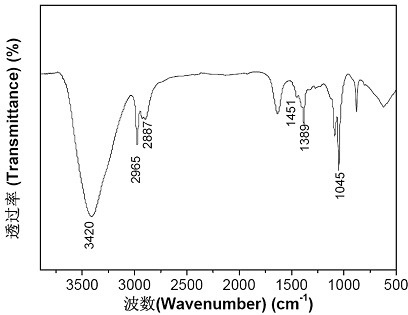
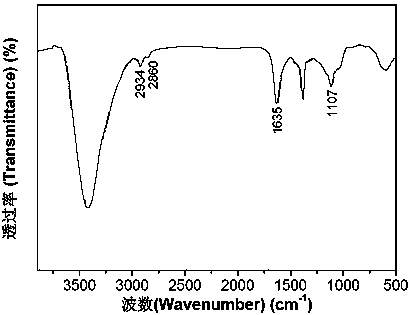
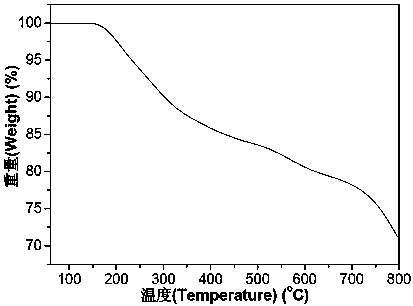
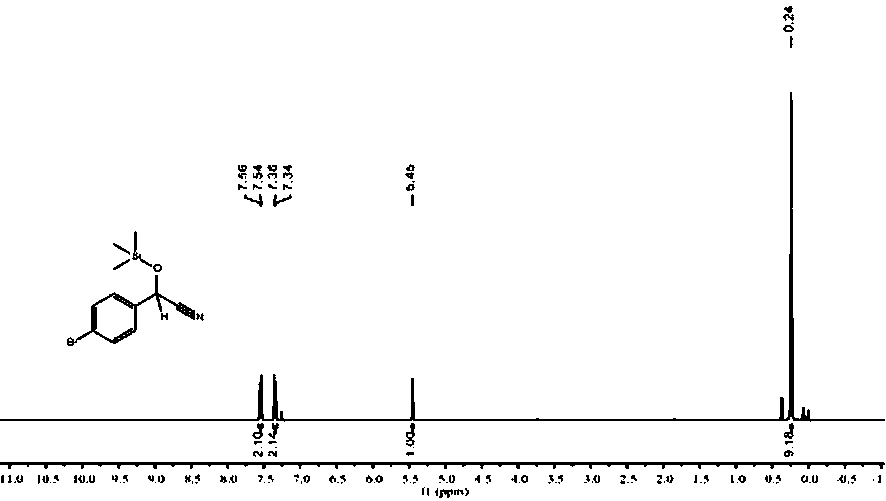
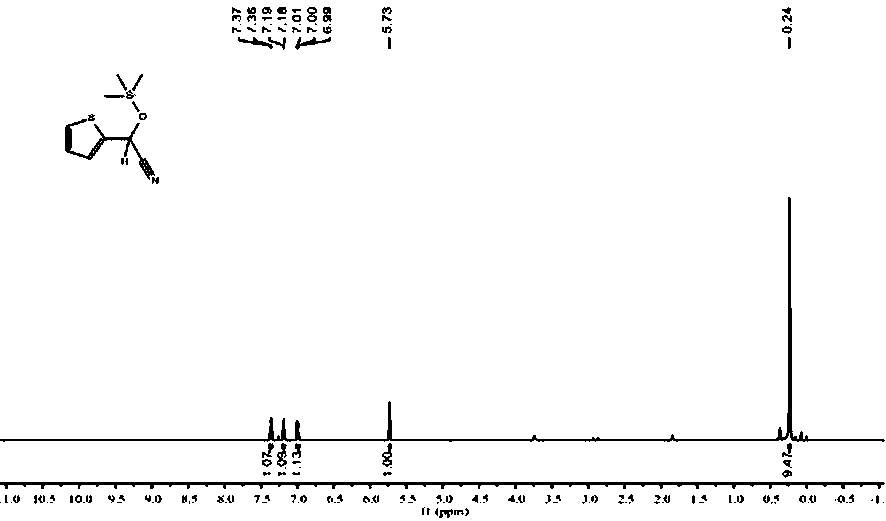
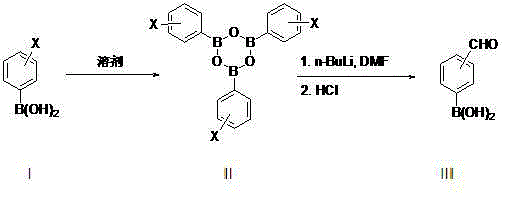
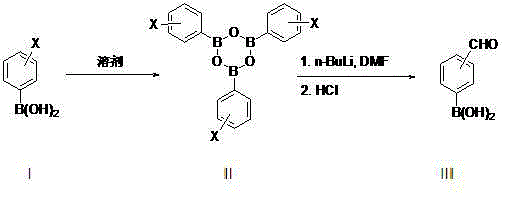
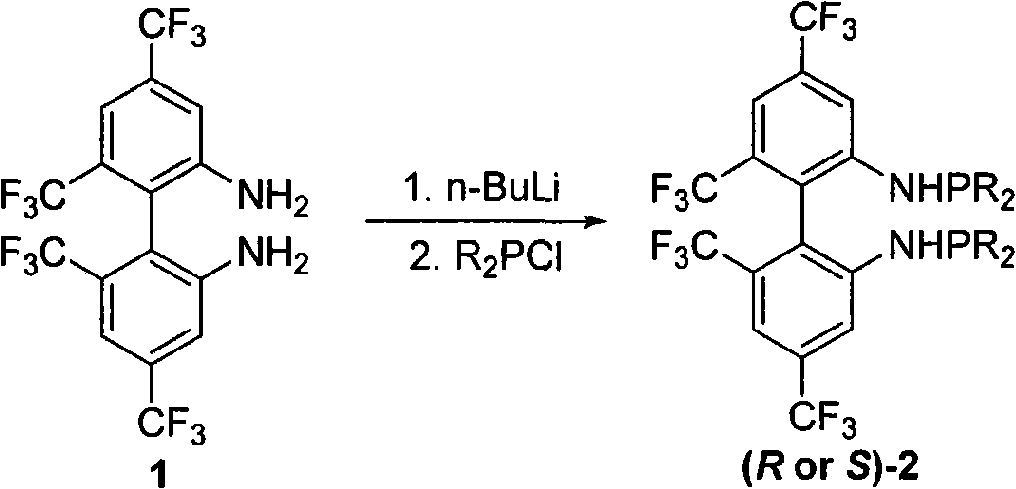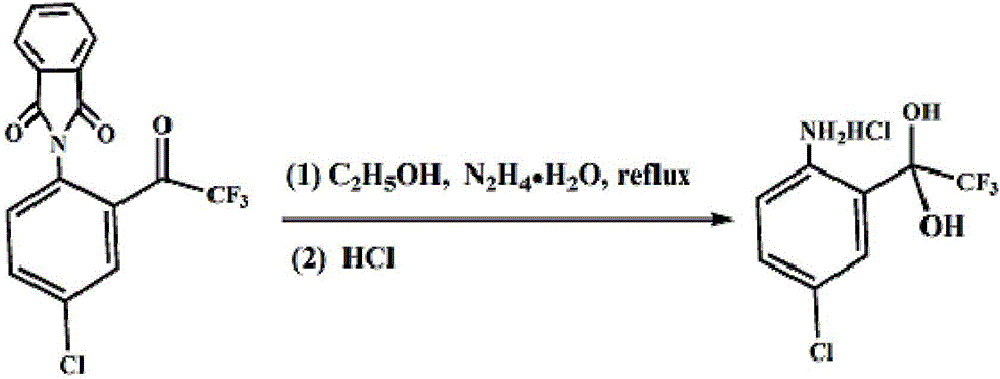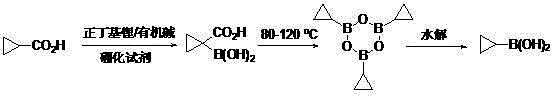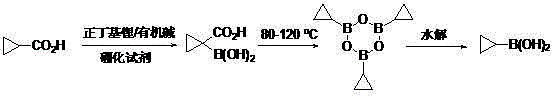Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
262 results about "Butyllithium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Butyllithium may refer to one of 5 isomeric organolithium reagents of which 3 are commonly used in chemical synthesis...
