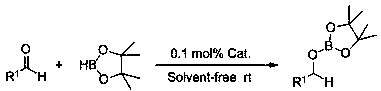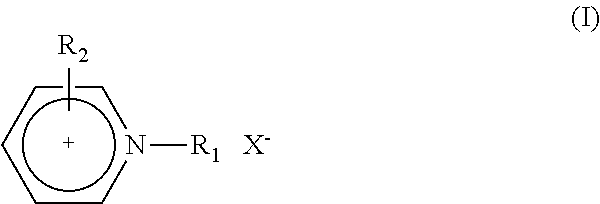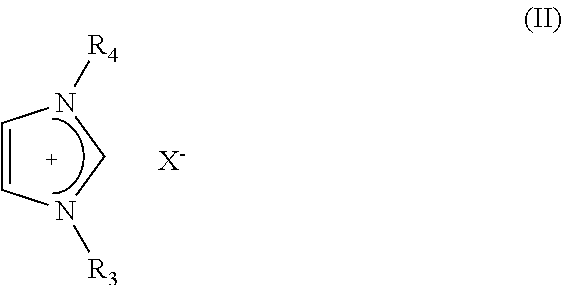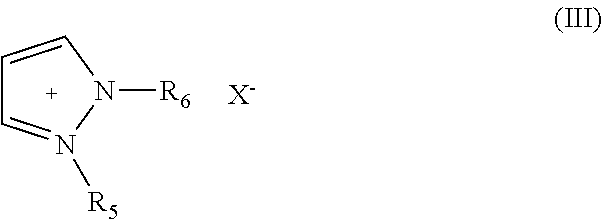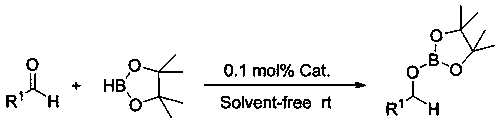Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
187 results about "Boranes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Boranes is the name given to the class of synthetic hydrides of boron with generic formula BₓHy. In the past, borane molecules were often labeled "electron-deficient" because of their multicenter bonding (in which a pair of bonding electrons links more than two atoms, as in 3-center-2-electron bonds); this was done in order to distinguish such molecules from hydrocarbons and other classically bonded compounds. However, this usage is incorrect, as most boranes and related clusters such as carboranes are actually electron-precise, not electron-deficient. For example, the extremely stable icosahedral B₁₂H₁₂²⁻ dianion, whose 26 cluster valence electrons exactly fill the 13 bonding molecular orbitals, is in no actual sense deficient in electrons; indeed it is thermodynamically far more stable than benzene.
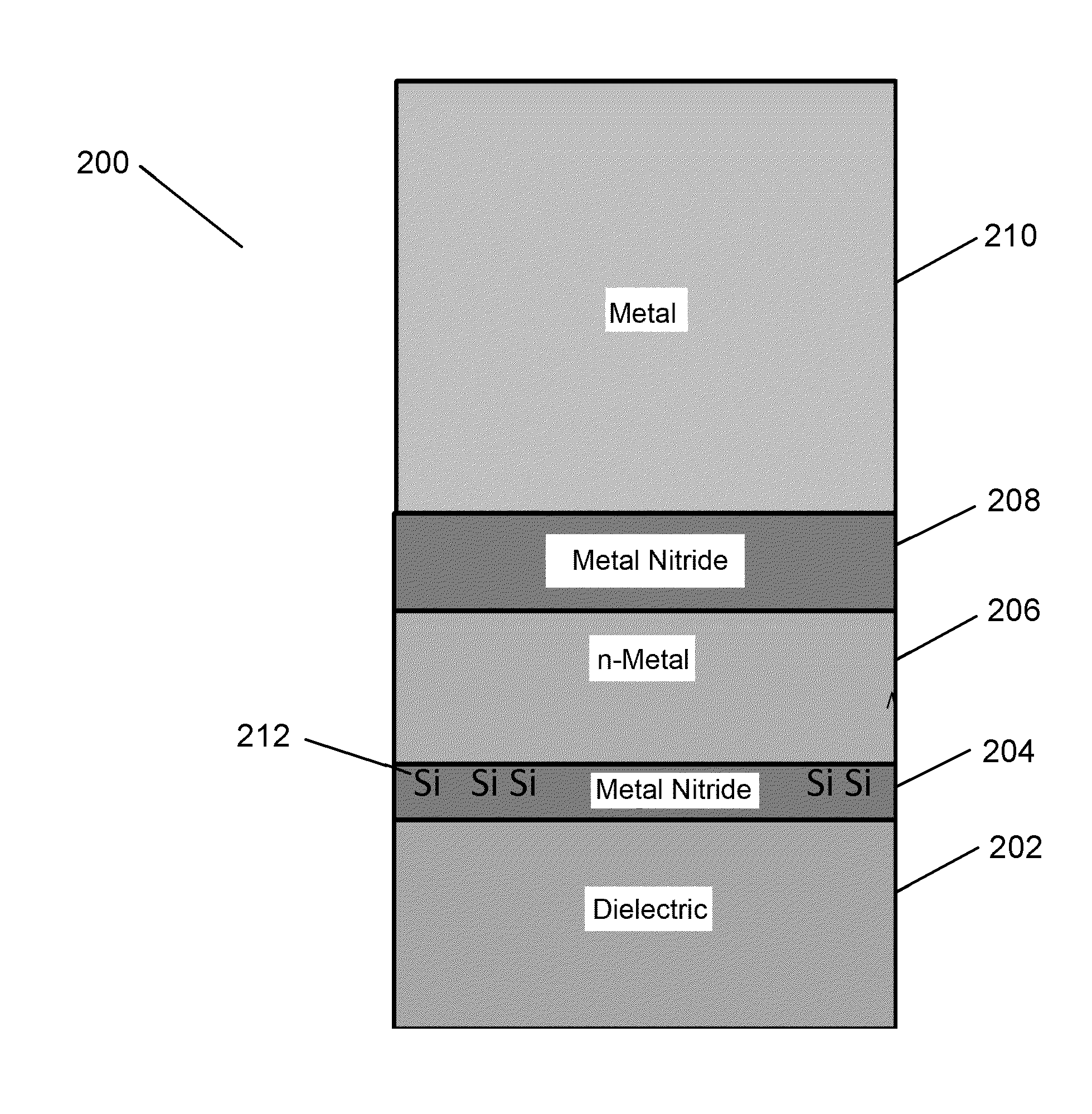
Silane or borane treatment of metal thin films
ActiveUS20140273428A1Semiconductor/solid-state device manufacturingChemical vapor deposition coatingSilanesGate stack
The negative effect of oxygen on some metal films can be reduced or prevented by contacting the films with a treatment agent comprising silane or borane. In some embodiments, one or more films in an NMOS gate stack are contacted with a treatment agent comprising silane or borane during or after deposition.
Owner:ASM IP HLDG BV
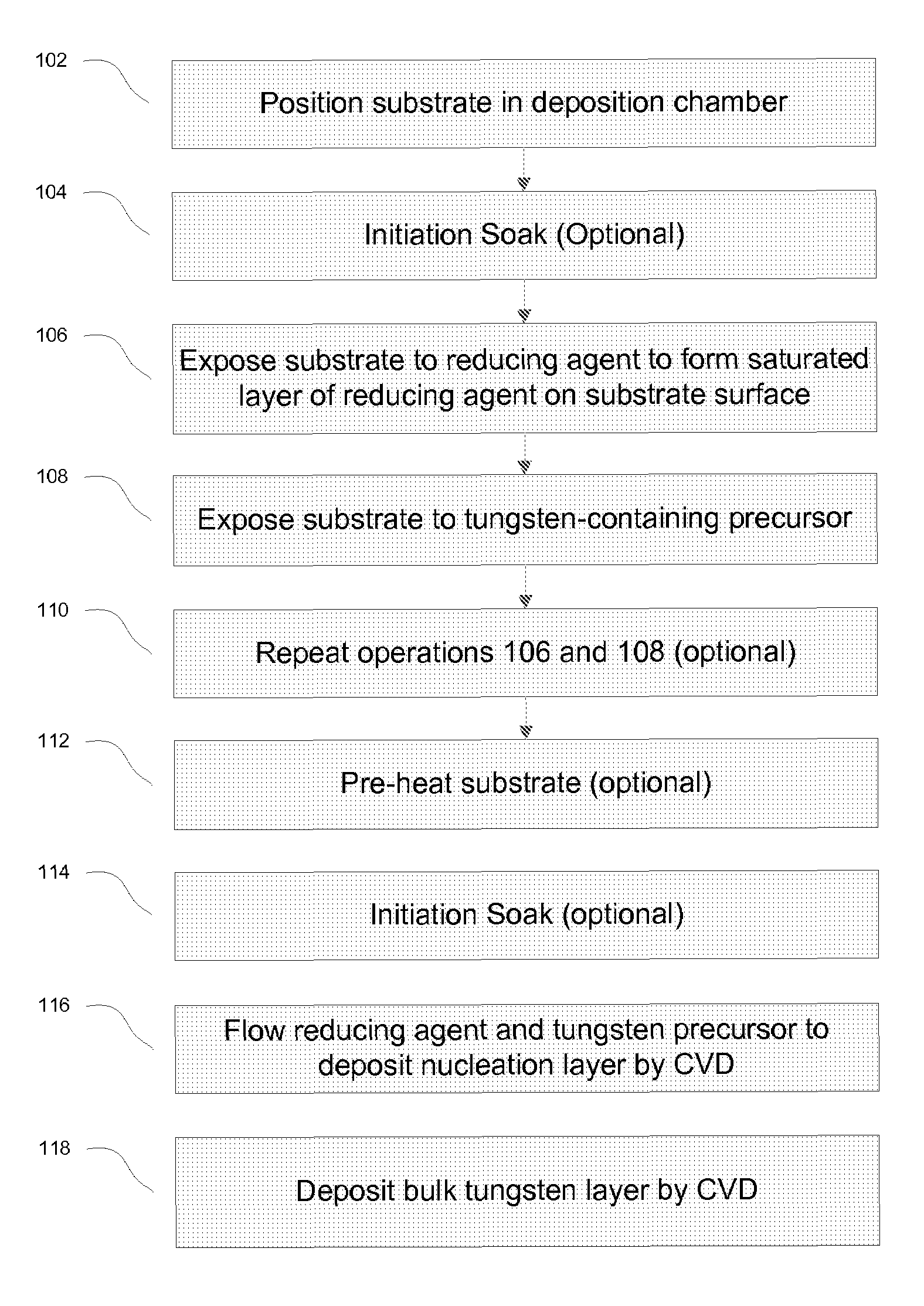
Methods for improving uniformity and resistivity of thin tungsten films
ActiveUS7655567B1Low resistivitySemiconductor/solid-state device detailsSolid-state devicesGas phaseNucleation
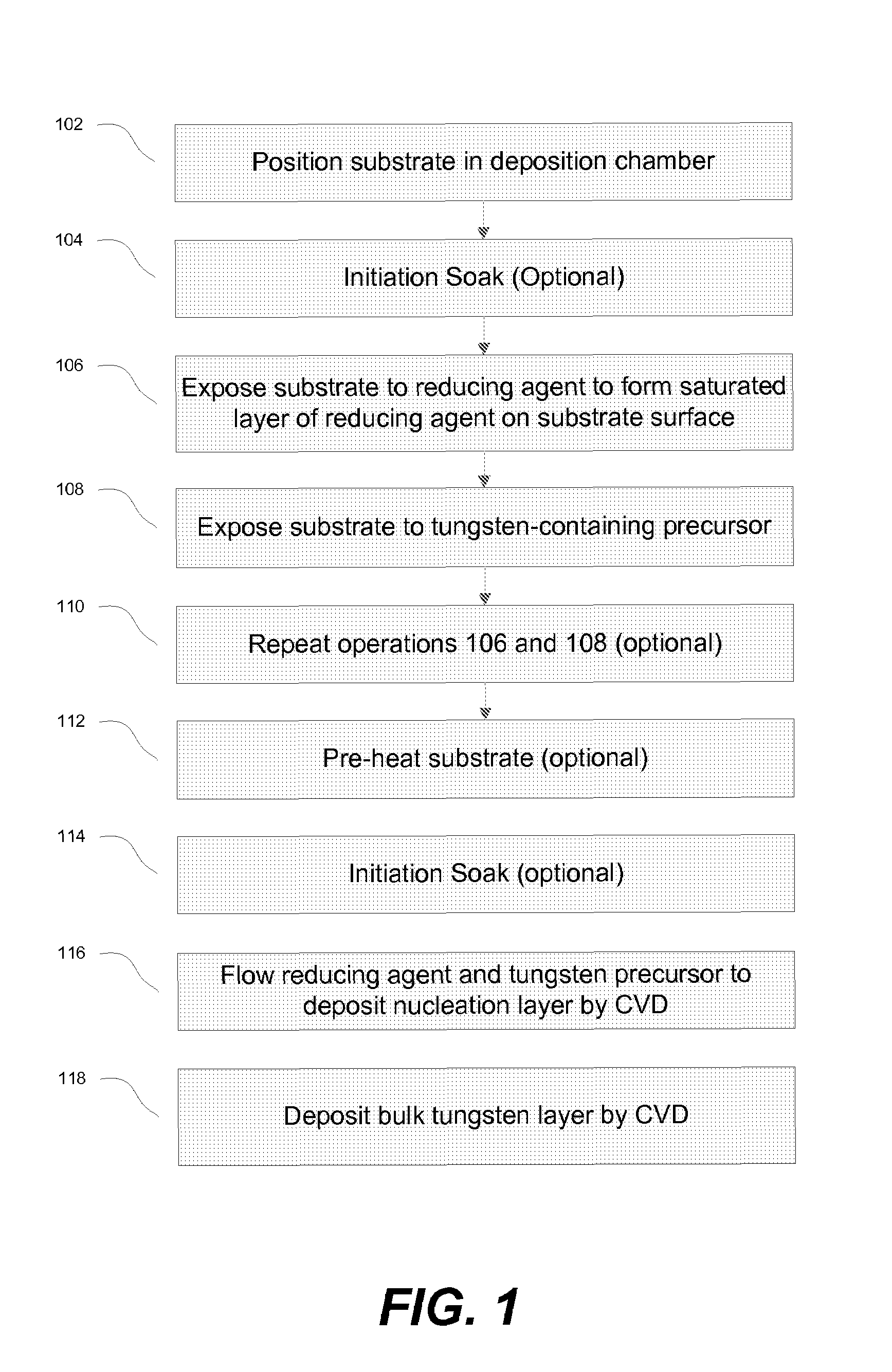
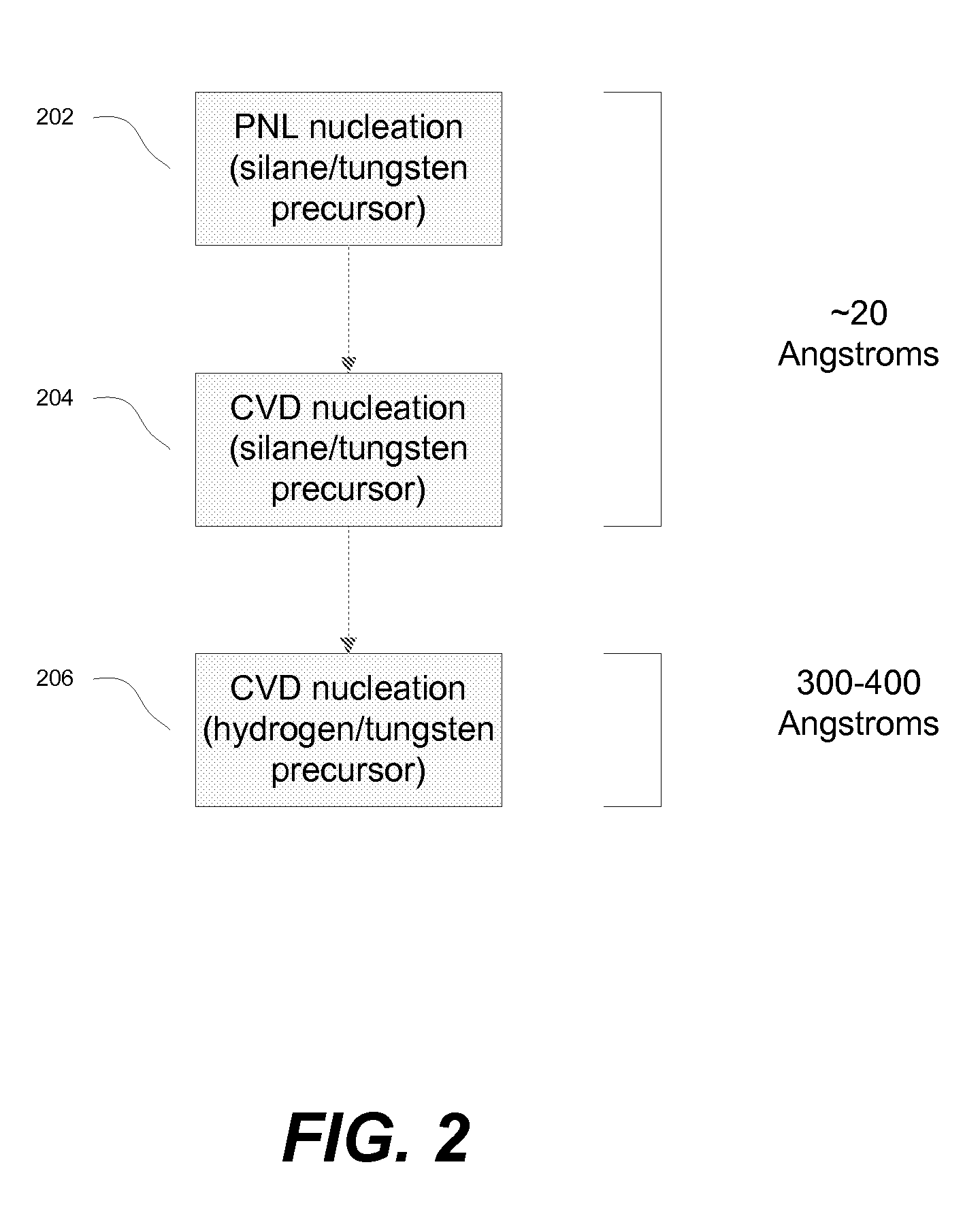
The methods described herein relate to deposition of low resistivity, highly conformal tungsten nucleation layers. These layers serve as a seed layers for the deposition of a tungsten bulk layer. The methods are particularly useful for tungsten plug fill in which tungsten is deposited in high aspect ratio features. The methods involve depositing a nucleation layer by a combined PNL and CVD process. The substrate is first exposed to one or more cycles of sequential pulses of a reducing agent and a tungsten precursor in a PNL process. The nucleation layer is then completed by simultaneous exposure of the substrate to a reducing agent and tungsten precursor in a chemical vapor deposition process. In certain embodiments, the process is performed without the use of a borane as a reducing agent.
