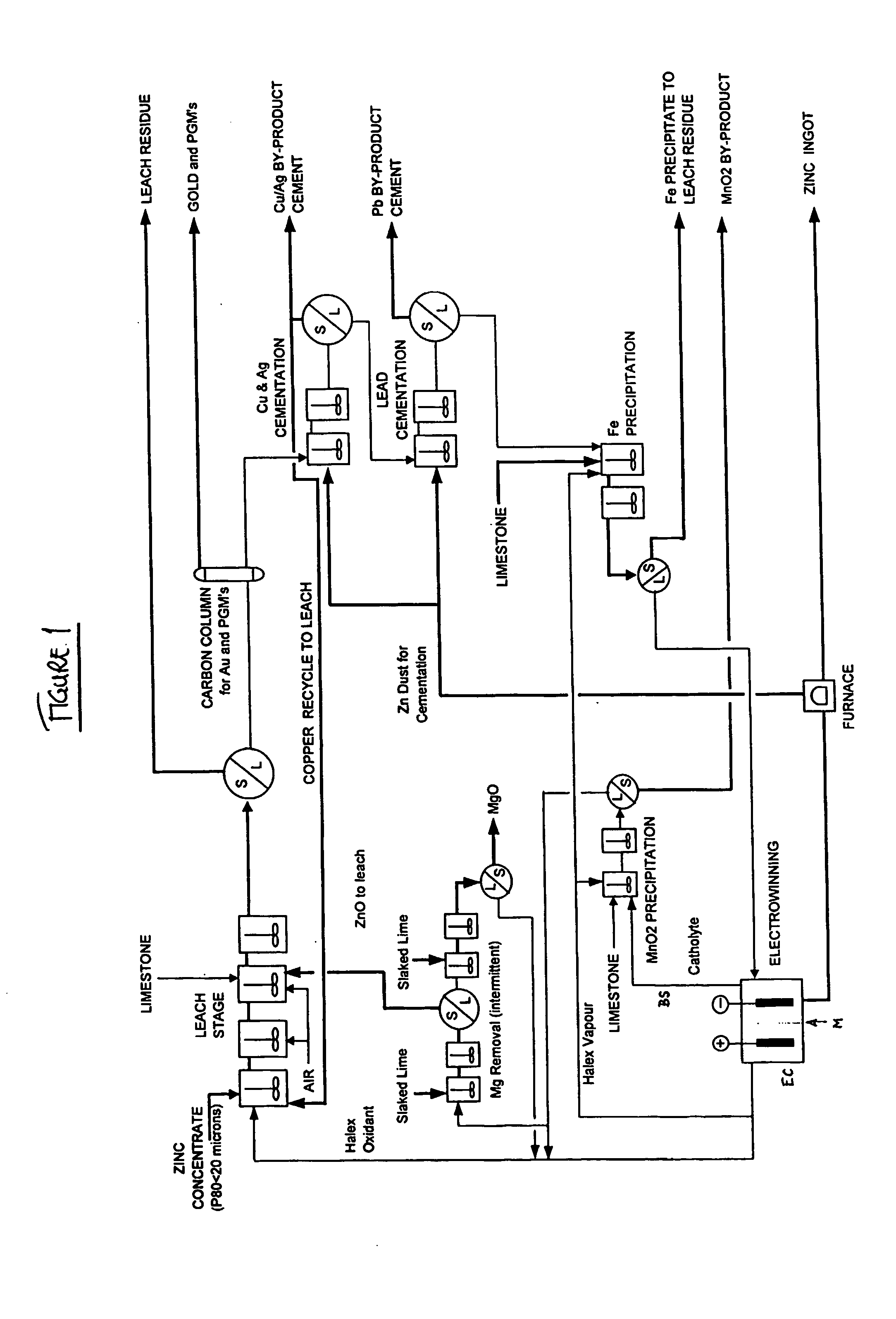
Zinc recovery process
InactiveUS20040237720A1Improve filtering effectEh is decreasedPhotography auxillary processesSolvent extractionZinc metalManganese
A process for the recovery of zinc metal from a zinc mineral includes the steps of leaching the zinc mineral in a solution including a halide species formed from two or more different halides, to leach the zinc into the solution. The zinc-bearing solution is then electrolysed to yield zinc metal and to generate the halide species. The electrolysed solution including the halide species is then returned to the leaching step. A portion of the electrolysed solution can be removed as a bleed stream from a cathode compartment of an electrolytic cell of the electrolysis process and processed to remove manganese as manganese dioxide precipitate by adding thereto limestone, and the halide species from an anode compartment of the electrolysis process. In this regard, the pH and Eh of the solution can regulated in a manner that favours the formation of the manganese dioxide precipitate over the formation of a precipitate of zinc.
Owner:INTEC INT PROJECTS
Methods and apparatus of electrochemical production of carbon monoxide, and uses thereof
The present invention relates to an electrolytic process, methods and apparatus for the preparation of carbon monoxide and in particular to electrolysis of molten carbonates to yield carbon monoxide which may be used for chemical storage of electrical energy and further as chemical feedstock for other organic products.
Owner:YEDA RES & DEV CO LTD
Electroplated abrasive tools, methods, and molds
InactiveUS20070128994A1Revolution surface grinding machinesGrinding devicesWear particleElectroplating
The present invention provides for a mold that can position and hold abrasive particles, which are to be electrolytically attached to an electrically conductive substrate during an electrolytic process. The mold can include an insulating material with a molding surface suitable for holding the abrasive particles in place during this process. Additionally, a method for making an abrasive tool using such a mold is provided, as well as abrasive tools made thereby. In one aspect of this invention, abrasive tools can have abrasive particle tips that are arranged in accordance with a predetermined vertical pattern and / or a predetermined horizontal pattern in a manner that requires little or no post electrodeposition processing.
