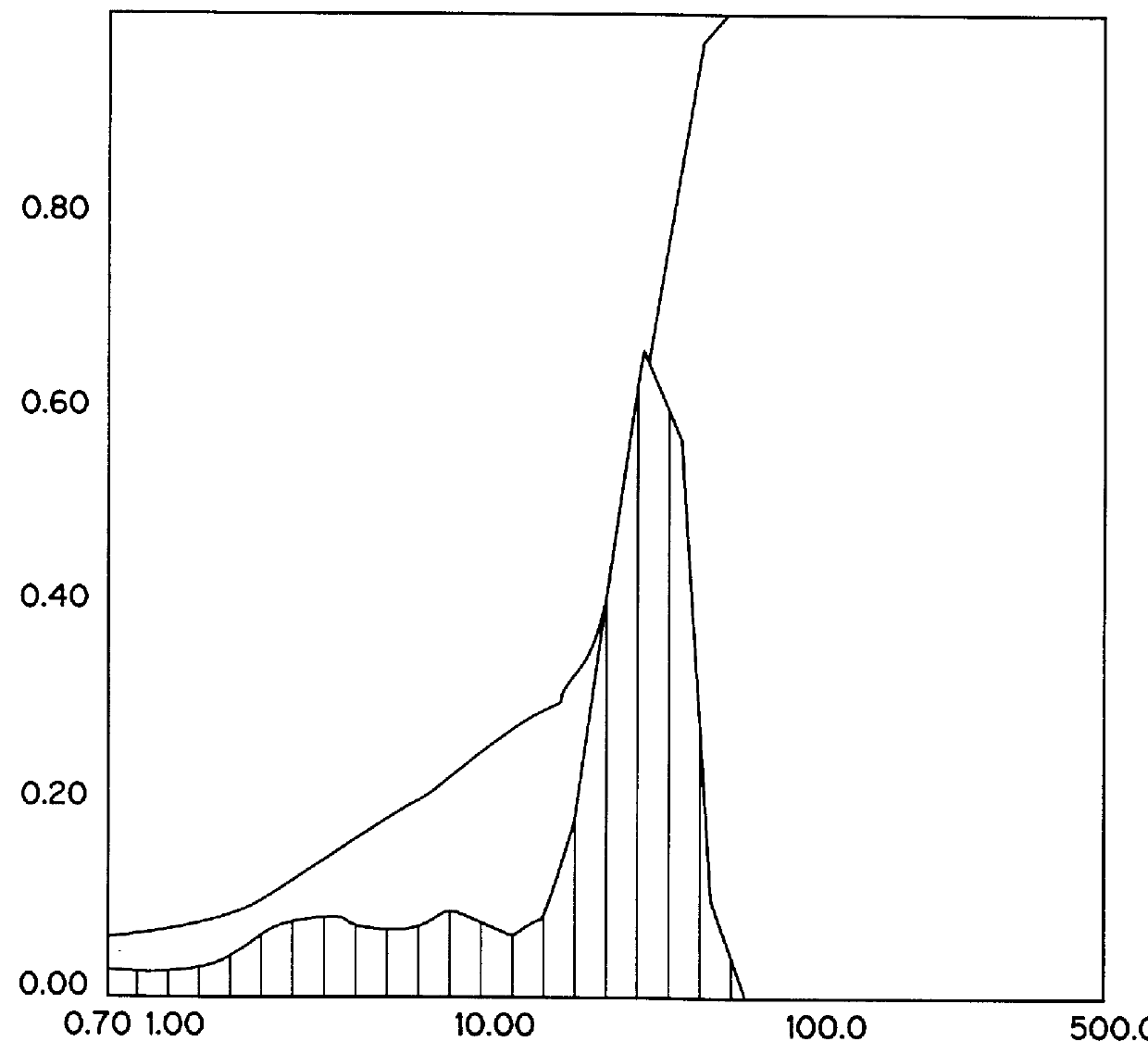
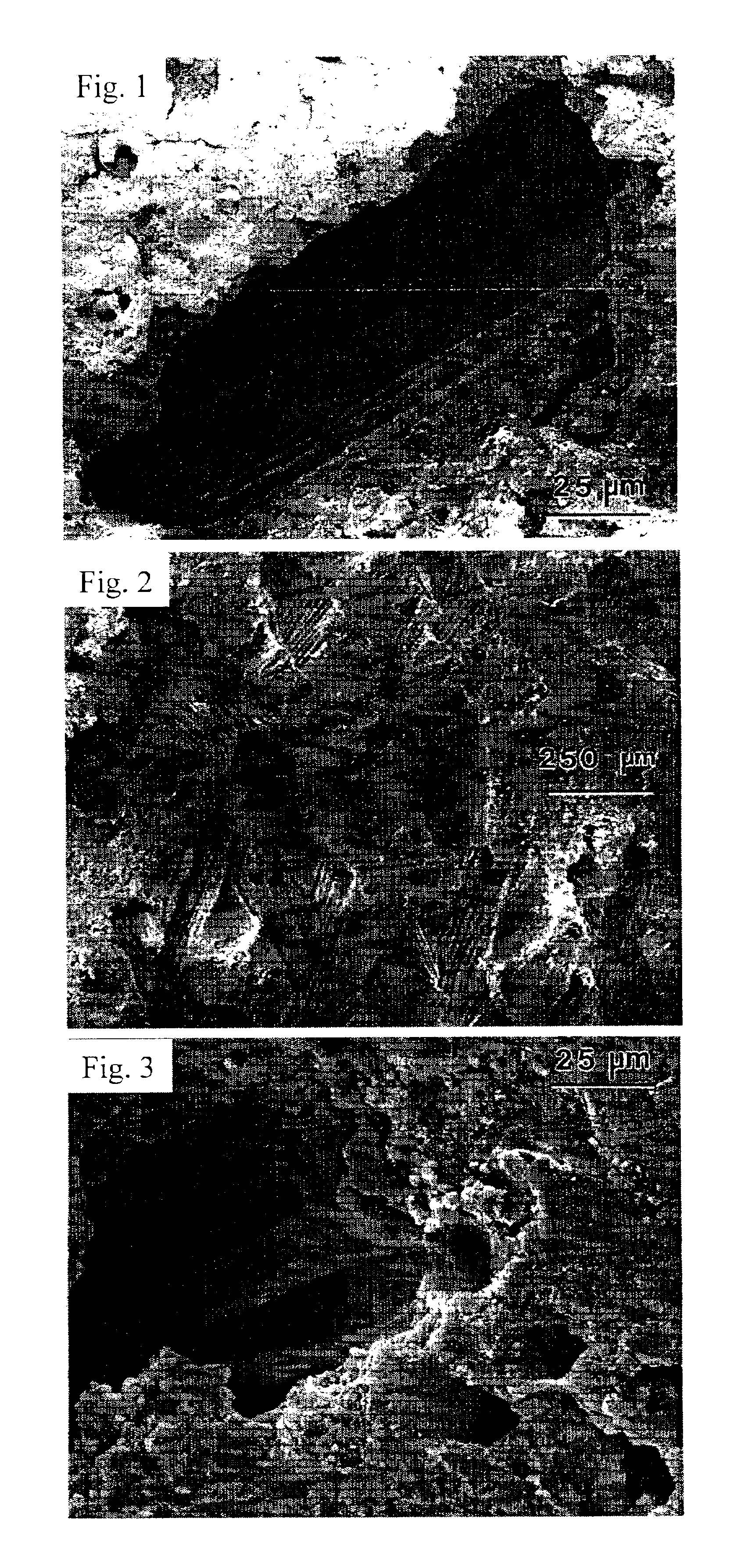
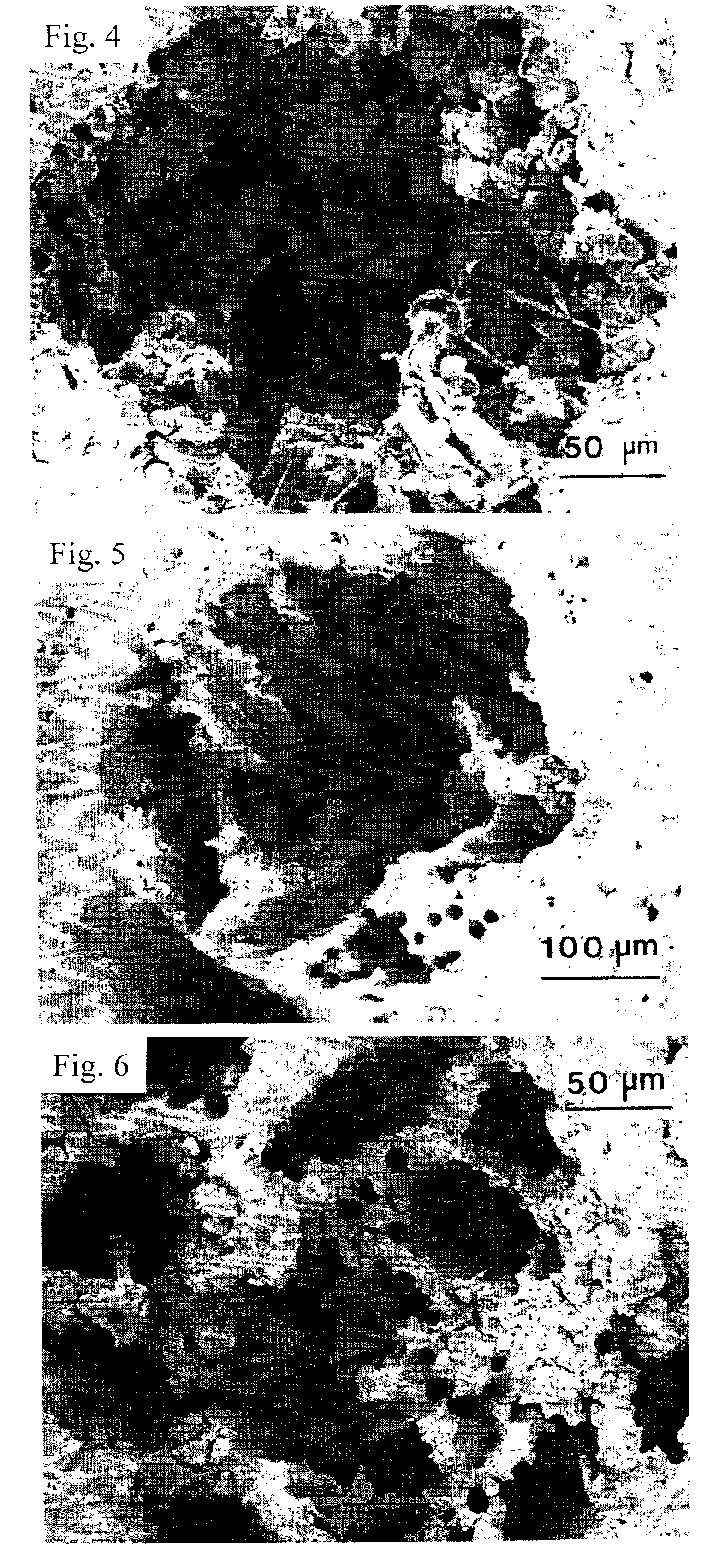
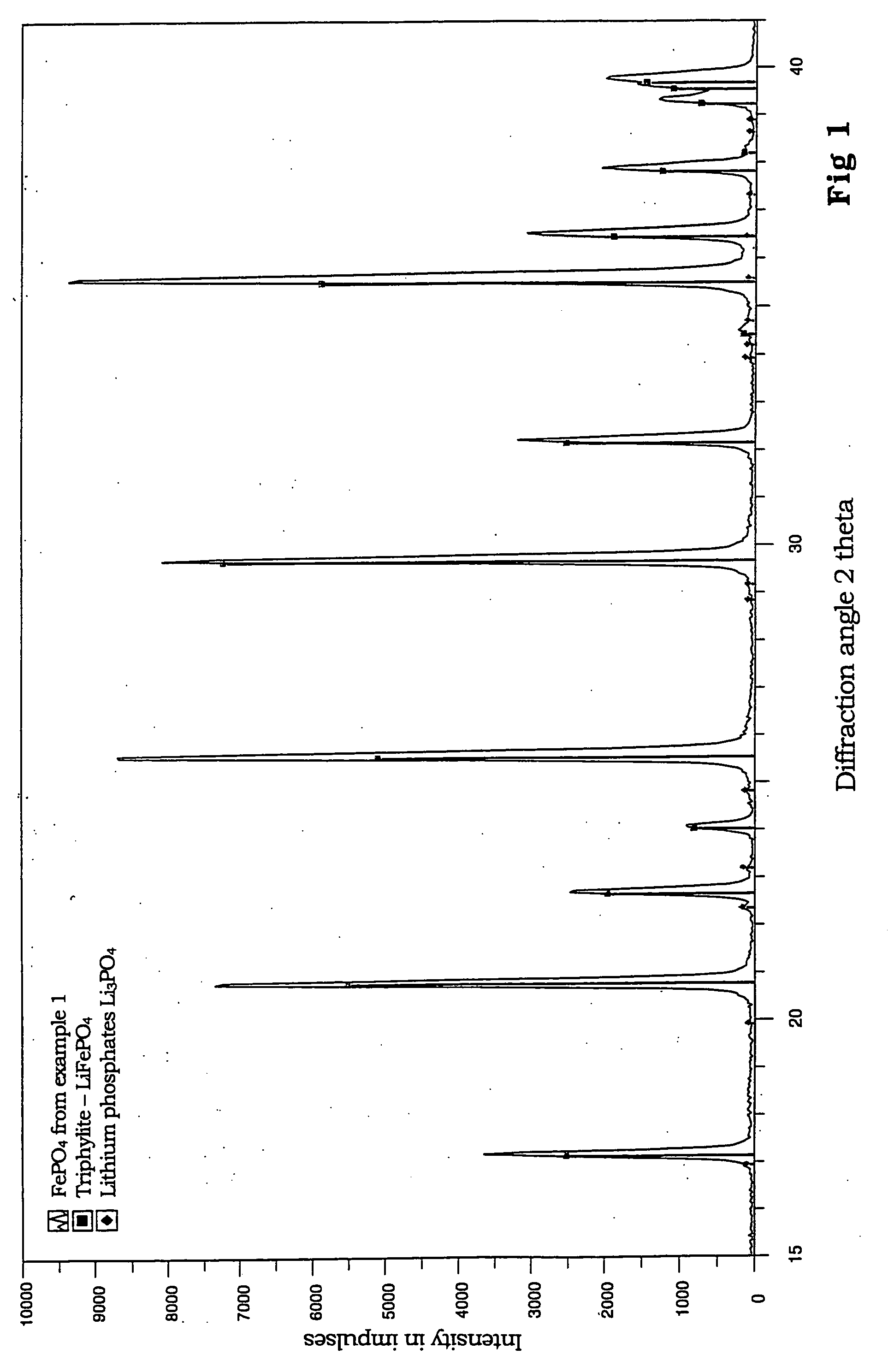
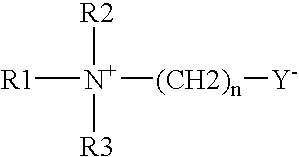Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2569results about "Peroxides/peroxyhydrates/peroxyacids/superoxides/ozonides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
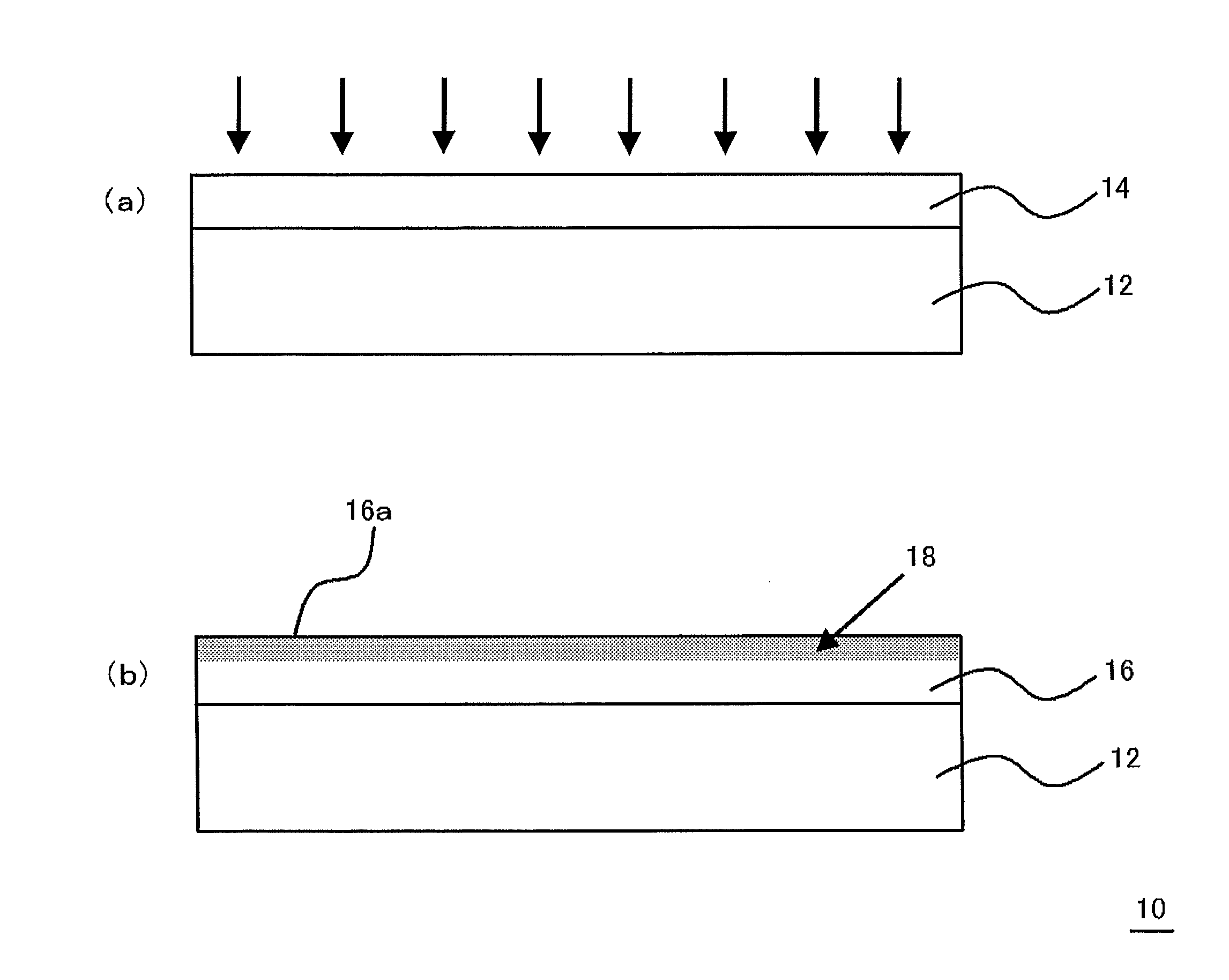
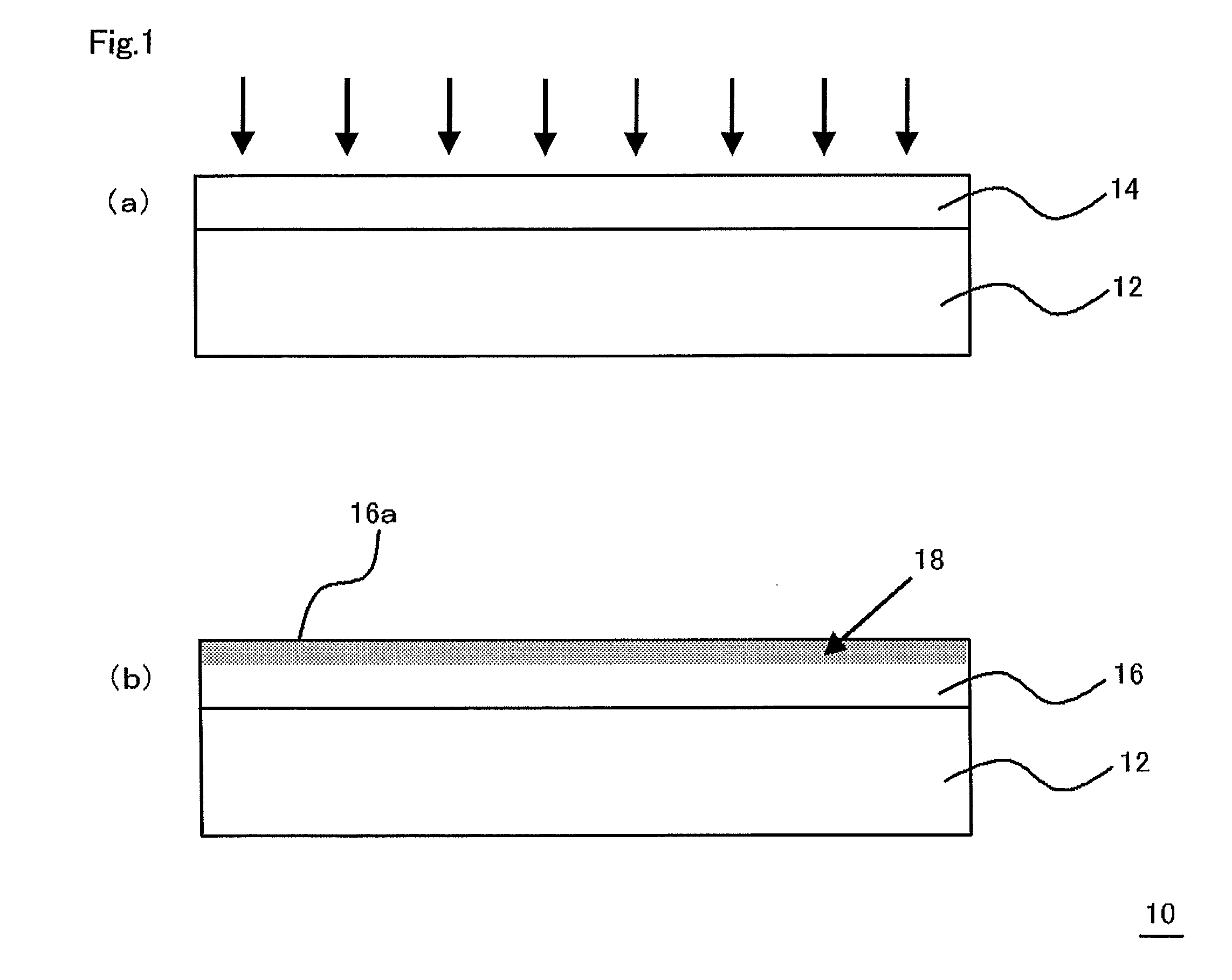
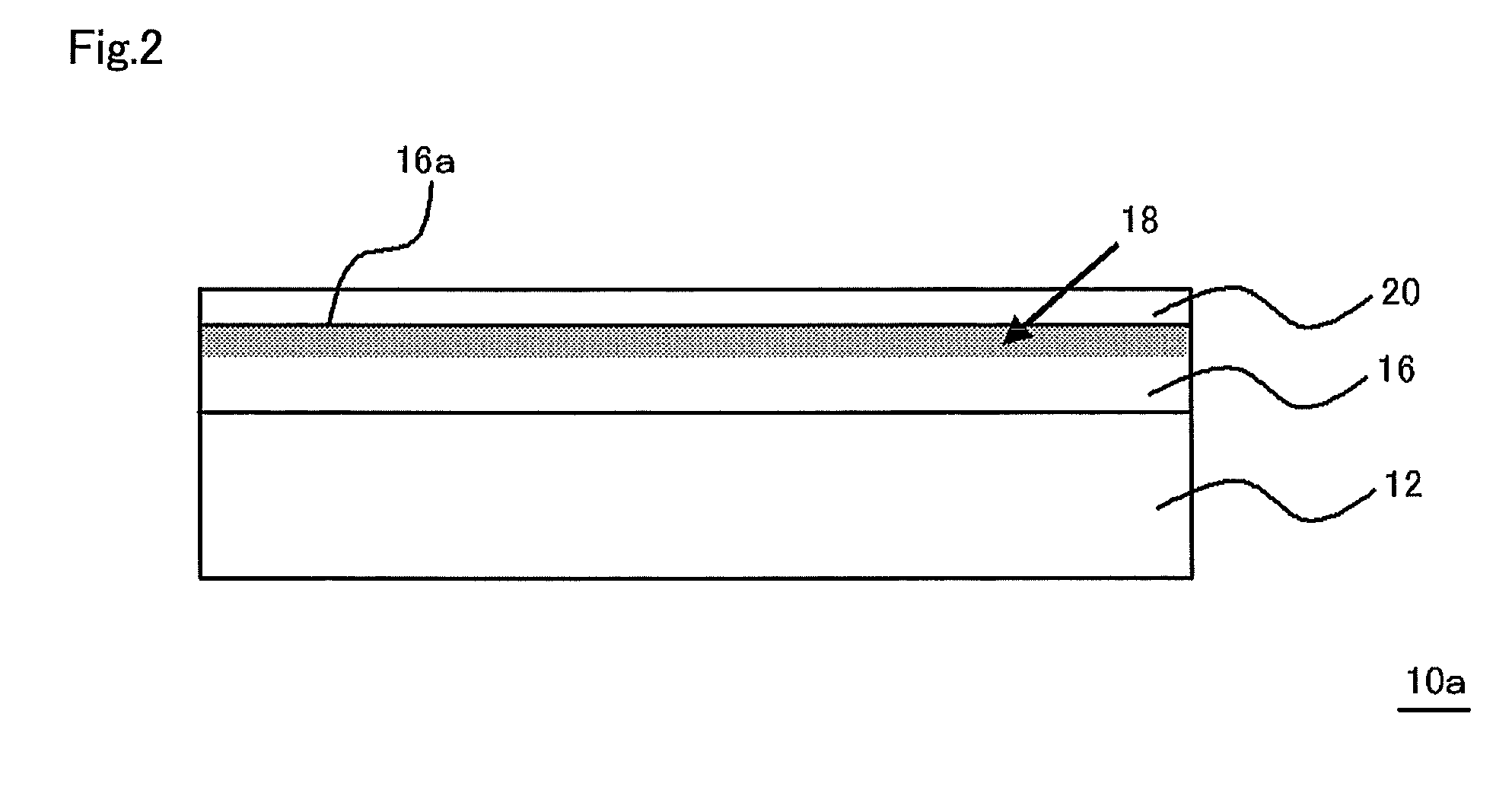
Multilayered material and method of producing the same
InactiveUS20120107607A1Reduce the impactIncrease production capacityRadiation applicationsPretreated surfacesWater vaporPolysilazane
A multilayered material is provided which includes a substrate and a silicon-containing film formed on the substrate, wherein the silicon-containing film has a nitrogen-rich area including silicon atoms and nitrogen atoms, or silicon atoms, nitrogen atoms, and an oxygen atoms and the nitrogen-rich area is formed by irradiating a polysilazane film formed on the substrate with an energy beam in an atmosphere not substantially including oxygen or water vapor and denaturing at least a part of the polysilazane film. A method of producing the multilayered material is also provided.
