Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36results about How to "Reasonable choice of reaction process" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
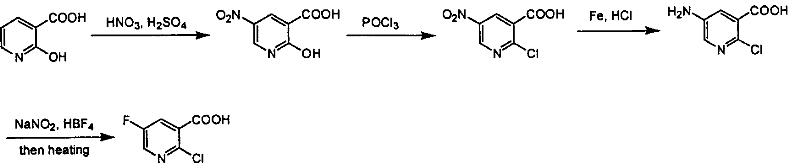
Preparation of 2-Cl-5-F-nicotinate and nicotonic acid
InactiveCN1613849AReasonable choice of reaction processShorten the synthesis processOrganic chemistryNiacinAcid production
Production of 2-chlorine-5-fluorine-nicotinate and acid is carried out by 2-chlorine-5-fluorine-nicotinate and related carboxylic acid selective dechlorinating, and 2-chlorine-5-fluorine-nicotinate hydrolyzing 2-chlorine-5-fluorine-niacin under the exist of alkaline substances. It can be used for 2-chlorine-5-fluorine-nicotinate and 2-chlorine-5-fluorine-niacin medicine intermediate.
Owner:上海药明康德新药开发有限公司
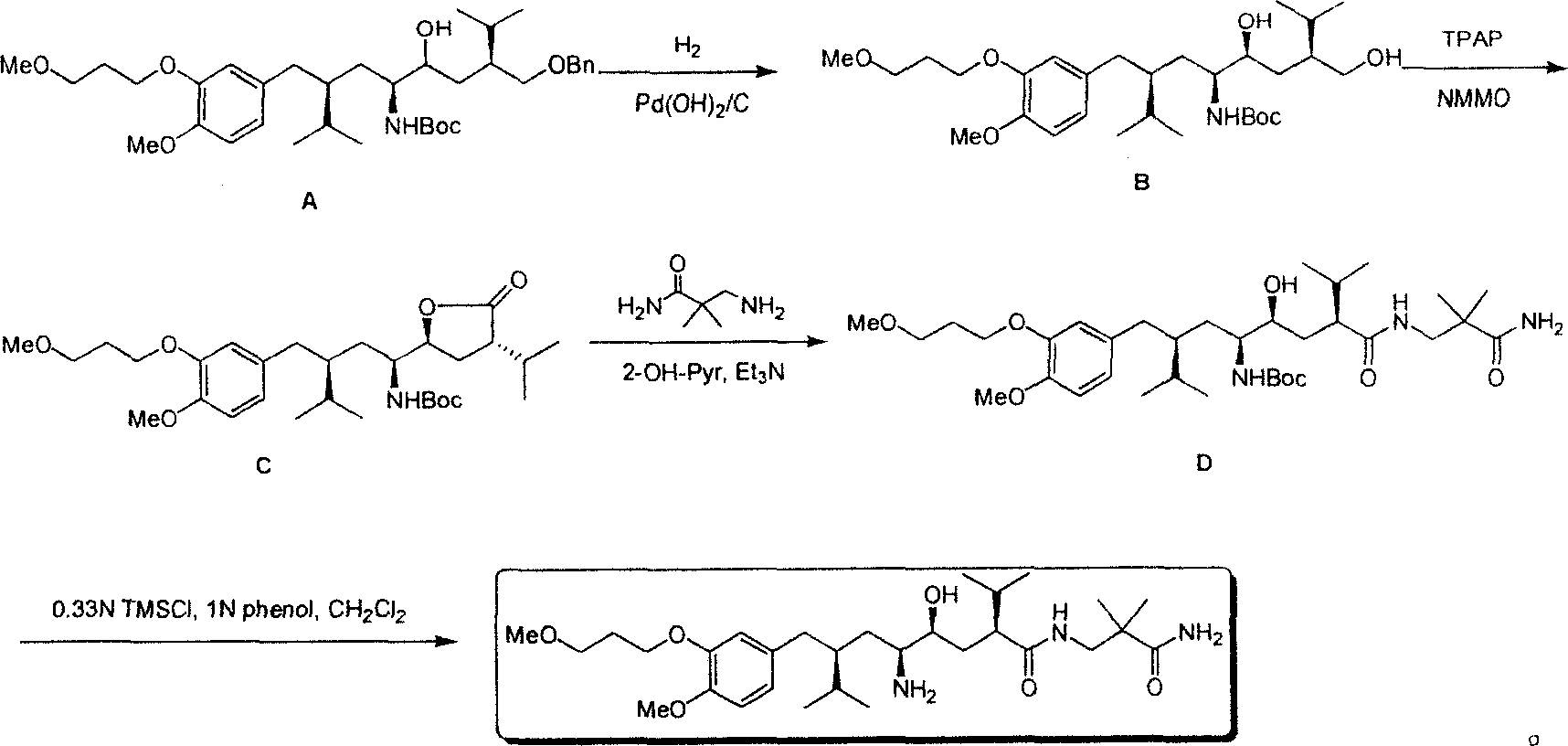
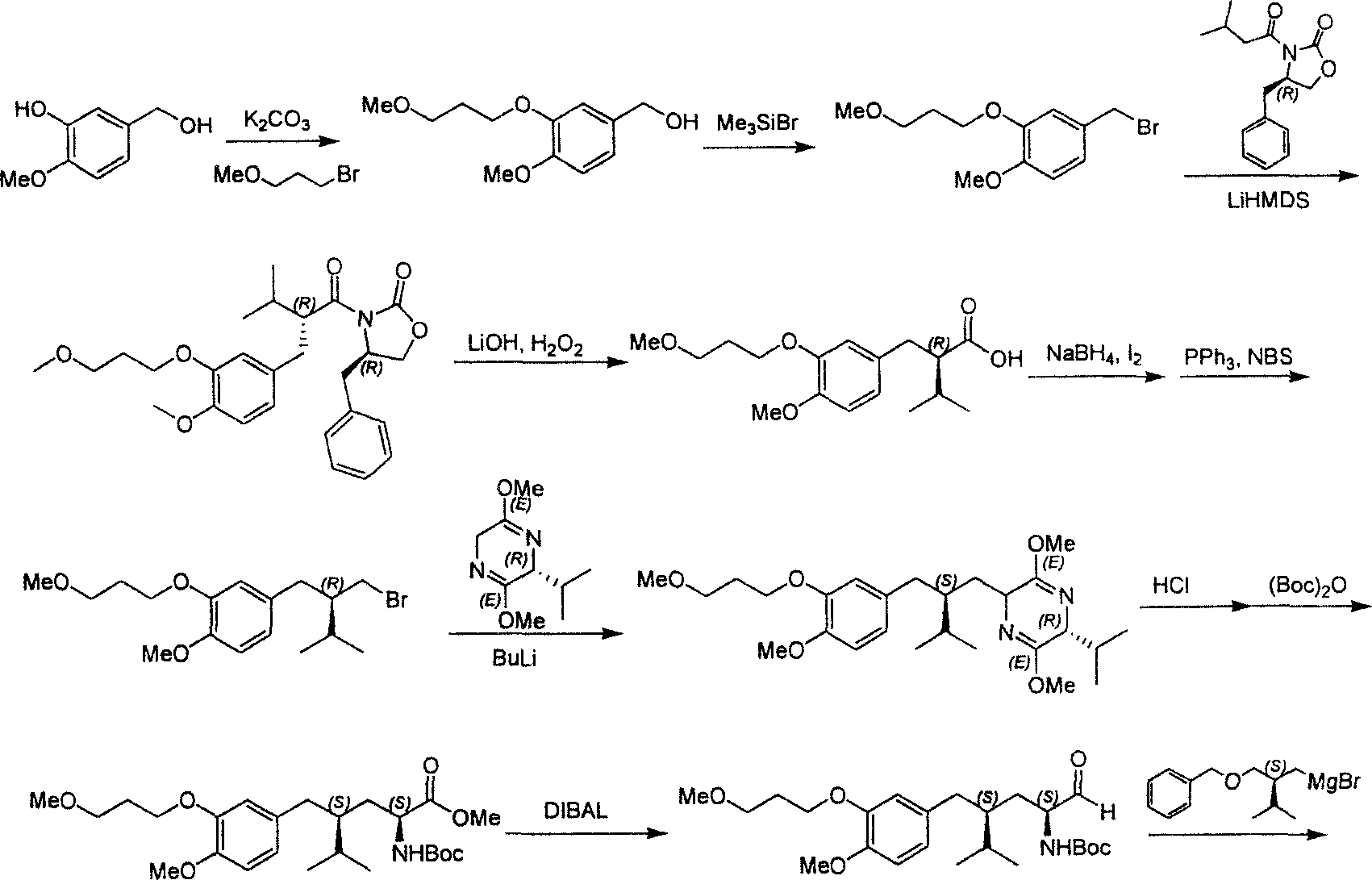
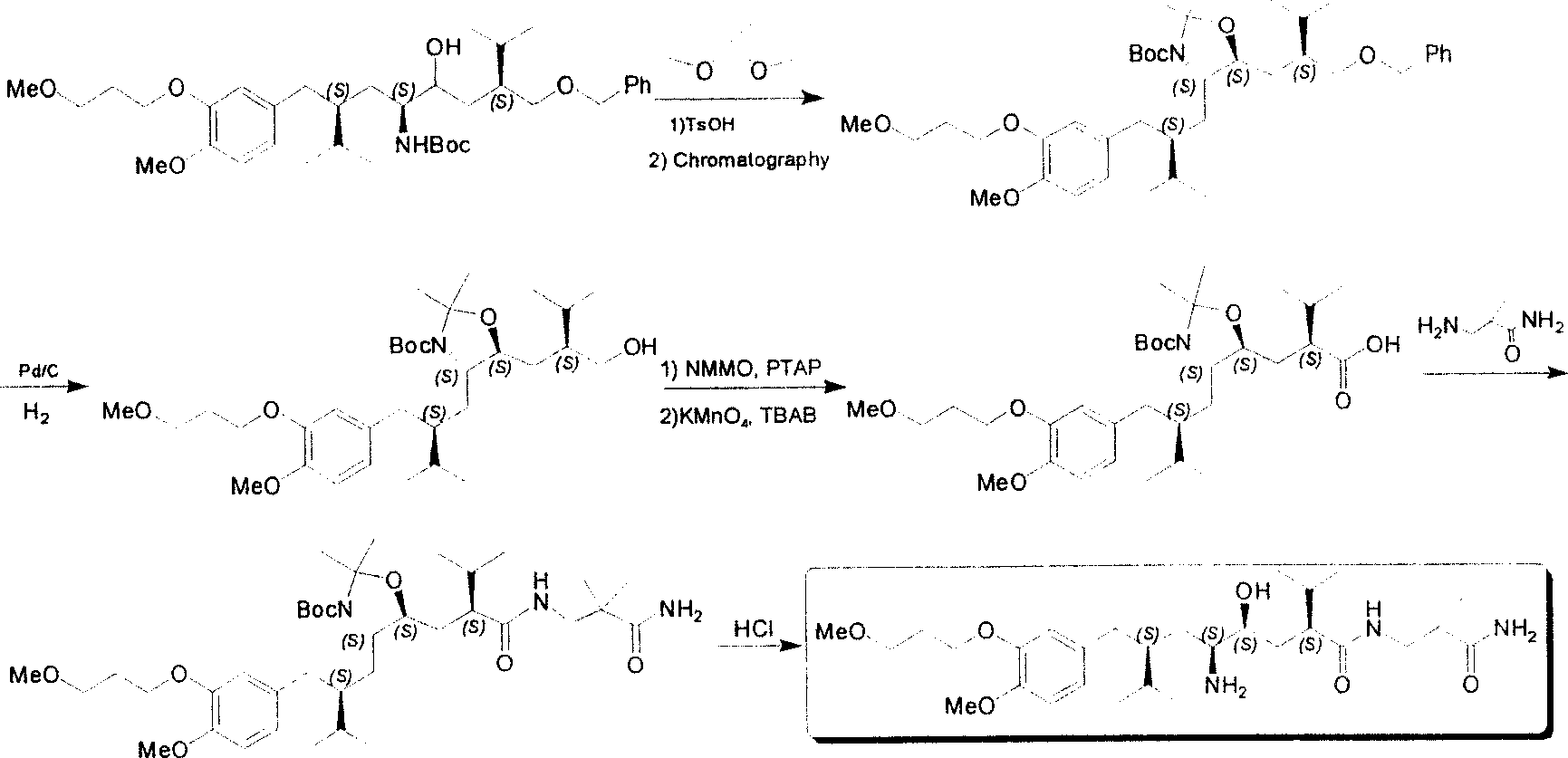
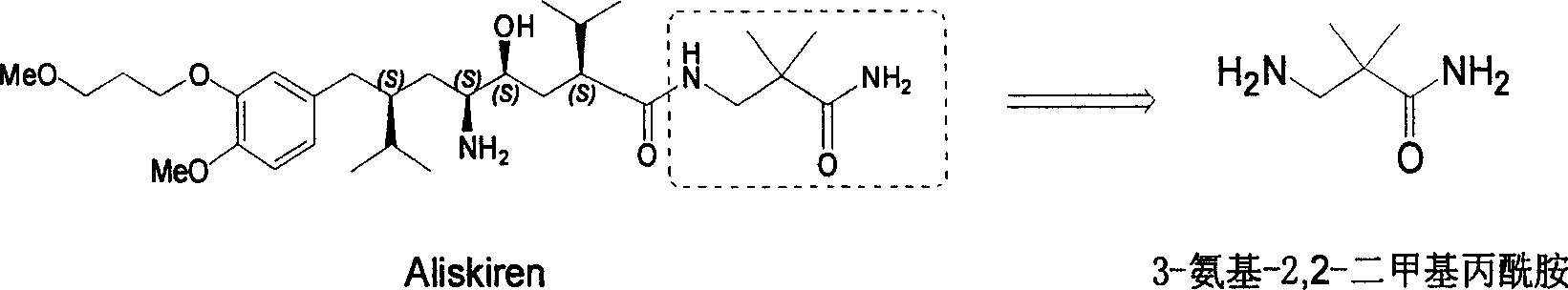
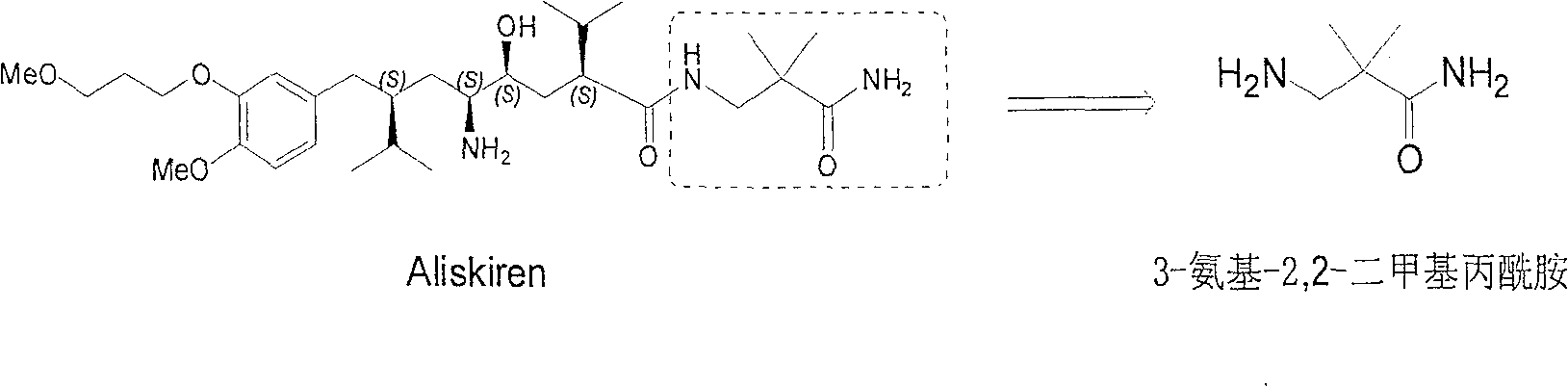
Practical synthesis method for feritin inhibitor aliskiren
InactiveCN101016253AReasonable choice of reaction processFew reaction stepsOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsTert-Butyloxycarbonyl protecting group
The invention discloses a synthesizing method of Alikelun of hypertension proteinogenase inhibitor, which comprises the following steps: adopting (1S, 4S, 2'S)-(4-benzyloxy methyl-2-hydroxy-1-{2-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-3-methyl-butyl}-5-methyl-hexyl)-carbonic tert-butyl as raw material; catalyzing; hydrogenating; removing benzyl; separating two R and S-typed diastereomers through column chromatography; obtaining (1S, 2S, 4S, 2'S)-(2-hydroxy-4-methylol-1-{2-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-3-methyl-butyl}-5-methyl-hexyl)-carbonic tert-butyl ester; oxidizing into (1S, 3S, 1'S, 4'S)-{1-(4-isopropyl-5-oxo-tetrahydrofuran-2-radical)-3-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-4-methyl-amyl}-carbonic tert-butyl ester; proceeding amide ester exchange to obtain (1 S, 3S, 1'S, 4'S)-(4-(2-amino formyl-2-methyl-propyl amino formyl)-2-hydroxy-1-{2-[4-methoxy-3-(3-methoxy-propoxy-benzyl]-3-methyl-butyl}-5-methyl-hexyl) carbonic tert-butyl ester; removing terbu-carbonyl to produce Aliskiren.
