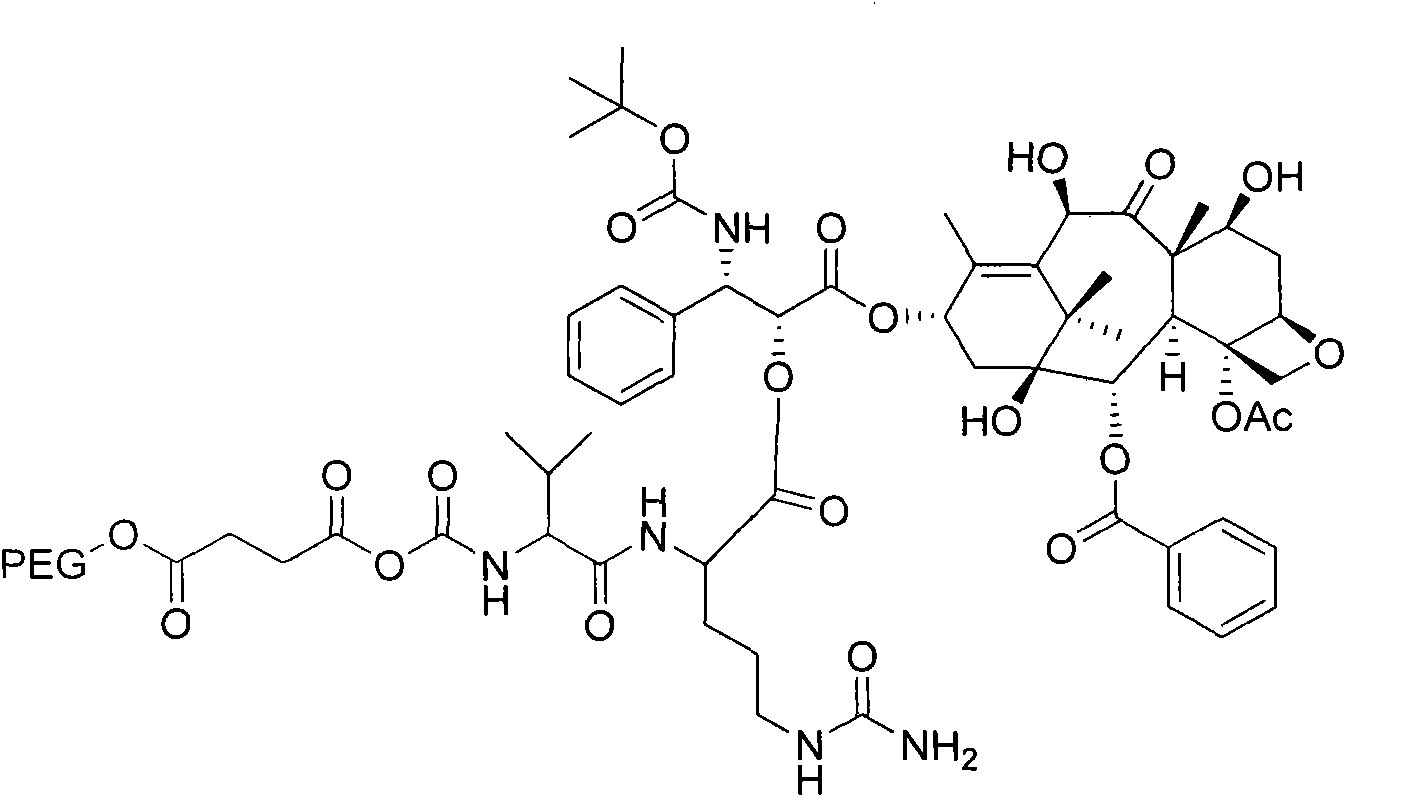
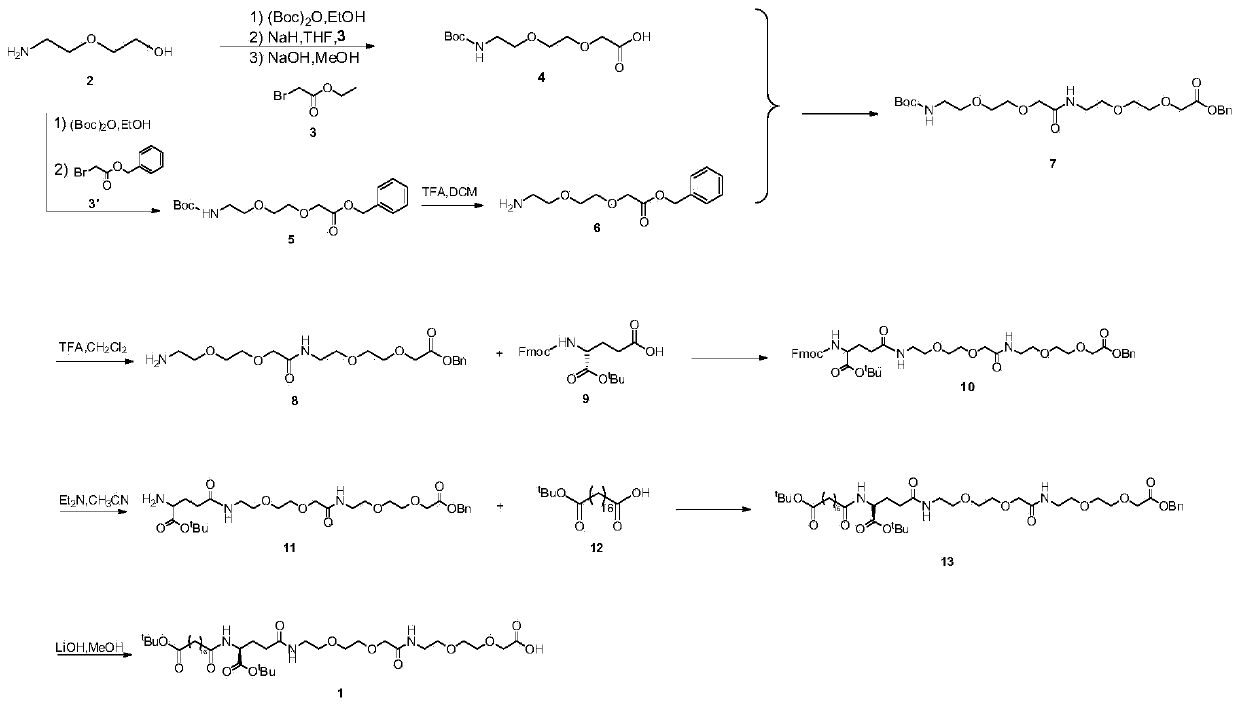
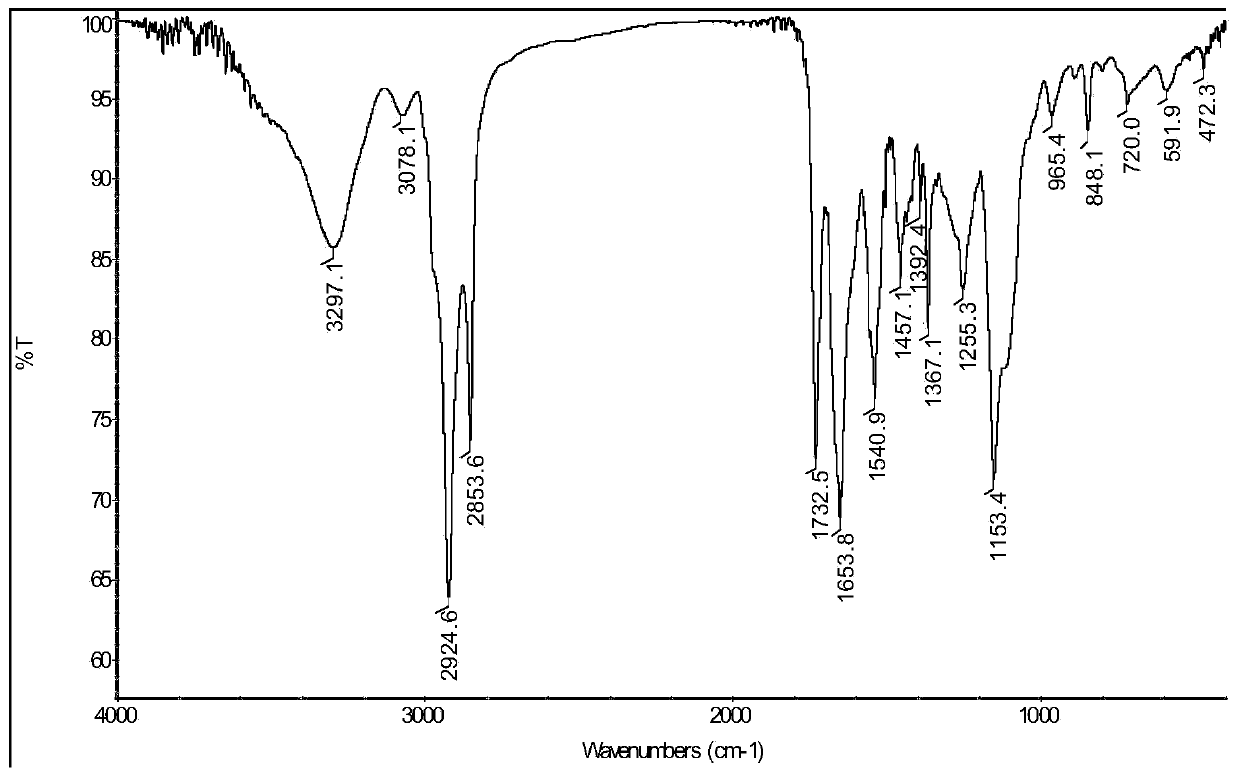
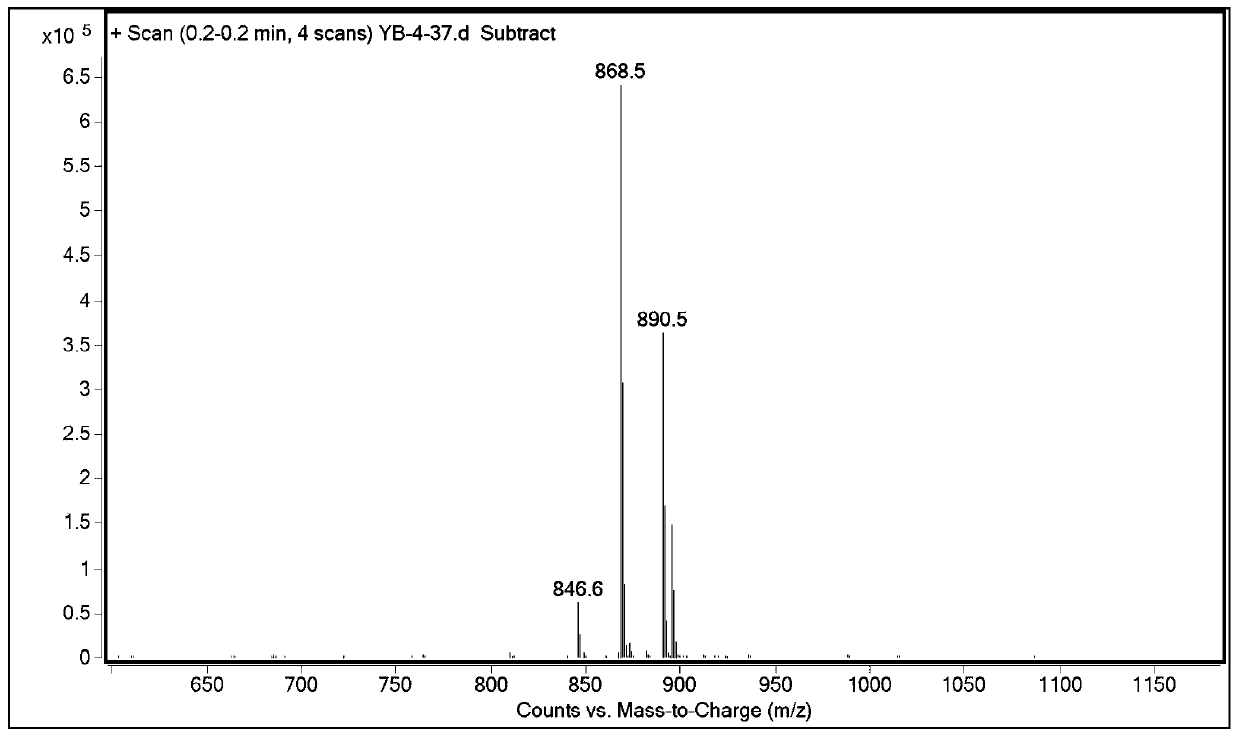
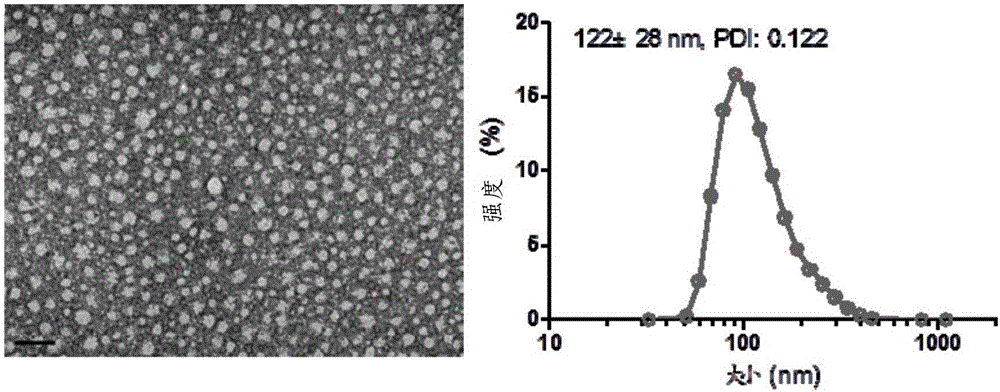
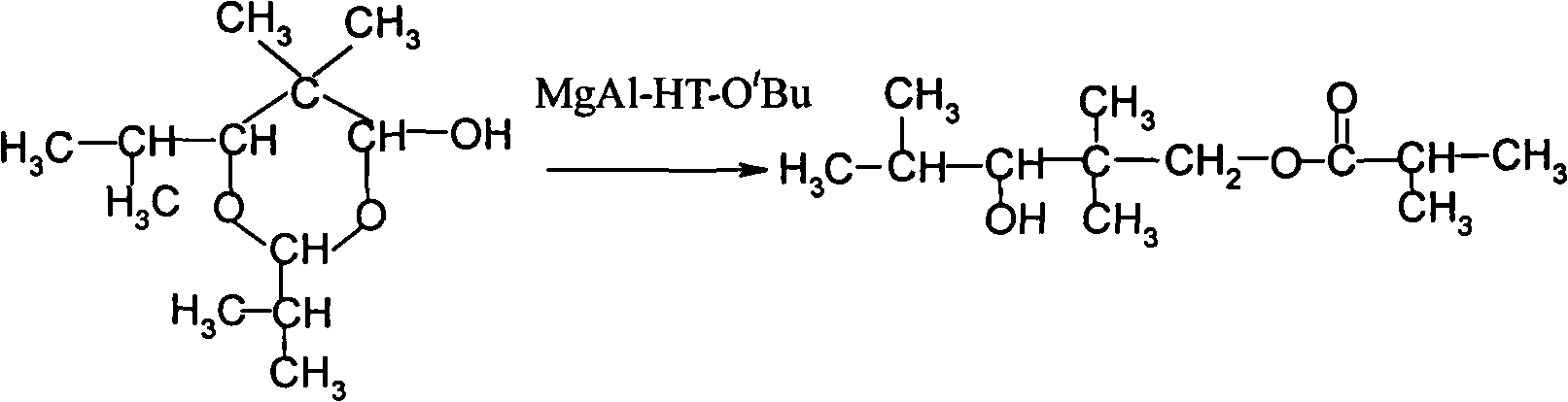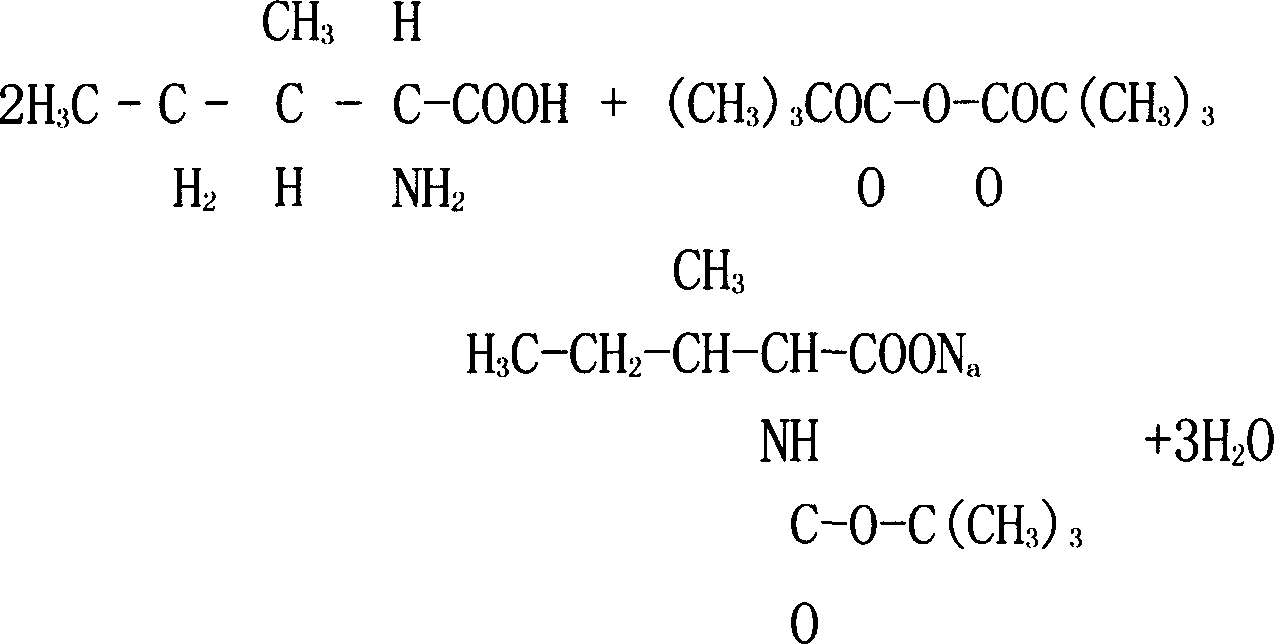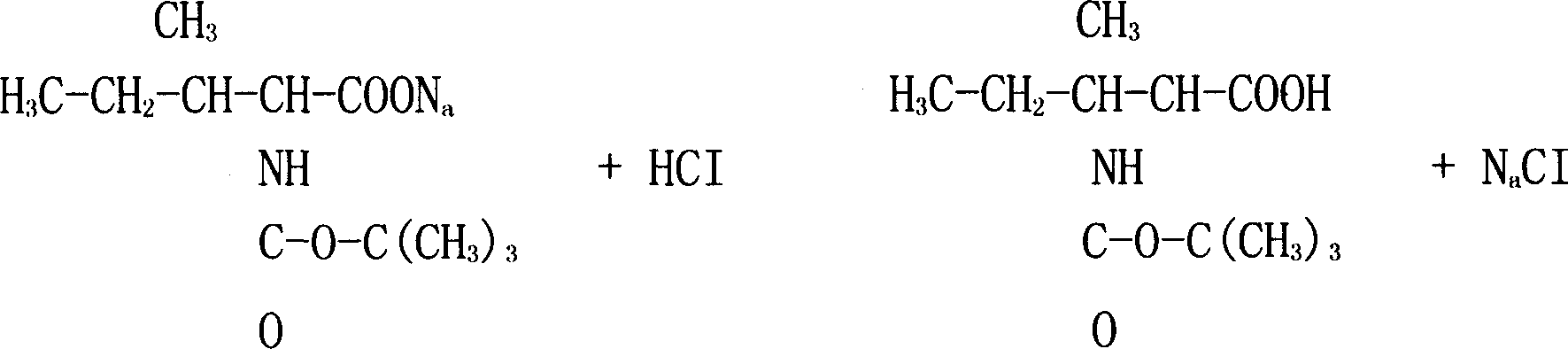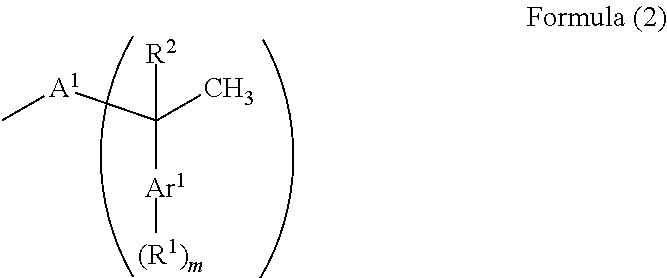Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
180 results about "Tert-butoxy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hyperbranched polymer, production method therefor and resist composition containing hyperbranched polymer
InactiveUS20070148585A1Improve flatnessImprove solubilityPhotosensitive materialsPhotomechanical apparatusResistPolymer science
The present invention provides a hyperbranched polymer suitable as a polymer material for nanofabrication, in particular, photolithography. The hyperbranched polymer of the present invention is characterized by having a core portion prepared by a living radical polymerization of chloromethyl styrene or the like, and an acid decomposition group such as p-tert-butoxy styrene, which is linked to the core portion and present at the end of a molecule of the polymer.
Owner:LION CORP
Vapor deposition of metal oxides, silicates and phosphates, and silicon dioxide
InactiveUS20050277780A1Good step coverageOxygen/ozone/oxide/hydroxideAluminium silicatesAlkylphosphateGas phase
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Folate-polyethylene glycol-polylactic acid segmented copolymer micelle encapsulated with hydrophobic anticancer drug and preparation method of segmented copolymer micelle
InactiveCN103520731AImprove stabilityAct as a slow releaseOrganic active ingredientsPharmaceutical non-active ingredientsPoly-L-lactidePolymer science
The invention discloses a folate-polyethylene glycol-polylactic acid segmented copolymer micelle encapsulated with a hydrophobic anticancer drug and a preparation method of the segmented copolymer micelle. The preparation method comprises the steps as follows: bis-amino polyethylene glycol polymer is prepared firstly; mono amino polyethylene glycol tert-butyl