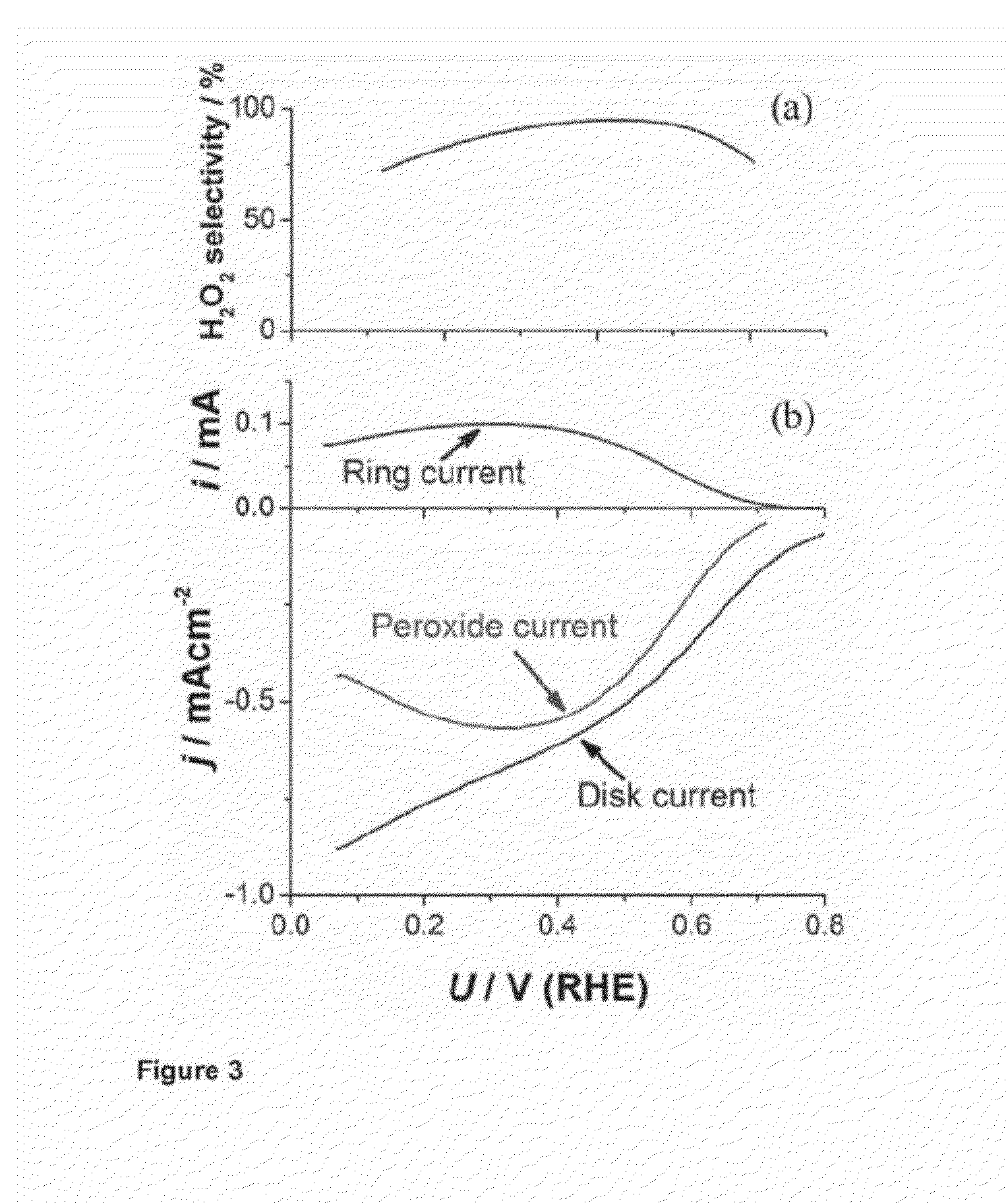
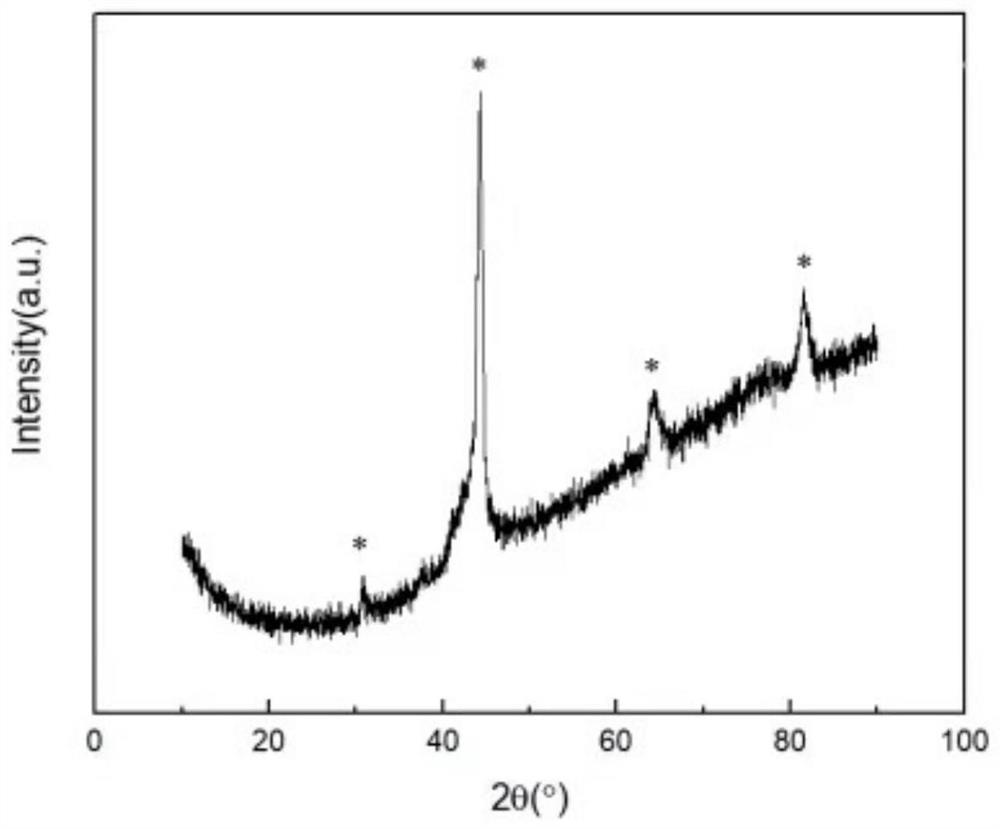
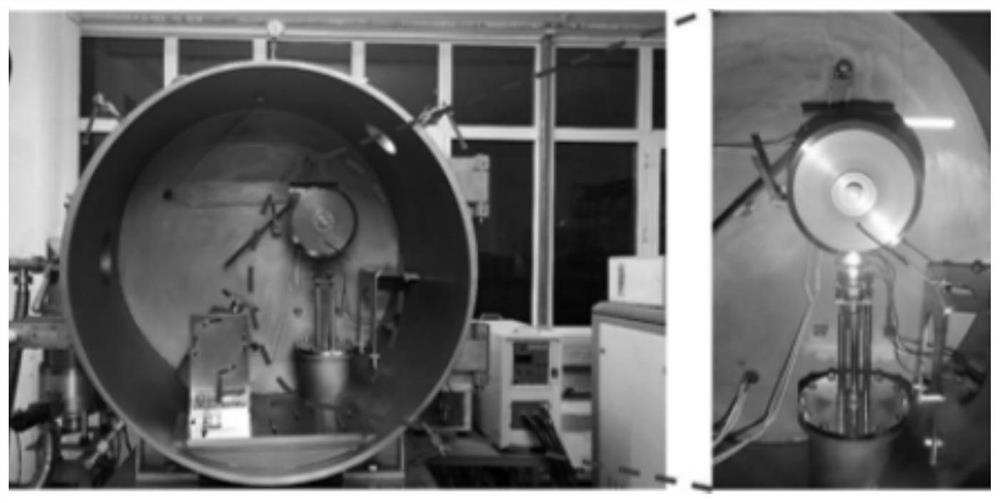
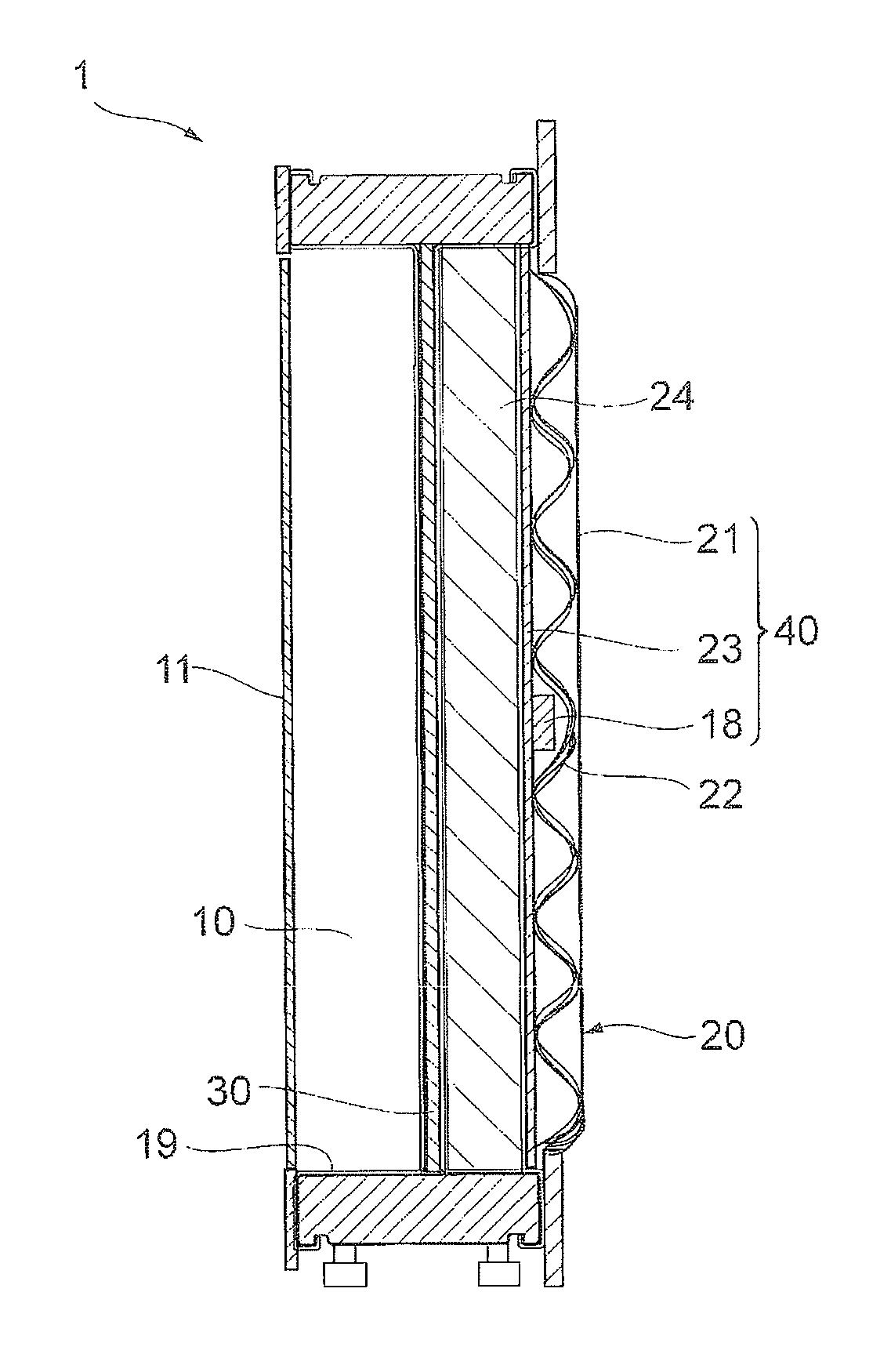
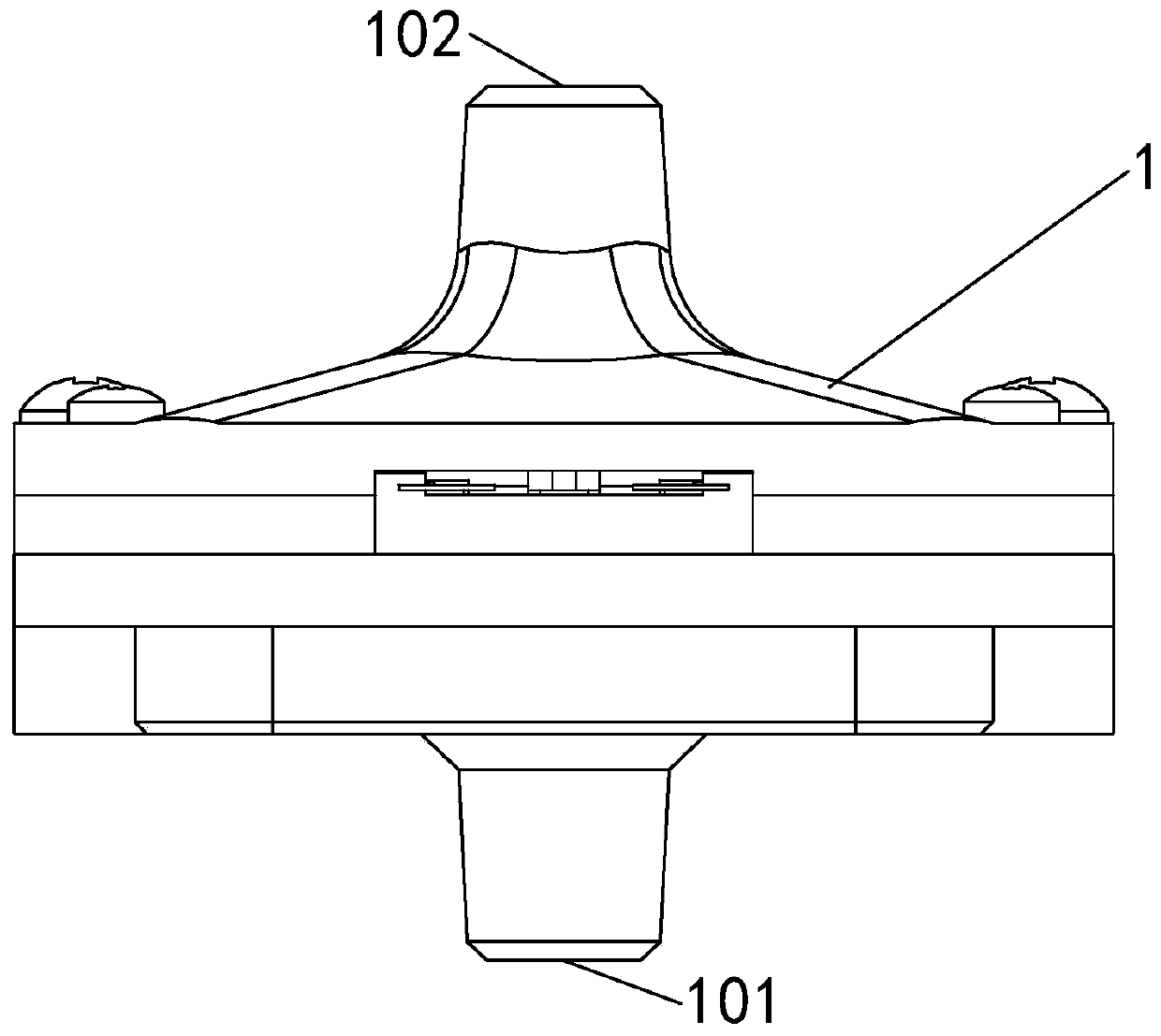
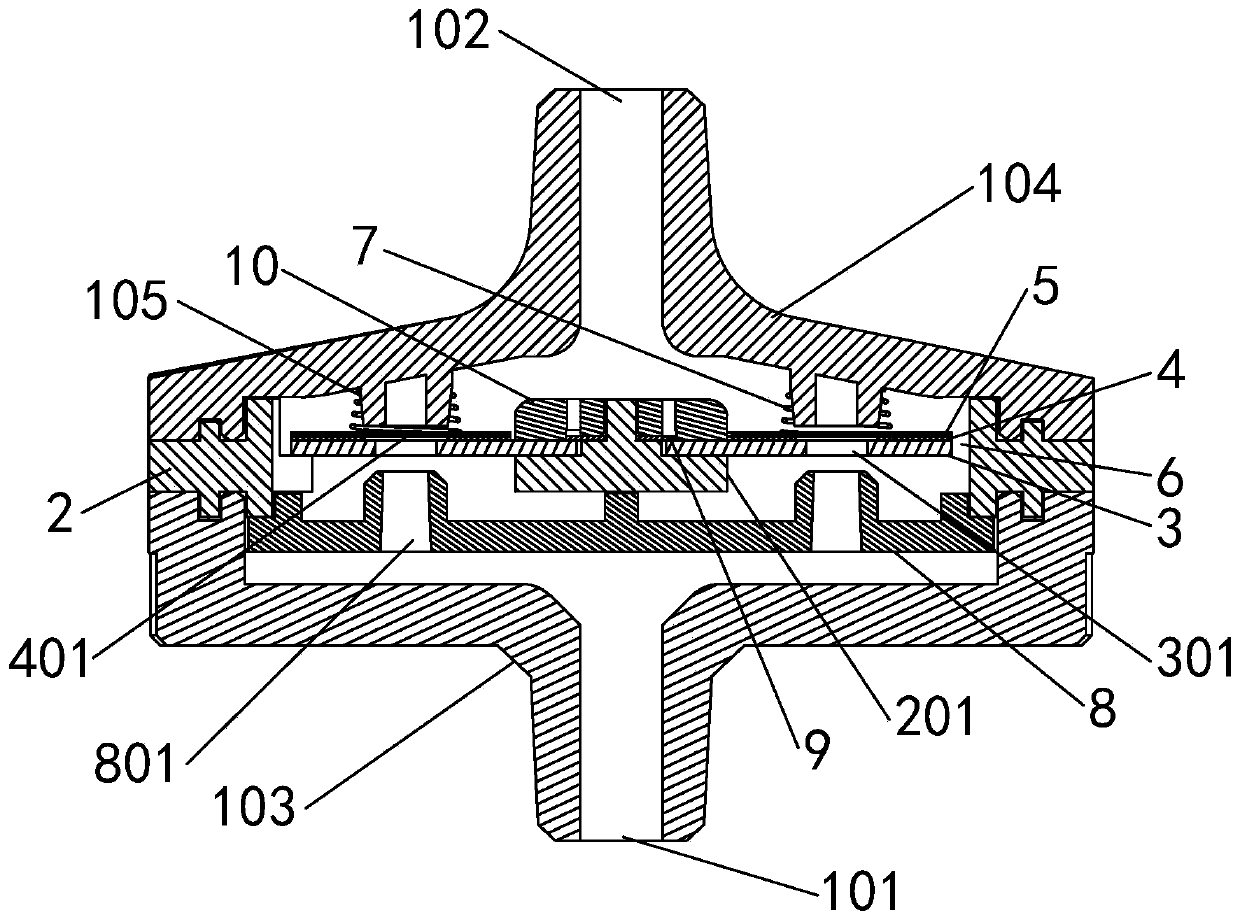
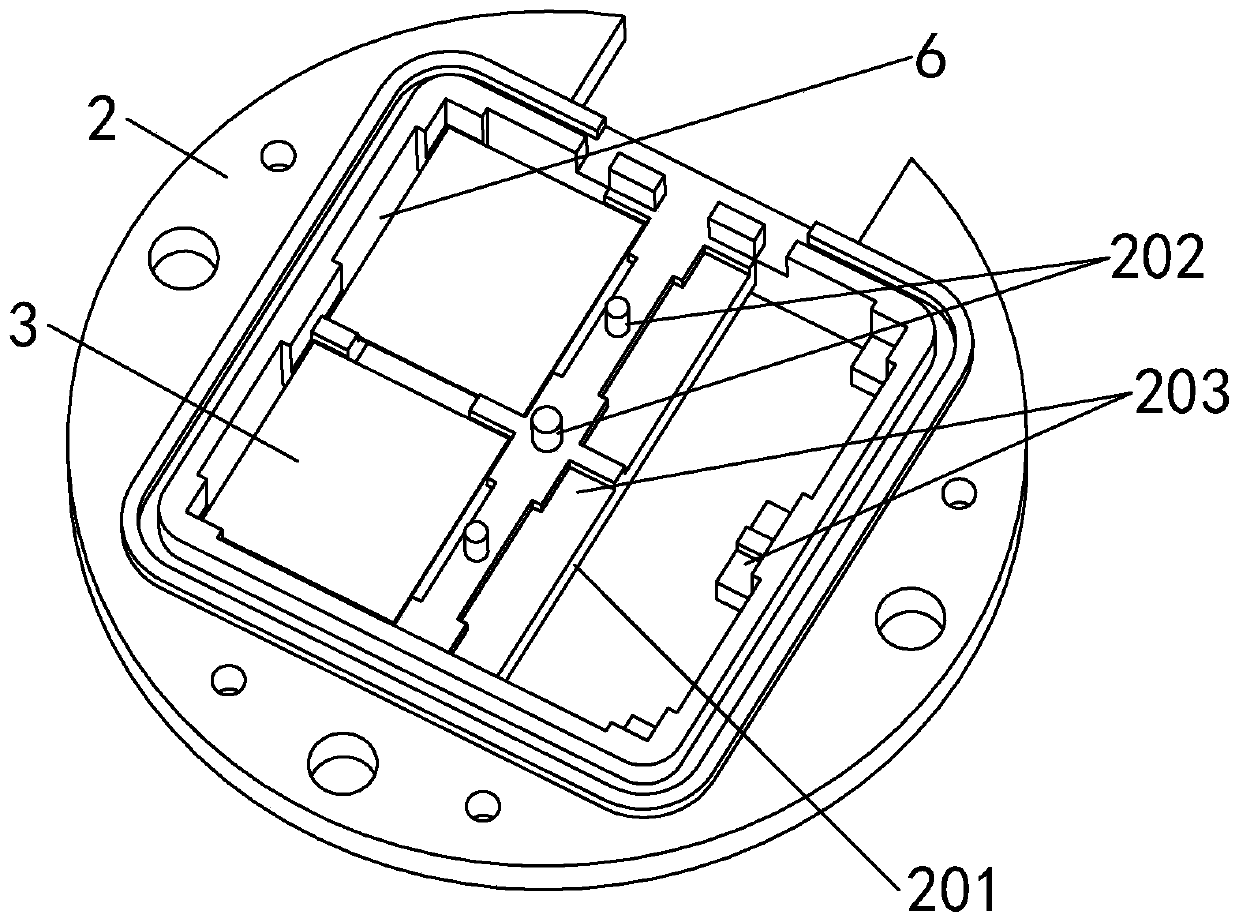
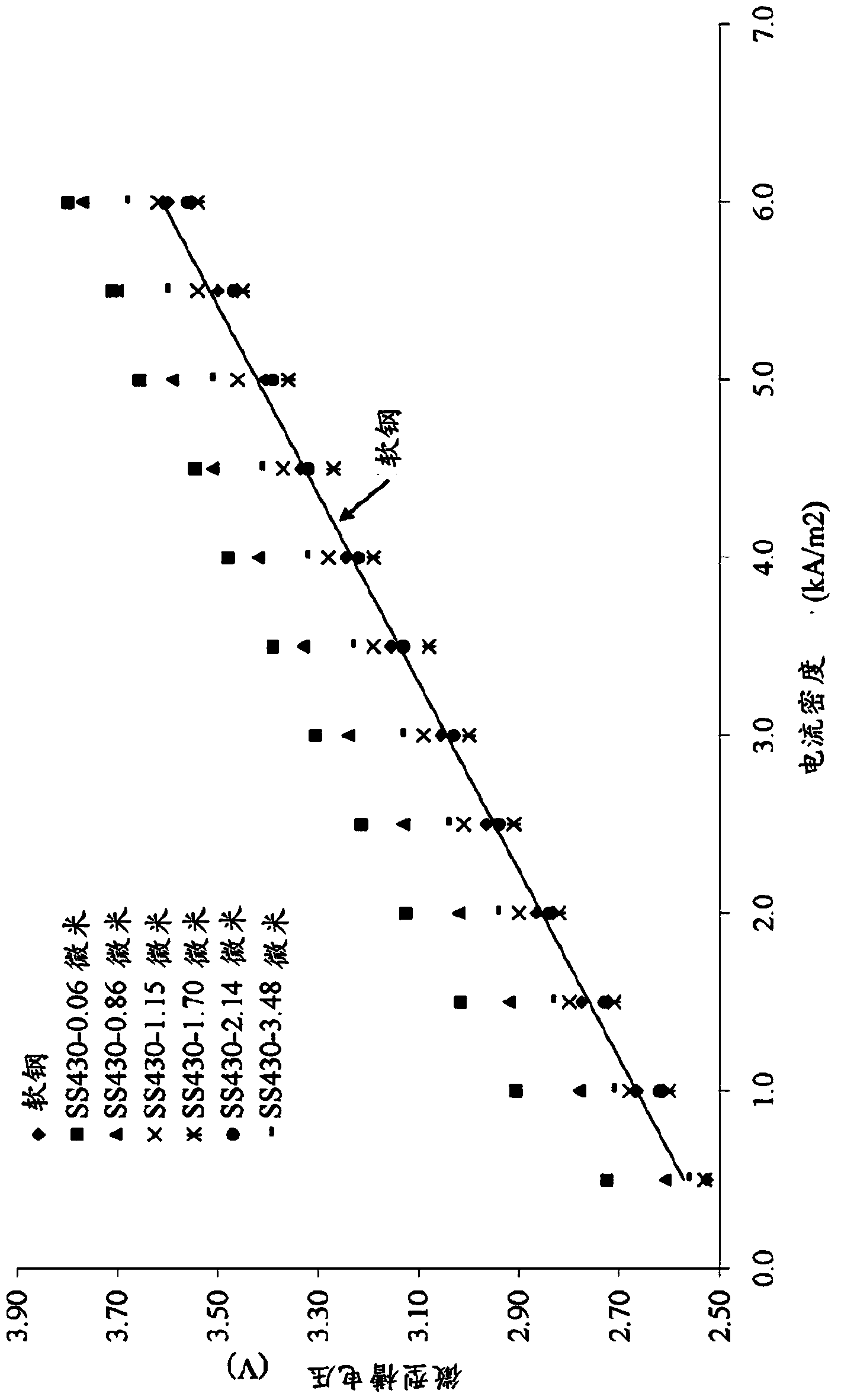
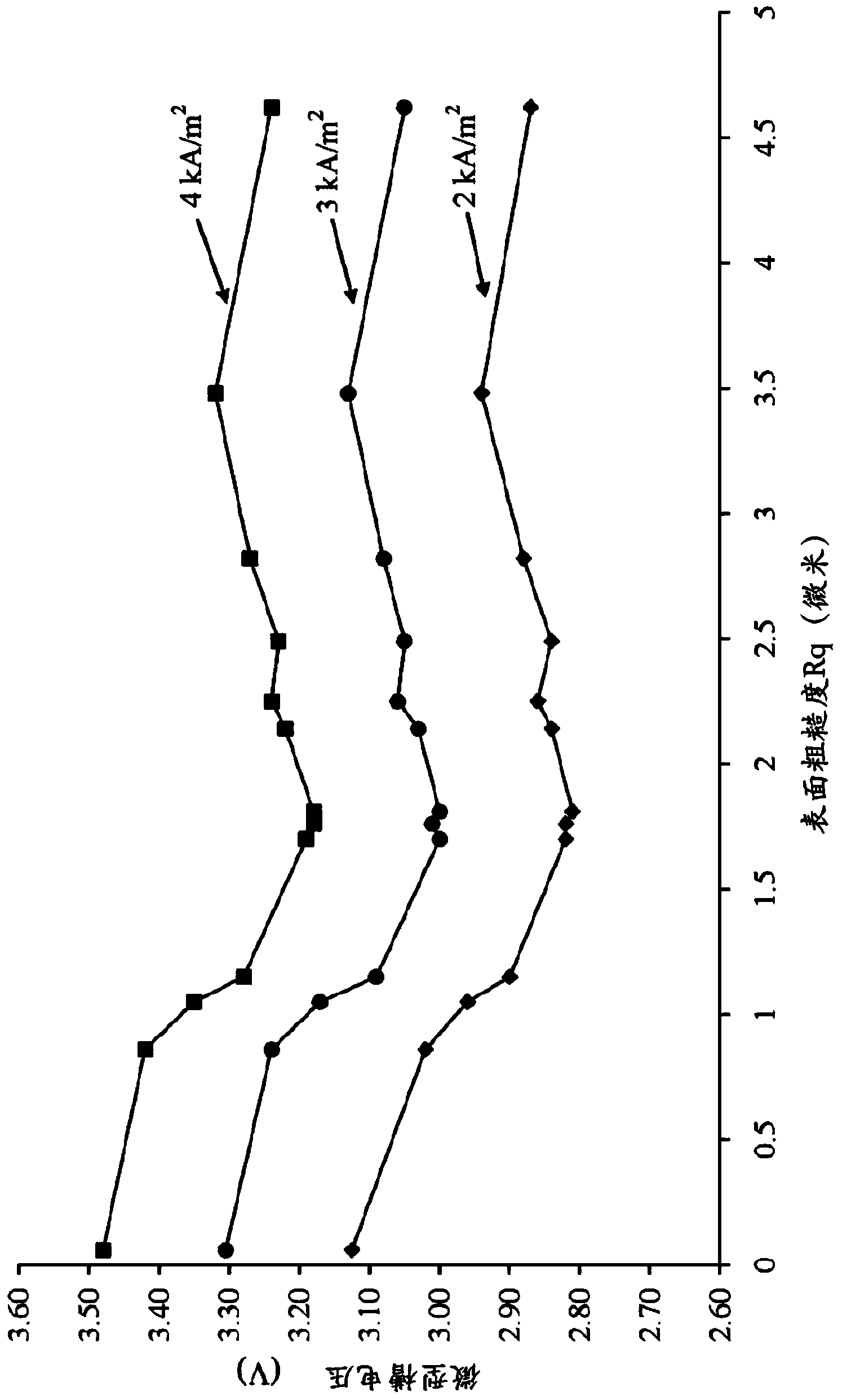
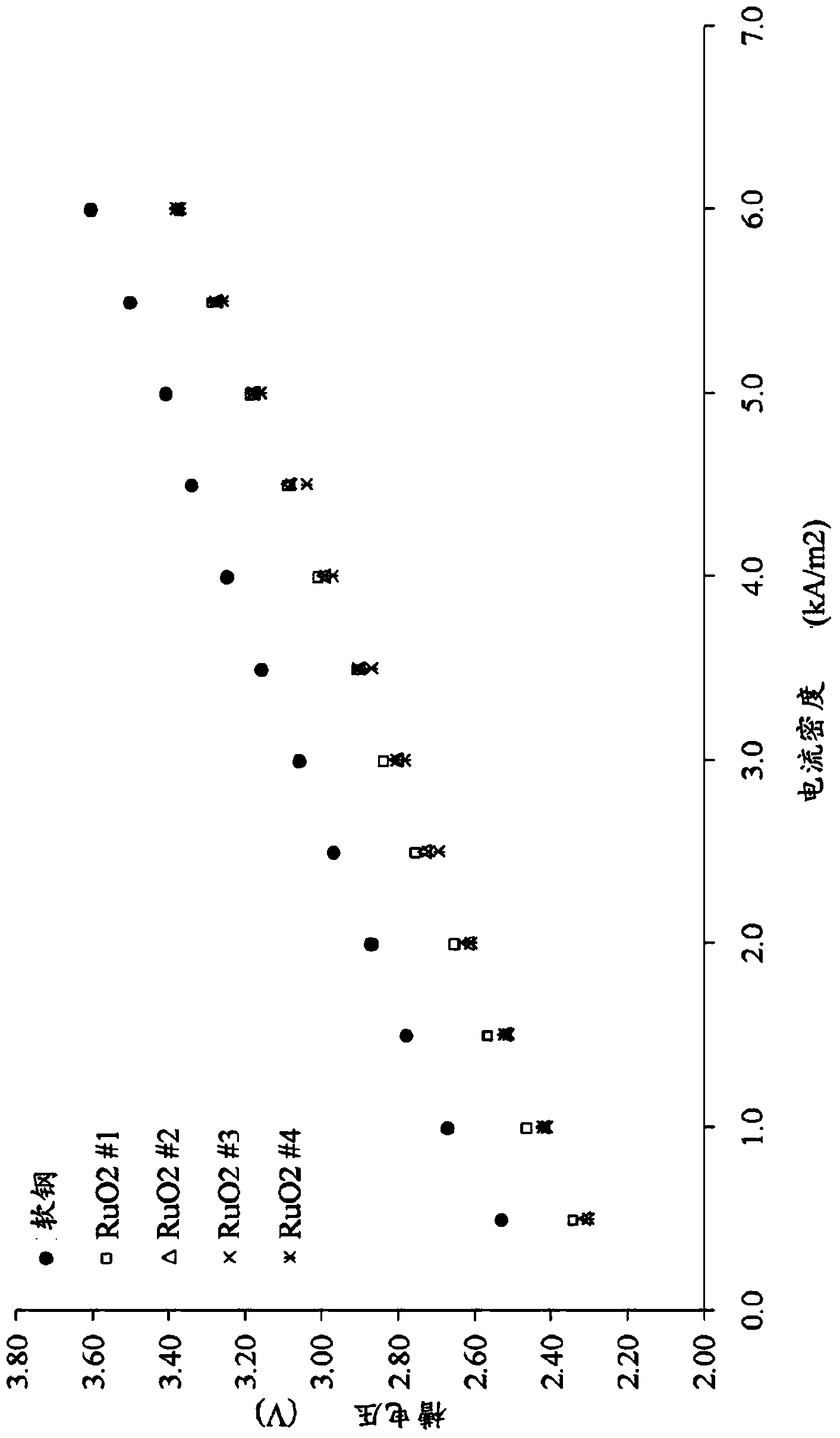
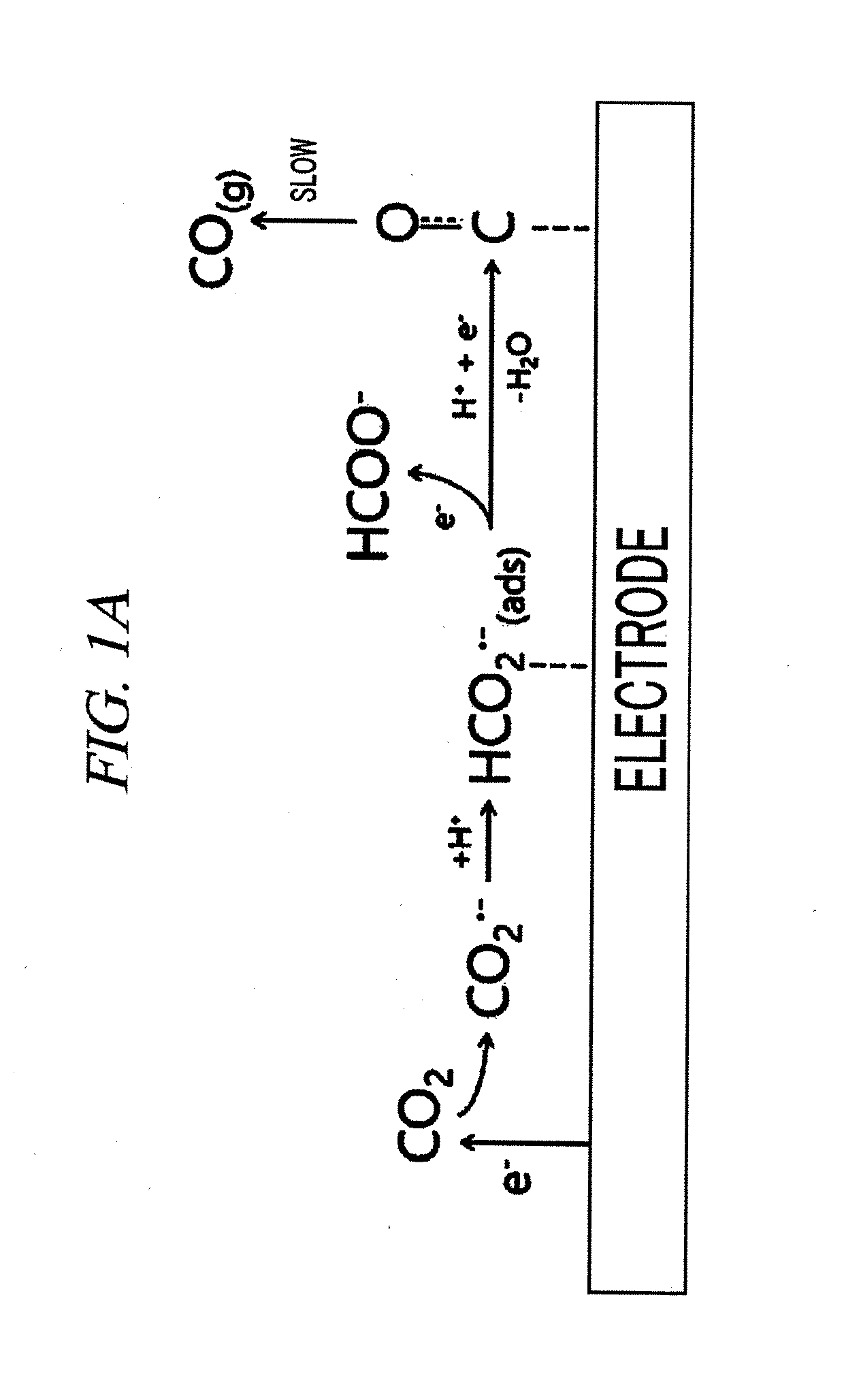
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
118results about "Non-noble metal oxide coatings" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
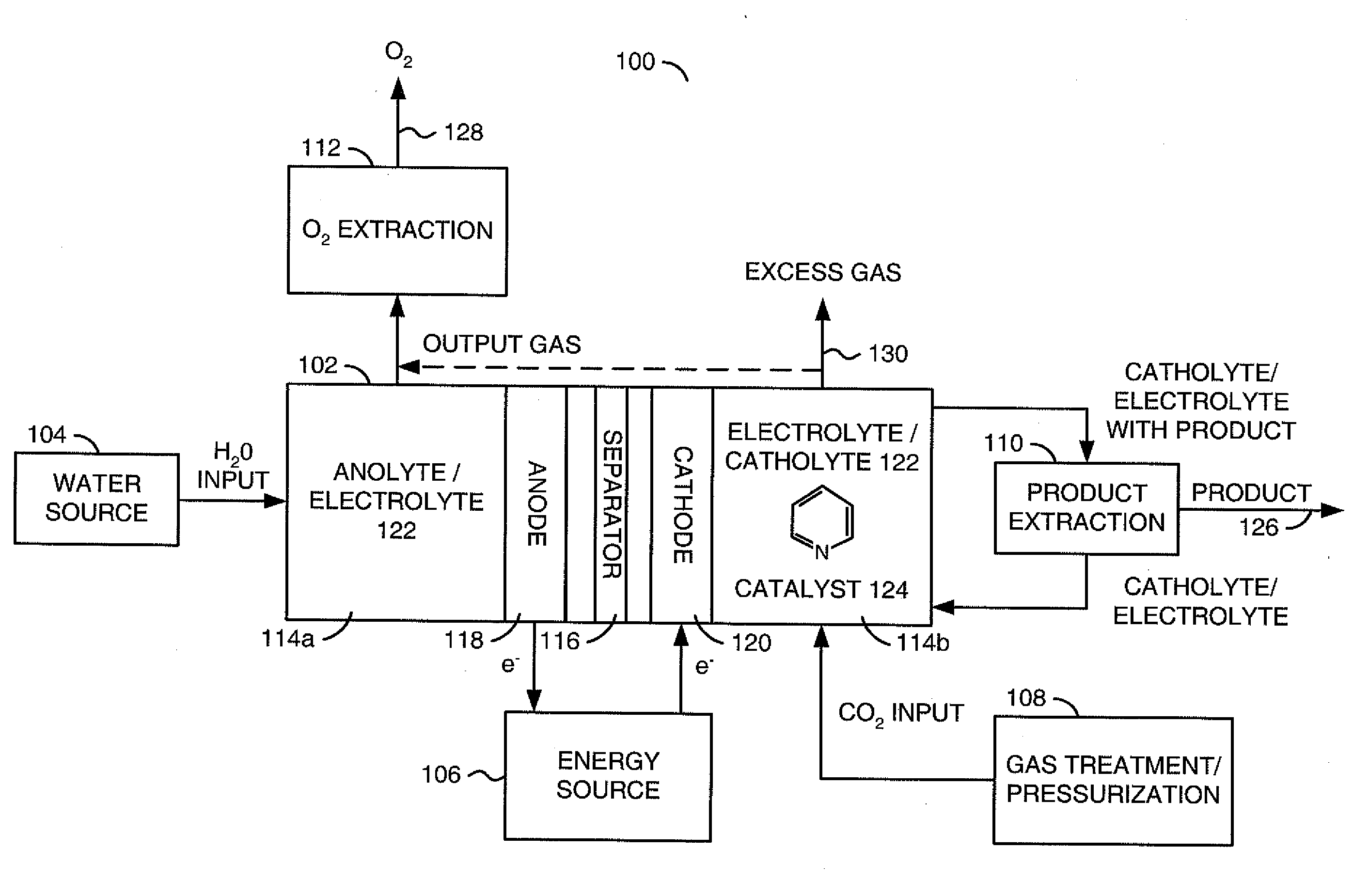
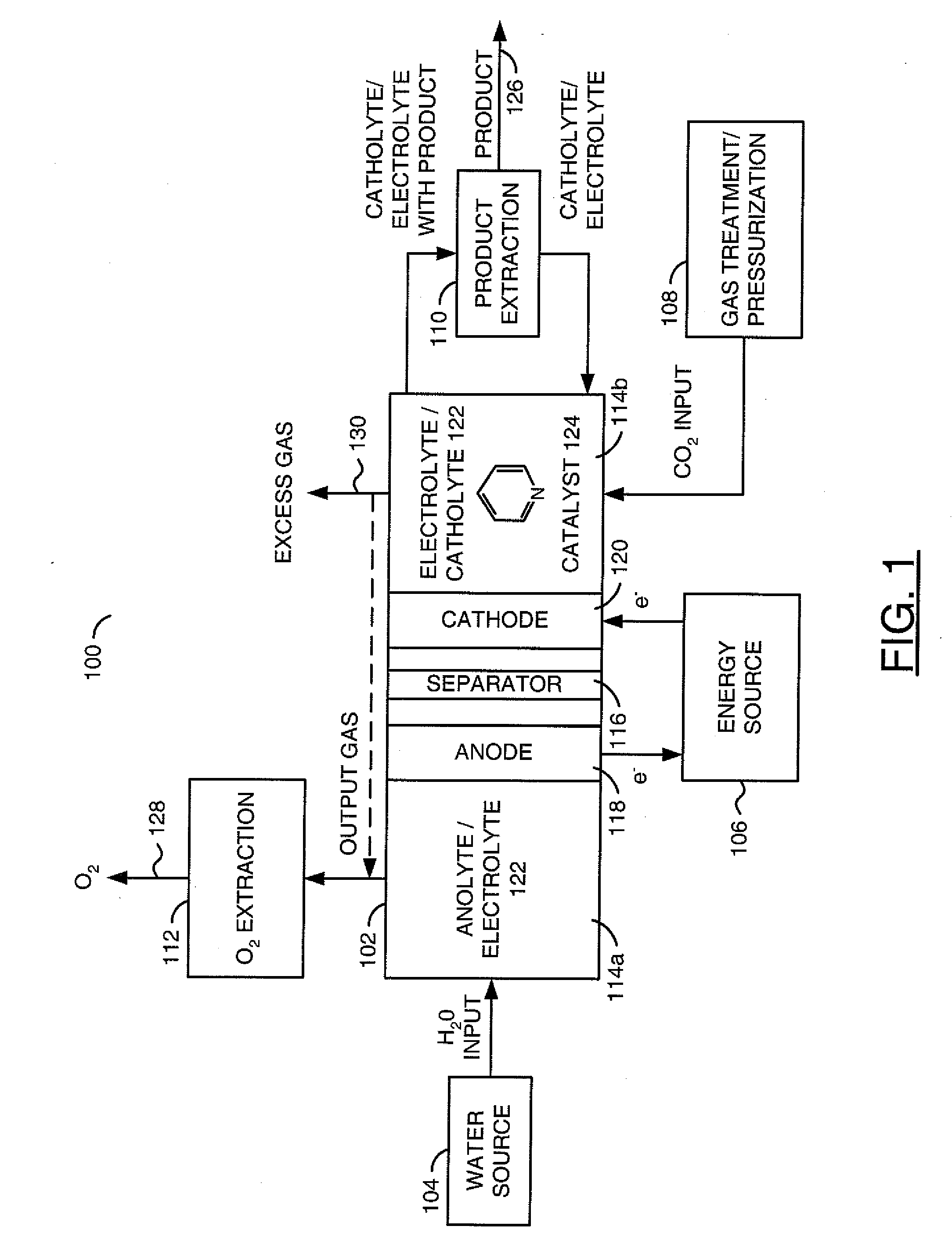
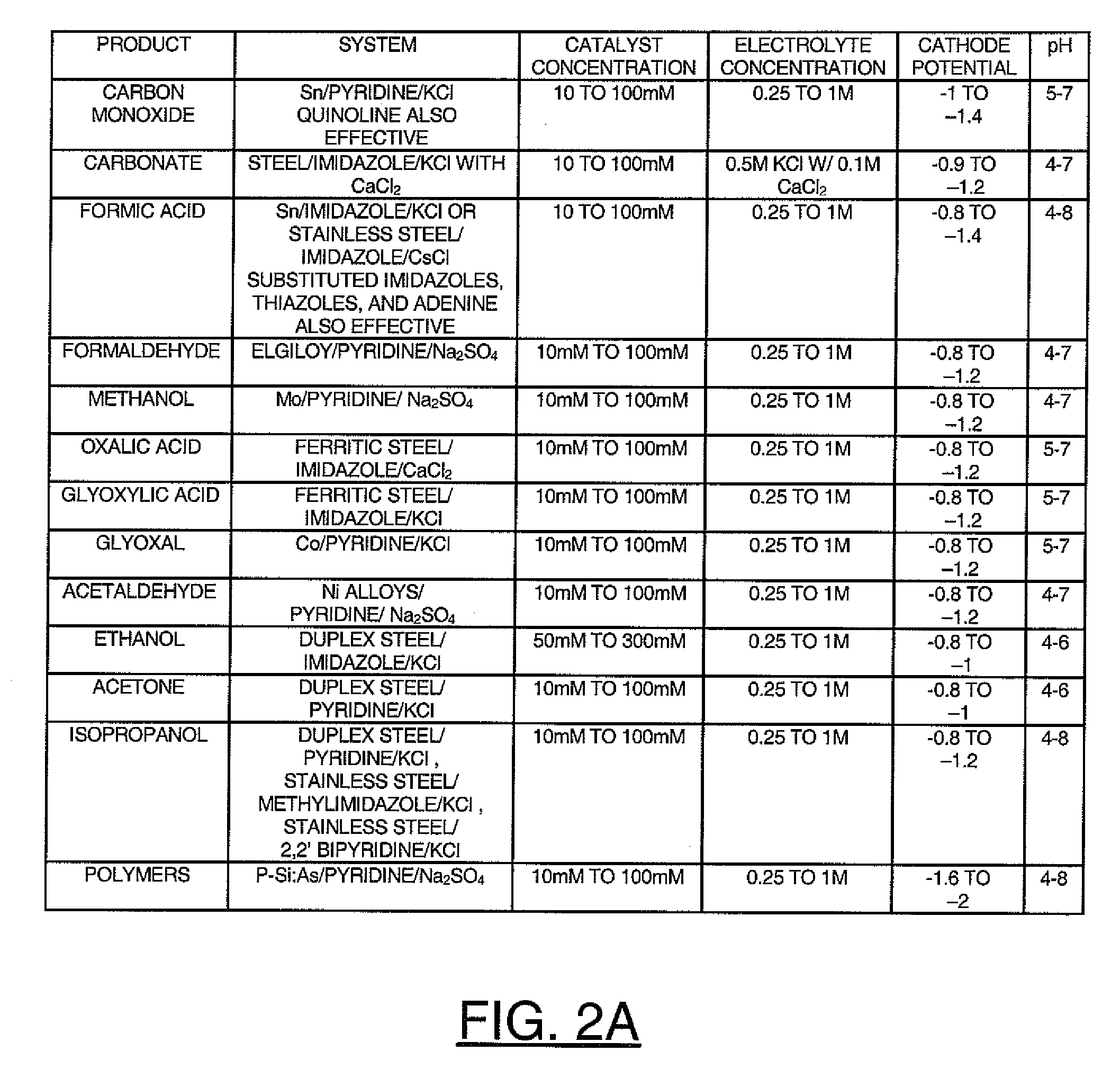
Reducing carbon dioxide to products
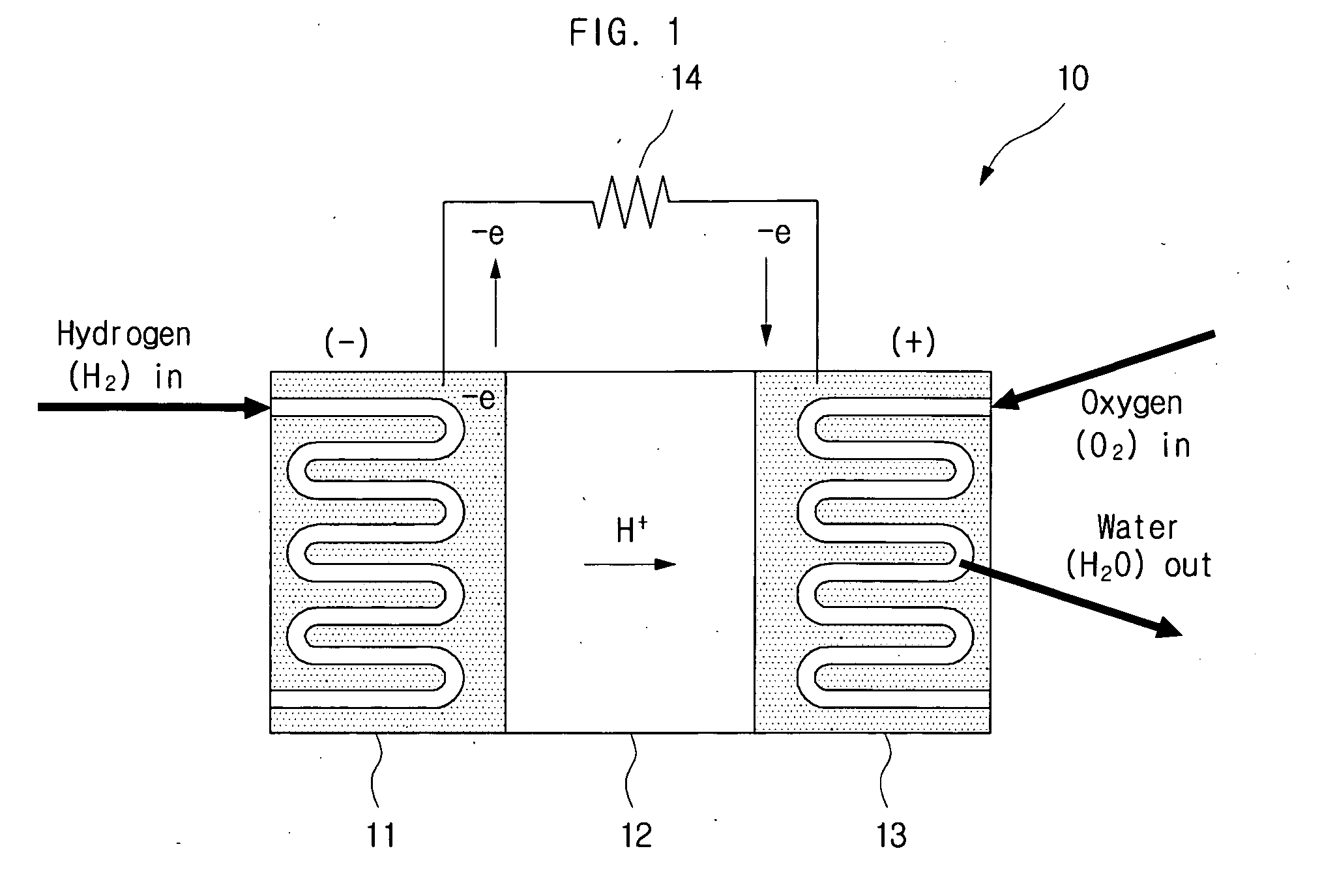
InactiveUS20110114502A1Provide stabile long-term reduction of carbon dioxideLow costCellsPhotography auxillary processesPtru catalystElectrical battery
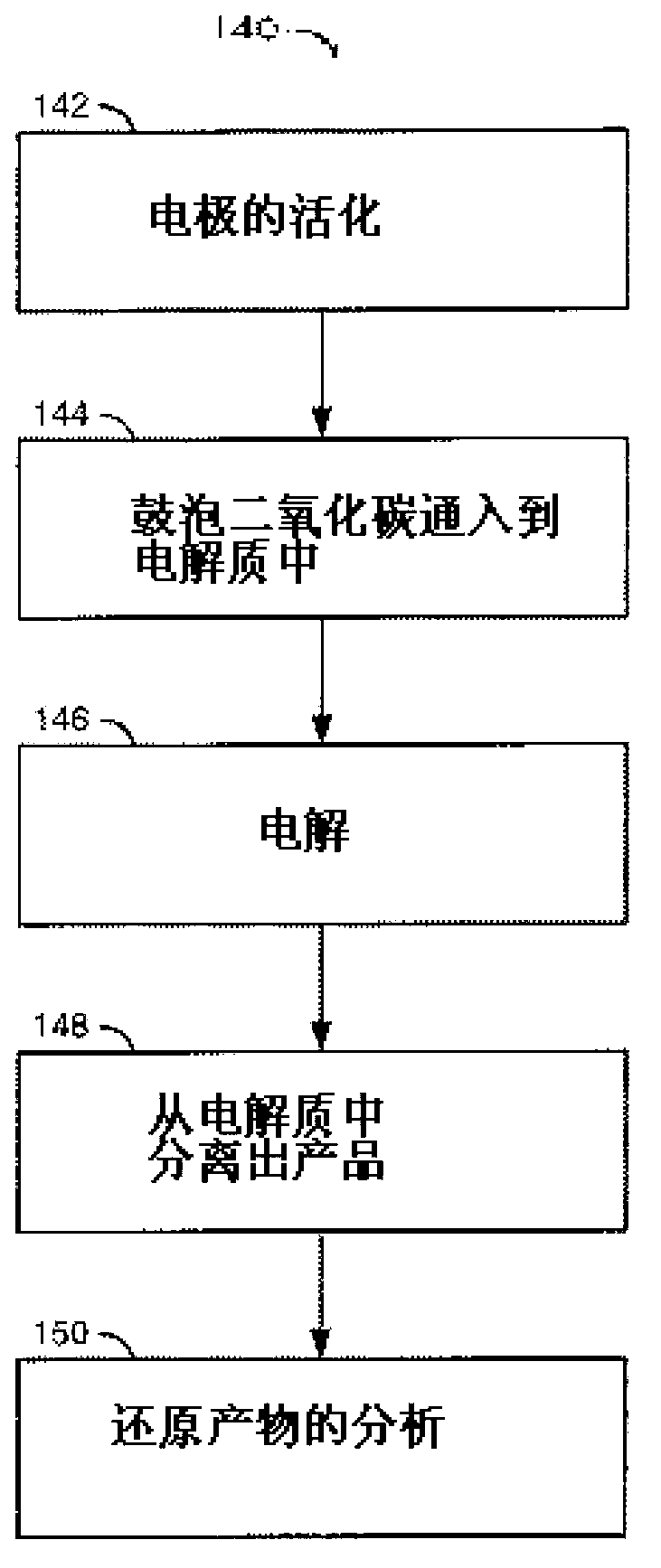
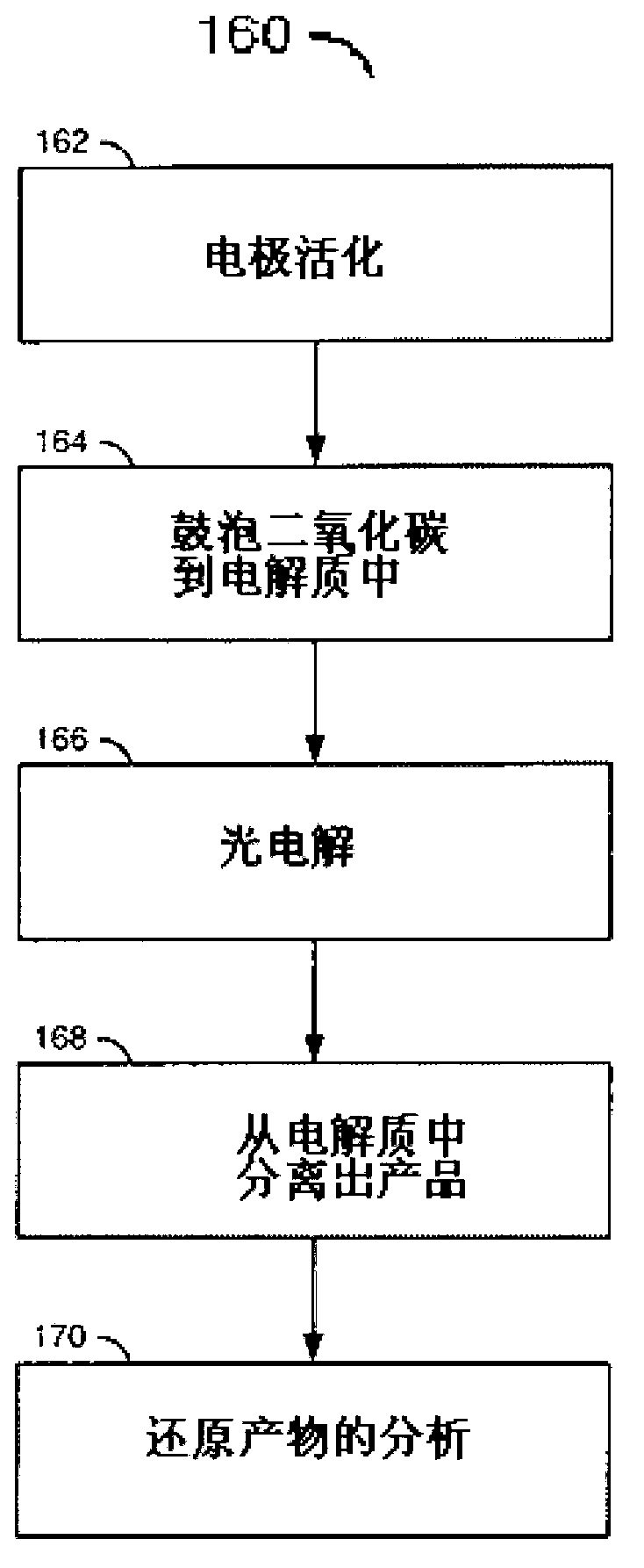
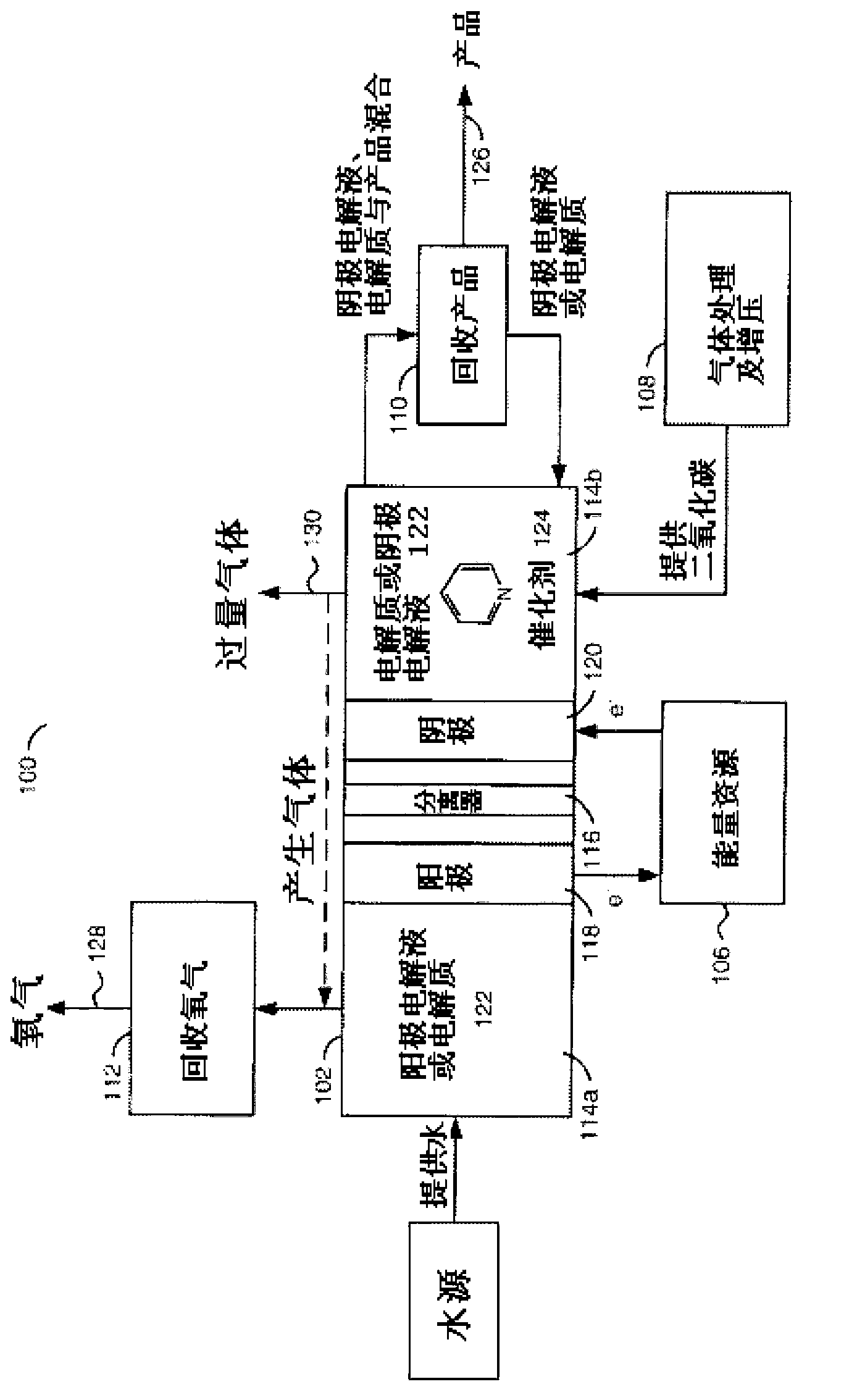
A method for reducing carbon dioxide to one or more products is disclosed. The method may include steps (A) to (C). Step (A) may bubble the carbon dioxide into a solution of an electrolyte and a catalyst in a divided electrochemical cell. The divided electrochemical cell may include an anode in a first cell compartment and a cathode in a second cell compartment. The cathode generally reduces the carbon dioxide into the products. Step (B) may vary at least