Quinolizinone compound and preparation method thereof
A compound, quinozinone technology, applied in the field of organic chemical synthesis, can solve the problems of high equipment cost, harsh reaction conditions, long production cycle, etc., and achieve the effect of high yield, short reaction time and less reaction equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of the quinazinone compound that the present invention proposes, concrete process is as follows:
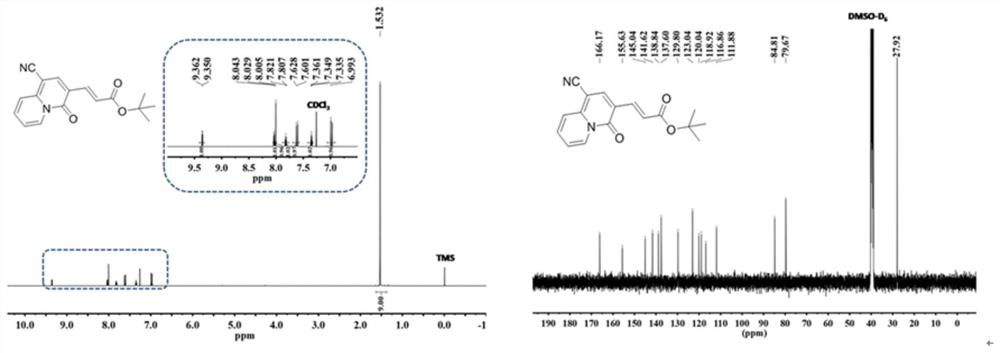
[0028] Using alkynyl ester propiolate compounds as raw materials, adding pyridine derivatives to the raw material, the added molar ratio is: alkynyl ester propiolates: pyridine derivatives=(2.5~3):1 to obtain a mixture , add ethyl acetate in the mixture, the mass ratio of adding is: mixture: ethyl acetate=1: (10~18), add alkali again, the add-on of alkali is identical with alkynes propiolates, at 60 ℃~80℃, react for 3~5 hours, cool to room temperature, distill under reduced pressure to remove the solvent in the reaction product, wash with water several times, remove the inorganic salt in the reaction product, adopt column chromatography to collect quinazone class of compounds.
[0029] In the preparation method of the above-mentioned quinazinone compounds, the base is any one of cesium carbonate, cesium hydroxide, potassium hydroxide, potassium carb...
Embodiment 1
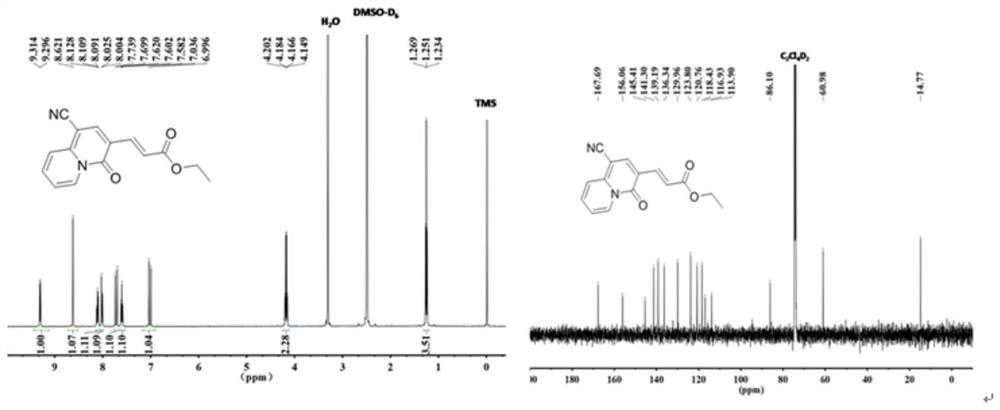
[0032] The molecular structural formula of the prepared quinazinone is as follows:
[0033]
[0034] The specific process is: taking ethyl propiolate (294mg, 3mmol) as raw material, adding 2-pyridineacetonitrile (118mg, 1mmol) to the raw material, the molar ratio added is: ethyl propiolate:2-pyridineacetonitrile=3 : 1, obtain mixture, add solvent in mixture, the mass ratio of adding is: mixture: solvent=1:15, described solvent is ethyl acetate, adds alkali again, and the add-on of alkali is identical with ethyl propiolate , the base is cesium carbonate (977.4mg, 3mmol), reacted at 80 degrees for 3 hours, cooled to room temperature, evaporated under reduced pressure to remove the solvent in the reaction product, washed with water several times, and removed the inorganic salt in the reaction product , using column chromatography to collect quinozinone compounds, the yield was 215 mg, and the yield was 80%. During the column chromatography, the eluent added was a mixed soluti...
Embodiment 2
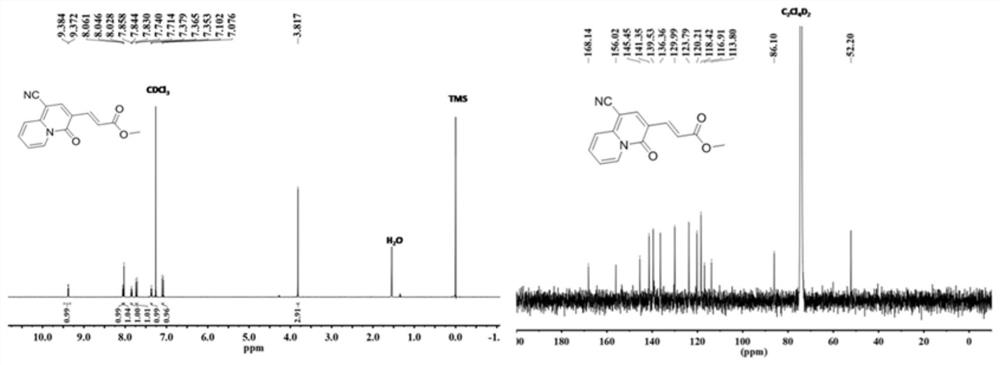
[0036] The molecular structural formula of the prepared quinazinone is as follows:
[0037]
[0038] The specific process is: taking ethyl propiolate (294mg, 3mmol) as raw material, adding 2-pyridineacetonitrile (118mg, 1mmol) to the raw material, the molar ratio added is: ethyl propiolate:2-pyridineacetonitrile=3 : 1, obtain mixture, add solvent in mixture, the mass ratio of adding is: mixture: solvent=1:15, described solvent is ethyl acetate, adds alkali again, and the add-on of alkali is identical with ethyl propiolate , the base is cesium hydroxide (450mg, 3mmol), reacted at 80 degrees for 3 hours, cooled to room temperature, distilled under reduced pressure to remove the solvent in the reaction product, washed with water several times, and removed the inorganic salt in the reaction product , using column chromatography to collect quinozinone compounds, the yield was 187 mg, and the yield was 70%. During the column chromatography, the eluent added was a mixed solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com