Preparation method for molybdenum disulfide nanosheet with reactive group-containing surface
ActiveCN103833081AGood dispersionReduce pollutionMaterial nanotechnologyMolybdenum sulfidesN-ButyllithiumHexane
The invention discloses a preparation method for a molybdenum disulfide nanosheet with a reactive group-containing surface. The preparation method comprises the following steps: adding one part by weight of molybdenum disulfide, 2-5 parts by weight of n-butyllithium and 10-20 parts by weight of n-hexane into a hydrothermal reaction kettle, heating at 50-150DEG C for 0.5-12 hours, cooling to room temperature, performing centrifugal separation and n-hexane washing for five times, drying at 50DEG C for 12 hours so as to obtain molybdenum disulfide powder with lithium ion intercalation, adding 1 part by weight of molybdenum disulfide powder with lithium ion intercalation, 0.05-10 parts by weight of sulihydryl reagents with reactive groups, and 100-1000 parts by weight of water into a flask for performing ultrasonic treatment for one minute to five hours under 50-100 watt, to obtain molybdenum disulfide nanosheet with the reactive group-containing surface. The method is easily available, mild to react, simple to operate, and small in environmental pollution; the prepared nanosheet can be further functionalized, thus having good application prospects.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
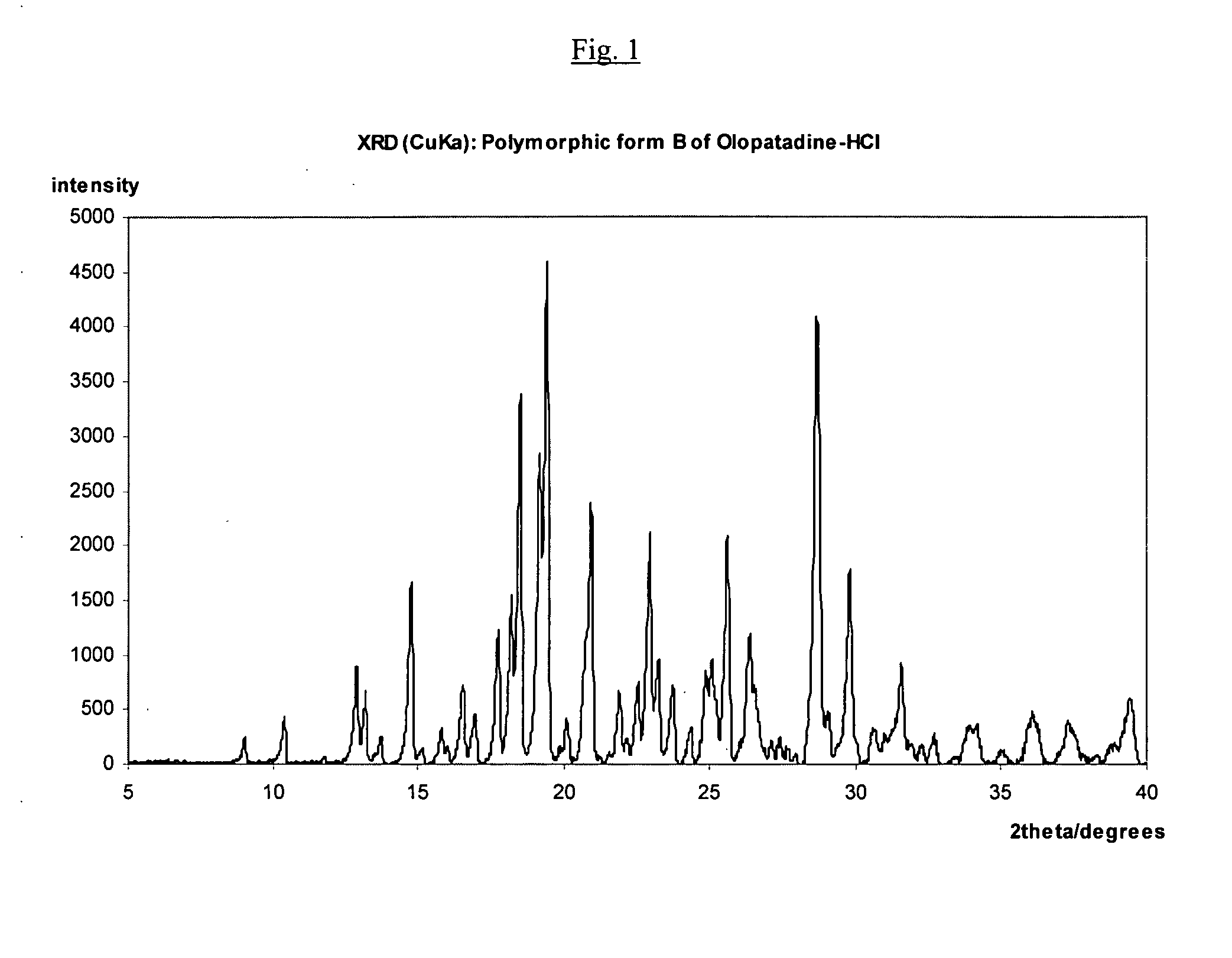
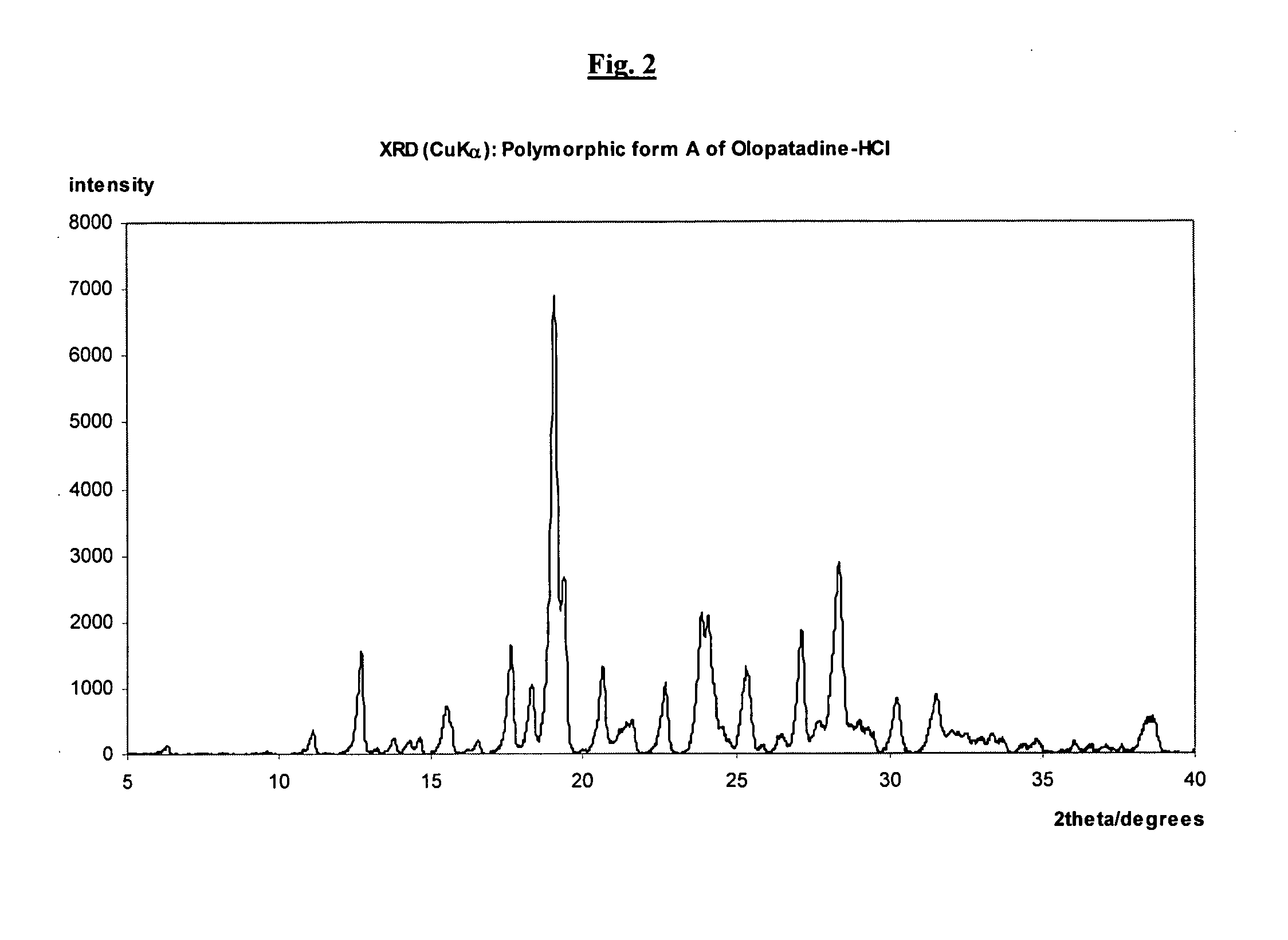
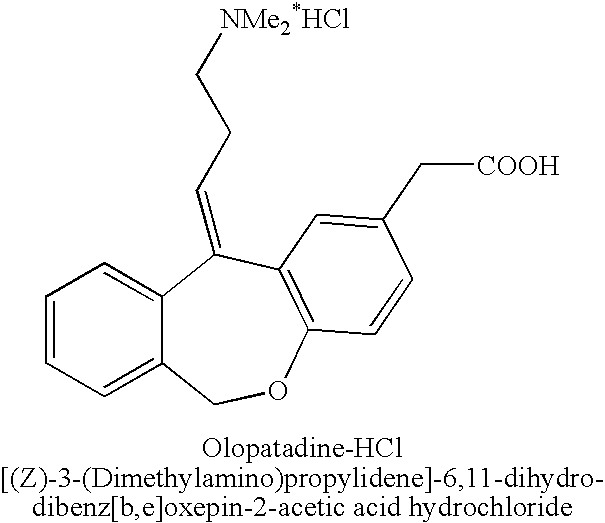
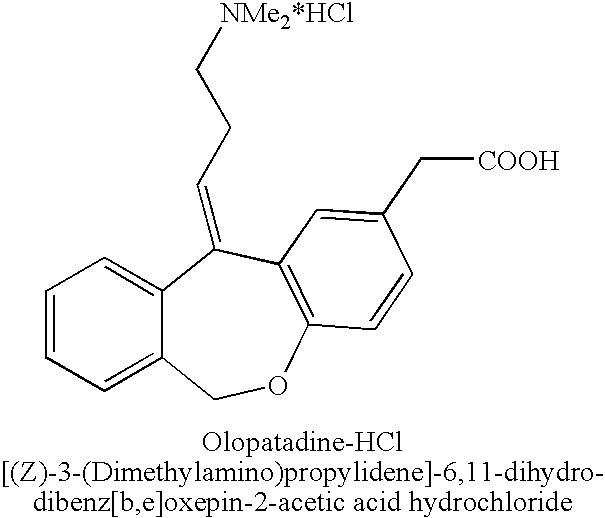
Polymorphic forms of olopatadine hydrochloride and methods for producing olopatadine and salts thereof
ActiveUS20070232814A1Senses disorderGroup 5/15 element organic compoundsHydrobromideSeasonal allergic conjunctivitis
The present invention provides a novel polymorphic form of olopatadine hydrochloride ([(Z)-3-(dimethylamino)propylidene]-6,11-dihydrodibenz[b,e]oxepin-2-acetic acid hydrochloride), a selective histamine H1-receptor antagonist that is used for the treatment of ocular symptoms of seasonal allergic conjunctivitis. The present invention also provides novel methods for producing olopatadine on a large scale, and in a manner that is cost effective, provides a low level of impurities and eliminates the need to use the costly and dangerous base, butyllithium, which is used in prior art reactions for making olopatadine. The present invention further provides novel processes for carrying out a large scale production of 3-dimethylaminopropyltriphenylphosphonium bromide and its corresponding hydrobromide salt, which are employed in the production of olopatadine, and pharmaceutically acceptable salts of olopatadine.
Owner:UNIV ZURICH
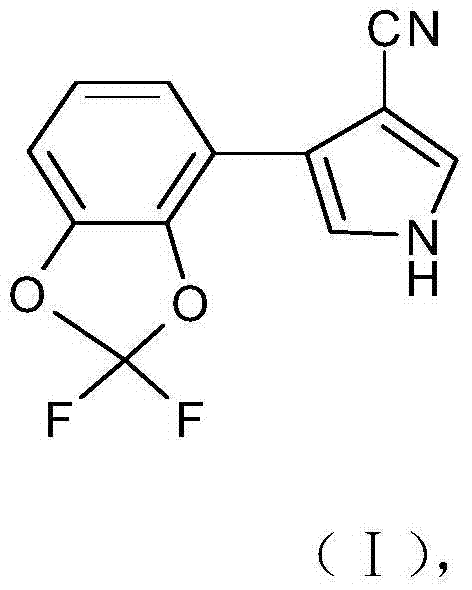
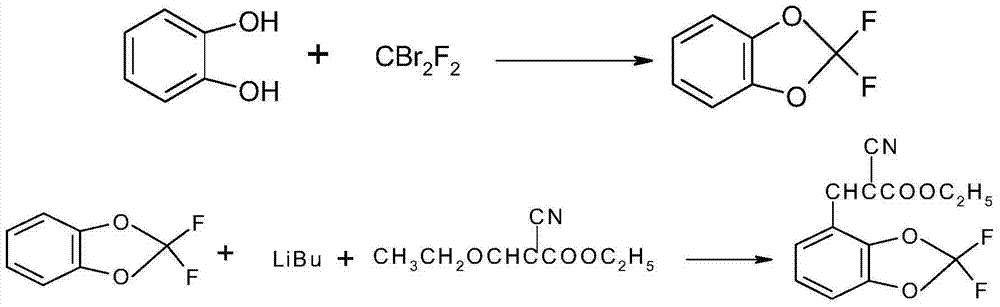
Synthetic method of 4-(2,2-difluoro-1,3-benzodioxole-4-yl)pyrrole-3-nitrile
The invention discloses a synthetic method of 4-(2,2-difluoro-1,3-benzodioxole-4-yl)pyrrole-3-nitrile. The method comprises the following steps of: preparing an intermediate 2,2-difluorobenz-1,3-dioxole through reacting catechol with dibromodifluoromethane, and preparing an intermediate 2-cyano-3-(2,2-difluorobenz-1,3-dioxole-4-yl)-2-acrylate. The fludioxonil prepared by the synthetic method provided by the invention has the purity of more than 99.0% and the total yield of more than 45.0%; the synthetic method has the advantages of cheap and easily available raw materials, simple process, high product yield in each step, good purity, low production cost, applicability to batch industrial production and the like.
