Reducing carbon dioxide to products
A carbon dioxide and product technology, applied in non-precious metal oxide coating, reduction electrolysis, electrolytic organic production, etc., can solve problems such as low reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0063] Example 1: General Electrochemical Methods
[0064] Chemicals and materials, the chemical substances used are all obtained directly from the supplier, the purity of the chemical substances is greater than 98%, no further purification is required, and the electrolyte aqueous solution is prepared using deionized water or high-purity water.
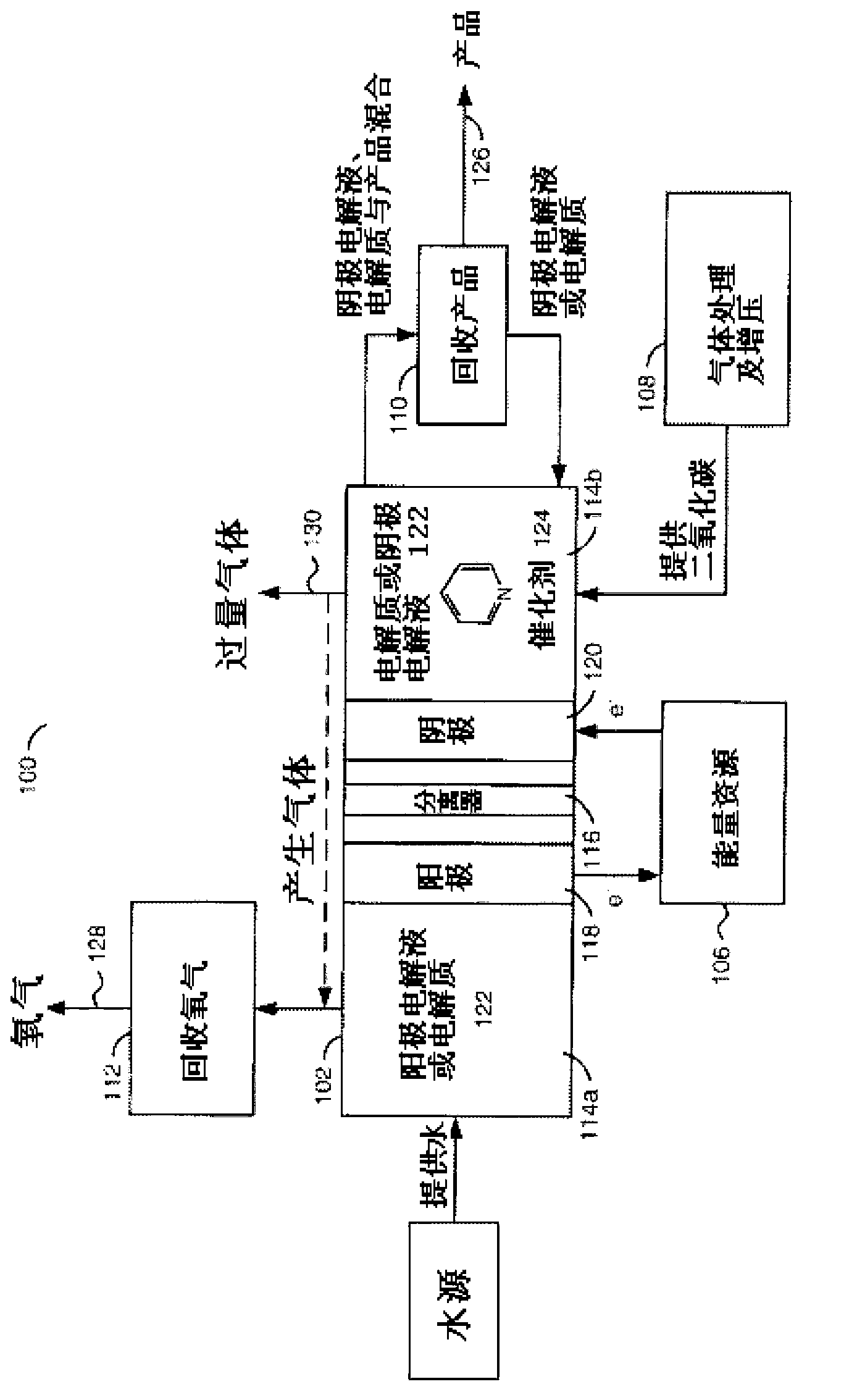
[0065] electrochemical system. The electrochemical system is a standard two-chamber electrolyzer 102 separated into an anode 118 and a cathode 120 within the cell separated by a porous glass or other ion-conducting bridge 116. The electrolyte 122 has a concentration range of 0.1 molar to 1 mole, the most typical concentration is 0.5 mole, the use range of catalyst concentration is 0.1 millimolar to 1 mole, select specific electrolyte 122 and specific catalyst 124 according to different products.
