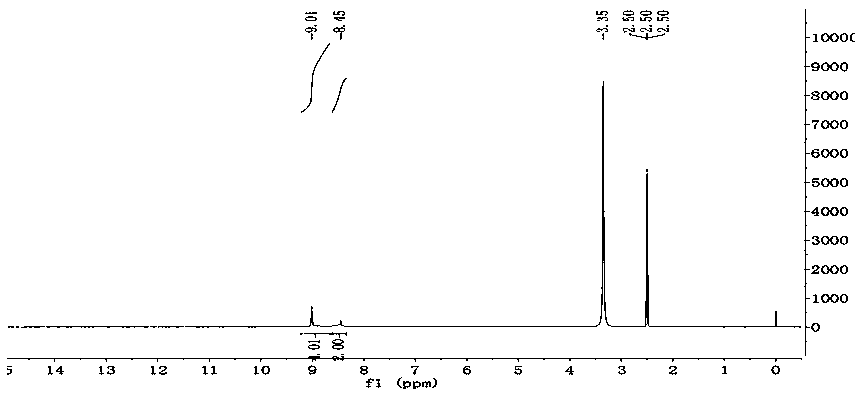
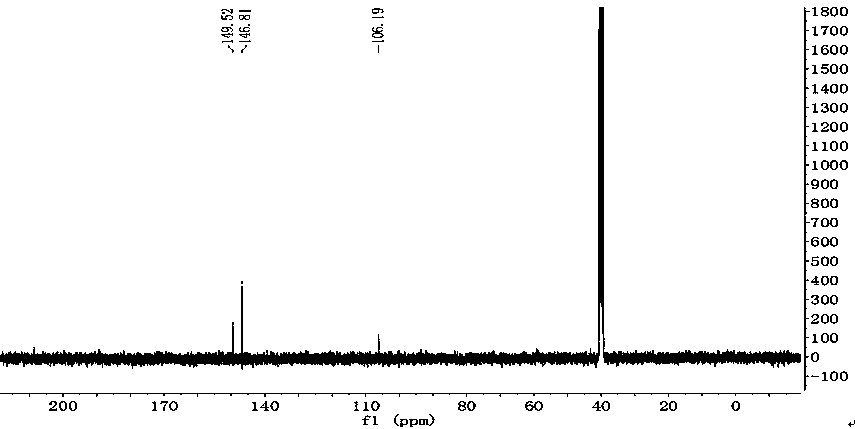
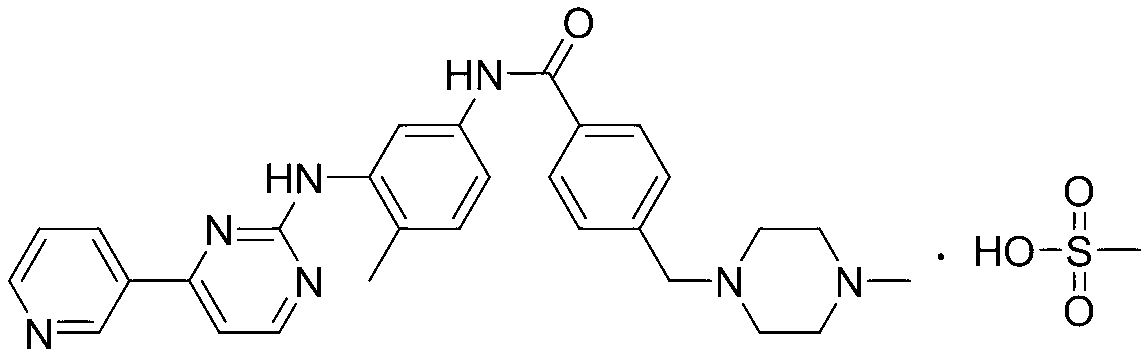
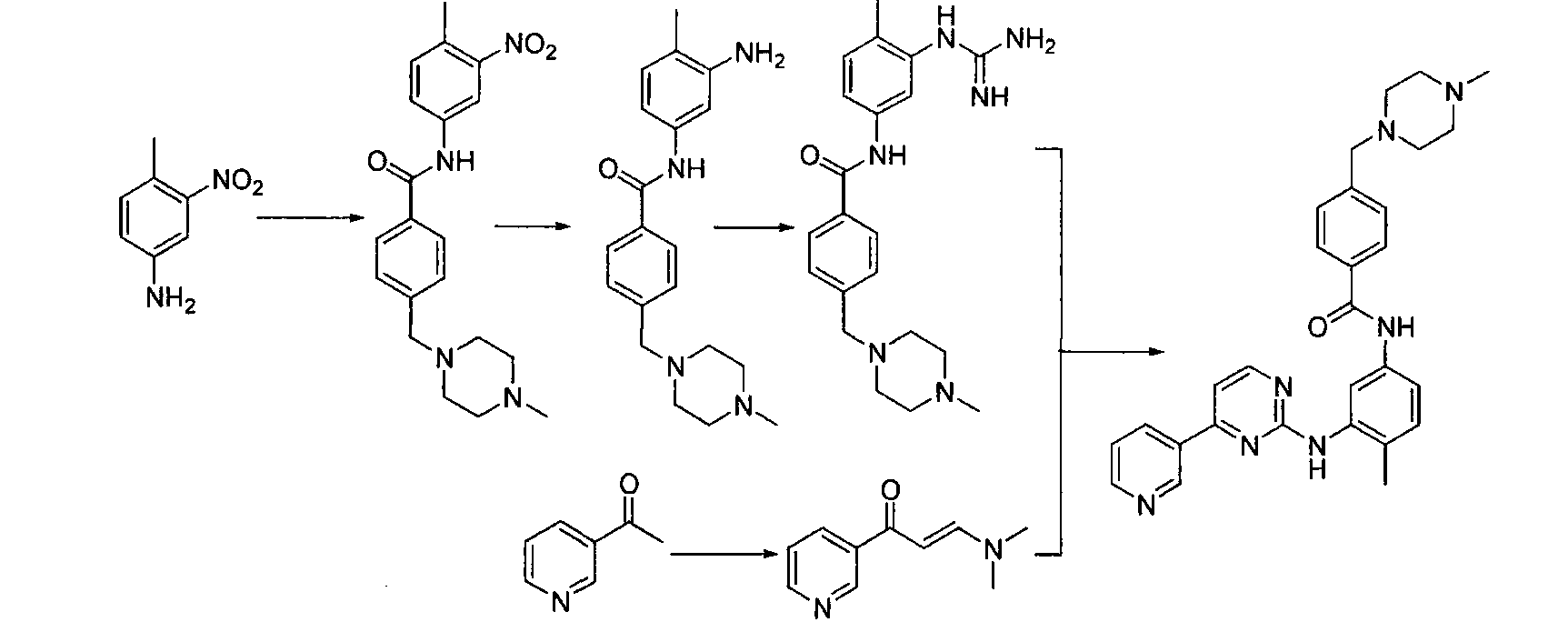
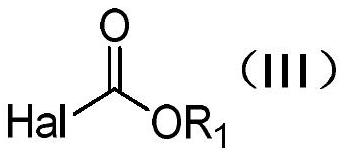
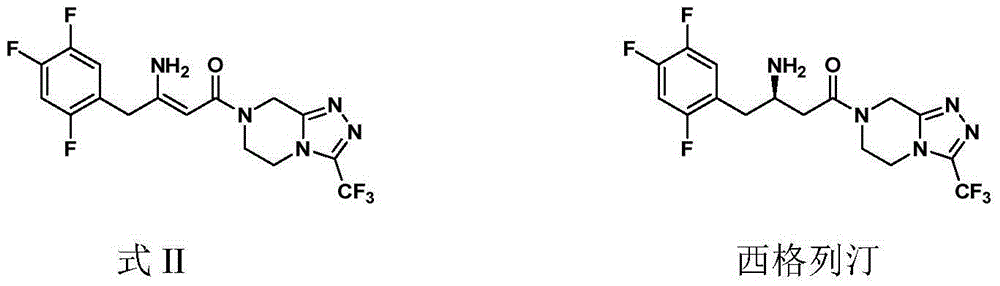Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
219results about How to "Short synthetic steps" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Synthesis and refinement of nelarabine
ActiveCN101348511AHigh yieldLow equipment requirementsOrganic active ingredientsSugar derivativesProduction lineCombinatorial chemistry
The invention discloses a method for preparing and refining a nucleotide compound, and particularly a method for preparing and refining Nalarabine. The preparation method has low requirements on facilities, with shorter synthesis process and simplified operation; the yielding rate of the whole production line is high with low production cost, and the method is economically viable and suitable for industrialized production. In addition, the invention also discloses a method for synthesizing an intermediate of Nalarabine.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Method for preparing praziquantel
InactiveCN101445507AShort synthetic stepsMild reaction conditionsOrganic chemistryHigh pressureSodium cyanide
