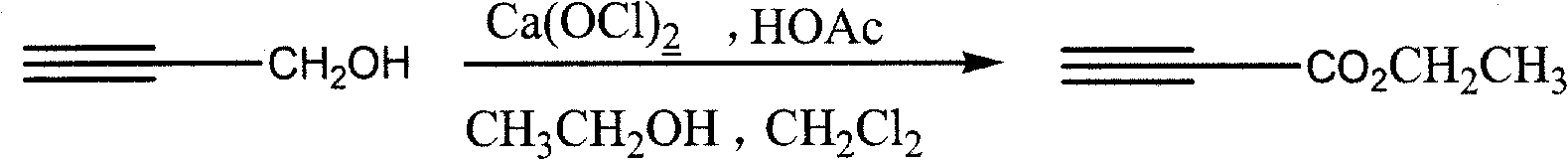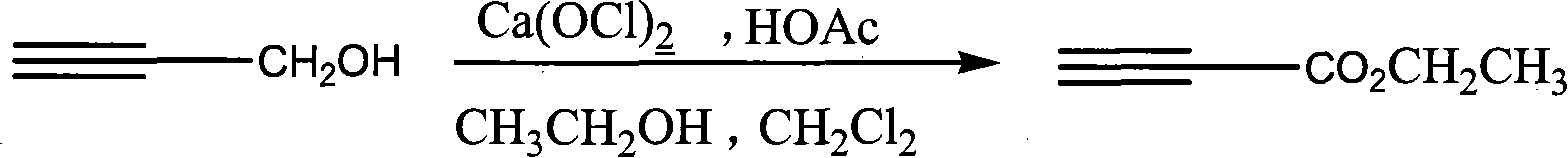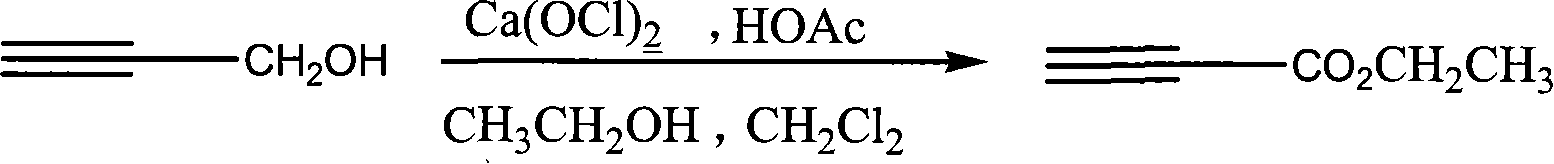Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
87 results about "Propiolic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
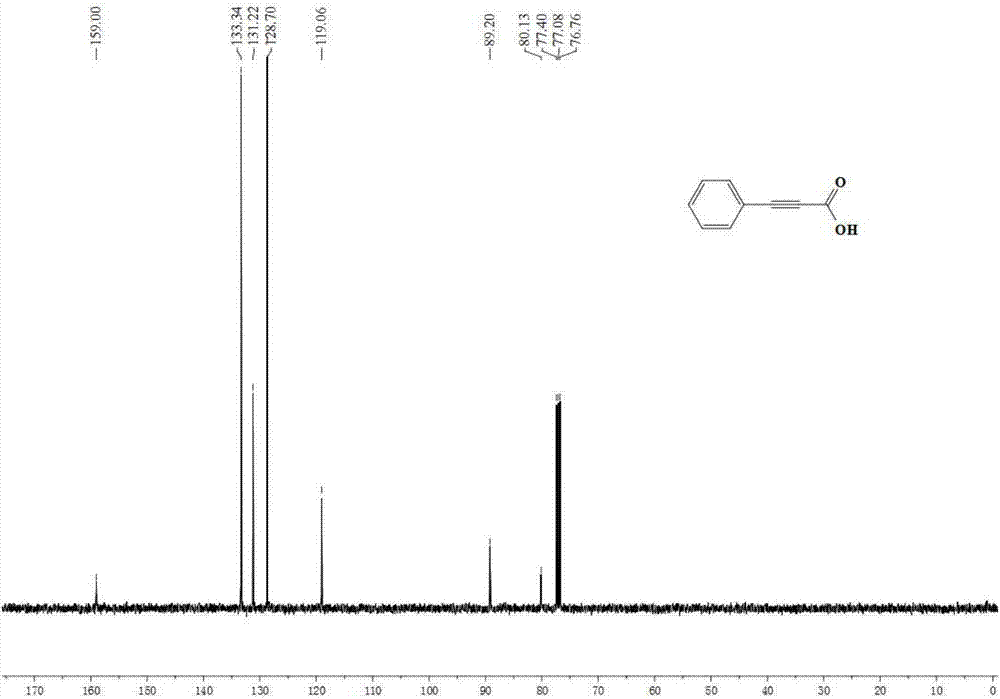
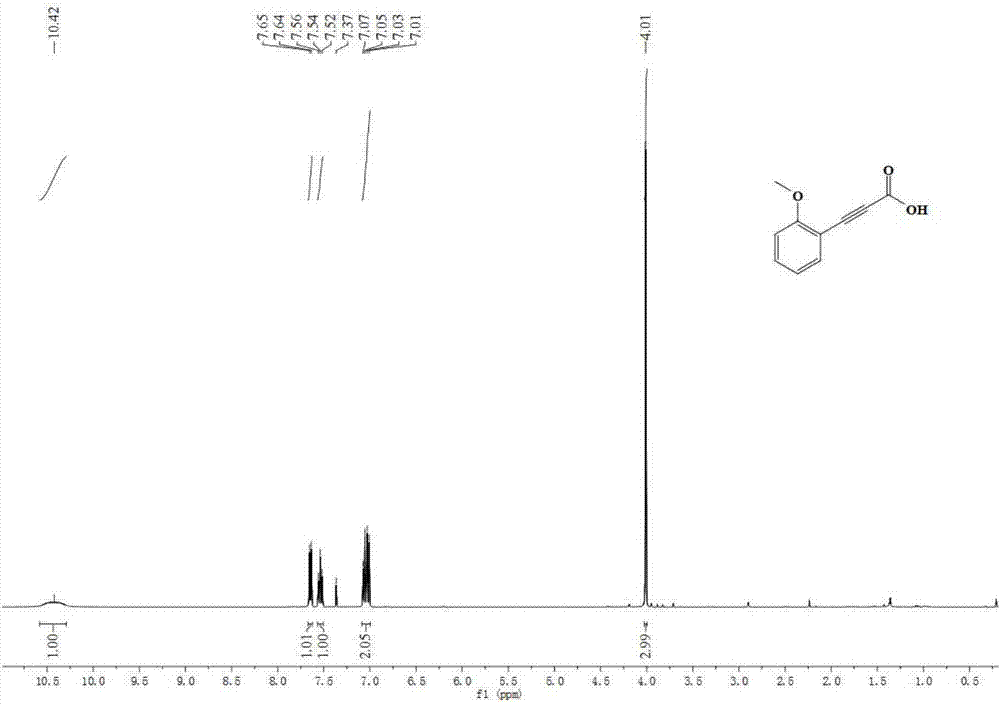
Propiolic acid is the organic compound with the formula HC₂CO₂H. It is the simplest acetylenic carboxylic acid. It is a colourless liquid that crystallises to give silky crystals. Near its boiling point, it decomposes.
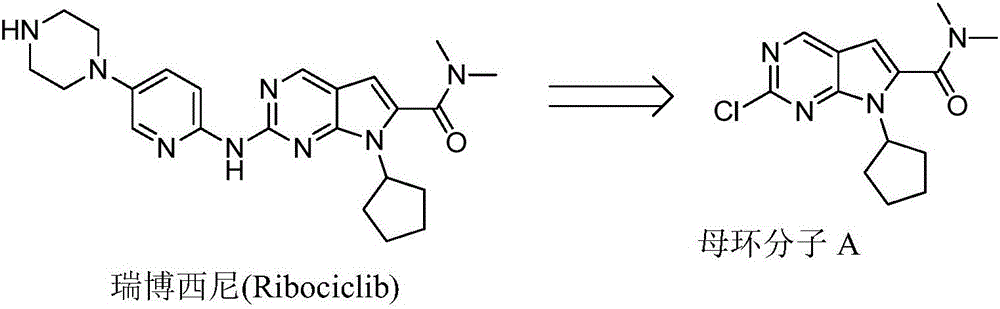
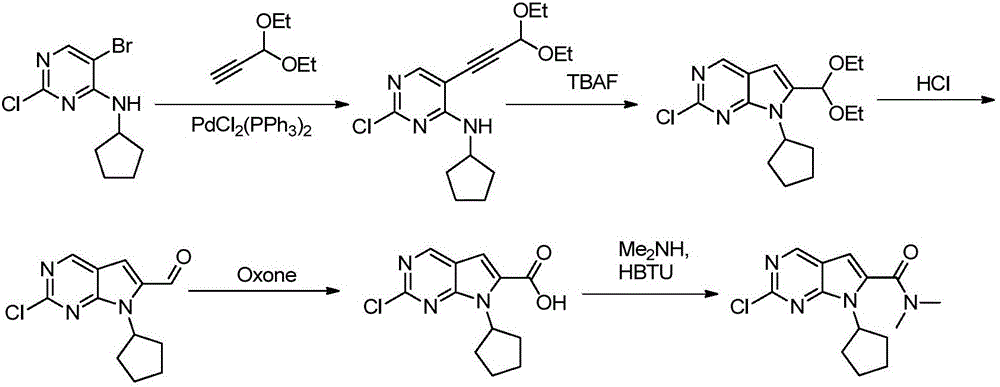
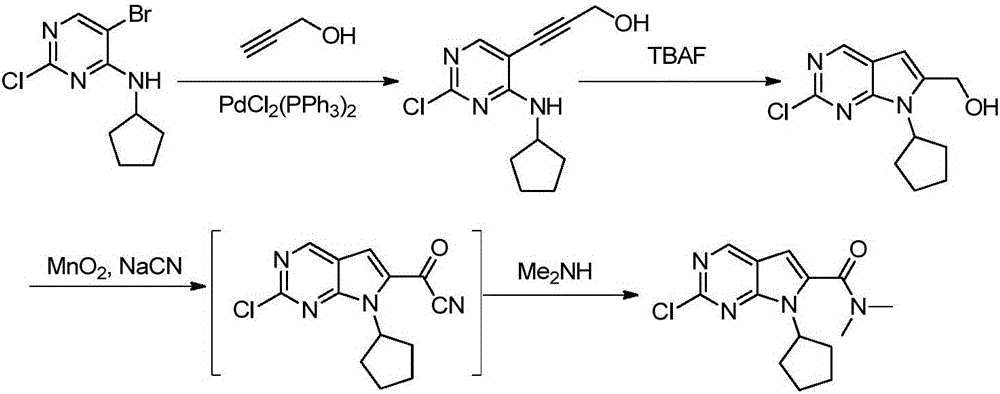
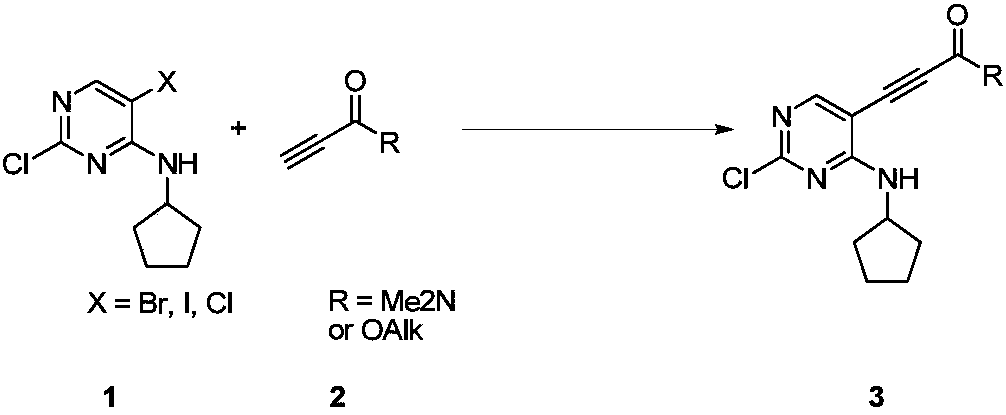
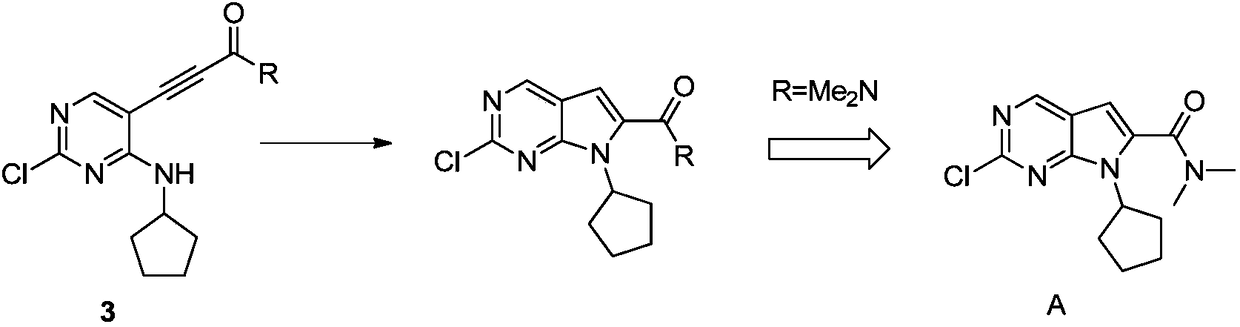
New synthesis method of ribociclib intermediate
ActiveCN106478641AEasy to operateImprove coupling conditionsOrganic chemistrySynthesis methodsSide chain
The invention discloses preparation of a ribociclib key intermediate A; propiolic acid ester or amide 2 is directly used as a Sonogashira coupling side chain, coupling conditions are optimized, an intermediate 3 is obtained with relatively high yield, the intermediate 3 is directly subjected to ring closing under simple conditions to complete construction of a mother ring molecule, to obtain a structural formula A or a precursor ester 4 of the structural formula A, and the precursor ester 4 is subjected to hydrolysis and condensation to obtain the structural formula A. The route has simple operation, reaction steps are shorted, the yield is relatively high, and the purity of the obtained product is relatively high, and the method is suitable for enlarged production, wherein the reaction route is described in the specification.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
Hyperbranched polytriazole formate as well as preparation method and application thereof
ActiveCN102585220AHigh StereoselectiveNo protectionFluorescence/phosphorescenceLuminescent compositionsFormateSolvent
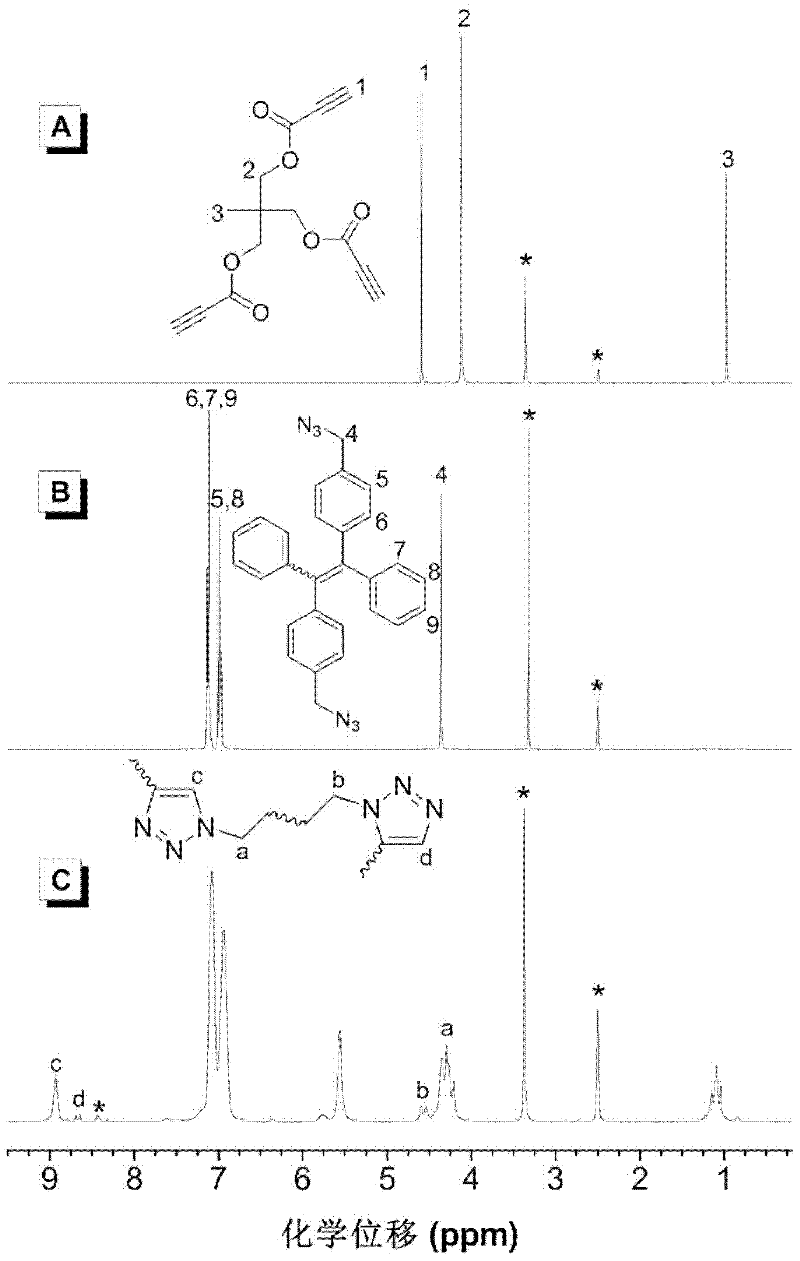
The invention discloses hyperbranched polytriazole formate as well as a preparation method and application thereof. The preparation method of hyperbranched polytriazole formate comprises the following steps: firstly, synthesizing binary azide containing a tetraphenyl ethylene unit; then synthesizing a ternary ester compound containing alkynyl based on ternary alcohol and propiolic acid as raw materials; and finally, carrying out non-metal-catalytic 'click' polymerization reaction under the heating condition in a polar solvent by utilizing the azide and the alkynyl-containing ester compound soas to obtain a target polymer in high yield. The hyperbranched polytriazole formate prepared by using the method is high in 1,4-stereoregularity, good in workability and high in thermal stability, degradability, illumination patterning and aggregation-induced emission property. The invention also discloses application of hyperbranched polytriazole formate in detection of a polynitroarene explosive.