Owner:上海药明康德新药开发有限公司
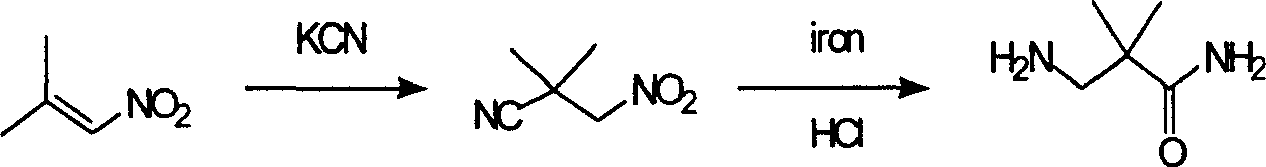
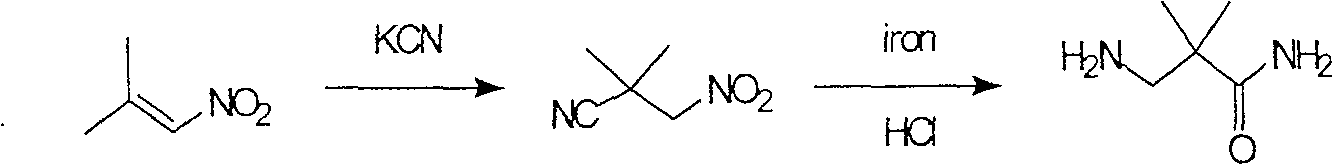
Industrial preparation method for 3-amino-2, 2-dimethyl propionamide
InactiveCN1990461AReasonable choice of reaction processReduce process stepsOrganic compound preparationCarboxylic acid amides preparationPotassium cyanideChemistry
The invention relates to an industrial method for preparing 3- amino- 2, 2- bimethyl propionamide, which is a segment in high pressure protein proenzyme inhibitor. The invention takes normal and cheap cyanoacetamide as raw material, alkalyating two nethyls, catalytic hydrogenating or adding reducant agent and getting 3- amnio- 2, 2- bimethyl propionamide. The chenmical reaction is shown in the graph. The invention solves problems of difficulty of getting raw material, low productivity and long process line, needs no column chromatography and purification, avoids toxic potassium cyanide and realizes large scale production.
Owner:上海药明康德新药开发有限公司
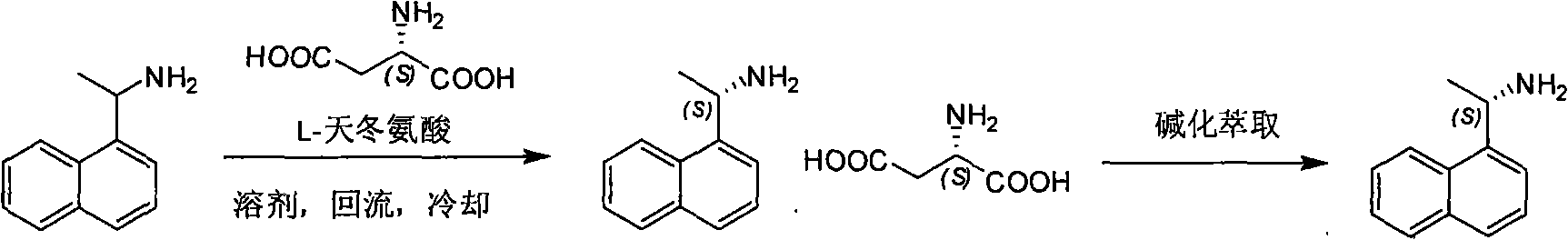
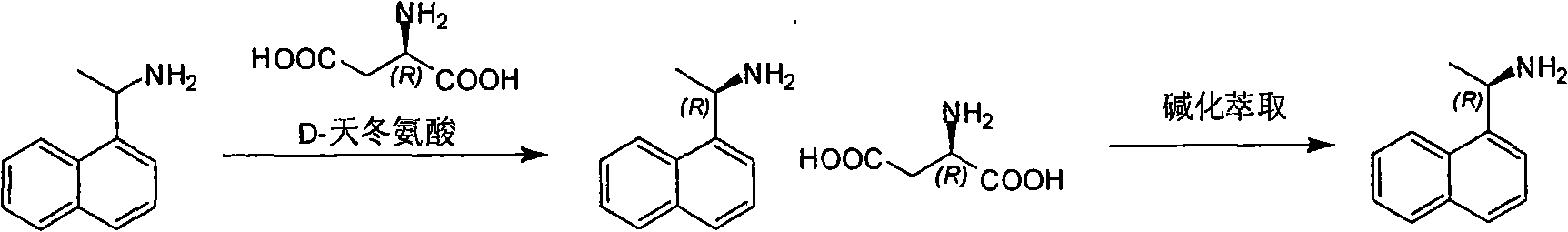
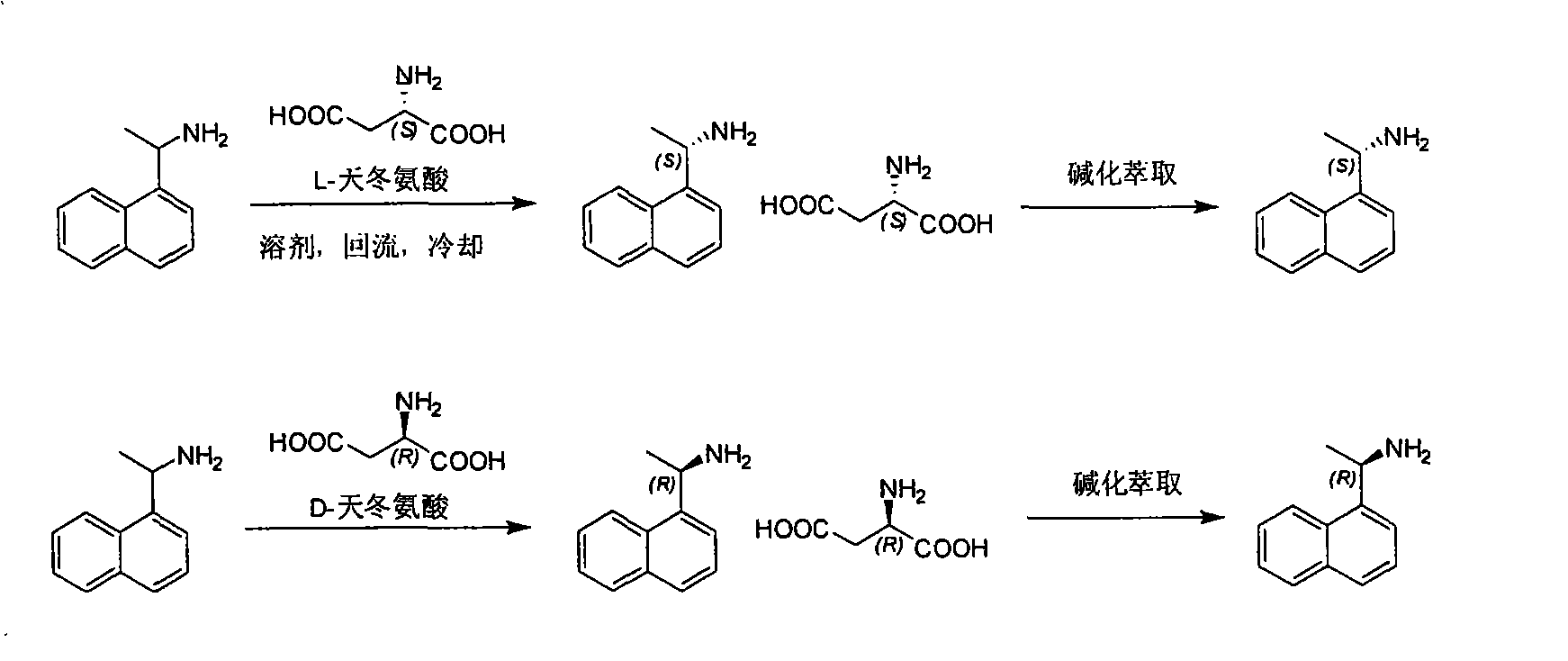
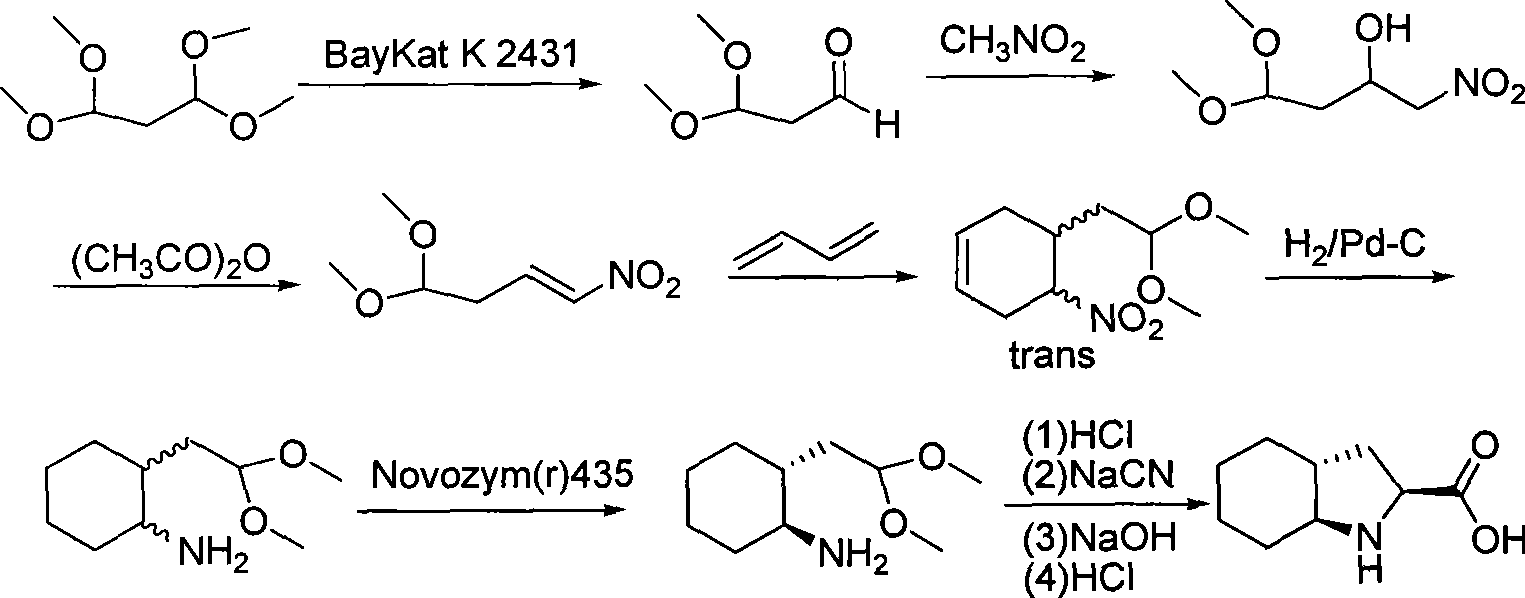
Method for preparing optical pure 1-(1-naphthyl)ethylamine by separation
ActiveCN101407465AReasonable choice of reaction processThe split method is simpleAmino compound purification/separationSolubilitySolvent
The invention relates to a method for preparing 1-(1-naphtaline) ethylamine possessing optical activity, which mainly solves problems of current process of expensive original raw material, long synthesizing process, low yield coefficient, and over-high cost and the like; the invention has chiral aspartic acid as chiral resolving agent, water or solution of water and dioxane as solvent; under a heating condition, racemate 1-(1-naphtaline) ethylamine is reacted with chiral aspartic acid to form enantiomeric salt. Then, according to different solubility of 1-(1-naphtaline) ethylamine to enantiomeric salt, splitting is carried out, and two configurations (S configuration and R configuration) of 1-(1-naphtaline) ethylamine are separated and prepared; chiral e.e. value for one time of splittingcan reach over 98 percent, with yield coefficient over 30 percent. The method is mainly used for preparing S configuration 1-(1-naphtaline) ethylamine and R configuration 1-(1-naphtaline) ethylamine drug intermediate possessing optical activity and template compound researched and developed by innovative small molecule drugs.
