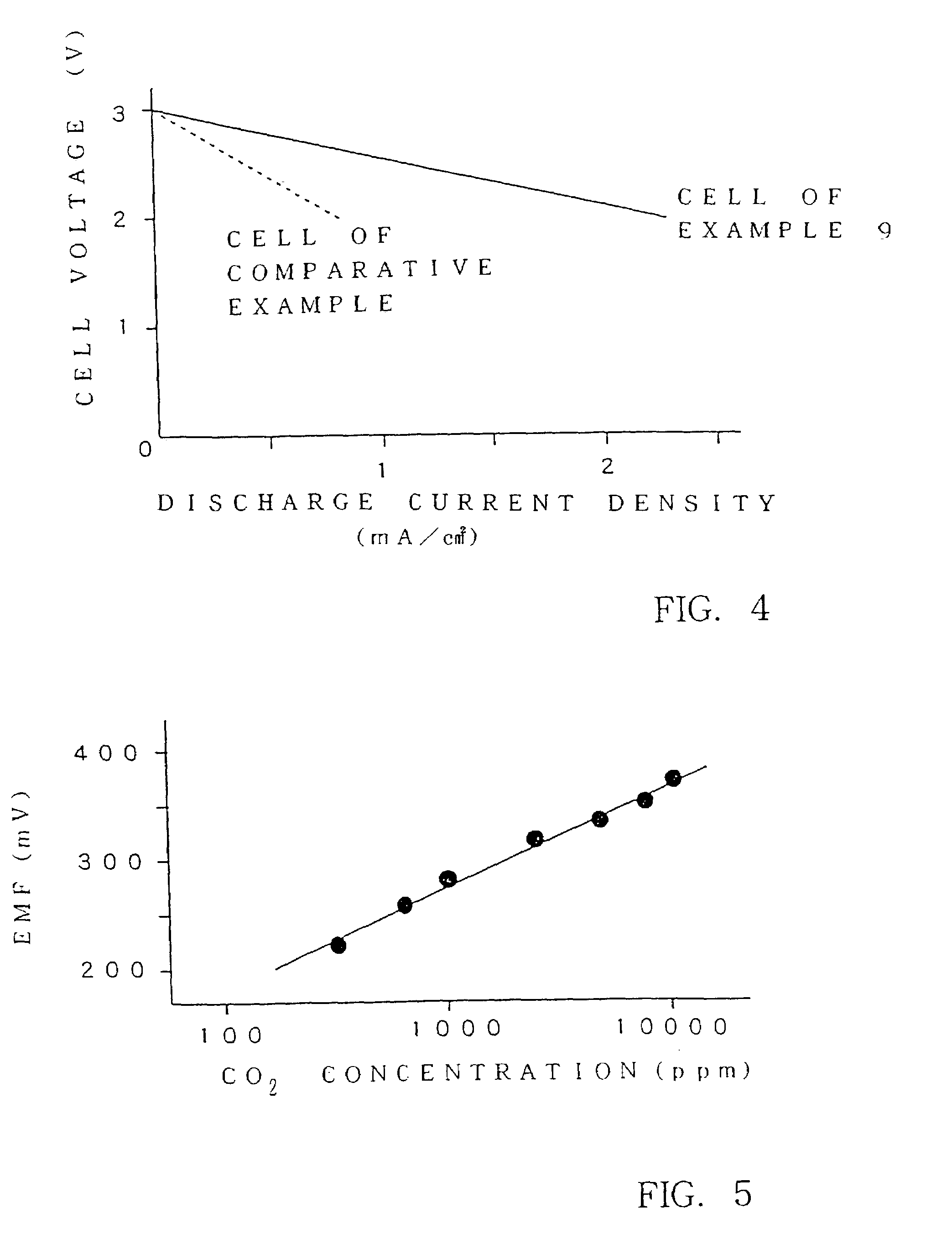
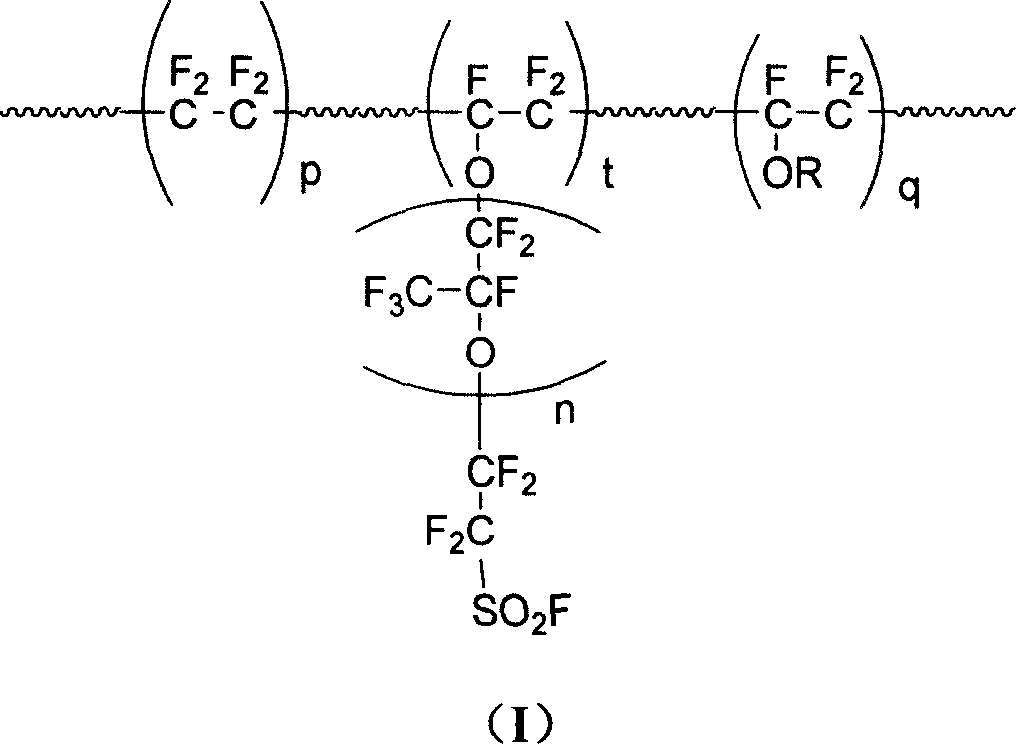
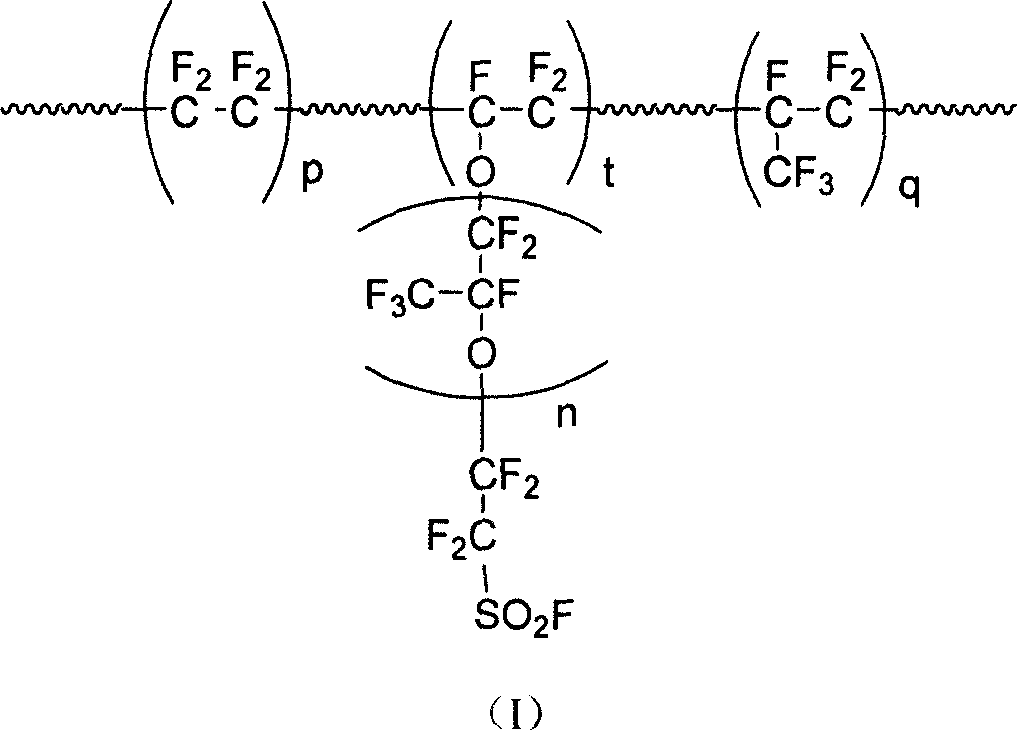
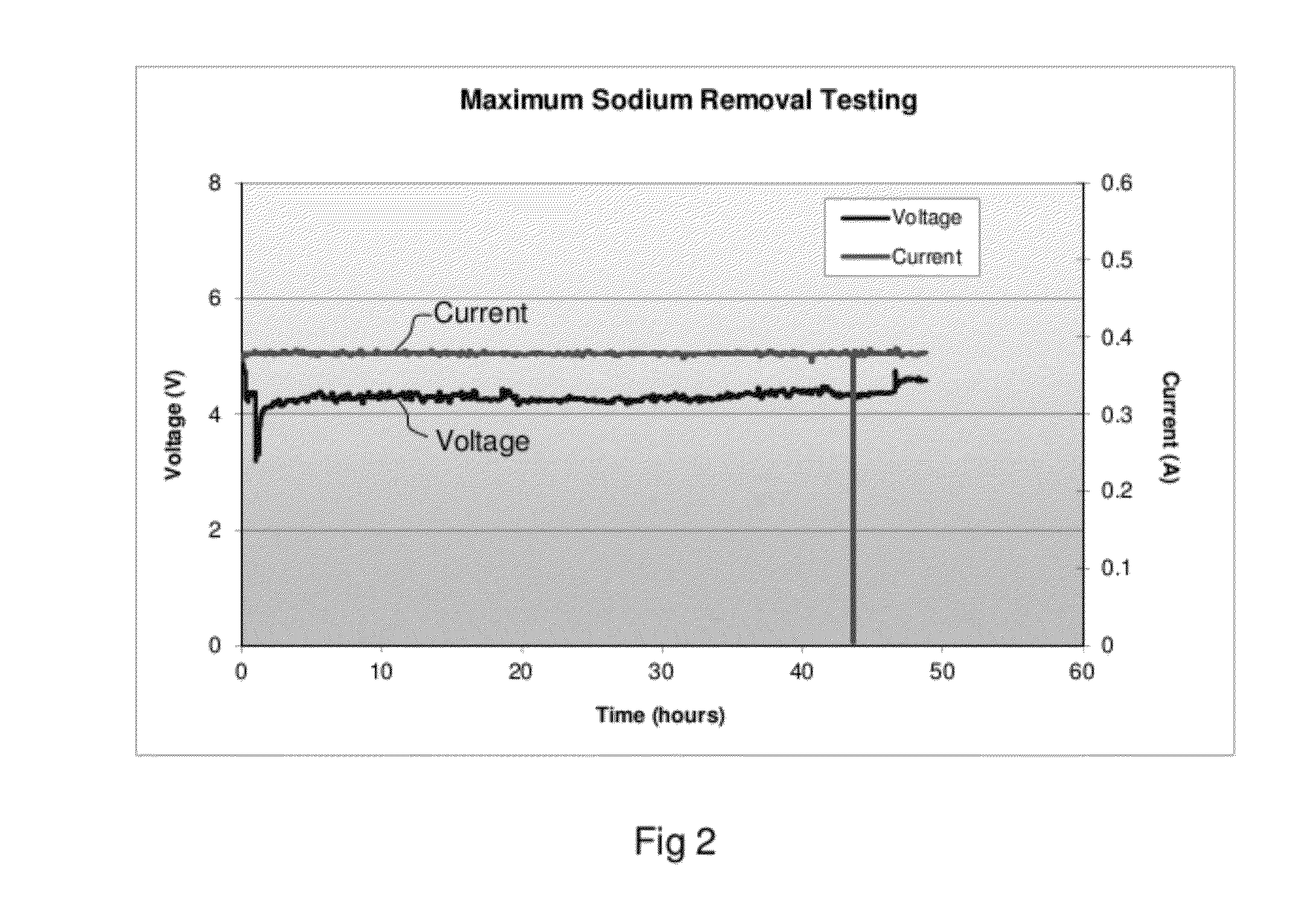
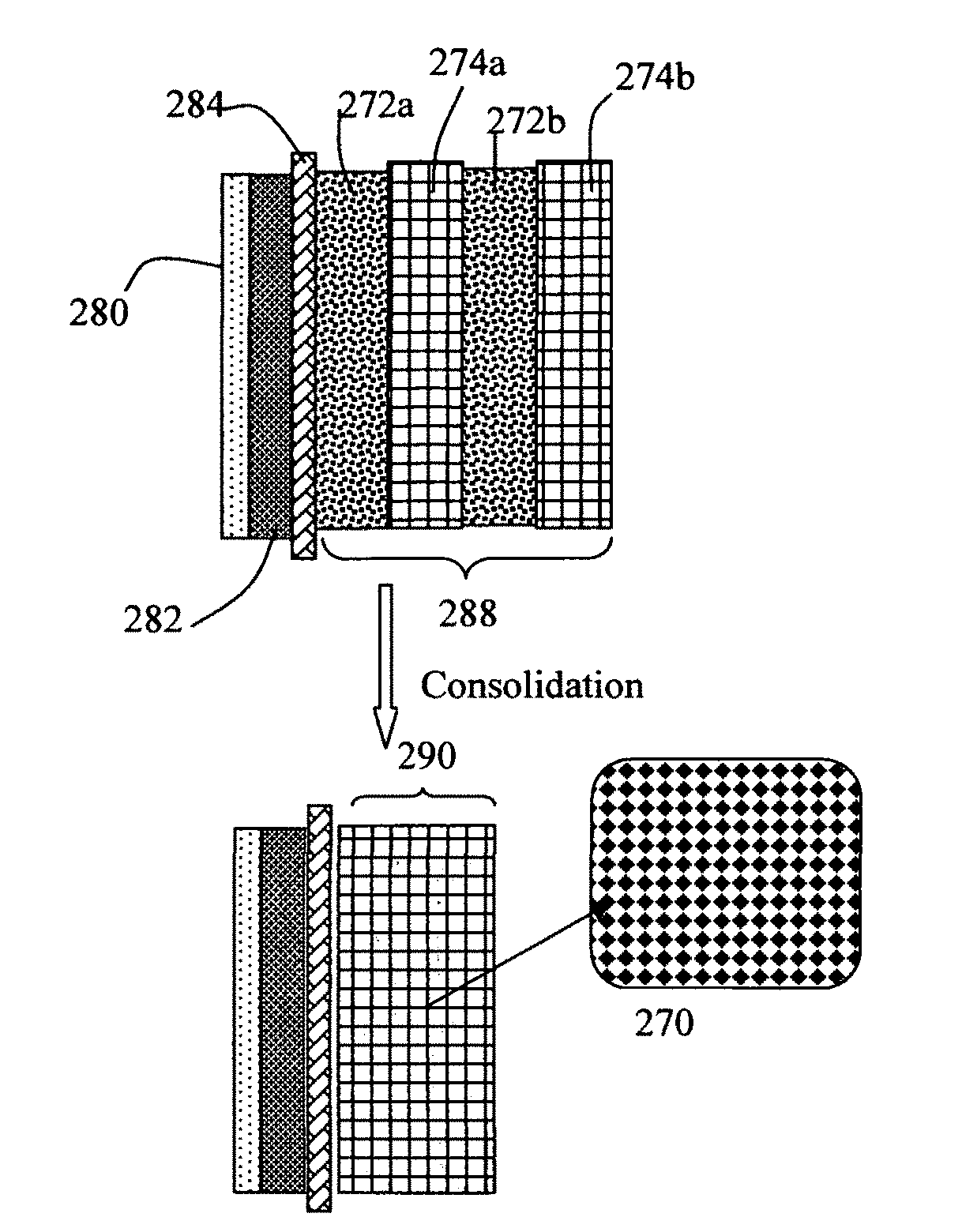
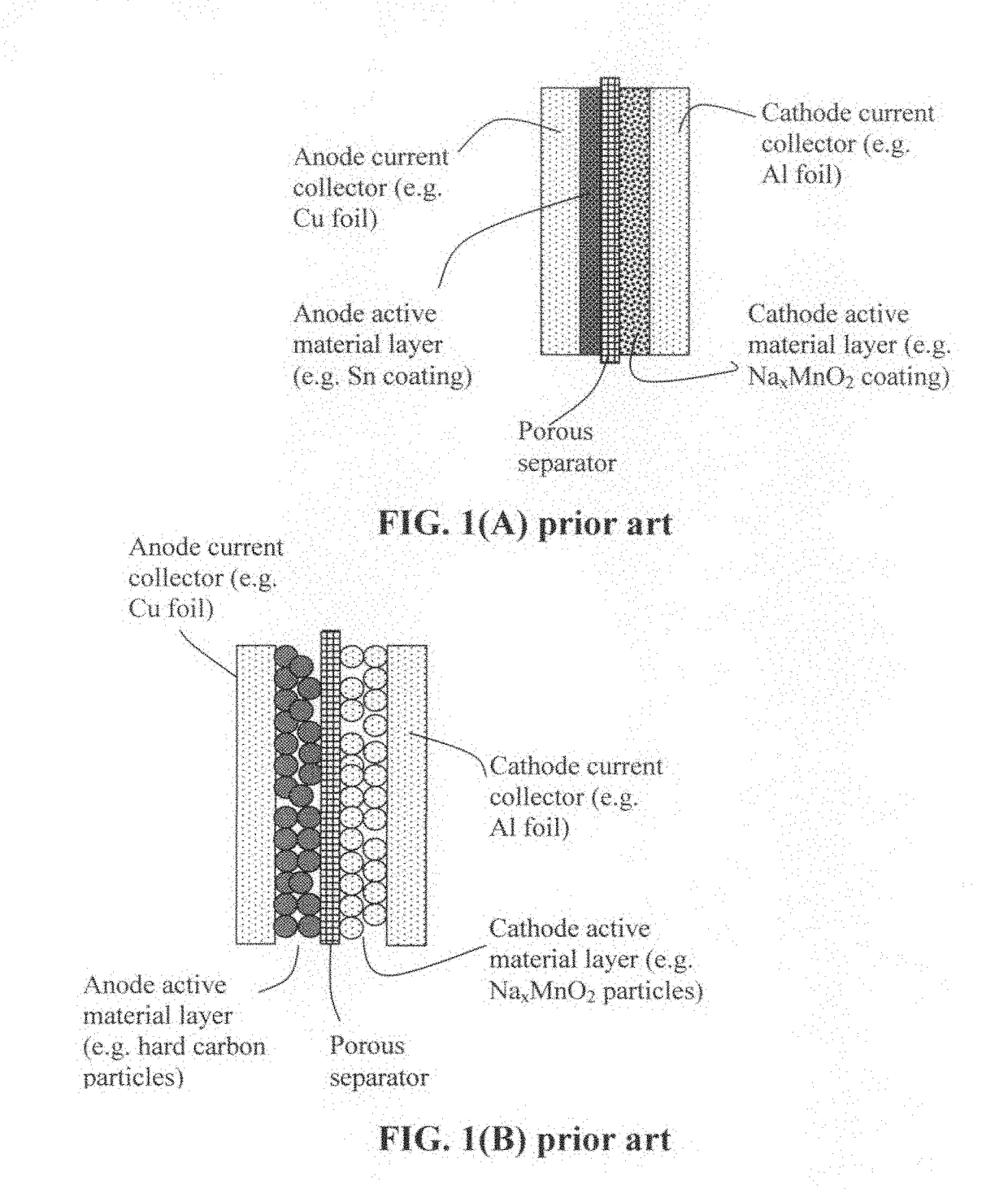
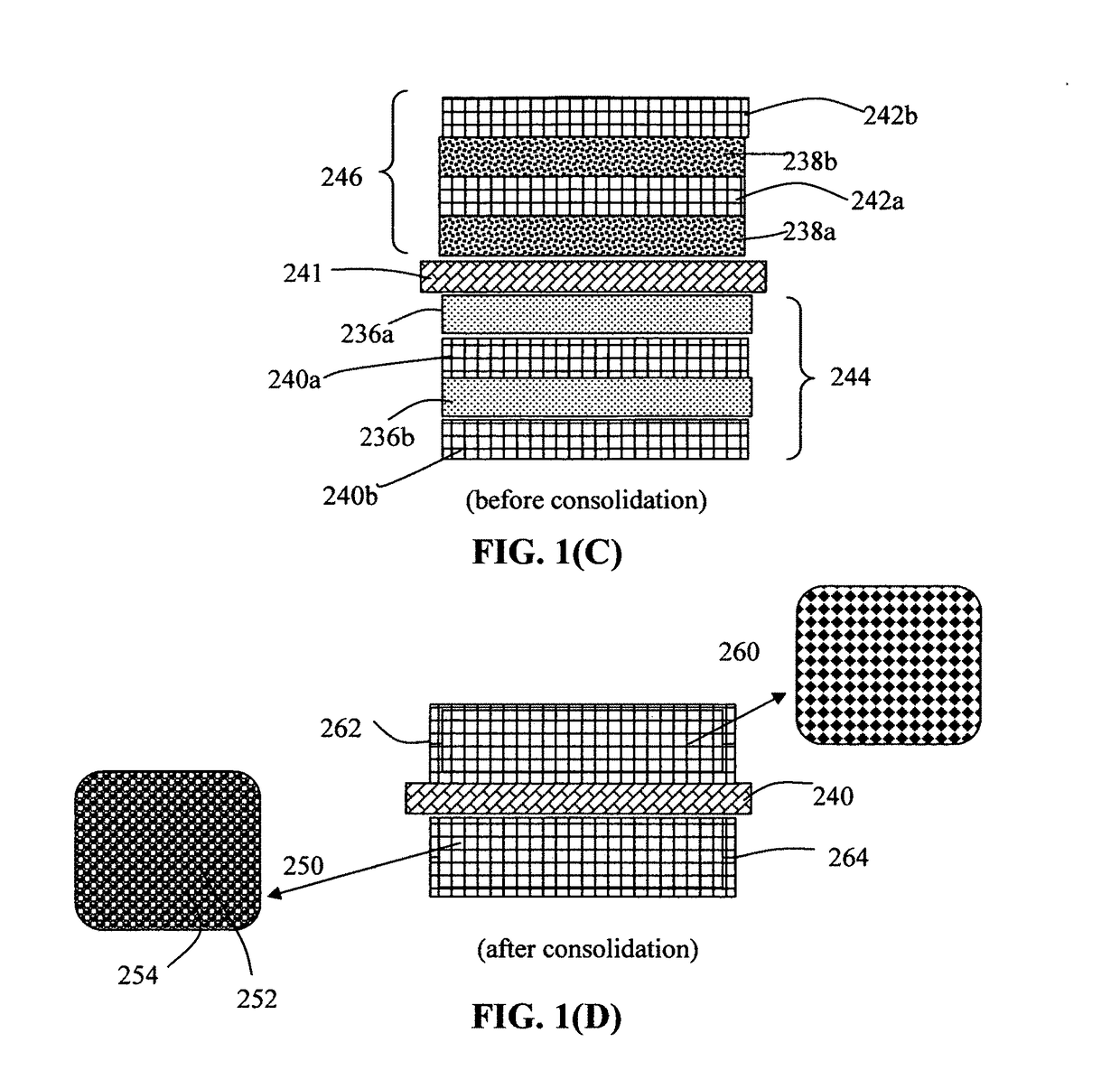
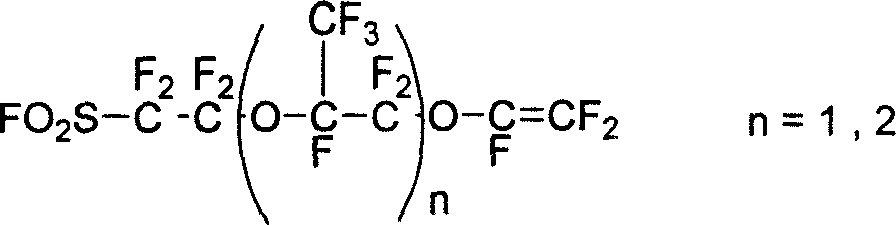Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
235 results about "Alkali ions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The ion found in all alkalis is hydroxide ion. That said, there is a group of metals called alkali metals (NOT ions) which are in group I of the periodic table. They get their name because they react with water to form alkaline solutions. The lithium hydroxide is an alkali as it is soluble and contains hydroxide ion.
Method of forming an oxide film
InactiveUS6960812B2Improve performanceReduce the temperatureSolid-state devicesVacuum evaporation coatingAlkali ionsSputtering
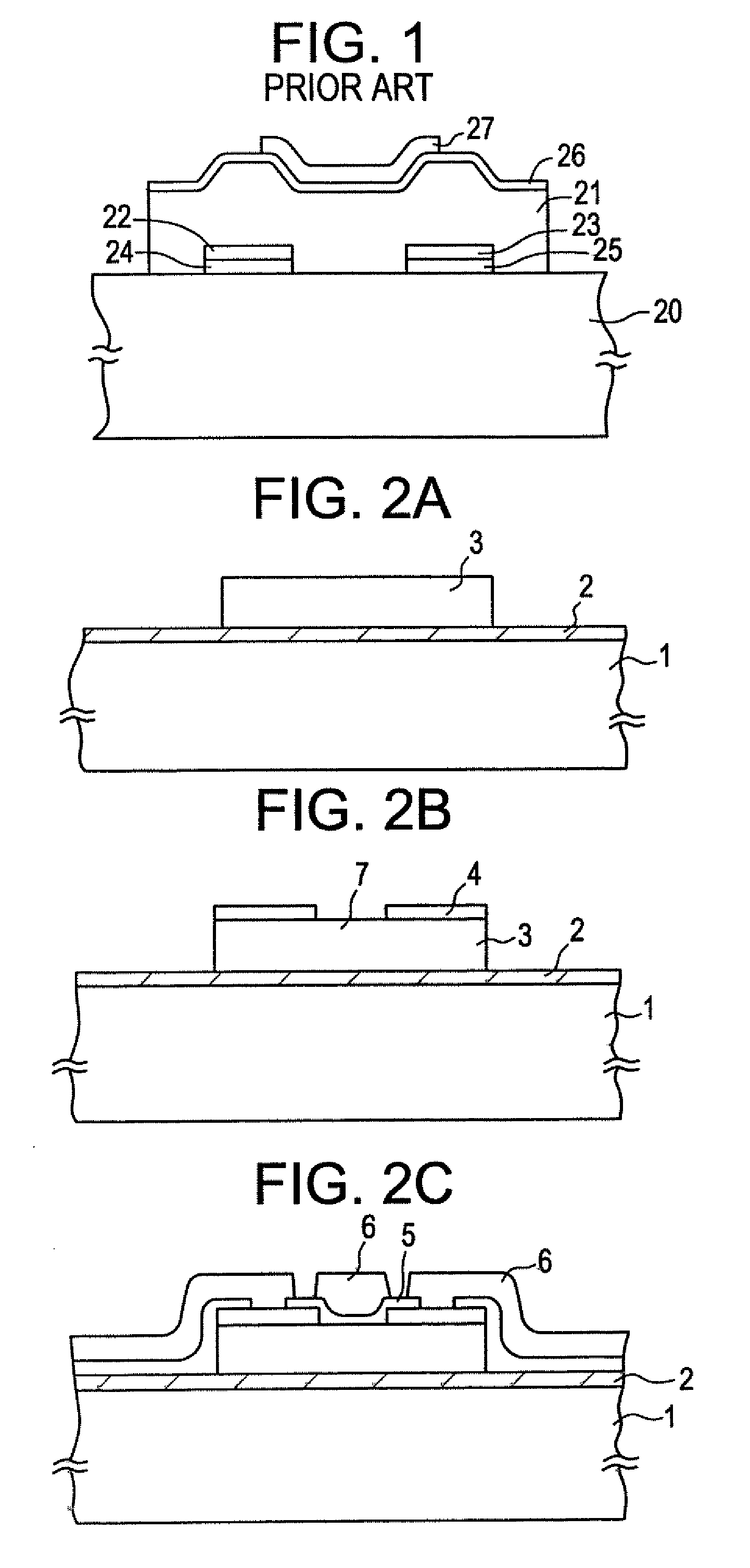
A method of forming an oxide film and a method of manufacturing an electronic device utilizing the oxide film is disclosed. A silicon oxide film is formed on a substrate by sputtering. Therefore, the film formation is carried out at a low temperature. The sputtering atmosphere comprises an oxidizing gas and an inert gas such as argon. In order to prevent fixed electric charges from being generated in the film and to obtain an oxide film of good properties, the proportion of argon is adjusted to 20% or less. Alternatively, a gas including halogen elements such as fluorine is added to the above sputtering atmosphere at a proportion less than 20%. Hereupon, alkali ions and dangling bonds of silicon in the oxide film are neutralized by the halogen elements, whereby a fine oxide film is obtained.