Owner:ZHEJIANG UNIV
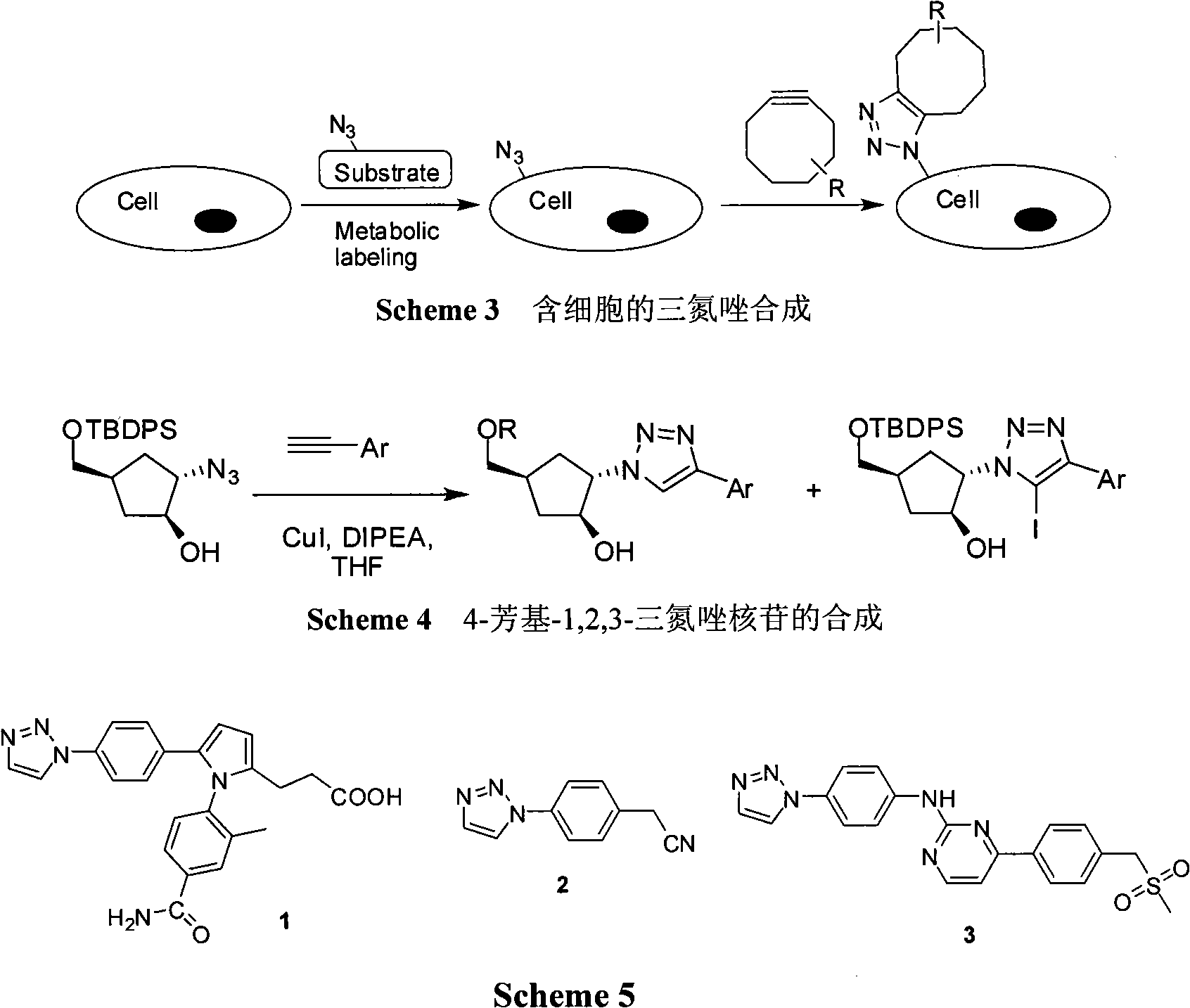
Method for synthesizing 1-substituted-1,2,3-tolyltriazole
InactiveCN101792438AEasy to operateHigh yieldOrganic chemistryChromatographic separationOrganic synthesis
The invention belongs to the technical field of organics and medicinal synthesis and particularly relates to a method for synthesizing 1-substituted-1,2,3-tolyltriazole, which comprises the following steps: adding cuprous halide or copper sulfate serving as a catalyst, sodium ascorbate, an organic trinitride compound and propiolic acid into a solvent, magnetically stirring the solution to perform reactions at the temperature of between room temperature and 200 DEG C below zero for 0.5 hour to 3 days, extracting the resulting product by using ethyl acetate or dichloromethane after the reactions are finished, washing an organic layer with saturated salt solution, drying the organic layer with anhydrous sodium sulfate, removing the solvent to obtain a coarse product, and subjecting the coarse product to column chromatographic separation and purification by using a mixture of ethyl acetate and petroleum ether as leacheate to obtain the required product, wherein the molar ratio of the propiolic acid to the organic trinitride compound is 1-2.0:1; the molar ratio of the cuprous halide or copper sulfate serving as the catalyst to the organic trinitride compound is 0.1-0.3:1; and the molar ratio of the sodium ascorbateto the organic trinitride compound is 0.2-0.4:1. The compound of the invention contains 1,2,3-tolyltriazole structural units with physiological activity and can be used for synthesizing and modifying organically synthesized intermediates and medicaments. Meanwhile, a novel and effective synthesis method is provided for medicament screening.
Owner:TONGJI UNIV
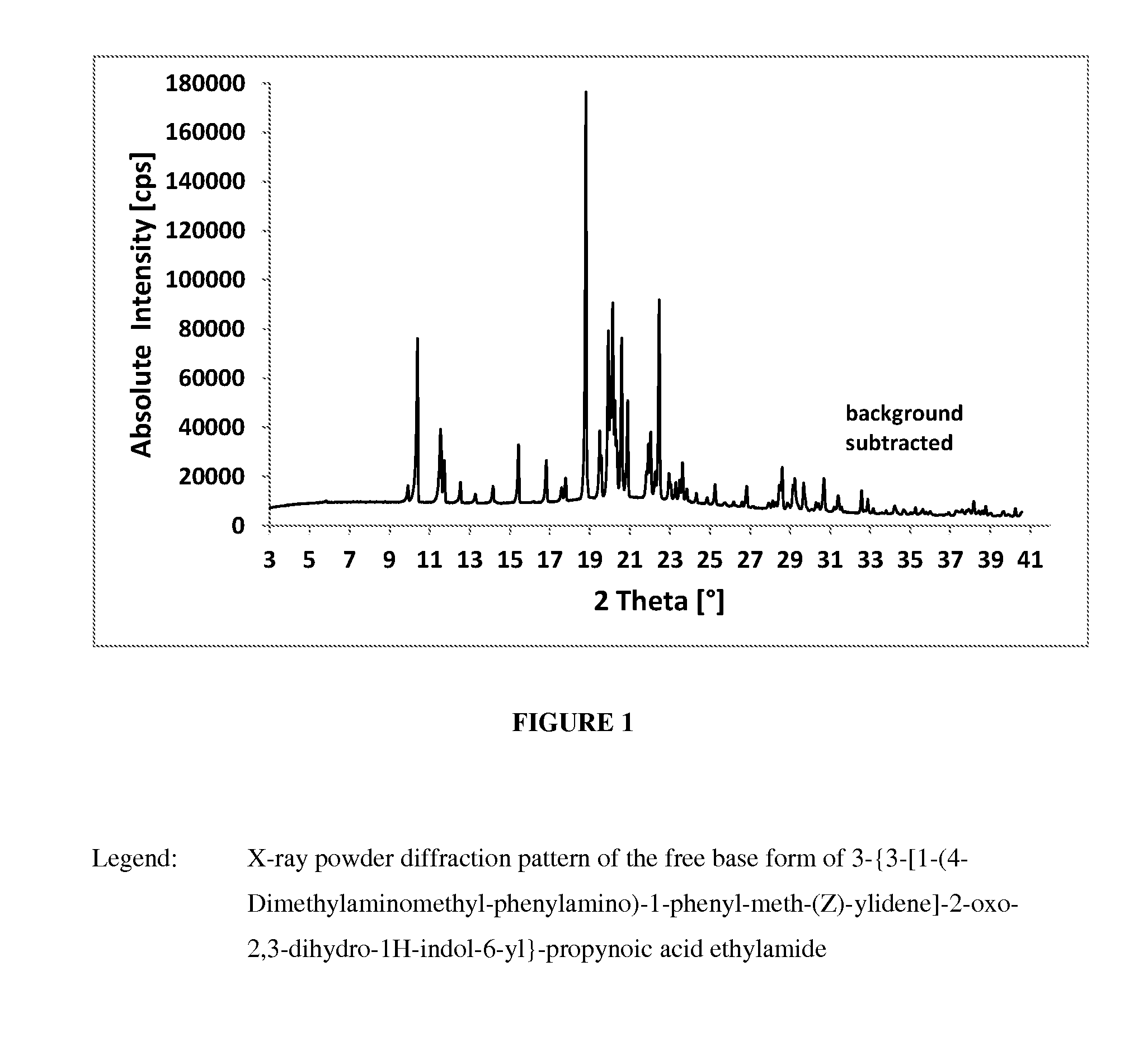
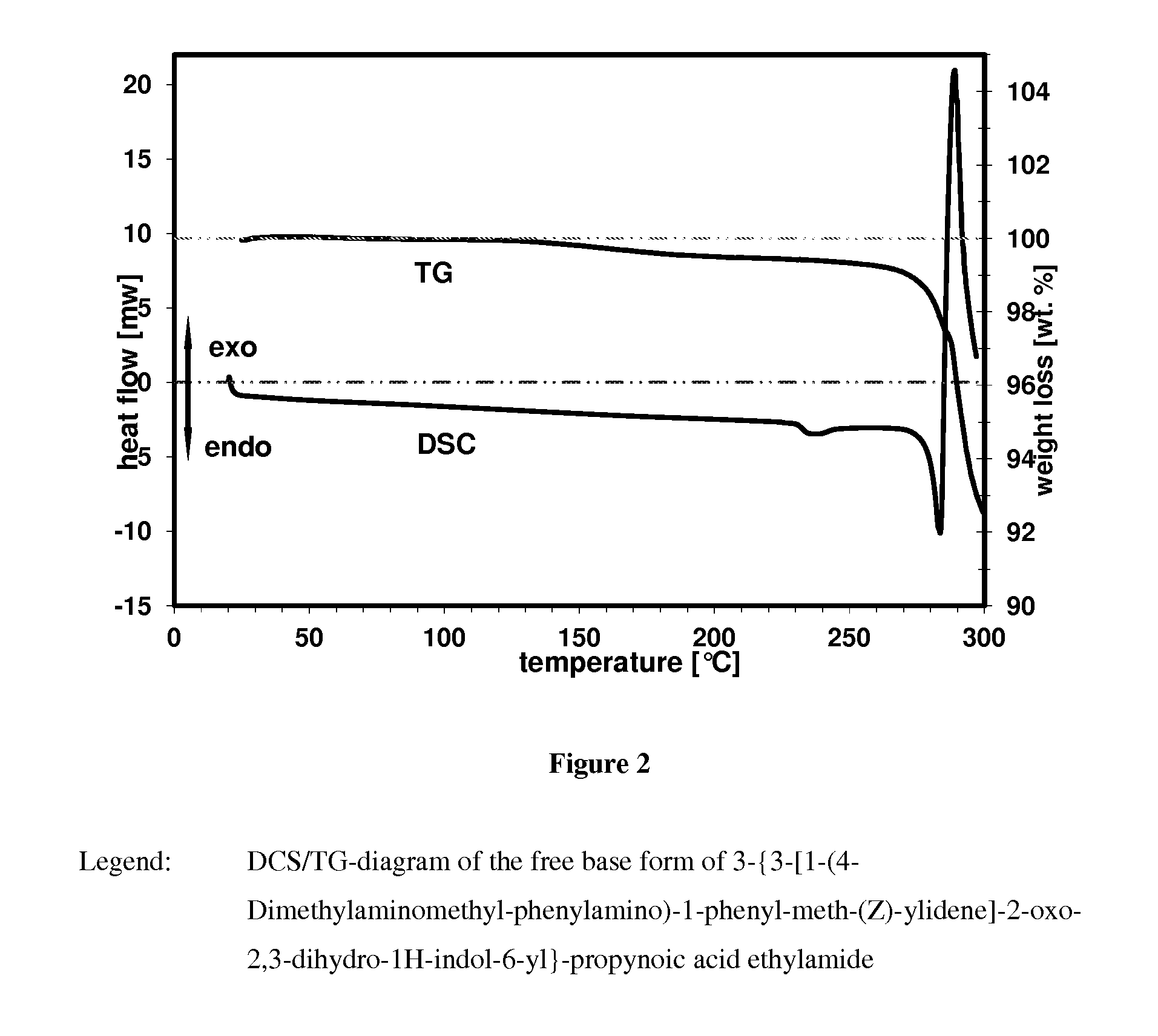
Crystalline form of a indolinone derivative and its use
InactiveUS20140018372A1Treating and preventing disorderAvoid adjustmentBiocideOrganic chemistryMeth-Propynoic acid
A crystalline form of 3-{3-[1-(4-Dimethylaminomethyl-phenylamino)-1-phenyl-meth-(Z)-ylidene]-2-oxo-2,3-dihydro-1H-indol-6-yl}-propynoic acid ethylamide.