Owner:NOVELLUS SYSTEMS
Method for preparing high-performance doped diamond-like film
InactiveCN101748381AImprove overall performanceImprove performanceChemical vapor deposition coatingIon beam depositionPhysics
The invention discloses a method for preparing a high-performance doped diamond-like film. The method is characterized by comprising the following steps: firstly, utilizing ultrasonic cleaning technology to remove a polluted layer on the surface of a substrate; utilizing ion beam assisted deposition technology to prepare a gradient transition layer; and finally utilizing ion beam deposition and magnetron sputtering to synthesize a multi-element doped DLC film, wherein except any one of carbonaceous gases, such as methane, acetylene, benzene, ethanol, acetone and the like, any gas containing non-carbon elements, such as silicon hydride, boron hydride, phosphorane, carbon tetrafluoride and the like, is simultaneously introduced, and a metal sputtering source is opened for the doping of metal elements. The method has the advantages of synthesizing the multi-element doped DLC film which is simultaneously doped with the metal elements and the nonmetal elements, fully developing the complementary advantages of the doped metal elements and the doped nonmetal elements and remarkably improving the combination properties of the DLC film.
Owner:CHINA UNIV OF GEOSCIENCES (BEIJING)
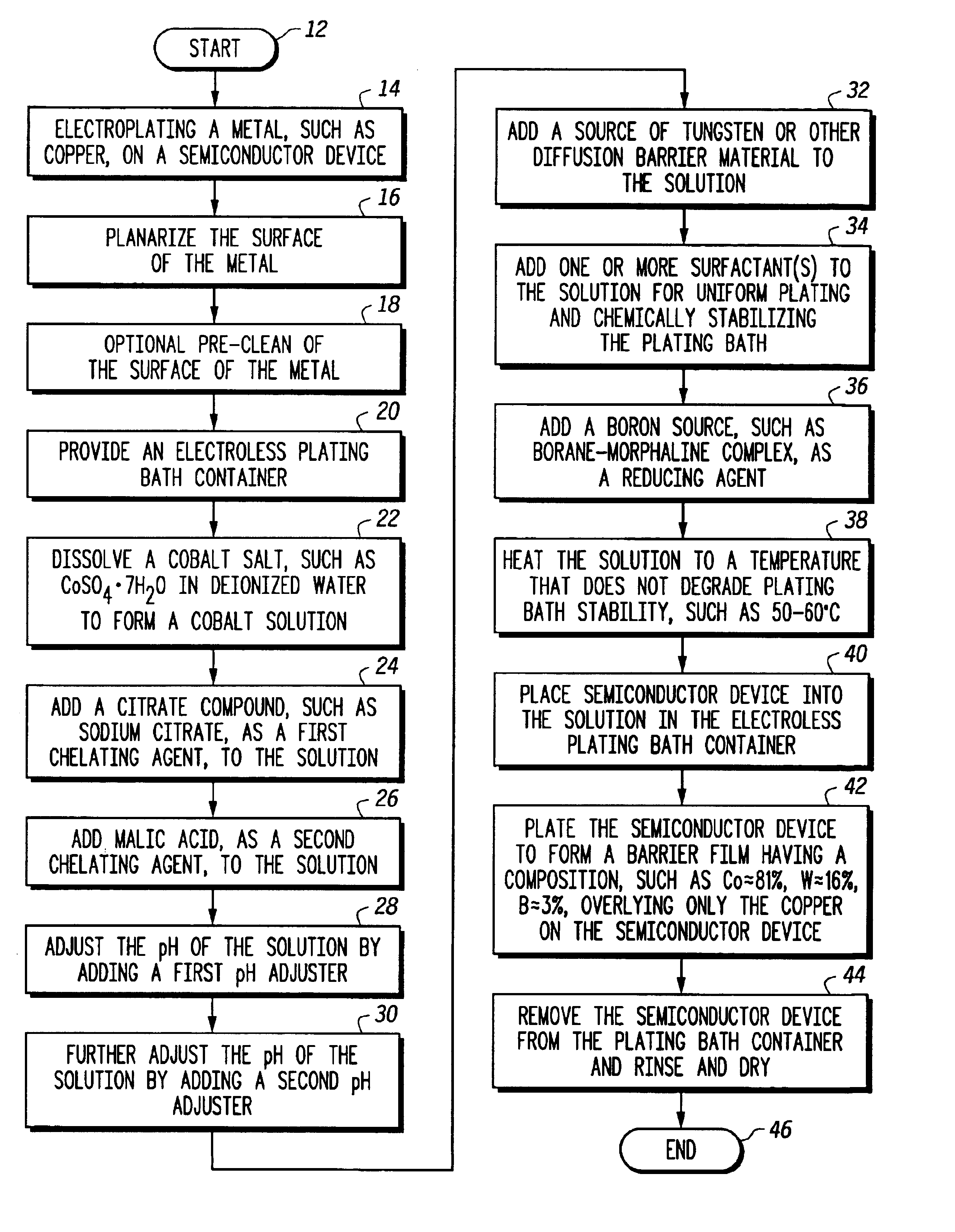
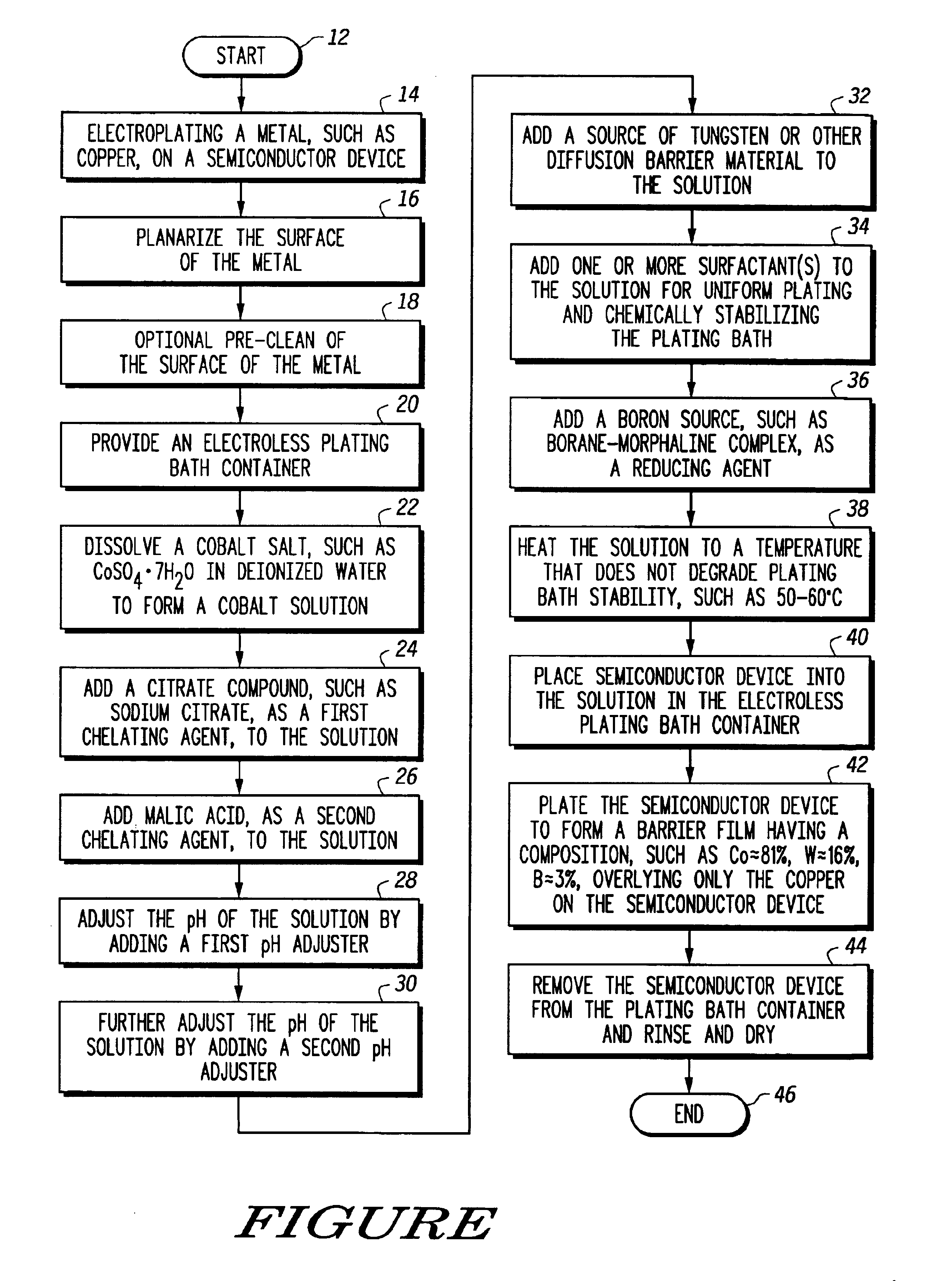
Semiconductor process and composition for forming a barrier material overlying copper
InactiveUS6924232B2Semiconductor/solid-state device detailsSolid-state devicesCopper interconnectMorpholine
Owner:APPLE INC
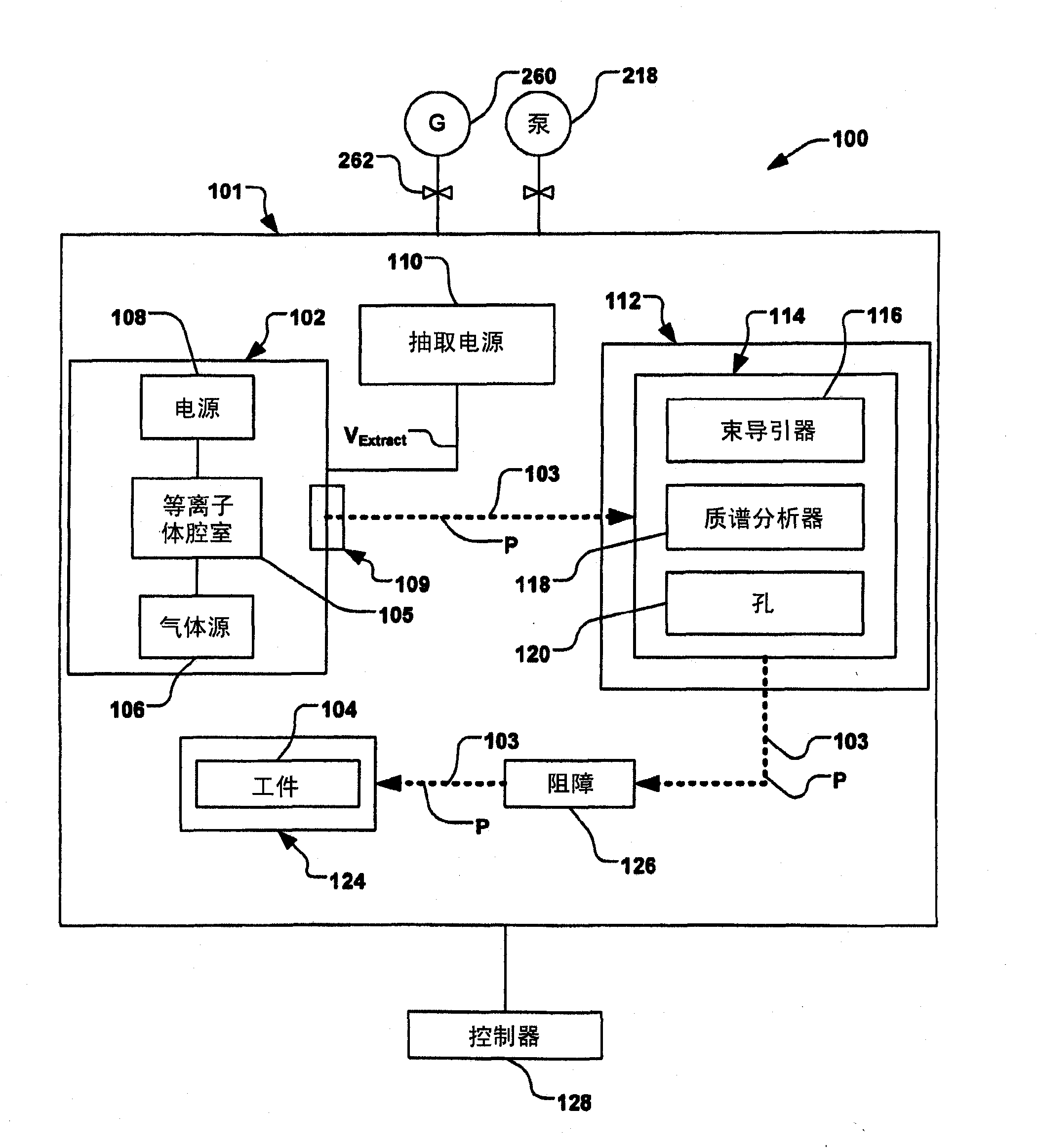
Methods for implanting B22Hx and its ionized lower mass byproducts
Methods for implanting an ionized polyhedral borane cluster or a selected ionized lower mass byproduct into a workpiece generally includes vaporizing and ionizing a polyhedral borane cluster molecule in an ion source to create a plasma and produce ionized polyhedral borane cluster molecules and its ionized lower mass byproducts. The ionized polyhedral borane cluster molecules and lower mass byproducts within the plasma are then extracted to form an ion beam. The ion beam is mass analyzed with a mass analyzer magnet to permit selected ionized polyhedral borane cluster molecules or selected ionized lower mass byproducts to pass therethrough and implant into a workpiece.