Owner:SEMICON ENERGY LAB CO LTD
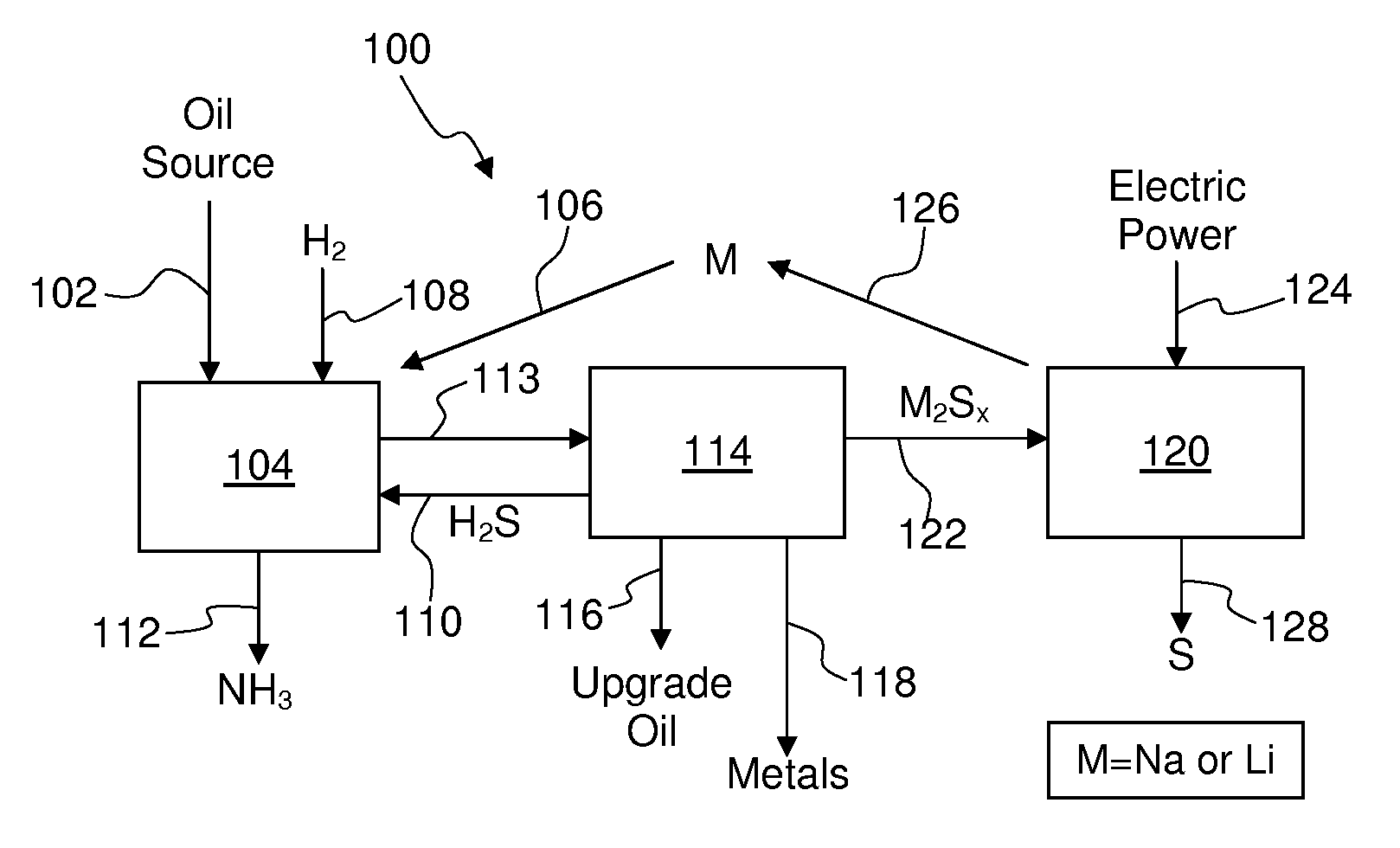
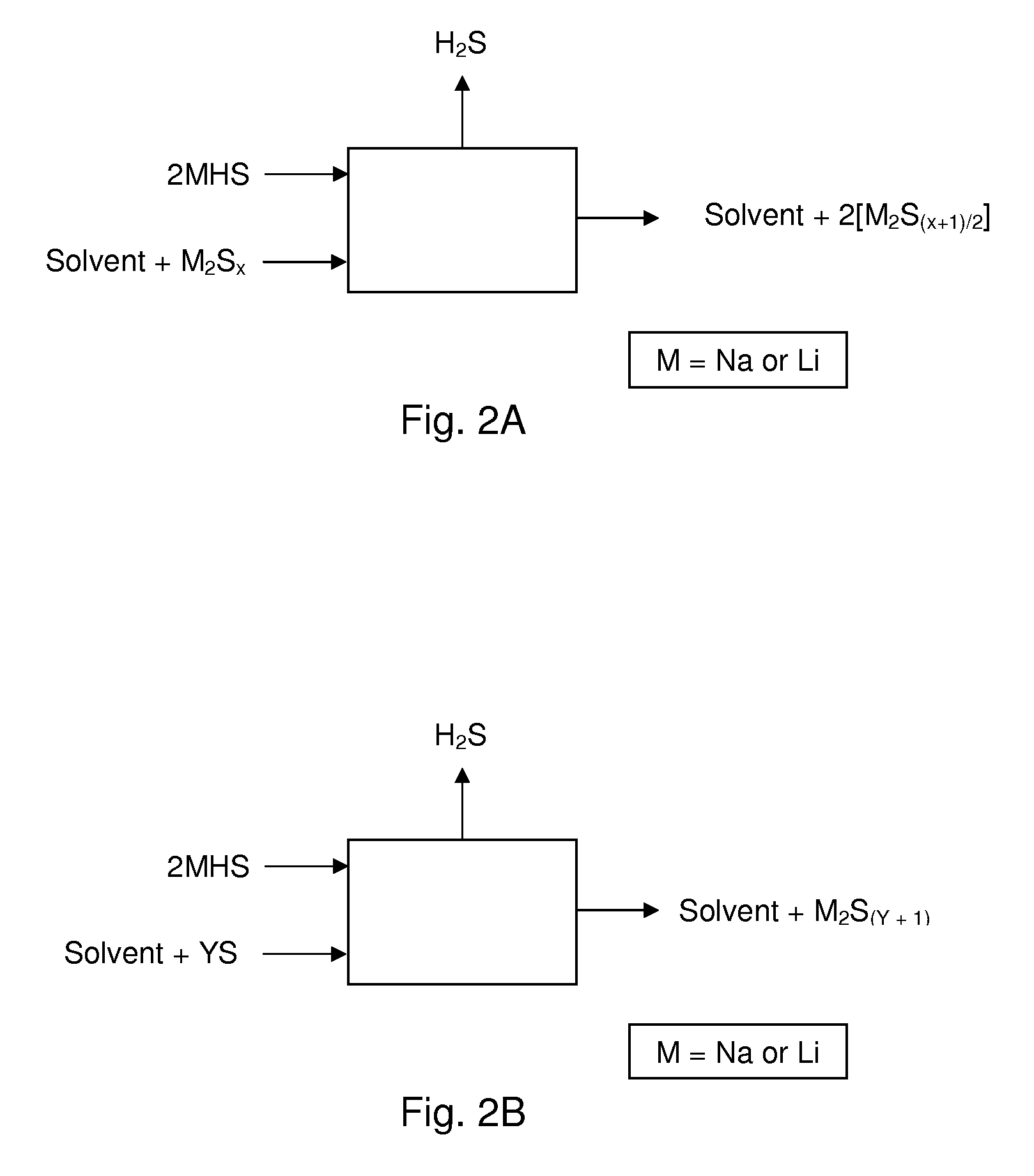
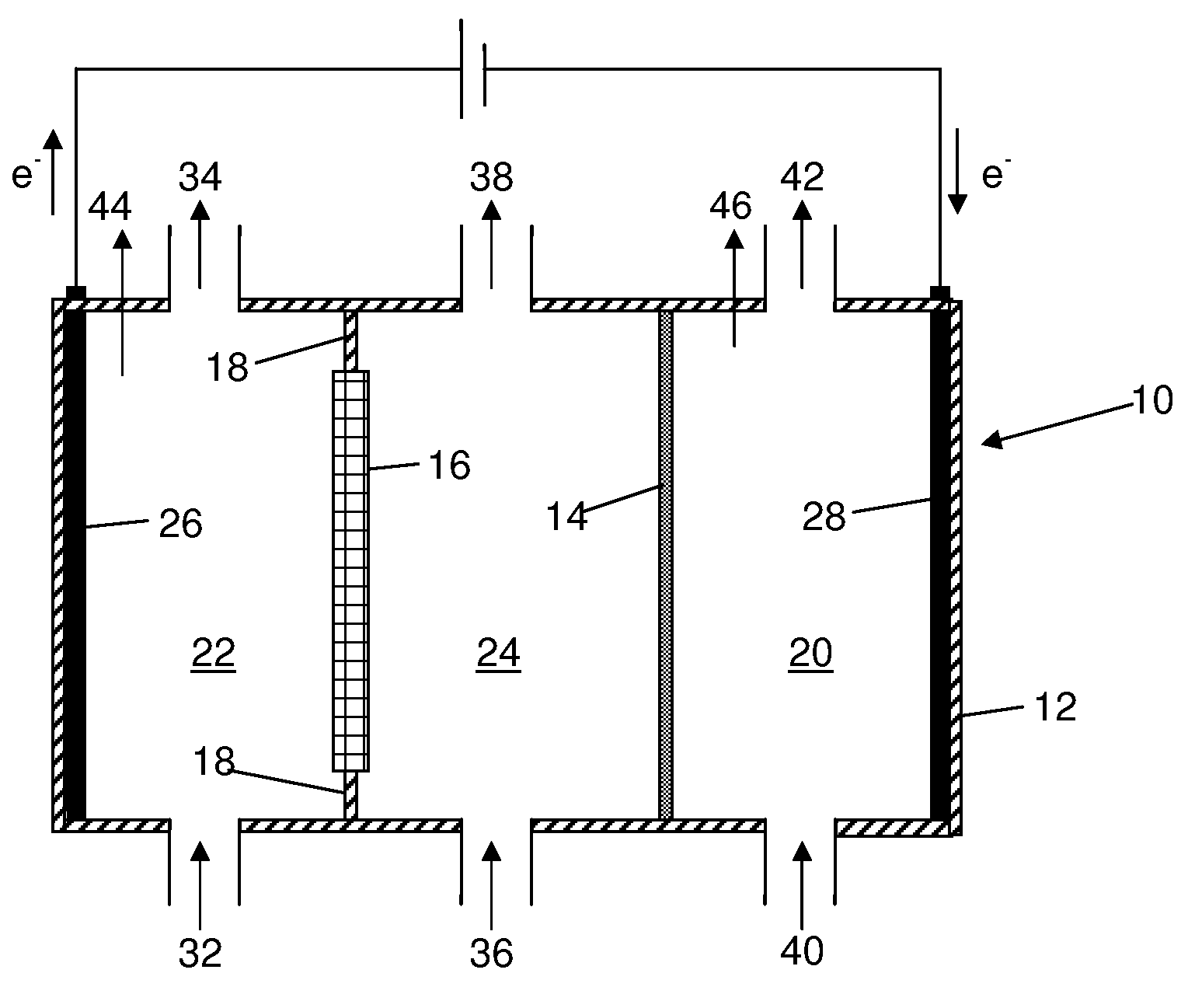
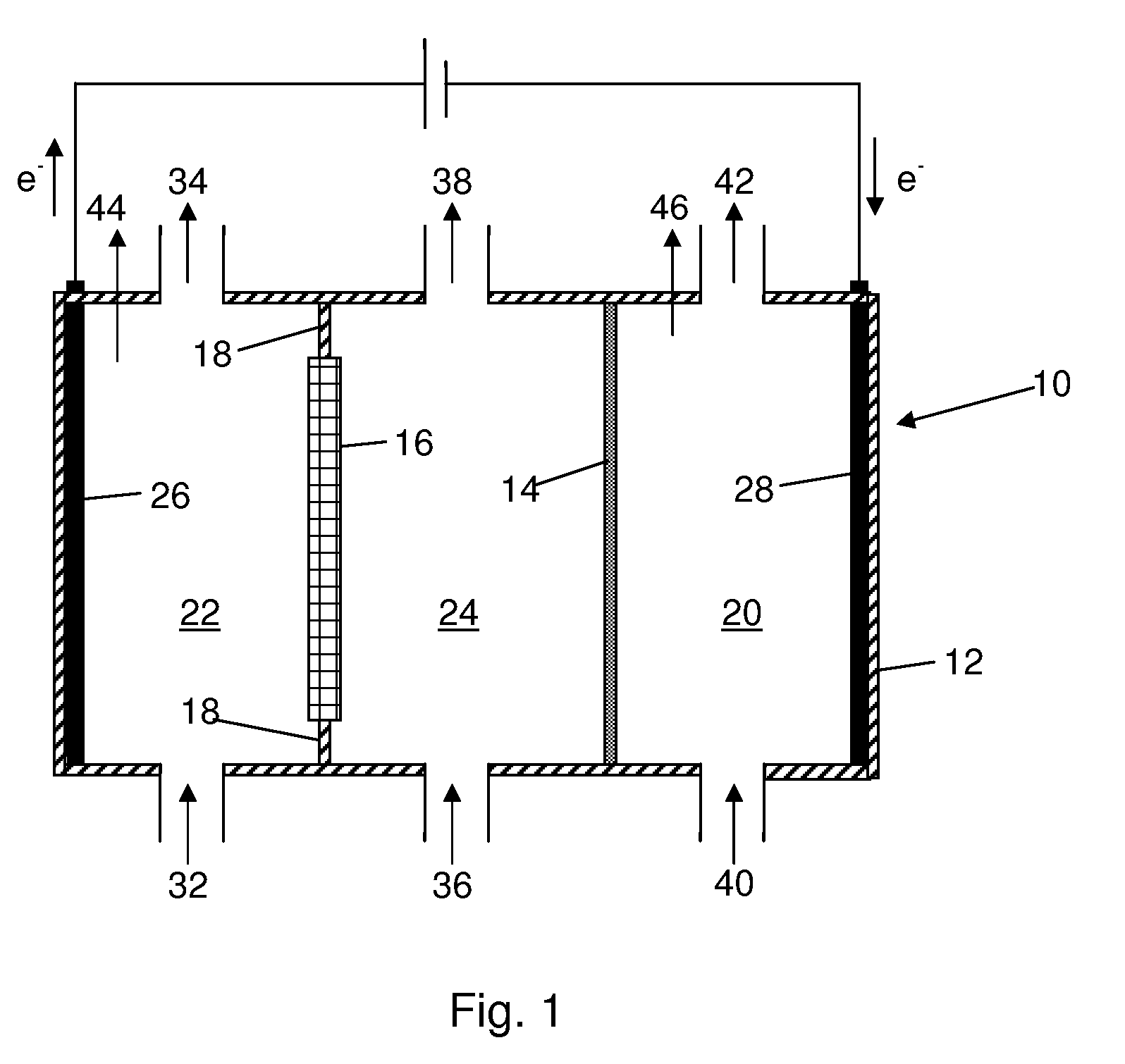
Process For Recovering Alkali Metals and Sulfur From Alkali Metal Sulfides and Polysulfides
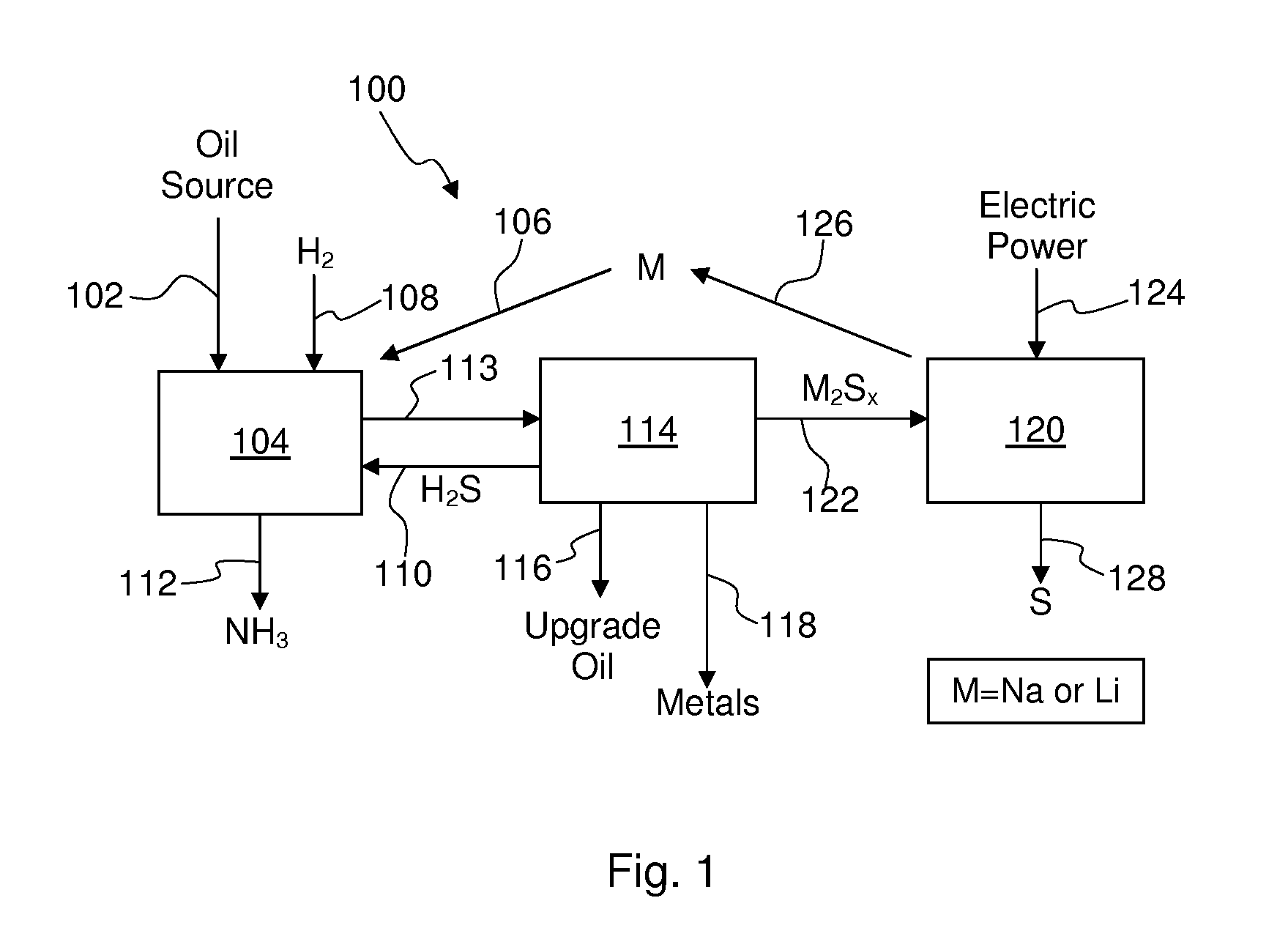
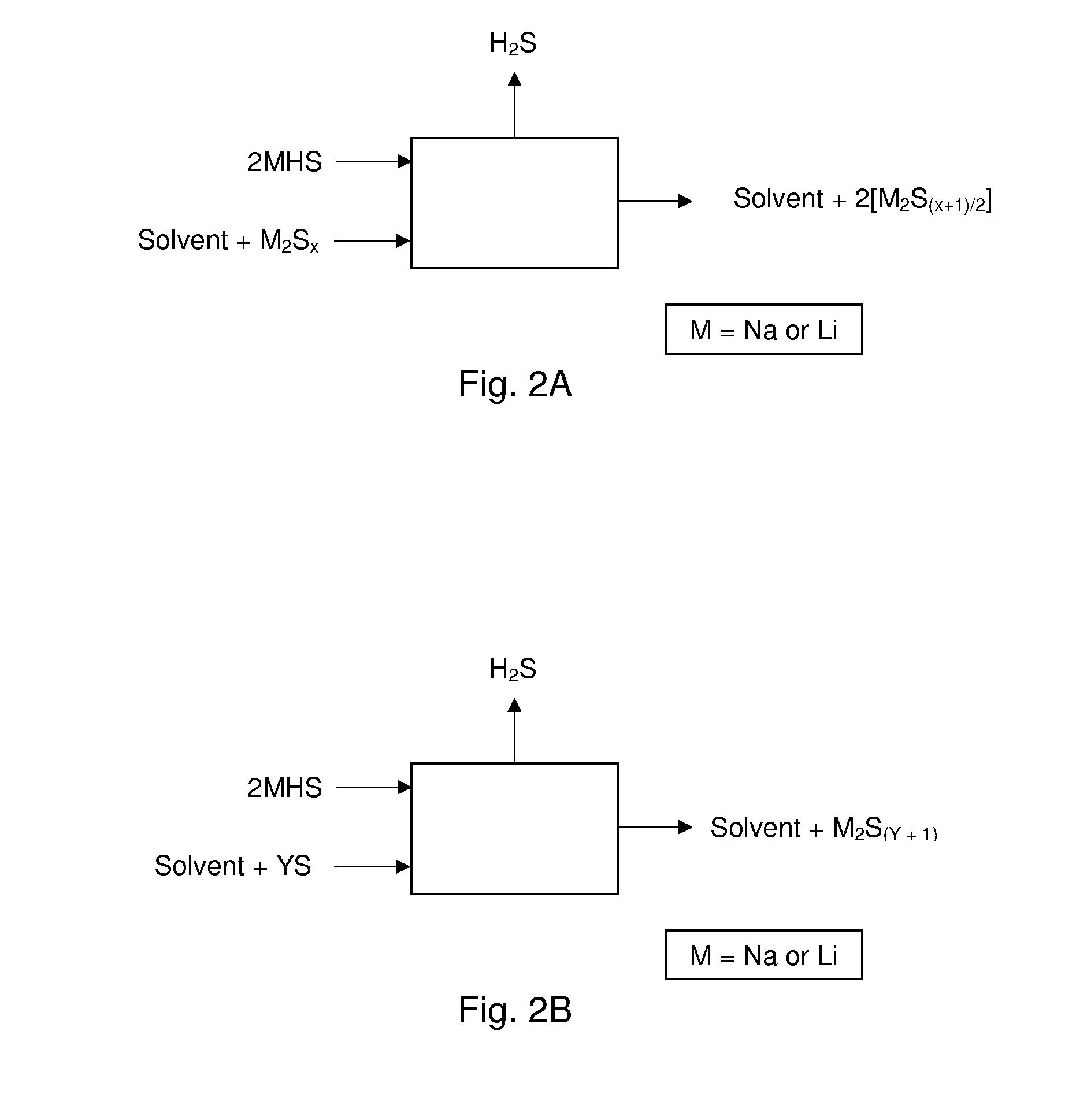
Alkali metals and sulfur may be recovered from alkali polysulfides in an electrolytic process that utilizes an electrolytic cell having an alkali ion conductive membrane. An anolyte solution includes an alkali polysulfide and a solvent that dissolves elemental sulfur. A catholyte solution includes alkali metal ions and a catholyte solvent. Applying an electric current oxidizes sulfur in the anolyte compartment, causes alkali metal ions to pass through the alkali ion conductive membrane to the catholyte compartment, and reduces the alkali metal ions in the catholyte compartment. Sulfur is recovered by removing and cooling a portion of the anolyte solution to precipitate solid phase sulfur. Operating the cell at low temperature causes elemental alkali metal to plate onto the cathode. The cathode may be removed to recover the alkali metal in batch mode or configured as a flexible band to continuously loop outside the catholyte compartment to remove the alkali metal.
Owner:ENLIGHTEN INNOVATIONS INC
Alkali ion conductive glass-ceramics and electric cells and gas sensors using the same
InactiveUS7211532B2Improve performanceImprove conductivityMaterial analysis by electric/magnetic meansSecondary cellsLithiumAlkali ions
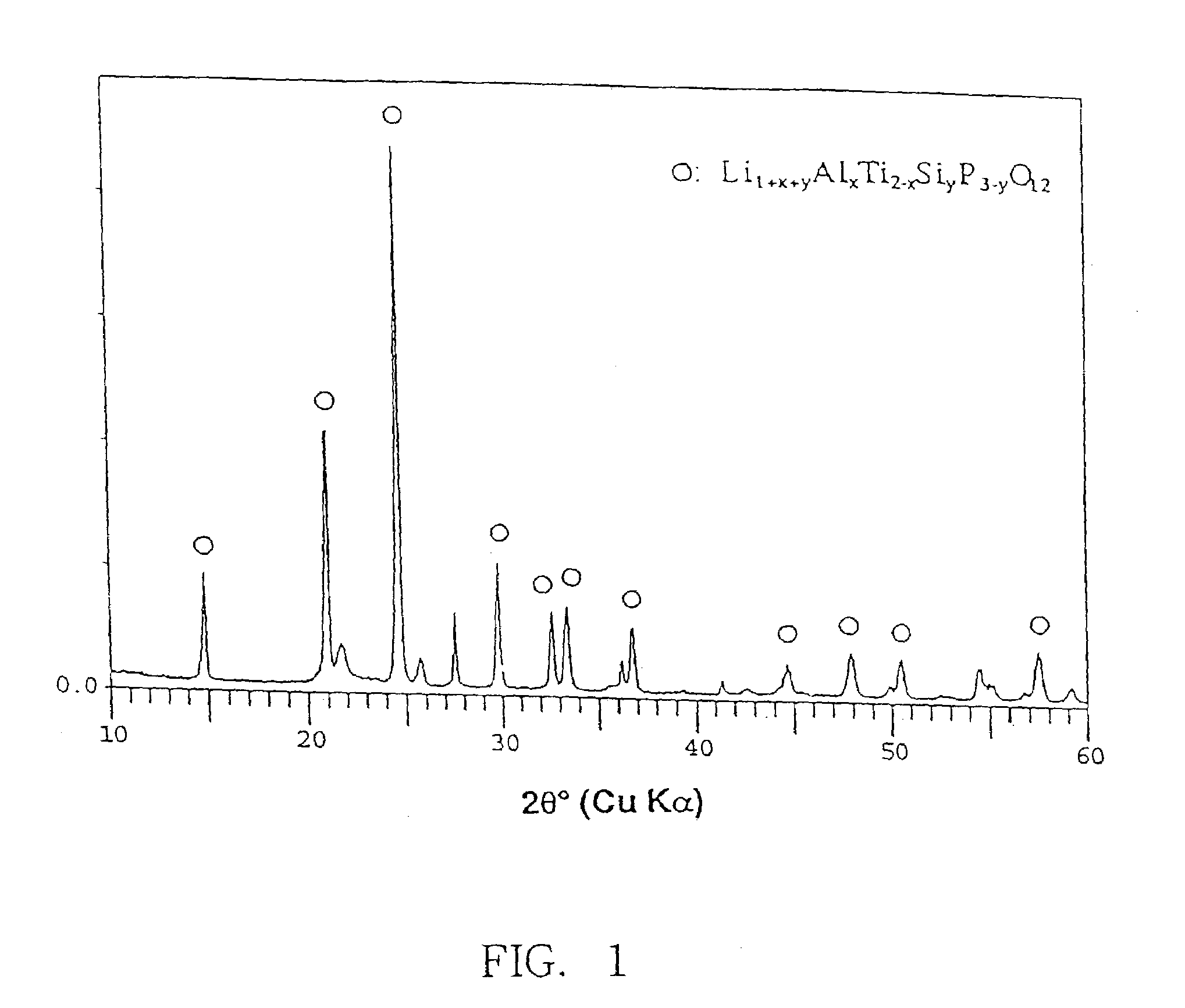
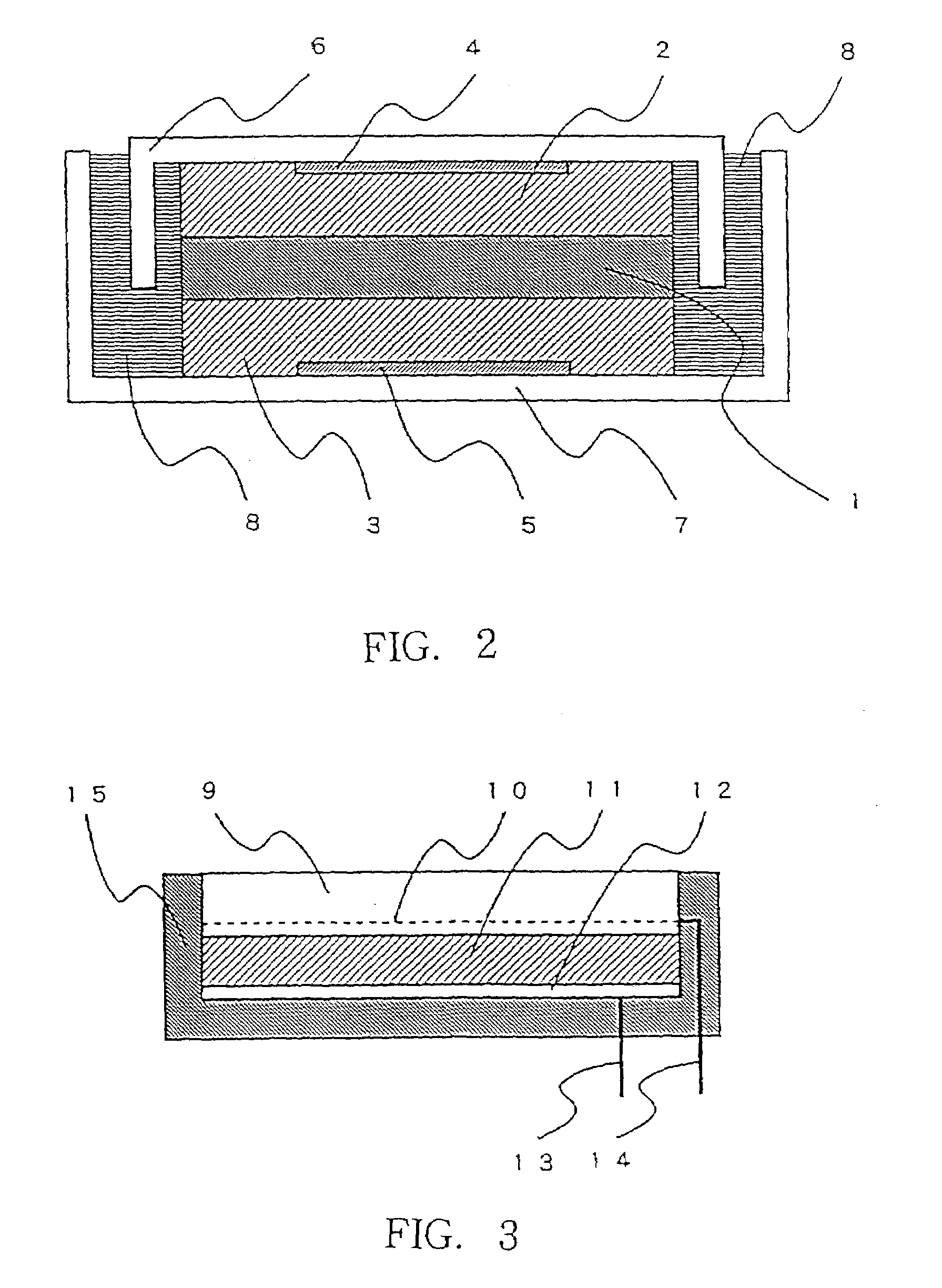
There are provided glass-ceramics having a high lithium ion conductivity which include in mol %:P2O538–40%TiO225–45%M2O3 (where M is Al or Ga) 5–15%Li2O10–20%and contain Li1+X(Al, Ga)XTi2−X(PO4)3 (where 0<X<0.8) as a main crystal phases. There are also provided glass-ceramics having a high lithium ion conductivity which include in mol %:P2O5 26–40%SiO20.5–12%TiO2 30–45%M2O3 (where M is Al or Ga) 5–10%Li2O 10–18%and contain Li1+X+YMXTi2−XSiYP3−YO12 (where 0<X≦0.4 and 0<Y≦0.6) as a main crystal phase. There are also provided solid electrolytes for an electric cell and a gas sensor using alkali ion conductive glass-ceramics, and a solid electric cell and a gas sensor using alkali ion conductive glass-ceramics as a solid electrolyte.
Owner:OHARA
Polymer of containing fluorin, and application as material of ion exchange fiber
InactiveCN101003588AIncrease the effective areaLower resistanceMelt spinning methodsVinyl etherAlkali ions
This invention discloses a fluorine-containing polymer and its application as ion exchange fiber material. The fluorine-containing polymer is a perfluoro resin containing sulfonylfluoride groups, and is shown in formula 1. The fluorine-containing polymer has ion exchange function, and is prepared by free radical copolymerization of perfluorosulfonyl vinyl ether, tetrafluoroethylene and hexafluoropropylene in the presence of dispersant, solvent and initiator. The dispersant / solvent is mixed solution of melamine derivative containing linear perfluoro hydrocarbon and water. The fluorine-containing polymer can be melt-spun into polymer fibers, which can be woven into fiber network that can be used as the reinforcing network material for ion exchange membranes and chlor-alkali ion membrane to reinforce and improve the ion exchange ability.