Owner:WUXI APPTEC (TIANJIN) CO LTD +1
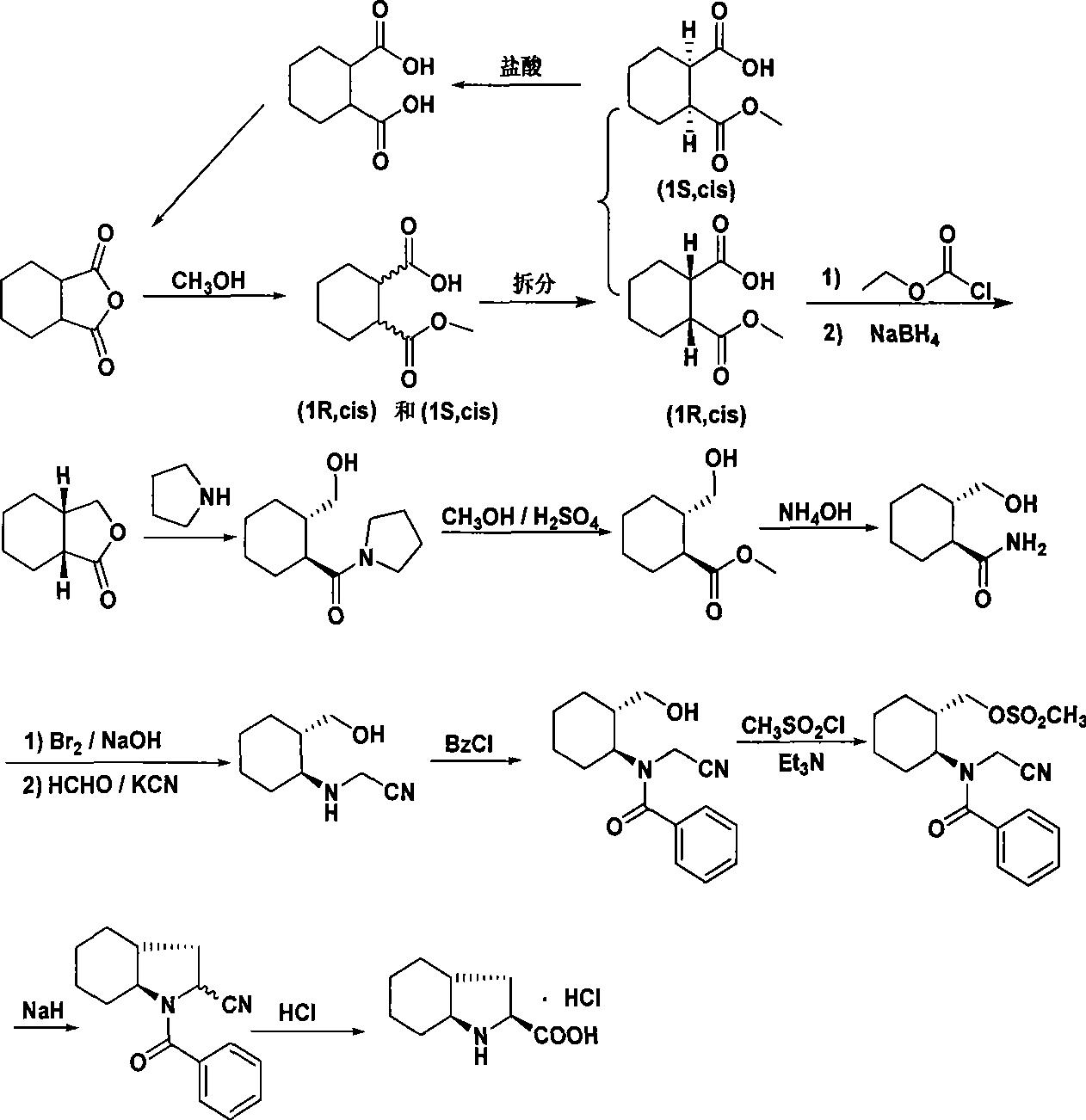
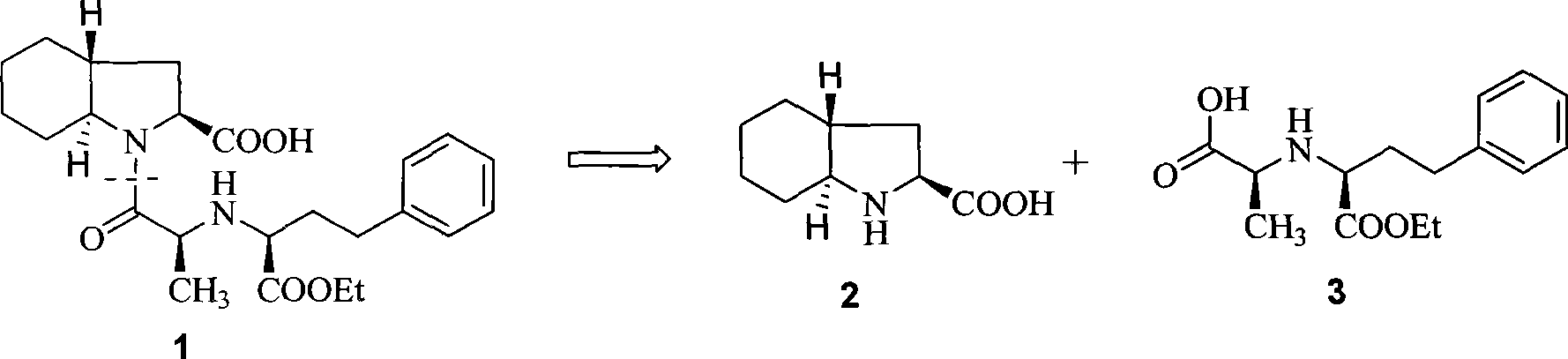
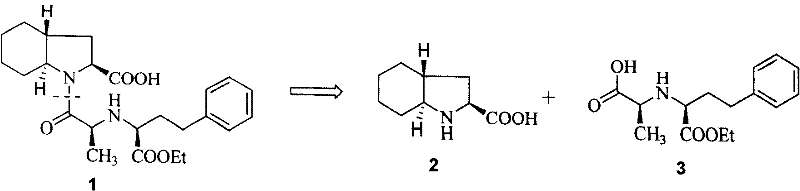
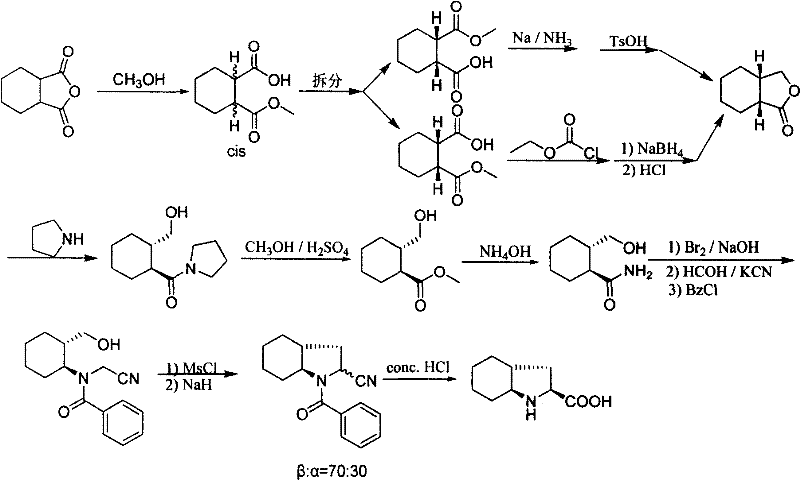
Synthetic method of trandolapril key intermediate (2S,3aR,7as)-octahydro-1H-indole-2-carboxylic acid
ActiveCN101423490AImprove utilization efficiencyHigh purityOrganic chemistryBulk chemical productionCarboxylic acidImpurity
The invention relates to a synthetic method for trandolapril key intermediate (2S, 3aR and 7as)-octahydro-1H-indole-2-carboxylic acid. The method comprises the following steps: obtaining a pair of cis 1, 2-cyclohexane dimethyl acid mono-methyl ester after refluxing hexahydrophthalic anhydride by methanol, splitting the cis 1, 2-cyclohexane dimethyl acid mono-methyl ester by (-) N, N-dimethyl amino diol to obtain 1, 2-cyclohexane dimethyl acid mono-methyl ester of (1R, cis) and (1S, cis) respectively; refluxing (1R, cis)-1, 2-cyclohexane dimethyl acid mono-methyl ester obtained by reclaiming mother liquor in hydrochloric acid to obtain cyclohexane o-dioctyl phthalate, and cyclizing the cyclohexane o-dioctyl phthalate to obtain an initial raw material of the hexahydrophthalic anhydride; and circulating the processes unceasingly to improve the impurity of the (1R, cis)-1, 2-cyclohexane dimethyl acid mono-methyl ester, and then directly entering a follow-up synthesizing process. The process effectively solves the problems of treatment of (1S, cis) 1, 2-cyclohexane dimethyl acid mono-methyl ester after racemization of (d1)1, 2-cyclohexane dimethyl acid mono-methyl ester and yield of (1R, cis)-1, 2-cyclohexane dimethyl acid mono-methyl ester, improves utilization efficiency of raw materials, reduces cost of products, is more suitable for industrialized production, can effectively improve follow-up reaction efficiency, and saves energy of a system.