Owner:SUNG CHIEN MIN
Hydrogen-assisted electrolysis processes
InactiveUS7108777B2Reduce cell potentialEfficient processingElectrolysis componentsPhotography auxillary processesHydrogenElectrical battery
Owner:MILLENNIUM CELL +1
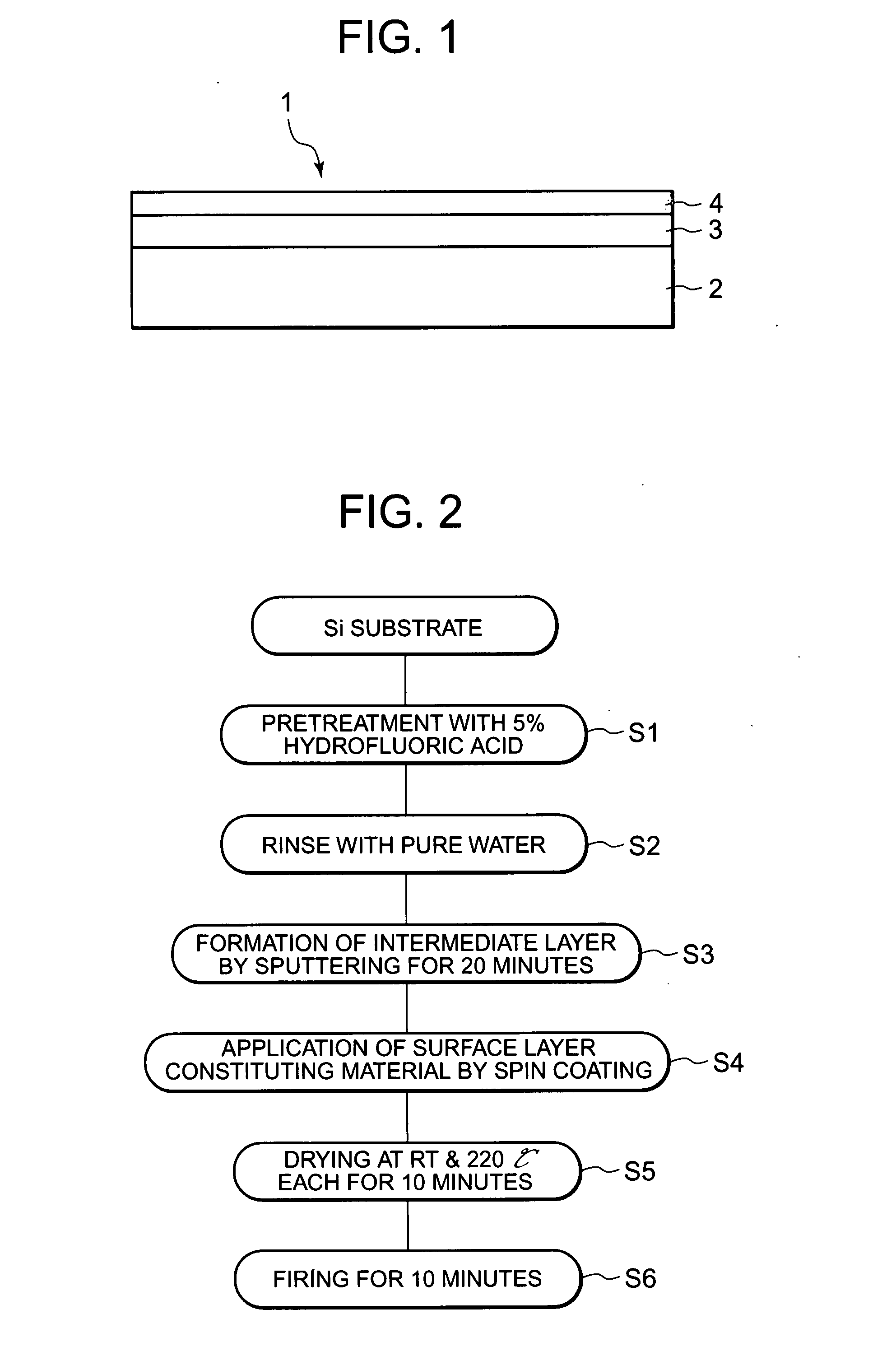
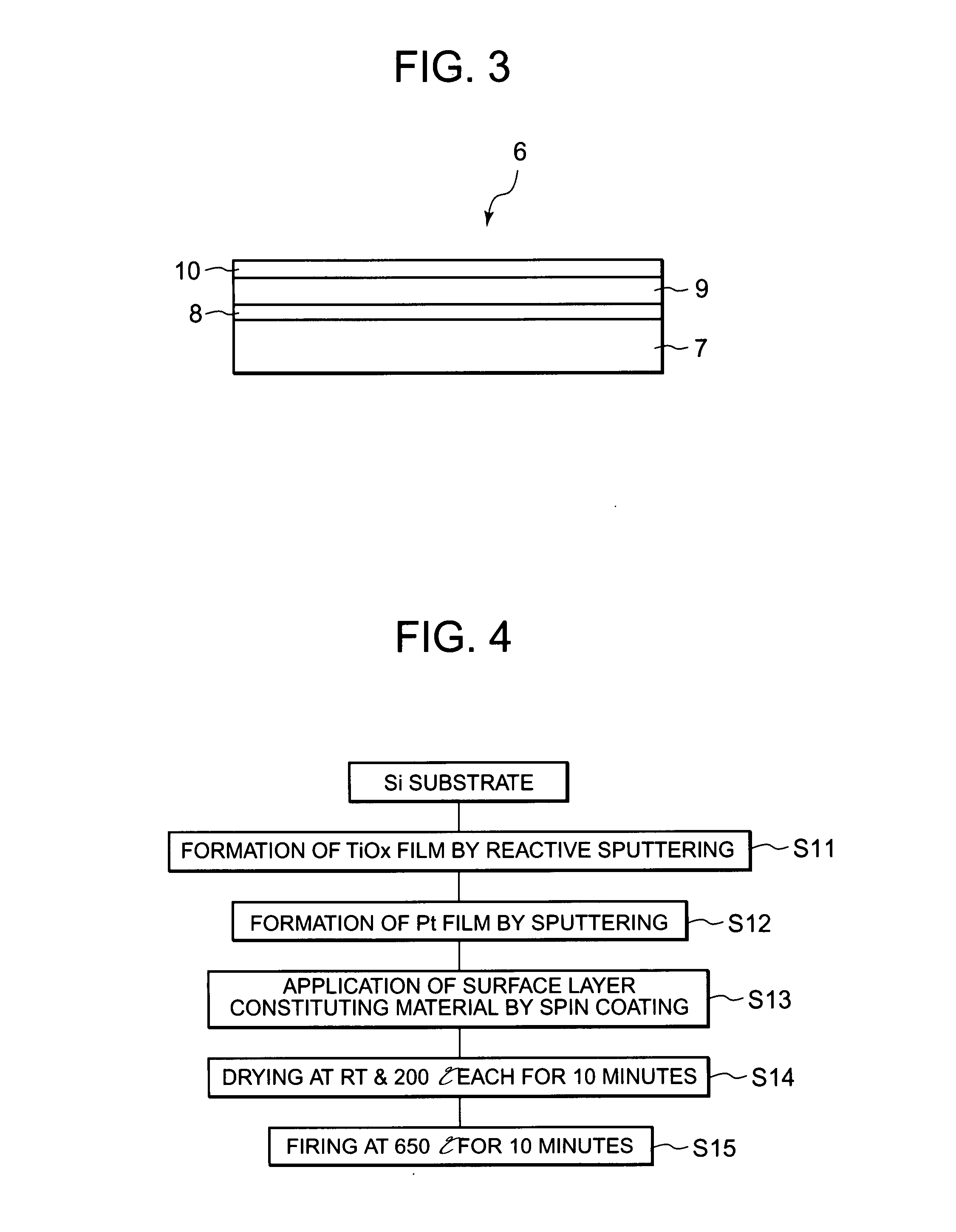
Electrode for electrolysis, electrolytic process using the electrode, and electrolytic apparatus using them
InactiveUS20080060947A1Efficient productionShort lifeMachining electrodesCellsManufacturing technologySurface layer
An object of the present invention is to provide an electrode for electrolysis which is available by an easy fabrication process, can produce ozone water at high efficiency and also can produce hydrogen peroxide and OH radicals having a high oxidizing power by the electrolysis of water at a low current density; an electrolytic process using the electrode; and an electrolytic apparatus using them. The electrode for electrolysis according to the present invention has a substrate and a surface layer formed on the surface thereof, the surface layer being made of anatase type titanium oxide.
Owner:PANASONIC INTELLECTUAL PROPERTY MANAGEMENT CO LTD
Manufacturing method of carbon nano tube paper
A carbon nanotube paper is prepared from carbon nanotubes through adding them to acid, heating or thermal reflux, and / or ultrasonic stirring, removing impurity, diluting in water, filtering, washing the filtered cake, dispersing the carbon nanotubes in water by ultrasonic stirring, spreading on carrier, and drying. It has regular structure, high uniformity, purity and electric conductivity, and enough mechanical strength.
