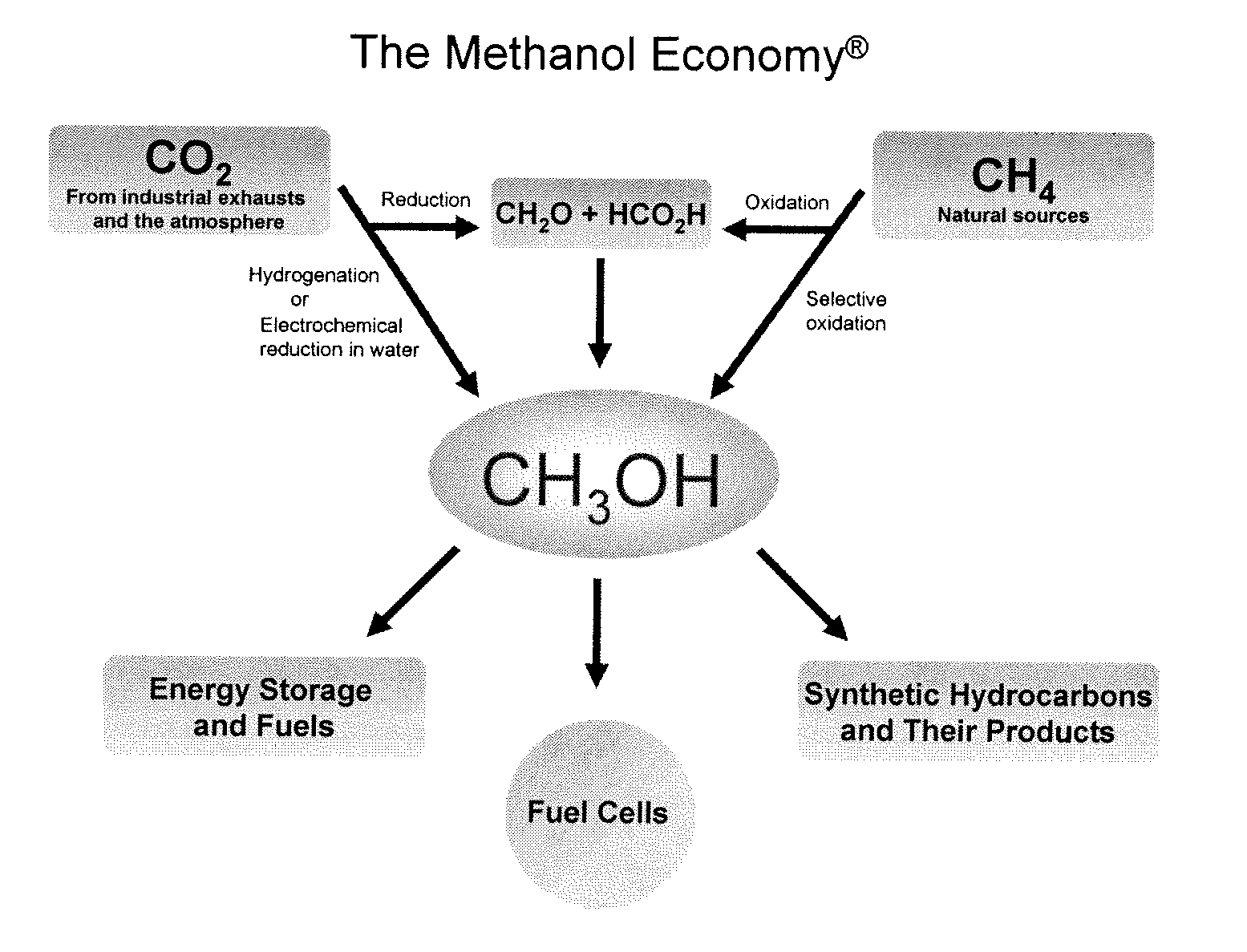
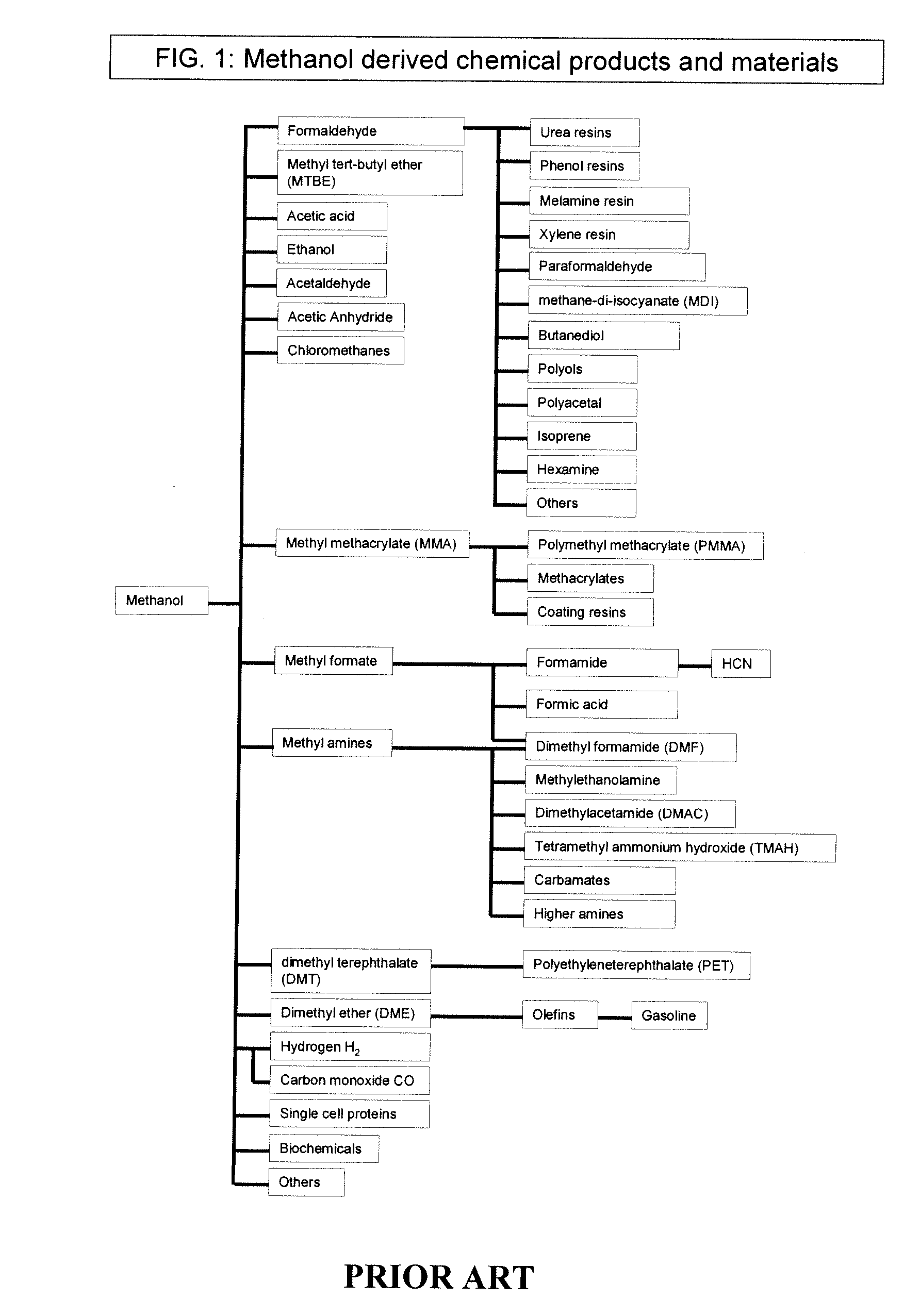
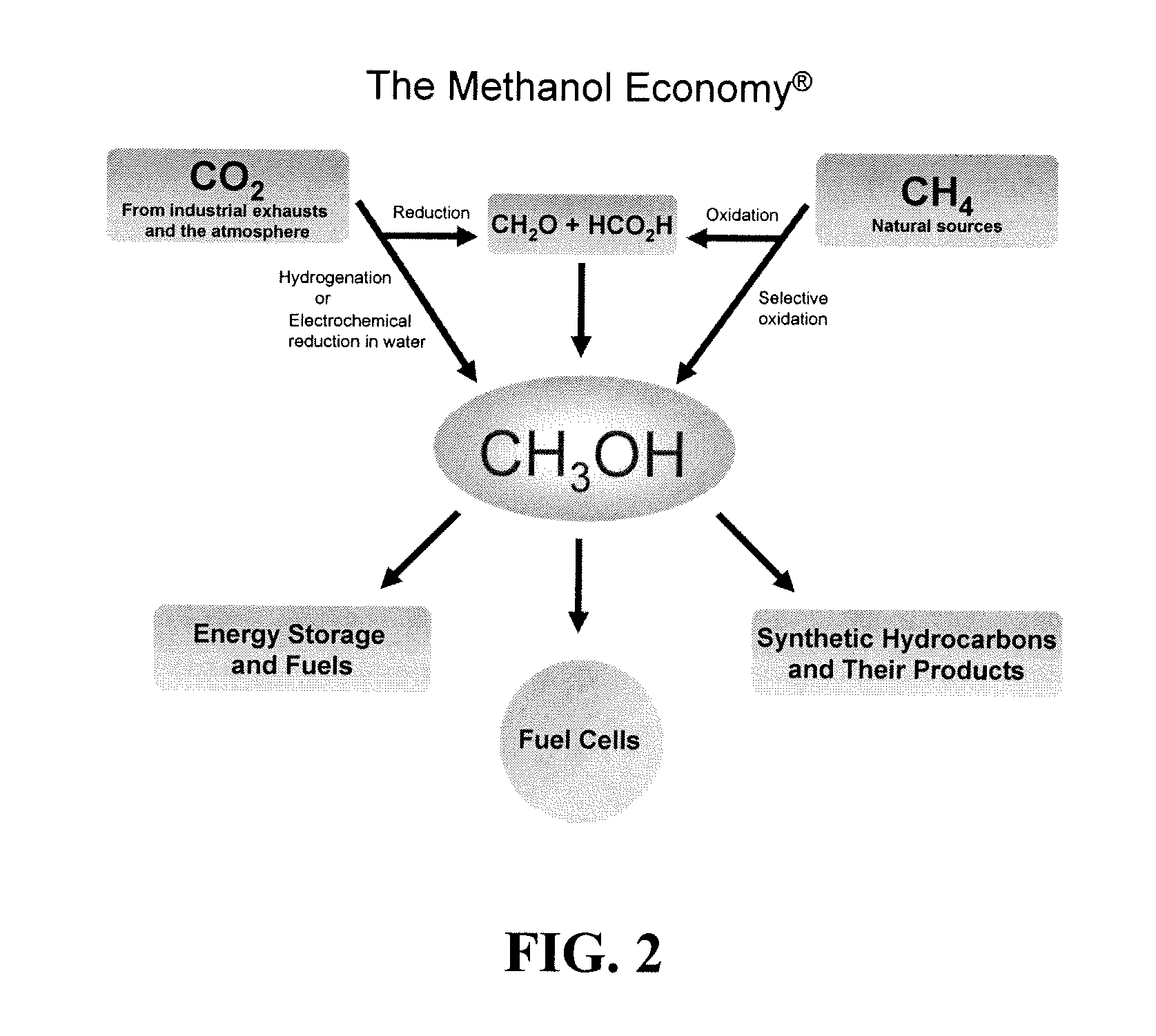
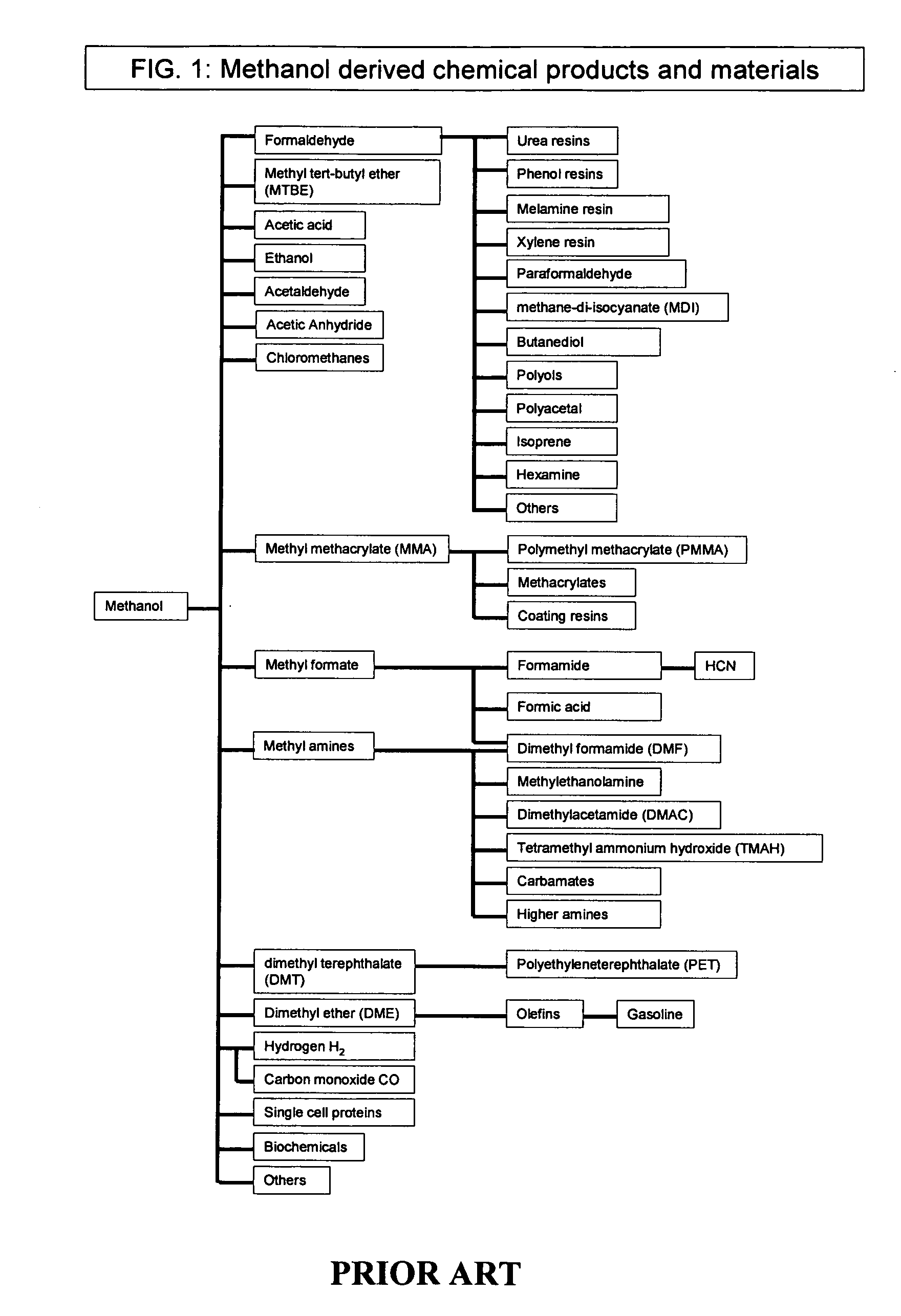
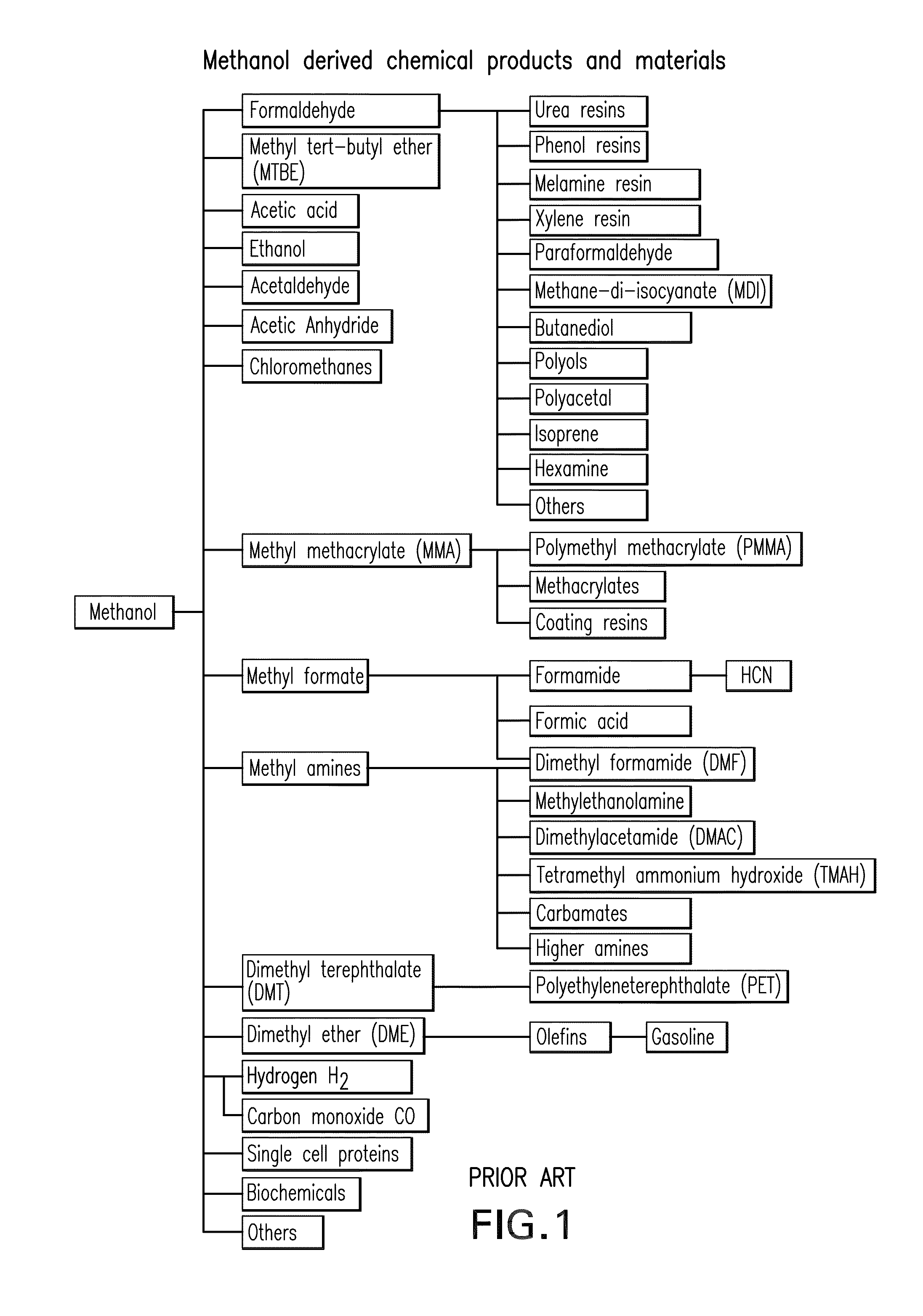
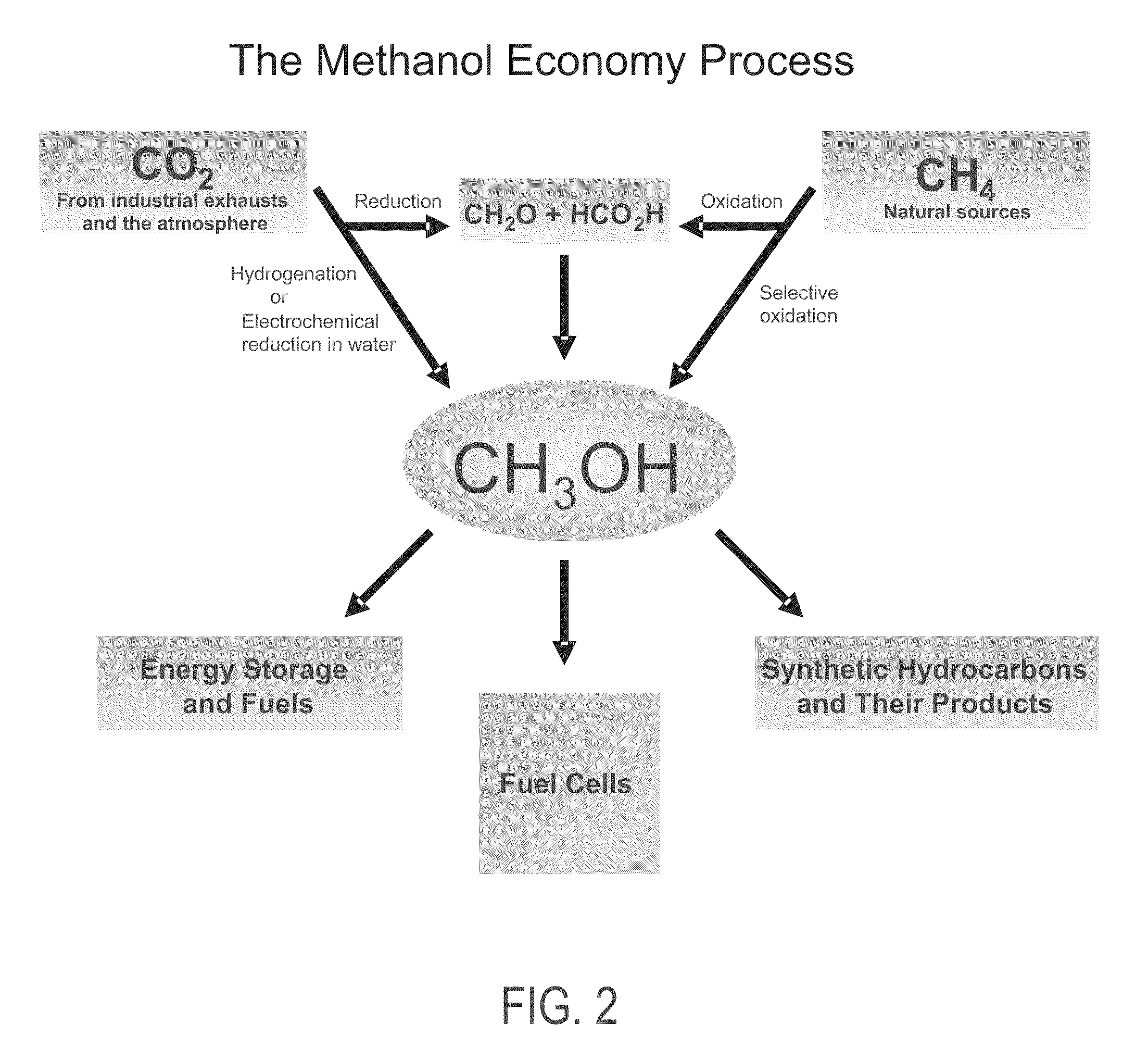
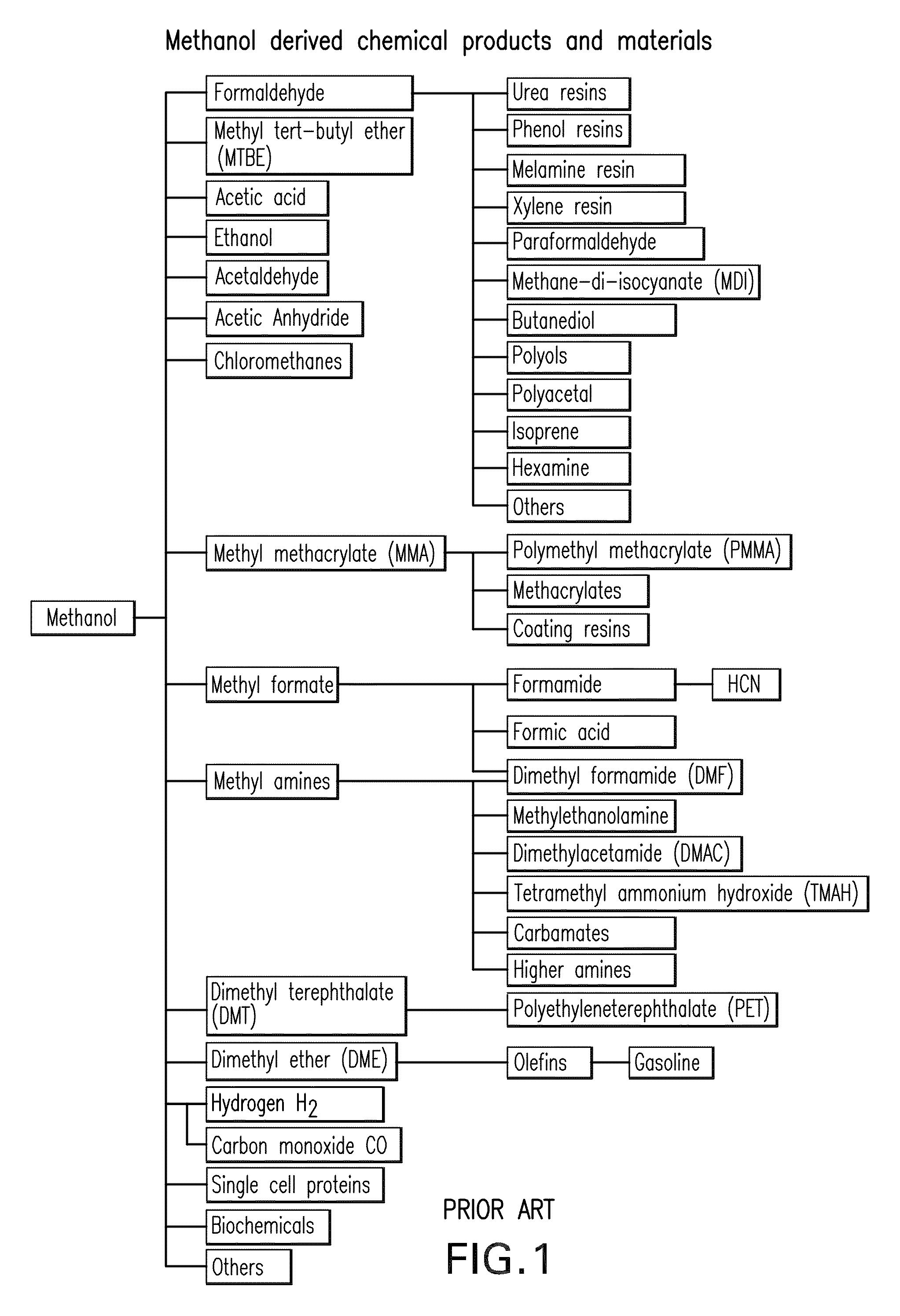
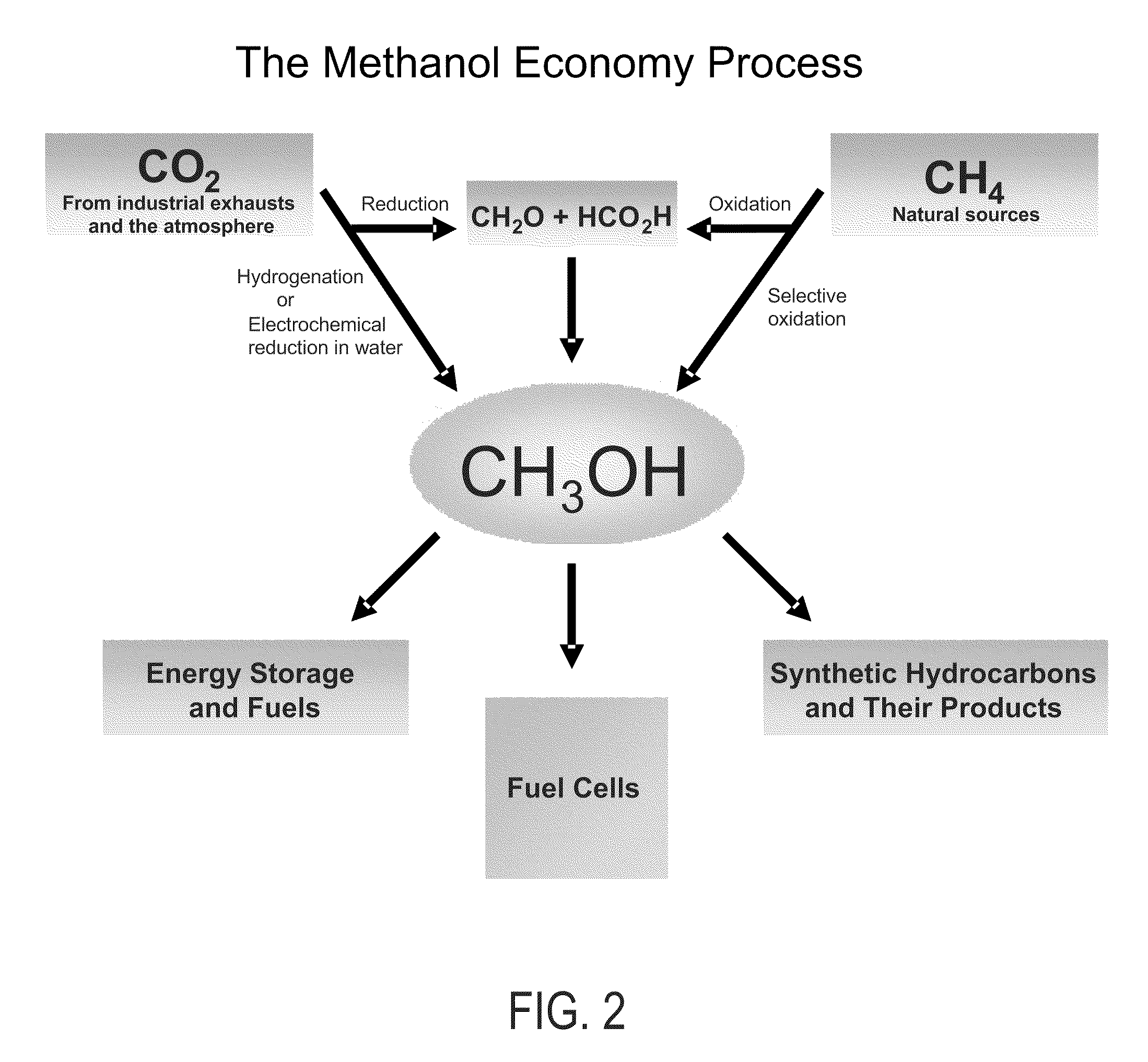
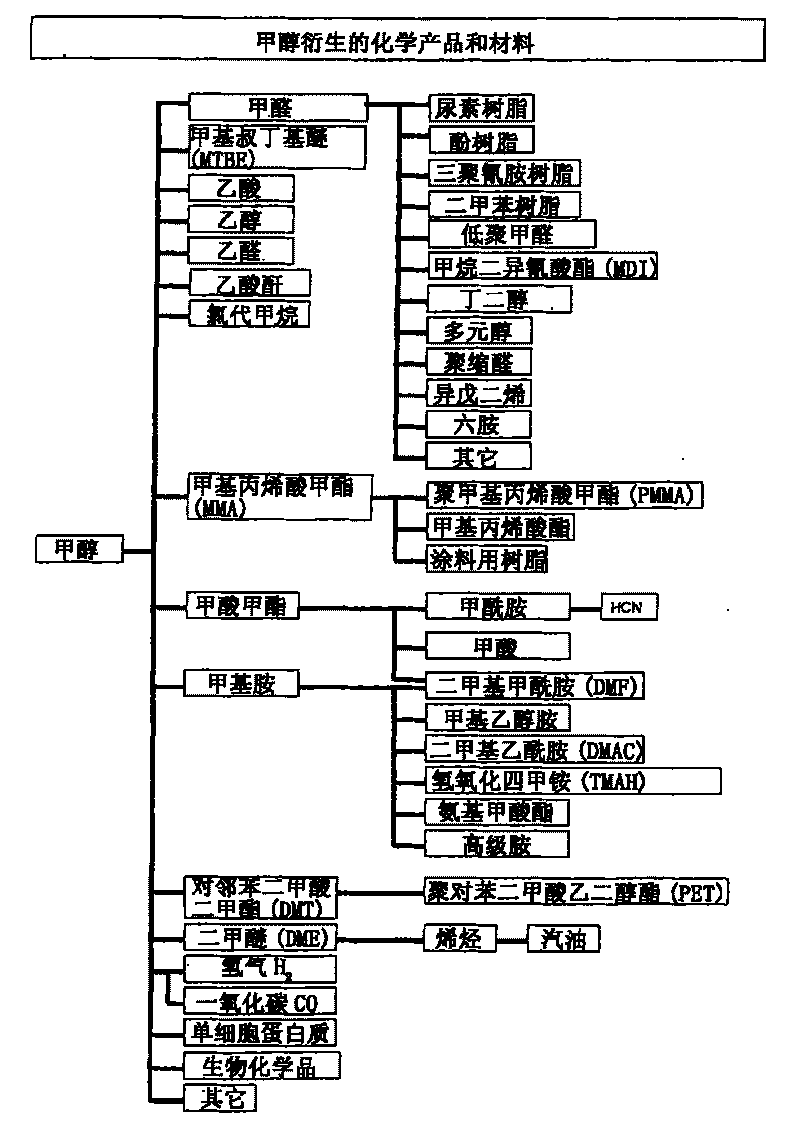
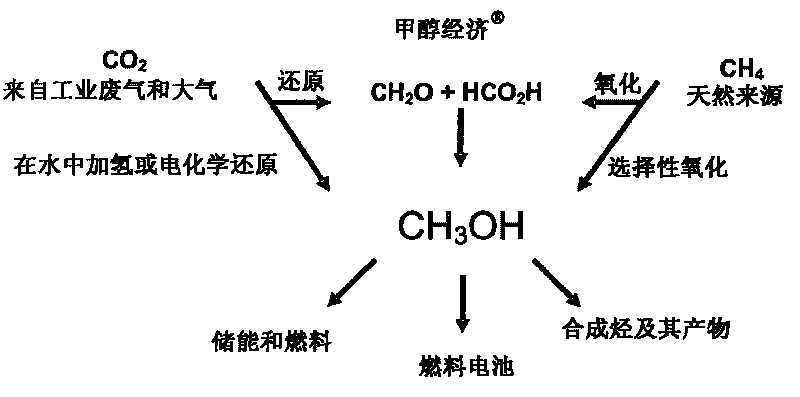
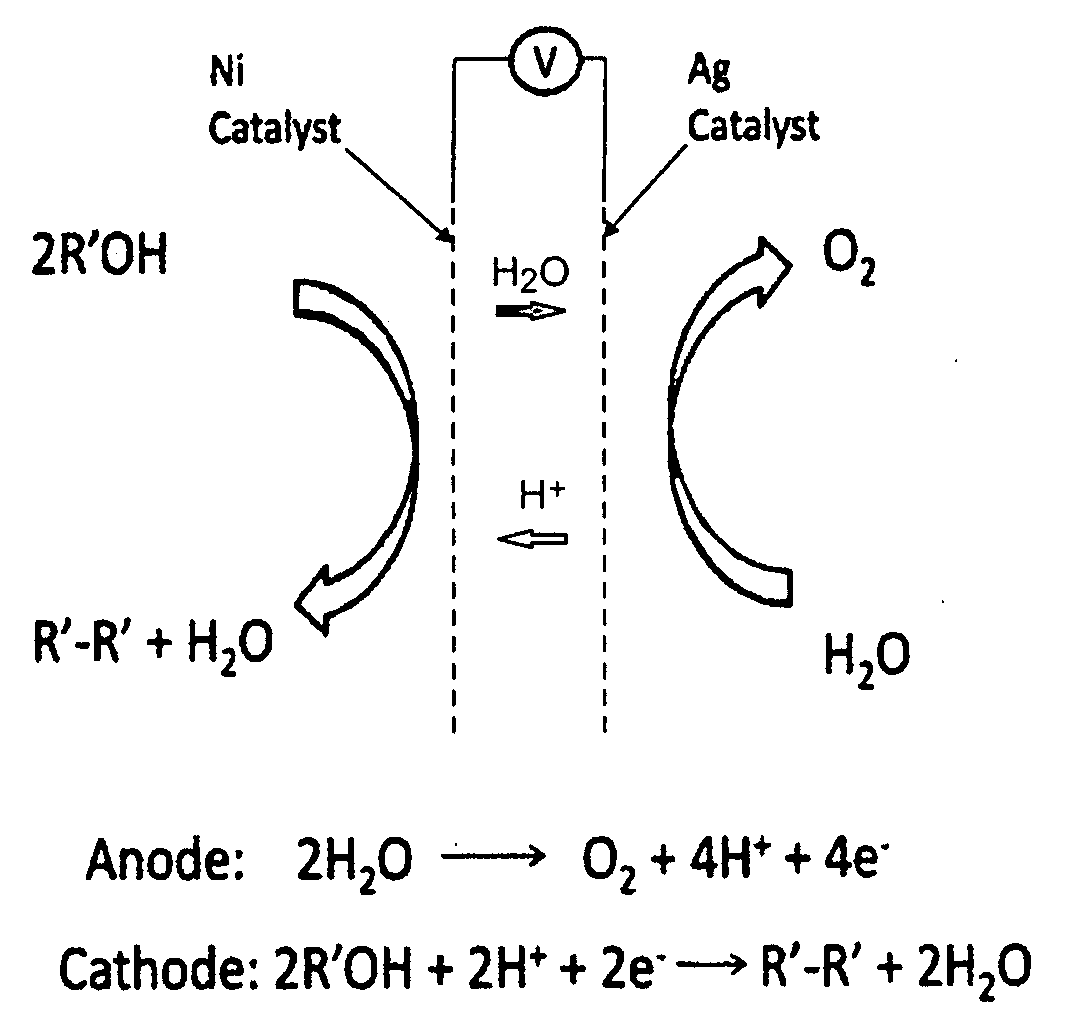
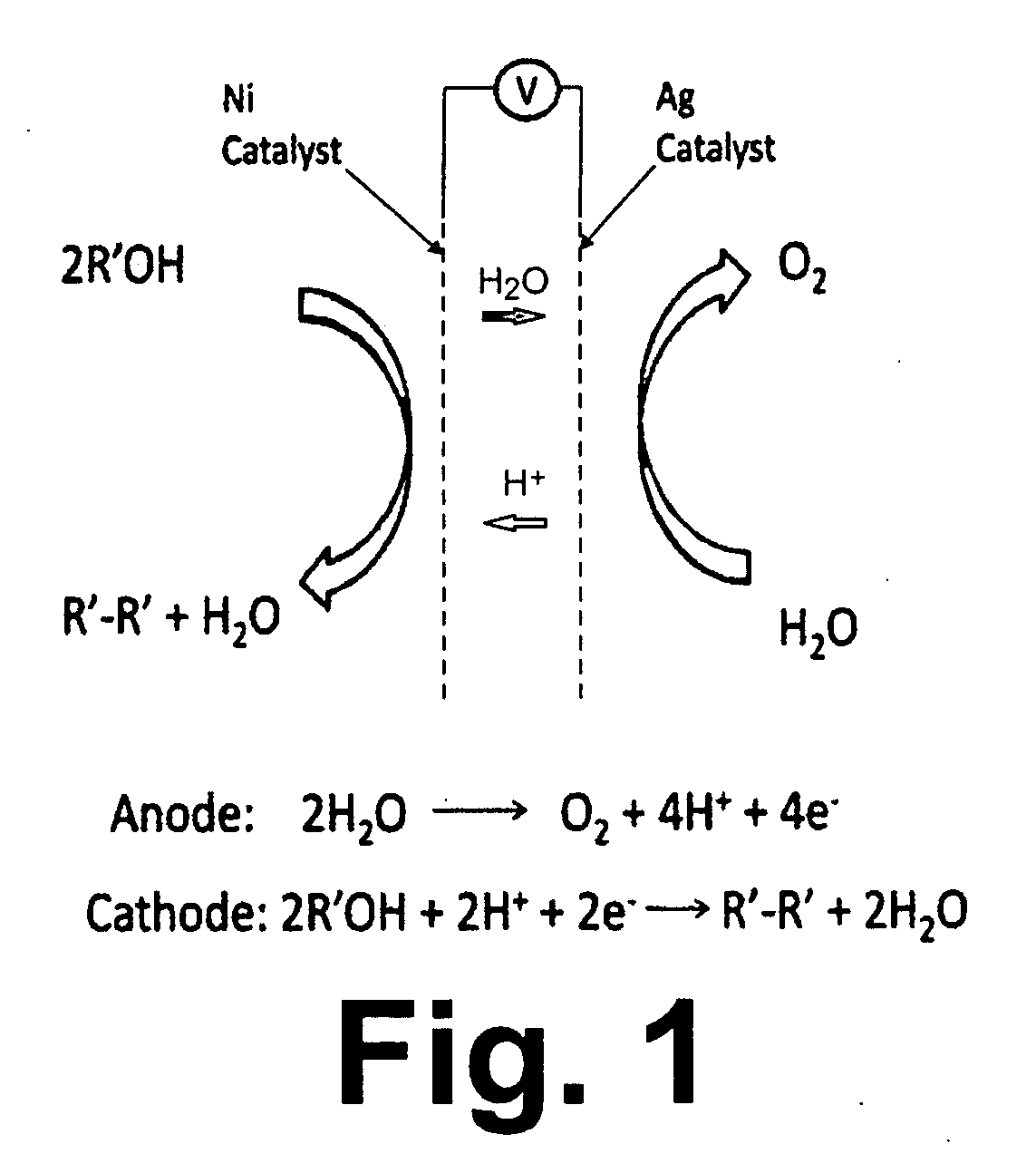
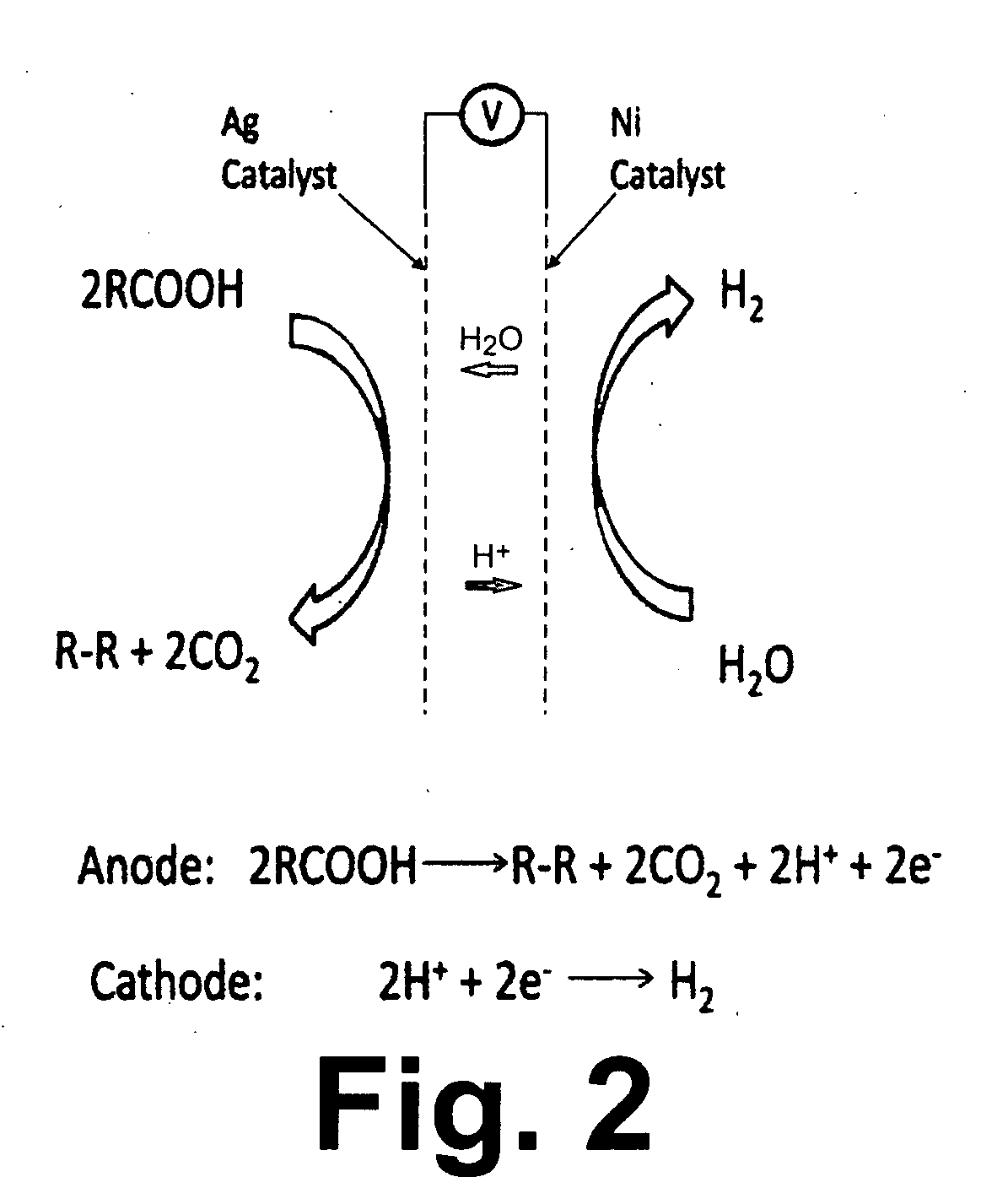
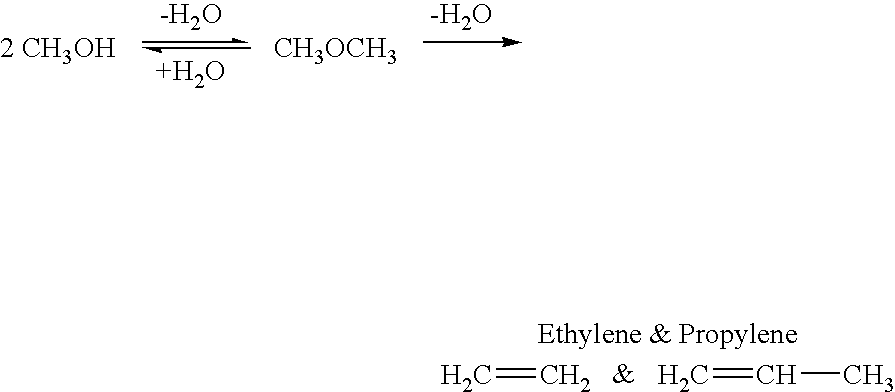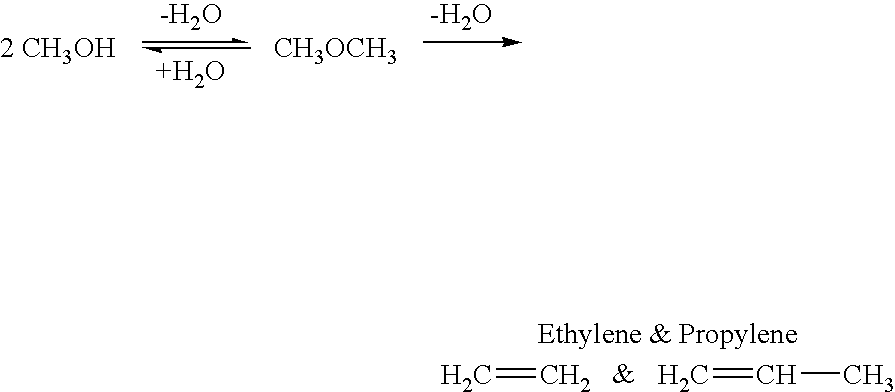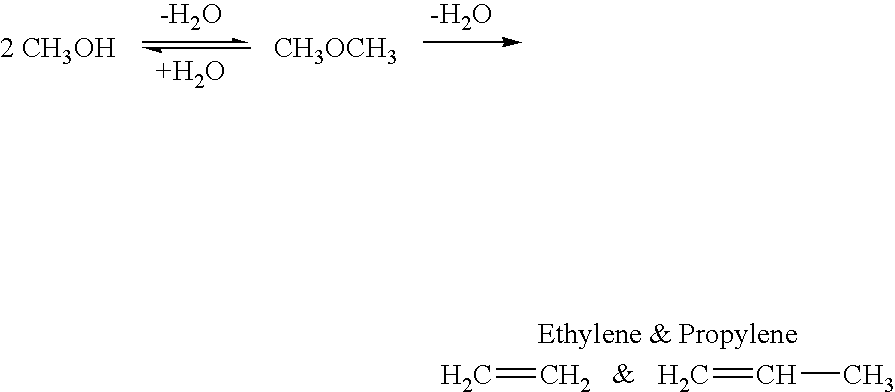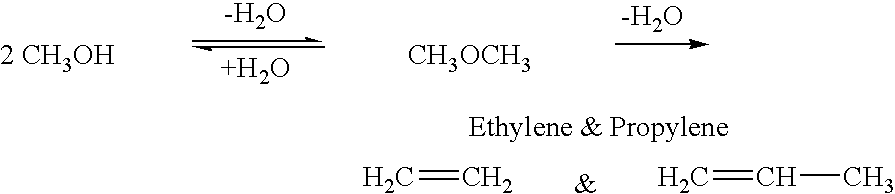Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
259results about "Electrolytic organic reduction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
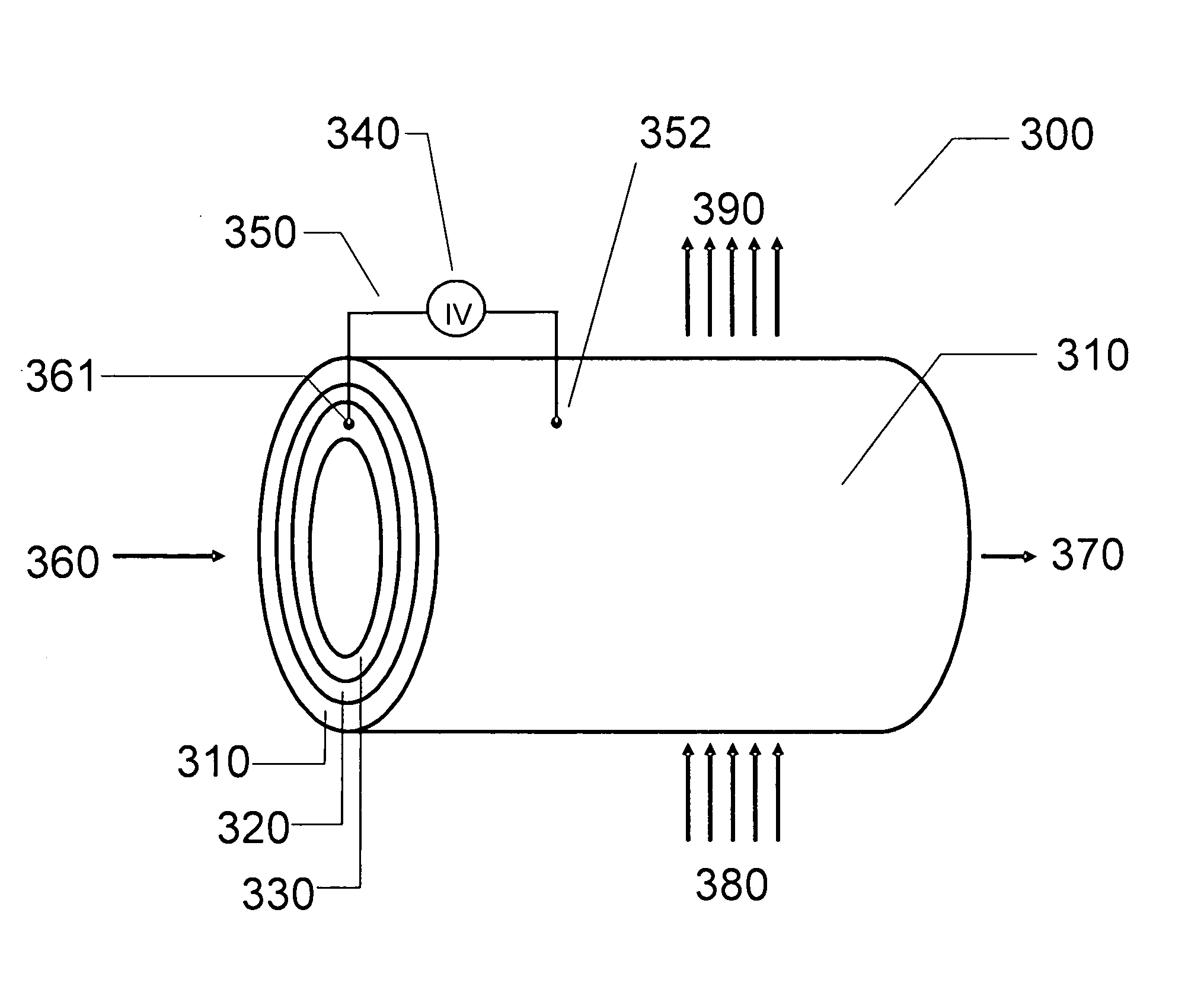
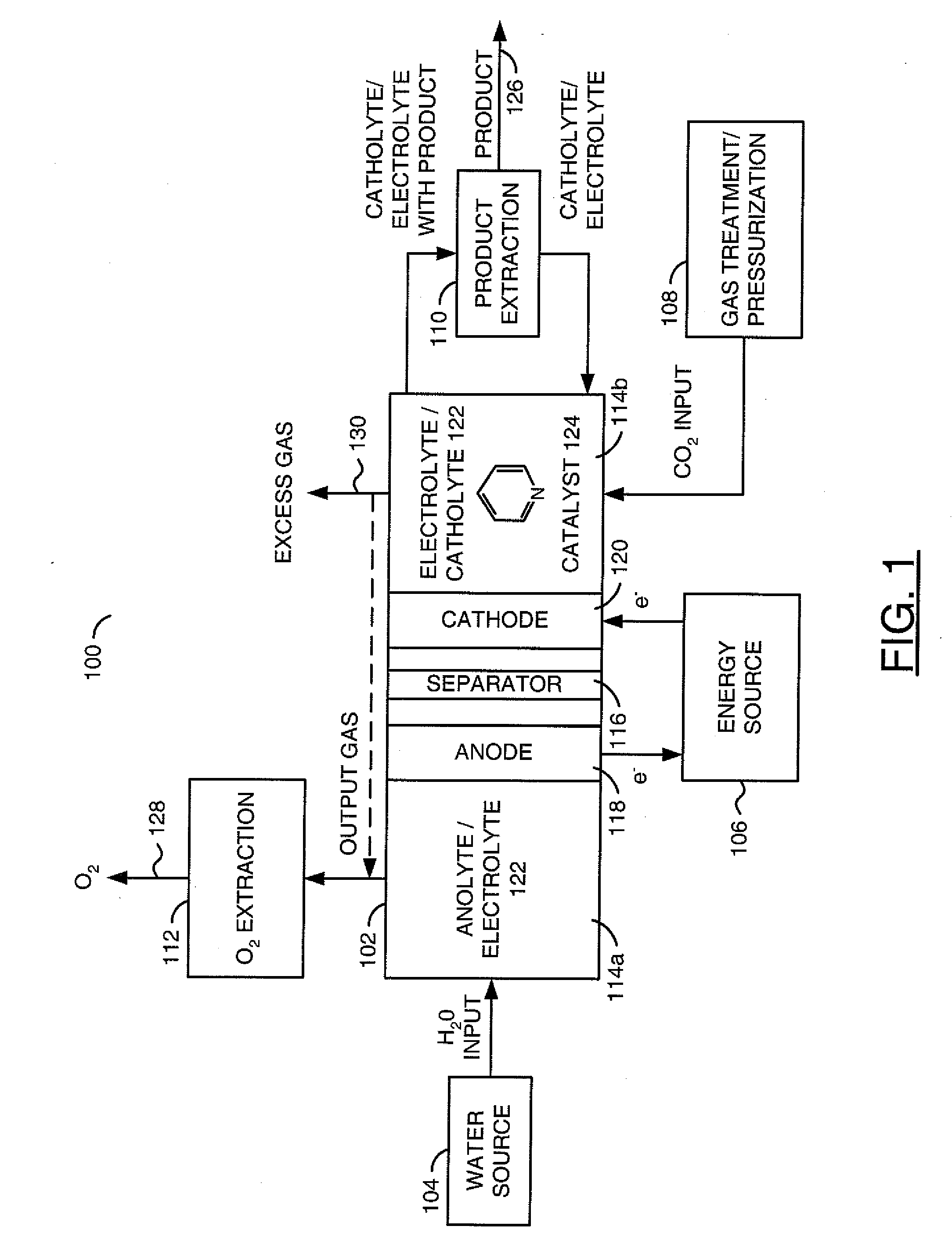
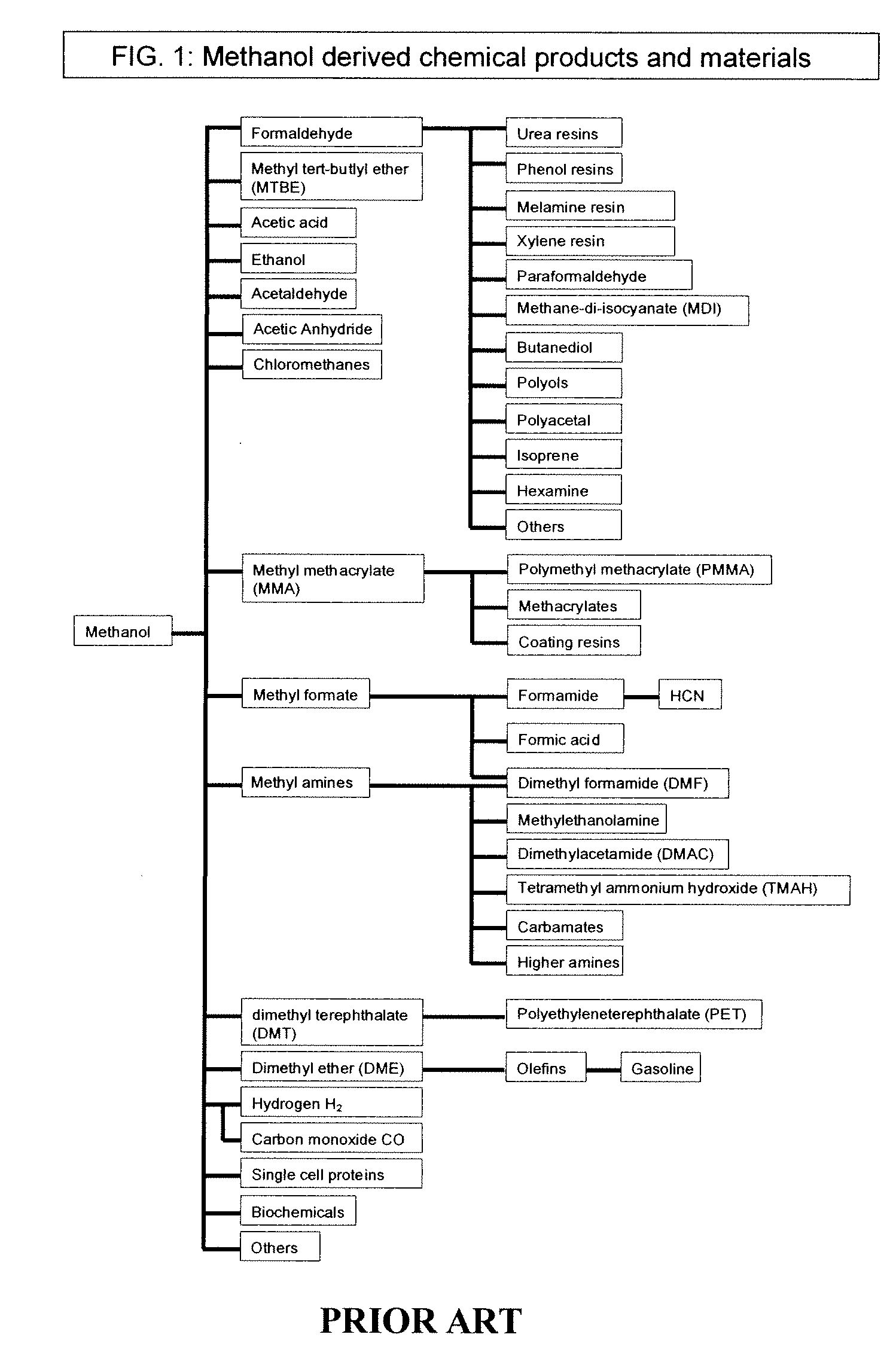
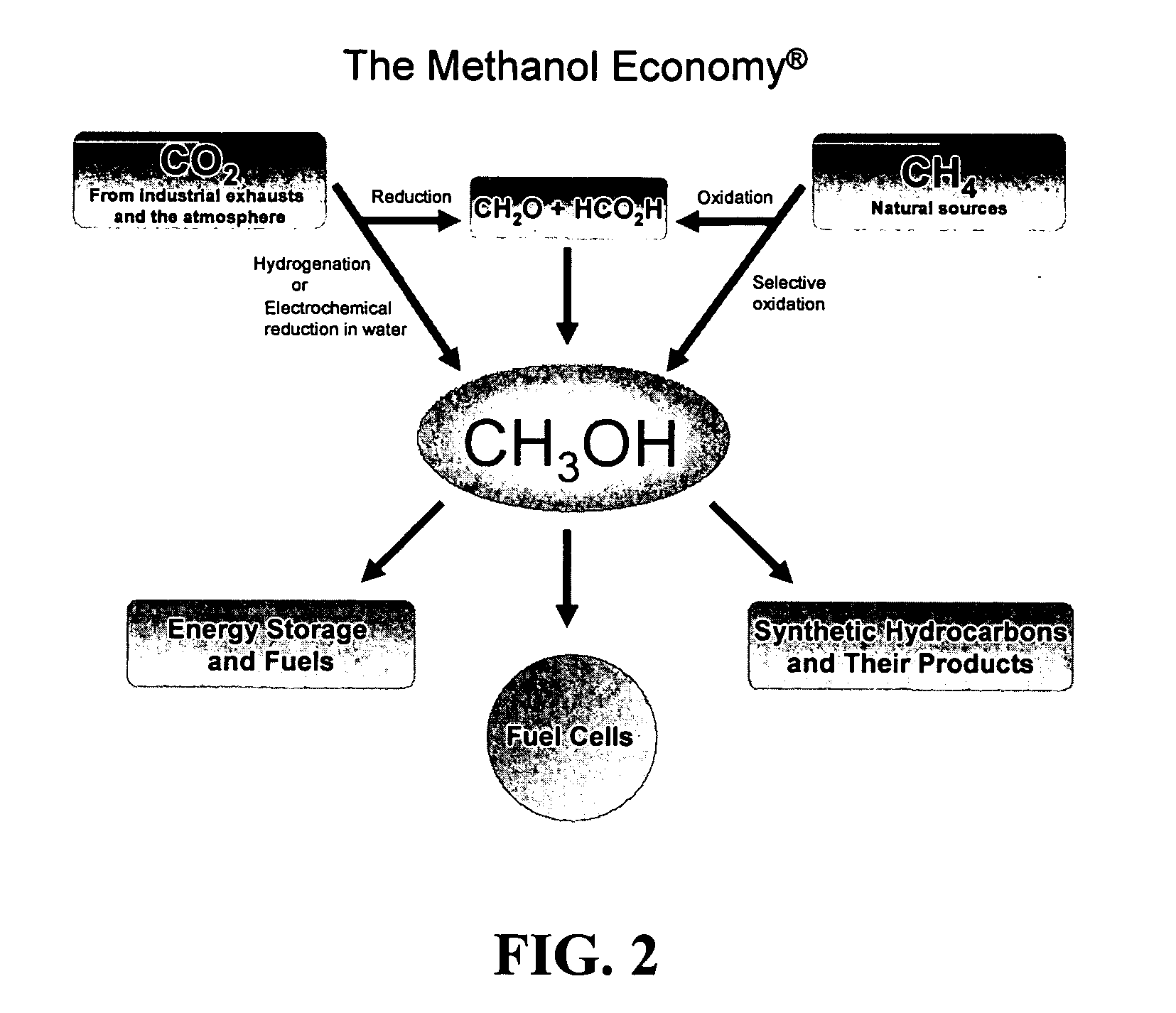
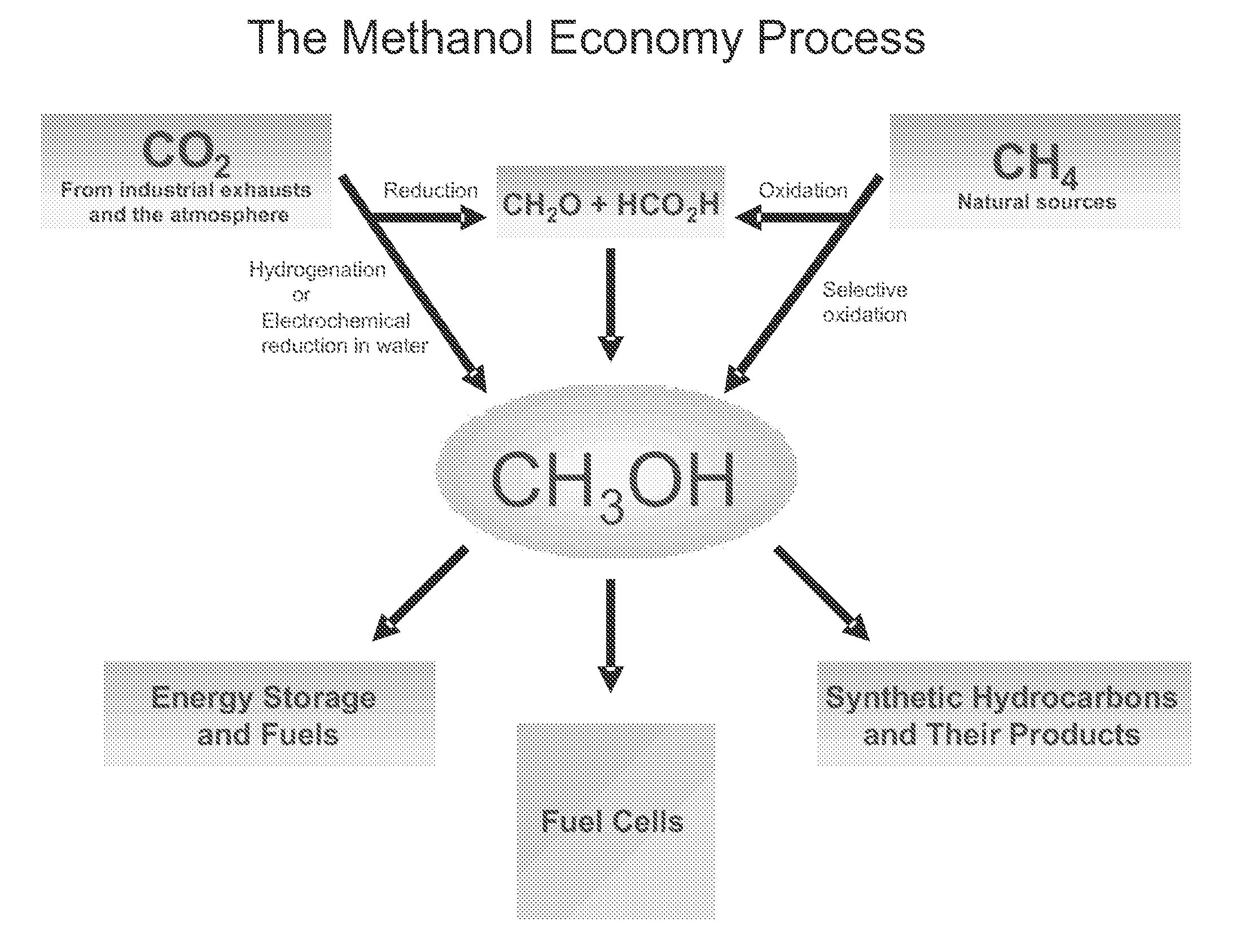
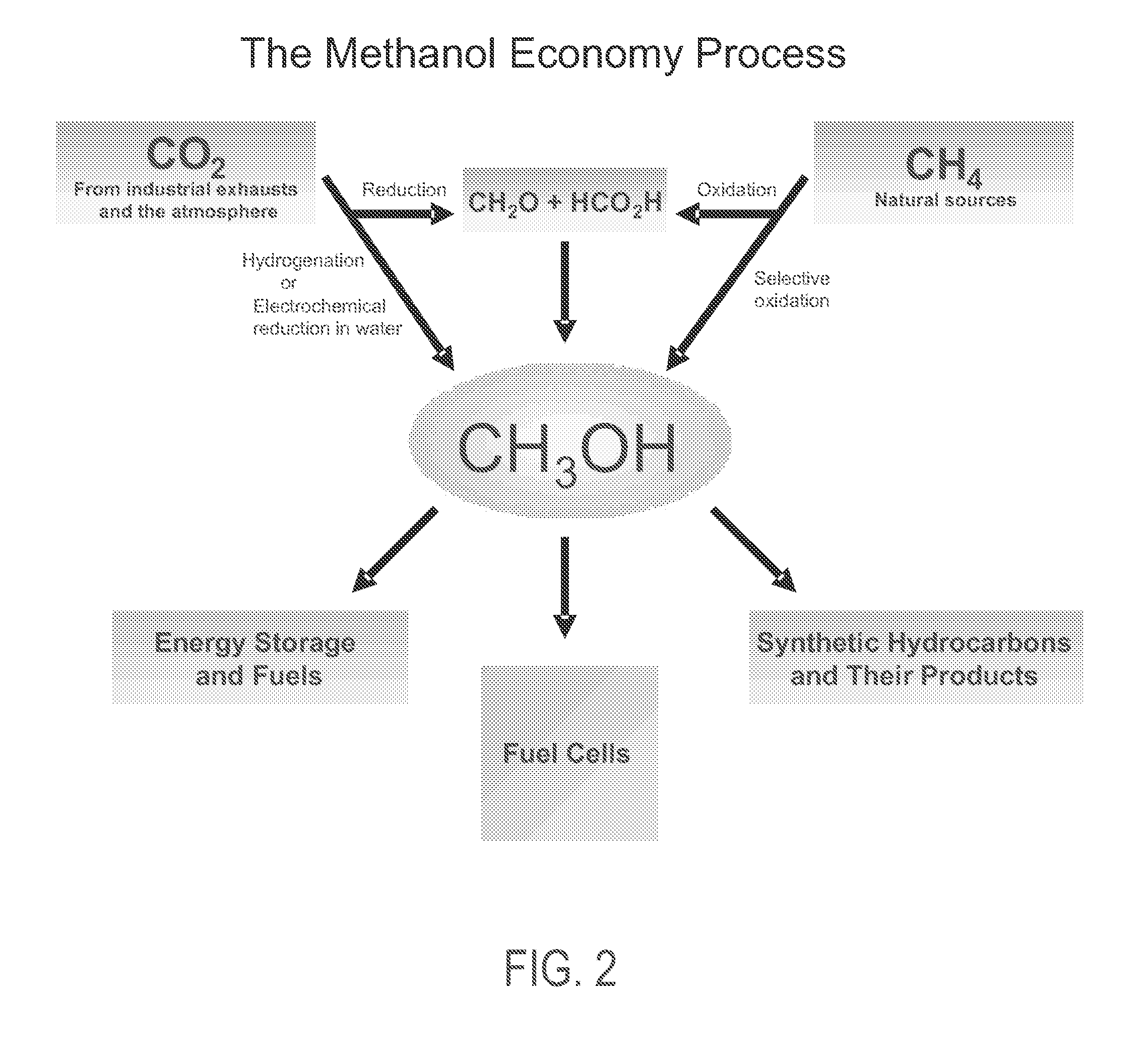