Owner:MITSUI CHEM INC
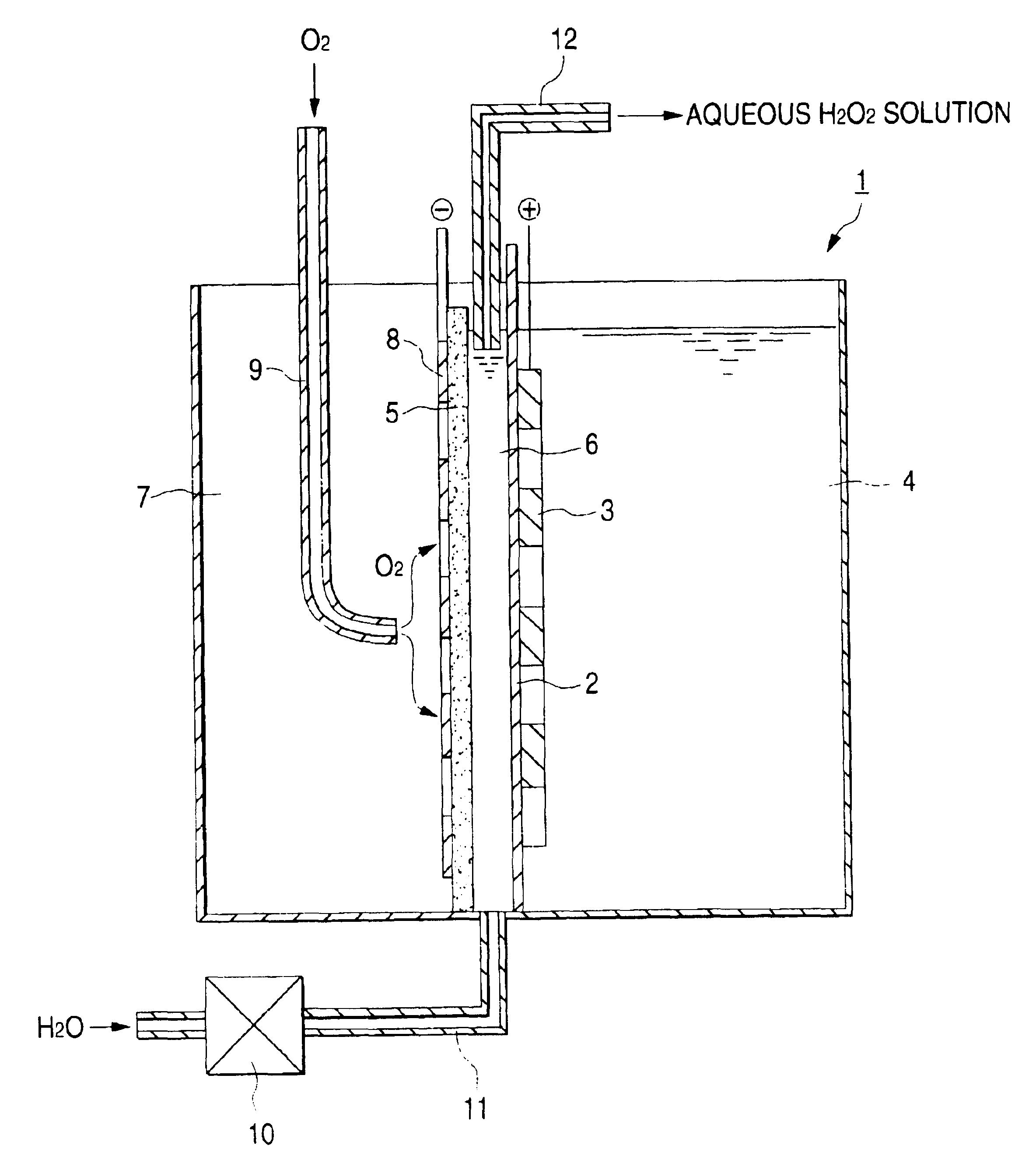
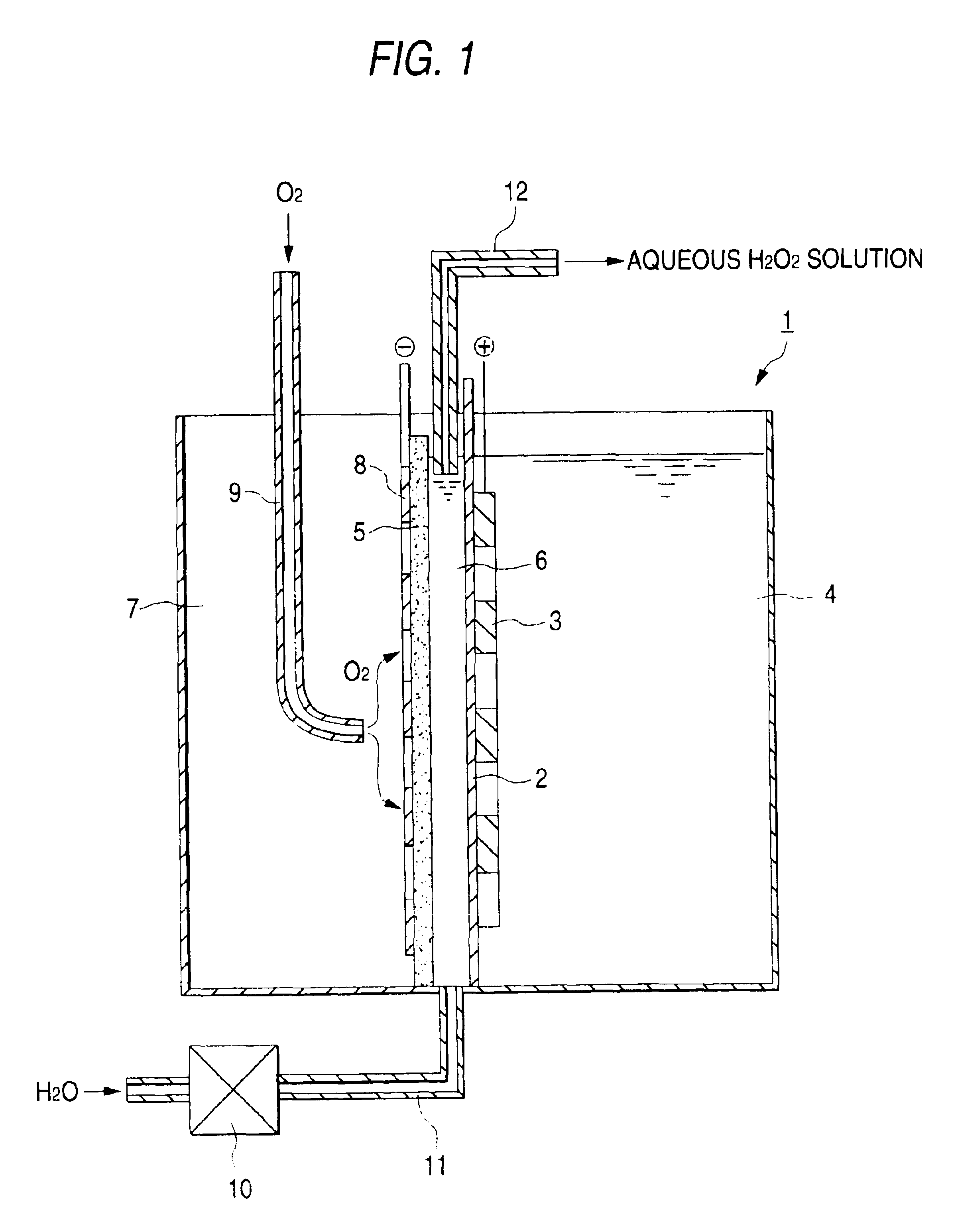
Electrolytic cell for hydrogen peroxide production and process for producing hydrogen peroxide
InactiveUS6767447B2CellsPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesSodium sulfateNuclear chemistry
An electrolytic cell and method of electrolysis for producing hydrogen peroxide at a moderate current density while preventing metal deposition on the cathode surface. A feed water from which multivalent metal ions have been removed and in which a salt of a univalent metal, e.g., sodium sulfate, has been dissolved in a given concentration is prepared with an apparatus for removing multivalent metal ions and dissolving a salt in low concentration. The feed water is supplied to an electrolytic cell. Even when electrolysis is continued, almost no deposition of a hydroxide or carbonate occurs on the cathode because multivalent metal ions are not present in the electrolytic solution. Due to the dissolved salt, a sufficient current density is secured to prevent an excessive load from being imposed on the electrodes, etc. Thus, stable production of hydrogen peroxide is possible over a long period of time.
Owner:DE NORA PERMELEC LTD
Conductive lithium storage electrode
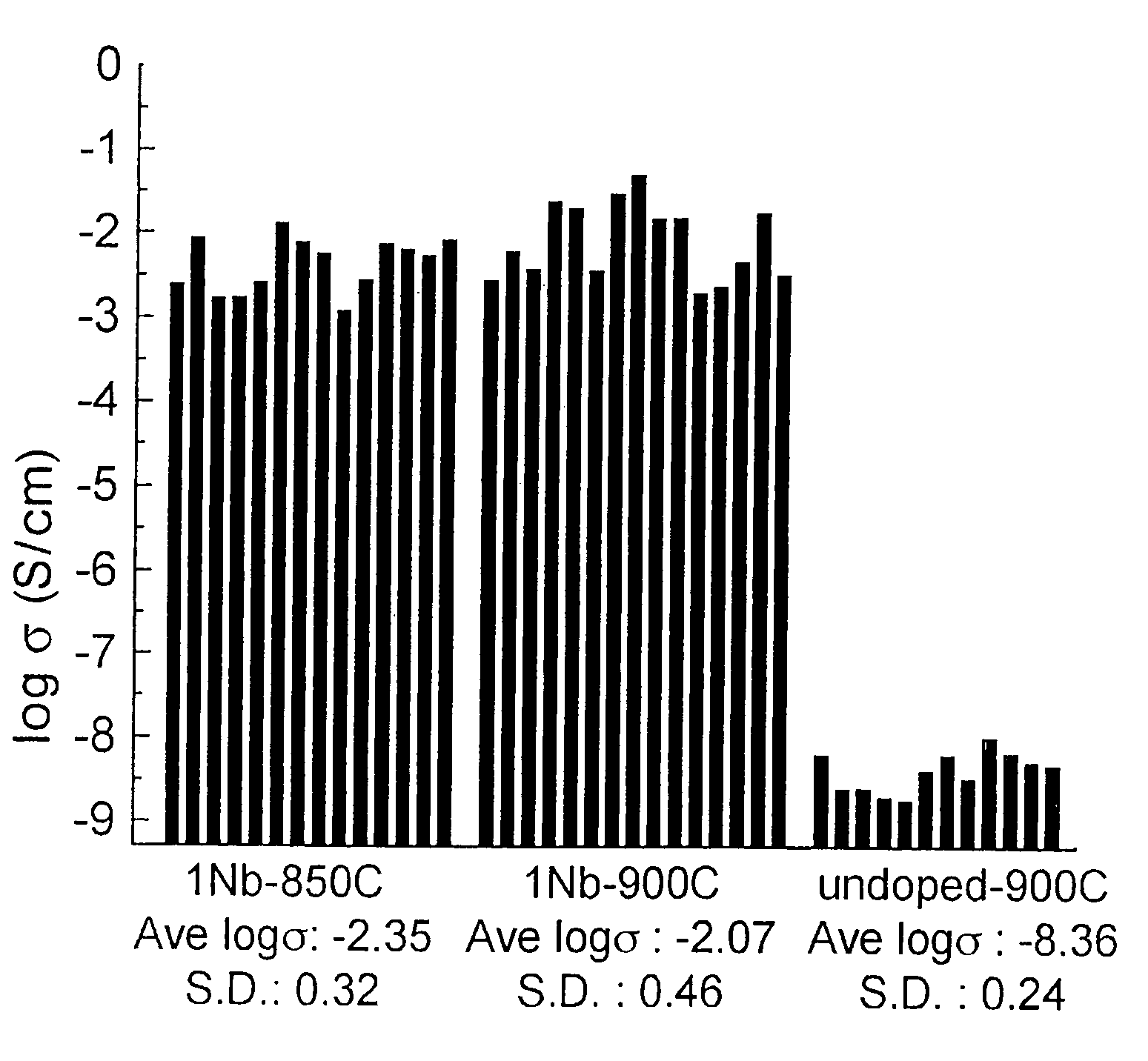
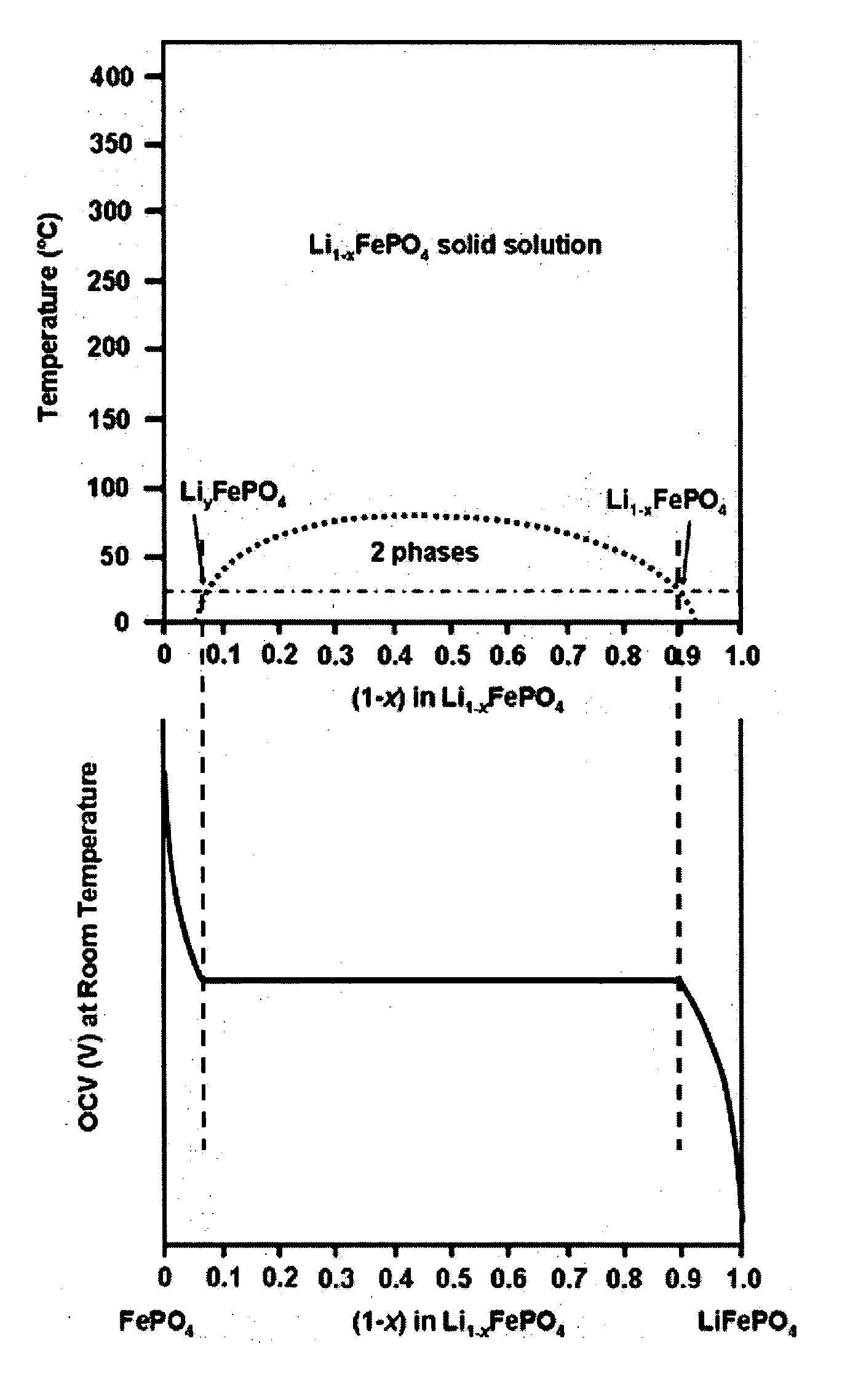
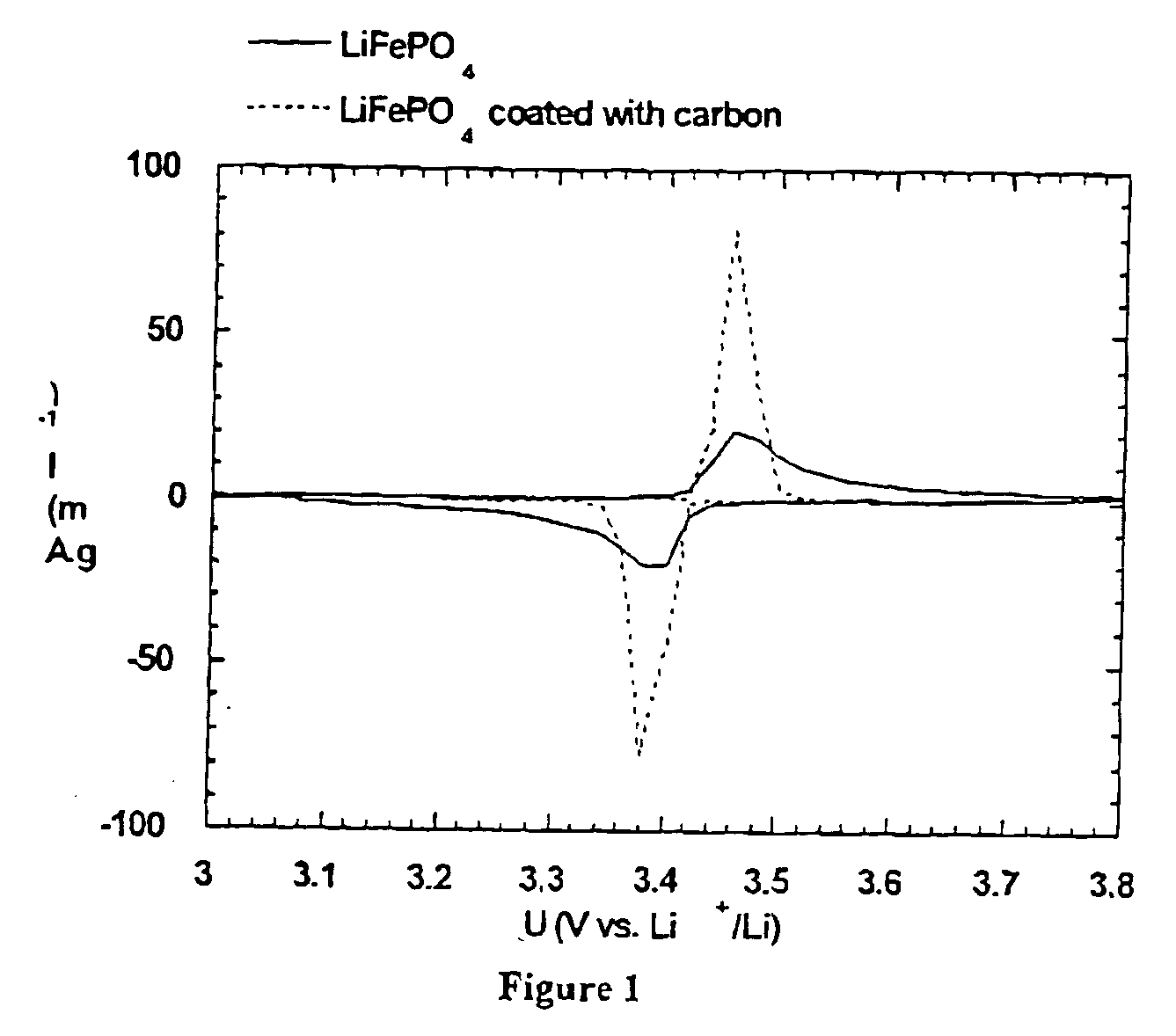
A compound comprising a composition Ax(M′1-aM″a)y(XD4)z, Ax(M′1-aM″a)y(DXD4)z, or Ax(M′1-aM″a)y(X2D7)z, and have values such that x, plus y(1-a) times a formal valence or valences of M′, plus ya times a formal valence or valence of M″, is equal to z times a formal valence of the XD4, X2D7, or DXD4 group; or a compound comprising a composition (A1-aM″a)xM′y(XD4)z, (A1-aM″a)xM′y(DXD4)z(A1-aM″a)xM′y(X2D7)z and have values such that (1-a)x plus the quantity ax times the formal valence or valences of M″ plus y times the formal valence or valences of M′ is equal to z times the formal valence of the XD4, X2D7 or DXD4 group. In the compound, A is at least one of an alkali metal and hydrogen, M′ is a first-row transition metal, X is at least one of phosphorus, sulfur, arsenic, molybdenum, and tungsten, M″ any of a Group IIA, IIIA, IVA, VA, VIA, VIIA, VIIIA, IB, IIB, IIIB, IVB, VB, and VIB metal, D is at least one of oxygen, nitrogen, carbon, or a halogen, 0.0001<a≦0.1, and x, y, and z are greater than zero. The compound can have a conductivity at 27° C. of at least about 10−8 S / cm. The compound can be a doped lithium phosphate that can intercalate lithium or hydrogen. The compound can be used in an electrochemical device including electrodes and storage batteries and can have a gravimetric capacity of at least about 80 mAh / g while being charged / discharged at greater than about C rate of the compound.
Owner:MASSACHUSETTS INST OF TECH
Ozone gas concentration measurement method, ozone gas concentration measurement system, and substrate processing apparatus
ActiveUS20100119439A1Improve concentrationEasy to measurePeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesSemiconductor/solid-state device manufacturingProduct gasGas concentration
An ozone gas concentration measurement method that can easily measure the concentration of ozone gas. A process gas containing ozone gas is produced from a raw gas containing oxygen gas. The number of moles of gas molecules contained in the process gas is measured. The concentration of the ozone gas contained in the process gas is calculated based on the number of moles of gas molecules contained in the process gas.