Owner:SHANDONG HUAXIA SHENZHOU NEW MATERIAL
Process for producing thin glass on roll
InactiveUS6092392ALow probability of fractureImprove productivityRibbon machinesDuplicating/marking methodsFiberglass meshFiber
This invention relates to a continuous process for producing a web of thin, chemically hardened glass that can be wound on a roll. The process comprises the steps of (i) drawing glass, containing original alkali ions, to form a web of glass having a thickness equal to or lower than 1.2 mm and having a first and second major surface; (ii) directly after or during said drawing, treating both said surfaces of said web with chemical hardening means during less than two hours, replacing said original alkali ions by alkali ions having a larger radius; and (iii) after treating both said surfaces, winding said web on a core.
Owner:AGFA-GEVAERT NV
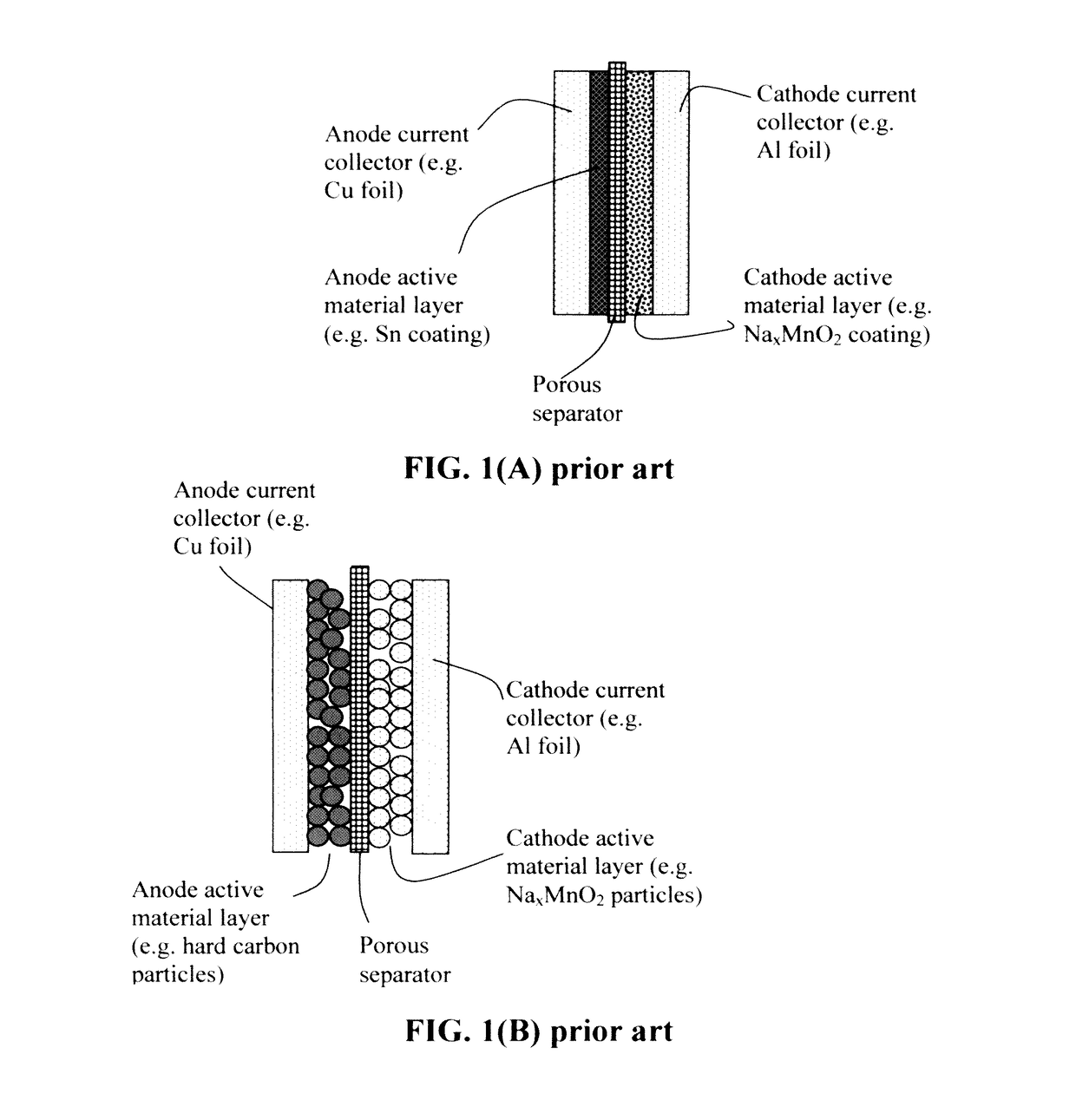
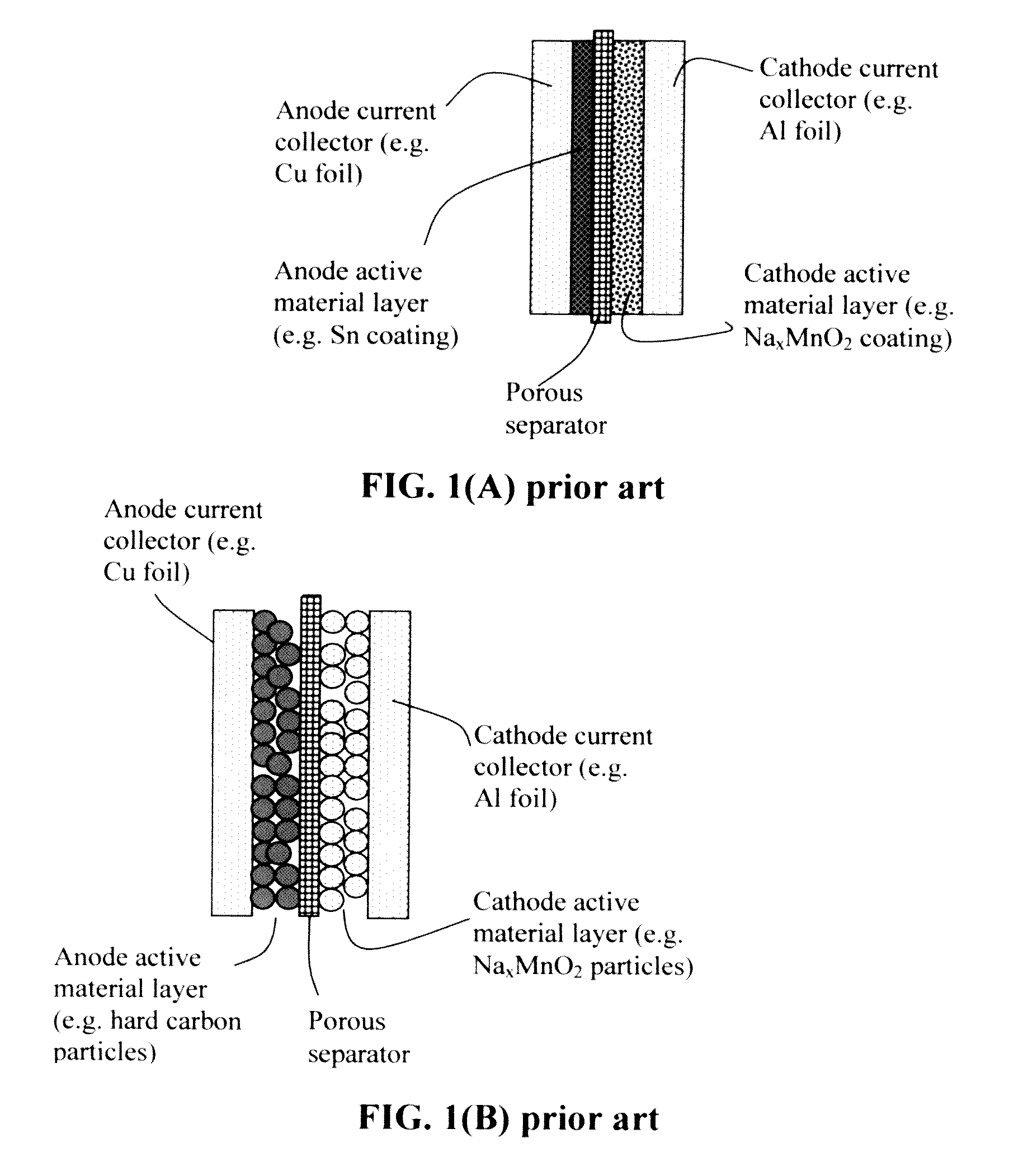
Alkali metal or Alkali-Ion batteries having high volumetric and gravimetric energy densities
ActiveUS20170077546A1Increase energy densityImprove power densityElectrode carriers/collectorsSecondary cellsAlkali ionsFluid electrolytes
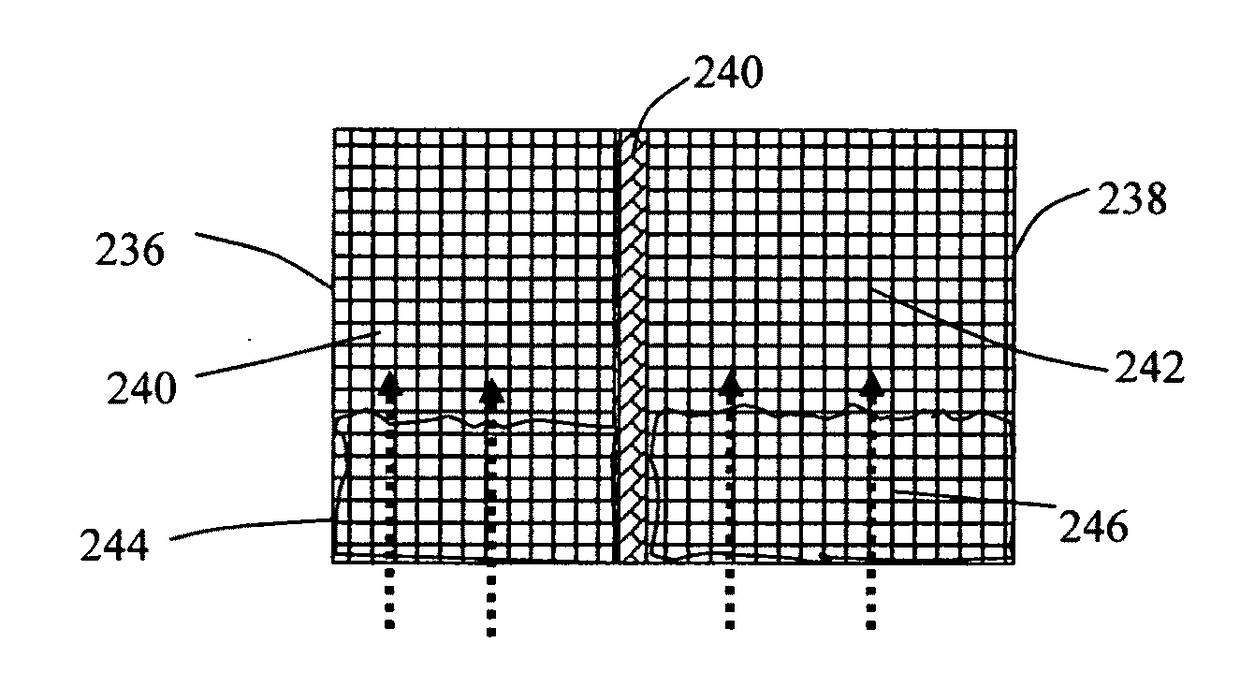
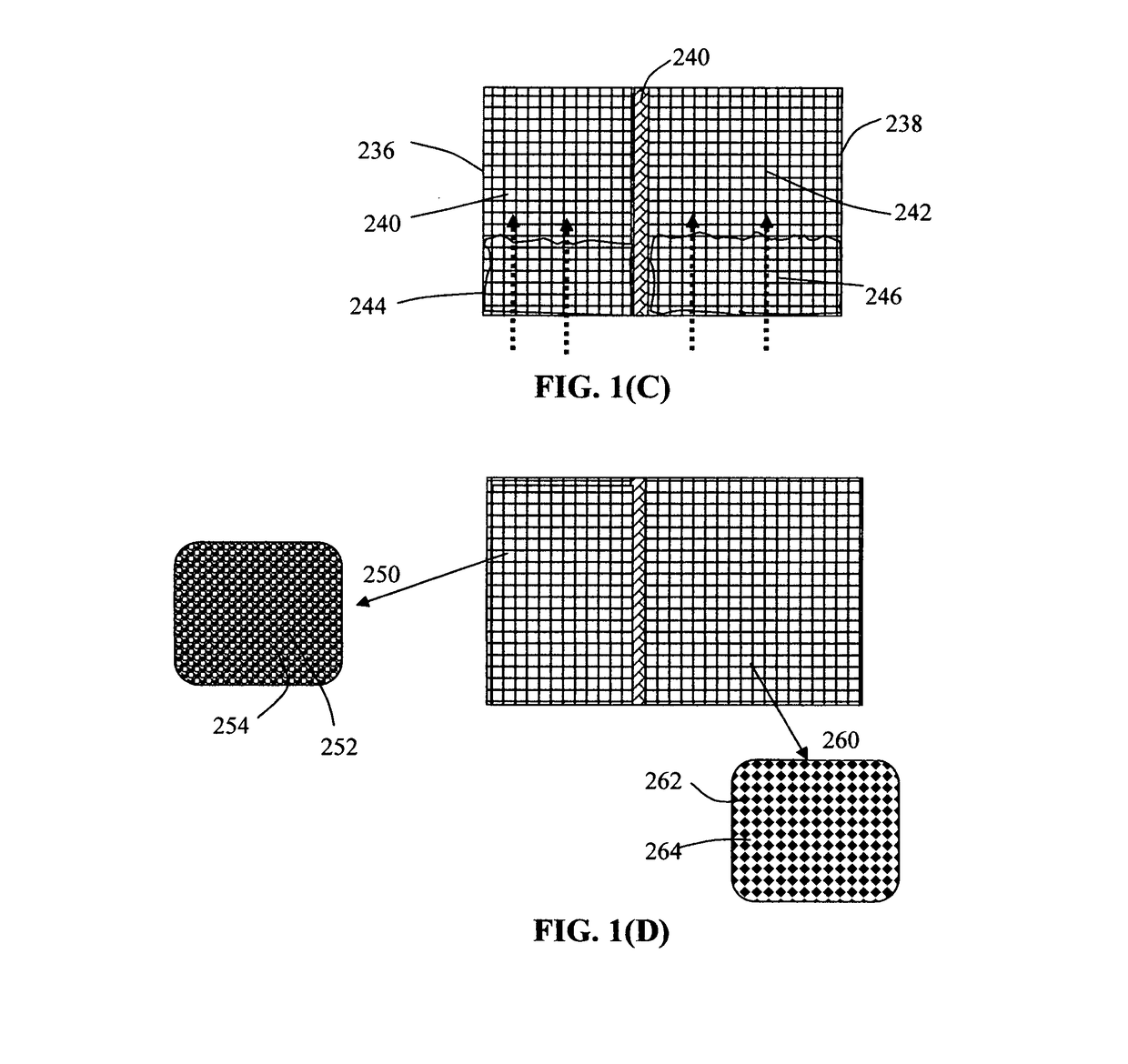
Provided is an alkali metal-ion battery, comprising: (a) an anode having an anode active material dispersed in a first liquid electrolyte disposed in pores of a 3D porous anode current collector having at least 80% by volume of pores; (b) a cathode having a cathode active material dispersed in a second liquid electrolyte disposed in pores of a 3D porous cathode current collector wherein the cathode thickness-to-current collector thickness ratio is from 0.8 / 1 to 1 / 0.8; (c) a separator disposed between the anode and the cathode; wherein the anode or cathode active material loading is greater than 10 mg / cm2, the anode and cathode active materials combined exceeds 40% by weight of the battery, and / or the 3D porous anode and / or cathode current collector has a thickness no less than 200 μm (preferably greater than 500 μm and more preferably greater than 700 μm) and is in physical contact with the separator.
Owner:GLOBAL GRAPHENE GRP INC
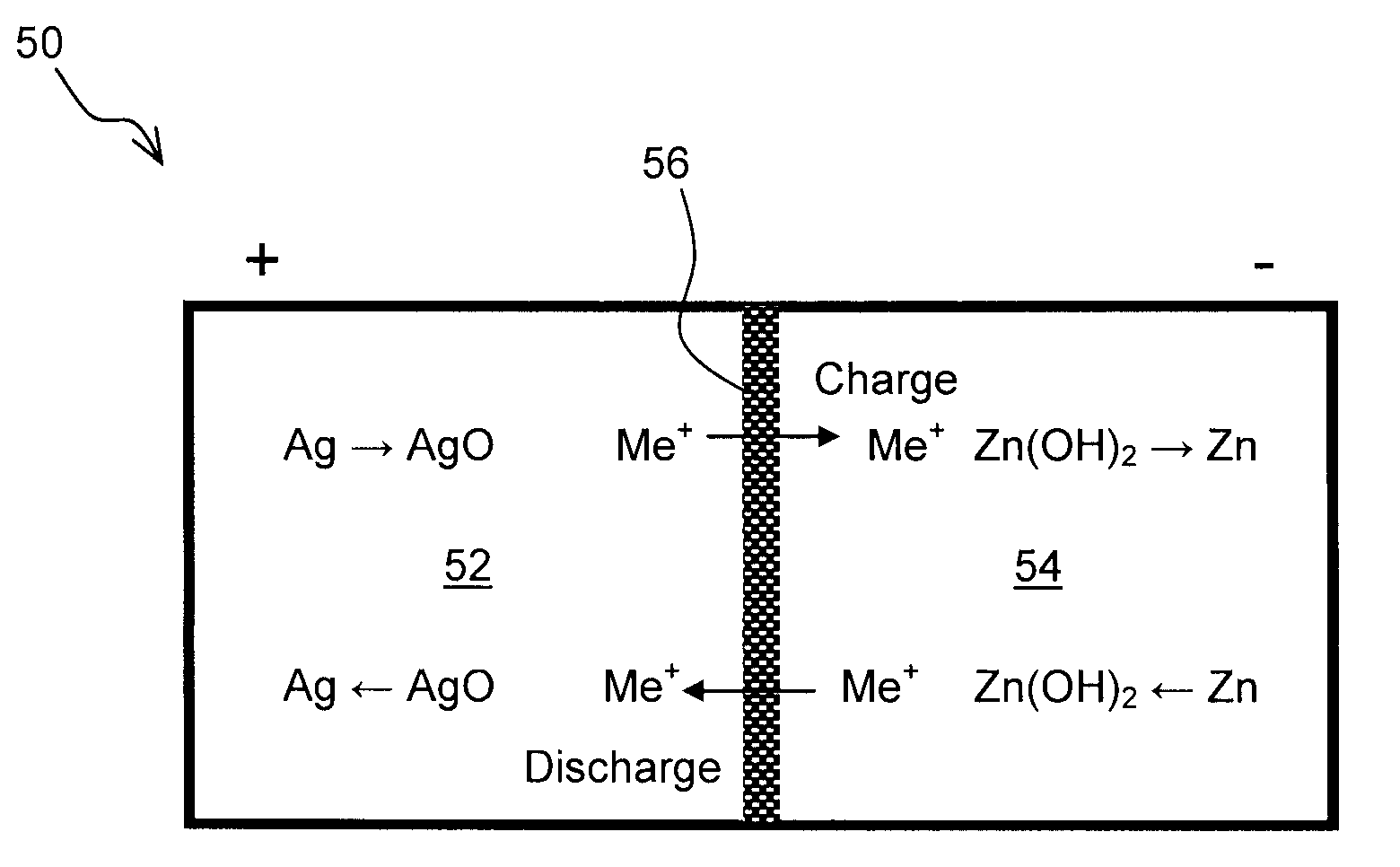
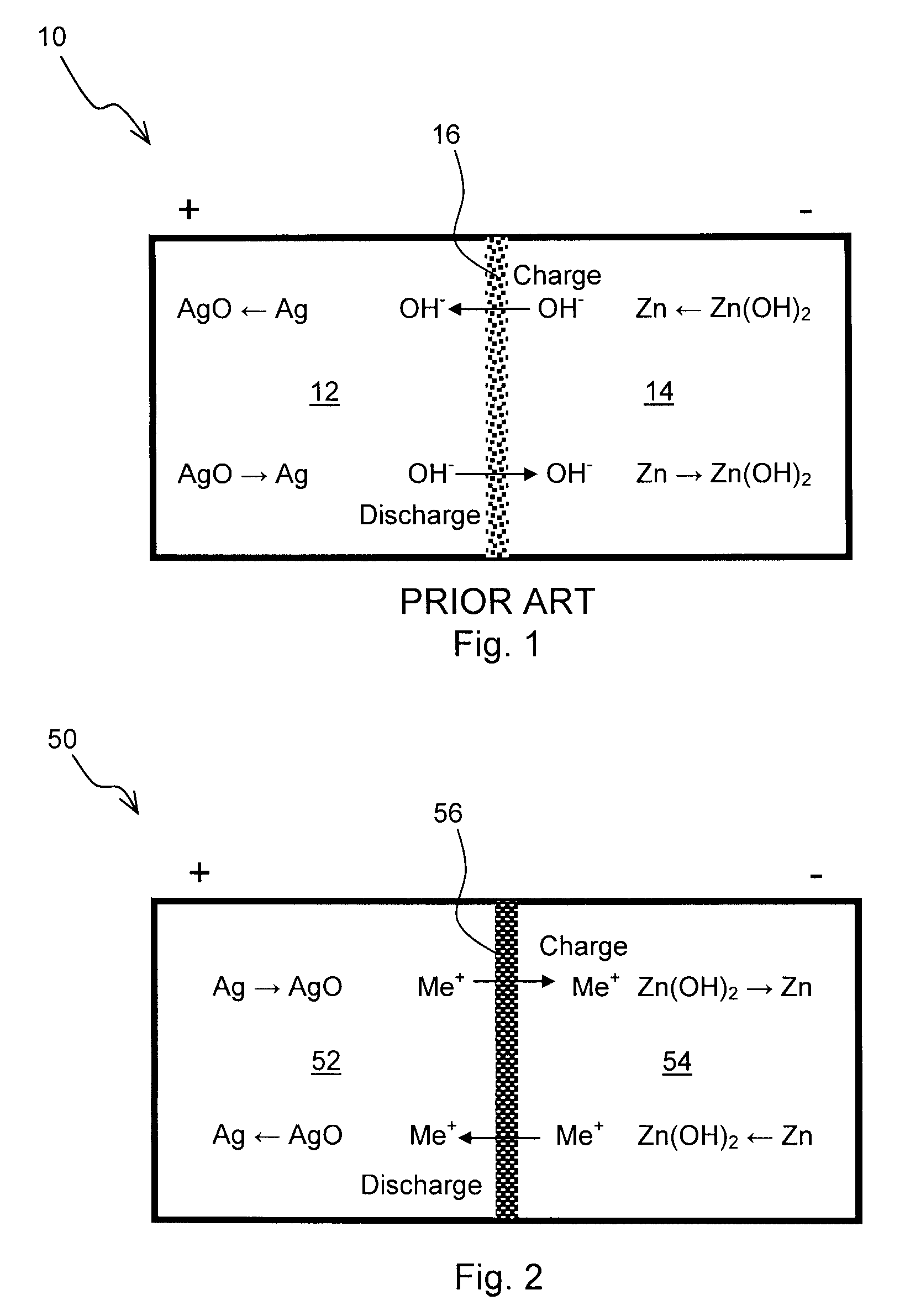
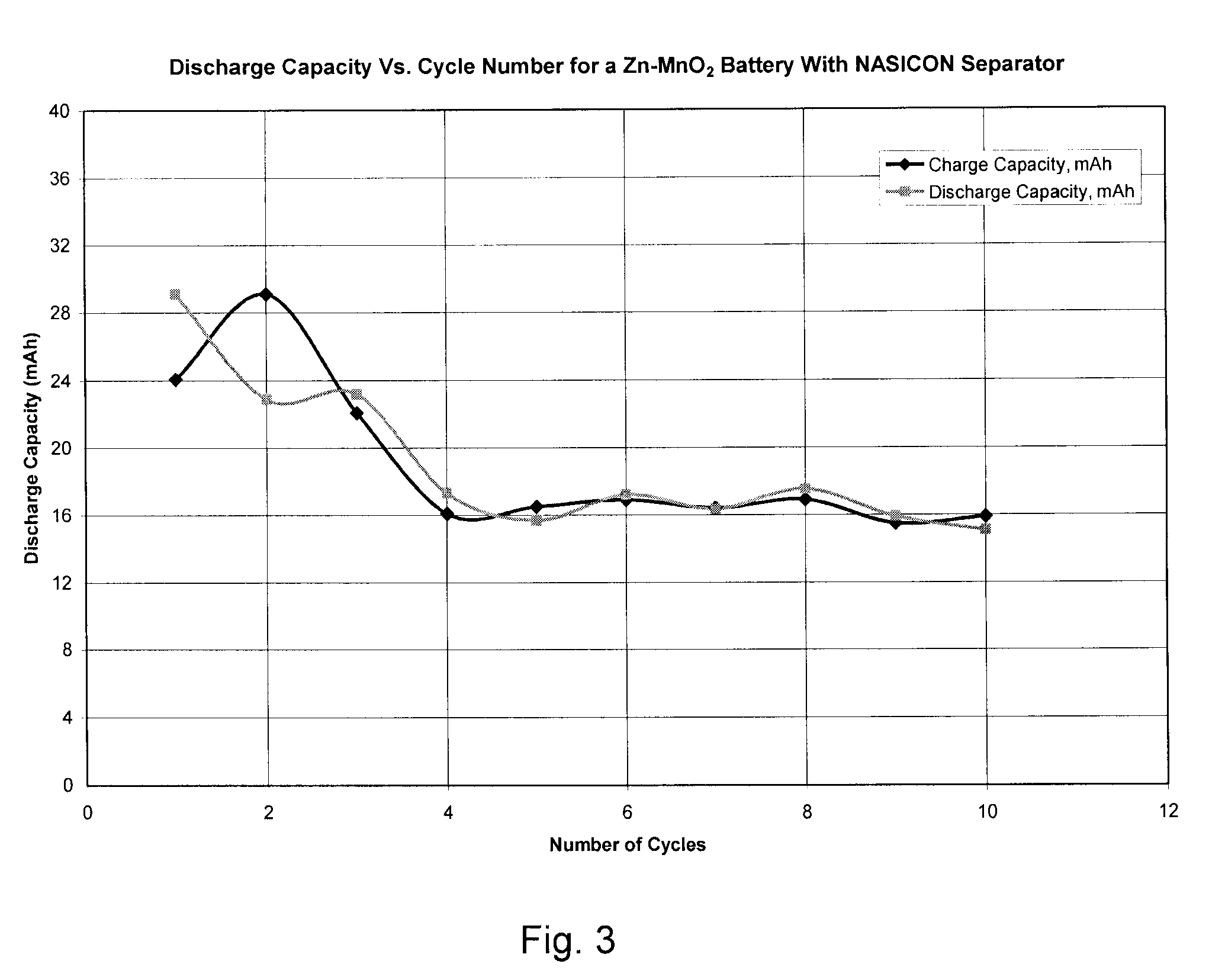
Zinc Anode Battery Using Alkali Ion Conducting Separator
InactiveUS20090189567A1Eliminates capacity lossEliminates self dischargeAlkaline accumulatorsBatteries circuit arrangementsAlkali ionsElectrolyte
A zinc anode storage battery comprising a first electrode containing zinc or a zinc alloy, a second electrode containing an oxidizing material capable of electrochemical reduction by zinc, an alkaline electrolyte, and a substantially non-porous, alkali-ion conducting separator provided between the first electrode and the second electrode. The alkali conducting separator may be a solid alkali metal ion super ion conducting material, wherein the alkali metal is Na, K, or Li.