Owner:CHONGQING NANSONG CHEMI TECH
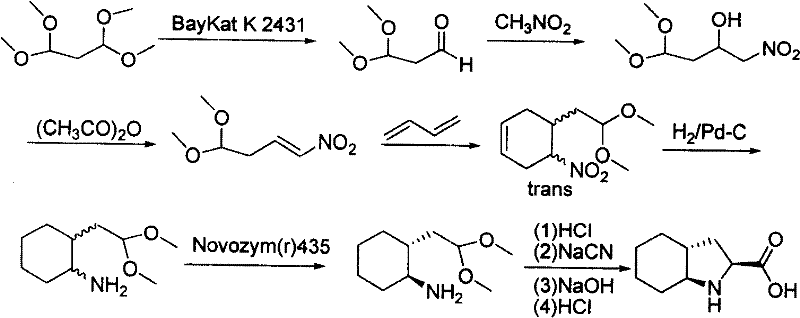
Method for quickly preparing cis-octahydropyrrolo[3,2-b]pyrrole
InactiveCN102086202AThe solution is not easy to obtainGet quicklyOrganic chemistryBulk chemical productionAlkyl transferSynthesis methods
The invention relates to a novel synthesis method for cis-octahydropyrrolo[3,2-b]pyrrole, and mainly solves the technical problems that raw materials are expensive, reaction conditions are harsh, a reaction route is long, the reaction operation is inconvenient, and a large number of isomers are generated through ring closure in the conventional cis-octahydropyrrolo[3,2-b]pyrrole preparation process. The method comprises the following steps of: performing alkylation reaction of industrially available 3-pyrrolidone serving as an initiative raw material and an allyl compound directly under the action of acid to obtain a 3-tetrahydro-pyrrolidone substitute; performing reductive amination reaction to obtain a 3-aminopyrrolidine derivative; performing protective group adding reaction; and performing ozonization reduction ring-closing reaction and dehydroxylation reaction on the 3-aminopyrrolidine derivative added with the protective group to obtain a corresponding cis-octahydropyrrolo[3,2-b]pyrrole derivative. The target product and important intermediates can be widely applied to synthesizing various pyrrole derivatives.
Owner:上海药明康德新药开发有限公司 +1
Industrial continuous preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptane
InactiveCN1986548AReasonable choice of reaction processReduce manufacturing costOrganic chemistryTert-butoxyTert-Butyloxycarbonyl protecting group
The present invention relates to continuous industrial preparation process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2, 2, 1] heptane. The present invention prepares N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2, 2, 1] heptane with facile L-hydroxy praline as material, and through methyl esterification, tert-butoxy carbonyl radical protection, paratoluene sulfonation, hydrolysis and lactonization. The present invention has lowered cost, raised yield, less environmental pollution and no need of column chromatographic purification, and may be used in industrial production.
Owner:上海药明康德新药开发有限公司
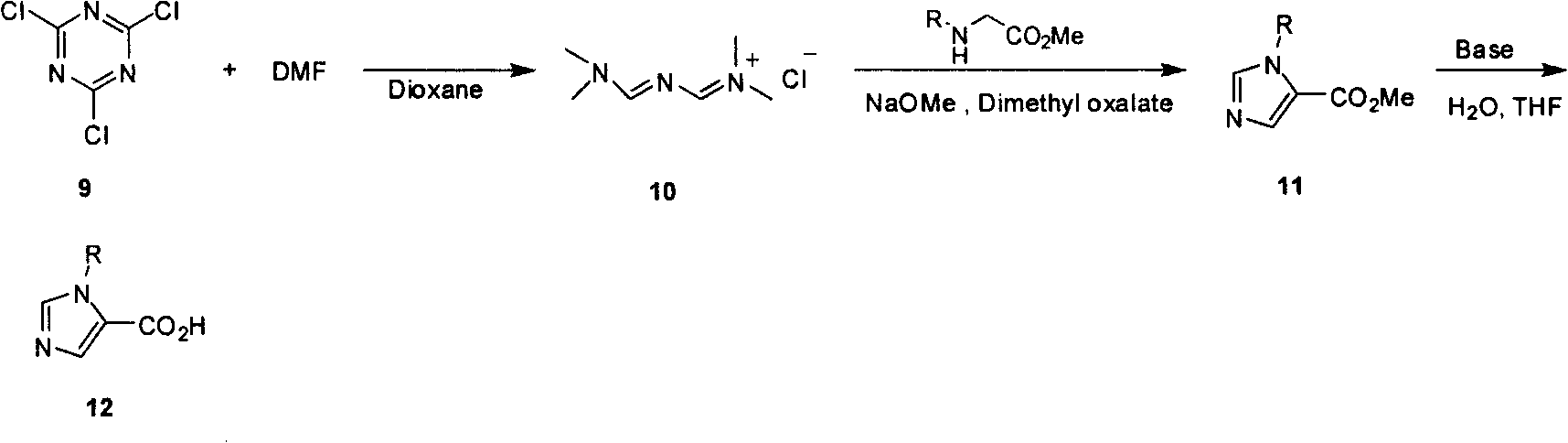
Industrialized preparation method of N-alkyl substituted-imidazole-5-carboxylic-acid/ester compound
InactiveCN101550106AReasonable choice of reaction processEasy to controlOrganic chemistryChlorideHydrolysis
The invention relates to an industrialized preparation method of an N-alkyl substituted-imidazole-5-carboxylic-acid / ester compound, which uses conventional and easily-obtained cranuric chloride as a raw material to react with DMF to generate a gold reagent, and react with alkyl-substituted glycinate to obtain N-alkyl substituted-imidazole-5-carboxylic ester under an alkali condition a loop closure way. N-alkyl substituted-imidazole-5-carboxylic acid can be obtained by hydrolysis. The invention solves the problems of long line, low yield, difficult purification and no scale production of the prior art and can realize the scale industrialized production.
Owner:上海药明康德新药开发有限公司
Method for preparing S-1-aminoindane
InactiveCN105061219AReasonable choice of reaction processThe split method is simpleAmino compound purification/separationSolubilityAlcohol
The invention discloses a method for preparing S-1-aminoindane through separation. D-mandelic acid is taken as a chirality resolving agent, a mixed solution of alcohol and water is taken as a solvent, under the condition of heating reflux, 1-aminoindane is dropwise added and racemized, the 1-aminoindane is reacted with the D-mandelic acid to form diastereomeric salt, and the D-mandelate of the S-1-aminoindane is obtained through crystallization and separation according to different solubility of the diastereomeric salt; the S-1-aminoindane is obtained by conducting purification and alkalization on the D-mandelate; all the solutions containing the D-mandelate are mixed, the alcohol is removed through steaming, acidification is conducted on the solution, and the D-mandelate can be recycled. The method for preparing the S-1-aminoindane through the separation has the advantages of being mild in condition, easy to operate, high in product yield, high in optical purity, capable of recycling and reusing the resolving agent and the like, and the method is extremely suitable for industrial production of the S-1-aminoindane.
Owner:吴玲
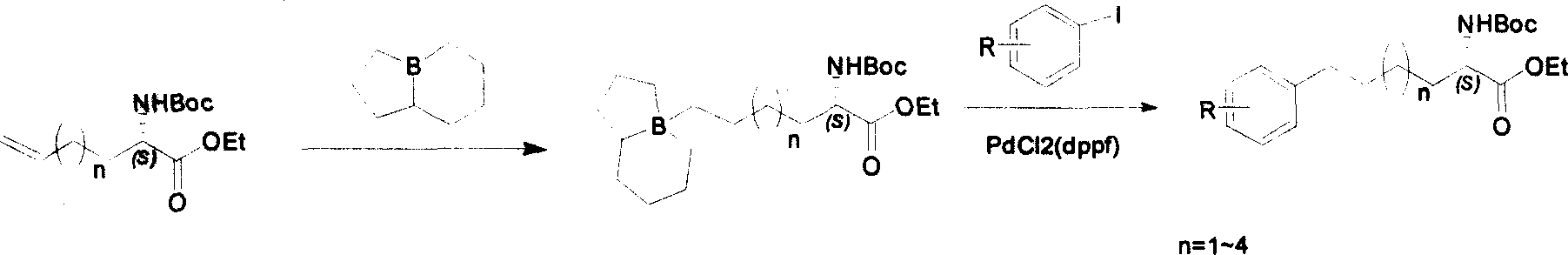
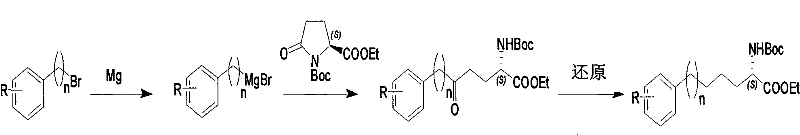
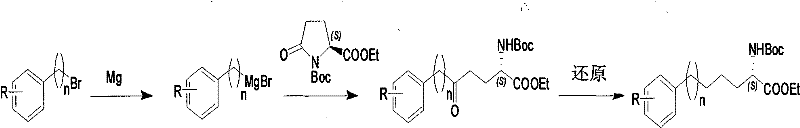
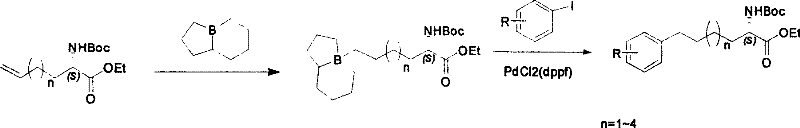
Method for synthesizing optically active derivative of omega - aryl ¿C (2S) ¿C N ¿C boc ¿Calpha amino acid
ActiveCN101092371AReduce usageReasonable choice of reaction processOrganic compound preparationAmino-carboxyl compound preparationArylGrignard reagent
This invention relates to a process for synthesizing optically active omega-aryl-(2S)-N-tert-butyloxycarbonyl-alpha-amino acid derivative, more specifically, omega-aryl-(2S)-N-tert-butyloxycarbonyl-alpha-amino acid ester. The process comprises: preparing omega-arylalkyl bromide into Grignard reagent, reacting with ethyl N-tert-butyloxycarbonyl-L-pyroglutamate to obtain omega-aryl-5-one-(S)-N-tert-butyloxycarbonyl-alpha-amino acid ethyl ester, and reducing to obtain omega-aryl-(2S)-N-tert-butyloxycarbonyl-alpha-amino acid ethyl ester. The process has such advantages as reasonable reaction process, abundant raw materials, short synthesis time, and no need for expensive enzyme reagents, and is suitable for mass production.