ester polymer is prepared by the bis-amino polyethylene glycol polymer; the mono amino polyethylene glycol tert-butyl ester polymer is cross-linked with lactide to obtain tert-butoxy acylamino polyethylene glycol-polylactic acid polymer, and the tert-butoxy acylamino polyethylene glycol-polylactic acid polymer reacts with trifluoroacetic acid to obtain amino-terminated polyethylene glycol-polylactic acid polymer; and the amino-terminated polyethylene glycol-polylactic acid polymer and activated folate react away from light to obtain the folate-polyethylene glycol-polylactic acid polymer. The compound has hydrophilia , biocompatibility and water solubility of the hydrophobic segment, material absorption and cell adhesion of protein can be reduced, the circulation time of the drug in blood is prolonged, and the formed copolymer has modificability; a tumor target compound with low molecular weight and hydrophilic polyethylene glycol-4000 segmer are bonded to form a hydrophilic segment; and the hydrophobic segment is polylactic acid, and the hydrophobic anticancer drug is embedded into the hydrophobic segment, so that the toxic and side effects are reduced.
Owner:SOUTH CHINA UNIV OF TECH
Method for preparing 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate
InactiveCN101863762AImprove one-way yieldHigh selectivityOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by aldehyde oxidation-reductionTert-butoxyTishchenko reaction
The invention provides an environmental-friendly method for preparing 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate, which comprises a condensation reaction and a Tishchenko reaction of isobutyraldehyde, wherein condensation products of the isobutyraldehyde are subjected to the Tishchenko reaction under the catalytic action of tert-butoxy anion-pillared layered magnesium-aluminum anionic clay to generate 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate. The preparation method has the advantages of high single-pass yield, high selectivity of target products, a few by-products, mild reaction conditions, catalyst recycling and environmental-friendly synthetic process.