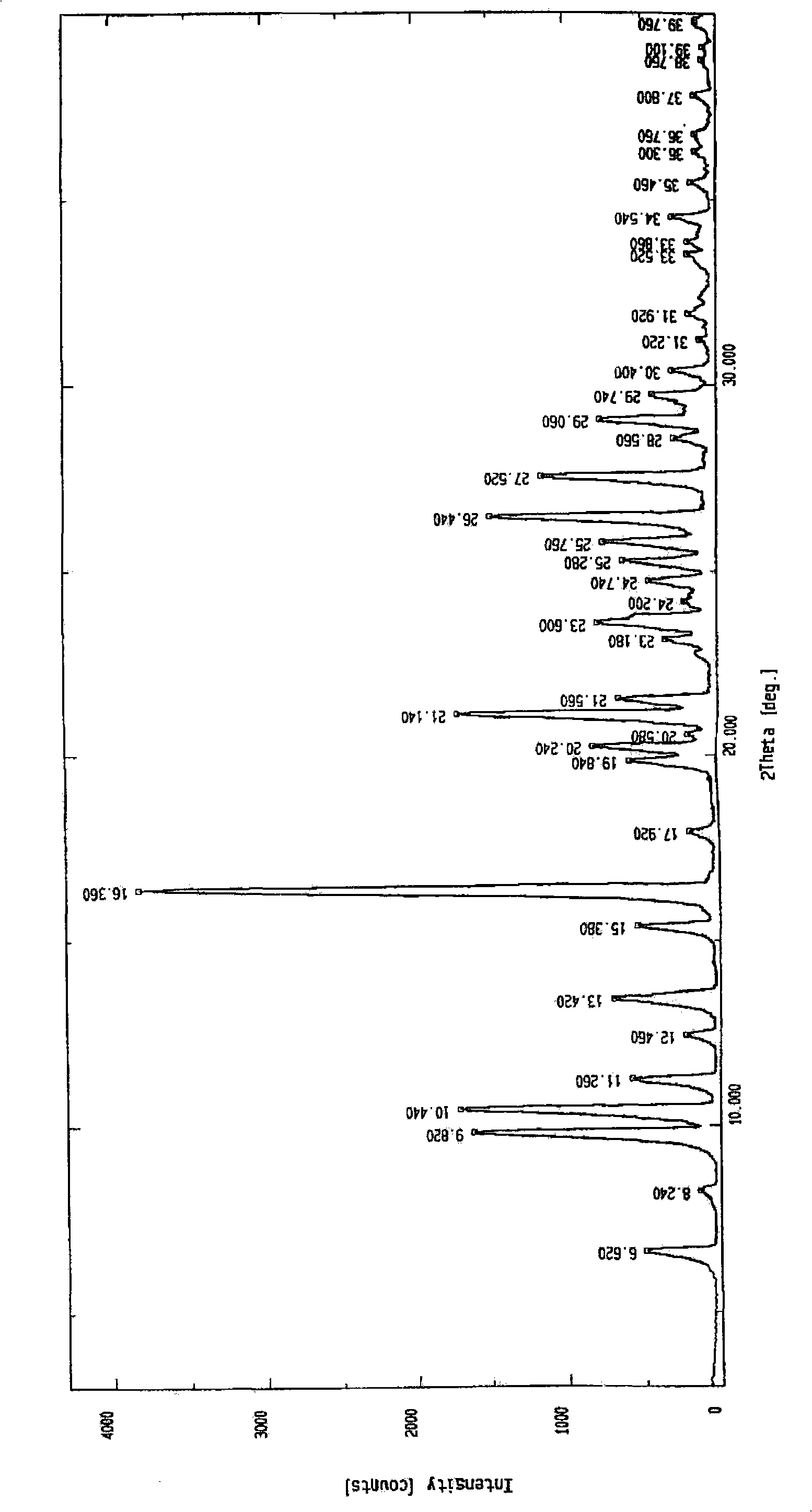
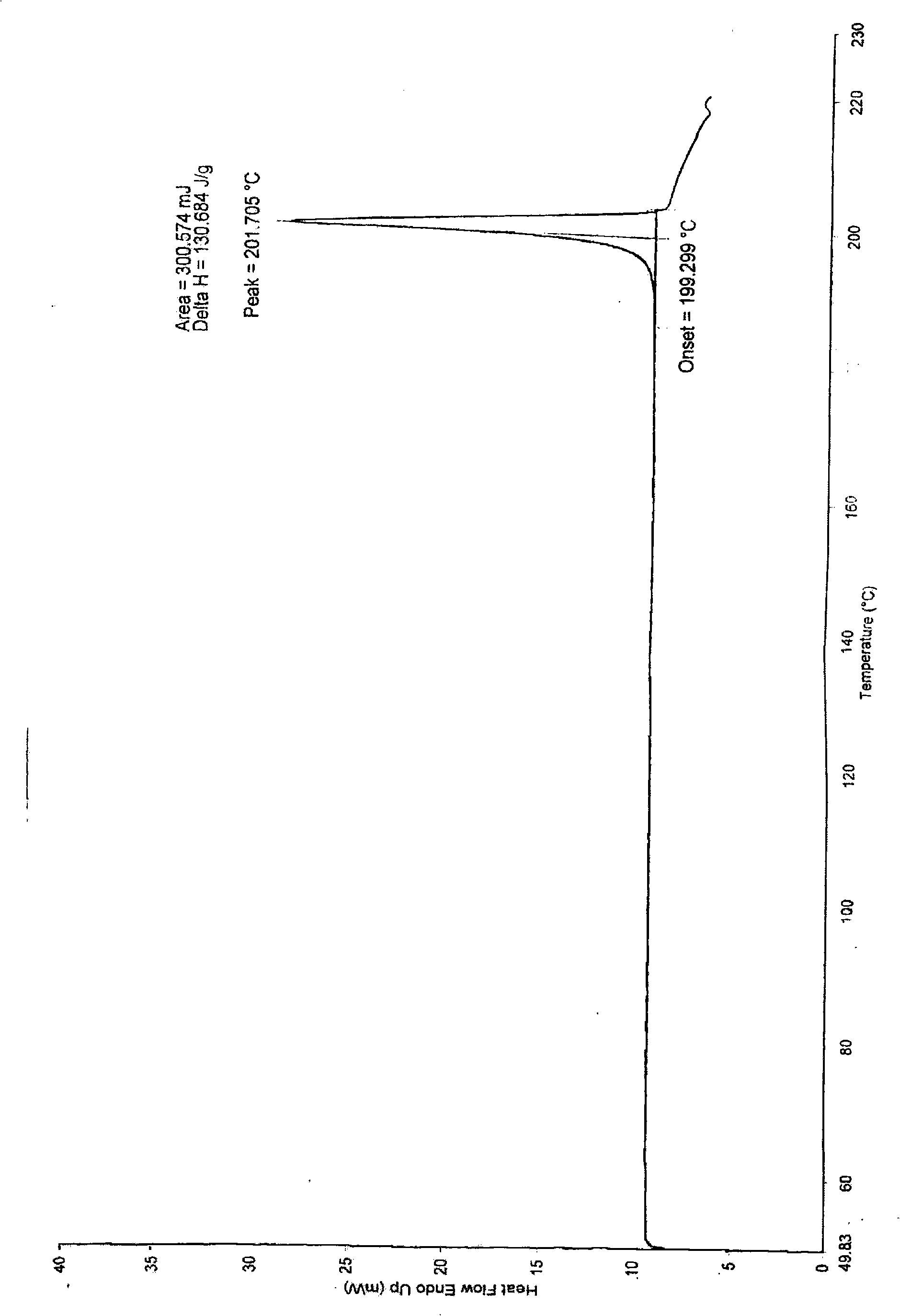
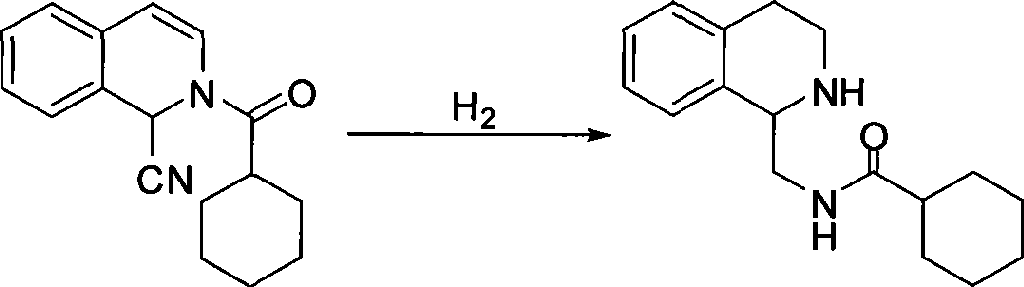
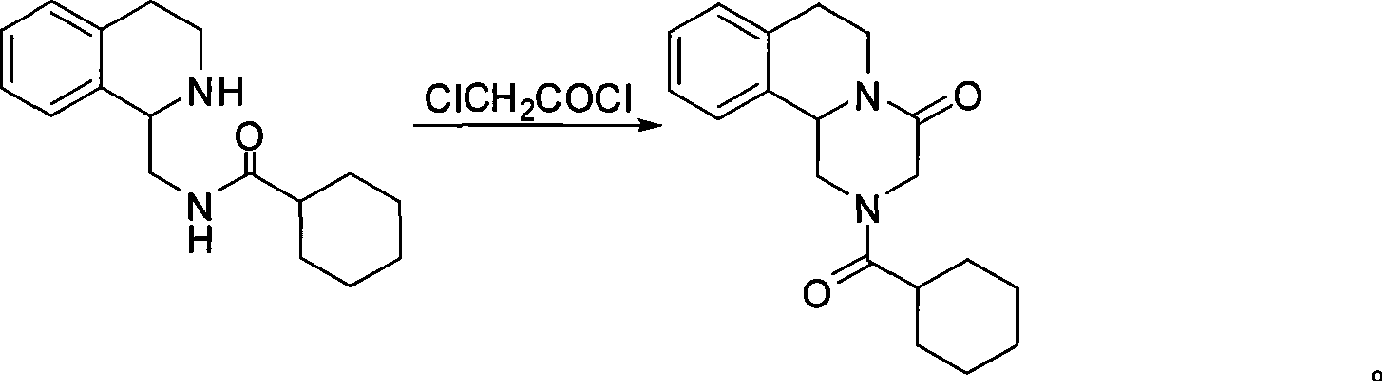
The invention relates to a method for preparing praziquantel, which is characterized by comprising the steps: (1) isoquinolin, sodium cyanide or potassuim cyanide and triethylamine or pyridine are added into a reactor to react, and yellow solid which is a first step product is obtained; (2) the first step product is added into a high-pressure hydrogenation kettle and filled with hydrogen to react under the action of catalyst, so that a second step product is obtained; (3) after being stirred and dissolved, the second step product and ethyl acetate is added with anhydrous potassium carbonate or anhydrous sodium carbonate, and then is stirred at the room temperature and dripped with chloracetyl chloride to be stirred at the room temperature to react, so that the solid obtained in the reaction is crude product of the praziquantel, and fine product is obtained by recrystallization of absolute methanol. Compared with the prior art, the method has the advantages of shortening the reaction process, reducing the energy consumption, improving the overall yield, etc.