Owner:上海药明康德新药开发有限公司
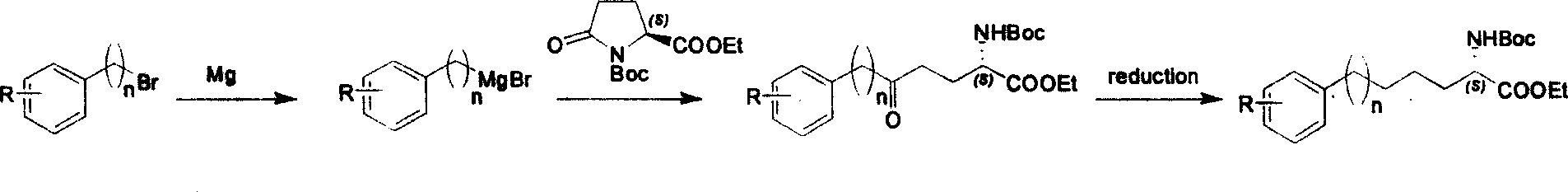
Method for synthesizing optically active derivative of 5 - aryl - (S) - N - boc - alpha amino pentanoic acid
ActiveCN101092373AReduce usageReasonable choice of reaction processOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisAryl
This invention relates to a method for synthesizing optically active 5-aryl-(S)-N-tert-butyloxycarbonyl-alpha-aminopentanoic acid derivative. The method comprises: preparing conventional and abundant phenyl bromide into Grignard reagent, reacting with ethyl N-tert-butyloxycarbonyl-L-pyroglutamate to obtain ethyl 5-aryl-5-one-(S)-N-tert-butyloxycarbonyl-alpha-pentanoate, and reducing to obtain ethyl 5-aryl-(S)-N-tert-butyloxycarbonyl-alpha-pentanoate. The method has such advantages as short reaction time and low cost, and is suitable for mass production. The method solves the problems of long synthesis time, unable industrial production, expensive Pd reagent or enzyme reagent, and high synthesis cost faced by the present technique.
Owner:上海药明康德新药开发有限公司
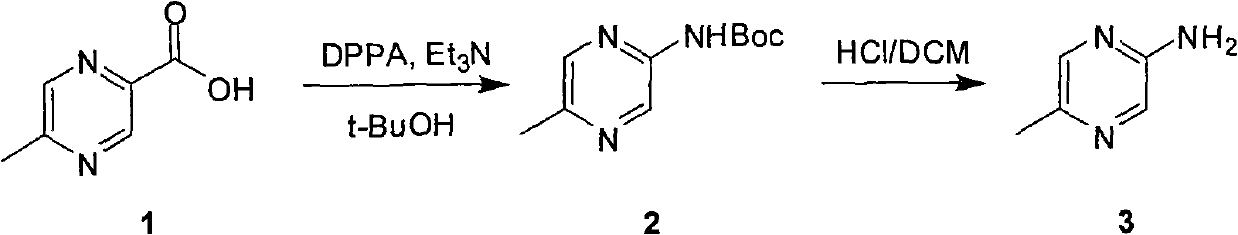
Industrial preparation method of 5-methylpyrazin-2-amine
InactiveCN101857575AReasonable choice of reaction processEasy to controlOrganic chemistryState of artChemical reaction
The invention relates to an industrial preparation method of 5-methylpyrazin-2-amine. In the invention, the 5-methylpyrazin-2-amine is prepared from general and easily obtained 5-methyl-2-pyrazinecarboxylic acid by the steps of azidation, Curtius rearrangement and Boc desorption. The chemical reaction formula is shown in the specification. The invention solves the problems of high cost, low yield, environment pollution and the like in the prior art, does not need column chromatography for purification, and can realize large-scale industrial production.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD +1
3,3-gem-difluoro cyclobutanecarboxylic acid industrial preparation method
InactiveCN105418406AReasonable choice of reaction processSolve the problem of limited laboratory preparation and no large-scale productionOrganic compound preparationCarboxylic acid esters preparationMeth-Chemical reaction
The invention relates to a 3,3-gem-difluoro cyclobutanecarboxylic acid industrial preparation method. The method mainly solves the technical problem of existing preparing methods that the price of the raw material 3-oxo-cyclobutylcarboxylic acid or 3-methylenecyclobutane-carbonitrile and the price of the fluoro reagent DAST are high, and therefore large-scale production can not be conducted. According to the 3,3-gem-difluoro cyclobutanecarboxylic acid industrial preparation method, conventional methyl acrylate and 1,1-dichloro-2,2-difluoroethylene which are easy to obtain are taken as raw materials, and 3,3-gem-difluoro cyclobutanecarboxylic acid is obtained through addition, hydrolysis and hydrogenation. Please see the description for the chemical equation. The 3,3-gem-difluoro cyclobutanecarboxylic acid industrial preparation method is simple, practical and quite low in preparing cost.
Owner:SHANGHAI STA PHARMA R&D CO LTD
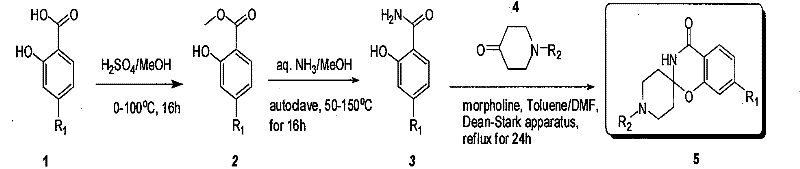
Industrial compounding method of mule (benzo (e) (1,3) oxazine-2, 4'-piperidine)-4(3H)-ketonic
ActiveCN101550143BReasonable choice of reaction processReaction is easy to controlOrganic chemistryMetaclazepamAcyl group
The invention relates to an industrial preparation method of spiral (benzo (e) (1,3) oxazine-2, 4'-piperidine)-4(3H)-ketonic, which uses economical, easily-obtained and scale-produced salicylic acid (and various para-orientation salicylic acids) and N-protecting piperidone (including Boc, Cbz, acyl groups and alkyl group protection) as raw materials, and spiral (benzo (e) (1,3) oxazine-2, 4'-piperidine)-4(3H)-ketonic is obtained after methyl esterification, aminolysis and the close under the catalysis of piperidine or morpholino. The invention solves the problems of complex reaction, low purity and yield, and the like in the prior art, does not need column chromatography purification and can realize scale industrial production.
Owner:上海药明康德新药开发有限公司 +1
Industrial preparation method for 3-amino-2, 2-dimethyl propionamide
InactiveCN100588646CReasonable choice of reaction processReduce process stepsOrganic compound preparationCarboxylic acid amides preparationChemical reactionPotassium cyanide
The invention relates to an industrial method for preparing 3- amino- 2, 2- bimethyl propionamide, which is a segment in high pressure protein proenzyme inhibitor. The invention takes normal and cheapcyanoacetamide as raw material, alkalyating two nethyls, catalytic hydrogenating or adding reducant agent and getting 3- amnio- 2, 2- bimethyl propionamide. The chenmical reaction is shown in the graph. The invention solves problems of difficulty of getting raw material, low productivity and long process line, needs no column chromatography and purification, avoids toxic potassium cyanide and realizes large scale production.
Owner:上海药明康德新药开发有限公司
Industrial preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptance in one cauldron
InactiveCN1986547AReasonable choice of reaction processHigh yieldOrganic chemistryTert-butoxyTert-Butyloxycarbonyl protecting group
The present invention relates to industrial preparation process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2, 2, 1] heptane in one kettle. The present invention prepares N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2, 2, 1] heptane with facile L-hydroxy praline as material, and through methyl esterification, tert-butoxy carbonyl radical protection, paratoluene sulfonation, hydrolysis and lactonization. The present invention has lowered cost, raised yield, less environmental pollution and no need of column chromatographic purification, and may be used in industrial production.
Owner:上海药明康德新药开发有限公司
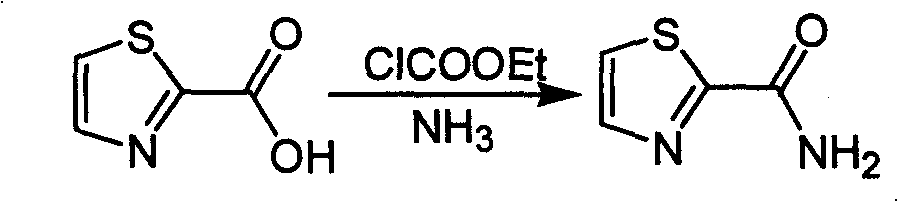
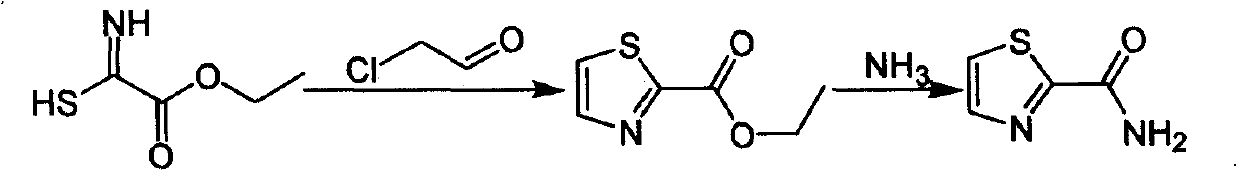
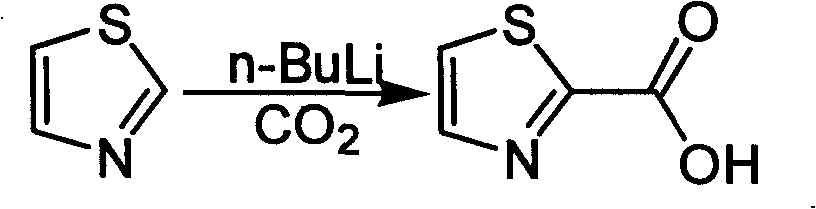
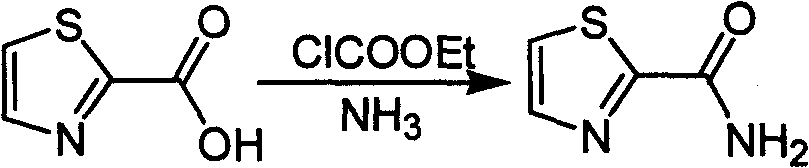
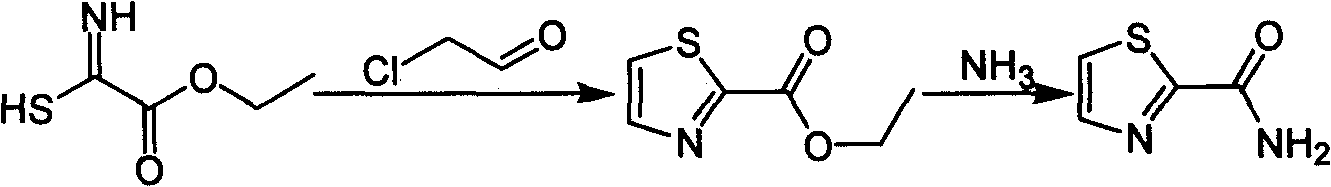
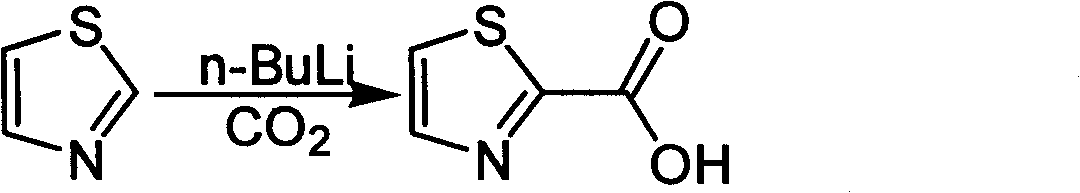
Industrialized preparation method of using 2-bromothiazole for compounding thiazole-2-methanamide in a one-boiler mode
ActiveCN101550113AReasonable choice of reaction processRaw materials are cheap and easy to getOrganic chemistryState of artChemical reaction
The invention relates to an industrialized preparation method of using 2-bromothiazole for compounding thiazole-2-methanamide in a one-boiler mode, which uses cheap and easily-obtained 2-bromothiazole as a raw material to obtain thiazole-2-methanamide after carbonyl-inserting reaction, chlorination and ammonolysis. The reaction formula is as above. The invention solves the problems of environmental pollution, safety danger in production, high cost, low yield, and the like in the prior art, the intermediate product is not required to be purified, the target product can be obtained in one step, the operation is simple, and the large-scale industrialized production can be realized.
