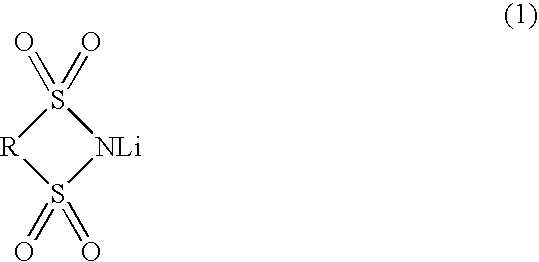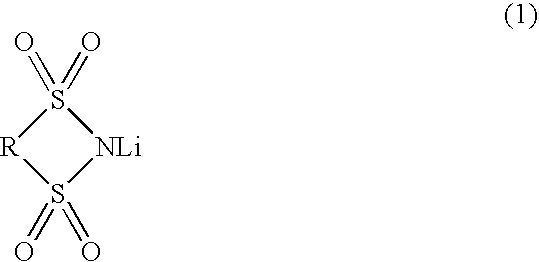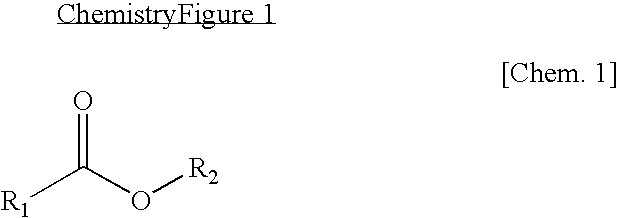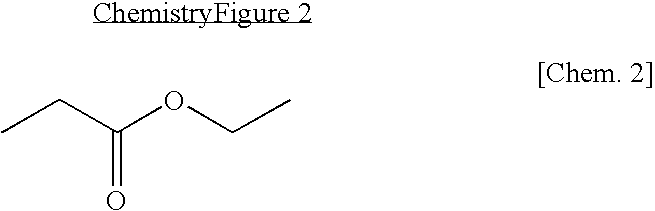Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
421results about How to "Improve discharge characteristics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
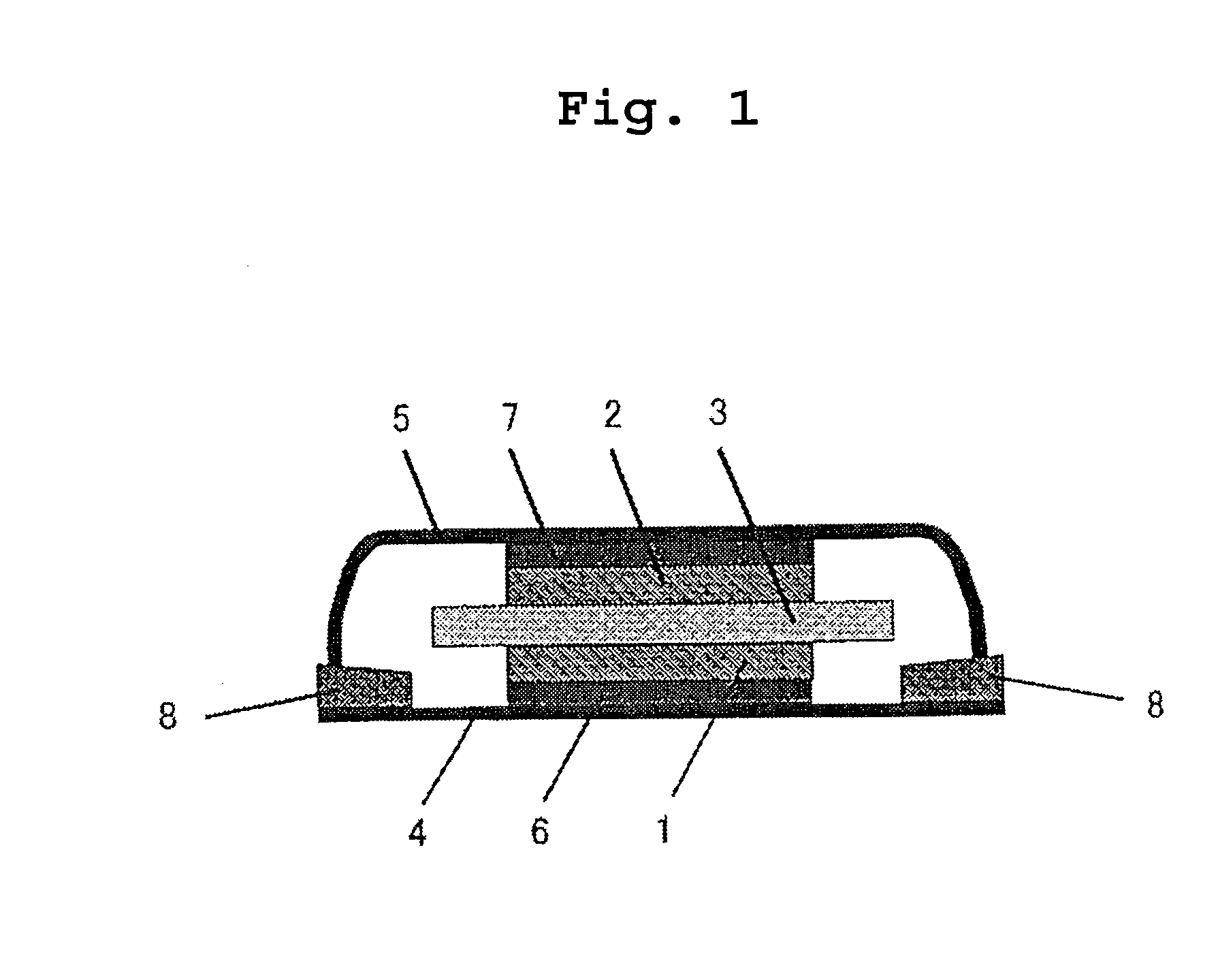
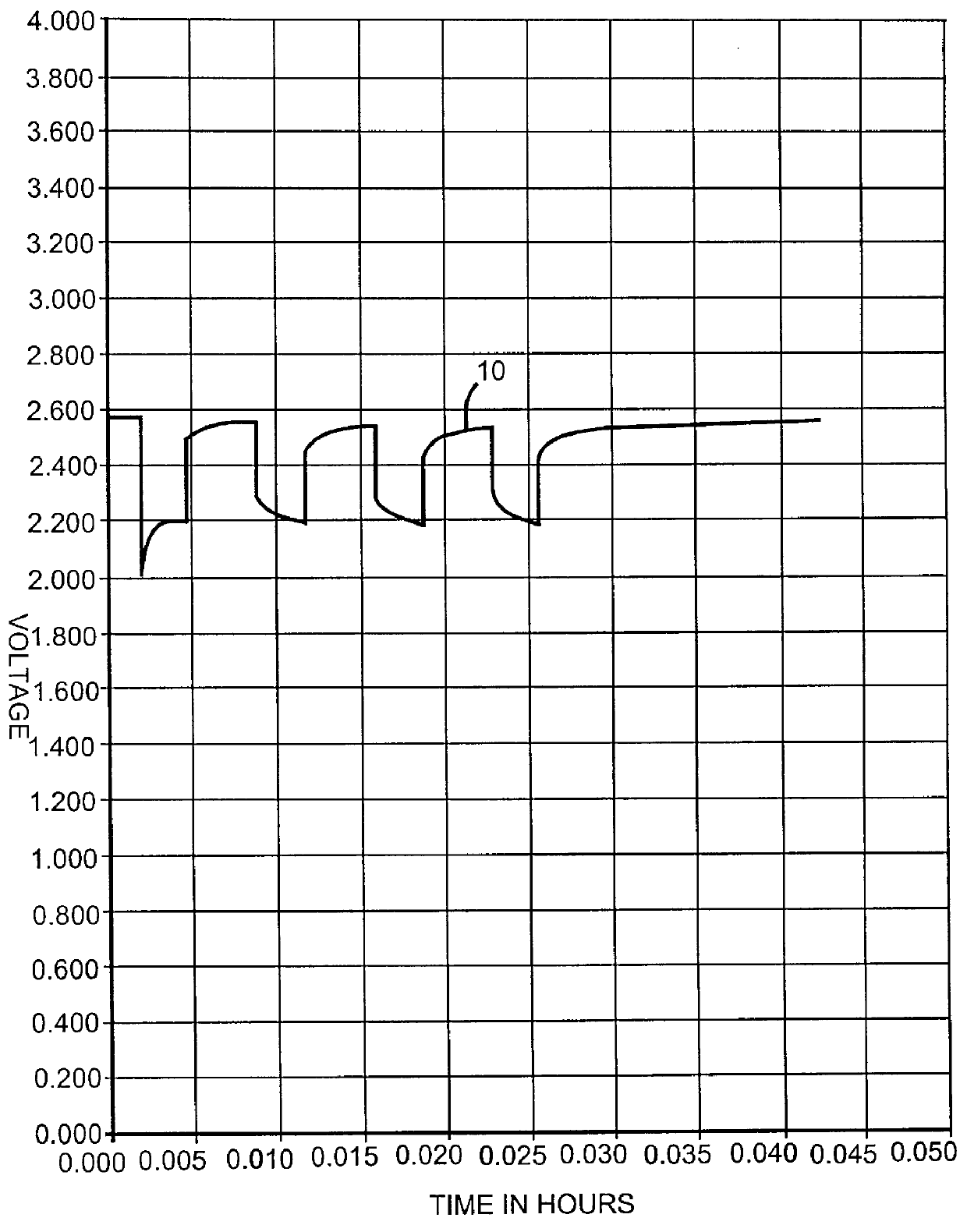
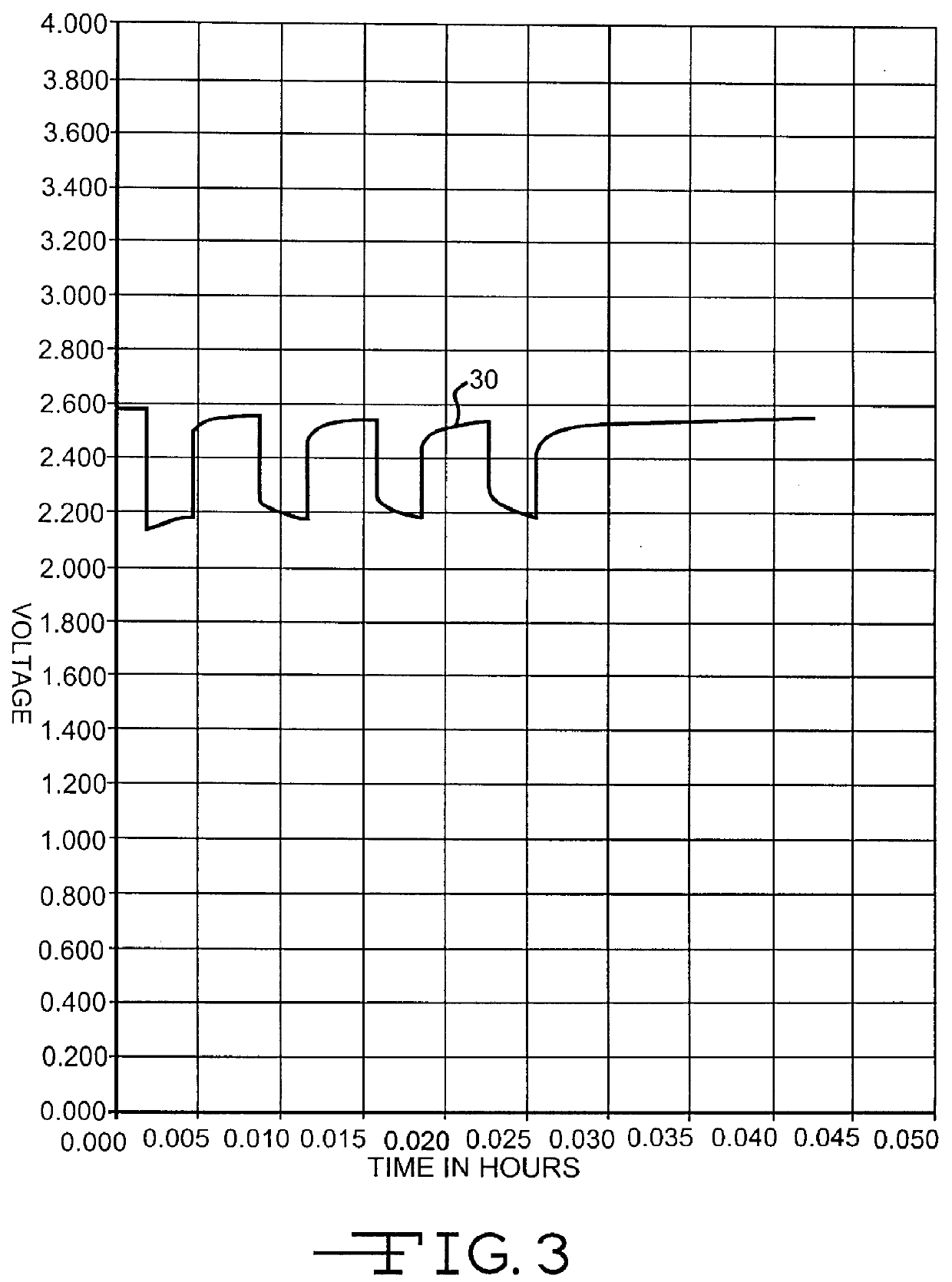
Inorganic and organic nitrate additives for nonaqueous electrolyte in alkali metal electrochemical cells
InactiveUS6060184AGood charge and discharge cycleImprove efficiencyOrganic electrolyte cellsSecondary cells charging/dischargingAlkaline earth metalOrganic nitrates
An alkali metal, solid cathode, nonaqueous electrochemical cell capable of delivering high current pulses, rapidly recovering its open circuit voltage and having high current capacity, is described. The stated benefits are realized by the addition of at least one nitrate additive to an electrolyte comprising an alkali metal salt dissolved in a mixture of a low viscosity solvent and a high permittivity solvent. A preferred solvent mixture includes propylene carbonate, dimethoxyethane and an alkali metal nitrate, alkaline earth metal nitrate and / or an organic alkyl nitrate additive.
Owner:WILSON GREATBATCH LTD
Phosphonate additives for nonaqueous electrolyte in alkali metal electrochemical cells
InactiveUS6096447AGood charge and discharge cycleImprove efficiencyOrganic electrolyte cellsHeart stimulatorsPermittivityPropylene carbonate
An alkali metal, solid cathode, nonaqueous electrochemical cell capable of delivering high current pulses, rapidly recovering its open circuit voltage and having high current capacity, is described. The stated benefits are realized by the addition of at least one phosphonate additive to an electrolyte comprising an alkali metal salt dissolved in a mixture of a low viscosity solvent and a high permittivity solvent. A preferred solvent mixture includes propylene carbonate, dimethoxyethane and an alkyl phosphonate additive.