Owner:TOKYO ELECTRON LTD
Method of synthesizing zirconium phosphate particles
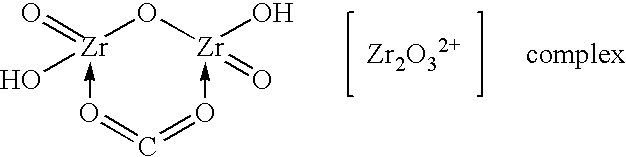
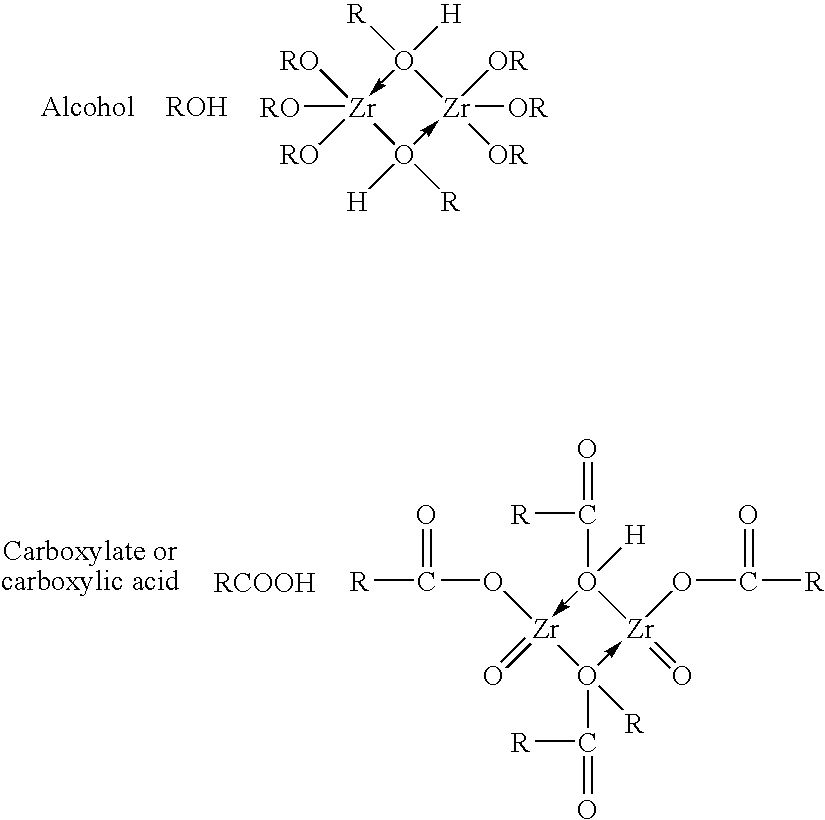
InactiveUS7566432B2Inhibition of agglomerationReduce moistureSemi-permeable membranesPhosphatesO-Phosphoric AcidZirconium oxychloride
Zirconium phosphate particles are synthesized by providing a solution of zirconium oxychloride in an aqueous solvent, adding at least one oxygen-containing additive to the solution, the oxygen-containing additive being selected to form a complex with zirconium ions in the solution of zirconium oxychloride and thereby reduce hydration of the zirconium ions, and combining this solution with phosphoric acid or a phosphoric acid salt to obtain zirconium phosphate particles by sol gel precipitation.
Owner:RENAL SOLUTIONS
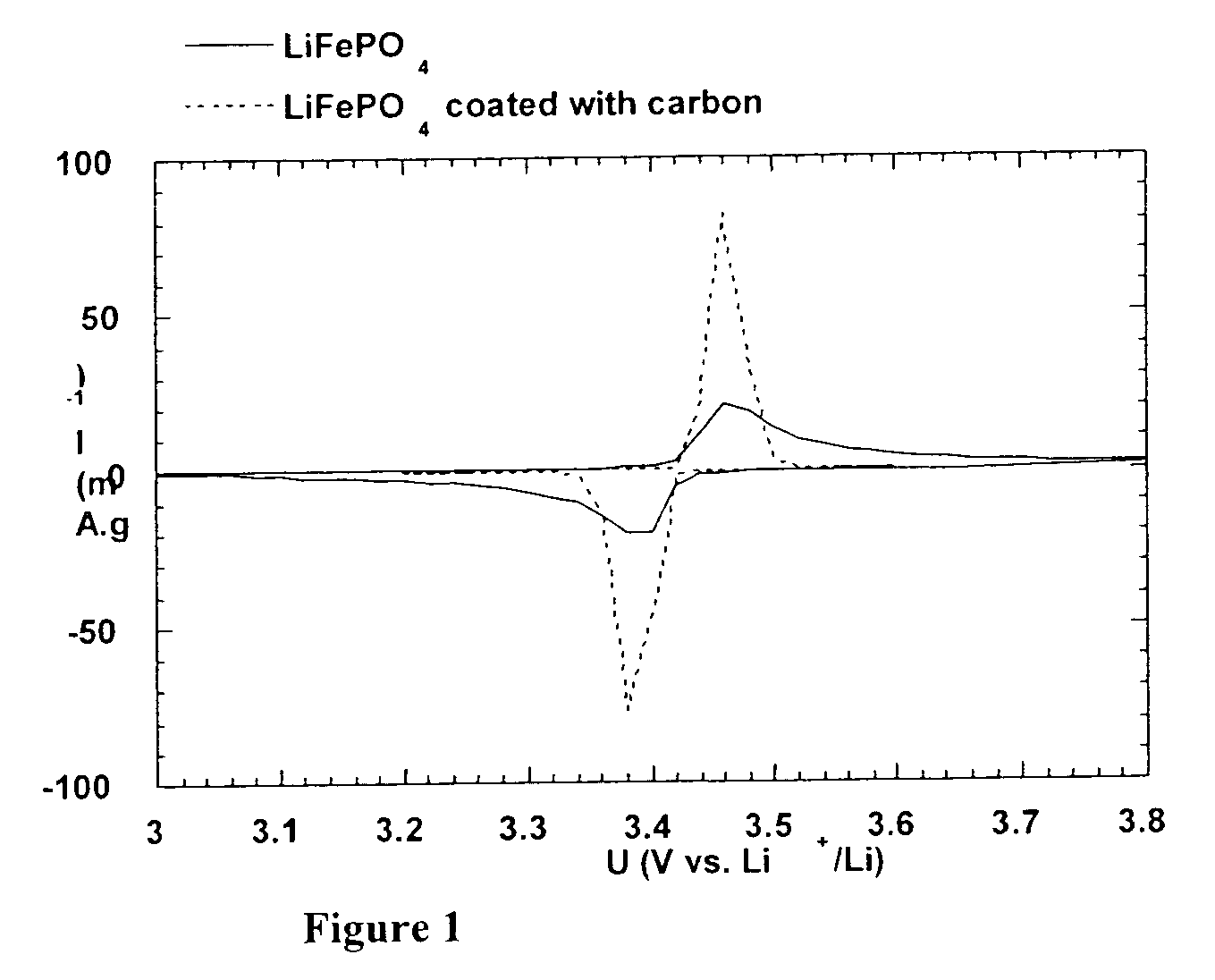
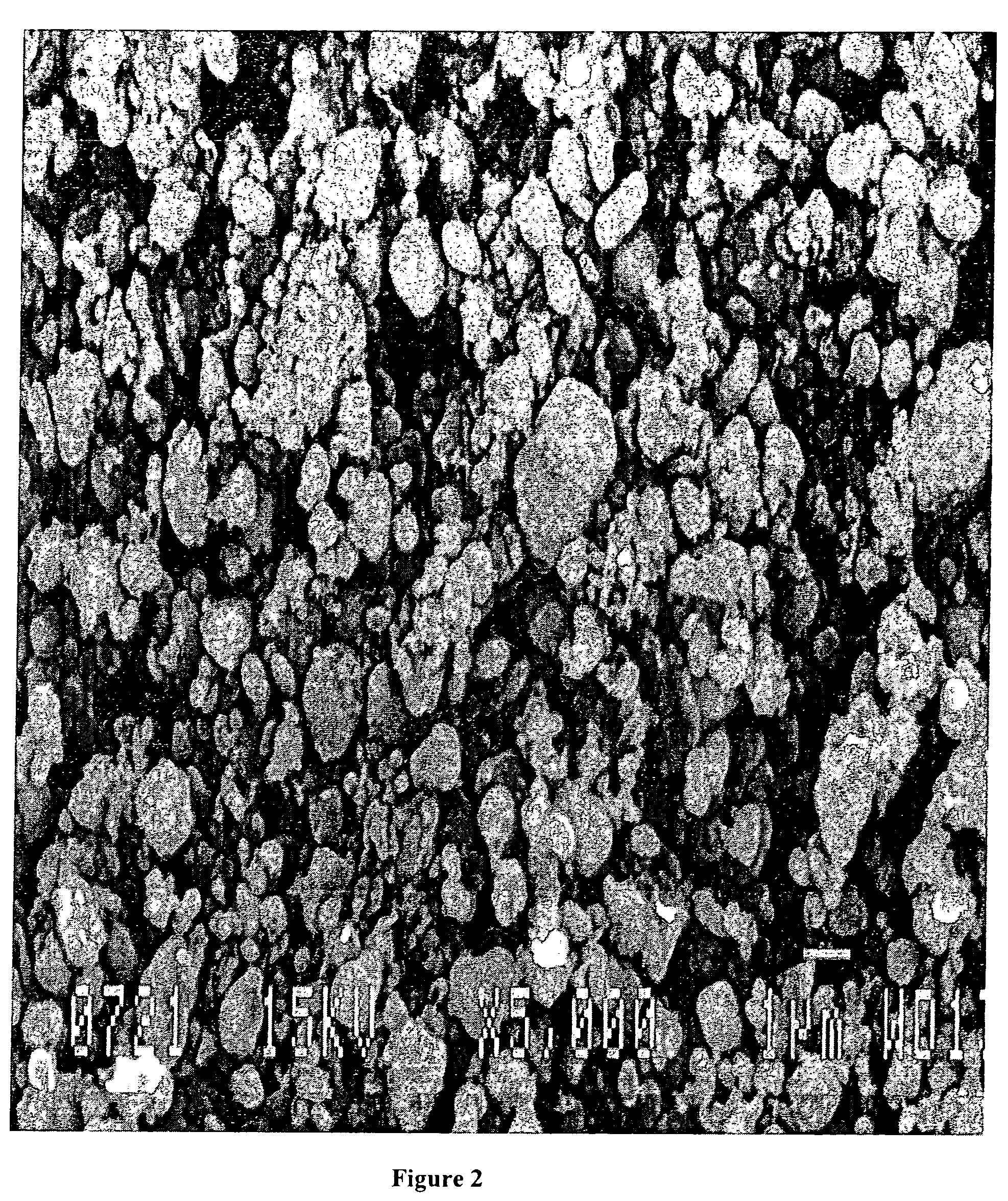
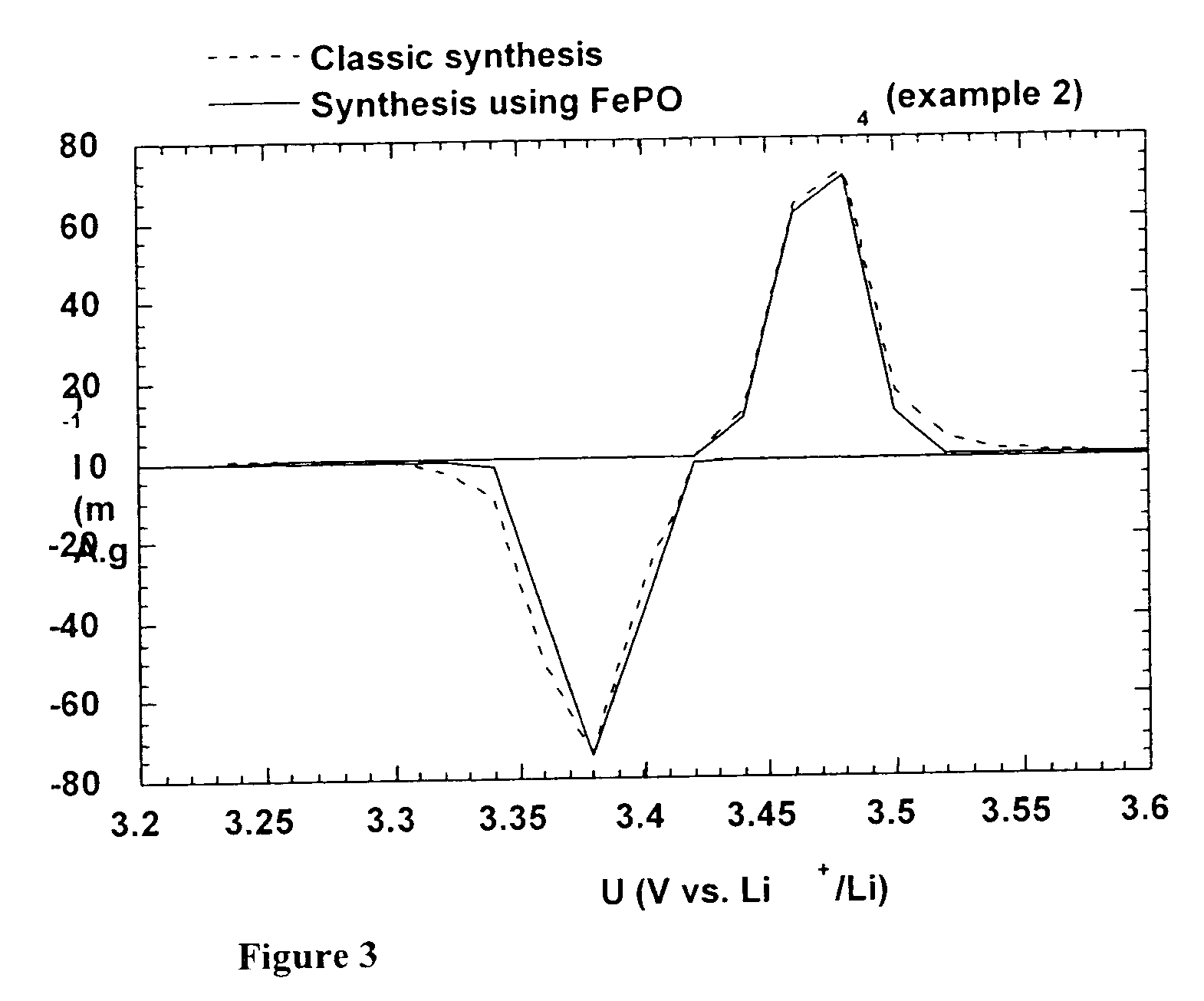
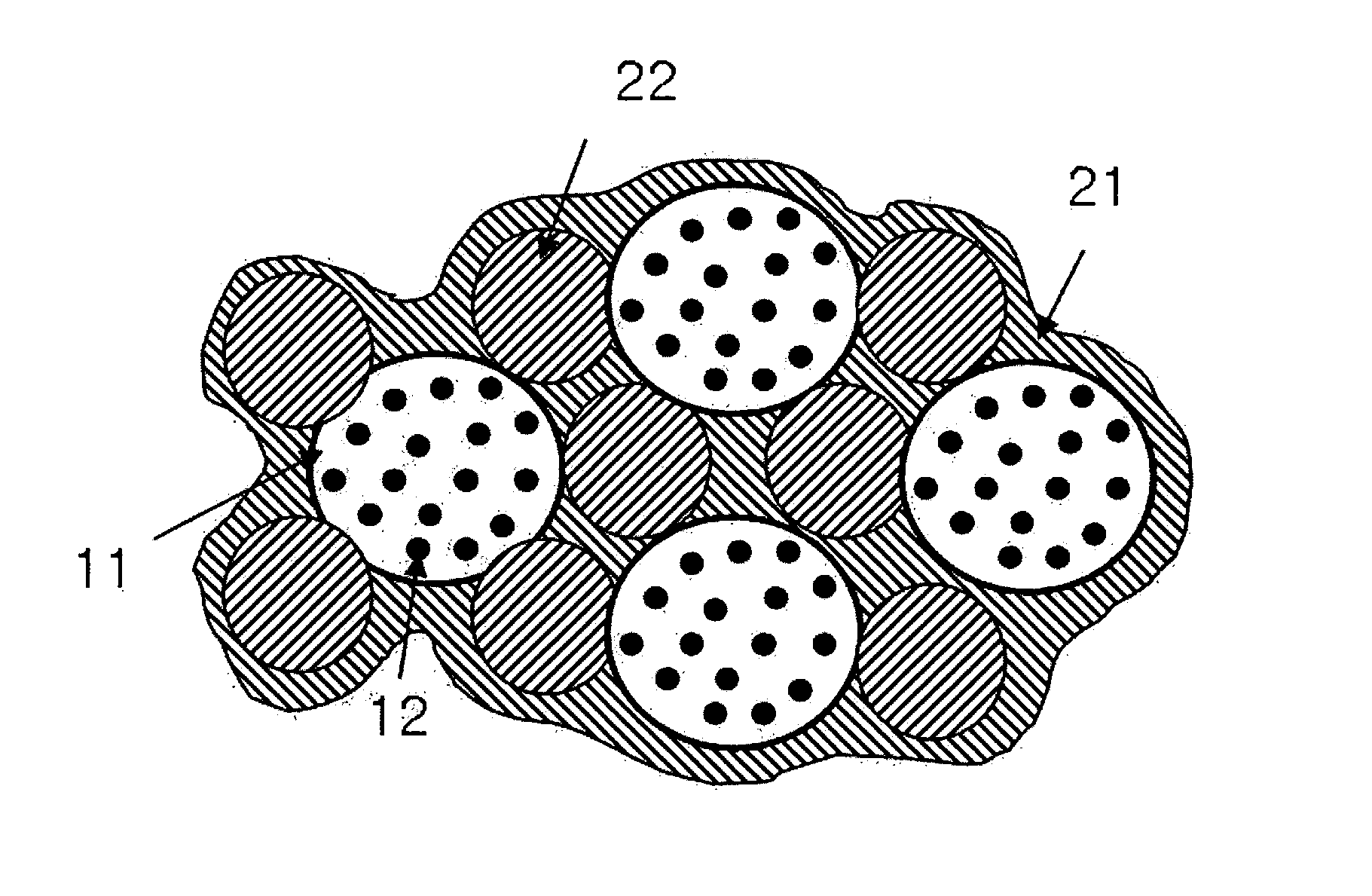
Method for synthesis of carbon-coated redox materials with controlled size
ActiveUS20040033360A1Low costReduce the numberMaterial nanotechnologyHybrid capacitorsCross-linkRedox
A method for the synthesis of compounds of the formula C-LixM1-yM'y(XO4)n, where C represents carbon cross-linked with the compound LixM1-yM'y(XO4)n, in which x, y and n are numbers such as 0<=x<=2, 0<=y<=0.6, and 1<=n<=1.5, M is a transition metal or a mixture of transition metals from the first period of the periodic table, M' is an element with fixed valency selected among Mg<2+>, Ca<2+>, Al<3+>, Zn<2+> or a combination of these same elements and X is chosen among S, P and Si, by bringing into equilibrium, in the required proportions, the mixture of precursors, with a gaseous atmosphere, the synthesis taking place by reaction and bringing into equilibrium, in the required proportions, the mixture of the precursors, the procedure comprising at least one pyrolysis step of the carbon source compound in such a way as to obtain a compound in which the electronic conductivity measured on a sample of powder compressed at a pressure of 3750 Kg.cm<-2 >is greater than 10<-8 >S.cm<-1>. The materials obtained have excellent electrical conductivity, as well a very improved chemical activity.
Owner:CENT NAT DE LA RECHERCHE SCI +2
Negative active material for a rechargeable lithium battery, a method of preparing the same, and a rechargeable lithium battery comprising the same
ActiveUS20050233213A1Improved cycle-life characteristic and charge and discharge characteristicIncrease chanceElectrode manufacturing processesNon-aqueous electrolyte accumulatorsHigh rateSilicon oxide
The present invention relates to a negative active material for a rechargeable lithium battery, which includes a silicon-based composite having a silicon oxide of the form SiOX where x≦1.5 and at least one element selected from the group consisting of B, P, Li, Ge, Al, and V, and a carbonaceous material. The negative active material of the present invention can improve the cycle-life and high-rate charge / discharge characteristics of a rechargeable lithium battery.
Owner:SAMSUNG SDI CO LTD
Thin film battery and electrolyte therefor
A solid amorphous electrolyte composition for a thin-film battery. The electrolyte composition includes a lithium phosphorus oxynitride material containing a sulfide ion dopant wherein the atomic ratio of sulfide ion to phosphorus ion (S / P) in the electrolyte ranges greater than 0 up to about 0.2. The composition is represented by the formula: where 2x+3y+2z=5+w, x ranges from about 3.2 to about 3.8, y ranges from about 0.13 to about 0.46, z ranges from greater than zero up to about 0.2, and w ranges from about 2.9 to about 3.3. Thin-film batteries containing the sulfide doped lithium oxynitride electrolyte are capable of delivering more power and energy than thin-film batteries containing electrolytes without sulfide doping.
Owner:OAK RIDGE MICRO ENERGY
Metal-containing compounds
The invention relates to a novel solid state process for the preparation of metal-containing compounds comprising the steps i) forming a reaction mixture comprising one or more metal-containing precursor compounds and optionally one or more non-metal-containing reactants, and ii) using one or more hypophosphite-containing materials as a reducing agent; wherein one or more of the hypophosphite-containing materials is used as an agent to reduce one or more of the metal-containing precursor compounds; and further wherein the process is performed in the absence of an oxidizing atmosphere. Materials made by such a process are useful, for example, as electrode materials in alkali metal-ion battery applications.
Owner:LITHIUM WERKS TECH BV
Lithium iron phosphate cathode materials with enhanced energy density and power performance
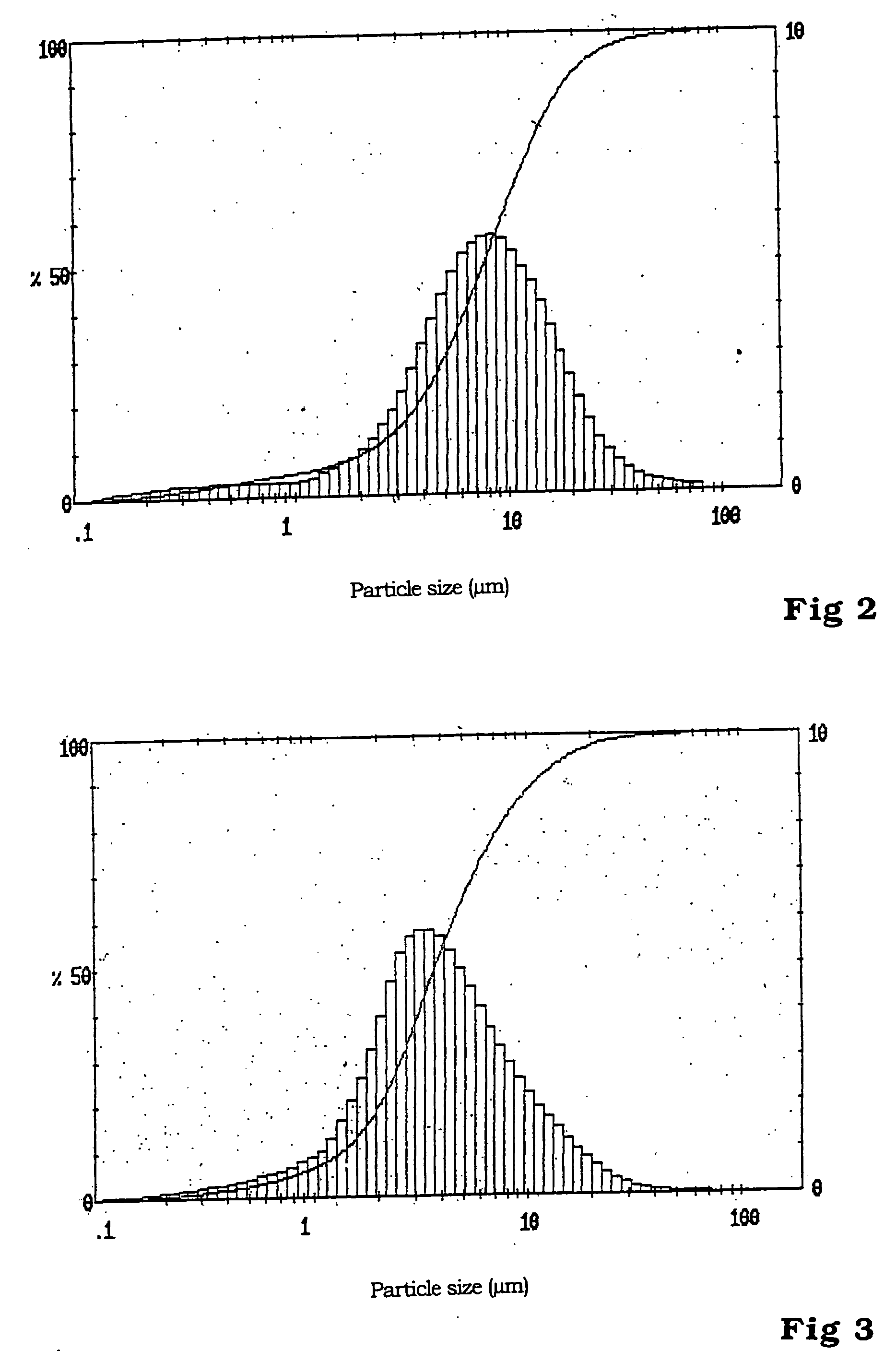
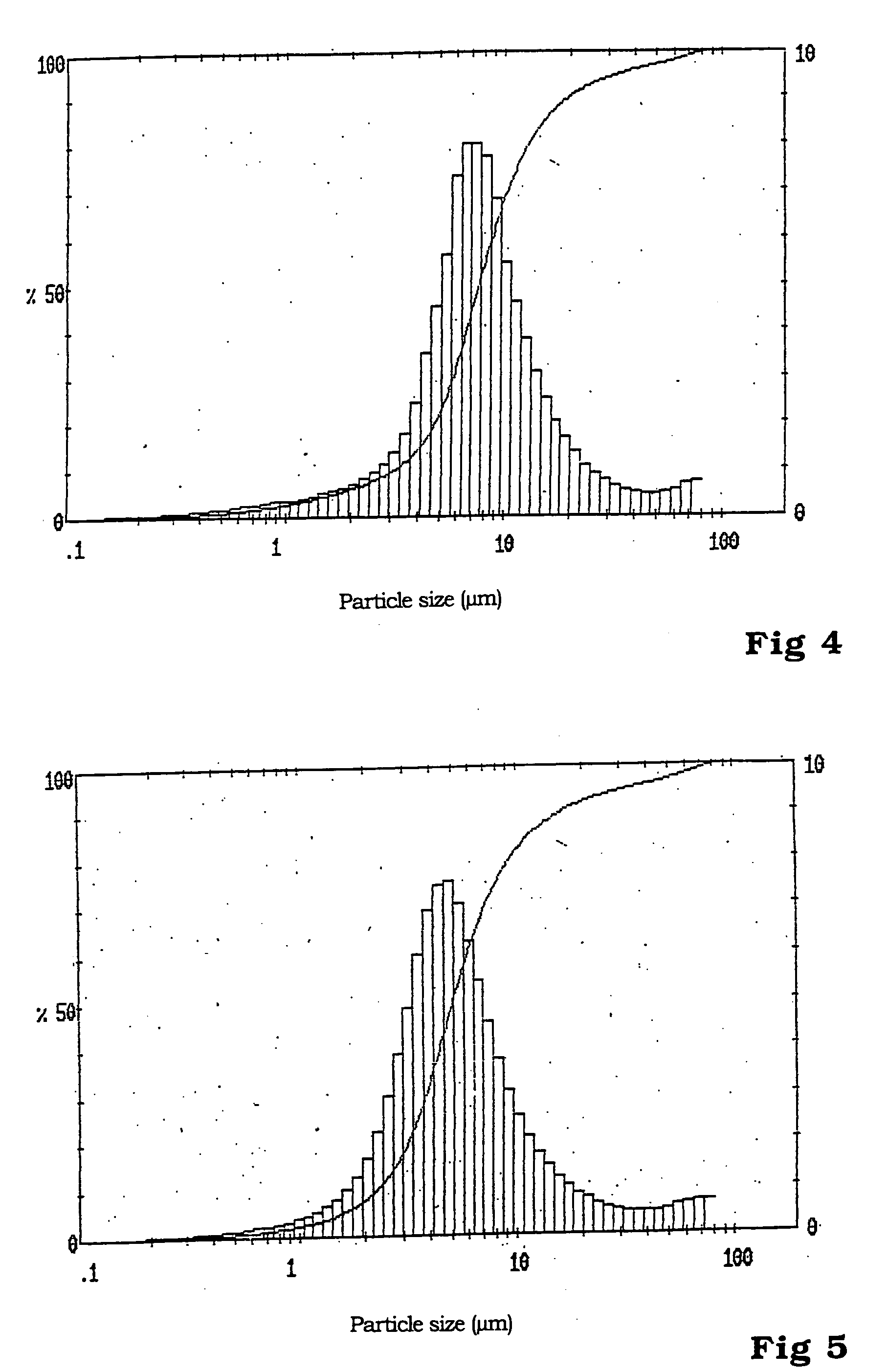
InactiveUS20090155689A1Powerful performanceHigh discharge ratePhosphatesPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesFiberPhosphate
The invention is related to a cathode material comprising particles having a lithium metal phosphate core and a pyrolytic carbon deposit, said particles having a synthetic multimodal particle size distribution comprising at least one fraction of micron size particles and one fraction of submicron size particles, said lithium metal phosphate having formula LiMPO4 wherein M is at least Fe or Mn.Said material is prepared by method comprising the steps of providing starting micron sized particles and starting submicron sized particles of at least one lithium metal phosphate or of precursors of a lithium metal phosphate; mixing by mechanical means said starting particles; making a pyrolytic carbon deposit on the lithium metal phosphate starting particles before or after the mixing step, and on their metal precursor before or after mixing the particles; optionally adding carbon black, graphite powder or fibers to the said lithium metal phosphate particles before the mechanical mixing.