Owner:江苏天音化工有限公司 +1
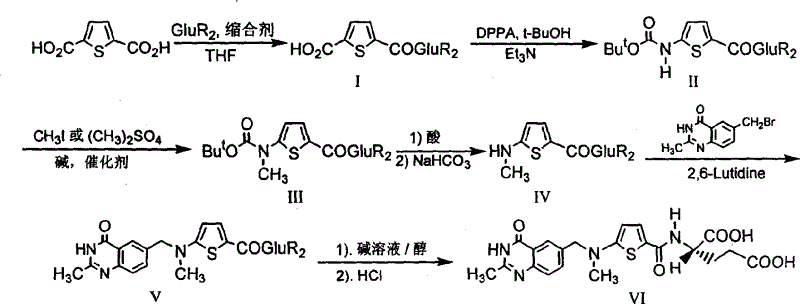
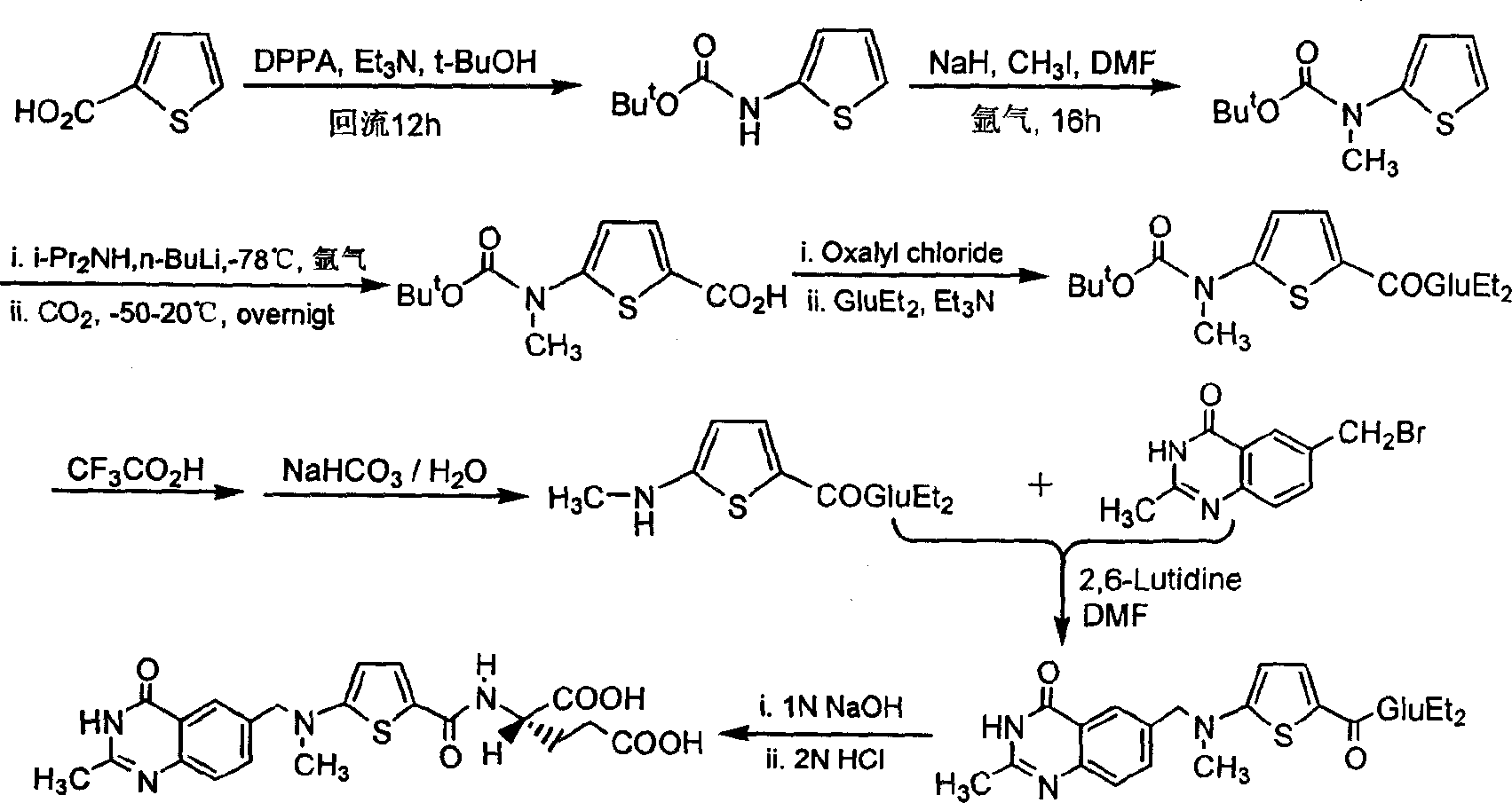
Synthesis of anticancer medicine Raltiprexed
InactiveCN1486985AShort processLow costOrganic chemistryAntineoplastic agentsTert-Butyloxycarbonyl protecting groupN methylation
The synthesis of anticancer medicine Raltitrexed with 2, 5-thienyl diformic acid and diethyl glutamate as initial material and through six reaction steps of monocondensation, rearrangement, N-methylation, e;limination of tert-butoxy carbonyl group, condensation with 6-bromomethyl-2-methyl-4-quinbolone and saponification. The present invention has total yield of 18.1%, higher than that of available synthesis line, less reaction steps, mild condition and simple operation, and is suitable for mass production.
Owner:CAPITAL NORMAL UNIVERSITY
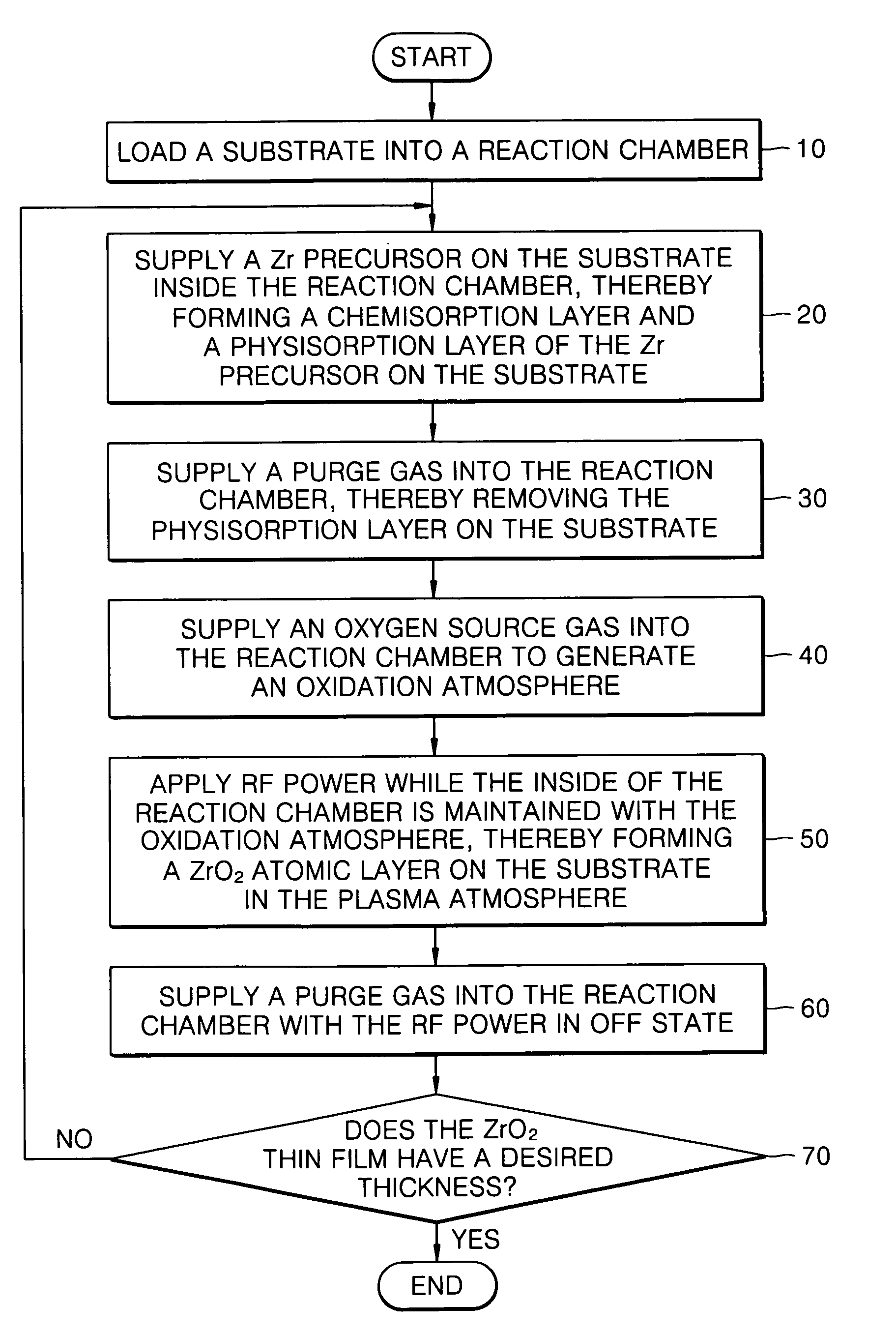
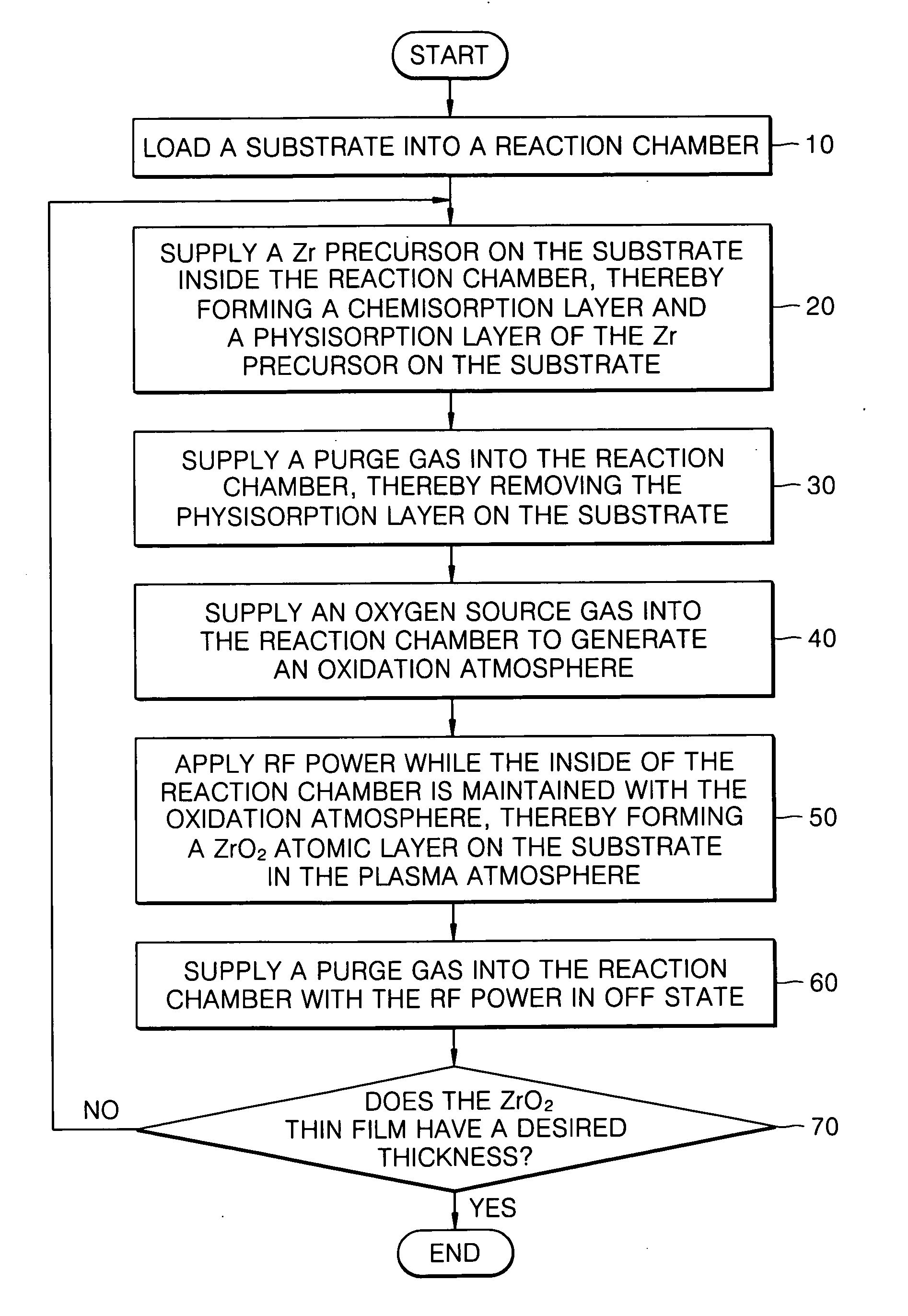
Method of forming a ZrO2 thin film using plasma enhanced atomic layer deposition and method of fabricating a capacitor of a semiconductor memory device having the thin film
InactiveUS7491654B2Improved thermal characteristic and electrical characteristicIncrease deposition rateSolid-state devicesSemiconductor/solid-state device manufacturingChemisorptionOptoelectronics
Example embodiments of the present invention relate to a method of forming a dielectric thin film and a method of fabricating a semiconductor memory device having the same. Other example embodiments of the present invention relate to a method of forming a ZrO2 thin film and a method of fabricating a capacitor of a semiconductor memory device using the ZrO2 thin film as a dielectric layer. A method of forming a ZrO2 thin film may include supplying a zirconium precursor on a substrate maintained at a desired temperature, thereby forming a chemisorption layer of the precursor on the substrate. The zirconium precursor may be a tris(N-ethyl-N-methylamino)(tert-butoxy) zirconium precursor. The substrate having the chemisorption layer of the precursor may be exposed to the plasma atmosphere of oxygen-containing gas for a desired time, thereby forming a Zr oxide layer on the substrate, and a method of fabricating a capacitor of a semiconductor memory device having the ZrO2 thin film.
