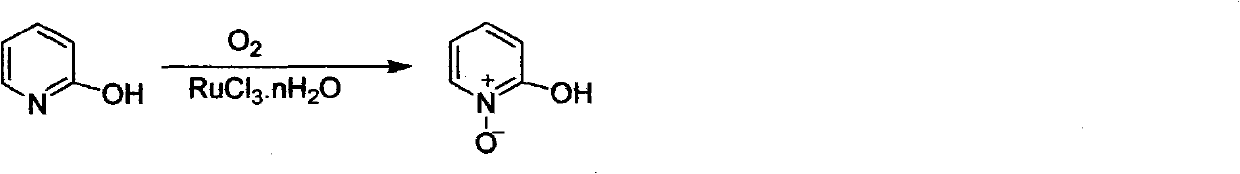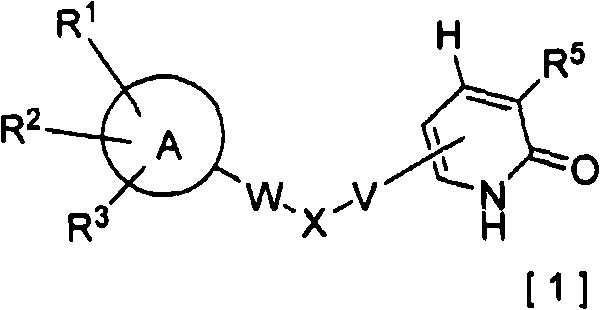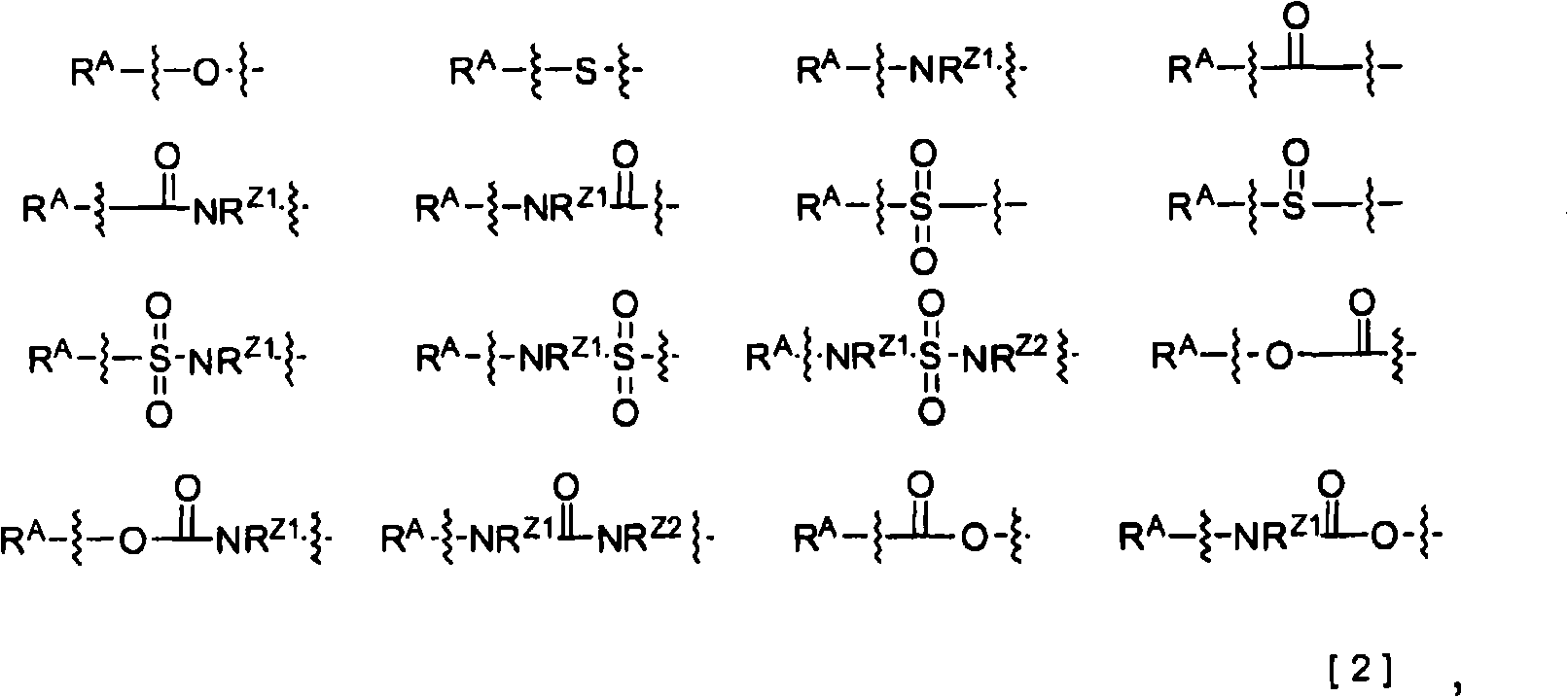Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
98 results about "2-Pyridone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
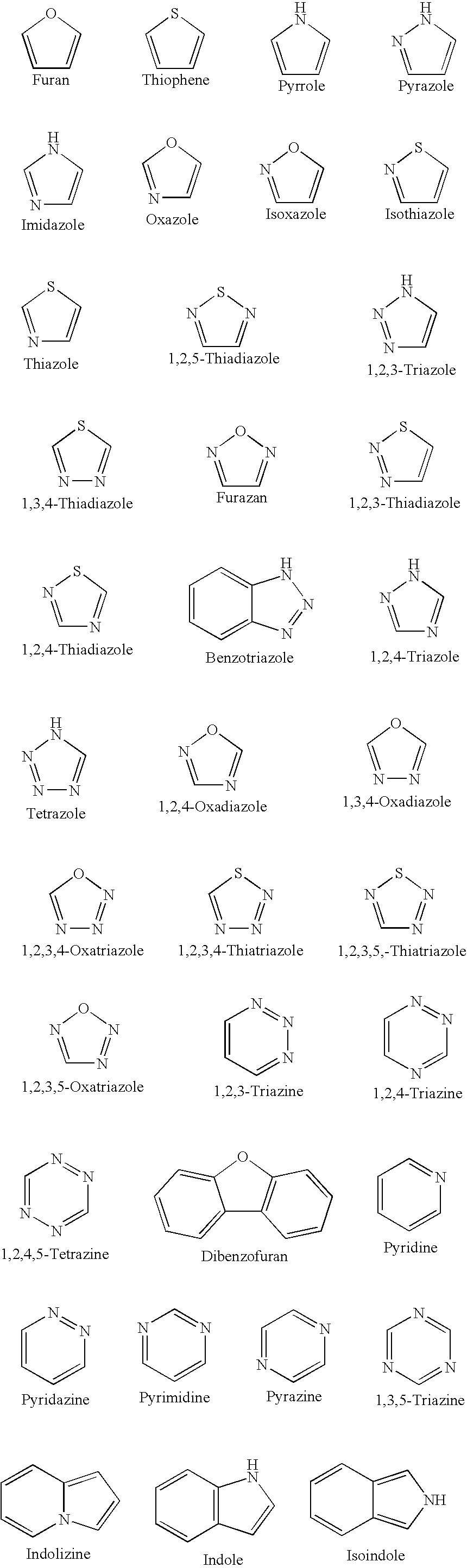
2-Pyridone is an organic compound with the formula C₅H₄NH(O). It is a colourless solid. It is well known to form hydrogen bonded dimers and it is also a classic case of a compound that exists as tautomers.
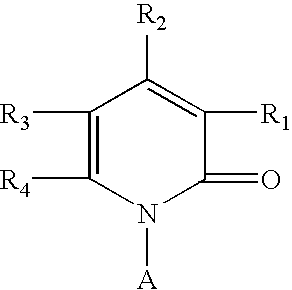
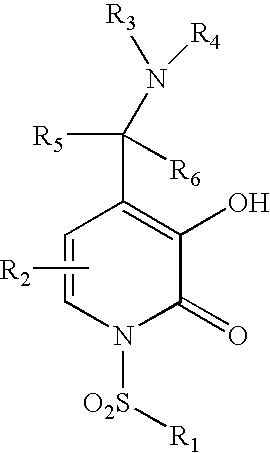
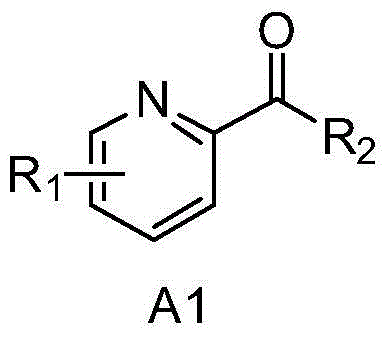
3-Carbamoyl-2-Pyridone Derivative
ActiveUS20080103139A1Excellent in transdermal absorbency absorbencyExcellent in absorbency oral absorbencyBiocideSenses disorderHydrogenExternal application
The present invention provides compounds having an agonistic activity to the cannabinoid receptor, which is represented by the formula (I): wherein R1 is optionally substituted C1-C8 alkyl and the like; R2 is C1-C6 alkyl; R3 is C1-C6 alkyl and the like; or R2 and R3 taken together with the adjacent carbon atoms may form an optionally substituted 5 to 10 membered non-aromatic carbon ring; R4 is hydrogen and the like; G is a group selected from the groups shown by the formula and the like: wherein R5 is hydrogen and the like; X1 is a single bond and the like; X2 is optionally substituted C1-C8 alkylene that may be replaced by one or two groups of -O-, or -N(R6)-, wherein R6 is hydrogen, and the like; X3 is a single bond and the like; a pharmaceutically acceptable salt or a solvate thereof, and pharmaceutical compositions, atopic dermatitis treating agents, and anti-pruritus agents, especially anti-pruritus agents for oral use and for external application, which each contains the said compound as an active ingredient.