Owner:XIAN MODERN CHEM RES INST
Triphenyl phosphorus oxide-based thermal excitation delayed fluorescent blue light guest material and its preparation method and use
ActiveCN105924472ABalance injectionBalanced transmissionGroup 5/15 element organic compoundsSolid-state devicesElectron donorTriplet state
The invention relates to a fluorescent blue light guest material and its preparation method and use and especially relates to a triphenyl phosphorus oxide-based thermal excitation delayed fluorescent blue light guest material and its preparation method and use. The triphenyl phosphorus oxide-based thermal excitation delayed fluorescent blue light guest material solves the problem that because of large difference of an electron donor and an acceptor, when the electron donor is increased, guest emission wavelength produces red shift so that stable and efficient blue guest luminescence cannot be realized. The blue light guest material has a structural formula shown in the description. The preparation method comprises 1, preparing PXZPhBr, PXZPhBr, PTZPhBr and DMACPhBr, 2, mixing the products obtained through the step 1 and tetrahydrofuran, adding n-butyllithium and diphenylphosphinous chloride into the mixture, adding hydrogen peroxide into the mixture, carrying out stirring and carrying out chromatography and recrystallization. The triphenyl phosphorus oxide-based thermal excitation delayed fluorescent blue light guest material is used for preparation of a thermal excitation delayed fluorescent electroluminescent device. The material utilizes short-axis modification strategy, effectively keeps a matrix triplet state energy level, has a donor-acceptor (D-A)-type molecular structure and balances carrier injection and transmission. The preparation method belongs to the field of fluorescent material preparation.
Owner:HEILONGJIANG UNIV
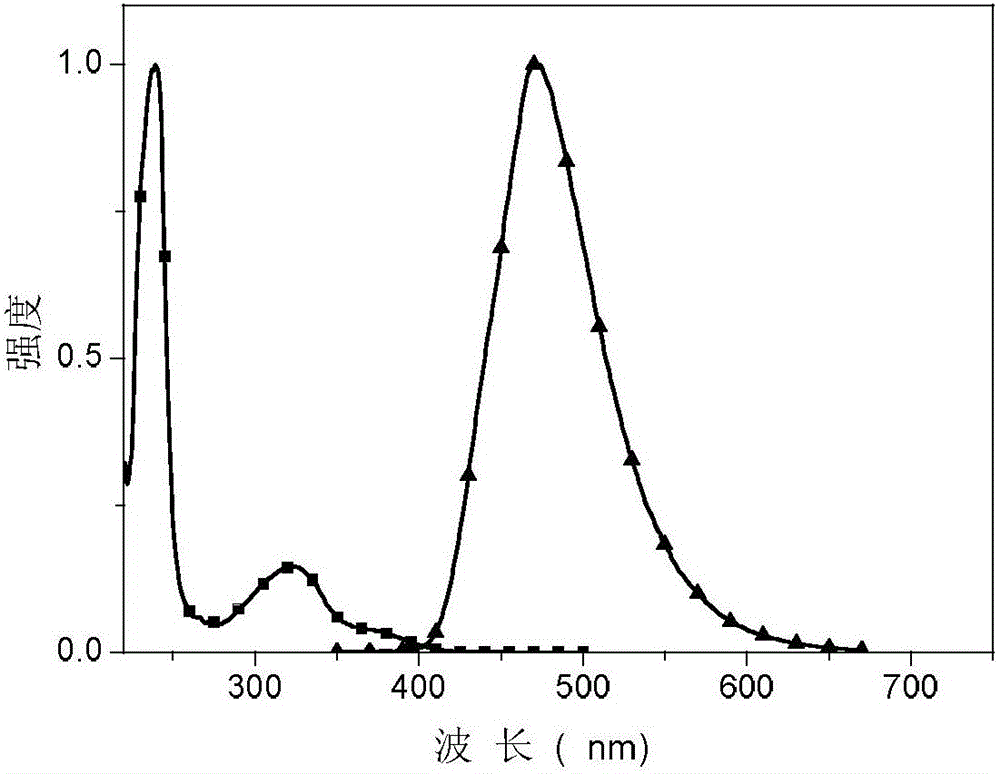
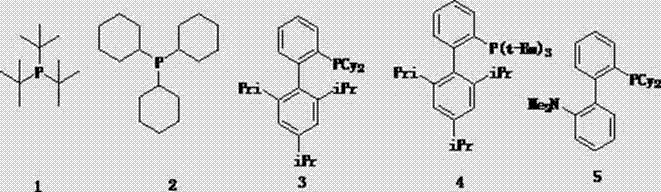
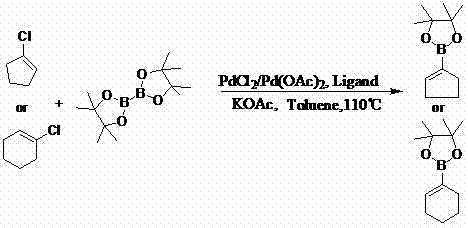
Method for preparing cyclopentene/cyclohexene-1-boronic acid pinacol ester
InactiveCN103044469AReduce dosageLow costGroup 3/13 element organic compoundsCyclopentenePtru catalyst
The invention discloses a method for preparing cyclopentene / cyclohexene-1-boronic acid pinacol ester. The method comprises the step of carrying out coupling reaction on 1-chlorine-cyclopentene / cyclohexene serving as a raw material in an organic solvent A in the presence of monophosphate ligand and a palladium catalyst based on potassium acetate as alkali so as to prepare the cyclopentene / cyclohexene-1-boronic acid pinacol ester. The method is characterized in that the physicochemical reaction at the n-butyllithium and ultralow temperature condition in the literature is avoided, and the palladium catalyst is adopted for catalyzing, so that the amount of the used catalyst is reduced, and the cyclopentene / cyclohexene-1-boronic acid pinacol ester can be prepared in relatively high yield at the acceptable temperature; the boronizing agent n-butyllithium / isopropoxy boronic acid pinacol ester system is replaced by the boronizing agent palladium catalyst / bis(pinacolato)diboron system, and therefore, the cost of the raw materials is greatly reduced, the reaction condition is relatively easy to realize, amplification is easy, and the technical operation is simpler and more convenient.
Owner:DALIAN NETCHEM CHIRAL TECH
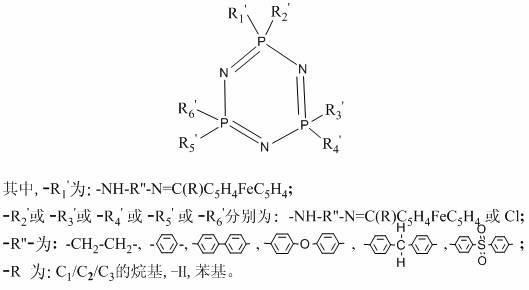
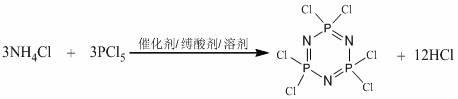
Monoacyl ferrocene Schiff base aminocyclotriphosphazene and its synthesis method
InactiveCN102276659ASpecial performanceLow costGroup 5/15 element organic compoundsMetallocenesPtru catalystAcyl group
The invention relates to a monoacyl ferrocene Schiff base aminocyclotriphosphazene and a synthesis method thereof. The structural formula of the compound is: the present invention makes the prepared monoacyl ferrocene Schiff base amino cyclotriphosphazene have the advantages of ferrocene group and cyclotriphosphazene matrix at the same time, and shows special performance. It has a wide range of application values in chemistry, medicine, catalyst, lubrication and other fields. Compared with the prior art, the method for introducing the ferrocene group into the cyclotriphosphazene matrix of the present invention avoids highly active reagents such as butyllithium, can be operated at normal temperature, and has a simple, safe and low-cost method. features.