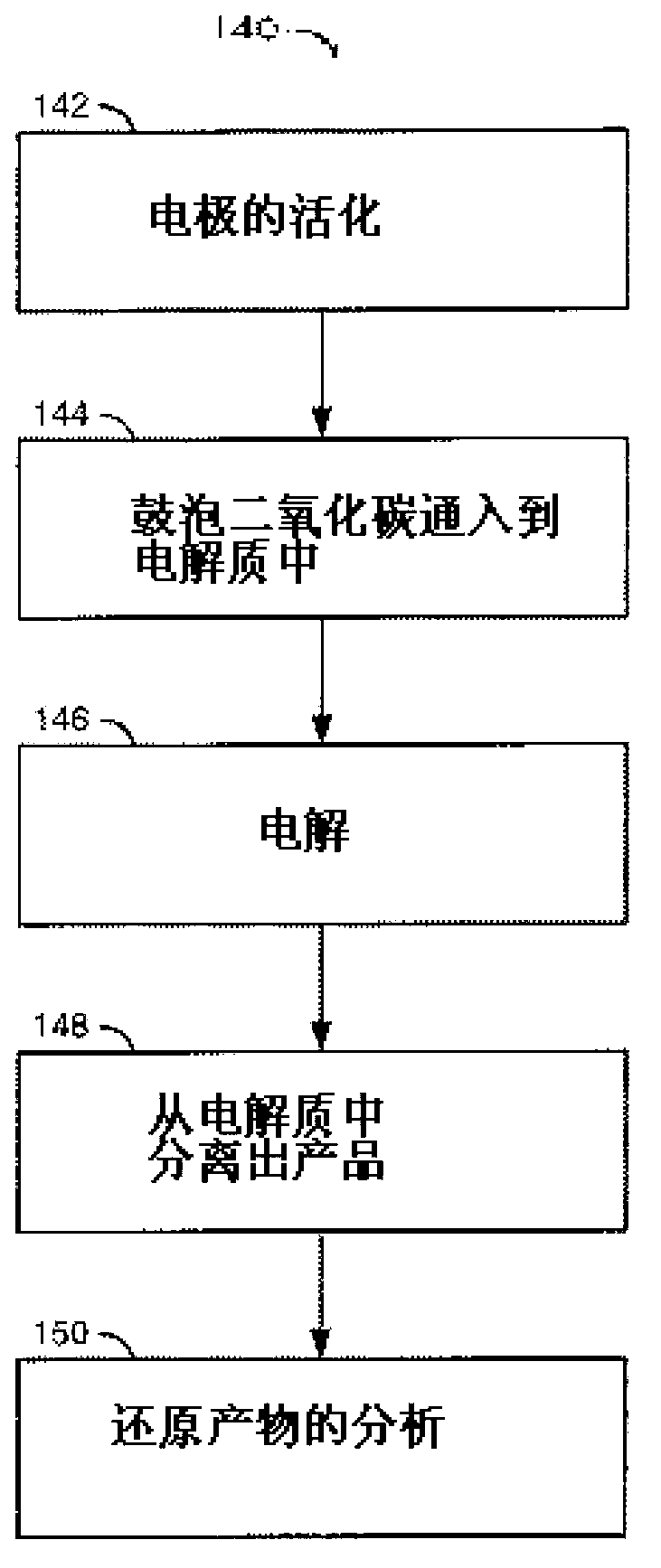
[0066] like Figure 7 Shown is an electrochemical flow chart of the method. The method (or process) 140 generally includes steps 142 , 14...