Owner:SHANGHAI WANXIANG INDAL
Method for preparing gefitinib and intermediate thereof
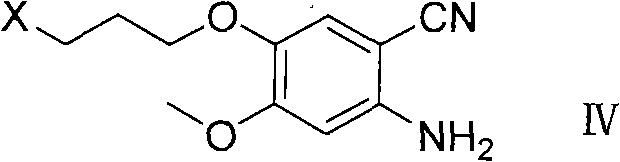
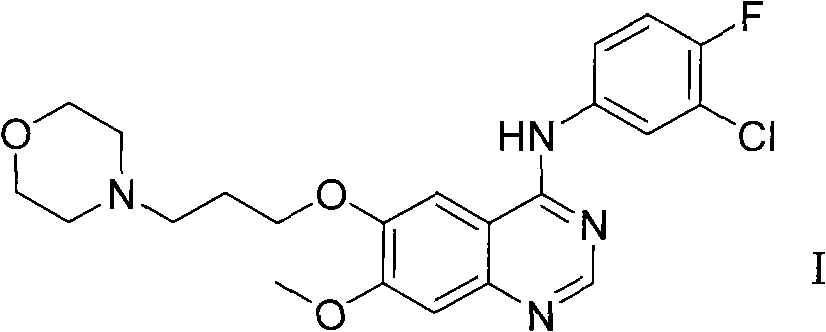
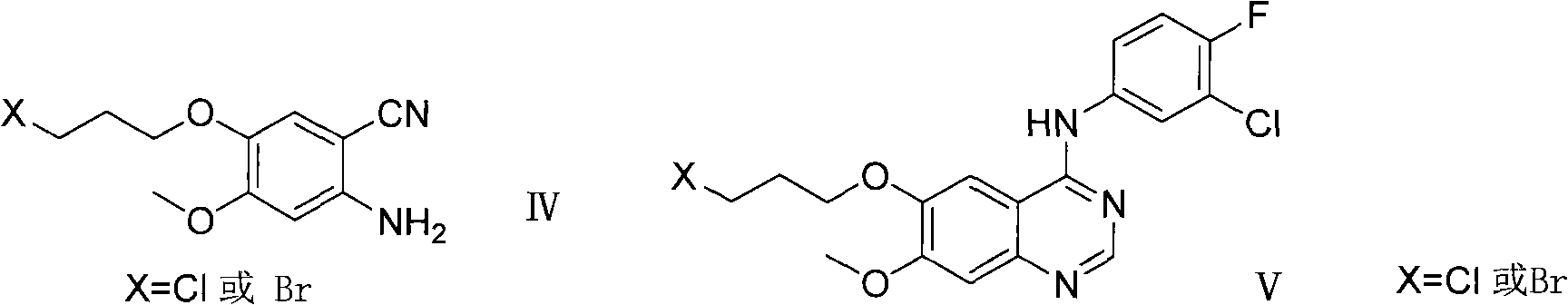
ActiveCN102146060AReduce pollutionFriendly production environmentCarboxylic acid nitrile preparationOrganic compound preparationPurification methodsSide chain
The invention relates to a preparation method of an anilinoquinazoIine compound, in particular to a method for preparing gefitinib and an intermediate thereof. The preparation method of the invention not only avoids the use of highly-pollutant halogenating agents to greatly reduce environmental pollution, but is also characterized by the connection with a 3-halogenated propyl side chain at first prior to the synthesis of a quinazoline parent ring and then introduction of a morpholine ring at the last step of the synthesis, thus repeated adjustment for pH value in the purification of a reaction product of every step is avoided, a purification method is simplified and the yield is raised.
Owner:SHAANXI NORMAL UNIV +2
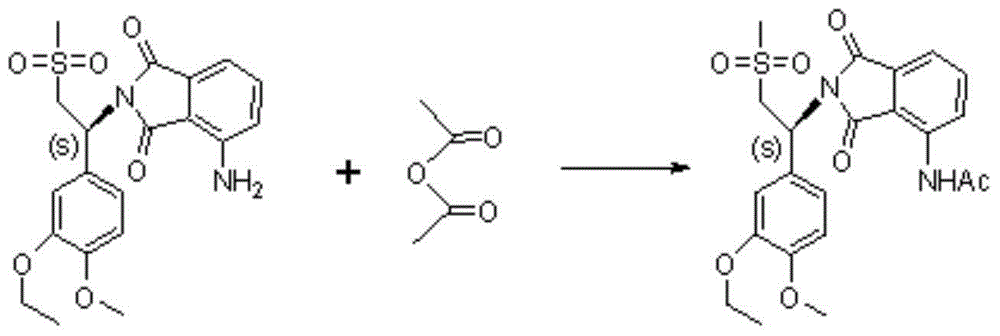
Preparation method of S-type apremilast
The invention discloses a preparation method of apremilast (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2-mesylethyl]-4-acetamidoisoindolinyl-1,3-dione. The method comprises the following steps: (1) converting a compound 1-(3-ethoxy-4-methoxyphenyl)-2-(methanesulfonyl)ethylamine racemate disclosed as Formula (II) into a salification substance disclosed as Formula (III) by using a resolving agent; and (2) connecting the compound disclosed as Formula (III) with 3-acetamidophthalic anhydride to obtain the compound disclosed as Formula (I). The method can be utilized to obtain the stable apremilast acceptable finished product, has the advantages of mild technological conditions, simple after-treatment, high purity and low reaction cost, and can easily implement industrial production.