Owner:TSINGHUA UNIV
Preparation method of foam electrode for water electrolysis
InactiveCN101748426AReduce precipitation overpotentialReduce energy consumptionElectrode shape/formsHigh activityEnergy consumption
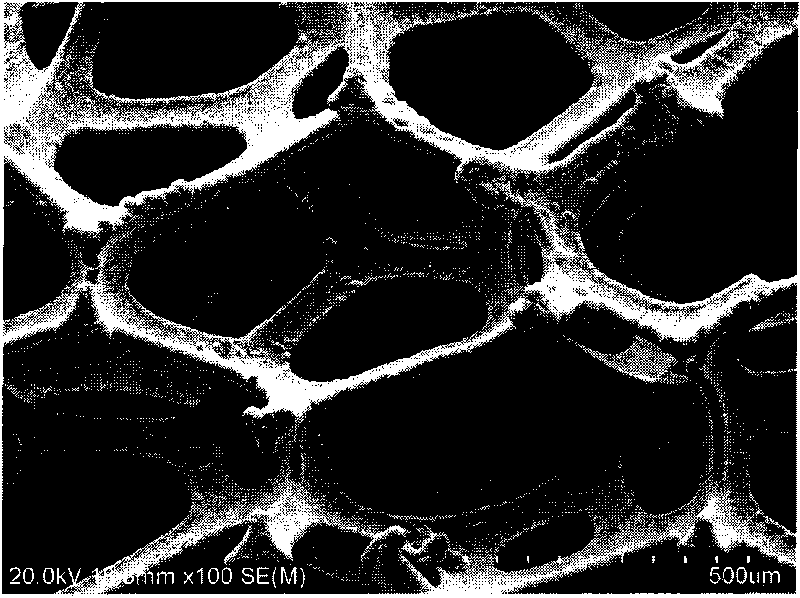
The invention discloses a preparation method of a foam electrode for water electrolysis, which belongs to the technical field of water electrolysis. Foam nickel is selected as the base material of the electrode material; oil stains on the surface of the foam nickel are removed so that the surface is clean; the foam nickel is soaked in diluted acid solution, and the surface of the foam nickel is activated; pure water is used to flush the activated foam nickel, and the foam nickel is quickly soaked into an electrodeposition tank as a cathode for electrodeposition; the active surface is prepared; the foam electrode with the active surface is thoroughly cleaned, dried and thermally treated; and the thermally treated foam material is cut to prepare the electrode material. The high-activity foam electrode material prepared through the method provided by the invention can be used for the electrode material in the electrolysis hydrogen production industry, can reduce hydrogen precipitation overpotential in the electrolysis process, reduce energy consumption, and improve the production efficiency.