Owner:SAMSUNG ELECTRONICS CO LTD
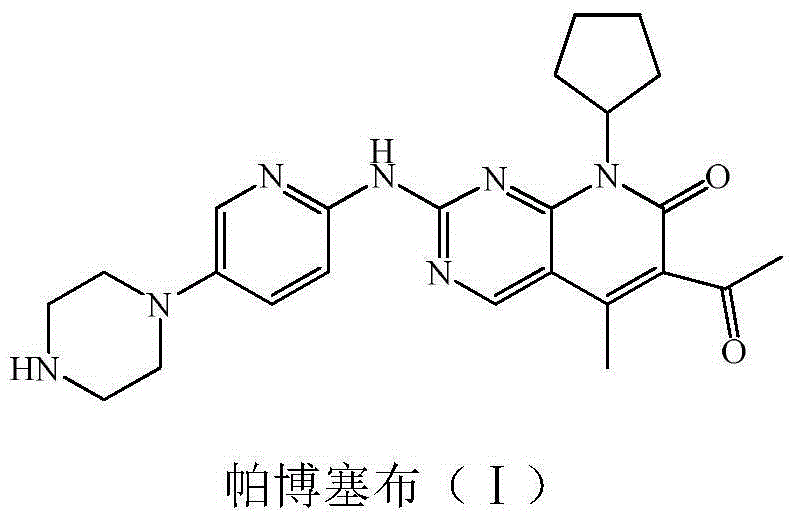
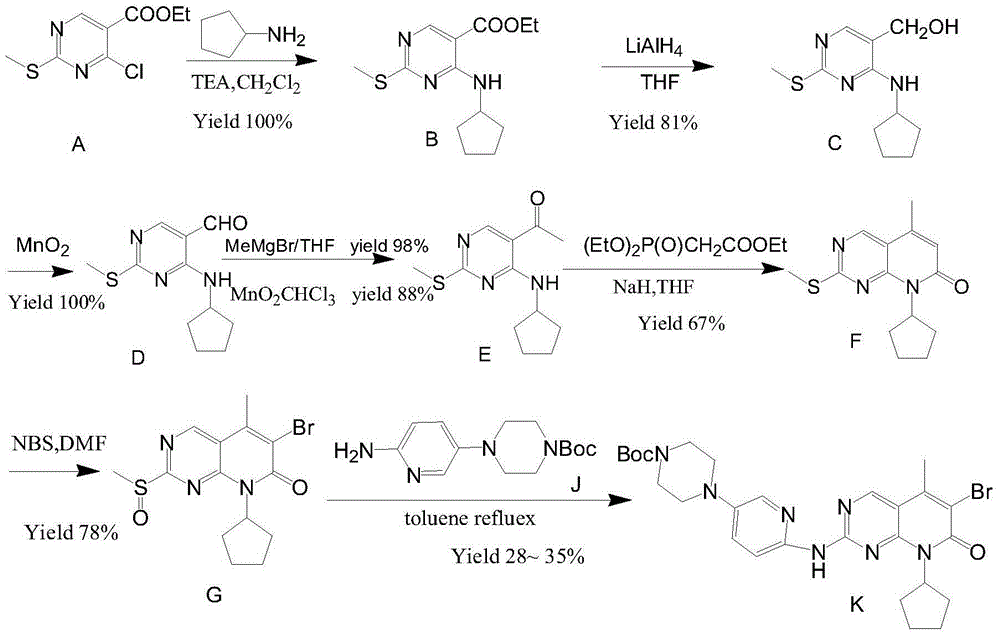
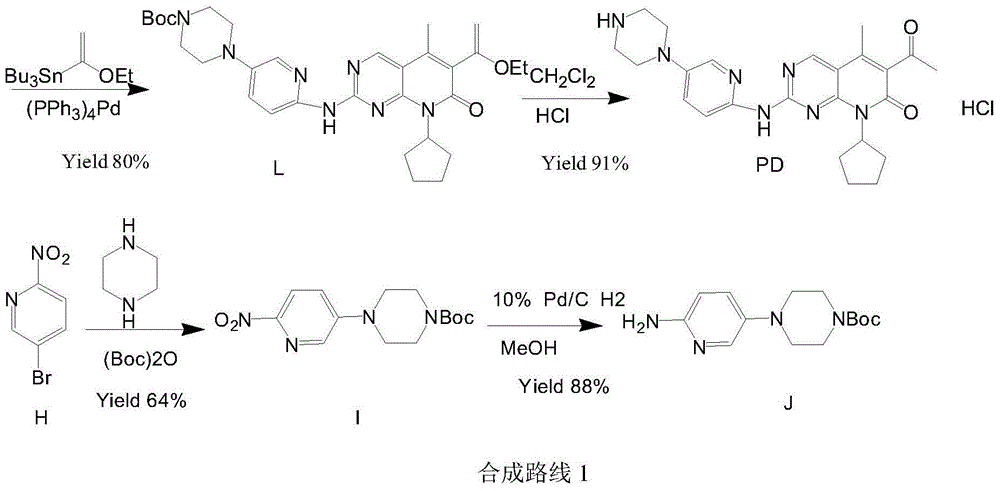
Preparation method of palbociclib
ActiveCN104478874AHigh yieldRaw materials are cheap and easy to getOrganic chemistry2-PyridoneMethyl group
The invention relates to a preparation method of palbociclib. The method comprises the following steps: carrying out N-acetoacetylation reaction by using cyclopentylamine and an acetoacetylation agent to obtain N,N-di(acetoacetyl) cyclopentylamine (II), carrying out intramolecular condensation on N,N-di(acetoacetyl) cyclopentylamine (II) in the presence of an alkaline reagent to obtain N-cyclopentyl-3-acetyl-4-methyl-6-hydroxy-2-pyridone (III), enabling N-cyclopentyl-3-acetyl-4-methyl-6-hydroxy-2-pyridone (III) to react with a formylation reagent to prepare N-cyclopentyl-3-acetyl-4-methyl-5-formyl-6-chlorine-2-pyridone (IV), carrying out pyrimidine ring reaction by using N-cyclopentyl-3-acetyl-4-methyl-5-formyl-6-chlorine-2-pyridone (IV) and N-(5-(4-tert-butoxy carbonyl-1-hexahydropyrazinyl)-2-pyridyl) guanidine sulfate (V), and then hydrolyzing under the alkaline condition to prepare palbociclib. The preparation method of palbociclib is easily available in raw materials, short in process, simple and convenient to operate and safe and environmentally friendly in process.
Owner:XINFA PHARMA
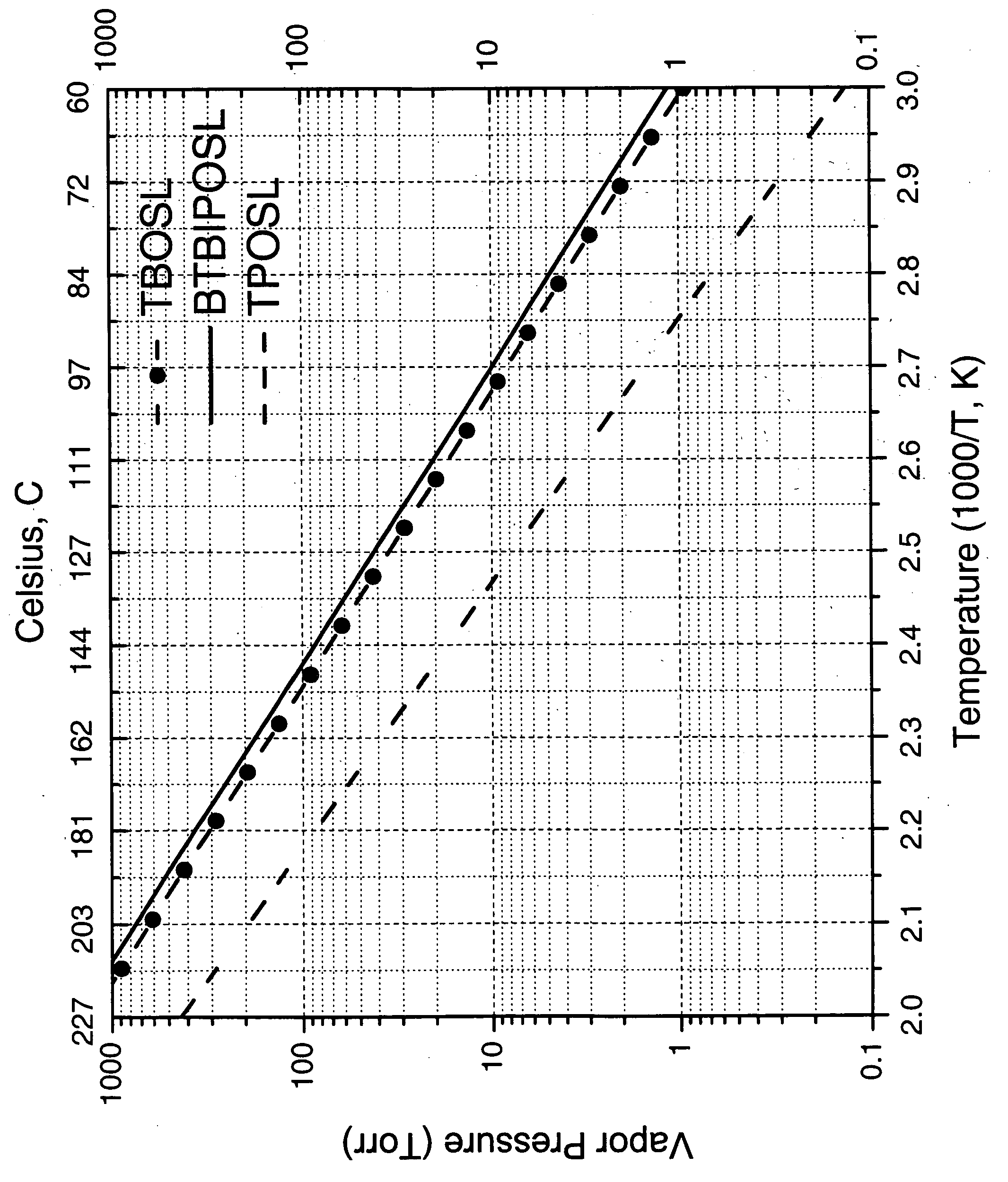
Precursors for silica or metal silicate films
A composition selected from the group consisting of bis(tert-butoxy)(isopropoxy)silanol, bis(isopropoxy)(tert-butoxy)silanol, bis(tert-pentoxy)(isopropoxy)silanol, bis(isopropoxy)(tert-pentoxy)silanol, bis(tert-pentoxy)(tert-butoxy)silanol, bis(tert-butoxy)(tert-pentoxy)silanol and mixtures thereof; its use to form a metal or metalloid silicate layer on a substrate and the synthesis of the mixed alkoxysilanols.
Owner:VERSUM MATERIALS US LLC
Method for preparing water-soluble taxane medicament and application thereof
InactiveCN101935336AImprove solubilityLow toxicityOrganic active ingredientsPeptidesHydrogenHydrogen atom
Owner:PEKING UNIV
Radiation-sensitive resin composition
A radiation-sensitive resin composition comprising (A) a photoacid generator such as 2,4,6-trimethylphenyldiphenylsulfonium 2,4-difluorobenzenesulfonate or 2,4,6-trimethylphenyldiphenylsulfonium 4-trifluoromethylbenzenesulfonate and (B) a resin having an acetal structure typified by a poly(p-hydroxystyrene) resin in which a part of hydrogen atoms of phenolic hydroxyl groups have been replaced by 1-ethoxyethyl groups, 1-ethoxyethyl groups and t-butoxycarbonyl groups, or 1-ethoxyethyl groups and t-butyl groups. The resin composition is sensitive to deep ultraviolet rays and charged particles such as electron beams, exhibits excellent resolution performance and pattern shape-forming capability, and suppresses a nano-edge roughness phenomenon to a minimal extent.