Owner:SHIONOGI & CO LTD
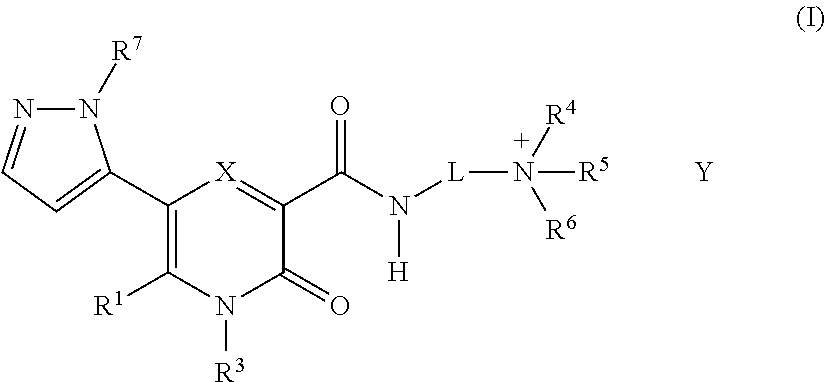
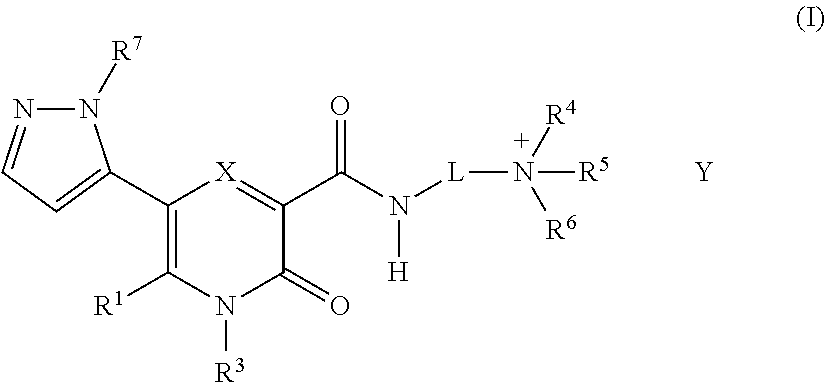
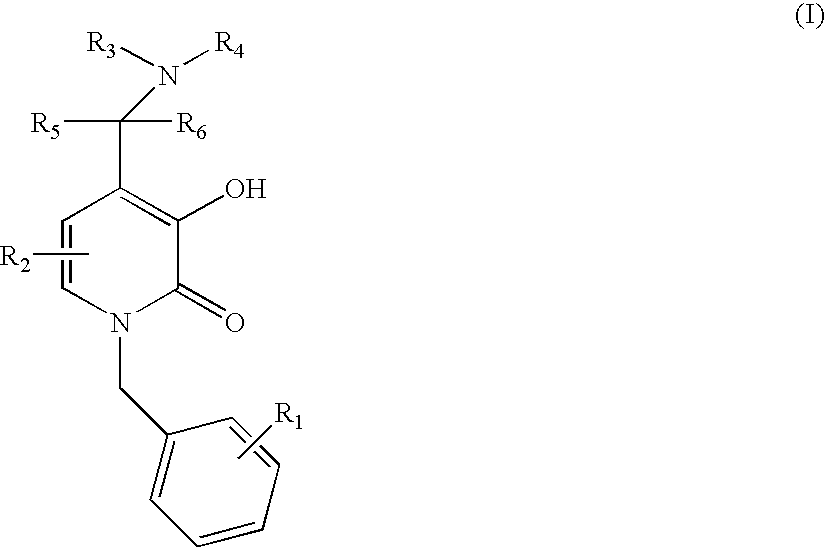
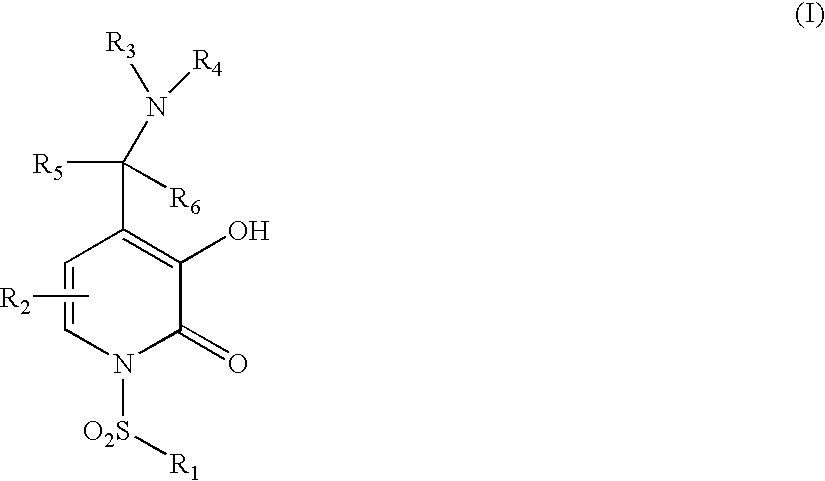
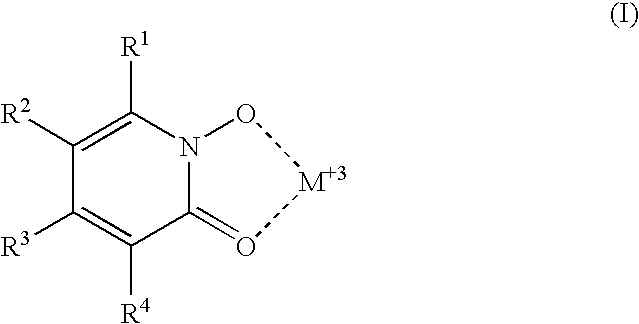
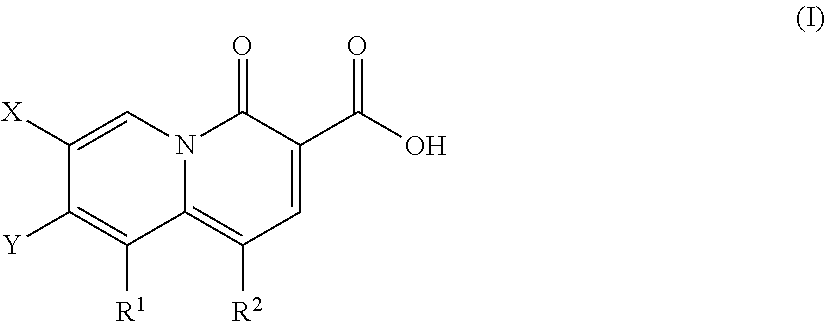
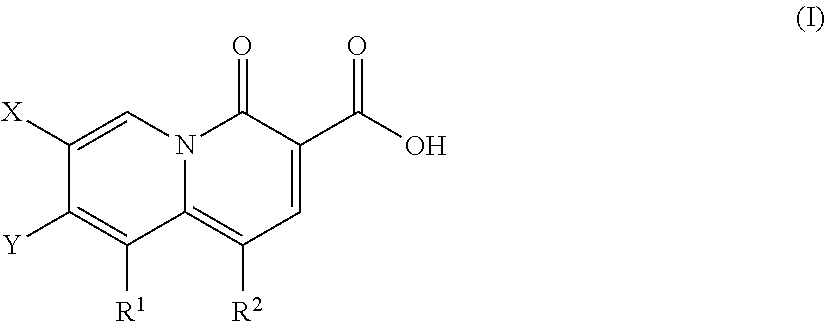
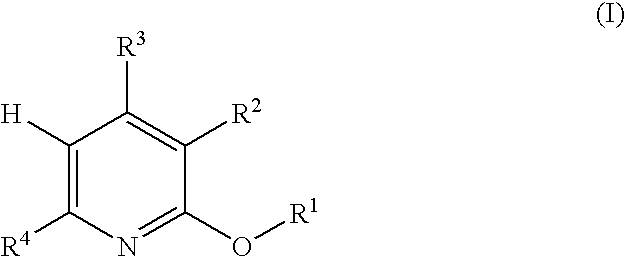
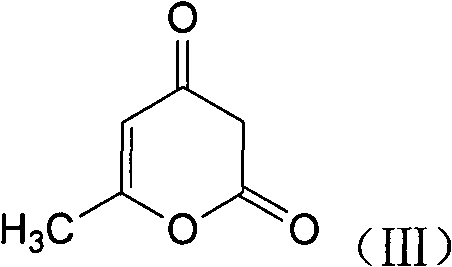
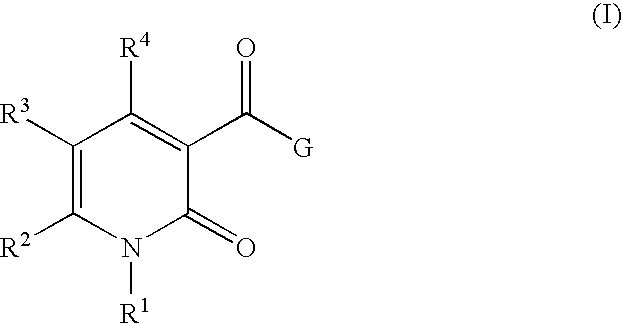
5-Substituted-4-[(substituted phenyl) amino]-2-pyridone derivatives
Owner:WARNER-LAMBERT CO +1
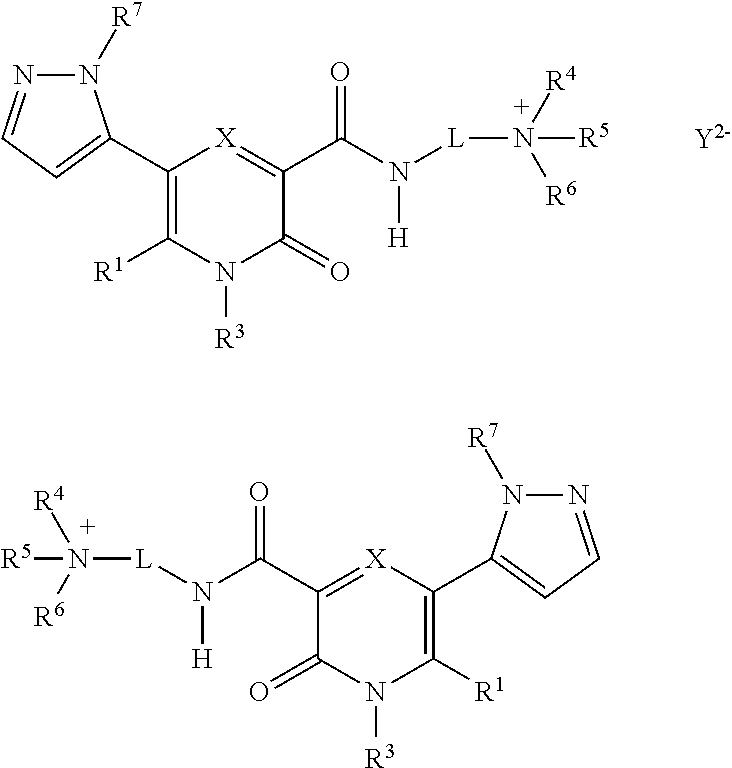
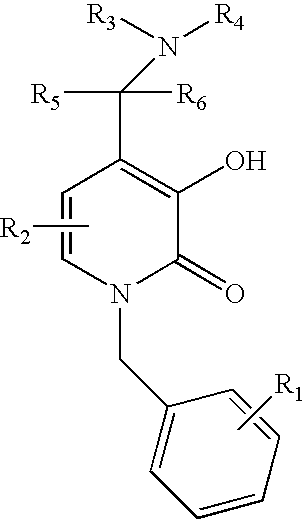
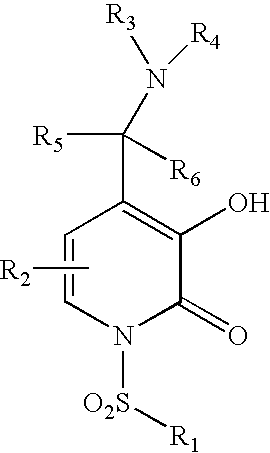
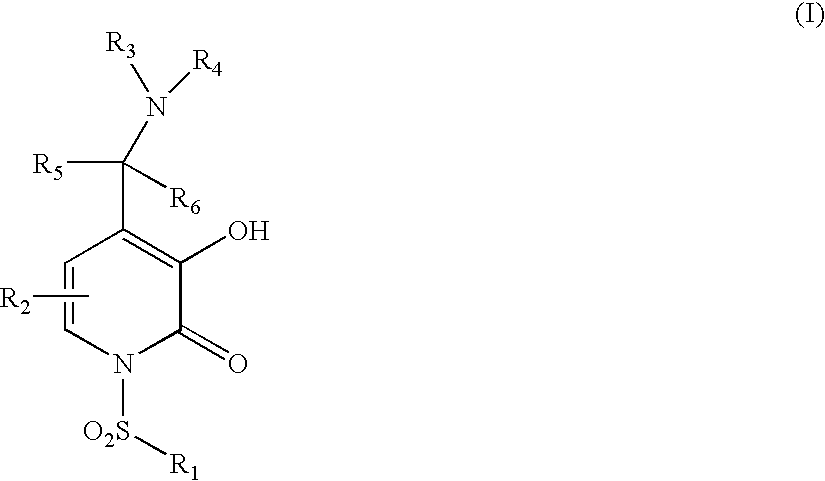
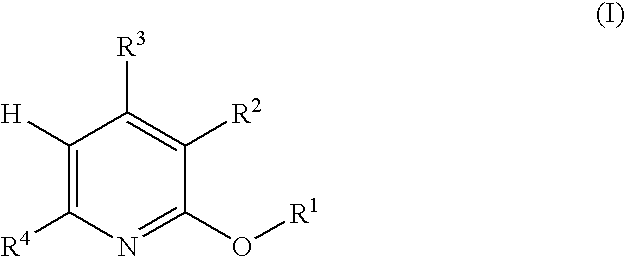
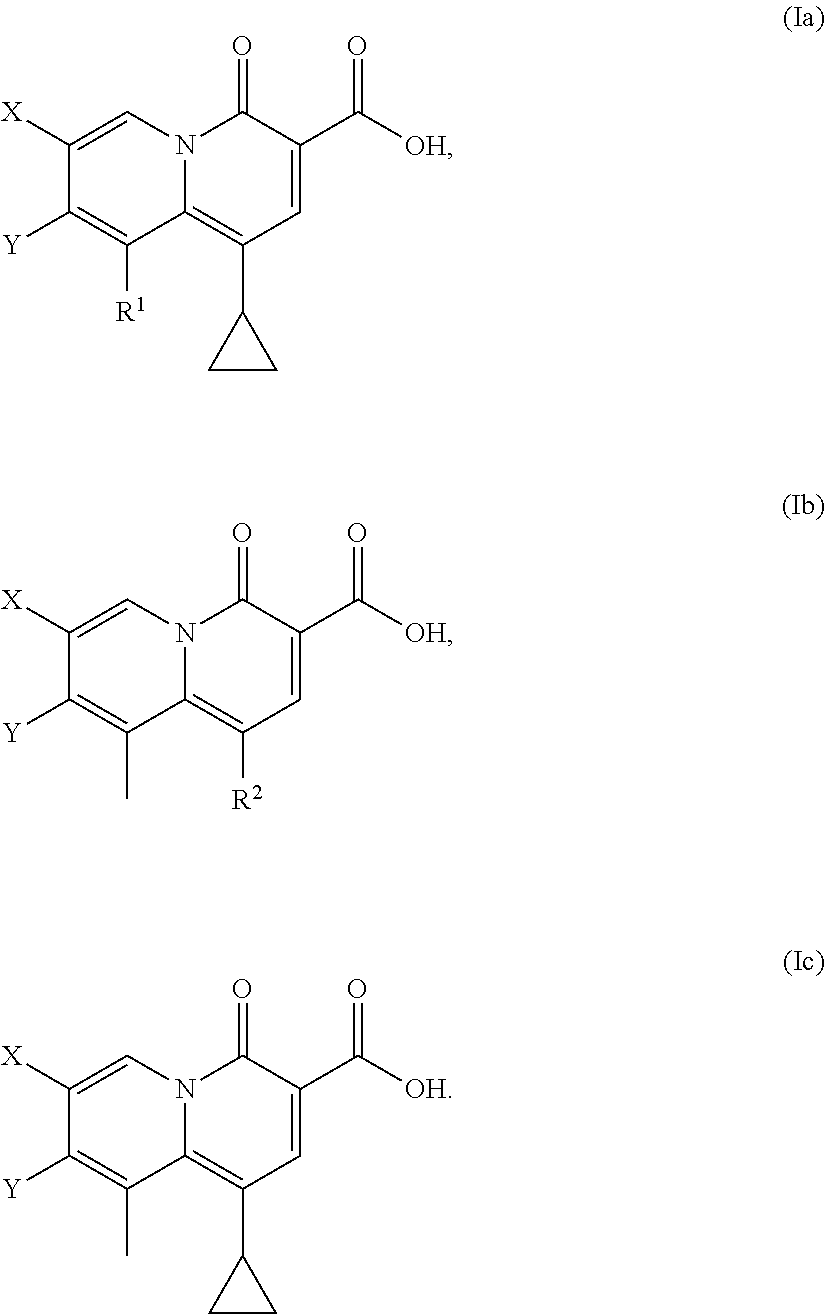
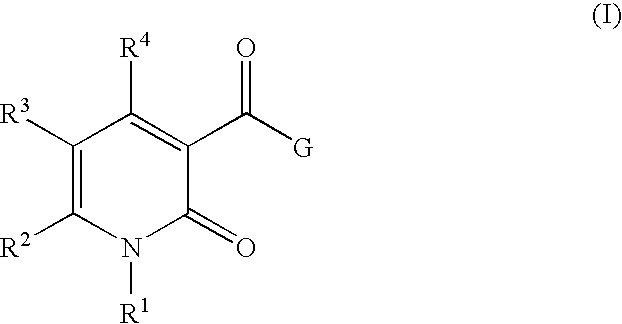
5-substituted-4-[(substituted phenyl) amino]-2-pyridone derivatives
Owner:WARNER LAMBERT CO LLC +1
Novel 2-Pyridone Compounds
InactiveUS20110082155A1Reduce riskGood physical propertiesBiocideOrganic chemistry2-PyridoneCombinatorial chemistry
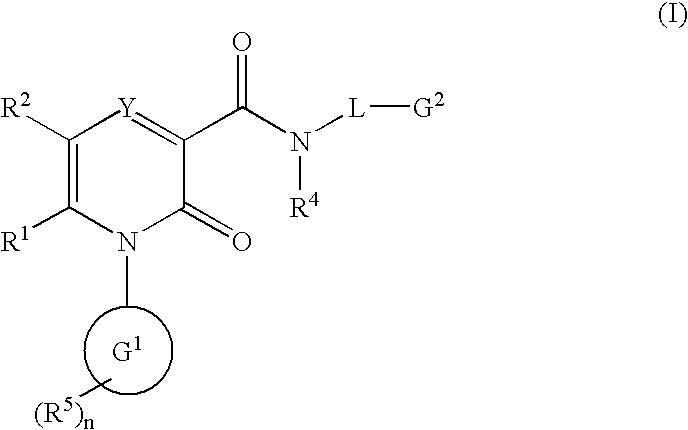
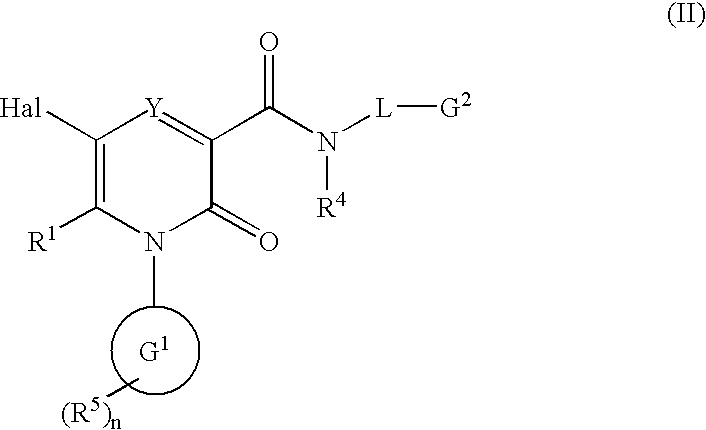
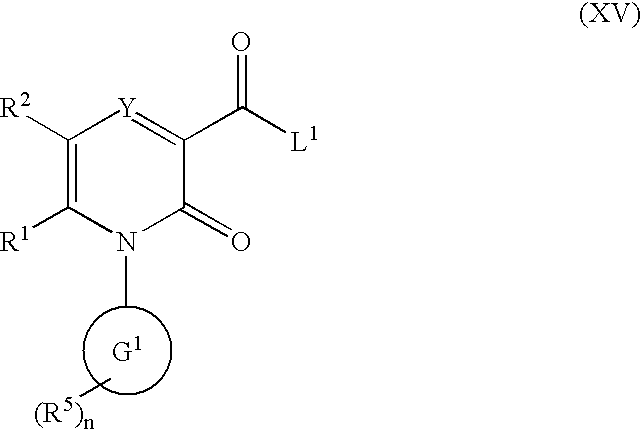
The invention provides compounds of formulawherein R1, R3, R4, R5, R6, R7, L, X and Y are as defined in the specification; together with processes and intermediates for their preparation, pharmaceutical compositions containing them and their use in therapy. The compounds are inhibitors of human neutrophil elastase.