Owner:NANJING CORE TECH CO LTD
Preparation method of crystalline nintedanib esylate
InactiveCN105418483AReduce usageShort synthetic stepsSulfonic acids salts preparationEthanesulfonic acidSulfonate
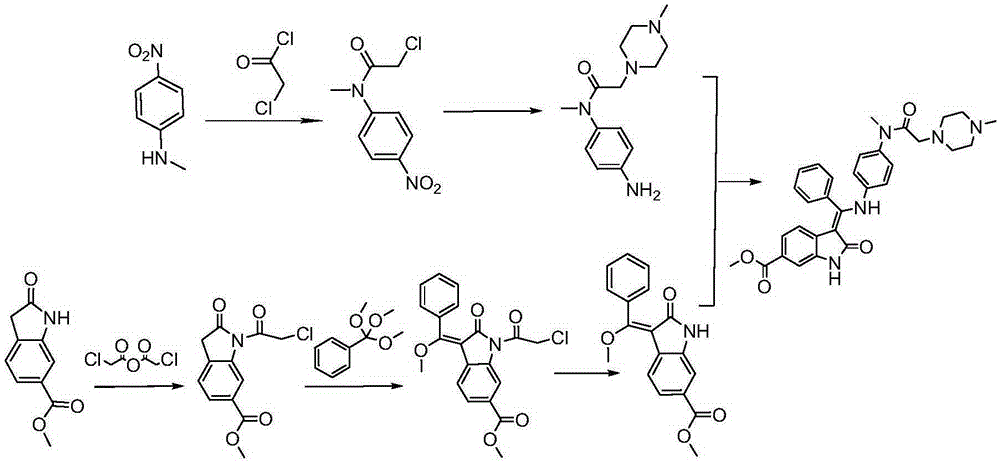
The invention discloses a preparation method of crystalline nintedanib esylate (3-Z-[1-(4-(N-((4-methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-phenylamino)-1-phenyl-methylene]-6-methoxycarbonyl-2-dihydroindolone monoethyl sulfonate). The method comprises steps as follows: (1) a compound represented in the formula (B) and acylating chlorination reagent chloroacetic anhydride react, and acyl chloride (C) is obtained; (2) the compound represented in the formula (C) and trimethyl orthobenzoate have a condensation reaction, and a compound represented in the formula (D) is obtained; (3) the compound represented in the formula (D) is deprotected, and a compound represented in the formula (E) is obtained; (4) the compound represented in the formula (E) and N-(4-aminophenyl)-N-methyl-2-(4-methylpiperazin-1-yl) acetamide have a condensation reaction, and a compound represented in the formula (F) is obtained; (5) the compound represented in the formula (F) and ethanesulfonic acid have a salification reaction, and a nintedanib esylate compound represented in the formula (A) is obtained. The stable crystalline nintedanib esylate can be obtained with the method, technological conditions are mild, aftertreatment is simple, the purity is high, the reaction cost is low, and industrial production is easy to realize.
Owner:NANJING CORE TECH CO LTD
The synthetic method of febuxostat
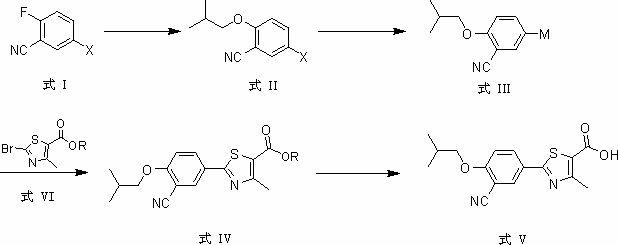
The invention provides a method for synthesizing febuxostat. The method comprises the following steps of: (a) performing aromatic ring substitution reaction on a compound of a formula I to obtain a compound of a formula II; (b) performing halogen-metal exchange reaction on the compound of the formula II to obtain a compound of a formula III; (c) performing coupling reaction on the compound of the formula III and the compound of a formula VI under the action of a metal catalyst to obtain a compound of a formula IV; and (d) performing hydrolysis reaction on the compound of the formula IV to obtain the compound of a formula V, namely febuxostat, wherein X is I or Br; M is selected from boric acid ester or SnBu3; and R is H or an alkyl group. In the method, a convergent synthesis strategy is adopted, and a carbon-carbon bond is formed by applying metal catalyzed aromatic ring coupling reaction in a key step, so that a system in which a benzene ring is coupled with a thiazole ring is established. The method has the advantages of simple and short steps, high yield and low environmental pollution and can be suitable for industrial production.
Owner:ARROMAX PHARMATECH
High-birefringence liquid crystal compound, and preparation method and composition thereof
ActiveCN106978192AHigh birefringenceLow melting enthalpyLiquid crystal compositionsSpatial light modulatorAlkoxy group
The invention discloses a high-birefringence liquid crystal compound, a preparation method of the high-birefringence liquid crystal compound and a composition containing the high-birefringence liquid crystal compound. The liquid crystal compound has a structure general formula (I) shown in the description, wherein X1, X2 and X3 is H or F; R is one of straight-chain alkenyl with a carbon atom number being 2 to 5 or fluoro alkenyl with a carbon atom number being 2 to 5. The liquid crystal compound comprises less than or equal to 40 percent (not zero) of compounds shown as the general formula (I), 1 to 40 percent of compounds shown as a general formula (II), 1 to 30 percent of compounds shown as a general formula (III) and 2 to 50 percent of compounds shown as a general formula (IV), wherein the general formulas are shown in the description; R1, R2 and R3 are respectively one of alkyl with a carbon atom number being 1 to 7, alkoxyl with a carbon atom number being 1 to 7 or fluoroalkyl with a carbon atom number being 1 to 5; X4 to X9 are respectively -H or -F. The high-birefringence liquid crystal compound has the advantages of high birefringence and low rotary viscosity, and is applicable to the fields such as space light modulators, laser detectors and 3D (3-dimensional) displays.
Owner:XIAN MODERN CHEM RES INST +1
Synthetic method of sitagliptin and salt thereof
The invention discloses a synthetic method of sitagliptin and a salt thereof. The synthetic method comprises the steps: carrying out an esterification reaction, a reduction reaction, an oxidizing reaction and a witting reaction on 2,4,5-trifluorophenylacetic acid as a starting raw material to obtain 4-(2,4,5-trifluorophenyl)-2-ethyl crotonate; then carrying out a hydroamination reaction on 4-(2,4,5-trifluorophenyl)-2-ethyl crotonate and chiral amine in the presence of butyl lithium or hexamethyldisilazane sodium to form a chiral hydroamination product; carrying out an esterolysis reaction, a condensation reaction and a hydrogenation reaction to obtain sitagliptin. The raw materials used in the synthetic method of sitagliptin are low in price and easy to obtain; the synthetic method of sitagliptin is less in step, easy to operate and capable of effectively reducing cost. By the use of the method, high-purity sitagliptin can be obtained, a sitgliptin phosphate obtained through salifying has an HPLC (High Performance Liquid Chromatography) and an ee (enantiomeric excess) value of more than 99% and can be applied to the field of medicine.
Owner:ZHEJIANG NHU CO LTD +1
Michlers ketone-cyano groups organic dyestuff and synthesis method thereof
InactiveCN101265365AShort synthetic stepsSimple processSolid-state devicesAzo dyesOrganic solar cellSynthesis methods
The invention provides a novel Michler ketone-cyan organic dye and a synthetic method thereof. The dye derives from Michler ketone, and has the structural formulae which represent Michler ketone-cyan organic dye compounds 1, 2, 3, 4 and 5, respectively. Each molecule of the dye comprises two nitrogen containing groups, namely, the structural feature of double electron-donating groups. Accordingly, the molecules can be oxidized and deacidized easily, and have better capability of donating electrons and the capability of trapping electrons from electrolyte more quickly. The dye extends the design thought of organic photosensitizers, and enriches the research content of dye sensitization solar batteries, thereby having very important significances for improving the photoelectric transformation efficiency of organic solar batteries and reducing the cost of batteries both in theory and practice. The synthetic step is short, the process is simple; the cost is low and the yield is high.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
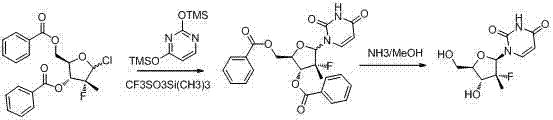
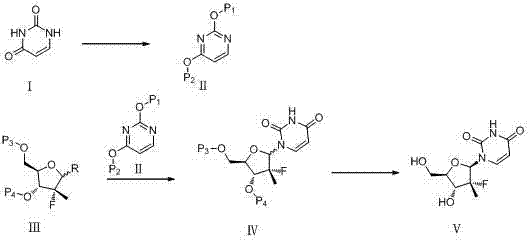
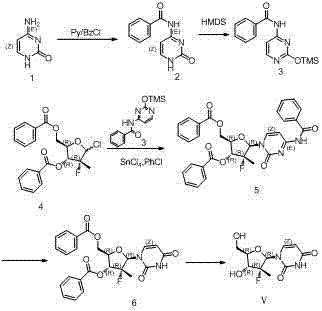
Synthesis method for (2'R)-2'-deoxy-2'-fluorine-2'-methyl uridine
InactiveCN104744539AShort synthetic stepsSimple and fast operationSugar derivativesSugar derivatives preparationFuranPyrimidine
The invention discloses a synthesis method for (2'R)-2'-deoxy-2'-fluorine-2'-methyl uridine, which comprises the following steps: in the presence of an organic solvent and Lewis acid, enabling (R)-3-F-3-methyl furan to react with 2,4-(di-hydroxyl protection) pyrimidine at 25-100DEG C; after the reaction is finished, processing a reaction product to obtain a compound (IV); and performing dehydroxylation protection to obtain the (2'R)-2'-deoxy-2'-fluorine-2'-methyl uridine (V). When adopted to synthesize the (2'R)-2'-deoxy-2'-fluorine-2'-methyl uridine, the method has short synthesis steps, is simple and convenient to operate and has important application values.