Owner:AXCELIS TECHNOLOGIES
Atomic Layer Deposition of Transition Metal Thin Films Using Boranes as the Reducing Agent
InactiveUS20130330473A1Solve problemsChemical vapor deposition coatingHydrogenAtomic layer deposition
A method for forming a metal comprises contacting a compound having formula 1 with a compound having formula 2 with an amine borane:wherein:M is Cu, Ni, Co, and Mn;R1R2, R3 are each independently C1-C6 alkyl; andR4-R6 are each independently hydrogen or C1-C6 alkyl. A method for making a metal film by an atomic layer deposition process using a compound having formula (1) is also provided.
Owner:WAYNE STATE UNIV
Methods of synthesis of isotopically enriched borohydride and methods of synthesis of isotopically enriched boranes
InactiveUS7641879B2High yieldPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesMonoborane/diborane hydridesIsotopeBoric acid
The invention provides new methods for the synthesis of isotopically enriched metal borohydrides, metal tetrahydroundecaborate salts, and decaborane from isotopically enriched 10B-boric acid or 11B-boric acid. The invention is particularly useful for synthesis of isotopically enriched sodium or lithium borohydride, MB11H14 (where M is Li, Na, K, or alkylammonium), and decaborane.
Owner:SEMEQUIP
Ezetimible intermediate and synthetic method of ezetimible
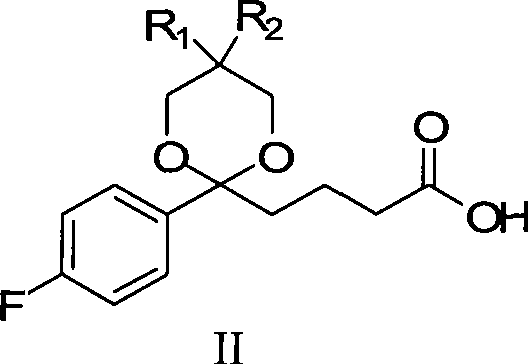
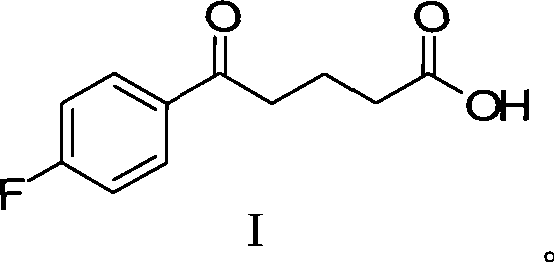
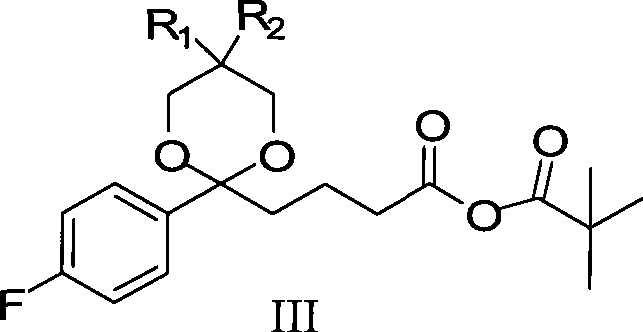
InactiveCN101423511AReduce pollutionEasy to operateOrganic chemistryMetabolism disorderCompound aSynthesis methods
The invention provides an intermediate for synthesizing ezetimibe and a preparation process thereof, and also provides a method for synthesizing the ezetimibe by the intermediate. The synthesizing method has short route, and comprises the following concrete steps: a compound I and substituted 1, 3-propanediol react to generate a compound II; the compound II and pivalyl chloride react to generate a compound III; the compound III and a compound A react to generate a compound IV; the compound IV and a compound V react to generate a compound VI under the condition of a titanium compound catalyst; the compound VI is re-ringed to generate a compound VII with beta-lactam; the compound VII is hydrolyzed to produce a compound VIII; and the compound VII is reduced to a compound IX ezetimibe by a borane chiral reducing agent. The synthesization has short route and mild reaction condition; and the produced intermediate and final product has high yield and high purity.
Owner:ENANTIOTECH CORP
Organic Electroluminescent Device
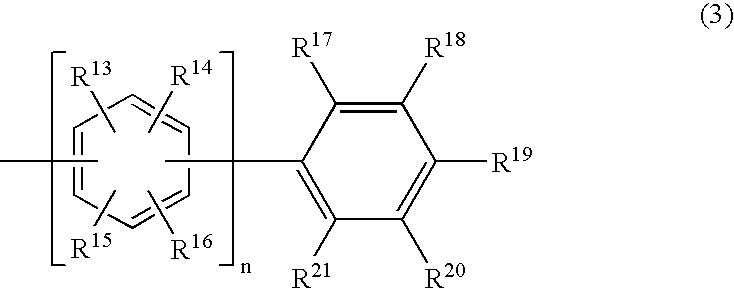
InactiveUS20070212568A1Improve emission efficiencyReduce the driving voltageDischarge tube luminescnet screensElectroluminescent light sourcesDopantAnthracene
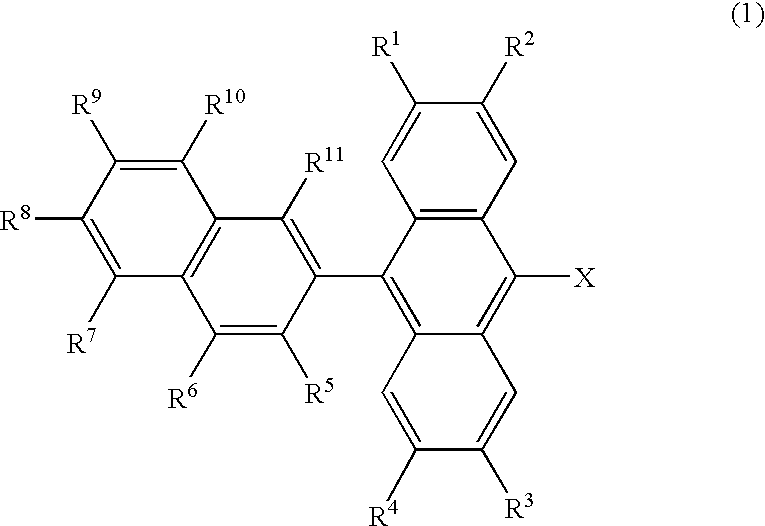
The present invention relates to an organic electroluminescent device comprising an anthracene derivative represented by Formula (1) shown below as a host and at least one selected from a perylene derivative, a borane derivative, a coumarin derivative, a pyran derivative, an iridium complex and a platinum complex as a dopant. The organic electroluminescent device of the present invention has a high efficiency, a long life, a low driving voltage and a high durability in storing and driving. wherein R1 to R4 and R12 are independently hydrogen or alkyl having 1 to 12 carbon atoms; R5 to R11 are independently hydrogen, alkyl having 1 to 12 carbon atoms, cycloalkyl having 3 to 12 carbon atoms or aryl having 6 to 12 carbon atoms; and Ar is non-condensed aryl represented by Formula (3); and m is an integer of 1 to 3; wherein n is an integer of 0 to 5; R13 to R21 are independently hydrogen, alkyl having 1 to 12 carbon atoms or aryl having 6 to 12 carbon atoms.
Owner:CHISSO CORP
Novel passivated perovskite solar cell and preparation method thereof
ActiveCN109888105AInhibition of phase transitionEnhanced light absorptionFinal product manufactureSolid-state devicesAnti solventPerovskite solar cell
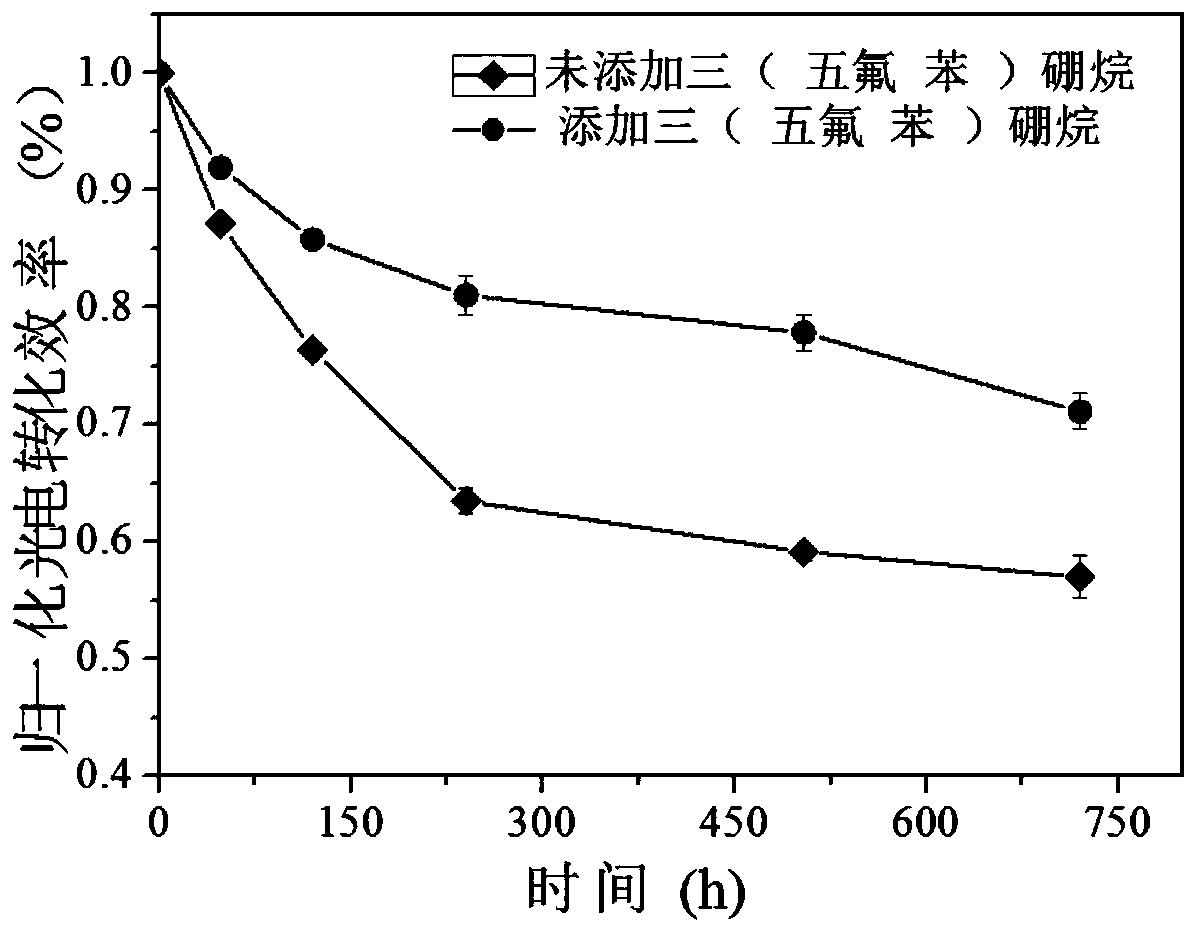
The invention discloses a novel passivated perovskite solar cell and a preparation method thereof. The perovskite solar cell optimizes the absorption of perovskite, so that a perovskite absorption layer contains an anti-solvent tris(pentafluorophenyl)borane to serve as a novel additive. On one hand, the addition of fluoride ions can change the crystallinity and the defect state of a perovskite film to form a high-quality perovskite film with a large grain size; the anti-solvent tris(pentafluorophenyl)borane can enhance the surface appearance of the perovskite and plays a passivation function at the grain boundary; and on the other hand, the fluoride ions can improve the hydrophobicity of the perovskite film and inhibit the phase change of the perovskite to better protect the perovskite from being destroyed by water, thereby further improving the air stability and the light stability of the cell to obtain a high-efficiency perovskite solar cell.