Owner:ASTRAZENECA AB
N-alkyl-4-methyleneamino-3-hydroxy-2-pyridones
Owner:AERPIO TERAPYUTIKS INK
N-sulfonyl-4-methyleneamino-3-hydroxy-2-pyridones
Owner:AERPIO TERAPYUTIKS INK
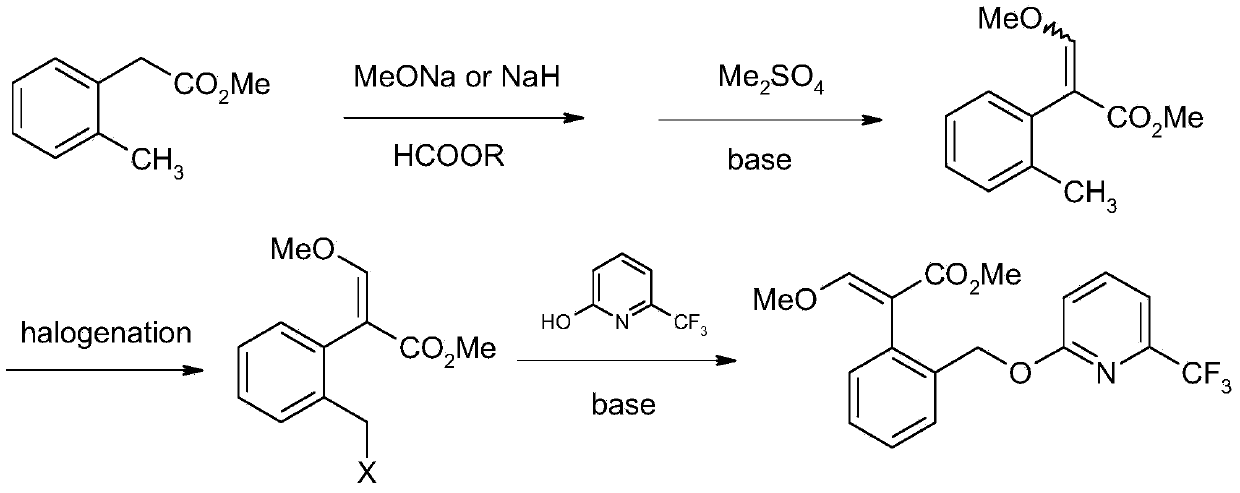
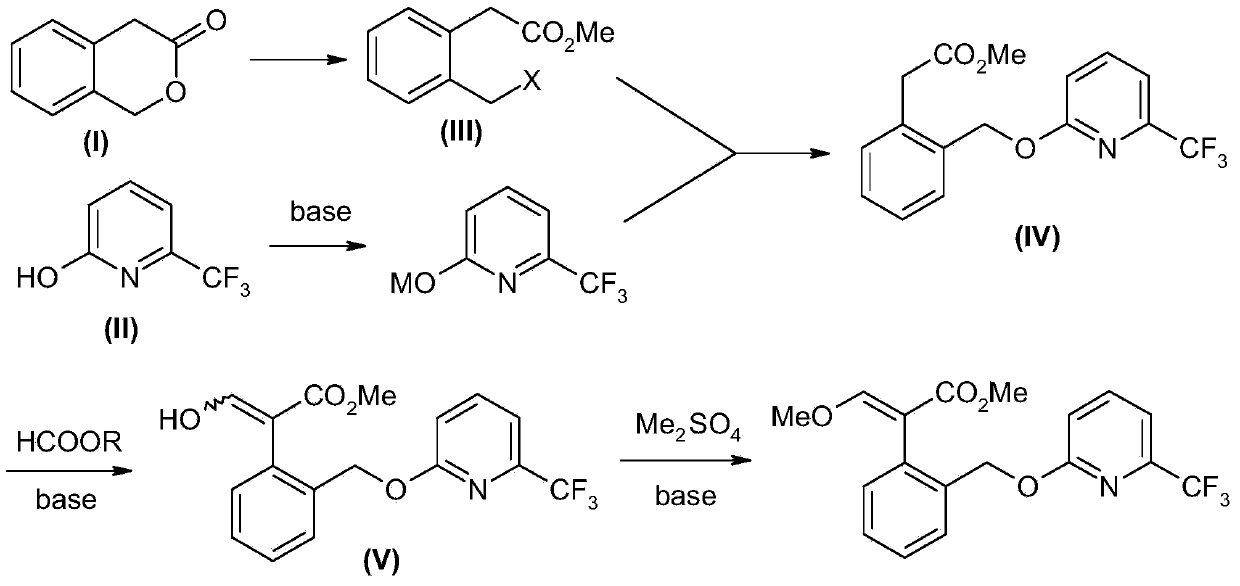
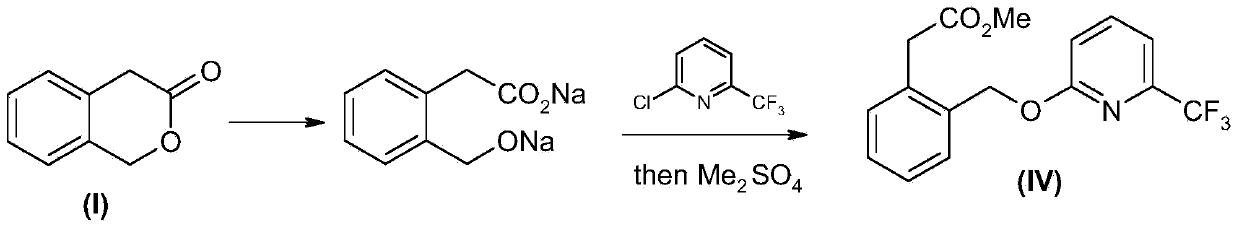
Picoxystrobin preparation method
The invention discloses a picoxystrobin preparation method. The picoxystrobin preparation method comprises the following steps: (1) dissolving a compound (I)3-isochromanone in methyl alcohol and first inert solvent, and reacting so as to obtain (III) (2-halogenated methyl phenyl) methyl acetate; (2) converting (II) 6-trifluoromethyl-2-pyridone into sodium salt, reacting with quaternary ammonium salt in a polar solvent, and then adding the (III) (2-halogenated methyl phenyl) methyl acetate, thus obtaining (IV) 2-[2'-(6'-tirfluoromethylpyridine-2-oxyl) methyl] phenyl methyl acetate; (3) carrying out aldolization on the (IV) 2-[2'-(6'-tirfluoromethylpyridine-2-oxyl) methyl] phenyl methyl acetate and a formamide compound, and hydrolyzing so as to obtain (V) 2-[2'-(6'-tirfluoromethylpyridine-2-oxyl) methyl] phenyl-3-hydroxyl methyl acetate; and (4) treating the (V) 2-[2'-(6'-tirfluoromethylpyridine-2-oxyl) methyl] phenyl-3-hydroxyl methyl acetate by using alkali and a methylation reagent so as to obtain picoxystrobin.
Owner:上海禾本药业股份有限公司
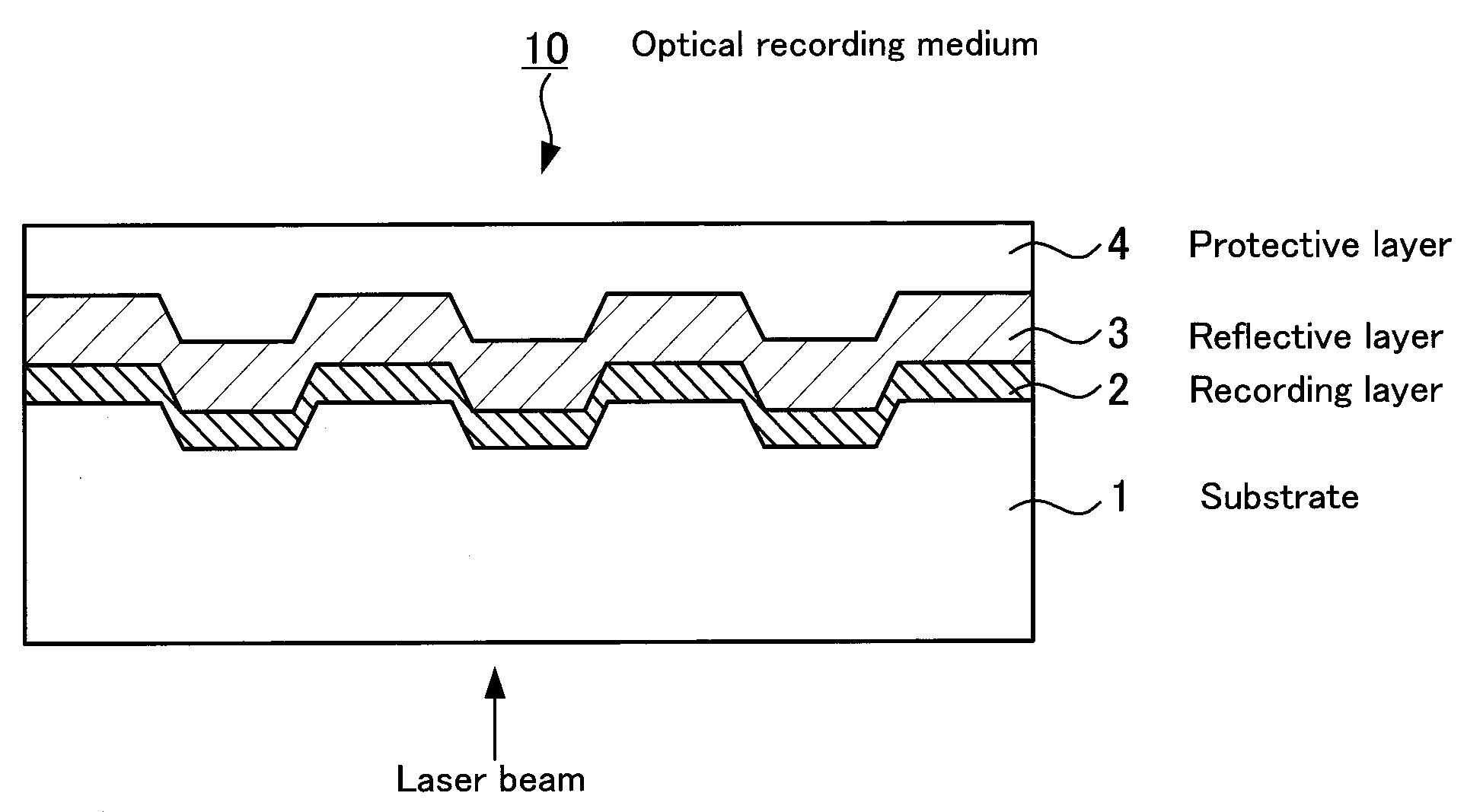
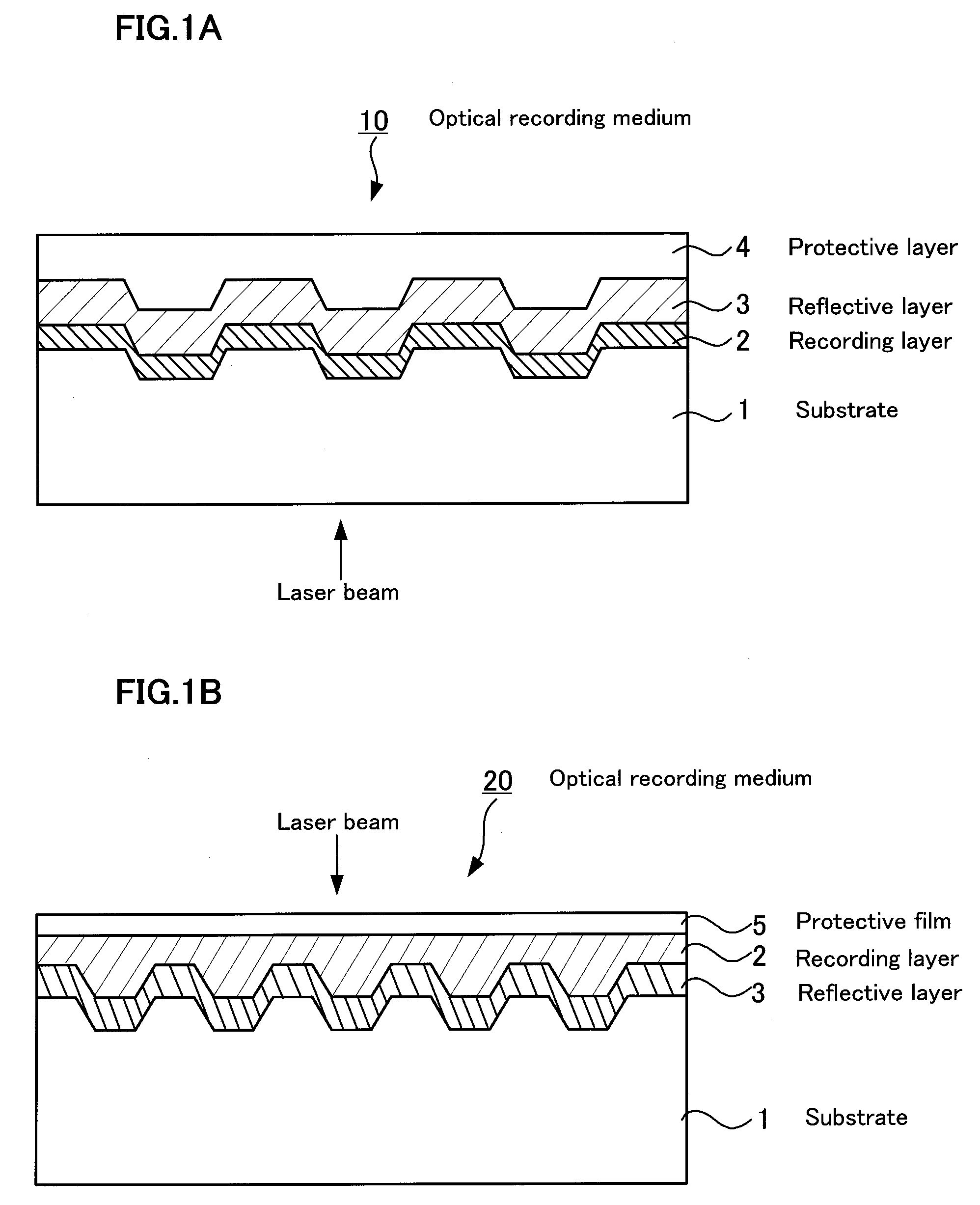
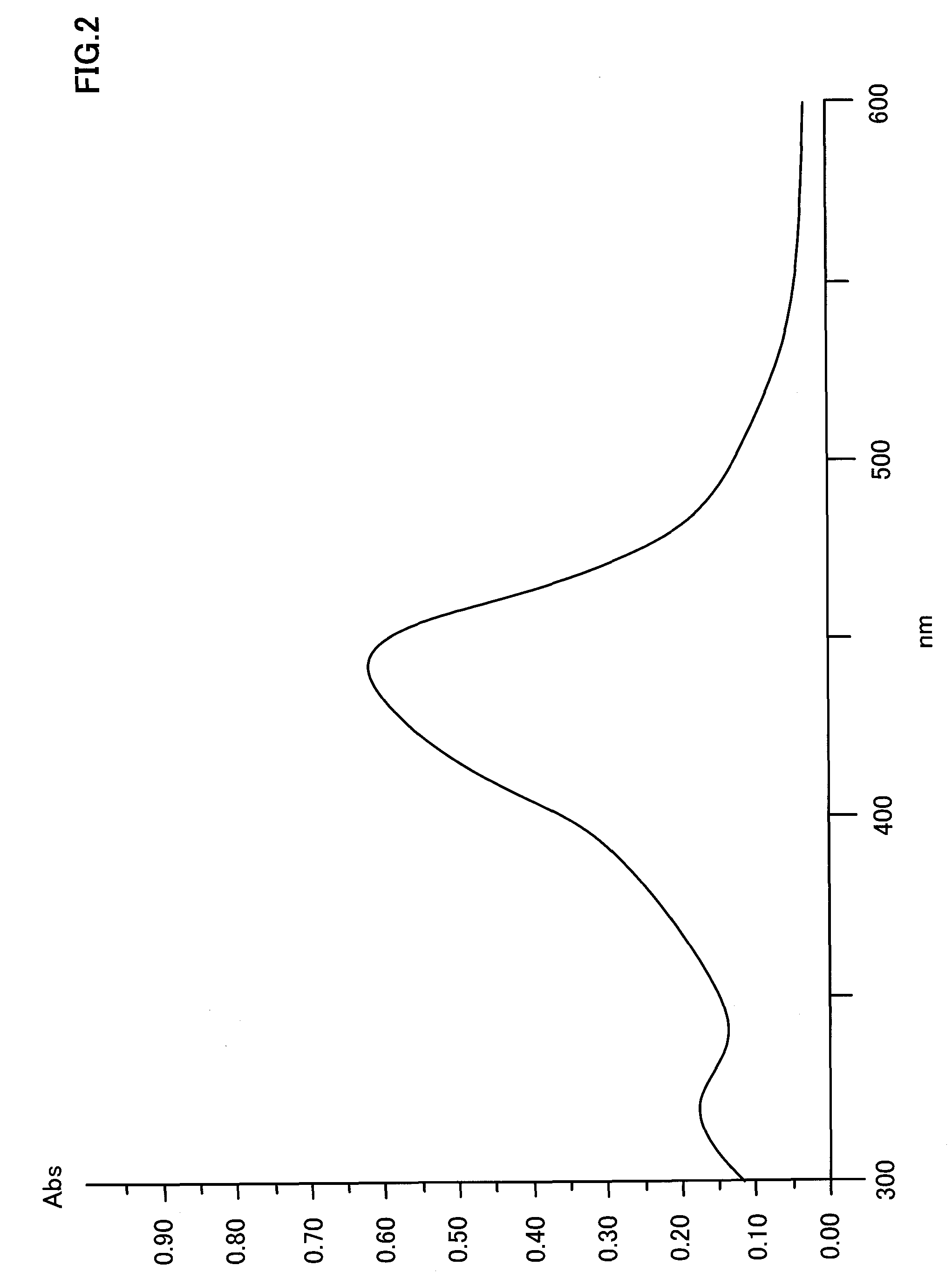
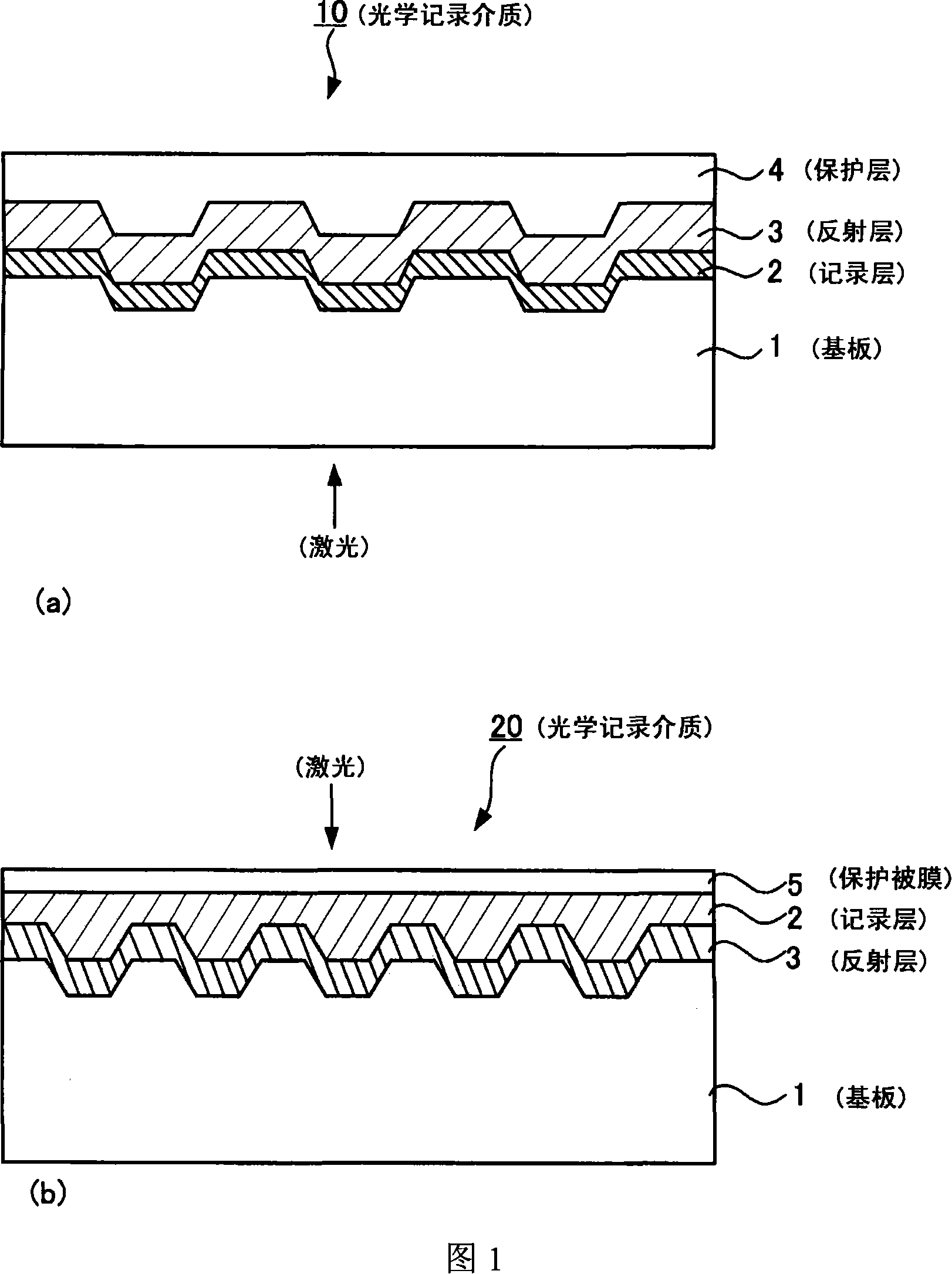
Optical recording medium, metal complex compound and organic dye compound
InactiveUS20090053455A1Optical informationHigh sensitivityMonoazo dyesLayered productsOrganic dye2-Pyridone
Problem: To provide an optical recording medium having a recording layer improved in light stability that is capable of writing and reading of high-density optical information by a short-wavelength laser beam.Means for Solving the Problems: There is provided an optical recording medium with a recoding layer comprising an organic dye compound, wherein the recording layer contains a metal complex compound composed of a pyridone azo compound represented by the following the general formulas [I] to [III] and a divalent ion of metal coordinated to it such as nickel, cobalt, iron, zinc, copper, manganese and the like, wherein the pyridone azo compound contains a 6-hydroxy-2-pyridone structure as a coupling component and isoxazole, 1,2,4-triazole or pyrazole structure as a diazo component.(In the general formulas [I] to [III], R1 to R10 each is independently a hydrogen atom or monovalent functional group.)