Owner:GRIPM ADVANCED MATERIALS CO LTD
Strontium containing hydroxyapatite biologically active film and preparation method thereof
InactiveCN1927410ANo side effectsHigh bonding strengthProsthesisElectrolytic agentPlasma electrolytic oxidation
The invention relates to the means of active biological film, especially the titanium radicel porous nanometer Strontium hydroxyapatite active biological film and the Process for the manufacture. The differential arc oxidation technology is used in the method, the film is generated in the surface of titanium and titanium alloy, a electrolyte solution with phosphate radical ion, calcium ion and Strontium ion is supplied; the titanium and titanium alloy is the anode and the rustless steel or the titanium is the cathode, the direct current power supply or the direct current impulsing power source is applied to differential arc oxygenize the titanium and titanium alloy; the oxidate time is 3-60min; the temperature of electrolyte is not above 50DEG C. There is no interface between the porous hydroxyapatite containing Strontium film produced by the invention and the basal body, the modulus of elasticity is approximate with the sclerotin, the porous hydroxyapatite containing Strontium film produced by the invention which has perfect bioactivity can be the alternate material of several parts with heavy load, such as femur, hip joint and teeth root.
Owner:SOUTH CHINA UNIV OF TECH
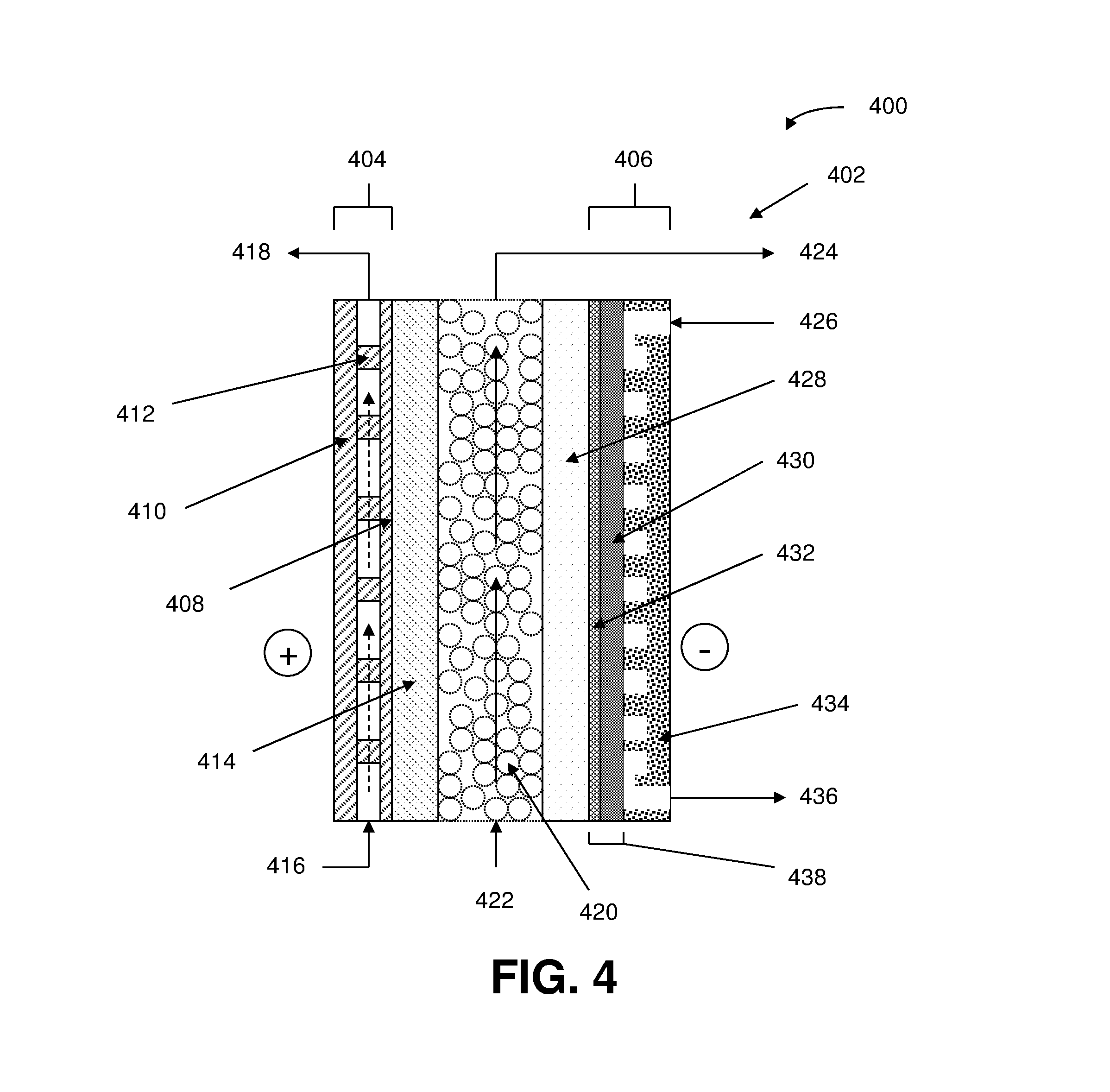
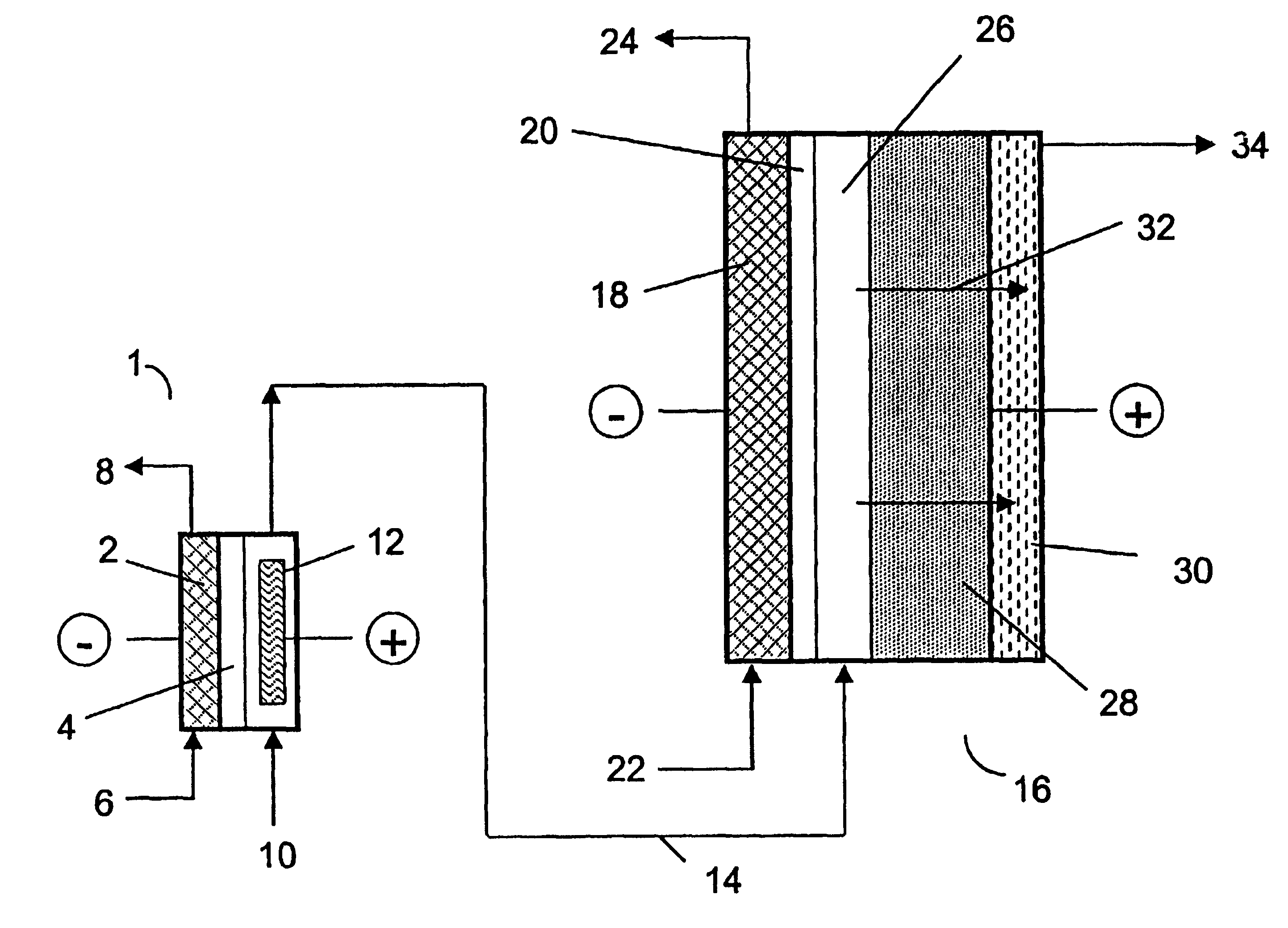
Method of generating electrolyzed water and electrolyzed water generation apparatus therefor
InactiveUS20080047844A1Easy to carryCellsWater treatment parameter controlElectrolysed waterGeneral family
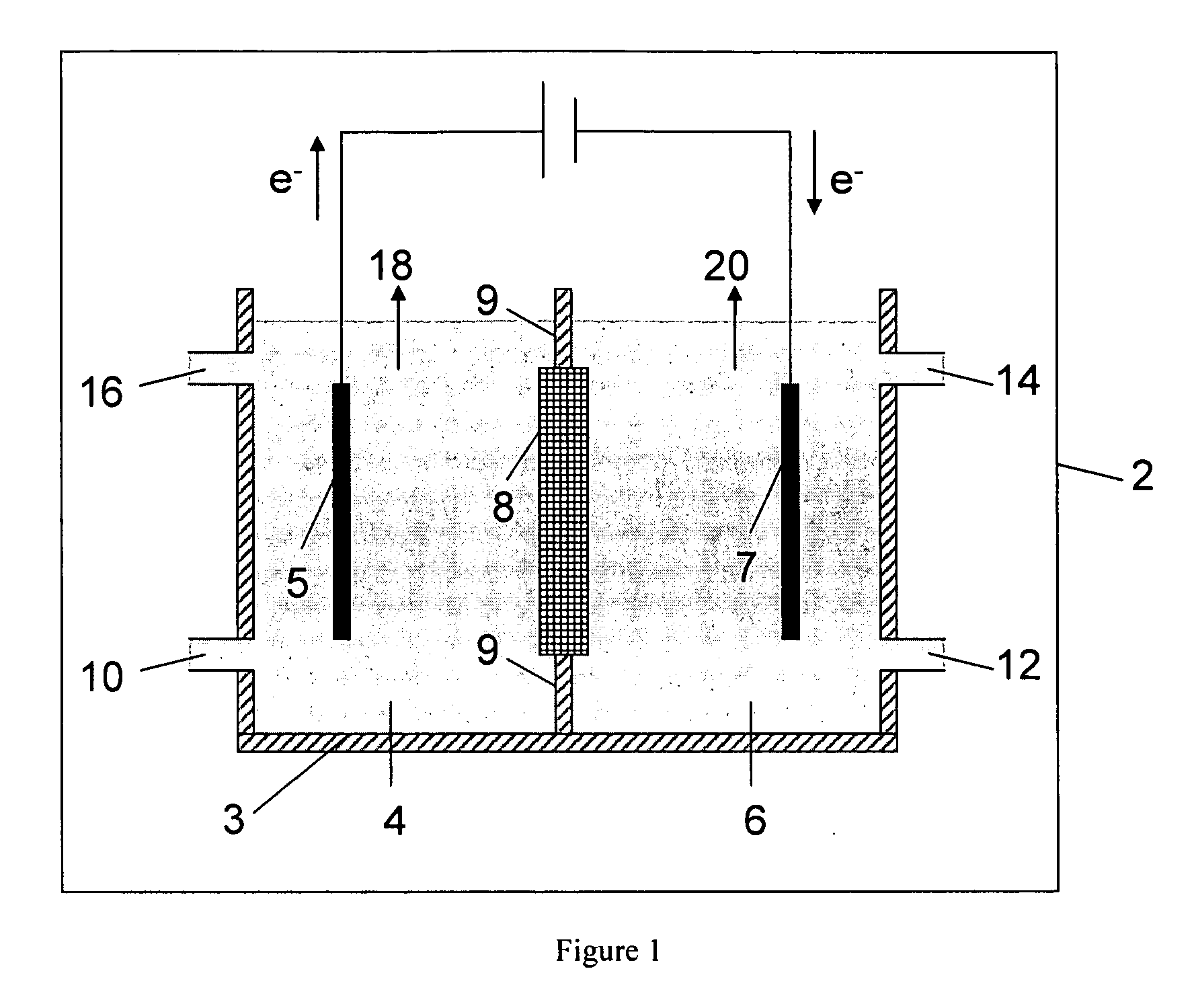
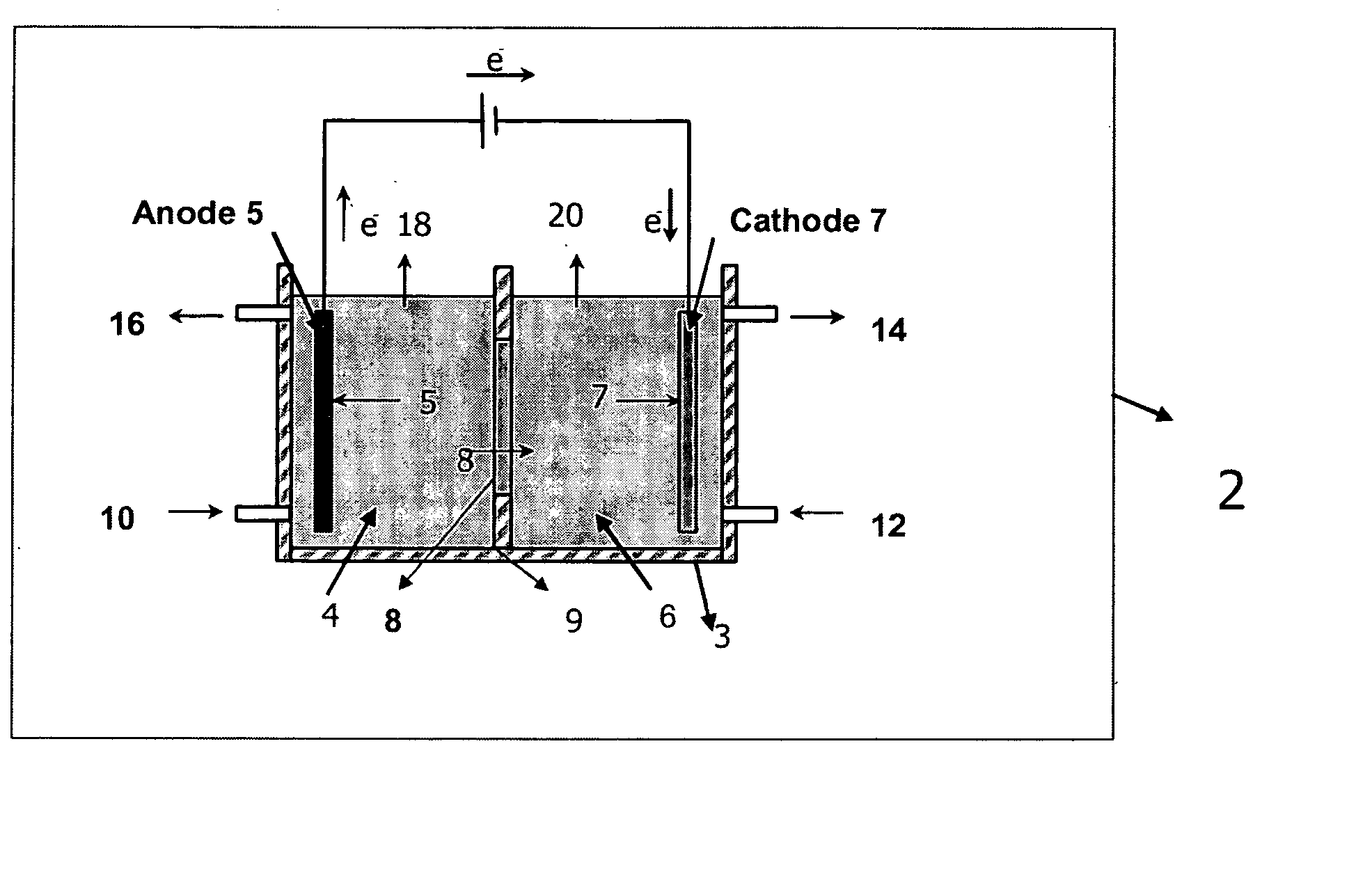
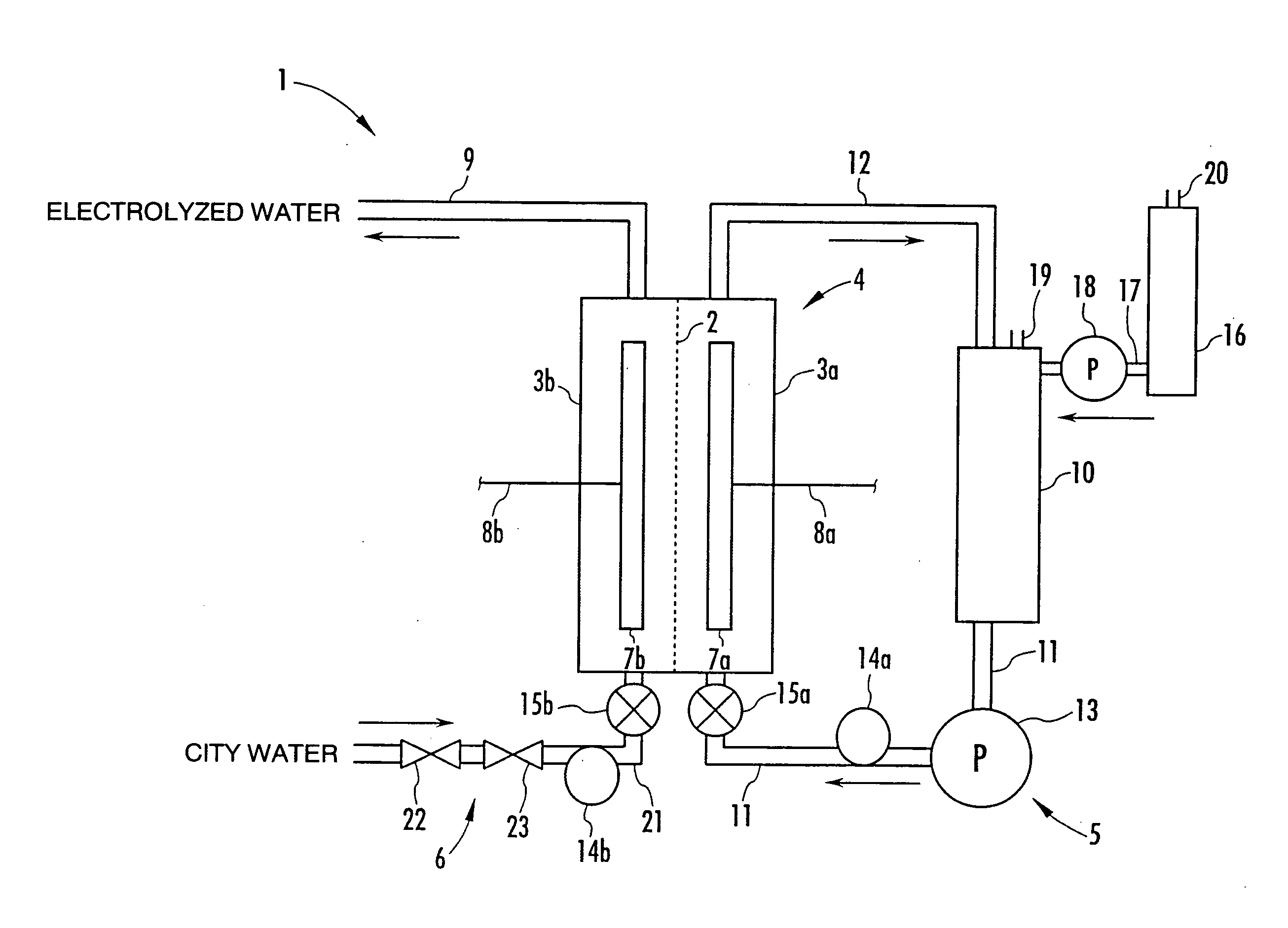
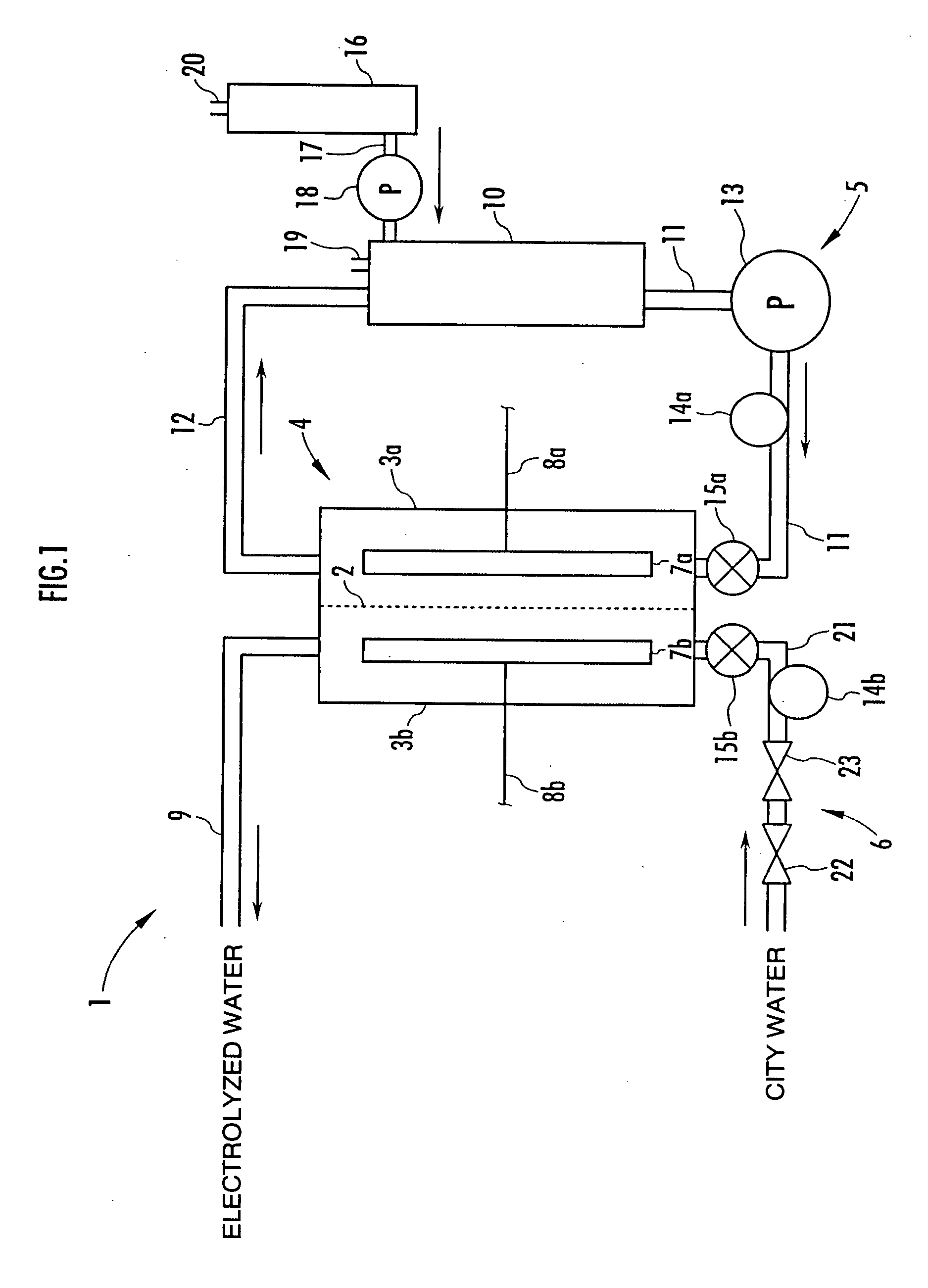
A method and apparatus for generating electrolyzed water easy to carry out or handle even in ordinary homes. An electrolyte aqueous solution is circuited through a first electrolysis chamber 3a of two electrolysis chambers placed on opposite sides of an ion-permeable membrane 2, and raw water is supplied only to the second electrolysis chamber 3b. A voltage is applied between electrodes 7a and 7b to cause electrolysis. Electrolyzed water generated in the second electrolysis chamber 3b is drawn out. The concentration of the electrolyte aqueous solution circulated through the first electrolysis chamber 3a is maintained within a predetermined range. The membrane 2 is an anion-exchange membrane. The electrolyte aqueous solution is circulated through the first cathode-side electrolysis chamber 3a; raw water is supplied only to the second anode-side electrolysis chamber 3b; and acid electrolyzed water generated in the anode-side electrolysis chamber 3a is drawn out. The electrolyte aqueous solution is NaCl or KCl aqueous solution. The concentration is maintained within the predetermined range by adding hydrochloric acid according to the pH of the NaCl or KCl solution or the amount of reaction of this solution computed from the amount of energization during the electrolysis.
Owner:HONDA MOTOR CO LTD
Electrolytic process for generating chlorine dioxide
An electrolytic process for generating chlorine dioxide. An aqueous feed stream of an alkali metal chlorite solution is treated with chlorine gas or a mixture of hydrogen chloride and hypochlorous acid formed in an anode compartment from, an aqueous alkali metal chloride solution and subsequently electrolyzed to form a chlorine dioxide effluent.