Owner:SHAANXI NORMAL UNIV
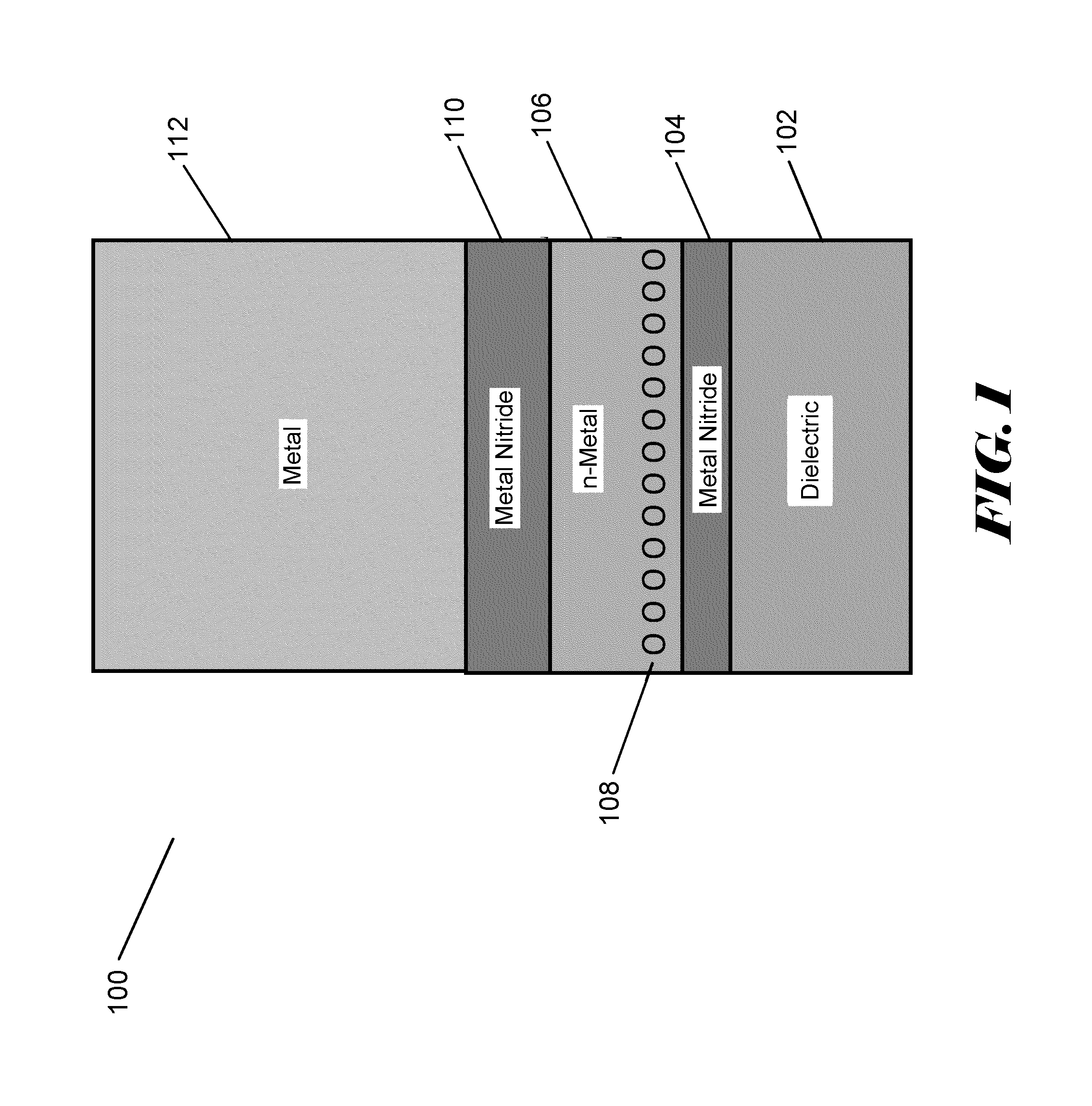
Silane or borane treatment of metal thin films
ActiveUS8846550B1Semiconductor/solid-state device manufacturingChemical vapor deposition coatingSilanesGate stack
The negative effect of oxygen on some metal films can be reduced or prevented by contacting the films with a treatment agent comprising silane or borane. In some embodiments, one or more films in an NMOS gate stack are contacted with a treatment agent comprising silane or borane during or after deposition.
Owner:ASM IP HLDG BV
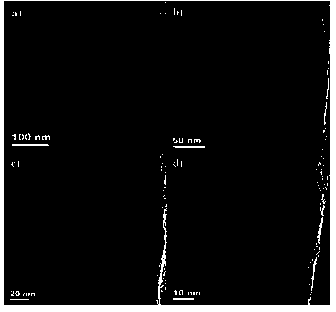
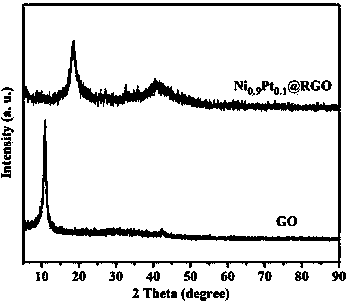
NiPt@RGO composite nano catalyst for producing hydrogen by using hydrazine borane and preparation method thereof
ActiveCN103949272AImprove catalytic performanceEasy to useHydrogen productionMetal/metal-oxides/metal-hydroxide catalystsNano catalystHydrogen
The invention provides a NiPt@RGO composite nano catalyst for producing hydrogen by using hydrazine borane and a preparation method thereof. The catalyst is prepared by using sodium borohydride (NaBH4) as a reducing agent to reduce precursors of metallic Ni and Pt and a graphene oxide (GO) carrier together. The catalyst shows very excellent catalytic performance and can catalyze the hydrazine borane at 50 DEG C, so that the hydrazine borane can be completely hydrolyzed, and hydrazine can be cracked to produce hydrogen, and the hydrogen turnover frequency (TOF) of the catalyst reaches 240 (molH2.mol<-1>metal.h<-1>). The method for preparing the catalyst is simple and convenient to operate, particularly the noble metal Pt content of the obtained optimal alloy catalyst is low (the molar ratio Ni / Pt is 9 / 1), and the catalyst is cheap and efficient.
Owner:JIANGXI NORMAL UNIV
Compositions containing borane or carborane cage compounds and related applications
Owner:HONEYWELL FED MFG & TECHNOLOGI
Control of particles on semiconductor wafers when implanting boron hydrides
InactiveCN102047376AElectric discharge tubesSemiconductor/solid-state device manufacturingPhysical chemistryIon beam
A method for reducing particle contamination during implantation of ions comprises providing an implantation system (200) for implanting ions into a workpiece (228) via an ion beam (210), wherein one or more components are under selective vacuum and have one or more contaminants in a first state disposed thereon. A gas is introduced to the implantation system (at 200), wherein the gas generally reacts with at least a portion of the one or more contaminants, therein transforming the at least a portion of the one or more contaminants into a second state. The at least a portion of the one or more contaminants in the second state remain disposed on the one or more components, and wherein the at least a portion of the second state of the one or more contaminants generally does not produce particle contamination on the one or more workpieces.
Owner:AXCELIS TECHNOLOGIES
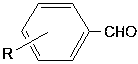
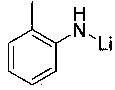
Method of preparing borate ester based on aldehyde
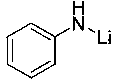
InactiveCN108409772AEfficient hydroboration reactionReduce pollutionOrganic-compounds/hydrides/coordination-complexes catalystsGroup 3/13 element organic compoundsLithiumBottle
The invention relates to application of anilino lithium, in particular to a method of preparing borate ester based on hydroboration reaction of aldehyde and borane. The method comprises the followingsteps of under the conditions without water and oxygen, in an inert gas atmosphere, adding the borane in a reaction bottle subjected to dehydration and deoxygenation treatment, then adding the anilinolithium as a catalyst, performing uniform mixing, then adding the aldehyde for hydroboration reaction, and performing exposure in the air for reaction termination to obtain the borate ester as a product, wherein the aldehyde is selected from fatty aldehydes. By adopting the method provided by the invention, the condition that the anilino lithium can extremely efficiently catalyze cyclohexanecarboxaldehyde, propionaldehyde and heptanal to generate hydroboration reaction with the borane is discovered for the first time, and a new scheme is provided to prepare the borate ester by adopting a carbonyl compound and the borane to generate the hydroboration reaction.
Owner:NANTONG TEXTILE & SILK IND TECH RES INST +1
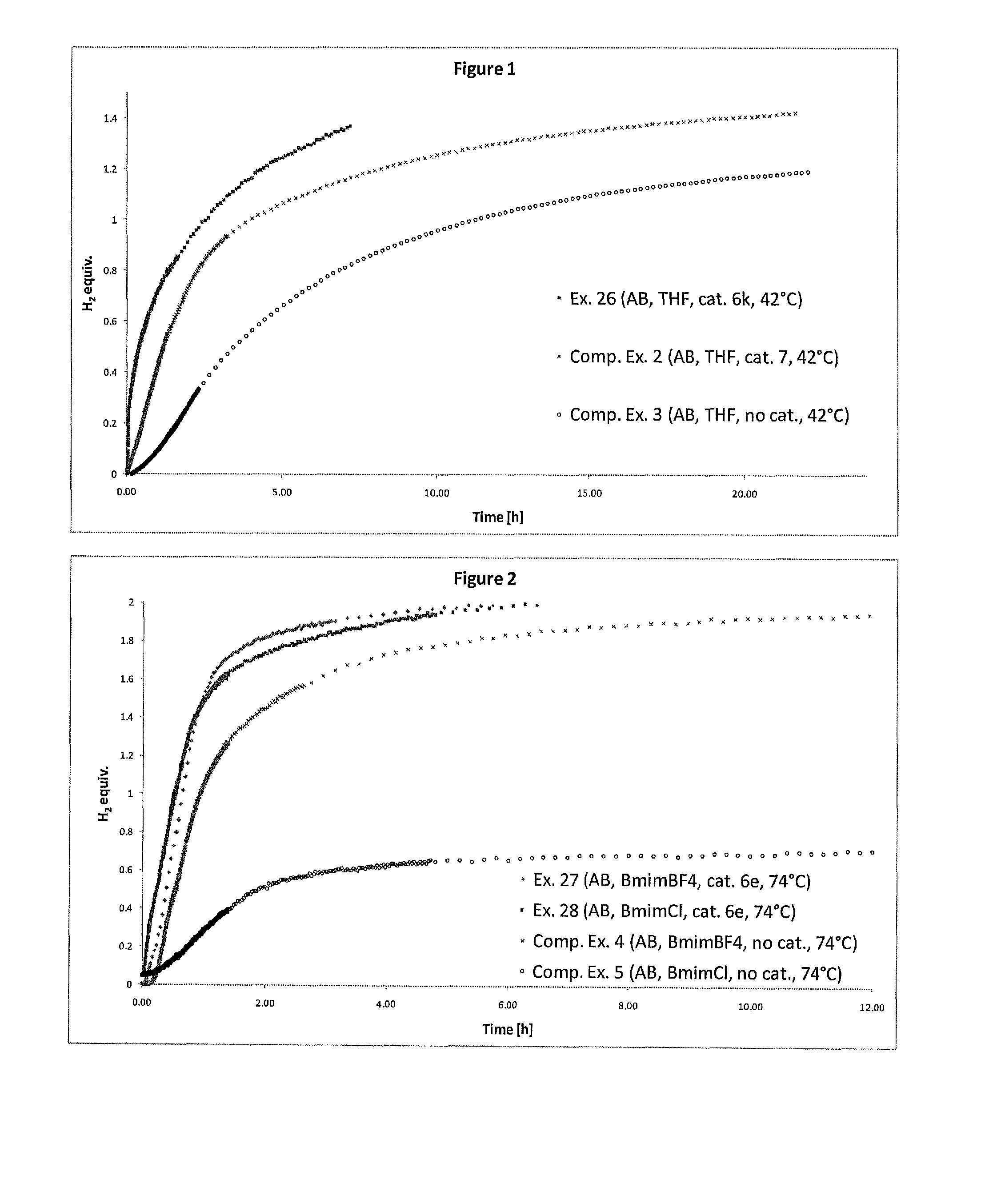
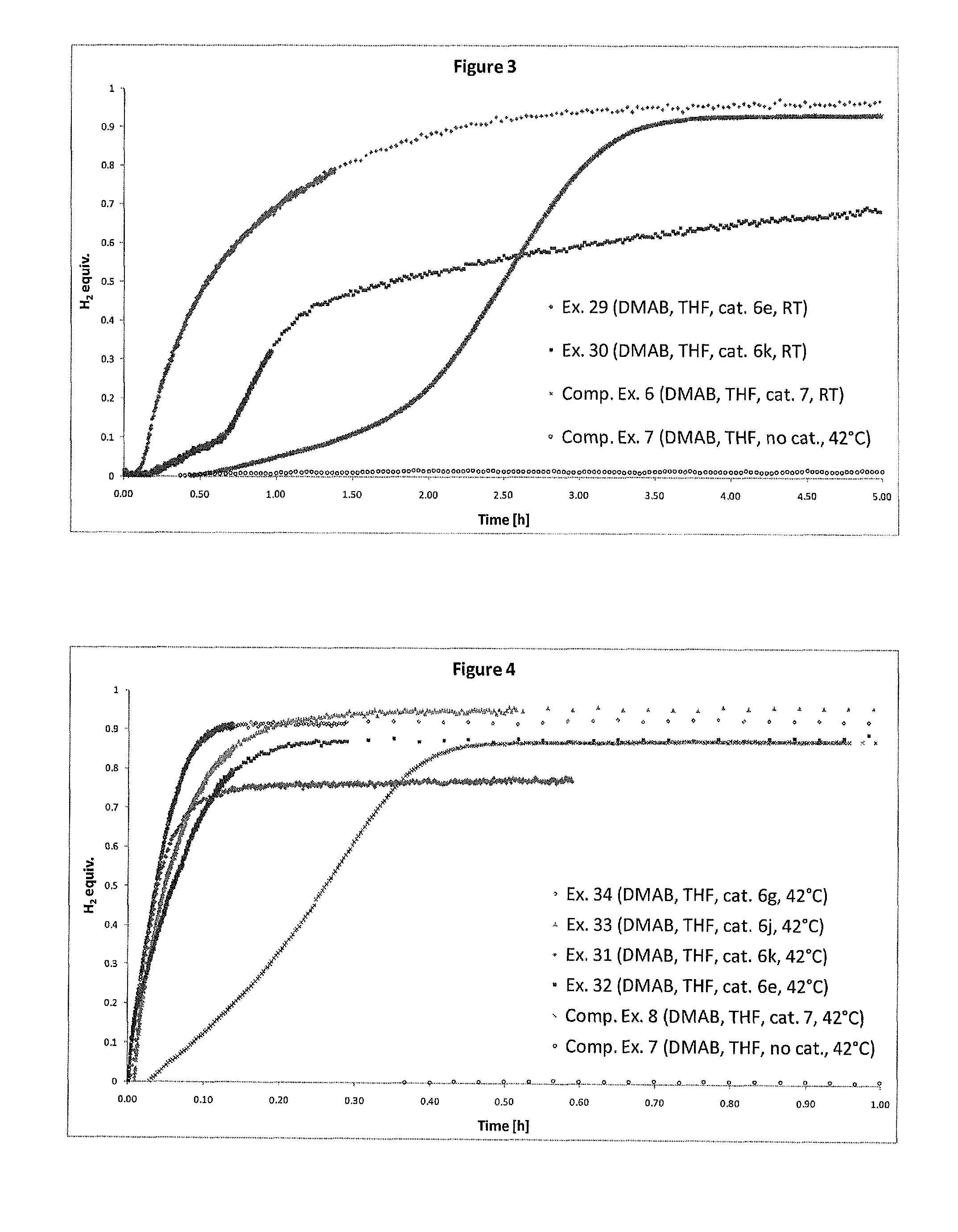
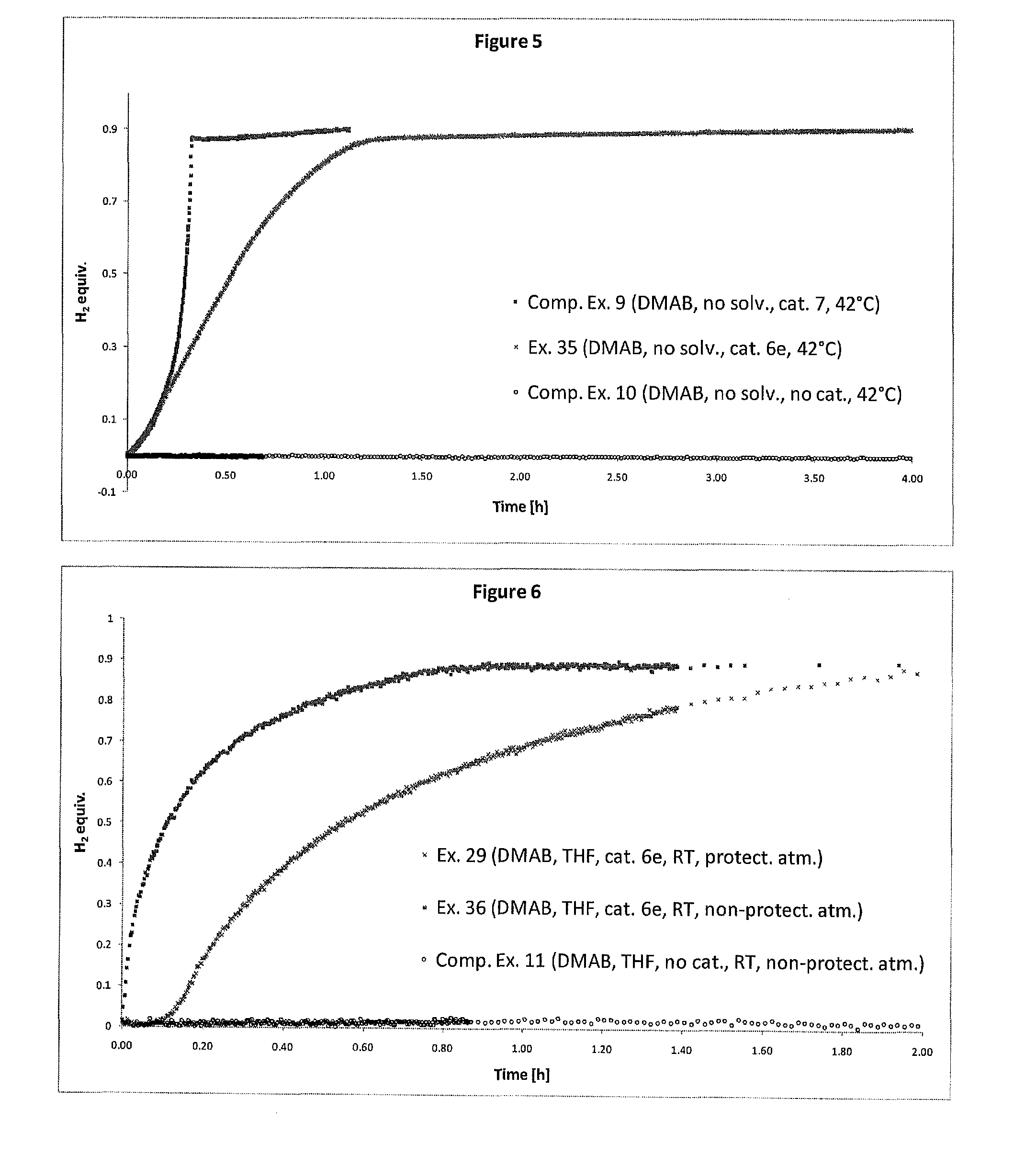
Catalyst and Process for the Production of Hydrogen from Ammonia Boranes
InactiveUS20160087295A1Eliminate needEnhanced dehydrogenation kineticsHydrogen productionHydrogen/synthetic gas productionDouble bondSolvent