Owner:SHANGHAI HAOYUAN MEDCHEMEXPRESS CO LTD +2
Preparation method of energetic compound 2,4,6-triamino-5-nitropyrimidine-1,3-dioxide
The invention discloses a preparation method of an energetic compound 2,4,6-triamino-5-nitropyrimidine-1,3-dioxide. The preparation method comprises the following steps: with 2,4,6-triaminopyrimidineand a substituent thereof as reaction raw materials, performing a nitration reaction; performing an oxidation reaction; performing an acid-alkali neutralization reaction to obtain the needed product,namely the 2,4,6-triamino-5-nitropyrimidine-1,3-dioxide. The preparation method has the advantages of short synthesis steps, low cost of used raw materials and used solvents, simple post treatment, small damage to people, equipment and the environment and the like; the compound synthesized by the preparation method provided by the invention has the characteristics of high density, high energy, insensitiveness to mechanical stimulation and the like, and has a relatively good market prospect in the field of application of energetic materials.
Owner:INST OF CHEM MATERIAL CHINA ACADEMY OF ENG PHYSICS
Preparation method of alpha-crystal form imatinib mesylate
InactiveCN103058991AShort synthetic stepsEasy to operateOrganic chemistryBenzoic acidOrganic solvent
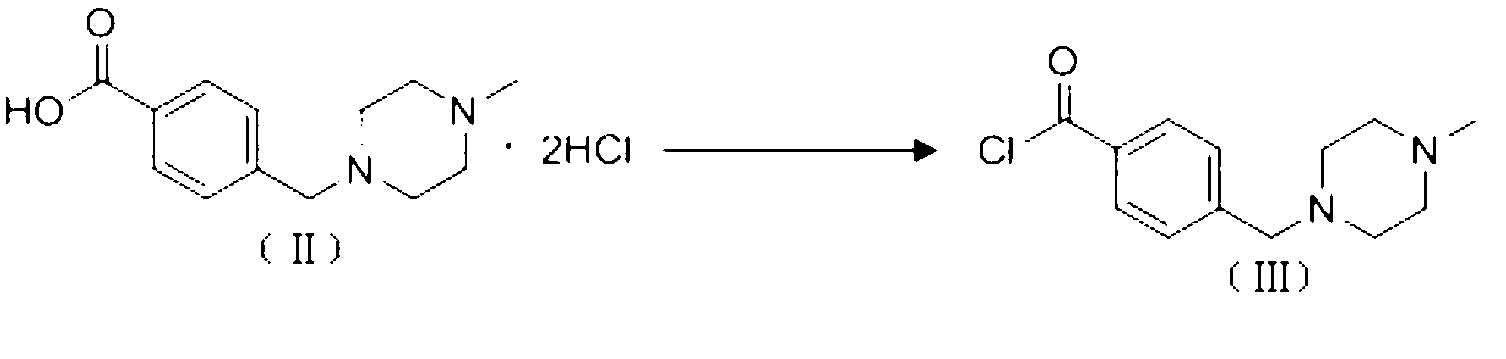
The invention discloses a preparation method of alpha-crystal form imatinib mesylate. The preparation method comprises the following steps that 1, 4-[(4-methyl piperazine-1-yl)methyl]benzoic acid dihydrochloride shown in the formula II is converted into an acyl chloride shown in the formula III by an acylating chlorination reagent; 2, the acyl chloride shown in the formula III and N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-aminopyrimidin undergo a condensation reaction in the presence of an alkali to produce a compound shown in the formula IV; and 3, the compound shown in the formula IV and methylsulfonic acid undergo a salt forming reaction in the presence of an organic solvent to produce a compound shown in the formula I. The preparation method can realize preparation of stable alpha-crystal form imatinib mesylate and allows mild process conditions. The stable alpha-crystal form imatinib mesylate can be after-treated simply and has high purity. The preparation method has a low reaction cost and is suitable for industrial production.
Owner:NANJING CORE TECH CO LTD
Preparation method of (S)-equol
The invention relates to a preparation method of (S)-equol, which aims to solve the problem of complex operation in the existing preparation method of (S)-equol. The preparation method comprises the following steps: by using daidzein as the main material, carrying out hydroxy protection, catalytic hydrogenation, dehydration and chiral catalytic reduction to prepare the (S)-equol. The invention has the advantages of cheap and accessible raw materials and low cost, is simple to operate, and is suitable for industrial scale-up production; and the yield is 60-70%. The invention is applicable to the field of synthesis of medicine compounds.
Owner:HEILONGJIANG UNIV
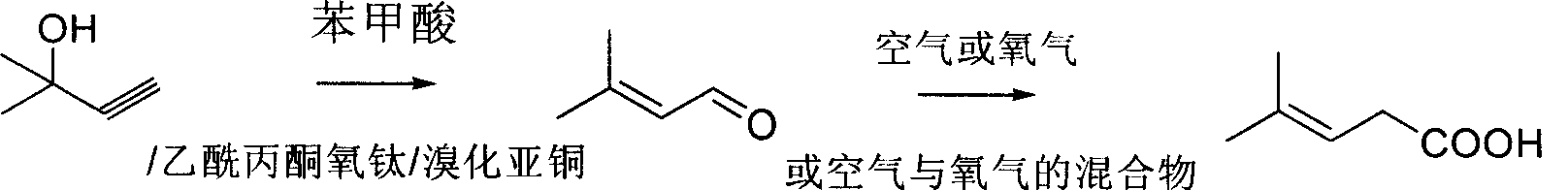
Method for preparing 3-methyl-2-butenoic acid
ActiveCN101391948AShort synthetic stepsLess side effectsOrganic compound preparationCarboxylic compound preparationCatalytic reformingBenzoic acid
The invention discloses a preparation method of 3-methyl-2-butenoic acid. The existing method has great amount of three wastes which pollute the environment heavily. The method is characterized in that: 2-methyl-3-butyne-2-alcohol is adopted as a raw material; 3-methyl-2-butenoic aldehyde is obtained by the complex system of acetylacetone titania-copper bromide-benzoic acid through catalytic reforming, and finally the 3-methyl-2-butenoic acid is obtained by oxidizing 3-methyl-2-butenoic aldehyde; and an oxidizer adopted is air or oxygen or the mixture of air and oxygen, wherein, the preference is air. The oxidizer adopted by the method is safe, avirulent and easy to be controlled, and has low cost; in addition, the reaction yield is high and the product purity is high on the premise of ensuring no pollution to the environment.
Owner:ZHEJIANG NHU CO LTD
Alkyne liquid crystal compound, preparation method, composition containing compound and high-frequency assembly containing liquid crystal medium
ActiveCN108517217ALow dielectric lossHigh quality factorLiquid crystal compositionsDielectric lossRadar
The invention discloses an alkyne liquid crystal compound, a preparation method, a composition containing the compound and a high-frequency assembly containing a liquid crystal medium in order to solve the problem that the comprehensive performance of the prior art is poor. The structure of the liquid crystal compound is shown in the description, and the liquid crystal composition includes a firstcompound the content of which is lower than or equal to 80% and higher than 0, a second compound the content of which is higher than 0 and lower than or equal to 65% and a third compound the contentof which is higher than 0 and lower than or equal to 60%. The liquid crystal compound has the advantages of low dielectric loss, high quality factors and a wide nematic phase temperature interval andis applicable to filters, phase shifters, phase-controlled array radars, 5G communication networks and other fields. The liquid crystal high-frequency assembly has a wide working temperature range, low loss and high tunability.
Owner:XIAN MODERN CHEM RES INST +1
1, 5, 9-trisubstituted coronene compound and synthesis method thereof
ActiveCN106565408AReduce pollutionImprove thermal stabilityOrganic compound preparationHydrocarbonsFuranCoronene
The present invention relates to a 1, 5, 9-trisubstituted coronene compound and a synthesis method thereof. The structural formula of the compound is shown in img file = 'dest _ path _ image 001. TIF 'wi = '109 'he = '108', wherein R represents H, C1-C18 alkyl, phenyl, 4-methylphenyl, 4-methoxy phenyl, benzyl, cyclohexyl, 4-trifluoromethylphenyl, thiophene, furan and the like. According to the technical scheme of the invention, the easily prepared 1, 5, 9-triamido triphenylene is subjected to diazotization and halogenation reaction to obtain the tri-halogenated triphenylene. After that, the tri-halogenated triphenylene is subjected to Sonogashira reaction with various alkynes to generate atriyne-triphenylene compounds. Finally, through the metal-catalyzed reaction and the cyclization reaction under the effect of an organic base, various 1, 5, 9-trisubstituted coronene compounds, novel in structure, can be obtained. According to the technical scheme of the invention, raw materials are easy for mass preparation. Meanwhile, the synthesis step is relatively short and the operation is convenient. The obtained trisubstituted coronene compound is good in thermal stability and chemical stability, and the trisubstituted coronene emits the relatively strong fluorescence within the range of 420-550 nm according to the fluorescence emission spectrum of the trisubstituted coronene compound. Therefore, the trisubstituted coronene is an excellent fluorescent material for preparing UV ultraviolet charge-coupled devices (UV-CCD) and organic light-emitting diodes (OLEDs), and has a wide application prospect in the field of electronic materials.