Owner:WILSON GREATBATCH LTD
Nonaqueous electrolyte secondary battery comprising carbon particles with a plural-layer structure
InactiveUS6403259B1High specific capacityImprove propertiesElectrode thermal treatmentActive material electrodesX-rayGraphite
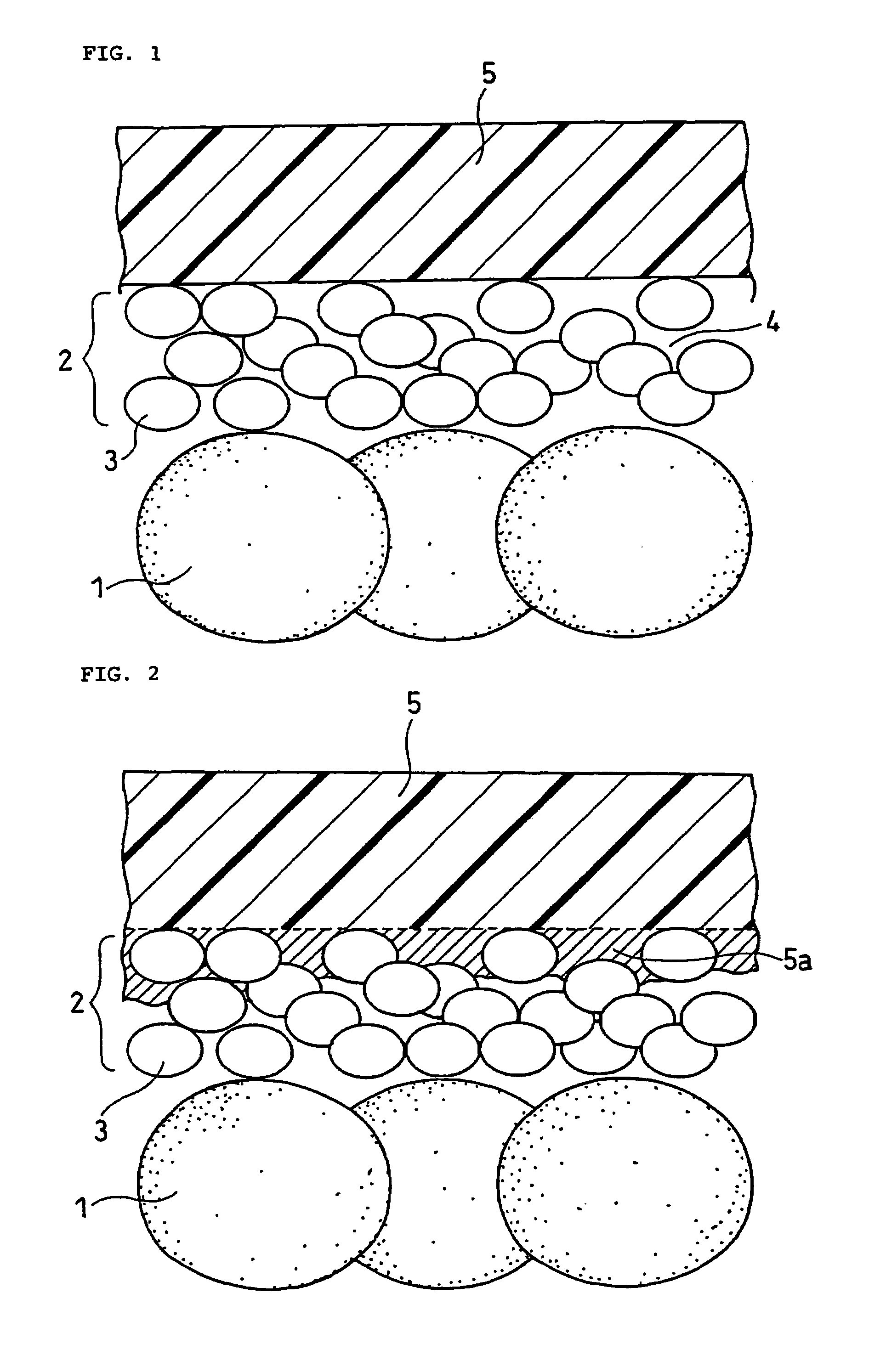
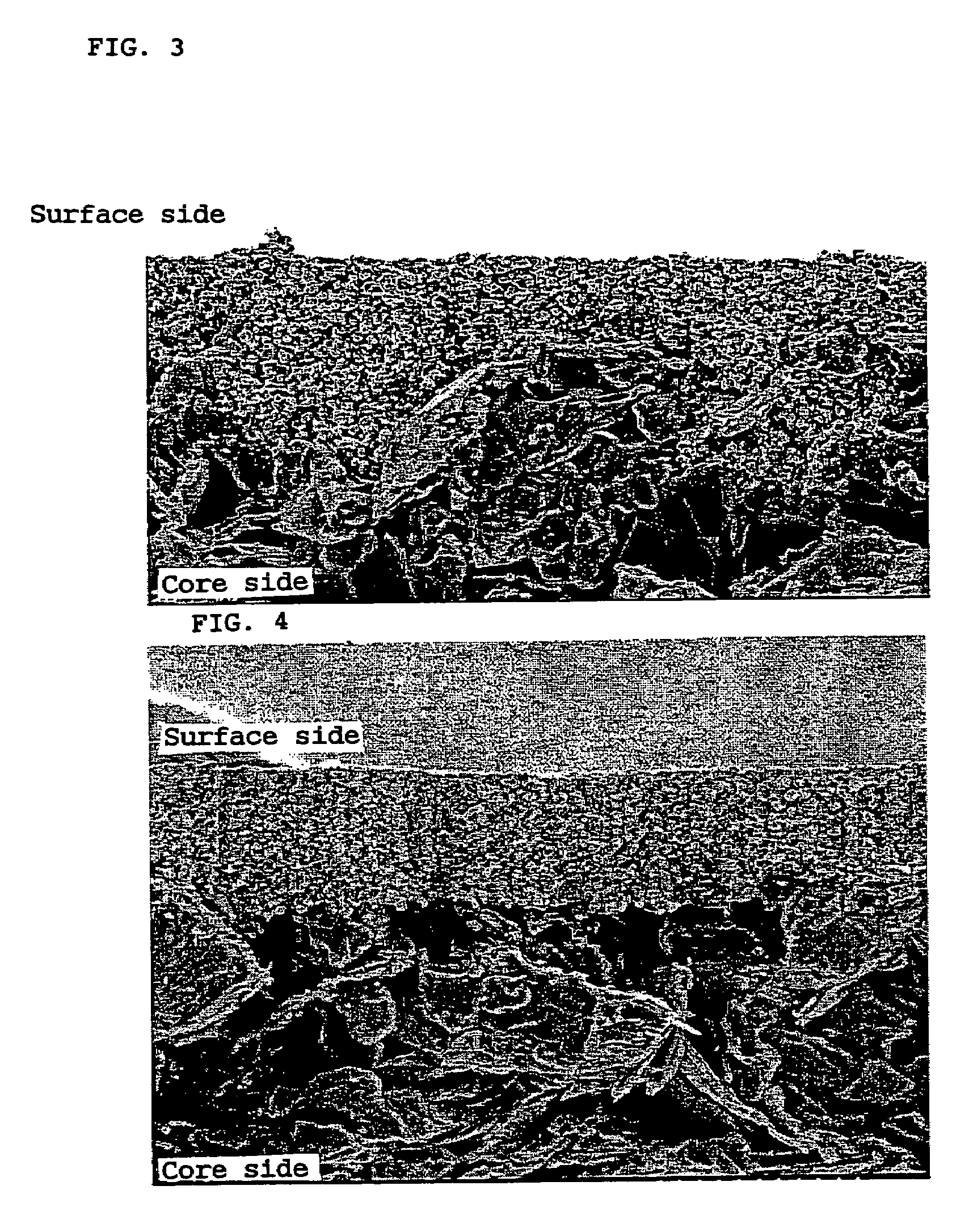
Enhancement of the storage property at a high temperature and discharge characteristics at a low temperature of a nonaqueous electrolyte secondary cell is intended. A negative electrode material which is prepared by covering the surface of a nucleus made of a graphite powder with a carbonaceous matter, the graphite powder having a specified plane interval, spectrum value, mean particle size, specific surface area, tapping density, and (110) / (004) X-ray peak intensity ratio, is used in the nonaqueous electrolyte secondary cell.
Owner:PANASONIC CORP +1
Alkaline battery
InactiveUS6566009B1Good heavy-loading discharge characteristicImprove discharge characteristicsGel electrodesAlkaline accumulator electrodesZinc compoundsTitanium
The present invention provides an alkaline battery having good heavy-loading discharge characteristics even after long-term storage at high temperatures. The alkaline battery of the present invention comprises a positive electrode containing manganese dioxide and nickel oxyhydroxide as an active material, a negative electrode containing zinc as an active material, and an alkaline electrolyte. The positive electrode further contains at least one compound selected from the group consisting of an oxygen-containing zinc compound, an oxygen-containing calcium compound, an oxygen-containing yttrium compound, and an oxygen-containing titanium compound.
Owner:PANASONIC CORP
Nonaqueous electrolyte secondary cell
InactiveUS20020061445A1High specific capacityImprove propertiesElectrode thermal treatmentLayered productsX-rayGraphite
Enhancement of the storage property at a high temperature and discharge characteristics at a low temperature of a nonaqueous electrolyte secondary cell is intended. A negative electrode material which is prepared by covering the surface of a nucleus made of a graphite powder with a carbonaceous matter, the graphite powder having a specified plane interval, spectrum value, mean particle size, specific surface area, tapping density, and (110) / (004) X-ray peak intensity ratio, is used in the nonaqueous electrolyte secondary cell.
Owner:MITSUBISHI CHEM CORP
Carbon powder suitable as a negative electrode material for nonaqueous secondary batteries
InactiveUS20090196816A1Excellent charge and discharge characteristicsImprove discharge performanceFixed capacitor electrodesGraphiteDesorptionNitrogen
Carbon powder having low temperature calcined carbon derived from pitch adhered to a portion of the surface of natural graphite powder is obtained by solids mixing of natural graphite powder and pitch powder as a carbon precursor followed by heat treatment at 900-1500° C. to carbonize the pitch. The amount of pitch powder is such that the ratio V2 / V1 of the pore volume V2 of pores having a diameter of 50-200 nm to the pore volume V1 of pores having a diameter of 2-50 nm in a pore size distribution curve obtained by analysis of the nitrogen desorption isotherm of the resulting carbon powder by the BJH method is at least 1. This carbon powder can be used as a negative electrode material for a nonaqueous secondary battery able to operate at low temperatures.
Owner:NIPPON DENKO CO LTD
Non-aqueous electrochemical apparatus
InactiveUS6958198B2Improve featuresImprove wettabilityNon-aqueous electrolyte accumulatorsFinal product manufactureFree energiesDyne
The invention relates to a non-aqueous electrochemical apparatus in which the difference (γl−γse) between the surface tension γl of non-aqueous electrolyte and the surface free energy γse of electrode is not more than 10 dynes / cm.
Owner:PANASONIC CORP +1
Nonaqueous electrolytic solution and lithium secondary battery
ActiveUS7083878B2Promote formationImprove featuresElectrolytic capacitorsOrganic electrolyte cellsPropylene carbonateSolvent