The present invention relates to a process for the production of hydrogen comprising contacting at least one complex of formula (I), (I) wherein: X− is an anion; M is a metal selected from Ru, Os, Fe, Co and Ni; D is optionally present and is one or more monodentate or multidentate donor ligands; Y1 is selected from CR13, B and N; Z1 and Z2 are each independently selected from ═N, ═P, NR14, PR15, O, S and Se; or Z2 is a direct bond between carbocyclic ring B and substituent R4; each of A and B is independently a saturated, unsaturated or partially unsaturated carbocyclic hydrocarbon ring; R3 and R4 are each independently selected from H, C1-6-alkyl, aryl and C1-6-haloalkyl, and a linker group optionally attached to a solid support; or R3 and R4 together form the following moiety: (AB) Y2 is a direct single bond or double bond, or is CR18; R1, R2, R5-13 and R16-18 are each independently selected from H, C1-6-alkyl, C2-6-alkenyl, C2-6-alkynyl, aryl, C1-6-haloalkyl, NR19R20 and a linker group optionally attached to a solid support; or two or more of said R1-13 and R16-18 groups are linked, together with the carbons to which they are attached, to form a saturated or unsaturated hydrocarbon group; R14, R15, R19 and R20 are each independently selected from H, C1-6-alkyl, C2-6-alkenyl, C2-6-alkynyl, aryl, C1-6-haloalkyl, and a linker group optionally attached to a solid support; with at least one substrate of formula (II), R21R22NH—BHR23R24, wherein R21 to R24 are each independently selected from H, C1-6-alkyl, fluoro-substituted C1-6-alkyl, C6-14-aryl and C6-14-aralkyl, or any two of R21, R22, R23 and R24 are linked to form a C3-10-alkylene group or C3-10-alkenylene group, which together with the nitrogen and / or boron atoms to which they are attached, forms a cyclic group; or a substrate comprising two, three or four substrates of formula (II) linked via one or more bridging groups so as to form a dimeric, trimeric or tetrameric species, and wherein the bridging group is selected from straight or branched C1-6-alkylene optionally substituted by one or more fluoro groups, boron, C6-14-aryl and C6-14-aralkyl; or a substrate comprising two, three or four substrates of formula (II) which are joined so as to form a fused cyclic dimeric, trimeric or tetrameric species. Further aspects of the invention relate to a hydrogen generation system comprising a complex of formula (I), a substrate of formula (II) and a solvent, and to the use of complexes of formula (I) in fuel cells. Another aspect of the invention relates to novel complexes of formula (I).
Owner:UNIV COLLEGE DUBLIN NAT UNIV OF IRELAND DUBLIN
Aluminum or aluminum alloy molten salt electroplating bath having good throwing power, electroplating method using the bath, and pretreatment method of the bath
The purpose of the present invention is to provide an electrical Al plating bath that poses little danger of exploding or igniting as a result of contacting air or water, and contains no benzene, toluene, xylene, naphthalene, or 1,3,5-trimethylbenzene, which have detrimental effects to humans. The present invention provides an electrical aluminum or aluminum alloy fused salt plating bath that is obtained by heat treatment of an electrical aluminum or aluminum alloy fused salt plating bath containing (A) a halogenated aluminum as the primary component and (B) at least one other type of halide after adding (C) one, two or more reducible compounds selected from the group consisting of hydrides of elements in Group 1 Periods 2 through 6 of the Periodic Table of Elements and / or hydrides of Group 13 Periods 2 through 6 of the Periodic Table of Elements and amine borane compounds.
Owner:DISPOL CHEMICALS CO LTD
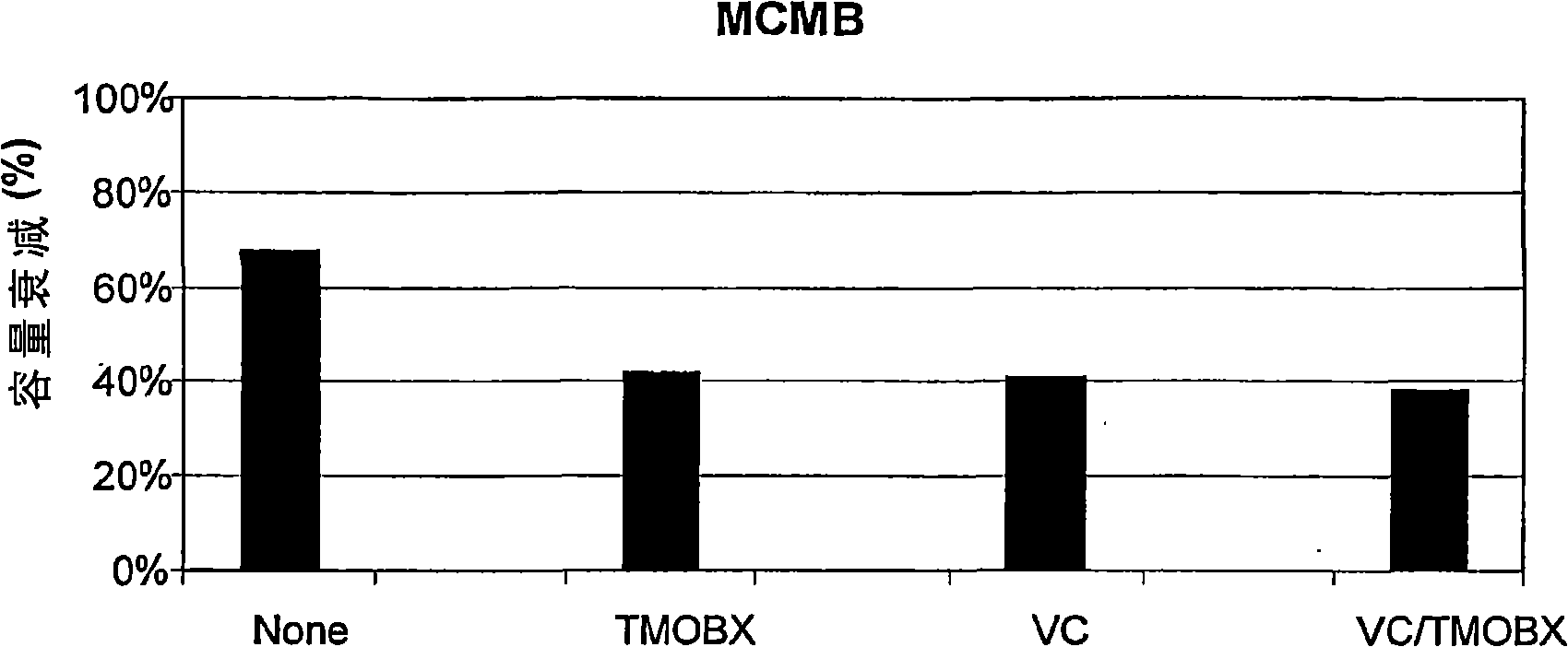
Electrolyte for lithium ion battery using silica-based material as negative electrode material and lithium ion battery
ActiveCN103413969AReduce consumptionExtend your lifeSecondary cellsSodium-ion batteryLithium-ion battery
The invention discloses an electrolyte for a lithium ion battery using a silica-based material as a negative electrode material and the lithium ion battery. The electrolyte comprises a lithium salt, a non-aqueous organic solvent and a film formation additive, wherein the non-aqueous organic solvent comprises ethylene carbonate; the film formation additive comprises TPFPB Tris(pentafluorophenyl) borane. The TPFPB in the electrolyte is used as the film formation additive of an SEI film, can facilitate the formation of the stable and integral SEI film on the surface of the negative electrode material, and can weaken an efflorescence phenomenon caused by a silicon volume effect when the silica-based material is used as the negative electrode material; the TPFPB can release freely moving lithium ions through the SEI film, so that part of lithium ions consumed during the formation process of the SEI film can be offset, lithium ion consumption can be lowered, and the charging and discharging efficiency and the cycle performance can be improved. The structural characteristic of the TPFPB determines that the TPFPB is relatively stable and not easy to decompose, so that the service life of the electrolyte is prolonged.