one of (i) which of the products is produced and (ii) a faradaic yield of the products by adjusting one or more of (a) a cathode material and (b) a surface morphology of the cathode. Step (C) may separate the products from the solution.
Owner:LIQUID LIGHT
Stainless steel electrolytic plates
ActiveUS20060201586A1Easy to disassembleDifficultly removed lower metal portionMachining electrodesNon-noble metal oxide coatingsElectrolysisLower grade
There is provided a substantially permanent stainless steel cathode plate suitable for use in electrorefining of metal cathodes, the cathode being composed of a low-nickel duplex steel or a lower grade “304” steel, wherein operational adherence of an electrodeposition thereon is enabled by altering various qualities of the cathode surface. There is also provided a method of producing the above duplex or Grade 304 cathode plates, such that the desired operational adherence of the deposit upon the plate is not so strong as to prevent the deposit being removed during subsequent handling.
Owner:XSTRATA QUEENSLAND
Electrochemical reduction for reducible dyes
InactiveCN1408037AHigh yieldHigh selectivityNon-noble metal oxide coatingsWater contaminantsElectrochemistryPhotochemistry
A process for an electrochemical reduction of a reducible dye by contacting said reducible dye with a cathode comprising a support of an electrically conductive material and an electrically conductive, cathodically polarized layer formed thereon in situ by alluviation comprises conducting said electrochemical reduction in the presence of a base.