An object of the invention is to provide such a battery that has a high capacity, is excellent in storage characteristics, cycle characteristics and continuous charging characteristics, and is small in gas generation amount, whereby size reduction and improvement in performance of a lithium secondary battery can be attained.The present invention relates to a nonaqueous electrolytic solution comprising a lithium salt and a nonaqueous solvent dissolving the same, wherein the electrolytic solution contains, as the lithium salt, LiPF6 in a concentration of from 0.2 to 2 mole / L, and LiBF4 and / or a compound represented by the following formula (1) in a molar ratio of from 0.005 to 0.4 with respect to LiPF6, and the nonaqueous solvent mainly comprises (1) ethylene carbonate and / or propylene carbonate, (2-1) a symmetric linear carbonate, (2-2) an asymmetric linear carbonate, and (3) vinylene carbonate.
Owner:MU IONIC SOLUTIONS CORP +1
Nonaqueous electrolyte secondary battery
ActiveUS20060188785A1Prevent deterioration of discharge characteristicImprove securityFinal product manufactureNon-aqueous electrolyte accumulator electrodesPorosityInorganic oxide
A non-aqueous electrolyte secondary battery including: a positive electrode; a negative electrode; a separator interposed between the positive electrode and the negative electrode; a non-aqueous electrolyte; and a porous insulating film adhered to a surface of at least one selected from the group consisting of the positive electrode and the negative electrode, the porous insulating film including an inorganic oxide filler and a film binder, wherein the ratio R of actual volume to apparent volume of the separator is not less than 0.4 and not greater than 0.7, and wherein the ratio R and a porosity P of the porous insulating film satisfy the relational formula: −0.10≦R−P≦0.30.
Owner:PANASONIC CORP
Negative electrode for lithium secondary battery and lithium secondary battery
ActiveUS20030235762A1Inhibit deteriorationImprove discharge characteristicsElectrode thermal treatmentElectrode carriers/collectorsMean diameterLithium
A negative electrode for a lithium secondary battery obtained by providing an active material layer containing particles of an active material and a binder on a surface of a current collector which is an electrically conductive metal foil, and sintering the layer under a non-oxidizing atmosphere; wherein the mean diameter of the particles of the active material is not smaller than 1 mum and not greater than 10 mum, and the particle size distribution of the particles is such that at least 60 volume % of the particles are in a range of not smaller than 1 mum and not greater than 10 mum.
Owner:PANASONIC ENERGY CO LTD
Organic nitrite additives for nonaqueous electrolyte in alkali metal electrochemical cells
InactiveUS6027827AGood charge and discharge cycleImprove efficiencyElectrotherapyPrimary cell maintainance/servicingPermittivitySolvent
Owner:WILSON GREATBATCH LTD
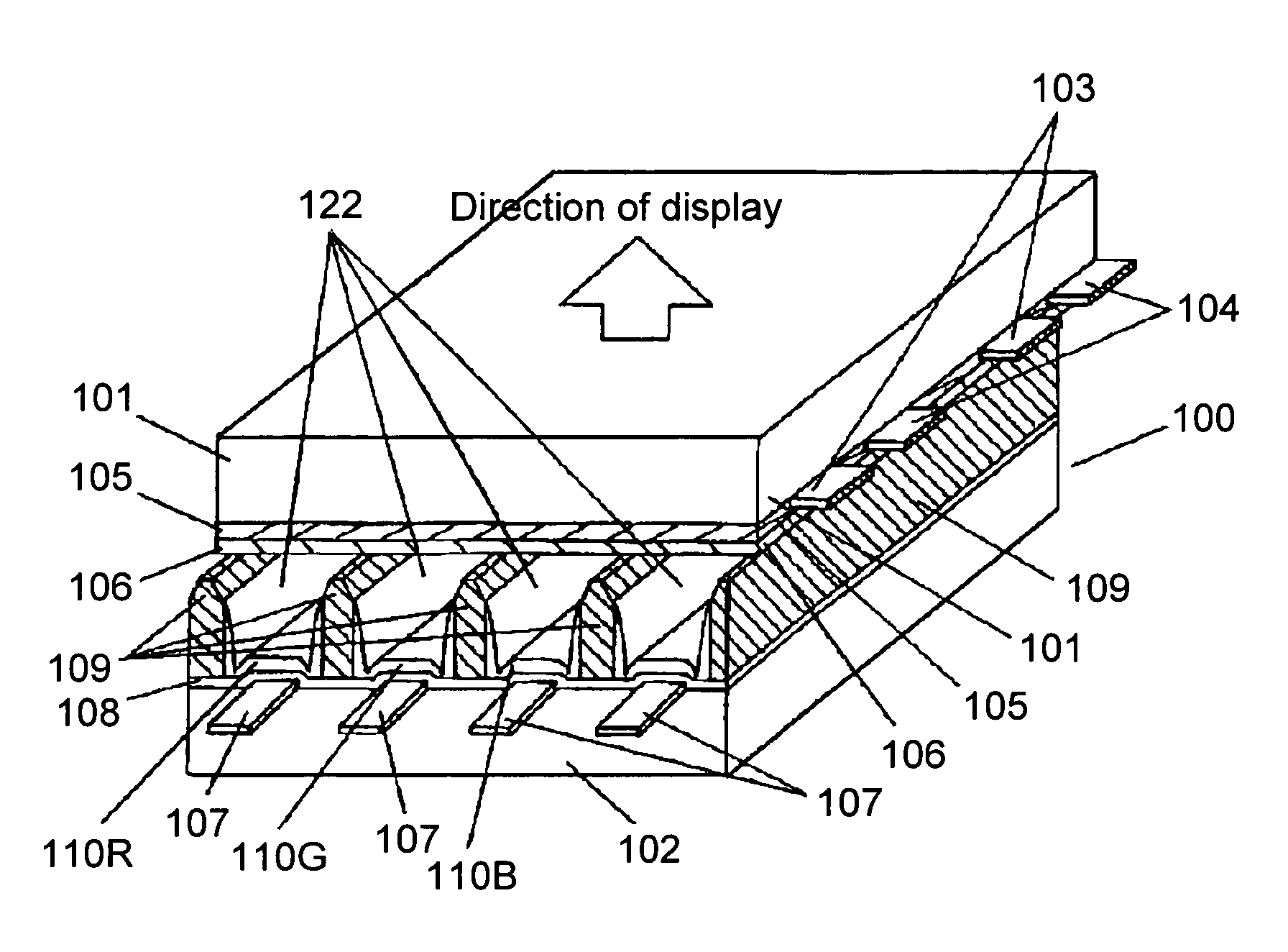
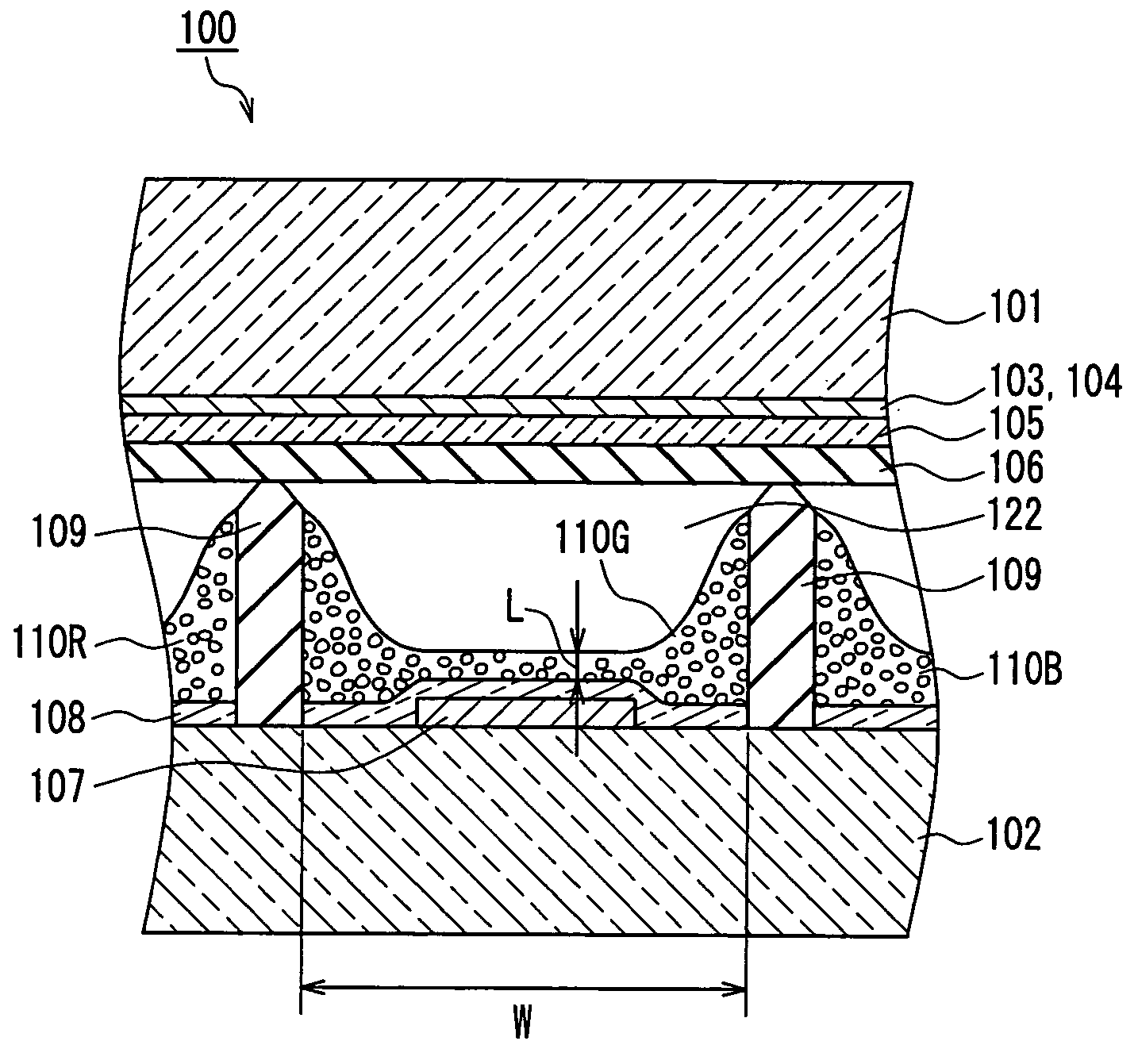
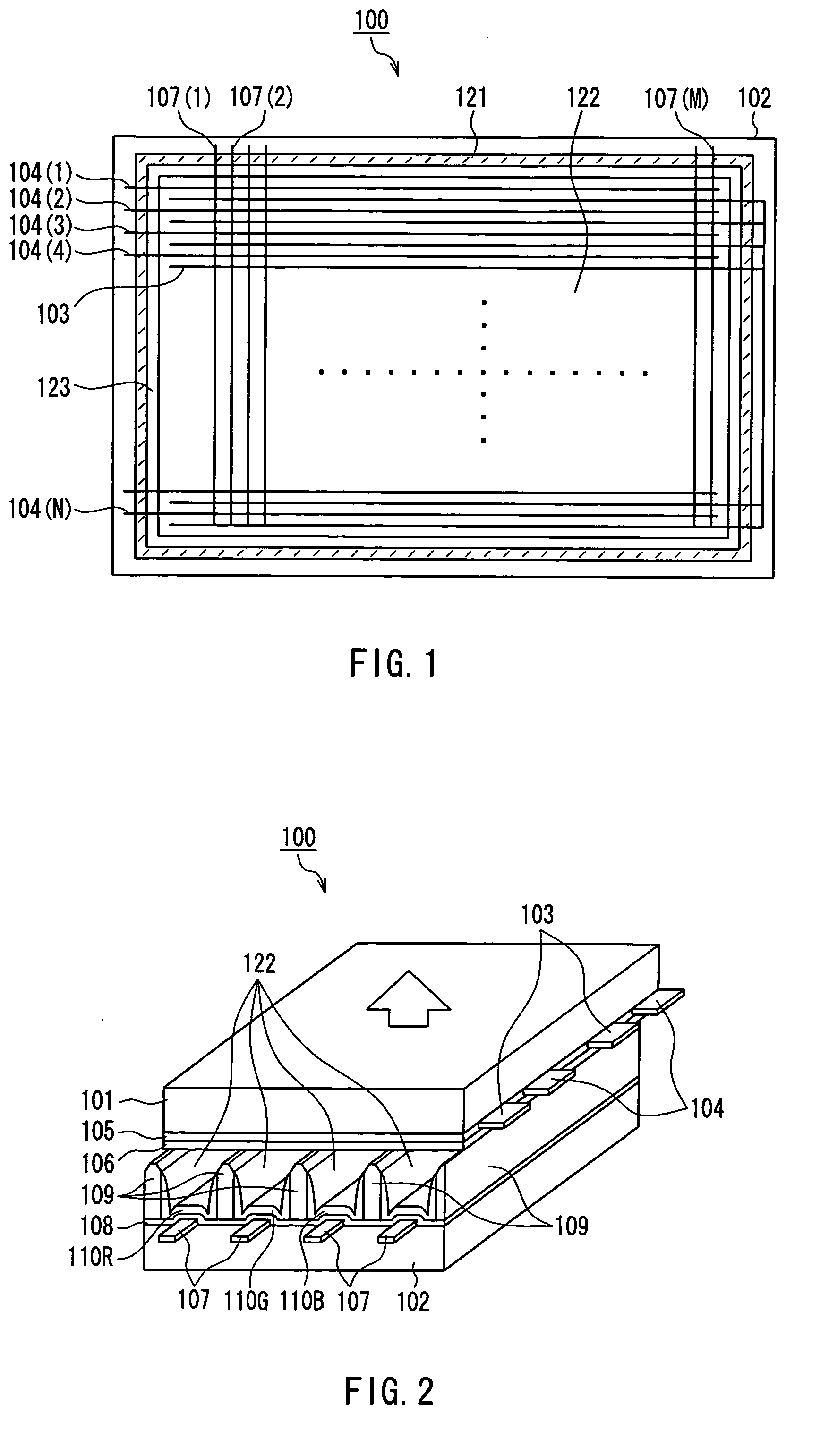
Plasma display unit, phosphor and process for producing phosphor
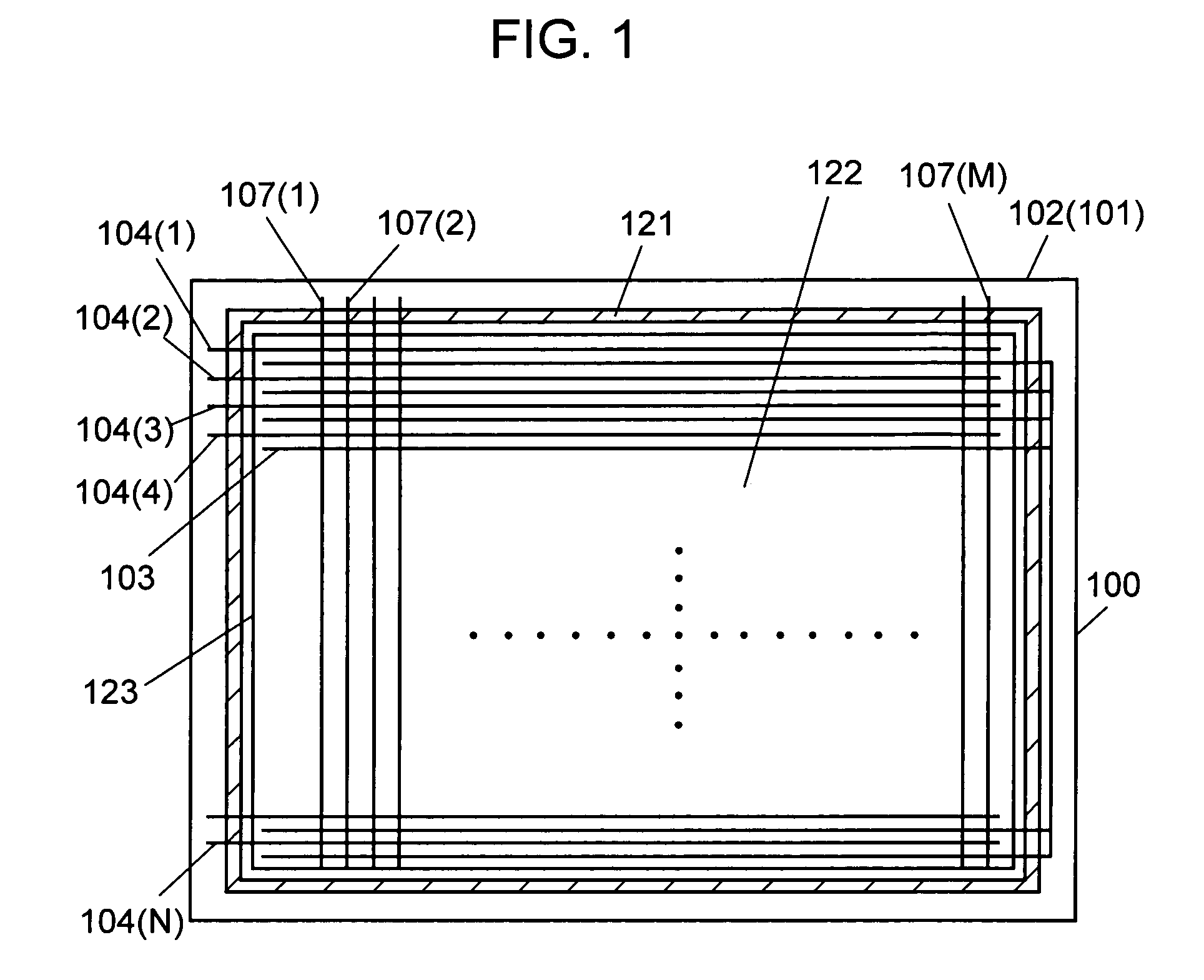
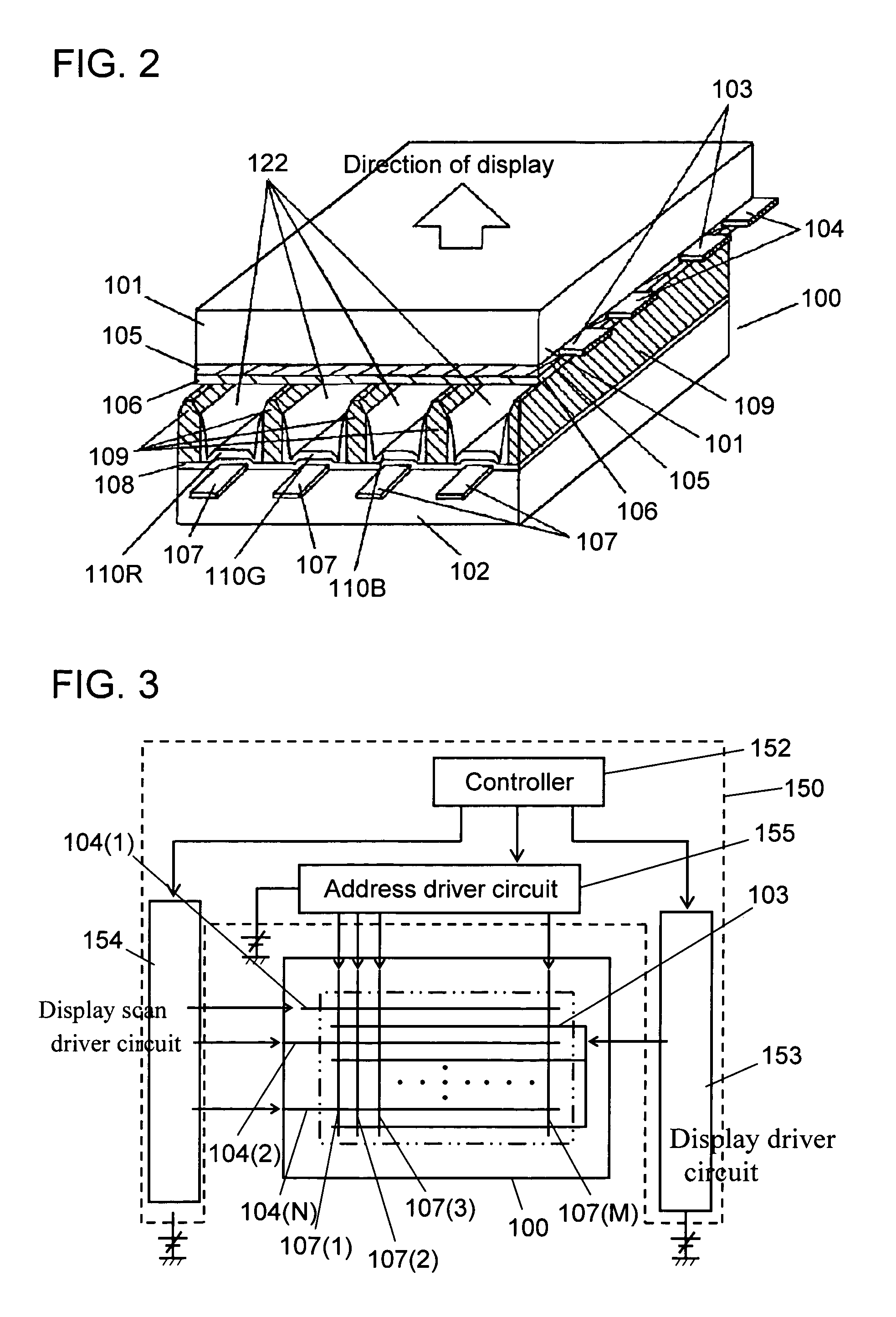
InactiveUS7208102B2Inhibition of adsorptionDecrease in luminanceAddress electrodesSustain/scan electrodesPhosphorFluorescence
A plasma display device exhibits suppressed luminance degradation of a phosphor, a suppressed change in chromaticity and improved discharge characteristics as a result of suppression of adsorption of water or hydrocarbon-containing gas on a surface of a blue phosphor. A blue phosphor layer used in the plasma display device is formed of a compound expressed by Ba1−XMgAl10O17:EuX or Ba1−x−ySryMgAl10O17:EuX and includes at least one element that is selected from Nb, Ta, Pr, P, As, Sb, Bi and Tm which substitutes for a part of its Al or Mg element.
Owner:PANASONIC CORP
Nonaqueous electrolyte and nonaqueous-electrolyte battery
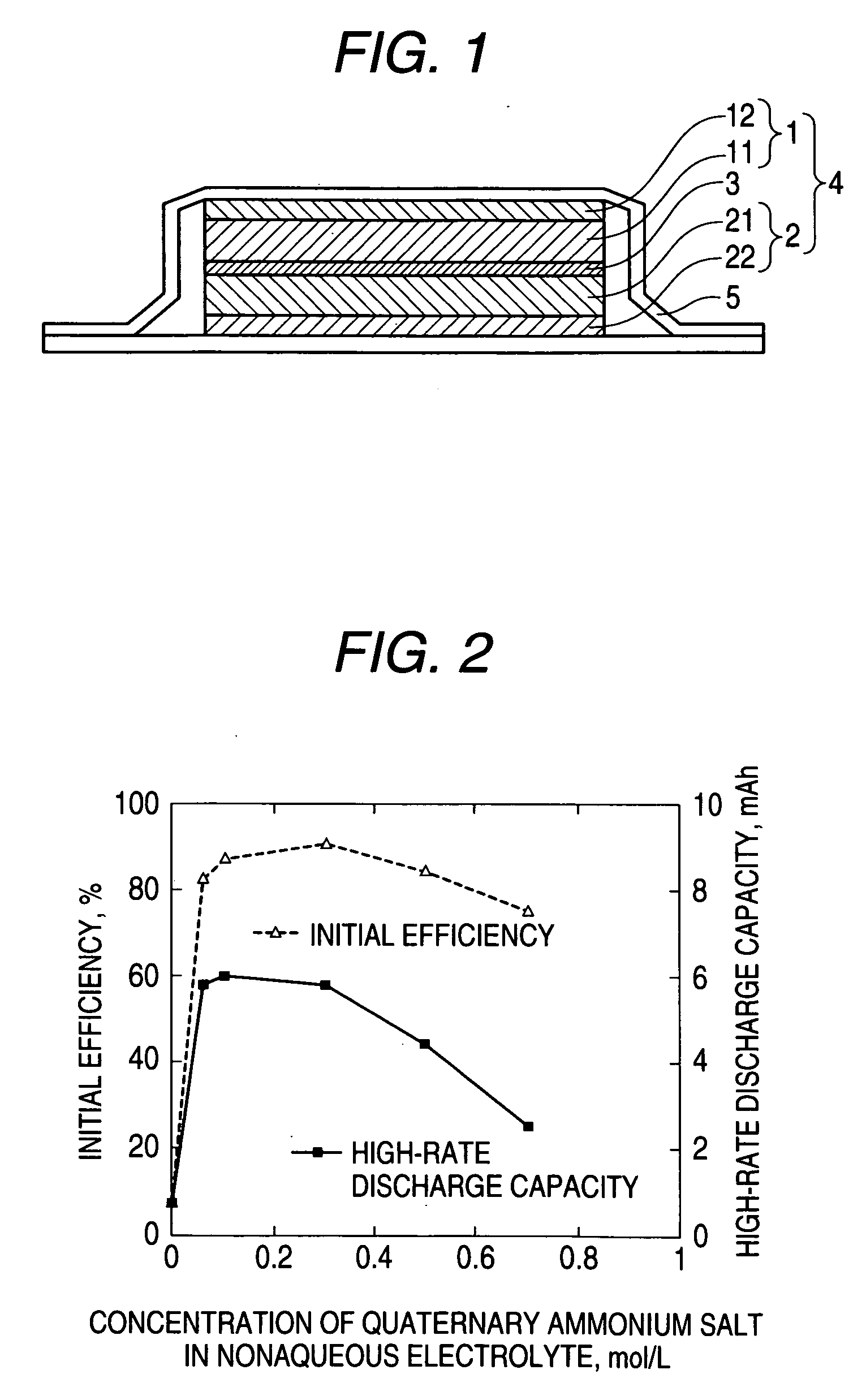
ActiveUS20060068296A1Avoid decompositionHigh rate discharge characteristicsHybrid capacitor electrolytesCell electrodesElectrode potentialDischarge efficiency
The object is to provide a nonaqueous-electrolyte battery having high charge / discharge efficiency and excellent high-rate performance. This subject is accomplished by using a nonaqueous electrolyte which comprises an organic solvent and a lithium salt dissolved therein and is characterized by containing at least one quaternary ammonium salt in an amount of 0.06 mol / L or larger and 0.5 mol / L or smaller. This effect is thought to be attributable to the following mechanism: in a relatively early stage (stage in which the negative-electrode potential is relatively noble) in a first charge step, a satisfactory protective coating film is formed on the negative electrode by the action of the quaternary ammonium salt and, hence, the organic solvent employed in the nonaqueous electrolyte is inhibited from decomposing.