Owner:BOEHRINGER INGELHEIM INT GMBH
Method for preparing propiolic acid compounds
ActiveCN105585473AClear structureSimple structureCarboxylic acid nitrile preparationOrganic compound preparationPhenyl-acetyleneAlkyne
The invention discloses a method for preparing propiolic acid compounds. An ionic type iron (III) complex containing monoimide functionalized imidazolium cations is taken as the single-component catalyst, carbon dioxide is taken as the carboxylation reagent, and various propiolic acid compounds are prepared through carboxylation reaction of terminal alkyne under normal pressure. The terminal alkyne substrate relates to phenylacetylene, substituted phenylacetylene, heterocyclic aryne, aromatic diyne or aliphatic series terminal alkyne. The method for preparing the propiolic acid compounds through carboxylation reaction of terminal alkyne and carbon dioxide under the catalysis of the iron-based catalyst is provided for the first time. Compared with the prior art, the method has the advantages that the catalyst is more environmentally friendly, synthesis is easier, reaction conditions are mild, and catalytic activity and functional group tolerance are unchanged or improved.
Owner:东营悦来湖园区运营管理有限公司
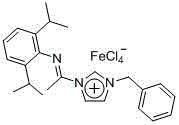
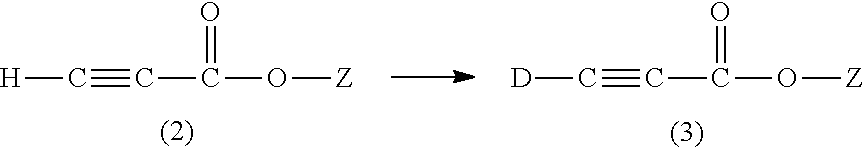
Methods for the synthesis of deuterated acrylate salts
ActiveUS20130079554A1Significant scaleLess-costly and more straightforwardOrganic compound preparationOrganic chemistry methodsPolymer sciencePalladium catalyst
A method for synthesizing a deuterated acrylate of the Formula (1), the method comprising: (i) deuterating a propiolate compound of Formula (2) to a methyne-deuterated propiolate compound of Formula (3) in the presence of a base and D2O: and (ii) reductively deuterating the methyne-deuterated propiolate compound of Formula (3) in a reaction solvent in the presence of deuterium gas and a palladium-containing catalyst to afford the deuterated acrylate of the Formula (1). The resulting deuterated acrylate compounds, derivatives thereof, and polymers derived therefrom are also described.
Owner:UT BATTELLE LLC
Circular polymer and preparation method thereof
The invention relates to a circular polymer and a preparation method thereof. The active anion polymerizing and end-capping techniques and the ring-opening polymerization (ROP) are adopted to synthesize a linear polymer with hydroxyls at both ends; sodium hydride (NaH) and propine bromide are adopted to modify chain-end hydroxyls into propargyls; the ROP is adopted to synthesize a linear polymer which is polycaprolactone (PCL) with hydroxyls at both ends; the propiolic acid is adopted to modify chain-end hydroxyls into propargyls; the atom transfer radical polymerization (ATRP) is adopted to synthesize linear polymer with bromine-based group at two ends; the nitrogen-oxygen free radical compound containing propargyls is adopted to modify chain-end bromine-based groups into propargyls; and the Glaser coupling reaction of the propargyls is adopted to synthesize the circular polymer in the presence of cuprous bromide (CuBr) and N, N, N', N'' and N''-pentamethyl diethylenetriamine (PMDETA).
Owner:FUDAN UNIV
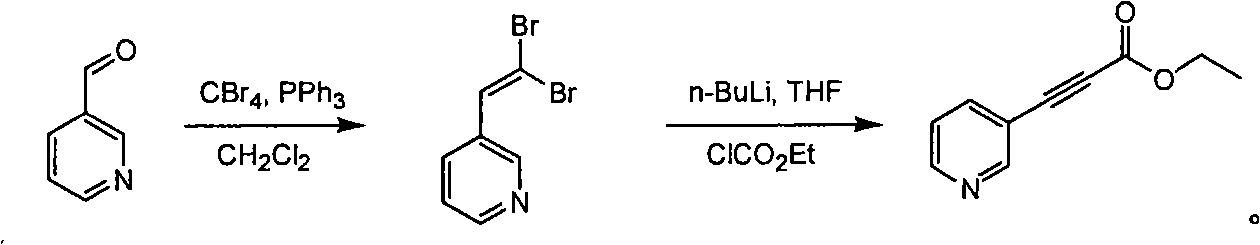
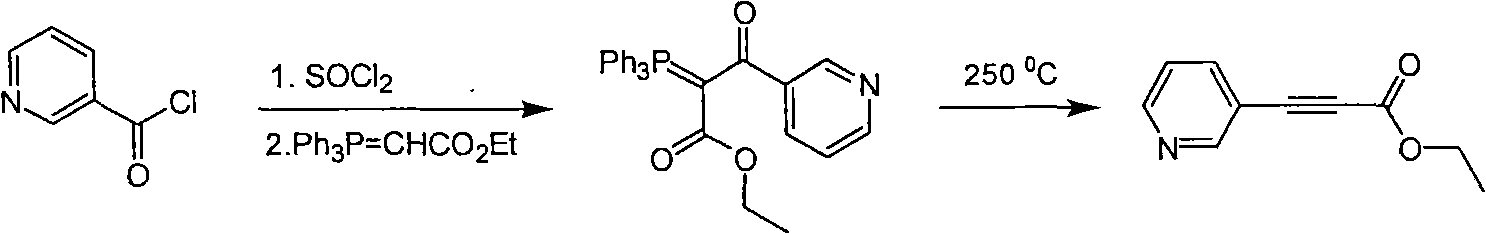
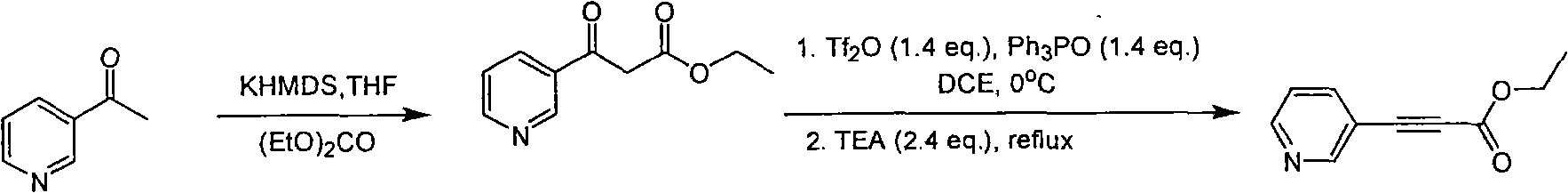
Method for synthesizing pyridine propiolic acid ester
InactiveCN101654431AReasonable choice of reaction processNo purification requiredOrganic chemistryChemical reactionChloroformate
The invention relates to a method for preparing pyridine propiolic acid ester and mainly solves the technical problem of high temperature requirement in the prior process for synthesizing the pyridinepropiolic acid ester. In the method, pyridylaldehyde serving as a readily available and large-scale production material undergoes a witting reaction, and the resulting product undergoes an addition-elimination reaction with chloroformate to obtain the corresponding pyridine propiolic acid ester. The chemical reaction formula is shown as above. The invention provides the method for synthesizing the pyridine propiolic acid ester, which has the advantages that: the raw materials are low in price and readily available, the total yield is 50 to 60 percent, and the intermediate is unnecessarily purified.
Owner:上海药明康德新药开发有限公司
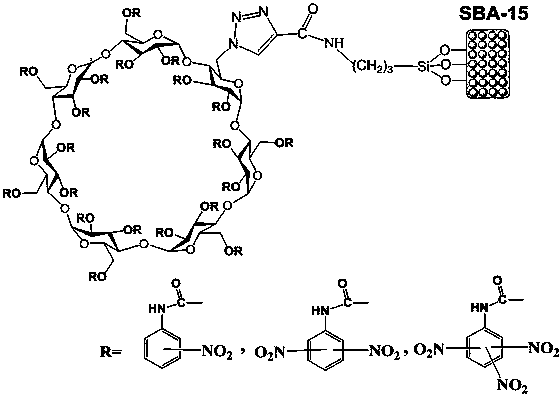
Preparation method of nitrophenylcarbamate full-derived beta-cyclodextrin bonded ordered mesoporous SBA-15 chiral stationary phase
InactiveCN103881107AHigh bonding capacitySimple methodOther chemical processesBond orderN dimethylformamide
The invention relates to a preparation method of a bonded SBA-15 silica gel chiral stationary phase. The preparation method comprises the major steps of firstly, preparing an azido-containing beta-cyclodextrin ligand from beta-cyclodextrin in an anhydrous solvent, secondly, preparing alkynyl-containing bonded ordered mesoporous SBA-15 silica gel from 3-aminopropyltriethoxysiloxane and propynoic acid, thirdly, bonding the azido-containing beta-cyclodextrin to the surface of the SBA-15 silica gel by virtue of a click reaction by taking anhydrous N,N-dimethylformamide as a solvent and CuI(PPh3) as a catalyst, and fourthly, modifying the product of the third step by using a nitro-containing phenyl isocyanate with anhydrous N,N-dimethylformamide as a solvent, and reacting to obtain the nitrophenylcarbamate full-derived beta-cyclodextrin bonded SBA-15 silica gel chiral stationary phase. The preparation method is simple and convenient, low in cost and wide in applicability.
Owner:NANCHANG UNIV
Method for preparing propiolic acid and derivatives thereof under mild condition
ActiveCN108424367AReduce usageMild reaction conditionsCarboxylic acid nitrile preparationOrganic compound preparationPropiolic acidFunction group
The invention provides a novel method for preparing propiolic acid compounds through a domino reaction. The method comprises a step of subjecting terminal alkyne compounds, hydrosilane and CO2 to thedomino reaction under the catalysis action of Lewis base so as to obtain propiolic acid compounds. According to the invention, common Lewis base is used as a promoter, and corresponding propiolic acidcompounds containing different function groups can be efficiently produced through a reaction of the terminal alkyne compounds with hydrosilane and normal-pressure CO2 under a mild condition (a temperature of 40 DEG D). According to the method, CO2 is used as a raw material; the cheap Lewis base is used as the promoter; usage of precious metals is avoided; the domino reaction is employed; purification and separation of intermediates are not needed; and reaction conditions are mild. Thus, the method is an efficient cheap green synthetic method and has good industrial application value.
Owner:INST OF CHEM CHINESE ACAD OF SCI
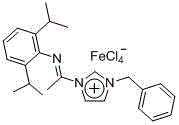
Preparation method of propiolic acid compounds
ActiveCN106946682ALower reaction costExperiment operation is simpleOrganic compound preparationCarboxylic compound preparationSynthesis methodsAlkyne
The invention relates to CO2 activation and conversion and related chemical technical fields, and specifically relates to a synthesis method of propiolic acid compounds. The method is characterized in that in the presence of an added additive and alkalis, terminal alkynes directly react with CO2 to generate propiolic acid compounds. The proper terminal alkyne substrate may be phenyl acetylene, substituted phenyl acetylene, heterocyclic aryl alkynes, or aliphatic terminal alkynes. Compared with the prior art, the invention mainly provides a novel and simple reaction system. According to the preparation method, potassium carbonate is taken as the alkali, quaternary ammonium salts are taken as the additive, and acetonitrile, which is convenient for the post treatment, is taken as the solvent. The preparation method is reported for the first time. The reaction system does not need any transition metal catalyst; the experiment operation is simple; the raw materials are cheap and easily available, and the preparation method is environment-friendly and generates great application value, social benefits, and economic benefits.