Owner:DYSTAR TEXITILFARBEN GMBH & CO
Reducing carbon dioxide to products
InactiveCN103140608ARestore stabilityRestoration stable long-termCellsNon-noble metal oxide coatingsElectrochemical cellCarbon dioxide
A method for reducing carbon dioxide to one or more products is disclosed. The method may include steps (A) to (C). Step (A) may bubble the carbon dioxide into a solution of an electrolyte and a catalyst in a divided electrochemical cell. The divided electrochemical cell may include an anode in a first cell compartment and a cathode in a second cell compartment. The cathode generally reduces the carbon dioxide into the products. Step {B} may vary at least one of (i) which of the products is produced and (ii) a faradaic yield of the products by adjusting one or more of (a) a cathode material and (b) a surface morphology of the cathode. Step (C) may separate the products from the solution.
Owner:AVANTIUM KNOWLEDGE CENT BV
Metal composite electrode for a hydrogen generating apparatus
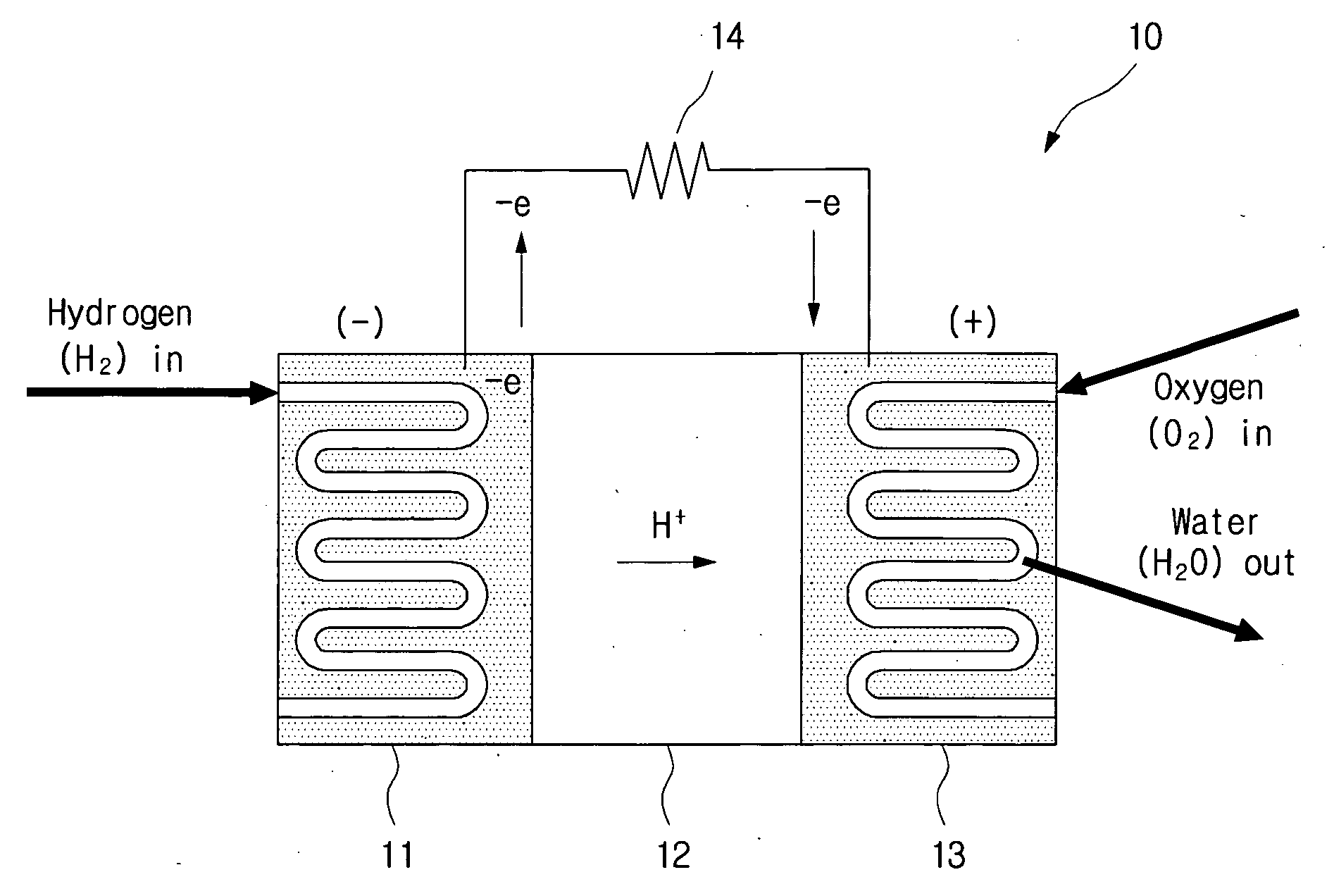
A metal composite electrode for a hydrogen generating apparatus and a hydrogen generating apparatus including the metal composite electrode are disclosed, where the metal composite electrode includes a metal and a metal hydride. The metal composite electrode used in a hydrogen generating apparatus can be utilized to increase the amount and duration of hydrogen generation.
Owner:SAMSUNG ELECTRO MECHANICS CO LTD
Stainless steel electrolytic plates