Owner:VERBATIM CORPORATION
4-oxoquinolizine antibacterial agent having 2-pyridone skeleton as partial structure
InactiveUS7223773B2Wide antibacterial spectrumHigh antibacterial activityAntibacterial agentsBiocideAcyl group2-Pyridone
The present invention provides a 4-oxoquinolizine antibacterial agent having a 2-pyridone skeleton as a partial structure and also having a strong antibacterial effect on gram-positive bacteria, gram-negative bacteria or anaerobic bacteria. The compound having the following formula (I) or a pharmaceutically acceptable salt thereof:wherein:R1 represents hydrogen atom or a carboxyl-protecting group,R2 represents hydrogen atom, a halogen atom, a lower alkyl group, a lower alkoxyl group or hydroxyl group,R3 represents phenyl group or an aromatic substituent selected from the group consisting of 5-membered and 6-membered heterocyclic groups and R3 has a substituent selected from the group consisting of a hydrogen atom, a lower alkyl group, a lower alkoxyl group, a nitro group, a cyano group, an amino group, an acyl group, a carbamoyl group and a ureido group, andR4 represents a hydrogen atom or a halogen atom.
Owner:SATO PHARMA
Cyanide-free silver plating solution additive
The invention relates to a cyanide-free silver plating solution additive which comprises the following components by ratio: 0.1-10g / l of brightener, 5-10g / l of leveling agent, 100-600g / l of complexing agent and the balance of plasma water, wherein the brightener is one or mixture of more in nitrogen-containing compound, triazole, benzotriazole, 2-hydroxypyridine, pyridine, 22 dipyridyl, 1, 10-phenanthroline, triethylene tetramine and diethylene triamine according to any ratio; the leveling agent is one or mixture of more in aromatic hydrocarbon compounds, naphthalene, 1-methylnaphthalene, 1, 4-naphthoquinone and 1-naphthol according to any ratio; the complexing agent is one or mixture of more in disodium ethylenediamine tetraacetate, niacin, aminosulfonic acid and potassium pyrophosphate according to any ratio. The cyanide-free silver plating solution additive has the beneficial effects that the plating solution is stable, low in toxicity and good in dispersing ability; the obtained plating layer is bright and fine as well as good in binding force; the technology adopts the environment-friendly organic additive which does not contain heavy metal and sulfide; the plating layer is good in corrosion resistance. Furthermore, the cyanide-free silver plating solution additive can be directly used for parts such as brass, copper, chemical nickel and the like, preplating is not needed, and the binding force is also guaranteed.