Owner:SHANGHAI UNIV
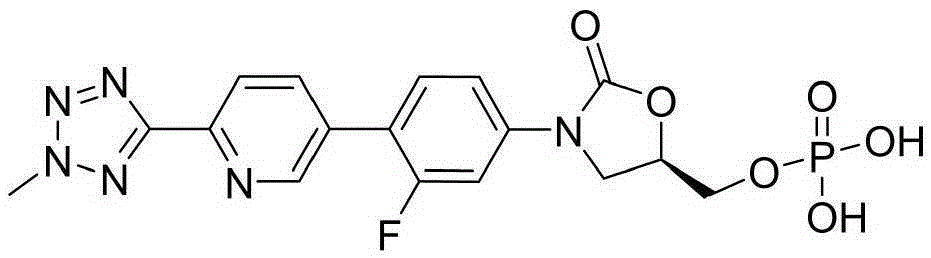
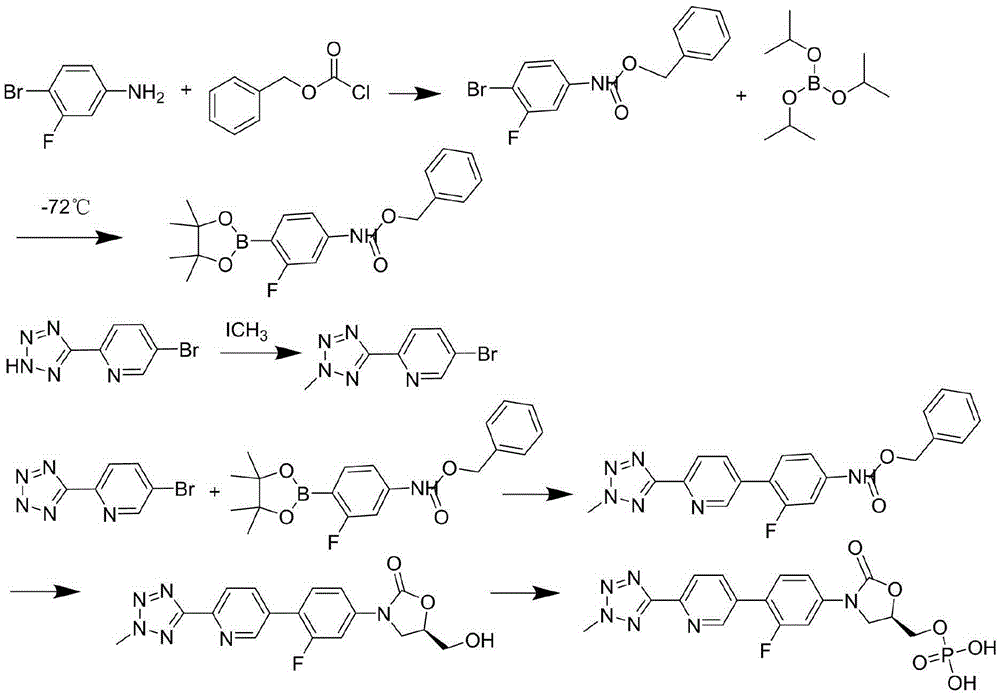
Preparation method of tedizolid phosphate
InactiveCN105418681AShort synthetic stepsEasy to operateGroup 5/15 element organic compoundsPhosphateBromine
The invention belongs to the technical field of synthesis of chemical medicines and particularly relates to a preparation method of tedizolid phosphate. (5R)-3-(4-bromo-3-fluorophenyl)-5-(hydroxymethyl)oxazolidin-2-one and 2-(2-methyl-2H-tetrazolyl-5-yl) pyridine-5-boronic acid pinacol ester are taken as raw materials, and (R)-3-(4-(2-(2-methyl tetrazole-5-yl) pyridine-5-yl)-3-fluorophenyl)-5-(hydroxymethyl)oxazolidin-2-ketone phosphate is prepared with a four-step method. The method has a mild process condition, aftertreatment is simple, the purity is high, the reaction cost is low, and industrial production is easy to realize.
Owner:NANJING CORE TECH CO LTD
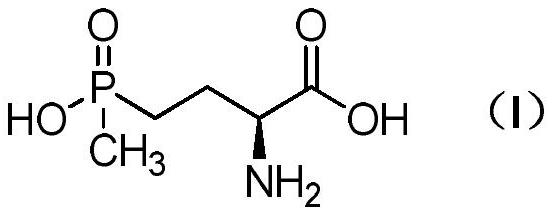
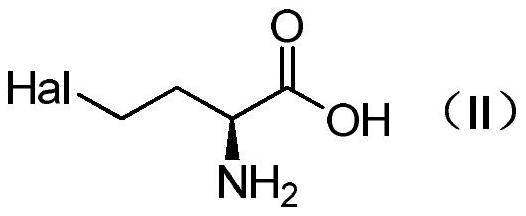
Method for preparing L-glufosinate-ammonium
ActiveCN111662324ALow costNo chiral catalysis requiredGroup 5/15 element organic compoundsOrganic chemistry methodsHomoserineCombinatorial chemistry
The invention relates to a method for preparing L-glufosinate-ammonium. According to the method, cheap and easily available L-homoserine is used as an initial raw material, L-glufosinate-ammonium witha high ee value is prepared through a three-step reaction, chiral catalysis is not needed, the cost is low, and the method has a potential industrial application value.
Owner:LIER CHEM CO LTD +1
Method for rapidly preparing remdesivir intermediate
The present invention relates to a method for rapidly preparing a remdesivir intermediate represented by a following formula (I) without complex purification and separation, wherein X represents a halogen element. The method is simple, high in yield and single in product, and can be obtained through simple filtration and separation; complicated separation operation such as column chromatography isnot needed; the remdesivir intermediate represented by the formula (I) can be rapidly, efficiently, and massively prepared; and sufficient intermediates are provided for large-scale preparation of remdesivir drugs that may be used to treat new corona viruses.
Owner:UNIV OF SCI & TECH OF CHINA
Method for synthesizing lorlatinib
InactiveCN109232607AShort synthetic stepsEasy to operateOrganic chemistry methodsKetoneChiral resolution
The invention belongs to the technical field of medicines, and relates to a method for synthesizing lorlatinib (PF-06463922), which is finally synthesized from 5-fluoro-3-methyl isobenzofuran-1(3H)-ketone and 1-methyl-3-((methylamino)methyl)-1H-pyrazol-5-nitrile through the reacting steps including aminolysis, substitution, coupling, chiral resolution and the like, or from (S)-5-fluoro-3-methyl isobenzofuran-1(3H)-ketone and 1-methyl-3-((methylamino)methyl)-1H-pyrazol-5-nitrile through the reacting steps including aminolysis, substitution, coupling and the like. According to the method, a novel method is provided to synthesis of an anti-tumor medicine lorlatinib (PF-06463922).
Owner:SHENYANG PHARMA UNIVERSITY
Preparation of Sitagliptin
ActiveCN105315286AHigh ee valueImprove recovery yieldOrganic chemistrySitagliptinAsymmetric hydrogenation
The invention belongs to the field of medicine synthesis and particularly provides a preparation method for Sitagliptin. According to the method, a cheap metal ruthenium complex and cheap R-BINAP serve as ligands, and the asymmetric hydrogenation reduction of an enamine intermediate is catalyzed, so that R-configuration Sitagliptin can be obtained in a high-selectivity manner; and the reaction time is short, and both the yield of reduction and the ee value of the product are relatively high, so that the method is applicable to industrialized amplified production.