Owner:PHOSTECH LITHIUM
Zirconium Phosphate Particles Having Improved Adsorption Capacity and Method Of Synthesizing The Same
InactiveUS20100084330A1Avoid disadvantagesHigh porosityPhosphatesDialysis systemsPhosphoric acidOxygen
Zirconium phosphate particles are synthesized by providing a solution of zirconium oxychloride in an aqueous solvent, adding at least one low molecular weight, oxygen containing, monofunctional, organic additive to the solution, and combining this solution with heated phosphoric acid or a phosphoric acid salt to obtain zirconium phosphate particles by sol gel precipitation.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Synthesis of nanoparticles
InactiveUS20030032192A1High sensitivityIncreasing efficiency of collectorOptical radiation measurementMaterial nanotechnologyDopantOrganic solvent
The present invention relates to methods for the preparation of inorganic nanoparticles capable of fluorescence, wherein the nanoparticles consist of a host material that comprises at least one dopant. The synthesis of the invention in organic solvents allows to gain a considerably higher yield compared to the prior art synthesis in water. All kinds of objects can advantageously be marked and reliably authenticated by using an automated method on the basis of a characteristic emission. Further, the size distribution of the prepared nanoparticles is narrower which renders a subsequent size-selected separation process superfluous.
Owner:CENT FUR ANGEWANDTE NANOTECH
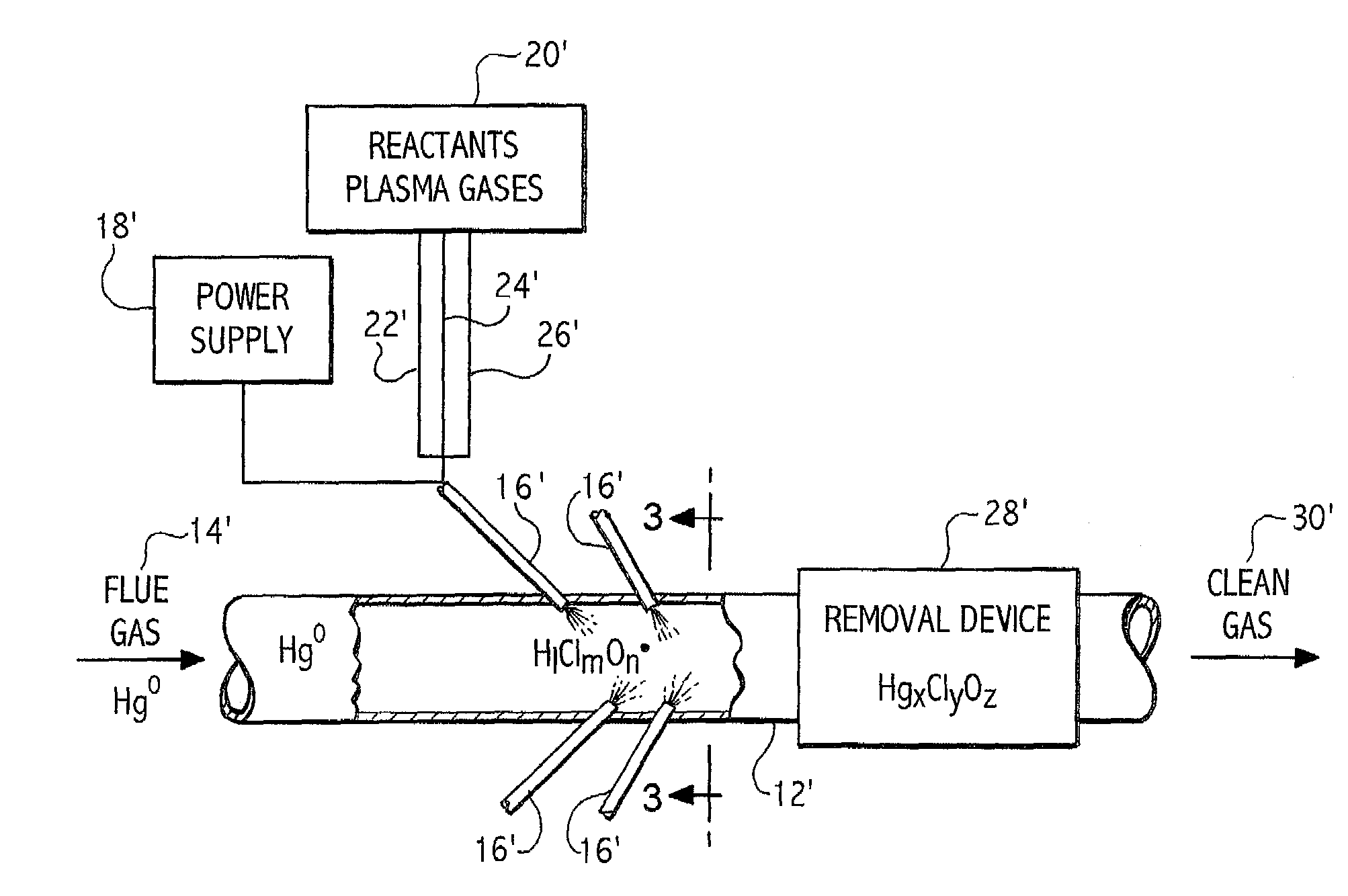
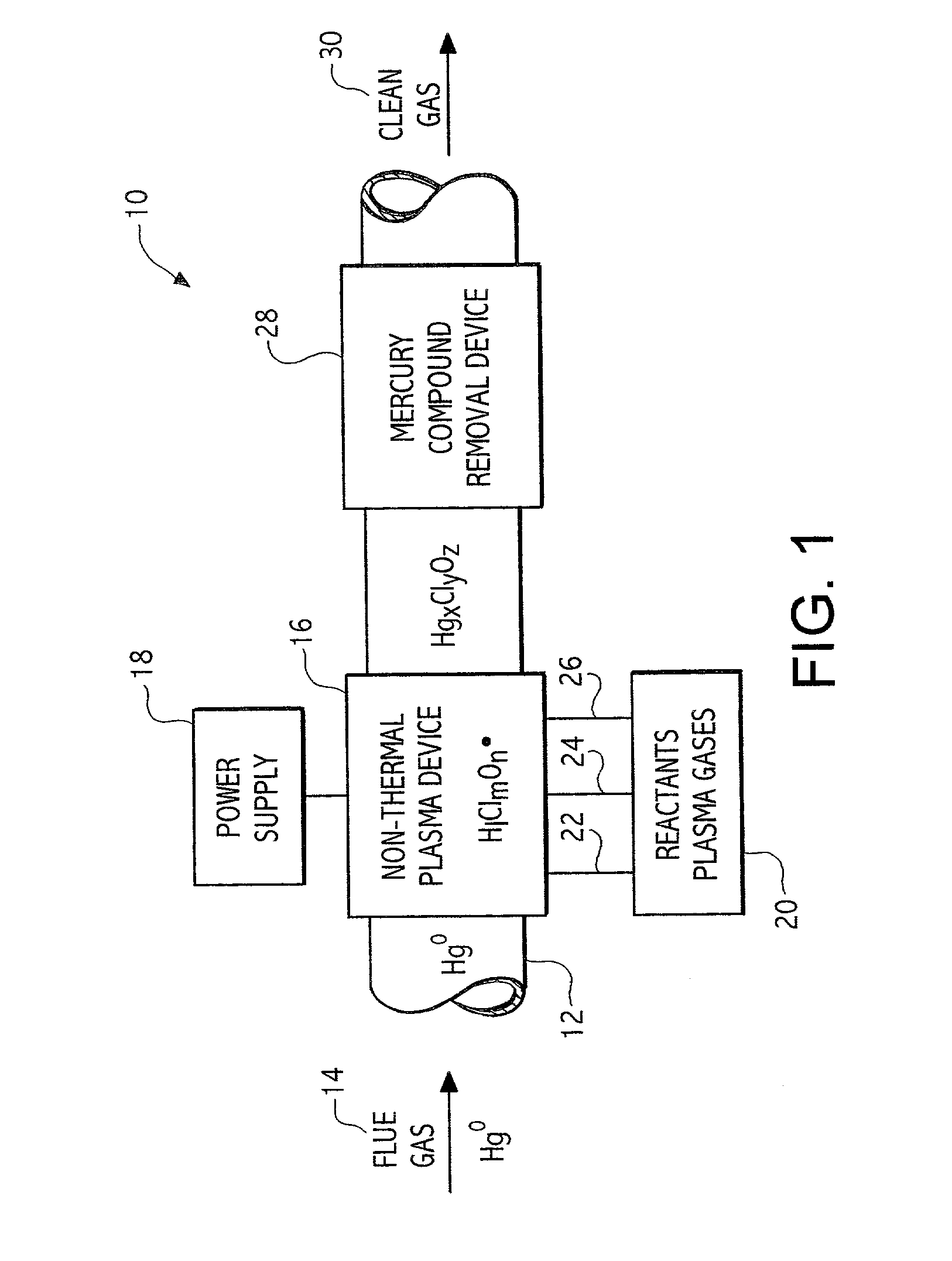
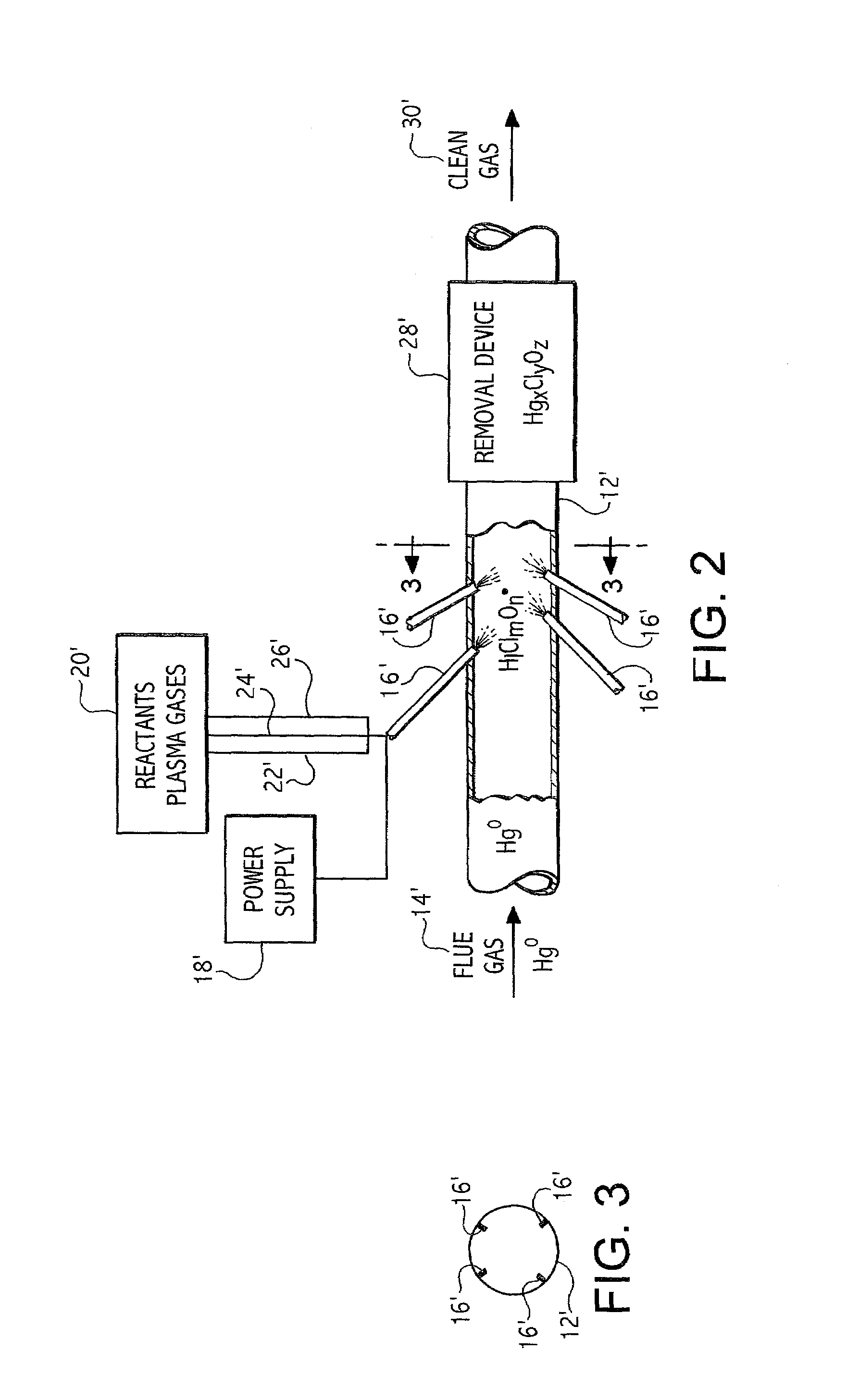
Plasma based trace metal removal apparatus and method
A system and method for the removal of metals such as mercury from a gas stream. The method involves contacting a gas stream containing the target metals with reactive chemical species generated in a plasma device. The metal to be removed is chemically converted into forms enabling capture in either conventional particulate removal devices or in a wet scrubber for the capture of a soluble chemical species.
Owner:CONTINENTAL RES & DEV
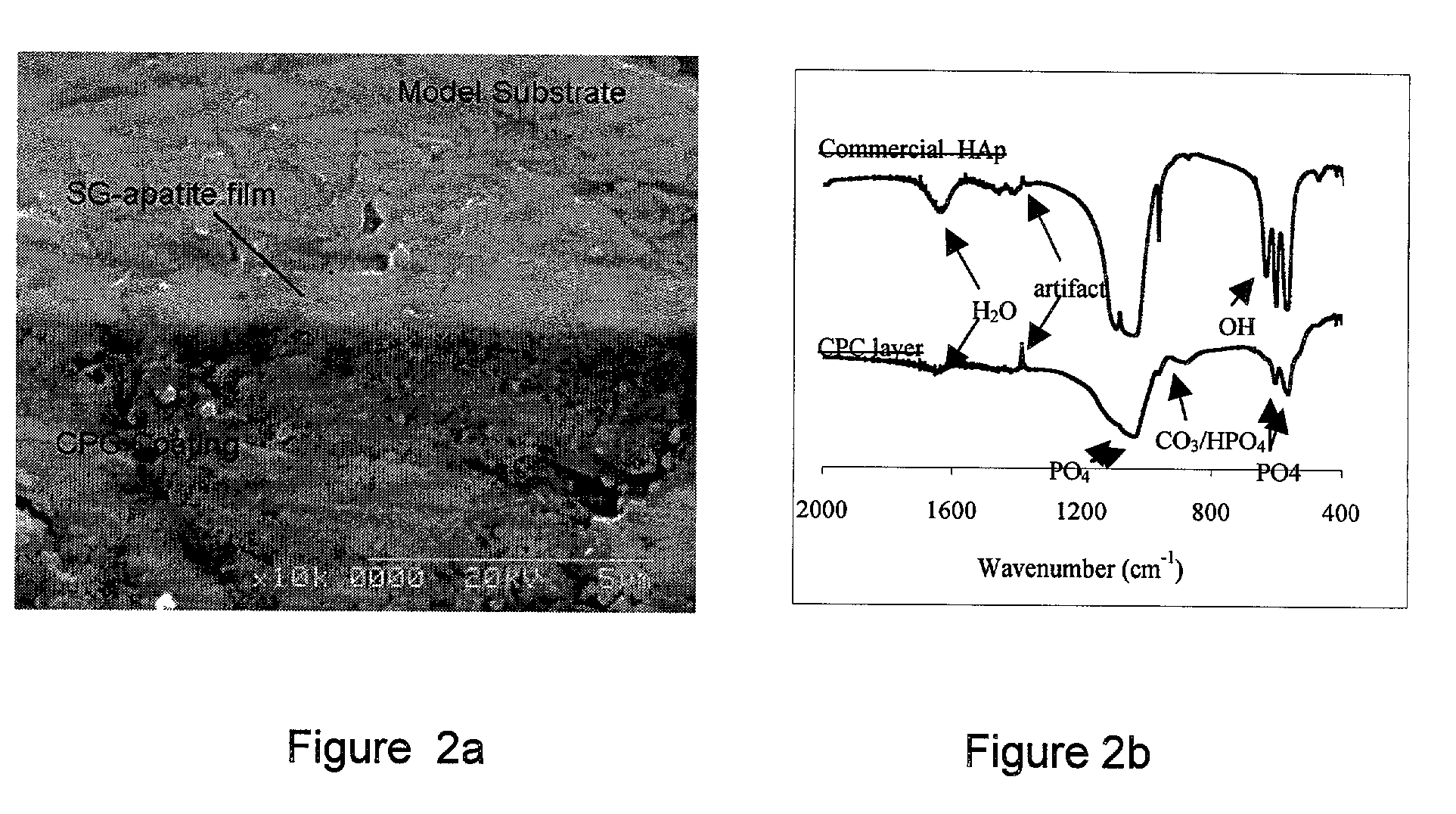
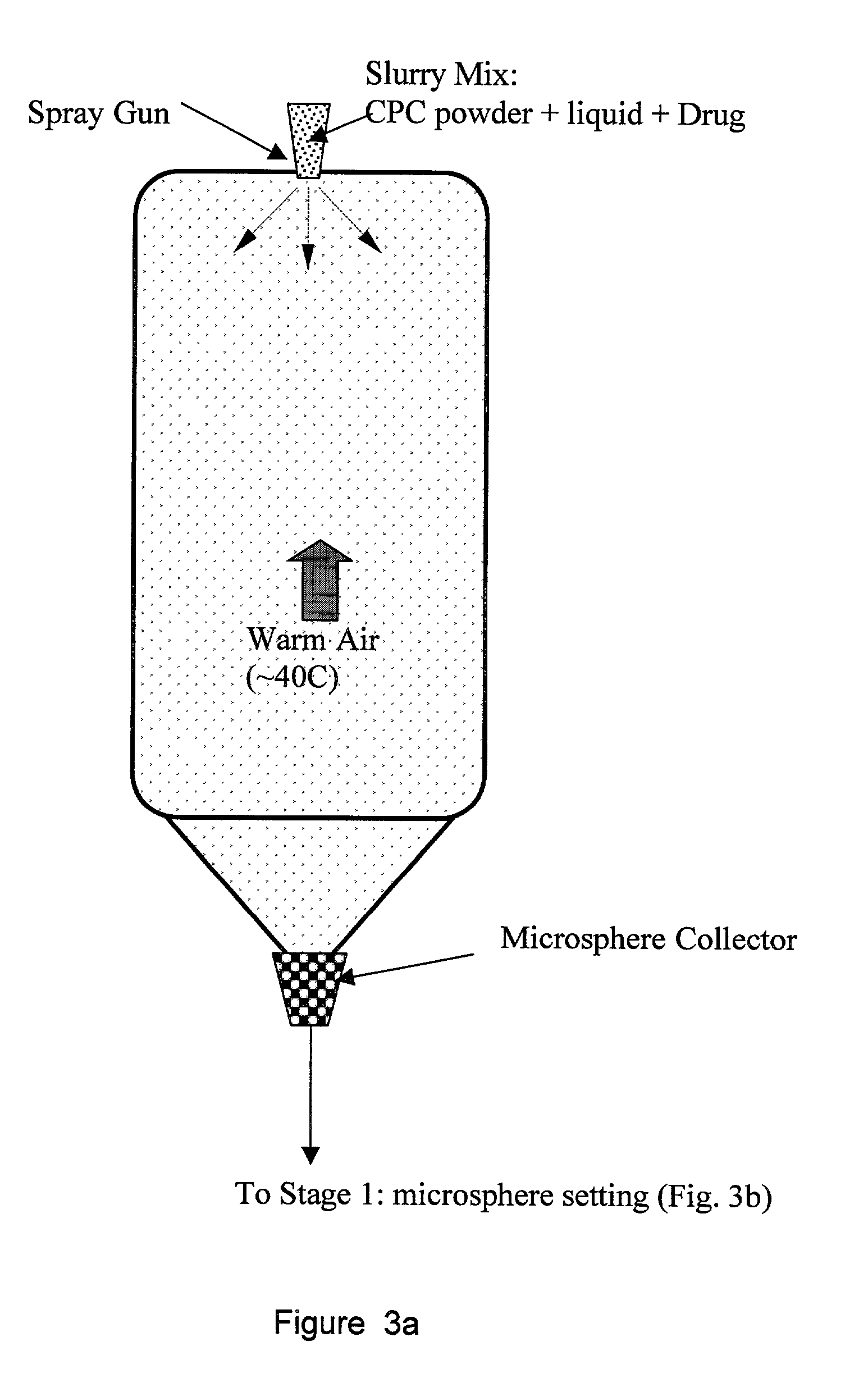
Biofunctional hydroxyapatite coatings and microspheres for in-situ drug encapsulation
InactiveUS20020155144A1Improve relationshipImprove interface strengthPowder deliveryOrganic active ingredientsGene deliverySide effect
Owner:THE UNIV OF BRITISH COLUMBIA
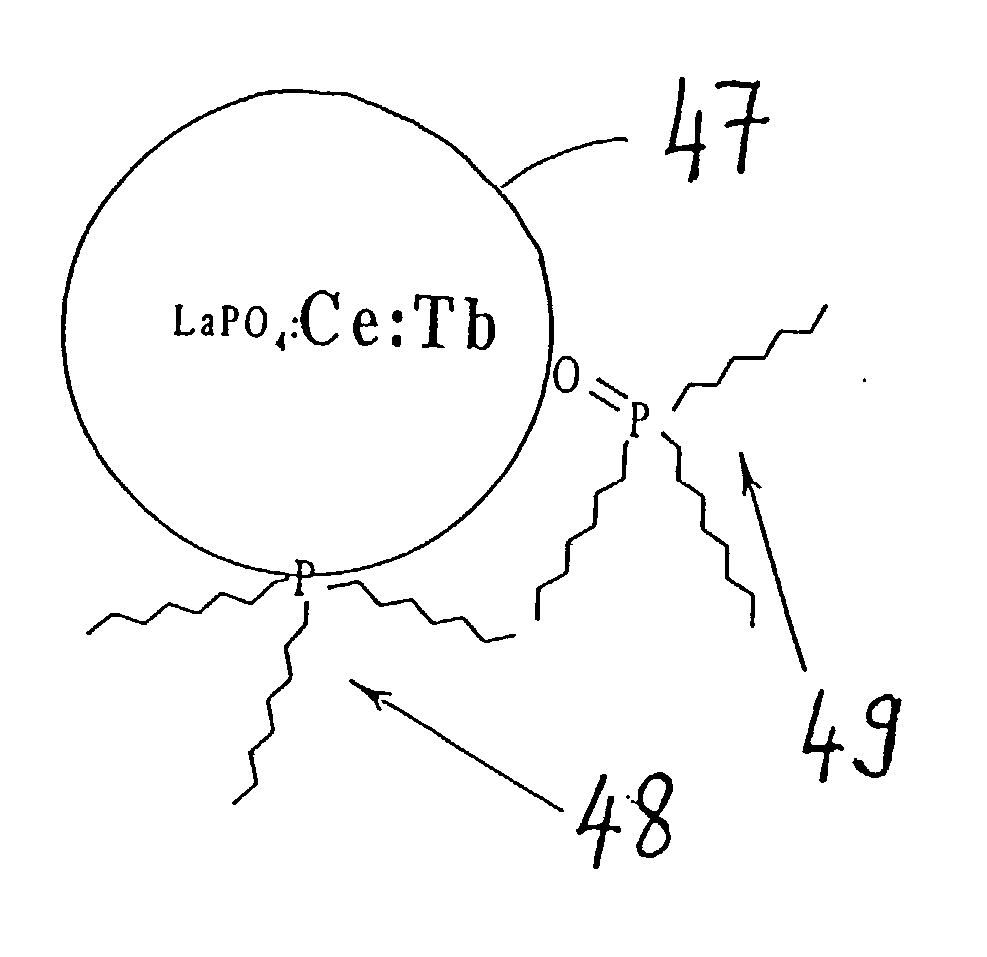
Phosphor mixture and light emitting device using the same
ActiveUS20060045832A1Discharge tube luminescnet screensPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesPhosphorHigh color
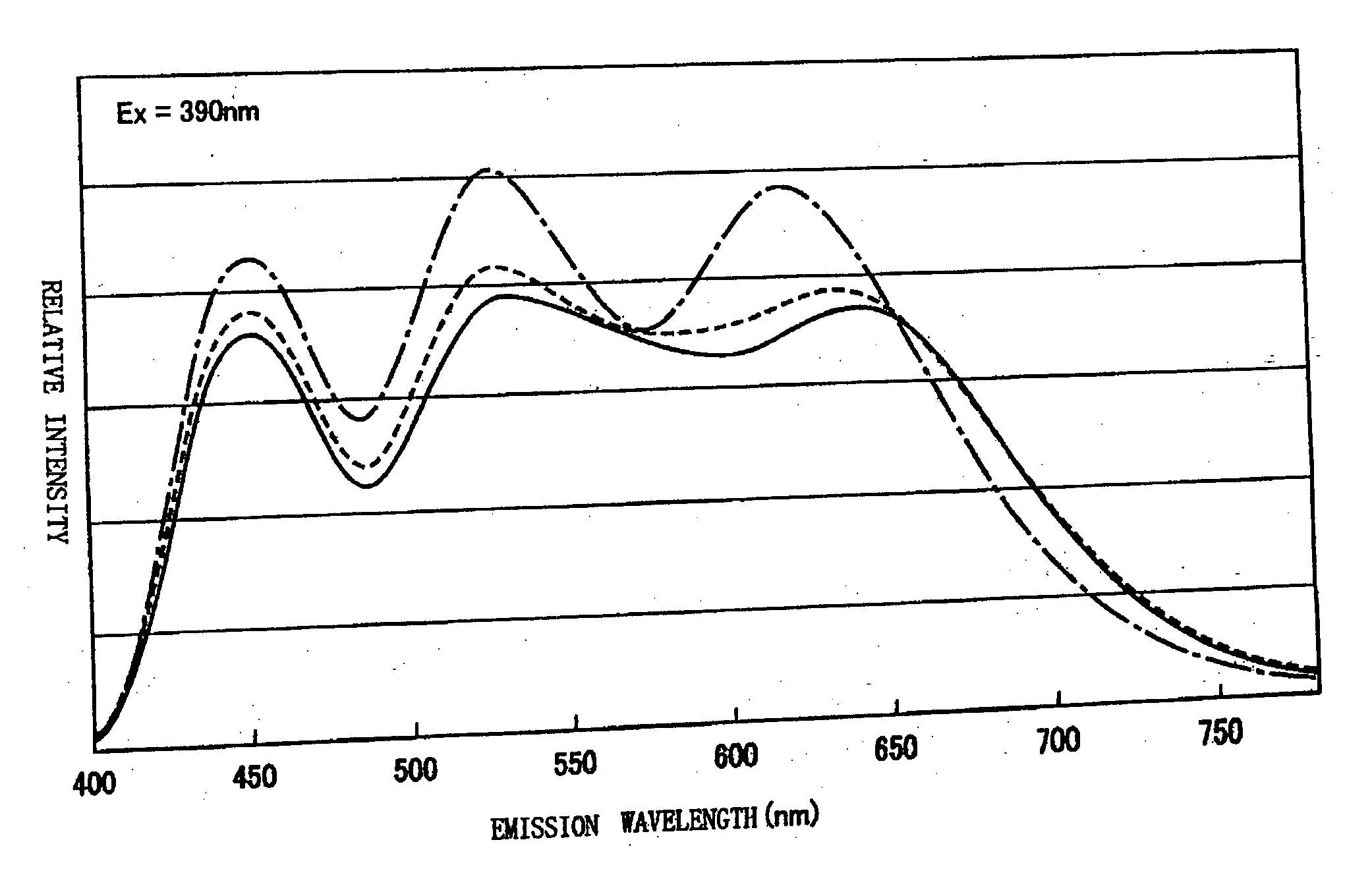
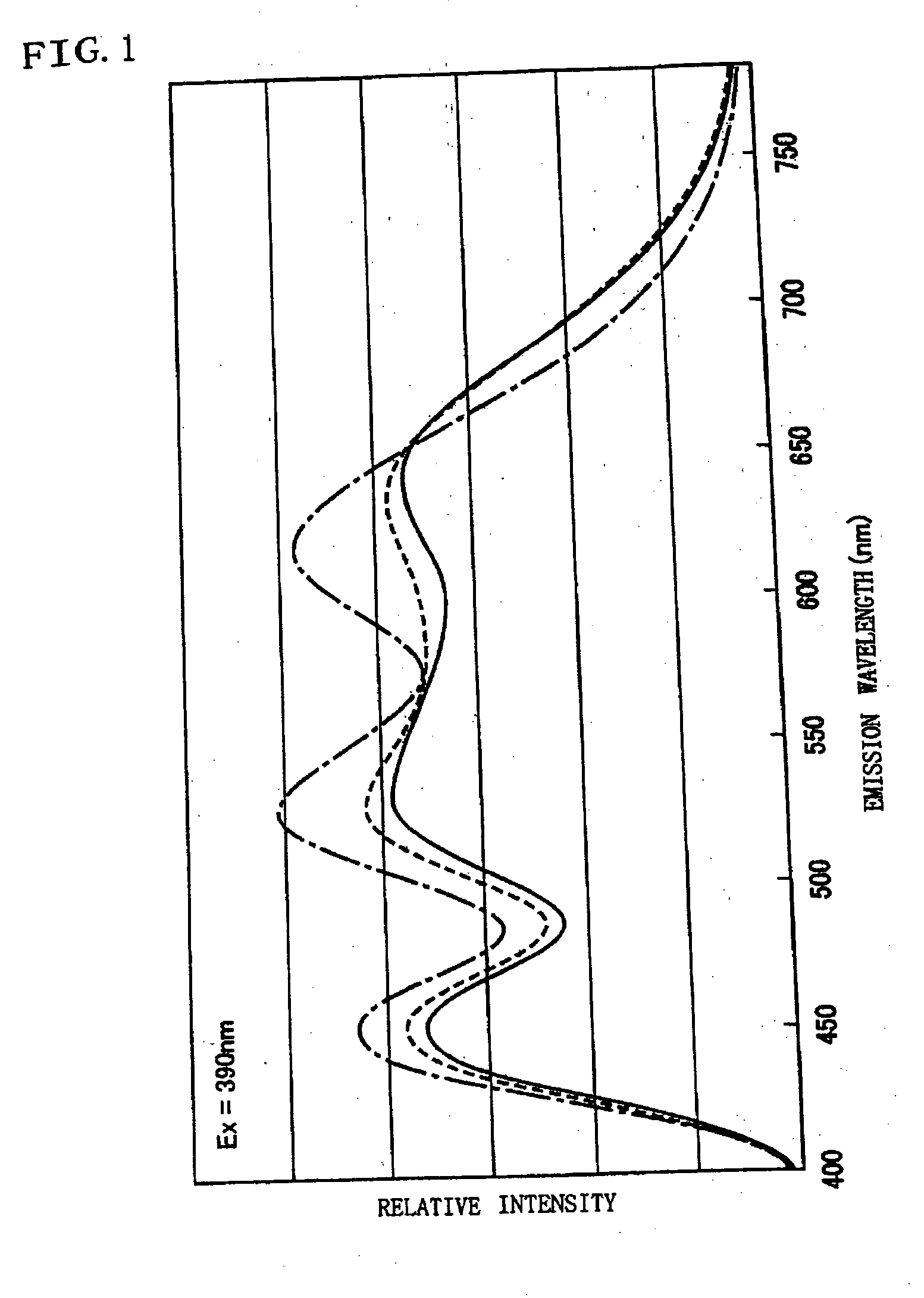
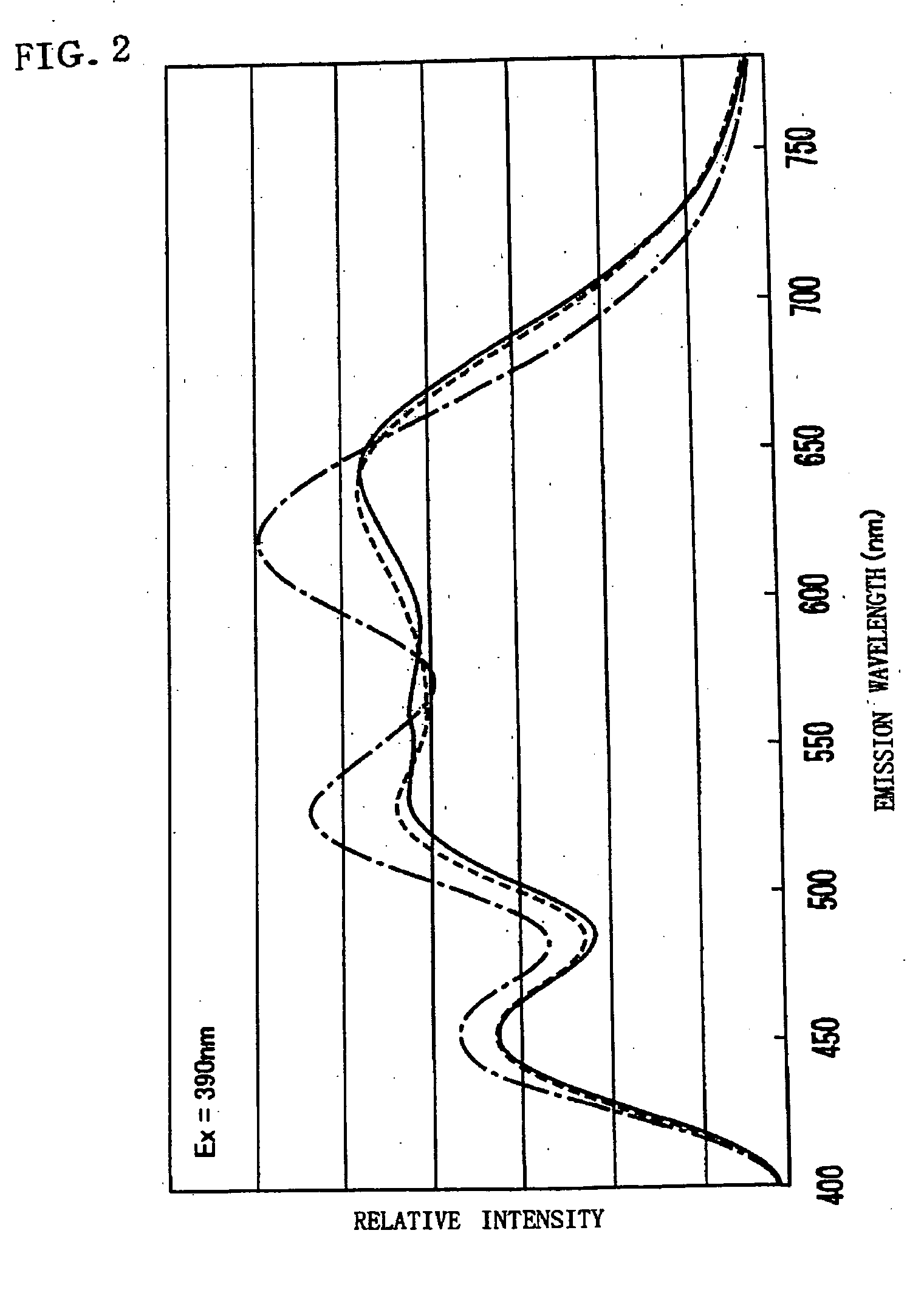