Owner:CERAMTEC
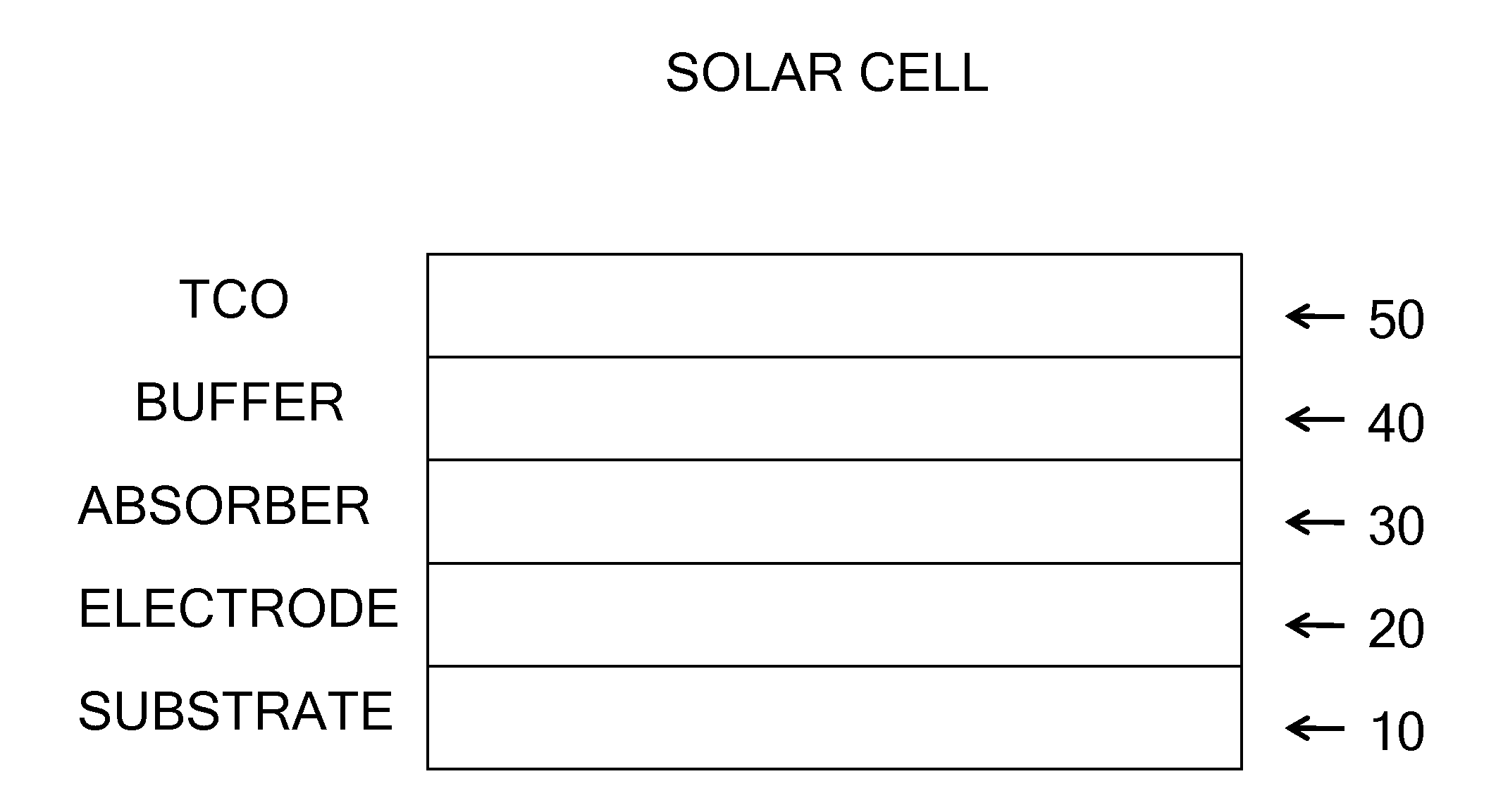
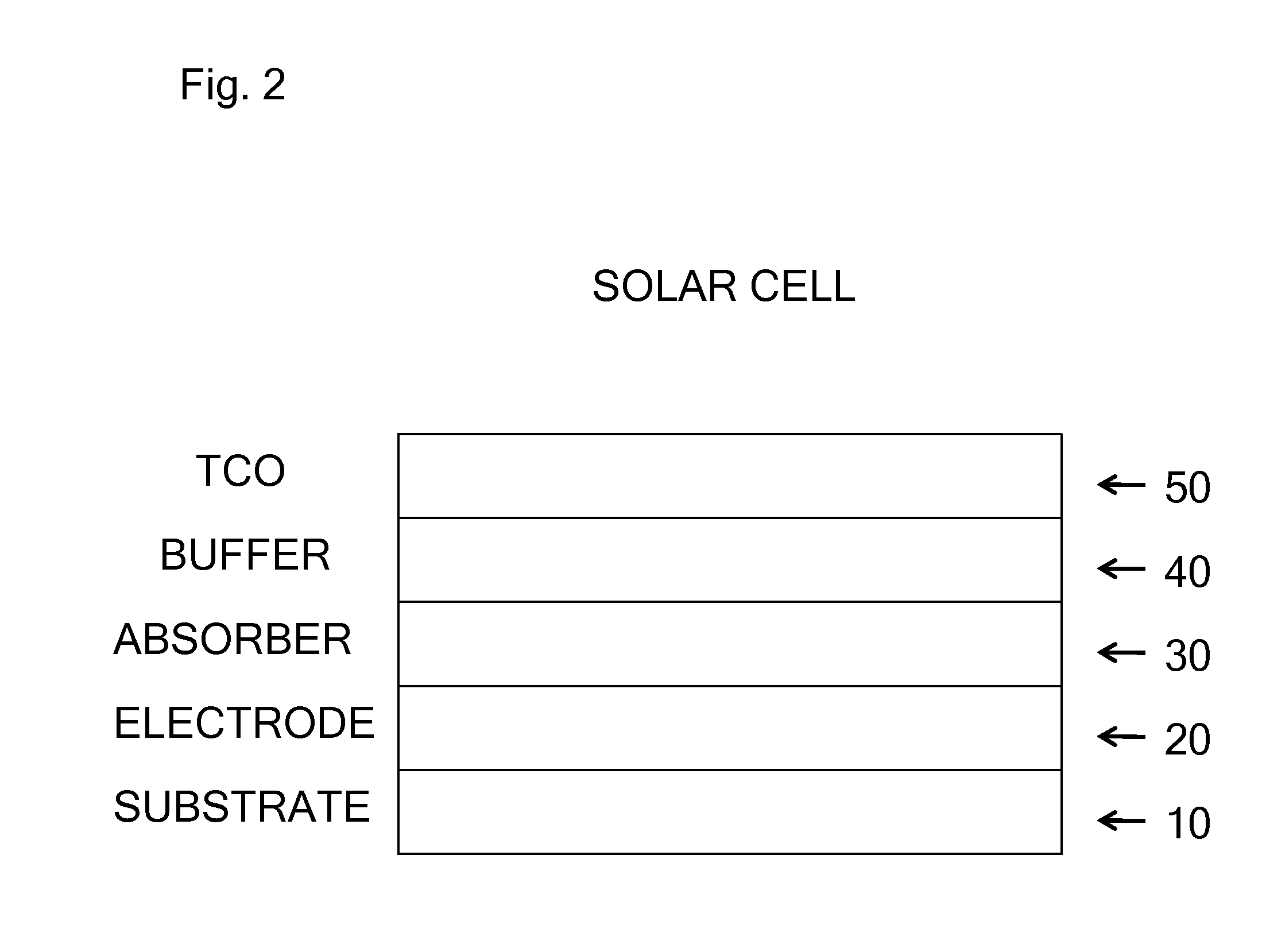
Thin Film Semi-Conductor-on-Glass Solar Cell Devices
InactiveUS20080308143A1Inhibit transport and deleterious actionLower performance requirementsSemiconductor/solid-state device manufacturingPhotovoltaic energy generationAlkali ionsRare earth
The present invention relates to semiconductor devices suitable for electronic, optoelectronic and energy conversion applications. In a particular form, the present invention relates to the fabrication of a thin film solar cells and thin film transistors through the advantageous combination of semiconductors, insulators, rare-earth based compounds and amorphous and / or ceramic and / or glass substrates. Crystalline or polycrystalline thin film semiconductor-on-glass formation using alkali ion impurity barrier layer(s) are disclosed. Example embodiment of crystalline or polycrystalline thin film semiconductor-on-glass formation using rare-earth based material as impurity barrier layer(s) is disclosed. In particular, thin film silicon-on-glass substrate is disclosed as the alternate embodiment, with impurity barrier designed to inhibit transport of deleterious alkali species from the glass into the semiconductor thin film.
Owner:IQE
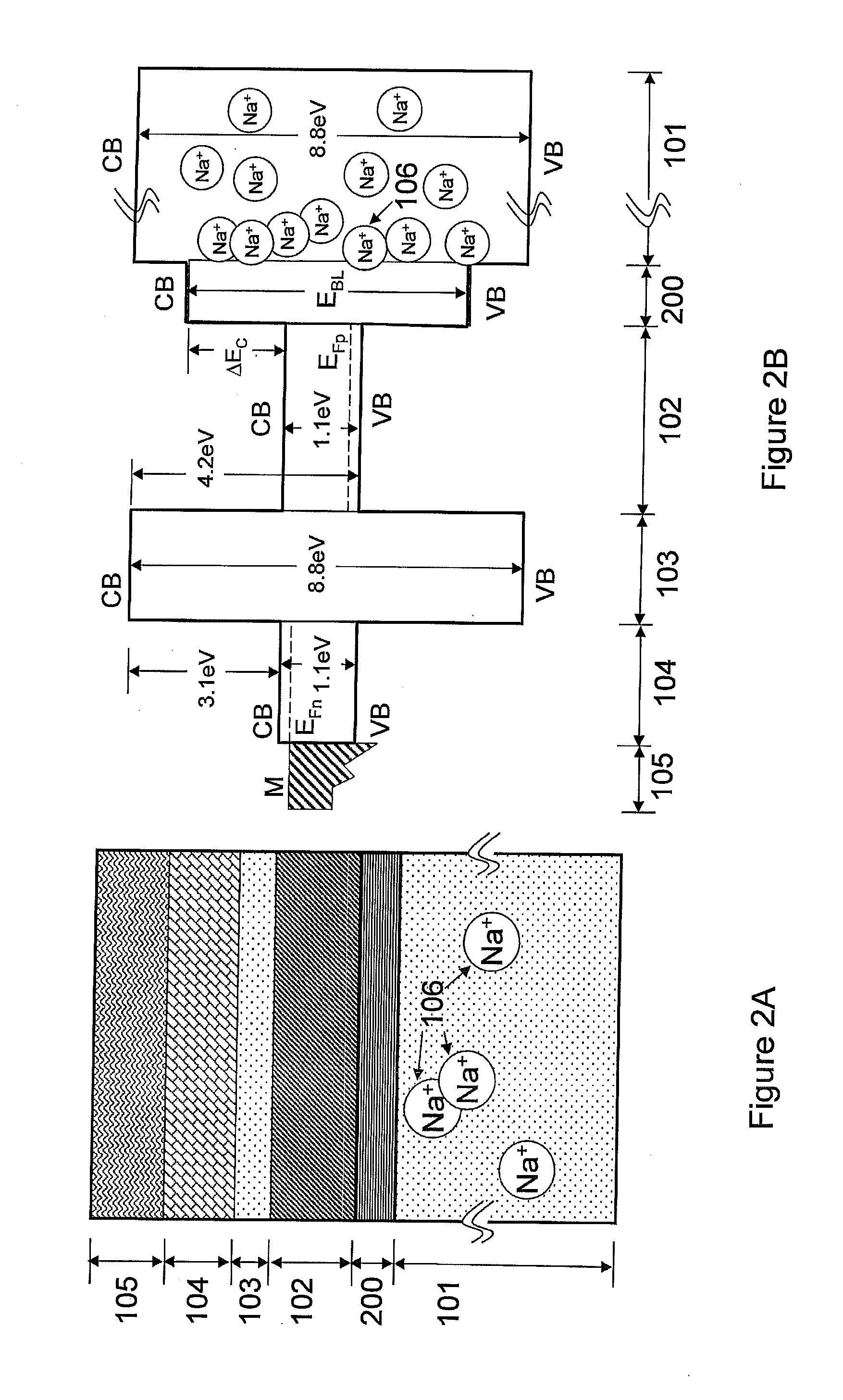
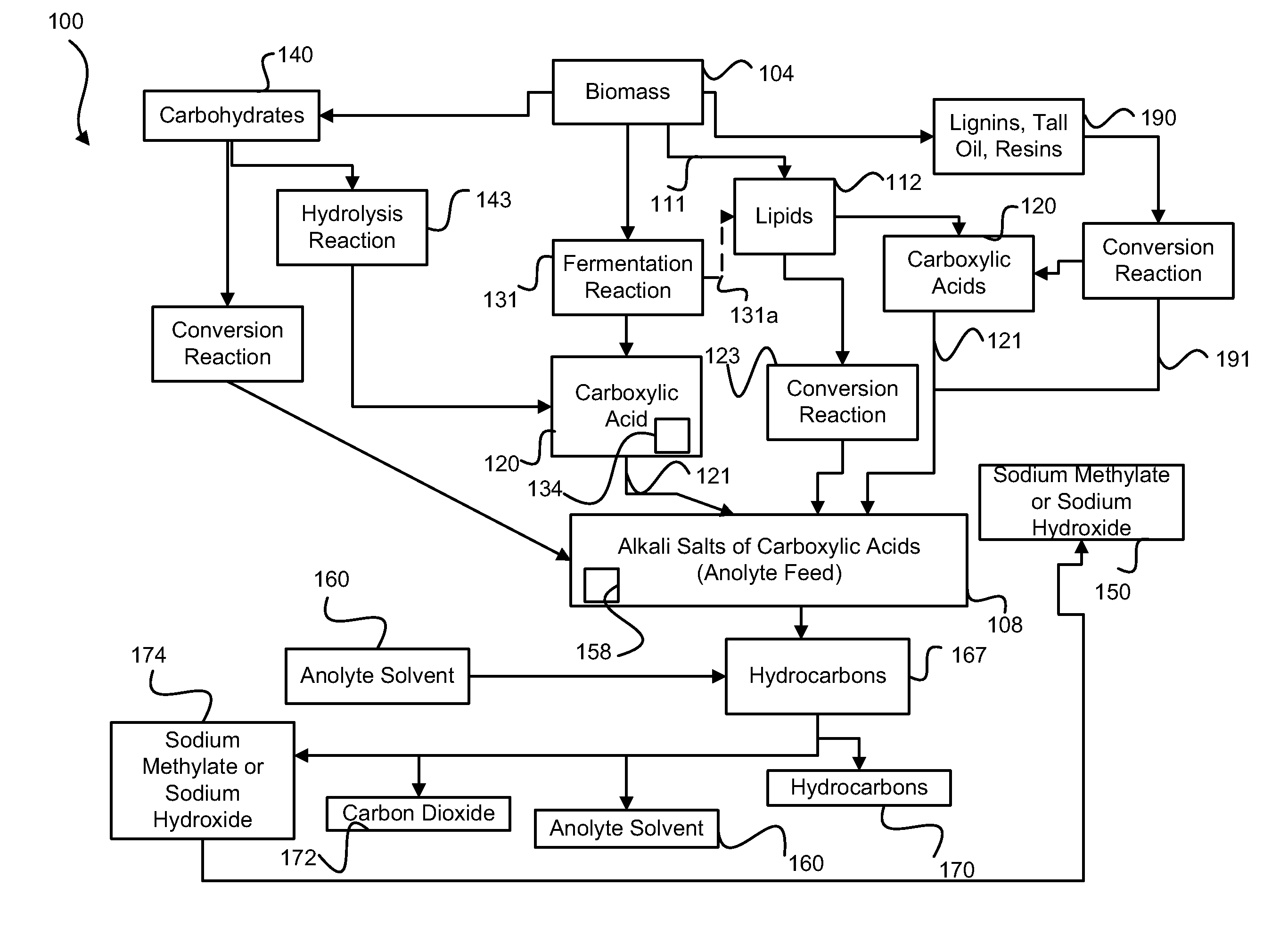
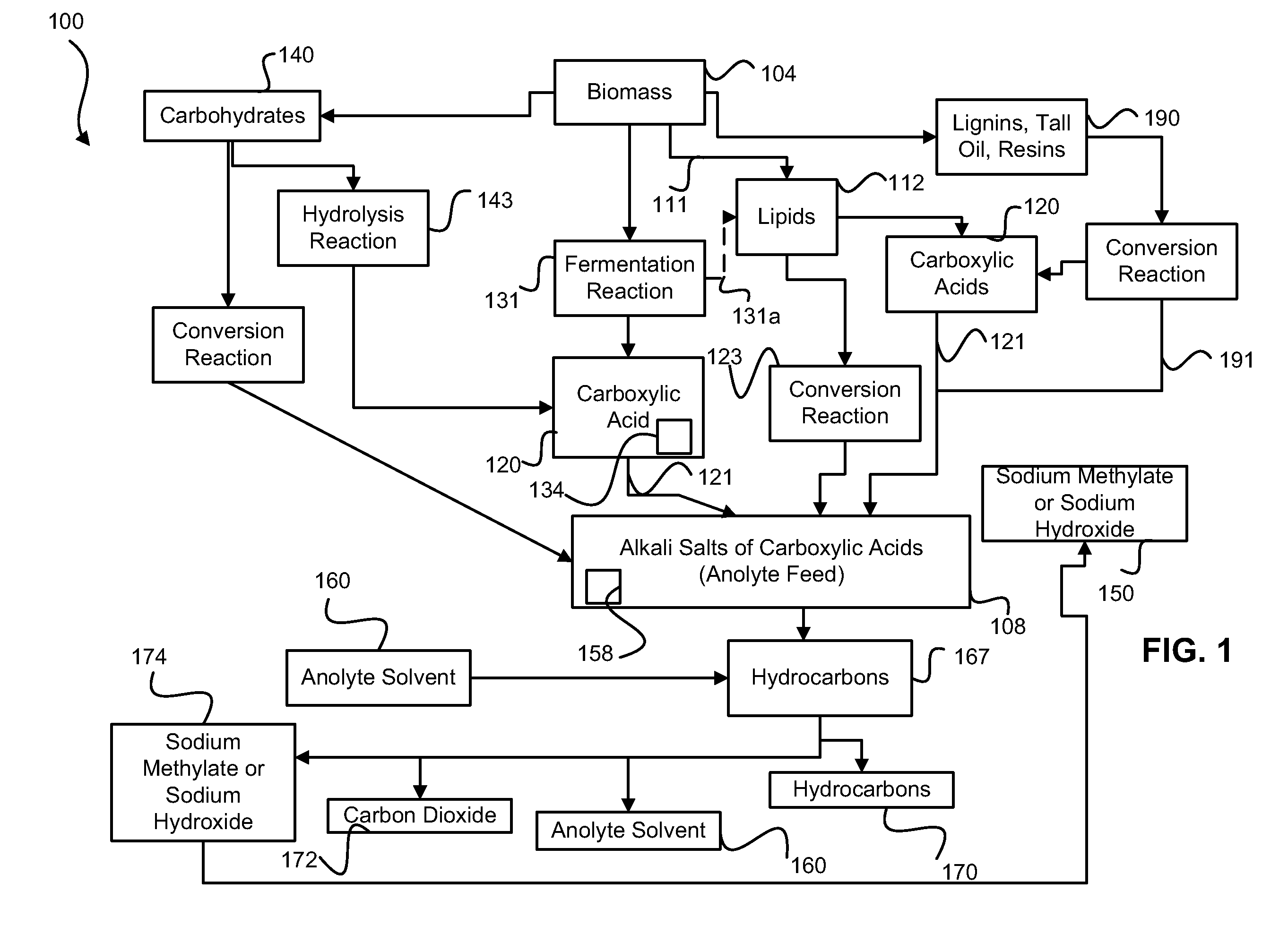
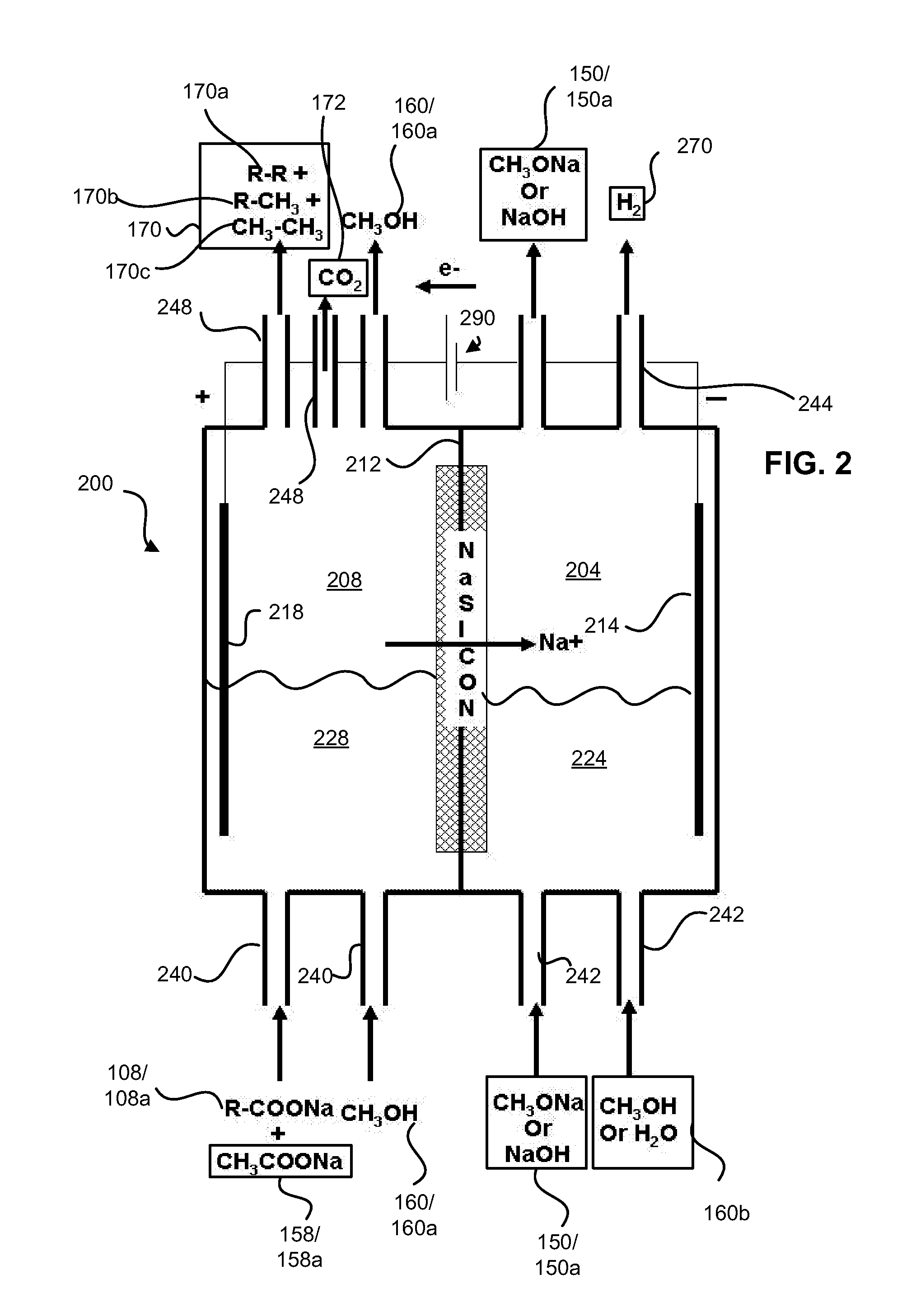
Decarboxylation cell for production of coupled radical products
InactiveUS20110024288A1Avoid mixingLow costCellsElectrolytic organic coupling reactionsAlkali ionsTG - Triglyceride
Owner:CERAMTEC
Chromium-free pickle for plastic surfaces
ActiveUS20110140035A1Decorative surface effectsLiquid/solution decomposition chemical coatingChromium freeAlkali ions
A pickling solution for the surface pre-treatment of plastic surfaces in preparation for metallization, the solution comprising a source of Mn(VII) ions; and an inorganic acid; wherein the pickling solution is substantially free of chromium (VI) ions, alkali ions, and alkaline-earth ions.
Owner:MACDERMID ENTHONE INC
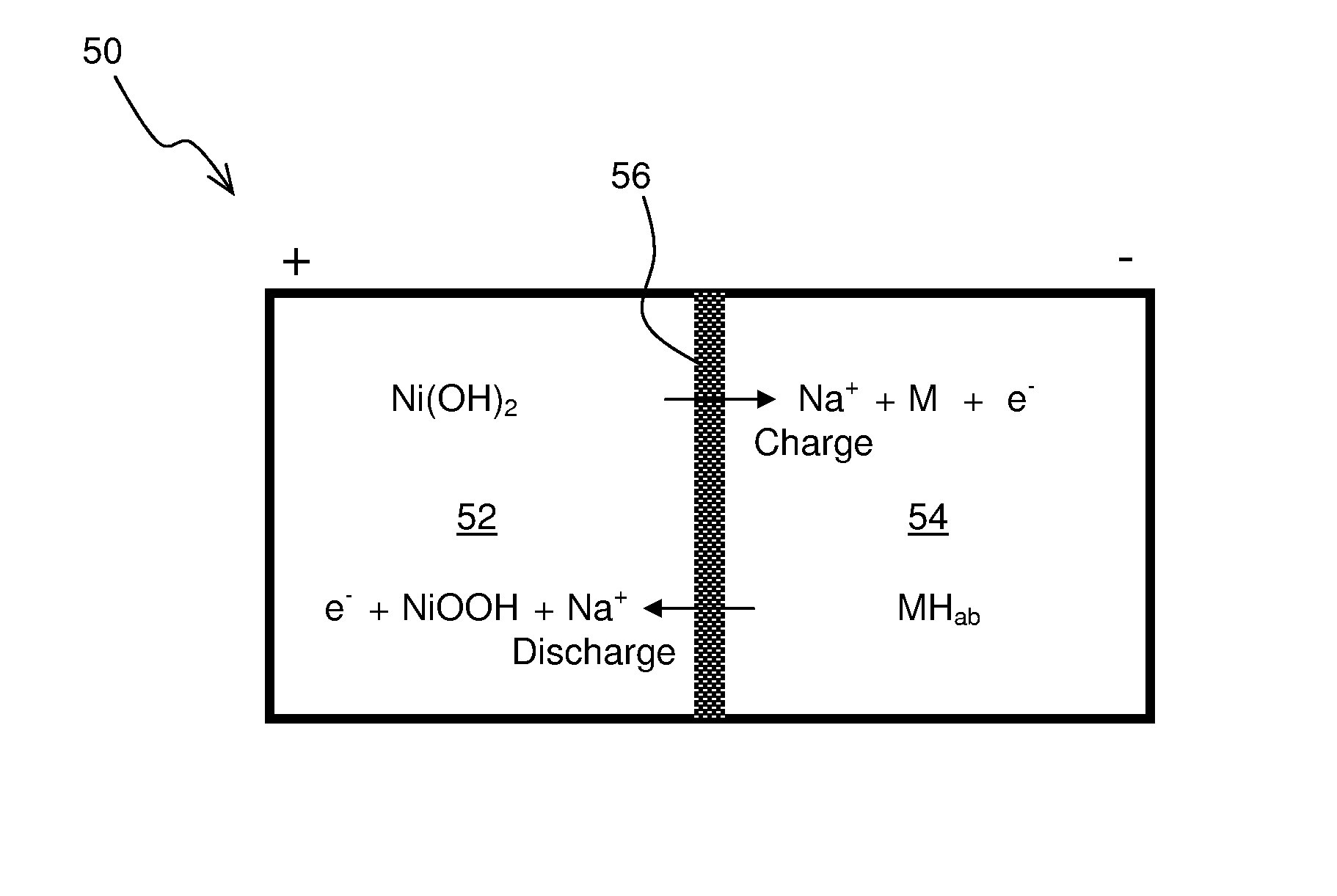
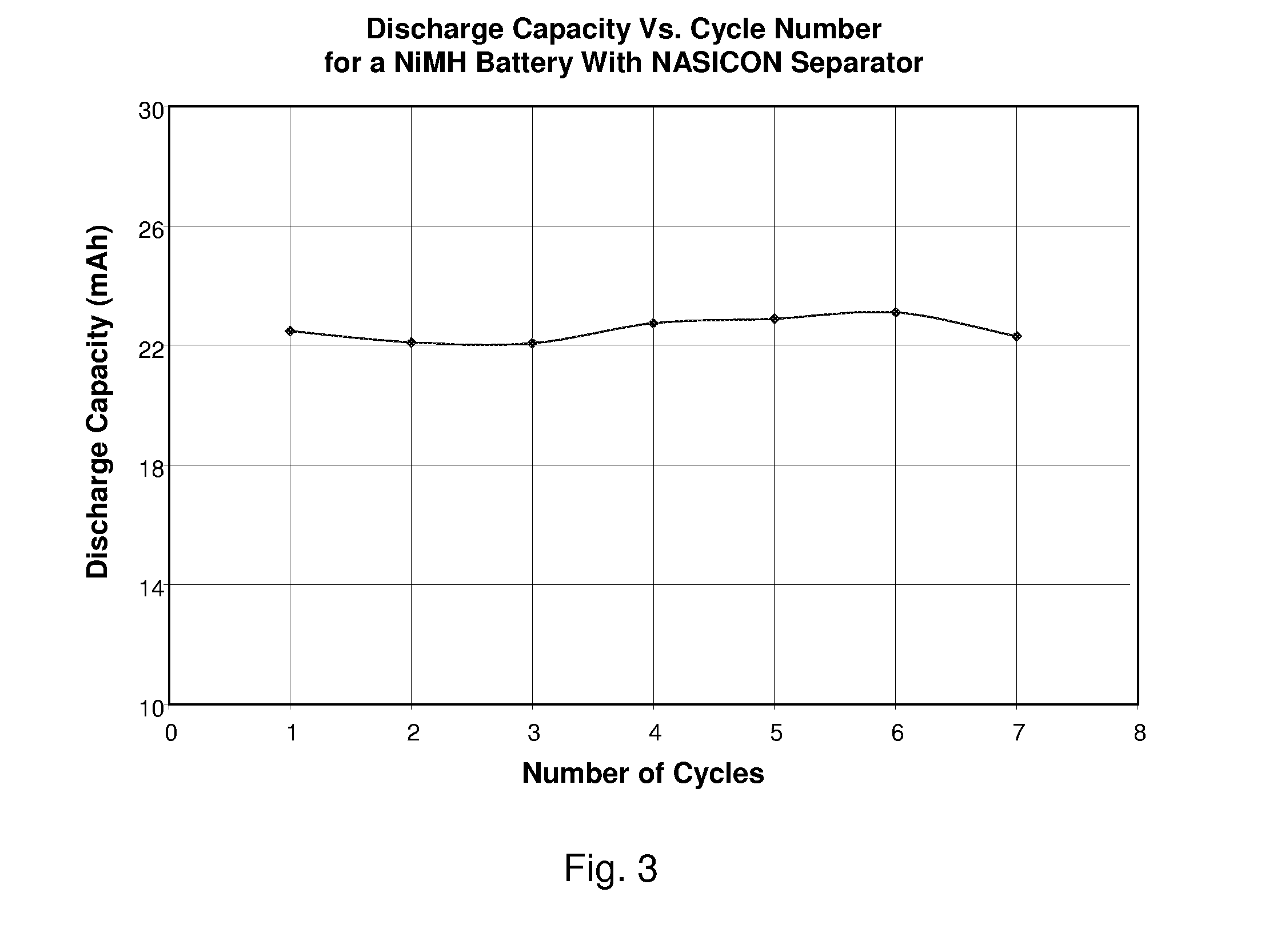
Nickel-Metal Hydride Battery Using Alkali Ion Conducting Separator
ActiveUS20090134842A1Eliminates capacity lossEliminates self dischargeBatteries circuit arrangementsAlkaline accumulatorsAlkali ionsNickel oxide hydroxide