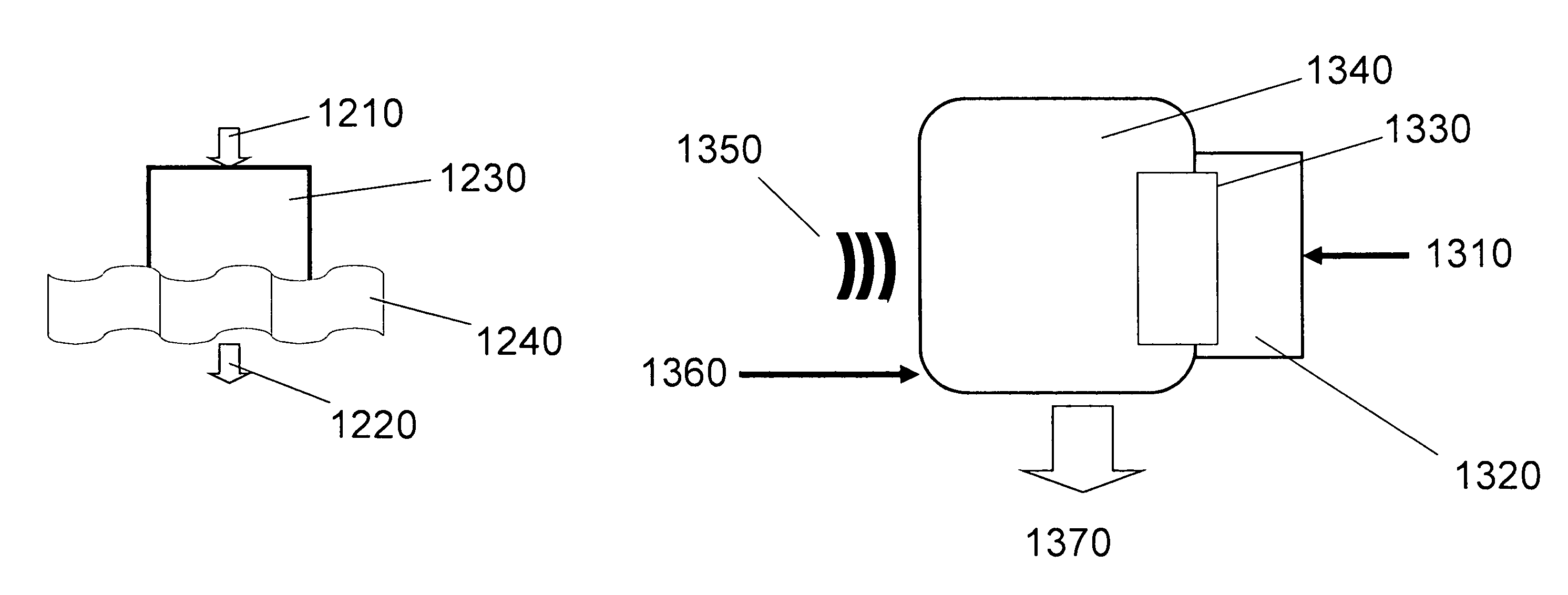
Methods and devices for the production of Hydrocarbons from Carbon and Hydrogen sources
InactiveUS20080283411A1Reduce the environmentEasy to controlPhotography auxillary processesInternal combustion piston enginesElectrolysisAtmospheric air
Devices and methods are described for converting a carbon source and a hydrogen source into hydrocarbons, such as alcohols, for alternative energy sources. The influents may comprise carbon dioxide gas and hydrogen gas or water, obtainable from the atmosphere for through methods described herein, such as plasma generation or electrolysis. One method to produce hydrocarbons comprises the use of an electrolytic device, comprising an anode, a cathode and an electrolyte. Another method comprises the use of ultrasonic energy to drive the reaction. The devices and methods and related devices and methods are useful, for example, to provide a fossil fuel alternative energy source, store renewable energy, sequester carbon dioxide from the atmosphere, counteract global warming, and store carbon dioxide in a liquid fuel.