Owner:上海药明康德新药开发有限公司 +1
Synthetic method of trandolapril key intermediate (2S,3aR,7as)-octahydro-1H-indole-2-carboxylic acid
ActiveCN101423490BImprove utilization efficiencyHigh purityOrganic chemistryBulk chemical productionHexahydrophthalic anhydrideCarboxylic acid
The invention relates to a synthetic method for trandolapril key intermediate (2S, 3aR and 7as)-octahydro-1H-indole-2-carboxylic acid. The method comprises the following steps: obtaining a pair of cis 1, 2-cyclohexane dimethyl acid mono-methyl ester after refluxing hexahydrophthalic anhydride by methanol, splitting the cis 1, 2-cyclohexane dimethyl acid mono-methyl ester by (-) N, N-dimethyl amino diol to obtain 1, 2-cyclohexane dimethyl acid mono-methyl ester of (1R, cis) and (1S, cis) respectively; refluxing (1R, cis)-1, 2-cyclohexane dimethyl acid mono-methyl ester obtained by reclaiming mother liquor in hydrochloric acid to obtain cyclohexane o-dioctyl phthalate, and cyclizing the cyclohexane o-dioctyl phthalate to obtain an initial raw material of the hexahydrophthalic anhydride; and circulating the processes unceasingly to improve the impurity of the (1R, cis)-1, 2-cyclohexane dimethyl acid mono-methyl ester, and then directly entering a follow-up synthesizing process. The process effectively solves the problems of treatment of (1S, cis) 1, 2-cyclohexane dimethyl acid mono-methyl ester after racemization of (d1)1, 2-cyclohexane dimethyl acid mono-methyl ester and yield of (1R, cis)-1, 2-cyclohexane dimethyl acid mono-methyl ester, improves utilization efficiency of raw materials, reduces cost of products, is more suitable for industrialized production, can effectively improve follow-up reaction efficiency, and saves energy of a system.
Owner:CHONGQING NANSONG CHEMI TECH
Industrialized method for preparing 2-chlorine-5-fluorin-nicotinic aicd
InactiveCN100355733CReasonable choice of reaction processLarge-scale industrial productionOrganic chemistryCombinatorial chemistryOrganic chemistry
Owner:CHANGZHOU HEQUAN PHARMA CO LTD
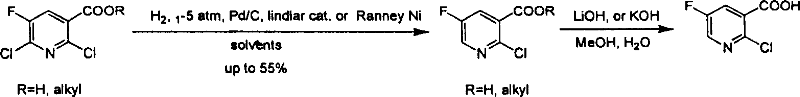
Preparation of 2-Cl-5-F-nicotinate and nicotonic acid
InactiveCN100355732CReasonable choice of reaction processShorten the synthesis processOrganic chemistryThiolNiacin
Production of 2-chlorine-5-fluorine-nicotinate and acid is carried out by 2-chlorine-5-fluorine-nicotinate and related carboxylic acid selective dechlorinating, and 2-chlorine-5-fluorine-nicotinate hydrolyzing 2-chlorine-5-fluorine-niacin under the exist of alkaline substances. It can be used for 2-chlorine-5-fluorine-nicotinate and 2-chlorine-5-fluorine-niacin medicine intermediate.
Owner:上海药明康德新药开发有限公司
Industrial preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptance in one cauldron
InactiveCN1986547BReasonable choice of reaction processHigh yieldOrganic chemistryTert-Butyloxycarbonyl protecting groupProcess engineering
The present invention relates to industrial preparation process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2, 2, 1] heptane in one kettle. The present invention prepares N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2, 2, 1] heptane with facile L-hydroxy praline as material, and through methyl esterification, tert-butoxy carbonyl radical protection, paratoluene sulfonation, hydrolysis and lactonization. The present invention has lowered cost, raised yield, less environmental pollution and no need of column chromatographic purification, and may be used in industrial production.
Owner:上海药明康德新药开发有限公司
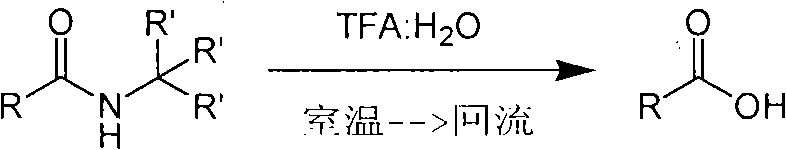
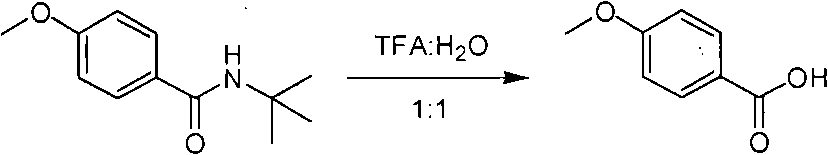
Method of de-alkyl amine
ActiveCN101550047AReasonable choice of reaction processAvoid hydrolytic conditionsPreparation from carboxylic acid amidesFunctional group formation/introductionSolventChemistry
The invention relates to a method of de-tert-alkyl amine, which mainly solves the technical problems of over strong reacting conditions and low yield of the prior method. The method of de-tert-alkyl amine is characterized in that tertiary tert-alkyl acid amide is reacted in the mixed solution of trifluoroacetic acid and water and is hydrolyzed into corresponding carboxylic acids, the volume ratio of the trifluoroacetic acid and water is 1-100: 0-5, and the reacting formula is as above. In the formula, R and R' are the alkyl group or the aromatic group of C1 to C20, and the reaction temperature is from the room temperature to the solvent reflux temperature. The invention hydrolyzes the tertiary tert-alkyl amine into corresponding carboxylic acids under a mild condition.
Owner:上海药明康德新药开发有限公司
Method for synthesizing optically active derivative of omega - aryl - (2S) - N - boc -alpha amino acid
ActiveCN101092371BReduce usageReasonable choice of reaction processOrganic compound preparationAmino-carboxyl compound preparationGrignard reagentTert-Butyloxycarbonyl protecting group
This invention relates to a process for synthesizing optically active omega-aryl-(2S)-N-tert-butyloxycarbonyl-alpha-amino acid derivative, more specifically, omega-aryl-(2S)-N-tert-butyloxycarbonyl-alpha-amino acid ester. The process comprises: preparing omega-arylalkyl bromide into Grignard reagent, reacting with ethyl N-tert-butyloxycarbonyl-L-pyroglutamate to obtain omega-aryl-5-one-(S)-N-tert-butyloxycarbonyl-alpha-amino acid ethyl ester, and reducing to obtain omega-aryl-(2S)-N-tert-butyloxycarbonyl-alpha-amino acid ethyl ester. The process has such advantages as reasonable reaction process, abundant raw materials, short synthesis time, and no need for expensive enzyme reagents, and is suitable for mass production.
Owner:上海药明康德新药开发有限公司
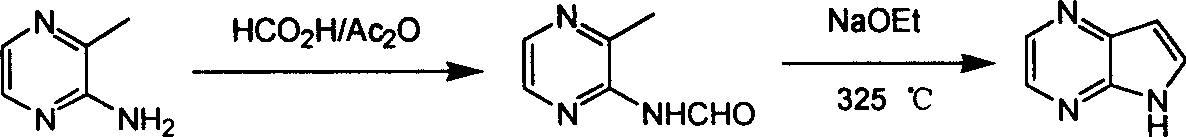
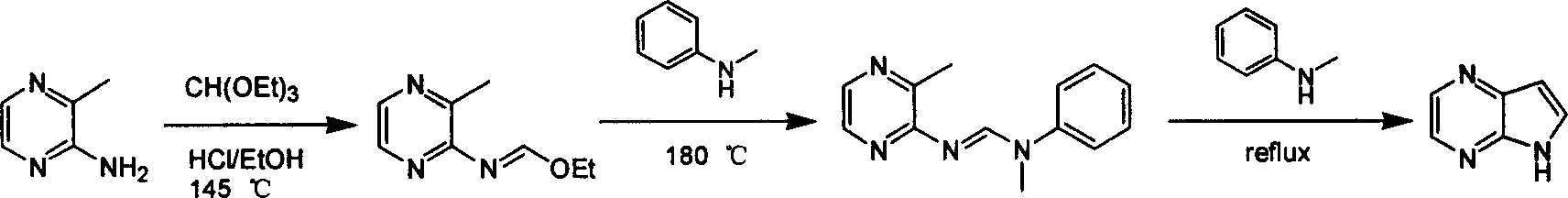
Preparing process of 4,7-diazaindole
ActiveCN100588658CReasonable choice of reaction processEasy to operateOrganic chemistryBulk chemical productionPyrazineAcid anhydride
Owner:CHANGZHOU HEQUAN PHARMA CO LTD
Method for synthesizing 3-aza-bicyclo[4.1.0]heptane-6-formic acid with protective group
ActiveCN102070526BEasy to removeGet quicklyOrganic chemistryBulk chemical productionHalohydrocarbonAlkaline hydrolysis
The invention relates to a method for preparing 3-aza-bicyclo[4.1.0]heptane-6-formic acid with a protective group, and mainly solves the technical problem that multi-step chromatographic purification is needed in the conventional process for preparing the 3-aza-bicyclo[4.1.0]heptane-6-formic acid. The method comprises the following steps of: performing reflux alkylation reaction on conventional and readily available ethyl isonicotinate serving as a raw material and halohydrocarbon in alcohol solution to obtain quaternary ammonium; performing cyanoborohydride reaction to obtain amino-protected 1,2,3,6-tetrahydropyridine-4-ethyl formate; not performing purification, and directly performing ring-closing reaction to obtain a three-membered ring; and performing alkaline hydrolysis reaction to obtain amino-protected 3-aza-bicyclo[4.1.0]heptane-6-formic acid. The 3-aza-bicyclo[4.1.0]heptane-6-formic acid with the protective group is an important medicinal intermediate.
Owner:上海药明康德新药开发有限公司 +2
Industrial continuous preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptane
InactiveCN1986548BReasonable choice of reaction processReduce manufacturing costOrganic chemistryTert-butoxyTert-Butyloxycarbonyl protecting group
The present invention relates to continuous industrial preparation process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2, 2, 1] heptane. The present invention prepares N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2, 2, 1] heptane with facile L-hydroxy praline as material, and through methyl esterification, tert-butoxy carbonyl radical protection, paratoluene sulfonation, hydrolysis and lactonization. The present invention has lowered cost, raised yield, less environmental pollution and no need of column chromatographic purification, and may be used in industrial production.
Owner:上海药明康德新药开发有限公司
Industrialized preparation method of using 2-bromothiazole for compounding thiazole-2-methanamide in a one-boiler mode
ActiveCN101550113BReasonable choice of reaction processRaw materials are cheap and easy to getOrganic chemistryState of artThiazole
The invention relates to an industrialized preparation method of using 2-bromothiazole for compounding thiazole-2-methanamide in a one-boiler mode, which uses cheap and easily-obtained 2-bromothiazoleas a raw material to obtain thiazole-2-methanamide after carbonyl-inserting reaction, chlorination and ammonolysis. The reaction formula is as below. The invention solves the problems of environmental pollution, safety danger in production, high cost, low yield, and the like in the prior art, the intermediate product is not required to be purified, the target product can be obtained in one step, the operation is simple, and the large-scale industrialized production can be realized.
Owner:上海药明康德新药开发有限公司 +1
5-bromo-2-furfural synthesis method
InactiveCN103275040AImprove protectionAvoid separation and purificationOrganic chemistryOrganic synthesisSynthesis methods
The invention relates to the organic synthesis field, and especially discloses a 5-bromo-2-furfural synthesis method. The 5-bromo-2-furfural synthesis method which treats furfural and bromine as raw materials is characterized in that the method comprises the following steps: placing the raw materials in an aprotic solvent, reacting at 25-70DEG C for 2-24h under the initiation of resorcinol and sulfur, concentrating, washing, drying to obtain an oily product, processing the oily product by using a mixed solution comprising water and alcohol to obtain a crude product, and recrystallizing to obtain pure 5-bromo-2-furfural. The method has the advantages of reasonable selection of the reaction technology, mild product condition, simple operation, stable product quality, high product purity, and avoiding of the aldehyde group protection and deprotection, and the separation and purification of an intermediate in a multistep reaction.