example 2
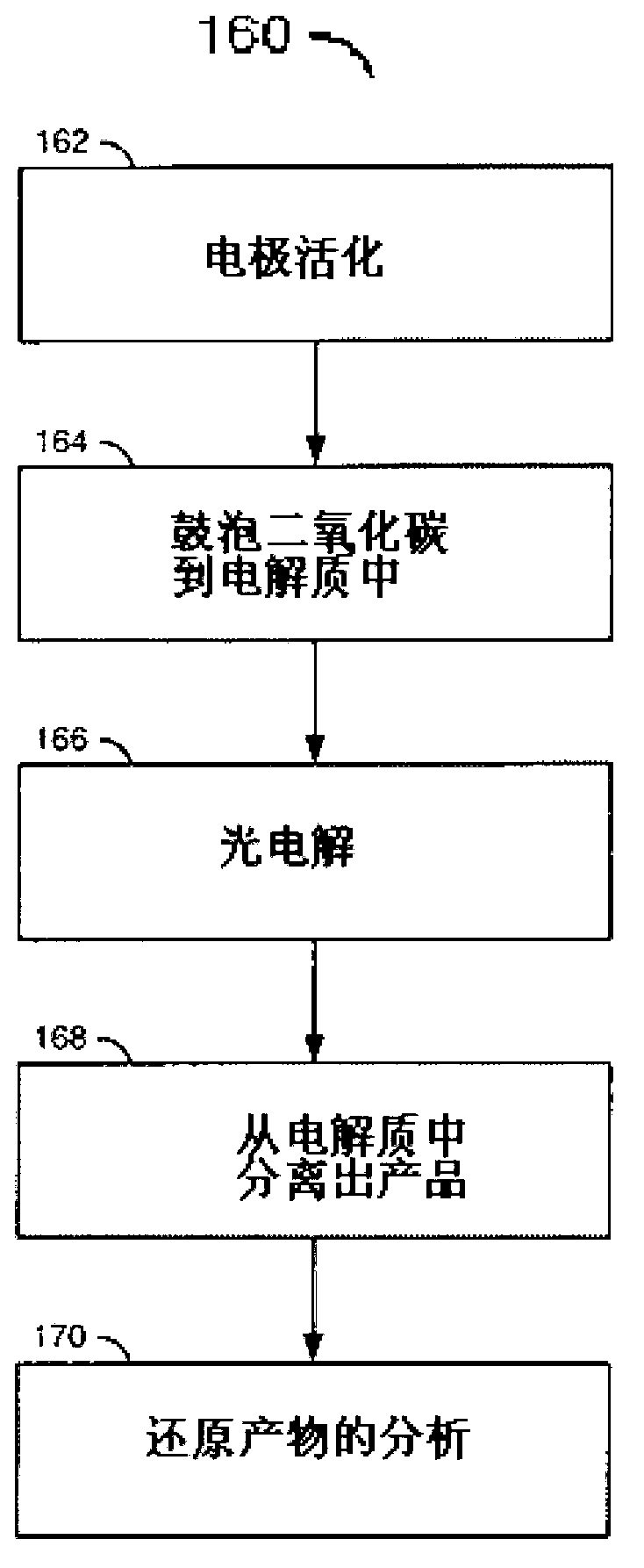
[0069] Example 2: General photoelectrochemical method
[0070] Chemicals and materials, use higher analytical grade chemicals, use deionized or high-purity water to prepare aqueous electrolyte solutions.
[0071] photochemical system. The photochemical system is composed of a three-necked flask containing 0.5 mmol potassium chloride as electrolyte and 1 mmol to 1 mole catalyst (for example, 10 mmol pyridine or pyridine derivatives), the cathode is a single crystal P-type semiconductor, before operation Concentrated nitric acid:concentrated hydrochloric acid = 2:1 Etching in bath for about 1 to 2 minutes, crystal soldered with indium-zinc (2% zinc by weight), conductive silver epoxy (epoxy technology) contacts to connect external leads, photography Cover the glass tube with insulating epoxy cement. Only the front side of the semiconductor is exposed in the aqueous solution. All potential values are measured by saturated calomel electrodes. The three electrode assemblies are ...
example 3
[0077] Example 3: Analysis of electrolysis products
[0078] Electrochemical experiments usually use a CH potentiostat or DC power supply with a current logger for electrolysis experiments, and the CH instrument potentiostat runs for 6 to 30 hours at the electrolytic potential of cyclic voltammetry until it reaches the threshold of each run. amount of charge.
[0079] Gas chromatography, a gas chromatograph (HP 5890 gas chromatograph) equipped with a Midland detector was used to electrolyze the sample, and the removal of salt in the electrolyte was achieved by ion exchange resin (firstly, the polymer was stirred in an aqueous solution with a volume fraction of 0.1%) Ethylene glycol octyl phenyl ether, before cleaning, make sure there are no organic products, and then rinse with plenty of water), dry and inject the sample into the gas chromatograph at a temperature of 60 degrees, 1 gram of resin can be used to remove For a sample of about 1ml of salt, the temperature of the in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com