Owner:JSR CORPORATIOON
Tumor-targeting pH- and redox-response macromolecule nano prodrug and preparation method and application thereof
InactiveCN109966507AHigh drug loadingHas pHOrganic active ingredientsPharmaceutical non-active ingredientsTumor targetHigh concentration
The invention belongs to the technical field of biological medical polymer materials and discloses a pH- and redox-response macromolecule nano prodrug based on CD44 receptor tumor targeting and preparation method and application thereof. The molecular structure of the macromolecule nano prodrug is as shown in general formula (1), wherein R is one of the structures as shown in the specifications; mand n are respectively side chain polymerization degree and peptide chain polymerization degree, and x is the repetitive unit number; when R1 is phenyl, R2 is acetyl; when R1 is tert-butoxy, R2 is hydrogen. The macromolecule nano prodrug is applicable to the precise delivery of antitumor drugs and the preparation of drugs for inhibiting tumor cell proliferation; after the macromolecule nano prodrug forms nano-micelles in water through self-assembling, tumor tissue is actively identified, the nano-micelles are devoured by cancer cells, the low-pH and high-concentration GSH environments in thecancer cells allow the acylhydrazone bond and disulfide bond in the structure of the nano-micelles to break, the nano-micelles disassemble to accelerate the release of chemotherapy drugs, and the cancer cells are finally killed.
Owner:GUANGZHOU MEDICAL UNIV
Process for synthesizing ritonavir
InactiveCN1554647AStrong response specificityLow costOrganic chemistryAntiviralsChloroformateHydrolysis
The present invention relates to the synthesis process of Ritonavir as one proteinase inhibitor for resisting AIDS. The synthesis process includes the condensation between benzylamino alcohol and valine NCA to obtain valyl benzylamino alcohol, the reaction between valyl benzylamino alcohol and ditert-butyl dicarbonate to obtain tert-butoxy acyl valyl benzylamino alcohol, the hydrogenolysis and debenzylation of tert-butoxy acyl valyl benzylamino alcohol in ammonium formate and Pd-C to obtain tert-butoxy acyl valyl amino alcohol, the active esterification reaction between tert-butoxy acyl valyl amino alcohol and 5-methylol thiazole, subsequent hydrolysis to obtain thiazolyl-5-methoxycarbonyl valyl amino alcohol, and the reaction of thiazolyl-5-methoxycarbonyl valyl amino alcohol and isopropyl thiazolyl methylamine under the action of BTC to obtain final product Ritonavir. The present invention has lowered cost and raised atomic utilization.
Owner:XIAMEN UNIV
Film-forming apparatus, film-forming method and recording medium
InactiveUS20090269494A1Satisfactory utilization efficiencyImprove productivitySemiconductor/solid-state device manufacturingRecord information storageTert-butoxyPhysical chemistry
A film forming apparatus comprises a processing chamber for holding therein a to-be-processed substrate, a first gas supplying means for supplying into the processing chamber a first vapor source including a metal alkoxide having a tertiary butoxyl group as a ligand, and a second gas supplying means for supplying into the processing chamber a second vapor source including a silicon alkoxide source, wherein the first gas supplying means and the second gas supplying means are connected to a pre-reaction means for causing pre-reactions of the first vapor source and the second vapor source, and the film forming apparatus is configured to supply the first vapor source and the second vapor source after the pre-reactions into the processing chamber.
Owner:TOKYO ELECTRON LTD
Method for synthesizing N-tert-butoxy-oxo-L-isoleucine
InactiveCN1814585ALow priceSuitable for industrial productionCarbamic acid derivatives preparationOrganic compound preparationTert-butoxyTert-Butyloxycarbonyl protecting group
The invention relates to N-boc-L-isoleucine synthetic method. It belongs to chemical engineering technique field. Its features are as follows: using BOC acid anhydride as raw material to increase production efficiency, reduce production cost; adding NaOH solution to shorten production cycle; the yield of L-isoleucine can reach 105.2% which is higher than literature disclosed for over 20%.
Owner:BAOSHENG BIOLOGICAL CHEM YANGZHOU
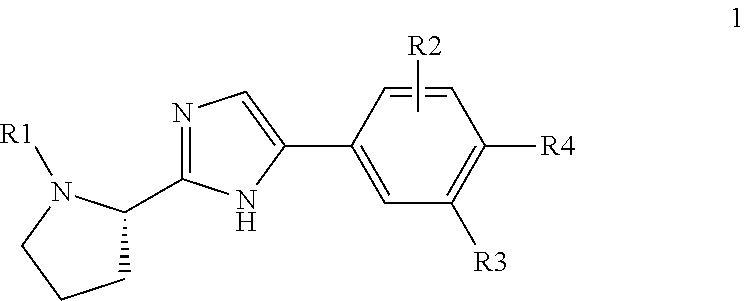
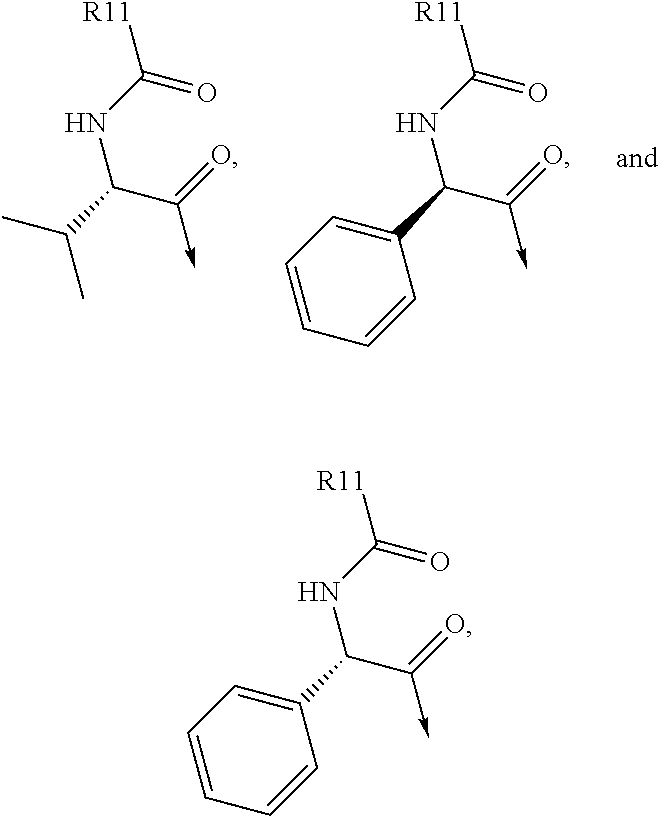
Pan-genomic inhibitors of NS5A protein encoded by HCV, pharmaceutical compositions, intermediates for inhibitor synthesis, and their synthesis and application methods
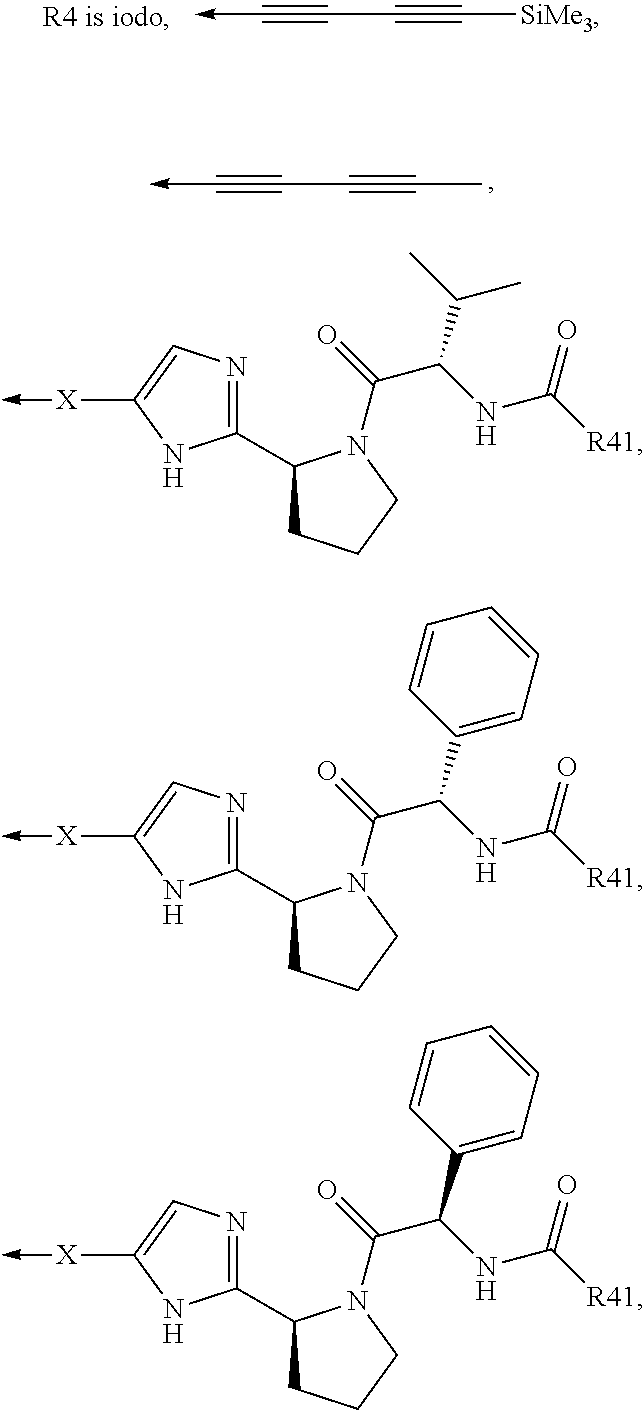
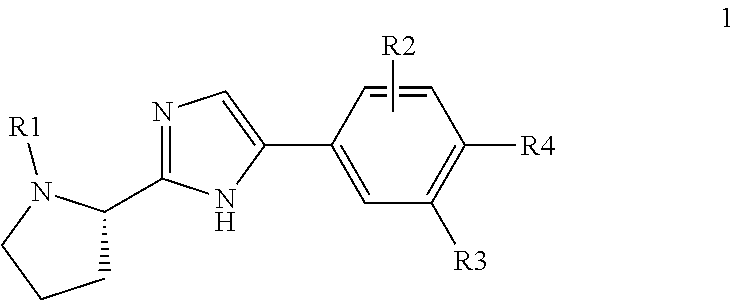
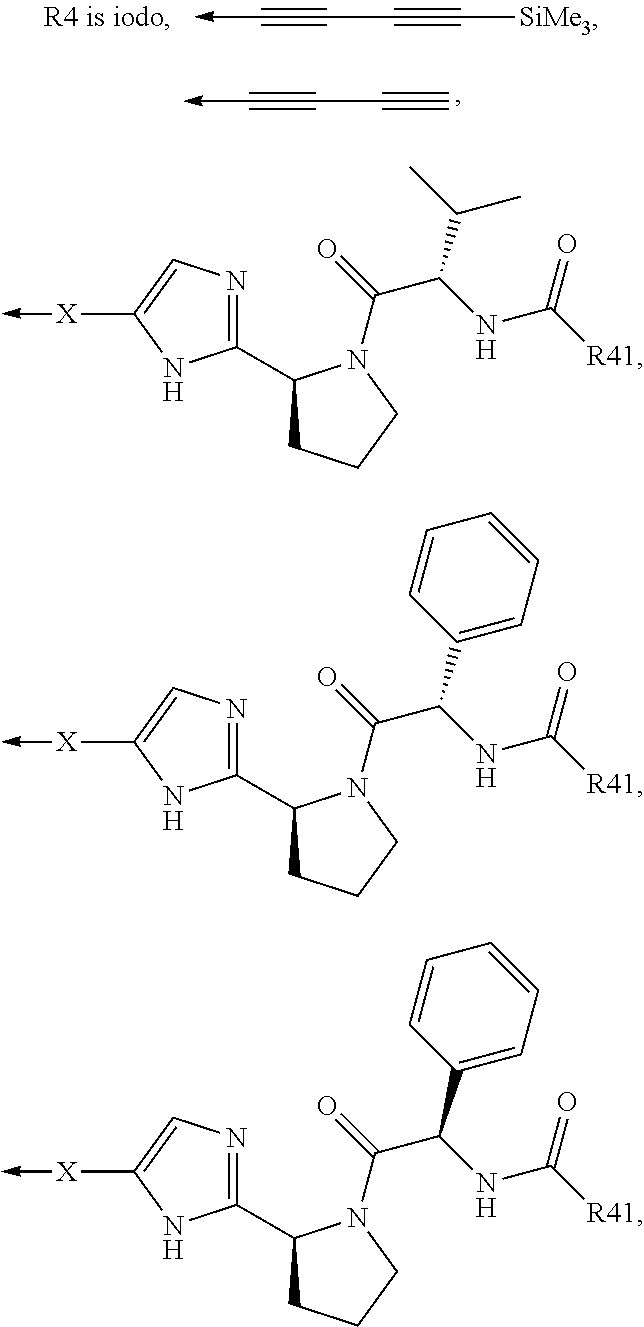
Compound represented by formula 1:or a pharmaceutically acceptable salt, a hydrate, a crystalline form, or a stereoisomer thereof, wherein:R1 is hydrogen, tert-butoxycarbonyl,where R11 is an optionally substituted C1-C6 alkyl, an optionally substituted C3-C6 cycloalkyl, or an optionally substituted C1-C6 alkyloxy, and arrows (←) indicate the position of substituents attachment;R2 is hydrogen, halogen, or C1-C4alkyl;R3 is an optionally substituted aryl, an optionally substituted aryloxy, an optionally substituted arylsulfanyl, an optionally substituted arylamino, or an optionally substituted nitrogen hetaryl;where R41 is an optionally substituted C1-C6 alkyl, an optionally substituted C3-C6 cycloalkyl, or an optionally substituted C1-C6 alkyloxy; X is buta-1,3-diynylene or 1,4-phenylene; arrows (←) indicate the position of substituents attachment.