Owner:HANGZHOU WIN WIN TECH CO LTD
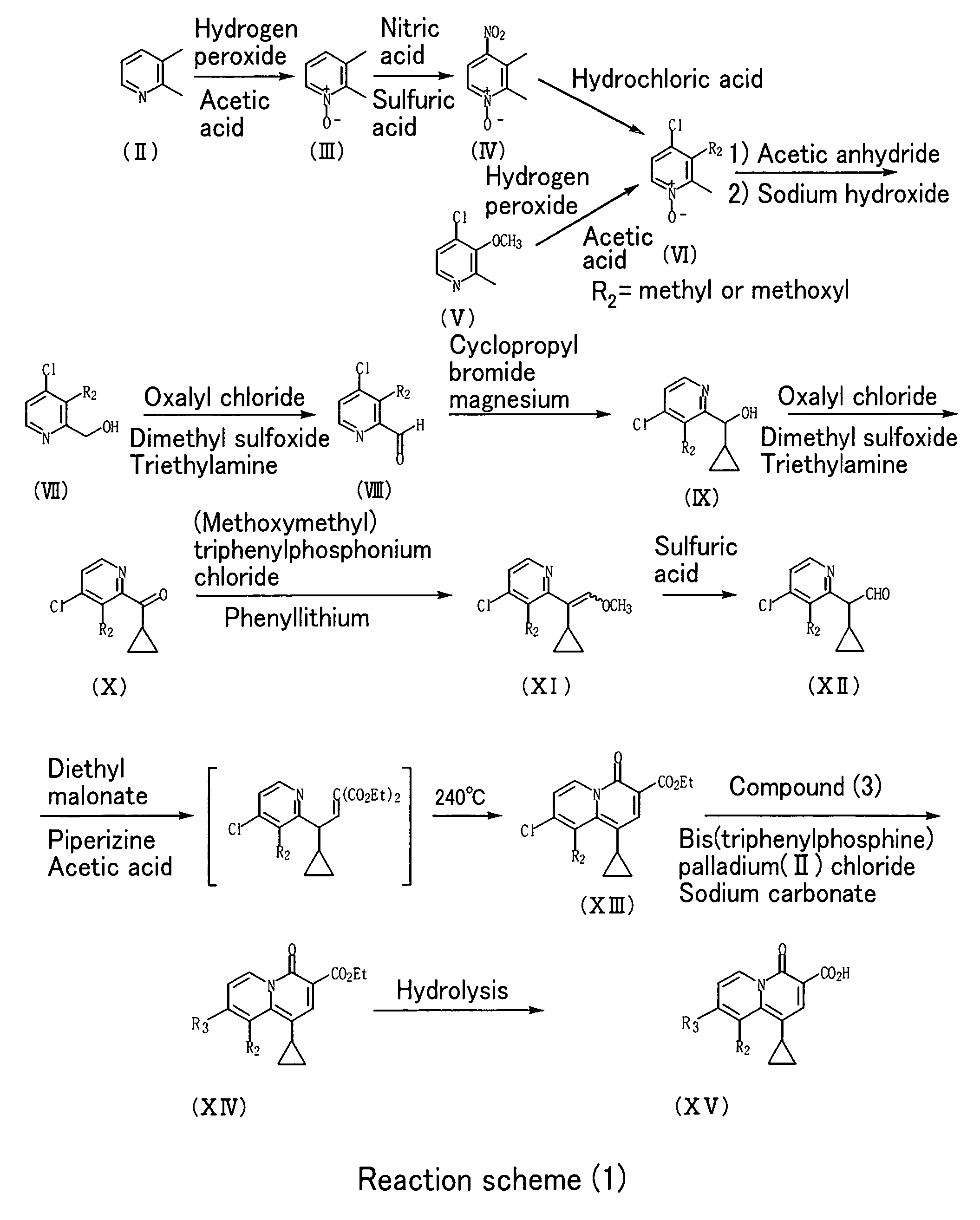
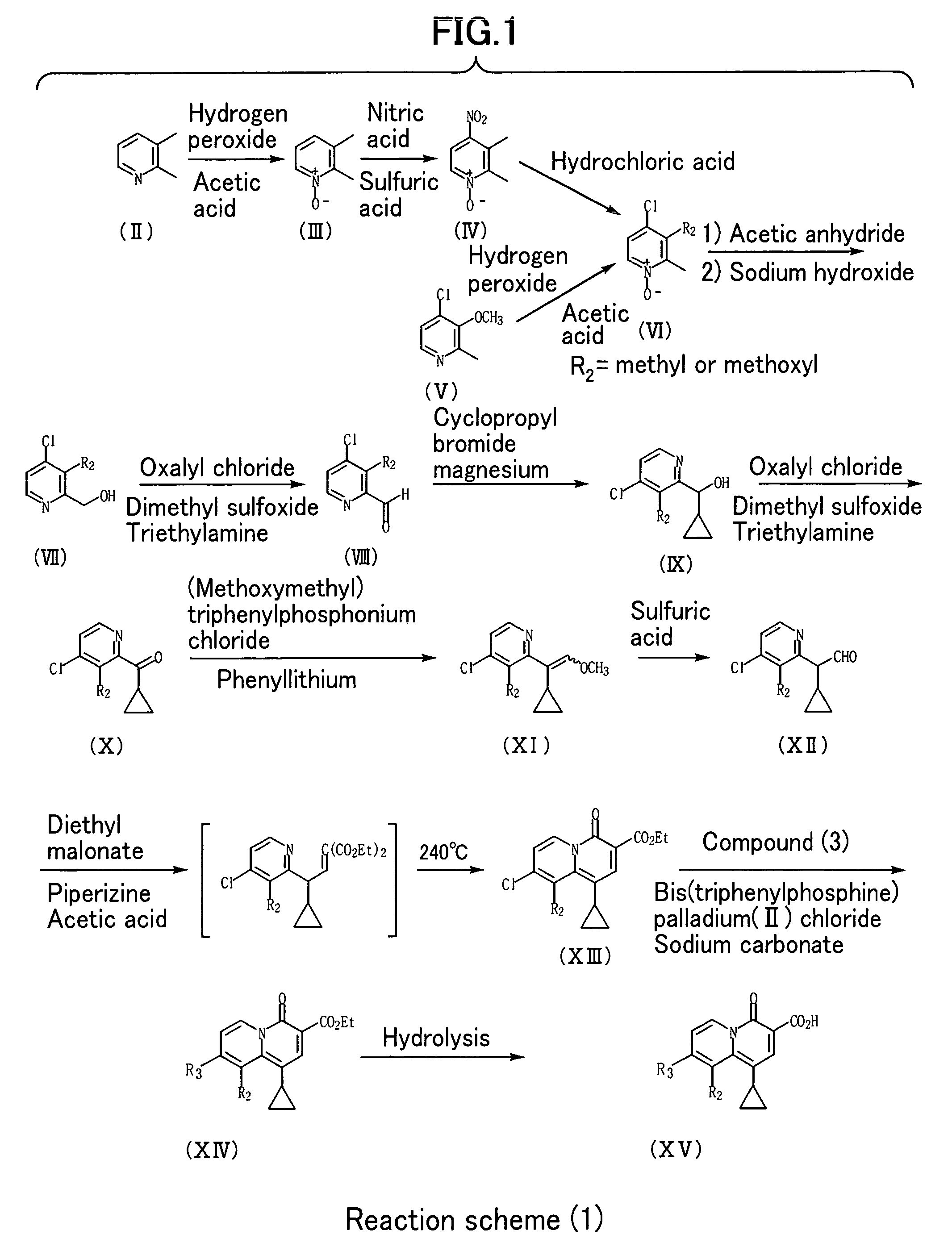
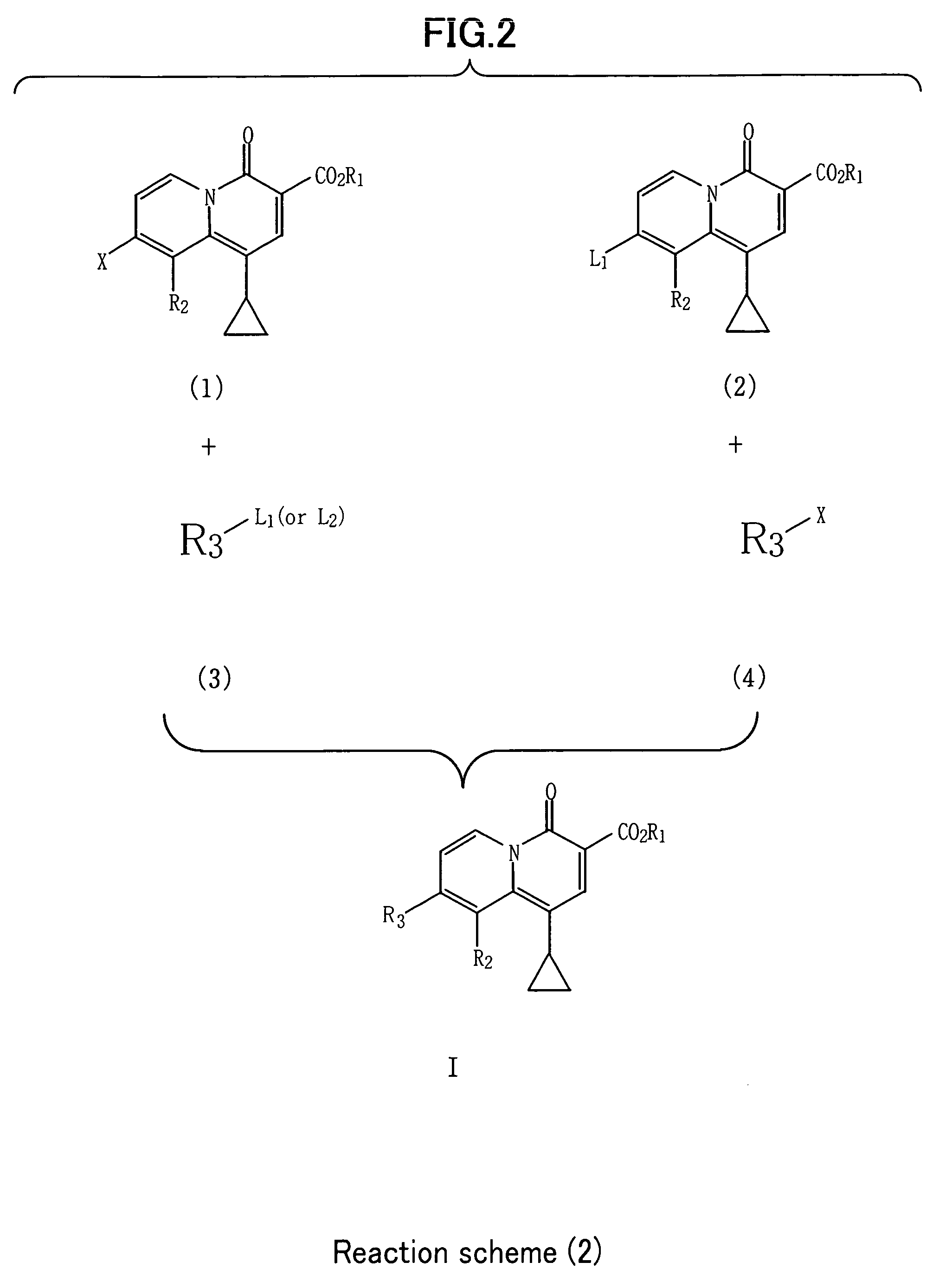
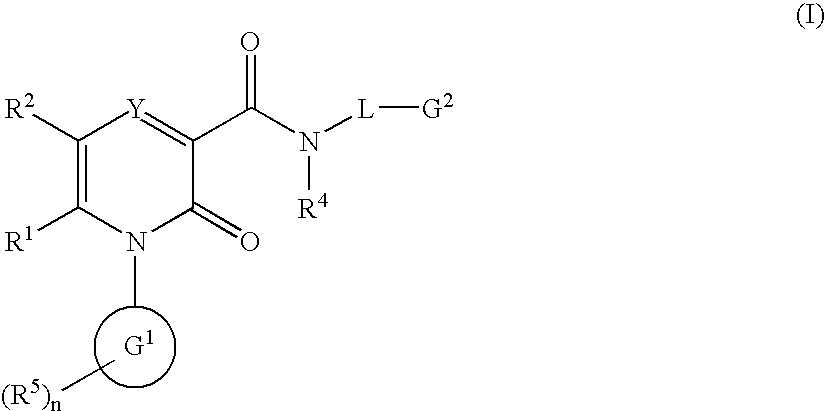
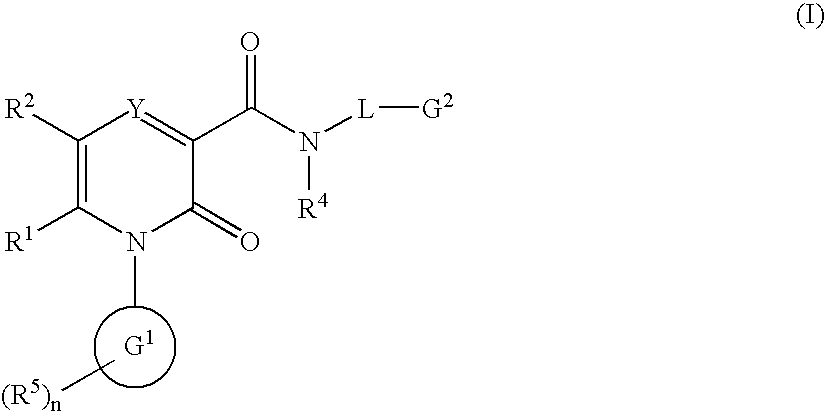
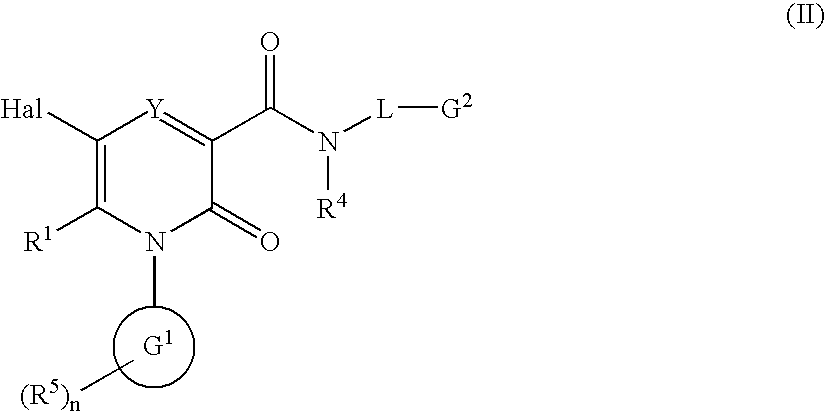
2-Pyridone Derivatives As Neutrophil Elastase Inhibitors And Their Use
There are provided novel compounds of formula (I), wherein R1, R2, R4, R5, G1, G2, L, Y and n are as defined in the Specification and optical isomers, racemates and tautomers thereof, and pharmaceutically acceptable salts thereof; together with processes for their preparation, compositions containing them and their use in therapy. The compounds are inhibitors of neutrophil elastase.
Owner:ASTRAZENECA AB
Optical recording medium, metal complex compound and organic dye compound
InactiveCN101151164AHigh sensitivityHigh densityMonoazo dyesNickel organic compounds2-PyridoneRecording layer
To provide an optical recording medium having a recording layer improved in light stability that is capable of recording and regeneration of high-density optical information by short-wavelength laser beams. There is provided an optical recording medium comprising a recording layer containing an organic dye compound wherein the recording layer contains a metal complex compound composed of any of pyridone azo compounds of the following general formulae [I] to [III] having a 6-hydroxy-2-pyridone structure as a coupler component and having an isoxazole, 1,2,4-triazole or pyrazole structure as a diazo component and, coordinating therewith, an ion of bivalent metal, such as nickel, cobalt, iron, zinc, copper or manganese. [I] [II] [III] In the general formulae [I] to [III], each of R1 to R10 independently is a hydrogen atom or a monovalent functional group.
Owner:CMC MAGNETICS CORPORATION
2-Pyridone derivatives as neutrophil elastase inhibitors and their use
There are provided novel compounds of formula (I), wherein R1, R2, R4, R5, G1, G2, L, Y and n are as defined in the Specification and optical isomers, racemates and tautomers thereof, and pharmaceutically acceptable salts thereof; together with processes for their preparation, compositions containing them and their use in therapy. The compounds are inhibitors of neutrophil elastase.