ActiveUS7807028B2Easy to disassembleAvoid easy removalMachining electrodesNon-noble metal oxide coatingsElectrolysisLower grade
There is provided a substantially permanent stainless steel cathode plate suitable for use in electrorefining of metal cathodes, the cathode being composed of a low-nickel duplex steel or a lower grade “304” steel, wherein operational adherence of an electrodeposition thereon is enabled by altering various qualities of the cathode surface.There is also provided a method of producing the above duplex or Grade 304 cathode plates, such that the desired operational adherence of the deposit upon the plate is not so strong as to prevent the deposit being removed during subsequent handling.
Owner:XSTRATA QUEENSLAND
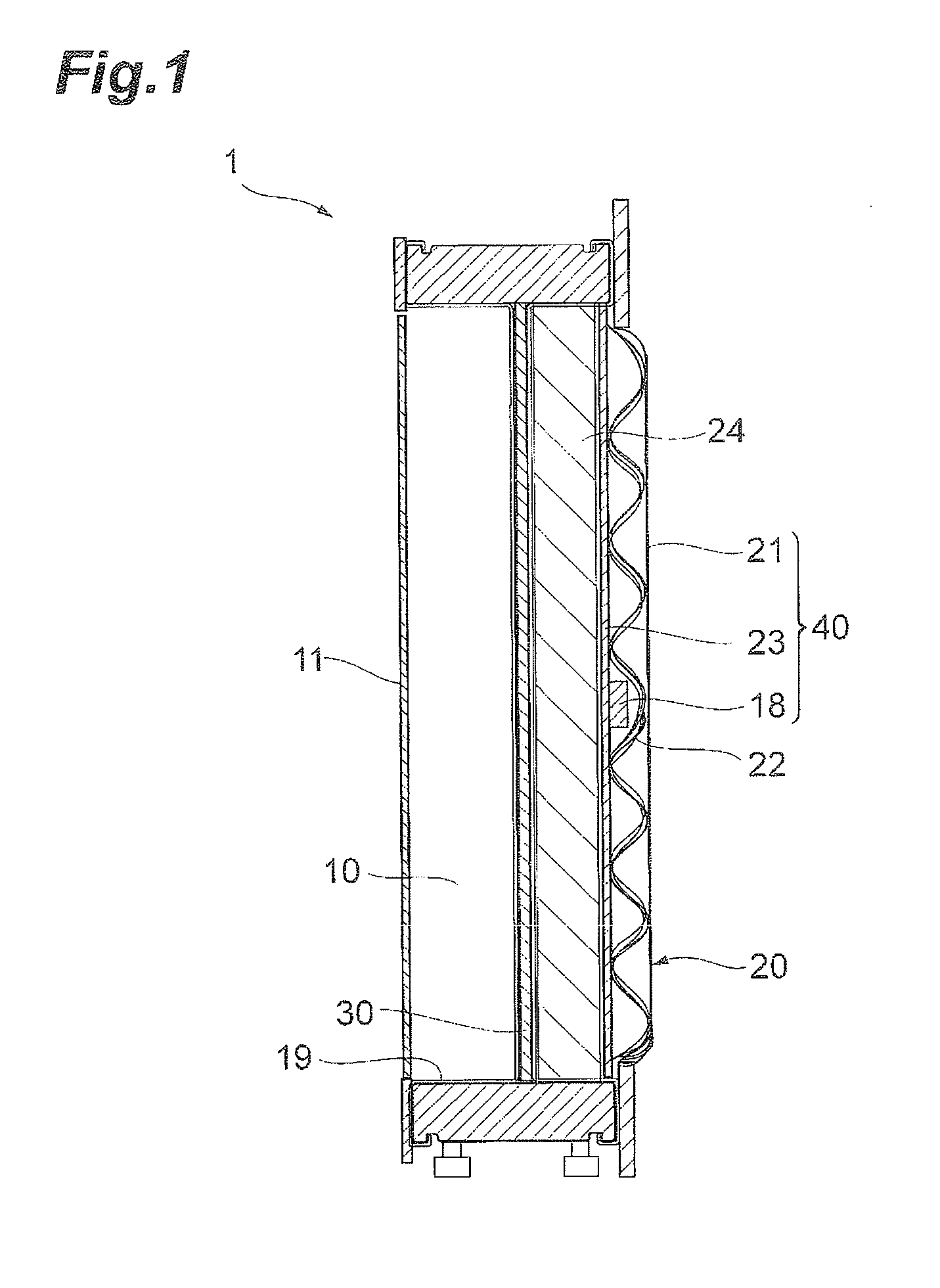
Electrolysis cell and electrolysis tank
Provided is an electrolysis cell capable of suppressing the degradation of a cathode by the reverse current at the time of stopping electrolysis. According to an aspect of the invention, there is provided an electrolysis cell comprising an anode chamber, a cathode chamber, a partition wall separating the anode chamber from the cathode chamber, an anode installed in the anode chamber, a cathode installed in the cathode chamber, and a reverse current absorbing body having a substrate and a reverse current absorbing layer formed on the substrate and installed in the cathode chamber, in which the anode and the cathode are electrically connected and the cathode and the reverse current absorbing layer are electrically connected.