Owner:PRINCIPLE ENERGY SOLUTIONS
Reducing carbon dioxide to products
InactiveUS20110114502A1Provide stabile long-term reduction of carbon dioxideLow costCellsPhotography auxillary processesPtru catalystElectrical battery
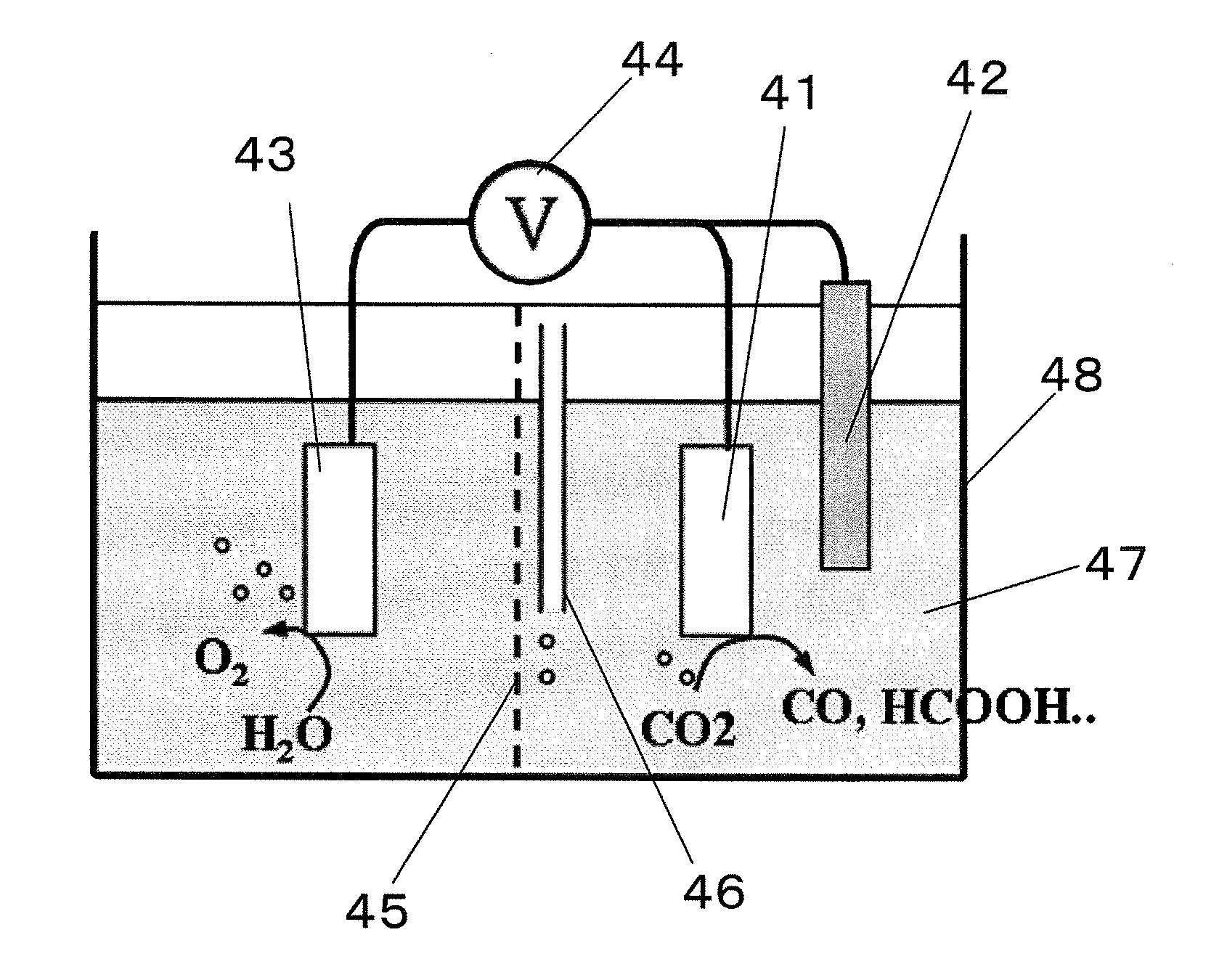
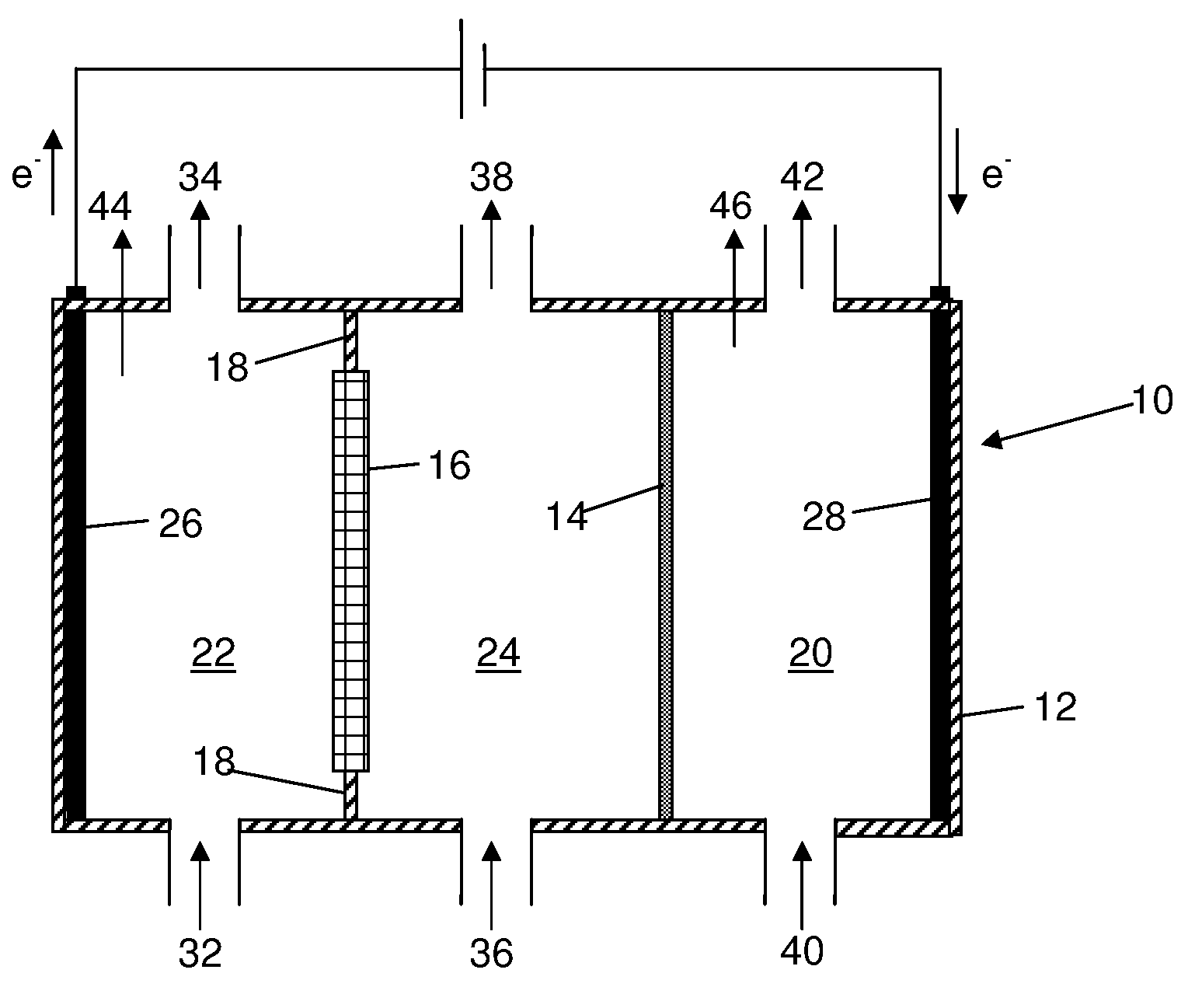
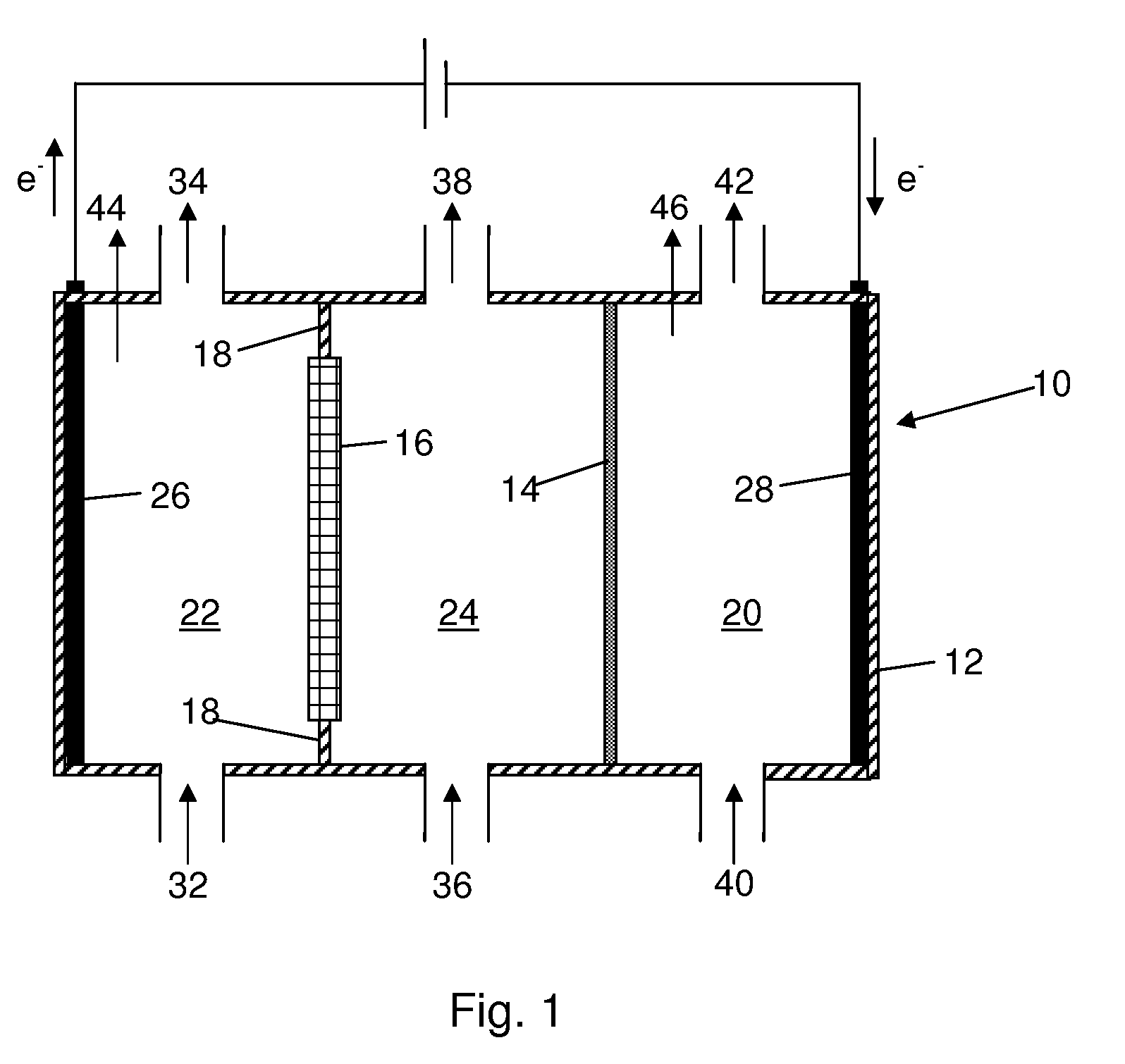
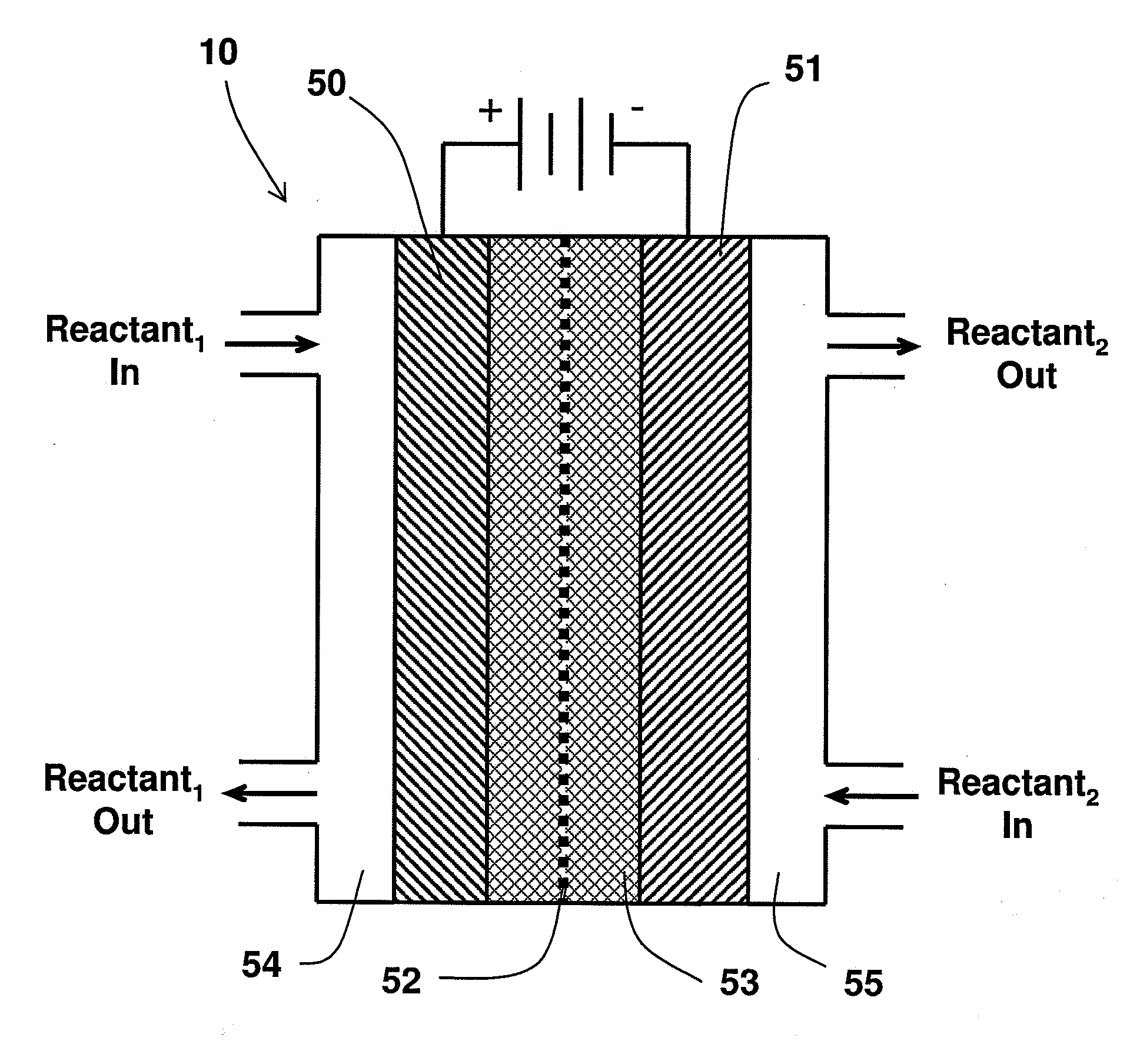
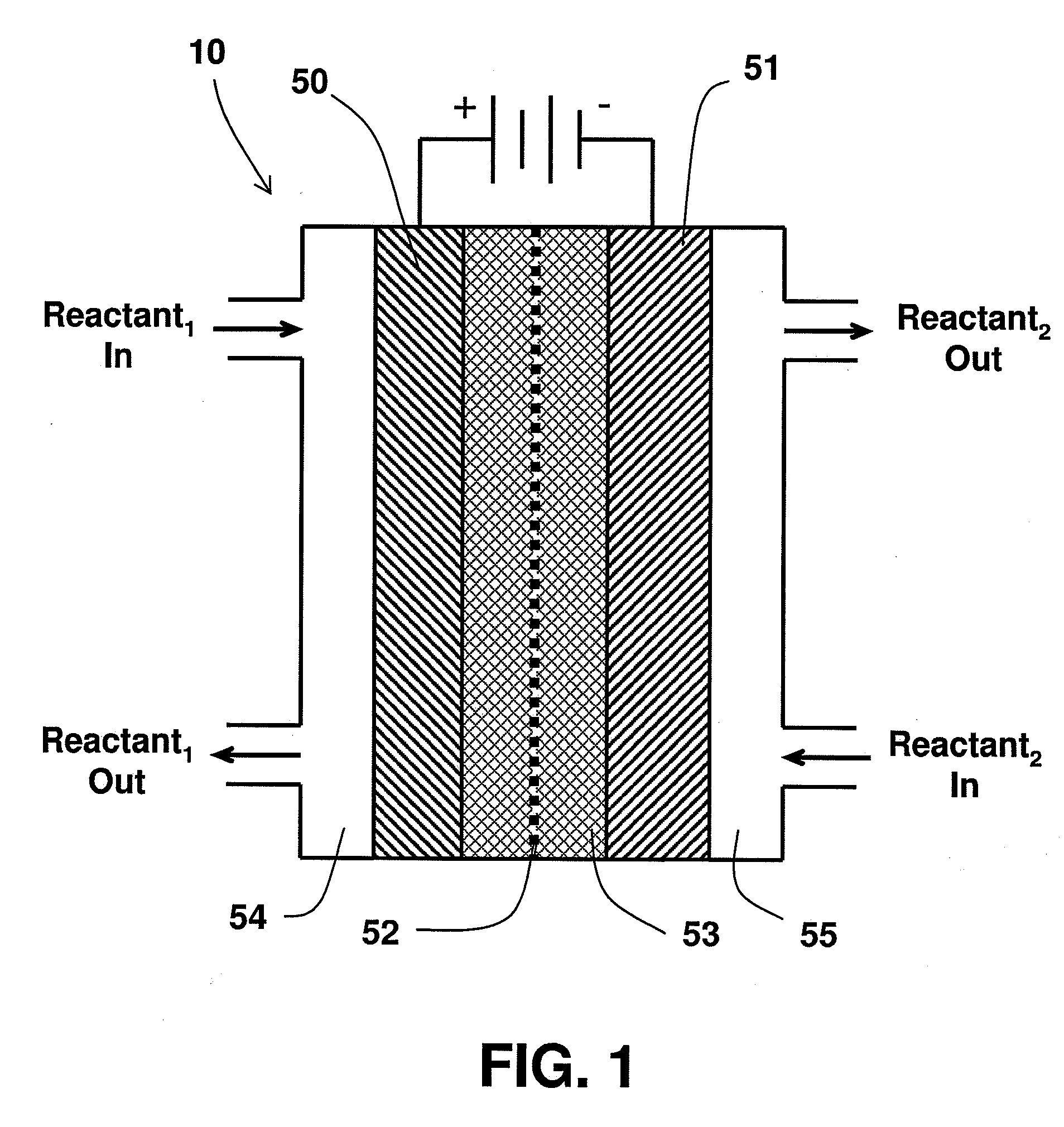
A method for reducing carbon dioxide to one or more products is disclosed. The method may include steps (A) to (C). Step (A) may bubble the carbon dioxide into a solution of an electrolyte and a catalyst in a divided electrochemical cell. The divided electrochemical cell may include an anode in a first cell compartment and a cathode in a second cell compartment. The cathode generally reduces the carbon dioxide into the products. Step (B) may vary at least one of (i) which of the products is produced and (ii) a faradaic yield of the products by adjusting one or more of (a) a cathode material and (b) a surface morphology of the cathode. Step (C) may separate the products from the solution.