Owner:GS YUASA INT LTD
Secondary battery of improved lithium ion mobility and cell capacity
ActiveUS20060204845A1Improve discharge characteristicsIncrease battery capacityPositive electrodesBedsLithiumHigh rate
Provided is a lithium secondary battery having improved discharge characteristics in a range of high-rate discharge while minimizing a dead volume and at the same time, having increased cell capacity via increased electrode density and electrode loading amounts, by inclusion of two or more active materials having different redox levels so as to exert superior discharge characteristics in the range of high-rate discharge via sequential action of cathode active materials in a discharge process, and preferably having different particle diameters.
Owner:LG ENERGY SOLUTION LTD
Nonaqueous electrolyte secondary battery
ActiveUS7422825B2Prevent deterioration of discharge characteristicImprove securityFinal product manufactureSolid electrolyte cellsPorosityInorganic oxide
A non-aqueous electrolyte secondary battery including: a positive electrode; a negative electrode; a separator interposed between the positive electrode and the negative electrode; a non-aqueous electrolyte; and a porous insulating film adhered to a surface of at least one selected from the group consisting of the positive electrode and the negative electrode, the porous insulating film including an inorganic oxide filler and a film binder, wherein the ratio R of actual volume to apparent volume of the separator is not less than 0.4 and not greater than 0.7, and wherein the ratio R and a porosity P of the porous insulating film satisfy the relational formula: −0.10≦R−P≦0.30.
Owner:PANASONIC CORP
Anode active material, method of preparing the same, and anode and lithium battery containing the material
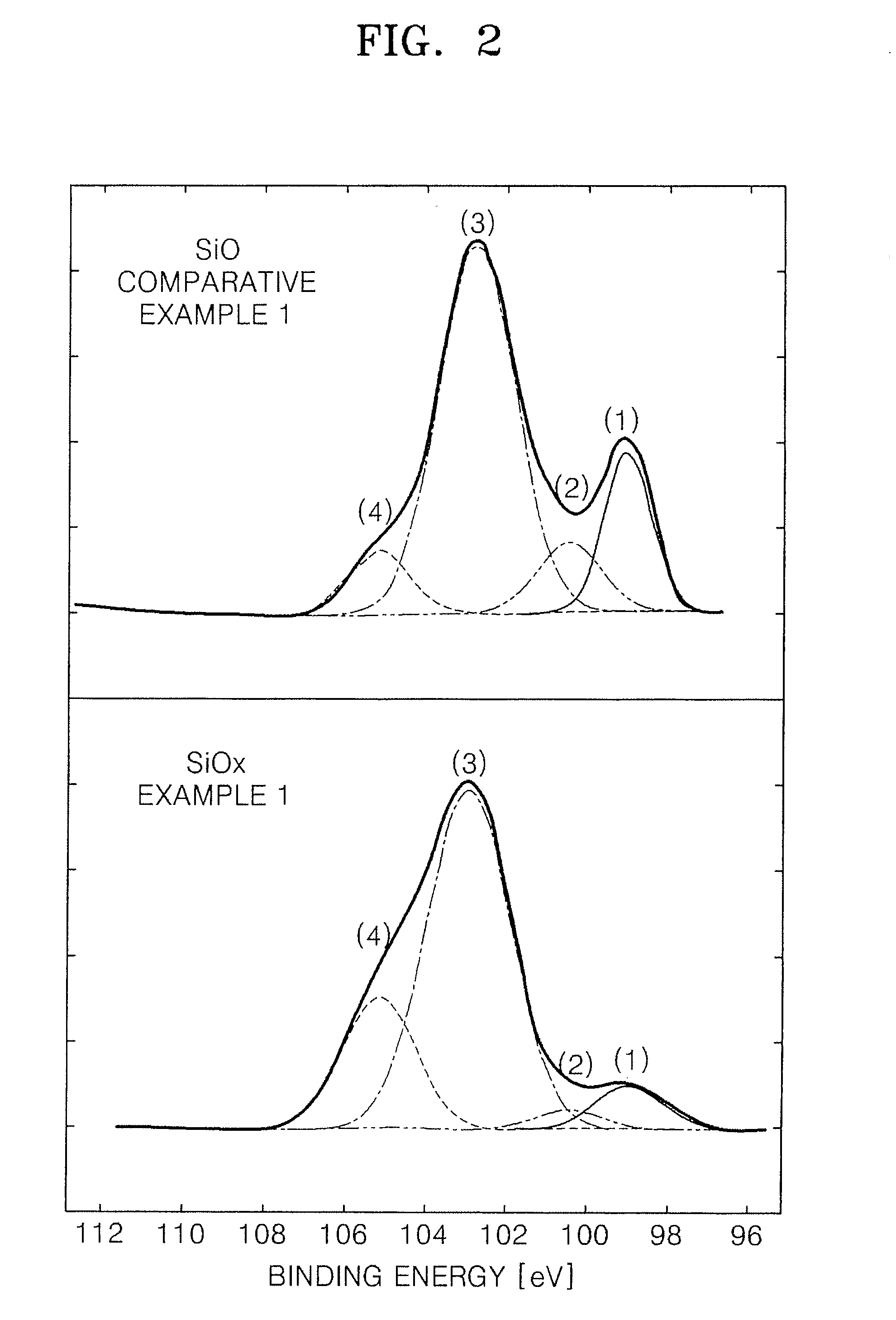
ActiveUS20080166634A1Improving initial chargeImprove discharge efficiencyBio-organic fraction processingSilicaFull width at half maximumX-ray
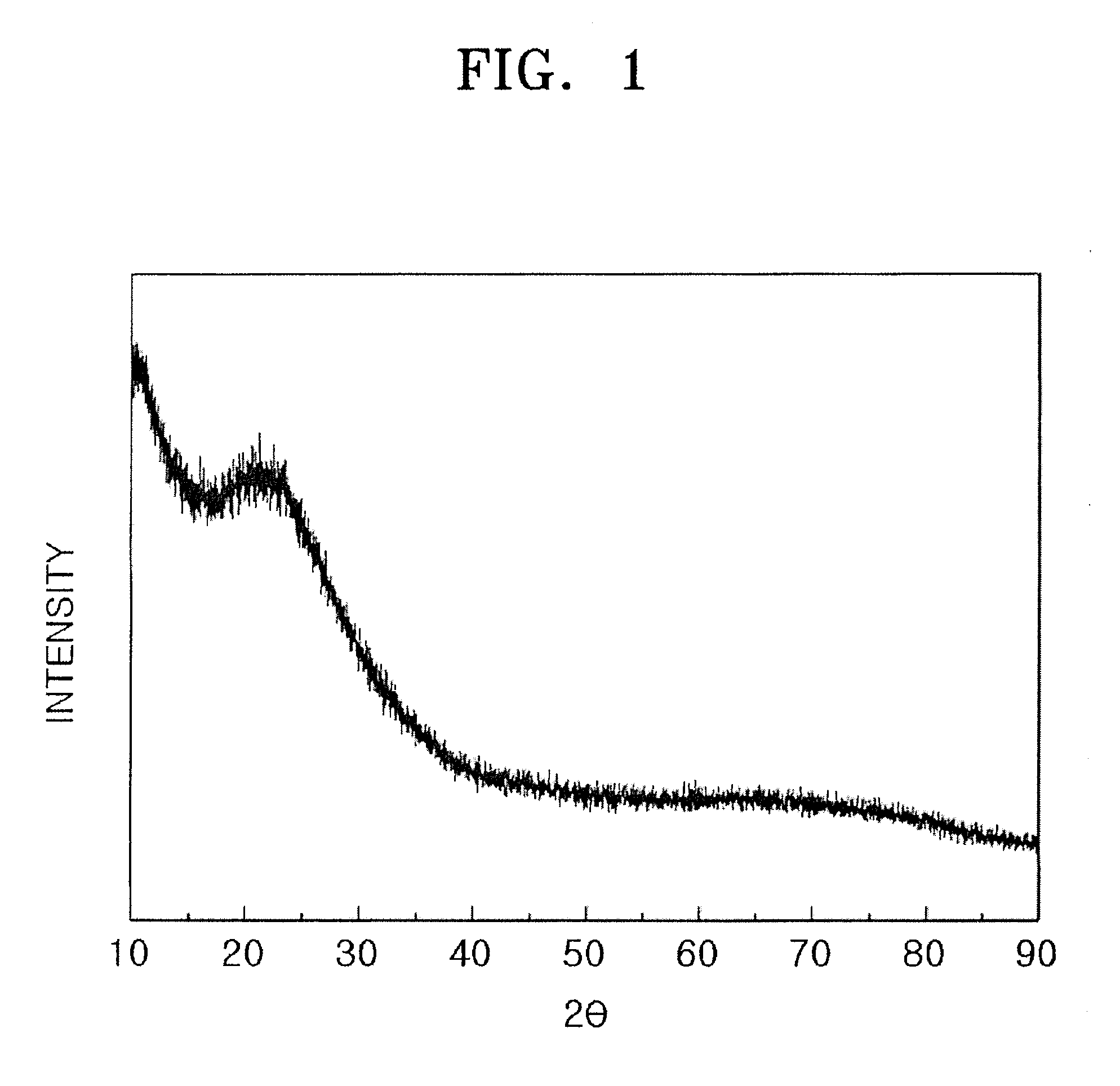
Silicon oxide based composite anode active materials including amorphous silicon oxides are provided. In one embodiment, the amorphous silicon oxide is represented by SiOx (where 0<x<2), has a binding energy of about 103 to about 106 eV, a silicon peak with a full width at half maximum (FWHM) ranging from about 1.6 to about 2.4 as measured by X-ray photoelectron spectrometry, and an atomic percentage of silicon greater than or equal to about 10 as calculated from an area of the silicon peak. The anode active material is a composite anode active material obtained by sintering hydrogen silsesquioxane (HSQ). Anodes and lithium batteries including the anode active material exhibit improved charge and discharge characteristics.
Owner:SAMSUNG SDI CO LTD
Gas discharge tube and method for forming electron emission layer in gas discharge tube
InactiveUS6932664B2Reduce unevennessImprove discharge characteristicsTube/lamp screens manufactureGas-filled discharge tubesEngineeringElectron
Owner:SHINODA PLASMA
All-solid-state lithium-ion secondary battery and production method thereof
InactiveUS20080241665A1Increase the effective surface areaImprove discharge characteristicsPrimary cell to battery groupingFinal product manufactureAll solid stateMaterials science
An all-solid-state lithium-ion secondary battery has an anode, a cathode, a solid electrolyte layer disposed between the anode and the cathode, and at least one of a first mixed region formed at an interface between the anode and the solid electrolyte layer and containing a constituent material of the anode and a constituent material of the solid electrolyte layer, and a second mixed region formed at an interface between the cathode and the solid electrolyte layer and containing a constituent material of the cathode and a constituent material of the solid electrolyte layer.
Owner:TDK CORPARATION
Lithium ion secondary battery
ActiveUS20060134526A1Easily meltedEasily contractedNon-aqueous electrolyte accumulatorsCell seperators/membranes/diaphragms/spacersLithiumPorous membrane
A lithium ion secondary battery is provided. The lithium ion secondary battery generally comprises an electrode assembly, a container for accommodating the electrode assembly; and an electrolyte. The electrode assembly comprises two electrodes having opposite polarities and a separator. The separator comprises a porous membrane comprising clusters of ceramic particles. The porous membrane is formed by bonding the particle clusters with a binder. Each particle cluster is formed either by sintering or by dissolving and re-crystallizing all or a portion of the ceramic particles. The ceramic particles comprise a ceramic material having a band gap. Each particle cluster may have the shape of a grape bunch or a lamina, and may be formed by laminating scale or flake shaped ceramic particles.