Owner:NALCO CO +1
Aluminum electrolytic MES system based on accurate perception and intelligent decision
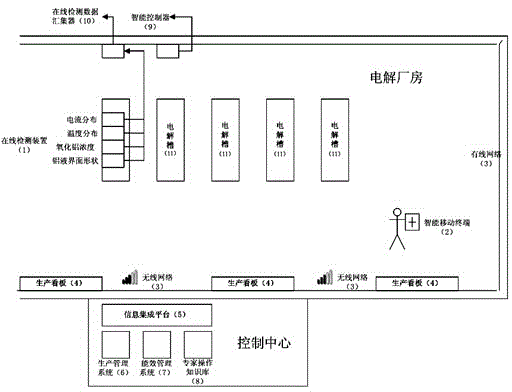
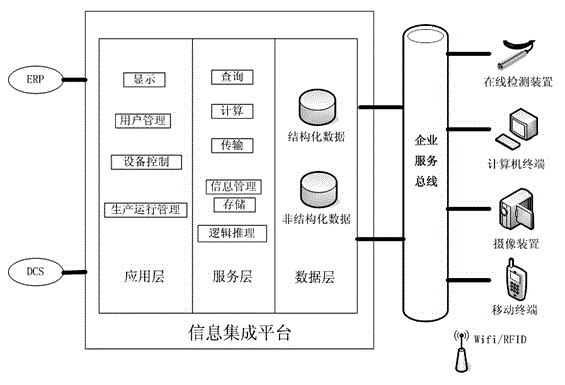
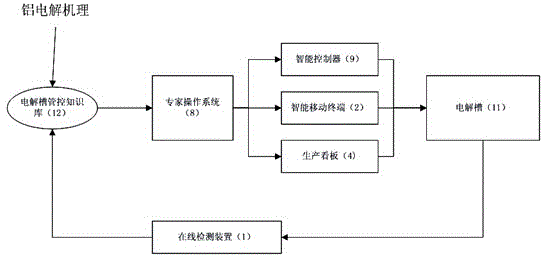
ActiveCN105467946ARealize precise perceptionReal-time detection of real-time production dataEnergy industryTotal factory controlManagement efficiencyInformation integration
The present invention discloses an aluminum electrolytic MES system based on an accurate perception and an intelligent decision. The system comprises an electrolytic tank online detection device (1) for detecting aluminum electrolytic real-time production data, an online detection data collection device (10) for collecting real-time production data, an intelligent mobile terminal (2) for obtaining the position information of an operator, a plant wireless or wired network (3), a production billboard (4) for information display, an information integration platform (5), a production management system (6) based on production process real-time data, an aluminum electrolytic process energy management system (7), an expert operation system (8) based on electrolytic tank (11) real-time production data, and an intelligent controller (9) for production control. According to the system, the degree of precision and the automation level of the aluminum electrolytic production management and control can be improved, the management efficiency is improved, and the influence on aluminum electrolytic production brought by personnel technology and management level differences can be reduced.
Owner:GUIYANG AL-MG DESIGN & RES INST
Method for preparing electronic-grade tetramethylammonium hydroxide (TMAH)
InactiveCN102206832ASimple processImprove current efficiencyElectrolysis componentsElectrolytic organic productionTetramethylammonium hydroxideBuffer tank
The invention discloses a method for preparing electronic-grade tetramethylammonium hydroxide (TMAH), belonging to the field of electrochemistry. In the method, tetramethylammonium bicarbonate is used as a raw material for electrolysis, a dual-film electrolyzer is used for preparing the electronic-grade TMAH, and the whole electrolyzer implements cyclic stirring of the electrolyte in the electrolytic process in an external cycle mode. The electrolytic conditions are as follows: the cathode tank uses a 0.2-0.5 mol / L TMAH solution; the buffer tank uses a 1.0-3.0 mol / L TMAHCO3 solution; the anode tank uses a 0.2-0.6 mol / L Na2SO4 solution; the temperature for electrolysis is 40-60 DEG C; and the current density is 400-1000 A / m<2>. The invention has the advantages of simple technological process, low raw material cost, high product yield (the content of metallic ions in the product is lower than 4ppm, achieving the standard for electronic-grade TMAH), high electrolytic-current efficiency (up to 80-90%), environmental protection, no pollution and high production safety.
Owner:ZHENGZHOU UNIV
Electrolytic copper foil with low-contour and high property and producing method thereof
ActiveCN101067210AReduce the roughness of the matte surfaceIncrease crystal densityElectroforming processesMetallic pattern materialsSurface roughnessCopper foil
The present invention discloses one kind of low roughness and high performance electrolytic copper foil with surface crystal grains in equiaxial pyramid form, surface roughness not greater than 2.5 micron, thickness of 18 micron, peel strength not lower than 0.45 kg / cm, tensile strength not lower than 330 MPa and elongation not lower than 6 %. The present invention discloses also the preparation process of the electrolytic copper foil, and prepares electrolytic copper foil through a DC electrodeposit process with electrolyte with proper composition and added mixed organic additive and reasonable electrodeposit technological parameters. The electrolytic copper foil has low roughness and stable and high performance, and may be used in preparing fine circuit.
Owner:湖北中科铜箔科技有限公司
Alloy material suitable for inert anode of metal fused-salt electrolysis cell
InactiveCN101717969AReduce consumptionEliminate emissionsElectrodesAluminium electrolysisMolten salt
The invention relates to an alloy material suitable for an inert anode of a metal fused-salt electrolysis cell, relating to metal fused-salt electrolysis, in particular to a carbon-free metal-base alloy inert anode of an aluminium electrolysis cell. The carbon-free metal-base alloy inert anode of the aluminium electrolysis cell is characterized in that the alloy material comprises the following components in percentage by weight: 5-30% of Ni, 5-20% of Al, and the balance of Cu. The alloy material suitable for the inert anode of the metal fused-salt electrolysis cell is suitable for a lower-temperature (700 DEG C-850 DEG C) electrolyte system; in addition, the alloy inert anode has good high-temperature oxidation resistance and electrolyte corrosion resistance in the electrolytic processes, and electrolyzed aluminium products reach the quality more than 99.7 percent.
Owner:GUIZHOU BRANCH CHINA ALUMINUM IND
Producing lithium
InactiveUS20150014184A1Energy consumptionLithium ion conducting composite layer is stableCellsPhotography auxillary processesLithium metalLithium carbonate
A electrolytic process for continuous production of lithium metal from lithium carbonate or other lithium salts by use of an aqueous acid electrolyte and a lithium producing cell structure which includes: a cell body with a cathode within the cell body; an electrolyte aqueous solution within the cell body, the solution containing lithium ion and an anion; and a composite layer intercalated between the cathode and the electrolyte aqueous solution, the composite layer comprising a lithium ion conductive glass ceramic (LI-GC) and a lithium ion conductive barrier film (LI-BF) that isolates cathode-forming lithium from the electrolyte aqueous solution.
Owner:CLEAN LITHIUM
Method for extracting steel superfine varia by electrolysis method
InactiveCN101074907ASimple and fast operationPreparing sample for investigationTesting metalsMetallurgyElectrochemistry
A method of applying electrolytic process to extract out superfine impurities in steel includes preparing organic electrolyte by 2wt% of tetramethylsalmiac, 8wt% of diacetone, 5wt% of glycerin, 6wt% of trolamine, allowance of pure methanol and 5g / L of DPG as per valum / weight; polishing and washing steel sample then placing steel sample in electrolytic tank; using steel sample as anode; leading inter-gas of argon in and regulating electrolytic potential, holding steel sample in said tank for 20-40hours then using vacuum filtering device to separate out superfine impurities in steel sample under vacuum condition.
Owner:SHANGHAI UNIV
Dynamic evolution modeling method for aluminum electrolysis process electrolytic bath technology energy consumption
InactiveCN103345559AStrong tracking abilityFast convergenceSpecial data processing applicationsRate of convergenceComputer science
Provided is a dynamic evolution modeling method for aluminum electrolysis process electrolytic bath technology energy consumption. The method is characterized by including the following steps of step 1, collecting data [XN, Y], step 2, carrying out normalization processing on the collected data, step 3, carrying out modeling on the data after the normalization processing by strongly tracking a square root trackless Kalman neural network, and step 4, estimating an electrolysis process energy consumption value by applying an established model to obtain a technology energy consumption value of the electrolysis process at the moment. The method has the advantages that advantages of strong tracking filtering and square root filtering are combined, convergence rates of the model and tracking ability on electrolytic bath mutation states are improved, the algorithm is stable, accuracy is high, tracking ability on the electrolytic bath mutation states is strong, therefore, real time estimation on the aluminum electrolysis process electrolytic bath technology energy consumption is achieved, technology operations on the aluminum electrolysis process can be optimized, and the purposes of saving energy and reducing emission can be achieved.