In order to provide a phosphor mixture which contains four or more kinds of phosphors comprising a red phosphor, an orange phosphor, a blue phosphor, and a green phosphor, and produce emission having excellent color rendering not only in the case of white light at a high color temperature, but also in the case of emission in warm white which is low in color temperature, and a light emitting device including the phosphor mixture and a light-emitting section, CaAlSiN3:Eu and CaAl2Si4N8:Eu are manufactured as a red phosphor and an orange phosphor respectively, and ZnS:Cu, Al, and BAM:Eu are prepared as a green phosphor and a blue phosphor respectively. Then the emission spectrums of these four kinds of phosphors are measured and a relative mixing ratio at which the correlated color temperature of the phosphor mixture becomes a targeted color temperature is determined from the emission spectrum by simulation, and a phosphor mixture is obtained by weighing and mixing the respective phosphors based on the simulation result.
Owner:NICHIA CORP +1
Nanoscale ion storage materials
ActiveUS20070031732A1Improve electronic conductivityImproved electromechanical stabilityMaterial nanotechnologyPhosphatesHigh ratePhosphate
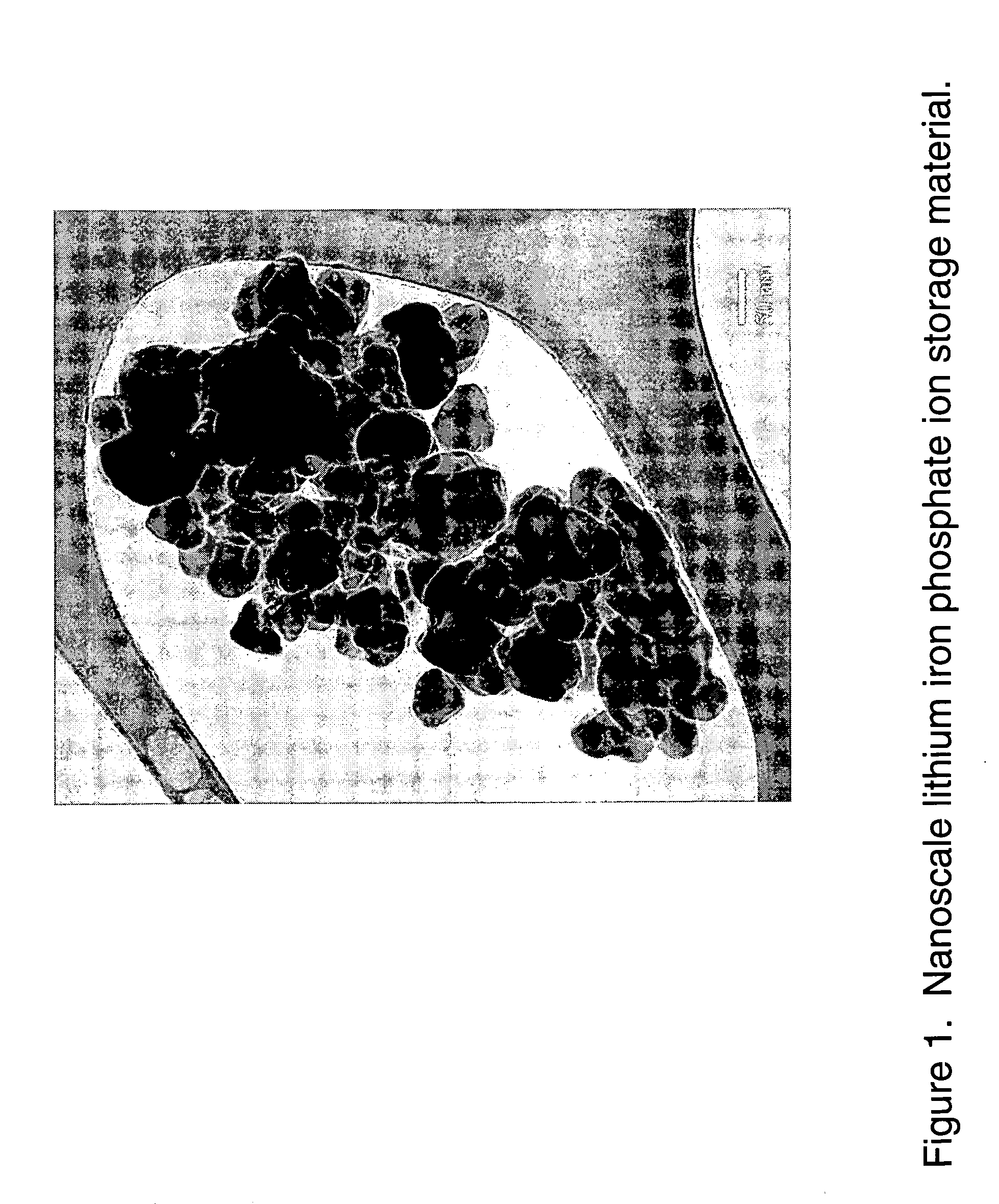
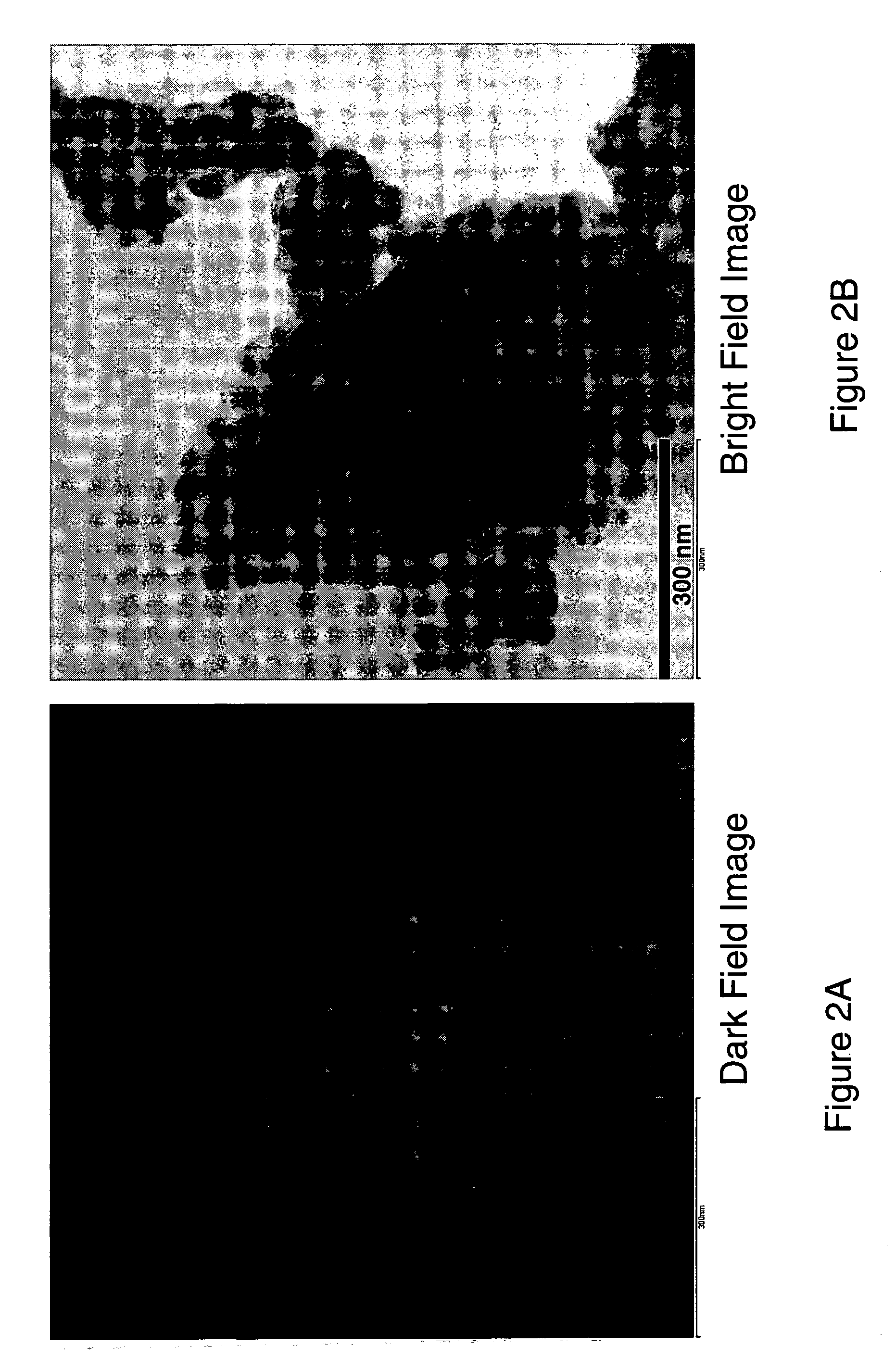
Nanoscale ion storage materials are provided that exhibit unique properties measurably distinct from their larger scale counterparts. For example, the nanoscale materials can exhibit increased electronic conductivity, improved electromechanical stability, increased rate of intercalation, and / or an extended range of solid solution. Useful nanoscale materials include alkaline transition metal phosphates, such as LiMPO4, where M is one or more transition metals. The nanoscale ion storage materials are useful for producing devices such as high energy and high power storage batteries, battery-capacitor hybrid devices, and high rate electrochromic devices.
Owner:RIL USA INC +1
Lithium ion conductive solid electrolyte and method for manufacturing the same
ActiveUS20070087269A1Increase battery capacityImprove discharge characteristicsSolid electrolytesPhosphatesLithiumPorosity
Owner:OHARA
Method for making a lithium mixed metal compound having an olivine structure
ActiveUS20070207080A1Mitigate such drawbackNon-metal conductorsLiquid surface applicatorsLithiumCarbon layer
Owner:AQUIRE ENERGY CO LTD
Process for the production of particles or powders
In a novel process for the production of particles or powders, a substance or mixture of substances to be treated is provided in a pressure vessel. A highly compressible fluid is dissolved under pressure in the substance or mixture of substances provided until a solution containing 5% to 90% by weight of said highly compressible fluid has formed. The melting point of said highly compressible fluid is at least around 40 K lower than the melting point of the substance or mixture of substances to be treated. The solution obtained by dissolving said highly compressible fluid in the substance or mixture of substances provided is adjusted to a temperature of up to around 50 K above or below the melting point under atmospheric pressure of the substance or mixture of substances to be treated and is then rapidly decompressed by means of a decompression device in such a way that the temperature falls, downstream of the decompression device, below the solidification point of the substance or mixture of substances to be treated and essentially all of the highly compressible fluid turns gaseous, whereby particles of the substance or mixture of substances provided form. Subsequently, the particles which have formed are removed from the stream of decompressed highly compressible fluid.