Owner:ASAHI KASEI KK
Organic compound hydrogenation apparatus and method for hydrogenating organic compound
InactiveUS20070000788A1Improve hydrogenation efficiencyIncrease contactCellsLiquid separation by electricityOrganic compoundHydrogen storage
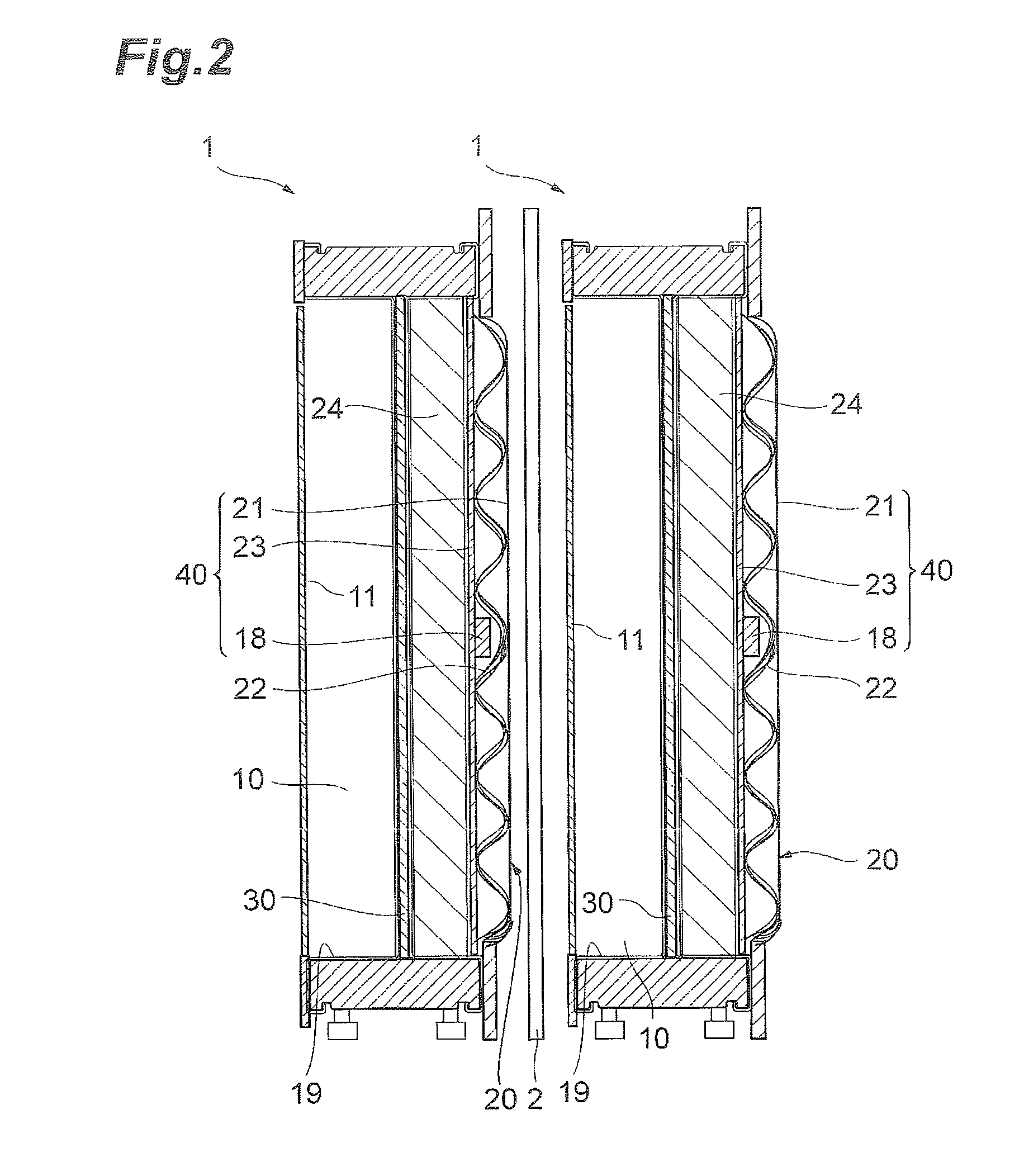
An organic compound hydrogenation apparatus 1 of the present invention includes a reaction cell 13 to which an electrolytic solution is supplied, and an anode 11 and a cathode 12 arranged in the reaction cell 13, in which the cathode 12 is made of a material including a hydrogen storage material, the cathode being arranged as a tubular member so that an organic compound as an object to be treated circulates thereinside. The present invention having the arrangement described above can provide a method for hydrogenating organic compounds and an organic compound hydrogenation apparatus that can enhance efficiency of hydrogenation of the organic compound.
Owner:IDEMITSU KOSAN CO LTD
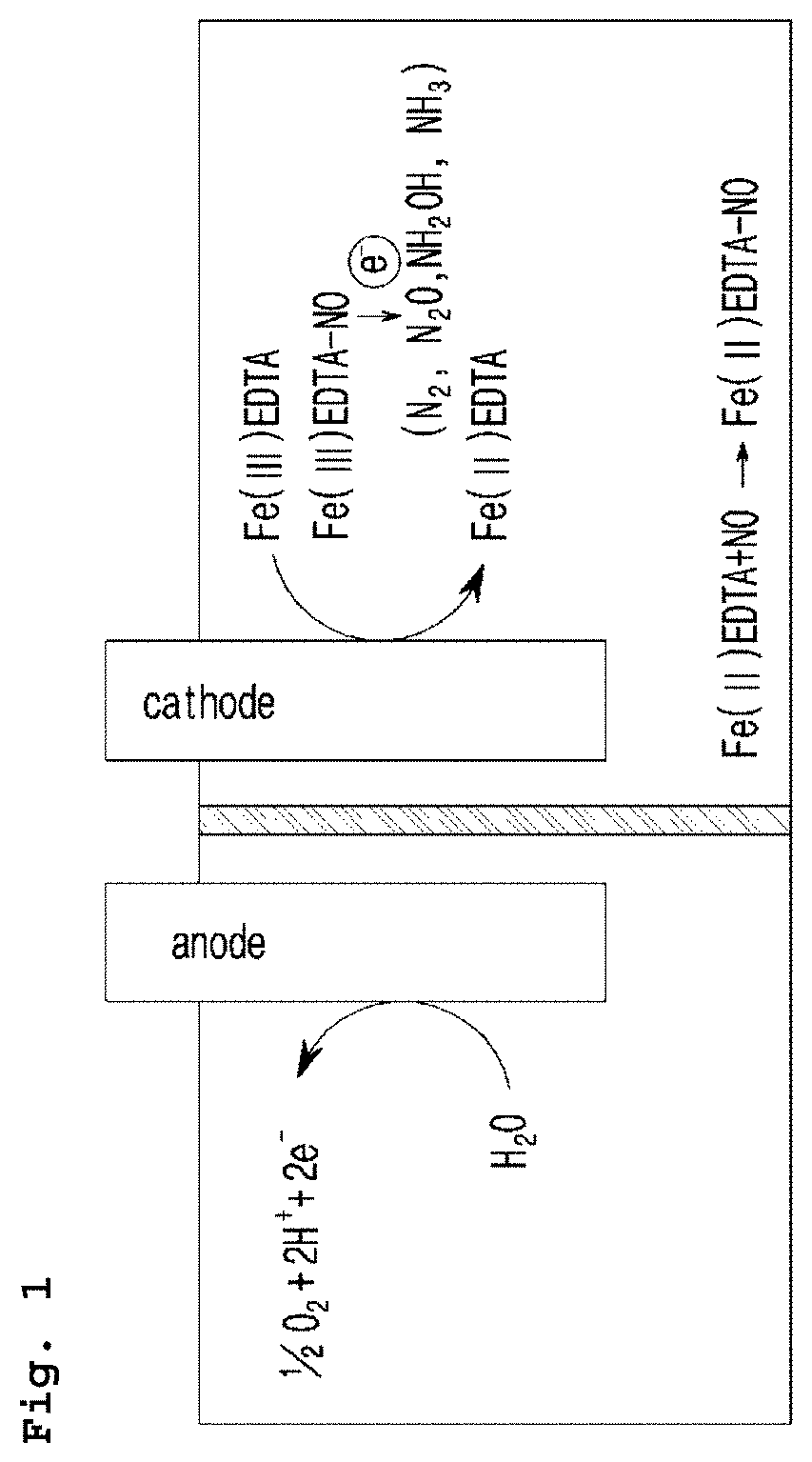
Electrochemical system for producing ammonia from nitrogen oxides and preparation method thereof
InactiveUS20200002180A1High selectivityEfficient productionCellsNon-noble metal oxide coatingsNitrogen oxidesCathode electrode
It is an object of the present invention to provide an electrochemical system for producing ammonia from nitrogen oxides which can perform the reaction at room temperature under normal pressure with high ammonia selectivity, and a preparation method thereof.To achieve the object above, the present invention provides an electrochemical system for producing ammonia from nitrogen oxides characteristically comprising a cathode electrode where the reduction reaction of a complex of nitrogen oxide and a metal complex compound occurs, an anode electrode, a reference electrode, an electrolyte including a metal complex compound, and a nitrogen oxide supply unit.The present invention also provides a method for producing ammonia from nitrogen oxides, which characteristically comprises the steps of introducing nitrogen oxide in the electrochemical system; forming a complex from the introduced nitrogen oxide and the metal complex compound included in the electrolyte; and performing an electrical reduction reaction of the formed complex. According to the present invention, ammonia can be produced from nitrogen oxides via electrochemical method under normal atmospheric pressure and at room temperature with a high selectivity.
Owner:KOREA RES INST OF CHEM TECH
Preparation method of hierarchical pore high-entropy alloy water electrolysis catalyst
ActiveCN113061925AImprove the oxygen production performance of electrolyzed waterLow costNon-noble metal oxide coatingsPtru catalystElectrolysed water
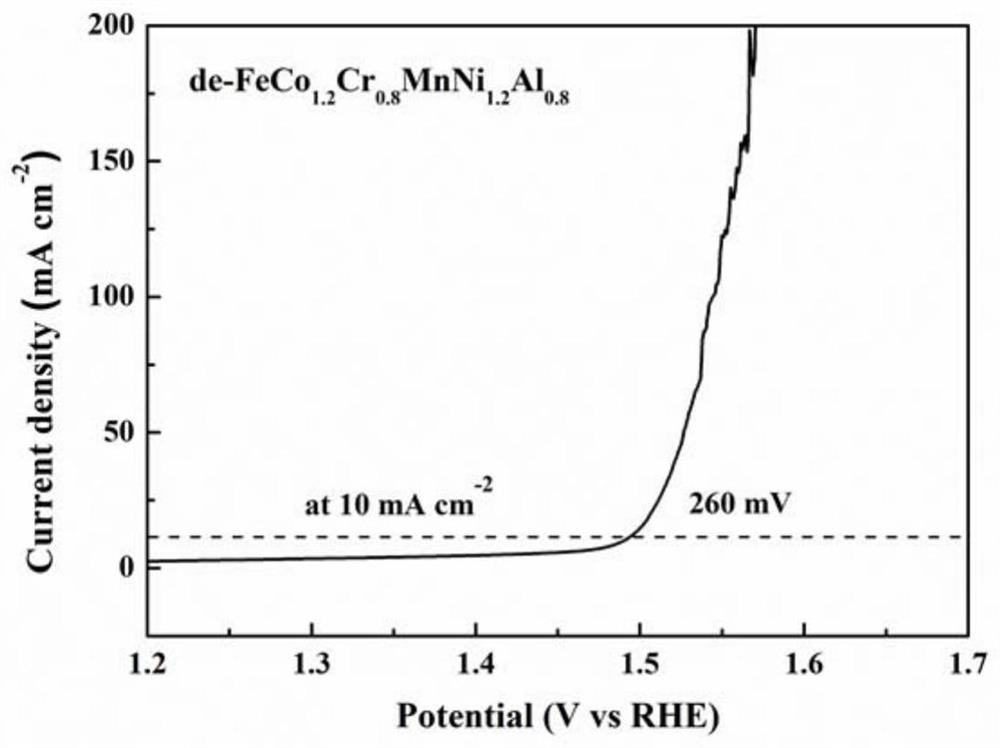
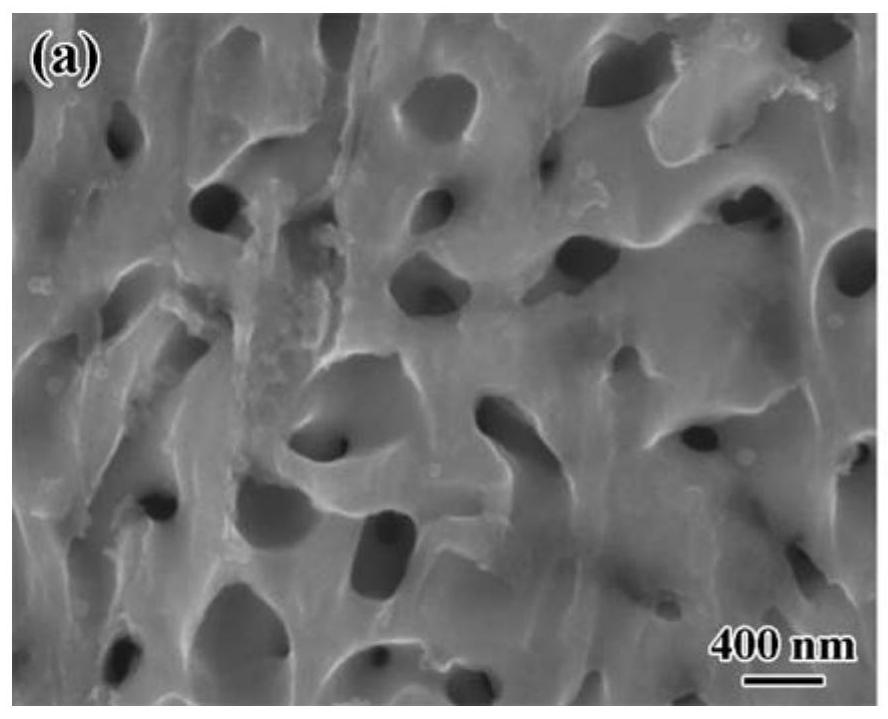
The invention belongs to the technical field of electro-catalysis application, and relates to a preparation method of a hierarchical pore high-entropy alloy water electrolysis catalyst, which comprises the following steps of: by utilizing the difference of mixing enthalpy between melt metal and pre-alloy components, putting the smelted high-entropy pre-alloy into molten metal M, and selectively reacting the molten metal M with elements with negative mixing enthalpy in the pre-alloy to form M-rich and M-poor multi-phase structures; and selectively removing a relatively active M-rich phase in the alloy by utilizing an acidic solution, and diffusing the remaining components to form a three-dimensional bicontinuous high-entropy alloy with a hierarchical pore structure, wherein the size of macropores is 500 nanometers to 2 micrometers, and the size of micropores is 40-200 nanometers. According to the present invention, the cost is reduced, the water electrolysis oxygen production performance of the material can be substantially improved through the synergistic effect among the multi-principal element transition group metals and the hierarchical pore structure, the required overpotential at 10 mA cm <-2 > is 260-300 mV, and the potential industrial application value is great.
Owner:UNIV OF SCI & TECH BEIJING
Sulfuric acid electrolytic cell and a sulfuric acid recycle type cleaning system applying the sulfuric acid electrolytic cell
ActiveUS8137513B2High mechanical strengthAvoid corrosionMachining electrodesCellsConductive pasteElectric power
In a sulfuric acid electrolytic cell to electrolyze sulfuric acid supplied to an anode compartment and a cathode compartment comprising a diaphragm, said anode compartment and said cathode compartment separated by said diaphragm, a cathode provided in said cathode compartment and a conductive diamond anode provided in said anode compartment, as said conductive diamond anode, a conductive diamond film is formed on the surface of said conductive substrate, the rear face of said conductive substrate is pasted, with conductive paste, on an current collector comprising a rigid body with size equal to, or larger than, said conductive substrate, an anode compartment frame constituting said anode compartment is contacted via gasket with the periphery on the side of the conductive diamond film of said diamond anode, said diaphragm is contacted with the front face of said anode compartment, further, with the front face of said diaphragm, the cathode compartment frame constituting said cathode compartment, a gasket, and said cathode are contacted in sequence, the rear face of said cathode is pasted with conductive paste to the current collector comprising a rigid body with size equal to, or larger than, said cathode and electric power is supplied from one current collector to the other current collector via said conductive paste.