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD
Method for synthesizing mitochondria targeted spinning scavenger MitoPBNs (spinning probe)
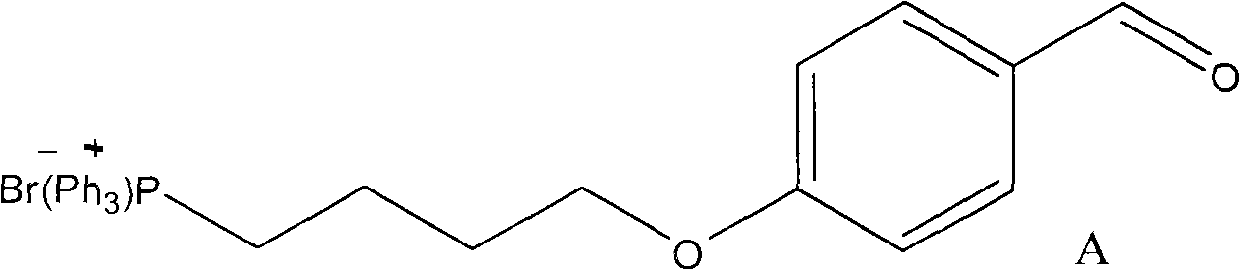
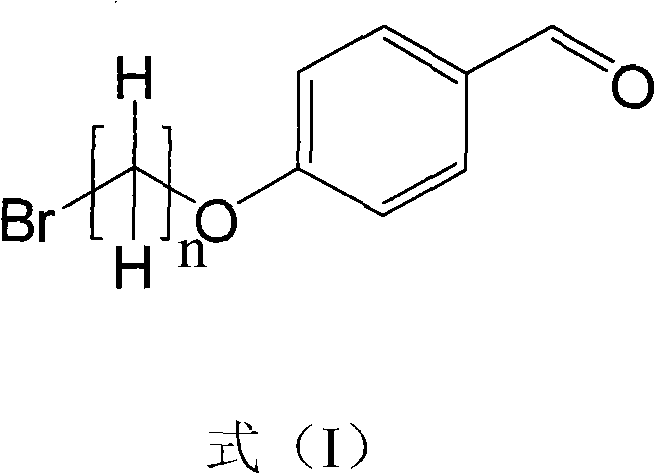
InactiveCN101906118AHigh purityRealize early warningGroup 5/15 element organic compoundsBiological testingScavengerStructural formula
The invention discloses a method for preparing a mitochondria targeted spinning scavenger MitoPBNs (spinning probe). The structural formula of the MitoPBN is shown in a formula (I). A synthesis flow is shown in the formula (II), and comprises the following steps of: performing elimination reaction on 4-hydroxybenzaldehyde and dibromo-straight-chain paraffin under alkali condition to prepare a compound 3; reacting the compound 3 with triphenylphosphine to prepare the compound 4; in ethanol, mixing the compound 4, 2-nitro-2-methylpropane, a 4A molecular sieve and zinc powder in a solvent, adding dropwise a glacial acetic acid, stirring and reacting the mixed solution at room temperature, placing the mixed solution in a refrigerator for refrigeration, and performing separation and purification to prepare the spinning probe MitoPBN; and performing liposome packing treatment. The method has the characteristics of relatively fewer synthesis steps, low raw material cost, readily available raw materials and relatively higher product purity. The probe for scavenging free radical signals can enter cell mitochondria.
Owner:刘珊林
Method for synthesizing repaglinide key intermediate
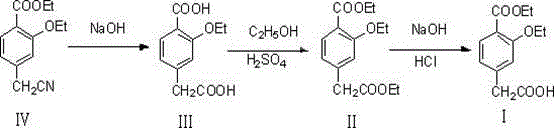
InactiveCN104628518AHigh yieldAvoid highOxygen-containing compound preparationOrganic compound preparationPhenylacetic acidFirst pass yield
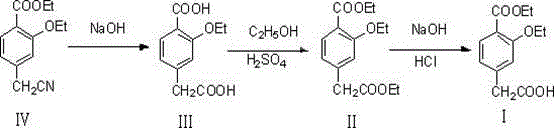
The invention relates to a method for synthesizing a repaglinide key intermediate, namely 4-ethoxycarbonyl-3-ethyoxy phenylacetic acid. The method comprises the following steps: by taking 3-ethyoxy-4-ethoxycarbonyl-benzyl cyanide as an initial raw material, performing hydrolysis, esterification, selective hydrolysis and the like, thereby obtaining the important intermediate 4-ethoxycarbonyl-3-ethyoxy phenylacetic acid of repaglinide. According to the key intermediate, the impurities of the product is less, the purity of the product is high and can reach over 99.7%, so that the quality of the subsequently synthesized repaglinide product is improved, the 100 percent first-pass yield of the subsequently synthesized repaglinide product can be basically reached, a refining step is avoided, and the synthetic yield is effectively improved. The process is easy and convenient to operate, the yield is high, the molar yield is 69.1 percent, the production cost is low, and the method is suitable for industrial production.
Owner:HUBEI YITAI PHARMA
Topiroxostat preparation technology
ActiveCN105566301AImprove solubilityIncreased rate of release of cyanide ionsOrganic chemistryPotassium ferrocyanideHydrazide
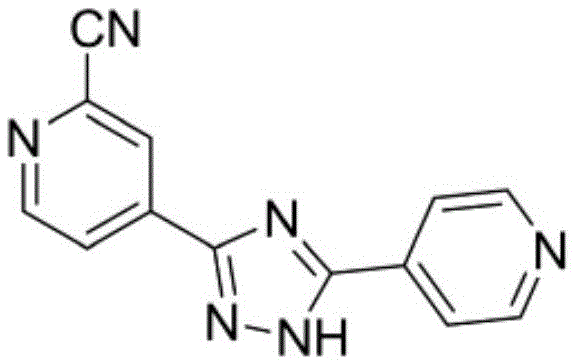
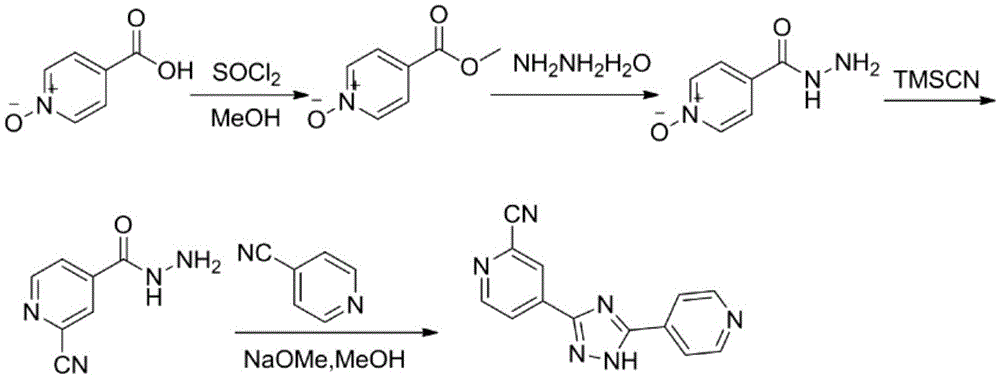
The invention discloses a topiroxostat preparation method. According to the method, 2-chloroisonicotinic acid which is cheap and easy to obtain serves as an initial material, potassium ferrocyanide serves as a green cyanogens source, AgI-KI-PEG generates 2-cyanoisonicotinate through cyanation of a mixed catalysis system and then directly acts with hydrazine hydrate to obtain 2-cyanoisonicotinate hydrazide under the action of an amide condensing agent phenyl dichlorophosphate (PDCP), and the product and 4-cyanopyridine are condensed to obtain topiroxostat. Through technological improvement of the reaction, reaction steps are shortened, the reaction yield is obviously increased by 90% or above, and the technological cost is obviously reduced. Meanwhile, a 2-chloroisonicotinic acid raw material which is cheaper and easy to obtain is used, use of corrosive thionyl chloride and other chlorinating agents is avoided, and the technology is suitable for industrial production. The method is short in synthesis step, easy to operate, mild in reaction condition, economical, environmentally friendly and suitable for industrial production, and the yield is obviously increased.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Preparation method of 2-(4-alkylphenyl) propanoic acid
InactiveCN102199085AShort synthetic stepsLower reaction conditionsPreparation from carboxylic acid esters/lactonesPropionateAlkylbenzenes
The invention discloses a synthesis method of 2-(4-alkylphenyl) propionic acid, which comprises the following steps of: carrying out Friedel-Crafts reaction between ethyl 2-chloro-propionate and alkyl benzene with anhydrous aluminum chloride as a catalyst to obtain an intermediate product ethyl 2-(4-alkylphenyl) propionate; and hydrolyzing the intermediate product to obtain 2-(4-alkylphenyl) propionic acid. The invention has the advantages of short synthesis step, low reaction condition, and no need for high temperature or high pressure; the atom utilization rate is high, and the invention can lower energy consumption and reduce waste discharge, meeting the requirement of green chemistry on chemical production; and inexpensive common anhydrous aluminum chloride is used as a catalyst, thus lowering the production cost; moreover, the anhydrous aluminum chloride is especially suitable for producing brufen.
Owner:SHANGHAI QIBAO HIGH SCHOOL
High-efficiency high-stereoselectivity semisynthesis method of harringtonine and allied alkaloids
ActiveCN102304132AHigh yieldHigh selectivityAntibacterial agentsGroup 4/14 element organic compoundsLewis acid catalysisAlkaloid
The invention relates to a high-efficiency high-stereoselectivity semisynthesis method of harringtonine and allied alkaloids, which uses optical pure cephalotaxin serving as a raw material to perform reactive synthesis with substituted silicon ketene in Lewis acid catalysis. The structural general formula of the compound is shown as (A), wherein the meaning of each group is shown as the specification. The method has the advantages of high chemical yield and diastereoselectivity of key reactions, convenient operation, short synthesis steps and the like. The method is a universal method for synthesizing the optical pure compound with the structural general formula shown as (A), and is suitable for massive preparation. The compound is widely applied to anti-tumor (malignant and non-malignant tumors), anti-parasitic, antifungal and antimicrobial chemotherapy medicaments.