Owner:SAMSUNG SDI CO LTD
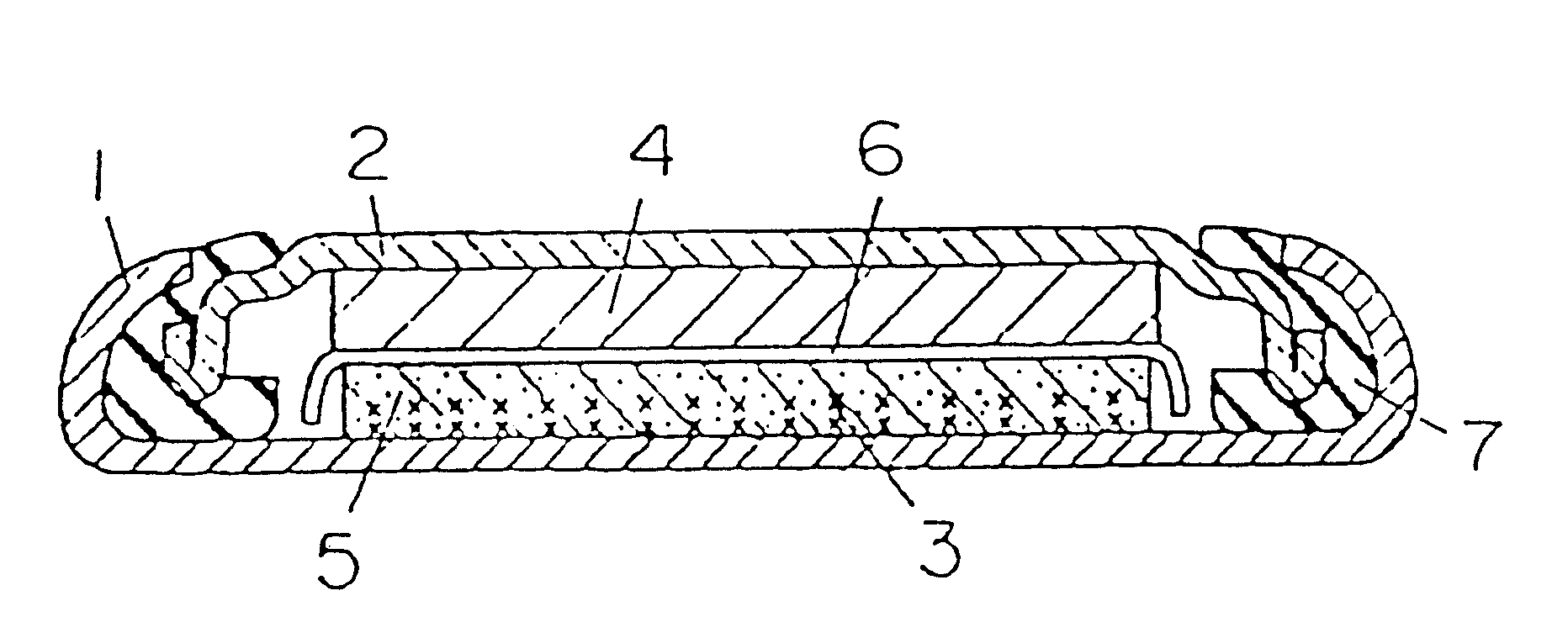
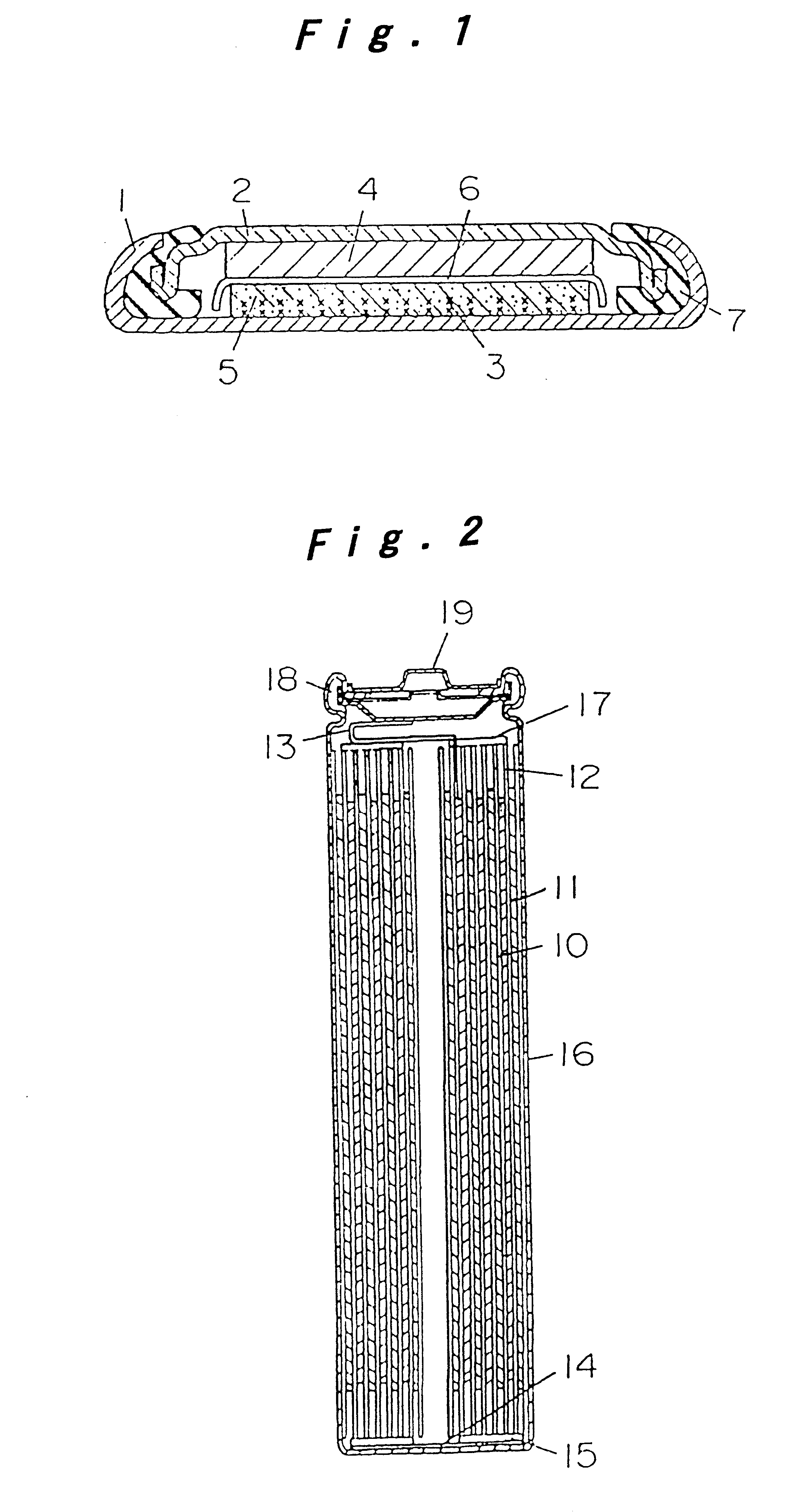
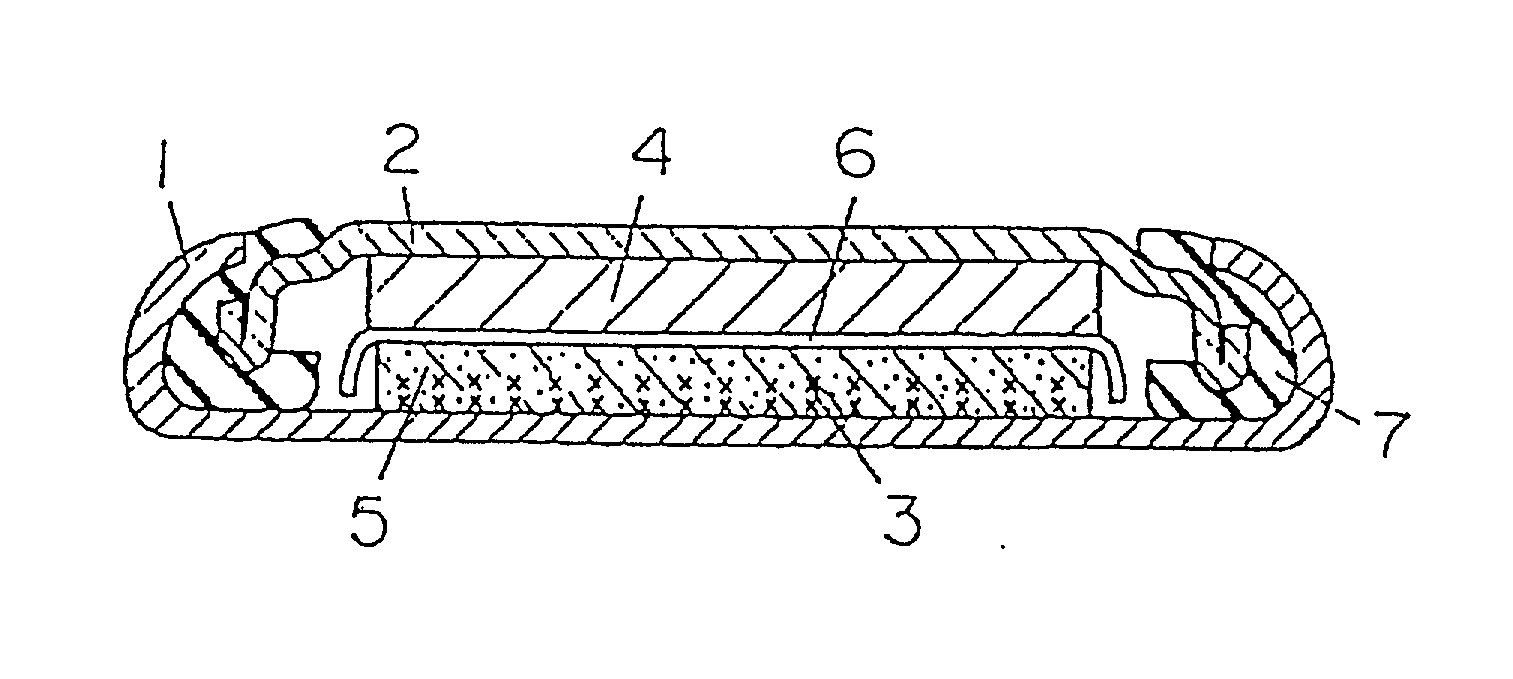
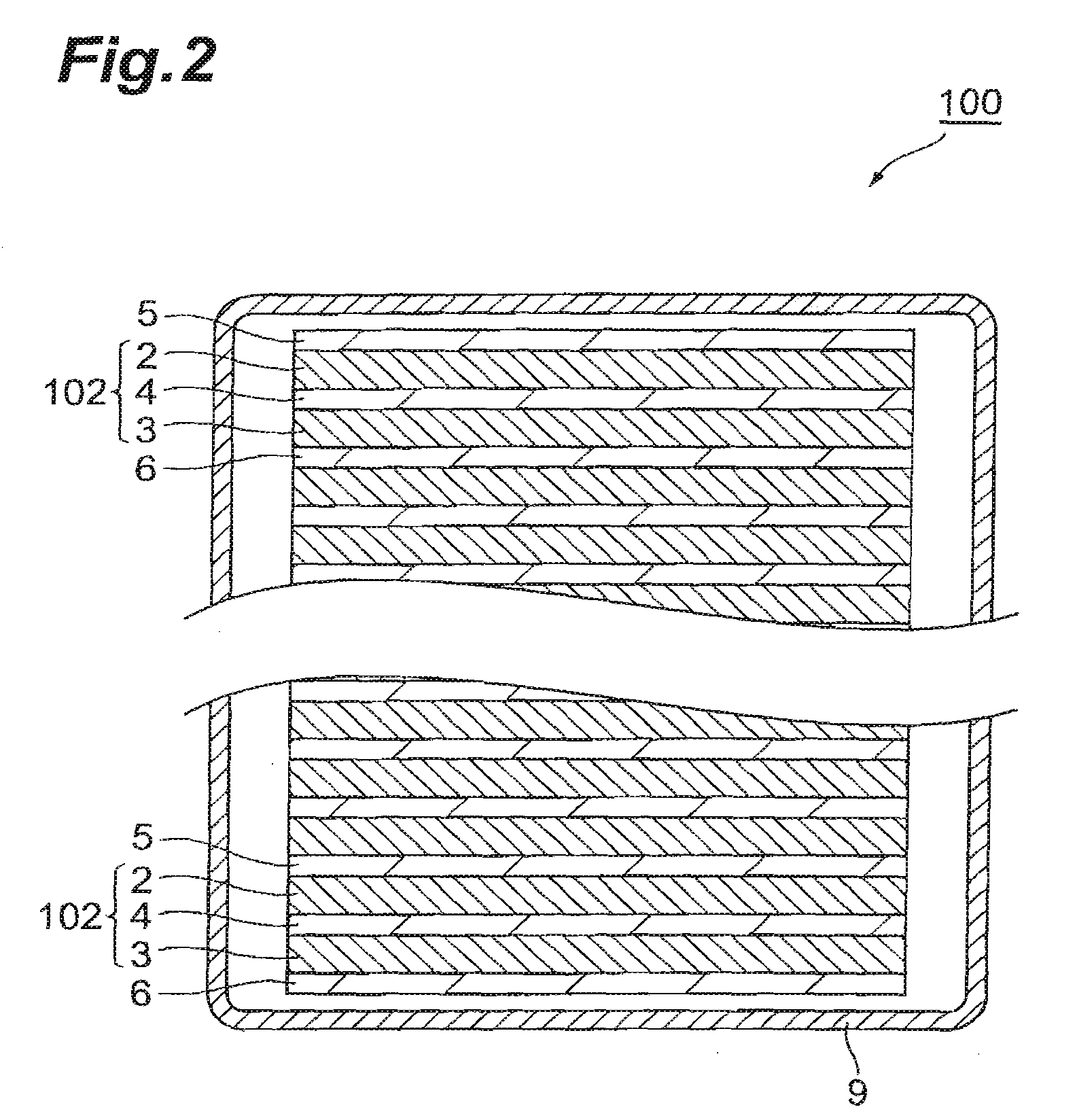
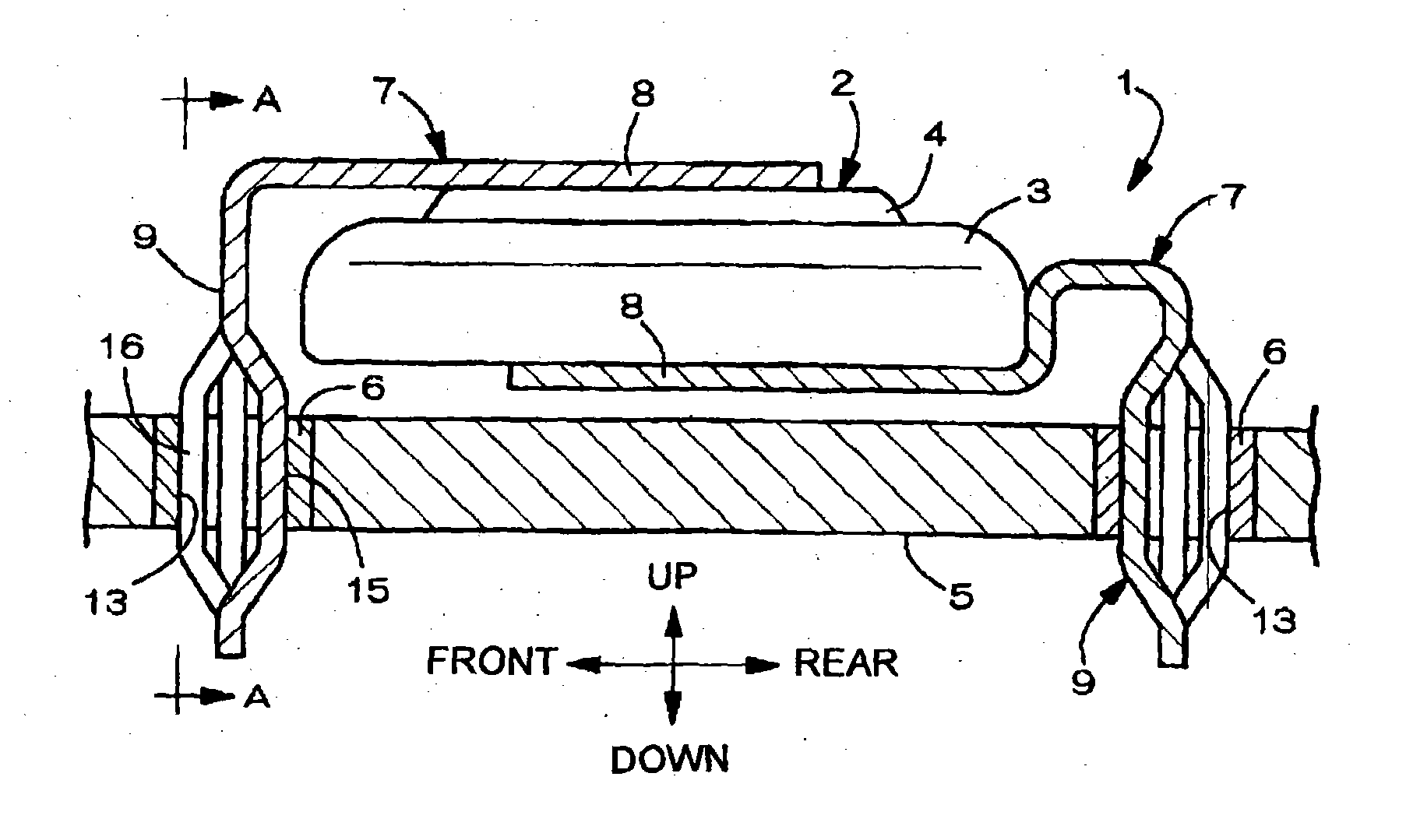
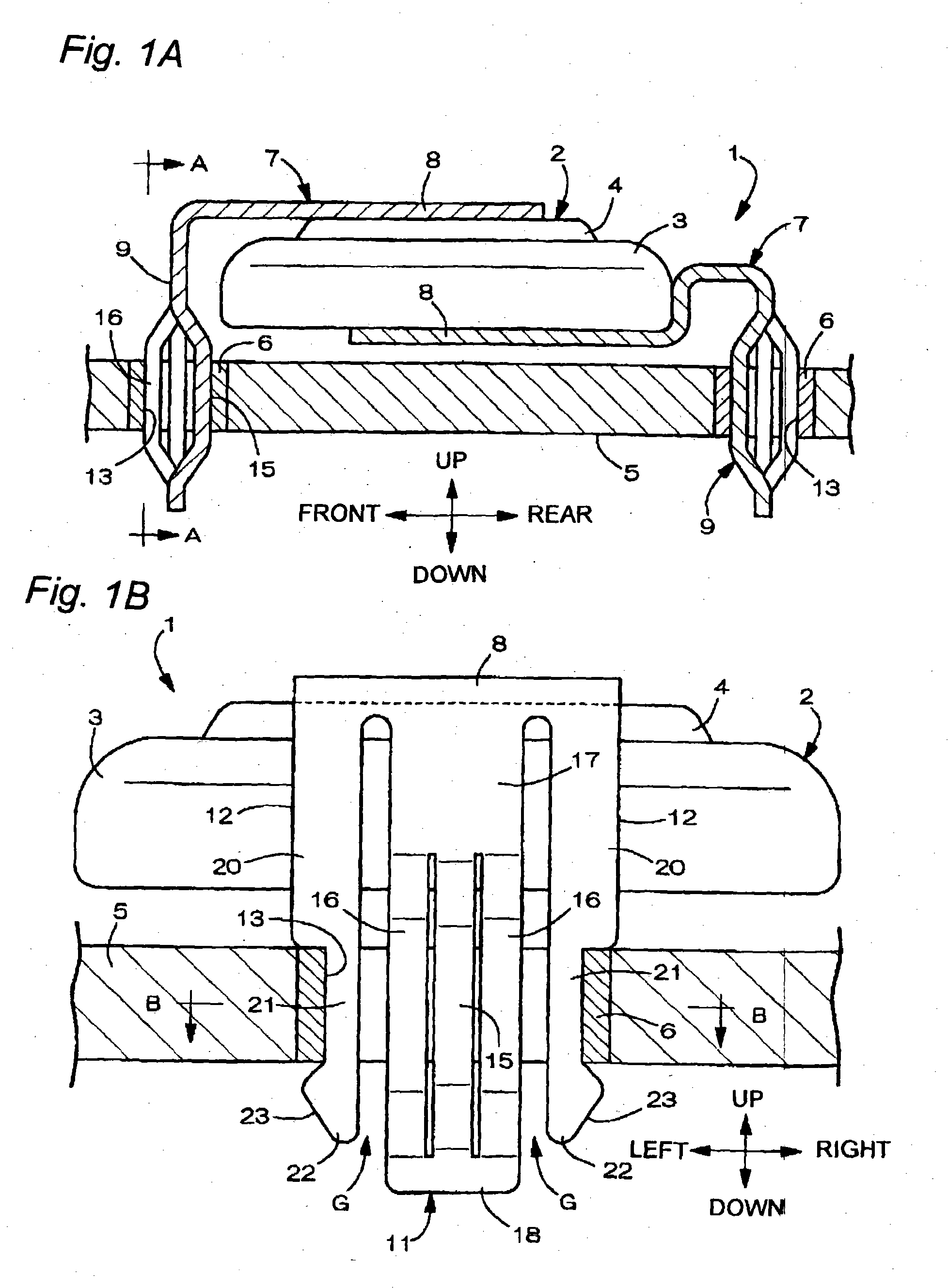
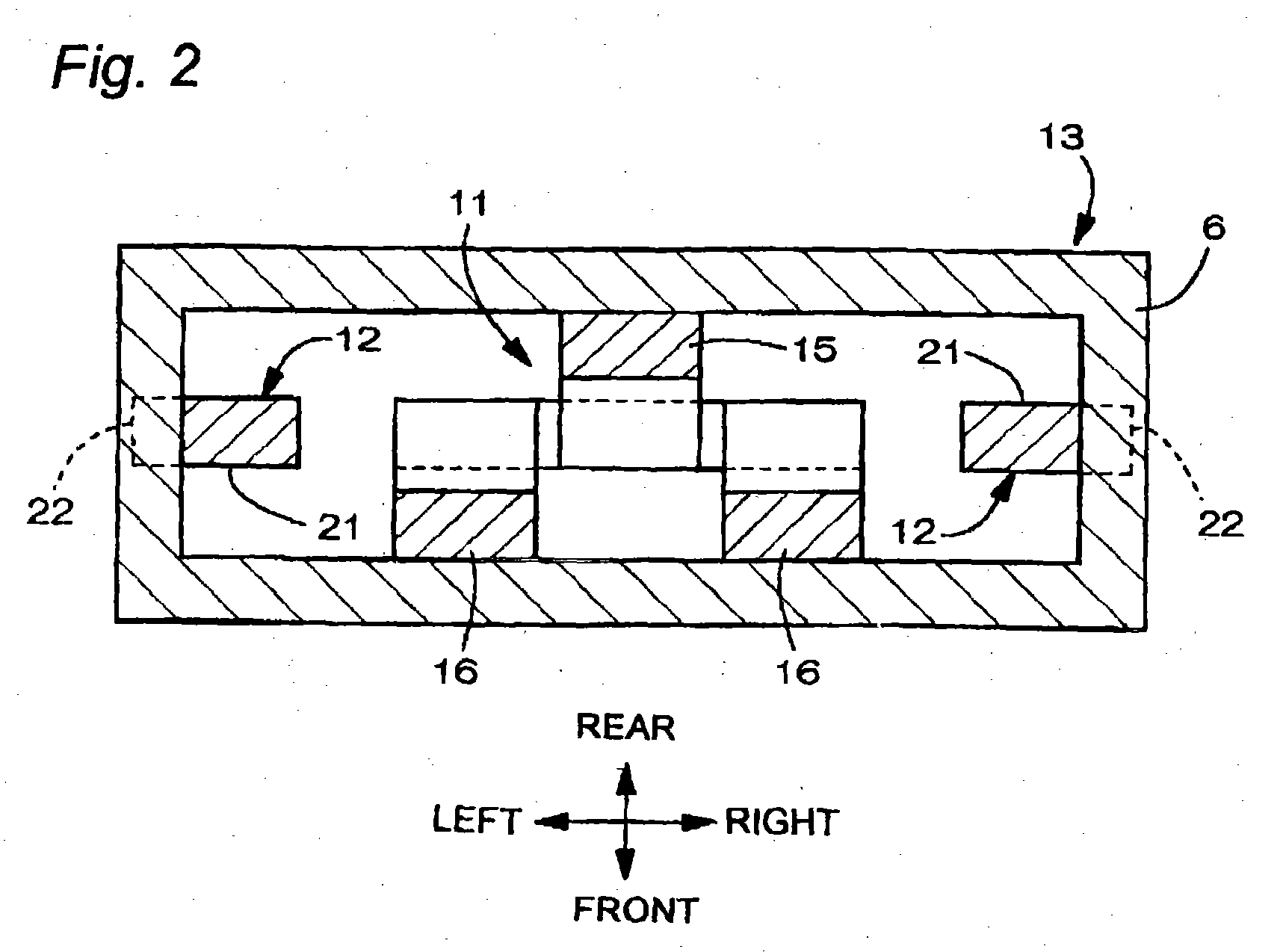
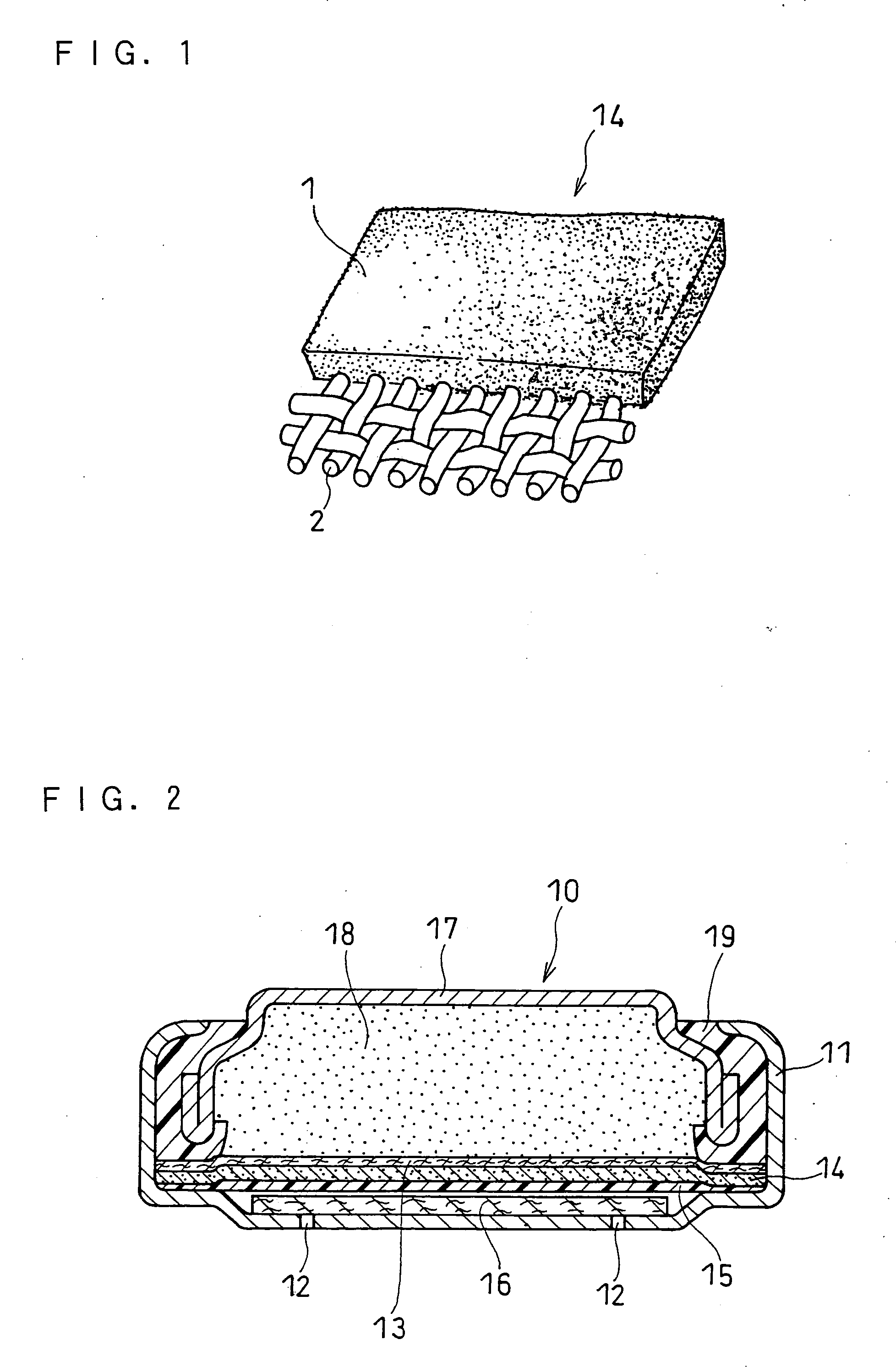
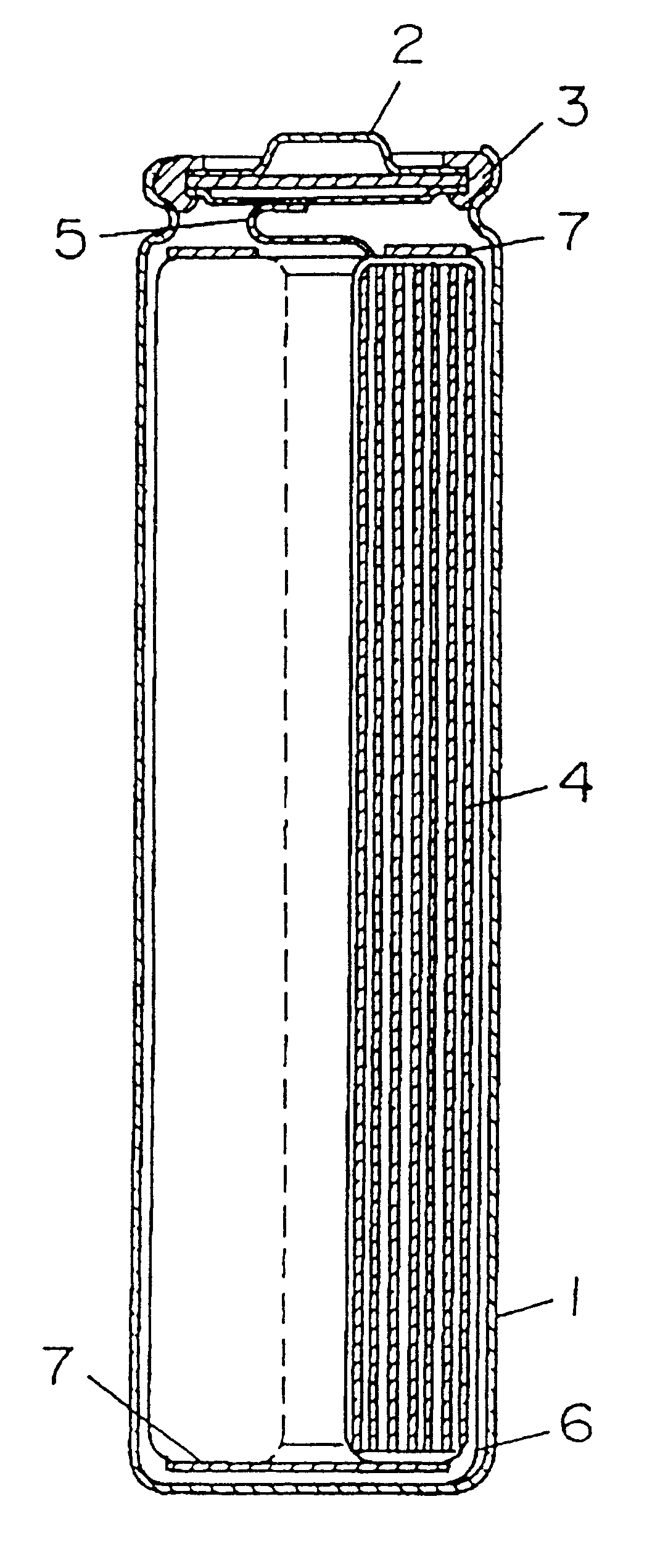
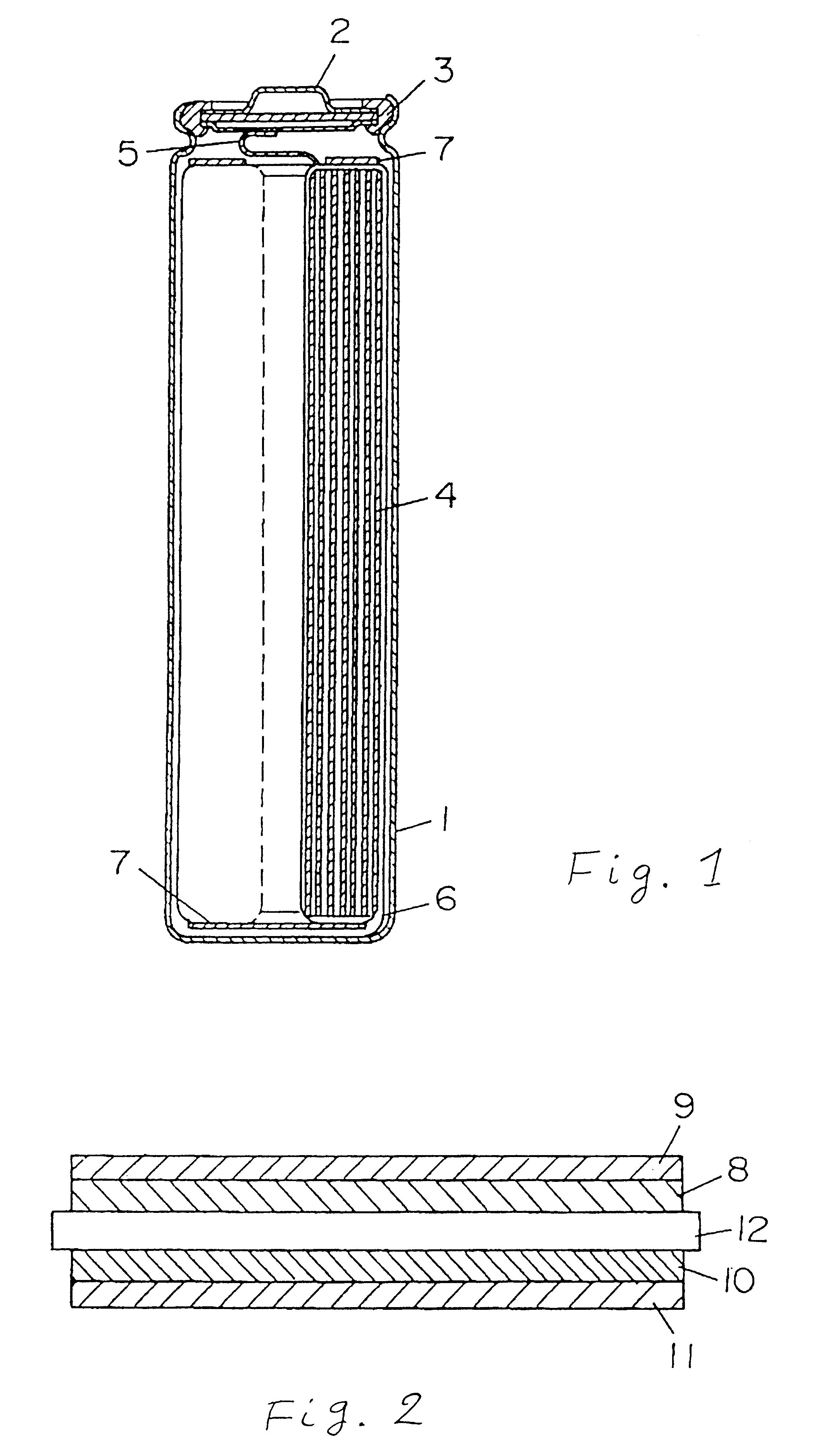
Battery provided with terminals
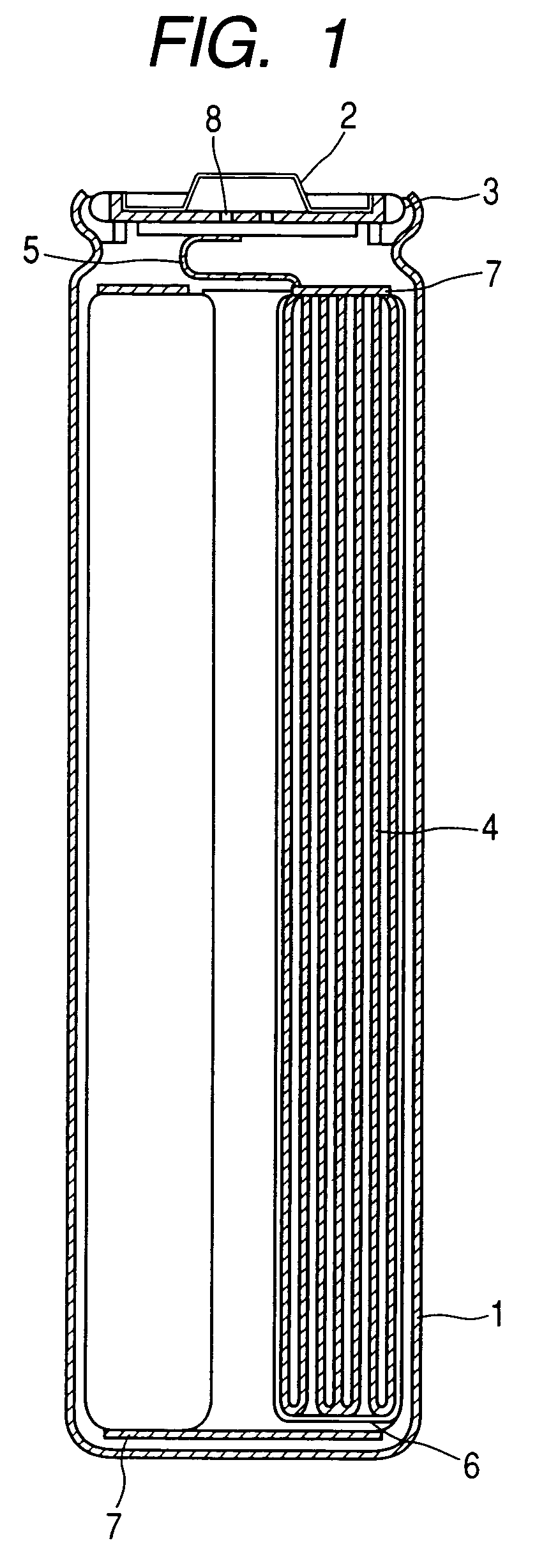
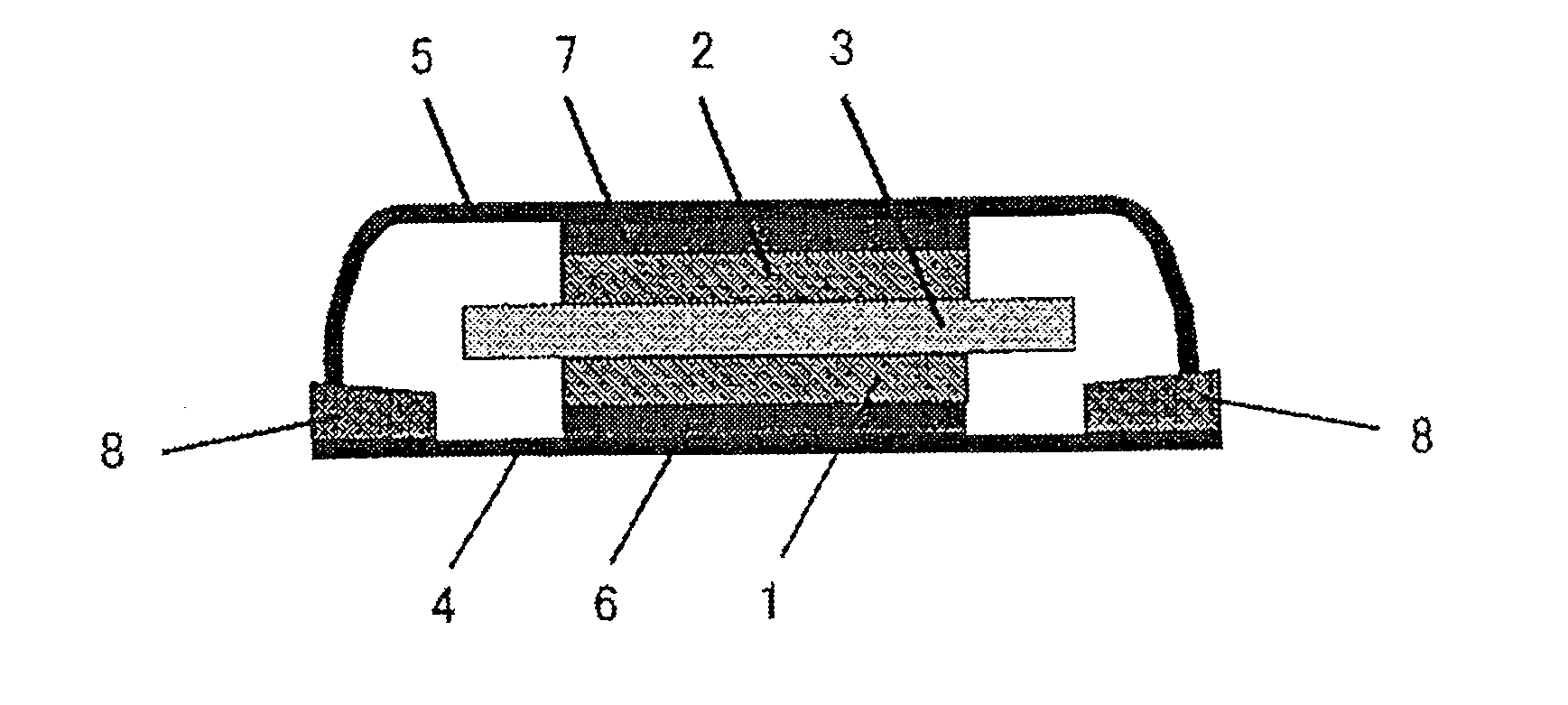
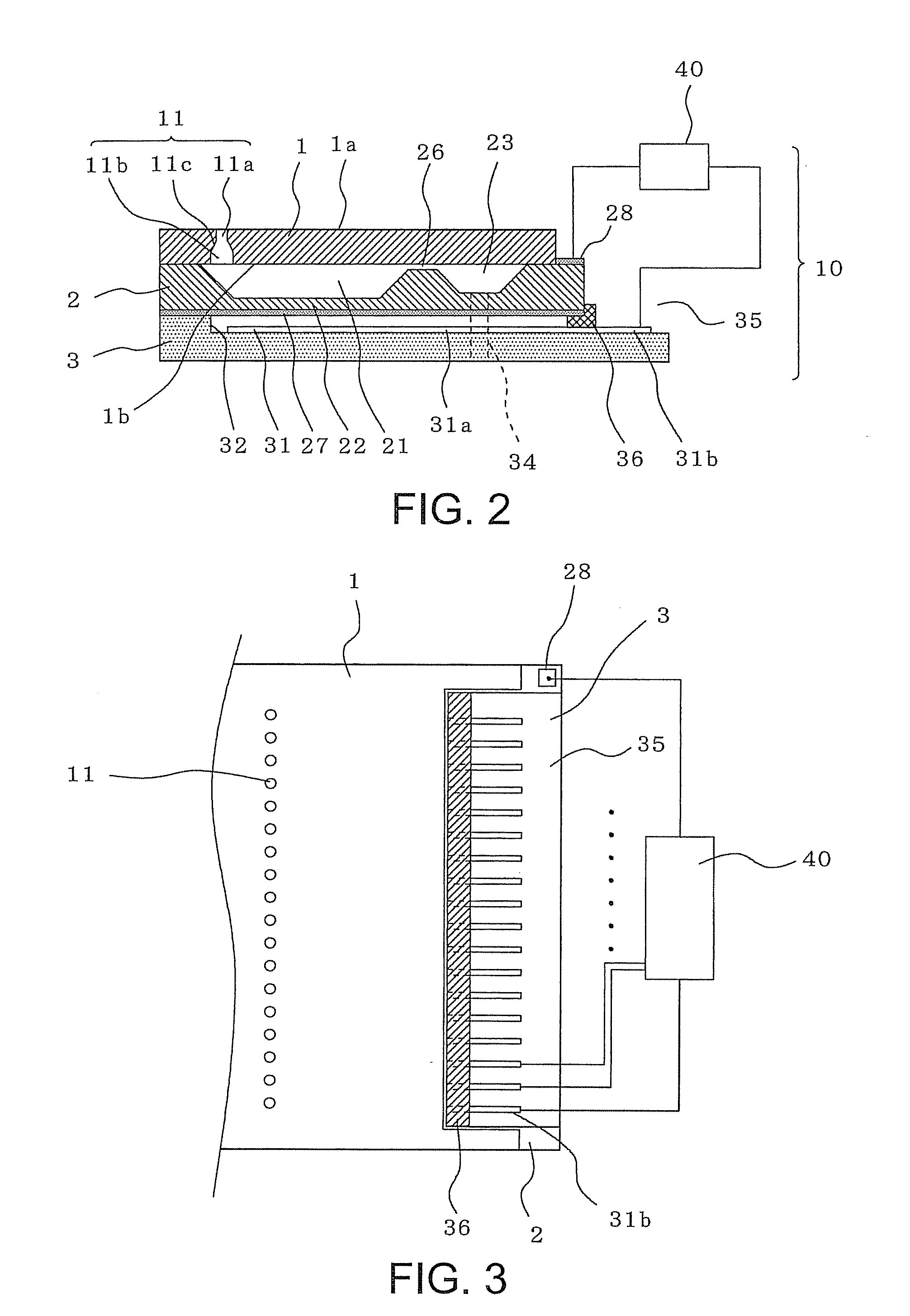
ActiveUS20050019654A1Solve the real problemReduce the amount requiredPrinted circuit assemblingFinal product manufactureSolderingElectrical and Electronics engineering
A battery provided with terminals, which is capable of being mounted on a circuit board without soldering, is disclosed. The battery includes a battery body 2, and terminals 7 for electrically connecting the battery body 2 to conductive portions 6 provided in a circuit board 5. A portion of each of the terminals 7 is a fixing portion 9 for fixing the battery body 2 to the circuit board 5. The fixing portion 9 of each of the terminals 7 is provided with engaging portions 12, which penetratingly engage a portion where the conductive portion 6 is provided or a portion in the vicinity of the conductive portion 6, and a contacting portion 11, which contacts the conductive portion 6 to electrically connect the conductive portion 6 to the battery body 2, so that the battery body 2 is fixed in a state of being electrically connected to the conductive portions 6 of the circuit board 5.