Owner:HEZE ASSET CHEM TECH
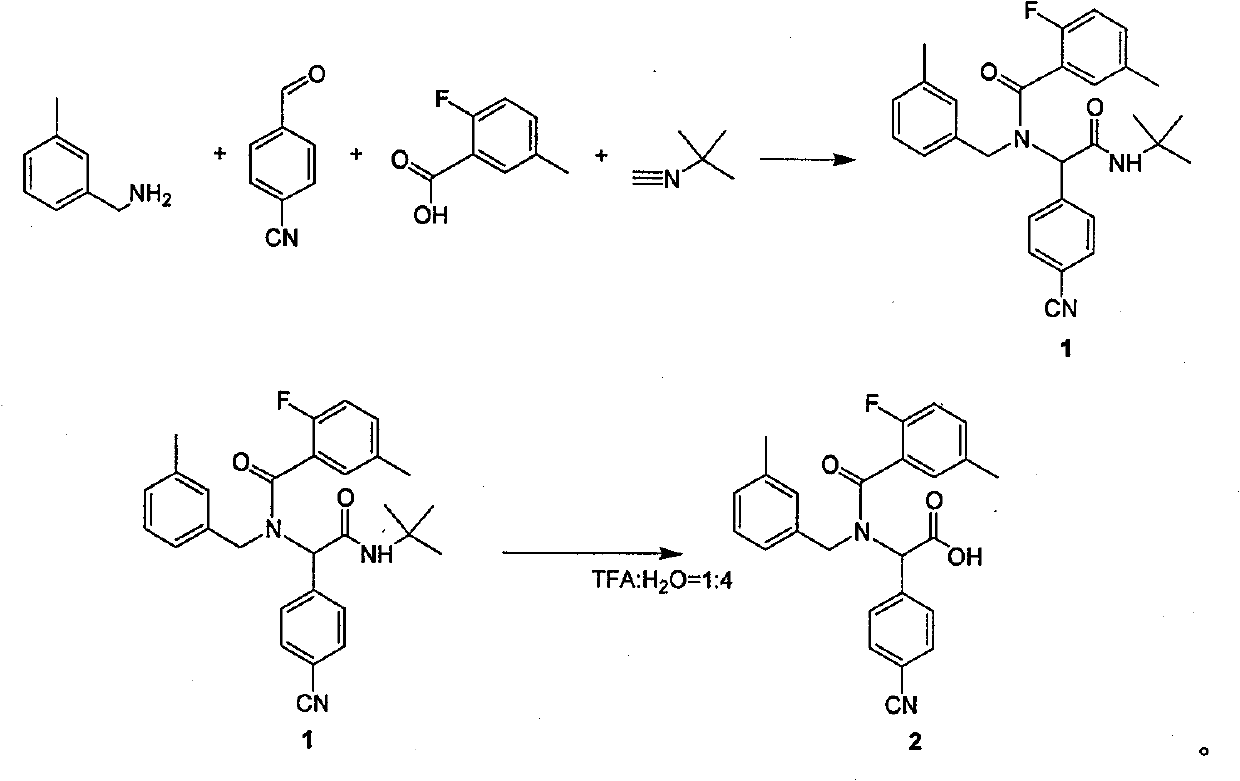
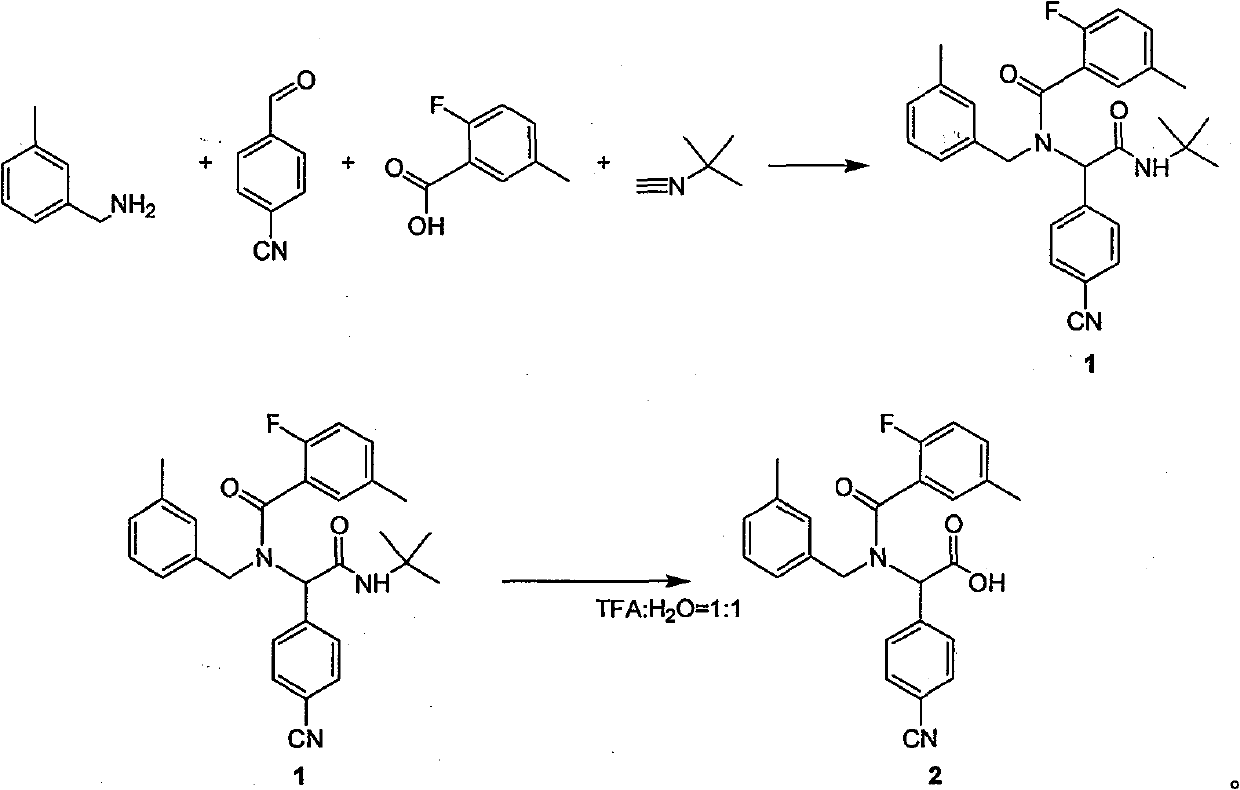
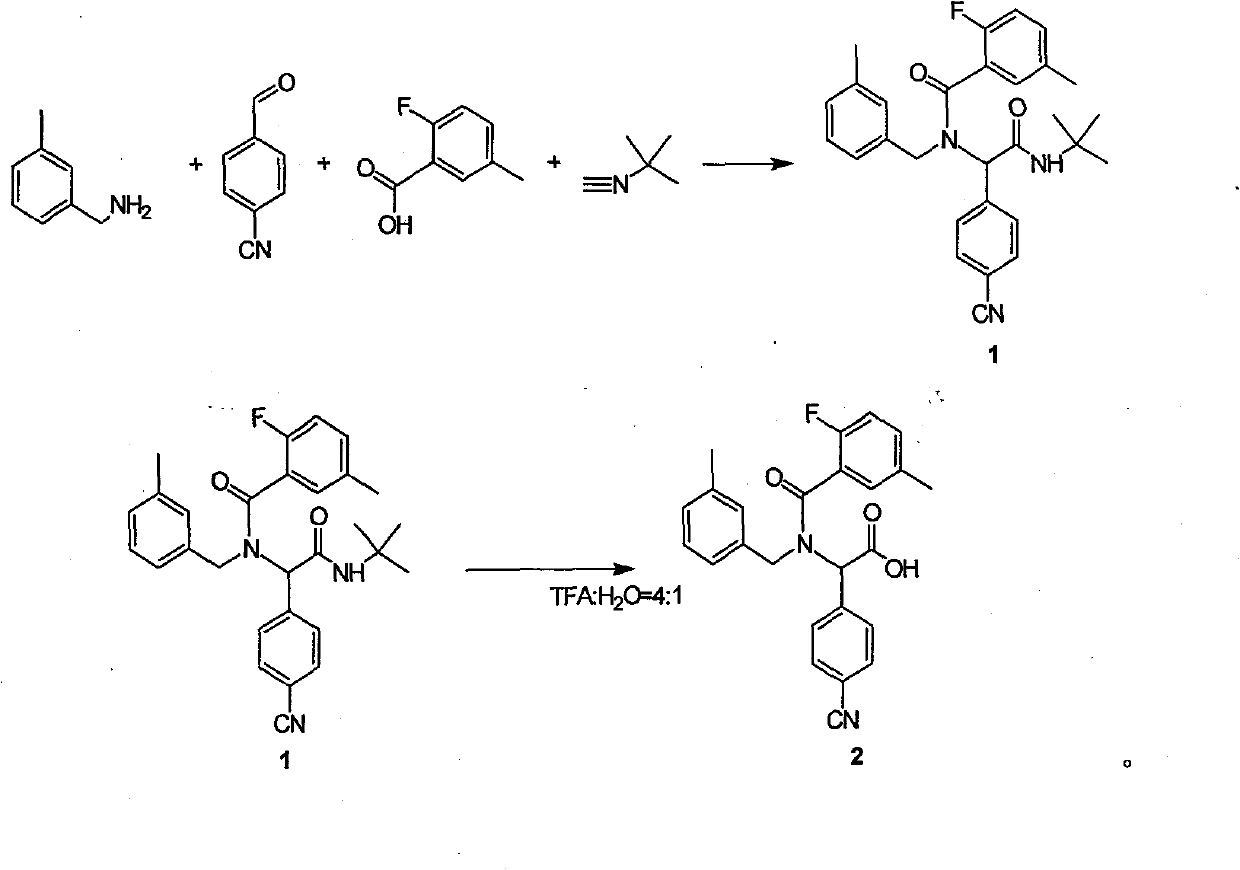
Method for synthesizing substituted amino carboxylic acid by ugi reaction
ActiveCN101468953BReasonable choice of reaction processAvoid disadvantages such as difficult purificationOrganic compound preparationCarboxylic acid amides preparationArylTrifluoroacetic acid
The invention relates to a method for synthesizing substituted amino carboxylic acid, in particular to a method for synthesizing the substituted amino carboxylic acid by utilization of Ugi reaction. The method mainly solves the technical problems of rigorous reaction conditions and low industrialization degree in the prior synthesis method. The method adopts the technical proposal that the method adopts tert-butyl isonitrile which can be obtained by commercialization as a raw material, obtains corresponding t-butyl carboxamide through the Ugi reaction, and obtains the substituted amino carboxylic acid by mildly hydrolyzing the t-butyl carboxamide under the action of trifluoroacetic acid and water. The synthesis technology is as shown in the formula. In the technology, R, R1 and R2 refer to alkyl groups or aryl groups between C1 and C20; the volume ratio of the trifluoroacetic acid to the water in the second step, namely TFA:H2O, is between 1:5 and 100:0; and the reaction temperature is between the room temperature and the solvent reflux temperature.