Owner:ASTRAZENECA AB
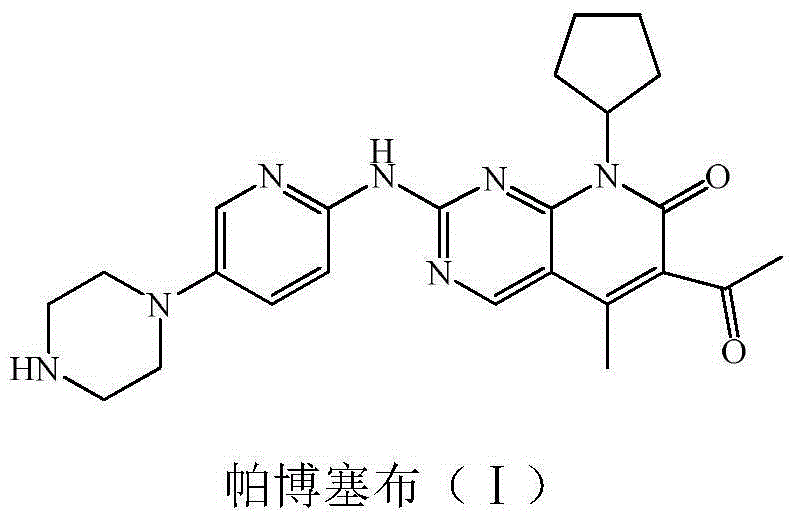
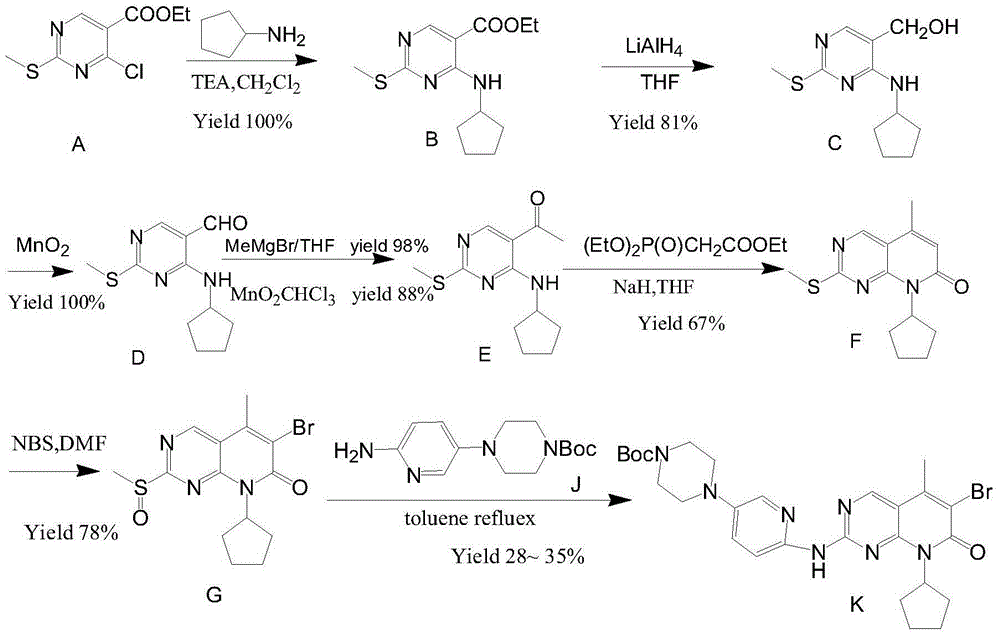
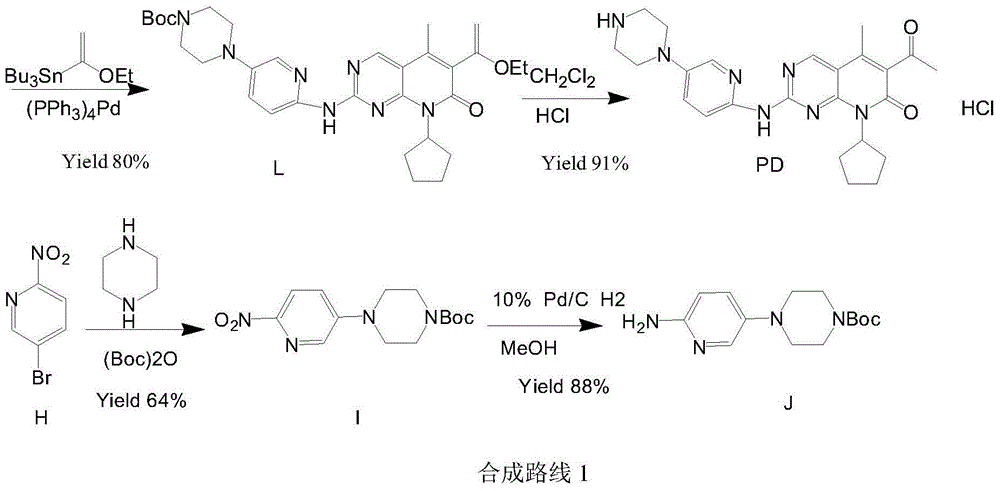
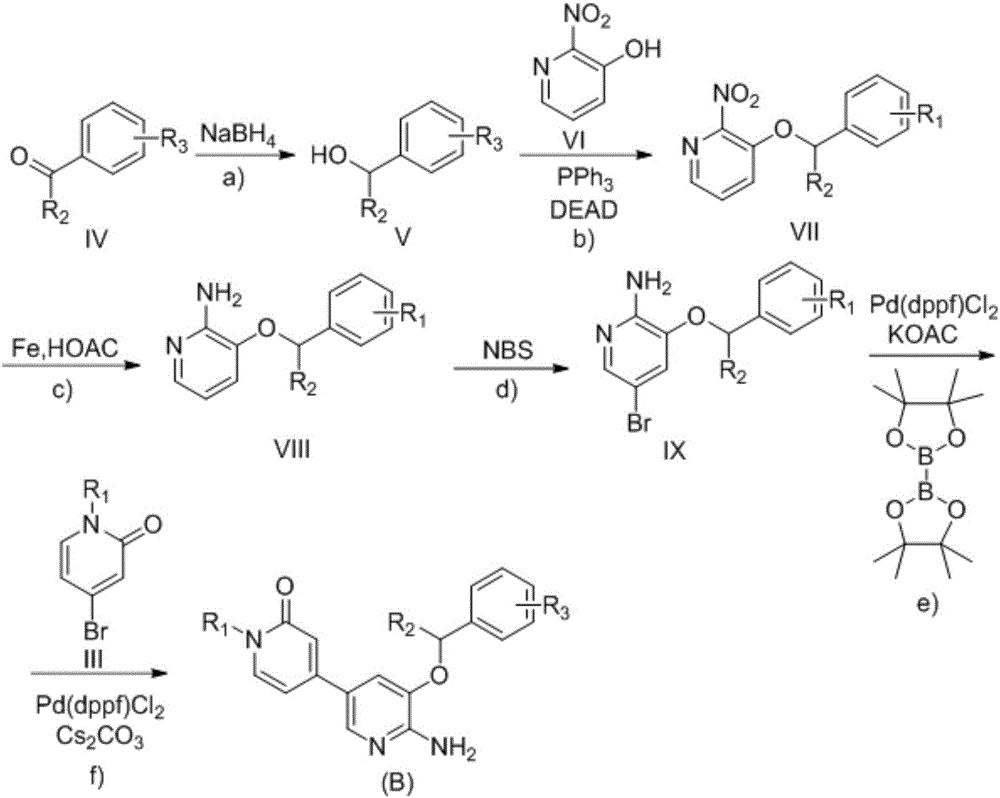
Preparation method of palbociclib
ActiveCN104478874AHigh yieldRaw materials are cheap and easy to getOrganic chemistry2-PyridoneMethyl group
The invention relates to a preparation method of palbociclib. The method comprises the following steps: carrying out N-acetoacetylation reaction by using cyclopentylamine and an acetoacetylation agent to obtain N,N-di(acetoacetyl) cyclopentylamine (II), carrying out intramolecular condensation on N,N-di(acetoacetyl) cyclopentylamine (II) in the presence of an alkaline reagent to obtain N-cyclopentyl-3-acetyl-4-methyl-6-hydroxy-2-pyridone (III), enabling N-cyclopentyl-3-acetyl-4-methyl-6-hydroxy-2-pyridone (III) to react with a formylation reagent to prepare N-cyclopentyl-3-acetyl-4-methyl-5-formyl-6-chlorine-2-pyridone (IV), carrying out pyrimidine ring reaction by using N-cyclopentyl-3-acetyl-4-methyl-5-formyl-6-chlorine-2-pyridone (IV) and N-(5-(4-tert-butoxy carbonyl-1-hexahydropyrazinyl)-2-pyridyl) guanidine sulfate (V), and then hydrolyzing under the alkaline condition to prepare palbociclib. The preparation method of palbociclib is easily available in raw materials, short in process, simple and convenient to operate and safe and environmentally friendly in process.
Owner:XINFA PHARMA
Stable antifouling paint composition containing metal salt of pyrithione and cuprous oxide
The present invention describes a composition for stabilizing a paint or paint base composition against viscosity change, gelation and agglomeration during formulation and storage. Illustratively, an antifouling paint or paint base composition is disclosed that contains a hydrolysable acrylate resin such as copper acrylate, zinc acrylate, or silyl acrylate, a metal salt of pyrithione, cuprous oxide, and a primary stabilizing agent selected from the group consisting of benzoic acid, hydroxypyridine, metal salts of benzoic acid, and hydroxypyridine, with the proviso that said composition is essentially free of 2-hydroxypyridine N-oxide, and is also essentially free of the sodium, zinc and copper salts of 2-hydroxypyridine-N-oxide.
Owner:ARCH CHEM INC
N-sulfonyl-4-methyleneamino-3-hydroxy-2-pyridones
Owner:AERPIO TERAPYUTIKS INK
Luminescent 1-hydroxy-2-pyridinone chelates of lanthanides
ActiveUS8557601B2High quantum yieldImprove solubilityPeptide/protein ingredientsGroup 5/15 element organic compoundsLanthanide2-Pyridone
Owner:RGT UNIV OF CALIFORNIA
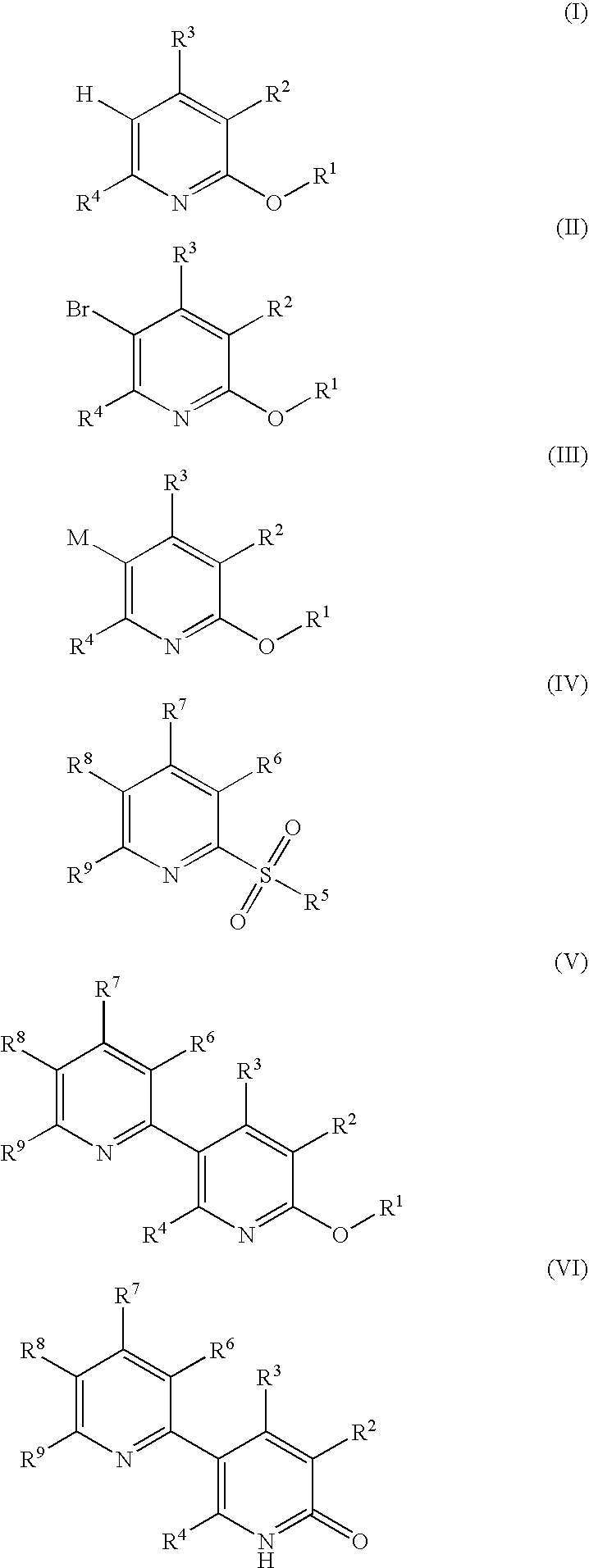
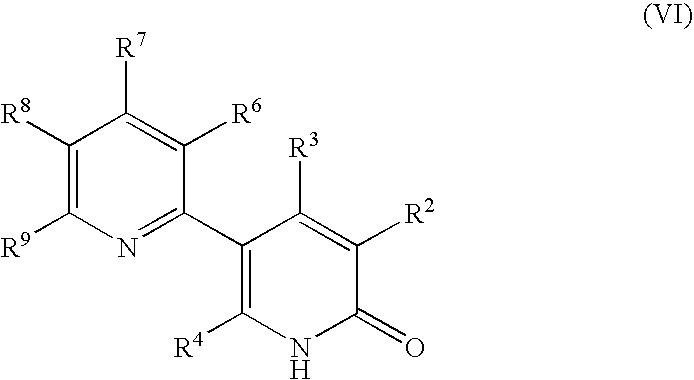
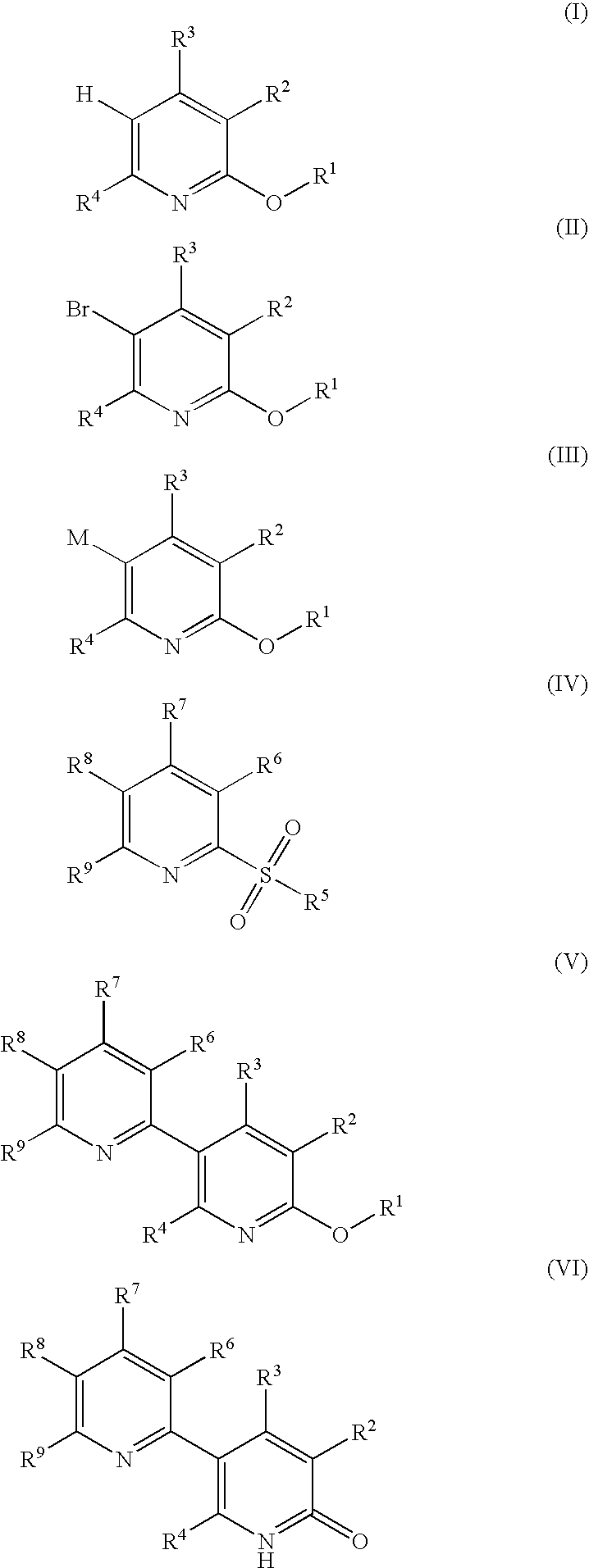
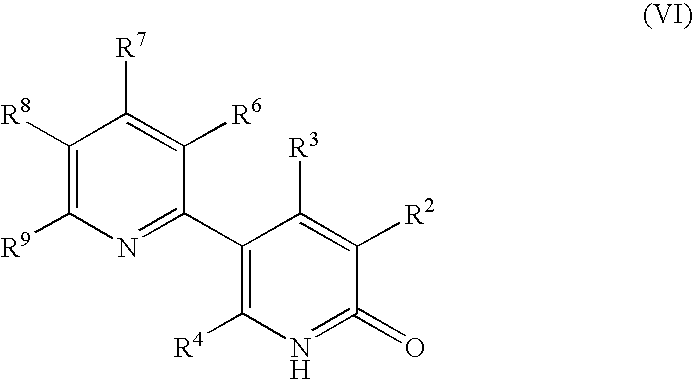
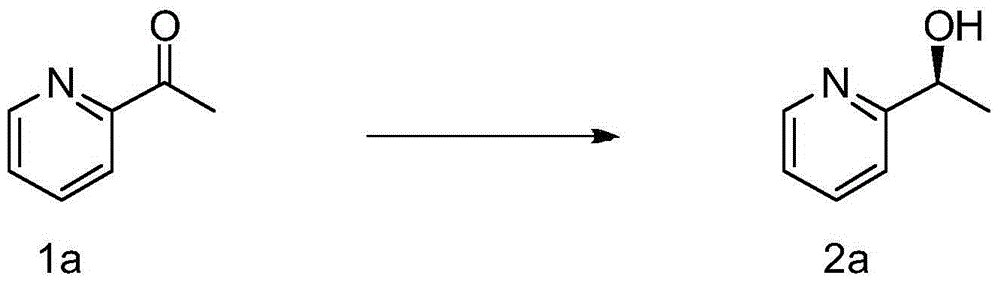
Process for producing 5-(2'-pyridyl)-2-pyridone derivative
The present invention provides a method of industrially and advantageously producing a 5-(2′-pyridyl)-2-pyridone derivative. The present invention relates to a production method of a 5-(2′-pyridyl)-2-pyridone derivative represented by the formula (VI), which includes reacting a pyridine derivative of the formula (I) with a brominating agent to give a 5-bromopyridine derivative of the formula (II), reacting the obtained 5-bromopyridine derivative with a metallizing agent to give an organometallic compound of the formula (III), reacting the obtained organometallic compound with a 2-sulfonylpyridine derivative of the formula (IV) to give a 6-alkoxy-3,2′-bipyridine derivative of the formula (V) and hydrolyzing the obtained 6-alkoxy-3,2′-bipyridine derivative:wherein each symbol is as defined in the Description.