Owner:MAXELL HLDG LTD
Nozzle plate, droplet discharge head, method for manufacturing the same and droplet discharge device
ActiveUS20080309718A1Improve discharge characteristicsIncreasing nozzle densityPrintingEngineeringSilicon
A nozzle plate includes a silicon substrate, and a nozzle hole formed in the silicon substrate for discharging a liquid droplet provided with: a first nozzle portion formed perpendicularly to a surface of the silicon substrate; a second nozzle portion formed on a same axis as an axis of the first nozzle portion and having a cross-sectional area that is larger than a cross-sectional area of the first nozzle portion; and an inclined portion having a cross-sectional area gradually increasing from the first nozzle portion to the second nozzle portion.
Owner:SEIKO EPSON CORP
Secondary battery and method for producing the same
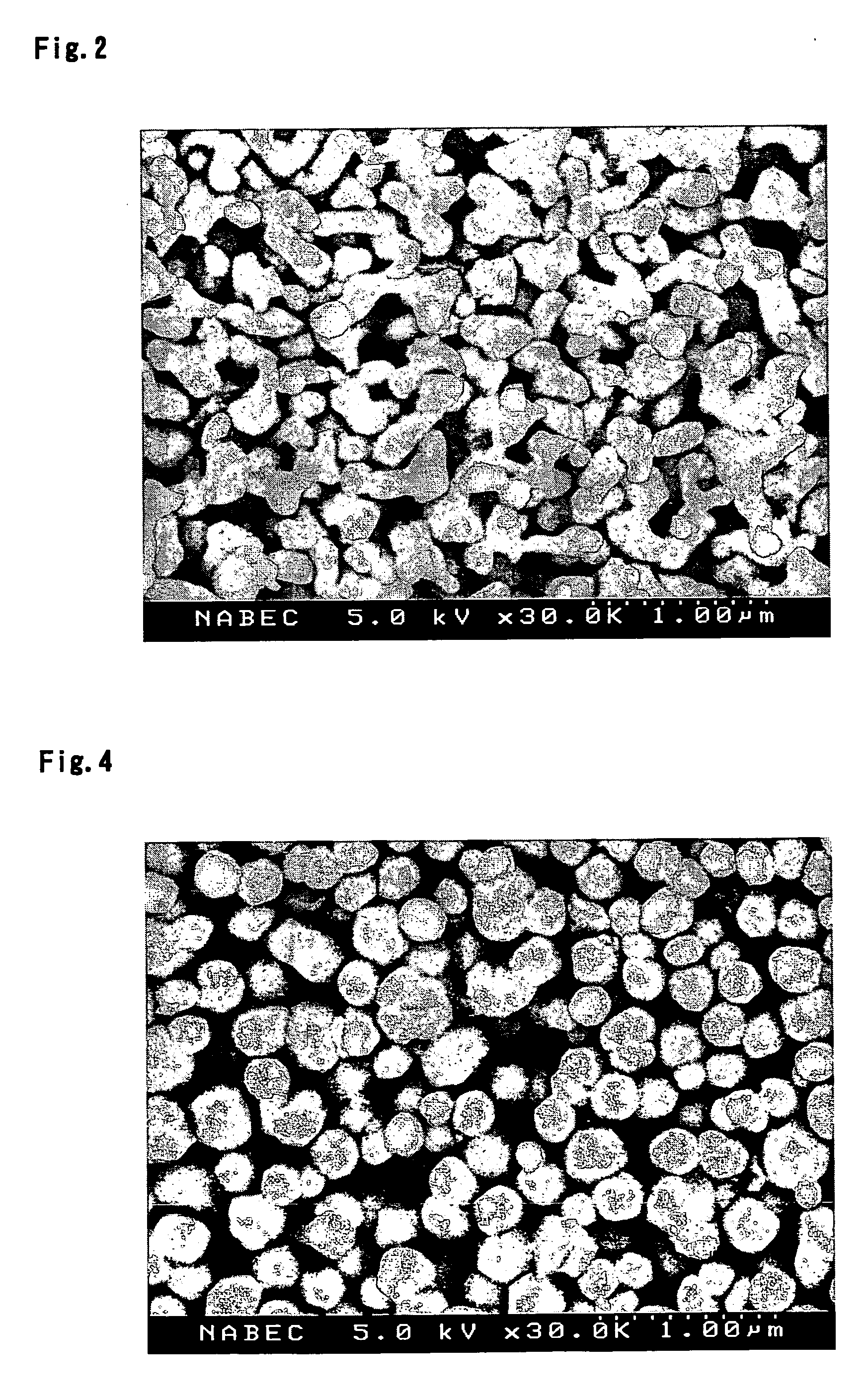
ActiveUS20060105245A1High porosityEasy to getAlkaline accumulatorsElectrode manufacturing processesSlurryBattery cell
In a secondary battery including a positive electrode, a negative electrode and a porous film bonded to the surface of at least one of the positive electrode and the negative electrode, the porous film includes ceramic particles and a binder, and the ceramic particles include polycrystalline particles obtained by mechanically crushing a fired material comprising a ceramic that is directly synthesized from a ceramic precursor. The porous film