Owner:SHANGHAI UNIV
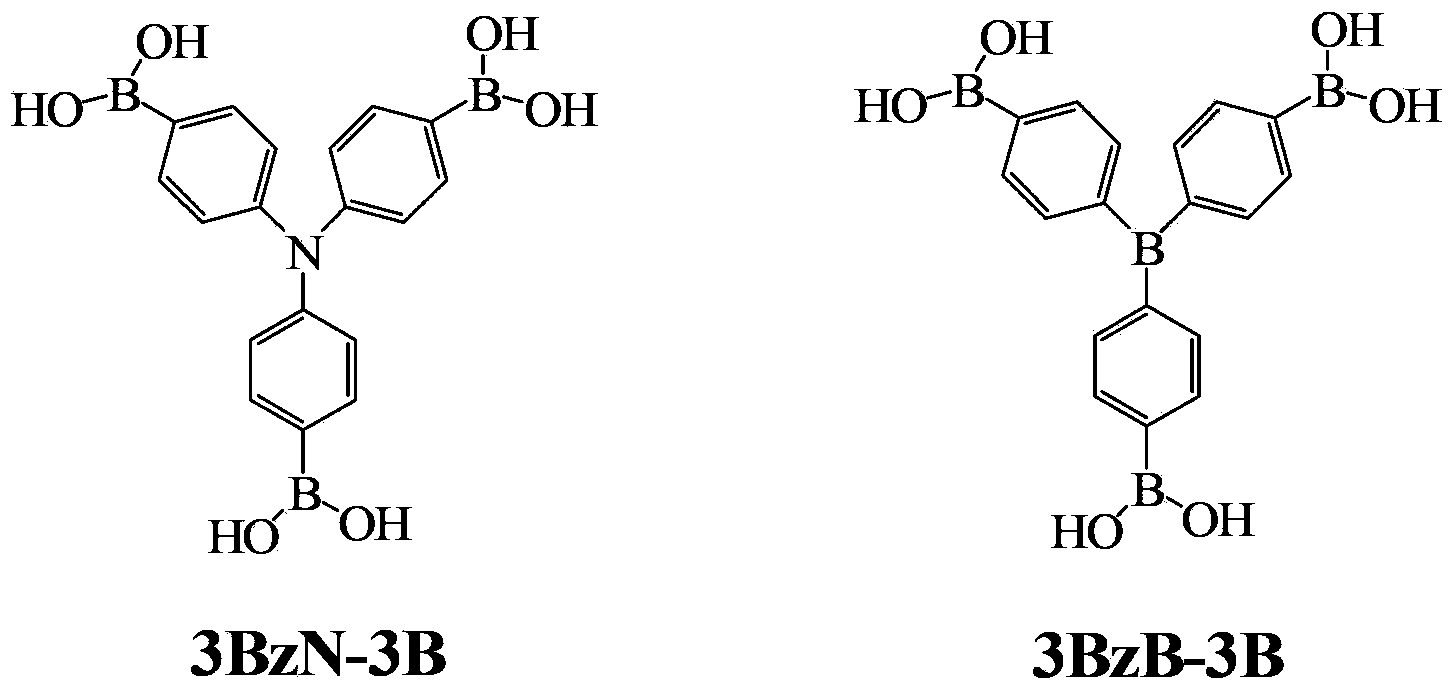
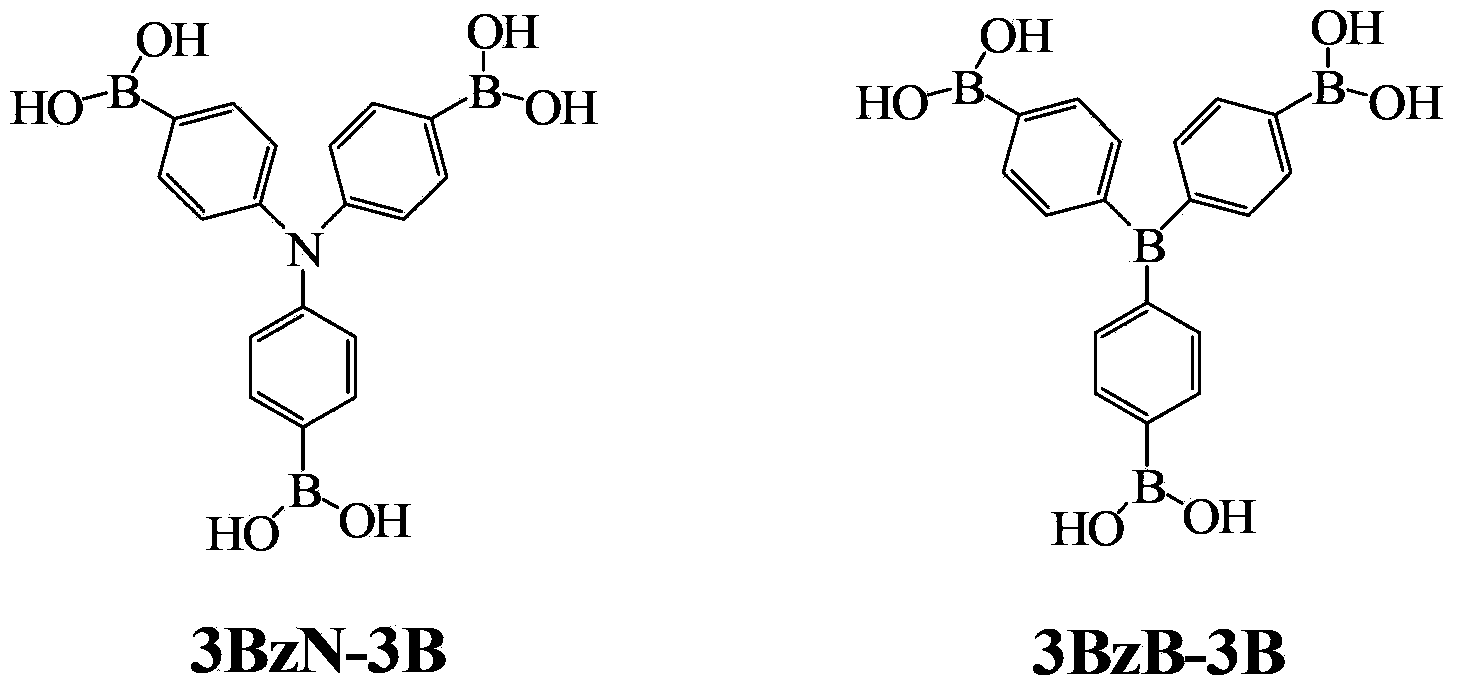
Arylboronic acid derivatives and preparation method thereof
ActiveCN103374024AStop burningTo solve the toxicGroup 3/13 element organic compoundsArylN-Butyllithium
The invention provides arylboronic acid derivatives and a preparation method thereof. The two arylboronic acid derivatives are tri(4,4',4'-triboric acid-phenyl)amino(3BzN-3B) and tri(4,4',4'-triboric acid-phenyl)boron(3BzB-3B), and the molecular structures are as shown in the specification. 3BzN-3B and 3BzB-3B are prepared by firstly, respectively reacting tri(4,4',4'-triboric acid-phenyl)amino and tri(4,4',4'-triboric acid-phenyl)boron with n-butyllithium, secondly, respectively carrying out nucleophilic substitution reaction with boric acid ester, and finally carrying out acidolysis by using diluted hydrochloric acid. The products of the arylboronic acid derivatives, namely, the 3BzN-3B and the 3BzB-3B are expected to be excellent environment-friendly organic fire retardants.
Owner:SOUTH CHINA UNIV OF TECH
Thermal excitation delayed fluorescence main material based on phosphonic aryl derivatives, preparation method and application thereof
ActiveCN106831874AImprove efficiencyImproving Electron Injection Transport CapabilityGroup 5/15 element organic compoundsSolid-state devicesChlorodiphenylphosphinePhenyl group
The invention provides a thermal excitation delayed fluorescence main material based on phosphonic aryl derivatives, a preparation method and an application thereof and relates to the thermal excitation delayed fluorescence main material, the preparation method and the application thereof. The invention aims to solve the technical problem of low device efficiency caused by the easiness in quenching of the molecules of the present planar thermal excitation delayed fluorescence dye. The structural formula of the thermal excitation delayed fluorescence main material provided by the invention is as shown in the specification. The preparation method comprises the following steps: adding an o-dibromo-benzene derivative, dichlorophenyl phosphine and n-butyllithium into tetrahydrofuran and reacting; extracting and drying, and then directly purifying or vulcanizing / and oxidizing and then re-purifying, thereby acquiring the product; or adding o-dibromo-benzene derivative, dichlorophenyl phosphine and n-butyllithium into tetrahydrofuran and reacting and then reacting with chlorodiphenylphosphine, re-oxidizing and purifying, thereby acquiring the product. The thermal excitation delayed fluorescence main material based on phosphonic aryl derivatives is applied to a thermal excitation delayed fluorescence electrofluorescence device.
Owner:HEILONGJIANG UNIV
Furanocoumarin-Tr*ger's Base derivative as well as synthesis method and application thereof
ActiveCN110551145AMild reaction conditionsShort reaction timeOrganic chemistryFluorescence/phosphorescenceFluorescenceSynthesis methods
The invention provides a furanocoumarin-Base derivative, wherein the structural formula of the derivative is as shown in the following formula 6 or 7; the derivative is synthesized by performing ringformation and coupling reaction on parabromoaniline,paraformaldehyde, 4-hydroxycoumarin, isonitrile and n-butyllithium; and the synthesis process is mild in reaction condition, short in reaction timeand high in yield. The Base derivative containing a coumarin fragment has excellent luminescent property and high bioactivity; some of the products have anti-tumor activity, show high-selectivity inhibition on human triple negative breast cancer cells (MDA-MB-231) and have research value of being further developed into anti-tumor medicines; and the other products can be applied to synchronous detection on neuroblastoma metabolites homovanillic acid (HVA) and vanilmandelic acid (VMA) and have the potential of being developed into a high-efficiency fluorescent probe for human neuroblastoma earlywarning and definite diagnosis.
Owner:XUZHOU NORMAL UNIVERSITY
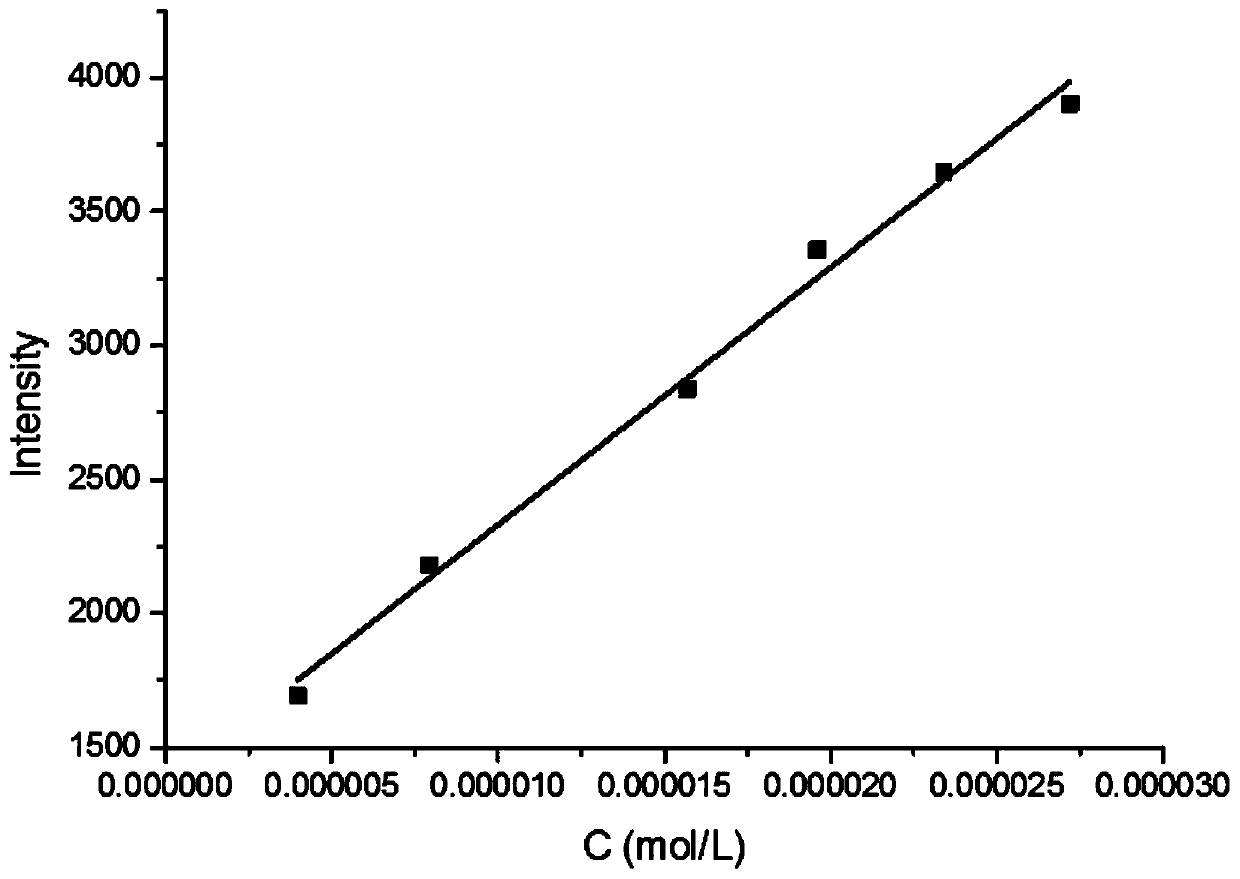
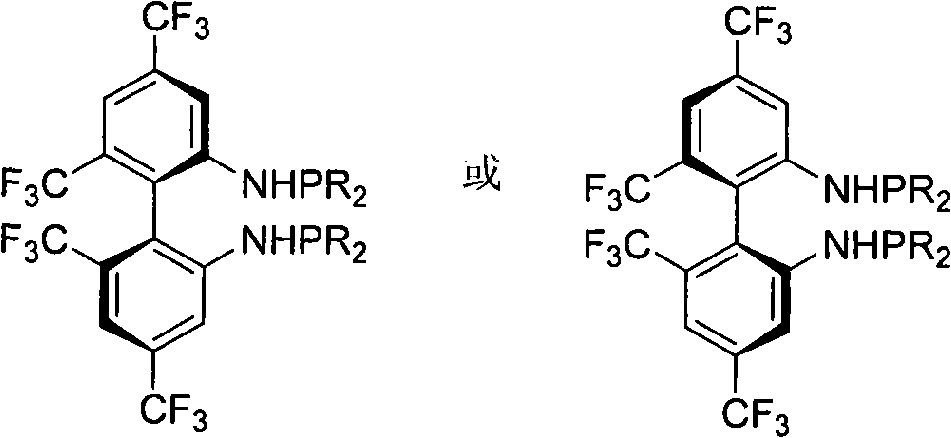
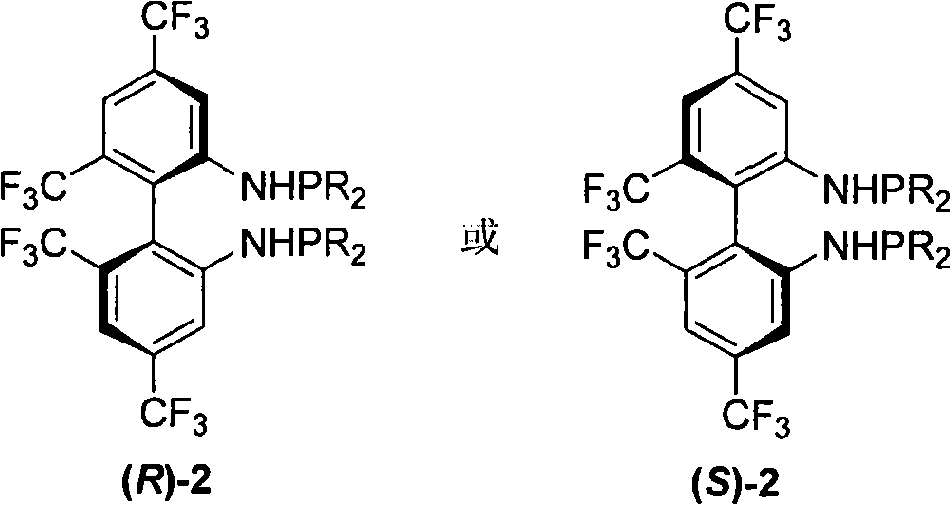
Phosphoramidite type diphosphine ligand, preparation method and application thereof
InactiveCN101333230AEasy to synthesizeOrganic compound preparationGroup 5/15 element organic compoundsEnantiomerDiphosphines
The invention provides a novel pure enantiomer amide type phosphinidene bidentate phosphine ligand with the structural formula shown on the right, wherein, the substituent R on the phosphorus atom of the bidentate phosphine ligand is alkyl or aryl. The synthetic methodsynthesis method is as follows: 4,4',6,6' -quadra (trifluoromethyl) biphenyl -2,2'- diamine is lithiated with n-butyllithium at -40 DEG C to 0 DEG C; then the lithiated 4,4',6,6' -quadra (trifluoromethyl) biphenyl-2,2'-diamine is reacted with dialkyl phosphonium chloride or diaryl phosphonium chloride, and heated naturally to room temperature and reacted for 12-24 hours to get the bidentate phosphine ligand through column chromatography. The invention takes chiral 4,4',6,6'-quadra(trifluoromethyl) - biphenyl -2,2'- diamine and various dialkyl phosphine chlorides or diphenyl(or substituted diphenyl group) phosphine chlorides as raw materials to prepare the amide type phosphinidene bidentate phosphine ligand. The invention enables the pure enantiomer amide type phosphinidene bidentate phosphine ligand to be applied to Rh-catalyzed ene amide and asymmetric hydrogenation of alpha-dehydro amino acid esters and achieves very good results, with the product enantioselectivity up to 99%.