Owner:CHERY AUTOMOBILE CO LTD
Application of o-methyl-anilino lithium in catalysis of aldehyde and borane hydroboration
InactiveCN108659027AEfficient hydroboration reactionReduce pollutionOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsLithiumBottle
The invention discloses application of o-methyl-anilino lithium in the catalysis of aldehyde and borane hydroboration. Borane is added into a reaction bottle that is dewatered and deoxidized in an inert gas atmosphere in an anhydrous oxygen-free environment; the catalyst, o-methyl-anilino lithium, is added; mixing is performed well before an aldehyde is added; hydroboration is carried out; the reaction is ended after exposure to air so as to obtain borates; the aldehyde is selected from aromatic aldehydes and heterocyclic aldehydes. The catalyst disclosed herein is well applicable to aromaticaldehydes and heterocyclic aldehydes having different substitution positions and electronic effects; more choices are provided to attain borates with different substituent structures.
Owner:SUZHOU UNIV
Preparation method of hydrogen storage material of borane ammonia compound
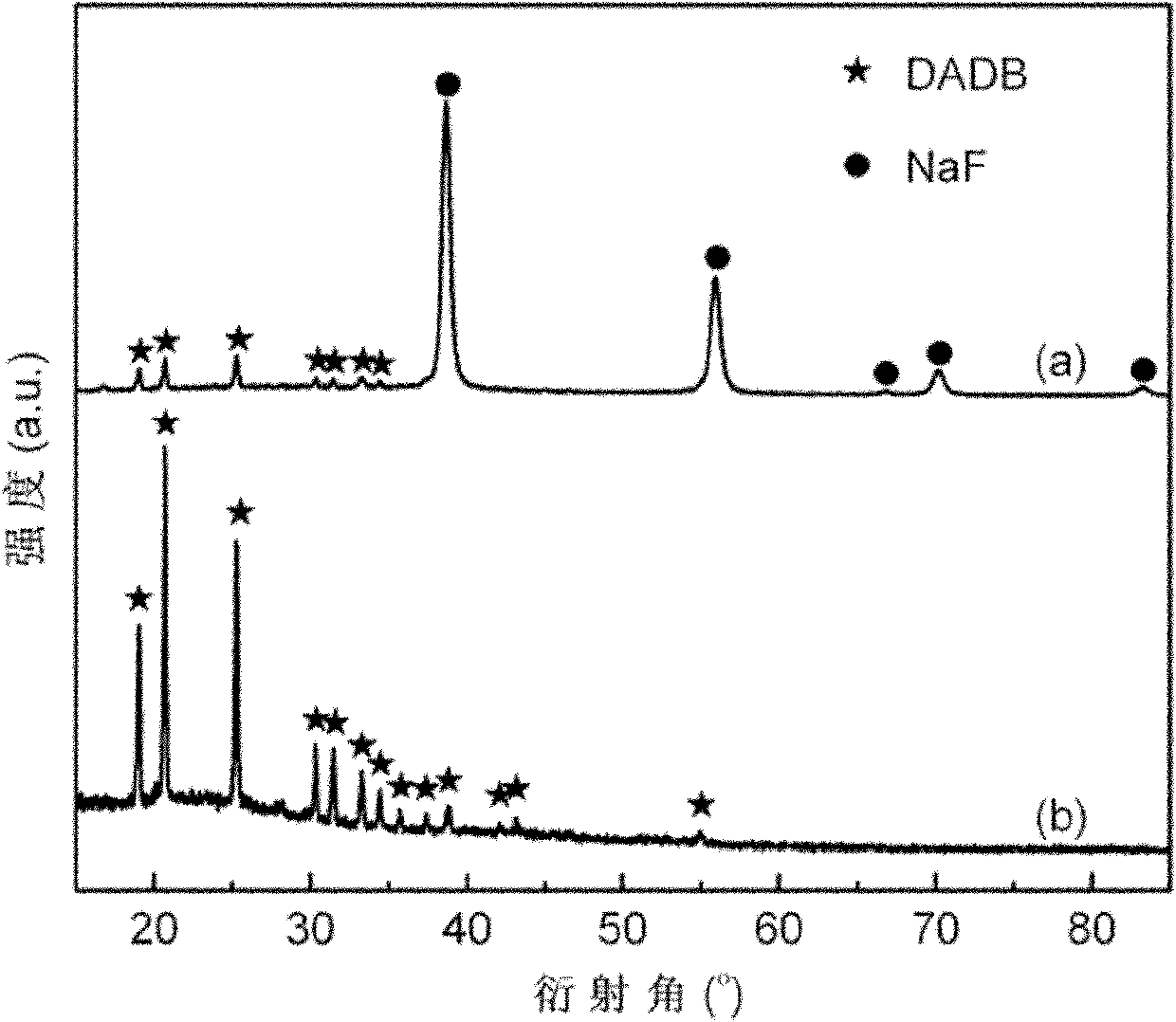
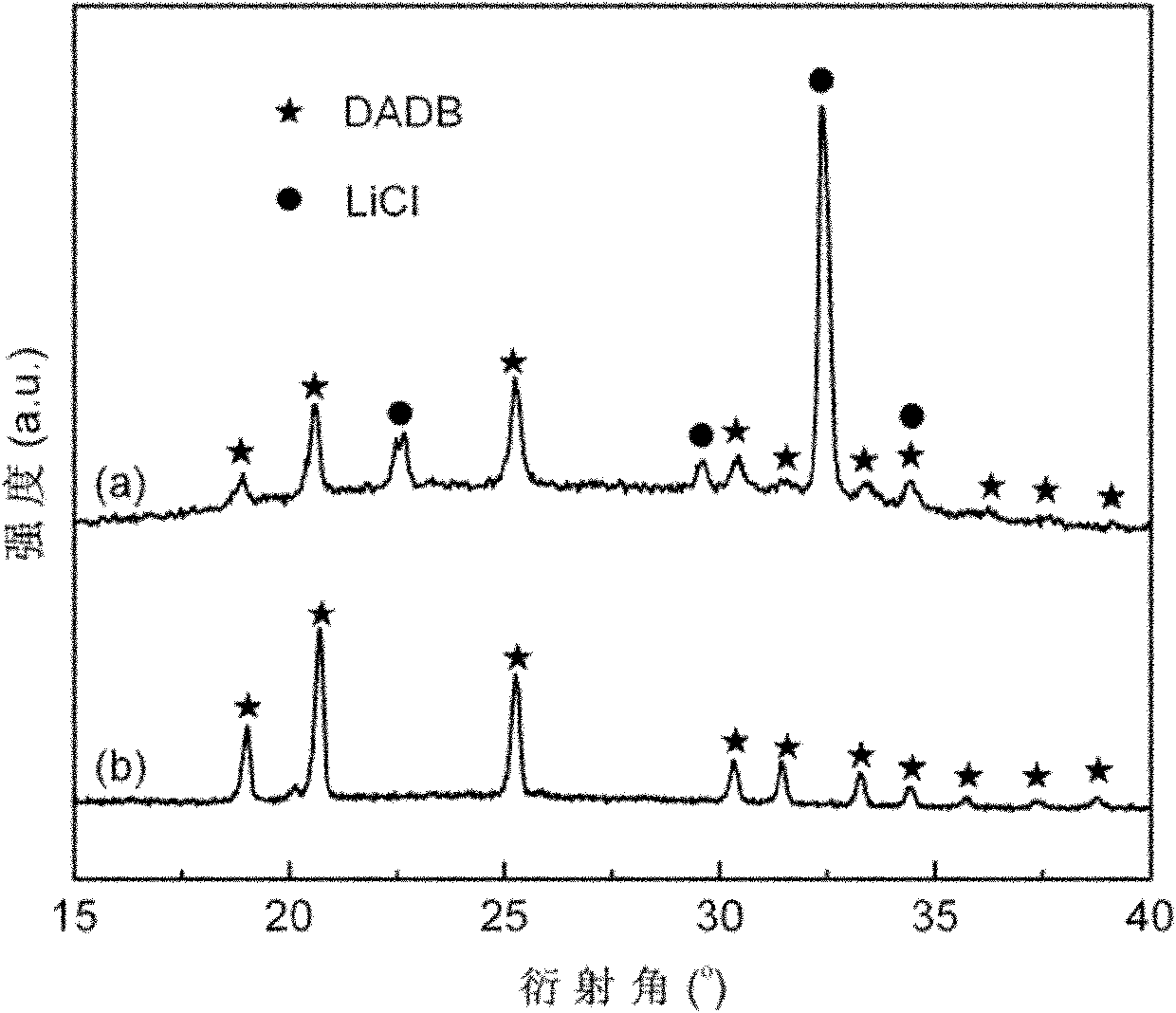
The invention relates to the field of hydrogen storage materials, in particular to a preparation method of a hydrogen storage material of a borane ammonia compound, which aims at resolving the problem in the prior art that the existing hydrogen storage material is complex in preparation process, utilizes toxic raw material B2H6, and is low in compounding rate, low in sample purity, not suitable for large-scale preparation and the like. The borane ammonia compound comprises boron, nitrogen and hydrogen, the molecular formula of the borane ammonia compound is [(NH3)2BH2](BH4), and the abbreviation is DADB. The special operation steps include utilizing the mixture of metal borohydride M(BH4)x and ammonium salt (NH4)yL as an initial raw material, adopting the mechanical ball milling method toprepare a mixture of DADB and MyLx; and (2) utilizing the mixture of the DADB and the MyLx as the initial raw material and adopting a liquid ammonia solvent to conduct dissolving, filtering and ammonia removing steps to remove by-product MyLx and obtain a pure DADB powder sample. The preparation method is simple and easy to implement, capable of preparing a high-purity DADB compound in high yieldand suitable for scale preparation, and the raw materials are low in cost and non-toxic.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
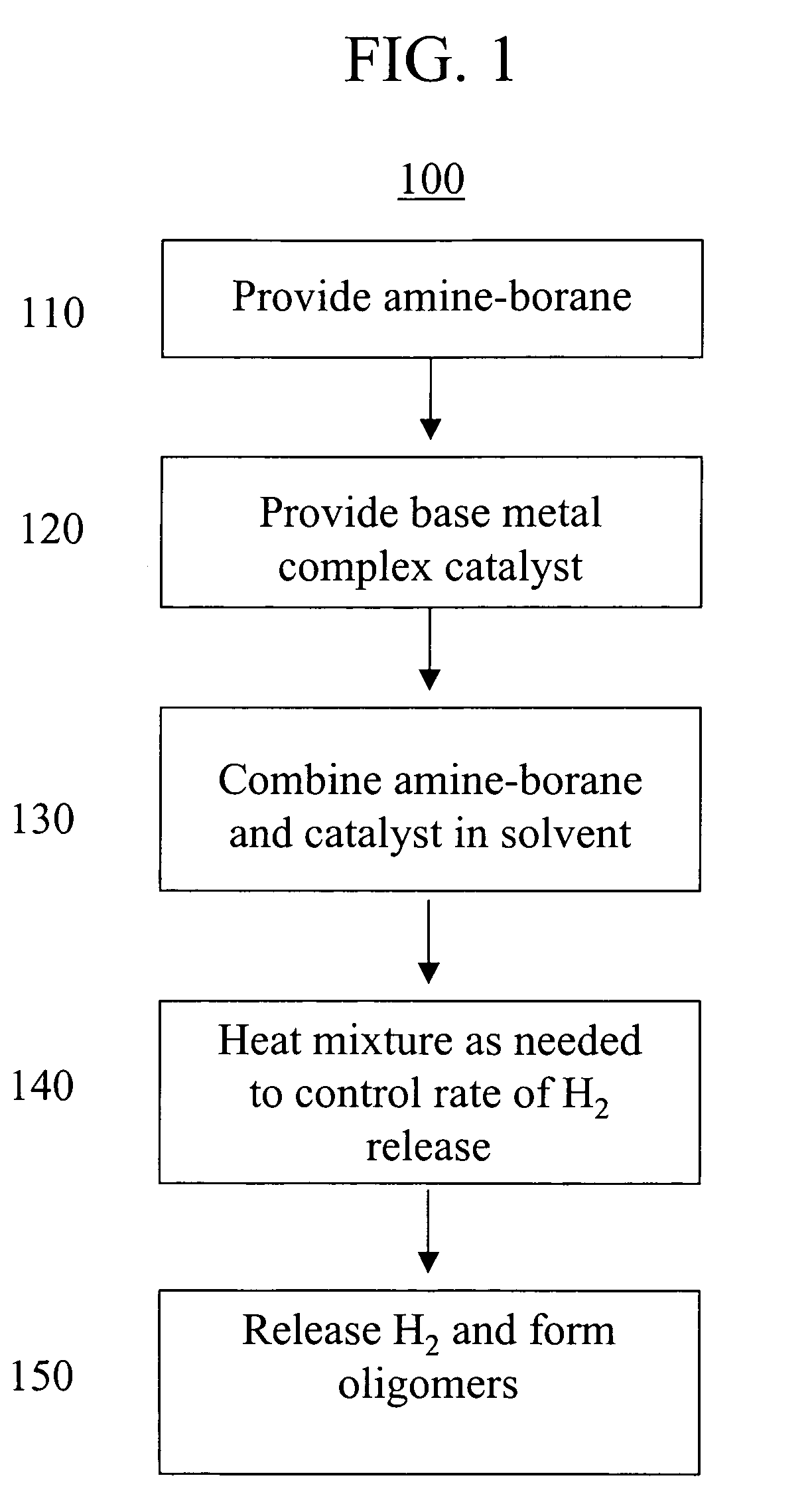
Base metal dehydrogenation of amine-boranes
A method of dehydrogenating an amine-borane having the formula R1H2N—BH2R2 using base metal catalyst. The method generates hydrogen and produces at least one of a [R1HN—BHR2]m oligomer and a [R1N—BR2]n oligomer. The method of dehydrogenating amine-boranes may be used to generate H2 for portable power sources, such as, but not limited to, fuel cells.