Owner:LIQUID LIGHT
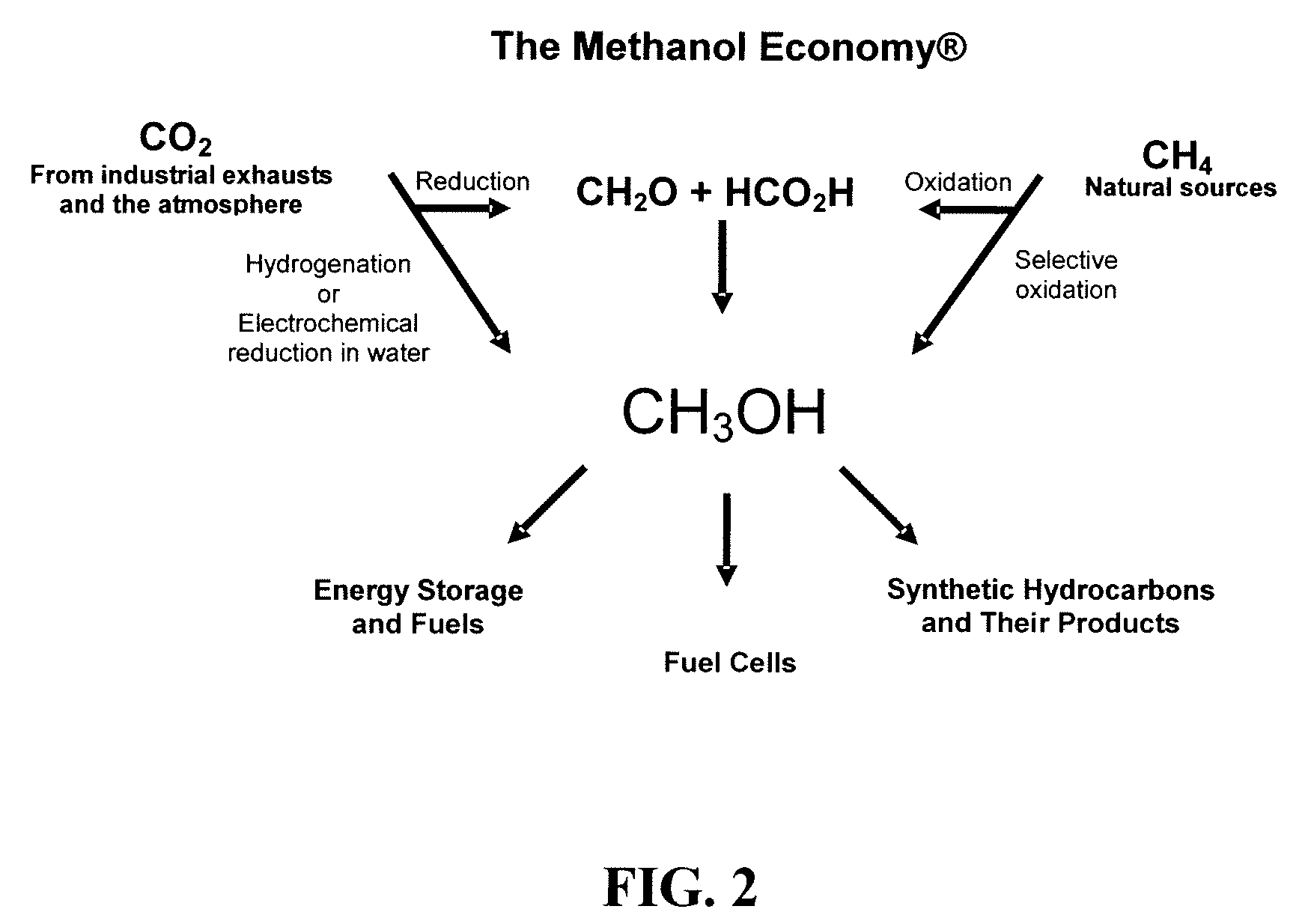
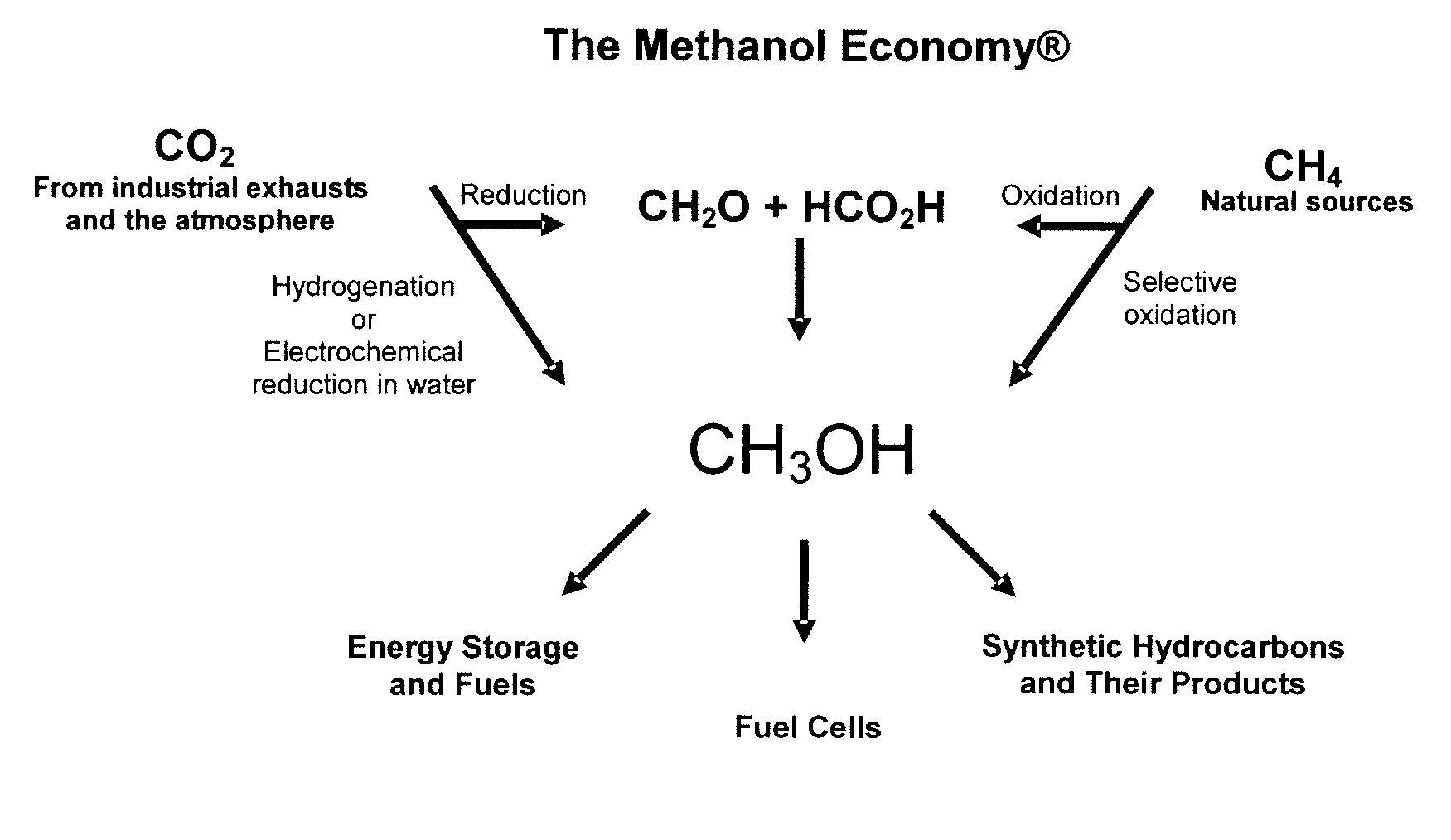
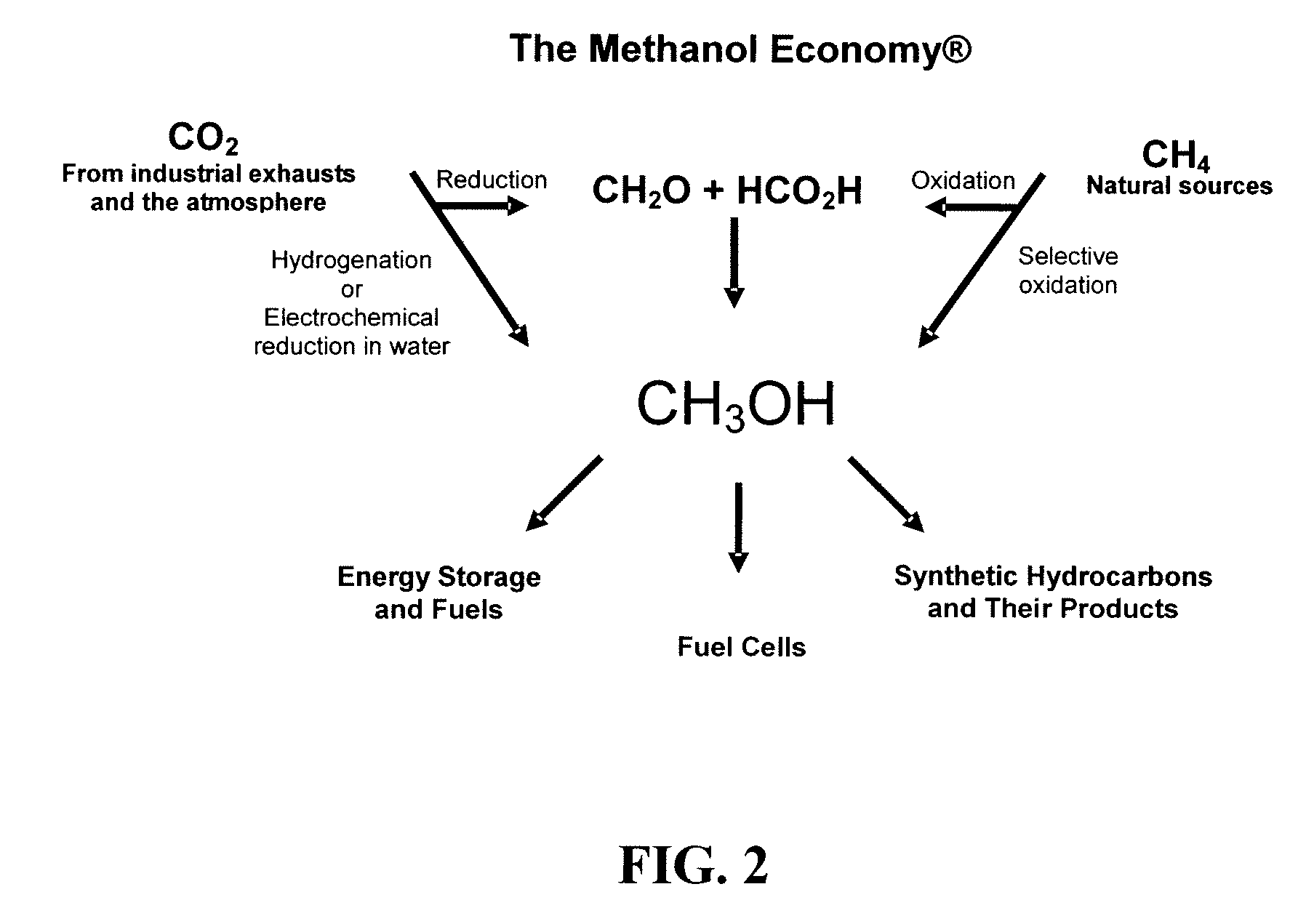
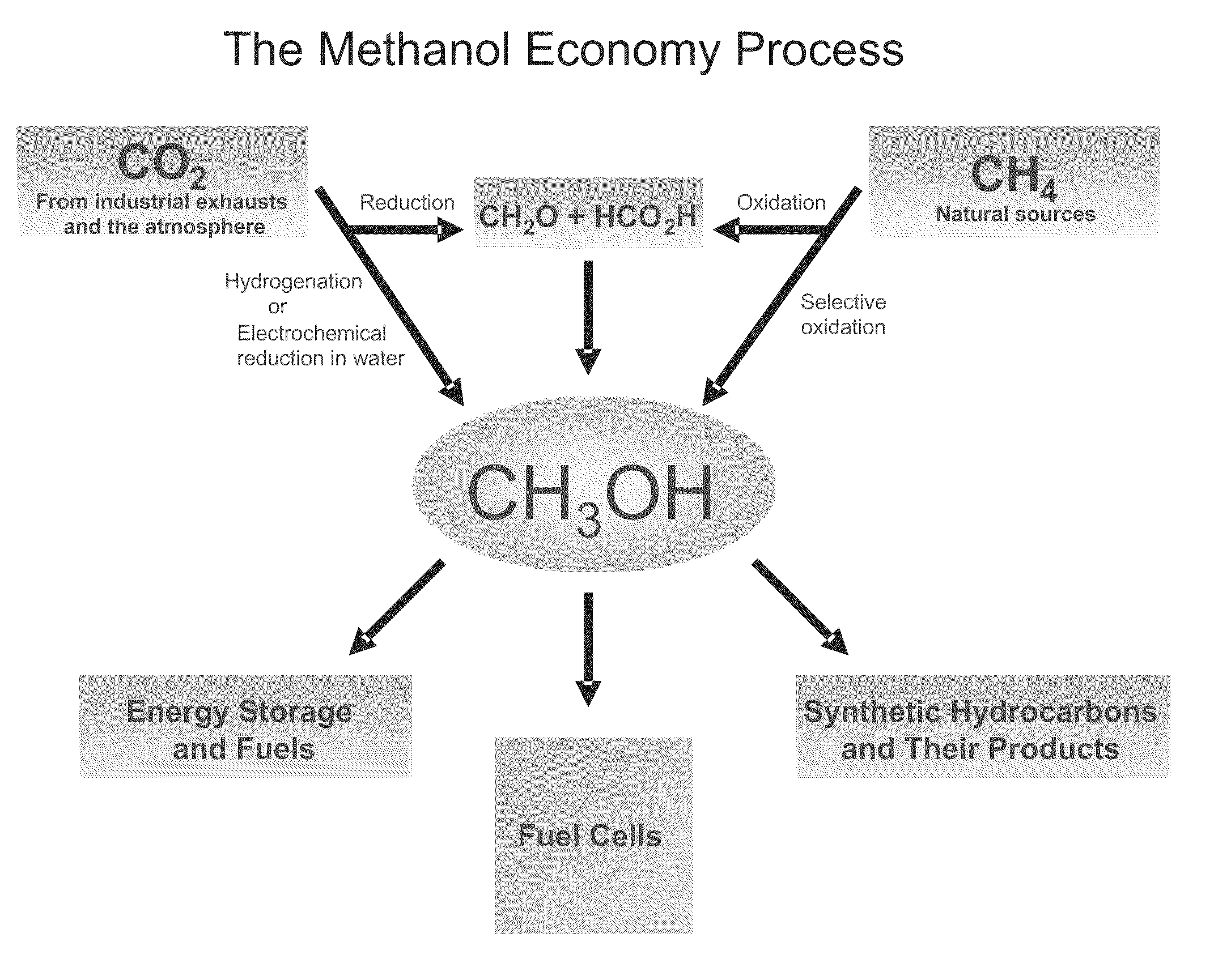
Electrolysis of carbon dioxide in aqueous media to carbon monoxide and hydrogen for production of methanol
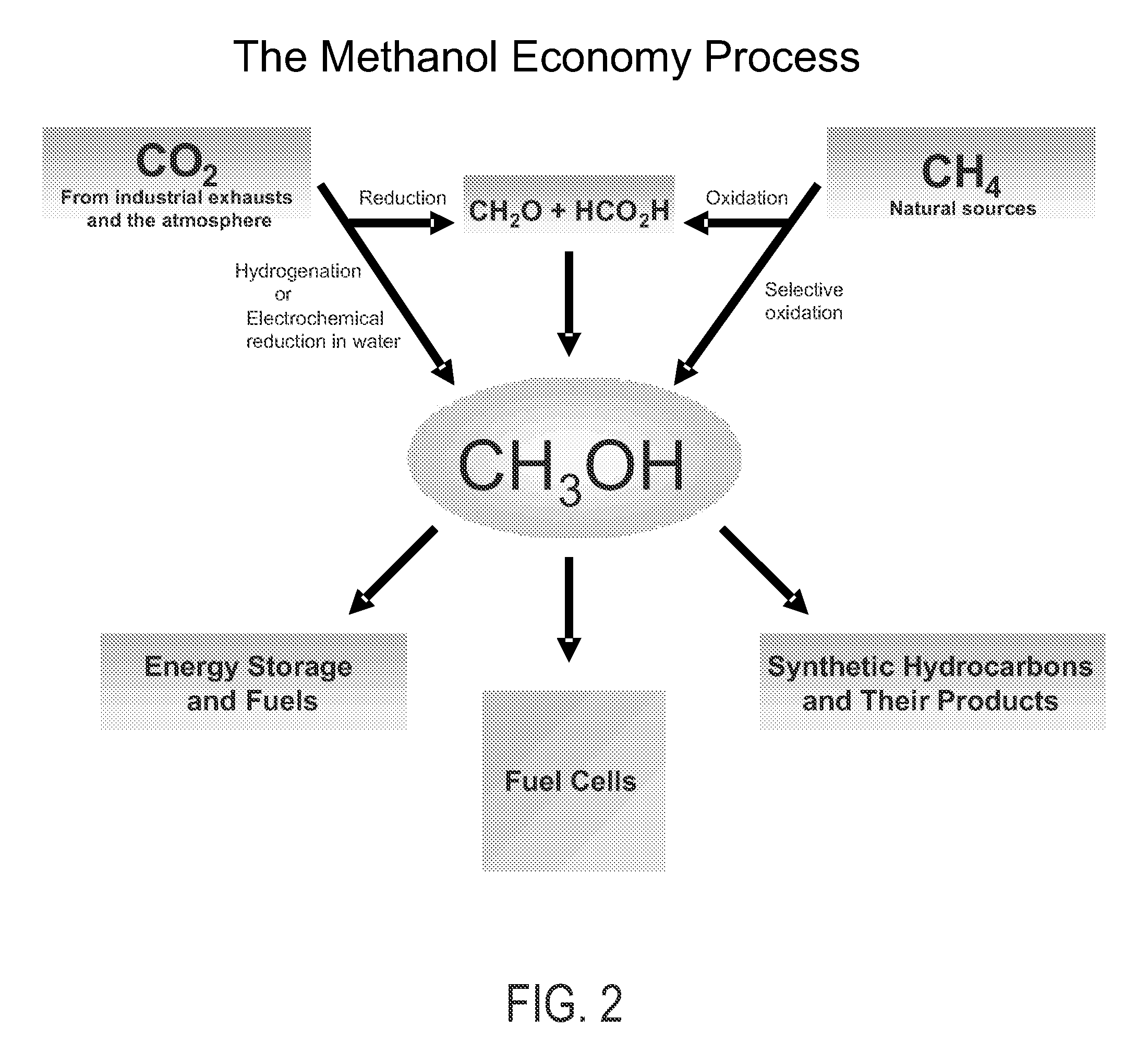
An environmentally beneficial method of producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning power plants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by an electrochemical reduction of carbon dioxide in a divided electrochemical cell that includes an anode in one cell compartment and a metal cathode electrode in another cell compartment that also contains an aqueous solution comprising methanol and an electrolyte of one or more alkyl ammonium halides, alkali carbonates or combinations thereof to produce therein a reaction mixture containing carbon monoxide and hydrogen which can be subsequently used to produce methanol while also producing oxygen in the cell at the anode.
Owner:UNIV OF SOUTHERN CALIFORNIA
Electrolysis of carbon dioxide in aqueous media to carbon monoxide and hydrogen for production of methanol
An environmentally beneficial method of producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning power plants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by an electrochemical reduction of carbon dioxide in a divided electrochemical cell that includes an anode in one cell compartment and a metal cathode electrode in another cell compartment that also contains an aqueous solution comprising methanol and an electrolyte of one or more alkyl ammonium halides, alkali carbonates or combinations thereof to produce therein a reaction mixture containing carbon monoxide and hydrogen which can be subsequently used to produce methanol while also producing oxygen in the cell at the anode.
Owner:UNIV OF SOUTHERN CALIFORNIA
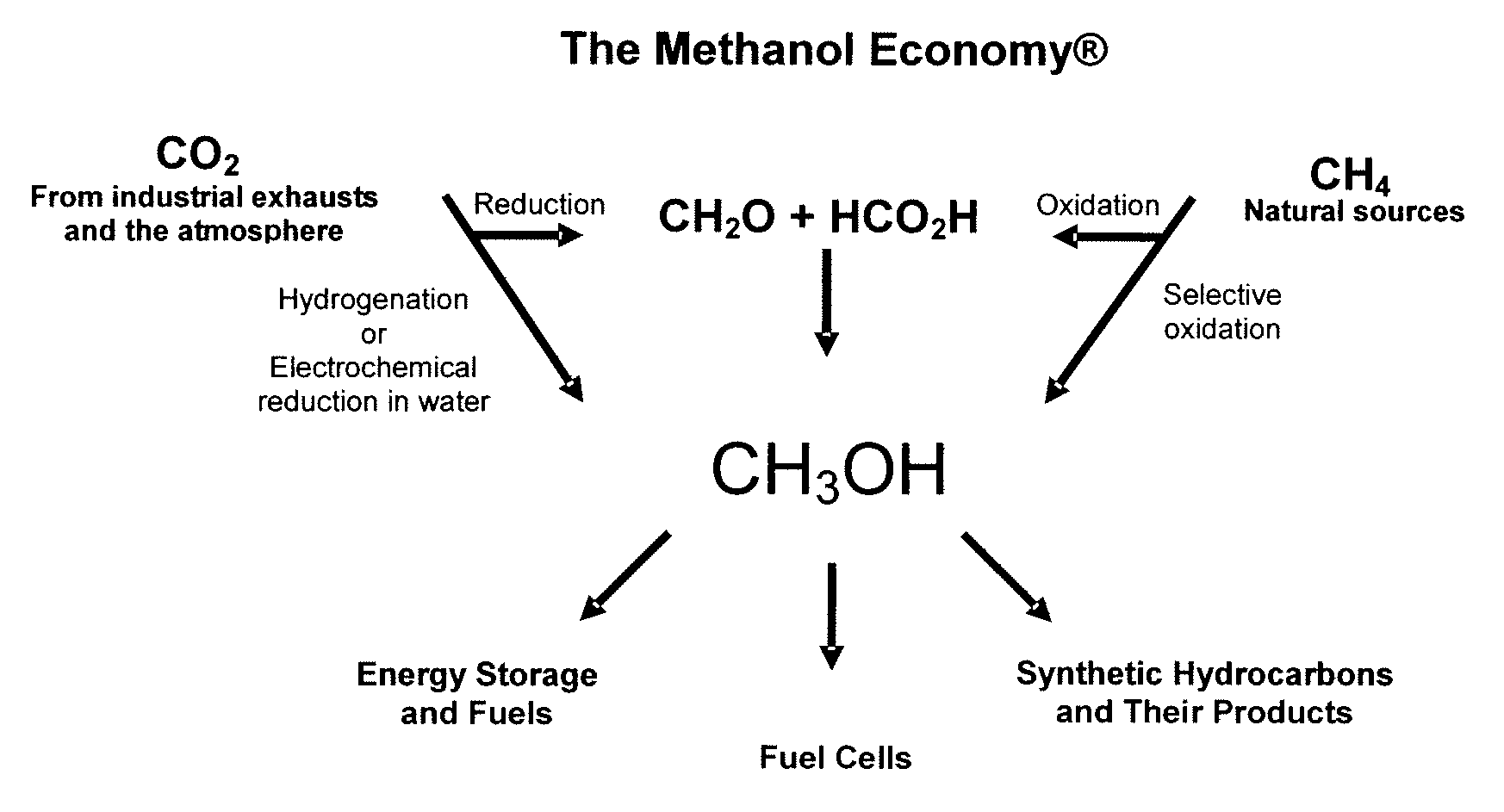
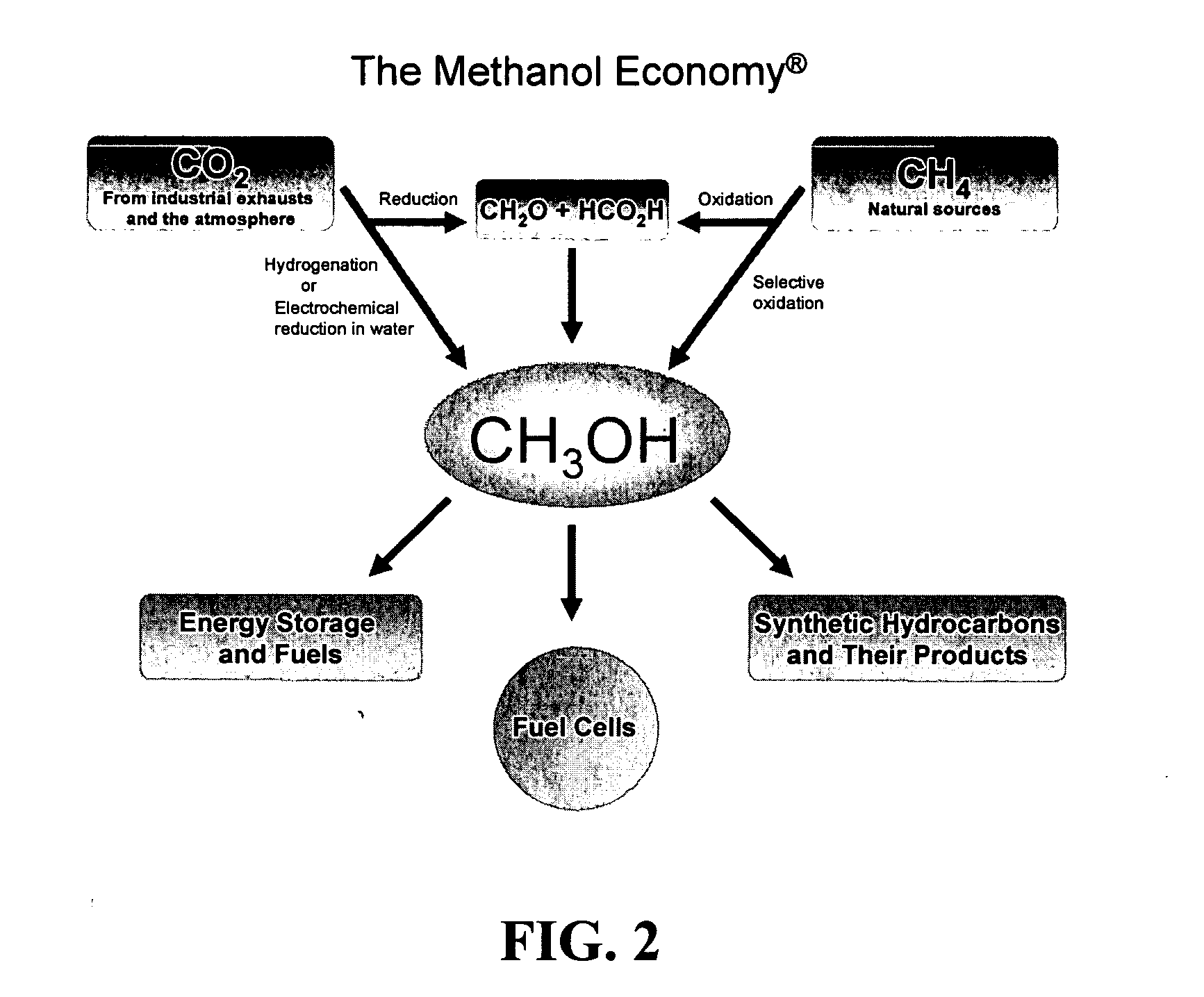
Efficient and selective chemical recycling of carbon dioxide to methanol, dimethyl ether and derived products
ActiveUS20070254969A1Avoid emissionsElectrolysis componentsOxygen compounds purification/separationElectrochemistryDimethyl ether
An efficient and environmentally beneficial method of recycling and producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning powerplants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by chemical or electrochemical reduction secondary treatment to produce essentially methanol, dimethyl ether and derived products.
Owner:UNIV OF SOUTHERN CALIFORNIA
Efficient and selective conversion of carbon dioxide to methanol, dimethyl ether and derived products
ActiveUS20060235091A1Minimize or eliminate the disadvantages or dangers inherentElectrolysis componentsCarbon compoundsHydrogenFlue gas
An environmentally beneficial method of producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning powerplants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by electrochemical reduction produces formic acid acid and some formaldehyde and methanol mixtures. The formic acid can be used as source of carbon as well as hydrogen to produce methanol, dimethyl ether and other products.
Owner:UNIV OF SOUTHERN CALIFORNIA
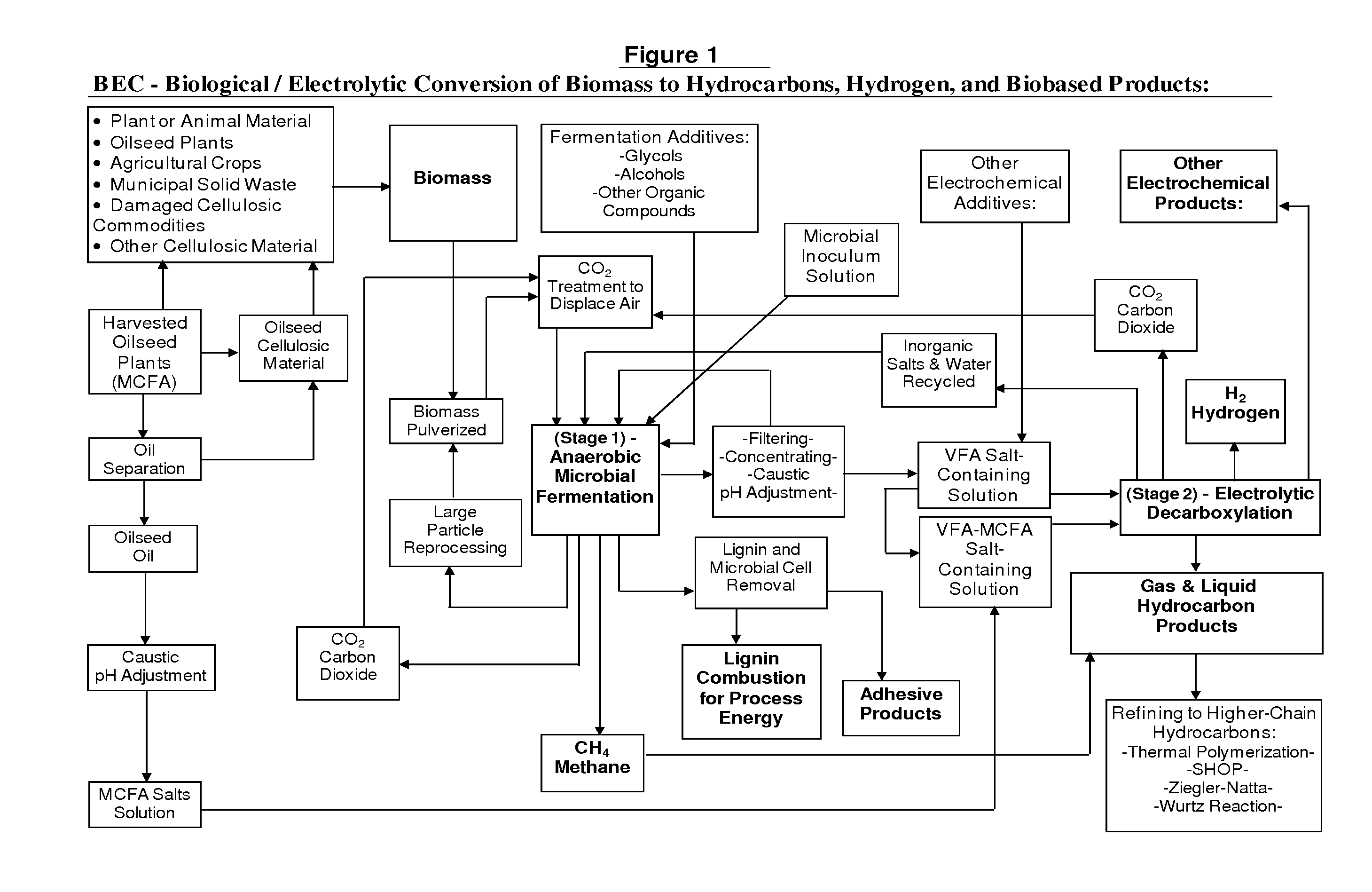
Biological/Electrolytic Conversion of Biomass to Hydrocarbons
ActiveUS20110111475A1Eliminate needInexpensive solutionElectrolysis componentsBacteriaElectrolysisSufficient time
Hydrocarbon and hydrogen fuels and other products may be produced by a process employing a combination of fermentation and electrochemical stages. In the process, a biomass contained within a fermentation medium is fermented with an inoculum comprising a mixed culture of microorganisms derived the rumen contents of a rumen-containing animal. This inoculated medium is incubated under anaerobic conditions and for a sufficient time to produce volatile fatty acids. The resultant volatile fatty acids are then subjected to electrolysis under conditions effective to convert said volatile fatty acids to hydrocarbons and hydrogen simultaneously. The process can convert a wide range of biomass materials to a wide range of volatile fatty acid chain lengths and can convert these into a wide range of biobased fuels and biobased products.