Owner:上海药明康德新药开发有限公司
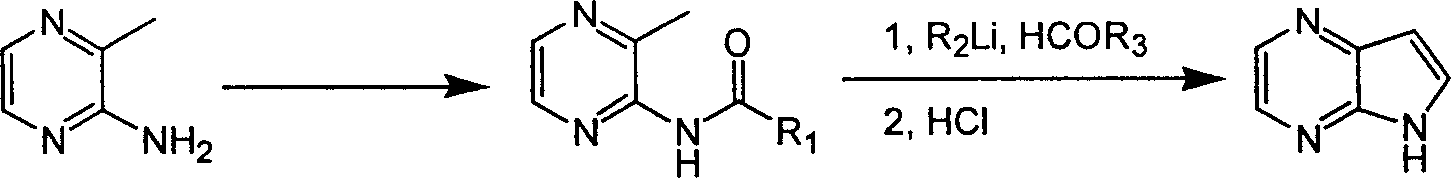
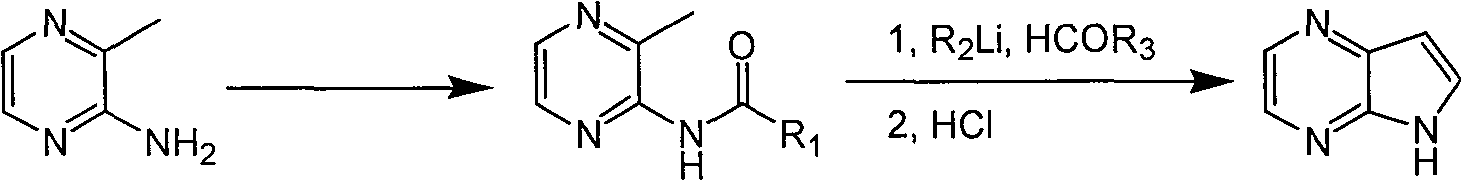
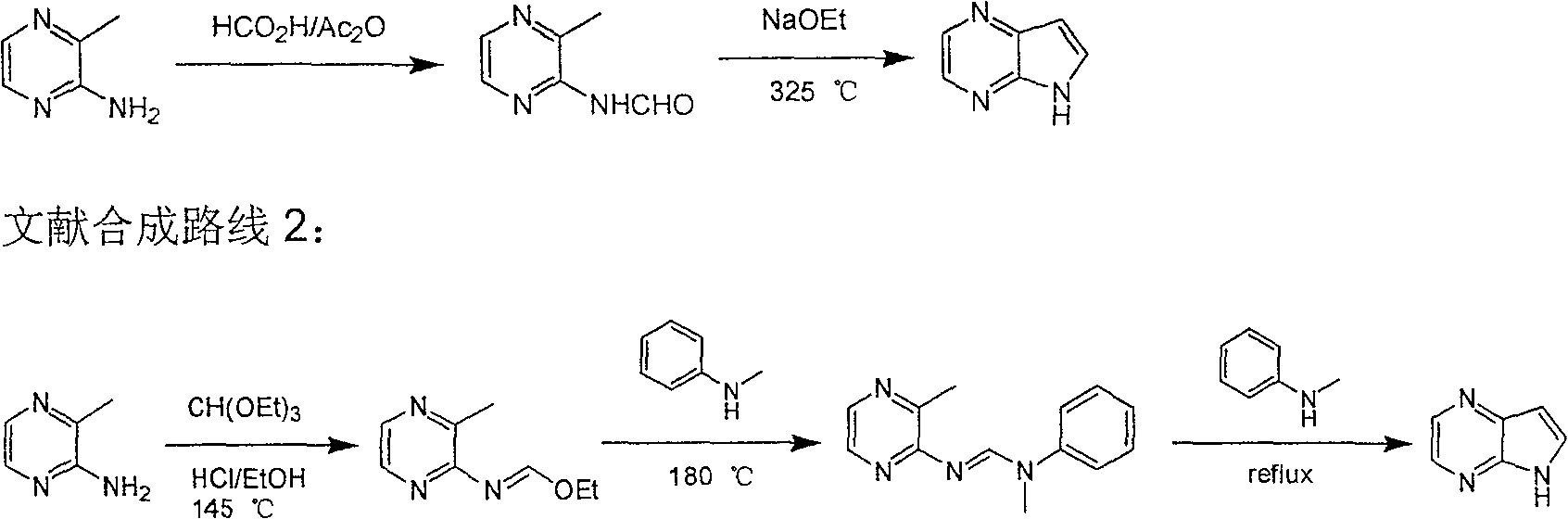
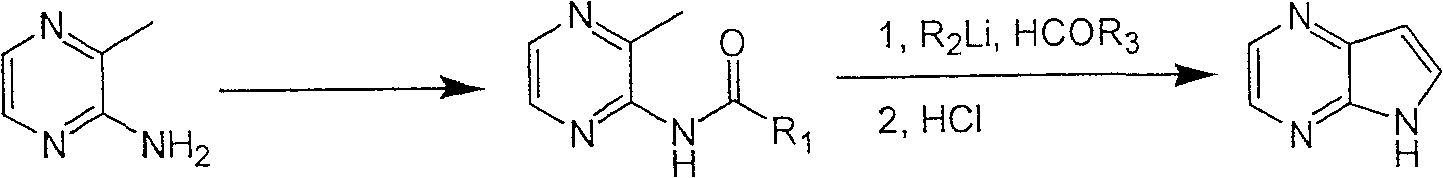
Preparing process of 4,4-diazaindole
ActiveCN1986544AReasonable choice of reaction processEasy to operateOrganic chemistryBulk chemical productionPyrazineAcid anhydride
The process of preparing 4, 7-diazaindole includes the following steps: 1. reaction of amino-3-methyl pyrazine and pivaloyl chloride or Boc anhydride to obtain 2-pivaloyl amino-3-methyl pyrazine or 2-tert-butyloxyformamido-3-methyl pyrazine; 2. reaction of the obtained 2-pivaloyl amino-3-methyl pyrazine or 2-tert-butyloxyformamido-3-methyl pyrazine with R2Li and HCOR3 to produce aldehyde intermediate; and 3. eliminating the protecting ring of the intermediate to obtain 4, 7-diazaindole. The present invention is one effective process of synthesizing 4, 7-diazaindole as one medicine intermediate.
Owner:CHANGZHOU HEQUAN PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



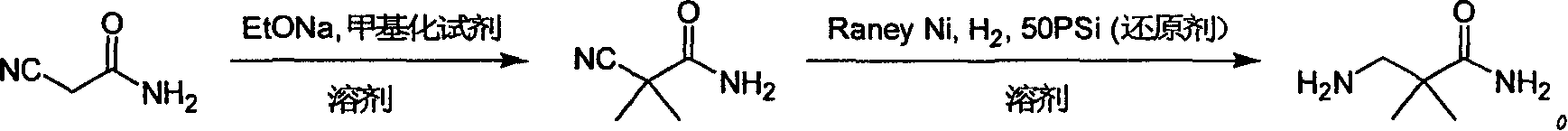
![Method for quickly preparing cis-octahydropyrrolo[3,2-b]pyrrole Method for quickly preparing cis-octahydropyrrolo[3,2-b]pyrrole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0bfe83ec-7fcb-4056-bae5-a3c36aa5f397/G200910201887XD00011.png)
![Method for quickly preparing cis-octahydropyrrolo[3,2-b]pyrrole Method for quickly preparing cis-octahydropyrrolo[3,2-b]pyrrole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0bfe83ec-7fcb-4056-bae5-a3c36aa5f397/G200910201887XD00012.png)
![Method for quickly preparing cis-octahydropyrrolo[3,2-b]pyrrole Method for quickly preparing cis-octahydropyrrolo[3,2-b]pyrrole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0bfe83ec-7fcb-4056-bae5-a3c36aa5f397/G200910201887XD00021.png)
![Industrial continuous preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptane Industrial continuous preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d88ca614-96d3-440c-9418-d717e1ecdbba/A2005101118530002C1.PNG)
![Industrial continuous preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptane Industrial continuous preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d88ca614-96d3-440c-9418-d717e1ecdbba/A20051011185300041.PNG)
![Industrial continuous preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptane Industrial continuous preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d88ca614-96d3-440c-9418-d717e1ecdbba/A20051011185300051.PNG)


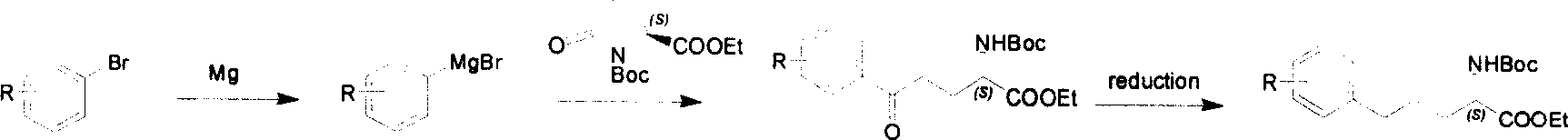
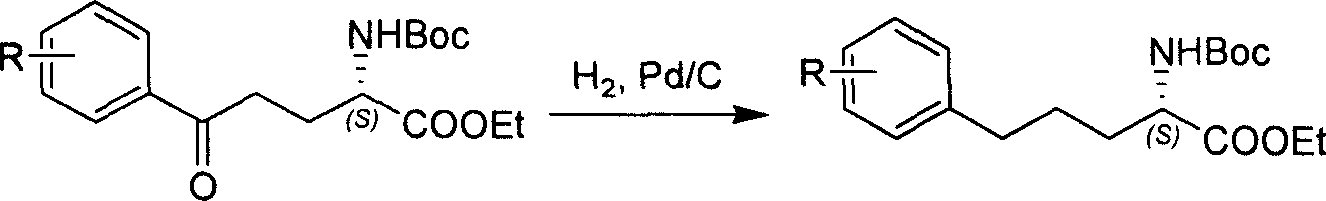


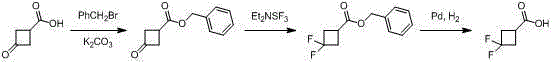

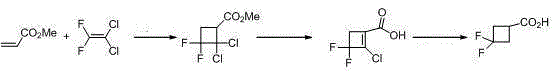
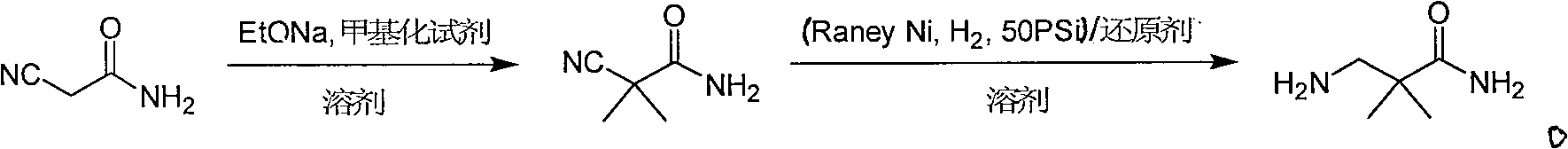
![Industrial preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptance in one cauldron Industrial preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptance in one cauldron](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b2bea058-5193-49c0-a316-2a3cdeeed038/A2005101118520002C1.PNG)
![Industrial preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptance in one cauldron Industrial preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptance in one cauldron](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b2bea058-5193-49c0-a316-2a3cdeeed038/A20051011185200041.PNG)
![Industrial preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptance in one cauldron Industrial preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptance in one cauldron](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b2bea058-5193-49c0-a316-2a3cdeeed038/A20051011185200051.PNG)




![Industrial preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptance in one cauldron Industrial preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptance in one cauldron](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/27fff49e-84da-40cf-af68-64e81c97d271/D2005101118529A00011.png)
![Industrial preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptance in one cauldron Industrial preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptance in one cauldron](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/27fff49e-84da-40cf-af68-64e81c97d271/F2005101118529C00011.png)
![Industrial preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptance in one cauldron Industrial preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptance in one cauldron](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/27fff49e-84da-40cf-af68-64e81c97d271/G2005101118529D00011.png)
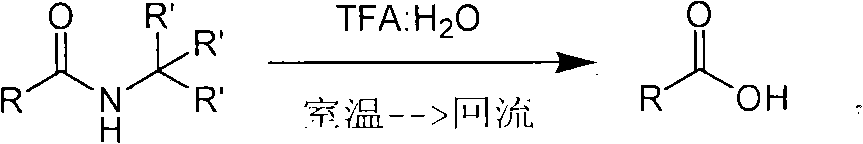
![Method for synthesizing 3-aza-bicyclo[4.1.0]heptane-6-formic acid with protective group Method for synthesizing 3-aza-bicyclo[4.1.0]heptane-6-formic acid with protective group](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7882b425-89ad-42fb-9250-4931ae01e932/G2009102018600D00011.PNG)
![Method for synthesizing 3-aza-bicyclo[4.1.0]heptane-6-formic acid with protective group Method for synthesizing 3-aza-bicyclo[4.1.0]heptane-6-formic acid with protective group](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7882b425-89ad-42fb-9250-4931ae01e932/G2009102018600D00021.PNG)
![Industrial continuous preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptane Industrial continuous preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a7694337-3632-4f6f-9a83-ea9fcfd6fd01/D2005101118533A00011.png)
![Industrial continuous preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptane Industrial continuous preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a7694337-3632-4f6f-9a83-ea9fcfd6fd01/F2005101118533C00011.png)
![Industrial continuous preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptane Industrial continuous preparing process of N-tert-butoxy carbonyl-5-aza-2-oxa-3-one-dicyclo-[2,2,1] heptane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a7694337-3632-4f6f-9a83-ea9fcfd6fd01/G2005101118533D00011.png)