Owner:DALIAN UNIV OF TECH
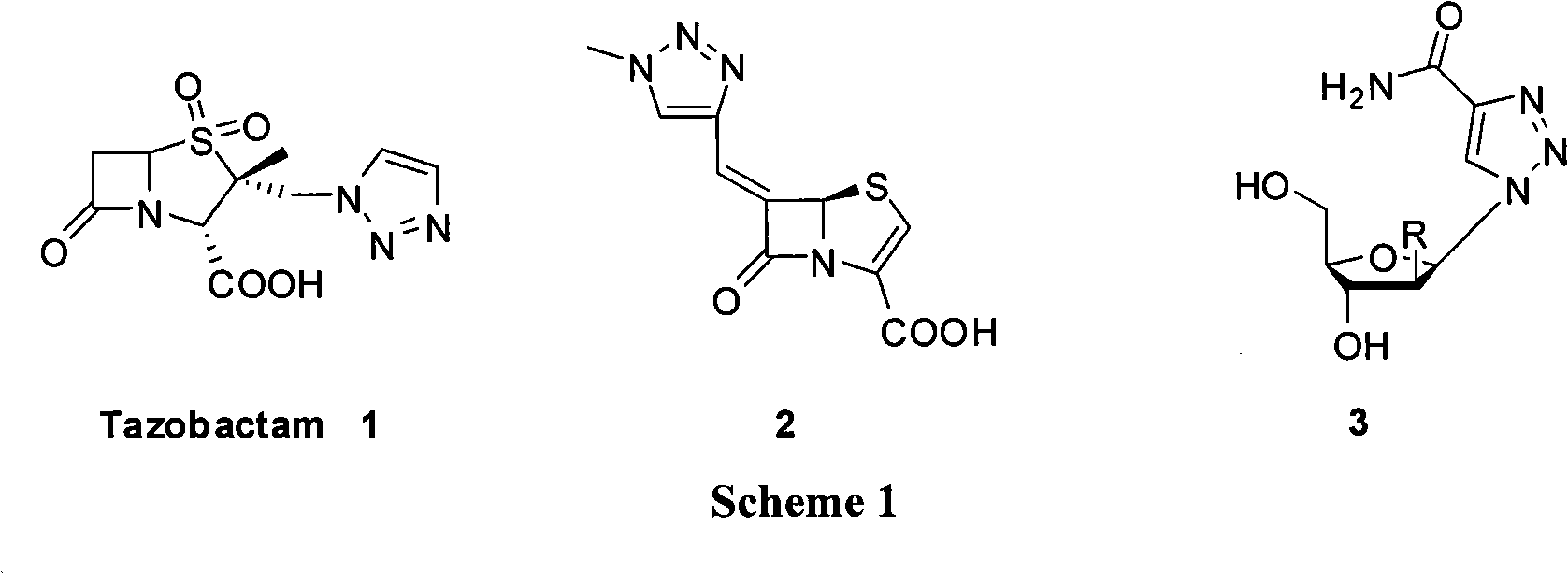
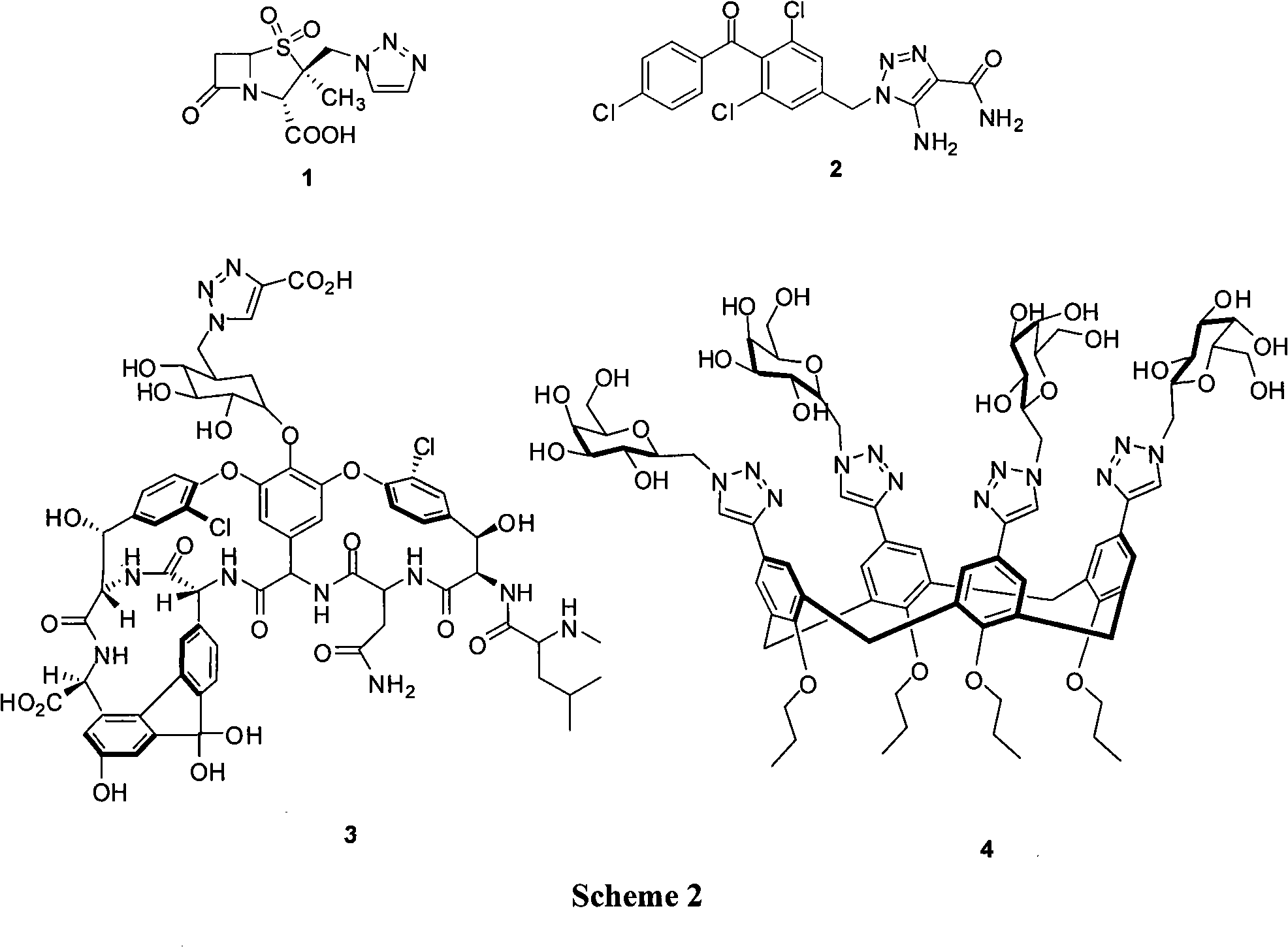
Method for synthesizing deuterated tazobactam
ActiveCN103012431AHigh yieldCarbon-deuterium bonds are stableOrganic chemistryDecompositionSodium ascorbate
The invention discloses a method for synthesizing deuterated tazobactam. The method comprises the following steps: adding 2beta-azomethylpenicillanic acid-1beta-oxide, propiolic acid, a catalyst containing copper or cuprous ion and sodium ascorbate in a deuterated solvent in turn, stirring at 25-200 DEG C for reaction for 1 to 48 hours, and after the reaction, performing extraction, filtering and column chromatography to obtain the deuterated tazobactam. Through the method, according to the method for synthesizing the deuterated tazobactam, the deuterated tazobactam is a novel beta-lactamase inhibitor of the penicillanic sulfone type and can be used for treating various bacterial infections; the method has the characteristics of mild reaction condition, short synthesis procedure, simple process and high yield, the safety during the reaction is effectively improved by using propiolic acid as the raw material, and the carbon-deuterium chain in the obtained deuterated tazobactam molecule is so stable that the medicine decomposition process can be effectively retained, therefore, the deuterated tazobactam has a longer action time in the body and is better than the normal tazobactam.
Owner:SUZHOU ROEING BIOPHARMACEUTICALS CO LTD
Synthesis process of beta-cyclopropylamino acrylate
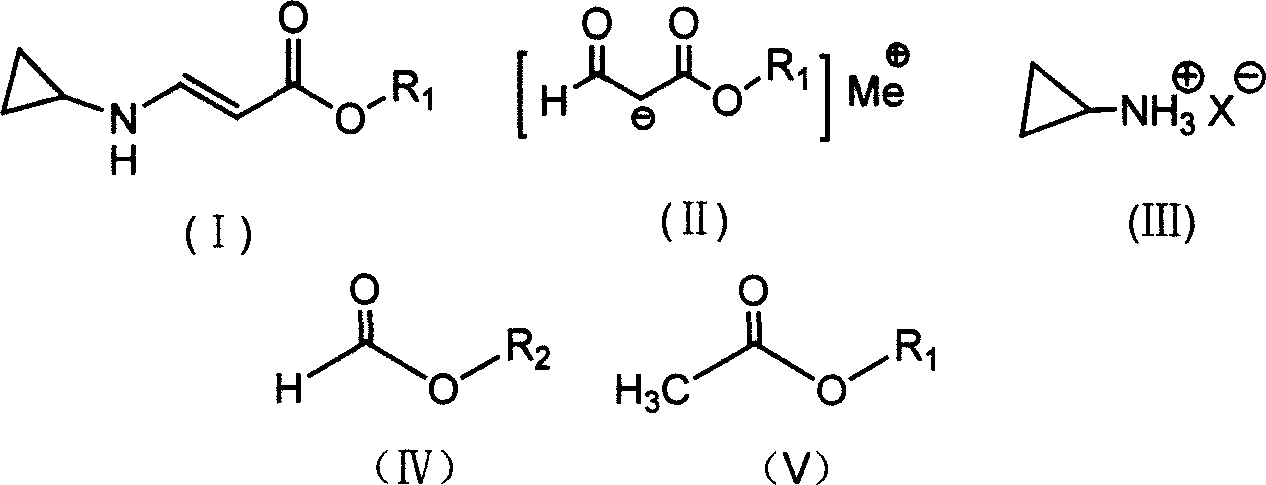
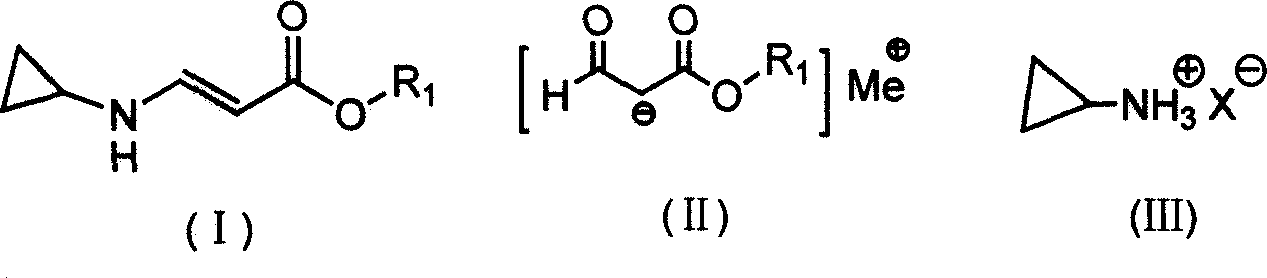
InactiveCN101020647AReduce usageEmission reductionOrganic compound preparationAmino-carboxyl compound preparationFormatePropiolic acid
The present invention provides synthesis process of beta-cyclopropylamino acrylate. The synthesis process includes the following steps: 1. the condensation reaction of formate and acetate in the presence of alkali metal or alkali metal hydride to produce alkali metal salt as shown; and 2. amine substituting and eliminating reaction of the alkali metal salt and ammonium salt in organic solvent to obtain beta-cyclopropylamino acrylate as shown. The present invention has simple synthesis process, convenient operation, facile material, low cost and less pollution.
Owner:HANGZHOU NORMAL UNIVERSITY +2
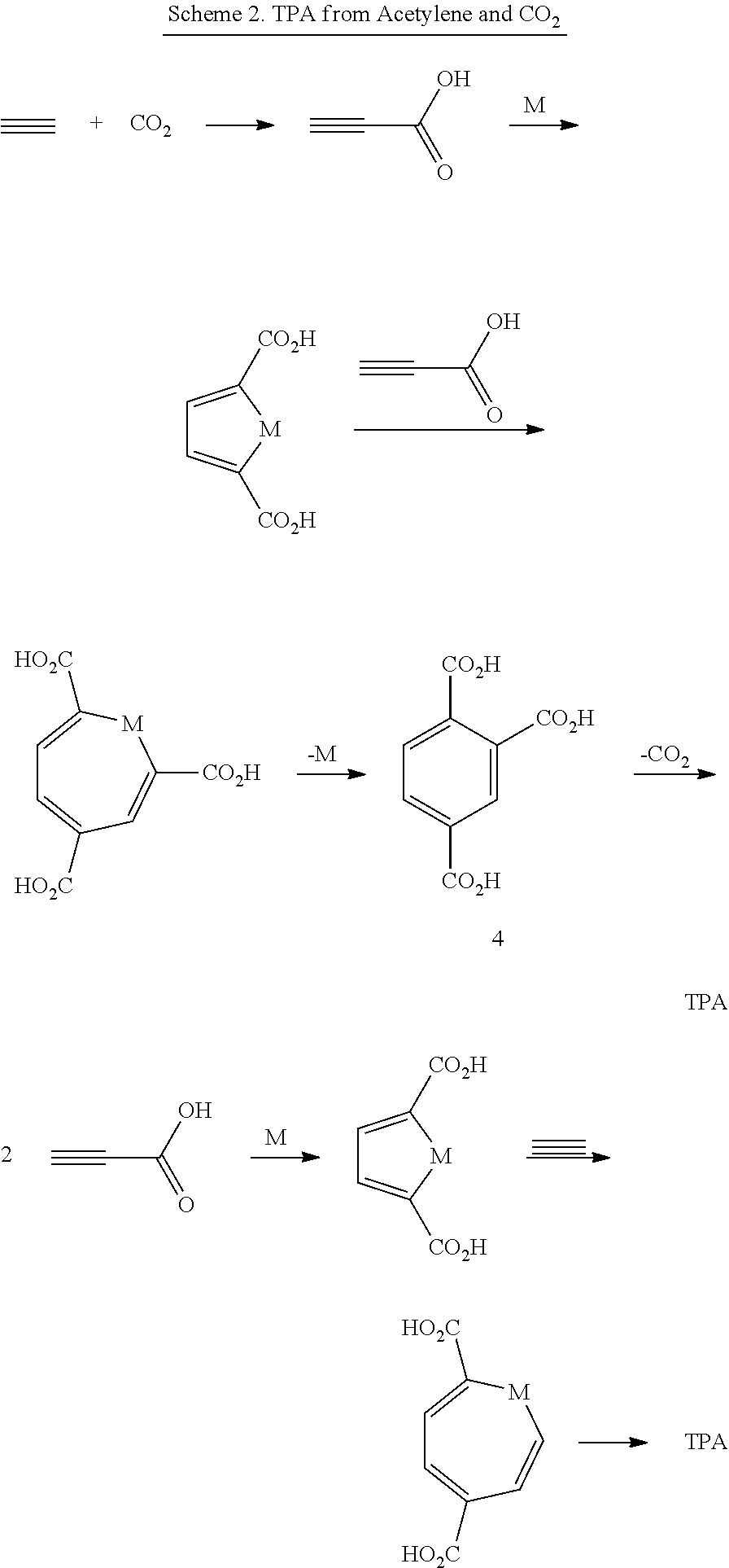
Production Of Terephthalic Acid Via Reductive Coupling Of Propiolic Acid Or Propiolic Acid Derivatives
ActiveUS20160264506A1Organic compound preparationCarboxylic preparation by ozone oxidationPropiolic acidTerephthalic acid
A method of making terephthalic acid via reductive coupling of two molecules of propiolic acid or propiolic acid derivatives is presented. The reductive coupling can be catalyzed by compounds comprising metals, and propiolic acid or propiolic acid derivatives can be produced from acetylene and carbon dioxide. At least 4 of the 8 carbons in the terephthalic acid are non-fossil-derived.