Owner:US SEC AGRI +1
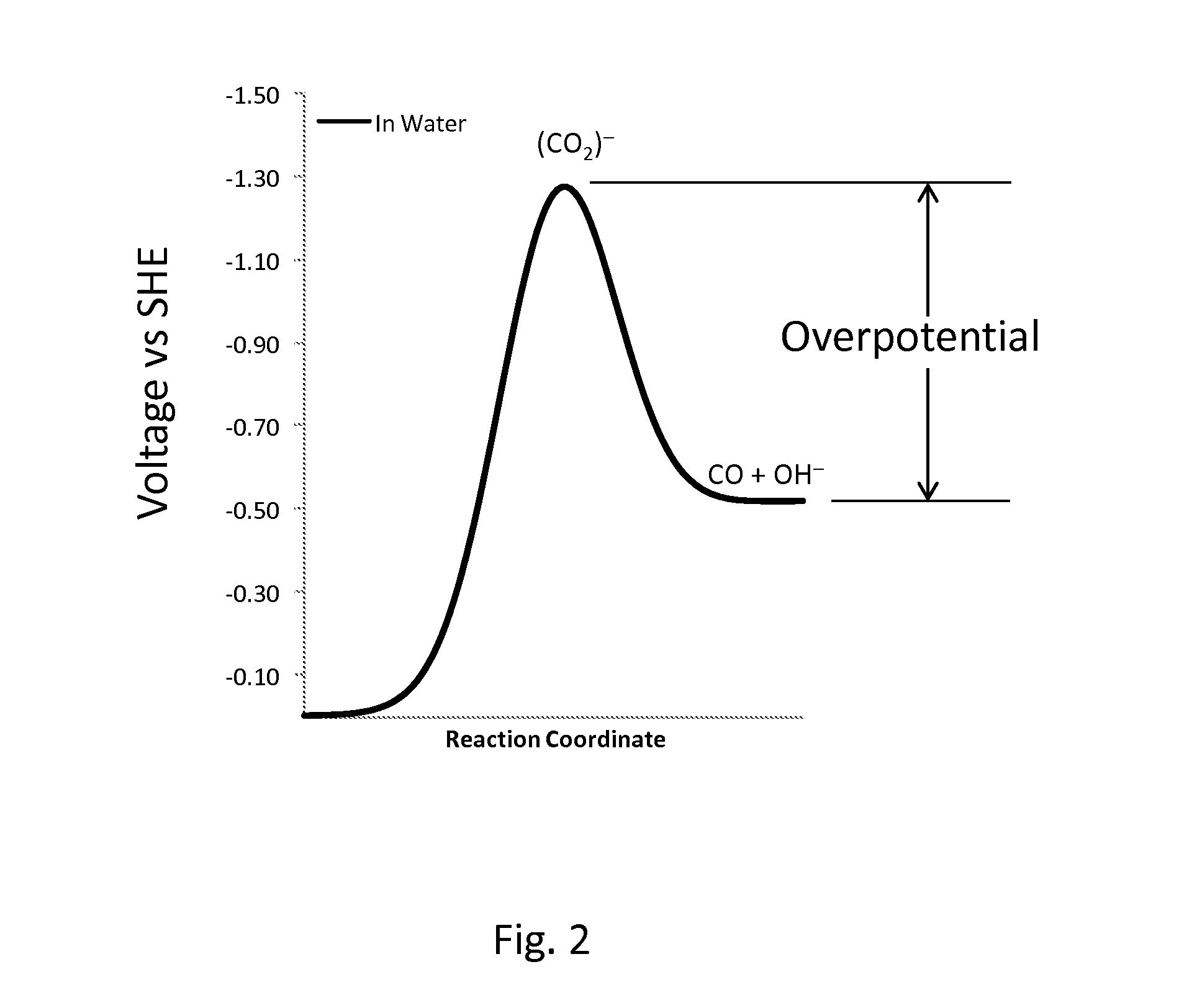
Novel catalyst mixtures
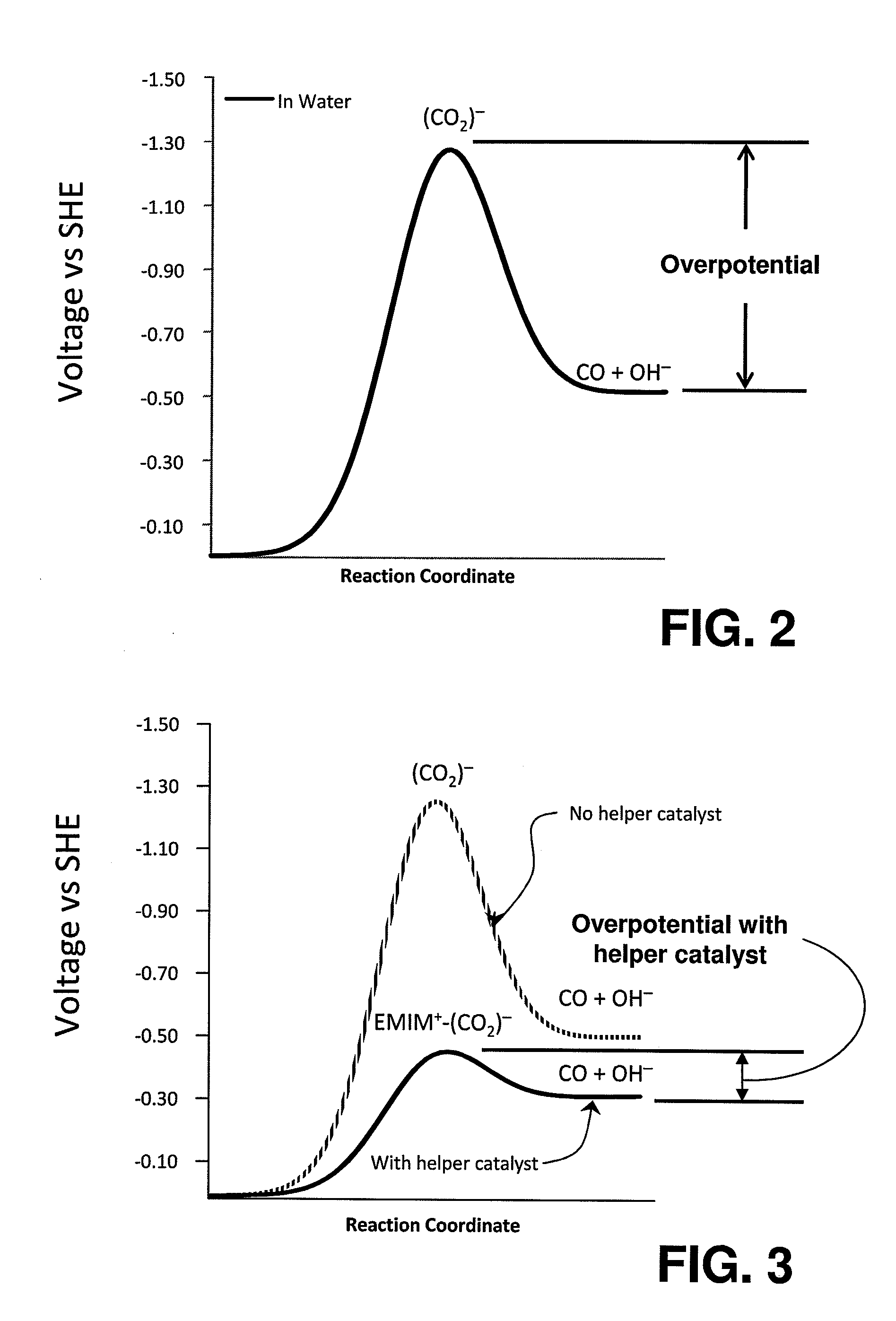
InactiveUS20110237830A1Low rateRaise the overpotentialOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsChemical reactionCompound (substance)
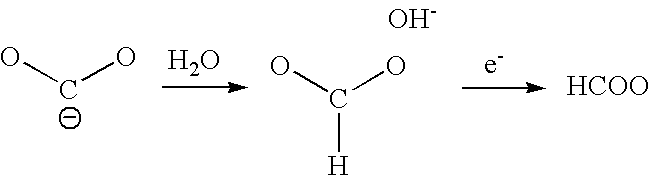
Catalysts comprised of at least one catalytically active element and at least one helper catalyst are disclosed. The catalysts may be used to increase the rate, the selectivity or lower the overpotential of chemical reactions. These catalysts may be useful for a variety of chemical reactions including in particular the electrochemical conversion of carbon dioxide to formic acid.
Owner:DIOXIDE MATERIALS
Method And System For Electrochemical Production Of Formic Acid From Carbon Dioxide
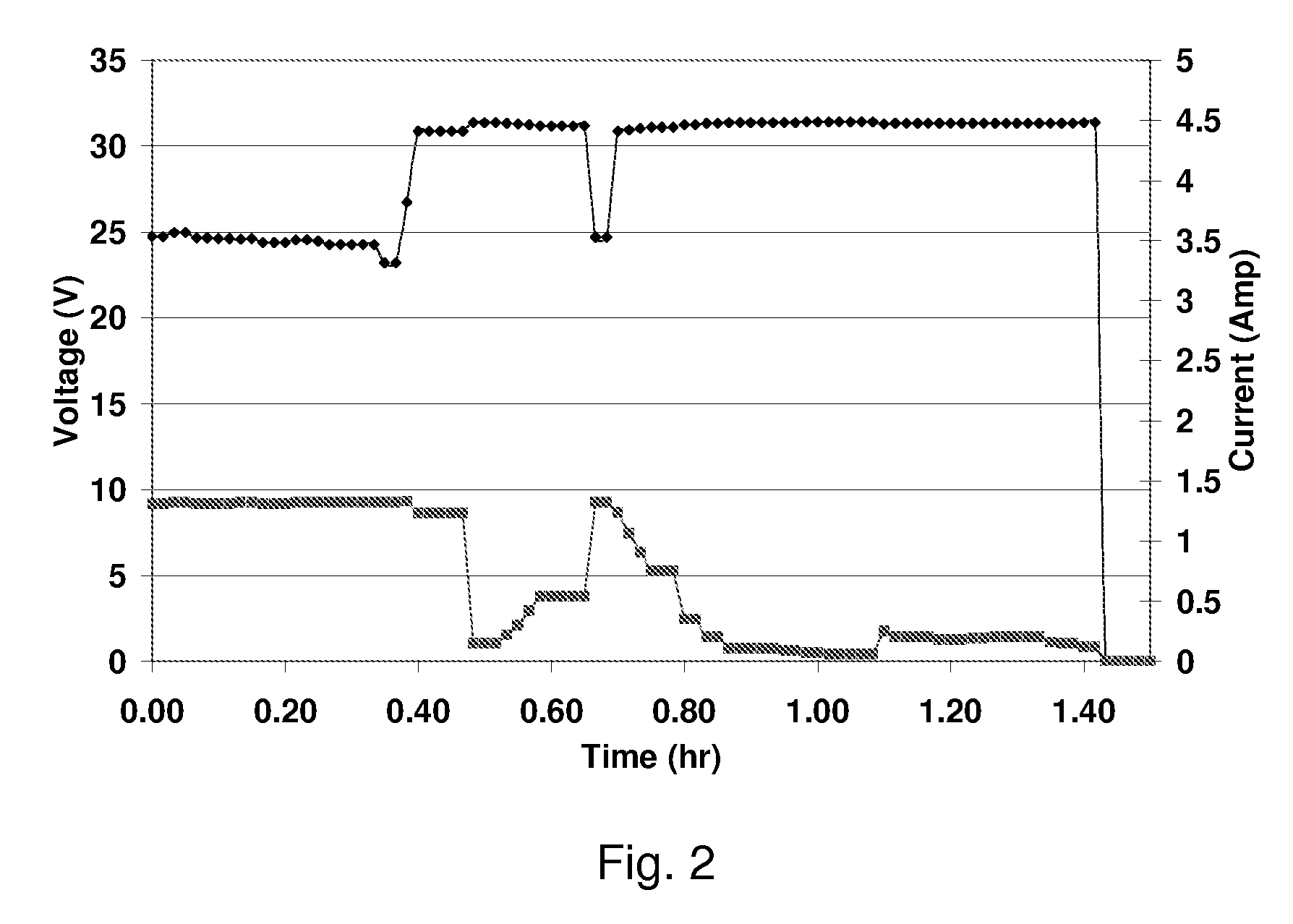
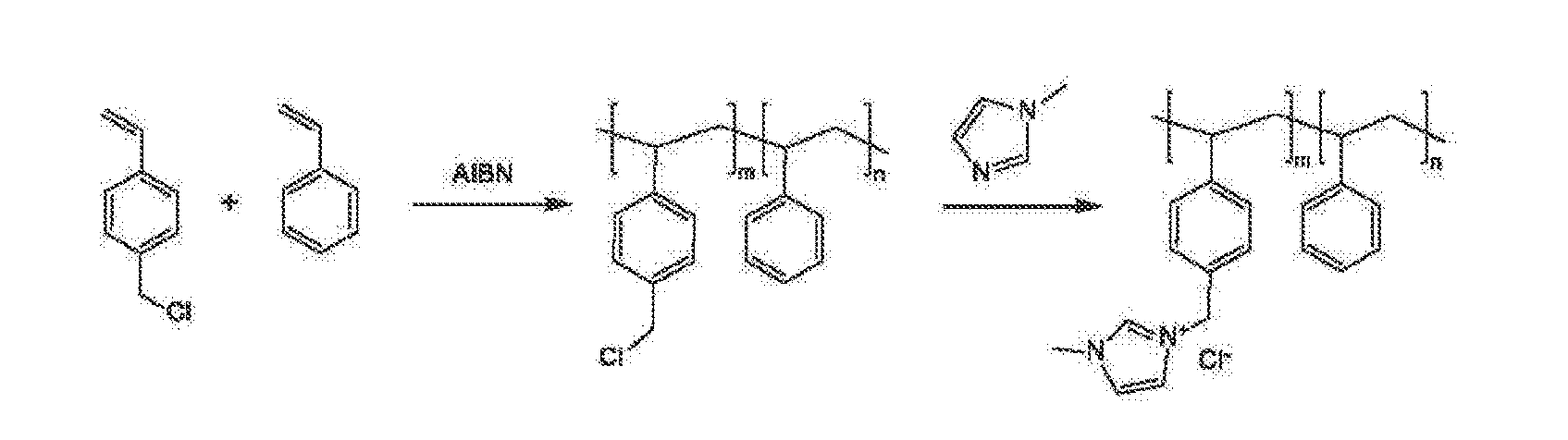
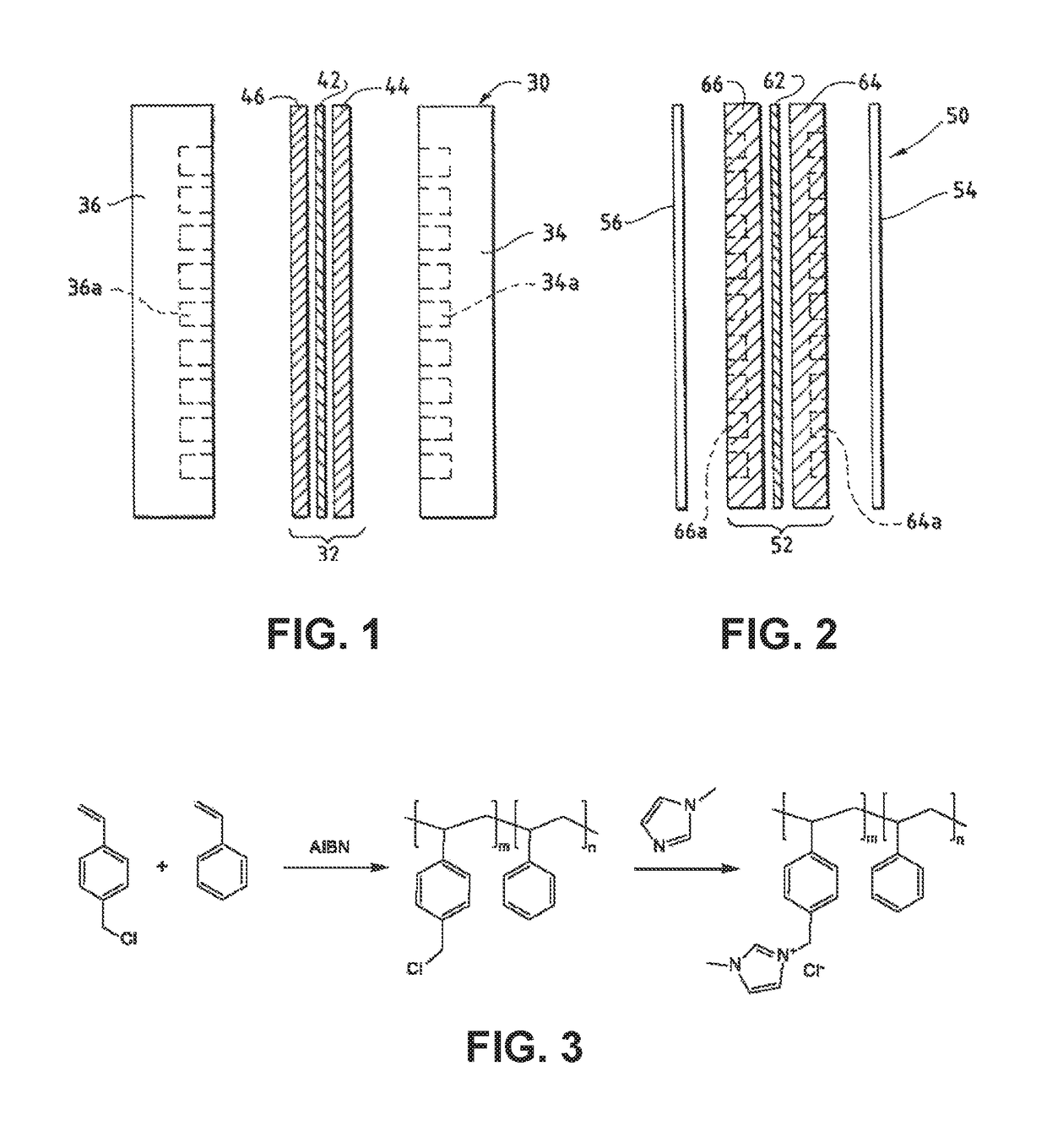
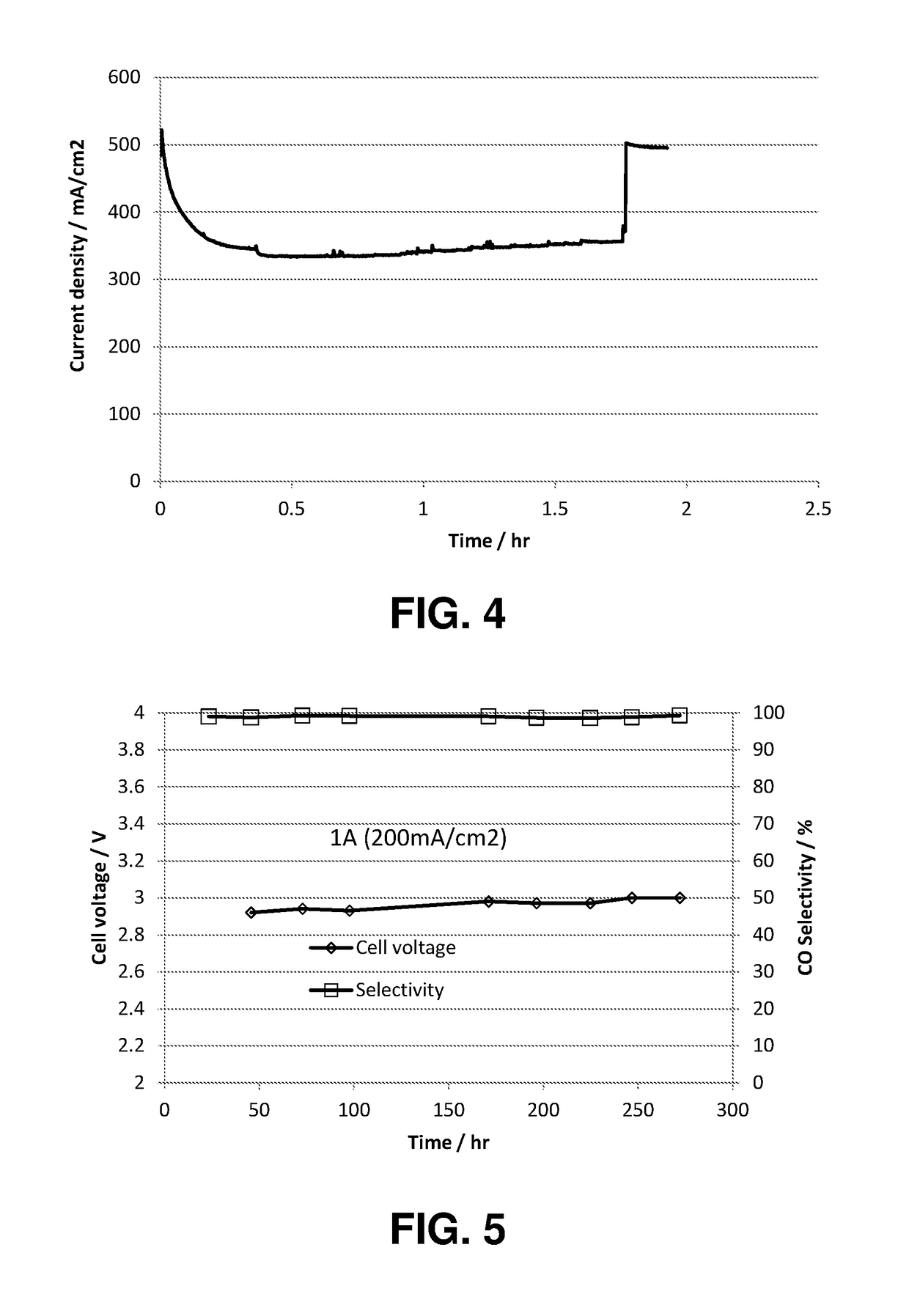
An electrochemical device converts carbon dioxide to a formic acid reaction product. The device includes an anode and a cathode, each comprising a quantity of catalyst. The anode and cathode each have reactant introduced thereto. Two membranes, a cation exchange polymer electrolyte membrane and an anion exchange polymer electrolyte membrane, are interposed between the anode and the cathode, forming a central flow compartment where a carbon dioxide reduction product, such as formic acid, can be recovered. At least a portion of the cathode catalyst is directly exposed to gaseous carbon dioxide during electrolysis. The average current density at the membrane is at least 20 mA / cm2, measured as the area of the cathode gas diffusion layer that is covered by catalyst, and formate ion selectivity is at least 50% at a cell potential difference of 3.0 V. In some embodiments, at least one polymer electrolyte membrane comprises a polymer in which a constituent monomer is (p-vinylbenzyl)-R, where R is selected from the group consisting of imidazoliums, pyridiniums and phosphoniums. In some embodiments, the polymer electrolyte membrane is a Helper Membrane comprising a polymer containing an imidazolium ligand, a pyridinium ligand, or a phosphonium ligand.
Owner:DIOXIDE MATERIALS
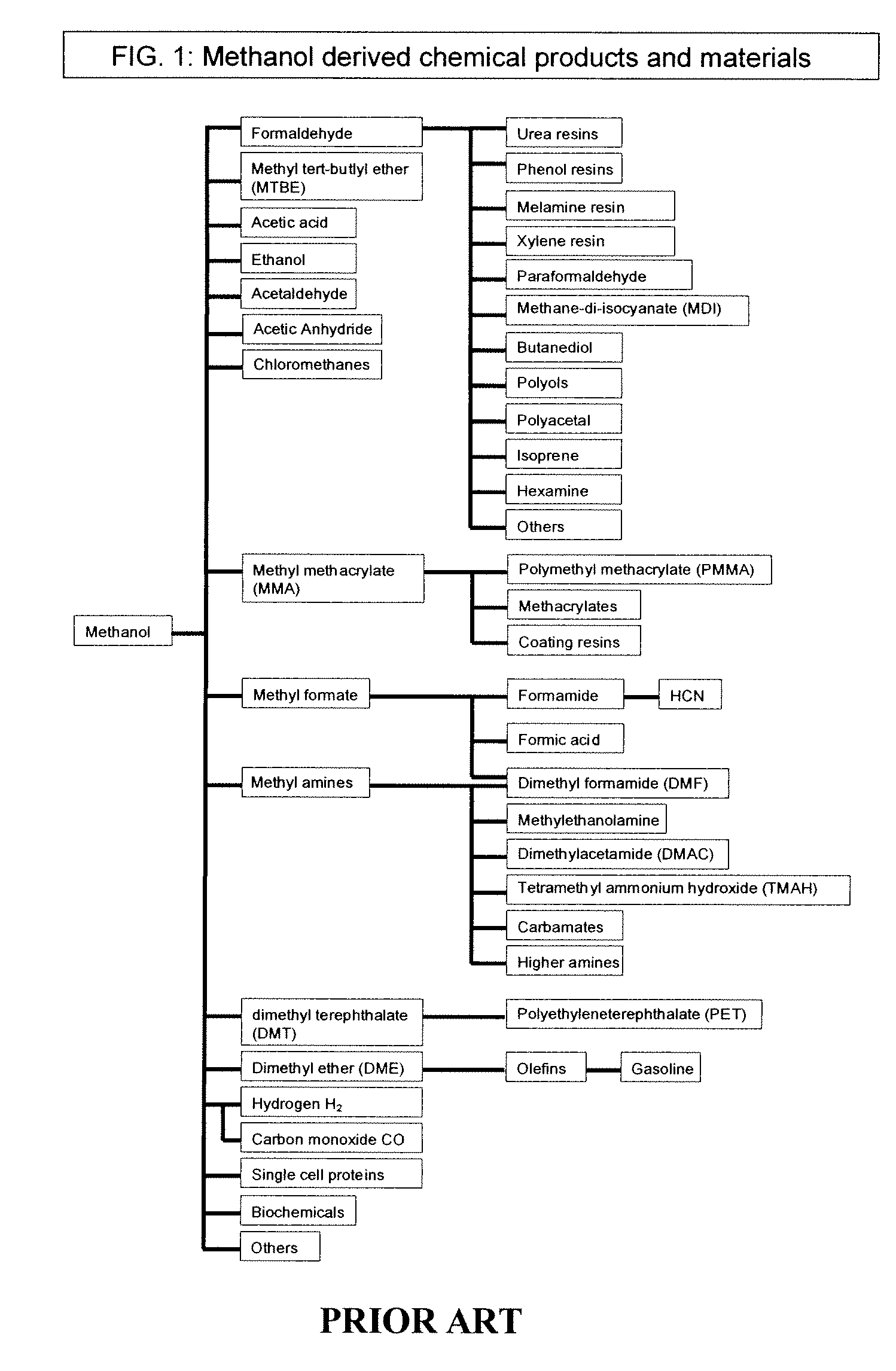
Selective oxidative conversion of methane to methanol, dimethyl ether and derived products
ActiveUS20060235088A1Minimize or eliminate the disadvantages or dangers inherentPreparation by oxidation reactionsElectrolysis componentsFormate EstersDimethyl ether
The present invention relates to a method of producing methanol from a methane source by oxidizing methane under conditions sufficient to a mixture of methanol and formaldehyde while minimizing the formation of formic acid and carbon dioxide. The oxidation step is followed by treatment step in which formaldehyde is converted into methanol and formic acid which itself can further be converted into methanol via catalytic hydrogenation of intermediately formed methyl formate.