Owner:EISIA R&D MANAGEMENT CO LTD +1
2-pyridone antimicrobial compositions
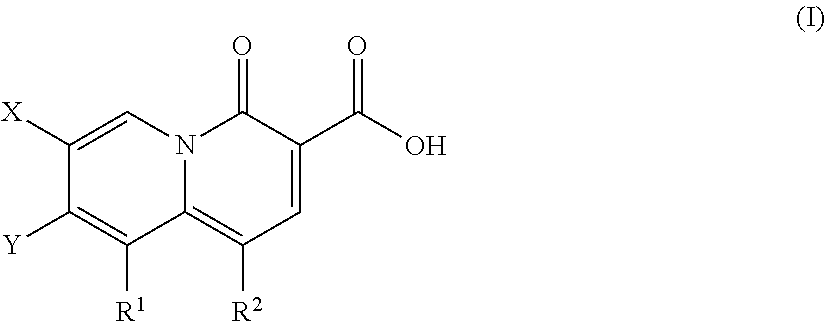
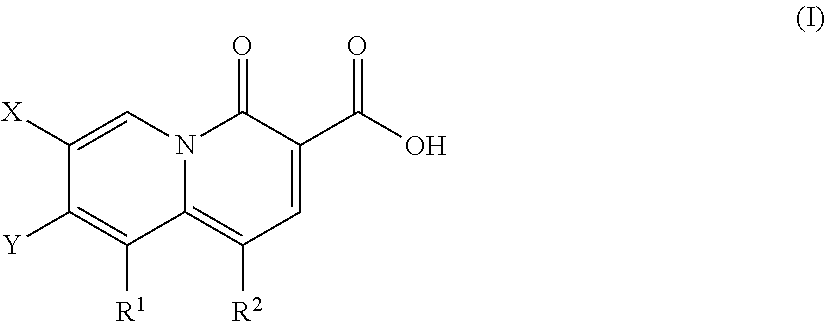
Described are a series of 2-pyridone compounds as a potent and selective new class of type II topoisomerase inhibitors with broad-spectrum antimicrobial activity having the general formula (I); where R1, R2, X, and Y are defined herein Such compounds can be used in methods for treating an infection caused by a gram-positive pathogen, a gram-negative pathogen, or a drug-resistant strains thereof.
Owner:EMERGENT PROD DEV GAITHERSBURG INC
Determination reagent of total bile acid
The invention relates to a determination reagent of a total bile acid. The reagent comprises two components, wherein the reagent 1 comprises a phosphate buffer, Thio-NAD (Nicotinamide Adenine Dinucleotide), Emulgen B-66 CTAB (Cetyltrimethyl Ammonium Bromide), NaCl, 2-hydroxypyridine-N-oxide; the reagent 2 comprises a glycinate buffer, dextran sulfate, EGTA (Ethylene Glycol Tetraacetic Acid), 3 alpha-HSD (Hydrogen Sterol Dehydrogenase), Emulgen B-66, NADH (Nicotinamide-adenine Dinucleotide Acid), and 2-hydroxypyridine-N-oxide. As the combination of the cation surfactant and the nonionic surfactant Emulgen B-66 is added, the interference of bilirubin can be effectively eliminated; the selected stabilizer is added in the product, so that the reagent can be stored for one year at 2-8 DEG C without influence on the properties; the substrate concentration and the ion strength can be adjusted to enable the recovery rate of the reagent to be more than 98%. The determination reagent disclosed by the invention has the advantages of high accuracy in determined results, strong anti-interference performance, good stability and the like.
Owner:武汉利弘生物科技有限责任公司
Process for producing 5-(2'-pyridyl)-2-pyridone derivative
The present invention provides a method of industrially and advantageously producing a 5-(2′-pyridyl)-2-pyridone derivative. The present invention relates to a production method of a 5-(2′-pyridyl)-2-pyridone derivative represented by the formula (VI), which includes reacting a pyridine derivative of the formula (I) with a brominating agent to give a 5-bromopyridine derivative of the formula (II), reacting the obtained 5-bromopyridine derivative with a metallizing agent to give an organometallic compound of the formula (III), reacting the obtained organometallic compound with a 2-sulfonylpyridine derivative of the formula (IV) to give a 6-alkoxy-3,2′-bipyridine derivative of the formula (V) and hydrolyzing the obtained 6-alkoxy-3,2′-bipyridine derivative: wherein each symbol is as defined in the Description.
Owner:EISIA R&D MANAGEMENT CO LTD +1
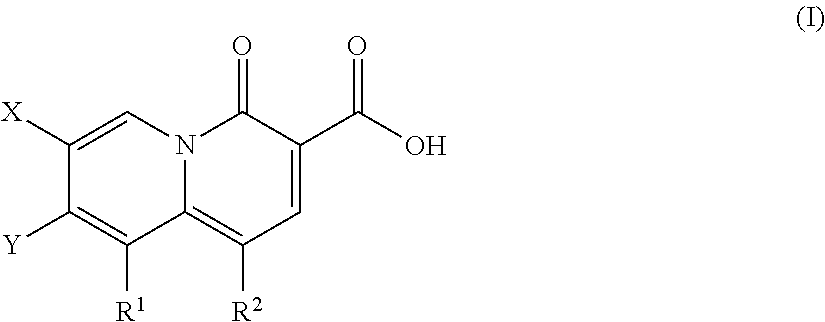
2-pyridone antimicrobial compositions
ActiveUS8962842B2Broad spectrum of antibacterial activityHigh activityAntibacterial agentsBiocideGram2-Pyridone
Described are a series of 2-pyridone compounds as a potent and selective new class of type II topoisomerase inhibitors with broad-spectrum antimicrobial activity having the general formula (I); where R1, R2, X, and Y are defined herein Such compounds can be used in methods for treating an infection caused by a gram-positive pathogen, a gram-negative pathogen, or a drug-resistant strains thereof.
Owner:EMERGENT PROD DEV GAITHERSBURG INC
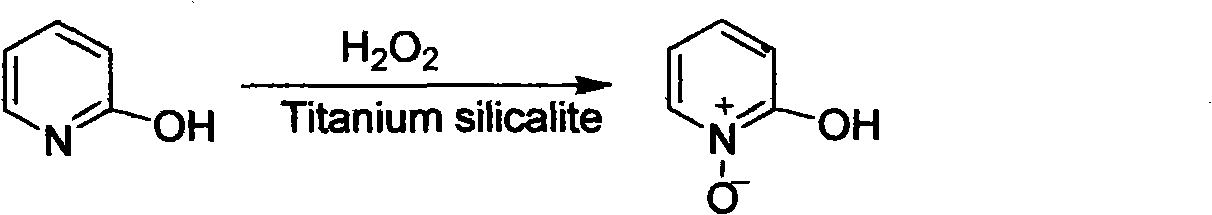
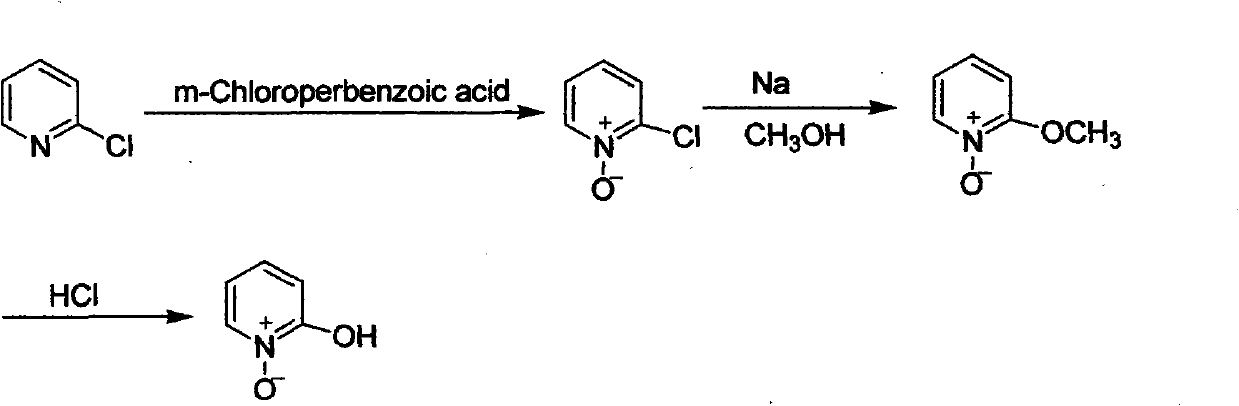
Preparation method of 2-hydroxypyridine-N-oxide
The invention discloses a preparation method of an important intermediate: 2-hydroxypyridine-N-oxide with the CAS Number of 13161-30-3. The invention reports a new synthetic route applicable to industrial production, which comprises the following steps: under catalysis of a catalyst generated in-situ, oxidizing 2-chloropyridine with hydrogen peroxide (30%) to generate an N-oxide, and then hydrolyzing under an alkaline condition to directly generate the target product. The preparation method greatly improves the yield, realizes one-pot reaction, greatly reduces generation of organic waste liquor and realizes environmental protection.
Owner:湖南欧亚药业有限公司
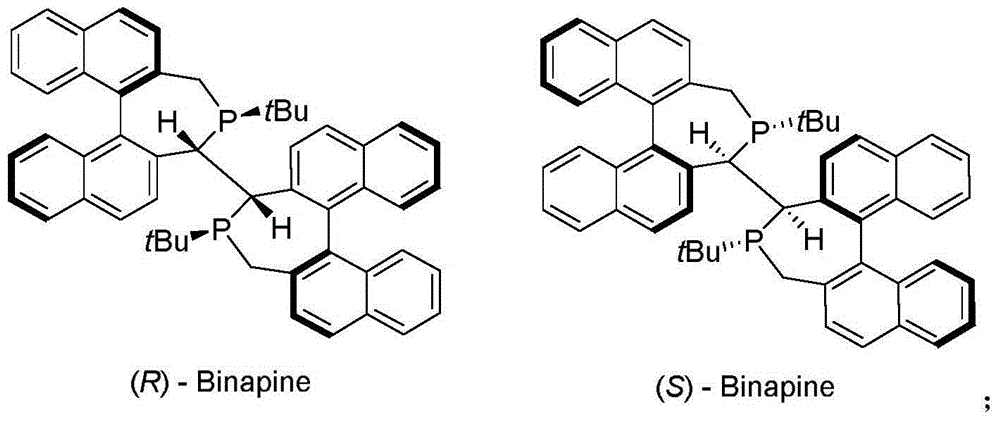
Asymmetric catalytic hydrogenation method for 2-pyridinone compounds
The invention belongs to the chemical field of medicine synthesis, and relates to an asymmetric catalytic hydrogenation method for 2-pyridinone compounds. The asymmetric catalytic hydrogenation method includes steps of firstly, enabling chiral ligand and metal rhodium precursors to react with one another in organic solvents for 0.5-2 hours at the temperatures of 20-40 DEG C to obtain in-situ catalysts; secondly, arranging reaction systems in an autoclave, adding the 2-pyridinone compounds into the autoclave, enabling the reaction systems and the 2-pyridinone compounds to react with one another for 20-24 hours in hydrogen atmosphere at the reaction temperatures of 0-50 DEG C under the hydrogen pressures of 2-50 atmospheric pressures. The chiral ligand is (R)-Binapine. The asymmetric catalytic hydrogenation method has the advantages that the asymmetric catalytic hydrogenation method is high in catalytic efficiency, and products can be quickly obtained within short time under low-pressure conditions; only a few catalysts are utilized; most 2-pyridone substrates can have conversion rates of 99% and the optimal stereoselectivity of 99%, and accordingly the asymmetric catalytic hydrogenation method has an excellent industrial prospect.