Owner:WUHAN UNIV
Thermal excitation delayed fluorescence main material based on phosphonic aryl derivatives, preparation method and application thereof
ActiveCN106831875AImproving Electron Injection Transport CapabilityGood carrier transport abilityGroup 5/15 element organic compoundsSolid-state devicesDichloromethaneChemistry
The invention provides a thermal excitation delayed fluorescence main material based on phosphonic aryl derivatives, a preparation method and an application thereof and relates to the thermal excitation delayed fluorescence main material, the preparation method and the application thereof. The invention aims to solve the technical problem of low device efficiency caused by the easiness in quenching of the molecules of the present planar thermal excitation delayed fluorescence dye. The structural formula of the thermal excitation delayed fluorescence main material provided by the invention is as follows. The preparation method comprises the following steps: adding an o-dibromo aryl compound, dichlorophenyl phosphine and n-butyllithium into tetrahydrofuran; uniformly mixing and then reacting under argon protection; pouring into water; extracting with dichloromethane, thereby acquiring an organic layer; after drying, directly purifying or vulcanizing / and oxidizing and then re-purifying, thereby acquiring the thermal excitation delayed fluorescence main material based on phosphonic aryl derivatives, wherein the o-dibromo aryl compound is o-dibromo-benzene or 2,3-dibromo naphthalene. The thermal excitation delayed fluorescence main material based on phosphonic aryl derivatives is applied to a thermal excitation delayed fluorescence electrofluorescence device.
Owner:HEILONGJIANG UNIV
Method for regulating and controlling structure of o-carborane derivative through preoccupation
The invention discloses a method for regulating and controlling the structure of an o-carborane derivative through preoccupation. The method comprises the following steps: (1) subjecting o-carborane to lithiation to form a monolithium salt and then reacting the monolithium salt with a CH group protecting agent so as to quantitatively convert the o-carborane into C-H protected carborane; (2) activating C-H by using butyllithium and then carrying out functionalization of a second carbon position; and (3) carrying out deprotection by using tetrabutyl ammonium fluoride. According to the invention, the CH group protecting agent is introduced at first, then the group is subjected to functionalization, and finally deprotection of the group is carried out, so the structure of the o-carborane derivative is regulated and controlled through preoccupation; and thus, a monosubstituted or disubstituted o-carborane derivative is purposefully and selectively synthesized. The obtained monosubstituted or disubstituted o-carborane derivative can be applied to field of aerospace materials like high-temperature-resistant bonding materials and composite materials and has great application prospect in the fields like biological medicines, catalysts, supramolecular systems and high energy fuels.
Owner:HARBIN INST OF TECH WUXI RES INST OF NEW MATERIALS +1
Method for preparing ionic liquid/transition metal disulphide gas separating membrane and application thereof
InactiveCN106902648AImprove separation performanceImprove stabilitySemi-permeable membranesDispersed particle separationSolubilityGas separation
The invention discloses a method for preparing an ionic liquid / transition metal disulphide gas separating membrane and an application thereof. The method comprises the following steps: 1 ) transition metal disulphide powder MS2 (M means corresponding transition metal) and n-butyllithium are mixed to obtain the lithium intercalated transition metal disulphide powder LixMS2; 2) adding the LixMS2 into ionic liquid for ultrasonic mixing to obtain the MS2 ionic liquid dispersion liquid; and 3) performing vacuum pumping filtration on the dispersion liquid to obtain the required ionic liquid / transition metal disulphide gas separating membrane which is used for gas separation. The LixMS2 is interacted with ionic liquid during an ultrasonic process to form a MS2 two-dimensional layered material, the ionic liquid is subjected to in-situ introduction to the intermediate of a two-dimensional sheet layer; LiBF4 generated in ultrasound can increase the CO2 separating performance of a composite film; and ionic liquid confined effect and difference of different gas solubility are used for realizing the gas separating. The prepared gas separating membrane has high application value as well as concise and efficient preparation process.