has a porosity of 40 to 80%, for example. The porous film can be formed by a method including the steps of: obtaining a fired material comprising a ceramic from a ceramic precursor; obtaining ceramic particles by mechanically crushing the fired material of the ceramic; obtaining a slurry including the ceramic particles and a binder; and applying the slurry onto the surface of an electrode, followed by drying.
Owner:PANASONIC CORP
Non-aqueous electrolyte lithium secondary battery
InactiveUS20100266905A1Improve discharge characteristicsSolid electrolytesAlkaline accumulatorsPropionateHigh rate
A lithium secondary battery has a cathode made of carbon material capable of occluding or emitting a lithium ion, a cathode made of lithium-contained oxide, and a non-aqueous electrolyte. The non-aqueous electrolyte includes a lithium salt containing LiPF6 and LiBF4; and a non-linear carbonate-based mixed organic solvent in which (a) a cyclic carbonate having ethylene carbonate or a mixture of ethylene carbonate and propylene carbonate and (b) a propionate-based ester such as ethyl propionate are mixed at a volume ratio (a:b) in the range from about 10:90 to about 70:30. This lithium secondary battery ensures excellent high-rate charging / discharging characteristics and improved life cycle and low-temperature discharging characteristics since it includes a predetermined mixed organic solvent not including a linear carbonate. Also, since gas generation is restrained at a high temperature, a battery set may be mounted in a more improved way.