Owner:UNIV OF SOUTHERN CALIFORNIA
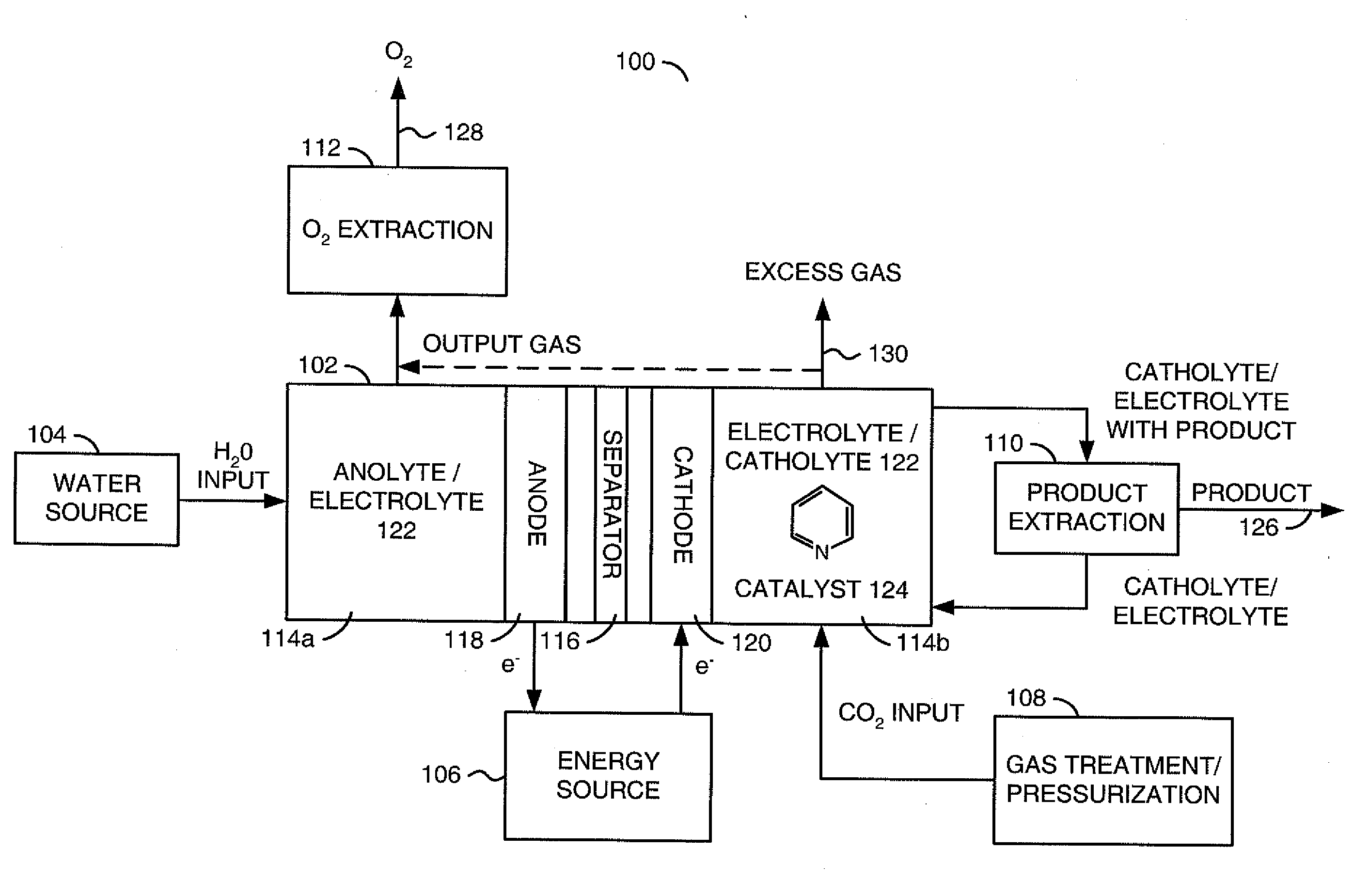
Conversion of carbon dioxide to organic products
ActiveUS20100187123A1Effective and stableReducing carbon dioxideCellsPhotography auxillary processesAqueous solutionElectrochemical cell
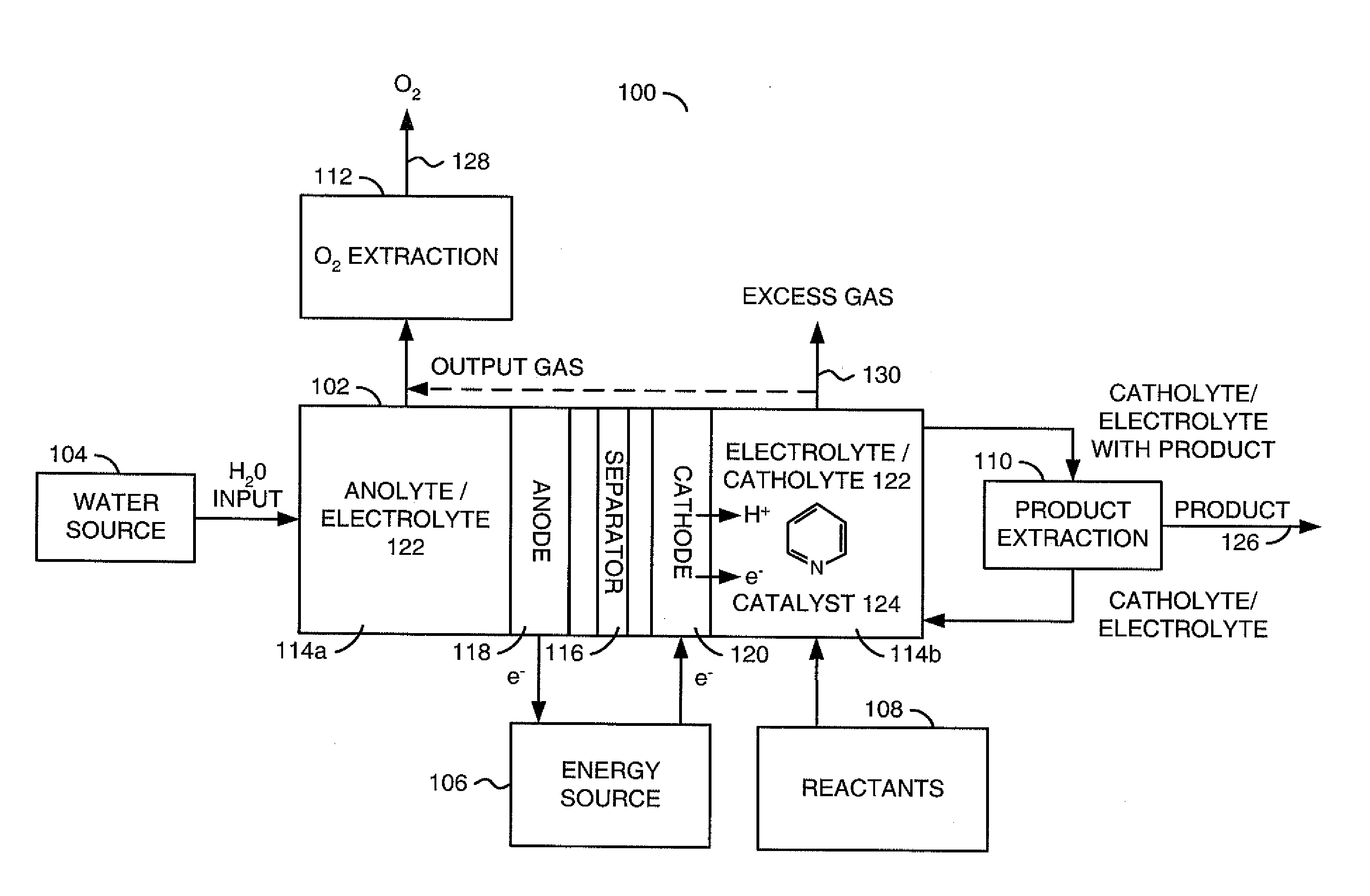
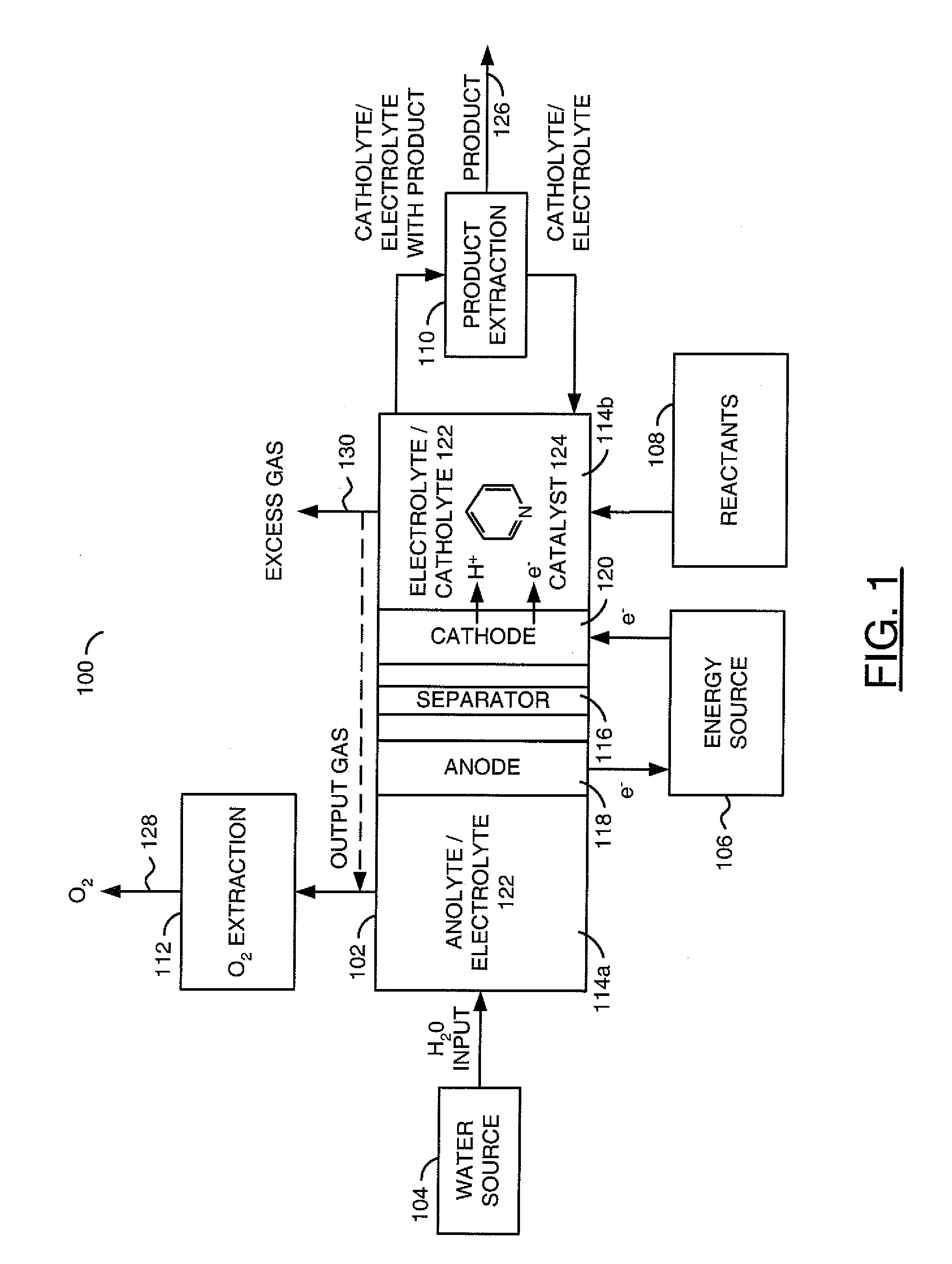
The invention relates to various embodiments of an environmentally beneficial method for reducing carbon dioxide. The methods in accordance with the invention include electrochemically or photoelectrochemically reducing the carbon dioxide in a divided electrochemical cell that includes an anode, e.g., an inert metal counterelectrode, in one cell compartment and a metal or p-type semiconductor cathode electrode in another cell compartment that also contains an aqueous solution of an electrolyte and a catalyst of one or more substituted or unsubstituted aromatic amines to produce therein a reduced organic product.
Owner:PRINCETON UNIV
Electrolysis of carbon dioxide in aqueous media to carbon monoxide and hydrogen for production of methanol
ActiveUS20100193370A1Efficient combustionElectrolysis componentsPhotography auxillary processesElectrolysisAtmospheric air
An environmentally beneficial method of producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning power plants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by an electrochemical reduction of carbon dioxide in a divided electrochemical cell that includes an anode in one compartment and a metal cathode electrode in a compartment that also contains an aqueous solution comprising methanol and an electrolyte. An anion-conducting membrane can be provided between the anode and cathode to produce at the cathode therein a reaction mixture containing carbon monoxide and hydrogen, which can be subsequently used to produce methanol while also producing oxygen in the cell at the anode. The oxygen produced at the anode can be recycled for efficient combustion of fossil fuels in power plants to exclusively produce CO2 exhausts for capture and recycling as the source of CO2 for the cell.
Owner:UNIV OF SOUTHERN CALIFORNIA
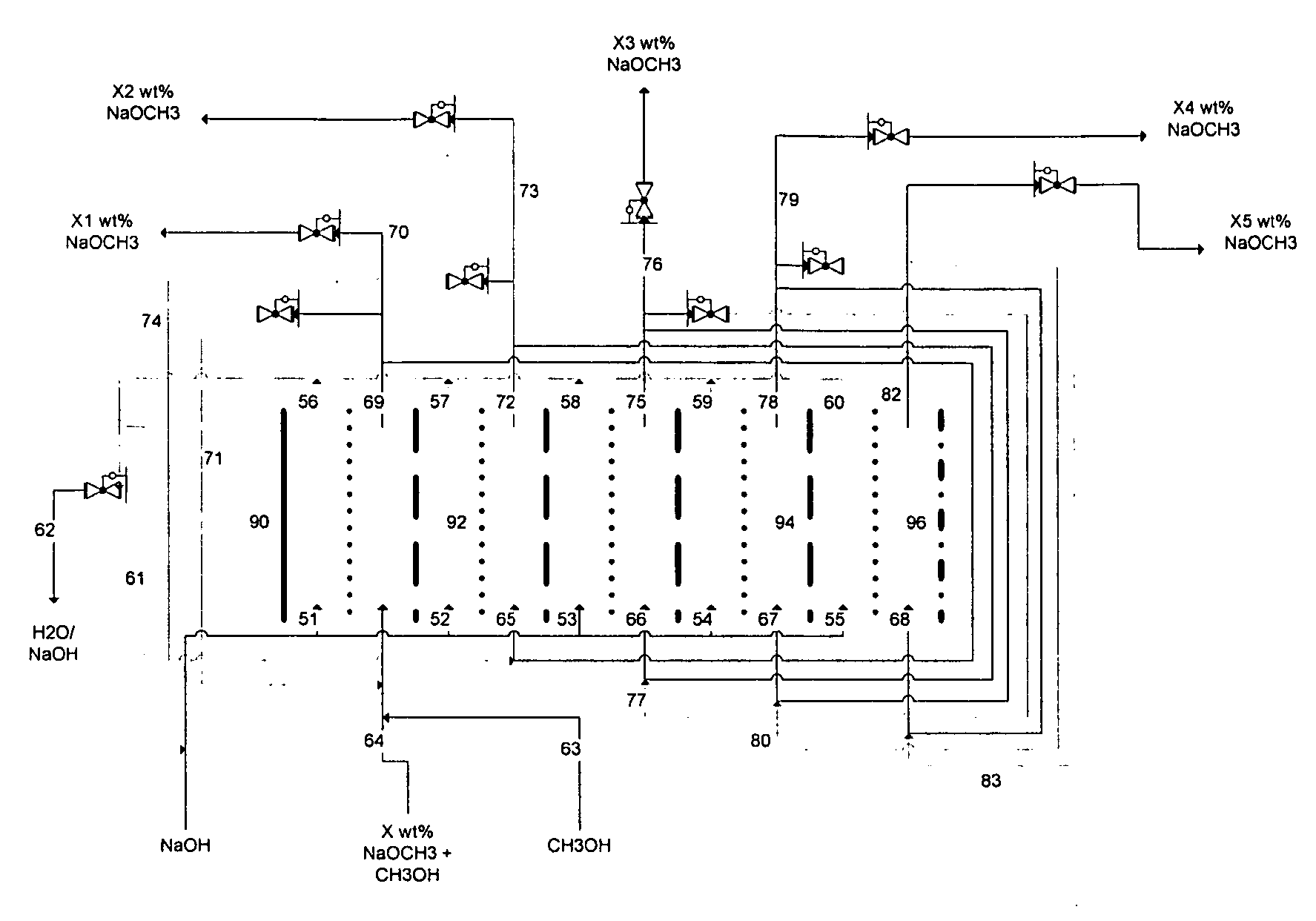
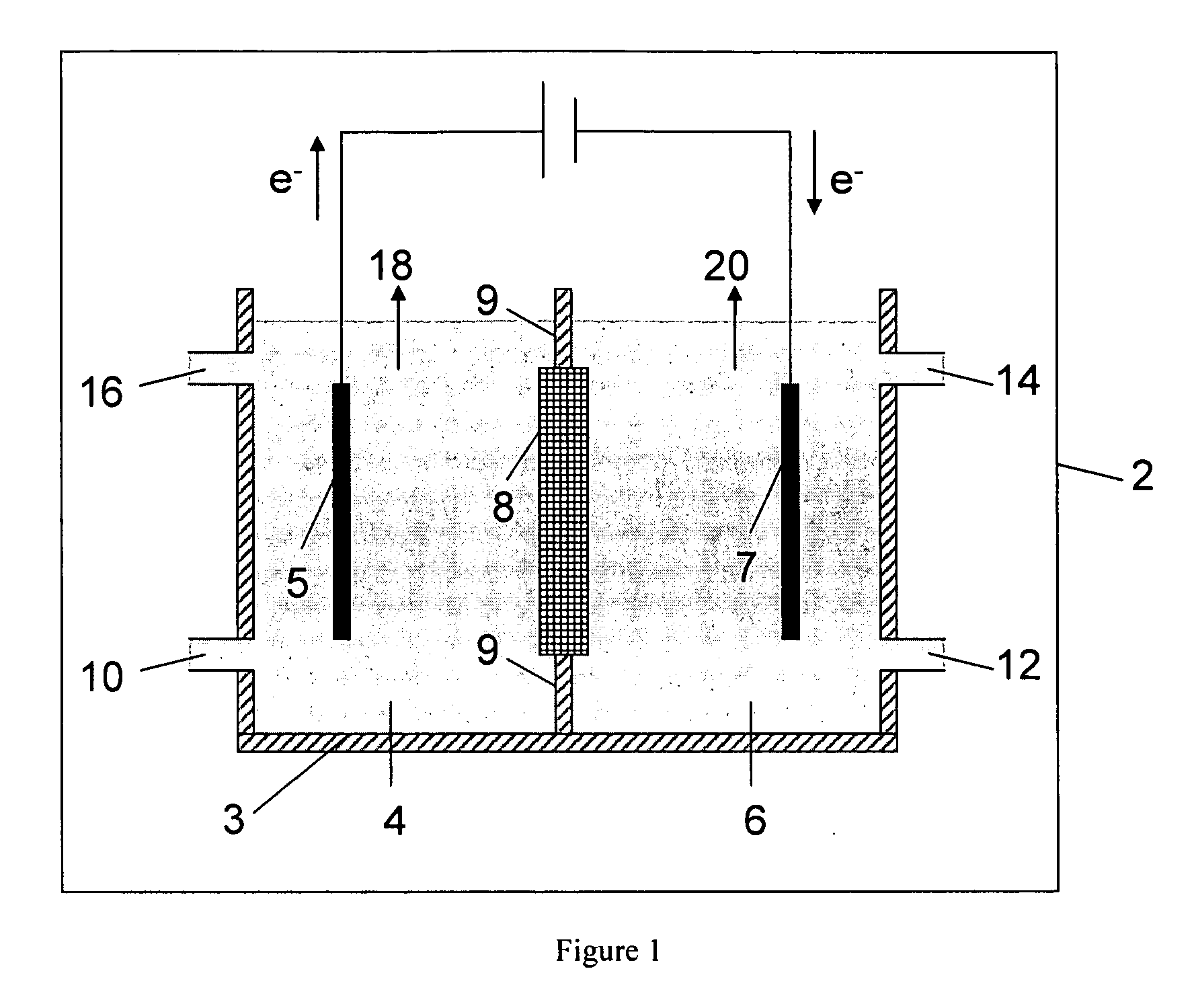
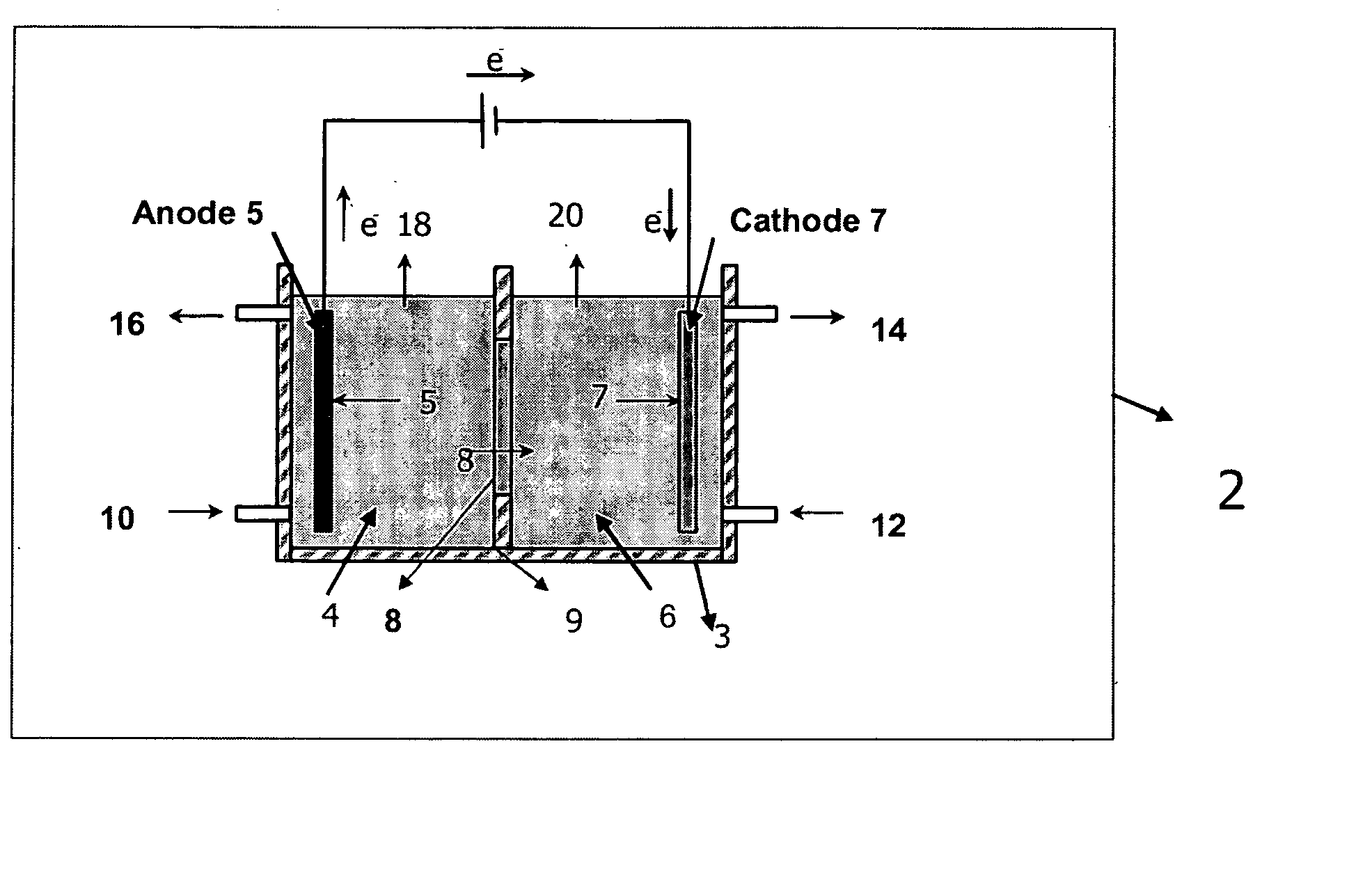
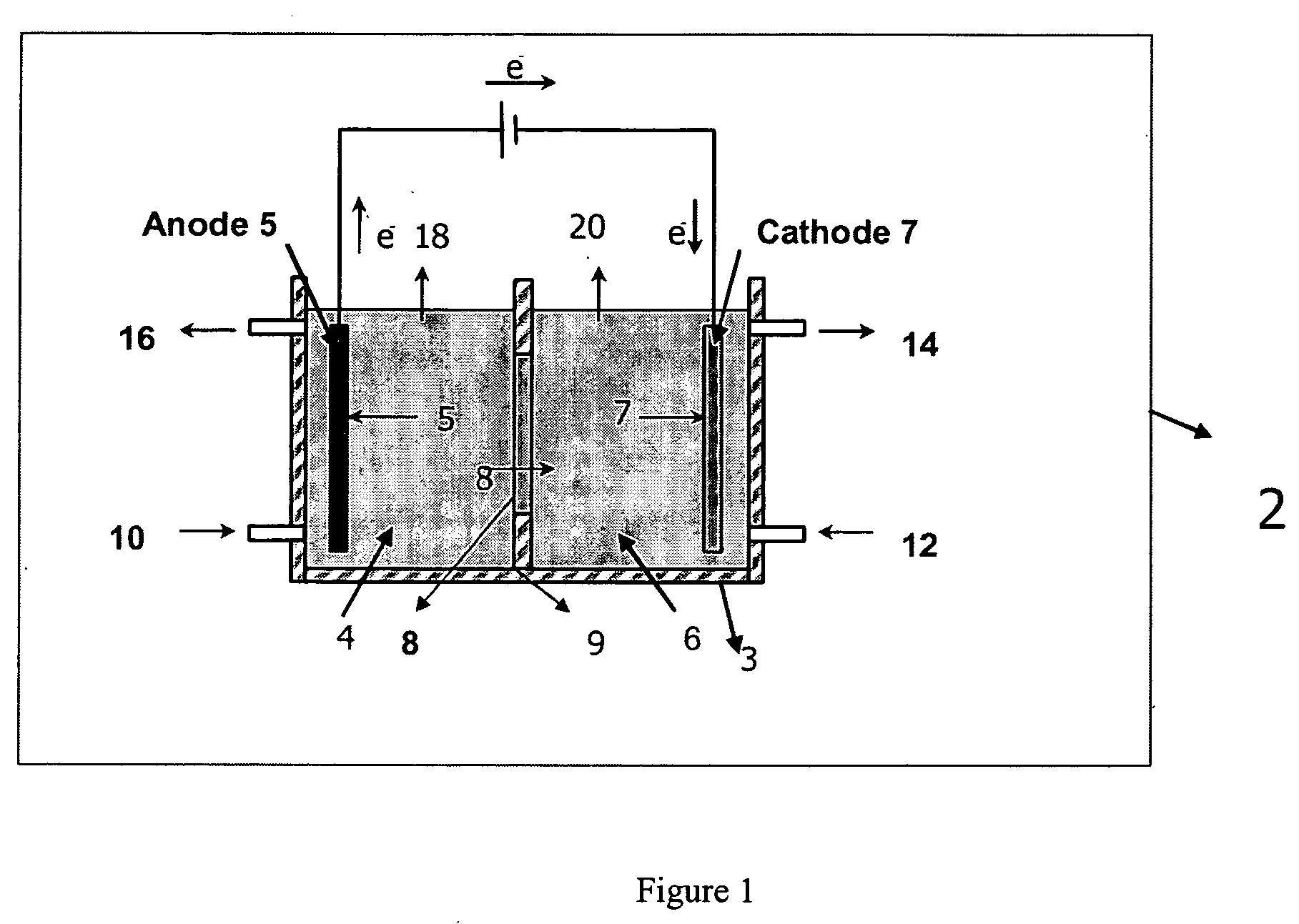
Electrolytic method to make alkali alcoholates using ceramic ion conducting solid membranes
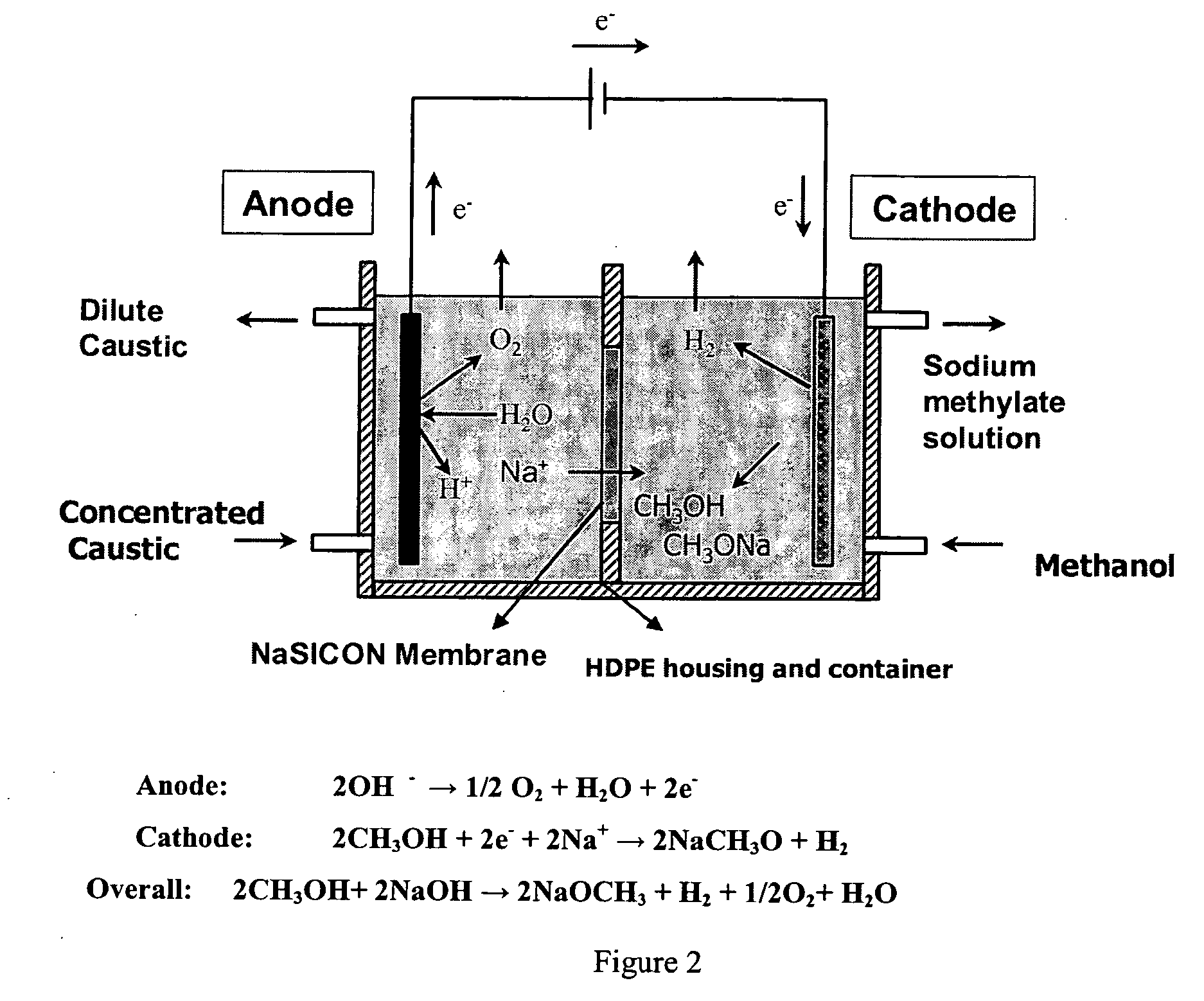
Disclosed are processes of making solutions of alkali alkoxides in their corresponding alcohols using an electrolytic process. In one embodiment, sodium methoxide in methanol is made from methanol and aqueous sodium hydroxide solution, where the aqueous sodium hydroxide solution is present in the anolyte compartment and a solution of sodium methoxide in methanol is present in the catholyte compartment, the two compartments are separated by a ceramic membrane that selectively transports sodium ions under the influence of an electric potential, and wherein the composition of the solution of sodium methoxide in methanol in the catholyte compartment of the electrolytic cell comprises between at least about 2% by weight sodium methoxide and at most about 20% by weight sodium methoxide.
Owner:ENLIGHTEN INNOVATIONS INC
Electrolytic method to make alkali alcoholates using ceramic ion conducting solid membranes
Disclosed are processes of making solutions of metal alcoholates in their corresponding alcohols using an electrolytic process. In a preferred embodiment, sodium methylate in methanol is made from methanol and sodium hydroxide solution. The sodium hydroxide solution is placed in the anolyte compartment and the methanol is placed in the catholyte compartment, and the two compartments are separated by a ceramic membrane that selectively transports sodium under the influence of current. In preferred embodiments, the process is cost-effective and not environmentally harmful.