Owner:WUHAN UNIV
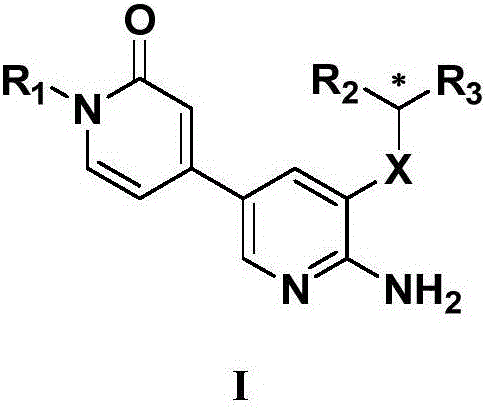
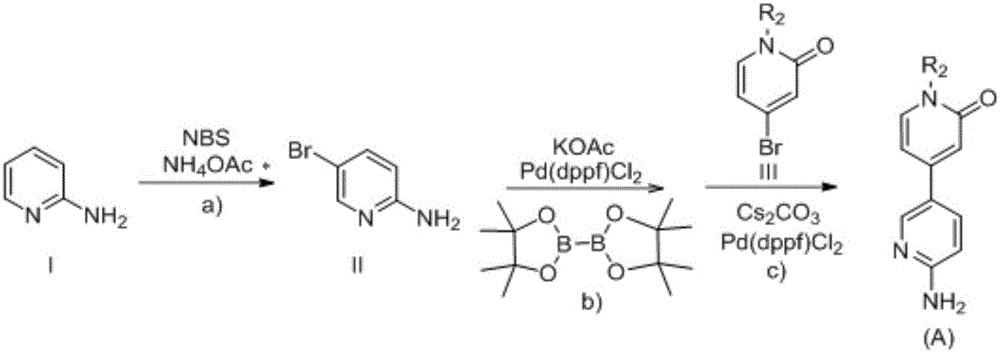
2-aminopyridine derivative containing 2-pyridone ring side chain, preparation and application
InactiveCN106083708AStrong growth inhibitory effectEnhanced inhibitory effectOrganic chemistryAntineoplastic agentsEnantiomerSide chain
The invention provides a 2-aminopyridine derivative containing a 2-pyridone ring side chain or an enantiomer of the 2-aminopyridine derivative. The target compound is obtained mainly by taking 2-aminopyridine as a parent nucleus to be coupled with a 4-bromo-2-pyridone ring. Experiments prove that the 2-aminopyridine derivative has a significant proliferation inhibition effect on tumor cells Karpas 299 (NPM-ALK positive cell lines) related to the ALK tyrosine kinase activity, NCI-H2228 (EML4-ALK positive cell lines), SKN-BE2 (ALK gene amplification cell lines) and SH-SY5Y (ALK F1174 mutant cell lines) and can be prepared into corresponding anti-tumor-cell drugs. The 2-aminopyridine derivative has a general structural formula I (please see the formula in the description).
Owner:ZHEJIANG UNIV
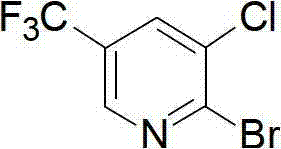
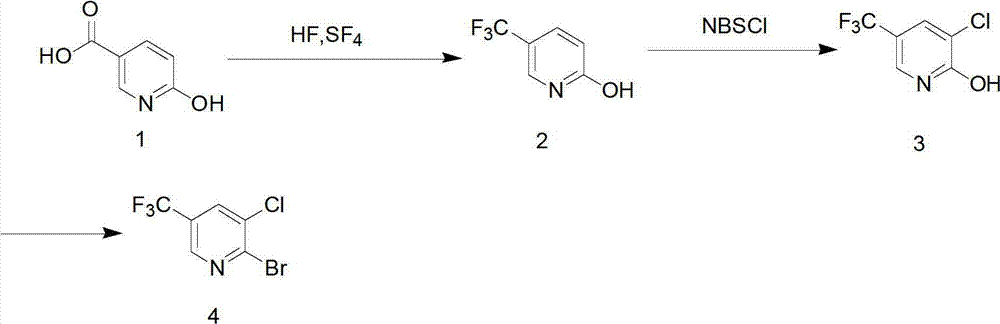
Preparation method for pyridine medical intermediate for synthesizing anti-cancer auxiliary medicines
The invention relates to the field of medical chemistry and discloses a method for synthesizing a pyridine medical intermediate, namely 2-bromo-3-chloro-5-trifluoromethyl pyridine, for synthesizing anti-cancer auxiliary medicines. The method comprises the following steps of: (1) reacting 6-hydroxynicotinic acid, hydrofluoric acid and sulfur tetrafluoride at the temperature of between 100 and 120DEG C and under the pressure of 0.1-0.3MPa, and adding water to obtain 2-hydroxy-5-trifluoromethyl pyridine; (2) reacting with N-chlorosuccinimide, and performing water precipitation to obtain 3-chloro-5-trifluoromethyl-2-hydroxypyridine; and (3) adding excessive phosphorus oxybromide, reacting at the temperature of between 145 and 160DEG C for 5 to 8 hours, cooling, violently stirring at the temperature of between -5 and 0DEG C, extracting, combining organic phases, drying, filtering, performing spin drying, and purifying by using a silica gel column. According to the method, raw materials are readily available, the cost is low, the method is suitable for industrial production and the yield exceeds 38 percent.
Owner:上海泰坦科技股份有限公司
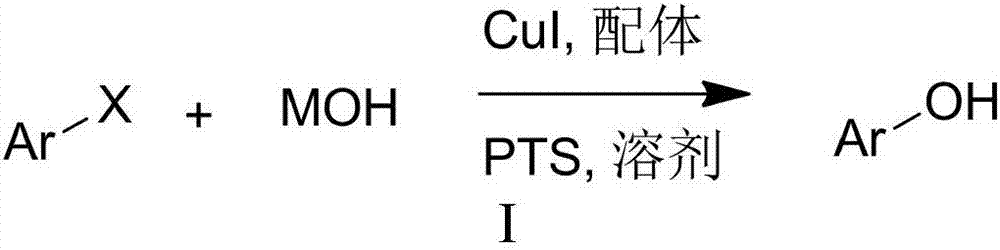
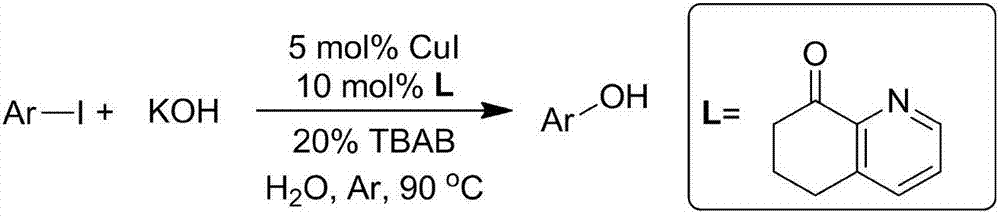
Hydroxylation method of halogenated aromatic compound
ActiveCN107253894APromote hydroxylation reactionHigh yieldOrganic compound preparationHydroxy group formation/introductionBromineReaction temperature
The invention relates to a hydroxylation method of a halogenated aromatic compound. The method takes a 2-pyridone compound as a ligand additive and takes CuI as a catalyst, under existence of a phase-transfer catalyst and a solvent, MOH and the halogenated aromatic compound are subjected to a hydroxylation reaction under mild condition, the reaction yield is high, and an application scope of a substrate is wide. Compared with same types of reactions reported in literature, the reaction condition of the provided method is mild, the yield is high, and the method has good application prospect. the hydroxylation reaction of an iodo aromatic compound can be carried out in an aqueous solution at the temperature of 90 DEG C, and a hydroxylated product with high yield can be obtained, the reaction temperature is averagely reduced by about 30 DEG C by comparing with a report in the literature; the hydroxylation reaction of a brominated aromatic compound can be carried out in the aqueous solution at the temperature of 120 DEG C, and the reaction temperature is averagely reduced by about 20 DEG C by comparing with the report in the literature.
Owner:HENGYANG NORMAL UNIV
2-pyridone compounds
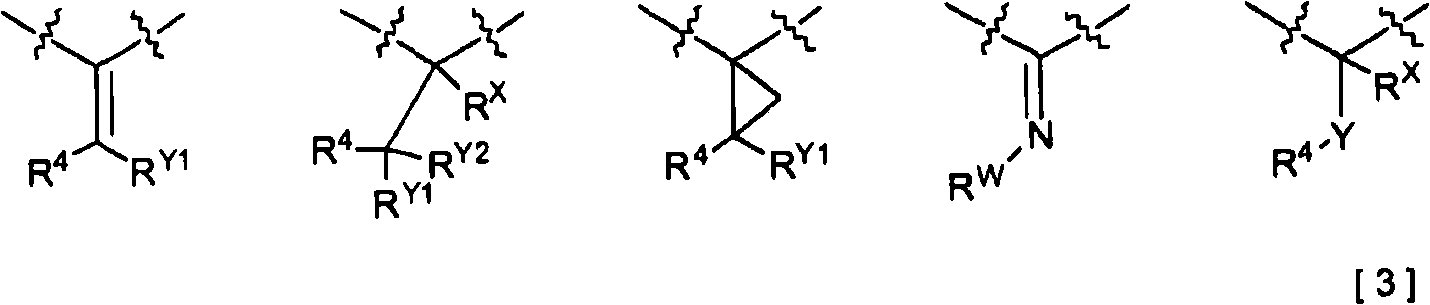
2-Pyridone compounds represented by general formula [1], tautomers or stereoisomer thereof, pharmaceutically acceptable salts of the same, or solvates thereof have excellent GK-activating activity and therefore are useful as drugs. In general formula [1], A is a benzene ring or a pyridine ring; X is a structure represented by general formula [3]; V is a single bond or lower alkylene; W is a single bond, an ether linkage, or lower alkylene (which may contain an ether linkage).
Owner:NISSAN CHEM IND LTD
1,4-dyhydroxy-6-methyl-2-pyridine ketone compound, and preparation method and use thereof
InactiveCN103360308AHas anti-HIV effectReduce phototoxicityAntibacterial agentsOrganic active ingredientsKetone2-Pyridone
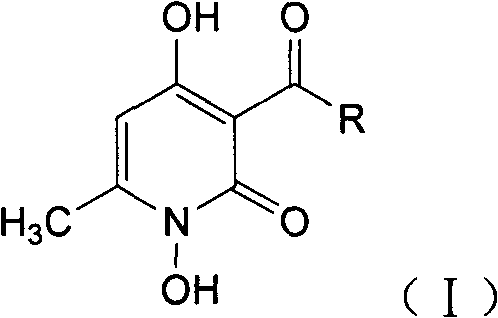
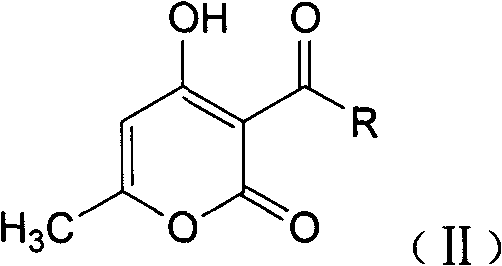
A 1,4-dihydroxy-6-methyl-2-pyridone compound having structural formula (I), and a preparation method and application thereof are provided. The compound has good antibacterial activity in vitro against bacteria such as staphylococcus aureus, streptococcus, Escherichia coli, and has a stronger antibacterial activity against Gram-positive bacteria than against Gram-negative bacteria; and also has good antibacterial activity against fungi such as Candida albicans and Candida Krusei, so that the compound can be used as a drug for preventing and curing bacterial and fungal infections.
Owner:孙伟新 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
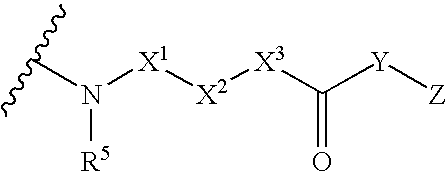
![5-Substituted-4-[(substituted phenyl) amino]-2-pyridone derivatives 5-Substituted-4-[(substituted phenyl) amino]-2-pyridone derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f0737eb4-9c61-41d7-9624-43facbeed6a7/US20050026964A1-20050203-C00001.png)
![5-Substituted-4-[(substituted phenyl) amino]-2-pyridone derivatives 5-Substituted-4-[(substituted phenyl) amino]-2-pyridone derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f0737eb4-9c61-41d7-9624-43facbeed6a7/US20050026964A1-20050203-C00002.png)
![5-Substituted-4-[(substituted phenyl) amino]-2-pyridone derivatives 5-Substituted-4-[(substituted phenyl) amino]-2-pyridone derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f0737eb4-9c61-41d7-9624-43facbeed6a7/US20050026964A1-20050203-C00003.png)
![5-substituted-4-[(substituted phenyl) amino]-2-pyridone derivatives 5-substituted-4-[(substituted phenyl) amino]-2-pyridone derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8141f24f-19d1-4c62-a1dc-7ec164b1b880/US07273877-20070925-C00001.png)
![5-substituted-4-[(substituted phenyl) amino]-2-pyridone derivatives 5-substituted-4-[(substituted phenyl) amino]-2-pyridone derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8141f24f-19d1-4c62-a1dc-7ec164b1b880/US07273877-20070925-C00002.png)
![5-substituted-4-[(substituted phenyl) amino]-2-pyridone derivatives 5-substituted-4-[(substituted phenyl) amino]-2-pyridone derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8141f24f-19d1-4c62-a1dc-7ec164b1b880/US07273877-20070925-C00003.png)