Owner:IVACHTCHENKO ALEXANDRE VASILIEVICH +3
Novel chiral iron complex, and preparation and use thereof
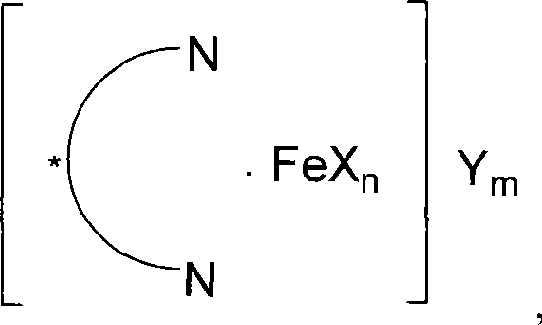
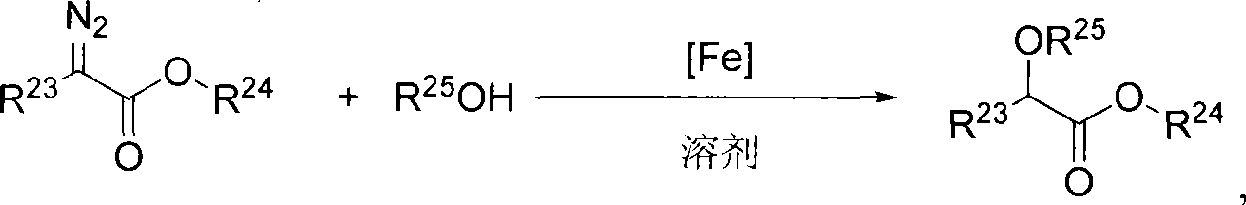
ActiveCN101412738AHigh activityHigh enantioselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAluminum IonSulfate radicals
The invention relates to a novel chiral iron complex, in particular to a novel axial chiral iron complex of a nitrogen ligand. The novel chiral iron complex has the following structural formula: wherein m+n is between 2 and 3; m is an integer from 0 to 3; n is an integer from 0 to 3; X and Y are halogens, C1-C8 carboxylate radials, sulfate radicals, perchlorate radicals, 4-(3,5-bi-trifluoromethylphenyl) borate radials, 4-(pentafluoro phenyl) borate radials, 4(perfluoro tert-butoxy ) aluminum ions, 4(hexafluoro isopropoxy) aluminum ions, hexafluoro phosphate radicals, hexafluoro antimonate radials, tetrafluoro borate radials or trifluoro methanesulfonic acid radials; and X and Y are the same or different. The invention also provides a method for preparing the novel chiral iron complex. The novel axial chiral iron complex of the nitrogen ligand can catalyze the insertion reaction of dissymmetrical O-H bonds of alpha-diazotate and show high activity and enantioselectivity.
Owner:瑞博(杭州)医药科技有限公司
Pan-genomic inhibitors of NS5A protein encoded by HCV, pharmaceutical compositions, intermediates for inhibitor synthesis, and their synthesis and application methods
ActiveUS20170066746A1Organic active ingredientsOrganic chemistryHalogenTert-Butyloxycarbonyl protecting group
Compound represented by formula 1:or a pharmaceutically acceptable salt, a hydrate, a crystalline form, or a stereoisomer thereof, wherein:R1 is hydrogen, tert-butoxycarbonyl,where R11 is an optionally substituted C1-C6 alkyl, an optionally substituted C3-C6 cycloalkyl, or an optionally substituted C1-C6 alkyloxy, and arrows (←) indicate the position of substituents attachment;R2 is hydrogen, halogen, or C1-C4alkyl;R3 is an optionally substituted aryl, an optionally substituted aryloxy, an optionally substituted arylsulfanyl, an optionally substituted arylamino, or an optionally substituted nitrogen hetaryl;where R41 is an optionally substituted C1-C6 alkyl, an optionally substituted C3-C6 cycloalkyl, or an optionally substituted C1-C6 alkyloxy; X is buta-1,3-diynylene or 1,4-phenylene; arrows (←) indicate the position of substituents attachment.
Owner:IVACHTCHENKO ALEXANDRE VASILIEVICH +3
Resist underlayer film-forming composition containing novolac resin to which aromatic vinyl compound is added
PendingUS20170097568A1Improve solubilityExcellent spin coating propertySemiconductor/solid-state device manufacturingPhotomechanical coating apparatusPolymer scienceLithography process
A resist underlayer film-forming composition has high solubility in a solvent used at a lithography process for exhibiting good coating film forming properties and able to decrease a sublime generated during formation of a film. A resist underlayer film-forming composition having a novolac resin having a structure group (C) obtained by a reaction of an aromatic ring structure of an aromatic ring-containing compound (A) with a vinyl group of an aromatic vinyl compound (B). The aromatic vinyl compound (B) is represented by Formula (1), and is specifically styrene, 2-vinylnaphthalene, 4-tert-butylstyrene, or 4-tert-butoxystyrene.The structure group (C) is represented by Formula (2).The aromatic ring-containing compound (A) is an aromatic amine compound or a phenolic hydroxy group-containing compound. The novolac resin is a resin produced by a reaction of the aromatic amine compound or the phenolic hydroxy group-containing compound with aldehyde or ketone.
Owner:NISSAN CHEM IND LTD
Polymer solid electrolyte and preparation method thereof
InactiveCN111613833AImprove thermal stabilityImprove electrochemical stabilityFinal product manufactureElectrolyte accumulators manufactureTert-butoxyALUMINUM HYDRIDE
The invention discloses a polymer solid electrolyte and a preparation method thereof. The polymer solid electrolyte comprises a polymer matrix, a lithium salt and an inorganic filler, wherein the polymer matrix is PVDF or a mixture of PVDF and PEO, the lithium salt comprises a lithium salt A, and the lithium salt A is lithium tri-tert-butoxy aluminum hydride (LTTBA) or lithium triethylborohydride(LTEB) or a mixture of the LTTBA and the LTEB. The invention also discloses a preparation method of the polymer solid electrolyte. A lithium battery containing the polymer solid electrolyte has the excellent cycle performance and rate capability and good interface stability.
Owner:DONGGUAN DONGYANG SOLAR SCI RES & DEV CO LTD
Tert-butoxy carbonyl dihydro artemisinin, preparation method and drug composition thereof
InactiveCN1405168AHigh activityLow toxicityOrganic active ingredientsOrganic chemistryDicarbonateTert-Butyloxycarbonyl protecting group
The invention provides a tert-butoxycarbonyl dihydroartemisine, which is characterized by that said invention provides its structural formula, and it is made up by using dihydroartemisine as initiation raw material, and making it and double tertiary butyl dicarbonate implement acidation reactino in organic solvent. The invented mecicine composition for resisting parasitic disease contains tert-butoxycarbonyl dihydroartemisine with therapeutic effective dose and pharmaceutically-acceptable carrier. Said invented product is high in therapeutical effect and low in toxicity, can be used for preventing and curing the parasitic diseases of schistosomiasis and malaria, etc.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