Owner:DE NORA PERMELEC LTD +2
Method for producing hydrogen by electrolyzing water step by step and device thereof
PendingCN113151843ATo achieve separate productionIncrease transfer rateCellsNon-noble metal oxide coatingsElectrolytic agentElectrolysed water
The invention discloses a method for producing hydrogen by electrolyzing water step by step and a device thereof. The device comprises an electrolytic bath, a hydrogen evolution catalysis cathode electrode, a nickel hydroxide anode electrode, a hydrogen outlet and an oxygen outlet. Wherein the electrolytic bath does not comprise a diaphragm, and a cavity is formed in the electrolytic bath. The method for producing hydrogen by electrolyzing water comprises the following steps: firstly, switching on an external direct-current power supply of an electrolytic bath, so that water molecules in electrolyte are electrochemically reduced on the surface of a hydrogen evolution catalytic cathode electrode to produce hydrogen, and a Ni (OH)2 anode electrode is electrochemically oxidized into a NiOOH anode electrode; and then, a direct-current power supply outside the electrolytic bath is switched off, high-temperature saturated steam is introduced into the electrolyte of the electrolytic bath, the temperature of the electrolyte is ensured to reach 90-110 DEG C, at the moment, due to the thermodynamic instability of NiOOH, the anode electrode is decomposed and reduced into a Ni (OH)2 electrode, and oxygen is generated around the electrode. The hydrogen and the oxygen are prepared by electrolysis through the two steps, so that the high-purity hydrogen and the high-purity oxygen can be prepared, and the cost of hydrogen production by electrolysis of water can be effectively reduced.
Owner:SHANGHAI SUN-HYDROGEN ENERGY TECH CO LTD
Metal self-supporting electrode, preparation method and application
PendingCN114395777AEasy to prepareReduce manufacturing costNon-noble metal oxide coatingsElectrolytic organic productionHollow fibreFiber
The invention provides a metal self-supporting electrode, a preparation method and application, and the preparation method comprises the following steps: S1, grinding and mixing metal or metal oxide powder, an organic solvent and a binder to obtain a slurry; s2, the slurry liquid is sprayed out through a spinning nozzle and enters solidification liquid through an air layer, and a hollow fiber tubular soft body is cast; s3, washing and shaping to obtain a green body; s4, roasting the green body in an oxidizing gas atmosphere to obtain an intermediate product; and S5, heating and reducing the intermediate product in a reducing gas atmosphere, and cooling to obtain the metal self-supporting electrode. The metal self-supporting electrode prepared by the preparation method is applied to electrocatalytic oxidation and reduction of CO2, N2, H2O, CH4, ethylene, propylene and glycerol. The preparation method is simple, convenient and low in cost, and the prepared metal self-supporting electrode has good electrocatalytic oxidation-reduction reaction activity, high product selectivity, high reproducibility and good stability.
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI
Alloy Catalyst Material
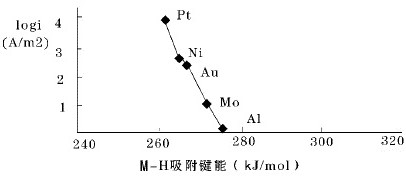
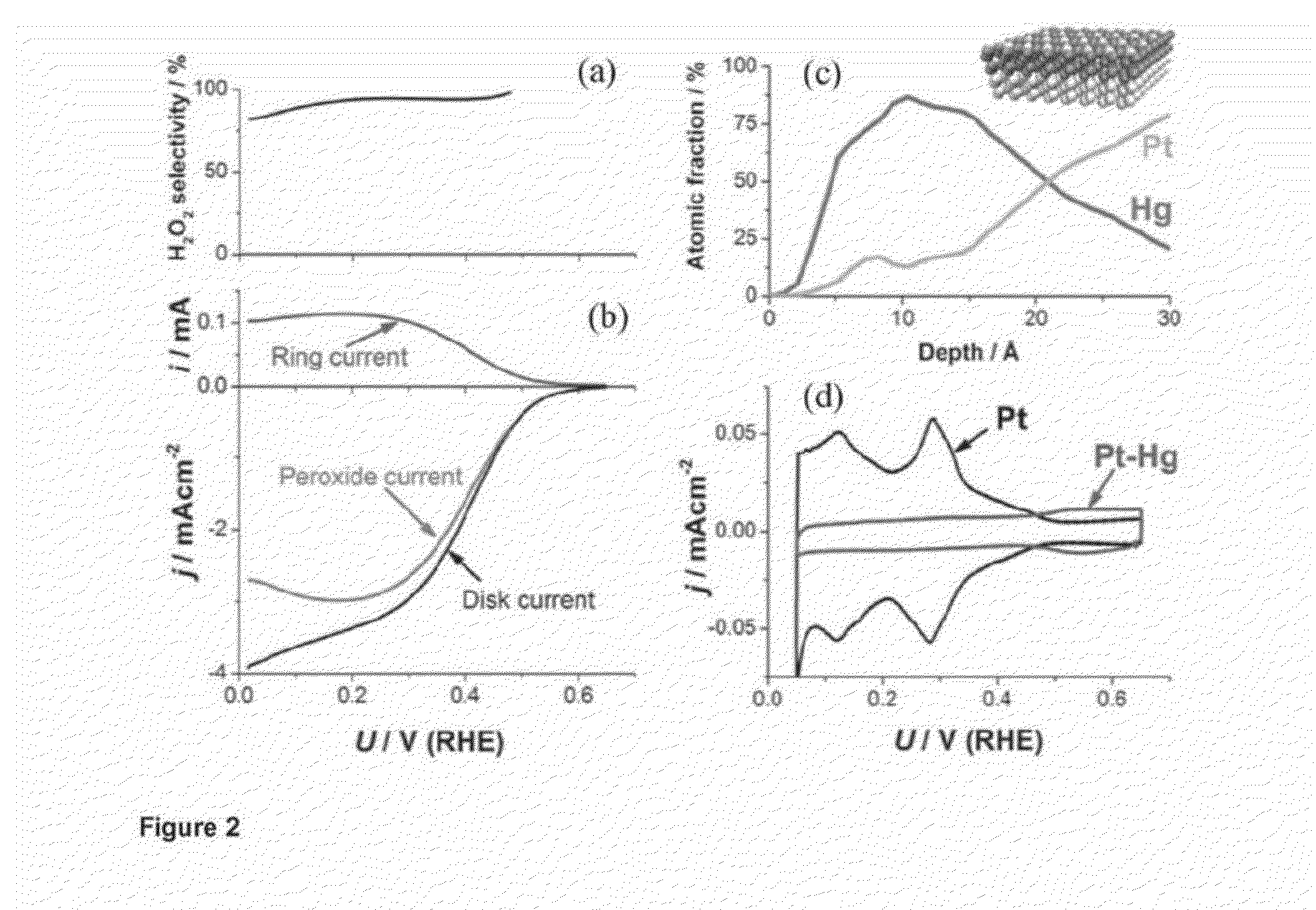
The present invention relates to a novel alloy catalyst material for use in the synthesis of hydrogen peroxide from oxygen and hydrogen, or from oxygen and water. The present invention also relates to a cathode and an electrochemical cell comprising the novel catalyst material, and the process use of the novel catalyst material for synthesising hydrogen peroxide from oxygen and hydrogen, or from oxygen and water.
Owner:DANMARKS TEKNISKE UNIV
High-entropy alloy fiber as well as preparation method and application thereof
The invention discloses a high-entropy alloy fiber as well as a preparation method and application thereof, relates to an electrochemical catalyst and a preparation method thereof, and aims to solve the problems that an existing platinum catalyst is high in price and low in platinum reserve, and the electrochemical catalysis performance and the stability need to be improved. The preparation method comprises the steps: 1, weighing metal raw materials; 2, smelting the mixed metal raw materials into a metal button ingot by adopting a high-vacuum electric arc smelting furnace, and then melting the button ingot to form a master alloy rod by suction casting; 3, performing drawing treatment on the master alloy rod by adopting melt drawing equipment to obtain alloy fibers; and 4, carrying out free dealloying treatment on the alloy fibers. After the high-entropy alloy fiber is subjected to dealloying treatment, a porous structure is formed on the surface, the overpotential of 230 mV can be achieved on electrochemical oxygen evolution catalysis, the Tafel slope is about 40, a good catalysis effect is achieved, meanwhile, a good electrochemical hydrogen evolution catalysis effect is achieved, and the overpotential of hydrogen evolution catalysis is about 180 mV.
Owner:HARBIN INST OF TECH
Electrolysis cell and electrolysis tank
Owner:ASAHI KASEI KK
Stainless steel electrolytic plates
ActiveUS20080095655A1Easy to disassembleAvoid easy removalMachining electrodesNon-noble metal oxide coatingsElectrolysisLower grade
There is provided a substantially permanent stainless steel cathode plate (1) suitable for use in electrorefining of metal cathodes, the cathode being composed of a low-nickel duplex steel or a lower grade “304” steel, wherein operational adherence of an electrode-position thereon is enabled by altering various qualities of the cathode surface. There is also provided a method of producing the above duplex or Grade 304 cathode plates, such that the desired operational adherence of the deposit upon the plate is not so strong as to prevent the metal deposit being removed during subsequent handling.
Owner:XSTRATA QUEENSLAND
Nickel and molybdenum alloy electrode material and preparation method thereof
InactiveCN112779440AExtend working lifeImprove conductivityNon-noble metal oxide coatingsPumping vacuumArgon gas
The invention discloses a nickel and molybdenum alloy electrode material which comprises 30-80% of Ni and 10-60% of Mo and further comprises 0.1-10% of Fe, Cr, W, Co, Mn and V. The preparation method of the electrode material comprises the following steps: drying a raw material after washing the same; placing dried Ni and Mo in a vacuum induction melting furnace to perform smelting at 10-30 kW; introducing argon into the vacuum induction melting furnace which smelts Ni and Mo; vacuumizing the vacuum induction melting furnace which smelts Ni and Mo; adding dried Fe, Cr, W, Co, Mn and V into the vacuumized vacuum induction melting furnace and heating the vacuum induction melting furnace in sequence at 10-30 kW and 30-60 kW; refining the melted material; and taking out the vacuum cooled material to obtain the nickel and molybdenum alloy electrode material. The preparation method is easy to operate, safe and reliable, short in production period and low in cost.
Owner:GRIMAT ENG INST CO LTD
Preparation method of nickel nanowire array electrode and application of nickel nanowire array electrode as electrochemical oxygen evolution active material
PendingCN112760676AEasy to makeSimple methodNon-noble metal oxide coatingsPtru catalystNanowire array
The invention discloses a preparation method of a nickel nanowire array electrode and an application of the nickel nanowire array electrode as an electrochemical oxygen evolution active material, and relates to the field of electrochemical oxygen evolution. The method comprises the steps: firstly, preparing the nickel nanowire array electrode with a high specific surface area by combining a deep reactive ion etching technology and an electroplating method; secondly, modifying a nickel nanowire array electrode substrate with doped metal atoms (Fe, Co and Mo) capable of strengthening or exciting the catalytic activity of the nickel nanowire array electrode substrate to prepare various composite material electrocatalysts, and further strengthening the catalytic activity of the nickel nanowire array electrode; and finally, deeply characterizing the crystal microstructure of the obtained material and testing the electrocatalytic oxygen evolution performance of the material, thereby exploring the application of the nickel nanowire array electrode as the efficient electrochemical oxygen evolution active material. The nickel nanowire array electrode prepared by the method has a high specific surface area and an excellent response signal, and shows relatively good electrocatalytic oxygen evolution activity and stability, so that direct scientific basis and technical means are provided for designing and constructing a stable and efficient electrocatalytic oxygen evolution active material.
Owner:BEIJING UNIV OF TECH
Ozone electrolysis chamber and ozone electrolysis chamber application module
PendingCN111254453AEasy to moveIncrease concentrationCellsNon-noble metal oxide coatingsElectrolysisEnvironmental engineering
Owner:GUANGZHOU DEPOSON ELECTRIC TECH
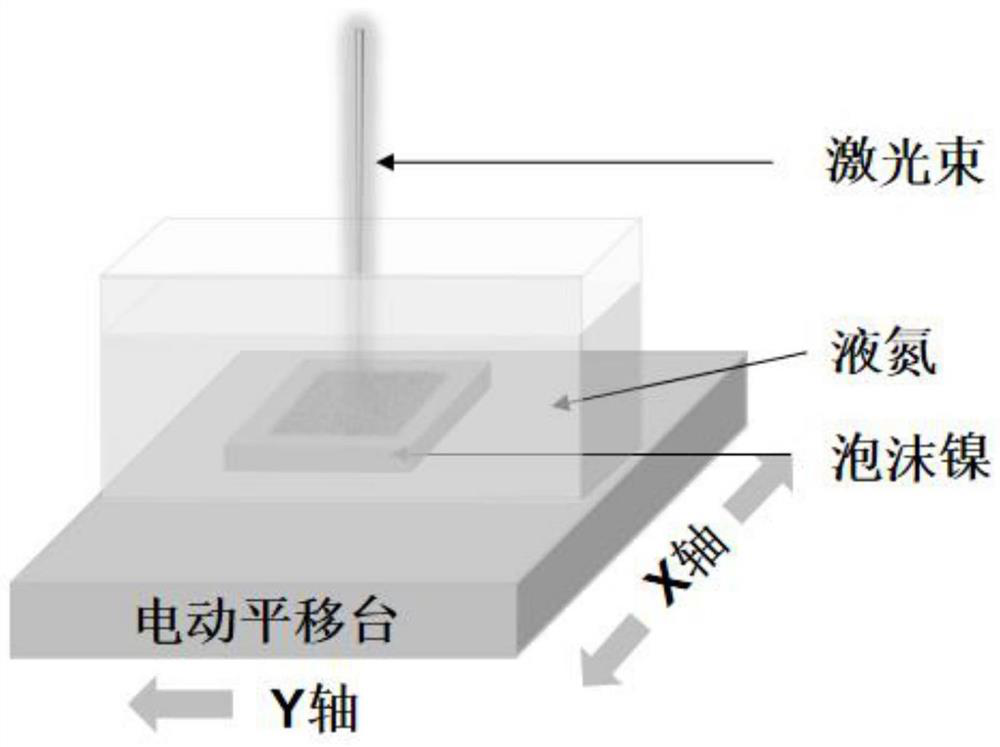
Method for preparing catalytic electrode rich in crystal defects through pulse laser direct writing in liquid nitrogen environment
ActiveCN113913858AHigh catalytic activityReduce dosageNon-noble metal oxide coatingsDirect writingCrystallographic defect
The invention relates to a method for preparing a catalytic electrode rich in crystal defects through pulse laser direct writing in a liquid nitrogen environment. The method comprises the following steps: ultrasonically cleaning foamed nickel in distilled water and absolute ethyl alcohol respectively, soaking in diluted hydrochloric acid for 15-30 minutes, taking out the material, cleaning the material with distilled water, and drying the material; soaking the treated foamed nickel in a chloroplatinic acid solution, taking out the material, cleaning the material with distilled water, and drying the material; fixing the prepared foamed nickel to the bottom of an open container, pouring liquid nitrogen into the open container, and placing the open container in the container containing the liquid nitrogen so that the liquid level of the liquid nitrogen can be kept higher than the foamed nickel; at normal temperature, setting the working range of a millisecond laser on a computer by adopting the millisecond laser, and carrying out direct writing by using pulse laser; and moving out the obtained foamed nickel, cleaning with deionized water, and drying the foamed nickel to obtain the platinum-nickel alloy catalytic electrode rich in defects. The preparation method is simple and easy to implement, high in controllability and capable of achieving large-scale production.