Owner:LOS ALAMOS NATIONAL SECURITY
Targeted boron preparation and preparation method
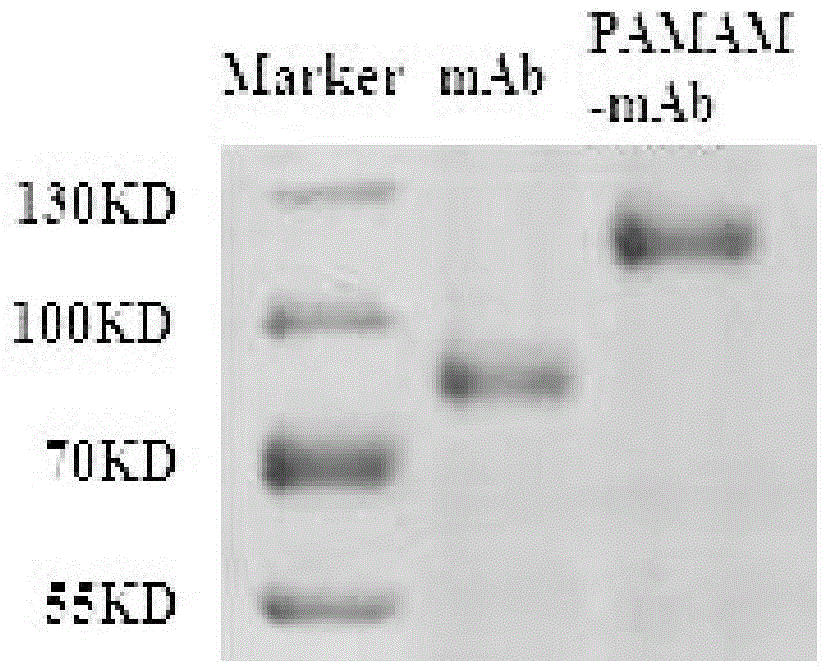
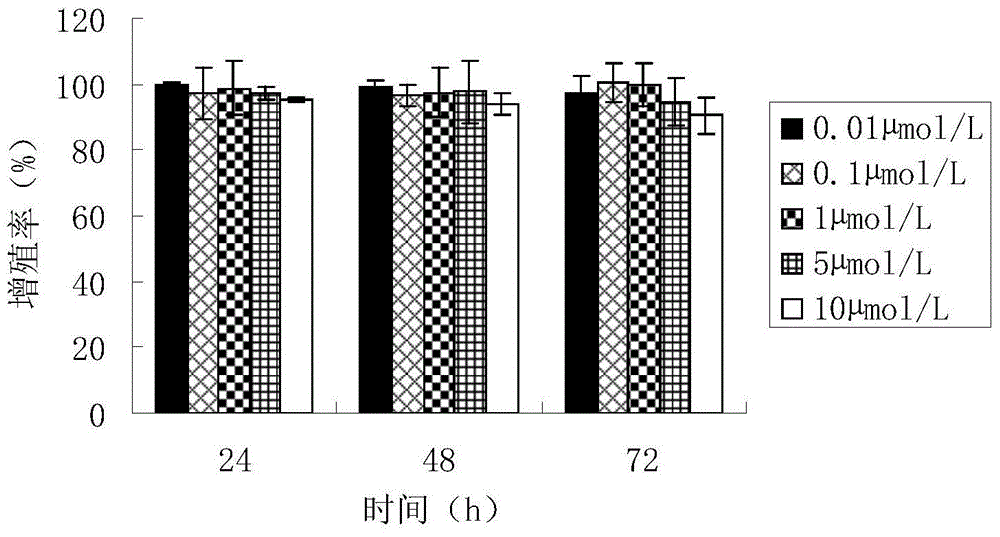
InactiveCN104399094AIncrease dosageMeet the dosage requirementsRadioactive preparation carriersAntineoplastic agentsDendrimerNeutron irradiation
The invention belongs to the biological medicine field, and discloses a targeted boron preparation and a preparation method and application. The target boron preparation comprises polyamidoamine-amine dendrimers, epidermal growth factor receptor antibody and polyhedral boranes, the polyamide amine dendrimers are connected with the epidermal growth factor receptor antibody, and are internally loaded with the polyhedral boranes. The targeted boron preparation is high in boron content, can meet the requirements of BNCT (boron neutron capture therapy) on the boron atom dose, is well targeted to tumor cells, has good stability and low cell toxicity, and can be efficiently uptaken by human glioma U87MG cells of high surface expression EGFR (epidermal growth factor receptor). The target boron preparation can effectively improve the content of boron in orthotopic transplantation tumor nude mice tumor tissues, can significantly prolong orthotopic transplantation tumor nude mice lifetime by neutron irradiation, and is suitable for boron neutron capture therapy for glioma. The preparation method of the targeted boron preparation has the advantages of simple operation, and the prepared targeted boron preparation has good stability.
Owner:THE FIRST AFFILIATED HOSPITAL OF SOOCHOW UNIV
High-energy welding-cutting gas
Owner:李铁锁 +1
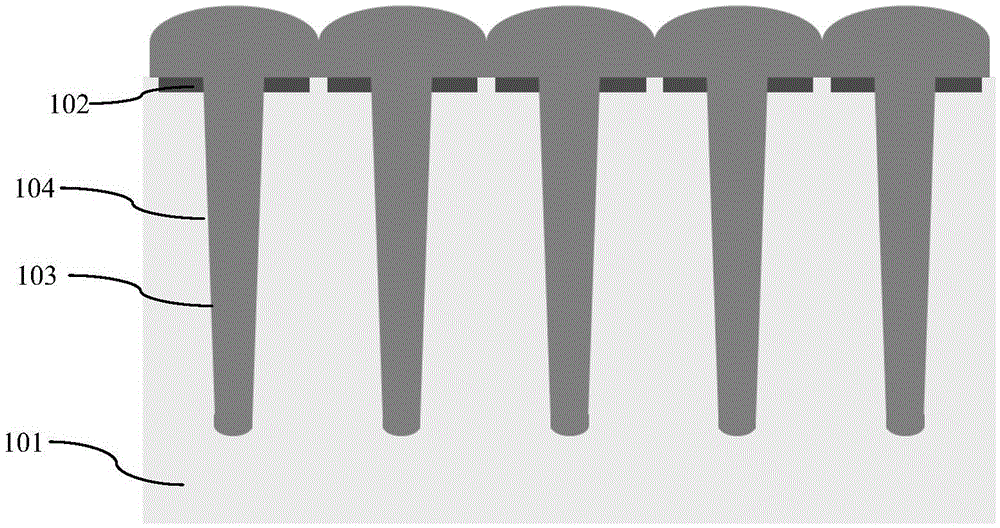
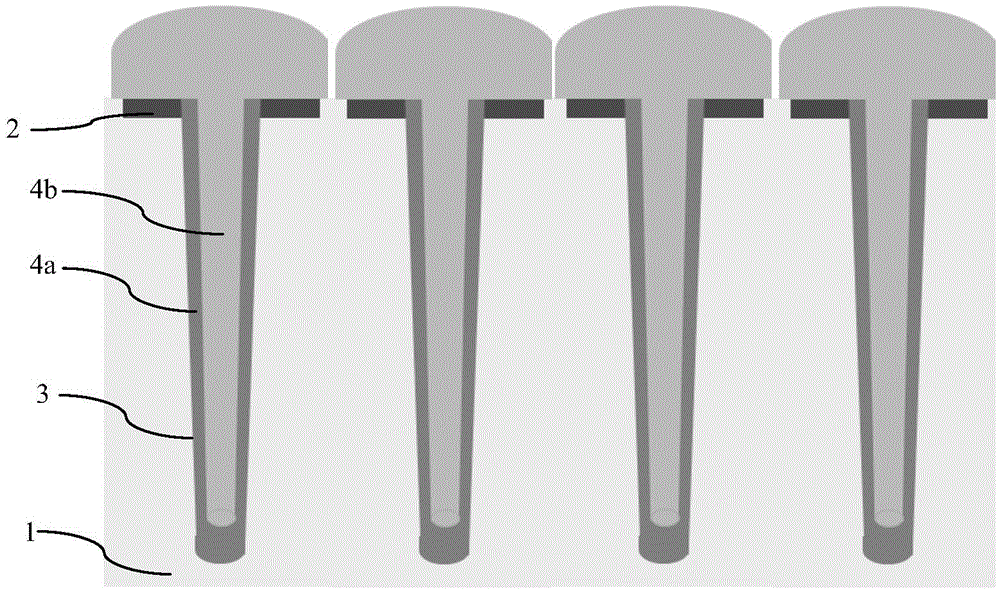
Groove type super junction epitaxial filling method
ActiveCN105529355AImproves time to full debiasReduce external electromagnetic interferenceSemiconductor/solid-state device detailsSolid-state devicesDevice formElectromagnetic interference
The invention discloses a groove type super junction epitaxial filling method. The groove type super junction epitaxial filling method comprises the following steps: a first step, providing a semiconductor substrate formed with an N type epitaxial layer; a second step, forming a plurality of grooves in the N type epitaxial layer; and a third step, filling a P type epitaxial layer with a hierarchical structure in the grooves by epitaxial growth, wherein the hierarchical structure is realized by adjusting the filling speed of a borane gas. By adopting the groove type super junction epitaxial filling method disclosed by the invention, the switching speed of a device formed by a super junction can be reduced, and external electromagnetic interference is reduced.
Owner:SHANGHAI HUAHONG GRACE SEMICON MFG CORP
Naphthenic hydrocarbon additives for diaryl phosphide salt formation
ActiveUS20100234642A1Organic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsAdductPhosphine
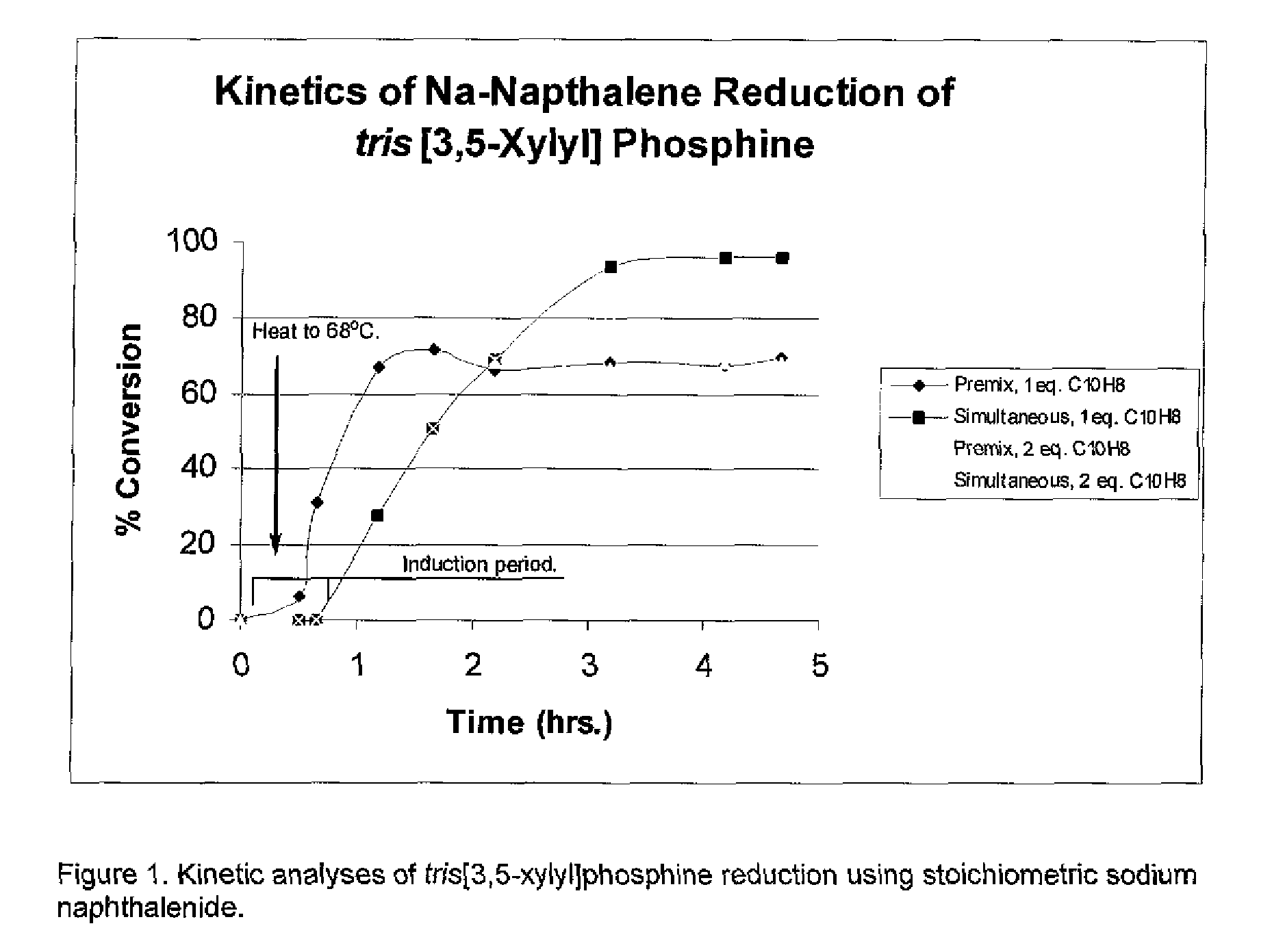
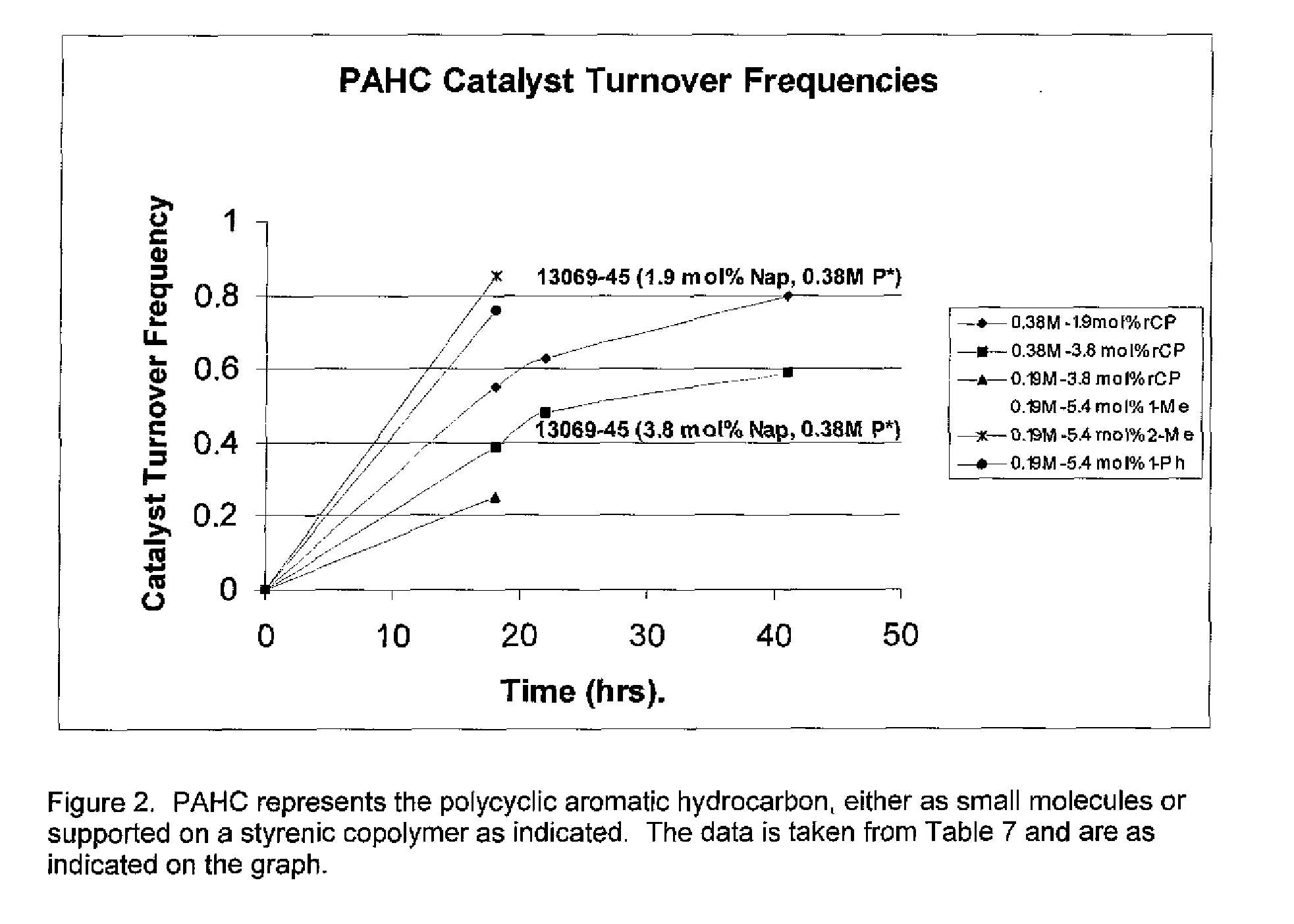
The invention relates to the use of polycyclic aromatic hydrocarbons (PAHs) such as naphthalene and its alkyl, aryl, or heteroatom substituted analogs, that act as catalysts in the presence of an alkali metal (Li, K, Na) for the reduction of electron-deficient and electron-rich triaryl phosphines to their corresponding alkali metal diaryl phosphide salts. The process is also useful for the catalysis of triaryl phosphine chalcogen adducts such as the sulfides, oxides, and selenides, diaryl(halo)phosphines, triaryl phosphine-borane adducts, and tetra-aryl bis(phosphines) that can also be reduced to their corresponding alkali metal diaryl phosphide salts. The invention also relates to small molecule PAHs and polymer tethered PAHs naphthenics.