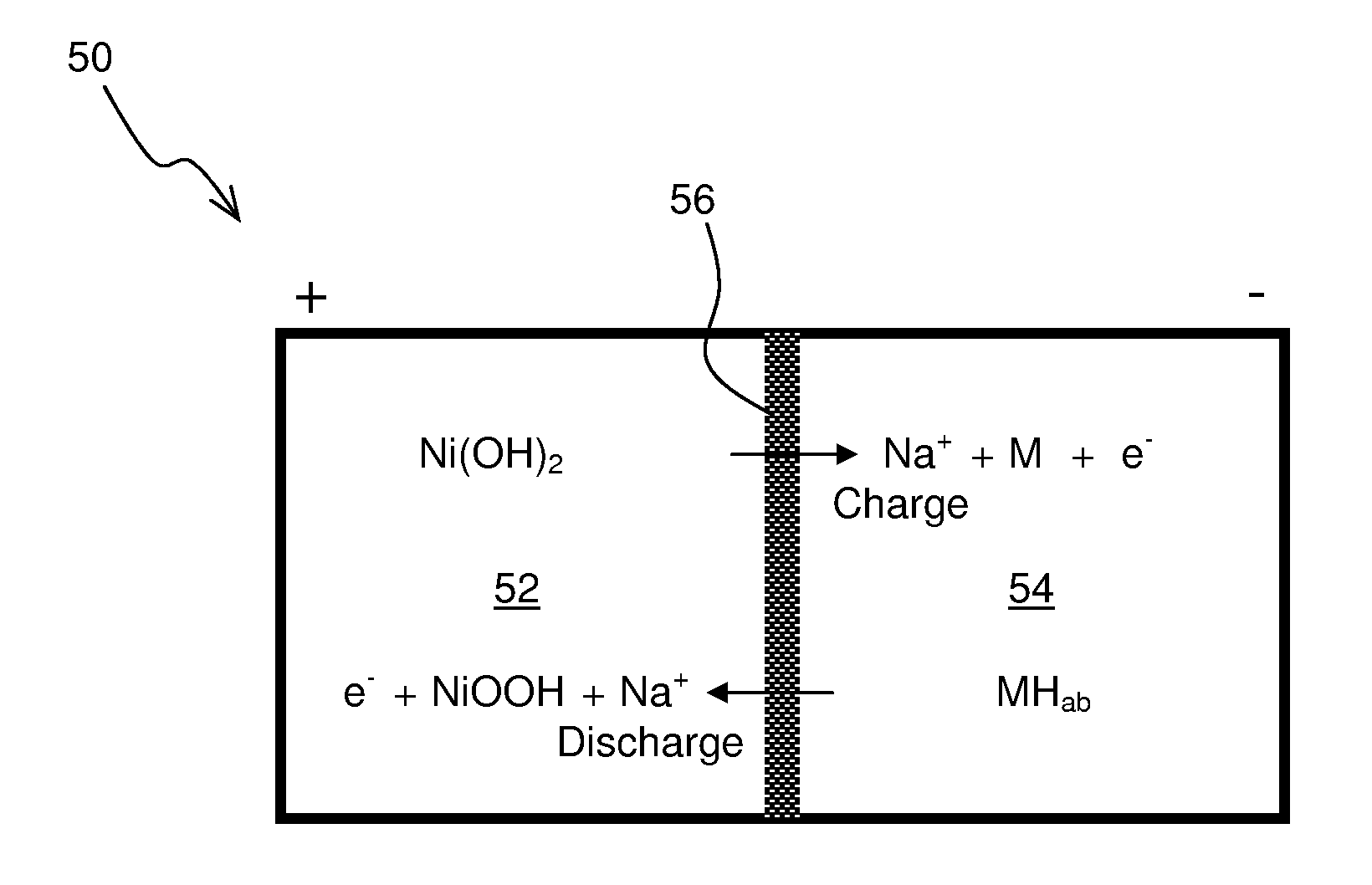
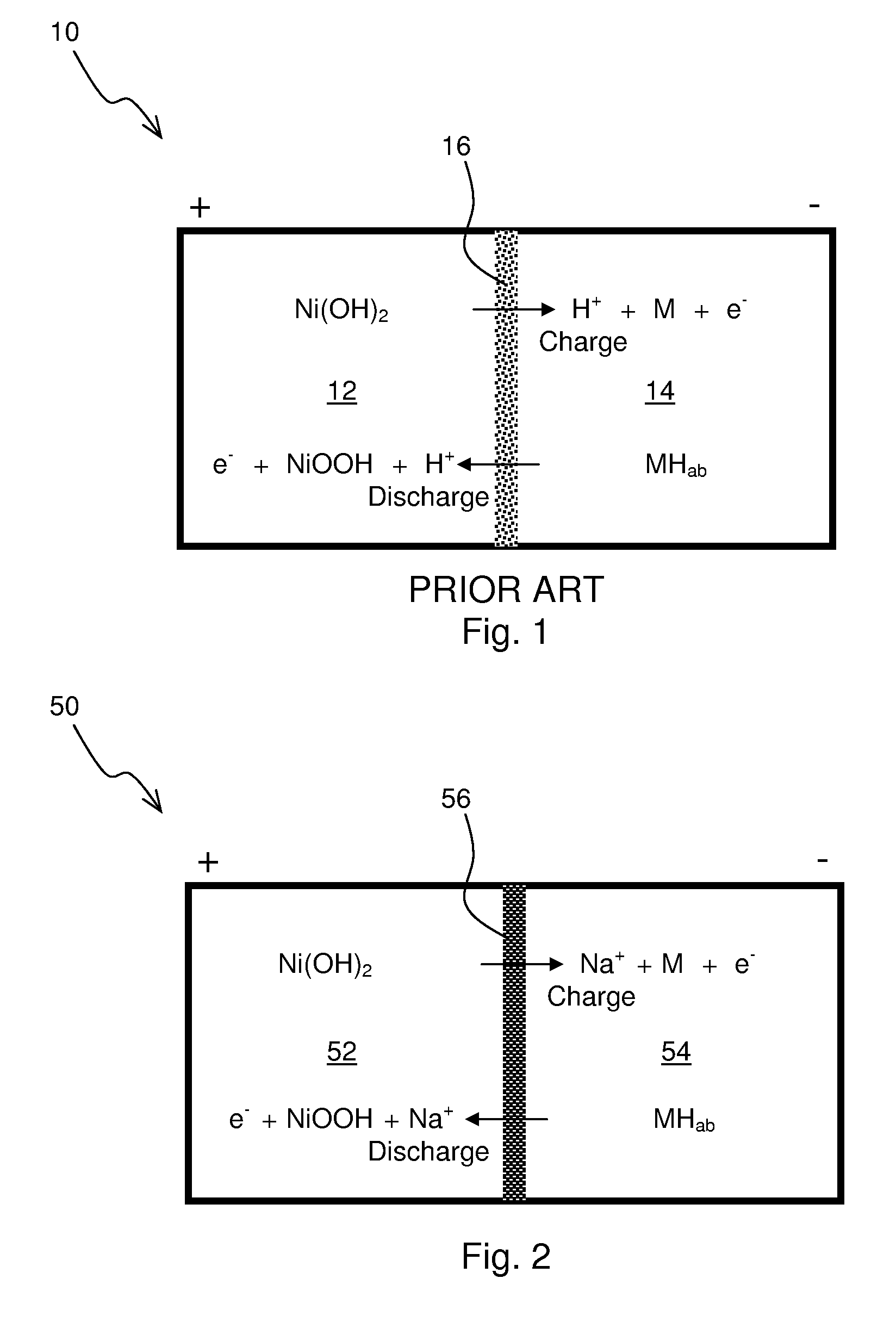
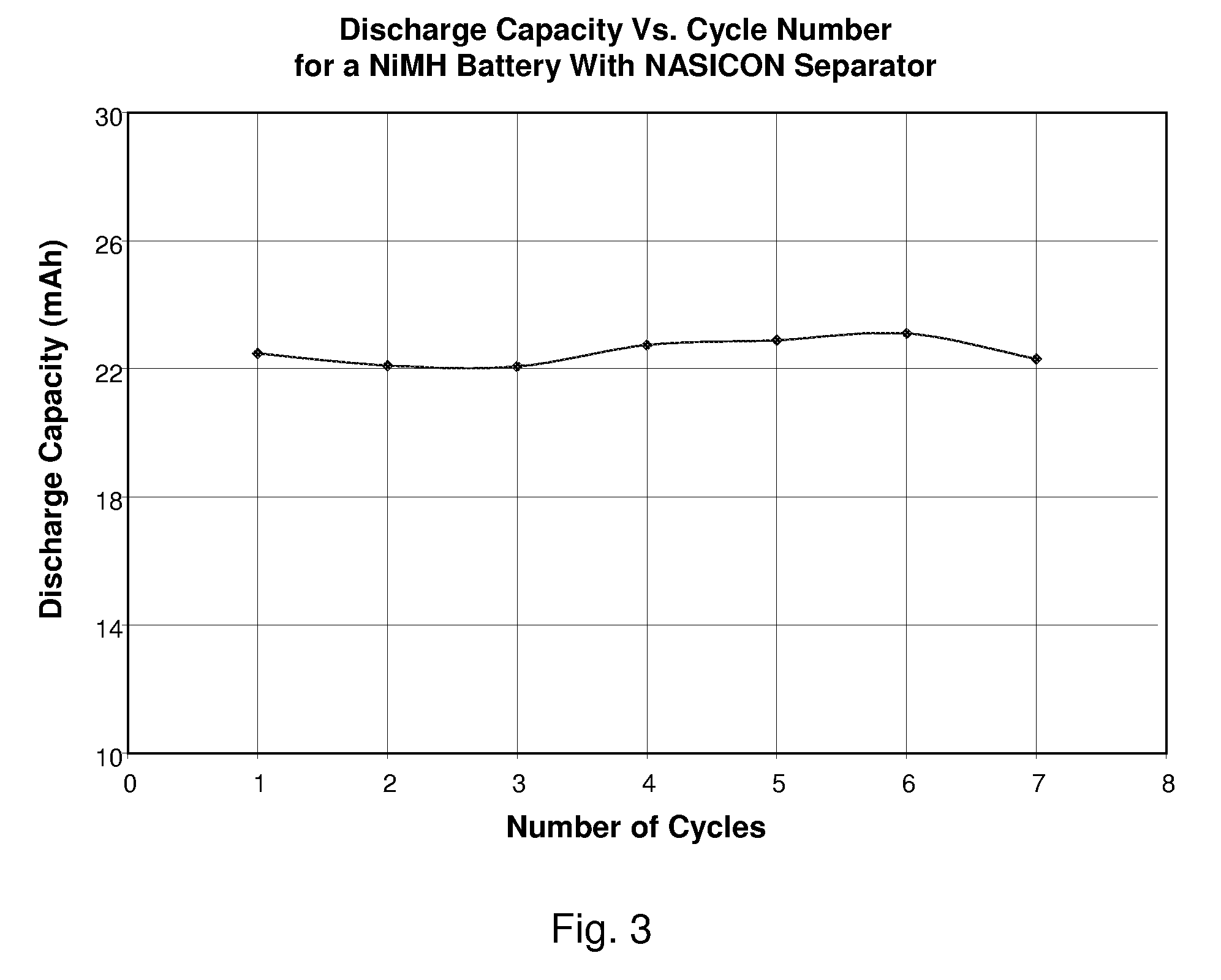
A nickel-metal hydride storage battery comprising a positive electrode containing nickel hydroxide, a negative electrode containing a hydrogen absorbing alloy, an alkaline electrolyte, and an alkali conducting separator provided between the positive electrode and the negative electrode. The alkali conducting separator may be a solid alkali metal ion super ion conducting material, wherein the alkali metal is Na, K, or Li
Owner:FIELD UPGRADING USA INC
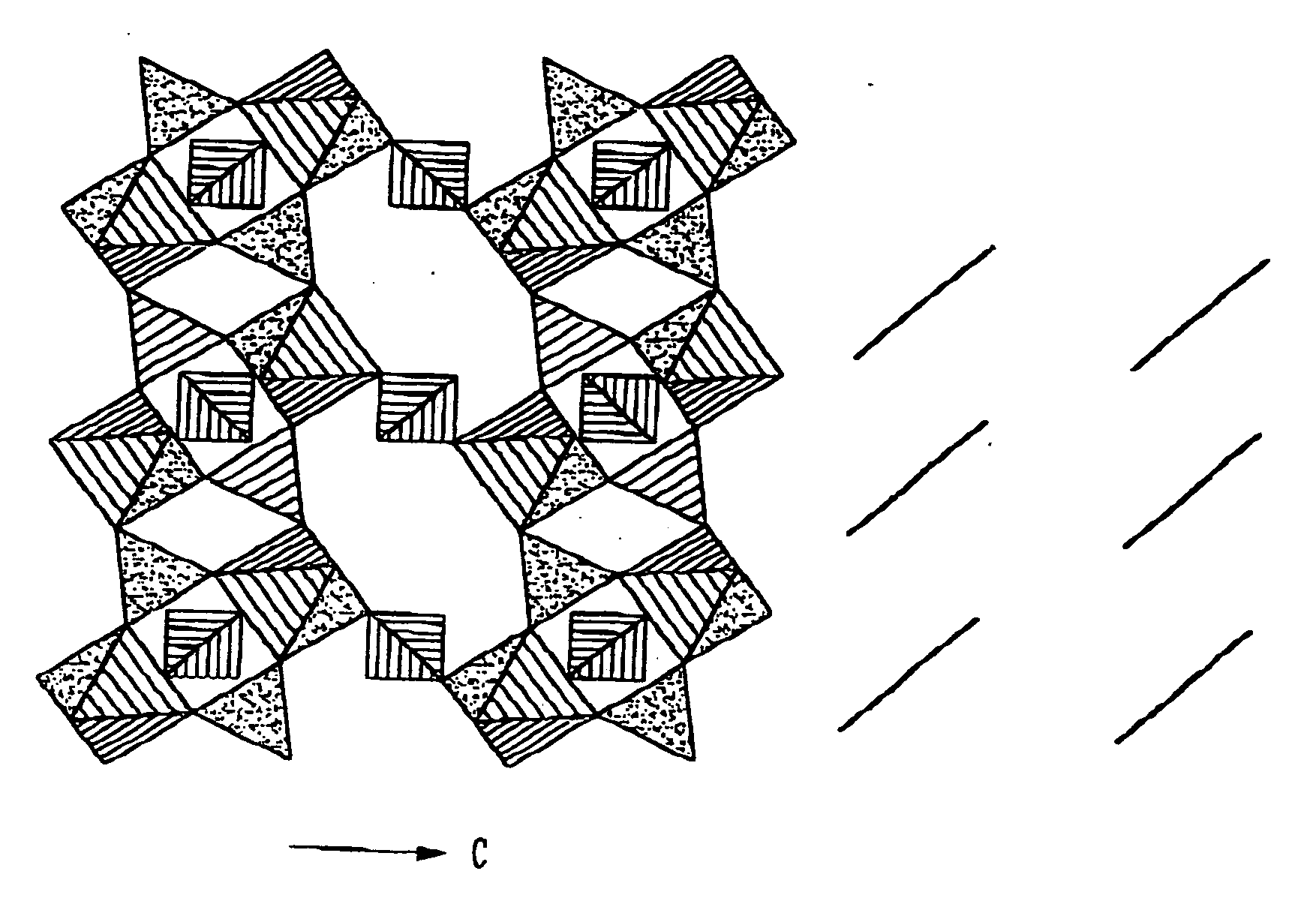
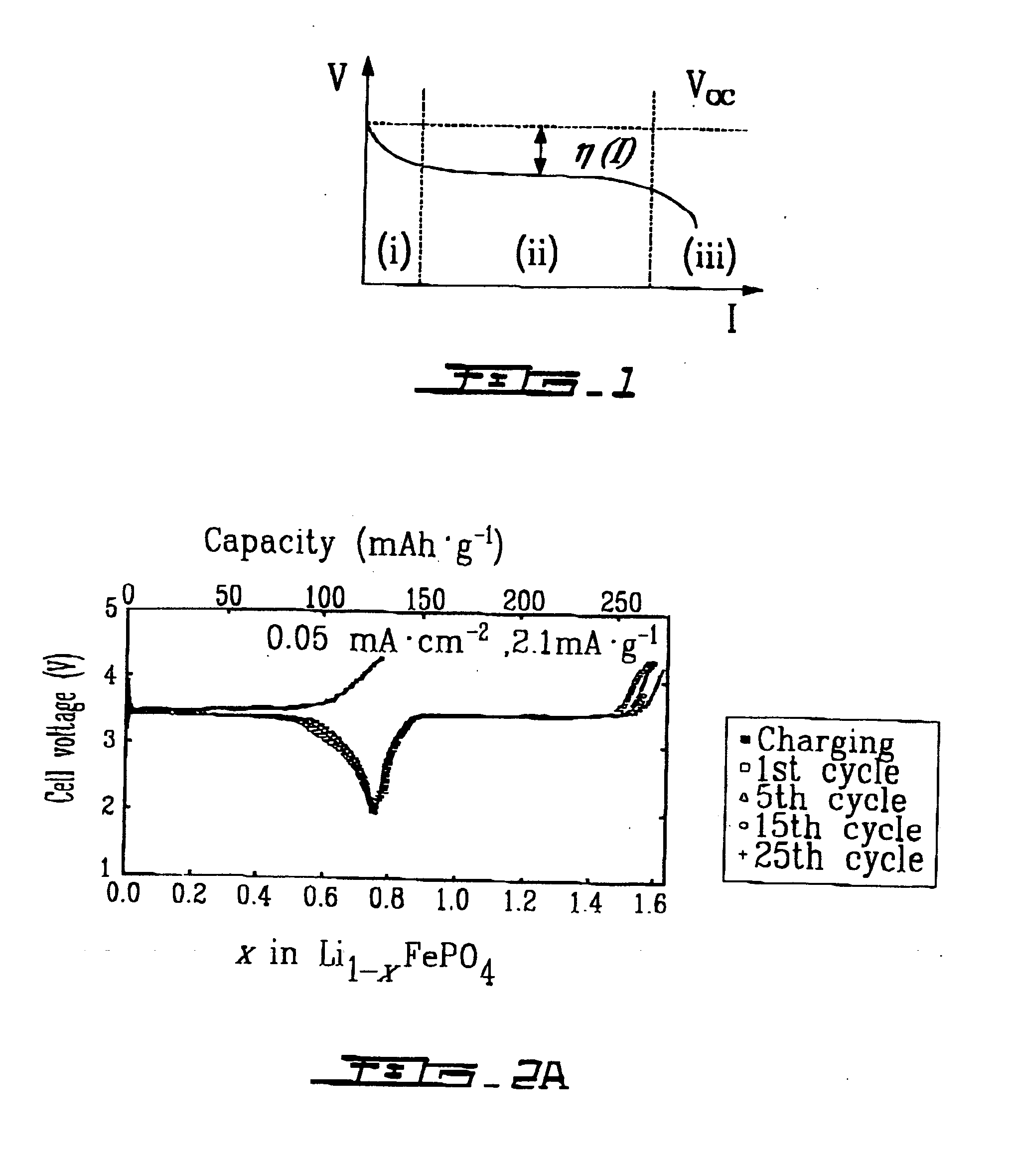
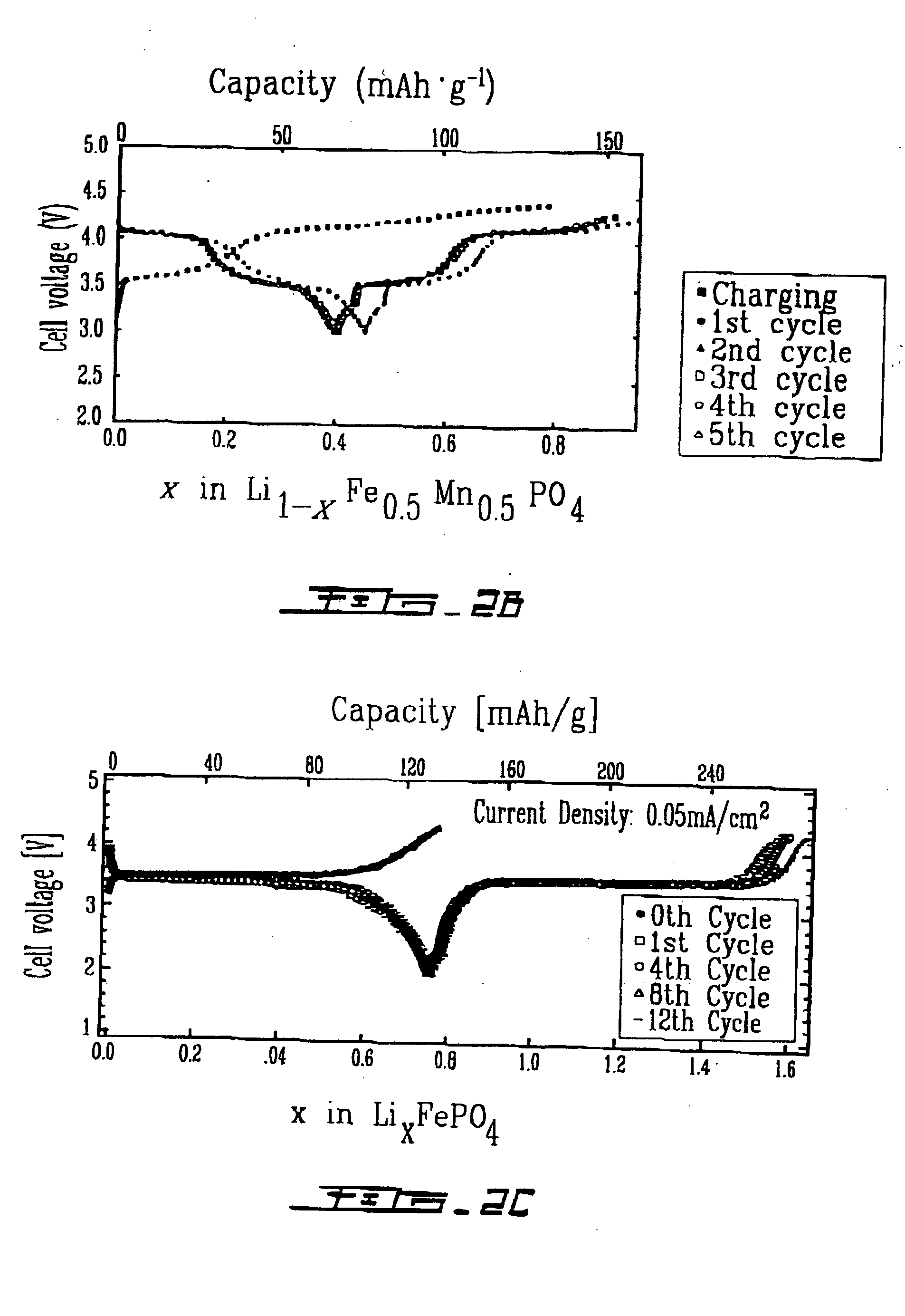
Cathode materials for secondary (rechargeable) lithium batteries
InactiveUS20050003274A1Cell electrodesPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesAlkali ionsRechargeable cell
The invention relates to materials for use as electrodes in an alkali-ion secondary (rechargeable) battery, particularly a lithium-ion battery. The invention provides transition-metal compounds having the ordered-olivine, a modified olivine, or the rhombohedral NASICON structure and the polyanion (PO4)3− as at least one constituent for use as electrode material for alkali-ion rechargeable batteries.
Owner:HYDRO QUEBEC CORP
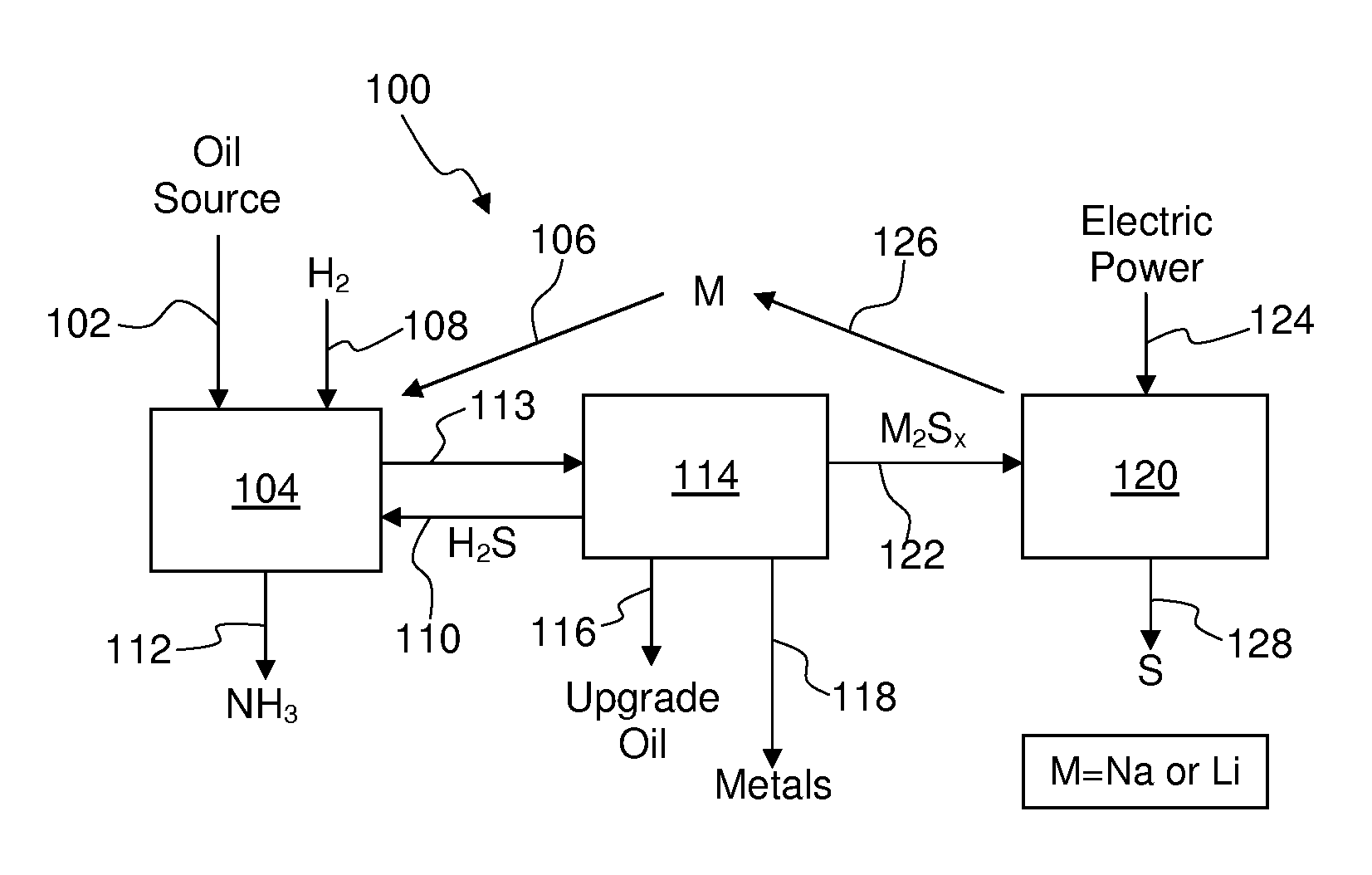
Process for recovering alkali metals and sulfur from alkali metal sulfides and polysulfides
Alkali metals and sulfur may be recovered from alkali polysulfides in an electrolytic process that utilizes an electrolytic cell having an alkali ion conductive membrane. An anolyte solution includes an alkali polysulfide and a solvent that dissolves elemental sulfur. A catholyte solution includes alkali metal ions and a catholyte solvent. Applying an electric current oxidizes sulfur in the anolyte compartment, causes alkali metal ions to pass through the alkali ion conductive membrane to the catholyte compartment, and reduces the alkali metal ions in the catholyte compartment. Sulfur is recovered by removing and cooling a portion of the anolyte solution to precipitate solid phase sulfur. Operating the cell at low temperature causes elemental alkali metal to plate onto the cathode. The cathode may be removed to recover the alkali metal in batch mode or configured as a flexible band to continuously loop outside the catholyte compartment to remove the alkali metal.