Owner:WEIDNER ECKHARD +2
Self-hardening calcium phosphate materials with high resistance to fracture, controlled strength histories and tailored macropore formation rates
InactiveUS6955716B2Strong and tough self-hardeningHigh strengthPhosphatesOther chemical processesFiberHigh resistance
A bone replacement material and therapy comprises the combination of calcium phosphate compounds and two or more soluble fillers in the form of fibers, mesh or other materials which have the dual functions of reinforcing an in vivo implant while dissolving at a programmed rate to form macropores capable of receiving natural bone ingrowth.
Owner:ADA FOUND
Binary, ternary and quaternary lithium phosphates, method for the production thereof and use of the same
ActiveUS20040151649A1High specific capacitySimple and inexpensivePhosphatesPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesRoom temperatureReducing atmosphere
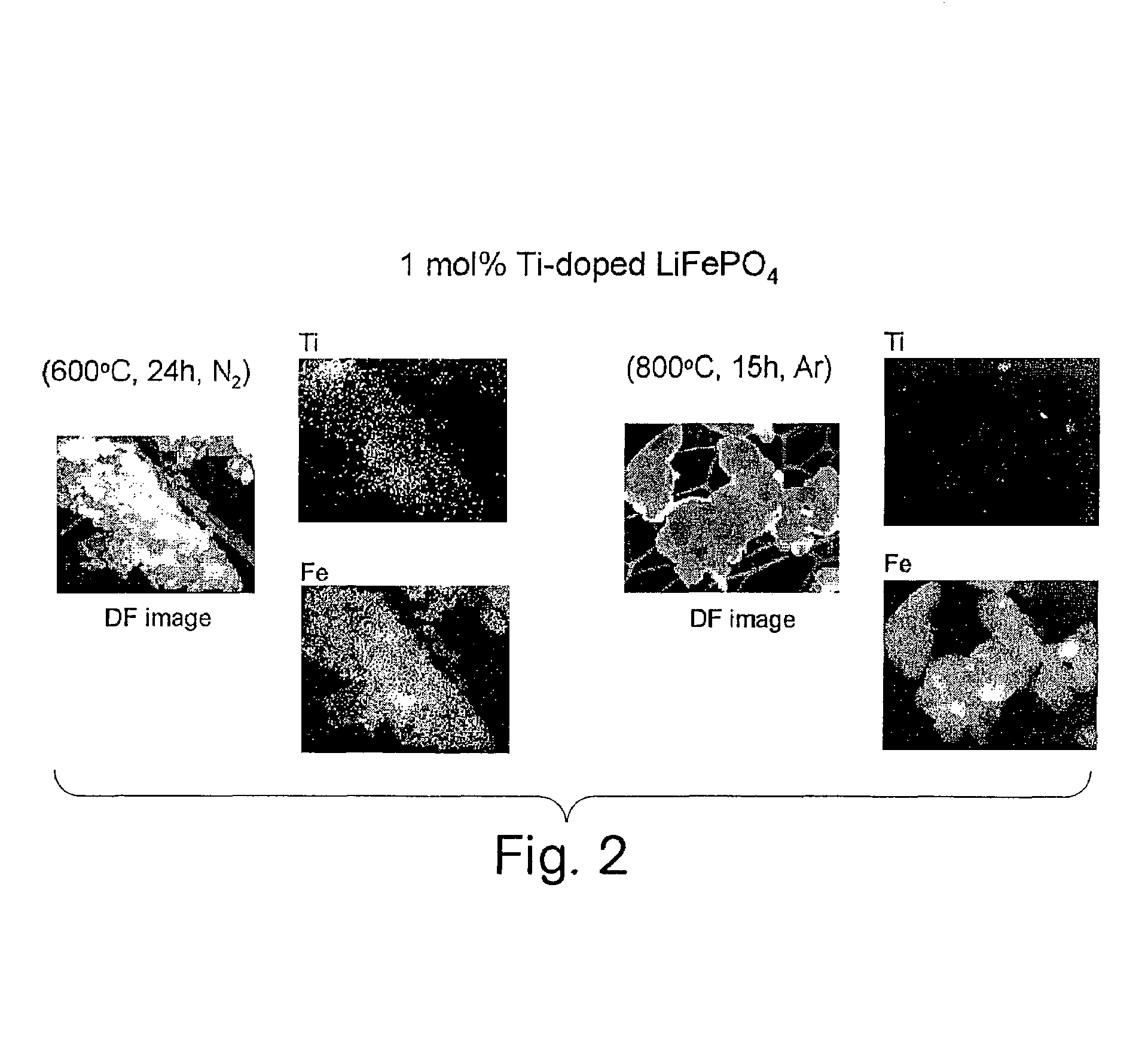
The invention relates to binary, ternary and quaternary lithium phosphates of general formula Li(FexM<1>yM<2>z)PO4 wherein M<1 >represents at least one element of the group comprising Sc, Ti, V, Cr, Mn, Co, Ni, Cu, Zn, Be, Mg, Ca, Sr, Ba, Al, Zr, and La; M<2 >represents at least one element of the group comprising Sc, Ti, V, Cr, Mn, Co, Ni, Cu, Zn, Be, Mg, Ca, Sr, Ba, Al, Zr, and La; x=between 0.5 and 1, y=between 0 and 0.5, z=between 0 and 0.5, provided that x+y+z=1, or x=0, y =1 and z=0. The said lithium phosphates can be obtained according to a method whereby precursor compounds of elements Li, Fe, M<1 >and / or M<2 >are precipitated from aqueous solutions and the precipitation product is dried in an inert gas atmosphere or a reducing atmosphere at a temperature which is between room temperature and approximately 200° C. and tempered at a temperature of between 300° C. and 1000° C. The inventive lithium phosphates have a very high capacity when used as cathode material in lithium accumulators.
Owner:ZENT FUR SONNENENERGIE & WASSERSTOFF FORSCHUNG BADEN WURTTEMBERG GEMEINNUTZIGE STIFTUNG
Synthesis method for carbon material based on lixm1-ym'(xo4)n
InactiveUS20040086445A1Improve performanceLow costHybrid capacitorsElectrolytic capacitorsElectrical conductorSynthesis methods
Method of synthesis for a material made of particles having a core and a coating and / or being connected to each other by carbon cross-linking, the core of these particles containing at least one compound of formula LixM1-yM'y(XO4)n, in which x,y and n are numbers such as 0<=x<=2, 0<=y<=0.6 and 1<=n<=1.5, M is a transition metal, M' is an element with fixed valency, and the synthesis is carried out by reaction and bringing into equilibrium the mixture of precursors, with a reducing gaseous atmosphere, in such a way as to bring the transition metal or metals to the desired valency level, the synthesis being carried out in the presence of a source of carbon called carbon conductor, which is subjected to pyrolysis. The materials obtained have excellent electrical conductivity as well as very improved chemical activity.
Owner:CENT NAT DE LA RECHERCHE SCI +2
Disinfecting composition and process for disinfecting surfaces
InactiveUS6841090B1Immediate and long lasting disinfectionBiocideOrganic detergent compounding agentsHydrogenEther
Liquid disinfecting compositions comprise an effective amount of a disinfecting material and a poly (alkylene glycol) ether having the following formula: R1—O—(CH2—CHR2O)n—R3, wherein R1 and R2 are each independently hydrogen or a substituted or unsubstituted, saturated or unsaturated, linear or branched hydrocarbon chain having from 1 to 30 carbon atoms or a hydroxy bearing linear or branched hydrocarbon chain having from 1 to 30 carbon atoms. R3 is a substituted or unsubstituted, saturated, or unsaturated, linear or branched hydrocarbon chain having from 1 to 30 carbon atoms or a hydroxy bearing linear or branched hydrocarbon chain having from 1 to 30 carbon atoms, and n is greater than 2. Processes of disinfecting and hard-surface employ such a composition.
Owner:THE PROCTER & GAMBLE COMPANY
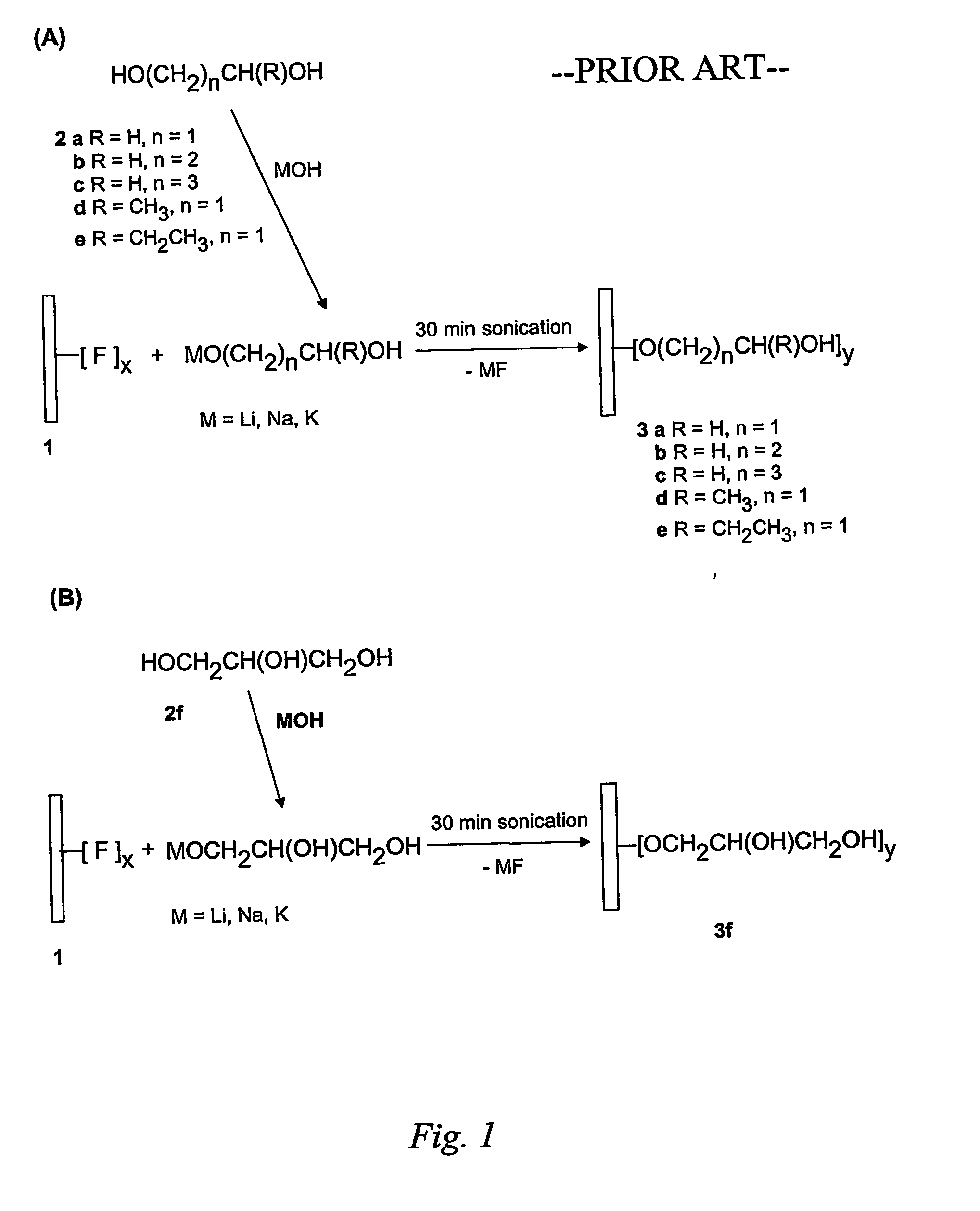
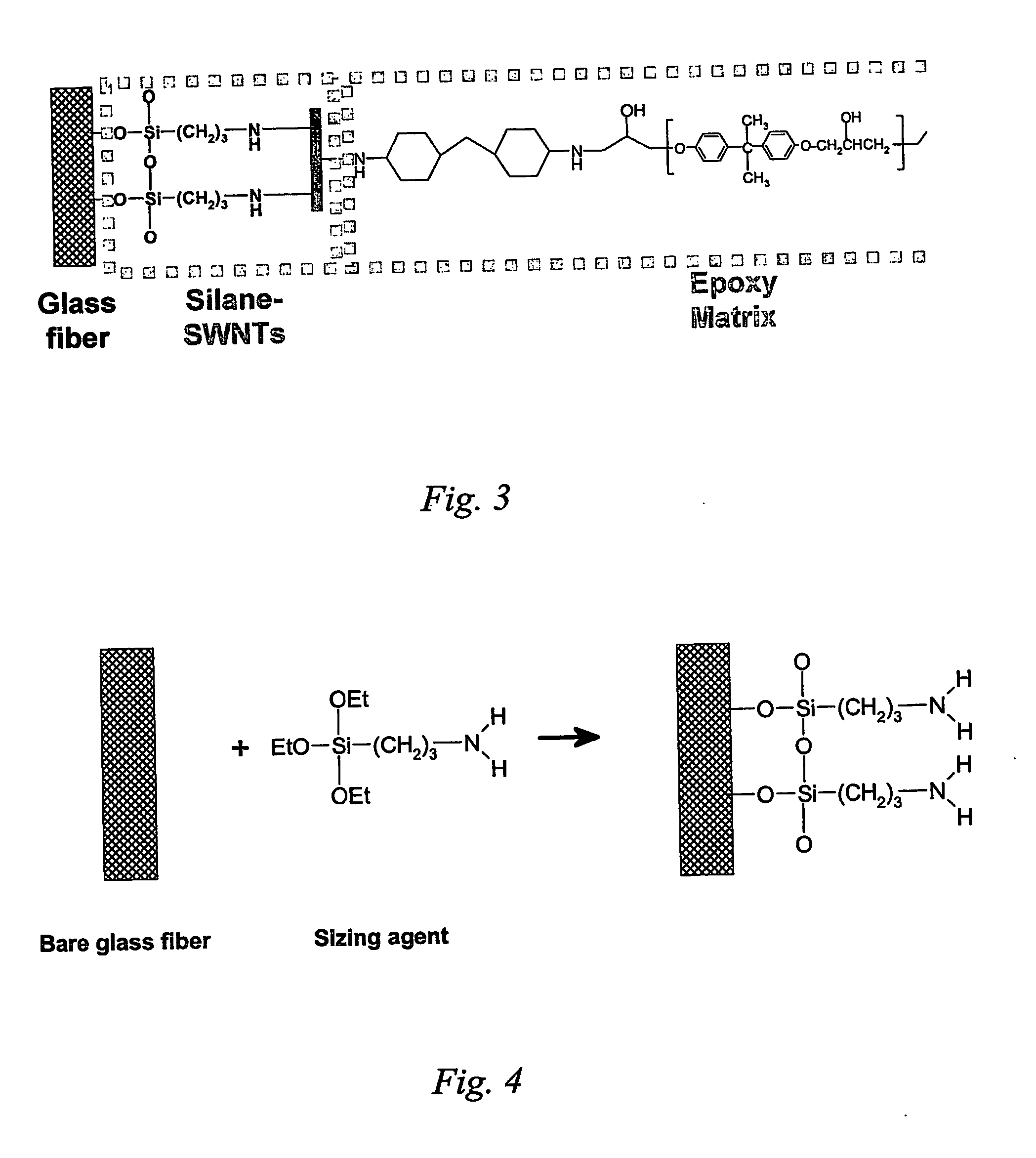
Sidewall Functionalization Of Carbon Nanotubes With Organosilanes For Polymer Composites
InactiveUS20070298669A1Improve the level ofStrong attachmentMaterial nanotechnologySilicon organic compoundsPolymer compositesPolymer chemistry
The present invention is directed to methods of functionalizing carbon nanotubes (CNTs), particularly single-wall carbon nanotubes (SWNTs), with organosilane species, wherein such functionalization enables fabrication of advanced polymer composites. The present invention is also directed toward the functionalized CNTs, advanced CNT-polymer composites made with such functionalized CNTs, and methods of making such advanced CNT-polymer composites.
Owner:RICE UNIV
Processes for preparing highly pure lithium carbonate and other highly pure lithium containing compounds
ActiveUS20110200508A1Electrolysis componentsLithium organic compoundsLithium carbonateLithium hydroxide
Owner:TERRALITHIUM LLC
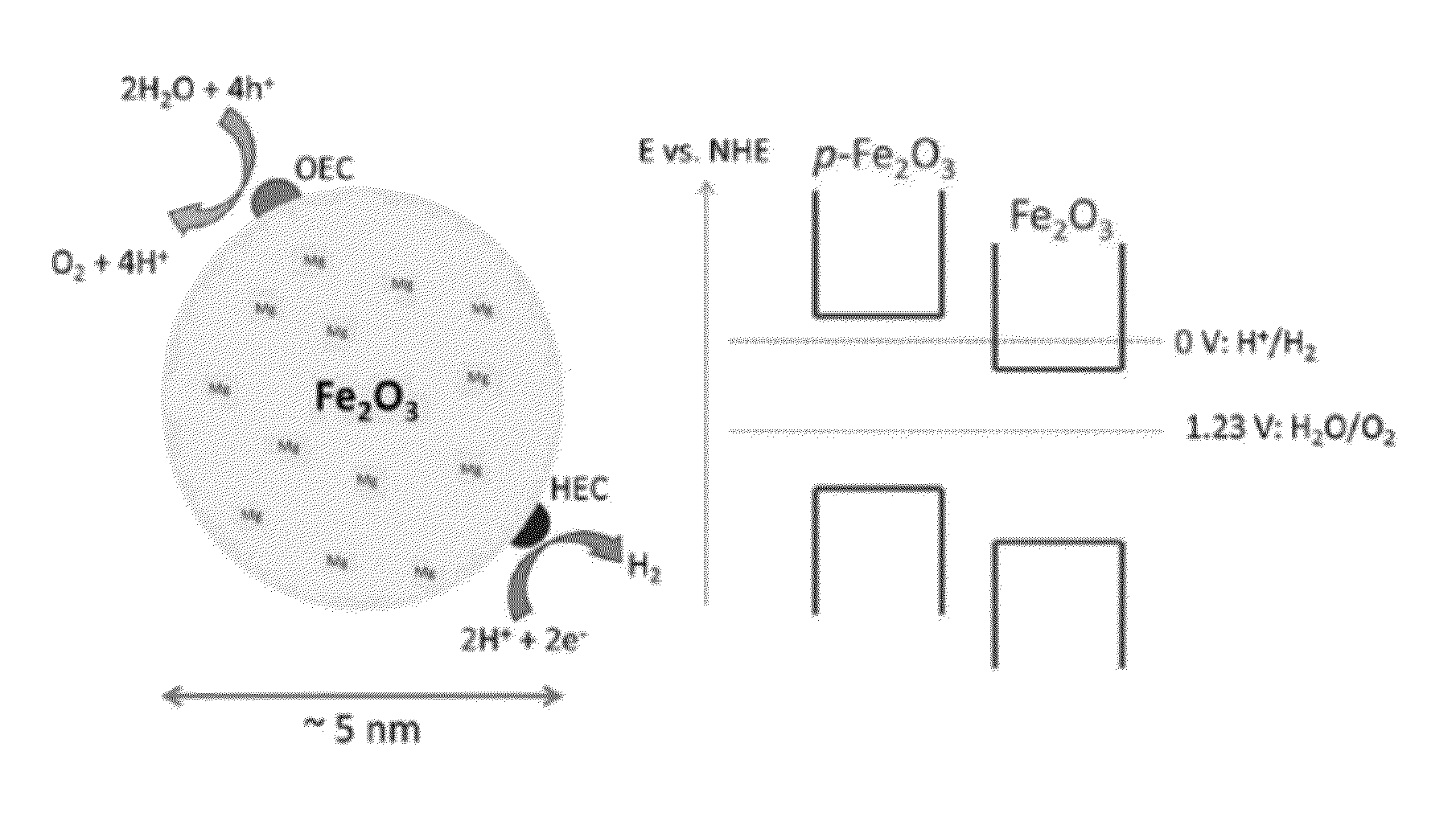
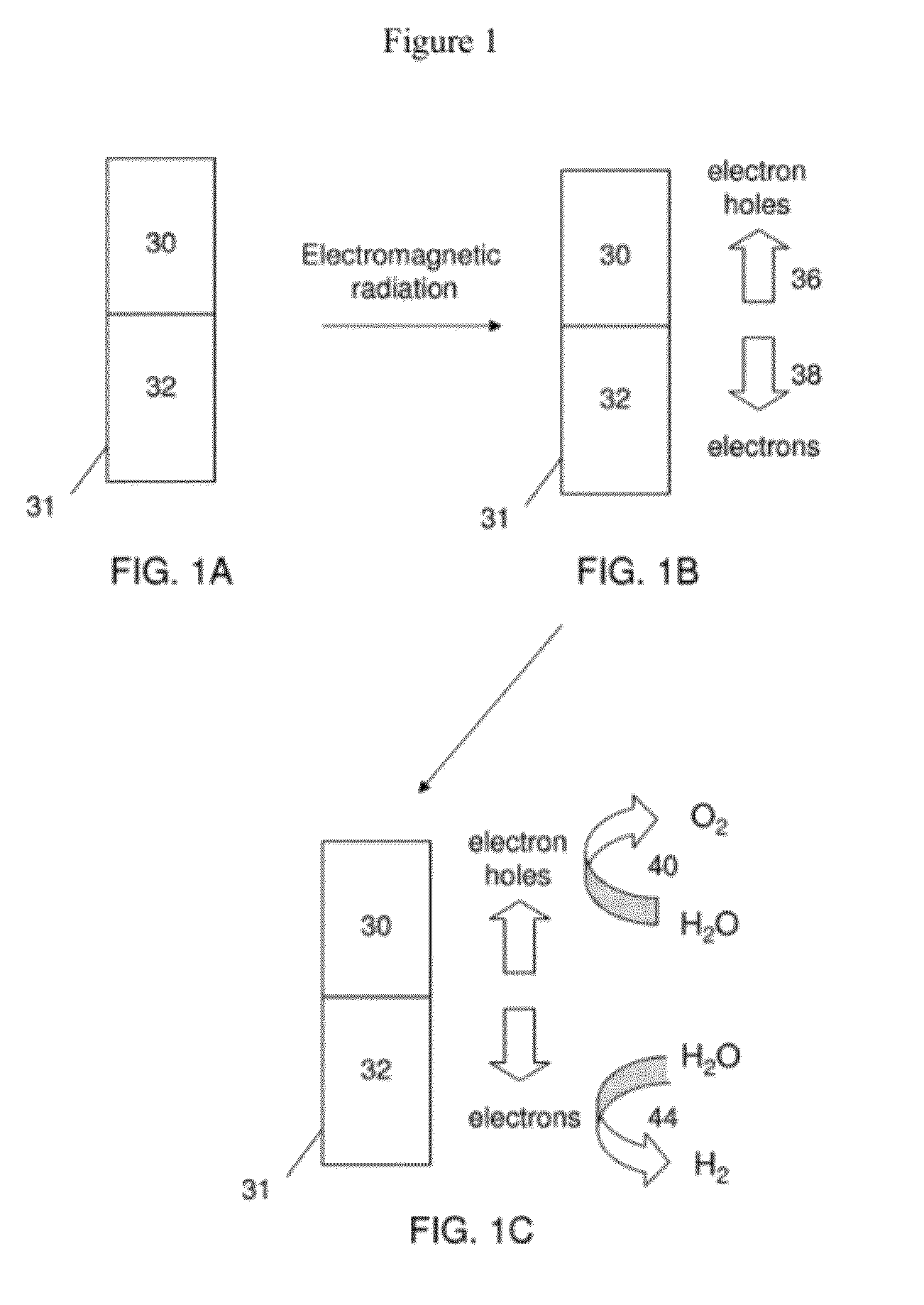
Nanostructures, Systems, and Methods for Photocatalysis
The present invention generally relates to nanostructures and compositions comprising nanostructures, methods of making and using the nanostructures, and related systems. In some embodiments, a nanostructure comprises a first region and a second region, wherein a first photocatalytic reaction (e.g., an oxidation reaction) can be carried out at the first region and a second photocatalytic reaction (e.g., a reduction reaction) can be carried out at the second region. In some cases, the first photocatalytic reaction is the formation of oxygen gas from water and the second photocatalytic reaction is the formation of hydrogen gas from water. In some embodiments, a nanostructure comprises at least one semiconductor material, and, in some cases, at least one catalytic material and / or at least one photosensitizing agent.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Enzymatic peracid generation formulation
ActiveUS20100086510A1Good storage stabilityGood mixing propertiesInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsDisinfectantAqueous solution
Disclosed herein are multi-component formulations for enzymatically producing aqueous solutions of peroxycarboxylic acids suitable for use in, e.g., disinfectant and / or bleaching applications. The multi-component peroxycarboxylic acid formulations comprise at least one carbohydrate esterase family 7 enzyme having perhydrolytic activity.
