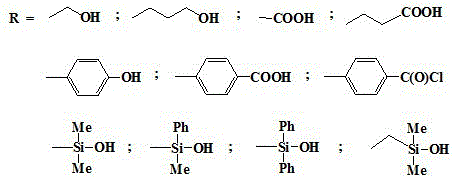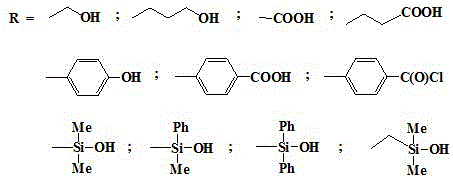Method for regulating and controlling structure of o-carborane derivative through preoccupation
A technology of carborane and derivatives, which is applied in the field of regulating the structure of carborane derivatives, can solve the problems of complex reaction system, increase the difficulty of product separation and purification, and reduce chemical selectivity, and achieve the effect of great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a nitrogen atmosphere, add 851.2mg (5.902mmol) of 1,2-carborane and 20ml of benzene / ether (2:1) mixed solvent to a 100ml three-necked flask respectively, stir, and add n-butyllithium (11.804 mmol) n-hexane 5.50ml solution, continue stirring for 2h, add dimethyl tert-butylchlorosilane (892.8mg, 5.902mmol), continue stirring at 35°C for 2h, add paraformaldehyde 420.3mg (14.01mmol) at room temperature, 3h Finally, it was acidified with 10% hydrochloric acid solution, the solvent was evaporated by rotary evaporation, extracted with ether, the organic layer was washed three times with deionized water and saturated brine, dried over anhydrous magnesium sulfate, and subjected to column chromatography of petroleum ether: ethyl acetate = 1:1 For purification, dissolve it in 15ml of ether solution at -35°C, add 5.902mmol of tetrabutylammonium fluoride, and stir for 60min. After rotary evaporation of the solvent, the final product was obtained after extraction and vacuum drying...
Embodiment 2
[0031] In a nitrogen atmosphere, add 851.2mg (5.902mmol) of 1,2-carborane and 18ml of benzene / ether (2:1) mixed solvent to a 100ml three-necked flask respectively, stir, and add n-butyllithium (11.804 mmol) n-hexane 5.50ml solution, continue stirring for 4h, add triphenylchlorosilane (1.735g, 5.902mmol), continue stirring at 35°C for 4h, add paraformaldehyde 420.3mg (14.01mmol) at room temperature, after 3h, use 10% hydrochloric acid solution was acidified, the solvent was evaporated by rotary evaporation, extracted with ether, the organic layer was washed three times with deionized water and saturated brine, dried over anhydrous magnesium sulfate, purified by petroleum ether: ethyl acetate = 1:1 column chromatography, in Dissolve it in 15ml of ether solution at 0°C, add 5.902mmol of tetrabutylammonium fluoride, and stir for 30min. After rotary evaporation of the solvent, extraction and vacuum drying gave a colorless solid 1-hydroxymethyl-2-H-carborane with a yield of 70%.
...
Embodiment 3
[0034]In a nitrogen atmosphere, add 851.2 mg (5.902 mmol) of 1,2-carborane and 15 ml of a mixed solvent of benzene / ether (2:1) to a 100 ml three-necked flask, stir, and add n-butyllithium (11.804 mmol) n-hexane 5.50ml solution, continue stirring for 3h, add dimethyl tert-butylchlorosilane (892.8mg, 5.902mmol), continue stirring for 3h at 35°C, add oxetane (0.87ml, 13.20mmol) at room temperature ), after 3 hours, acidify with 10% hydrochloric acid solution, spin evaporate the solvent, extract with ether, wash the organic layer three times with deionized water and saturated brine, dry with anhydrous magnesium sulfate, pass through petroleum ether: ethyl acetate = 1:1 Purify by column chromatography, dissolve it in 15ml of ether solution at -76°C, add 5.902mmol of tetrabutylammonium fluoride, and stir for 30min. After rotary evaporation of the solvent, extraction and vacuum drying gave a colorless solid 1-hydroxypropyl-2-H-carborane with a yield of 70%.
[0035] Infrared analysi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com