Owner:TIANJIN UNIV
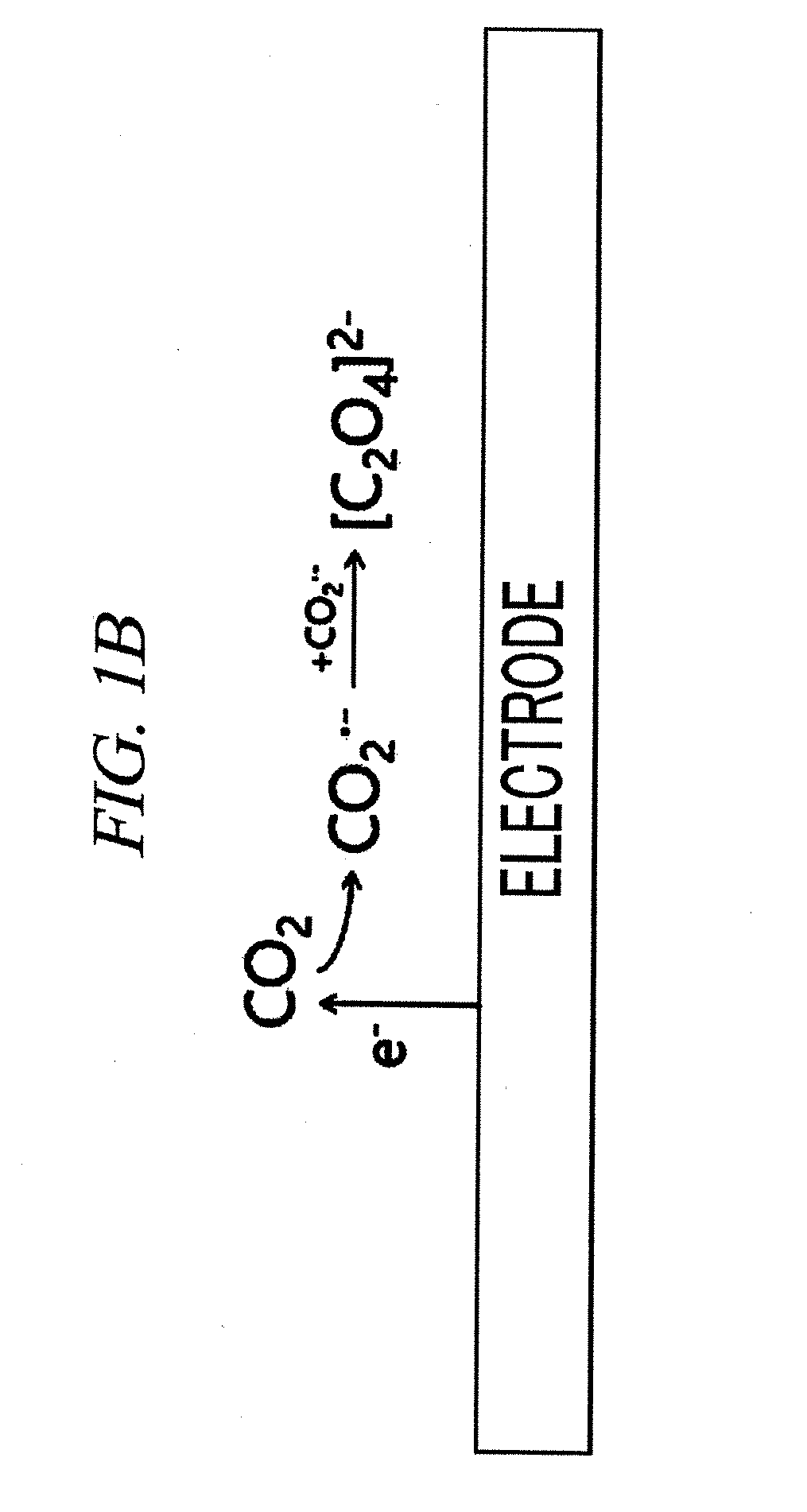
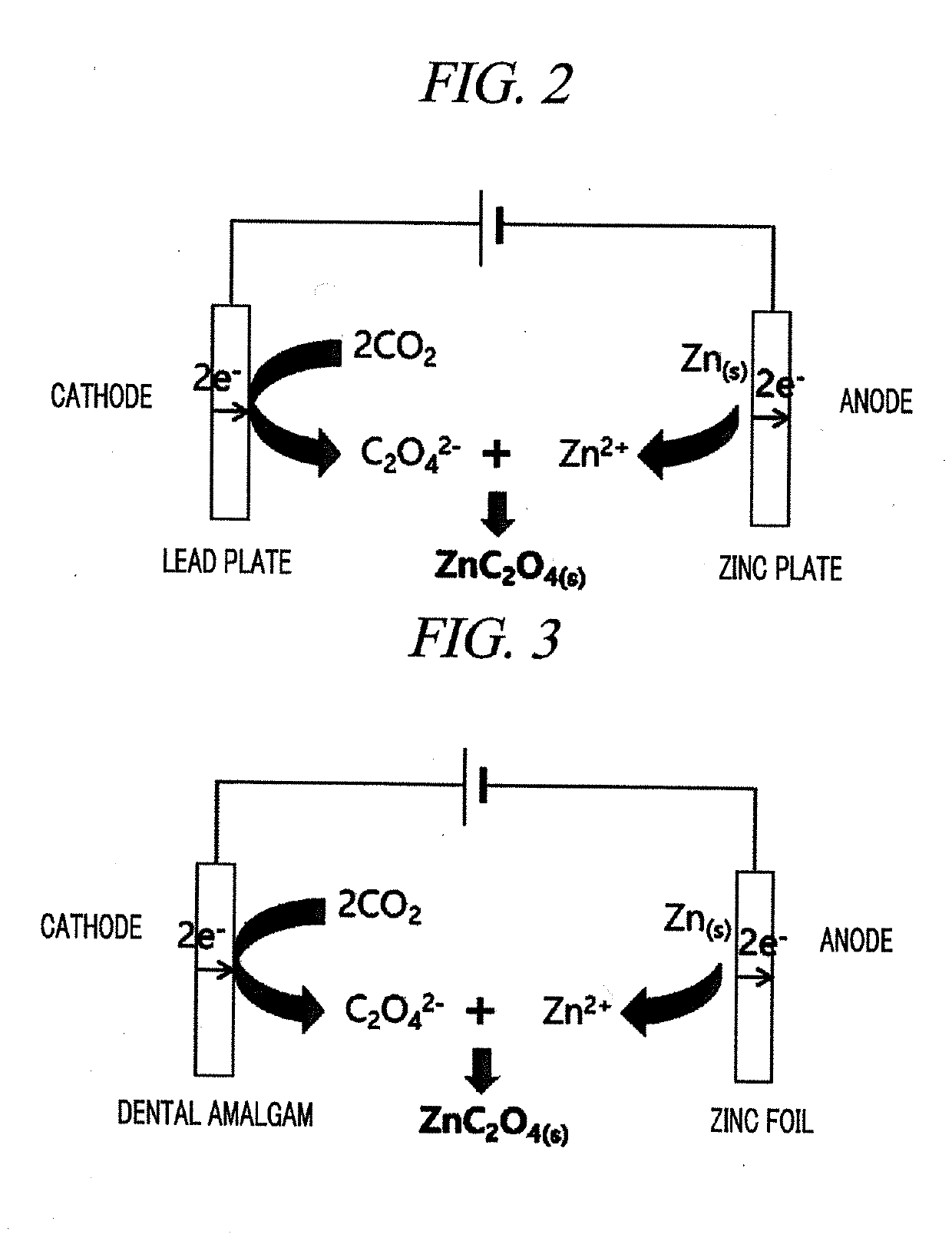
Amalgam electrode, method for manufacturing same, and method for electrochemical reduction of carbon dioxide using same
ActiveCN105765109AIncrease surface areaEfficient electrochemical conversionNon-noble metal oxide coatingsElectrolytic organic productionElectrochemistryElectrochemical reduction of carbon dioxide
The present invention relates to a method for manufacturing an amalgam electrode, an amalgam electrode manufactured by the method, and a method for electrochemical reduction of carbon dioxide using the amalgam electrode.
Owner:IND UNIV COOP FOUND SOGANG UNIV
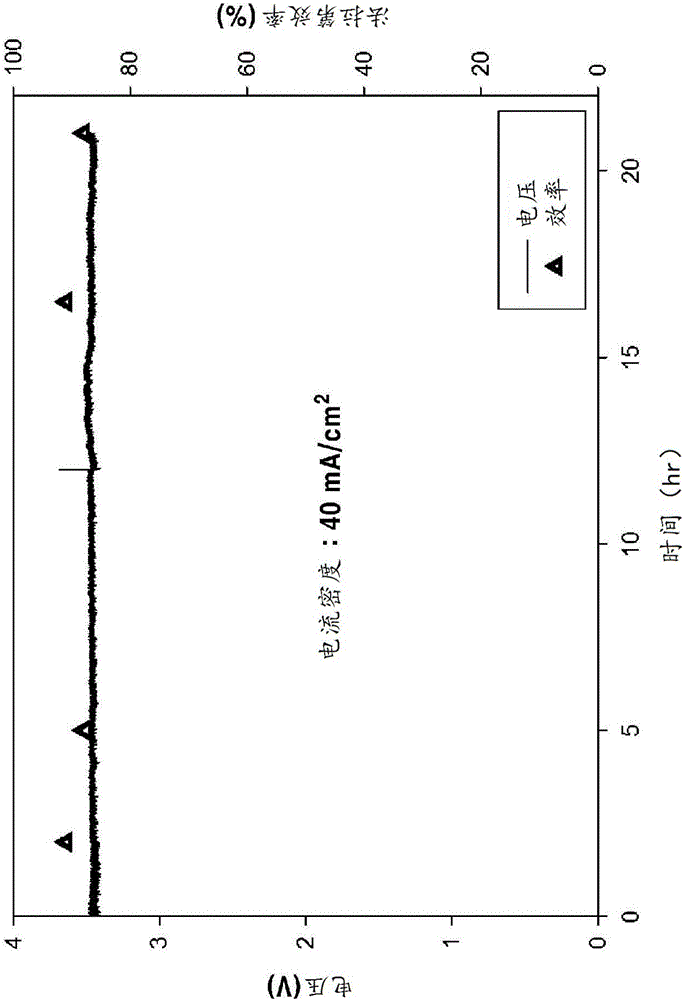
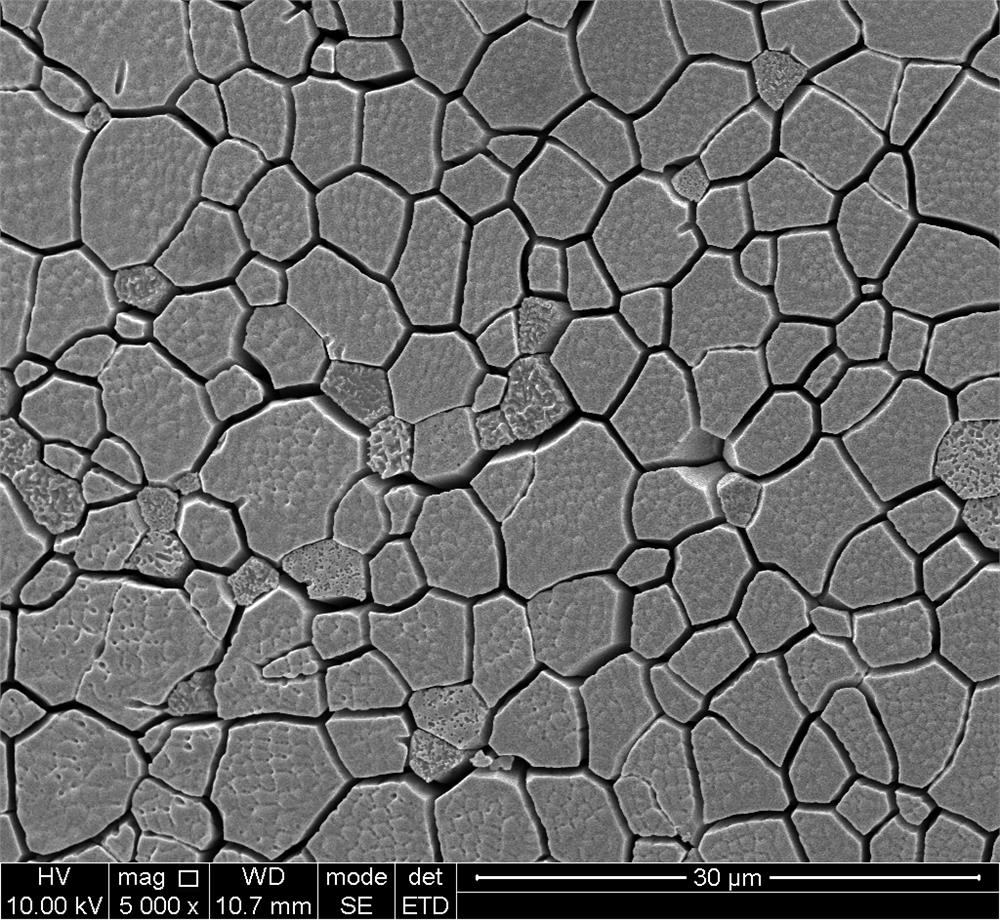
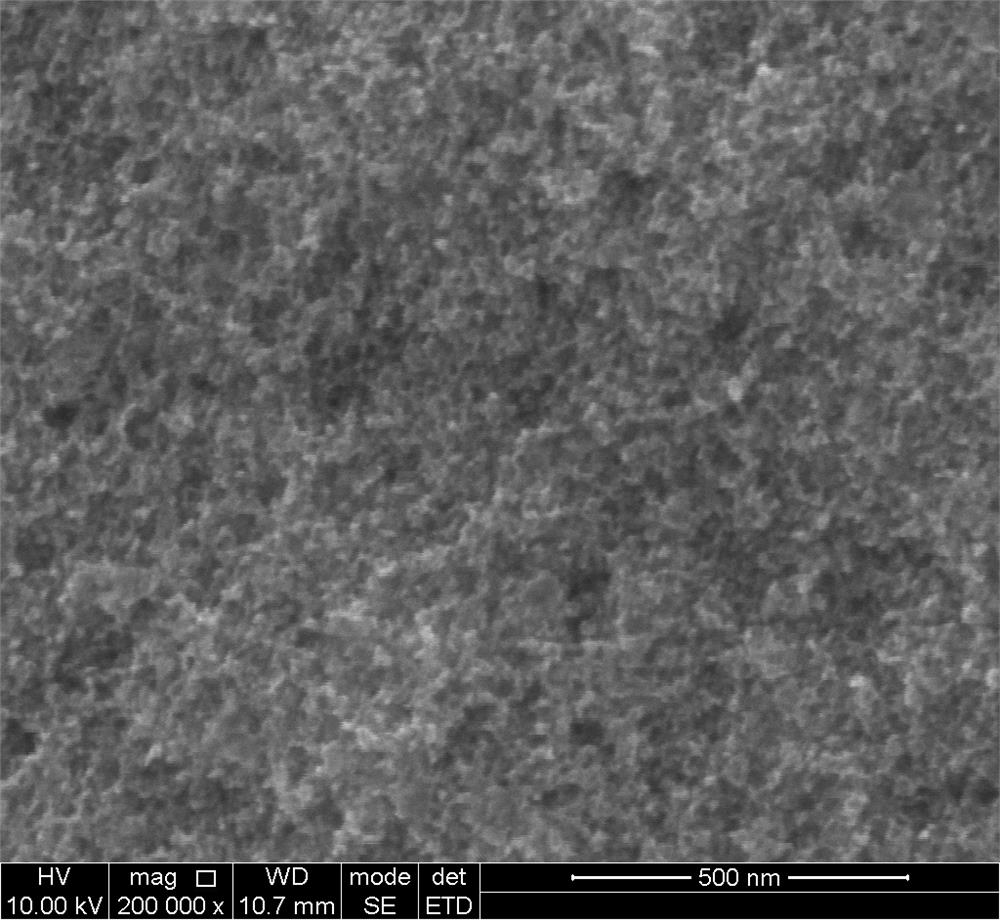
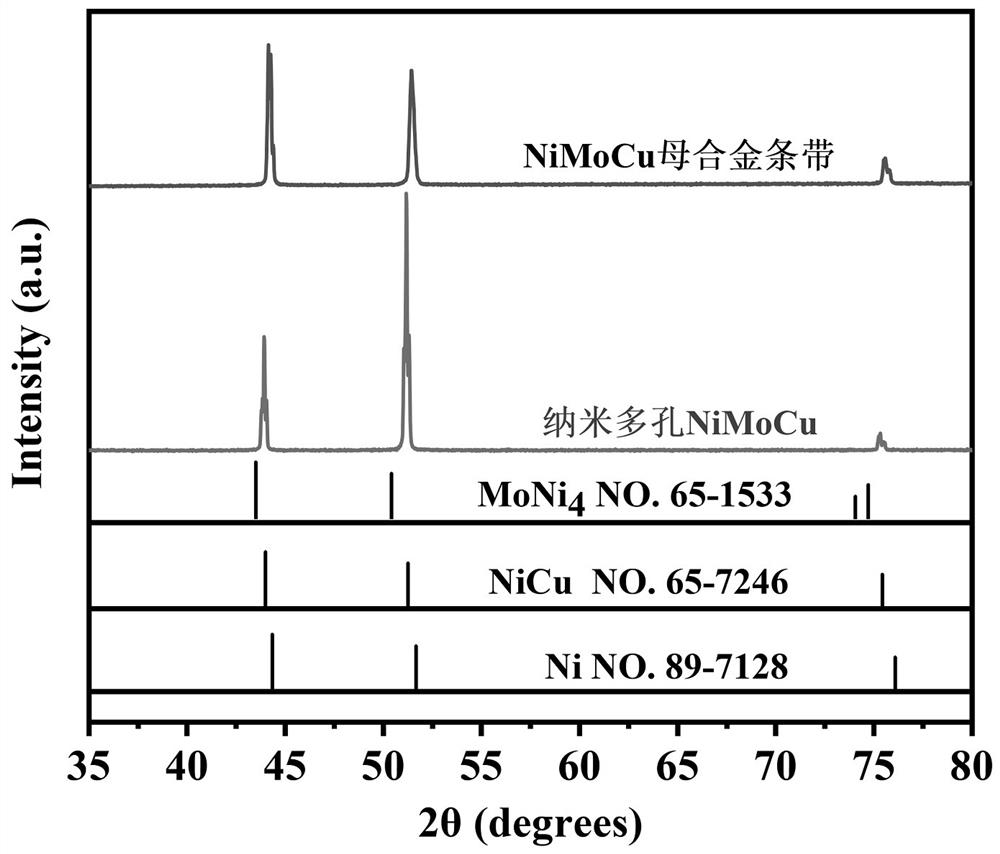
Preparation method of NiMoCu catalyst with nano-porous surface
The invention provides a preparation method of a surface nano-porous NiMoCu catalyst capable of being applied to water electrolysis hydrogen production, and belongs to the technical field of catalyst material preparation. The preparation method comprises the following steps: firstly, preparing a Ni74Mo6Cu20 master alloy strip by utilizing an electric arc melting technology, and preparing the surface nano-porous NiMoCu catalyst by adopting an electrochemical etching method on the basis of the Ni74Mo6Cu20 master alloy strip. The NiMoCu catalyst prepared by the method shows good HER catalytic activity in an alkaline medium, a spongy nano-porous microstructure is obtained after etching, and the active specific surface area is optimized, so that more active sites are exposed, and the purpose of improving the activity of the catalyst is achieved. Meanwhile, the preparation process is simple, raw materials are rich and cheap, expensive and scarce noble metal-based catalysts can be replaced, and commercial application of hydrogen production through water electrolysis is promoted.
Owner:SOUTHWEST PETROLEUM UNIV
Surface modified stainless steel cathode for electrolyser
InactiveCN104271809AGuaranteed corrosion resistanceReduce overvoltageNon-noble metal oxide coatingsAbrasion apparatusOvervoltageElectrolysis
Sodium chlorate is produced industrially via electrolysis of brine and is thus an energy intensive process. An improved cathode for this and other industrial processes is a low nickel content stainless steel whose surface has been suitably modified. With an appropriate amount of surface roughening, the cathode provides for improved overvoltages during electrolysis while still maintaining corrosion resistance.