Owner:DUPONT US HLDG LLC
Process for metals recovery from spent catalyst
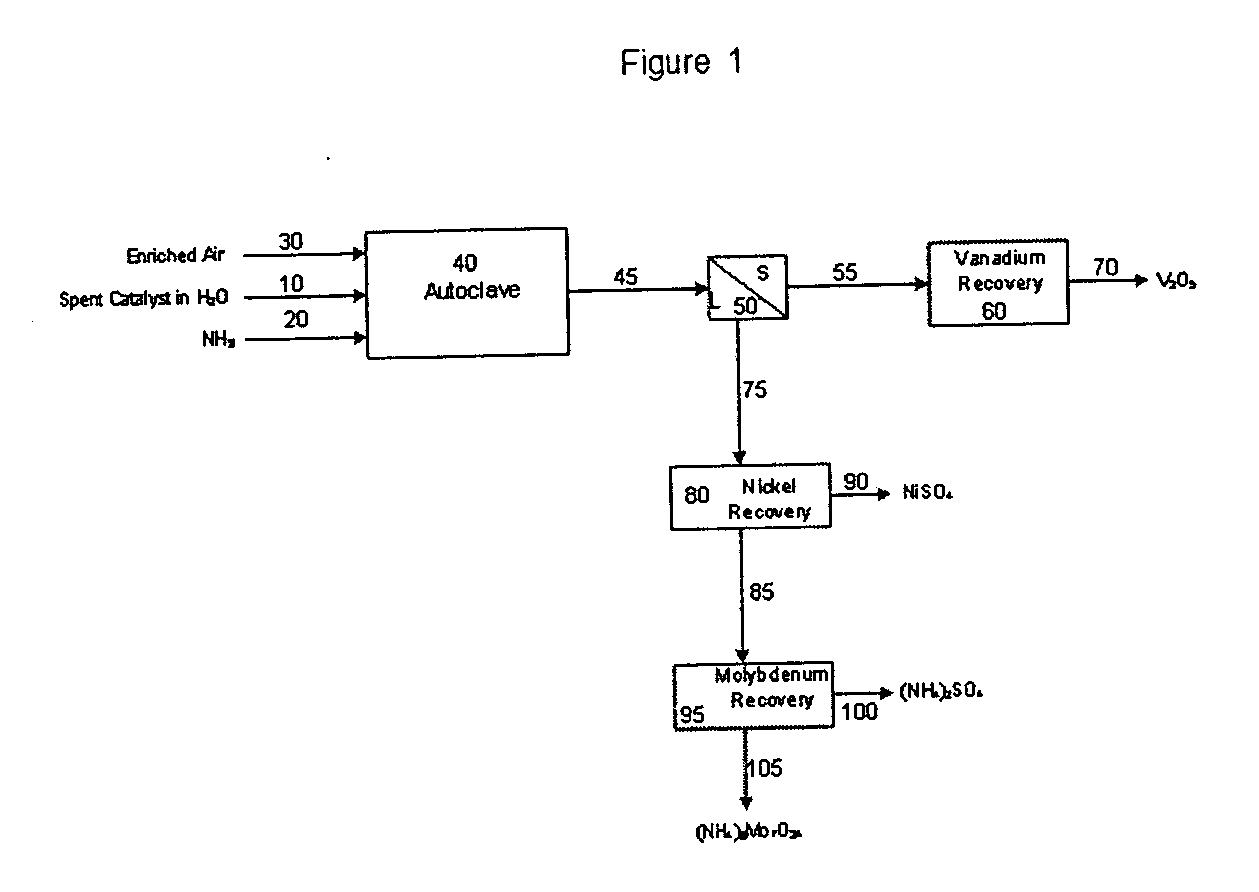
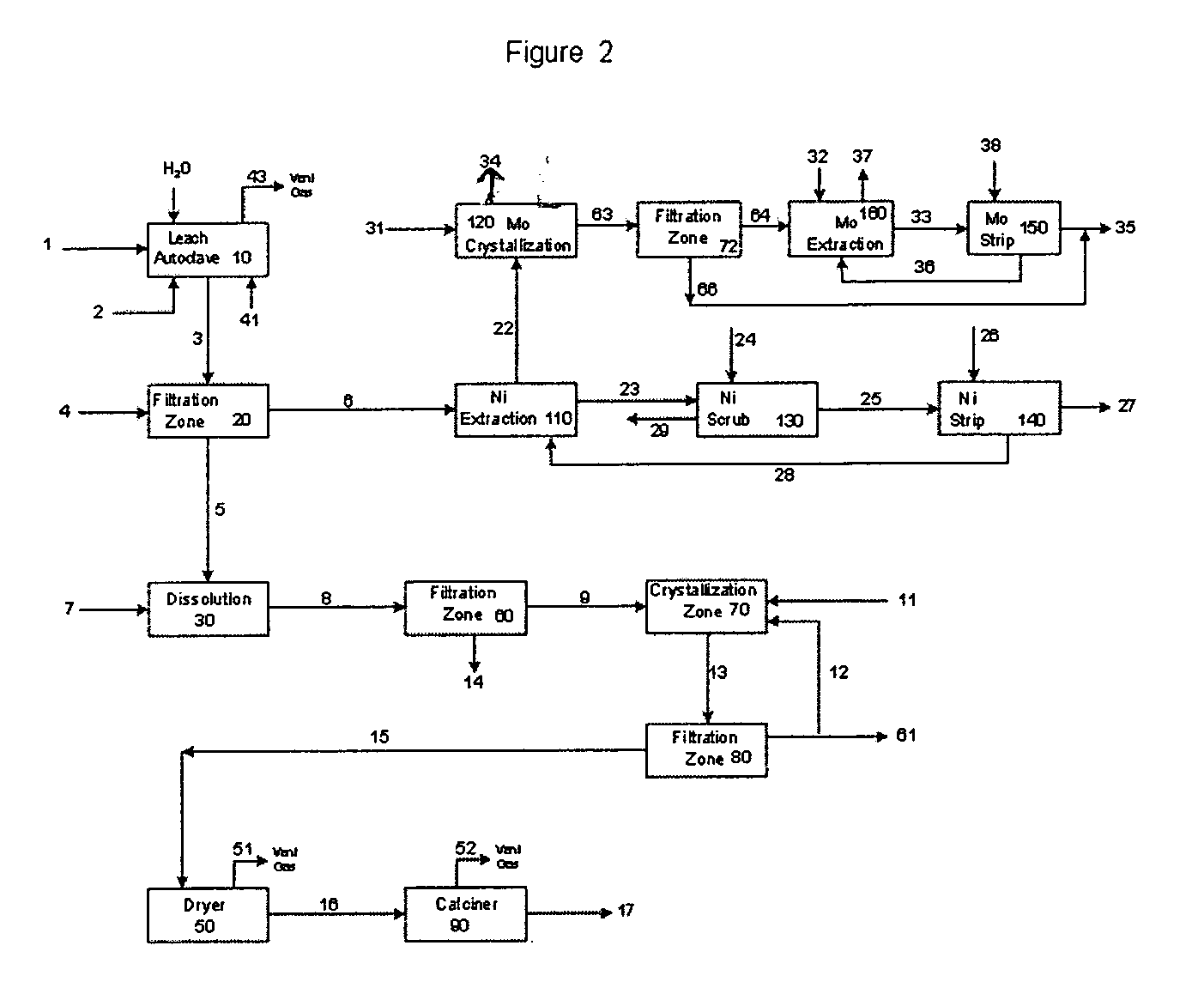
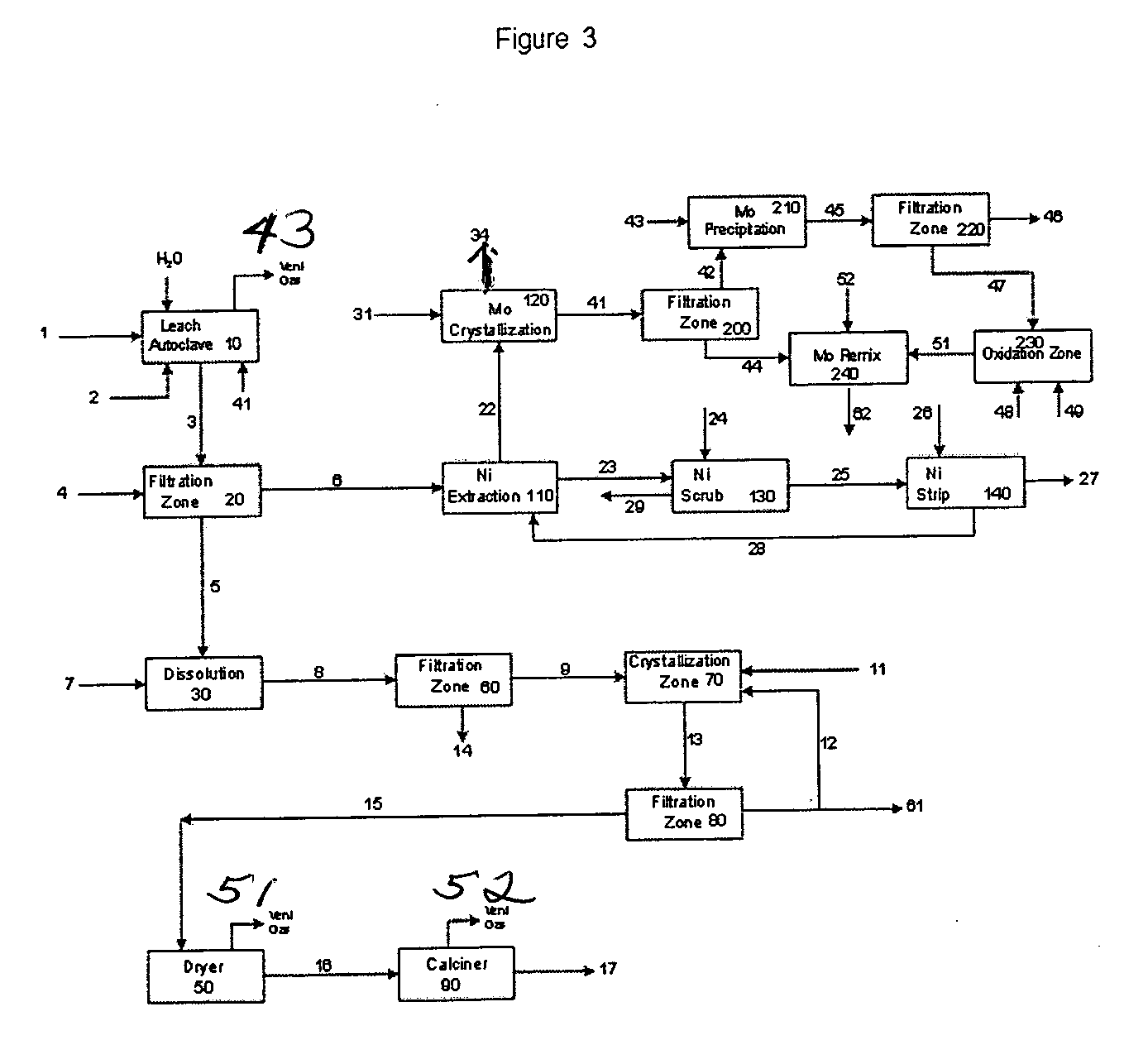
The process of this invention is directed to the removal of metals from an unsupported spent catalyst. The catalyst is subjected to leaching reactions. Vanadium is removed as a precipitate, while a solution comprising molybdenum and nickel is subjected to further extraction steps for the removal of these metals. Molybdenum may alternately be removed through precipitation.
Owner:CHEVROU USA INC
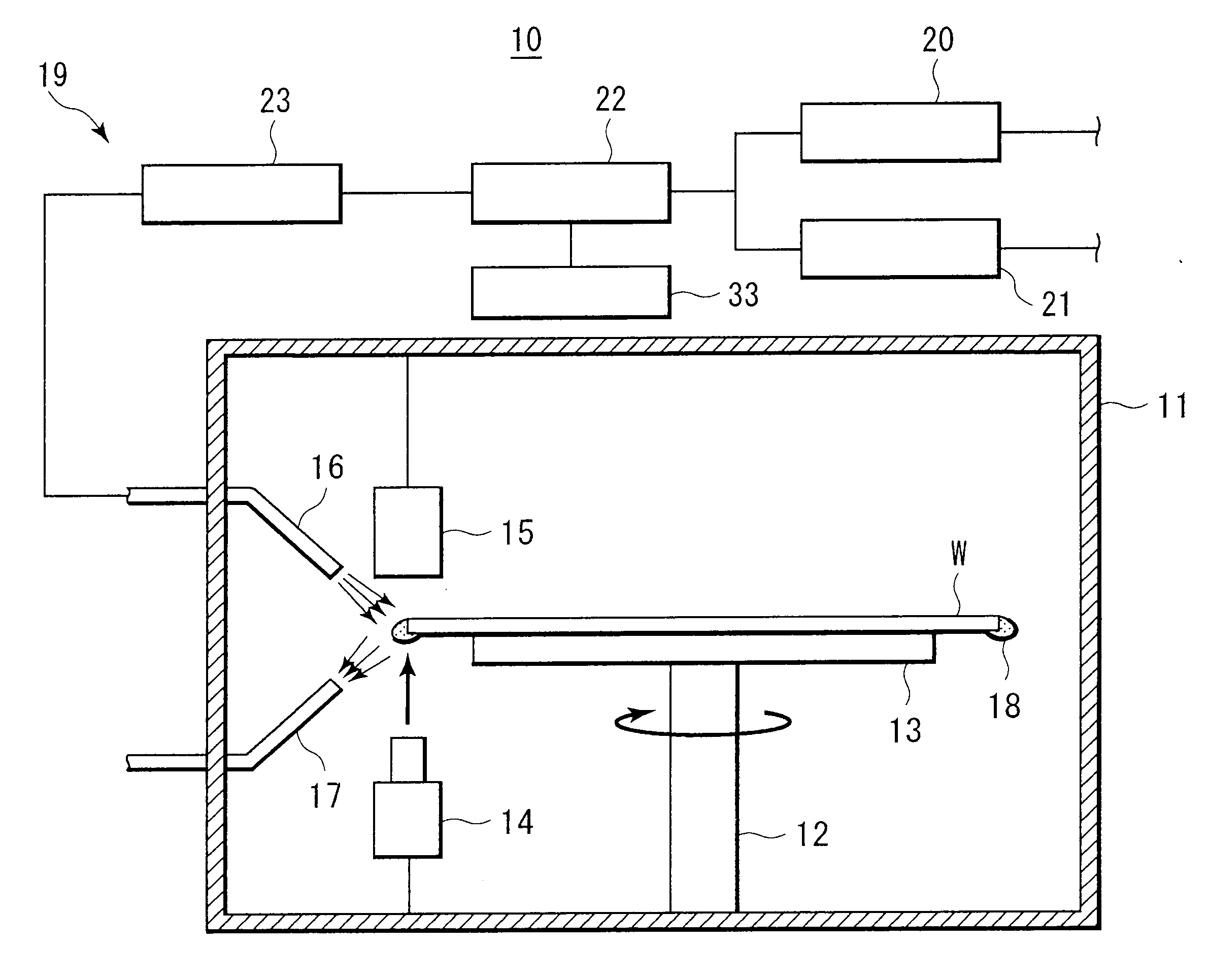
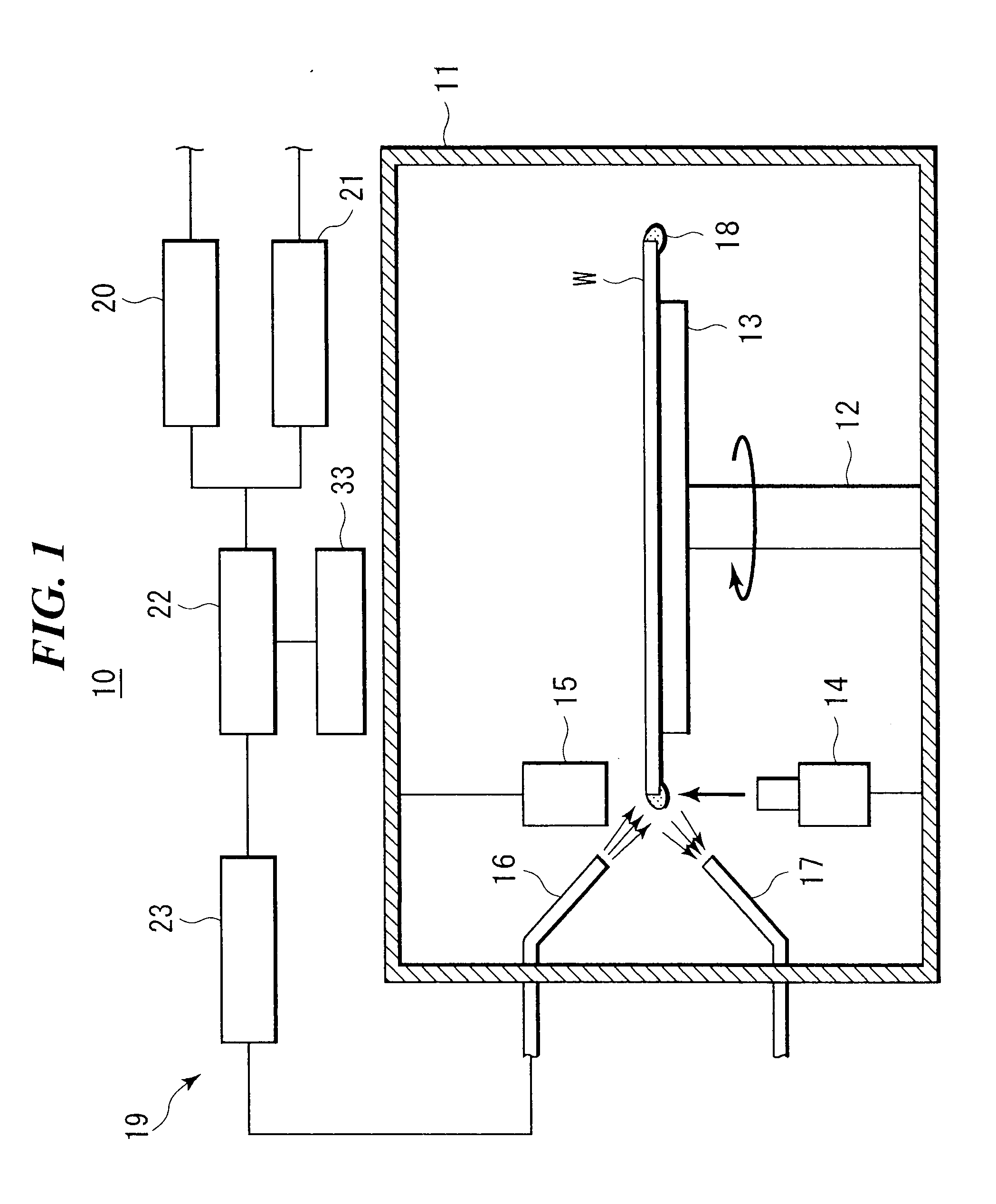
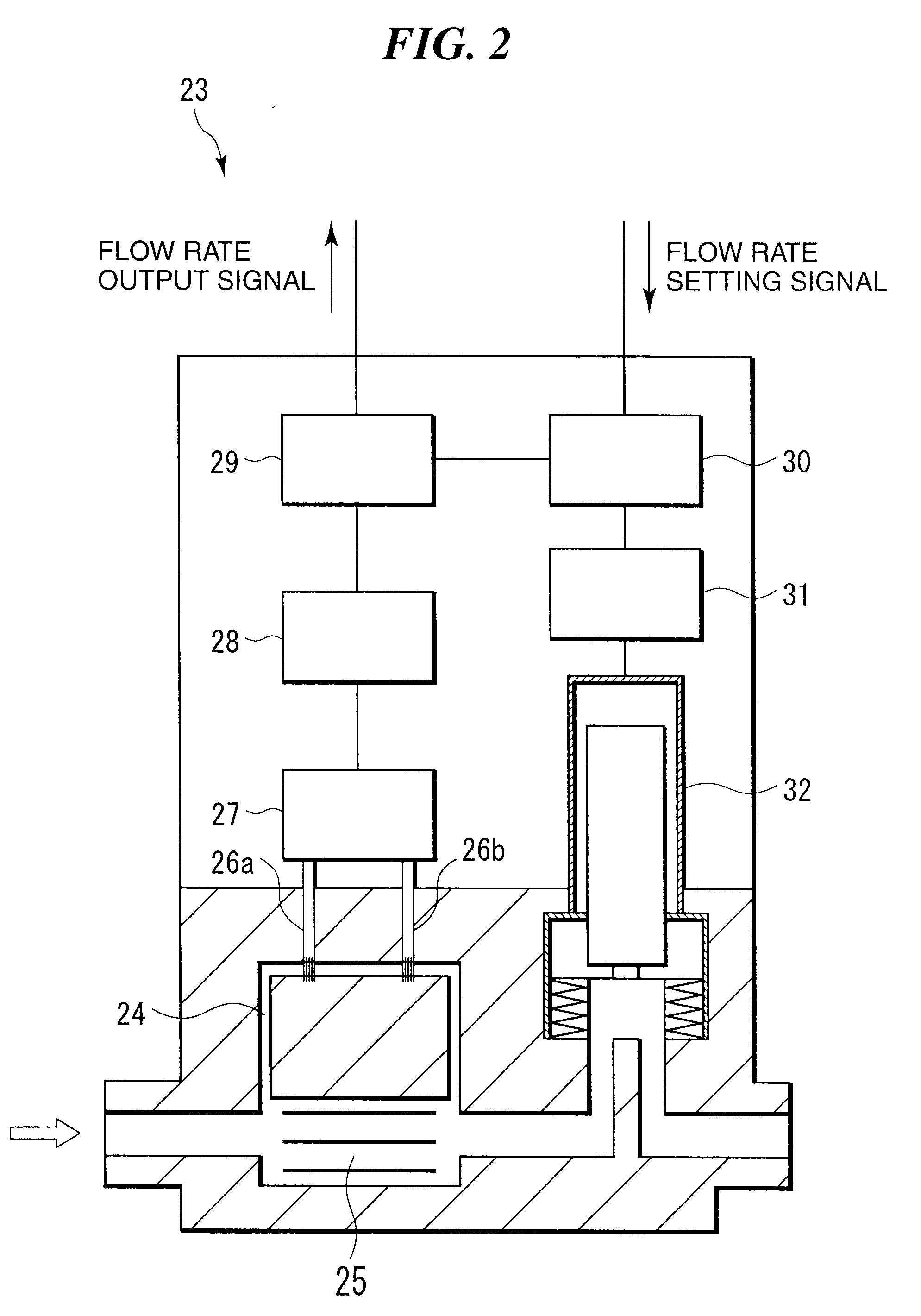
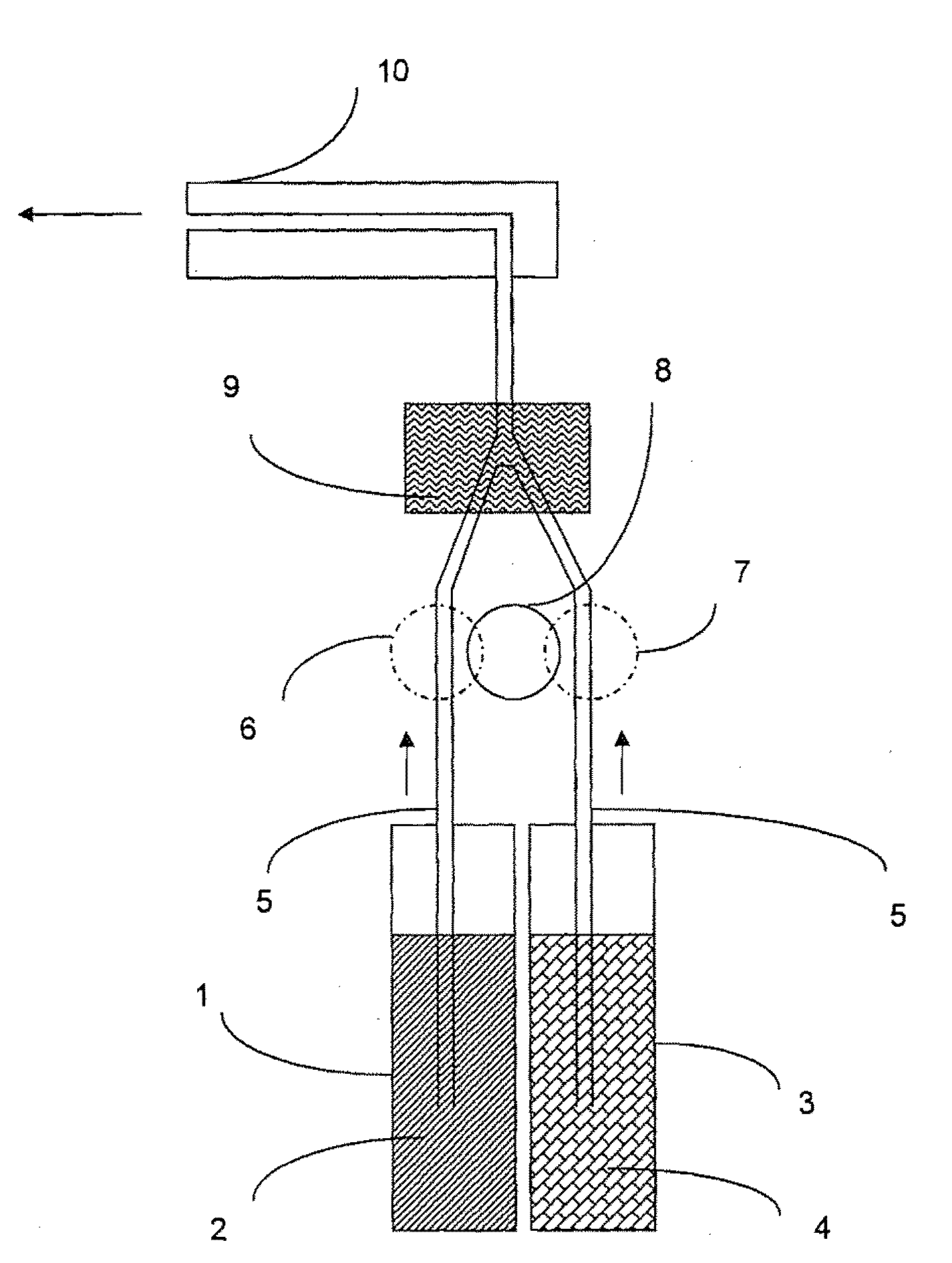
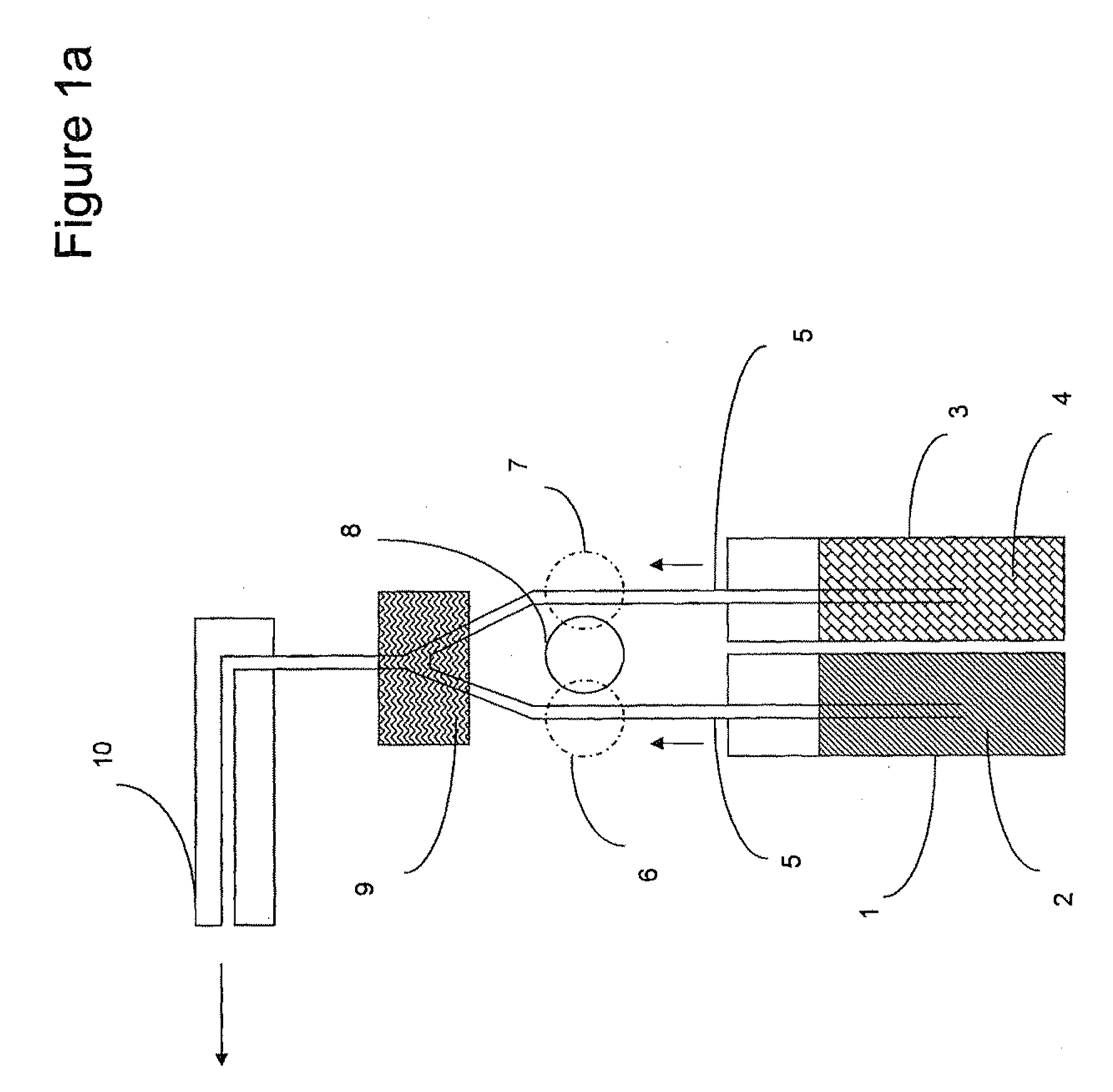
System and method of producing thin-film electrolyte
InactiveUS6852139B2PhosphatesPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesLithiumHydrogen
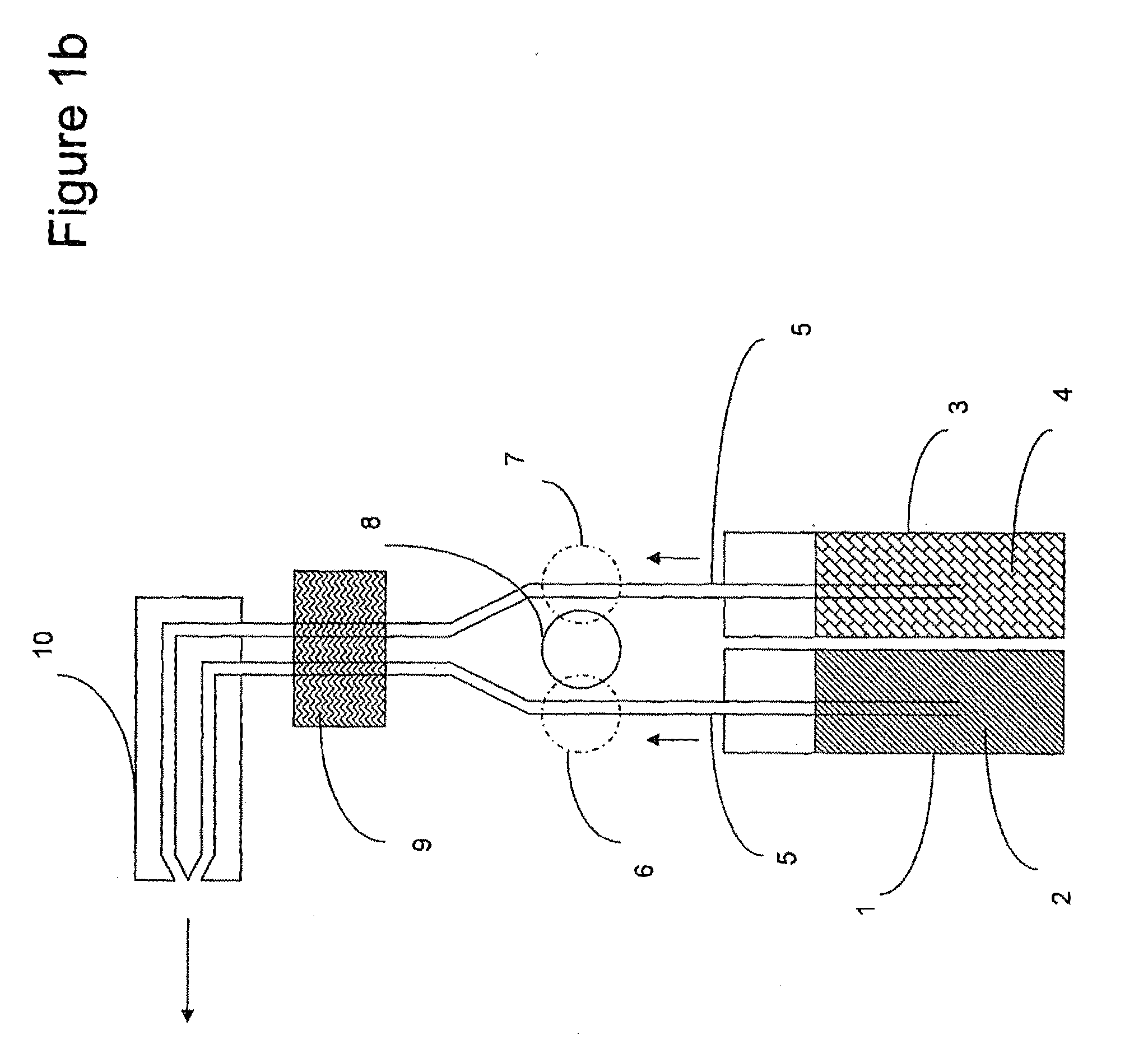
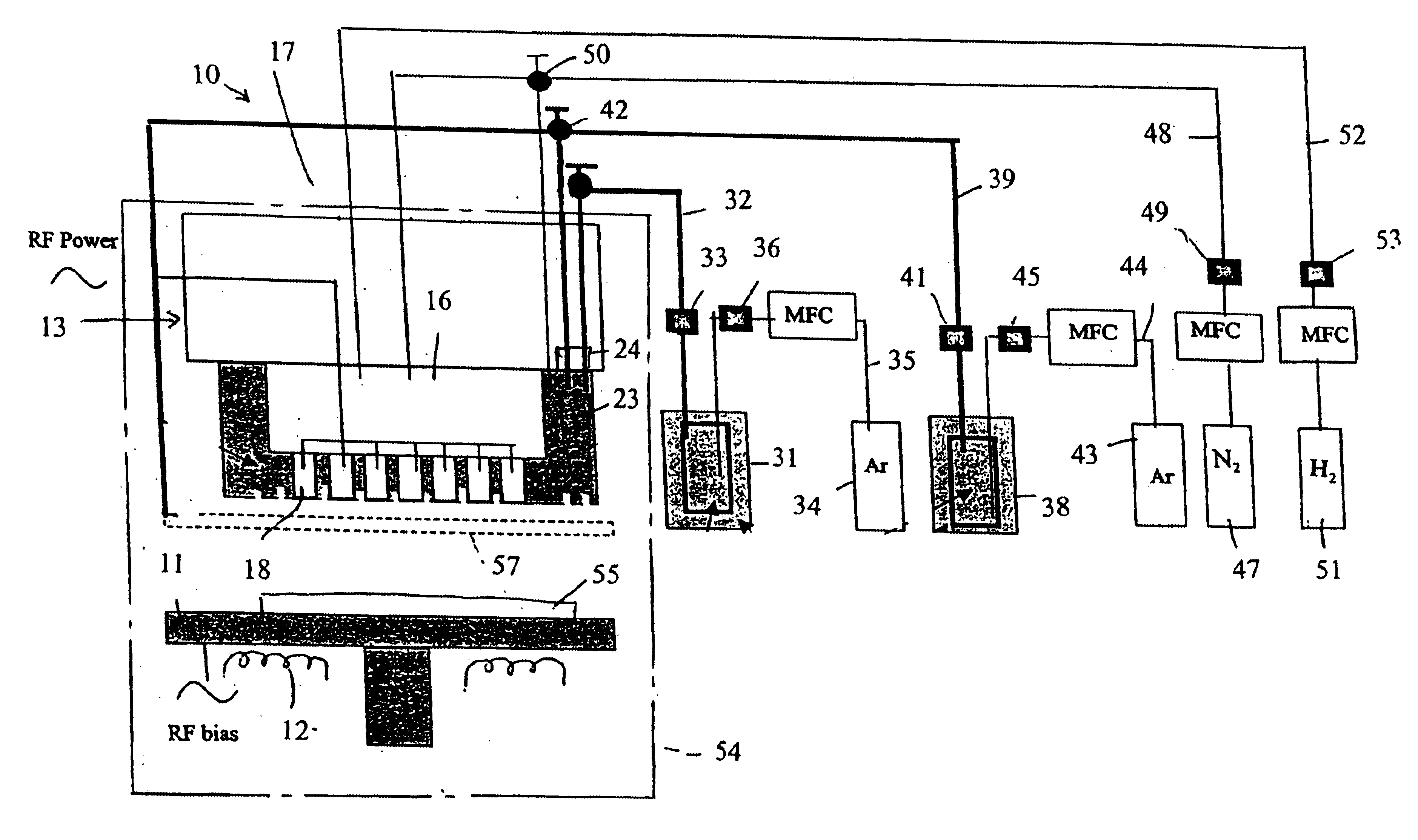
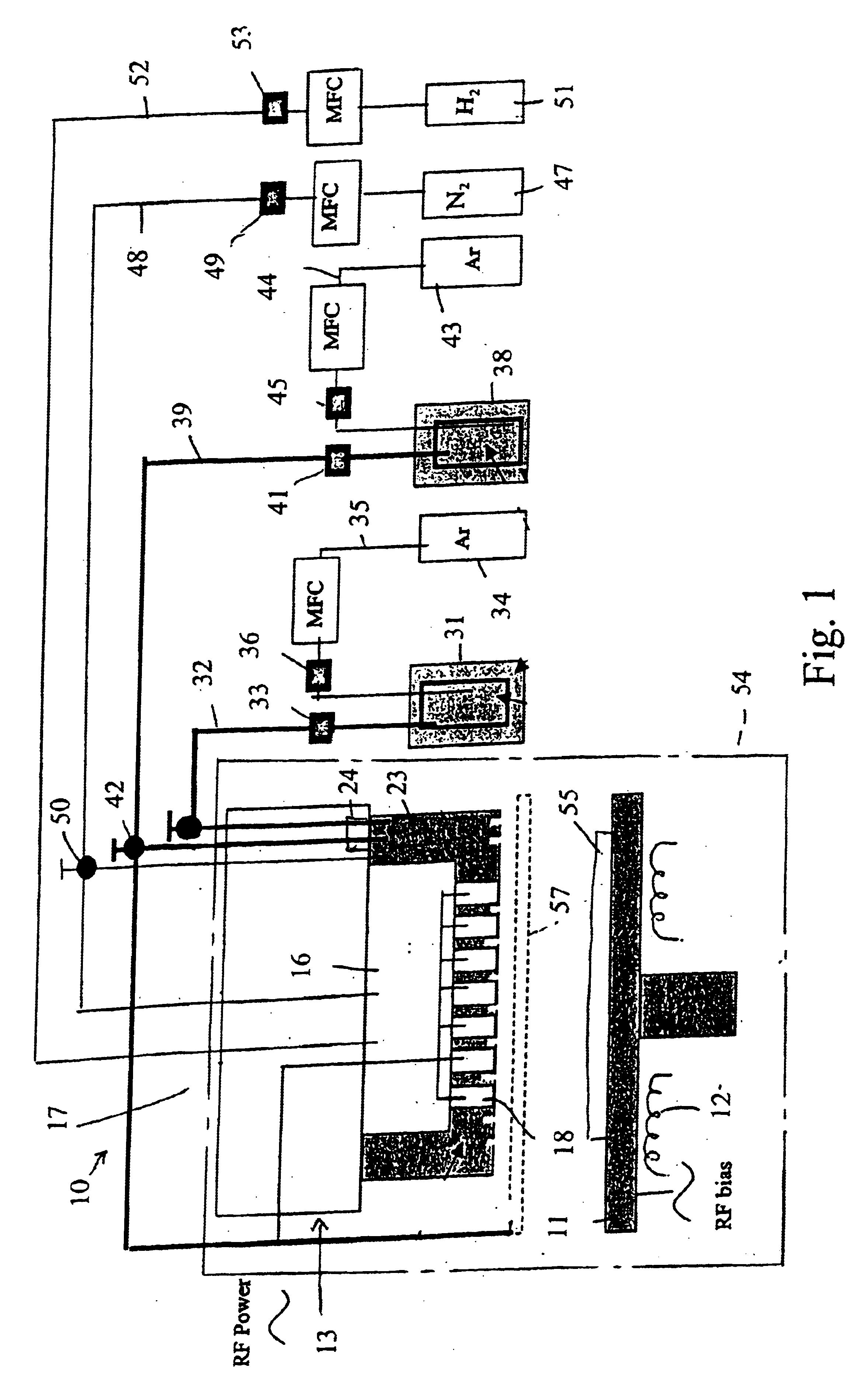
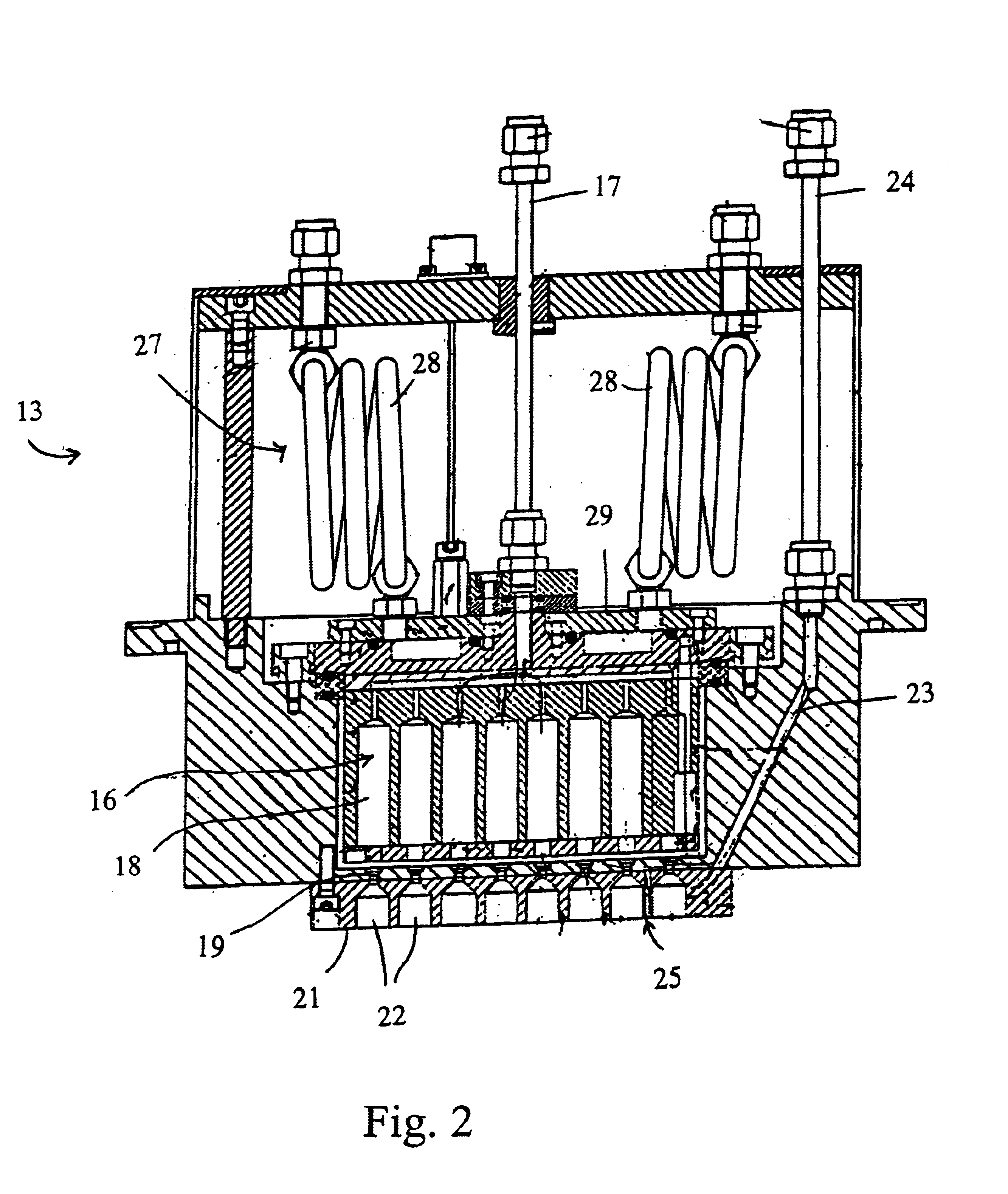
A process of producing a thin film electrolyte is provided wherein a volatile lithium-containing precursor and a volatile phosphate-containing precursor are mixed into a plasma generated from a plasma source. The mixture is then deposited upon a substrate. The process