Owner:ZHEJIANG UNIV
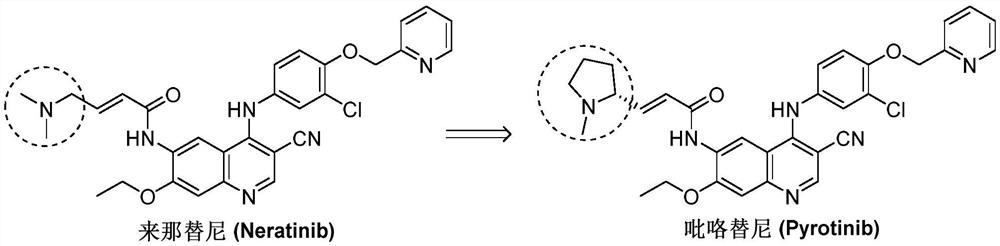
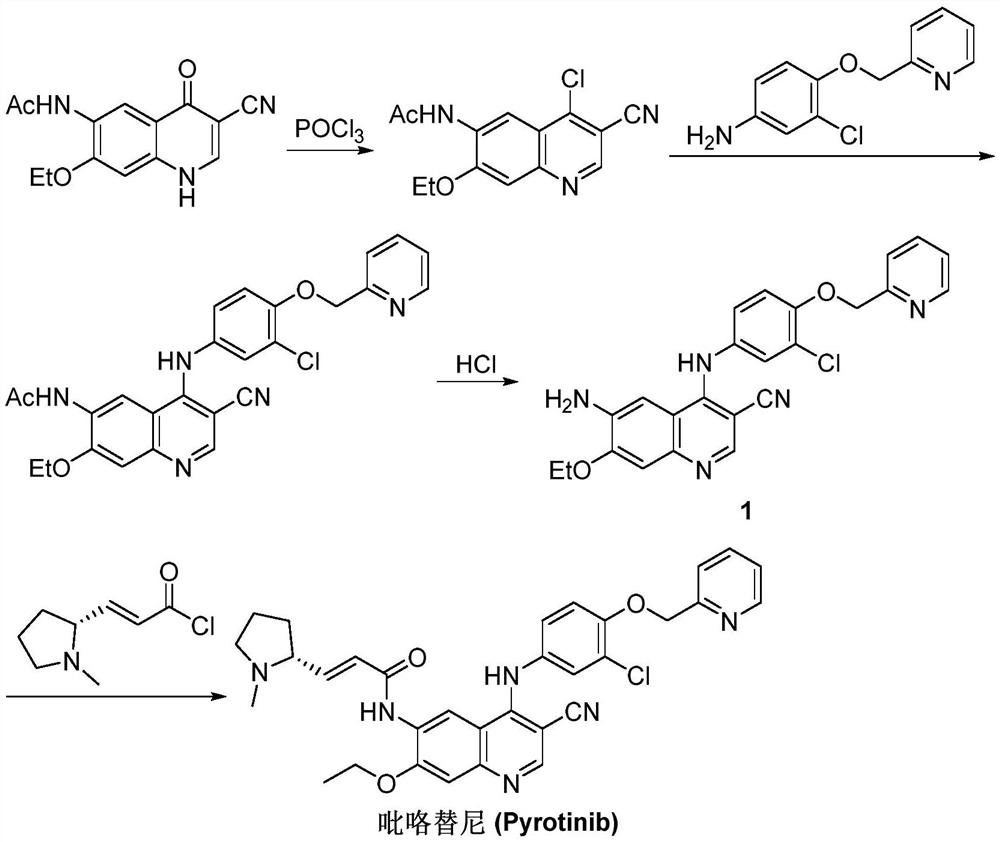
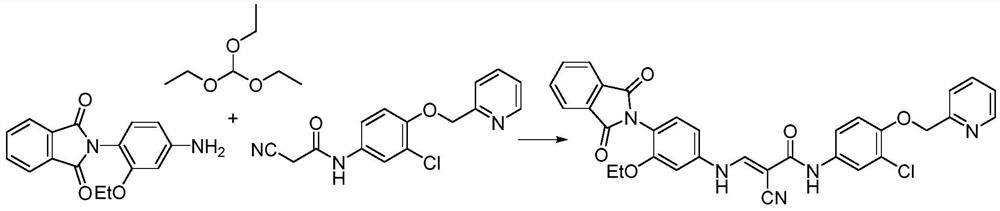
Synthesis method of pyrotinib
ActiveCN112110899ARaw materials are easy to getSimple processOrganic chemistry methodsChemical synthesisMeth-
The invention discloses a synthesis method of pyrotinib, which belongs to the technical field of chemical synthesis of medicines. The preparation method comprises the following steps of firstly, reacting 2-[(4-bromo-2-chlorophenoxy)methyl]pyridine with n-butyllithium at a low temperature, and then carrying out a boronation reaction with an organic boron reagent to obtain [3-chloro-4-(pyridine-2-ylmethoxy)phenyl]boric acid, enabling (2E)-N-(4-amino-3-cyano-7-ethoxy quinoline-6-yl)-3-[(2R)-1-methyl pyrrolidine-2-yl] acrylamide and the obtained [3-chloro-4-(pyridine-2-yl methoxy)phenyl]boric acid to be subjected to a catalytic coupling reaction in a system of a copper catalyst, a base catalyst and a solvent, and acquiring pyrotinib. The method has the characteristics of readily available rawmaterials, simple process, economy, environmental protection and the like, is beneficial to quality control and improvement of final product raw material medicines, is suitable for industrial production and is beneficial to promoting cheap development of the raw material medicines.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
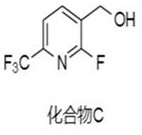
Synthesis method of (2-fluoro-6-(trifluoromethyl) pyridine-3-yl) methanol
Owner:阿里生物新材料(常州)有限公司
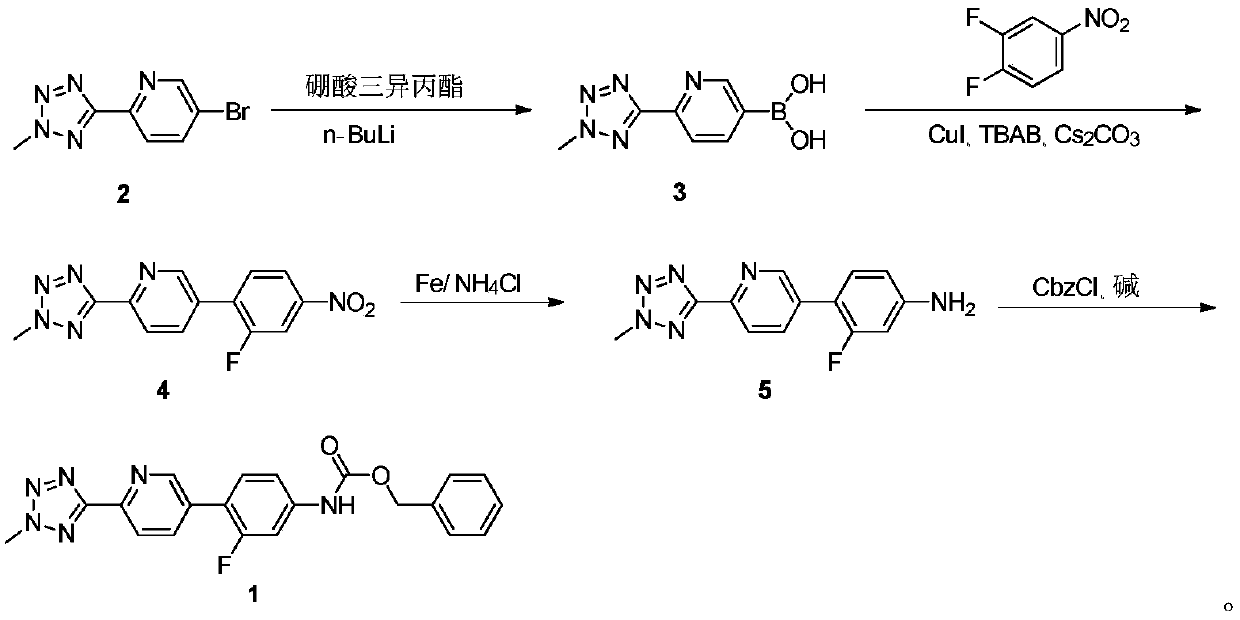
Preparation method of oxazolidinone antibiotic intermediate
ActiveCN110938058ARaw materials are easy to getSimple processOrganic chemistryBiotechnologyCarbamate
The invention provides a preparation method of a tedizolid phosphate intermediate compound 3-fluoro-4-(6-(2-methyltetrazole-5-yl)pyridine-3-yl)phenyl benzyl carbamate. The method comprises the following steps: carrying out a reaction on 5-bromo-2-(2-methyltetrazole-5-yl)pyridine as a raw material and triisopropyl borate under the action of n-butyllithium to prepare a boric acid intermediate, coupling the boric acid intermediate and 1,2-difluoro-4-nitrobenzene, and finally performing nitro reduction and amidation to obtain the required target product. Compared with the method in the prior art,the preparation method disclosed by the invention has the advantages of easily available raw materials, simple process, economy, environmental protection and suitability for industrial production.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES +3
Fluorescent nanoprobe, preparation method thereof and application thereof in biosensor
ActiveCN106908424AEasy to prepareLow toxicityFluorescence/phosphorescenceFluorescenceHigh selectivity
The invention relates to a preparation method of a fluorescent nanoprobe based on a two-dimensional lanthanum metal organic skeleton MOF-La, a prepared probe material and application of the prepared probe in a biosensor. The preparation method comprises the following preparation steps: (1) in an alkali solution, adding 2,2'-thiodiacetic acid or 2,2'-thiodiacetin, La<3+> lanthanum ion inorganic salt, and reacting for 10 to 30 h at the temperature of 120 to 180 DEG C; adding an obtained crystal into an organic solvent, carrying out ultrasonic treatment, performing centrifugation, dispersing into an organic solution of n-butyllithium, and reacting for 15 to 25 h at the temperature of 20 to 30 DEG C under the protection of inert gas, so as to obtain a two-dimensional MOF-La nanomaterial; dispersing the two-dimensional MOF-La nanomaterial in a buffer solution, adding fluorescently-labeled nucleotide chains, and performing centrifugation after reaction at room temperature to obtain the material. The preparation method is simple in preparation conditions, convenient in operation and low in cell toxicity, and the obtained probe has the characteristics of high selectivity and high sensitivity, so that a false positive result is avoided, qualitative performance and quantitative performance are more accurate, and the probe has a good application prospect.
Owner:NANJING UNIV
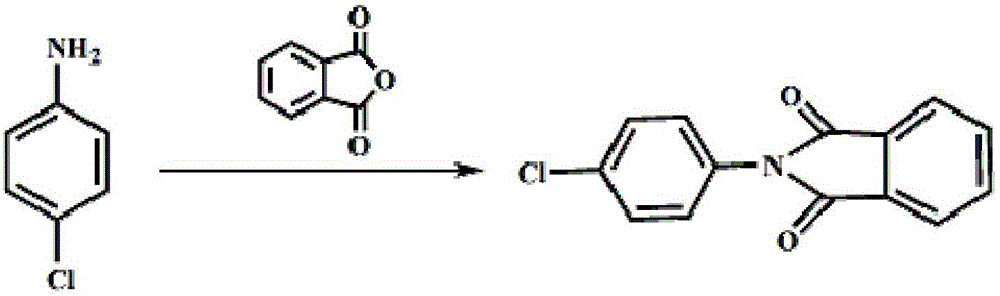
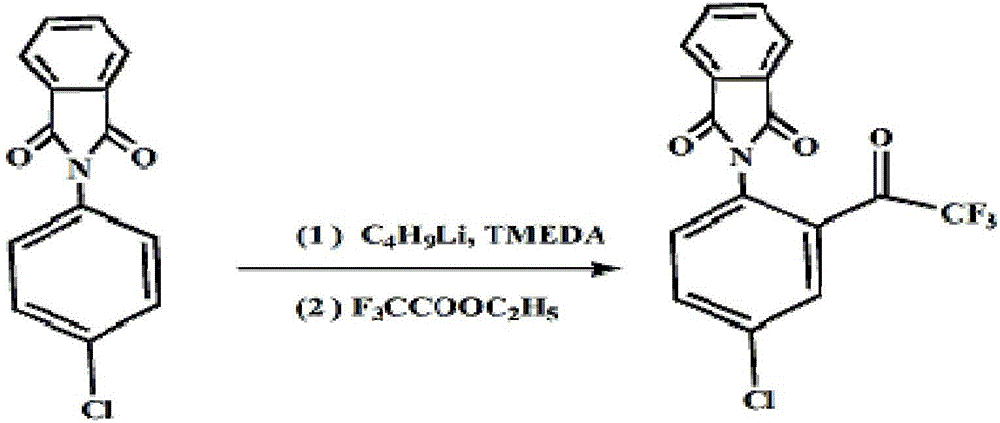
Method for preparing 4-chlorine-2-(trifluoroacetyl) aniline hydrochloride hydrate
ActiveCN105777610ALow costImprove product qualityOrganic compound preparationAmino-hyroxy compound preparationHydrazine compoundAniline
The invention discloses a method for preparing a 4-chlorine-2-(trifluoroacetyl) aniline hydrochloride hydrate, and belongs to the field of compound synthesis. P-chloroaniline serves as a raw material, the method includes the following steps that 1, the p-chloroaniline and acid anhydride are reacted to obtain 4-chlorine-N-phthaloyl aniline; 2, the 4-chlorine-N-phthaloyl aniline and trifluoroacetic acid ethyl ester are reacted under the effect of butyl lithium to obtain N-(4-chlorine-2 trifluoroacetyl phenyl)-phthalic diamide; 3, after the N-(4-chlorine-2 trifluoroacetyl phenyl)-phthalic diamide and hydrazine hydrate are heated and subjected to reflux in absolute ethyl alcohol, hydrochloric acid and glacial acetic acid are added, and the 4-chlorine-2-(trifluoroacetyl) aniline hydrochloride hydrate is obtained. The method is easy to operate, low in cost and suitable for industrial production, and the total yield is up to 82.2%.