Owner:ENLIGHTEN INNOVATIONS INC
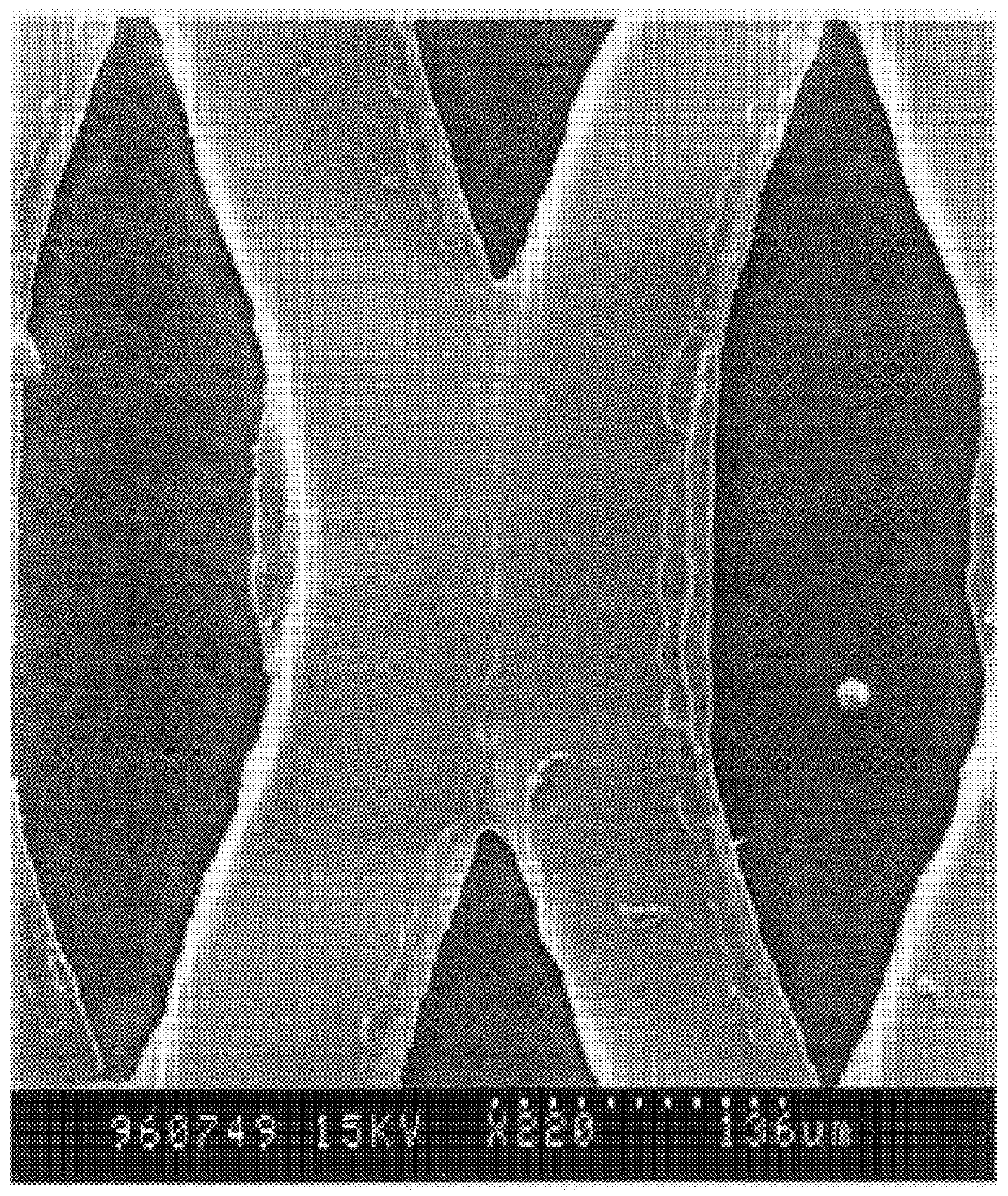
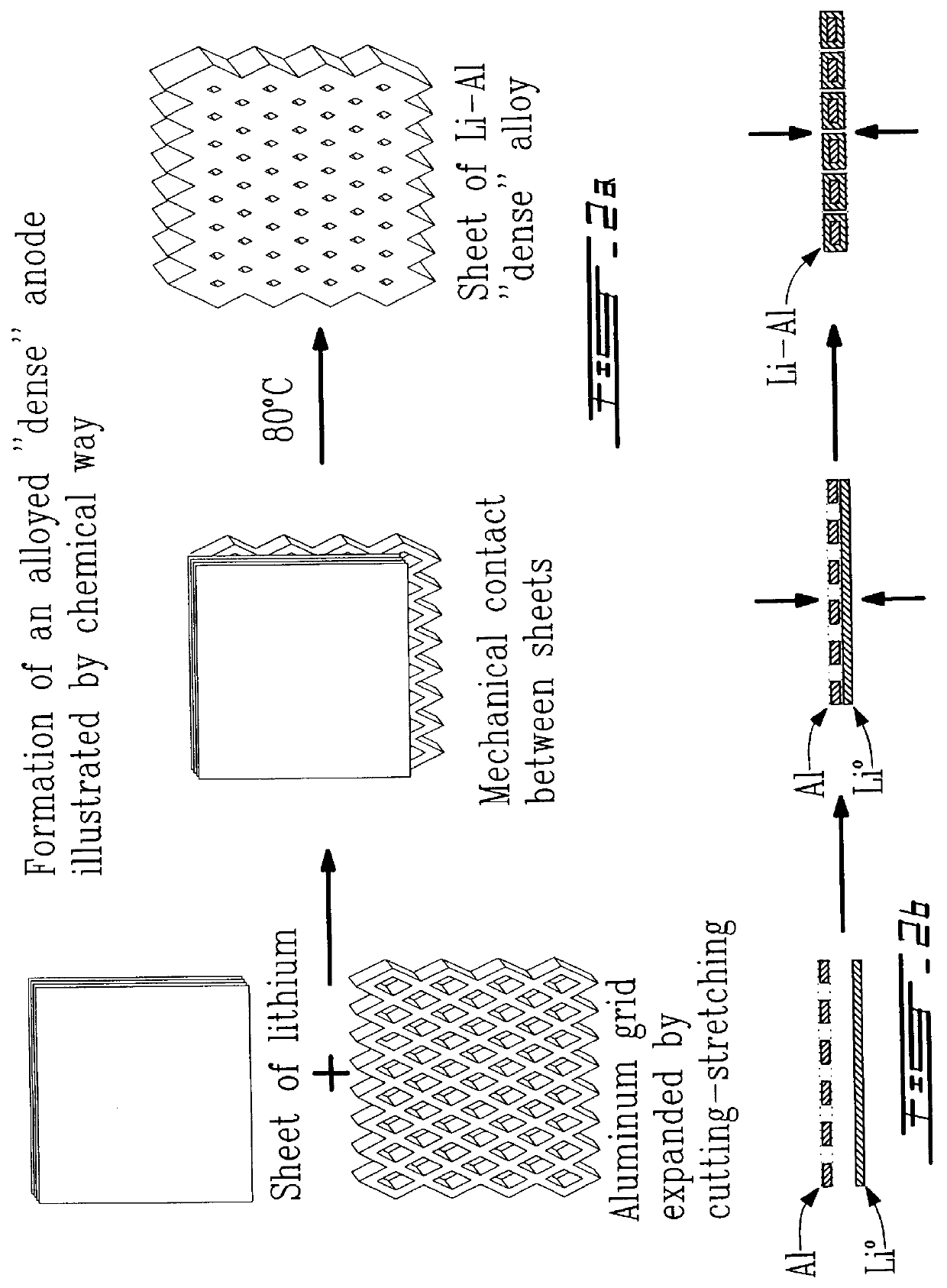
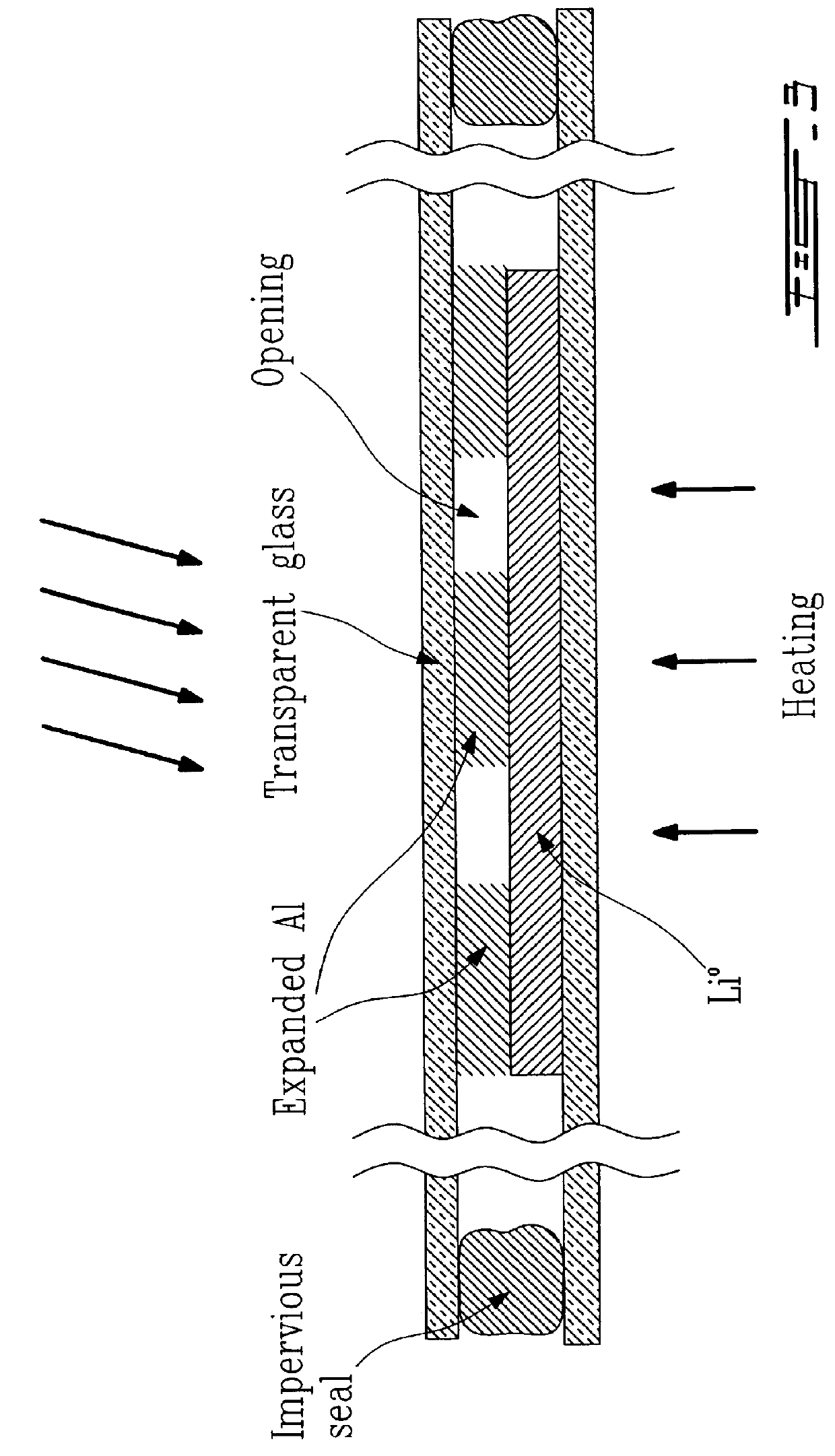
Alloyed and dense anode sheet with local stress relaxation
InactiveUS6265099B1Avoid changeMaximize ionic exchangeFinal product manufactureElectrode carriers/collectorsAlkali ionsEngineering
An electrochemical generator comprising thin films including a positive electrode and its collector, and a sheet of a host metal intended to later on constitute a negative electrode, as well as an electrolyte which is conductive towards alkaline ions and also a source of alkali ions. The sheet of the host metal has voids whose quantity and arrangement are capable of locally absorbing any lateral expansion of the sheet of host metal and thereby substantially preventing all cumulative change in the plane of the sheet of host metal when there is an initial formation of alloy in the sheet between the host metal and an alkali metal which is brought about by the alkaline ions. A method of manufacturing such a generator is also described.
Owner:BATHIUM CANADA
Process for producing alkali metal or alkali-ion batteries having high volumetric and gravimetric energy densities
ActiveUS9564656B1Increase energy densityImprove power densityFinal product manufactureCell electrodesAlkali ionsMass loading
Provided is a process for producing an alkali metal battery, comprising: (A) Preparing an anode material suspension and a cathode active material suspension; (B) Assembling a porous cell framework composed of a first conductive foam structure, a second conductive foam structure, and a porous separator disposed between said first and said second conductive foam structure; and (C) Injecting the anode suspension into pores of the first conductive foam structure to form an anode and injecting cathode suspension into pores of the second conductive foam structure to form a cathode, wherein the anode active material has a material mass loading no less than 20 mg / cm2 or the cathode active material has a material mass loading no less than 15 mg / cm2 for an organic or polymer material or no less than 40 mg / cm2 for an inorganic material. The resulting batteries exhibit exceptional gravimetric and volumetric energy densities and long cycle life.
Owner:GLOBAL GRAPHENE GRP INC
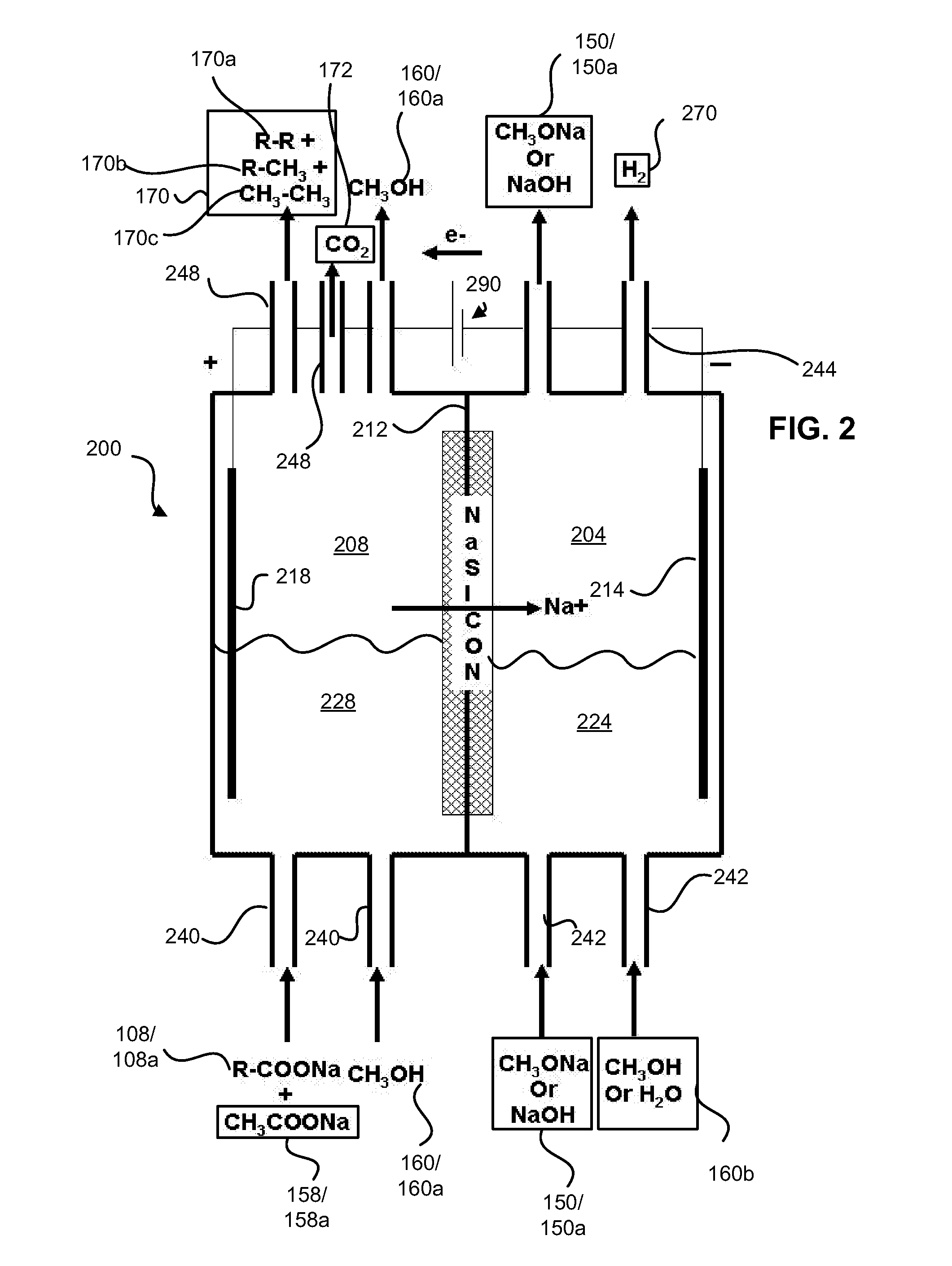
Electrolytic method to make alkali alcoholates using ion conducting alkali electrolyte/seperator
ActiveUS20080142373A1Efficient executionElectrolysis componentsElectrolytic organic reductionAlkali ionsAlcohol
Alkali alcoholates, also called alkali alkoxides, are produced from alkali metal salt solutions and alcohol using a three-compartment electrolytic cell. The electrolytic cell includes an anolyte compartment configured with an anode, a buffer compartment, and a catholyte compartment configured with a cathode. An alkali ion conducting solid electrolyte configured to selectively transport alkali ions is positioned between the anolyte compartment and the buffer compartment. An alkali ion permeable separator is positioned between the buffer compartment and the catholyte compartment. The catholyte solution may include an alkali alcoholate and alcohol. The anolyte solution may include at least one alkali salt. The buffer compartment solution may include a soluble alkali salt and an alkali alcoholate in alcohol.
Owner:ENLIGHTEN INNOVATIONS INC
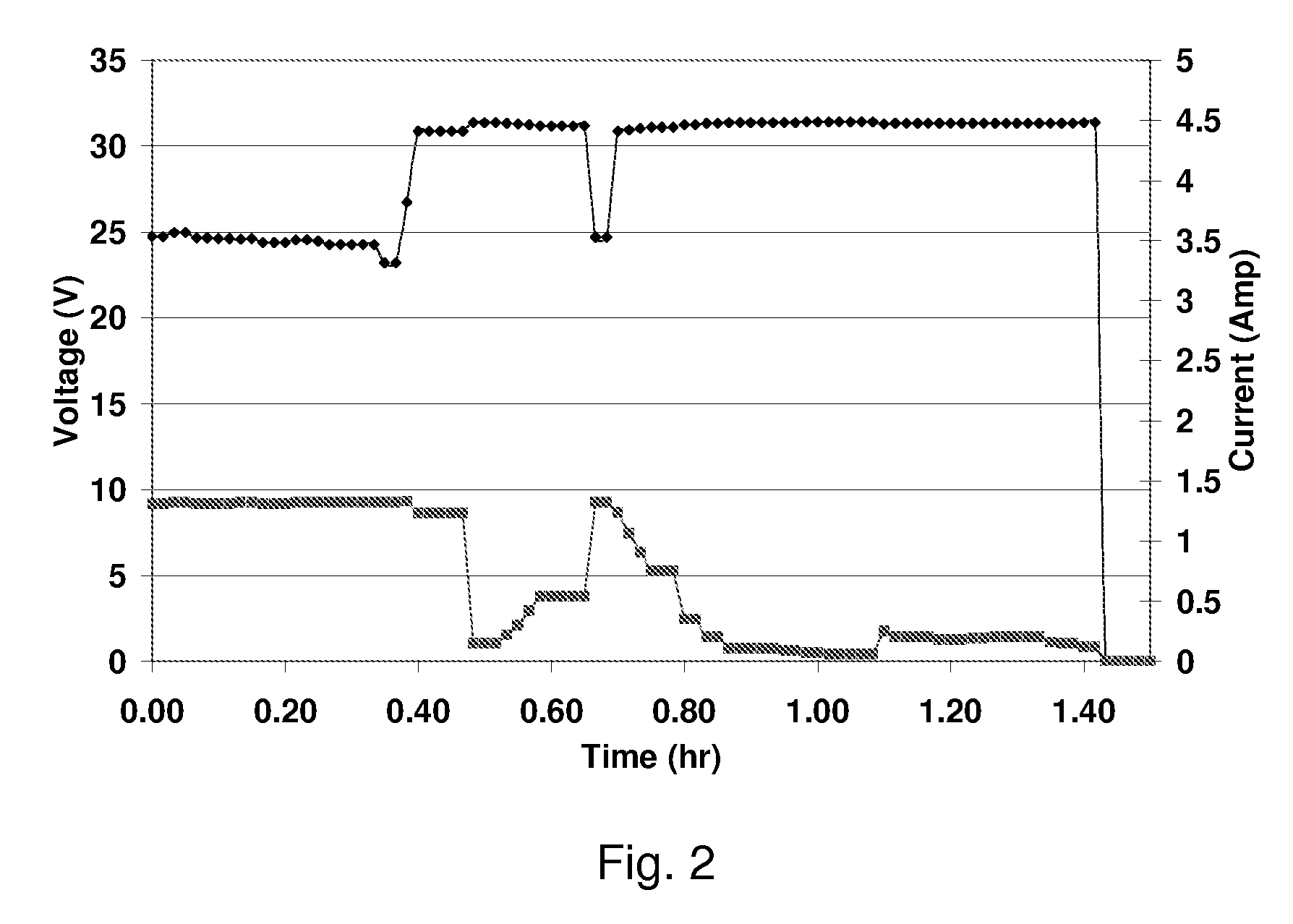
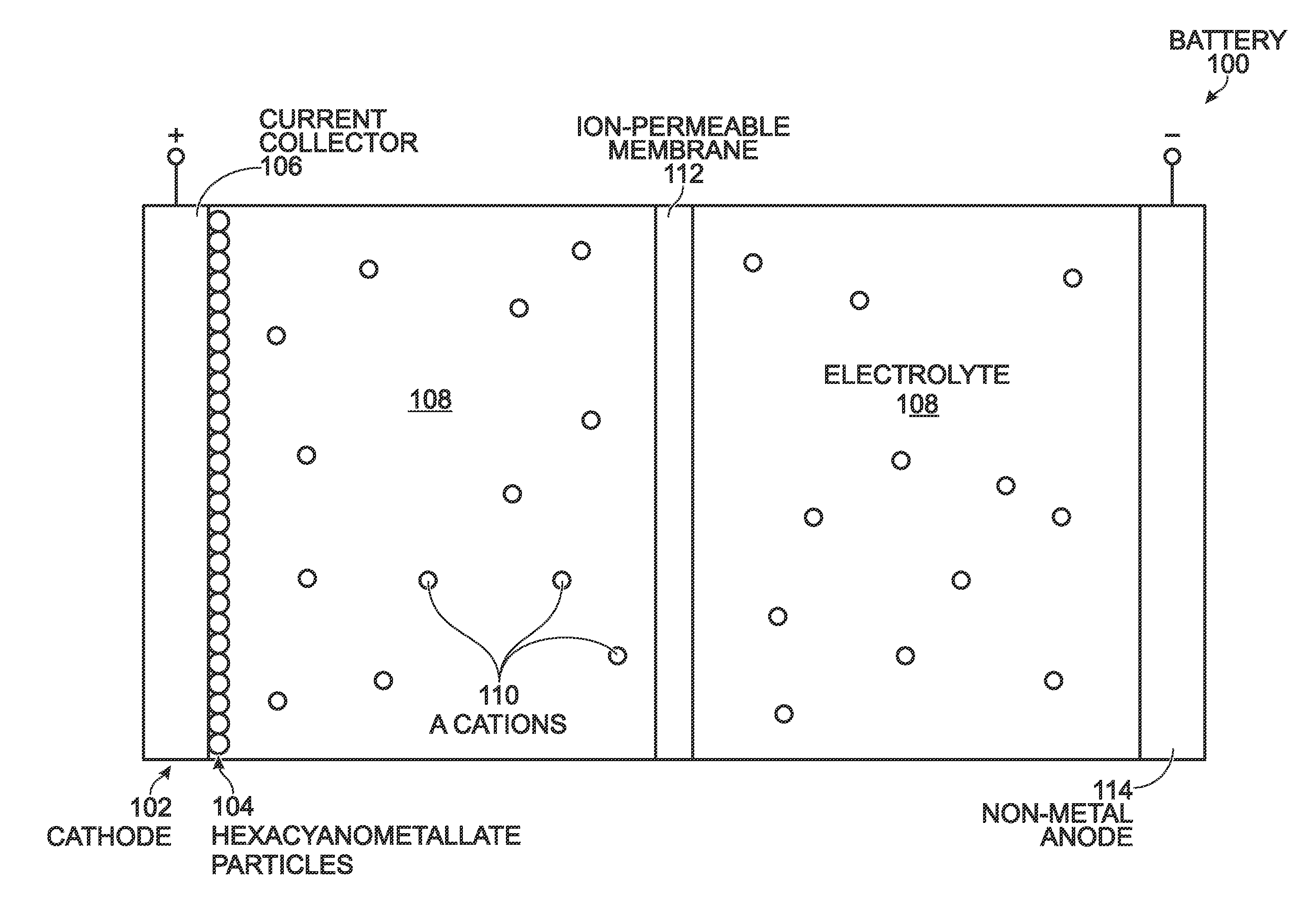
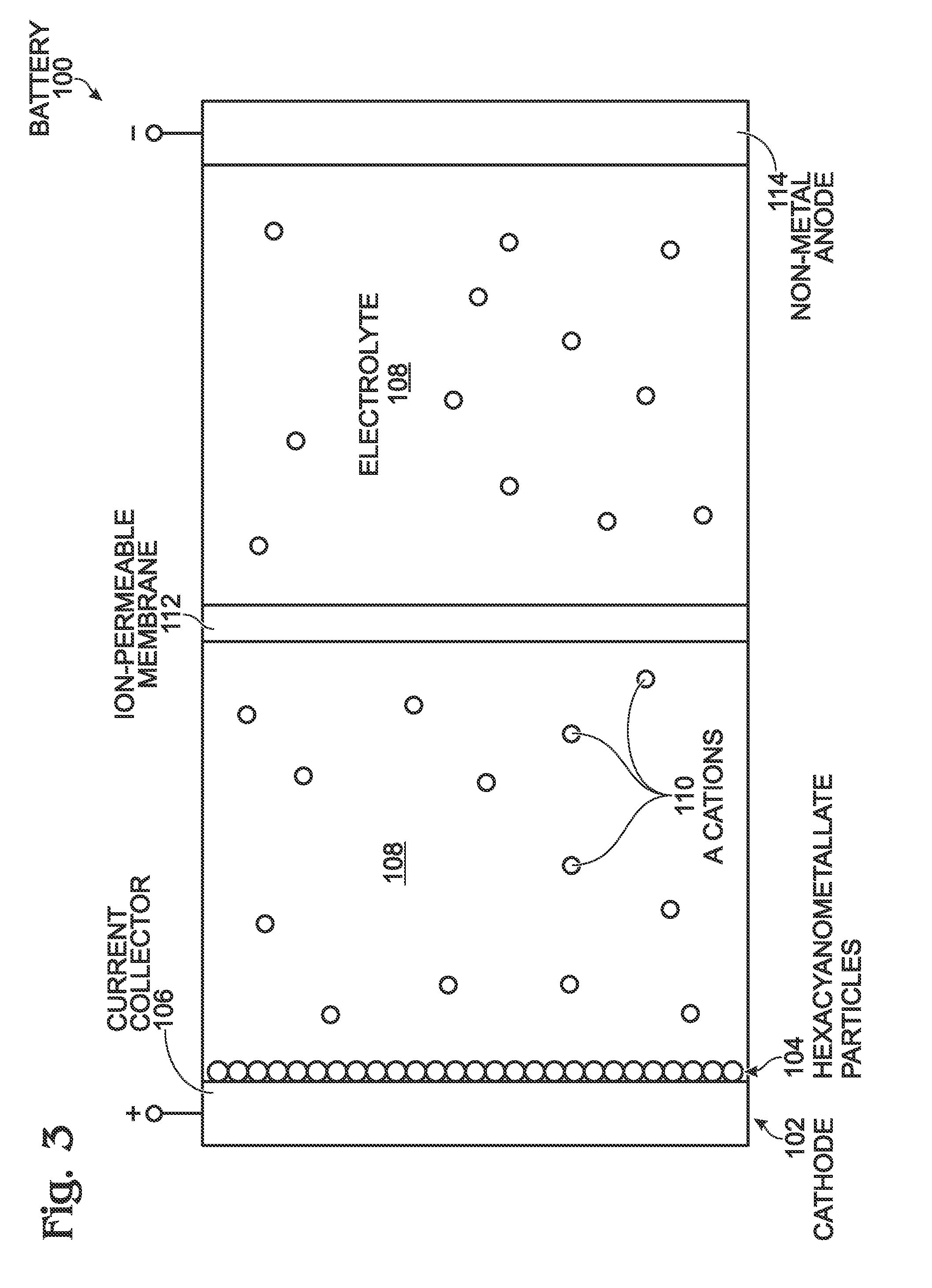
Alkali and Alkaline-Earth Ion Batteries with Hexacyanometallate Cathode and Non-Metal Anode
ActiveUS20130260232A1High energyImprove cycle lifeElectrochemical processing of electrodesElectrode carriers/collectorsAlkali ionsAlkaline earth metal
A battery structure is provided for making alkali ion and alkaline-earth ion batteries. The battery has a hexacyanometallate cathode, a non-metal anode, and non-aqueous electrolyte. A method is provided for forming the hexacyanometallate battery cathode and non-metal battery anode prior to the battery assembly. The cathode includes hexacyanometallate particles overlying a current collector. The hexacyanometallate particles have the chemical formula A′n, AmM1xM2y(CN)6, and have a Prussian Blue hexacyanometallate crystal structure.
Owner:SHARP KK
Purification method of ultra-pure silicon dioxide sol
InactiveCN101475180AHigh purityGood removal effectSilicon compoundsPolishing compositions with abrasivesAlkali ionsPurification methods
The invention discloses a method for purifying a silica sol with superhigh purity in the technical field of chemical mechanical polishing. The purification process comprises taht: strong acid type cation exchange resin, strong alkali type anion exchange resin and strong acid and strong alkali mixed-bed resin are filled to an ion exchange column and are subjected to regeneration treatment; the temperature of the silica sol is controlled between 0 and 60 DEG C and inversely flows through a strong acid type cation exchange bed; the obtained acid silica sol inversely flows through a strong alkali type anion exchange bed and is added with a chelator so that pH of the silica sol is between 1.0 and 3.0; and the silica sol added with the chelator inversely flows through a strong acid and strong alkali ion exchange resin mixed bed to obtain the purified silica sol. The method has good purification effect, can obtain the silica sol of which the superhigh purity meets the requirement of the chemical mechanical polishing, has short purification time and high efficiency, and can be used for continuous industrialized production.
Owner:TSINGHUA UNIV +1
Glass article and glass substrate for display panel
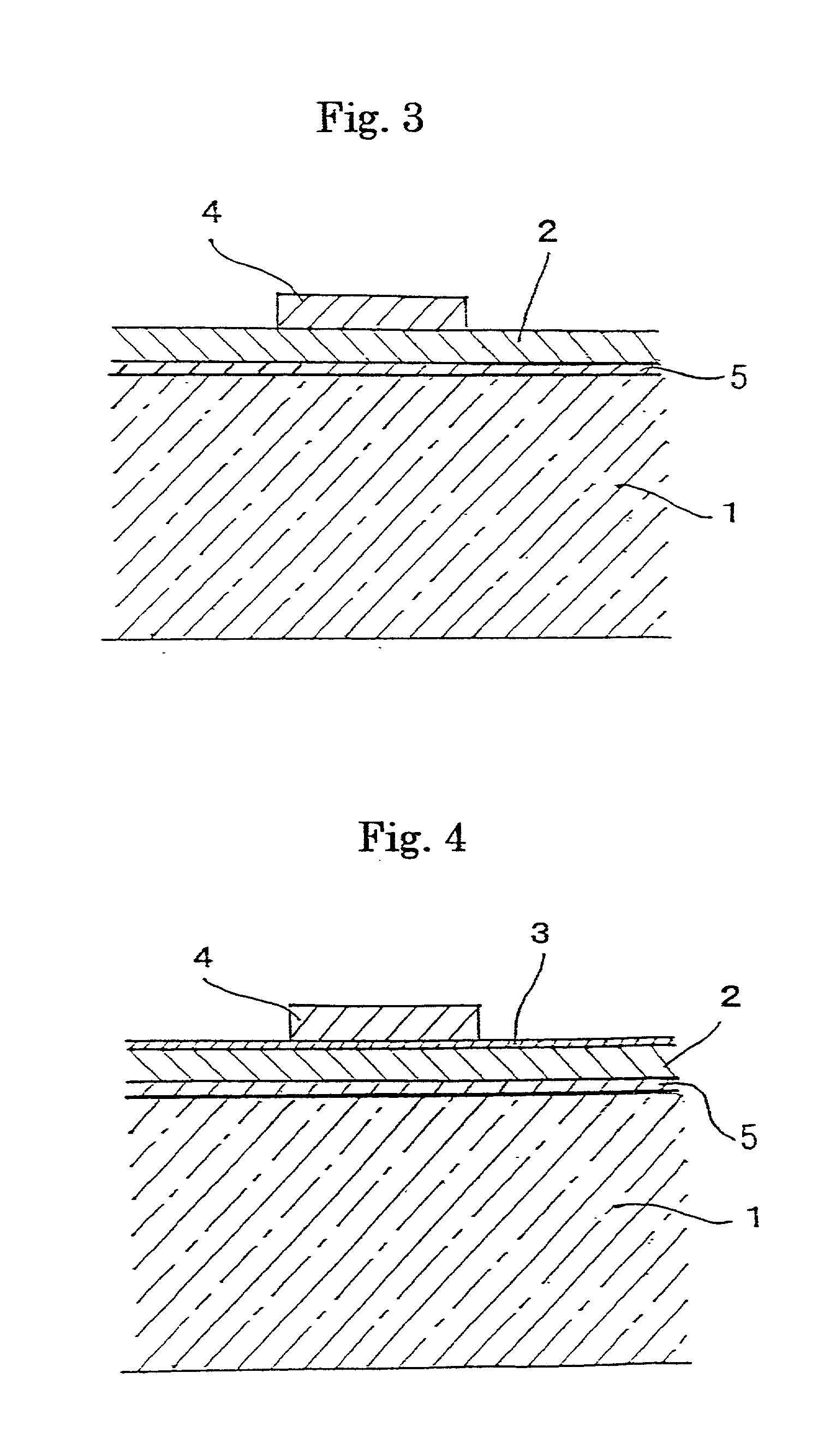
InactiveUS20010016253A1Excellent efficiency of preventing diffusion of metal ionInhibited DiffusionGas discharge vessels/containersLayered productsAlkali ionsElectrical resistance and conductance
A glass article having no problem of stain due to metal colloids because of its excellent efficiency of preventing the diffusion of metal ions, and a glass substrate for a high-quality display comprising the aforementioned glass article are provided. The glass article comprises an alkali-containing glass substrate 1, and a barrier film 2 formed on a surface of the alkali-containing glass substrate 1. The metal ion diffusion barrier film 2 mainly contains indium oxide and / or tin oxide. A glass substrate for a display comprises: an alkali-containing glass substrate 1; an alkali ion diffusion barrier film 5 formed on a surface of said alkali-containing glass substrate 1; a barrier film 2 mainly containing indium oxide and / or tin oxide; an insulating film 3; and an electrode film 4. The surface electrical resistance of the insulating film is kept in a range from 1.0x106 OMEGA / square to 1.0x1016 OMEGA / square even after heating process at 550° C. for 1 hour.