Owner:NANKAI UNIV
Method for synthesizing 1-BOC-3-piperidone
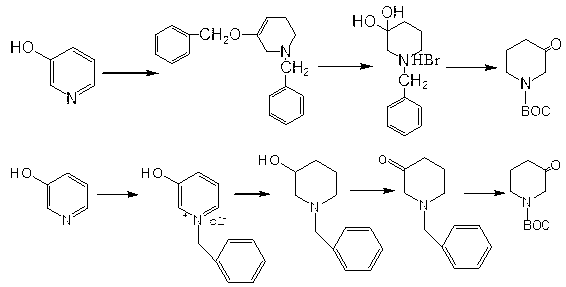
The invention relates to a method for synthesizing 1-BOC-3-piperidone and relates to a chemical synthesis method of 1-BOC-3-piperidone, belonging to the technical field of medicines. The method comprises the following steps of: with 3-hydroxypyridine as a raw material, reducing the 3-hydroxypyridine, protecting piperidine cyclo-nitrogen BOC, and oxidizing oppenauer to prepare a crude product of the 1-BOC-3-piperidone; and then, carrying out reduced pressure distillation to obtain a fine product of the 1-BOC-3-piperidone. The invention provides the high-efficiency and low-pollution method for synthesizing the 1-BOC-3-piperidone; and the method is simple and convenient to operate, short in synthesis step, mild in condition, environment-friendly in reaction process, high in product yield, good in purity and suitable for industrial production.
Owner:YANGZHOU TIANHE PHARM CO LTD
Novel preparation method of insensitive explosive TATB
ActiveCN111995527AImprove solubilityThe synthesis process is matureOrganic compound preparationCarboxylic acid amides preparationChemical industryAcetic anhydride
The invention provides a novel preparation method of an insensitive explosive TATB. The method comprises the following steps: 1, with 2,4,6-trinitrotoluene as a raw material, subjecting 2,4,6-trinitrotoluene to reacting with hydrogen and acetic anhydride in sequence to obtain 2,4,6-triacetylaminotoluene; 2, subjecting the obtained 2,4,6-triacetylaminotoluene o oxidation and a decarboxylation reaction to obtain 1,3,5-triacetylaminobenzene; and 3, carrying out nitrification and hydrolysis reactions on the obtained 1,3,5-triacetylaminobenzene to obtain 2,4,6-trinitro-1,3,5-triaminobenzene. According to the invention, the raw material used in the preparation method is the cheap 2,4,6-trinitrotoluene, and used reactants or catalysts are commonly used products in the chemical industry, so the preparation method has characteristics of low cost and usage of simple and easily available raw materials. In addition, the preparation method also has the characteristics of short synthesis steps, simple operation of each step, high yield, high reaction rate, easy separation and collection of intermediate and final products and the like, and is beneficial for realization of mass production of TATB.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
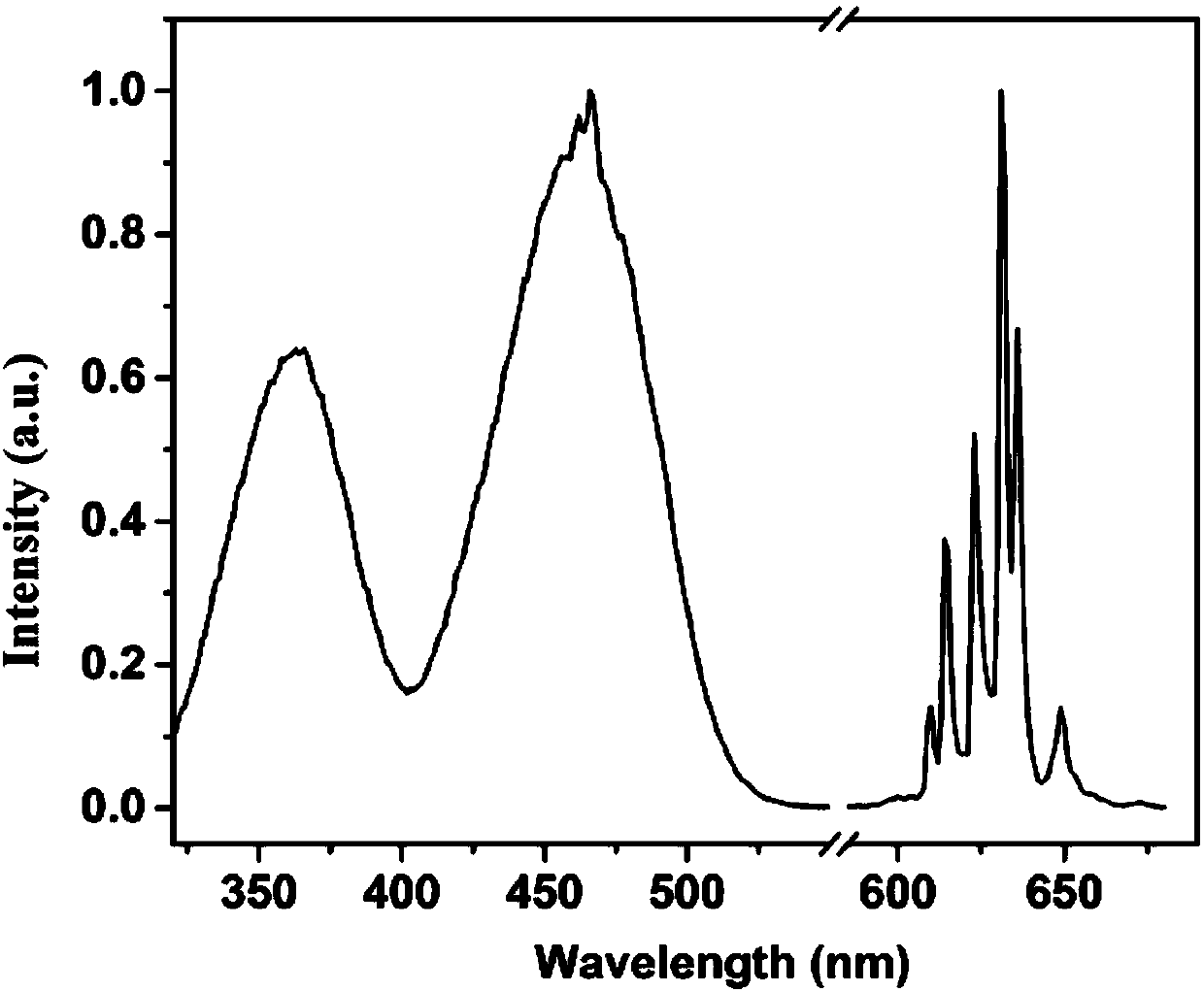
Preparation method of Mn<4+>-activated fluoride fluorescent powder
ActiveCN109957400AReduced dosage requirementsHigh Luminescence Quantum YieldLuminescent compositionsLuminescence quantum yieldOptical property
The invention provides a preparation method of Mn<4+>-activated fluoride fluorescent powder. The preparation method provided by the invention does not need to form a saturated solution of A2MF6, so that the requirement of the use amount of raw materials is greatly reduced; in the preparation process, the target product can be synthesized at room temperature and is convenient to synthesize; a precipitating agent is added, so that the preparation is more convenient, the operation is simpler, the synthetic steps are simplified, and the prepared fluorescent powder has better optical properties; the fluoride fluorescent powder can be well excited by ultraviolet light to blue light, especially the blue light, and has strong red light emission, wherein the red light emission peak is in the wavelength range from 600 to 650 nm; the method has the advantages of a simple preparation process and short consumed time; and the prepared material has a high luminescence quantum yield and is suitable for industrial large-scale preparation.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Method for synthesizing deuterated tazobactam
ActiveCN103012431AHigh yieldCarbon-deuterium bonds are stableOrganic chemistryDecompositionSodium ascorbate
The invention discloses a method for synthesizing deuterated tazobactam. The method comprises the following steps: adding 2beta-azomethylpenicillanic acid-1beta-oxide, propiolic acid, a catalyst containing copper or cuprous ion and sodium ascorbate in a deuterated solvent in turn, stirring at 25-200 DEG C for reaction for 1 to 48 hours, and after the reaction, performing extraction, filtering and column chromatography to obtain the deuterated tazobactam. Through the method, according to the method for synthesizing the deuterated tazobactam, the deuterated tazobactam is a novel beta-lactamase inhibitor of the penicillanic sulfone type and can be used for treating various bacterial infections; the method has the characteristics of mild reaction condition, short synthesis procedure, simple process and high yield, the safety during the reaction is effectively improved by using propiolic acid as the raw material, and the carbon-deuterium chain in the obtained deuterated tazobactam molecule is so stable that the medicine decomposition process can be effectively retained, therefore, the deuterated tazobactam has a longer action time in the body and is better than the normal tazobactam.
Owner:SUZHOU ROEING BIOPHARMACEUTICALS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com