Owner:THE PROCTER & GAMBLE COMPANY
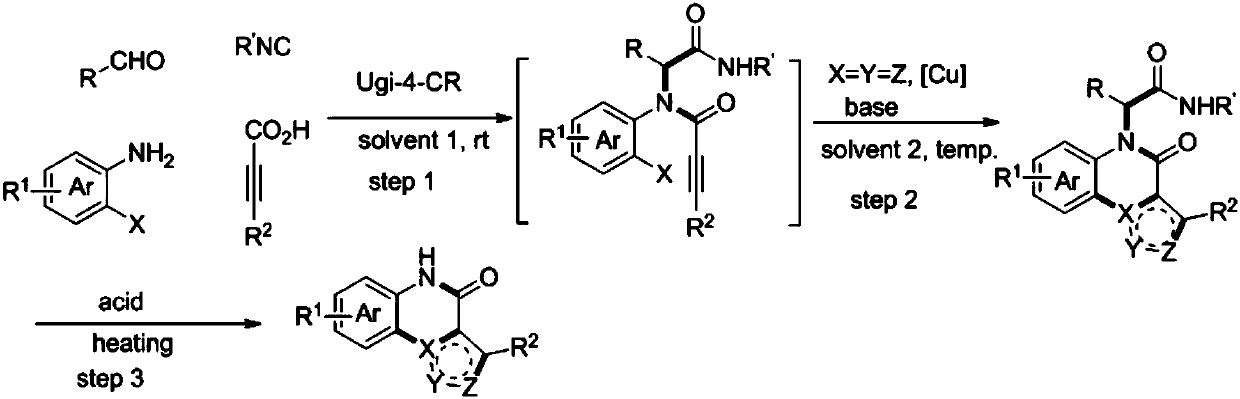
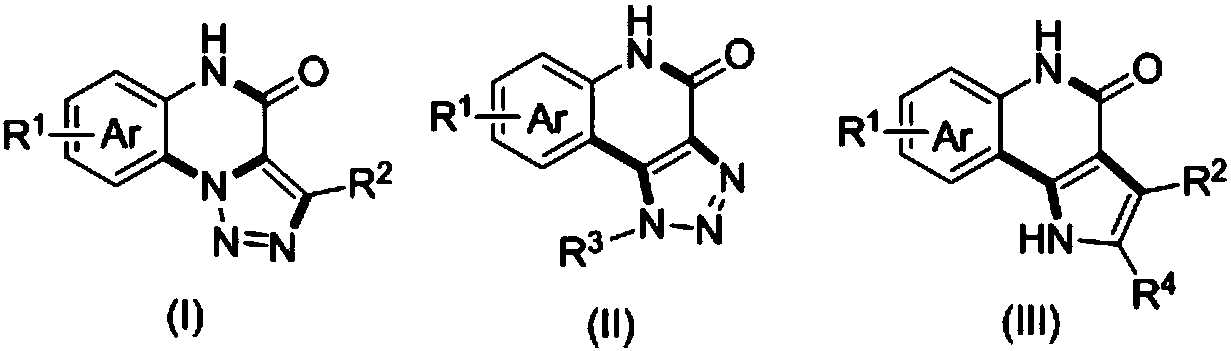
Method for compounding nitrogenous polynary heterocyclic compound
ActiveCN107602570AImprove compatibilityRaw materials are easy to obtainOrganic chemistryChemical synthesisCatalytic effect
The invention belongs to the field of chemical synthesis and discloses a method for compounding a nitrogenous polynary heterocyclic compound. The method comprises the following steps: (1) performing Ugi four-component reaction on aldehyde RCHO, iso-nitrile compound R'NC, o-halide arylamine and substituted propiolic acid R2-C is equivalent to C-CO2H under the solvent existence condition and removing the solvent after ending the reaction, thereby acquiring a product of the step (1); (2) performing consecutive reaction on the product acquired in the step (1) and 1,3-dipole reaction reagent underthe catalytic effect of cupric salt under the solvent existence condition and the inert atmosphere and then purifying the acquired reaction solution, thereby acquiring the product acquired in the step(2); (3) performing acid promotion reaction on the product acquired in the step (2) and the acid under a heating condition with or without solvent and purifying the acquired reaction solution after ending the reaction, thereby acquiring a target product. The method has wide substrate applicability; the preparation method is simple and efficient; the nitrogenous polynary heterocyclic compound hashigh functional group compatibility and wide popularizing prospect.
Owner:JINAN UNIVERSITY
2,3-disubstituted benzimidazo[1,2-alpha]pyrimidine compound and preparation method and application thereof
ActiveCN108250202AEasy to operateWide range of substratesOrganic chemistryFluorescence/phosphorescence2-aminobenzimidazoleSynthesis methods
The invention discloses a 2,3-disubstituted benzimidazo[1,2-alpha]pyrimidine compound and a preparation method and the application thereof. The compound adopts a structural formula as shown in the specification, wherein R represents any one of phenyl, C1-C4 alkyl-substituted phenyl, C1-C4 alkoxy-substituted phenyl, halogenated phenyl, cyano-substituted phenyl, thienyl and naphthyl. The compound isprepared from aromatic aldehyde, 2-aminobenzimidazole and propiolic acid as raw materials by a one-pot method under the coaction of a copper catalyst and an alkaline additive. A synthesis method is simple, intermediate separation is not required, operating steps are simplified, and the application range of a substrate is relatively wide. The compound has fluorescence performance and can be applied to selective fluorescent detection of low-concentrations of Fe<3+>.
Owner:SHAANXI NORMAL UNIV
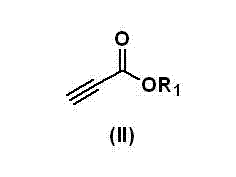
Method for preparing 1,4-dihydropyridine by taking acidic ionic liquid as catalyst
InactiveCN103044315AMethod environmentally friendlyReduce pollutionOrganic chemistryChemical recyclingPtru catalystDihydropyridine
The invention discloses a method for preparing 1,4-dihydropyridine by taking an acidic ionic liquid as a catalyst, comprising the following steps of: taking an amine compound shown in formula (I), a propiolate compound shown in formula (II) and a benzaldehyde compound shown in formula (III) as raw materials in a solvent-free condition, wherein the ratio of the amounts of substance of the raw materials is 1: (2 to 4): 1; reacting for 0.5 to 2 hours at a reaction temperature of 50-100 DEG C under the catalysis of the acidic ionic liquid; and performing separation treatment on the reaction solution to obtain the 1,4-dihydropyridine shown in formula (IV). According to the method, no solvent is used during the preparation process, thus simplifying operation process and reducing cost; the used catalyst is the acidic ionic liquid and capable of being cyclically used, and the technical process is environment-friendly; and the method is moderate in reaction conditions, simple and convenient to operate, easy to separate products, good in product quality and high in yield.
Owner:SHIJIAZHUANG UNIVERSITY
Synthetic method for tazobactam
The invention discloses a synthetic method for tazobactam. The method comprises the following steps: adding propiolic acid, 2 beta-azidomethyl penicillanic acid-1 beta-oxide, sodium ascorbate and a catalyst containing cuprous ions or copper ions to a solvent in sequence; stirring and reacting these materials for 0.5-72 h at 20-180 DEG C; and after the reaction is finished, extracting a reactant and carrying out column chromatography on the reactant so as to obtain the tazobactam. Through the method, the synthetic method for the tazobactam, disclosed by the invention, has the advantages as follows: the tazobactam is a novel sulbactam type beta-lactamase inhibitor and can be used for treating a plurality of bacterial infections; and compared with a method with acetylene as raw material, the method has the advantages as follows: through using the propiolic acid as the raw material, the safety in the reaction process is enhanced and an electricity absorbing carboxyls on a molecule is beneficial for carrying out cycloaddition reaction and preferably compatible with a reaction substrate; the reaction condition is mild; the reaction can be conducted at normal temperature; the operation process is convenient and simple; the yield of obtained products is high; and industrial production can be carried out on a large scale.
Owner:SUZHOU ROEING BIOPHARMACEUTICALS CO LTD
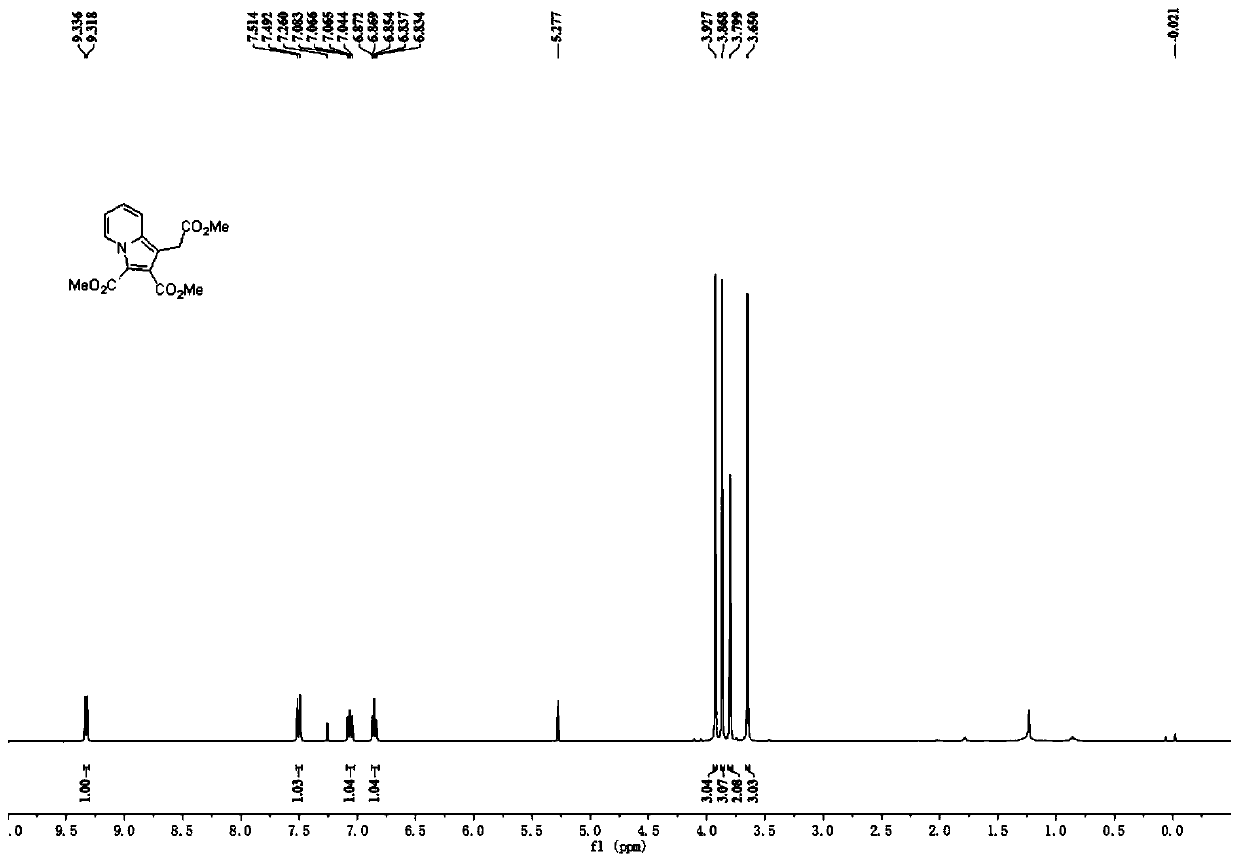
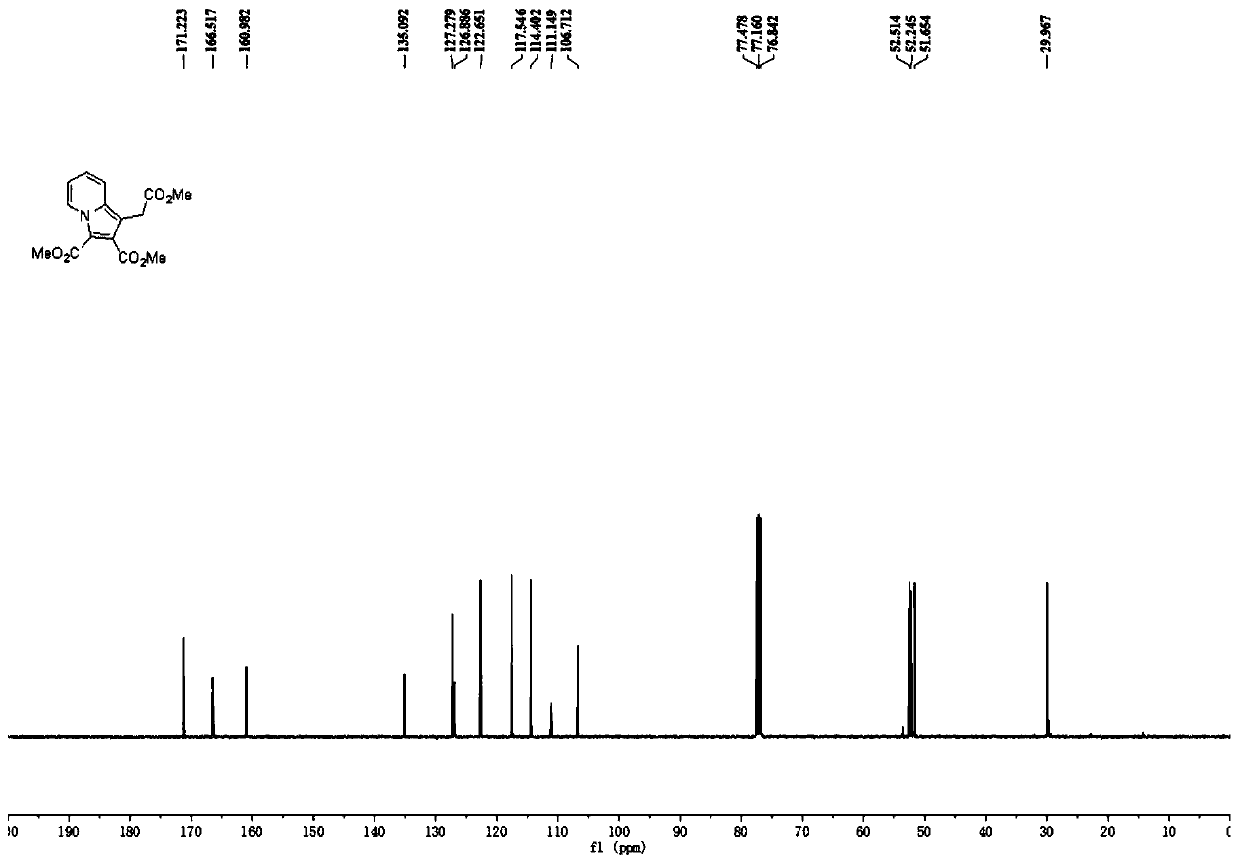
Polysubstituted indazine derivative and preparation method thereof
InactiveCN111440166AEasy to manufactureNo pollution in the processOrganic chemistryCombinatorial chemistryPerylene derivatives
The invention mainly aims to provide a polysubstituted indazine derivative and a preparation method thereof. The method realizes one-step simple and efficient synthesis of indazine derivatives from propiolate compounds and pyridine sulfur-containing ylide salt at 30 DEG C by using triethylamine and potassium carbonate as a mixed alkali and using common solvent dichloromethane as a solvent. The method has the advantages of simple reaction operation, mild conditions, no metal pollution, and easy realization of large-scale preparation; and a new method for synthesizing indazine without transitionmetal catalysis is developed.