Owner:NIPPON SHEET GLASS CO LTD
Inks with alkali metals for thin film solar cell processes
InactiveUS20120073633A1Final product manufactureSemiconductor/solid-state device manufacturingAlkali ionsElectrical battery
Processes for making a thin film solar cell on a substrate by providing a substrate coated with an electrical contact layer, depositing an ink onto the contact layer of the substrate, wherein the ink contains an alkali ion source compound suspended or dissolved in a carrier along with photovoltaic absorber precursor compounds, and heating the substrate. The alkali ion source compound can be MalkMB(ER)4 or Malk(ER). The processes can be used for CIS or CIGS.
Owner:PRECURSOR ENERGETICS
Choline-based crosslinker compositions for fracturing fluids
InactiveUS20140305650A1Readily hydratableControllable viscosity propertyFluid removalFlushingParticulatesHigh concentration
Disclosed are compositions derived of mixtures of choline ion salts (typically choline chloride) in aqueous solution with suspended particulates of sparingly soluble borate minerals or with alkali or alkaline earth borate salts, boric acid and its ester derivatives and salts, or other aqueous soluble borate forms. These compositions are useful as cross-linkers for polysaccharides and other biopolymers and particularly as used in subterranean treatment fluids for completion and stimulation of oil and gas wells. Advantages of the compositions are the combination into a single package of the properties of clay stabilizing actives (choline ion) and crosslinking actives (borates, etc.), in relatively high concentrations, and these compositions are easy to handle, being stable and pumpable at low temperatures, and with attractive environmental profiles. Also disclosed are the analogous choline solutions mixed with metallic cross-linking ions know in the art such as Zr+, Ti4+, Al3+, & Fe3+.
Owner:CHAMPIONX USA INC
Thin-film transistor
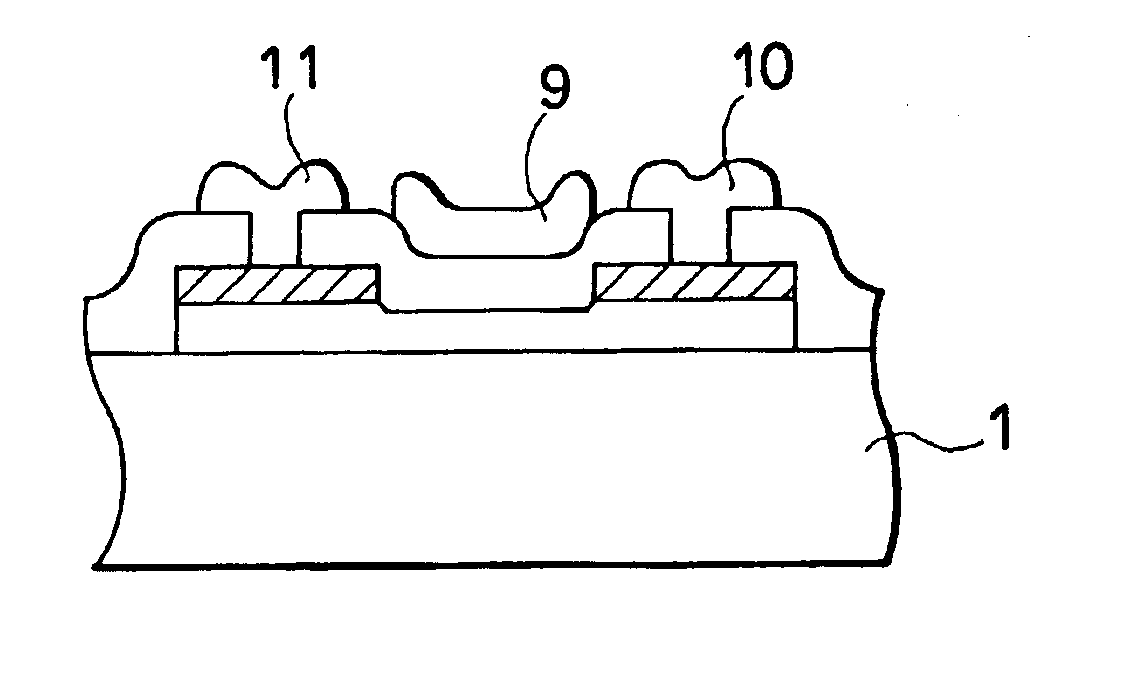
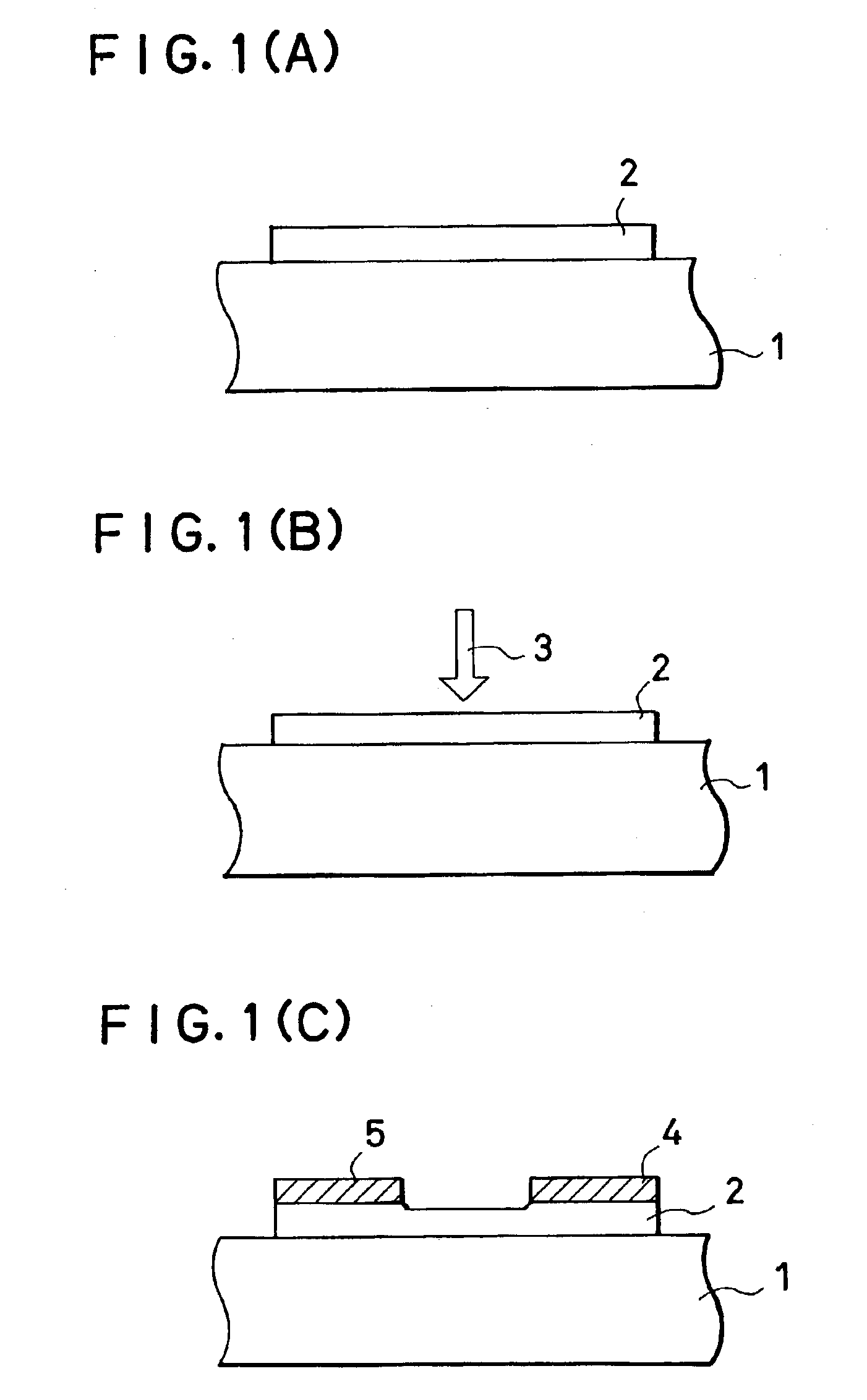
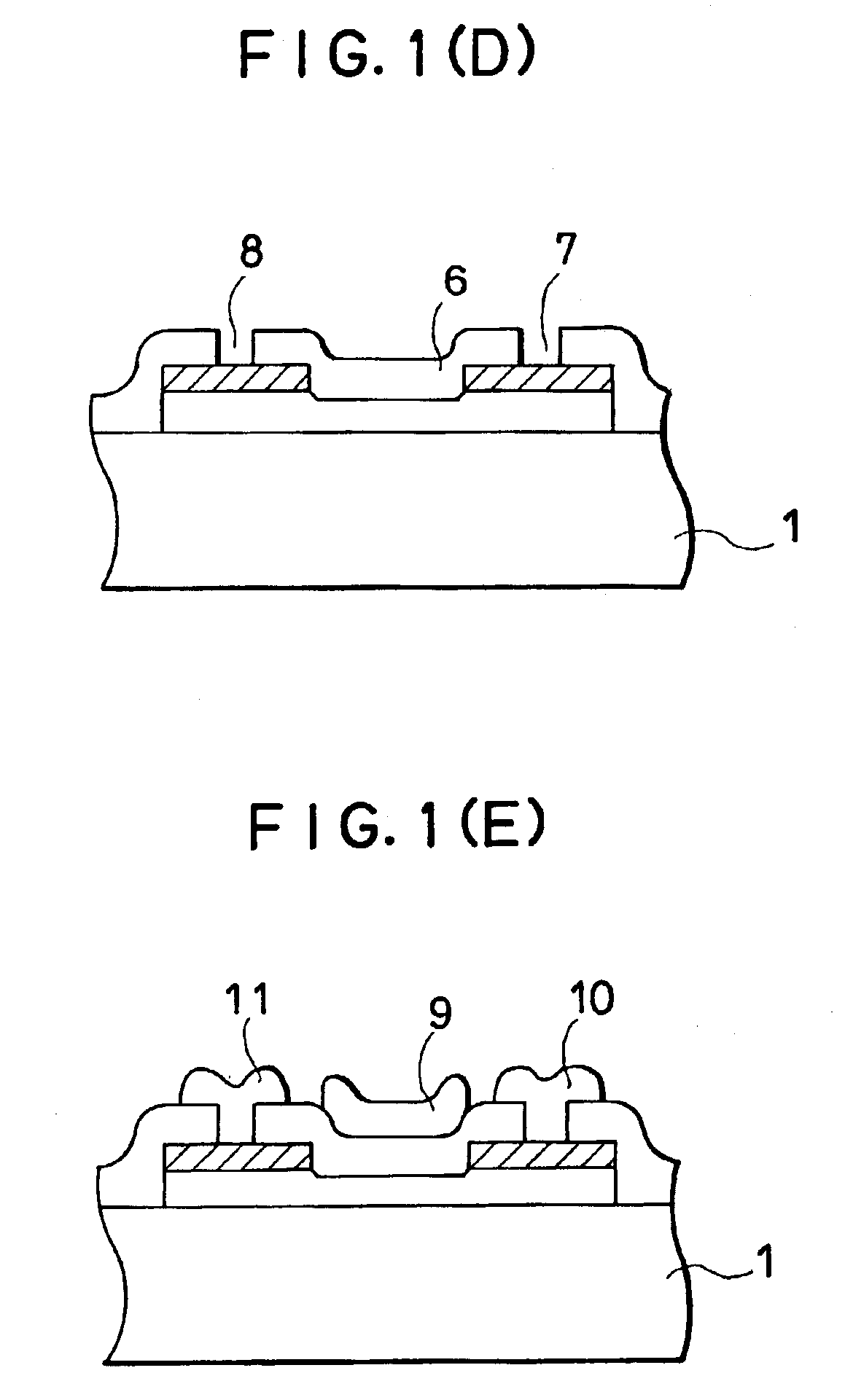
InactiveUS7355202B2High mutual conductanceSuppresses diffusion of impuritySolid-state devicesSemiconductor/solid-state device manufacturingAlkali ionsGate insulator
A gate-insulated thin film transistor is disclosed. One improvement is that the thin film transistor is formed on a substrate through a blocking layer in between so that it is possible to prevent the transistor from being contaminated with impurities such as alkali ions which exist in the substrate. Also, a halogen is added to either or both of the blocking layer and a gate insulator of the transistor.
Owner:SEMICON ENERGY LAB CO LTD
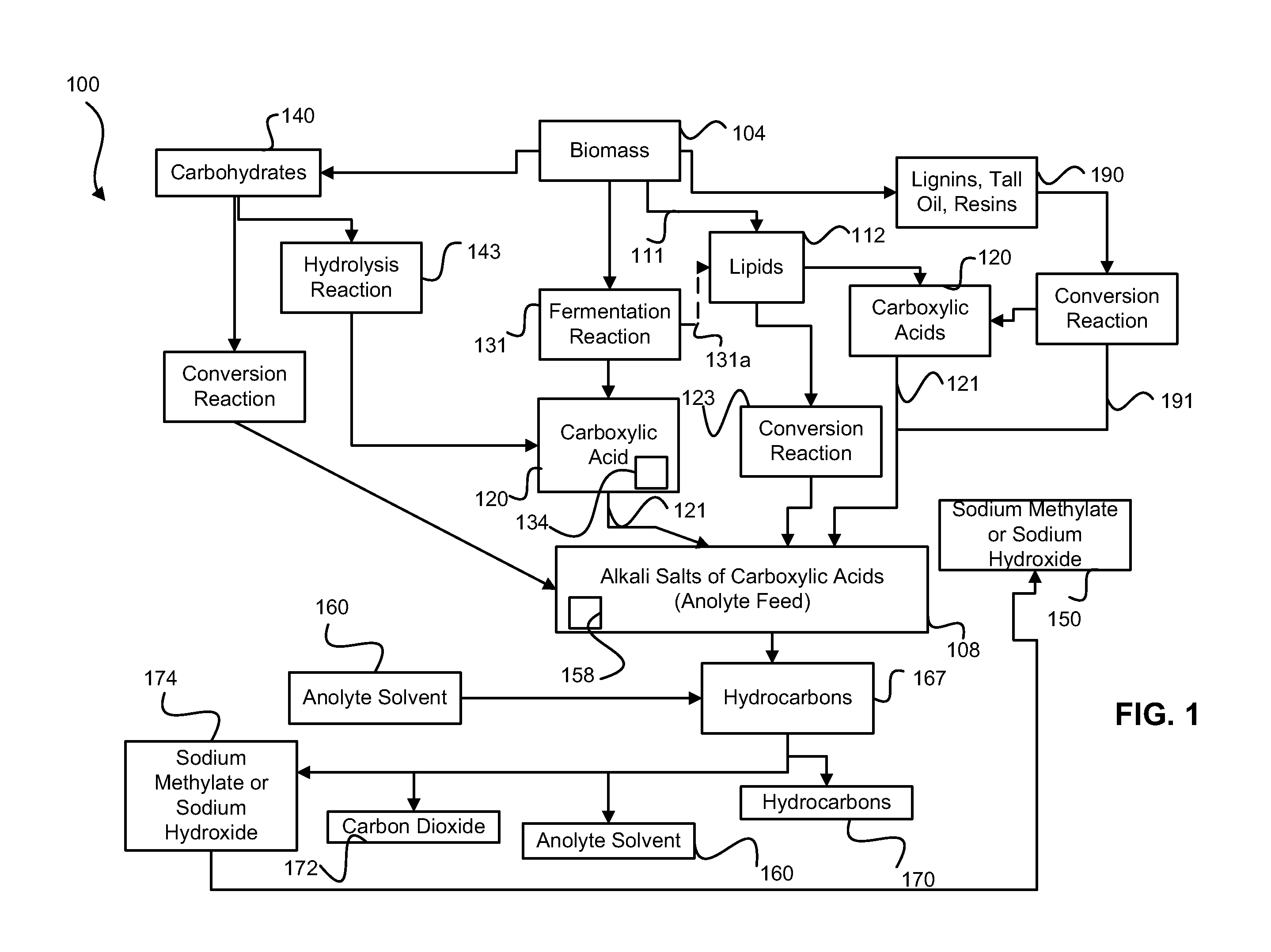
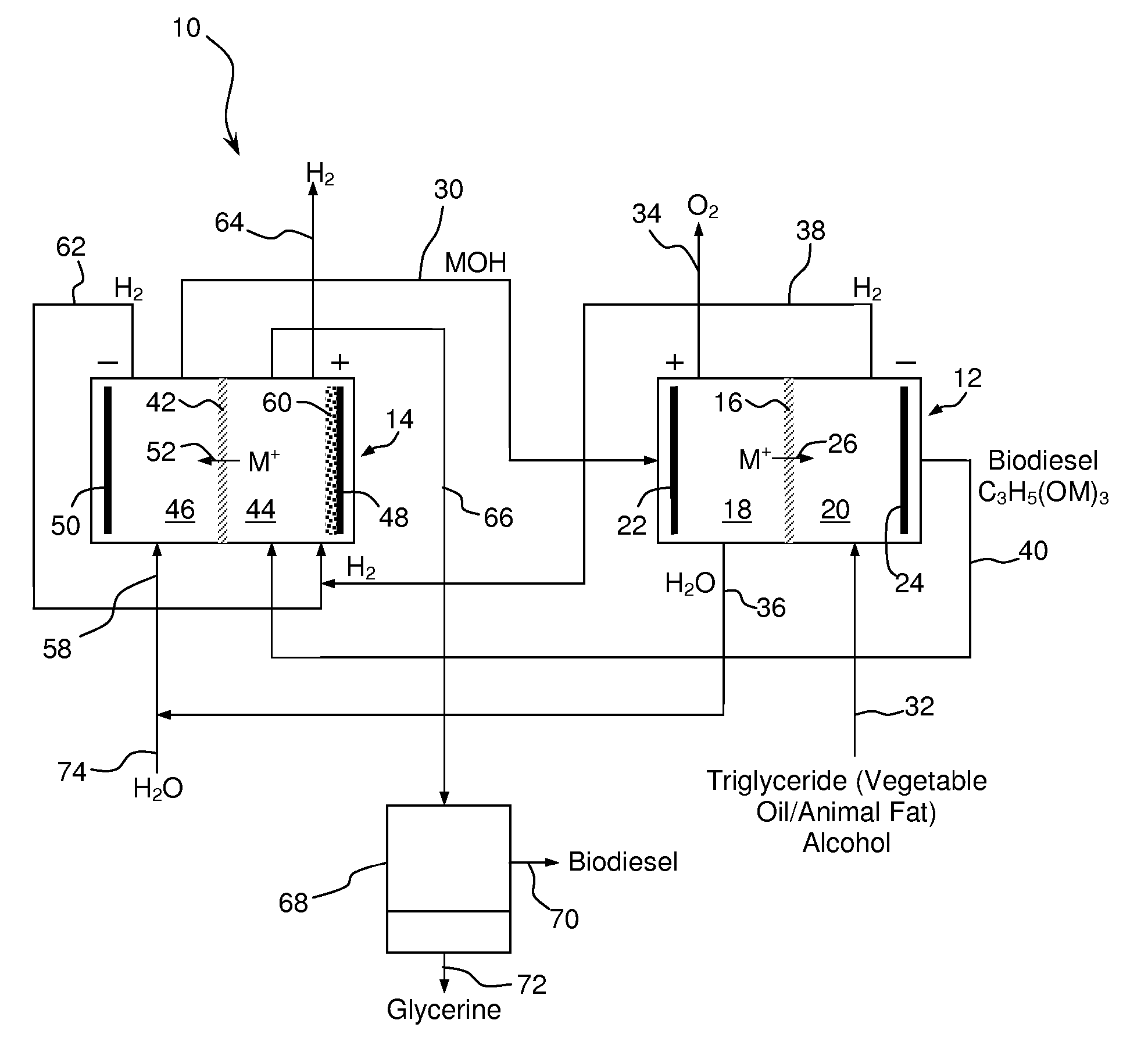
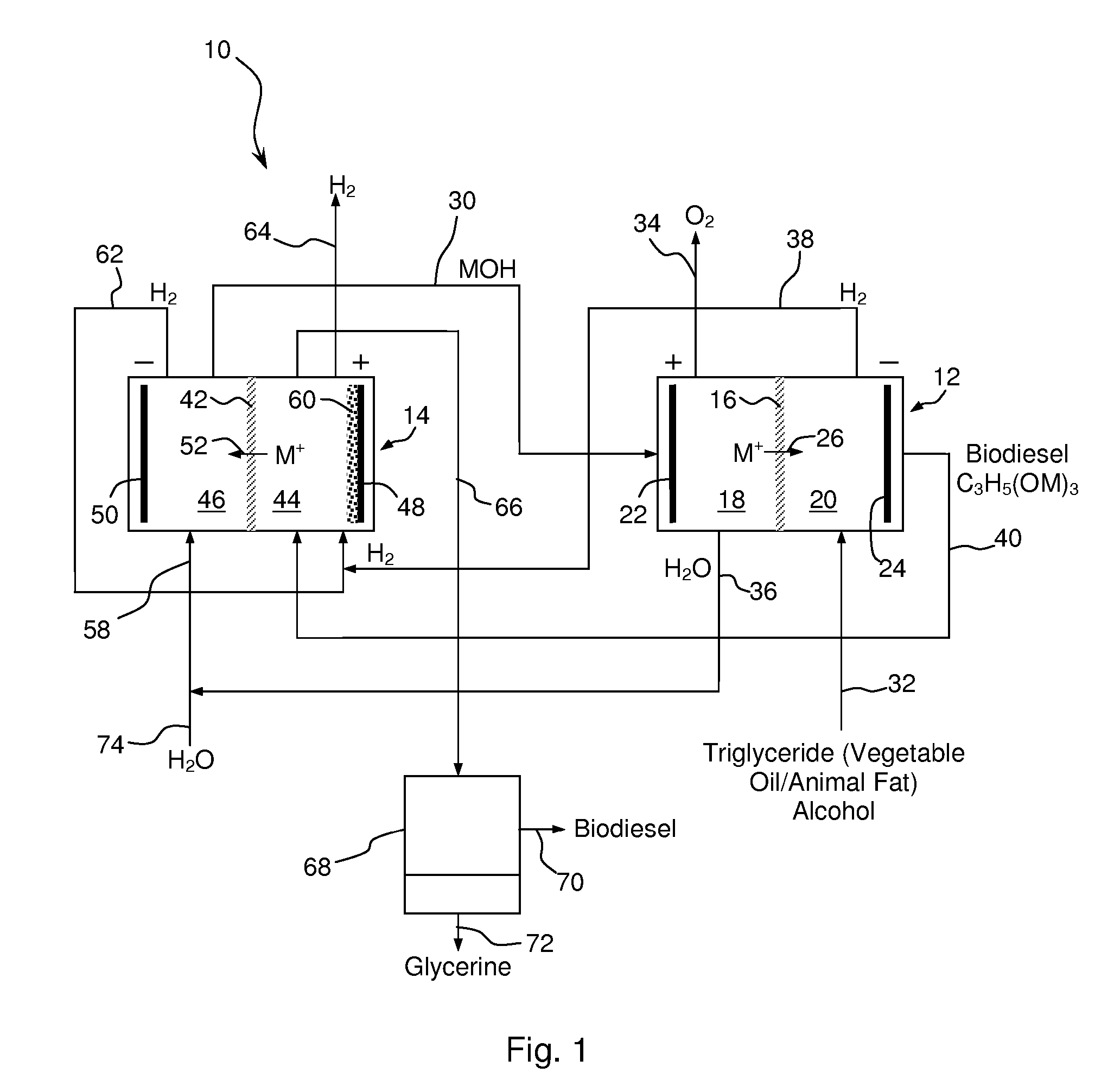
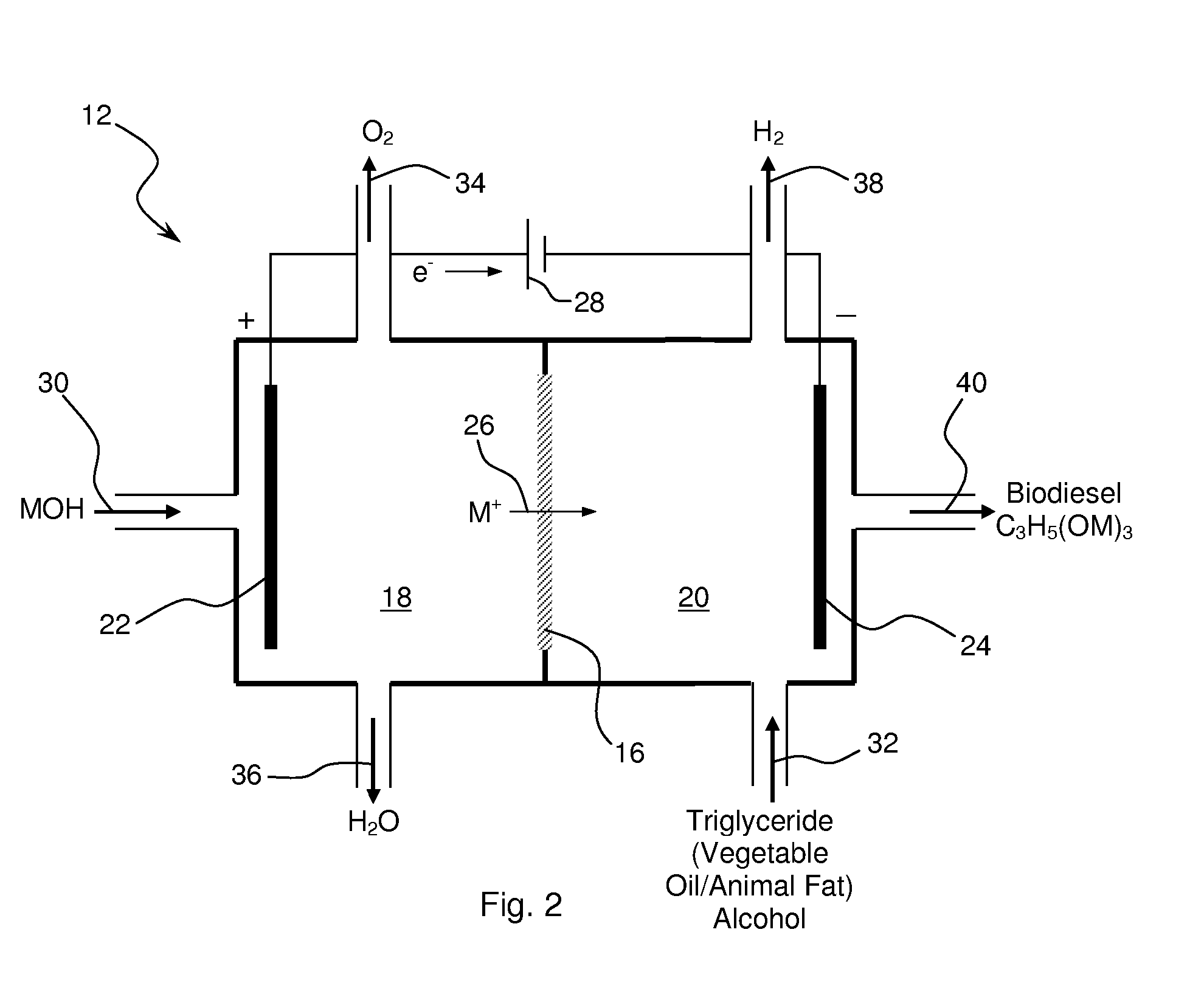
Synthesis of Biodiesel Using Alkali Ion Conductive Ceramic Membranes
InactiveUS20070158205A1Easy to separateImprove responseCellsFinal product manufactureAlkali ionsBiodiesel
Methods and apparatus for synthesizing biodiesel using alkali alkoxide generated on-site using an electrochemical process are disclosed. The apparatus and methods are disclosed to converting alkali salts of glycerine into glycerine and thereby facilitate the separation of clean glycerine from biodiesel. These methods are enabled by the use of alkali ion conductive ceramic membranes in electrolytic cells.