Owner:LG CHEM LTD
Electrical energy storage device
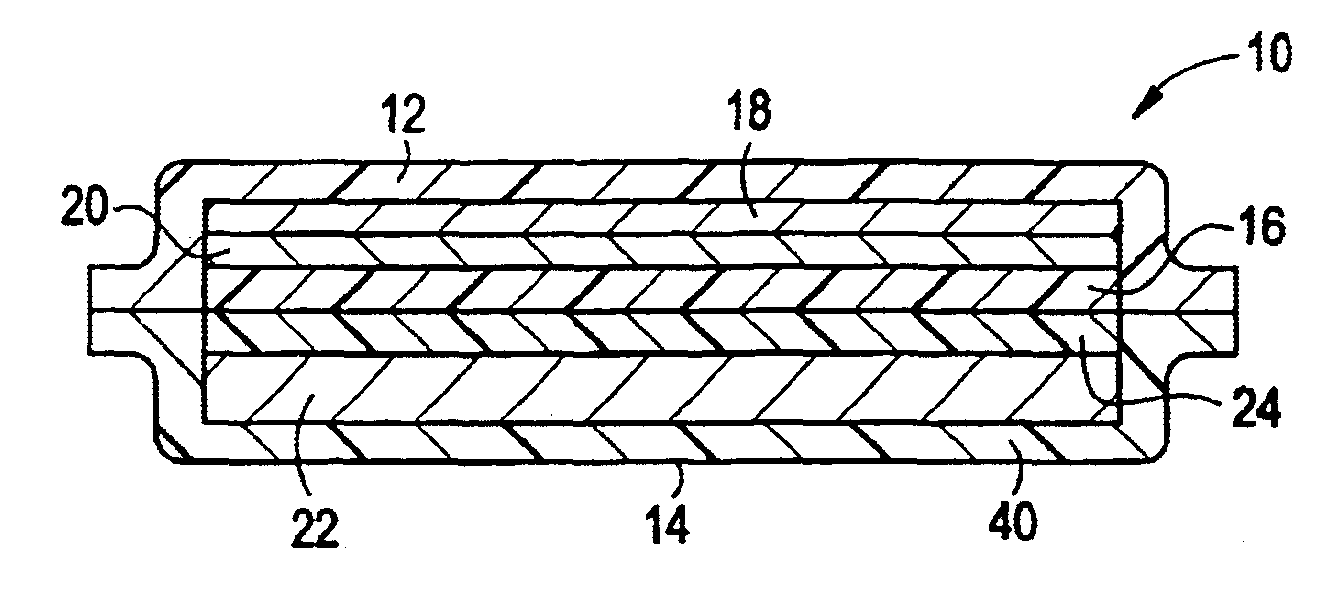
InactiveUS20110317333A1Difference in thicknessIncrease rangeLiquid electrolytic capacitorsCurrent conducting connectionsHigh rateElectrical polarity
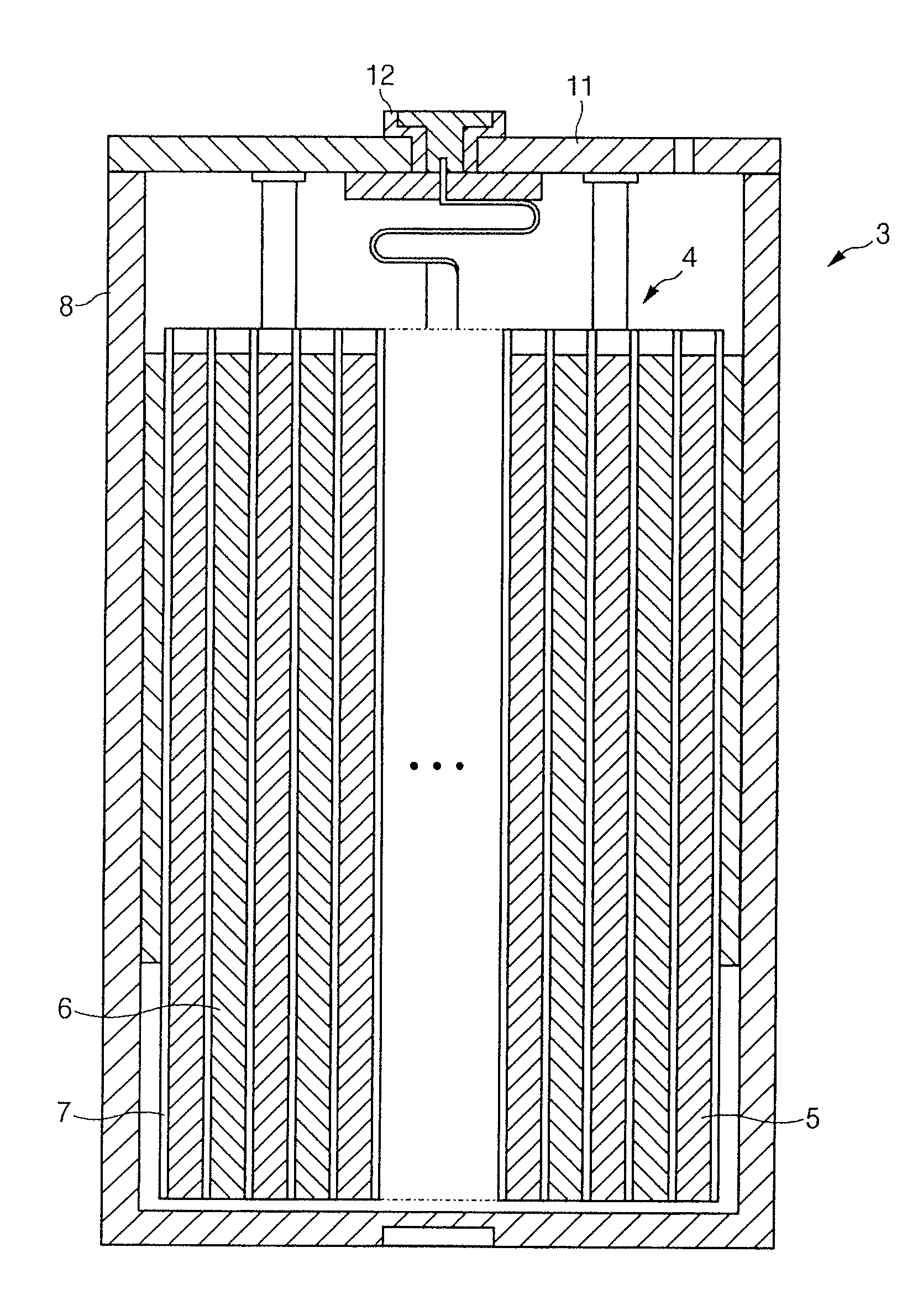
Provided is an electrical energy storage device including an electrode winding body, which includes a positive electrode generating electrons by oxidation and reduction, a negative electrode for absorbing the generated electrons, and separation layers for physically separating the negative electrode from the positive electrode, which are sequentially wound around a winding core, and an electrolyte provided between the positive electrode and the negative electrode, the electrical energy storage device including: a terminal plate for externally connecting the electrode winding body to an external electrode connecting member such as an external resistor; a cylindrical can for accommodating the electrode winding body connected to the terminal plate; and a conductive interconnecting member for connection between the terminal plate and polarity-leads on one side of the electrode winding body by a method selected from the group consisting of plasma-spraying, welding, soldering and adhesion using a conductive adhesive material. According to the present invention, in which an interconnecting member for reducing a difference in thickness between objects to be welded is employed, it is possible to prevent welding failure between the polarity-leads of the electrode winding body and the terminal plate, thereby improving high-rate discharge (large current discharge) efficiency.
Owner:MAXWELL TECH KOREA CO LTD
Air battery and method for producing air electrode for air battery
InactiveUS20070160898A1Excellent in heavy-load discharge characteristicImprove storage characteristicsFuel and primary cellsPrimary cellsCompound (substance)Three-phase
An air battery that is excellent in heavy-load discharge characteristics and storage characteristics is provided by ensuring the stability of the three-phase interface of a catalyst layer of an air electrode. The air battery has an air electrode including a catalyst layer that is bonded under pressure to a current collector and a water-repellent film. The catalyst layer includes a catalyst for reducing oxygen, a promoter for decomposing a product of the oxygen reduction, conductive carbon, and a binder. A non-polymeric fluorine compound is added to the catalyst layer in order to enhance the water repellency of the catalyst layer.
Owner:PANASONIC CORP
Cathode active material for lithium secondary batteries with high safety, method of preparing the same and lithium secondary batteries comprising the same
ActiveUS20100310940A1Excellent characteristicsImprove securityMaterial nanotechnologyAlkaline earth titanatesOxideMetal
A cathode active material for a lithium secondary battery includes a lithium metal oxide secondary particle core formed by agglomerating lithium metal oxide primary particles; and a shell formed by coating the secondary particle core with barium titanate and metal oxide. This cathode active material allows making a lithium secondary battery having improved safety, particularly in thermal stability and overcharging characteristics.
Owner:KOKAM LTD
Phosphor and plasma display device
InactiveUS20050062417A1Excellent initial characteristicDeterioration in brightnessSustain/scan electrodesDischarge tube luminescnet screensAluminateAlkaline earth metal
Phosphor and a plasma display device are provided whose deterioration in brightness of phosphors and a degree of change in chromaticity are alleviated and whose discharge characteristics are improved and that has excellent initial characteristics. Phosphor of the present invention is an alkaline-earth metal aluminate phosphor containing an element M (where M denotes at least one type of element selected from the group consisting of Nb, Ta, W and B). In this phosphor, a concentration of M in the vicinity of a surface of the phosphor particles is higher than the average concentration of M in the phosphor particles as a whole. A plasma display device according to the present invention includes a plasma display panel in which a plurality of discharge cells in one color or in a plurality of colors are arranged and phosphor layers are arranged so as to correspond to the discharge cells in colors and in which light is emitted by exciting the phosphor layers with ultraviolet rays. The phosphor layers include blue phosphor, where the afore-mentioned phosphor is used as the blue phosphor.
Owner:PANASONIC CORP
Lithium-ion power battery electrolyte for high/low temperature environment
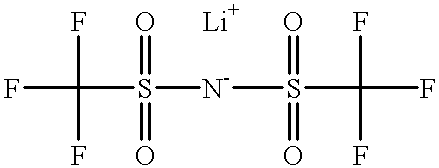
InactiveCN104810551AImprove high temperature performanceImprove discharge characteristicsSecondary cellsImidePower battery
The invention discloses a lithium-ion power battery electrolyte for a high / low temperature environment. The electrolyte comprises a lithium salt, an organic solvent and a negative electrode film forming additive. The lithium salt is a mixture of lithium hexafluorophosphate and lithium dis(trifluoromethanesulfonyl)imide or a mixture of lithium hexafluorophosphate and lithium difluoro(oxalato)borate. The organic solvent is a mixture of a carbonic ester solvent and a carboxylate solvent and a volume ratio of the carbonic ester solvent to the carboxylate solvent is 7-9: 1-3. Through modification of the lithium salt, the organic solvent and the film forming additive, charging-discharging, cycle and storage performances of the lithium-ion battery under condition of high / low temperature are obtained, and the problem that the existing lithium-ion power battery electrolyte has a low charging-discharging capacity, a short cycle life and poor storage performances in a high / low temperature environment is solved.
Owner:WANXIANG 123 CO LTD +2
Nonaqueous electrolyte secondary battery and its anode
InactiveUS6420070B1Improve featuresHigh rate discharge characteristicsSecondary cellsNon-aqueous electrolyte accumulator electrodesLithiumHigh rate
Using a graphite material capable of intercalating and de-intercalating lithium ions as the anode, the peak intensity ratio R(=I(110) / I(004)) corresponding to lattice planes (110) and (004) of graphite material obtained by wide angle X-ray diffraction measurement of this anode is in a range of 0.05 to 0.5. Hence, edges of graphite crystals exist adequately on the electrode surface at the interface to the electrolyte, and therefore intercalation of lithium proceeds smoothly, polarization at the time of charging and discharging is suppressed, and a nonaqueous electrolyte secondary battery excellent in high rate discharging characteristic is obtained. The obtained battery is small in deterioration if charging and discharging are repeated.
Owner:PANASONIC CORP
Stabilized electrochemical cell active material
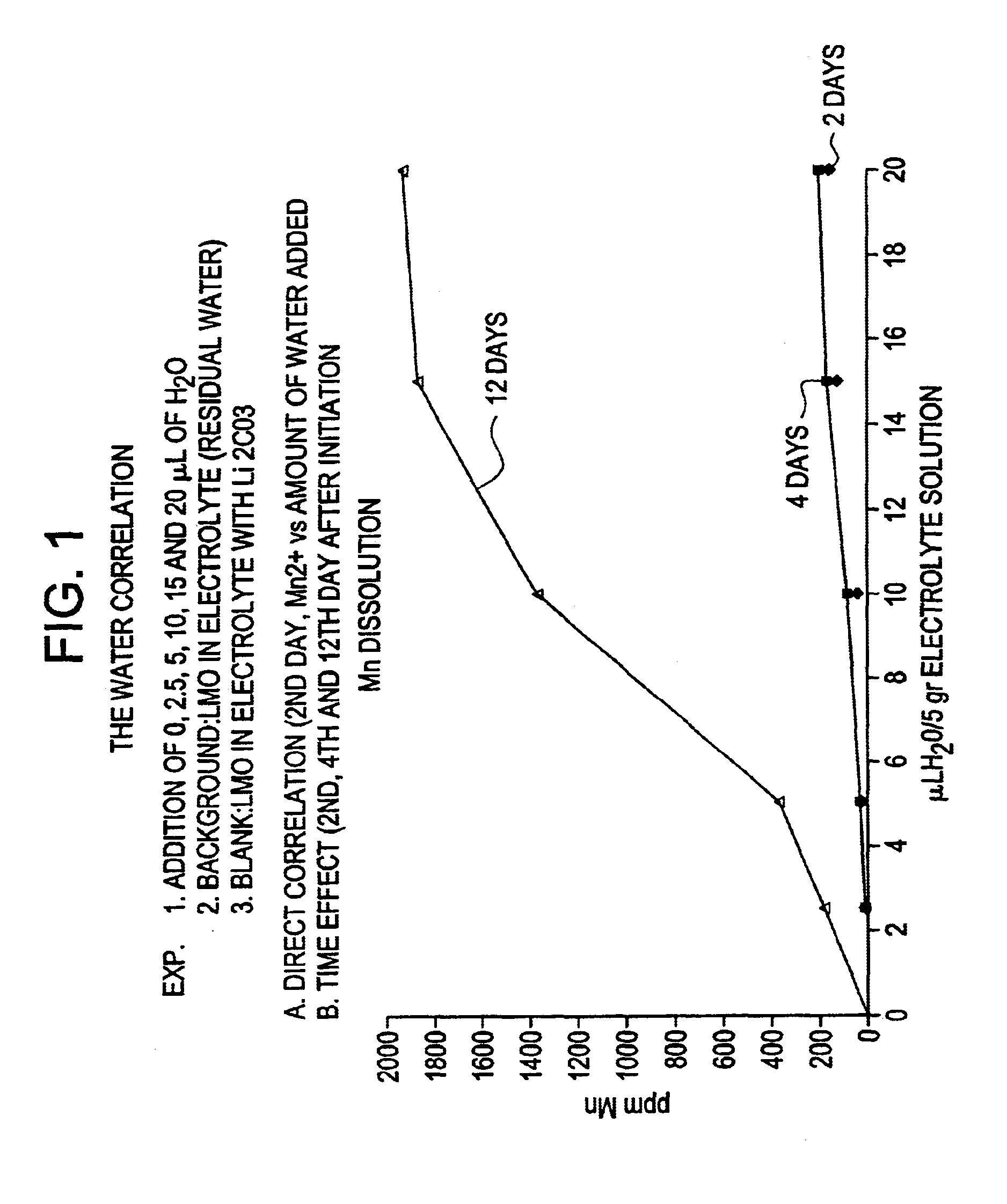
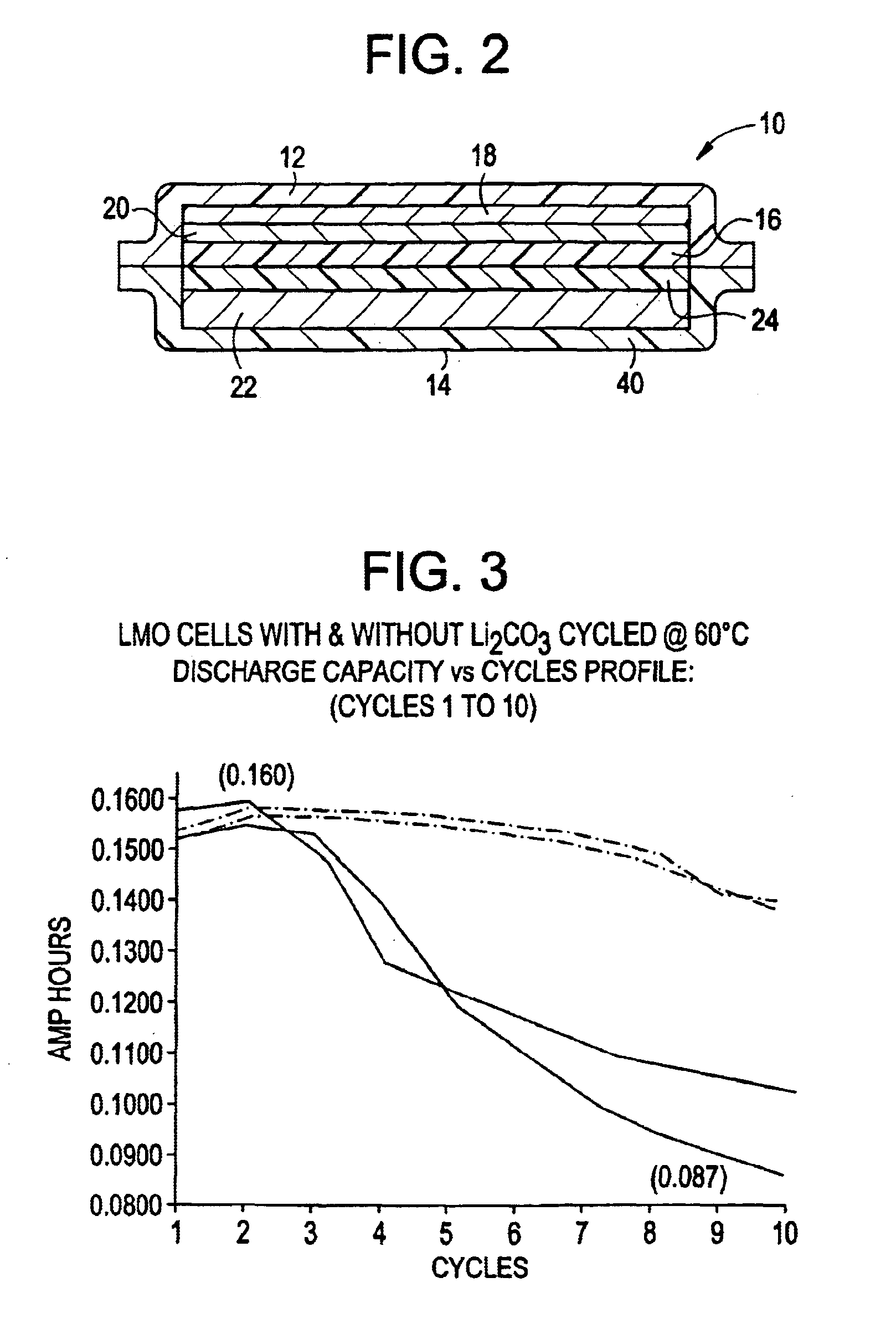
InactiveUS6869547B2Improve discharge characteristicsMaintain integrityConductive materialLithium compoundsDecompositionAlkali metal
Stabilized lithiated manganese oxide (LMO) is prepared by reacting cubic spinel lithium manganese oxide particles and particles of an alkali metal compound in air for a time and at a temperature sufficient to decompose at least a portion of the alkali metal compound, providing a treated lithium manganese oxide. The reaction product is characterized as particles having a core or bulk structure of cubic spinel lithium manganese oxide and a surface region which is enriched in Mn+4 relative to the bulk. X-ray diffraction data and x-ray photoelectron spectroscopy data are consistent with the structure of the stabilized LMO being a central bulk of cubic spinel lithium manganese oxide with a surface layer or region comprising A2MnO3, where A is an alkali metal. Electrochemical cells containing the stabilized LMO of the invention have improved charging and discharging characteristics and maintain integrity over a prolonged life cycle. The electrochemical cells are stabilized against decomposition of cell components, including electrode and electrolyte components.
Owner:VALENCE TECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com