Owner:SHENZHEN POLYTECHNIC
Preparation method of propiolic acid compounds
ActiveCN107353194ALower reaction costExperiment operation is simpleOrganic compound preparationCarboxylic compound preparationAlkyneBottle
The invention belongs to the technical fields of carbon dioxide activation and conversion and related chemistry, and discloses a preparation method of propiolic acid compounds. The preparation method comprises the following steps: (1) adding a copper catalyst, an additive, an alkali, and solid terminal alkynes into a reactor, adding an organic solvent under the protection of nitrogen gas, and filling CO2 into the reactor; or adding a copper catalyst, an additive, and an alkali into a reactor, adding an organic solvent and liquid terminal alkynes, and filling CO2 into the reactor; (2) sealing the reactor, and placing the reactor in an oil bath to carry out reactions; and (3) after reactions, opening a valve of the reactor to slowly release residual gas in the reactor, transferring the reaction liquid to a one-mouth bottle, carrying out concentration, diluting the concentrate by deionized water, adding n-hexane to carry out extraction, adding hydrochloric acid to carry out acidification, adding diethyl ether to carry out extraction, collecting the organic phase, washing the organic phase by a saturated saline solution, drying the organic phase by anhydrous sodium sulfate, filtering, and removing the solvent in vacuum to obtain the target material. The preparation method has the advantages of low reaction cost, simple experiment operation, mild reaction conditions, and easiness for industrialization.
Owner:DALIAN UNIV OF TECH
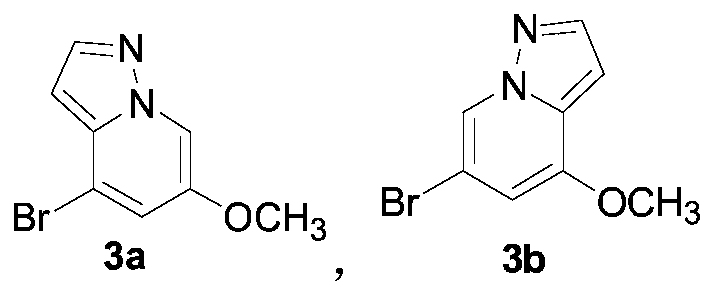
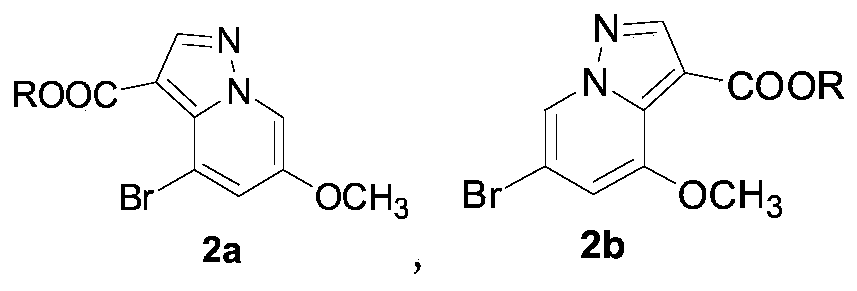
RET kinase inhibitor intermediates and preparation method thereof
The invention discloses RET kinase inhibitor intermediates and a preparation method thereof, and relates to the field of medicinal chemistry, wherein the method for synthesizing RET kinase inhibitorsrepresented by a formula (5a) and a formula (5b) comprises the steps: (a) carrying out a reaction of compounds represented by a formula (4a) and a formula (4b) with hydroxylamine sulfonic acid to formthe compounds represented by the formula (5a) and the formula (5b); and (b) carrying out a reaction of the compounds represented by a formula (3a) and a formula (3b) with phosphorus oxychloride to form compounds represented by the formula (4a) and the formula (4b); and (c) allowing compounds represented by a formula (2a) and a formula (2b) to form the compounds represented by the formula (3a) andformula (3b) under the action of an acid; and (d) after the reaction of the mixture of 3-bromo-5-methoxypyridine, hydroxylamine sulfonic acid and water is finished at the temperature of 70-100 DEG C,adding an alcoholic solution of alkali at room temperature, dropwise adding propiolate, and after the reaction is finished at room temperature, obtaining the compounds represented by the formula (2a)and the formula (2b), wherein R is methyl, ethyl, propyl or butyl.
Owner:厦门云凡医药科技有限公司
Preparation method of 4-aryl coumarin-3-phosphonate derivatives
InactiveCN103254236ARich typeRaw materials are easy to getGroup 5/15 element organic compoundsAntineoplastic agentsPhosphoric Acid EstersMolecular sieve
The invention discloses a preparation method of 4-aryl coumarin-3-phosphonate derivatives, which is characterized comprising the following steps: adding one of 3-aryl propiolic acid aryl ester derivatives, one of phosphite esters and a manganite octahedral molecular sieve into a solvent; and carrying out a reaction at a temperature of 20-150 DEG C so as to obtain a 4-aryl coumarin-3-phosphonate derivative. According to the invention, 3-aryl propiolic acid aryl ester derivatives are used as a starting material, so that raw materials are easily obtained and diverse in type; products obtained by using the method disclosed by the invention is diverse in type, both can be directly used, and can be applied to other further reactions; and the method is short in synthetic route, mild in reaction conditions, simple in reaction operation and post-treatment process, and high in yield.
Owner:SUZHOU UNIV
N-heterocyclic condensed tryptamine-beta-lactam derivative as well as preparation method and application thereof
InactiveCN111533732AHas antitumor activityEasy to operateOrganic chemistryAntineoplastic agentsCyanide compoundOrganic synthesis
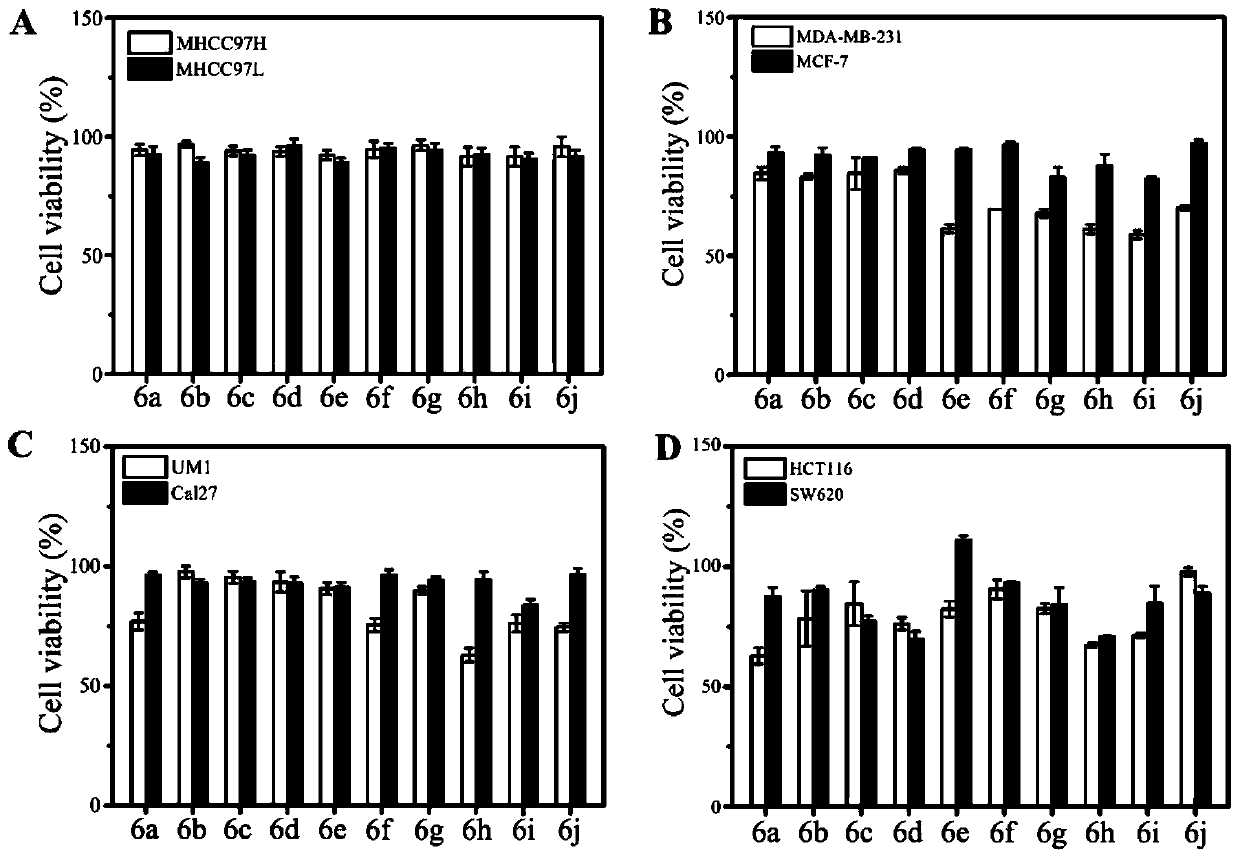
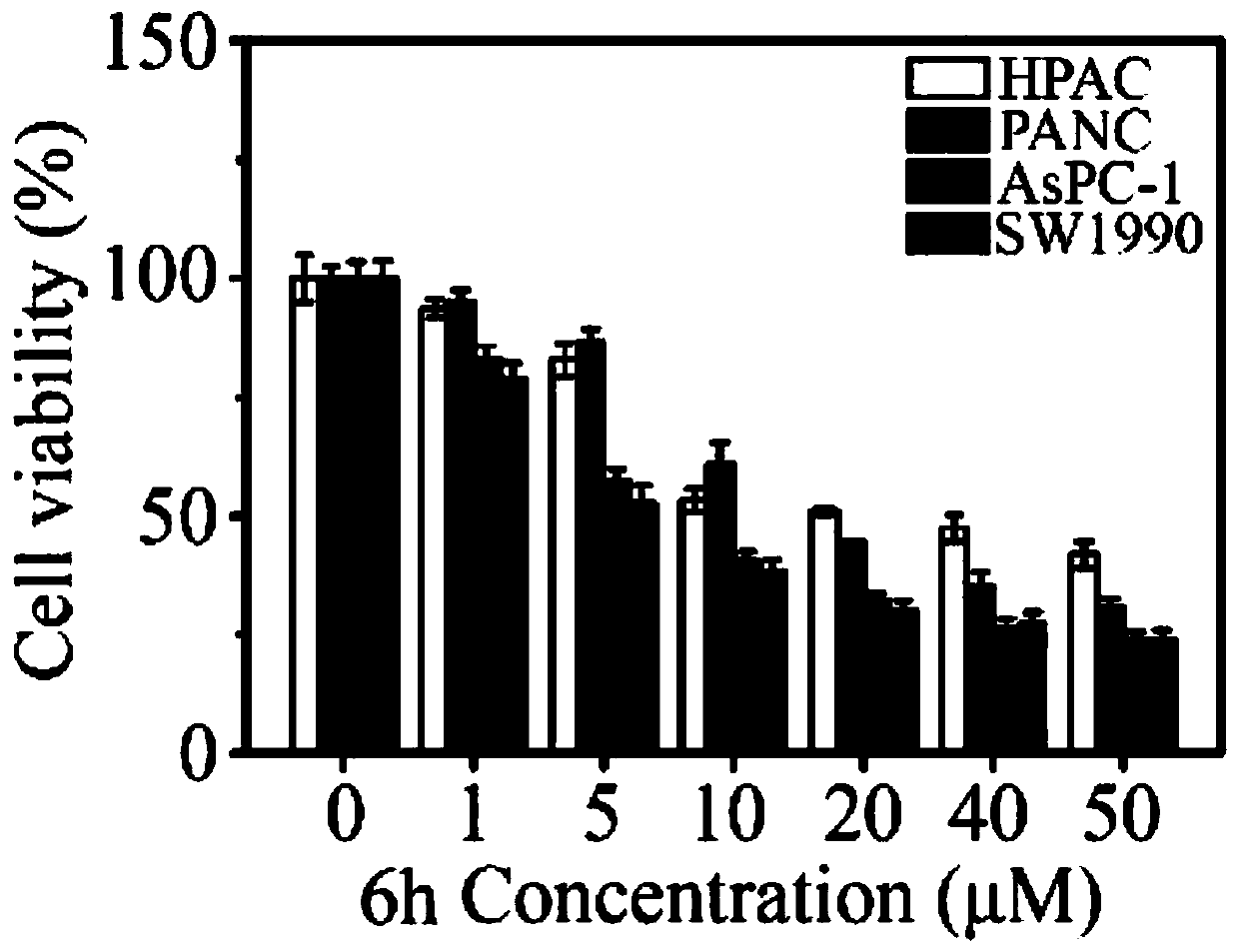
The invention relates to the technical field of organic synthesis, in particular to an N-heterocyclic condensed tryptamine-beta-lactam derivative as well as a preparation method and application thereof. The preparation method comprises the following steps: adding an aldehyde compound and propiolic acid into methanol and stirring; then adding tryptamine and isocyanide, and reacting under stirring;removing the methanol in a nitrogen atmosphere; dissolving obtained residues in acetonitrile, adding K2CO3, sealing the mixture, heating the mixture in a microwave oven, removing the acetonitrile, diluting with ethyl acetate, washing with saline water, drying with sodium sulfate, concentrating, purifying the obtained concentrate by silica gel column chromatography, and drying to obtain the N-heterocyclic condensed tryptamine-beta-lactam derivative. The N-heterocyclic fused tryptamine-beta-lactam derivative has a certain inhibition effect on the cell viability of liver cancer, breast cancer, head and neck squamous cell carcinoma and colon cancer cell lines, can inhibit or kill tumor cells, has antitumor activity, and can be applied to preparation of antitumor drugs.