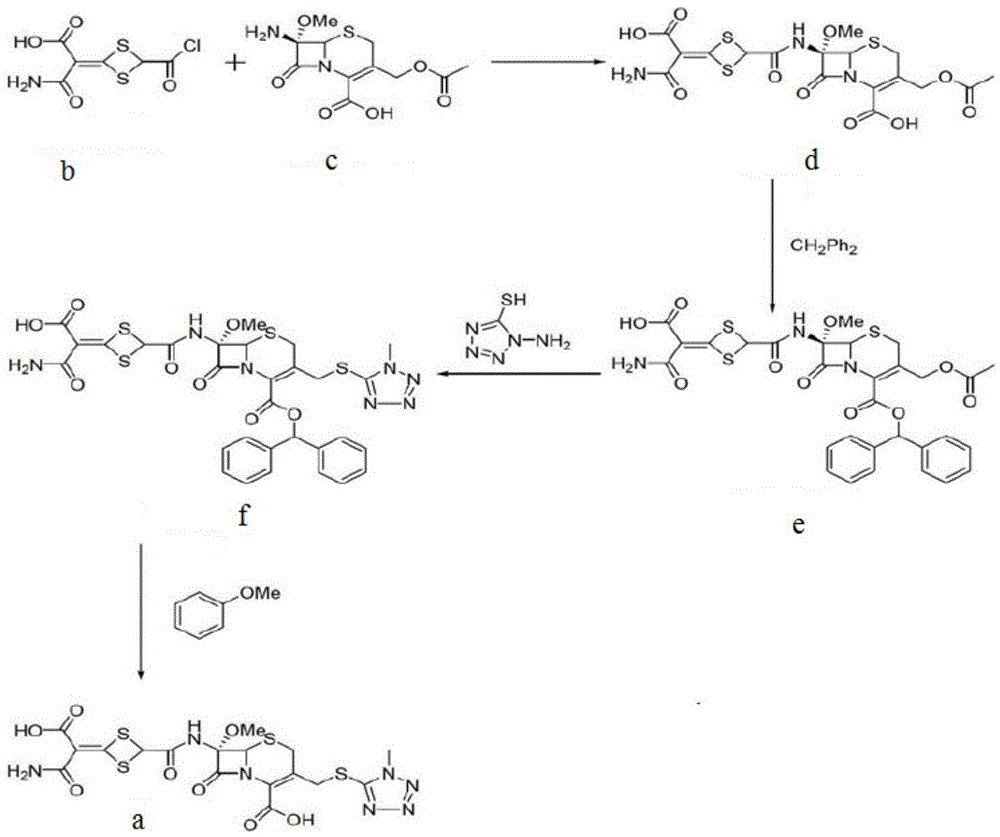
Synthesis method of new cephalosporin side-chain intermediate compound
ActiveCN103232405AIncrease profitImprove conversion rateOrganic compound preparationCarboxylic acid esters preparationSide chainDistillation
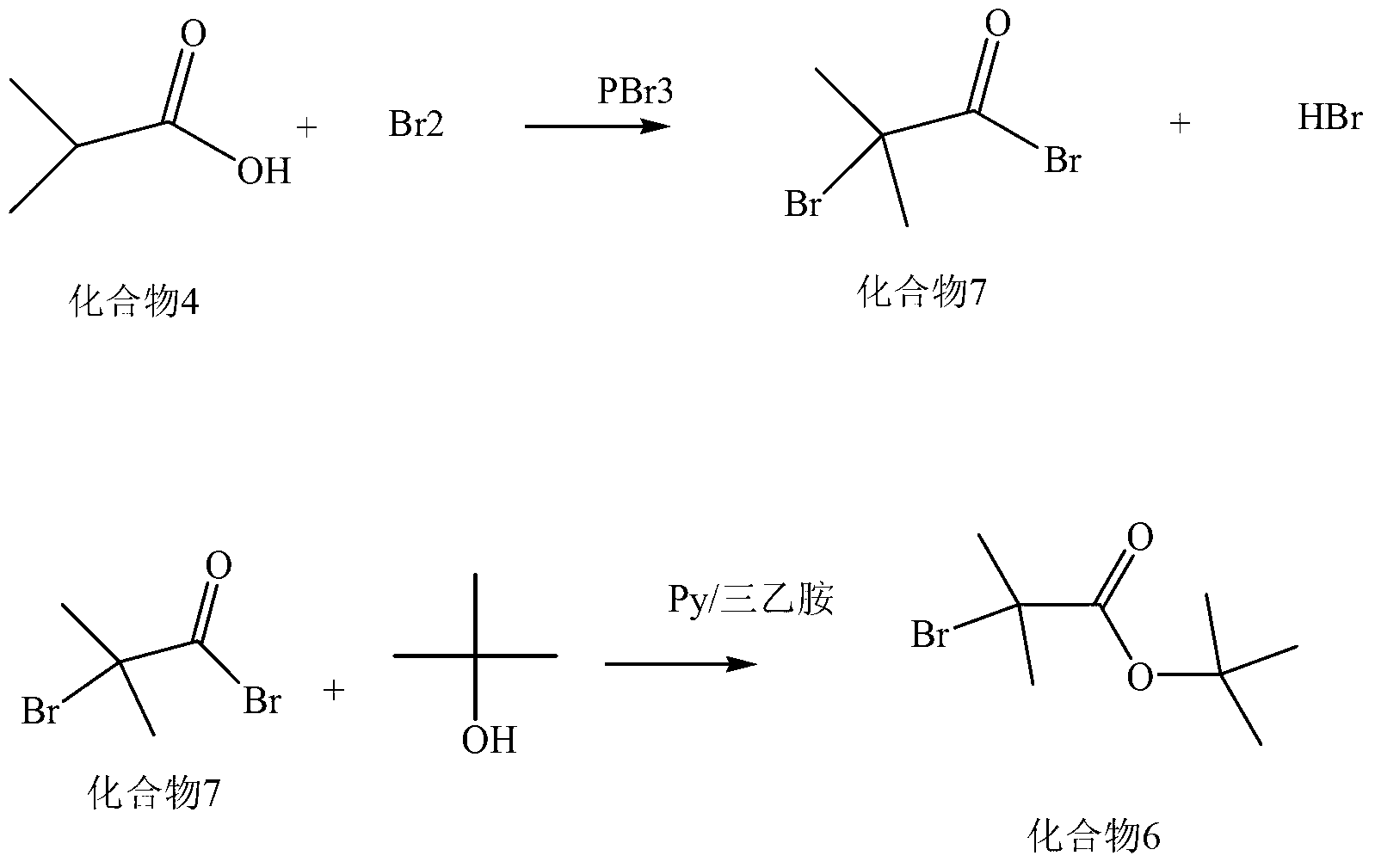
The invention discloses a synthesis method of a new cephalosporin side-chain intermediate compound, which comprises the following steps: by taking isobutyric acid and element bromine as raw materials, dropwisely adding the element bromine more than once, reacting to obtain alpha-bromoisobutyric acid, wherein after the element bromine is dropwisely added each time, chlorine gas is introduced to further react, so that the HBr byproduct is converted into element bromine which can be used for reaction with the isobutyric acid; reacting the obtained alpha-bromoisobutyric acid and isobutylene under the action of a protonic acid strong catalyst, and performing direct distillation to obtain tert-butyl alpha-bromoisobutyrate; performing alkylation reaction on the tert-butyl alpha-bromoisobutyrate and ethyl 2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetate under the catalytic action of a phase-transfer catalyst by taking acetone as solvent; and hydrolyzing to obtain (Z)-2-amino-alpha-[[2-(tert-butoxy)-1,1-demethyl-2-oxoethoxy]imino]-4-thiazol acetic acid. The production process disclosed by the invention has the advantages of simple process, high raw material conversion rate and yield, environment friendliness and low cost.
Owner:APELOA PHARM CO LTD +2
Cefotetan disodium and preparation method of intermediate of cefotetan disodium
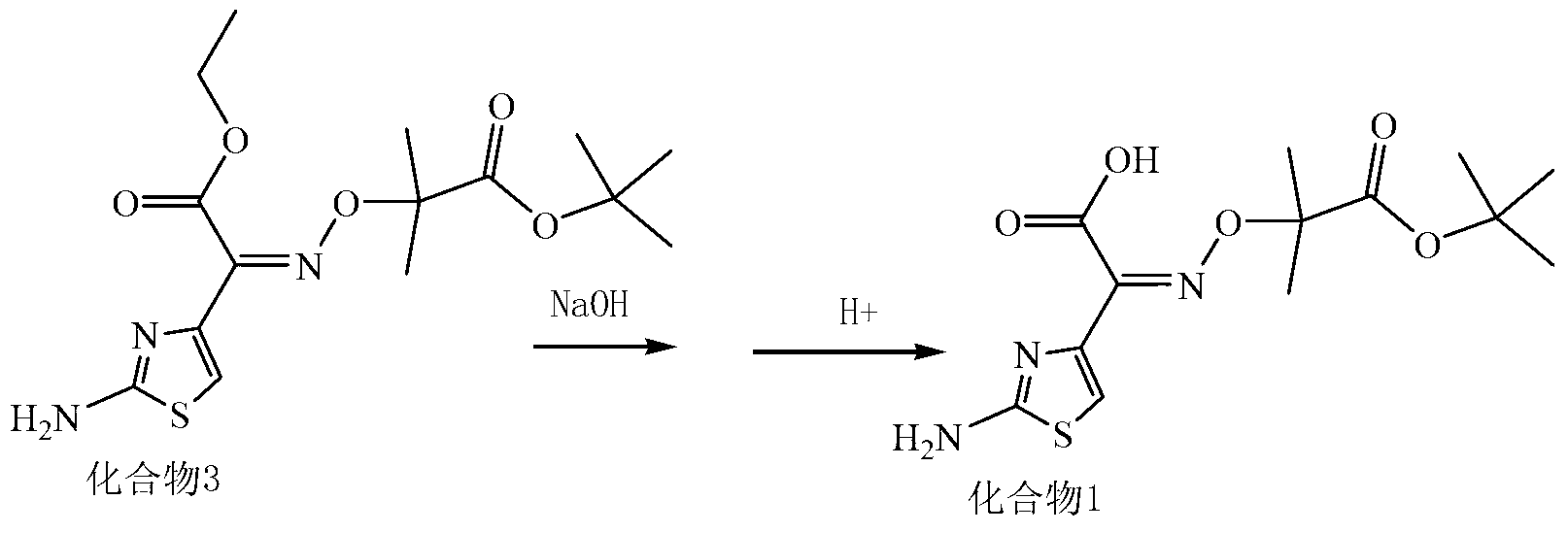
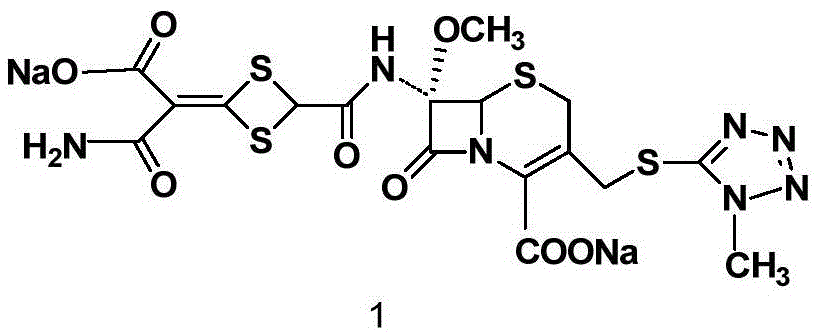
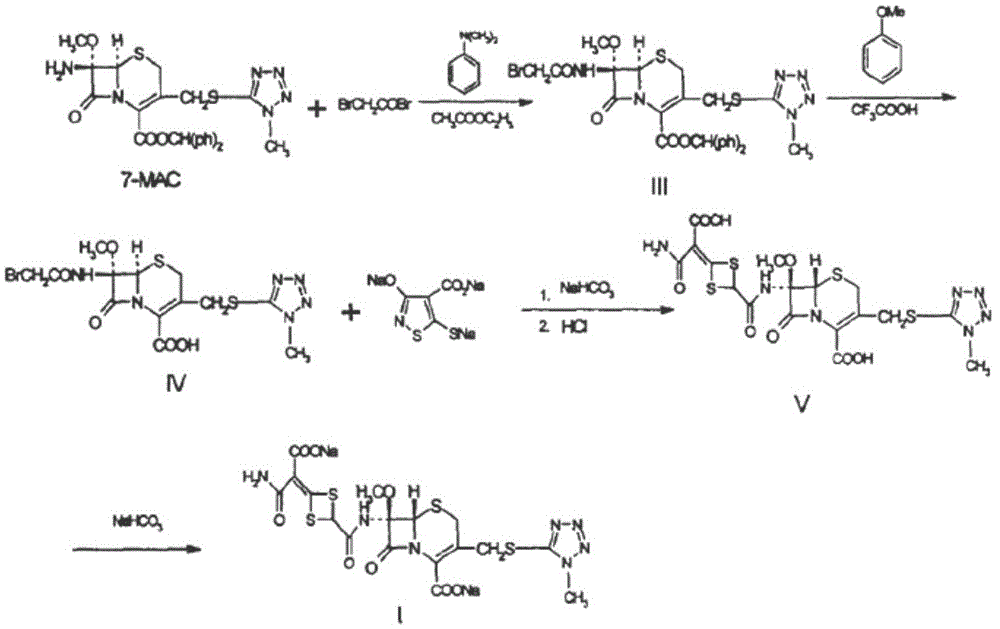
The invention discloses cefotetan disodium and a preparation method of an intermediate of cefotetan disodium. Commercially-available raw materials, namely, 4-(1-amino-3-tert-butoxy-1,3-dioxo-2-alkenyl)-1,3-dithietane-2-carboxylic acid (2) and 7-MAC (3) are subjected to a condensation reaction to obtain a compound (4), and then the compound (4) is subjected to deprotection and salification to obtain cefotetan disodium (1). The invention further provides a preparation method of the compound (2). In the route, no chloride agent contaminating the environment in a conventional route is used, and the preparation method has the advantages of being simple in process route and environmentally friendly, lowering the process cost and increasing the total product yield and is suitable for industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Method of forming a ZrO2 thin film using plasma enhanced atomic layer deposition and method of fabricating a capacitor of a semiconductor memory device having the thin film
InactiveUS20070026688A1Excellent electrical propertiesIncrease deposition rateSolid-state devicesSemiconductor/solid-state device manufacturingChemisorptionOxygen
Example embodiments of the present invention relate to a method of forming a dielectric thin film and a method of fabricating a semiconductor memory device having the same. Other example embodiments of the present invention relate to a method of forming a ZrO2 thin film and a method of fabricating a capacitor of a semiconductor memory device using the ZrO2 thin film as a dielectric layer. A method of forming a ZrO2 thin film may include supplying a zirconium precursor on a substrate maintained at a desired temperature, thereby forming a chemisorption layer of the precursor on the substrate. The zirconium precursor may be a tris(N-ethyl-N-methylamino)(tert-butoxy) zirconium precursor. The substrate having the chemisorption layer of the precursor may be exposed to the plasma atmosphere of oxygen-containing gas for a desired time, thereby forming a Zr oxide layer on the substrate, and a method of fabricating a capacitor of a semiconductor memory device having the ZrO2 thin film.
Owner:SAMSUNG ELECTRONICS CO LTD
M20 methanol gasoline power performance improver
ActiveCN103865595AImprove combustion efficiencyAddress lack of motivationLiquid carbonaceous fuelsChemistryTert-butoxy
The invention discloses an M20 methanol gasoline power performance improver which is prepared by mixing 18.0-31.0 percent of 1,1-dimethyl cyclopentane, 15.0-29.5 percent of 4-trifluorometoxybenzene methanol, 15.0-24.5 percent of bi(diethylamine) phenyl phosphine, 13.0-24.0 percent of 3-amino-N-methyl benzylamine, 10.0-20.5 percent of tert-butoxy bi(dimethylamino) methanol and 9.0-19.5 percent of 2-acetyl benzofuran at normal temperature. Additives adopted by the M20 methanol gasoline power performance improver have a remarkable effect on improvement of the engine power of M20 methanol gasoline, and the common M20 methanol gasoline is added with 0.3-1.0 percent of the additives, and thus the power performance like acceleration is obviously better than that of the same model number of common methanol gasoline.