Owner:SHAXING CHEM TAIZHOU CITY
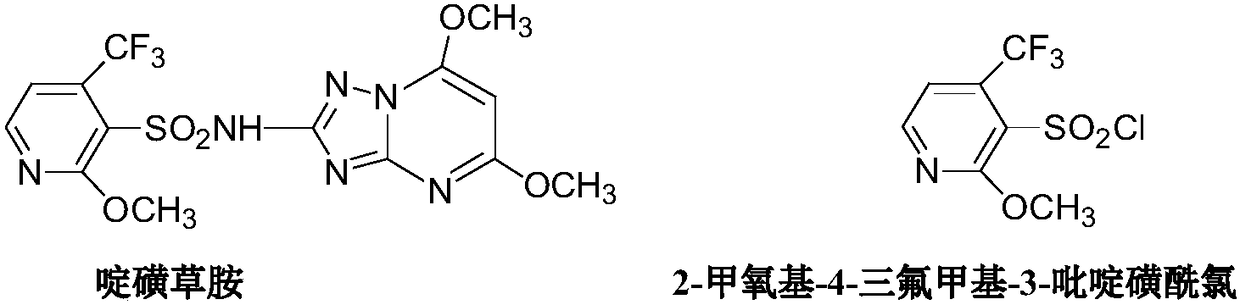
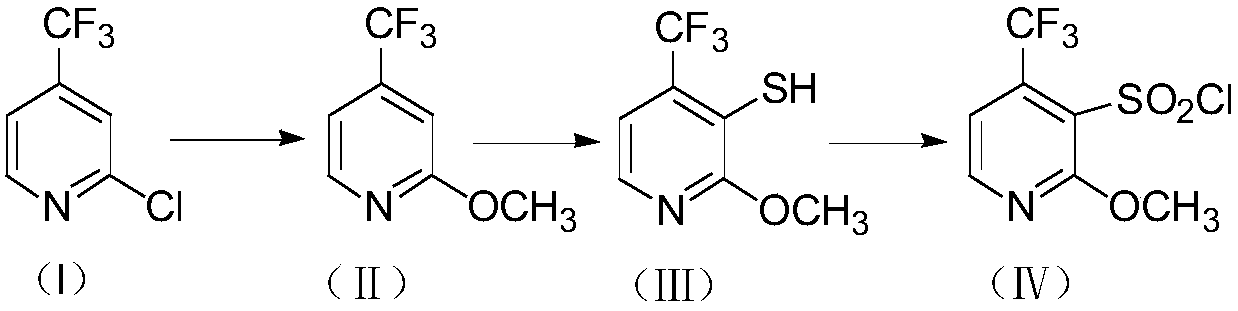
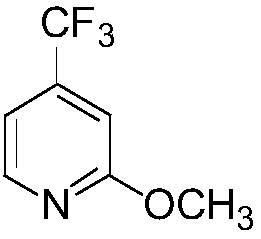
Method for preparing 2-methoxy-4-trifluoromethyl-3-pyridinesulfonyl chloride
The invention provides a method for preparing 2-methoxy-4-trifluoromethyl-3-pyridinesulfonyl chloride, and belongs to the field of fine chemical engineering. The method comprises the following steps:2-chloro-4-trifluoromethylpyridine used as a starting material is methoxylated to obtain a 2-methoxy-4-trifluoromethyl-3-pyridine compound, the 2-methoxy-4-trifluoromethyl-3-pyridine compound reacts with sulfur powder under the action of butyllithium to prepare a 2-methoxy-3-mercapto-4-trifluoromethyl-3-pyridine compound, and the 2-methoxy-3-mercapto-4-trifluoromethyl-3-pyridine compound is chlorinated and oxidized to obtain the final product 2-methoxy-4-trifluoromethyl-3-pyridinesulfonyl chloride compound. The sulfur powder is used the preparation process of compound III, and thioether compounds are avoided, so odorous odor brought in the production process is eliminated, and environmental pollution is reduced. Hydrogen peroxide is adopted to carry out oxidation, thionyl chloride is adopted to carry out chlorination, and chlorine is avoided, so dangers in the preparation and synthesis process are effectively reduced, and the safety production coefficient is improved.
Owner:CHANGZHOU UNIV
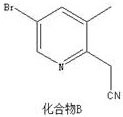
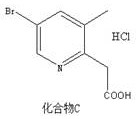
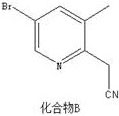
Preparation method of 2-(5-bromo-3-methylpyridine-2-yl) acetic acid hydrochloride
The invention belongs to the technical field of medical intermediates, and particularly relates to a preparation method of 2-(5-bromine- 3-methylpyridine-2-yl) acetic acid hydrochloride. According tothe preparation method, n-butyllithium and acetonitrile are subjected to a reaction so as to increase protection of bromine in a compound A, and then the compound A is added for a reaction so as to form a compound B so that the yield and the purity of the product are improved, side reactions are avoided, and the compound B and concentrated hydrochloric acid are subjected to a reaction, and the 2-(5-bromine-3-methyl pyridine-2-yl) acetic acid hydrochloride with high purity and high yield is obtained; the synthetic route of the 2-(5-bromine-3-methyl pyridine-2-yl) acetic acid hydrochloride is short, the design is reasonable, the operation is simple, the control is easy, and the product yield is higher.
Owner:埃法姆药物研发(宁夏)有限公司
Integrated rubber SIBR (Styrene Isoprene Butadiene Rubber) and preparation method thereof
The invention relates to an integrated rubber SIBR (Styrene Isoprene Butadiene Rubber) and a preparation method thereof. The integrated rubber SIBR is prepared from 10-30 parts by weight of styrene, 20-60 parts by weight of isoprene, 10-30 parts by weight of butadiene, 0.1-1 part by weight of cyclohexane and 0.1-1 part by weight of n-butyllithium. By adopting a method of once charging and reaction extruding, the content of entire alkadiene is increased to above 75 percent; and mechanical properties of tensile strength, bending strength, impact strength, tearing strength and the like of the prepared integrated rubber SIBR are superior to those of an existing product, and important research significance and application value are obtained.
Owner:HEFEI GENIUS NEW MATERIALS
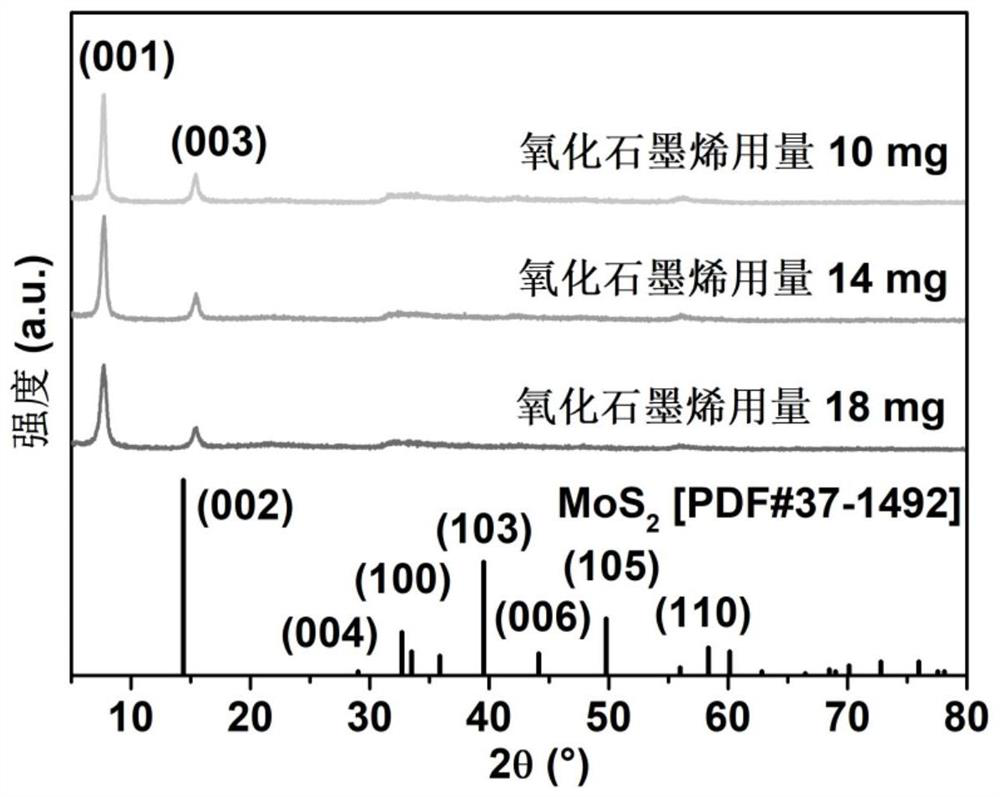
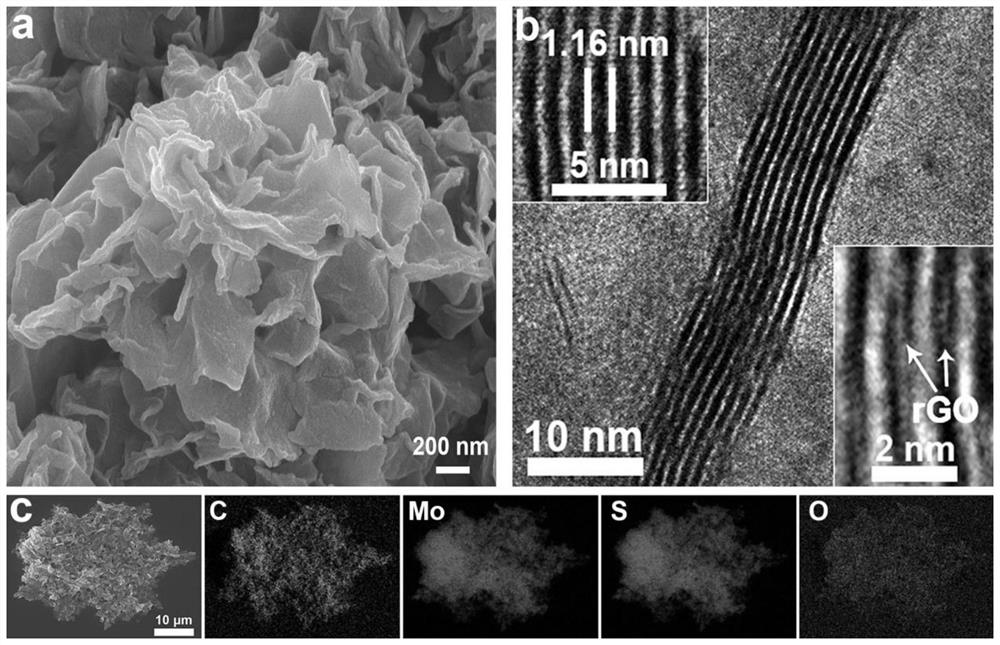
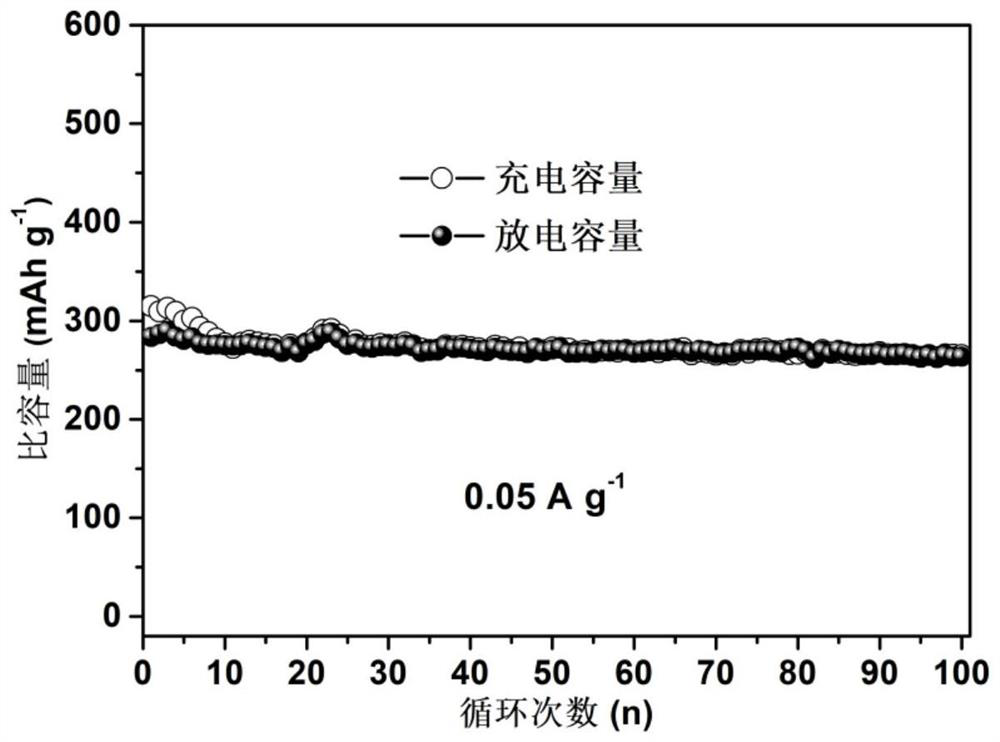
Preparation and application method of graphene intercalated molybdenum disulfide composite material
ActiveCN112875754AEasy to controlImprove hydrophilicityMaterial nanotechnologyCell electrodesFreeze-dryingActive agent
The invention discloses a preparation and application method of a graphene intercalated molybdenum disulfide composite material. The preparation method comprises the following steps: soaking commercial molybdenum disulfide in an n-butyllithium solution, stirring in an argon environment, cleaning, drying to obtain lithium molybdenum sulfide, adding water, and violently reacting to obtain a stripped molybdenum disulfide dispersion liquid A; adding a cationic surface active agent and a single-layer graphene oxide dispersion liquid into the solution A, wherein the molybdenum disulfide layer and the graphene oxide layer are mutually adsorbed; adding a reducing agent, carrying out hydro-thermal treatment, reducing the graphene oxide into graphene, and embedding the graphene into molybdenum disulfide layers; and washing and freeze-drying to obtain the graphene intercalated molybdenum disulfide composite material. The method has the advantages that repeatability is good, and reaction conditions are easy to control. The obtained material is a micron flower assembled by composite nanosheets with a sandwich structure, wherein the thickness of the nanosheets is 10-20 nm, the interlayer spacing of molybdenum disulfide is expanded to 1.16 nm, the hydrophilicity is good, rapid and stable deintercalation of hydrated zinc ions is facilitated, and the zinc storage capacity, the rate capability and the cycling stability are remarkably improved.