Owner:PMC ORGANOMETALLIX INC
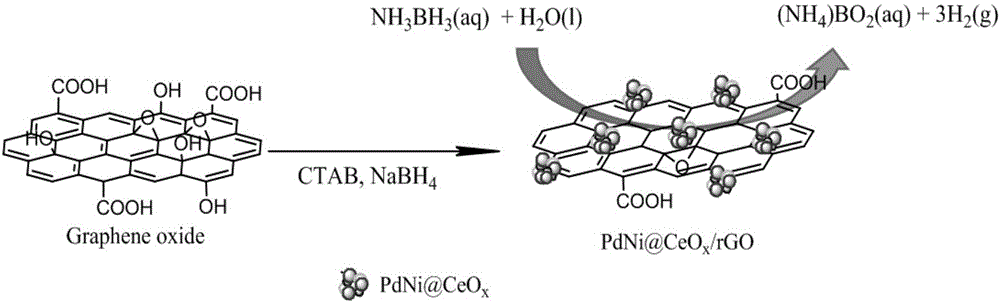
Graphene loaded palladium-nickel/cerium oxide nano composite material, preparation method and amino borane catalytic decomposition method
InactiveCN106378150AEfficient decompositionGood catalyticHeterogenous catalyst chemical elementsCatalyst activation/preparationCatalytic decompositionCerium
The invention discloses a graphene loaded palladium-nickel / cerium oxide nano composite material, a preparation method and an amino borane catalytic decomposition method. The preparation method comprises the following steps of: 1) mixing graphene oxide, hexadecyl trimethyl ammonium bromide and water so as to obtain a graphene oxide activating system; and 2) dispersing a nickel source, a palladium source and a cerium source into the graphene oxide activating system, and adding a reducing agent into the system for a reduction reaction, thereby obtaining the graphene loaded palladium-nickel / cerium oxide nano composite material. By adopting the preparation method, the graphene loaded palladium-nickel / cerium oxide nano composite material prepared by using a one-step method has an excellent catalysis effect on amino borane, and meanwhile the preparation method is simple in step, gentle and controllable in condition and good in environment-friendliness. In addition, by adopting the graphene loaded palladium-nickel / cerium oxide nano composite material, decomposition of the amino borane can be efficiently catalyzed.
Owner:ANHUI NORMAL UNIV
Lithium-ion battery
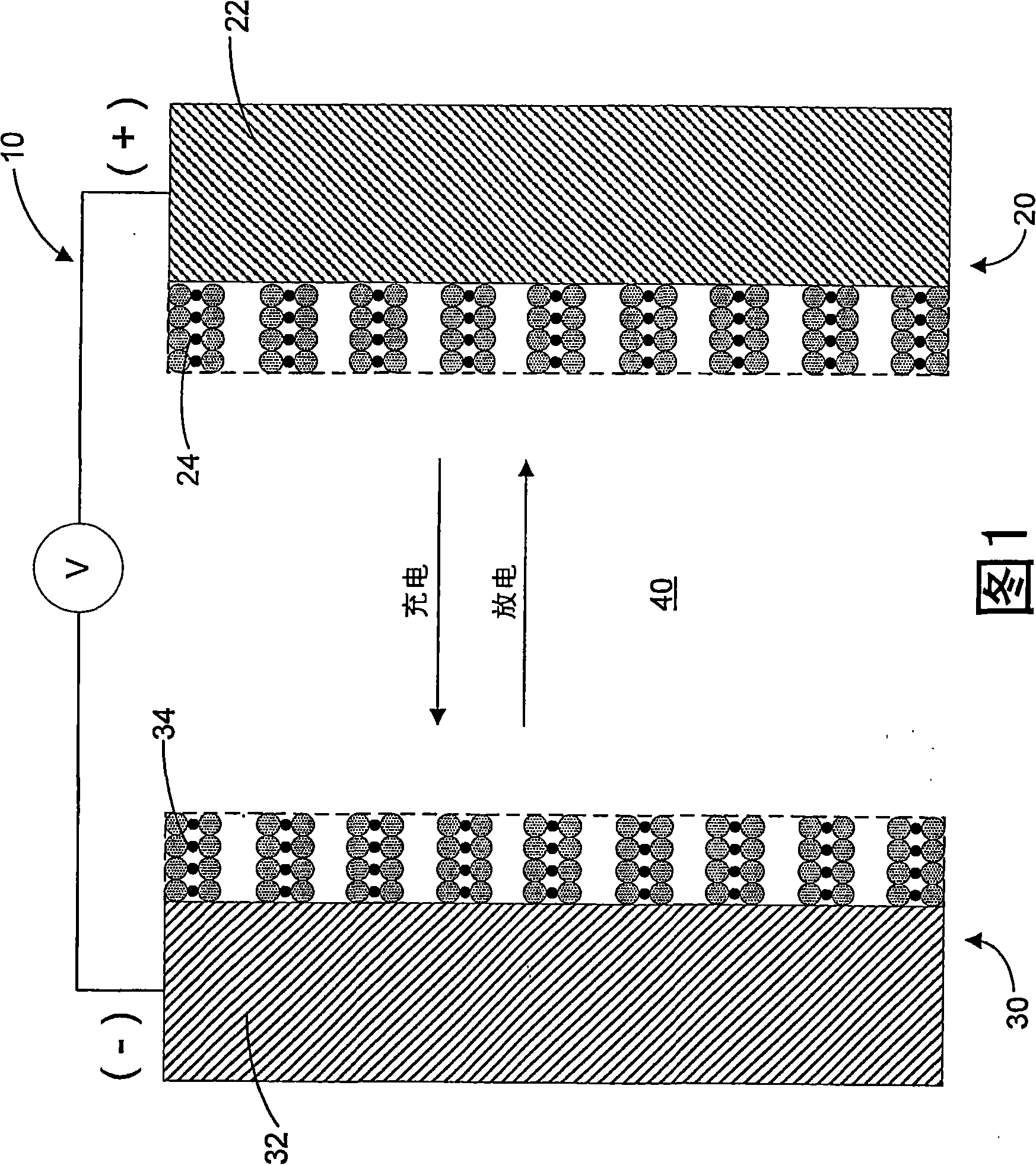
A lithium-ion battery (100) includes a positive electrode (110), a negative electrode (120) comprising carbon, and an electrolyte (130) containing a first and second additive. The first additive includes a borane or borate compound that acts as an ion receptor and the second additive includes an alkene capable of reacting on a surface of the negative electrode (120) to form an ionically conductive layer.
Owner:MEDTRONIC INC
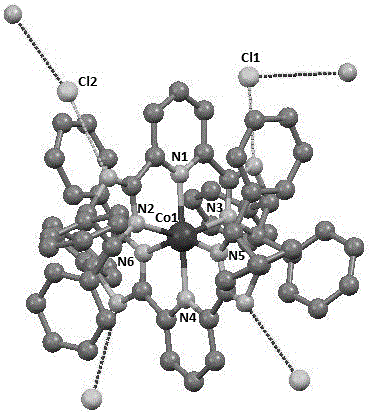
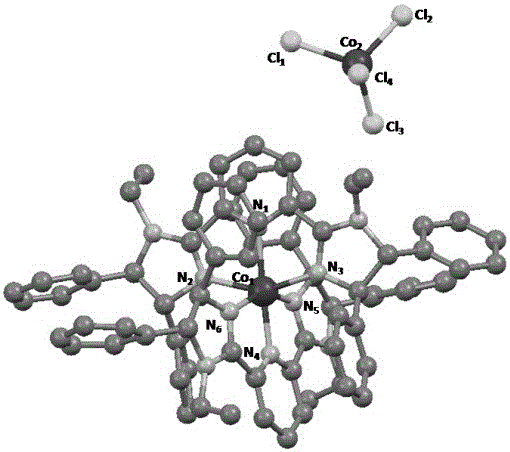
Chiral pyridine biimidazole ligand transition metal complex catalyst and preparation method thereof
InactiveCN105854947AEasy to prepareRaw materials are cheap and easy to getOrganic-compounds/hydrides/coordination-complexes catalystsIron group organic compounds without C-metal linkagesMetal saltsRacemization
The invention relates to a chiral pyridine biimidazole ligand transition metal complex catalyst and a preparation method thereof. The general structure of the compound is shown in the description, wherein M represents transition metal, R<1>, R<2>, R<3>, R<4>, R<5>, R<6>, R<7>, R<8> and R<9> represent hydrogen atoms or alkane groups of C1-C30 or aryl groups of C6-C30, X represents a halogen atom or halogenated metal salt ion, the asterisk place is racemization or chirality, and when the asterisk place is chirality, the asterisk place represents an R configuration or S configuration. An applied chiral pyridine biimidazole ligand can change the electronic property and steric hindrance of the ligand through functional group modification, and as a catalyst precursor, asymmetric hydroboration of alkenyl borane has medium catalytic activity and stereoselectivity. According to the chiral pyridine biimidazole ligand transition metal complex catalyst and the preparation method thereof, the preparation method is simple, the raw materials are cheap and easy to obtain, environmental friendliness is achieved, the reaction condition is mild, the yield is high, and synthetic operation is easy and convenient.
Owner:SHANGHAI UNIV
Process and device for the production of polyhedral boranes
The present invention provides methods and devices for producing polyhedral boron compounds. The process is generally an anhydrous, one-pot process that comprises a pyrolytic reaction of a tetraborohydride with a quaternary amine salt to form the polyhedral borane. In another aspect of the present invention, polyhedral boranes are produced, without isolation of the Lewis base-borane complex.
Owner:UNIVERSITY OF MISSOURI
Application of n-butyllithium in catalyzing hydroboration of aldehyde and borane
InactiveCN108404984AReduce electropositivityWeakened nucleophilic addition activityOrganic-compounds/hydrides/coordination-complexes catalystsGroup 3/13 element organic compoundsN-ButyllithiumOxygen
The invention discloses application of n-butyllithium in catalyzing hydroboration of aldehyde and borane, a method of hydroboration comprises the following steps of in a water-free and oxygen-free environment, under the inert gas atmosphere, adding borane into a reaction bulb subjected to dehydration and deoxidization treatment, then adding a catalyst n-butyllithium, uniformly mixing, then addingaldehyde to perform hydroboration. Exposing to the air to stop the reaction after the reaction is finished, so as to obtain a product. The catalyst disclosed by the invention has better universality to aromatic aldehyde with different replace positions and different electronic effects as well as to heterocyclic aldehydes and fatty aldehyde, and more choice can be provided for obtaining boric acidester compounds with different substituent group structures.
Owner:SUZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com