Owner:CERAMTEC
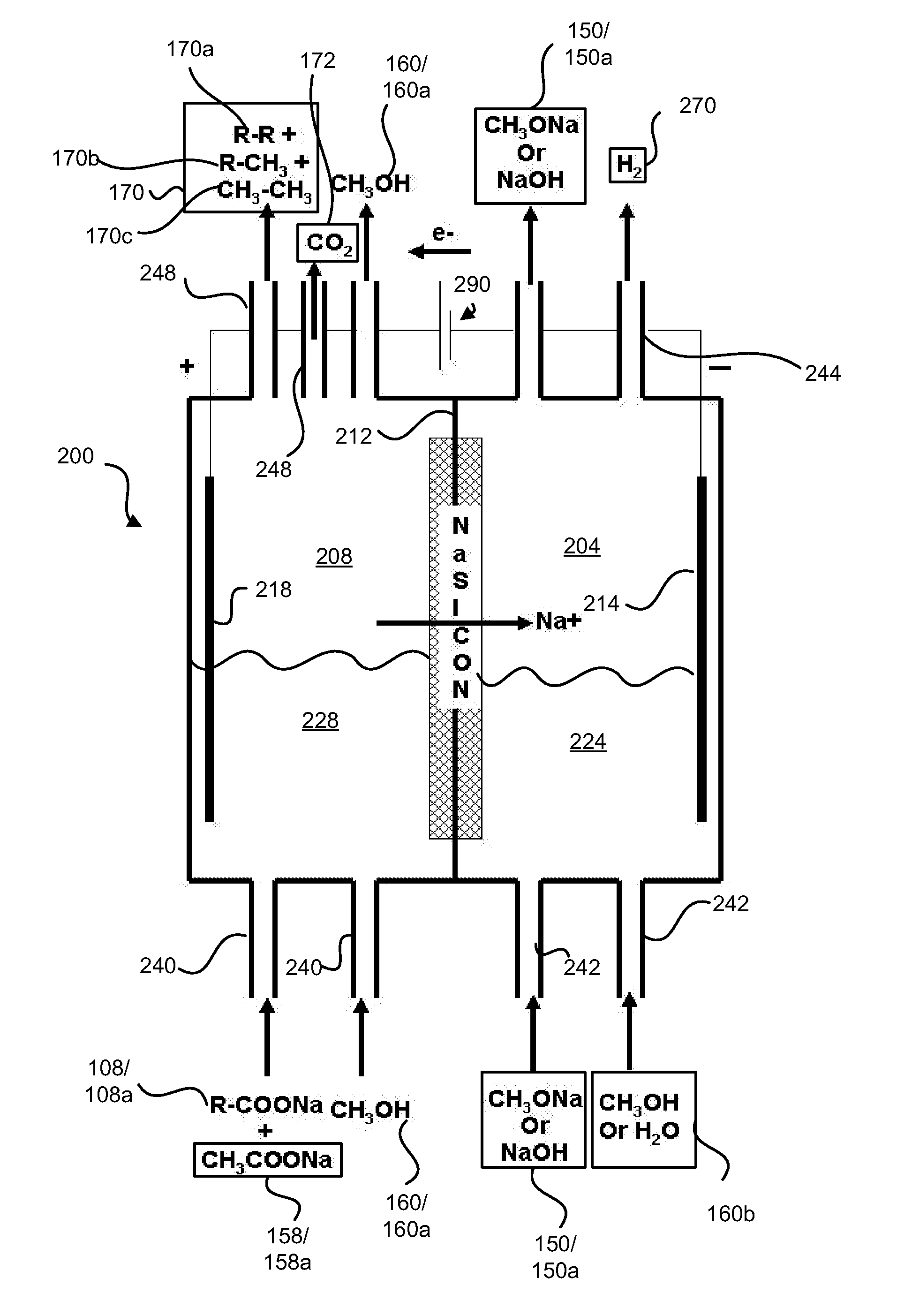
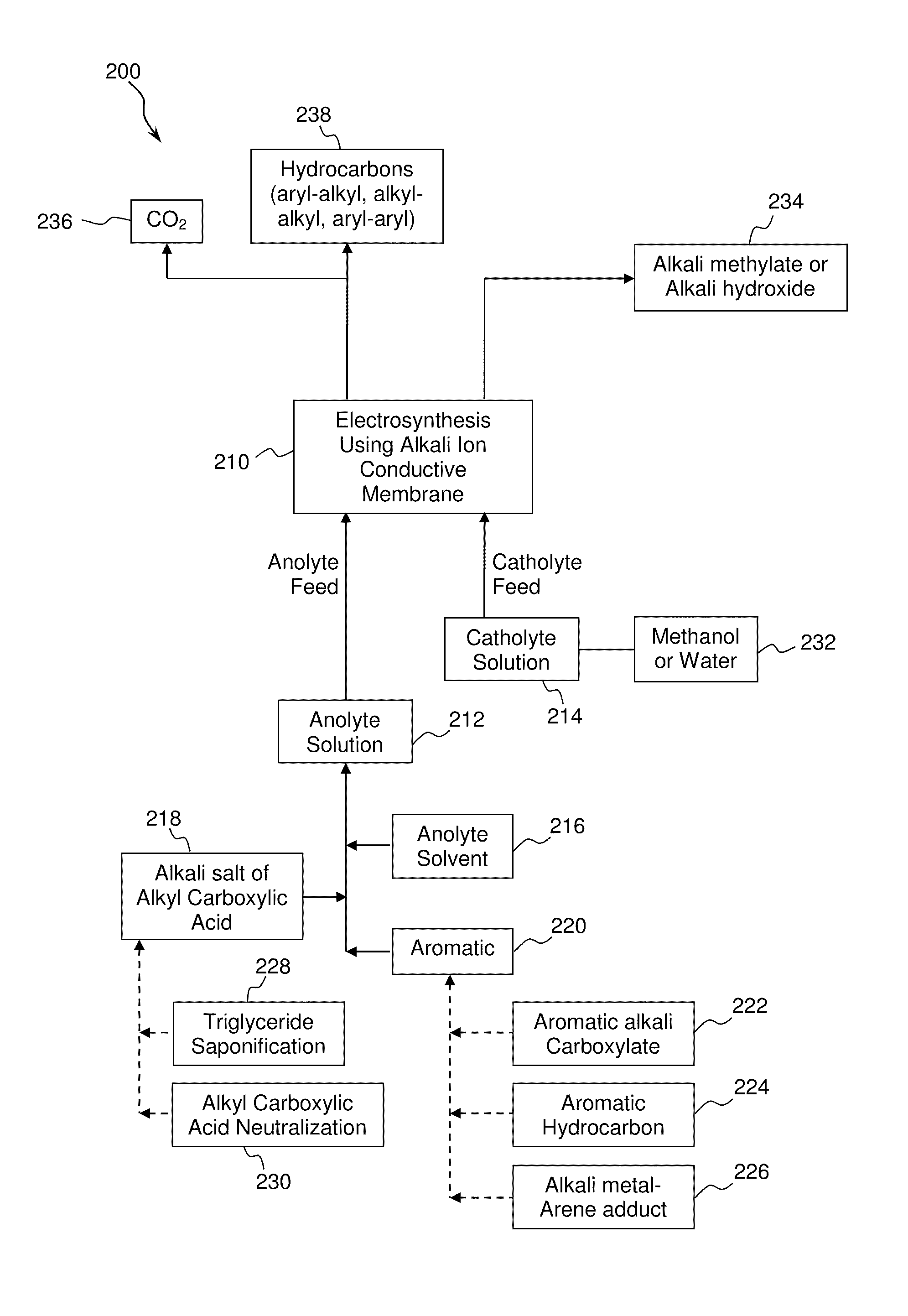
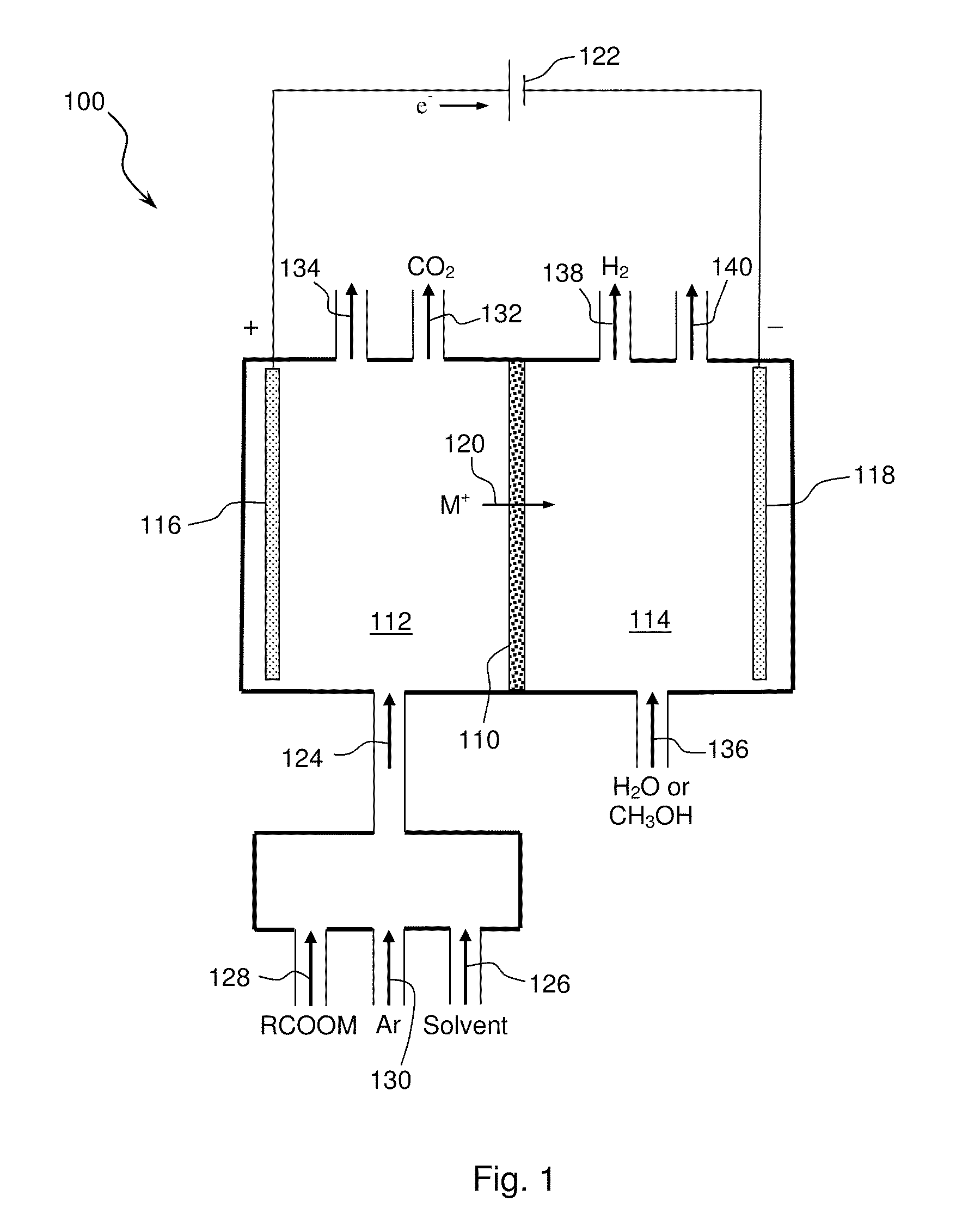
Electrochemical synthesis of aryl-alkyl surfacant precursor
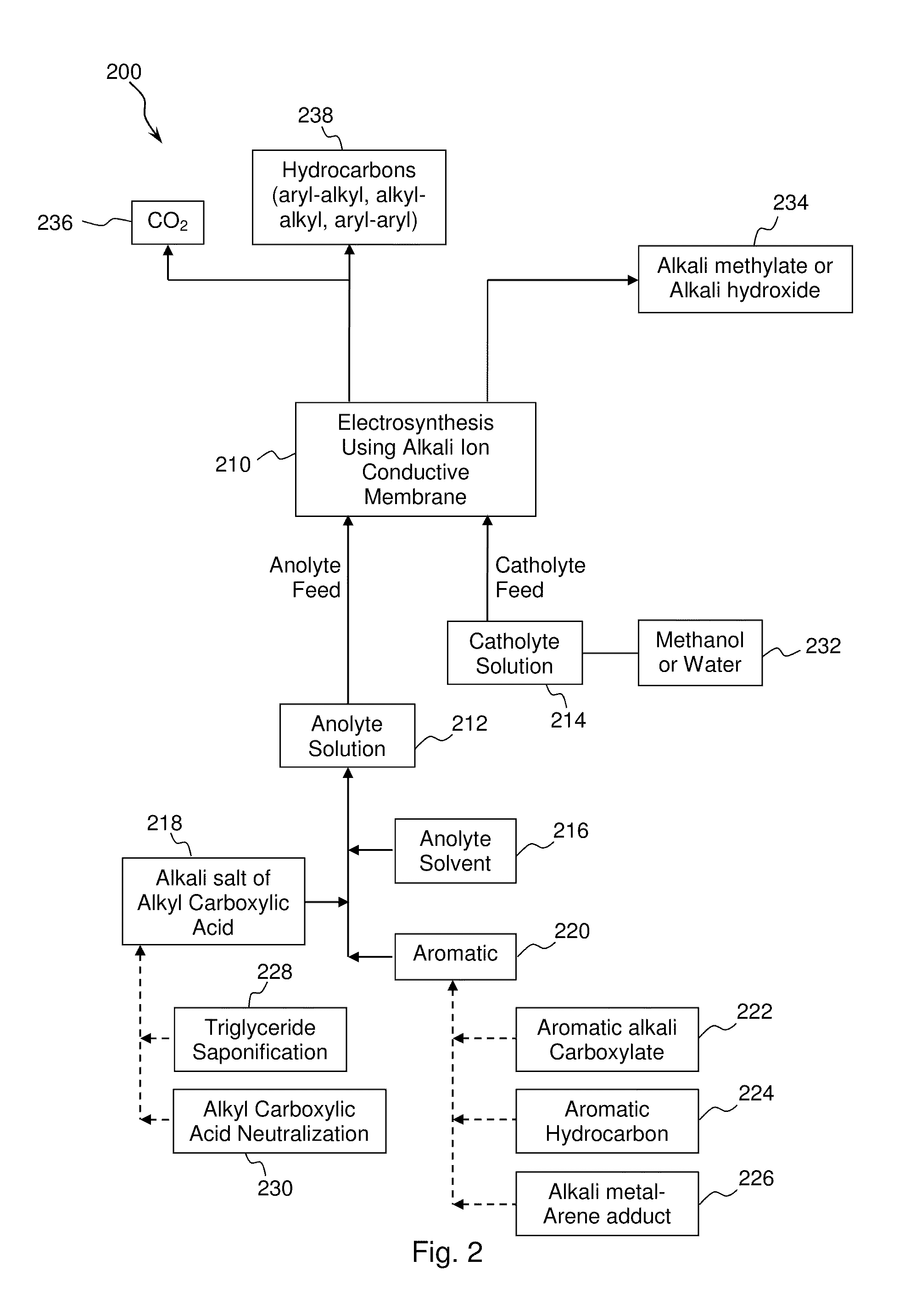
An aryl-alkyl (R—Ar) hydrocarbon is prepared by an electrosynthesis process in an electrolytic cell having an alkali ion conductive membrane positioned between an anolyte compartment configured with an anode and a catholyte compartment configured with a cathode. An anolyte solution containing an alkali metal salt of an alkyl carboxylic acid and an aryl compound is introduced into the anolyte compartment. The aryl compound may include an alkali metal salt of an aryl carboxylic acid, an arene (aromatic) hydrocarbon, or an aryl alkali metal adduct (Ar−M+). The anolyte solution undergoes electrolytic decarboxylation to form an alkyl radical. The alkyl radical reacts with the aryl compound to produce the aryl-alkyl hydrocarbon.
Owner:ENLIGHTEN INNOVATIONS INC
Nickel-metal hydride battery using alkali ion conducting separator
ActiveUS8012621B2Reduce capacityEliminate dischargeFinal product manufactureAlkaline accumulator electrodesAlkali ionsElectrolyte
A nickel-metal hydride storage battery comprising a positive electrode containing nickel hydroxide, a negative electrode containing a hydrogen absorbing alloy, an alkaline electrolyte, and an alkali conducting separator provided between the positive electrode and the negative electrode. The alkali conducting separator may be a solid alkali metal ion super ion conducting material, wherein the alkali metal is Na, K, or Li.
Owner:FIELD UPGRADING USA INC
Thin-film transistor
InactiveUS20090101910A1High mutual conductanceSuppresses diffusion of impurityTransistorSolid-state devicesAlkali ionsGate insulator
Owner:ZHANG HONGYONG +1
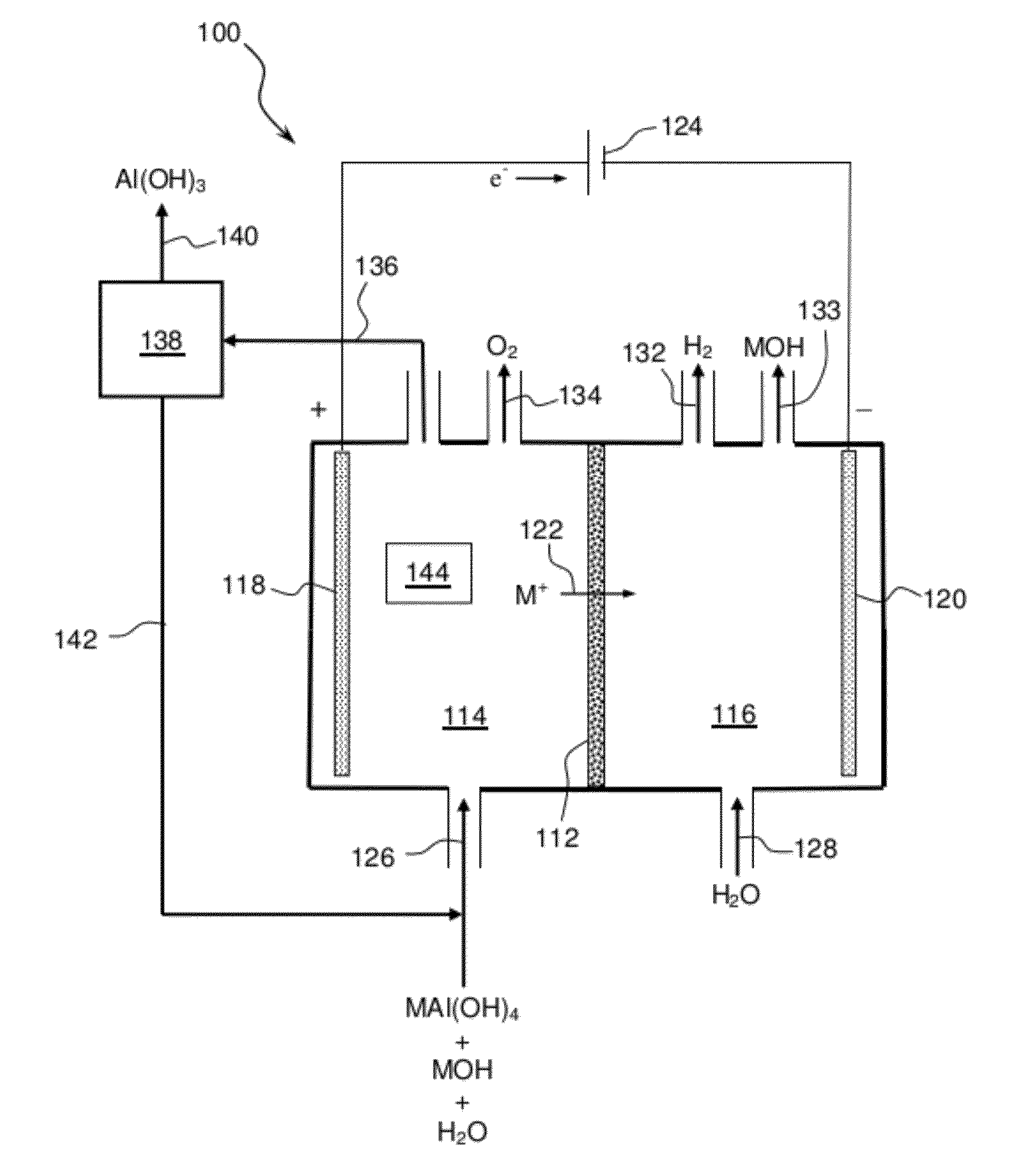
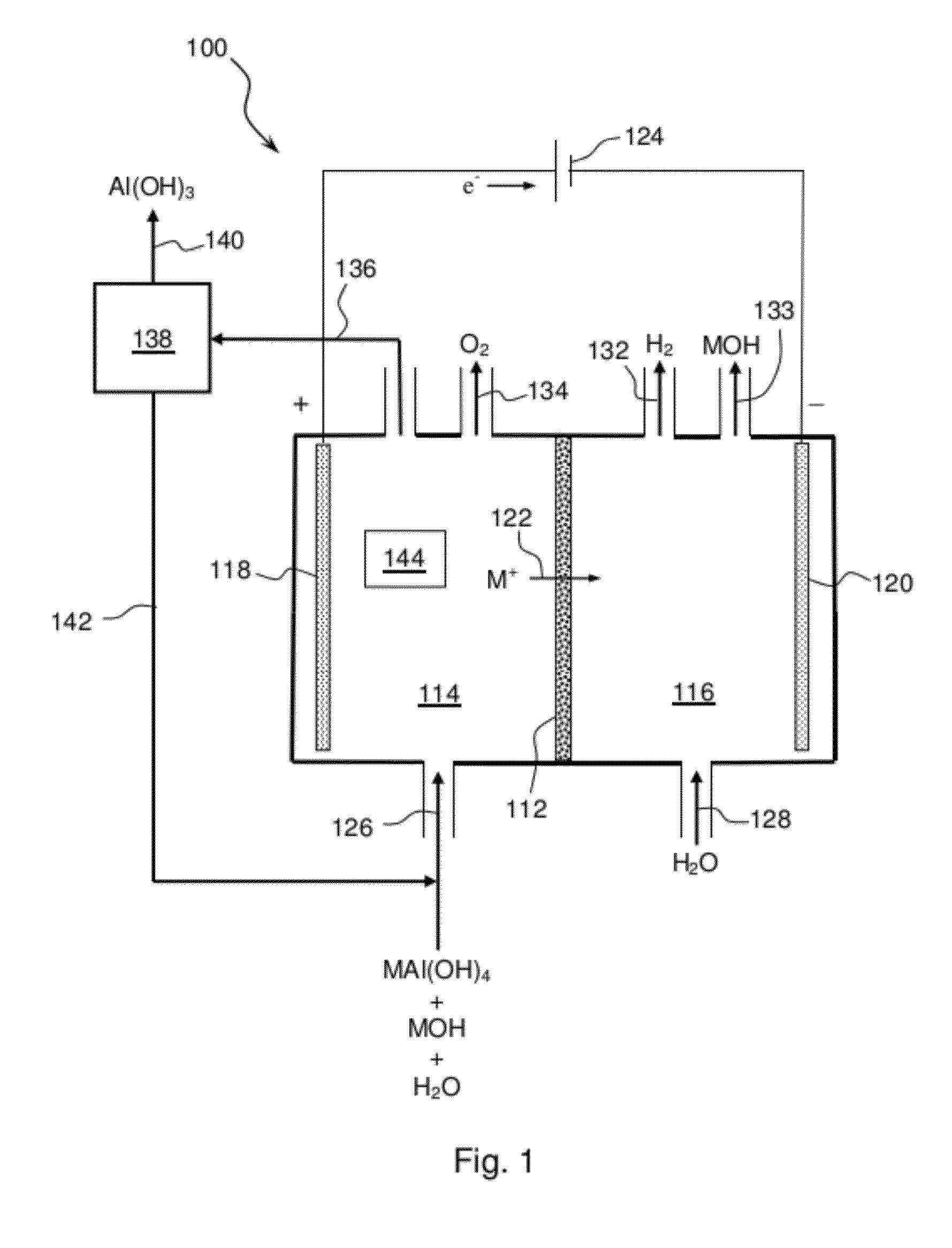
Electrolytic process to produce aluminum hydroxide
Methods and apparatus for separating aqueous solution of alkali aluminate into alkali hydroxide and aluminate hydroxide are disclosed. These methods are enabled by the use of alkali ion conductive membranes in electrolytic cells that are chemically stable and alkali ion selective. The alkali ion conductive membrane includes a chemically stable ionic-selective cation membrane.
Owner:CERAMTEC
Method of producing alkali metal or alkali-ion batteries having high volumetric and gravimetric energy densities
ActiveUS20170207489A1Increase energy densityImprove power densityFinal product manufactureElectrode carriers/collectorsAlkali ionsElectrical battery
A process for producing an alkali metal battery, comprising: (a) preparing multiple conductive porous layers (having at least 80% by volume of pores), multiple wet anode layers of an anode active material mixed with a liquid electrolyte, and multiple wet cathode layers of a cathode active material mixed with a liquid electrolyte; (b) stacking and consolidating a desired number of the porous layers and a desired number of wet anode layers to form an anode electrode; (c) placing a porous separator layer in contact with the anode electrode; (d) preparing a cathode electrode in a similar manner than anode; and (e) assembling all the components in a housing to produce the battery; wherein the anode active material has a material mass loading no less than 20 mg / cm2 in the anode and / or the cathode active material has a material mass loading no less than 30 mg / cm2 in the cathode electrode.
Owner:GLOBAL GRAPHENE GRP INC
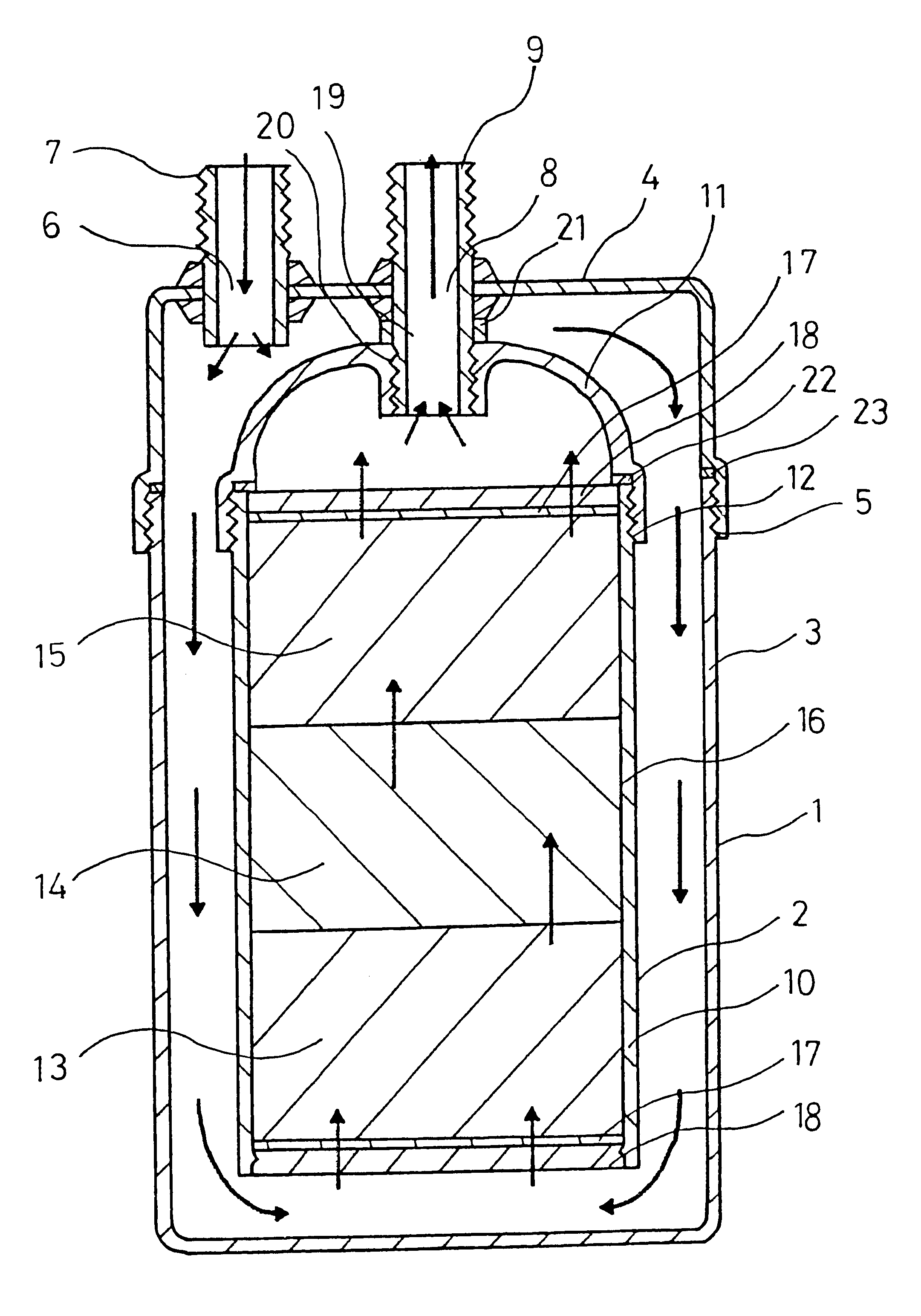
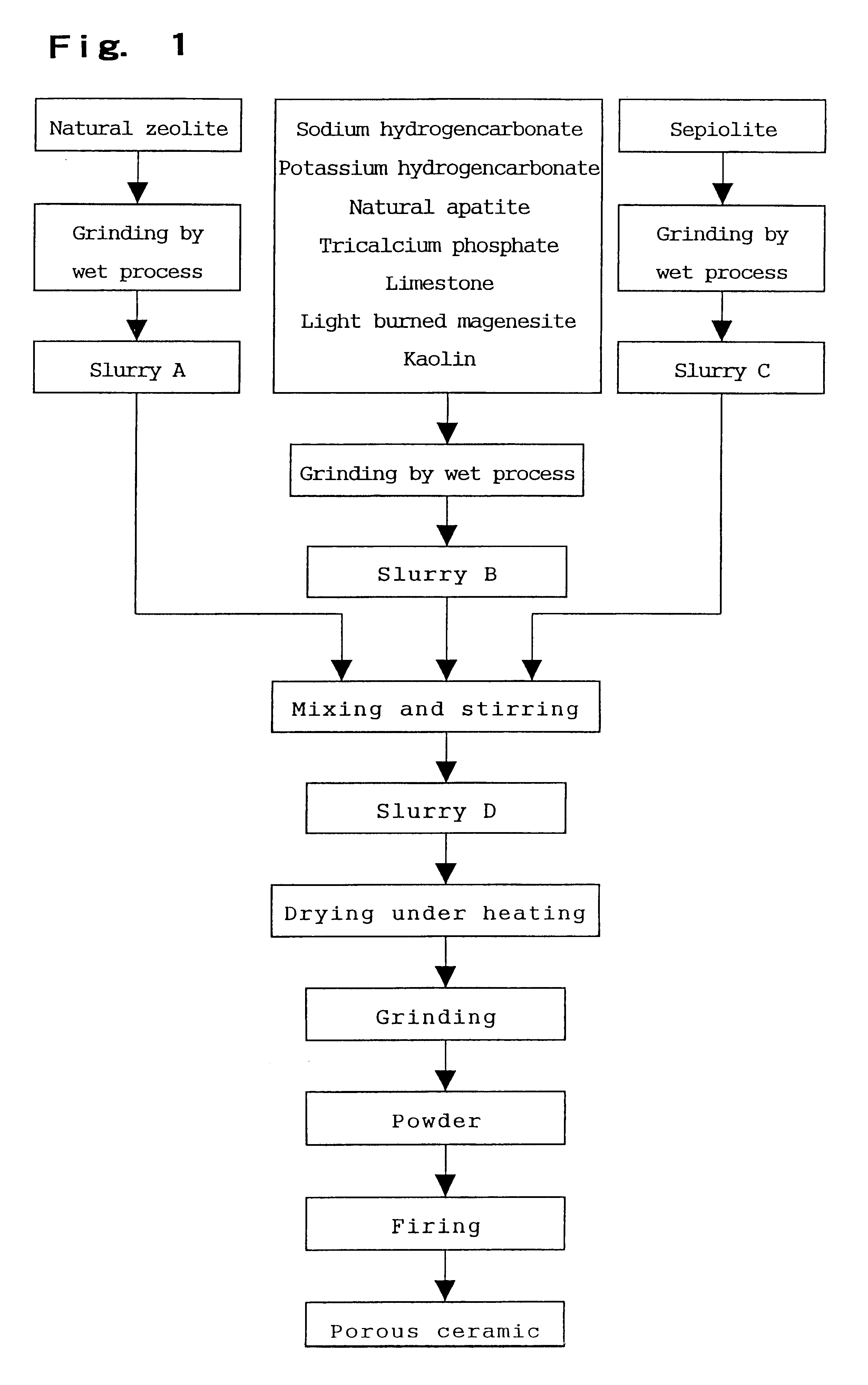
Porous ceramic for producing alkali ion water, method for producing the porous ceramic and device for producing the alkali ion water
InactiveUS6245701B1Dissolved easily and efficientlyEasy and efficientAluminium compoundsTreatment involving filtrationAlkali ionsClay minerals
The invention relates to a porous ceramic used for producing alkali ion water. The ceramic of the invention includes finely particulate zeolite having an average particle size of 0.1 to 40 mum and an alkali ion producing material as components. Besides the above components, the invention preferably comprises a fibrous mineral and / or a clay mineral. To produce the ceramic of the invention, a slurry obtained by grinding zeolite by a wet process, a slurry obtained by grinding the alkali ion producing material and the clay mineral by a wet process and a slurry obtained by grinding the fibrous mineral are first mixed and stirred to prepare a mixed slurry. This mixed slurry is then dried and fired to obtain a sintered body.
Owner:JAPAN ZEOLITE
Method of producing coupled radical products from biomass
ActiveUS20110027848A1Avoid mixingLow costCellsPhotography auxillary processesAlkali ionsTG - Triglyceride
Owner:ENLIGHTEN INNOVATIONS INC
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com