Owner:CHEMETICS
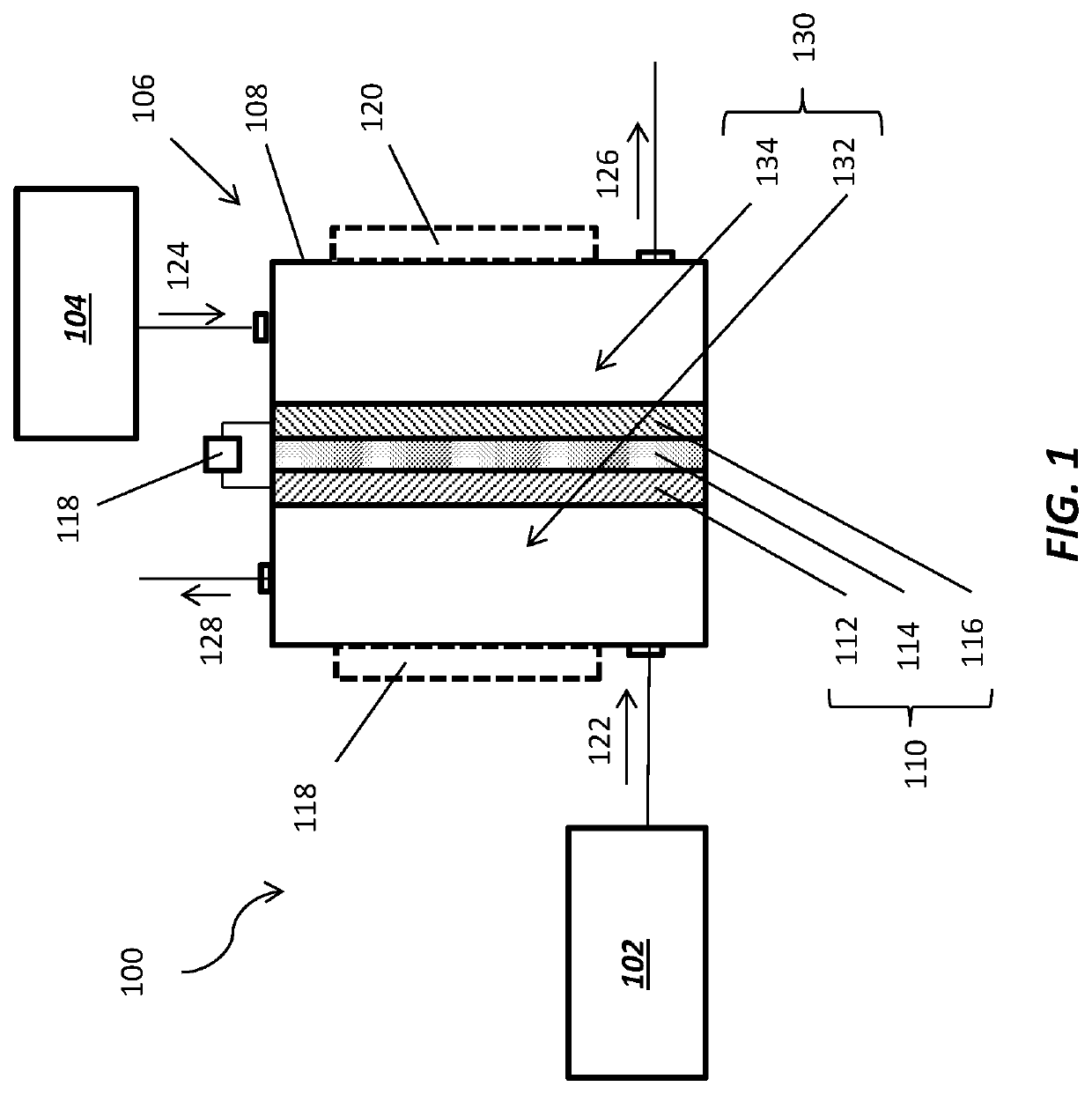
System for electrochemical of carbon dioxide
ActiveUS20190153606A1Low production costReduce volatilityCellsMercury/amalgum electrodesOrganic solventElectrochemistry
The present disclosure provides a system for electrochemical conversion of carbon dioxide, including: a reduction electrode unit to which carbon dioxide is supplied and including a metal-containing electrode; an oxidation electrode unit including a sacrificial electrode; and an electrolyte unit including an aprotic polar organic solvent and an auxiliary electrolyte, which is in contact with the reduction electrode unit and the oxidation electrode unit, and the carbon dioxide supplied to the reduction electrode unit is electrochemically reduced so as to produce an oxalate salt.
Owner:SOGANG UNIV RES & BUSINESS DEV FOUND
Methods and systems for co-producing hydrocarbon products and ammonia, and related electrochemical cells
ActiveUS20200039896A1Improve reaction speedRate of reactionCellsNon-noble metal oxide coatingsHydrocotyle bowlesioidesPotential difference
A method of a hydrocarbon product and ammonia comprises introducing C2H6 to a positive electrode of an electrochemical cell comprising the positive electrode, a negative electrode, and a proton-conducting membrane between the positive electrode and the negative electrode. The proton-conducting membrane comprising an electrolyte material having an ionic conductivity greater than or equal to about 10−2 S / cm at one or more temperatures within a range of from about 150° C. to about 600° C. N2 is introduced to the negative electrode of the electrochemical cell. A potential difference is applied between the positive electrode and the negative electrode of the electrochemical cell. A system for co-producing higher hydrocarbons and NH3, and an electrochemical cell are also described.
Owner:BATTELLE ENERGY ALLIANCE LLC
Compact hydrogen-oxygen generator
ActiveCN111075612ALarge specific surface areaFacilitated DiffusionCellsNon-fuel substance addition to fuelFlame arresterElectrolysis
The invention discloses a compact hydrogen-oxygen generator. On a fluid channel, a water circulation outlet of a water box communicates with a water pump through a one-way throttle valve, the water pump communicates with a hydrogen-oxygen generator electrolytic bath, the hydrogen-oxygen generator electrolytic bath communicates with a water circulation inlet of the water box through another one-waythrottle valve, and a gas outlet of the water box communicates with an engine inlet body through a steam-water separation device and a dry type flame arrester in sequence; on a circuit, the water pump and the hydrogen-oxygen generator electrolytic bath are connected to the positive pole end and the negative pole end of a vehicle power source in a parallel connection mode; and a switch, a fuse andthe hydrogen-oxygen generator electrolytic bath are connected with the vehicle power source in a series connection mode. According to the compact hydrogen-oxygen generator, through the compact designthat a porous electrode bar and a stainless steel sleeve are closely nested, efficient electrolysis is achieved, on the premise that the gas production quantity is met, the size and weight of the electrolytic bath are decreased, assembling of a single electrolytic chamber of the on-board hydrogen-oxygen generator is achieved, the hydrogen-oxygen generator is directly connected with the single sealed electrolytic chamber in the circuit and the fluid channel, and the problems caused by series connection of multiple electrolytic chambers are effectively solved.
Owner:SOUTH CHINA UNIV OF TECH
Device and method for degrading gaseous organic pollutants by electrochemical method
PendingCN112870931AEfficient degradationExtended service lifeGas treatmentDispersed particle separationElectrochemical responseElectrochemistry
The invention discloses a device and method for degrading gaseous organic pollutants by an electrochemical method. The device for degrading the gaseous organic pollutants through the electrochemical method comprises an electrochemical reactor, the electrochemical reactor comprises a power source, an anode, a cathode, a proton exchange membrane, an anode airflow channel and a cathode airflow channel, the anode is arranged in the anode airflow channel, the cathode is arranged in the cathode airflow channel, the proton exchange membrane is arranged between the anode and the cathode, the anode, the proton exchange membrane and the cathode are arranged in a clamping manner, and a titanium black material coating is arranged on the surface of the anode. According to the technical scheme, gaseous organic pollutants can be efficiently degraded.
Owner:SHENZHEN PUREMATE TECH
Alloy electrocatalyst for ultra-stable PEM oxygen evolution reaction and preparation method thereof
ActiveCN112626539AIncrease intrinsic activityImprove stabilityNon-noble metal oxide coatingsEnergy inputElectric arc furnaceElectrolysis
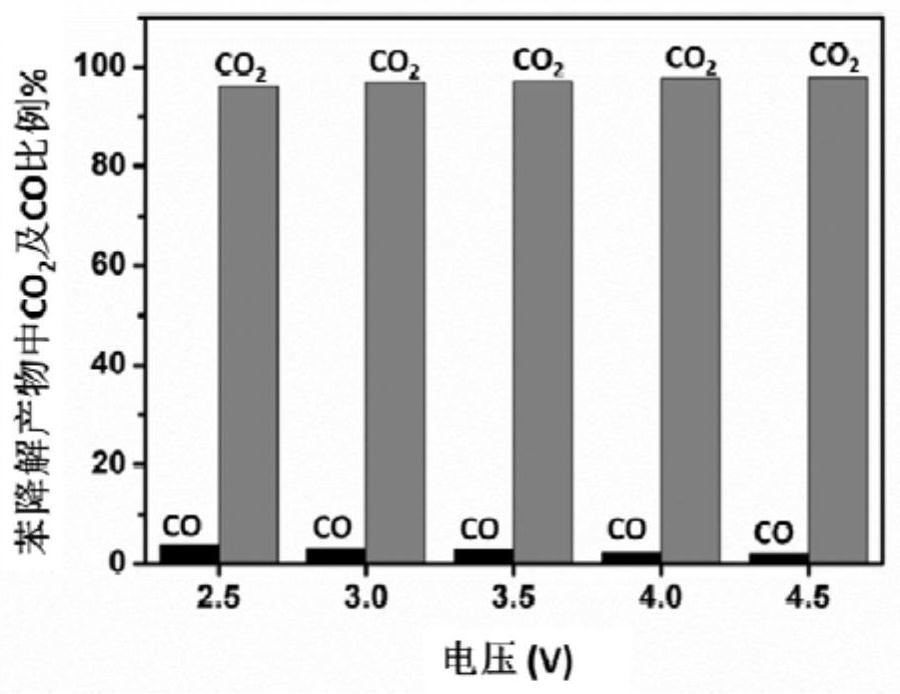
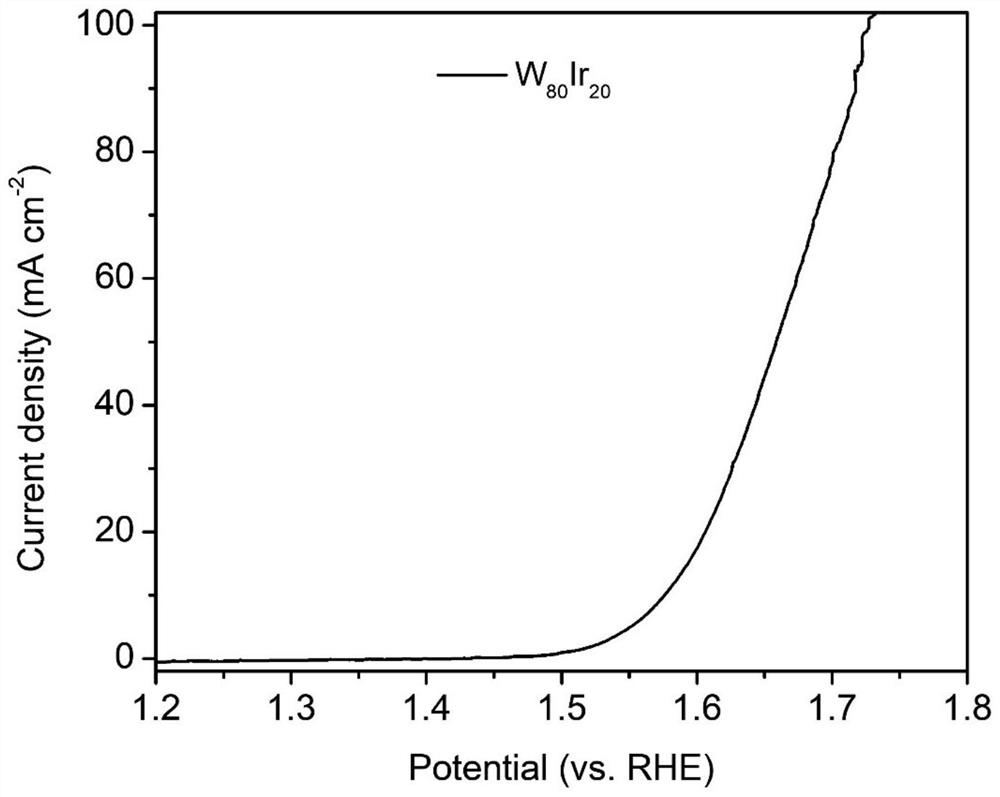
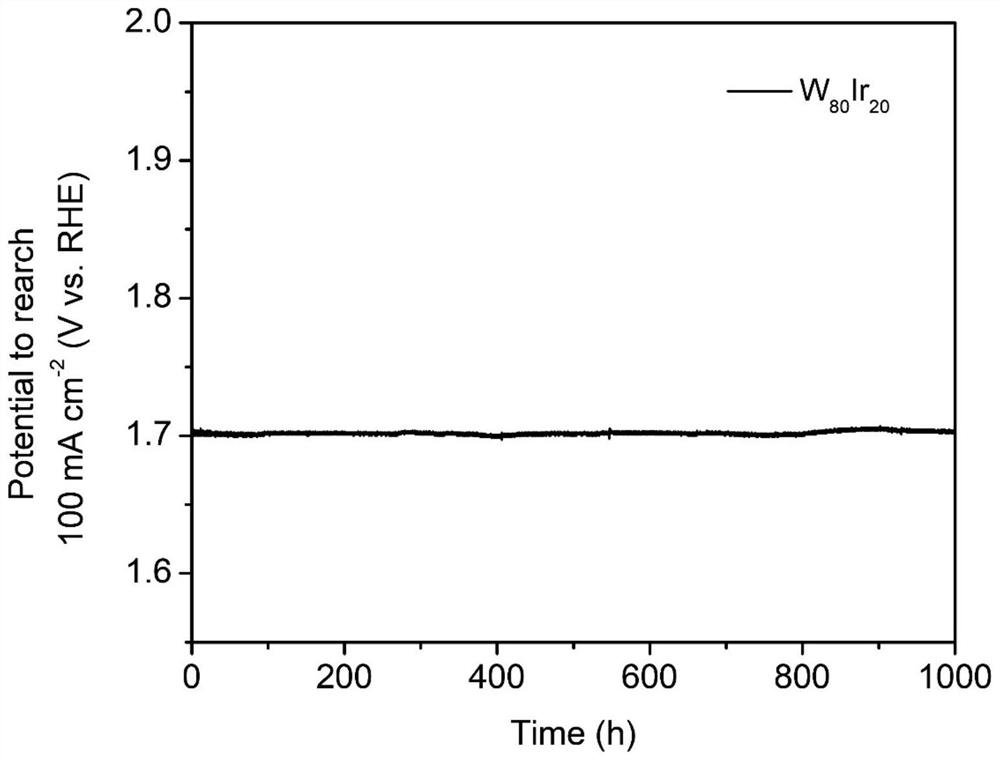
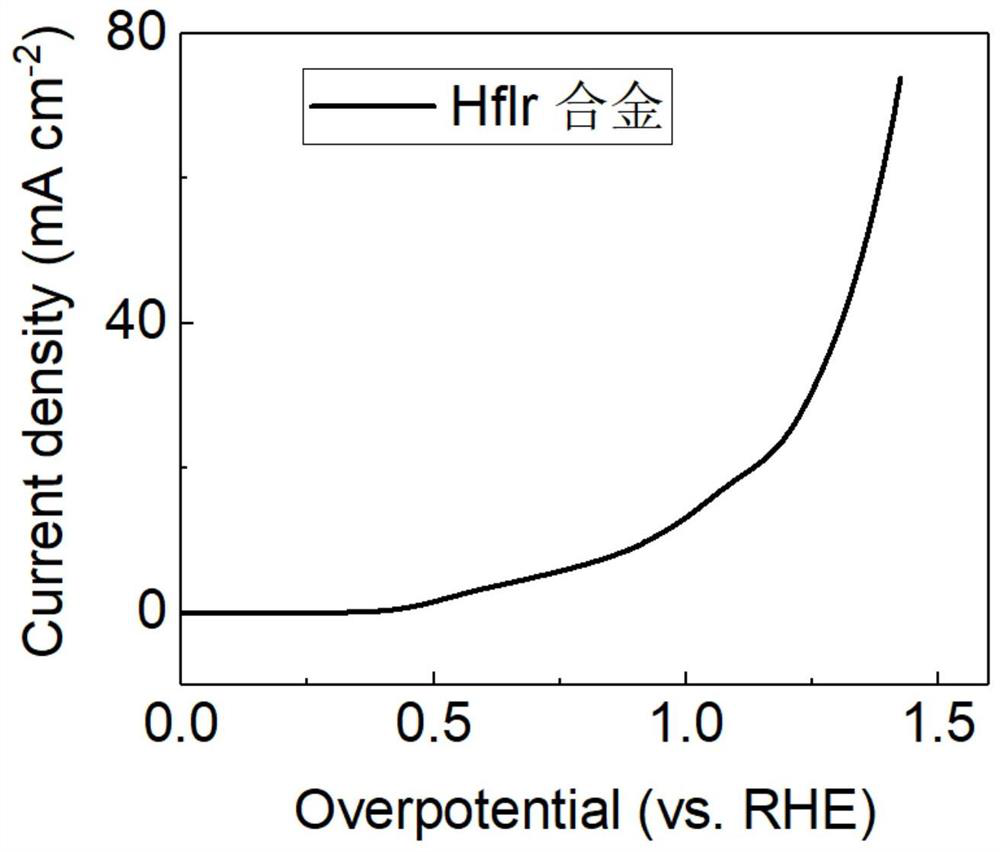
The invention discloses an alloy electrocatalyst for an ultra-stable PEM oxygen evolution reaction. The alloy electrocatalyst is a (W, Mo, Nb, Ta, Zr, Hf, Re, Os)-Ir block alloy material, the specific component of the alloy is (W, Mo, Nb, Ta, Zr, Hf, Re, Os)<x>-Ir<y>, x is greater than or equal to 60 and less than or equal to 80, y is greater than or equal to 20 and less than or equal to 40, and x and y are atomic percentages of the elements. The invention further discloses a preparation method of the alloy electrocatalyst for the ultra-stable PEM oxygen evolution reaction. An electric arc furnace is adopted for smelting and casting molding, and the method is simple, easy to operate and suitable for large-scale industrial production. The material can be directly used as an oxygen evolution reaction electrode, shows excellent electrochemical oxygen evolution activity in an acid medium, and can stably work for more than 1000 hours under the condition of high current density. The material can be used as an anode of an industrial acidic water electrolysis hydrogen production device, and is beneficial to large-scale application of a proton exchange membrane water electrolysis technology.
Owner:新余市金通科技有限公司 +1
Amalgam electrode, producing method thereof, and method of electrochemical reduction of carbon dioxide using the same
ActiveUS20160032470A1Stably electrochemically reduce carbon dioxideLow costMachining electrodesMercury/amalgum electrodesPhysical chemistryElectrochemistry
The embodiments described herein pertain generally to an amalgam electrode, and a producing method of the amalgam electrode, and an electrochemical reduction method of carbon dioxide using the amalgam electrode.
Owner:SOGANG UNIV RES FOUND +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com