Owner:CHONGQING UNIVERSITY OF SCIENCE AND TECHNOLOGY
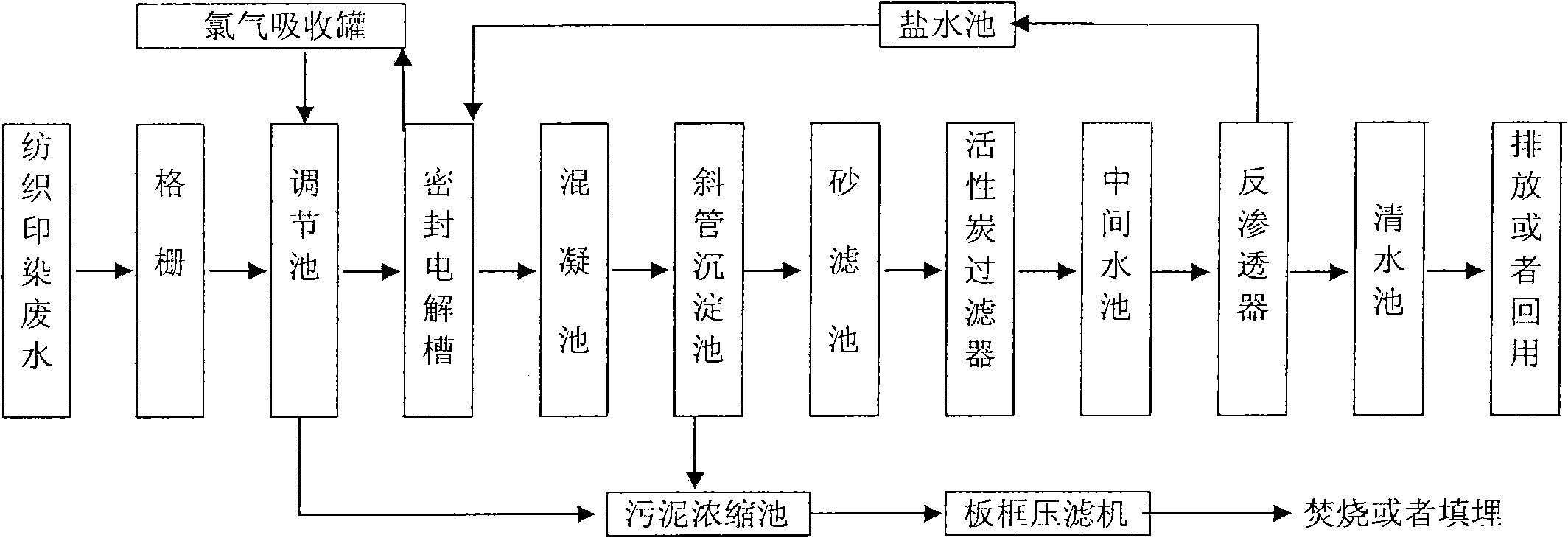
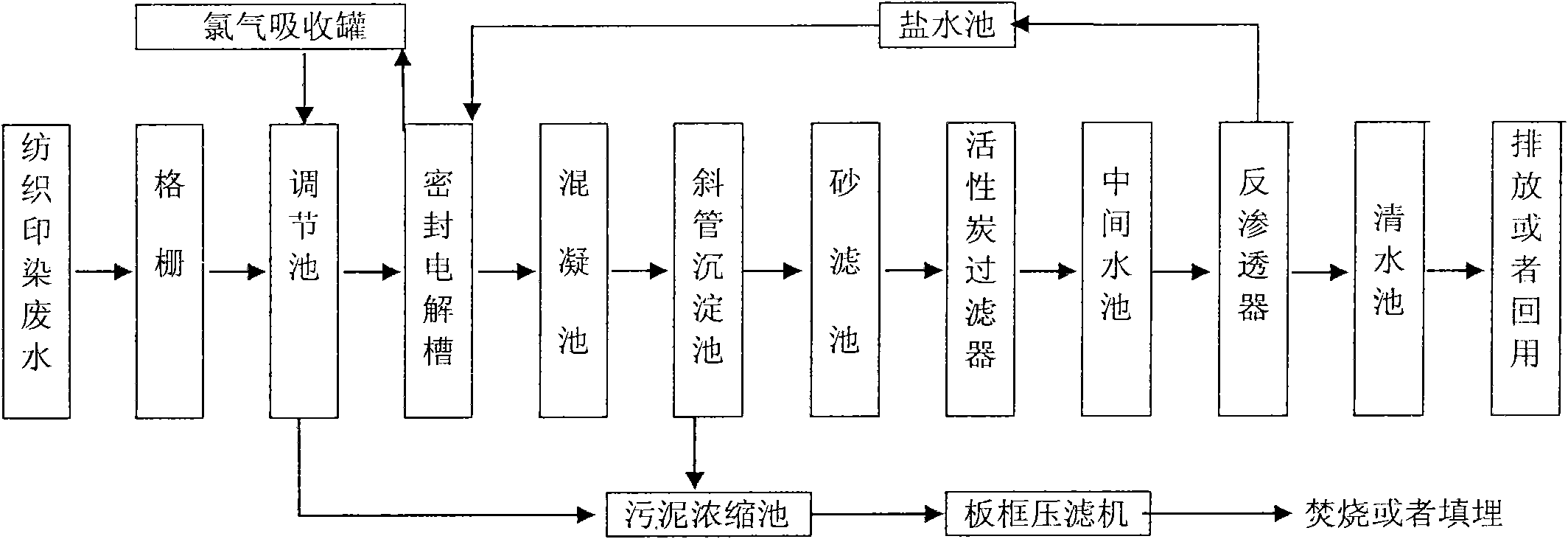
Spinning printing and dyeing waste water reclamation method, device and application thereof
InactiveCN101857328AEfficient removalReduce work stressWater/sewage treatment bu osmosis/dialysisMultistage water/sewage treatmentAluminium chlorideActivated carbon filtration
The invention discloses a spinning printing and dyeing waste water reclamation method, a device and the application thereof. The method comprises the steps of: filtering spinning printing and dyeing waste water; sequentially carrying out homogenizing, equalizing quantity and primary sedimentation on filtrate; adding sodium chloride into waste water supernate, and evenly mixing for indirect electro-catalysis oxidation; sequentially evenly mixing the waste water treated by indirect electro-catalysis oxidation with basic aluminium chloride and polyacrylamide; removing the precipitated supernate, and sequentially treating by activated carbon adsorption and reverse osmosis; removing trace organic chloride generated in the electrolytic process and the rest COD and color; and obtaining recyclable water resource. The device for realizing the method comprises a grate, a regulating reservoir, a sealed electrolytic cell, a flocculation tank, an inclined tube sedimentation tank, a sand filter tank, an active carbon filter, an intermediate water tank, a reverse osmosis unit, a clear water reservoir, a brine tank, a chlorine absorbing tank, a sludge concentration tank and a plate-and-frame filter press. The invention can effectively treat spinning printing and dyeing waste water, and has the advantages of high treatment efficiency, stable effluent quality, simple technological process and the like.
Owner:DONGGUAN HONGJIE ENVIRONMENTAL PROTECTION TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com