Owner:ENLIGHTEN INNOVATIONS INC
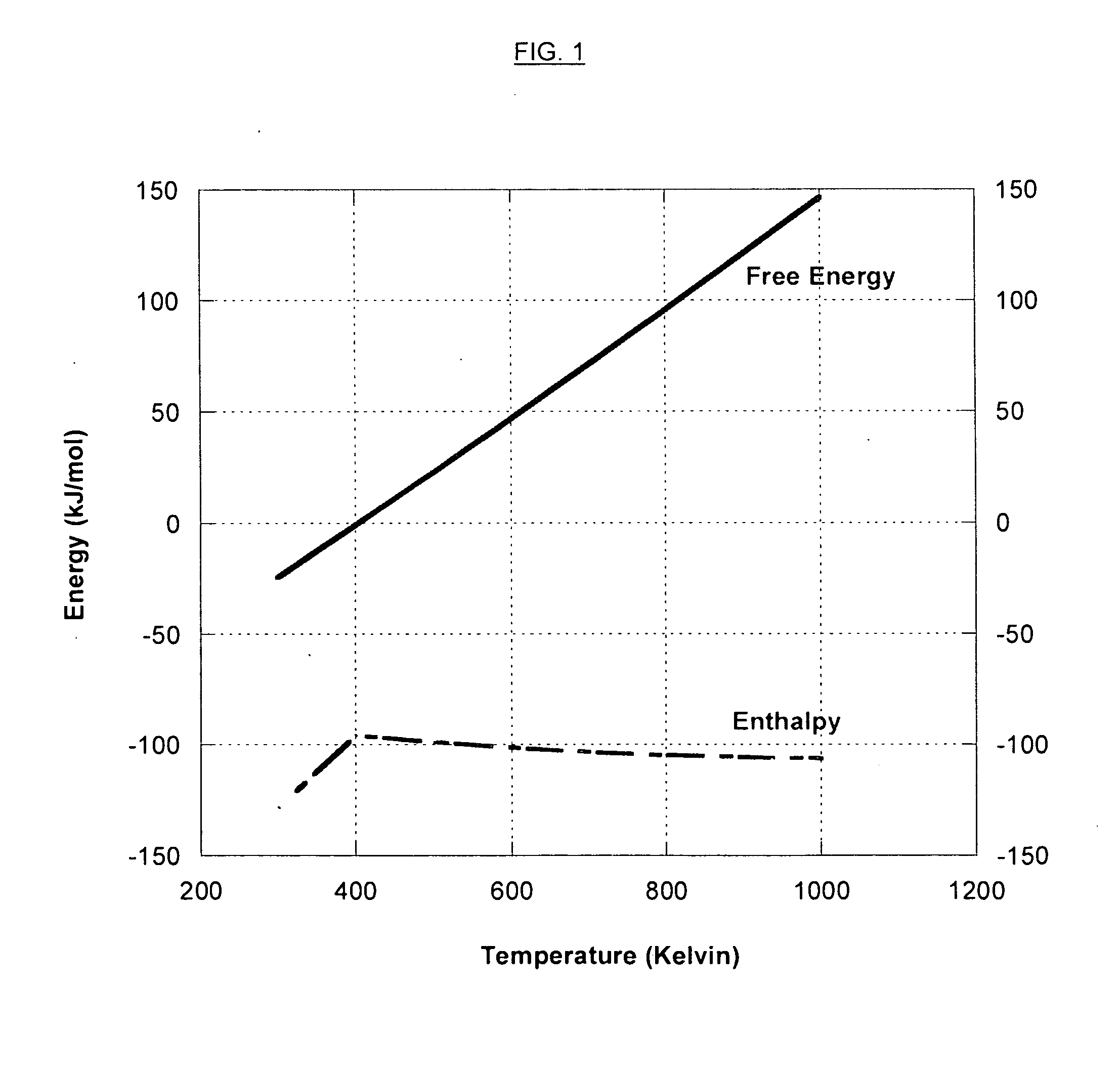
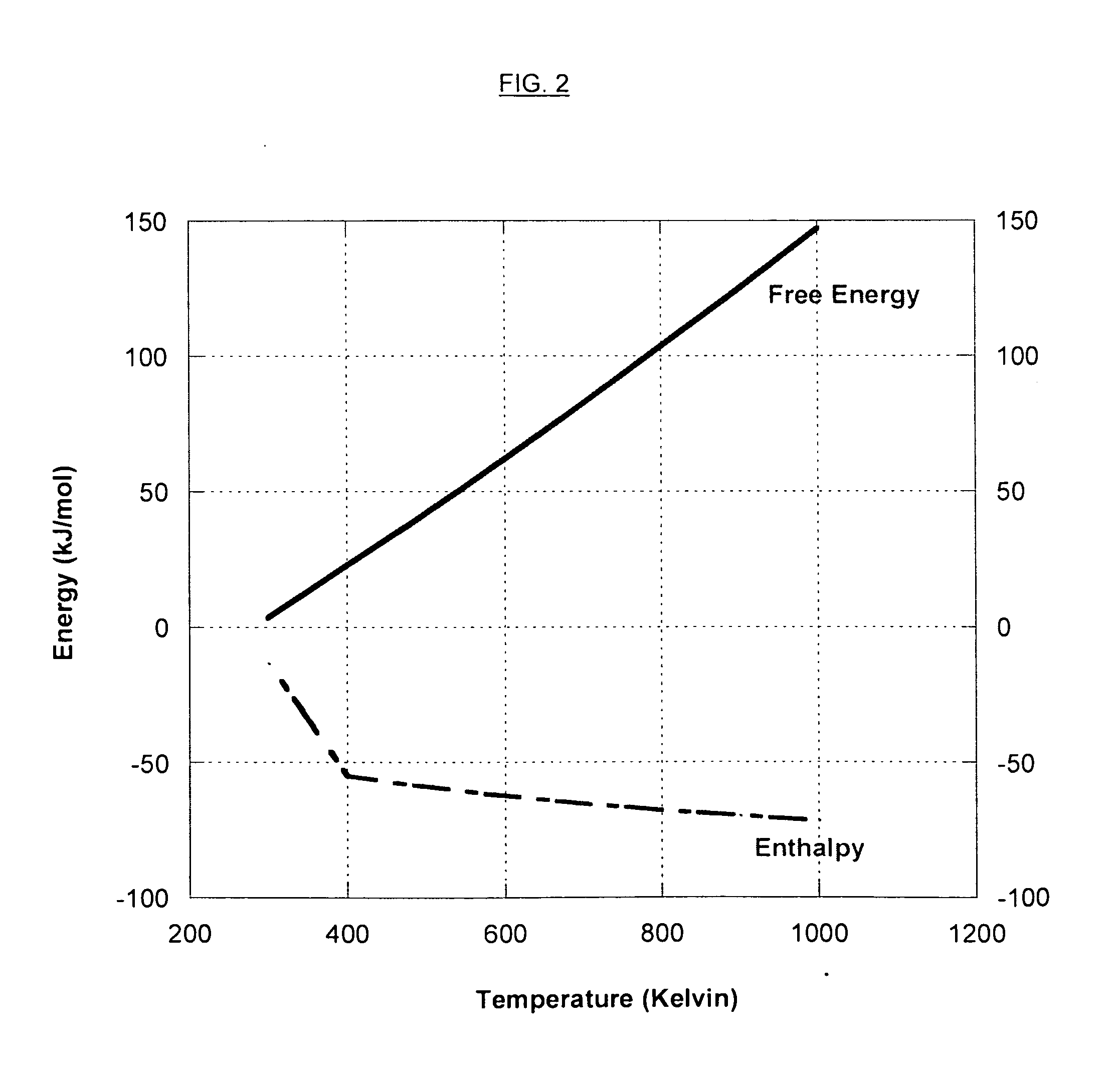
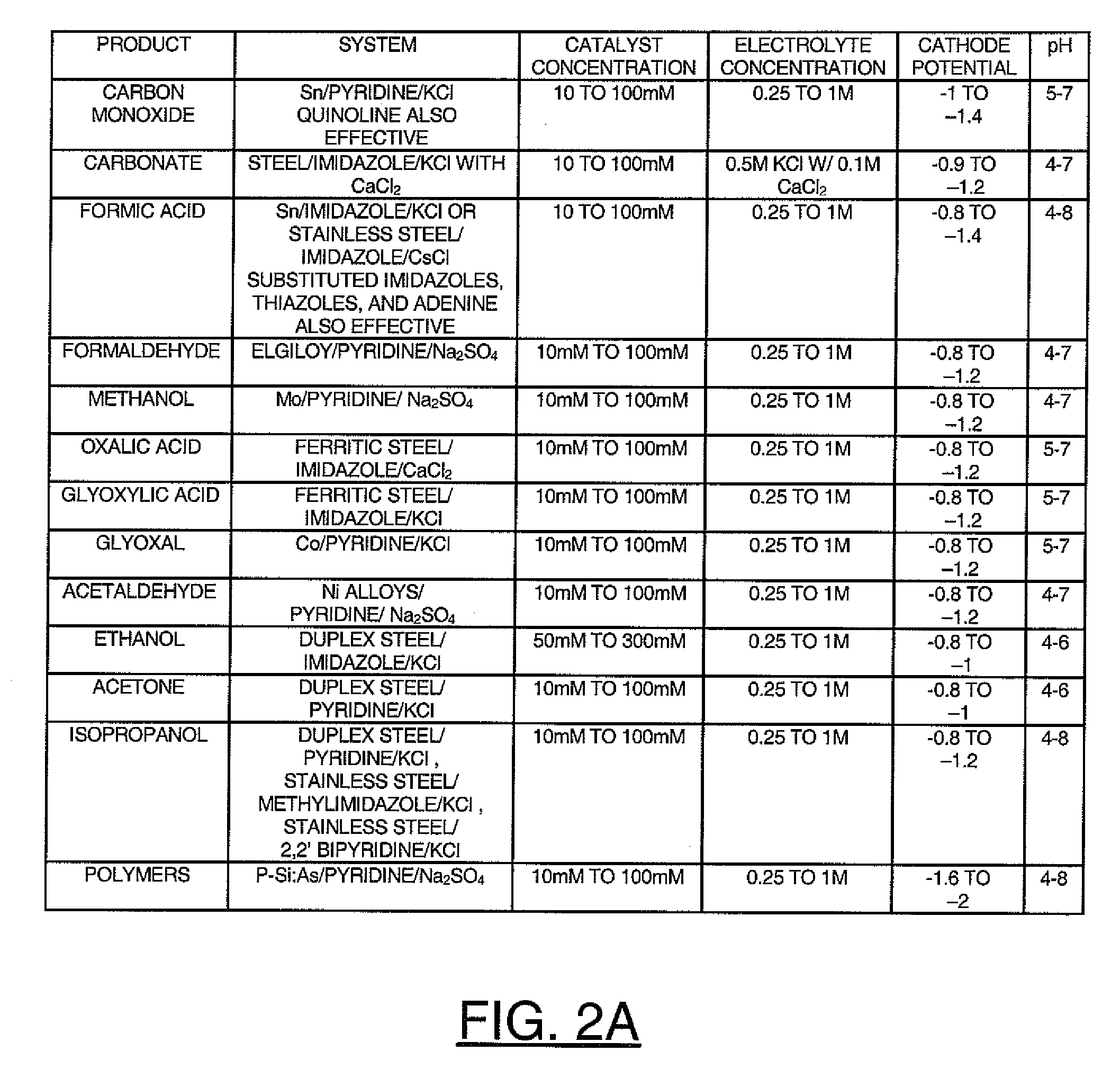
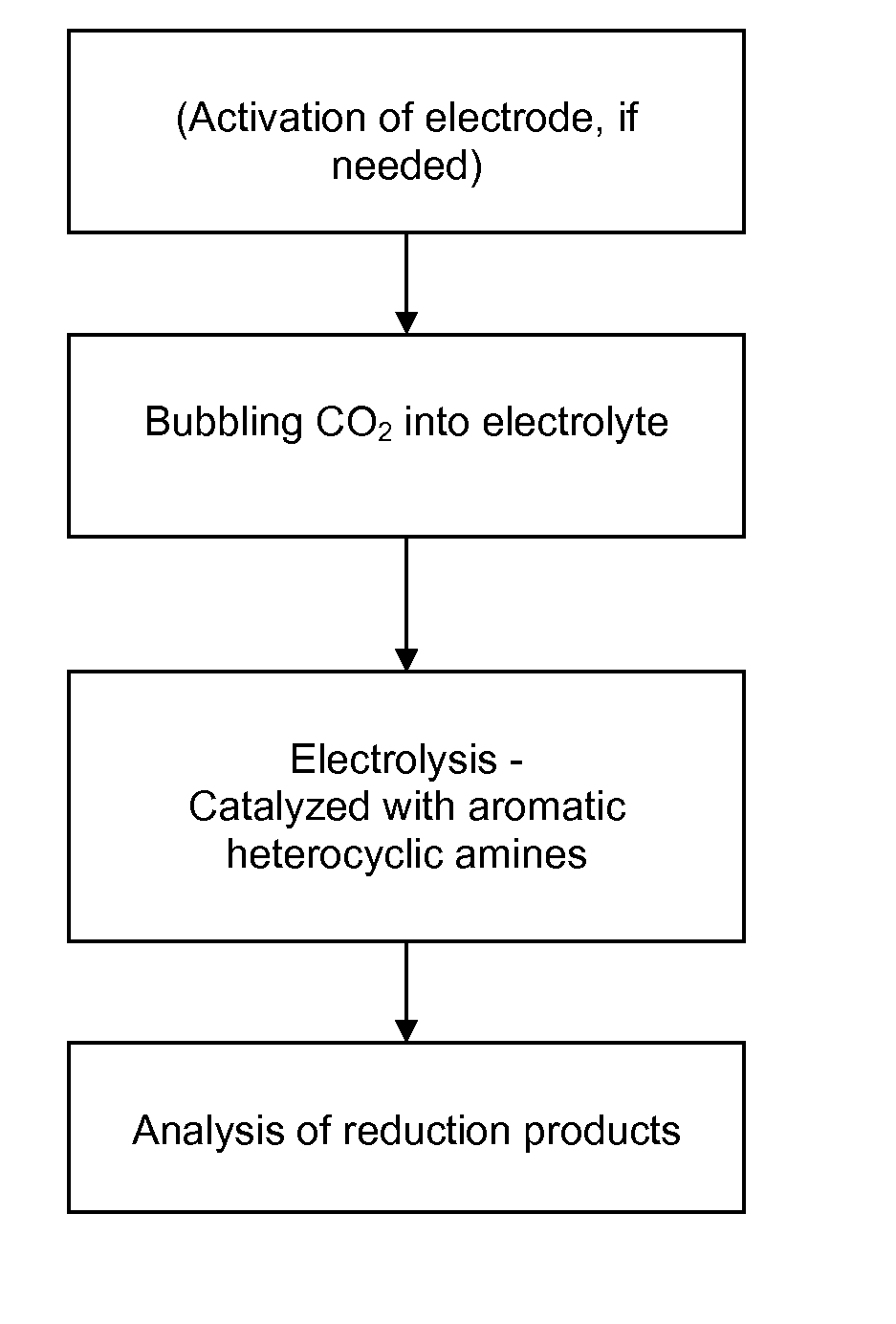
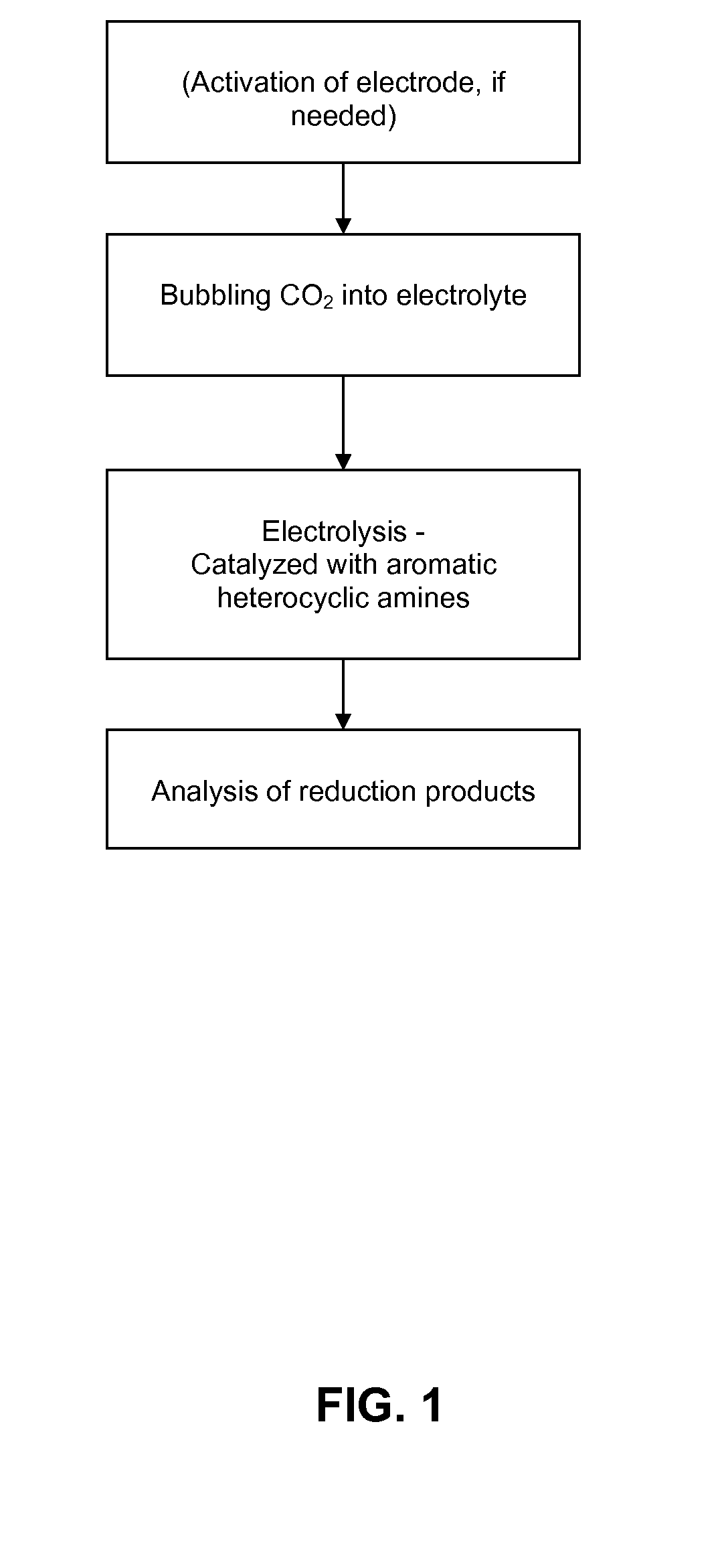
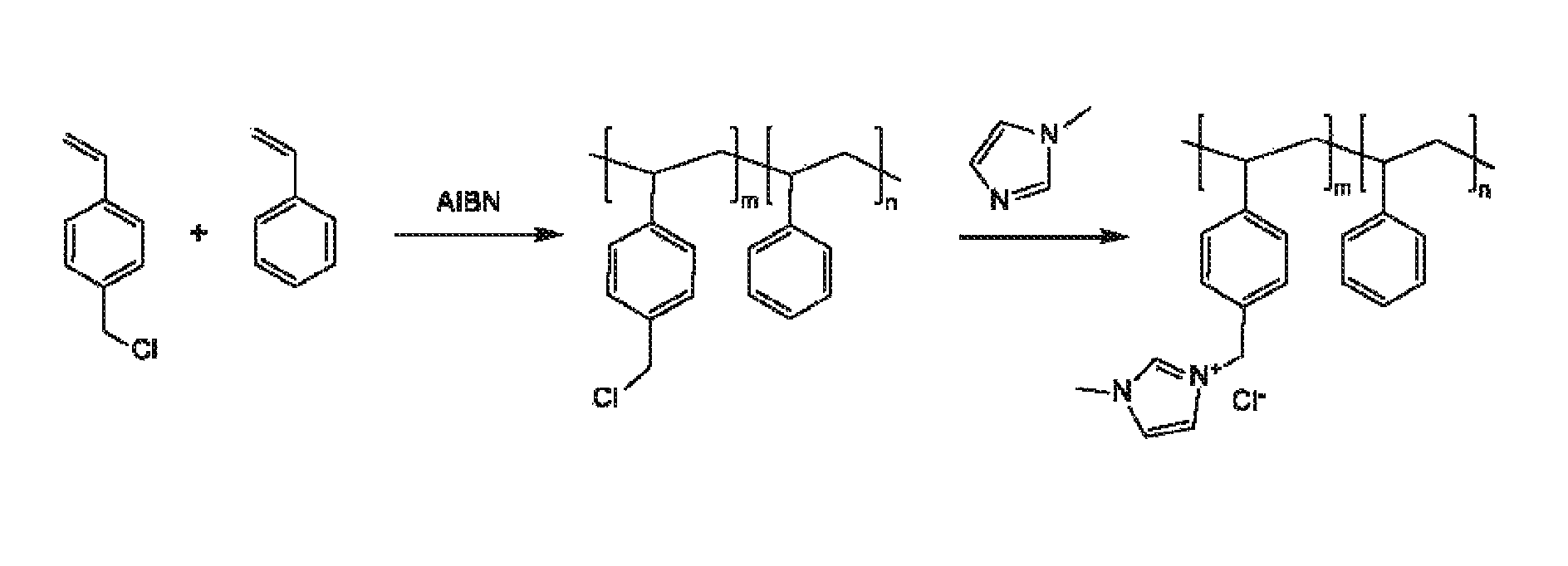
Heterocycle catalyzed electrochemical process
ActiveUS20110226632A1Process stabilityReduce carbonyl groupsCellsElectrolytic organic reductionPtru catalystElectrical battery
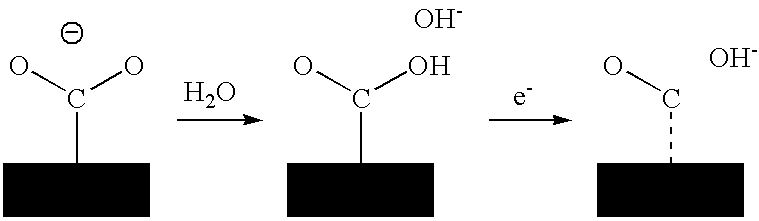
A method for heterocycle catalyzed electrochemical reduction of a carbonyl compound is disclosed. The method generally includes steps (A) to (C). Step (A) may introduce the carbonyl compound into a solution of an electrolyte and a heterocycle catalyst in a divided electrochemical cell. The divided electrochemical cell may include an anode in a first cell compartment and a cathode in a second cell compartment. The cathode generally reduces the carbonyl compound to at least one aldehyde compound. Step (B) may vary which of the aldehyde compounds is produced by adjusting one or more of (i) a cathode material, (ii) the electrolyte, (iii) the heterocycle catalyst, (iv) a pH level and (v) an electrical potential. Step (C) may separate the aldehyde compounds from the solution.
Owner:AVANTIUM KNOWLEDGE CENT BV +1
Methods and devices for the production of hydrocarbons from carbon and hydrogen sources
InactiveUS8277631B2Reduce impactEasy to controlPhotography auxillary processesInternal combustion piston enginesElectrolysisCo2 storage
Devices and methods are described for converting a carbon source and a hydrogen source into hydrocarbons, such as alcohols, for alternative energy sources. The influents may comprise carbon dioxide gas and hydrogen gas or water, obtainable from the atmosphere for through methods described herein, such as plasma generation or electrolysis. One method to produce hydrocarbons comprises the use of an electrolytic device, comprising an anode, a cathode and an electrolyte. Another method comprises the use of ultrasonic energy to drive the reaction. The devices and methods and related devices and methods are useful, for example, to provide a fossil fuel alternative energy source, store renewable energy, sequester carbon dioxide from the atmosphere, counteract global warming, and store carbon dioxide in a liquid fuel.
Owner:PRINCIPLE ENERGY SOLUTIONS
Electrolysis of carbon dioxide in aqueous media to carbon monoxide and hydrogen for production of methanol
ActiveUS8138380B2Efficient combustionPhotography auxillary processesElectrolysis componentsElectrolysisOxygen
An environmentally beneficial method of producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning power plants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by an electrochemical reduction of carbon dioxide in a divided electrochemical cell that includes an anode in one compartment and a metal cathode electrode in a compartment that also contains an aqueous solution comprising methanol and an electrolyte. An anion-conducting membrane can be provided between the anode and cathode to produce at the cathode therein a reaction mixture containing carbon monoxide and hydrogen, which can be subsequently used to produce methanol while also producing oxygen in the cell at the anode. The oxygen produced at the anode can be recycled for efficient combustion of fossil fuels in power plants to exclusively produce CO2 exhausts for capture and recycling as the source of CO2 for the cell.
Owner:UNIV OF SOUTHERN CALIFORNIA
Efficient and selective conversion of carbon dioxide to methanol, dimethyl ether and derived products
ActiveUS7605293B2Minimize or eliminate the disadvantages or dangers inherentElectrolysis componentsCarbon compoundsHydrogenFlue gas
An environmentally beneficial method of producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning powerplants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by electrochemical reduction produces formic acid acid and some formaldehyde and methanol mixtures. The formic acid can be used as source of carbon as well as hydrogen to produce methanol, dimethyl ether and other products.
Owner:UNIV OF SOUTHERN CALIFORNIA
Electrolysis of carbon dioxide in aqueous media to carbon monoxide and hydrogen for production of methanol
An environmentally beneficial method of producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning power plants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by an electrochemical reduction of carbon dioxide in a divided electrochemical cell that includes an anode in one cell compartment and a metal cathode electrode in another cell compartment that also contains an aqueous solution comprising methanol and an electrolyte of one or more alkyl ammonium halides, alkali carbonates or combinations thereof to produce therein a reaction mixture containing carbon monoxide and hydrogen which can be subsequently used to produce methanol while also producing oxygen in the cell at the anode.
Owner:UNIV OF SOUTHERN CALIFORNIA
Process For Sequestrating Carbon In The Form Of A Mineral In Which The Carbon Has Oxidation Number +3
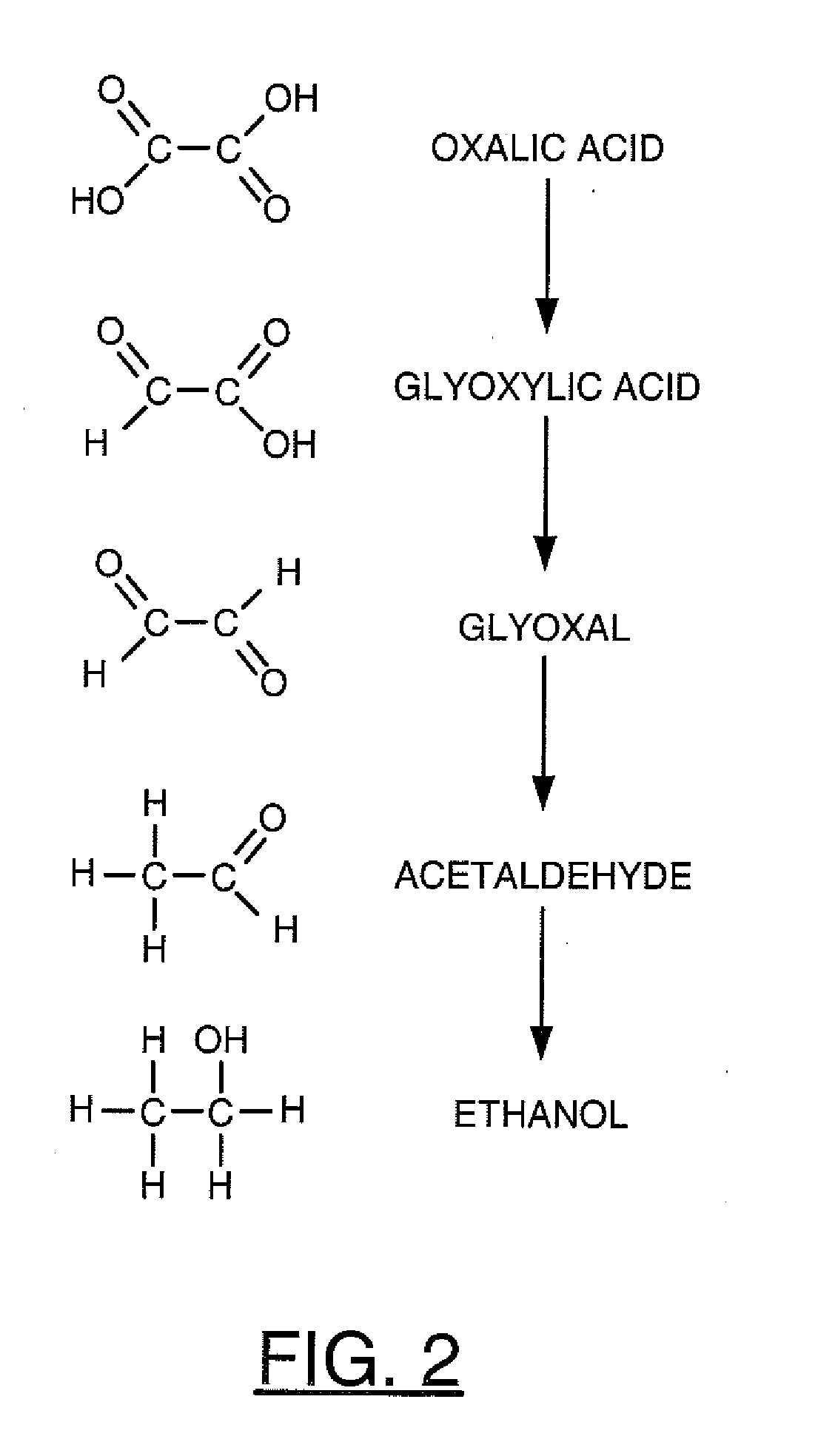
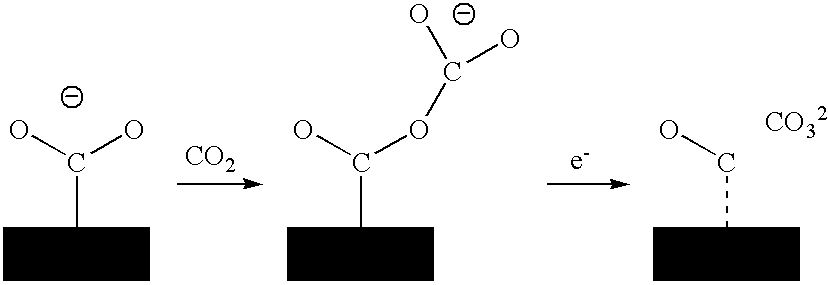
A process for sequestrating carbon emitted into the atmosphere in the form of CO2 comprises:a) a step for concentrating CO2 in the liquid phase;b) a step for electro-reduction in an aprotic medium to a compound in which the carbon changes to oxidation number +3 in the form of oxalic acid or formic acid;c) if appropriate, a step for re-extracting oxalic or formic acid in the aqueous phase; andd) a step for mineralization by reaction with a compound of an element M, resulting in a stable compound in which the atomic ratio C / M is about 2 / 1.
Owner:INST FR DU PETROLE
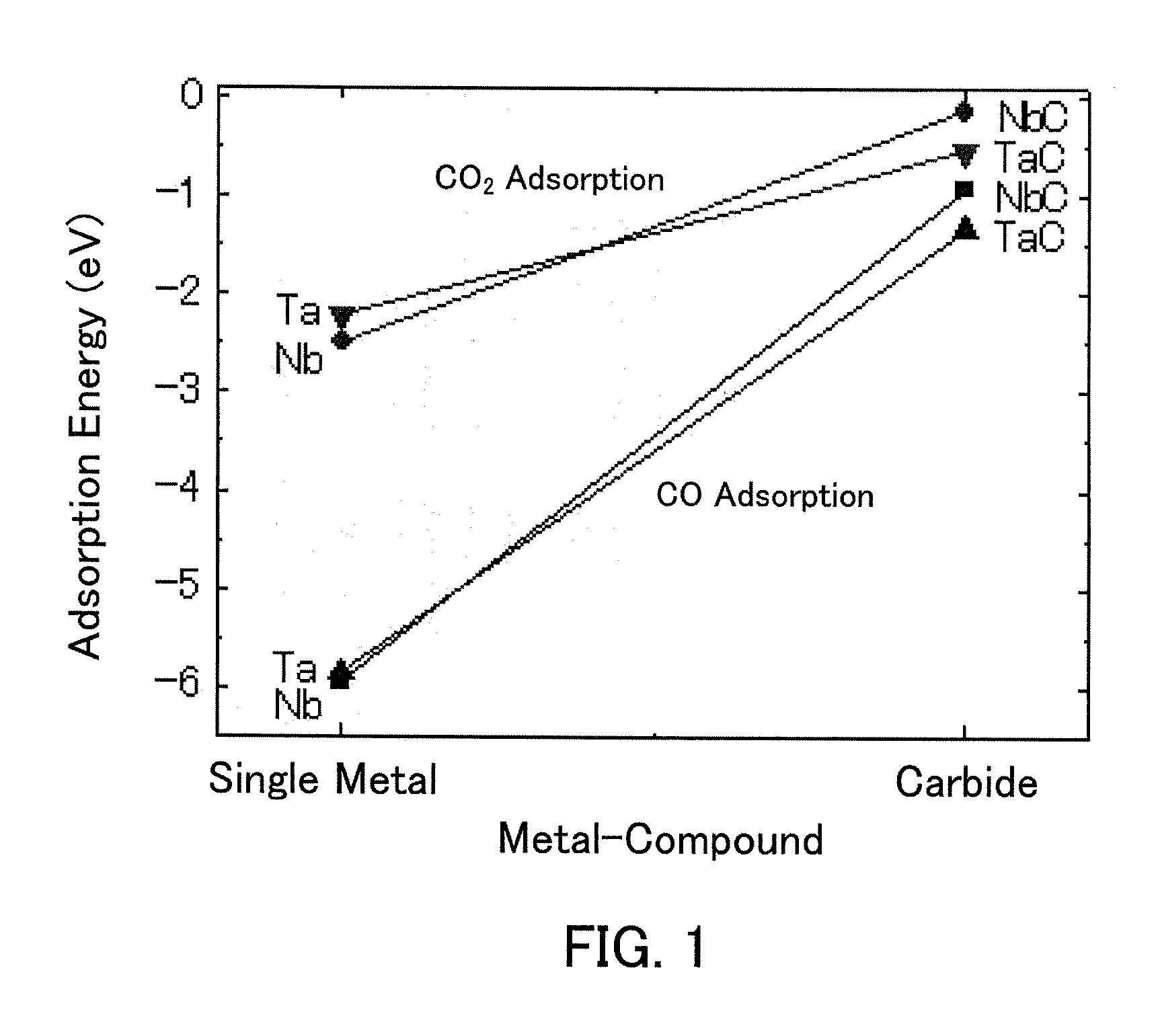
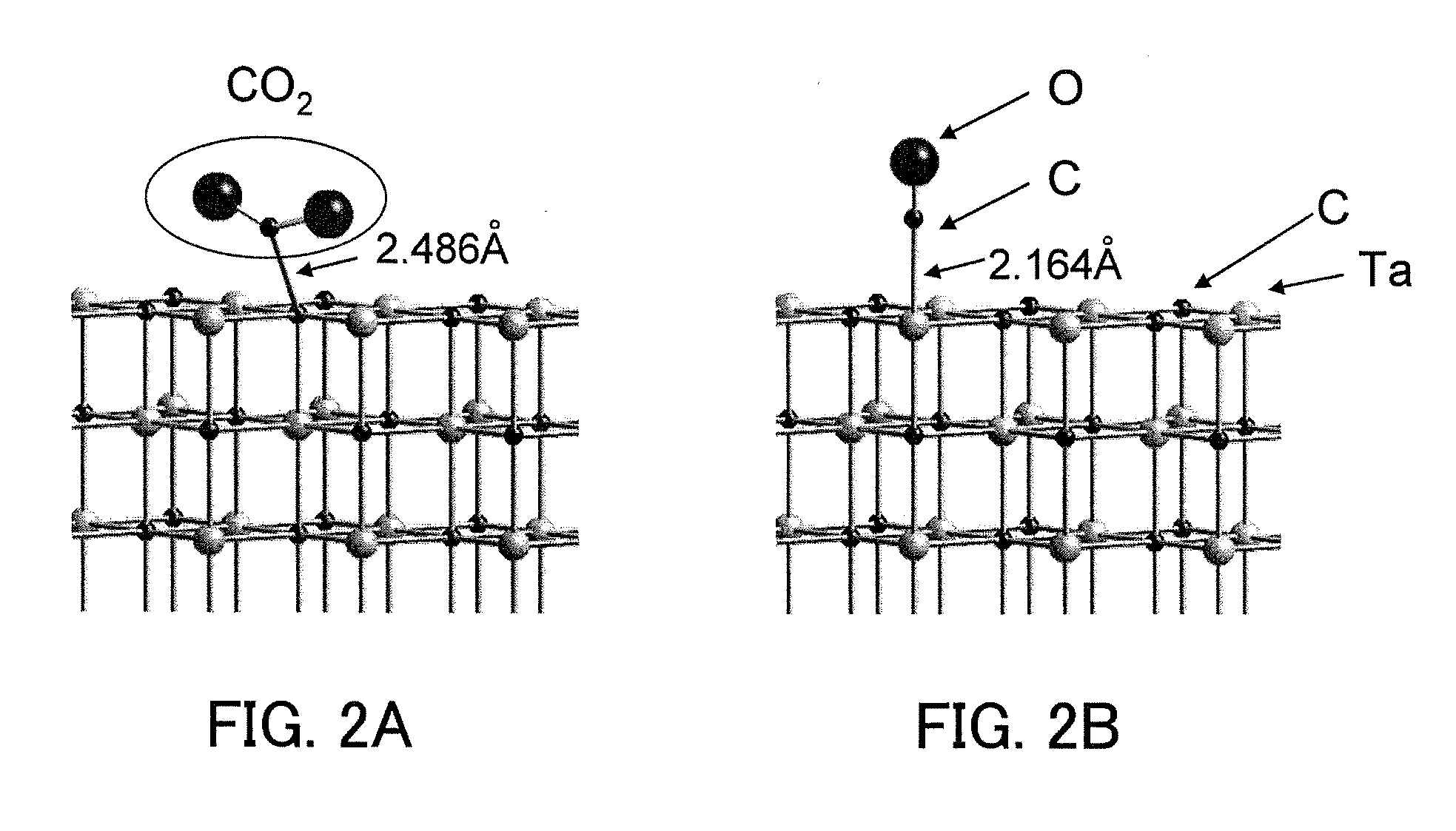
Carbon dioxide reduction method, and carbon dioxide reduction catalyst and carbon dioxide reduction device used for the method
The carbon dioxide reduction method of the present invention is a method including steps of: bringing an electrode (working electrode) containing a carbide of at least one element selected from Group V elements (vanadium, niobium, and tantalum) into contact with an electrolytic solution; and introducing carbon dioxide into the electrolytic solution to reduce the introduced carbon dioxide by the electrode. The material contained in the electrode, that is, the material containing a carbide of at least one element selected from Group V elements (vanadium, niobium, and tantalum) is the carbon dioxide reduction catalyst of the present invention.