is conducted with the use of a system (11) having a plasma source (13) having a primary plenum (16) and a secondary plenum (23). The primary plenum is in fluid communication with a source of nitrogen gas (47) and a source of hydrogen gas (51). The secondary plenum is in fluid communication with a first bubbler (31) and a second bubbler (38).
Owner:JOHNSON IP HLDG LLC
Method of hydrothermal liquid phase sintering of ceramic materials and products derived therefrom
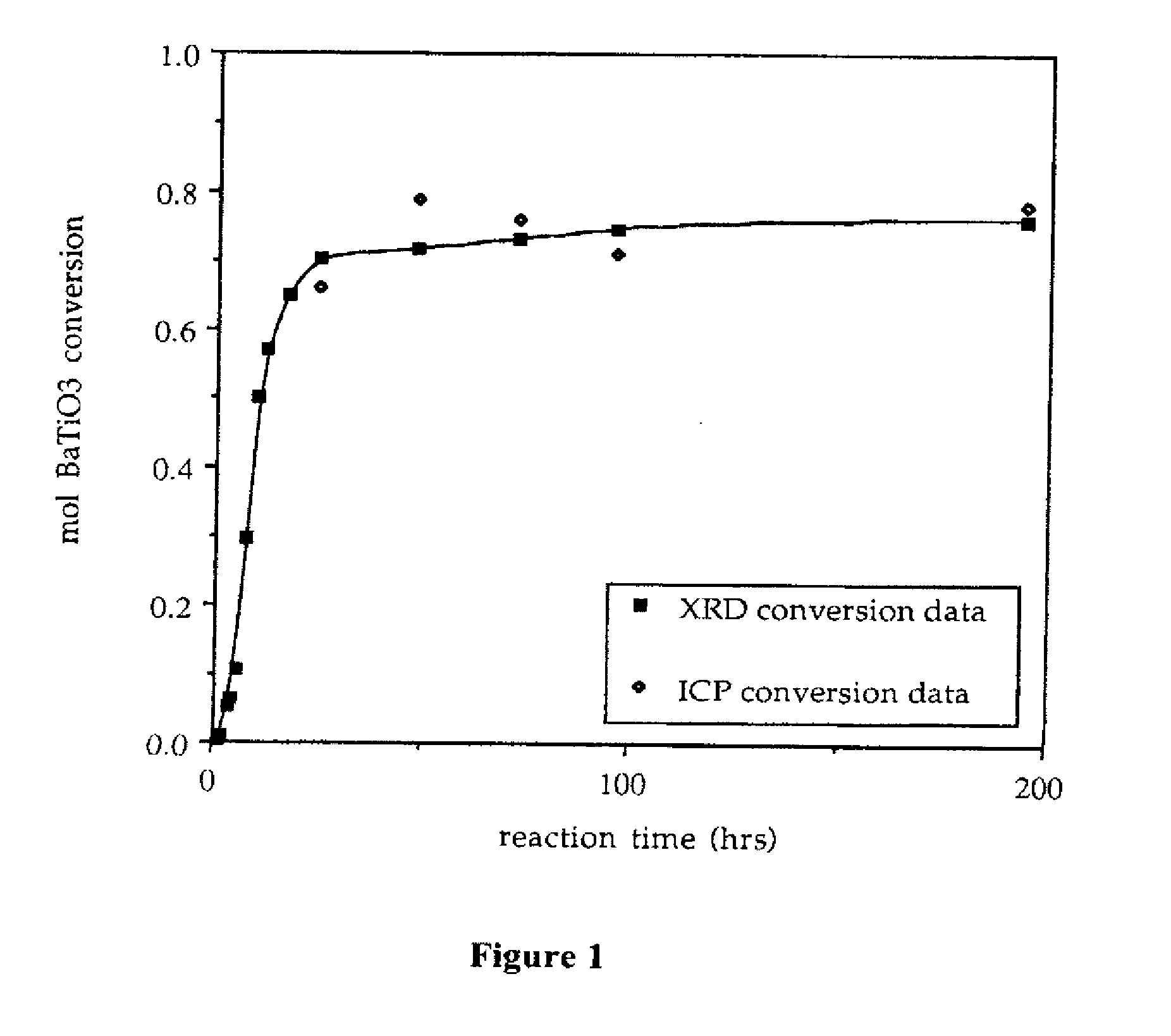
Provided here is a method of producing a monolithic body from a porous matrix, comprising: (i) providing a porous matrix having interstitial spaces and comprising at least a first reactant; (ii) contacting the porous matrix with an infiltrating medium that carries at least a second reactant; (iii) allowing the infiltrating medium to infiltrate at least a portion of the interstitial spaces of the porous matrix under conditions that promote a reaction between the at least first reactant and the at least second reactant to provide at least a first product; and (iv) allowing the at least first product to form and fill at least a portion of the interstitial spaces of the porous matrix, thereby producing a monolithic body, wherein the monolithic body does not comprise barium titanate.
Owner:RUTGERS THE STATE UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com