Owner:CHONGQING UNIV OF ARTS & SCI
Alkynyl-containing conjugated polymers and synthetic method thereof
The invention discloses alkynyl-containing conjugated polymers and a synthetic method thereof, and belongs to the field of macromolecular compound synthesis. The prior triple bond-containing conjugated polymers and synthesis have the problems of low conversion rate of a monomer, poor dissolution of products and the like. The polymers are shown as a general formula (I). The monomer and an initiator are added into a reactor in a molar ratio of 2-100:1, and reacts at a temperature of between 20 DEG C below zero and 70 DEG C for 1 to 24 hours; and the products are dissolved by hydrochloric acid, separated, and dried in vacuum to obtain the alkynyl-containing conjugated polymers, wherein the monomer is methyl acetylenecarboxylate, ethyl acetylenecarboxylate or butyl acetylenecarboxylate; and the initiator is solid sodium hydride, potassium hydride, sodium methoxide, sodium alcoholate or organolithium. The alkynyl-containing conjugated polymers and the method have the advantages of wide reaction temperature range, high conversion rate of the monomer and the like. In the formula, R is -CH3, -CH2CH3, or -CH2CH2CH2CH3, and n is between 10 and 500.
Owner:BEIJING UNIV OF CHEM TECH
High-performance antibacterial preservative
InactiveCN105028518AGood antibacterial effectPlay a role in antiseptic preservationBiocideFungicidesBiotechnologyQuisqualis indica
The invention relates to a high-performance antibacterial preservative. The high-performance antibacterial preservative is prepared from the following raw materials in parts by weight: 7-11 parts of isopropanol, 6-8 parts of dimethyl ether, 11-15 parts coconut oil, 9-16 parts of chitosan, 11-18 parts of mandragora, 2-4 parts of propiolic acid, 7-9 parts of n-butyl alcohol, 4-8 parts of cyclohexanol, 3-9 parts of sodium dodecyl benzene sulfonate, 8-10 parts of polyacrylate, 6-11 parts of alkyl dimethyl benzyl ammonium chloride, 6-7 parts of Chinaberry seed, 0.5-2 parts of antiseptic corrosion inhibitor, 8-14 parts of rangooncreeper fruit and 2-5 parts of Eusteralis stellata. The high-performance antibacterial preservative has the beneficial effects that the antibacterial preservative has good antibacterial and bacteria-inhibitory effects, is capable of well playing preservative and fresh-keeping roles and has a remarkable killing effect on bacteria, fungi and alga.
Owner:QINGDAO MAIKE 3D HI TECH CO LTD
The synthetic method of ribociclib intermediate
The invention discloses preparation of a ribociclib key intermediate A; propiolic acid ester or amide 2 is directly used as a Sonogashira coupling side chain, coupling conditions are optimized, an intermediate 3 is obtained with relatively high yield, the intermediate 3 is directly subjected to ring closing under simple conditions to complete construction of a mother ring molecule, to obtain a structural formula A or a precursor ester 4 of the structural formula A, and the precursor ester 4 is subjected to hydrolysis and condensation to obtain the structural formula A. The route has simple operation, reaction steps are shorted, the yield is relatively high, and the purity of the obtained product is relatively high, and the method is suitable for enlarged production, wherein the reaction route is described in the specification.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
Quinolizinone compound and preparation method thereof
InactiveCN112174949ARaw materials are easy to getHigh yieldOrganic chemistryChemical synthesisDistillation
The invention relates to a quinolizinone compound and a preparation method thereof, and belongs to the technical field of organic chemical synthesis. The molecular structural formula of the quinolizidone compound is shown in the specification. The preparation method comprises the following steps: by taking an alkyne ester propiolate compound as a raw material, adding a pyridine derivative into theraw material, adding ethyl acetate into the mixture, then adding alkali, reacting at a proper temperature, cooling to room temperature, carrying out reduced pressure distillation to remove a solventin a reaction product, washing with water for multiple times, removing the inorganic salt in a reaction product, and collecting to obtain the quinolizinone compound by adopting a column chromatographymethod. According to the method, the raw materials required by the reaction are easy to obtain, the yield is high, the reaction conditions are mild, the reaction time is short, and the reaction stepsare few, so that the used reaction equipment is few, and the post-treatment is simple and convenient.
Owner:BEIJING NORMAL UNIVERSITY
Synthesis method of racemic-(3aR,7aR)-5-(t-butyloxycarboryl)-octahydro furan [3,2-c] pyridine-3a- carboxylic acid
InactiveCN107383034AReasonable reaction process designMethod route shortOrganic chemistry methodsFuranLithium hydroxide