Owner:UNIV OF SCI & TECH BEIJING
Application of n-butyllithium in catalyzing cyanosilylation reaction of ketone and silane
PendingCN111303201AMethods for Enriching Synthetic ReactionsSimple structureGroup 4/14 element organic compoundsPtru catalystSilanes
The invention discloses an application of n-butyllithium in catalyzing cyanosilylation reaction of ketone and silane. The n-butyllithium has high catalytic activity, low catalyst dosage and good substrate application range. After n-butyllithium, ketone and silane are mixed, the cyanosilylation reaction is carried out to obtain a cyanohydrin compound. The n-butyllithium reagent disclosed by the invention has the advantages of commercialization, easiness in obtaining, low cost, no need for a solvent, environmental friendliness and the like, has a very good catalytic effect on ketone, and is widein substrate application range. Commercial n-butyllithium is used for efficiently catalyzing the cyanosilylation reaction of ketone by using a relatively low dosage of catalyst.
Owner:SUZHOU UNIV
Synthetic method of 1-methyl 4-pyrazole pinacol ester
InactiveCN102690281AAvoid the disadvantages of being unable to realize industrialized productionSimple processGroup 3/13 element organic compoundsN-ButyllithiumPharmaceutical drug
The invention relates to a synthetic method of 1-methyl 4-pyrazole pinacol ester. The biggest advantage of the invention is that the technology is greatly improved; and the problem that the preparation technology of 1-methyl 4-pyrazole pinacol ester is limited in a laboratory and there is no large-scale production is solved. The technical scheme provided by the invention is as follows: the synthetic method of 1-methyl 4-pyrazole pinacol ester comprises The following steps of: Step 1, performing a reaction between 1-methyl 4-pyrazole and triisopropyl borate under the action of n-butyllithium to obtain an intermediate A; and Step 2, performing a reaction between the intermediate A and pinacol under the action of magnesium sulfate to obtain the target product 1-methyl 4-pyrazole pinacol ester. The obtained 1-methyl 4-pyrazole pinacol ester is an important intermediate commonly-used in pharmaceutical chemistry.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD +2
Application of n-butyllithium in catalyzing cyanosilylation reaction of aldehyde and silane
PendingCN111253427ASimple structureLow costGroup 4/14 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystN-Butyllithium
The invention discloses an application of n-butyllithium in catalyzing cyanosilylation reaction of aldehyde and silane. The n-butyllithium has high catalytic activity, low catalyst dosage and good substrate application range, and after n-butyllithium, aldehyde and silane are mixed, cyanosilylation reaction is carried out to obtain a cyanohydrin compound. The n-butyllithium reagent disclosed by theinvention has the advantages of commercialization, easiness in obtaining, low cost, no solvent, environmental friendliness and the like, has a very good catalytic effect on aldehyde, and is wide in substrate application range. Commercial n-butyllithium can efficiently catalyze the cyanosilylation reaction of aldehyde by using a relatively low catalyst dosage.
Owner:SUZHOU UNIV
Method for preparing formyl phenylboronic acid
ActiveCN105037408AShort synthetic stepsSimple and fast operationGroup 3/13 element organic compoundsPhenylboronic acidBoronic acid
The invention discloses a method for preparing formyl phenylboronic acid, which comprises the following steps: heating halogenated phenylboronic acid in toluene or heptane under reflux for dewatering to form a tripolymer, mixing the tripolymer with dimethylformamide, dropwisely adding n-butyllithium at low temperature to react by a one-pot process, hydrolyzing with hydrochloric acid, and recrystallizing to obtain the formyl phenylboronic acid. The method is simple to operate, avoids the process of separating the intermediate after formyl protection in the conventional technique, has high universality, can obtain favorable yield for ortho-, meta- and para- formyl phenylboronic acids, and is beneficial to scale-up production.
Owner:CANGZHOU PURUI DONGFANG SCI & TECH
Preparation method of hydroxyphenylboronic acid
ActiveCN111072698AEasy to protectEasy to realize industrial scale-upGroup 3/13 element organic compoundsBulk chemical productionGrignard reagentHydrolysis
The invention discloses a preparation method of hydroxyphenylboronic acid, which belongs to the technical field of boric acid synthesis in medical intermediates. The method comprises the following steps: starting from bromophenol, carrying out BOC, trimethylsilyl or benzyl protection, forming a Grignard reagent, reacting with borate, or carrying out one-pot reaction with borate and n-butyllithium,and hydrolyzing to obtain hydroxyphenylboronic acid. According to the invention, cheap and easily available protecting groups are adopted, so that the protecting groups are easy to remove during boronation reaction hydrolysis, industrial amplification is easy to realize, batch production is carried out on the scale of dozens of kilograms, and the process stability is good.
Owner:CANGZHOU PURUI DONGFANG SCI & TECH
Silicon-containing macromolecule cationic photoinitiator and preparation method thereof
The invention synthesizes a silicon-containing macromolecule cationic photoinitiator for gradient polymerization and a preparation method thereof. The method comprises the following steps that hexamethyl cyclotrisiloxane, dimethylchlorosilane and n-butyllithium are added into a reaction kettle to obtain a product 1; the product 1 and methyl methacrylate take a reaction to obtain a product 2; the product 2 and a 6M sodium hydroxide water solution take a reaction to obtain a product 3; a cationic photoinitiator 1187 and a catalyst are added into the product 3 for taking a reaction to obtain a product 4. The silicon-containing macromolecule cationic photoinitiator has the beneficial effects that (1) the synthesized photoinitiator has self floating capability, and forms gradient distribution in a photopolymerization system, and a gradient polymer is obtained after once photopolymerization; (2) the photoinitiator has good solubility in cationic resin or monomers, so that the addition of organic solvents in the photo-curing process is not needed; the photoinitiator can be used for preparing materials with little harm to environment and human bodies; (3) the photoinitiator cannot be easily volatilized; decomposition fragments cannot easily migrate; the compatibility with the system is good.
Owner:BEIJING UNIV OF CHEM TECH
Polymorphic forms of olopatadine hydrochloride and methods for producing olopatadine and salts thereof
The present invention provides a novel polymorphic form of olopatadine hydrochloride ([(Z)-3-(dimethylamino)propylidene]-6,11-dihydrodibenz[b,e]oxepin-2-acetic acid hydrochloride), a selective histamine H1-receptor antagonist that is used for the treatment of ocular symptoms of seasonal allergic conjunctivitis. The present invention also provides novel methods for producing olopatadine on a large scale, and in a manner that is cost effective, provides a low level of impurities and eliminates the need to use the costly and dangerous base, butyllithium, which is used in prior art reactions for making olopatadine. The present invention further provides novel processes for carrying out a large scale production of 3-dimethylaminopropyltriphenylphosphonium bromide and its corresponding hydrobromide salt, which are employed in the production of olopatadine, and pharmaceutically acceptable salts of olopatadine.
Owner:UNIV ZURICH
Method for preparing cyclopropyl boronic acid
ActiveCN105001249AReduce usageEasy to operateGroup 3/13 element organic compoundsOrganic baseBoronic acid
The invention discloses a method for preparing cyclopropyl boronic acid. Cyclopropyl methanoic acid is adopted as a raw material and added into a solution obtained after n-butyllithium reacts with organic alkali at the low temperature, and then a boronizing reagent is added into the mixture; after boronation is finished, acid is added for quenching to obtain 1-carboxyl cyclopropyl boronic acid; the intermediate is added into high-boiling-point solvent and heated until the temperature is 80 DEG C-150 DEG C, after reaction deacidification, methylbenzene is added into the mixed solution for dehydration to form cyclopropyl boronic acid trimer, and the cyclopropyl boronic acid trimer is hydrolyzed to obtain the cyclopropyl boronic acid; the melting point of the obtained cyclopropyl boronic acid is 90 DEG C-95 DEG C, and the HNMR purity of the obtained cyclopropyl boronic acid is over 98%. The method is easy to operate and suitable for industrial scale-up production, and no highly toxic chemical is used in the whole process.
Owner:CANGZHOU PURUI DONGFANG SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com