Owner:PANASONIC CORP
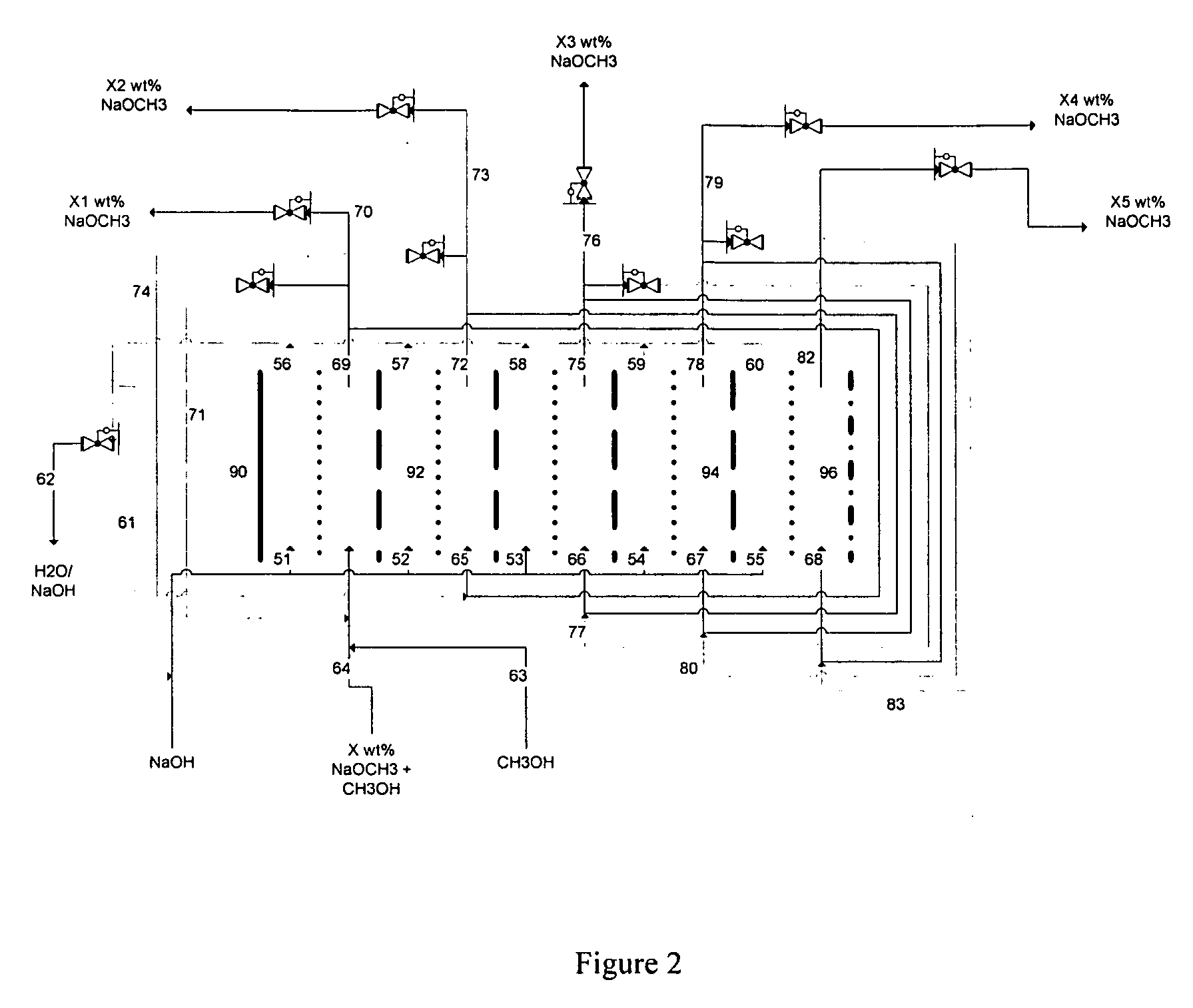
Electrolytic method to make alkali alcoholates using ion conducting alkali electrolyte/seperator
ActiveUS20080142373A1Efficient executionElectrolysis componentsElectrolytic organic reductionAlkali ionsAlcohol
Alkali alcoholates, also called alkali alkoxides, are produced from alkali metal salt solutions and alcohol using a three-compartment electrolytic cell. The electrolytic cell includes an anolyte compartment configured with an anode, a buffer compartment, and a catholyte compartment configured with a cathode. An alkali ion conducting solid electrolyte configured to selectively transport alkali ions is positioned between the anolyte compartment and the buffer compartment. An alkali ion permeable separator is positioned between the buffer compartment and the catholyte compartment. The catholyte solution may include an alkali alcoholate and alcohol. The anolyte solution may include at least one alkali salt. The buffer compartment solution may include a soluble alkali salt and an alkali alcoholate in alcohol.
Owner:ENLIGHTEN INNOVATIONS INC
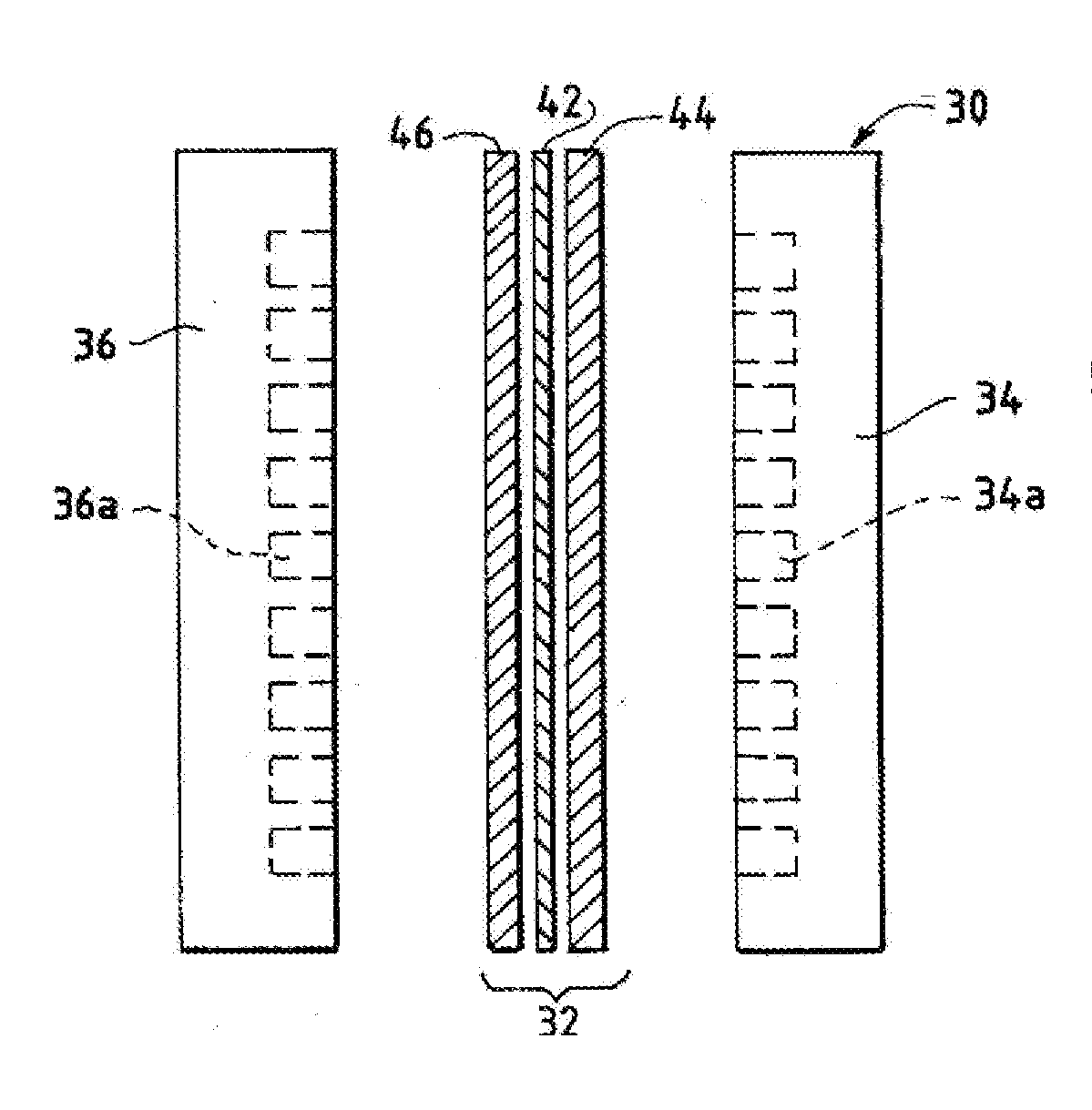
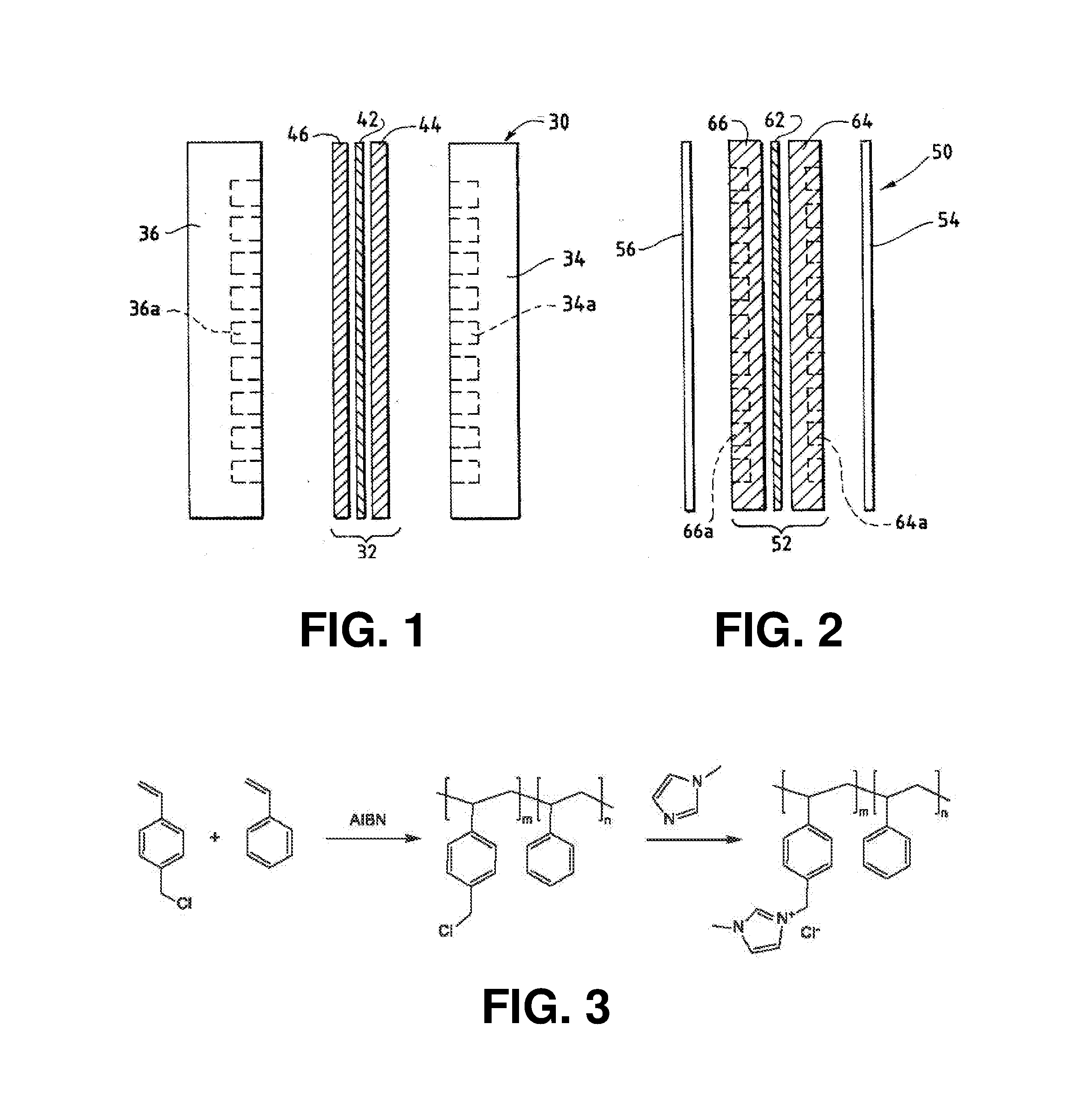
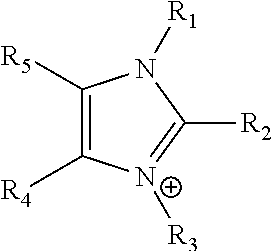
Ion-conducting membranes
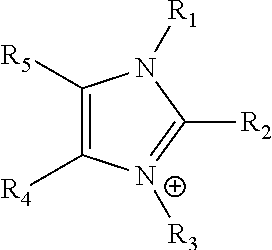
An ion conducting polymeric composition mixture comprises a copolymer of styrene and vinylbenzyl-Rs. Rs is selected from the group consisting of imidazoliums, pyridiniums, pyrazoliums, pyrrolidiniums, pyrroliums, pyrimidiums, piperidiniums, indoliums, and triaziniums. The composition contains 10%-90% by weight of vinylbenzyl-Rs. The composition can further comprise a polyolefin comprising substituted polyolefins, a polymer comprising cyclic amine groups, a polymer comprising at least one of a phenylene group and a phenyl group, a polyamide, and / or the reaction product of a constituent having two carbon-carbon double bonds. The composition can be in the form of a membrane. In a preferred embodiment, the membrane is a Helper Membrane that increases the faradaic efficiency of an electrochemical cell into which the membrane is incorporated, and also allows product formation at lower voltages than in cells without the Helper Membrane.
Owner:DIOXIDE MATERIALS
Method for Producing Fuel from Captured Carbon Dioxide
InactiveUS20080072496A1Electrolysis componentsHydrocarbon from carbon oxidesHydrogenCarbon dioxide production
The invention provides a method for producing combustible fuels from a gaseous mixture containing carbon dioxide, which comprises: (i) capturing CO2 from said gaseous mixture by means of K2CO3, thus forming KHCO3; (ii) releasing the CO2 from said KHCO3; and (iii) subsequently producing fuel from the released CO2 by reaction with hydrogen.
Owner:ENGINEUITY RES & DEV +1
Ion-conducting membranes
An ion conducting polymeric composition mixture comprises a copolymer of styrene and vinylbenzyl-Rs. Rs is selected from the group consisting of imidazoliums and pyridiniums. The composition contains 10%-90% by weight of vinylbenzyl-Rs. The composition can further comprise a polyolefin comprising substituted polyolefins, a polymer comprising cyclic amine groups, a polymer comprising at least one of a phenylene group and a phenyl group, a polyamide, and / or the reaction product of a constituent having two carbon-carbon double bonds. The composition can be in the form of a membrane. In a preferred embodiment, the membrane is a Helper Membrane that increases the faradaic efficiency of an electrochemical cell into which the membrane is incorporated, and also allows product formation at lower voltages than in cells without the Helper Membrane.
Owner:DIOXIDE MATERIALS
Efficient and selective chemical recycling of carbon dioxide to methanol, dimethyl ether and derived products
ActiveUS7608743B2Avoid emissionsElectrolysis componentsOxygen compounds purification/separationFlue gasExhaust fumes
An efficient and environmentally beneficial method of recycling and producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning powerplants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by chemical or electrochemical reduction secondary treatment to produce essentially methanol, dimethyl ether and derived products.
Owner:UNIV OF SOUTHERN CALIFORNIA
Novel Catalyst Mixtures
ActiveUS20120308903A1Raise the overpotentialLow rateWeather/light/corrosion resistanceCell electrodesChemical reactionCompound (substance)
Catalysts that include at least one catalytically active element and one helper catalyst can be used to increase the rate or lower the overpotential of chemical reactions. The helper catalyst can simultaneously act as a director molecule, suppressing undesired reactions and thus increasing selectivity toward the desired reaction. These catalysts can be useful for a variety of chemical reactions including, in particular, the electrochemical conversion of CO2 or formic acid. The catalysts can also suppress H2 evolution, permitting electrochemical cell operation at potentials below RHE. Chemical processes and devices using the catalysts are also disclosed, including processes to produce CO, OH−, HCO−, H2CO, (HCO2)−, H2CO2, CH3OH, CH4, C2H4, CH3CH2OH, CH3COO−, CH3COOH, C2H6, O2, H2, (COOH)2, or (COO−)2, and a specific device, namely, a CO2 sensor.
Owner:DIOXIDE MATERIALS
Ion-conducting membranes
An anion-conducting polymeric membrane comprises a terpolymer of styrene, vinylbenzyl-Rs and vinylbenzyl-Rx. Rs is a positively charged cyclic amine group. Rx is at least one constituent selected from the group consisting Cl, OH and a reaction product between an OH or Cl and a species other than a simple amine or a cyclic amine. The total weight of the vinylbenzyl-Rx groups is greater than 0.3% of the total weight of the membrane. In a preferred embodiment, the membrane is a Helper Membrane that increases the faradaic efficiency of an electrochemical cell into which the membrane is incorporated, and also allows product formation at lower voltages than in cells without the Helper Membrane.
Owner:DIOXIDE MATERIALS
Biofuel production by high temperature non-faradaic electrochemical modification of catalysis
InactiveUS20100258447A1High calorific valueEasy to separateElectrolysis componentsEnergy inputMetallurgyBiofuel
A method for producing biofuels from biomass in which a refined biomass material is introduced into a non-Faradaic electrochemical device, preferably at a temperature greater than or equal to about 150° C., and deoxygenated and / or decarboxylated in said device to produce an increased carbon chain fuel.
Owner:GAS TECH INST
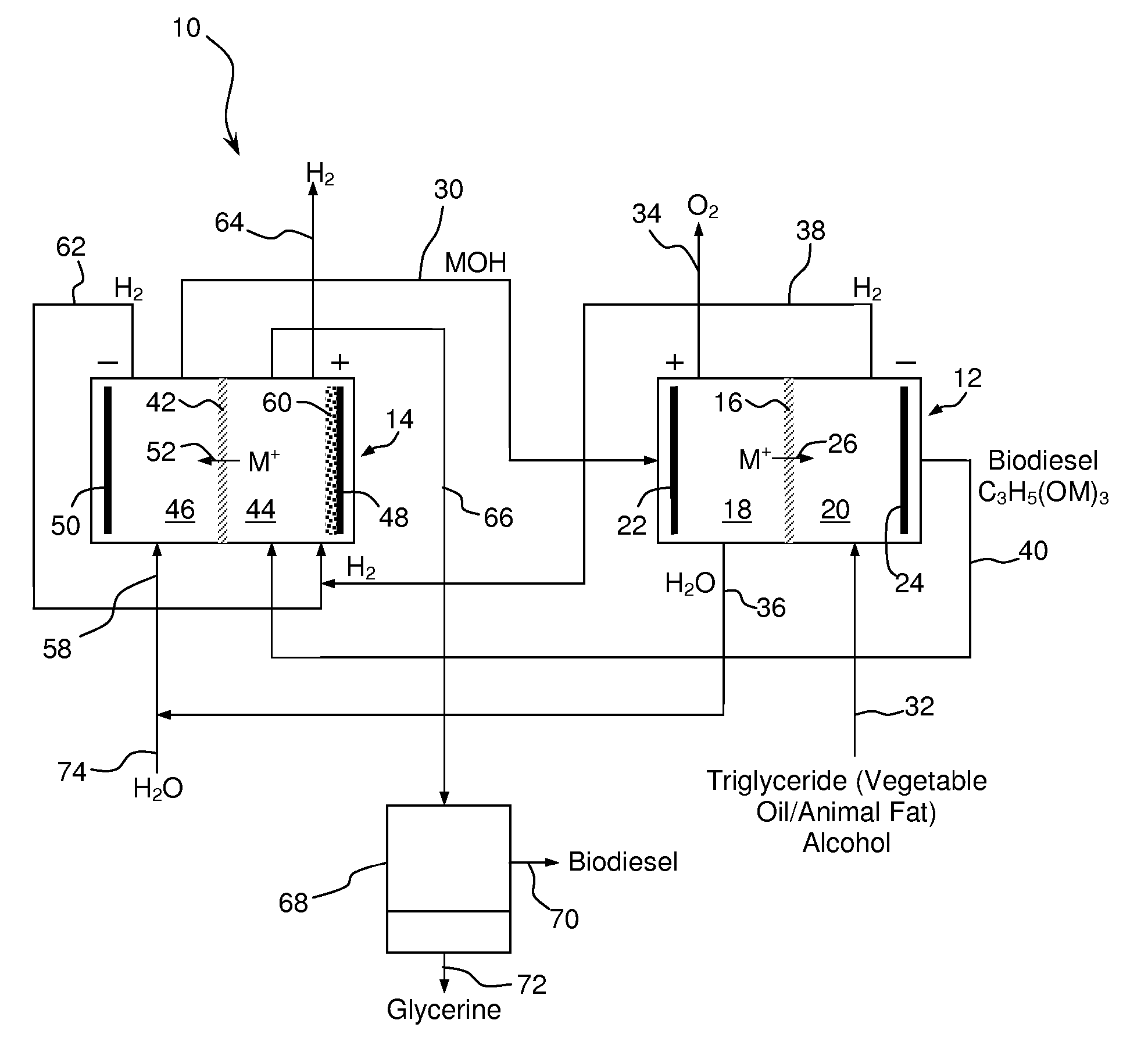
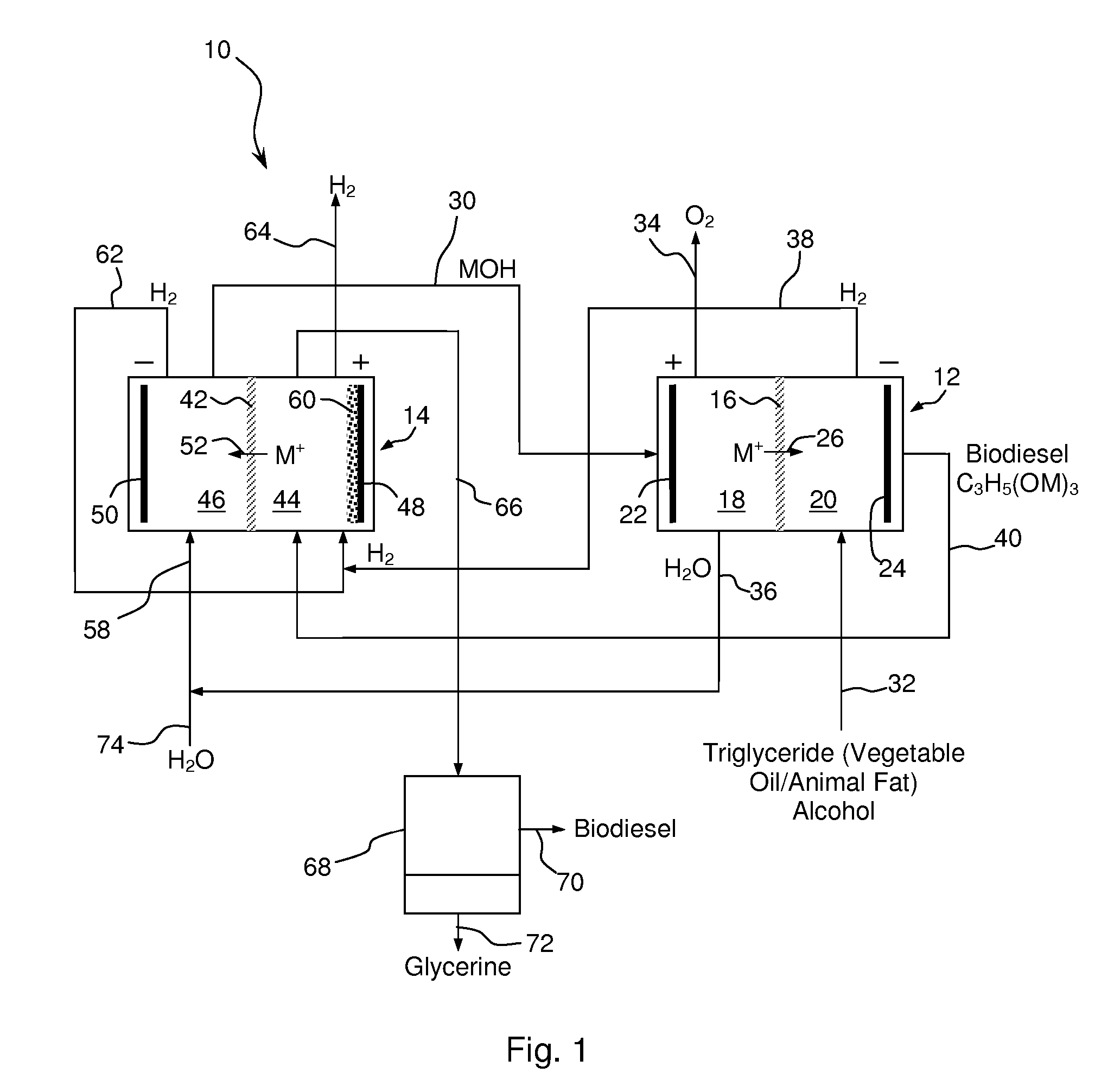
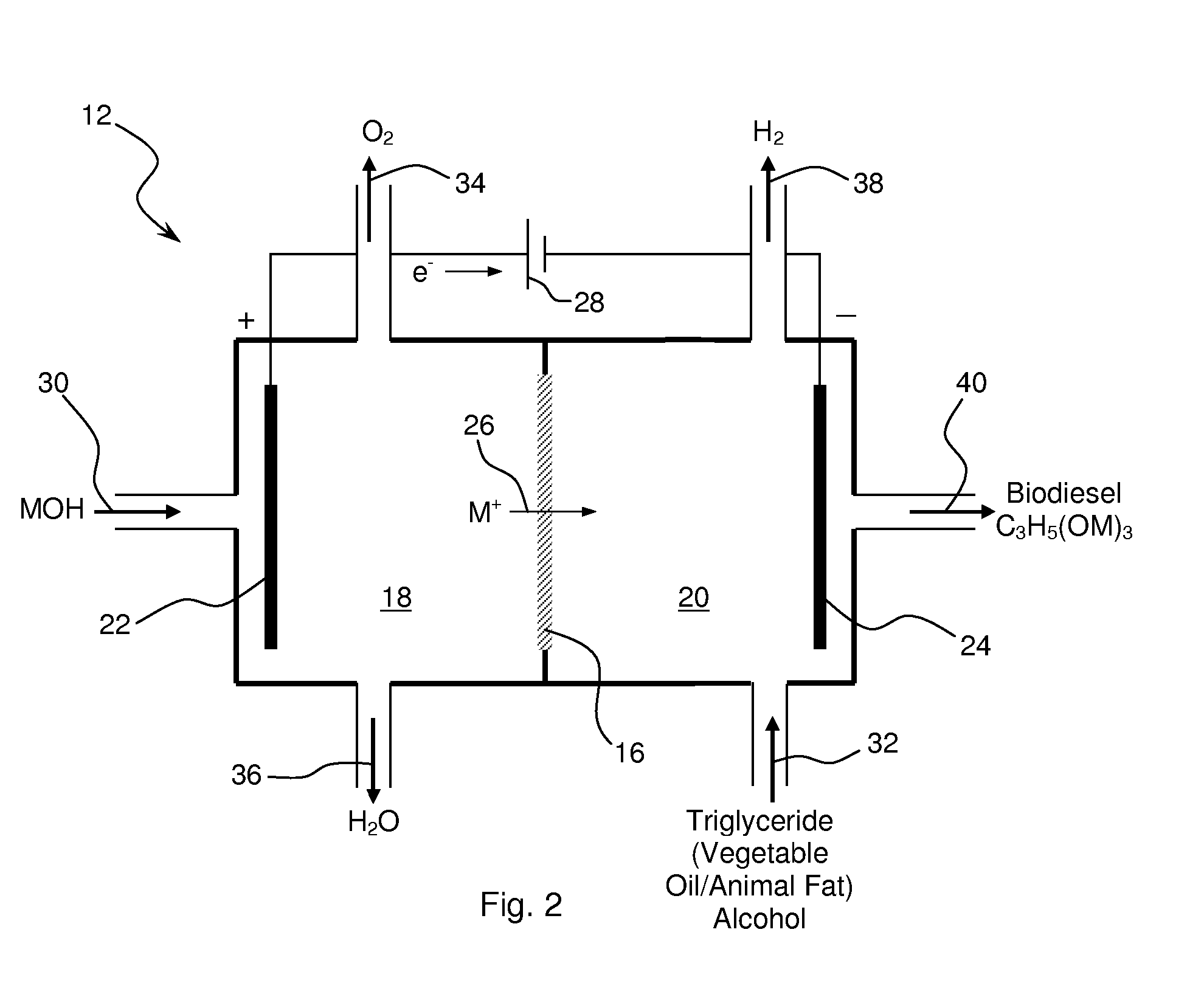
Synthesis of Biodiesel Using Alkali Ion Conductive Ceramic Membranes
InactiveUS20070158205A1Easy to separateImprove responseCellsFinal product manufactureAlkali ionsBiodiesel
Methods and apparatus for synthesizing biodiesel using alkali alkoxide generated on-site using an electrochemical process are disclosed. The apparatus and methods are disclosed to converting alkali salts of glycerine into glycerine and thereby facilitate the separation of clean glycerine from biodiesel. These methods are enabled by the use of alkali ion conductive ceramic membranes in electrolytic cells.
Owner:CERAMTEC
Popular searches
Liquid hydrocarbon mixture production Hydrocarbon oils treatment Gas treatment Non-fuel substance addition to fuel Dispersed particle separation Combustion-air/fuel-air treatment Petrochemical industry Energy based chemical/physical/physico-chemical processes By chemical separation Non-noble metal oxide coatings
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com