The invention relates to a synthesis method of racemic-(3aR,7aR)-5-(t-butyloxycarboryl)-octahydro furan [3,2-c] pyridine-3a-carboxylic acid. The method mainly solves the technical problem that no proper industrial synthesis method exists in the prior art. The method comprises seven steps that: firstly, 2-bromoethanol and ethyl propiolate react in a solvent of methylene dichloride at the room temperature to obtain a compound 2; then, the compound 2 and ethyl cyanoacetate react in a solvent of N, N-dimethyl formamide at the room temperature over the night to obtain a compound 3; the compound 3 is subjected to catalytic hydrogenation to reduce the cyano groups to obtain amino groups; meanwhile, the exchange with the self ester is performed to obtain a compound 4; a compound 5 is obtained through carbobenzoxy chloride on the compound 4; the compound 5 is reduced by borane to obtain a compound 6; finally, the compound 6 uses a palladium hydroxide as a catalyst in ethanol; meanwhile, Boc estolide is added; hydrogen gas is introduced for overnight reaction to obtain a target compound 7; the compound 7 is hydrolyzed by water and lithium hydroxide in a mixed solution of methanol and water; a final target compound 8 is obtained.
Owner:上海药明康德新药开发有限公司 +4
Composition for preventing and eliminating malignancy rank grass in cornfield
ActiveCN101313677ACephalonBroad herbicidal spectrumBiocideAnimal repellantsNoxious weedComposition B
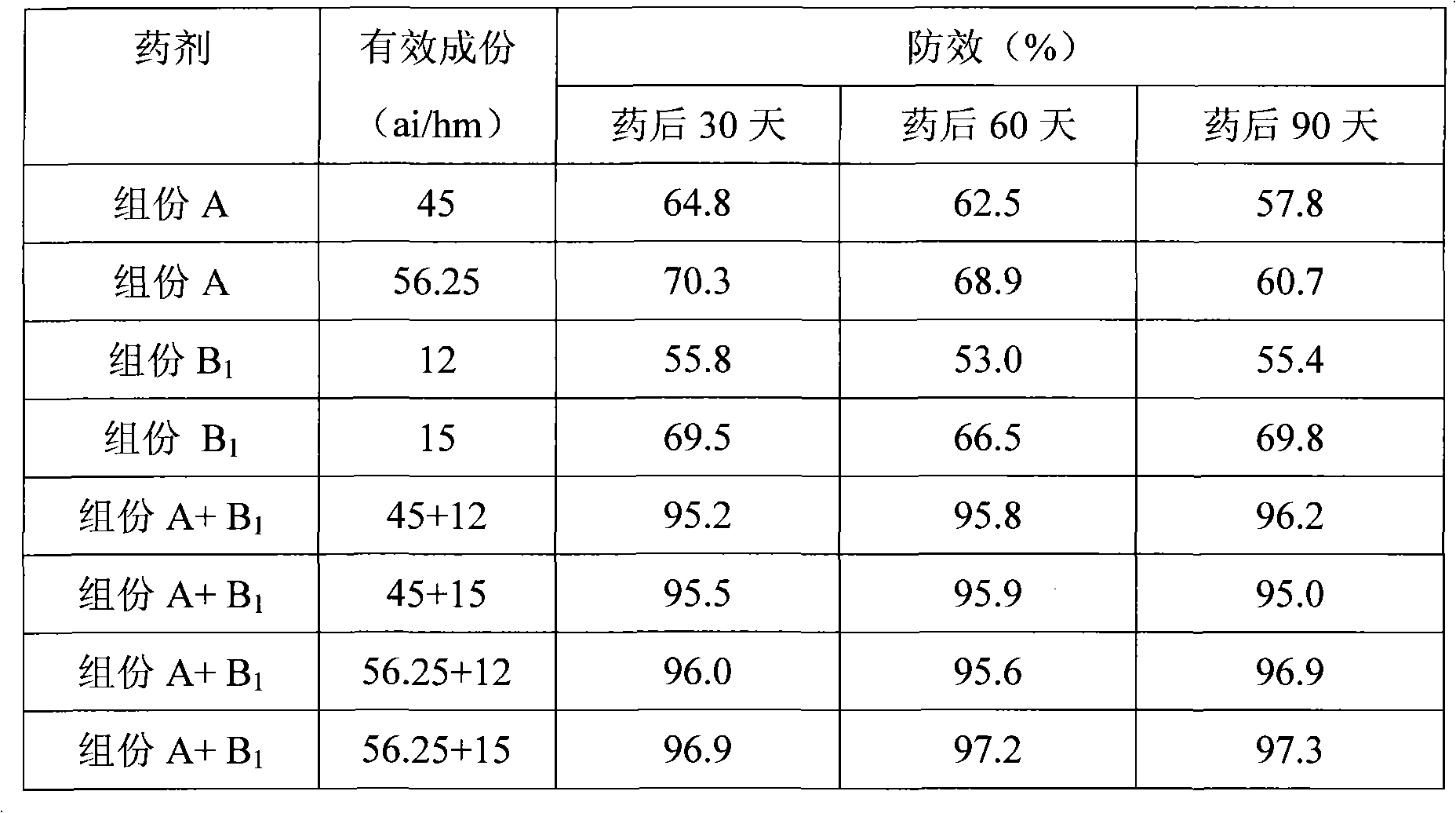
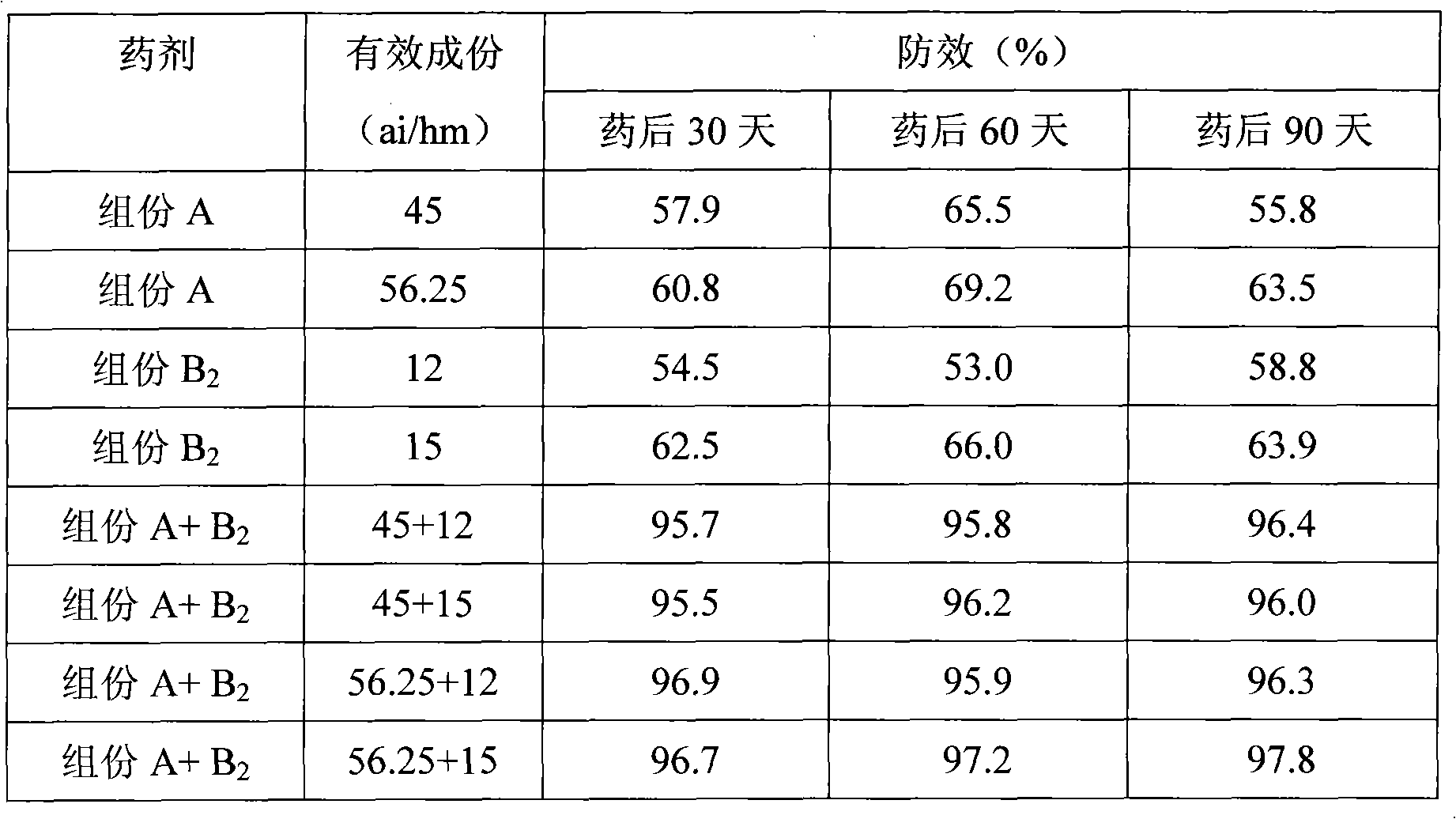
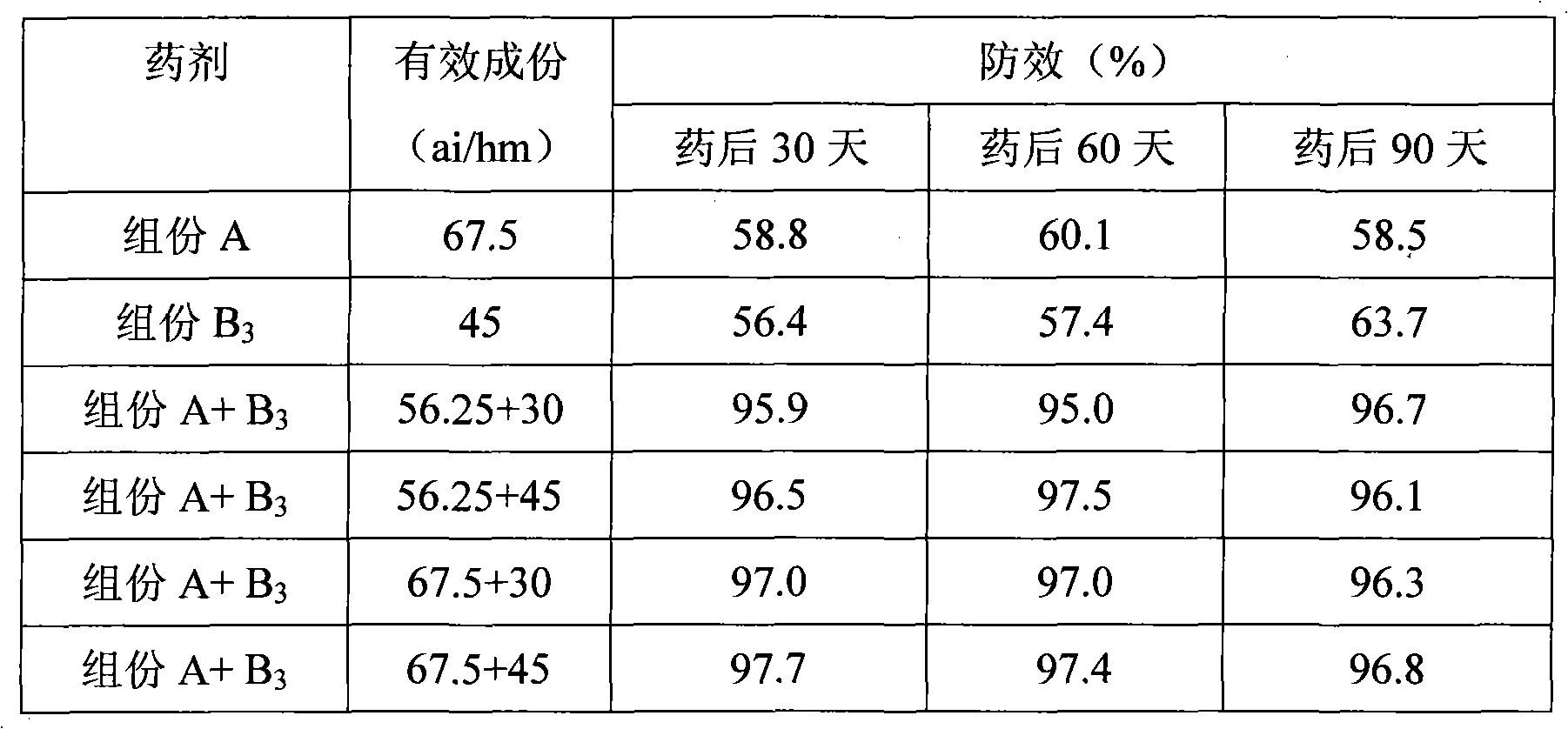
The invention discloses a combination used for preventing and removing cornfield noxious weed. The combination is characterized in that: the combination comprises active ingredient composition A and composition B, wherein the composition A is R-2-[4-(5-chloro-3 fluoro-2-oxylpyridine)- phenoxy]-propiolic acid; and the composition B is one of or more than one of 2-[4-methoxyl-6-methyl-1, 3, 5-triazine-2-methyl amido methyl amido sulfonyl] methyl benzoate, thifensulfuron methyl and bensulfuron methyl. The combination provided by the invention has the advantages of wide weed control spectrum, high activity against noxious weed, safety, low toxicity and low residue for crops, safety for following rotation crops, resistance to adverse environments such as low temperature and drought, long working life and convenient use, etc. Meanwhile, the weed control effect of the combination is better than the control effect when the compound A or the compound B is used independently, and the application dose of each active substance is reduced; moreover, the combination is used for just one time and can control the damages caused by various weeds during the entire growing period of wheat, thereby having ideal economic benefit and ecological benefit.
Owner:ANHUI HUAXING CHEM IND CO LTD
Process for synthesizing ethyl propiolate
InactiveCN101318900BNo pollution in the processRaw materials are easy to getOrganic compound preparationCarboxylic acid esters preparationReaction temperatureEthyl propiolate
The invention relates to a method for synthesizing ethyl propiolate, comprising the following steps of: using propargyl alcohol and ethanol as reaction raw materials; using dichloromethane as a solvent with a reaction temperature between 0 and 40 DEG C; and carrying out a one-step synthesis of the ethyl propiolate by using the reaction raw materials under actions of calcium hypochlorite and an acetic acid. The method has the advantages of easy acquisition of raw materials, convenient method, mild reaction conditions and no pollution to environment, and is capable of performing the reaction under a room temperature.
Owner:SUZHOU YUANFANG CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


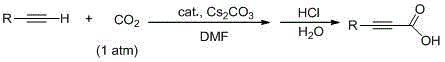
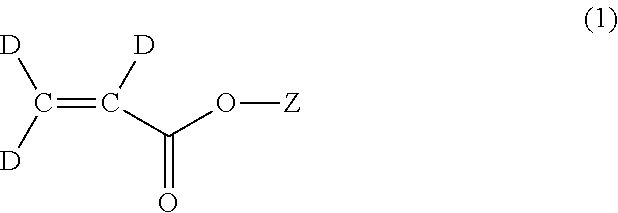
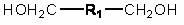




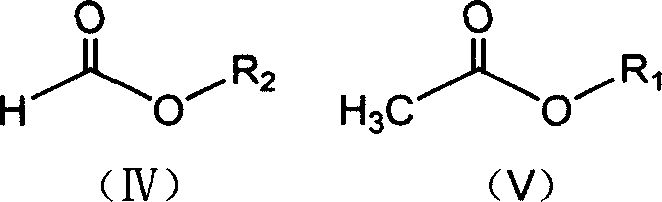
![2,3-disubstituted benzimidazo[1,2-alpha]pyrimidine compound and preparation method and application thereof 2,3-disubstituted benzimidazo[1,2-alpha]pyrimidine compound and preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7e47ec74-3d5d-481d-8851-70f851845329/HDA0001547978490000011.png)
![2,3-disubstituted benzimidazo[1,2-alpha]pyrimidine compound and preparation method and application thereof 2,3-disubstituted benzimidazo[1,2-alpha]pyrimidine compound and preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7e47ec74-3d5d-481d-8851-70f851845329/HDA0001547978490000012.png)
![2,3-disubstituted benzimidazo[1,2-alpha]pyrimidine compound and preparation method and application thereof 2,3-disubstituted benzimidazo[1,2-alpha]pyrimidine compound and preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7e47ec74-3d5d-481d-8851-70f851845329/HDA0001547978490000021.png)

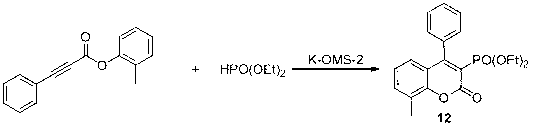

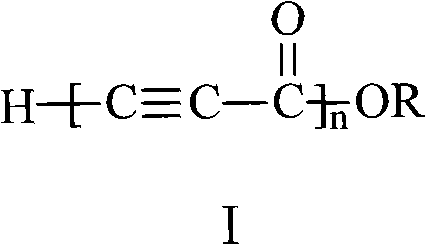
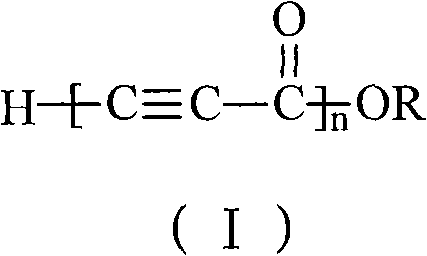
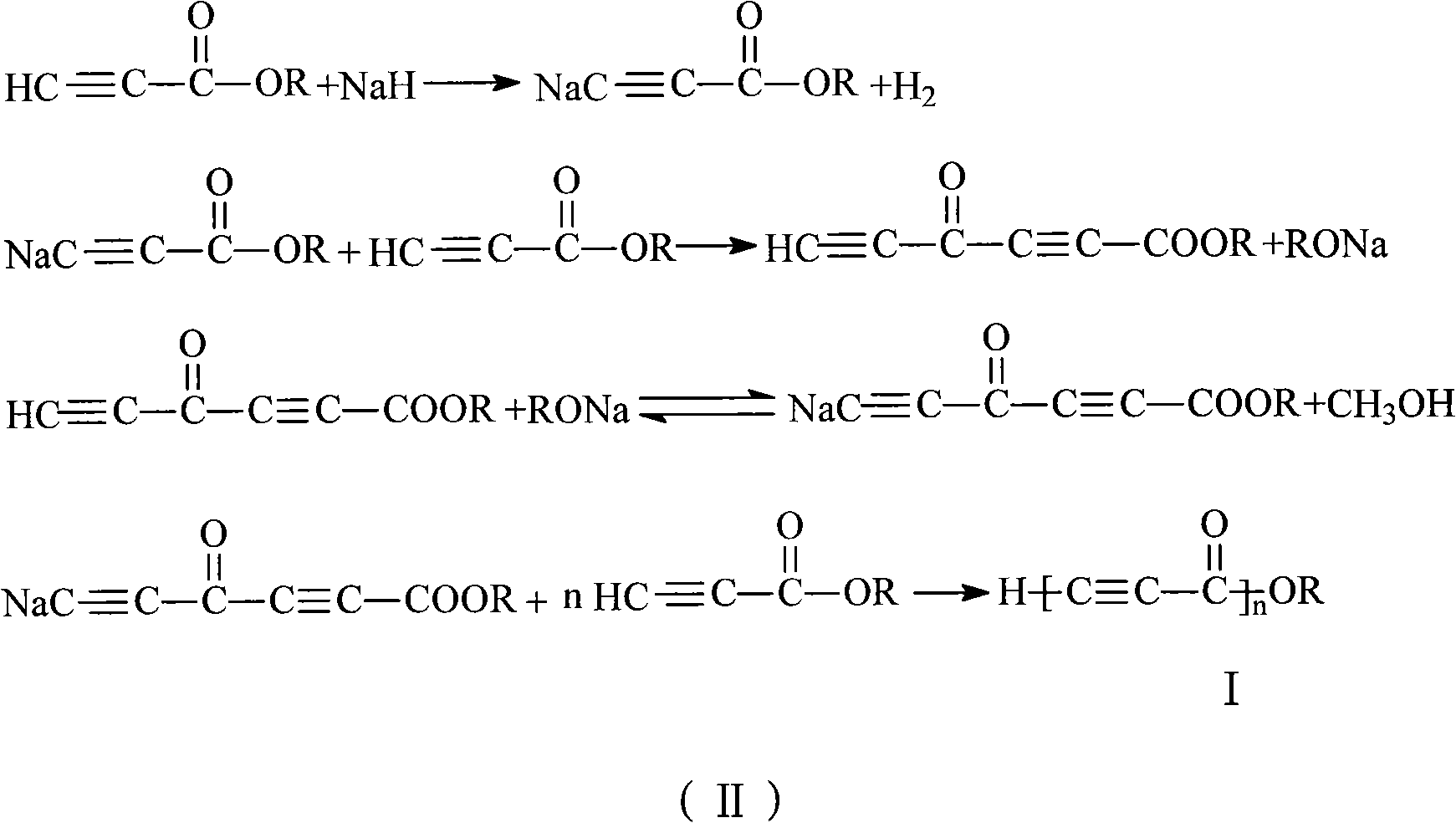

![Synthesis method of racemic-(3aR,7aR)-5-(t-butyloxycarboryl)-octahydro furan [3,2-c] pyridine-3a- carboxylic acid Synthesis method of racemic-(3aR,7aR)-5-(t-butyloxycarboryl)-octahydro furan [3,2-c] pyridine-3a- carboxylic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/985c4940-3413-4eb4-ac6c-89b233e25011/972576DEST_PATH_IMAGE002.png)
![Synthesis method of racemic-(3aR,7aR)-5-(t-butyloxycarboryl)-octahydro furan [3,2-c] pyridine-3a- carboxylic acid Synthesis method of racemic-(3aR,7aR)-5-(t-butyloxycarboryl)-octahydro furan [3,2-c] pyridine-3a- carboxylic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/985c4940-3413-4eb4-ac6c-89b233e25011/DEST_PATH_IMAGE001.png)