Owner:甘肃桑田清洁能源开发有限公司
Arbekacin synthesis method
InactiveCN104447908AMild removal conditionsEasy to separate and purifySugar derivativesSugar derivatives preparationSynthesis methodsHydrazine compound
The present invention relates to an arbekacin synthesis method. According to the arbekacin synthesis method, di-tert-butyl dicarbonate is adopted as a protection agent, tert-butyloxycarbonyl protection is performed on three amino groups on the sites C3, C2' and C6' of 3',4'-dideoxy-3',4' didehydro-kanamycin B, difference between the remaining free amino groups on the site 1 and the site 3' is adopted to directly and selectively introduce the side chain on the amino group on the site 1, the amino-protected 1-tert-butoxy amide-3-hydroxybutyric acid is directly adopted as an acylation reagent of the amino group on the site 1, and hydrolysis with an acid is adopted to remove the tert-butyloxycarbonyl protection. According to the present invention, the operations of the method are simple, the reaction condition and the protection group removing condition are mild, the separation purification of the product obtained from the reaction is easy compared with the separation purification of the product obtained by adopting other types of the amino acid protection agents, the one-pot reaction is adopted, the concurrent deprotection is adopted, the product yield is high, the production cost is reduced, the industrial production is easily achieved, and hydrazine hydrate and other hazardous compounds are not used so as to provide the advantages of low environment pollution.
Owner:CHANGZHOU FANGYUAN PHARMA
Precursors for silica or metal silicate films
A composition selected from the group consisting of bis(tert-butoxy)(isopropoxy)silanol, bis(isopropoxy)(tert-butoxy)silanol, bis(tert-pentoxy)(isopropoxy)silanol, bis(isopropoxy)(tert-pentoxy)silanol, bis(tert-pentoxy)(tert-butoxy)silanol, bis(tert-butoxy)(tert-pentoxy)silanol and mixtures thereof; its use to form a metal or metalloid silicate layer on a substrate and the synthesis of the mixed alkoxysilanols.
Owner:VERSUM MATERIALS US LLC
Method for preparing semaglutide side chain by liquid phase method
InactiveCN111269137AEfficient synthesisWide choiceCarbamic acid derivatives preparationOrganic compound preparationSide chainAklanonic acid
The invention discloses a method for preparing a semaglutide side chain. The preparation method comprises the following steps: protecting amino of an initial raw material 2-(2-aminoethoxy) ethanol byusing R1; then carrying out nucleophilic substitution reaction with alpha halogenated ester to prolong a carbon chain; preparing an aliphatic chain with two protected ends by a one-pot method; removing a protecting group at one end of each aliphatic chain and condensing to obtain a compound 7; removing the R1 protecting group to obtain a compound 8, performing condensation reaction on the compound8 and fluorenylmethoxycarbonyl-L-glutamic acid 1-tert-butyl ester to obtain a compound 10, removing the fluorenylmethoxycarbonyl, performing amidation condensation reaction on the compound 10 and 18-(tert-butoxy)-18-oxooctadecanoic acid to obtain a compound 13, and removing the R2 protecting group to obtain a target product chain 1. Compared with solid-phase synthesis, the method disclosed by theinvention is lower in cost and wider in selection of protecting groups, and has industrial production and application prospects.
Owner:ZHEJIANG UNIV OF TECH
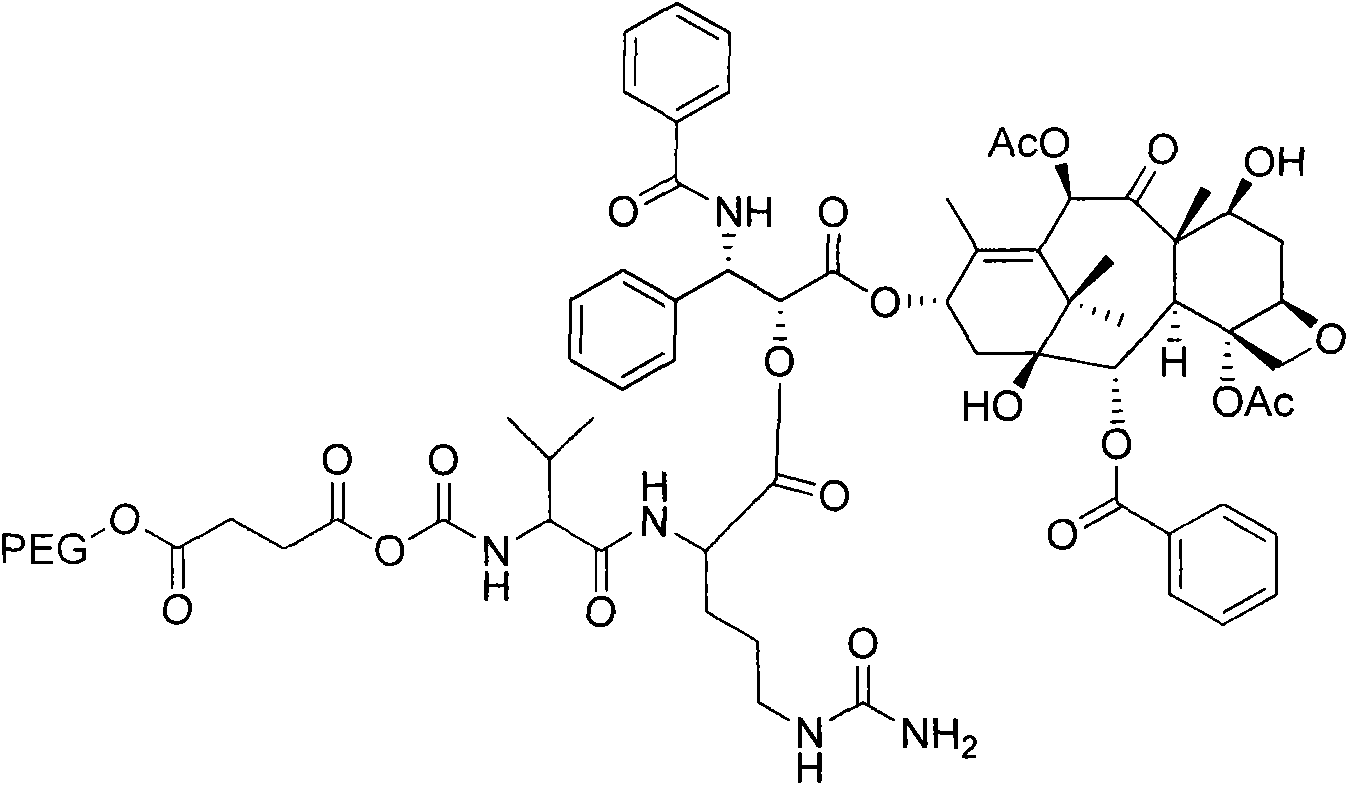
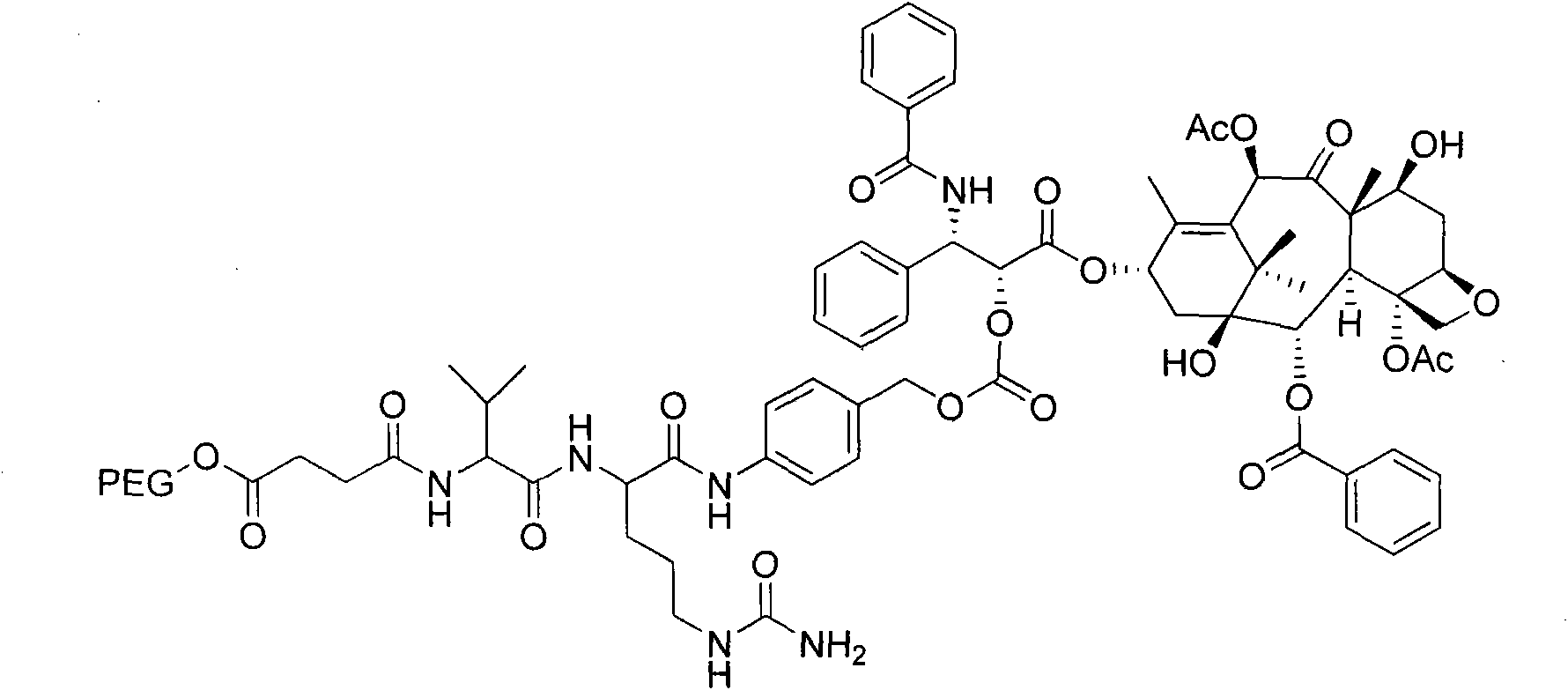
Anti-tumor medicine conjugate, preparation method, preparation and application
InactiveCN106317067AGood anti-tumor effectSimple stepsOrganic active ingredientsOrganic chemistryTert-butoxyIn vitro test
The invention discloses an anti-tumor medicine conjugate, a preparation method, nano-micelle preparation thereof and application. The structural formula of the conjugate provided by the invention is represented by the formula (I), and the conjugate is formed by connecting 7-ethyl-10-hydroxy camptothecin with a paclitaxel anti-tumor medicine through a connection bond, wherein L is the connection bond, R1 is phenyl or tert-butoxy, R2 is acetyl, H or methyl, and R3 is H or methyl. The steps are simple, the preparation cost is low, the stability is high, and the safety is high; the requirement on clinical medication is met; the anti-tumor medicine conjugate is suitable for large-scale industrial production. The invention further discloses nano-micelle consisting of the anti-tumor medicine conjugate represented by the formula (I) and an amphiphilic polymer and application of the nano-micelle in tumor resistance; an in-vitro test shows that the nano-micelle is good in tumor resisting effect and has relatively high market prospect and value.
Owner:ZHEJIANG UNIV
Entecavir derivative and preparation method thereof
The invention discloses an entecavir derivative and a preparation method thereof. The chemical name of the entecavir derivative is 2-amino-9-[(1S, 3R, 4S)-4-hydroxy-2-methylene cyclopentyl]-1,9-dihydro-6H-purine-6-one-3-methyl hydroxyl-L-valine ester, namely valerian entecavir for short. The preparation method comprises the following steps: reacting 0.5-5 equivalent of (S)-2-(tert-butoxy carbonyl-amino)-3-methylbutyric acid, 0.5-5 equivalent of dicyclohexyl carbodiimide, and 0.01-0.5 equivalent of 4-dimethylamino-pyridine with 0.5-5 equivalent of entecavir in dimethylformamide for 0.5-240 hours; separating and purifying by silica gel column chromatography after reaction, and then deprotecting to obtain a target product. The valerian entecavir prepared by the method has the advantages of being good in water solubility and targeting property, and low in toxicity on other organs, can achieve high liver distribution, and is a stable prodrug of the entecavir.
Owner:FUJIAN TIANQUAN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com